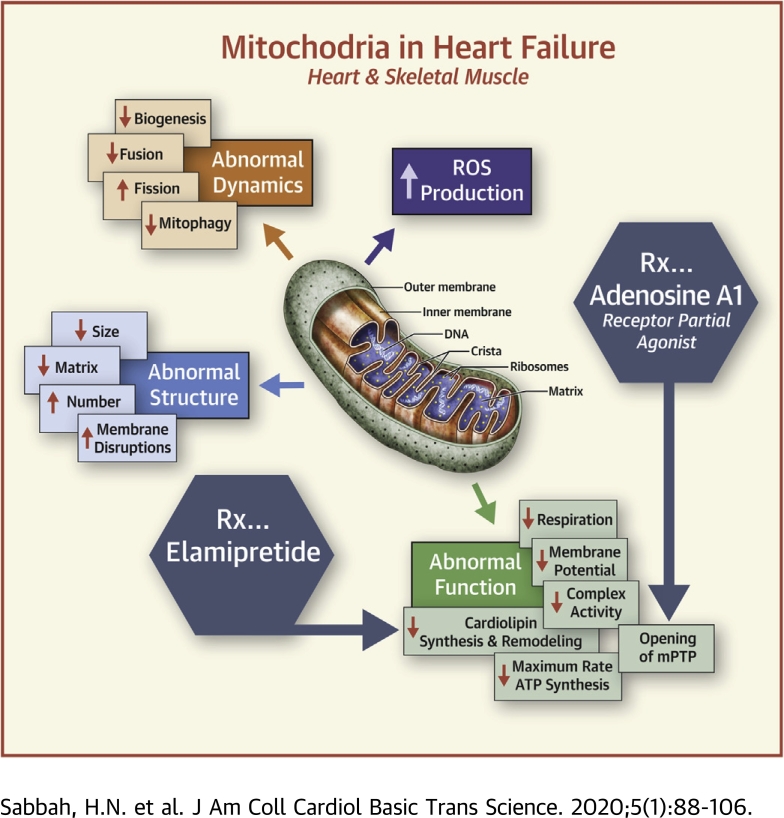
Visual Abstract
Key Words: cardiolipin, heart failure, mitochondria, myocardial energetics, oxidative phosphorylation
Abbreviations and Acronyms: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CI (to V), complex I (to V); Drp, dynamin-related protein; ETC, electron transport chain; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; Mfn, mitofusin; MPTP, mitochondrial permeability transition pore; mtDNA, mitochondrial deoxyribonucleic acid; OPA, optic atrophy; PGC, peroxisome proliferator-activated receptor coactivator; PINK, phosphatase and tensin homolog–inducible kinase; ROS, reactive oxygen species; TAZ, tafazzin
Highlights
-
•
Cardiac energy deprivation due to mitochondrial dysfunction is characteristic of heart failure.
-
•
Mitochondrial dysfunction contributes to worsening of the heart failure state.
-
•
Pharmacologic targeting of mitochondria in heart failure is an unmet need.
-
•
Mitochondrial dysfunction in heart failure can be reversed with novel experimental drugs.
Summary
The burden of heart failure (HF) in terms of health care expenditures, hospitalizations, and mortality is substantial and growing. The failing heart has been described as “energy-deprived” and mitochondrial dysfunction is a driving force associated with this energy supply-demand imbalance. Existing HF therapies provide symptomatic and longevity benefit by reducing cardiac workload through heart rate reduction and reduction of preload and afterload but do not address the underlying causes of abnormal myocardial energetic nor directly target mitochondrial abnormalities. Numerous studies in animal models of HF as well as myocardial tissue from explanted failed human hearts have shown that the failing heart manifests abnormalities of mitochondrial structure, dynamics, and function that lead to a marked increase in the formation of damaging reactive oxygen species and a marked reduction in on demand adenosine triphosphate synthesis. Correcting mitochondrial dysfunction therefore offers considerable potential as a new therapeutic approach to improve overall cardiac function, quality of life, and survival for patients with HF.
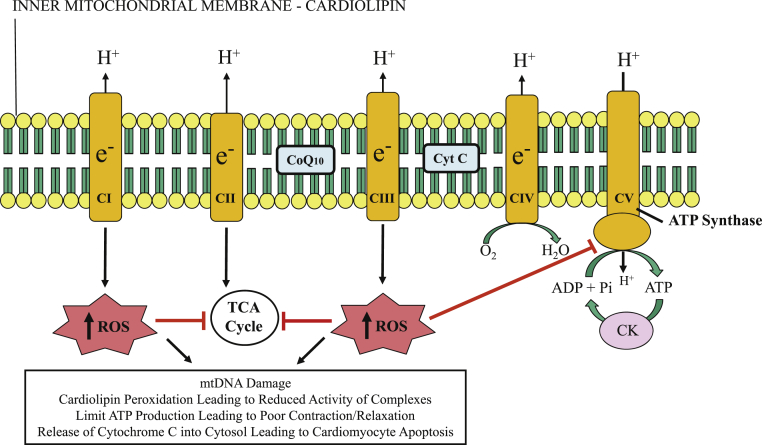
Mitochondria are intracellular double-membraned organelles that are considered the “power houses” of eukaryotic cells and, as such, are most abundant in cardiac muscle cells and in skeletal muscle type-1 fibers, where high-energy–requiring processes take place. The heart, being the most metabolically active organ in the body, possesses the highest content of mitochondria of any tissue (1), comprising about 25% of cell volume in human myocardium 2, 3. The primary role of mitochondria is the generation of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) using macromolecular complexes that form the electron transport chain (ETC): nicotinamide-adenine dinuculeotide dehydrogenase (complex I [CI]), succinate dehydrogenase (CII), cytochrome bc1 (CIII), and cytochrome c oxidase (CIV) (4). Protons (H+) are pumped from the matrix to the intercristae space during these reactions, creating a proton gradient; ATP synthesis from inorganic phosphate and ADP is driven by the enzyme ATP synthase (CV) as a result of protons diffusing back along this gradient (Figure 1) 5, 6. The coupling of substrate oxidation and ATP formation in the mitochondria (oxidative phosphorylation) is central to tissue and organ health (4). Cardiolipin is a key phospholipid expressed exclusively on the inner mitochondrial membrane that is required for ETC activity and is especially important for anchoring soluble cytochrome c to the inner mitochondrial membrane to facilitate electron transfer from CIII to CIV (7).
Figure 1.
Mitochondrial Inner Membrane and Electron Transport Chain
Depiction of mitochondrial inner membrane and electron transport chain consisting of complexes I through V (CI to CV). Reactive oxygen species (ROS) are generated at CI and CIII. Excessive ROS production can lead to mitochondrial and cardiomyocyte dysfunction by inhibiting the tricarboxylic acid (TCA) cycle enzymes and adenosine triphosphate (ATP) synthase, and by damaging mitochondrial deoxyribonucleic acid (mtDNA). CK = creatine kinase; CoQ10 = coenzyme Q10; Cyt C = cytochrome c; e− = electrons; Pi = inorganic phosphate.
Reprinted with permission from Sabbah (6) and adapted with permission from Okonko and Shah (5).
Humans produce and consume about 65 kg of ATP every day, with the heart accounting for about 8% of ATP consumption daily or about 6 kg (8). About 90% of cellular ATP within the myocardium is used to meet the enormous energy requirements for contraction and relaxation (both active processes and both ATP-dependent) (9). Mitochondrial dysfunction therefore plays a central role in a wide variety of metabolic and cardiac disorders, including heart failure (HF) (10). Dysfunctional mitochondria in skeletal muscle has been implicated in HF-associated exercise intolerance (11) and in the pathology of chronic metabolic disorders such as obesity and type 2 diabetes 12, 13.
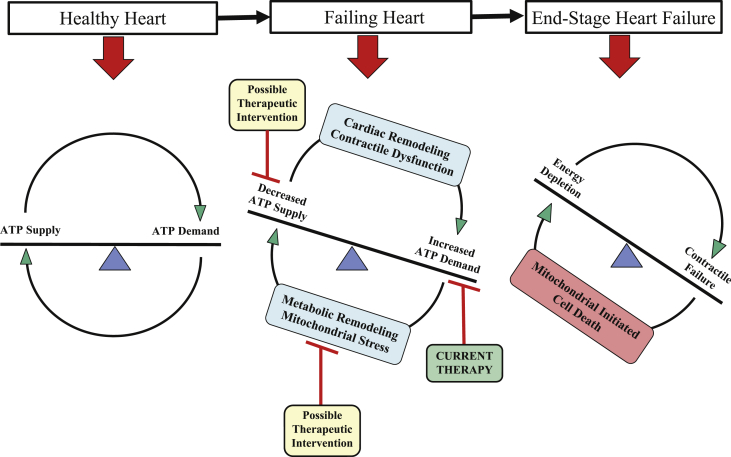
Because ATP cannot be stored, it is critical that the rate of ATP synthesis matches the rate of ATP consumption on a beat-to-beat basis (14). This process is accomplished by mitochondrial oxidative phosphorylation within the ETC using fatty acids as the primary fuel source (15). Although there are numerous reasons why a human heart can fail, the worsening of the HF state can be attributed, in part, to a mismatch between ATP supply and demand, also described as an engine “out of fuel” (8). Pathologic left ventricular (LV) remodeling including chamber dilation and hypertrophy causes inefficiencies that increase energy demand but concomitantly reduce the capacity for energy supply (Figure 2) (14). The subsequent altered bioenergetics attempt to regain energy homeostasis in the failing heart and are characterized by changes in substrate utilization, including an initial up-regulation and subsequent reduction of glucose utilization and a decrease in fatty acid utilization (14). Changes in oxidative phosphorylation are characterized by decreased energy production, with reductions in oxygen utilization, and respiratory-chain and ATP synthase activity. Changes in high-energy phosphate metabolism associated with HF include impaired creatine kinase energy-transfer mechanism, increased free ADP levels, and, in advanced HF, reduced ATP content (8). Mitochondrial dysfunction also contributes to skeletal muscle performance limitations by reducing the ability of the mitochondria to meet the ATP demand of aerobic, slow-twitch, fatigue-resistant working muscles (16). Lack of availability of energy at rest and during activity leads to progressive LV dysfunction and worsening of the HF state along with abnormalities of skeletal muscle composition that culminates in exercise intolerance.
Figure 2.
Imbalance Between Energy Supply and Demand in the Development of HF
Schematic of the imbalance between energy supply and demand in the development of heart failure (HF). ATP = adenosine triphosphate.
Reprinted with permission from Zhou and Tian (14).
Current treatments for chronic HF such as beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor antagonists, and aldosterone antagonists rely primarily on energy-sparing maneuvers such as heart rate reduction and reductions of preload and afterload that help decrease overall cardiac workload for their therapeutic effect. Few strategies that can potentially increase ATP supply have emerged recently and are likely to undergo clinical assessment in the short term. The aim of this review is to describe the role and specific pathophysiologic mechanisms of mitochondria dysfunction in HF, as well as potential agents that target mitochondrial dysfunction in HF.
Abnormalities of Mitochondria in the Failing Heart
Ultrastructural abnormalities
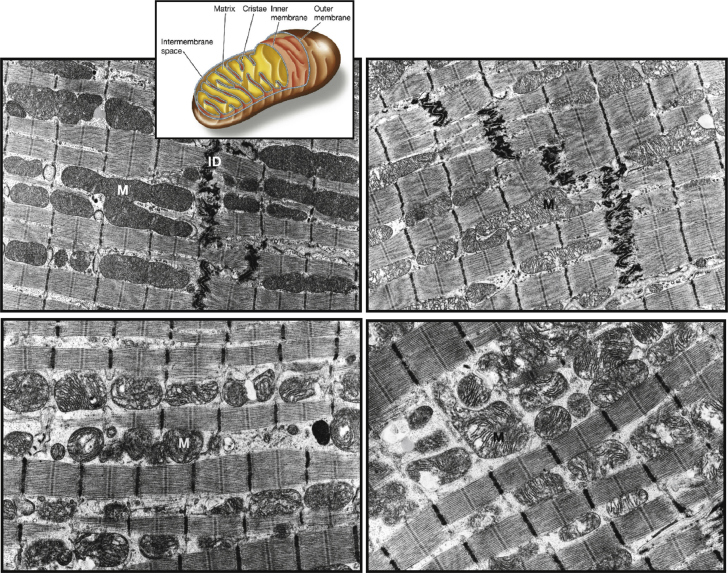
It is well known that structural abnormalities of mitochondria in HF are present in animal models and in humans. These abnormalities include hyperplasia, reduced organelle size or fragmentation, and structural injury including loss of electron-dense matrix and disruption of inner and outer membranes (Figure 3) 17, 18, 19. In dogs with experimentally induced HF, there was a significant increase in the overall number of interfibrillar mitochondria (hyperplasia) and a significant decrease in the overall average size of mitochondria relative to normal dogs (17), resulting in impaired mitochondrial respiratory activity (18). Abnormal mitochondrial morphology is also observed in cardiomyocytes of humans with HF 20, 21, 22. Human ischemic and idiopathic dilated cardiomyopathy are associated with functional mitochondrial abnormalities characterized by impaired respiration that can potentially adversely affect overall energy production and contribute to myocardial energy depletion (19).
Figure 3.
LV Mitochondria in Normal Dogs and Dogs With HF
Transmission electron micrographs of left ventricular (LV) mitochondria in normal dogs and dogs with heart failure (HF). (Top left) Normal dog showing predominantly normal, large mitochondria with tightly packed cristae and electron-dense matrix, with insert depicting various structural components of mitochondria. (Top right) Coronary microembolization-induced HF showing mild abnormalities of mitochondria in the form of clearance of electron-dense matrix. (Bottom left) Coronary microembolization-induced HF showing moderate abnormalities of mitochondria in the form of reduced organelle size and marked disorganization of cristae. (Bottom right) Coronary microembolization-induced HF showing severe mitochondrial injury with inner and outer membrane disruption and myelinization. ID = intercalated disk; M = mitochondrion.
Reprinted with permission from Sabbah (6) and adapted with permission from Sabbah et al. (17).
In dogs with HF, mild to severe mitochondrial injury, ranging from electron-dense matrix depletion to myelinization, and mitochondrial membrane disruption, was observed in 27% of mitochondria compared with only 3% in normal dogs (p < 0.001) (17). Electron micrographs showed predominantly large mitochondria with tightly packed cristae and electron-dense matrix in cardiac tissue from normal dogs, whereas tissue from dogs with HF showed clearance of mitochondrial matrix and marked disorganization of mitochondrial cristae and severe mitochondrial injury including inner and outer membrane disruption and myelinization (17). Accordingly, the overall mitochondrial injury index (ratio of the number of injured mitochondria to the total number of mitochondria multiplied by the average score of the injury) was significantly higher in dogs with HF versus normal dogs. The extent of mitochondrial injury and injury index was similar across all the regions of the heart assessed (LV free wall, interventricular septum, and right ventricular free wall), and also correlated with increased plasma norepinephrine, suggesting a role for sympatho-adrenergic hyperactivity in mitochondrial dysfunction in HF (17).
Mitofilin, an inner mitochondrial membrane structural protein, maintains cristae morphology and structure. Down-regulation of mitofilin in HeLa cells using specific small interfering ribonucleic acid resulted in mitochondria with abnormal morphology characterized by disorganized inner membranes, increased apoptosis, elevated reactive oxygen species (ROS) production, and overall loss of mitochondrial function and structure (23). Mitofilin levels were also reported to be significantly reduced in LV tissues from explanted failed human hearts of both ischemic and idiopathic dilated cardiomyopathic etiologies compared with normal donor hearts (24).
Cardiolipin, a key phospholipid located in the inner mitochondrial membrane, is required to support energy production. It functions as a cofactor for mitochondrial transport proteins, including stabilization of supercomplexes (CI, CIII, CIV, and CV) and mitochondrial cristae and retention of cytochrome c to the inner mitochondrial membrane 25, 26. Cytochrome c is a key electron carrier for electron transport and also triggers cellular apoptosis when released from mitochondria into the cellular cytoplasm. Peroxidation of cardiolipin leads to the release of cytochrome c into the cytoplasm, a process that triggers cellular apoptosis by activation of caspase-3 27, 28. Loss of cardiolipin in HF amplifies the production of ROS, which, in turn, aggravates the peroxidation of cardiolipin. This vicious cycle leads to further mitochondrial dysfunction and ultimately to cardiomyocyte death (26). Cardiolipin is also a key regulator of mitochondrial biogenesis and fission and fusion dynamics. In dogs with HF, compared with normal dogs, cardiolipin synthase-1, a key enzyme in the synthesis of cardiolipin was significantly reduced (29). The same study in dogs with HF also showed that the cardiolipin remodeling enzymes tafazzin-1 (TAZ-1) and acyl-CoA:lysocardiolipin acyltransferase-1 are dysregulated in HF. Specifically, TAZ-1 was significantly reduced and acyl-CoA:lysocardiolipin acyltransferase-1 was significantly increased in dogs with HF compared with normal dogs (29). It is well known that certain diseases arise from genetic mutations that adversely impact the ability of cardiolipin to perform key functional processes. Barth syndrome, for example, is caused by genetic mutations of TAZ-1 and is clinically manifested as cardiomyopathy, skeletal myopathy, neutropenia, and growth retardation (25). Cardiolipin deficiency has been observed in both animal models of HF and in explanted failed human hearts 30, 31, 32. Gene expression of TAZ-1 and cytidine diphosphate-diacylglycerol synthetase-2, a rate-limiting enzyme in cardiolipin biosynthesis, were shown to be significantly reduced in failing relative to nonfailing human hearts (30).
Abnormalities of mitochondrial dynamics
Mitochondrial biogenesis
Mitochondria have their own deoxyribonucleic acid (mtDNA) with a genetic code that is distinct from the host-cellular DNA. Mutations in mtDNA cause a variety of disorders characterized by altered energy homeostasis (reduced ATP synthesis) in high-energy tissues such as cardiac muscle. Mitochondrial turnover or biogenesis and respiration in both skeletal and cardiac muscle are regulated by the transcriptional factor peroxisome proliferator-activated receptor coactivator (PGC)-1α and PGC-1β 33, 34. Expression of these key regulators of energy metabolism is reduced in animal models of HF 35, 36, 37, 38. PGC-1α was significantly down-regulated in LV myocardium of dogs with HF, and phosphorylation was markedly reduced, a dysregulation suggestive of abnormal biogenesis (39). Patients with HF of diverse etiologies also showed reductions in mtDNA contents accompanied by reductions in mtDNA-encoded proteins. Impaired mitochondrial biogenesis was also reported in failing human hearts, which is possibly the result of defective mtDNA replication (40). Down-regulation of estrogen-related receptor α is required for the activation of mitochondrial genes and increased mitochondrial biogenesis was also observed as well as reduced expression of a number of mitochondrial genes under transcriptional control of estrogen-related receptor α. DNA microarray alterations in PGC-1α and estrogen-related receptor α target gene sets correlated with LV ejection fraction and predicted failing versus nonfailing genetic phenotypes (41). The observations overall suggest that mitochondrial biogenesis signaling is reduced in HF and implicate the transcriptional energy metabolic regulator PGC-1α and its target genes in the pathogenesis of this maladaptation.
Mitochondrial fusion and fission
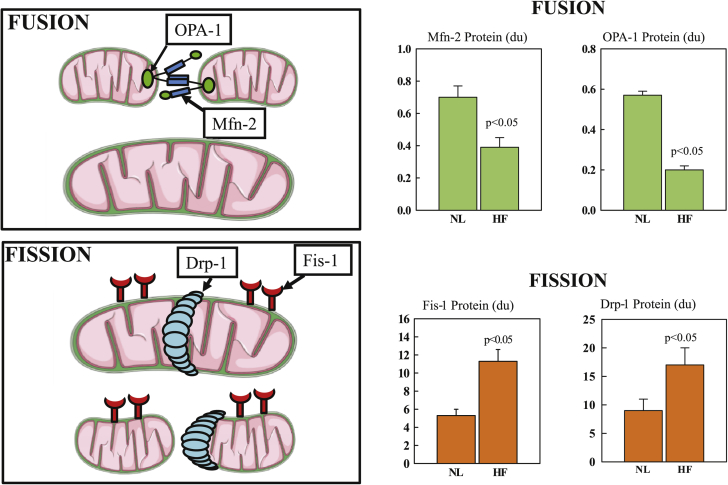
The dynamic physiological process of mitochondrial biogenesis is governed, in part, by fusion and fission of the organelles (Figure 4) (24). Mitochondria undergo fusion to form elongated interconnected mitochondrial networks and fission to produce discrete fragmented mitochondria (42). These processes, both regulated by specific mitochondrial fission and fusion proteins, ensure maintenance of mtDNA integrity by eliminating mitochondria with damaged DNA and promoting functional mtDNA content (6). Mitochondrial fission is facilitated by dynamin-related protein (Drp)-1 and fission (Fis)-1 and ensures equal division of mitochondrial numbers during cell division and mediates removal of damaged mitochondria (mitophagy) 29, 43. Mitochondrial fusion, mediated primarily by mitofusin (Mfn)-2 in the outer membrane and by optic atrophy (OPA)-1 in the inner membrane, allows mixing of intra-mitochondrial proteins and replacement of damaged mtDNA 29, 43. Fusion maintains the structural integrity of the inner mitochondrial membrane and matrix, preserving oxidative phosphorylation and avoiding formation of cytotoxic oxidizing molecules generated via interruption of the ETC (43). In animal models of HF, Mfn-1 and Mfn-2 ablation results in a lethal cardiomyopathy 44, 45, whereas a reduction of OPA-1 expression with short hairpin ribonucleic acid results in increased apoptosis and fragmentation of the mitochondria and overexpression of OPA-1 that results in increased mitochondrial tubularity (46). Mfn-1 or OPA-1 ablation induces LV hypertrophy 47, 48. In LV myocardium of dogs with chronic HF, Mfn-2 and OPA-1 were significantly down-regulated whereas Drp-1 and fission-1 were significantly up-regulated, indicating a marked dysregulation of fission and fusion dynamics (Figure 4) (24). Measures of mitochondrial dynamics in humans with HF (LV tissue from 12 explanted failing human hearts, 6 each failing due to ischemic and nonischemic etiologies, with comparisons made with LV tissue from 6 normal donor hearts) were consistent with those observed in dogs with experimentally induced HF (24). PGC-1α protein levels were significantly lower in the failing hearts (both etiologies) relative to the healthy donor hearts, and a marked dysregulation in the mitochondrial fission and fusion proteins (fission-1 and Drp-1 were significantly increased and Mfn-2 and OPA-1 were significantly decreased), and of the mitochondrial inner membrane protein mitofilin (which was significantly decreased) (24).
Figure 4.
Fusion- and Fission-Mediating Proteins in Normal and HF Dogs
(Left) Fusion-mediating proteins include dominant optic atrophy (OPA)-1 and mitofusin (Mfn)-2 and fission-mediating proteins include fission (Fis)-1 and dynamin-related protein (Drp)-1. (Right) Levels of fusion-mediating proteins and fission-mediating proteins in left ventricular myocardium of normal dogs (NL) and dogs with coronary microembolization–induced heart failure (HF). Data are shown as mean ± SEM. du = densitometric units.
Adapted with permission from Sabbah et al. (24).
Mitophagy
Mitophagy is a central step in maintaining the mitochondrial quality control process and overall health of the cellular mitochondrial pool by removing mitochondria with damage too severe for correction through biogenic or fusion-mediated repair (Figure 5) (49). Mitochondrial fission suppression accelerates mitophagy by lowering the threshold for mitochondrial removal, a maladaptation likely to promote the elimination of functioning mitochondria, whereas mitochondrial fusion inhibition suppresses mitophagy, thus reducing the removal of toxic, ROS-producing mitochondria. Studies in mitochondrial fission- and fusion-defective murine hearts and cells showed that Drp-1–mediated mitochondrial fission is essential to properly target mitophagy and restrain mitochondrial permeability transition pore (MPTP)-mediated cell necrosis (50). Mfn-1 and/or Mfn-2 deletion caused accumulation of defective mitochondria without appropriately increasing mitophagy, whereas Drp-1 ablation interrupted mitochondrial fission by increased mitophagy, causing a generalized loss of mitochondria (50). MPTP opening in Drp-1–null mitochondria was associated with mitophagy, cardiomyocyte necrosis, and dilated cardiomyopathy (50). As eluded to earlier, reduced myocardial levels of fusion-regulating proteins and increased levels of fission-regulating proteins are present in LV myocardium of dogs and humans with HF 29, 51. These observations underscore the importance of the ability of mitochondria in the myocardium to serially execute fission and fusion followed by mitophagy, which is essential for maintenance of mitochondrial health and survival.
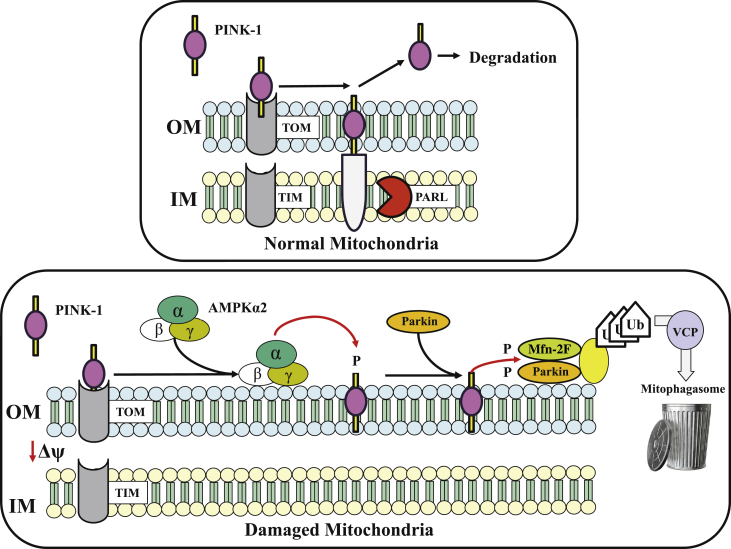
Figure 5.
Mitophagy Signaling in Normal Healthy and Damaged Mitochondria
Schematic diagrams depicting mitophagy signaling in normal healthy mitochondria (top) and in damaged mitochondria (bottom). Activation of phosphatase and tensin homolog–inducible kinase (PINK)-1 by adenosine monophosphate–activated protein kinase (AMPK) α-2 regulation of PINK-1. (Top) Import of PINK-1 into healthy mitochondria via translocase of the outer membrane–translocase of the inner membrane (TOM/TIM) import complex. PINK-1 undergoes proteolytic cleavage by presenilins-associated rhomboid-like (PARL) and cleaved PINK-1 retro-translocates to the cytosol where it is degraded by proteasome. (Bottom) When mitochondria are damaged, import of PINK-1 is abrogated and it accumulates on the outer membrane, which leads to its phosphorylation by AMPKα2. Phosphorylated PINK-1 recruits the E3 ubiquitin ligase Parkin to the mitochondria from the cytosol. PINK-1 phosphorylates both Parkin and mitofusion (Mfn)-2 promoting ubiquitination (Ub) of mitochondrial substrates. The valosin-containing protein (VCP) transports ubiquinated mitochondria to the mitophagosome for their degradation.
Adapted with permission from Shires and Gustafsson (49).
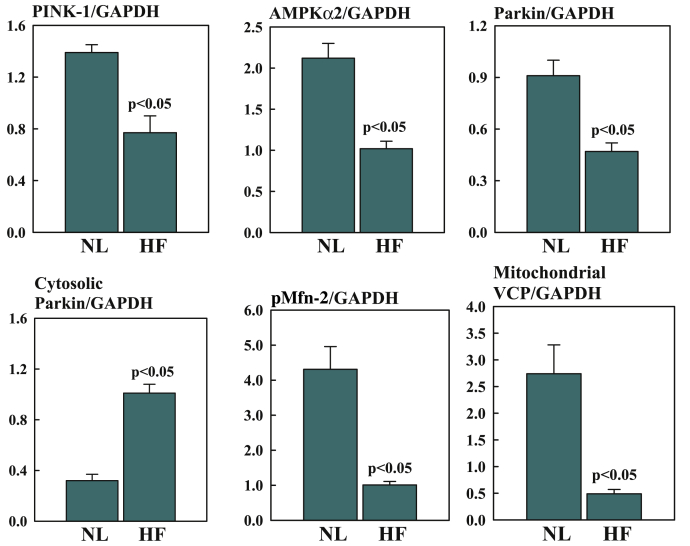
Abnormalities of mitochondria occur in cardiomyocytes of the failing heart and, as mentioned earlier, include hyperplasia, reduced organelle size, and disruption of the inner membrane. The underlying causes of these abnormalities are not fully understood but are believed to result from abnormalities associated with mitochondrial fission, fusion, and mitophagy. The selective degradation of mitochondria is an essential process for mitochondrial quality control in both health and disease (49). When mitochondria are normal, phosphatase and tensin homolog–inducible kinase (PINK)-1 is imported into the mitochondria via the translocase of the outer membrane–translocase of the inner membrane import complex where it is cleaved by presenilin-associated rhomboid-like protein (Figure 5). Cleaved PINK-1 is then retro-translocated to the cytosol and degraded by proteasome (49). However, when mitochondria are damaged, import of PINK-1 is terminated and the protein accumulates on the outer mitochondrial membrane. PINK-1 is then phosphorylated by adenosine monophosphate–activated protein kinase α-2. Phosphorylated PINK-1 recruits the E3 ubiquitin ligase Parkin to the mitochondria from the cytosol. PINK-1 phosphorylates both Parkin- and Mfn-2–promoting ubiquitination of mitochondrial substrates 49, 52. The valosin-containing protein transports ubiquinated mitochondria to the mitophagosome for their degradation (Figure 5) 49, 53. In HF, this pathway is dysregulated, resulting in partial deactivation of mitophagy and, hence, the accumulation of damaged mitochondria. Recent studies in sodium dodecyl sulfate–extracts of LV tissue, extracts of mitochondria isolated from LV tissue, as well as cytosolic fractions of LV tissue of dogs with HF, showed significant down-regulation of PINK-1, adenosine monophosphate–activated protein kinase α-2, phosphorylated Mfn-2, Parkin, and valosin-containing protein in HF dogs compared with in normal dogs (F. Last, unpublished observations, April 2019) (Figure 6).
Figure 6.
Down-Regulation of PINK-1, AMPKα2, Parkin, Cytosolic Parkin, pMfn-2, and VCP
Bar graphs depicting down-regulation of PINK-1, AMPKα2, Parkin (E3 ubiquitin ligase), cytosolic Parkin, phosphorylated (p) Mfn-2, and mitochondrial VCP in left ventricular myocardium of NL dogs and HF dogs. All proteins were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are shown as mean ± SEM. Abbreviations as in Figures 3, 4, and 5.
Mitochondrial functional abnormalities
Mitochondria in the failing heart also manifest functional abnormalities highlighted by excessive production of ROS with reduced ATP production 18, 19, 54. Studies conducted in dogs with chronic HF showed significant reductions in mitochondrial membrane potential, maximum rate of ATP synthesis, ATP-ADP ratio, and of 18:2 cardiolipin (a key structural lipid of the inner mitochondrial membrane on which the mitochondrial ETC resides), and a significant increase in cytochrome c in cardiomyocyte cytosolic fraction 55, 56. Measures of key subunits of mitochondrial complexes showed that CI and CIV activities were also significantly reduced in dogs with HF relative to normal control animals (57).
Increased opening of the MPTP, decreased mitochondrial membrane potential, and decreased mitochondrial cytochrome c oxidase and state-3 (ADP-stimulated) respiration have been shown in cardiomyocytes isolated from dogs with chronic HF compared with cardiomyocytes isolated from normal control dogs (55). Treatment of failing cardiomyocytes with cyclosporine A, a potent inhibitor of MPTP opening, improving membrane potential, preserved expression of cytochrome c oxidase, improved mitochondrial cytochrome c oxidase–dependent respiration, and tended to enhance the maximum rate of ATP synthesis 55, 56.
Mitochondrial ROS production is dependent on the mitochondrial membrane potential. The combination of ROS with elevated calcium concentrations leads to MPTP opening and subsequently reduced membrane potential, which decreases the extramitochondrial phosphorylation potential, adversely impacting cell function 58, 59; a surge in ROS production leads to cytochrome c release, which initiates cell death via apoptosis 60, 61. Mitochondrial damage to cardiomyocytes from excess ROS production limits ATP production to a level insufficient to support the H+ pump function during times of high oxidative energy requirements (62).
Mitochondrial production of ROS occurs at sites along the inner mitochondrial membrane by ETC components and in the mitochondrial matrix by components of the Krebs cycle (63). Under normal physiological conditions, ROS production is typically low and is maintained by intracellular and intramitochondrial scavenging systems. Pathological ROS levels in the heart typically occur with excessive ROS production and reduced endogenous scavenging capacity, leading to protein and lipid damage, cell death cascades, and synchronized breakdowns in the cellular energy grid 64, 65. ROS can damage the mitochondrial ETC (66), trigger lipid peroxidation (67), oxidize proteins (68), and cause DNA strand breaks (69), all of which can lead to mitochondrial dysfunction (70). Elevated mitochondrial ROS production and downstream ROS-mediated damage; mtDNA damage; decreased enzymatic activity of CI, CIII, and CIV; and defects in electron transport function have been reported in animal models of HF 71, 72, 73.
Abnormalities of Skeletal Muscle in Heart Failure and Role of Mitochondria
Fiber type composition
Exercise intolerance is a hallmark of chronic HF. Observations of a poor correlation between central hemodynamics and exercise intolerance in patients with HF shifted interest to peripheral factors, including skeletal muscle structure and metabolism 74, 75, 76, 77, 78, 79. Exercise intolerance in HF has been attributed to skeletal muscle atrophy, a shift from slow-twitch type 1 (oxidative) to fast-twitch type 2 (glycolytic) muscle fibers, mitochondrial abnormalities, and increased inducible nitric oxide synthase expression with resulting increase of nitric oxide and causing a decrease in mitochondrial creatine kinase, a key enzyme necessary for the transfer of high-energy phosphates from mitochondria to cytosol 80, 81, 82, 83, 84. A decrease in the relative composition of type-1 fibers and an increase in type-2 fibers has been shown in dogs with coronary microembolization-induced HF (83). This shift from type-1 to type-2 muscle fibers was associated with reduced exercise tolerance but not with atrophy of either fiber type (83).
The changes in composition of skeletal muscle fiber type are have also been described in patients with HF and are also associated with exercise intolerance 77, 81, 85. Patients with chronic HF have a reduced proportion of slow twitch type-1 fibers and a higher proportion of type-2 fast twitch fibers in vastus lateralis skeletal muscle biopsies 74, 77. These patients also manifest a reduction in myosin heavy-chain type I (85), an isoform that is more abundant in skeletal muscle type-1 aerobic fibers. In HF, the shift in fiber-type composition may be partly due to skeletal muscle mitochondrial abnormalities and the associated reduction of ATP synthesis needed by aerobic type-1 fibers (86); reduction in ATP production can lead to an adaptation of slow-twitch type-1 fibers to utilize glycogen as energy source and thus shifting fiber-type composition toward a fast-twitch phenotype.
Mitochondrial abnormalities in skeletal muscle
Under normal physiologic conditions, ATP production by oxidative phosphorylation in the mitochondria fulfills most of the ATP demands of skeletal muscle at rest and during exercise (16). Mitochondrial dysfunction, therefore, can contribute to decrements in skeletal muscle performance via loss of mitochondrial capacity to generate ATP, or reduced ability to meet ATP demand of working skeletal muscle.
Studies using skeletal muscle biopsies obtained from the leg vastus lateralis muscle showed marked functional abnormalities of mitochondria in dogs with coronary microembolization-induced HF compared with normal dogs (11). Mitochondria from skeletal muscle of HF dogs showed significant reductions in ADP-stimulated respiration, membrane potential, and CIV activity compared with skeletal muscle from normal dogs (11). Levels of inducible nitric oxide synthase were also significantly increased in HF dogs compared with in normal dogs (11). In this same study, measurements of cytochrome c and HtrA serine peptidase-2, a proapoptotic mitochondrial serine protease involved in caspase-dependent and caspase-independent cell death, in isolated mitochondria showed a decrease of cytochrome c and HtrA serine peptidase-2 in HF-control dogs compared with normal dogs, indicating translocation of cytochrome c and HtrA serine peptidase-2 from the mitochondria to the cytosol, the latter is a known trigger for activation of caspase-3–dependent and caspase-3–independent apoptotic pathways (11).
A variety of alterations specific to skeletal muscle, including muscle atrophy, myocyte apoptosis, fiber-type changes, defects in oxidative metabolism, and decreased mitochondrial volume density have been described in patients with HF (87). Studies using phosphorus P 31 nuclear magnetic resonance spectroscopy clearly demonstrated intrinsic skeletal muscle metabolic abnormalities in patients with chronic HF (74). These metabolic abnormalities likely contribute to skeletal muscle contractile dysfunction and, consequently, to the reduced exercise capacity observed in patients with HF.
Aging Mitochondria as a Model of Mitochondrial Abnormalities in Heart Failure
The majority of patients with chronic HF are 65 years of age or older. This is particularly true for patients with heart failure with preserved ejection fraction (HFpEF) whose average age is well into the 70s. Mitochondria are important regulators of the aging process, and abnormalities in mitochondrial quality control, including biogenesis and mitophagy, are considered a major driver of cellular senescence with aging. Replicative and degradative processes continually turn over mtDNA within the mitochondrial matrix. The incidence and frequency of mtDNA mutations increase markedly with age, contributing to cellular senescence 88, 89. In mice expressing error-prone mtDNA polymerase γ defective for proofreading activity (which causes increased mutation frequency), loss of function of DNA proofreading activity produced faulty respiratory chain proteins and premature aging 90, 91, 92. In addition, in transgenic mice, these mitochondrial defects (rapid accumulation of mtDNA mutations) caused premature aging, dilated cardiac hypertrophy, and death by congestive HF within 6 months (93). Fusion of healthy mitochondria with mitochondria possessing mtDNA mutations during replication can have an impact on the quality of the mitochondrial pool within the cell 94, 95, 96. As the age-related accumulation of somatic mtDNA mutations increases, mitochondrial function is eroded (i.e., mitochondrial energetic output declines, ROS production increases, as does the propensity for apoptosis), cells are progressively lost through apoptosis and organ function declines, which ultimately leads to symptoms when the number of cells in a given organ declines below the minimum necessary to maintain normal function (97).
Mitochondrial efficiency and capacity to generate ATP in skeletal muscle is particularly susceptible to advancing age. The theoretical maximum capacity for mitochondrial ATP production and mitochondrial volume density in the human quadriceps are reduced in sedentary versus active subjects, as is the flux per mitochondrial volume, indicating that the observed capacity loss is due to both reduced mitochondrial content and quality (98). Mitochondrial respiratory capacity and coupling control of skeletal muscle mitochondria also decline with advancing age; specifically, respiratory capacity and the proportion of respiration coupled to phosphorylation are lower in older adults (99). These findings suggest that the capacity of skeletal muscle mitochondria to produce electrochemical potential and to couple this to ADP phosphorylation is diminished with advancing age (99).
In addition to loss of mitochondrial capacity, mitochondrial quality is reduced in aged skeletal muscle. Coupling of oxidative phosphorylation declines with age in human (100) and mouse 86, 101, 102 skeletal muscles. Reduced coupling of oxidative phosphorylation was also observed in young sedentary adults compared with age-matched control subjects, supporting a vital role for physical activity in the maintenance of mitochondrial quality (103). In aging skeletal muscle, controllers of skeletal muscle biogenesis and quality are disrupted, especially PGC-1α, a key regulator of mitochondrial biogenesis 104, 105, 106. Age-related declines in PGC-1α expression in skeletal muscle have been demonstrated in human and rodent models 107, 108, 109, 110. Deterioration in mitochondrial quality control is another important aspect of aging and HF on mitochondrial dysfunction in skeletal muscle. The efficiency of mitophagy in skeletal muscle is impaired with age 111, 112, 113.
Potential Therapeutic Interventions
A number of therapeutic breakthroughs in the treatment of HF have substantially improved patient outcomes; however, there have been few breakthroughs in HF treatment over the past decade. The current treatment paradigm for patients with heart failure with reduced ejection fraction (HFrEF) involves systemic blockade of maladaptive neurohormonal systems to attenuate progressive cardiac remodeling and improve clinical outcomes. Nearly all of these treatments have relied on reducing cardiac workload through a decrease in heart rate, preload, and afterload. The concurrent hemodynamic effects of these therapies such as bradycardia and decreased preload and/or afterload limit the achievement of optimal therapy intensity in some patients and rarely achieve improvements in quality-of-life indicators such as an improvement in exercise tolerance. Furthermore, existing therapies provide symptom relief and clinical benefit but do not adequately address the molecular abnormalities of HF, underscoring the unmet need to develop more effective and complimentary therapy for these patients, including treatments that target myocardial and skeletal muscle energetics through modulation of mitochondrial function in the failing heart. What follows is a brief description of some novel therapies that seek to derive benefits in the treatment of HF by targeting or partially targeting the mitochondria.
Elamipretide
Elamipretide (SS-31, MTP-131, Bendavia, Stealth BioTherapeutics, Newton, Massachusetts) is a water-soluble, aromatic-cationic mitochondria-targeting tetrapeptide that readily penetrates and transiently localizes to the inner mitochondrial membrane and associates with cardiolipin to restore mitochondrial bioenergetics 7, 114. As discussed, mitochondrial cardiolipin is essential for the proper assembly and stability of the ETC to ensure optimal mitochondrial function, including biogenesis, fusion and fission, respiration, regulation of cristae formation, mtDNA stability and segregation, protein import, as well as the function and organization of the respiratory complexes into supercomplexes for oxidative phosphorylation 25, 115, 116.
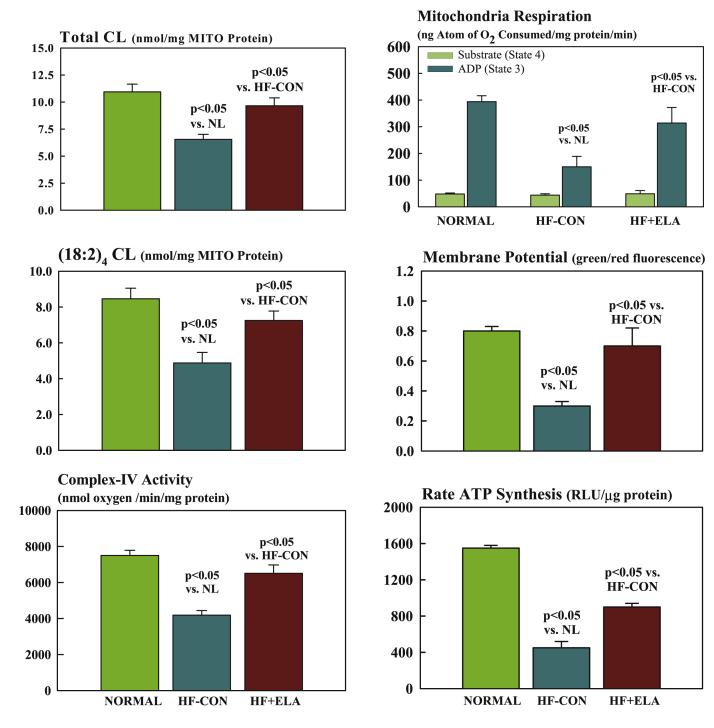
Studies in dogs with coronary microembolization-induced chronic HF showed that 3 months’ treatment with daily subcutaneous injections of elamipretide improved LV systolic function and prevented progressive LV dilation without affecting heart rate, blood pressure, or systemic vascular resistance (57). Elamipretide also elicited a normalization of mitochondrial function evidenced by improved respiration, normalization of membrane potential, reduced ROS formation, and improved maximum rate of ATP synthesis (Figure 7) (57). These improvements were accompanied by reduction of plasma biomarkers of N-terminal pro–B-type natriuretic peptide and proinflammatory cytokines including tumor necrosis factor-α, interleukin-6, and C-reactive protein. In LV tissue obtained from these dogs, chronic therapy with elamipretide reversed the dysregulation of mitochondrial fission (fission-1 and Drp-1) and fusion (Mfn-2 and OPA-1) proteins and PGC-1α protein levels, supporting observations of improved mitochondrial respiration, maximum rate of ATP synthesis, and LV function (51). The dysregulation in cardiolipin and determinants of cardiolipin synthesis and remodeling, including cardiolipin synthase-1, TAZ-1, and acyl-CoA:lysocardiolipin acyltransferase-1 were also normalized after 3 months’ therapy with elamipretide (24).
Figure 7.
Measures of Mitochondrial Function
(Left) Bar graphs depicting total cardiolipin level (CL) normalized to mitochondrial (MITO) protein (top), total (18:2)4 CL normalized to MITO protein level (middle), and MITO complex IV activity (bottom) in left ventricular myocardium of NL dogs, untreated HF control dogs (HF-CON), and dogs with HF treated with elamipretide (HF+ELA). (Right) MITO states 3 and 4 respiration (top), MITO membrane potential (middle), and maximum rate of ATP synthesis (bottom) in left ventricular myocardium of NL dogs, untreated HF-CON dogs, and HF+ELA dogs. All bar graphs are depicted as mean ± SEM.
Adapted with permission from Sabbah et al. (57). ADP = adenosine diphosphate; RLU = relative light units.
Long-term treatment with elamipretide also restored skeletal muscle fiber-type composition to a more normal distribution (increased proportion of skeletal muscle type-1 fibers relative to skeletal muscle type-2 fibers) in dogs with HF. Elamipretide also improved mitochondrial function in skeletal muscle myofibers isolated from dogs with chronic HF and reduced levels of inducible nitric oxide synthase (11), changes that would be expected to lead to improved exercise tolerance (81). In a recent study, elamipretide treatment rapidly improved mitochondrial oxygen flux, CI and CIV activities, and supercomplex-associated CIV activity in freshly explanted failing ventricular tissue from children and adults (117). These effects were independent of cardiolipin remodeling and suggest that the improvement in mitochondrial function was likely mediated through improved coupling of the mitochondrial supercomplex.
A phase 1/2 ascending single-dose study of elamipretide (4-h infusions of 0.005, 0.05, and 0.25 mg/kg/h) in 36 patients with HFrEF showed that elamipretide was well tolerated and significantly reduced LV end-diastolic (–18 ml; p = 0.009) and end-systolic (–14 ml; p = 0.005) volumes in the highest dose cohort (118). No serious adverse events were reported in any of the cohorts and blood pressure and heart rate remained stable. Two phase 2 clinical trials examining the effects of elamipretide in patients with HFrEF (The Effect of Multiple Injections of Elamipretide on Various Measures of Heart Function in Patients With Chronic Heart Failure With a Reduced Ejection Fraction; NCT02788747) and in patients with HFpEF (A Study to Evaluate the Effects of 4 Weeks’ Treatment With Subcutaneous Elamipretide on Left Ventricular Function in Subjects With Stable Heart Failure With Preserved Ejection Fraction; NCT02814097) are underway.
Partial adenosine A1 receptor agonists
Adenosine is a purine nucleoside that exerts a variety of physiological actions by binding to adenosine cell surface receptor subtypes A1, A2a, A2b, and A3, acting as a cytoprotective modulator that links cardiac function to metabolic demand (119). The cardioprotective effects of adenosine are primarily mediated by activation of the A1 receptor subtype 120, 121. Potential ways that adenosine A1 receptor activation might improve HF include enhancement of cardiomyocyte energetics, calcium homeostasis, and cardiac structure and function (119). Adenosine A1 receptor activation results in downstream inhibition of adenyl cyclase and reduction in intracellular levels of cyclic adenosine monophosphate (122), promoting a cardioprotective state by reducing sympathetic overactivation and enhancing atrial natriuretic peptide release 123, 124. Adenosine A1 receptors also modulate mitochondrial KATP channels via activation of protein kinase C, which reduces mitochondrial protein transition pore opening, improving mitochondrial function under hypoxic conditions (125). Activation of the adenosine A1 receptor using the selective agonist N6-cyclopentyladenosine similarly prevented phenylephrine-mediated cardiomyocyte hypertrophy and cardiac fibrosis, and up-regulated adenosine A1 receptor expression in a rat model of compensated hypertrophy (126). The results suggest that adenosine A1 receptor agonism might mitigate α1-adrenoceptor–stimulated cardiac remodeling and subsequent cardiac dysfunction (126).
Activation of adenosine A1 receptors using full agonists is limited by adverse effects, including bradycardia, atrioventricular block, vasoconstriction, negative inotropy and dromotropy, sedation, and antidiuretic effects 127, 128. These undesirable effects can be overcome by the use of partial A1 receptor agonists (129). The benefits of partial adenosine A1 receptor agonists for the treatment of HF, therefore, lie in their ability to protect the failing myocardium by limiting triggers of cell injury and death and potentially by providing the necessary energy to the working myocardium (130).
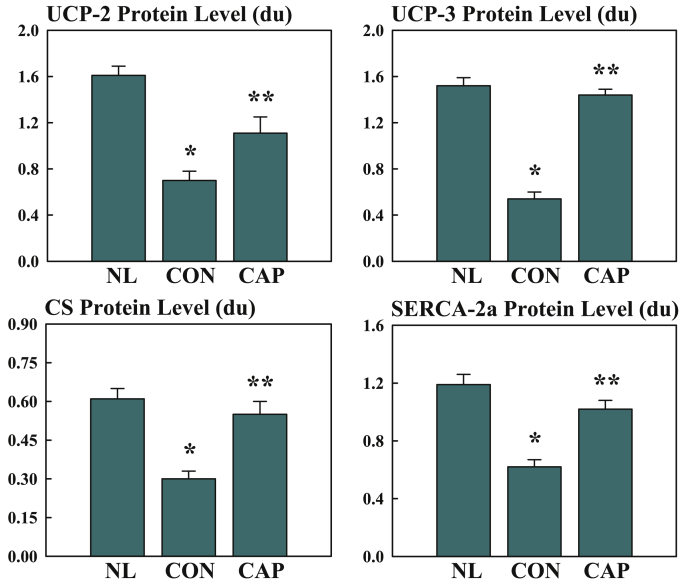
In dogs with microembolization-induced chronic systolic HF, 12 weeks of therapy with oral capadenoson, a partial adenosine A1 receptor agonist, improved LV systolic function and prevented progressive LV enlargement, as evidenced by an improvement in LV ejection fraction and a reduction of LV end-systolic volume (130). Treatment with capadenoson also significantly decreased plasma norepinephrine and plasma N-terminal pro–B-type natriuretic peptide. Histomorphometric findings showed that capadenoson significantly decreased volume fraction of interstitial fibrosis, oxygen diffusion distance, and myocyte cross-sectional area and increased capillary density (124). Capadenoson also normalized expression of mitochondrial uncoupling proteins-2 and -3, mitochondrial citrate synthase, and sarcoplasmic reticulum calcium ATPase-2a activity (Figure 8) (130). Capadenoson treatment did not induce bradycardia or lower systemic blood pressure.
Figure 8.
Changes in LV Myocardium Protein Levels of Various Proteins in NL, HF-CON, and HF-CAP Dogs
Bar graphs depicting changes in LV myocardium protein levels of various metabolic and sarcoplasmic reticulum proteins in NL dogs, untreated HF-CON dogs CON, and dogs with HF treated with capadenoson (CAP). Data are shown as mean ± SEM. *p < 0.05 versus NL; **p < 0.05 versus CON. CS = citrate synthase; SERCA-2a = sarcoplasmic reticulum calcium adenosine triphosphatase; UCP = uncoupling protein; other abbreviations as in Figures 3, 4, and 7.
Adapted with permission from Sabbah et al. (130).
Issues with central adverse effects of vertigo and dizziness observed with capadenoson in a clinical trial in patients with stable angina pectoris (suggesting that the degree of adenosine A1 receptor agonism was too high) (131), as well as low solubility, which hampered tablet development (132), led to the identification and development of neladenoson bialanate (Bay1067197, Bayer HealthCare Pharmaceuticals, Montville, New Jersey), a prodrug currently being evaluated in clinical trials for the treatment of HF (132). In 2 small pilot trials in patients with HFrEF, oral neladenoson bialanate treatment was well tolerated and no atrioventricular conduction disorders or neurological adverse effects were observed (133). In the first study that assessed single-dose neladenoson bialanate 30 mg in 11 patients receiving background beta-blocker therapy, the drug was well tolerated and no second- or third-degree atrioventricular block was detected on 48-h ambulatory electrocardiographic monitoring (133). In the second study, the double-blind, placebo-controlled PARSiFAL (Partial Adenosine Receptor Agonist in Heart Failure) study, 7 days’ treatment with either 10 mg or 20 mg daily in 31 patients receiving optimal HF therapy (all patients were receiving beta-blockers) was also well tolerated and no episodes of second- or third-degree atrioventricular block, detrimental effects on heart rate or blood pressure, or clinically relevant neurological adverse effects were observed (133). There was no significant difference, however, in ejection fraction among study groups. Two phase 2 dose-finding trials, PANTHEON (A Trial to Study Nelandenoson Bialanate Over 20 Weeks in Patients With Chronic Heart Failure With Reduced Ejection Fraction; NCT02992288) and PANACHE (A Trial to Study Nelandenoson Bialanate Over 20 Weeks in Patients With Chronic Heart Failure With Preserved Ejection Fraction; NCT03098979), are underway to assess the efficacy and safety of neladenoson bialanate (dose range 5 to 40 mg/day) in patients with HFrEF and HFpEF, respectively (134).
Conclusions
The burden of HF in terms of health care expenditures, hospitalizations, and mortality is growing in the United States and worldwide and will likely continue to increase with the aging of the population 1, 6. Current therapies for HF produce benefit by reducing cardiac workload by lowering heart rate and loading conditions, which reduces myocardial energy demands. The recent recognition that the failing heart is “energy deprived” and that mitochondrial dysfunction is a driving force associated with this energy imbalance in the heart has led to the evaluation of mitochondria as therapeutic target in HF. Mitochondrial dysfunction in the heart leads to reduced ATP synthesis and excessive formation of damaging ROS. In this review, we have described abnormalities of mitochondrial structure, function, and dynamics in the failing heart and briefly discussed studies of novel agents that target or partially target mitochondrial dysfunction in HF. The structural, dynamic, and functional abnormalities described herein, coupled with excessive ROS production, are undoubtedly instrumental in mediating progressive mitochondrial cellular injury and dysfunction that culminate in progressive worsening of HF. Correcting mitochondrial dysfunction to enhance the energy supply of the failing heart to meet the desired energy needs offers considerable potential to improve cardiac function, reduce symptoms, and improve exercise tolerance in HF, and ultimately offer improved quality of life and survival for patients, and reduce the overall economic burden of this condition.
Translational outlook
Although our understanding of mitochondrial dysfunction in HF has markedly improved over the past several years, application of this newly gained knowledge toward the development of drugs that target mitochondrial dysfunction in HF remains in its early stages. Questions remain as to what particular aspects of mitochondrial dysfunction should be targeted, such as biogenesis, mitophagy, fission, and fusion, or the ETC complexes that are likely to yield the best outcomes. Another issue is the conditions under which the therapy is applied and likely to lead to a positive outcome in a given HF patient population. Should one expect improvements under resting conditions or, more likely, during exercise when energy demands on mitochondria are highest? Outcomes may vary depending on the stage of the disease and its form (HFrEF or HFpEF). Answers to these and many other questions will be helpful in developing target-specific drugs and in designing appropriate clinical trials.
Acknowledgments
The author thanks Rick Davis, RPh, and Jim Shiffer, RPh, CCP, for their assistance in the preparation of this manuscript.
Footnotes
Supported in part by research grants from Stealth BioTherapeutics, Inc. and National Heart Lung and Blood Institute (1RO1HL132154-01A1). Dr. Sabbah has received research grants from Stealth BioTherapeutics, Inc.; and is a consultant for Stealth BioTherapeutics, Inc. and Bayer AG.
The author attests he is in compliance with human studies committees and animal welfare regulations of the author's institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Brown D.A., Perry J.B., Allen M.E. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth E., Stammler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 3.Schaper J., Meiser E., Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. doi: 10.1161/01.res.56.3.377. [DOI] [PubMed] [Google Scholar]
- 4.Johannsen D.L., Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okonko D.O., Shah A.M. Heart failure: mitochondrial dysfunction and oxidative stress in CHF. Nat Rev Cardiol. 2015;12:6–8. doi: 10.1038/nrcardio.2014.189. [DOI] [PubMed] [Google Scholar]
- 6.Sabbah H.N. Targeting mitochondrial dysfunction in the treatment of heart failure. Expert Rev Cardiovasc Ther. 2016;14:1305–1313. doi: 10.1080/14779072.2016.1249466. [DOI] [PubMed] [Google Scholar]
- 7.Birk A.V., Chao W.M., Bracken C., Warren J.D., Szeto H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171:2017–2028. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 9.Opie L.H., Heusch G. The Heart: Physiology, From Cell to Circulation. 3rd edition. Lippincott-Raven; Philadelphia, PA: 1997. Fuels: aerobic and anaerobic metabolism; pp. 295–342. [Google Scholar]
- 10.Marin-Garcia J., Goldenthal M.J. Mitochondrial centrality in heart failure. Heart Fail Rev. 2008;13:137–150. doi: 10.1007/s10741-007-9079-1. [DOI] [PubMed] [Google Scholar]
- 11.Sabbah H.N., Gupta R.C., Singh-Gupta V., Zhang K. Effects of elamipretide on skeletal muscle in dogs with experimentally induced heart failure. ESC Heart Fail. 2019;6:328–335. doi: 10.1002/ehf2.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper M.E., Dent R., Monemdjou S. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 2002;51:2459–2466. doi: 10.2337/diabetes.51.8.2459. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C., Tremblay A., Despres J.P. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolwicz S.C., Jr., Purohit S., Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S.Z., Marcinek D.J. Skeletal muscle bioenergetics in aging and heart failure. Heart Fail Rev. 2017;22:167–178. doi: 10.1007/s10741-016-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbah H.N., Sharov V., Riddle J.M., Kono T., Lesch M., Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 18.Sharov V.G., Goussev A., Lesch M., Goldstein S., Sabbah H.N. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 19.Sharov V.G., Todor A.V., Silverman N., Goldstein S., Sabbah H.N. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 20.Baandrup U., Florio R.A., Roters F., Olsen E.G. Electron microscopic investigation of endomyocardial biopsy samples in hypertrophy and cardiomyopathy: a semiquantitative study in 48 patients. Circulation. 1981;63:1289–1298. doi: 10.1161/01.cir.63.6.1289. [DOI] [PubMed] [Google Scholar]
- 21.Ferrans V.J., Morrow A.G., Roberts W.C. Myocardial ultrastructure in idiopathic hypertrophic subaortic stenosis: a study of operatively excised left ventricular outflow tract muscle in 14 patients. Circulation. 1972;45:769–792. doi: 10.1161/01.cir.45.4.769. [DOI] [PubMed] [Google Scholar]
- 22.Perennec J., Hatt P. Myocardial morphology in cardiac hypertrophy and failure: electron microscopy in man. In: Swynghedaw B., editor. Cardiac Hypertrophy and Failure. John Libbey and Company; London, England: 1988. pp. 267–276. [Google Scholar]
- 23.John G.B., Shang Y., Li L. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabbah H.N., Gupta R.C., Singh-Gupta V., Zhang K., Lanfear D.E. Abnormalities of mitochondrial dynamics in the failing heart: normalization following long-term therapy with elamipretide. Cardiovasc Drugs Ther. 2018;32:319–328. doi: 10.1007/s10557-018-6805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Z., Ye C., McCain K., Greenberg M.L. The role of cardiolipin in cardiovascular health. Biomed Res Int. 2015;2015:891707. doi: 10.1155/2015/891707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolinsky V.W., Cole L.K., Sparagna G.C., Hatch G.M. Cardiac mitochondrial energy metabolism in heart failure: role of cardiolipin and sirtuins. Biochim Biophys Acta. 2016;1861:1544–1554. doi: 10.1016/j.bbalip.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Ott M., Robertson J.D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajith T.A., Jayakumar T.G. Mitochondria-targeted agents: future perspectives of mitochondrial pharmaceutics in cardiovascular diseases. World J Cardiol. 2014;6:1091–1099. doi: 10.4330/wjc.v6.i10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah H., Gupta R.C., Rastogi S., Wang M. Dyregulation of mitochondria fission and fusion proteins in explanted failure human hearts. J Heart Lung Transplant. 2011;30(4S):S137. [Google Scholar]
- 30.Saini-Chohan H.K., Holmes M.G., Chicco A.J. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparagna G.C., Chicco A.J., Murphy R.C. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Sparagna G.C., Lesnefsky E.J. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 33.Lai L., Leone T.C., Zechner C. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin O.J., Lai L., Soundarapandian M.M. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riehle C., Wende A.R., Zaha V.G. PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bugger H., Schwarzer M., Chen D. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010;85:376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 37.Garnier A., Fortin D., Delomenie C., Momken I., Veksler V., Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arany Z., Novikov M., Chin S., Ma Y., Rosenzweig A., Spiegelman B.M. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta R.C., Szekely K., Wang M. Long-term therapy with partial adenosine A1 receptor agonist capadenoson, improves peroxisome proliferators-activated receptor coactivator-1α phosphorylation and protein expression in left ventricular myocardium of dogs with chronic heart failure. J Am Coll Cardiol. 2013;61(Suppl):E702. [Google Scholar]
- 40.Karamanlidis G., Nascimben L., Couper G.S., Shekar P.S., del Monte F., Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sihag S., Cresci S., Li A.Y., Sucharov C.C., Lehman J.J. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb R.A., Bernstein D. Mitochondrial remodeling: rearranging, recycling, and reprogramming. Cell Calcium. 2016;60:88–101. doi: 10.1016/j.ceca.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong S.B., Kalkhoran S.B., Cabrera-Fuentes H.A., Hausenloy D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur J Pharmacol. 2015;763:104–114. doi: 10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Liu Y., Dorn G.W., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papanicolaou K.N., Kikuchi R., Ngoh G.A. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L., Gong Q., Stice J.P., Knowlton A.A. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., Csordas G., Jowdy C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papanicolaou K.N., Khairallah R.J., Ngoh G.A. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shires S.E., Gustafsson A.B. Regulating renewable energy: connecting AMPKalpha2 to PINK1/Parkin-mediated mitophagy in the heart. Circ Res. 2018;122:649–651. doi: 10.1161/CIRCRESAHA.118.312655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M., Mihara K., Chen Y., Scorrano L., Dorn G.W., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–286. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabbah H., Gupta R.C., Szekely K. Bendavia (MTP-131), a mitochondria targeting peptide, normalizes dysregulation of mitochondria fission and fusion proteins in myocardium of dogs with chronic heart failure (abstr) Circulation. 2014;130:A12903. [Google Scholar]
- 52.Chen Y., Dorn G.W., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo X., Sun X., Hu D. VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington's disease. Nat Commun. 2016;7:12646. doi: 10.1038/ncomms12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song M., Chen Y., Gong G., Murphy E., Rabinovitch P.S., Dorn G.W., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharov V.G., Todor A., Khanal S., Imai M., Sabbah H.N. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharov V.G., Todor A.V., Imai M., Sabbah H.N. Inhibition of mitochondrial permeability transition pores by cyclosporine A improves cytochrome C oxidase function and increases rate of ATP synthesis in failing cardiomyocytes. Heart Fail Rev. 2005;10:305–310. doi: 10.1007/s10741-005-7545-1. [DOI] [PubMed] [Google Scholar]
- 57.Sabbah H.N., Gupta R.C., Kohli S., Wang M., Hachem S., Zhang K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gellerich F.N., Khuchua Z.A., Kuznetsov A.V. Influence of the mitochondrial outer membrane and the binding of creatine kinase to the mitochondrial inner membrane on the compartmentation of adenine nucleotides in the intermembrane space of rat heart mitochondria. Biochim Biophys Acta. 1993;1140:327–334. doi: 10.1016/0005-2728(93)90073-o. [DOI] [PubMed] [Google Scholar]
- 59.Halestrap A.P., Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Whelan R.S., Kaplinskiy V., Kitsis R.N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 61.Huttemann M., Pecina P., Rainbolt M. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion. 2011;11:369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikeda Y., Shirakabe A., Brady C., Zablocki D., Ohishi M., Sadoshima J. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol. 2015;78:116–122. doi: 10.1016/j.yjmcc.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orr A.L., Ashok D., Sarantos M.R., Shi T., Hughes R.E., Brand M.D. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic Biol Med. 2013;65:1047–1059. doi: 10.1016/j.freeradbiomed.2013.08.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Aon M.A., Cortassa S., Akar F.G., O'Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korge P., Ping P., Weiss J.N. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paradies G., Petrosillo G., Paradies V., Ruggiero F.M. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Stadtman E.R. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 69.Maynard S., de Souza-Pinto N.C., Scheibye-Knudsen M., Bohr V.A. Mitochondrial base excision repair assays. Methods. 2010;51:416–425. doi: 10.1016/j.ymeth.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andreadou I., Iliodromitis E.K., Farmakis D., Kremastinos D.T. To prevent, protect and save the ischemic heart: antioxidants revisited. Expert Opin Ther Targets. 2009;13:945–956. doi: 10.1517/14728220903039698. [DOI] [PubMed] [Google Scholar]
- 71.Goh K.Y., Qu J., Hong H. Impaired mitochondrial network excitability in failing guinea-pig cardiomyocytes. Cardiovasc Res. 2016;109:79–89. doi: 10.1093/cvr/cvv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ide T., Tsutsui H., Hayashidani S. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 73.Ide T., Tsutsui H., Kinugawa S. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 74.Mancini D.M., Coyle E., Coggan A. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 75.Mancini D.M., Walter G., Reichek N. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 76.Minotti J.R., Christoph I., Oka R., Weiner M.W., Wells L., Massie B.M. Impaired skeletal muscle function in patients with congestive heart failure: relationship to systemic exercise performance. J Clin Invest. 1991;88:2077–2082. doi: 10.1172/JCI115537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan M.J., Green H.J., Cobb F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 78.Wilson J.R., Mancini D.M., Dunkman W.B. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- 79.Drexler H., Riede U., Munzel T., Konig H., Funke E., Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 80.Hambrecht R., Niebauer J., Fiehn E. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25:1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 81.Hambrecht R., Fiehn E., Yu J. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067–1073. doi: 10.1016/s0735-1097(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 82.Hambrecht R., Adams V., Gielen S. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol. 1999;33:174–179. doi: 10.1016/s0735-1097(98)00531-2. [DOI] [PubMed] [Google Scholar]
- 83.Sabbah H.N., Hansen-Smith F., Sharov V.G. Decreased proportion of type I myofibers in skeletal muscle of dogs with chronic heart failure. Circulation. 1993;87:1729–1737. doi: 10.1161/01.cir.87.5.1729. [DOI] [PubMed] [Google Scholar]
- 84.De Sousa E., Veksler V., Bigard X., Mateo P., Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation. 2000;102:1847–1853. doi: 10.1161/01.cir.102.15.1847. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan M.J., Duscha B.D., Klitgaard H., Kraus W.E., Cobb F.R., Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29:860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Marcinek D.J., Schenkman K.A., Ciesielski W.A., Lee D., Conley K.E. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavine K.J., Sierra O.L. Skeletal muscle inflammation and atrophy in heart failure. Heart Fail Rev. 2017;22:179–189. doi: 10.1007/s10741-016-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haendeler J., Hoffmann J., Diehl J.F. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 89.Wei Y.H., Wu S.B., Ma Y.S., Lee H.C. Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging. Chang Gung Med J. 2009;32:113–132. [PubMed] [Google Scholar]
- 90.Trifunovic A., Wredenberg A., Falkenberg M. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 91.Kujoth G.C., Hiona A., Pugh T.D. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 92.Trifunovic A., Hansson A., Wredenberg A. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang D., Mott J.L., Chang S.W., Denniger G., Feng Z., Zassenhaus H.P. Construction of transgenic mice with tissue-specific acceleration of mitochondrial DNA mutagenesis. Genomics. 2000;69:151–161. doi: 10.1006/geno.2000.6333. [DOI] [PubMed] [Google Scholar]
- 94.Kraytsberg Y., Nekhaeva E., Bodyak N.B., Khrapko K. Mutation and intracellular clonal expansion of mitochondrial genomes: two synergistic components of the aging process? Mech Ageing Dev. 2003;124:49–53. doi: 10.1016/s0047-6374(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 95.Katada S., Mito T., Ogasawara E., Hayashi J., Nakada K. Mitochondrial DNA with a large-scale deletion causes two distinct mitochondrial disease phenotypes in mice. G3 (Bethesda) 2013;3:1545–1552. doi: 10.1534/g3.113.007245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farmer T., Naslavsky N., Caplan S. Tying trafficking to fusion and fission at the mighty mitochondria. Traffic. 2018;19:569–577. doi: 10.1111/tra.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conley K.E., Jubrias S.A., Esselman P.C. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526 Pt 1:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porter C., Hurren N.M., Cotter M.V. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309:E224–E232. doi: 10.1152/ajpendo.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amara C.E., Shankland E.G., Jubrias S.A., Marcinek D.J., Kushmerick M.J., Conley K.E. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegel M.P., Wilbur T., Mathis M. Impaired adaptability of in vivo mitochondrial energetics to acute oxidative insult in aged skeletal muscle. Mech Ageing Dev. 2012;133:620–628. doi: 10.1016/j.mad.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siegel M.P., Kruse S.E., Percival J.M. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–771. doi: 10.1111/acel.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conley K.E., Amara C.E., Bajpeyi S. Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary versus active subjects. J Clin Endocrinol Metab. 2013;98:129–136. doi: 10.1210/jc.2012-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kelly D.P., Scarpulla R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 105.Lehman J.J., Barger P.M., Kovacs A., Saffitz J.E., Medeiros D.M., Kelly D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.St-Pierre J., Drori S., Uldry M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Johnson M.L., Robinson M.M., Nair K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. 2013;24:247–256. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ji L.L., Kang C. Role of PGC-1alpha in sarcopenia: etiology and potential intervention—a mini-review. Gerontology. 2015;61:139–148. doi: 10.1159/000365947. [DOI] [PubMed] [Google Scholar]
- 109.Chabi B., Ljubicic V., Menzies K.J., Huang J.H., Saleem A., Hood D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 110.Ghosh S., Lertwattanarak R., Lefort N. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. doi: 10.2337/db11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cuervo A.M., Bergamini E., Brunk U.T., Droge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 112.Coleman R., Silbermann M., Gershon D., Reznick A.Z. Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology. 1987;33:34–39. doi: 10.1159/000212851. [DOI] [PubMed] [Google Scholar]
- 113.Brunk U.T., Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 114.Szeto H.H., Birk A.V. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014;96:672–683. doi: 10.1038/clpt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luevano-Martinez L.A., Forni M.F., dos Santos V.T., Souza-Pinto N.C., Kowaltowski A.J. Cardiolipin is a key determinant for mtDNA stability and segregation during mitochondrial stress. Biochim Biophys Acta. 2015;1847:587–598. doi: 10.1016/j.bbabio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Birk A.V., Liu S., Soong Y. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24:1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chatfield K.C., Sparagna G.C., Chau S. Elamipretide improved mitochondrial function in the failing human heart. J Am Coll Cardiol Basic Trans Science. 2019;4:147–157. doi: 10.1016/j.jacbts.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daubert M.A., Yow E., Dunn G. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of elamipretide. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004389. pii:e004389. [DOI] [PubMed] [Google Scholar]
- 119.Greene S.J., Sabbah H.N., Butler J. Partial adenosine A1 receptor agonism: a potential new therapeutic strategy for heart failure. Heart Fail Rev. 2016;21:95–102. doi: 10.1007/s10741-015-9522-7. [DOI] [PubMed] [Google Scholar]
- 120.Mustafa S.J., Morrison R.R., Teng B., Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009;193:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 122.Akbar M., Okajima F., Tomura H., Shimegi S., Kondo Y. A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol Pharmacol. 1994;45:1036–1042. [PubMed] [Google Scholar]
- 123.Yuan K., Cao C., Han J.H., Kim S.Z., Kim S.H. Adenosine-stimulated atrial natriuretic peptide release through A1 receptor subtype. Hypertension. 2005;46:1381–1387. doi: 10.1161/01.HYP.0000190041.61737.fd. [DOI] [PubMed] [Google Scholar]
- 124.Schutte F., Burgdorf C., Richardt G., Kurz T. Adenosine A1 receptor-mediated inhibition of myocardial norepinephrine release involves neither phospholipase C nor protein kinase C but does involve adenylyl cyclase. Can J Physiol Pharmacol. 2006;84:573–577. doi: 10.1139/y06-007. [DOI] [PubMed] [Google Scholar]
- 125.Xiang F., Huang Y.S., Zhang D.X., Chu Z.G., Zhang J.P., Zhang Q. Adenosine A1 receptor activation reduces opening of mitochondrial permeability transition pores in hypoxic cardiomyocytes. Clin Exp Pharmacol Physiol. 2010;37:343–349. doi: 10.1111/j.1440-1681.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 126.Puhl S.L., Kazakov A., Muller A. Adenosine A1 receptor activation attenuates cardiac hypertrophy and fibrosis in response to alpha1 -adrenoceptor stimulation in vivo. Br J Pharmacol. 2016;173:88–102. doi: 10.1111/bph.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fredholm B.B., AP IJ, Jacobson K.A., Klotz K.N., Linden J. International Union of Pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 128.Dinh W., Albrecht-Kupper B., Gheorghiade M., Voors A.A., van der Laan M., Sabbah H.N. Partial adenosine A1 agonist in heart failure. Handb Exp Pharmacol. 2017;243:177–203. doi: 10.1007/164_2016_83. [DOI] [PubMed] [Google Scholar]
- 129.Albrecht-Kupper B.E., Leineweber K., Nell P.G. Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal. 2012;8:91–99. doi: 10.1007/s11302-011-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sabbah H.N., Gupta R.C., Kohli S. Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure. Circ Heart Fail. 2013;6:563–571. doi: 10.1161/CIRCHEARTFAILURE.112.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tendera M., Gaszewska-Zurek E., Parma Z. The new oral adenosine A1 receptor agonist capadenoson in male patients with stable angina. Clin Res Cardiol. 2012;101:585–591. doi: 10.1007/s00392-012-0430-8. [DOI] [PubMed] [Google Scholar]
- 132.Meibom D., Albrecht-Kupper B., Diedrichs N. Neladenoson bialanate hydrochloride: a prodrug of a partial adenosine A1 receptor agonist for the chronic treatment of heart diseases. ChemMedChem. 2017;12:728–737. doi: 10.1002/cmdc.201700151. [DOI] [PubMed] [Google Scholar]
- 133.Voors A.A., Dungen H.D., Senni M. Safety and tolerability of neladenoson bialanate, a novel oral partial adenosine a1 receptor agonist, in patients with chronic heart failure. J Clin Pharmacol. 2017;57:440–451. doi: 10.1002/jcph.828. [DOI] [PubMed] [Google Scholar]
- 134.Voors A.A., Shah S.J., Bax J.J. Rationale and design of the phase 2b clinical trials to study the effects of the partial adenosine A1-receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced (PANTHEON) and preserved (PANACHE) ejection fraction. Eur J Heart Fail. 2018;20:1601–1610. doi: 10.1002/ejhf.1295. [DOI] [PubMed] [Google Scholar]