Summary
The management of hepatocellular carcinoma (HCC) has become ever more demanding. To evaluate the available evidence and to give clinicians the best guidance, all major hepatology societies have developed guidelines for HCC. Recently, updated versions have been published by the American, the Asian Pacific, and the European societies. This article presents a comparison of these three guidelines summarising both common ground and differences. Moreover, it highlights areas of ongoing research which will make yet another round of updates of the guidelines necessary in the near future.
Keywords: Hepatocellular carcinoma, cirrhosis, guidelines, diagnosis, treatment, systemic, resection, liver transplant
Key points
The major international guidelines on the management of patients with HCC share many similarities, but also a few striking differences.
Patients at risk of developing HCC (i.e. patients with liver cirrhosis) should undergo surveillance.
CT and MRI should be employed for the diagnosis of HCC.
Liver transplantation, liver resection and ablation are treatments of choice when available and feasible because of their curative potential.
TACE is the treatment of choice for unresectable, large/multifocal HCCs with no vascular invasion or extrahepatic spread (BCLC-B).
Systemic therapy should be offered to patients with advanced HCC (tumours with macrovascular invasion or extrahepatic metastasis; BCLC-C).
Alt-text: Unlabelled Box
Introduction
Hepatocellular carcinoma (HCC) is a growing global health burden, and both basic and clinical research has intensified in the past decades to tackle the challenge posed by HCC. International guidelines on the management of HCC have been published to give clinicians an overview of the available evidence in order to support them in delivering state of the art care to their patients. So far, the most important guidelines have been put forward by the American Association for the Study of Liver Diseases (AASLD), the Asian Pacific Association for the Study of the Liver (APASL), and the European Association for the Study of the Liver (EASL). While physicians and scientists deal with HCC as one and the same cancer, it is not surprising that there are regional differences in the management of HCC. The guidelines published in the years 2010-2012[1], [2], [3] were extensively reviewed by Bolondi and colleagues.4 Recently, the three major hepatology societies have published updates of their guidelines.[5], [6], [7] This review article presents a comparison of the current international guidelines on the management of HCC, highlighting both common ground and differences.
At first glance, the guidelines differ in concept and focus. The new AASLD guidelines have taken a radically different approach by selecting 10 key questions which the authors deemed most relevant for today’s clinical decision-making.6 This makes the AASLD guidelines the most focused but also the shortest recommendations (see Table 1). The APASL7 and EASL guidelines5 are similar in length and number of references. However, the EASL guidelines are particularly broad, covering topics such as response assessment, palliative care, and trial design in designated chapters, which is a first among HCC guidelines.
Table 1.
Differences in concept and focus between the international HCC guidelines.
| AASLD | APASL | EASL | |
|---|---|---|---|
| Structure | 10 key questions | 5 chapters | 14 chapters |
| Length | - 23 pages | - 54 pages | - 55 pages |
| - 98 references | - 605 references | - 636 references | |
| Concept | Focused on most relevant issues | Overview of the available evidence with focus on Asia-Pacific | Extensive overview of the available evidence |
AASLD, American Association for the Study of Liver Diseases; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; HCC, hepatocellular carcinoma.
Methodology
All three guidelines relied on the GRADE approach[8], [9] for rating the quality of evidence and strength of recommendations. The differences here are only subtle (Table 2): AASLD used four categories for the quality of evidence (high – moderate – low – very low), while APASL and EASL used only three. Regarding the grading of recommendations, all three guidelines distinguished two strengths with slightly different terminologies.
Table 2.
Methodologies of rating the quality of evidence and the strength of recommendation.
| AASLD | APASL | EASL | |
|---|---|---|---|
| Quality of evidence | - High | - High | - High |
| - Moderate | - Moderate | - Moderate | |
| - Low | - Low or very low | - Low | |
| - Very low | |||
| Strength of recommendation | - Strong | - Strong | - Strong |
| - Conditional | - Weaker | - Weak |
AASLD, American Association for the Study of Liver Diseases; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver.
Prevention
Considering the increasing global incidence of HCC, its prevention is a public health issue of high relevance. However, the AASLD guidelines did not include this topic because of their explicit focus on patients with liver cirrhosis. In contrast, the APASL and EASL guidelines cover the topic of prevention and recommend hepatitis B virus vaccination for either all infants (APASL) or all newborns and high-risk groups (EASL). Furthermore, both guidelines elaborate on antiviral therapy; here, the APASL guidelines recommend therapy for patients with chronic hepatitis B infection and active liver disease but do not give an explicit recommendation for treating hepatitis C virus infected patients with direct-acting antivirals. The EASL guidelines recommend antiviral therapy for patients with chronic viral hepatitis (including B and C). In terms of general prevention, the EASL guidelines are the first to explicitly encourage patients with chronic liver disease to drink coffee.
Surveillance
All three guidelines recommend surveillance for patients with cirrhosis because of their high risk of developing HCC. The APASL and EASL guidelines extend surveillance to certain non-cirrhotic high-risk groups, while the AASLD guidelines do not address the issue of HCC in non-cirrhotic livers. Regarding the surveillance mode, all three guidelines agree on the use of ultrasound but differ concerning the utilisation of alpha-fetoprotein (AFP). AFP in surveillance is obligatory in the APASL guidelines, optional in the AASLD guidelines, and not recommended by the EASL guidelines. The main reason for this discrepancy is that the addition of AFP has been shown to improve sensitivity and specificity of ultrasound alone (in one recent study from Asia10 from 92.0/72.4% to 99.2/68.3%), while EASL finds that the available, though insufficient, data on biomarkers such as AFP show that they are suboptimal in terms of cost-effectiveness for routine surveillance.11
Diagnosis
Three subtopics regarding the diagnosis of HCC can be distinguished: i) the recommended imaging modalities, ii) the management of indeterminate nodules, and iii) the use of biopsy.
As for i), all guidelines agree on using multiphasic computed tomography (CT) or magnetic resonance imaging (MRI) to diagnose HCC. However, the use of contrast-enhanced ultrasound (CEUS) is controversial: AASLD does not recommend CEUS, APASL considers it to be as sensitive as CT or MRI, and EASL considers it to be sufficient for the diagnosis of nodules ≥1 cm in size in cirrhotic patients. In general, several studies have confirmed the utility of CEUS for diagnosing HCC.[12], [13], [14] However, some have cautioned against using just CEUS to diagnose HCC, because intrahepatic cholangiocellular carcinoma (iCCA) might display the same vascular pattern as HCC.[15], [16] Subsequent studies have demonstrated that the washout in iCCA begins earlier (less than 60 s) after contrast injection than in HCC,[17], [18], [19], [20] which has led to a slightly modified definition of the typical hallmark of HCC at CEUS, i.e. arterial phase hyperenhancement followed by late (>60 s) washout of mild degree.[21], [22]Nevertheless, CT and MRI are the methods of choice for diagnosing HCC, because they enable examination of the liver as a whole.
As for ii), i.e. the management of indeterminate nodules, AASLD does not have a preference but suggests follow-up imaging, employing an alternative imaging modality, using an alternative contrast agent, or performing a biopsy. Similarly, APASL recommends further examination. In contrast, EASL recommends a defined approach comprising the use of a different imaging modality and – in case of a still inconclusive finding – a liver biopsy.
Similarly, the approach to nodules <1 cm also differs slightly: AASLD considers these nodules indeterminate, APASL recommends CT/MRI every 3 to 6 months, and EASL suggests ultrasound at ≤ 4-month intervals in the first year after which the patient can return to regular surveillance if there has been no increase in the size or number of nodules.
As for iii), AASLD is against the routine use of biopsy for every indeterminate nodule, while APASL advocates it for indeterminate nodules ≥1 cm in size, and EASL requires it as confirmation of HCC in non-cirrhotic patients (deeming it optional in cirrhotic patients). The previous version of the AASLD guidelines had recommended biopsy for all indeterminate lesions initially detected by surveillance ultrasound.1 The updated guidelines now weigh the benefits and risks of a biopsy differently, since the procedure may cause harm (pain, bleeding and seeding of cancer cells) on the one hand and false negative results on the other. However, they also agree with performing biopsies as part of an individualized diagnostic workup based on clinical context and imaging findings. EASL’s decision to require a biopsy for pathologic proof of HCC in patients without cirrhosis is based on the decreased specificity of HCC’s imaging hallmarks in non-cirrhotic livers as alternative diagnoses are more frequent (e.g. hepatocellular adenoma and hypervascular metastases).
Treatment
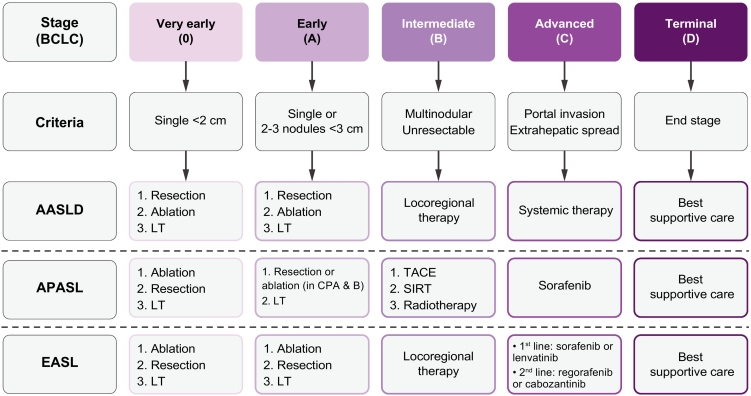
Fortunately, various treatment modalities with a proven therapeutic benefit are available for patients with HCC today. Generally, there are three kinds of therapy: surgical (liver transplantation [LT] and liver resection), locoregional (ablation, transarterial chemoembolisation [TACE]), and systemic (e.g., sorafenib). The three HCC guidelines discuss these in detail and give stage-dependent recommendations (summarised in Fig. 1).
Fig. 1.
Summary of stage-dependent recommendations on the treatment of HCC by the international guidelines.
AASLD, American Association for the Study of Liver Diseases; APASL, Asian Pacific Association for the Study of the Liver; BCLC, Barcelona Clinic Liver Cancer; BSC, best supportive care; CPA & B, Child-Pugh class A and B; EASL, European Association for the Study of the Liver; LRT, locoregional therapy; LT, liver transplantation; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolisation.
Resection
AASLD starts by recommending resection over radiofrequency ablation for adults with Child-Pugh class A cirrhosis and resectable T1 or T2 HCC. APASL recommends liver resection as first-line treatment for Child-Pugh class A patients with resectable HCC. For EASL, liver resection is the treatment of choice in non-cirrhotic patients and can be considered for single HCCs of any size, as well as for tumours > 2 cm in patients with liver cirrhosis, if feasible. The three guidelines agree in not recommending adjuvant treatment after liver resection.
Liver transplantation
The AASLD guidelines do not provide an explicit recommendation regarding LT. Instead, they recommend observation with follow-up imaging over treatment for patients with cirrhosis and T1 HCC awaiting LT. APASL and EASL remark on the use of LT, recommending it as first-line treatment for Child-Pugh class B and C patients, and HCCs within the Milan criteria, respectively.
Concerning bridging, AASLD supports it for patients within the Milan criteria (OPTN T2); APASL does not give an explicit recommendation; EASL recommends it when feasible. As for downstaging, the AASLD guidelines state that patients beyond the Milan criteria “should” be considered for LT after successful downstaging, while the EASL guidelines propose that such patients “can” be considered. The APASL guidelines do not address this issue but recommend TACE as first-line therapy in this setting. The reason for the different recommendations lies in the regional differences in the availability of treatment modalities and LT protocols, while there is insufficient data to recommend one form of treatment over another.
The concept of salvage LT is only a minor topic in the guidelines. AASLD does not mention it at all; APASL and EASL allude to it as a second-line treatment for recurrent/persistent HCC after potentially curative treatment provided that the tumour burden remains within the LT criteria.
Locoregional treatment
AASLD recommends locoregional treatment over no treatment in cirrhotic HCC patients (T2 or T3, no vascular involvement) who are not candidates for resection or transplantation. However, AASLD does not recommend one form of locoregional treatment over another. Here, the APASL and EASL guidelines are more comprehensive: APASL appraises ablation as an alternative to resection for Child-Pugh class A or B patients with ≤3 tumours, each of ≤3 cm in size. Furthermore, it even recommends ablation as first-line treatment for HCCs ≤2 cm in this group of patients. EASL recommends ablation as the standard of care for unresectable BCLC 0 and A tumours, an alternative to resection in single tumours 2 to 3 cm in size, and even a potential first-line therapy for resectable BCLC-0 HCCs when they are in a favourable location. The appraisal of ablation as an alternative to resection can be explained by similar outcomes despite a different balance of risk and efficacy (liver resection and ablation are associated with higher rates of serious adverse events and HCC recurrence, respectively).23 In addition, real-world experience has shown that only a limited number of cases are equally suitable for both techniques, since the suitability of these methods varies depending on the tumour location.24
As for percutaneous ethanol injection, APASL deems it the treatment of choice when ablation cannot be performed safely, while EASL suggests it as an option in cases where ablation is not technically feasible (especially when the tumours are <2 cm in size).
Concerning TACE, the APASL and EASL guidelines agree on recommending it as first-line treatment for unresectable, large/multifocal HCCs with no vascular invasion or extrahepatic spread (BCLC-B). Furthermore, APASL supports selective TACE for patients with small tumours where ablation is difficult and advises changing the treatment strategy for patients with HCC who are not suitable for or do not response to repeated TACE (a concept known as treatment stage migration).
Regarding selective internal radiation therapy (SIRT), the APASL guidelines consider it as an alternative treatment for unresectable HCC. The EASL guidelines do not give a recommendation but comment that it has shown a good safety profile and local tumour control but no overall survival benefit. Therefore, the subgroup of patients benefitting from SIRT remains to be defined.
Radiotherapy
The APASL guidelines view radiotherapy as a reasonable option for patients who have failed other locoregional treatment, while the EASL guidelines did not find enough evidence to support this approach for HCC.
Systemic therapy
Systemic therapy is the area where the most relevant developments for clinical practice have taken place in recent years. This is highlighted by the positive phase III studies for the multikinase inhibitors regorafenib,25 lenvatinib26 and cabozantinib,27 as well as the monoclonal antibody ramucirumab,28 and the FDA approval of nivolumab (based on the CheckMate 040 trial29) and pembrolizumab (based on the KEYNOTE-224 trial30) as second-line treatment of advanced HCC following treatment with sorafenib. Given the speed of these developments, it is obvious that any HCC guidelines will quickly become outdated. This explains the AASLD’s decision to simply recommend systemic therapy – without specifying an agent – over no therapy for patients with Child-Pugh class A cirrhosis or well-selected patients with Child-Pugh class B cirrhosis plus advanced HCC with macrovascular invasion and/or metastatic disease. Here, the APASL and EASL guidelines are more comprehensive again, with EASL covering most of the recently presented drugs. Both APASL and EASL recommend sorafenib as first-line treatment for patients with advanced HCCs (BCLC-C) who are not suitable for locoregional treatment and who have Child-Pugh class A liver function. APASL adds that sorafenib may be used with caution in patients with Child-Pugh class B liver function, and EASL states that it may also be prescribed for earlier stage tumours progressing upon or unsuitable for locoregional treatment (as treatment stage migration). While the APASL guidelines do not cover other systemic agents, the EASL guidelines recommend lenvatinib as a non-inferior alternative to sorafenib. Furthermore, the EASL recommends regorafenib as second-line treatment for patients progressing on sorafenib and with Child-Pugh class A liver function and good performance status. In addition, EASL notes that cabozantinib has shown a survival benefits vs. placebo as a second-line treatment following sorafenib and summarises the data which were available for ramucirumab when the guidelines went to press. Concerning immunotherapy of HCC, EASL concedes that the data are not mature enough to give a clear recommendation for nivolumab. Furthermore, it does not discuss any data on pembrolizumab.
Outlook
Our comparison of the international HCC guidelines has revealed many commonalities but also a few striking differences (summarised in Table 3). These stretch from differences in concept and focus to subtle alternatives for managing patients with HCC. However, several paradigms are shared among the three guidelines:
-
1.
Patients at risk of developing HCC (i.e. patients with liver cirrhosis) should undergo surveillance.
-
2.
CT and MRI should be employed for the diagnosis of HCC.
-
3.
Liver transplantation, liver resection and ablation are treatments of choice when available and feasible because of their curative potential.
-
4.
TACE is the treatment of choice for unresectable, large/multifocal HCCs with no vascular invasion or extrahepatic spread (BCLC-B).
-
5.
Systemic therapy should be offered to patients with advanced HCC (tumours with macrovascular invasion or extrahepatic metastasis; BCLC-C).
Table 3.
Differences in recommendations between the international HCC guidelines.
| AASLD | APASL | EASL | |
|---|---|---|---|
| Surveillance | US every 6 months, AFP optional | US + AFP every 6 months | US every 6 months |
| CEUS | Not recommended | As sensitive as CT/MRI | Suitable for nodules ≥ 1 cm in cirrhosis |
| Biopsy | No routine use | For indeterminate nodules ≥ 1 cm | Required in non-cirrhotic HCC |
| Bridging | Recommended for T2 | No recommendation | Recommended if feasible |
| LT after downstaging | Recommended | No recommendation | Possible |
| LRT | - Recommended in cirrhotic non-surgical patients (T2 or T3, no vascular involvement) - No preference regarding modality |
- Ablation: For HCCs ≤2 cm in CP-A/B - TACE: For unresectable, large/multifocal HCCs - SIRT: Alternative to TACE |
- Ablation: or unresectable BCLC 0 and A + selected surgical patients - TACE: For BCLC B - SIRT: Good safety profile, efficacy not yet proven |
| Radiotherapy | No recommendation | Option when other LRTs have failed | Insufficient evidence |
| Systemic therapy | - For patients with CP-A cirrhosis or well-selected patients with CP-B cirrhosis plus advanced HCC with macrovascular invasion and/or metastatic disease - No preference regarding drug |
- Sorafenib for advanced HCC with CP-A liver function (possible with caution in CP-B) | - Sorafenib & lenvatinib: 1st line for BCLC-C - Treatment stage migration - Regorafenib: 2nd line - Cabozantinib: Benefit as 2nd line - Nivolumab: No recommendation yet |
AASLD, American Association for the Study of Liver Diseases; AFP, alpha-fetoprotein; APASL, Asian Pacific Association for the Study of the Liver; BCLC, Barcelona Clinic Liver Cancer; CEUS, contrast-enhanced ultrasound; CP, Child-Pugh class; CT, computed tomography; EASL, European Association for the Study of the Liver; LRT, locoregional therapy; LT, liver transplantation; MRI, magnetic resonance imaging; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolisation; US, ultrasound.
The AASLD, APASL and EASL guidelines are not the only comprehensive recommendations on the management of HCC but several other (regional) guidelines have been published. Covering all the available guidelines is beyond the scope of this article. However, one example of regional guidelines which deserves particular attention due to the country’s size is the Chinese current edition.31 As we wrote recently,32 these guidelines deviate from international consensus with regard to treatment recommendations by introducing a high degree of flexibility in the choice of therapy. Such differences and the ones mentioned in this article will hopefully stir the debate among international HCC experts and result in inter-societal exchange to define the best protocols for managing patients with HCC. In light of the recent developments, it is clear that all guidelines will require an update in the near future.
Financial support
The authors did not receive any financial support for the conduct of the research or the preparation of the article.
Conflicts of interest
FF: no conflict of interest to declare. PRG: Consultant and lecturing: AstraZeneca, Bayer Schering Pharma, Bristol-Myers Squibb, Eisai, Eli Lily and Company, Ipsen, Merck, MSD, Roche, Sillagen, Sirtex Medical.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
FF and PRG reviewed the literature and wrote the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.04.005.
Supplementary data
Supplementary material
References
- 1.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver, European Organisation For Research and Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tovoli F, Negrini G, Bolondi L. Comparative analysis of current guidelines for the treatment of hepatocellular carcinoma. Hepat Oncol. 2016;3:119–136. doi: 10.2217/hep-2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Am J Gastroenterol. 2015;110:836–844. doi: 10.1038/ajg.2015.100. quiz 845. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–110. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 12.Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41:71–90. doi: 10.1007/s00261-015-0592-8. [DOI] [PubMed] [Google Scholar]
- 13.Niu Y, Huang T, Lian F, Li F. Contrast-enhanced ultrasonography for the diagnosis of small hepatocellular carcinoma: a meta-analysis and meta-regression analysis. Tumour Biol. 2013;34:3667–3674. doi: 10.1007/s13277-013-0948-z. [DOI] [PubMed] [Google Scholar]
- 14.Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K. Contrast-enhanced ultrasound using SonoVue(R) (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1–243. doi: 10.3310/hta17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilana R, Forner A, Bianchi L, Garcia-Criado A, Rimola J, de Lope CR. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020–2029. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 16.Galassi M, Iavarone M, Rossi S, Bota S, Vavassori S, Rosa L. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771–779. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 17.de Sio I, Iadevaia MD, Vitale LM, Niosi M, Del Prete A, de Sio C. Optimized contrast-enhanced ultrasonography for characterization of focal liver lesions in cirrhosis: A single-center retrospective study. United European Gastroenterol J. 2014;2:279–287. doi: 10.1177/2050640614538964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu GJ, Wang W, Lu MD, Xie XY, Xu HX, Xu ZF. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer. 2015;4:241–252. doi: 10.1159/000367738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildner D, Bernatik T, Greis C, Seitz K, Neurath MF, Strobel D. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med. 2015;36:132–139. doi: 10.1055/s-0034-1399147. [DOI] [PubMed] [Google Scholar]
- 20.Wildner D, Pfeifer L, Goertz RS, Bernatik T, Sturm J, Neurath MF. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med. 2014;35:522–527. doi: 10.1055/s-0034-1385170. [DOI] [PubMed] [Google Scholar]
- 21.Piscaglia F, Kudo M, Han KH, Sirlin C. Diagnosis of Hepatocellular Carcinoma with Non-Invasive Imaging: a Plea for Worldwide Adoption of Standard and Precise Terminology for Describing Enhancement Criteria. Ultraschall Med. 2017;38:9–11. doi: 10.1055/s-0042-124204. [DOI] [PubMed] [Google Scholar]
- 22.Piscaglia F, Wilson SR, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med. 2017;38:320–324. doi: 10.1055/s-0042-124661. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD011650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leoni S, Piscaglia F, Serio I, Terzi E, Pettinari I, Croci L. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig Liver Dis. 2014;46:549–555. doi: 10.1016/j.dld.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 27.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 29.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) Liver Cancer. 2018;7:235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foerster F, Galle PR. Hepatocellular carcinoma: one world, one cancer-different guidelines? Hepatobiliary Surg Nutr. 2018;7:41–43. doi: 10.21037/hbsn.2018.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material