Summary
The worldwide prevalence of non-alcoholic fatty liver disease (NAFLD) is estimated to have reached 25% or more in adults. NAFLD is prevalent in obese individuals, but may also affect non-obese insulin-resistant individuals. NAFLD is associated with a 2- to 3-fold increased risk of developing type 2 diabetes (T2D), which may be higher in patients with more severe liver disease – fibrosis increases this risk. In NAFLD, not only the close association with obesity, but also the impairment of many metabolic pathways, including decreased hepatic insulin sensitivity and insulin secretion, increase the risk of developing T2D and related comorbidities. Conversely, patients with diabetes have a higher prevalence of steatohepatitis, liver fibrosis and end-stage liver disease. Genetics and mechanisms involving dysfunctional adipose tissue, lipotoxicity and glucotoxicity appear to play a role. In this review, we discuss the altered pathophysiological mechanisms that underlie the development of T2D in NAFLD and vice versa. Although there is no approved therapy for the treatment of NASH, we discuss pharmacological agents currently available to treat T2D that could potentially be useful for the management of NASH.
Key points
NAFLD is associated with a 2- to 3-fold increased risk of developing T2D, while patients with T2D have a higher prevalence of steatohepatitis, liver fibrosis and end-stage liver disease.
Individuals with NAFLD and T2D are insulin resistant not only at the level of the muscle and the liver but also in adipose tissue.
Dysfunctional adipose tissue and an increased rate of lipolysis contribute to ectopic fat accumulation, development of insulin resistance, lipotoxicity and impaired beta-cell function.
Excess FFA availability is also an important cause of inflammation, mitochondrial dysfunction, increased oxidative stress and uncoupled oxidative phosphorylation, activating a fibrogenic response in hepatic stellate cells that can promote the progression to NASH and cirrhosis.
Glucotoxicity is referred as to the harmful effect of chronic hyperglycaemia that alters intracellular glucose and lipid pathways and promotes cellular dysfunction and eventually death.
Glucotoxicity and lipotoxicity are closely interrelated and both contribute to the deterioration of insulin resistance and impaired insulin secretion in T2D.
Among the pharmacological agents currently available to treat T2D, pioglitazone, GLP-1RAs and SGLT-2 inhibitors are promising for the management of NAFLD or NASH, although more work is needed to fully understand their clinical potential.
Alt-text: Unlabelled Box
Introduction
The estimated prevalence of non-alcoholic fatty liver disease (NAFLD) has now reached an average of 25%, ranging from 13% in Africa to over 30% in South America.1 It is of no surprise that NAFLD prevalence is very similar to that of obesity, since increased caloric intake (mainly from fat), sedentary lifestyle and consequent development of obesity are major risk factors for the development of NAFLD. Several studies have now shown that individuals with NAFLD/non-alcoholic steatohepatitis (NASH) are at a higher risk of developing type 2 diabetes (T2D).2,3 A recent meta-analysis, including 296,439 individuals (30% with NAFLD) and nearly 16,000 cases of incident diabetes over a median of 5 years from 19 observational studies, has shown that the risk of incident diabetes is more than 2-fold higher in individuals with NAFLD.4
The pathophysiological mechanisms underlying the development of NAFLD are mainly the alterations in glucose and lipid metabolism, insulin resistance (IR) and insulin secretion, explaining the close association between NAFLD and T2D. Moreover, both patients with NAFLD and T2D often share the comorbidities associated with the metabolic syndrome, namely fasting hyperglycaemia, hypertension, hypertriglyceridemia, low high-density lipoprotein-cholesterol and/or abdominal fat accumulation.5,6
From NAFLD/NASH to diabetes
Increased insulin resistance in NAFLD and risk of diabetes
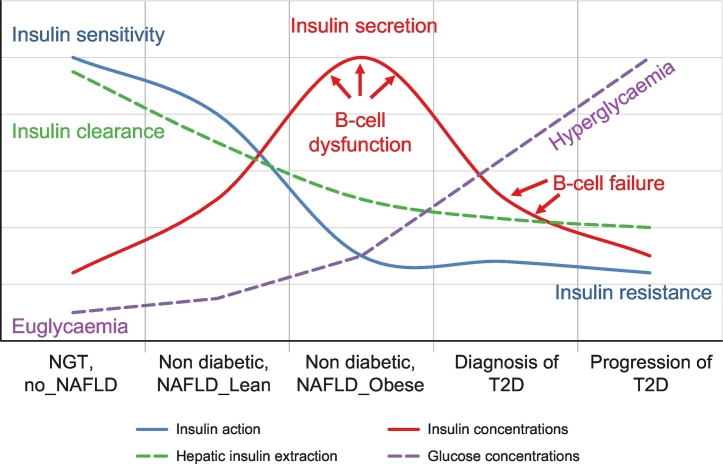
It is well established that individuals with NAFLD are more insulin resistant than those without NAFLD, even if they are lean and without diabetes.7 Both longitudinal and cross-sectional studies have demonstrated that increased IR is the earliest detectable abnormality in both prediabetes and overt T2D.[8], [9], [10] The pancreas responds to increased IR by secreting more insulin and the liver decreases insulin clearance in order to increase peripheral insulin concentrations and prevent the development of diabetes (Fig. 1) (i.e., below 126 mg/dl after an overnight fast and below 200 mg/dl 2 hours after a glucose load).8,11
Fig. 1.
Natural history of T2D in the context of NAFLD.
T2D results from an imbalance between insulin sensitivity and insulin secretion. In the progression from normal to impaired glucose tolerance to T2D, insulin secretion increases to overcome insulin resistance. At the same time there is a decrease in insulin clearance (mainly hepatic), especially in individuals with NAFLD, that determines higher peripheral insulin concentrations. However, despite high concentrations, insulin secreted is insufficient (beta-cell dysfunction) and therefore there is an increase in both fasting and postprandial glucose concentrations. Subjects become T2D only when the beta cells are unable to increase insulin secretion (beta-cell failure) and overcome peripheral IR. Redrawn from ref 8,11,13.
IR, insulin resistance; NAFLD, non-alcoholic fatty liver disease; NGT, normal glucose tolerance; T2D, type 2 diabetes
In NAFLD, IR is present in muscle, liver and adipose tissue.7,12,13 As a consequence, hepatic glucose production and adipose tissue lipolysis are only in part suppressed by insulin, resulting in higher fasting glucose and free fatty acid (FFA) concentrations,7,12,13 increasing the risk of T2D in these patients. IR can be assessed directly using the euglycaemic-hyperinsulinaemic clamp, which involves infusing insulin at a constant rate and maintaining glycaemia close to the normal fasting concentrations by infusing glucose intravenously. The rate of glucose infusion gives an estimate of peripheral insulin sensitivity, i.e., the individual is highly sensitive if the glucose infusion rate is high, or IR if the individual is resistant to the insulin action and thus less glucose is taken up by peripheral tissues (largely muscle). Indirectly IR is estimated using the HOMA-IR (homeostatic model assessment for insulin resistance) index (given by the product of fasting insulin x glucose/22.5)14 or from oral glucose tolerance test (OGTT) indexes like the Matsuda or oral glucose insulin sensitivity indexes.15
In an IR state more insulin is required to obtain the same metabolic effects, namely glucose uptake in the muscle, suppression of lipolysis and of hepatic glucose production. IR also affects glucose tolerance, since post OGTT glucose levels depend on the balance between muscle glucose clearance and hepatic glucose flux. To overcome IR, the pancreas is stimulated to secrete more insulin. Thus, as individuals progress from normal (NGT) to impaired glucose tolerance (IGT) (see Table 1 for criteria of diagnosis16) they need more insulin in the periphery to overcome the postprandial hyperglycaemia[9], [10], [11] and this explains the higher insulin concentrations observed in individuals with IR (Fig. 1). Higher peripheral insulin concentrations occur not only because of increased pancreatic insulin secretion, but also because of decreased insulin clearance by the liver, which allows more insulin to reach the peripheral circulation. However, in a state of IR the workload of pancreatic beta cells is increased, leading to beta-cell dysfunction and a reduction of beta-cell mass over time, a major risk factor for the development of hyperglycaemia and T2D.17,18
Table 1.
Screening for and diagnosis of prediabetes and type 2 diabetes, according to American Diabetes Association guidelines 2018.16
| Fasting plasma glucose⁎ | Glucose tolerance (2-hour PG)# | Haemoglobin A1C§ | |
|---|---|---|---|
| Normal (NGT#) |
FPG ≪100 mg/dl (5.6 mmol/L) | 2-hour PG ≪140 mg/dl (7.8 mmol/L) during 75 g OGTT | ≪5.7% (39 mmol/mol) |
| Prediabetes (IFG and/or IGT#) |
FPG from 100 mg/dl (5.6 mmol/L) to 125 mg/dl (6.9 mmol/L) (IFG) | 2-hour PG from 140 mg/dl (7.8 mmol/L) to 199 mg/dl (11.0 mmol/L) (IGT#) | 5.7 to 6.4% (39–47 mmol/mol) |
| Type 2 diabetes | FPG ≥126 mg/dl (7.0 mmol/L). | 2-hour PG ≥200 mg/dl (11.1 mmol/L) during OGTT | ≥6.5% (48 mmol/mol) |
Fasting is defined as no caloric intake for at least 8 h.
The OGTT should be performed as described by the WHO, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. In a patient with classic symptoms of hyperglycaemia or hyperglycaemic crisis, a random plasma glucose ≥200 mg/dl (11.1 mmol/L). In the absence of unequivocal hyperglycaemia, results should be confirmed by repeat testing.
Glucose tolerance measured after OGTT: NGT or IGT.
The test should be performed in a laboratory using a method that is NGSP certified and standardised to the DCCT assay.
DCCT, diabetes control and complications trial; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; PG, plasma glucose; WHO, World Health Organization.
Impaired insulin secretion in NAFLD and risk of diabetes
Initial studies on the natural history of T2D indicate that hyperglycaemia results from an imbalance between insulin sensitivity and insulin secretion.9,10,19 There is no doubt that the majority of patients with NAFLD are insulin resistant, even if they are non-obese.7,17 However, most of individuals with NAFLD/NASH do not develop hyperglycaemia despite displaying higher insulin concentrations.15 This is because the liver, as it accumulates triglycerides and toxic lipid-derived metabolites, becomes resistant to the effect of insulin and decreases its capacity to clear insulin,13,20 thus increasing peripheral insulin concentrations. In response to changes in glucose concentrations, insulin is initially secreted rapidly in a quantity proportional to the rise in glucose concentration (first phase, early minutes) and then as a dose response to plasma glucose concentration (second phase).21 Since insulin is secreted in the portal vein, the first phase should have a major effect on decreasing hepatic glucose production, while the second phase acts on the periphery, mainly muscle but also adipose tissue.
In the progression from NGT to IGT, the first phase is already reduced, and the total amount of insulin delivered to the periphery is still insufficient to clear glucose, despite high insulin secretion rates and plasma insulin concentrations; this is referred to as impaired beta-cell function.8,21,22 Together with increased IR the impaired beta-cell function explains the high postprandial glucose concentrations. Both ourselves and others have shown that individuals with IGT have lost almost 80% of their beta-cell capacity8,21,22 and about half of their beta-cell mass23 compared to those with NGT.
Although an impaired pancreatic beta-cell insulin response to a glucose challenge is already present in normal glucose-tolerant patients with NAFLD, they only develop diabetes when pancreatic beta cells are unable to increase insulin secretion to match their severe peripheral IR11,21 (Fig. 1).
Hepatic insulin resistance and risk of type 2 diabetes
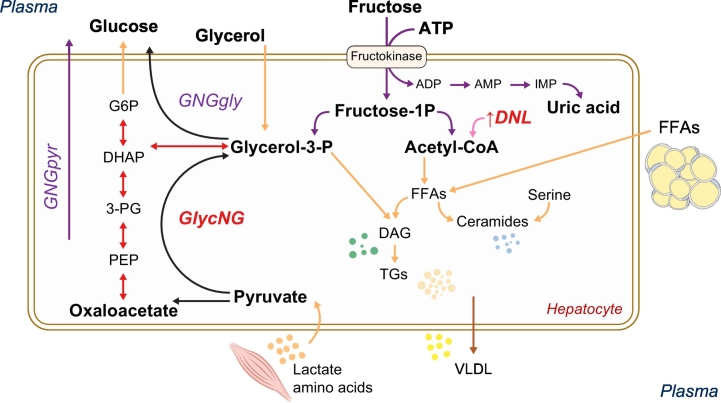
Hepatic steatosis is associated with alterations in both lipid and glucose metabolism. The liver is the main site of glucose production during fasting conditions.22,24 Glucose is produced from hepatic glycogenolysis (i.e., from hydrolysis of the glycogen stored after a meal) or gluconeogenesis, i.e., synthesised from amino acids, glycerol or lactate (Fig. 2). Endogenous glucose production is tightly regulated by the pancreatic hormones, insulin and glucagon, which maintain glucose concentrations within the normal range. In patients with hepatic steatosis, fasting glucose production, and in particular gluconeogenesis are elevated, despite high insulin levels, leading to fasting and postprandial hyperglycaemia.17,22,25 Glucagon stimulates gluconeogenesis and glycogenolysis, while insulin suppresses hepatic glucose production mainly by suppressing gluconeogenesis.26 Both individuals with NAFLD and T2D are insulin resistant at the level of the liver, i.e., insulin is unable to properly suppress glucose production.17,27 Moreover, they both tend to have increased glucagon concentrations that exacerbate hepatic IR.8,28 Patients with either T2D and/or NAFLD have increased gluconeogenesis29,30 that contributes to excessive glucose production and hepatic IR. However, increased gluconeogenesis does not seem to be related to the amount of hepatic triglycerides.13 Thus, excess gluconeogenesis is probably a consequence rather than a cause of hepatic IR, since increased gluconeogenesis is closely associated with excess gluconeogenic substrates that overload the liver, rather than with increased hepatic fat accumulation.13
Fig. 2.
Insulin resistance causes excess substrate (amino acids, glycerol, FFAs) to be transported to the liver.
Excess substrate (amino acids, glycerol, FFAs) stimulate GNG, glucose fluxes, de novo glycerol synthesis, TG synthesis and DNL. Moreover, the synthesis of lipotoxic compounds like DAGs and ceramides is enhanced. Modified from 32. DAGs, diacylglycerols; DNL, de novo lipogenesis; FFAs, free fatty acids; GNG, gluconeogenesis; TG, triglyceride; VLDL, very low-density lipoprotein.
In the postprandial state, hepatic glucose production is suppressed by glucose stimulated insulin secretion and the glucose ingested is in part stored as glycogen, and in part oxidised, used to produce pyruvate, lactate and amino acids like alanine (that are also gluconeogenic substrates), but also re-routed to de novo lipogenesis (DNL) and glycerol synthesis (Fig. 2). Excess gluconeogenic substrates (and glucose itself) can also be used to synthesise glycerol de novo, via glyceroneogenesis,31 that in turn is used for triglyceride synthesis (Fig. 2). This pathway, previously not considered, is indeed accelerated in individuals with NAFLD.32 This is very important because glycerol availability is a limiting step for hepatic triglyceride synthesis.
DNL, i.e., the synthesis of fatty acids from acetyl-CoA subunits derived mainly from carbohydrate catabolism, is much higher after a carbohydrate-rich meal (Fig. 2). Carbohydrate-rich meals together with hyperinsulinemia stimulate the activity of lipogenic enzymes in the liver, the production of newly synthesised fatty acids, and very low-density lipoprotein (VLDL) secretion.[33], [34], [35] DNL is driven mainly by fructose, with very little glucose used for DNL (Fig. 2). However, the western diet is rich in fructose and sucrose (composed of 1 glucose and 1 fructose molecule). For this reason, excess sugar consumption is associated with increased DNL, which is exacerbated by a diet high in saturated fat, but not by a diet rich in unsaturated fat.36 DNL is increased more than 3-fold in NAFLD[37], [38], [39] and characterised by an increased production of saturated fatty acids (especially palmitate) and a reduction of unsaturated fat.38 Increased saturated fatty acids are known to be lipotoxic, not only in liver cells but also in pancreatic beta cells, endothelial cells, skeletal muscle and cardiomyocytes.40,41 Palmitate is also a precursor of ceramides and other lipotoxic lipids.42 Excess fructose is only in part used for DNL and can also be metabolised as uric acid through the purine cycle (Fig. 2).
DNL is tightly regulated both by hormone signalling and transcription factors, such as sterol response element binding protein 1c (SREBP1c) and carbohydrate response element binding protein (ChREBP), which regulate the expression of the key lipogenic genes acetyl CoA carboxylase (ACC), fatty acid synthase (FAS) and ATP-citrate lyase (ACL).43,44 Insulin also promotes lipogenesis and adiposity. However, ectopic fat accumulates only when fatty acid oxidation is impaired, and subcutaneous adipose tissue becomes insulin resistant and unable to store excess fat and glucose, typically from excess rates of adipose tissue lipolysis.7,[45], [46], [47] This has also been supported by genetic studies.48,49 Individuals with NAFLD tend to accumulate ectopic fat not only in the liver and skeletal muscle, but also in the pancreas, likely contributing to beta-cell dysfunction. A recent study showed that the loss of hepatic and pancreatic fat was associated with improved control of hyperglycaemia and of beta-cell function in patients with T2D.50
However, the progression from NAFLD to NASH with liver injury, i.e., fibrosis, cirrhosis and hepatocellular carcinoma, is strongly associated with metabolic changes in both glucose and lipid metabolism. A recent study has shown that decreased glucose tolerance is most closely associated with the presence and severity of liver fibrosis rather than the degree of steatosis or presence of obesity.15
From diabetes to NAFLD/NASH
Role of dysfunctional adipose tissue and lipotoxicity
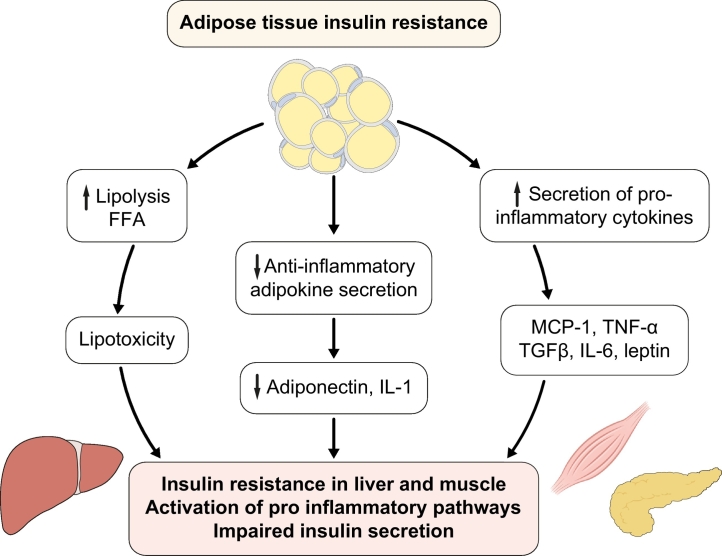
Increased adiposity, as often found in NAFLD and T2D, is associated with adipocyte IR and dysfunction.7,13,51 This results in excess FFA release into the blood stream and predisposes to lipotoxicity, i.e. when FFA overflow from adipose tissue leads to excess lipid uptake by tissues such as the liver, pancreas or muscle (Fig. 3). FFAs, both from unsuppressed lipolysis and increased DNL in hepatocytes, can either be oxidised through mitochondrial beta oxidation, or re-esterified to triglycerides that are either secreted as VLDL, used to synthesise other toxic lipid metabolites or stored as ectopic fat. Lipotoxicity is associated with increased peripheral IR,27,52 hepatic glucose production and gluconeogenesis leading to hyperglycaemia,13,22 as well as pancreatic beta-cell dysfunction with impaired insulin secretion.53,54
Fig. 3.
Relationship between adipose tissue insulin resistance and dysfunction and insulin resistance in liver and muscle.
Dysfunctional adipose tissue displays resistance to the antilipolytic effect of insulin with increased lipolysis and release of FFAs and glycerol that in turn are responsible for triglyceride accumulation and lipotoxicity in the liver, muscle and pancreas, impairing insulin secretion. Moreover, dysfunctional adipose tissue releases adipokines that activate pro-inflammatory pathways in these organs. FFAs, free fatty acids.
Overflow of fatty acids to the liver has also been associated with increased cellular levels of toxic lipids such as diacylglycerols, ceramides (Fig. 2), and long-chain fatty acyl-coenzyme A (CoA), which are involved in inflammatory pathways17,27,[55], [56], [57], [58], [59] (Fig. 4). Excess FFAs also promote mitochondrial dysfunction, an increase in oxidative stress and uncouple oxidative phosphorylation. They also activate a fibrogenic response in hepatic stellate cells that can promote the progression to NASH and cirrhosis,[60], [61], [62] and the production of reactive oxygen species63 (Fig. 3, Fig. 4). Adipose tissue dysfunction and IR lead to the increased release of pro-inflammatory cytokines (e.g., MCP-1, TNF-α TGF-β, PAI-1, IL-6) and reduced release of anti-inflammatory adipokines (e.g. adiponectin) by adipose tissue64 (Fig. 3). These molecules can directly damage the liver, or act indirectly, by increasing oxidative stress, hepatocellular damage, liver fibrosis and tumour development, e.g., through the activation of the oncogenic factor STAT3.61,65 Ceramides, diacylglycerols and saturated fatty acids not only trigger inflammation but also apoptosis.27,31,59 They also alter insulin signalling and action,27,52 decrease muscle ATP synthesis66 and nitric oxide production (as well as endothelial nitric oxide synthase or eNOS),67 impair insulin-stimulated activation of phosphoinositol-3 kinase (PI3K), pyruvate dehydrogenase kinase, isozyme 1, and RAC-alpha serine/threonine-protein kinase (also known as proto-oncogene c-Akt).67
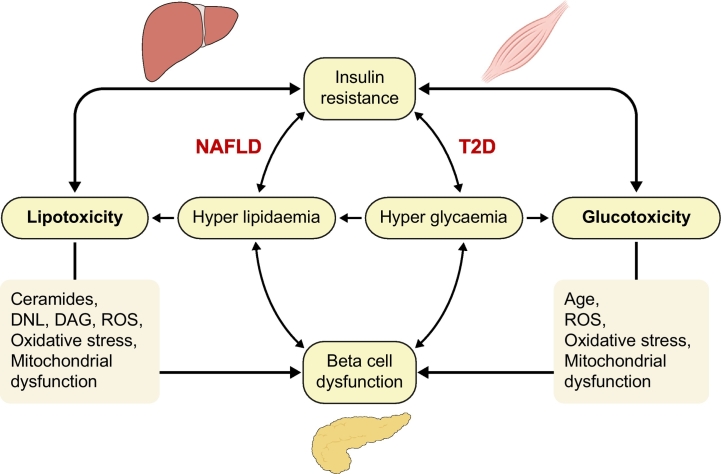
Fig. 4.
Relationship between lipo- and glucotoxicity, insulin resistance and beta-cell function.
Both lipotoxicity and glucotoxicity contribute to insulin resistance, ectopic fat accumulation and beta-cell dysfunction and vice versa. In NAFLD, lipotoxicity and insulin resistance have been recognised as pathophysiological mechanisms responsible of development and progression to a more severe form of this disease. T2D is a chronic condition of glucotoxicity, although also lipotoxicity is often present and are responsible not only of insulin resistance but also of impaired insulin secretion. DAG, diacylglycerol; DNL, de novo lipogenesis; NAFLD, non-alcoholic fatty liver disease; ROS, reactive oxygen species; T2D, type 2 diabetes.
Adipose tissue IR can be estimated simply by calculating the Adipo-IR index, as the product of fasting plasma insulin x fasting plasma FFA. Adipo-IR is a reliable marker of dysfunctional adipose tissue and is increased in NAFLD13,17,[68], [69], [70] and in T2D.13
Role of glucotoxicity in promoting NASH and disease progression
Glucotoxicity is defined as chronically elevated glucose concentrations causing glucose-induced IR, cellular dysfunction and a cycle of progressive metabolic deterioration. T2D is a chronic condition of glucotoxicity, although lipotoxicity is also often present (Fig. 4). Glucotoxicity in T2D often develops from either excess glucose production or an excess consumption of carbohydrates and sugars in the presence of decreased glucose clearance. In particular, fructose and sucrose are considered particularly lipotoxic to hepatocyte function since they have been shown to stimulate DNL and ectopic fat accumulation. However, glucotoxicity might be particularly important in individuals with IGT, where the risk of progression from IGT to diabetes is closely related to the 2-hour plasma glucose test, HbA1c and adipose tissue IR.71
Glucotoxicity and lipotoxicity are closely interrelated and both contribute to worsening IR and impaired insulin secretion72 (Fig. 4). DNL is increased in the presence of hyperglycaemia and/or as a consequence of excess carbohydrate intake, and can favour the synthesis of palmitate.36 Chronically elevated plasma glucose concentrations promote DNL through 2 distinct mechanisms: 1) directly, by increasing TCA cycle activity and synthesis of Acyl CoA that acts as a substrate of both gluconeogenesis and DNL31; and 2) indirectly, by activating the expression of ChREBP and liver X receptor α (LXRα) which in turn promote gene transcription of ACL, FAS and SCD-1.73 Glucotoxicity also stimulates the activation of ChREBP in the pancreas, kidney, and skeletal muscle, and exacerbates lipotoxicity, impairing insulin secretion (pancreas) and worsening IR in these tissues. On the other hand, ChREBP expression is decreased in adipocytes, which may exacerbate the state of IR by affecting the release of specific adipokines and lipid species.73 Chronically elevated concentrations of plasma glucose lead to functional and structural damage in beta cells, promoting oxidative stress and production of reactive oxygen species,74 cytoplasmic DNA fragmentation, alteration of mitochondrial morphology and activation of pro-apoptotic pathways.75
Genetics: links to NAFLD in diabetes
Genetic studies also support the hypothesis that IR promotes lipogenesis and adiposity.48,49 Using integrative genomic approaches, these authors48,49 have identified a cluster of genes associated with IR, of which the most important is PPARG, which is also associated with the reduced capacity of subcutaneous adipose tissue to expand, resulting in ectopic fat accumulation, NAFLD and a higher visceral-to-subcutaneous adipose tissue ratio.
However, not all individuals with NAFLD are equally resistant to insulin and it is now established that NAFLD has a dual “genetic” and “metabolic” origin. Individuals with mutations in PNPLA3, TM6SF2, MBOAT7, DGAT, or hypo-betaliproteinaemia, are at an increased risk of NASH and severe liver disease.2 They are usually overweight or obese and IR, but compared to individuals without NAFLD with similar anthropometric characteristics they have similar IR in muscle and/or liver.17,[76], [77], [78] Moreover, patients with PNPLA3-148MM mutations showed a better response to a 6-day hypocaloric low-carbohydrate diet, with a greater decrease in liver fat content and better improvement in insulin sensitivity.79 Thus, it is still unclear why patients with “genetic NAFLD” accumulate hepatic fat. Different factors appear to act as the primary drivers, although obesity further increases the risk of NASH associated with PNPLA3, TM6SF2 and GCKR genotype.80
Other genes have been associated with an increased risk of developing NAFLD/NASH (reviewed in76). They include genes associated with glucose metabolism and IR, as well as insulin secretion (i.e., ENPP1; IRS1; KLF6; GCKR; SLC2A1; TCF7L2; PPARG), hepatic lipid metabolism (synthesis: DGAT2, SLC25A13; export and oxidation: PEMT, MTTP, APOC3, APOE, TM6SF2; hepatic triglyceride hydrolysis: ATGL, LIPA), and increased risk of oxidative stress and inflammation (SOD2, NOS2, GCLC, TLR4; CD14; TNF; IL6; ADIPOQ, ADIPOR1, ADIPOR2, STAT3). However, only a few of the aforementioned genetic polymorphisms are associated with an increased risk of T2D.81 The most important is probably transcription factor 7–like 2 (TCF7L2), which increases the risk of both T2D82,83 and of NAFLD.84 The risk T allele is associated with beta-cell dysfunction due to both impaired insulin secretion and the incretin response, as well as increased hepatic glucose production.83
Also, genes involved in lipid metabolism are associated with an increased risk of both T2D and NAFLD. Reported polymorphisms include SREBP-2 (involved in cholesterol metabolism), SREBF-2 (regulator of uptake and fatty acid biosynthesis),85 and ADIPOQ that modulates fatty acid oxidation and glucose metabolism.86,87 Genes controlling apolipoprotein B (APOB), apolipoprotein C-III (APOC3), and microsomal triglyceride transfer protein (MTTP) have also been associated with NAFLD.76 APOB and APOC3 are important since they are involved in the mobilisation of hepatic fat through the secretion of VLDL and thus have an impact on fasting plasma concentration of triglycerides. The association between APOC3 and NAFLD has been shown by some but not confirmed by others, indicating that it is probably not specific for NAFLD but only for increased VLDL secretion. The MTTP gene has been implicated in lipoprotein synthesis and secretion by the liver. The polymorphism 493 G/T is associated with liver steatosis and low plasma concentrations of total and low-density lipoprotein-cholesterol (LDL-c), probably because of reduced VLDL secretion.76
TM6SF2 has been linked to the secretion of VLDL, with some polymorphisms resulting in hepatic fat accumulation (but lower circulating atherogenic lipids) and hepatic fibrosis progression.88 Dongiovanni et al.89 confirmed the severity of histological damage associated with the TM6SF2 E167K variant and also showed that TM6SF2 E167K is associated with a lower prevalence of carotid plaques in histologically confirmed NAFLD and reduced cardiovascular events in the SOS study, indicating that mutations in the TM6SF2 gene are a risk factor for NAFLD but at the same time might reduce the risk of cardiovascular disease (CVD). The GCKR (glucokinase regulator) gene regulates the enzyme glucokinase, responsible both for hepatic and pancreatic glucose metabolism. The polymorphism rs780094 is associated with a higher incidence of both NAFLD and diabetes,76,90 while the polymorphism rs1260326-P446L protects against T2D despite high circulating triglyceride levels.76,91 Other genes like KLF6 are also implicated in the regulation of glucokinase as we have shown.92
Pharmacological treatment: Management of NASH with existing T2D medications
Lifestyle is the cornerstone of treatment for patients with obesity, T2D, and NAFLD. Many trials have reported a decrease in the rate of onset of T2D with lifestyle modification in patients with IGT. Patients with obesity, T2D, and/or NAFLD have an increased cardiovascular risk. However, a reduction in CVD in long-term lifestyle intervention trials, where weight loss was overall rather modest (≤5%), has proven difficult to demonstrate in patients with prediabetes.[93], [94], [95] Cardiovascular risk reduction has been reported in bariatric surgery studies where there is a sustained and significant reduction in body weight (≥20%).96
Glucagon-like peptide-1 receptor agonists for the treatment of NAFLD
After metformin, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have become the second-line therapy for patients with T2D (along with SGLT inhibitors) because they help restore normoglycaemia as well as ameliorate the risk of CVD or chronic kidney disease.97 Their modified chemical structure, compared to native GLP-1, makes them more resistant to enzymatic degradation by DPP-4, allowing for a prolonged duration of action. Exenatide and lixisenatide are examples of rather short-acting GLP-1RAs, while longer-acting formulations include liraglutide and weekly formulations of exenatide, albiglutide, dulaglutide and semaglutide. They promote satiety and weight loss through direct effects on the central nervous system. They also reverse abnormal insulin and glucagon secretion in T2D. They also have a myriad of other beneficial metabolic effects,98,99 many relevant to the pathophysiology of NAFLD. In vitro and in vivo studies have suggested that GLP-1RAs induce hepatic gene expression of pathways that improve autophagy/endoplasmic reticulum stress, macrophage recruitment, and enhance mitochondrial function and hepatocyte fatty acid oxidation, resulting in a decrease in steatosis and inflammation.[100], [101], [102], [103], [104], [105], [106], [107] However, their mechanism of action in humans is less clear. GLP-1RAs induce weight loss,108 although there are clear differences in their potency, with minimal weight loss with albiglutide in contrast to the significant weight loss of about 10% observed with semaglutide.109,110
While earlier studies suggested that GLP-1RAs could directly improve hepatic glucose/lipid metabolism and decrease steatosis through GLP-1 signalling,111,112 this has not been confirmed in more recent studies.102,113 It has been postulated that the hepatic effects of GLP-1 and GLP-1RAs are mediated mainly by indirect pathways, such as modulation of insulin and glucagon levels, or amelioration of IR by changes in body weight or other mechanisms. However, this does not fully explain how the infusion of GLP-1 results in a decrease in hepatic glucose production when insulin and glucagon secretion are clamped at basal levels using somatostatin and hormone replacement.114,115 The hepatic effect of a short-acting GLP-1RA, like exenatide, is acute and observable from the first dose.116 Exenatide acutely diminishes hepatic IR by decreasing hepatic glucose production and increasing hepatic glucose uptake during an OGTT, independent of glucagon.116 A recent study in an animal model of NASH reported that exenatide reversed lipotoxicity and mitochondrial dysfunction, but given mild weight loss, it is difficult to establish the relative role of direct vs. indirect hepatic effects.117 Amelioration of hepatic IR after GLP-1RA treatment has been shown not only in individuals with T2D,118 but also in those with NASH119 or with morbid obesity.120 GLP-1RA therapy has also resulted in a reduction in the risk of CVD and chronic kidney disease. However, the mechanisms are still unclear121 and likely complex given that GLP-1 receptors are widely distributed across many tissues.102,113 However, their metabolic and cardiovascular benefit is likely to go beyond that of weight loss alone, as albiglutide (a GLP-1RA that does not induce significant weight loss) has recently been associated with a reduction in CVD.122
A number of pooled analyses from randomised-controlled trials (RCTs) in patients with T2D indicate that GLP-1RAs reduce plasma aminotransferases, interpreted as a surrogate endpoint of hepatocyte improvement in NAFLD.108,[123], [124], [125] Consistent with these findings many small, uncontrolled pilot studies have reported a lowering of plasma aminotransferases[126], [127], [128], [129], [130], [131], [132] or intrahepatic triglyceride (IHTG)127,128 (BI)130,132 with GLP-1RAs. Adding a GLP-1RA to basal insulin appears to be more effective in lowering plasma alanine aminotransferase (ALT) levels or IHTG than basal-bolus insulin therapy alone.[131], [132], [133], [134] Liraglutide induces resolution of NASH in patients with biopsy-proven NASH135 and an improvement in hepatic and adipose tissue insulin sensitivity.119 This was only in part mediated by reductions in body weight.135 Still, weight loss may be an important factor, especially for improvement in hepatic steatosis, although this has not been observed in all studies.136,137 It is a challenge to interpret the effect of liraglutide separately from weight loss as it occurs in most studies (10 out of 11 studies).138 Petit et al.139 treated 68 patients with uncontrolled T2D and NAFLD with liraglutide and reported that the reduction in IHTG was highly correlated with baseline liver fat accumulation and the magnitude of weight loss (both p ≪0.0001), and much less with the change in HbA1c (p = 0.034). Liver fat content was unchanged in those without a significant decrease in body weight.139 Also in a recent study by Matikainen et al.,140 where a dietary-induced weight loss control group was included to match the weight change induced by liraglutide, the decrease in IHTG was numerically greater but not significantly different between both groups, especially when multiple regression analysis and weight change was included as an independent variable. In general, GLP-1RAs are well tolerated and the gastrointestinal side effects minimised with careful titration. Ongoing studies will clarify the role of these agents in the management of NASH. Finally, “dual agonists” combining GLP-1RAs with glucagon141 are also under intense investigation for their potential role in T2D, obesity and NASH.
Dipeptidyl peptidase-IV inhibitors for the treatment of NAFLD
Dipeptidyl peptidase (DPP)-IV inhibitors have been available for the treatment of T2D for about a decade. They are not particularly potent glucose-lowering agents (HbA1c decreases only about 0.5–0.7%) but have become popular among clinicians given their ease of use, oral once daily dosing, potential for combination in the same tablet with metformin, relative low cost and excellent safety record.142 They primarily lower the postprandial glucose level, as they inhibit the plasma degrading enzyme DPP-IV, leading to higher plasma GLP-1 levels.98 There is a good rationale for their use to treat NAFLD as many studies in vitro143 and in animal models of NAFLD/NASH[144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154] have reported marked beneficial effects.
Several uncontrolled studies have reported that treatment with DPP-IV inhibitors lowers plasma aminotransferase levels.[155], [156], [157], [158], [159], [160], [161] In one study, a lower plasma ALT was only observed in those with the most uncontrolled diabetes at baseline.162 Kato et al.163 observed that while sitagliptin reduced IHTG compared to glimepiride, this was also associated with significant weight loss, possibly from concomitant lifestyle changes. Other studies have also reported a modest reduction of hepatic steatosis.164,165 Of note, DPP-IVs per se are believed to be weight neutral.98,142 Liver histological improvement has been reported with sitagliptin in an open-label study in 40 patients with biopsy-proven NASH (also biopsied after treatment), but again results were confounded by a strong correlation between weight loss and histological changes. Others have failed to see any decrease in plasma aminotransferases or IHTG.137,[166], [167], [168], [169] Of note, in all 3 controlled studies treatment with sitagliptin resulted in negative findings.137,167,168 Taken together, while a number of mechanisms have been proposed for DPP-IV inhibitors in animal models of NASH,98,170 strong clinical evidence for their clinical use in NAFLD/NASH is still lacking.
Metformin and pioglitazone
Among insulin-sensitizers, metformin does not significantly impact resolution of NASH or fibrosis, as reviewed elsewhere171 and is considered neutral by current guidelines.2,172
Pioglitazone has shown beneficial effects in NASH.[173], [174], [175], [176] It activates peroxisome proliferation-activated receptor gamma (PPAR-γ), a nuclear receptor that adopts a heterodimer configuration with retinoid X receptor (RXRα).177,178 Its activation reverses IR and improves glucose and lipid metabolism, with redistribution of excess triglyceride accumulation from the liver (as well as skeletal muscle and other tissues) to a “metabolically healthier” adipose tissue, explaining the frequent weight gain observed in patients treated with pioglitazone. Other likely mechanisms by which pioglitazone reverses the state of “lipotoxicity” in NASH include an increase in plasma adiponectin (2- to 3-fold) and an amelioration of subclinical inflammation.179 Part of the clinical benefit of pioglitazone in NASH has also been linked to its partial PPAR-α agonism180,181, which enhances hepatocyte fatty acid oxidation and lipoprotein changes leading to a reduction in plasma triglyceride concentrations and an increase in high-density lipoprotein-cholesterol concentrations. This is in contrast to a pure PPAR-γ agonist, such as rosiglitazone, which has limited effect on necroinflammation in NASH, no effect on plasma triglyceride levels and increases LDL-c levels.182
Four RCTs[173], [174], [175], [176] have provided the evidence supporting the clinical practice guidelines in Europe2 and the United States172 that recognise that pioglitazone may be prescribed for patients with biopsy-proven NASH. Resolution of NASH ranges from ~47% (or 29% placebo-subtracted) in patients without diabetes treated with pioglitazone 30 mg/day for 2 years,175 to ~60% (or 40% placebo-subtracted) in those with T2D treated with pioglitazone 45 mg/day for up to 3 years.176 It reverses IR in adipose tissue, liver, and skeletal muscle, and improves hepatocyte steatosis, necrosis and lobular inflammation, halting rapid fibrosis progression in T2D.183,184 It may reduce fibrosis,174,176 perhaps more in those with severe fibrosis,185 but its greatest value may reside in preventing fibrosis progression (rather than regression) by turning off the metabolic drivers of liver fibrosis. These metabolic benefits go beyond the liver and the reduction of hyperglycaemia (i.e., reduction of HbA1c ~1.0–1.5%), translating into a decrease in cardiovascular risk in patients with or without T2D.[186], [187], [188], [189]
One would expect pioglitazone to be used more widely in patients with NASH, with or without T2D, given the positive results from RCTs,[173], [174], [175], [176] the guideline recommendations,2,172 and the difficulty of treating NAFLD with significant long-term weight loss. However, outside endocrinology, many clinicians are not familiar with the results of recent RCTs, or misunderstand the metabolic significance of pioglitazone-induced weight gain and other potential side effects, leading to its underuse. It is also not FDA-approved for NASH, only holding an indication for the treatment of T2D. Pioglitazone-induced weight gain ranges from 3% to 5% in patients with NASH, in RCTs lasting 6 months to 3 years[173], [174], [175], [176] or longer in patients with T2D.[186], [187], [188], [189] It tends to occur predominantly in the first 6–12 months of therapy. Clinicians may naturally steer away from a therapy that may be associated with weight gain given the embedded medical training that any weight gain is harmful, or the belief that to improve liver histology weight loss is mandatory. However, by restoring normal adipocyte biology, pioglitazone causes a radical switch from insulin resistant adipose tissue to a metabolically “healthy” obese state (i.e., leading to near-normal or at least significantly improved insulin sensitivity). Indeed, weight gain during pioglitazone treatment often indicates an improvement in insulin action in insulin-sensitive tissues (fat, liver, muscle) that is associated with an increase in plasma adiponectin, reduction in plasma FFA and “lipotoxicity”, amelioration of subclinical inflammation (i.e., high-sensitivity C-reactive protein and other adipokines) and a reduction in visceral fat (most metabolically harmful), as reported in long-term clinical trials in NASH175,176 and diabetes.[186], [187], [188], [189] Weight gain may be ameliorated with proper nutritional counselling, but worsened if combined with high-dose insulin regimens, so careful assessment is needed in this setting. An alternative approach that is gaining momentum among hepatologists is to prescribe low-dose pioglitazone (15 mg/day) and titrate over time as needed. Dose-response studies show that lower dose pioglitazone (15 mg/day) retains significant biological activity with minimal weight gain (≪1 kg) and rarely oedema.[190], [191], [192], [193] However, the efficacy of low-dose pioglitazone (15 mg/day) in NASH remains to be established.
Significant misunderstanding exists about the effects of pioglitazone on cardiac function. Pioglitazone reduces cardiovascular events in patients with and without T2D.[186], [187], [188], [189] However, because it may cause oedema, and there are reports of congestive heart failure (CHF) associated with pioglitazone use,186 many practitioners believe that it causes it. Indeed, pioglitazone improves left ventricular diastolic/systolic function as well as cardiac muscle insulin sensitivity.194,195 While lower extremity oedema may develop in a minority of treated patients, more often when associated with high doses of insulin, the thiazolidinedione does not cause CHF per se. The confusion arises because undiagnosed heart failure with preserved ejection fraction (i.e., left ventricular diastolic dysfunction) is common in patients with NAFLD or T2D. In a recent study in 2,813 middle-aged patients with T2D screened by echocardiography, the prevalence of mild/moderate left ventricular diastolic dysfunction was 35% (95% CI 24%–46%),196 and even higher in patients with diabetes and NAFLD.197 Pioglitazone is contraindicated in patients with clinical evidence of CHF. If fluid retention occurs during pioglitazone therapy in patients who are unaware that they have NAFLD and left ventricular diastolic dysfunction, it would seem to be causing CHF, rather than unmasking it. One way to avoid such a scenario is to obtain a careful history, and if CHF is suspected, perform complementary studies (i.e., plasma brain natriuretic peptide, echocardiogram, other cardiac testing), and if unsure, withhold pioglitazone or start it at the lowest possible dose (15 mg/day).
The effects of pioglitazone on bone metabolism remain poorly understood198 but call for close monitoring during long-term treatment, which could be achieved by taking a baseline bone mineral density measurement, followed by repeat tests every 2 years (or sooner in patients with significant bone disease).A recent study in patients with NASH did not observe increased fractures, but the study was relatively small.199 An increase in lumbar spine and femoral neck fracture risk, in both males and females, was shown quite conclusively in a large long-term (4 years) RCT.200 Finally, mixed results in the literature have been reported about the relationship between thiazolidinediones and bladder cancer, which remains uncertain, although most studies have failed to observe such an association, particularly if low quality studies are excluded.201
In summary, many general practitioners and specialists remain unaware that pioglitazone has the potential to induce resolution of NASH in ~60% of patients, as well as conferring extrahepatic benefits (i.e., reduction of new-onset diabetes or CVD). However, it is likely that its use will continue to increase in patients with NASH to prevent fibrosis progression. Of interest, many novel insulin-sensitizers are being investigated for the treatment of NASH, with the aim of achieving an improved safety and efficacy profile.170
SGLT2 inhibitors for the treatment of NAFLD
From a broad spectrum of animal models to large multicentre RCTs, SGLT2 inhibitors have been shown to improve glucose homeostasis, as well as conferring cardiorenal protection via multiple mechanisms.202 This building body of evidence has led to the recent diabetes management guidelines16 that place SGLT2 inhibitors (along with GLP-1RAs) as second-line agents for the management of T2D.97 There is also a growing expectation that this class of agents will assist in the treatment of NASH. As recently reviewed,170 a number of recent in vivo studies report significant benefits, including the reversal of liver steatosis, hepatocyte necrosis, inflammation and/or fibrosis. Most clinical studies have reported a reduction in plasma aminotransferases with the SGLT2 inhibitors empagliflozin,203,204 dapagliflozin,[205], [206], [207] canagliflozin,[207], [208], [209], [210] luseogliflozin,211 and ipragliflozin[212], [213], [214], [215], [216] in patients with T2D. Typically, the reduction in plasma ALT concentration is proportional to the magnitude of weight loss, with higher baseline plasma aminotransferases resulting in a more significant reduction with treatment.
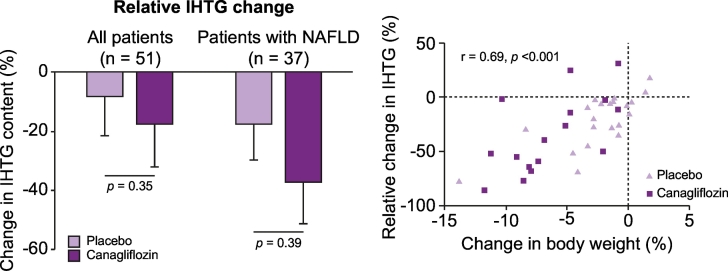
While the assumption is that a reduction in plasma aminotransferases reflects a reduction in hepatic steatosis and necroinflammation, there are no controlled trials with liver histology as the primary endpoint. Liver imaging trials have led to more mixed results. Several small, uncontrolled 6-month studies have reported a decrease in IHTG, either using the liver-to-spleen attenuation ratio (a rather qualitative measurement of liver fat)211,213,216 or proton magnetic resonance spectroscopy (1H-MRS).215 However, placebo-controlled studies have been negative despite reductions in subcutaneous and visceral fat, as observed in an early study of dapagliflozin where IHTG was measured by 1H-MRS.217 Recently, a 24-week RCT treated 56 patients with uncontrolled T2D with canagliflozin 300 mg or placebo, where hepatic insulin sensitivity was measured by a 2-step euglycaemic-insulin clamp and IHTG by 1H-MRS.218 In patients with NAFLD (n = 37), canagliflozin significantly improved hepatic insulin sensitivity. Canagliflozin also improved pancreatic beta-cell function and insulin clearance. However, the relative decrease in IHTG, while numerically greater with canagliflozin than placebo, did not reach statistical significance (-38% vs. -20%, respectively, p = 0.09; Fig. 5, left panel). Also, a weight loss ≥5% associated with a ≥30% relative reduction in IHTG, was more common in patients on canagliflozin than placebo (38% vs. 7%, respectively, p ≪0.01). The investigators concluded that while canagliflozin improved hepatic insulin sensitivity, reduction in IHTG appeared to fundamentally depend on the magnitude of weight loss (Fig. 5, right panel), which was greater and more frequent with the SGLT2 inhibitor.
Fig. 5.
Effect of SGLT2 inhibitor on IHTG and body weight.
Left panel: Effect of the SGLT2 inhibitor canagliflozin vs. placebo on relative IHTG content among all patients (left) or only patients with a diagnosis of NAFLD (right) at baseline. Right panel: Relationship between changes in body weight after canagliflozin or placebo treatment and change in IHTG. Reproduced with permission from 218.
IHTG, intrahepatic triglyceride; NAFLD, non-alcoholic fatty liver disease.
In summary, SGLT2 inhibitors decrease plasma ALT concentrations, and often hepatic steatosis, if accompanied by weight loss. The clinical translation of these findings to the treatment of NASH is unknown in the absence of studies with histological outcomes. Only a case report,219 and an uncontrolled 12-week study in 10 patients with biopsy-confirmed NASH and T2D,210 have suggested some benefit with canagliflozin on liver fat and fibrosis biomarkers, but both studies were too small to offer any firm conclusions. If improvement of steatohepatitis is only dependent on weight loss, one would anticipate the effect to be rather modest. Usually weight loss with SGLT2 inhibitors is in the range of 3 to 5% while resolution of NASH and of fibrosis calls for a weight loss of ~7 to 10%.172 Of note, “positive studies” with SGLT2 inhibitors in NAFLD have been open-label, short-term trials (12 to 24-week duration) and not placebo-controlled RCTs. SGLT2 inhibitors are usually well tolerated but physicians should educate patients about their potential risks, such as osmotic diuresis/volume depletion that may lead to orthostatic hypotension and falls, vulvovaginitis/balanitis and related genital mycotic infections, urinary tract infections, osteoporosis and lower extremity amputations (canagliflozin) and diabetic ketoacidosis (although rare in patients with T2D).220
Insulin for the treatment of NAFLD
Insulin remains a valuable agent for many patients with T2D that remain uncontrolled on a combination of oral agents or have comorbidities that preclude a given patient from using them. The effect of insulin has not been extensively studied in NAFLD and there are no studies examining its effect on liver histology. One study reported that 76% of patients with T2D not previously treated with insulin had hepatic steatosis, measured by magnetic resonance imaging, compared to 62% of those treated with insulin, while the median hepatic fat fraction was 13% compared to 10.2%, respectively (both p ≪0.01).221 Basal exogenous insulin decreases hepatic fat accumulation222,223 likely by suppressing FFA and glucose flux to the liver in patients with T2D and NAFLD.
Conclusions
NAFLD in T2D is a disease with unclear mechanisms and an individual genetic susceptibility that promotes the development and progression from fatty liver disease to NASH, cirrhosis and hepatocellular carcinoma. Not only the close association with obesity, but also with IR and lipotoxicity, increases the risk of T2D and related comorbidities in NAFLD. Screening in the primary care setting, diabetes clinics and among hepatologists is more relevant than ever, as effective therapies are available, including lifestyle changes that induce weight loss, bariatric surgery, and medications currently available to treat diabetes and proven to be effective in NASH (i.e., pioglitazone, liraglutide). The American Diabetes Association in 2019 recommended universal screening for advanced fibrosis in all patients with prediabetes or T2D with steatosis or elevated ALT, in an effort to identify and treat those at the highest risk of developing advanced fibrosis.224
Of note, the target of screening and treatment is not steatosis per se, but NASH-induced liver fibrosis as it has been associated with increased morbidity and mortality.2,172 Many new compounds are undergoing phase IIB/III RCTs. It is critically important to increase awareness among primary care physicians, specialists and health policy makers about the risks associated with this disease, since early diagnosis and treatment will be the only way to tackle the looming epidemic of NASH.
Financial support
This work was partially supported by the European Union (EU) Program Horizon H2020 under grant agreement no. 634413 for the project EPoS-“Elucidating Pathways of Steatohepatitis” (A.G.) and funds of the University of Florida (K.C.).
Conflict of interest
AG is a consultant for Eli Lilly, Inventiva, Genentech, Menarini, Gilead, outside the submitted work. KC has received research support as principal investigator from the National Institutes of Health (NIH), Cirius, Echosens, Inventiva, Novartis, Novo Nordisk, Poxel and Zydus. KC is a consultant for Allergan, Astra-Zeneca, BMS, Boehringer Ingelheim, Coherus, Deuterex, Eli Lilly, Genentech, Gilead, Janssen, Pfizer, Poxel, Novo Nordisk, and Sanofi-Aventis.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Both authors contributed equally to this manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.07.002.
Contributor Information
Amalia Gastaldelli, Email: amalia@ifc.cnr.it.
Kenneth Cusi, Email: Kenneth.Cusi@medicine.ufl.edu.
Supplementary data
Supplementary material
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.EASL, Marchesini G, Day CP, Dufour J-F, Canbay A, Nobili V. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 5.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166–174. doi: 10.2337/diabetes.54.1.166. [DOI] [PubMed] [Google Scholar]
- 10.Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- 11.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 13.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 14.Isokuortti E, Zhou Y, Peltonen M, Bugianesi E, Clement K, Bonnefont-Rousselot D. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017 doi: 10.1007/s00125-017-4340-1. [DOI] [PubMed] [Google Scholar]
- 15.Rosso C, Mezzabotta L, Gaggini M, Salomone F, Gambino R, Marengo A. Peripheral insulin resistance predicts liver damage in nondiabetic subjects with nonalcoholic fatty liver disease. Hepatology. 2016;63:107–116. doi: 10.1002/hep.28287. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association A 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 17.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017;6:943–957. doi: 10.1016/j.molmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 20.Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:2178–2187. doi: 10.1002/hep.26988. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, Defronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 22.Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 23.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 24.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 25.Gastaldelli A, Miyazaki Y, Pettiti M, Santini E, Ciociaro D, Defronzo RA. The effect of rosiglitazone on the liver: decreased gluconeogenesis in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:806–812. doi: 10.1210/jc.2005-1159. [DOI] [PubMed] [Google Scholar]
- 26.Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quinones-Galvan A, Sironi AM. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50:1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junker AE, Gluud L, Holst JJ, Knop FK, Vilsboll T. Diabetic and nondiabetic patients with nonalcoholic fatty liver disease have an impaired incretin effect and fasting hyperglucagonaemia. J Intern Med. 2016;279:485–493. doi: 10.1111/joim.12462. [DOI] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes. 2000;49:1367–1373. doi: 10.2337/diabetes.49.8.1367. [DOI] [PubMed] [Google Scholar]
- 30.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients. 2015;7:9453–9474. doi: 10.3390/nu7115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyotylainen T, Jerby L, Petaja EM, Mattila I, Jantti S, Auvinen P. Genome-scale study reveals reduced metabolic adaptability in patients with non-alcoholic fatty liver disease. Nat Commun. 2016;7:8994. doi: 10.1038/ncomms9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourgeois CS, Wiggins D, Hems R, Gibbons GF. VLDL output by hepatocytes from obese Zucker rats is resistant to the inhibitory effect of insulin. Am J Phys. 1995;269:E208–E215. doi: 10.1152/ajpendo.1995.269.2.E208. [DOI] [PubMed] [Google Scholar]
- 34.Wiggins D, Hems R, Gibbons GF. Decreased sensitivity to the inhibitory effect of insulin on the secretion of very-low-density lipoprotein in cultured hepatocytes from fructose-fed rats. Metabolism. 1995;44:841–847. doi: 10.1016/0026-0495(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 35.Park J, Lemieux S, Lewis GF, Kuksis A, Steiner G. Chronic exogenous insulin and chronic carbohydrate supplementation increase de novo VLDL triglyceride fatty acid production in rats. J Lipid Res. 1997;38:2529–2536. [PubMed] [Google Scholar]
- 36.Luukkonen PK, Sadevirta S, Zhou Y, Kayser B, Ali A, Ahonen L. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotronen A, Seppanen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepaa AL. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolan CJ, Larter CZ. Lipotoxicity: why do saturated fatty acids cause and monounsaturates protect against it? J Gastroenterol Hepatol. 2009;24:703–706. doi: 10.1111/j.1440-1746.2009.05823.x. [DOI] [PubMed] [Google Scholar]
- 41.Suvitaival T, Bondia-Pons I, Yetukuri L, Poho P, Nolan JJ, Hyotylainen T. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism. 2018;78:1–12. doi: 10.1016/j.metabol.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Chavez JA, Summers SA. A Ceramide-Centric View of Insulin resistance. Cell Metab. 2012;15:585–591. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefan N, Schick F, Haring HU. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26:292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gastaldelli A. Insulin resistance and reduced metabolic flexibility: cause or consequence of NAFLD? Clin Sci. 2017;131:2701–2704. doi: 10.1042/CS20170987. [DOI] [PubMed] [Google Scholar]
- 48.Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaghootkar H, Scott RA, White CC, Zhang W, Speliotes E, Munroe PB. Genetic evidence for a normal-weight "metabolically obese" phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63:4369–4377. doi: 10.2337/db14-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for beta Cell Recovery. Cell Metab. 2018 doi: 10.1016/j.cmet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Gastaldelli A, Gaggini M, DeFronzo RA. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes. 2017;66:815–822. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 52.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gastaldelli A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;93:S60–S65. doi: 10.1016/S0168-8227(11)70015-8. [DOI] [PubMed] [Google Scholar]
- 54.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 55.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 56.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389–1397. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong MJ, Hazlehurst JM, Hull D, Guo K, Borrows S, Yu J. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes Metab. 2014;16:651–660. doi: 10.1111/dom.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelsey MM, Forster JE, Van Pelt RE, Reusch JE, Nadeau KJ. Adipose tissue insulin resistance in adolescents with and without type 2 diabetes. Pediatr Obes. 2014;9:373–380. doi: 10.1111/j.2047-6310.2013.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725.e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801:299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Sunny NE, Bril F, Cusi K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol Metab. 2017;28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Grasselli E, Voci A, Demori I, De Matteis R, Compalati AD, Gallo G. Effects of binge ethanol on lipid homeostasis and oxidative stress in a rat model of nonalcoholic fatty liver disease. J Physiol Biochem. 2014;70:341–353. doi: 10.1007/s13105-013-0308-x. [DOI] [PubMed] [Google Scholar]
- 64.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013 doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. S0016-5085(05)01788-9 [pii] [DOI] [PubMed] [Google Scholar]
- 66.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. doi:55/1/136 [pii] [PubMed] [Google Scholar]
- 67.Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54:1640–1648. doi: 10.2337/diabetes.54.6.1640. doi:54/6/1640 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65:1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 70.Brouwers B, Schrauwen-Hinderling VB, Jelenik T, Gemmink A, Havekes B, Bruls Y. Metabolic disturbances of non-alcoholic fatty liver resemble the alterations typical for type 2 diabetes. Clin Sci (Lond) 2017;131:1905–1917. doi: 10.1042/CS20170261. [DOI] [PubMed] [Google Scholar]
- 71.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care. 2013;36:3607–3612. doi: 10.2337/dc13-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gastaldelli A, Ferrannini E. Chapter 3: Pathophysiology of Prediabetes: Role of Lipotoxicity? In: Bergman M, editor. Global health perspectives in prediabetes and diabetes prevention. Volume 1. World Scientific; New Jersey: 2014. pp. 31–48. [Google Scholar]
- 73.Abdul-Wahed A, Guilmeau S, Postic C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Eriksson JW. Metabolic stress in insulin's target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. S0014-5793(07)00701-6 [pii] [DOI] [PubMed] [Google Scholar]
- 75.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med. 2009;26:1185–1192. doi: 10.1111/j.1464-5491.2009.02847.x. DME2847 [pii] [DOI] [PubMed] [Google Scholar]
- 76.Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645–655. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Y, Llaurado G, Oresic M, Hyotylainen T, Orho-Melander M, Yki-Jarvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J Hepatol. 2015;62:657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sevastianova K, Kotronen A, Gastaldelli A, Perttila J, Hakkarainen A, Lundbom J. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr. 2011;94:104–111. doi: 10.3945/ajcn.111.012369. [DOI] [PubMed] [Google Scholar]
- 80.Stender S, Kozlitina J, Nordestgaard BG, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842–847. doi: 10.1038/ng.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grarup N, Sandholt CH, Hansen T, Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia. 2014;57:1528–1541. doi: 10.1007/s00125-014-3270-4. [DOI] [PubMed] [Google Scholar]
- 82.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. ng1732 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musso G, Gambino R, Pacini G, Pagano G, Durazzo M, Cassader M. Transcription factor 7-like 2 polymorphism modulates glucose and lipid homeostasis, adipokine profile, and hepatocyte apoptosis in NASH. Hepatology. 2009;49:426–435. doi: 10.1002/hep.22659. [DOI] [PubMed] [Google Scholar]
- 85.Musso G, Cassader M, Bo S, De Michieli F, Gambino R. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes. 2013;62:1109–1120. doi: 10.2337/db12-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naik A, Kosir R, Rozman D. Genomic aspects of NAFLD pathogenesis. Genomics. 2013;102:84–95. doi: 10.1016/j.ygeno.2013.03.007. S0888-7543(13)00060-8 [pii] [DOI] [PubMed] [Google Scholar]
- 87.Ramya K, Ayyappa KA, Ghosh S, Mohan V, Radha V. Genetic association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population. Gene. 2013;532:253–262. doi: 10.1016/j.gene.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 90.Li H, Xu R, Peng X, Wang Y, Wang T. Association of glucokinase regulatory protein polymorphism with type 2 diabetes and fasting plasma glucose: a meta-analysis. Mol Biol Rep. 2013;40:3935–3942. doi: 10.1007/s11033-012-2470-6. [DOI] [PubMed] [Google Scholar]
- 91.Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, Tichet J, Marre M, Balkau B. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes. 2008;57:2253–2257. doi: 10.2337/db07-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bechmann LP, Gastaldelli A, Vetter D, Patman GL, Pascoe L, Hannivoort RA. Glucokinase links Kruppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology. 2012;55:1083–1093. doi: 10.1002/hep.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Look ARG, Wing RR, Bolin P, Brancati FL. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 97.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20:5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 99.Gastaldelli A, Marchesini G. Time for Glucagon like peptide-1 receptor agonists treatment for patients with NAFLD? J Hepatol. 2016;64:262–264. doi: 10.1016/j.jhep.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 100.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samson SL, Sathyanarayana P, Jogi M, Gonzalez EV, Gutierrez A, Krishnamurthy R. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia. 2011;54:3093–3100. doi: 10.1007/s00125-011-2317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panjwani N, Mulvihill E, Longuet C, Yusta B, Campbell J, Brown T. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154:127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 103.Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes. 2014;63:3637–3646. doi: 10.2337/db14-0263. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka K, Masaki Y, Tanaka M, Miyazaki M, Enjoji M, Nakamuta M. Exenatide improves hepatic steatosis by enhancing lipid use in adipose tissue in nondiabetic rats. World J Gastroenterol. 2014;20:2653–2663. doi: 10.3748/wjg.v20.i10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He Q, Sha S, Sun L, Zhang J, Dong M. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun. 2016;476:196–203. doi: 10.1016/j.bbrc.2016.05.086. [DOI] [PubMed] [Google Scholar]
- 106.Yamamoto T, Nakade Y, Yamauchi T, Kobayashi Y, Ishii N, Ohashi T. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in non-obese mice. World J Gastroenterol. 2016;22:2512–2523. doi: 10.3748/wjg.v22.i8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ao N, Yang J, Wang X, Du J. Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stress-associated pathway. Hepatol Res. 2016;46:343–353. doi: 10.1111/hepr.12551. [DOI] [PubMed] [Google Scholar]
- 108.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pratley RE, Aroda VR, Lingvay I, Ludemann J, Andreassen C, Navarria A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 110.O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 111.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 112.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 113.Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 114.Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2013;56:156–161. doi: 10.1007/s00125-012-2738-3. [DOI] [PubMed] [Google Scholar]
- 115.Prigeon RL, Quddusi S, Paty B, D'Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–E707. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 116.Gastaldelli A, Gaggini M, Daniele G, Ciociaro D, Cersosimo E, Tripathy D. Exenatide improves both hepatic and adipose tissue insulin resistance: A dynamic positron emission tomography study. Hepatology. 2016;64:2028–2037. doi: 10.1002/hep.28827. [DOI] [PubMed] [Google Scholar]
- 117.Kalavalapalli S, Bril F, Guingab J, Vergara A, Garrett TJ, Sunny NE. Impact of exenatide on mitochondrial lipid metabolism in mice with nonalcoholic steatohepatitis. J Endocrinol. 2019;241:293–305. doi: 10.1530/JOE-19-0007. [DOI] [PubMed] [Google Scholar]
- 118.Cersosimo E, Gastaldelli A, Cervera A, Wajcberg E, Sriwijilkamol A, Fernandez M. Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects. J Clin Endocrinol Metab. 2011;96:1763–1770. doi: 10.1210/jc.2010-2146. [DOI] [PubMed] [Google Scholar]
- 119.Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64:399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Camastra S, Astiarraga B, Tura A, Frascerra S, Ciociaro D, Mari A. Effect of exenatide on postprandial glucose fluxes, lipolysis, and ss-cell function in non-diabetic, morbidly obese patients. Diabetes Obes Metab. 2017;19:412–420. doi: 10.1111/dom.12836. [DOI] [PubMed] [Google Scholar]
- 121.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 122.Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Sr., Granger CB, Jones NP. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 123.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrond B, Gough SC. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–242. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]
- 124.Gluud LL, Knop FK, Vilsboll T. Effects of lixisenatide on elevated liver transaminases: systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cusi K, Sattar N, Garcia-Perez LE, Pavo I, Yu M, Robertson KE. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med. 2018;35:1434–1439. doi: 10.1111/dme.13697. [DOI] [PubMed] [Google Scholar]
- 126.Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 127.Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bi Y, Zhang B, Xu W, Yang H, Feng W, Li C, Tong G., Li M., Wang X., Shen S., Zhu B., Weng J., Zhu D. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol. 2014;51(5):865–873. doi: 10.1007/s00592-014-0638-3. http://www.ncbi.nlm.nih.gov/pubmed/25118999. [DOI] [PubMed] [Google Scholar]
- 129.Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702–708. doi: 10.1590/s0004-27302013000900005. [DOI] [PubMed] [Google Scholar]
- 130.Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol Res. 2015;45:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 131.Savvidou S, Karatzidou K, Tsakiri K, Gagalis A, Hytiroglou P, Goulis J. Circulating adiponectin levels in type 2 diabetes mellitus patients with or without non-alcoholic fatty liver disease: Results of a small, open-label, randomized controlled intervention trial in a subgroup receiving short-term exenatide. Diabetes Res Clin Pract. 2016;113:125–134. doi: 10.1016/j.diabres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 132.Feng W, Gao C, Bi Y, Wu M, Li P, Shen S. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800–809. doi: 10.1111/1753-0407.12555. [DOI] [PubMed] [Google Scholar]
- 133.Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521–529. doi: 10.1002/dmrr.2561. [DOI] [PubMed] [Google Scholar]
- 134.Bouchi R, Nakano Y, Fukuda T, Takeuchi T, Murakami M, Minami I. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64:269–281. doi: 10.1507/endocrj.EJ16-0449. [DOI] [PubMed] [Google Scholar]
- 135.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 136.Tang A, Rabasa-Lhoret R, Castel H, Wartelle-Bladou C, Gilbert G, Massicotte-Tisluck K. Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: A randomized trial. Diabetes Care. 2015;38:1339–1346. doi: 10.2337/dc14-2548. [DOI] [PubMed] [Google Scholar]
- 137.Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588–2593. doi: 10.1007/s00125-016-4100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cusi K. Incretin-Based Therapies for the Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes. Hepatology. 2019;69:2318–2322. doi: 10.1002/hep.30670. [DOI] [PubMed] [Google Scholar]
- 139.Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The Lira-NAFLD study. J Clin Endocrinol Metab. 2017;102:407–415. doi: 10.1210/jc.2016-2775. [DOI] [PubMed] [Google Scholar]
- 140.Matikainen N, Soderlund S, Bjornson E, Pietilainen K, Hakkarainen A, Lundbom N. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: A single-centre randomized controlled study. Diabetes Obes Metab. 2019;21:84–94. doi: 10.1111/dom.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soni H. Peptide-based GLP-1/glucagon co-agonists: A double-edged sword to combat diabesity. Med Hypotheses. 2016;95:5–9. doi: 10.1016/j.mehy.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Scheen A. The safety of gliptins: updated data in 2018. Expert Opin Drug Saf. 2018;17:387–405. doi: 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- 143.Hwang HJ, Jung TW, Kim BH, Hong HC, Seo JA, Kim SG. A dipeptidyl peptidase-IV inhibitor improves hepatic steatosis and insulin resistance by AMPK-dependent and JNK-dependent inhibition of LECT2 expression. Biochem Pharmacol. 2015;98:157–166. doi: 10.1016/j.bcp.2015.08.098. [DOI] [PubMed] [Google Scholar]
- 144.Maiztegui B, Borelli MI, Madrid VG, Del Zotto H, Raschia MA, Francini F. Sitagliptin prevents the development of metabolic and hormonal disturbances, increased beta-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin Sci (Lond) 2011;120:73–80. doi: 10.1042/CS20100372. [DOI] [PubMed] [Google Scholar]
- 145.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–1257. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kern M, Kloting N, Niessen HG, Thomas L, Stiller D, Mark M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aroor AR, Habibi J, Ford DA, Nistala R, Lastra G, Manrique C. Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 2015;64:1988–2001. doi: 10.2337/db14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014;47:137–149. doi: 10.1007/s00795-013-0053-9. [DOI] [PubMed] [Google Scholar]
- 149.Fukuda-Tsuru S, Kakimoto T, Utsumi H, Kiuchi S, Ishii S. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. Eur J Pharmacol. 2014;723:207–215. doi: 10.1016/j.ejphar.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 150.Ohyama T, Sato K, Yamazaki Y, Hashizume H, Horiguchi N, Kakizaki S. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol. 2014;20:16227–16235. doi: 10.3748/wjg.v20.i43.16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ideta T, Shirakami Y, Miyazaki T, Kochi T, Sakai H, Moriwaki H. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int J Mol Sci. 2015;16:29207–29218. doi: 10.3390/ijms161226156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi SH, Leem J, Park S, Lee CK, Park KG, Lee IK. Gemigliptin ameliorates Western-diet-induced metabolic syndrome in mice. Can J Physiol Pharmacol. 2017;95:129–139. doi: 10.1139/cjpp-2016-0026. [DOI] [PubMed] [Google Scholar]
- 153.Kim MK, Chae YN, Ahn GJ, Shin CY, Choi SH, Yang EK. Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models. Arch Pharm Res. 2017;40:268–281. doi: 10.1007/s12272-016-0864-z. [DOI] [PubMed] [Google Scholar]
- 154.Nakamura K, Fukunishi S, Yokohama K, Ohama H, Tsuchimoto Y, Asai A. A long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, as a preventive drug for the development of hepatic steatosis in high-fructose diet-fed ob/ob mice. Int J Mol Med. 2017;39:969–983. doi: 10.3892/ijmm.2017.2899. [DOI] [PubMed] [Google Scholar]
- 155.Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S. Sitagliptin as a novel treatment agent for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus. Hepato-Gastroenterology. 2011;58:2103–2105. doi: 10.5754/hge11263. [DOI] [PubMed] [Google Scholar]
- 156.Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci World J. 2012;2012 doi: 10.1100/2012/496453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jung YA, Choi YK, Jung GS, Seo HY, Kim HS, Jang BK. Sitagliptin attenuates methionine/choline-deficient diet-induced steatohepatitis. Diabetes Res Clin Pract. 2014;105:47–57. doi: 10.1016/j.diabres.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 158.Kanazawa I, Tanaka K, Sugimoto T. DPP-4 inhibitors improve liver dysfunction in type 2 diabetes mellitus. Med Sci Monit. 2014;20:1662–1667. doi: 10.12659/MSM.890989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asakawa M, Mitsui H, Akihisa M, Sekine T, Niitsu Y, Kobayashi A. Efficacy and safety of sitagliptin for the treatment of diabetes mellitus complicated by chronic liver injury. Springerplus. 2015;4:346. doi: 10.1186/s40064-015-1135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med. 2018;10:23–31. doi: 10.2147/HMER.S158053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T. Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology. Jun 2019;69(6):2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepato-Gastroenterology. 2014;61:323–328. [PubMed] [Google Scholar]
- 163.Kato H, Nagai Y, Ohta A, Tenjin A, Nakamura Y, Tsukiyama H. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;109:199–205. doi: 10.1016/j.diabres.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 164.Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab. 2015;100:1578–1585. doi: 10.1210/jc.2014-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kosi-Trebotic L, Thomas A, Harreiter J, Chmelik M, Trattnig S, Kautzky-Willer A. Gliptin therapy reduces hepatic and myocardial fat in type 2 diabetic patients. Eur J Clin Investig. 2017;47:829–838. doi: 10.1111/eci.12817. [DOI] [PubMed] [Google Scholar]
- 166.Arase Y, Kawamura Y, Seko Y, Kobayashi M, Suzuki F, Suzuki Y. Efficacy and safety in sitagliptin therapy for diabetes complicated by non-alcoholic fatty liver disease. Hepatol Res. 2013;43:1163–1168. doi: 10.1111/hepr.12077. [DOI] [PubMed] [Google Scholar]
- 167.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yabiku K, Mutoh A, Miyagi K, Takasu N. Effects of Oral Antidiabetic Drugs on Changes in the Liver-to-Spleen Ratio on Computed Tomography and Inflammatory Biomarkers in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Clin Ther. 2017;39:558–566. doi: 10.1016/j.clinthera.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 170.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. Aug 2018;70(2):711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 171.Portillo-Sanchez P, Cusi K. Treatment of Nonalcoholic Fatty Liver Disease (NAFLD) in patients with Type 2 Diabetes Mellitus. Clin Diabetes Endocrinol. 2016;2 doi: 10.1186/s40842-016-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 173.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 174.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 175.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 177.Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 178.Liss KH, Finck BN. PPARs and nonalcoholic fatty liver disease. Biochimie. 2017;136:65–74. doi: 10.1016/j.biochi.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol. 2008;52:869–881. doi: 10.1016/j.jacc.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kintscher U. Pharmacological differences of glitazones: does peroxisome proliferator-activated receptor-alpha activation make the difference? J Am Coll Cardiol. 2008;52:882–884. doi: 10.1016/j.jacc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 182.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 183.Bril F, Cusi K. Nonalcoholic Fatty Liver Disease: The New Complication of Type 2 Diabetes Mellitus. Endocrinol Metab Clin N Am. 2016;45:765–781. doi: 10.1016/j.ecl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 184.Bril F, Kalavalapalli S, Clark VC, Lomonaco R, Soldevila-Pico C, Liu IC. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs. without type 2 diabetes. Clin Gastroenterol Hepatol. 2018;16:558–566.e552. doi: 10.1016/j.cgh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 185.Musso G, Cassader M, Paschetta E, Gambino R. Pioglitazone for advanced fibrosis in nonalcoholic steatohepatitis: New evidence, new challenges. Hepatology. 2017;65:1058–1061. doi: 10.1002/hep.28960. [DOI] [PubMed] [Google Scholar]
- 186.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 187.Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB., Sr. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 188.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 189.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rajagopalan S, Dutta P, Hota D, Bhansali A, Srinivasan A, Chakrabarti A. Effect of low dose pioglitazone on glycemic control and insulin resistance in Type 2 diabetes: A randomized, double blind, clinical trial. Diabetes Res Clin Pract. 2015;109:e32–e35. doi: 10.1016/j.diabres.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 191.Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Pioglitazone 014 Study G. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251–257. [PubMed] [Google Scholar]
- 192.Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25:517–523. doi: 10.2337/diacare.25.3.517. [DOI] [PubMed] [Google Scholar]
- 193.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 194.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 195.Clarke GD, Solis-Herrera C, Molina-Wilkins M, Martinez S, Merovci A, Cersosimo E. Pioglitazone improves left ventricular diastolic function in subjects with diabetes. Diabetes Care. 2017;40:1530–1536. doi: 10.2337/dc17-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab Vasc Dis Res. 2018;15:477–493. doi: 10.1177/1479164118787415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Pichiri I. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep. 2013;13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 199.Portillo-Sanchez P, Bril F, Lomonaco R, Barb D, Orsak B, Bruder JM. Effect of pioglitazone on bone mineral density in patients with nonalcoholic steatohepatitis: A 36-month clinical trial. J Diabetes. 2019;11:223–231. doi: 10.1111/1753-0407.12833. [DOI] [PubMed] [Google Scholar]
- 200.Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL. Pioglitazone and Risk for Bone Fracture: Safety Data From a Randomized Clinical Trial. J Clin Endocrinol Metab. 2017;102:914–922. doi: 10.1210/jc.2016-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Filipova E, Uzunova K, Kalinov K, Vekov T. Pioglitazone and the risk of bladder cancer: A meta-analysis. Diabetes Ther. 2017;8:705–726. doi: 10.1007/s13300-017-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 203.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 204.Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME(R) trial. Diabetologia. 2018;61:2155–2163. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 206.Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: A prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bajaj HS, Brown RE, Bhullar L, Sohi N, Kalra S, Aronson R. SGLT2 inhibitors and incretin agents: Associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018;44:493–499. doi: 10.1016/j.diabet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 208.Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32. doi: 10.1016/j.diabet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 209.Seko Y, Sumida Y, Sasaki K, Itoh Y, Iijima H, Hashimoto T. Effects of canagliflozin, an SGLT2 inhibitor, on hepatic function in Japanese patients with type 2 diabetes mellitus: pooled and subgroup analyses of clinical trials. J Gastroenterol. 2018;53:140–151. doi: 10.1007/s00535-017-1364-8. [DOI] [PubMed] [Google Scholar]
- 210.Seko Y, Nishikawa T, Umemura A, Yamaguchi K, Moriguchi M, Yasui K. Efficacy and safety of canagliflozin in type 2 diabetes mellitus patients with biopsy-proven nonalcoholic steatohepatitis classified as stage 1-3 fibrosis. Diabetes Metab Syndr Obes. 2018;11:835–843. doi: 10.2147/DMSO.S184767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
- 212.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig. 2016;36:313–319. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 213.Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: A randomized, 24-week, open-label, active-controlled trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 214.Takase T, Nakamura A, Miyoshi H, Yamamoto C, Atsumi T. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocr J. 2017;64:363–367. doi: 10.1507/endocrj.EJ16-0295. [DOI] [PubMed] [Google Scholar]
- 215.Ohta A, Kato H, Ishii S, Sasaki Y, Nakamura Y, Nakagawa T. Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother. 2017;18:1433–1438. doi: 10.1080/14656566.2017.1363888. [DOI] [PubMed] [Google Scholar]
- 216.Bando Y, Ogawa A, Ishikura K, Kanehara H, Hisada A, Notumata K. The effects of ipragliflozin on the liver-to-spleen attenuation ratio as assessed by computed tomography and on alanine transaminase levels in Japanese patients with type 2 diabetes mellitus. Diabetol Int. 2017;8:218–227. doi: 10.1007/s13340-016-0302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 218.Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. Nov 16 2019;21(4):812–821. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 219.Takeda A, Irahara A, Nakano A, Takata E, Koketsu Y, Kimata K. The improvement of the hepatic histological findings in a patient with non-alcoholic steatohepatitis with type 2 diabetes after the administration of the sodium-glucose cotransporter 2 inhibitor ipragliflozin. Intern Med. 2017;56:2739–2744. doi: 10.2169/internalmedicine.8754-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia. 2018;61:2118–2125. doi: 10.1007/s00125-018-4663-6. [DOI] [PubMed] [Google Scholar]
- 221.Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017;19:1630–1634. doi: 10.1111/dom.12973. [DOI] [PubMed] [Google Scholar]
- 222.Juurinen L, Tiikkainen M, Hakkinen AM, Hakkarainen A, Yki-Jarvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E829–E835. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]
- 223.Cusi K, Sanyal AJ, Zhang S, Hoogwerf BJ, Chang AM, Jacober SJ. Different effects of basal insulin peglispro and insulin glargine on liver enzymes and liver fat content in patients with type 1 and type 2 diabetes. Diabetes Obes Metab. 2016;18:50–58. doi: 10.1111/dom.12751. [DOI] [PubMed] [Google Scholar]
- 224.American Diabetes A 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S34–S45. doi: 10.2337/dc19-S004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material