Abstract
Cell–cell and cell–substrate adhesion are essential to the proper formation and maintenance of tissue patterns during development, and deregulation of these processes can lead to invasion and metastasis of cancer cells. Cell surface adhesion and signaling molecules are key players in both normal development and cancer progression. One set of cell surface proteins, the Eph receptor tyrosine kinases and their membrane-bound ligands, ephrins, are significant regulators of these processes. During embryonic development, the Eph/ephrin signaling system is involved in cell–cell contact events that result in cell sorting and boundary formation between receptor and ligand bearing cells. When migrating cells that display the membrane bound ligands or receptors come in contact with cells bearing the cognate partner, the response may be adhesion or repulsion, ultimately leading to the proper positioning of these cells. During cancer progression, the signaling between these receptor/ligand pairs is often deregulated, leading to increased invasion and metastasis. To gain mechanistic insight into the pathways that mediate Eph receptor and ephrin signaling we have relied upon a very tractable system, the frog Xenopus. This model system has proven to be extremely versatile, and represents a relatively quick and manipulable system to explore signaling events and the in vivo processes affected by these signals.
Keywords: Xenopus, Eph, development
1 ∣. EPHB/EPHRINB SIGNALING
The EphB/ephrinB signaling system sends signals affecting cell–cell junctions and cell movement. This signaling system affects morphogenesis during development and has been shown to play an instructive role in angiogenesis, as well as invasion of tumors (Pasquale, 2010). Eph receptors are transmembrane receptor tyrosine kinases and the intracellular domain contains a number of tyrosine phosphorylation sites, a single kinase domain, and a Sterile Alpha Motif and a C-terminal PDZ binding motif. These receptors are divided into two subclasses (A & B) determined by the sequence, and binding affinities to either A or B-type ligands, termed ephrins. The ephrins are all membrane-bound proteins with the A subclass being glycosylphosphatidylinositol (GPI)-linked to the membrane, and the B-type are transmembrane proteins with a short cytoplasmic domain. Generally, the A-type receptors have specificity toward A-type ligands, while B-type bind B receptors, but there are limited exceptions to this rule (Pasquale, 2010). Activation of the Eph receptors upon ligand binding, is referred to as ‘forward’ signaling, while ‘reverse’ signaling may occur within the ephrin residing cell as a result of this interaction. Similar to other receptor tyrosine kinases, ligand binding induces trans-phosphorylation and activation that often results in downstream regulation of Rho family of GTPases, but phosphorylation-independent signaling has also been described (Singh, Winterbottom, & Daar, 2012).
Eph receptors and ephrin ligands signal in a bi-directional manner, where both molecules transmit intracellular signals upon cell–cell contact. These interactions induce cell repulsive or attractive responses in several cell types. Unlike the receptors, the B-type transmembrane ephrin ligands do not possess intrinsic catalytic activity for signaling, and depend upon a scaffolding activity that recruits signaling molecules to transmit an effect on cell function. EphrinB signaling encompasses both phosphorylation-dependent and -independent signaling pathways. Upon binding a cognate Eph receptor, ephrinBs become tyrosine phosphorylated in the intracellular domain mediated by Src, which leads to the recruitment of signaling molecules that exert a functional effect. Unphosphorylated ephrinB can also transduce signals through associating with proteins of a signaling complex, but upon tyrosine phosphosphorylation, the interaction of ephrinB with the signaling complex is disrupted or modulated (Daar, 2012). Since it is the ability to regulate cell–cell adhesion and cell motility that makes Eph/ephrin signaling pathways impressive systems for regulating tissue separation and morphogenesis, we have continued to explore how these signaling pathways interact.
In this minireview, we will first describe a few characteristics of EphB/ephrinB signaling events that our laboratory and others have uncovered using the Xenopus system. Subsequently, we will provide specific examples of how different techniques employed in the frog system have been able to assist us in understanding some of these signaling mechanisms.
2 ∣. ADVANTAGES OF XENOPUS AS A SYSTEM TO EXPLORE EPHRIN SIGNALING
We often employ the Xenopus system since we have found it to be a powerful tool for understanding the contribution of several signaling molecules to developmental and cellular processes. An advantage of this system stems from its well-characterized and reliable cell fate map, where cell lineage can be easily traced during experiments. Translation of specific endogenous proteins can be inhibited, and mutant proteins ectopically expressed in embryos with great facility and biochemical or developmental effects examined within 2–3 days. In addition, one can use the CRISPR/Cas system to generate gene specific knockouts in embryos. Another very useful application of the Xenopus system is the ability to make explants of embryonic tissues from control or gene-specific knockdown embryos and culture them alone, or in combination with other tissue specific explants. With these approaches in hand, signal transduction and differentiation processes can be assessed morphologically, histologically, as well as biochemically in a developing vertebrate.
3 ∣. WHAT WE HAVE LEARNED ABOUT EPHRIN SIGNALING FROM XENOPUS
Approxmately twenty years ago, the Eph/ephrin signaling field began to make inroads in understanding this important receptor ligand system through overexpressing wild type and mutant ephrinB ligands and EphB receptors in the developing Xenopus embryo. Smith and colleagues injected two-cell stage embryos with RNA encoding truncated Eph receptors, and showed that disrupting EphA4 or EphB1 function led to anomalous migration of neural crest cells into adjacent branchial arch territories (Smith, Robinson, Patel, & Wilkinson, 1997). Moreover, ectopic overexpression of ephrinB2 caused scattering of neural crest cells into adjacent regions (Smith et al., 1997). Heibling and colleagues studied the function of EphB4 and its ligands during intersomitic vein development. Expressing a dominant negative EphB4 receptor and ephrinB ligands in embryos, these authors found that intersomitic veins would grow aberrantly and invade adjacent somitic tissue (Helbling, Saulnier, & Brandli, 2000). Mann and colleagues used an in vivo lipofection technique, where a DNA-lipid complex was injected into the lateral portion of the eye field that will populate the dorsal retina (Mann, Ray, Harris, & Holt, 2002). A truncated ephrinB ligand (lacking ‘reverse’ signaling capabilities) was expressed in the dorsal retina and led to in vivo targeting errors of retinal ganglion cell axons in the optic tectum (Mann et al., 2002).
Several years ago, our laboratory provided evidence that overexpression of ephrinB1 in Xenopus embryos causes the blastomeres of ectodermal tissue to dissociate (Jones et al., 1998). It is not likely that this effect is simply a result of the adhesive properties of the Eph receptor/ephrin interaction since adhesion was disrupted by overexpressing an ephrinB1 ligand mutant incapable of binding the cognate receptor (Jones et al., 1998). Genetic evidence Indicates that the intracellular domain of ephrinBs is critical for neural crest movement, and vascular morphogenesis, consistent with a signaling function for this domain (Adams et al., 2001; Davy, Aubin, & Soriano, 2004; Makinen et al., 2005). This de-adhesive activity can be modulated by tyrosine phosphorylation of ephrinB1 initiated by binding to the extracellular domain of a cognate Eph receptor or by an interaction with an activated FGF receptor (Chong, Park, Latimer, Friesel, & Daar, 2000). Using the epithelial cells of early stage Xenopus embryos, we previously showed that loss-of-function of ephrinB1 through the use of antisense morpholino oligonucleotides, as well as gain-of-function via over expression can disrupt cell–cell contacts and tight junctions (Lee, Nishanian, Mood, Bong, & Daar, 2008). Thus, a mechanism was identified in which ephrinB1 competes with active Cdc42 for binding to Par-6, a scaffold protein central to the Par polarity complex (Par-3/Par-6/Cdc42/aPKC), and disrupts the localization of tight junction-associated proteins (ZO-1, Cingulin). This competition affects formation of tight junctions, and is regulated by tyrosine phosphorylation of ephrinB1. This study serves as a good example of some of the powerful tools that allow one to identify associated signaling proteins, and to determine the role of these signaling molecules in a specific morphogenetic event, and to place these signaling molecules in the hierarchy of a signaling pathway.
4 ∣. OOCYTES AS EPHRIN PROTEIN INTERACTION TEST TUBES
As mentioned previously, we found that overexpression of ephrinB1, in the absence of cognate receptor, was able to affect cell–cell adhesion in the embryo. The Xenopus laevis oocyte and embryo are wonderful in vivo test tubes, where one can easily express a protein of interest by injecting in vitro transcribed RNA encoding the proteins of interest. For example, RNAs encoding ephrinB1 and Grb4 (growth-factor-receptor-bound protein 4), an adaptor protein that is known to increase FAK (focal adhesion kinase) activity and cell rounding (Cowan & Henkemeyer, 2001) were injected into Xenopus oocytes and co-immunoprecipitation (Co-IP) analysis performed. EphrinB1 interacts with Grb4 in the presence of an activated FGFR1. Amino acid substitutions were generated in Grb4, and the resulting mutants were expressed along with ephrinB1 and an activated FGFR in Xenopus oocytes (Bong et al., 2004). Co-IP analysis identified the tyrosine residue at position 298 of ephrinB1 as being required for the physical interaction with Grb4, a signaling molecule that can mediate or modulate ephrinB ‘reverse’ signaling (Bong et al., 2004; Cowan & Henkemeyer, 2001).
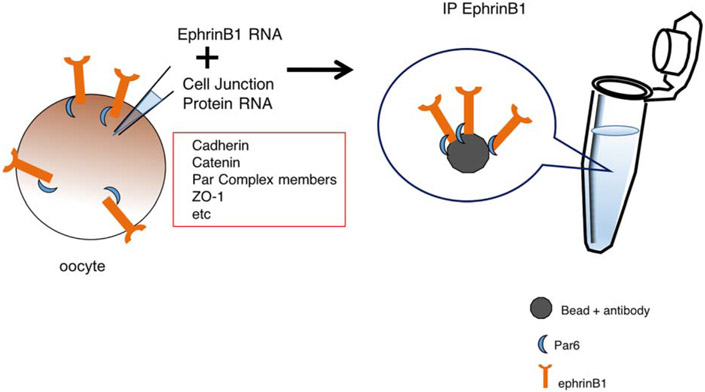
To ascertain which other cell–cell adhesion molecules may be involved in ephrinB signaling, we undertook a limited screen for possible binding proteins. Either singularly or in groups, RNAs encoding possible cell border and cell adhesion molecules were injected into the same oocyte. The proteins were allowed to be expressed over several hours of incubation, and then the oocytes were lysed and ephrinB1 immunoprecipitated. Western analysis was performed to determine which (if any) of the candidate cell border and adhesion molecules were found in the ephrinB1 immune-complexes (Figure 1). Thus, Par-6 was identified as an ephrinB1 interactor. Subsequently, serial deletions and various mutants were generated to determine interaction domains and phosphorylation sites that could affect the ephrinB1/Par-6 association. Using this method, the interaction between ephrinB1 and Par-6 was revealed, and shown to be disrupted by phosphorylation of tyrosine 310 in the intracellular domain of ephrinB1 (Lee et al., 2008).
FIGURE 1.
A limited screen for candidate interacting proteins. This schematic depicts the use of isolated stage 6 Xenopus oocytes to test whether a protein of interest (ephrinB1) is able to interact with possible candidate proteins (cell–cell adhesion and cell boundary proteins). Oocytes are injected with in vitro transcribed mRNA encoding ephrinB1 and individual candidate gene mRNAs, cultured for several hours, lysates prepared and ephrinB1 immune-complexes collected. Western analysis should then be performed to detect whether a candidate associates with the ephrinB1 protein
5 ∣. USING XENOPUS EMBRYOS FOR TARGETED KNOCK DOWN AND REEXPRESSION OF EPHRIN INTERACTORS
The beauty of the Xenopus embryonic system is that one can carefully titrate and control the expression of an exogenously expressed protein. This allows for the use of “replacement strategies”, where an antisense morpholino (MO) is injected into an embryo to knockdown a specific protein by preventing its translation. Then one injects an MO-resistant RNA encoding this same protein to rescue the biochemical and phenotypic changes that occur with the MO knockdown of the protein. In addition, one can express a mutant protein at the same level required for rescuing a phenotype with the wildtype version of the protein. This allows one to ascertain whether the mutation affects the biochemical or developmental pathways in which the protein of interest is involved. Another excellent example of the value of this replacement strategy is demonstrated in this same study of ephrinB1 and Par-6 interactions (Lee et al., 2008).
Both blastomeres of 2-cell-stage embryos were injected with ephrinB1MO. MOs that block translation of the endogenous ephrinB1 caused a redistribution of tight junction-associated proteins (ZO1 and Cingulin), but left intact the lateral expression of lethal giant larvae 2 (LGL2) (Lee et al., 2008). Thus, the localization of ZO-1 and Cingulin to tight junctions was prominently reduced, as evidenced by immunofluorescence microscopy. In embryos, the introduction of an appropriate level of EphrinB1WTΔUTR RNA (anephrinB1MO-resistant RNA) was capable of rescuing the expression of wildtype ephrinB1, resulting in restoration of the proper localization of tight junction-associated proteins. In contrast, this was not the case when an MO-resistant mutant RNA of ephrinB1 (ephrinB1Y310FΔUTR) that lacks the tyrosine necessary for disengaging Par-6 from the ephrinB1 molecule was introduced and expressed at levels equivalent to that which allowed ephrinB1WT to rescue the localization of tight junction-associated proteins (Lee et al., 2008). These experiments assisted in revealing a mechanistic model where unphosphorylated ephrinB1 associates with Par-6, and upon tyrosine phosphorylation, Par-6 is released from ephrinB1, allowing active Cdc42 to bind Par-6 and maintain cell–cell boundaries and adhesion (Lee et al., 2008).
In addition to cell–cell adhesion, there is a role for ephrinB1 in cell movement. A study in Xenopus conducted by Tanaka and colleagues, found that Dishevelled, a scaffold central to the Wnt signaling pathway, can interact with ephrinB1 (Tanaka, Kamo, Ota, & Sugimura, 2003). They also revealed that Dishevelled could mediate cell repulsion between receptor and ligand bearing cells. This study took advantage of another wonderful cell biological technique imparted by Xenopus embryos; using in vitro re-aggregation assays, it was shown that coexpression of a dominant-negative mutant of Dishevelled affected the sorting of EphB2-expressing cells from those expressing ephrinB1. These sorting assays entail excising ectodermal explants from gastrula stage embryos that have been injected with a fluorescent dye and RNA encoding ephrinB1 or its EphB2 receptor along with a Dishevelled mutant. The explants are dissociated in Ca+-free medium and the cells are re-aggregated, and the ability of the cells (having different colored fluorescent dyes) to sort examined by epifluorescence microscopy (Tanaka et al., 2003). This cell sorting assay represents a very easy and informative ex vivo technique, that placed Dishevelled as a mediator in the Eph/ephrin signaling system (Tanaka et al., 2003).
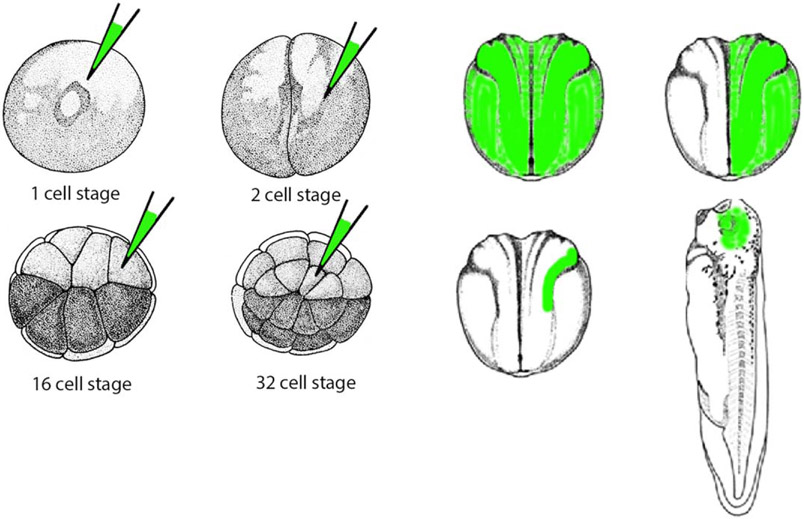
We along with our collaborators reported that morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Moreover, we found that Dishevelled mediates ephrinB1 signaling that controls retinal progenitor cell movement into the eye field (Lee et al., 2006). The mechanism by which Eph or FGFR modulates signaling from the ephrinB1 molecule was revealed in a series of studies showing that FGFR or Eph-induced phosphorylation of ephrinB1 disrupts the ephrinB1/Dishevelled interaction, leading to a loss of ephrinB1-induced planar cell polarity (PCP) signaling (Moore, Mood, Daar, & Moody, 2004; Lee et al., 2006, 2009). Thus, crosstalk between FGF signaling and the ephrinB1/Dsh/PCP pathway can regulate the movements and positioning of specific progenitor cells during embryogenesis. To determine how retinal progenitors are affected by ephrinB1 signaling entailed targeting specific regions within the embryos by injecting an mRNA encoding a tracer molecule along with mRNA encoding our protein of interest (i.e., ephrinB1) or an antisense ephrinB1 MO into a specific blastomere of a 16 or 32 cell stage embryo. Using this approach, one can achieve the spatially restricted and specific knockdown of the ephrinB1 protein (Figure 2). Effects on cell–cell adhesion, cell movement, and endogenous or exogenous protein localization can be assessed at later stages in specific targeted tissues using epifluorescence microscopy and immunolocalization. This study also took full advantage of the replacement experiments (described above) using ephrinB1 mutants, as well as epistasis experiments where an MO for an upstream or downstream signaling protein was introduced to determine the requirement for that protein in ephrinB-driven signaling events (Moore et al., 2004; Lee et al., 2006, 2009). These studies showed that FGFR-induced phosphorylation of ephrinB1 on tyrosines 324 and 325 in the C-terminus of ephrinB1 disrupts the ephrinB1/Dishevelled interaction, leading to a loss of ephrinB1-induced activation of planar cell polarity (PCP).
FIGURE 2.
Schematic depicting spatial control of mRNA or MO location in a Xenopus embryo. GFP mRNA or fluorescent dye can be injected along with a particular mRNA or MO in specific blastomeres at various stages of development to spatially restrict protein expression or knockdown, respectively. The target area can be assessed by epifluorescence microscopy
Other studies involving ephrinBs in Xenopus used adroit techniques to interrogate neuronal signaling. For example, Lim and colleagues showed that in the optic tectum of a Xenopus laevis embryo, ephrinB ‘reverse’ signaling promotes the morphological maturation of retinotectal synapses (Lim, Matsuda, & Poo, 2008). One of the techniques used in this report is electroporation into late stage embryos. Electroporation is an advantageous method to introduce mRNA or MOs into a specific set or group of cells at various stages of development, but is most often used for late stages of development to circumvent early stage injections that might cause undesirable or obfuscating developmental defects (Chernet & Levin, 2012). In this study, plasmids encoding the wild-type or mutant ephrinB1 constructs, are electroporated into retinal ganglion cells along with a plasmid for EGFP fused to synaptobrevin, a synaptic vesicle membrane protein. Another experiment was performed where wildtype or mutant EphB2 receptor constructs were electroporated into tadpole tectal neurons. These constructs were designed to disrupt the endogenous interactions with ephrinB1. Using these two different sets of constructs in different tissues, they were able to gauge the role of ephrinB1 reverse signaling in synapse formation (Lim et al., 2008). Using live imaging in vivo, they found that increased ephrinB1 signaling enhanced presynaptic maturation, but not axonal growth (Lim et al., 2008).
6 ∣. USE OF EXPLANTS TO DEFINE EPH RECEPTOR/LIGAND SIGNALING
Rohani and colleagues took advantage of another crafty tool available to researchers using the Xenopus system; explants from developing tissues. These authors showed that ephrinBs are required at the mesoderm/ectoderm border during gastrulation for repulsion or separation between these tissues to occur properly (Rohani, Canty, Luu, Fagotto, & Winklbauer, 2011). This separation event occurs very early in development between the dorsal ectoderm (which is fated to form the nervous system and skin) and the mesoderm (which will become muscle and connective tissues). They subsequently built upon this discovery, using the explant system to cleverly determine the individual roles of the Eph receptors and ligands in tissue separation at the mesoderm and ectoderm border in developing embryos. The authors used knockdown and replacement experiments to define the Eph/ephrin pairings that drive the separation of these two tissues (Rohani, Parmeggiani, Winklbauer, & Fagotto, 2014). Using an ex vivo explant system (Gorny & Steinbeisser, 2012; Wacker, Grimm, Joos, & Winklbauer, 2000), the authors test the requirement for individual EphB receptors and B-ligands for maintaining tissue separation (Rohani et al., 2014). Strikingly, individual loss or replacement experiments show that each receptor or ligand is required at the dorsal ectoderm/mesoderm borders for separation. Chimeric receptors were used to show that the specificity resides in the extracellular domain - not the cytoplasmic kinase domain. Using Morpholinos and Eph extracellular domains fused to immunoglobulin Fc domain (to impart the ability to first oligomerize the fusion protein in vitro) in the explants, the authors recognized specific ligand receptor pairings that signal tissue separation (ectodermal ephrinB3/mesodermal EphA4; ectodermal EphB2 & B4/mesodermal ephrinB2) (Rohani et al., 2014). Using replacement and expression swapping experiments with ephrinB3 and EphA4, it was shown that complementary expression regardless of tissue type allows for the separation of tissues with this pairing. They used dissociated and mixed aggregates of tissues to show phosphorylation of EphB or EphA, as well as phosphorylation of myosin at the ectoderm/mesoderm border. To test the role of Eph/ephrin signaling in the balance between adhesion and repulsion, the levels of Cadherin or Eph receptors were modulated by MO or expression of ephrins and Eph receptors. For example, in mesoderm cells (single cells mixed), stable cell contact between mesoderm cells occurs when Cadherin is reduced along with Eph reduction (Rohani et al., 2014). In addition, in vivo experiments examining the influence of ephrins and Ephs on the notochord boundary was examined by targeted depletion (via MOs) in a restricted region of the embryo to verify the model (Rohani et al., 2014). Also of interest, using similar explants and embryo injections, Park and colleagues found that EphA4 and the ephrinA1 ligand are expressed in a mutually exclusive manner in the involuting mesoderm and noninvoluting ectoderm of early gastrulae. MO-mediated inhibition of EphA4 or ephrinA1 function inhibited the tissue separation of mesoderm and ectoderm, indicating a role for A-type ligands and receptors in this process (Park, Cho, Kim, Choi, & Han, 2011).
7 ∣. IN VIVO MOSAICS TO DEFINE EPH RECEPTOR/LIGAND SIGNALING
Experiments examining the notochord boundary strengthen earlier work by Fagotto and colleagues showing that increased actomyosin contractility and inhibition of cadherin clustering along the notochord boundary are a consequence of ephrin-Eph signaling (Fagotto, Rohani, Touret, & Li, 2013). To demonstrate this, the authors took advantage of the ability to easily create mosaic expression regions in a spatially defined manner in Xenopus embryos. The mosaic assay used by the authors is one where a Lef-VP16 fusion DNA construct is injected into an embryo and a small number of cells expressing Lef-VP16 are efficiently excluded from the notochord and accumulate in the adjacent presomitic mesoderm. In contrast, GFP-expressing control cells evenly distribute between the tissues. The LefVP16-expressing cells were unable to sort in embryos injected with antisense MOs against ephrinB2, EphB4, or EphA4. This interference with ephrin/Eph signaling correlated with the loss of myosin activation at the notochord boundary, and loss of notochord separation (Fagotto et al., 2013). Thus, increased actomyosin contractility and inhibition of cadherin clustering along the boundary are a result of Eph/ephrin signaling. This implicates a more widespread role for the Eph/ephrin signaling system in vertebrate tissue separation.
8 ∣. IMMUNOPRECIPITATION AND MASS SPECTROMETRIC ANALYSIS TO IDENTIFY EPHRIN INTERACTORS
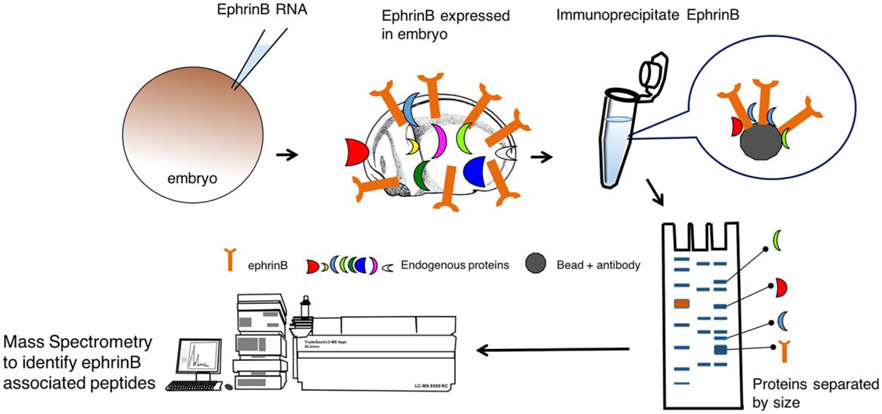
Although many studies provided mechanistic insight into how ephrinBs regulate cell movement and cell–cell boundaries, until recently, we understood little of how ephrinBs were regulated during embryogenesis. Since the regulation of ephrinB proteins was likely to be a critical process in controlling morphogenetic events, the post-translational regulation of ephrinBs was studied in Xenopus. In one study, it was revealed that a system of differential interactions between ephrinB1 and the E3 ubiquitin ligases, Smurf1 and Smurf2, regulates the maintenance of tissue boundaries through the control of ephrinB protein levels (Hwang et al., 2013). This study made use of another beneficial technique for identifying proteins that associate with the ephrinB1 protein; immunoprecipitation and mass spectrometric analyses (Figure 3). We previously described a study that used a biased approach in the Xenopus system to perform a screen of candidate ephrinB1 interacting proteins (Figure 1). In another study, an unbiased approach was employed, where ephrinB1 was overexpressed in Xenopus embryos by injecting a C-terminally tagged version of ephrinB1 RNA into both blastomeres of a two-cell stage embryo (Hwang et al., 2013). After culturing the embryos to the early neurula stage, lysates were prepared and ephrinB1 immunoprecipitated. Mass spectrometric analysis of proteins that co-immunoprecipitate (Co-IP) with ephrinB1 was performed. From this analysis, Smurf2 was identified as an ephrinB1-interacting protein (Hwang et al., 2013). To assess whether endogenous ephrinB1 and Smurf1 or Smurf2 proteins interact, a Co-IP analysis of lysates was performed from HT29 colon carcinoma cells that express abundant levels of all three proteins. To determine regions within the ephrinB1 protein necessary for the interaction with Smurfs, a series of deletion mutants within ephrinB1 were generated, co-expressed in embryos, and Co-IP analyses performed. An interaction was found between ephrinB1 and the Smurf2 WW domains, which are usually associated with substrates of the ubiquitin ligase (Rotin & Kumar, 2009). In addition, co-expression of Smurf2 with ephrinB1 in embryos resulted in a marked decrease in ephrinB1 expression, while overexpression of Smurf1 blocked this reduction. A number of biochemistry experiments were performed in embryos and oocytes indicating that there was competition between Smurf1 and Smurf2 for binding to ephrinB1, but only Smurf2 targeted ephrinB1 for ubiquitination and degradation (Hwang et al., 2013).
FIGURE 3.
Schematic depicting the use of immunoprecipitation and mass spectrometry to identify possible ephrinB-associated proteins. Xenopus embryos are injected with ephrinB mRNA, cultured until later stages, lysates prepared, and ephrinB immune-complexes collected. Proteins are separated by gel electrophoresis, gel slices removed and prepared for mass spectrometric analysis to identify proteins in the immune-complex
9 ∣. USE OF EXPLANTS TO UNDERSTAND REGULATION OF EPHRIN AND ITS OUTCOME
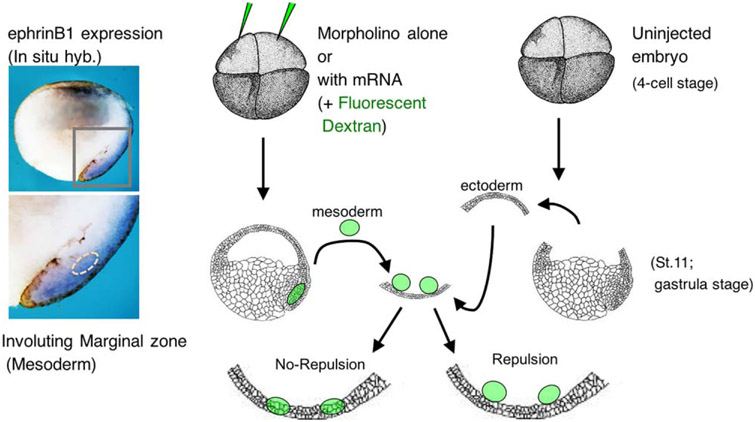
To test the possible effects of this competition in vivo can be difficult due to overlapping expression of ephrinB1, Smurf1, and Smurf2 in neuroectoderm, and both mesoderm and ectoderm. However, an ex vivo approach (explants) can be used that represents another powerful technique in the Xenopus model system arsenal (Figure 4). In these experiments, embryos were injected with MOs against Smurf1 or Smurf2, or ephrinB1, along with fluorescent dextran to label the tissue. Ectoderm was removed from the blastocoel roof (BCR) of uninjected late gastrula stage embryos and used as a substrate for excised mesoderm from injected embryos (Figure 4; Hwang et al., 2013). Control MO-injected mesoderm explants placed on the BCR (ectoderm) substrate maintained tissue separation as evidenced by the lack of integration or mixing of these mesoderm cells into the BCR. However, the introduction of the Smurf1 MO or the ephrinB1 MO into the mesoderm tissue caused a loss of tissue separation from the BCR, while the mesoderm containing the Smurf2 MO separated from the BCR. Re-expressing ephrinB1 rescued tissue separation in the presence of a Smurf1 MO indicating that loss of Smurf1 rendered ephrinB1 susceptible to ubiquitination and degradation via Smurf2. Using MOs against both Smurf1 and Smurf2 in the mesoderm explant led to a restoration of tissue separation, and confirmed a model in which Smurf1 protects ephrinB1 from Smurf2-targeted degradation (Hwang et al., 2013).
FIGURE 4.
Use of explants to determine the role of proteins in tissue repulsion. The left side of the figure shows a wholemount in situ hybridization of a bisected Xenopus embryo with a probe for ephrinB1 expression; showing expression in the involuting marginal zone. The right side is a schematic depicting the BCR/mesoderm repulsion assay. Four cell stage embryos are injected with either mRNA or MOs or both, along with fluorescent dextran as a tracer. The mesoderm (involuting marginal zone) is removed and placed upon the blastocoel roof (BCR) explant from an uninjected embryo. Light and epifluorescence microscopy allows for visualization of whether the two explants mix or repulse one another
In another study examining regulation of ephrinBs, IP mass spectrometry analysis was performed to identify new ephrinB interacting proteins as mentioned above (Figure 3). Flotillins were identified in ephrinB immunecomplexes, and it was found that the presence of flotillin-1 is critical to maintain ephrinB2 protein levels (Ji et al., 2014). Reduction in ephrinB2 protein due to the loss of flotillin-1, led to the failure of neural tube closure, an important morphogenetic event. Using biochemistry along with loss-of-function (via morpholino oligonucleotides) and gain-of-function or rescue experiments in the amphibian system, evidence was provided in vivo that loss of flotillin-1 leads to the cleavage of the ephrinB2 ectodomain specifically through the ADAM-10 metalloprotease, and ephrinB2 is subsequently degraded. The reduction in ephrinB2 in the absence of flotillin-1 leads to failure of apical constriction in the developing neural tube. Thus, ephrinB2 protein levels are sustained by a lipid raft protein (flotillin-1) that interacts with ephrinB2, and inhibits cleavage and processing by ADAM-10. This report identified ADAM-10 as the protease that targets ephrinB2 for cleavage in vivo, and links this regulatory mechanism of ephrinB2 to proper neural tube closure (Ji et al., 2014).
10 ∣. USING CRISPR/CAS TECHNOLOGY CAN BE VERY EFFECTIVE AND LESS EXPENSIVE THAN MOS
In this section, we will only briefly discuss using clustered regularly interspaced short palindromic repeats (CRISPRs) gene-editing in Xenopus (Banach, Edholm, & Robert, 2016; Bhattacharya, Marfo, Li, Lane, & Khokha, 2015; Blitz, Biesinger, Xie, & Cho, 2013; Guo et al., 2014; Naert et al., 2016; Nakayama et al., 2013). Although use of CRISPR/Cas has not been reported in Eph/ephrin signaling in Xenopus yet, our laboratory has been using the technology to confirm the validity of phenotypic and biochemical effects observed with MOs against specific proteins found to be involved in ephrin signal transduction (Hwang YS, Yoon J and Daar I, unpublished). Moreover, in Xenopus it appears that MOs generally provide similar phenotypes as the CRISPR/Cas system (Bhattacharya et al., 2015). CRISPR/Cas based gene editing is generally very effective in both frequently used frog systems, Xenopus laevis (nondiploid) and tropicalis (diploid). Briefly, using the CRISPR/Cas9 system, a site-specific small guide RNA (sgRNA) targets the genomic sequence adjacent to a protospacer adjacent motif (PAM) sequence, and recruits the Cas9 endonuclease to generate a double-stranded break. Nonhomologous end joining repairs the break, but often causes insertions and deletions (indels) leading to frame shifts, and cause premature termination of the target protein translation. Xenopus tropicalis is usually used to derive null frog lines due to its diploid genome and faster generation time. Regardless, CRISPR/Cas gene editing is an extremely useful technology due to its inexpensive and efficient nature, and it can be used to disrupt genes in both Xenopus laevis and tropicalis for examining effects in F0 embryos of either species. Determining the efficacy of the gene knockout with the CRISPR/Cas system can be easily determined by PCR.
This opens a new avenue for examining Eph/ephrin signaling. Like MOs, one can still block endogenous protein production and spatially restrict the regions affected, while allowing the collection of sufficient embryos to perform biochemistry and live cell imaging, which is easier in Xenopus than in mammalian embryos. There are various strategies for circumventing embryonic lethality (reviewed in Tandon, Conlon, Furlow, & Horb, 2016), but one recent method takes advantage of the ease of transplants in the system (Blitz, Fish, & Cho, 2016). One can transplant primordial germ cells (located in vegetal hemisphere of the developing embryo) from a mutant embryo into a wildtype host embryo to generate germ cell-specific mutations (Blitz et al., 2016). Currently, different protocols are being devised for creating knock-ins of specific genes (Shi et al., 2015). As the techniques become more refined to allow easy tagging of endogenous proteins, and thus alleviating the need for antibodies, biochemistry with endogenous Eph/ephrin signaling molecules will advance rapidly in the amphibian system. Moreover, as the technology for specific knock-in mutations within genes become more facile, understanding the protein-protein interactions as well as the biochemical and signaling alterations of disease mutations will be vastly more accessible in this versatile and useful model organism.
ACKNOWLEDGMENTS
We wish to apologize to all of our colleagues whose work was not cited in this minireview. Many have contributed greatly to our understanding of the role of the Eph-ephrin system in biology, but space considerations prevented the inclusion of their work. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
REFERENCES
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, & Klein R (2001). The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell, 104, 57–69. [DOI] [PubMed] [Google Scholar]
- Banach M, Edholm ES, & Robert J (2016). Exploring the functions of nonclassical MHC class Ib genes in Xenopus laevis by the CRISPR/Cas9 system. Developmental Biology. doi: 10.1016/j.ydbio.2016.05.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Marfo CA, Li D, Lane M, & Khokha MK (2015) CRISPR/Cas9: An inexpensive, efficient loss of function tool to screen human disease genes in Xenopus. Developmental Biology 408,196 – 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Biesinger J, Xie X, & Cho KW (2013). Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis, 51, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Fish MB, & Cho KW (2016). Leapfrogging: primordial germ cell transplantation permits recovery of CRISPR/Cas9-induced mutations in essential genes. Development, 143, 2868–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bong YS, Park Y, Lee HS, Mood K, Ishimura A, & Daar IO (2004). Tyr-298 in ephrinB1 is critical for an interaction with the Grb4 adaptor protein. Biochemistry Journal, 377, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, & Levin M (2012). A versatile protocol for mRNA electroporation of Xenopus laevis embryos. Cold Spring Harbor Protocol, 2012, 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, & Daar IO (2000). Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Molecular Cell Biology, 20, 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, & Henkemeyer M (2001). The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature, 413, 174–179. [DOI] [PubMed] [Google Scholar]
- Daar IO (2012). Non-SH2/PDZ reverse signaling by ephrins. Seminars in Cell Developmental Biology, 23, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Aubin J, & Soriano P (2004). Ephrin-B1 forward and reverse signaling are required during mouse development. Genes & Development, 18, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Rohani N, Touret AS, & Li R (2013). A molecular base for cell sorting at embryonic boundaries: Contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Developmental Cell, 27, 72–87. [DOI] [PubMed] [Google Scholar]
- Gorny AK, & Steinbeisser H (2012). Brachet’s cleft: a model for the analysis of tissue separation in Xenopus. Wiley Interdisciplinary Review in Developmental Biology, 1, 294–300. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, … Chen Y (2014). Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development, 141, 707–714. [DOI] [PubMed] [Google Scholar]
- Helbling PM, Saulnier DM, & Brandli AW (2000). The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development, 127, 269–278. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, … Stainier DY (2009). Arterial-venous segregation by selective cell sprouting: An alternative mode of blood vessel formation. Science, 326, 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YS, Lee HS, Kamata T, Mood K, Cho HJ, Winterbottom E, Ji YJ, … Daar IO (2013). The Smurf ubiquitin ligases regulate tissue separation via antagonistic interactions with ephrinB1. Genes & Development, 27, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji YJ, Hwang YS, Mood K, Cho HJ, Lee HS, Winterbottom E, … Daar IO (2014). EphrinB2 affects apical constriction in Xenopus embryos and is regulated by ADAM10 and flotillin-1. Nature Communications, 5, 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL, Chong LD, Kim J, Xu RH, Kung HF, & Daar IO (1998). Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proceedings of National Academic Science USA, 95, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, & Daar IO (2006). Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nature & Cell Biology, 8, 55–63. [DOI] [PubMed] [Google Scholar]
- Lee HS, Mood K, Battu G, Ji YJ, Singh A, & Daar IO (2009). Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Molecular Biolgy & Cell, 20, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Nishanian TG, Mood K, Bong YS, & Daar IO (2008). EphrinB1 controls cell–cell junctions through the Par polarity complex. Nature & Cell Biology, 10, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Matsuda N, & Poo MM (2008). Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nature & Neuroscience, 11, 160–169. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, … Wilkinson GA (2005). PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes & Development, 19, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, & Holt C (2002) Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron 35, 461–473. [DOI] [PubMed] [Google Scholar]
- Moore KB, Mood K, Daar IO, & Moody SA (2004). Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Developmental Cell, 6, 55–67. [DOI] [PubMed] [Google Scholar]
- Naert T, Colpaert R, Van Nieuwenhuysen T, Dimitrakopoulou D, Leoen J, Haustraete J, … Vleminckx K (2016). CRISPR/Cas9 mediated knockout of rb1 and rbl1 leads to rapid and penetrant retinoblastoma development in Xenopus tropicalis. Scientific Reports, 6, 35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, & Grainger RM (2013). Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis, 51, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Cho GS, Kim GH, Choi SC, & Han JK (2011). The involvement of Eph-Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Developmental Biology, 350,441–450. [DOI] [PubMed] [Google Scholar]
- Pasquale EB (2010). Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nature Review of Cancer, 10, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N, Parmeggiani A, Winklbauer R, & Fagotto F (2014). Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biology, 12, e1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N, Canty L, Luu O, Fagotto F, & Winklbauer R (2011). EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biology, 9, e1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, & Kumar S (2009). Physiological functions of the HECT family of ubiquitin ligases. Nature Reviews in Molecular Cell Biology, 10, 398–409. [DOI] [PubMed] [Google Scholar]
- Shi Z, Wang F, Cui Y, Liu Z, Guo X, Zhang Y, … Chen Y (2015). Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis. FASEB Journal, 29, 4914–4923. [DOI] [PubMed] [Google Scholar]
- Singh A, Winterbottom E, & Daar IO (2012). Eph/ephrin signaling in cell–cell and cell–substrate adhesion. Frontiers in Bioscience, 17,473–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, & Wilkinson DG (1997). The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Current Biology, 7, 561–570. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kamo T, Ota S, & Sugimura H (2003). Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO Journal, 22, 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon P, Conlon F, Furlow JD, & Horb ME (2016). Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Developmental Biology. doi: 10.1016/j.ydbio.2016.04.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker S, Grimm K, Joos T, & Winklbauer R (2000). Development and control of tissue separation at gastrulation in Xenopus. Developmental Biology, 224, 428–439. [DOI] [PubMed] [Google Scholar]