Abstract
Objective:
The endocannabinoid (eCB) system is involved in diverse aspects of human physiology and behavior but little is known about the impact of circadian rhythmicity on the system. The two most studied endocannabinoids, AEA (ananamide) and 2-AG, can be measured in peripheral blood however the functional relevance of peripheral eCB levels is not clear. Having previously detailed the 24-hr profile of serum 2-AG, here we report the 24-hr serum profile of AEA to determine if these two endocannabinoids vary in parallel across the biological day including a nocturnal 8.5-h sleep period. Further, we assessed and compared the effect of a physiological challenge, in the form of sleep restriction to 4.5-h, on these two profiles.
Methods:
In this randomized crossover study, we examined serum concentrations of AEA across a 24-hr period in fourteen young adults. Congeners of AEA, the structural analogs oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) were simultaneously assayed. Prior to 24-hr blood sampling, each participant was exposed to two nights of normal (8.5 hrs) or restricted sleep (4.5 hrs). The two sleep conditions were separated by at least one month. In both sleep conditions, during the period of blood sampling, each individual ate the same high-carbohydrate meal at 0900, 1400, and 1900.
Results:
Mean 24-hr concentrations of AEA were 0.697 ± 0.11 pmol/ml. A reproducible biphasic 24-h profile of AEA was observed with a first peak occurring during early sleep (0200) and a second peak in the mid-afternoon (1500) while a nadir was detected in the mid-morning (1000). The 24-hr profiles for both OEA and PEA followed a similar pattern to that observed for AEA. AEA, OEA, and PEA levels were not affected by sleep restriction at any time of day, contrasting with the elevation of early afternoon levels previously observed for 2-AG.
Conclusions:
The 24-hr rhythm of AEA is markedly different from that of 2-AG, being of lesser amplitude and biphasic, rather than monophasic. These observations suggest distinct regulatory pathways of the two eCB and indicate that time of day needs to be carefully controlled in studies attempting to delineate their relative roles. Moreover, unlike 2-AG, AEA is not altered by sleep restriction, suggesting that physiological perturbations may affect AEA and 2-AG differently. Similar 24-hr profiles were observed for OEA and PEA following normal and restricted sleep, further corroborating the validity of the wave-shape and lack of response to sleep loss observed for the AEA profile. Therapeutic approaches involving agonism or antagonism of peripheral eCB signaling will likely need to be tailored according to time of day.
Keywords: Cannabinoid, anandamide, circadian rhythm, 24-hr profile
1. Introduction
Humans have long been using Cannabis sativa with the noted effects of euphoria, decreased stress, increased hunger, and potential alterations in anxiety. The active component of cannabis, Δ9-tetrahydrocannabinol (THC) was isolated in 1964 (Gaoni and Mechoulam, 1964) but the major components of the endogenous cannabinoid (eCB) system were not identified until the early 1990’s. It is now well established that the endocannabinoid system is comprised of the cannabinoid receptors, the endogenous ligands of these receptors, including 2 – arachidonoylglycerol (2-AG) and N – arachidonylethanolamine (AEA or anandamide), and the enzymes responsible for the biosynthesis and degradation of the eCBs (Devane et al., 1992; Gerard et al., 1991; Mechoulam et al., 1995; Munro et al., 1993; Sugiura et al., 1995). Components of the eCB system are located both centrally and peripherally and the ubiquity of the system lends itself to involvement in multiple aspects of human physiology (Mazier et al., 2015). A large body of literature has documented that the eCB system is not only involved in mediating feeding behavior, reward, stress and anxiety but also in glucose metabolism, pain, immune response, neurological disorders, and depression (Hillard, 2018; Iannotti et al., 2016; Turcotte et al., 2015). Endocannabinoids can be measured in blood from lipid extracts of plasma or serum, although the specific origin of peripheral concentrations of serum eCBs remains unclear (Hillard, 2018). Some data suggests that circulating eCBs are derived from the multiple tissues in which the enzymatic machinery to synthesize eCBs are located (Hillard, 2018). These tissues include, but are not limited to brain, gut, muscle, pancreas, and adipose tissue (Mazier et al., 2015). The eCB like compounds N-acyl ethanolamines (NAEs), oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), are structurally similar to AEA but do not bind cannabinoid receptors. These lipids are produced by similar enzymatic machinery as for AEA and can also be measured in circulation (Hillard, 2018). They are frequently measured in conjunction with AEA as they may produce similar physiological effects as AEA, without binding eCB receptors (Lam et al., 2010). Whether these eCBs and NAEs are purposefully released into the circulation as a physiological signal with explicit function or are simply a marker of tissue endocannabinoid tone remains to be seen.
Much attention has been paid in recent years to the ability of the eCB system to control feeding, body weight, and peripheral metabolism in diet-induced or genetically obese animals (Chen and Pang, 2013; Fong et al., 2007; Jbilo et al., 2005; Poirier et al., 2005; Ravinet Trillou et al., 2003); thus this system has been the target of efforts to develop new anti-obesity drugs (Chen and Pang, 2013; Despres et al., 2005; Kipnes et al., 2010; Proietto et al., 2010; Scheen et al., 2006; Van Gaal et al., 2008). Most notably, rimonabant, a selective CB1 receptor blocker was approved in Europe as an appetite-suppressant in 2006 and was shown to have beneficial metabolic effects beyond those mediated by weight loss (Despres et al., 2005; Scheen et al., 2006; Van Gaal et al., 2005; Van Gaal et al., 2008). The drug had to be withdrawn in 2008 due to serious psychiatric adverse effects (Sam et al., 2011). These notable side-effects have sidelined drug development, but the role of the eCB system in the regulation of energy balance, glucose and lipid metabolism, and food intake remains nonetheless a putative target of the pharmacological treatment of obesity. The eCB system is indeed involved in modulating not only homeostatic (energy balance) pathways but also hedonic and reward mechanisms that govern food intake (Coccurello and Maccarrone, 2018). The endocannabinoid system is also known to affect other reward-related behaviors as well as reinforcement, mood, anxiety, and cognition (Wenzel and Cheer, 2018). There is also evidence that the eCB system is involved in the inflammatory response; eCBs can alter macrophage migration, and macrophages along with T and B cells have the capacity to release eCBs (Cabral and Griffin-Thomas, 2009; Di Marzo et al., 1999; Sido et al., 2016). Moreover, reports suggest that the eCB system can mediate stress responses and, alternatively, can be altered by stress (Hillard, 2014), suggesting their role as regulators of endocrine response to stress (Hillard et al., 2016). Surprisingly, the vast literature on the functions of the eCB system and the efforts to target the eCB system in drug development have largely ignored the role of the circadian system and of sleep, both major modulators of mammalian metabolism, mood and behavior.
Indeed, only a limited number of reports have examined circadian fluctuations in the eCB system or regulation of circadian rhythms by the eCB system (reviewed in (Prospero-Garcia et al., 2016)). An early study by Perron and colleagues (Perron et al., 2001), revealed that discontinuation of treatment with an exogenous cannabinoid (THC), inverted the circadian rhythm of brain temperature suggesting a role for the eCB system in modulating brain temperature rhythm. More recent studies have shown diurnal variation in CB1 receptor and protein levels, as well as in the CBR ligands AEA and 2-AG, in rat brain (Martinez-Vargas et al., 2013; Martinez-Vargas et al., 2003; Rueda-Orozco et al., 2008; Valenti et al., 2004), and in rat liver (Bazwinsky-Wutschke et al., 2017). However, to date, only a few studies have examined the rhythm of circulating endocannabinoid levels in humans (Hanlon et al., 2015; Vaughn et al., 2010). Whether the impact of the circadian system and sleep may differ for the two major eCB ligands, AEA and 2-AG, has not been examined. Delineating the 24-hr variation in the activity of the eCB system may help unravel the links between eCBs and disturbances of circadian and sleep regulation, and their behavioral and physiological implications.
We therefore examined the 24-hr profile of serum AEA under normal sleep conditions and during sleep restriction. We contrast the findings with our previously published characterization of the 24-hr profile of 2-AG (Hanlon et al., 2016).
2. Material and Methods
2.1. Participants
Healthy men and women between the ages of 18 to 30 years, with a body mass index (BMI; in kg/m2) less than 27 for women, 28 for men were recruited for participation in this study. Individuals had self-reported habitual sleep duration of 7.5–8.5 hours between the hours of 2300 and 0900. All participants had an overnight polysomnography in the laboratory to exclude sleep disorders, as well as a standard 75-g oral glucose tolerance test and fasting blood sample collection for routine laboratory analyses. A 12-lead electrocardiogram was also obtained. Healthy individuals who had normal glucose tolerance and no sleep disorders were included. Further exclusion criteria included: irregular sleep schedule, habitual daytime naps, shift work, travel across time zones in last 4 weeks, chronic medical condition, acute illness, use of any prescription medications, use of over-the-counter medications or supplements known to affect sleep or glucose metabolism, smoking, marijuana usage, excessive alcohol (>2 drinks per day) or caffeine (>300mg per day) consumption, history of psychiatric disorders, or abnormal findings on medical history, physical examination or routine laboratory testing. Individuals were administered both the Center for Epidemiologic Studies Depression Scale (CESD; cut off of >16 for clinical depression) and the Beck Inventory for Depression (cut off of >10). Only those with both scores below the cut off were included. Only non-pregnant women were studied, and data collection was scheduled during the follicular phase of the menstrual cycle. All research volunteers gave written informed consent and were paid for their participation.
2.2. Study Protocol
The protocol was approved by the Institutional Review Board of the University of Chicago. All study procedures took place in the University of Chicago Clinical Resource Center. During the week preceding each in laboratory session, participants were instructed to maintain a standardized schedule of bedtimes (2300 – 0730) and to not deviate from this schedule by more than 30 minutes. To verify adherence, sleep-wake cycles of the participants were continuously monitored by wrist activity (Actiwatch; Philips/Respironics). Each participant was tested under two sleep conditions (normal sleep duration: 8.5h/night; restricted sleep duration: 4.5h/night), spaced by at least four weeks, and conducted in randomized order. Each condition involved four consecutive inpatient days with either 8.5 hours in bed (2300 to 0730, normal sleep-NS), or4.5 hours in bed (0100 to 0530, restricted sleep-RS). Experimental nights in each condition were preceded by one night of acclimation to the laboratory environment with 8.5 hours in bed. In the afternoon following the 2nd experimental night of each sleep condition, an intravenous sterile heparin-lock catheter was inserted in a forearm vein. Blood sampling was initiated at 2130 and continued for 24 hours. eCBs were assayed at 60-min intervals. Serum samples were frozen at - 80 C° until assay. Further specifics regarding room and sleep/wake conditions as well as blood sampling procedures were as previously described (Hanlon et al., 2016).
2.3. Controlled Caloric Intake
Caloric intake was identical under both sleep conditions and was calculated to meet individual participant’s caloric requirements for sedentary conditions (Schofield, 1985). A registered dietitian from the Clinical Resource Center Metabolic Kitchen at the University of Chicago supervised meals to ensure they met protocol specifications. Foods or beverages that were not provided by the metabolic kitchen were not allowed. During the 24-hour period of blood sampling, participants ate three identical carbohydrate-rich meals (20% fat, 68% carbohydrate, 12% protein), served at 0900, 1400 and 1900. These meals were consumed in their entirety within 20 minutes.
2.4. Sleep Recording
Sleep was recorded by polysomnography (Neurofax EEG-1100A, Nihon Kohden, Foothill Ranch, California) each night. Recordings were visually scored in 30-second epochs as wake, non- rapid eye movement (REM) sleep stages N1, N2, and N3 or REM sleep according to standardized criteria (Iber et al., 2007). The following summary variables were calculated: sleep period time (i.e. time interval separating sleep onset from morning awakening), total sleep time (i.e. SP – duration of intra-sleep wake periods), sleep efficiency (i.e. total sleep time / time in bed *100), duration of REM sleep, duration of light non-REM sleep (i.e. stages N1+N2), and duration of deep non-REM sleep (i.e. stage N3).
2.5. Assays
Serum concentrations of the endocannabinoids AEA were extracted from serum using Bond Elut C18 solid phase extraction columns (Varian Inc, Lake Forest, CA), as previously described (Hill et al., 2008). These extractions for AEA were done concurrent to those previously reported for 2-AG, on the same samples. The lipid was quantified in the lipid extracts by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS; Agilent LC-MSD 1100 series) and quantified by isotope dilution as described previously (Patel et al., 2005). Congeners of AEA, the structural analogs oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) were simultaneously assayed.
2.6. Analysis of individual profiles of serum concentrations of AEA, OEA, and PEA
Isolated AEA values that represented a relative change of more than 100% in comparison with both the preceding and following value and were not concomitant with a similar change in levels of congeners OEA and/or PEA were assumed to represent assay error and were replaced by linear interpolation. In the present study a total of 672 samples were measured. Nine AEA values (1.3%) were interpolated, as well as for OEA and PEA. To quantify the 24-hour profiles of serum levels of AEA, a best-fit curve was calculated for each individual profile using a robust locally weighted nonlinear regression procedure with a window of 2 hours (Cleveland, 1979). The peaks and the nadir were defined as the maximums and minimum of the regression curve, respectively. The amplitudes were defined as half of the difference between the peak and the nadir. The 24-hr profile was further characterized with the timing of the nadir and timing of the peaks (often referred to as acrophases).
2.7. Statistical analysis
All group values are expressed as mean ± SEM. The present study rigorously tests the hypothesis that circulating levels of AEA undergo a 24hr variation with a non-zero amplitude and a reproducible timing of nadir and acrophases. Amplitude was tested against the null hypothesis of zero using a t-test. The reproducibility of the timings of the acrophases and nadir was tested using the Rao Spacing Test (Russell and Levitin, 1995), a statistic that examines the clustering of reference time points (e.g. peaks and nadirs) against the hypothesis of uniform distribution across the 24-hour cycle. Summary measures extracted from the 24-hr profile characteristics of AEA and the corresponding measures extracted from the 2-AG profile (Hanlon et al., 2016) were compared by a paired t-test. Differences between the normal sleep and restricted sleep conditions for summary measures extracted from the 24-hr profiles of serum concentrations of AEA were also tested with paired t-tests. Similar analysis regarding 24-hr profile characteristics were conducted for OEA and PEA. Comparisons with corresponding measures extracted from AEA were compared with paired t-tests. Correlations were calculated using the Pearson coefficient.
3. Results
3.1. Demographics
Fourteen individuals, 3 women and 11 men, with a mean BMI of 23.9 ± 0.7 kg/m2 and mean age of 23.4 ± 0.8 years participated in this study. Eight of the 14 participants were tested under the Restricted Sleep (RS) condition first, and the remaining 6 participants began with Normal Sleep (NS). All demographic information and sleep characteristics have been reported in our previous publication (Hanlon et al., 2016).
3.2. 24-hr Profiles of AEA under normal sleep conditions
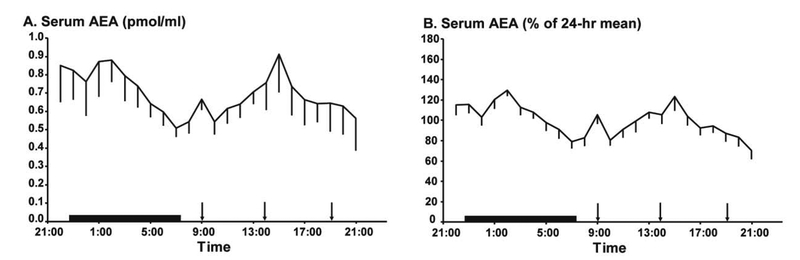
As expected, mean 24-hr serum concentrations of AEA were approximately 100-fold lower than concentrations of 2-AG (previously reported; Hanlon et al., 2016) measured in the same assay run, in the same samples (AEA 0.697 ± 0.11 pmol/ml vs 2-AG 237 ± 64 pmol/ml, p = 0.0019) (Figure 1A, Table 1). Interestingly, despite this large difference in circulating concentrations of AEA and 2-AG, individual mean 24-h levels of these two eCBs were highly correlated in the normal sleep condition (r2 = 0.84, p < 0.0001). Individuals with low mean 24hr concentrations of AEA also had low mean 24hr concentrations of 2-AG, and vice versa. Thus, irrespective of independent synthesis pathways, widely different concentrations and potential differences in physiological function, on average, across the 24-hr cycle, the mean concentrations of the two eCBs seem strongly related.
Figure 1:
Mean 24-h profiles of serum AEA concentrations in pmol/ml (A) in healthy lean participants (n=14). To facilitate the comparison of relative changes around the 24-hr mean, the profiles of serum AEA levels were expressed in % of the respective individual 24-h mean (B) in the same participants (n=14). In both panels, the standard error of the mean (SEM) is represented as a vertical line at each time point, black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900 and the black bars represent the time in bed.
Table 1.
Summary measures extracted from the 24-hr profile characteristics of AEA and the corresponding measures extracted from the previously published 2-AG profile. Values reported are mean ± SEM for qualitative variables. Profile characteristics were compared by a paired t-test.
| 2-AG | AEA | p-level | |
|---|---|---|---|
| 24hr Mean (pmol/ml) | 237 ± 64 | 0.697 ± 0.11 | 0.0019 |
| Amplitude from Morning Nadir to Daytime Peak (% of mean) | 67.5 ± 4.4 | 40.9 ± 4.3 | 0.0006 |
| Amplitude from Morning Nadir to Nighttime Peak (% of mean) | NA | 44.6 ± 4.0 | NA |
| Time of Daytime Acrophase (time ± min) | 12h34 ± 20 | 14h56 ± 42 | 0.0047 |
| Time of Nighttime Acrophase (time ± min) | NA | 02h04 ± 46 | NA |
| Time of Nadir (time ± min) | 04h09 ± 27 | 10h17 ± 41 | <0.0001 |
There was a wide inter-individual variability in the mean levels of AEA, similar to what was previously reported for 2-AG (Hanlon et al., 2016). Therefore, each individual AEA profile was expressed as a percentage of the individual 24-hour mean concentration (Figure 1B). When temporal variations of AEA are expressed in % of their mean level, it becomes apparent that the 24-hr profile of serum concentrations of AEA is clearly distinct from that observed for 2-AG as the profile of AEA is biphasic with both a nocturnal peak and a daytime peak while the profile of 2-AG is characterized by a single early afternoon peak. Quantitative comparisons are summarized in Table 1 and the two profiles are contrasted in Figure 2. Relative to the 24-hr variation in 2-AG, the amplitude of the AEA rhythm is markedly blunted (Table 1). The mean 24-hour profile of AEA is of lower amplitude (Table 1) and is clearly bi-phasic with a significant nadir in the mid-morning and two peaks, the first occurring shortly after bedtime and the second in the afternoon. The Rao spacing test revealed that the two individual AEA acrophases, as well as the mid-morning nadir, were significantly clustered around these times (p < 0.001). The timing of the nadir of the AEA rhythm was significantly later than that of the 2-AG nadir. Moreover, neither of the two daytime peaks of AEA was coincident with the single 2-AG peak (Table 1). The two AEA peaks were similar to each other in amplitude (p = 0.2984).
Figure 2:
Alignment of the relative 24-hr profiles of AEA (black line) with the relative 24-hr profile of 2-AG (grey line). For each profile, all data are shown as % of the individual 24-h mean. Meals times are denoted with arrows and time in bed with a black bar. For both AEA and 2-AG, the standard error of the mean (SEM) is represented as a vertical line at each time point. The bi-phasic rhythm of AEA is apparent in contrast to the single peak observed for 2-AG.
3.3. Effect of sleep restriction on 24-hr profiles of AEA
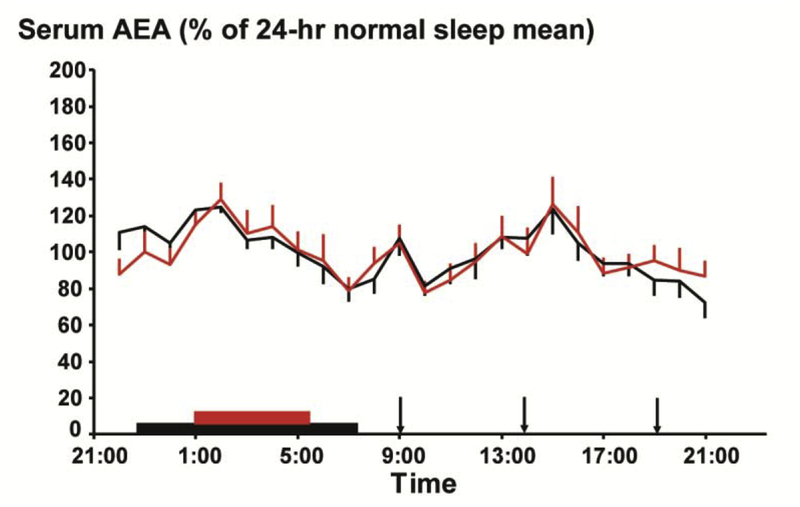
Thirteen of the 14 participants were considered for analysis of AEA following sleep restriction because overnight samples from the sleep restriction condition of one participant were inadvertently mishandled. Sleep restriction had no detectable effect on the 24-hr AEA profile which was remarkably reproducible in the two conditions (Figure 4A), in contrast to the dynamic alterations previously observed for 2-AG (Hanlon et al., 2016). The amplitude of the two AEA peaks remained similar between normal and restricted sleep (n.s.). Moreover, the timings of the nadir and acrophases were also unaffected by sleep restriction (n.s.) (Figure 3). Not only were the AEA serum concentrations over the 24-hr period comparable between the normal sleep and sleep restriction conditions, they were virtually superimposable. Indeed, the mean 24-hr concentrations of AEA following the two sleep conditions were significantly correlated (r2 = 0.85, p < 0.0001), showing the high reproducibility within a given subject.
Figure 4.
Mean 24-h profiles of serum OEA (A) and PEA (B) concentrations in pmol/ml in healthy lean participants during the restricted 4.5 hour (red) or normal 8.5 hour (black) sleep condition (n=13). In both panels, the standard error of the mean (SEM) is represented as a vertical line at each time point, black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900. The wave-shapes of the 24-hr profiles OEA and PEA were similar to that observed for AEA with two peaks and a nadir in the morning. Similar to AEA, the 24-hr profiles of OEA and PEA were not affected by restricted sleep. The sleep period is denoted with red or black bar for restricted 4.5 hour (red) or normal 8.5 hour (black) sleep condition, respectively.
Figure 3:
Mean 24-hour profiles of AEA (n=13), expressed as % of 24-hour mean of the normal sleep condition during the restricted 4.5 hour (red) or normal 8.5 hour (black) sleep condition. Vertical lines at each time point represent the SEM. There are no significant differences in AEA levels between the two sleep conditions at any time point. The sleep period is denoted with red or black bars. Closed arrows represent the identical carbohydrate-rich meals, presented at 0900, 1400, 1900.
3.4. 24-hr Profiles of OEA and PEA under normal and restricted sleep conditions
As expected, the wave-shape of the 24-hr profile of both OEA and PEA resembled that observed for AEA; the profiles were biphasic with a nocturnal as well as a diurnal peak and a nadir in the morning. While the 24-hr mean absolute concentrations were higher than those of AEA for both OEA (OEA:3.65 ± 0.85 pmol/ml v AEA 0.697 ± 0.11 pmol/ml, p = 0.004) and PEA (PEA:19.4 ± 4.12 pmol/ml v AEA 0.697 ± 0.11 pmol/ml, p = 0.001), both congeners had serum concentrations significantly lower than those reported for 2-AG (OEA v 2-AG, p = 0.002; PEA v 2-AG, p = 0.003). Moreover, analogous with the characteristics of the 24-hr profile for AEA, the amplitudes of the nocturnal and daytime peaks were similar to each other for both OEA and PEA (n.s.), and significantly smaller than the amplitude of the robust single peak observed for 2-AG (for all peak amplitude comparisons, p < 0.001). Most notably, the timing of the two acrophases observed for the 24-hr profile of OEA and PEA were coincident with those peaks observed for AEA (n.s.). Further, the timing of the nadirs of AEA, OEA, and PEA were all similar (n.s. for all comparisons). Lastly, comparable to what was observed with AEA, sleep restriction did not affect the 24-hr profiles of OEA or PEA (Figure 4).
4. Discussion
The current study examined 24-hr rhythms of serum concentrations of one of the most studied endogenous ligands of the cannabinoid receptors, AEA, also known as anandamide. As expected (Hillard et al., 2012), mean 24-hr concentrations of AEA were significantly lower than those previously reported for 2-AG (Hanlon et al., 2016). Despite the vast difference in synthesis pathways and serum concentrations, 24-hr mean values of AEA were highly correlated with values for 2-AG. Most interestingly, the novel and important finding of this study is that the 24-hr profile of AEA was qualitatively distinct from that of 2-AG and of lower amplitude. In contrast to the large monophasic rhythm of 2-AG with a nadir around mid-sleep and an early afternoon peak, serum concentrations of AEA fluctuate consistently in a bi-phasic pattern with both a nocturnal peak in early sleep and a daytime peak in the mid-afternoon. These two peaks are separated by a mid-morning nadir. Similar 24-hr profile characteristics were observed for both OEA and PEA. This is in stark contrast to the observed 24-hr rhythm of 2-AG. Moreover, we did not detect any impact of sleep restriction on the 24-hr profile of serum concentrations of AEA, OEA, or PEA, in contrast to previously reported elevation of peak daytime level of 2-AG (Hanlon et al., 2016).
The protocol optimized over 4 consecutive days, conditions known to promote alignment in the circadian system: constant light-dark cycle, constant sleep-wake cycle, constant overnight fast, fixed timing of meals. Nonetheless, we found major differences in the temporal variation of AEA as compared to 2-AG over the 24-hr cycle. The differences in both amplitude and timing of the AEA versus 2-AG variations suggest that the circadian control of the production of peripheral levels of AEA may be less robust than for 2-AG. This could reflect a greater heterogeneity of tissues and cell types releasing AEA versus 2-AG in the peripheral circulation, and/or a weaker control of these tissues or cell types by the circadian signal. Besides the difference in amplitude of the daily wave shape of the two eCB, the contrast in the overnight pattern is particularly striking, as in the healthy lean young adults, AEA serum concentration peaked early in the night at a time when concomitant 2-AG concentrations were declining. The functional relevance of these relative overnight fluctuations in serum eCB concentrations is unclear at this time but suggests a temporal segregation of the actions of the two eCB during the habitual rest period. In contrast, from mid-morning to mid-afternoon, the concentrations of both eCB seem to increase in concert, albeit much more briskly for 2-AG than AEA. Some studies have suggested that AEA and 2-AG may serve different functions in the periphery although further experimental studies will be required to elucidate the functional significance of the patterns of eCB fluctuations observed in the present study.
Food intake was carefully controlled in the present study; all meals were isocaloric and over the period of 24-hr sampling, within a subject, the same carbohydrate-rich meals were ingested in both sleep conditions. As we have previously noted, the wave shape of 2-AG suggests that the stimulation of appetite and food intake mediated by activation of the eCB system may increase substantially from early morning to midafternoon (Hanlon et al., 2016). The rise of AEA is initiated later than what was observed for 2-AG and is not as robust, suggestive of a differential underlying mechanism. In regards to food intake, since circulating 2-AG is thought to be more involved in perceived reward or hedonic value of food, while peripheral AEA may be concerned with the overall homeostatic control of energy intake (Hillard, 2018), the blunted (in comparison to 2-AG) 24-hr rhythm of AEA may indicate subtle fluctuations in homeostatic control of energy need and storage over the 24-hr period.
This hypothesis is further supported by the lack of effect of sleep restriction on the AEA 24-hr profile. Sleep restriction causes a robust increase in hunger, appetite, and food intake particularly for energy dense, highly palatable, snack foods (Hanlon et al., 2016; Nedeltcheva et al., 2009; St-Onge et al., 2011). This increase occurs despite the fact that changes in energy needs are much smaller (Jung et al., 2011; Markwald et al., 2013; Shechter et al., 2013). Thus, increased energy intake in the presence of ad lib feeding appears to exceed the energy demands of extended wakefulness under sedentary conditions (Brondel et al., 2010; Markwald et al., 2013; Nedeltcheva et al., 2009; Spiegel et al., 2004; St-Onge et al., 2011). The previously reported observed increase in circulating 2-AG following sleep restriction suggests that this arm of the eCB system may be involved in the observed increases in hedonically driven snack intake (Hanlon et al., 2016) while the unchanged profiles of circulating AEA reported here may reflect unchanged homeostatic energy need. The duration and severity of sleep restriction in the present study may not have been sufficient to produce alterations in homeostatic energy need, and thus alter serum concentrations of AEA, similar to what was previously reported for the leptin profiles which were minimally affected by sleep restriction (Hanlon et al., 2016). Lastly, previous studies have indicated that circulating AEA may be involved in the anticipatory aspect of food intake. In normal weight individuals, circulating AEA was found to increase before food ingestion, and decrease post-prandially (Gatta-Cherifi et al., 2012). In the current study, a sharp rise in AEA is evident from 0800 to 0900, and the 0900 sample was taken prior to ingestion of the morning meal (Figure 1A and B), consistent with a pre-prandial anticipatory rise in circulating AEA. However, the temporal resolution (q60 min) does not allow for adequate analysis or interpretation of pre- or post-prandial excursions in circulating serum eCB concentrations.
Diurnal variation in OEA and PEA has been previously reported in rat brain (Murillo-Rodriguez et al., 2006) and human plasma (Cedernaes et al., 2016). The characteristics of the 24-hr profiles of OEA and PEA in the current report differ from these previous descriptions, although this is not surprising considering the differences in species (rats vs humans) and in study conditions (exercise vs no exercise). Consistent with the current observations, in the study by Cedernaes and colleagues, eCB and NAE plasma concentrations were measured on morning fasting samples, following a period of normal and restricted sleep and no difference was observed in AEA, OEA, and PEA levels (Cedernaes et al., 2016). In contrast, OEA has been observed to be increased in cerebral spinal fluid and marginally increased in serum following a 24-hr sleep deprivation in humans, while AEA was not affected (Koethe et al., 2009). The pharmacologic and physiologic actions of OEA and PEA may differ to that of AEA (Karwad et al., 2017), thus further investigation regarding the functional significance of the 24-hr observed fluctuations in OEA and PEA is warranted.
Besides the low frequency of sampling (i.e. hourly) of eCB serum concentrations, the relatively small sample size and short duration of sleep restriction are other limitations of the present study. Nonetheless, the findings clearly show a dissimilarity in 24-hr rhythm of the two most studied endocannabinoids suggesting a differential impact of the circadian system on these two components of the eCB system.
In conclusion, the present study shows that serum concentrations of the ligand of the endocannabinoid receptors, AEA have a reproducible 24-hr rhythm distinct from that observed for 2-AG levels despite the fact that, on average over the entire 24-hr cycle, concentrations of AEA and 2-AG are highly correlated. Moreover, unlike 2-AG, AEA is not altered by sleep restriction, revealing that sleep disturbances may affect AEA and 2-AG differentially. The present findings indicate that time of day needs to be carefully controlled in future studies attempting to delineate the relative roles of the two eCBs.
Acknowledgements:
The author would like to thank Kara L Stuhr, Elizabeth Doncheck, and Harry Whitmore for their assistance in data collection. Thanks are also due to her collaborators on the initial project and publications, including Rachel Leproult, PhD and Esra Tasali, MD, and Harriet de Wit, PhD. Special thanks to Cecilia J. Hillard, PhD and Eve Van Cauter, PhD for their critical review of the final draft of the manuscript.
Funding Sources: This study was supported by Grant Number KL2RR025000 from the National Center for Research Resources, contract W81XWH-07-2-0071 from the Department of Defense Peer Reviewed Medical Research Program, and the University of Chicago Institute for Translational Medicine supported by UL1 RR024999. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Center for Research Resources, the Department of Defense or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: The author reports no conflicts of interest.
References
- Bazwinsky-Wutschke I, Zipprich A, Dehghani F, 2017. Daytime-Dependent Changes of Cannabinoid Receptor Type 1 and Type 2 Expression in Rat Liver. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D, 2010. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 91, 1550–1559. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L, 2009. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedernaes J, Fanelli F, Fazzini A, Pagotto U, Broman JE, Vogel H, Dickson SL, Schioth HB, Benedict C, 2016. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 74, 258–268. [DOI] [PubMed] [Google Scholar]
- Chen G, Pang Z, 2013. Endocannabinoids and obesity. Vitam Horm 91, 325–368. [DOI] [PubMed] [Google Scholar]
- Cleveland W, 1979. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association 74, 829–836. [Google Scholar]
- Coccurello R, Maccarrone M, 2018. Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back. Frontiers in neuroscience 12, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L, Rimonabant in Obesity-Lipids Study, G., 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353, 2121–2134. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R, 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G, 1999. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem 264, 258–267. [DOI] [PubMed] [Google Scholar]
- Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LH, Goulet MT, Hagmann WK, Lin LS, Lanza TJ Jr., Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, Shearman LP, 2007. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(t rifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther 321, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R, 1964. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society 86, 1646–1647. [Google Scholar]
- Gatta-Cherifi B, Matias I, Vallee M, Tabarin A, Marsicano G, Piazza PV, Cota D, 2012. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes (Lond) 36, 880–885. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M, 1991. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J 279 (Pt 1), 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E, 2015. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab 100, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E, 2016. Sleep Restriction Enhances the Daily Rhythm of Circulating Levels of Endocannabinoid 2-Arachidonoylglycerol. Sleep 39, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ, 2008. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 41, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, 2014. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol 26, 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, 2018. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 43, 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Beatka M, Sarvaideo J, 2016. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr Physiol 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, Stuhr KL, 2012. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience 204, 207–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA, Di Marzo V, Petrosino S, 2016. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog Lipid Res 62, 107–128. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AJ, Quan SF, 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine, Westchester, Illinois. [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P, 2005. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19, 1567–1569. [DOI] [PubMed] [Google Scholar]
- Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP, 2011. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwad MA, Macpherson T, Wang B, Theophilidou E, Sarmad S, Barrett DA, Larvin M, Wright KL, Lund JN, O’Sullivan SE, 2017. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARalpha. FASEB J 31, 469–481. [DOI] [PubMed] [Google Scholar]
- Kipnes MS, Hollander P, Fujioka K, Gantz I, Seck T, Erondu N, Shentu Y, Lu K, Suryawanshi S, Chou M, Johnson-Levonas AO, Heymsfield SB, Shapiro D, Kaufman KD, Amatruda JM, 2010. A one-year study to assess the safety and efficacy of the CB1R inverse agonist taranabant in overweight and obese patients with type 2 diabetes. Diabetes Obes Metab 12, 517–531. [DOI] [PubMed] [Google Scholar]
- Koethe D, Schreiber D, Giuffrida A, Mauss C, Faulhaber J, Heydenreich B, Hellmich M, Graf R, Klosterkotter J, Piomelli D, Leweke FM, 2009. Sleep deprivation increases oleoylethanolamide in human cerebrospinal fluid. J Neural Transm 116, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PM, Marczylo TH, Konje JC, 2010. Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 398, 2089–2097. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP Jr., 2013. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A 110, 5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vargas M, Morales-Gomez J, Gonzalez-Rivera R, Hernandez-Enriquez C, Perez-Arredondo A, Estrada-Rojo F, Navarro L, 2013. Does the neuroprotective role of anandamide display diurnal variations? Int J Mol Sci 14, 23341–23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vargas M, Murillo-Rodriguez E, Gonzalez-Rivera R, Landa A, Mendez-Diaz M, Prospro-Garcia O, Navarro L, 2003. Sleep modulates cannabinoid receptor 1 expression in the pons of rats. Neuroscience 117, 197–201. [DOI] [PubMed] [Google Scholar]
- Mazier W, Saucisse N, Gatta-Cherifi B, Cota D, 2015. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab 26, 524–537. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. , 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50, 83–90. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M, 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Desarnaud F, Prospero-Garcia O, 2006. Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life sciences 79, 30–37. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD, 2009. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 89, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ, 2005. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res 46, 342–349. [DOI] [PubMed] [Google Scholar]
- Perron RR, Tyson RL, Sutherland GR, 2001. Delta9 -tetrahydrocannabinol increases brain temperature and inverts circadian rhythms. Neuroreport 12, 3791–3794. [DOI] [PubMed] [Google Scholar]
- Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O’Connor SE, Janiak P, Herbert JM, 2005. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 7, 65–72. [DOI] [PubMed] [Google Scholar]
- Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, Jones ME, Johnson-Levonas AO, Heymsfield SB, Kaufman KD, Amatruda JM, 2010. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes (Lond) 34, 1243–1254. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Amancio-Belmont O, Becerril Melendez AL, Ruiz-Contreras AE, Mendez-Diaz M, 2016. Endocannabinoids and sleep. Neurosci Biobehav Rev 71, 671–679. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P, 2003. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284, R345–353. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Soria-Gomez E, Montes-Rodriguez CJ, Martinez-Vargas M, Galicia O, Navarro L, Prospero-Garcia O, 2008. A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology (Berl) 198, 565–576. [DOI] [PubMed] [Google Scholar]
- Russell GS, Levitin DJ, 1995. An expanded table of probability values for rao’s spacing test. Communications in Statistics - Simulation and Computation 24, 879–888. [Google Scholar]
- Sam AH, Salem V, Ghatei MA, 2011. Rimonabant: From RIO to Ban. J Obes 2011, 432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, Group RI-DS, 2006. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368, 1660–1672. [DOI] [PubMed] [Google Scholar]
- Schofield WN, 1985. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39 Suppl 1, 5–41. [PubMed] [Google Scholar]
- Shechter A, Rising R, Albu JB, St-Onge MP, 2013. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr 98, 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido JM, Nagarkatti PS, Nagarkatti M, 2016. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur J Immunol 46, 1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E, 2004. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141, 846–850. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, Jones PJ, 2011. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 94, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K, 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215, 89–97. [DOI] [PubMed] [Google Scholar]
- Turcotte C, Chouinard F, Lefebvre JS, Flamand N, 2015. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97, 1049–1070. [DOI] [PubMed] [Google Scholar]
- Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V, 2004. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cellular and molecular life sciences : CMLS 61, 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, Group RI-ES, 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O, Group RI-ES, 2008. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 29, 1761–1771. [DOI] [PubMed] [Google Scholar]
- Vaughn LK, Denning G, Stuhr KL, de Wit H, Hill MN, Hillard CJ, 2010. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol 160, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Cheer JF, 2018. Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling. Neuropsychopharmacology 43, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]