Abstract
Whether marine omega-3 fatty acid (n-3 FA) or vitamin D supplementation can prevent cardiovascular disease (CVD) in general populations at usual risk for this outcome is unknown. A major goal of the VITamin D and OmegA-3 TriaL (VITAL) was to fill this knowledge gap. In this article, we review the results of VITAL, discuss relevant mechanistic studies regarding n-3 FAs, vitamin D, and vascular disease, and summarize recent meta-analyses of the randomized trial evidence on these agents. VITAL was a nationwide, randomized, placebo-controlled, 2×2 factorial trial of marine n-3 FAs (1 g/d) and vitamin D3 (2000 IU/d) in the primary prevention of CVD and cancer among 25,871 U.S. men aged ≥50 and women aged ≥55, including 5,106 African Americans. Median treatment duration was 5.3 years. Supplemental n-3 FAs did not significantly reduce the primary cardiovascular endpoint of major CVD events (composite of myocardial infarction [MI], stroke, and CVD mortality; hazard ratio [HR]=0.92 [95% confidence interval 0.80–1.06]) but was associated with significant reductions in total MI (HR=0.72 [0.59–0.90]), percutaneous coronary intervention (HR=0.78 [0.63–0.95]), and fatal MI (HR=0.50 [0.26–0.97]) but not stroke or other cardiovascular endpoints. For major CVD events, a treatment benefit was seen in those with dietary fish intake below the cohort median of 1.5 servings/week (HR=0.81 [0.67–0.98]) but not in those above (p, interaction=0.045). For MI, the greatest risk reductions were in African Americans (HR=0.23 [0.11–0.47]; p, interaction by race=0.001). Vitamin D supplementation did not reduce major CVD events (HR=0.97 [0.85–1.12]) or other cardiovascular endpoints. Updated meta-analyses that include VITAL and other recent trials document coronary risk reduction from supplemental marine n-3 FAs but no clear CVD risk reduction from supplemental vitamin D. Additional research is needed to determine which individuals may be most likely to derive net benefit from supplementation.
(VITAL (clincialtrial.gov identifier: )
Keywords: cardiovascular disease, diet and nutrition, epidemiology, race and ethnicity, primary prevention, marine n-3 fatty acids, randomized controlled trial, vitamin D
INTRODUCTION
Marine n-3 FA supplementation has been recommended for heart health in patients with coronary heart disease (CHD) who do not meet target intakes for n-3 FA-rich fatty fish,1, 2 and vitamin D supplementation is an established intervention for the prevention and treatment of bone disorders.3 A decade ago, these supplements were also increasingly being used for the possible prevention of a first cardiovascular event or cancer, and their U.S. sales soared.4–6 Indeed, n-3 FAs and vitamin D remain among the most widely used supplements today.7, 8
Ecologic, laboratory, and observational study data supporting these potential new indications were promising but inconclusive and insufficient to establish causality.3, 9, 10 For n-3 FAs, some11–13 though not all14–16 trials in secondary prevention or high-risk settings had found cardiovascular disease (CVD) risk reductions, but no large trial in a general population unselected for elevated cardiovascular risk had been conducted. For vitamin D, trials that had assessed CVD or cancer outcomes in secondary or post hoc analyses had shown generally null results, but low doses, inadequate statistical power, short treatment durations, and/or lack of rigorous endpoint adjudication precluded definitive assessments.3 Large trials of vitamin D in doses adequate to produce meaningful changes in 25-hydroxyvitamin D (25(OH)D) levels and designed to assess CVD or cancer as primary outcomes were lacking. The Institute of Medicine3 in 2011 concluded that the effectiveness and benefit-risk balance of vitamin D supplementation for CVD or cancer prevention could not be established with existing data (as did the U.S. Preventive Services Task Force17 in 2014) and highlighted the need for trials of vitamin D in doses at least twice the current recommended dietary allowance of 600–800 IU/d for bone health to understand the benefit-risk balance, including in diverse populations.
We conducted the VITamin D and OmegA-3 TriaL (VITAL), an investigator-initiated study, to fill these knowledge gaps. To date, VITAL is the only trial of n-3 FA supplementation for prevention of CVD in a general population selected only on age and not on high risk for CVD. VITAL is also the first of only two large (N≥10,000) trials of moderate- to high-dose vitamin D for the prevention of CVD and cancer, and the only such trial with a significant number of black individuals, for whom these issues may be particularly salient because of their lower cutaneous synthesis of vitamin D in response to solar radiation.18 In this article, we summarize the design and primary results19, 20 of the trial; discuss the findings in the context of relevant research; report on recent meta-analyses of clinical trial evidence; summarize mechanistic data; and suggest avenues for further investigation. The focus is on the trial’s cardiovascular findings, but cancer and all-cause mortality are also discussed as these outcomes are important contributors to the benefit-risk balance of supplementation.
OVERVIEW OF VITAL
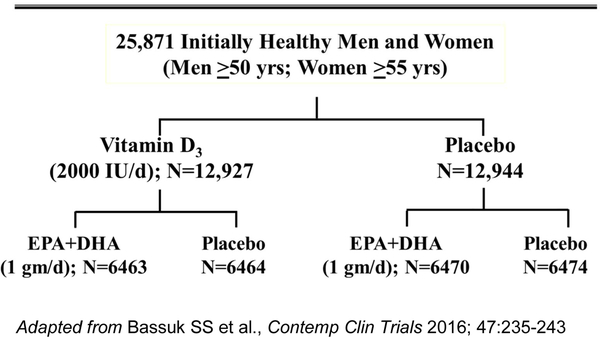
VITAL was a randomized, double-blind, placebo-controlled trial of the benefits and risks of supplemental marine n-3 FAs (1 g/d Omacor® fish-oil capsule with 840 mg of n-3 FAs, including eicosapentaenoic acid [EPA, 460 mg] + docosahexaenoic acid [DHA, 380 mg]) and vitamin D3 (2000 IU/d) in the primary prevention of CVD and cancer among 25,871 men and women, aged ≥50 and ≥55, respectively.9, 19–21 Participants were recruited throughout the U.S., balanced by sex, and with oversampling of African Americans (n=5,106). Eligible participants had no prior myocardial infarction (MI), stroke, transient ischemic attack, coronary revascularization, or cancer (except non-melanoma skin cancer) at study entry. They needed to agree to forego the use of fish-oil supplements and to limit their daily intake of vitamin D and calcium from all supplemental sources, including multivitamins, to no more than 800 IU and 1200 mg, respectively (the recommended dietary allowances [RDAs] for older adults3). Safety exclusions included renal failure or dialysis, severe liver disease (cirrhosis), use of anticoagulants, history of hypercalcemia or parathyroid disorders, or other conditions that would preclude participation. After successful completion of a 3-month placebo run-in, participants were randomized in a 2×2 factorial design to vitamin D, n-3 FAs, both active agents, or both placebos (Figure 1). Randomization took place from November 2011 to March 2014. Randomized treatment ended as planned on December 31, 2017, yielding a median intervention period of 5.3 years (range 3.8–6.1 years).
Figure 1:
VITAL Factorial Design.
Baseline questionnaires collected data on clinical and lifestyle risk factors for CVD, cancer, and other conditions; a food frequency questionnaire ascertained intake of fish, dairy products, and other foods. Annual follow-up questionnaires assessed treatment compliance and side effects, risk factor updates, and endpoint occurrence. Participant-reported endpoints were confirmed/disconfirmed by medical record review using accepted criteria22–24 by physicians blinded to treatment assignment, and deaths were ascertained through the National Death Index-Plus and other sources. Baseline blood samples were collected during the run-in from all willing participants (16,956 of 25,871 randomized participants [66%]), and follow-up samples were collected in years 1–5 from ~6000 participants in ancillary studies. Some Boston-area participants (n=1,054) underwent detailed in-clinic assessments at a local Clinical and Translational Science Center (CTSC) clinic at baseline and years 1, 2, and 4. Except for the CTSC component and selected ancillary studies, VITAL has been conducted primarily by postal and electronic communication.
Baseline characteristics of the study population are in Table 1. During the 5.3-year intervention period, response rates to yearly questionnaires averaged 93%, and mortality follow-up was >98%.19 Adherence to randomized treatment was high.19, 20 Among the ~15,500 participants with analyzable baseline blood samples, the mean plasma n-3 index (EPA+DHA as a percent of total fatty acids25) was 2.6% (SD, 0.9%) and the mean serum total 25(OH)D level was 30.8 ng/mL (SD, 10.0) ng/mL, with 12.7% and 32.2% having levels <20 ng/mL and 20-<30 ng/mL, respectively. There was a large post-randomization difference in the n-3 index between the active n-3 FA and placebo groups (55% increase) and in 25(OH)D levels between the active vitamin D and placebo groups (40% increase), which was evident throughout the trial. In the subgroup with follow-up measurements, the achieved plasma n-3 index with active n-3 FA was ~4.1% and the achieved serum 25(OH)D with active vitamin D exceeded 40 ng/mL (100 nmol/L), without changes over time in the placebo groups.
Table 1.
Baseline characteristics of the 25,871 VITAL participants, according to randomized treatment assignment
| Baseline characteristic | All participants | n-3 fatty acids | Vitamin D | ||||
|---|---|---|---|---|---|---|---|
| Active | Placebo | Active | Placebo | ||||
| Total number | 25,871 | 12,933 | 12,938 | 12,927 | 12,944 | ||
| Female sex—number (%) | 13,085/25,871 (50.6) | 6547 (50.6) | 6538 (50.5) | 6547 (50.6) | 6538 (50.5) | ||
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 | 67.1 ± 7.0 | 67.1 ± 7.1 | ||
| Race/ethnicity—number (%)a | |||||||
| Non-Hispanic White | 18,046/25,304 (71.3) | 9044 (71.5) | 9002 (71.2) | 9013 (71.3) | 9033 (71.4) | ||
| African American | 5106/25,304 (20.2) | 2549 (20.1) | 2557 (20.2) | 2553 (20.2) | 2553 (20.2) | ||
| Hispanic (not African American) | 1013/25,304 (4.0) | 491 (3.9) | 522 (4.1) | 516 (4.1) | 497 (3.9) | ||
| Asian/Pacific Islander | 388/25,304 (1.5) | 200 (1.6) | 188 (1.5) | 188 (1.5) | 200 (1.6) | ||
| Native American | 228/25,304 (0.9) | 120 (0.9) | 108 (0.9) | 118 (0.9) | 110 (0.9) | ||
| Other or unknown | 523/25,304 (2.1) | 249 (2.0) | 274 (2.2) | 259 (2.0) | 264 (2.1) | ||
| Body mass index, kg/m2, mean ± SDb | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 | 28.1 ± 5.7 | 28.1 ± 5.8 | ||
| Current smoking—number (%) | 1836/25,485 (7.2) | 920 (7.2) | 916 (7.2) | 921 (7.2) | 915 (7.2) | ||
| Hypertension treated with medication—number (%) | 12,791/25,698 (49.8) | 6338 (49.3) | 6453 (50.2) | 6352 (49.5) | 6439 (50.1) | ||
| Cholesterol-lowering medication, current use—number (%) | 9524/25,428 (37.5) | 4788 (37.7) | 4736 (37.2) | 4822 (38.0) | 4702 (36.9) | ||
| Diabetes—number (%) | 3549/25,828 (13.7) | 1799 (13.9) | 1750 (13.5) | 1812 (14.0) | 1737 (13.4) | ||
| Current regular aspirin use—number (%)c | 11,570/25,497 (45.4) | 5771 (45.3) | 5799 (45.5) | 5756 (45.2) | 5,814 (45.6) | ||
Abbreviations: SD = standard deviation. There were no significant differences in the baseline characteristics between the groups.
Race and ethnic group were reported by participants.
For body mass index, data were missing for 2.4% of participants.
At least monthly.
SUPPLEMENTAL n-3 FAs
VITAL Results
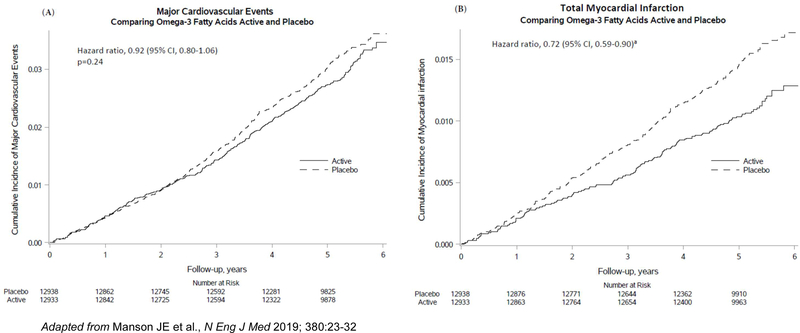
Supplemental n-3 FAs did not significantly reduce the primary endpoint of major CVD events (a composite of MI, stroke, and CVD mortality; hazard ratio=0.92 [95% confidence interval 0.80–1.06]) but did reduce total MI (a prespecified secondary endpoint) by a significant 28% (HR=0.72 [0.59–0.90]) (Table 2). This benefit emerged after the first year and persisted throughout the trial (Figure 2). Significant reductions in risk of percutaneous coronary intervention (PCI; HR=0.78 [0.63–0.95]), fatal MI (HR=0.50 [0.26–0.97]), and total CHD (HR=0.83 [0.71–0.97]) were also found. In contrast, there were no significant reductions in risk of coronary artery bypass grafting (CABG), stroke, CVD mortality, or an expanded CVD endpoint (major CVD events plus coronary revascularization [PCI or CABG]). In analyses that excluded the first 2 years of follow-up, the HR for major CVD events was 0.89 (0.76–1.05). In analyses that censored for noncompliance, the HRs were similar to those in intention-to-treat analyses.
Table 2.
Hazard ratios (HR) and 95% confidence intervals (CI) of primary, secondary, and other outcomes by randomized assignment to n-3 fatty acids (n-3 FAs)a
| Endpoint | n-3 FAs (N =12,933) | Placebo (N =12,938) | HR | 95% CI |
|---|---|---|---|---|
| # of participants w/event | ||||
|
Cardiovascular disease (CVD), primary and secondary outcomes |
||||
| Major CVD eventb,c | 386 | 419 | 0.92 | 0.80–1.06 |
| Expanded CVD eventd | 527 | 567 | 0.93 | 0.82–1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59–0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83–1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76–1.21 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63–0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73–1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26–0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49–1.16 |
| Total CHDf | 308 | 370 | 0.83 | 0.71–0.97 |
| Ischemic stroke | 111 | 116 | 0.96 | 0.74–1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72–2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60–2.01 |
| Total invasive cancerb | 820 | 797 | 1.03 | 0.93–1.13 |
| Cancer mortality | 168 | 173 | 0.97 | 0.79–1.20 |
| All-cause mortality | 493 | 485 | 1.02 | 0.90–1.15 |
| Excluding the first two years of follow-up: | ||||
| Cardiovascular outcomes | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76–1.05 |
| Total myocardial infarction | 94 | 131 | 0.72 | 0.55–0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70–1.20 |
| Total invasive cancer | 536 | 476 | 1.13 | 1.00–1.28 |
| Cancer mortality | 126 | 135 | 0.93 | 0.73–1.19 |
| All-cause mortality | 371 | 381 | 0.97 | 0.84–1.12 |
Analyses were from Cox regression models that were controlled for age, sex, and randomization group in the vitamin D portion of the trial. Analyses were not adjusted for multiple comparisons.
Primary outcomes
A composite of MI, stroke, and cardiovascular mortality
A composite of MI, stroke, cardiovascular mortality, and coronary revascularization (CABG, PCI)
Not prespecified as primary or secondary outcomes.
A composite of MI, coronary revascularization (CABG, PCI), and CHD death
Figure 2.
Cumulative incidence rates of major CVD events and total MI by year of follow-up, in the n-3 fatty acid group and the placebo group.
With respect to noncardiovascular endpoints, n-3 FA supplementation was not associated with incidence of total cancer (co-primary endpoint) or breast, prostate, or colorectal cancers; cancer mortality; or all-cause mortality in analyses of the full trial period (Table 2). However, a signal for increased cancer incidence (HR=1.13 [1.00–1.28]), though not for cancer mortality (HR=0.93 [0.73–1.19]) or all-cause mortality (HR=0.97 [0.84–1.12]), emerged in analyses that excluded the first two years of follow-up. With regard to side effects, the n-3 FA intervention was well tolerated, with no treatment-associated increase in bleeding or gastrointestinal symptoms.
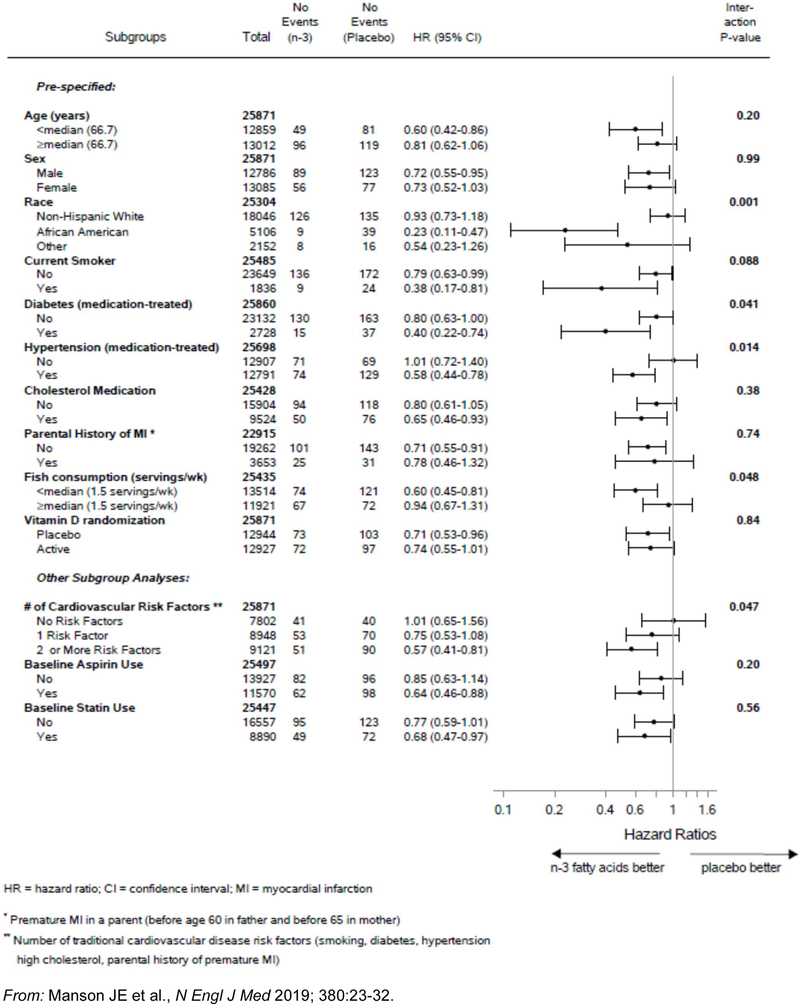
The following characteristics, assessed at baseline, were examined as potential modifiers of n-3 FA treatment effects: age; sex; race/ethnicity; traditional cardiovascular risk factors; aspirin use; statin use; dietary fish intake, plasma n-3 index, and concurrent randomization to the active vitamin D group. Of these, only baseline dietary fish intake significantly influenced the effect of n-3 FA supplementation on both major CVD events (Online Figure I) and total MI (Figure 3) (p, interaction<0.05 for each endpoint). This characteristic also significantly modified the intervention’s effect on all-cause mortality (p, interaction=0.02) and tended to modify its effect on cancer (p, interaction=0.09). In individuals with low fish intake (below the median of 1½ servings/week), n-3 FA supplementation was associated with a 19% reduction in major CVD events (HR=0.81 [0.67–0.98]), including a 40% reduction in MI (HR=0.60 [0.45–0.81]), and a trend toward a reduction in all-cause mortality (HR=0.87 [0.73–1.04]) and no indication of increased cancer risk (HR=0.96 [0.84–1.09]). In contrast, for individuals with higher fish intake (≥1½ servings/week), n-3 FAs offered no protection against major CVD events, MI, all-cause mortality, or cancer. No other characteristic significantly affected the association between the intervention and risk of major CVD events or all-cause mortality. For MI, however, race/ethnicity and the presence of traditional cardiovascular risk factors also significantly modified the effect of n-3 FA supplementation. African Americans experienced a significant 77% treatment-associated reduction in MI (HR=0.23 [0.11–0.47]) while other racial/ethnic groups had smaller reductions (p, interaction=0.001) (Figure 3). Of note, African Americans derived an MI benefit irrespective of fish intake, but non-Hispanic whites did so only when fish intake was low. African Americans also had significant treatment-associated reductions in coronary revascularization (HR=0.51 [0.28–0.92]) and total CHD (HR=0.61 [0.43–0.88]) and no treatment-associated increase in cancer risk (HR=1.02 [0.79–1.33]) or all-cause mortality (HR=0.84 [0.64–1.11]). Participants with a larger number of traditional cardiovascular risk factors also derived an MI benefit from n-3 FA supplementation, but those without these risk factors did not (p, interaction=0.047) (Figure 3). The aforementioned treatment benefit for MI in African Americans did not diminish after adjustment for cardiovascular risk factors (HR=0.19 [0.07–0.50]) and was more apparent than in non-Hispanic whites across all cardiovascular risk-factor strata. For all-cause mortality, no factor other than dietary fish intake significantly modified the treatment effect.
Figure 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of total myocardial infarction according to subgroups, comparing n-3 fatty acid and placebo groups. (From Cox regression models controlling for age, sex, and vitamin D randomization group.)
We recently analyzed Intervention effects on lipids and inflammatory markers in participants with 1-year follow-up blood samples. n-3 FA supplementation was associated with a small but significant reduction in triglycerides but had no significant effect on other lipids. Treatment-associated reductions in high-sensitivity C-reactive protein levels were greater in those with low fish intake (p, interaction=0.06), supporting the finding that such individuals were more likely to experience reduced risk of major CVD events with n-3 FAs.
Discussion and Context of Other Research
VITAL is the only large trial of n-3 FAs for the primary prevention of CVD in a usual-risk population. However, several earlier n-3 FA trials have been conducted in patients with or at high risk for CVD. Various meta-analyses have aggregated these findings, with some reporting benefit26 but most27–29 concluding that supplementation has no or at most a weak preventive effect limited to coronary death but not stroke or other cardiovascular outcomes. In a 2018 meta-analysis by the Omega-3 Treatment Trialists’ Collaboration of 10 trials with 12,001 incident major vascular events among 77,917 participants, n-3 FA supplementation (EPA dose range, 226–1800 mg/d; all but one trial12 tested an EPA-DHA combination) for a mean of 4.4 years (range, 1.0–6.2 years) did not reduce the incidence of major vascular events, major CHD events, stroke, or revascularization,28 although the subdivision of major CHD events into nonfatal MI and CHD death revealed a suggestive 7% reduction in the latter outcome (relative risk [RR]=0.93 ([99% confidence interval 0.83–1.03]; p=0.053).30 Although the 2018 meta-analysis did not consider total MI, an earlier meta-analysis27 of 13 trials found a HR of 0.89 (0.76–1.04) for this endpoint, and a HR of 0.91 (0.85–0.98) for cardiac death. The results of the ASCEND trial, which tested a median of 7.4 years of supplementation with a 1 g/d fish-oil capsule (containing the same EPA-DHA dose and ratio as in VITAL) in 15,480 UK adults with diabetes, were published shortly before those of VITAL. ASCEND investigators reported null results for the primary endpoint of serious vascular events (HR=0.97 [0.87–1.08]).31 With regard to coronary outcomes, treatment-associated HRs for coronary death, nonfatal MI, and the composite of these two endpoints were 0.79 (0.61–1.02), 0.93 (0.76–1.14), and 0.89 (0.75–1.04), respectively. For vascular death, the HR was 0.81 (0.67–0.99). Although the marked treatment benefit on MI and PCI in VITAL contrasts with the more modest coronary benefits suggested by the collective findings of these secondary-prevention or high-risk trials, neither VITAL nor these other trials indicate a consistent benefit for stroke or composite cardiovascular endpoints, suggesting that future investigations should be designed and powered to analyze potential effects of n-3 FAs on coronary outcomes as distinct endpoints. That said, recent results from the 4.9-year REDUCE-IT trial, which tested high-dose synthetic EPA (icosapent ethyl [4 g/d]) in 8,179 statin users with elevated triglyceride levels (70% with prior CVD, and the rest with diabetes plus other cardiovascular risk factors) indicate a significant 26% reduction in major CVD events, including significant reductions of 31%, 28%, and 20% in total MI, total stroke, and cardiovascular death, respectively.32 Whether these greater risk reductions are due to the higher dose, specific formulation, and/or other factors requires further study.
There are several potential explanations for the apparently stronger coronary benefits of n-3 FAs in VITAL than those seen in aggregated analyses of secondary prevention trials. Two early open-label trials—GISSI11 in Italy, which tested 3.5 years of EPA+DHA (1 g/d) in 11,324 recent MI patients, and JELIS12 in Japan, which tested 4.6 years of EPA (1.8 g/d) in 18,645 hypercholesterolemic patients on statins—found significant coronary protection. However, all but one13 (two, if REDUCE-IT32 is included) subsequent placebo-controlled trials13–16, 31, 33, 34 (some with smaller sample sizes13–16 and some testing lower doses14, 15) failed to find benefit. The divergent results may be partly attributable to differences in these design parameters. In addition, the use of cardiovascular medications, including statins, β-blockers, anticoagulants, and angiotensin-converting enzyme inhibitors, was more prevalent among participants in recent trials than among those in earlier trials, perhaps reducing the opportunity for n-3 FAs to provide incremental benefit. The Omega-3 Treatment Trialists’ meta-analysis, ASCEND, and VITAL found no variation in results by statin use, but attenuation of a potential n-3 FA effect by other medications remains a possibility. This attenuation would likely be greater in secondary prevention settings, where medication use is more prevalent. (In REDUCE-IT, where statin use was an entry requirement, there was a significant protective effect of the icosapent ethyl intervention in participants with high- or moderate-intensity statin use but not in those with low-intensity use [p, interaction=0.12]. However, interpretation of the findings is complicated by the trial’s use of a mineral-oil placebo, which may have interfered with the absorption of, and thus the salutary effects of, statins.35) Another possible explanation for different effects in primary vs. secondary prevention is the more advanced atherosclerotic disease in the latter, which may require more powerful interventions than n-3 FAs—or as suggested by REDUCE-IT, significantly higher doses of n-3 FAs—to forestall clinical events. Indeed, in the Omega-3 Treatment Trialists’ meta-analysis,28 a greater n-3 FA benefit for major vascular events was found in participants without (HR=0.92 [0.84–1.01]) than with (HR=1.07 [0.95–1.20]) prior stroke (p, interaction=0.06). Similarly, the Age-Related Eye Disease Study 2,36 a trial of n-3 FAs for progression of macular degeneration or vision loss in ophthalmology patients, reported a greater treatment benefit for CVD risk reduction in participants without (HR=0.81 [0.62–1.06]) than in those with (RR=1.25 [0.91–1.72]) prior CVD (p, interaction=0.04). Differences in dietary fish intakes across study populations may have also influenced findings. The VITAL finding of a substantial treatment benefit on MI (and also a reduction in the primary composite CVD endpoint) in those with low fish intake is a novel finding requiring confirmation (few trials have examined this variable as a potential effect modifier) and suggests that larger benefits may be observed in populations with very low n-3 FA intakes. Finally, the secondary prevention trials enrolled few black participants, who as a group derived a greater coronary benefit from n-3 FA supplementation than members of other racial/ethnic groups in VITAL.
A 2019 meta-analysis of 30 n-3 FA trials with a total of >130,000 participants that included VITAL and ASCEND, but not REDUCE-IT, reported modest but statistically significant risk reductions for MI (RR=0.92 [0.85–0.99]) and total CHD (RR=0.93 [0.89–0.98]); a nonsignificant reduction in CVD mortality (RR=0.93 [0.86–1.01]); and no reduction in stroke (RR=1.05 [0.97–1.13]).37 Inclusion of the REDUCE-IT results would be expected to magnify the risk reductions. However, results of meta-analyses that combine VITAL with trials in higher-risk cohorts may be of limited use in formulating public-health guidelines regarding n-3 FA supplementation in usual-risk populations. Moreover, VITAL’s promising findings in African Americans are of interest due to the potential of n-3 FAs for reducing health disparities.
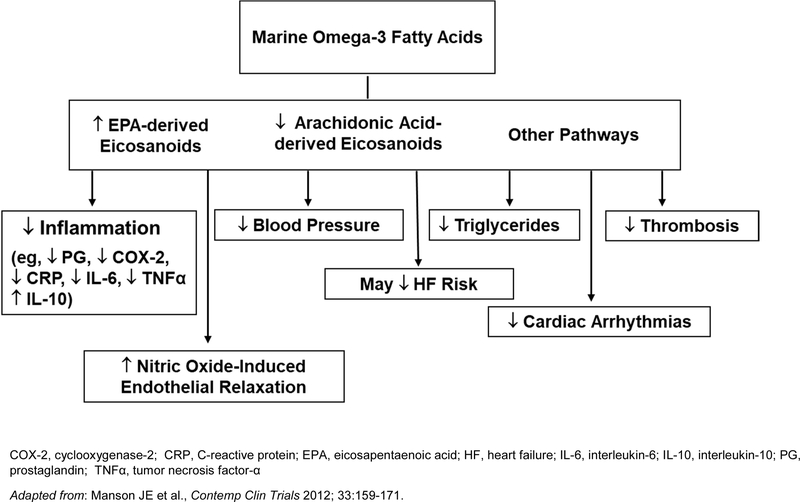
Coronary benefits of supplemental n-3 FAs are consistent with data from laboratory investigations, animal studies, and/or small trials of intermediate cardiovascular endpoints in humans, which suggest that n-3 FAs reduce inflammation, low-density lipoprotein oxidation, triglycerides, blood pressure, heart rate, thrombosis, and atherosclerotic plaque growth and instability; and improve endothelial function (Figure 4).9, 38–44 Experimental studies indicate relevant molecular and gene-regulatory effects.38 The dose-response curve for some effects appears to plateau at n-3 FA doses of 1 g/d or lower.45 Observational studies indicate that healthy individuals who regularly eat fish, a rich dietary source of marine n-3 FAs, experience significant reductions in fatal CHD and possibly nonfatal MI.46 Such studies also report significant inverse associations between dietary intakes of EPA+DHA from food or supplements and incident CHD events,26, 47 and between selected n-3 FA biomarkers and CHD mortality,48 nonfatal MI,48 and total CHD.47 The data for stroke are less compelling; high fish intakes49, 50 show more consistent inverse associations with ischemic stroke than do high levels of n-3 FA biomarkers.50–53
Figure 4. Mechanisms by which marine omega-3 fatty acids may lower cardiovascular disease risk.
COX-2, cyclooxygenase-2; CRP, C-reactive protein; EPA, eicosapentaenoic acid; HF, heart failure; IL-6, interleukin-6; IL-10, interleukin-10; PG, prostaglandin; TNFα, tumor necrosis factor-α
For cancer, the VITAL findings agree with those from trials of n-3 FAs for secondary prevention of CVD, which have reported neutral effects or slight (but nonsignificant) elevations in cancer incidence28, 54 and neutral effects or borderline significant reductions in cancer mortality.13, 31, 55 For all-cause mortality, the absence of a significant treatment effect in VITAL agrees with results of meta-analyses of earlier trials27, 29 and ASCEND.31 A 2019 meta-analysis of n-3 FA trials (41 trials, 10,707 deaths, 134,034 participants) that included VITAL and ASCEND reported a RR of 0.98 (0.93–1.02) for this endpoint.37 However, longer follow-up may be needed to detect a benefit, should such an effect exist.
Future Directions
Racial considerations.
We did not anticipate that African American participants in VITAL would experience a greater treatment-associated reduction in coronary risk than non-Hispanic whites, given that both groups entered the trial with comparable EPA+DHA blood levels and fish intakes. This finding requires replication in future trials. As noted above, n-3 FA supplementation trials for CVD prevention have, with few exceptions,12 been conducted in non-Hispanic white populations, precluding an assessment of treatment effects by race.28 However, a recent pooling project of 19 observational cohorts from 16 countries reported racial variation in associations of marine- and plant-derived n-3 FA biomarkers with incident coronary disease, including a significantly stronger inverse relationship between α-linolenic acid and nonfatal MI in blacks than whites.48 Observational studies also suggest that genetic variation in genes encoding key enzymes in FA metabolism, including the FA desaturase genes FADS1 and FADS2, the 5-lipooxygenase gene ALOX5, the 5-lipoxygenase activating protein gene ALOX5AP, and the cyclooxygenase COX-2 gene, may interact with dietary FA intakes to influence risk of CHD and other health outcomes,56–58 and that people with African ancestry differ from those with European ancestry with respect to FADS variants.58–60 Clarifying the role of genetic factors may be key to understanding the significant n-3 FA-associated reduction in MI in African American participants. In addition, although treatment-associated benefits remained more pronounced in African Americans than in non-Hispanic whites across risk strata defined by the presence or absence of traditional cardiovascular risk factors, it is possible that racial/ethnic differences in other clinical, dietary, or socioenvironmental factors may help to explain the comparatively stronger benefit in African Americans. For example, it has been postulated that n-3 FAs may ameliorate the adverse impact of air pollution,61 an exposure that disproportionately affects African Americans62 and increases cardiovascular risk.63 Elucidating the reasons for possible racial differences in n-3 FA supplementation effects is of interest.
Dose, formulation considerations.
VITAL tested only one n-3 FA dose and formulation and thus could not assess whether the effectiveness of supplementation varies according to these parameters. However, the tested dose has been recommended by the American Heart Association for cardioprotection in patients with prior CHD1, 2 and, based on fish consumption (1–2 servings/week), is at least twice the dose recommended by this organization for cardiovascular protection in healthy individuals.2, 46 That coronary benefits of n-3 FA supplementation were limited to participants with low baseline fish intake (in non-Hispanic white individuals and in the total cohort) suggests that further benefits may not accrue beyond a threshold dose. However, as noted above, REDUCE-IT found significant benefits with a higher-dose formulation in patients with CVD or at high risk for it. Results from the ongoing STRENGTH trial,64 which is testing whether 3 to 5 years of high-dose n-3 FAs (n-3 carboxylic acids [4 g/d], DHA-EPA ratio of 1:2.75) reduce major CVD events in 13,000 statin users with hypertriglyceridemia and low high-density lipoprotein cholesterol and who have established atherosclerotic disease or are at high risk for CVD, will be useful for further assessing the efficacy of a high-dose intervention. However, the generalizability of these findings to primary prevention populations is unknown. Future trials of higher doses and/or alternative formulations of supplemental n-3 FAs in primary prevention settings are warranted.
Other cardiovascular endpoints.
In VITAL, results of ancillary studies addressing effects of n-3 FA supplementation on heart failure, cardiac structure and function (2D-echocardiograms), atrial fibrillation, hypertension, diabetes, and other endpoints will soon be available to provide a fuller picture of the impact of n-3 FA supplementation on cardiovascular health and perhaps to help clarify underlying mechanisms for protective coronary effects. With respect to heart failure, n-3 FAs may be effective in reducing the risk of this condition or its cardiovascular sequelae. The placebo-controlled GISSI-HF trial, conducted among 6,975 patients with heart failure (>90% with reduced ejection fraction), found that supplemental n-3 FAs (1 g/d), added to standard therapy, had a favorable effect on clinical outcomes, reducing cardiovascular-related hospitalizations and CVD mortality by 7% (1–13%) and 10% (1%−19%), respectively, over a median follow-up of 3.9 years.13 Meta-analyses of small, short-term trials of n-3 FA supplementation in patients with heart failure65, 66, showed favorable changes in cardiac function, ventricular remodeling, inflammatory markers, and/or fibrosis, as did a 6-month trial in 358 post-MI patients.67 Supplemental EPA prevented contractile dysfunction and fibrosis in a heart-failure mouse model.68, 69 Among a racially/ethnically diverse cohort of 6,562 US adults aged 45–84 followed for 13 years in the observational Multi-Ethnic Study of Atherosclerosis, high baseline plasma EPA levels were associated with a reduced incidence of heart failure.70 However, in REDUCE-IT, high-dose EPA supplementation did not significantly lower heart failure risk (HR=0.95 [0.77–1.17]).32 VITAL is the first large trial of n-3 FAs for prevention of this endpoint in a population unselected for elevated cardiovascular risk. Regarding atrial fibrillation, observational studies of fish or n-3 FA intakes or n-3 FA biomarkers in relation to this endpoint in initially healthy populations have yielded generally neutral results.71–75 Trials of n-3 FAs have not found benefit for the prevention of recurrent atrial fibrillation76–78 or postoperative atrial fibrillation in cardiac surgery patients.79, 80 In REDUCE-IT, n-3 FA supplementation was associated with a significant increase in risk of atrial fibrillation as compared with placebo (5.3 vs. 3.9%, p=0.003).32 With respect to hypertension, a 2014 meta-analysis40 of small, short-term n-3 FA trials found that the interventions significantly reduced blood pressure, with the greatest reductions in participants with untreated hypertension. Regarding diabetes, meta-analyses of small, short-term n-3 FA trials in patients with this condition81 or healthy individuals82 have shown neutral or inconsistent treatment effects on glucose or insulin-related biomarkers. A 2012 meta-analysis of 18 observational studies in generally healthy populations found that neither fish, seafood, nor EPA+DHA intake, nor circulating EPA+DHA levels, predicted incident diabetes.83
Benefit-risk balance.
Forthcoming results of VITAL ancillary studies of noncardiovascular outcomes, including cognition, depression, macular degeneration, infections, pulmonary health, autoimmune disorders, fractures, and falls, will help to inform the overall benefit-risk balance of n-3 FA supplementation. Post-intervention follow-up of the VITAL cohort is ongoing to capture potential latent and long-term treatment effects and to increase statistical power, especially for assessment of secondary endpoints and subgroup effects.
SUPPLEMENTAL VITAMIN D
VITAL Results
Vitamin D supplementation did not reduce the primary composite endpoint of major CVD events (HR=0.97 [0.85–1.12]), nor did it affect the risk of secondary cardiovascular endpoints or all-cause mortality (Table 3). Analyses that excluded the first year or two years of follow-up or that censored for noncompliance did not materially change these results. Vitamin D had no effect on 1-year changes in lipid profiles or inflammatory markers.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of primary, secondary, and other outcomes by randomized assignment to vitamin Da
| Endpoint | Vitamin D (N = 12,927) | Placebo (N = 12,944) | HR | 95% CI |
|---|---|---|---|---|
| no. of participants w/event | ||||
|
Cardiovascular disease (CVD),
primary and secondary outcomes |
||||
| Major CVD eventb,c | 396 | 409 | 0.97 | 0.85–1.12 |
| Expanded CVD eventd | 536 | 558 | 0.96 | 0.86–1.08 |
| Total myocardial infarction | 169 | 176 | 0.96 | 0.78–1.19 |
| Total stroke | 141 | 149 | 0.95 | 0.76–1.20 |
| Cardiovascular mortality | 152 | 138 | 1.11 | 0.88–1.40 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 182 | 188 | 0.97 | 0.79–1.19 |
| Coronary artery bypass graft (CABG) | 73 | 98 | 0.75 | 0.55–1.01 |
| Fatal MI | 24 | 15 | 1.60 | 0.84–3.06 |
| Fatal stroke | 19 | 23 | 0.84 | 0.46–1.54 |
| Total invasive cancerb | 793 | 824 | 0.96 | 0.88–1.06 |
| Cancer mortality | 154 | 187 | 0.83 | 0.67–1.02 |
| All-cause mortality | 485 | 493 | 0.99 | 0.87–1.12 |
|
Excluding the first two years of follow-up: |
||||
| Major CVD event | 274 | 296 | 0.93 | 0.79–1.09 |
| Total invasive cancer | 490 | 522 | 0.94 | 0.83–1.06 |
| Cancer mortality | 112 | 149 | 0.75 | 0.59–0.96 |
| All-cause mortality | 368 | 384 | 0.96 | 0.84–1.11 |
Analyses were from Cox regression models controlling for age, sex, and n-3 fatty acid randomization group. Analyses were not adjusted for multiple comparisons.
Primary outcome.
A composite of myocardial infarction, stroke, and cardiovascular mortality.
A composite of major cardiovascular events plus coronary revascularization (CABG + PCI).
Not prespecified as primary or secondary outcomes.
Vitamin D did not reduce total cancer incidence (HR=0.96 [95% 0.88–1.06]) but showed a promising signal for reduction in cancer mortality (HR=0.83 [0.67–1.02]), especially in analyses accounting for latency by excluding the first year (HR=0.79 [0.63–0.99]) or first two years (HR=0.75 [0.59–0.96]) of follow-up. The intervention was well tolerated, with no significant treatment-associated increases in risk of hypercalcemia, kidney stones, or gastrointestinal symptoms.
The association between vitamin D and risk of CVD endpoints or all-cause mortality did not significantly vary by age, sex, race/ethnicity, cardiovascular risk factors, serum 25(OH)D level, or concurrent randomization to n-3 FAs (results of the latter two analyses are in Online Table I); nor did vitamin D significantly reduce CVD endpoints or all-cause mortality in any subgroup. Intriguingly, individuals with normal BMI (<25 kg/m2) experienced a significant treatment-associated reduction in cancer risk (HR=0.76 [0.63–0.90]), but overweight or obese individuals did not (p, interaction=0.002). African Americans assigned to vitamin D also had a suggestive reduction in cancer risk (HR=0.77 [0.59–1.01]), although the p-value for interaction by race/ethnicity was not significant (p, interaction=0.21).
Discussion and Context of Other Research
VITAL is the first large (N≥10,000) trial of moderate- or high-dose vitamin D for CVD and cancer prevention. Although VITAL was designed to overcome methodologic limitations of earlier randomized trials, the observed absence of cardiovascular benefit in VITAL is consistent with results of other trials. Among the largest of these trials are the Women’s Health Initiative (WHI) calcium-vitamin D trial,84, 85 the Randomized Evaluation of Calcium or vitamin D (RECORD) trial,86, 87 and a UK trial by Trivedi et al.88 The WHI randomized >36,000 US postmenopausal women to 7 years of daily calcium (1000 mg) plus vitamin D3 (400 IU) or to placebo; the intervention did not affect the incidence of CHD84 or stroke84 or mortality from these outcomes,85 but the below-RDA dose is a limitation. In RECORD, 5,292 UK adults aged ≥70 were randomized to daily vitamin D3 (800 IU), calcium (1000 mg), both, or placebo for 2–5.2 years for secondary fracture prevention and then followed observationally for 3 years; vitamin D did not reduce the incidence of MI (HR=0.97 [0.75–1.26]),86 stroke (HR=1.06 [0.85–1.32]),86 or vascular disease mortality (HR=0.91 [0.79–1.05]).87 Trivedi et al.88 randomized 2,686 older adults to vitamin D3 (100,000 IU every 4 months [~833 IU/d]) or placebo for up to 5 years and found nonsignificant reductions in CHD incidence (0.94 [0.77–1.15]), CHD mortality (0.84 [0.56–1.27]), CVD incidence (0.90 [0.77–1.06]), and CVD mortality (0.84 [0.65–1.10]); however, the trial’s modest size and intermittent bolus dosing, which has been associated with nonphysiological fluctuations in vitamin D blood levels,89 are limitations. Given such results, it is not surprising that meta-analyses of these (and smaller) vitamin D trials, even those restricted to trials of RDA-level (800 IU/d) or higher doses,90 have not found cardiovascular benefit.86, 90–93 More recently, the 3.3-year Vitamin D Assessment Study (ViDA), which tested high-dose vitamin D (100,000 IU/month [~3300/d]) vs. placebo for CVD prevention in 5,110 New Zealanders, also reported null results (MI: RR=0.90 [0.54–1.50]; stroke: RR=0.95 [0.55–1.62]),94 although the short duration and intermittent bolus dosing limit definitive conclusions.89 A 2019 meta-analysis of vitamin D trials that included VITAL and ViDA found that, compared with placebo, vitamin D supplementation did not reduce major adverse cardiovascular events (10 trials, 6243 events, 79,111 participants; RR=1.00 [0.95–1.06]), MI (18 trials, 2550 events, 82,576 participants; RR=1.00 [0.93–1.08]), stroke (15 trials, 2354 events, 82,239 participants; RR=1.06 [0.98–1.15]), or CVD mortality (10 trials, 2202 events, 76,783 participants; RR=0.98 [0.90–1.07]).95 Results did not significantly vary by baseline 25(OH)D level, vitamin D dose or administration frequency, or presence or absence of calcium co-administration. Supplemental calcium, often administered concurrently with vitamin D, raises blood calcium levels more rapidly than dietary calcium and could theoretically raise cardiovascular risks. However, results of calcium or calcium-plus-vitamin D trials do not clearly support this hypothesis.91, 96–98 In the WHI, for example, a 22% increase in MI risk occurred in participants who first started calcium supplements as part of the trial but not in those already taking them at baseline.99 As noted earlier, there was no treatment-associated elevation in MI or stroke risk in the total study population. In addition, the intervention did not increase coronary artery calcification at trial’s end.100 Large trials of calcium plus high-dose vitamin D supplementation are lacking, however.
As did VITAL, ViDA found that vitamin D failed to reduce all-cause mortality.94 Lower-dose vitamin D trials have also shown neutral effects or at most modest reductions in this endpoint.91–93 A 2019 meta-analysis of 20 vitamin D trials (6,502 deaths among 83,059 participants) that included VITAL and ViDA reported a RR of 0.97 (0.93–1.02) for all-cause mortality.95 However, longer follow-up may be needed to detect a benefit.
The lack of benefit of vitamin D for cancer incidence and the suggestive benefit for cancer mortality in the overall VITAL cohort is also consistent with findings from previous vitamin D trials. In a 2019 meta-analysis of vitamin D trials including VITAL and ViDA, treatment-associated RRs for cancer mortality (5 trials, 6547 cancer deaths) and cancer incidence (10 trials, 6,547 incident cancers) were 0.87 (0.79–0.96) and 0.98 (0.93–1.03), respectively.101 The VITAL findings for cancer mortality are also supported by laboratory research suggesting that vitamin D decreases tumor invasiveness and metastatic propensity102 and by observational studies showing that higher 25(OH)D levels at diagnosis predict longer survival in cancer patients.103–105
Although the reasons for the significant treatment-associated cancer reductions among normal-weight participants and suggestive reductions among African Americans require further exploration, these benefits sharply contrast with the null cardiovascular findings in these groups. It is possible that the divergence may be explained by differing vitamin D requirements for CVD and cancer prevention. In observational studies, the 25(OH)D levels associated with lowest risks tend to be between 20–25 ng/mL for CVD106 but above 30 ng/mL for cancer (at least colorectal cancer).107 Thus, it is possible that many participants entered VITAL (and other contemporary clinical trials) with their vitamin D requirements for cardiovascular health already met. Although neither VITAL nor ViDA found significant cardiovascular benefit for vitamin D among participants with low 25(OH)D at baseline, a trial among individuals with vitamin D levels well below the 20 ng/mL recommended for bone health3 might show stronger risk reductions. However, it would be neither ethical nor feasible to target patients with vitamin D deficiency and maintain them in this state for 5 or more years (as would be the case for the 50% assigned to placebo).
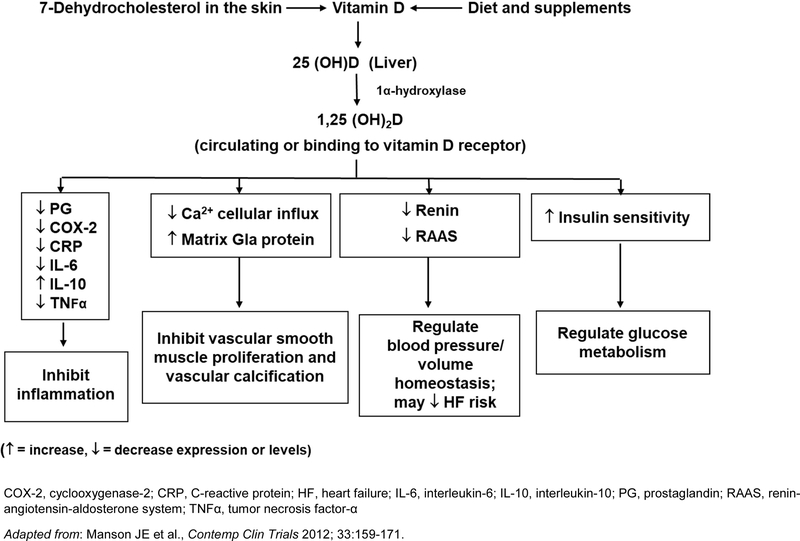
There are several plausible mechanisms by which vitamin D may prevent CVD (Figure 5). Vitamin D, obtained from diet, supplements, or conversion of 7-dehydrocholesterol in the skin by ultraviolet-B radiation, is hydroxylated in the liver to 25(OH)D, the major circulating vitamin D metabolite, which is then further hydroxylated to the active metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D), primarily in the kidneys.108, 109 Vascular smooth muscle cells, endothelial cells, cardiomyocytes, and macrophages also express the vitamin D receptor and/or produce 1α-hydroxylase, allowing for extra-renal production of 1,25(OH)2D. Laboratory and animal study data suggest that 1,25(OH)2D inhibits vascular smooth muscle cell proliferation and vascular calcification, controls volume homeostasis and blood pressure via regulation of the renin-angiotensin-aldosterone system, exerts anti-inflammatory effects, and improves insulin sensitivity and secretion.9, 110–112 In addition, observational studies have found inverse associations between 25(OH)D levels and cardiovascular risk factors and/or incident CVD.106, 113, 114 However, meta-analyses of mostly small, short-term trials in humans have found null or mixed results for effects of supplemental vitamin D on intermediate cardiovascular endpoints,115 including blood pressure,116–119 glucose or insulin homeostasis and type 2 diabetes,119–122 inflammation,123–125 and markers of vascular function,118, 126–129 as well as lipid profiles.90, 130 Of interest, a hypotensive effect of supplemental vitamin D in normal-weight individuals but a hypertensive effect in those who are overweight has been noted.117 Most recently, the Vitamin D and Type 2 Diabetes Study, in which 2,423 adults with mean baseline 25(OH)D of 28.0 ng/mL were randomized to 4000 IU/d of vitamin D vs. placebo for a median of 2.5 years, also found differences in the effect of supplemental vitamin D by BMI, with a significant treatment-associated reduction in risk of diabetes in those without, but not with, obesity.131
Figure 5. Mechanisms by which vitamin D may lower cardiovascular disease risk.
COX-2, cyclooxygenase-2; CRP, C-reactive protein; HF, heart failure; IL-6, interleukin-6; IL-10, interleukin-10; PG, prostaglandin; RAAS, renin-angiotensin-aldosterone system; TNFα, tumor necrosis factor-α
Future Directions
Alternative vitamin D biomarkers.
Although total 25(OH)D has traditionally been viewed as the optimal marker of clinical vitamin D status, most 25(OH)D circulates bound to vitamin D binding protein (DBP, ~85%) or albumin (~15%). Some studies have suggested that alternative biomarkers of vitamin D status, including DBP; bioavailable 25(OH)D, defined as 25(OH)D not bound to DBP; free 25(OH)D, defined as 25(OH)D not bound to either DBP or albumin; and parathyroid hormone may be potentially useful adjuncts to—or more biologically relevant indicators than—total 25(OH)D in characterizing vitamin D status132, 133 in relation to various clinical outcomes, including CHD134–136 and cancer.137–139 However, the data are not entirely consistent.133, 140 Whether baseline levels or changes in novel vitamin D markers influence the likelihood of deriving CVD benefit or harm from supplemental vitamin D requires further examination.
Obesity.
Obesity is associated with lower levels of both free and total 25(OH)D,141 may disproportionately lower the former,142 and may affect the correlation between the two markers.143 In a recent mouse study, diet-induced obesity decreased free 25(OH)D but not total 25(OH)D, and increased expression of CYP2R1, a key vitamin D-related gene.142 Among patients with CHD, higher levels of free 25(OH)D predicted reduced cardiovascular and all-cause mortality in those with normal BMI but not in those who were overweight/obese.136 These data suggest that vitamin D bioactivity is altered in obesity and lend credence to the aforementioned more favorable treatment effects on blood pressure,117 diabetes,131 and cancer incidence19 in normal-weight than in overweight/obese participants observed in some vitamin D trials.
Vitamin K.
Vitamin K may optimize the benefit-risk ratio of vitamin D supplementation. Vitamin K-vitamin D interactions appear to promote bone and cardiovascular health and may also protect against possible adverse effects of high-dose vitamin D such as kidney stones and vascular calcification.144 Vitamin D stimulates the transcription and translation of osteocalcin (OC), matrix Gla protein (MGP), and other proteins in bone, the vasculature, and other tissues. Vitamin K then helps to carboxylate and activate these proteins. Carboxylated OC is involved in calcium binding in bone, and carboxylated MGP inhibits calcium deposition in the vasculature, kidney, and soft tissues. High-dose vitamin D supplementation may increase synthesis of OC and MGP, resulting in the need for higher levels of vitamin K to fully activate these proteins and achieve maximal bone and cardiovascular benefits. Whether vitamin K status modifies effects of supplemental vitamin D on CVD warrants further study in a large trial.
Magnesium.
Magnesium plays a key role in vitamin D synthesis and metabolism,145, 146 suggesting that adequate magnesium is required for optimal response to vitamin D supplementation. Among US National Health and Nutrition Examination Survey participants, magnesium intake significantly interacted with vitamin D intake to affect vitamin D status, and also interacted with circulating 25(OH)D to influence risk of CVD mortality.147 An inverse association between 25(OH)D and mortality was primarily seen in individuals with above-median magnesium intakes. In a trial in people without overt magnesium deficiency, supplemental magnesium raised vitamin D levels in those with low levels and lowered vitamin D levels in those with high levels—a pattern suggestive of this mineral’s importance in optimizing vitamin D status.148 In mice with induced chronic kidney disease, co-administration of magnesium blunted the adverse impact of vitamin D on vascular calcification.149 Whether magnesium status affects the relation between vitamin D supplementation and cardiovascular endpoints has yet to be tested in a large trial.
Dose, administration frequency.
VITAL examined only one vitamin D dose and administration frequency and therefore could not address dose-response issues or the relative efficacy of daily vs. less frequent dosing. Ongoing vitamin D trials150 may help clarify these uncertainties. D-Health151—the only large (N≥10,000) trial of high-dose vitamin D other than VITAL—is testing a bolus vitamin D dose of 60,000 IU/month for 5 years in 21,315 Australians aged 60 and older; the primary endpoints are cancer and all-cause mortality, but CVD will also be examined. Results are expected in 2021.
Heart failure.
In VITAL, results of ancillary studies of supplemental vitamin D on incident heart failure, cardiac structure and function, and other CVD-related endpoints will soon be available. Vitamin D deficiency may lead to heart failure through deleterious effects on the renin-angiotensin-aldosterone system and cardiac morphology. In the RECORD trial, supplemental vitamin D (800 IU/d) was associated with a significant reduction in incident heart failure (HR=0.75 [0.58–0.97]).86 A 2014 meta-analysis of seven vitamin D trials, including RECORD and six smaller trials, also reported a significant reduction in this endpoint (HR=0.79 [0.59–0.99]).86 However, ViDA did not find a benefit of monthly high-dose vitamin D on incident heart failure (HR=1.19 [0.84–1.68]).94 Small trials in heart failure patients suggest that daily high-dose vitamin D (4000 IU) for 1–3 years may improve left ventricular structure and/or function,152, 153 although a reduction in all-cause mortality has not been found.154
Impaired kidney function.
Individuals with chronic kidney disease, even at early stages, have lower vitamin D levels (in part because of reduced conversion to its active metabolite) and higher CVD rates than members of the general population. Adequately powered randomized trials testing the effect of vitamin D supplementation on cardiovascular outcomes in such patients are lacking155 but challenging to conduct because such supplementation is routinely prescribed for bone health maintenance in this population. Thus, investigation of potential modification of the effect of supplemental vitamin D on cardiovascular outcomes according to baseline markers of kidney function in a general population is of interest; such analyses are underway and will be soon be reported in VITAL.
Benefit-risk balance.
Forthcoming results from VITAL ancillary studies of noncardiovascular outcomes (e.g., fractures, falls, cognition, depression, and infections) will also inform the overall benefit-risk balance of vitamin D supplementation. As noted earlier for n-3 FAs, post-intervention follow-up of the VITAL cohort to capture potential latent and long-term treatment effects and to increase statistical power, especially for assessment of secondary endpoints and subgroup effects, is in progress.
CONCLUSION
In VITAL, n-3 FA supplementation among initially healthy adults led to a small but statistically nonsignificant reduction in a composite endpoint of major CVD events, a statistically significant 28% reduction in total MI, reductions in other coronary outcomes, but no reduction in stroke or cardiovascular deaths not related to heart disease. The reduction in total MI supports a possible cardioprotective role for n-3 FAs in a usual-risk setting, especially in people with low dietary fish intake or with cardiovascular risk factors, and in African Americans. Daily high-dose vitamin D supplementation did not reduce the incidence of major CVD events or secondary CVD endpoints in initially healthy adults but showed a promising signal for reducing cancer death. VITAL and other recent trials have contributed to updated meta-analyses of these interventions and suggest that the benefit-risk pattern may vary by subgroup. Additional research is needed to determine which individuals may be most likely to derive a net benefit from these supplements.
Supplementary Material
ACKNOWLEDGMENTS
VITAL Investigators, Staff, and Study Participants
The authors thank the VITAL investigators, staff, and study participants for their dedication and commitment to the trial. A list of VITAL Research Group members is in the Supplemental Materials.
Funding/Support: VITAL is an investigator-initiated trial supported by grants U01 CA138962 and R01 CA138962, which include support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others.
Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured the serum 25(OH)D levels and plasma omega-3 index at no cost to the study.
Role of the Sponsor: The NIH sponsors of VITAL had a role in the design and conduct of the study and interpretation of the data. Final decisions concerning the above, however, as well as data collection, management, analysis, manuscript review or approval, and decision to submit the manuscript for publication resided with VITAL investigators and the VITAL research group. The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/National Institutes of Health.
VITAL has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital. The study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration.
Non-standard abbreviations and acronyms:
- ASCEND
A Study of Cardiovascular Events in Diabetes
- CABG
coronary artery bypass grafting
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CTSC
Clinical and Translational Science Center
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- HR
hazard ratio
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- n-3 FA
n-3 fatty acid
- RDA
recommended dietary allowance
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl—Intervention Trial
- RR
relative risk
- SD
standard deviation
- ViDA
Vitamin D Assessment Study
- VITAL
VITamin D and OmegA-3 TriaL
- 25(OH)D
25-hydroxyvitamin D
Footnotes
A complete list of the members of the VITAL Research Group is provided in the Supplemental Materials.
VITAL is registered at (clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
Conflict of Interest Disclosures: All authors will submit a completed ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
JoAnn E. Manson, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
Shari S. Bassuk, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Nancy R. Cook, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
I-Min Lee, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
Samia Mora, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Christine M. Albert, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Cardiology, Cedars-Sinai Medical Center, Los Angeles, CA.
Julie E. Buring, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, and the Department of Cardiology, Cedars-Sinai Medical Center, Los Angeles, CA (CMA).
VITAL Research Group, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
References
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–2757. [DOI] [PubMed] [Google Scholar]
- 2.Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D, American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 4.Stipp D Fish-oil doses can be hard to swallow. Wall Street Journal. January 8, 2008:D1–D2. [Google Scholar]
- 5.Greider K Has vitamin D been oversold? AARP Bulletin. July 5, 2012. Available at http://www.aarp.org/health/drugs-supplements/info-07-2012/how-much-vitamin-d-is-enough.1.html. Accessed July 31, 2019. [Google Scholar]
- 6.Kupferschmidt K Uncertain verdict as vitamin D goes on trial. Science 2012;337:1476–1478. [DOI] [PubMed] [Google Scholar]
- 7.Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use was very high among older adults in the United States in 2011–2014. J Nutr 2017;147:1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grebow J 75% of Americans take dietary supplements, latest CRN survey finds. Nutritional Outlook. October 19, 2018. Available at http://www.nutritionaloutlook.com/trends-business/75-americans-take-dietary-supplements-latest-crn-survey-finds Accessed July 31, 2019. [Google Scholar]
- 9.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA 2015;313:1311–1312. [DOI] [PubMed] [Google Scholar]
- 11.GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999;354:447–455. [PubMed] [Google Scholar]
- 12.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 13.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 14.Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 15.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J, OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 17.Moyer VA, U.S. Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:558–564. [DOI] [PubMed] [Google Scholar]
- 18.Harris SS. Vitamin D and African Americans. J Nutr 2006;136:1126–1129. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44.30415629 [Google Scholar]
- 20.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassuk SS, Manson JE, Lee IM, Cook NR, Christen WG, Bubes VY, Gordon DS, Copeland T, Friedenberg G, D’Agostino DM, Ridge CY, MacFadyen JG, Kalan K, Buring JE. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 2016;47:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz AG, Percy C, Jack A, Shanmugaratham K, Sobin LH, Parkin MD, Whelan S. International Classification of Diseases for Oncology (ICD-O), Third Edition Geneva: World Health Organization; 2000. [Google Scholar]
- 23.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–220. [DOI] [PubMed] [Google Scholar]
- 26.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc 2017;92:15–29. [DOI] [PubMed] [Google Scholar]
- 27.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 28.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R, Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, AlAbdulghafoor FK, Summerbell CD, Worthington HV, Song F, Hooper L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;7:CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke RJ, Aung T, Armitage J. Questioning the associations of omega-3 fatty acid supplement use with cardiovascular disease risks-reply. JAMA Cardiol 2018. [DOI] [PubMed] [Google Scholar]
- 31.ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, Investigators R-I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 33.Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S, ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 34.Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R, Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 35.Kastelein JJP, Stroes ESG. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med 2019;380:89–90. [DOI] [PubMed] [Google Scholar]
- 36.Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY, Writing Group for the AREDS Research Group. Effect of long-chain omega-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med 2014;174:763–771. [DOI] [PubMed] [Google Scholar]
- 37.Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, Okunrintemi V, Riaz IB, Khan MS, Kaluski E, Murad MH, Blaha MJ, Guallar E, Michos ED. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015;1851:469–484. [DOI] [PubMed] [Google Scholar]
- 40.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens 2014;27:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis 2015;242:357–366. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis 2012;221:536–543. [DOI] [PubMed] [Google Scholar]
- 43.Burke MF, Burke FM, Soffer DE. Review of cardiometabolic effects of prescription omega-3 fatty acids. Curr Atheroscler Rep 2017;19:60. [DOI] [PubMed] [Google Scholar]
- 44.Colussi G, Catena C, Novello M, Bertin N, Sechi LA. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: relevance for cardiovascular outcomes. Nutr Metab Cardiovasc Dis 2017;27:191–200. [DOI] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–1899. [DOI] [PubMed] [Google Scholar]
- 46.Rimm EB, Appel LJ, Chiuve SE, Djousse L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH, American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018;138:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 48.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateau P, Campos H, Chaves PH, Djousse L, Giles GG, Gomez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Riserus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, van Dam RM, Voutilainen S, Willett WC, Woodward M, Mozaffarian D, Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe). Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xun P, Qin B, Song Y, Nakamura Y, Kurth T, Yaemsiri S, Djousse L, He K. Fish consumption and risk of stroke and its subtypes: accumulative evidence from a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2012;66:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, Franco OH. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ 2012;345:e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veno SK, Bork CS, Jakobsen MU, Lundbye-Christensen S, McLennan PL, Bach FW, Overvad K, Schmidt EB. Marine n-3 polyunsaturated fatty acids and the risk of ischemic stroke. Stroke 2019;50:274–282. [DOI] [PubMed] [Google Scholar]
- 52.Saber H, Yakoob MY, Shi P, Longstreth WT Jr., Lemaitre RN, Siscovick D, Rexrode KM, Willett WC, Mozaffarian D. Omega-3 fatty acids and incident ischemic stroke and its atherothrombotic and cardioembolic subtypes in 3 US cohorts. Stroke 2017;48:2678–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yaemsiri S, Sen S, Tinker LF, Robinson WR, Evans RW, Rosamond W, Wasserthiel-Smoller S, He K. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke 2013;44:2710–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YF, Gao HF, Hou AJ, Zhou YH. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bordeleau L, Yakubovich N, Dagenais GR, Rosenstock J, Probstfield J, Chang Yu P, Ryden LE, Pirags V, Spinas GA, Birkeland KI, Ratner RE, Marin-Neto JA, Keltai M, Riddle MC, Bosch J, Yusuf S, Gerstein HC, ORIGIN Trial Investigators. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37:1360–1366. [DOI] [PubMed] [Google Scholar]
- 56.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med (Maywood) 2010;235:785–795. [DOI] [PubMed] [Google Scholar]
- 57.Corella D, Ordovas JM. Interactions between dietary n-3 fatty acids and genetic variants and risk of disease. Br J Nutr 2012;107 Suppl 2:S271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chilton FH, Dutta R, Reynolds LM, Sergeant S, Mathias RA, Seeds MC. Precision nutrition and omega-3 polyunsaturated fatty acids: a case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathias RA, Sergeant S, Ruczinski I, Torgerson DG, Hugenschmidt CE, Kubala M, Vaidya D, Suktitipat B, Ziegler JT, Ivester P, Case D, Yanek LR, Freedman BI, Rudock ME, Barnes KC, Langefeld CD, Becker LC, Bowden DW, Becker DM, Chilton FH. The impact of FADS genetic variants on omega-6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet 2011;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, Ainsworth HC, Vaidya D, Case LD, Langefeld CD, Freedman BI, Bowden DW, Mathias RA, Chilton FH. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr 2012;107:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietary Tong H. and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta 2016;1860:2891–2898. [DOI] [PubMed] [Google Scholar]
- 62.Tessum CW, Apte JS, Goodkind AL, Muller NZ, Mullins KA, Paolella DA, Polasky S, Springer NP, Thakrar SK, Marshall JD, Hill JD. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci U S A 2019;116:6001–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 64.Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Pedersen TR, Ridker PM, Ray K, Karlson BW, Lundstrom T, Wolski K, Nissen SE. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol 2018;41:1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xin W, Wei W, Li X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart 2012;98:1620–1625. [DOI] [PubMed] [Google Scholar]
- 66.Xin W, Wei W, Li X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2012;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL randomized clinical trial. Circulation 2016;134:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O’Connell TD, Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 2011;123:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eclov JA, Qian Q, Redetzke R, Chen Q, Wu SC, Healy CL, Ortmeier SB, Harmon E, Shearer GC, O’Connell TD. EPA, not DHA, prevents fibrosis in pressure overload-induced heart failure: potential role of free fatty acid receptor 4. J Lipid Res 2015;56:2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block RC, Liu L, Herrington DM, Huang S, Tsai MY, O’Connell TD, Shearer GC. Predicting risk for incident heart failure with omega-3 fatty acids: from MESA. JACC Heart Fail 2019. [DOI] [PubMed] [Google Scholar]
- 71.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J 2006;151:857–862. [DOI] [PubMed] [Google Scholar]
- 72.Berry JD, Prineas RJ, van Horn L, Passman R, Larson J, Goldberger J, Snetselaar L, Tinker L, Liu K, Lloyd-Jones DM. Dietary fish intake and incident atrial fibrillation (from the Women’s Health Initiative). Am J Cardiol 2010;105:844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA, Pandey S, Levy D, Vasan RS, Quatromoni PA, Junyent M, Ordovas JM, Benjamin EJ. Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr 2011;93:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gronroos NN, Chamberlain AM, Folsom AR, Soliman EZ, Agarwal SK, Nettleton JA, Alonso A. Fish, fish-derived n-3 fatty acids, and risk of incident atrial fibrillation in the Atherosclerosis Risk in Communities (ARIC) study. PLoS One 2012;7:e36686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larsson SC, Wolk A. Fish, long-chain omega-3 polyunsaturated fatty acid intake and incidence of atrial fibrillation: A pooled analysis of two prospective studies. Clin Nutr 2017;36:537–541. [DOI] [PubMed] [Google Scholar]
- 76.Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, Tognoni G, Macchia A. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2013;2:e005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, Jones P, Ramprasath VR, O’Hara G, Kopecky S, Brophy JM, Tardif JC, Investigators A. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol 2014;64:1441–1448. [DOI] [PubMed] [Google Scholar]
- 78.Darghosian L, Free M, Li J, Gebretsadik T, Bian A, Shintani A, McBride BF, Solus J, Milne G, Crossley GH, Thompson D, Vidaillet H, Okafor H, Darbar D, Murray KT, Stein CM. Effect of omega-3 polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol 2015;115:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O’Gara PT, Page RL, Tavazzi L, Tognoni G, Investigators O. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012;308:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O’Gara PT, Page RL, Silletta MG, Tavazzi L, Marchioli R. Fish oil and post-operative atrial fibrillation: a meta-analysis of randomized controlled trials. J Am Coll Cardiol 2013;61:2194–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartweg J, Perera R, Montori V, Dinneen S, Neil HA, Farmer A. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008:CD003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009;12:138–146. [DOI] [PubMed] [Google Scholar]
- 83.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse L, Hu FB, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107 Suppl 2:S214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–854. [DOI] [PubMed] [Google Scholar]
- 85.LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, Manson JE, Stefanick ML, Wactawski-Wende J. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009;64:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, Record Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr 2014;100:746–755. [DOI] [PubMed] [Google Scholar]
- 87.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 2012;97:614–622. [DOI] [PubMed] [Google Scholar]
- 88.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Bmj 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013;98:4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:1931–1942. [DOI] [PubMed] [Google Scholar]
- 91.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2014;2:307–320. [DOI] [PubMed] [Google Scholar]
- 92.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014:CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rejnmark L, Bislev LS, Cashman KD, Eiriksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips P, Pilz S, van Schoor NM, Kiely M, Jorde R. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS One 2017;12:e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study : a randomized clinical trial. JAMA Cardiol 2017;2:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, Yelangi A, Sundus S, Bachuwa G, Alkotob ML, Manson JE. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs 2012;12:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manson JE, Bassuk SS. Calcium supplements: do they help or harm? Menopause 2014;21:106–108. [DOI] [PubMed] [Google Scholar]
- 98.Chin K, Appel LJ, Michos ED. Vitamin D, calcium, and cardiovascular disease: A”D”vantageous or “D”etrimental? An era of uncertainty. Curr Atheroscler Rep 2017;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, Hsia J, Hunt JR, Lewis CE, Margolis KL, Robinson JG, Rodabough RJ, Thomas AM. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause 2010;17:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol 2019;30:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–357. [DOI] [PubMed] [Google Scholar]
- 103.Vaughan-Shaw PG, O’Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG, Zgaga L. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer 2017;116:1092–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maalmi H, Walter V, Jansen L, Chang-Claude J, Owen RW, Ulrich A, Schottker B, Hoffmeister M, Brenner H. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol 2017;32:961–971. [DOI] [PubMed] [Google Scholar]
- 105.Xu J, Yuan X, Tao J, Yu N, Wu R, Zhang Y. Association of circulating 25-hydroxyvitamin D levels with colorectal cancer: an updated meta-analysis. J Nutr Sci Vitaminol (Tokyo) 2018;64:432–444. [DOI] [PubMed] [Google Scholar]
- 106.Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 2017;105:810–819. [DOI] [PubMed] [Google Scholar]
- 107.McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, Eliassen AH, Zeleniuch-Jacquotte A, Agnoli C, Albanes D, Barnett MJ, Buring JE, Campbell PT, Clendenen TV, Freedman ND, Gapstur SM, Giovannucci EL, Goodman GG, Haiman CA, Ho GYF, Horst RL, Hou T, Huang WY, Jenab M, Jones ME, Joshu CE, Krogh V, Lee IM, Lee JE, Mannisto S, Le Marchand L, Mondul AM, Neuhouser ML, Platz EA, Purdue MP, Riboli E, Robsahm TE, Rohan TE, Sasazuki S, Schoemaker MJ, Sieri S, Stampfer MJ, Swerdlow AJ, Thomson CA, Tretli S, Tsugane S, Ursin G, Visvanathan K, White KK, Wu K, Yaun SS, Zhang X, Willett WC, Gail MH, Ziegler RG, Smith-Warner SA. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2019;111:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial 2005;18:266–275. [DOI] [PubMed] [Google Scholar]
- 109.Giovannucci E The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 2005;16:83–95. [DOI] [PubMed] [Google Scholar]
- 110.Bassuk SS, Manson JE. Does vitamin D protect against cardiovascular disease? J Cardiovasc Transl Res 2009;2:245–250. [DOI] [PubMed] [Google Scholar]
- 111.Pilz S, Tomaschitz A, Marz W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, Holick MF, Dekker JM. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–584. [DOI] [PubMed] [Google Scholar]
- 112.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One 2015;10:e0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grubler MR, Marz W, Pilz S, Grammer TB, Trummer C, Mullner C, Schwetz V, Pandis M, Verheyen N, Tomaschitz A, Fiordelisi A, Laudisio D, Cipolletta E, Iaccarino G. Vitamin-D concentrations, cardiovascular risk and events - a review of epidemiological evidence. Rev Endocr Metab Disord 2017;18:259–272. [DOI] [PubMed] [Google Scholar]
- 115.Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol 2017;70:89–100. [DOI] [PubMed] [Google Scholar]
- 116.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD, D-PRESSURE Collaboration. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med 2015;175:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Golzarand M, Shab-Bidar S, Koochakpoor G, Speakman JR, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: An updated meta-analysis. Nutr Metab Cardiovasc Dis 2016;26:663–673. [DOI] [PubMed] [Google Scholar]
- 118.Veloudi P, Jones G, Sharman JE. Effectiveness of vitamin D supplementation for cardiovascular health outcomes. Pulse (Basel) 2017;4:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swart KM, Lips P, Brouwer IA, Jorde R, Heymans MW, Grimnes G, Grubler MR, Gaksch M, Tomaschitz A, Pilz S, Eiriksdottir G, Gudnason V, Wamberg L, Rejnmark L, Sempos CT, Durazo-Arvizu RA, Dowling KG, Hull G, Skrabakova Z, Kiely M, Cashman KD, van Schoor NM. Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr 2018;107:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jamka M, Wozniewicz M, Jeszka J, Mardas M, Bogdanski P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep 2015;5:16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc 2018;2:687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang H, Li D, Li Y, Zhang X, Song Y, Li X. Effects of vitamin D supplementation on glucose and insulin homeostasis and incident diabetes among nondiabetic adults: a meta-analysis of randomized controlled trials. Int J Endocrinol 2018;2018:7908764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jamka M, Wozniewicz M, Walkowiak J, Bogdanski P, Jeszka J, Stelmach-Mardas M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr 2016;55:2163–2176. [DOI] [PubMed] [Google Scholar]
- 124.Mousa A, Naderpoor N, Teede H, Scragg R, de Courten B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 2018;76:380–394. [DOI] [PubMed] [Google Scholar]
- 125.Yu Y, Tian L, Xiao Y, Huang G, Zhang M. Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab 2018;73:62–73. [DOI] [PubMed] [Google Scholar]
- 126.Stojanovic M, Radenkovic M. Vitamin D versus placebo in improvement of endothelial dysfunction: a meta-analysis of randomized clinical trials. Cardiovasc Ther 2015;33:145–154. [DOI] [PubMed] [Google Scholar]
- 127.Rodriguez AJ, Scott D, Srikanth V, Ebeling P. Effect of vitamin D supplementation on measures of arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf) 2016;84:645–657. [DOI] [PubMed] [Google Scholar]
- 128.Hussin AM, Ashor AW, Schoenmakers I, Hill T, Mathers JC, Siervo M. Effects of vitamin D supplementation on endothelial function: a systematic review and meta-analysis of randomised clinical trials. Eur J Nutr 2017;56:1095–1104. [DOI] [PubMed] [Google Scholar]
- 129.Beveridge LA, Khan F, Struthers AD, Armitage J, Barchetta I, Bressendorff I, Cavallo MG, Clarke R, Dalan R, Dreyer G, Gepner AD, Forouhi NG, Harris RA, Hitman GA, Larsen T, Khadgawat R, Marckmann P, Mose FH, Pilz S, Scholze A, Shargorodsky M, Sokol SI, Stricker H, Zoccali C, Witham MD. Effect of vitamin D supplementation on markers of vascular function: a systematic review and individual participant meta-analysis. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M, D2d Research Group. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsuprykov O, Chen X, Hocher CF, Skoblo R, Lianghong Y, Hocher B. Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol 2018;180:87–104. [DOI] [PubMed] [Google Scholar]
- 133.Jukic AMZ, Hoofnagle AN, Lutsey PL. Measurement of vitamin D for epidemiologic and clinical research: shining light on a complex decision. Am J Epidemiol 2018;187:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robinson-Cohen C, Zelnick LR, Hoofnagle AN, Lutsey PL, Burke G, Michos ED, Shea SJC, Tracy R, Siscovick DS, Psaty B, Kestenbaum B, de Boer IH. Associations of vitamin D-binding globulin and bioavailable vitamin D concentrations with coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Endocrinol Metab 2017;102:3075–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qi L, Ma W, Heianza Y, Zheng Y, Wang T, Sun D, Rimm EB, Hu FB, Giovannucci E, Albert CM, Rexrode KM, Manson JE. Independent and synergistic associations of biomarkers of vitamin D status with risk of coronary heart disease. Arterioscler Thromb Vasc Biol 2017;37:2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu C, Xue H, Wang L, Chen Q, Chen X, Zhang Y, Hu G, Ling W. Serum bioavailable and free 25-hydroxyvitamin D levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ Res 2018;123:996–1007. [DOI] [PubMed] [Google Scholar]
- 137.Tagliabue E, Raimondi S, Gandini S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer Epidemiol Biomarkers Prev 2015;24:1758–1765. [DOI] [PubMed] [Google Scholar]
- 138.Mondul AM, Weinstein SJ, Parisi D, Um CY, McCullough ML, Albanes D. Vitamin D-binding protein and risk of renal cell carcinoma in the Cancer Prevention Study-II cohort. Cancer Epidemiol Biomarkers Prev 2018;27:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan C, Shui IM, Wilson KM, Stampfer MJ, Mucci LA, Giovannucci EL. Circulating 25-hydroxyvitamin D, vitamin D binding protein and risk of advanced and lethal prostate cancer. Int J Cancer 2019;144:2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song M, Konijeti GG, Yuan C, Ananthakrishnan AN, Ogino S, Fuchs CS, Giovannucci EL, Ng K, Chan AT. Plasma 25-hydroxyvitamin D, vitamin D binding protein, and risk of colorectal cancer in the Nurses’ Health Study. Cancer Prev Res (Phila) 2016;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Walsh JS, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I, Jacques RM, Eastell R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr 2016;103:1465–1471. [DOI] [PubMed] [Google Scholar]
- 142.Bonnet L, Hachemi MA, Karkeni E, Couturier C, Astier J, Defoort C, Svilar L, Martin JC, Tourniaire F, Landrier JF. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J Steroid Biochem Mol Biol 2019;185:39–46. [DOI] [PubMed] [Google Scholar]
- 143.Schwartz JB, Gallagher JC, Jorde R, Berg V, Walsh J, Eastell R, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I, Holick M, Orwoll E, Nielson C, Kaufmann M, Jones G, Bouillon R, Lai J, Verotta D, Bikle D. Determination of free 25(OH)D concentrations and their relationships to total 25(OH)D in multiple clinical populations. J Clin Endocrinol Metab 2018;103:3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Ballegooijen AJ, Pilz S, Tomaschitz A, Grubler MR, Verheyen N. The synergistic interplay between vitamins D and K for bone and cardiovascular health: a narrative review. Int J Endocrinol 2017;2017:7454376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosanoff A, Dai Q, Shapses SA. Essential nutrient interactions: does low or suboptimal magnesium status interact with vitamin D and/or calcium status? Adv Nutr 2016;7:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc 2018;118:181–189. [DOI] [PubMed] [Google Scholar]
- 147.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med 2013;11:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan L, Murff H, Ness RM, Seidner DL, Yu C, Shrubsole MJ. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr 2018;108:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zelt JG, McCabe KM, Svajger B, Barron H, Laverty K, Holden RM, Adams MA. Magnesium modifies the impact of calcitriol treatment on vascular calcification in experimental chronic kidney disease. J Pharmacol Exp Ther 2015;355:451–462. [DOI] [PubMed] [Google Scholar]
- 150.Bassuk SS, Manson JE. Chapter 66: Randomized clinical trials of vitamin D, with a focus on the VITamin D and OmegA-3 TriaL (VITAL) In: Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D, Volume 2: Health, Disease, and Therapeutics, Fourth Edition San Diego CA: Academic Press; 2018:167–176. [Google Scholar]
- 151.Neale RE, Armstrong BK, Baxter C, Duarte Romero B, Ebeling P, English DR, Kimlin MG, McLeod DS, RL OC, van der Pols JC, Venn AJ, Webb PM, Whiteman DC, Wockner L. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials 2016;48:83–90. [DOI] [PubMed] [Google Scholar]
- 152.Witte KK, Byrom R, Gierula J, Paton MF, Jamil HA, Lowry JE, Gillott RG, Barnes SA, Chumun H, Kearney LC, Greenwood JP, Plein S, Law GR, Pavitt S, Barth JH, Cubbon RM, Kearney MT. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J Am Coll Cardiol 2016;67:2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zittermann A, Ernst JB, Prokop S, Fuchs U, Gruszka A, Dreier J, Kuhn J, Knabbe C, Berthold HK, Gouni-Berthold I, Pilz S, Gummert JF, Paluszkiewicz L. Vitamin D supplementation of 4000IU daily and cardiac function in patients with advanced heart failure: The EVITA trial. Int J Cardiol 2019;280:117–123. [DOI] [PubMed] [Google Scholar]
- 154.Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, Knabbe C, Birschmann I, Schulz U, Berthold HK, Pilz S, Gouni-Berthold I, Gummert JF, Dittrich M, Borgermann J. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J 2017;38:2279–2286. [DOI] [PubMed] [Google Scholar]
- 155.Junarta J, Jha V, Banerjee D. Insight into the impact of vitamin D on cardiovascular outcomes in chronic kidney disease. Nephrology (Carlton) 2019;24:781–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.