Abstract
The circadian system generates endogenous rhythms of approximately 24 h, the synchronisation of which are vital for healthy bodily function. The timing of many physiological processes, including glucose metabolism, are coordinated by the circadian system, and circadian disruptions that desynchronise or misalign these rhythms can result in adverse health outcomes. In this review, we cover the role of the circadian system and its disruption in glucose metabolism in healthy individuals and individuals with type 2 diabetes mellitus. We begin by defining circadian rhythms and circadian disruption and then we provide an overview of circadian regulation of glucose metabolism. We next discuss the impact of circadian disruptions on glucose control and type 2 diabetes. Given the concurrent high prevalence of type 2 diabetes and circadian disruption, understanding the mechanisms underlying the impact of circadian disruption on glucose metabolism may aid in improving glycaemic control.
Keywords: Beta cell function, Circadian disruption, Circadian misalignment, Circadian rhythm, Diabetes, Glucose control, Glucose metabolism, Glucose tolerance, Insulin sensitivity, Review, Type 2 diabetes mellitus
Introduction
Diabetes is pandemic, with 8.6% of American adults (21 million) in 2016 [1] living with type 2 diabetes mellitus and 8.4% of adults worldwide (451 million) in 2017 [2] living with type 1 or type 2 diabetes. The prevalence of type 1 and type 2 diabetes is projected to increase to 693 million adults worldwide by 2045 [2]. Type 2 diabetes makes up approximately 95% of all diabetes cases; this review covers type 2 diabetes only and refers to type 2 diabetes when the term ‘diabetes’ is used. The high and increasing prevalence of type 2 diabetes may be explained in part by lifestyle risk factors, such as physical inactivity, smoking and poor diet. In recent decades, an additional lifestyle risk factor has become common in modern society: circadian disruption. Notably, approximately 20% of individuals are involved in shift work [3], 33% sleep no more than 6 h per night [4] and 69% experience social jet lag [5]. Accumulating epidemiological studies have demonstrated significant associations between circadian disruption-related lifestyles and an increased risk of type 2 diabetes (e.g. shift work has been associated with a 10–40% increased risk of diabetes) [6]. Given the possible links between circadian disruptions and diabetes, there is cause for investigating the role of the circadian system and circadian disruption in glucose control and type 2 diabetes risk. As such, this review provides an overview of the circadian regulation of glucose metabolism and the impact of circadian disruption on glucose control.
Defining circadian rhythms and circadian disruption
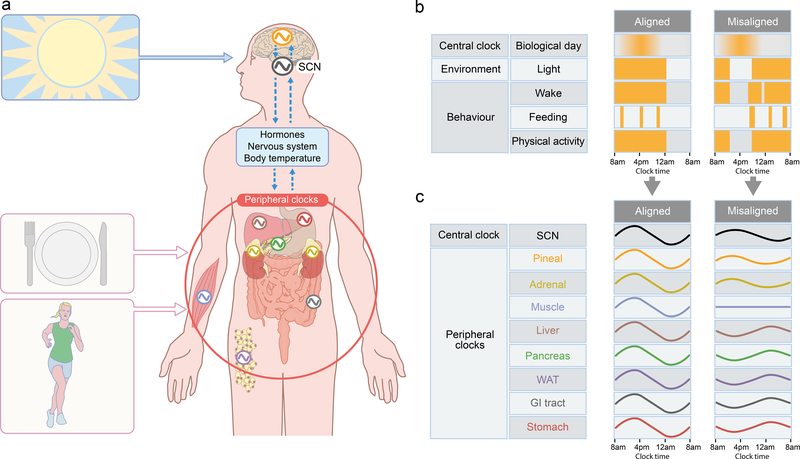
The circadian system evolved to generate daily rhythms in physiological functions and biological processes. These rhythms are synchronised to and, importantly, can anticipate the ~24 h environmental cycles brought about by the Earth’s rotation. Twenty-four-hour rhythms in physiology, as experienced in everyday life, result from a combination of the influence of circadian rhythms (endogenous ~24 h rhythms that persist under constant conditions) and behavioural and environmental influences [7, 8]. The endogenous circadian rhythms are produced by a multi-oscillator system composed of the central clock, located in the hypothalamic suprachiasmatic nucleus (SCN), as well as peripheral clocks in virtually every organ, tissue and cell [8]. The molecular clock mechanism is comprised of feedback loops, with the main transcription–translation negative feedback loop involving core clock genes including CLOCK, BMAL1 (also known as ARNTL), PER and CRY [8]. The SCN is entrained primarily by light signals via the retinohypothalamic tract. Through neural and/or hormonal pathways, the SCN relays the timing signals to other brain areas and peripheral organs, such as the pineal gland, adrenal gland, liver, pancreas, muscle, adipose tissue and gastrointestinal tract (Fig. 1a). In addition to influencing organ function rapidly through ‘classical’ neuroendocrine control [7], the SCN can also influence organ function through synchronisation of the molecular clocks within these peripheral organs [8]. These molecular clocks subsequently can influence organ function through their impact on clock-controlled genes. In addition to synchronisation by neuroendocrine signals from the SCN, peripheral clocks can also be shifted by non-photic stimuli, such as exercise [9] and feeding, with feeding being the strongest Zeitgeber (time cue) for many metabolic-related organs, such as the liver [7].
Fig. 1.
Circadian timekeeping and the alignment between environmental/behavioural rhythms and central/peripheral clocks. (a) Timing signals from the environment (e.g. light, as indicated by the image of the sun) and from behaviours (e.g. food intake, as indicated by the plate with knife and fork, and physical activity, as indicated by the runner) affect the rhythms of the central clock (i.e. the SCN) and/or peripheral clocks (shown for the pineal gland [orange], liver [brown], adrenal gland [yellow], pancreas [green], stomach [red], muscle [blue], white adipose tissue [purple] and gastrointestinal tract [grey]). Rhythms in the clock oscillators are represented by the cosine waves. Through hormonal/humoral, neural and temperature pathways (shown by the dashed blue arrows), temporal signals are also transmitted between the central clock and peripheral clocks. Through this relay of timing signals, the body’s rhythms entrain to external environmental and behavioural rhythms while, internally, the central and peripheral clocks maintain synchrony. (b) Alignment and misalignment between central clock and environmental/behavioural rhythms. Timing of the central clock, environment light exposure and behaviours are shown as yellow bars across a 24 h period. In the ‘aligned’ condition, the central clock is aligned with light exposure, wake, feeding and physically active period. In the ‘misaligned’ condition, the light exposure, wake, feeding and physical activity are shifted so that they are not aligned with the central clock. This misalignment between the central clock and the environmental and behavioural rhythms is a type of circadian disruption. (c) Alignment and misalignment between central and peripheral clocks. Timing of the central and peripheral clock rhythms are shown schematically as cosine waves across a 24 h period. In the aligned condition, the rhythms of the central and peripheral clocks are aligned. In the misaligned condition, the rhythms are dampened or flat and some rhythms are shifted such that not all are aligned. This misalignment between central and peripheral clocks is another form of circadian disruption, also called ‘internal misalignment’ or ‘internal desynchrony’. Note that the phases of the cosine waves do not necessarily show the rhythmic expression of specific clock genes but illustrate the concept of alignment (when the timing of different clocks occur at an optimal phase relationship, i.e. the timing within each rhythm’s cycle are in alignment with one another) vs misalignment (when these relationships are abnormal). GI, gastrointestinal; WAT, white adipose tissue. This figure is available as part of a downloadable slideset.
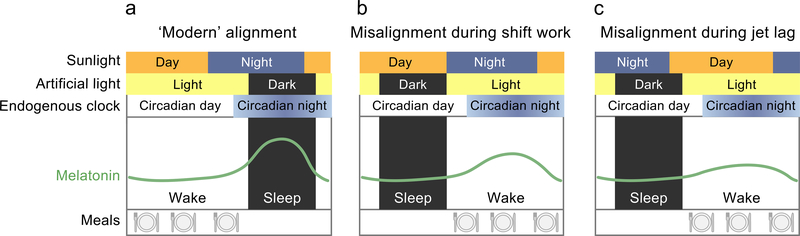
Circadian misalignment can occur between the central clock and environmental (i.e. light–dark; ‘environmental misalignment’) or behavioural (i.e. feeding–fasting, wake–sleep, activity–rest; ‘behavioural misalignment’) cycles (Fig. 1b) or between the central clock and peripheral clocks throughout the body (‘internal misalignment’; Fig. 1c) [10]. Circadian misalignment is common in modern industrialised societies: artificial light exposure coupled with work and social demands often lead to environments and behaviours mismatched in time with endogenous circadian rhythms. For example, it frequently occurs during shift work and jet lag (Fig. 2), where there is misalignment between the central circadian clock (that can be assessed by melatonin levels under low light conditions), environmental rhythms (e.g. light–dark) and behavioural rhythms (e.g. meals).
Fig. 2.
Comparison of the alignment of environmental, circadian and behavioural rhythms in a modern diurnal lifestyle (a), during night-shift work (b) and during jet lag (c). Environmental light exposure patterns are determined by sunlight and artificial light. Sunlight is shown as varying by day and night; artificial light is shown as light (i.e. ‘on’) or dark (i.e. ‘off’). Endogenous circadian rhythms are illustrated by the endogenous clock and profiles of melatonin, varying by circadian day (when there is no circadian drive for melatonin production and melatonin levels are low) and circadian night (when there is circadian drive for melatonin production and melatonin levels are high). The green curve represents endogenous melatonin levels across the wake and sleep episodes, with behavioural rhythms shown as the wake and sleep episodes and feeding patterns (meals; illustrated by the plate with knife and fork). In the modern lifestyle (a), there is relative alignment between the daylight, artificial light, circadian day, wake period and meal timing. However, note that the sleep episode and, therefore, the artificial light exposure and circadian phase are typically delayed relative to the solar night, because of the choice by most to remain awake many hours after sun set and often awakening after sunrise. The melatonin curve shows robust rhythm and is aligned to the environmental and behavioural rhythms. During shift work (b), the wake period and meal ingestion occur during the circadian night, with artificial lighting during the wake episode. With this acute misalignment, there is a dampened melatonin rhythm. Jet lag (c) occurs in response to rapid shifts in time zone. While the sunlight and artificial light align with the wake period and meal ingestion in this scenario, they are inverted compared with the circadian day–night pattern and the melatonin profile is dampened. This figure is available as part of a downloadable slideset.
Diurnal patterns of glucose metabolism in healthy individuals and those with type 2 diabetes
Diurnal rhythms are driven in part by endogenous rhythms and in part by environmental and behavioural rhythms. The external rhythms mask the endogenous rhythms, making it difficult to distinguish the contributions of each to the diurnal rhythm. Diurnal variation in glucose control is well established in healthy individuals. In the 1960s, glucose tolerance and insulin sensitivity were shown to be lower in the evening than in the morning [11]. Subsequent studies performed at the end of the 20th century confirmed this glucose tolerance pattern using oral glucose tolerance tests (OGTTs), identical meal tests, glucose infusions and enteral nutrition [12]. The evening decrease in glucose tolerance seems to be due to a decrease in both insulin sensitivity and beta cell function [12]. In individuals with type 2 diabetes, the hyperglycaemic clamp technique has revealed an inverted glucose tolerance profile, with glucose tolerance improving across the waking day [13]. Additionally, in contrast to stable blood glucose levels in normal-weight non-diabetic humans, people with type 2 diabetes exhibit a night-to-morning elevation of blood glucose across the sleep period [14]. In this well-known ‘dawn phenomenon’, there is an increase in blood glucose levels and decreased insulin sensitivity in the early morning fasted hours. An increase in endogenous glucose production overnight contributes to this fasting/morning hyperglycaemia seen in type 2 diabetes [15]. These diurnal patterns in glucose control prompted investigators to perform intensive in-laboratory studies to isolate endogenous circadian patterns of glucose control (i.e. those that are not driven by the sleep–wake, fasting–feeding or dark–light cycles). As described below, these studies have been performed primarily in healthy adults; studies in those with diabetes are needed, especially given the differences in diurnal rhythms of glycaemic control between individuals with and without diabetes.
Circadian patterns of glucose metabolism in healthy individuals
To determine whether the circadian system contributes to the diurnal rhythms of glucose metabolism discussed above, behavioural/environmental influences, such as from sleep, food intake and physical activity, must be controlled for. Experimental in-laboratory protocols have been undertaken to control for such influences, thereby allowing for assessment of endogenous circadian patterns of glucose metabolism.
One such protocol, designed to remove the influence of the sleep–wake cycle and the fasting–feeding cycle, has studied glucose control during extended wakefulness and constant glucose infusion. Under these conditions, healthy adults showed a peak in glucose and insulin levels during the time when habitual sleep would have occurred [16], thus providing evidence for circadian influence on glucose metabolism. When such a protocol is conducted with participants in a semi-recumbent posture and with isoenergetic snacks at regular intervals during extended wakefulness in a time-free, dim-light environment, it is known as a ‘constant routine’ protocol. Constant routine protocols aim to remove 24 h cycling in all environmental and behavioural inputs to minimise their cyclic influence, thus enabling measurement of circadian rhythms independent from those masking effects. Consistent with results reported with the constant glucose infusion protocol, constant routine experiments show circadian rhythmicity of glucose levels with a peak in the circadian night [17, 18].
The aforementioned experimental protocols involve sleep deprivation, which is known to adversely impact glucose metabolism [19]. The ‘forced desynchrony’ protocol is an approach that limits sleep deprivation while still disentangling circadian rhythms from behavioural/environmental cycles. In a forced desynchrony protocol, participants are studied in time-free conditions under dim light during a recurrent short or long sleep–wake cycle (e.g. 20 h or 28 h day; including fasting–feeding, rest–activity and postural cycles with the same period). The circadian system cannot entrain to these very short/long days under dim light and, thus, expresses its endogenous period. The forced desynchrony protocol has the added advantage that behaviours including fasting–feeding and sleep–wake are uniformly distributed across all circadian phases. This enables the separate influences of circadian and behavioural factors to be studied, as well as their alignment and misalignment, the latter of which will be discussed in the following section on circadian disruptions. As in the two experimental approaches discussed above, glucose exhibits a circadian rhythm with peak in the circadian night during the forced desynchrony protocol [20].
Another experimental approach to assess the influence of the circadian system and circadian misalignment uses a protocol that simulates a rapid and large shift of the behavioural and environmental cycles, such as commonly experienced in shift work. This study design has been used to investigate circadian and behavioural/environmental contributions to morning vs evening glucose tolerance. In a randomised crossover protocol with rapid 12 h shift, identical mixed meals were given in the circadian morning or evening when the behavioural/environmental cycle was either aligned or misaligned to the central clock [21]. The results indicated that the circadian system made a larger contribution to the difference in glucose tolerance between morning and evening than the combined behavioural/environmental factors. Specifically, the circadian phase accounted for 17% higher postprandial glucose in the circadian evening vs morning, independent of effects from behavioural cycles. The early-phase postprandial insulin was also 27% lower in the circadian evening vs morning. Thus, this circadian influence on glucose tolerance is, at least in part, a result of stronger pancreatic beta cell response in the circadian morning [21, 22]. Taken together, data from these experimental protocols indicate that glucose metabolism in healthy individuals is under strong circadian control, with a key role for pancreatic beta cell function. Future experimental protocols are required to disentangle circadian contributions to the diurnal rhythms of glucose metabolism in individuals with type 2 diabetes. It is possible that differences between glucose metabolism in individuals with and without type 2 diabetes may be linked to circadian rhythm contributions given recent findings that the central biological clock in the human SCN is affected in individuals with type 2 diabetes; specifically, there are significantly decreased numbers of arginine vasopressin immunoreactive (AVP-ir) neurons, vasoactive intestinal polypeptide (VIP-ir) neurons and glial fibrillary acidic protein immunoreactive (GFAP-ir) astroglial cells in the SCN of individuals with type 2 diabetes as compared with healthy control individuals [23].
Impact of circadian disruption on glucose control in healthy individuals and those with type 2 diabetes
Epidemiological and genetic studies on effects of circadian disruption
Epidemiological studies on lifestyles associated with misalignment between environmental, behavioural and/or circadian cycles suggest that circadian disruptions contribute to type 2 diabetes risk. One prevalent form of circadian disruption is shift work, with over 20% of the US workforce engaged in night shift, rotating shift or other schedules besides regular day/evening shift [3]. A meta-analysis of observational studies indicates that individuals exposed to shift work have a 9% increased risk of diabetes compared with individuals with no shift-work experience [24]. According to a longitudinal study using the Nurses’ Health cohorts, this risk is also positively associated with duration of exposure to shift work, with a 5% increase in risk for every 5 years of shift work [25]. Importantly, it has also been shown that workers on a rotating shift have even higher odds of diabetes than workers on fixed night shift [6, 24]. As rotating shift work involves constantly switching between combinations of morning/day, evening and/or night shifts (possibly a more severe form of circadian disruption than fixed night work), these findings together suggest that circadian disruption increases risk of type 2 diabetes in a dose-dependent manner.
Another prevalent circadian disruption is social jet lag, quantified as sleep and wake timing discrepancies between work and free days, as an estimate of misalignment between biological and social time [26]. It has been reported that people with social jet lag of greater than 1 h (approximately 69% of the population [5]) have 1.75 times the prevalence of diabetes (defined as fasting plasma glucose levels >6.5 mmol/l and HbA1c levels >6.5% or >48 mmol/mol) or prediabetes (defined as fasting plasma glucose levels >6.1 mmol/l and HbA1c levels >6.0% or >44 mmol/mol) compared with people with less than 1 h of social jet lag [27]. The degree of social jet lag is partially dependent on a person’s chronotype (a person’s ‘morning’ or ‘evening’ preference), which has been correlated with body temperature rhythm [28]. Later chronotypes, as compared with earlier chronotypes, have an odds ratio of about 2–2.5 for diabetes independent of sleep duration [29, 30].
In addition to their role in predisposing people to diabetes, lifestyle factors associated with circadian disruption also have deleterious health consequences in individuals with diabetes. Diabetic individuals working night shifts present with higher HbA1c levels than those not working night shifts, irrespective of rotating or non-rotating shifts and after adjusting for age, BMI, insulin use, sleep duration, morning/evening preference and percentage daily carbohydrate intake [31]. One form of behavioural misalignment that is of particular interest for individuals with diabetes is the mistiming of eating (Fig. 1) [32]. For example, it has been shown recently in a randomised, crossover study that late dinner timing, concurrent with elevated night-time melatonin concentrations, impairs glucose tolerance in MTNR1B risk variant carriers [33]. A causal role for melatonin is suggested by the observation that the significant impairment of glucose tolerance with delayed dinner is only observed in carriers of the G allele of the MTNR1B risk variant [33], which has been associated with increased risk for diabetes [34, 35]. This conclusion is further supported by placebo-controlled studies showing that exogenous melatonin administration both in the morning and in the evening impairs glucose tolerance [36] and that this effect is six times larger in MTNR1B risk carriers than in non-carriers [37]. Mistiming of food intake may be a particular concern in the diabetic population, in which those who night-eat (consume >25% of their daily energy intake after regular dinnertime) report less compliance with glucose monitoring, higher HbA1c levels and greater number of diabetes complications [38, 39]. Late circadian meal timing may impair glucose tolerance due to a number of reasons: (1) eating at an adverse circadian phase; (2) eating concurrent with elevated melatonin concentrations and/or (3) late meal timing causing internal misalignment (although this is primarily a working hypothesis). According to this last hypothesis, food mistiming can uncouple peripheral clocks in metabolic tissues from the central clock, causing internal misalignment [40]. However, to date, there is no direct evidence that internal desynchrony per se adversely affects glucose control [41], suggesting that the first two mechanisms may be most important.
It should be noted that shift workers and people with social jet lag, late chronotype and genetic risk often have characteristics related to circadian disruption that may predispose them to higher risk of type 2 diabetes. Insufficient sleep or poor sleep quality, for instance, are often a by-product of the circadian misalignment experienced by shift workers and are known to adversely impact glucose metabolism [42, 43]. Physical inactivity and higher rates of obesity are also commonly associated with these lifestyles. Thus, sleep, activity, and other lifestyle factors correlated with circadian disruption may contribute to the increased risk of diabetes seen in the aforementioned epidemiological studies, making it difficult to isolate the impacts of circadian disruption in these studies [44].
Human experimental studies on the effects circadian disruption
As mentioned, it is difficult to disentangle the impact of circadian disruption from the impact of other factors in real-life scenarios like shift work and social jet lag. Additionally, although these associations suggest that circadian disruptions adversely impact glucose metabolism, a causal effect cannot be demonstrated. Human experimental work has been conducted to tease out independent effects of circadian disruptions and possible causal relationships.
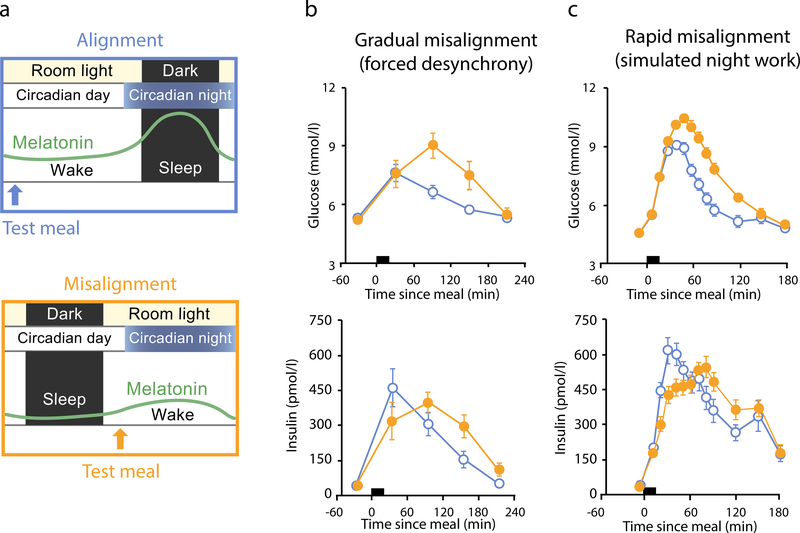
In a forced desynchrony protocol with a 28 h day, participants displayed a 6% increase in glucose during the circadian misalignment portion (when they ate/slept 12 h out of phase from habitual schedules) in spite of a 22% increase in insulin across the whole sleep–wake cycle; the effects were driven by the postprandial phases, demonstrating reduced glucose tolerance potentially due to reduced insulin sensitivity (Fig. 3b) [20]. Since shift work is often accompanied by sleep restriction, another forced desynchrony protocol examined the impact of circadian misalignment combined with sleep restriction. When sleep was restricted to 6.5 h during a 28 h day (equivalent to 5.6 h per 24 h day), combined with a recent history of misalignment, fasting and postprandial glucose were increased by 8% and 14%, respectively, while fasting and postprandial insulin decreased by 12% and 27%, respectively, suggesting decreased beta cell function [45]. An elegant experimental design has also separated the effects of circadian misalignment from effects of sleep restriction. When sleep was restricted to 5 h, participants sleeping mostly during the day (circadian misalignment) had 47% greater reduction in insulin sensitivity compared with 34% reduction in insulin sensitivity of participants sleeping during the night [46].
Fig. 3.
Effect of misalignment between the central clock and the behavioural/environmental cycle on glucose tolerance. (a) In-laboratory experimental conditions designed to test the effect of alignment vs misalignment on glucose tolerance. In the alignment condition, room light and wake occur during the circadian day, when melatonin is low. A test meal (blue arrow) is given during the wake period to assess glucose tolerance during alignment. In the misalignment condition, room light and wake occur during the circadian night, when circadian drive for melatonin production is high. A test meal (orange arrow) is given during the wake period to assess glucose tolerance during misalignment. (b, c) Glucose tolerance is impaired in misaligned vs aligned conditions during in-laboratory experimental protocols of both gradual and rapid misalignment. In a forced desynchrony protocol (b), in which misalignment is gradual (see text for more details), postprandial glucose and late-phase postprandial insulin (timing of meal indicated by black bar) were higher in the misaligned (orange closed circles) vs aligned (blue open circles) condition (n = 10; mean ± SEM; p<0.001 and p<0.06, respectively, with statistical significance for effect of misalignment). In a simulated shift-work protocol (c), in which misalignment occurred rapidly (see text for more details), postprandial glucose and insulin were both higher in the misaligned (orange closed circles) vs aligned (blue open circles) condition (n = 14; mean ± SEM; p=0.013 and p=0.001, respectively). In both experimental protocols, the results indicated that circadian misalignment impaired glucose tolerance, in part via reduced insulin sensitivity. Data in (b, c) adapted from [20] and [21] under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium. This figure is available as part of a downloadable slideset.
To see how this may apply in shift work, one study used a rapid shift protocol and found that postprandial glucose was 6% higher (with 14% higher late-phase postprandial insulin) during circadian misalignment compared with alignment (Fig. 3c) [21]. To determine whether these findings persist in the shift-work population, a randomised, crossover laboratory study gave healthy chronic shift workers identical test meals under conditions of simulated night-shift work (with circadian misalignment of 12 h inverted behavioural/environmental cycles) and simulated day-shift work (with circadian alignment). Under circadian misalignment, postprandial glucose was 6% higher and postprandial late-phase insulin was 10% higher compared with circadian alignment conditions [47]. In a field study of shift workers in Antarctica, mixed meals resulted in higher postprandial glucose, insulin and triacylglycerol levels during night shift compared with dayshift [48]. These findings indicate that real-world shift work has a similar impact on glycaemic outcomes when compared with laboratory-simulated shift-work protocols. Furthermore, these experimental findings corroborate those from epidemiological studies on shift work and provide causal evidence for the contribution of circadian misalignment to adverse glycaemic outcomes.
Insight into potential glucoregulatory mechanisms leading to impaired glycaemic outcomes from circadian disruption has also been gleaned from experimental induction of circadian misalignment using rapid shift and forced desynchrony protocols (Fig. 3a). In a 12 h rapid shift protocol with euglycaemic–hyperinsulinaemic clamp technique [49], circadian misalignment was shown to decrease muscle insulin sensitivity. Biopsies of muscle also indicated that the molecular circadian clock was not able to synchronise and align to the rapid shift. A subsequent study using the oral minimal model further separated effects of circadian misalignment from effects of circadian phase and environmental/behavioural factors on glucose control. The reduction of glucose tolerance in the circadian evening compared with circadian morning was due primarily to beta cell responsivity, while the impaired glucose tolerance under circadian misalignment was mainly due to decreased insulin sensitivity [22].
All together, these experimental studies provide substantial evidence that circadian misalignment contributes to impaired glucose metabolism primarily through effects on muscle insulin sensitivity in healthy, young populations as well as in chronic shift workers. In addition, the endogenous circadian phase has a robust effect on glucose control, with relatively impaired glucose tolerance in the biological evening, primarily due to a decrease in beta cell function.
Implications
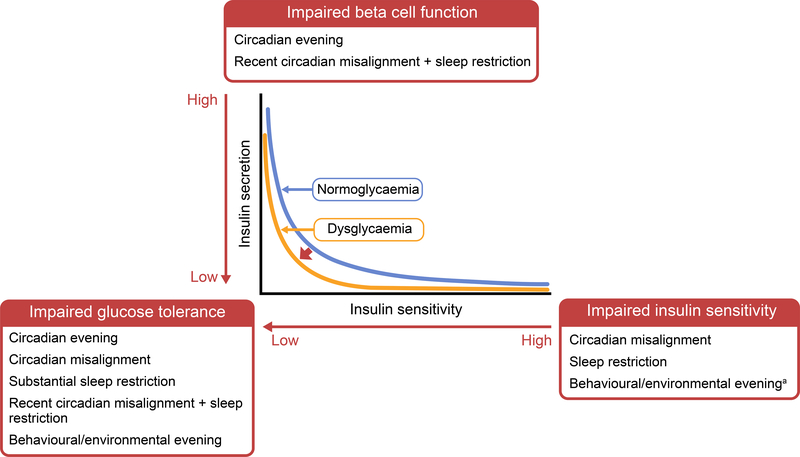
Taken together, these human epidemiological, genetic and experimental studies support the contribution of the circadian system to glucose metabolism (for a summary, see Table 1). Experimental evidence indicates that circadian disruption impairs beta cell function and insulin sensitivity, resulting in impaired glucose tolerance (Fig. 4). Circadian disruptions acutely impact glycaemic control and thereby may increase the risk for impaired glucose tolerance and the transition to diabetes. Healthy individuals as well as those with type 2 diabetes may support glycaemic control by appropriately aligning the endogenous, behavioural (i.e. sleep–wake, meals) and environmental (i.e. light exposure) rhythms. There is burgeoning evidence, for instance, that time-restricted feeding may have a beneficial effect on metabolic health and glucose control [50, 51]. However, long-term randomised controlled trials investigating the impact of aligning endogenous and environmental rhythms on glycaemic control in individuals improve glycaemic control for healthy individuals and those with type 2 diabetes who are exposed to misalignment and circadian disruption.
Table 1.
Summary of experimental evidence of impact of circadian and behavioural components on insulin sensitivity, beta cell function and glucose tolerance
| Circadian/behavioural component | Insulin sensitivity | Beta cell function | Glucose tolerance |
|---|---|---|---|
| Circadian evening | ↔a | ↓ | ↓ |
| Behavioural evening | ↓/↔ | ↔ | ↓ |
| Circadian | ↓ | ↔ | ↓ |
| misalignmentb | |||
| Sleep restriction | ↓ | ↓/↔ | ↓ |
| Recent circadian | No data | ↓ | ↓ |
| misalignment + sleep | |||
| restriction |
Evidence [22] requires further confirmation
Misalignment between the central clock and the environmental and behavioural cycles
↓, reduction; ↔, no change
Fig. 4.
Diagram illustrating the impact of circadian and behavioural influences on glycaemic control and the potential transition to diabetes. The relationship between insulin secretion and insulin sensitivity is shown by the two hyperbolae for normoglycaemia (blue line) and dysglycaemia (orange line). When insulin sensitivity decreases, insulin secretion increases to maintain normoglycaemia (moving from right to left along the blue line). If and when insulin secretion is unable to compensate for decreased insulin sensitivity, the curve shifts to the left and impaired glucose tolerance develops (moving from right to left along the yellow line, insulin secretion does not rise sufficiently to counter the decrease in insulin sensitivity). Experimental evidence indicates that insulin sensitivity is impaired by circadian misalignment, sleep restriction and, with less strong evidencea, behavioural/environmental evening. Beta cell function is impaired by circadian evening and recent circadian misalignment with sleep restriction. Glucose tolerance has been shown to be impaired by circadian evening, circadian misalignment, substantial sleep restriction, recent circadian misalignment with sleep restriction, and behavioural/environmental evening. The circadian and behavioural impact on glycaemic control can, thus, affect the shift from normoglycaemia to dysglycaemia and the transition to diabetes. This figure is available as part of a downloadable slideset.
Concluding remarks
Here, we have compiled evidence that glucose metabolism is regulated by the circadian system and that circadian disruptions adversely impact glycaemic control. Improved understanding of mechanisms by which circadian disruption affects the development and progression of impaired insulin secretion and impaired insulin sensitivity will potentially contribute to improving glycaemic control and managing type 2 diabetes.
Supplementary Material
Chronobiology terminology.
Chronobiology
An area of biology concerned with biological events that cycle over time
Circadian rhythm
Term derived from the Latin phrase ‘circa dies’, meaning ‘about a day’; endogenous biological rhythm with a period of ~24 h that is self-sustaining and that persists independent of environmental (i.e. light–dark, day–night) and behavioural (i.e. sleep–wake, fasting–feeding cycles) influences
Constant routine
Experimental protocol in which participants are kept in a semi-recumbent posture and given isoenergetic snacks at regular intervals during extended wakefulness in a time-free, dim-light environment; designed to remove 24 h cycling in all environmental and behavioural inputs, thus enabling measurement of circadian rhythms independent from those masking effects
Diurnal or day–night
A rhythm that is driven partly by an endogenous circadian rhythm and partly by environmental and behavioural rhythms, such as the sleep–wake, fasting–feeding and light–dark cycles; the external rhythms mask the endogenous rhythm, making it difficult to distinguish the contributions of each to the day–night rhythm
Entrainment
Synchronisation of one cyclical pattern to another cyclical pattern (e.g. synchronisation of the circadian system to the light–dark cycle)
Forced desynchrony
Experimental protocol in which participants are studied in time-free conditions under dim light during a recurrent short or long sleep–wake cycle (e.g. 20 h or 28 h days, including fasting–feeding, rest–activity and postural cycles of the same period); disentangles circadian rhythms from behavioural/environmental cycles and allows for measurements of biological processes in response to behavioural/environmental cycles across all circadian phases, including investigation of alignment/misalignment
Jet lag
Transient misalignment of biological rhythms with the local light–dark cycle associated with rapid eastward or westward travel across one or more time zones
Masking
Obscuring of endogenous rhythm by conditions that induce acute effects on the measure of interest
Misalignment
The incorrect timing of a rhythm in relation to another rhythm
Period
Cycle length, or the time it takes to cycle from one circadian phase to the same phase again
Phase
Time within a cycle when an event occurs (e.g. minimum, maximum)
Zeitgeber
A German word meaning ‘time-giver’; a time cue that can entrain rhythms
Tests to assess glycaemic control.
Hyperglycaemic clamp
Plasma glucose concentration is raised above basal levels by a continuous infusion of glucose and maintained by adjustment of a variable glucose infusion; as the glucose concentration is held constant, the glucose infusion rate is an index of insulin secretion and glucose metabolism
Hyperinsulinaemic–euglycaemic clamp
Plasma insulin concentration is raised and maintained by a continuous infusion of insulin while the plasma glucose concentration is held constant at basal levels by a variable glucose infusion; when steady state is achieved, the glucose infusion rate is equal to glucose uptake by all tissues in the body and is a measure of tissue insulin sensitivity
IVGTT
Glucose is injected i.v. and blood insulin levels are measured before and after the injection, often with high frequency (frequently sampled IVGTT [FSIGT]),at time points immediately following glucose injection
Mixed meal tolerance test
Blood samples are taken just before and after oral ingestion of a mixed meal that contains fats, protein and carbohydrates; blood samples indicate how blood glucose and insulin respond to the meal
OGTT
Baseline blood sample is taken before oral ingestion of a liquid containing a certain amount of glucose (commonly 75 g) after which blood is sampled every 10–30 min for up to 3 h
Future directions.
Experimental studies in those with diabetes to distinguish circadian rhythmicity from diurnal variations of glucose patterns
Prospective studies on the role of the circadian system and circadian disruptions in the development and severity of diabetes
Mechanistic studies on how circadian disruptions impact glycaemic control and diabetes development
Intervention studies to ameliorate adverse gly- caemic consequences of circadian disruption stemming from shift work, jet lag and other common occurrences in modern lifestyles
More comprehensive research on circadian control of glucose metabolism in diverse populations, such as different ethnic/racial groups and patient populations, to increase accuracy and generalisability
Investigations on circadian biomarkers of central and peripheral clocks, and of circadian misalignment, in healthy individuals and those with diabetes
Intervention studies on chronotherapies for those with diabetes
Translation of current and future research findings to evidence-based prevention/treatment of diabetes
Acknowledgments
Funding ICM was supported in part by National Institutes of Health (NIH) grants R01 HL140574 and T32 HL7901–20 and American Heart Association grant 19POST34380188. JQ was supported in part by American Diabetes Association grant no. 1–17-PDF-103 and NIH grant R01 DK102696. GKA was supported in part by NIH grant K24 HL103845. FAJLS was supported in part by NIH grants R01 DK099512, R01 HL118601, R01 DK102696, R01 DK105072 and R01 HL140574.
Abbreviation
- SCN
Suprachiasmatic nucleus
Footnotes
Duality of interest FAJLS received lecture fees from Bayer HealthCare, Sentara HealthCare, Philips, Vanda Pharmaceuticals and Pfizer Pharmaceuticals. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Bullard K, Cowie C, Lessem S, et al. (2018) Prevalence of diagnosed diabetes in adults by diabetes type — United States, 2016. MMWR Morb Mortal Wkly Rep 67: 359–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cho N, Shaw J, Karuranga S, et al. (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes research and clinical practice 138: 271–281 [DOI] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention. 2010. National Health Interview Survey (NHIS). Public-use data file and documentation. Available from ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2010/samadult_freq.pdf Accessed 17 May 2019
- [4].Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA (2018) Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep 42(2): zsy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Roenneberg T, Allebrandt KV, Merrow M, Vetter C (2012) Social jetlag and obesity. Current Biology 22(10): 939–943 [DOI] [PubMed] [Google Scholar]
- [6].Vetter C, Dashti HS, Lane JM, et al. (2018) Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 41(4): 762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gamble KL, Berry R, Frank SJ, Young ME (2014) Circadian clock control of endocrine factors. Nature Reviews Endocrinology 10(8): 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annual review of neuroscience 35: 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harfmann BD, Schroder EA, Esser KA (2015) Circadian rhythms, the molecular clock, and skeletal muscle. Journal of biological rhythms 30(2): 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qian J, Scheer FA (2016) Circadian system and glucose metabolism: implications for physiology and disease. Trends in Endocrinology & Metabolism 27(5): 282–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jarrett R, Keen H (1969) Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J 2(5653): 341–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Cauter E, Polonsky KS, Scheen AJ (1997) Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine reviews 18(5): 716–738 [DOI] [PubMed] [Google Scholar]
- [13].Boden G, Chen X, Urbain JL (1996) Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45(8): 1044–1050 [DOI] [PubMed] [Google Scholar]
- [14].Shapiro ET, Polonsky KS, Copinschi G, et al. (1991) Nocturnal elevation of glucose levels during fasting in noninsulin-dependent diabetes. The Journal of Clinical Endocrinology & Metabolism 72(2): 444–454 [DOI] [PubMed] [Google Scholar]
- [15].Radziuk J, Pye S (2006) Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia 49(7): 1619–1628 [DOI] [PubMed] [Google Scholar]
- [16].Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS (1991) Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. The Journal of clinical investigation 88(3): 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morgan L, Arendt J, Owens D, et al. (1998) Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. Journal of endocrinology 157(3): 443–452 [DOI] [PubMed] [Google Scholar]
- [18].Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS (2005) Independent circadian and sleep/wake regulation of adipokines and glucose in humans. The Journal of Clinical Endocrinology & Metabolism 90(5): 2537–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nedeltcheva AV, Scheer FA (2014) Metabolic effects of sleep disruption, links to obesity and diabetes. Current opinion in endocrinology, diabetes, and obesity 21(4): 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences 106(11): 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morris CJ, Yang JN, Garcia JI, et al. (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences 112(17): E2225–E2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer FA (2018) Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes, Obesity and Metabolism 20(10): 2481–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hogenboom R, Kalsbeek MJ, Korpel NL, et al. (2019) Loss of arginine vasopressin- and vasoactive intestinal polypeptide-containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes. Diabetologia 62(11): 2088–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gan Y, Yang C, Tong X, et al. (2015) Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 72(1): 72–78 [DOI] [PubMed] [Google Scholar]
- [25].Pan A, Schernhammer ES, Sun Q, Hu FB (2011) Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS medicine 8(12): e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wittmann M, Dinich J, Merrow M, Roenneberg T (2006) Social jetlag: misalignment of biological and social time. Chronobiology international 23(1–2): 497–509 [DOI] [PubMed] [Google Scholar]
- [27].Koopman AD, Rauh SP, van ‘t Riet E, et al. (2017) The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new hoorn study. Journal of biological rhythms 32(4): 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Horne JA, Östberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology 4(2): 97–110 [PubMed] [Google Scholar]
- [29].Finn L, Young E, Mignot E, Young T, Peppard P (2013) Associations of eveningness chronotype with adverse metabolic indications in the Wisconsin Sleep Cohort. Sleep 36: 188 [Google Scholar]
- [30].Merikanto I, Lahti T, Puolijoki H, et al. (2013) Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiology international 30(4): 470–477 [DOI] [PubMed] [Google Scholar]
- [31].Manodpitipong A, Saetung S, Nimitphong H, et al. (2017) Night shift work is associated with poorer glycaemic control in patients with type 2 diabetes. Journal of sleep research 26(6): 764–772 [DOI] [PubMed] [Google Scholar]
- [32].Dashti HS, Scheer FA, Saxena R, Garaulet M (2019) Timing of food intake: identifying contributing factors to design effective interventions. Advances in Nutrition 10(4): 606–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lopez-Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M (2018) Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clinical Nutrition 37(4): 1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lyssenko V, Nagorny CL, Erdos MR, et al. (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nature genetics 41(1): 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Prokopenko I, Langenberg C, Florez JC, et al. (2009) Variants in MTNR1B influence fasting glucose levels. Nature genetics 41(1): 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M (2014) Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37(10): 1715–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garaulet M, Gómez-Abellán P, Rubio-Sastre P, Madrid JA, Saxena R, Scheer FA (2015) Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 64(12): 1650–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB (2006) Isn’t this just bedtime snacking?: The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care 29(8): 1800–1804 [DOI] [PubMed] [Google Scholar]
- [39].Reutrakul S, Hood MM, Crowley SJ, et al. (2013) Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes care 36(9): 2523–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wehrens SM, Christou S, Isherwood C, et al. (2017) Meal timing regulates the human circadian system. Current Biology 27(12): 1768–1775. e1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van der Vinne V, Swoap SJ, Vajtay TJ, Weaver DR (2018) Desynchrony between brain and peripheral clocks caused by CK1δ/ε disruption in GABA neurons does not lead to adverse metabolic outcomes. Proceedings of the National Academy of Sciences 115(10): E2437–E2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK (2010) Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59(9): 2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. The lancet 354(9188): 1435–1439 [DOI] [PubMed] [Google Scholar]
- [44].Vetter C, Scheer FA (2019) A healthy lifestyle—reducing T2DM risk in shift workers? Nature Reviews Endocrinology 15(4): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Buxton OM, Cain SW, O’Connor SP, et al. (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Science translational medicine 4(129): 129ra143–129ra143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Leproult R, Holmbäck U, Van Cauter E (2014) Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63(6): 1860–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morris CJ, Purvis TE, Mistretta J, Scheer FA (2016) Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. The Journal of Clinical Endocrinology & Metabolism 101(3): 1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lund J, Arendt J, Hampton S, English J, Morgan L (2001) Postprandial hormone and metabolic responses amongst shift workers in Antarctica. Journal of Endocrinology 171(3): 557–564 [DOI] [PubMed] [Google Scholar]
- [49].Wefers J, van Moorsel D, Hansen J, et al. (2018) Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proceedings of the National Academy of Sciences 115(30): 7789–7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chaix A, Manoogian EN, Melkani GC, Panda S (2019) Time-restricted eating to prevent and manage chronic metabolic diseases. Annual review of nutrition 39: 291–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell metabolism 27(6): 1212–1221. e1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.