Abstract
The discovery that patients with Gaucher Disease (GD), a rare lysosomal storage disorder, were developing symptoms similar to Parkinson Disease (PD) led to investigation of the relationship between the two seemingly unrelated pathologies. GD, an autosomal recessive disorder, is the result of a biallelic mutation in the gene GBA1, which encodes for the enzyme glucocerebrosidase (GCase). Since the observation of its relation to PD, GBA1 mutations have become recognized as the most common genetic risk factor for development of synucleinopathies such as PD and dementia with Lewy bodies. Although the exact mechanism by which GBA1 mutations promote PD is unknown, current understanding suggests that impaired GCase inhibits lysosomal activity and decreases the overall ability of the cell to degrade proteins, specifically the neuronal protein α -synuclein. Decreased elimination of α-synuclein can lead to its abnormal accumulation and aggregation, an important component of PD development. Further understanding of how decreased GCase activity increases risk for α-synuclein pathology can assist with the development of clinical biomarkers for early detection of synucleinopathies, as well as promote novel treatments tailored for people with a GBA1 mutation. Historically, α-synuclein has not been a reliable biomarker for PD. However, recent research on α-synuclein content within exosomes, which are small vesicles released by cells that carry specific cellular cargo, has yielded promising results. Moreover, decreased GCase activity has been shown to influence exosomal contents. Exosomes have emerged as a promising new avenue for the identification of novel biomarkers and therapeutic targets aimed at improving neuronal GCase function and limiting the development of synucleinopathies.
Keywords: Gaucher Disease, GBA1, carriers, alpha-synuclein, exosomes, biomarker
1. Introduction
Gaucher Disease (GD), an autosomal recessive disorder with an incidence of approximately 1 in every 40,000 – 60,000 live births, is the most prevalent of the over 50 lysosomal storage disorders. These disorders are characterized by genetic deficiencies in lysosomal enzymes [1]. The enzyme affected in GD is glucocerebrosidase (GCase), encoded by the gene GBA1, which catalyzes the breakdown of glucosylceramide (GlcCer) and its deacylated derivative glucosylsphingosine (GlcSph) [1]. The occurrence of GD is uniquely high in the Ashkenazi Jewish population, where it occurs in 1 out of every 800 births [2]. Symptom severity is highly variable, although the presence or absence of CNS manifestations allows classification into three clinical types [2]. The most common is type 1 GD (GD1), which is considered non-neuronopathic and is characterized by symptoms of hepatosplenomegaly, anemia, and osteological issues such as bone pain and osteoporosis [3]. However, symptomatology and severity are heterogeneous, and genotype is often an unreliable predictor of phenotype and prognosis [4]. Types 2 and 3 GD are the more severe, less common neuronopathic forms and due to differences in the rate of progression are denoted as acute neuronopathic and chronic neuronopathic, respectively [4]. Type 2 GD typically results in death in infancy, while type 3 has slower onset of neurologic symptoms such as ocular muscle apraxia (a defect in horizontal eye movement), ataxia, myoclonic epilepsy, and variable learning impairments, in addition to the skeletal and visceral symptoms found in GD1 [3]. While biallelic mutations in GBA1 causes GD, in the last decade it has become apparent that presence of even a single GBA1 mutation (designated carriers) is a significant risk factor for development of Parkinson disease (PD). PD is a neurological disease that initially presents as motor symptoms such as resting tremor and muscle rigidity, resulting from the degeneration of substantia nigra dopaminergic neurons, and progresses to more widespread neurodegeneration, severe motor impairment, non-motor symptoms such as cognitive and sleep dysfunction, and eventually death [5, 6]. Both patients with GD, including those with very little to no symptoms, and carriers that will include relatives of GD patients are at an increased risk for PD and an investigation of the relationship between these two disease states is warranted.
In this review, we will provide an overview of the impact of GBA1 mutations on the development of α-synuclein pathologies. Pathological α-synuclein is significant in PD, as α-synuclein aggregates are a major component of Lewy bodies - insoluble protein bodies that are a neuropathological hallmark of many synucleinopathies, as well as other neurological disorders [7]. Although α-synuclein aggregates were classically considered the primary component of Lewy bodies, recent findings indicate that they contain high quantities of dysmorphic organelles and lipid membranes as well [8]. Teasing apart the relationship between α-synuclein pathology and organelle dysfunction is a crucial step to understanding PD pathophysiology. It should be noted that many of the findings linking GBA1 mutations and PD have also been observed in other synucleinopathies, such as dementia with Lewy bodies. However, for the purposes of this review we will focus only on GBA1 and PD. To date there are no validated clinical biomarkers for PD development. Circulating α-synuclein levels, though biologically relevant, have not been shown to be clinically viable as a biomarker due to inconsistent trends and the invasive techniques involved. The biology of exosomes, small extracellular vesicles that carry specific cellular cargo, is an emerging area of investigation in neurodegenerative disorders due to their ability to reflect changes in the central nervous system (CNS) [9]. Determining how GBA1 mutations affect α-synuclein aggregation, exosome secretion, and exosomal contents can lead to a better understanding of PD development as well as identify novel biomarkers and therapeutic targets.
1.1. Association between GBA1 mutations and PD risk
More than 350 different mutations have been characterized in the GBA1 gene [10]. However, most patients with GD in North America and Europe have at least one of the two mutated alleles – N370S or L444P [10]. The most common GBA1 mutation is N370S, making up approximately 80% of mutations in the Ashkenazi Jewish population [11]. Homozygous and compound heterozygous N370S mutations are associated only with GD1 symptomology [12]. Homozygous and compound non-N370S heterozygous L444P mutations are commonly found in patients with GD2 or GD3 [13]. Additionally, the GBA1 mutations T369M and E326K, which are generally not associated with GD, are common variants found in the Caucasian population that increase the risk for PD [14]. For a full review of GBA1 mutation prevalence by ethnicity, see Migdalski & Shapiro, 2016 [15].
The effect of decreased GCase activity in GD1 is particularly evident in macrophages, which take on an enlarged foamy characteristic and are eponymously denoted Gaucher cells [16]. In addition to their enlarged size, these cells also present with their nuclei pushed to the cell boundary due to lysosomal build-up of GlcCer and take on a “crumpled tissue paper” appearance under light microscopy [16]. Gaucher cells primarily infiltrate the spleen, liver, and bone, resulting in the common visceral symptoms of GD1 [12]. Although the effects of GCase deficiency on the blood, bone, and viscera are relatively well characterized, the effect on neurons is not well known [12]. A clear understanding of how decreased GCase activity impairs neural function in both homozygous and heterozygous GBA1 mutations is crucial to unravel the link between GBA1 and PD.
The relationship between PD and GD was first observed in the late 1990s, when patients with GD1 were noticed to develop PD-like symptoms [17]. Subsequent studies corroborated these findings, suggesting increased PD risk for GD patients [18, 19]. It was then discovered that GBA1 mutations were also common in PD patients without GD [20, 21]. One of these studies also found that PD patients of Ashkenazi Jewish heritage had GBA1 mutations more frequently than non-Ashkenazi PD, demonstrating the increased mutation prevalence in this population. Additionally, risk to relatives of GD patients was quantified. A study done in the US and Israel population found approximately 25% of GD patients had a close relative with PD [22]. Following these preliminary studies, large cohort studies were undertaken that solidified this relationship, and GBA1 mutations are now considered to pose the greatest risk for PD development among all known genetic causes [23, 24].
Although there are no immediately apparent clinical differences between idiopathic PD (iPD) and GBA-associated PD (GBA-PD), several differences in disease progression have been noted in the literature. The most prominent difference is that PD develops 1.7 – 6 years earlier in GBA-PD vs. iPD (average age of onset ~60 years), with the more severe GBA1 mutations resulting in onset in the early 50s [25–28]. Additionally, the overall risk of developing GBA-PD appears to increase with the severity of mutation,, although the N370S allele is commonly observed among patients with GD who develop parkinsonian symptoms [26, 29]. Increased cognitive impairment and dementia has also been reported in GBA-PD [27, 30]. Further, histological investigation of patient brain samples revealed those with GBA-PD display a more diffuse neocortical Lewy body-type pathology compared to iPD, suggesting a potential mechanism for the increase in dementia and cognitive deficits [23]. Psychiatric symptoms in GBA-PD appear to be more severe as well, with hallucinations, depression, and psychosis appearing earlier and more often than iPD [30, 31]. Severe GBA1 mutations also seem to increase the severity of all motor and non-motor symptoms [32, 33]. Collectively, understanding the basis for the reported differences in disease onset and manifestations between iPD and GBA-PD can provide insights related to pathophysiology of PD, leading to novel treatment strategies.
1.2. Role of glucocerebrosidase in α-synuclein pathology
The association between GBA1 mutations and PD is well established. However, the mechanisms that promote PD development in the presence of lower GCase activity are still relatively unknown. Although GBA1 mutations increase risk for developing PD, reduced GCase activity appears insufficient to cause PD. Indeed, only about 10% of GD patients will develop PD before the age of 80, leaving a vast majority unaffected [34]. This suggests lower GCase activity alone likely requires other downstream defects to progress to PD development. One theory explaining this discrepancy involves the protein α-synuclein.
Αlpha-synuclein is a 140 amino-acid protein predominantly present in neurons, although some can be detected in the periphery, such as red blood cells, platelets and plasma [35–37]. While not fully understood, under normal conditions α-synuclein is believed to play a role in presynaptic signaling and membrane trafficking [7, 35]. Based on the nature of its structure, α-synuclein is predisposed to abnormal aggregation [38]. Initial aggregation results in soluble oligomers, which appear to be the toxic component of PD pathogenesis and thus the most relevant therapeutic target [39]. These oligomers slowly coalesce into insoluble fibrils and eventually Lewy bodies [7, 39]. This abnormal aggregation appears to play a crucial role in the pathogenesis of PD, although the process is not completely understood. Moreover, loss of α-synuclein function may contribute to PD development, the extent of which is still unclear. Silencing of α-synuclein function in rats has been shown to lead to dopaminergic neuron degeneration [40]. Additionally, knocking out α-synuclein in apoE-TR mice enhanced the neuropathological effects of apoE4 neurodegenerative pathways [41]. However, α-synuclein knockout models do not appear to develop PD symptoms, such as altered motor activity [42]. It is likely that both pathological α-synuclein accumulation and aggregation as well as decreased function contribute to PD development. Decreased degradation has been hypothesized as one of the potential mechanisms by which α-synuclein accumulates and aggregates [43]. GBA1 mutations appear to promote α-synuclein accumulation, however the mechanism by which this occurs is still debated [44].
1.2.1. Loss-of-Function
The loss-of-function hypothesis suggests that low GCase activity decreases lysosomal function, impairing α-synuclein degradation and increasing its accumulation and aggregation (Fig. 1). This was demonstrated in a study where neuronal GCase knockdown models had a 40% decrease in the rate of lysosomal proteolysis and an increase in steady-state α -synuclein levels [45]. The authors further observed GD mouse models to have increased monomeric and oligomeric α-synuclein levels, potentially the consequence of the decreased proteolysis. Decreased lysosomal activity and a corresponding increase in α-synuclein has been demonstrated in human neuroblastoma cells with GBA1 nonsense mutations as well as following GCase inhibition via conduritol beta-epoxide [46, 47]. Additionally, in vivo GCase inhibition through conduritol beta-epoxide was shown to lead to α-synuclein aggregate accumulation and subsequent neuroinflammation and neurodegeneration [48]. Interestingly, the contrary has also been shown to be true; increased GCase activity in mouse models via either direct enzyme injection or gene therapy led to a decrease in oligomeric α-synuclein levels [49, 50].
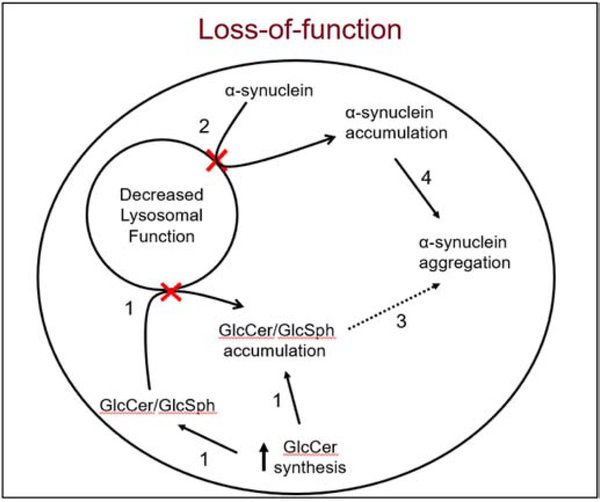
Fig. 1: Role of GCase in α-synuclein pathology -the loss-of-function hypothesis.
1. Decreased GCase activity (shown with red X) leads to decreased degradation and accumulation of the substrates GlcCer and GlcSph. Up-regulation of GlcCer synthase also adds to the substrate load [51, 52]. 2. Decreased GCase (shown with red X) also affects the rate of total proteolysis, causing a build-up of α-synuclein [45, 48]. 3. The accumulated GlcCer and GlcSph can act as a seed for α-synuclein aggregation [45, 53]. 4. Accumulated α-synuclein increases the propensity to aggregate [54]. Evidence that has been experimentally proven in vivo is indicated with a solid arrow, while what has not yet been demonstrated is shown with a dashed arrow.
Decreased GCase activity leads to accumulation of GlcCer and GlcSph, both of which can promote aggregation of α-synuclein by acting as a seed for oligomer formation [45, 53]. Further, these sphingolipid substrates can decrease autophagosome clearance [55]. While neuronal GCase knockdown models and neurons of GD mouse models show accumulation of these lipids, it is still unclear whether heterozygous GBA1 mutations increase neuronal GlcCer and GlcSph levels [45, 51, 52]. A study conducted in postmortem neurons of GBA-PD patients found no difference in either substrate [56]. Experiments looking at GlcCer levels in iPS-derived neurons heterozygous for GBA1 mutations have shown mixed results [57, 58]. Elevated GlcCer substrate levels have also been reported in the blood of GBA-PD patients [59]. Interestingly, decreased GCase activity and increased GlcSph levels were observed in the substantia nigra and hippocampus in iPD patients, indicating GCase substrate accumulation can occur in the absence of a GBA1 mutation [60]. Dysfunction in GCase also suggests a potential decrease in the level of its product, ceramide. Increasing ceramide levels was shown to lower α-synuclein accumulation, indicating a role for ceramide [61]. However, GD mouse models failed to show decreased ceramide levels [45]. This may be due to synthesis of intracellular ceramide from alternate sources such as sphingomyelin. Future research in GBA1 carrier models should further investigate how the neuronal levels of these substrates are impacted as well as how the ratio of substrates to products affects α-synuclein accumulation.
1.2.2. Toxic Gain-of-Function
The previous studies provide evidence for a loss-of-function mechanism to explain GBA-PD. However, only a small fraction of people with GBA1 mutations develop PD, indicating a more complex scenario. N-glycosylation and proper folding of GCase takes place in the endoplasmic reticulum (ER), after which it is exported via the Golgi bodies to the lysosome. If a protein is misfolded, it is “tagged” in the ER, transported back into the cytosol, and eliminated via the ubiquitin-proteasome system (UPS). This process is known as Endoplasmic Reticulum Associated Degradation (ERAD) and has been demonstrated to be involved in the elimination of misfolded mutant GCase proteins [62]. The toxic gain-of-function hypothesis states that increased GCase misfolding puts more load and stress on the ER and UPS, resulting in impaired degradation of other misfolded proteins (Fig. 2). Mutant GCase associates with parkin, a ubiquitin E3 ligase which has also been implicated in PD pathophysiology [62]. Binding of parkin with mutated GCase decreases its ability to degrade other protein targets, whose accumulation is toxic to dopaminergic neurons [63, 64]. Interestingly, the degree of misfolded protein being eliminated via ERAD depends on the severity of GBA1 mutations, with L444P resulting in the most degradation [62, 65]. This may help explain the earlier onset of GBA-PD in severe mutations. The constant effort necessary to process misfolded GCase was shown to increase ER stress in a study using iPSC-derived dopaminergic neurons with an N370S mutation [58]. Multiple studies have also demonstrated increased ER calcium release in cell lines derived from GBA-PD patients [57, 66]. Deregulation of calcium homeostasis can alter the function of various cellular components and has been implicated in both PD and GD [66]. Lastly, mutated GCase may itself promote α-synuclein aggregation and inclusion body formation, as GCase is significantly more prevalent in the Lewy bodies of GBA-PD patients than in iPD [67]. One finding challenging the gain-of-function hypothesis is that heterozygous GD patients with a null allele mutation still develop PD and appear to have an earlier age of onset [68]. This is contradictory, as it shows PD still develops even in the absence of misfolded GCase and any associated increased ER stress. Understanding of the complex interplay of glycosphingolipid metabolism, GBA1 mutations, structural and conformational enzyme abnormalities, lysosomal and autophagic dysfunction, UPS, ERAD stress and α-synuclein accumulation continues to be a work in progress.
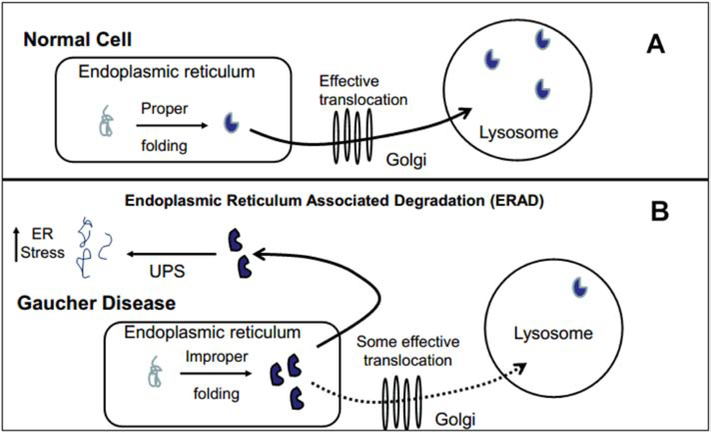
Fig. 2: Role of GCase in α-synuclein pathology -the gain-of-function hypothesis.
A) In a wild-type cell, GCase is properly folded in the endoplasmic reticulum (ER) and successfully translocated through the Golgi into the lysosome. B) In the presence of a GBA1 mutation, the ER cannot consistently fold GCase correctly [62]. While some are appropriately configured and translocated, many are misfolded and marked for degradation. These proteins are then exported into the cytosol and degraded via the ubiquitin-proteasome system (UPS). This process is called Endoplasmic Reticulum Associated Degradation (ERAD). The gain-of-function hypothesis states that GBA1 mutations and transcription/translation of aberrant GCase greatly increases utilization of ERAD, creating and sustaining ER and UPS stress and thus impairing degradation of the total cell proteome and specifically α-synuclein oligomers [58, 66]. This can result in accumulation of these oligomers and aggregation into Lewy bodies. All evidence of this hypothesis has only been demonstrated in vitro.
1.2.3. Additional Mechanisms
Although both mechanisms could lead to lysosomal and cellular dysfunction, it is more likely that both the loss-of-function and gain-of-function pathways, as well as other mechanisms, act in concert to bring about the cell death seen in GBA-PD. In the absence of a GBA1 mutation, iPD patients appear to have lower GCase activity compared to controls [69, 70]. Mazzulli et al., speculated that high α-synuclein levels may impair GCase translocation to the lysosome and lower enzyme activity [45]. This would result in a circular feedback loop in GD and GBA-PD, where loss of GCase function increases α-synuclein levels and aggregation, which in turn can decrease GCase activity [71, 72]. However, to date this relationship has not been experimentally demonstrated and more research is required, especially since some mouse models expressing increased levels of α-synuclein have failed to show decreased GCase activity [73, 74].
Given that in all previously discussed literature GCase dysfunction preceded α-synuclein accumulation, a more likely explanation is that an upstream mechanism in PD development results in decreased GCase activity, which could then contribute to α-synuclein accumulation. Further evidence of this is that there is a significant age-related risk for PD, and GCase activity in the substantia nigra and putamen appears to naturally decrease with age [60]. This age-related decrease was also demonstrated in wild-type mice, and coincided with increased GlcCer and GlcSph levels. Thus, it is possible that the presence of a GBA1 mutation can contribute to α-synuclein accumulation in both an upstream and downstream manner, either giving rise to initial GCase dysfunction or exacerbating any spontaneous dysfunction arising from a different mechanism. It should be noted that some studies have failed to find decreased GCase activity in iPD, which may be due to the extent of PD progression during sample collection, and is an area for future research [75, 76]. In addition to GCase deficiency, decreased activity in other lysosomal enzymes has been observed in iPD as well [77]. In fact, a recent study found that over 50% of PD cases in their sample contained at least one damaging mutation in a known lysosomal storage disorder gene [78]. These findings further highlight the importance of lysosomal dysfunction in PD pathophysiology and suggest impaired lysosomal flux is likely an important contributor to iPD development.
Lastly, mitochondrial dysregulation is believed to play a role in both GD pathophysiology and in the etiology of GBA-PD [79, 80]. Increased cytosolic calcium levels via ER stress produce mitochondrial dysfunction, causing reactive oxygen species (ROS) release and increasing oxidative stress [81]. These impaired mitochondria are funneled through the lysosomal-autophagy system and eliminated in a process called mitophagy [82]. The lysosomal impairment associated with GBA1 mutations potentially disrupts mitophagy, resulting in accumulation of defective mitochondria and increased ROS levels. This has been demonstrated in GD mouse models [83]. High α-synuclein levels can also induce mitochondrial dysfunction [84]. Additionally, oxidative stress has been demonstrated to promote α-synuclein aggregation, suggesting a potential positive feedback loop [85]. Although the exact mechanism is still unknown, GD models have demonstrated increased inflammation in affected neurons, leading to increased nitric oxide release, mitochondrial damage, and increased oxidative stress [86]. Taken together, these processes are thought to play a role in the dopaminergic cell death in GBA-PD, although the relative contribution is still controversial. For a more extensive review of mitochondrial dysfunction in GBA-PD, see Gegg et al. 2016 [86].
2. Alpha-synuclein as a biomarker
Despite rigorous efforts ongoing for decades to identify a biomarker for PD development, no reliable indicators have been discovered to date. This void prevents development of prophylactic interventions and restricts current PD treatment to symptom management. Unfortunately, symptoms do not typically develop until 60–80% of striatal dopaminergic neurons have already been lost [87]. This leaves a large gap between asymptomatic disease progression and treatment initiation to the detriment of patient quality of life and overall survival. Due to its well documented pathological role, α-synuclein has long been viewed as a potential biomarker for PD development. However, research regarding this hypothesis has generated inconsistent and at times conflicting results (Table 1).
Table 1:
Summary of α-synuclein biomarker studies done in PD
| Authors (year) [reference number] | Matrix | Measured | PD (n) | Control (n) | Methods | α-synuclein PD vs. controls (p-value) |
|---|---|---|---|---|---|---|
| Mollenhauer et al. (2013) [89] | CSF | Total α-synuclein | 78 | 48 | ELISA | Lower in PD (p=0.002) |
| Kang et al. (2013) [90] | CSF | Total α-synuclein | 63 | 39 | ELISA | Lower in PD (p=0.01) |
| Hong et al. (2010) [91] | CSF | Total α-synuclein | 126 | 137 | Luminex | Lower in PD (p<0.001) |
| Ohrfelt etl a. (2009) [92] | CSF | Total α-synuclein | 15 | 55 | Western Blot | NS |
| Park et al. (2011) [93] | CSF Plasma | Total α-synuclein Oligomeric α-synuclein Total α-synuclein Oligomeric α-synuclein |
23 | 29 | ELISA | NS Higher in PD (p=0.005) NS NS |
| Foulds et al. (2012) [94] | CSF | Total α-synuclein Oligomeric α-synuclein |
39 | 20 | ELISA | NS NS |
| Gorostidi et al. (2012) [96] | Plasma | Total α-synuclein | 134 | 109 | ELISA | Lower in PD (p=0.010) |
| Duran et al. (2010) [97] | Plasma | Total α-synuclein | 95 | 60 | ELISA | Higher in PD (p=0.0229) |
| Lee et al. (2006) [98] | Plasma | Total α-synuclein | 105 | 51 | ELISA | Higher in PD (p<0.001) |
| Foulds et al. (2013) [99] | Plasma | Total α-synuclein | 189 | 91 | ELISA | NS |
| Foulds et al. (2011) [100] | Plasma | Total α-synuclein Oligomeric α-synuclein |
32 | 30 | ELISA | NS NS |
| Malec-Litwinowisz et al. (2018) [101] | Plasma | Total α-synuclein | 58 | 38 | ELISA | NS |
| Caranci et al. (2013) [102] | Plasma | Total α-synuclein | 69 | 110 | ELISA | NS |
| El-Agnaf et al. (2006) (103] | Plasma | Oligomeric α-synuclein | 34 | 27 | ELISA | Higher in PD (p=0.002) |
| Vincente et al. (2017) [105] | Erythrocyte | α-synuclein posttranslational modifications pY125 PY39 AGE SUMO |
58 | 30 | Western Blot |
Higher in PD (p<.0001) (p=0.004) (p<0.0001) Lower in PD (p=0.0082) |
| Wang et al. (2015) [106] | Erythrocyte | Oligomeric α-synuclein/tot al protein | 100 | 102 | ELISA | Higher in PD (p<0.001) |
| Shi et al. (2014) [142] | CNS-derived plasma exosomes Plasma |
Total α-synuclein (exosome) Total α-synuclein (exosome + plasma) |
267 | 215 | Luminex | Higher in PD (p<0.0001) NS |
| Zhao et al. (2019) [143] | CNS-derived plasma exosomes Plasma |
Total α-synuclein (exosome) Total α-synuclein (exosome + plasma) |
39 | 40 | ELISA | Higher in PD (P=0.018) NS |
| Cerri et al. (2018) [144] | Plasma exosomes Plasma |
Total α-synuclein (exosome) Total α-synuclein (exosome + plasma) |
39 | 33 | ELISA | Higher in PD (p<0.001) NS |
| Si et al. (2019) [145] | CNS- derived serum exosomes |
Total α-synuclein (exosome) | 38 | 39 (18 healthy controls & 21 essential tremor patients) | ELISA | Lower in PD (p=0.009) |
Researchers first explored cerebrospinal fluid (CSF) for potential diagnostic surrogate biomarkers [88]. Some studies found total CSF α-synuclein levels to be lower in PD compared to healthy controls [89–91]. However, other studies failed to show this difference, leading to ambiguity [92, 93]. CSF α-synuclein oligomers have shown similar variability, with one study finding elevated levels in PD while another showed no difference [93, 94]. One meta-analysis concluded that total CSF α-synuclein is likely decreased in PD and suggested the observed discrepancies are due to differences in sample preparation and variable antibody characteristics [95]. Taken together, total CSF α-synuclein appears to be a potential biomarker for PD, but further optimization and standardization is needed to confirm its clinical utility.
A major limitation of employing CSF is that it requires an invasive procedure. Detectable α-synuclein differences in more accessible matrices would provide a more viable biomarker for PD monitoring. This has led to investigation of whole blood and blood plasma for biomarkers. Observations of total plasma α-synuclein have been controversial, with one study finding decreased α-synuclein [96] and others showing an increase [97, 98]. Furthermore, a third set of studies found no alterations [93, 99–102]. This inconsistency suggests total plasma α-synuclein is likely a poor choice for biomarker development. Similarly, plasma α-synuclein oligomer studies have yielded inconclusive results [93, 100, 103]. It should be noted that in addition to neuronal synthesis, α-synuclein is also synthesized peripherally, mainly within red blood cells (RBC) [104]. This can interfere plasma α-synuclein measurements with red cell contamination, contributing to ambiguous values. There is evidence that RBC α-synuclein could potentially act as a biomarker for PD. One study showed that post-translational modification of blood derived α-synuclein is altered in PD and that these changes can be measured [105]. An increased ratio of α-synuclein oligomer/total RBC proteins has also been demonstrated in PD [106]. These results indicate blood derived α-synuclein as an avenue for further study.
Until recently, most biomarker studies were done in PD patients with no attention to disease etiology. While there is currently no way to predict who will develop iPD, risk for GBA-PD is recognizable and GBA1 mutation carriers are concerned about their risk. Since only a small proportion of patients with a GBA1 mutation develop PD, development of a predictive clinical biomarker is crucial. With a clear relationship between GCase activity and α-synuclein, it is prudent to study α-synuclein levels in GD1 and GBA-PD patients. Currently, only a few studies have investigated α-synuclein in these populations (Table 2). Pchelina et al. conducted a study with 41 GD patients and found higher oligomeric α-synuclein in the plasma of GD patients compared to controls [107]. Additionally, the red blood cell α-synuclein dimer to monomer ratio was observed to be elevated in both GD and GBA-PD patients [108, 109]. It should be noted that in patients with GD, with or without PD, plasma α-synuclein levels may be affected by variable platelet counts and splenic platelet sequestration. Nonetheless, the more concrete nature of these findings suggests GBA1 mutations may result in more detectable differences in α-synuclein oligomer levels. This represents a potential biomarker for development of GBA-PD and should be an area for continued study.
Table 2:
Summary of α-synuclein biomarker studies done in GD and GBA-PD.
| Authors (year) (reference number) | Matrix | Protein Measured | GD/GBAPD | Control | Methods | α-synuclein in GBA vs. controls (p-value) |
|---|---|---|---|---|---|---|
| Pchelina et. al (2014) [107] | Plasma | Oligomeric α-synuclein | GD: 41 | 40 | ELISA | Higher in GD (p<0.0001) |
| Papagiannakis et. al (2018) [108] | Erythrocyte | Monomeric α-synuclein Dimeric α-synuclein α-synuclein Dimer/Monomer ratio |
GBA-PD: 18 | 56 | Western Blot | NS Higher in GBA-PD (p<0.01) Higher in GBA-PD (p<0.01) |
| Argyriou et al. (2012) [109] | Erythrocyte | Monomeric α-synuclein α-synuclein Dimer/Monomer ratio |
GD: 27 | 32 | Western Blot | NS Higher inGD (p=0.0135) |
3. Extracellular Vesicles – a tool for biomarker discovery and target identification
Throughout their lifespan, cells release an abundance of extracellular vesicles (EVs) into the environment, a discovery that has been recognized in recent years and received the 2013 Nobel Prize in Medicine [110]. These EVs typically contain a mixture of nucleic acids, lipids, and proteins, and this is now considered to be a common route by which cells communicate and exchange materials, among other functions [111, 112]. They are found extensively throughout the body, can be isolated from all bodily fluids, and appear to be released by most, if not all cell types [113, 114]. In recent years, a specific subset of EVs known as exosomes have gained increased attention. Exosomes are classically defined as EVs of 30–150nm in diameter and originate from the invagination of an endosomal wall, resulting in an inward budding and formation of an intraluminal vesicle [111, 114]. The formation of multiple intraluminal vesicles within an endosome results in the formation of a multivesicular body (MVB), the functions of which have been extensively characterized [115, 116]. Once formed, an MVB can fuse with the cell membrane and release its intraluminal vesicles into the extracellular space, at which point they are deemed exosomes [111, 117]. Additionally, an MVB can fuse with the lysosome, resulting in the degradation of its contents [116]. Initially, it was believed the only role of exosomes was the removal of cellular waste from cells. However, they have since been discovered to carry out a diverse range of biological functions, such as proper immune function, and inflammatory signaling, as well as potentially contributing to the propagation of pathologies and cancer growth [110, 111, 118]. Exosomes have been found to contain a diverse yet specific array of molecules, including lipids, mRNA, miRNA, proteins, and metabolites, as well as recognizable surface markers such as CD9, CD63, and CD81 [110, 117]. The molecular variety seen both within and on exosomes helps explain the broad yet targeted functionality they accomplish. For a complete review of exosome biogenesis and composition, see Raposo & Stoorvogel, 2013 [110].
Due to their intracellular origin, variety of biochemical content, and ubiquitous distribution throughout the body, exosomes present an attractive avenue for detecting subtle changes in cellular function. A recent study showed that the presence of a pathology can alter exosomal content, suggesting potential diagnostic utility [119]. This would be especially useful in the early detection of neuronal pathologies, as exosomes pass through the blood-brain-barrier (BBB) and can be harvested from blood samples [114, 120]. Aberrant neural functioning could therefore result in recognizable changes in the content of neuronally derived plasma exosomes, allowing for noninvasive measurement of neuronal function and potential disease detection. This has already been demonstrated in Alzheimer’s disease and frontotemporal dementia, where patients were shown to have elevated levels of relevant pathogenic proteins [121]. Additionally, neuronal-derived exosomes isolated from blood samples of Alzheimer’s patients collected prior to disease onset showed elevated levels of pathogenic proteins as early as 10 years prior to diagnosis [121–123]. Exosomes also contribute to cell-to-cell transmission, as demonstrated in a recent study describing the mechanism by which occupational exposure to manganese, historically considered a risk factor for PD, can contribute to α-synuclein aggregation and neuroinflammation in welders [124]. This is another example of how studying exosomes peripherally can provide insight on CNS changes. Exosomes can also harbor promising markers for treatment response in the CNS. This was demonstrated in a study examining exenatide use in PD, where neuronally derived exosomes had altered contents related to insulin signaling following initiation of therapy [125]. Lastly, since exosomes can cross the BBB, they are being investigated as a CNS drug delivery mechanism [120]. Modification of the exosome surface is also being studied as a method to target specific cell types, potentially increasing treatment precision and limit off target effects [120]. The field of exosome biology is evolving rapidly and shows immense potential in the areas of novel biomarker discovery and therapeutic targets. One major bottle-neck hindering its development is the lack of an optimal, reproducible methodology for exosome isolation, an area that is still being debated and researched [126].
3.1. Exosomes: the missing link in GBA-PD?
Interest in the relationship between exosomes and PD initially stemmed from the question of how α-synuclein aggregation propagates to different neurons, which was demonstrated both in vivo and in vitro [127–129]. The discovery of α-synuclein in exosomes presented a potential means of transportation [130]. This hypothesis was further strengthened by the finding that encapsulation into exosomes further promotes α-synuclein misfolding and aggregation, increasing its pathogenicity [131]. It was also demonstrated that exosomal α-synuclein oligomers were more likely to be taken up by recipient neurons compared to free oligomers [132]. Interestingly, α-synuclein secretion via exosomes increases in the presence of a lysosomal inhibitor [133]. This increase is likely an overcompensation by the cell due to its inability to properly dispose of waste, leading to MVBs normally targeted towards the lysosome being redirected to release their contents at the cell surface [134]. Furthermore, it has been demonstrated that genetically or chemically lowered GCase activity results in increased exosome secretion, as well as increased secretion of exosome-associated α-synuclein oligomers [135–137]. These results suggest that the lysosomal dysfunction present in GBA1 mutations could potentially act to increase the propagation of α-synuclein aggregates via exosomes (Fig. 3).
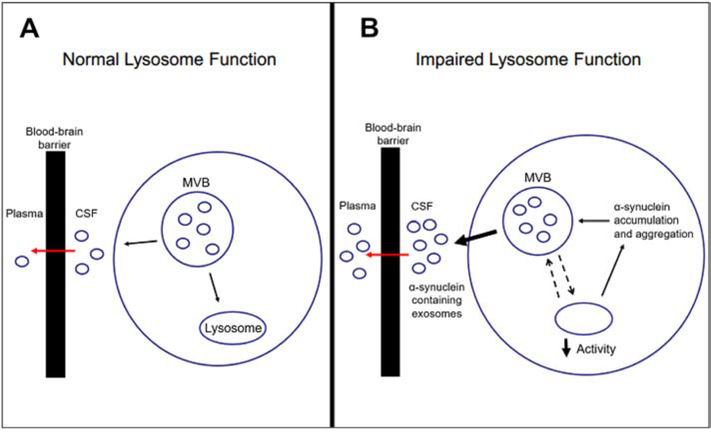
Fig. 3: Plasma exosomes as potential matrix for α-synuclein biomarker studies.
A) In normal conditions, a cell removes all waste being stored in a multi-vesicular body (MVB) by either transferring vesicles to the lysosome for degradation or exporting the vesicles via exocytosis into the CSF, at which point they are deemed exosomes [115]. These exosomes are then actively transported across the blood-brain barrier and enter the plasma [111]. B) If lysosome function is impaired, as when GBA1 mutations are present, the lysosome is not able to degrade the cellular wastes at the necessary rate [45, 47]. To compensate, the cell increases the number of vesicles that are being exported [134]. Additionally, decreased lysosomal function leads to α-synuclein accumulation, which gets re-packaged into vesicles and exported [135, 137]. These findings suggest that in the presence of lysosomal dysfunction, there is an increase in α-synuclein containing exosomes exported into the CSF, which can cross the blood-brain barrier and enter the plasma.
It is important to note that there is currently no in vivo evidence showing that neuronal uptake of exosomes is a vehicle for transmission of α-synuclein aggregates, and no linkage between exosomal α-synuclein and PD has been established. It is also unclear whether direct cell-to-cell transmission of α-synuclein oligomers is a mechanism for PD progression. Nevertheless, exosomes and the variety of its contents may still contribute to PD through the initiation of other harmful triggers. For example, α-synuclein accumulation and aggregation can activate microglia [138]. This activation may in part be due to intracellular accumulation of α-synuclein in the microglia, which has been shown to increase the release of exosomes containing high levels of inflammatory factors that promote neuronal cell death [139, 140]. Additionally, neuronal α-synuclein accumulation in the substantia nigra has been shown to result in neuroinflammation and subsequent neurodegeneration in post-synaptic brain regions [141]. Taken together, these findings suggest that intraneuronal α-synuclein accumulation may result in release of α-synuclein, either through exosomes or exocytosis, into the extracellular space. This α-synuclein can then be taken up by microglia, leading to microglial α-synuclein accumulation, release of inflammatory factor containing exosomes and neuroinflammation. Further research is needed to better determine whether exosomal transport of α-synuclein aggregates contributes to PD as well as to better understand other roles that exosomes may play in PD development.
PD biomarker investigation has only recently begun focusing on exosomal content. To date, only three studies have looked at plasma exosomal α-synuclein in PD (Table 1). Two of these studies isolated neuronally derived plasma exosomes and collectively showed increased levels of both α-synuclein and DJ-1 in PD patients compared to controls [142, 143]. A third study by Cerri et al. found higher plasma exosomal α-synuclein levels in PD patients while also showing that the ratio of exosomal α-synuclein/total plasma α-synuclein in PD patients is directly correlated with disease severity and inversely correlated with GCase activity [144]. Furthermore, Cerri et al. measured total plasma exosomes and still showed increased exosomal α-synuclein in PD, indicating neuronal isolation might not be necessary. Of note, this study excluded GBA1 mutation carriers and to date there has been no investigation of plasma exosomal α-synuclein in this population or in GD. The above studies found no difference in total plasma α-synuclein between PD patients and controls, suggesting exosomal α-synuclein is a better biomarker candidate. Interestingly, a recent study investigating α-synuclein levels in CNS-derived exosomes in serum found that it was lower in PD patients than in controls [145], although there were no other markers, such as DJ-1, measured to confirm this finding. Moreover, no correlation was observed with disease duration or severity.
As shown in Figure 3, decreased lysosomal activity may act to increase the level of α-synuclein containing exosomes in the plasma. Going forward, it will be interesting to examine how plasma and serum exosomal α-synuclein levels compare between GBA-PD and iPD, as well as how exosomal content in synucleinopathies changes in response to treatment. Further, exosomes offer the opportunity to investigate other glycosylated lipids or cholesterol esters that are known to be increased due to reduced GCase activity. For example, sterylglucosides are impacted by GBA mutations and are known to trigger synucleinopathies [146]. Investigation of the overall contents of exosomes isolated from GBA1 mutation carriers followed by pathway analyses to identify affected molecular signaling networks could further explain the link between lysosomal dysfunction and development of PD.
4. Therapeutic Strategies for GBA-PD
With better understanding of how GBA1 mutations lead to GBA-PD, there is increased need to consider therapeutic implications. One potential treatment strategy is to increase GCase function in neurons, thereby increasing lysosomal function and potentially lowering α-synuclein aggregation. This is an attractive route, as medications enhancing intra-lysosomal GCase activity are already in routine clinical use for GD1. Enzyme replacement therapy (ERT) refers to periodic intravenous infusions of pharmacologic recombinant GCase engineered to target macrophage mannose receptors leading to targeted cellular uptake and increased functional lysosomal GCase. This medication has good efficacy for alleviating the peripheral symptoms of GD1. However, long term intravenous ERT treatment neither prevents development of GBA-PD nor ameliorates any neurologic manifestations [147]. This is due to the inability of these medications to cross the BBB. However, intracerebroventricular delivery of recombinant human GCase in neuronopathic GD mouse models resulted in widespread distribution throughout the brain as well as reduced GlcCer and GlcSph and increased life span [148]. This indicates that available ERTs are functionally incorporated by neuronal cells and could provide a basis for treating GBA-PD if the obstacle of the BBB can be overcome. Formulating the GCase enzyme within a vehicle that can cross the BBB, such as by sequestering into an exosome, is being investigated [149]. Another approach to overcome the BBB and increase neural GCase activity is through the use of gene therapy. In a study by Rocha et al., vectors containing the coding sequence for human GBA1 were injected into various brain regions of mouse models overexpressing α-synuclein, resulting in increased GCase activity, decreased α-synuclein, and decreased dopaminergic neuron degradation [50]. It should be noted that the mouse models did not have dysfunctional GCase, yet still demonstrated decreased α-synuclein with increased GCase activity. This finding provides early evidence for targeted GCase gene therapy as a potential treatment for PD, even in the absence of GCase deficiencies, and is an area for future study.
A second medication class currently used to treat GD1 is substrate reduction therapy (SRT). These therapies inhibit glucosylceramide synthase, lowering the amount of GCase substrate being synthesized and thus decreasing the load for any residual enzyme activity. Furthermore, accumulating GlcCer stabilizes α-synuclein oligomers, which then bind to mutated GCase and inhibit enzymatic activity, block trafficking of mutant GCase from the ER to the Golgi, and augment the unfolded protein stress response [12]. Thus, inhibition of glucosylceramide synthase also has the potential to interrupt this bidirectional pathogenic loop. SRT is also available as an oral formulation, eliminating the need for infusions and improving the quality of life for patients. Currently there are two FDA approved SRT medications for GD1: eliglustat and miglustat. These medications have both shown to improve α-synuclein pathology in vitro [150, 151]. However neither are effective in vivo: eliglustat, because therapeutic CNS concentrations cannot be achieved due to a drug-specific P-glycoprotein efflux pump; and miglustat, because of its relatively weak glucosylceramide synthase Ki and an unfavorable side effect profile at higher doses [152, 153]. Recently, the glucosylceramide synthase inhibitor ibiglustat (Genz-682452) was shown to effectively penetrate the CNS, decrease GlcCer levels, and improve neurologic symptoms in GD mouse models [154]. A phase 2 clinical trial designated as the MOVES-PD trial is currently investigating ibiglustat in GBA-PD patients [154, 155].
In recent years a greater emphasis has been placed on small molecular chaperones for potential neurologic GD treatment. Many GBA1 mutations result in misfolding of GCase, leading to premature degradation via ERAD and UPS [62]. These small chemical chaperones assist with protein folding and increase enzyme stability, lysosomal translocation, and catalytic activity [156]. Additionally, the improved translocation decreases ER stress and improves UPS functioning. Many candidate protein chaperones have been identified thus far and fall into two classes: inhibitory and non-inhibitory small molecules [157].
The inhibitory class is the larger of the two and is defined as a small molecule which binds at the active site and inhibits GCase function [158]. When a destabilizing mutation is present, binding of the chaperone in the active site can stabilize the enzyme and facilitate proper folding and lysosomal translocation. Once in the acidic environment of the lysosome, GlcCer can outcompete the small molecule and hopefully the residual enzyme activity will allow for adequate substrate catalyzation. Unfortunately, the competitive binding kinetics of this drug class makes development and dosing difficult, as the molecule can bind to GCase too tightly to be competed off at an effective rate. They are also ineffective if the mutation alters the active site. Initially, the drug isofagomine held promise, as it increased GCase activity in mouse models of both N370S and L444P mutations and improved both neurologic and non-neurologic symptoms [159, 160]. However, in phase 2 clinical trials it failed to show clinical benefit, even for systemic manifestations in GD1 patients [158].
Ambroxol (ABX), historically used outside the United States as an expectorant, is a promising inhibitory small molecular chaperone of GCase [161]. Initial studies showed ABX increased both lysosomal translocation of GCase as well as overall enzyme activity in GD fibroblasts [65]. These results, plus reduced α-synuclein, have since been shown in GBA-PD fibroblasts. [162]. In fruit flies expressing mutated GBA, treatment with ABX (and with isofagomine) lowered ER stress and prevented loss of motor function [163]. Ambroxol also increased GCase activity in healthy nonhuman primates and decreased α-synuclein in GD mouse models [15, 164]. A pilot study on the efficacy of ABX in GD1 patients demonstrated improvements in at least one disease parameter but did not improve symptoms globally, indicating a potentially insufficient dose [165]. A subsequent open-label pilot study was conducted using a higher dose of ABX in five patients with either type 2 or 3 GD presenting neurological symptoms [166]. Ambroxol was found to be safe and tolerable, increased lymphocyte GCase activity, crossed the BBB, decreased CNS GlcSph levels, and improved neurologic symptoms. Although studies involving ABX have been positive, a recent study in mouse cortical neurons suggests it may also block macroautophagy flux and increase total α-synuclein levels, as well as promote exocytosis into the CSF via exosomes [167]. This was in contrast to a previous study showing increased autophagy and may likely be due to be dosing differences [167, 168]. If this is the case, certain dosage regimens may act to improve GD symptoms yet simultaneously trigger the development of GBA-PD. Clinical trials for high dose ABX use in PD are currently ongoing (ClinicalTrials.gov identifiers and ).
The second class of small molecular chaperones is non-inhibitory small molecules, which bind at areas of the enzyme other than the active site. This class is more attractive than the inhibitory class, as it leaves the active site open for substrates and permits the possibility of increasing residual enzyme activity through positive allosteric interaction. So far, thirty potential non-inhibitory small protein chaperones have been identified, from which additional protein analogues were constructed [158, 169]. Using iPSC-derived dopaminergic neurons from GD patients, two of these proteins (NCGC607 and NCGC00188758) have been shown to effectively increase GCase activity, decrease lipid storage, and decrease elevated α-synuclein [170–172]. N-acetylcysteine also acts as a non-inhibitory chaperone and can increase activity of mutated α-glucosidase, the enzyme implicated in the lysosomal storage disorder Pompe disease [173]. This enzyme and GCase are both glycoside hydrolase enzymes and have similar structures. We are currently conducting a clinical trial to examine whether oral N-acetylcysteine can alter measures of oxidative stress and inflammation in the blood and brain of patients with GD1 (ClinicalTrials.gov Identifier: ) [174]. Further, our preliminary investigations in GD1 fibroblasts show increased GCase activity following N-acetylcysteine exposure, demonstrating the potential chaperone activity of this antioxidant (unpublished results). It will be prudent to also examine its effect on α-synuclein levels to evaluate its benefit for GBA-PD. Nevertheless, these small molecules present a promising avenue for future drug development and warrant further study.
5. Knowledge Gaps in the Field
While multiple studies have demonstrated increased levels of α-synuclein within plasma exosomes, more research is required to further confirm its potential as a biomarker as well as eventually determine its predictability and utility for clinical use. One initial issue that needs to be solved is how to best isolate exosomes, as there is still considerable debate on the most effective protocol [126]. Additionally, there are multiple methodologies for isolating CNS-derived exosomes, and more work needs to be done to determine what methodology provides the most consistent isolation [114, 142]. On this note, results from Cerri et al. suggest that it may not be necessary to isolate CNS-derived exosomes [144]. Future research should investigate whether measuring α-synuclein in CNS-derived exosomes provides benefit over total plasma exosomes. It would also be beneficial to investigate plasma exosomal α-synuclein levels in patients with GD, GBA1 carriers, and GBA-PD compared to iPD. If similar levels are observed in these groups, the next step would be a longitudinal study evaluating exosomal α-synuclein levels in middle-adulthood GBA1 carriers (40–50 years old) and following them for 20–30 years to see if high initial levels predict PD development. This could provide an avenue for early treatment of PD in these populations and would justify the need to monitor these levels in the general population as well. Lastly, other pathologies have been shown to cause measurable changes in exosomal content [119]. Further investigation into exosomal protein and lipid concentrations in various disease states is needed, as it may provide avenues for future biomarker research.
5. Conclusion
Although the mechanistic relationship between GBA1 mutations and PD development is not yet fully understood, current research is beginning to uncover the underlying biochemistry and pathophysiology. So far, it appears that dysfunctional GCase can trigger interactions between a number of different pathways, culminating in PD. With the advent of new technologies and increasing interdisciplinary collaborations, researchers will continue to expand the knowledge of the complex biology behind these two different disease states. As personal genetic information becomes readily available to patients and their providers, a tailored approach can be taken to manage their total healthcare and specific morbidities. Hence it is imperative for individuals with GBA1 mutations as well as those with iPD that reliable, sensitive, inexpensive and less invasive GBA-PD biomarkers be discovered. Drugs designed to modulate GCase activity also present a unique opportunity for personalized or patient centric treatments, acting to specifically treat GBA-PD. Although this is a relatively new area of research, many great strides have already been made, and the future looks promising for this population.
Limitations
We have attempted to provide a concise review of this highly heterogenous topic. Each section of this paper warrants its own review article and thus only relevant research was discussed. With the long history of PD research, and rapidly appearing new developments, we realize that some remarkable studies were likely either overlooked or not cited. The purpose of this article is to give an overview of our best current understanding of how GBA1 mutations alter α-synuclein metabolism and its implications for detection, treatment, and prevention of GBA-PD. Additional information, such as other potential biomarkers or a more in-depth physiology discussion, was not included to maintain the focus of this review.
Acknowledgements
Authors acknowledge funding from the Lysosomal Disease Network fellowship to RVK. The Lysosomal Disease Network (U54NS065768) is a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), and National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS). This consortium is funded through a collaboration between NCATS, NINDS, and NIDDK. This research was also supported by the NCATS, grant UL1TR002494 that offered a scholarship to PJ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s NCATS.
RVK have received grants from NIH, Pfizer Inc. and Sanofi-Genzyme outside of this work.
JCC have received grants from NIH, Pfizer Inc. and Sanofi-Genzyme outside of this work.
PJT has received grants from NIH, Parkinson Study Group, Northwestern University, Biogen Inc., and Bristol-Myers Squibb
NJW has received grants from Sanofi-Genzyme and Takeda-Shire, personal fees from Sanofi-Genzyme, Takeda-Shire and Pfizer Inc, and non-financial support from Sanofi-Genzyme.
Abbreviations
- GD
Gaucher Disease
- PD
Parkinson’s Disease
- GCase
Glucocerebrosidase
- GlcCer
Glucosylceramide
- GlcSph
Glucosylsphingosine
- GD1
Type 1 Gaucher Disease
- CNS
Central Nervous System
- iPD
Idiopathic Parkinson’s Disease
- GBA-PD
GBA-associated Parkinson’s Disease
- ER
Endoplasmic Reticulum
- UPS
Ubiquitin-proteasome System
- ERAD
Endoplasmic Reticulum Associated Degradation
- ROS
Reactive Oxygen Species
- CSF
Cerebrospinal Fluid
- RBC
Red Blood Cell
- EV
Extracellular Vesicles
- MVB
Multivesicular Body
- BBB
Blood-brain-barrier
- ERT
Enzyme Replacement Therapy
- SRT
Substrate Reduction Therapy
- ABX
Ambroxol
Footnotes
Disclosures:
PJ declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler E (1992) Gaucher disease: new molecular approaches to diagnosis and treatment. Science 256:794–799 [DOI] [PubMed] [Google Scholar]
- 2.Grabowski GA (2008) Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 372:1263–1271. 10.1016/S0140-6736(08)61522-6 [DOI] [PubMed] [Google Scholar]
- 3.Pastores GM, Hughes DA (1993) Gaucher Disease In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews®. University of Washington, Seattle, Seattle (WA) [PubMed] [Google Scholar]
- 4.Nalysnyk L, Rotella P, Simeone JC, et al. (2017) Gaucher disease epidemiology and natural history: a comprehensive review of the literature. Hematology 22:65–73. 10.1080/10245332.2016.1240391 [DOI] [PubMed] [Google Scholar]
- 5.Goker-Alpan O, Schiffmann R, LaMarca ME, et al. (2004) Parkinsonism among Gaucher disease carriers. J Med Genet 41:937–940. 10.1136/jmg.2004.024455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 7.Malek N, Swallow D, Grosset KA, et al. (2014) Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease - a systematic review. Acta Neurol Scand 130:59–72. 10.1111/ane.12247 [DOI] [PubMed] [Google Scholar]
- 8.Shahmoradian SH, Lewis AJ, Genoud C, et al. (2019) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. 10.1038/s41593-019-0423-2 [DOI] [PubMed] [Google Scholar]
- 9.Shi M, Sheng L, Stewart T, et al. (2019) New windows into the brain: Central nervous system-derived extracellular vesicles in blood. Prog Neurobiol 175:96–106. 10.1016/j.pneurobio.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Regan G, deSouza R-M, Balestrino R, Schapira AH (2017) Glucocerebrosidase Mutations in Parkinson Disease. J Parkinsons Dis 7:411–422. 10.3233/JPD-171092 [DOI] [PubMed] [Google Scholar]
- 11.Fairley C, Zimran A, Phillips M, et al. (2008) Phenotypic heterogeneity of N370S homozygotes with type I Gaucher disease: an analysis of 798 patients from the ICGG Gaucher Registry. J Inherit Metab Dis 31:738–744. 10.1007/s10545-008-0868-z [DOI] [PubMed] [Google Scholar]
- 12.Stirnemann J, Belmatoug N, Camou F, et al. (2017) A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int J Mol Sci 18:. 10.3390/ijms18020441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koprivica V, Stone DL, Park JK, et al. (2000) Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet 66:1777–1786. 10.1086/302925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Shu L, Sun Q, et al. (2018) Integrated Genetic Analysis of Racial Differences of Common GBA Variants in Parkinson’s Disease: A Meta-Analysis. Front Mol Neurosci 11:43 10.3389/fnmol.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migdalska-Richards A, Schapira AHV (2016) The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 139 Suppl 1:77–90. 10.1111/jnc.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastores GM (1997) Gaucher’s Disease. Pathological features. Baillieres Clin Haematol 10:739–749 [DOI] [PubMed] [Google Scholar]
- 17.Neudorfer O, Giladi N, Elstein D, et al. (1996) Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM 89:691–694 [DOI] [PubMed] [Google Scholar]
- 18.Bembi B, Zambito Marsala S, Sidransky E, et al. (2003) Gaucher’s disease with Parkinson’s disease: clinical and pathological aspects. Neurology 61:99–101 [DOI] [PubMed] [Google Scholar]
- 19.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W (1999) Parkinson’s syndrome preceding clinical manifestation of Gaucher’s disease. Am J Hematol 61:216–217 [DOI] [PubMed] [Google Scholar]
- 20.Lwin A, Orvisky E, Goker-Alpan O, et al. (2004) Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab 81:70–73 [DOI] [PubMed] [Google Scholar]
- 21.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R (2004) Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med 351:1972–1977. 10.1056/NEJMoa033277 [DOI] [PubMed] [Google Scholar]
- 22.Halperin A, Elstein D, Zimran A (2006) Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis 36:426–428. 10.1016/j.bcmd.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 23.Neumann J, Bras J, Deas E, et al. (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132:1783–1794. 10.1093/brain/awp044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidransky E, Nalls MA, Aasly JO, et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361:1651–1661. 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tayebi N, Callahan M, Madike V, et al. (2001) Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab 73:313–321. 10.1006/mgme.2001.3201 [DOI] [PubMed] [Google Scholar]
- 26.Gan-Or Z, Amshalom I, Kilarski LL, et al. (2015) Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 84:880–887. 10.1212/WNL.0000000000001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Sun Q-Y, Zhao Y-W, et al. (2015) Effect of GBA Mutations on Phenotype of Parkinson’s Disease: A Study on Chinese Population and a Meta-Analysis. Parkinsons Dis 2015:916971 10.1155/2015/916971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark LN, Ross BM, Wang Y, et al. (2007) Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology 69:1270–1277. 10.1212/01.wnl.0000276989.17578.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böttcher T, Rolfs A, Meyer B, et al. (2013) Clinical, genetic, and brain sonographic features related to Parkinson’s disease in Gaucher disease. J Neurol 260:2523–2531. 10.1007/s00415-013-7011-2 [DOI] [PubMed] [Google Scholar]
- 30.Oeda T, Umemura A, Mori Y, et al. (2015) Impact of glucocerebrosidase mutations on motor and nonmotor complications in Parkinson’s disease. Neurobiol Aging 36:3306–3313. 10.1016/j.neurobiolaging.2015.08.027 [DOI] [PubMed] [Google Scholar]
- 31.Barrett MJ, Shanker VL, Severt WL, et al. (2014) Cognitive and Antipsychotic Medication Use in Monoallelic GBA-Related Parkinson Disease. JIMD Rep 16:31–38. 10.1007/8904_2014_315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thaler A, Bregman N, Gurevich T, et al. (2018) Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 10.1016/j.parkreldis.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Cilia R, Tunesi S, Marotta G, et al. (2016) Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann Neurol 80:662–673. 10.1002/ana.24777 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbloom B, Balwani M, Bronstein JM, et al. (2011) The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis 46:95–102. 10.1016/j.bcmd.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiglieri V, Calabrese V, Calabresi P (2018) Alpha-Synuclein: From Early Synaptic Dysfunction to Neurodegeneration. Front Neurol 9:295 10.3389/fneur.2018.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakai M, Fujita M, Waragai M, et al. (2007) Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun 358:104–110. 10.1016/j.bbrc.2007.04.108 [DOI] [PubMed] [Google Scholar]
- 37.Shi M, Zabetian CP, Hancock AM, et al. (2010) Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neuroscience Letters 480:78–82. 10.1016/j.neulet.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breydo L, Wu JW, Uversky VN (2012) Α-synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta 1822:261–285. 10.1016/j.bbadis.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 39.Dehay B, Bourdenx M, Gorry P, et al. (2015) Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14:855–866. 10.1016/S1474-4422(15)00006-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benskey MJ, Sellnow RC, Sandoval IM, et al. (2018) Silencing Alpha Synuclein in Mature Nigral Neurons Results in Rapid Neuroinflammation and Subsequent Toxicity. Front Mol Neurosci 11:36 10.3389/fnmol.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bar R, Boehm-Cagan A, Luz I, et al. (2018) The effects of apolipoprotein E genotype, α-synuclein deficiency, and sex on brain synaptic and Alzheimer’s disease-related pathology. Alzheimers Dement (Amst) 10:1–11. 10.1016/j.dadm.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abeliovich A, Schmitz Y, Fariñas I, et al. (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252 [DOI] [PubMed] [Google Scholar]
- 43.Balestrino R, Schapira AHV (2018) Glucocerebrosidase and Parkinson Disease: Molecular, Clinical, and Therapeutic Implications. Neuroscientist 1073858417748875 10.1177/1073858417748875 [DOI] [PubMed] [Google Scholar]
- 44.Cullen V, Sardi SP, Ng J, et al. (2011) Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol 69:940–953. 10.1002/ana.22400 [DOI] [PubMed] [Google Scholar]
- 45.Mazzulli JR, Xu Y-H, Sun Y, et al. (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146:37–52. 10.1016/j.cell.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manning-Boğ AB, Schüle B, Langston JW (2009) Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology 30:1127–1132. 10.1016/j.neuro.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 47.Bae E-J, Yang NY, Lee C, et al. (2015) Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp Mol Med 47:e153 10.1038/emm.2014.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha EM, Smith GA, Park E, et al. (2015) Sustained Systemic Glucocerebrosidase Inhibition Induces Brain α-Synuclein Aggregation, Microglia and Complement C1q Activation in Mice. Antioxid Redox Signal 23:550–564. 10.1089/ars.2015.6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sardi SP, Clarke J, Viel C, et al. (2013) Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci USA 110:3537–3542. 10.1073/pnas.1220464110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha EM, Smith GA, Park E, et al. (2015) Glucocerebrosidase gene therapy prevents α-synucleinopathy of midbrain dopamine neurons. Neurobiology of Disease 82:495–503. 10.1016/j.nbd.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 51.Westbroek W, Nguyen M, Siebert M, et al. (2016) A new glucocerebrosidase-deficient neuronal cell model provides a tool to probe pathophysiology and therapeutics for Gaucher disease. Dis Model Mech 9:769–778. 10.1242/dmm.024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farfel-Becker T, Vitner EB, Kelly SL, et al. (2014) Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum Mol Genet 23:843–854. 10.1093/hmg/ddt468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taguchi YV, Liu J, Ruan J, et al. (2017) Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J Neurosci 37:9617–9631. 10.1523/JNEUROSCI.1525-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning-Bog AB, McCormack AL, Li J, et al. (2002) The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem 277:1641–1644. 10.1074/jbc.C100560200 [DOI] [PubMed] [Google Scholar]
- 55.Tamboli IY, Hampel H, Tien NT, et al. (2011) Sphingolipid storage affects autophagic metabolism of the amyloid precursor protein and promotes Abeta generation. J Neurosci 31:1837–1849. 10.1523/JNEUROSCI.2954-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gegg ME, Sweet L, Wang BH, et al. (2015) No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov Disord 30:1085–1089. 10.1002/mds.26278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schöndorf DC, Aureli M, McAllister FE, et al. (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028 10.1038/ncomms5028 [DOI] [PubMed] [Google Scholar]
- 58.Fernandes HJR, Hartfield EM, Christian HC, et al. (2016) ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s iPSC-Derived Dopamine Neurons. Stem Cell Reports 6:342–356. 10.1016/j.stemcr.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sardi P, Liu G, Scherzer A, et al. Glucosylceramide Blood Test for GBA-associated Parkinson’s Disease: A Potential Companion For Precision Therapeutics. AD/PD 2019. program [Google Scholar]
- 60.Rocha EM, Smith GA, Park E, et al. (2015) Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann Clin Transl Neurol 2:433–438. 10.1002/acn3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MJ, Jeon S, Burbulla LF, Krainc D (2018) Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function. Hum Mol Genet 27:1972–1988. 10.1093/hmg/ddy105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ron I, Horowitz M (2005) ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet 14:2387–2398. 10.1093/hmg/ddi240 [DOI] [PubMed] [Google Scholar]
- 63.Ron I, Rapaport D, Horowitz M (2010) Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet 19:3771–3781. 10.1093/hmg/ddq292 [DOI] [PubMed] [Google Scholar]
- 64.Bendikov-Bar I, Rapaport D, Larisch S, Horowitz M (2014) Parkin-mediated ubiquitination of mutant glucocerebrosidase leads to competition with its substrates PARIS and ARTS. Orphanet J Rare Dis 9:86 10.1186/1750-1172-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bendikov-Bar I, Ron I, Filocamo M, Horowitz M (2011) Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis 46:4–10. 10.1016/j.bcmd.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 66.Kilpatrick BS, Magalhaes J, Beavan MS, et al. (2016) Endoplasmic reticulum and lysosomal Ca2+ stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium 59:12–20. 10.1016/j.ceca.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E (2010) Glucocerebrosidase is present in α-synuclein inclusions in Lewy body disorders. Acta Neuropathol 120:641–649. 10.1007/s00401-010-0741-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gan-Or Z, Giladi N, Rozovski U, et al. (2008) Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 70:2277–2283. 10.1212/01.wnl.0000304039.11891.29 [DOI] [PubMed] [Google Scholar]
- 69.Alcalay RN, Levy OA, Waters CC, et al. (2015) Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain 138:2648–2658. 10.1093/brain/awv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy KE, Gysbers AM, Abbott SK, et al. (2014) Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 137:834–848. 10.1093/brain/awt367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pchelina S, Emelyanov A, Baydakova G, et al. (2017) Oligomeric α-synuclein and glucocerebrosidase activity levels in GBA-associated Parkinson’s disease. Neurosci Lett 636:70–76. 10.1016/j.neulet.2016.10.039 [DOI] [PubMed] [Google Scholar]
- 72.Nuzhnyi E, Emelyanov A, Boukina T, et al. (2015) Plasma oligomeric alpha-synuclein is associated with glucocerebrosidase activity in Gaucher disease. Mov Disord 30:989–991. 10.1002/mds.26200 [DOI] [PubMed] [Google Scholar]
- 73.Richter F, Fleming SM, Watson M, et al. (2014) A GCase chaperone improves motor function in a mouse model of synucleinopathy. Neurotherapeutics 11:840–856. 10.1007/s13311-014-0294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rockenstein E, Clarke J, Viel C, et al. (2016) Glucocerebrosidase modulates cognitive and motor activities in murine models of Parkinson’s disease. Hum Mol Genet 25:2645–2660. 10.1093/hmg/ddw124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papagiannakis N, Xilouri M, Koros C, et al. (2015) Lysosomal alterations in peripheral blood mononuclear cells of Parkinson’s disease patients. Mov Disord 30:1830–1834. 10.1002/mds.26433 [DOI] [PubMed] [Google Scholar]
- 76.Kim H-J, Jeon B, Song J, et al. (2016) Leukocyte glucocerebrosidase and β-hexosaminidase activity in sporadic and genetic Parkinson disease. Parkinsonism Relat Disord 23:99–101. 10.1016/j.parkreldis.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 77.Alcalay RN, Wolf P, Levy OA, et al. (2018) Alpha galactosidase A activity in Parkinson’s disease. Neurobiol Dis 112:85–90. 10.1016/j.nbd.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robak LA, Jansen IE, van Rooij J, et al. (2017) Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140:3191–3203. 10.1093/brain/awx285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de la Mata M, Cotán D, Villanueva-Paz M, et al. (2016) Mitochondrial Dysfunction in Lysosomal Storage Disorders. Diseases 4:. 10.3390/diseases4040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zancan I, Bellesso S, Costa R, et al. (2015) Glucocerebrosidase deficiency in zebrafish affects primary bone ossification through increased oxidative stress and reduced Wnt/β-catenin signaling. Hum Mol Genet 24:1280–1294. 10.1093/hmg/ddu538 [DOI] [PubMed] [Google Scholar]
- 81.Burchell VS, Gandhi S, Deas E, et al. (2010) Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin Ther Targets 14:369–385. 10.1517/14728221003652489 [DOI] [PubMed] [Google Scholar]
- 82.Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253. 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osellame LD, Duchen MR (2013) Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy 9:1633–1635. 10.4161/auto.25878 [DOI] [PubMed] [Google Scholar]
- 84.Luth ES, Stavrovskaya IG, Bartels T, et al. (2014) Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem 289:21490–21507. 10.1074/jbc.M113.545749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scudamore O, Ciossek T (2018) Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J Neuropathol Exp Neurol 77:443–453. 10.1093/jnen/nly024 [DOI] [PubMed] [Google Scholar]
- 86.Gegg ME, Schapira AHV (2016) Mitochondrial dysfunction associated with glucocerebrosidase deficiency. Neurobiol Dis 90:43–50. 10.1016/j.nbd.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson’s disease. Lancet Neurol 5:75–86. 10.1016/S1474-4422(05)70285-4 [DOI] [PubMed] [Google Scholar]
- 88.Moors T, Paciotti S, Chiasserini D, et al. (2016) Lysosomal Dysfunction and α-Synuclein Aggregation in Parkinson’s Disease: Diagnostic Links. Mov Disord 31:791–801. 10.1002/mds.26562 [DOI] [PubMed] [Google Scholar]
- 89.Mollenhauer B, Trautmann E, Taylor P, et al. (2013) Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 532:44–48. 10.1016/j.neulet.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 90.Kang J-H, Irwin DJ, Chen-Plotkin AS, et al. (2013) Association of cerebrospinal fluid β-amyloid 1–42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 70:1277–1287. 10.1001/jamaneurol.2013.3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong Z, Shi M, Chung KA, et al. (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133:713–726. 10.1093/brain/awq008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohrfelt A, Grognet P, Andreasen N, et al. (2009) Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett 450:332–335. 10.1016/j.neulet.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 93.Park MJ, Cheon S-M, Bae H-R, et al. (2011) Elevated levels of α-synuclein oligomer in the cerebrospinal fluid of drug-naïve patients with Parkinson’s disease. J Clin Neurol 7:215–222. 10.3988/jcn.2011.7.4.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foulds PG, Yokota O, Thurston A, et al. (2012) Post mortem cerebrospinal fluid α-synuclein levels are raised in multiple system atrophy and distinguish this from the other α-synucleinopathies, Parkinson’s disease and Dementia with Lewy bodies. Neurobiol Dis 45:188–195. 10.1016/j.nbd.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao L, Tang H, Nie K, et al. (2015) Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int J Neurosci 125:645–654. 10.3109/00207454.2014.961454 [DOI] [PubMed] [Google Scholar]
- 96.Gorostidi A, Bergareche A, Ruiz-Martínez J, et al. (2012) Αlpha-synuclein levels in blood plasma from LRRK2 mutation carriers. PLoS ONE 7:e52312 10.1371/journal.pone.0052312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duran R, Barrero FJ, Morales B, et al. (2010) Plasma alpha-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord 25:489–493. 10.1002/mds.22928 [DOI] [PubMed] [Google Scholar]
- 98.Lee PH, Lee G, Park HJ, et al. (2006) The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J Neural Transm (Vienna) 113:1435–1439. 10.1007/s00702-005-0427-9 [DOI] [PubMed] [Google Scholar]
- 99.Foulds PG, Diggle P, Mitchell JD, et al. (2013) A longitudinal study on α-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci Rep 3:2540 10.1038/srep02540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foulds PG, Mitchell JD, Parker A, et al. (2011) Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J 25:4127–4137. 10.1096/fj.10-179192 [DOI] [PubMed] [Google Scholar]
- 101.Malec-Litwinowicz M, Plewka A, Plewka D, et al. (2017) The relation between plasma α-synuclein level and clinical symptoms or signs of Parkinson’s disease. Neurologia i Neurochirurgia Polska. 10.1016/j.pjnns.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 102.Caranci G, Piscopo P, Rivabene R, et al. (2013) Gender differences in Parkinson’s disease: focus on plasma α-synuclein. J Neural Transm (Vienna) 120:1209–1215. 10.1007/s00702-013-0972-6 [DOI] [PubMed] [Google Scholar]
- 103.El-Agnaf OMA, Salem SA, Paleologou KE, et al. (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J 20:419–425. 10.1096/fj.03-1449com [DOI] [PubMed] [Google Scholar]
- 104.Barbour R, Kling K, Anderson JP, et al. (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5:55–59. 10.1159/000112832 [DOI] [PubMed] [Google Scholar]
- 105.Vicente Miranda H, Cássio R, Correia-Guedes L, et al. (2017) Posttranslational modifications of blood-derived alpha-synuclein as biochemical markers for Parkinson’s disease. Sci Rep 7:13713 10.1038/s41598-017-14175-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Yu S, Li F, Feng T (2015) Detection of α-synuclein oligomers in red blood cells as a potential biomarker of Parkinson’s disease. Neurosci Lett 599:115–119. 10.1016/j.neulet.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 107.Pchelina SN, Nuzhnyi EP, Emelyanov AK, et al. (2014) Increased plasma oligomeric alpha-synuclein in patients with lysosomal storage diseases. Neurosci Lett 583:188–193. 10.1016/j.neulet.2014.09.041 [DOI] [PubMed] [Google Scholar]
- 108.Papagiannakis N, Koros C, Stamelou M, et al. (2018) Alpha-synuclein dimerization in erythrocytes of patients with genetic and non-genetic forms of Parkinson’s Disease. Neurosci Lett 672:145–149. 10.1016/j.neulet.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 109.Argyriou A, Dermentzaki G, Papasilekas T, et al. (2012) Increased dimerization of alpha-synuclein in erythrocytes in Gaucher disease and aging. Neuroscience Letters 528:205–209. 10.1016/j.neulet.2012.08.069 [DOI] [PubMed] [Google Scholar]
- 110.Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baixauli F, López-Otín C, Mittelbrunn M (2014) Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5:403 10.3389/fimmu.2014.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harding CV, Heuser JE, Stahl PD (2013) Exosomes: looking back three decades and into the future. J Cell Biol 200:367–371. 10.1083/jcb.201212113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beer KB, Wehman AM (2017) Mechanisms and functions of extracellular vesicle release in vivo-What we can learn from flies and worms. Cell Adh Migr 11:135–150. 10.1080/19336918.2016.1236899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mustapic M, Eitan E, Werner JK, et al. (2017) Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci 11:278 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 93:313–340. 10.1016/j.pneurobio.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piper RC, Katzmann DJ (2007) Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23:519–547. 10.1146/annurev.cellbio.23.090506.123319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kowal J, Tkach M, Théry C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125. 10.1016/j.ceb.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 118.Chan BD, Wong W-Y, Lee MM-L, et al. (2019) Exosomes in inflammation and inflammatory disease. Proteomics e1800149 10.1002/pmic.201800149 [DOI] [PubMed] [Google Scholar]
- 119.Sharma P, Mesci P, Carromeu C, et al. (2019) Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA. 10.1073/pnas.1902513116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalani A, Tyagi A, Tyagi N (2014) Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 49:590–600. 10.1007/s12035-013-8544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fiandaca MS, Kapogiannis D, Mapstone M, et al. (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11:600–607.e1. 10.1016/j.jalz.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kapogiannis D, Boxer A, Schwartz JB, et al. (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29:589–596. 10.1096/fj.14-262048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goetzl EJ, Boxer A, Schwartz JB, et al. (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85:40–47. 10.1212/WNL.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harischandra DS, Rokad D, Neal ML, et al. (2019) Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci Signal 12:. 10.1126/scisignal.aau4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Athauda D, Gulyani S, Karnati H, et al. (2019) Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial. JAMA Neurol. 10.1001/jamaneurol.2018.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li P, Kaslan M, Lee SH, et al. (2017) Progress in Exosome Isolation Techniques. Theranostics 7:789–804. 10.7150/thno.18133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Desplats P, Lee H-J, Bae E-J, et al. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106:13010–13015. 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ulusoy A, Musgrove RE, Rusconi R, et al. (2015) Neuron-to-neuron α-synuclein propagation in vivo is independent of neuronal injury. Acta Neuropathol Commun 3:13 10.1186/s40478-015-0198-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luk KC, Kehm V, Carroll J, et al. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee H-J, Patel S, Lee S-J (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25:6016–6024. 10.1523/JNEUROSCI.0692-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grey M, Dunning CJ, Gaspar R, et al. (2015) Acceleration of α-synuclein aggregation by exosomes. J Biol Chem 290:2969–2982. 10.1074/jbc.M114.585703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Danzer KM, Kranich LR, Ruf WP, et al. (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42 10.1186/1750-1326-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alvarez-Erviti L, Seow Y, Schapira AH, et al. (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42:360–367. 10.1016/j.nbd.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eitan E, Suire C, Zhang S, Mattson MP (2016) Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev 32:65–74. 10.1016/j.arr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Papadopoulos VE, Nikolopoulou G, Antoniadou I, et al. (2018) Modulation of β-glucocerebrosidase increases α-synuclein secretion and exosome release in mouse models of Parkinson’s disease. Hum Mol Genet 27:1696–1710. 10.1093/hmg/ddy075 [DOI] [PubMed] [Google Scholar]
- 136.Bae E-J, Yang N-Y, Song M, et al. (2014) Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun 5:4755 10.1038/ncomms5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thomas RE, Vincow ES, Merrihew GE, et al. (2018) Glucocerebrosidase deficiency promotes protein aggregation through dysregulation of extracellular vesicles. PLoS Genet 14:e1007694 10.1371/journal.pgen.1007694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Halliday GM, Stevens CH (2011) Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord 26:6–17. 10.1002/mds.23455 [DOI] [PubMed] [Google Scholar]
- 139.Chang C, Lang H, Geng N, et al. (2013) Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett 548:190–195. 10.1016/j.neulet.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 140.Khandelwal PJ, Herman AM, Moussa CE-H (2011) Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol 238:1–11. 10.1016/j.jneuroim.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chung CY, Koprich JB, Siddiqi H, Isacson O (2009) Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 29:3365–3373. 10.1523/JNEUROSCI.5427-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi M, Liu C, Cook TJ, et al. (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128:639–650. 10.1007/s00401-014-1314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Z-H, Chen Z-T, Zhou R-L, et al. (2018) Increased DJ-1 and α-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front Aging Neurosci 10:438 10.3389/fnagi.2018.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cerri S, Ghezzi C, Sampieri M, et al. (2018) The Exosomal/Total α-Synuclein Ratio in Plasma Is Associated With Glucocerebrosidase Activity and Correlates With Measures of Disease Severity in PD Patients. Front Cell Neurosci 12:125 10.3389/fncel.2018.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Si X, Tian J, Chen Y, et al. (2019) Central Nervous System-Derived Exosomal Alpha-Synuclein in Serum May Be a Biomarker in Parkinson’s Disease. Neuroscience. 10.1016/j.neuroscience.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 146.Franco R, Sánchez-Arias JA, Navarro G, Lanciego JL (2018) Glucocerebrosidase Mutations and Synucleinopathies. Potential Role of Sterylglucosides and Relevance of Studying Both GBA1 and GBA2 Genes. Front Neuroanat 12:52 10.3389/fnana.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chetrit EB, Alcalay RN, Steiner-Birmanns B, et al. (2013) Phenotype in patients with Gaucher disease and Parkinson disease. Blood Cells Mol Dis 50:218–221. 10.1016/j.bcmd.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 148.Cabrera-Salazar MA, Bercury SD, Ziegler RJ, et al. (2010) Intracerebroventricular delivery of glucocerebrosidase reduces substrates and increases lifespan in a mouse model of neuronopathic Gaucher disease. Exp Neurol 225:436–444. 10.1016/j.expneurol.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 149.Choi K, Choi H, Yim N, et al. (2018) Exosome-based delivery of glucocerebrosidase lysosomal enzyme for treatment of Gaucher disease. Molecular Genetics and Metabolism 123:S31–S32. 10.1016/j.ymgme.2017.12.060 [DOI] [Google Scholar]
- 150.Zunke F, Moise AC, Belur NR, et al. (2017) Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron 10.1016/j.neuron.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim S, Yun SP, Lee S, et al. (2018) GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc Natl Acad Sci USA 115:798–803. 10.1073/pnas.1700465115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Larsen SD, Wilson MW, Abe A, et al. (2012) Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J Lipid Res 53:282–291. 10.1194/jlr.M021261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Platt FM, Jeyakumar M, Andersson U, et al. (2003) Substrate reduction therapy in mouse models of the glycosphingolipidoses. Philos Trans R Soc Lond, B, Biol Sci 358:947–954. 10.1098/rstb.2003.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marshall J, Sun Y, Bangari DS, et al. (2016) CNS-accessible Inhibitor of Glucosylceramide Synthase for Substrate Reduction Therapy of Neuronopathic Gaucher Disease. Mol Ther 24:1019–1029. 10.1038/mt.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sardi SP, Cedarbaum JM, Brundin P (2018) Targeted Therapies for Parkinson’s Disease: From Genetics to the Clinic. Mov Disord 33:684–696. 10.1002/mds.27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lieberman RL, Wustman BA, Huertas P, et al. (2007) Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol 3:101–107. 10.1038/nchembio850 [DOI] [PubMed] [Google Scholar]
- 157.Zheng W, Padia J, Urban DJ, et al. (2007) Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci USA 104:13192–13197. 10.1073/pnas.0705637104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jung O, Patnaik S, Marugan J, et al. (2016) Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev Proteomics 13:471–479. 10.1080/14789450.2016.1174583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khanna R, Benjamin ER, Pellegrino L, et al. (2010) The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. FEBS J 277:1618–1638. 10.1111/j.1742-4658.2010.07588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun Y, Ran H, Liou B, et al. (2011) Isofagomine in vivo effects in a neuronopathic Gaucher disease mouse. PLoS ONE 6:e19037 10.1371/journal.pone.0019037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maegawa GHB, Tropak MB, Buttner JD, et al. (2009) Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem 284:23502–23516. 10.1074/jbc.M109.012393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McNeill A, Magalhaes J, Shen C, et al. (2014) Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 137:1481–1495. 10.1093/brain/awu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanchez-Martinez A, Beavan M, Gegg ME, et al. (2016) Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci Rep 6:31380 10.1038/srep31380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Migdalska-Richards A, Ko WKD, Li Q, et al. (2017) Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse 71:. 10.1002/syn.21967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zimran A, Altarescu G, Elstein D (2013) Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells, Molecules, and Diseases 50:134–137. 10.1016/j.bcmd.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 166.Narita A, Shirai K, Itamura S, et al. (2016) Ambroxol chaperone therapy for neuronopathic Gaucher disease: A pilot study. Ann Clin Transl Neurol 3:200–215. 10.1002/acn3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Magalhaes J, Gegg ME, Migdalska-Richards A, Schapira AH (2018) Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons. Sci Rep 8:1385 10.1038/s41598-018-19479-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang S-Y, Beavan M, Chau K-Y, et al. (2017) A Human Neural Crest Stem Cell-Derived Dopaminergic Neuronal Model Recapitulates Biochemical Abnormalities in GBA1 Mutation Carriers. Stem Cell Reports 8:728–742. 10.1016/j.stemcr.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goldin E, Zheng W, Motabar O, et al. (2012) High throughput screening for small molecule therapy for Gaucher disease using patient tissue as the source of mutant glucocerebrosidase. PLoS ONE 7:e29861 10.1371/journal.pone.0029861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aflaki E, Borger DK, Moaven N, et al. (2016) A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J Neurosci 36:7441–7452. 10.1523/JNEUROSCI.0636-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mazzulli JR, Zunke F, Tsunemi T, et al. (2016) Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. J Neurosci 36:7693–7706. 10.1523/JNEUROSCI.0628-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aflaki E, Stubblefield BK, Maniwang E, et al. (2014) Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med 6:240ra73 10.1126/scitranslmed.3008659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Porto C, Ferrara MC, Meli M, et al. (2012) Pharmacological enhancement of α-glucosidase by the allosteric chaperone N-acetylcysteine. Mol Ther 20:2201–2211. 10.1038/mt.2012.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kartha RV, Joers J, Tuite P, et al. (2017) Role of oxidative stress and inflammation in type 1 Gaucher disease (GD1): Potential use of antioxidant/anti-inflammatory medications. Molecular Genetics and Metabolism 120:S74 10.1016/j.ymgme.2016.11.175 [DOI] [Google Scholar]