Abstract
Mesenchymal stromal cells (MSCs) have potent immunomodulatory abilities to regulate most of the immune cells. Not only the tissue origin of MSCs can affect their functions, but also their microenvironment can strongly influence their biology, particularly through toll-like receptors (TLR)/ligands interaction. In the present study, we compared MSCs derived from two different sources, i.e. human olfactory ecto-mesenchymal stem cells (OE-MSCs) and adipose tissue (AT-MSCs), in terms of their immunosuppressive effects before and after TLR3 and TLR4 stimulation through low-level and short-term TLR-priming protocol. After isolation and characterization of OE-MSCs and AT-MSCs, flow cytometry analyses were used to assess the expression of TLR3, TLR4 by MSCs. Secretion and expression levels of immune-related genes were analyzed using ELISA and RT-qPCR techniques. Based on the results, the proliferation potential of OE-MSCs was significantly higher than that of AT-MSCs. The gene expression and also protein levels of both TLR3 and TLR4 were significantly higher in OE-MSCs, compared to AT-MSCs. Among the examined cytokines and chemokines, OE-MSCs exhibited significantly higher levels of CCL5, IL-8, and TGF-β production, in comparison with AT-MSCs. However, IL-6 secretion by AT-MSCs was considerably more than that by OE-MSCs. OE-MSCs were only affected by the TLR4 ligand, and IL-8 and IL-6 production levels increased after LPS treatment. However, only IL-8 significantly increased after adding LPS or Poly (I:C) to the AT-MSC media. According to the obtained data, OE-MSCs exhibited a higher proliferative potential and greater expression levels of TLR3 and TLR4 genes, compared to AT-MSCs. However, unlike AT-MSCs, the expression of TLR3 by OE-MSCs was nonfunctional. Finally, based on our findings, OE-MSCs have a stronger secretion of immunosuppressive cytokines both before and after LPS or PIC treatment, compared to AT-MSCs.
Keywords: Mesenchymal stromal cells, Toll-like receptors, Human olfactory mucosa, Adipose tissue, Immunomodulation
Introduction
Mesenchymal stromal cells (MSCs) were first isolated from mouse bone marrow (BM) by Friedenstein et al. in the 1970s. The authors reported that the isolated mesenchymal cells were able to form colonies [fibroblast colony-forming units (CFU-F)] and differentiate into various mesodermal lineages including adipogenic, osteogenic and chondrogenic cells (Friedenstein et al. 1974). MSCs are multipotent cells that are considered attractive candidates for application in tissue regeneration and repair. Moreover, due to their progenitor characteristics, MSCs have desirable immunomodulatory capabilities that enable them to modulate most immune cells, particularly T lymphocytes (Bartholomew et al. 2002; Zappia et al. 2005). Recently, the reported expression of Toll-Like Receptors (TLRs) by MSCs has encouraged scientists and clinicians to study the potential link between TLR signaling and MSC-mediated immunoregulatory functions (Liotta et al. 2008). These immunologic characteristics make MSCs exciting choices for cellular therapy. In this regard, several previous studies on experimental models of inflammatory diseases have demonstrated an efficient protection against graft-versus-host disease, allograft rejection, collagen-induced arthritis, experimental autoimmune encephalomyelitis, autoimmune myocarditis, and sepsis (Baharlou et al. 2017; Gonzalez-Rey et al. 2009; González et al. 2009; Le Blanc et al. 2004; Németh et al. 2009; Ohnishi et al. 2007; Zappia et al. 2005).
Bone marrow (BM) represents the main source of human MSCs for both experimental and clinical studies. However, utilization of BM-derived MSCs is associated with several restrictions which have encouraged researchers to find new sources of MSCs, such as adipose, dental tissue, placenta, amniotic fluid, umbilical cord, Wharton’s jelly, and olfactory mucosa. Among these tissues, adult human adipose tissue that contains mesenchymal stem cells (AT-MSCs) has been considered as the best potential alternative. On the other hand, olfactory ecto-mesenchymal stem cells (OE-MSCs) have been characterized as new types of resident stem cells in the olfactory lamina propria (Delorme et al. 2009). This readily accessible peripheral tissue contains highly proliferative stem cells that eliminates all ethical concerns and also technical issues associated with other stem cell types (Girard et al. 2011; King and Perrin 2014). OE-MSCs are mainly derived from neural crest cells and exhibit a high proliferation rate, self-renewal capability and multiple lineage differentiation abilities (Hauser et al. 2011).
According to early experimental and preclinical studies, OE-MSC transplantation can induce neurogenesis and restore hippocampal neuronal networks (Nivet et al. 2011). However, the immunoregulatory properties of OE-MSCs have been poorly defined. The current study has been designed to investigate and compare the cytokine secretion of OE-MSCs and AT-MSCs, following stimulation of TLR3 and TLR4 via low-level and short-term TLR-priming protocols.
Materials and methods
All samples were obtained with informed consent. The study was approved by the Ethics Committee of Iran University of Medical Sciences (reference number: IR.IUMS.REC1395.9221126204) and performed according to the declaration of Helsinki.
Study population
Isolation and culture of human OE-MSCs and AT-MSCs
Olfactory mucosa (N = 8) and adipose tissue (N = 8) samples were obtained from discarded nasal mucosa and adipose tissue, respectively, during surgery of patients in Rasoul-e-Akram Hospital, Tehran, Iran. It is necessary to mention that samples were successfully collected from 10 patients. But during analysis, two sample were contaminated.
To isolate OE-MSCs, biopsies of nasal olfactory mucosa from the root of the medial aspect of middle turbinate or the septum in dorsomedial area were obtained from patients who referred to ENT and Head and Neck Research Center for skull base surgery. The samples were washed with Dulbecco modified Eagle medium/Ham F-12 (DMEM/F12) and digested for 1 to 2 h using 1 mL of collagenase IA (5 mg/mL) to complete tissue dissociation. After centrifugation, the cell pellet was re-suspended in DMEM/F12 culture medium supplemented with 15% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin (all obtained from Gibco, USA). The isolated cells were incubated at 37 °C, 5% CO2 under 95% humidified atmosphere. After 72 h, non-adherent cells were removed and adherent cells were thoroughly washed twice with PBS. The culture media was changed twice a week.
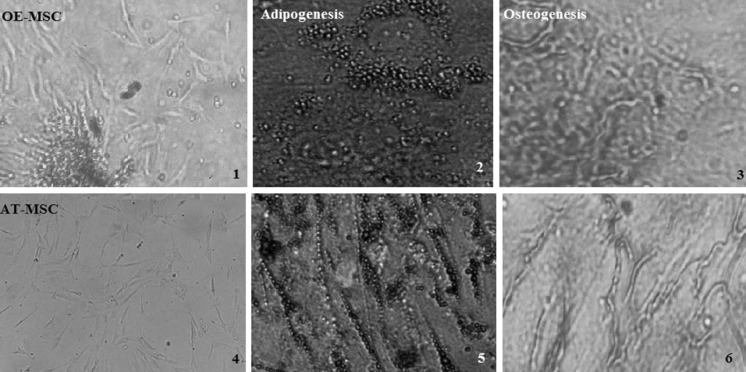
Adipose tissue samples were harvested from normal healthy patients undergoing elective cosmetic abdominoplasty. AT-MSCs were isolated according to a previously published protocol (Hanson et al. 2010). Briefly, subcutaneous adipose tissues were rinsed with phosphate-buffered saline (PBS), minced and then digested with collagenase IA (1 mg/mL) under gentle agitation for 90 min at 37 °C. The enzyme was deactivated with an equal volume of DMEM/F12 culture medium containing 15% FBS. After centrifugation at 400 RCF for 5 min, the supernatant was discarded and the pellet cells were re-suspended in DMEM/F12 supplemented with 15% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin. The results of full characterization of OE-MSCs and AT-MSC have been shown in Fig. 1.
Fig. 1.
MSCs were characterized by their differentiation potential to adipogenic and osteogenic lineages by Oil red O and Alizarin red S staining, respectively. 70% confluent MSCs at passage 3–5 from three individuals of each group were seeded and incubated with the appropriate adipogenic and osteogenic media. After incubation (21 day in adipogenic and 16 day in osteogenic media) stained with Oil Red O or Alizarin Red S to evaluate differentiation to adipocyte and osteocyte, respectively. The cells displayed a fibroblast-like phenotype. Cells in the images are passage 3 (1,4). Adipogenesis of OE-MSCs and AT-MSCs. Red color indicates the staining of lipid vesicle-forming adipocytes (2,5). Osteogenesis of OE-MSCs and AT-MSCs. Deposition of calcified nodules was visualized by Alizarin red staining. The ‘red cloudsʼ demonstrates the mineral deposits in some of the MSCs (3,6). All the images have a ×400 magnification. AT-MSC adipose tissue-mesenchymal stem cell, OE-MSC olfactory ecto-mesenchymal stem cell. (Color figure online)
Immunophenotyping
MSCs were cultured to 70% confluence, harvested and then stained with the following monoclonal antibodies against human markers: CD14-FITC, CD31-PE, CD34-PE, CD45-FITC, CD44-FITC, CD73-FITC, CD90-FITC, CD105-PE, HLA-DR-PerCP.Cy5.5, TLR3-PE, TLR4-PE (all from Biolegend, USA). All experiments were performed on cells at passages 3 to 5.
For cell staining, about 105 MSCs were incubated with the selected monoclonal antibody in PBS for 20 min at room temperature. To perform intracellular TLR3 detection, MSCs were washed, fixed and permeabilized using the Cytofix/Cytoperm kit (Immunostep, Spain). The cells were then analyzed using a FACS calibur flow cytometer (BD Biosciences, USA).
MSC differentiation potential
To confirm the multilineage differentiation potential of isolated cells, OE-MSCs and AT-MSCs were cultured under suitable conditions to assess adipogenic and osteogenic differentiation. Briefly, 70% confluent MSCs at passage 3–5 from three individuals of each group were seeded into 24 well plates (1.2 × 104 cells/well).
To induce cells toward adipogenic phenotype, MSCs were incubated with the appropriate adipogenic media (α-MEM supplemented with 10% FBS, 1 µM dexamethasone, 100 µg/mL 3-isobutyl-1-methylxanthine, 5 µg/mL insulin, and 60 µM indomethacin). The culture medium was replaced every 2–3 days. After 3 weeks, the medium was removed, cells were rinsed once with PBS and fixed with 4% formaldehyde solution for 30 min. Thereafter, they were washed twice with PBS, stained with Oil Red O for 15 min, rinsed twice with PBS and visualized under a light microscope (Olympus, Japan).
Osteogenic differentiation was induced using α-MEM supplemented with 0.1 mM dexamethasone, 10 mM glycerol-2-phosphate and 50 mM ascorbate-2-phosphate. The medium was changed every 3–4 days. After 16 days, the medium was removed, cells were washed twice with PBS and fixed with 4% formaldehyde solution for 30 min. The cells were then rinsed with distilled water and stained with 2% Alizarin Red S solution for 5 min.
TLR priming protocol
In order to activate TLRs, we used a low-level, short-term TLR-priming protocol. TLR3 and TLR4 were activated through incubating MSCs with fresh growth medium containing poly (I:C) (polyinosinic–polycytidylic acid, TLR3 agonist, 1 µg/mL, Sigma-Aldrich) and LPS (lipopolysaccharide TLR4 agonist, 10 ng/mL, Sigma-Aldrich) for 1 h, as previously suggested (Waterman et al. 2010). The cells were then washed twice with growth media to remove residual ligands before utilization in subsequent experiments.
Cytokine level measurements
After seeding 1 × 105 OE-MSCs or AT-MSCs on each well of 24-well plates, they were allowed to adhere overnight and then primed with TLR agonists for 1 h, as described in the previous section. The levels of IL1β, IL4, IL6, IL8, IL10, IL17, IFN-γ, IDO, IL1Ra, TGF-β, CCL5 in the cell culture supernatant were measured through enzyme-linked immunosorbent assay (ELISA). We purchased IL1β and IL6 from Mabtech (Sweden), IDO, IL1Ra and TGF-β from R&D system (USA), CCL5, IL17 from Biolegend (USA), all other cytokines from BD Bioscience (USA) and used them according to the manufacturer’s instructions. The experiments were performed at least three times on six individual MSC donors for each group of OE-MSCs or AT-MSCs.
Proliferative potential of MSCs
OE-MSCs and AT-MSCs at passage 1 were trypsinized and seeded into 6-well plates at a density of 105/well. Every 4 days, the cells were detached using 0.25% Trypsin-EDTA (2 min at 37 °C and 5% CO2) and counted using a hemacytometer. The average level of cell counts was calculated from each cell population. The mean population doubling time was obtained according to the following formula: population doubling time = T × log 2/(log Nt – log N0), where T is the culture time, N0 is the number of initially seeded cells and Nt is the number of harvested cells.
Quantitative real time PCR (qRT-PCR)
To determine the expression of TLR3, TLR4, Nestin, Vimentin and N-cadherin by MSCs, total RNA was extracted using RNx reagent according to the manufacturer's guidelines (SinColon, IRAN). Total RNA (1 µg) was reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) according to the supplier's instructions. We used 25 ng of cDNA in qRT-PCRs with SYBR Green PCR Master Mix (BIOFACT, Korea) and 0.32 mM of gene-specific forward and reverse primers. The used primers are listed in Table 1. qRT-PCR was performed using a Rotor gene Q instrument (Qiagen, the Netherlands). The thermal cycler parameters for amplification of these genes were as follows: 1 cycle at 95 °C for 15 min followed by 40 cycles at 95 °C for 15 s and finally, 60 °C for 40 s. GAPDH was used as the housekeeping gene for normalization of the cDNA amounts in qRT-PCR. NTC (No template control) and NAC (No amplification control) were included in the reactions. The relative level of gene expression was calculated according to the 2−ΔΔCT method. All measurements were carried out in triplicate.
Table 1.
Forward and reverse primers of GAPDH, TLR3, TLR4, Nestin, Vimentin and N-cadherin genes for real-time PCR amplification
| Gene name | Primers | Sequences | Product size (bp) |
|---|---|---|---|
| TLR3 | Forward | 5′-GCTGCAGTCAGCAACTTCAT-3′ | 144 |
| Reverse | 5′-AGGAAAGGCTAGCAGTCATCC-3′ | ||
| TLR4 | Forward | 5′-GATGAGGACTGGGTAAGGAATG-3′ | 102 |
| Reverse | 5′-GGCCACACCGGGAATAAA-3′ | ||
| Nestin | Forward | 5′-GTAGCTCCCAGAGAGGGGAA-3′ | 206 |
| Reverse | 5′-CTCTAGAGGGCCAGGGACTT-3′ | ||
| Vimentin | Forward | 5′-GGTGGACCAGCTAACCAACGA-3′ | 183 |
| Reverse | 5′-TCAAGGTCAAGACGTGCCAG-3′ | ||
| N-Cadherin | Forward | 5′-GGACAGTTCCTGAGGGATCA-3′ | 253 |
| Reverse | 5′-GGATTGCCTTCCATGTCTGT-3′ | ||
| GAPDH | Forward | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 138 |
| Reverse | 5′-TGGTGAAGACGCCAGTGGA-3′ |
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA). All data were presented as mean ± standard error. Multiple group comparisons were performed through one-way analysis of variance (ANOVA). Comparison between any two groups was performed using the two-tailed Student’s t-test. P values less than 0.05 were considered to indicate a statistically significant difference
Results
Characterization of MSCs
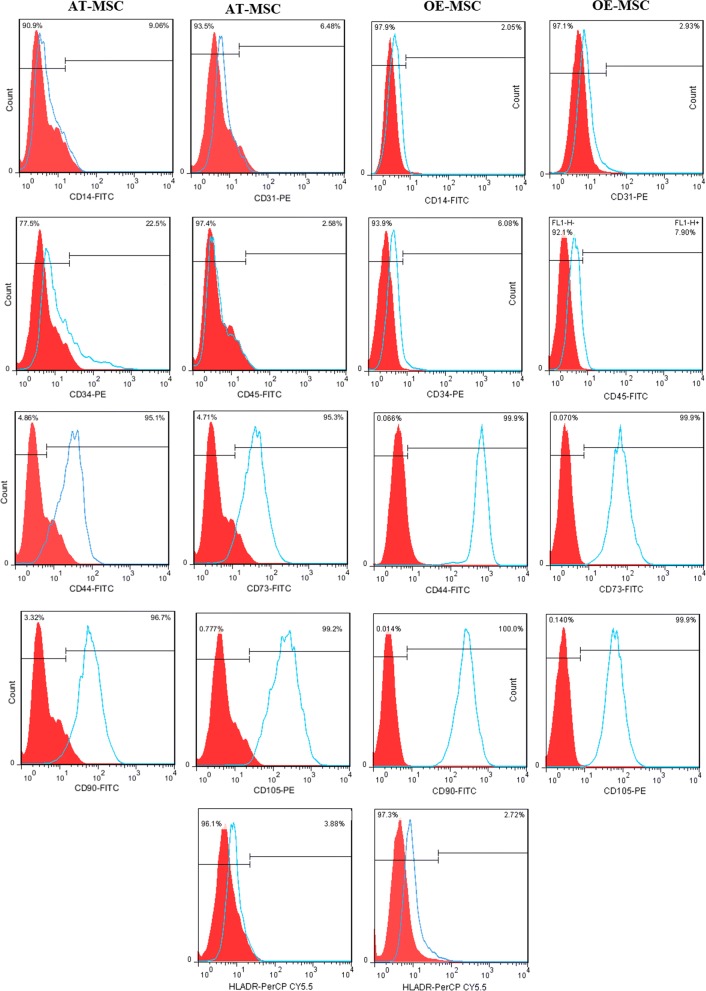
MSCs were isolated from human olfactory mucosa and adipose tissue, as described in “Materials and methods” section. The isolated cells presented the typical morphology of spindle-like cells and displayed a high capacity to adhere to the plastic disc. Both AT-MSCs and OE-MSCs exhibited osteogenic and adipogenic differentiation potentials (Fig. 1). Cell surface markers of OE-MSCs and AT-MSCs were examined through flowcytometric analysis. Both cell types were positive for MSC-specific surface markers (CD44, CD73, CD90 and CD105) and negative for hematopoietic lineage markers (CD14, CD31, CD34, CD45 or HLADR) (Fig. 2).
Fig. 2.
Flow cytometric analysis of mesenchymal stem cell (MSC) makers. MSC isolated from two different sources. MSC were harvested at passage 3–5, labeled with monoclonal antibodies against CD14, CD31, CD34, CD45-, CD44, CD73, CD90-, CD105, HLA-DR, and analyzed by flow cytometry. One representative experiment is reported on AT-MSC and OE-MSC that obtained after the third passage. Filled red histogram shows negative control and blue line displays indicated marker. For flow cytometric analysis of MSC surface markers (CD44, CD73, CD90, and CD105) that have expression more than 75%, unstained cell was used as negative control. However, for analysis of CD14, CD31, CD34, CD45, and HLA-DR markers that have low expression, isotype control was used as negative control. Meanwhile, unspecific binding was removed by three time washing and by FC receptor blacking. (Color figure online)
Proliferative differences in MSCs
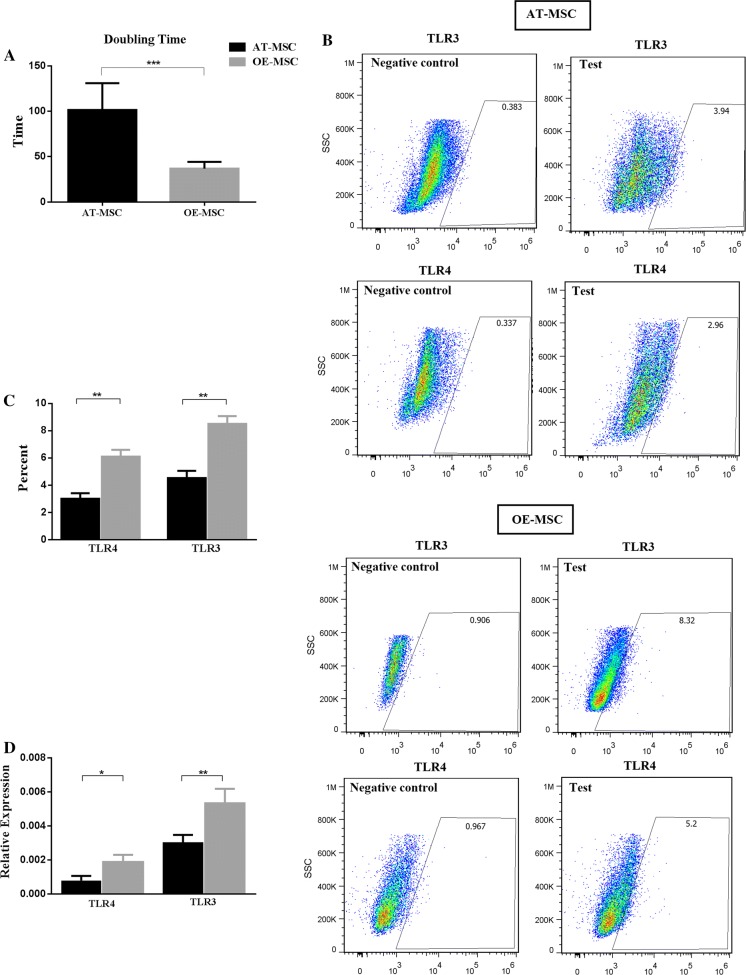
During cell proliferation, the population doubling time (PDT) was measured for AT-MSCs and OE-MSCs, and the obtained results for the two cell populations were significantly different. PDT of OE-MSCs was around 36 h, while that of AT-MSCs was approximately 101 h (Fig. 3a).
Fig. 3.
Population doubling time (PDT) (a), TLR3 and TLR4 protein expression (b, c), and change in TLR3 and TLR4 mRNA expression (d) of OE-MSCs and AT-MSCs. The number of MSCs was counted following subculture from first passage and after calculation of average of cell count, the mean population doubling time was obtained according to the formula that is mentioned in “Materials and methods”. For evaluation of TLRs expression, MSCs were cultured to 70% confluence, harvested and then stained with the monoclonal antibodies against TLR3-PE and TLR4-PE. Isotype control was used as negative control. In addition, unspecific binding was removed by three time washing and by FC receptor blacking. The cells were then analyzed using flow cytometer. For TLR3 and TLR4 measurement at mRNA level, MSCs were grown to 70% confluence, total RNA was extracted and reverse transcribed. Real time was performed and the relative level of gene expression was calculated according to the 2−ΔΔCT method. The experiment was performed in triplicate for each sample of OE-MSC and AT-MSC were in the same passage. Data shown represent mean ± SE of MSCs from 8 donners. *p < 0.05; **p < 0.01; ***p < 0.001. TLR toll like receptor
Expression of TLR3 and TLR4 by MSCs
The expression of TLR3 and TLR4 by OE-MSCs and AT-MSCs were evaluated through flow cytometry. The TLR3 and TLR4 expression levels in OE-MSCs were 8.5 ± 1.5% and 6.1 ± 1.2%, respectively, whereas AT-MSCs exhibited 4.5 ± 1.5% and 3 ± 1.2% expression levels of these molecules, respectively (Fig. 3b, c). Additionally, gene expressions of these TLRs were assessed using qRT-PCR. Consistent with the flow cytometry results, mRNA levels of TLR3 and TLR4 were significantly higher in OE-MSCs, in comparison with AT-MSCs (Fig. 3d).
Expression of Nestin, Vimentin, and N-cadherin in MSCs
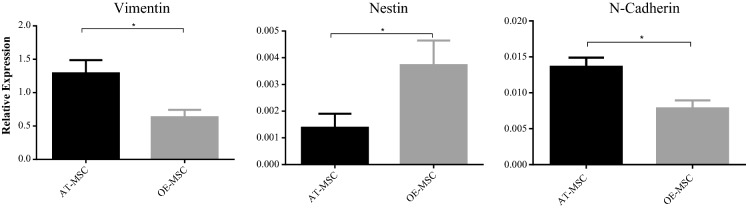
The expression levels of Nestin, Vimentin, and N-cadherin genes by AT-MSC and OE-MSC were different. While OE-MSCs exhibited a higher expression level of Nestin, the expressions of Vimentin and N-cadherin by these cells were significantly lower in comparison with AT-MSCs (Fig. 4).
Fig. 4.
OE-MSCs had higher expression of Nestin, but lower expression of Vimentin and N-cadherin in comparison to AT-MSCs. To determine the expression of Nestin, Vimentin and N-cadherin, MSCs were grown to 70% confluence, total RNA was extracted and reverse transcribed. Real time was performed and the relative level of gene expression was calculated according to the 2−ΔΔCT method. The experiment was performed in triplicate for each sample. Data shown represent mean ± SE of MSCs from 8 donners. *p < 0.05.
Comparison of cytokine and chemokine secretion following exposure to TLRs agonist
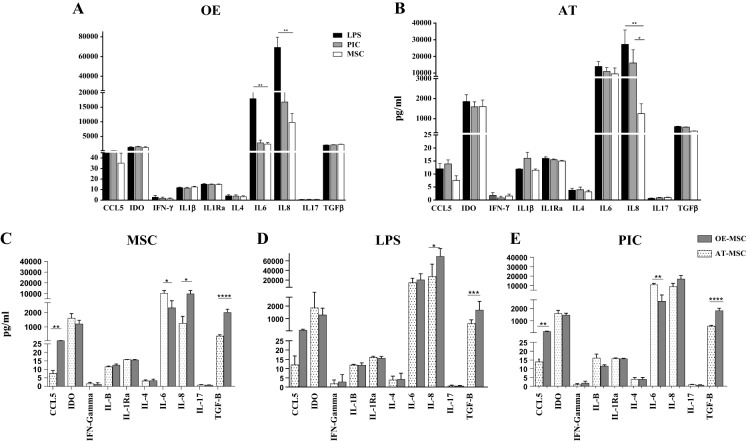
The production levels of cytokines and chemokines including IL-1B, IL-4, IL-6, IL-8, IL-10, IL-17, IL-1Ra, RANTES (CCL5), IFN-γ, TGF-β, and also IDO enzyme were assessed in the cell culture supernatant (Fig. 5). OE-MSCs and AT-MSCs exhibited different patterns of cytokine production after TLR3 and TLR4 activation. IL-6 and IL-8 levels for the LPS-treated OE-MSCs were significantly higher than those for untreated OE-MSCs (Fig. 5a). However, LPS- and PIC-treated AT-MSCs only exhibited a significantly higher secretion of IL-8, compared to untreated AT-MSCs (Fig. 5b).
Fig. 5.
OE-MSCs and AT-MSCs have different patterns of cytokine production. a, b Data demonstrate the effect of LPS and PIC on cytokine secretion of AT-MSC and OE-MSC separately. c–e Data present the comparison of cytokine secretion in OE-MSCs and AT-MSCs media before and after LPS and PIC treatment. MSCs were pre-treated for 1 h with LPS or Poly(I:C), washed and cultured for an additional 48 h prior to harvesting the spent medium and analyzed with ELISA kits following the manufacturer’s instructions. Error bars indicate SEM. Data shown represent mean ± SE of MSCs from 8 donners. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. LPS lipopolysaccharide, PIC polyinosinic:polycytidylic acid
Before any treatment, OE-MSCs showed significantly greater levels of CCL5, IL-8, and TGF-β in comparison with AT-MSCs. However, AT-MSCs exhibited a significantly higher secretion of IL-6, compared to OE-MSCs (Fig. 5c). After LPS treatment, the secretion of TGF-β, IL-8 and CCL5 remained significantly higher in OE-MSCs, compared to AT-MSCs (Fig. 5d). In addition, after PIC treatment, the expression patterns of TGF-β, IL-6 and CCL5 were similar to those for untreated MSCs (Fig. 5e).
Discussion
Activation of Toll-like receptors enhances the therapeutic potency of MSCs. Several studies have addressed these effects and reported them to be important for prospective cell-based therapy (Liotta et al. 2008). Many researches on MSCs have been focused on the expression of TLRs by these cells as well as modulation of their differentiation, migration, proliferation, survival, and immunosuppression capacities (DelaRosa and Lombardo 2010). TLRs are members of a large family of receptors (e.g. TLR1-10), among which TLR3 and TLR4 are highly expressed by human mesenchymal stem cells (Raicevic et al. 2011). Therefore, many researchers have surveyed the role of TLR3 and TLR4 ligands on the ability of human MSCs to modulate differentiation, proliferation, and immune responses.
Bone-marrow-derived MSCs (BM-MSCs) are the most extensively characterized and commonly-used MSCs in clinical treatments. However, utilizing BM-MSCs is associated with some limitations, such as the significant decline in cell number and proliferation/differentiation ability with increasing the donor age and the painful and invasive procedure required to obtain BM from patients (Wakitani et al. 1995; Young et al. 1998). Therefore, other sources of MSCs such as OE-MSCs, which are easily accessible and exhibit an extensive proliferation rate, has been considered for use by various researchers. It has been reported that the age of donor and cell passage number does not affect the characteristics of OE-MSCs, in contrast to other human MSCs (Raicevic et al. 2011). Based on our results, the population doubling time of OE-MSCs was significantly less than AT-MSCs, in agreement with the findings of previous studies (Hwang et al. 2014; Li et al. 2014). In this study, we assessed whether there is a significant difference in TLR3 and TLR4 expression between OE-MSCs and AT-MSCs. We also evaluated whether the exposure of TLR agonists such as LPS and poly(I:C) could induce a significant difference in cytokine and chemokine production by these cells.
We observed significant differences in the expression of TLR3 and TLR4 in terms of mRNA and protein levels between OE-MSCs and AT-MSCs. These data are in agreement with the findings of Hwang et al., who showed that TLR3 and TLR4 are expressed by human turbinated MSCs (Hwang et al. 2014). In addition, Liotta et al. reported that TLR3 and TLR4 mRNAs are highly expressed by MSCs in BM-MSCs (Liotta et al. 2008). The expression of TLRs at the protein level seems to be low and is often difficult to detect by flow cytometry, despite the high expression of TLRs by MSCs from different sources at the mRNA level (DelaRosa et al. 2012). Here, we observed relatively weak expressions of TLR3 and TLR4 at the protein level in both OE-MSCs and AT-MSCs.
Nestin has been widely considered a marker for central nervous system (CNS) progenitor cells in different contexts. In this study, we observed that OE-MSCs exhibited a significantly higher expression of Nestin, compared to AT-MSCs. This was in agreement with the findings of Lindsay et al. (2013). Nestin-positive neural MSCs have beneficial immunoregulatory functions over immune cells, in comparison with Nestin-negative MSCs (Xu et al. 2013b).
Vimentin is the most ubiquitous intermediate filament protein, and the first protein expressed during cell differentiation. All primitive cell types, including BM-, Warton Jelly (WJ)-, AT- and cord blood-derived non-hematopoietic (i.e. mesenchymal and stromal) progenitor cells express Vimentin (Li et al. 1995; Rosada et al. 2003; Secunda et al. 2015). In the current study, AT-MSCs had a significantly higher expression of Vimentin, compared to OE-MSCs, which may be due to the low degree of differentiation of OE-MSCs.
N-Cadherin is a calcium-dependent cellular adhesive protein that inhibits osteogenesis, but promotes migration of MSCs (Xu et al. 2013a). This molecule is also expressed by cardiomyocyte progenitor cells during mouse development (Radice et al. 1997). In this study, AT-MSCs showed a considerably higher expression of N-cadherin, in comparison with OE-MSCs. Our results reveal that AT-MSCs may be more suitable for regenerative medicine in cardiovascular diseases, compared to OE-MSCs.
To compare the immunomodulatory functions of OE-MSCs and AT-MSCs, the secretion levels of cytokines and chemokines were analyzed before and after exposure to TLR agonists. Among IL-1B, IL-4, IL-6, IL-8, IL-10, IL-17, IL-1Ra, RANTES (CCL5), IFN-γ, TGF-β, and also IDO enzyme, only the production of IL-6 and IL-8 by the LPS-treated OE-MSCs was significantly higher than untreated OE-MSCs. However, in PIC- and LPS-treated AT-MSCs, only the secretion level of IL-8 was significantly higher than that by untreated AT-MSCs. These findings follow a pattern of cytokine secretion similar to previous studies on nasal mucosal MSCs and AT-MSCs (Hwang et al. 2014; Jakob et al. 2009; Raicevic et al. 2011). Waterman et al. have demonstrated that the TLR3-primed effect on BM-MSC secretion of IL-4, IL-10, IP-10, CCL-5 and TLR4 signaling was the upstream of IL-6 and IL-8 secretion, after low-level and short-term TLR-priming protocol (the same protocol as our study) (Waterman et al. 2010). While OE-MSCs expressed TLR3, this receptor appeared to be non-functional as its ligation did not trigger the secretion of different tested cytokines. Our results could be explained by the findings of Hwang et al. who suggested that OE-MSCs would express non-functional TLR3 (Hwang et al. 2014).
OE-MSCs exhibited significantly higher levels of IL-8, TGF-β, and CCL5 secretion in comparison with AT-MSCs before any treatment. However, in this situation, only IL-6 secretion was significantly higher in AT-MSCs, compared to OE-MSCs. These findings reveal that the immunomodulatory function of OE-MSCs is higher than AT-MSCs. In this way, immune cells are attracted by IL-8 and CCL-5 in a close proximity of OE-MSCs. These chemokines provide a direct cell-to-cell contact as well as a possible paracrine immunoregulatory effect of other effector molecules that are also secreted by OE-MSCs such as TGF-β (Kyurkchiev et al. 2014). This cytokine can inhibit T-cell proliferation, differentiation and effector functions. TGFβ is continuously produced in large quantities by OE-MSCs. Therefore, application of OE-MSC to ameliorate of various autoimmune disease can be more helpful than application of AT-MSC.
Similarly, after PIC treatment, secretion of TGF-β, IL-6 and CCL5 followed the pattern observed for untreated MSCs, when we compared OE-MSCs and AT-MSCs. After LPS treatment, the levels of IL-8, TGF-β, and CCL5 secretion remained significantly higher in OE-MSC media, in comparison with AT-MSCs. However, secretion of IL-6 was higher in the supernatant of OE-MSCs media. Given the fact that IL-6 has an anti-inflammatory function along with pro-inflammatory effects (Opal and Depalo 2000; Steensberg et al. 2003; Tilg et al. 1994; Xing et al. 1998), this finding can support the idea that pro-inflammatory stimuli enhance the immunosuppressive functions of MSCs (Domenis et al. 2018). Our results were consistent with the findings of Hwang et al. and Waterman et al. on the role of TLR4 agonist on IL-6 secretion in OE-MSCs and BM-MSCs, respectively (Hwang et al. 2014; Waterman et al. 2010).
Conclusions
According to the results of this study, the proliferative potential of OE-MSCs is higher than that of AT-MSCs. OE-MSCs exhibit a greater expression of TLR3 and TLR4 genes, compared to AT-MSCs. However, unlike AT-MSCs, the expression of TLR3 by OE-MSCs appear to be nonfunctional. Our data reveal that OE-MSCs have a stronger secretion of immunosuppressive cytokines than AT-MSCs. Therefore, these properties make OE-MSCs one of the best sources of MSCs, both for regenerative medicine and cell therapy in autoimmune disorders. However, additional studies need to be performed in order to compare other aspects of immunomodulatory properties of OE-MSCs and AT-MSCs after exposure to TLR agonists, to offer a novel target in the improvement of stem cell-based therapeutic strategies.
Acknowledgements
We are grateful to the entire staff in the Department of Immunology, School of Medicine, Iran University of Medical Sciences, for their continued involvement in this project.
Funding
This study was financially supported by Grant (Grant Number: 3933), given by Iran University of Medical Sciences (IUMS).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baharlou R, et al. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: regulatory effects on peripheral blood mononuclear cells activation. Int Immunopharmacol. 2017;47:59–69. doi: 10.1016/j.intimp.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010 doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelaRosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182. doi: 10.3389/fimmu.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme B, et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2009;19:853–866. doi: 10.1089/scd.2009.0267. [DOI] [PubMed] [Google Scholar]
- Domenis R, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8:13325. doi: 10.1038/s41598-018-31707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Girard SD, Devéze A, Nivet E, Gepner B, Roman FS, Féron F. Isolating nasal olfactory stem cells from rodents or humans. J Vis Exp. 2011;22:2762. doi: 10.3791/2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheumatol. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, Thibeault SL. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–551. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S, et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2011;21:742–756. doi: 10.1089/scd.2011.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, et al. Toll like receptor 3 & 4 responses of human turbinate derived mesenchymal stem cells: stimulation by double stranded RNA and lipopolysaccharide. PLoS ONE. 2014;9:e101558. doi: 10.1371/journal.pone.0101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob M, Hemeda H, Janeschik S, Bootz F, Rotter N, Lang S, Brandau S. Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells Develop. 2009;19:635–644. doi: 10.1089/scd.2009.0245. [DOI] [PubMed] [Google Scholar]
- King NM, Perrin J. Ethical issues in stem cell research therapy. Stem Cell Res Ther. 2014;5:85. doi: 10.1186/scrt474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Li J, Sensebe L, Herve P, Charbord P. Nontransformed colony-derived stromal cell lines from normal human marrows. II Phenotypic characterization differentiation pathway. Exp Hematol. 1995;23:133–141. [PubMed] [Google Scholar]
- Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695–704. doi: 10.3892/ijmm.2014.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay SL, Johnstone SA, Mountford JC, Sheikh S, Allan DB, Clark L, Barnett SC. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia. 2013;61:368–382. doi: 10.1002/glia.22440. [DOI] [PubMed] [Google Scholar]
- Liotta F, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Németh K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivet E, et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Investig. 2011;121:2808–2820. doi: 10.1172/JCI44489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Opal SM, Depalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Develop Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Raicevic G, et al. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell Immunol. 2011;270:207–216. doi: 10.1016/j.cellimm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- Secunda R, Vennila R, Mohanashankar A, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793–807. doi: 10.1007/s10616-014-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metabol. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor. Blood. 1994;55:113–118. doi: 10.1182/blood.V83.1.113.113. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve Off J Am Assoc Electrodiagn Med. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Investig. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Meng F, Ni M, Lee Y, Li G. N-Cadherin regulates osteogenesis and migration of bone marrow-derived mesenchymal stem cells. Mol Biol Rep. 2013;40:2533–2539. doi: 10.1007/s11033-012-2334-0. [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Akiyama K, Chai Y, Le A, Wang Z, Shi S. Gingivae contain neural-crest-and mesoderm-derived mesenchymal stem cells. J Dental Res. 2013;92:825–832. doi: 10.1177/0022034513497961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- Zappia E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]