Abstract
HaCaT cells have been widely used as undifferentiated epidermal keratinocytes, since these non-tumorigenic cells can be readily maintained in conventional medium and partly retain epidermal differentiation potential upon stimulation with high concentration of calcium. In contrast to primary epidermal keratinocytes, however, these cells never form tight junction (TJ), a specific structure in highly differentiated keratinocytes, solely by the differentiation stimulation. Here, we show that HaCaT cells secrete a considerable amount of high mobility group box-1 protein (HMGB1), one of major inflammatory mediator, which appeared to be responsible, at least in part, for such aberrant differentiation response. So far, inhibition of c-Jun N-terminal kinase (JNK) in high calcium medium has been supposed to be the only way to induce TJ formations in HaCaT cells; however, SP600125, a potent inhibitor of JNK showed cytostatic effects and clearly attenuated epidermal differentiation and stratification. In contrast, dipotassium glycyrrhizate (GK2), a soluble analogue of HMGB1-blocker Glycyrrhizin, down-regulated interferon-β, a typical inflammatory cytokine induced by secreted HMGB1, and accelerated differentiation responses to the calcium treatment in these cells. In addition, GK2-treatmenrt resulted in the formation of double cell layers in cultured HaCaT cells, where the stratified upper cells transiently accumulated TJ proteins at the cell–cell contact sites. These results highlight the importance of attenuation of secreted HMGB1-signals in cultured HaCaT cells for studies of functional keratinocytes.
Electronic supplementary material
The online version of this article (10.1007/s10616-019-00367-6) contains supplementary material, which is available to authorized users.
Keywords: Glycyrrhizin, Dipotassium glycyrrhizate, HaCaT, Tight junction, High mobility group box-1 protein, Epidermal differentiation
Introduction
The stratified epidermal structure in the skin is formed by the outward proliferation of anaplastic keratinocytes and their successive differentiation. The epidermal barrier function is maintained and exerted by the strict control of cornification in the stratum corneum and, the tight junction (TJ) formation in the underlying granular cell layer (Brandner et al. 2015; Ishida-Yamamoto et al. 2018). Upon TJ formation, the junctional elements, such as zonula occludens (ZO) proteins, Claudins, and junction adhesion molecules (JAMs), accumulate and associate at the cell–cell contact sites, completely sealing the paracellular pathway (Brandner et al. 2015).
The epidermis of the skin is the outermost tissue of the body, and disruption of the barrier function readily elicits inflammatory responses. Skin inflammation activates a number of molecular mediators that attract immune cells to the affected area, which, in turn, lead to parakeratosis and disruption of the epithelial barrier (Brady 2004; Huet et al. 2018). Such skin inflammation and barrier dysfunctions are common features in many skin diseases including atopic dermatitis and psoriasis (Engebretsen and Thyssen 2016; Miyagaki and Sugaya 2015).
For in vitro studies, primary cultured normal human epidermal keratinocytes (NHEK) have been frequently used as undifferentiated epidermal keratinocytes; however, these cells are mortal, display considerable lot-to-lot variations, and need to be maintained in low calcium and serum-free medium to prevent spontaneous differentiation. As alternative model cells, HaCaT keratinocytes have also been used, since these non-tumorigenic and immortal cells can be readily maintained in conventional serum-containing medium and partly retain their differentiation potential upon stimulation with high concentration of calcium (Colombo et al. 2017; Micallef et al. 2009). However, the HaCaT cells show aberrant differentiation responses relative to NHEK cells and the formation of TJ is scarcely induced simply by the activated calcium influx, implying the existence of some molecular elements causing such deviant behaviors (Aono and Hirai 2008; Micallef et al. 2009).
Recently, high mobility group box-1 (HMGB1) has garnered special attention as a master proinflammatory mediator (Castiglioni et al. 2011). While HMGB1 regulates several transcription factors in the nucleus, leading to cell proliferation and migration of keratinocytes (Ranzato et al. 2009; Shin et al. 2015), a number of studies have reported extracellular presence of HMGB1 via a non-canonical secretion pathway (Radisky et al. 2009; Vijayakumar et al. 2019). Extracellular HMGB1 binds to receptors such as advanced glycation end products (RAGE) and activates several signaling pathways including the extracellular signal-regulated Kinase (ERK) pathway or the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway (Karuppagounder et al. 2015). Several inflammatory cytokines are then up-regulated, leading to perturbation of the epidermal differentiation process, leading to a defect in the epidermal barrier function (Nygaard et al. 2017). It is also notable that HMGB1 has been shown to contribute to wound healing processes in the skin, where differentiation programs and intercellular adhesion systems in the epidermis were temporally and severely perturbed (Ranzato et al. 2010; Sinagra et al. 2015; Straino et al. 2008). Clinically, serum HMGB1 levels, that reflect the amount of extracellularly released HMGB1, correlate with the severity of psoriasis (Bergmann et al. 2016).
An active component of licorice, glycyrrhizin, is readily hydrolyzed to glycyrrhetinic acid, has anti-inflammatory function (Huang et al. 2016) that is believed to be mediated by several possible mechanisms, such as suppression of the cyclooxygenase-2/thromboxane A2 (COX2/TxA2) pathway, regulation of corticosteroid metabolism, or inactivation of DNA polymerase (Huang et al. 2016; Ishida et al. 2012; Tanahashi et al. 2002). However, recent studies revealed that glycyrrhizin directly binds to HMGB1 to ablate inflammatory signaling, thereby ameliorating atopic dermatitis-like symptoms (Wang et al. 2018). Herein, we report that dipotassium glycyrrhizate (GK2), highly soluble analogue of glycyrrhizin facilitates epidermal differentiation and TJ formation in HaCaT keratinocytes, by abrogating proinflammatory pathways activated by endogenously secreted HMGB1. From this point of view, HaCaT cells maintained with a HMGB1 blocker would be a reliable model for the study of functional keratinocytes.
Materials and methods
Cells and reagents
The normal human epidermal keratinocytes (NHEK) obtained from Kurabo LTD were maintained in HuMedia-KG2 medium with growth supplement as instructed in the protocol (Kurabo), and those in low passage number (less than four) were used in this study. HaCaT keratinocytes were grown and maintained in DMEM/HamF12’s medium (Wako Chemicals) supplemented with 10% FCS (DH10). To test TJ formation, these cells were incubated in DH10 medium containing 10 mM CaCl2 and either SP600125 (40 μM), GK2 (30 μM), or potent steroid aldosterone (1 μM) for 1 or 3 days. Concentration of aldosterone in treated cells was ten times higher than the functional concentration for keratinocytes (Boix et al. 2017), which gave non-toxic effect to HaCaT cells. The SP600125, GK2, and aldosterone were purchased from Sigma-Aldrich, Maruzen Pharm, and CIL, respectively. Antibodies against Keratin 10 (K10) (Abcam), type I transglutaminase (TGase1) (Protein Tech), HMGB1 (Abcam), c-Jun N-terminal kinases (JNK), phosphorylated JNK, ERK, phosphorylated ERK (Cell signaling), β-actin (Sigma-Aldrich) and Claudin-1 (Invitogen) were used in this study. Monoclonal antibody against ZO-1 (Itoh et al. 1993) was a generous gift from Dr. Itoh and Dr. Nagafuchi while HRP-, Alexa488- and Cy3-labeled secondary antibodies were purchased from Sigma-Aldrich, Invitrogen and GE healthcare, respectively.
Immunodetection
Western blotting and immunocytochemistry were carried out according to standard protocols. A 10 × –concentrated conditioned media was used for western blotting supernatant samples. The conditioned media was prepared from supernatant of NHEK or HaCaT cells incubated in appropriate media without growth supplements (for NHEK) or serum (for HaCaT) for 48 h. The culture supernatant was then collected, centrifuged to remove cell debris and concentrated to ten times by ammonium sulfate precipitation. For western blotting, signal intensities relative to β-actin in the same sample were quantified with ImageJ (Schneider et al. 2012). Cells were counterstained with DAPI for immunocytochemistry.
Assessment of TJ formation
Cells cultured on chamber slides (SPL life sciences) were stained for ZO-1, and to measure signal intensities, three random lines (200 μm each) from three randomly selected photographs were scanned using LASX (Leica), a microscope software program. The number of signal peaks having more than 150 relative fluorescence units (r-fu) with a > 45 r-fu gap with the neighboring pixel was determined as the number of TJs.
Analyses of cell viability
The relative cell viability was evaluated using Alamar blue reagent, following the manufacturer’s protocol (Invitrogen). In brief, the Alamar blue reagent (1/10) was added to the subconfluent cells in the culture, incubated for 90 min, the absorbance ratio at 570 and 600 nm was then analyzed and compared to the control.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells that have been treated with or without GK2 for 4 h, using Miniprep system (Viogene). The RNA was subsequently reverse transcribed with ReverTra Ace (TOYOBO) using random primer (Takara). The cDNA template (1 μg) was then applied to qRT-PCR performed using FastStart Essential DNA Green Master on a LightCycler Nano system (Roche), according to the manufacturer’s protocol. The primers used for qRT-PCR were 5′-AGCCACATCGCTCAGACA-3′ and 5′-GCCCAATACGACCAAATC-3′ (for GAPDH), 5′-CCAAACCGATAGGAAACGAG-3′ and 5′-CATAAAACTTTGCTCCAAAGAGG-3′ (for HMGB1), 5′-AGGTCTTCCCGACGATGA-3′ and 5′-GTCTTTCCGTGCTCCAAAAC-3′ (for luciferase), 5′-CTTTGCTATTTTCAGACAAGATTCA-3′ and 5′-GCCAGGAGGTTCTCAACAAT-3′ (for interferon-β). Data were analyzed with the double delta Ct method using the expression of GAPDH as a control.
Statistical analyses
The results are expressed as the mean ± SD. of at least three independent experiments. Data were analyzed using Mann–Whitney t test and a p-value of < 0.05 was considered statistically significant.
Results and discussion
TJ formation by the JNK inhibitor SP600125
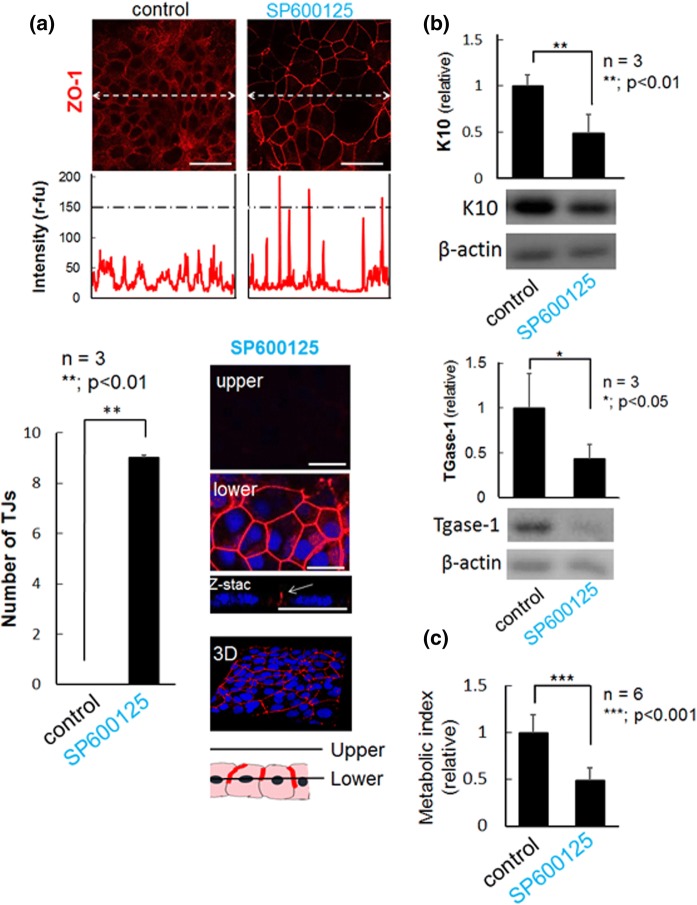
Although the formation of TJ is induced only by high concentration of calcium in NHEK cells, inhibition of JNK with SP600125 is additionally required in HaCaT cells (Aono and Hirai 2008; Kitagawa et al. 2014). Without SP600125, HaCaT cells express ZO-1, a scaffold protein for TJ sealing proteins, with diffusible or dot-like distribution pattern throughout their cell bodies or at membrane-proximal regions (Fig. 1a). As previously reported, in response to SP600125, ZO-1 protein evidently accumulates at the cell–cell contact sites in the cells (Aono and Hirai 2008), indicating the formation of watertight TJ seals (Fig. 1a). Even though undifferentiated keratinocytes do not have the ability to form TJ, considerable number of differentiation markers, Keratin 10 (K10) and TGase1, were detectable in untreated HaCaT cells, which were conversely down-regulated upon induction of TJ formation (Fig. 1b). It is noteworthy that TGase1 is a specific marker for the differentiated/stratified granular layer, the only cell layer forming epidermal TJs in vivo. A previous study showed that SP600125-treated HaCaT cells formed TJs without augmentation of typical differentiation markers including Keratin 1/10 (K 1/10), albeit some anaplastic markers seemed to be down-regulated (Kitagawa et al. 2014). In addition, SP600125 showed significant cytostatic activity, without causing morphological changes and stratification of the cells (Fig. 1a, c). Thus, SP600125-induced TJ formation in HaCaT cells could be considered as a distinct phenomenon from epidermal differentiation, and its physiological relevance remained to be further elucidated.
Fig. 1.
Induction of TJ formation by SP600125 in HaCaT cells. a On day 1, the upper, TJ protein ZO-1 (red) was immunostained in cells treated with (SP600125) and without (control) SP600125. The central portion of the photographs were line scanned (dotted lines) and the signal intensities were plotted. Scale bars, 50 μm. Lower left, total number of TJs on 600 μm scanned line (200 μm × 3 lines) from each cell image. Lower right, a representative confocal image of cells treated with SP600125. Images from upper (9 μm from culture dish) and lower (4.5 μm from culture dish) planes, and that of Z-stack were shown. Scale bars, 25 μm. b Western blot analyses of expression of Keratin 10 (K10) and TGase-1. Cells treated with SP600125 significantly down-regulated these differentiation markers. Experiments were repeated three times. c Relative cell viability was measured via a metabolic assay using Alamar-blue, as described previously (Rampersad 2012). SP600125 exhibited a cytostatic or toxic effect in all the six experiments
HaCaT cells produce and secrete a proinflammatory mediator HMGB1
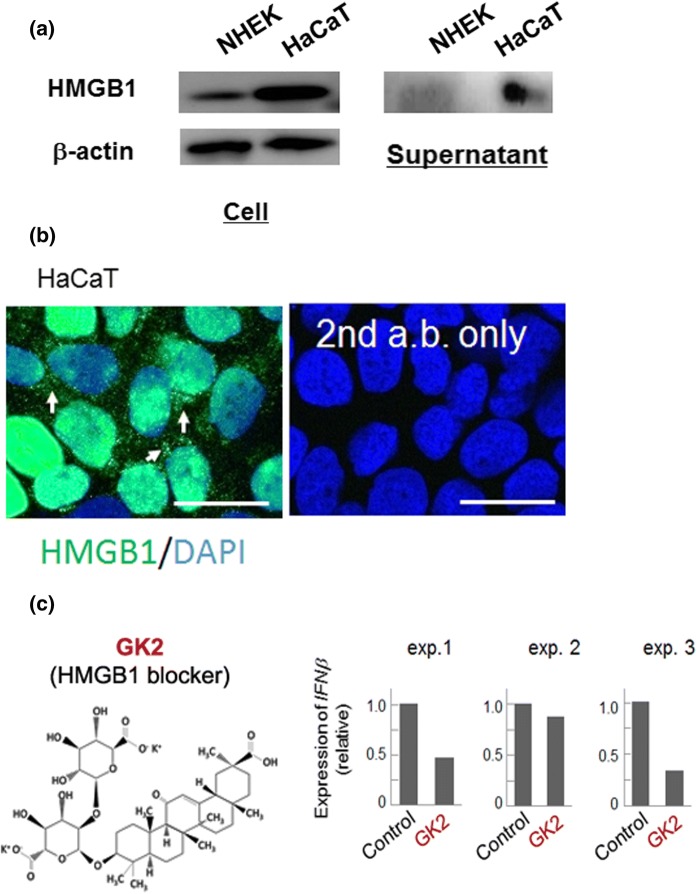
In contrast to NHEK cells, HaCaT cells neither undergo normal differentiation nor form TJ structures in response solely to the high-calcium treatment, despite that this cell line has been used widely as a suitable model of anaplastic keratinocyte (Matsui et al. 1992). We assumed that some molecular elements have conferred stable characteristics to HaCaT cells in conventional medium, which might compensate for defects in normal differentiation potential. We found that HaCaT cells endogenously produce much higher amount of HMGB1, a major inflammatory mediator (Castiglioni et al. 2011), than NHEK cells (Fig. 2a), which is detected in nuclei, cytoplasm, and even in culture supernatant (Fig. 2a, b). These results indicate that aberrant differentiation responses with the defect in TJ formation are possibly attributed to the HMGB1-induced inflammation in HaCaT cells, and led us to pursue the effect of functional ablation of HMGB1 in these cells.
Fig. 2.
HaCaT cells express considerable amount of a proinflammatory mediator HMGB1. a Expression of HMGB1 in NHEK and HaCaT cells. Compare to primary cultured keratinocyte, HaCaT cells actively produce and secrete HMGB1 in Supernatant, concentrated conditioned medium. Experiments were repeated three times and the representative blot images were shown. b Immunodetection of expression and distribution of HMGB1. HGM1 was detectable in both the nuclei and cytosol (arrows) of HaCaT cells. Experiments were repeated three times and the representative images were shown. Bars, 25 μm. c Effect of GK2 on the mRNA expression of interferon-β (IFNβ), one of the most sensitive HMGB1 downstream elements in keratinocytes. All the independent experiments (exp1, 2 and 3) demonstrated clear down-regulation of IFNβ by the treatment with GΚ2, albeit its expression was varied depending on the culture
As a HMGB1-blocker, glycyrrhizin, which has been shown to directly bind to HMGB1and exert anti-inflammatory function, recently garnered special attention (Wang et al. 2018). Since this natural compound shows low solubility in physiological medium and is readily permeable to the cell membrane, it could act in both intra- and extracellular environment. Considering the proinflammatory effect of extracellularly supplied HMGB1 and dramatic alleviation of psoriasis-like lesions by extracellularly added anti-HMGB1 antibodies (Zhang et al. 2017), we focused on the extracellular activity of HMGB1. For this, we tested the effect of GK2, a highly soluble analogue of glycyrrhizin that might not be intracellularly accessible (Fig. 2c). Inactivation of ERK and the signaling attenuation of NFκB were not clear in the HaCaT cells treated with GK2 for 24 h in a medium containing 10% serum (Supplementary Fig. S1). However, these cells evidently down-regulated a proinflammatory cytokine interferon-β (Fig. 2c), which acts as one of the most sensitive downstream elements of extracellular HMGB1 in keratinocytes (Mori et al. 2018). The variation in the production of interferon-β appeared depending on the experiments was noteworthy, and we did not test other proinflammatory cytokines such as IL-1β and IL-8 (Mori et al. 2018). Higher concentrations of GK2 presumably show stronger inhibitory effects, given that much higher concentrations of GK2 (up to 400 μM) were reportedly avirulent and showed higher anti-inflammatory effects (Lee et al. 2019).
Effect of GK2 on HaCaT cell activities
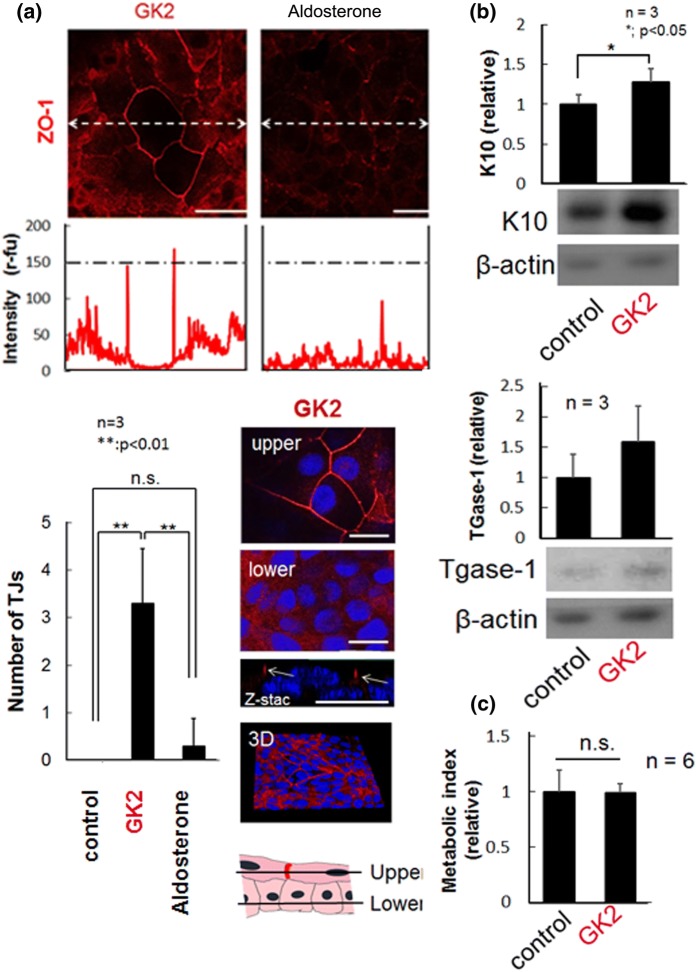
Upon treatment with GK2 only at a concentration of 30 μM, certain cell populations exhibited enlarged or flattened morphology with sharp distribution of ZO-1 protein exclusively at the cell–cell boundaries, indicating a causal effect of GK2 on TJ formation (Fig. 3a). A similar expression pattern was observed for another TJ protein, claudin-1 (data not shown). Given that excess administration of glycyrrhizin often cause pseudoaldosteronism (Morinaga et al. 2018; Ohtake et al. 2007), one might consider this phenomenon to be attributed to its feeble aldosterone-like activity or indirect activation of mineralocorticoid receptor, a primary target of aldosterone (Stewart et al. 1987); however, a potent steroid aldosterone treatment gave no appreciable effect on TJ formation (Fig. 3a). Compared to the cells treated with SP600125, neither JNK inactivation nor cytostatic effects were detected in cells treated with GK2 (Supplementary Fig S2 and Fig. 3c), suggesting that GK2-induced TJ formation is independent of JNK-mediated signaling pathway. Confocal microscopic analyses revealed that HaCaT cells deliver daughter cells upward to form a new flattened cell layer and only upper cells, but not the basal cells, form TJ (Fig. 3a). In addition, analyses of epidermal differentiation markers revealed that the cells treated with GK2 underwent onset of differentiation (Fig. 3b).
Fig. 3.
Induction of TJ formation by GK2 in HaCaT cells. a On day 1, the upper, TJ protein ZO-1 (red) was immunostained in cells treated with GK2 (30 μM) or aldosterone (1 μM). Central portion of the photographs were line scanned (dotted lines) and the signal intensities were plotted. Scale bars, 50 μm. Lower left, total number of TJs on 600 μm scanned line (200 μm × 3 lines) from each cell image. Lower right, a representative confocal image of cells treated with GK2. Images from upper (9 μm from culture dish) and lower (4.5 μm from culture dish) planes, and that of Z-stack were shown. Arrows, accumulation of ZO-1 at cell–cell contact sites only in the stratified upper layer. Scale bars, 25 μm. b Western blot analyses of Keratin 10 (K10) and TGase-1. Cells treated with GK2 significantly up-regulated K10 and tend to increase TGase-1. Experiments were repeated three times and the representative blot data were shown. c Relative cell viability measured with Alamar-blue reagent. While the experiments were repeated six times, cytostatic or toxic effect was not detected in GK2
GK2-induced TJs are different from those induced by SP600125
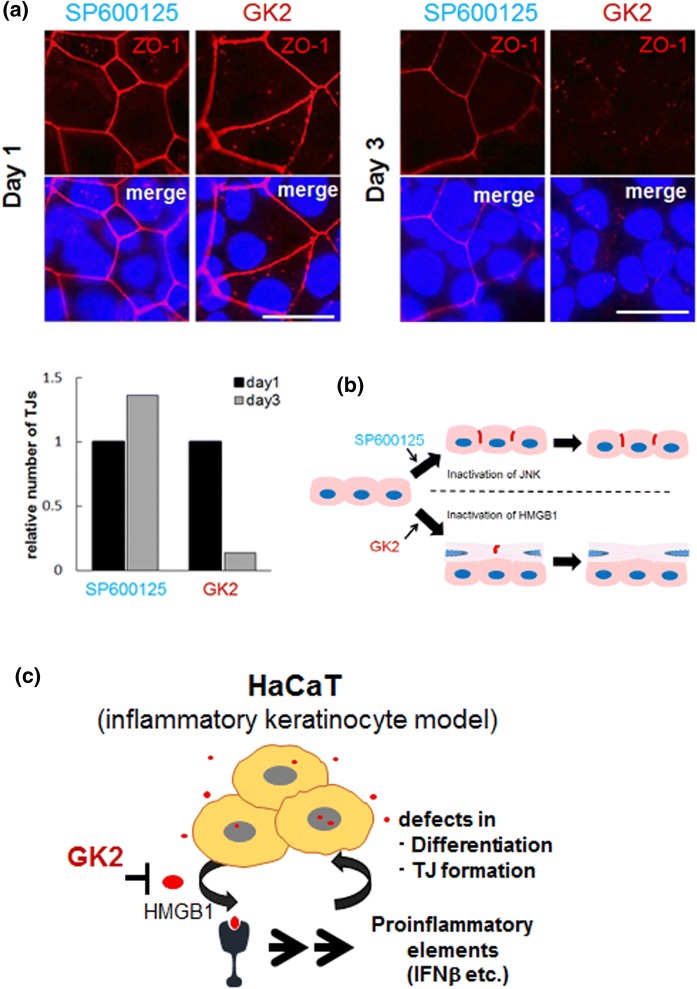
While TJs formed by SP600125 were stable for at least 3 days, those formed only in the upper cell layer by GK2 appeared to be disrupted on day 2 and were almost undetectable on day 3 (Fig. 4a, b). In vivo, nascent keratinocytes undergo upward differentiation in the epidermis, where TJ structures are transiently formed when cells reach the granular layer, but are disrupted in the subsequent horny layer (Basler and Brandner 2017). Thus, HMGB1 signal could dysregulate spatio-temporal TJ dynamics, even though multicellular stratification into three or more layers did not occur in HaCaT cells treated with GK2.
Fig. 4.
Distinct effects of SP600125 and GK2 on TJ formation in HaCaT cells. a upper, Accumulation of a TJ component ZO-1 (red) in cells with SP600125 and GK2 on day 1 (left) and day3 (right). The nuclei were counterstained with DAPI. Bars, 25 μm. Lower, quantification of relative number of TJs on day 1 and 3. Average numbers of TJs from three line scan assays (n = 3 for each category, as shown in Figs. 1 and 3) were calculated and relative numbers to those of day1 are shown. TJs induced by SP600125 appeared to be stable, whereas those by GK2 were almost disrupted on day 3. Bars, 50 μm. b Comparative diagram of TJ-formed cell populations in HaCaT keratinocytes treated with SP600125 and GK2. Cells treated with GK2 construct double cell layers and only upper cells formed TJs (see Figs. 1 and 3). c Schema of the effect of GK2 on HaCaT cells that produce an inflammatory mediator HMGB1. GK2 abrogates the inflammatory action of HMGB1, leading to restoration of the differentiation potential and of the TJ formation program
Conclusion
In conclusion, GK2-induced TJ formation may be involved in restoration of the differentiation program that has been perturbed, at least in part, by HMGB1 in HaCaT cells. Thus, the disruption of secreted HMGB1-signals might be an important element for cultivation of HaCaT cells as undifferentiated keratinocyte model cells (Fig. 4c). Given that the effect of GK2 is reminiscent of ameliorating inflammation in the skin epidermis, our results may also provide new insight on the anti-inflammatory effects of GK2 in the skin. Analyses of molecular elements to identify the functional link between HMGB1-induced inflammation and defects in differentiation/TJ formation are now underway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs. Itoh and Nagafuchi for anti-ZO-1 antibodies. We are grateful to members of Healthcare division in Kobayashi Pharmaceutical for information on GK2, and all members of Hirai laboratory for helpful discussions. Part of this work was supported by KG Special Research Fund (to Yo. H) and Grant-in Aid for Scientific Research (KAKENHI) (to Yu. H. and Yo. H.).
Funding
Funding was provided by Grant-in Aid for Scientific Research (Grant Nos. 19J22142, 17K09739), KG Special Research Fund (Grant No. 167AB0177a).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aono S, Hirai Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp Cell Res. 2008;314:3326–3339. doi: 10.1016/j.yexcr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Basler K, Brandner JM. Tight junctions in skin inflammation. Pflugers Arch. 2017;469:3–14. doi: 10.1007/s00424-016-1903-9. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Strohbuecker L, Lotfi R, Sucker A, Joosten I, Koenen H, Koerber A. High mobility group box 1 is increased in the sera of psoriatic patients with disease progression. J Eur Acad Dermatol Venereol. 2016;30:435–441. doi: 10.1111/jdv.13564. [DOI] [PubMed] [Google Scholar]
- Boix J, et al. Primary aldosteronism patients show skin alterations and abnormal activation of glucocorticoid receptor in keratinocytes. Sci Rep. 2017;7:15806. doi: 10.1038/s41598-017-16216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SP. Parakeratosis. J Am Acad Dermatol. 2004;50:77–84. doi: 10.1016/s0190-9622(03)02801-9. [DOI] [PubMed] [Google Scholar]
- Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, De Benedetto A. Epidermal tight junctions in health and disease. Tissue Barriers. 2015;3:e974451. doi: 10.4161/21688370.2014.974451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–199. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- Colombo I, et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediators Inflamm. 2017;2017:7435621. doi: 10.1155/2017/7435621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen KA, Thyssen JP. Skin barrier function and allergens. Curr Probl Dermatol. 2016;49:90–102. doi: 10.1159/000441548. [DOI] [PubMed] [Google Scholar]
- Huang QC, Wang MJ, Chen XM, Yu WL, Chu YL, He XH, Huang RY. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget. 2016;7:1193–1202. doi: 10.18632/oncotarget.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet F, et al. Reconstructed human epidermis for in vitro studies on atopic dermatitis: a review. J Dermatol Sci. 2018;89:213–218. doi: 10.1016/j.jdermsci.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Ishida T, et al. Inhibitory effects of glycyrrhetinic acid on DNA polymerase and inflammatory activities. eCAM. 2012;2012:650514. doi: 10.1155/2012/650514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Igawa S, Kishibe M. Molecular basis of the skin barrier structures revealed by electron microscopy. Exp Dermatol. 2018;27:841–846. doi: 10.1111/exd.13674. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder V, et al. Modulation of HMGB1 translocation and RAGE/NFkappaB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp Dermatol. 2015;24:418–423. doi: 10.1111/exd.12685. [DOI] [PubMed] [Google Scholar]
- Kitagawa N, Inai Y, Higuchi Y, Iida H, Inai T. Inhibition of JNK in HaCaT cells induced tight junction formation with decreased expression of cytokeratin 5, cytokeratin 17 and desmoglein 3. Histochem Cell Biol. 2014;142:389–399. doi: 10.1007/s00418-014-1219-9. [DOI] [PubMed] [Google Scholar]
- Lee SH, et al. Ameliorating effect of dipotassium glycyrrhizinate on an IL-4- and IL-13-induced atopic dermatitis-like skin-equivalent model. Arch Dermatol Res. 2019;311:131–140. doi: 10.1007/s00403-018-1883-z. [DOI] [PubMed] [Google Scholar]
- Matsui MS, Chew SL, DeLeo VA. Protein kinase C in normal human epidermal keratinocytes during proliferation and calcium-induced differentiation. J Invest Dermatol. 1992;99:565–571. doi: 10.1111/1523-1747.ep12667411. [DOI] [PubMed] [Google Scholar]
- Micallef L, Belaubre F, Pinon A, Jayat-Vignoles C, Delage C, Charveron M, Simon A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp Dermatol. 2009;18:143–151. doi: 10.1111/j.1600-0625.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89–94. doi: 10.1016/j.jdermsci.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Mori H, et al. Reduced-HMGB1 suppresses poly(I:C)-induced inflammation in keratinocytes. J Dermatol Sci. 2018;90:154–165. doi: 10.1016/j.jdermsci.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Morinaga O, et al. Isolation of a novel glycyrrhizin metabolite as a causal candidate compound for pseudoaldosteronism. Sci Rep. 2018;8:15568. doi: 10.1038/s41598-018-33834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard U, van den Bogaard EH, Niehues H, Hvid M, Deleuran M, Johansen C, Vestergaard C. The “Alarmins” HMBG1 and IL-33 downregulate structural skin barrier proteins and impair epidermal growth. Acta Derm-Venereol. 2017;97:305–312. doi: 10.2340/00015555-2552. [DOI] [PubMed] [Google Scholar]
- Ohtake N, Kido A, Kubota K, Tsuchiya N, Morita T, Kase Y, Takeda S. A possible involvement of 3-monoglucuronyl-glycyrrhetinic acid, a metabolite of glycyrrhizin (GL), in GL-induced pseudoaldosteronism. Life Sci. 2007;80:1545–1552. doi: 10.1016/j.lfs.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Stallings-Mann M, Hirai Y, Bissell MJ. Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat Rev Mol Cell Biol. 2009;10:228–234. doi: 10.1038/nrm2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad SN. Multiple applications of Alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol Cell Biochem. 2009;332:199–205. doi: 10.1007/s11010-009-0192-4. [DOI] [PubMed] [Google Scholar]
- Ranzato E, Patrone M, Pedrazzi M, Burlando B. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys. 2010;57:9–17. doi: 10.1007/s12013-010-9077-0. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JU, et al. In vivo relative quantitative proteomics reveals HMGB1 as a downstream mediator of oestrogen-stimulated keratinocyte migration. Exp Dermatol. 2015;24:478–480. doi: 10.1111/exd.12713. [DOI] [PubMed] [Google Scholar]
- Sinagra T, Merlo S, Spampinato SF, Pasquale RD, Sortino MA. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front Pharmacol. 2015;6:126. doi: 10.3389/fphar.2015.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet. 1987;2:821–824. doi: 10.1016/s0140-6736(87)91014-2. [DOI] [PubMed] [Google Scholar]
- Straino S, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128:1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- Tanahashi T, et al. Glycyrrhizic acid suppresses type 2 11 beta-hydroxysteroid dehydrogenase expression in vivo. J Steroid Biochem Mol Biol. 2002;80:441–447. doi: 10.1016/S0960-0760(02)00033-X. [DOI] [PubMed] [Google Scholar]
- Vijayakumar EC, Bhatt LK, Prabhavalkar KS. High mobility group box-1 (HMGB1): a potential target in therapeutics. Curr Drug Targets. 2019 doi: 10.2174/1389450120666190618125100. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Peng G, Han X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int Immunopharmacol. 2018;60:9–17. doi: 10.1016/j.intimp.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. Proinflammatory effect of high-mobility group protein B1 on keratinocytes: an autocrine mechanism underlying psoriasis development. J Pathol. 2017;241:392–404. doi: 10.1002/path.4848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.