Abstract
Human dental pulp stem cells (hDPSCs) are promising source of cells for numerous and varied regenerative medicine applications as those possess high proliferation potential with multilineage differentiation capacity compare to other sources of adult stem cells; therefore, hDPSCs could be the good source for autologous transplantation in tissue engineering and regenerative medicine. In this study stem cells were isolated from dental pulp and were characterised by flowcytometry and immunocytochemistry. The controlled cells as well as, 7-day cultured cells were positive for transcription factors, OCT 4 and SOX 2 thatconfirmed isolated cellsasmesenchymal stem cells (MSCs). These cells showed positive expression for CD 19, CD 73, CD 90, CD 105 and are negative for CD 34, CD 45. Viability of hDPSCS were studied by trypan blue (TB) staining and fluorescent microscopic study. After 7 days of passaging by using several growth factors, cells express neural cell markers oligodendrocyte and glial fibrillary acidic protein. Specifically, osteocytes were grown from dental pulp MSCSsin vitro with the help of growth factors, dexamethasone, ascorbic acid-2- phosphate and β-glycerophosphate whereas, adipocytes were grown with indomethacin, 3-isobutyl-1-methylxanthine and insulin. Osteocytes and adipocytes were characterized by von Kossa and Oil red O staining, respectively. Chromosomal analysis of dental pulp-MSCs was done for qualitative assessment of MSCs. Karyotyping indicated diploid chromosome number in dental pulp derived MSCs. In vitro grown osteocytes could be used for bone fracture reunion cases, and adipocytes could be used for further research purposes.
Keywords: Biological sciences, Cell biology, Stem cells research, Biotechnology, Health sciences, Dentistry, Dental pulp, Stem cells, Neural cells, Flow cytometry, Immunocytochemistry
Biological sciences; Cell biology; Stem cells research; Biotechnology; Health sciences; Dentistry; Dental pulp; Stem cells; Neural cells; Flow cytometry; Immunocytochemistry.
1. Introduction
Stem cells are ontogenically primitive unspecialized cells that can be differentiated to any type of cells. According to the origin stem cells can be divided into two categories – embryonic and adult stem cells. Embryonic stem cells are pluripotent in nature as the differentiation of cell types depend on adequate stimulations for a specific cell type, sometimes even with feeder layers, such as mouse embryonic fibroblast (MEF), stroma cells (STO), fetal muscle, skin and foreskin cells, adult fallopian tube cells, adult marrow cells or dishes coated with animals celled condition medium or MEF [1, 2]. However, embryonic stem cells have both moral and ethical problems - as these cells will later develop into a human being, taking these cells will require the destruction of an embryo. Adult stem cells are not totipotent, and they can be further classified depending on their origin and differentiation potential. Adult stem cells are non-hematopoietic multipotent stromal cells with capabilities of self-renewal by proliferation into multiple cell lineages/types, such as, adipocytes, osteocytes, hepatocytes, cardiomyocytes, chondrocytes and neural cells, etc., guided by specific growth factors during cell growth in vitro [3, 4, 5]. Dental stem cells are considered to be an appealing source for mesenchymal stem cells, since they are non-controversial, readily accessible, have a large donor pool, and pose no risk of discomfort for the donor.
Contemporary research with human stem cells is focused for regenerative medicine, for which stem cells are isolated from different sources, bone marrow (BM), peripheral blood, umbilical cords (UC), neural tissues, liver, gastrointestinal tract, skin, muscle, tissues and dental pulp, etc. [6, 7]. Indeed, Moreover, MSCs isolated from any source exhibit the common features as the fibroblastic (or fibroblast-like) phenotype, with a multi-potent differentiation potential and the expression of typical MSC surface markers [8]. Moreover, the use of BM in regenerative medicine is a common place strategy for the richness of MSCs; nonetheless the collection of BM is a highly invasive and remains as a painful procedure. Furthermore, the number and multi-lineage differentiating potentiality of BM-MSCs declines with donor's age [9], which renders it being selectively suitable for autologous corrective measures. Thus any alternate source of MSCs could be exploited.
Human dental pulp stem cells (hDPSCs) are the cells possessing several applications in regenerative medicine. As compare to other sources, hDPSCs have greater proliferation potential with multilineage differentiation capacity, which allows hDPSCs for autologous transplantation in tissue engineering [10]. Due to mesenchymal stem cell (MSC) features, hDPSCs can be used for allograft transplantation too [11]. hDPSCs are potential sources for bone tissue engineering. hDPSCs are replacement of embryonic stem cells (ESC) by expressing several pluripotency markers, and too display multipotency characteristics by differentiating to chondrocytes, osteocytes and neural cells [12]. Cell therapy treatments for liver disease require effective stem-cell derived hepatocytes. DPSCs have been differentiated to produce hepatocyte-like cells (HLCs) with acquired hepatocyte functions, such as glycogen storage and urea production [13].
Herein, isolation and characterization of in vitro cultured hDPSCs have been described in terms of phenotypic profiles using flowcytometry and fluorescence microscope. Moreover, neural cells, adipocytes and osteocytes were grown from hDPSCs in vitro, separately, and were characterized by suitable staining procedures. Neural cells could be used for treatment of neurodegenerative diseases. Osteocytes grown from hDPSCs could be used for therapeutic purposes for bone fracture reunion. Adipocytes, being fat-forming cells, have a limited clinical application but could lend themselves in further molecular research. Chromosomal analysis of hDPSCs was also done for qualitative assessment of isolated stem cells.
2. Material and methods
2.1. Collection and isolation of dental pulp stem cells
Immediately extracted dental pulp tissues were used for the study. Pulp tissues were washed thoroughly in Dulbecco's modified eagles medium (DMEM) containing antibiotics. Pulp tissues were cut into1–2 mm2 pieces. Small pieces were immersed in digestive solution which contains collagenase and dispase dissolved in DMEM. Pulp tissues were kept for 1 h at 37 °C. After enzymatic disaggregation, pulp was dissociated and then filtered in a 100 μm Falcon Cell Strainers, in order to obtain a cell suspension. All experimental protocols were approved by the institutional ethical committee, Ajman University. Informed consent was obtained from the patient. This study was approved by institutional ethical committee, Ajman University.
2.2. Cell counting, viability testing and in vitro culture
The isolated cells were subjected to cell counting using hemocytometer. Cell viability was studied by trypan blue staining and fluorescent microscopic study. Further, the enumeration of DPSCs was done by culturing 1 × 106 viable DPSCs in 6 well culture plates in DMEM with 15 % Foetal Bovine Serum, 100 μl penicillin streptomycin solution. Medium was changed in each alternative day depending on the confluency and monitored for 21 days.
2.2.1. Trypan blue staining
Trypan blue solution was prepared in Phosphate buffered saline at the concentration of 0.4 g/mL. For the study of cell viability to the in vitro grown mass of dental pulp stem cells, TB solution was added at the 1:1 ratio; the mixture was kept in an incubator for 2 min at 37 °C and cells in triplicates were observed under a phase-contrast microscope at the 400X magnification. The live cells remained unstained, whereas the nuclei of dead cells appeared blue, as TB is a membrane permeable dye that enters dead cells and stains the nuclei to blue, where as the viable cells remain unstained.
2.2.2. Fluorescent microscopic study
For fluorescent microscopic study, the AO/EB solution was prepared in PBS at the concentration of 100 μg/mL and is applied to in vitro cultured cells. When observed under the fluorescent microscope at 400X, green colour indicated live cells, whereas cells with orange and red colour were recorded as apoptotic and necrotic cells, respectively. AO is taken up by both live and dead cells and emits green fluorescence where as EB is only taken up by dead cells, as the integrity cytoplasmic membrane is lost and it stains nucleus orange. Hence live cells, apoptotic cells and necrotic cells were green, orange and red in appearance, respectively.
2.3. Characterisation of DPSC by flowcytometry
After 21 days, trypsinized cells were resuspended in PBS at 1 × 106 cells/mL, from which aliquots of 100 μL of the cell suspension was taken in each Falcon polystyrene fluorescent activated cell sorter tube and were incubated at 4 °C in dark for 30 min with fluorescein-conjugated antibody against following antigens, CD 29, CD 34, CD 45, CD 73, CD 90 and CD 105. Thereafter, cells were washed with PBS, centrifugation was done. Each pellet was resuspended in an aliquot of 100 μL PBS and was run through a flow cytometer (Becton-Dickinson, San Jose, CA).
2.4. Immunocytochemistry of in vitro differentiated cells
In vitro differentiated cells as controls were cultured on sterile cover slips and cells were washed thrice with PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. The fixed cells were incubated for 25 min with normal goat serum in PBS at the 1:9 dilution. Cells were incubated in 3% H2O2 in PBS for 15 min to block endogenous peroxidase and were incubated with anti OCT4, SOX 2 antibodies overnight at 4 °C. The slides were washed thrice with PBS and incubated with goat anti-rabbit IgG-FITC for 1 h at room temperature. Cells were washed and incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min to detect the nuclei. Cells were observed under a fluorescent microscope.
2.5. Neural cell induction
DPSCs from passage 3 to 4 were further induced with serum free human neural proliferation medium (StemCell Technologies, Canada). Mitogenic factors such as Epidermal growth factor (20 ng/ml) and basic Fibroblast growth factor (10 ng/ml) were used with1X antibiotic solution to stimulate neurogenic cells at 37°Cand 5% CO2 atmosphere. Fresh medium was replenished every after 3rd day, and cells were maintained for 21 days.
2.6. Osteogenic differentiation
MSCs with 85 % confluence were transferred to a 6-well culture plate using DMEM supplemented with 10% FBS, 1% PS, 10 nM dexamethasone, 0.1 mM ascorbic acid-2- phosphate and 10 mM β-glycerophosphate solution. The medium was changed thrice a week for 21 days. Deposition of phosphates was determined by vonKossa staining: PBS-washed cells were fixed in 10% formalin, rewashed and were stained with 2% AgNO3. Further, the cells were washed with distilled water and were exposed to bright light for 15 min. To check the accumulation of calcium on MSCs microscopically, 2% alizarin red solution at PH 4.2 for 10 min at room temperature was treated.
2.7. Adipogenic differentiation
After an 85 % confluence approximately, MSCs were grown in 6-well culture plates containing DMEM supplemented with 10% FBS, 1% PS, 100 nM dexamethasone, 0.25 mM indomethacin, 0.1 mM 3-isobutyl-1-methylxanthine and 0.01 M insulin. Growth supporting media were changed thrice a week for 21 days. The accumulation of lipids was determined by the oil red-O staining. Cells were washed with PBS and fixed in 10% formalin for 30 min. After being fixed, cells were washed with 60 % isopropanol and were stained with oil red-O stain [14].
2.8. Chromosomal analysis
Stem cells at the passage 7 were used for chromosomal analysis. The cells were incubated with colchicine (Sigma, USA) at the final concentration of 10 μg/mL for 2 h. Thereafter colchicine was removed by centrifugation at 1000 rpm and 75 mM KCl was added and incubated for 18 min as a hypotonic treatment. Sensitive swollen (hypotonic) cells were fixed in cold Canoy's fixative and the washing with PBS was repeated. The fixative containing cell pellet was flushed by repeated centrifugations. Cells were put on to an inclined air dried clean slide with a pipette from around one meter distance for the bursting of swollen cells and spreading of metaphase chromosomes. Finally slides with chromosomes were air dried, stained with 5% Giemsa (pH 6.8) for 20 min and more than 100 metaphasic plates were scored to check chromosomal stability.
3. Results
3.1. Dental pulp stem cells isolation
Stem cells were isolated from dental pulp in sterile environment and were cultured in suitable culture conditions. DPSCs formed colonies after 7 days of growth. These cell populations were cultured up to 12 passages; doubling time of the population was 45.3 ± 7.0 h during the first seven passages.
3.2. Viability study
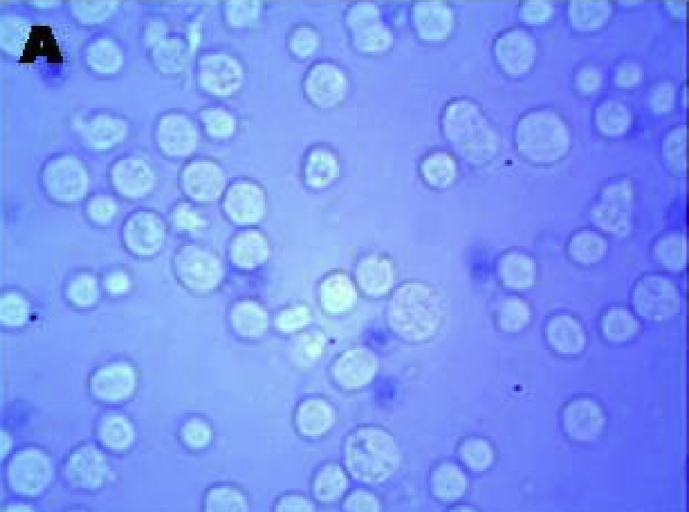
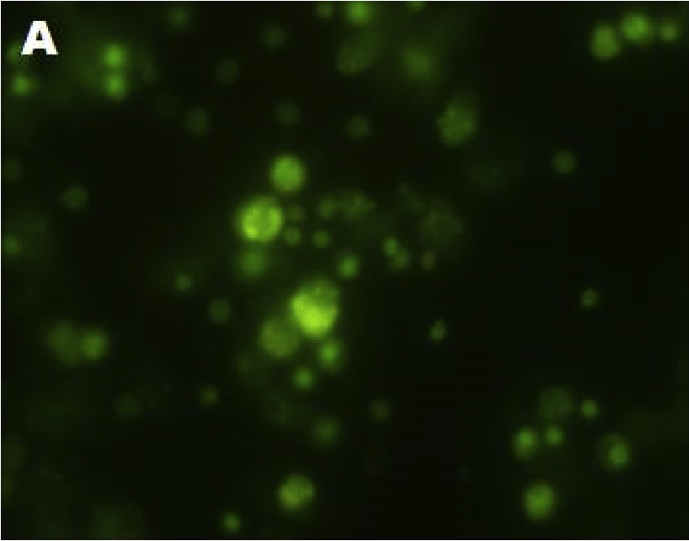
Stem cells isolated from dental pulp are mostly viable. Trypan blue staining clearly indicated there are no dead cells. All the cells are viable (Figure 1). Fluorescent microscopic study by AO/EB staining showed that stem cells isolated from dental pulp are live cells after 7 passages of growth. All the cells emit green fluorescence hence are live cells (Figure 2).
Figure 1.
A- Viable cells by trypan blue staining.
Figure 2.
A- Fluorescent microscopic study indicated viable cells isolated from dental pulp.
3.3. Characterisation of DPSC by flowcytometry
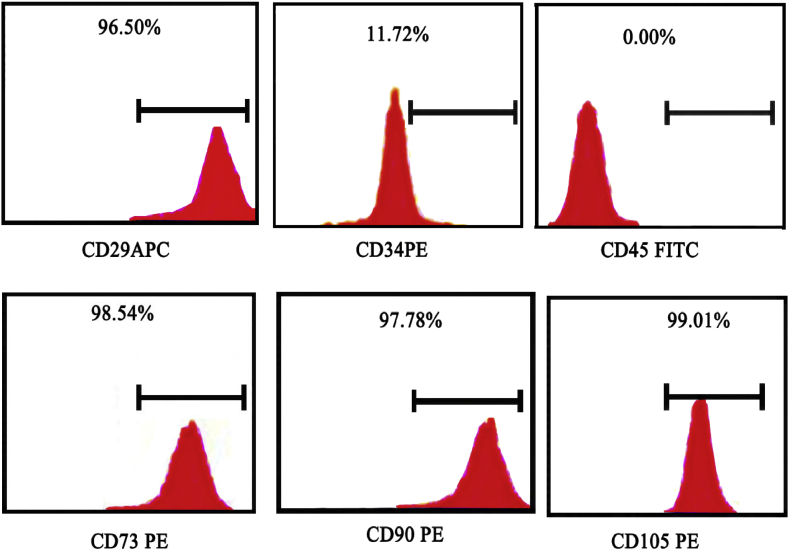
To ascertain phenotypes of stem cells, the cultured cells were positive for antibodies, CD 29, CD 90, CD 73 and CD 105, and were negative for CD 34, CD 45. This result shows that the cells isolated from dental pulp were mesenchymal stem cells (Figure 3). The cells which express the said markers are stem cells, so are the dental pulp stem cells.
Figure 3.
Flow cytometric analysis of cultured stem cells with monoclonal antibodies against CD 29, CD 34, CD 45, CD 73, CD 90 and CD 105 with 10,000 events. The bar is a mark for the region of cells that are positive for the specific marker.
3.4. Immunocytochemistry of differentiated cells
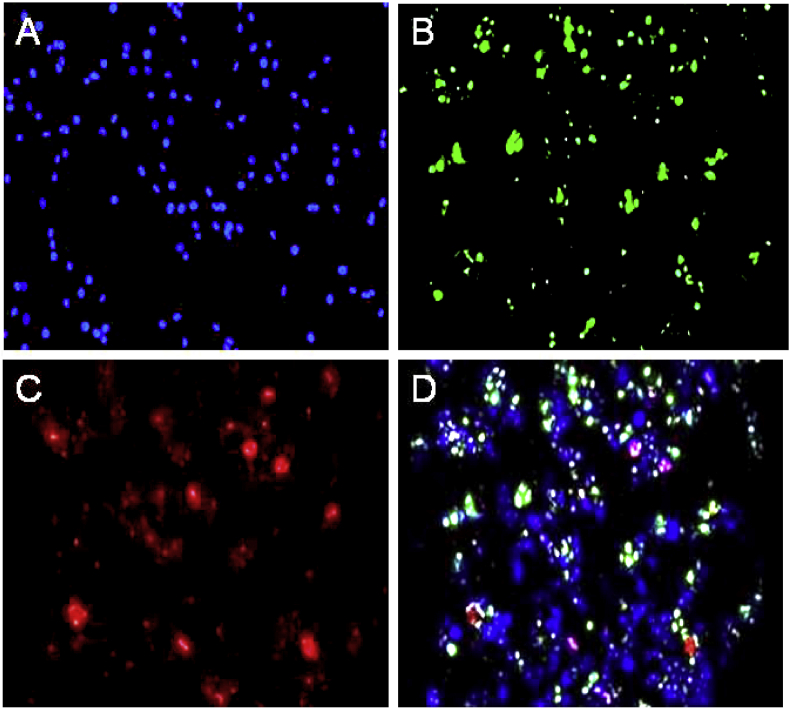
Different markers typical for characterization of stem cells were tested by immunocytochemical staining, for the presence of transcription factors, Oct-4 and SOX 2 which are stem cell markers (Figure 4). In control cells, those are isolated and cultured from dental pulp and also the cells at seventh passage were mesenchymal stem cells, due to the presence of both factors.
Figure 4.
(A) DAPI staining of control cells, (B) Expression of OCT 4 in 7 days hDPSCs culture, (C) Expression of SOX 2 in 7 days hDPSCs culture, (D) Expression of OCT 4 and SOX 2 in 7 days hDPSCs culture along with DAPI staining.
3.5. Neurogenic induction
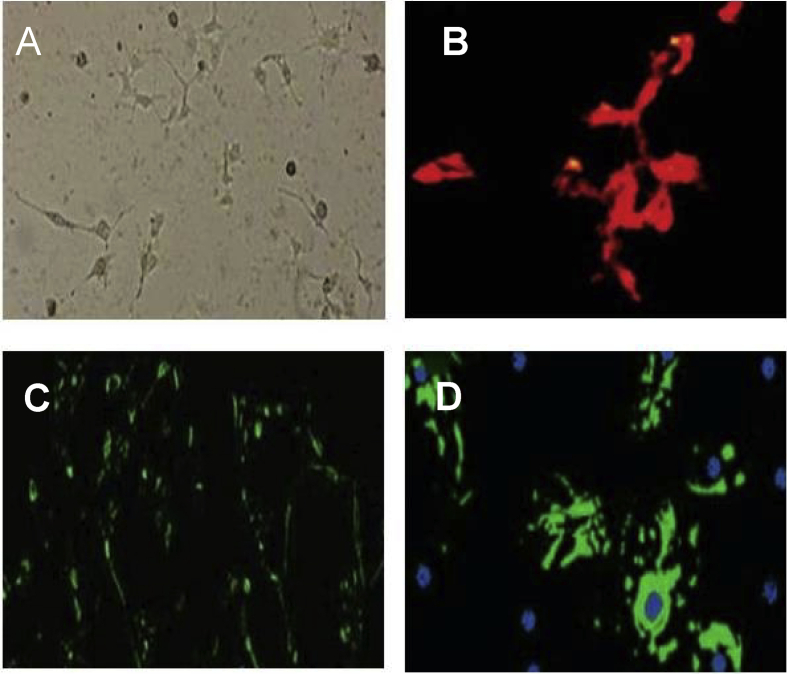
Passage 7 cells when treated with basic fibroblast growth factor and epidermal growth factor, showed positive response for glial fibrillary acidic protein, tubulin and oligodendrocyte markers (Figure 5). These markers are present in neural cells. Hence this result clearly indicated that the cells isolated from human dental pulp were developed to neural cells after treating with appropriate growth factor. Human dental pulp stem cells have the ability to produce neural cells.
Figure 5.
A- Neural cells after 7 days of passaging, B- Neural cells expressing oligodendrocyte, C- Neural cells expressing tubulin, D- Neural cells expressing Glial fibrllary acidic protein (GFAP).
3.6. Osteogenic differentiation
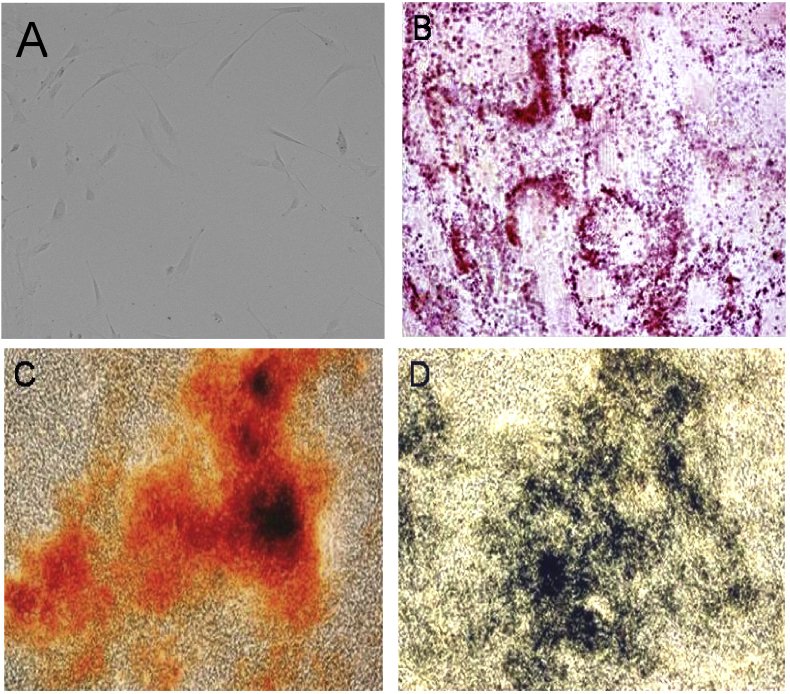
hDPSCs were differentiated to osteogenic cells during growth in specified media in vitro. The accumulation of calcium and phosphate depositions, feature of osteocytes was determined by alzarin red staining. The cells with calcium and phosphate deposits were red and black in appearance, respectively (Figure 6).
Figure 6.
(A) hDPSCs after 7 days of culture (B) Accumulation of lipid vacuoles stained with oil red o after the growth of hDPSCs to adipocytes, (C) Accumulation of calcium stained with alizarin red staining after growth of hDPSCs to osteocytes, (D) Accumulation of phosphates stained von kossa staining after growth of hDPSCS to osteocytes.
3.7. Adipogenic differentiation
During in vitro growth in specified media, hDPSCs were differentiated to adipocytes. The accumulation of lipids, a unique feature of adipocytes was determined by oil red O staining (Figure 6) indicating hDPCs can grow to adipocytes under suitable conditions.
3.8. Chromosomal analysis
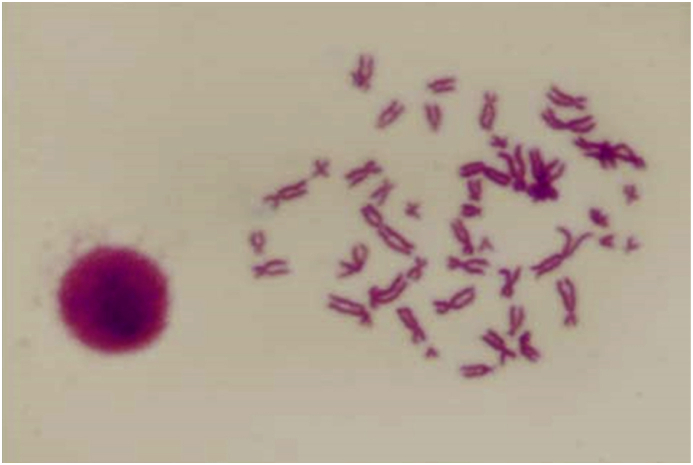
Cytogenetic analysis of in vitro cultured cells is a qualitative assessmentof stem cells. In dental pulp derived stem cells, chromosome analysis of 100 metaphasic plates of serial passages showed a modal peak at 46 (2n) chromosomes in maximum cases, depicts diploid karyotype, where homologous chromosomes are aligned (Figure 7).
Figure 7.
Chromosomal analysis of hDPSCs.
4. Discussion
MSCs grown from dental pulps have high proliferative capacity and were differentiated to neural cells, osteocytes and adipocytes in vitro and provides new approach in cell therapies in neurodegenerative diseases and active bone reunion. In a study, large bone defects were treated with vascular endothelial growth factor (VEGF) transfected BM stem cells using a coralline scaffold [15]. In tissue engineering and regenerative medicine, to stimulate angiogenesis and bone formation, VEGF and a bone morphogenetic proteins have been used [16]. Additionally, MSCs contribute to understanding the background of age-dependant changes about adipogenesis [17] and reduced bone mineral density [18]. Cardiomyogenic potential of UCB stem cells have been demonstrated in 1–3 day old Sprague-Dawley rats, which had doxorubicin, induced cardiac hypertrophy [19]. As it is, human UC stem cells have been used in animal models in correcting spinal cord injury by improving axonal regeneration [20]. Moreover, cartilage defects were shown to be corrected by UCB derived multilineage progenitor cells [21]
The present study aimed to isolate stem cells from human dental pulp and to study in vitro proliferation, neurogenic lineage differentiation, osteocyte and adipocyte differentiation of DPSCs. Currently, the functional involvement of other source of cells in neurotransmission is not clearly defined. We hypothesized that DPSCs whichoriginate from the neural crest cells might be a valuable candidate to repair neurological damage and treat several devastating neurological disorders. However, these cells were previously expected to be utilized for repairing the damaged dental nerve tissues [22]. Various other studies have demonstrated the DPSCs characteristics and their potential to make neurogenic cells in vitro under various micro environments. However, for clinical translation of these cells requires a significant quantity of cells with similar growth potential and molecular profile and can be maintained long term without any potential risk. In addition, the differentiation protocols should be easy and acceptable which could provide desired cell type for different applications.
As stem cells derived from dental pulp too are capable of giving rise to hematopoietic, epithelial, endothelial and neural progenitor cells [5], those have become a promising tool helping to understand how neurological disorders, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and spinal cord injury are expressed and ultimately to develop stem cell-based therapies [23]. Osteocytes and adipocytes were grown in vitro from UC-MSCs in specified media and the chromosomal analysis of UC-MSCs confirmed the diploid karyotypic pattern. Prima facie, in the murine pre-adipocyte cell lines 3T3-L1 and 3T3-F442A, the characteristics and molecular mechanism underlying adipocyte differentiation have been extensively investigated [24, 25]. Increased adipose tissue mass associated with obesity is due to the increased number and size of adipocytes differentiated from MSCs [26, 27]. Thus the role of MSCs in obesity could help further in proper management of the malady. Moreover, those are potential to contribute to tissue homeostasis by replenishment of cells or regeneration of tissue after an injury [28].
5. Conclusions
In this study stem cells were isolated from dental pulp. Viability of isolated DPSCs wwere studied by several viability methods indicated that cells were live after culturing in DMEM. DPSCs expressed stem cell markers CD 19, CD 73, CD 90, CD 105 and are negative for CD 34, CD 45. Immunocytochemical staining showed cells were positive for transcription factors Oct-4 and SOX 2 in both control cells and cells after 7 days of culture, confirming those as MSCs.After 7 days of passaging cells express neural cell markers oligodendrocyte and glial fibrillary acidic protein. osteocytes and adipocytes were too grown from dental pulp-MSCs. Those can be used for the known therapeutic purposes and also for further research.
Declarations
Author contribution statement
A. Luke, S, Kuriadom and S. Abu-Fanas: Conceived and designed the experiments; Performed the experiments.
R. Patnaik: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. Mathew and K. Shetty: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by a grant given by AJMAN UNIVERSITY (IRG-2018-A-DEN-02).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Dean, Ajman University, for giving us facility to carry out the research work. We appreciate the help of research committee.
References
- 1.Park J.H., Kim S.J., Oh E.J., Moon S.Y., Roh S.I., Yoon H.S. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol. Reprod. 2003;69:2007–2014. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- 2.Rosler E.S., Fisk G.J., Ares X., Irving J., Miura T., Rao M.S., Carpenter M.K. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev. Dynam. 2004;229:259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M., BlancK L., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells, the international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Liu L., Chai J., Han Y., Sun T., Li D., Zhao J. Research progress of biological characteristics and advantages of Wharton’s jelly mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:745–749. [Article in Chinese] [PubMed] [Google Scholar]
- 5.Achyut B.R., Varma N.R., Arbab A.S. Application of umbilical cord blood derived stem cells in diseases of the nervous system. J. Stem Cell Res. Ther. 2014;4:5. doi: 10.4172/2157-7633.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prentice D.A. Adult stem cells. Issues Law Med. 2004;19:265–294. [PubMed] [Google Scholar]
- 7.Wang R., Zhu C.Z., Qiao P., Liu J., Zhao Q., Wang K.J., Zhao T.B. Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells. Asian Pac. J. Trop. Med. 2014;7:262–266. doi: 10.1016/S1995-7645(14)60034-1. [DOI] [PubMed] [Google Scholar]
- 8.LV F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concisereview: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 9.Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 10.Ullah I. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016;154:39–51. doi: 10.1016/j.lfs.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Alkhalil M., Smajilagic A., Redzic A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med. Glas. 2015;12:27–32. [PubMed] [Google Scholar]
- 12.Tatullo M., Marrelli M., Shakesheff K.M., White L.J. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J. Tissue Eng. Regenerat. Med. 2015;9:1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- 13.Khanna-Jain R. Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. Stem Cell Res. Ther. 2012;2:4. [Google Scholar]
- 14.Scott M.A., Nguyen V.T., Levi B., James A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Res. Dev. 2011;20:1793–1804. doi: 10.1089/scd.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger F. Bone substitute effect on vascularization and bone remodeling after application of phVEGF165 transfected BMSC. J. Funct. Biomater. 2012;3:313–326. doi: 10.3390/jfb3020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur. J. Cell Mat. 2014;27:1–12. doi: 10.22203/ecm.v027a01. [DOI] [PubMed] [Google Scholar]
- 17.Gregoire F.M. Adipocyte differentiation: from fibroblast to endocrine cell. Exp. Biol. Med. (Maywood). 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- 18.Gimble J.M., Zvonic S., Floyd Z.E., Kassem M., Nuttall M.E. Playing with bone and fat. J. Cell. Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath S., Vanamala S.K., Gondi C.S., Rao J.S. Human umbilical cord blood derived stem cells repair doxorubicin-induced pathological cardiac hypertrophy in mice. Biochem. Biophys. Res. Commun. 2010;395:367–372. doi: 10.1016/j.bbrc.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues L.P., Iglesias D., Nicola F.C., Steffens D., Valentim L., Witczak A., Zanatta G., Achaval M., Pranke P., Netto C.A. Transplantation of mononuclear cells from human umbilical cord blood promotes functional recovery after traumatic spinal cord injury in Wistar rats. Braz. J. Med. Biol. Res. 2012;45:49–57. doi: 10.1590/S0100-879X2011007500162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi Y.S., Im M.W., Kim C.S., Lee M.H., Noh S.E., Lim S.M., Kim S.L., Cho C.G., Kim D.I. Chondrogenic differentiation of human umbilical cord blood-derived multilineage progenitor cells in a telocollagen. Cytotherapy. 2008;10:165–173. doi: 10.1080/14653240701817002. [DOI] [PubMed] [Google Scholar]
- 22.Fatima N., Khan A.A., Vishwakarma S.K. Immunophenotypic and molecular analysis of human dental pulp stem cells potential for neurogenic differentiation. Contemp. Clin. Dent. 2017;8:81–89. doi: 10.4103/ccd.ccd_998_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra M., Brito C., Costa E.M., Sousa M.F., Alves P.M. Integrating human stem cell expansion and neuronal differentiation in bioreactors. BMC Biotechnol. 2009;9:82. doi: 10.1186/1472-6750-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- 25.Moseti D., Regassa A., Kim W.K. Molecular regulation of adipogenesis and potentialanti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016;19(1):17. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd P.R. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 27.Wu C.L., Diekman B.O., Jain D., Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int. J. Obes. 2013;37:1079–1087. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodward W.A., Chen M.S., Behbod F., Rosen J.M. On mammary stem cells. J. Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]