Abstract
Ovarian hormones are associated with risk for binge eating in women. Recent animal and human studies suggest that food-related reward processing may be one set of neurobiological factors that contribute to these relationships, but additional studies are needed to confirm and extend findings.
Keywords: binge eating, estrogen, ovarian hormones, progesterone, reward
Introduction
Binge eating [BE; consuming an objectively large amount of food in a short period of time accompanied by loss of control over eating (2)] is a core symptom of most eating disorders (e.g., bulimia nervosa, binge eating disorder, other specified feeding or eating disorders) (2). Sex differences in BE are robust and substantial, with female-to-male ratios ranging from ∼2:1 to 4:1 (56). Recent research suggests that ovarian hormones likely influence food intake and BE behaviors in adult females across species (6, 56, 72). Ovarian hormones are steroid hormones that include both estrogens and progestogens, although estradiol and progesterone are the most typically studied in relation to behavior, including food intake and BE. In general, overall levels of food intake (6, 29), BE (62, 114), and emotional eating (i.e., overconsumption of food in response to negative emotions, a strong correlate and precursor to BE) (57–59, 84) decrease when estradiol levels are high across the menstrual cycle (i.e., during pre-ovulation) but increase when estradiol levels are lower (i.e., during post-ovulation) (37, 61, 64, 66, 83). Progesterone alone does not consistently alter BE or eating behaviors (20, 52, 115); however, progesterone indirectly influences food intake and BE by antagonizing the anorexic effects of estrogen, which leads to increases in both consummatory behaviors in post-ovulatory periods when progesterone levels peak (5, 6, 69) (FIGURE 1). Similar effects of both estrogen and progesterone on food intake have been observed across the reproductive cycle and through direct hormone manipulations (e.g., via ovariectomies and exogenous hormone administration) in animals (5).
FIGURE 1.
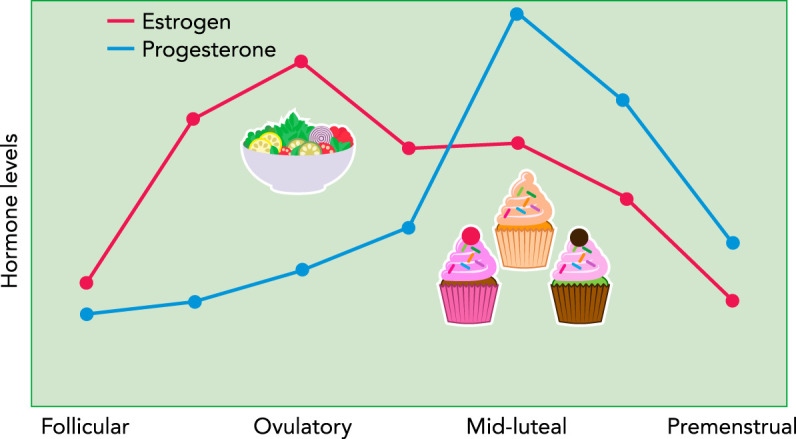
Changes in ovarian hormones and binge eating across the menstrual cycle in women
Hormone levels are depicted as z scores to show changes from baseline (i.e., a level of 0) across the cycle. Rates of binge eating are low during pre-ovulation (i.e., follicular and ovulatory phases) when estradiol levels are increasing and progesterone levels are low. Binge eating significantly increases during post-ovulation, particularly during the mid-luteal phase when estradiol levels are lower and progesterone levels are high.
Moving forward, it will be important to identify the neural substrates involved in the effects of ovarian hormones on BE. The purpose of the present review is to summarize emerging evidence examining the mesolimbic dopaminergic pathway as one system involved in these hormone effects. The mesolimbic dopaminergic pathway projects from the ventral tegmental area (VTA) to the ventral striatum [including the nucleus accumbens (NAcc)] (82), as well as the hippocampus, amygdala, and medial prefrontal cortex, which in turn sends inputs to the NAcc (82) (FIGURE 2). Similar to BE, the dopaminergic circuits are sexually differentiated (24, 28, 112) and regulated by ovarian hormones (12, 33, 46, 71, 81, 103, 108). Changes in dopaminergic functioning have been observed across the reproductive cycle in women (e.g., Ref. 88), non-human primates (e.g., Ref. 30), and other animals (e.g., Ref. 79), and ovarian hormones are known to influence dopaminergic neurotransmission during synthesis, release, turnover and degradation on both pre- and postsynaptic receptors and transporters (12). Despite some mixed findings (see Ref. 12), most researchers currently agree that estrogen facilitates dopaminergic neurotransmission (50, 89, 97, 109), whereas the effects of progesterone may partly depend on the presence of estrogen (13, 23, 34).
FIGURE 2.
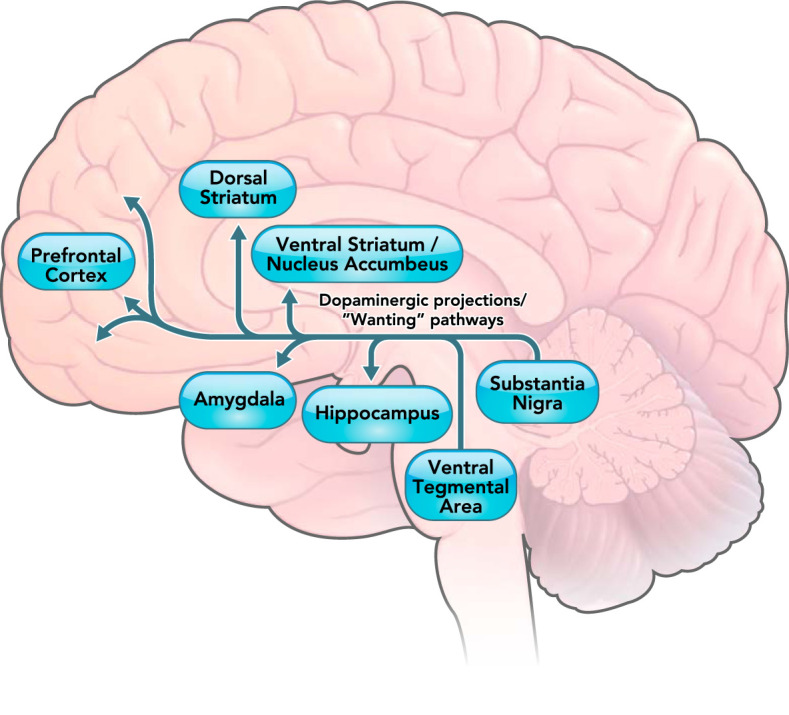
The mesolimbic dopaminergic pathway involved in “wanting” of rewards in humans
The mesolimbic dopaminergic pathway projects from the ventral tegmental area to the ventral striatum, hippocampus, amygdala, and prefrontal cortex. The projection from prefrontal cortex to the nucleus accumbens is not a dopaminergic pathway and is thus not shown in the figure. Previous research (18) has demonstrated that projections shown in the figure are associated with motivational “wanting” of rewards.
Importantly, palatable food (PF; as opposed to nutritionally balanced food) is a natural reinforcer that reliably activates these neural pathways (18). BE is marked by the overconsumption of PF (rather than nutritionally balanced foods) (60) and may therefore stem, in part, from disruptions in these pathways. Understanding the role of ovarian hormones/dopamine in PF intake is therefore likely to inform understanding of BE, much like studies of hormones/dopamine effects on excessive alcohol/drug intake inform etiological models of addiction (14, 65). Although very few studies have examined ovarian hormone regulation of these reward system alterations for BE per se, many more have examined these processes for PF intake.
Given the above, the purpose of this narrative review is to summarize studies examining ovarian hormone influences on dopaminergic reward pathways in PF intake.1 We begin the review with a short description of the types of reward processes (i.e., reward “wanting”) that are likely important for hormone-dopaminergic pathway effects on PF intake. We then review studies directly examining these hormone/reward associations in both animals and humans. We end the review by discussing directions for future research and the implications of the findings for understanding ovarian hormone effects on BE. Notably, we primarily focus our review on studies in adulthood, since only one study has examined the effects of puberty on ovarian hormone/reward/PF intake associations [see discussion of Reichelt et al. (87) below].
Dopaminergic Reward Processes in PF Intake and BE
Overconsumption of PF is a key feature of BE (78). The vast majority of food consumed during a binge episode is high in sugar, fat, or both [e.g., cake, cookies, French fries (43, 51)]. BE on nutritionally balanced or low-calorie foods is very rare (40, 51, 94). Likewise, rats that are identified as BE prone (BEP) overconsume PF (e.g., vanilla frosting) but do not overconsume nutritionally balanced food (i.e., chow) (56, 60).
As noted above, PF intake activates circuits in the mesolimbic dopaminergic pathway that are regulated by hormones and are sexually differentiated (85, 86). Dopamine neurotransmission in this pathway is associated with reward “wanting” (16–18, 21, 75, 91, 92) (FIGURE 2), which is the motivation to approach a salient stimulus. In animals, reward “wanting” is typically measured by motor responses to the stimulus [e.g., lever pressing during progressive-ratio (PR) schedules, which require an increasing number of responses for the delivery of a reinforcer, using the break-point, or the amount of work the animal is willing to engage in to obtain a reward as a measure for motivation] (e.g., see Ref. 26). In humans, reward “wanting” is often measured by participants’ self-report rating for how much they want the food or by measuring neural activation in key dopaminergic areas while presenting food images (e.g., Ref. 4). Reward “wanting” is not the same as reward “liking,” defined as the pleasant/hedonic experience associated with reward receipt/consumption, which is often measured in animals by the amount of licking after having a small taste of food (18, 104), and in humans by having participants rate how much they like a food after tasting it (39). Reward “liking” is associated with the activation of the opioid circuitry in the brain stem, ventral pallidum, NAcc, and prefrontal cortex (18). Very few studies have examined links between hormones, reward “liking,” and PF intake or BE. In contrast, more studies have examined associations between hormones/“wanting”/PF intake, and substantial research has shown phenotypic associations between BE and “wanting” of PF (75), as well as disruptions to the dopaminergic reward neural circuitry in BE (7, 8, 35, 48, 53, 68, 105). Indeed, researchers have coined the term “food addiction” to highlight conceptual parallels between processes of reward “wanting” in BE and substance dependence (49, 101, 102). Thus this review focuses on dopaminergic pathways that may underlie “wanting” due to the limited data available for hormones, PF intake, BE, and reward “liking.”
Ovarian Hormones, Dopaminergic Reward Processes, and PF Intake
Animal Studies
Initial findings suggest that estrogen may reduce motivational “wanting” of PF in animals. Although some studies show no effects of ovarian hormones on lever-pressing to receive PF during PR schedules (70), others show significant differences in reward motivation across estrous phases. For example, Contini and colleagues (26) found that intact female rats in proestrus and estrus had fewer lever responses under a FR schedule and consumed less chocolate-flavored beverage than those in diestrus and metestrus. Intact female rats in proestrus and estrus also had attenuated increases in extracellular dopamine dialysate from the NAcc shell during both anticipation and consumption of the beverage, which may reflect lower sensitivity to motivational values of conditioned stimuli and reduced motivational drive for obtaining the beverage reward (26). The authors interpreted their results as reflecting an effect of estrogen on the motivational properties of PF across phases, since they noted that estradiol is relatively higher during proestrus and estrus (which should cause fewer lever presses) than during diestrus and metestrus (26). Notably, however, it is possible that results reflect an extended and delayed effect of the initial increase of estradiol during diestrus or the peak estradiol levels that occur during proestrus on later beverage intake.
Richard and colleagues (90) also found significant estrogen effects on motivation for PF. In naturally cycling female rats, motivation for sucrose pellets (measured on a PR schedule) was lower during estrus than diestrus, which they interpreted as reflecting the delayed behavioral effects of peak estradiol levels during proestrus. Subcutaneous estradiol treatment in OVX rats also decreased motivation for sucrose reward after a delay (i.e., 24-h post-injection), and injections of estradiol directly into the VTA of OVX rats resulted in reduced motivation for PF reward after 1 h (but not after 24 h). The authors noted that delayed effects on motivation for PF across estrous phases and after subcutaneous estradiol treatment suggest that estrogen may function at other neural sites in addition to the VTA and/or through slower mechanisms (e.g., genomic, transcription, and translation processes) to control food-reward response (90). Overall, the attenuated motivation for PF reward after peak estradiol levels (either during estrus or due to exogenous injection) is consistent with previous research showing decreased BE with high estradiol levels in both animals (6) and women (56). Importantly, no differences in chow intake were observed between intra-VTA estradiol-treated OVX rats and oil-treated OVX controls (90), suggesting that estrogen may act directly in the mesolimbic reward circuitry to specifically control PF intake.
These results mirrored findings from an earlier study by Uban and colleagues (109), who examined estradiol modulation of female rats’ effort-based decision-making for obtaining sugar pellet rewards. When faced with the option to lever press once to receive two pellets (i.e., low effort and low reward) or lever press more times to receive four pellets (i.e., high effort and high reward), OVX, food-restricted rats were significantly more likely to choose the high-effort and high-reward option post-OVX compared with pre-OVX. Higher (but not lower) doses of exogenously administered estradiol significantly reduced this tendency, as did joint injections of an estrogen receptor α agonist [propyl-pyrazole triol (PPT)] and an estrogen receptor β agonist [diarylpropionitrile (DPN)]. These decision-making patterns may suggest that removal of ovarian hormones increases motivation for PF reward and that exogenous estradiol or activation of estrogen receptors in OVX rats decreases motivation. All of these findings were more pronounced on the day after the administrations/injections rather than the day of, suggesting that the effects may be genomic in nature (109). Significant changes in decision-making were not observed in intact female rats across their estrous cycle; however, the consistent effects in OVX rats across experimental manipulations suggest estrogen may attenuate wanting for PF reward.
Finally, Reichelt and colleagues (87) found that adult female rats that had been exposed to cherry-flavored sucrose liquid during adolescence showed higher breakpoints on a PR schedule than adult control females. Adult male rats that had been exposed to sucrose had lower breakpoints than control males (87). Although sample sizes were too small to examine estrous-cycle differences in adulthood in this study, findings suggested that ovarian hormones may work synergistically with early reward experiences to alter responses to food reward in adulthood.
In summary, animal studies provide evidence of hormone effects on motivation for PF. Findings across estrous phases and with ovarian hormone removal suggest that estrogen may attenuate the “wanting” of PF reward in female animals (FIGURE 1). Nonetheless, studies were relatively few in number, and the use of different types of PF makes it somewhat difficult to compare results. Most animal studies used sucrose as the food reinforcer, but caloric content and mode of delivery (i.e., beverage vs. pellets) varied. Because different types of foods (e.g., high-sugar, high-fat, or high-fat and -sugar) may produce different reward responses and act differently on the gustatory and oral somatosensory systems (106), all of which are regulated by ovarian hormones (64), future research should examine whether PF content impacts ovarian hormone/reward response associations. Last, although some studies measured dopaminergic activity along with behavioral responses, causal relationships between changes in dopamine measures (e.g., concentrations of dopamine release, concentrations of dopamine metabolites, dopamine utilization rates) and motivated behavior toward food have yet to be examined/identified.
Human Studies
In humans, neuroimaging methods have been the primary tool for studying ovarian hormone effects on brain reward circuitry. Most studies have used menstrual cycle phase as a proxy for ovarian hormone levels, and they have often scanned women when fasting and sated to examine differential mechanisms related to homeostatic (fasting state) versus non-homeostatic (sated state) feeding (1, 25). Some (1) have argued that studies examining fasting states may tap homeostatic systems and be less readily interpretable in the context of PF reward. However, because caloric restriction is common in eating disorders involving BE (22, 45), examining neural activation when presented with food stimuli during fasting may lend additional insights into neural mechanisms of BE. Importantly, all of the studies used visual images of PF, control foods, or non-food controls (e.g., jewelry), and did not deliver actual food reward to the participants. Some studies asked women to indicate whether they “wanted” the object in the image (4); these studies were more clearly assessing reward wanting. However, some studies asked women to rate how “appealing” the object seemed to them (1, 110) or to imagine eating the food (41). These prompts are more difficult to categorize as wanting versus liking, although they likely tap anticipation for actual food reward, which is associated with motivational “wanting” of a reward rather than the hedonic experience of reward “liking” at receipt of the reward. The concept of food embedded in the images may indeed trigger sudden urges and thoughts to consume PF and BE (17, 18). Nonetheless, the variability in visual prompts/instructions should be kept in mind when reviewing the studies.
Four studies have used functional magnetic resonance imaging (fMRI) to examine neural responses to food across menstrual cycle phases, and all four obtained some evidence for ovarian hormone effects on motivational “wanting” of PF. Arnoni-Bauer and colleagues (4) examined cycle changes in neural responses to images of PF (e.g., cake, pizza) and non-food objects (e.g., hammer) in 18 normal-weight women without eating disorders. In both the fed and the fasting (i.e., 10-h overnight fast) states, women showed significantly stronger activation to PF in the amygdala and ACC during the mid-luteal phase (when estradiol levels have decreased and progesterone levels are high) compared with the follicular phase (when estradiol levels are high and progesterone levels are low). Visual regions (i.e., calcarine and lateral occipital cortex) also showed greater activation during the mid-luteal phase than the follicular phase and were more activated in the fasting versus the fed state in both phases. As the authors suggested, greater activation in visual regions during the luteal phase may reflect heightened attention to visual food cues (54), which may be mediated by regions in the reward system (e.g., amygdala) that project to the visual cortex (63). Eleven women on monophasic combined oral contraceptives (COCs) were also studied to examine exogenous hormone effects. Monophasic COCs suppress endogenous ovarian hormones but provide exogenous levels of estrogens and progestins that achieve an estradiol-to-progestin ratio reminiscent of the luteal phase of the menstrual cycle (4, 100). Importantly, the COC group showed neural activation to food cues equivalent to that of normally cycling women in the mid-luteal phase but significantly greater activation in the amygdala than cycling women in the follicular phase. This latter effect would be expected, given that the constant levels of exogenous estrogen and progestins in women taking COCs would lead to elevated activation in reward areas across all phases, whereas the naturally cycling women would be expected to have lower activation during the follicular phase when estradiol levels are high and progesterone levels are low. Finally, in the fasting state, there was stronger activation in the insula and hypothalamus during the mid-luteal compared with the follicular phase. The authors interpreted these findings as increased sensitivity to food stimuli and homeostatic hunger during the mid-luteal phase (4). This interpretation extends previous findings showing significantly higher levels of BE during menstrual cycle phases marked by higher progesterone and lower estradiol levels; it suggests that ovarian hormones may affect PF intake (and possibly BE) through changes in neural processing of stimuli that may possess motivational value.
Frank et al. (41) also found some evidence for differential activation across menstrual cycle phase in their study of 12 normal-weight women (screened to be free of eating disorder symptoms) who were tested in a fasting state (i.e., 6–8 h after breakfast). Findings revealed greater activation in the right orbitofrontal cortex (OFC) and the mid-cingulum, structures involved in reinforcement learning, in response to images of PF (e.g., pasta, cake) versus low-calorie (e.g., fruit) food during the luteal compared with the periovulatory (i.e., when estradiol peaks and progesterone is low) phase. Although activation in other major reward-related structures (e.g., NAcc, hippocampus) was greater in the periovulatory versus the luteal phase regardless of contrast [i.e., PF vs. control images (e.g., flowers), PF vs. low-calorie images, low-calorie vs. control images], differential clusters of activation were observed within and across phase that suggested a hormone effect on PF reward. Specifically, 33 clusters of contiguous voxels in the corticolimbic brain regions showed activation in response to both PF and low-calorie food cues during the periovulatory phase, but only 10 clusters were activated during the luteal phase, and only in response to PF cues (41). Because more neural clusters were activated in the periovulatory versus the luteal phase in this study, a corticolimbic neural network analysis could possibly reveal a more segregated (and thus complex/efficient) neural network in the periovulatory phase, which would suggest network disruption in the luteal phase for PF intake that may create risk for BE (96). Future studies should investigate this possibility by examining brain regions as functioning in an interactive network in addition to their individual activities.
Finally, both Alonso-Alonso et al. (1) and Van Vugt (110) found greater activation in visual regions involved in reward processing across menstrual cycle phases. Importantly, both studies asked women to rate how “appealing” the food and non-food images were, and these prompts may have led to increased activation in these visual regions relative to studies using other prompts [e.g., rating how much the women “want” the food (1, 110)]. In terms of study findings, no significant menstrual cycle phase differences were observed in the hippocampus, amygdala, or NAcc; however, Alonso-Alonso et al. (1) found that neural activation to food images (PF and low-calorie foods) versus non-food images (e.g., jewelry) was significantly greater in the fusiform gyrus during the early follicular (when estradiol and progesterone levels are low) compared with the late follicular (when estradiol levels are high and progesterone levels are low) phase after an overnight fast. Importantly, this study also directly assessed estradiol levels before the fasting-state scan and observed significant inverse associations between estradiol levels and fusiform gyrus activation (i.e., stronger activation at lower vs. higher estradiol levels). The authors noted that the fusiform gyrus belongs to the ventral visual pathway involved in reward processing (1), and it interacts with the OFC to alter reward valuation (74). Thus the authors concluded that estrogen may partially exert its anorexigenic effects by reducing the salience of visual food cues in a fasting state. Notably, Van Vugt (110) also found that the fusiform was the only region activated in response to food during the luteal phase, whereas several brain regions directly involved in reward processing (e.g., lateral OFC, prefrontal cortex, hippocampus, amygdala) along with the fusiform were activated during the periovulatory phase (110). Interestingly, the insula was the only region showing differential response to PF versus low-calorie food cues in this study; the insula was stimulated only by low-calorie food cues in the late follicular phase, and only by PF cues in the luteal phase (110).
Overall, findings from human imaging studies are suggestive of significant hormone effects on major reward pathways, including dopaminergic pathways, for PF stimuli. All studies showed some increased activation in brain reward pathways during “high risk” hormonal milieus (e.g., lower estradiol or higher estradiol with higher progesterone). However, non-significant results were also obtained (1), and, in some cases, the opposite effects were observed (4, 110). Somewhat mixed findings may be due, in part, to methodological shortcomings. Sample sizes were quite small, leading to instability of findings and the potential for type II errors. The vast majority of studies did not examine estrogens or progestogens but instead relied on menstrual cycle phase as a proxy for these hormones. Because ovarian hormone levels vary between individuals even within phase, it is difficult to confirm that phase differences reflect actual estrogen and progestogen effects, and it is also difficult to disentangle estrogen versus progestogen influences. Moreover, there may be nonlinear effects of ovarian hormones on the reward system. Specifically, ovarian hormones may exert an inverted U-shape effect on reward processes, where higher concentrations of the hormones inhibit dopamine activity (31, 113).
The menstrual cycle phases examined also varied significantly between studies, and not all of them examined the luteal phase when PF intake and BE are elevated in women (1). Neural activation was measured in response to visual food cues rather than actual food stimuli, and studies differed in the instructions/verbal prompts for the scanning tasks, the prandial states examined, and different requirements for the type (e.g., overnight vs. skipping lunch) and length of fasting. These methodological limitations and differences make it more difficult to compare results across studies and identify replicable effects.
Finally, it is important to note that the variability in fMRI results may be due to the complexity involved in neural processing of motivational stimuli, which are difficult to disentangle given the resolution of functional imaging techniques. For instance, subjective valuation and attentional salience of reward both contribute to motor response toward reward (21). Although neurons coding for motivational salience are strongly excited in the presence of both rewarding and aversive stimuli (21), other neurons may show more selective activation in response to reward value (93). In addition, one function of dopaminergic neurons is to signal surprise/prediction errors (98), so when stimuli are presented in random order, as is often done in imaging studies, it is possible that areas with high concentrations of dopamine neurons are merely responding to the constant unpredictability. Additional studies using adequately large samples, assessments of actual hormone levels, standardization of prandial state and verbal prompts/instructions, and a variety of random and non-random food cues (e.g., visual images, actual food) are needed to clarify results from extant studies.
Concluding Remarks
Research investigating ovarian hormone regulation of reward pathways for PF intake is in its nascent stage. There are strong theoretical and conceptual underpinnings to the work, since estrogen and progesterone are associated with significant changes in PF intake and BE in women (37, 44, 56, 61, 66, 83) and female animals (5, 6, 69, 111). Understanding the extent to which functioning in the mesolimbic dopaminergic pathway mediates these associations could substantially advance the field and enhance etiological models of basic appetitive processes as well as pathological eating.
Existing data are promising in suggesting that neural systems involved in dopaminergic reward “wanting” may be involved in associations between ovarian hormones and PF intake in women (see FIGURE 3). Findings from animal studies suggest that estrogen may act on the mesolimbic dopaminergic system to reduce motivation for reward from PF, and progesterone may exert the opposite effect, potentially through attenuating the effects of estrogen. Results from imaging studies generally show changes in functional activation in brain regions involved in motivation under different ovarian hormonal milieus. In combination with previous studies showing associations between ovarian hormones and BE/PF intake, these results provide initial, preliminary support for a potential role of neural systems involved in processing motivational rewards (i.e., stimuli that may trigger motivational wanting) in hormone-PF associations. However, additional studies that address the methodological limitations above and more clearly target motivational “wanting” and the dopaminergic systems involved in these reward processes are needed.
FIGURE 3.
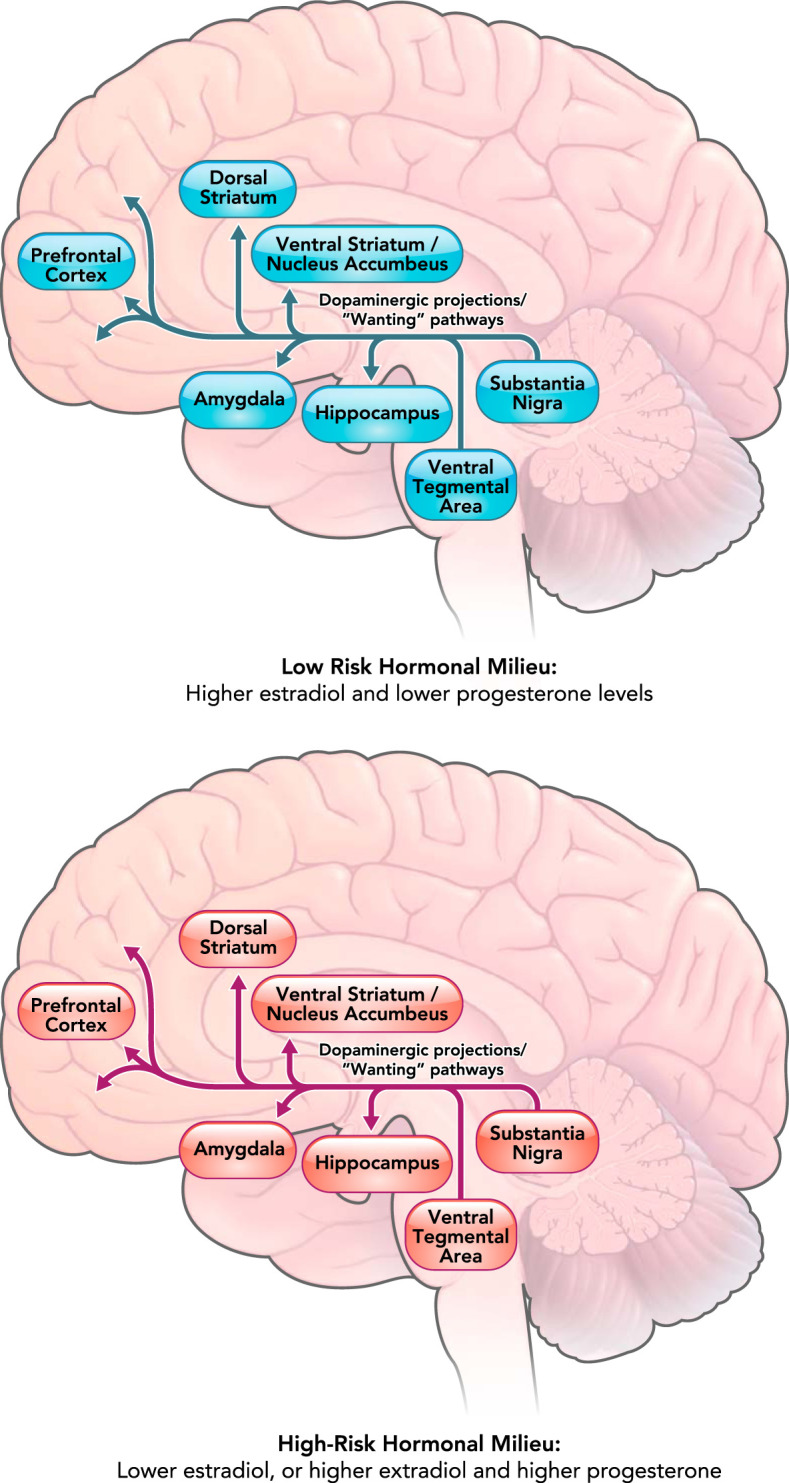
Differential activation of the mesolimbic dopaminergic pathway involved in “wanting” of rewards across low-risk versus high-risk hormonal milieus
The mesolimbic dopaminergic pathway projects from the ventral tegmental area to the ventral striatum, hippocampus, amygdala, and prefrontal cortex. The projection from prefrontal cortex to the nucleus accumbens is not a dopaminergic pathway and is thus not shown in the figure. The dopaminergic circuitry has been shown to be associated with motivational “wanting” of rewards (18) and may be more sensitive to palatable food when estradiol levels are low and/or when estradiol and progesterone levels are both high. Differential activation of the dopaminergic system in different hormonal milieus may lead to increased “wanting” of palatable food and potentially binge eating in women when estradiol is low or when estradiol and progesterone are both high.
There is one neuroimaging study that directly examined associations between ovarian hormones and a BE phenotype (i.e., emotional eating) (15), although sample sizes were quite small (n = 10 women) and the study examined resting-state function rather than task-/cue-elicited wanting of PF. Nonetheless, findings revealed significant associations between estradiol levels and connectivity in the default mode network, such that women who were higher on emotional eating showed weaker connectivity from the right to left lateral parietal cortices and weaker associations between this connectivity and estradiol levels than women who scored lower on emotional eating. No significant associations were observed for the reward network, although the absence of a cue-elicited probe for PF may have contributed to these non-significant results. Nonetheless, this study provides a model for the type of projects that are needed to understand hormone-neural-BE associations in women. Notably, although additional imaging studies would be ideal and provide key neural levels of analysis, purely behavioral studies of changes in “wanting” of PF across estrous/menstrual cycle phases and hormone levels are also needed and could provide important corroborating data in support of a role for reward systems and mesolimbic dopaminergic pathways in hormone-BE associations. Findings from Frank et al. (41) showed decreased “appeal” ratings for PF during weeks of the menstrual cycle marked by high levels of estradiol (i.e., week 2, the late follicular phase/ovulation); although it is unclear whether appeal ratings tap “wanting” or “liking” of PF, differential responses to PF across menstrual cycle phase provide initial support for behavioral studies of hormone effects on reward processes in PF intake and BE.
Another important area for future research is understanding how and whether the homeostatic system alters associations between ovarian hormones, reward/dopaminergic systems, and BE. Unlike other types of rewards (e.g., alcohol, drug, non-food reward), PF intake activates homeostatic processes that likely interact with or impact reward-driven behavior and networks. These interactions and processes may make hormone-reward-BE associations different from those for other types of rewards. In fact, estrogen appears to have opposite effects on non-food reward responses compared with its effects on BE. Although estrogen tends to decrease BE (6, 56) and motivation for food reward (see review above), substantial evidence indicates that estrogen enhances subjective and physiological responses to alcohol/drug rewards (14, 73) and, less consistently, monetary rewards (9, 36, 67, 77, 107). Progesterone, in contrast, is associated with increases in BE but attenuated responses to substances (80) and other non-food rewards (32, 36, 67, 107). These findings suggest differential mechanisms of ovarian hormone influences on BE and PF intake versus non-food rewards.
One explanation could be ovarian hormone regulation of neural functions in regions traditionally placed in the homeostatic system (64), which controls food intake in concert with systems controlling motivational reward processing (76). Substances and other non-food rewards presumably would not activate homeostatic mechanisms to the same extent as food reward, and the influence or interaction between reward and homeostatic mechanisms may make ovarian hormone influences categorically different for BE and food reward versus other types of rewarding stimuli.
This may be particularly true given anatomical and functional neural evidence that areas traditionally thought to separately control homeostasis and motivation for reward work synergistically to influence food intake (19, 95). Moreover, recent evidence also suggests that homeostasis may be maintained through allostasis (i.e., regulation through change, for example, by anticipating the body’s needs and preparing for the maintenance of stability before the needs arise) (10, 11, 99). Different rewards (e.g., food vs. substances) may therefore possess different allostatic value to the individual, depending on homeostatic state and hormonal status, particularly since ovarian hormones may alter both interoceptive signaling of homeostatic state and allostatic control (3, 42). Women who engage in BE have been shown to have altered interoceptive awareness for physiological states (38) (e.g., hunger and satiety states), and these alterations, coupled with the dietary restriction that is common with BE (22, 45), may substantially impact the dopaminergic system (25) and differentially alter motivation for PF and its associations with ovarian hormones. Interestingly, one of the neural networks important for allostatic-interoceptive functions is the default mode network (55) that has been shown to be differentially associated with estrogen in women who engage in high versus low levels of emotional eating [see description of Beltz et al. (15) above]. The Beltz et al. (15) data linking hormones and the default node network are preliminary, and hypotheses about hormone-homeostatic-reward associations are speculative, but they highlight a promising area for future research. Indeed, elucidating the extent to which homeostatic and interoceptive processes may alter hormone/reward functioning across different types of rewards could increase understanding of the etiology of a range of “addictive-like” behaviors in women.
Acknowledgments
Current affiliation of K. M. Culbert: Department of Family Medicine & Public Health Sciences, Wayne State University School of Medicine, Detroit, Michigan.
No conflicts of interest, financial or otherwise, are declared by the author(s).
R.M. and K.L.K. drafted manuscript; R.M., M.E.M., K.M.C., A.J., C.L.S., and K.L.K. edited and revised manuscript; K.L.K. approved final version of manuscript.
Footnotes
Notably, although this is a narrative review, we feel it is important to include our search strategy so that others can replicate and extend our work. We used combinations of the following terms in our literature search, with no restrictions placed on the study publication year or any other parameters: “ovarian hormones,” “estrogen,” “estradiol,” “progesterone,” “progestogen,” “food,” “eating,” “binge eating,” “reward,” and “wanting.” We also reviewed all reference sections and included any relevant additional papers.
References
- 1.Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, Rifkin-Graboi A, Yannakoulia M, Rojas R, Pascual-Leone A, Mantzoros CS. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr 94: 377–384, 2011. doi: 10.3945/ajcn.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 3.Andreano JM, Touroutoglou A, Dickerson B, Barrett LF. Hormonal cycles, brain network connectivity, and windows of vulnerability to affective disorder. Trends Neurosci 41: 660–676, 2018. doi: 10.1016/j.tins.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnoni-Bauer Y, Bick A, Raz N, Imbar T, Amos S, Agmon O, Marko L, Levin N, Weiss R. Is it me or my hormones? Neuroendocrine activation profiles to visual food stimuli across the menstrual cycle. J Clin Endocrinol Metab 102: 3406–3414, 2017. doi: 10.1210/jc.2016-3921. [DOI] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251–1263, 2006. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305: R1215–R1267, 2013. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology 63: 87–96, 2012. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balodis IM, Grilo CM, Potenza MN. Neurobiological features of binge eating disorder. CNS Spectr 20: 557–565, 2015. doi: 10.1017/S1092852915000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banis S, Lorist MM. The combined effects of menstrual cycle phase and acute stress on reward-related processing. Biol Psychol 125: 130–145, 2017. doi: 10.1016/j.biopsycho.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Barrett LF, Quigley KS, Hamilton P. An active inference theory of allostasis and interoception in depression. Philos Trans R Soc Lond B Biol Sci 371: 20160011, 2016. doi: 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci 16: 419–429, 2015. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci 9: 37, 2015. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118: 169–171, 1990. doi: 10.1016/0304-3940(90)90618-J. [DOI] [PubMed] [Google Scholar]
- 14.Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res 95: 136–147, 2017. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltz AM, Moser JS, Zhu DC, Burt SA, Klump KL. Using person-specific neural networks to characterize heterogeneity in eating disorders: Illustrative links between emotional eating and ovarian hormones. Int J Eat Disord 51: 730–740, 2018. doi: 10.1002/eat.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25, 1996. doi: 10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- 17.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 97: 537–550, 2009. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge KC. Evolving concepts of emotion and motivation. Front Psychol 9: 1647, 2018. doi: 10.3389/fpsyg.2018.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthoud H-R, Münzberg H, Morrison CD. Blaming the brain for obesity: Integration of hedonic and homeostatic mechanisms. Gastroenterology 152: 1728–1738, 2017. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielert C, Busse C. Influences of ovarian hormones on the food intake and feeding of captive and wild female chacma baboons (Papio ursinus). Physiol Behav 30: 103–111, 1983. doi: 10.1016/0031-9384(83)90045-8. [DOI] [PubMed] [Google Scholar]
- 21.Bissonette GB, Roesch MR. Neurophysiology of reward-guided behavior: correlates related to predictions, value, motivation, errors, attention, and action. In: Behavioral Neuroscience of Motivation, edited by Simpson E, Balsam P. Cham: Springer, 2015, p. 199–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewerton TD, Dansky BS, Kilpatrick DG, O’Neil PM. Which comes first in the pathogenesis of bulimia nervosa: dieting or bingeing? Int J Eat Disord 28: 259–264, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera R, Díaz A, Pinter A, Belmar J. In vitro progesterone effects on 3H-dopamine release from rat corpus striatum slices obtained under different endocrine conditions. Life Sci 53: 1767–1777, 1993. doi: 10.1016/0024-3205(93)90164-X. [DOI] [PubMed] [Google Scholar]
- 24.Caldú X, Dreher JC. Hormonal and genetic influences on processing reward and social information. Ann N Y Acad Sci 1118: 43–73, 2007. doi: 10.1196/annals.1412.007. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy RM, Tong Q. Hunger and satiety gauge reward sensitivity. Front Endocrinol (Lausanne) 8: 104, 2017. doi: 10.3389/fendo.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contini A, Sanna F, Maccioni P, Colombo G, Argiolas A. Comparison between male and female rats in a model of self-administration of a chocolate-flavored beverage: Behavioral and neurochemical studies. Behav Brain Res 344: 28–41, 2018. doi: 10.1016/j.bbr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol 16: 376–385, 2008. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 29.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav 6: 329–349, 1975. doi: 10.1016/0018-506X(75)90003-3. [DOI] [PubMed] [Google Scholar]
- 30.Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34: 548–554, 2009. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- 31.Diekhof EK. Estradiol and the reward system in humans. Curr Opin Behav Sci 23: 58–64, 2018. doi: 10.1016/j.cobeha.2018.03.010. [DOI] [Google Scholar]
- 32.Diekhof EK, Ratnayake M. Menstrual cycle phase modulates reward sensitivity and performance monitoring in young women: Preliminary fMRI evidence. Neuropsychologia 84: 70–80, 2016. doi: 10.1016/j.neuropsychologia.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Dluzen D, Horstink M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine 21: 67–75, 2003. doi: 10.1385/ENDO:21:1:67. [DOI] [PubMed] [Google Scholar]
- 34.Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology 39: 149–155, 1984. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly B, Touyz S, Hay P, Burton A, Russell J, Caterson I. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J Eat Disord 6: 3, 2018. doi: 10.1186/s40337-018-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 104: 2465–2470, 2007. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med 37: 131–141, 2007. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 38.Fassino S, Pierò A, Gramaglia C, Abbate-Daga G. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology 37: 168–174, 2004. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- 39.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev 31: 987–1002, 2007. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgibbon ML, Blackman LR. Binge eating disorder and bulimia nervosa: differences in the quality and quantity of binge eating episodes. Int J Eat Disord 27: 238–243, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 1363: 81–92, 2010. doi: 10.1016/j.brainres.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 42.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294: E817–E826, 2008. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- 43.Gendall KA, Sullivan PE, Joyce PR, Carter FA, Bulik CM. The nutrient intake of women with bulimia nervosa. Int J Eat Disord 21: 115–127, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry 144: 1592–1595, 1987. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 45.Grilo CM, Masheb RM. Onset of dieting vs binge eating in outpatients with binge eating disorder. Int J Obes Relat Metab Disord 24: 404–409, 2000. doi: 10.1038/sj.ijo.0801171. [DOI] [PubMed] [Google Scholar]
- 46.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170: 1045–1055, 2010. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutson PH, Balodis IM, Potenza MN. Binge-eating disorder: clinical and therapeutic advances. Pharmacol Ther 182: 15–27, 2018. doi: 10.1016/j.pharmthera.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, Jacobs WS, Kadish W, Manso G. Refined food addiction: a classic substance use disorder. Med Hypotheses 72: 518–526, 2009. doi: 10.1016/j.mehy.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci 31: 5286–5293, 2011. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav 48: 837–840, 1990. doi: 10.1016/0031-9384(90)90236-W. [DOI] [PubMed] [Google Scholar]
- 52.Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm Behav 23: 235–250, 1989. doi: 10.1016/0018-506X(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev 63: 223–238, 2016. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Killgore WDS, Yurgelun-Todd DA. Positive affect modulates activity in the visual cortex to images of high calorie foods. Int J Neurosci 117: 643–653, 2007. doi: 10.1080/00207450600773848. [DOI] [PubMed] [Google Scholar]
- 55.Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav 1: 0069, 2017. doi: 10.1038/s41562-017-0069 . New results for this article can be found at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klump KL, Culbert KM, Sisk CL. Sex differences in binge eating: Gonadal hormone effects across development. Annu Rev Clin Psychol 13: 183–207, 2017. doi: 10.1146/annurev-clinpsy-032816-045309. [DOI] [PubMed] [Google Scholar]
- 57.Klump KL, Keel PK, Burt SA, Racine SE, Neale MC, Sisk CL, Boker S. Ovarian hormones and emotional eating associations across the menstrual cycle: an examination of the potential moderating effects of body mass index and dietary restraint. Int J Eat Disord 46: 256–263, 2013. doi: 10.1002/eat.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med 38: 1749–1757, 2008. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol 122: 131–137, 2013. doi: 10.1037/a0029524. An erratum for this article was published in J Abnorm Psychol 122: 137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klump KL, Racine S, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord 46: 729–736, 2013. doi: 10.1002/eat.22139. [DOI] [PubMed] [Google Scholar]
- 61.Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK. Influences of ovarian hormones on dysregulated eating: a comparison of associations in women with versus women without binge episodes. Clin Psychol Sci 2: 545–559, 2014. doi: 10.1177/2167702614521794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav 59: 585–593, 2011. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology 35: 199–210, 1998. doi: 10.1111/1469-8986.3520199. [DOI] [PubMed] [Google Scholar]
- 64.Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update 23: 300–321, 2017. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenz B, Müller CP, Stoessel C, Sperling W, Biermann T, Hillemacher T, Bleich S, Kornhuber J. Sex hormone activity in alcohol addiction: integrating organizational and activational effects. Prog Neurobiol 96: 136–163, 2012. doi: 10.1016/j.pneurobio.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med 33: 51–60, 2003. doi: 10.1017/S0033291702006815. [DOI] [PubMed] [Google Scholar]
- 67.Macoveanu J, Henningsson S, Pinborg A, Jensen P, Knudsen GM, Frokjaer VG, Siebner HR. Sex-steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology 41: 1057–1065, 2016. doi: 10.1038/npp.2015.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: evidence for general rather than specific differences. Psychol Rec 61: 561–582, 2011. doi: 10.1007/BF03395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marrone BL, Roy EJ, Wade GN. Progesterone stimulates running wheel activity in adrenalectomized-ovariectomized rats. Horm Behav 6: 231–236, 1975. doi: 10.1016/0018-506X(75)90010-0. [DOI] [PubMed] [Google Scholar]
- 70.Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro 3: ENEURO.0140-16.2016, 2016. doi: 10.1523/ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–604, 1996. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Micioni Di Bonaventura MV, Lutz TA, Romano A, Pucci M, Geary N, Asarian L, Cifani C. Estrogenic suppression of binge-like eating elicited by cyclic food restriction and frustrative-nonreward stress in female rats. Int J Eat Disord 50: 624–635, 2017. doi: 10.1002/eat.22687. New findings for this article can be found at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moran-Santa Maria MM, Flanagan J, Brady K. Ovarian hormones and drug abuse. Curr Psychiatry Rep 16: 511, 2014. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci 1121: 273–296, 2007. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- 75.Novelle MG, Diéguez C. Food addiction and binge eating: lessons learned from animal models. Nutrients 10: 71, 2018. doi: 10.3390/nu10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novelle MG, Diéguez C. Unravelling the role and mechanism of adipokine and gastrointestinal signals in animal models in the nonhomeostatic control of energy homeostasis: Implications for binge eating disorder. Eur Eat Disord Rev 26: 551–568, 2018. doi: 10.1002/erv.2641. [DOI] [PubMed] [Google Scholar]
- 77.Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernández G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 55: 345–352, 2011. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 78.Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord 44: 203–211, 2011. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Paolo T, Falardeau P, Morissette M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci 43: 665–672, 1988. doi: 10.1016/0024-3205(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 80.Peltier MR, Sofuoglu M. Role of exogenous progesterone in the treatment of men and women with substance use disorders: a narrative review. CNS Drugs 32: 421–435, 2018. doi: 10.1007/s40263-018-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220: 2415–2422, 2015. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30: 215–238, 2006. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics 28: 378–379, 1987. doi: 10.1016/S0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- 84.Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord 45: 333–344, 2012. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A high-fat meal, or intraperitoneal administration of a fat emulsion, increases extracellular dopamine in the nucleus accumbens. Brain Sci 2: 242–253, 2012. doi: 10.3390/brainsci2020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134: 737–744, 2005. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 87.Reichelt AC, Abbott KN, Westbrook RF, Morris MJ. Differential motivational profiles following adolescent sucrose access in male and female rats. Physiol Behav 157: 13–19, 2016. doi: 10.1016/j.physbeh.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 88.Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Hum Reprod 11: 2799–2805, 1996. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- 89.Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology 39: 1282–1289, 2014. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richard JE, López-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP. Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78: 193–202, 2017. doi: 10.1016/j.psyneuen.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 91.Robinson MJF, Fischer AM, Ahuja A, Lesser EN, Maniates H. Roles of “wanting” and “liking” in motivating behavior: gambling, food, and drug addictions. In: Behavioral Neuroscience of Motivation, edited by Simpson E, Balsam P. Cham: Springer, 2015, p. 105–136. [DOI] [PubMed] [Google Scholar]
- 92.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291, 1993. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 93.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science 304: 307–310, 2004. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 94.Rosen JC, Leitenberg H, Fisher C, Khazam C. Binge-eating episodes in bulimia nervosa: The amount and type of food consumed. Int J Eat Disord 5: 255–267, 1986. doi:. [DOI] [Google Scholar]
- 95.Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27: 42–56, 2018. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52: 1059–1069, 2010. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Sánchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16: e43–e71, 2010. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultz W, Carelli RM, Wightman RM. Phasic dopamine signals: from subjective reward value to formal economic utility. Curr Opin Behav Sci 5: 147–154, 2015. doi: 10.1016/j.cobeha.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seth AK, Tsakiris M. Being a beast machine: the somatic basis of selfhood. Trends Cogn Sci 22: 969–981, 2018. doi: 10.1016/j.tics.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Siekmann L, Siekmann A, Bidlingmaier F, Brill K, Albring M. Gestodene and desogestrel do not have a different influence on concentration profiles of ethinylestradiol in women taking oral contraceptives--results of isotope dilution mass spectrometry measurements. Eur J Endocrinol 139: 167–177, 1998. doi: 10.1530/eje.0.1390167. [DOI] [PubMed] [Google Scholar]
- 101.Small DM, DiLeone RJ. An introduction to the special issue. Biol Psychiatry 73: 799–801, 2013. doi: 10.1016/j.biopsych.2013.03.007. [DOI] [Google Scholar]
- 102.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry 73: 804–810, 2013. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 103.Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct 215: 187–194, 2011. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept 1973: 254–278, 1973. [PubMed] [Google Scholar]
- 105.Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, Fernandez-Aranda F. Neural network alterations across eating disorders: A narrative review of fMRI studies. Curr Neuropharmacol 16: 1150–1163, 2018. doi: 10.2174/1570159X15666171017111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr 98: 1377–1384, 2013. doi: 10.3945/ajcn.113.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas J, Météreau E, Déchaud H, Pugeat M, Dreher JC. Hormonal treatment increases the response of the reward system at the menopause transition: a counterbalanced randomized placebo-controlled fMRI study. Psychoneuroendocrinology 50: 167–180, 2014. doi: 10.1016/j.psyneuen.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 133: 53–59, 2018. doi: 10.1016/j.steroids.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology 37: 390–401, 2012. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update 16: 276–292, 2010. doi: 10.1093/humupd/dmp051. [DOI] [PubMed] [Google Scholar]
- 111.Wade G. Sex hormones, regulatory behaviors, and body weight. In: Advances in the Study of Behavior, edited by Rosenblatt JS, Hinde RA, Shaw E. New York: Academic Press, Inc, 1976, p. 201–279. [Google Scholar]
- 112.Yeung AWK. Sex differences in brain responses to food stimuli: a meta-analysis on neuroimaging studies. Obes Rev 19: 1110–1115, 2018. doi: 10.1111/obr.12697. [DOI] [PubMed] [Google Scholar]
- 113.Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem 14: 83–89, 2014. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol Behav 95: 501–507, 2008. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu Z, Geary N, Corwin RL. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol Behav 104: 687–693, 2011. doi: 10.1016/j.physbeh.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


