Abstract
The etiology and disease patterns of hepatocellular carcinoma (HCC) significantly vary among regions. Modern standard treatments commonly require multidisciplinary approaches, including applications of up-to date medicine and advanced procedures, and necessitate the support of socioeconomic systems. For these reasons, a number of clinical guidelines for HCC from different associations and regions have been presented. External beam radiation therapy was contraindicated for HCC until a few decades ago, but with the development of new technologies, its application has rapidly increased as selective irradiation for tumorous lesions became possible. Most of the guidelines had been opposed or indifferent to radiotherapy in the past, but several guidelines have introduced indications and recommendations for radiotherapy in their updated versions. This review will discuss the characteristics of important guidelines and their contents regarding radiotherapy and will also provide guidance to physicians who are considering applications of locoregional modalities that include radiotherapy.
Keywords: Hepatocellular carcinoma, Clinical guideline, Radiotherapy, Radiation therapy, Liver neoplasm, Stereotactic body radiotherapy
Core tip: Hepatocellular carcinoma vary in incidence and disease characteristics by region. This review systematically organizes a number of hepatocellular carcionma treatment guidelines from a radiation oncological perspective, providing helpful information for physicians considering local treatment, including radiotherapy.
INTRODUCTION
Liver cancer is the fourth leading cause of all cancer-related deaths globally; its mortality rate (8.2%) is similar to that of stomach (8.2%) and colorectal cancers (9.2%), which are the third and second leading causes, respectively. Liver cancer incidence is predominant in males (second leading cause with a proportion of 10.2%) and has the highest incidence in East Asia, with a calculated age-standardized rate of 26.8 per 100000 among males. Its incidence is relatively rare in Western countries, including the United States and other European countries, except for southern European nations such as Spain or Italy, which have an age-standardized rate of 10.9 per 100000 among males[1]. Among all liver cancers, hepatocellular carcinoma (HCC) comprises the vast majority (up to 85%).
Although the vast majority of medicinal clinical guidelines are from the United States or European countries[2], a significant portion of HCC guidelines are from East Asian and South European countries and are based on abundant experiences and tailored standards[3]. The most common cause of HCC in East Asian countries, including China and Korea, is chronic hepatitis B virus (HBV) infection; patients in these countries tend to be diagnosed at younger ages and with more advanced disease[4,5]. Chronic hepatitis C virus (HCV) is the most common cause of HCC among patients in Western countries; such patients commonly have decompensated liver function at diagnosis. In Japan, chronic HCV is also the most common cause (unlike other Asian countries), and more than 60% of patients are diagnosed with early-stage disease partly because of a successful surveillance program[6]. Multidisciplinary approaches have become common for treating HCC[7], and updated systemic agents and complex interventions are commonly applied as modern standard treatments[8]. HCC is a disease that is prevalent in developing countries such as Southeast Asia or sub-Saharan Africa[9]. Hence, the socioeconomic status of regions should also be considered for clinical decisions in practice. Owing to the many differences mentioned above, numerous clinical guidelines for treating HCC have been presented by associations in various countries. In a recent review performed by the Chinese Cochrane Center, as many as 30 clinical guidelines for treating HCCs from different associations were included[10].
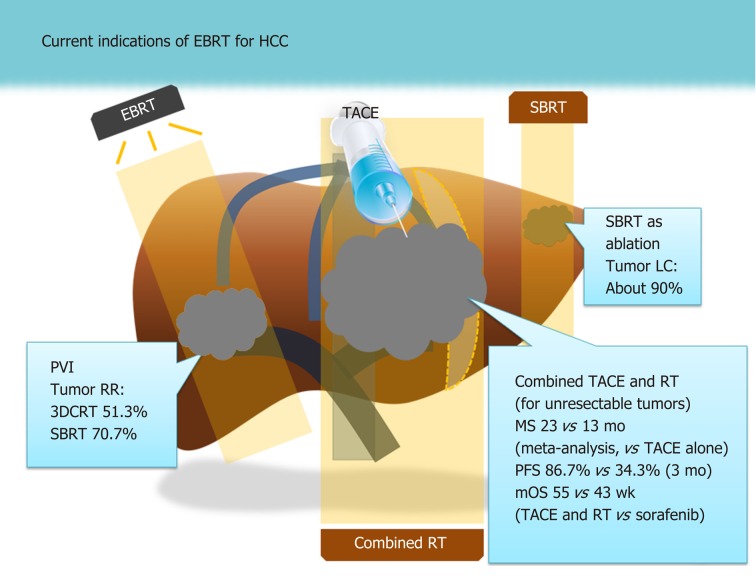
The mainstay of treatment for HCC has been the surgical approach, but other locoregional modalities such as transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) are also commonly applied[11,12]. Although external beam radiotherapy (EBRT) is not well-accepted as a standard treatment like other locoregional modalities are, it has been increasingly applied in clinical practice, especially in East Asian countries[13,14]. Patients in these areas are commonly found to have locally advanced diseases, such as tumors involving major vessels[15,16]. Transplantation is less commonly performed in East Asian countries than in Western countries owing to the shortage of donors; moreover, liver function is less deteriorated in East Asian patients than it is in Western patients who have HCV- or alcohol-based etiologies[17]. Although EBRT was previously contraindicated for HCC owing to the high risk of whole-liver toxicity[18], pioneering researchers showed the feasibility and efficacy of EBRT in the 1990s with computed tomography planning, which enables the selective irradiation of tumorous lesions[19,20]. Currently, the clinical indications of EBRT for HCC range from curative treatment for early HCC with the use of stereotactic body radiotherapy (SBRT)[21] to palliation of intractable cases such as those with major vessel invasion or extrahepatic metastases[15,16,22,23]. We summarize the indications and efficacies of EBRT for HCC based on previous major studies and meta-analyses in Figure 1.
Figure 1.
Current indications based on recent meta-analyses and major studies[15,21,27,31]. 3DCRT: 3-dimensional conformal radiation therapy; EBRT: External beam radiotherapy; LC: Local control; mOS: Median overall survival; MS: Median survival; PFS: Progression-free survival; PVI: Portal vein invasion; RCT: Randomized controlled trial; RR: Response rate; RT: Radiotherapy; SBRT: Stereotactic body radiotherapy; TACE: Transarterial chemoembolization.
Although the efficacy of EBRT is known to be potent, several clinical guidelines commented negatively on this method or did not describe it at all[24], mostly owing to a lack of high-level evidence. However, several updated guidelines changed their stances on EBRT, as its clinical efficacy and feasibility have been proven in recent studies and experiences[24]. For example, the National Cancer Comprehensive Network (NCCN) guidelines upgraded their recommendation level for radiotherapy as a locoregional modality for unresectable HCC from 2B to 2A in early 2018, which is the same grade as that for arterial-directed therapy and ablation[25].
Multidisciplinary treatment is increasingly important for treating HCC. The role of systemic treatment is significant owing to the high metastatic and recurrence potential of this disease, although the response rate remains unsatisfactory[26]. Locoregional modalities are possible curative options, but the best results can be achieved with an optimized combination of modalities[14,27]. The present review will investigate the indications and perspectives of EBRT based on the guidelines for HCC treatment. This should consequently provide helpful information for clinical decision-making, including applications of EBRT.
Table 1 summarizes the categorization of evidence and recommendations of the guidelines discussed below to improve comprehension[28-32].
Table 1.
Categorization of evidences and recommendations of the clinical guidelines1
| Oxford system level of evidences2 | |
| 1A | Systematic review of randomized clinical trials |
| 1B | Individual RCTs with narrow confidence intervals |
| 1C | All or none studies |
| 2A | Systematic reviews of cohort studies |
| 2B | Individual cohort study including low-quality RCTs |
| 2C | Outcomes research; ecological studies |
| 3A | Systematic review of case-control studies |
| 3B | Individual case-control studies |
| 4 | Case series and poor-quality cohort and case-control studies |
| 5 | Expert opinion without explicit critical appraisal or descriptive epidemiology |
| GRADE system3 | |
| Quality of evidence criteria | |
| High | (1) Further research is unlikely to change confidence in the estimate of the clinical effect. |
| Moderate | (2) Further research may change confidence in the estimate of the clinical effect. |
| Low | (3) Further research is very likely to impact confidence on the estimate of clinical effect. |
| Strength of recommendation criteria | |
| Strong | (1) Factors influencing the strength of the recommendation included the quality of the evidence, presumed patient-important outcomes, and cost. |
| Weak | (2) Variability in preferences and values, or more uncertainty. Recommendation is made with less certainty, higher cost, or resource consumption. |
| NCCN categories of evidence and consensuses4 | |
| Category 1 | Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate. |
| Category 2A | Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate. |
| Category 2B | Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate. |
| Category 3 | Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate. |
1Most clinical guidelines used their simplified adaptation of Oxford and GRADE system; above are selected examples.
Data from clinical guidelines by Canadian Association for the Study of the Liver.
Data from clinical guidelines by Korean Liver Cancer Study Group.
Data from the NCCN formal website (Available from: https://www.nccn.org/professionals/physician_gls/categories_of_consensus.aspx). RCT: Randomized controlled trials; NCCN: National Comprehensive Cancer Network.
LANDMARK GUIDELINES FOR INTERNATIONAL USAGE
According to a systematic review by Alonso-Coello et al[2], the vast majority of clinical guidelines are developed in Western countries, including the United States and Europe. The subject of HCC treatment is an exception in that many of the published guidelines are from Asian countries, owing to the relatively high incidences and abundant clinical experiences. Currently, the most well-known and internationally used guidelines appear to include those from the European Association for the Study of the Liver (EASL)[13], NCCN[11], and Asian Pacific Association for the Study of the Liver (APASL)[33], which represent their respective global regions. In this section, we will discuss the characteristics of these guidelines that focus on the perspective of EBRT, which were somewhat changed in their recently updated versions.
The EASL formed a panel of board members who devised the Barcelona Clinic of Liver Cancer (BCLC) staging system, which is most commonly used among hepatologists, and validated its efficacy in numerous studies[14,34,35]. The guideline emphasizes the importance of high-level evidence from randomized trials; in its previous version in 2012, it stated the following: “In oncology, the benefits of treatments should be assessed through randomized controlled trials and meta-analyses. Few medical interventions have been thoroughly tested in HCC, in contrast with other cancers with a high prevalence”[25]. The EASL has not been favorable toward the application of EBRT. The previous version of the guideline stated that there was no scientific evidence that EBRT can treat HCCs and that any benefits are outweighed by liver toxicity, and the guidelines only suggested the use of EBRT for palliating bone metastases. Both the levels of evidence and recommendation were evaluated to be lowest. Although the updated version[13] first indicated the lack of well-conducted prospective trials, several studies of combination EBRT and TACE[27], as well as those of first-line treatment for portal vein thrombosis (PVT), were referenced[36,37]. Literally, only 3 sentences were allotted to discussing EBRT in the previous version; however, more than 4 paragraphs were presented in the updated version. The level of evidence was not changed; however, the recommendation level was increased up to the borderline of “negative” and “weak”, which is the same level as internal radiotherapy using yttrium.
The NCCN guidelines are issued by a coalition of 28 major cancer centers in the United States; their authentic and comprehensive flowchart system makes them popular among clinical physicians. Frequent and regular updates, which are performed at least once a year, are another merit. They use their own system for evidence evaluation and recommendation. The NCCN guidelines highly consider the special aspects of oncology that are directly related to survival, and cases are commonly intractable; the guidelines state that: “…much of the clinical evidence available is primarily based on data from indirect comparisons among randomized trials, phase II or non-randomized trials, limited data from multiple smaller trials, retrospective studies, or clinical observations… in the field of oncology, it becomes critical and necessary to include input from the experience and expertise of cancer or other experts...”[30]. They use the Child-Pugh score and United Network for Organ Sharing criteria to evaluate liver function and resectability, whereas the BCLC system is not referenced. Among the 3 international guidelines mentioned in this section, only the NCCN used a panel of radiation oncologists to devise the guidelines. EBRT is indicated as one of the locoregional modalities for unresectable HCCs, and the grade of recommendation was increased from 2B to 2A in the 2018 version[25], which is same for those of arterial-directed therapy and ablation. In addition to its main indication, modern modalities such as intensity-modulated radiotherapy, SBRT, and proton therapy were introduced. The term “EBRT” was changed to “radiation therapy” in that version to cover not only EBRT using linear accelerators but also internal radiotherapy using radioisotopes.
The guidelines of the APASL, which might be the only ones based in the Asia-Pacific region and known internationally[30], have comprehensively described different epidemiologies and social circumstances among countries in the region[33]. Although most of the clinical guidelines were issued by authors from developed countries, the authors of the APASL guidelines encompassed experts from developing countries such as Pakistan, the Philippines, and Indonesia. No radiation oncology specialist was included as a formal author in their recent version. In the version updated in 2017, the evidence and recommendation levels were as low as C2 (low quality of evidence and weak recommendation); however, the guidelines allotted a significant volume of text to EBRT. Modern EBRT modalities such as SBRT and proton therapy were introduced and suggested as reasonable options when other locoregional modalities failed or portal vein involvement was present. The APASL guidelines described EBRT as follows: “Even though strong evidence is lacking, radiotherapy may be one of the promising treatment options for HCC.” They maintained a relatively neutral stance by referencing EBRT indications from multiple guidelines, including the EASL, American Association for the Study of Liver Disease (AASLD), and NCCN, rather than directly expressing their opinion.
All the 3 guidelines mentioned above, which have been used internationally, describe EBRT indications with wider and more positive perspectives in their recent versions. However, criticism for the lack of high-level evidence remains a hindrance and necessitates future randomized controlled studies to define roles for EBRT.
CLINICAL GUIDELINES FROM MAJOR OR NATIONAL ASSOCIATIONS
Among the national clinical guidelines that mention radiotherapy, those issued by AASLD are probably the most well-known and widely used. AASLD mainly assigns a panel of hepatologists and hepatic surgeons and produces guidelines for many major liver-related diseases, including viral or other hepatitis, as well as liver cancer (https://www.aasld.org/publications/practice-guidelines). In its previous version, there was little mention of EBRT indications for HCCs[38]. In the version updated in 2018, EBRT along with TACE or internal radiation was introduced as one of the locoregional modalities that can be applied to unresectable or advanced cases that involve macrovascular invasion or metastatic disease[39]. AASLD recommends locoregional treatment over no treatment for unresectable HCC but does not recommend a specific type of modality. For advanced disease, neither a preferred type of locoregional treatment nor whether to recommend systemic therapy over locoregional treatment is suggested owing to the heterogeneity of the disease and limited evidence. AASLD also states that “…the results for use of EBRT and internal radiotherapy is emerging and encouraging, but inadequate to make a re-commendation.” Survival benefits observed owing to the combined use of TACE and EBRT compared to TACE alone for unresectable cases and owing to EBRT after TACE application compared to sorafenib alone for cases with PVT involvement were major results mentioned in the referenced studies[27,40]. The guidelines of the Canadian Association for the Study of the Liver are another set from North America. Radiation oncologists were included as panelists, and indications of SBRT for palliating PVT or bridging liver transplantation (LT) were introduced with a modest level of recommendation (Level 5: Expert opinion without explicit critical appraisal or descriptive epidemiology)[29].
Because HCC is particularly prevalent in East Asia, several developed countries in that region have published their own HCC treatment guidelines. A recent set of guidelines from China were created by a large number of experts from various fields, including radiation oncologists[17,41]. These guidelines mentioned most known indications of EBRT, including palliation of major vessel invasion or extrahepatic metastases, bridging LT, symptomatic palliation, and postoperative adjuvant therapy. In addition to indications, the methodologic information for targeting tumors and the required dose and hepatic reserve for treatment safety were presented in detail. The guidelines from the Korean Liver Cancer Study Group (KLCSG) also included a large number of experts from various fields, including radiation oncologists, as board members[28]. Using the modified Union for International Cancer Control staging system, possible roles of EBRT were suggested for all stages of HCC as the best or alternative options. The guidelines also encompass methodologic information such as target dose and recommended hepatic reserve. Recommended indications are for cases with portal vein invasion, incomplete TACE, and palliation of symptoms and metastases. The guidelines from the National Cancer Center of Singapore[17] are known for their own flowchart algorithm, which is similar to that of NCCN. The included indications are as an alternative for LT or RFA for early HCCs and as a local modality for cases with vascular invasion. The guidelines consider the evidence level of EBRT for the former indication (alternative for LT or RFA for early HCCs) as 1B, which is one of the most well-regarded grades among the HCC clinical guidelines. In the updated guidelines from the Japanese hepatic society[42], we could not find any content regarding EBRT. Causes of uncommon EBRT applications in Japan might include chronic HCV but not HBV as the major cause of disease. Unlike other East Asian countries, most patients are diagnosed as having early-stage disease, and locally advanced cases such as those involving major vessels are rarely encountered[6].
Some guidelines have been developed that take local conditions into greater account, although the incidence of HCC is modest. The guidelines of the Latin American Association for the Study of the Liver were written by various experts from countries, including Mexico, Argentina, Brazil, Chile, Columbia, and Venezuela, although no radiation oncologists were included as authors. The introduced indication of EBRT was limited to symptomatic palliation[43]. The guideline of the Indian National Association for Study of the Liver[44], in its introduction, stated that the treatments recommended in the guidelines from the United States, Europe, and East Asia are difficult to be applied in India for economic reasons. Although there was no radiation oncologist among the authors, the guidelines well-described the efficacy of EBRT, stating that “HCCs are indeed radiosensitive. Sustained local control rates ranging from 71% to 100% have been reported following 30–90 Gy delivered over 1-8 wk”. They indicated that EBRT is a promising tool for some unresectable HCCs (evidence level 2b), but at the same time, they stated that the primary or definitive use of EBRT and/or other modalities cannot be recommended outside of clinical trials (evidence level 5). The guidelines from the Egyptian Society for Liver Cancer, while lacking information of systematic evidence grading and literature reviews, are the only guidelines from the African region[23]. Throughout the introduction, socioeconomic status, absence of uniform health insurance systems, and unavailability of cadaveric LT were emphasized as locally specific situations. Among the contents of the short guidelines, the only stated indication of EBRT was palliation of bone metastases additional to sorafenib application.
The indication of EBRT for HCC as a locoregional modality, such as palliating PVT, bridging LT, and combined use with TACE, was suggested in many guidelines from North America and East Asia. Guidelines from developing countries mostly mentioned a limited role, i.e., palliating extrahepatic metastases. Evidence and recommendation levels were significantly different among the guidelines. Table 2 summarizes the key components of the guidelines discussed in the present and previous sections.
Table 2.
Key information of major clinical guidelines
| Affiliation | Country | Publication, year | Staging system | Evidence stratification | (Possible) RT indication for HCC | Radiation oncologist panelists | Practical contents of EBRT | Quote | Level of Recom-mendation |
| EASL | Multinational (Europe) | J Hepatol, 2018 | BCLC | GRADE | Palliating PVT | No | None | Many series or some trials have reported efficacy and tolerability of EBRT, but no well-conducted prospective trial to consider EBRT as proven option | C2 (under investigation, no proven role for treating HCC) |
| Combined use with TACE | |||||||||
| SBRT bridging LT | |||||||||
| NCCN | United States | Own publication | Child-Pugh score, UNOS criteria | Own system | For unresectable HCC | Yes | Limited information on dose/fracti-onations of SBRT | Case series and single-arm studies demonstrate safety and efficacy of radiation therapy in selected cases | 2A (LRT for unresectable HCC) |
| Alternative to other LRT (e.g., TACE or RFA) | |||||||||
| APASL | Multinational (Asia) | Liver cancer, 2015 | Own system considering Child-Pugh score, resectability, macrovas-cular invasion, number and size of tumors | GRADE | For unresectable HCC | No | None | Even though strong evidence is lacking, RT may be one of the promising treatment options for HCC | None (HCC) C2 (bone metastasis) |
| SBRT or proton therapy as alternatives to other LRT | |||||||||
| Charged particle RT for PVT | |||||||||
| AASLD | United States | Hepatol, 2018 | AJCC staging, Milan criteria | GRADE | For unresectable HCC | No | None | The results to date are encouraging but inadequate to make a recommen-dation | C1 (for inoperable HCCs) |
| Combined use with TACE | |||||||||
| CASL | Canada | Can J Gastroenterol Hepatol, 2015 | BCLC | OXFORD | SBRT palliating PVT and bridging LT | Yes | None | Phase I and II trials have shown efficacy in achieving disease control; again, there has not been any direct comparison between radiotherapy and any other form of treatment | Evidence level 5 |
| National Health & Family Planning Commission | China | Liver Cancer, 2018 | Own system considering Child-Pugh score, extrahepatic metastases, tumor number and size, vessel invasion | OXFORD | Palliating vessel invasion or extrahepatic metastases bridging LT postoperative RT for close margin | Yes | Dose and fractionations, normal organ constraints, targeting, respiratory gating methods | Evidence level 3 for all indications | |
| KLCSG | South Korea | Gut Liver, 2019 | Modified UICC system | GRADE | Combined use with TACE palliating PVT palliating bone, brain, lung, lymphatic metastases | Yes | Dose and fractionations, normal organ constraints | EBRT for the treatment of HCC is commonly used for lesions that are surgically unresectable and not amenable to other local modalities | B2 (combined use with TACE, for PVT); B1 (palliating metastases) |
| NCC Singapore | Singapore | Liver Cancer, 2016 | Own system using Child-Pugh score, Milan criteria, tumor size, vessel invasion | OXFORD | Alternative for cases neither suitable for LT or RFA (early HCC) cases with vascular invasion | Yes | None | Evidence level 1B (alternative for LT or RFA); 2A (vascular invasion) | |
| LAASL | Multinational (Latin America) | Ann Hepatol, 2014 | BCLC | Modified OXFORD and GRADE | Palliation of symptoms, mass effect, bone metastasis | No | None | Primary symptoms should be treated with less invasive alternatives… radiotherapy may be used on a case-by-case basis | 1C (symptomatic palliation) |
| INASL | India | J Clin Exp Hepatol, 2014 | BCLC | OXFORD | For some unresectable HCCs | No | None | EBRT is a promising tool for some unresectable HCC. EBRT alone or in combination with other modalities cannot be recom-mended outside of clinical trials | Evidence level 2B (for some unresectable HCCs), 5 (definitive use) |
| ESLC | Egypt | Own publication, 2011 | BCLC, CLIP | None | Bone metastasis | N/A | None | Addition of EBRT is amenable in case of bone metastasis together with sorafenib | N/A |
EBRT: External beam radiotherapy; EASL: European Association for the Study of the Liver; BCLC: Barcelona Clinic Liver Cancer; PVT: Portal vein thrombosis; TACE: Transarterial chemoembolization; SBRT: Stereotactic body radiotherapy; LT: Liver transplantation; NCCN: National Comprehensive Cancer Network; UNOS: United Network for Organ Sharing; HCC: Hepatocellular carcinoma; LRT: Locoregional treatment; RFA: Radiofrequency ablation; APASL: Asia-Pacific Association for the Study of the Liver; RT: Radiotherapy; AASLD: American Association for the Study of Liver Disease; AJCC: American Joint Committee on Cancer; CASL: Canadian Association for the Study of the Liver; KLCSG: Korea Liver Cancer Study Group; NCC: National Cancer Center; LAASL: Latin America Association for the Study of the Liver; INASL: Indian National Association for the Study of the Liver; ESLC: Egyptian Study of Liver Cancer; CLIP: Cancer of Liver Italian Program.
SO WHICH GUIDELINES SHOULD BE REFERENCED?
From a practical perspective, clinical guidelines are good methods to suggest indications for EBRT, which is yet to become familiar to physicians other than radiation oncologists. They can be easy and quick references for physicians who devote themselves to clinical practice rather than academic concerns, such as some of those from Asia and other developing countries. In order to find suitable guidelines for the clinical practice that each physician participates in, the quality of the guidelines themselves will be important, and whether the contents of the guideline reflect the local circumstances should be also considered. For poorly standardized modalities such as EBRT for HCCs, consideration should be given to whether sufficient practical contents (e.g., dose of radiation, normal organ constraints, and targeting practice) are included in the guidelines.
Quantitative evaluation of clinical guidelines can be difficult and subjective. The Appraisal of Guidelines for Research and Evaluation (AGREE) tool is internationally validated and the only clinical guideline evaluation tool endorsed by the World Health Organization advisory boards[45]. The AGREE tool evaluates guidelines using 23 items across 6 domains, namely “Scope and purpose”, “Stakeholder involvement”, “Rigor of development”, “Clarity and presentation”, “Applicability”, and “Editorial independence”. Based on a few systemic reviews using the AGREE tool published after 2010, globally known guidelines such as those from EASL, NCCN, and AASLD were generally well evaluated and recommended[3,10,46]. These guidelines not only analyzed the most important literature and suggested evidence and recommendations in a systematic manner but also provided good descriptions regarding items such as objectives, target population, and editorial independence, which could be easily ignored during the developmental process.
The evaluations of guidelines published by several national associations, which are relatively less known than the major guidelines above, vary somewhat among appraisers. Nevertheless, the clinical guidelines contributed by Japanese hepatic society[42] have received strong overall recommendations in systematic reviews by Gavriilidis et al[3] and the Chinese Cochrane group[10]. Unfortunately, they do not contain any content regarding EBRT; hence, they might not be a good clinical reference for radiation oncologists. Among the guidelines that include contents regarding EBRT, those by KLCSG were classified as “recommended to use without modification” in the review by Gavriilidis et al[3] and also received the highest scores in the evaluation study by Holvoet et al[46]. In terms of the guidelines we analyzed in the previous section, we assessed whether radiation oncologists were included as panelists and if the guidelines included practical contents regarding EBRT. In terms of having sufficient practical contents on EBRT, the guidelines that included radiation oncologists as panelists were the Chinese guidelines[41] and those of KLCSG[28]. The Chinese guidelines included information on suggested dose and fractionations, normal organ constraints, tumor targeting, and even respiratory gating methods. Suggested doses and fractionations, as well as normal organ constraints, were also well covered in the guidelines of KLCSG.
Guidelines from China and Korea are based on abundant clinical experiences, which represent the highest incidences of HCC globally[9]. The common etiology is chronic HBV infection, which results in frequent cases of locally advance HCCs but with relatively preserved liver functions compared to HCCs caused by HCV infection or alcoholic hepatitis[4]. Hence, guidelines of the national associations of these 2 countries can be recommended for radiation oncologists who encounter these types of patients. For referencing trends of standard treatments or establishing overall treatment strategies, the clinical guidelines that are internationally and widely used, such as those of EASL, NCCN, and AASLD, could be recommended.
CONCLUSION
The causes and characteristics of HCCs vary significantly among regions, and modern treatment modalities have necessitated multimodality approaches and socioeconomic support. A single standard guideline cannot provide all the necessary information to treat all HCCs in the world. Radiation oncologists should consider both the latest research trends and the socioeconomic status of their societies and obtain the necessary information from various guidelines. Key guidelines such as those by EASL or NCCN can play a major role in understanding the flow of international standards and in communicating with physicians from other disciplines. The guidelines that encompass practical information supporting the application of EBRT, such as those from China or KLCSG, as well as those specifically considering economic or other situations of the relevant regions, should be also referenced.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All the authors have nothing to disclose.
Peer-review started: November 5, 2019
First decision: December 23, 2019
Article in press: January 11, 2020
P-Reviewer: Lin Q S-Editor: Dou Y L-Editor: A E-Editor: Zhang YL
Contributor Information
Sunmin Park, Department of Radiation Oncology, Ansan Hospital, Korea University Medical College, Ansan 15355, Gyeonggi-do, South Korea.
Won Sup Yoon, Department of Radiation Oncology, Ansan Hospital, Korea University Medical College, Ansan 15355, Gyeonggi-do, South Korea.
Chai Hong Rim, Department of Radiation Oncology, Ansan Hospital, Korea University Medical College, Ansan 15355, Gyeonggi-do, South Korea. crusion3@naver.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, Tort S, Bonfill X, Burgers J, Schunemann H. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19:e58. doi: 10.1136/qshc.2010.042077. [DOI] [PubMed] [Google Scholar]
- 3.Gavriilidis P, Roberts KJ, Askari A, Sutcliffe RP, Huo TL, Liu PH, Hidalgo E, Compagnon P, Lim C, Azoulay D. Evaluation of the current guidelines for resection of hepatocellular carcinoma using the Appraisal of Guidelines for Research and Evaluation II instrument. J Hepatol. 2017;67:991–998. doi: 10.1016/j.jhep.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016;122:3430–3446. doi: 10.1002/cncr.30237. [DOI] [PubMed] [Google Scholar]
- 5.Sinn DH, Gwak GY, Cho J, Paik SW, Yoo BC. Comparison of clinical manifestations and outcomes between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma: analysis of a nationwide cohort. PLoS One. 2014;9:e112184. doi: 10.1371/journal.pone.0112184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4:39–50. doi: 10.1159/000367727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, Kang TW, Lee MW, Rhim H, Hyun D, Cho SK, Shin SW, Jeong WK, Kim SH, Yu JI, Ha SY, Lee SJ, Lim HY, Kim K, Ahn JH, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Joh JW, Lim HK, Paik SW. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14:e0210730. doi: 10.1371/journal.pone.0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rim CH, Yoon WS. Leaflet manual of external beam radiation therapy for hepatocellular carcinoma: a review of the indications, evidences, and clinical trials. Onco Targets Ther. 2018;11:2865–2874. doi: 10.2147/OTT.S164651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Luo Q, Li Y, Wang H, Deng S, Wei S, Li X. Quality assessment of clinical practice guidelines on the treatment of hepatocellular carcinoma or metastatic liver cancer. PLoS One. 2014;9:e103939. doi: 10.1371/journal.pone.0103939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Pai JT, Loong CC, Chiou YY, Lee RC, Lee FY, Huo TI, Lee SD. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19:842–849. doi: 10.1245/s10434-011-2060-1. [DOI] [PubMed] [Google Scholar]
- 12.Thamtorawat S, Hicks RM, Yu J, Siripongsakun S, Lin WC, Raman SS, McWilliams JP, Douek M, Bahrami S, Lu DS. Preliminary Outcome of Microwave Ablation of Hepatocellular Carcinoma: Breaking the 3-cm Barrier? J Vasc Interv Radiol. 2016;27:623–630. doi: 10.1016/j.jvir.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. Electronic address: European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34:160–167. doi: 10.3857/roj.2016.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rim CH, Kim CY, Yang DS, Yoon WS. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother Oncol. 2018;129:112–122. doi: 10.1016/j.radonc.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Rim CH, Kim CY, Yang DS, Yoon WS. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: A meta-analysis and systemic review. Radiother Oncol. 2018;129:123–129. doi: 10.1016/j.radonc.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Chow PK, Choo SP, Ng DC, Lo RH, Wang ML, Toh HC, Tai DW, Goh BK, Wong JS, Tay KH, Goh AS, Yan SX, Loke KS, Thang SP, Gogna A, Too CW, Irani FG, Leong S, Lim KH, Thng CH. National Cancer Centre Singapore Consensus Guidelines for Hepatocellular Carcinoma. Liver Cancer. 2016;5:97–106. doi: 10.1159/000367759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 19.Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, Lawrence TS. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 20.Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO, Kim GE. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393–397. doi: 10.1016/s0360-3016(98)00415-5. [DOI] [PubMed] [Google Scholar]
- 21.Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144. doi: 10.1016/j.radonc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Rim CH, Kim CY, Yang DS, Yoon WS. The role of external beam radiotherapy for hepatocellular carcinoma patients with lymph node metastasis: a meta-analysis of observational studies. Cancer Manag Res. 2018;10:3305–3315. doi: 10.2147/CMAR.S175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rim CH, Choi C, Choi J, Seong J. Establishment of a Disease-Specific Graded Prognostic Assessment for Hepatocellular Carcinoma Patients with Spinal Metastasis. Gut Liver. 2017;11:535–542. doi: 10.5009/gnl16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rim CH, Yim HJ, Park S, Seong J. Recent clinical applications of external beam radiotherapy for hepatocellular carcinoma according to guidelines, major trials and meta-analyses. J Med Imaging Radiat Oncol. 2019;63:812–821. doi: 10.1111/1754-9485.12948. [DOI] [PubMed] [Google Scholar]
- 25.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 27.Huo YR, Eslick GD. Transcatheter Arterial Chemoembolization Plus Radiotherapy Compared With Chemoembolization Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2015;1:756–765. doi: 10.1001/jamaoncol.2015.2189. [DOI] [PubMed] [Google Scholar]
- 28.Korean Liver Cancer Association. National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burak KW, Sherman M. Hepatocellular carcinoma: Consensus, controversies and future directions. A report from the Canadian Association for the Study of the Liver Hepatocellular Carcinoma Meeting. Can J Gastroenterol Hepatol. 2015;29:178–184. doi: 10.1155/2015/824263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foerster F, Galle PR. Comparison of the current international guidelines on the management of HCC. J Hep Reports. 2019;1:114–119. doi: 10.1016/j.jhepr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D'Amico F, Ciarleglio FA, Boccagni P, Brolese A, Zanus G, D'Amico DF. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124–131. doi: 10.1016/j.jhep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 36.Im JH, Yoon SM, Park HC, Kim JH, Yu JI, Kim TH, Kim JW, Nam TK, Kim K, Jang HS, Kim JH, Kim MS, Yoon WS, Jung I, Seong J. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int. 2017;37:90–100. doi: 10.1111/liv.13191. [DOI] [PubMed] [Google Scholar]
- 37.Cha H, Park HC, Yu JI, Kim TH, Nam TK, Yoon SM, Yoon WS, Kim JW, Kim MS, Jang HS, Choi Y, Kim JH, Kay CS, Jung I, Seong J. Clinical Practice Patterns of Radiotherapy in Patients with Hepatocellular Carcinoma: A Korean Radiation Oncology Group Study (KROG 14-07) Cancer Res Treat. 2017;49:61–69. doi: 10.4143/crt.2016.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 40.Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. doi: 10.1186/1471-230X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) Liver Cancer. 2018;7:235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) Hepatol Res. 2015:45. doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 43.Méndez-Sánchez N, Ridruejo E, Alves de Mattos A, Chávez-Tapia NC, Zapata R, Paraná R, Mastai R, Strauss E, Guevara-Casallas LG, Daruich J, Gadano A, Parise ER, Uribe M, Aguilar-Olivos NE, Dagher L, Ferraz-Neto BH, Valdés-Sánchez M, Sánchez-Avila JF. Latin American Association for the Study of the Liver (LAASL) clinical practice guidelines: management of hepatocellular carcinoma. Ann Hepatol. 2014;13 Suppl 1:S4–40. [PubMed] [Google Scholar]
- 44.Kumar A, Acharya SK, Singh SP, Saraswat VA, Arora A, Duseja A, Goenka MK, Jain D, Kar P, Kumar M, Kumaran V, Mohandas KM, Panda D, Paul SB, Ramachandran J, Ramesh H, Rao PN, Shah SR, Sharma H, Thandassery RB (The INASL Task-Force on Hepatocellular Carcinoma) The Indian National Association for Study of the Liver (INASL) Consensus on Prevention, Diagnosis and Management of Hepatocellular Carcinoma in India: The Puri Recommendations. J Clin Exp Hepatol. 2014;4:S3–S26. doi: 10.1016/j.jceh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holvoet T, Raevens S, Vandewynckel YP, Van Biesen W, Geboes K, Van Vlierberghe H. Systematic review of guidelines for management of intermediate hepatocellular carcinoma using the Appraisal of Guidelines Research and Evaluation II instrument. Dig Liver Dis. 2015;47:877–883. doi: 10.1016/j.dld.2015.07.005. [DOI] [PubMed] [Google Scholar]