Abstract
Chemerin is a novel adipokine that regulates immune responses, adipocyte differentiation, and glucose metabolism. However, the role of chemerin in pancreatogenic diabetes mellitus (PDM) remains unknown. PDM is recognized as DM occurring secondary to chronic pancreatitis or pancreatic resection due to the loss of the loss of islet cell mass. The aim of the present study was to investigate the role of chemerin in PDM by collecting blooding samples from DM patients and establishing in vivo PDM model. The present study demonstrated that chemerin levels are decreased in the serum of patients with PDM and are negatively associated with the insulin resistance (IR) status. Chemerin levels also decreased during the development of PDM in C57BL/6 mice, together with increasing serum levels of interleukin-1 and tumor necrosis factor-α and decreasing mRNA expression levels of glucose transporter 2 (GLUT2) and pancreatic and duodenal homeobox 1 (PDX1). Treatment of PDM model mice with chemerin chemokine-like receptor 1 (CMKLR1) agonist, chemerin-9, elevated the serum levels of chemerin and mRNA expression levels of GLUT2 and PDX1, leading to the alleviation of glucose intolerance and IR in these animals. Together, the accumulated data indicated that chemerin may exert a protective function in PDM, perhaps by regulating perhaps by regulating GLUT2 and PDX1 expression, and that the restoration of the chemerin/CMKLR1 pathway may represent a novel therapeutic strategy for PDM.
Keywords: chemerin, pancreatogenic diabetes mellitus, insulin resistance
Introduction
Chemerin is a novel 16-kDa adipokine that is implicated in the regulation of innate and adaptive immunity, adipocyte differentiation and metabolism (1,2). It can act as a chemoattractant agent promoting the recruitment of immune cells to lymphoid organs and sites of tissue damage (1). Knockdown of chemerin expression impaired differentiation of 3T3-L1 cells into adipocytes, reduces the expression of genes involved in glucose and lipid homeostasis, and alters metabolic functions in mature adipocytes (2). It is expressed in numerous types of tissues, including white adipose tissue, liver, and lung (3,4), and it is secreted as an inactive prochemerin that, following activation, binds to the chemerin chemokine-like receptor 1 (CMKLR1) to exert its biological functions (5). CMKLR1 is coupled G proteins, and the interaction between chemerin and CMKLR1 inhibits cAMP production and promotes phospholipase C activation, IP3 release, and activation of PI3K and mitogen-activated protein kinase pathways (6). It has been demonstrated that the genetic knockdown of chemerin or CMKLR1 in preadipocytes results in the downregulation of genes controlling glucose and lipid metabolism (2), and chemerin can induce insulin resistance (IR) in cardiomyocytes by modulating the ERK1/2 pathway (7). Furthermore, chemerin reportedly promotes insulin signaling in 3T3-L1 adipocytes and enhances glucose uptake (8) and circulating chemerin is associated with inflammation and metabolic syndrome (9,10). The baseline levels of chemerin in Type 2 diabetes mellitus (T2DM) group were significantly higher compared with the normal control group (10). However, the role of chemerin in regulating IR remains unclear.
DM is a metabolic disease characterized by the presence of chronic hyperglycemia (11). The diagnosis of DM is based on the glucose criteria including fasting plasma glucose levels, postprandial plasma glucose levels and hemoglobin A1C (12). The mechanisms underlying different types of DM include impaired insulin secretion, IR, or a combination of the two (13). Type 1 DM is associated with absolute insulin deficiency. T2DM is characterized by insulin resistance and relative insulin deficiency (12). Pancreatogenic DM (PDM) is recognized as DM occurring secondary to chronic pancreatitis or pancreatic resection due to the loss of the loss of islet cell mass. A retrospective study enrolling nearly 1,900 patients indicated that PDM accounted for approximately 9% of all diabetics (14). Exocrine pancreatic diseases underlying PDM include benign and malign conditions such as acute or chronic pancreatitis of any etiology, cystic fibrosis, fibrocalculous pancreatopathy, hemochromatosis, pancreatectomy, pancreatic agenesis and pancreatic cancer (15). However, the relationship between chemerin and IR in PDM remains to be investigated. The aim of the present study was to determine the association between chemerin levels and PDM in patients and a mouse PDM model; which was characterized as the simultaneous presence of impaired glucose tolerance and IR. In addition, the efficacy of the CMKLR1 agonist, chemerin-9, in alleviating the impaired glucose tolerance and IR was investigated in the PDM mouse model.
Materials and methods
Patient studies
Patients with T2DM (n=110) or PDM (n=113) were recruited between January 2016 and December 2019 in the Department of Endocrinology and the Center for Severe Acute Pancreatitis (SAP) at the Jinling Hospital, Medical school of Nanjing University. Blood samples from healthy populations, which are difficult to collect, were not included since the aim of this study was to investigate the role of chemerin-9 in DM. T2DM patients were 69 male and 41 female with a mean age of 48.6±2.1 years. PDM patients were 82 male and 31 female with a mean age of 45.3±1.8 years. The diagnosis of T2DM or PDM was verified according to the Expert Committee on Diagnosis and Classification of Diabetes Mellitus (12). The underlying cause of PDM was either acute or chronic pancreatitis. To evaluate the association between chemerin levels and IR status, the patients with T2DM or PDM were further divided into two groups, with (IR>1) and without (IR≤1) IR, and serum samples (10 ml) were collected for subsequent ELISA analysis. Written informed consent for the use of serum samples was obtained from all patients enrolled in this study. The study was approved by The Ethics Committee of the Jinling Hospital (Nanjing, China).
ELISA assay
Serum levels of chemerin (ml063020 for mice, ml058526 for human) and serum glucose (ml057865 for mice, ml063205 for human) and insulin (DCM076-8) were analyzed using ELISA kit from Shanghai Enzyme-linked Biotechnology Co., Ltd Serum levels of IL-1 (SEA057Mu) and TNF-α (SEA133Mu) were analyzed ELISA kits from Cloud-Clone Corp.
Animal studies
C57BJ/6J mice (age, 8 weeks; weight, 18–22 g; female; n=24) obtained from the Experimental Animal Institute of Jinling Hospital were used to establish a model of PDM. Mice were housed under a 12-h light/dark cycle at 23±1°C with a relative humidity of 50±5%. Mice in the PDM model group (n=8) were injected peritoneally with arginine (16) (350 µg/g/day) for 42 days. Mice in PDM group or the control diet group (n=8) were fed with a normal diet for 42 days. Mice in the high-fat diet group (n=8) to model T2DM were fed with a high-fat diet, for 42 days; the composition of the control diet and high-fat diet is listed in Table I. The health and behavior of mice were checked every 3 days. Mice were anesthetized with an intraperitoneal injection of chloral hydrate (250 mg/kg) prior to collecting blood (200 µl/mouse) through the tail vein at day 0 and at 21 and 42 days post-modeling to measure fasting serum glucose (FPG) and postprandial serum glucose (PPG), the levels of chemerin, interleukin (IL)-1, and tumor necrosis factor (TNF)-α, as well as the homeostatic model assessment of insulin resistance (HOMA-IR). Following blood collection at 42 days, the mice were euthanized by 100% CO2 inhalation administered at 30% volume/minute. Death was verified by cervical dislocation and the end-point weight of the mice was 25–27 g. Subsequently, pancreatic head tissue was collected for RNA extraction and hematoxylin and eosin (H&E) staining.
Table I.
Composition of the normal (control) and high-fat diet for C57BL/6 mice.
| Composition | Normal food (%) | High-fat food (%) |
|---|---|---|
| Starch | 52.4 | 0 |
| Sucrose | 4.9 | 45.0 |
| Protein | 18.9 | 23.0 |
| Fat | 6.0 | 20.0 |
| Cellulose | 3.8 | 5.0 |
| Vitamins | 5.8 | 1.5 |
| Minerals | 8.2 | 5.5 |
To evaluate the impact of the CMKLR1 agonist, chemerin-9, on PDM, peritoneal injections of phosphate buffer solution (PBS, 200 µl, Control group, n=8) or chemerin-9 (4 µg in 200 µl PBS, n=8) or were performed every day for 42 days, as previously described (17). Blood samples were collected at day 0 and 42. The in vivo animal experimental protocols were approved by the Ethics Committee of Jinling Hospital (Nanjing, China).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from pancreatic head tissue samples (40 mg) was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. qPCR was subsequently performed using the SYBR Green PCR Master mix including reverse transcriptase, buffer, dNTPs (Applied Biosystems; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Thermal cycling of RT-qPCR was set as: 95°C for 5 min, 95°C for 10 sec and 60°C for 20 sec, repeated for 40 cycles. Primers were obtained from Tiangen Biotech Company. The following primer pairs were used for qPCR: PDX1 forward, 5′-GCGAGATGCTGGCAGACCTCT-3′ and reverse, 5′-GGCAGACCTGGCGGTTCACAT-3′; GLUT2 forward, 5′-CAATTTCATCATCGCCCTCT-3′ and reverse, 5′-TGCAGCAATTTCGTCAAAAG-3′; and β-actin forward, 5′-TCACTGAGGATGAGGTGGAAC-3′ and reverse, 5′-TCAGTCGCTCCAGGTCTTCACG-3′. The 2−ΔΔCq method was used to analyze the relative expression of mRNAs (18). β-actin was used as an internal reference control. The relative levels of mRNAs was normalized to the internal reference gene β-actin.
H&E staining
The pancreatic tissues were fixed in 10% formalin solution for 24 h at room temperature, and were processed by routine histological tissue preparation. All specimens were embedded in wax and sectioned at 4 µm. The sections were stained with hematoxylin (0.5%, 5 mins at room temperature) and eosin (0.5%, 1 min at room temperature) for pathological histological examination.
FPG and insulin levels, postprandial glucose and homeostatic model assessment of insulin resistance (HOMA-IR) calculation
Glucose and insulin levels were measured in the mice on day 21 and 42 following fasting for 16 h. For postprandial glucose, fasting mice were injected with 1–1.5 mg/g of glucose and blood samples were collected after 120 min. The concentrations of serum glucose and insulin were determined using ELISA assays. Following obtaining the fasting glucose and insulin levels, the HOMA-IR was calculated according to the following formula: HOMA-IR=[fasting glucose (nmol/l) × fasting insulin (µU/l)]/22.5.
Statistical analysis
Statistical analyses were performed using the SPSS version 22.0 (IBM Corp.) and GraphPad Prism version 5.0 (GraphPad Software, Inc.) software. Quantitative data are presented as the mean ± SEM with at least three independent repeats. Statistical significance between groups was determined using the Student's t-test or one-way ANOVA with post hoc Tukey HSD (Honestly Significant Difference) Test. P<0.05 was considered to indicate a statistically significant difference.
Results
Chemerin levels are low in the serum of patients with PDM and is negatively associated with the IR status of DM patients
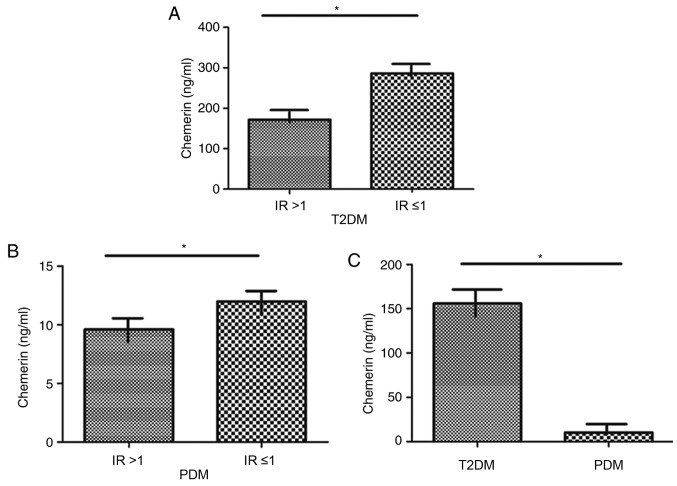
The role of chemerin in IR in patients with T2DM or PDM was explored by determining whether an association between the level of chemerin and IR status is present in these subjects. Compared with patients with T2DM with an IR≤1, patients with an IR>1 exhibited a significantly decreased level of chemerin (P<0.05; Fig. 1A). Patients with PDM with an IR>1 had a significantly lower level of chemerin compared with those with IR ≤1 (P<0.05; Fig. 1B). The level of chemerin in patients with PDM was significantly lower compared with levels in patients with T2DM (P<0.05; Fig. 1C). Together, these data suggested a close association of chemerin levels and IR in patients with DM (T1/2), and suggested that chemerin may serve a crucial role in the pathogenesis of PDM, which is characterized by impaired glucose tolerance and IR.
Figure 1.
Serum levels of chemerin in patients with T2DM and PDM and its association with IR status. Serum levels of chemerin in patients with (A) T2DM or (B) PDM with (IR>1) or without (IR≤1) IR. (C) Serum levels of chemerin in PDM compared with T2DM patients. *P<0.05. IR, insulin resistance; PDM, pancreatogenic diabetes mellitus; T2DM, type 2 diabetes mellitus.
Successful establishment of a mouse PDM model
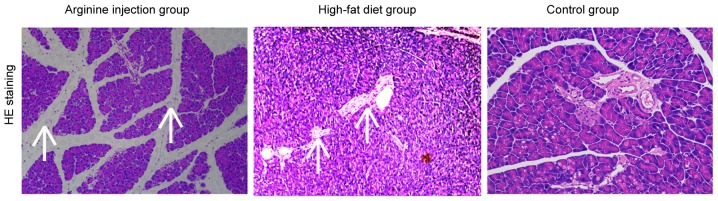
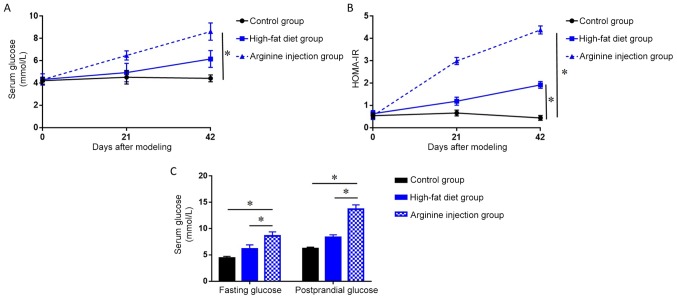
A mouse model of PDM was generated to further evaluate the role between chemerin and IR. Subsequent H&E staining revealed that compared with mice fed the high-fat diet or the control group, mice in the arginine group exhibited a focal enlargement of the interlobular septum in the pancreatic head and a minor increase in the number of white blood cells in, or around, the pancreatic lobules (data not shown). Compared with control group, the high-fat diet group showed pancreatic alveolar atrophy and interstitial fibrosis. Necrosis of glandular cells and bleeding were also absent in the pancreatic head region (Fig. 2). PDM mice had significantly higher fasting blood glucose levels and HOMA-IR at 42 days post-modeling compared to the high-fat diet and control group (P<0.05; Fig. 3A and B). Notably, following days, PDM model mice demonstrated a significant increase in the levels of FPG and PPG (P<0.05; Fig. 3C) compared with the high-fat diet or control group. Together, these data indicated that arginine injection for 42 days successfully induced PDM in mice, resulting in higher FPG levels, impaired glucose tolerance, and enhanced IR.
Figure 2.
Representative hematoxylin & eosin staining of the pancreatic head tissue in C57BL/6 mice. Mice were injected with arginine intraperitoneally to establish a PDM model, or fed a high-fat diet to induce T2DM or control (normal diet). The arrows indicate the focal enlargement of the interlobular septum and interstitial fibrosis. Magnification, ×40. PDM, pancreatogenic diabetes mellitus.
Figure 3.
Serum glucose levels and HOMA-IR in a PDM mouse model established by arginine injection. (A) Changes in fasting serum glucose level concentrations measured by ELISA over a 42 day period in the PDM group compared with high-fat diet and control groups. (B) Changes in HOMA-IR over a 42 day period in the PDM group compared with the high-fat diet and control groups. (C) FPG and PPG levels were measured by ELISA over a 42 day period in the PDM group compared with the high-fat diet and control groups. *P<0.05. PDM, pancreatogenic diabetes mellitus, FPG, fasting plasma glucose; PPG, postprandial plasma glucose.
Chemerin levels decrease in PDM mice
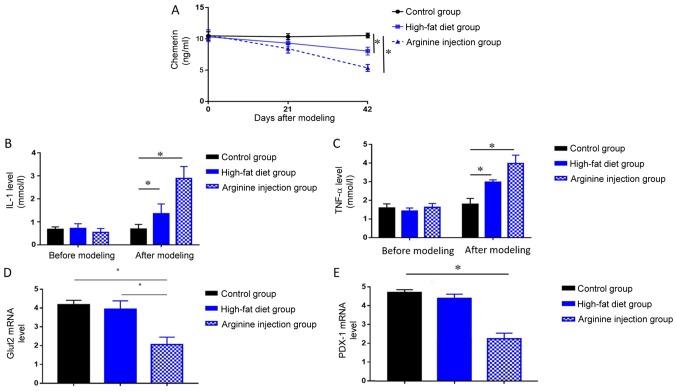
The levels of circulating chemerin, IL-1, and TNF-α in PDM mice, high-fat diet mice and the control group were measured by ELISA. PDM mice exhibited significantly reduced levels of chemerin at 42 days compared with mice fed a high-fat or control diet (P<0.05; Fig. 4A), in addition to significantly elevated levels of IL-1 and TNF-α compared with the high-fat diet and control group at 42 days (both P<0.05; Fig. 4B and C). Compared with control group, mice in high-fat diet group also showed decreased level of chemerin, as well as increased level of IL-1 and TNF-a (P<0.05, Fig. 4A-C). Subsequent RT-qPCR analysis of GLUT2 and PDX1 mRNA expression levels in the pancreatic head tissues collected from each group revealed that PDM mice exhibited significantly lower levels of both genes compared with mice in the high-fat diet and control group (P<0.05; Fig. 4D and E). These results indicated chemerin may exert protective role in the pathogenic process of PDM.
Figure 4.
Levels of chemerin, IL-1, TNF-α, GLUT2, and PDX1 in mice. Changes in serum levels of (A) chemerin, (B) IL-1, (C) TNF-α were detected in the PDM, high-fat diet and control groups after modeling at 42 days using ELISA. Expression levels of (D) GLUT2 or (E) PDX1 were detected at 42 days in the PDM group, high-fat diet or control group by reverse transcription-quantitative PCR. *P<0.05. GLUT2, glucose transporter 2; IL, interleukin; PDX1, pancreatic and duodenal homeobox; PDM, pancreatogenic diabetes mellitus; TNF, tumor necrosis factor.
CMKLR1 agonist chemerin-9 alleviates glucose intolerance and IR in PDM mice
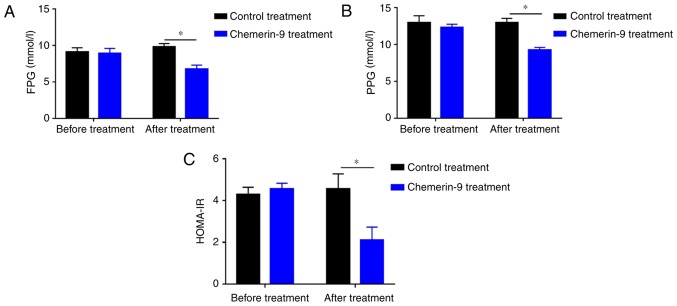
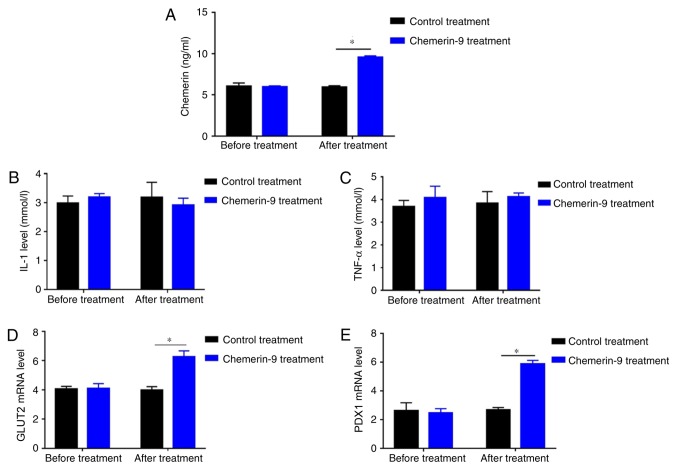
To further clarify the role of chemerin in PDM, the model mice were treated with chemerin-9, a classical agonist of CMKLR1. Before treatment, mice in control and chemerin-9 group showed similar level in fasting glucose, postprandial glucose and HOMA-IR (Fig. 5A-C). The treatment significantly decreased the FPG levels, PPG levels and HOMA-IR compared with the mice in the control group (all P<0.05; Fig. 5A-C). In addition, chemerin-9 treatment significantly increased the levels of chemerin compared with the control treatment group (P<0.05; Fig. 6A). No measurable change was reported in the serum levels of IL-1 or TNF-α between the chemerin-9 treatment and the control before or after treatment (Fig. 6B and C). In addition, the mRNA expression levels of GLUT2 and PDX1 were significantly increased following chemerin-9 treatment PDM mice compared with the control treatment (both P<0.05; Fig. 6D and E). These results indicate targeting chemerin may represent a novel therapeutic strategy for PDM.
Figure 5.
Effect of chemerin-9 treatment on FPG, PPG, and HOMA-IR in PDM model mice. Levels of (A) FPG and (B) PPG were measured by ELISA in the chemerin-9 and control treatment groups before and after 42 days of treatment. (C) HOMA-IR was measured in the chemerin-9 and control treatment before and after treatment. *P<0.05. HOMA-IR, homeostatic model assessment for insulin resistance; FPG, fasting plasma glucose; PPG, postprandial plasma glucose; PDM, pancreatogenic diabetes mellitus.
Figure 6.
Effect of chemerin-9 treatment on the plasma levels of chemerin, IL-1, TNF-α, GLUT2, and PDX1 in PDM model mice. Changes in (A) chemerin, (B) IL-1 and (C) TNF-α levels were detected in the chemerin-9 and control treatment groups following ELISA before and 42 days after treatment. Expression levels of (D) GLUT2 or (E) PDX1 were detected in the chemerin-9 and control groups by reverse transcription-quantitative PCR before and after treatment. *P<0.05. GLUT2, glucose transporter 2; IL, interleukin; PDX1, pancreatic and duodenal homeobox; TNF, tumor necrosis factor.
Discussion
Chemerin, a novel adipokine, regulates innate and adaptive immunity, adipocyte differentiation and metabolism (9). The role of chemerin in T2DM and IR has gained increasing attention (19,20). Chemerin levels were found to be markedly increased in patients with T2DM with hypertension compared with patients with T2DM and normal controls (19). In gestational DM, chemerin significantly and positively correlated with HOMA-IR (20). One previous study reported that chemerin levels in T2DM were significantly higher compared with the expression in the control group, and the level of chemerin positively correlated with HOMA-IR (10). However, another study of T2DM indicated that chemerin levels were not significantly different between subjects with T2DM and normal controls (9). These indicate that the role of chemerin in DM remains controversial. In addition, the function of chemerin in PDM remains unknown. The present study demonstrated that serum levels of chemerin in patients with PDM were significantly lower compared with patients with T2DM, and chemerin levels were negatively associated with the HOMA-IR status of patients with T2DM or PDM. These findings indicated that the function of chemerin, and its underlying mechanisms, may be different in PDM compared with T2DM, and it may affect the pathogenesis of PDM by serving a protective role through alleviating IR, thus, revealing a potential novel molecular mechanism and therapeutic strategy for PDM. It is worth mentioning here that the data from the present study differs from that in T2DM and gestational DM (10,20), indicating that the molecular mechanism underlying different types of DM is different.
To further validate the role of chemerin in PDM, a mouse model of the disease was established using the arginine injection method (16). Following 42 days of arginine administration, the treated mice exhibited mild inflammatory changes in the pancreas, whereas the animals in the control and high-fat groups failed to exhibit this pathological feature. The inflammatory changes were not so clear in the high-fat group. Notably, arginine injections resulted in an increased concentration of FPG, PPG, and HOMA-IR, demonstrating the successful establishment of the mouse model of PDM (21,22). The levels of chemerin were significantly decreased in PDM mice, which was consistent with the patient data. The administration of chemerin-9, a classical agonist of CMKLR1, resulted in increased levels of chemerin in the PDM mice, decreased the concentrations of FPG and PPG, and alleviated the IR of these animals. Thus, increasing the level of chemerin may represent a novel therapeutic strategy for PDM. However, the molecular mechanism underlying these biological functions of chemerin in PDM remains to be elucidated.
In the present study, the concentrations of two inflammatory mediators, IL-1 and TNF-α, were found to be increased during PDM development. These data were consistent with previous studies reporting that elevated TNF-α, IL-1β, and IL-6 in type 1 DM and T2DM (23,24) and indicated that chronic inflammation may be involved in PDM and may be a common underlying mechanism for different types of DM. However, the administration of chemerin-9 did not have an effect on the level of IL-1 and TNF-α, which suggested that chronic inflammation may not be mediating the therapeutic effect of chermin-9 in PDM.
GLUT2, the major mediator of glucose uptake by hepatocytes and pancreatic β-cells is decreased in patients with DM (25–27). The present study demonstrated that PDM mice had decreased levels of GLUT2 mRNA, which led to the impaired uptake of glucose and secretion of insulin. Upon administration of chemerin-9, GLUT2 expression was increased, which was accompanied by decreased IR in the PDM mice. Thus, decreased GLUT2 levels may be implicated in the pathogenesis of PDM, and chemerin-9 may alleviate the IR associated with PDM by increasing the expression of GLUT2 (28).
PDX1 is a crucial transcription factor regulating the transcription of the insulin gene (29). The present study revealed that PDX1 mRNA levels decreased during the development of PDM but significantly increased following the administration of chemerin-9, which alleviated IR. This is consistent with previously published studies reporting that PDX1 expression was decreased in patients with T2DM (30,31) and that the activation of the PDX1/JAK signal transduction cascade in C57BL/6 mice ameliorates the IR of DM (32). Thus, this supports the notion that the impaired proliferation of pancreatic β-cells resulting from decreased PDX1 expression may be causally related to the pathogenesis of PDM, and that restoring PDX1 signaling using chemerin-9 may explain the protective role against IR in PDM.
One limitation of the present study is a lack of a healthy cohort. Including healthy individuals will be beneficial for better interpretation of chemerin levels and IR. Another limitation of this study is that the mechanism underlying the function of chemerin in PDM remains unknown. In conclusion, the present study demonstrated that chemerin levels are decreased in the serum of patients with PDM, and are negatively associated with IR in this population. In vivo experiments utilizing a mouse model of PDM revealed that chemerin levels decreased during the development of the disease, together with a concomitant increase in the levels of IL-1 and TNF-α, and decreased mRNA expression levels of GLUT2 and PDX1. Administration of the CMKLR1 agonist, chemerin-9, caused an increased expression of chemerin, GLUT2, and PDX1, which led to the alleviation of glucose intolerance and IR in PDM model mice. Together, these data indicated that chemerin may exert a protective function against PDM, and the restoration of the chemerin/CMKLR1 pathway may represent a novel therapeutic strategy for the treatment of PDM.
Acknowledgements
Not applicable.
Funding
This study was supported by The Natural Science Foundation of Zhejiang Provincial (grant. no. LY18H150005), The Science and Technology Foundation of Zhejiang Province (grant. no. 2013C37022) and The National Natural Science Foundation of China (grant. nos. 81670588 and 81570584).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JT, WL and QX designed the study. YY, JZ, GL, LK and ZT performed the in vivo experiments and collected the data. MK, DH and QX performed the in vivo experiments and the statistical analysis. QX and JT wrote the manuscript.
Ethics approval and consent to participate
The experimental protocol was approved by the Ethics Committee of the Jinling Hospital (Nanjing, China). Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 3.Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, Gualillo O. Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37:413–420. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst MC, Sinal CJ. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Liu S, Guo B, Chang L, Li Y. Chemerin induces insulin resistance in rat cardiomyocytes in part through the ERK1/2 signaling pathway. Pharmacology. 2014;94:259–264. doi: 10.1159/000369171. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–578. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 10.Yu S, Zhang Y, Li MZ, Xu H, Wang Q, Song J, Lin P, Zhang L, Liu Q, Huang QX, et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl) 2012;125:3440–3444. [PubMed] [Google Scholar]
- 11.Chatterjee S, Davies MJ. Accurate diagnosis of diabetes mellitus and new paradigms of classification. Nat Rev Endocrinol. 2018;14:386–387. doi: 10.1038/s41574-018-0025-1. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.S5. [DOI] [PubMed] [Google Scholar]
- 14.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–342. doi: 10.1002/dmrr.2260. [DOI] [PubMed] [Google Scholar]
- 15.Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)-are we neglecting an important disease? Eur J Intern Med. 2013;24:203–206. doi: 10.1016/j.ejim.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Weaver C, Bishop AE, Polak JM. Pancreatic changes elicited by chronic administration of excess L-arginine. Exp Mol Pathol. 1994;60:71–87. doi: 10.1006/exmp.1994.1007. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy AJ, Yang P, Read C, Kuc RE, Yang L, Taylor EJ, Taylor CW, Maguire JJ, Davenport AP. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J Am Heart Assoc. 2016;5(pii):e004421. doi: 10.1161/JAHA.116.004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Yang G, Dong J, Liu Y, Zong H, Liu H, Boden G, Li L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med. 2010;58:883–886. doi: 10.2310/JIM.0b013e3181ec5db2. [DOI] [PubMed] [Google Scholar]
- 20.Pfau D, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M, Fasshauer M. Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Horm Res Paediatr. 2010;74:56–61. doi: 10.1159/000282114. [DOI] [PubMed] [Google Scholar]
- 21.Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226–237. doi: 10.1016/S2468-1253(16)30106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen DK. The practical importance of recognizing pancreatogenic or type 3c diabetes. Diabetes Metab Res Rev. 2012;28:326–328. doi: 10.1002/dmrr.2285. [DOI] [PubMed] [Google Scholar]
- 23.Araya AV, Pavez V, Perez C, Gonzalez F, Columbo A, Aguirre A, Schiattino I, Aguillón JC. Ex vivo lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2 secretion in whole blood from Type 1 diabetes mellitus patients with or without aggressive periodontitis. Eur Cytokine Netw. 2003;14:128–133. [PubMed] [Google Scholar]
- 24.Saxena M, Srivastava N, Banerjee M. Association of IL-6, TNF-α and IL-10 gene polymorphisms with type 2 diabetes mellitus. Mol Biol Rep. 2013;40:6271–6279. doi: 10.1007/s11033-013-2739-4. [DOI] [PubMed] [Google Scholar]
- 25.Hassani-Nezhad-Gashti F, Rysa J, Kummu O, Näpänkangas J, Buler M, Karpale M, Hukkanen J, Hakkola J. Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochem Pharmacol. 2018;148:253–264. doi: 10.1016/j.bcp.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Beamish CA, Zhang L, Szlapinski SK, Strutt BJ, Hill DJ. An increase in immature β-cells lacking Glut2 precedes the expansion of β-cell mass in the pregnant mouse. PLoS One. 2017;12:e0182256. doi: 10.1371/journal.pone.0182256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khandelwal P, Sinha A, Jain V, Houghton J, Hari P, Bagga A. Fanconi syndrome and neonatal diabetes: Phenotypic heterogeneity in patients with GLUT2 defects. CEN Case Rep. 2018;7:1–4. doi: 10.1007/s13730-017-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathinam A, Pari L. Myrtenal ameliorates hyperglycemia by enhancing GLUT2 through Akt in the skeletal muscle and liver of diabetic rats. Chem Biol Interact. 2016;256:161–166. doi: 10.1016/j.cbi.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, Ding D, Wang T, Liu Q, Lin Y. MiR-338 controls BPA-triggered pancreatic islet insulin secretory dysfunction from compensation to decompensation by targeting Pdx-1. FASEB J. 2017;31:5184–5195. doi: 10.1096/fj.201700282R. [DOI] [PubMed] [Google Scholar]
- 30.Shi S, Zhao L, Zheng L. NSD2 is downregulated in T2DM and promotes β cell proliferation and insulin secretion through the transcriptionally regulation of PDX1. Mol Med Rep. 2018;18:3513–3520. doi: 10.3892/mmr.2018.9338. [DOI] [PubMed] [Google Scholar]
- 31.Yang BT, Dayeh TA, Volkov PA, Kirkpatrick CL, Malmgren S, Jing X, Renström E, Wollheim CB, Nitert MD, Ling C. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol. 2012;26:1203–1212. doi: 10.1210/me.2012-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao T, Zhang H, Li S, Tian H. Glucagon-like peptide 1 receptor agonist ameliorates the insulin resistance function of islet β cells via the activation of PDX-1/JAK signaling transduction in C57/BL6 mice with high-fat diet-induced diabetes. Int J Mol Med. 2017;39:1029–1036. doi: 10.3892/ijmm.2017.2910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.