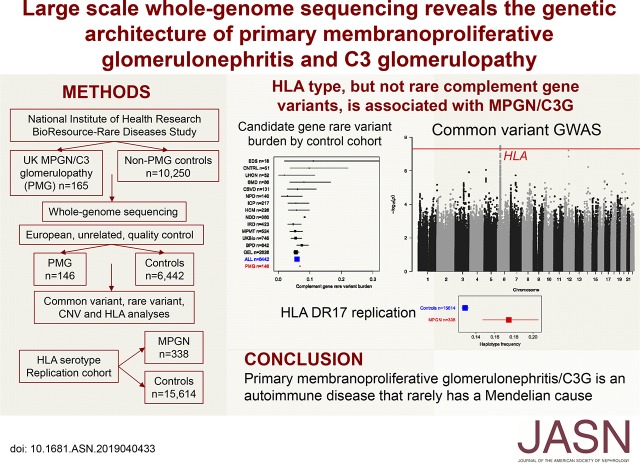
Significance Statement
A minority of cases of primary membranoproliferative GN are familial, caused by mutations in complement genes, and nonfamilial cases have also been reported to harbor such mutations. To characterize the genetic factors contributing to this disease, the authors analyzed whole-genome data from 165 cases of primary membranoproliferative GN and 10,250 control individuals, including 146 cases and 6442 controls who were unrelated and of European ancestry. Although they observed no significant enrichment of rare variants in complement genes or exome-wide among cases compared with controls, they found that the HLA locus was strongly associated with primary membranoproliferative GN, a finding replicated in an independent cohort. These findings imply that in most cases, primary membranoproliferative GN is driven by autoimmunity rather than an underlying monogenic disorder of complement regulation.
Keywords: C3 glomerulopathy, Human leukocyte antigen, membranoproliferative glomerulonephritis (MPGN), Genome-wide association study, complement
Visual Abstract
Abstract
Background
Primary membranoproliferative GN, including complement 3 (C3) glomerulopathy, is a rare, untreatable kidney disease characterized by glomerular complement deposition. Complement gene mutations can cause familial C3 glomerulopathy, and studies have reported rare variants in complement genes in nonfamilial primary membranoproliferative GN.
Methods
We analyzed whole-genome sequence data from 165 primary membranoproliferative GN cases and 10,250 individuals without the condition (controls) as part of the National Institutes of Health Research BioResource–Rare Diseases Study. We examined copy number, rare, and common variants.
Results
Our analysis included 146 primary membranoproliferative GN cases and 6442 controls who were unrelated and of European ancestry. We observed no significant enrichment of rare variants in candidate genes (genes encoding components of the complement alternative pathway and other genes associated with the related disease atypical hemolytic uremic syndrome; 6.8% in cases versus 5.9% in controls) or exome-wide. However, a significant common variant locus was identified at 6p21.32 (rs35406322) (P=3.29×10−8; odds ratio [OR], 1.93; 95% confidence interval [95% CI], 1.53 to 2.44), overlapping the HLA locus. Imputation of HLA types mapped this signal to a haplotype incorporating DQA1*05:01, DQB1*02:01, and DRB1*03:01 (P=1.21×10−8; OR, 2.19; 95% CI, 1.66 to 2.89). This finding was replicated by analysis of HLA serotypes in 338 individuals with membranoproliferative GN and 15,614 individuals with nonimmune renal failure.
Conclusions
We found that HLA type, but not rare complement gene variation, is associated with primary membranoproliferative GN. These findings challenge the paradigm of complement gene mutations typically causing primary membranoproliferative GN and implicate an underlying autoimmune mechanism in most cases.
Membranoproliferative GN (MPGN) refers to inflammatory kidney disease in which there is increased glomerular mesangial matrix and cellularity, thickening of the capillary walls, and deposition of immunoglobulins (Igs) and/or complement. Such appearances can be seen when the immune system is chronically activated; the term primary membranoproliferative GN (PMG) refers to those cases in which an underlying infectious, neoplastic, or autoimmune disorder is not identified. PMG is divided into immune complex primary membranoproliferative GN (IC-PMG), where there is positive immunostaining for Igs and complement, and complement 3 glomerulopathy (C3G), where complement 3 (C3) is the predominant immunoprotein deposited. C3G is subdivided by electron microscopic appearances into C3 glomerulonephritis (C3GN) and dense deposit disease (DDD), in which there is characteristic dense transformation of the glomerular basement membrane.1
PMG is rare, with incidence estimated at 3–5 per million population.2–4 In most cases the cause is not known but familial C3G has been linked to genomic rearrangements in the Complement Factor H Related genes (CFHR1–5),5–8 biallelic loss of function variants of Complement Factor H (CFH),9 and an activating mutation of C3.10 In addition, studies of nonfamilial cases of PMG have identified rare variants in these and other complement genes (previously associated with atypical hemolytic uremic syndrome; aHUS) in up to 40% of patients.11–14 These findings, together with the almost invariable presence of C3 in the glomerulus, have implicated complement alternative pathway activation as a key causal mechanism and testing for complement gene mutations is currently recommended in C3G, especially where living related renal transplantation is considered.15
However, the current paradigm, in which the disease is frequently assumed to result from a rare genetic defect of complement regulation, seems incompatible with the following observations: first, the disease is usually not familial; second, a C3 nephritic factor (C3NeF), an autoantibody that activates the complement alternative pathway in the blood, is detectable in a substantial proportion of patients, including those in whom a rare variant in a complement gene is identified11; and third, there is a recognized association of MPGN with other autoimmune diseases16–18 including a very substantially increased rate of type 1 diabetes mellitus in relatives of patients with DDD.19
Here, we use whole-genome sequencing to investigate the role of genetic variation in the causation of PMG in the United Kingdom (UK) population, and resolve all three of these anomalous observations: although rare genetic variation in the a priori candidate genes was not enriched in PMG (or the subset with C3G), there is a strong association with common variation at the HLA locus, explaining the phenotypic association with established autoimmune diseases and implicating autoimmunity as the key causal mechanism.
Methods
Abbreviated Methods Follow
Detailed methods are provided in Supplemental Appendix 1.
National Institute for Health Research BioResource Rare Diseases Study
This study is a part of the National Institute for Health Research BioResource Rare Diseases study (BR-RD),20 in which whole-genome sequencing has been undertaken on 13,342 individuals: 12,525 across 16 rare disease domains and 817 apparently healthy individuals (see Supplemental Table 1). Given the potential for a shared genetic cause with PMG, cohorts with diseases with a known immunologic basis (pulmonary artery hypertension [PAH] and primary immunodeficiencies [PID]) and steroid-resistant nephrotic syndrome (SRNS) were excluded. Clinical phenotypic data for all participants was encoded using Human Phenotype Ontology,21 SNOMED CT, and ORPHANET codes. Among those without PMG, three participants with the phenotypes microangiopathic hemolytic anemia, thrombocytopenia and acute kidney injury, or SNOMED CT or ORPHANET codes compatible with hemolytic uremic syndrome, were identified and excluded from the control cohort, as were eight participants with evidence of retinal drusen or macular degeneration.
A summary of the analytic workflow, number of samples analyzed, and main findings is provided in Supplemental Figure 1.
PMG Cohort
Recruitment of patients with PMG was undertaken from ten British pediatric (64 patients) and 18 adult centers (120 patients, of whom 21 had pediatric onset of disease). Patients with histologically confirmed MPGN either with or without immune-complex deposition (IC-PMG or C3G, respectively) in the absence of a known or suspected underlying systemic cause22 were considered eligible. No genetic prescreening was applied. Clinical data were extracted from the UK Rare Renal Disease Registry (http://rarerenal.org/radar-registry). Where available, kidney biopsies were reviewed centrally to confirm the histologic diagnosis and to classify as IC-PMG, C3GN, or DDD. Serum C3NeF and C3 and C4 levels were measured using standard, clinically validated assays.
Whole-Genome Sequencing: Data Generation, Variant Calling, Annotation, Relatedness, and Ancestry
The methods used for data generation and variant calling have been previously described20 and are further detailed, along with information on quality control, variant annotation, and the identification of a subset of unrelated individuals of European ancestry, in Supplemental Appendix 1.
Structural and Copy Number Variants
The occurrence of previously described rare structural variants and copy number variants for PMG5–7,23,24 was examined by manually inspecting all structural variants and copy number variants involving the genes of relevance in unrelated PMG individuals of all ethnicity. Subsequent analyses were restricted to the unrelated European cohort of cases and controls. A genome-wide comparison of the frequency of deletions per gene between PMG and controls was undertaken, with P values calculated by permutation testing (n=100,000).
Comparison with Previously Described PMG and aHUS Variants
We examined the occurrence of common and rare variants in C3, CD46, CFB, CFH, CFHR1, CFHR3, CFHR5, CFI, DGKE, and THBD previously observed in patients with aHUS, age-related macular degeneration, C3G, or thrombotic microangiopathy, as per the Database of Complement Gene Variants—a compilation of rare variant data from 3128 patients with aHUS and 443 with C3G tested in six national reference laboratories (http://www.complement-db.org)14 and a further study.11
Rare Variant Candidate Gene and Exome-wide Coding Variant Burden Analysis
Rare coding variants (gnomAD-Non-Finnish European [NFE] minor allele frequency [MAF] <0.0001) of moderate or high impact were extracted. Per-gene rare variant burden was enumerated as the proportion of individuals (cases versus controls) with at least one alternate allele in each gene with significance calculated using the exactCMC function in RVTESTS,25 which uses the Fisher exact test. Analyses were also conducted filtering variants on the basis of their predicted deleteriousness, using CADD scores.26
Common Variant Genome-Wide Association Study
Common, high-quality variants (MAF≥0.05 in gnomAD-NFE and BR-RD) were retained. Standard quality-control procedures27 were undertaken (detailed in Supplemental Appendix 1). The final data set included 5,897,512 variants with the call rate across the samples exceeding 0.999. A genome-wide association study was undertaken with PLINK v1.9, assuming additive allele effects using logistic regression with the first five principal components as covariates.
HLA Imputation
HLA genotyping was performed using BWAKIT/BWAMEM v0.7.15 (https://github.com/lh3/bwa/tree/master/bwakit) and HLA-HD v1.2.0.128. Alleles with MAF<0.05 in controls were excluded. Logistic regression with the first five principal components as covariates was performed using PLINK v1.9. Haplotype association analysis was performed using PLINK v1.07.
Replication
HLA serotypes from the UK National Health Service Blood and Transplant (NHSBT) service were utilized as an independent replication cohort. The analyzed cohort was a subset of data from all White individuals listed for a kidney transplant in the UK within the past 25 years. HLA serotype data were available for HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ. Only those serotypes observed at a frequency >0.05 in controls were analyzed for association (n=28). Chi-squared allelic tests and logistic regression were performed using PLINK v1.9. Serotypes were converted to molecular subtypes using the HLA Dictionary.29
Results
The initial PMG cohort comprised 184 participants. After centralized biopsy review, 19 were excluded, most because the biopsy showed mesangial proliferative GN rather than MPGN, leaving 165 (47.9% male). Histologic subtypes, complement abnormalities, and clinical features are summarized in Table 1. Full clinical details for all individuals with C3G (C3GN and DDD) are provided in Supplemental Table 2. A C3NeF was more likely to have been observed in those with DDD (Supplemental Table 2), and almost all of those with a C3NeF or DDD had exhibited a low C3 level on at least one occasion. Consistent with previous reports,30 transiently low serum C4 was documented in some patients with DDD and C3GN, but this almost always normalized within weeks of initial presentation.
Table 1.
Histologic categorization and clinical details
| Category | Total, % | C3NeF Detected (%) | Low C3 (<0.68 g/L), % | Low C4 (<0.18 g/L), % |
|---|---|---|---|---|
| All participants | 165 (100) | 25 (36.2) | 58 (69.0) | 38 (45.2) |
| IC-PMG | 53 (46.5) | 6 (24.0) | 19 (63.3) | 19 (63.3) |
| C3GN | 39 (34.2) | 8 (33.3) | 19 (67.9) | 8 (28.6) |
| DDD | 22 (19.3) | 10 (58.8) | 16 (84.2) | 9 (47.4) |
| Pediatric onset | 85 (51.5) | 23 (40.4) | 51 (77.2) | 33 (50.0) |
| Immunosuppression | 75 (56.0) | 18 (38.3) | 42 (77.8) | 27 (50.0) |
| ESKD | 42 (25.4) | 5 (41.7) | 6 (42.9) | 1 (7.1) |
| Renal transplant | 30 (18.2) | 4 (57.1) | 4 (44.4) | 0 (0.0) |
| C3NeF detected | 25 (36.2) | — | 23 (92.0) | 11 (44.0) |
Ultrastructural and immunostaining data to allow sub-classification into IC-PMG, C3GN, or DDD were available for 114 participants. Results of clinically accredited C3NeF assays were available for 69 and serum C3 and C4 levels were available for 84 participants, but only a small number of those with ESKD.
The total number of individuals in each BR-RD cohort is shown in Supplemental Table 1. Excluding PID, PAH, and SRNS, and control individuals with phenotypic codes compatible with aHUS or age-related macular degeneration (n=11), there were 10,250 individuals for use as controls. Of these, 6491 were unrelated and of European ancestry. All the cases were genetically unrelated with the exception of one sibling pair and the majority (n=146) were genetically classified as of European ancestry (Supplemental Figure 2). After further quality control measures, the final data set comprised 146 PMG cases and 6442 non-PMG controls.
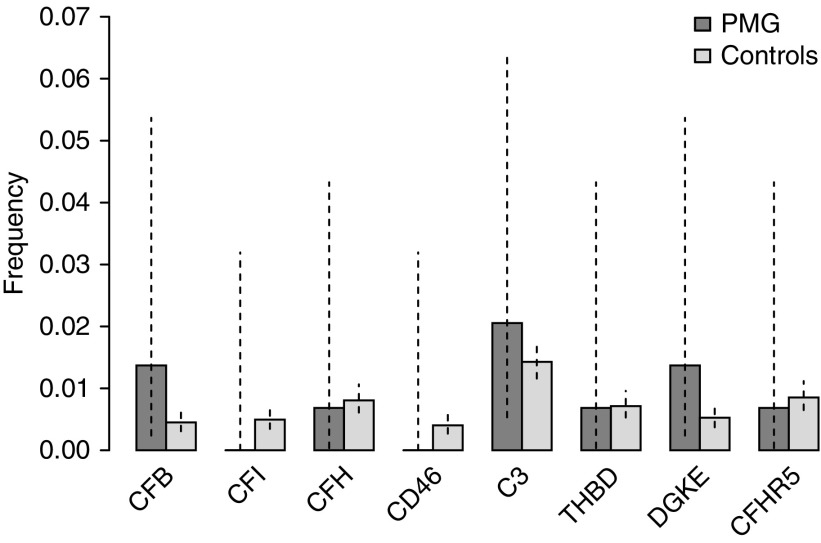
In the genes typically screened in patients with C3G and aHUS (namely C3, CD46, CFB, CFH, CFHR5, CFI, DGKE, and THBD), there was no enrichment of rare (gnomAD-NFE MAF <0.0001) variants of moderate or high predicted impact in the PMG cohort (Figure 1). The number of individuals with at least one such variant was 10 (6.8%; 95% confidence interval [95% CI], 3.5% to 12.6%) in the PMG cohort, compared with 381 (5.9%; 95% CI, 5.4% to 6.5%) in non-PMG controls (P=0.37, one-tailed Fisher exact test), consistent across each of the control cohorts (Supplemental Figure 3). Among the PMG cohort, there was no difference in the candidate gene rare variant burden between the histologic subgroups C3GN, DDD, IC-PMG, and PMG unclassified, between those with and without C3NeF, and those with low C3 (Supplemental Figure 3). The details of the 11 and 318 variants identified in the PMG participants and in non-PMG controls, respectively, are provided in Supplemental Tables 3 and 4. Analyses were also performed imposing a variable CADD threshold (none to ≥20) and control allele frequency (gnomAD-NFE MAF <0.0001 to <0.01); however, in none of these permutations was there a significant difference between PMG and controls (Supplemental Figure 4). Furthermore, there was no enrichment of rare variants previously classified as pathogenic or likely pathogenic in the Database of Complement Gene Variants, with one and 13 such variants identified in PMG and non-PMG individuals, respectively (Supplemental Table 5).
Figure 1.
Similar burden of rare variants with moderate or high predicted impact in candidate genes comparing unrelated European PMG cases (n=146) with controls (n=6442). Vertical dotted lines indicate 95% CIs.
Sixteen previously described common complement gene variants (gnomAD-NFE MAF ≥0.05) were identified (Supplemental Table 6). Computing pairwise linkage disequilibrium demonstrated that these variants represented ten independent signals at r2<0.8. Association analysis using logistic regression including principal components as covariates identified four variants in the genes C3 and CFH, representing two independent signals, that were statistically significant after correction for multiple testing (Bonferroni threshold for ten loci, P<0.005) (Table 2). The full association statistics for all 16 variants are provided in Supplemental Table 6. There was no evidence of epistasis between the associated variants (P>0.05).
Table 2.
Association statistics comparing PMG with controls for four common variants in complement genes previously described at altered frequency in individuals with aHUS/MPGN
| Chr | Position | Ref | Alt | rsID | Gene | HGVSp | Case | Control | gnomAD-NFE | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 196654324 | A | C | rs1061147 | CFH | p.A307A | 0.534 | 0.620 | 0.617 | 0.71 (0.56 to 0.89) | 3.5×10−3 |
| 1 | 196659237 | C | T | rs1061170 | CFH | p.H402Y | 0.534 | 0.620 | 0.616 | 0.71 (0.56 to 0.89) | 3.4×10−3 |
| 19 | 6713262 | G | A | rs1047286 | C3 | p.P314L | 0.284 | 0.213 | 0.200 | 1.47 (1.13 to 1.90) | 3.9×10−3 |
| 19 | 6718387 | G | C | rs2230199 | C3 | p.R102G | 0.295 | 0.218 | 0.206 | 1.49 (1.16 to 1.93 | 2.1×10−3 |
The two chromosome 19 variants and two chromosome 1 variants are in linkage disequilibrium (r2=0.844 and r2=0.999, respectively). Chr, chromosome; Ref, reference allele; Alt, alternate allele; rsID, dbSNP identifier; HGVSp, HGVS protein sequence change; gnomAD-NFE, allele frequency in non-Finish Europeans in the gnomAD database; Position, reference and alternate alleles are given with reference to Build 37 of the human genome.
Across the whole exome, there was no enrichment of rare variants with a moderate or high predicted impact per gene in PMG (Supplemental Figure 5). The minimum P value across the exome was 1.9×10−4 as compared with the exome-wide significance threshold, correcting for 28,252 genes, of P<1.77×10−6. The QQ plot showed no evidence of deviation from the null (Supplemental Figure 6). When filtering the data using a CADD threshold of ≥15, the minimum P value was also 1.9×10−4.
The only previously reported rare structural variant observed in PMG cases was the 6.3 kbp CFHR5 tandem duplication (chr1:196950207–196956508) known to cause CFHR5 nephropathy,5 present in a single individual of Cypriot ancestry. The common CFHR3-CFHR1 deletion was observed at a similar frequency in European PMG cases and controls at 0.164 and 0.201, respectively (Fisher exact test P=0.14), similar to that in the UK population.31 Across all the candidate genes, a total of 65 structural variants and copy number variants were identified, of which only one was seen in a PMG case: a 128.3 kbp heterozygous deletion involving exon 1 of CFH (chr1:196498350–196626665). Genome-wide, there was no enrichment of deletions in PMG cases either in total or per gene, after correcting for multiple testing by permutation analysis.
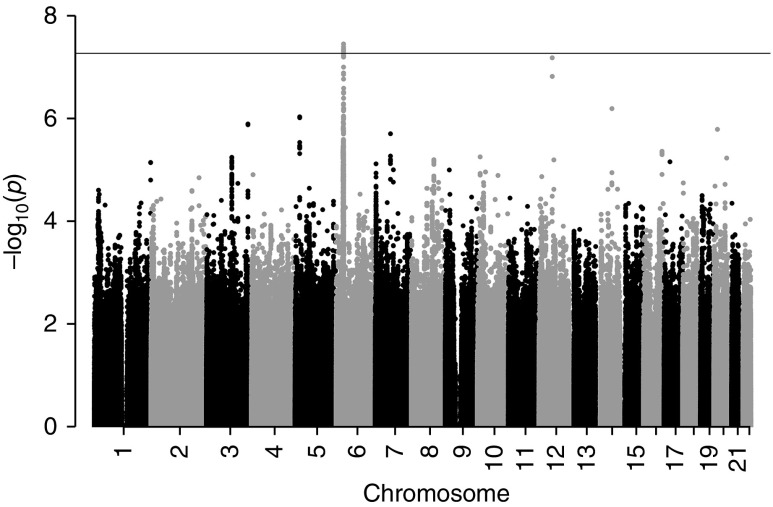
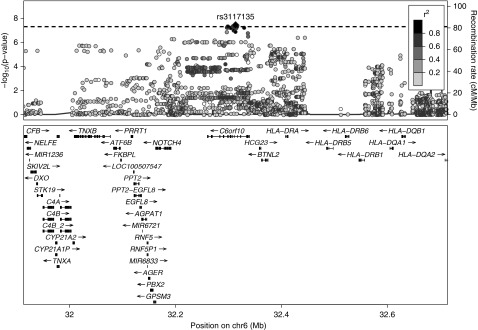
A common-variant genome-wide association study examining unrelated individuals of European ancestry identified one locus achieving genome-wide significance (P<5×10−8) at 6p21.32 (Figure 2). The genomic inflation (λ) was 1.017 (QQ plot in Supplemental Figure 7). Association statistics for all variants achieving P<5×10−8 are provided in Supplemental Table 7. At the 6p21.32 locus, the lead variant (rs35406322) was associated at P=3.29×10−8 (odds ratio [OR], 1.93; 95% CI, 1.53 to 2.44). Significance was maximal for variants within the gene C6orf10 (Figure 3). The control allele frequency of the lead variant (0.361) approximated to that in gnomAD-NFE (0.376) and was consistent across all of the BR-RD control cohorts (Supplemental Figure 8). There was no statistically significant difference in the frequency of the lead variants by PMG histologic subtype, C3NeF status, or in those with low C3 (Supplemental Figure 8). Conditioning on the lead variant abrogated the signal. The second lead variant (rs3117135) is a known eQTL for multiple genes in multiple tissue types at genome-wide significance, including HLA-DRB5, CYP21A1P, C4A, and NOTCH4 (Supplemental Table 8). There was no evidence of epistasis (P>0.05) between the 6p21.32 variants and the nominally associated common candidate complement gene variants. Testing dominant and recessive models for the lead variant showed weaker evidence of association, suggesting an additive genetic model best explains the association. A second locus at 12q14.1 was not statistically significant at the genome-wide level (lead variant rs61938185, P=6.14×10−8).
Figure 2.
Genome-wide association study comparing unrelated European PMG cases (n=146) with controls (n=6442) at 5,897,512 common variants identifies a single locus surpassing the genome-wide significance threshold (P<5×10−8, indicated by the horizontal line) on chromosome six.
Figure 3.
LocusZoom plot for the chromosome 6 locus associated with PMG at genome-wide significance shows the signal to reside within the gene C6orf10 within the HLA complex. Variants are colored on the basis of their linkage disequilibrium (LD), using 1000 Genomes (November 2014) European data. As there were no reference LD data available for the lead marker, the second most significant marker was used (chr6:32313531, rs3117135). A horizontal dotted line indicates P=5×10−8.
Fully imputed HLA genotypes at all six loci were available for all 146 of the PMG cases and 6386 of the controls. A total of 39 HLA alleles were observed with a frequency >0.05. The strongest association was with DQA1*05:01 at P=2.09×10−8 (OR, 1.94; 95% CI, 1.54 to 2.45), followed by DRB1*03:01 and DQB1*02:01 (Table 3). Full association details for all alleles tested are provided in Supplemental Table 9. The association with both DQB1*02:01 and DRB1*03:01 was abrogated by conditioning on DQA1*05:01. The DQA1*05:01|DQB1*02:01|DRB1*03:01 haplotype was observed in cases at a frequency of 0.233 compared with 0.122 in controls (P=1.21×10−8; OR, 2.19; 95% CI, 1.66 to 2.89). The control frequency of this haplotype approximates to that observed in 1899 European American individuals (0.131).32 These analyses were repeated using HLA types imputed using HLA-HD, yielding similar results (Supplemental Table 9).
Table 3.
Association statistics comparing PMG and controls for the three most significant HLA alleles imputed using BWAKIT/BWAMEM
| Allele | Case | Control | OR (95% CI) | P Value |
|---|---|---|---|---|
| DQA1*05:01 | 0.291 | 0.154 | 1.94 (1.54 to 2.45) | 2.09×10−8 |
| DRB1*03:01 | 0.264 | 0.146 | 1.94 (1.51 to 2.50) | 2.46×10−7 |
| DQB1*02:01 | 0.264 | 0.144 | 1.81 (1.43 to 2.29) | 7.69×10−7 |
HLA serotypes from the NHSBT were available from 338 individuals with MPGN (both primary and secondary) and 15,614 non-MPGN controls with renal failure of nonimmune or unknown cause, the largest groups of which were unknown (n=6836), polycystic kidney disease (n=4442), and pyelonephritis/interstitial nephritis (n=1958). Using a Bonferroni threshold of P<1.8×10−3 (n=28), three serotypes were statistically significantly associated with MPGN, with an OR of approximately 1.4: DR17 (corresponding to DRB1*03:01/04), B8 (B*08), and DQ2 (DQB1*02:01/02/03/04/05) (Table 4). The frequency of the most significant serotype, DR17, was approximately consistent across each of the control cohorts, particularly those with larger sample sizes (Supplemental Figure 9), and equal to the frequency in 1043 UK blood cell donors.33 The significance of B8 and DQ2 was abrogated by conditioning on DR17. The DR17|DQ2 haplotype was observed in cases at a frequency of 0.175 compared with 0.129 in controls (P=4.55×10−4; OR, 1.43; 95% CI, 1.17 to 1.75).
Table 4.
Association statistics for the HLA serotypes associated with MPGN in the NHSBT data after correcting for multiple testing (P<1.8×10−3)
| Serotype | Case | Control | OR (95% CI) | P Value |
|---|---|---|---|---|
| DR17 | 0.188 | 0.137 | 1.46 (1.20 to 1.78) | 1.4×10−4 |
| B8 | 0.186 | 0.140 | 1.42 (1.16 to 1.72) | 4.7×10−4 |
| DQ2 | 0.283 | 0.228 | 1.33 (1.12 to 1.58) | 9.3×10−4 |
Discussion
In this study we have examined the genetics of PMG using whole-genome sequence data generated from a UK-wide collection of cases and a large number of genetic ancestry–matched, non-PMG controls. The high prevalence of C3NeFs and reduced serum C3 levels, especially in patients with DDD and/or a C3NeF, is consistent with previous literature11,13 and suggests that the cohort under study was comparable with previously reported PMG cohorts. Although we did observe rare, protein-altering variants in the candidate genes (encoding components of the complement alternative pathway and other genes observed in the related disease aHUS), PMG cases were not enriched for such variants, which occurred at a frequency of approximately 6% across all cohorts. Our study of 146 European cases and 6442 European controls had >92% power to detect a >15% burden of rare complement gene variants in PMG. We also observed association of PMG with common alleles of the candidate complement genes and, although not statistically significant at the genome-wide level, this is consistent with previous data and provides evidence that variation in genes encoding components of the complement alternative pathway affects susceptibility to PMG.
Power calculation, which we performed before recruitment to this study (using previously described methods)34 indicated that, using whole-exome analysis, 100 individuals would have provided >80% power to detect association with rare variants in a novel gene accounting for 20% of unexplained cases under a dominant model (power would be >95% under a recessive model). We recruited a greater number than this, which suggests that any currently unrecognized monogenic disorders (caused by coding mutations) are unlikely to account for a significant proportion of PMG in the UK population.
Analysis of the frequency of common genetic variants across the genome identified a single locus achieving genome-wide significance of P<5×10−8. Numerous markers at the HLA locus were strongly associated with PMG, and imputation identified an associated haplotype containing DQA1*05:01, DQB1*02:01, and DRB1*03:01. This finding was replicated in an independent cohort that included both primary and secondary MPGN, in which we observed association with the corresponding HLA serotypes DQ2 (DQB1*02:01) and DR17 (DRB1*03:01), which are associated with a number of immune-mediated disorders. These genes encode components of the MHC class 2 molecule that are found on the surface of antigen-presenting cells and are important in initiation of the adaptive immune response, including antibody production.35 This suggests that a key step in the pathogenesis of these disorders is an aberrant adaptive immune response,36 which is consistent with the high frequency of autoantibodies (i.e., C3NeFs) in these patients. However, the possibility has not been excluded that the observed associations are mediated by one of the non-HLA genes spanned by this haplotype, including those encoding complement components C2, Factor B, and C4. Variation in dose of C4A and C4B, which encode isotypes of C4 that preferentially bind antibody-protein or antibody-cell surface complexes, respectively,37 is known to affect serum C4 activity and has previously been implicated in SLE and schizophrenia.38,39 However, direct comparison of the copy number of each of these genes showed no significant differences between PMG cases and controls. Examining C4NeF levels and the relationship with the observed variants may be informative.
We observed shared genetic risk factors in all the subgroups of PMG (IC-PMG, C3GN, and DDD), as well as those with and without C3NeF, and those with low C3 (Supplemental Figure 5) in this study, implying shared underlying disease mechanisms. We did not observe any significant genetic differences between these subgroups and it is likely larger studies would be needed to identify such differences, if they exist. Tests for the presence of C4 nephritic factors or autoantibodies against complement regulators (which have been reported in patients with PMG40) were not available for the whole cohort, so we are unable to determine the proportion of patients in whom an autoantibody was present; however, the HLA association we observed is consistent with other reports in which multiple autoantibodies are detectable in cohorts of patients with PMG40 and previous observations showing an association between MPGN/C3G and autoimmune disorders.16–19 Some of the variants and HLA alleles that we observed to be associated with PMG have previously been associated with a number of immune mediated diseases, including membranous nephropathy,41 rheumatoid arthritis,42 myasthenia gravis,43 asthma,44 celiac disease,45 and type 1 diabetes mellitus,46,47 potentially explaining the observed phenotypic association between these different disorders. Together, these findings imply that, rather than resulting from a primary genetic disorder of complement alternative pathway regulation, in most cases PMG is actually an autoimmune disease.
Disclosures
Dr. Carss reports personal fees from AstraZeneca, outside the submitted work. Dr. Gale reports personal fees from Alexion, personal fees from Novartis, outside the submitted work. Dr. Harris reports other from Admirx, other from GlaxoSmithKline, other from Gyroscope Therapeutics, grants from RaPharma, other from Roche, outside the submitted work. Dr. Marchbank reports other from Gemini Therapeutics LTD, outside the submitted work; In addition, Dr. Marchbank has a patent “Modified Complement Proteins and Uses Thereof” pending. Dr. Megy reports grants from National Institute for Health Research, during the conduct of the study.
Funding
This work was funded by the National Institute for Health Research (NIHR; grant numbers: RG65966, BH141504, and PD00400) and Kids Kidney Research. Dr. Gale is supported by a Medical Research Council Clinician Scientist Fellowship and St Peter’s Trust. Dr. Chan is supported by a Kidney Research UK Clinical Training Fellowship. Dr. Sadeghi-Alavijeh is supported by an NIHR clinical fellowship.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of all National Institute for Health Research (NIHR) BioResource volunteers, and thank the NIHR Cambridge BioResource Centre and the NIHR Newcastle Biomedical Research Centre for their contributions. We also acknowledge the UK National Health Service Blood and Transplant (NHSBT) Service for kindly providing HLA serotype data. Genomic data from the NIHR BioResource Rare Diseases study has been deposited in the European Genome Archive under accession number EGAS00001001012.
Dr. Gale conceived and designed the study. Dr. Levine, Dr. Chan, and Dr. Sadeghi-Alavijeh analyzed the data with the assistance of Dr. Carss, Dr. Penkett, and Dr. Tuna. Dr. Johnson directed the establishment of the primary membranoproliferative GN cohort. Prof. Cook undertook the centralized biopsy review. Dr. Gale and Dr. Levine drafted the manuscript. All other authors were responsible for recruitment of patients to the study and data acquisition.
The members of the MPGN/DDD/C3 Glomerulopathy Rare Disease Group and the NIHR BioResource may be found in the Supplemental Material.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019040433/-/DCSupplemental.
Supplemental Appendix 1. Consortia, supplemental methods, results, detailed legends for tables and references.
Supplemental Figure 1. Sample and analytic workflow.
Supplemental Figure 2. Principal component analysis.
Supplemental Figure 3. Candidate gene rare variant cumulative burden by cohort and PMG subphenotype.
Supplemental Figure 4. Candidate gene rare variant cumulative burden with variable filtering (CADD and frequency).
Supplemental Figure 5. Exome-wide rare variant burden analysis Manhattan plot.
Supplemental Figure 6. Exome-wide rare variant burden analysis QQ plot.
Supplemental Figure 7. Genome-wide association study QQ plot.
Supplemental Figure 8. Allele frequency of chromosome 6 lead variant by cohort and PMG subphenotype.
Supplemental Figure 9. Frequency of HLA DR17 by cohort in the NHSBT data.
Supplemental Table 1. BR-RD cohorts and sample sizes with filtering.
Supplemental Table 2. Histological characterization and clinical details of C3G participants.
Supplemental Table 3. Prioritized rare variants in candidate genes in PMG participants.
Supplemental Table 4. Prioritized rare variants in candidate genes in non-PMG participants.
Supplemental Table 5. Rare variants in candidate genes in the Database of Complement Gene Variants.
Supplemental Table 6. Association statistics for common variants in candidate genes previously identified in aHUS/MPGN.
Supplemental Table 7. Association statistics for all variants achieving P<5×10−8 in the genome-wide association study.
Supplemental Table 8. eQTL results for rs3117135.
Supplemental Table 9. Association statistics for imputed HLA alleles using BWAKIT/BWAMEM and HLA-HD.
References
- 1.Sethi S, Nester CM, Smith RJ: Membranoproliferative glomerulonephritis and C3 glomerulopathy: Resolving the confusion. Kidney Int 81: 434–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, et al.: The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16: 1364–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Leung N, Lager DJ, Melton LJ 3rd, Bergstralh EJ, Rohlinger A, et al.: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Zand L, Leung N, Smith RJ, Jevremonic D, Herrmann SS, et al.: Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol 5: 770–782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, et al.: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik TH, Lavin PJ, Goicoechea de Jorge E, Vernon KA, Rose KL, Patel MP, et al.: A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol 23: 1155–1160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, et al.: Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 124: 145–155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, et al.: C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 123: 2434–2446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ault BH, Schmidt BZ, Fowler NL, Kashtan CE, Ahmed AE, Vogt BA, et al.: Human factor H deficiency. Mutations in framework cysteine residues and block in H protein secretion and intracellular catabolism. J Biol Chem 272: 25168–25175, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, et al.: Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 120: 3702–3712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey M-A, et al.: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bu F, Borsa NG, Jones MB, Takanami E, Nishimura C, Hauer JJ, et al.: High-throughput genetic testing for thrombotic microangiopathies and C3 glomerulopathies. J Am Soc Nephrol 27: 1245–1253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iatropoulos P, Noris M, Mele C, Piras R, Valoti E, Bresin E, et al.: Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol 71: 131–142, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Osborne AJ, Breno M, Borsa NG, Bu F, Frémeaux-Bacchi V, Gale DP, et al.: Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol 200: 2464–2478, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al. Conference Participants : Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 91: 539–551, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Dizdar O, Kahraman S, Gençtoy G, Ertoy D, Arici M, Altun B, et al.: Membranoproliferative glomerulonephritis associated with type 1 diabetes mellitus and Hashimoto’s thyroiditis. Nephrol Dial Transplant 19: 988–989, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Chihara J, Takebayashi S, Taguchi T, Yokoyama K, Harada T, Naito S: Glomerulonephritis in diabetic patients and its effect on the prognosis. Nephron 43: 45–49, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Srikanta S, Malaviya AN, Rajagopalan P, Bhuyan UN, Ahuja MM: Association of type I (insulin-dependent) diabetes mellitus, autoimmunity, antinuclear antibody, and membranoproliferative glomerulonephritis. Diabetes Care 6: 71–74, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Lu DF, Moon M, Lanning LD, McCarthy AM, Smith RJH: Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol 27: 773–781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The NIHR BioResource, on behalf of the 100,000 Genomes Project. Whole-genome sequencing of rare disease patients in a national healthcare system. bioRxiv. Available at: . Accessed January 01, 2019. [DOI]
- 21.Köhler S, Carmody L, Vasilevsky N, Jacobsen JOB, Danis D, Gourdine JP, et al.: Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res 47: D1018–D1027, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al.: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togarsimalemath SK, Sethi SK, Duggal R, Le Quintrec M, Jha P, Daniel R, et al.: A novel CFHR1-CFHR5 hybrid leads to a familial dominant C3 glomerulopathy. Kidney Int 92: 876–887, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Xiao X, Ghossein C, Tortajada A, Zhang Y, Meyer N, Jones M, et al.: Familial C3 glomerulonephritis caused by a novel CFHR5-CFHR2 fusion gene. Mol Immunol 77: 89–96, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ: RVTESTS: An efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics 32: 1423–1426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M: CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 47: D886–D894, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT: Data quality control in genetic case-control association studies. Nat Protoc 5: 1564–1573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi S, Higasa K, Shimizu M, Yamada R, Matsuda F: HLA-HD: An accurate HLA typing algorithm for next-generation sequencing data. Hum Mutat 38: 788–797, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Holdsworth R, Hurley CK, Marsh SG, Lau M, Noreen HJ, Kempenich JH, et al.: The HLA dictionary 2008: A summary of HLA-A, -B, -C, -DRB1/3/4/5, and -DQB1 alleles and their association with serologically defined HLA-A, -B, -C, -DR, and -DQ antigens. Tissue Antigens 73: 95–170, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Vargas R, Thomson KJ, Wilson D, Cameron JS, Turner DR, Gill D, et al.: Mesangiocapillary glomerulonephritis with dense “deposits” in the basement membranes of the kidney. Clin Nephrol 5: 73–82, 1976 [PubMed] [Google Scholar]
- 31.Holmes LV, Strain L, Staniforth SJ, Moore I, Marchbank K, Kavanagh D, et al.: Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS One 8: e60352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klitz W, Maiers M, Spellman S, Baxter-Lowe LA, Schmeckpeper B, Williams TM, et al.: New HLA haplotype frequency reference standards: High-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens 62: 296–307, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Pingel J, Solloch UV, Hofmann JA, Lange V, Ehninger G, Schmidt AH: High-resolution HLA haplotype frequencies of stem cell donors in Germany with foreign parentage: How can they be used to improve unrelated donor searches? Hum Immunol 74: 330–340, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Zhi D, Chen R: Statistical guidance for experimental design and data analysis of mutation detection in rare monogenic mendelian diseases by exome sequencing. PLoS One 7: e31358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein J, Sato A: The HLA system. First of two parts. N Engl J Med 343: 702–709, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Dendrou CA, Petersen J, Rossjohn J, Fugger L: HLA variation and disease. Nat Rev Immunol 18: 325–339, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Law SK, Dodds AW, Porter RR: A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J 3: 1819–1823, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jüptner M, Flachsbart F, Caliebe A, Lieb W, Schreiber S, Zeuner R, et al.: Low copy numbers of complement C4 and homozygous deficiency of C4A may predispose to severe disease and earlier disease onset in patients with systemic lupus erythematosus. Lupus 27: 600–609, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium : Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noris M, Donadelli R, Remuzzi G: Autoimmune abnormalities of the alternative complement pathway in membranoproliferative glomerulonephritis and C3 glomerulopathy. Pediatr Nephrol 34: 1311–1323, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, et al.: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. RACI consortium; GARNET consortium : Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506: 376–381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregersen PK, Kosoy R, Lee AT, Lamb J, Sussman J, McKee D, et al.: Risk for myasthenia gravis maps to a (151) Pro→Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol 72: 927–935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, et al.: Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet 43: 893–896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, et al.: A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 39: 827–829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al.: A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 448: 591–594, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. Type 1 Diabetes Genetics Consortium : HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: Analysis of the type 1 diabetes genetics consortium families. Diabetes 57: 1084–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.