Abstract
Background
Treatment of metastatic pancreatic adenocarcinoma (mPAC) relies on chemotherapeutic regimens. We investigated patterns of first-line and second-line treatment choices, their geographical variation between European countries, and alignment with current European recommendations.
Methods
This retrospective, observational chart review study was conducted between July 2014 and January 2016. Physicians were recruited from nine European countries. Patient data were collected in electronic patient record forms (PRFs) by physicians managing patients with mPAC. Patients with a current mPAC diagnosis aged ≥18 years old who had completed first-line therapy during the study period were included.
Results
Participating physicians (n=225) completed 2565 PRFs. The vast majority of PRFs were from France, Germany, Italy, Spain and the UK. Most patients (86.6%) had stage IV disease at diagnosis. The most common first-line treatments were FOLFIRINOX (5-fluorouracil, leucovorin/folinic acid, irinotecan and oxaliplatin) (35.6%), gemcitabine+nab-paclitaxel (25.7%) and gemcitabine monotherapy (20.5%). Physicians in France and the UK prescribed FOLFIRINOX more frequently than gemcitabine+nab-paclitaxel. Gemcitabine-based therapies were more widely used at second-line, although 5-fluorouracil-based therapies were preferred in Italy and Spain, where gemcitabine-based treatments were more frequently selected for first-line. For patients receiving first-line modified FOLFIRINOX, second-line gemcitabine monotherapy was preferred in the overall population (45.9%).
Conclusion
Although treatment choices for patients with mPAC varied between countries, they align with current European guidelines. Factors including drug availability, reimbursement, patient characteristics, physician preference and prior first-line therapy affect treatment choices. Approved, recommended therapies for patients who progress following first-line treatment are lacking. These findings may influence the development of effective treatment plans, potentially improving future patient outcomes.
Keywords: 5-fluorouracil, albumin-bound paclitaxel, gemcitabine, pancreatic cancer, Western Europe
Key questions.
What is already known about this subject?
Very little data have been published regarding first-line and second-line treatment choices for European patients with metastatic pancreatic adenocarcinoma.
What does this study add?
Although treatment choices varied between different European countries due to influencing factors such as drug availability and physician preference, these choices were aligned with the European Society for Medical Oncology guidelines.
How might this impact on clinical practice?
A clearer understanding of current treatment patterns within Europe may help in the development of effective treatment plans in the future.
Introduction
Pancreatic cancer is predicted to become the second most common cause of cancer-related death by 2030 in the USA,1 2 despite overall falls in cancer-related mortality.1 3 The disease is estimated as the fourth most common cause in Europe,4 and pancreatic adenocarcinoma accounts for 85% of pancreatic cancer cases.5
Surgery is the sole potentially curative option for patients with pancreatic cancer. This is only possible in 15%–20% of patients6 as non-specific symptoms and disease aggressiveness lead to late diagnosis.7 Most patients (~80%) have locally advanced or metastatic pancreatic adenocarcinoma (mPAC) at diagnosis,8 9 and 60%–90% of resected patients will develop locally recurrent or metastatic disease despite surgery and adjuvant treatment.10 11
The European Society for Medical Oncology (ESMO) guidelines recommend patients with mPAC and European Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1 receive first-line treatment with gemcitabine combined with albumin-bound paclitaxel (nab-paclitaxel), or with infusional 5-fluorouracil (5-FU), leucovorin/folinic acid (LV), irinotecan and oxaliplatin (FOLFIRINOX). Less fit patients (ECOG PS 2) should generally receive gemcitabine monotherapy, or best supportive care in those with worse PS or comorbidities,6 although certain patients with PS 2 may be able to receive gemcitabine+nab-paclitaxel if their poor performance status is due to a heavy tumour load.6 12
Until recently, there has been no approved standard of care for second-line treatment13; however, liposomal irinotecan (nal-IRI) in combination with 5-FU+LV (nal-IRI+5-FU/LV) is now approved in several countries following the results of the NAPOLI-1 study.14 Data from the CONKO-003 and PANCREOX studies evaluating oxaliplatin-based regimens yielded conflicting results.15 16 This, along with the NAPOLI-1 results, led the ESMO to conclude that ‘For fit patients, nal-IRI combined with 5-FU and LV may constitute an active and tolerable second-line treatment option’.17 Recommendations were also made in recent National Comprehensive Cancer Network (NCCN) guidelines and American Society of Clinical Oncology (ASCO) treatment guidelines for pancreatic cancer.18 19 First-line FOLFIRINOX and gemcitabine-based treatments can extend survival by several months in patients with mPAC.6 20 Nevertheless, survival remains poor and there is a clear need to develop new treatment strategies and concepts to improve survival while minimising adverse events.
Patient characteristics, comorbidities and safety profile affect treatment choices, which may also vary depending on country, treatment guidelines, treatment goals, physician preference and reimbursement status.21 Published data regarding the European mPAC treatment landscape are very limited. Therefore, this study aimed to investigate patterns of first-line and second-line treatment choices, their geographical variation between countries, and alignment with current ESMO continental recommendations. Information regarding safety and detailed baseline characteristics will be reported elsewhere.
Methods
Data for this retrospective, observational, chart review study were collected between July 2014 and January 2016 using online patient record forms (PRFs; online supplementary appendix A) in France, Germany, Italy, Spain, the UK, the Netherlands, Denmark, Norway and Sweden. Due to small sample size, the Netherlands, Denmark, Norway and Sweden were grouped into one region (North).
esmoopen-2019-000587supp001.pdf (759.1KB, pdf)
Physicians completed PRFs electronically, with respondents recruited from a range of regions and settings ensuring a balanced representation of each country (eg, university and general hospitals, cancer and reference centres, office-based specialists). Respondents were asked to confirm patient eligibility and to report each patient only once. Programming rules were implemented in the PRF system to avoid extreme or incoherent values at data collection. Completed PRFs were reviewed by a statistician (Genactis SAS, Mougins, France) on behalf of the study sponsor, and inaccurate data were removed prior to analysis. The data clean-up process included reviewing outliers and contacting physicians to confirm values. The data presented in this manuscript are descriptive only.
Study population
This study was conducted and data were collected by Genactis SAS on behalf of the study scientific committee and sponsor. It included patients aged ≥18 years with a current diagnosis of mPAC who had completed a first-line anticancer treatment during the review period. Physicians were encouraged to enter patients who underwent second-line metastatic treatment to obtain data on first-line and second-line treatment patterns. Physicians were included if they were certified medical oncologists or gastroenterologists with an oncology specialty (France and Germany) currently treating patients with mPAC and were involved in treatment choices. Verified physicians from the panel were randomly invited and underwent a screening process (telephone or online) of seven questions (online supplementary appendix B) to confirm their practice and experience. Those passing screening were invited to join the study. Genactis SAS provided support during the study in the physicians’ native language by email or telephone, via inhouse medical recruiters.
esmoopen-2019-000587supp002.pdf (187.7KB, pdf)
The minimum number of patients with mPAC treated by each physician was set at 15, with at least 10 patients having received first-line treatment in the last 24 months.
Outcomes
Information collected in PRFs included diagnostic details, baseline patient characteristics and treatment history (additional details in online supplementary appendix A). Physicians reported age, gender, date and method of diagnosis, disease stage at diagnosis, and tumour location, grade and resectability. Baseline characteristics included weight, height, ECOG PS, comorbidities, and serum levels of carbohydrate antigen (CA) 19-9, albumin and bilirubin (reported at initial diagnosis (any stage) and metastatic diagnosis). Data were collected regarding all therapies received (including resection and radiotherapy), treatment outcome, chemotherapy doses, dose modifications/discontinuations and associated reasons. Information regarding clinical trial involvement, current treatment status and last known therapy was also collected.
Results
Patient characteristics at initial and metastatic diagnoses
Between July 2014 and January 2016, 2565 online PRFs were completed by 225 physicians across nine European Union countries (n=500–504 PRFs per country for France, Germany, Italy, Spain and the UK), including 10 gastroenterologists with an oncology specialty (6 from France, 4 from Germany). As few PRFs were finally collected from the North region (n=49 in total for the Netherlands, Denmark, Norway and Sweden), this individual region is not included in the analysis reported here. Physician recruitment and patient record flow are detailed in online supplementary figure 1.
esmoopen-2019-000587supp003.pdf (1.2MB, pdf)
Patient demographics and characteristics at initial and metastatic diagnoses are summarised in table 1 and online supplementary table 1, respectively. At initial diagnosis, 57.7% of patients were male. This proportion was similar across countries. The median patient age at initial diagnosis was 64 years, with 56.8% patients aged ≤65 years and 43.2% aged >65 years. This proportion varied between countries, with more patients ≥65 years in France, Germany and Italy than in Spain and the UK.
Table 1.
Patient demographics and characteristics at initial diagnosis
| Total (N=2565) |
France (n=504) |
Germany (n=504) |
Italy (n=500) |
Spain (n=504) |
UK (n=504) |
|
| Age (years), n (%) | ||||||
| ≤65 | 1457 (56.8) | 244 (48.4) | 263 (52.2) | 276 (55.2) | 319 (63.3) | 322 (63.9) |
| >65 | 1108 (43.2) | 260 (51.6) | 241 (47.8) | 224 (44.8) | 185 (36.7) | 182 (36.1) |
| Gender, n (%) | ||||||
| Male | 1479 (57.7) | 303 (60.1) | 293 (58.1) | 278 (55.6) | 281 (55.8) | 295 (58.5) |
| Female | 1086 (42.3) | 201 (39.9) | 211 (41.9) | 222 (44.4) | 223 (44.2) | 209 (41.5) |
| Primary tumour location, n (%) | ||||||
| Head | 1030 (40.2) | 196 (38.9) | 212 (42.1) | 200 (40.0) | 195 (38.7) | 207 (41.1) |
| Body | 594 (23.2) | 114 (22.6) | 119 (23.6) | 98 (19.6) | 115 (22.8) | 142 (28.2) |
| Tail | 259 (10.1) | 55 (10.9) | 46 (9.1) | 37 (7.4) | 67 (13.3) | 51 (10.1) |
| Head/body | 417 (16.3) | 88 (17.5) | 75 (14.9) | 79 (15.8) | 90 (17.9) | 71 (14.1) |
| Body/tail | 246 (9.6) | 46 (9.1) | 51 (10.1) | 83 (16.6) | 35 (6.9) | 26 (5.2) |
| Unknown | 19 (0.7) | 5 (1.0) | 1 (0.2) | 3 (0.6) | 2 (0.4) | 7 (1.4) |
| ECOG performance status, n (%) | ||||||
| 0 | 522 (20.4) | 71 (14.1) | 98 (19.4) | 165 (33.0) | 71 (14.1) | 94 (18.7) |
| 1 | 1432 (55.8) | 264 (52.4) | 276 (54.8) | 260 (52.0) | 288 (57.1) | 325 (64.5) |
| 2 | 561 (21.9) | 151 (30.0) | 112 (22.2) | 73 (14.6) | 140 (27.8) | 78 (15.5) |
| 3 | 41 (1.6) | 18 (3.6) | 16 (3.2) | 2 (0.4) | 5 (1.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 9 (0.4) | 0 (0.0) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 7 (1.4) |
| Median CA19-9, U/mL | 387 (N=2151) |
478 (n=393) |
534 (n=458) |
211 (n=443) |
425 (n=456) |
345 (n=362) |
| Median albumin, g/L | 34.0 (N=1764) |
33.0 (n=391) |
37.0 (n=296) |
35.0 (n=280) |
31.0 (n=333) |
32.0 (n=418) |
| Median bilirubin, mg/dL | 1.8 (N=2138) |
2.0 (n=398) |
2.0 (n=451) |
1.8 (n=397) |
1.3 (n=430) |
2.5 (n=416) |
CA19-9, carbohydrate antigen 19-9; ECOG, Eastern Cooperative Oncology Group.
Patients with ECOG PS 2 were more frequent in France (30%) and Spain (27.8%) than in Italy (14.6%) and the UK (15.5%). Tumours were mostly located in the pancreatic head (40.2%) or body (23.2%). The median CA19-9 levels at diagnosis ranged from 211 to 534 U/mL across the five countries. The median bilirubin and albumin levels were generally similar across the surveyed countries (bilirubin range 1.3–2.5 mg/dL; albumin range 31.0–37.0 g/L).
Most patients (86.6%) had stage IV disease at diagnosis, with 2.9%, 8.5% and 1.6% of patients initially diagnosed at stage III, II and I, respectively. Patient characteristics of the 86.6% who were metastatic at diagnosis are presented in online supplementary table 1. Of those patients who did not have metastatic disease at diagnosis, 92.4% had their primary tumour resected, with 68% of resected patients receiving adjuvant therapy following surgery. FOLFIRINOX and gemcitabine-based regimens were most commonly used to treat patients who had locally advanced unresectable disease at diagnosis (30.8% and 53.8%, respectively), with 9.2% of these patients treated with another 5-FU-based regimen.
First-line metastatic treatment patterns
Most first-line treatment choices could be split between 5-FU-based (43.4%) and gemcitabine-based treatments (55.4%; online supplementary table 2). FOLFIRINOX (35.6%, including full dose and modified dose), gemcitabine+nab-paclitaxel (25.7%) and gemcitabine monotherapy (20.5%) were the most commonly used first-line treatments overall. Other 5-FU or gemcitabine combinations were less frequently used (online supplementary figure 2).
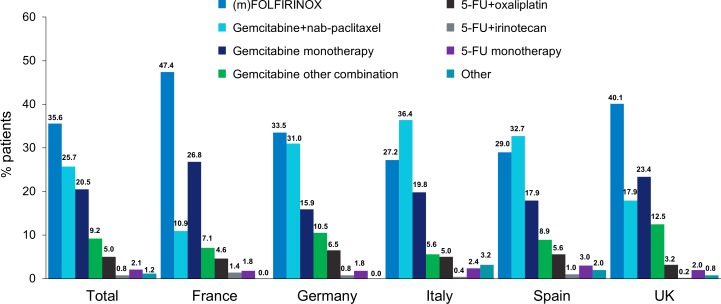
First-line treatment preferences varied across Europe, with FOLFIRINOX used more frequently in France and the UK (47.4% and 40.1%, respectively) than in Spain and Italy (29.0% and 27.2%, respectively; online supplementary table 2, figure 1). Gemcitabine-based therapies were less frequently used in France (44.8%) than in Italy, Spain, Germany and the UK (61.8%, 59.5%, 57.3% and 53.8%, respectively). Gemcitabine+nab-paclitaxel was used less frequently in France and the UK versus other countries (online supplementary figure 2B). Conversely, gemcitabine monotherapy was used more often in France and the UK versus Germany, Italy and Spain. Patients treated with FOLFIRINOX were more likely to be ≤65 years old and have an ECOG PS score 0–1 than those treated with gemcitabine+nab-paclitaxel (table 2). Similar trends were observed in the individual countries (online supplementary table 3).
Figure 1.
Geographical distribution of first-line treatment regimen choices5-FU, 5-fluorouracil; FOLFIRINOX, folinic acid, fluorouracil, irinotecan and oxaliplatin; m, modified.
Table 2.
Patients’ demographics and characteristics in first-line gemcitabine+nab-paclitaxel or FOLFIRINOX treatment
| Patients (%) | First-line gemcitabine+nab-paclitaxel (n=660) | First-line FOLFIRINOX | ||||
| All (n=912) | mFOLFIRINOX (n=164) | fFOLFIRINOX | ||||
| All (n=748) | Never modified (n=553) |
Modified in cycle ≥2 (n=195) | ||||
| Total population | ||||||
| With dose adjustment | 20.5 | 22.6 | 6.7 | 26.1 | NA | 100.0 |
| >65 years old | 49.4 | 29.9 | 25.0 | 31.0 | 29.3 | 35.9 |
| Female | 43.2 | 36.3 | 41.5 | 35.2 | 32.7 | 42.1 |
| ECOG PS 0–1 | 76.5 | 87.7 | 89.6 | 87.3 | 86.7 | 89.2 |
| Received second-line treatment | 67.4 | 78.1 | 78.0 | 78.1 | 78.9 | 75.9 |
| Median OS/PFS (months) | 12/7 | 15/10 | 16/10 | 15/10 | 14/10 | NR* |
*OS/PFS not calculated due to small sample size.
ECOG, Eastern Cooperative Oncology Group; fFOLFIRINOX, full-dose FOLFIRINOX; FOLFIRINOX, folinic acid, fluorouracil, irinotecan and oxaliplatin; mFOLFIRINOX, modified FOLFIRINOX; NA, not applicable; NR, not reported; OS, overall survival; PFS, progression-free survival; PS, performance status.
Overall, patients were more likely to receive full-dose FOLFIRINOX (fFOLFIRINOX; 28.1%) at first-line than modified FOLFIRINOX (mFOLFIRINOX; 7.4%) (online supplementary figure 3). Similar trends were observed across countries. In general, fewer patients in this study were female (36.3% of patients receiving FOLFIRINOX and 43.2% of patients receiving gemcitabine+nab-paclitaxel; table 2).
FOLFIRINOX and gemcitabine+nab-paclitaxel tended to be dose-modified later during the treatment course (online supplementary figure 4). Dose adjustments were more common in patients treated with fFOLFIRINOX (26.1%) and gemcitabine+nab-paclitaxel (20.5%) than with mFOLFIRINOX (6.7%). Patients treated with mFOLFIRINOX (modified at any stage) had a median overall survival (mOS) of 15 months (95% CI 14 to 17). Patients receiving gemcitabine+nab-paclitaxel and gemcitabine monotherapy had an mOS of 12 months (95% CI 12 to 13) and 9 months (95% CI 9 to 10), respectively (online supplementary figure 5). mOS was 16 and 15 months, respectively, in patients receiving mFOLFIRINOX and fFOLFIRINOX at treatment start (online supplementary figure 6).
Second-line metastatic treatment patterns
Information regarding second-line treatment was available for 1666 of the 2565 patients evaluated in this study (table 3). In total, 691 of patients (26.9%) completed first-line treatment and did not continue treatment. After completing second-line treatment, 574 patients (22.4%) received no further active therapy, and 133 (5.2%) completed second-line therapy and were waiting to start third-line therapy. Patients receiving first-line gemcitabine monotherapy were less likely to receive a second-line therapy (online supplementary figure 7). Overall, gemcitabine monotherapy was the most frequently used second-line therapy (27.1%), followed by gemcitabine+nab-paclitaxel (17.8%), 5-FU+oxaliplatin (17.6%) and 5-FU monotherapy (16.7%) (online supplementary figure 8).
Table 3.
Geographical variation in second-line treatment regimen choices
| Second-line treatment/% of patients | Total (N=1666) | France (n=358) | Germany (n=333) | Italy (n=279) | Spain (n=352) | UK (n=311) |
| 5-FU-based | 44.9 | 31.8 | 37.2 | 58.1 | 52.3 | 47.6 |
| FOLFIRINOX | 3.7 | 2.2 | 2.7 | 3.2 | 4.0 | 7.1 |
| Full-dose FOLFIRINOX | 2.5 | 2.0 | 1.8 | 2.9 | 3.1 | 2.9 |
| Modified FOLFIRINOX | 1.3 | 0.3 | 0.9 | 0.4 | 0.9 | 4.2 |
| Other 5-FU-based | 41.2 | 29.6 | 34.5 | 54.8 | 48.3 | 40.5 |
| 5-FU+oxaliplatin | 17.6 | 10.9 | 17.7 | 20.8 | 17.9 | 20.9 |
| 5-FU+irinotecan | 6.9 | 9.2 | 7.8 | 9.7 | 6.5 | 1.3 |
| 5-FU monotherapy | 16.7 | 9.5 | 9.0 | 24.4 | 23.9 | 18.3 |
| 5-FU infusional | 2.1 | 4.5 | 3.0 | 0.4 | 1.7 | 0.6 |
| 5-FU oral | 14.6 | 5.0 | 6.0 | 24.0 | 22.2 | 17.7 |
| Gemcitabine-based | 53.2 | 67.0 | 61.6 | 38.7 | 44.9 | 51.1 |
| Gemcitabine+nab-paclitaxel | 17.8 | 12.8 | 34.8 | 11.1 | 22.4 | 4.5 |
| Gemcitabine monotherapy | 27.1 | 50.0 | 16.2 | 22.6 | 12.5 | 34.1 |
| Other gemcitabine combinations | 8.3 | 4.2 | 10.5 | 5.0 | 9.9 | 12.5 |
| Gemcitabine+erlotinib | 2.7 | 0.6 | 8.4 | 0.0 | 4.3 | 0.0 |
| Gemcitabine+capecitabine | 3.2 | 0.6 | 0.9 | 2.5 | 2.0 | 10.6 |
| Gemcitabine+oxaliplatin | 1.7 | 3.1 | 0.3 | 1.4 | 3.7 | 0.0 |
| Gemcitabine+cisplatin | 0.7 | 0.0 | 0.9 | 1.1 | 0.0 | 1.9 |
| Other | 1.9 | 1.1 | 1.2 | 3.2 | 2.8 | 1.3 |
FOLFIRINOX, folinic acid, fluorouracil, irinotecan and oxaliplatin; 5-FU, 5-fluorouracil.
More patients who received first-line treatment with FOLFIRINOX (78.0% and 78.1% of patients received modified and full dose, respectively) went on to receive second-line treatment than those who received first-line gemcitabine+nab-paclitaxel (67.4%; table 2). Gemcitabine-based therapies were most commonly used at second-line (53.2%), followed by 5-FU-based regimens (44.9%). FOLFIRINOX was rarely used as second-line (3.7%) versus first-line (35.6%) treatment.
Second-line treatment regimens varied between countries, with 5-FU-based treatments used more commonly in Italy, Spain and the UK than in France and Germany (table 3). 5-FU-based therapies other than FOLFIRINOX were more frequently used in second-line (41.2%) treatment than in first-line treatment (7.9%; online supplementary table 2 and table 3), with 5-FU+oxaliplatin and 5-FU monotherapy used most often (online supplementary figure 9A).
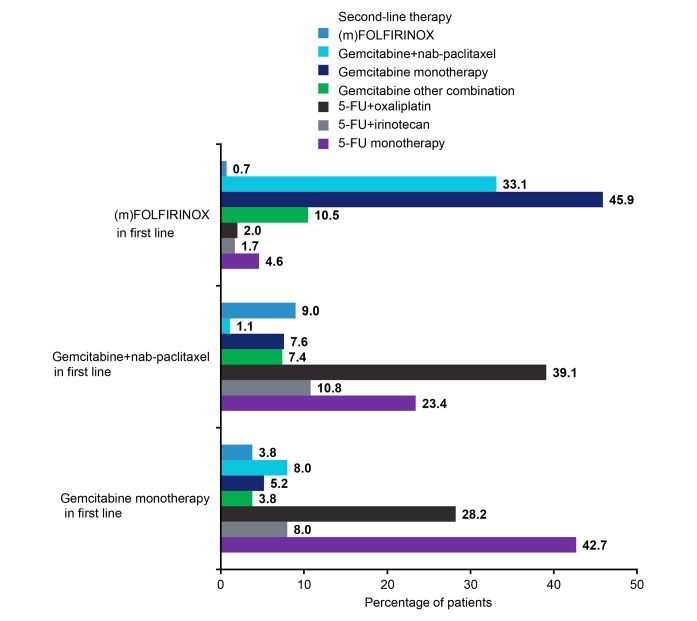
Gemcitabine-based second-line therapies were used more often in France, Germany and the UK (table 3). Gemcitabine monotherapy was more frequently prescribed in France, Italy and the UK, versus gemcitabine+nab-paclitaxel in Germany and Spain (online supplementary figure 9B). Patients who received mFOLFIRINOX as first-line often received second-line gemcitabine monotherapy (45.9%; figure 2). Gemcitabine+nab-paclitaxel was the next most common treatment (33.1%). Other 5-FU-based options were used less often.
Figure 2.
Second-line treatment choices according to first-line treatment5-FU, 5-fluorouracil; FOLFIRINOX, folinic acid, fluorouracil, irinotecan and oxaliplatin; m, modified.
Conversely, patients who received gemcitabine+nab-paclitaxel or gemcitabine monotherapy as first-line treatment tended to receive a 5-FU-based second-line treatment, with 5-FU+oxaliplatin and 5-FU monotherapy used most frequently. Patients who received gemcitabine monotherapy, gemcitabine+nab-paclitaxel, 5-FU+oxaliplatin and 5-FU/LV as second-line treatment had mOS of 6 (95% CI 5 to 6), 8 (95% CI 8 to 9), 6 (95% CI 5 to 7) and 5 months (95% CI 4 to 6), respectively.
Treatment availability
During this study, most mPAC treatments were available in the countries surveyed. Gemcitabine, 5-FU, irinotecan, oxaliplatin, capecitabine and cisplatin were available for patients with mPAC in all participating countries (online supplementary table 4). nab-paclitaxel was available in France, Germany, Italy and Spain; however, it was not reimbursed in the UK and was difficult to prescribe in France despite its registration due to limited reimbursement.22
Discussion
This observational study reviewed first-line and second-line treatment patterns for patients with mPAC across five European countries and investigated the geographical variation between them. FOLFIRINOX, gemcitabine+nab-paclitaxel and gemcitabine monotherapy were most frequently used. FOLFIRINOX was the most widely used in the first-line setting, possibly reflecting its lower basic cost versus gemcitabine+nab-paclitaxel,23 although this does not account for additional costs relating to toxicity management. Gemcitabine+nab-paclitaxel was the most frequently used first-line therapy in Spain and Italy. Treatment reimbursement in the respondents’ countries likely influenced geographical variation in treatment choices. As anticipated, more elderly and ECOG PS 2 patients received gemcitabine+nab-paclitaxel versus FOLFIRINOX.
In contrast to our study, a web-based physician questionnaire study of first-line treatment trends in 19 European countries in 2015 found that gemcitabine+nab-paclitaxel was used more frequently than FOLFIRINOX.21 It should be noted that this study reported more data from Germany, Italy and Spain than from France and the UK, both of which represent a total of 120 million inhabitants and were less likely to use gemcitabine+nab-paclitaxel in the current study. This was most likely due to reimbursement issues at the time of the study in both France and the UK.22 24 Moreover, in the above-mentioned study, only 213 responses were received of 5420 questionnaires sent (participation rate <4%). Finally, the reported cohort has a large proportion of younger patients (median age 64 years), allowing patients to receive intensified treatment in the first-line setting. These factors may account for the observed differences. Additionally, the current study focused on patients who received second-line treatment.
Although FOLFIRINOX was the most frequently used treatment in the first-line setting in our study, gemcitabine was also a common choice for first-line treatment. Recent results from the PRODIGE 24 study revealed that first-line treatment with FOLFIRINOX resulted in a 19.4-month improvement in overall survival compared with gemcitabine monotherapy, although grade 3/4 adverse events were more frequent in the FOLFIRINOX arm.25 These results could potentially influence first-line treatment choices across European countries in the future, although it should be noted that this study was conducted in an adjuvant setting rather than in patients with metastatic disease.25 Results from the POLO study could also influence future treatment choices for patients with mPAC, with the poly(ADP–ribose) polymerase inhibitor olaparib significantly improving progression-free survival compared with placebo as maintenance therapy.26 However, this treatment would be an option for a small proportion of patients with mPAC, due to its antitumour activity in those with BRCA mutations only.26
As anticipated, first-line choices influenced second-line treatment in the current study, with the most commonly used therapies in the second-line setting being gemcitabine monotherapy following FOLFIRINOX, and 5-FU-based therapies other than FOLFIRINOX following gemcitabine+nab-paclitaxel. Similar second-line treatment trends were observed in our study and the study by Le and colleagues.21 It is interesting to note that over a quarter of patients did not continue past first-line treatment; this may reflect the shortage of approved later-line regimens for patients with pancreatic adenocarcinoma.
Although treatment choices in the current study varied between countries, they largely followed ESMO recommendations. The ESMO guidelines recommend first-line treatment with gemcitabine monotherapy, FOLFIRINOX or gemcitabine+nab-paclitaxel, guided by performance status, for patients with mPAC.6 The majority (81.8%) of patients in this study received one of these treatments at first-line. ESMO guidelines also recommend consideration of nal-IRI+5-FU/LV as a second-line treatment for patients with mPAC, which was not approved during the present study17; ASCO and NCCN made similar recommendations.18 19 Following completion of this study, nal-IRI+5-FU/LV was approved in Europe for second-line treatment of patients with mPAC that has progressed following gemcitabine-based therapy and is the first second-line therapy approved for mPAC.27
Some treatment choices may be influenced by treatment availability and reimbursement costs. In 2014, nab-paclitaxel was available and reimbursed in Germany, Italy and Spain, but not in the UK and with limited reimbursement in France,22 reflecting the higher cost of this treatment.28 Since the time of the current study, gemcitabine+nab-paclitaxel has been approved for reimbursement for treatment of patients with mPAC in the UK,29 so this trend may change.
Study limitations include the descriptive, retrospective nature of the data and their collection via physician reports, the lack of weighting or adjustment of the data, and a bias towards recruiting patients who received second-line treatment. This last factor may have resulted in over-representation of certain first-line treatments and younger patients with a better PS qualifying for second-line treatment following disease progression.
Importantly, this study was not designed to compare treatments and there was no approved second-line treatment option available during the study period. Patients treated with gemcitabine+nab-paclitaxel received second-line therapy less frequently compared with those receiving first-line FOLFIRINOX. However, patients receiving first-line FOLFIRINOX were younger with better PS, which may have influenced treatment and subsequent outcomes.
A strength of this study was the large data set, including over 2500 patients from nine European countries and significant numbers of patient records from five of the countries. This allows presentation of data that represent treatment trends in these countries. Building on these data, a similar study with a target recruitment of 6000 patients has recently been completed. This will provide an update to the results presented here.
This study revealed that although treatment choices for patients with mPAC varied between countries, they aligned with current ESMO guidelines. The differences between European countries suggest that factors including patient characteristics, drug availability, physician preference and prior first-line therapy affect treatment choices. Variability in second-line treatment regimens reflects the lack of approved and recommended therapies at the time of this study for patients who progress following first-line treatment. Insights from this study into the use and efficacy of treatments in a real-world setting may influence the development of effective treatment plans, potentially improving patient outcomes in the future.
Acknowledgments
Medical writing assistance was provided by Carol McNair of Physicians World Europe (Mannheim, Germany). The study and data analyses were carried out by Genactis SAS (Mougins, France).
Footnotes
Twitter: @none
Contributors: JT, GP, DM, CBW, ND, AF, AC and TM contributed to study design and conception. ND and AF were responsible for the acquisition and quality control of the data, and also the statistical analysis. JT, GP, DM, CBW, ND, AF, AC and TM were all involved in the data analysis and interpretation. All authors contributed to the preparation, editing and review of this manuscript.
Funding: The study was funded by Shire (Zug, Switzerland). Medical writing assistance was initially funded by Shire and subsequently by Servier Global Medical Affairs (Suresnes, France). Although employees of the sponsor were involved in the design, collection, analysis, interpretation, fact checking of information, and coordination and collation of comments, the content of this manuscript, the interpretation of the data and the decision to submit the manuscript for publication in ESMO Open were made by the authors independently.
Competing interests: JT has acted as a consultant or speaker for Amgen, Roche, Merck Serono, Celgene, Shire, MSD, Lilly, Pierre Fabre, Sanofi, Sirtex and Servier. GP has acted as a consultant or speaker for Shire, Celgene, Merck Serono, Roche, Amgen, Sanofi, Lilly, Bayer, Servier, Bristol-Myers Squibb, MSD, Taiho and Halozyme. DM has received research funding from Shire, Incyte, Evotec and Celgene; and acted as a consultant for Lilly, Shire, Evotec, Servier, Baxter and Incyte. AC has acted as a consultant or advisor for Roche, Merck, MSD, Servier, Shire, Celgene and Bayer; and has received travel expenses from Merck, Celgene and Bristol-Myers Squibb. CBW has received research funding from Roche; and has acted as a consultant or advisor for Roche, Shire/Baxalta, Bayer, Ipsen, Rafael Pharmaceuticals, Celgene and Redhill. ND and AF are employees of Genactis, a company that received funding from Shire to support data collection for this study. TM has acted as an advisor or speaker for Baxalta, Celgene, Genzyme, H3 Biomedicine, QED, Roche, Sanofi and Shire; and has received support for travel and accommodation from Bayer, H3 Biomedicine, Merck and Sanofi. She has received research funding from Agios, Aslan, AstraZeneca, Bayer, Celgene, Genentech, Halozyme, Immunomedics, Lilly, Merrimack, Millennium, Novartis, Novocure, Pfizer, Pharmacyclics and Roche.
Patient consent for publication: Not required.
Ethics approval: This study was a retrospective, anonymised patient survey, and adhered to the ethical and legal codes of conduct of the European Society for Opinion and Marketing Research, European Pharmaceutical Market Research Association and British Healthcare Business Intelligence Association. Ethics approval was not necessary.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Where patient data can be anonymised, Servier will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to Servier via https://clinicaltrials.servier.com/data-request-portal/ and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data will be available.
References
- 1. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 2. Quante AS, Ming C, Rottmann M, et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med 2016;5:2649–56. 10.1002/cam4.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol 2018;29:1016–22. 10.1093/annonc/mdy033 [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356–87. 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 5. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 6. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56–68. 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 7. Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318–48. 10.3322/caac.21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163–72. 10.1038/nrclinonc.2009.236 [DOI] [PubMed] [Google Scholar]
- 9. Melisi D, Calvetti L, Frizziero M, et al. Pancreatic cancer: systemic combination therapies for a heterogeneous disease. Curr Pharm Des 2014;20:6660–9. 10.2174/1381612820666140826154327 [DOI] [PubMed] [Google Scholar]
- 10. Puleo F, Marechal R, Demetter P, et al. New challenges in perioperative management of pancreatic cancer. WJG 2015;21:2281–93. 10.3748/wjg.v21.i8.2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamburrino A, Piro G, Carbone C, et al. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front Pharmacol 2013;4:56 10.3389/fphar.2013.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hidalgo M, Pazo-Cid R, Guillen-Ponce C, et al. A phase I and randomized phase II trial to evaluate the efficacy and safety of nab-paclitaxel (nab-P) in combination with gemcitabine (G) for the treatment of patients with ECOG 2 advanced pancreatic cancer (PDAC). Ann Oncol 2017;28 10.1093/annonc/mdx369.007 [DOI] [Google Scholar]
- 13. Aprile G, Negri FV, Giuliani F, et al. Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol 2017;115:1–12. 10.1016/j.critrevonc.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 14. Wang-Gillam A, Li C-P, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545–57. 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 15. Oettle H, Riess H, Stieler JM, et al. Second-Line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;32:2423–9. 10.1200/JCO.2013.53.6995 [DOI] [PubMed] [Google Scholar]
- 16. Gill S, Ko Y-J, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016;34:3914–20. 10.1200/JCO.2016.68.5776 [DOI] [PubMed] [Google Scholar]
- 17. ESMO Guidelines Committee Appendix 6: cancer of the pancreas: MCBS eUpdate published online 20 June 2017 (www.esmo.org/Guidelines/Gastrointestinal-Cancers). Ann Oncol 2017;28 10.1093/annonc/mdx244 [DOI] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network Us NCCN clinical practice guidelines in oncology (NCCN Guidelines®) pancreatic adenocarcinoma version 1.2019, 2019. Available: www.nccn.org [Accessed 25th June 2019].
- 19. Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:2545–56. 10.1200/JCO.2018.78.9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manji GA, Olive KP, Saenger YM, et al. Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res 2017;23:1670–8. 10.1158/1078-0432.CCR-16-2319 [DOI] [PubMed] [Google Scholar]
- 21. Le N, Vinci A, Schober M, et al. Real-World clinical practice of intensified chemotherapies for metastatic pancreatic cancer: results from a pan-European questionnaire study. Digestion 2016;94:222–9. 10.1159/000453257 [DOI] [PubMed] [Google Scholar]
- 22. Cherny N, Sullivan R, Torode J, et al. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol 2016;27:1423–43. 10.1093/annonc/mdw213 [DOI] [PubMed] [Google Scholar]
- 23. Goldstein DA, Krishna K, Flowers CR, et al. Cost description of chemotherapy regimens for the treatment of metastatic pancreas cancer. Med Oncol 2016;33 10.1007/s12032-016-0762-8 [DOI] [PubMed] [Google Scholar]
- 24. Sarabi M, Mais L, Oussaid N, et al. Use of gemcitabine as a second-line treatment following chemotherapy with Folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett 2017;13:4917–24. 10.3892/ol.2017.6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conroy T, Hammel P, Hebbar M, et al. Folfirinox or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–406. 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 26. Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA -Mutated Metastatic Pancreatic Cancer. N Engl J Med Overseas Ed 2019;381:317–27. 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. European Medicines Agency Summary of product characteristics: Onivyde. EMEA/H/C/004125, 2017. Available: https://www.ema.europa.eu/documents/product-information/onivyde-epar-product-information_en.pdf [Accessed 25 Jun 2019].
- 28. Gharaibeh M, McBride A, Bootman JL, et al. Economic evaluation for the UK of nab-paclitaxel plus gemcitabine in the treatment of metastatic pancreas cancer. Br J Cancer 2015;112:1301–5. 10.1038/bjc.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer, 2017. Available: https://www.nice.org.uk/guidance/ta476/resources/paclitaxel-as-albuminbound-nanoparticles-with-gemcitabine-for-untreated-metastatic-pancreatic-cancer-pdf-82604969382085 [Accessed 25 Jun 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000587supp001.pdf (759.1KB, pdf)
esmoopen-2019-000587supp002.pdf (187.7KB, pdf)
esmoopen-2019-000587supp003.pdf (1.2MB, pdf)