Abstract
Intraspecific trait variation is an important determinant of fundamental ecological interactions. Many of these interactions are mediated by behaviour. Therefore, interindividual differences in behaviour should contribute to individual niche specialization. Comparable with variation in morphological traits, behavioural differentiation between individuals should limit similarity among competitors and thus act as a mechanism maintaining within-species variation in ecological niches and facilitating species coexistence. Here, we aimed to test whether interindividual differences in boldness covary with spatial interactions within and between two ecologically similar, co-occurring rodent species (Myodes glareolus, Apodemus agrarius). In five subpopulations in northeast Germany, we quantified individual differences in boldness via repeated standardized tests and spatial interaction patterns via capture–mark–recapture (n = 126) and automated VHF telemetry (n = 36). We found that boldness varied with space use in both species. Individuals of the same population occupied different spatial niches, which resulted in non-random patterns of within- and between-species spatial interactions. Behavioural types mainly differed in the relative importance of intra- versus interspecific competition. Within-species variation along this competition gradient could contribute to maintaining individual niche specialization. Moreover, behavioural differentiation between individuals limits similarity among competitors, which might facilitate the coexistence of functionally equivalent species and, thus, affect community dynamics and local biodiversity.
Keywords: animal personality, competition, individual niche specialization, movement ecology, coexistence, small mammals
1. Introduction
Individuals of the same species do not occupy identical ecological niches [1]. Individual niche specialization extends the classical concept of ecological niche partitioning between species to the within-species level [2]. Variation in how individuals of a population interact with abiotic and biotic components of the environment will affect fundamental ecological interactions, such as within- and between-species competition, and predator–prey relationships [2–7]. Many aspects of these interactions are directly mediated by behaviour (e.g. foraging, anti-predator behaviour and decision-making). Therefore, interindividual differences in behaviour (i.e. animal personality) should be a key determinant of individual niche specialization and ecological interactions between individuals.
Partitioning of space and time is an important means of ecological niche differentiation [8,9]. Home range size and utilization patterns, as well as the distribution of individuals over microhabitats, can vary with personality within populations (e.g. [10–14]). Consequently, interindividual variation in behaviour should contribute to ecological niche specialization [15,16]. If individuals are not distributed randomly within a population's habitat, their ecological environment, including other interacting individuals, is not random as well [12,17]. Consequently, interactions within and between species may be biased towards certain behavioural types [18,19].
Although we know much about how individual behavioural differences affect within-species interaction (e.g. summarized in [17]), fewer studies explored whether and how they modify interactions between species. For example, in habitat-forming, semi-social spiders changes in the behavioural-type composition of the founding group, from mainly aggressive individuals to mainly docile ones, altered the interactions with heterospecific web associates from amensalism to commensalism or mutualism [20]. Similarly, the strength of mutualism between anemonefish (Amphiprion percula) and sea anemones (Entacmaea quadricolor) scaled positively with the shyness of fish individuals [21]. Few studies consider individual differences in both interacting species. For example, more active predatory old field jumping spiders (Phidippus clarus) consumed inactive house crickets more often (Acheta domesticus) than active crickets and vice versa [22]. Personality types of both predator and prey were therefore determining the outcome of this interaction. Similarly, the mutualism between magpies (Pica pica) and rocky mountain elk (Cervus canadensis) is influenced by variation in boldness in both species; bold birds were more likely to land on and groom elks compared with shy conspecifics, while shy elks were more likely to let birds groom them [23].
One of the most important interspecific interactions is competition over resources, like food, shelter and predator-free area, because the outcome of competition ultimately determines species coexistence, community composition and, therefore, biodiversity. Within-species variation in behavioural types might affect the competitive ability of a species and, thus, may play a vital part in the outcome of competition between ecologically similar species. Although highlighted in many opinion papers (e.g. [17,24]), these effects have rarely been demonstrated. For example, indirect competitive interactions of two stickleback species (Gasterosteus aculeatus and Pungitius pungitius) in a foraging context, bolder individuals consumed more prey, irrespective of species, resulting in a stronger competitive ability compared with shyer individuals [25].
Most studies on the effects of consistent individual differences in behaviour on interspecific interactions were performed as short-term experiments under laboratory conditions, creating novel surroundings for the observed individuals and relying mostly on staged dyadic encounters that allow only direct interactions (e.g. [22,25]). Alternatively, focusing on established spatial patterns in natural communities might provide crucial insights into indirect interactions of whole communities as they reflect the outcome of long-term within- and between-species interactions. Empirical evidence for individual niches in co-occurring species and their reciprocal effects on species interaction patterns are scarce. Costa-Pereira et al. [7] demonstrated that individual trophic niches in thin toed frogs (Leptodactylus spp.) are temporally and spatially flexible, and connected to community composition and dynamics. Consequently, individual niche variation might play a vital part in interspecific competitive interactions and crucially influence species coexistence at the landscape scale, as well as regional biodiversity patterns.
Simultaneously assessing spatial patterns of individuals of different species in natural communities might provide crucial insights into how individual niche specialization affects ecological interactions. Since movement and space use are indispensable for finding and defending resources (i.e. food, shelter, predator-free area) in mobile organisms, they determine temporal and spatial aspects of resource competition [9]. Both movement and space use are the outcomes of a series of behavioural decisions (e.g. where to move, how to move, when to move [26]) and these decisions are modified by interindividual differences in boldness, which reflects risk-taking [27], and in exploration, which reflects reaction to new situations [28], as demonstrated for sleepy lizards (Tiliqua rugosa) [13] and bank voles (Myodes glareolus) [29]. Whether and how within-species variation in these two behavioural traits also affects between-species spatial interactions remains unknown.
Inferring patterns and strengths of spatial interactions within and between species is difficult, however, because it requires sufficient information on space use of all potential interaction partners. Small, ground-dwelling rodents are a suitable study system that allows to overcome these challenges because ecologically similar species co-occur in high densities, their spatial interactions are on an easily trackable scale and they inhabit areas with vegetation cover that can be quantified as a proxy for predation risk [30,31].
Here, we studied spatial interaction patterns of two ecologically similar and naturally co-occurring rodent species, the bank vole (Myodes glareolus) and the striped field mouse (Apodemus agrarius), in established natural communities, and aimed to test whether individual differences in space use facilitate the occupation of individual spatial niches and, thus, affect intra- and interspecific interactions. Both species commonly co-occur in various habitats in parts of central and eastern Europe, are similar in habitat choice and diet and have partially overlapping temporal activity patterns [32–34]. Thus, both species are equivalent functional types within an ecological community and are assumed to occupy similar ecological niches, suggesting high levels of resource competition, which mainly happens indirectly via exploitation competition [35]. Previously, we showed personality-dependent space-use patterns in bank voles that facilitated the occupation of individual spatial niches [36] (electronic supplementary material, figures SA1 and SA2). Specifically, we identified boldness as the key behavioural trait affecting space use, movement and spatial interactions in bank voles, while exploration had only marginal effects [36]. Based on the ecological similarity of our two study species, we assume similar patterns for striped field mice.
The main focus of our study was whether behavioural-dependent individual spatial niche occupation in both species covary with spatial interactions with con- and heterospecifics. Therefore, we investigated small mammal community composition at several study sites, measured interindividual differences in behaviour within species and quantified spatial interactions within and between species. We hypothesized that individual differences in behaviour are functionally integrated with intra- and interspecific spatial interactions of co-occurring bank voles and striped field mice. We predicted that irrespective of species, boldness positively covaries with the overlap of home ranges and core areas of heterospecific individuals due to the positive relationship between boldness and home range size. For the overlap of home ranges and core areas of conspecific individuals, we predicted a negative covariance with boldness, due to the higher spatial exclusivity on the intraspecific scale [36] (electronic supplementary material, figure SA1). Based on the assumption of a link between boldness and competitive ability [25], we predicted that bolder individuals of both species spatially interact more with heterospecific individuals and less with conspecific individuals. Specifically, we expected a positive covariance between boldness and the number of neighbours (intra- and interspecific) within their home ranges and core areas, as well as a positive covariance between boldness and the distances between the home range centre of a focus individual and those of neighbouring individuals (con- or heterospecific). We did not expect exploration to covary with spatial interactions within and between species based on the previous study [36].
2. Material and methods
(a). Study sites
Data were collected on five study sites within the AgroScapeLabs in northwestern Brandenburg, Germany (53°21′56.2″ N, 13°48′17.3″ E). This region is characterized by intensive agriculture on large fields with small, island-like fallow lands and hedges. These interspersed fallow lands served as study sites (size: 0.85 to 1.66 ha) and were characterized by heterogeneous vegetation of grasses, streaked with nettles (Poaceae 40%, Urtica spp. 21%, Ballota spp. 9%), bushes and trees. These confined habitat islands are an ideal setting to investigate patterns and mechanisms of competition at a local scale. Study sites were visited consecutively and on each study site, experimental procedures—capture–mark–recapture (CMR), individual difference testing, VHF telemetry (see details below)—were done within a continuous time period of 13 ± 3 days.
(b). Capture–mark–recapture
Between August and November 2016, we conducted CMR with Ugglan live traps (Grahnab Sweden, special no. 2). At each study site, traps were set up in a grid consisting of 55 traps with ca. 10 m distance between them, grid shape depended on the shape of the habitat remnant. Traps were baited with rolled oats and apples and, prior to the commencement of trapping, pre-baited for 24 h. Upon initial capture, individuals were temporarily marked with a unique fur cut. Individuals used for repeated behavioural testing were permanently marked with a passive integrated transponder (Euro ID, Trovan ID100) after their first test. Trapping continued on each site until greater than 95% of the captured animals were marked. We captured between 75 and 103 rodents on each of the five study sites, representing a density of 84 to 234 rodents ha−1, with bank vole and striped field mouse being the two most abundant species (51–81% of the captured individuals; 108–179 individuals ha−1; details on the number of animals per study site in electronic supplementary material, table SA1). Other species included common vole (Microtus arvalis), field vole (M. agrestis), yellow-necked mouse (Apodemus flavicollis) and wood mouse (A. sylvaticus; species are presented with declining abundances).
(c). Individual difference test
To quantify interindividual differences in behaviour in both species, adult individuals (greater than 17 g) were subjected to an individual difference test directly after capture. Tests were performed at the capture site at comparable locations on each study site. The test set-up unites a dark–light test with an open-field test within one mobile set-up. Test details are described in Schirmer et al. [36]. Briefly, in the dark–light test, individuals moved from the trap into an opaque plastic pipe (10.5 × 32 cm) with a swing door at each end, attached to a round arena (diameter 1.30 m, 30 cm height). Within 300 s, we quantified the latency to stick the head out of the pipe (latency head) and the latency to leave the pipe with the whole body, excluding the tail (latency body). If an individual did not leave the pipe during the test, the latency was set to 300 s. Once the individual entered the round arena, the open-field part of the test started. In the open-field test, behaviour is assessed based on the assumption that the middle of the arena (open, exposed; risky) and the border area (covered, not exposed; safe) represent different levels of perceived predation risk. In this test part, we quantified the latency to enter the centre of the arena for the first time, the number of crossings of the arena centre, the number of sections of the arena entered and the activity. All variables were quantified via direct observations in the field by one observer (A.S.). We tested only on rainless days with low wind speed. Tests were repeated for recaptured individuals (n = 57) 1–7 days later.
(d). Automated radio telemetry
To assess movement and space use on a finer spatio-temporal scale, we equipped individuals at three study sites with VHF radio telemetry transmitters (1.1 g, BD-2C, Holohil Systems, Canada) on a collar, and tracked them for four days. At each site and for each radio-tracked individual, tracking commenced 4.6 ± 3.5 days after the last individual difference test was performed. Individuals were selected based on their recapture probability (greater than two captures before collaring) and body mass (greater than 20 g, i.e. transmitter weight less than or equal to 5% of body mass). We excluded females in the last stages of gestation, based on visual inspection. In total, we radio-tracked 21 bank voles (9 females, 12 males) and 15 striped field mice (6 females, 9 males). All individuals on a respective site were tracked simultaneously during four consecutive days.
The automated VHF tracking system consisted of eight omnidirectional antennas (GP 150 Winkler-Spezialantennen, Annaberg, Germany) placed around the trapping grid at ground level, connected to two automated receiving units (ARU; JDMC Corp, Illinois, US; four antennas/ARU). ARUs logged the signal strengths the antennas received from transmitters carried by animals. We calculated two-dimensional location points of radio-collared individuals based on two perpendicular isolines of distributions of signal strengths, creating an x- and y-dimension following the border lines of the site, respectively. Isolines were calibrated with transmitters at known locations within each grid prior to data collection (for more details, see [36]). We sampled on average 96 location points per day for each individual with an average location accuracy of 9.4 ± 7.3 m varying with vegetation density and air moisture. We are aware that location accuracy might affect our space-use estimates and therefore combined several methods (see below) and interpret all findings conservatively. Given the small body size and ground-dwelling habits of our study species, there are—to the best of our knowledge—no other suitable and more accurate methods available.
(e). Spatial analyses
Based on CMR data, we calculated for each individual tested for behavioural differences a proxy for its centre of spatial activity as the arithmetic mean trapping point (mean ± s.d.: 4.3 ± 3.9 captures per individual; n = 227; for sensitivity analysis, see electronic supplementary material, figure SA3). As a measure of the strength of the interaction between spatially interacting individuals, we calculated the distance between each individual and its nearest neighbour (con- and heterospecific). Spatial analyses were conducted in the program QGIS (version 2.18.14). Since we are interested in general longer-term interaction patterns, we restricted subsequent statistical analyses of these spatial interactions to adult individuals of known behavioural type because we assume that only these residential individuals (n = 126) have temporary stable home ranges within our study sites. Hence, we excluded juveniles and transient individuals (captured only once).
Given the resolution of our tracking data, we concentrated on static spatial interactions patterns by calculating intra- and interspecific home range overlaps for 95% and 50% kernel density estimations, representing the home ranges and core areas, with the package adehabitat (version 1.8.18 [37]) in the program R (version 3.3.0 [38]). For each individual, these spatial overlap metrics were obtained by calculating its home range overlap with every other simultaneously tracked individual at the study site. Repeated sampling of individuals was corrected for in statistical models.
Additionally, using QGIS, we combined detailed data of spatio-temporal space use of radio-tracked individuals (n = 36) with intensive CMR data of the vast majority of individuals present at each study site and counted the number of conspecific and heterospecific neighbours that had their mean trapping point (based on CMR data) in the home range and the core area of each radio-tracked individual.
(f). Statistical analyses
(i). Individual differences
We estimated repeatability for each behavioural variable observed during the individual difference test (§2c) using the R package rptR (version 0.6.405; electronic supplementary material, table SA2 [39]). Repeatable behavioural variables were entered into a principal component analysis (PCA) with oblimin rotation to combine correlated variables into meaningful components. We used species as a categorical grouping factor in the PCA. PCA rendered two meaningful components, which were interpreted as a measure for exploration and for boldness (electronic supplementary material, table SA3). Individual scores on the components were used as repeated measures for exploration and boldness in the subsequent analyses.
In earlier analyses, only boldness was identified as a strong predictor of space use in bank voles [36]. Therefore, we focused on this behavioural trait and present all results for exploration in the supplements (electronic supplementary material, table SA4 and figure SA4).
(ii). Individual differences and interspecific spatial interactions
We used bivariate Bayesian mixed-effects models, run with the package MCMCglmm [40], to assess covariance between boldness and spatial interaction parameters. We followed the procedures described in [40–42]. Models were calculated with a bivariate structure with boldness and the respective spatial interaction variable (kernel overlap, number of neighbours, distance to neighbours) as dependent variables. The random structure of the models always incorporated the ID of each individual, to account for repeated measures of the same individual, as well as the factor study site, to account for variation among sites. We set slightly informative priors by dividing the total phenotypic variance of the dependent variable by the number of random effects in the model and set a low degree of belief (nu = 1), since we do not have much information regarding the posterior distribution of the data [40]. Spatial parameters were only calculated once for each individual, with the exception of kernel overlaps; therefore, the within-individual trait variation is zero. As variances have to be positive, we fixed the within-individual trait variance to 0.0001 for spatial variables in the prior specification according to Houslay & Wilson [42]. Since boldness and spatial variables were not assed at the same time, we fixed the within-individual covariance to zero [41]. Error structures of the data were modelled via the underlying distribution families of the response variables. We used 101 000 iterations, a thinning interval of 100 and a burnin of 1000, which resulted in low temporal autocorrelation between estimates of subsequent models. Based on the posterior distributions, we extracted covariances between pairs of response variables and their credibility intervals. Covariances were interpreted significant if the credibility intervals did not include zero [42].
To test whether interindividual differences in boldness vary with intra- and interspecific home range and core area overlaps between individuals, we used bivariate Bayesian mixed-effects models with boldness and the intra- or interspecific overlap (home range or core area) as response variables. These overlap metrices were calculated for each radio-tracked dyad of individuals per study site (intraspecific dyads: n = 202, interspecific dyads: n = 216). As fixed factors, we entered species and sex in these models. Additionally, we included the difference in boldness between individuals (based on mean values) of a dyad as a fixed factor to assess whether spatial interactions are biased towards individuals of similar or different behavioural types and a factor defining whether individuals were of similar or different sex. The random structure of these models included both the ID of the focus individual and the ID of the dyadic partner individual to account for multiple measurements of each individual as a partner, as well as the study site.
To test whether interindividual differences in boldness covary with the number of neighbours in home ranges and core areas of individuals, we calculated bivariate Bayesian mixed-effects models with boldness and the number of mean trapping points of con- or heterospecifics in a radio-tracked individual's (n = 36) home range or core area as response variables. As fixed effects, we included species and sex. As random effects, we included study site and individual ID.
To test whether interindividual differences in boldness covary with spatial distances between con- or heterospecific individuals' centres of activity (i.e. mean trapping points), we applied bivariate Bayesian mixed-effects model with boldness and the distance to the next con- or heterospecific as the response variables. Since this analysis was based on the trapping data, the larger sample size (n = 126 individuals) allowed us to include species, sex as well as the difference in boldness scores between individuals and whether individuals were of the same sex or of different sexes (electronic supplementary material, table SA5) as fixed factors to assess a potential bias of spatial interactions. The random structure of these models included the study site and the individual ID.
For completeness, we ran all models also with exploration instead of boldness as a response variable in bivariate models. Since we already demonstrated that exploration does not explain variation in space use in our study species [36] and none of these models revealed a significant covariance between exploration and spatial overlap patterns, we present these results only in electronic supplementary material, table SA4 and figure SA4.
3. Results
(a). Interindividual differences in behaviour
Almost all variables observed in the individual difference test were repeatable (electronic supplementary material, table SA2). Species had no effect in the PCA. Two meaningful components were extracted, which cumulatively explained 83% of the variance in the data. The first component, representing exploration, explained 56% of the variance, and was comprised of the variables from the open-field test part (eigenvalue 3.15, all loadings greater than 0.7) and was repeatable over time (R = 0.17, 95% CIs = [0.04, 0.37], p = 0.04). The second component, representing two latencies measured in the dark–light test part (eigenvalue 1.51, all loadings greater than 0.7, electronic supplementary material, table SA3), explained 27% of the variance and was interpreted as boldness. This component was repeatable over time (R = 0.39, 95% CIs = [0.15, 0.59], p = 0.001). Both components did not correlate at the phenotypic level (ρ = −0.09, p = 0.346).
(b). General spatial interaction patterns
Based on radio-tracking of selected individuals of known behavioural type, we previously demonstrated that boldness scales positively with home range (mean ± s.d.: 2125 ± 1812 m2) and core area (mean ± s.d.: 530 ± 472 m2) sizes, as well as microhabitat characteristics of home ranges in bank voles [36]. This pattern also pertains in striped field mice (A. agrarius, n = 15; home range size mean ± s.d.: 2737 ± 2046 m2, core area size mean ± s.d.: 600 ± 446 m2; electronic supplementary material, figures SA1 and SA2).
(c). Intraspecific spatial interaction patterns
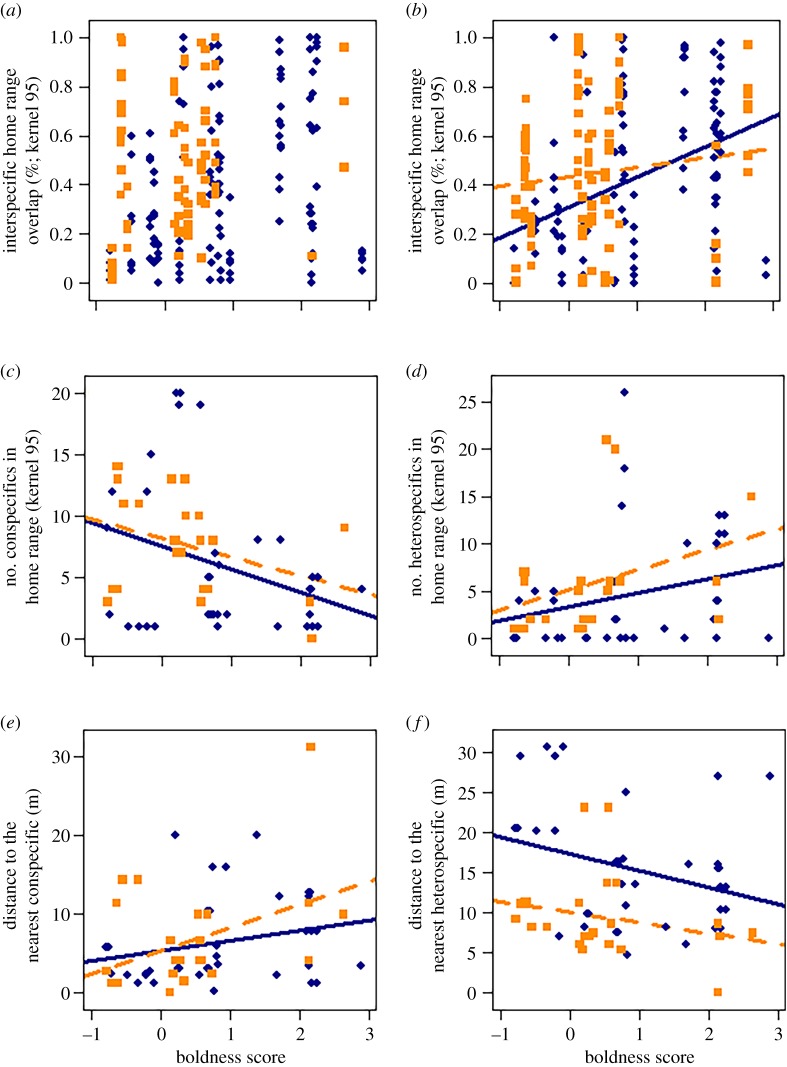
Intraspecific overlap of home ranges and core areas (n = 202 dyads based on tracking data) did not covary with individual boldness (table 1; figure 1a, electronic supplementary material, figure SA5a). Contrarily, the number of conspecific neighbours negatively covaried with the boldness of the focal individual on the home range scale. Bolder individuals had fewer conspecific neighbours in their home range compared with shy individuals (figure 1c; table 1; based on tracking data, n = 36). Similarly, we obtained a positive covariance between boldness and the distance to the nearest conspecific (trapping data, n = 126). Bolder individuals had larger distances to the nearest conspecific compared with shy individuals (figure 1e; table 1). The fixed factors species and sex, as well as dyad sex and difference in boldness scores in the models concerning spatial overlap patterns, had no effect (electronic supplementary material, table SA5).
Table 1.
Covariances between boldness and spatial interaction variables. Represented are covariances and their credibility intervals based on posterior distributions of bivariate Bayesian mixed-effects models. Additionally, we present the correlation coefficients (based on the ratio between covariances and standard deviations of variables) for easier interpretation of the strengths of associations between variables. Covariances with credibility intervals excluding zero are indicated in italics.
| variable | correlation coefficient | covariance | CI low | CI high |
|---|---|---|---|---|
| intraspecific overlap of core areas | 0.05 | 0.43 | −0.206 | 0.636 |
| intraspecific overlap of home ranges | 0.16 | 0.36 | −0.002 | 0.672 |
| no. of conspecific neighbours in core area | 0.16 | 0.37 | −0.227 | 0.686 |
| no. of conspecific neighbours in home range | −0.37 | −0.16 | −0.466 | −0.054 |
| distance to the nearest conspecific | 0.32 | 0.49 | 0.017 | 0.663 |
| interspecific overlap of core areas | 0.10 | 0.18 | −0.373 | 0.463 |
| interspecific overlap of home ranges | 0.23 | 0.48 | 0.070 | 0.693 |
| no. of heterospecific neighbours in core area | −0.04 | 0.09 | −0.430 | 0.459 |
| no. of heterospecific neighbours in home range | 0.25 | 0.31 | 0.041 | 0.713 |
| distance to the nearest heterospecific | −0.17 | −0.22 | −0.250 | −0.006 |
Figure 1.
Covariance between boldness and intra- and interspecific overlap of home ranges (a,b), the number of con- and heterospecific neighbours in an individual's home range (c,d) and the distance to the nearest con- and heterospecific neighbour (e,f) of bank voles (M. glareolus, solid line and points) and striped field mice (A. agrarius, dashed line and squares). Lines represent covariances with a credibility interval different from zero. Individuals are always represented multiple times within the plots due to the repeated measurements of boldness scores. (Online version in colour.)
(d). Interspecific spatial interaction patterns
The bolder an individual, the higher its home range overlap with individuals of the other species (based on tracked individuals, n = 216 dyads), the larger the number of heterospecific neighbours (figure 1b,d, table 1) in its home range, and the lower the distance of its mean trapping point to that of its nearest heterospecific neighbour (figure 1f, table 1). None of the fixed effects predicted variation in interspecific interaction variables (electronic supplementary material, table SA5). At the core area scale, neither the interspecific overlap nor the number of heterospecific neighbours covaried with boldness (table 1; electronic supplementary material, figure SA5b and SA5d).
4. Discussion
Combining behavioural phenotyping, automated radio-tracking and intensive CMR, we found that consistent individual differences in boldness covary with space use of two ecologically similar rodent species. As a result, spatial interaction patterns between individuals were not random. Behavioural types mainly differed in the relative importance of intra- versus interspecific competition. As we discuss in detail below, within-species variation along this competition gradient could contribute to maintaining individual niche specialization and facilitate species coexistence.
(a). Personality-dependent spatial interactions within species
In both species, individuals did not interact randomly in space with conspecifics. Although not apparent for overlap between home ranges of selected radio-tracked individuals, bolder individuals appeared to maintained high spatial exclusivity at the within-species level, indicated by lower numbers of conspecific neighbours in their home range and larger distances to nearest neighbours. In both study species, individuals compete over resources predominantly indirectly via exploitation competition and direct competitive interactions are rare [35]. Under this competitive regime, the strength of spatial interactions might serve as a proxy for the intensity of resource competition. Consequently, bolder individuals are probably facing reduced intraspecific competition compared with shy individuals. Boldness also scales positively with home range and core area size and covaries with microhabitat characteristics associated with predation risk on the home range scale [36] (electronic supplementary material, figures SA1 and SA2). These patterns suggest that in both study species, consistent individual differences in boldness facilitated the occupation of individual spatial niches, which resulted in reduced interactions with conspecifics. Behavioural-type-dependent niche differentiation should reduce intraspecific competition by decreasing the similarity of conspecifics, facilitating the maintenance of different behavioural types in natural populations [17,43].
Based on the positive relationship between boldness and competitive ability [25], it would be plausible that bolder individuals occupy microhabitats of better quality and/or have higher access to resources (e.g. food, shelter, predator-free area). With the data at hand, we found a positive covariance between boldness and percentage of ground cover in home ranges but a negative covariance with maximum vegetation height in home ranges (electronic supplementary material, figure SA2). These differences in microhabitat occupation of bold and shy individuals could be connected to different predation risks since the accessibility of predators might differ between microhabitats. Microhabitats with higher levels of maximum vegetation height might be more accessible for ground predators, while those with the high ground cover but less maximum vegetation height might be more accessible for avian predators. Whether behavioural types also occupy ranges differing in food resource quality/quantity remains open due to the tremendous difficulty of quantifying the distribution of all components of an omnivorous diet at the home range scale.
(b). Personality-dependent spatial interactions between species
Spatial interactions between species were not random but varied with behavioural type. Irrespective of species, bolder individuals shared their home ranges with more heterospecific neighbours compared with shy individuals. Similar to the within-species pattern, indirect competition via exploitation is the main form of resource competition between the two study species [35]. Hence, spatial interactions among heterospecifics could indicate the strength of competition between them. In contrast to the within-species pattern, we found that boldness varied positively with interspecific overlap of home ranges and number of heterospecific neighbours in an individual's home range. Additionally, the bolder an individual, the shorter its distance to the centre of activity of its nearest heterospecific neighbour. Thus, reduced intraspecific competition in bolder individuals appears to come at the cost of increased interspecific competition. This behavioural-type-dependent pattern of intra- and interspecific competition could equalize potential competitive advantages between both types within species and facilitate their maintenance in the natural population. We could not detect any connection between behavioural-type and spatial overlap or the number of neighbours on the core area scale. These areas represent nest or refuge sites in ground-dwelling rodents [44,45] and might thus be kept exclusive independent of behavioural type.
(c). Can spatial niche specialization facilitate species coexistence?
Overall, our results support the hypothesis of within-species spatial niche specialization [36]. Moreover, depending on their behavioural type, individuals occupy spatial niches varying in the relative importance of intra- versus interspecific resource competition. Given their high intraspecific competitive ability, bolder individuals, which are also dominant in staged encounter tests (Microtus arvalis; J.A.E. 2010, unpublished data), can maintain large areas with more exclusive resource access. Particularly bold individuals of the other species might move into these areas of reduced resource competition, ultimately creating non-random distribution patterns of behavioural types and biased patterns of intra- versus interspecific resource competition. Furthermore, behavioural types differed in their distribution over microhabitats varying in vegetation cover (electronic supplementary material, figure SA2) [36]; thus, similar types experienced comparable microhabitat conditions. Studying whether different behavioural types choose different microhabitats and interaction environments (i.e. niche choice), or the environment drives the emergence of different behavioural types and associated interaction patterns (i.e. niche conformity) might ultimately explain why each individual only realizes a small fraction of a species's ecological niche and should be an interesting avenue of future research.
Currently, only a few quantitative analyses exist that predict whether and how intraspecific trait variation affects species coexistence and their results are equivocal [46,47]. Including intraspecific variation in a simple competition model between non-moving, semelparous organisms did not facilitate coexistence [46]. By contrast, a recent game-theoretical approach explicitly allowing heritability of phenotypic traits demonstrated that intraspecific trait variation promotes species coexistence [47]. Similarly, results of experimental manipulations of intraspecific variation in life-history and resource preference traits in two weevil species increased or decreased species coexistence depending on interacting effects of within-population variation [48]. Comparable with individual dietary niche specialization [2,49], our results suggest that heterospecific interactions are biased towards individuals of one, similar behavioural type (figure 1b,d,f; table 1). Consequently, resource competition might be increased for more similar behavioural types but decreased for less similar ones due to differential spatial interactions. In this context, consistent individual differences in behaviour could be a largely overlooked aspect of limiting similarity [8] because it contributes to lowering the strength of competition between phenotypically different individuals (i.e. the niche complementarity [3]). Our results therefore support that species coexistence is promoted by intraspecific trait variation rather than prevented. Additionally, boldness has been proposed to be positively connected to the competitive ability of individuals [25], therefore the observed restriction of heterospecific interactions to mainly bold individuals of both species might mean a restriction of interactions to individuals of similar competitive ability. Consistent interindividual differences and behavioural variation could therefore, based on our results, also act as an equalizing mechanism of species coexistence, balancing competitive differences on the individual level.
In our observed natural communities, we found substantial behavioural variation in both species suggesting high spatial niche differentiation and complementarity between individuals at the local scale. An interesting avenue of future research will be to quantify whether high levels of intraspecific behavioural variation are also maintained in allopatry and to test whether reducing intraspecific behavioural variation diminishes competitive release for behavioural types of the other species. Ultimately, only such experimental manipulations can clarify whether interindividual behavioural variation could act as a mechanism of character displacement and/or a mode of separation facilitating the coexistence of ecologically similar species in sympatry [50].
5. Conclusion
In two ecological generalist and widely distributed rodent species, interindividual differences in behaviour within species also pertain to interactions between species. Boldness varied with space-use patterns of individuals which consequently affected spatial interactions between individuals, and ultimately led to the occupation of individual spatial niches. Based on those niche differences, the competitive environment varies between individuals. Individual spatial niche specialization might facilitate the coexistence of species by restricting the interspecific interactions to a set of individuals from a population whose competitive strengths are balanced and by reducing the limiting similarity between individuals to a degree that allows stable coexistence. Hence, interindividual differences in behaviour might mediate fine-scale niche partitioning between equivalent functional types within a trophic guild, possibly increasing local biodiversity.
Further research is needed on the fitness consequences deriving from individual niche occupation and the resulting interactions and spatial patterns. Moreover, experimental approaches need to clarify the importance of within-species behavioural variation for spatial interaction patterns, for mediating community compositions, and as a mechanism of character displacement. Additionally, natural communities are much more complex than our simplified view based on the two most immediate competitors. To understand the full extent of individual differences and individual niches for natural community structure and processes, future research needs to incorporate more than just a few focus species and concentrate on all interactions within a community. Since this is logistically challenging for empirical studies, further advances might also rely on individual-based modelling [51]. As suggested here, interindividual variation in behaviour and resulting individual spatial niche specialization might affect species interactions beyond direct encounters and might constitute a significant component of intraspecific trait variation, a key driver of community dynamics and local biodiversity [52]. Since intraspecific variation in behavioural types, their competitive ability and microhabitat preferences contribute to the ecological and selective environment of individuals of other species, they are also key for understanding eco-evolutionary dynamics.
Supplementary Material
Acknowledgements
We thank Antje Herde, Lisa Teckentrup, Maureen Schuster and Lina Mey for indispensable help during fieldwork, and Angela Puschmann and Elke Seydewitz for technical support. We thank Florian Jeltsch for helpful comments on an earlier draft and for unflagging support. We thank the Animal Ecology group at Potsdam University and the BioMove team for fruitful discussions.
Ethics
Experiments were conducted under the permission of the Landesamt fuer Umwelt, Verbraucherschutz und Gesundheit, Brandenburg (LUGV_7RO-4610/34+5#86908/2011; V3-2347-44-2011). All applicable institutional and national guidelines for the care and use of animals were followed.
Data accessibility
Data are available at the open research data portal of the Leibniz Center for Agricultural Landscape Research (ZALF) and can be accessed via the following links: https://www.doi.org/10.4228/ZALF.DK.129; https://www.doi.org/10.4228/ZALF.DK.131.
Authors' contributions
A.S., J.A.E. and M.D. developed and designed the study, J.A.E. developed the tracking methodology. A.S., J.H. and M.D. collected the data. A.S. analysed the data, all authors discussed the results. A.S. wrote the first draft of the manuscript. All co-authors contributed to the final draft of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding for A.S. was provided by the German Research Foundation (DFG) within the BioMove research training group (grant no. DFG-GRK 2118/1). J.H. was funded by the German Federal Environmental Foundation (DBU) (grant no. 20015/374). During manuscript preparation, M.D. was funded by the German Research Foundation (grant no. DA 1377/4-1).
References
- 1.Van Valen L. 1965. Morphological variation and width of ecological niche. Am. Nat. 99, 377–390. ( 10.1086/282379) [DOI] [Google Scholar]
- 2.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 3.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araújo MS, Bolnick DI, Layman CA. 2011. The ecological causes of individual specialisation. Ecol. Lett. 14, 948–958. ( 10.1111/j.1461-0248.2011.01662.x) [DOI] [PubMed] [Google Scholar]
- 5.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. ( 10.1016/j.tree.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 7.Costa-Pereira R, Rudolf VHW, Souza FL, Araújo MS. 2018. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 87, 1452–1464. ( 10.1111/1365-2656.12879) [DOI] [PubMed] [Google Scholar]
- 8.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 9.Jeltsch F, et al. 2013. Integrating movement ecology with biodiversity research-exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 1, 1–13. ( 10.1186/2051-3933-1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT, Moore AEAJ. 2001. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135. ( 10.1086/321307) [DOI] [PubMed] [Google Scholar]
- 11.Boon AK, Réale D, Boutin S. 2008. Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117, 1321–1328. ( 10.1111/j.0030-1299.2008.16567.x) [DOI] [Google Scholar]
- 12.Kobler A, Klefoth T, Mehner T, Arlinghaus R. 2009. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia 161, 837–847. ( 10.1007/s00442-009-1415-9) [DOI] [PubMed] [Google Scholar]
- 13.Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM. 2015. When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc. R. Soc. B 282, 20151768 ( 10.1098/rspb.2015.1768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtmann B, Santos ESA, Lara CE, Nakagawa S. 2017. Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance. Proc. R. Soc. B 284, 20170943 ( 10.1098/rspb.2017.0943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearish S, Hostert L, Bell AM. 2013. Behavioral type-environment correlations in the field: a study of three-spined stickleback. Behav. Ecol. Sociobiol. 67, 765–774. ( 10.1007/s00265-013-1500-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel O, Leu ST, Bull CM, Sih A. 2017. What's your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol. Lett. 20, 3–18. ( 10.1111/ele.12708) [DOI] [PubMed] [Google Scholar]
- 17.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 18.Bergmüller R, Taborsky M. 2010. Animal personality due to social niche specialisation. Trends Ecol. Evol. 25, 504–511. ( 10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 19.Laskowski KL, Pruitt JN. 2014. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B 281, 20133166 ( 10.1098/rspb.2013.3166) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Pruitt JN, Ferrari MCO. 2011. Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology 92, 1902–1908. ( 10.1890/11-0701.1) [DOI] [PubMed] [Google Scholar]
- 21.Schmiege PFP, D'Aloia CC, Buston PM. 2017. Anemonefish personalities influence the strength of mutualistic interactions with host sea anemones. Mar. Biol. 164, 24 ( 10.1007/s00227-016-3053-1) [DOI] [Google Scholar]
- 22.Sweeney K, Cusack B, Armagost F, O'Brien T, Keiser CN, Pruitt JN. 2013. Predator and prey activity levels jointly influence the outcome of long-term foraging bouts. Behav. Ecol. 24, 1205–1210. ( 10.1093/beheco/art052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Found R. 2017. Interactions between cleaner-birds and ungulates are personality dependent. Biol. Lett. 13, 20170536 ( 10.1098/rsbl.2017.0536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 25.Webster MM, Ward AJW, Hart PJB. 2009. Individual boldness affects interspecific interactions in sticklebacks. Behav. Ecol. Sociobiol. 63, 511–520. ( 10.1007/s00265-008-0685-2) [DOI] [Google Scholar]
- 26.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dammhahn M, Almeling L. 2012. Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim. Behav. 84, 1131–1139. ( 10.1016/j.anbehav.2012.08.014) [DOI] [Google Scholar]
- 28.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 29.Korpela K, Sundell J, Ylönen H. 2011. Does personality in small rodents vary depending on population density? Oecologia 165, 67–77. ( 10.1007/s00442-010-1810-2) [DOI] [PubMed] [Google Scholar]
- 30.Morris DW. 1997. Optimally foraging deer mice in prairie mosaics: a test of habitat theory and absence of landscape effects. Oikos 80, 31–42. ( 10.2307/3546513) [DOI] [Google Scholar]
- 31.Arlettaz R, Krähenbühl M, Almasi B, Roulin A, Schaub M. 2010. Wildflower areas within revitalized agricultural matrices boost small mammal populations but not breeding barn owls. J. Ornithol. 151, 553–564. ( 10.1007/s10336-009-0485-0) [DOI] [Google Scholar]
- 32.Kozakiewicz A. 1987. Spatial distribution and interspecific interactions in small rodent community of a lake coastal zone. Acta Theriol. (Warsz.) 32, 433–447. ( 10.4098/AT.arch.87-30) [DOI] [Google Scholar]
- 33.Jancewicz E, Gliwicz J. 2017. Niche dynamics and biodiversity: many rodent species on one marshy meadow. Pol. J. Ecol. 65, 371–379. ( 10.3161/15052249PJE2017.65.4.006) [DOI] [Google Scholar]
- 34.Gliwicz J. 1981. Competitive interactions within a forest rodent community in central Poland. Oikos 37, 353–362. ( 10.2307/3544128) [DOI] [Google Scholar]
- 35.Kozakiewicz A, Boniecki P. 1994. Intra-and interspecific behaviours in the bank vole and striped-field mouse under enclosure conditions. Acta Theriol. (Warsz.) 39, 29–36. ( 10.4098/AT.arch.94-4) [DOI] [Google Scholar]
- 36.Schirmer A, Herde A, Eccard JA, Dammhahn M. 2019. Individuals in space: personality-dependent space use, movement and microhabitat use facilitate individual spatial niche specialization. Oecologia 189, 647–660. ( 10.1007/s00442-019-04365-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calenge C. 2006. The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197, 516–519. ( 10.1016/j.ecolmodel.2006.03.017) [DOI] [Google Scholar]
- 38.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 39.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185x.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 40.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 41.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 42.Houslay TM, Wilson AJ. 2017. Avoiding the misuse of BLUP in behavioural ecology. Behav. Ecol. 28, 948–952. ( 10.1093/beheco/arx023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eccard JA, Ylönen H. 2002. Direct interference or indirect exploitation? An experimental study of fitness costs of interspecific competition in voles. Oikos 99, 580–590. ( 10.1034/j.1600-0706.2002.11833.x) [DOI] [Google Scholar]
- 45.Koskela E, Mappes T, Ylonen H. 1997. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. J. Anim. Ecol. 66, 341–349. ( 10.2307/5980) [DOI] [Google Scholar]
- 46.Hart SP, Schreiber SJ, Levine JM. 2016. How variation between individuals affects species coexistence. Ecol. Lett. 19, 825–838. ( 10.1111/ele.12618) [DOI] [PubMed] [Google Scholar]
- 47.Maynard DS, Serván CA, Capitán JA, Allesina S. 2019. Phenotypic variability promotes diversity and stability in competitive communities. Ecol. Lett. 22, 1776–1786. ( 10.1111/ele.13356) [DOI] [PubMed] [Google Scholar]
- 48.Hausch S, Vamosi SM, Fox JW. 2018. Effects of intraspecific phenotypic variation on species coexistence. Ecology 99, 1453–1462. ( 10.1002/ecy.2346) [DOI] [PubMed] [Google Scholar]
- 49.Toscano BJ, Gownaris NJ, Heerhartz SM, Monaco CJ. 2016. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia 182, 55–69. ( 10.1007/s00442-016-3648-8) [DOI] [PubMed] [Google Scholar]
- 50.Schreier BM, Harcourt AH, Coppeto SA, Somi MF. 2009. Interspecific competition and niche separation in primates: a global analysis. Biotropica 41, 283–291. ( 10.1111/j.1744-7429.2008.00486.x) [DOI] [Google Scholar]
- 51.Grimm V. 1999. Ten years of individual-based modelling in ecology: what have we learned and what could we learn in the future? Ecol. Model. 115, 129–148. ( 10.1016/S0304-3800(98)00188-4) [DOI] [Google Scholar]
- 52.Raffard A, Santoul F, Cucherousset J, Blanchet S. 2018. The community and ecosystem consequences of intraspecific diversity: a meta-analysis. Biol. Rev. 94 648–661. ( 10.1111/brv.12472) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the open research data portal of the Leibniz Center for Agricultural Landscape Research (ZALF) and can be accessed via the following links: https://www.doi.org/10.4228/ZALF.DK.129; https://www.doi.org/10.4228/ZALF.DK.131.