Abstract
Background:
Environmental reward-predictive stimuli provide a major source of motivation for adaptive reward pursuit behavior. This cue-motivated behavior is known to be mediated by the nucleus accumbens core (NAc). The cholinergic interneurons in the NAc are tonically active and densely arborized and, thus, well-suited to modulate NAc function. But their causal contribution to adaptive behavior remains unknown. Here we investigated the function of NAc cholinergic interneurons in cue-motivated behavior.
Methods:
To do this, we used chemogenetics, optogenetics, pharmacology, and a translationally analogous Pavlovian-to-instrumental transfer behavioral task designed to assess the motivating influence of a reward-predictive cue over reward-seeking actions in male and female rats.
Results:
The data show that NAc cholinergic interneuron activity critically opposes the motivating influence of appetitive cues. Chemogenetic inhibition of NAc cholinergic interneurons augmented cue-motivated behavior. Optical stimulation of acetylcholine release from NAc cholinergic interneurons prevented cues from invigorating reward-seeking behavior, an effect that was mediated by activation of β2-containing nicotinic acetylcholine receptors.
Conclusions:
Thus, NAc cholinergic interneurons provide a critical regulatory influence over adaptive cue-motivated behavior and, therefore, are a potential therapeutic target for the maladaptive cue-motivated behavior that marks many psychiatric conditions, including addiction and depression.
Keywords: Pavlovian-to-instrumental transfer, acetylcholine, tonically-active neurons, optogenetics, chemogenetics, biosensors, dopamine, motivation
Environmental reward-predictive stimuli provide a major source of motivation for adaptive reward pursuit behaviors (1). This incentive motivational value can become dysfunctional in many psychiatric disease states (2). Indeed, it can become amplified allowing cues to become potent triggers for maladaptive compulsive overeating (3), alcohol abuse (4–7), or drug seeking (8–12). Stress, anxiety, and depression (13–16) can also disrupt the motivating influence of appetitive cues, resulting in dampened or inappropriate motivation. The nucleus accumbens core (NAc) has been implicated in cue-motivated behavior (17–19). But little is known about the function of the major NAc neuromodulator acetylcholine. Such information is crucial given the purported importance of cholinergic signaling in many mental illnesses (20, 21).
Cholinergic interneurons provide the primary, though not exclusive (22), source of acetylcholine in the NAc (23). Despite comprising only 1–2% of the population, these large-bodied, tonically active neurons are densely arborized (24–29), making them ideally suited to modulate NAc function and associated behaviors. Cholinergic interneurons have also been shown to locally regulate striatal dopamine release (30–32). NAc cholinergic signaling is elevated under conditions that discourage vigorous reward seeking, such as satiety (33, 34), and has been implicated in anxiety- and depression-like states (35, 36) marked by blunted motivation. Cholinergic interneurons are also transiently activated by informative environmental stimuli. Cues that discourage motivated behavior transiently activate the cholinergic interneurons (37, 38), whereas reward-predictive cues that encourage motivated behavior cause a characteristic pause in cholinergic interneuron activity (29, 37, 39–46). Yet still, very little is known of the causal contribution of NAc cholinergic interneurons to motivation.
We sought to fill this gap in knowledge by assessing the function of NAc cholinergic interneurons in cue-motivated behavior. Working from the evidence that cholinergic interneurons increase their activity when vigorous motivated behavior is disadvantageous and pause when active reward pursuit is encouraged, we tested the hypothesis that NAc cholinergic interneuron activity functions to oppose the motivating influence of appetitive cues. Chemogenetic and optogenetic methods were used to selectively manipulate NAc cholinergic interneuron activity. We used the Pavlovian-to-instrumental transfer (PIT) test to measure cue-motivated behavior. This test is translationally analogous to that used in humans in health and disease (5, 11, 17, 47–55) and assesses the invigorating influence of an environmental reward-predictive stimulus over instrumental reward-seeking activity. Because the Pavlovian and instrumental components are trained separately, PIT isolates the incentive motivational value of the cue from other processes through which cues trigger action, such as via discriminative control or a stimulus-response relationship.
MATERIALS AND METHODS
Subjects.
Adult (3–5 months) male and female ChAT::Cre+ bacterial artificial chromosome (BAC) transgenic rats (Long-Evans background) (56) were used for all experiments. Although BAC transgenic ChAT::Cre+ mice have been shown to overexpress the vesicular acetylcholine transporter, which can lead to behavioral and electrophysiological changes (57), we found normal expression of the behaviors of interest, similar to our prior reports in wild-type rats (58–60). Pups were weaned at postnatal day 21 and group housed until experiment onset. Handling occurred daily, beginning at postnatal day 60. Training and test were performed during the dark phase of a 12:12 hr reverse dark/light cycle. Rats were food-restricted to ~85% free-feeding body weight and water was provided ad libitum in the home cage. All procedures were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by UCLA’s Institutional Animal Care and Use Committee.
Surgery.
Standard surgical procedures, described previously (58, 61, 62), were used for infusion of adeno-associated viruses (AAVs) and implantation of optical fiber or microinfusion injector/optical fiber guide cannula into the NAc core. Rats were anesthetized with isoflurane and a nonsteroidal anti-inflammatory agent was administered pre- and post-operatively to minimize pain and discomfort. Surgical details for each experiment are provided in the Supplemental Methods. Expression and placement was verified with standard histological procedures (see Supplemental Methods).
Behavioral Procedures.
General training and testing.
Training.
Rats received Pavlovian and instrumental training in Med Associates conditioning chambers, as described previously (58–60).
Pavlovian conditioning.
Rats first received 8 days of Pavlovian training in which 1 of 2 auditory stimuli (75 dB tone or white noise; counterbalanced across rats) was paired with non-contingent delivery of 45 mg chocolate-flavored, grain-based pellets (Bio-Serv, Frenchtown, NJ). During each 2-min presentation of the conditional stimulus (CS+), pellets were presented on a random time (RT)-30s schedule. The CS+ was presented 6x/session with a random 2–4 min inter-trial interval (mean=3 min). The lever was never present during these sessions.
Instrumental conditioning.
All rats then received 8 days of instrumental training in which lever pressing earned delivery of a single chocolate pellet. Each session lasted until 20 outcomes had been earned, or 30 min elapsed. Rats received one day each of continuous, random interval (RI)-15 s, and RI-30 s schedules of reinforcement, followed by 5 days on the final RI-60 s schedule. The CS+ was never present during this training.
CSØ habituation.
Rats received 1 session of habituation to the neutral control stimulus (CSØ), which consisted of 6, 2-min presentations of the CSØ (opposite stimulus as the CS+), with a 2–4 min inter-trial interval. No rewards were delivered during this session.
Pavlovian-to-instrumental transfer test.
On the day prior to each PIT test, rats were given a single 30-min instrumental extinction session in which no cues were present and the lever was available, but presses were unrewarded. During each PIT test the lever was continuously available, but pressing was not reinforced. Responding was extinguished for 5 min to establish a low rate of baseline performance, after which each CS was presented 4 times in pseudorandom order, also without accompanying reward. Each CS lasted 2 min with a 4-min fixed inter-trial interval. Rats received 1 Pavlovian and 2 instrumental retraining sessions identical to those above in between subsequent PIT tests. In all cases, testing commenced at least 4 weeks post-viral infusion to allow construct expression.
Chemogenetic inactivation of NAc cholinergic interneurons.
Prior to training, ChAT::Cre+ rats were bilaterally infused with a cre-inducible AAV vector to express the inhibitory designer receptor human M4 muscarinic receptor (hM4D(Gi)) or control fluorophore mCherry selectively in cholinergic interneurons of the NAc. Following training, rats received PIT tests, counterbalanced for order, one following vehicle and one following i.p. injection of the hM4D(Gi) ligand clozapine-N-oxide (CNO; 5mg/kg; see Supplemental Methods). These experiments were run in two separate cohorts and data were collapsed across cohorts following analyses indicating no interaction between Cohort and any of the variables of primary interest (hM4D(Gi): highest F=3.23, P=0.09; mCherry highest F=3.876, P=0.07). Final hM4D(Gi) N=19 (8 female; 2 rats were excluded due to off target viral spread) and mCherry N=16 (8 female). Following PIT testing, a subset of subjects were tested for the influence of NAc cholinergic interneuron inactivation on food consumption and lever pressing on a progressive ratio response requirement (see Supplemental Methods).
Optical stimulation of NAc cholinergic interneurons.
Prior to training, ChAT::Cre+ rats were bilaterally infused with a cre-inducible AAV vector to express the excitatory opsin channelrhodopsin-2 (ChR2) or control fluorophore eYFP selectively in NAc cholinergic interneurons. Optical fibers were implanted bilaterally in the NAc. From the last 2 days of instrumental training and for a single additional Pavlovian retraining session, rats were tethered to the patchcord, but no light was delivered to allow habituation to the optical tether. Following training, rats received 4 PIT tests, counterbalanced for order, with intervening retraining. During each test, optical fibers were connected via ceramic sleeves to patch cords attached to a commutator. Blue light (473 nm, 10 Hz, 10 mW, 5 ms pulse width, 120 s duration; see also Supplemental Methods) was delivered for optical activation of ChR2-expressing NAc cholinergic interneurons. For the main experimental condition, light was delivered concurrent with each of the 4 CS+ presentations, with light and CS+ onset and offset synced. There were 3 separate control conditions: light delivered concurrent with each CSØ presentation, light delivered during the CS-free 2-min baseline period immediately prior to each CS+ presentation, or light delivered during the CS-free 2-min baseline period immediately prior to each CSØ presentation. There were no significant differences in performance between the preCS+ and preCSØ stimulation tests and, thus, data were collapsed across these tests into a single ‘baseline stimulation’ control condition (see Supplemental Figure 4). Final ChR2 N=9 (5 female; 5 subjects excluded for lack of expression and/or optical fiber misplacement), eYFP N=8 (5 female).
Optical stimulation of NAc cholinergic interneurons and inactivation of NAc β2-containing nAChRs.
Prior to training, ChAT::Cre+ rats were bilaterally infused with a cre-inducible AAV vector to express ChR2 selectively in NAc cholinergic interneurons. Microinfusion injector/optical fiber guide cannula were implanted bilaterally above the NAc. Following training, rats received 4 PIT tests, counterbalanced for order with intervening retraining. Prior to each test, rats were bilaterally infused with either the selective α4β2-containing nicotinic receptor competitive antagonist dihydro-β-erythroidine (DhβE; 15 μg/0.5 μl/side; see Supplemental Methods) or artificial cerebral spinal fluid (ACSF) vehicle via an injector inserted through the guide cannula designed to protrude 2.5 mm to just above the NAc (−6.5 mm). Following infusion, injectors were removed and optical fibers, also designed to protrude 2.5 mm and, thus target the NAc, were placed through guide cannula and secured via ceramic sleeves. During 2 of the tests, one each following vehicle or DhβE, blue light (473 nm, 10 Hz, 10 mW, 5 ms pulse width, 120 s duration) was delivered for optical activation of ChR2-expressing NAc cholinergic interneurons concurrent with each CS+ presentation. During the other two tests, an optical fiber was attached but no light was delivered. Thus, each rat received 4 tests: Vehicle/No stimulation, Vehicle/stimulation during CS+, DhβE/no stimulation, DhβE/stimulation during CS+. Following the PIT tests, optical fibers were removed and dummies were placed in the guide cannula. Final N=11 (all male, 1 rat was excluded due to a clogged cannula).
Data analysis.
Behavioral analysis.
Lever pressing and entries into the food-delivery port were the primary behavioral output measures for the PIT test. These measures were counted for each 2-min CS period, with behavioral output during the 2-min periods prior to each CS serving as the baseline. For both the chemogenetic inhibition and optical stimulation experiments there was no interaction between trial and any of the other variables on lever pressing during the test (highest F=1.84, P=0.13). Thus, in all cases, data were collapsed across trials.
Sex differences.
Approximately half the subjects in the chemogenetic and optical manipulation experiments were female. In neither case was there a main effect of Sex (hM4D(Gi): F1,7=2.72, P=0.12; ChR2: F1,7=0.71, P=0.43) and Sex did not significantly interact with the effect of CS and/or Drug or Stimulation period on lever pressing (highest F=3.41, P=0.08). Thus, all data were collapsed across sexes. Because sex did not influence results of the initial optogenetic experiment, the follow-up experiment assessing the influence of intra-NAc DhβE on the behavioral effect of optical stimulation included only males.
Statistical analysis.
Data were processed with Microsoft Excel (Redmond, WA). Statistical analyses were conducted with GraphPad Prism (La Jolla, CA) and SPSS (IBM Corp, Chicago, IL). Data were analyzed with Student’s t tests, one-, two-, and three-way repeated-measures analysis of variance (ANOVA; Geisser-Greenhouse correction). Corrected post-hoc comparisons were used to clarify main effects and interactions. All datasets met equal covariance assumptions, justifying ANOVA interpretation (63). Alpha levels were set at P<0.05.
Approach validation.
Optical stimulation and chemogenetic inhibition of NAc cholinergic interneurons was validated in vivo with electroenzymatic choline biosensors and constant-potential amperometry as detailed in the Supplemental Methods. Briefly, to confirm chemogenetic inhibition of NAc cholinergic interneurons, silicon wafer-based platinum microelectrode array choline biosensors packaged with an optical fiber affixed to the back surface of the probe (to reduce the photovoltaic artifact) were lowered into the NAc of anesthetized rats expressing ChR2 and hM4D(Gi) in cholinergic interneurons. The ability of blue light (473 nm, 20 Hz, 5–30 mW, 10-ms pulse width, 5-s duration) to evoke acetylcholine release continuously monitored by the sensor was assessed following injection of vehicle or CNO (5 mg/kg, i.p.). Final N=4 recording locations in 2 subjects. To confirm stimulation of NAc cholinergic interneurons with the exact light parameters used in the behavioral experiments, choline biosensors/optical fibers were lowered into the NAc of anesthetized rats expressing ChR2 or eYFP in cholinergic interneurons. Choline fluctuations were monitored and blue light (473 nm, 10 Hz, 10 mW, 5-ms pulse width, 120-s duration) was delivered to evaluate its ability to evoke acetylcholine release in ChR2-expressing subjects. Final ChR2 N=5 recording locations in 4 subjects, eYFP N=5 recording locations in 3 subjects.
RESULTS
Chemogenetic inhibition of NAc cholinergic interneurons augments cue-motivated behavior.
To evaluate the contribution of NAc cholinergic interneurons to cue-motivated behavior, we first chemogenetically inactivated these cells during a PIT test. Inactivation was achieved by using ChAT::Cre+ rats and a cre-inducible AAV vector to express the inhibitory designer receptor hM4D(Gi) selectively in cholinergic interneurons of the NAc (Figure 1A–C). In separate subjects expressing both hM4D(Gi) and ChR2 in cholinergic interneurons, CNO (5 mg/kg, i.p.) activation of hM4D(Gi) in cholinergic interneurons was found to effectively attenuate optically-evoked NAc acetylcholine release in vivo (Figure 1D).
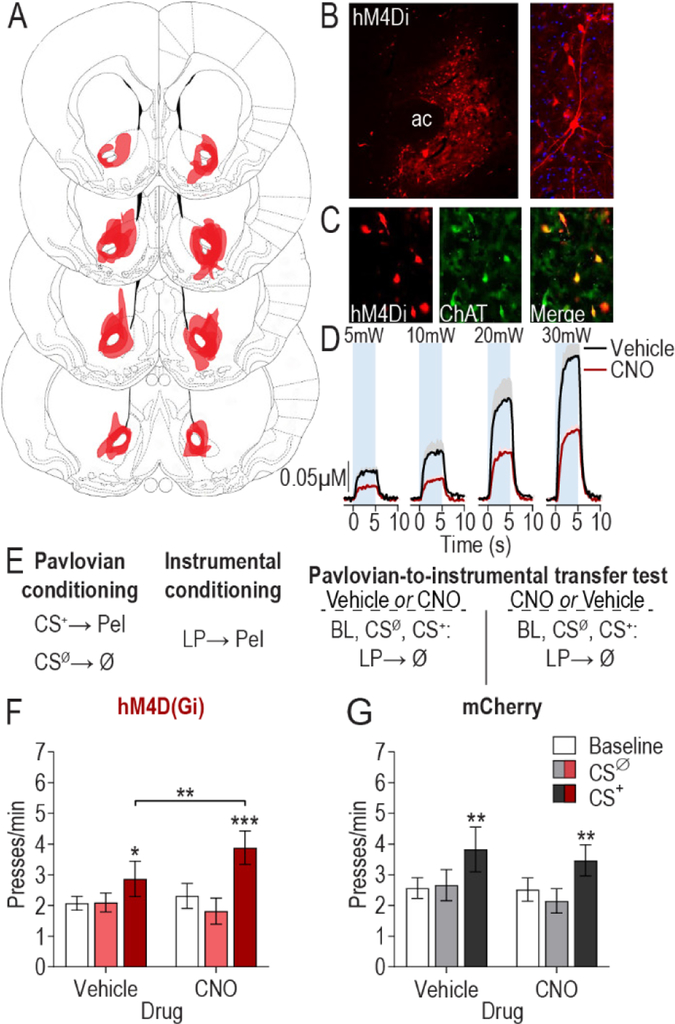
Figure 1. Chemogenetic inhibition of nucleus accumbens cholinergic interneurons augments cue-motivated behavior.
(A) Schematic representation of hM4D(Gi)-mCherry expression in the NAc for all subjects. Slides represent 0.7 – 1.7 mm anterior to bregma. Images taken from (101). (B) Representative immunofluorescent images of hM4D(Gi)-mcherry expressing cholinergic interneurons in the NAc. AC, anterior commissure. (C) Colocalization of ChAT staining and hM4D(Gi)-mcherry expression in the NAc. (D) CNO:hM4D(Gi) attenuation of optically-evoked (473 nm, 20 Hz, 5–30 mW, 10-ms pulse width, 5-s duration) acetylcholine release in the NAc in vivo (see Supplemental Figure 1 for histology demonstrating hM4D(Gi) and ChR2 expression in cholinergic interneurons; N=4). Mean +1 s.e.m. (E) Procedure schematic. CS+, reward-predictive cue; CSØ, neutral control stimulus; Pel, pellet reward; LP, lever press; Ø, no reward; Veh, Vehicle; CNO, Clozapine N-oxide. (F-G) Lever press rate during each 2-min period of the PIT test, averaged across trials compared between the CS-free (baseline), neutral stimulus (CSØ), and reward-predictive cue (CS+) periods for the vehicle- and CNO-treated conditions in hM4D(Gi) (N=19) (F) or mCherry control (N=16) (G) subjects. Mean ±1 s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Rats received Pavlovian training to pair a 2-min auditory conditional stimulus (CS+) with food pellet reward (Figure 1E). An alternate 2-min auditory stimulus was presented unpaired with reward and served as a control (CSØ). Rats were then instrumentally conditioned, in the absence of the stimuli, to lever press to earn food rewards (see Supplemental Table 1 for training data). At the PIT test, the lever was available and each CS was presented in pseudorandom order to assess the motivating influence of the CS+ over lever-pressing activity. No rewards were delivered during this test. Increased lever-press rate during the CS+ provided the measure of cue-motivated behavior (i.e., expression of PIT). Each rat was tested twice, once following injection of vehicle and once following CNO, counterbalanced for order (Figure 1E).
Inactivation of NAc cholinergic interneurons augmented the expression of PIT (CS period: F2,36=8.15, P=0.001; Drug: F1,18=0.78, P=0.39; CS × Drug: F2,36=5.2, P=0.01; Figure 1F). Demonstrating PIT, the CS+ elevated lever pressing relative to both the baseline and CSØ periods under vehicle control conditions (P<0.05). Inactivation of NAc cholinergic interneurons enhanced the invigorating influence of the CS+ relative to the vehicle control condition (P<0.01). NAc cholinergic interneuron inactivation predominantly influenced CS+-invigorated responding; neither baseline, nor CSØ lever-press rate were significantly altered in the CNO condition (P>0.05). There was no effect of CNO on the expression of PIT in subjects lacking the hM4D(Gi) transgene (CS period: F2,30=4.47, P=0.02; Drug: F1,15 =0.31, P=0.58; CS × Drug: F2,30=0.45, P=0.64; Figure 1G). Inactivation of NAc cholinergic interneurons did not alter the expression of Pavlovian conditional food-port approach responses during the PIT test. It also did not alter lever pressing during a progressive ratio test or basic food consumption (Supplemental Figure 2). Thus, inactivation of NAc cholinergic interneurons selectively enhanced the motivating influence of a reward-predictive cue over instrumental behavior.
Optical stimulation of NAc cholinergic interneurons concurrent with reward cue presentation blunts cue-motivated behavior.
The chemogenetic inactivation results suggest that NAc cholinergic interneurons function to oppose cue-motivated behavior. To further test this, we next evaluated the influence of activation of NAc cholinergic interneurons on expression of PIT. We used optical stimulation to provide temporal specificity. The excitatory opsin ChR2 was selectively expressed in NAc cholinergic interneurons (Figure 2A–C) of ChAT::Cre+ rats. Optical stimulation (473 nm, 10 Hz, 10 mW, 2 min) of these cells at a frequency in the upper range of their normal firing rate (64, 65) was found to increase acetylcholine release in vivo. This increase was restricted to the light-on period (F2,8=15.15, P=0.01) and did not occur in subjects lacking the ChR2 transgene (Figure 2D). Following Pavlovian and instrumental training, during the PIT test, we used a within-subject design to stimulate NAc cholinergic interneurons either concurrent with each 2-min CS+ presentation, or, in separate control tests, each CSØ presentation, or an equivalent number and duration of CS-free baseline periods (Figure 2E).
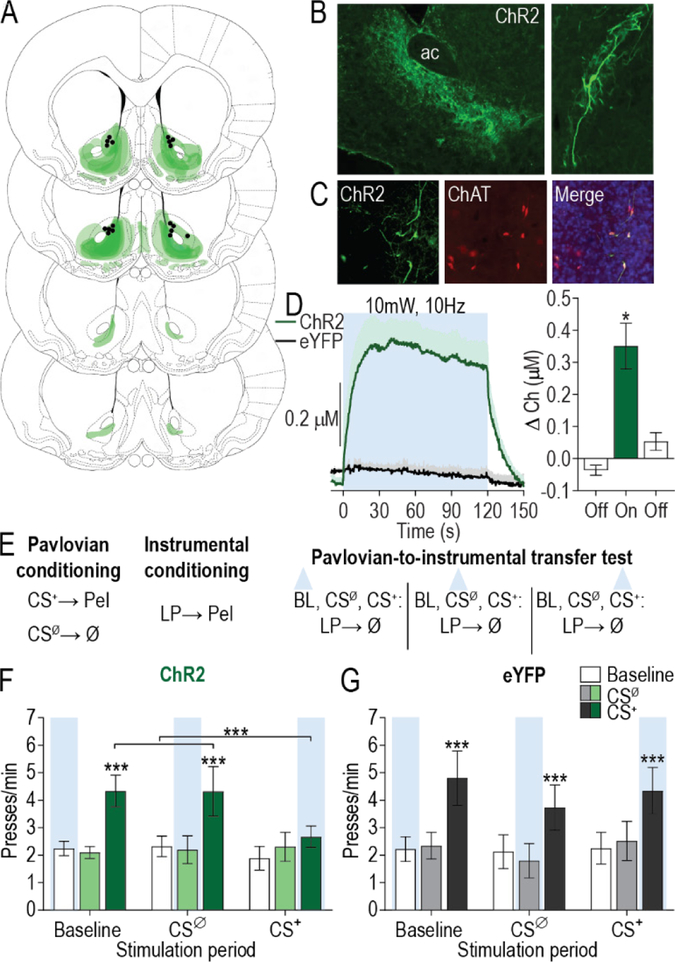
Figure 2. Optical stimulation of nucleus accumbens cholinergic interneurons concurrent with reward-predictive cue blunts cue-motivated behavior.
(A) Schematic representation of ChR2-eYFP expression and fiber tips the NAc for all subjects. Slides represent 0.7 – 1.7 mm anterior to bregma. (B) Representative immunofluorescent images of ChR2-eYFP expressing cholinergic interneurons in the NAc. AC, anterior commissure. (C) Colocalization of ChAT staining and ChR2-eYFP expression in the NAc. (D) Optically-evoked acetylcholine release in vivo by blue light delivery (473 nm, 10 Hz, 10 mW, 5-ms pulse width, 120-s duration) to ChR2-expressing cholinergic interneurons in the NAc (see Supplemental Figure 3 for histology; N=5/group). Mean +1 s.e.m. (E) Procedure schematic. CS+, reward-predictive cue; CSØ, neutral control stimulus; Pel, pellet reward; LP, lever press; Ø, no reward; blue triangle, light delivery. (F-G) Lever press rate during each 2-min period of the PIT test, averaged across trials compared between the CS-free (baseline), neutral stimulus (CSØ), and reward-predictive cue (CS+) periods for tests in which optical stimulation occurred during the baseline stimulation, CSØ, and CS+ periods in ChR2 (N=9) (F) or eYFP control (N=8) (G) subjects. Mean ±1 s.e.m. ***P<0.001.
Optical stimulation of NAc cholinergic interneurons during CS+ presentation blunted the expression of PIT (CS period: F2,16=8.07, P=0.004; Stimulation period: F2,16=0.71, P=0.50; CS × Stimulation period: F4,32=3.79, P=0.01; Figure 2F). Neither baseline nor CSØ period stimulation altered lever pressing during those periods (P>0.05) or the significant enhancement in such pressing induced by the CS+ (P<0.001). However, stimulation of NAc cholinergic interneurons concurrent with CS+ presentation prevented that cue from increasing lever pressing (P>0.05). Light delivery had no effect on the expression of PIT in subjects lacking the ChR2 transgene (CS period: F2,14=8.656, P=0.004; Stimulation period: F2,14=0.27, P=0.77; CS × Stimulation period: F4,28=1.04, P=0.41; Figure 2G). Optical stimulation of NAc cholinergic interneurons did not prevent the CS+ from eliciting Pavlovian conditional food-port approach responses (Supplemental Figure 5), suggesting no deficit in CS+ recognition. Thus, optical stimulation of NAc cholinergic interneurons blunted the expression of cue-motivated behavior.
Acetylcholine release from NAc cholinergic interneurons works via β2-containing nicotinic receptors to blunt cue-motivated behavior.
These data suggest that cholinergic interneuron activity tempers the motivating influence of reward-predictive cues over reward-seeking actions. Acetylcholine receptors are broadly distributed in the NAc and consist of two major subtypes: metabotropic muscarinic (mAChR) and ionotropic nicotinic (nAChR). We previously found that activity of the NAc nAChRs, in particular, works to restrain the expression of cue-motivated behavior (58). Moreover, nAChRs containing the β2 subunit have been shown to be located exclusively on dopamine axons and terminals (66) where they regulate phasic dopamine release (67–72), which has itself, in the NAc, been shown to track and mediate cue-motivated behavior (9, 59, 60, 73–76). Thus, we next asked whether the attenuating effect of optical stimulation of NAc cholinergic interneurons over cue-motivated behavior is mediated via these β2-containing nAChRs. To achieve this, we again selectively expressed ChR2 in NAc cholinergic interneurons (Figure 3A–C) and evaluated the influence of intra-NAc infusion of dihydro-β-erythroidine (DhβE; 15μg/side), a selective α4β2-containing nAChR antagonist, on the suppressive influence of NAc cholinergic interneuron stimulation over PIT expression (Figure 3D).
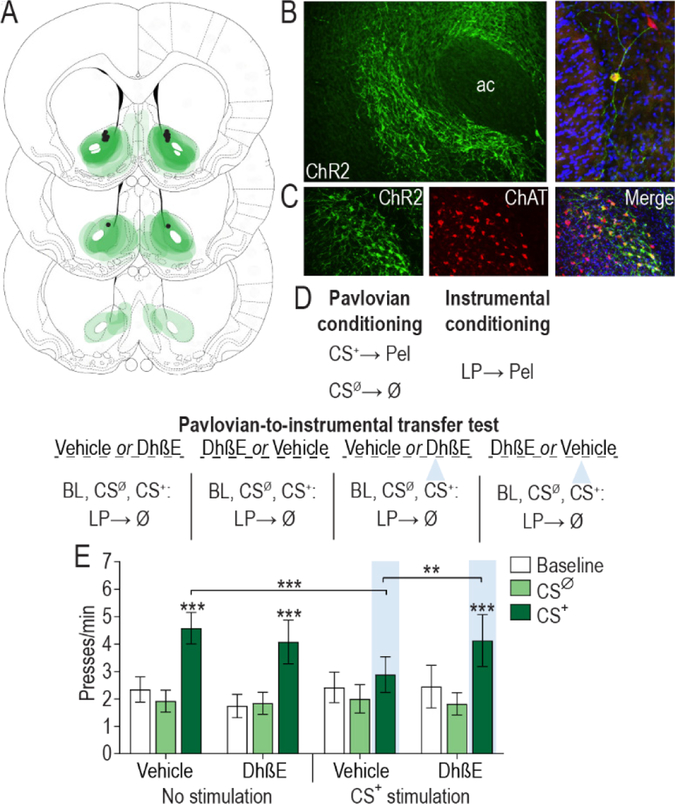
Figure 3. Acetylcholine release from nucleus accumbens cholinergic interneurons works via β2-containing nicotinic receptors to blunt cue-motivated behavior.
(A) Schematic representation of ChR2-eYFP expression and fiber/injector tips the NAc for all subjects. Slides represent 0.7 – 1.7 mm anterior to bregma. (B) Representative immunofluorescent images of ChR2-eYFP expressing cholinergic interneurons in the NAc. AC, anterior commissure. (C) Colocalization of ChAT staining and ChR2-eYFP expression in the NAc. (D) Procedure schematic. CS+, reward-predictive cue; CSØ, neutral control stimulus; Pel, pellet reward; LP, lever press; Ø, no reward; Veh, Vehicle; DhβE, Dihydro-β-erythroidine; blue triangle, light delivery. (E) Lever press rate during each 2-min period of the PIT test, averaged across trials compared between the CS-free (baseline), neutral stimulus (CSØ), and reward-predictive cue (CS+) periods for the tests with either intra-NAc vehicle or DhβE with or without optical stimulation during the CS+ (N=11). Mean ±1 s.e.m. **P<0.01, ***P<0.001.
Blockade of β2-containing nAChRs recovered the impairment of PIT induced by optical stimulation of NAc cholinergic interneurons during the CS+ (CS period: F2,22=22.69, P<0.0001; Optical stimulation: F1,11=0.082, P=0.78; Drug: F1,11=0.003, P=0.96; CS × Stimulation: F2,22 =5.19, p=0.02; CS × Drug × Stimulation: F2,22 =5.10, P=0.02; Figure 3E). We replicated the suppressive effect of optical stimulation of NAc cholinergic interneurons during CS+ presentation on the expression of PIT relative to a non-stimulated control condition (P>0.001). Whereas intra-NAc infusion of DhβE alone at this dose did not influence PIT expression relative to the vehicle-infused control condition (P>0.05), it did alleviate the suppressive effect of cholinergic interneuron stimulation (P<0.01), allowing subjects to show a significant PIT effect (P<0.001). These data demonstrate that acetylcholine release from NAc cholinergic interneurons acts via β2-containing nAChRs to blunt the motivating influence of cues. Secondarily, they indicate that the effect of optical stimulation of cholinergic interneurons was not due to nAChR receptor desensitization.
DISCUSSION
Using a combination of chemogenetic, optogenetic, and pharmacological approaches, we investigated the function of NAc cholinergic interneurons in cue-motivated behavior. The data revealed that cholinergic interneuron activity in the NAc functions to limit the motivational influence of reward-predictive cues over reward-seeking actions. Chemogenetic inactivation of NAc cholinergic interneurons augmented cue-motivated behavior, whereas optical stimulation of these cells temporally restricted to cue presentation prevented cues from motivating action. This mitigating function is achieved via acetylcholine activation of β2-containing nAChRs.
These data accord well with evidence of the activity patterns of striatal cholinergic interneurons collected in non-human primates and rodents. Striatal cholinergic interneurons can both tonically and phasically increase their activity when vigorous motivated behavior is discouraged, for example in states of satiety (33, 34), or when cues signal unfavorable (e.g., high effort, low reward) conditions (37). Cholinergic interneurons also transiently increase their activity when cues signal that reward is available contingent upon a no-go response (38), i.e., when motivated movement must be withheld. Striatal cholinergic interneurons transiently pause their activity in response to cues signaling that vigorous reward seeking is advantageous. For example, cholinergic interneurons will pause in response to reward-predictive cues (29, 37, 39–46) and when cues signal favorable low effort/high reward conditions (37). The current data provide an important causal addition to this literature and reveal that increases in NAc cholinergic interneuron activity function to oppose cue-motivated behavior and that decreases or pauses in such activity are permissive to cue-motivated action. These results also indicate that the NAc inputs that regulate cholinergic interneuron excitability, activity, or synchrony, such as thalamostriatal projections (69), are well-positioned to influence cue-motivated behavior. Indeed, recent evidence from the dorsal striatum indicates that stimulation of rostral intralaminar thalamic inputs can regulate motivated behavior by triggering a rapid burst then pause in cholinergic interneuron activity (77).
We found the suppressive effect of optical stimulation over cue-motivated behavior to depend on activity of β2-containing nAChRs. Acetylcholine release from NAc cholinergic interneurons acts at β2-containing nAChRs receptors to curtail the motivating influence of appetitive cues. This is consistent with our previous evidence that general nAChR, but not mAChR, blockade augments cue-motivated behavior (58). Moreover, that inactivation of β2-containing nAChRs completely recovered the suppressive influence of optical stimulation of NAc CINs over cue-motivated behavior, suggests that, although other acetylcholine receptor subtypes may contribute, β2-containing nAChR receptors are a critical locus of action for cholinergic regulation of cue-motivated behavior.
NAc core dopamine release is a major substrate of cue-motivated behavior. Its activity correlates with (58, 60, 74, 78) and is necessary (59, 76, 79) and sufficient (75, 80, 81) for the motivational influence of reward-predictive cues. β2-containing nAChRs are located exclusively on NAc DA axons and terminals (66), where they have been found to modulate dopamine release (67–72). The present data may be considered surprising in light of evidence that optical stimulation of striatal cholinergic interneurons can evoke dopamine release from terminals via action at β2-containing nAChRs (69, 70). But a growing body of literature indicates that cholinergic regulation of dopamine release depends on the activity state of the dopamine cells (82, 83). β2-containing nAChR activity facilitates low probability (32, 67, 84) and tonic dopamine release (85), but will actually suppress dopamine release that results from high-frequency stimulation, which mimics dopamine neuron burst firing (32, 67, 84). Indeed, inactivation of β2-containing nAChRs in the NAc will augment dopamine release induced by high frequency stimulation ex vivo (68, 86) and general nAChR inactivation in the NAc will potentiate the phasic dopamine release response to reward-predictive cues in awake-behaving animals (58). Thus, we speculate that NAc cholinergic interneuron activity may restrain the motivating influence of reward-predictive cues via attenuating their ability to elicit dopamine release, with pausing in their signaling being permissive to such release and associated motivation.
The suppressive function of NAc cholinergic interneurons over cue-motivated behavior is interesting in light of how these cells are regulated. NAc cholinergic interneurons are controlled by several factors that mediate food-related motivation and responsivity to food cues. For example, they express receptors for the adiposity and satiety signal insulin, activation of which increases their activity and modulates NAc dopamine signaling through a nAChR-dependent mechanism (87). They also express receptors for corticotropin releasing factor (CRF), which mediates the positive and negative effects of stress (88–91). NAc CRF receptor activation increases cholinergic interneuron activity (92) and acetylcholine release (93), and regulates dopamine release (92). Moreover, serotonin, a neuromodulator long linked to motivation and mood, and recently in the NAc linked to adaptive social behavior (94), attenuates the excitability of NAc cholinergic interneurons via presynaptic 5-HT1A and postsynaptic 5-HT1B receptors (95).
Thus, NAc cholinergic interneurons are well-positioned to mitigate cue-motivated behavior when vigorous motivated action would not be beneficial and to promote cue-motivated behavior when it is adaptive. Dysfunction in this mechanism could, therefore, lead to the dysregulated motivation underlying some mental illnesses. Indeed, cues can become unnaturally strong motivators of drug-seeking behavior in addiction (4, 8, 96, 97) and NAc cholinergic interneurons have been linked to addiction-like behaviors (98, 99). Depression can be characterized by avolitional symptoms (96, 100), and NAc cholinergic interneurons have been linked to depression-like behavior (35). These results, therefore, have implications for the understanding and treatment of these and other diseases marked by maladaptive motivation.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH grants MH106972 to KMW and SBO, AG045380, DK098709, and DA029035 to SBO, DA035443 to KMW, NS087494 to HGM and KMW, and T32 DA024635 to ALC. It was also supported by a UCLA dissertation year fellowship to ALC and a UCLA Integrated and Interdisciplinary Undergraduate Research Program Fellowship to TJA. The authors would like to thank Dr. Melissa Malvaez for helpful comments on the manuscript and Ana Sias for her assistance with histology.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Corbit LH, Balleine BW (2015): Learning and Motivational Processes Contributing to Pavlovian-Instrumental Transfer and Their Neural Bases: Dopamine and Beyond. Curr Top Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- 2.Cartoni E, Balleine B, Baldassarre G (2016): Appetitive Pavlovian-instrumental Transfer: A review. Neurosci Biobehav Rev. 71:829–848. [DOI] [PubMed] [Google Scholar]
- 3.Johnson AW (2013): Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 36:101–109. [DOI] [PubMed] [Google Scholar]
- 4.Garbusow M, Schad DJ, Sebold M, Friedel E, Bernhardt N, Koch SP, et al. (2015): Pavlovian-to-instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addict Biol. [DOI] [PubMed] [Google Scholar]
- 5.Garbusow M, Schad DJ, Sommer C, Jünger E, Sebold M, Friedel E, et al. (2014): Pavlovian-to-instrumental transfer in alcohol dependence: a pilot study. Neuropsychobiology. 70:111–121. [DOI] [PubMed] [Google Scholar]
- 6.Corbit LH, Janak PH (2016): Changes in the Influence of Alcohol-Paired Stimuli on Alcohol Seeking across Extended Training. Front Psychiatry. 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbit LH, Janak PH (2007): Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res. 31:766–774. [DOI] [PubMed] [Google Scholar]
- 8.Robinson MJ, Robinson TE, Berridge KC (2013): Incentive salience and the transition to addiction In: Miller PM, editor. Biological Research on Addiction 2: Academic Press, pp 391–399. [Google Scholar]
- 9.Ostlund SB, LeBlanc KH, Kosheleff AR, Wassum KM, Maidment NT (2014): Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology. 39:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc KH, Ostlund SB, Maidment NT (2012): Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci. 126:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogarth L, Maynard OM, Munafò MR (2014): Plain cigarette packs do not exert Pavlovian to instrumental transfer of control over tobacco-seeking. Addiction. 110:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leblanc KH, Maidment NT, Ostlund SB (2013): Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 8:e61355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quail SL, Morris RW, Balleine BW (2016): Stress associated changes in Pavlovian-instrumental transfer in humans. Q J Exp Psychol (Hove).1–29. [DOI] [PubMed] [Google Scholar]
- 14.Morgado P, Silva M, Sousa N, Cerqueira JJ (2012): Stress Transiently Affects Pavlovian-to-Instrumental Transfer. Front Neurosci. 6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pielock SM, Sommer S, Hauber W (2013): Post-training glucocorticoid receptor activation during Pavlovian conditioning reduces Pavlovian-instrumental transfer in rats. Pharmacol Biochem Behav. 104:125–131. [DOI] [PubMed] [Google Scholar]
- 16.Huys QJ, Gölzer M, Friedel E, Heinz A, Cools R, Dayan P, et al. (2016): The specificity of Pavlovian regulation is associated with recovery from depression. Psychol Med. 46:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talmi D, Seymour B, Dayan P, Dolan RJ (2008): Human pavlovian-instrumental transfer. J Neurosci. 28:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbit LH, Balleine BW (2011): The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 31:11786–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbit LH, Muir JL, Balleine BW (2001): The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 21:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarr E, Gibbons AS, Neo J, Udawela M, Dean B (2013): Cholinergic connectivity: it’s implications for psychiatric disorders. Front Cell Neurosci. 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulawa SC, Janowsky DS (2018): Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, et al. (2014): A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. 34:4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou FM, Wilson CJ, Dani JA (2002): Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 53:590–605. [DOI] [PubMed] [Google Scholar]
- 24.Rymar VV, Sasseville R, Luk KC, Sadikot AF (2004): Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol. 469:325–339. [DOI] [PubMed] [Google Scholar]
- 25.Descarries L, Gisiger V, Steriade M (1997): Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 53:603–625. [DOI] [PubMed] [Google Scholar]
- 26.Descarries L, Mechawar N (2000): Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res. 125:27–47. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CJ, Chang HT, Kitai ST (1990): Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 10:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inokawa H, Yamada H, Matsumoto N, Muranishi M, Kimura M (2010): Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience. 168:395–404. [DOI] [PubMed] [Google Scholar]
- 29.Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M (1994): Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 14:3969–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cachope R, Cheer JF (2014): Local control of striatal dopamine release. Front Behav Neurosci. 8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cragg SJ (2006): Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 29:125–131. [DOI] [PubMed] [Google Scholar]
- 32.Sulzer D, Cragg SJ, Rice ME (2016): Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mark GP, Rada P, Pothos E, Hoebel BG (1992): Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 58:2269–2274. [DOI] [PubMed] [Google Scholar]
- 34.Helm KA, Rada P, Hoebel BG (2003): Cholecystokinin combined with serotonin in the hypothalamus limits accumbens dopamine release while increasing acetylcholine: a possible satiation mechanism. Brain Res. 963:290–297. [DOI] [PubMed] [Google Scholar]
- 35.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, et al. (2012): Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 109:11360–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoebel BG, Avena NM, Rada P (2007): Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 7:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nougaret S, Ravel S (2015): Modulation of Tonically Active Neurons of the Monkey Striatum by Events Carrying Different Force and Reward Information. J Neurosci. 35:15214–15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee IH, Seitz AR, Assad JA (2006): Activity of tonically active neurons in the monkey putamen during initiation and withholding of movement. J Neurophysiol. 95:2391–2403. [DOI] [PubMed] [Google Scholar]
- 39.Ravel S, Legallet E, Apicella P (2003): Responses of tonically active neurons in the monkey striatum discriminate between motivationally opposing stimuli. J Neurosci. 23:8489–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H (2004): Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 43:133–143. [DOI] [PubMed] [Google Scholar]
- 41.Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H (2008): Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 28:11673–11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apicella P, Scarnati E, Schultz W (1991): Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res. 84:672–675. [DOI] [PubMed] [Google Scholar]
- 43.Shimo Y, Hikosaka O (2001): Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J Neurosci. 21:7804–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura M, Rajkowski J, Evarts E (1984): Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci U S A. 81:4998–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apicella P (2007): Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 30:299–306. [DOI] [PubMed] [Google Scholar]
- 46.Aosaki T, Kimura M, Graybiel AM (1995): Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 73:1234–1252. [DOI] [PubMed] [Google Scholar]
- 47.Bray S, Rangel A, Shimojo S, Balleine B, O’Doherty JP (2008): The neural mechanisms underlying the influence of pavlovian cues on human decision making. J Neurosci. 28:5861–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prévost C, Liljeholm M, Tyszka JM, O’Doherty JP (2012): Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: a high-resolution fMRI study. J Neurosci. 32:8383–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allman MJ, DeLeon IG, Cataldo MF, Holland PC, Johnson AW (2010): Learning processes affecting human decision making: An assessment of reinforcer-selective Pavlovian-to-instrumental transfer following reinforcer devaluation. J Exp Psychol Anim Behav Process. 36:402–408. [DOI] [PubMed] [Google Scholar]
- 50.Nadler N, Delgado MR, Delamater AR (2011): Pavlovian to instrumental transfer of control in a human learning task. Emotion. 11:1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trick L, Hogarth L, Duka T (2011): Prediction and uncertainty in human Pavlovian to instrumental transfer. J Exp Psychol Learn Mem Cogn. 37:757–765. [DOI] [PubMed] [Google Scholar]
- 52.Martinovic J, Jones A, Christiansen P, Rose AK, Hogarth L, Field M (2014): Electrophysiological responses to alcohol cues are not associated with Pavlovian-to-instrumental transfer in social drinkers. PLoS One. 9:e94605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lovibond PF, Colagiuri B (2013): Facilitation of voluntary goal-directed action by reward cues. Psychological Science. 24:2030–2037. [DOI] [PubMed] [Google Scholar]
- 54.Seabrooke T, Le Pelley ME, Hogarth L, Mitchell CJ (2017): Evidence of a goal-directed process in human Pavlovian-instrumental transfer. J Exp Psychol Anim Learn Cogn. 43:377–387. [DOI] [PubMed] [Google Scholar]
- 55.Lehner R, Balsters JH, Herger A, Hare TA, Wenderoth N (2016): Monetary, Food, and Social Rewards Induce Similar Pavlovian-to-Instrumental Transfer Effects. Front Behav Neurosci. 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. (2011): Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 72:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, et al. (2018): Altered Baseline and Nicotine-Mediated Behavioral and Cholinergic Profiles in ChAT-Cre Mouse Lines. J Neurosci. 38:2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins AL, Aitken TJ, Greenfield VY, Ostlund SB, Wassum KM (2016): Nucleus Accumbens Acetylcholine Receptors Modulate Dopamine and Motivation. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wassum KM, Ostlund SB, Balleine BW, Maidment NT (2011): Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 18:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wassum KM, Ostlund SB, Loewinger GC, Maidment NT (2013): Phasic Mesolimbic Dopamine Release Tracks Reward Seeking During Expression of Pavlovian-to-Instrumental Transfer. Biol Psychiatry. 73:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, et al. (2017): Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malvaez M, Shieh C, Murphy M, Greenfield V, Wassum K (2018): Distinct cortical-amygdala projections drive reward value encoding and retrieval. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabachnick BG, Fidell LS, Osterlind SJ (2001): Using multivariate statistics.
- 64.Bennett BD, Wilson CJ (1999): Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 19:5586–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson CJ, Goldberg JA (2006): Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol. 95:196–204. [DOI] [PubMed] [Google Scholar]
- 66.Jones IW, Bolam JP, Wonnacott S (2001): Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol. 439:235–247. [DOI] [PubMed] [Google Scholar]
- 67.Exley R, Cragg SJ (2008): Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 153 Suppl 1:S283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ (2008): Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 33:2158–2166. [DOI] [PubMed] [Google Scholar]
- 69.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012): Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 75:58–64. [DOI] [PubMed] [Google Scholar]
- 70.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. (2012): Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rice ME, Cragg SJ (2004): Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 7:583–584. [DOI] [PubMed] [Google Scholar]
- 72.Zhou FM, Liang Y, Dani JA (2001): Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 4:1224–1229. [DOI] [PubMed] [Google Scholar]
- 73.Berridge KC (2007): The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl). 191:391–431. [DOI] [PubMed] [Google Scholar]
- 74.Aitken TJ, Greenfield VY, Wassum KM (2016): Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J Neurochem. 136:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saunders BT, Richard JM, Margolis EB, Janak PH (2018): Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci. 21:1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lex A, Hauber W (2008): Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 15:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cover KK, Gyawali U, Kerkhoff WG, Patton MH, Mu C, White MG, et al. (2019): Activation of the Rostral Intralaminar Thalamus Drives Reinforcement through Striatal Dopamine Release. Cell Rep. 26:1389–1398.e1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collins AL, Greenfield VY, Bye JK, Linker KE, Wang AS, Wassum KM (2016): Dynamic mesolimbic dopamine signaling during action sequence learning and expectation violation. Sci Rep. 6:20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saunders BT, Robinson TE (2012): The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 36:2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peciña S, Berridge KC (2013): Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wyvell CL, Berridge KC (2000): Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 20:8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA (2009): Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 76:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Sulzer D (2004): Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 7:581–582. [DOI] [PubMed] [Google Scholar]
- 84.Threlfell S, Cragg SJ (2011): Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim SA, Kang UJ, McGehee DS (2014): Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rice ME, Cragg SJ (2004): Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 7:583–584. [DOI] [PubMed] [Google Scholar]
- 87.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, et al. (2015): Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koob GF (1999): Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 46:1167–1180. [DOI] [PubMed] [Google Scholar]
- 89.Koob GF, Bloom FE (1985): Corticotropin-releasing factor and behavior. Fed Proc. 44:259–263. [PubMed] [Google Scholar]
- 90.Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, et al. (2012): Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 490:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, et al. (1993): The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 172:277–289; discussion 290–275. [DOI] [PubMed] [Google Scholar]
- 92.Lemos J, Shin J, Ingebretson A, Dobbs L, Alvarez V (2018): Cholinergic interneurons as a novel target of CRF in the striatum that is spared by repeated stress. BioRxiv. [Google Scholar]
- 93.Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG (2012): Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 206:155–166. [DOI] [PubMed] [Google Scholar]
- 94.Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013): Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 501:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Virk MS, Sagi Y, Medrihan L, Leung J, Kaplitt MG, Greengard P (2016): Opposing roles for serotonin in cholinergic neurons of the ventral and dorsal striatum. Proc Natl Acad Sci U S A. 113:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olney JJ, Warlow SM, Naffziger EE, Berridge KC (2018): Current perspectives on incentive salience and applications to clinical disorders. Curr Opin Behav Sci. 22:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinson TE, Berridge KC (1993): The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 18:247–291. [DOI] [PubMed] [Google Scholar]
- 98.Hikida T, Kitabatake Y, Pastan I, Nakanishi S (2003): Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 100:6169–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, et al. (2001): Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci U S A. 98:13351–13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Treadway MT, Zald DH (2013): Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr Dir Psychol Sci. 22:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paxinos G, Watson C (1998): The rat brain in stereotaxic coordinates. 4th ed.: Academic Press. [DOI] [PubMed] [Google Scholar]
- 102.Stefanik MT, Kalivas PW (2013): Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malvaez M, Greenfield VY, Wang AS, Yorita AM, Feng L, Linker KE, et al. (2015): Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci Rep. 5:12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016): DREADDS: Use and application in behavioral neuroscience. Behav Neurosci. 130:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wassum KM, Ostlund SB, Maidment NT, Balleine BW (2009): Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 106:12512–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corrigall WA, Coen KM, Adamson KL (1994): Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653:278–284. [DOI] [PubMed] [Google Scholar]
- 107.Wassum KM, Tolosa VM, W J, Walker E, Monbouquette HG, Maidment NT (2008): Silicon Wafer-Based Platinum Microelectrode Array Biosensor for Near Real-Time Measurement of Glutamate In Vivo. Sensors. 8:5023–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wassum KM, Tolosa VM, Tseng TC, Balleine BW, Monbouquette HG, Maidment NT (2012): Transient Extracellular Glutamate Events in the Basolateral Amygdala Track Reward-Seeking Actions. J Neurosci. 32:2734–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burmeister JJ, Moxon K, Gerhardt GA (2000): Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 72:187–192. [DOI] [PubMed] [Google Scholar]
- 110.Giuliano C, Parikh V, Ward JR, Chiamulera C, Sarter M (2008): Increases in cholinergic neurotransmission measured by using choline-sensitive microelectrodes: enhanced detection by hydrolysis of acetylcholine on recording sites? Neurochem Int. 52:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP (2004): Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 20:1545–1554. [DOI] [PubMed] [Google Scholar]
- 112.Parikh V, Sarter M (2006): Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. Journal of neurochemistry. 97:488–503. [DOI] [PubMed] [Google Scholar]
- 113.Wassum KM, Tolosa VM, Wang J, Walker E, Monbouquette HG, Maidment NT (2008): Silicon Wafer-Based Platinum Microelectrode Array Biosensor for Near Real-Time Measurement of Glutamate in Vivo. Sensors (Basel). 8:5023–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hersman S, Cushman J, Lemelson N, Wassum K, Lotfipour S, Fanselow MS (2017): Optogenetic excitation of cholinergic inputs to hippocampus primes future contextual fear associations. Sci Rep. 7:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marshall AT, Ostlund SB (2018): Repeated cocaine exposure dysregulates cognitive control over cue-evoked reward-seeking behavior during Pavlovian-to-instrumental transfer. Learn Mem. 25:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halbout B, Marshall A, Azimi A, Liljeholm M, Mahler S, Wassum K, et al. Ventral tegmental dopamine inputs to the nucleus accumbens mediates cue-triggered motivation but not reward expectancy. BioRxiv. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.