Abstract
The revised WHO classification newly defined the entities “High‐grade B‐cell lymphoma with MYC and BCL2, and/or BCL6 rearrangements (HGBL‐DH/TH)” and “HGBL, NOS.” Standard immunochemotherapy for diffuse large B‐cell lymphoma (DLBCL), R‐CHOP, is insufficient for HGBL patients, and there are currently no optimized therapeutic regimens for HGBL. We previously reported that CCND3, which encodes cyclin D3, harbored high mutation rates in Burkitt lymphoma (BL), HGBL and a subset of DLBCL. Furthermore, the knockdown of cyclin D3 expression was toxic to germinal center (GC)‐derived B‐cell lymphomas. Thus, the fundamental function of cyclin D3 is important for the pathogenesis of GC‐derived B‐cell lymphoma. We herein used two structurally different CDK4/6 inhibitors, palbociclib and abemaciclib, and examined their suppressive effects on cell proliferation and their ability to induce apoptosis in various aggressive B‐cell lymphoma cell lines. The results obtained demonstrated that abemaciclib more strongly suppressed cell proliferation and induced apoptosis in GC‐derived B‐cell lymphoma cell lines than the control, but only slightly inhibited those features in activated B‐cell (ABC)‐like DLBCL cell lines. Palbociclib exerted partial or incomplete effects compared with the control and the effect was intermediate between abemaciclib and the control. Moreover, the effects of abemaciclib appeared to depend on cyclin D3 expression levels based on the results of the expression analysis of primary aggressive B‐cell lymphoma samples. Therefore, abemaciclib has potential as a therapeutic agent for aggressive GC‐derived B‐cell lymphomas.
Keywords: B‐cell lymphoma, CDK4/6 inhibitor, cyclin D3, high‐grade B‐cell lymphoma, MYC
The effects of CDK4/6 inhibitors on cell proliferation in aggressive B‐cell lymphoma cell lines were investigated. Abemaciclib completely suppressed cell growth irrespective of the CCND3 mutation status in GCB‐like DLBCL cell lines. Abemaciclib has potential as a therapeutic agent for aggressive GC‐derived B‐cell lymphomas, including high‐grade B‐cell lymphomas.

1. INTRODUCTION
MYC‐positive aggressive B‐cell lymphoma has a broad spectrum in the current WHO classification, and includes Burkitt lymphoma (BL), high‐grade B‐cell lymphoma (HGBL) with MYC and BCL2, and/or BCL6 rearrangements (HGBL‐DH/TH), and diffuse large B‐cell lymphoma (DLBCL).1 The WHO classification newly listed HGBL‐DH/TH and HGBL, NOS, and these two categories have very poor outcomes with aggressive clinical courses. Although some clinical trials have been conducted to establish standard therapeutic strategies, including intensified protocols, there are currently no optimized therapeutic regimens, including hematopoietic cell transplantation (HCT), for HGBL patients.2 Furthermore, because the mean age of HGBL patients is greater than 60 years, intense immunochemotherapy is often not tolerated by these patients. Massive parallel sequencing technology recently revealed the genetic landscape of MYC‐positive aggressive B‐cell lymphomas, including HGBL, and recurrent mutations, such as ID3, TCF3, MYC, TP53, SMARCA4 and CCDN3, were identified in BL, HGBL and DLBCL cases.3, 4, 5, 6
CCND3 is a member of the D‐type cyclin gene family and forms a complex with CDK4/6, which phosphorylates RB1, leading to the release of E2F and subsequent promotion of cell cycle progression in the early G1‐to‐S phase.7, 8 CCND3 mutations enhance proliferation and stabilize the mutated cyclin D3 protein.5 Furthermore, the formation of germinal centers (GC) was shown to be markedly impaired in Ccnd3 null mice.9 These findings indicate that cyclin D3 plays important roles in both normal GC formation and GC B‐cell (GCB)‐derived lymphomagenesis.
In current clinical practice, three CDK4/6 inhibitors, including palbociclib, ribociclib, and abemaciclib, are approved for clinical use in the USA and other countries.10 Although these agents are used in the treatment of estrogen receptor (ER)‐positive breast cancer, the clinical outcome for malignant lymphoma has not been established. Mantle cell lymphoma (MCL) has been proposed as a good candidate disease entity due to its overexpression of cyclin D1 and is the only disease entity that is currently in an ongoing clinical trial on CDK4/6 inhibitors.11
We herein examined the effects of two CDK4/6 inhibitors, palbociclib and abemaciclib, on cell proliferation and the induction of apoptosis, and the results obtained demonstrated that abemaciclib, but not palbociclib, completely suppressed cell proliferation and induced apoptosis in GCB‐derived aggressive B‐cell lymphoma cell lines, including HGBL‐DH cell lines. These results suggest the potential of abemaciclib as a therapeutic reagent for HGBL‐DH cases that have a poor outcome.
2. MATERIALS AND METHODS
2.1. Cell culture and drug treatment
The following cell lines were used: Burkitt lymphoma (BL2, 29, 30, 41, 64, 65, 67, 70, 74, Gumbus, Namalwa and Raji), HGBL, DHL (SU‐DHL 4, 6 and 10),12 DLBCL, GCB type (HT, OCI‐Ly7, SU‐DHL 5, 8 and 16) and DLBCL, ABC type (HBL‐1, SUDHL2 and OCI‐Ly10). SU‐DHL 2, 4, 5, 6, 8, 10 and 16 were purchased from ATCC. BL2, 30, 41, 70, Raji, Gumbus, Namalwa and OCI‐Ly10 were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). HBL‐1 was kindly provided by Dr Yuko Hashimoto (Fukushima). BL64, 65, 67, and 74 were generously provided by Dr Georg Bornkamm (München, Germany). BL2, 29, 30, 41, 64, 65, 67 and 74 were maintained in IMDM medium 1640 containing 10% FBS (purchased from PAN Biotech, Aidenbach, Germany or Sigma‐Aldrich Japan), 1% penicillin/streptomycin, 50 μmol/L 1‐thioglycerol (Sigma, M6145) and 20 nmol/L bathocuproinedisulfonic acid (Sigma, B1125) at 37°C under 5% CO2. Other cell lines were maintained in RPMI1640 (Gibco, Life Technologies) containing 10%‐20% heat‐inactivated FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Two CDK4/6 inhibitors, palbociclib (PD0332991) and abemaciclib (LY2835219), were obtained from Sigma‐Aldrich Japan and AdooQ BioScience, respectively. Both compounds were dissolved in DMSO.
2.2. Antibodies and western blot analysis
Cells were lysed in RIPA buffer (1% Triton X‐100/1% sodium deoxycholate/0.1% NaDodSO4/150 mmol/L NaCl/10 mmol/L Tris HCl, pH 7.2) followed by centrifugation at 12 000 × g at 4°C for 30 minutes. Protein concentrations were measured with Bio‐Rad Protein Assay Dye Reagent (Bio‐Rad). Western blots were performed on SDS‐PAGE gels of appropriate concentrations, followed by immunodetection using CDK4 (DCS156), CDK6 (DCS83), cyclin D1 (DCS6), cyclin D2 (D52F9), cyclin D3 (DCS22), RB1 (4H1), phospho‐RB1 (S780) (D59B7) and β‐tubulin (9F3), which were all purchased from Cell Signaling Technology. Relative protein expression was analyzed by Image Lab Software (Bio‐Rad).
2.3. Sanger sequencing
In CCND3 mutation screening, the hot spot region of CCND3 (exon 5) was amplified by PCR and PCR products were sequenced with the Big Dye Terminator Cycle Sequencing Kit (Life Technologies) using the 3130xl Genetic Analyzer (Life Technologies). Primers were designed using Primer 3 and are described in Table S1. The amino acid positions and substitutions of the CCND3 protein are shown in Table S2 according to protein accession NP_001751.
2.4. Cell proliferation assay, cell cycle analysis and apoptosis assays
Cells were seeded at an optimized density (0.5‐2.0 × 105/mL) with a concentration of 0.5 μmol/L for both inhibitors and control DMSO and then examined using cell viability, cell cycle and apoptosis assays. In the cell proliferation assay, cells were counted with a Countess II FL Automated Cell Counter (Thermo Fisher Scientific). In the cell cycle analysis, cells were fixed in 70% ethanol, washed with PBS and labeled with propidium iodide (Sigma–Aldrich) as previously described.13 Samples were then run on a BD FACSVerse flow cytometer (Becton‐Dickinson), and the percentages of cells within each phase of the cell cycle were analyzed using BD FACSuite software (Becton‐Dickinson). In the apoptosis assay, cells were stained with Annexin V‐FITC and propidium iodide and also analyzed using BD FACSuite software.
2.5. ROS measurements
The OxiSelect In Vitro ROS Assay Kit (cat. STA‐347, Cell Biolabs) was used to measure ROS in cell lines according to the manufacturer's protocol. Green fluorescence was detected using the Varioskan LUX multimode microplate reader (Thermo Fisher Scientific).
2.6. Gene expression analysis
We analyzed two independent microarray datasets that included both BL and DLBCL (the Hummel dataset, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4475, and the Dave dataset, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4732),14, 15 and intermediate B‐cell lymphomas from the Hummel dataset. In both datasets, data regarding morphological and molecular diagnoses were collected directly from the GSE file. Gene probes were identified based on the reported platform data: CDK4 (202246_s_at), CDK6 (207143_at for Hummel et al and 224851_at for Dave et al), CYCLIN D1 (208712_at for Hummel et al and 208711_s_at for Dave et al), CYCLIN D2 (200953_s_at for Hummel et al and 200951_s_at for Dave et al) and CYCLIN D3 (201700_at) transcript levels among molecular BL (mBL), intermediate BL/DLBCL, and DLBCL (GCB, unclassifiable and ABC type) in data from Hummel et al, and BL and DLBCL (GCB, unclassifiable and ABC type) in data from Dave et al. We compared each gene's expression level among aggressive B‐cell lymphoma subtypes according to the former WHO classification criteria (WHO 2008) for the following reasons. “Intermediate between BL and DLBCL (BCLU)” is described as synonymous with high‐grade B‐cell lymphoma (including HGBL‐DH/TH and HGBL, NOS) according to the current WHO classification and many BCLU cases correspond to HGBL. However, a subset of morphologically diagnosed DLBCL also correspond to HGBL‐DH/TH in the presence of MYC and BCL2, and/or BCL6 rearrangements in the current WHO classification. In addition, in some cases the diagnosis was indefinite, such as for aggressive B‐cell lymphoma. Thus, we cannot re‐diagnose all cases using the current criteria of WHO classification. Therefore, we applied the original criteria that were used in both data sets (mBL, intBL/DLBCL and DLBCL). The relationships between gene expression and morphological and molecular diagnoses were evaluated using the Kruskal‐Wallis rank‐sum test.
2.7. Statistical analysis
P‐values < 0.05 were considered to be significant. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, ver.1.33).16
3. RESULTS
3.1. Expression of CCNDs, CDKs and RB1 in aggressive B‐cell lymphoma cell lines
To assess the protein expression levels of CCND and CDK in aggressive B‐cell lymphoma cell lines, we performed an immunoblotting analysis of the BL, HGBL‐DH and DLBCL cell lines (Figure 1A). No significant differences were observed in the expression levels of CDK4 or CDK6 among each disease category. We did not detect cyclin D1 expression in any cell lines investigated in our panel. Regarding cyclin D2 expression, most cell lines were under the detection sensitivity limit, except for BL74, HT, HBL‐1 and OCI‐ly10. Concerning cyclin D3, the size of the cyclin D3 product differed from that of the wild‐type cyclin D3 product in some BL cell lines (BL41, 67, 70 and Gumbus). Because BL harbors a recurrent mutation in CCND3, we performed mutational screening on all of the cell lines described above and detected mutations in BL41 (S259fs), BL67 (A269fs), BL70 (K268fs) and Gumbus (T283A) (Table S2) (Protein Data Bank accession number NP_001751; the CCND3 mutations in BL41, BL70 and Gumbus were also described previously).5 Schmitz et al previously reported that a mutation in CCND3 provided protein stability along with oncogenic properties5; therefore, we compared the protein expression levels of CCND3 between wild‐type and mutated CCND3 in BL cell lines. The expression level of CCND3 was normalized with the amount of β‐tubulin. Cyclin D3 protein expression levels were significantly higher in CCND3‐mutated cell lines than in CCND3 wild‐type cell lines (Figure 1B, P = 0.0196, the Mann‐Whitney U test).
Figure 1.
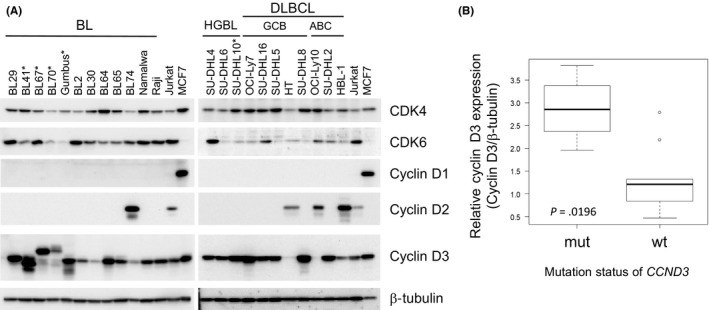
The protein expression of CCND, CDK4/6 and RB1 in aggressive B‐cell lymphoma cell lines. A, Whole cell lysates were subjected to a western blot analysis for cyclin D1, D2 and D3 and CDK 4 and 6. β‐tubulin was loaded as a control. The asterisk indicates the CCND3‐mutated cell line. HGBL, high‐grade B‐cell lymphoma with MYC and BCL2 rearrangements. Jurkat and MCF are shown as a reference. B, Cyclin D3 expression levels between CCND3 wild‐type and mutated‐type BL cell lines. The expression level of cyclin D3 normalized to total β‐tubulin is indicated (P = 0.0196, Mann‐Whitney U test)
To clarify the mutational spectrum of CCND in aggressive B‐cell lymphomas, we searched databases and previous literature 5, 6, 17, 18, 19, 20, 21, 22, 23 (Table S3). The mutation rates of CCND3 in BL ranged from 14.6% to 35.4%. Although only a few case series were examined for HGBL‐DH/TH, HGBL, NOS and BCL‐U, the mutation rates in these categories ranged from 14.3% to 33.3%. In DLBCL, the mutation rates of CCND1 and CCND2 in two large cohorts were less than 1%.15, 20 Furthermore, the mutation rates of CCND3 in DLBCL differed among subtypes defined by the cell of origin (COO). Mutation rates were generally higher in ABC‐type DLBCL (ABC‐like DLBCL) than in GCB‐type DLBCL (GCB‐like DLBCL) in each study.
3.2. Gene expression levels of CDK4/6 and CCND in primary aggressive B‐cell lymphoma cases
To investigate the expression levels of CDK4/6 and CCND in primary samples of aggressive B‐cell lymphoma, we analyzed two large cohorts that defined the expression profiles of BL and DLBCL (Figure 2A).14, 15 The gene expression level of CDK4 was significantly higher in molecular BL (mBL) than in either type of other categories: DLBCL (GCB, ABC and the unclassified [Un] type) in both datasets or B‐cell lymphoma unclassifiable, intermediate between BL and DLBCL (BCLU) in the Hummel dataset (P < 0.001, the Kruskal‐Wallis test). However, the expression level of CDK6 did not significantly differ among each category in both studies.
Figure 2.
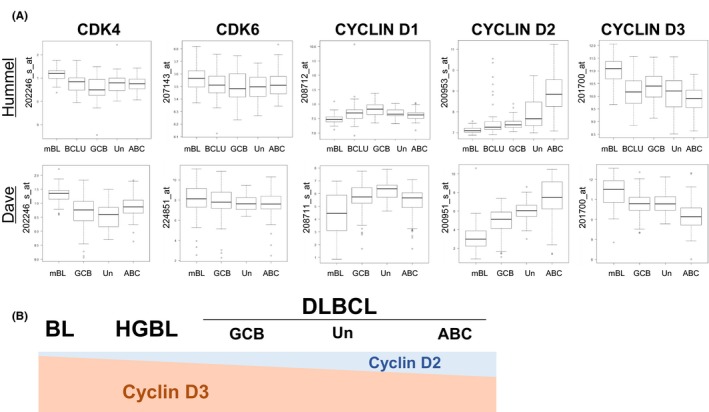
Gene expression levels of CCND and CDK4/6 from microarray data. A, Microarray data from two independent studies were analyzed to compare CDK4 and 6 and cyclin D1, D2 and D3 transcript levels among mBL, intermediate BL/DLBCL and DLBCL (GCB, unclassifiable and ABC type) in data from Hummel et al (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4475), and BL and DLBCL (GCB, unclassifiable and ABC type) in data from Dave et al (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4732). Normalized log2 expression was indicated for all gene sets. Gene probes are indicated on the right side. B, Schematic representation of cyclin D2 and cyclin D3 gene expression levels among aggressive B‐cell lymphomas. ABC, activated B‐cell‐like type; BCLU, B‐cell lymphoma unclassifiable, intermediate between BL and DLBCL; DLBCL, diffuse large B‐cell lymphoma; GCB, germinal center B‐cell‐like type; HGBL, high‐grade B‐cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements; mBL, molecular Burkitt lymphoma; Un, unclassifiable type
In CCND, expression differed among cyclin D1, D2 and D3 in primary aggressive B‐cell lymphoma cases. The lowest expression level of cyclin D1 was observed in mBL among all groups in both datasets. However, there was no common group other than mBL that showed significant gene expression differences in cyclin D1 between the Hummel and Dave datasets. The lowest gene expression level of cyclin D2 was observed in mBL in both datasets and the highest gene expression level of cyclin D2 was noted in ABC‐like DLBCL. There were significant gene expression differences among groups, except for the relationship between BCLU and GCB‐like DLBCL in cyclin D2. In contrast, the highest gene expression level of cyclin D3 was observed in mBL, while the lowest was noted in ABC‐like DLBCL. The gene expression level of cyclin D3 in GCB‐like DLBCL was intermediate between mBL and ABC‐type DLBCL in both datasets. Among the expression of CCND, a reverse expression pattern was found between cyclin D2 and cyclin D3. Furthermore, an expression analysis of cell lines indicated that cell lines with detectable cyclin D2 expression levels had lower cyclin D3 expression levels than those with undetectable cyclin D2 expression levels (Figure 1A). These results supported microarray data obtained from primary samples. The expression of cyclin D3 gradually decreased from mBL to DLBCL, and the same result was observed between two different COO: from GCB to the ABC type.
Therefore, a common feature of both datasets was that CDK4 expression levels were higher in mBL than in other groups and no significant difference was observed for CDK6. The expression level of cyclin D3 was inversely proportional to that of cyclin D2, suggesting that each gene compensates for the expression of other genes (Figure 2B).
3.3. Growth suppressive effects of CDK4/6 inhibitors on aggressive B‐cell lymphoma cell lines
The higher expression levels of CDK4/6 and cyclin D in malignant neoplasms are effective targets for CDK4/6 inhibitors.8 Although a small number of in vitro and in vivo studies reported the utility of CDK4/6 inhibitors for aggressive B‐cell lymphomas,5, 17, 24 a systemic evaluation of the effects of different CDK4/6 inhibitors on aggressive B‐cell lymphoma cell lines has not yet been conducted. Therefore, we examined the effects of palbociclib and abemaciclib, two structurally different CDK4/6 inhibitors, on aggressive B‐cell lymphoma cell lines. Although previous findings showed that palbociclib suppressed cell growth and induced apoptosis in aggressive B‐cell lymphoma cell lines, the concentration of CDK4/6 inhibitors applied was mainly 1 μmol/L or higher,5 which is higher than tolerated blood concentrations. Therefore, we set the concentration of palbociclib to 0.5 μmol/L, which is still higher than the maximum tolerated dose but lower than that in previous studies.5 Furthermore, the concentration of abemaciclib was set to 0.5 μmol/L which was the maximum tolerated blood concentration.
We initially investigated growth suppressive effects using a treatment with palbociclib and abemaciclib.
Abemaciclib completely suppressed growth in all lines irrespective of the CCND3 mutation status, except for three ABC‐like DLBCL cell lines (HBL‐1, SU‐DHL2 and OCI‐Ly10) (Figure 3A,B). However, no cell lines showed complete growth suppression following the addition of palbociclib. Palbociclib exerted weaker effects than abemaciclib in all cell lines (Figure 3B). Moreover, we compared the effects of CDK4/6 inhibitors on each cell line subtype. No significant differences were observed in the suppression of cell proliferation in BL cell lines between the CCND3‐mutated group and CCND3 wild‐type group (Figure 3C). Similar results were obtained for HGBL cell lines and GCB‐like DLBCL cell lines. However, suppressive effects on cell proliferation were slightly weaker in ABC‐like DLBCL cell lines than in other cell lines. When wild‐type CCND3 and mutated CCND3 in BL cell lines were combined and compared with other cell lines, similar suppressive effects on cell proliferation were observed among GCB‐derived aggressive lymphoma cell lines (BL, HGBL and GCB‐like DLBCL) (Figure 3D).
Figure 3.
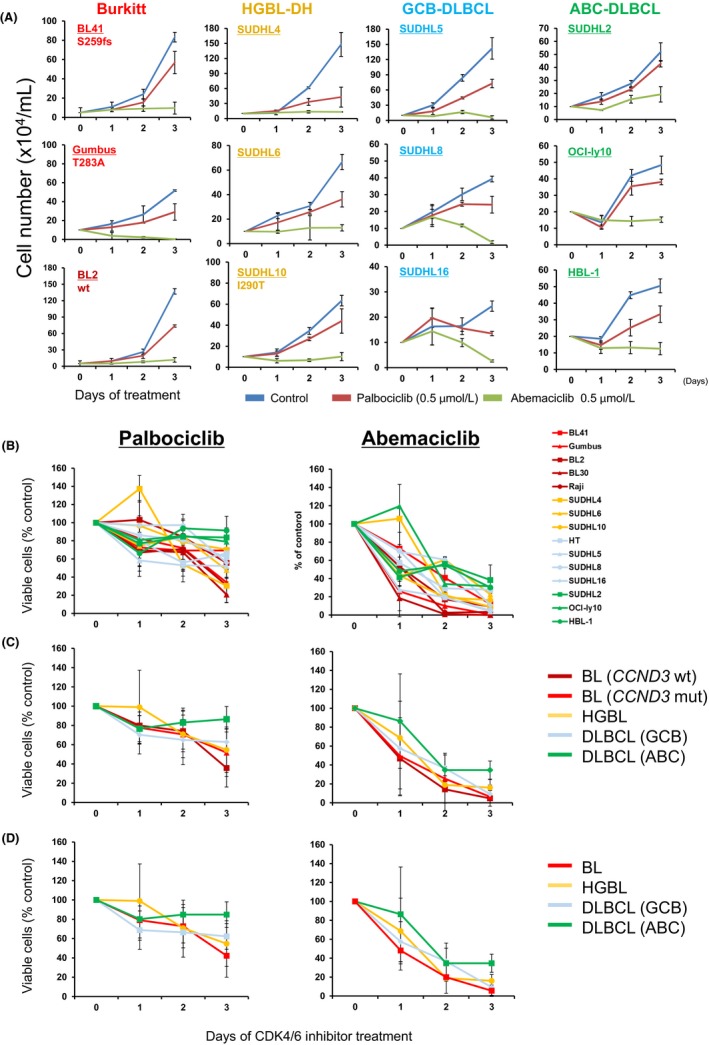
Effects of CDK4/6 inhibitors on cell proliferation in aggressive B‐cell lymphoma cell lines. A, Treatment of aggressive B‐cell lymphoma cell lines with two CDK4/6 inhibitors, palbociclib (0.5 μmol/L) and abemaciclib (0.5 μmol/L), or vehicle control DMSO for 1‐3 d and subsequent evaluations. Data are representative of triplicate experiments. B, The fraction of viable cells relative to CDK4/6 inhibitor non–treated cells is shown at the indicated times and normalized to control viable cells on each day. C and D, Data from (B) were summarized by each category, indicated on the right side
We then investigated the effects of these inhibitors on cell cycles (Figure 4A,B). Abemaciclib induced cell cycle arrest in all cell lines examined. However, the effects of palbociclib on the cell cycle differed among cell lines. Palbociclib did not affect the cell cycle in BL41 (CCND3‐mutated) or SUDHL4. The other cell lines showed a partial response or similar trend for abemaciclib compared with the control and this tendency did not depend on the expression of either cyclin D3 or CDK4/6. Because RB1 is a major target of cyclin D‐CDK4/6 and the direct phosphorylation of RB1 by cyclin D‐CDK4/6 inactivates RB1, which ultimately induces early G1‐to‐S transition in the cell cycle, we performed immunoblotting to analyze RB1 expression and its phosphorylation status. Consistent with growth suppression and the induction of cell cycle arrest (Figures 3A,B and 4B), abemaciclib induced a decrease in RB1 expression and the suppression of RB1 phosphorylation (Ser780) (Figure 5C). The expression level and phosphorylation status of RB1 following the treatment with palbociclib were similar to those with the control in all cell lines.
Figure 4.
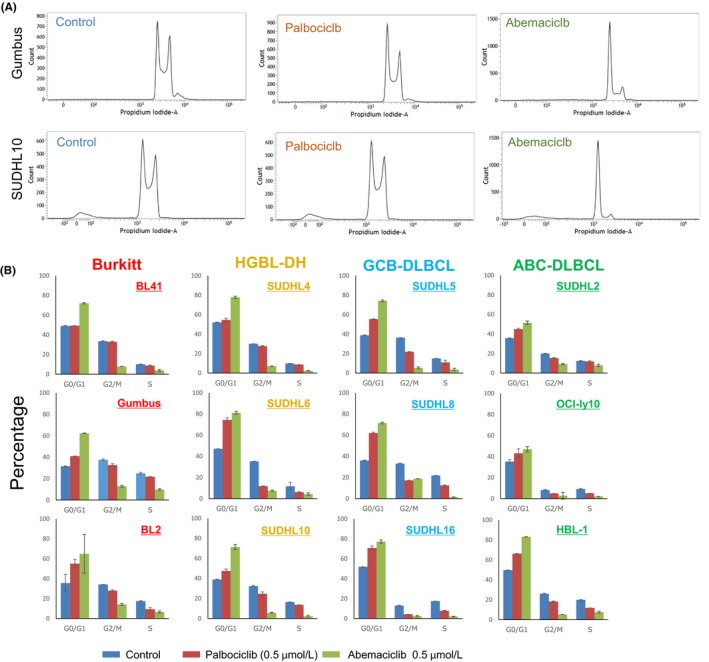
Effects of CDK4/6 inhibitors on cell cycles in aggressive B‐cell lymphoma cell lines. A, Representative data from a cell cycle analysis 24 h after the addition of palbociclib (0.5 μmol/L) and abemaciclib (0.5 μmol/L) or DMSO controls. B, A cell cycle analysis of aggressive B‐cell lymphoma cell lines was performed at 24 h. Data are representative of triplicate experiments
Figure 5.
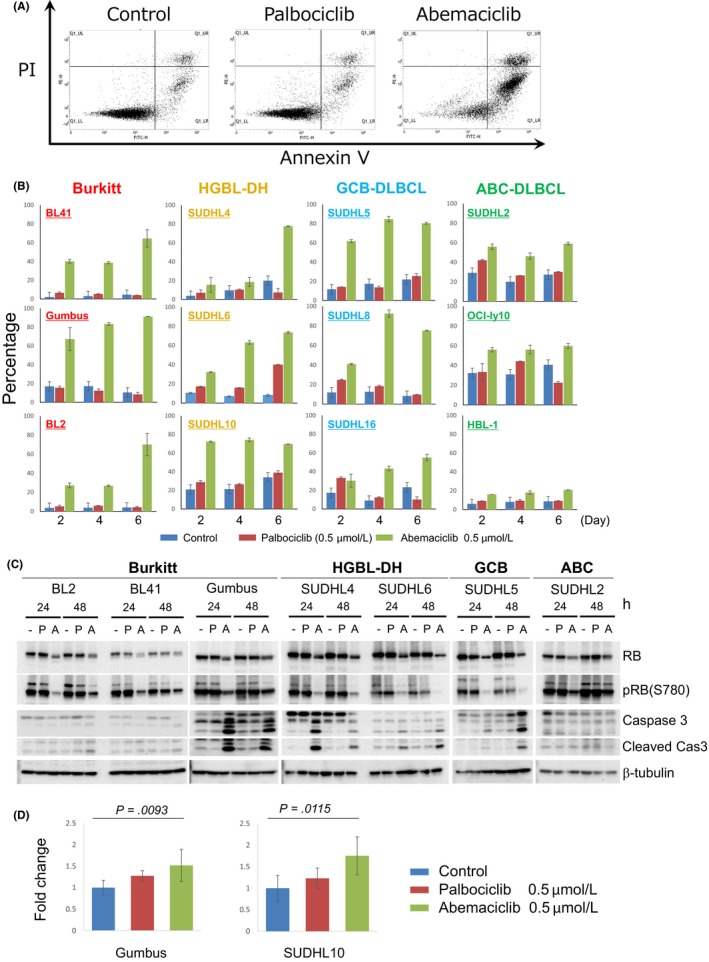
Effects of CDK4/6 inhibitors on apoptosis in aggressive B‐cell lymphoma cell lines. A, Representative data from an apoptosis induction analysis 48 h after the addition of palbociclib (0.5 μmol/L), abemaciclib (0.5 μmol/L) and DMSO controls. B, The proportion (%) of apoptosis‐induced cells treated with CDK4/6 inhibitors and DMSO controls was indicated for 2, 4 and 6 d after the treatment. C, The expression level of RB1 and the phosphorylation status of RB1 (Ser780) were indicated by immunoblotting 24 and 48 h after the treatment with palbociclib (P) (0.5 μmol/L) and abemaciclib (A) (0.5 μmol/L) or DMSO controls (−) in each cell line. In the evaluation of the induction of apoptosis, the expression levels of caspase 3 and induced cleaved caspase 3 were indicated at the same time points described above. β‐tubulin was indicated as a loading control. D, Relative levels of ROS production in Gumbus and SUDHL10 upon treatment with CDK4/6 inhibitors and DMSO controls are indicated. Fold changes were calculated relative to CDK4/6 inhibitor non–treated controls
3.4. Effects of CDK4/6 inhibitors on the induction of apoptosis in aggressive B‐cell lymphoma cell lines
We investigated the effects of CDK4/6 inhibitors on the induction of apoptosis. The apoptotic fraction detected by flow cytometry was strongly induced by abemaciclib in all cell lines by day 6 (Figure 5B). Some cell lines (BL2, 41, Gumbus, SUDHL5 and SUDHL 10) showed early apoptosis by day 2. Consistent with the modest effects on growth suppression and the induction of cell cycle arrest of abemaciclib in ABC‐like DLBCL cell lines (HBL‐1, SUDHL2 and OCI‐ly10), the induction of apoptosis by abemaciclib treatment was also partial compared with GCB‐derived cell lines. In contrast, the induction of apoptosis by palbociclib was weaker or negligible. We also assessed the expression status of caspase 3 and the expression level of cleaved caspase 3 (Figure 5C). Consistent with abemaciclib‐induced apoptosis detected by flow cytometry, the cleavage of caspase 3 and induction of cleaved caspase 3 were observed in the cell lines of Burkitt (BL2, 41 and Gumbus), HGBL (SUDHL4 and 6) and GCB‐type DLBCL (SUDHL5); however, we did not detect cleaved caspase 3 in an ABC‐type DLBCL cell line (SUDHL2). Furthermore, palbociclib did not significantly cleave caspase 3 in the cell lines examined.
Collectively, these results demonstrated that abemaciclib strongly suppressed cell growth and induced apoptosis in GCB‐derived lymphoma cell lines, and to a lesser extent in ABC‐type DLBCL. Palbociclib exerted only partial or no effects in all cell lines examined.
Wang et al reported that the cyclin D3/CDK6 complex phosphorylated 6‐phosphofructokinase (PFK1) and pyruvate kinase M2 (PKM2) in T‐cell acute lymphoblastic leukemia (T‐ALL) cell lines,25 and the inhibition of CDK6 by palbociclib depleted the levels of the antioxidants NADPH and glutathione, increased ROS and, ultimately, induced apoptosis. Therefore, we speculated that the overexpression of cyclin D3 in GCB‐derived B‐cell lymphoma (BL, HGBL and GCB‐like DLBCL) in combination with CDK6 expression may modulate metabolic pathways and, thus, we measured ROS production in two representative cell lines (Gumbus and SUDHL10). Abemaciclib increased ROS levels over those with the control in both cell lines (P < 0.05, Bonferroni) (Figure 5D). These results indicated that the ability to induce apoptosis by CDK4/6 inhibitors in GCB‐derived cell lines was strong due to ROS production via metabolic reprogramming.
4. DISCUSSION
The revised WHO classification (4th edition) newly describes the disease entity “High‐grade B‐cell lymphoma (HGBL)” and differentiates this disease entity from preexisting related WHO entities because of its poor outcome. Although several clinical trials on HGBL‐DH/TH are ongoing, there is currently no established therapeutic regimen and an intensified chemotherapeutic regimen other than R‐CHOP may be preferable for these patients due to the aggressive clinical course of HGBL‐DH/TH.26 Furthermore, because the median age of HGBL‐DH/TH patients is greater than 60 years,1 high dose chemotherapy may not be tolerated by some of these patients due to their advanced age. Therefore, a specific and tolerable therapeutic regimen based on the pathophysiology of HGBL‐DH/TH is needed.
Most HGBL cases are derived from GC‐originated B cells, and GC‐originated aggressive B‐cell lymphoma cells are addicted to cyclin D3.5 However, previous studies that validated CDK4/6 inhibitors administered palbociclib concentrations that were two‐fold higher than the tolerated blood concentration.27, 28 We herein obtained results from experiments that are applicable to clinical settings by adjusting the doses of CDK4/6 inhibitors administered to more tolerable blood concentrations.
The suppressive effects of palbociclib on cell growth in most cell lines were mild and weaker than those of abemaciclib even when its concentration in the cell culture medium was nearly two‐fold the tolerable concentration (Figures 3A‐D and 4B). Abemaciclib completely suppressed cell proliferation and markedly induced apoptosis. However, these effects of abemaciclib were limited to GCB‐derived B‐cell lymphoma cell lines and only partial responses were observed in ABC‐derived B‐cell lymphoma cell lines. These results may be explained by the expression level of cyclin D3 in aggressive B‐cell lymphoma cell lines and primary samples (Figures 1A and 2A). The expression level of cyclin D1 was generally lower in aggressive B‐cell lymphoma cases than those of cyclin D2 and cyclin D3. Furthermore, the expression levels of cyclin D2 and cyclin D3 were shown to be inversely related based on the GEP data of primary samples (Figure 2A,B). This phenomenon was observed in the cell lines examined in the present study (Figure 1A). A previous study reported that BCL6 suppressed the gene expression of CCND2 and induced that of CCND3 9, 29; therefore, the presence of an inverse expression relationship in the spectrum of aggressive B‐cell lymphomas was expected. Although the effects of abemaciclib mainly depended on the expression level of CCND3, we cannot exclude the possibility that cyclin D2 can increase the reduced expression level of cyclin D3 that provides the compensatory effects of CDK4/6 inhibitors in aggressive B‐cell lymphoma cell lines. Nevertheless, because the expression level of cyclin D2 was lower than that of cyclin D3, even in ABC‐like DLBCL cases, the effects of abemaciclib appear to mainly depend on the expression level of cyclin D3. Furthermore, the genetic analysis revealed that higher mutation rates of CCND3 were observed in ABC‐like DLBCL than in GCB‐like DLBCL (Table S2); however, cyclin D3 expression levels were significantly higher in GCB‐like DLBCL than in ABC‐like DLBCL. Abemaciclib also suppressed cell growth and induced apoptosis in BL and HGBL cell lines, irrespective of the CCND3 mutation status, suggesting that an investigation of the expression levels of CCND3 rather than a mutational analysis is more important for these disease entities. Collectively, the present results indicate that the expression level of cyclin D3 or COO of DLBCL rather than the mutational status of CCND3 is more important for the application of abemaciclib in the treatment of aggressive B‐cell lymphoma cases.
We herein demonstrated that CDK4/6 inhibitors, particularly abemaciclib, strongly induced apoptosis in GCB‐derived B‐cell lymphoma cell lines in combination with the suppression of cell proliferation, and also showed that the induction of apoptosis may be partially attributed to the production of ROS by CDK4/6 inhibitors (Figure 5D). CDK4/6 inhibitors generally exert their growth inhibitory effects in the presence of RB1; however, in T‐ALL cell lines, CDK4/6 inhibitors suppressed cell proliferation and induced apoptosis even though they did not express the RB1 protein.30 A recent study demonstrated that the cyclin D3‐CDK6 complex acted as a kinase against PFK1 and PKM2, which regulate the glycolytic pathway. Thus, high cyclin D3 and CDK6 expression levels increased the amounts of the glycolytic intermediates, NADPH and glutathione, and reduced ROS concentrations in T‐ALL cell lines.25 These findings suggest that metabolic modifications by high expression levels of cyclin D3‐CDK6 not only accelerate cell cycle division but also reprogram the glycolytic pathway to suppress ROS production and the induction of apoptosis. Common features between T‐ALL and BL/HGBL are high proliferation properties and strong cyclin D3 expression. Because BL and HGBL cell lines both express CDK6 and cyclin D3, similar metabolic modulations may be provided by the CDK6/cyclin D3 complex in BL and HGBL; however, genetic aberrations in the RB1 gene account for approximately 20%, and are not a frequent event in BL or HGBL.17
Although the present results showed strong suppressive effects on cell proliferation and induction of apoptosis by abemaciclib and, to a lesser extent, palbociclib, the underlying mechanisms remain unclear. There are currently three CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) that have been approved by the FDA. Palbociclib and ribociclib have similar structures and are specific to CDK4 and CDK6. Abemaciclib is structurally different from the former two CDK4/6 inhibitors and acts as a kinase inhibitor not only for CDK4/6 but also other substrates, such as cyclin T1/CDK9, cyclin E2/CDK2, p25/CDK5 and p35/CDK5.8 Therefore, the effects of abemaciclib may be attributed to inhibition of another kinase that can also suppress cell proliferation and induce apoptosis.
The present results showed that abemaciclib more effectively suppressed cell proliferation and induced apoptosis in GCB‐derived B‐cell lymphoma cell lines than palbociclib. These effects were more prominent in GCB‐derived B‐cell lymphomas than in ABC‐derived B‐cell lymphomas. Therefore, abemaciclib has potential as a therapeutic agent for aggressive GC‐derived B‐cell lymphomas, especially for HGBL, which has currently no standardized therapeutic regimen.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
The authors thank Kazuko Takaku and Chika Nakabayashi for their excellent technical support. This work was supported by Kamoda grant 001.
Tanaka Y, Momose S, Tabayashi T, et al. Abemaciclib, a CDK4/6 inhibitor, exerts preclinical activity against aggressive germinal center‐derived B‐cell lymphomas. Cancer Sci. 2020;111:749–759. 10.1111/cas.14286
Tanaka and Momose contributed equally to this work.
Andreas Rosenwald, Masahiro Kizaki and Jun‐ichi Tamaru share senior authorship.
REFERENCES
- 1. Swerdlow SH, Campo E, Harris NL, Pileri SA. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Revised, 4th edn Lyon, France: IARC; 2017. [Google Scholar]
- 2. Ohmoto A, Fuji S. Double‐hit and double‐expressor B‐cell lymphomas: Current treatment strategies and impact of hematopoietic cell transplantation. Adv Cell Gene Ther. 2018;1(2):e13. [Google Scholar]
- 3. Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richter J, Schlesner M, Hoffmann S, et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet. 2012;44:1316‐1320. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Momose S, Weissbach S, Pischimarov J, et al. The diagnostic gray zone between Burkitt lymphoma and diffuse large B‐cell lymphoma is also a gray zone of the mutational spectrum. Leukemia. 2015;29:1789‐1791. [DOI] [PubMed] [Google Scholar]
- 7. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558‐572. [DOI] [PubMed] [Google Scholar]
- 8. Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34:9‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peled JU, Yu JJ, Venkatesh J, et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 2010;20:631‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goel S, DeCristo MJ, McAllister SS, Zhao JJ. CDK4/6 inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol. 2018;28:911‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin P, Bartlett NL, Blum KA, et al. A phase 1 trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood. 2019;133:1201‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chong LC, Ben‐Neriah S, Slack GW, et al. High‐resolution architecture and partner genes of MYC rearrangements in lymphoma with DLBCL morphology. Blood Adv. 2018;2:2755‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sagawa M, Ohguchi H, Harada T, et al. Ribonucleotide reductase catalytic subunit M1 (RRM1) as a novel therapeutic target in multiple myeloma. Clin Cancer Res. 2017;23:5225‐5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2431‐2442. [DOI] [PubMed] [Google Scholar]
- 15. Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419‐2430. [DOI] [PubMed] [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bouska A, Bi C, Lone W, et al. Adult high‐grade B‐cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood. 2017;130:1819‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:1290‐1291. [DOI] [PubMed] [Google Scholar]
- 19. Ennishi D, Jiang A, Boyle M, et al. Double‐hit gene expression signature defines a distinct subgroup of germinal center B‐cell‐like diffuse large B‐cell lymphoma. J Clin Oncol. 2019;37:190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karube K, Enjuanes A, Dlouhy I, et al. Integrating genomic alterations in diffuse large B‐cell lymphoma identifies new relevant pathways and potential therapeutic targets. Leukemia. 2018;32:675‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481.e15‐494.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohde M, Bonn BR, Zimmermann M, et al. Relevance of ID3‐TCF3‐CCND3 pathway mutations in pediatric aggressive B‐cell lymphoma treated according to the non–Hodgkin Lymphoma Berlin‐Frankfurt‐Munster protocols. Haematologica. 2017;102:1091‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B‐cell lymphoma. N Engl J Med. 2018;378:1396‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caro P, Kishan AU, Norberg E, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Nicolay BN, Chick JM, et al. The metabolic function of cyclin D3‐CDK6 kinase in cancer cell survival. Nature. 2017;546:426‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies A. Double‐hit lymphoma: so what? Hematol Oncol. 2019;37(Suppl 1):19‐23. [DOI] [PubMed] [Google Scholar]
- 27. Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose‐escalation trial of the oral cyclin‐dependent kinase 4/6 inhibitor PD 0332991, administered using a 21‐day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568‐576. [DOI] [PubMed] [Google Scholar]
- 28. Tamura K, Mukai H, Naito Y, et al. Phase I study of palbociclib, a cyclin‐dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107:755‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaffer AL, Rosenwald A, Hurt EM, et al. Signatures of the immune response. Immunity. 2001;15:375‐385. [DOI] [PubMed] [Google Scholar]
- 30. Sawai CM, Freund J, Oh P, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


