Abstract
Systematic classification and determination of various cells in normal peripheral blood of artificially feeding Tupaia belangeri chinensis of different ages and genders and evaluation of the effectiveness of an automatic blood cell classification counter for measuring tree shrew blood cells. Child, young and adult tree shrews (forty for each group) were randomly selected, half male and half female. After the animals were stable, the peripheral blood of each group was collected through the femoral vein, and the morphology of various blood cells of the tree shrew was observed and classified by the manual microscopic counting method and by an automatic blood cell classification counter. The Reference intervals of the normal peripheral blood cell absolute count, cell diameter and white blood cell percentage in tree shrews of different ages and genders has been calculated. White blood cell count and neutrophil relative count increased with age, while lymphocyte relative count decreased. The white blood cell count, neutrophil relative count, and lymphocyte relative count in the child group, as well as lymphocyte relative count in the young group, significantly differed according to gender (P<0.05), and the differences in other indicators were not significant. The Bland-Altman plot and the Passing-Bablok scattergram showed that the change trend of each indicator was consistent but exhibited large systematic differences between methods. Differences in peripheral blood cells exist among different age groups and different genders. An automatic blood cell classification counter is not suitable for the absolute count of blood cells in the tree shrew.
Keywords: artificial feeding, hemogram, tree shrew
Introduction
The tree shrew (Tupaia belangeri chinensis), a small mammal resembling a squirrel, is mainly distributed in Southeast Asia north of the Kela Strait. This animal has the characteristics of small size, easy feeding, a short reproductive cycle, and a brain-to-body mass ratio similar to that in other mammals [26, 42, 43]. A series of recent studies on comparative genomic analysis of the tree shrew [8, 14, 39] show that compared with traditional rodent experimental animals, the gene homology of the tree shrew is higher than that of humans; furthermore, the physiological and biochemical metabolism is similar to that in humans [8, 15, 16, 40], which is conducive to the construction of a low-cost and high-efficiency human pathophysiological model. Based on the above advantages, the tree shrew is increasingly favored by researchers, and some progress has been made in cancer, diabetes, infectious diseases, visual impairment, psychological stress and other fields [3, 9, 17, 34, 41].
In mammals, the occurrence of diseases is often accompanied by hemogram changes, and the occurrence, development and prognosis of such diseases can be understood by monitoring hemograms. Change is relative to the normal reference range, so defining the physiological hemogram is the premise of exploring the relationship between host and disease. Although the tree shrew has proven to be a potential experimental animal, systematic research on the normal hematology of the tree shrew is still lacking. Only Hunt et al. [12] have performed a rough measurement of the peripheral hemogram of the tree shrew, using such animals captured from the wild in 1967. However, because the specific subspecies, age and health conditions are not mentioned, the results lack systematicness and universality. To explore the normal hemogram in artificially feeding tree shrews (chinensis) of different ages and genders, this study used four generations of artificially feeding tree shrews in a closed population at the Kunming Institute of Zoology, Kunming, China. For the first time, traditional microscopic examination and manual counting were employed to systematically count peripheral blood cells in this subspecies of tree shrew, and the accuracy of using an automatic blood cell classification counter for analyzing the tree shrew was explored. This study provides some basic information for biomedical research and animal disease models utilizing the tree shrew.
Materials and Methods
Animals
This study used four generations of artificially feeding tree shrews in a closed population (Kunming Institute of Zoology) that had been stable for 2 weeks at the experimental animal center of Guangxi Medical University. The experiment was conducted during the nonbreeding period of the tree shrew. The usual life span of the tree shrew is eight to ten years in artificial feeding condition, sexual maturity is four to six months [26], so we choose forty child tree shrews aged 3 months (body weight: male 74.3 ± 9.7 g, female 81.4 ± 10.5 g), 40 young tree shrews aged 6 months (body weight: male 101.2 ± 9.6 g, female 93.7 ± 8.4 g) and 40 adult tree shrews aged 16 months (body weight: male 142.7 ± 13.4 g, female 124.7 ± 11.6 g) were selected, and each group of tree shrews was half male and half female. The use of experimental animals in this study was approved by the Animal Ethics Review Committee of Guangxi Medical University (Approval No. 201812031). Humanitarian care was given according to the 3R principle during this study.
Feeding condition
Tree shrews are reared in the general tree shrew feeding house of the experimental animal center of Guangxi Medical University. All tree shrews were kept in single cages (length, width, and height of 40 cm, 30 cm and 35 cm, respectively). Each cage was equipped with a food box and drinking bottle and had a cassette connected to the cage for sleep (length, width, and height of 15 cm, 12 cm and 12 cm, respectively). The feeding temperature was 24 ± 1°C, and the relative humidity was 40–60%, along with a light and dark cycle of 12 h to 12 h and noise ≤60 dB.
Sample collection
After fasting for 12 h, each tree shrew was placed in a special device for fixation. Under the nonanesthetic state, 1 ml of blood was collected through the femoral vein, and the sampling time was controlled within 30 s. Samples were put into 2 anticoagulation tubes with EDTA-K2 and mixed thoroughly, one for the automatic blood cell classification counter and the other for the Neubauer blood-cell counter and blood smear.
Sample detection methods
A URIT-3010 impedance counter automatic blood cell classification counter (Unit, Guilin, China) measures the red blood cell (RBC) absolute count, white blood cell (WBC) absolute count, lymphocyte (LYM) absolute count, neutrophilic granulocyte (GRAN) absolute count, middle blood cell (MID, the sum of monocytes, eosinophils, and basophils) absolute count, lymphocyte relative count (LYM%), MID relative count (MID%), neutrophilic granulocyte relative count (GRAN%) and so on. Platelets often blocked the measuring hole and caused large numerical oscillation and low reliability. Therefore, the results of platelets were not included in this study. The reagent and cleaning solution matched with the instrument is used during measurement.
The erythrocyte diluent was prepared with sodium chloride, sodium citrate, formaldehyde and distilled water, and the leukocyte diluent was composed of glacial acetic acid and methylene blue. The prepared diluent was transferred into a dry test tube, and the blood was pipetted into the diluent and mixed. Then, a small amount of the mixed diluent was added to the Neubauer blood-cell counter; the slides were covered and held for 15 min. Next, the numbers of RBCs and WBCs were counted under a direct optical microscope (Olympus, Tokyo, Japan) and converted into absolute counts of RBCs and WBCs according to a certain formula.
A pipette was used to mix the blood in the anticoagulation tube, and 6 µl was drawn and dropped onto one end of the glass slide. The cover glass was held at a 45° angle to the slide and smoothly pushed to the other end. The slide was air-dried at room temperature for 30 min and then soaked in absolute ethanol for 15 min. After air-drying again, the blood smear was stained by the Wright-Giemsa stain (Solebao, Beijing, China). Under a direct optical microscope, a well-stained area of the blood smear was selected for observation. Differential leukocytes were counted under an oil lens, and the cell morphology (cell diameter, heteromorphic cells, vacuoles, pseudopods, band neutrophils, etc.) were described. Each sample was continuously counted for 200 WBCs and RBCs.
Statistical analysis
Statistical analyses were conducted using SPSS statistical software for Windows (ver. 25.0; SPSS, Chicago, IL, USA). Each hematologic variable is expressed as the mean ± SD (median). Reference intervals (RIs) were calculated using the 2.5th and 97.5th percentiles provided by Tukey’s Hinges test. The differences between different genders and age groups were compared by the t-test for normally distributed variables and the Mann-Whitney U-test for nonnormally distributed variables and the Kruskal-Wallis H-test. P<0.05 was considered statistically significant.
Bland-Altman plots and Passing-Bablok scattergrams were drawn by Medcalc statistical analysis software (MedCalc Software bv, Ostend, Belgium) and Graphpad Prism (ver. 7.0; GraphPad Software, San Diego, CA, USA) to evaluate the consistency between different methods. The consistency between difference methods is evaluated by the intercept and slope obtained through the Passing-Bablok scattergram: if the 95% confidence interval (CI) of the intercept and slope includes 0 and 1, respectively, the consistency is good; if the 95% CI of the intercept and slope does not include 0 and 1, respectively, then the consistency is poor. Based on the distribution of points on the Bland-Altman plot in the limits of agreement (LoA), it is determined whether there are systematic differences between the different methods, and whether the 95% CI of the total mean difference, total intercept and slope of the two schemes includes 0 is evaluated as to whether there are proportional differences between the different methods.
Results
Results of automatic blood cell classification counter
The results of the hemogram indexes of different age group tree shrews in different genders and the differences between age and gender are shown in Table 1. There were significant differences in WBC count, MID count and Lymphocyte% among the different age groups (P<0.05); the WBC count increased with age, while Lymphocyte% decreased. The WBC and lymphocyte count in the child group and Lymphocyte% in the adult group were significantly different between different genders (P<0.05). It is speculated that the incomplete development of the immune system of the child tree shrew may lead to large fluctuations in various indicators.
Table 1. Measurement value of hematological physiological indexes by the automatic hematology analyzer*.
| Hematologic Analyte | Age Group | Gender Merger (n=120) |
P (Age Group) |
Male (n=60) | Female (n=60) |
P (Gender) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | ||||
| RBC (×1012/L) | Child | 6.15 ± 1.46 (6.22) | 5.60–6.76 | 0.065 | 5.57 ± 1.71 (5.62) | 4.45–6.74 | 6.63 ± 1.16 (6.82) | 5.96–7.20 | 0.878 |
| Young | 5.82 ± 1.32 (5.84) | 5.36–6.32 | 5.43 ± 1.39 (5.49) | 4.70–6.24 | 6.17 ± 1.21 (6.19) | 5.55–6.79 | 0.205 | ||
| Adult | 6.15 ± 1.41 (6.12) | 5.62–6.61 | 5.66 ± 1.55 (5.66) | 4.87–6.46 | 6.55 ± 1.18 (6.58) | 5.94–7.10 | 0.14 | ||
| WBC (×109/L) | Child | 2.79 ± 2.35 (2.88) | 2.01–3.65 | <0.001* | 1.59 ± 0.85 (1.62) | 1.21–2.12 | 3.78 ± 2.77 (4.14) | 2.48–5.24 | 0.033* |
| Young | 3.08 ± 1.76 (3.16) | 2.52–3.79 | 3.28 ± 1.87 (3.29) | 2.24–4.32 | 3.03 ± 1.71 (3.03) | 2.14–3.91 | 0.668 | ||
| Adult | 4.00 ± 2.64 (3.98) | 2.93–5.09 | 2.83 ± 2.75 (2.94) | 1.58–3.99 | 4.74 ± 2.95 (5.02) | 3.20–6.35 | 0.06 | ||
| LYM (×109/L) | Child | 1.94 ± 2.11 (2.02) | 1.26–2.73 | 0.145 | 1.02 ± 0.65 (1.09) | 0.72–1.41 | 2.81 ± 2.59 (2.95) | 1.53–4.11 | 0.027* |
| Young | 2.17 ± 1.40 (2.12) | 1.64–2.65 | 2.18 ± 1.29 (2.09) | 1.44–2.87 | 2.10 ± 1.53 (2.15) | 1.34–2.92 | 0.058 | ||
| Adult | 2.65 ± 2.33 (2.65) | 1.74–3.54 | 1.67 ± 0.98 (1.95) | 0.97–2.28 | 3.35 ± 2.73 (3.35) | 1.89–4.80 | 0.57 | ||
| GRAN (×109/L) | Child | 1.01 ± 0.75 (1.09) | 0.73–1.32 | 0.136 | 0.92 ± 0.64 (0.88) | 0.54–1.39 | 1.62 ± 0.83 (1.30) | 0.63–1.51 | 0.129 |
| Young | 0.76 ± 0.54 (0.81) | 0.60–0.99 | 0.89 ± 0.68 (0.89) | 0.49–1.28 | 0.75 ± 0.37 (0.73) | 0.51–0.90 | 0.11 | ||
| Adult | 0.61 ± 0.37 (0.69) | 0.54–0.80 | 0.48 ± 0.27 (0.48) | 0.35–0.61 | 0.87 ± 0.37 (0.90) | 0.66–1.02 | 0.722 | ||
| MID (×109/L) | Child | 0.35 ± 0.25 (0.38) | 0.23–0.43 | 0.017* | 0.31 ± 0.19 (0.27) | 0.15–0.41 | 0.34 ± 0.29 (0.49) | 0.20–0.51 | 0.059 |
| Young | 0.22 ± 0.17 (0.22) | 0.17–0.28 | 0.26 ± 0.16 (0.28) | 0.15–0.32 | 0.23 ± 0.18 (0.16) | 0.12–0.30 | 0.185 | ||
| Adult | 0.16 ± 0.08 (0.14) | 0.14–0.19 | 0.12 ± 0.07 (0.13) | 0.09–0.16 | 0.20 ± 0.07 (0.15) | 0.17–0.23 | 0.116 | ||
| LYM% (%) | Child | 65.71 ± 13.80 (65.71) | 60.04–71.38 | 0.043* | 65.22 ± 11.93 (64.31) | 58.31–71.52 | 65.14 ± 15.63 (67.11) | 57.42–73.49 | 0.382 |
| Young | 63.98 ± 11.93 (64.34) | 60.06–64.38 | 63.21 ± 9.88 (62.87) | 57.56–68.09 | 64.74 ± 13.66 (65.81) | 58.66–72.25 | 0.224 | ||
| Adult | 60.88 ± 16.60 (61.47) | 55.36–68.49 | 56.98 ± 14.39 (57.42) | 47.64–66.98 | 65.33 ± 17.70 (65.52) | 55.67–74.53 | 0.029* | ||
| GRAN% (%) | Child | 26.94 ± 11.51 (27.13) | 22.79–31.09 | 0.575 | 26.73 ± 10.80 (27.44) | 21.37–33.34 | 27.51 ± 12.42 (26.82) | 20.19–36.96 | 0.76 |
| Young | 27.87 ± 9.83 (28.30) | 24.79–31.65 | 29.10 ± 9.19 (29.10) | 23.87–34.32 | 27.51 ± 10.51 (27.51) | 21.93–33.08 | 0.643 | ||
| Adult | 29.11 ± 14.09 (29.42) | 23.58–34.73 | 30.11 ± 9.50 (30.84) | 24.23–36.99 | 26.78 ± 14.18 (28.00) | 18.72–33.83 | 0.878 | ||
| MID% (%) | Child | 7.51 ± 4.88 (7.64) | 5.86–9.26 | 0.11 | 7.87 ± 3.23 (7.46) | 6.03–9.47 | 7.22 ± 6.08 (7.78) | 4.36–10.41 | 0.16 |
| Young | 7.62 ± 3.46 (7.62) | 6.34–8.89 | 7.51 ± 3.76 (7.96) | 5.65–9.82 | 7.63 ± 3.27 (7.28) | 5.70–9.06 | 0.329 | ||
| Adult | 8.87 ± 3.81 (8.72) | 7.41–10.43 | 9.65 ± 3.36 (8.92) | 7.10–11.61 | 8.13 ± 4.17 (8.52) | 6.40–10.85 | 0.186 | ||
*P<0.05: considered to be significantly different. Reference interval (RI) was calculated using the 2.5th and 97.5th percentiles. a) RBC, red blood cell; WBC, white blood cell; LYM and LYM%, absolute and relative lymphocyte count, respectively; GRAN and GRAN%, absolute and relative neutrophilic granulocyte count, respectively; MID and MID%, absolute and relative sum, respectively, of monocytes, eosinophils, and basophils. b) The number of each age group=40.
Manual blood cell count
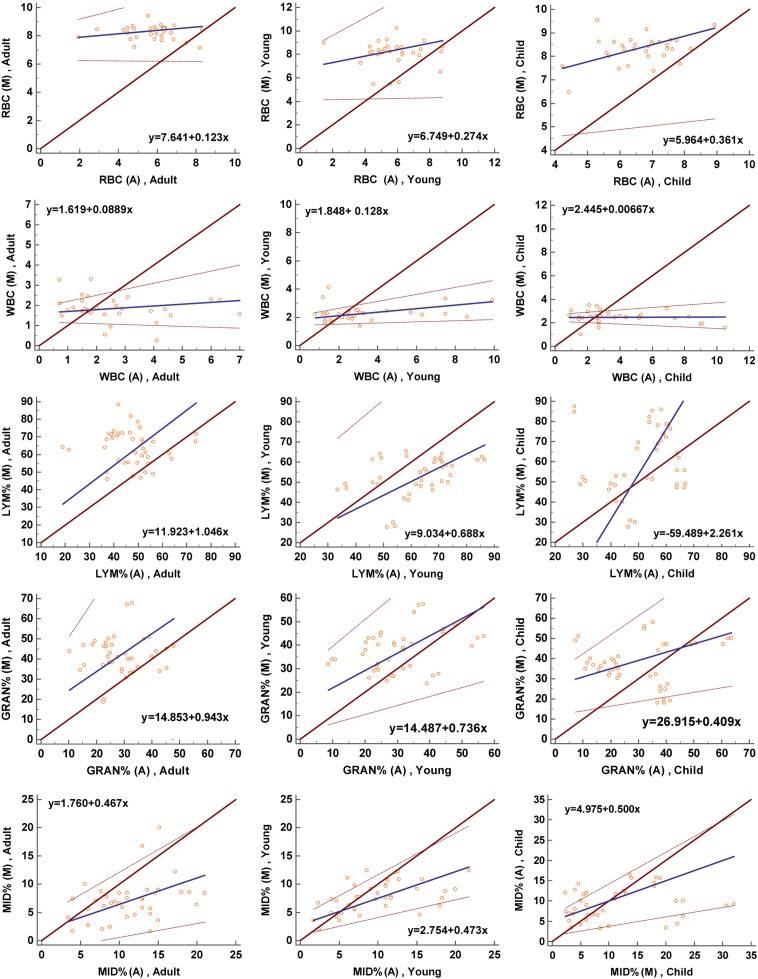
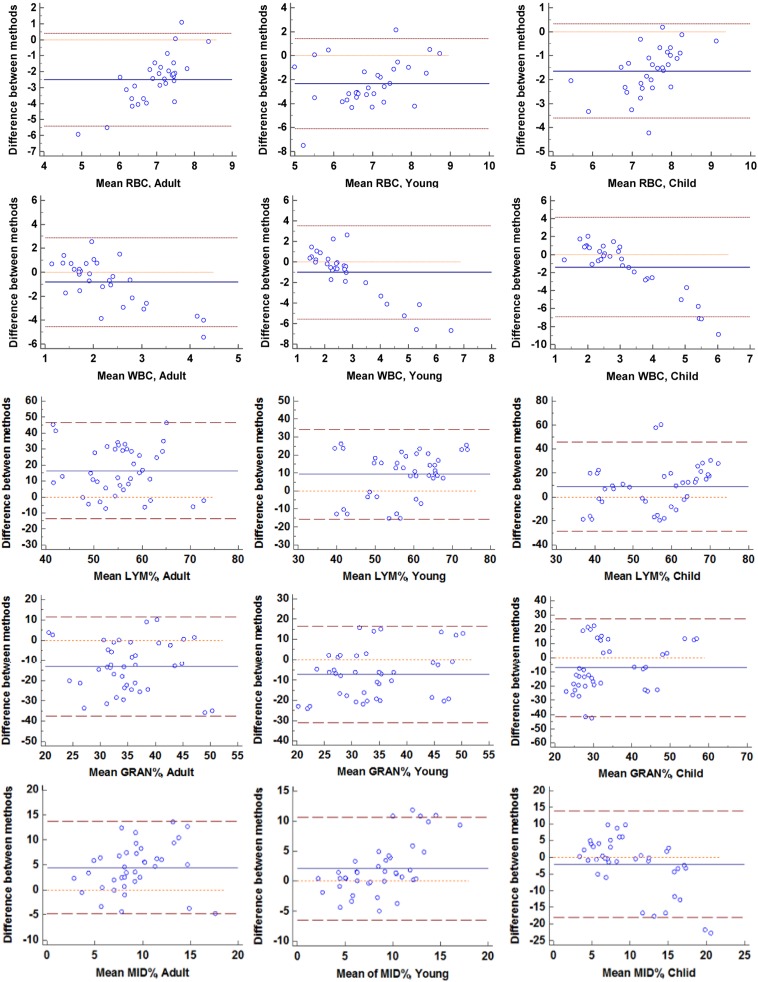
The results of manual blood cell counts in different gender and age groups are shown in Table 2. There was no significant difference in the number of RBCs between the different age groups (P=0.786), but there was a significant difference in the WBC count (P<0.001), which increased with age. In the child group, the WBC count was higher in females than in males (P=0.016), but there was no significant difference in other age groups. A Passing-Bablok scattergram was used to analyze between the results of the automatic blood cell classification counter and those of manual blood cell counting in order to evaluate the consistency of the two methods (Fig. 1); the results showed that there was poor consistency between the RBC count and WBC count. The Bland-Altman plot indicated that there was a large systematic difference between the two methods (Fig. 2).
Table 2. Manual blood cell counting and leukocyte difference count of normal tree shrew.
| Hematologic Analyte | Age Group | Gender Merger (n=120) |
P (Age Group) |
Male (n=60) | Female (n=60) |
P (Gender) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | ||||
| RBC (×1012/L) | Child | 8.23 ± 0.49 (8.43) | 8.05–8.41 | 0.786 | 8.26 ± 0.62 (8.44) | 7.93–8.61 | 8.20 ± 0.36 (8.25) | 8.01–8.36 | 0.452 |
| Young | 8.08 ± 1.06 (8.12) | 7.69–8.47 | 7.95 ± 1.23 (8.24) | 7.27–8.63 | 8.20 ± 0.89 (8.18) | 7.72–8.68 | 0.627 | ||
| Adult | 8.29 ± 0.60 (8.31) | 8.07–8.51 | 8.32 ± 0.56 (8.33) | 8.00–8.63 | 8.27 ± 0.65 (8.37) | 7.92–8.61 | 0.247 | ||
| WBC (×109/L) | Child | 1.84 ± 0.64 (1.80) | 1.61–2.07 | <0.001* | 1.51 ± 0.74 (1.75) | 1.10–1.92 | 2.16 ± 0.52 (1.91) | 1.88–2.44 | 0.016* |
| Young | 2.28 ± 0.57 (2.25) | 2.08–2.49 | 2.44 ± 0.67 (2.25) | 2.07–2.80 | 2.14 ± 0.44 (2.18) | 1.91–2.38 | 0.271 | ||
| Adult | 2.47 ± 0.53 (2.48) | 2.27–2.66 | 2.53 ± 0.62 (2.48) | 2.18–2.87 | 2.41 ± 0.45 (2.43) | 2.17–2.65 | 0.652 | ||
| Neutrophil% (%) | Child | 36.93 ± 7.22 (37.26) | 31.15–42.26 | 0.032* | 33.82 ± 6.97 (35.21) | 30.36–40.68 | 39.32 ± 7.47 (39.31) | 33.48–44.01 | 0.021* |
| Young | 40.08 ± 6.48 (41.43) | 33.65–46.83 | 39.14 ± 6.22 (39.14) | 33.06–45.21 | 41.23 ± 6.83 (43.72) | 34.71–47.94 | 0.875 | ||
| Adult | 43.47 ± 4.72 (42.58) | 38.79–47.11 | 42.71 ± 5.04 (44.53) | 39.08–46.37 | 44.02 ± 4.13 (40.63) | 30.76–47.69 | 0.382 | ||
| Lymphocyte% (%) | Child | 51.63 ± 8.26 (51.63) | 44.36–58.87 | 0.018* | 52.14 ± 8.16 (51.76) | 46.74–59.20 | 50.46 ± 7.93 (51.50) | 43.63–57.52 | 0.517 |
| Young | 49.19 ± 6.58 (48.33) | 43.60–55.73 | 46.57 ± 4.32 (44.31) | 43.34–50.69 | 52.72 ± 8.19 (52.53) | 45.35–58.43 | 0.03* | ||
| Adult | 45.05 ± 6.32 (46.37) | 41.01–50.55 | 47.04 ± 5.84 (48.72) | 43.75–51.81 | 44.02 ± 6.95 (44.02) | 39.48–48.57 | 0.243 | ||
| Eosinophil% (%) | Child | 3.25 ± 0.27 (3.49) | 3.08–3.34 | 0.278 | 3.04 ± 0.23 (3.11) | 2.94–3.31 | 3.42 ± 0.29 (3.87) | 3.16–3.58 | 0.124 |
| Young | 2.21 ± 0.48 (2.21) | 1.98–2.45 | 2.44 ± 0.37 (2.44) | 2.15–2.73 | 2.01 ± 0.57 (1.98) | 1.74–2.42 | 0.269 | ||
| Adult | 3.82 ± 0.21 (3.74) | 3.66–3.93 | 3.58 ± 0.26 (3.46) | 3.31–3.73 | 3.96 ± 0.18 (4.02) | 3.83–4.01 | 0.745 | ||
| Basophil% (%) | Child | 0.43 ± 0.13 (0.46) | 0.39–0.51 | 0.475 | 0.32 ± 0.08 (0.42) | 0.27–0.35 | 0.44 ± 0.14 (0.44) | 0.35–0.52 | 0.533 |
| Young | 0.34 ± 0.07 (0.41) | 0.29–0.35 | 0.29 ± 0.09 (0.34) | 0.23–0.34 | 0.38 ± 0.06 (0.48) | 0.33–0.42 | 0.378 | ||
| Adult | 0.14 ± 0.15 (0.12) | 0.18–0.22 | 0.17 ± 0.12 (0.13) | 0.09–0.21 | 0.12 ± 0.17 (0.11) | 0.08–0.24 | 0.492 | ||
| Monocyte% (%) | Child | 9.25 ± 2.33 (9.34) | 8.15–10.77 | 0.134 | 11.34 ± 3.27 (11.49) | 9.14–14.36 | 7.62 ± 1.58 (7.19) | 6.24–9.03 | 0.014* |
| Young | 8.63 ± 1.89 (8.57) | 7.84–10.63 | 9.04 ± 1.93 (8.41) | 8.02–10.74 | 8.30 ± 1.74 (8.73) | 7.25–9.83 | 0.542 | ||
| Adult | 8.15 ± 1.53 (8.15) | 6.99–9.32 | 7.60 ± 1.26 (7.47) | 6.51–8.89 | 8.56 ± 1.87 (8.83) | 7.60–9.82 | 0.697 | ||
*P<0.05: considered to be significantly different. Reference interval (RI) was calculated using the 2.5th and 97.5th percentiles. a) RBC, red blood cell; WBC, white blood cell. b) The number of each age group=40.
Fig. 1.
Passing-Bablok scattergrams showing the agreement between the data obtained with the manual counting and classification (M) with the automatic blood cell classification counter (A).
Fig. 2.
Bland-Altman plots showing the mean values obtained with manual counting and with classification by the automatic blood cell classification counter or manual differential results on the x-axes and the difference between the results of the 2 methods on the y-axes.
Manual WBC differential count and morphological observation
The differential counts of WBCs in different genders and age groups are shown in Table 2. The percentage of neutrophils and lymphocytes in the different age groups was significantly different (P<0.05). As age increased, Neutrophil% gradually increased, and Lymphocyte% gradually decreased. This finding is consistent with the automatic blood cell classification counter. Between different genders, there were significant differences in the percentages of lymphocytes, neutrophils and monocytes (P<0.05). In the child group, the percentage of neutrophils in females was higher than that of males, with opposite results for monocytes. Females’ Lymphocyte% in the young group was higher than that of males, and there was no significant difference in the adult group. The Passing-Bablok scattergram showed that different methods had consistency only at Lymphocyte% and Neutrophil% in the adult group, while the remaining items did not show good consistency (Fig. 1). The Bland-Altman plot shows that in addition to Lymphocyte% and Neutrophil% in the adult group, there were systematic and proportional differences between the two methods in the remaining analyses (Fig. 2).
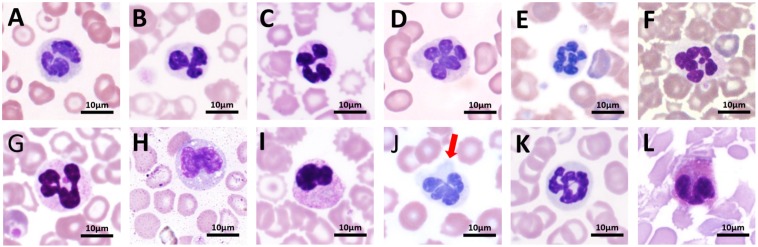
The ratio of band neutrophils to segmented neutrophils in normal tree shrews was between 1:10.48 to 14.69. The percentage of band neutrophils in the child group was higher than that in the young and adult groups. The distribution of the number of nuclei in neutrophils of each age group is shown in Table 3. The nuclear number was highest in 3 and 4 lobules, generally 2 to 5 lobules, and 7 or more lobules were rare. Occasionally abnormal neutrophils were found in normal tree shrew blood smears, mainly vacuoles, toxic particles and pseudopods (Table 4 and Fig. 3). Occasionally, nuclear extrusion and Pelger-Huet-like abnormalities were found. The proportion of heteromorphic neutrophils in total neutrophils was less than 3%. The RI of the neutrophil diameter ranged from 15.74 to 15.96 µm in the child group, 15.37 to 15.55 µm in the youth group and 15.30 to 15.50 µm in the adult group. No significant difference between age groups was observed.
Table 3. Distribution of the nuclear number in neutrophils.
| Age Group |
Reference item |
Band neutrophil (%) |
2 lobules (%) |
3 lobules (%) |
4 lobules (%) |
5 lobules (%) |
6 lobules (%) |
7 lobules and more (%) |
|---|---|---|---|---|---|---|---|---|
| Child (n=40) | Mean ± SD (Median) |
8.79 ± 9.41 (8.43) | 8.46 ± 8.02 (8.72) | 31.01 ± 10.26 (30.68) | 33.41 ± 8.03 (34.27) | 13.28 ± 8.51 (13.28) | 4.73 ± 5.92 (4.31) | 1.11 ± 1.49 (1.07) |
| RI | 7.58–14.77 | 5.78–11.85 | 26.68–35.34 | 31.02–37.80 | 9.68–16.87 | 2.23–7.23 | 0.48–1.34 | |
| Young (n=40) | Mean ± SD (Median) |
7.91 ± 10.23 (7.42) | 6.87 ± 10.56 (6.98) | 27.16 ± 10.30 (25.33) | 34.28 ± 8.07 (34.65) | 17.71 ± 8.87 (16.83) | 5.54 ± 4.47 (5.41) | 0.92 ± 1.88 (1.01) |
| RI | 5.24–11.39 | 4.14–8.45 | 24.20–34.13 | 30.49–38.27 | 14.44–22.99 | 3.38–7.69 | 0–1.82 | |
| Adult (n=40) | Mean ± SD (Median) |
7.52 ± 8.76 (8.21) | 8.29 ± 4.47 (9.45) | 31.33 ± 9.84 (30.97) | 31.54 ± 10.22 (33.56) | 14.89 ± 9.44 (14.89) | 5.01 ± 5.64 (5.12) | 0.45 ± 1.35 (0.55) |
| RI | 6.53–12.46 | 6.77–14.96 | 27.49–35.10 | 27.55–37.46 | 11.22–18.54 | 2.82–7.91 | 0–0.97 |
Table 4. Comparison of the proportion of heteromorphic neutrophils.
| Age Group | Gender | Vacuoles (%) | Toxic particles (%) | Pseudopod (%) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | Mean ± SD (Median) | RI | ||
| Child (n=40) | Merge | 1.93 ± 1.01 (1.87) | 1.38–2.47 | 0.34 ± 0.09 (0.34) | 0.28–0.40 | 1.09 ± 0.46 (1.13) | 0.83–1.31 |
| Male (n=20) | 1.84 ± 0.97 (1.76) | 1.44–2.34 | 0.28 ± 0.07 (0.29) | 0.24–0.32 | 0.93 ± 0.39 (0.95) | 0.79–1.22 | |
| Female (n=20) | 1.02 ± 0.58 (1.04) | 0.73–1.32 | 0.41 ± 0.15 (0.41) | 0.38–0.44 | 1.25 ± 0.43 (1.34) | 1.08–1.33 | |
| Young (n=40) | Merge | 1.27 ± 0.62 (1.30) | 0.89–1.53 | 2.41 ± 0.80 (2.53) | 2.03–2.84 | 1.14 ± 0.37 (1.14) | 0.98–1.30 |
| Male (n=20) | 1.82 ± 0.77 (1.44) | 1.41–2.03 | 2.05 ± 0.74 (2.09) | 1.81–2.57 | 1.03 ± 0.22 (0.99) | 0.89–1.14 | |
| Female (n=20) | 1.71 ± 0.51 (1.71) | 1.50–1.92 | 2.79 ± 0.93 (2.84) | 2.42–3.35 | 1.24 ± 0.45 (1.24) | 1.01–1.46 | |
| Adult (n=40) | Merge | 1.37 ± 0.48 (1.29) | 1.10–1.51 | 1.23 ± 0.26 (1.30) | 1.16–1.32 | 1.69 ± 0.31 (1.71) | 1.50–1.81 |
| Male (n=20) | 1.25 ± 0.33 (1.25) | 1.12–1.38 | 1.31 ± 0.27 (1.31) | 1.20–1.41 | 1.63 ± 0.29 (1.63) | 1.48–1.77 | |
| Female (n=20) | 1.53 ± 0.54 (1.61) | 1.22–1.86 | 1.14 ± 0.24 (1.15) | 1.08–1.29 | 1.77 ± 0.34 (1.85) | 1.49–1.92 | |
The result is expressed as the ratio of heteromorphic neutrophils to total neutrophils. RI, reference interval.
Fig. 3.
Normal and heteromorphic neutrophils under a light microscope (100×).
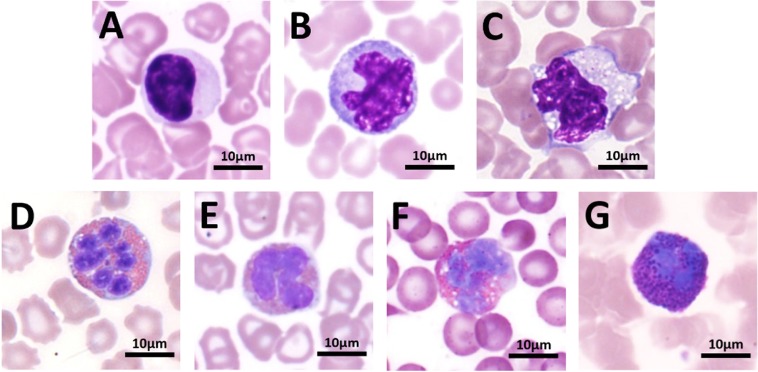
Each age group’s lymphocyte cell diameter is shown in Table 5. Ninety percent of lymphocytes were less than or equal to 9 µm in diameter, and no significant difference was observed between different genders. The morphology of the tree shrew lymphocytes is similar to that of humans, in which the cytoplasm is very sparse, the chromatin is distributed in a coarse cluster, and the diameter is approximately 7.5 µm. Occasionally, large lymphocytes and immature lymphocytes larger than 9 µm in diameter can be found. There was no significant difference in cell morphology between the different age groups. In the normal tree shrew blood smear, atypical lymphocytes were found occasionally (Fig. 4), accounting for 2.28% of the total lymphocyte count. The morphology roughly conformed to Downey’s classification, with type I predominant and type II and type III being rare.
Table 5. Cell diameter of various blood cells.
| Hemocyte Type | Age Group | Diameter (μm) | Minimum (μm) | Maximum (μm) | |
|---|---|---|---|---|---|
| Mean ± SD (Median) | RI | ||||
| Neutrophils | Child | 15.85 ± 1.71 (15.93) | 15.74–15.86 | 7 | 19 |
| Young | 15.46 ± 1.24 (15.33) | 15.27–15.64 | 8 | 18 | |
| Adult | 15.40 ± 1.75 (16.11) | 15.30–15.50 | 9 | 19 | |
| Lymphocyte | Child | 7.60 ± 1.38 (7.80) | 7.72–7.58 | 4 | 13 |
| Young | 7.23 ± 1.27 (6.99) | 7.15–7.34 | 5 | 12 | |
| Adult | 7.68 ± 1.44 (7.68) | 7.61–7.76 | 4 | 13 | |
| Eosinophil | Child | 15.56 ± 2.39 (15.52) | 15.09–16.10 | 12 | 22 |
| Young | 14.75 ± 1.90 (14.55) | 14.11–15.32 | 12 | 24 | |
| Adult | 15.02 ± 1.91 (15.02) | 14.65–15.38 | 10 | 21 | |
| Basophil | Child | 15.33 ± 1.45 (15.51) | 14.51–16.23 | 14 | 18 |
| Young | 14.93 ± 1.14 (15.03) | 14.27–14.54 | 14 | 17 | |
| Adult | 15.30 ± 1.53 (15.30) | 14.52–16.01 | 13 | 18 | |
| Monocyte | Child | 17.64 ± 3.10 (17.58) | 17.27–18.09 | 13 | 25 |
| Young | 17.02 ± 2.90 (17.11) | 16.51–17.43 | 12 | 26 | |
| Adult | 16.17 ± 3.09 (15.80) | 15.78–16.51 | 11 | 23 | |
| RBC | Child | 6.22 ± 2.46 (6.71) | 5.14–7.85 | 3 | 11 |
| Young | 6.81 ± 1.97 (6.84) | 5.41–8.17 | 5 | 11 | |
| Adult | 6.55 ± 2.01 (6.49) | 5.22–7.98 | 4 | 12 | |
The cell diameter was measured according to the reference line provided by the system under a light microscope (100×). a) All measurements are expressed as integers. b) For irregularly shaped cells, the cell diameter is the mean of the sum of long and short paths.
Fig. 4.
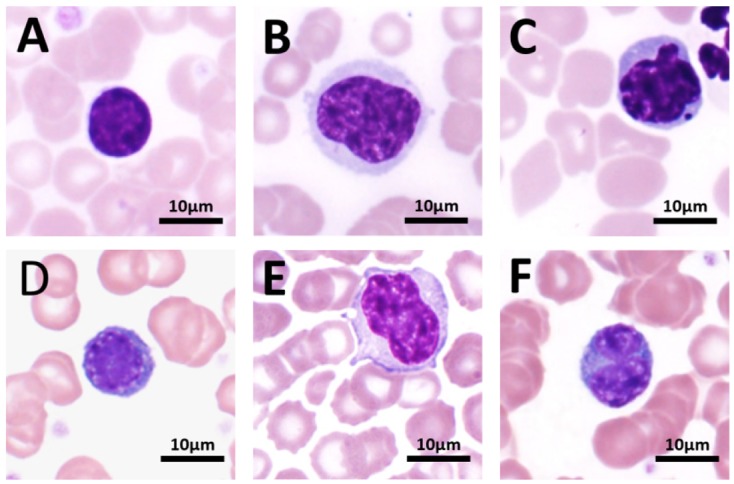
Various lymphocytes under a light microscope (100×).
The diameters of monocytes, eosinophils and basophils in each age group are shown in Table 5. There were no significant differences among genders. The morphology of mononuclear cells in the peripheral blood of normal tree shrews is variable, with rounded or serrate edges, abundant cytoplasm and occasional macrophages differentiated from mononuclear cells, with cell diameters up to 26 µm. Heteromorphic mononuclear cells (Fig. 5) were occasionally seen in the blood smear of normal tree shrews, and the majority of them exhibited abnormal nuclear morphology and vacuoles, accounting for approximately 3.11% of all mononuclear cells. The size of eosinophils in the peripheral blood of normal tree shrews is similar to that of neutrophils, with a large number of uniformly sized orange-red granules in the cell. The proportion of band eosinophils to segmented eosinophils ranged from 1:6.42 to 8.37, with no significant difference among different age groups. However, no band basophils were found. Heteromorphic eosinophils and heteromorphic basophils were rare and are mainly vacuolar.
Fig. 5.
Various monocytes, eosinophils and basophils under a light microscope (100×).
RBC morphological observation
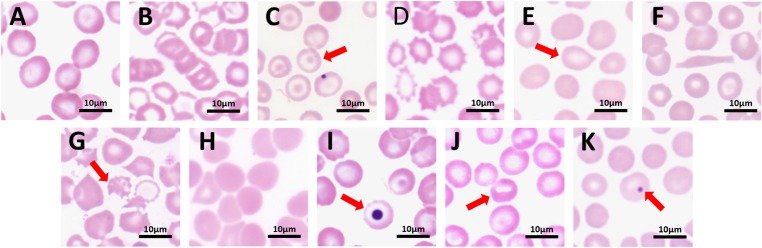
The erythrocytes in the peripheral blood of normal tree shrews were double concave discs, and there was no statistically significant difference in cell size between different age groups and different genders. The overall mean diameter was 6.73 ± 2.31 µm. The proportion of poikilocytes in the peripheral blood of 95% of normal tree shrews ranged from 2.41% to 5.83%, and the target cell was the main type, accounting for 1.01% to 3.93% of the total RBCs. Burr cells, target cells, and schistocytes accounted for 0.22–0.87%, 0.68–1.75% and 0.16–0.53%, respectively. Other poikilocytes (with poikilocytosis, stomatocytes and sickle cells) accounted for less than 1%. Nucleated erythrocytes, basophilic stippling cells and Howell-Jolly bodies are rarely seen in normal peripheral blood, and their morphology is shown in Fig. 6.
Fig. 6.
Normal and heteromorphic red blood cells (RBCs) under light microscope (100×).
Discussion
Traditional manual counting under a microscope is the most accurate method to count blood cells [13], but it is time-consuming and laborious, which makes it unsuitable for large sample size operation. Although the automatic blood cell classification counter is convenient and fast [23], its reliability in the peripheral blood cell count of tree shrews has not been verified [19]. In this study, the peripheral blood of the tree shrew was measured by an automatic blood cell classification counter and traditional manual method, and the results showed that although the trends of the two methods were consistent among different age groups, the RBC count, Neutrophil% and MID% of the results obtained by the automatic blood cell classification counter were lower overall, while the WBC count and Lymphocyte% were relatively high. By drawing Bland-Altman plots and Passing-Bablok scattergrams and comparing the two methods, it was found that compared with the traditional method, the automatic blood cell classification counter only showed consistency in the neutrophil count and lymphocyte count of the adult group but showed poor consistency in other groups of data, with large systematic differences, indicating that an automatic blood cell classification counter cannot be used as an alternative to the manual method in peripheral blood analysis of the tree shrew, but can only be used as a trending device. The reasons are manifold. First, the standard deviation of the results obtained by the automatic blood cell classification counter was large, indicating that the results obtained by this instrument exhibit large fluctuations and low credibility. Second, in the actual observation, the number of giant platelets that appear in the peripheral blood of the tree shrew is much higher than that in humans, and a few of these cells can even reach 9 µm in diameter. During the measurement process, these giant platelets may be mistaken for lymphocytes, which could increase Lymphocyte% in the final result [20, 21]. Third, the hematopoietic system and immune system of child and young tree shrews are not fully developed, their bone marrow metabolism is active, and the probability of atypical cells is higher than that of adult tree shrews, which will affect the results of the automatic blood cell classification counter. It can be learned from this study that the neutrophils in the peripheral blood of normal tree shrews account for approximately 30–50% of the total number of WBCs, and the neutrophil percentage and cell diameter of the tree shrew are closer to those of humans than are those of rats, mice, hamsters and other rodents [18, 28, 32]. With increasing age, the percentage of neutrophils increased gradually among the different age groups, and the difference was statistically significant; Neutrophil% in the peripheral blood of child tree shrews was 31.15–42.26%, and the adult tree shrews reached 38.79–47.11%. From a physiological point of view, this result is reasonable. Neutrophils play an important role in the body’s nonspecific cellular immunity [11, 37]. The increase in their percentage indicates that the tree shrew’s immune system tends to be complete, which is similar to the second crossover of neutrophils and lymphocytes in human development [38]. Regarding gender, the percentage of neutrophils in female child tree shrews was higher than that in males (P=0.021), but there was no significant difference between the young and adult groups, which might be related to the secretion of estrogen. Estrogen is a class of steroid compounds with broad biological activities that impact the proliferation and differentiation of hematopoietic stem cells. With an increase in estrogen, the ability of hematopoietic stem cells to differentiate into granulocyte/macrophage clonal units is enhanced [1, 4, 29, 38]. One of the possible reasons for this phenomenon is that the individual development of young females occurs earlier than that of males.
Neutrophils originate from bone marrow progenitor cells and precursor cells, which are released into the peripheral blood circulation after hyperplasia and maturation [25, 27, 33]. The more nuclear lobules segmented neutrophils have, the higher their maturity [5, 38]. Band neutrophils are the precursors of segmented neutrophils and are immature cells. Therefore, the ratio of band neutrophils to segmented neutrophils in peripheral blood can indirectly reflect the maturity of the bone marrow hematopoietic system. It can be inferred from Table 3 that with increasing age, the ratio of band neutrophils to segmented neutrophils tends to decrease, and the hematopoietic system of the tree shrew gradually matures. Compared with the increase in the percentage of neutrophils, the percentage of lymphocytes in the peripheral blood of normal tree shrews decreased with age, and there were significant differences among different age groups, with approximately 40% to 50% in adulthood, slightly higher than in humans (20% to 40%) but lower than in rodents (Rat 50% to 85%, Mice 60% to 83%) [18, 30]. The Lymphocyte% in female tree shrews in the young group was higher than that in males (P=0.030), which may be the reason Neutrophil% in males increased greatly from childhood to youth, leading to a large decrease in Lymphocyte%, while the fluctuation in females was less than that in males, resulting in a numerical difference.
Mono-macrophages not only exhibit strong phagocytosis and digestion but also play an important role in maintaining homeostasis and resisting foreign pathogenic agents [24, 35]. The percentage of monocytes in child male tree shrews was higher than that in females (P=0.014), but this difference was not obvious in other age groups, which may be because female tree shrews develop earlier than males and have a more mature immune system. More mature monocytes have a smaller cell diameter and nuclear size [36, 38]. Normal adult tree shrew monocytes account for approximately 7–10% of WBCs in peripheral blood, slightly higher than that in humans (3–8%) and far more than the 0–4% in rodents [2, 18, 22].
Similarly to neutrophils, the normal tree shrew’s eosinophils have both a band and segmented nucleus [7]. However, band basophils were not observed. One possible reason is that the cytoplasm of basophils in the tree shrew contains a large number of basophilic particles and substances. After staining, the cytoplasm appears a thick blue-purple color, making the nucleus blurred and difficult to recognize.
The morphology and size of erythrocytes in normal tree shrews are similar to those of human erythrocytes, and there is no significant difference in quantity and morphology between different ages and genders. There are occasional poikilocytic RBCs on blood smears (Fig. 6); the most common type is the target cell. This is because aged RBCs will actively bind IgG to the membrane surface. When WBCs come into contact with red blood cells, the membrane protein is lost, and the morphology of the cell gradually becomes globular, which makes it easier to adhere to monocytes and macrophages from the spleen and peritoneum and be phagocytized [6, 10, 31]. Other poikilocytes are very rare in normal peripheral blood.
This study determined the reference ranges for the blood cell count and WBC diameter and percentage and systematically revealed the morphology of WBCs and RBCs in the peripheral blood of Tupaia Belangeri Chinensis of different ages and genders, providing a hematological basis for future studies on the disease model of tree shrews.
Conflict of Interest
This study was funded by the Guangxi Science and Technology Research Project (No. 14124003-2).
Acknowledgments
We would like to thank professor the Experimental Animal Center of Guangxi Medical University for their help in this study.
References
- 1.Abdelbaset-Ismail A., Suszynska M., Borkowska S., Adamiak M., Ratajczak J., Kucia M., Ratajczak M.Z.2016. Human haematopoietic stem/progenitor cells express several functional sex hormone receptors. J. Cell. Mol. Med. 20: 134–146. doi: 10.1111/jcmm.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Hur H., Mor G., Insler V., Blickstein I., Amir-Zaltsman Y., Sharp A., Globerson A., Kohen F.1995. Menopause is associated with a significant increase in blood monocyte number and a relative decrease in the expression of estrogen receptors in human peripheral monocytes. Am. J. Reprod. Immunol. 34: 363–369. doi: 10.1111/j.1600-0897.1995.tb00965.x [DOI] [PubMed] [Google Scholar]
- 3.Chen B., Qin M.C., Huang J.L., Wu D.P., Guo E.C., Liu Z.P., Xu Z.H., Guo X.X., Zhong Z.G.2017. [Preliminary establishment of integration of Alzheimer’s disease and blood stasis syndrome tree shrew model and evaluation of intervention of Panax notoginseng saponins]. Zhongguo Zhongyao Zazhi 42: 1175–1182. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 4.Calvanese V., Lee L.K., Mikkola H.K.2014. Sex hormone drives blood stem cell reproduction. EMBO J. 33: 534–535. doi: 10.1002/embj.201487976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmel R., Green R., Jacobsen D.W., Qian G.D.1996. Neutrophil nuclear segmentation in mild cobalamin deficiency: relation to metabolic tests of cobalamin status and observations on ethnic differences in neutrophil segmentation. Am. J. Clin. Pathol. 106: 57–63. doi: 10.1093/ajcp/106.1.57 [DOI] [PubMed] [Google Scholar]
- 6.da Costa R.M.1992. Constitution and behaviour of the spherocyte membrane. Br. J. Haematol. 81: 624–625. doi: 10.1111/j.1365-2141.1992.tb03009.x [DOI] [PubMed] [Google Scholar]
- 7.Egesten A., Malm J.1999. New Light Shed On The Enigmatic Eosinophil Granulocyte; A Versatile Cell Of The Immune System. EJIFCC 11: 6–25. [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y., Huang Z.Y., Cao C.C., Chen C.S., Chen Y.X., Fan D.D., He J., Hou H.L., Hu L., Hu X.T., Jiang X.T., Lai R., Lang Y.S., Liang B., Liao S.G., Mu D., Ma Y.Y., Niu Y.Y., Sun X.Q., Xia J.Q., Xiao J., Xiong Z.Q., Xu L., Yang L., Zhang Y., Zhao W., Zhao X.D., Zheng Y.T., Zhou J.M., Zhu Y.B., Zhang G.J., Wang J., Yao Y.G.2013. Genome of the Chinese tree shrew. Nat. Commun. 4: 1426. doi: 10.1038/ncomms2416 [DOI] [PubMed] [Google Scholar]
- 9.Feng Y., Feng Y.M., Lu C., Han Y., Liu L., Sun X., Dai J., Xia X.2017. Tree shrew, a potential animal model for hepatitis C, supports the infection and replication of HCV in vitro and in vivo. J. Gen. Virol. 98: 2069–2078. doi: 10.1099/jgv.0.000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias M.G.2017. Advances in laboratory diagnosis of hereditary spherocytosis. Clin. Chem. Lab. Med. 55: 944–948. doi: 10.1515/cclm-2016-0738 [DOI] [PubMed] [Google Scholar]
- 11.Grechowa I., Horke S., Wallrath A., Vahl C.F., Dorweiler B.2017. Human neutrophil elastase induces endothelial cell apoptosis by activating the PERK-CHOP branch of the unfolded protein response. FASEB J. 31: 3868–3881. doi: 10.1096/fj.201700012R [DOI] [PubMed] [Google Scholar]
- 12.Hunt R.D., Chalifoux L.1967. The hemogram of the tree shrew (Tupaia glis). Folia Primatol. (Basel) 7: 34–36. doi: 10.1159/000155094 [DOI] [PubMed] [Google Scholar]
- 13.Kim S.J., Kim Y., Shin S., Song J., Choi J.R.2012. Comparison study of the rates of manual peripheral blood smear review from 3 automated hematology analyzers, Unicel DxH 800, ADVIA 2120i, and XE 2100, using international consensus group guidelines. Arch. Pathol. Lab. Med. 136: 1408–1413. doi: 10.5858/arpa.2010-0757-OA [DOI] [PubMed] [Google Scholar]
- 14.Liu X.H., Yao Y.G.2013. Characterization of 12 polymorphic microsatellite markers in the Chinese tree shrew (Tupaia belangeri chinensis). Zool. Res. 34:(E2): E62–E68. doi: 10.3724/SP.J.1141.2013.E02E62 [DOI] [PubMed] [Google Scholar]
- 15.Lin J., Chen G., Gu L., Shen Y., Zheng M., Zheng W., Hu X., Zhang X., Qiu Y., Liu X., Jiang C.2014. Phylogenetic affinity of tree shrews to Glires is attributed to fast evolution rate. Mol. Phylogenet. Evol. 71: 193–200. doi: 10.1016/j.ympev.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 16.Lindblad-Toh K., Garber M., Zuk O., Lin M.F., Parker B.J., Washietl S., Kheradpour P., Ernst J., Jordan G., Mauceli E., Ward L.D., Lowe C.B., Holloway A.K., Clamp M., Gnerre S., Alföldi J., Beal K., Chang J., Clawson H., Cuff J., Di Palma F., Fitzgerald S., Flicek P., Guttman M., Hubisz M.J., Jaffe D.B., Jungreis I., Kent W.J., Kostka D., Lara M., Martins A.L., Massingham T., Moltke I., Raney B.J., Rasmussen M.D., Robinson J., Stark A., Vilella A.J., Wen J., Xie X., Zody M.C., Baldwin J., Bloom T., Chin C.W., Heiman D., Nicol R., Nusbaum C., Young S., Wilkinson J., Worley K.C., Kovar C.L., Muzny D.M., Gibbs R.A., Cree A., Dihn H.H., Fowler G., Jhangiani S., Joshi V., Lee S., Lewis L.R., Nazareth L.V., Okwuonu G., Santibanez J., Warren W.C., Mardis E.R., Weinstock G.M., Wilson R.K., Delehaunty K., Dooling D., Fronik C., Fulton L., Fulton B., Graves T., Minx P., Sodergren E., Birney E., Margulies E.H., Herrero J., Green E.D., Haussler D., Siepel A., Goldman N., Pollard K.S., Pedersen J.S., Lander E.S., Kellis M., Broad Institute Sequencing Platform and Whole Genome Assembly TeamBaylor College of Medicine Human Genome Sequencing Center Sequencing TeamGenome Institute at Washington University.2011. A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478: 476–482. doi: 10.1038/nature10530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R., Zanin M., Xia X., Yang Z.2018. The tree shrew as a model for infectious diseases research. J. Thorac. Dis. 10:(Suppl 19): S2272–S2279. doi: 10.21037/jtd.2017.12.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindstrom N.M., Moore D.M., Zimmerman K., Smith S.A.2015. Hematologic assessment in pet rats, mice, hamsters, and gerbils: blood sample collection and blood cell identification. Vet. Clin. North Am. Exot. Anim. Pract. 18: 21–32. doi: 10.1016/j.cvex.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K., Ochiai T., Sekita K., Kawasaki Y., Yasuhara K., Furuya T.1983. [Application of an automatic blood cell counter, MICROX, to experimental animals. II. Leukocyte differential count in rabbits, rats, and mice]. Jikken Dobutsu 32: 115–122. (in Japanese) [PubMed] [Google Scholar]
- 20.Milton J.G., Hutton R.A., Tuddenham E.G., Frojmovic M.M.1985. Platelet size and shape in hereditary giant platelet syndromes on blood smear and in suspension: evidence for two types of abnormalities. J. Lab. Clin. Med. 106: 326–335. [PubMed] [Google Scholar]
- 21.Mhawech P., Saleem A.2000. Inherited giant platelet disorders. Classification and literature review. Am. J. Clin. Pathol. 113: 176–190. doi: 10.1309/FC4H-LM5V-VCW8-DNJU [DOI] [PubMed] [Google Scholar]
- 22.Munn D.H., Garnick M.B., Cheung N.K.1990. Effects of parenteral recombinant human macrophage colony-stimulating factor on monocyte number, phenotype, and antitumor cytotoxicity in nonhuman primates. Blood 75: 2042–2048. doi: 10.1182/blood.V75.10.2042.2042 [DOI] [PubMed] [Google Scholar]
- 23.Novis D.A., Walsh M., Wilkinson D., St Louis M., Ben-Ezra J.2006. Laboratory productivity and the rate of manual peripheral blood smear review: a College of American Pathologists Q-Probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch. Pathol. Lab. Med. 130: 596–601. [DOI] [PubMed] [Google Scholar]
- 24.Ni H.X., Yu N.J., Yang X.H.2010. The study of ginsenoside on PPARgamma expression of mononuclear macrophage in type 2 diabetes. Mol. Biol. Rep. 37: 2975–2979. doi: 10.1007/s11033-009-9864-0 [DOI] [PubMed] [Google Scholar]
- 25.Nefzger C.M., Rossello F.J., Chen J., Liu X., Knaupp A.S., Firas J., Paynter J.M., Pflueger J., Buckberry S., Lim S.M., Williams B., Alaei S., Faye-Chauhan K., Petretto E., Nilsson S.K., Lister R., Ramialison M., Powell D.R., Rackham O.J.L., Polo J.M.2017. Cell Type of Origin Dictates the Route to Pluripotency. Cell Rep. 21: 2649–2660. doi: 10.1016/j.celrep.2017.11.029 [DOI] [PubMed] [Google Scholar]
- 26.Peng Y., Ye Z., Zou R.1991. Biology of Chinese tree shrews (Tupaia belangeri chinensis). Yunnan Science and Technology Press. [Google Scholar]
- 27.Pruchniak M.P., Arazna M., Demkow U.2013. Life of neutrophil: from stem cell to neutrophil extracellular trap. Respir. Physiol. Neurobiol. 187: 68–73. doi: 10.1016/j.resp.2013.02.023 [DOI] [PubMed] [Google Scholar]
- 28.Restell T.I., Porfirio L.C., Souza A.S., Silva I.S.2014. Hematology of Swiss mice (Mus musculus) of both genders and different ages. Acta Cir. Bras. 29: 306–312. doi: 10.1590/S0102-86502014000500004 [DOI] [PubMed] [Google Scholar]
- 29.Ray R., Novotny N.M., Crisostomo P.R., Lahm T., Abarbanell A., Meldrum D.R.2008. Sex steroids and stem cell function. Mol. Med. 14: 493–501. doi: 10.2119/2008-00004.Ray [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rugh R., Somogyi C.1968. Pre- and postnatal normal mouse blood cell counts. Proc. Soc. Exp. Biol. Med. 127: 1267–1271. doi: 10.3181/00379727-127-32926 [DOI] [PubMed] [Google Scholar]
- 31.Rumsby M.G., Trotter J., Allan D., Michell R.H.1977. Recovery of membrane micro-vesicles from human erythrocytes stored for transfusion: a mechanism for the erythrocyte discocyte-to-spherocyte shape transformation. Biochem. Soc. Trans. 5: 126–128. doi: 10.1042/bst0050126 [DOI] [PubMed] [Google Scholar]
- 32.Suckow M.A., Stevens K.A., Wilson R.P.2012. The laboratory rabbit, guinea pig, hamster, and other rodents: Academic Press. [Google Scholar]
- 33.Schrimpf C., Wrede C., Glage S., Hegermann J., Backhaus S., Blasczyk R., Heuft H.G., Müller T.2017. Differentiation of induced pluripotent stem cell-derived neutrophil granulocytes from common marmoset monkey (Callithrix jacchus). Transfusion 57: 60–69. doi: 10.1111/trf.13909 [DOI] [PubMed] [Google Scholar]
- 34.Tong Y., Hao J., Tu Q., Yu H., Yan L., Li Y., Lv L., Wang F., Iavarone A., Zhao X.2017. A tree shrew glioblastoma model recapitulates features of human glioblastoma. Oncotarget 8: 17897–17907. doi: 10.18632/oncotarget.15225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y., Wu Y., Liu L., He L., Gao J., Zhou L., Yu F., Yu S., Wang H.2019. The structural characteristics of mononuclear-macrophage membrane observed by atomic force microscopy. J. Struct. Biol. 206: 314–321. doi: 10.1016/j.jsb.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 36.Terry R.L., Miller S.D.2014. Molecular control of monocyte development. Cell. Immunol. 291: 16–21. doi: 10.1016/j.cellimm.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venge P.2018. Human neutrophil lipocalin (HNL) as a biomarker of acute infections. Ups. J. Med. Sci. 123: 1–8. doi: 10.1080/03009734.2017.1420112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wintrobe M.M.2008. Wintrobe’s clinical hematology: Lippincott Williams & Wilkins. [Google Scholar]
- 39.Xu L., Chen S.Y., Nie W.H., Jiang X.L., Yao Y.G.2012. Evaluating the phylogenetic position of Chinese tree shrew (Tupaia belangeri chinensis) based on complete mitochondrial genome: implication for using tree shrew as an alternative experimental animal to primates in biomedical research. J. Genet. Genomics 39: 131–137. doi: 10.1016/j.jgg.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Xu L., Fan Y., Jiang X.L., Yao Y.G.2013. [Molecular evidence on the phylogenetic position of tree shrews]. Zool. Res. 34: 70–76 (in Chinese). doi: 10.3724/SP.J.1141.2013.02070 [DOI] [PubMed] [Google Scholar]
- 41.Xiao J., Liu R., Chen C.S.2017. Tree shrew (Tupaia belangeri) as a novel laboratory disease animal model. Zool. Res. 38: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Y.G.2017. Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zool. Res. 38: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y., Yao Y., Xu L. 2014. Basic biology and disease models of tree shrews. Technol. Press K. 10: 156–174. [Google Scholar]