Abstract
Biological evaluation of exopolysaccharides (EPS) produced by wild type and mutant Lactobacillus delbureckii (EPSWLD and EPSMLD) was investigated. Varying degrees of functional groups associated with polysaccharides were present thus confirming the EPS. The EPSs had strong antioxidant potential in a dose dependent (0.5–10 mg/mL) manner. EPSWLD and EPSMLD exhibited the highest 1,1-diphemy 1-2-picryl-hydrazyl (DPPH) activity (73.4 % and 65.6 %), total antioxidant activity (1.80 % and 1.42 %), H2O2 scavenging activity (88.5 % and 78.6 %) and Ferric Reducing Antioxidant Power (FRAP) (1.89 % and1.81 %) at 10 mg/mL respectively. WLD and MLD were highly susceptible to chloramphenicol, cotrimoxazole, tetracycline, erythromycin and ceftazidine and resistant to cefuroxime, gentamicin and cloxacillin. The EPSs had antibacterial activity against the test pathogens. B. subtilis and S. aureus had the highest susceptibility (26.0 mm and 23.0 mm). EPSMLD modulate the highest IgG, IgA and IgM production (68–126 mg/dL and 67–98 mg/dL and 64–97 mg/dL) in the treated tumor induced mice (TTIM). EPSWLD and EPSMLD exhibited reduction capability on the CEA level (3.99–4.35 ng/L and 4.12–4.23 ng/L) of the TTIM. EPSWLD TTIM had the highest amount of RBC, WBC and PCV (5.6 × 1012%, 68000% and 42%). The EPS increased the lifespan of TTIM. In conclusion EPSWLD and EPSMLD had strong biological potential with pharmacological and neutraceutical activity.
Keywords: Microbiology, Exopolysaccharides, Antioxidants activity, Antimicrobial potential, Hematological, Immune system
Microbiology; Exopolysaccharides; Antioxidants activity; Antimicrobial potential; Hematological; Immune system
1. Introduction
Lactobacillus delbrueckii subsp bulgaricus is a nonpathogenic microorganism that produces lactic acid which is largely used in dairy industries, especially in cheese-making and yoghurt production. It has the ability of changing the intestinal milieu, reduces lactose intolerance and also improves the immune system (Piard and Desmazeaud, 1992). Strains of Lactic Acid Bacteria (LAB) such as Streptococcus, Lactococcus, Pediococcus, Lactobacillus, Leuconostoc and Weissella species are frequently known to produce (EPSs (Patel and Prajapati, 2013; Adebayo-Tayo et al., 2018). EPSs that are naturally produced are highly susceptible to biodegradation and are less harmful than synthetic polymers (Boudjek et al., 2015; Wang et al., 2014; Ngan et al., 2014).
Microbial EPSs refers to all forms of bacterial polysaccharide, both slime and capsule, found outside the cell wall (Prathima et al., 2001). LABs are able to produce EPSs in the surrounding medium as slime or on the surface of bacterial cells to form a capsule (Ramchandran and Shah, 2009). EPSs producing LAB such as Streptococcus thermophilus, L. delbrueckii subsp. bulgaricus, Lactococcuslactis subsp. cremoris isolated from dairy products and fermented milk have been extensively studied (Patel and Prajapati, 2013) and may provide physiological benefits which include antioxidant activities, antitumor, immunomodulation and cholesterol lowering ability (Zhang et al., 2013a, 2013b; Li et al., 2014; Shao et al., 2014; Adebayo-Tayo et al., 2018).
Free radicals are harmful to living organisms (Mahapatra and Banerjee, 2013). To reduce the damage caused by free radicals, both synthetic and natural antioxidants are used. Butylated hydroxyanisole (BHA), butylated hydroxyto-luene (BHT) and n-propyl gallate (PG) are examples of synthetic antioxidants with potent antioxidant activity against several oxidation systems which results in carcinogenesis and liver damage thereby posing potential risks in human system (Luo and Fang, 2008; Liu et al., 2009). This results in the development of natural nontoxic antioxidants to protect humans from free radicals. Exploitation of safe natural antioxidants from bio-resources such as exopolysaccharides that can replace synthetic antioxidants has gained great importance in science and medicine because of their ability to maintain human health as well as treatment of diseases (Li, 2012). LAB exhibit antioxidant activity in four major ways; they may reinforce the inherent cellular antioxidant defense by secreting enzymes like superoxide dismutase (SOD). They also release and promote the production of the major non-enzymatic antioxidant and free radical scavenger glutathione (GSH). Moreover, they promote the production of certain antioxidant biomolecules, such as the exopolysaccharides (EPSs). Finally, they exhibit metal chelating activity. Superoxide anion and hydrogen peroxide degradability potential and reduction of Rreactive Oxygen Species (ROS) accumulation risk through indigestion of food by LAB has been reported (Liu and Pan, 2010).
Antimicrobials have been used increasingly as a primary intervention for inhibition or inactivation of pathogenic microorganisms in foods (Davidson and Zivanovic, 2003). EPSs from LAB also produces antimicrobial compounds including hydrogen peroxide, CO2, diacetyl, acetaldehyde, D-isomers of amino acids, reuterin and bacteriocins (Cintas et al., 2001). Generally, food antimicrobial agents are not used alone to control foodborne pathogens, but are included as components of the multiple approaches to microbial control.
EPS from LAB are good candidates for immunotherapeutic agent against cancer because they usually have low side effect and are less cytotoxic (Yu et al., 2009; Osuntoki and Korie, 2010). EPS from Lactobacillus delbrueckii strains can enhance humoral immunity mediated by immunoglobulins produced by the bone marrow lymphocytes (B lymphocytes). The B lymphocytes are responsible for specific recognition and removal of antigens that are extracellular located. The EPS from LAB that can also enhance cell-mediated immune responses such as natural killer cell tumoricidal activity, T-lymphocyte proliferation and mononuclear cell phagocytic capacity has been reported (LeBlanc et al., 2002). This research work is therefore aimed at determining the antioxidant, immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild and mutant Lactobacillus delbureckii subsp bulgaricus.
2. Materials and methods
2.1. Culture collection
The wild and mutant Lactobacillus delbrueckii and the test pathogens (Bacillus subtilis, S. typhi, E. coli, K. pneumonia, S. dysentriae and S. aureus) used in this experiment were collected from the culture collection of our previous work in Microbial Physiology Unit, Department of Microbiology, University of Ibadan (Ishola and Adebayo-Tayo, 2018). The isolates was maintained in De Man, Rogosa and Sharpe (MRS) broth (De Man et al., 1960) and stored at 28 °C for three days.
2.2. Production, extraction and quantification of EPSWLD and EPSMLD
Exopolysaccarides was produced using wild and mutant Lactobacillus delbrueckii using Modified Exopolysaccharide Selection Medium (De Man et al., 1960). The inoculated medium was incubated at 30 °C for 16 h (Adebayo-Tayo and Onilude, 2008). The supernatant from the fermentation medium was precipitated at 4 °C by the addition of 2 volume of ethanol (100%). Total sugar of the precipitate was determined using phenol-sulphuric acid method (Dubois et al., 1956). The amount of sugar present and their levels were calculated using glucose as standard.
2.3. Characterization of the EPSWLD and EPSMLD
2.3.1. Fourier Transform Infrared (FTIR) analysis of the EPSWLD and EPSMLD samples
The major structural groups of the purified EPS were detected using Fourier Transform Infrared (FT-IR) spectroscopy. The spectrum of the EPS was obtained using a KBr method. The polysaccharide samples were pressed into KBr pellets at a sample ratio of 1:100. The FT-IR spectra were recorded on a Bruker Tensor 27 instrument with a resolution of 4 cm−1 in the region of 4, 000–400 cm−1 (Fontana et al., 2015).
2.3.2. Determination of the monosaccharide composition of the EPSWLD and EPSMLD using high performance liquid chromatography
The monosaccharide composition of EPSWLD and EPSMLD was determined using HPLC (Honda et al., 1989; Yang et al., 2012). The rehydrated EPSWLD and EPSMLD samples was hydrolyzed uing 1 mL of 2 M trifluoroacetic acid (TFA) at 120 °C for 2 h, derivatized with 1-phenyl-3-methyl-5-pyrazolone and subsequently analyzed by high-performance liquid chromatography (HPLC) with a four-unit pump (Agilent Technologies, Wilmington, USA) and a Shim-pak VPODS column (4.6 × 150 mm) with detection by absorbance monitoring at 245 nm. The mobile phase consisted of 82% sodium phosphate (50 mM, pH 7.0) and 18% acetonitrile (v/v), and the sample was eluted at a flow rate of 1.0 mL/min with the use of refractive index detector. An integrator was used to measure the area under the curves and by comparing different sugar standard with a known retention time of the EPS sample.
2.4. Determination of antibiotic susceptibility of WLD and MLD
The susceptibility of the isolates to different types of antibiotics was done using Agar Disc Diffusion method (Bauer et al., 1966). Commercially available antibiotics disc (Oxoid) containing ceftazidine (30 μg), cefuroxine (30 μg), erythromycin (5 μg), gentamycine (10 μg), cefixime (5 μg), ofloxacin (5 μg), augmentin (30 μg), chloramphenicol (30 μg), tetracycline (10 μg), ciprofloxacine (5 μg), amoxycillin (25 μg), cotrimoxazole (25 μg), nitrofurantion (300 μg) and cloxacillin (5μg) were used. Young culture of WLD and MLD were aseptically swab on MRS agar plates and the antibiotics disc were placed on the surface of the agar plates. The inoculated plates were incubated at 37 °C for 24 h and zone of inhibition were measured and recorded in diameter (Vlkova et al., 2006).
2.5. Antibacterial potential of the EPSWLD and EPSMLD
The antimicrobial potential of the EPSWLD and EPSMLD was determined using Agar Well Diffusion method (Honda et al., 1989; Sarrazin et al., 2012) using Bacillus subtilis, S. typhi, E. coli, K. pneumonia, S. dysentriae and S. aureus as test pathogens. The suspension of the tested microorganism (1 × 106 CFU/ml) was seeded on Mueller - Hinton Agar (Lab M Ltd., UK) plates. Uniform wells were cut on the dried agar plate using a sterile cork borer of diameter 7 mm. Each well was filled with 10 μL of the EPSWLD and EPSMLD. The inoculated plates were incubated at 37 °C for 24 h. After incubation the plates were observed for zones of inhibition (ZOI) around the wells and the ZOI diameters (mm) were recorded.
2.6. Determination of the antioxidant potential of the EPSWLD and EPSMLD
The free radical scavenging activity of the exopolysaccharide was measured by 1-1-diphenyl-2-picrylhydrazyl (DPPH) radicals according to Shimada et al. (1992). Five milliliters of 0.1 of DPPH ethanol solution (freshly prepared) were added to different concentration of EPS (50, 100, 250, 500 and 1000 μL). The reaction was kept in the dark for 30 min. Absorbance was measured at 517 nm using Spectrophotometer UV-visible 2401PC (Shimadzu, Japan). Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The experiment was carried out in triplicate and averaged (inhibition percentage plotted against concentration of tested polysaccharide). The capability to scavenge the DPPH radical was calculated using the following equation:
| Scavenging activity (%) = [1–(Asample–Ablank)/Acontrol] × 100 |
Hydrogen Peroxide scavenging potential was done following the method of Thampi and Shalini (2015). 0.1M Phosphate buffer (pH 7.4) was prepared in 10mM solution of Hydrogen Peroxide.1mL of different concentration of EPS (50, 100, 250 and 1000 mg/mL) was added to 2 mL of the Hydrogen Peroxide solution. Absorbance of the reaction mixture was taken at 230nm after 10 min of incubation at 30 °C against blank solution using UV- spectrophotometer. Ascorbic acid was used as standard.
| Scavenging activity (%) = (Asample–Ablank)/Acontrol × 100 |
Total Antioxidant Activity of the EPS samples was determined (Dilna et al., 2015). Total antioxidant capacity of the EPS samples was determined by mixing 7.45mL Sulfuric acid (0.6M), 1.235g of Ammonium Molybdate (4mM), 0.9942g of Sodium Sulphate (28mM) in 250mL distilled water (Total antioxidant capacity reagent). 0.1 mL of different concentration of EPS (50,100, 250, 500 and 1000 mg/mL) was dissolved in 1 mL of total antioxidant capacity and absorbance was taken at 695 nm after 15 min. Ascorbic acid was used as standard.
The reducing power of the EPS was evaluated using the method modified by Gülçin et al. (2010). 2.5 mL of 2 mM Phosphate buffer (pH 6.6), 1% of Potassium Ferricyanide (2.5 mL) and 1 mL distilled water was mixed with different concentration of EPS (200, 400, 600, 800 and 1000 mg/mL). The mixture was kept for 20 min at 50 °C. 2.5mL of 10% Trichloroacetic acid was added to the reaction and spinned at 3000 rpm for 10 min. 1% ferric chloride (0.1mL) and 2.5 mL distilled water was added. The reaction mixture was allowed to stay for 10 min after which the absorbance was taken at 700 nm. Ascorbic acid was used as standard.
2.7. Determination of antitumor potential of the EPSWLD and EPSMLD
2.7.1. Rearing condition of the animals and exposure method and experimental design
All procedures used for the animal study conforms to the guidelines for Animal Care Use and Research Ethics Committee (ACUREC), University of Ibadan. 8–10 weeks old weighing 20–24 g female Swiss albino mice (20) maintained in the Animal Breeding Unit, University of Ibadan was used. The mice were fed with pelletized rat feed and water ad libitum, permitted to adapt to the laboratory surroundings for two weeks prior to the experiment.
Smoking of cigarette was done two times in a day for five days for four month in an enclosed compartment. The side stream smoke was created by burning Little London cigarettes in a smoking compartment. The cigarettes were ignited and allowed to flare in the chamber. Whole body of the four experimental groups of mice was subjected to the side stream ignited cigarette.
Mice were randomly grouped into five different groups (five mice in each); Grp 1 (mice that are not exposed to cigarette), Grp 2 (mice exposed to the cigarettes smoke and was not treated); Grp 3 (mice exposed to the smoke of cigarettes and treated with 20 mg/kg/day 5-fluorouracil); Grp 4 (mice exposed to the cigarettes smoke and treated with 500 nM aqueous solution of EPSWLD through intra-peritoneal (IP) injection) and Grp 5: (mice exposed to the cigarettes smoke and treated with 500 nM aqueous solution of EPSMLD through intra-peritoneal (IP) injection). All treatments were administered for 15 days. The antitumor efficiency of EPS was juxtapose with that of 20 mg/kg/day 5-fluorouracil IP for nine days (Muthlu et al., 2009).
2.7.2. Determination of immunoglobulin
The assay for antibodies was based on turbidimetric measurement. Turbidity was caused by the formation of antigen-antibody insoluble immune complexes. For the determination of IgG, IgA and IgM, the following reagents were used: Blood serum, (phosphate buffer saline-pH 7.4), IgG, IgA and IgM Antibody, Sodium azide and distilled water. The IgG, IgA and IGM of the treated and untreated mice were determined by diluting the blood serum samples and the control in 0.9% saline (1:10). 20μL of the diluted samples was added to 900 μL of phosphate buffer and labeled sample A2. The absorbance of sample A1 was taken at 340 μL. 100 μL antibody reagent was added into the prepared samples and mixed properly. The reaction mixture was incubated for 15mins. Absorbance of A2 and control was taken at 340nm.
2.7.3. Determination of hematology analysis of the EPSWLD and EPSMLD
The mice were anaesthetized with chloroform after which blood samples were taken by intracardiac puncture. Blood sample was promptly transferred into ethylenediaminetetra-acetic-coated vials (EDTA) for determination of possible hematological test. Hematological assay (white blood cell (WBC), red blood cell (RBC), platelet level, percentage Packed Cell Volume (PCV)) as well as Measurement of Mean Corpuscular Hemoglobin Concentration (MCHC) of each sample was determined using standard laboratory procedures.
2.7.4. IchromatmCEA test
Plasma and serum were separated from the whole blood obtained from the tumor induced mice within 3hrs by centrifugation. 150 μL of plasma and control sample was transferred into a test tube containing the detection buffer and thoroughly shaking for 10 min before dispensing on the test cartridge through its well. The cartridge was allowed to stay at room temperature for 12 min and then inserted into the holder. The cartridge was scanned by the ichroma™ reader.
2.8. Statistical analysis
All experiments were performed in triplicates and the results were statistically subjected to Analysis of Variance (ANOVA) using SPSS (version 11.0, Chicago, IL). Probability values (P = 0.05) was considered significant to indicate difference. Duncan's Multiple Range Test (P = 0.05) were used to determine the significant difference.
3. Results and discussion
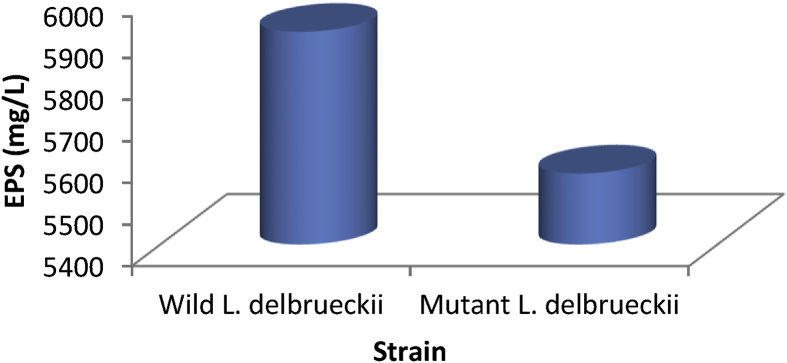
Production of EPS by the wild and mutant L. delbrueckii.
Production of EPS by the wild and mutant L. delbrueckii is shown in Figure 1. The EPS produced by the wild and mutant L. delbrueckii ranged from 5570.34 – 5910.62 mg/L. Wild L. delbrueckii (WLD) had the highest amount of EPS.
Figure 1.
Production of EPS by the wild and mutant Lactobacillus delbrueckii.
This result is in line with our previous work in which wild Lactobacillus delbrueckii (WLDYG2) had the highest EPS production (Ishola and Adebayo-Tayo, 2018). Glucose and glucose moiety was reported as source of sugar for biosynthesis of heteropolysaccharides (Escalante et al., 1998).
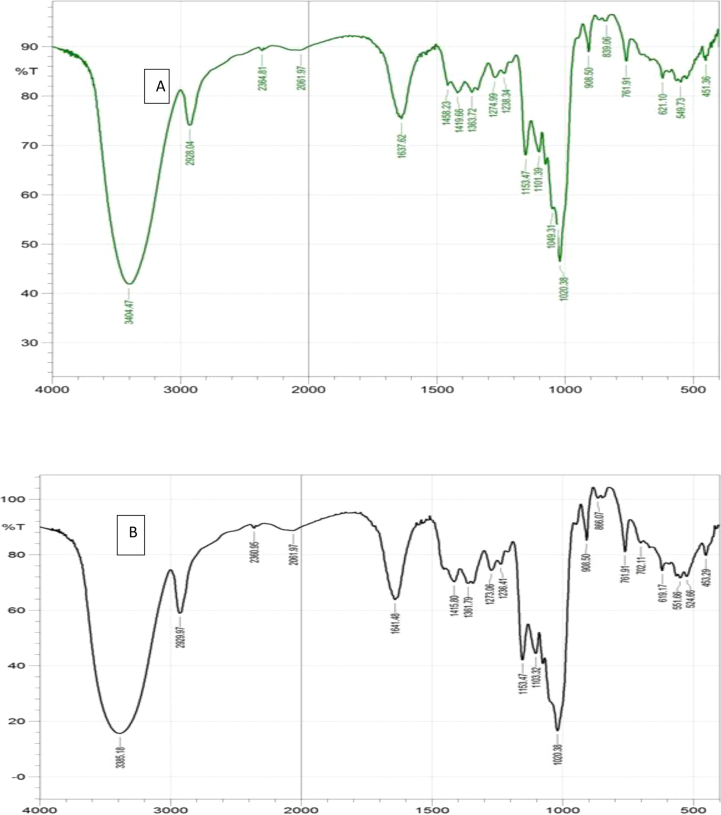
Figure 2a – b showed the infrared spectra of the major functional groups of EPSWLD and EPSMLD. The distribution of functional groups present in EPSWLD and EPSMLD is shown in the Table 1. The EPS showed that peaks at 3404.47 cm−1 and 3385.18 cm−1 were characteristics of O–H which existed in hydrogen bond of polymer indicating that the compound is likely to be unsaturated or aromatic and a weak peak at 2364.81 cm−1 and 2360.95 cm−1 indicate an asymmetrical C–H stretching bond. Unsaturation of the EPS was more highlighted at peaks 1637.62 and 1641.48 cm−1 and these peaks were assigned to the starching vibration of the carboxyl group (C=O).
Figure 2.
a–b: FTIR spectrum of (a) EPSWLD and (b) EPSMLD.
Table 1.
Distribution of functional group of the FTIR spectra of EPSWLD and EPSMLD.
| S/N | Peak (cm−1) | Functional group | EPSWLD | EPSMLD |
|---|---|---|---|---|
| 1 | 453.29 | Chloroalkanes | + | + |
| 2 | 524.66. 549.73, 551.66 | Alkyl halide | + | + |
| 3 | 619.17, 621.1 | Alkynes | + | + |
| 4 | 702.11, 761.91 | Alkanes | + | + |
| 5 | 839.06, 866.07 | Alkyl halide | + | + |
| 6 | 908.5 | Aromatics | + | + |
| 7 | 1020.38, 1049.31 | Alkenes | + | + |
| 8 | 1101.39, 1103.32 | Alkenes | + | + |
| 9 | 1153.41 | Alkenes | + | + |
| 10 | 1274.99 | Alkanes | + | - |
| 11 | 1236.41 | Aliphatic amines | - | + |
| 12 | 1363.72, 1273.06 | Alkanes | + | + |
| 13 | 1419.66, 1415.8 | Ester | + | + |
| 14 | 1637.62, 1641.48 | Un-Saturated Ester | + | + |
| 15 | 1658 | Amide | + | - |
| 16 | 2061.96 | Alcohol | + | + |
| 17 | 2364.81, 2360.95 | Alcohol | + | + |
| 18 | 2928.04, 2929.97 | Aldehyde | + | - |
| 19 | 3404.47, 3385.18 | Hydroxyl/alcohol | + | + |
The FTIR spectra of EPSWLD and EPSMLD are shown in Figure 2a and b.
Water associated with this peak indicates the presence of organic substances such as the ring stretching of mannose or galactose (Wang et al., 2015). The strong absorption peak at 1, 020.38 and 1,049.31 cm−1 indicated the presence of carbohydrates (Abdhul et al., 2014). The EPSWLD differ from EPSMLD in three peaks less in the regions 1, 236.41 cm−1; 1,274.99 cm−1; 2,928. 04–2929.97 cm−1 which indicates lesser OH presence in the EPSWLD as this region is characteristics of alcohol and aromatic bonds (Adebayo-Tayo et al., 2017, 2018). Absence of peaks in the range of 260–290 cm−1 clearly indicated that the sample did not have any proteins and nucleic acids (Bai et al., 2016).
3.1. Monosaccharide composition of the EPS
The retention time, area under curve and sugar concentration of the EPSWLD and EPSMLD samples is shown in Table 2. There was a significant difference (P ≤ 0.05) in sugar concentration of the EPS produced by EPSWLD and EPSMLD. Sugars such as ribose, xylose, arabinose, rhamnose, fructose, glucose, mannose and galactose were present in the EPS samples produced by both wild and mutant Lactobacillus delbrueckii. The sugar concentration ranged from 2.09938 – 39.8584 mg/100g and 1.48119–23.85038 mg/100g respectively. Glucose had the highest concentration in EPSWLD contained higher glucose compared to EPSMLD which contained higher concentration of galactose. EPSMLD contained less Ribose compared to EPSWLD.
Table 2.
Sugar concentration and monosaccharide composition of the EPSWLD and EPSMLD.
| Monosaccharide Name | EPSWLD |
EPSMLD |
||||
|---|---|---|---|---|---|---|
| Retention time | Area under Curve | Amount (mg/100g) | Retention time | Area under curve | Amount (mg/100g) | |
| Ribose | 8.656h | 1.62f | 2.06638f | 8.92h | 5.71c | 1.48119h |
| Xylose | 10.842g | 6.19b | 4.47902d | 10.65g | 4.43d | 1.82028g |
| Arabinose | 12.266f | 1.22g | 3.23573e | 12.27f | 4.46d | 2.25737f |
| Rhamnose | 13.660e | 1.06h | 3.55212e | 13.66e | 2.82e | 6.10709e |
| Fructose | 14.695d | 5.81c | 9.58819c | 14.69d | 8.10a | 7.73321d |
| Glucose | 16.094c | 1.93e | 39.8584a | 16.04c | 1.34f | 12.86967b |
| Mannose | 17.094b | 4.81d | 9.50729c | 17.09b | 7.16b | 8.70846c |
| Galactose | 18.056a | 6.50a | 20.44084b | 18.06a | 1.17g | 23.85038a |
Mean of values with the same superscript are not significantly different (P ≤ 0.05).
This is in line with the result of Li et al. (2014) that EPS from L. helveticus was mainly composed of galactose, glucose, and mannose (Li et al., 2015). The present of eight different sugar moieties present in the EPSWLD and EPSMLD indicates that the EPS was heteropolysaccharide. It is also in line with the work of Dolyeres who reported that galactose and glucose are the dominant sugars.
3.2. Determination of Antioxidant potential of the EPSWLD and EPSMLD
Table 3 shows the different radical scavengers (DPPH, OH-, Total antioxidant capacity and reducing power capacity) used to determine the antioxidant properties of EPSWLD and EPSMLD. The DPPH scavenging activity ranged from 38.5 to 73.4% and 37.5–65.6%, and total antioxidant capacity ranged from 1.21% to 1.80% and 0.41% to 1.42 % for EPSWLD and EPSMLD. H2O2 and FRAP ranged from 50.6 % to 88.5 % and 33.1% to 78.6 % and 1.23–1.89% and 1.0 to1.81 %for EPSWLD and EPSMLD. There was a significant difference (P ≤ 0.05) in the DPPH, OH−, Total antioxidant capacity and FRAP activity. Generally the antioxidant activity increased with increase in EPS concentration. Both EPSWLD and EPSMLD exhibited higher scavenging activity than ascorbic acid which was used as control.
Table 3.
Antioxidant potential of the EPSWLD and EPSMLD.
| Conc. (mg/L) | DPPH (%) |
Total Antioxidant Capacity (%) |
H2O2 (%) |
FRAP (%) |
||||
|---|---|---|---|---|---|---|---|---|
| EPSWLD | EPSMLD | EPSWLD | EPSMLD | EPSWLD | EPSMLD | EPSWLD | EPSMLD | |
| 0.5 | 38.5e | 37.5e | 1.21e | 0.41e | 50.6e | 33.1e | 1.23e | 1.00e |
| 1.0 | 42.5d | 46.3d | 1.47d | 0.71d | 70.8d | 57.8d | 1.68d | 1.04d |
| 2.5 | 59.4c | 53.3c | 1.64c | 0.82c | 80.7c | 64.2c | 1.75c | 1.26c |
| 5.0 | 68.6b | 64.6b | 1.75b | 0.87b | 81.0b | 65.7b | 1.86b | 1.72b |
| 10 | 73.4a | 65.6a | 1.80a | 1.42a | 88.5a | 78.6a | 1.89a | 1.81a |
| Ascorbic acid (10 mg/L) | 27.9f | 30.8f | 0.87f | 0.25f | 37.9f | 21.8f | 0.59f | 0.66f |
Mean of values with the same superscript on the same column are not significantly different (P ≤ 0.05).
EPSWLD exhibited the highest H2O2 and FRAP scavenging activity compared to EPSMLD. EPSWLD and EPSMLD had higher H2O2 and FRAP scavenging potential compared to ascorbic acid. Generally, the highest antioxidant scavenging activity was recorded at 10 mg/mL EPSWLD and EPSMLD concentration. At all concentrations the EPSWLD and EPSMLD showed higher activities than ascorbic acid which was used as the control. The EPSWLD exhibited higher antioxidant capacity than EPSMLD.
Higher antioxidant activity exhibited by the EPSWLD and EPSMLD could be as a result of the bioactive component of the polysaccharides. These bioactive components could be influenced by chemical components, molecular weight and method of extraction of the EPS. EPSWLD and EPSMLD also donated hydrogen ions which react with the DPPH radicals (Adebayo-Tayo et al., 2018). When DPPH and proton-donating substances are connected such as an antioxidant, the absorbance is reduced thereby scavenging the free radical. Antioxidant potential of EPS produced by Weisella confusa has been reported by Adebayo-Tayo et al. (2018). Zheng et al. (2006) reported that the total antioxidant activity of polysaccharide depends primarily on the configuration of the glycosidic bond and its structural characteristic. The sensitivity of free ferrous iron to oxygen which results in superoxide and ferric iron has been reported by Sahu and Gray (1997). Sahu and Gray (1997) also reported that the reaction of ferrous ion with superoxide hydrogen peroxide results in Fenton reaction which leads into a hydroxyl radical by oxidizing biomolecules in the surroundings. The hydroxyl radical production is directly related to the concentration of copper or iron. The variation in antioxidant capacity of the EPSWLD and EPSMLD may be due to genetic modifications L. delbrueckii during mutagenesis (Saarela et al., 2011). This result shows that ferric ions had reducing ability for neutralizing free radicals to form a more stable product. Liu and Pan (2010) reported the reductive capability of L. paracasei and L. plantarum EPS.
The sensitivity of the WLD and MLD to some antibiotics is shown in Table 4. There was a significant difference (P ≤ 0.05) in the antimicrobial sensitivity of the WLD and MLD to the antibiotics. 78.57% (11) of the WLD and MLD were susceptible while 18.75% (3) were resistant to the antibiotics. The susceptibility of WLD and MLD to the tested antibiotics ranged from 14f 0.00d–28.0amm and 8f 0.00–24.0a mm. The highest susceptibility was exhibited towards erythromycin, tetracycline and chloramphenicol. Generally, wild and mutant Lactobacillus delbrueckii were highly susceptible to chloramphenicol, cotrimoxazole, tetracycline, erythromycin and ceftazidine and were resistant to cefuroxime, gentamicin and cloxacillin.
Table 4.
Sensitivity test of the WLD and MLD to some antibiotics.
| Antibiotics | Zone of Inhibition (mm) |
|
|---|---|---|
| WLD | MLD | |
| Ceftazidine (30μg) | 26 | 20 |
| Cefuroxime(30μg) | 0 | 0 |
| Gentamicine (10μg) | 0 | 0 |
| Cefixime (5μg) | 18 | 14 |
| Ofloxacin (5μg) | 22 | 20 |
| Augmentin (30μg) | 24 | 20 |
| Nitrofurantoin (300μg) | 14 | 8 |
| Ciprofloxacine (5μg) | 22 | 16 |
| Amoxycillin (25μg) | 18 | 14 |
| Erythromycin (5μg) | 28 | 22 |
| Tetracycline (10μg) | 26 | 24 |
| Cloxacillin (5μg) | 0 | 0 |
| Cotrimoxazole (25μg) | 24 | 20 |
| Chloramphenicol (30μg) | 26 | 24 |
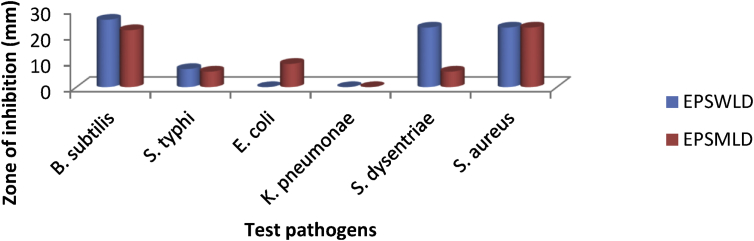
The antibacterial activity of EPS produced by the LABs against some selected pathogenic microorganism is shown in Figure 3. There was a significant difference (P ≤ 0.05) in the susceptibility of the test pathogen. The antibacterial activity ranged from 0.0 - 26.0 mm and 0.0–23.0 mm for EPSWLD and EPSMLD. B. subtilis and S. aureus had the highest susceptibility while K. pneumonia and E. coli were resistant to the EPS. B. subtilis was highly susceptible to all the EPS produced by the LAB strains.
Figure 3.
Antibacterial activity of EPSWLD and EPSMLD against some test pathogens.
The antimicrobial potential can be affiliated with the collection of organic acids, fatty acids, diacetyl and hydrogen peroxide (Dunne, 2001). Okereke et al. (2012) also reported that LAB isolated in their study prevents the growth of S. aureus, E. coli and B. cereus. Abdel-Bar et al. (1987) also reported that L. bulgaricus produces antimicrobial substance different from lactic acid and these substances are effective against to S. aureus. Lactobacilli are able to produce antimicrobial agents, such as organic acids, hydrogen peroxide, diacetyl, short chain fatty acids and bacteriocins, against pathogens (Servin, 2004).
The immunomodulatory potential of tumor induced mice treated with EPSWLD and EPSMLD is shown in Table 5. There was a significant difference (P ≤ 0.05) in immunoglobulins production by the treated mice. Group 5 (tumor induced mice treated with EPSMLD) had the highest IgG (126 ± 0.03 mg/ dL.), IgA level (98 ± 0.24 mg/dL) and IgM level (97 ± 0.05 mg/dL). This was followed in order by the IgG (122 mg/ dL), IgA level (95 mg/ dL) and IgM level (89 mg/ dL) of Group 4 (tumor induced mice treated with EPSWLD) and Group 3 (tumor induced mice treated with 5 –flurouracil acid). IgA level (67 mg/ dL) of Group 1(mice not administered with treatment) and Group 2(Tumor induced mice and not treated) are the same. The least IgM was recorded in group 2 (Tumor induced mice and not treated). There was no significant difference (P ≤ 0.05) in the IgM level of Group 1 and Group 2.
Table 5.
Antitumor and Immunomodulatory potential of EPSWLD and EPSMLD.
| Groups | Cigarrete | EPSWLD | EPSMLD | % Flurouracil | Immunoglobulins (mg/dL) |
||
|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | |||||
| Grp 1 | - | - | - | - | 68 | 67 | 64 |
| Grp 2 | + | - | - | - | 105 | 67 | 63 |
| Grp 3 | + | - | - | + | 108 | 84 | 70 |
| Grp 4 | + | + | - | - | 122 | 95 | 89 |
| Grp 5 | + | - | + | - | 126 | 98 | 97 |
Key: Florouracil is a standard drug for cancer treatment.
Ability of EPSWLD and EPSMLD to stimulate IgG in the immune system of the mice may be due to the engulfment of macrophages by the EPS. There is a stimulation of serum glycoproteins to produce a subpopulation of white blood cells called lymphocytes. Host immunity improvement based on Ig A production as a result of intake of LAB has been reported (Zhu et al., 2012; Qiu et al., 2012). Stimulation of immunoglobulin's in broiler chickens fed with LAB has been reported (Yang et al. (2012). Qiu et al., 2012) reported the immnomodulatory potential of one day old broilers fed with lactic acid bacteria strains and control diet has been reported.
The amount of carciniembryonic antigen was also investigated in the treated and untreated tumor induced mice (Table 6). Induced and untreated mice displayed high level of CEA. Reduction in the CEA level of the induced and treated mice using 5-flurouracil acid as well as EPSWLD and EPSMLD was observed. The CEA level of the mice ranged from 0.00-0.01 ng/L in group 1. Group 2 had CEA which ranged from 6.29 – 6.42 ng/L. CEA level in Group 3 ranged from 4.79 - 5.02 ng/L. The CEA level of the mice in group 4 and 5 (3.99–4.35 ng/L and 4.12–4.23 ng/L) reduced compared to the CEA level of mice in group 2 and 3.
Table 6.
Carcinoembryonic antigen (CEA) of the untreated and treated tumor induced mice.
| Group | Treatments | CEA |
|---|---|---|
| 1 | CNT 1 | 0.01 |
| CNT 2 | 0.00 | |
| CNT 3 | 0.00 | |
| CNT 4 | 0.00 | |
| 2 | CNT+1 | 6.41 |
| CNT+2 | 6.29 | |
| CNT+3 | 6.42 | |
| 3 | 5F1 | 4.79 |
| 5F2 | 4.97 | |
| 5F3 | 5.02 | |
| 4 | EPSWLD01 | 4.35 |
| EPSWLD02 | 4.06 | |
| EPSWLD03 | 3.99 | |
| EPSWLD04 | 4.11 | |
| 5 | EPSMLD01 | 4.23 |
| EPSMLD02 | 4.19 | |
| EPSMLD03 | 4.17 | |
| EPSMLD04 | 4.12 |
EPSMLD had the highest inhibitory potential on the tumor cell. This result is in agreement with the report of Yoo et al. (2004) on the CEA Inhibitory potential of EPS on tumor induced mice. The systolic effect in recovery of infection disease and tumor sing EPS has been reported by Zheng et al. (2006).
Ruiz-Brevo et al. (2001) reported that some bacterial EPS have been identified as biological Response Modifier (BMRs) with tumor rejection stimulatory activity. BMRs are agents that change the usual immune response and whose mode of action is induction of cytokines. Although, varieties of microbial EPS are immunomodulators, with activities for T-cells and macrophages while some polysaccharides are considered to be T-cell-independent antigens (Tzianabos, 2000). Doleyres and Laeroix (2005) reported the advantageous physiological effects of EPS on human health, such as antitumours activity, immunomodulating bioactivity and antimutagenicity. The ability of certain EPS from LAB on cytotoxic effect on cancer cells and their synergistically influence on the action of some chemotherapy drugs, such as camptothecin, to kill cancer cells has been reported (Cousin et al., 2012).
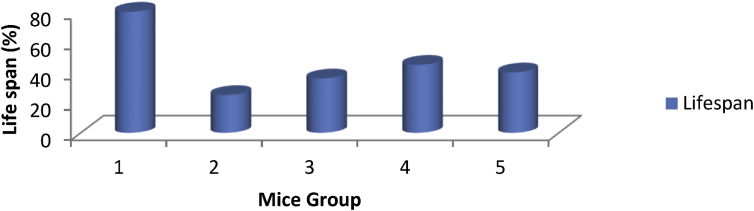
The lifespan of the tumor induced mice treated with EPSWLD and EPSMLD is shown in Figure 4. It was observed that Group 1 (non-tumor induced mice and untreated) had the highest lifespan (80 %) followed in order by Group 4 (tumor induced mice treated with EPSWLD) had a lifespan of 45 %. Group 5 (tumor induced mice treated with EPSMLD) had a lifespan of 40% while the least was observed in Group 3(tumor induced mice and treated with 5-flourouracil acid) had lifespan of 36% and Group 2 (tumor induced mice that is not treated) with life span of 25%. This may be due to the inhibition of DNA and RNA synthesis by the standard drug.
Figure 4.
Life span of the untreated and treated tumor induced mice; Group 1 (non-tumor induced mice and untreated); Group 2 (tumor induced mice that is not treated); Group 3 (tumor induced mice treated with 5-fluorouracil acid); Group 4 (tumor induced mice treated with EPSWLD) and Group 5 (tumor induced mice treated with EPSMLD).
The hematological parameter of the mice induced with tumor is shown in Table 7. RBC, WBC, Lymphocyte, neutrophil, PCV and haemoglobulin of the untreated and treated mice ranged from 3.0–5.6 × 1012 %, 37000–68000 %, 42–77%, 58–72%, 17–42% and 5.66–13.1 d/dl respectively. Group 4 had the highest RBC, WBC, Lymphocyte, neutrophil, PCV and haemoglobulin level.
Table 7.
Hematology Parameters of the untreated and treated mice.
| Hematology parameters | Group 1 (c1) | Group 2 (c2) | Group 3 (c3) | Group 4 | Group 5 |
|---|---|---|---|---|---|
| RBCx1012 | 4.7b | 3.0d | 3.7c | 5.6a | 3.8c |
| WBC | 37000c | 52000b | 65000a | 68000a | 51000b |
| Platelet | 21700b | 23400a | 15500c | 21300b | 11000d |
| Lymph | 70b | 42g | 53c | 77a | 70b |
| Neut | 70b | 58d | 61c | 72a | 64c |
| PCV (%) | 39.0b | 17.0d | 29.0c | 42.0a | 27c |
| HB d/dl | 12.9b | 5.66d | 9.68c | 13.1a | 10.67b |
| MCH | 2.74a | 1.89d | 2.61b | 2.34c | 2.80a |
| MCHC | 33.07b | 33.3b | 33.4b | 31.1c | 39.5a |
| MCV | 84.2a | 56.7d | 78.3b | 82.5a | 68.4c |
Mean of values with the same superscript are not significantly different (P ≤ 0.05).
The Mean corpuscular Haemoglobin (MCH) and Mean Corpuscular Haemoglobin Concentration (MCHC) of the experimental mice ranged from 1.89 - 2.80 and 31.1–39.5. The highest was recorded in group 1 and 4 respectively while the least was recorded in group 2 and group 5. The Mean corpuscular volume value ranged from 56.7 - 84.2 of which group 1 had the highest MCV value followed by group 4 while the least was found in group 2. The result showed that EPSWLD had significant increase in the numbers of RBC, PCV, Hb, Platelet and MCHC of mice exposed to tumor and treated with EPS.
PCV (Packed Cell Volume), RBC (Red Blood Cell), WBC (White Blood Cell), Hb (Haemoglobulin), PLT (Platelet), MCH (Mean corpuscular haemoglobin), MCHC (Mean Corpuscular haemoglobin Concentration) and MCV (Mean corpuscular volume).
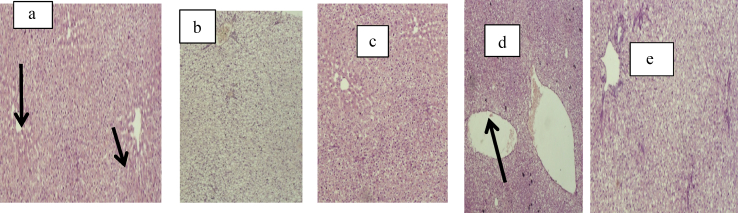
The photomicrograph of the liver section of the untreated and treated mice with EPSWLD and EPSMLD is shown in Figure 5 (a–e).
Figure 5.
(a–e): Photomicrograph of the liver section of (a) non-tumor induced mice and untreated; (b) mice induced tumor and not treated; (c) tumor induced mice treated with 5-fluorouracil acid; (d) tumor induced mice treated with EPSWLD and (e) tumor induced mice treated with EPSMLD.
The histology of liver in non-tumor induced mice and untreated is shown in Figure 5a. Pathological damage, abnormal lines and visible lesions were not observed. The histology of liver in mice induced with tumor and not treated is shown in Figure 5b. Peripotal inflammation was observed. Predominant abnormalities observed in the liver include vascular degeneration, necrosis of hepatocytes and severe interstitial Oedema. The histology of mice induced tumor and treated with 5-fluorouracil acid is shown in Figure 5c. There was a severe degeneration in the liver. The histology of mice induced tumor and treated with EPSWLD and EPSMLD is shown in Figure 5d and e. Moderate inflammation was observed. The visible and microscopic observation of the haematoxylin and eosin stained sections of liver of the trial animals revealed that the exposure of mice to cigarette caused pathological damage to their organs. These result into visible lesion and abnormality in the organs of the mice.
The photomicrograph of the lung section of tumor induced mice treated with EPSWLD and EPSMLD shown in Figure 6 (a–e). Figure 6a shows the histology of lung in non-tumor induced mice and untreated. There was a physiological damage in the lung. The photomicrograph of mice induced tumor and not treated is shown in Figure 6b. Abnormalities observed in the lung shows severe intestinal oedema and diffuse vascular degeneration. Figure 6c shows the micrograph of tumor induced mice treated with 5-fluorouracil acid. Diffused pulmonary oedema was observed. Histology of tumor induced mice treated with EPSWLD and EPSMLD is shown in Figure 6d and e. Moderate diffused pulmonary oedema was observed.
Figure 6.
(a–e): Photomicrograph of the lung section of (a) non-tumor induced mice and untreated; (b) mice induced tumor and not treated; (c) tumor induced mice treated with 5-fluorouracil acid; (d) tumor induced mice treated with EPSWLD and (e) tumor induced mice treated with EPSMLD.
The serum glycoprotein stimulatory capability of the EPSWLD and EPSMLD on the mice which causes production of lymphocytes may be as a result of engulfment of macrophages by the exopolysaccharides. This stimulation lead to improvement in the heamatological parameter (BC, PCV, Hb, Platelet and MCHC) of the mice exposed to tumor and treated with EPSWLD and EPSMLD.
From the result Tumor induced mice shows the heamatopoietic function of the EPSWLD and EPSMLD. Effect of Lactobacillus and food on immune response has been evaluated Solis-Pereyra et al. (1997) and Gerritse et al. (1990). The values obtained in this were comparatively higher than that of Kabir et al. (2011) and Galip and Seyidoolu (2012). The result conformed to the observation of Ezema and Eze (2015) and Wallace et al. (2012) who reported that probiotics LAB producing EPS caused increase in total erythrocyte and leukocyte cell counts as well as marked increase in the percentage of lymphocytes and monocytes. Reticulocytosis occur as the initial response to stimulation of the erythropoietic system and is often characterized by macro cytic and hypochromic red blood cells with increased MCV and decreased MCH and MCHC values (Adamson and Longo, 2001). Therefore, EPSWLD and EPSMLD had stimulatory effect on immune system and it can be used to boost immune status.
4. Conclusion
FT-IR of the EPS produced by the wild type and mutant Lactobacillus delbrueckii shows varying degree of functional group which indicate the presence of EPS. The EPS had different sugar moiety and composition indicating heteropolysaccharide. The antioxidant activity of the EPSWLD and EPSMLD increases in a dose dependent manner. The EPS produced by wild type and mutant Lactobacillus delbrueckii had antibacterial and antitumor potential against the tumor induced mice. Tumor induced mice treated with EPS produced by EPSWLD and EPSMLD increased the RBC, platelet and WBC. The CEA level of the tumor induced mice treated with EPSWLD reduced compare to the tumor induced mice that is not treated.
Declarations
Author contribution statement
Bukola Adebayo-Tayo: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Racheal Fashogbon: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors want to acknowledge Titiloye Oyewunmi and Ayodeji O. Salami of Immunology and Chemical Pathology Units, College of Medicine, University of Ibadan, Nigeria for their assistance during this work.
References
- Abdel-Bar N., Harris N.D., Rill R.L. Purification and properties of an antimicrobial substance produced by Lactobacillus bulgaricus. J. Food. Sci. 1987;52(2):411–415. [Google Scholar]
- Abdhul K., Ganesh J., Lee M., Shanmughapriya S., Kanagavel M., Anbarasu K., Natarajaseenivasan K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecum (BDU7) from Ngari. Int. J. Biol. Macromol. 2014;70:450–454. doi: 10.1016/j.ijbiomac.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Adamson A., Longo F. Anaemia and polycythemia. In: Braunwald E., Fauci A.S., Kasper D.>L., Hauser S.L., Longo D.L., Jameson J.L., editors. Harrison’s principles of Internal Medicine. fifteenth ed. McGraw-Hill; New York: 2001. pp. 348–354. [Google Scholar]
- Adebayo-Tayo B., Agidigbi O., Alao S. Comparative influence of immobilization medium and mutation on EPS-production by L. plantarum MK 02 isolated from fermented milk. Trakia J. Sci. 2017;1:30. [Google Scholar]
- Adebayo-Tayo B., Ishola R., Oyewunmi T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018:e00271. doi: 10.1016/j.btre.2018.e00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebayo-Tayo B.C., Onilude A.A. Screening of lactic acid bacteria strains isolates from some Nigeria fermented foods for EPS production. World Appl. Sci. J. 2008;4(5):741–747. [Google Scholar]
- Bai L., Wang L., Ji S. Structural elucidation and antioxidant activities of exopolysaccharide from L. helveticus SMN2-1. Chem. Eng. Trans. 2016;55 [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- Boudjek T., Megnekou R., Woguia A.L., Kegne1 F.M., Ngomoyogoli J.E.K., Tchapoum C.D.N., Koum O. Antioxidant and immunomodulatory properties of polysaccharides from Allanblackia floribunda Oliv stem bark and Chromolaena odorata (L.) King and H.E. Robins leaves. BMC Res. Notes. 2015;8:759. doi: 10.1186/s13104-015-1703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintas L.M., Casaus M.P., Herranz C., Nes I.F., Hernández P.E. Review: bacteriocins of lactic acid bacteria. Food. Sci. Technol. Int. 2001;7(4):281–305. [Google Scholar]
- Cousin F.J., Jouan-Lanhouet S., Dimanche-Boitrel M.T., Corcos L., Jan G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastri cancer cells. PLoS One. 2012;7:31892. doi: 10.1371/journal.pone.0031892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.M., Zivanovic S. Food antimicrobials. In: Davidson P.M., Sofos J.N., Branen A.L., editors. Antimicrobials in Foods. CRC press; USA: 2003. [Google Scholar]
- De Man J.C., Rogosa M., Sharpe M.E. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960;23:130–135. [Google Scholar]
- Dolyeres Y., Schaub L., Lacroix C. Comparison of the functionality of exopolysaccharides produced in situ or added as bioingredients on yoghurt properties. J. Dairy Sci. 2005;88:4146–4156. doi: 10.3168/jds.S0022-0302(05)73100-3. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Dunne C. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 2001;73.2:386S–392S. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- Dilna S.V., Surya H., Aswathy R.G., Varsha K.K., Sakthikumar D.N., Pandey A., Nampoothiri K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT Food Sci. Technol. 2015;64(2):1179–1186. [Google Scholar]
- Escalante A., Wacher-Rodarte C., Garcia-Garibay M., Farres A. Enzymes involved in carbohydrate metabolism and their role on exopolysaccharide production in Streptococcus thermophiles. J. Appl. Microbiol. 1998;84:108–114. doi: 10.1046/j.1365-2672.1997.00330.x. [DOI] [PubMed] [Google Scholar]
- Ezema C., Eze D.C. Probiotics effect of yeast (saccharomyces cerevisiae) on hen-day egg performance, serum and egg cholesterol levels in laying chicken. Pak. J. Nutr. 2015;14(1):44–46. [Google Scholar]
- Fontana C., Li S., Yang Z. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr. Res. 2015;402:7–94. doi: 10.1016/j.carres.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Galip N., Seyidoolu N. Effect of yeast culture on serum lipid and meat lipid values of rabbits. J. Anim. Vet. Adv. 2012;11(22):4115–4120. [Google Scholar]
- Gerritse K., Posno M., Schellekens M., Boersna W., Claassen E. Oral administration of TNP-Lactobacillus con- jugates in mice: a model for evaluation of mucosal and systemic immune response and memory formation elicited by transformed Lactobacilli. Res. Microbiol. 1990;141:955–962. doi: 10.1016/0923-2508(90)90135-d. [DOI] [PubMed] [Google Scholar]
- Gülçin I., Bursal E., Ehitolu H.M., Bilsel M., Gören A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum Turkey. Food. Chem. Toxicol. 2010;48(8-9):2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- Honda S., Akao E., Suzuki S., Okuda M., Kakehi K., Nakamura J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Chem. 1989;180:351–357. doi: 10.1016/0003-2697(89)90444-2. [DOI] [PubMed] [Google Scholar]
- Ishola R.O., Adebayo-Tayo B.C. Mutagenesis and immobilization effect on exopolysaccharide production by Weissella confusa and Lactobacillus delbrueckii. J. Adv. Microbiol. 2018;10(2):1–10. [Google Scholar]
- Kabir S.R., Zubair M.A., Nurujjaman M., Haque M.A., Hassan J., Islam M.F., Hossain M.T., Hossain M.A., Rakib M.A., Alam M.T., Shaha R.K., Kumura Y., Absar N. Purification and characterization of a Ca (2+)-dependent novel lectin from Nymphea nouchali tuber with antopoliferative activities. Biosci. Rep. 2011;31(6):465–475. doi: 10.1042/BSR20100126. [DOI] [PubMed] [Google Scholar]
- LeBlanc J.G., Matar C., Valdez J.C., LeBlanc J., Perdigon G. Immunomodulating effects of peptidic fractions issued from milk fermented with Lactobacillus helveticus. J. Dairy Sci. 2002;85:2733–2742. doi: 10.3168/jds.S0022-0302(02)74360-9. [DOI] [PubMed] [Google Scholar]
- Li W., Ji J., Chen X., Jiang M., Rui X., Dong M. Structural elucidation and antioxidant activities of exopolysachharide from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014;102:351–359. doi: 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Li W., Xia X., Tang W., Ji J., Rui X., Chen X., Jiang M., Zhou J., Zhang Q., Dong M. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015;63:3454–3463. doi: 10.1021/acs.jafc.5b01086. [DOI] [PubMed] [Google Scholar]
- Li Y. Effect of perioperative intestinal probiotics on intestinal flora and immune functioning patients with colorectal cancer (abstract) J. South. Med. Univ. 2012;32:1190–1193. [PubMed] [Google Scholar]
- Liu C.F., Pan T.M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010;77:86. [Google Scholar]
- Liu J., Luo J., Ye H., Sun Y., Lu Z., Zeng X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbonhydr. Polym. 2009;78:275–281. [Google Scholar]
- Luo D., Fang B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydr. Polym. 2008;72:376–381. [Google Scholar]
- Mahapatra S., Banerjee D. Evaluation of in vitro antioxidant potency of exopolysaccharide from endophytic Fusarium solani SD5. Int. J. Biol. Macromol. 2013;53:62–66. doi: 10.1016/j.ijbiomac.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Muthlu B.G.A., Mutlu M., Mutlu S.A., Aziz K. Influence of different exopolysaccharide-producing strains on the physicochemical, sensory and syneresis characteristics of reduced-fat stirred yoghurt. Int J. Dairy Technol. 2009;2:422–430. [Google Scholar]
- Ngan L.T.K., Wang S.L., Hiep I.M., Luong P.M., Vui N.T., Dinh T.M., Dzung N.A. Preparation of chitosan nanoparticles by spray drying and their antibacterial activity. Res. Chem. Intermed. 2014;40:2165–2175. [Google Scholar]
- Okereke H.C., Achi O.K., Ekwenye U.N., Orji F.A. Antimicrobial properties of probiotics bacteria from various sources. Afr. J. Biotechnol. 2012;11(39):9416–9421. [Google Scholar]
- Osuntoki A., Korie I. Antioxidant activity of whey from milk fermented with Lactobacillus Species isolated from Nigerian Fermented foods. Fd. Technol. Biotechnol. 2010;48(4):505–511. [Google Scholar]
- Patel A., Prajapati J.B. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013;1:107. [Google Scholar]
- Piard J.C., Desmazeaud M. Inhibiting factors produced by lactic acid bacteria. 2. Bacteriocins and other antibacterial substances. Lait. 1992;72:113–142. [Google Scholar]
- Prathima P.C., Lule V.V.K., Tomar S.K., Singh A.K. Optimization of exopolysaccharide by Lactococcus lactis 191 by response surface methodology. Inter. J. Cur. Microbiol. App. Sci. 2001;3(5):835–854. [Google Scholar]
- Qiu R., Croom J., Ali R.A., Ballou A.L., Smith C., Ashwell C.M., Hassan H.M., Chiang C.C., Koci M.D. Direct fed microbial supplementation repartitions host energy to the immune system. J. Anim. Sci. 2012;90:2639–2651. doi: 10.2527/jas.2011-4611. [DOI] [PubMed] [Google Scholar]
- Ramchandran L., Shah N.P. Effect of exopolysaccharides on the proteolytic and angiotensin-I converting enzymes-inhibiting activities and textural and rheological properties of low-fat yoghurt during refrigerated storage. J. Dairy Sci. 2009;92(3):895–906. doi: 10.3168/jds.2008-1796. [DOI] [PubMed] [Google Scholar]
- Ruiz-Bravo A.M., Jimenez-Valeri E., Moreno V.A., Ramos C. Biological response modifier activity of an exopolysaccharide Paenibacillus jamilae CP. Clin. Diagn. Lab. Immunol. 2001;8(4):706–710. doi: 10.1128/CDLI.8.4.706-710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela M., Alakomi H.L., Matto J., Ahonen A.M., Puhakka A., Tynkkynen S. Improving the storage stability of Bifidobacterium breve in Low pH fruit juice. Int. J. Fd. Microbiol. 2011;149:106–110. doi: 10.1016/j.ijfoodmicro.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Sahu S.C., Gray G.C. Lipid peroxidation and DNA damage induced by morin and naringenin in isolated rat liver nuclei. Food. Chem. Toxicol. 1997;35:443–447. doi: 10.1016/s0278-6915(97)00011-2. [DOI] [PubMed] [Google Scholar]
- Sarrazin S.L.F., Oliveira R.B., Barata L.E.S., Maourao R.,H.V. Chemical composition and antimicrobial activity of the essential oil of Lippia Schauer (Verbenaceae) from the western Amazon. Food. Chem. 2012;134:1474–1478. doi: 10.1016/j.foodchem.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Servin A.L. Antagonistic activities of lactobacilli and bifidobacterium against microbial pathogens. FEMS Microbiol. Rev. 2004;28(4):405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Shao L., Wu Z., Zhang H., Chen W., Ai L., Guo B. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014;107:51–56. doi: 10.1016/j.carbpol.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. [Google Scholar]
- Solis-Pereyra B., Aattouri N., Lemonnier D. Role of food in the stimulation of cytokine production. Am. J. Clin. Nutr. 1997;66:521–525. doi: 10.1093/ajcn/66.2.421S. [DOI] [PubMed] [Google Scholar]
- Thampi N., Shalini V.J. Bio-prospecting the in-vitro antioxidant and anti-cancer activities of silver nanoparticles synthesized from the leaves of Syzygium samarangense. Int. J. Pharm. Sci. 2015;7(7):269–274. [Google Scholar]
- Tzianabos A.O. Polysaccharide immunomodulators as therapeutic agents: structural aspect and biological function. Clin. Microbiol. Rev. 2000;13:523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlkova E., Rada V., Popelarova P., Trojan I., Killer J. Antimicrobial susceptibility of bifidobacteria isolated from gastrointestinal tract of calves. Livest. Sci. 2006;105:253–259. [Google Scholar]
- Wallace P.A., Osei D.Y., Aseidu P., Amoah K.O., Asafu-adjaye A. Influence of the probiotic, Re 3 on nutritional performance, hematological, immune status and carcass characteristics of Rabbit reared under tropical conditions. J. Anim. Feed. Res. 2012;2(5):450–456. [Google Scholar]
- Wang C.L., Chen C.J., Nguyen A.D., Liang T.W., Twu Y.K., Huang S.Y., Wang S.L. Environmental chitinous materials as adsorbents for the one-step purification of protease and chitosanase. Res. Chem. Intermed. 2014;40:2363–2370. [Google Scholar]
- Wang J., Zhao X., Zhao A., Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015;74:119–126. doi: 10.1016/j.ijbiomac.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Zhou L., Zhang L.,, Xiao Y.P., Chen A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Yoo S.H., Yoon E.J., Cha E., Lee H.G. Antitumor activity of levan polysaccharides from selected microorganisms. Int. J. Biol. Macromol. 2004;34:37–41. doi: 10.1016/j.ijbiomac.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Yu G.H., He P.J., Shao L.M. Chracterisation of extracellular of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability. Bioresour. Technol. 2009;100:3193–3198. doi: 10.1016/j.biortech.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu C., Li D., Zhao Y., Zhang X., Zeng X., Yang Z., Li S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013;54:270–275. doi: 10.1016/j.ijbiomac.2012.12.037. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang X., Liu C., Li C., Li S., Li T., Li D., Zhao Y., Yang Z. Manufacture of Cheddar cheese using probiotic Lactobacillusplantarum K25 and its cholesterol-lowering effects in a mice model. Wrld. J. Microbiol. Biotechnol. 2013;29:127–135. doi: 10.1007/s11274-012-1165-4. [DOI] [PubMed] [Google Scholar]
- Zheng W., Chen C., Cheng Q., Wang Y., Chu C. Oral administration of exopolysaccharide from Aphanothece halophytica (Choococcales) significantly inhibits influenza virus (H1N1)-induced pneumonia in mice. Int. Immunopharmacol. 2006;67:1093–1099. doi: 10.1016/j.intimp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Zhu D.J., Chen X.W., Wu J.H., Ju Y.L., Feng J., Lu G.S., Ouyang M.Z., Ren B.J., Li Y. Effect of perioperative intestinal probiotics on intestinal flora and immune functioning patients with colorectal cancer (abstract) J. South. Med. Univ. 2012;32:1190–1193. [PubMed] [Google Scholar]