Summary
The development of non-alcoholic fatty liver disease is closely linked to lifestyle factors, namely excessive caloric intake coupled with reduced physical activity and exercise. This review aims to examine the evidence behind lifestyle change as a tool to improve hepatic steatosis and liver histology in patients with non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. Furthermore, potential barriers to adopting lifestyle changes and strategies to overcome these barriers in the clinical setting are discussed.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, lifestyle, weight loss, diet, physical activity/exercise
Key points
In the absence of approved drug therapy, lifestyle interventions are key in the clinical management of NAFLD across the disease spectrum
Lifestyle treatment resulting in weight loss leads to significant improvements in steatosis, liver injury and fibrosis in patients with NASH
A menu of options should be available for both dietary and physical activity/exercise interventions to allow tailoring for individual patients
Lifestyle interventions offer a holistic approach to managing our patients and not only improve liver health, but also reduce cardiovascular disease and type 2 diabetes risks
Alt-text: Unlabelled Box
Introduction
The development of non-alcoholic fatty liver disease (NAFLD) is intimately related to lifestyle factors, namely the excessive intake of calorie-dense food coupled with reduced physical activity and exercise. Global urbanisation and modernisation in the 20th and 21st centuries have been linked to unhealthy lifestyle changes. Consequently, the last 3 decades have seen significant increases in the mean global body mass index (BMI) and the prevalence of obesity, which are the pathophysiological drivers of NAFLD.1 This is exemplified by the rapid increase in the prevalence of NAFLD in Asia over the past 15 years – related to urbanisation and the adoption of ‘Western’ type foods.2 The driving forces behind unhealthy lifestyle habits and choices are complex and multi-faceted, however they can be successfully combated to induce significant health benefits. When successful, lifestyle changes that lead to weight loss are highly effective at reducing fibrosis and the necro-inflammatory changes of non-alcoholic steatohepatitis (NASH), surpassing the efficacy of drugs currently being evaluated in phase III trials. However, sustained lifestyle changes and weight loss are difficult to achieve and, unfortunately, lifestyle changes alone are not successful in every individual. In this review, we aim to examine the evidence behind dietary change, weight loss, physical activity and exercise as tools to improve hepatic steatosis and liver histology in patients with NAFLD. Furthermore, we discuss potential barriers to adopting lifestyle changes and strategies to overcome these barriers in the clinical setting.
Lifestyle-induced weight loss
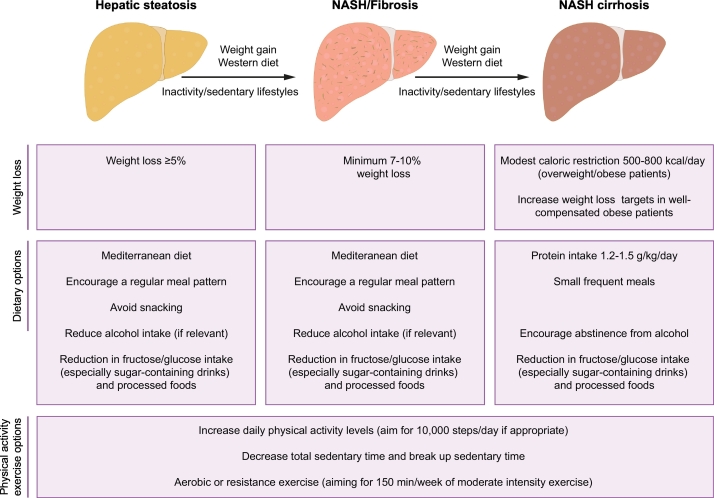
Randomised-controlled trials demonstrate that lifestyle intervention in patients with NAFLD reduces body weight; improves hepatic triglyceride content, as determined by magnetic resonance techniques; and improves NAFLD activity score (a composite of steatosis, inflammation and hepatocyte ballooning), as determined by liver biopsy (outlined in Table 1).[3], [4], [5], [6], [7], [8], [9] The majority of studies also demonstrate that lifestyle change is accompanied by concomitant improvement in cardiovascular disease (CVD) risk factors, such as serum lipid levels and insulin resistance. Fig. 1 summarises the lifestyle treatment options that can be incorporated into clinical care through the course of NAFLD.
Table 1.
Randomised clinical trials examining lifestyle interventions of diet and exercise in adult patients with NAFLD.
| Author | N | Lifestyle intervention (vs. control) | Duration, weeks | Mean weight loss | Hepatic triglyceride | Liver histology | Cardiovascular risk |
|---|---|---|---|---|---|---|---|
| Promrat7 | 31 NASH | Low-fat (25%) diet + 200 min/week moderate-intensity PA + CBT | 48 | -8.7 kg | n.a. | Improved steatosis, NAS | No difference in glucose or HOMA-IR |
| Eckard8 | 41 NAFLD | Low-fat (20%) diet + moderate exercise vs. low-carbohydrate (50%) diet + moderate exercise vs. moderate PA/exercise | 26 | -0.2 lbs vs. -3.0 lbs vs. 0.1 lbs | n.a. | Improved NAS vs. Improved NAS vs. No improvement | n.a. |
| Ueno9 | 25 NAFLD | Low (30%) fat diet + 210 min/week vigorous PA | 12 | n.a. | n.a. | Improved steatosis | Improved cholesterol and triglyceride |
| Wong6 | 145 NAFLD | Low-fat, low GI diet + 210 mins/week moderate PA | 52 | -5.6 kg | -6.7% (MRS) | n.a. | Improved LDL-cholesterol |
| Gepner3 | 278 Obese or dyslipidaemia (53% NAFLD) | Low-fat diet vs. low-carbohydrate/med. diet ± 180 min/week moderate PA | 78 | -3.2%⁎ | -5.8% vs. -7.3% | n.a. | Improved HbA1c |
| Sun5 | 1,024 NAFLD | Low-fat (30%), low-sugar diet + 27 MET/hr/week PA/exercise | 52 | -7 kg | No difference (CT) | n.a. | Improved HOMA-IR and cholesterol |
| St George4 | 152 elevated ALT and HOMA-IR | Low saturated fat, caloric restricted diet + 150 min/week moderate PA + 3 vs. 6 counselling sessions | 12 | -1.9 kg vs. -2.8 kg | n.a. | n.a. | Improved cholesterol and triglyceride |
No difference between groups. ALT, alanine aminotransferase; GI, glycaemic index; HbA1c, glycated haemoglobin; HOMA-IR, homeostasis model of assessment - insulin resistance; MET, metabolic equivalent of tasks; NAFLD, non-alcoholic fatty liver disease; n.a., not assessed; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; PA, physical activity.
Fig. 1.
Summary of the lifestyle treatment options through the course of NAFLD (modified from115). NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
A single-arm clinical trial of 261 patients with biopsy-proven NASH who underwent repeat liver biopsies after 12 months of low-fat hypocaloric diet (750 kcal less than daily requirement) in association with 200 min/week of low intensity exercise (walking), has provided important information about the impact of lifestyle-induced weight loss on liver histology.10 The likelihood of NASH resolution and fibrosis regression was proportional to the degree of weight loss, being 10% and 16%, respectively, in those who lost ≪5% of total body weight, compared to 90% and 45%, respectively, in individuals who lost ≥10% of total body weight. The correlation between weight loss induced by lifestyle change and NAFLD improvement was further supported by a 12-month randomised clinical trial involving an American Dietetic Association low-fat, low-glycaemic index diet in association with 90–150 min/week of moderate-intensity exercise.6 Resolution of NAFLD (determined by magnetic resonance spectroscopy [MRS]) occurred in 50% of those losing 5.0–6.9% of body weight, 60% of those losing 7.0–9.9% of body weight and 97% of those losing ≥10% of total body weight. Thus, resolution of NAFLD and NASH, and even fibrosis reversal can be achieved with significant lifestyle-induced weight loss. Importantly, baseline BMI influences the likelihood of NAFLD resolution (assessed by MRS), with a 3–5% body weight reduction sufficient for resolution in 50% of non-obese individuals, compared to the 7–10% body weight reduction required to achieve a similar outcome in obese individuals.11 Thus, less weight loss may be required for non-obese individuals undergoing lifestyle therapy.
Although lifestyle-induced weight loss has the potential to be highly efficacious, clinical trials based in tertiary centres, community-based interventions and novel internet-based interventions consistently demonstrate that only 10–20% of individuals are able to lose ≥10% of their body weight over a 1–2 year period.6,10,12 Patient and disease factors influence the efficacy of lifestyle-induced weight loss, with NASH resolution less likely if individuals are morbidly obese (BMI ≥35 kg/m2), have type 2 diabetes (T2DM) or severe NASH demonstrated by significant hepatocyte ballooning.10 Interestingly, the PNPLA3 rs738409 gene polymorphism which is associated with an increased risk of developing NAFLD, was also associated with a 3-fold greater reduction in hepatic triglyceride in response to lifestyle intervention, suggesting that these individuals may be more sensitive to the benefits of lifestyle treatment.13
The majority of clinical trials examining lifestyle treatment in NAFLD are relatively short (3–12 months), raising concern about the long-term efficacy of this approach given the well-established rebound of weight, which often occurs following the cessation of an intervention. Nonetheless, persistent effects have been observed in patients with NAFLD for up to 5 years after 6–12 months of lifestyle intervention, with less weight gain and a greater likelihood of alanine aminotransferase normalisation and sustained improvement in hepatic fat content than those not undergoing lifestyle therapy.11,14 Thus, the impact of a well-designed lifestyle treatment may last for several years despite a lack of ongoing prescribed intervention.
Lifestyle therapy for NASH-related cirrhosis
A lack of clinical data limits firm recommendations regarding the optimal lifestyle programme for patients with NASH-related cirrhosis. Nevertheless, careful consideration is given to avoid excess caloric restriction due to concerns about exacerbating sarcopenia, which is a predictor of poorer outcomes in obese cirrhotic patients.15 Current European Guidelines recommend a daily protein intake of 1.2–1.5 g/kg of body weight/day for all with NASH-related cirrhosis and modest caloric reductions of 500–800 kcal/day in combination with a physical activity/exercise programme in obese patients.16 In addition, small frequent meals are advised with screening for micronutrient and vitamin deficiency. A short-term (16-week) lifestyle intervention in overweight/obese cirrhotic patients with mild portal hypertension (25% of whom had NASH) led to a ≥20% reduction in hepatic venous portal gradient in 24% of individuals, which was associated with a reduction in body fat and improvement in cardiorespiratory fitness and quality of life.17 Longer studies, with adequate patient numbers, are required to assess the impact on liver outcomes such as decompensation.
Impact of different diets on NAFLD
Altering the patient’s nutrition, with or without weight loss, is a key component of an effective lifestyle programme. The impact of the macronutrient composition of a dietary intervention is dependent upon whether the diet is isocaloric or hypocaloric. Saturated fats appear to be particularly problematic with hypercaloric intakes preferentially increasing de novo lipogenesis and liver fat content in comparison to unsaturated fat and carbohydrates.18 Short-term (2–4 weeks) studies of isocaloric diets demonstrate that a high-fat (43–56%)/low-carbohydrate (30–38%) intake increases hepatic triglyceride content in comparison to a low-fat (16–23%)/high-carbohydrate (57–65%) intake.19 A more extreme isocaloric very low-carbohydrate (4%) diet in association with high-fat (72%) and -protein (24% of energy) was shown to reduce hepatic de novo lipogenesis, increase fatty acid oxidation and significantly reduce (-43%) hepatic fat over 2 weeks.20 Interestingly, this diet altered gut microbiota composition and function, suggesting it may mediate diet-induced changes in hepatic fat in the absence of significant weight loss. This short-term study used a diet which is not palatable over the longer term, however, it provides proof of concept of the role of diet and gut microbiome modulation in lifestyle treatment of NAFLD. Lastly, the role of dietary protein is unclear, with cross-sectional studies suggesting an association between high protein intake and NAFLD in the elderly.21 However, short-term interventional studies have demonstrated that increasing dietary animal or plant protein to 30% of energy requirements (with carbohydrates 40% and fat 30%) reduces hepatic fat by 36–48% over 6 weeks, perhaps by altering peripheral adipose metabolism.22 Similarly, in weight stable patients with T2DM, a high-protein (30%) diet with 30% carbohydrate and 40% fat led to a reduction in hepatic steatosis over 6 weeks,23 suggesting that replacing fat or carbohydrate with protein intake may be a successful strategy to reduce hepatic steatosis.
It is notable that longer duration (3–6 months) hypocaloric diets, resulting in weight loss, lead to equivalent reductions in hepatic triglyceride content regardless of whether they are low carbohydrate (10–30%) or low fat (20%).24,25 Among habitual consumers of sugar-containing drinks, reducing intake from 10% to ≤1% of daily calories may be an important component of nutritional treatment, as this reduces hepatic triglyceride content independently of weight loss.26 This may in part be due to a reduction in fructose, a type of sugar which upregulates de novo hepatic lipogenesis. In keeping with this, short-term isocaloric fructose restriction reduces hepatic lipid content in obese children.27 However, a study in overweight men demonstrated equivalent increases in hepatic triglyceride content with either a high-fructose or high-glucose diet, suggesting excessive amounts of both fructose and glucose should be minimised in patients with NAFLD.28
Beyond the characterisation of diets by their fat or carbohydrate content, the Mediterranean diet is rich in mono-unsaturated fatty acids, with high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains and fish.29 It has been associated with a reduction in cardiovascular morbidity and mortality and is thus an attractive lifestyle adjunct for patients with NAFLD, in whom the leading cause of death is CVD.30 Two randomised-controlled studies have demonstrated that the Mediterranean diet reduces hepatic triglyceride to a greater degree than a low-fat (≪30%) diet, whereas a third study using ad libitum diets demonstrated equivalence.3,31,32 Notably, the latter trial which focussed upon altering dietary macronutrient composition rather than weight loss per se, demonstrated that a 25–30% relative reduction in hepatic steatosis can be achieved using this approach. The Mediterranean diet was easier to adhere to than a low-fat diet (88% vs. 64%) and improved CVD risk factors, including lipids and glycated haemoglobin, to a greater degree, supporting its inclusion in the European Guideline recommendations.33
Role of alcohol
NAFLD, by definition, requires the absence of significant alcohol ingestion, however low levels of consumption are ‘allowed’, typically falling within an average of less than 30 g/day for men and 20 g/day for women. It remains unclear whether low-level consumption is harmful in terms of the development of NAFLD, NASH or fibrosis. Cross-sectional studies have suggested low-level (≤20 g/day) consumption, particularly wine in a non-binge fashion, are associated with reduced risk of liver fibrosis.34,35 A longitudinal study of 285 patients with NAFLD undergoing paired liver biopsies found that baseline alcohol consumption was associated with a lower likelihood of NASH resolution but not an increased risk of fibrosis progression.36 A recent population-based study found an increased risk of advanced liver disease, but a reduced risk of CVD and no impact on all-cause mortality, in patients consuming more than 10 g/day of alcohol.37 In contrast, it is clear that any form of alcohol consumption should be avoided in cirrhotic patients due to the increased risk of HCC.38
Vitamins and antioxidants
Micronutrients, including vitamins D and E, have antioxidant and immune-modulatory effects and have been implicated in the pathogenesis of NAFLD and NASH. Although observational studies have implicated low vitamin D levels in the development of liver injury, fibrosis and incident NAFLD,39,40 small clinical trials have not demonstrated any impact on liver histology.41,42 In contrast, vitamin E, which has potent antioxidant properties, has been shown to lead to NASH resolution in 36–58% of adults and children, respectively, though not fibrosis improvement.43,44 EASL and AASLD guidelines recommend considering vitamin E (800 IU/day) for the treatment of non-diabetic non-cirrhotic patients with NASH, although, a recent trial also demonstrated efficacy in patients with T2DM.45 However, a meta-analysis of clinical trials not specifically targeting patients with NAFLD has suggested a small increased risk of mortality with vitamin E supplementation, raising concern about its long-term use.46
Coffee and tea have antioxidant effects, in part related to polyphenol compounds, with green tea and coffee having similar and slightly higher antioxidant content than black tea.47 Cross-sectional studies in selected populations of patients with biopsy-proven NAFLD, suggest that coffee is associated with less liver fibrosis, particularly in those with lower degrees of insulin resistance.48 Nonetheless, population studies have been conflicting regarding whether tea or coffee ingestion is associated with hepatic steatosis,49,50 although others have demonstrated a reduction in chronic liver disease (all-cause) and HCC.51 However, clinical trial data are lacking and thus it is premature to recommend coffee as an effective treatment for NASH, although it appears not to be detrimental. Interestingly, a small human cross-over trial of patients with NASH found that ingestion of 40 g/day of dark chocolate (≫85% cocoa) improved serum markers of oxidative stress and apoptosis, suggesting it may be of some benefit.52
Diet plus exercise versus diet alone
The additive impact of exercise with nutritional intervention on hepatic steatosis is not well-established in patients with NAFLD, with studies limited to patients with metabolic risk factors such as obesity, T2DM or dyslipidaemia rather than NAFLD per se. A recent 18-month study of 278 individuals with dyslipidaemia or central obesity (of which half had NAFLD), found no additive impact of exercise on top of dietary intervention on hepatic fat content or cardiovascular risk parameters.3 Similarly, an 8-week trial of 45 patients with T2DM (with and without NAFLD) found that exercise had no additional impact on hepatic triglyceride content when used on top of a diet high in mono-unsaturated fat, or a high-carbohydrate/low-glycaemic index diet.53 In contrast, in a study of 130 severely obese individuals (BMI ≫35 kg/m2), adding a physical activity programme to a dietary intervention resulted in greater weight loss and hepatic fat reduction than the dietary intervention alone over 6 months.54 A small 6-month trial in elderly (≫65) obese adults found similar reductions in body weight and hepatic triglyceride content in diet vs. diet plus exercise groups, however, the diet and exercise group benefited from significant reductions in serum lipids and blood pressure.55 Lastly, a small (n = 41) 6-month study of patients with NAFLD who underwent paired liver biopsies, only found significant improvements in the NAFLD activity score in patients undergoing dietary intervention plus exercise, but not in those who underwent an exercise intervention alone.8 Thus, exercise may only have a marginal additional effect on hepatic steatosis in the setting of significant diet-induced weight loss, however, it is likely to lead to cardiovascular benefits, which remain an important goal of treatment for patients with NAFLD.
Sedentary behaviour, physical activity and exercise in NAFLD
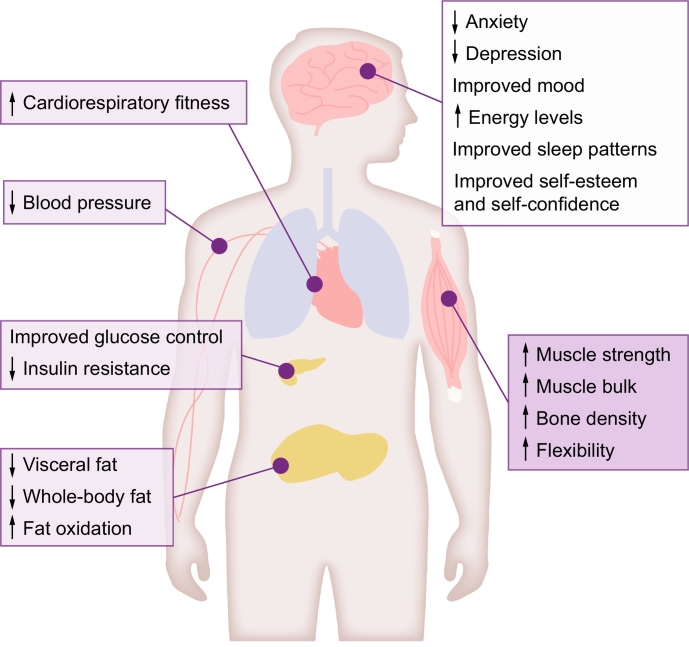
Physical activity and exercise are key regulators of metabolism and are recommended for people with NAFLD, usually alongside weight loss and dietary modification. Physical activity and exercise have both been shown to improve hepatic steatosis in NAFLD and should be included as part of the clinical care of all patients, regardless of where they sit on the NAFLD disease spectrum.33,56 Physical activity and exercise confer benefits, independent of weight loss, and are useful for patients struggling to lose weight through dietary change, as well as being a key tool in weight loss maintenance. Both aerobic and resistance training effectively reduce hepatic steatosis57,58 and arguably more importantly in this patient group, reduce CVD risk.59 The type of exercise programme should be tailored to a patient’s preference, physical fitness and comorbidities, to facilitate maintenance in the long-term and should be used as an adjunct to dietary change. Reducing or breaking up sedentary time should also be a key therapeutic target for all patients. Fig. 2 summarises the extrahepatic benefits of physical activity and exercise for patients with NAFLD.
Fig. 2.
Extrahepatic benefits of physical activity and exercise for patients with NAFLD. Physical activity and exercise offer a host of extrahepatic benefits for patients with NAFLD across the disease spectrum and are useful tools to aid weight-loss maintenance. Increasing cardiorespiratory fitness and muscle strength can help to maintain and improve the ability to perform day-to-day activities and thus improve quality of life. NAFLD, non-alcoholic fatty liver disease.
Sedentary behaviour and NAFLD
Sedentary behaviour refers to “any waking activity characterised by a low level of energy expenditure (≪1.5 metabolic equivalent of tasks or METs) and a sitting or reclining posture” (e.g. watching the TV, using a computer, travelling by car or bus).60 Sitting for ≥3 h a day has been linked to an increase in all-cause mortality (relative risk 1.30; 95% CI 1.06–1.56)61 and sedentary behaviour in general is reported to be higher in people predisposed to developing metabolic syndrome, obesity, T2DM62 and NAFLD.60 One study reported that with every 1 h increase in television viewing per day, there was a 26% rise in the prevalence of metabolic syndrome.63 A more recent study found a 1.15% (95% CI 1.14–1.50%) increase in hepatic triglyceride content was associated with each additional hour of time spent sedentary per day.64 Prospective cohort studies have identified sedentary behaviour as an independent risk factor for NAFLD,65 thus, increases in sedentary behaviour play a potential role in the development and progression of the disease.
It is not just the total amount of sedentary time that is important, it is how this is accumulated over the course of the day and if/how this is broken up with activity. Interruptions in sedentary time have been shown to be beneficial for weight control, glucose and triglyceride levels.66 To date, it is not clear whether a minimum duration and/or intensity of activity is required for the interruption of sedentary behaviour to have beneficial metabolic effects. However, decreasing overall sedentary time and increasing breaks throughout the day is a useful therapeutic message to relay to all people with NAFLD/NASH, and may be perceived as being more achievable by patients initially than increasing physical activity levels. Any means of increasing energy expenditure, whether it be at home, work or during leisure time, may exert positive metabolic benefits and aid weight loss/weight loss maintenance.
Physical activity and NAFLD
Physical activity is any activity that requires use of more energy than the body requires at rest, and incorporates many of the activities carried out as part of the daily routine (e.g. housework, gardening, walking up/down stairs).60 General health guidelines promote at least 150 min/week of moderate-vigorous leisure time physical activity or 10,000 steps per day for the primary prevention of CVD and to decrease the risks of metabolic diseases.59,67,68 However, most people in the general population do not reach this target, which may be contributing to the rising numbers of people affected by obesity, T2DM and NAFLD.
Cross-sectional studies, using self-reporting methodologies, suggest that physical activity levels are lower in people with NAFLD than those without.[69], [70], [71] Krasnoff et al. found that ≫80% of people with NAFLD did not meet recommended physical activity guidelines of 30 min of moderate-intensity exercise ≥3 times/week.72 A recent study that objectively measured activity levels revealed that people with NAFLD not only carried out less physical activity, but also undertook less moderate and vigorous activity than people without NAFLD on a day-to-day basis.73 Lower levels of these higher intensity activities may have general health implications, as the intensity of the activity/exercise could also play an important role in reducing CVD risk and improving metabolic control.59,74
A recent longitudinal follow-up study evaluated the impact of physical activity on the natural course of NAFLD in 5,860 adults.75 After multivariate adjustments, individuals without NAFLD (based on ultrasonography) who were already active or became physically active were less likely to develop NAFLD compared with those that remained inactive (OR = 0.75, p = 0.03 and 0.75, p = 0.04, respectively), irrespective of BMI. Of those with NAFLD at baseline, remaining active or becoming physically active improved NAFLD status (i.e. there was a disappearance of hepatic steatosis on ultrasound) (OR = 0.66, p = 0.01 and 0.64, p = 0.01, respectively). However, the significance was lost when adjusted for changes in BMI, reinforcing the importance of weight loss alongside physical activity in people with established NAFLD.
There is a dose-response relationship between NAFLD and physical activity (both prevalence and disease severity),[76], [77], [78] therefore, the more physical activity performed throughout the day, the better! Evidence suggests that multiple short bouts of physical activity throughout the day can be just as good as long periods of activity and may be more achievable for some patients with NAFLD.79 A recent randomised study investigated the effects of different doses and intensities of physical activity on hepatic steatosis and found even the group with the lowest physical activity dose and intensity (low-to-moderate intensity, low dose 50% VO2 peak, 45 min, 3 days/week) showed a reduction in hepatic and visceral fat independent of weight loss.80 Increasing daily walking has also been shown to improve fat oxidation81 and is a way of increasing physical activity levels at no cost and without additional equipment. Encouraging patients to be as active as possible within their daily routine is often a good place to start, prior to discussing structured exercise, especially with deconditioned patients or those with comorbidities. Patients should be encouraged to self-monitor their daily physical activity levels (using pedometers/activity trackers/activity diaries) as this has been shown to improve long-term adherence to physical activity/lifestyle interventions and increases the likelihood of patients achieving their activity-related goals.82
Exercise and NAFLD
Exercise is a subcategory of physical activity in which “planned, structured and repetitive movements are performed to maintain or improve fitness” (e.g. running, strength training, Pilates, yoga).60 To date, most exercise studies have focussed on the mild end of the disease spectrum (i.e. non-fibrotic/non-cirrhotic NAFLD), with changes in hepatic steatosis as the primary outcome.
A systematic review in 201757 identified 24 exercise-only studies in NAFLD and revealed that exercise produced a 20–30% relative reduction in hepatic steatosis, independent of weight loss. The data suggests that different types of exercise (aerobic, resistance/strength training, or high-intensity interval training [HIIT]) have a relatively similar effect on liver fat.57,[83], [84], [85], [86] This is good news for our patients as it offers them an element of choice with regards to the type of exercise they prefer to undertake to improve their liver health. Similarly, patients may have comorbid conditions (e.g. arthritis, CVD) which suit either an aerobic or resistance training regime.
Recent research has focussed on the effects of different intensities of exercise in NAFLD. Exercise intensity refers to the amount of effort that must be invested in a specific workout. The American College of Sports Medicine generally recommends a target heart rate of 55–69 HRmax% for moderate-intensity and 70–89 HRmax% for vigorous-intensity aerobic exercise.60 A recent meta-analysis found continuous high-intensity training more effective at reducing liver fat than moderate-intensity training or HIIT.86 However, this type of exercise may be unsuitable and unsustainable for the majority of patients with NAFLD. Other studies have demonstrated that the intensity of exercise has little effect on the level of improvement in liver fat – moderate-intensity exercise is equally as effective in reducing liver fat as vigorous-intensity exercise80,87 and more people are likely to adhere to a lower intensity exercise programme.88 However, vigorous-intensity exercise was shown to have a greater impact on cardiac risk factors in people with NAFLD, including reductions in whole body fat, visceral fat and blood pressure.87,89 Clinically, we should be encouraging patients to undertake exercise at an intensity suitable for their current fitness level and matched to their personal goals.
It should be acknowledged that, to date, most of the exercise studies in NAFLD have been relatively small, in terms of the number of recruits, and have a wide range of variability in terms of their protocol intensities and duration (usually between 8–12 weeks). A longer term study shows that if patients continue to exercise for 12 months the benefits continue,87 and improvements in liver fat, abdominal obesity and blood pressure persist 1 year after the exercise intervention has ended, although the benefits are reduced.89 However, another study in NAFLD found that if patients do not continue to exercise regularly, the benefits gained during the intervention are lost.90 This supports the need for patients to be able to maintain any lifestyle changes they make in the long-term to improve liver, cardiac and metabolic health.
The optimal frequency, intensity, duration and type of physical activity/exercise for NAFLD/NASH management remains unclear. This means that the recommendations within the clinical guidelines are non-specific.33,56 Ultimately, the most effective physical activity/exercise prescription for NAFLD/NASH should be tailored to the individual patient’s clinical characteristics, comorbidities, current fitness and preference.
Aerobic exercise and NAFLD
Aerobic exercise, sometimes referred to as “cardio” or cardiovascular exercise, is “any activity that uses large muscle groups and can be maintained continuously over a period of time”.60 Aerobic exercise overloads the heart and lungs, causing them to work harder than at rest and, if performed regularly, improves cardiorespiratory fitness. One of the key benefits of regular aerobic exercise, is a reduction in CVD risk – this is important to remember in our NAFLD/NASH patient group, as only 9% will die a liver-related death, compared with 38% that will die from CVD.91
Multiple studies and reviews have highlighted the benefits, independent of weight loss, of aerobic exercise in NAFLD.57,58,83 The protocols used in these studies largely follow the guidelines for physical activity prescription in the general population of 150 min moderate-to-vigorous intensity exercise per week67,68 and utilise a combination of static cycling, walking/jogging and circuit-based exercise. These exercise levels may be beyond the initial targets that a large proportion of patients with NAFLD should be aiming for, as their baseline exercise levels will be significantly lower.72,73
One barrier to exercise people often cite is lack of time. HIIT is a relatively new method of exercising. HIIT consists of aerobic exercise divided into high-intensity bouts interspersed with recovery periods; it can offer equivalent or superior benefits to cardiorespiratory fitness than continuous moderate-intensity exercise of a longer duration.92 Studies have found that some volunteers prefer HIIT to continuous exercise routines as it is less time consuming.93,94 HIIT has also been shown to improve hepatic steatosis (27% relative reduction) and cardiac function in patients with NAFLD85 and is another option to offer patients in the clinical setting.
It is worth noting that more vigorous-intensity aerobic exercise does not hold additional benefit for reducing hepatic steatosis compared with moderate-intensity aerobic exercise.80,87 However, vigorous exercise is of benefit for CVD risk management60,87,89 – the majority of patients with NAFLD would benefit from a combined exercise approach that targets not only liver health but also reduced T2DM and CVD risk. For weight maintenance it is important to focus on increasing the volume of exercise; to improve cardiorespiratory fitness and glycaemic control it is more important to increase the intensity of exercise.74
Resistance exercise and NAFLD
Resistance exercise, often known as strength or weight training, works the muscles against a load. Resistance exercise offers an alternative to aerobic exercise and can improve muscular strength, muscle mass and metabolic control, safely and effectively.95 Resistance exercise has been shown to modestly decrease (13% relative reduction) liver fat in NAFLD and increase whole body fat burning capacity, independent of weight loss.96 It places less demand on the cardiorespiratory system and may be accessible to more patients,97 proving a particularly useful tool in the management of our patients with NAFLD and multiple comorbidities.
Resistance exercise could also be beneficial for weight control, not only because of the direct caloric cost of the activity and the residual elevation of the post-exercise VO2 but also because of the greater post-exercise fat oxidation. Energy expenditure has been found to be elevated for up to 48 h after an acute bout of heavy resistance exercise.98 Metabolic activity was increased by 21% in the first 24 h period following exercise and by 19% in the subsequent 24 h period. These differences could equate to 404 kcal and 369 kcal increases in extra energy expenditure per day, respectively, for average build individuals.98 Thus, the energy required to recover from a resistance training session may be of significant use in a weight control/weight loss programme.
Evidence for NASH and cirrhosis
Most clinical trials have focussed on the effects of physical activity/exercise on hepatic triglyceride content, assessed by MRS or imaging, without knowledge of the underlying degree of liver inflammation or fibrosis. A retrospective, cross-sectional analysis by the NASH Clinical Research Network showed that people who self-reported meeting vigorous-intensity exercise targets (≥6 MET, minimum target 75 min/week) were less likely to develop NASH than those that reported meeting moderate-intensity activity targets (3.0–5.9 MET, minimum target 150 min/week) (OR 0.65; 95% CI 0.43–0.98 vs. OR 0.53; 95% CI 0.29–0.97, respectively).76 The total amount of time spent exercising per week was not associated with NASH or stage of fibrosis.
A randomised-controlled trial including 24 patients with biopsy-proven NASH showed that a 12-week programme combining aerobic and resistance exercise (without weight loss), reduced liver and visceral fat but had no effect on circulating inflammatory markers or non-invasive markers of fibrosis.99 O’Gorman et al. recently reported significant regression in fibrosis, demonstrated by paired liver biopsies, following a 12-week aerobic exercise intervention in 16 patients with NAFLD.100 However, the exercise group lost a significant amount of weight, which makes it difficult to decipher whether it was the effect of exercise per se or the weight loss which conferred the anti-fibrotic effect.
There is even less evidence for the effects of physical activity/exercise in NASH-related cirrhosis. Patients with cirrhosis are at risk of increasing debilitation and frailty, and obese, particularly older cirrhotics may be at increased risk of sarcopenia.101 Maintaining functional status and improving quality of life are important in this patient group. Supervised and home exercise programmes, combining aerobic and resistance exercise in small groups of cirrhotic patients, including some with NASH, have been shown to be safe with no apparent increased risk of variceal haemorrhage or encephalopathy; these programmes have also been shown to be effective, leading to increases in lean body mass, reductions in fat mass, greater mobility and a marginal reduction in hepatic venous pressure gradient.[102], [103], [104], [105]
Barriers and facilitators to implementing lifestyle change
Although the evidence demonstrates lifestyle interventions can be effective tools for managing NAFLD and clinical guidelines recommend lifestyle changes, there is a substantial disconnect between these ideals and how care is delivered in routine clinical practice.106,107 Many clinicians feel they lack the training, tools and time to effectively deliver lifestyle interventions,106 and report the need for a multidisciplinary team to adequately manage NAFLD including a range of lifestyle intervention options to support maintenance of long-term behaviour change.106,108,109 This is a luxury not afforded in most clinical setups.
Patients have reported insufficient provision of information following a diagnosis of NAFLD and a lack of support to help them address their condition.106,109,110 A patient’s understanding and comprehension of their diagnosis is vital in order to motivate effective and lasting lifestyle change. A lack of understanding and acknowledgement of the link between current lifestyle habits and their NAFLD/NASH will hinder any meaningful change - patients need to understand that NAFLD/NASH is potentially reversible if they make and sustain these changes.106,[109], [110], [111]
There are multiple behaviour change techniques (BCTs) that have been shown to be effective in the management of patients with chronic diseases.[112], [113], [114] These BCTs aid clinicians to support patients in making informed decisions about their behaviour(s) and encourage patients to take a level of responsibility for their own health. Motivational interviewing techniques are also useful during consultations in a bid to empower patients to make their own health-related decisions.111 Fig. 3 illustrates practical examples of inexpensive tools that can be integrated into consultations with patients in the clinical setting and are useful techniques to encourage patients to think about lifestyle changes. Box 1 lists some practical tips for clinicians on how to support patients with NAFLD to make lifestyle changes.
Fig. 3.
Useful techniques to support patients to make and sustain lifestyle changes110,112,114,116
Box 1. Practical tips on how to support patients to make lifestyle changes for clinicians.111.
-
1.
Explain what NAFLD is and that it is reversible with lifestyle change; address any misconceptions e.g. alcohol being the cause of NAFLD
-
2.
Explain energy balance in relation to body weight changes
-
3.
Set a SMART (see Fig. 3) weight loss target
-
4.
Use appropriate interventions e.g. regular meal patterns, reduced snacking, portion control
-
5.Encourage use of self-regulation/self-monitoring tools e.g.
-
a.Regular weighing
-
b.Count daily calories to track food intake using a diary, smartphone app or internet websites
-
c.Use of pedometers/activity trackers
-
d.Read nutrition information labels to check, compare & choose healthier options
-
e.Develop skills in meal planning, shopping, food preparation & cooking
-
a.
-
6.
Signposting to local Exercise Schemes, community gyms, weight management programmes, walking groups
Alt-text: Box 1
Conclusion
Lifestyle interventions can be highly effective in treating NAFLD across the disease spectrum and offer a holistic way of managing not only liver health, but also cardiovascular and metabolic health. Often changing lifestyle behaviours can be difficult for our patients to achieve, but with individualised support, significant long-term changes are possible. Further investigation is required to determine the optimal nutritional therapy in combination with physical activity/exercise which improves liver injury and fibrosis; additional evidence is needed to determine which patients are most likely to gain from lifestyle intervention and in particular, whether baseline fibrosis stage influences the efficacy of lifestyle modification. However, if lifestyle change is achieved and sustained, the benefits to liver, cardiac and metabolic health can surpass the efficacy of the drugs currently being evaluated in phase III trials. Thus, lifestyle modification should remain the primary focus for all patients with NAFLD.
Abbreviations
ALT, alanine aminotransferase; BMI, body mass index; CVD, cardiovascular disease; GI, glycaemic index; HbA1c, glycated haemoglobin; HIIT, high-intensity interval training; HOMA-IR, homeostasis model of assessment - insulin resistance; MET, metabolic equivalent of tasks; MRS, magnetic resonance spectroscopy; NAS, NAFLD activity score; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PA, physical activity.
Financial support
KH is funded by a Clinical Lectureship (grant number CAT CL-2013-04-010) supported by the National Institute for Health Research and Health Education England. The views expressed in this manuscript are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
KH and LA drafted the manuscript and both authors contributed to the writing and critical review for important intellectual content of the final manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.10.008.
Supplementary data
Supplementary material
References
- 1.Collaboration NCDRF Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 3.Gepner Y, Shelef I, Komy O, Cohen N, Schwarzfuchs D, Bril N. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71:379–388. doi: 10.1016/j.jhep.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 4.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24:399–407. doi: 10.1111/j.1440-1746.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 5.Sun WH, Song MQ, Jiang CQ, Xin YN, Ma JL, Liu YX. Lifestyle intervention in non-alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol. 2012;4:224–230. doi: 10.4254/wjh.v4.i7.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Ther Adv Gastroenterol. 2013;6:249–259. doi: 10.1177/1756283X13484078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 10.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. e365. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349–1356. doi: 10.1016/j.jhep.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–1163. doi: 10.1016/j.jhep.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Wong GL, Chan HL, Chan HY, Yeung DK, Chan RS. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther. 2014;39:532–539. doi: 10.1111/apt.12609. [DOI] [PubMed] [Google Scholar]
- 14.Haufe S, Haas V, Utz W, Birkenfeld AL, Jeran S, Bohnke J. Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care. 2013;36:3786–3792. doi: 10.2337/dc13-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706–1717. doi: 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 18.Luukkonen PK, Sadevirta S, Zhou Y, Kayser B, Ali A, Ahonen L. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H. Nutritional Modulation of Non-Alcoholic Fatty Liver Disease and Insulin Resistance. Nutrients. 2015;7:9127–9138. doi: 10.3390/nu7115454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Rasanen SM. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27:559–571.e555. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alferink LJ, Kiefte-de Jong JC, Erler NS, Veldt BJ, Schoufour JD, de Knegt RJ. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: the Rotterdam Study. Gut. 2018;68:1088–1098. doi: 10.1136/gutjnl-2017-315940. [DOI] [PubMed] [Google Scholar]
- 22.Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology. 2017;152:571–585.e578. doi: 10.1053/j.gastro.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Skytte MJ, Samkani A, Petersen AD, Thomsen MN, Astrup A, Chabanova E. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;Jul 23 doi: 10.1007/s00125-019-4956-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haufe S, Engeli S, Kast P, Bohnke J, Utz W, Haas V. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 26.Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. 2019;321:256–265. doi: 10.1001/jama.2018.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology. 2017;153:743–752. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145:1016–1025.e1012. doi: 10.1053/j.gastro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37:936–949. doi: 10.1111/liv.13435. [DOI] [PubMed] [Google Scholar]
- 30.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 31.Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology. 2018;68:1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 32.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 33.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell T, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Ching H. Type and Pattern of Alcohol Consumption is Associated With Liver Fibrosis in Patients With Non-alcoholic Fatty Liver Disease. Am J Gastroenterol. 2018;113:1484–1493. doi: 10.1038/s41395-018-0133-5. [DOI] [PubMed] [Google Scholar]
- 35.Hagström H, Nasr P, Ekstedt M, Kechagias S, Önnerhag K, Nilsson E. Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2017;52:159–165. doi: 10.1080/00365521.2016.1239759. [DOI] [PubMed] [Google Scholar]
- 36.Ajmera V, Belt P, Wilson LA, Gill RM, Loomba R, Kleiner DE. Among Patients With Nonalcoholic Fatty Liver Disease, Modest Alcohol Use Is Associated With Less Improvement in Histologic Steatosis and Steatohepatitis. Clin Gastroenterol Hepatol. 2018;16:1511–1520. doi: 10.1016/j.cgh.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Åberg F, Puukka P, Salomaa V, Männistö S, Lundqvist A, Valsta L. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology. 2019, Jul 19 doi: 10.1002/hep.30864. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443–457. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-κB? Am J Gastroenterol. 2016;111:852–863. doi: 10.1038/ajg.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black LJ, Jacoby P, She Ping-Delfos WC, Mori TA, Beilin LJ, Olynyk JK. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol. 2014;29:1215–1222. doi: 10.1111/jgh.12541. [DOI] [PubMed] [Google Scholar]
- 41.Geier A, Eichinger M, Stirnimann G, Semela D, Tay F, Seifert B. Treatment of non-alcoholic steatohepatitis patients with vitamin D: a double-blinded, randomized, placebo-controlled pilot study. Scand J Gastroenterol. 2018;53:1114–1120. doi: 10.1080/00365521.2018.1501091. [DOI] [PubMed] [Google Scholar]
- 42.Kitson MT, Pham A, Gordon A, Kemp W, SK R. High-dose vitamin D supplementation and liver histology in NASH. Gut. 2016;65:717–718. doi: 10.1136/gutjnl-2015-310417. [DOI] [PubMed] [Google Scholar]
- 43.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 46.Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. 2013;8:e74558. doi: 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yashin A, Yashin Y, Wang JY, Nemzer B. Antioxidant and Antiradical Activity of Coffee. Antioxidants. 2013;2:230–245. doi: 10.3390/antiox2040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bambha K, Wilson LA, Unalp A, Loomba R, Neuschwander-Tetri BA, Brunt EM. Coffee consumption in patients with NAFLD with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014;34:1250–1258. doi: 10.1111/liv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alferink LJM, Fittipaldi J, Kiefte-de Jong JC, Taimr P, Hansen BE, Metselaar HJ. Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: The Rotterdam study. J Hepatol. 2017;67:339–348. doi: 10.1016/j.jhep.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Graeter T, Niedermayer PC, Mason RA, Oeztuerk S, Haenle MM, Koenig W. Coffee consumption and NAFLD: a community based study on 1223 subjects. BMC Res Notes. 2015;3:640. doi: 10.1186/s13104-015-1645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118–125. doi: 10.1053/j.gastro.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loffredo L, Del Ben M, Perri L, Carnevale R, Nocella C, Catasca E. Effects of dark chocolate on NOX-2-generated oxidative stress in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2016 Aug;44:279–286. doi: 10.1111/apt.13687. Epub 2016 Jun 6 2016;44:279-286. [DOI] [PubMed] [Google Scholar]
- 53.Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35:1429–1435. doi: 10.2337/dc12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 57.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 2017;66:142–152. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 60.ACSM . 5th ed. Lippincott Williams and Wilkins; 2006. ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 61.Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30:516–522. doi: 10.2337/dc06-1996. [DOI] [PubMed] [Google Scholar]
- 63.Dunstan D, Salmon J, Owen N, Armstrong T, Zimmet P, Welborn T. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia. 2005;48:2254–2261. doi: 10.1007/s00125-005-1963-4. [DOI] [PubMed] [Google Scholar]
- 64.Bowden Davies KA, Sprung VS, Norman JA, Thompson A, Mitchell KL, Harrold JOA. Physical Activity and Sedentary Time: Association with Metabolic Health and Liver Fat. Med Sci Sports Exerc. 2019;51:1169–1177. doi: 10.1249/MSS.0000000000001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–1237. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ. Breaks in Sedentary Time: Beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 67.ACSM American College of Sports Medicine Position Stand. Appropriate physical activity intervention for weight loss and weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 68.Department of Health UK Physical Activity Guidelines. 2011. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_127931 [cited 2012 February]; Available from:
- 69.Hsieh SD, Yoshinaga H, Muto T, Sakurai Y. Regular Physical Activity and Coronary Risk Factors in Japanese Men. Circulation. 1998;97:661–665. doi: 10.1161/01.cir.97.7.661. [DOI] [PubMed] [Google Scholar]
- 70.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I. Role of leisure-time physical activity in nonalcoholic fatty liver disease: A population-based study. Hepatology. 2008;48:1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 71.Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A. Habitual Physical Activity Is Associated With Intrahepatic Fat Content in Humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 72.Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–1166. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51. doi: 10.1136/flgastro-2014-100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keating SE, George J, Johnson NA. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:1247–1250. doi: 10.1586/17474124.2015.1075392. [DOI] [PubMed] [Google Scholar]
- 75.Gerage AM, Ritti-Dias RM, Balagopal PB, Conceição RDO, Umpierre D, Santos RD. Physical activity levels and hepatic steatosis: A longitudinal follow-up study in adults. J Gastroenterol Hepatol. 2018;33:741–746. doi: 10.1111/jgh.13965. [DOI] [PubMed] [Google Scholar]
- 76.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–468. doi: 10.1038/ajg.2010.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–1215. doi: 10.1002/hep.27544. [DOI] [PubMed] [Google Scholar]
- 78.Kwak MS, Kim D, Chung GE, Kim W, Kim YJ, Yoon JH. Role of physical activity in nonalcoholic fatty liver disease in terms of visceral obesity and insulin resistance. Liver Int. 2015;35:944–952. doi: 10.1111/liv.12552. [DOI] [PubMed] [Google Scholar]
- 79.Kwak MS, Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 2018;33:64–74. doi: 10.3904/kjim.2017.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Trenell MI, Hollingsworth KG, Lim EL, Taylor R. Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care. 2008;31:1644–1649. doi: 10.2337/dc08-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carels RA, Darby LA, Rydin S, Douglass OM, Cacciapaglia HM, O'Brien WH. The relationship between self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Ann Behav Med. 2005;30:182–190. doi: 10.1207/s15324796abm3003_2. [DOI] [PubMed] [Google Scholar]
- 83.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 85.Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129:1097–1105. doi: 10.1042/CS20150308. [DOI] [PubMed] [Google Scholar]
- 86.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:1074–1082. doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 88.Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452–458. [PubMed] [Google Scholar]
- 89.Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF, Sun Q. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: A 1-year follow-up study. Diabetes Obes Metab. 2016;19:284–289. doi: 10.1111/dom.12809. [DOI] [PubMed] [Google Scholar]
- 90.Pugh CJ, Sprung VS, Jones H, Richardson P, Shojaee-Moradie F, Umpleby AM. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes. 2016;40:1927–1930. doi: 10.1038/ijo.2016.123. [DOI] [PubMed] [Google Scholar]
- 91.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397. e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guiraud T, Nigam A, Juneau M, Meyer P, Gayda M, Bosquet L. Acute Responses to High-Intensity Intermittent Exercise in CHD Patients. Med Sci Sports Exerc. 2011;43:211–217. doi: 10.1249/MSS.0b013e3181ebc5de. [DOI] [PubMed] [Google Scholar]
- 94.Coquart JB, Lemaire C, Dubart AE, Luttembacher DP, Douillard C, Garcin M. Intermittent versus continuous exercise: effects of perceptually lower exercise in obese women. Med Sci Sports Exerc. 2008;40:1546–1553. doi: 10.1249/MSS.0b013e31816fc30c. [DOI] [PubMed] [Google Scholar]
- 95.Larose J, Sigal RJ, Boule NG, Wells GA, Prud'Homme D, Fortier MS. Effect of Exercise Training on Physical Fitness in Type II Diabetes Mellitus. Med Sci Sports Exerc. 2010;42:1439–1447. doi: 10.1249/MSS.0b013e3181d322dd. [DOI] [PubMed] [Google Scholar]
- 96.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res Clin Pract. 2009;83:157–175. doi: 10.1016/j.diabres.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 98.Schuenke M, Mikat R, McBride J. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur J Appl Physiol. 2002;86:411–417. doi: 10.1007/s00421-001-0568-y. [DOI] [PubMed] [Google Scholar]
- 99.Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients With Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2017;15:96–102. doi: 10.1016/j.cgh.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Gorman P. Abstract presented at ILC. 2019. Significant regression in fibrosis in paired liver biopsies following a 12-week aerobic exercise intervention in NAFLD. [Google Scholar]
- 101.Spengler EK, O'Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation. 2017;101:2288–2296. doi: 10.1097/TP.0000000000001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, Moreno-Tavarez E, Weber-Sangri L, González-Arellano MF. Exercise prescription in patients with cirrhosis: Recommendations for clinical practice. Rev Gastroenterol Mex. 2019;Jun 28 doi: 10.1016/j.rgmx.2019.02.011. [pii: S0375-0906(19)30066-7. Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Wallen MP, Skinner TL, Pavey TG, Hall A, Macdonald GA, Coombes JS. Safety, adherence and efficacy of exercise training in solid-organ transplant candidates: A systematic review. Transplant Rev (Orlando) 2016;30:218–226. doi: 10.1016/j.trre.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Williams FR, Vallance A, Faulkner T, Towey J, Durman S, Kyte D. Home-Based Exercise in Patients Awaiting Liver Transplantation: A Feasibility Study. Liver Transpl. 2019;25:995–1006. doi: 10.1002/lt.25442. [DOI] [PubMed] [Google Scholar]
- 105.Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol. 2016;7:180. doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Avery L, Exley C, McPherson S, Trenell MI, Anstee QM, Hallsworth K. Lifestyle Behavior Change in Patients With Nonalcoholic Fatty Liver Disease: A Qualitative Study of Clinical Practice. Clin Gastroenterol Hepatol. 2017;15:1968–1971. doi: 10.1016/j.cgh.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 107.Zelber-Sagi S. Minding the Gap Between Clinical Trials and Treatment With the Mediterranean Dietary Pattern for Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:1248–1250. doi: 10.1016/j.cgh.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 108.Bellentani S, Grave R, Suppini A, Marchesini G, (FLIN) FLIN Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 109.Haigh L, Bremner S, Houghton D, Henderson E, Avery L, Hardy T. Barriers and Facilitators to Mediterranean Diet Adoption by Patients With Nonalcoholic Fatty Liver Disease in Northern Europe. Clin Gastroenterol Hepatol. 2019;17:1364–1371. doi: 10.1016/j.cgh.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 110.Hallsworth K, Dombrowski SU, McPherson S, Anstee QM, Avery L. Using the theoretical domains framework to identify barriers and enabling factors to implementation of guidance for the diagnosis and management of nonalcoholic fatty liver disease: a qualitative study. Transl Behav Med. 2019 doi: 10.1093/tbm/ibz080. [pii:ibz080.Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hallsworth K, Avery L, Trenell MI. Targeting Lifestyle Behavior Change in Adults with NAFLD During a 20-min Consultation: Summary of the Dietary and Exercise Literature. Curr Gastroenterol Rep. 2016;18:11. doi: 10.1007/s11894-016-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avery L, Flynn D, Dombrowski SU, van Wersch A, Sniehotta FF, Trenell MI. Successful behavioural strategies to increase physical activity and improve glucose control in adults with Type 2 diabetes. Diabet Med. 2015;32:1058–1062. doi: 10.1111/dme.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care. 2012;35:2681–2689. doi: 10.2337/dc11-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Atkins L, Michie S. Designing interventions to change eating behaviours. Proc Nutr Soc. 2015;74:164–170. doi: 10.1017/S0029665115000075. [DOI] [PubMed] [Google Scholar]
- 115.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 116.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material