Abstract
Purpose:
ZIP4 is overexpressed in human pancreatic cancer and promotes tumor growth. However, little is known about the role of ZIP4 in advanced stages of this dismal neoplasm. Our goal is to study the underlying mechanism and define a novel signaling pathway controlled by ZIP4 modulating pancreatic tumor metastasis.
Experimental Design:
The expression of ZIP4, ZO-1, claudin-1, and ZEB1 in human pancreatic cancer tissues, genetically engineered mouse model, xenograft tumor model and pancreatic cancer cell lines were examined, and the correlations between ZIP4 and those markers were also analyzed. Functional analysis of ZO-1, claudin-1, and ZEB1 was investigated in pancreatic cancer cell lines and orthotopic xenografts.
Results:
Genetic inactivation of ZIP4 inhibited migration and invasion in pancreatic cancer and increased the expression of ZO-1 and claudin-1. Conversely, overexpression of ZIP4 promoted migration and invasion, and increased the expression of ZEB1 and downregulation of the aforementioned epithelial genes. ZIP4 downregulation of ZO-1 and claudin-1 requires the transcriptional repressor ZEB1. Further analysis demonstrated that ZIP4-mediated repression of ZO-1 and claudin-1 lead to upregulation of their targets FAK and Paxillin. Silencing of ZIP4 caused reduced phosphorylation of FAK and Paxillin, which was rescued by simultaneous blocking of ZO-1 or claudin-1. Clinically, we demonstrated that ZIP4 positively correlates with the levels of ZEB1 and inversely associates with the expression of ZO-1 and claudin-1.
Conclusions:
These findings suggest a novel pathway activated by ZIP4 controlling pancreatic cancer invasiveness and metastasis, which could serve as a new therapeutic target for this devastating disease.
Keywords: ZIP4, pancreatic cancer, migration, invasion, metastasis, ZO-1, claudin-1, ZEB1
Introduction
Pancreatic cancer is the third leading cause of cancer death in the United States (1). Over 90% of patients with pancreatic cancer are diagnosed at a late stage (2) and the five-year survival rate for patients with distant metastasis is only 7% (3). Pancreatic cancer is a highly invasive and metastatic disease, 60% of patients have distant metastasis within the first 24 months after surgery (4–6), and it is considered one of the primary cause of mortality in these patients (7). Disassembly and perturbation of the tight junction structure with loss of cell-to-cell associations is an essential event in the invasion process. The basement membrane degrades, leading to tumor cell migration and invasion (8). Peripheral or plaque anchoring protein (ZO-1) and integral transmembrane protein (claudin-1) are important tight junction proteins that prevent cell migration in pancreatic cancer (9,10). Previous studies suggest that zinc might regulate the integrity of ZO-1 and claudin-1. Zinc deficiency is known to result in epithelial barrier leak in the pancreas (11) and correlates to reduced levels of the tight junction proteins ZO-1 and claudin-1 through damaging the integrity of the cell connection structure (12). Both molecules are downregulated in pancreatic cancer, understanding the molecular events controlling the aberrant expression of ZO-1 and claudin-1 is of paramount importance, as it could lead to the development of new treatments for this dismal disease.
In the present study, we define a novel mechanism regulating the expression of ZO-1 and claudin-1 in pancreatic cancer cells mediated by ZIP4, a membrane zinc importer encoded by SLC39A4 playing an important role in maintaining cellular zinc concentration by taking up dietary zinc into cells and releasing zinc from cytosol vesicular compartments (13–18). Interestingly, zinc availability is important for tumor invasiveness and metastasis because it modifies tight junction proteins and selectively enhances the epithelial barrier (19). Previously we found that ZIP4 is overexpressed in human pancreatic cancer and promotes tumor growth and metastasis (15–17). Aberrant ZIP4 expression in pancreatic cancer at diagnosis correlates with the expression level of ZIP4 in the corresponding surgical samples, suggesting that ZIP4 may serve as a novel prognostic and predictive marker for pancreatic cancer. Although the biological effects of intracellular zinc and zinc transporters are demonstrated in pancreatic cancer, the molecular mechanisms controlling this phenomenon and the translational significance of this oncogenic axis remain elusive. Here we defined a role for the zinc-dependent transcription factor ZEB1 in the repression of ZO-1 and claudin-1 induced by ZIP4 and in regulating pancreatic cancer migration and metastasis. Decreased levels of ZO-1 and claudin-1 enhance tumor migration and invasion both in vitro and in vivo through targeting their downstream markers FAK and Paxillin. These findings indicate a novel molecular link between ZIP4 and pancreatic cancer invasiveness and metastasis and could serve as foundation for new treatment options for this devastating disease.
Materials and Methods
Chemicals, cell culture, and clinical sample collection.
Human pancreatic cancer cells AsPC-1, Panc-1 and MIA PaCa-2 were recently purchased from the American Type Culture Collection (ATCC, Rockville, MD) in 2016, and were cultured in RPMI 1640 medium or DMEM medium with 10% fetal bovine serum (FBS). The pancreatic cancer tissue microarray contains 72 cases of ductal pancreatic cancer from patients who underwent primary tumor resection at the University of Oklahoma Health Sciences Center (OUHSC) and Peking Union Medical College (PUMC) Hospital (The clinic-pathology data of the pancreatic cancer patients was shown in supplemental table 1). No patients in this study received chemotherapy or radiotherapy before surgery. All patients were given informed consent according to an IRB-approved human protocol and in accordance with NIH guidelines.
Stable cell line construction.
Stable cells with ZO-1 and claudin-1 silenced were selected in AsPC-shZIP4 cells with vector of psi-LVRH1GH (Genecopoeia). ZO-1 and claudin-1 shRNA were cloned into psi-LVRH1GH vector and recombinant plasmid was transfected into 293T cells. Viral supernatants were collected and transduced to the target cells. Stable cells expressing shZO-1 and shcldn-1 (AsPC-shZO-1, AsPC-shcldn-1 or empty vector (AsPC-shV) were selected by adding 400 μg/ml hygromycin into the medium.
RNA extraction and real-time PCR.
ZIP4, ZO-1, claudin-1, and ZEB1 mRNA were analyzed by real-time (RT)-PCR. Briefly, RT-PCR was performed using the SYBR supermix kit (Roche). The PCR reaction included the following components: 100 nM each primer, diluted cDNA templates, and iQ SYBR Green supermix, running for 40 cycles at 95°C for 20 s and at 60°C for 1 m. PCR efficiency was examined by serially diluting the template cDNA. The melting curve data were collected to check PCR specificity. Each cDNA sample was run in triplicate and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The β-actin primer was included in every plate to avoid sample variations. The relative mRNA level was presented as unit values of 2^ [Ct(β-actin) – Ct (gene of interest)]. The primer sequences for human ZIP4 and EMT genes are:
| Gene | Sequence | |
|---|---|---|
| ZIP4 | F | ATGTCAGGAGCGGGTCTTGC |
| R | GCTGCTGTGCTGCTGGAAC | |
| ZO-1 | F | CAACATACAGTGACGCTTCACA |
| R | CACTATTGACGTTTCCCCACTC | |
| claudin-1 | F | GCTGCGAACTGGAAACCATC |
| R | CCTCCTTCTGACCACACATTTGAA | |
| ZEB1 | F | GATGATGAATGCGAGTCAGATGC |
| R | ACAGCAGTGTCTTGTTGTTGT |
Western blot analysis.
Cells were lysed with lysis buffer (Roche), with 10% phosphor STOP and 10% protease inhibitor cocktail. Cell lysates were then collected after centrifugation at 12,000 rpm for 10 m at 4°C. 30μg of lysate protein was loaded and total cellular protein was separated with 8% SDS-polyacrylamide gel electrophoresis, then transblotted onto NC membrane (Life Technology). The membrane was probed with anti-ZIP4 (Proteintech, 1:2000), ZO-1, claudin-1, ZEB1(Cell signaling Technology 1:500), pFAK, FAK (Abcam, 1:1000), pPaxillin, Paxillin (Life tech, 1:1000), and anti-β-actin (Abcam, 1:10000) antibody at 4 °C overnight, and then washed three times with 0.1% Tween 20-TBS and incubated with horseradish peroxidase-linked or NIR-coupled secondary antibody (1:5000) for 2 h at room temperature. The membrane was washed three times with 0.1% Tween 20-TBS. The immunoreacted bands were detected using an enhanced chemiluminescent (ECL) plus reagent kit. We are using the Li-COR Odyssey Fc machine to detect both ECL and the NIR-labeled 2nd antibodies.
In vitro migration and invasion assays.
The wound healing assay was performed as described below. The cells were pretreated with 10 μg/ml mitomycin C(Sigma, M0503) for 2h, wounds were made in confluent monolayer cells, and wound healing was detected at 0, 24h within the scraped lines, and representative fields at different time points were photographed. The cell migration/invasion was determined using a modified Boyden chamber assay. Uncoated polycarbonate inserts (Costar) with 8-μm pores, either uncoated or coated with 100 μg/mL of Matrigel (Corning) were used. Cells were pretreated with 10 μg/ml mitomycin C for 2 h, then were trypsinized and re-suspended in growth medium or medium without FBS (5×104 cells/100 μl) added into the upper compartment of an invasion (Matrigel-coated insert) or migration (uncoated insert) chamber, with 600 μl of the growth medium added into the lower chamber. After 24 h, cells were fixed with 4% PFA at room temperature for 15 m. Cells that had migrated or invaded onto the lower surface of the membrane were counted.
Immunofluorescence.
AsPC-1 and Panc-1 cells were fixed with 4% PFA, blocked with PBS containing 4% BSA for 30 m at room temperature, and then incubated overnight at 4°C with the primary antibodies for ZIP4 (Proteintech), ZO-1, and claudin-1 (Cell Signaling Technology) diluted in the same buffer. After three washes, slides were incubated at room temperature for 1 h with secondary antibodies (1:200) and Hoechst (1 μg/ml) and were mounted with Prolong diamond antifade mounting (Life Technology). Sections were photographed using EVOS FLC (Life Technology).
Immunohistochemical (IHC) staining.
Human pancreatic adenocarcinoma and surrounding benign tissues were collected and processed into 5-μm slides. Fixed tissue slides were incubated in 3% hydrogen peroxide solution to quench endogenous peroxidase activity for 15 m and were subsequently washed with PBS. The slides were then incubated in blocking buffer for 30 m at room temperature and stained with anti-hZIP4 antibody (Proteintech, 1:500), ZO-1 (NOVUS,1:100), claudin-1 (1:100, Cell Signaling Technology), ZEB1 (1:250, Sigma) and incubated overnight at 4°C. After washing with PBS, the section was incubated with polymer secondary antibody for 30 m (Vector Laboratories). Immune complexes were detected with diaminobenzidine (DAB) under a phase-contrast microscope. The sections were then mounted and observed under a phase-contrast microscope. The slides were scanned using Aperio scanning software and the positivity was analyzed automatically. The KPC mouse sections were obtained from Dr. Courtney Houchen. ZIP4, ZO-1 (Proteintech), claudin-1 (Proteintech), ZEB1 (Sigma), pFAK (Abcam) and pPaxillin (Lifetech) were stained in pancreatic cancer tissue of KPC mouse sections as above.
Pancreatic cancer xenograft mouse model.
AsPC-shV, AsPC-shZO-1, AsPC-shcldn-1 cells were harvested by trypsinization and were resuspended in RPMI 1640. In a volume of 50 μL, 3 ×106 tumor cells were injected into the pancreases of 5-to-6-week-old male nude mice. The peritoneum and skin were closed with a 4.0 surgical sutures. After 4 weeks, all surviving mice were euthanized with an overdose of CO2 exposure. Mice were evaluated macroscopically for the presence of orthotopic tumors and metastases in the abdominal cavity. All mice were cared for in accordance with the IACUC and Animal Welfare Act guidelines.
Statistical analysis.
Quantitative results are shown as means ± SD. Paired t-test was used to analyze paired data (ZIP4 and EMT markers in tumor and adjacent normal pancreas tissues). Student’s t-test/ANOVA test was used for data from control and treated groups. Multiple testing was adjusted using Bonferroni’s method. Analysis based on the Pearson correlation was employed to investigate the correlation between the expression of ZIP4 and EMT markers in human pancreatic cancer tissue pairs and xenograft tumor tissue. A P value of <0.05 was considered statistically significant. All tests were two-sided.
Results
ZIP4 represses ZO-1 and claudin-1 in a ZEB1-dependent manner in pancreatic cancer cells.
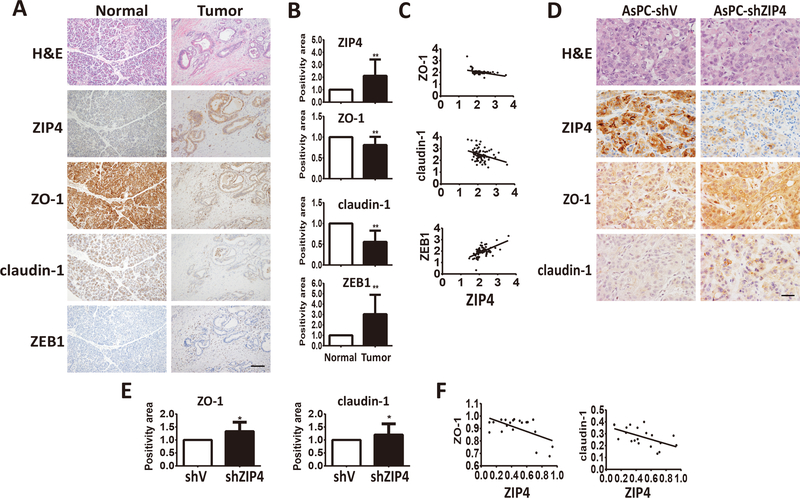
Previously we demonstrated that ZIP4 promotes pancreatic cancer migration and invasion (16,17). We next investigated whether ZIP4 promotes those cellular functions through the modulation of known regulators of cell migration and invasion. IHC analysis of the ZIP4 expression revealed an association with several important invasion markers, including ZO-1, claudin-1, and ZEB1 in 72 paired human TMA sections. ZIP4 and ZEB1 were overexpressed in the majority of the human pancreatic cancer tumor tissues, compared with their matched benign tissues, while ZO-1 and claudin-1 showed lower levels in tumor tissues than the benign ones (Figure 1A and 1B). ZIP4 expression was positively correlated with mesenchymal marker ZEB1 (P<0.0001, r=0.5641) but was negatively associated with epithelial markers ZO-1 (P=0.003, r=−0.4148) and claudin-1 (P=0.0062, r=−0.3197) (Figure 1C) in human specimens. Further analysis in the orthotopic xenograft tissues revealed that ZIP4 was also negatively associated with ZO-1 (P=0.0071, r=−0.5824) and claudin-1 (P=0.0095, r=−0.5648; Figure 1D–1F). Similarly to the human tumors, in a genetically engineered mouse model of pancreatic cancer (KPC) (20), we found that ZIP4 was negatively correlated with ZO-1 and claudin-1 but positively associated with ZEB1, FAK and Paxillin, targets of ZO-1 and claudin-1, in the tumor tissues of these mice (Figure S1).
Figure 1. Correlation between ZIP4 and EMT markers in human and mouse pancreatic cancer tissues.
(A) Representative pictures of IHC staining for ZIP4 and EMT markers ZO-1, claudin-1, ZEB1 in 72 pairs of human pancreatic cancer and adjacent normal pancreas tissues. The scale bar is 100 μm. (B) Positivity of ZIP4, ZO-1, claudin-1, ZEB1 IHC staining results in 72 pairs of human pancreatic cancer and adjacent normal pancreas tissues. (C) Correlations of ZIP4 with ZO-1, claudin-1, ZEB1 in 72 human pancreatic cancer tissues. Pearson correlation coefficient was employed to quantify the correlation between the expression of ZIP4 and EMT markers in human pancreatic cancer tissue pairs. * means P value <0.05, ** means P value <0.01 which were considered statistically significant. (D) H&E staining, IHC staining of ZIP4, EMT markers ZO-1, claudin-1 in orthotopic pancreatic cancer xenografts. n=10. The scale bar is 50μm. (E) Positivity of ZIP4, ZO-1, claudin-1 in 20 xenograft tumor tissues. (F) Correlations of ZIP4 and EMT markers ZO-1, claudin-1 in orthotopic pancreatic cancer xenografts. n=10. Pearson correlation coefficient was employed to quantify the correlation between the expression of ZIP4 and EMT markers in xenograft tumor tissue. * means P value <0.05, ** means P value <0.01 which were considered statistically significant.
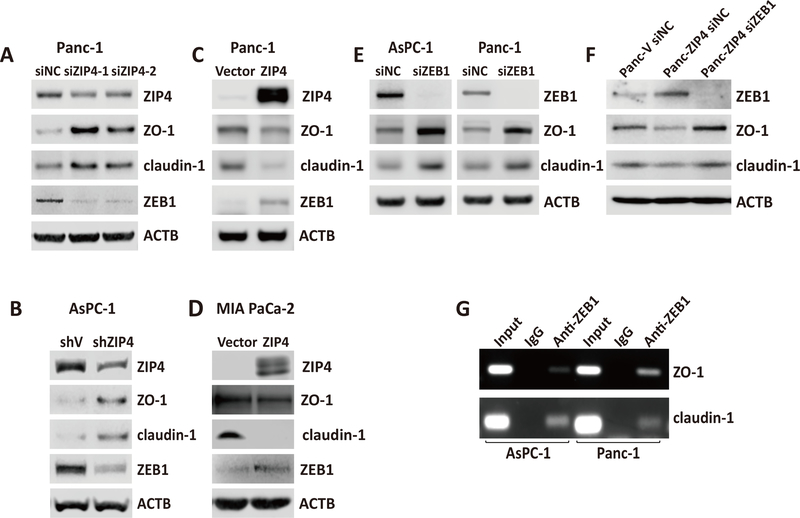
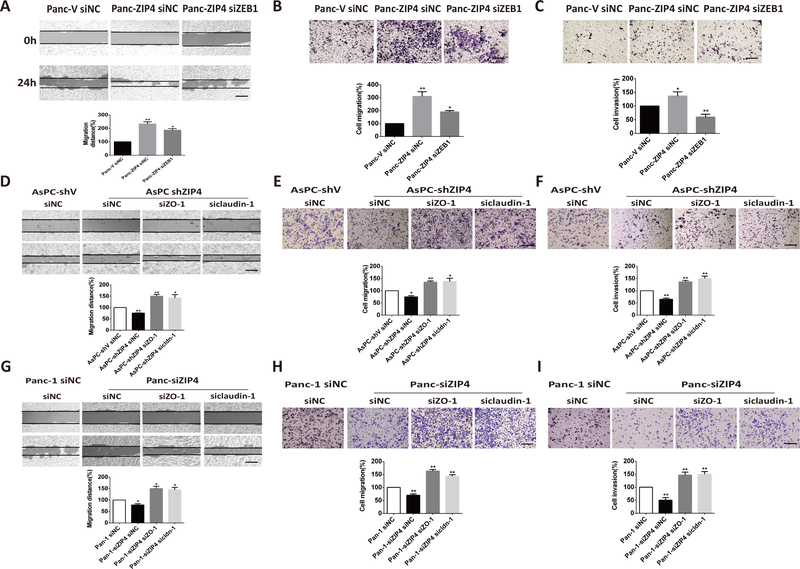
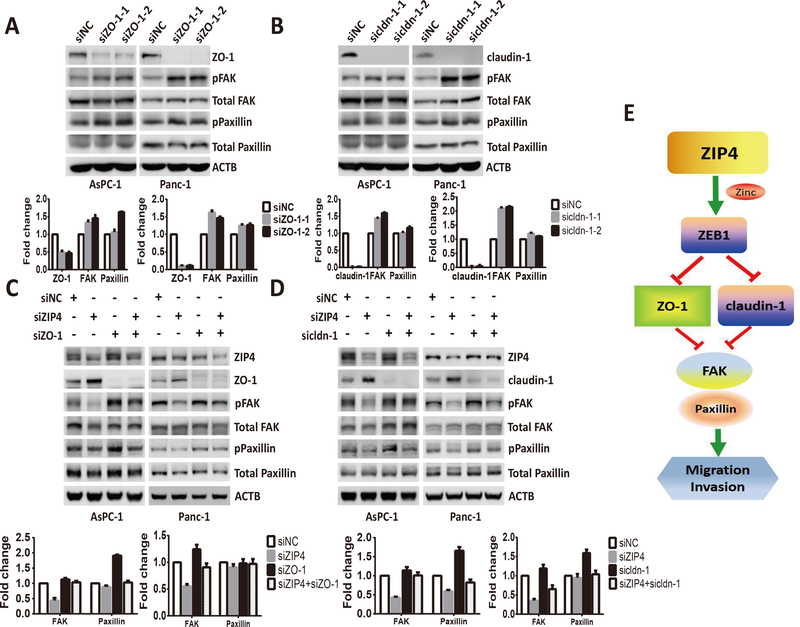
Furthermore, we investigated the effect of ZIP4 on the expression of those invasion markers in pancreatic cancer cell lines and found that knocking down ZIP4 in Panc-1 and AsPC-1 cells decreased the expression of mesenchymal markers ZEB1 and increased the expression of epithelial markers ZO-1 and claudin-1 (Figure 2A and 2B). Similar results showing that downregulation of ZIP4 elevated the expression of ZO-1 and claudin-1 were confirmed with immunofluorescence staining in AsPC-1 and Panc-1 cells (Figure S2). RT-PCR was used to validate the altered expression of the invasion markers in those two pancreatic cancer cells (Figure S3). Finally, we examined the effect of ZIP4 overexpression on the aforementioned markers. As shown in Figure 2C and 2D, overexpression of ZIP4 upregulated mesenchymal markers ZEB1 and lowered the levels of epithelial markers ZO-1 and claudin-1 in both MIA PaCa-2 and Panc-1 cells. Next we sought to mechanistically investigate the interplay between ZIP4, ZEB1 and epithelial genes ZO-1 and claudin-1. Knowing that ZEB1 is a zinc finger protein and homeodomain transcription factor and it is upregulated by ZIP4 (Figure 2A–2D), we aimed to determine whether ZEB1 is an effector of ZIP4 that regulates the expression of ZO-1 and claudin-1. ZIP4 overexpression downregulates ZO-1 and claudin-1 while knocking down ZEB1 enhances ZO-1 and claudin-1 expression in AsPC-1 and Panc-1 cells. Moreover, we found knocking down ZEB1 attenuates the downregulation role of ZIP4 on ZO-1 and claudin-1 (Figure 2E, 2F). To elucidate whether ZO-1 and claudin-1 are direct transcriptional targets of ZEB1, we performed ChIP assay in AsPC-1 cells, which confirmed the binding of ZEB1 to the promoter regions of ZO-1 and claudin-1 genes (Figure 2G). To determine the biological significance of these findings we investigated whether ZEB1 could regulate tumor migration and invasion induced by ZIP4. We performed a wound healing assay, transwell migration and invasion assay in Panc-V and Panc-ZIP4 cells in which ZEB1 was silenced using RNAi. We found that ZIP4 increased tumor migration and invasion was impaired by ZEB1 inactivation (Figure 3A–3C). Interestingly this effect of the ZIP4-ZEB1 axis was independent of CREB. Previously we reported that ZIP4 induces changes in gene expression through the activation of this transcription factor (17,21); expression studies in multiple pancreatic cancer cells showed no effect on ZO-1 nor claudin-1 levels in cells with CREB knockdown (Figure. S4). Taken together our results identified a novel transcriptional effector of ZIP4 controlling gene expression and migration of pancreatic cancer cells.
Figure 2. ZIP4 regulated expression of EMT markers in multiple pancreatic cancer cell lines.
(A) Western blot. Protein levels of ZIP4 and EMT markers detected in Panc-1 siNC and Panc-1 siZIP4 cells. (B) Western blot. Protein levels of ZIP4 and EMT markers detected in AsPC-shV and AsPC-shZIP4 cells. (C) Western blot. Protein levels of ZIP4 and EMT markers detected in Panc-1 vector and Panc-1 ZIP4 cells. (D) Western blot. Protein levels of ZIP4 and EMT markers detected in MIA-V and MIA-ZIP4 cells. All the immunoblotting was repeated at least three times, and the average values were presented. (E) Western blot. Protein levels of ZO-1 and claudin-1 detected in AsPC-1 or Panc-1 cells with ZEB1 blocked. (F) Western blot. Protein levels of ZO-1 and claudin-1 detected in Panc-V and Panc-ZIP4 cells with ZEB1 blocked. (G) CHIP PCR. CHIP binding assay with anti-ZEB1 in AsPC-1 and Panc-1 cells confirmed the binding of ZEB1 to the ZO-1 and claudin-1 promoter regions.
Figure 3. Knocking down ZO-1 and claudin-1 restored ZIP4 function in migration and invasion.
(A). Wound healing assay. The view of wound healing was captured at 0 and 24 h with Panc-V siNC, Panc-ZIP4 siNC, Panc-ZIP4 siZEB1 cells (P <0.05, t-test, n=10). (B). Transwell migration assay. Relative cell migration was detected in Panc-V siNC, Panc-ZIP4 siNC, Panc-ZIP4 siZEB1 cells (P <0.05, n=5). (C). Transwell invasion assay. Relative cell invasion was detected in Panc-V siNC, Panc-ZIP4 siNC, Panc-ZIP4 siZEB1 cells (P <0.01, n=5). (D) Wound healing assay. The view of wound healing was captured at 0 and 24 h with AsPC-shV siNC, AsPC-shZIP4 siNC, AsPC-shZIP4 siZO-1, and AsPC-shZIP4 siclaudin-1 cells (P <0.01, t-test, n=10). (E) Transwell migration assay. Relative cell migration was detected in AsPC-shV siNC, AsPC-shZIP4 siNC, AsPC-shZIP4 siZO-1, and AsPC-shZIP4 siclaudin-1 cells (P <0.05, t-test, n=10). (F) Transwell invasion assay. Relative cell invasion was detected in AsPC-shV siNC, AsPC-shZIP4 siNC, AsPC-shZIP4 siZO-1, and AsPC-shZIP4 siclaudin-1 cells (P <0.05, t-test, n=10). (G) Wound healing assay. The view of wound healing was captured at 0 and 24 h with Panc-1 siNC, Panc-1-siZIP4 siNC, Panc-1-siZIP4 siZO-1, and Panc-1-siZIP4 siclaudin-1 cells (P <0.05, t-test, n=10). (H) Transwell migration assay. Relative cell migration was detected in Panc-1 siNC, Panc-1-siZIP4 siNC, Panc-1-siZIP4 siZO-1, and Panc-1-siZIP4 siclaudin-1 cells (P <0.01, t-test, n=10). (I) Transwell invasion assay. Relative cell invasion was detected in Panc-1 siNC, Panc-1-siZIP4 siNC, Panc-1-siZIP4 siZO-1, and Panc-1-siZIP4 siclaudin-1 cells (P <0.01, t-test, n=10). All data are mean ± SE. The scale bar is 100 μm. * means P value <0.05, ** means P value <0.01 which were considered statistically significant.
Downregulation of ZO-1 and claudin-1 is required for ZIP4-dependent pancreatic cancer cell migration and invasion.
ZO-1 and claudin-1 play vital roles in tumor migration and invasion (9,10), we sought to determine whether ZIP4 could regulate these cellular events using the wound-healing assay in AsPC-shZIP4 and Panc-1 siZIP4 cells. As shown Fig. 3D–3I, knocking down ZIP4 decreased pancreatic cancer migration and invasion in AsPC-1 and Panc-1 cells. Similarly, blocking ZO-1 or claudin-1 by RNAi increased the migration distances in AsPC-shZIP4 and Panc-1 siZIP4 cells compared to cells transfected with siNC (Figure 3D and 3G). Similarly, transwell migration and Matrigel invasion assays also showed that knockdown of ZO-1 and claudin-1 rescued the invasive and migratory capacities of AsPC-shZIP4 and Panc-1 siZIP4 cells, in which ZIP4 was silenced (Figure 3E–3I). To further investigate whether ZO-1 and claudin-1 contribute to tumor metastasis in vivo, we constructed stable cells lines with ZO-1 and claudin-1 silenced by shRNA in AsPC-shZIP4 cells and implanted these cells in an orthotopic xenograft tumor model. The knocking down efficiency of ZO-1 and claudin-1 in AsPC-shZIP4 cells was confirmed by RT-PCR and Western blot (Figure S5). Four weeks after the tumor implantation all the mice were sacrificed. AsPC-shZO-1 and AsPC-shclaudin-1 groups showed increased tumor weight (P<0.05, n=6, Figure 4A), liver metastasis, colon/intestine obstruction and peritoneal dissemination (Figure 4B–4C), compared to the mice injected with AsPC-shV cells. There were also higher blue-stained bands of collagen in AsPC-shZO-1 and AsPC-shcldn-1 groups compared to AsPC-shV group (Figure. 4D–4E). Repressed ZO-1 and claudin-1 was found to increase tumor migration and invasion in pancreatic cancer both in vitro and in vivo.
Figure 4. ZO-1 and claudin-1 contribute to tumor migration and invasion in xenograft tumor.
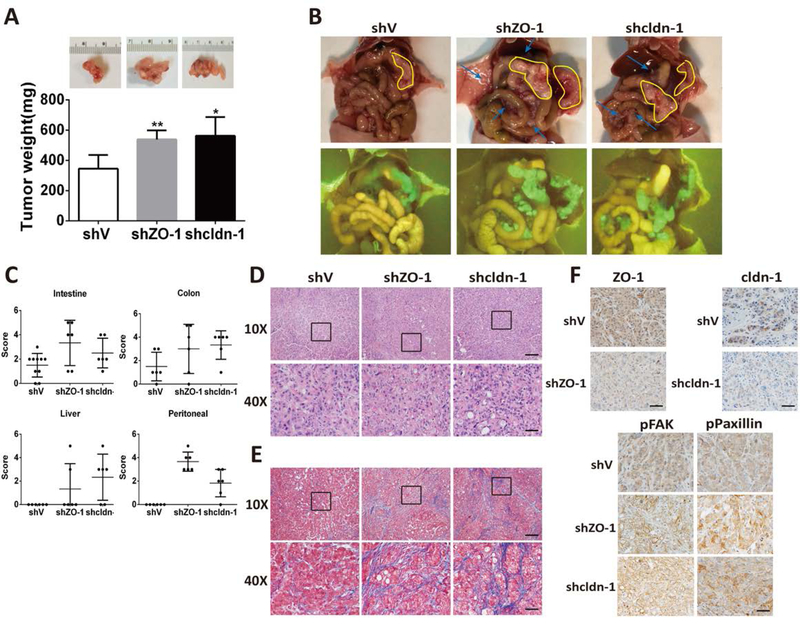
(A) Tumor weight. ASPC-shV, AsPC-shZO-1, AsPC-shcldn-1 were orthotopically inoculated into the pancreas of nude mice (n = 6/group). A representative picture from each group is shown in the inserts. (B) Primary tumor in the pancreas and metastatic tumors in small intestine, colon, liver and peritoneal. GFP fluorescence indicated the tumor cells in ASPC-shV, AsPC-shZO-1, AsPC-shcldn-1 groups. (C) Number and size of metastatic tumors in small intestine, colon, liver and peritoneal was calculated as 0–5. (D-E) The orthotopic tumors were collected 4 weeks post tumor implantation and processed for H&E staining (D) or Masson-trichrome staining (E) The scale bar is 200μm (10X) or 50μm (40X), IHC staining (F) of ZO-1, claudin-1, pFAK, pPaxillin in xenograft tumor tissues. n=6. The scale bar is 50μm.
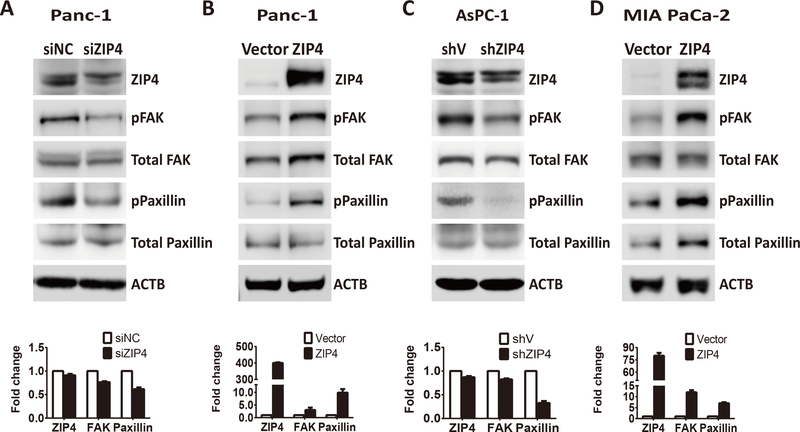
Finally we examined whether ZIP4 promotes migration and invasion by regulating the downstream genes FAK and Paxillin which are targeted by ZO-1 and claudin-1. Blocking ZIP4 decreased FAK and Paxillin phosphorylation in AsPC-1 and Panc-1 cells (Figure 5A and 5C), while ZIP4 upregulation increased FAK and Paxillin phosphorylation in MIA PaCa-2 and Panc-1 cells (Figure 5B and 5D). Next, we examined whether blocking ZO-1 and claudin-1 could activate FAK and Paxillin phosphorylation in AsPC-1 and Panc-1 cells. We found that FAK and Paxillin phosphorylation increased with knock down of ZO-1 and claudin-1 (Figure 6A, and 6B). We also found knocking down ZO-1 and claudin-1 led to enhanced pFAK and pPaxillin in xenograft tumor tissue (Figure 4F) which was consistent with the in vitro data. We have shown that knocking down ZIP4 decreased FAK and Paxillin phosphorylation, while blocking ZO-1 and claudin-1 increased FAK and Paxillin phosphorylation. Next, we investigated whether ZIP4 regulates FAK and Paxillin by directly targeting ZO-1 and claudin-1. We performed a rescue experiment by blocking ZIP4, ZO-1, and claudin-1, individually or in combination. We found that silencing of ZIP4 caused reduced phosphorylation of FAK and Paxillin, which was rescued by simultaneous blocking of ZO-1 or claudin-1 in both AsPC-1 and Panc-1 cells. When ZIP4 and ZO-1 or claudin-1 were blocked simultaneously, the phosphorylation levels of FAK and Paxillin were higher than those observed in the ZIP4 knockdown group, but lower than those in the ZO-1 or claudin-1 blocked groups (Figure 6C and 6D). Together, our results showed that knocking down ZIP4 decreased FAK and Paxillin phosphorylation by activating ZO-1 and claudin-1 in pancreatic cancer cells.
Figure 5. ZIP4 regulated downstream genes FAK and Paxillin in multiple pancreatic cancer cell lines.
(A) Protein levels of ZIP4 and pFAK, total FAK, pPaxillin, and total Paxillin detected in Panc-1 siNC, and Panc-1-siZIP4 cells. (B) Protein levels of ZIP4 and pFAK, total FAK, pPaxillin, and total Paxillin detected in Panc-1 vector and Panc-1 ZIP4 cells. (C) Protein levels of ZIP4 and pFAK, total FAK, pPaxillin, and total Paxillin detected in AsPC-shV and AsPC-shZIP4 cells. (D) Protein levels of ZIP4 and pFAK, total FAK, pPaxillin, and total Paxillin detected in MIA-V and MIA-ZIP4 cells. For Figure. 5A–5D group comparisons in FAK and Paxillin was based on the ratio of pPFAK/total FAK and the ratio of pPaxillin/total Paxillin.
Figure 6. Knocking down ZIP4 and ZO-1 or claudin-1 simultaneously rescued FAK and Paxillin phosphorylation levels.
(A) Protein levels of ZO-1, pFAK, total FAK, pPaxillin, and total Paxillin detected in AsPC-1 and Panc-1 cells transfected with ZO-1 siRNAs. (B) Protein levels of claudin-1, pFAK, total FAK, pPaxillin, and total Paxillin detected in AsPC-1 and Panc-1 cells transfected with claudin-1 siRNAs. (C) Protein levels of pFAK, total FAK, pPaxillin, and total Paxillin detected in AsPC-1 and Panc-1 cells transfected with ZIP4, ZO-1 siRNAs, or ZIP4 plus ZO-1. (D) Protein levels of pFAK, total FAK, pPaxillin, and total Paxillin detected in AsPC-1 and Panc-1 cells transfected with ZIP4, claudin-1 siRNAs, or ZIP4 plus claudin-1. For Figure. 6A–6D group comparisons in FAK and Paxillin was based on the ratio of pPFAK/total FAK and the ratio of pPaxillin/total Paxillin. (E) Schematic diagram of ZIP4-ZEB1-ZO-1, cldn-1-FAK, Paxillin pathway in pancreatic cancer.
Discussion
This study provided a novel ZIP4-ZEB1 dependent mechanism controlling expression of ZO-1, claudin-1 pathway that activates pancreatic cancer migration and invasion through targeting downstream genes FAK and Paxillin (Figure. 6E). Knocking down ZIP4 inhibited pancreatic cancer invasion and migration in pancreatic cancer cell lines and mouse models through repressing invasion markers. Meanwhile, blocking ZIP4 upregulated epithelial markers and reduced the levels of mesenchymal markers. We also demonstrated that ZIP4-mediated transcription factor ZEB1 which could modulate ZO-1 and claudin-1 repression further regulates downstream targets FAK and Paxillin. Silencing of ZIP4 caused reduced phosphorylation of FAK and Paxillin, which was rescued by simultaneous blocking of ZO-1 or claudin-1 in pancreatic cancer cells. These findings support the existence of a novel pathway downstream of zinc importer ZIP4 participating in pancreatic cancer invasiveness and metastasis.
Pancreatic cancer has a high propensity to metastasize. Cell migration and invasion play a critical role in facilitating the process of cancer metastasis (22). A remarkable connection between tumor invasiveness and metastasis exists in many types of cancers including pancreatic cancer (23). These cellular events are characterized by loss of E-cadherin, ZO-1, claudin-1 (8,24) and increase of mesenchymal markers such as N-cadherin, Vimentin, and transcription factors like ZEB1. Previous studies on tumor invasion have been largely focused on the transcriptional regulation by core regulators such as ZEB1, Twist and snail, which are induced to activate EMT by repressing the epithelial gatekeeper E-cadherin and driving tumor migration and invasion and contribute to early pathogenesis and metastatic potential in pancreatic cancer and lung cancer (25–27). Many of those transcription factors are zinc-dependent, and contain four to six zinc finger domains corresponding to the C2H2 type, which coordinate the zinc ion (28). Zinc plays a central role on the activation of the transcription factors, and zinc transporters such as ZIP4 may regulate the expression or activity of zinc dependent transcription factors through the intracellular zinc. But little is known about the underlying mechanism. Recent studies indicated that zinc and zinc transporters promote tumor migration, invasion, and EMT processes. ZIP6 (LIV-1) was linked with the transcription factors STAT3 and Snail in zebrafish embryogenesis (29). ZIP5 is involved in esophageal cancer progression through COX2 and E-cadherin (30). ZIP4 has been shown to enhance migration, invasion, and suppress apoptosis in hepatocellular carcinoma (31). However, how zinc and zinc transport are involved in the transcriptional regulation of invasion in pancreatic cancer remains unclear. In the present study, we investigated how a key zinc transporter, ZIP4, regulates core invasion markers in pancreatic cancer and therefore promotes cell migration, invasion, and metastasis. Our group has recently demonstrated an important role of ZIP4 in pancreatic cancer proliferation, invasiveness and tumor growth (16). We have shown that ZIP4 is the major zinc importer upregulated in pancreatic cancer, and overexpression of ZIP4 caused activation of the IL-6/STAT3 (21) pathway and led to increased VEGF and MMP2 expression (32). However, the detailed mechanism how ZIP4 activates the downstream signaling pathways to promote pancreatic cancer migration and invasion is still unclear.
ZO-1 and claudin-1 are important tight junction proteins that are downregulated in pancreatic cancer. In normal cells, ZO-1 and Caudin-1 form specialized structures in order to maintain the tight associations with each other in epithelial cells (9). They are expressed at low levels and suppress cell invasion and metastasis in pancreatic cancer (9,10). Studies suggest that zinc might regulate the integrity of ZO-1 and claudin-1. Zinc deficiency is known to result in epithelial barrier leak in the pancreas (11,33) and is closely related to reduced levels of the tight junction proteins ZO-1 and claudin-1 through damaging the integrity of the cell connection structure (12). Caco-2 cells grown in zinc-deficient media showed altered expression of ZO-1 and occludin integrity (34). How zinc levels affect those tight junction proteins, such as ZO-1 and claudin-1 in pancreatic cancer cells remains unclear. We hypothesize that ZIP4 regulates the expression of ZO-1 and claudin-1 through a zinc-dependent signaling cascade in pancreatic cancer cells. We found that knocking down ZIP4 led to upregulation of ZO-1 and claudin-1 but over-expression of ZIP4 led to decreased cell-to-cell connections via reduced ZO-1 and claudin-1. Our previous studies indicated that ZIP4 activates oncogenic miR-373 (17) and IL-6-STAT3 (21) pathways through a zinc-dependent transcriptional factor CREB. To further investigate whether ZIP4 also regulates ZO-1 and claudin-1 through CREB, we knocked down CREB with siRNA in ZIP4 high pancreatic cancer cells MIA-ZIP4, Panc-ZIP4 and AsPC-1 cells, and did not find upregulated expression of ZO-1 and claudin-1, suggesting that ZIP4 repressed ZO-1 and claudin-1 is independent of CREB. We also found that ZIP4 regulates ZEB1, key players in EMT. ZEB1 is a zinc-dependent transcriptional factor that is highly involved in tumor cell migration, invasion, and EMT through TGF-β, BMP, NF-kB and Notch signaling pathway (35,36). ZEB1 downregulates ZO-1 during the EMT process (37,38). Our results indicate that ZEB1 suppressed the expression of ZO-1 and claudin-1 in pancreatic cancer cells, and knocking down ZEB1 upregulated ZO-1 and claudin-1 in both AsPC-1 and Panc-1 cells, suggesting a novel zinc dependent regulatory pathway through which ZIP4 repressed ZO-1 and claudin-1 in human pancreatic cancer cells (Figure. 6E).
In this study we found ZIP4 promotes pancreatic cancer migration and invasion through targeting ZO-1 and claudin-1. Next, we further investigated the downstream genes of ZO-1 and claudin-1 directly involved in tumor cell migration and invasion. ZO-1 controls cell proliferation through regulating cyclinD1, erbB2, PCNA in many types of cancers (39,40). However, limited information exists about the activities of downstream targets of ZO-1 and claudin-1 involved in tumor migration and invasion. FAK and Paxillin have recently been shown to be the downstream targets of ZO-1 and claudin-1 and are highly involved in tumor migration and invasion (41). FAK is an intracellular non-receptor tyrosine kinase that is associated with adhesion, migration, and invasion. Multiple cancers including pancreatic cancer demonstrate FAK overexpression (41,42). Coll IV adhesion and integrin stimulation enhance phosphorylation of FAK (43). RTK mediated activation of c-Src and related kinases Fyn and Lck results in the phosphorylation of FAK (41). FAK knockout studies elucidated an early embryonic lethal phenotype that exhibited the defects in migration, providing the direct evidence for FAK’s involvement in migration (44). Paxillin is a major component of focal adhesions which can be regulated through Src and FAK mediated phosphorylation (44). Targeting FAK-Paxillin interactions can reduce FAK signaling which then reduces adhesion, migration and invasion processes (44). Increased Paxillin expression or activation of Paxillin leads to enhanced tumor invasion (42,45). FAK co-localizes with ZO-1 at the cell interface (46). ZO-1 depletion leads to pronounced activation of FAK and pPaxillin which were concentrated in ZO-1 knock down cells (47). Claudin-7 indirectly regulates the integrin/FAK signaling pathway in human colon cancer tissue (48). In this study, we found that ZIP4 activated phosphorylation of FAK and Paxillin in pancreatic cancer cells. This positive activation by ZIP4 may be mediated by reduced expression of ZO-1 and claudin-1, and these results were validated in both ZIP4 overexpression and knockdown pancreatic cancer cells, which are derived from different origins and with different phenotypes.
Presently, we describe a novel signaling pathway in which ZIP4 inhibits ZEB1 and then suppresses ZO-1 and claudin-1, leading to phosphorylation of FAK and Paxillin, which further causes increased cell migration, invasion and tumor metastasis. In ZIP4-high cells such as AsPC-1 and Panc-1, knock down of ZIP4 upregulated ZO-1 and claudin-1 and reduced FAK and Paxillin phosphorylation. In ZIP4-low cells such as MIA PaCa-2 cells, overexpression of ZIP4 led to reduced ZO-1 and claudin-1 and activation of FAK and Paxillin. Furthermore, the reduced phosphorylation of FAK and Paxillin by silencing of ZIP4 can be rescued by simultaneous blocking of ZO-1 or claudin-1 in both AsPC-1 and Panc-1 cells, suggesting a direct link of ZIP4, ZO-1, claudin-1, FAK, and Paxillin in pancreatic cancer. The interruption of this ZIP4-ZEB1-ZO-1-claudin-1-FAK-Paxillin pathway in pancreatic cancer may serve as a novel therapeutic strategy.
Supplementary Material
Translational Relevance.
Changes in zinc levels have been correlated with cancer risk, however, the biological role of zinc and zinc transporters in cancer carcinogenesis is still largely unknown. Here we report a novel role for ZIP4, a zinc importer commonly overexpressed in human cancer, in pancreatic tumor progression. ZIP4 modulates pancreatic cancer cells migration and invasion. Analysis of the mechanism shows that ZIP4 promotes invasion and metastasis by transcriptionally repressing ZO-1 and claudin-1 in a ZEB1-dependent manner. These findings identify a previously uncharacterized role of ZIP4 in pancreatic cancer progression, and define a new pathway controlling pancreatic cancer metastasis. Targeting ZIP4 might be a novel treatment strategy for pancreatic cancers with elevated expression of this transporter.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grants R01 CA138701, R01 CA186338–01A1, R01 CA203108–01 (M. Li) and the William and Ella Owens Medical Research Foundation (M. Li). M.E.F.-Z was supported by Mayo Clinic Pancreatic SPORE P50 CA102701, and Mayo Clinic Center for Cell Signaling in Gastroenterology P30 DK84567. We thank the Peggy and Charles Stephenson Cancer Center (SCC) at the University of Oklahoma, Oklahoma City, OK and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under grant number P20 GM103639 for the use of Histology and Immunohistochemistry Core, which provided immunohistochemistry and image analysis services.
Grant support: This work was supported in part by the National Institutes of Health (NIH) grants R01 CA138701, R01 CA186338–01A1, R01 CA203108–01 (M. Li), the William and Ella Owens Medical Research Foundation (M. Li), Mayo Clinic Pancreatic SPORE P50 CA102701 and Mayo Clinic Center for Cell Signaling in Gastroenterology P30 DK84567 (M.E.F.-Z), and Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under grant number P20 GM103639 for the use of Histology and Immunohistochemistry Core.
Footnotes
Conflict of interest: None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians 2017;67(1):7–30 doi 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022 doi 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64(1):9–29 doi 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nature cell biology 2016;18(5):549–60 doi 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257(4):731–6 doi 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364(19):1817–25 doi 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Metastasis-suppressor genes: a review and perspective on an emerging field. J Natl Cancer Inst 2000;92(21):1717–30. [DOI] [PubMed] [Google Scholar]
- 8.Martin TA. The role of tight junctions in cancer metastasis. Semin Cell Dev Biol 2014;36:224–31 doi 10.1016/j.semcdb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, et al. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas 2001;23(3):259–65. [DOI] [PubMed] [Google Scholar]
- 10.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci 2013;14(9):18148–80 doi 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012;4(8):875–903 doi 10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi Y, Tanabe S, Suzuki T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am J Physiol Gastrointest Liver Physiol 2016;311(1):G105–16 doi 10.1152/ajpgi.00405.2015. [DOI] [PubMed] [Google Scholar]
- 13.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem 2007;282(10):6992–7000 doi 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 14.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem 2004;279(6):4523–30 doi 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007;104(47):18636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res 2009;15(19):5993–6001 doi 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Yang J, Cui X, Chen Y, Zhu VF, Hagan JP, et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol Med 2013;5(9):1322–34 doi 10.1002/emmm.201302507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liuzzi JP, Guo L, Chang SM, Cousins RJ. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. American journal of physiology Gastrointestinal and liver physiology 2009;296(3):G517–23 doi 10.1152/ajpgi.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Valenzano MC, Mercado JM, Zurbach EP, Mullin JM. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci 2013;58(1):77–87 doi 10.1007/s10620-012-2328-8. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7(5):469–83 doi 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Bharadwaj U, Logsdon CD, Chen C, Yao Q, Li M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clinical cancer research: an official journal of the American Association for Cancer Research 2010;16(5):1423–30 doi 10.1158/1078-0432.CCR-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, Hopt UT, et al. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol 2015;30 Suppl 1:78–84 doi 10.1111/jgh.12752. [DOI] [PubMed] [Google Scholar]
- 23.Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res 2015;21(5):962–8 doi 10.1158/1078-0432.CCR-13-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 2009;1788(4):872–91 doi 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Wei SC, Yang J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol 2016;26(2):111–20 doi 10.1016/j.tcb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeyama Y, Sato M, Horio M, Hase T, Yoshida K, Yokoyama T, et al. Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells. Cancer letters 2010;296(2):216–24 doi 10.1016/j.canlet.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nature cell biology 2017;19(5):518–29 doi 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 28.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002;3(3):155–66 doi 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 29.Taylor KM, Hiscox S, Nicholson RI. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol Metab 2004;15(10):461–3 doi 10.1016/j.tem.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Jin J, Liu J, Wang L, He Y. Knockdown of Zinc Transporter ZIP5 by RNA Interference Inhibits Esophageal Cancer Growth In Vivo. Oncol Res 2016;24(3):205–14 doi 10.3727/096504016X14648701447896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Guo HJ, Xie HY, Li J, Zhuang RZ, Ling Q, et al. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J Biol Sci 2014;10(3):245–56 doi 10.7150/ijbs.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Chen C, Yao Q, Li M. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther 2010;9(3):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Sun X, Yang J, Ding H, LeBrun D, Ding K, et al. ZIP4 silencing improves bone loss in pancreatic cancer. Oncotarget 2015;6(28):26041–51 doi 10.18632/oncotarget.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr 2008;138(9):1664–70. [DOI] [PubMed] [Google Scholar]
- 35.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol 2012;105(7):655–61 doi 10.1002/jso.23020. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun 2016;7:10498 doi 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178–96 doi 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010;29(24):3490–500 doi 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 39.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta 2009;1788(4):761–7 doi 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Qiao X, Roth I, Feraille E, Hasler U. Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion. Cell Cycle 2014;13(19):3059–75 doi 10.4161/15384101.2014.949091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanteti R, Batra SK, Lennon FE, Salgia R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget 2016;7(21):31586–601 doi 10.18632/oncotarget.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Z, Zhang J, Bi M, Han X, Han Z, Wang H, et al. SRPX2 promotes cell migration and invasion via FAK dependent pathway in pancreatic cancer. Int J Clin Exp Pathol 2015;8(5):4791–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, et al. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer 2005;4:37 doi 10.1186/1476-4598-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011;63(8):610–5 doi 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao Y, Mu G, Zhang L, Zhou W, Zhang J, Yu H. Lysophosphatidic acid stimulates activation of focal adhesion kinase and paxillin and promotes cell motility, via LPA1–3, in human pancreatic cancer. Dig Dis Sci 2013;58(12):3524–33 doi 10.1007/s10620-013-2878-4. [DOI] [PubMed] [Google Scholar]
- 46.Deramaudt TB, Dujardin D, Noulet F, Martin S, Vauchelles R, Takeda K, et al. Altering FAK-paxillin interactions reduces adhesion, migration and invasion processes. PLoS One 2014;9(3):e92059 doi 10.1371/journal.pone.0092059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, et al. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 2015;208(6):821–38 doi 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding L, Wang L, Sui L, Zhao H, Xu X, Li T, et al. Claudin-7 indirectly regulates the integrin/FAK signaling pathway in human colon cancer tissue. J Hum Genet 2016;61(8):711–20 doi 10.1038/jhg.2016.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.