Abstract
Purpose
This review focuses on studies among pregnant women that used biomarkers to assess air pollution exposure, or to understand the mechanisms by which it affects perinatal outcomes.
Methods
We searched PubMed and Google scholar databases to find articles
Results and conclusions
We found 29 articles, mostly consisting of cohort studies. Interpolation models were most frequently used to assess exposure. The most consistent positive association was between polycyclic aromatic hydrocarbon (PAH) exposure during entire pregnancy and cord blood PAH DNA adducts. Exposure to particulate matter (PM) and nitrogen dioxide (NO2) showed consistent inverse associations with mitochondrial DNA (mtDNA) content, particularly in the third trimester of pregnancy. No single pollutant showed strong associations with all the biomarkers included in this review. C-reactive proteins (CRPs) and oxidative stress markers increased, whereas telomere length decreased with increasing air pollution exposure. Placental global DNA methylation and mtDNA methylation showed contrasting results with air pollution exposure, the mechanism behind which is unclear. Most studies except those on PAH DNA adducts and mtDNA content provided insufficient evidence for characterizing a critical exposure window. Further research using biomarkers is warranted to understand the relationship between air pollution and perinatal outcomes.
Keywords: Biomarkers, pregnancy, mechanisms, oxidative stress, inflammation
Introduction
Exposure to environmental toxins during pregnancy is associated with various perinatal as well as adult health outcomes, as suggested by Barker’s hypothesis (Bell et al., 2007, Gouveia et al., 2004, Hansen et al., 2007, Woodruff et al., 2009, Salam et al., 2005, Leech et al., 1999, Barker et al., 1989, Feldt et al., 2007). Fetuses are particularly susceptible to these toxins because they are still under physiological development (Perera et al., 1999). Human studies indicate that exposure to air pollution is associated with several adverse perinatal outcomes, including spontaneous abortions, intrauterine growth retardation, preterm births, and low birth weight (LBW) (Dejmek et al., 1999, Hannam et al., 2014, Liu et al., 2003, Poirier et al., 2015, Yorifuji et al., 2015). However, there is no clear understanding of the underlying mechanisms of these biologic processes.
Biomarkers have been widely used in animal models to gain insights into the mechanisms by which air pollution is associated with perinatal outcomes. Mouse models have shown that prenatal exposure to diesel exhaust is associated with placental hemorrhage, oxidative stress, inflammatory cell infiltration, as well as increased fetal brain cytokine and chemokine levels (Bolton et al., 2012, Weldy et al., 2014).
Recent studies have increasingly used biomarkers to measure personal exposure to specific pollutants, to understand the potential mechanisms by which air pollution exposure is related to various outcomes, and to predict health outcomes. This paper presents a comprehensive review of studies that utilized biomarkers to measure air pollution exposure and to explore the related mechanisms among pregnant women.
Clinical Significance
Telomeres protect chromosomal degradation, and telomere lengths at birth predict those in adulthood
mtDNA content is associated with low birth weight
Oxidative stress is related to preeclampsia and miscarriage
DNA methylation is a mechanism that relates air pollution exposure in early life to health events in adulthood
Polycyclic aromatic hydrocarbons act as teratogens and are associated with reduced growth among children; PAH-DNA adducts are markers of PAH exposure
The use of biomarkers of these processes help in understanding mechanisms underlying the associations between air pollution exposure during pregnancy and various health outcomes
Trimester specific associations can indicate critical exposure windows
Methods
We searched PubMed and Google Scholar databases, and used several combinations of keywords specified in Table 1. We also used the ‘related articles’ option in Google Scholar, and the ‘similar articles’ option in PubMed, and searched the bibliography of the included articles. The inclusion criteria constituted studies (i) carried among pregnant women, (ii) involving the use of biomarkers, (iii) published after the year 2000, and (iv) published in English. We excluded studies linking biomarkers and clinical outcomes if they did not assess the association between air pollution exposure and biomarkers. We also excluded studies on smoking, tobacco, conference abstracts, review papers, and book chapters. We categorized the studies into two groups: those that used intermediate biomarkers, and those that used markers of exposure. This strategy yielded 21 articles in the category of intermediate biomarkers, and 8 in the exposure category.
Table 1.
Keywords used in literature search
| Air pollution related words |
Pregnancy related words |
Biomarker related words |
|---|---|---|
| Air pollution | Pregnant | Biomarker |
| Traffic | Pregnancy | Telomere |
| Particulate matter | Perinatal | Methylation |
| Sulfur dioxide | Newborns | Adducts |
| Nitrogen dioxide | Inflammation | |
| PAH | Oxidative stress | |
| Diesel | Folate | |
| Phthalates | Mitochondrial | |
| Naphthalene | DNA | |
| Hydroxyvitamin |
Results and Discussion
The first section of the results includes studies using intermediate biomarkers such as telomere length, mitochondrial DNA (mtDNA) content, oxidative stress markers, inflammation markers, and DNA methylation. The second section includes studies using exposure biomarkers such as urinary PAH metabolites and PAH related DNA adducts. Tables 2 and 3 summarize the articles included in this review. Figure 1 illustrates trimester/pollutant specific findings.
Table 2.
Association between air pollution exposure and intermediate biomarkers
| Author, year | Study design |
Population | Pollutant | Exposure assessment |
Biospecimen, collection time |
Biomarker, assessment |
Major Results: Estimate (95%CI) | Controlled in the analyses |
|---|---|---|---|---|---|---|---|---|
| Telomere length | ||||||||
| Bijnens et al., 201527 | Cohort-R | 231 Caucasian twins, 1975–1982, Belgium | Traffic exp | Traffic densities | Placentas, 24h post delivery | Telomere length (TL):qPCR |
|
nb's sex, gstl age, BW, birth yr, zygosity and chorionicity, mat age, SES and smo during prg |
| mtDNA content | ||||||||
| Janssen et al., 201233 | Cohort-P | 178 nb, 02/05/2010–04/03/ 2011, Belgium | PM10 | Kriging interpolation | Placentas, cord leukocytes at delivery | mtDNA content: qPCR |
|
nb’s sex, mat and gstl age, parity, ethnicity, smo status, season, and time-sp apparent temp. Cord bl adj for bl cell count as well |
| Janssen et al., 201534 | Cohort-R | 381 mat-nb pairs, 2010 –2013, Belgium | PM2.5 | Kriging interpolation | Placentas at birth | Placental mtDNA meth |
|
Gender, mat and gstl age, smo, mat edu, parity, ethnicity, and season at conception |
| Clemente et al., 201538 | Cohort-R | 390 mat-nb pairs, 2004 –2008, Spain; 556 pw, 2010 – 2013, Belgium | NO2 | Spain: LUR passive samplers | Placentas at/after delivery | mtDNA content: qPCR |
|
nb’s sex, mat age, mat smo status, gstl age, pre-pregnancy BMI, parity, ethnicity, season of birth, and edu, and interaction term sex and mtDNA content |
| Belgium: Kriging interpolation | ||||||||
| Oxidative stress | ||||||||
| Nagiah et al., 201545 | Cohort-P | Pw, T3, 50 from South Durban (SD), 50 from North Durban (ND) | Air poln from industrialized locale | Not mentioned | PBMCs and serum: T3 | MDA: TBARS assay; mt function, DNA integrity, SOD2, Nrf2, UCP2;GSH: GSH-Glo™ GSH assay |
|
Not applicable |
| Grevendonk et al., 201646 | Cohort-R | 293 cord bl, 224 mat bl samples, pw, 2010–2013, Belgium | PM10 and PM2.5 | Kriging interpolation | Mat and venous cord bl samples at delivery | 8-OHdG: qPCR |
|
Models for mat bl: mat and gstl age, smo status, mat edu, alc intake during prg, season at conception. Models for cord bl : gender and date of delivery as well |
| Ambroz et al., 201648 | Cross-Sec | Mat bl, mat urine, cord bl, nb’s urine: Karvina : Mat bl and urine-288; Ceske Budejovice: 398 (CB) 2013–2014 | PM2.5, BaP | High volume air sampler | Mat bl, mat urine, cord bl, nb’s urine at delivery | 8-oxodG, 15-F2t-IsoP |
|
Not applicable |
| Saenen et al., 201647 | Cohort-R | 330 mat-nb pairs, Feb 2010 – May 2013, Belgium | PM2.5, NO2, BC | Interpolation, monitoring stations | Placentas at delivery | 3-NTp: Bio-Rad protein assay |
|
Gstl age, mat age, mat edu, pregestational BMI, smo status, nb’s sex, nb’s ethnicity, seasonality |
| Inflammation markers | ||||||||
| Lehmann et al., 200274 | Cohort-P | 85 mat-nb pairs, Dec 1997 – Jan 1999, Germany | VOC | Questionnaire, passive sampling | Cord bl at delivery | T cells: intracellular cytokine staining |
|
Family atopy history, gender, mat smo |
| Hertz-Picciotto et al., 200271 | Cross-Sec | Mat-nb pairs: 303-Teplice; 215-Prachatice, 1994–1996 | PM10, SO2, NOx | Monitoring stations | Mat and cord bl at delivery | Lymphocyte subsets |
|
Time of birth, mat alc intake, father’s alc intake, employment status of father, father’s edu, use of infertility treatments |
| Lee et al., 201164 | Cohort-R | 1696 pw, 1997–2001, Pennsylvania | PM10, CO, PM2.5, O3, NO2, SO2, | Kriging interpolation, monitoring stations | Mat nonfasting bl samples before 22 wks of gest | CRP: ELISA on the SpectraMax Me analyzer |
|
Gstl wk, season of sample collection, mat BMI at enrollment/bl draw, age, race, edu, cigarette exp in early prg, parity, household income, yr entering the study |
| Latzin et al., 201175 | Cohort-P | 265 neonates, 1999 –2005, Switzerland | PM10 and indoor air poln | Monitoring stations, distance to major road, questionnaire | Cord bl serum after delivery | Cytokines, chemo: human Cytokine Multiplex Assay Kit |
|
Gender, gstl wt and mat smo during prg, parental edu mat atopy and gstl age. |
| Baiz et al., 201155 | Cohort-R | 370 mat-nb pairs, Sept 2003–Dec 2005, France | PM10 and NO2 | Monitoring stations; benzene, toluene, ethylbenzene and xylenes: diffusive air sampler | Venous cord bl at delivery | Lymphocyte subsets |
|
Mat age at delivery and BMI, mat history of allergy, active and passive smo, perinatal infections, mode of delivery, season of the birth, nb's sex, wt and gstl age at birth |
| van den Hooven et al., 201266 | Cohort-P | 5067 pw for mat and 4450 for fetal cord bl, 2001–2005, Netherlands | PM10 and NO2 | Dutch national standard methods, monitoring stations | Mat venous bl: 4.5–17.9 wks gest; Venous cord bl: delivery | hs CRP: immunoturbidimetric assay on the Architect System |
|
Gstl age at birth, season of birth, mat age, BMI, parity, ethnicity, edu, smo, alc consumption, and noise exp |
| DNA methylation | ||||||||
| Herbstman et al., 201282 | Cohort-R | 164 African-American and Dominican pw, New York | PAH | PAH in air: personal monitors | Cord bl at delivery; urine specimens of some women to assess PAH conc | DNA adducts: HPLC-F, DNA meth: MethylampTM, Global DNA Meth Quant Kit |
|
No covariates were found to be confounders |
| Dietary PAH exp: questionnaire | ||||||||
| B. G. Janssen et al., 201383 | Cohort-P | 240 mat-nb pairs, 02/05/2010–01/21/2012, Belgium | PM2.5 | Kriging interpolation | Placentas at birth | DNA meth levels |
|
Nb's gender, mat and gstl age, parity, mat edu, smo, prenatal acetaminophen use, season at conception and trimester-sp apparent temp |
| B. G. Janssen et al., 201534 | Cohort-R | 381 mat-nb pairs, 2010 –2013, Belgium | PM2.5 | Kriging interpolation | Placentas at birth | Placental mtDNA meth |
|
Gender, mat and gstl age, smo, mat edu, parity, ethnicity, and season at conception |
| Other biomarkers | ||||||||
| Pedersen et al., 200997 | Cross-Sec | 75 pw, 12/13/2006–12/20/2007, Denmark | Traffic poln, indoor NO2 | Traffic: database, NO2: passive diffusive sampler cartridges | Mat peripheral bl and paired cord bl at C section | Micronuclei |
|
ETS exp, use of open fireplace, pre prg wt, folate, vit B12 levels, mat edu, season of delivery, dietary & genetic factors modulating adducts and micronuclei |
| Baiz et al., 201289 | Cohort-P | 375 mat-nb pairs, Feb 2003 – May 2005, Sept 2003 – Dec 2005, France | PM10 and NO2 | Atmospheric Dispersion Modelling | Cord bl serum at delivery | 25(OH)D cord bl serum level: ICLI |
|
Mat age and BMI, mat history of allergy, active and passive smo, number of siblings, household income, city, nb’s sex and wt, PTB, and season of birth |
| Yorifuji et al., 201291 | Cohort-R | 14,189 pw+nb singletons (≥ 22 wks), Jan 1997 – Dec 2008, Japan. | Traffic exp | Major roads: >50,000 vehicles, Road Traffic Census | Placentas at birth | Placenta/birth weight ratio (PBWR) |
|
Mat age, mat BMI, mat occupation, mat alc consumption, paternal smo, area level SES, and mat smo. |
| E. H. van den Hooven et al., 201295 | Cohort-P | 7,801 pw, 2001–2005, Netherlands | PM10 and NO2 | Dutch national standard methods for air quality modeling | T1 and T2 mat bl; cord bl samples at delivery | PGF and sFIt-1 |
|
Mat age, BMI, parity, ethnicity, edu, smo, alc, infant's sex, folic acid suppln, gstl age at measurement, noise exp, season of conception, mat height |
| O'Callaghan-Gordo et al., 201598 | Cohort-P | 181 mothers, 183 nb, Feb 2007–Feb 2008, Greece | PM2.5, PM10, NO2, NOx, | Land use regression | Cord bl at delivery | Micronuclei |
|
Mat age, mat educ, mat residence, mat origin, season of delivery, gestl age |
Abbreviations: 25 (OH)D=25-hydroxyvitamin D, 8-OHdG=8-hydroxy-2'-deoxyguanosine, 8-oxodG=8-oxo-7,8-dihydro-2_-deoxyguanosine, 15-F2t-IsoP=15-F2t-isoprostane, 3-NTp=3-nitrotyrosine, adj=adjusted, alc=alcohol, asso=association, ATP=adenosine triphosphate, avg=averaged, BaP=benzo(a)pyrene, bl=blood, BMI=body mass index, BC=black carbon, BW=birth weight, C2Cl4= tetrachloroethylene, chemo=chemokines, CI=confidence interval, CO=carbon monoxide, cohort-P=prospective cohort, cohort-R=retrospective cohort, conc=concentration, cross-sec=cross sectional, CRP=C reactive protein, Dec=December, diff=difference, ECOD=7-ethoxycoumarin O-deethylase, edu=education, ELISA=Enzyme Linked Immuno sorbent Assay, ETS=environmental tobacco smoke, exp=exposure, Feb=February, gest=gestation, GSH=glutathione, GST=glutathione-S-transferase, gstl=gestational, HPLC-F=high performance liquid chromatography-fluorescence, hs=high sensitivity, ICLI=immunochemiluminescent immunoassay, IFN-γ=interferon γ, IL=interleukin, IQ=interquartile, IQR=interquartile range, Jan=January, LUR=land use regression, mat=maternal, MDA=malondialdehyde, meth=methylation, mo=month, mRNA=mitochondrial RNA, mtDNA=mitochondrial DNA, MT-RNR1=mitochondrial RNR1 sequence, NA=no association, nb=newborn, NK=natural killer, NO2=nitrogen dioxide, nrf2=nuclear factor erythroid 2-related factor, O3=ozone, OGG1=oxoguanine glycosylase, OR=odds ratio, PAH=polycyclic aromatic hydrocarbons, PBMC=peripheral blood mononuclear cells, PBWR=placenta/birth weight ratio, PEMS=Personal Environmental Monitoring Sampler, PGF=placental growth factor, PM2.5=particulate matter ≤2.5µm diameter, PM10=particulate matter ≤10 µm diameter, poln=pollution, prg=pregnancy, PTB=preterm birth, pw=pregnant women, Q4=4th quartile, qPCR=quantitative polymerase chain reaction, quant=quantification, ref=reference, Sept=September, SES=socioeconomic status, sFlt-1=soluble fms-like tyrosine kinase 1, signi=significant, smo=smoking, SO2=Sulphur dioxide, SOD2=superoxide dismutase 2, sp=specific, suppln=supplementation, T1=1st trimester, T2=2nd trimester, T3=3rd trimester, TBARS=thiobarbituric reactive substances, temp=temperature, TEOM=tapered element oscillating microbalance, TNF-α=Tumor Necrosis Factor α, UCP2=uncoupling protein 2, vit=vitamin, VOC=volatile organic compounds, WBCs=white blood cells, wks=weeks, wt=weight, yr=year
Table 3.
Association between air pollution exposure and exposure biomarkers
| Author, year |
Study design |
Population | Pollutant | Exposure assessment |
Biospecimen, collection time |
Biomarker | Major Results: Estimate (95%CI) | Controlled in the analyses |
|---|---|---|---|---|---|---|---|---|
| PAH metabolites and DNA adducts | ||||||||
| Nethery et al., 2012102 | Cohort – P | 9 pregnant women from high pollution and 10 pregnant women from low pollution area, Canada | PAH, PM2.5, NO2 and NOX | Personal Monitors, PM2.5: PEMS, TEOMs, NOx: Ogawa samplers | urine samples once in each trimester | Urinary metabolites of PAHs |
|
Not applicable |
| Perera et al., 2005108 | Cohort – R | pregnant women: WTC area (268), Manhattan (468), Poland (191), China (136) | PAH | Monitoring stations | mat and cord blood at delivery | BaP DNA adducts |
|
Not applicable |
| Tang et al., 2006109 | Cohort – P | 150 newborns, 03/04/2002–06/19/2002, China | PAH | month of pregnancy overlapping with the period of coal fired plant | mat blood within 1 day postpartum, cord blood at delivery | PAH-DNA adducts |
|
ETS, sex, mat ht and wt, gestational age added for birth outcome analysis, and mat HC and cesarean status added for all analyses involving HC |
| Pedersen et al., 200997 | Cross-sectional | 75 pregnant women, 12/13/2006–12/20/2007, Denmark | Traffic pollution, indoor NO2 | Traffic: database, NO2: passive diffusive sampler cartridges | mat peripheral blood and paired cord blood at c-section | Bulky DNA adducts |
|
ETS exposure, use of open fireplace, pre pregnancy wt, folate levels, vitamin B12 levels, mat education, season of delivery, dietary & genetic factors modulating adducts and MN |
| Wu et al., 2010114 | Case-control | 81 cases: mscg <14 weeks; 81 controls: requesting abortion, 04/2007–11/2007, China | PAH | Mat interviews | aborted tissue and mat blood one hour post abortion | BaP DNA adducts: HPLC-F method |
|
Mat educational attainment and household income (and gestational age where applicable) |
| Jedrychowski et al., 2013112 | Cohort – R | 362 pregnant women, 01/2001–02/2004, Poland | PAH, BaP | PEMS | mat blood within 1 day postpartum, cord blood at delivery | PAH-DNA adducts |
|
Mat DNA adducts, season of birth |
| Tang et al., 2014113 | Cohort – P | 150 newborns, 03/2002–06/2002, China; 158 newborns, 03/2005–05/2005, same hospitals | PAH | Mini-Vol samplers | mat blood - 1 day postpartum, cord blood at delivery | PAH-DNA adducts: HPLC-F |
|
Models with BW and BL adjusted for ETS, gender, mat ht & wt before pregnancy and gestational age. Models with birth HC also adjusted for mat HC and caesarian status |
| Other biomarkers | ||||||||
| Mohorovic, 2003118 | Cohort – P | 260 pregnant women, Croatia | SO2 | Not mentioned | Blood & urine −3× each in clean & dirty periods, 1 month between each test | mHb |
|
Not applicable |
Abbreviations: BaP=Benzo(a)pyrene, BL=birth length, BW=birth weight, CI=confidence interval, cohort-P=prospective cohort, cohort-R=retrospective cohort, conc=concentration, CPF=chlorpyrifos, dist=distance, ETS=environmental tobacco smoke, ht=height, HC=head circumference, HPLC-F=high performance liquid chromatography fluorescence, mat=maternal, mHb=methemoglobin, MN=micronuclei, mscg=miscarriage, NA=no association, NO2=nitrogen dioxide, NOx=nitrogen oxides, nonpara=non parametric, OR=odds ratio, PAH=polycyclic aromatic hydrocarbons, PEMS=Personal Environmental Monitoring Sampler, PM2.5=particulate matter ≤2.5µm diameter, prg=pregnancy, PTB=preterm birth, SO2=sulfur dioxide, TEOM= tapered element oscillating microbalance, wt=weight, WTC=World Trade Center
Figure 1.
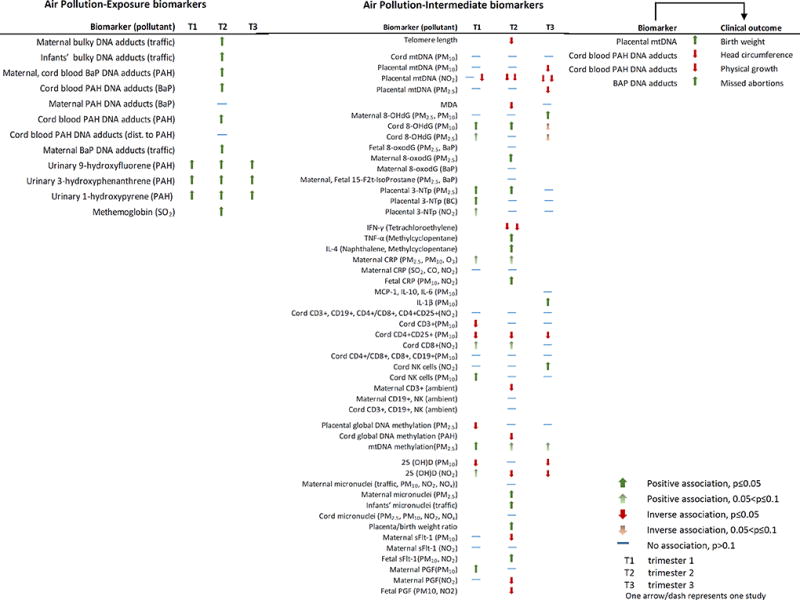
Association of biomarkers with air pollution exposure and clinical outcomes.
Abbreviations: 15-F2t-IsoP=15-F2t-isoprostane, 25 (OH)D=25-hydroxyvitamin D, 3-NTp=3-nitrotyrosine, 8-OHdG=8-hydroxy-2'-deoxyguanosine, BaP=benzo(a)pyrene, BC=black carbon, CD=cluster of differentiation, CO=carbon monoxide, CRP=C reactive protein, IFN-γ=interferon γ, IL=interleukin, MCP-1=monocyte chemoattractant protein-1, mtDNA=mitochondrial DNA, NK=natural killer, NO2=nitrogen dioxide, O3=ozone, PAH=polycyclic aromatic hydrocarbon, PGF=placental growth factor, PM10=particulate matter (diameter ≤ 10µm), PM2.5=particulate matter (diameter ≤ 2.5µm), sFlt-1=soluble fms-like tyrosine kinase 1, SO2=sulphur dioxide, TNF-α=tumor necrosis factor α
I. Intermediate Biomarkers
1. Telomere length (TL)
Telomeres are protein structures at each end of chromosomes, which prevent their degradation and preserve genomic information (Shammas, 2011). TL at birth is hypothesized to predict the TL in adulthood (Heidinger et al., 2012). Exposure to cigarette smoke, under nutrition, and maternal stress is associated with LBW, and TL has been hypothesized to be involved in this fetal programming (Entringer et al., 2012, Ko et al., 2014, Lee et al., 2011a, Schulz, 2010, Torche, 2011). The degradation of telomeres occurs at a faster rate during the first four years of life, suggesting that environmental exposures during this phase may have more pronounced effects on health outcomes (Frenck et al., 1998).
Among non-pregnant populations, air pollution has been associated with TL shortening, while there is limited evidence of the same among pregnant women (Hoxha et al., 2009, McCracken et al., 2010, Bijnens et al., 2015). A cohort study among 231 Caucasian twins in Belgium reported that mothers living closer to major roads (<252m) had 14% (95% CI: 1%, 24%) lower placental TL compared to those living farther (≥252m) (Bijnens et al., 2015). Additionally, doubling the distance to the major road increased the placental TL by 5.32% (95% CI: 1.90%, 8.86%). Similarly, in a surrounding 5000m buffer, for an interquartile range (IQR) increase (22%) in greenness, TL increased by 3.62% (95% CI: 0.20%, 7.15%), while it decreased by 4.90% (95% CI: −9.35%, −0.22%) for an IQR increase (5%) in industrial areas. Although the study was conducted among twins, 62% (95% CI: 47%, 74%) of the variation in placental TL was attributed to environmental factors, whereas that attributed to genetic factors was minimal. Despite being the only study among pregnant women, it provides strong evidence that air pollution exposure is associated with reduced placental TL.
2. Mitochondrial DNA (mtDNA) content
Mitochondria are intracellular structures involved in the production of energy in the form of adenosine triphosphate through oxidative phosphorylation (Montier et al., 2009). Mitochondrial respiration produces reactive oxygen species (ROS), which are hypothesized to be involved in the mechanism by which air pollution leads to adverse events (Li et al., 2008). mtDNA is prone to damage caused by ROS because it lacks the protective mechanisms present in nuclear DNA (Lee and Wei, 2000, Plaza, 2002). Altered mtDNA content has been associated with adverse pregnancy outcomes such as LBW (Gemma et al., 2006).
PM and mtDNA content: A study in Belgium found that every 10 µg/m3 increase in exposure to PM10 (PM ≤10 µm in diameter) in the third trimester decreased the placental mtDNA content by 17.40% (95% CI: −31.80%, −0.10%) (Janssen et al., 2012). Furthermore, doubling the residential distance to a major road increased the placental mtDNA content by 4% (95% CI: 0.40%, 7.80%). Cord blood mtDNA content was associated with neither PM10 exposure nor residential distance to a major road. Another study among 400 mother-newborn pairs in Belgium supported these findings and showed that placental mtDNA content decreased by 15.60% (95% CI: −23.92%, −6.38%) for every IQR increase in exposure to PM2.5 (PM ≤2.5 µm in diameter) during entire pregnancy (Janssen et al., 2015). This association was the strongest in the third trimester, −23.58% (95% CI: −36.27%, −8.37%).
NO2 and mtDNA content: Prenatal NO2 exposure has been associated with reduced fetal growth and adverse pregnancy outcomes (Ballester et al., 2010, Maroziene and Grazuleviciene, 2002, van den Hooven et al., 2012c). Analyses from two birth cohorts – the INMA (INfancia y Medio Ambiente; Environment and Childhood) and ENVIRONAGE (ENVIRonmental influence ON AGEing) showed that for every 10 µg/m3 increase in NO2 exposure, the placental mtDNA content decreased by 5.50% (95% CI: −8.80%, −2.10%) in the INMA cohort during the entire pregnancy and by 10.1% (95% CI: −20.10%, 1.24%) in the ENVIRONAGE cohort during the second and third trimester (Clemente et al., 2015). Additionally, mediation analyses showed that placental mtDNA might explain 10% (95% CI: 6.60%, 13%) of the association between average NO2 exposure and birth weight.
These studies provide consistent evidence that PM and NO2 exposure particularly in the third trimester of pregnancy might alter mtDNA content. The mediation analysis supports the role of mtDNA content as a precursor to adverse pregnancy outcomes.
3. Oxidative stress
Oxidative stress refers to the disruption in the levels of free radicals in the body and the corresponding antioxidant defenses (Betteridge, 2000). Although oxidative stress can be assessed using various biomarkers, only a few have been used in relation to air pollution among pregnant women. Oxidative stress during pregnancy is a hypothesized mechanism related to miscarriage, preeclampsia, fetal growth restriction, preterm birth, and LBW (Al-Gubory et al., 2010, Jauniaux et al., 2006, Jauniaux et al., 2000). Air pollution exposure has been associated with oxidative stress in several non-pregnant populations (Chahine et al., 2007, Kelly, 2003, Liu et al., 2003). In a study among pregnant women in South Africa, oxidative stress markers were found to be higher among women in high pollution areas compared to those in low pollution areas (Nagiah et al., 2015). In Belgian study, maternal mitochondrial 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels were found to increase by 18.30% (95% CI: 5.60%, 32.40%) and 13.9% (95% CI: 0.40%, 29.40%) for an IQR increase in PM10 and PM2.5 exposure respectively during the entire pregnancy (Grevendonk et al., 2016). Additionally, cord blood 8-OHdG was positively associated with PM10 exposure during the first (23% increase) and second trimester (16.60% increase). In another study in the same population, black carbon exposure in the first trimester, and PM2.5 exposure in the first and second trimester was associated with increased placental 3-nitrotyrosine (3-NTp) levels, indicating increased oxidative stress (Saenen et al., 2016). In a study in the Czech Republic, 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG), a marker of oxidative DNA damage, was found to be increased with increasing exposure to PM2.5 in the winter, and 15-F2t-isoprostane (15-F2t-IsoP), a marker of lipid peroxidation, increased with exposure to PM2.5 and benzo[a]pyrene (BaP) (Ambroz et al., 2016). While these studies show a positive association between air pollution exposure and oxidative stress, the specific etiologic time window of exposure needs to be identified through future longitudinal studies.
4. Inflammation markers
Inflammation is a critical mechanism through which air pollution is associated with various disease outcomes and is characterized by altered levels of cytokines, chemokines, and pattern recognition receptors (Brook et al., 2010, Brunekreef and Holgate, 2002, Demetriou et al., 2012, Hoek et al., 2013, Ruckerl et al., 2007, Challis et al., 2009). Inflammation among pregnant women is well studied, although not in relation to air pollution (Challis et al., 2009).
C-Reactive Proteins (CRPs): CRPs are markers of acute systemic inflammation that represent the presence and intensity of inflammation (Baiz et al., 2011, Delfino et al., 2008, Peters et al., 2001, Smith et al., 2008). Studies have shown that high CRP levels are associated with adverse pregnancy outcomes (Ernst et al., 2011, Guven et al., 2009, Lohsoonthorn et al., 2007, Pitiphat et al., 2005, Tjoa et al., 2003). In a US study, an interquartile increase in PM10 exposure was positively associated with increased maternal CRP (>8 ng/mL) levels at 22-day [odds ratio (OR) =1.23, 95% CI: 0.97, 1.57] and 29-day average periods (OR =1.18, 95% CI: 0.91, 1.53) respectively (Lee et al., 2011c). Exposure to PM2.5 showed similar results (Lee et al., 2011c, Lee et al., 2011b). Another study in Netherlands reported that the highest quartile of PM10 exposure during entire pregnancy was associated with elevated fetal CRP (>1 mg/L) levels at delivery (OR = 2.18; 95% CI: 1.08, 4.38) (van den Hooven et al., 2012a). Since CRP does not cross the placenta, the elevated fetal CRP levels are probably due to hepatic synthesis by the fetus (Jaye and Waites, 1997, Raio et al., 2003). The underlying mechanism relating maternal air pollution exposure to elevated fetal CRP levels is unclear. Further, maternal CRP levels are usually elevated because of inflammatory response to pregnancy, which makes it difficult to estimate the exact levels attributable to air pollution exposure (Thornton, 2010, von Versen-Hoeynck et al., 2009). However, studies were conducted among pregnant women, which increases comparability and validates the positive associations between air pollution exposure and elevated CRP levels to some extent.
Immune response: A study in France found that PM10 exposure before and during the entire pregnancy was associated with decreased levels of cord blood CD4+CD25+ T cells, and NO2 exposure in the first and second trimester was associated with decreased CD8+ cells (Baiz et al., 2011). In a study in the Czech Republic, maternal CD3+, CD4+, and CD4+/CD8+ cells were found to be lower, and cord blood NK cell levels were higher in high pollution areas (Hertz-Picciotto et al., 2002).
Cytokines: Cytokines are intercellular signaling polypeptides that play a central role in regulating tissue remodeling and cell signaling (Gabay and Kushner, 1999, Nathan and Sporn, 1991). A German study on maternal exposure to volatile organic compounds (VOCs) observed a positive association between IL-4 producing type-2 T cells and exposure to naphthalene (OR=2.9, 95%CI: 1.0, 8.2) and methylcyclopentane (OR=3.3, 95%CI: 1.1, 9.6), whereas tetrachloroethylene exposure was associated with decreased Interferon γ (IFN γ) producing type-1 T cells (Lehmann et al., 2002). Among pregnant women in Switzerland, PM10 exposure during the third trimester was associated with elevated cord blood IL-1b levels, OR = 3.00 (95%CI 1.30, 6.91) (Latzin et al., 2011). Additionally, PM10 exposure during the last three days of pregnancy was associated with decreased cord blood IL-10 levels, OR = 0.66 (95%CI: 0.45, 0.97). Adequate IL-10 levels are necessary during pregnancy, given their hypothesized function to suppress active maternal immunity to accept fetal allograft (Thaxton and Sharma, 2010). This study shows the changes in the cytokine levels in relation to PM10 exposure during various time windows in late pregnancy.
A disruption of the delicate balance of innate immune responses during pregnancy induces parturition prematurely, the underlying biologic process, however, is not clearly understood (Challis et al., 2009, Peltier, 2003, Silver et al., 2004). Regulatory T cells play an important role in allergy development, and altered levels of CD4+CD25+ T cells in response to air pollution exposure, as seen in the above study, might increase the newborns’ susceptibility to allergies (Smith et al., 2008). Overall, the above studies show an insufficient understanding of the relationship between air pollution exposure and inflammatory responses, necessitating further research.
5. DNA Methylation
DNA methylation plays a key role in maintaining genomic stability and expression of genes, and is hypothesized to be one of the mechanisms by which air pollution exposure in early life may be associated with adverse health outcomes in adulthood (Ohgane et al., 2008, Zhu et al., 2011, Koukoura et al., 2012).
Global DNA methylation: Air pollution exposure has been observed to be inversely associated with cord blood and placental global DNA methylation during pregnancy, starting from implantation stage (Herbstman et al., 2012, Janssen et al., 2013). In a prospective study among 240 mother-newborn pairs in Belgium, every 5 µg/m3 PM2.5 exposure during implantation (6–21 days) was associated with decreased placental global DNA methylation at birth by 1.08% (95% CI: −1.80%, −0.36%) (Janssen et al., 2013). Furthermore, for every 5 µg/m3 increase in PM2.5 exposure during the entire pregnancy, first trimester, and second trimester, placental global DNA methylation decreased by 2.19%, 2.41%, and 1.51% respectively (Janssen et al., 2013). In a study among nonsmoking African-American and Dominican women in New York city, prenatal exposure to PAH during the third trimester was inversely associated with global methylation in the cord white blood cells (Herbstman et al., 2012). However, among newborns, high levels of genomic methylation were positively associated with detectable levels of cord blood BaP-DNA adducts, OR = 2.35 (95% CI: 1.35, 4.09). It is hypothesized that benzo[a]pyrene diolepoxide (BPDE) binds to DNA, which might encourage DNA methylation (Subach et al., 2006). However, the temporal sequence of the formation of BaP-DNA adducts and methylation is not clear (Herbstman et al., 2012). A potential mechanism is that air pollution exposure alters global DNA methylation which in turn influences BaP-DNA adduct formation (Herbstman et al., 2012).
mtDNA methylation: Another study by Janssen et al. (2015) in the Belgian birth cohort reported that an IQR increase in PM2.5 exposure in the first trimester was associated with an increase in mtDNA methylation of 1.27% (95% CI: 0.23%, 2.32%) in the MT-RNR1 region and 0.44% (95% CI: 0.12%, 0.75%,) in the D-loop respectively (Janssen et al., 2015). Further analyses showed that the inverse association between PM2.5 exposure and placental mtDNA content was mediated by placental MT-RNR1 methylation [54% (95% CI: 31%, 60%)]. Significant inverse association between placental mtDNA content and methylation levels were observed among newborns (MT-RNR1: β = −0.04 ± 0.002, p<0.01; D-loop: β = −0.10 ± 0.01, p<0.01), however, the mechanism behind this is unclear. One hypothesis is that abnormal methylation at MTRNR1 and D loop region interferes with mtDNA biogenesis (Janssen et al., 2015). This is the first study to report epigenetic mitochondrial modifications in relation to early environmental exposures.
Although there is limited evidence, global DNA methylation levels showed consistent inverse association, whereas placental mtDNA methylation showed a positive association with air pollution exposure. mtDNA methylation at the D-loop is hypothesized to influence its replication or transcription, whereas methylation of the MT-RNR1 region impacts mitochondrial ribosomes and halts translation of mtDNA-encoded RNAs into proteins (Aloni and Attardi, 1971, Janssen et al., 2015, Metodiev et al., 2009). Environmental effects on placental DNA methylation may lead to epigenetic alterations and fetal programming of diseases (Koukoura et al., 2012). Placental DNA methylation is prone to high variability compared to methylation in other tissues (Houseman et al., 2008). More research is warranted in this area to understand the role of DNA methylation in the relationship between air pollution exposure and adverse pregnancy outcomes.
6. Other studies using intermediate biomarkers
This review also includes studies using 25-hydroxyvitamin D [25(OH)D], placenta/birth weight ratio, placental growth factors, and micronuclei. 25(OH)D is formed when vitamin D undergoes hydroxylation in the liver, and is an efficient marker of vitamin D (Crew et al., 2009). Only one study conducted among pregnant women showed that for every 10 µg/m3 increase in NO2 and PM10 levels, 25(OH)D levels decreased by 0.15 units (p = 0.047) and 0.41 units (p = 0.037) respectively (Baiz et al., 2012). This inverse association was the strongest during the third trimester of the pregnancy (NO2: β = −0.21, p <0.01; PM10: β = −0.43, p <0.01). It is hypothesized that maternal exposure to air pollution impedes the intestinal absorption of vitamin D, thereby affecting the cord blood 25(OH)D levels (Baiz et al., 2011). Another plausible mechanism is decrease in levels of vitamin D binding proteins (DBP) as a result of air pollution exposure, which leads to reduced serum 25(OH)D levels (Baiz et al., 2011).
Placenta/birth weight ratio (PBWR) is a marker of placental oxygen and nutrient transport efficiency, and a high PBWR is associated with adverse pregnancy outcomes (Naeye, 1987). A study in Japan found that living within 200m to a major road increased the PBWR by 0.48% (95%CI: 0.15%, 0.80%) (Yorifuji et al., 2012). Exposure to traffic related air pollution might lead to impaired placental transport through inflammation or oxidative stress. As a potential compensatory mechanism, placental weight may increase relative to the weight of the fetus, thereby resulting in poor pregnancy outcomes (Barker et al., 1993, Yorifuji et al., 2012).
Placental growth factor (PGF) is a pro-angiogenic growth factor necessary for placental growth, however, its distinct role is unclear. Soluble fms-like tyrosine kinase (sFlt-1) is an anti-angiogenic protein that reduces the development of new blood vessels and halts the maturation of existing ones (Jacobs et al., 2011). Both PGF and sFlt-1 levels are used as biomarkers to predict pregnancy outcomes, particularly preeclampsia (Zhu et al., 2016). A prospective study found that PM10 and NO2 exposure during the entire pregnancy period was associated with higher sFlt-1 and lower PIGF levels in cord blood (van den Hooven et al., 2012b). This was the only study to assess the relationship between air pollution exposure and angiogenic factors.
Micronuclei (MN) are small, extranuclear bodies that are left behind in the anaphase stage of cell division, and are not included in the daughter nuclei in the telophase stage due to direct and indirect DNA damage (Mateuca et al., 2006). A study in Denmark reported increased cord blood MN frequencies among those residing near high traffic density areas (Pedersen et al., 2009). Another study provided similar evidence and reported a 53% increase (RR = 1.53, 95%CI: 1.02, 2.29) in maternal MN frequencies for every 5 µg/m3 increase in PM2.5 exposure (O'Callaghan-Gordo et al., 2015).
II. Exposure biomarkers
1. PAH metabolites and DNA adducts
PAHs are persistent organic pollutants that are released in the atmosphere through incomplete combustion of fossil fuels, wood, and charcoal (Bostrom et al., 2002). Urinary PAH metabolites are markers of recent exposure and can be chemically reduced to their parent compounds (Becher and Bjorseth, 1983, Strickland et al., 1996). In Hamilton, Canada, pregnant women living near the downtown area had significantly higher levels of urinary 9-hydroxyfluorene, 3-hydroxyphenanthrene, and 1-hydroxypyrene compared to those living in the suburbs (Nethery et al., 2012).
Being lipophilic in nature, PAHs can enter the placenta where they may act as teratogens (Kim et al., 2013). PAH DNA adducts are formed when PAH metabolites covalently bind to DNA, and act as markers of PAH exposure (Shuker, 2002, Tang, 2008). Bulky DNA adducts, like PAH DNA adducts, reflect individual differences in exposure, absorption, activation, metabolism, and the ability to repair DNA damage (Godschalk et al., 2002, Pedersen et al., 2013). Although PAH DNA adducts from maternal blood have been used as markers of fetal PAH exposure, the adduct levels in newborns have been comparable or greater than the maternal levels (Perera et al., 2005, Tang et al., 2006, Whyatt et al., 2001). This is in spite of the transplacental PAH dose to the fetus being only 10% that of the mother (Yi et al., 2015). Cord blood PAH DNA adducts are markers of fetal PAH exposure of the preceding 4 months (Jedrychowski et al., 2013, Yi et al., 2015).
Previous studies have indicated that increasing levels of PAH exposure were associated with increased cord blood levels of PAH DNA adducts, BaP-DNA adducts, and bulky DNA adducts (Pedersen et al., 2009, Perera et al., 2005, Tang et al., 2014, Jedrychowski et al., 2013). Cord blood PAH DNA adduct levels have been associated with reduced head circumference and reduced physical growth among children, and maternal BaP-DNA adduct levels are a risk factor for missed abortions (Tang et al., 2006, Wu et al., 2010). The ratio of the fetal to maternal blood adduct levels (FMR) indicates susceptibility to fetal DNA damage, and has been used only in one study so far, which reported an FMR of 1.28 (95%CI: 1.10, 1.43) for high levels of PAH exposure (Perera et al., 2005). These results provide evidence of the teratogenic action of PAHs. The effects of PAH exposure on fetal growth are of particular concern because reduced head circumference and body growth are suggestive of poor neurodevelopmental and cognitive outcomes (Desch et al., 1990, Heinonen et al., 2008, Veena et al., 2010).
2. Other studies using exposure biomarkers
Methemoglobinemia is a condition characterized by increased methemoglobin levels, wherein hemoglobin cannot act as an oxygen transporter because the iron is in the ferric state instead of the ferrous state, thereby causing hypoxia (Mohorovic, 2003). In Croatia, ground level sulphur dioxide (SO2) concentrations were found to be positively correlated with methemoglobin levels among pregnant women during the operation of a coal powered thermoelectric power plant, whereas this correlation was found to be negative when the plant was closed (Mohorovic, 2003).
Limitations
Responses from each group of biomarkers might depend on the critical window of exposure before and during pregnancy. Although we identified critical exposure windows for certain pathways, many studies measured exposure only during one specific time period during pregnancy. This limited our ability to draw definite conclusions regarding the critical windows of exposure. Furthermore, high collinearity among various pollutant exposures makes it difficult to tease out their individual effects. Finally, some studies had small sample sizes, which could have affected the power of detecting weak associations, if any.
Conclusion
From this review, we conclude that biomarkers are useful in understanding the biologic mechanisms underlying the relationship between with air pollution and perinatal outcomes. We found the most consistent positive association between PAH exposure and cord blood DNA adducts. We also found strong evidence of decreasing mtDNA content with increasing air pollution exposure, particularly in the third trimester. We observed that global DNA methylation levels decreased, while levels of oxidative stress markers increased in response to air pollution exposure. However, no critical time window of exposure was identified for these pathways. Placental TL decreased with increasing exposure to traffic related air pollution. Although limited, this evidence was promising. Cytokine levels were altered in response to air pollution exposure throughout the entire pregnancy period, and also to third trimester exposure. Overall, we found that the use of biomarkers in relation to air pollution exposure among pregnant women is a promising but understudied area, with need for future research.
Acknowledgments
Funding source
This work is partially supported by the Community of Excellence in Global Health Equity, University at Buffalo, The State University of New York, USA, and partially by NIEHS grant (R21ES026429).
Footnotes
Declaration of interest
The authors report no declarations of interest.
Contributor Information
Li Chu, Email: chuli19740805@sina.com.
Yanjun Guo, Email: gyj_w@126.com.
Lina Mu, Email: linamu@buffalo.edu.
References
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. The international journal of biochemistry & cell biology. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Aloni Y, Attardi G. Expression of the mitochondrial genome in HeLa cells: II. Evidence for complete transcription of mitochondrial DNA. Journal of molecular biology. 1971;55:251–267. doi: 10.1016/0022-2836(71)90195-1. [DOI] [PubMed] [Google Scholar]
- Ambroz A, Vlkova V, Rossner P, Rossnerova A, Svecova V, Milcova A, Pulkrabova J, Hajslova J, Veleminsky M, Solansky I. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. International Journal of Hygiene and Environmental Health. 2016 doi: 10.1016/j.ijheh.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Baiz N, Dargent-Molina P, Wark JD, Souberbielle JC, Slama R, Annesi-Maesano I. Gestational exposure to urban air pollution related to a decrease in cord blood vitamin d levels. J Clin Endocrinol Metab. 2012;97:4087–95. doi: 10.1210/jc.2012-1943. [DOI] [PubMed] [Google Scholar]
- Baiz N, Slama R, Bene MC, Charles MA, Kolopp-Sarda MN, Magnan A, Thiebaugeorges O, Faure G, Annesi-Maesano I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn's cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Estarlich M, Iñiguez C, Llop S, Ramón R, Esplugues A, Lacasaña M, Rebagliato M. Air pollution exposure during pregnancy and reduced birth size: a prospective birth cohort study in Valencia, Spain. Environmental Health. 2010;9:1. doi: 10.1186/1476-069X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Becher G, Bjorseth A. Determination of exposure to polycyclic aromatic hydrocarbons by analysis of human urine. Cancer Lett. 1983;17:301–11. doi: 10.1016/0304-3835(83)90168-4. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environmental Health Perspectives. 2007:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Bijnens E, Zeegers MP, Gielen M, Kicinski M, Hageman GJ, Pachen D, Derom C, Vlietinck R, Nawrot TS. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int. 2015;79:1–7. doi: 10.1016/j.envint.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–54. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–88. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. The lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environmental health perspectives. 2007:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reproductive Sciences. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Clemente DB, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, Fernandez MF, Fierens F, Gyselaers W, Iniguez C, Janssen BG, Lefebvre W, Llop S, Olea N, Pedersen M, Pieters N, Santa Marina L, Souto A, Tardon A, Vanpoucke C, Vrijheid M, Sunyer J, Nawrot TS. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRAGE (Belgium) Birth Cohorts. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prevention Research. 2009;2:598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek J, Selevan SG, Benes I, Solanský I, Srám RJ. Fetal growth and maternal exposure to particulate matter during pregnancy. Environmental Health Perspectives. 1999;107:475. doi: 10.1289/ehp.99107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environmental health perspectives. 2008;116 doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou CA, Raaschou-nielsen O, Loft S, Møller P, Vermeulen R, Palli D, Chadeau-Hyam M, Xun WW, Vineis P. Biomarkers of ambient air pollution and lung cancer: a systematic review. Occupational and environmental medicine, oemed-2011-100566. 2012 doi: 10.1136/oemed-2011-100566. [DOI] [PubMed] [Google Scholar]
- Desch LW, Anderson SK, Snow JH. Relationship of head circumference to measures of school performance. Clin Pediatr (Phila) 1990;29:389–92. doi: 10.1177/000992289002900705. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci Signal. 2012;5:pt12. doi: 10.1126/scisignal.2003580. [DOI] [PubMed] [Google Scholar]
- Ernst GD, De Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA, Jaddoe VW. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. American journal of obstetrics and gynecology. 2011;205:132. e1–132. e12. doi: 10.1016/j.ajog.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Feldt K, Raikkonen K, Eriksson JG, Andersson S, Osmond C, Barker DJ, Phillips DI, Kajantie E. Cardiovascular reactivity to psychological stressors in late adulthood is predicted by gestational age at birth. J Hum Hypertens. 2007;21:401–10. doi: 10.1038/sj.jhh.1002176. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gemma C, Sookoian S, Alvarinas J, García SI, Quintana L, Kanevsky D, González CD, Pirola CJ. Mitochondrial DNA Depletion in Small-and Large-for-Gestational-Age Newborns. Obesity. 2006;14:2193–2199. doi: 10.1038/oby.2006.257. [DOI] [PubMed] [Google Scholar]
- Godschalk RW, Feldker DE, Borm PJ, Wouters EF, van Schooten FJ. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:790–3. [PubMed] [Google Scholar]
- Gouveia N, Bremner S, Novaes H. Association between ambient air pollution and birth weight in São Paulo, Brazil. Journal of Epidemiology and Community Health. 2004;58:11–17. doi: 10.1136/jech.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevendonk L, Janssen BG, Vanpoucke C, Lefebvre W, Hoxha M, Bollati V, Nawrot TS. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ Health. 2016;15:10. doi: 10.1186/s12940-016-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven MA, Coskun A, Ertas IE, Aral M, Zencırcı B, Oksuz H. Association of maternal serum CRP, IL-6, TNF-α, homocysteine, folic acid and vitamin b12 levels with the severity of preeclampsia and fetal birth weight. Hypertension in pregnancy. 2009;28:190–200. doi: 10.1080/10641950802601179. [DOI] [PubMed] [Google Scholar]
- Hannam K, Mcnamee R, Baker P, Sibley C, Agius R. Air pollution exposure and adverse pregnancy outcomes in a large UK birth cohort: use of a novel spatio-temporal modelling technique. Scand J Work Environ Health. 2014;40:518–30. doi: 10.5271/sjweh.3423. [DOI] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G, Simpson R. Low levels of ambient air pollution during pregnancy and fetal growth among term neonates in Brisbane, Australia. Environmental Research. 2007;103:383–389. doi: 10.1016/j.envres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–8. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen K, Räikkönen K, Pesonen A-K, Kajantie E, Andersson S, Eriksson JG, Niemelä A, Vartia T, Peltola J, Lano A. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121:e1325–e1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjodin A, Li Z, Camann D, Perera FP. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 2012;120:733–8. doi: 10.1289/ehp.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Dostál M, Dejmek J, Selevan SG, Wegienka G, Gomez-Caminero A, Srám RJ. Air pollution and distributions of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology. 2002;13:172–183. doi: 10.1097/00001648-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Christensen BC, Yeh R-F, Marsit CJ, Karagas MR, Wrensch M, Nelson HH, Wiemels J, Zheng S, Wiencke JK. Model-based clustering of DNA methylation array data: a recursive-partitioning algorithm for high-dimensional data arising as a mixture of beta distributions. BMC bioinformatics. 2008;9:1. doi: 10.1186/1471-2105-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha M, Dioni L, Bonzini M, Pesatori AC, Fustinoni S, Cavallo D, Carugno M, Albetti B, Marinelli B, Schwartz J. Association between leukocyte telomere shortening and exposure to traffic pollution: a cross-sectional study on traffic officers and indoor office workers. Environ Health. 2009;8:10.1186. doi: 10.1186/1476-069X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Nassar N, Roberts CL, Hadfield R, Morris JM, Ashton AW. Levels of soluble fms-like tyrosine kinase one in first trimester and outcomes of pregnancy: a systematic review. Reprod Biol Endocrinol. 2011;9:77. doi: 10.1186/1477-7827-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W. Placental mitochondrial DNA content and particulate air pollution during in utero life. 2012 doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Byun H-M, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIR ON AGE birth cohort study. Epigenetics. 2015:00–00. doi: 10.1080/15592294.2015.1048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, Fierens F, Penders J, Plusquin M, Gyselaers W. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:1–11. doi: 10.1186/1743-8977-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Human reproduction update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. The American journal of pathology. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. The Pediatric infectious disease journal. 1997;16:735–747. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Tang D, Rauh V, Majewska R, Mroz E, Flak E, Stigter L, Spengler J, Camann D, Jacek R. The relationship between prenatal exposure to airborne polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts in cord blood. J Expo Sci Environ Epidemiol. 2013;23:371–7. doi: 10.1038/jes.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occupational and environmental medicine. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Jahan SA, Kabir E, Brown RJ. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment international. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Ko TJ, Tsai LY, Chu LC, Yeh SJ, Leung C, Chen CY, Chou HC, Tsao PN, Chen PC, Hsieh WS. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55:20–7. doi: 10.1016/j.pedneo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review) Molecular medicine reports. 2012;5:883–889. doi: 10.3892/mmr.2012.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, Schaub B. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PloS one. 2011;6:e23130. doi: 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BE, Ha M, Park H, Hong YC, Kim Y, Kim YJ, Ha EH. Psychosocial work stress during pregnancy and birthweight. Paediatric and perinatal epidemiology. 2011a;25:246–254. doi: 10.1111/j.1365-3016.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- Lee P-C, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology (Cambridge, Mass.) 2011b;22:524. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011c;22:524–31. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech S, Richardson GA, Goldschmidt L, Day N. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicology and teratology. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Lehmann I, Thoelke A, Rehwagen M, Rolle-Kampczyk U, Schlink U, Schulz R, Borte M, Diez U, Herbarth O. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environmental toxicology. 2002;17:203–210. doi: 10.1002/tox.10055. [DOI] [PubMed] [Google Scholar]
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Biology and Medicine. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environmental health perspectives. 2003;111:1773. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clinical biochemistry. 2007;40:330–335. doi: 10.1016/j.clinbiochem.2006.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroziene L, Grazuleviciene R. Maternal exposure to low-level air pollution and pregnancy outcomes: a population-based study. Environmental Health. 2002;1:1. doi: 10.1186/1476-069X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie. 2006;88:1515–31. doi: 10.1016/j.biochi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Mccracken J, Baccarelli A, Hoxha M, Dioni L, Melly S, Coull B, Suh H, Vokonas P, Schwartz J. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans Affairs Normative Aging Study. Environmental health perspectives. 2010;118:1564. doi: 10.1289/ehp.0901831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metodiev MD, Lesko N, Park CB, Cámara Y, Shi Y, Wibom R, Hultenby K, Gustafsson CM, Larsson N-G. Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell metabolism. 2009;9:386–397. doi: 10.1016/j.cmet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Mohorovic L. The level of maternal methemoglobin during pregnancy in an air-polluted environment. Environmental health perspectives. 2003;111:1902. doi: 10.1289/ehp.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montier LLC, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. Journal of genetics and genomics. 2009;36:125–131. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeye RL. Do placental weights have clinical significance? Hum Pathol. 1987;18:387–91. doi: 10.1016/s0046-8177(87)80170-3. [DOI] [PubMed] [Google Scholar]
- Nagiah S, Phulukdaree A, Naidoo D, Ramcharan K, Naidoo RN, Moodley D, Chuturgoon A. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum Exp Toxicol. 2015;34:838–47. doi: 10.1177/0960327114559992. [DOI] [PubMed] [Google Scholar]
- Nathan C, Sporn M. Cytokines in context. J Cell Biol. 1991;113:981–6. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC, Foster W, Arbuckle TE. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. Journal of Exposure Science and Environmental Epidemiology. 2012;22:70–81. doi: 10.1038/jes.2011.32. [DOI] [PubMed] [Google Scholar]
- O'callaghan-Gordo C, Fthenou E, Pedersen M, Espinosa A, Chatzi L, Beelen R, Chalkiadaki G, Decordier I, Hoek G, Merlo DF. Outdoor air pollution exposures and micronuclei frequencies in lymphocytes from pregnant women and newborns in Crete, Greece (Rhea cohort) Environmental research. 2015;143:170–176. doi: 10.1016/j.envres.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Ohgane J, Yagi S, Shiota K. Epigenetics: the DNA methylation profile of tissue-dependent and differentially methylated regions in cells. Placenta. 2008;29:29–35. doi: 10.1016/j.placenta.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Schoket B, Godschalk RW, Wright J, von Stedingk H, Tornqvist M, Sunyer J, Nielsen JK, Merlo DF, Mendez MA, Meltzer HM, Lukacs V, Landstrom A, Kyrtopoulos SA, Kovacs K, Knudsen LE, Haugen M, Hardie LJ, Gutzkow KB, Fleming S, Fthenou E, Farmer PB, Espinosa A, Chatzi L, Brunborg G, Brady NJ, Botsivali M, Arab K, Anna L, Alexander J, Agramunt S, Kleinjans JC, Segerback D, Kogevinas M. Bulky dna adducts in cord blood, maternal fruit-and-vegetable consumption, and birth weight in a European mother-child study (NewGeneris) Environ Health Perspect. 2013;121:1200–6. doi: 10.1289/ehp.1206333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Wichmann J, Autrup H, Dang DA, Decordier I, Hvidberg M, Bossi R, Jakobsen J, Loft S, Knudsen LE. Increased micronuclei and bulky DNA adducts in cord blood after maternal exposures to traffic-related air pollution. Environmental research. 2009;109:1012–1020. doi: 10.1016/j.envres.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Peltier MR. Immunology of term and preterm labor. Reproductive Biology and Endocrinology. 2003;1:1. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo [a] pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiology Biomarkers & Prevention. 2005;14:709–714. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environmental Health Perspectives. 1999;107:451. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Fröhlich M, Döring A, Immervoll T, Wichmann H-E, Hutchinson W, Pepys M, Koenig W. Particulate air pollution is associated with an acute phase response in men. Results from the MONICA–Augsburg Study. European heart journal. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. American journal of epidemiology. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza SM. Mitochondrial factors in the pathogenesis of diabetes: a hypothesis for treatment. Altern Med Rev. 2002;7:94–111. [PubMed] [Google Scholar]
- Poirier A, Dodds L, Dummer T, Rainham D, Maguire B, Johnson M. Maternal Exposure to Air Pollution and Adverse Birth Outcomes in Halifax, Nova Scotia. J Occup Environ Med. 2015;57:1291–8. doi: 10.1097/JOM.0000000000000604. [DOI] [PubMed] [Google Scholar]
- Raio L, Ghezzi F, Mueller MD, Mcdougall J, Malek A. Evidence of Fetal C-Reactive Protein Urinary Excretion in Early Gestation. Obstetrics & Gynecology. 2003;101:1062–1063. doi: 10.1016/s0029-7844(02)02251-2. [DOI] [PubMed] [Google Scholar]
- Ruckerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Kuchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–80. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen ND, Vrijens K, Janssen BG, Madhloum N, Peusens M, Gyselaers W, Vanpoucke C, Lefebvre W, Roels HA, Nawrot TS. Placental Nitrosative Stress and Exposure to Ambient Air Pollution During Gestation: A Population Study. American Journal of Epidemiology. 2016:kww007. doi: 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- Salam MT, Millstein J, Li Y-F, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children's Health Study. Environmental health perspectives. 2005:1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A. 2010;107:16757–8. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas MA. Telomeres, lifestyle, cancer, and aging. Current opinion in clinical nutrition and metabolic care. 2011;14:28. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker DE. The enemy at the gates? DNA adducts as biomarkers of exposure to exogenous and endogenous genotoxic agents. Toxicology letters. 2002;134:51–56. doi: 10.1016/s0378-4274(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Silver RM, Peltier MR, Branch DW. The immunology of pregnancy. Maternal-Fetal Medicine: Principles and Practice. Philadelphia, Pa: WB Saunders. 2004:89–109. [Google Scholar]
- Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/−) regulatory T cell function. J Allergy Clin Immunol. 2008;121:1460–6. 1466.e1–7. doi: 10.1016/j.jaci.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Strickland P, Kang D, Sithisarankul P. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ Health Perspect. 1996;104(Suppl 5):927–32. doi: 10.1289/ehp.96104s5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subach OM, Baskunov VB, Darii MV, Maltseva DV, Alexandrov DA, Kirsanova OV, Kolbanovskiy A, Kolbanovskiy M, Johnson F, Bonala R. Impact of benzo [a] pyrene-2'-deoxyguanosine lesions on methylation of DNA by SssI and HhaI DNA methyltransferases. Biochemistry. 2006;45:6142–6159. doi: 10.1021/bi0511639. [DOI] [PubMed] [Google Scholar]
- Tang D, Li TY, Chow JC, Kulkarni SU, Watson JG, Ho SS, Quan ZY, Qu LR, Perera F. Air pollution effects on fetal and child development: a cohort comparison in China. Environ Pollut. 2014;185:90–6. doi: 10.1016/j.envpol.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Tang D, Li TY, Liu JJ, Chen YH, Qu L, Perera F. PAH-DNA adducts in cord blood and fetal and child development in a Chinese cohort. Environ Health Perspect. 2006;114:1297–300. doi: 10.1289/ehp.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. Optical Spectroscopic and NMR Studies of Covalent Polycyclic Aromatic Hydrocarbon-DNA Adducts: Influence of Base Sequence Context and Carcinogen Topology. 2008 ProQuest. [Google Scholar]
- Thaxton JE, Sharma S. REVIEW ARTICLE: Interleukin-10: A Multi-Faceted Agent of Pregnancy. American Journal of Reproductive Immunology. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton CA. Immunology of pregnancy. Proceedings of the Nutrition Society. 2010;69:357–365. doi: 10.1017/S0029665110001886. [DOI] [PubMed] [Google Scholar]
- Tjoa M, van Vugt J, Go A, Blankenstein M, Oudejans C, van Wijk I. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. Journal of reproductive immunology. 2003;59:29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Torche F. The effect of maternal stress on birth outcomes: exploiting a natural experiment. Demography. 2011;48:1473–1491. doi: 10.1007/s13524-011-0054-z. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, Lindemans J, Russcher H, Steegers EA, Miedema HM. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environmental health perspectives. 2012a;120:746. doi: 10.1289/ehp.1104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, Russcher H, Lindemans J, Miedema HM, Steegers EA, Jaddoe VW. Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Perspect. 2012b;120:1753–9. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Willemsen SP, Hofman A, van Ratingen SW, Zandveld PY, Mackenbach JP, Steegers EA, Miedema HM. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environmental health perspectives. 2012c;120:150. doi: 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena SR, Krishnaveni GV, Wills AK, Kurpad AV, Muthayya S, Hill JC, Karat SC, Nagarajaiah KK, Fall CH, Srinivasan K. Association of birthweight and head circumference at birth to cognitive performance in 9-to 10-year-old children in South India: prospective birth cohort study. Pediatric research. 2010;67:424–429. doi: 10.1203/PDR.0b013e3181d00b45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen-Hoeynck FM, Hubel CA, Gallaher MJ, Gammill HS, Powers RW. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. American journal of hypertension. 2009;22:687–692. doi: 10.1038/ajh.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldy CS, Liu Y, Liggitt HD, Chin MT. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS One. 2014;9:e88582. doi: 10.1371/journal.pone.0088582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Jedrychowski W, Hemminki K, Santella RM, Tsai WY, Yang K, Perera FP. Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol Biomarkers Prev. 2001;10:581–8. [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell ML, Choi H, Glinianaia S, Hoggatt KJ, Karr CJ, Lobdell DT. Methodological issues in studies of air pollution and reproductive health. Environmental research. 2009;109:311–320. doi: 10.1016/j.envres.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hou H, Ritz B, Chen Y. Exposure to polycyclic aromatic hydrocarbons and missed abortion in early pregnancy in a Chinese population. Science of the total environment. 2010;408:2312–2318. doi: 10.1016/j.scitotenv.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Yuan Y, Jin L, Zhou G, Zhu H, Finnell RH, Ren A. Levels of PAH-DNA adducts in cord blood and cord tissue and the risk of fetal neural tube defects in a Chinese population. Neurotoxicology. 2015;46:73–8. doi: 10.1016/j.neuro.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Doi H. Outdoor air pollution and term low birth weight in Japan. Environ Int. 2015;74:106–11. doi: 10.1016/j.envint.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Naruse H, Kashima S, Murakoshi T, Tsuda T, Doi H, Kawachi I. Residential proximity to major roads and placenta/birth weight ratio. Science of the Total Environment. 2012;414:98–102. doi: 10.1016/j.scitotenv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2016;311:R505–21. doi: 10.1152/ajpregu.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z-Z, Sparrow D, Hou L, Tarantini L, Bollati V, Litonjua AA, Zanobetti A, Vokonas P, Wright RO, Baccarelli A. Repetitive element hypomethylation in blood leukocyte DNA and cancer incidence, prevalence, and mortality in elderly individuals: the Normative Aging Study. Cancer Causes & Control. 2011;22:437–447. doi: 10.1007/s10552-010-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


