Abstract
Objective
This study was done for collection of real world data of Aripiprazole Once Monthly (AOM) in patients with schizophrenia.
Methods
The observation was up to 12 months from the first use of AOM in patients with antipsychotic polypharmacy (APpoly)/other long acting injectable antipsychotics (LAIs) for treatment of schizophrenia in daily practice. Demographics and available clinical information such as The Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression-severity (CGI-S) scores were retrieved from the electronic medical record (EMR). Adverse events were also noted as described in EMR.
Results
Eighteen patients were found to be switched from APpoly/LAIs. Mean numbers of previous APs treatment failure and immediate prior APs were 2.2 and 2.4, respectively; most commonly used APs before AOM were aripiprazole, blonanserin, quetiapine, and risperidone. Mean number of combined APs before AOM significantly decreased from 2.4 use to 0.7 at month 12 (p < 0.0001). The PANSS total (71.7 to 62.1, p = 0.000) and CGI-S (3.4 to 3.1, p = 0.008) scores were also significantly decreased from baseline (first use of AOM) to month 12, respectively. Other various psychotropics including anxiolytics were also significantly and substantially decreased at some point from baseline throughout the observation period as well. Mild hand tremor and akathisia were developed in 3 patients.
Conclusion
The present observation study clearly confirmed the use of AOM should be also effective and tolerable treatment option for patients with APpoly/LAIs in the real world practice. Subsequent, adequately-powered, and well-controlled clinical trials are warranted in near future.
Keywords: Long acting injectable antipsychotic, Aripiprazole once monthly, Polypharmacy, Schizophrenia, Effectiveness, Tolerability
INTRODUCTION
Long-acting injectable antipsychotics (LAIs) were developed to overcome poor adherence to antipsychotic therapy since it reduces relapse/rehospitalization as well as providing clear monitoring of adherence, stable plasma concentration, simplification of treatment, patients’ active participation in treatment, avoidance of overuse, regular interaction between patients and health professionals rates, and better relationship between dosing and blood level [1–3]. In these contexts, aripiprazole once monthly (AOM) has been also developed and approved by the U.S. Food and Drug Administration for the treatment of schizophrenia in 2013 [4–6].
The efficacy, safety, and lowering rehospitalization of AOM have been clearly demonstrated in a number of well-controlled, placebo-controlled, clinical trials and open, mirror-image studies for schizophrenia patients with acute, maintenance, and long-term treatment phase [7–13]. However, in pivotal trials [7,8], switching to AOM from prior antipsychotics were conservative approach since they were registration trials. Thus, switching to and stabilization with oral aripiprazole monotherapy over 4 to 6 weeks had to be done, after which AOM and oral aripiprazole (OARP) combination were also applied for 2 weeks at minimum. Furthermore, switching from other oral antipsychotics (OAPs) to AOM have not been well evaluated in large clinical trials. That is, there have been no supporting clinical trial data regarding switching from prior APs polypharmacy (APpoly)/LAIs which can be frequently met in routine practice, which is also similar limitation in switching to different LAI, such as paliperidone palmitate (PP) [14]. In fact APpoly ranges from 4 to 70% in routine practice depending study methodologies [15], such trend was also replicated in many claim-data and cohort studies [16,17].
Therefore, the present study tried to investigate the effectiveness and tolerability of switching to AOM in patients who were on APpoly/other LAIs in routine practice (SWAOM).
METHODS
This was a non-interventional, 12-month, retrospective, observational study. Study assessments were done during regular AOM injection visits that were part of routine care procudure.
Data were collected from January 2017 to August 2019 based on patients’ electronic medical records (EMR). Eighteen patients were enrolled based on the following criteria: 1) any type of the Diagnostic and Statistical Manual of Mental Disorders 5th edition schizophrenia; 2) patients who were on 2 or more APs or different LAIs (≥ 3 months) before the first injection of AOM (baseline); 3) patients should have at least 3 shots of AOM after baseline; 4) patients should have stable diagnosis of schizophrenia at least for more than 2 year, and 5) otherwise, no exclusion criteria were applied to reflect naturalistic treatment setting. Included data were age, sex, education level, family history, comorbidity, duration of illness, number of admission, number of treatment failure, pharmacological information (AOM/OARP doses at baseline, number of combined psychotropics, etc.). The Positive and Negative Syndrome Scale (PANSS) total, positive, negative, and general scores as well as Clinical Global Impression-Severity (CGI-S) score were also collected at baseline, month 3, month 6, and month 12. All adverse events (AEs) reported in EMR were also presented throughout the observation period.
Patients who had at least 3 shots of AOM were included for the outcome and tolerability analyses as priori definition of inclusion. Last observation carried forward was applied to impute missing or short follow-up data due to study period. Descriptive statistics were performed using the mean (standard deviation) and frequency (%) for continuous and categorical variables for basic demographics and clinical information. To compare changes in psychopathology (the PANSS and CGI-S scores) and clinical factors from baseline to month 12, nonparametric tests were done due to small sample size where appropriate. For exploratory purposes, a one-way general linear model was also conducted to see the trend of time-effect on psychopathology change. Group differences by follow-up periods (up to 12 months vs. < 12 months; ≥ 6 months vs. < 6 months) in psychopathology and other clinical factors were also compared by nonparametric tests where appropriate. All statistical tests were two-sided and a p value of ≤ 0.05 was considered significant.
The study was approved by the institutional review board at Bucheon St. Mary’s Hospital and was conducted in compliance with the Declaration of Helsinki (IRB approval number. HC19RESE0089).
REUSLTS
A total of 18 patients were enrolled as defined selection criteria. Briefly, gender distribution was equal (male, 50%) and mean age was approximately 40. The duration of illness was approximately 8 years. The previous admission and treatment failure numbers were 1.2 and 2.2, respectively. The PANSS total and CGI-S scores at baseline were 71.9 and 3.4, respectively.
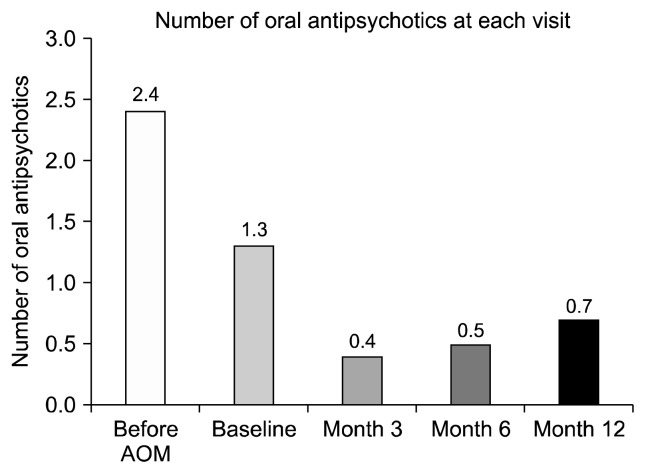
Table 1 demonstrates patients’ whole baseline demographic characteristics. The most frequently combined APs before AOM use were, aripiprazole, blonanserin, quetiapine, and risperidone, while PP was the only LAI. The mean numbers of APs and antianxiety medications before AOM were 2.4 and 0.9, respectively. The combined mood stabilizers (MSs) and antiparkinsonian drugs (APDs) before AOM were used in 11.1% and 44.4% of patients, respectively. The mean dose of AOM and OARP at baseline were 344.4 mg and 7.2 mg/d, respectively. The mean number of APs before AOM (2.4) to month 12 (0.7) was significantly decreased (p = 0.000, Fig. 1, Table 2, Supplementary Table 1). Furthermore, the linear decrease trend was substantial in change of combined APs during the study (p = 0.000, Fig. 1). Numerical decreases of concomitant MS, antianxiety drugs, and APDs before AOM to month 12 were also noted (Supplementary Table 1). There were no significant differences in proportion of conversion from AOM monotherapy at baseline to AOM/APpoly at month 12, and vice versa (from AOM monotherapy to AOM/APpoly n = 5, from AOM/APpoly to AOM monotherapy n = 2, p = 0.453, Supplementary Fig. 1).
Table 1.
Baseline demographics of the sample (n = 18)
| Variable | Value |
|---|---|
| Age (yr) | 39.6 ± 13.4 |
| Sex, male | 9 (50.0) |
| Education | |
| ≤ Middle school | 4 (22.2) |
| > Middle school, ≤ high school | 6 (33.3) |
| > High school, ≤ college | 8 (44.5) |
| Family history, yes | 1 (5.6) |
| Comorbidity, yes | 6 (33.3) |
| Diabetes mellitus | 3 (16.7) |
| Hypertension | 2 (11.1) |
| Hypothyroidism | 1 (5.6) |
| Duration of illness (yr) | 7.9 ± 7.2 |
| Number of admission | 1.2 ± 2.6 |
| Number of treatment failure | 2.2 ± 2.0 |
| PANSS total score | 71.9 ± 9.2 |
| PANSS positive | 19.9 ± 3.6 |
| PANSS negative | 24.0 ± 6.1 |
| PANSS general | 28.9 ± 7.4 |
| CGI-S | 3.4 ± 0.7 |
Values are presented as mean ± standard deviation or number (%).
PANSS, The Positive and Negative Syndrome Scale; CGI-S, Clinical Global Impression-Severity.
Fig. 1.
Combined oral antipsychotic pattern during the study. p = 0.000, p = 0.000, p = 0.414, p = 0.083, and p = 0.000 before aripiprazole once monthly (AOM) vs. baseline, baseline vs. month 3, month 3 vs. month 6, month 6 vs. month 12, and before AOM vs. month 12, respectively, Wilcoxon Signed Rank test; Time effect, df = 4, F = 44.129, p = 0.000, one way General Linear Model.
Table 2.
Antipsychotic administration pattern during the study (n = 18)
| Variable | Value |
|---|---|
| Antipsychotic combination immediate before AOM | |
| Amisulpride | 3 (16.7) |
| Aripiprazole | 16 (88.9) |
| Blonanserin | 6 (33.3) |
| Olanzapine | 3 (16.7) |
| Quetiapine | 6 (33.3) |
| Risperidone | 4 (22.2) |
| Haloperidole | 1 (5.6) |
| Ziprsidone | 1 (5.6) |
| Paliperidone palmitate | 3 (16.7) |
| First AOM dose | 344.4 ± 51.1 |
| Antipsychotic combination immediate before AOM | 2.4 ± 0.6 |
| Antianxiety drugs immediate before AOM | 0.9 ± 1.0 |
| Oral aripiprazole dose at baseline | 7.2 ± 4.6 |
| Duration of oral aripiprazole combination at baseline | 16.7 ± 10.0 |
| Duration of antipsychotic combination during the study | 114.1 ± 133.7 |
| Total AOM injection | 10.3 ± 3.1 |
| Antipsychotic combination at the first time of AOM (baseline) | |
| Aripiprazole | 16 (88.9) |
| Blonanserin | 1 (5.6) |
| Quetiapine | 3 (16.7) |
| Risperidone | 2 (11.1) |
| Antipsychotic combination at month 3 | |
| Aripiprazole | 1 (5.6) |
| Blonanserin | 1 (5.6) |
| Quetiapine | 3 (16.7) |
| Risperidone | 2 (11.1) |
| Antipsychotic combination at month 6 | |
| Aripiprazole | 1 (5.6) |
| Blonanserin | 1 (5.6) |
| Quetiapine | 5 (27.8) |
| Risperidone | 2 (11.1) |
| Antipsychotic combination at month 12 | |
| Aripiprazole | 1 (5.6) |
| Blonanserin | 1 (5.6) |
| Quetiapine | 4 (22.2) |
| Risperidone | 3 (16.7) |
| Olanzapine | 1 (5.6) |
Values are presented as number (%) or mean ± standard deviation.
AOM, aripiprazole once monthly; patients can take one or more antipsychotics.
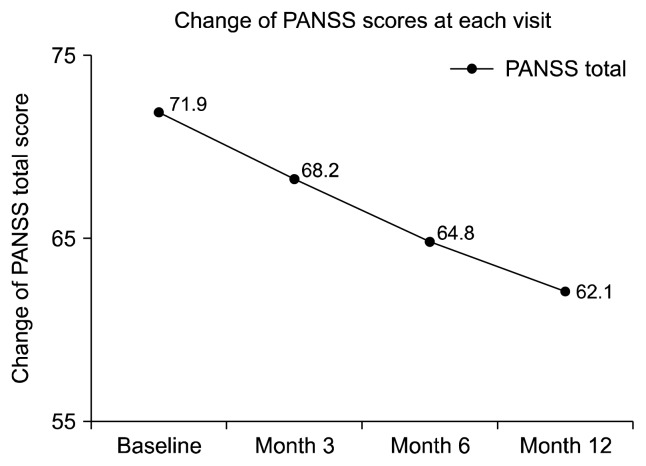
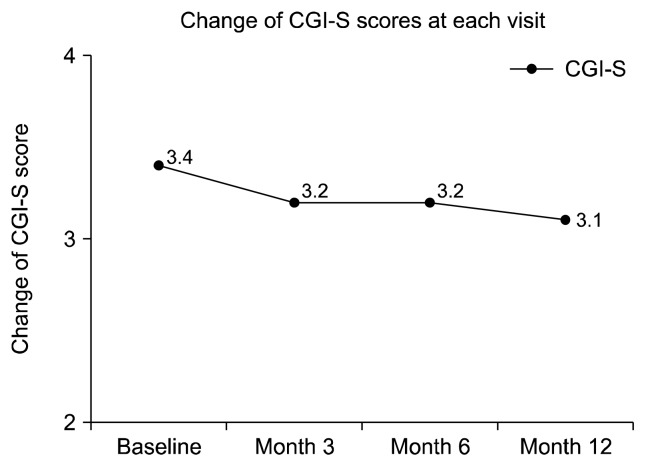
The PANSS total score was significantly decreased by 13.6% from baseline to month 12 (−9.8, p = 0.001, Fig. 2). When it comes to subscores of the PANSS, the PANSS positive, negative, and general scores all significantly decreased by 10.7% (p = 0.015), 21.3% (p = 0.001), and 13.6% (p = 0.001), respectively from baseline to month 12 (Supplementary Fig. 2). The CGI-S score was also significantly decreased by 8.8% from baseline to month 12 (−0.3, p = 0.008, Fig. 3). Furthermore, the linear decrease trend was substantial in change of all PANSS total (p = 0.001) and all subscores as depicted in Figure 2 and Supplementary Figure 2 (p = 0.038, p = 0.001, and p = 0.003, respectively), however, statistically marginal linear trend was noted in change of CGI-S score (p = 0.055) during the study.
Fig. 2.
The change of the Positive and Negative Syndrome Scale (PANSS) total score during the study. p = 0.005, p = 0.001, p = 0.003, and p = 0.001 between baseline to month 3, month 3 to month 6, month 6 to month 12, and baseline to month 12, respectively, Wilcoxon Signed Rank test; Time effect, df = 3, F = 9.915, p = 0.001, one way General Linear Model.
Fig. 3.
The change of Clinical Global Impression-severity (CGI-S) score during the study. p = 0.046, p = 0.317, p = 0.157, and p = 0.008 between baseline to month 3, month 3 to month 6, month 6 to month 12, and baseline to month 12, respectively, Wilcoxon Signed Rank test; Time effect, df = 3, F = 3.182, p = 0.055, one way General Linear Model.
Among 18 patients, 12 (66.7%) completed 12 month full observation, while 6 failed (33.3%). When compared the two groups, completers showed significantly greater improvements in PANSS total and negative scores than non-completers (Supplementary Fig. 3), while there were no group differences in scores of PANSS positive general and CGI-S. In addition, when subdividing patients completed 6 months observation and less than 6 months, there were no group differences in all psychopathology scores (Supplementary Fig. 4).
Regarding AEs, hand tremor, akathisia, headache, and sedation were presented in 5, 2, 2, and 1 patients, respectively during the study, mainly early phase of AOM use. All AEs were mild and disappeared spontaneously. When dividing 12 months completers and noncompleters, hand tremor, akathisia, headache, and sedation were presented in 3, 2, 0, and 0 in 12 months completers, while they were 2, 0, 2, and 1 in noncompleteres, during the study.
DISCUSSION
Overall the effectiveness and tolerability of AOM in the present study are in line with the positive findings of AOM use from a number of previous well-controlled clinical trials [7–13]. The present study tried to test the effectiveness and tolerability of switching to AOM from APpoly/other LAIs (SWAOM) in patients with schizophrenia. To our best knowledge, the present study is the first one proving the effectiveness and tolerability of SWOAM in patients with schizophrenia in daily practice. It is intriguing results since there have been no such supporting clinical data like our study. In all acute, maintenance, and long-term treatment studies using AOM [7–13]. AOM was switched from AP monotherapy (APmono) patients but not from APpoly patients who are commonly seen in routine practice; AOM studies were done in controlled and selected patients population, which is difficult to reflect the real world treatment setting. Therefore, the present study may firstly elaborate the potential utility of SWAOM in routine practice.
In the present study, after SWAOM, the total number of combined APs significantly and substantially decreased at month 12 than before AOM, as well as showing similar numerical reduction of other combined psychotropics throughout the study. Simplification of treatment regimen is essential in improving drug adherence/compliance which is strongly associated with later treatment outcomes, by which the present study may propose the advantage and usefulness of AOM in patients with APpoly in daily practice.
In addition, the SWAOM substantially reduced various domains of psychotic symptoms demonstrated by significant reduction of PANSS total and all subscores of PANSS throughout the study. Significant reduction of CGI-S score also support such treatment effects of AOM. Our present psychopathology data are also meet the findings from previous studies investigating the correlation of PANSS and CGI-S scores [18]. A ≥ 20% reduction of the PANSS score was approximately found to correspond to one point of CGI-S score, in fact 13.6% (−9.8) reduction of PANSS total score and −0.3 point decrease of CGI-S were noted in the present study which are in line with the prior study. In addition, approximately 15 points of PANSS total score was also found to correspond to 0.8 to 1 point of CGI-S score [19]. Given the previous study findings, the changes of PANSS and CGI-S scores in the present study are highly correlated each other and thereby we may assume reliability of present findings. Furthermore 12 months completers had better clinical outcomes than noncompleters, proposing an advocacy of long-term use of AOM in routine clinical practice.
The AEs were presented in 8 patients with mild-intensity and there were no observation of any movement disorder including tardive dyskinesia, which support a tolerability of AOM use in patients with APpoly/other LAIs.
Finally, our study has many shortcoming to be corrected in future well-designed studies for better generalization. The sample size was not sufficient to detect a large effect size, however, we have to consider that SWAOM has not been studied yet. All the patients were recruited in one teaching-hospital. Naturalistic and retrospective design could influence outcome results due to recall bias and lack of control group.
In conclusion, the present study potentially indicates that AOM may be beneficial and useful treatment option for patients with currently being treated by APpoly/other LAIs in routine practice without worsening of psychopathology or causing serious AEs. Adequately powered and well-controlled clinical trials are warranted to support the present findings in near future.
Supplementary Information
Footnotes
Conflicts of Interest
All authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author Contributions
Conceptualization: Pae CU, Han C. Protocol development: Pae CU. Draft writing: Pae CU, Han C. Intellectual comments and critics on the content: Lee SJ, Patkar AA, and Masand PS. Data acquisition: Pae CU, Han C. Data analysis: Pae CU, Han C, Patkar AA, and Masand PS.
SUPPLEMENTARY MATERIALS
Supplementary data are available online.
REFERENCES
- 1.Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219. doi: 10.1177/2045125314540297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pae CU, Wang SM, Han C, Bahk WM, Lee SJ, Patkar AA, et al. Comparison between long-acting injectable aripiprazole versus paliperidone palmitate in the treatment of schizophrenia: systematic review and indirect treatment comparison. Int Clin Psychopharmacol. 2017;32:235–248. doi: 10.1097/YIC.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 3.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Schizophrenia relapse and the clinical usefulness of once-monthly aripiprazole depot injection. Neuropsychiatr Dis Treat. 2014;10:1605–1611. doi: 10.2147/NDT.S52486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otsuka Pharmaceutical Co., Ltd. The manual of Abilify Maintena Kit. Tokyo: Otsuka Pharmaceutical Co., Ltd; 2013. [Google Scholar]
- 5.Han C, Wang SM, Bahk WM, Lee SJ, Patkar AA, Masand PS, et al. The potential utility of aripiprazole augmentation for major depressive disorder with mixed features specifier: a retrospective study. Clin Psychopharmacol Neurosci. 2019;17:495–502. doi: 10.9758/cpn.2019.17.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, Hong JS, Kim SM, Min KJ, Chung US, Han DH. Effects of amisulpride adjunctive therapy on working memory and brain metabolism in the frontal cortex of patients with schizophrenia: a preliminary positron emission tomography/computerized tomography investigation. Clin Psychopharmacol Neurosci. 2019;17:250–260. doi: 10.9758/cpn.2019.17.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205:135–144. doi: 10.1192/bjp.bp.113.134213. [DOI] [PubMed] [Google Scholar]
- 8.Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73:617–624. doi: 10.4088/JCP.11m07530. [DOI] [PubMed] [Google Scholar]
- 9.Ishigooka J, Nakamura J, Fujii Y, Iwata N, Kishimoto T, Iyo M, et al. Efficacy and safety of aripiprazole once-monthly in Asian patients with schizophrenia: a multicenter, randomized, double-blind, non-inferiority study versus oral aripiprazole. Schizophr Res. 2015;161:421–428. doi: 10.1016/j.schres.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Potkin SG, Raoufinia A, Mallikaarjun S, Bricmont P, Peters-Strickland T, Kasper W, et al. Safety and tolerability of once monthly aripiprazole treatment initiation in adults with schizophrenia stabilized on selected atypical oral antipsychotics other than aripiprazole. Curr Med Res Opin. 2013;29:1241–1251. doi: 10.1185/03007995.2013.821973. [DOI] [PubMed] [Google Scholar]
- 11.Kane JM, Peters-Strickland T, Baker RA, Hertel P, Eramo A, Jin N, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75:1254–1260. doi: 10.4088/JCP.14m09168. [DOI] [PubMed] [Google Scholar]
- 12.Fleischhacker WW, Sanchez R, Johnson B, Jin N, Forbes RA, McQuade R, et al. Long-term safety and tolerability of aripiprazole once-monthly in maintenance treatment of patients with schizophrenia. Int Clin Psychopharmacol. 2013;28:171–176. doi: 10.1097/YIC.0b013e3283615dba. [DOI] [PubMed] [Google Scholar]
- 13.Kane JM, Sanchez R, Zhao J, Duca AR, Johnson BR, McQuade RD, et al. Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Med Econ. 2013;16:917–925. doi: 10.3111/13696998.2013.804411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiner A, Caspi A, Bergmans P, Cherubin P, Keim S, Lara E, et al. Switching from oral atypical antipsychotic monotherapy to paliperidone palmitate once-monthly in non-acute patients with schizophrenia: a prospective, open-label, interventional study. Psychopharmacology (Berl) 2017;234:3–13. doi: 10.1007/s00213-016-4445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2014;17:1083–1093. doi: 10.1017/S1461145712000399. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J, le Quach P, Emborg C, Foldager L, Correll CU. 10-year trends in the treatment and outcomes of patients with first-episode schizophrenia. Acta Psychiatr Scand. 2010;122:356–366. doi: 10.1111/j.1600-0447.2010.01576.x. [DOI] [PubMed] [Google Scholar]
- 17.Gilmer TP, Dolder CR, Folsom DP, Mastin W, Jeste DV. Antipsychotic polypharmacy trends among Medicaid beneficiaries with schizophrenia in San Diego County, 1999–2004. Psychiatr Serv. 2007;58:1007–1010. doi: 10.1176/ps.2007.58.7.1007. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz J, Mehnert A, Eerdekens M. To what extent do the PANSS and CGI-S overlap? J Clin Psychopharmacol. 2006;26:303–307. doi: 10.1097/01.jcp.0000218407.10362.6e. [DOI] [PubMed] [Google Scholar]
- 19.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.