Abstract
A highly infectious tick‐borne virus causes Kyasanur Forest disease (KFD), which has been expanding in recent decades in India. Current studies do not provide an updated understanding of the disease trends and its expansion in India. We address this gap in the literature through a detailed review to reveal the annual historic expansion of KFD cases across the span of years from 1957 to 2017. In addition, we explore the factors that may have led to the geographic expansion of KFD. The annual numbers of cases of KFD among humans are estimated using peer‐reviewed journal articles, Pro‐MED database, historical and archived newspapers, and government reports, technical reports, publications, and medical websites. From 1957 to 2017, there were an estimated 9,594 cases of KFD within 16 districts in India. The most significant human outbreaks of the disease were in the years 1957–1958 (681 cases), 1983–1984 (2,589 cases), 2002–2003 (1,562 cases), and 2016–2017 (809 cases). In 2015, KFD appeared in Goa. In 2016, new cases emerged in Belgaum, a district in Karnataka state, and in the Sindhudurg district in Maharashtra state. The processes by which KFD persists and spreads are not clear, but demographic, socioeconomic, political, and environmental factors seem to play a role.
Keywords: Kyasanur Forest disease, retrospective analysis
Key Points
Kyasanur Forest disease is expanding across states in India
From 1957 to 2017, there were over 9,000 human cases of Kyasanur Forest disease in India
Demographic, socioeconomic, political, and environmental factors play a role in the persistence and spread of Kyasanur Forest disease, but more research is needed
1. Introduction
Kyasanur Forest disease (KFD) is caused by a highly infectious tick‐borne virus. KFD results in illness in both human and nonhuman primates. The disease has been geographically focused in southern India, but its area has been expanding in recent decades. Since its discovery in 1957, KFD was seen primarily in and around the Sagar and Sorab taluks in the Shimoga district of Karnataka (Bhatt et al., 1966; Work et al., 1959; Work & Trapido, 1957). However, KFD has now spread to as far as Sindhudurg district in Maharashtra to the north and Palakkad district in Kerala to the south, with recent news indicating that the virus has spread to new villages in Sindhudurg (Awate et al., 2016; Sadanandane et al., 2018). Antibodies against the virus have also been detected in humans in areas of Gujrat state, parts of West Bengal, and in Andaman and Nicobar islands (Pattnaik, 2006). Some authors believe that other strains of the KFD virus probably exist in other parts of India, even though their presence has not yet been recognized (Gould et al., 2004).
There is no cure in sight for KFD, and the available vaccine has a limited coverage in affected areas and is not fully efficacious to prevent new outbreaks (Kasabi et al., 2013; Kiran et al., 2015). Prevention, early detection, supportive care, and management of symptoms are the primary responses to reduce the effects of illness caused by this virus.
The etiological agent of KFD, the Kyasanur Forest disease virus (KFDV), belongs to the family Flaviviridae and genus Flavivirus. Serologic studies and phylogenetic sequencing indicate that it is part of a group of tick‐borne viruses of mammals associated with hemorrhagic fever and is closely related to Alkhurma virus in Saudi Arabia and Egypt (Dodd et al., 2011; LaSala & Holbrook, 2010; Pattnaik, 2006). Other viruses of note that are part of this complex circulate in different regions of the world and include Omsk hemorrhagic fever virus in Siberia and Powassan virus in the United States and Russia (Centers for Disease Control & Prevention, CDC, 2014; Morse et al., 1962).
Shortly after the discovery of Omsk hemorrhagic fever and KFD in 1950s, theories circulated about the origin of KFD in India. Scientists pondered that the migratory birds that flew from Soviet Russia to India might have carried infected ticks and thus the virus. Scientists at Bharatpur National Bird Sanctuary, Rajasthan, reported KFD antibodies in some birds (Lewis, 2002, Times of India, December 1978; Times of India, June 1979). However, there are no reports of disease among humans or monkeys in the Kyasanur forest region prior to December 1955 (Work et al., 1959). Nonetheless, it is possible that KFDV may have been present in India before 1957, but cases, if existent, may not have been identified or reported (Pattnaik, 2006). Both casual observations and the scientific literature point toward the effect of extensive deforestation to make way for plantations and climatic trends conducive to the viral transmission, which may have led to the development of cases (Ajesh et al., 2017; Pattnaik, 2006). Clearly, a complex set of processes account for the initial outbreak of KFD among monkeys and subsequent illnesses in humans and recent spread of the virus (Shah et al., 2018).
KFD is frequently fatal among nonhuman primates and is known to affect two South Indian species; Macaca radiata (bonnet macaque) and langurs (e.g., gray langur) in the genus Semnopithecus (Pattnaik, 2006). Monkeys are important reservoir hosts of KFDV, but they are often unable to withstand the onslaught of the virus and die shortly after infection (Goverdhan, 1974; Kenyon et al., 1992; Mourya et al., 2012). Cattles are also hosts for the primary vectors of KFDV, but they do not amplify the virus. Though cattle may act as maintenance hosts, the role of cattle in KFD transmission needs to be studied (Anderson & Singh, 1971; Rajagopalan & Sreenivasan, 1981). In addition to larger mammals, field studies have revealed that many small forest mammals can maintain the KFDV and have the potential to infect ticks. These include Blanford's rat, jungle striped squirrel, field mice, Indian gerbil, frugivorous and insectivorous bats, and the common house shrew (Banerjee, 1988; Mourya & Yadav, 2016; Pattnaik, 2006). Mammals of different sizes are usually involved at different stages of the tick life cycle (Pattnaik, 2006). Randolph and Rogers (2006) argue that virus transmission when ticks co‐feed on a single host is even more likely to transmit the virus than when ticks bite a host with low‐level viremia as found in cattle, produced by the bites of infected ticks on large mammals, primates and humans.
It is possible that the virus can be transmitted through contact with carcasses or feces, but the primary transmission path of KFDV is through a bite from an infected tick (Work, 1958). The primary vector of KFDV is Haemaphysalis spinigera, a hard‐bodied multihost tick species prevalent in India and Sri Lanka. KFDV also circulates in several other tick species, including Haemaphysalis turturis, Haemaphysalis kinneari, Haemaphysalis kyasanurensis, Haemaphysalis wellingtoni, Haemaphysalis minuta, Haemaphysalis cuspidata, Ixodes petauristae, Ix.sceylonensis, Dermacentor auratus, and Rhipicephalus haemaphysaloides, as well as is capable of being transmitted by soft ticks of the Ornithodoros genus (Holbrook, 2012; Mourya & Yadav, 2016). In India, these tick species have been reported in eleven states out of 29 states and 7 Union territories (Ghosh et al., 2007).
Under laboratory conditions with a temperature range of 18–35 °C, the H. spinigera life cycle is completed within 118–160 days (Ghalsasi & Dhanda, 1974). Unfed male and female adults feed on cattle or other large animals for 8–13 days. Upon full engorgement, they drop and the female oviposit after 2–5 days, postdetachment. During oviposition, females can lay a variable number of eggs depending on the host species. For example, if the female fed on a calf, it would lay 1,804–3,536 eggs and if it fed on a rabbit, it would lay 388–3,136 eggs (Ghalsasi & Dhanda, 1974). Larvae hatch after 25–30 days, and after 5–7 days they usually feed on small mammals, monkeys, and birds. Following feeding, larvae molt into nymphs within 13–16 days after detachment. At the nymph stage, the H. spinigera can feed on an array of species ranging from humans to monkeys, small mammals, and birds and feed for 25–30 days post molting (Ghalsasi & Dhanda, 1974; Pattnaik, 2006). Murhekar et al. (2015) argue that since monkeys serve as sentinel animals for KFD, monkey deaths can be used as event‐based surveillance system that would indicate the need to apply precautionary measures in areas where such deaths have been observed (Murhekar et al., 2015). Singh et al. (1963) have shown experimentally that KFDV can be transmitted transovarially among H. spinigera ticks.
The KFD presents in the form of high fever, chills, myalgia, local or generalized lymphadenopathy, suffusion of the conjunctiva, photophobia, petechial hemorrhages on the mucous membranes, and bleeding from the nose, mouth, or gastrointestinal tract (Pavri, 1989; Webb & Lakshmana Rao, 1961). These symptoms begin 3–8 days after exposure. Within a period of 2 weeks, patients begin to recover (Holbrook, 2012). However, in some cases there is a 7‐ to 14‐day period of remission, which is followed by a second phase characterized by neurologic manifestations. These may include severe headaches, mental disturbances, tremors, rigidity, photophobia, eye pain, and defective vision (CDC, 2014; Holbrook, 2012). According to the U.S. Centers for Disease Control & Prevention there are about 400–500 cases of KFD per year in India and case fatality is about 3–5%. More recently, Mourya and Yadav (2016) reported that the mortality rate for KFD is between 2% and 20%; however, Tandale et al. (2015) stated that case fatality is between 2% and 10% and Shah et al. (2018) reported case fatality between 3% and 10%.
People who work or reside in locations where ticks infected with KFDV are present have a high likelihood of acquiring the infection (Mourya & Yadav, 2016; Patil et al., 2017). India has been working on the distribution of preventative vaccines for KFD that have been shown to have moderate efficacy. Nevertheless, the disease continues to spread in spite of the vaccine (Chari, 2018; Kasabi et al., 2013; National Center for Disease Control Bulletin, 2018; Times of India, 1978). The disease has greatest socioeconomic impact on the livelihood of groups of people such as agriculturists, migrant workers, and indigenous tribal folk, who are dependent on the forests for survival (Ajesh et al., 2017; Patil et al., 2017; Sadanandane et al., 2017). Lack of awareness about the disease, limited access to healthcare, and the highly pathogenic nature of the virus further increased the need for better understanding of KFD and its spread.
Current studies do not provide an updated understanding of the disease trends and expansion in India. We address this gap through a detailed literature review to reveal the annual historic expansion of KFD cases across the span of years from 1957 to 2017. In addition, we explore the factors that may have led to the geographic expansion of KFD through maps, graphs, and review of historical trends.
2. Materials and Methods
The annual number of cases of KFD in humans was estimated using peer‐reviewed journal articles, the Pro‐MED database, historical and archived newspapers, and other gray literature sources such as government reports, technical reports, publications, and medical websites. All the necessary information was first evaluated and then stored in a comprehensive database that was used to construct a data matrix of the number of KFD cases by district and by year from 1957 to 2017.
In the first stage of the data compilation, peer‐reviewed articles were identified using keywords in the PubMed database in the U.S. National Library of Medicine, the Web of Science, a service provided by Clarivate Analytics, and the Cochrane Library database, which comprises collections from an independent network of clinicians and scientists to inform healthcare decision making. In addition, we performed targeted searches of articles online and through the Indian Journal of Medical Research, which published the bulk of KFD articles from 1957 to 1989. The comprehensive search was carried out from 13 June 2017 to 21 May 2018. Duplicates were removed, and any articles that did not provide information on KFD cases were excluded. The search keywords were “Kyasanur forest disease OR monkey fever OR KFD.”
Second, we searched the Program for Monitoring Emerging Diseases (known as Pro‐MED‐mail, abbreviated Pro‐MED). Pro‐MED is one of the largest publicly available emerging diseases and outbreak reporting systems in the world. Third, we carried out a careful review of available information from historical archives of newspapers indexed in the ProQuest database where contemporaneous reports of outbreaks are mentioned. These sources included the Times of India (the largest print paper in the nation), Deccan Chronicle, and The Hindu. Finally, we examined the Government of India health and welfare service websites of Karnataka, Kerala, Goa, and Maharashtra states as well as the same for Integrated Disease Surveillance Program (IDSP) (2018).
Information on the number of KFD cases reported in each year from all the sources was recorded first in a database with fields for year, number, and location of cases (state, district, and village), year of source publication, authors, nature of source, and any additional relevant information that was extracted. The database information was then reorganized into a matrix that was used to assess spatial and temporal trends supporting information data). The search was focused on 16 districts that had cases reported from any source or that lie geographically in a contiguous pattern with those that did have cases reported up to 21 May 2018. We considered this contiguous region of 16 districts to represent the KFD potential area. The 16 districts were the following: Shimoga, Uttar Kannada, Dakshina Kannada, Chikmagalur, Chamarajanagar, Udupi, Belgaum, The Nilgiris, Wayanad, Palakkad, Mallappuram, North Goa, South Goa, Kodagu, Mysore, and Sindhudurg (Figure 1).
Figure 1.
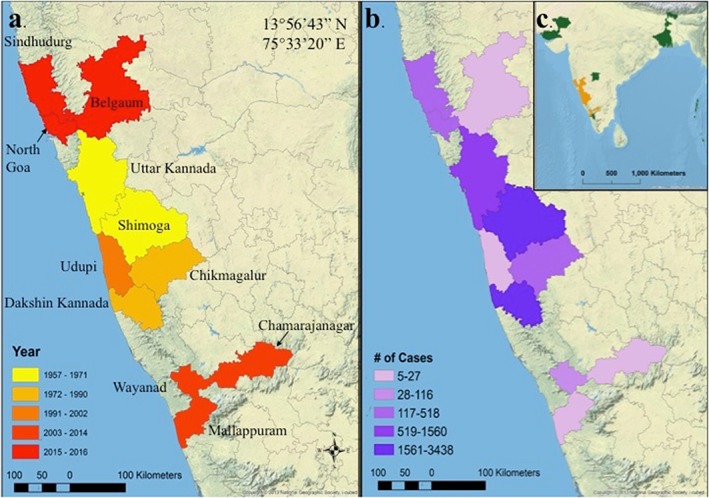
Cases of Kyasanur Forest disease in India depicted by (a) year of the first case in each district (n = 16), (b) number of human cases (n = 9594), and (c) and all seroprevalence antibodies discovered outside of this study's region of interest (n = 6).
The information in the full database by year and by district was reviewed to estimate the final number of cases for each district/year combination. The following strategy was used to remove duplicates and determine the authoritative source and number of cases for each outbreak:
Peer‐reviewed journal articles that reported case data were used whenever possible. If journal articles did not have information for a specific district/year, the Pro‐MED database, followed by historical newspapers and then other gray literature sources, was consulted. This order was used because the peer‐reviewed papers offered the most coverage over time and had the benefit of being vetted during the peer‐review process. The Pro‐MED database has a very consistent history of case documentation, but for KFD the information on Pro‐MED is available only from 1998 onward. Historical newspapers had important information for some years in which other sources were not available, but the overall coverage was less systematic and less consistent with other sources.
If data were given in the form of an aggregate number across a multiyear period and several locations were provided, the total number of cases was divided equally between the districts to estimate cases for individual district for individual years. For example, Dandawate et al. (1994) provided an aggregate case number for three years (1990–1993) in three Karnataka districts. For the final case estimates, the number of cases was divided by 3 to obtain a total annual number and then further divided by the three districts.
-
In some cases, the source mentioned the occurrence of outbreaks in certain time periods with some description of the magnitude of the outbreak, but with no specific number of cases. In this situation, case numbers were determined from descriptive text that inferred whether the outbreak appeared to be large, medium, or small.
We used the initial review of the literature to categorize outbreaks as large if there were 400 or more cases, medium if there were 100–399 cases, and small if there were 1–99 cases.
For the large outbreaks, we consistently found case estimates in the literature, so there was no need to estimate these. For medium and small outbreaks, we assigned conservative numbers, 110 for medium number of cases and 20 for small number of cases.
If there was no mention of any data or occurrence of a case in a district for a given year, we assigned 0 as the number of cases for that district/year.
Finally, the same set of literature used to create the data matrix was supplemented by additional peer‐reviewed publications, which were used to identify factors and explanations regarding the emergence and spread of KFD in India. Graphs were used to depict trends in the number of cases. Data of the number of the cases per year by district were mapped using Arc GIS v. 10.5.1 (Redland, California).
3. Results
The annual number of cases of KFD in humans was estimated using peer‐reviewed journal articles on four databases. The keyword searches yielded 153,689 articles on PubMed, 507 articles on Web of Science and 17 articles on Cochrane Library. We were left with 48 peer‐reviewed articles, which met our criteria of reporting cases, and we added it to the full database. The keyword search on the Pro‐MED (2018) database yielded 76 articles from 1998 to 2018 for both humans and monkeys. Similar search on newspapers resulted in 24 out of 100 articles being found that were included from “The Times of India” and 141 articles from “The Hindu.” Of the websites reviewed, the Directorate of Health and Welfare Services website of the Government of Karnataka had information for 5 years, that is, from 2013 to February 2017 via annual reports of 2015–2016 and 2016–2017 (Directorate of Health and Family Welfare Services, Govt of Karnataka (2016a, 2016b)). Additionally, the annual report of 2017 published by IDSP provided a general overview of KFD in the affected states (IDSP annual report, 2017).
Based on the data matrix (supporting information data), the first appearance of KFD in each of the selected districts was determined (Figure 1 and Table 1). After the initial outbreak in 1957, KFD spread to different districts within Karnataka and eventually to other states across the span of years from 1957 to 2017.
Table 1.
Characteristics of Kyasanur Forest Disease Cases in 16 Districts of India During the Period From 1957 to 2017
| District | First year reported | Number of years with cases | Total number of cases | Number of monkey deaths (and no. of years) |
|---|---|---|---|---|
| Shimoga | 1957 | 38 | 3,336 | 318 (21) |
| Uttar Kannada | 1971 | 22 | 1,560 | 34 (4) |
| Dakshina Kannada | 1982 | 10 | 3,438 | Unknown |
| Chikmagalur | 1990 | 14 | 367 | Unknown |
| Udupi | 2002 | 2 | 27 | Unknown |
| Chamarajanagar | 2012 | 2 | 7 | 10 |
| Wayanad | 2013 | 4 | 116 | 114 (1) |
| Mallappuram | 2014 | 1 | 5 | 1 (1) |
| North Goa | 2015 | 3 | 265 | 41 (1) |
| Belgaum | 2016 | 1 | 16 | 28 (1) |
| Sindhudurg | 2016 | 2 | 456 | 61 (1) |
| Nilgiris | NA | 0 | 0 | 1 (1) |
| Palakkad | NA | 0 | 0 | Unknown |
| Kodagu | NA | 0 | 0 | Unknown |
| South Goa | NA | 0 | 0 | Unknown |
| Mysore | NA | 0 | 0 | Unknown |
| Gulbargaa | 2006 | 1 | 1 | 11 (1) |
| Parts in state of West Bengala , b | 1962 | 1 | Not applicable | Unknown |
| Jalore & Barmer districts of Rajasthana , b | 1979 | 1 | Not applicable | Unknown |
| Kutch district of Gujarata , b | 1979 | 1 | Not applicable | Unknown |
| Andaman & Nicobar Islandsa , b | 2002 | 1 | Not applicable | Unknown |
| Palakkad district of Keralaa , b | 2014 | 1 | Not applicable | 18(1) |
Note. NA = not applicable.
Regions not part of the 16 districts Kyasanur Forest disease area of interest and thus not included in analysis.
Reports of human haemagglutination inhibition antibody presence against Kyasanur Forest disease in humans.
We identified outbreaks that included an estimated 9,594 cases within the 16 districts region from 1957 to 2017 (Figure 2). Information about KFD cases prior to 1957 is not available in the reviewed literature. Boshell (1969) considered that there may have been KFDV or similar infective agent that may have been inconspicuously present in other parts of India and that unknown changes in the environment might have led to the major flare‐up in 1957. Hence, we only offer KFD case information 1957 onward. After the first outbreak in 1957 at Shimoga, the number of cases dropped from around 500 to less than 200 in most years, except for 1975, with 226 cases. Cases remained restricted mostly to Shimoga, with some cases in Uttar Kannada. The next large outbreak occurred in 1981 at Shimoga with over 500 cases reported. In 1982, new cases were reported in Dakshina Kannada, which was followed by the largest outbreak since 1957, with 1,984 cases in the single year 1983. In 1984, cases were reported solely in Dakshina Kannada, but the number dropped to around 600. In the following years, the disease spread geographically to Chikmagalur and Udupi districts, but no major outbreaks were reported. Then in 2001, over 400 cases were reported at Shimoga and Uttar Kannada. In 2002, the number of cases increased to over 600. The growth in the number of cases continued in 2003 reaching over 900 cases. In 2013, for the first time KFD was observed in a new state—Kerala. Subsequently, the disease spread to other states of Goa and Maharashtra in 2015 and 2016. In recent years, the largest outbreak was seen in 2017 (462 cases). The most striking aspect of the recent outbreaks is that the cases are now being seen most often outside the traditional region near Shimoga.
Figure 2.
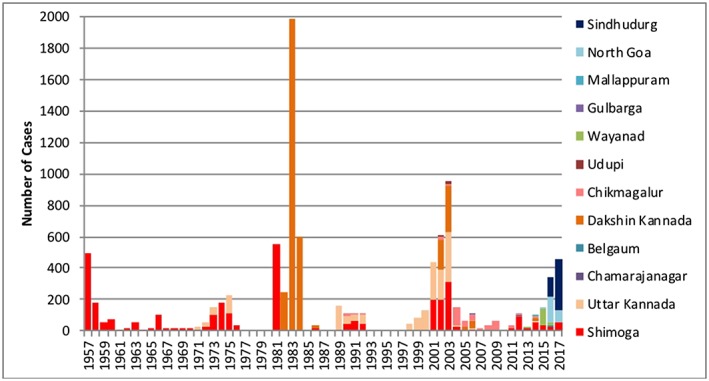
Distribution of Kyasanur Forest disease cases by districts in India (1957–2017).
Although we do not have adequate information on the death rate due to KFD among humans, the Pro‐MED data indicated 93 confirmed deaths compared to 3,836 total cases from 1999 to 2017, which would indicate a case fatality rate (CFR) of 2.42%. Compared to the CFR reported by Tandale et al. (2015), Shah et al. (2018), and Mourya and Yadav (2016), our CFR closely matches estimates provided by the CDC (2013). It has to be kept in mind that we provide only population level estimates reported via surveillance systems like Pro‐MED and journal articles instead of clinical cases; thus, the CFR is variable. As Gibbons et al. (2014) stated, cases reported at the top of the morbidity surveillance pyramid are often subject to underestimation due to the failure to capture all the infectious cases in a given population. Therefore, we need individual level data in order to truly assess CFRs in which individuals are followed through time.
Based on the information obtained in this retrospective analysis, we noted nine distinct epochs from 1957 to 2017 (Table 2). The most significant human outbreaks of the disease were in the years 1957–1958 (681 cases), 1983–1984 (2,589 cases), 2002–2003 (1,562 cases), and 2016–2017 (809 cases).
Table 2.
Summary of Important Events Within the Identified Periods in KFD Expansion
| KFD main periods | Important events within each period |
|---|---|
| 1956–1960 | 1956: States were reorganized based on linguistic and other criteria. The Kannada speaking population came together to form the present day Karnataka under the name of Mysore. |
| First KFD outbreak observed. 681 cases from 1957 to 1958 in Karnataka | 1958: Rockefeller Foundation donated $100,000 to build vaccines for KFDV in the United States |
| 1961–1971 | 1960: Bombay bifurcated into Gujarat and Maharashtra. |
| 1962: First KFD vaccine developed by the Walter Reed Army Institute Research Laboratory, Washington D.C., USA. | |
| Major outbreaks not observed, small number of cases reported throughout this period | 1964: Karnataka Land Revenue Act passed |
| 1966: Advent of the Green Revolution in India | |
| 1972–1976 | 1970s: Second phase of Green Revolution in Karnataka through the University of Agriculture Science, Bangalore |
| Major outbreaks not observed, moderate number of cases reported throughout this period | |
| 1977–1980 | 1980: National Forest Conservation Act passed |
| No cases reported | |
| 1981–1984 | 1980s: Health insurance launched, in the form of introduction of healthcare finance |
| Largest outbreak of KFD ever reported | |
| 1985–1997 | 1985: The 4th Land Reforms Act led to imposition of ceiling by reduction in size of landholding from 3.2 to 2.4 ha to allow equitable distribution of agrarian land. |
| Major outbreaks not observed, medium number of cases reported throughout this period | 1986: Environment Protection Act passed after Bhopal Gas tragedy. |
| 1990s: First licensed KFDV formalin inactivated chick fibroblasts vaccine produced and administered. | |
| 1998–2006 | 2000: India reaches 1 billion population mark. |
| 2004: Asian Tsunami hits southern most parts of India and the Andaman & Nicobar Islands, | |
| Major outbreaks not observed, large number of cases reported throughout this period in four districts of Karnataka | 2004: Integrated Disease Surveillance Programme (IDSP) (2018) launched. |
| 2005: Indian government launched the National Rural Health Mission (NRHM). | |
| 2007–2014 | 2011: Crimean‐Congo Hemorrhagic fever outbreak in Gujarat (a tick‐borne disease) |
| Major outbreaks not observed, medium number of cases reported throughout this period. KFD expanded to Kerala | |
| 2015–2017 | 2015: Largest recorded outbreak of dengue in India. |
| Large number of cases reported throughout this period. KFD expanded to three states | 2017: Severe floods in several parts of India including in Tamil Nadu and Maharashtra |
In order to provide context to these outbreak periods, we identified contemporaneous events that occurred during the study period from 1957 to 2017. Events were chosen based on their possible relevance to KFD, important changes in the key states of interest, and any political and environmental changes of note. When the first KFD outbreak occurred in 1957, Rockefeller Foundation scientists came down to India on the assumption that it was yellow fever but only after their investigations did they determine it to be a completely new disease (Rao, 2016). The Rockefeller Foundation later funded the development of the first KFD vaccines, which, although ineffective, set in motion the chain of developing better vaccines for KFD. Postindependence, several states underwent changes in geography as well as had to be reorganized based on the dominant languages spoken in the area as well as for ease of administrative purposes. Significant agrarian laws were passed such as Land Reforms Act and Land Revenue Act in order to provide equitably distributed land to the people and increase income generated from land holding (Table 2). The successful phase of the Green Revolution helped to increase the agricultural output from Karnataka and the passing of the Forest Conservation Act made conservation of forests important. Similarly, the Environment Protection Act was another key law passed during this time. Other important events that occurred were the launch of the IDSP for monitoring of diseases in India in 2004 (Reddy et al., 2005), which expanded previous efforts carried by the National Surveillance Programme for Communicable Diseases created in 1997–1998 (Thakur, 2006). There were outbreaks of other infectious diseases such as dengue fever and Crimean Congo hemorrhagic fever in and around the KFD area of interest. Lastly, other landmark events were noted that may have had an impact in the KFD prevalent states (Table 2; Agricultural Census of India, 2018; Besley & Burgess, 2000; British Broadcasting Corporation, 2018; Claire, 2015; Cousins, 2015; Express Healthcare, n.d.; Flood list‐ India, 2017; History of Karnataka Agriculture Policy, n.d.; Integrated Disease Surveillance Program, 2018; Times of India: U.S., 1958).
4. Discussion
This study provided an overview of the geographical expansion and trends of KFD from 1957 to 2017. Data were obtained through a systematic review of peer‐reviewed articles, Pro‐MED, historical newspapers, and government websites that allowed us to provide clear documentation of the spatiotemporal pattern of KFD cases both within and outside the Shimoga region. These data are the first record of the spatiotemporal dynamics of KFD based on a novel and thorough review as described.
We estimated that the total number of KFD cases that occurred within the period of 1957 to 2017 was over 9,000, with an average of about 160 cases per year (compared to 400 cases estimated by CDC, 2013) across the 61‐year study period. Our data indicate, however, that KFD has intermittent outbreaks with much higher numbers of cases during a few focal periods. So, the average number of cases per year is not the best way to represent the risk annually. Based on our data of 61 years, 37 years had fewer than 100 cases, 16 years had cases between 100 and 399 cases, and 8 years had 400 or more cases. This is another instance where underreporting of cases as well as differential reporting systems can provide inconsistent case estimates as well as CFRs (Atkins et al., 2015; Gibbons et al., 2014).
Several places outside the 16 districts of interest have evidence of KFD. For example, Gulbarga district reported one case in 2006, along with evidence of monkey infections, but this district lies outside the contiguous pattern with the other districts of interest (Table 1 and Figure 1). Some studies point toward silent KFD foci present in the Andaman and Nicobar Islands (Padbidri et al., 2002), Gujarat's Kutch region (Rao, 1971), parts of West Bengal (Sarkar & Chatterjee, 1962), and areas in Rajasthan (Rao, 1971). The silent foci were identified during studies where haemagglutination inhibition antibodies in people against different arthropod borne viruses was studied, and evidences of haemagglutination inhibition antibodies against KFDV were noted, despite no reports of outbreaks of the disease in these places.
The processes by which KFD persists and spreads are not clear. The presence of readily available vector competent mammalian reservoirs in the forests is definitely the basis for the circulation of the virus and the persistent infection of ticks (Banerjee & Bhat, 1977; Pattnaik, 2006; Work et al., 1959). Movement of monkeys to new areas in search of food may facilitate the expansion of infected ticks (Banerjee, 1988). Both deforestation and reforestation contribute to changes in population dynamics of forest‐dwelling animals, including hosts of vector ticks, but the precise mechanism by which this might affect KFD transmission is not clear. The largest human outbreak of the disease during the early 1980s occurred during a period of large‐scale deforestation as reported by Nichter (Nichter, 1987). News published in June 1983 reported on the deforestation of 400 ha of virgin forests in the Western Ghats, which was implemented for cashew plantations (Times of India, 1983).
The Western Ghats where KFD is generally located is a mountain chain rich in biodiversity and is deemed a United Nations Educational, Scientific and Cultural Organization World Heritage site (United Nations Educational, Scientific and Cultural Organization, 2012). Forest coverage in the Western Ghats has declined over the past century, from 73.1% in 1920 to 47.1% in 2013 (Reddy et al., 2016). But forest cover has not declined everywhere with some districts experiencing gains and other districts had losses in forest cover from 1991 to 2017 as per the State of Forest Reports from Forest Survey of India (Forest Survey of India (Listing of Reports), 2018) Based on these State of Forest Reports, 7 of the states in the 16 districts area of interest have had declines in forest cover and 9 have had increases in forest area (Forest Service of India, 2017).
Changes in the land use may increase the number of mammalian hosts and the spatial overlap with human activity, wildlife, and ticks. Singh and Gajadhar (2014) stated that due to this overlap, utilization of wildlife as indicators could facilitate efficient and rapid disease outbreak response in the area. Paul et al. (2016) opined that forest coverage may not be as important as the combination of factors that increase this interaction and thus lead to higher chances of disease transmission. Morris et al. (2016) noted that a decrease in generality of basal hosts in natural environments led to an increase in abundance of pathogens specific to a host, thereby increasing the load of the pathogen in the said environment. Similarly, Walsh et al. (1993) stated that deforestation implemented to make way for mining operations, plantations, urbanization, and fuelwood collections can definitely impact vector ecology and modify human behavior, resulting in increased disease occurrence.
Boshell (1969) argued that the development of the timber and firewood businesses and encroachment of paddy fields on forested areas increase the potential for KFD. Pattnaik (2006) noted that paddy fields provide a suitable habitat for the vector ticks to quest and thrive. However, data from the Agricultural Census of India indicated the total cultivated paddy area in Karnataka state decreased from 15,717,384 ha in 1995 to 3,729,120 ha in 2011. If paddy fields increased risk, then this decrease should be associated with fewer ticks and fewer KFD cases, not the reverse. It is possible, however, that the infected ticks have infiltrated diverse biotypes including those associated with paddy fields such as cultivated clearings, plantations, and grasslands (Mourya & Yadav, 2016).
The deep encroachment of previously untouched habitats such as cashew plantations, search for game meat, activities such as trench digging and fire line works in summer months, dwellings built closer to forest areas, and regular entry by villagers and tribal folk dependent on forests have led to more cases of KFD (Gurav et al., 2018; Holbrook, 2012; Kasabi et al., 2013; Murhekar et al., 2015; Patil et al., 2017; Sadanandane et al., 2017). However, these dynamics are not limited to the current KFD region only; similar conditions exist in every region in India (Times of India, October 1975).
Population growth may have played a role in the KFDV transmission and the gradual expansion of the disease. Boshell (1969) and Holbrook (2012) argued that the increase in human population in Shimoga (from 46,524 in 1951 to 1,755,512 in 2011; Census of India, 2011), and the consequent human migration into sylvatic territories may have led to humans coming into contact with the KFDV infected ticks. This dynamic was particularly intense in the Shimoga taluks of Sagar and Sorab, which saw both high increase in human population and the large numbers of KFD cases.
Handling of cows or contact with cattle has been hypothesized to play a role in the transmission cycle of KFD, but the exact mechanism (if any) continues to be uncertain. Randolph et al. (1996) stated that ticks co‐feeding on cattle or other mammalian hosts may transmit KFDV between ticks, even if the host is not infected. However, a study by Rajagopalan and Sreenivasan (1981) on tick infestations on cattle and water buffaloes showed that H. spinigera adult ticks only comprise 2.72% of the tick population on cattle and 4.2% on buffaloes, so this species is not the dominant tick on cattle. Additionally, Boshell (1969) stated that even though the role of domestic cattle is unclear, they are responsible for the maintenance, propagation and concentration of H. spinigera.
Climate change has also been posed as a plausible factor behind the rising numbers of KFD cases and its gradual expansion. A comprehensive 1951–2010 report from the Indian Ministry of Earth Science (Rathore et al., 2013) by state for the entire country found that temperature has increased by 0.60 °C. Furthermore, increases in annual mean maximum temperature trends in all KFD‐affected states were described as “most significant.” Precipitation has decreased in all KFD‐affected states, with Maharashtra, Goa, Karnataka, and Kerala recording annual decreases of −0.71, −3.82, −0.05, and −1.43 mm per year, respectively. Most KFD cases occur between December and May each year following the end of monsoons (Kasabi et al., 2013). Therefore, changes in the monsoon season may reflect changes in the transmission of KFD as well.
Even though there is no evidence of human‐to‐human transmission of KFDV, the virus has been found to be highly infectious and is a class 4 biosafety level pathogen. Morse et al. (1962) noted even under highly controlled conditions at the Walter Reed Army hospital in Washington D.C., three out of seven experienced laboratory personnel got ill even after having been immunized against Russian Spring Summer Encephalitis vaccine (Morse et al., 1962). The patients recovered, but this incident illustrates the highly infectious nature of KFDV. Wadia (1975) also reported two laboratory‐exposed cases of KFD in India (Wadia, 1975). Patil et al. (2017) have also noted the potential of infection for people who handle monkeys who have died with KFD. Alkhurma hemorrhagic fever virus (which is a variant genotype of KFDV) has been found to be transmissible through consumption of raw unpasteurized camel milk (Charrel et al., 2005; Memish et al., 2014). Nonetheless, the KFD transmission via consumption of contaminated milk has not been confirmed (CDC, 2013), but further studies are needed to examine this potential mode of transmission.
Vaccine development has been important since the first reports of KFD. Walter Reed Army Research Laboratory in Washington D.C. in the 1960s developed a vaccine but was not very effective (Morse et al., 1962). Subsequently, different vaccine development processes were started between the 1960s and 1980s such as formalin‐inactivated vaccine from mouse brain source (Mansharamani et al., 1965), a tissue culture vaccine (Mansharamani et al., 1967; Mansharamani & Dandawate, 1967), and a live attenuated vaccine through serial tissue culture passages (Bhatt & Anderson, 1971). The live attenuated vaccine was also tested among langurs, and although the vaccine prevented death, it could not protect against challenge infection (Bhatt & Dandawate, 1974; Pattnaik, 2006). Additionally, an attenuated Langat virus vaccine (Langat virus is related to KFDV as they are both part of the tick‐borne encephalitis virus complex) was also tested among langurs and was found to show protection against KFDV among monkeys (Shah et al., 2012; Thind, 1981).
Ultimately, the formalin‐inactivated virus that was produced in chick embryo fibroblasts was licensed and used in India in the endemic areas as routine vaccination since 1991 (Kasabi et al., 2013; Pattnaik, 2006). Two vaccine doses are provided to the extent possible to anyone aged 7–65 years in endemic or outbreak areas at an interval of a month. The first booster dose is recommended after 6–9 months. Then, further booster doses are recommended after 5 years depending on the last confirmed case in the area (Kasabi et al., 2013; Kiran et al., 2015). Field evaluation studies (Dandawate et al., 1994; Kasabi et al., 2013; Kiran et al., 2015) concluded that the vaccination strategy was poorly conducted, coverage was not sufficient, and there was low uptake of the vaccine. Though the vaccine has shown protective effect under ideal conditions, it still does not provide a high percentage of immunity against KFD.
Our data are limited to cases found through our sources. We often had to make assumptions about the disease distribution, which resulted in more defensible estimates for some years and districts than for others. The past issues of Deccan Chronicle, a major newspaper serving the states of Southern India, were not available for our review, with the exception of some issues available in the archives for only recent years. The lack of seroprevalence data was also a general limiting factor; thus, we focused our search on human cases of KFD. There is also limited data on KFDV in monkeys, so it is difficult to provide a complete retrospective case history of the disease in nonhuman primates.
In sum, we provided a 61‐year comprehensive review of KFD in India and examined the possible factors responsible for the transmission of KFDV and its continued expansion. The results from this retrospective analysis will be critical in developing further understanding of the key elements of the ecoepidemiology of this emerging infectious disease.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Data Set S1
Data Set S2
Data Set S3
Acknowledgments
The authors declare that they have no competing financial interests. The data set cited in the main article has been provided as part of the supporting information. References in the supporting information data provide information needed to access the data and other sources. The tables and figures included in the manuscript are the source for the authors' conclusions. The authors would like to thank J. J. Pionke, Social Sciences Librarian from the University of Illinois, Urbana Champaign, for help on information sources. Access to all the articles and archived newspapers was obtained through the University of Illinois Library system. The authors did not receive any extramural funding for this work.
Chakraborty, S. , Andrade, F. C. D. , Ghosh, S. , Uelmen, J. , & Ruiz, M. O. (2019). Historical expansion of Kyasanur Forest disease in India from 1957 to 2017: A retrospective analysis. GeoHealth, 3, 44–55. 10.1029/2018GH000164
This article was corrected on 15 JUL 2019. The online version of this article has been modified to include a Conflict of Interest statement.
References
- Agricultural Census of India (2018). Agricultural census database. Retrieved May 21, from http://agcensus.dacnet.nic.in/DistCharacteristic.aspx
- Ajesh, K. , Nagaraja, B. K. , & Sreejith, K. (2017). Kyasanur Forest disease virus breaking the endemic barrier: An investigation into ecological effects on disease emergence and future outlook. Zoonoses and Public Health, 64(7), e73–e80. 10.1111/zph.12349 [DOI] [PubMed] [Google Scholar]
- Anderson, C. R. , & Singh, K. R. P. (1971). The reaction of cattle to Kyasanur Forest disease virus. Indian Journal of Medical Research, 59(2), 195–198. [PubMed] [Google Scholar]
- Atkins, K. E. , Wenzel, N. S. , Ndeffo‐Mbah, M. , Altice, F. L. , Townsend, J. P. , & Galvani, A. P. (2015). Under‐reporting and case fatality estimates for emerging epidemics. BMJ, 350(3). 10.1136/bmj.h1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awate, P. , Yadav, P. , Patil, D. , Shete, A. , Kumar, V. , Kore, P. , Dolare, J. , Deshpande, M. , Bagde, S. , Sapkal, G. , Gurav, Y. , & Mourya, D. T. (2016). Outbreak of Kyasanur Forest disease (monkey fever) in Sindhudurg, Maharashtra State, India, 2016. The Indian Journal of Medical Research, 62(4), 497–510. [DOI] [PubMed] [Google Scholar]
- Banerjee, K. (1988). Kyasanur Forest disease. The Arboviruses: Epidemiology and Ecology, 3, 93–116. [Google Scholar]
- Banerjee, K. , & Bhat, H. R. (1977). Correlation between the number of persons suffering from Kyasanur Forest disease and the intensity of infection in the tick population. Indian Journal of Medical Research, 66(2), 175–179. [PubMed] [Google Scholar]
- Besley, T. , & Burgess, R. (2000). Land reform, poverty reduction, and growth: Evidence from India. The Quarterly Journal of Economics, 115(2), 389–430. 10.1162/003355300554809 [DOI] [Google Scholar]
- Bhatt, P. N. , & Anderson, C. R. (1971). Attenuation of a strain of Kyasanur Forest disease virus for mice. Indian Journal of Medical Research, 59(2), 199–205. [PubMed] [Google Scholar]
- Bhatt, P. N. , & Dandawate, C. N. (1974). Studies on the antibody response of a formalin inactivated Kyasanur Forest disease virus vaccine in langurs “Presbytis entellus”. Indian Journal of Medical Research, 62(6), 820–826. [PubMed] [Google Scholar]
- Bhatt, P. N. , Work, T. H. , Varma, M. G. , Trapido, H. , Murthy, D. P. , & Rodrigues, F. M. (1966). Tracing of viruses of tick‐borne and Japanese encephalitis in cultures of transplanted cells using the fluorescent antibody method. Indian Journal of Medical Sciences, 20(5), 316–320. [PubMed] [Google Scholar]
- Boshell, J. (1969). Kyasanur Forest disease. The American Journal of Tropical Medicine and Hygiene, 18(1), 67–80. [PubMed] [Google Scholar]
- British Broadcasting Corporation (2018). India profile—Time Accessed on May 1 from http://www.bbc.com/news/world-south-asia-12641776
- Census of India (2011). Census data. Retrieved May 21, 2018, From http://www.censusindia.gov.in/2011-Common/CensusData2011.html
- Centers for Disease Control & Prevention (2014). Tick borne encephalitis (TBE). Retrieved on May 22nd, 2018 from https://www.cdc.gov/vhf/tbe/index.html
- Centers for Disease Control & Prevention (2013). Kyasanur Forest disease. Retrieved on May 22nd 2018 from https://www.cdc.gov/vhf/kyasanur/index.html
- Chari, B. (2018, February 21). January witnessed 12 KFD cases, all in Sattari taluka. Times of India Retrieved from https://timesofindia.indiatimes.com/city/goa/january-witnessed-12-kfd-cases-all-in-sattari-taluka/articleshow/63004713.cms
- Charrel, R. N. , Zaki, A. M. , Fakeeh, M. , Yousef, A. I. , de Chesse, R. , Attoui, H. , & de Lamballerie, X. (2005). Low diversity of Alkhurma hemorrhagic fever virus, Saudi Arabia, 1994–1999. Emerging Infectious Diseases, 11(5), 683–688. 10.3201/eid1105.041298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claire, P. (2015). Dengue challenges India's health system. Lancet, 385, 453–465. [DOI] [PubMed] [Google Scholar]
- Cousins, S. (2015). Death toll from swine flu in India exceeds 2500. BMJ [British Medical Journal], 351, h4966. [DOI] [PubMed] [Google Scholar]
- Dandawate, C. N. , Desai, G. B. , Achar, T. R. , & Banerjee, K. (1994). Field evaluation of formalin inactivated Kyasanur Forest disease virus tissue culture vaccine in three districts of Karnataka state. The Indian Journal of Medical Research, 99, 152–158. [PubMed] [Google Scholar]
- Directorate of Health and Family Welfare Services, Govt of Karnataka (2016a). Annual report 2015–2016. Retrieved online on Feb 25th, 2018 from http://www.karnataka.gov.in/hfw/kannada/Documents/Annual%20Report%20of%20HFWS%20-2015-16-English%20.pdf
- Directorate of Health and Family Welfare Services, Govt of Karnataka (2016b). Annual report 2015–2016. Retrieved online on Feb 25th, 2018 from http://www.karnataka.gov.in/hfw/kannada/Documents/English%20Annual%20Report%20of%202016-17.pdf
- Dodd, K. A. , Bird, B. H. , Khristova, M. L. , Albariño, C. G. , Carroll, S. A. , Comer, J. A. , Erickson, B. R. , Rollin, P. E. , & Nichol, S. T. (2011). Ancient ancestry of KFDV and AHFV revealed by complete genome analyses of viruses isolated from ticks and mammalian hosts. PLoS Neglected Tropical Diseases, 5(10), e1352 10.1371/journal.pntd.0001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Express Healthcare (n.d.) Milestones in Indian healthcare—Cover story retrieved on May 1, 2018 from http://www.expressbpd.com/healthcare/cover-story-healthcare/milestones-in-indian-healthcare/191620/
- Flood list‐ India (2017). List of floods in India. Retrieved on May 22nd, 2018 from http://floodlist.com/tag/india
- Forest Service of India (2017). State of forest report. Accessed on 21st May, 2018 from http://fsi.nic.in/isfr2017/isfr-forest-cover-2017.pdf
- Forest Survey of India (Listing of Reports) (2018). State of forest report. Accessed on 3rd July, 2018 from http://fsi.nic.in/details.php?pgID=sb_64
- Ghalsasi, G. R. , & Dhanda, V. (1974). Taxonomy and biology of Haemaphysalis (Kaiseriana) Spinigera (Acarina: Ixodidae). Oriental Insects, 8(4), 505–520. 10.1080/00305316.1974.10434886 [DOI] [Google Scholar]
- Ghosh, S. , Bansal, G. C. , Gupta, S. C. , Ray, D. , Khan, M. Q. , Irshad, H. , Shahiduzzaman, M. , Seitzer, U. , & Ahmed, J. S. (2007). Status of tick distribution in Bangladesh, India and Pakistan. Parasitology Research, 101(2), 207–216. 10.1007/s00436-007-0684-7 [DOI] [PubMed] [Google Scholar]
- Gibbons, C. L. , Mangen, M. J. , Plass, D. , Havelaar, A. H. , Brooke, R. J. , Kramarz, P. , Peterson, K. L. , Stuurman, A. L. , Cassini, A. , Fevre, E. M. , & Kretzschmar, M. E. (2014). Measuring underreporting and under‐ascertainment in infectious disease datasets: A comparison of methods. BMC Public Health, 14(1). 10.1186/1471-2458-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, E. A. , Moss, S. R. , & Turner, S. L. (2004). Evolution and dispersal of encephalitic flaviviruses In Emergence and control of zoonotic viral encephalitides (pp. 65–84). Vienna: Springer. [DOI] [PubMed] [Google Scholar]
- Goverdhan, M. K. (1974). Epizootiology of Kyasanur Forest disease in wild monkeys of Shimoga district, Mysore State (1957‐1964). Indian Journal of Medical Research, 62(4), 497–510. [PubMed] [Google Scholar]
- Gurav, Y. K. , Yadav, P. D. , Gokhale, M. D. , Chiplunkar, T. R. , Vishwanathan, R. , Patil, D. Y. , Jain, R. , Shete, A. M. , Patil, S. L. , Sarang, G. D. , Sapkal, G. N. , Andhare, M. D. , Sale, Y. R. , Awate, P. S. , & Mourya, D. T. (2018). Kyasanur Forest disease prevalence in Western Ghats proven and confirmed by recent outbreak in Maharashtra, India, 2016. Vector Borne and Zoonotic Diseases, 18(3), 164–172. [DOI] [PubMed] [Google Scholar]
- History of Karnataka Agriculture Policy (n.d.). Retrieved May 1, 2018, from http://shodhganga.inflibnet.ac.in/bitstream/10603/18567/13/13_chapter1.pdf
- Holbrook, M. R. (2012). Kyasanur Forest disease. Antiviral Research, 96(3), 353–362. 10.1016/j.antiviral.2012.1010.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Disease Surveillance Program (n.d.). Annual report 2017. Retrieved on 21st may, 2018 from http://idsp.nic.in/showfile.php?lid=3947
- Kasabi, G. S. , Murhekar, M. V. , Sandhya, V. K. , Raghunandan, R. , Kiran, S. K. , Channabasappa, G. H. , & Mehendale, S. M. (2013). Coverage and effectiveness of Kyasanur Forest disease (KFD) vaccine in Karnataka, South India, 2005–10. PLoS Neglected Tropical Diseases, 7(1), e2025 10.1371/journal.pntd.0002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon, R. H. , Rippy, M. K. , McKee, K. T. Jr. , Zack, P. M. , & Peters, C. J. (1992). Infection of Macaca radiata with viruses of the tickborne encephalitis group. Microbial Pathogenesis, 13, 399–409. [DOI] [PubMed] [Google Scholar]
- Kiran, S. K. , Pasi, A. , Kumar, S. , Kasabi, G. S. , Gujjarappa, P. , Shrivastava, A. , Mehendale, S. , Chauhan, L. S. , Laserson, K. F. , & Murhekar, M. (2015). Kyasanur Forest disease outbreak and vaccination strategy, Shimoga District, India, 2013–2014. Emerging Infectious Diseases, 21(1), 146 10.3201/eid2101.141227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSala, P. R. , & Holbrook, M. (2010). Tick‐borne flaviviruses. Clinics in Laboratory Medicine, 30, 221–235. 10.1016/j.cll.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Lewis, M. (2002). Scientists or spies? Ecology in a climate of Cold War suspicion. Economic and Political Weekly, 2323–2332. [Google Scholar]
- Mansharamani, H. J. , & Dandawate, C. N. (1967). Experimental vaccine against Kyasanur Forest disease (KFD) virus from tissue culture source. II. Safety testing of the vaccine in cortisone sensitized Swiss albino mice. Indian Journal of Pathology & Bacteriology, 10(1), 25–32. [PubMed] [Google Scholar]
- Mansharamani, H. J. , Dandawate, C. N. , & Krishna, M. (1967). Experimental vaccine against Kyasanur Forest disease (KFD) virus from tissue culture source. I. Some data on the preparation and antigenicity tests of vaccines. Indian Journal of Pathology & Bacteriology, 10(1), 9–24. [PubMed] [Google Scholar]
- Mansharamani, H. J. , Dandawate, C. N. , Krishnamurthy, B. G. , & Jhala, H. (1965). Experimental vaccine against Kyasanur Forest disease (KFD) virus from mouse brain source. Indian Journal of Pathology & Bacteriology, 8(3), 159–177. [PubMed] [Google Scholar]
- Memish, Z. A. , Fagbo, S. F. , Ali, A. O. , AlHakeem, R. , Elnagi, F. M. , & Bamgboye, E. A. (2014). Is the epidemiology of alkhurma hemorrhagic fever changing?: A three‐year overview in Saudi Arabia. PLoS One, 9(2), e85564 10.1371/journal.pone.0085564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, A. L. , Guégan, J. F. , Andreou, D. , Marsollier, L. , Carolan, K. , Le Croller, M. , Sanhueza, D. , & Gozlan, R. E. (2016). Deforestation‐driven food‐web collapse linked to emerging tropical infectious disease, Mycobacterium ulcerans. Science Advances, 2(12), e1600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, L. J. , Russ, S. B. , Needy, C. F. , & Buescher, E. L. (1962). Studies of viruses of the tick borne encephalitis complex: II. Disease and immune responses in man following accidental infection with Kyasanur Forest disease virus. The Journal of Immunology, 88(2), 240–248. [PubMed] [Google Scholar]
- Mourya, D. T. , & Yadav, P. D. (2016). Recent scenario of emergence of Kyasanur Forest disease in India and public health importance. Current Tropical Medicine Reports, 3(1), 7–13. [Google Scholar]
- Mourya, D. T. , Yadav, P. D. , Mehla, R. , Barde, P. V. , Yergolkar, P. N. , Kumar, S. R. , Thakare, J. P. , & Mishra, A. C. (2012). Diagnosis of Kyasanur Forest disease by nested RT‐PCR, real‐time RT‐PCR and IgM capture ELISA. Journal of Virological Methods, 186(1–2), 49–54. 10.1016/j.jviromet.2012.1007.1019 [DOI] [PubMed] [Google Scholar]
- Murhekar, M. V. , Kasabi, G. S. , Mehendale, S. M. , Mourya, D. T. , Yadav, P. D. , & Tandale, B. V. (2015). On the transmission pattern of Kyasanur Forest disease (KFD) in India. Infectious Diseases of Poverty, 4(1), 37 10.1186/s40249-40015-40066-40249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Disease Control Bulletin. Directorate General of Health Services (2018). Kyasanur Forest disease: A public health concern. Retrieved online on 8th June, 2018 from http://idsp.nic.in/showfile.php?lid=3946
- Nichter, M. (1987). Kyasanur Forest disease: An ethnography of a disease of development. Medical Anthropology Quarterly, 1(4), 406–423. [Google Scholar]
- Padbidri, V. S. , Wairagkar, N. S. , Joshi, G. D. , Umarani, U. B. , Risbud, A. R. , Gaikwad, D. L. , Bedekar, S. S. , Divekar, A. D. , & Rodrigues, F. M. (2002). A serological survey of arboviral diseases among the human population of the Andaman and Nicobar Islands, India. Southeast Asian Journal of Tropical Medicine and Public Health, 33(4), 794–800. [PubMed] [Google Scholar]
- Patil, D. Y. , Yadav, P. D. , Shete, A. M. , Nuchina, J. , Meti, R. , Bhattad, D. , Someshwar, S. , & Mourya, D. T. (2017). Occupational exposure of cashew nut workers to Kyasanur Forest disease in Goa, India. International Journal of Infectious Diseases, 61, 67–69. 10.1016/j.ijid.2017.1006.1004 [DOI] [PubMed] [Google Scholar]
- Pattnaik, P. (2006). Kyasanur Forest disease: An epidemiological view in India. Reviews in Medical Virology, 16(3), 151–165. 10.1002/rmv.1495 [DOI] [PubMed] [Google Scholar]
- Paul, R. E. , Cote, M. , Le Naour, E. , & Bonnet, S. I. (2016). Environmental factors influencing tick densities over seven years in a French suburban forest. Parasites & Vectors, 9(1), 309 10.1186/s13071-016-1591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri, K. (1989). Clinical, clinicopathologic, and hematologic features of Kyasanur Forest disease. Reviews of Infectious Diseases, 11(Supplement_4), S854–S859. [DOI] [PubMed] [Google Scholar]
- ProMED Mail International Society for Infectious Diseases (2018). Kyasanur Forest disease cases. Retrieved on May 22nd 2018 from https://www.promedmail.org/
- Rajagopalan, P. K. , & Sreenivasan, M. A. (1981). Ixodid ticks on cattle and buffaloes in the Kyasanur Forest disease area of Karnataka state. Indian Journal of Medical Research, 73, 880–889. [Google Scholar]
- Randolph, S. E. , Gern, L. , & Nuttall, P. A. (1996). Co‐feeding ticks: Epidemiological significance for tick‐borne pathogen transmission. Parasitology Today, 12(12), 472–479. [DOI] [PubMed] [Google Scholar]
- Randolph, S. E. , & Rogers, D. J. (2006). Tick‐borne disease systems: Mapping geographic and phylogenetic space. Advances in Parasitology, 62, 263–291. [DOI] [PubMed] [Google Scholar]
- Rao, N. (2016, November 19). The seven‐decade transnational hunt for the origins of a strange Indian disease. The Wire Retrieved from https://thewire.in/health/kyasanur-kfd-rajagopalan-boshell
- Rao, T. R. (1971). Immunological surveys of arbovirus infections in South‐East Asia, with special reference to dengue, chikungunya, and Kyasanur Forest disease. Bulletin of the World Health Organization, 44(5), 585–591. [PMC free article] [PubMed] [Google Scholar]
- Rathore, L.S. , Attri, S.D. , and Jaswal, A.K. (2013). State level climate change trends in India. India Meterological Department. Meteorological Monograph No. ESSO/IMD/EMRC/02/2013
- Reddy, C. S. , Jha, C. S. , Dadhwal, V. K. , Krishna, P. H. , Pasha, S. V. , Satish, K. V. , Dutta, K. , Saranya, K. R. L. , Rakesh, F. , Rajashekar, G. , & Diwakar, P. G. (2016). Quantification and monitoring of deforestation in India over eight decades (1930–2013). Biodiversity and Conservation, 25(1), 93–116. [Google Scholar]
- Reddy, K. S. , Shah, B. , Varghese, C. , & Ramadoss, A. (2005). Responding to the threat of chronic diseases in India. The Lancet, 366(9498), 1744–1749. [DOI] [PubMed] [Google Scholar]
- Sadanandane, C. , Elango, A. , Marja, N. , Sasidharan, P. V. , Raju, K. H. K. , & Jambulingam, P. (2017). An outbreak of Kyasanur Forest disease in the Wayanad and Malappuram districts of Kerala, India. Ticks and tick‐borne diseases, 8(1), 25–30. 10.1016/j.ttbdis.2016.1009.1010. [DOI] [PubMed] [Google Scholar]
- Sadanandane, C. , Gokhale, M. D. , Elango, A. , Yadav, P. , Mourya, D. T. , & Jambulingam, P. (2018). Prevalence and spatial distribution of Ixodid tick populations in the forest fringes of Western Ghats reported with human cases of Kyasanur Forest disease and monkey deaths in South India. Experimental and Applied Acarology, 75(1), 135–142. 10.1007/s10493-018-0223-5 [DOI] [PubMed] [Google Scholar]
- Sarkar, J. K. , & Chatterjee, S. N. (1962). Survey of antibodies against arthropod‐borne viruses in the human sera collected from Calcutta and other areas of West Bengal. Indian Journal of Medical Research, 50(6), 833–841. [PubMed] [Google Scholar]
- Shah, K. V. , Dandawate, C. N. , & Bhatt, P. N. (2012). Kyasanur Forest disease virus: Viremia and challenge studies in monkeys with evidence of cross‐protection by Langat virus infection. F1000Research, 1, 61 10.12688/f1000research.1-61.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. Z. , Jabbar, B. , Ahmed, M. , Rehman, A. , Nasir, H. , Nadeem, S. , Jabbar, I. , Rahman, Z. U. , & Azam, S. (2018). Epidemiology, pathogenesis, and control of a tick‐borne disease‐ Kyasanur Forest disease: Current status and future directions. Frontiers in Cellular and Infection Microbiology, 8 10.3389/fcimb.2018.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. B. , & Gajadhar, A. A. (2014). Role of India's wildlife in the emergence and re‐emergence of zoonotic pathogens, risk factors and public health implications. Acta Tropica, 138, 67–77. 10.1016/j.actatropica.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, K. R. P. , Pavri, K. , & Anderson, C. R. (1963). Experimental transovarial transmission of Kyasanur Forest disease virus in Haemaphysalis spinigera . Nature, 199(4892), 513. [DOI] [PubMed] [Google Scholar]
- Tandale, B. V. , Balakrishnan, A. , Yadav, P. D. , Marja, N. , & Mourya, D. T. (2015). New focus of Kyasanur Forest disease virus activity in a tribal area in Kerala, India, 2014. Infectious Diseases of Poverty, 4(1), 12 10.1186/s40249-40015-40044-40242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, J. (2006). Integrated disease surveillance—A key step to improve public health in India. Indian Journal of Community Medicine, 31(4), 10–12. [Google Scholar]
- Thind, I. S. (1981). Attenuated Langat E5 virus as a live virus vaccine against Kyasanur Forest disease virus. The Indian Journal of Medical Research, 73, 141–149. [PubMed] [Google Scholar]
- Times of India (1983, June 23). Denudation of forests caused monkey epidemic, p. 7
- Times of India: U.S. (1958, February 10). Foundation's grant to India, p. 3
- Times of India (1978, December 27). Japanese virus killed over 1,600 Indians this year. p. 16.
- Times of India (1975, October 19). Plague pockets still exist in South India, p. 5.
- United Nations Educational, Scientific and Cultural Organization (2012). Western Ghats. Accessed 21st May 2018 from https://whc.unesco.org/en/list/1342
- Wadia, R. S. (1975). Neurological involvement in Kyasanur Forest disease. Neurology India, 23(3), 115. [PubMed] [Google Scholar]
- Walsh, J. F. , Molyneux, D. H. , & Birley, M. H. (1993). Deforestation: Effects on vector‐borne disease. Parasitology, 106(S1), S55–S75. [DOI] [PubMed] [Google Scholar]
- Webb, H. E. , & Lakshmana Rao, R. (1961). Kyasanur Forest disease: A general clinical study in which some cases with neurological complications were observed. Transactions of the Royal Society of Tropical Medicine and Hygiene, 55(3), 284–298. [DOI] [PubMed] [Google Scholar]
- Work, T. H. (1958). Russian spring‐summer virus in India: Kyasanur Forest disease. Progress in medical virology. Fortschritte der medizinischenVirusforschung. PRO, 1, 248–279. [PubMed] [Google Scholar]
- Work, T. H. , Roderiguez, F. R. , & Bhatt, P. N. (1959). Virological epidemiology of the 1958 epidemic of Kyasanur Forest disease. American Journal of Public Health and the Nations Health, 49(7), 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work, T. H. , & Trapido, H. (1957). Kyasanur Forest disease. A new virus disease in India. Summary of preliminary report of investigations of the virus research centre on an epidemic disease affecting forest villagers and wild monkeys of Shimoga District, Mysore. Indian Journal of Medical Sciences, 11(5), 341–342. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Set S1
Data Set S2
Data Set S3


