Abstract
Background
Fracture of the distal radius is a common clinical problem. A key method of surgical fixation is percutaneous pinning, involving the insertion of wires through the skin to stabilise the fracture. This is an update of a Cochrane Review published in 2007.
Objectives
To assess the effects (benefits and harms) of percutaneous pinning versus cast immobilisation alone and of different methods and techniques of percutaneous pinning, modalities or duration of immobilisation after pinning, and methods or timing of pin or wire removal for treating fractures of the distal radius in adults. Our primary focus was on dorsally displaced fractures.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, trial registers, conference proceedings and reference lists of articles up to June 2019.
Selection criteria
Randomised or quasi‐randomised controlled clinical trials involving adults with a fracture of the distal radius, which compared percutaneous pinning with non‐surgical treatment or different aspects of percutaneous pinning. Our main outcomes were patient‐reported function at the short term (up to three months), medium term (three up to 12 months) and long term (greater than 12 months); overall numbers of participants with complications requiring secondary treatment and any complication; grip strength and health‐related quality of life at 12 months.
Data collection and analysis
At least two review authors independently performed study screening and selection, 'Risk of bias' assessment and data extraction. We pooled data where appropriate and used GRADE for assessing the quality of evidence for each outcome.
Main results
We included 21 randomised controlled trials (RCTs) and five quasi‐RCTs, involving 1946 generally older and female adults with dorsally displaced and potentially or evidently unstable distal radial fractures. Trial populations varied but the majority of studies reported mean ages in the sixth decade or older. All trials were at high risk of bias, invariably performance bias ‐ which for most trials reflected the impracticality of blinding care providers or participants to treatment allocation ‐ and often detection bias and selective reporting bias. Allocation concealment was secure in one trial only. All trials reported outcomes incompletely. The studies tested one of 10 comparisons. In the following, we report on those of the main outcomes for which evidence was available. No subgroup analysis, such as by pinning methods, was viable.
Eleven heterogeneous trials involving 917 participants compared percutaneous pinning with plaster cast immobilisation after closed reduction of the fracture. The quality of the evidence was very low for all reported outcomes. Thus, we are uncertain if percutaneous pinning compared with plaster cast alone makes any difference to patient‐reported function, measured using the DASH questionnaire, at six weeks or six months (incomplete data from one trial). Overall numbers of participants with complications were not reported. Redisplacement resulting in secondary treatment occurred on average in 12% (range 3.3% to 75%) of participants treated by cast alone (six trials) whereas pin tract infection requiring antibiotics and, often, early wire removal, occurred on average in 7.7% (range 0% to 15%) of pinning group participants (seven trials). We are uncertain whether pinning makes a difference to the incidence of complex regional pain syndrome, reported in four studies. Although two studies found finger stiffness after cast removal was less common after pinning (20% versus 36%), the treatment implications were not reported. Other reported complications were mainly surgery‐related. Based on incomplete data or qualitative statements from only four studies, we are uncertain of the effects of pinning on grip strength at 12 months. We are uncertain if percutaneous pinning compared with plaster cast alone makes any difference to patient‐reported quality of life at four months (one study).
Five comparisons of different pinning methods were made by six trials in all. One of these trials, which reported results for 96 participants, compared Kapandji intrafocal pinning (2 or 3 wires) with early mobilisation versus trans‐styloid fixation (2 wires) with six weeks cast immobilisation. We are uncertain whether Kapandji pinning slightly increases the risk of superficial radial nerve symptoms or complex regional pain syndrome, or whether it makes a difference in grip strength at 12 months (very low‐quality evidence).
Two small trials using two distinct pinning techniques compared biodegradable pins versus metal pins in 70 participants. Although very low‐quality evidence, the extra demands at surgery of insertion of biodegradable pins and excess of serious complications (e.g. severe osteolytic reactions) associated with biodegradable material are important findings.
Three poorly‐reported trials involving 168 participants compared burying of wire ends versus leaving them exposed. We are uncertain whether burying of wires reduces the incidence of superficial infection (very low‐quality evidence). There is low‐quality evidence that burying of wires may be associated with a higher risk of requiring more invasive treatment for wire removal.
Four small trials compared different types or duration of postoperative immobilisation. Very low‐quality evidence of small between‐group differences in individual complications and grip strength at 17 weeks, means we are uncertain of the effects of positioning the wrist in dorsiflexion versus palmar flexion during cast immobilisation following pinning of redisplaced fractures (one trial; 60 participants). Three small heterogeneous trials compared cast immobilisation for one week (early mobilisation) versus four or six weeks after percutaneous pinning in 170 people. Although we note one trial using Kapandji pinning reported more complications in the early group, the very low‐quality evidence means there is uncertainty of the effects of early mobilisation on overall and individual complications, or grip strength at 12 months.
No trials tested different methods for, or timing of, pin/wire removal.
Authors' conclusions
Overall, there is insufficient RCT evidence to inform on the role of percutaneous pinning versus cast immobilisation alone or associated treatment decisions such as method of pinning, burying or not of wire ends, wrist position and duration of immobilisation after pinning. Although very low‐quality evidence, the serious complications associated with biodegradable materials is noteworthy. We advise waiting on the results of a large ongoing study comparing pinning with plaster cast treatment as these could help inform future research.
Plain language summary
Through the skin pinning for treating wrist fractures in adults
Background
Broken wrists are fractures at the lower end of the radius, one of the two forearm bones. Typically in older people, these can occur when people put out a hand to break a fall. The broken bones are often pushed apart, resulting in a 'displaced' fracture.
For most broken wrists, the bones can be moved back together (reduced) and held in place without an operation by a plaster cast for a few weeks. When fractures are unstable, the parts do not stay together. Another way of managing broken wrists is to hold the bones back in place with a surgical procedure, such as percutaneous pinning. This involves the insertion of pins or wires through the skin (percutaneous) to hold the bones in a proper position while they heal. In most pinning methods, pins or wires are used to fix the fragments together. In Kapandji pinning, the wires are placed to support the distal (lower end) fragment.
Methods
This is an update of a Cochrane review first published in 2007. We looked at the evidence from randomised controlled trials testing the use of percutaneous pinning or comparing aspects of pinning. Beforehand, we set out the main outcomes: patient‐reported function, numbers of participants with any complication and with complications requiring further treatment, grip strength and health‐related quality of life. We searched medical databases up to June 2019.
Results of the search
We included 26 randomised studies involving 1946 generally older and female adults with potentially unstable wrist fractures. The studies tested one of 10 comparisons and overall under‐reported outcomes. Below, we report only the main outcomes for which evidence was available.
Key results
Eleven trials compared percutaneous pinning with plaster cast immobilisation. We are uncertain whether pinning makes a difference to patient‐reported function at six weeks or six months (data from one study). There were no data on the numbers of participants who had any complication. Redisplacement of the fracture resulting in further treatment occurred on average in one of eight people who had cast alone (six studies). Of the surgery‐related complications, infection requiring antibiotics and sometimes early wire removal occurred on average in one of 13 people in the pinning group (seven studies). We are uncertain whether there is a difference between the two treatments in the risk of other complications. We are uncertain of the effects of pinning on grip strength at 12 months (four trials) or quality of life at four months (one study).
Of the six studies comparing different techniques of pinning, one compared Kapandji pinning with an across‐fracture method. We are uncertain whether Kapandji pinning increases the risk of complications and of the finding of little between‐group difference in grip strength.
Two studies compared biodegradable (dissolvable) pins versus metal wires, using two very different pinning techniques. Both found an excess of complications associated with the use of biodegradable material.
Three studies compared burying the wire ends under the skin with leaving them exposed. We are uncertain whether burying wires reduces the risk of superficial infection. However, burying of wires may require more invasive treatment for removal.
Usually, the wrist is placed in a cast after pinning. We are uncertain whether the position of the wrist in the cast makes a difference to the risk of complications or grip strength (one study). Three studies compared early mobilisation after one week with four or six weeks of cast immobilisation. One study using Kapandji pinning reported more complications for early mobilisation but we are uncertain of the effect of early mobilisation on overall complications or grip strength.
No trials tested different methods or timing of pin removal.
Quality of the evidence
All 26 studies had weaknesses that could affect the reliability of their results. We considered that the evidence for all reported outcomes and for all comparisons was low or, usually, very low quality. This means we are unsure of the results.
Conclusions
The review concluded that there is insufficient evidence to inform on the role of percutaneous pinning and associated treatment decisions. While further research is needed, we advise waiting on the results of a large ongoing study comparing pinning with plaster cast treatment.
Summary of findings
Summary of findings for the main comparison. Summary of findings: percutaneous pinning versus cast or brace immobilisation only.
| Percutaneous pinning compared with cast or brace immobilisation only for treating distal radius fractures in adults | ||||||
|
Patient or population: adults with dorsally displaced distal radius fractures Settings: emergency department, hospital Intervention: reduction and percutaneous pinning, usually supplemented by cast immobilisation Comparison: reduction and cast (or brace) immobilisation alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| cast only | percutaneous pinning | |||||
| Short‐term patient‐reported hand/wrist/upper limb function. DASH scores (30 to 150; worst disability) At 1.5 months | Median 97 (study result) | Median 85 (study result) | Not available | 60 (1 study) | ⊕⊝⊝⊝ very lowa | There are very limited data for this outcome. The study reported the difference was not statistically significant. |
| Medium‐term patient‐reported hand/wrist/upper limb function. DASH scores (30 to 150; worst disability) At 6 months | Median 84 | Median 67 | Not available | 60 (1 study) | ⊕⊝⊝⊝ very lowa | There are very limited data for this outcome. The study reported the difference was statistically significant. However, it may not be clinically important.b Another study (43 participants) reported similar functional results in the two groups, probably at 12 months. |
| Long‐term patient‐reported hand/wrist/upper limb function. Over 12 months follow‐up | See comment | See comment | ‐ | ‐ | ‐ | This was not reported in any of the 11 studies (917 participants) for this comparison. |
| Number of people incurring one or more complications requiring substantive treatment | See comment | See comment | ‐ | 799 (9 studies) | ⊕⊝⊝⊝ very lowc | Data are available for the most common individual complications in this category: treated redisplacement, pin tract infection, CRPS type 1 and persistent finger stiffness.d |
| Overall number of people incurring one or more complications | See comment | See comment | ‐ | 799 (9 studies) | ⊕⊝⊝⊝ very lowc | As well as those complications listed above, data were available for other surgery‐related complications (e.g. K‐wire migration) and other complications occurring in both groups, particularly those related to the median nerve (e.g. carpal tunnel syndrome).e |
|
Grip strength At 12 months |
See comment | See comment | ‐ | 238 (4 studies) | ⊕⊝⊝⊝ very lowf | None of the four studies provided data for pooling. Results were reported as similar in the two groups in three trials and favouring pinning in the fourth trial. |
|
Medium‐term quality of life measured via the Short Form 36 ‐ Physical score (0: worst to 100: best health) At 4 months |
Mean SF‐36 in the plaster cast group was 38.2 | Mean SF‐36 in the intervention group was 4.00 higher (1.59 lower to 9.59 higher) | 54 (1 study) | ⊕⊝⊝⊝ very lowg | A similar lack of between‐group difference was found for mental scores (MD 0.62, 95% CI ‐5.32 to 6.56). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;MD: Mean difference; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision.
b Based on a scoring scheme of 0 to 100 (worst outcome), a minimal clinically important difference (MCID) of 15 is recommended in DASH/QuickDASH; this translates to 18 for a scale of 30 to 150; which is greater than the 16 difference in median scores at six months.
c All the evidence for individual complications was considered very low quality, downgraded two levels for very serious risk of bias and one or two levels for serious or very serious imprecision.
d Redisplacement resulting in secondary treatment only occurred in the nonsurgical treatment group: 38/316 (12%); range 3.3% to 75% (reported in 6 studies). Pin tract infection requiring antibiotics and, often, early wire removal only occurred in the pinning group: 22/285 (7.7%); range 0% to 15% (reported in 7 studies). There was one case of persistent deep infection. Incidence of RSD (nowadays, complex regional pain syndrome type 1), reported in 4 studies, was similar in the two groups: 13/123 (10.6%) versus 17/125 (13.6%); this was dominated by the results for short‐term RSD in one study: 12/48 versus 15/50. Persistent finger stiffness was less common after pinning versus cast alone after cast removal or at 12 weeks: 29/147 versus 53/149; RR 0.52, 95% CI 0.36 to 0.76; 2 studies; the treatment implications of this were not stated.
e Reported events for other outcomes were generally few and reported in single trials only. These were mainly surgery‐related. The exception was complications relating to the median nerve, such as carpal tunnel syndrome, which occurred in both groups but were uncommon overall, amounting to 14 cases in 383 participants (3.7%) reported in five studies.
f The quality of the evidence was very low given the qualitative and incomplete nature of the reported results and the very serious risk of bias.
g Very low quality, downgraded one level for serious risk of bias and two levels for very serious imprecision.
Background
Description of the condition
Fractures of the distal radius, often referred to as "wrist fractures", are common in both children and adults. They are usually defined as occurring within three centimetres of the radiocarpal joint, where the distal (lower) end of the radius meets with two (the lunate and the scaphoid) of the eight bones forming the carpus (wrist). The majority are closed injuries, the overlying skin remaining intact.
In this review, we consider the treatment of distal radial fracture in adults only, in whom they are one of the most common fractures, predominantly in white and older populations in high‐income countries (MacIntyre 2016; Sahlin 1990; Singer 1998; Van Staa 2001). In women, the incidence of these fractures increases with age, starting at around 40 years of age. Before this age, the incidence is higher in men (Mosenthal 2019; Singer 1998). In contrast, between 60 to 94 years of age, females predominate. A multicentre study in the United Kingdom of patients aged 35 years and above with Colles' fracture (see below) reported an annual incidence of 9/10,000 in men and 37/10,000 in women (O'Neill 2001). This is just one of the 22 epidemiological studies describing the incidence in five global regions presented in MacIntyre 2016. Although MacIntyre 2016 warns against direct comparisons, they point out that the incidence of distal radius fracture is higher in Scandinavia, New Zealand and North America, than in regions of Asia and Africa. MacIntyre 2016 considered that incidence rates were increasing, whereas a study of the Swedish National Patient Registry found a decline in annual incidence in women from 77/10,000 in 2005 to 63/10,000 in 2013 and 18/10,00 to 15/10,000 in men; both populations were aged over 50 (Mellstrand Navarro 2019).
Young adults more often sustain this injury as a result of high‐energy trauma, such as a traffic accident or sports. In older adults, especially females, the fracture usually results from low‐energy or moderate trauma, such as falling from standing height. This reflects the greater fragility of the bone, resulting from postmenopausal osteoporosis or disuse. It has been estimated that, at 50 years of age, a white woman in the USA or Northern Europe has a 15% lifetime risk of a distal radius fracture whereas a man has a lifetime risk of just over two per cent (Cummings 1985). More recent estimates (Van Staa 2001) of the lifetime risks of radius or ulna fracture at 50 years of age remain consistent with these figures: 16.6% for women versus 2.9% for men.
Distal radial fractures are usually treated on an outpatient basis and nonsurgically but often require hospital admission, including when undergoing surgery. The number of people receiving surgery for these fractures has been increasing over the years in many countries: for example, Mellstrand Navarro 2019 reported the proportion of patients undergoing surgery for these fractures in Sweden had increased by 6.7% for women and 4.2% for men between 2005 and 2013.
Classification
Surgeons have classified fractures by anatomical configuration or fracture pattern, to help in their management. Simple classifications were based on clinical appearance and often named after those who described them. The term "Colles' fracture" (Figure 1) is still used for a fracture of the distal radius in which there is an obvious and typical clinical deformity, commonly referred to as a dinner fork deformity, due to the shape of the resultant forearm (Colles 1814). The introduction of X‐rays and other imaging methods made it clear that the characteristic deformity (dorsal angulation, dorsoradial displacement, radial shortening) may be associated with a range of different patterns of fracture which may be important in determining the outcome of treatment, and therefore the way in which treatment is conducted. For example, the fracture through the distal radius in a Colles' fracture may be extra‐articular (leaving the joint surface of the radius intact) or intra‐articular (the joint surface is disrupted, sometimes in a complex manner). Numerous classifications have been devised to define and group different fracture patterns. One of the most commonly used is that of Frykman, which distinguishes between extra‐articular and intra‐articular fractures of the radiocarpal and distal radio‐ulnar joints, and the presence or absence of an associated distal ulnar fracture (Frykman 1967). Another commonly used system is the AO (Arbeitsgemeinschaft fur Osteosynthesefragen) system (Muller 1991; AO 2018), which divides the fractures into three major groups: group A (extra‐articular), group B (simple/partial intra‐articular), and group C (complex/complete intra‐articular). These three groups are then subdivided yielding 27 different fracture types. Other classification systems have attempted to link fracture type more directly with fracture management. For instance, Cooney 1993 proposed a 'Universal Classification' based on fracture displacement, articular involvement, reducibility (whether the fracture can be reduced; that is whether the bone fragments can be put back in place) and stability (whether, once reduced, the fragments will remain so).
1.
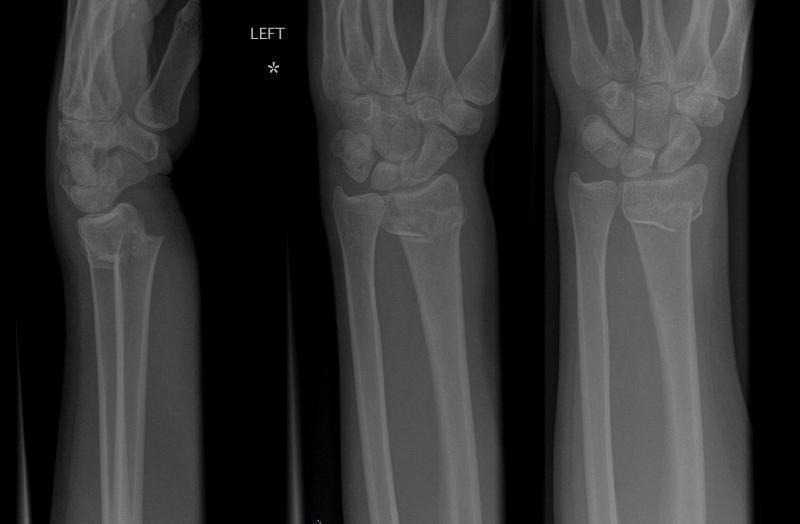
Example of a dorsally displaced "Colles'" distal radius fracture
Description of the intervention
For the purposes of this review, the nonsurgical treatment of distal radial fractures in adults involves reduction of the fracture, when displaced, and stabilisation in a plaster cast or other external brace. However, for fractures considered to be unstable (i.e. likely to lose position once reduced), the results of such treatment are not deemed consistently satisfactory. This has resulted in the development of other strategies involving surgery (insertion of metalwork into the bone) aiming to provide an improved position and more reliable stabilisation of the fracture.
One such surgical treatment strategy is percutaneous pinning, which involves the percutaneous (through the skin) insertion of pins or wires, which may or may not be threaded. This is considered less invasive, quicker or often less technically demanding than open surgery, where the fractured bone is exposed to direct view. In percutaneous pinning, the reduction of the fracture is closed (see Handoll 2003b); although pins ‐ such as Kirschner wires ‐ may be used to manipulate the fracture fragments. In a comprehensive account of percutaneous pinning of fractures of the distal radius, Rayhack 1993 refers to a "myriad of options, decisions and questions that must be addressed". These decisions include: the reduction technique; the method and extent of skin incision; the use and type of radiographic control; the configuration of the pins; the number, size and type of pins; whether the pin ends are left exposed out of the skin or not; and the type and duration of immobilisation after pinning. Later decisions involve the timing and methods for pin removal. A variety of different pinning techniques or methods have been described in the literature (Fernandez 1999; Rayhack 1993). In many of these, pins are placed in the bone and used to fix the distal radial fragment(s). In contrast, in Kapandji's double intrafocal pinning (Kapandji 1988), and subsequent developments of this technique, pins or wires are used to manipulate and then support or 'buttress' the distal radial fragment(s).
How the intervention might work
We consider three key comparisons below.
As indicated above, the attraction of surgery, compared with nonsurgical treatment involving cast immobilisation, is that the reduction of the fracture could be more accurate and the pins help hold the fracture in place while it heals. Surgery, however, is more invasive and comes with the risk of additional complications, notably pin track infection (see below). It is also a more costly primary intervention.
Various techniques of wiring are likely to have different complication profiles but also may result in differentially more stable or accurate fracture constructs. However, while still under debate, the implications of moderate deviation in anatomical (radiographic) parameters from average normal values on long‐term patient‐rated function remain uncertain (Downing 2008; Johnson 2019b).
Immobilisation after pinning can help rest the wrist and relieve pain in the first few days after intervention; beyond this, prolonged immobilisation for a number of weeks after surgery is aimed at supplementing the stability of the fracture and wire construct. A longer duration of immobilisation carries the additional risk of stiffness and could hamper pin‐site management.
Complications
Complications from this injury are diverse and frequent (Altissimi 1986; Atkins 1989; Cooney 1980).
Some are associated with the injury itself. As well as concomitant injuries to soft tissues, fracture displacement can further compromise blood vessels, tendons or nerves, with median nerve dysfunction being the most common early complication (Belsole 1993). Complex regional pain syndrome type 1, often termed reflex sympathetic dystrophy (RSD), but also referred to as algodystrophy, Sudeck's atrophy or shoulder‐hand syndrome (Fernandez 1996), is a major complication requiring many months of physiotherapy in serious cases to alleviate symptoms (pain and tenderness, impairment of joint mobility, swelling, dystrophy, vasomotor instability). The aetiology is often unclear.
Malunion is a complication that is poorly defined, but reflects the loss of anatomical alignment of the injured bones, either as a result of the injury or after treatment. The question of whether anatomical restoration of the distal radius is necessary to achieve good long term function and how much of a deformity can be accepted has not been answered. There is some consensus that intra‐articular step‐offs should generally be corrected, as they predispose to the development of radiologically identified degenerative changes (Downing 2008). However, an evidenced‐based radiographic threshold for intervention in extra‐articular malunion has not been defined; the clinical decision‐making is also dependent on patient factors such as mental capacity, functional demands, comorbidities and age (Johnson 2019a). Post‐traumatic arthritis can occur several months or years after injury (Knirk 1986; Taleisnik 1984), with varying impact on wrist and hand function. Furthermore, studies have failed to investigate thresholds linked to patient‐reported satisfaction with appearance.
Complications can also directly result from treatment interventions. Examples of such include residual finger stiffness, which may be due to faulty application of plaster casts (Gartland 1951) and pin track infection, or nerve or tendon injury, as a result of percutaneous pinning. Loss of fracture position can occur with any treatment modality and may or may not require further intervention.
Why it is important to do this review
Percutaneous pinning is one of the key methods for surgical treatment of distal radial fractures. It is attractive because it is less invasive and mostly relatively simple and quicker to perform compared with other fixation methods involving an open surgical approach to the bone, such as plating. Since the previous version of this review (Handoll 2007), studies have illustrated a rapid increase in the use of plate fixation and often a decline in percutaneous pinning (e.g. Mellstrand Navarro 2019 reporting trends in Sweden between 2005 and 2013; and Mosenthal 2019 reporting trends between in USA between 2007 and 2014). Huetteman 2019, reporting trends in USA between 2010 and 2015, found that the decline in pinning was across all age groups in adults. In the UK, this trend has been countered to some extent by the findings of DRAFFT 2014, a large multicentre pragmatic randomised controlled trial, involving 461 adults, set in the United Kingdom National Health Service. DRAFFT 2014 concluded that there was no difference in patient‐reported functional outcome in participants with dorsally displaced distal radius fractures which could be reduced closed, when treated with Kirschner wires or volar locking plates. Costa 2016 reported that for patients undergoing surgery in England, the proportion of patients having K‐wire fixation rose to 42% with a concurrent fall in the proportion having fixation with a plate to 48% following publication of the trial. Though this review does not compare percutaneous pinning with other modalities of surgical fixation such as plating, the possibility of an increased relevance of percutaneous pinning to current clinical practice worldwide for these fractures, necessitated an update of the available evidence on this group of interventions.
The previous version of this review, published in 2007, concluded that "Though there is some evidence to support its use, the precise role and methods of percutaneous pinning are not established". As well as need for a search update and to incorporate the new evidence, we also considered it important to update the review methods, including systematically appraising the quality of the evidence using GRADE (Guyatt 2008; Schünemann 2019).
Objectives
To assess the effects (benefits and harms) of percutaneous pinning versus cast immobilisation alone and of different methods and techniques of percutaneous pinning, modalities or duration of immobilisation after pinning, and methods or timing of pin or wire removal for treating fractures of the distal radius in adults. Our primary focus was on dorsally displaced fractures.
We aimed to compare the relative effects of the following for adults with these injuries.
Any method of percutaneous pinning versus nonsurgical treatment involving plaster cast or brace use alone.
Any method of percutaneous pinning versus any other method of percutaneous pinning.
Any technique or type of material or device used for percutaneous pinning versus any other technique or type of material or device at surgery or postoperatively.
Any type or duration of postoperative immobilisation versus any other type or duration of immobilisation including none.
Any method or timing of pin or wire removal versus any other method or timing of pin or wire removal including no removal.
We considered these effects primarily in terms of patient‐rated functional outcome and the incidence of people with complications which resulted in secondary treatment and overall.
We planned to study the outcomes in different age groups and for different types of fracture patterns; in particular, whether extra‐articular or intra‐articular.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised or quasi‐randomised (method of allocating participants to a treatment which is not strictly random e.g. by date of birth, hospital record number, alternation) controlled clinical trials of percutaneous pinning for treating distal radial fractures in adults were considered.
Types of participants
Patients of either sex who had completed skeletal growth, with a fracture of the distal radius. Our primary focus was on dorsally displaced fractures. Percutaneous pinning may be considered as primary treatment or may take place after the failure of initial nonsurgical management, generally within two to three weeks. Trials with a mixed population of adults and children were included provided the proportion of children was clearly small (< 5%); otherwise, they would have been excluded unless separate data for adults could have been obtained.
Types of interventions
This included the following comparisons.
Surgical interventions involving percutaneous pinning by itself versus nonsurgical (sometimes referred to as "conservative") interventions such as plaster cast immobilisation.
Different methods of percutaneous pinning in the treatment of fractures of the distal radius. Thus, comparisons evaluating:
different types of devices used for pinning;
use of different surgical techniques associated with percutaneous pinning, including type and extent of skin incision and measures to assist pin removal;
type and duration of immobilisation after percutaneous pinning;
type and timing of pin or wire removal.
Trials were excluded that compared percutaneous pinning with other methods of surgical fixation, such as external fixation, trials evaluating the use of supplementary percutaneous pinning in addition to another method of surgical fixation, and surgical versus nonsurgical treatment trials where the type of surgery was chosen by the surgeon, where percutaneous pinning was one of different surgical fixation methods used. We also excluded trials on pin site maintenance or other measures to prevent wound infection (already covered in Lethaby 2013).
Types of outcome measures
We aimed to divide all outcomes into short‐term (within three months), medium‐term (greater than three months and up to 12 months) and long‐term (greater than 12 months).
Primary outcomes
-
Function
Patient‐reported outcome measure (PROM) scores of hand/wrist/upper limb function (short‐, medium‐, long‐term), e.g. Patient Rated Wrist Evaluation (PRWE) Questionnaire (MacDermid 1998; MacDermid 2000), Disability of the Arm, Shoulder and Hand (DASH) (Hudak 1996) and QuickDASH Outcome Measures (Beaton 2005), Patient Evaluation Measure (PEM) (Macey 1995), Michigan Hand Questionnaire (MHQ) (Chung 1998) and Short MHQ (Waljee 2011).
-
Complications
Numbers of participants who incurred any complication that would typically receive substantive treatment (e.g. secondary surgical intervention, antibiotics for pin‐track infection; physiotherapy for Complex Regional Pain Syndrome (CRPS)). Finger stiffness during cast use, for which specific additional treatment, such as an unscheduled cast change or physiotherapy, was not undertaken, would generally not fall into this category.
Overall number of participants with complications.
Numbers of participants with individual complications
As found in the previous version of this review, the reporting of complications was incomplete and trials reported on the numbers of participants with individual complications, such as infection, rather than providing the total numbers of participants with complications requiring treatment. We have thus presented all data on complications as well as producing, where not reported, overall totals for participants with complications, provided unit of analyses and selective outcome reporting issues could be avoided. Our assumptions and interpretation, where reported data were incomplete, are documented in the review.
With regards to Complex Regional Pain Syndrome, CRPS Type 1 is now generally considered the equivalent of the older 'reflex sympathetic dystrophy' (RSD) and other terms such as 'algodystrophy', 'Sudeck’s atrophy' and 'shoulder hand syndrome'. In clinical practice, the term CRPS is often used inconsistently and, as the trials did not provide definitions, we have taken the approach to maintain the terminology used in source documents when reporting individual trial reports.
Secondary outcomes
-
Return to function as expressed by:
Return to (previous) occupation;
Return to patient key activities that are important to them.
-
Clinical:
Grip strength;
Range of motion;
Hand or wrist function performance scores, e.g. Jebsen‐Taylor (Jebsen 1969).
-
Quality of life:
Measures of health‐related quality of life (short‐, medium‐, long‐term) e.g. EQ‐5D (EuroQol Group 1990; Rundgren 2018), SF‐36 (Ware 1993), SF‐12 (Kosinski 2007).
-
Pain (short‐, medium‐, long‐term):
Visual analogue scales (VAS) or other stand‐alone pain scores;
Reports of life or function‐affecting pain measured as the pain component of a composite score, e.g. Gartland and Werley score (Gartland 1951).
-
Patient‐reported satisfaction:
Overall satisfaction;
Satisfaction with cosmetic appearance (often reported in terms of dissatisfaction).
-
Physician‐reported and/or composite outcome scores:
Total scores of physician‐reported and/or composite outcomes scores (short‐, medium‐, long‐term) e.g. Gartland and Werley score (Gartland 1951). In preference, for this outcome, we planned to report on 'poor and fair' categories.
-
Anatomical:
Radiographic parameters: e.g. dorsal angulation, radial length, radial inclination, ulnar variance and for intra‐articular fractures: the presence or absence of intra‐articular step or gap of the articular surface (Fernandez 1996; Kreder 1996a), based on plain radiographs. Definitions of four of the most commonly reported radiological parameters are presented in Table 2.
-
Economic outcomes:
Healthcare costs;
Resource use.
1. Definition of radiological parameters.
| Parameter | Definition (Radiographic view) | Approximate normative values |
| Dorsal angulation (dorsal or volar or palmar tilt) | Angle between a) the line which connects the most distal points of the dorsal and volar cortical rims of the radius and b) the line drawn perpendicular to the longitudinal axis of the radius Lateral view |
11–12 degrees, where positive values indicate a palmar angulation and negative values indicate angulation dorsally beyond neutral |
| Radial length | Distance between a) a line drawn at the tip of the radial styloid process, perpendicular to the longitudinal axis of the radius and b) a second perpendicular line at the level of the distal articular surface of the ulnar head Posteroanterior (PA) view |
11‐12 mm |
| Radial angle or radial inclination | Angle between a) the line drawn from the tip of the radial styloid process to the ulnar corner of the articular surface of the distal end of the radius and b) the line drawn perpendicular to the longitudinal axis of the radius. Posteroanterior (PA) view. |
22‐23 degrees |
| Ulnar variance | Vertical distance between a) a line drawn parallel to the proximal surface of the lunate facet of the distal radius and b) a line parallel to the articular surface of the ulnar head. Posteroanterior (PA) view obtained with the wrist in neutral forearm rotation, the elbow flexed 90° and the shoulder abducted 90° |
Varies in different populations, with a mean usually neutral to slightly negative (e.g. to ‐1 mm) |
Summary of findings table
The main findings to be included in the 'Summary of findings' tables were as follows.
Function: PROM scores ‐ short‐, medium‐, long‐term. Preference would have been given to PRWE (MacDermid 2000) if multiple PROM scores reported.
Complications: overall numbers of participants with complications that would typically receive substantive treatment; overall numbers of participants with one or more complications.
Clinical: grip strength in the medium term, ideally 12 months.
Quality of life: in the medium term, ideally 12 months.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (11 June 2019), the Cochrane Central Register of Controlled Trials (CENTRAL) (CRS Web 11 June 2019, Issue 6), MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R)) (1946 to 12 June 2019), and Embase (1974 to 12 June 2019). For this update, the search results were limited from 2006 onwards. Details of the search strategies used for the previous version of the review are given in Handoll 2007. We did not apply any language restrictions.
In MEDLINE (Ovid Online), a subject‐specific strategy was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (sensitivity‐maximising version) (Lefebvre 2011). Search strategies for CENTRAL, MEDLINE, and Embase are reported in Appendix 1.
We also searched ClinicalTrials.gov (12 June 2019) and the WHO International Clinical Trials Registry Platform (WHO ICTRP) (12 June 2019) for ongoing and recently completed trials (Appendix 1).
Searching other resources
We checked reference lists of articles.
We searched abstracts of the following conferences:
American Academy of Orthopaedic Surgeons (AAOS) annual meeting (2014, 2015, 2016 (posters only), 2017, 2018*, 2019),
American Orthopaedic Trauma Association (AOTA) annual meetings abstracts (1996 to 2018),
Bone and Joint Journal (BJJ) Orthopaedic Proceedings (April 2018),
British Society for Surgery of the Hand (BSSH) (2012 to April 2019),
British Trauma Society (BTS) annual scientific meeting (2014, 2015, 2016, 2018),
Société Internationale de Chirurgie Orthopédique et de Traumatologie (SICOT) meetings in 2014 (SICOT 2014), 2015 (SICOT 2015), 2016 (SICOT 2016), 2017 (SICOT 2017) and 2018 (SICOT 2018),
Federation of the European Societies for Surgery of the Hand (FESSH) XXI meeting June 2016 (published in Journal of Hand Surgery. European Volume 2016 41 Suppl 1: FESSH 2016); Federation of the European Societies for Surgery of the Hand (FESSH) XXII and EFSHT XII combined meeting June 2017 (published in Journal of Hand Surgery. European Volume 2017 42E Suppl 1: FESSH 2017); Federation of the European Societies for Surgery of the Hand (FESSH) XXIII meeting June 2018 (published in Journal of Hand Surgery. European Volume 2018 43 Issue 2 suppl: FESSH 2018).
* The 2018 AAOS proceedings were searched by the editorial base and included in the CRS.
Data collection and analysis
None of the authors were investigators of any of the trials eligible for this review. Should this have occurred, measures would have been taken to ensure independent assessment of eligibility and, if included, processing.
Selection of studies
For this update, pairs of review authors (AK and HH; AK and AS) independently screened search results and assessed potentially eligible studies for inclusion. The initial decisions of trial eligibility were based on citations and, where available, abstracts and indexing terms. We obtained full articles and, where necessary to ascertain trial methods and status, individual review authors, mainly AK, sent requests for information to trial investigators. Study inclusion was by consensus. Titles of journals, names of authors or supporting institutions were not masked at any stage.
Data extraction and management
For newly included trials, pairs of review authors (AK and HH; AK and AS) extracted trial details and data using a data extraction form. All disagreements were resolved by discussion. Where possible, we contacted trialists of trials that were not reported in full journal publications for additional information and data.
Results were collected for the final follow‐up time point for which these were available. We also recorded where clinically important differences had been reported at intermediate follow‐up assessments, but were no longer apparent at final follow‐up.
All three authors entered the data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
The same pairs of review authors as for data extraction independently assessed risk of bias for newly included trials. Two authors (AK and HH) assessed risk of bias for trials that had been included in the previous version of the review. All differences were resolved by discussion. We used the tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). This tool incorporates assessment of randomisation (sequence generation and allocation concealment), blinding (of participants and treatment providers, and outcome assessment), completeness of outcome data, selection of outcomes reported and other sources of bias. We considered 'subjective' outcomes (e.g. patient‐rated functional outcome scores, pain) and 'objective' outcomes (e.g. functional impairment, complications) separately in our assessment of blinding (performance bias). In our assessment of blinding (detection bias), we further split objective outcomes into those of functional impairment (e.g. grip strength) and the rest (e.g. complications). We considered short‐term (up to three months follow‐up) and longer‐term (three months or longer follow‐up) outcomes in our assessment of completeness of outcome data. We assessed two additional sources of bias: bias resulting from major imbalances in key baseline characteristics (e.g. age, gender, type of fracture, type of definitive treatment); and performance bias, particularly 'differential expertise' bias resulting from lack of comparability in clinician's experience with the interventions under test.
Additionally, we assessed four other aspects of trial design and reporting that would help us judge the applicability of the trial findings. The four aspects were: definition of the study population; description of the interventions; definition of primary outcome measures; and length of follow‐up.
Measures of treatment effect
Where available, quantitative data, both dichotomous and continuous, that were reported in individual trial reports for outcomes listed in the inclusion criteria were presented in the analyses. Risk ratios and 95% confidence intervals (CIs) were calculated for dichotomous outcomes and mean differences and 95% CIs were calculated for continuous outcomes.
Unit of analysis issues
We remained aware of potential unit of analysis issues arising from inclusion of participants with bilateral fractures, and presentation of outcomes, such as total complications, by the number of events rather than participants with these outcomes. We did not identify any trial reporting the inclusion of bilateral fractures, which, if few in number, would be unlikely to make much difference to study findings. We avoided the second stated unit of analysis issue, mainly by reporting on incidences of individual complications.
Dealing with missing data
Where appropriate for binary outcomes, we performed intention‐to‐treat analyses to include all people randomised to the intervention groups. In our protocol for this review, we indicated that we would, where appropriate, investigate the effect of missing data (dropouts and exclusions) by conducting best and worst case scenarios. So far, we have found no included data set where this would have been appropriate. We were alert to the potential mislabelling or nonidentification of standard errors and standard deviations. We calculated missing standard deviations from exact P values where provided. We did not assume values in order to present standard deviations in the analyses.
Assessment of heterogeneity
The decision to pool the results of individual studies depended on an assessment of clinical and methodological heterogeneity. If we considered studies sufficiently homogeneous for data pooling, we assessed statistical heterogeneity by visual inspection of the forest plots, and by using the Chi2 test with a significance level of P value less than 0.1, and the I2 statistic. We based our interpretation of the I2 statistic results on those suggested by Higgins 2011a (Section 9.5.2):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: considerable (very substantial) heterogeneity
Assessment of reporting biases
There were insufficient data (a minimum of 10 trials is recommended) to assess publication bias, for example, by preparing a funnel plot.
Data synthesis
Where appropriate, results of comparable groups of trials were pooled. As stipulated in the protocol, we used the fixed‐effect model and 95% confidence intervals.
Subgroup analysis and investigation of heterogeneity
There were no data available to carry out our prespecified subgroup analyses by age and gender and type of fracture (primarily extra‐articular versus intra‐articular fractures). While we planned to present separate subgroups according to whether there was a fundamental difference in pinning method (such as transfixation versus Kapandji's intrafocal method), the availability and nature of the outcome data did not support this. To investigate whether the results of subgroups were significantly different, we planned to inspect the overlap of CIs and perform the test for subgroup differences available in Review Manager 5 (RevMan 2014).
Sensitivity analysis
There were insufficient data available to carry out prespecified sensitivity analyses examining various aspects of trial and review methodology, including the effects of missing data, study quality (specifically allocation concealment, outcome assessor blinding and reportage of surgical experience), and inclusion of trials only reported in abstracts.
'Summary of findings' tables and quality assessment of the evidence
We produced 'Summary of findings' tables for one comparison for which a more substantive body of evidence had accrued. We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures for each comparison (see the Cochrane Handbook for Systematic Reviews of Interventions Section 12.2, Schunemann 2011).
Results
Description of studies
Results of the search
The search was updated from January 2006 to June 2019. A total of 1604 records resulted from searches of the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (24), the Cochrane Central Register of Controlled Trials (435), MEDLINE (632), Embase (265), ClinicalTrials.gov (86) and the WHO ICTRP (162). After deduplication of the main databases, performed by the BJMT Information Specialist, we screened 1297 records from these. For the search results from conference proceedings, we did not usually record the overall number of potentially eligible studies for those conferences proceedings where we used more general search terms or we had searched the whole conference proceedings, such as for the BSSH, where search facilities were unavailable. For the purposes of compiling the PRISMA diagram, we noted that we identified 647 potentially eligible studies from other sources, primarily conference proceedings, and articles relating to the six studies awaiting assessment in the previous version of this review (Handoll 2007).
The search update (to June 2019 for the main databases) identified a total of 44 articles for potential inclusion, for which full reports were obtained, where possible. We linked any references pertaining to the same study under a single study ID. Upon further analysis, 13 studies were included (Delgado 2009; Gravier 2006; Hargreaves 2004; Mardani 2011; Murphy 2008; Saddiki 2012; Shannon 2003; Snow 2007; Soleiman pour 2011; Venkatesh 2016; Waheed 2004, Wong 2010; Zyluk 2007), seven were excluded (Chung 2019; Gunay 2015; IRCT2013120814271N2; IRCT2016061828510N1; ISRCTN37842313; NCT02353338; Tomaszuik 2017), and five are ongoing studies (DRAFFT 2; IRCT20160508027797N4; IRCT2016112727797N1;NCT03311633; RBR‐8mq8bs). A further three studies (Kanakeshwar 2017; Mirhamidi 2013; Russe 2000) await classification. Five of the six studies awaiting classification in Handoll 2007 are now included: Gravier 2006 (formerly Gravier 2005), Hargreaves 2004, Shannon 2003, Snow 2007 (formerly Snow 2006), and Waheed 2004; the sixth study remains in this category (Russe 2000).
Overall, there are now 26 included studies, 12 excluded studies, three studies awaiting classification and five ongoing trials. A flow diagram summarising the study selection process is shown in Figure 2. The results from the previous search (up to 2006) are given in Appendix 2.
2.
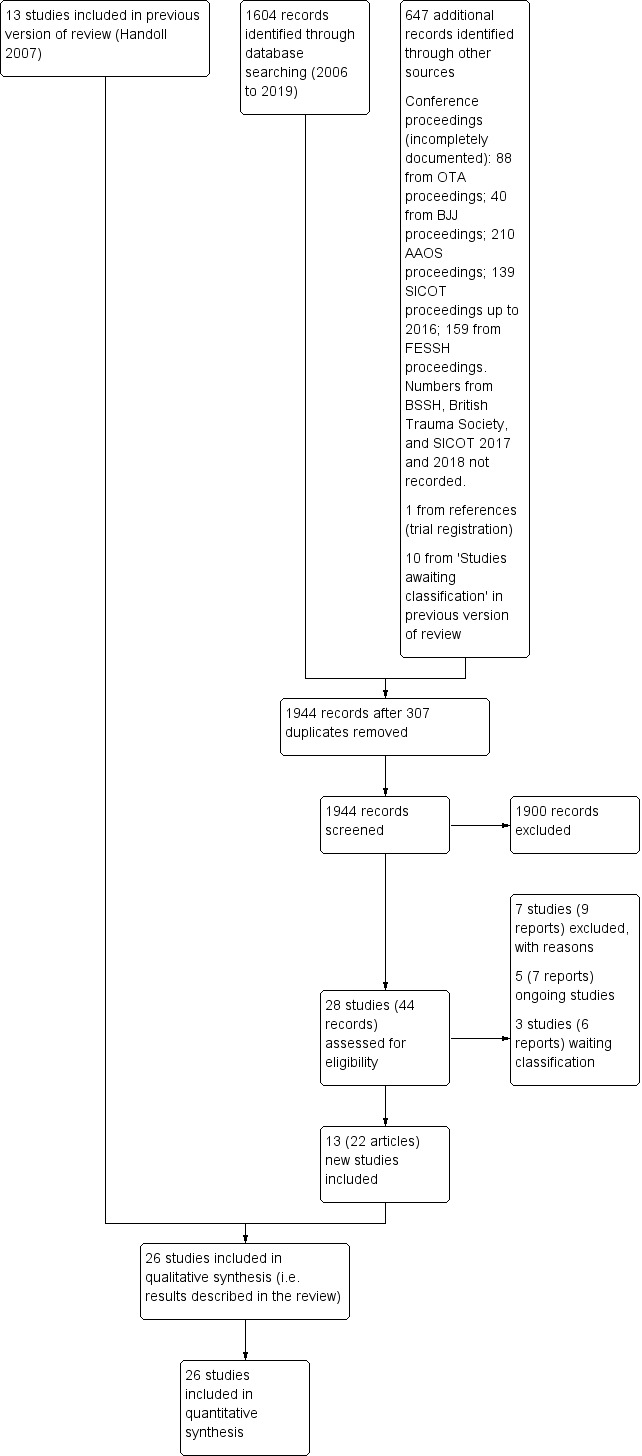
Study flow diagram for updated review
Included studies
Twenty of the 26 included studies were fully reported in medical journals. Trial reports of the other six trials (Delgado 2009; Korner 1999; Murphy 2008; Shannon 2003; Verhulst 1990; Waheed 2004) were only available as conference abstracts.
In this update and the first version of the review, we sent requests for further information, including publication status, on 12 trials and received responses for seven trials (Azzopardi 2005; Gravier 2006; Gupta 1999; Hargreaves 2004; Snow 2007; Strohm 2004; Zyluk 2007). However, additional unpublished information was obtained for only two trials (Gupta 1999; Zyluk 2007), as detailed in the Notes section for each trial in Characteristics of included studies.
Details of the methods, participants, interventions and outcomes of individual trials are provided in Characteristics of included studies.
Setting
The publication dates of the main reports of these trials span 16 years, Verhulst 1990 being the earliest. Aside from Lenoble 1995, which had two centres, the studies were all single centre studies, mainly in teaching hospitals. The trials each took place in one of 11 countries (Belgium (3), China (1), France (5), Germany (2), India (2), Iran (2), Ireland (3), Morocco (1), Poland (1), Spain (2), UK (4)). Translations were obtained for five trials, were from French in three trials (Fikry 1998; Gravier 2006; Milliez 1992), from Persian for Soleiman pour 2011 and from Polish for Zyluk 2007.
Participants
Sex and age
The 26 included trials involved a total of 1946 participants, most of whom were female. Three studies, reported in conference abstracts only, provided no information on sex or age (Korner 1999; Murphy 2008; Verhulst 1990); although Verhulst 1990 referred to an "elderly" population. Two further studies, both published in full articles, did not report on sex (Venkatesh 2016) or age (Soleiman pour 2011). It is noteworthy that the data for Stoffelen 1998 were best guesses because of the disparities in the baseline characteristics data provided in the three papers for this trial. The majority of participants were female in 16 trials, with the percentages ranging from 67% to 89%. Of the six trials featuring more male participants, the percentages of female participants were 21% in Delgado 2009, which focused on heavy labour workers in Spain, 25% in Fikry 1998, and between 42% to 46% in the other four trials (Hargreaves 2004; Mardani 2011; Soleiman pour 2011; Stoffelen 1998). The trial populations in the populations of three of these trials were markedly younger, with the mean age being 34 years in Fikry 1998, 35 years in Hargreaves 2004 and 40 years in Delgado 2009. Otherwise, where reported, the mean ages ranged from 48 years in Venkatesh 2016 (no sex data provided) to 71.5 years in Azzopardi 2005. The youngest participant was 7 years in Hargreaves 2004 and oldest was 92 years in Strohm 2004. It is clear that the vast majority of participants in the included trials were skeletally mature; with confirmation based on trial inclusion criteria or baseline characteristics information available for 22 trials. The four exceptions are Hargreaves 2004, where an unknown proportion were children; Korner 1999 and Murphy 2008, where the absence of any information on age means inclusion of children cannot be ruled out; and, to a very limited extent, Strohm 2004, where the youngest participant was 15 years of age. Three trials (Azzopardi 2005; Rodriguez‐Merchan 1997; Wong 2010) further restricted the trial population to more mature adults: above 60, 45 and 65 years respectively. An upper age limit of 65 years was applied in Rodriguez‐Merchan 1997, and 80 years in Gravier 2006 and Stoffelen 1998.
Fractures
A variety of descriptions was used to define the types of fracture in the included trials. There was explicit reference to inclusion of dorsally displaced or Colles' fractures (or exclusion of anterior displacement) in 16 trials (Allain 1999; Azzopardi 2005; Fikry 1998; Gupta 1999Milliez 1992; Rodriguez‐Merchan 1997; Saddiki 2012; Shankar 1992; Soleiman pour 2011; Stoffelen 1998; Strohm 2004; Verhulst 1990; Wong 2010; Zyluk 2007). There was no explicit inclusion of volar displaced fractures such as Barton's (Smith 1988). Trials often used descriptive terms such as displaced, unstable and comminuted, and referred to classification systems such as Frykman (Frykman 1967) or the AO system (Muller 1991). While Hargreaves 2004 and Strohm 2004 included a very few people with open fractures, it is likely that all the fractures in the other trials were closed, and certain in those 11 trials (Allain 1999; Azzopardi 2005; Casteleyn 1992; Lenoble 1995; Mardani 2011; Milliez 1992; Saddiki 2012; Soleiman pour 2011; Venkatesh 2016; Wong 2010; Zyluk 2007) which stipulated the exclusion of open fractures. Six trials described 'unstable' fractures, of which four trials described radiological criteria for defining an unstable fracture, thus defining the extent of displacement required for trial entry (Azzopardi 2005; Rodriguez‐Merchan 1997; Snow 2007; Wong 2010); whereas the other two did not (Delgado 2009; Shannon 2003). Mardani 2011 limited inclusion to stable fractures. Only Snow 2007 explicitly and solely included fractures that had redisplaced after a primary reduction. Of the 18 trials applying or reporting fracture type according to an established (not own) classification system, three trials grouped fractures according to more than one classification system. The Frykman system was used by 10 trials, the AO system by nine trials and Castaing system and Fernandez classification used by one trial each. Ten trials (Allain 1999; Casteleyn 1992; Korner 1999; Lenoble 1995; Milliez 1992; Saddiki 2012; Soleiman pour 2011; Snow 2007; Strohm 2004; Zyluk 2007) explicitly included both extra‐articular and intra‐articular fractures, although the extent of intra‐articular involvement was often limited; such as the exclusion of radiocarpal joint articular fractures in Casteleyn 1992, of fractures with more than two articular fractures in Allain 1999 and Lenoble 1995; and of comminuted fractures in Soleiman pour 2011. Seven trials only included or involved extra‐articular fractures (Azzopardi 2005; Gravier 2006; Gupta 1999; Mardani 2011; Stoffelen 1998; Venkatesh 2016; Wong 2010), whereas two trials (Rodriguez‐Merchan 1997; Shankar 1992) included intra‐articular fractures only. The involvement of the articular surface of the distal radius fracture was not clear in seven trials: Delgado 2009 (there was mention of intra‐articular fracture, however); Fikry 1998, where dislocated radiocarpal joint fractures were excluded; Hargreaves 2004; Murphy 2008; Shannon 2003; Verhulst 1990, which only referred to Colles' type fractures; or Waheed 2004.
Explicit exclusion of other injuries and conditions
Ten trials explicitly excluded people with multiple trauma, including other fractures (Allain 1999; Casteleyn 1992; Fikry 1998; Lenoble 1995; Mardani 2011; Milliez 1992; Snow 2007; Stoffelen 1998; Wong 2010; Zyluk 2007). Seven trials, including five of the first group, explicitly excluded people with previous wrist, elbow or upper limb fracture or malunion (Allain 1999; Azzopardi 2005; Lenoble 1995; Mardani 2011; Milliez 1992; Saddiki 2012; Snow 2007). Nine trials, only two of which did not appear in one or both of other categories, excluded for other conditions or reasons: dementia or psychiatric illness or problems (Azzopardi 2005; Mardani 2011; Wong 2010); severe brain injury (Stoffelen 1998); vascular or nerve complications (Milliez 1992; Soleiman pour 2011; Venkatesh 2016); unable or unwilling to participate in rehabilitation (Fikry 1998; Snow 2007: unable to physically or mentally participate in follow‐up). In all, 12 trials including all six trials only reported in abstracts did not report on other exclusion criteria.
Comparisons
The 26 included trials have been grouped according to the comparisons addressed by each trial. While most of the trials evaluated aspects of definitive treatment, three trials (Allain 1999; Milliez 1992; Soleiman pour 2011) tested the duration of immobilisation after surgery and Snow 2007 compared cast immobilisation of wrist in dorsiflexion versus palmar flexion after intrafocal pinning in people whose fractures had redisplaced.
Any method of percutaneous pinning versus nonsurgical treatment involving plaster or brace use
Eleven trials involving 917 participants compared closed reduction and internal fixation involving percutaneous pinning with closed reduction and plaster cast immobilisation (Azzopardi 2005; Delgado 2009; Gupta 1999; Mardani 2011; Rodriguez‐Merchan 1997; Shankar 1992; Stoffelen 1998; Venkatesh 2016; Verhulst 1990; Wong 2010; Zyluk 2007). A concise summary of the participants, fracture type, timing and details of the interventions for the 11 trials is given in Table 3. There was explicit reference to inclusion of dorsally displaced or Colles' fractures (or exclusion of anterior displacement) in eight trials (Azzopardi 2005; Gupta 1999Rodriguez‐Merchan 1997; Shankar 1992; Stoffelen 1998; Verhulst 1990; Wong 2010; Zyluk 2007). In Mardani 2011 and Venkatesh 2016 the inclusion of dorsally displaced fractures could confidently be implied as dorsal by the context of the study report. One trial, reported solely in abstract form (Delgado 2009), did not report on fracture direction or displacement. There was no explicit inclusion of volar displaced fractures, such as Smith's or Barton's (Smith 1988) in any trial.
2. Percutaneous pinning versus nonsurgical treatment: participants & interventions.
| Trial | Participants | Fracture type and classification | Timing of surgery and reduction method | Fixation | Nonsurgical treatment |
| Azzopardi 2005 | 57; 89% female (of 54); mean age 71.5 years | Unstable (dorsal angulation 20+ degrees) extra‐articular fractures. AO type A3a; Frykman I and II | Timing not stated. Closed reduction | Crossed pins, one from the radial styloid and the other from the dorso‐ulnar side of the distal fragment into the distal radial shaft. Cast immobilisation for 5 weeks | Closed reduction under general anaesthesia and fluoroscopy with three‐point fixation obtained in a "well‐moulded" short‐arm cast for 5 weeks |
| Delgado 2009 | 43; 21% female; mean age 40 years | Unstable fractures of distal radius (intra‐articular fracture mentioned in abstract report), no further details | Timing not stated. Closed reduction |
No details on wiring type or duration of immobilisation | Closed reduction. No details on cast type or duration of immobilisation |
| Gupta 1999 | 50; 74% female; mean age 56 years | Colles'. All extra‐articular. Frykman I and II | Timing not stated. Closed reduction | Crossed pins, one from the radial styloid and the other from the dorso‐ulnar side of the distal fragment into the distal radial shaft. Cast immobilisation for 6 weeks | Closed reduction under traction and plaster cast for 6 weeks (change in position and new plaster at 3 weeks) |
| Mardani 2011 | 198; 44% female; mean age 50.8 years | Displaced distal radius fracture with congruous joint with less than 2 mm joint gap [described as Fernandez classification type 1]. Dorsal displacement can be inferred by content of study report. | Timing not stated. Closed reduction | Percutaneous pinning with smooth 1.5 mm or 2 mm pin and immobilised with short arm cast. Cast immobilisation for 6 or 8 weeks | Closed reduction under general anaesthesia and long arm cast applied by the same orthopaedist. Cast immobilisation for 6 or 8 weeks |
| Rodriguez‐Merchan 1997 | 40; 73% female; mean age 57 years | Comminuted unstable fractures. All intra‐articular. Displaced (10+ degrees dorsal angulation/3+ mm radial shortening). Frykman III to VIII | Probably next day after presentation at hospital. Closed reduction | Crossed pins, two pins from the radial side and one from the ulnar side of the distal fragment into the distal shaft. Cast immobilisation for 7 weeks. | Closed reduction under local anaesthesia and plaster cast for 7 weeks |
| Shankar 1992 | 45; 88% female; age range 17‐88 years | Comminuted Colles', Frykman IV to VIII intra‐articular fractures | Timing not stated. Closed reduction | Two pins inserted obliquely from the radial side across the inferior radio‐ulnar joint and into the ulnar medial cortex. Pins incorporated into plaster. Cast immobilisation for 5 to 6 weeks | Closed reduction under general anaesthesia and image intensifier control plaster with cast for 5 to 6 weeks |
| Stoffelen 1998 | 98; % female unknown (discrepancies between trial reports but fewer females); mean age 58 years | Colles'. Extra‐articular fractures with dorsal displacement. Frykman I and II | Timing not stated. Probably closed reduction | Triple intrafocal Kapandji pinning: 3 pins, inserted at the fracture site and driven into the radial shaft, act as buttresses to the distal fragment. Cast immobilisation for 1 week | Closed reduction. Above‐elbow plaster cast for 3 weeks; below‐elbow for 3 weeks |
| Venkatesh 2016 | 70; % female unknown; mean age 47.75 years | Extra‐articular fractures of distal radius (AO types 23‐A2, 23‐A3a). Dorsal displacement can be inferred by content of study report and treatment in Colles' cast | Not older than 2 weeks. Closed reduction | 5 mm K‐wire through the radial styloid process piercing the far medial cortex of proximal fragment. An additional K‐wire was passed through the ulnar side of the radius engaging the opposite cortex when deemed necessary by the operating surgeon. Below elbow cast in neutral for 6 weeks | Closed reduction under general anaesthesia. Below elbow cast maintaining the wrist in palmar flexion and ulnar deviation (Colles' cast) for 6 weeks |
| Verhulst 1990 | 130; % female unknown; "elderly" | Colles' | Timing not stated. Closed reduction | Single Kirschner wire (pin) inserted through radial styloid, across fracture and into the distal radial shaft. Cast immobilisation for 4 weeks (mean) | Closed reduction and plaster cast for 5 weeks (mean) |
| Wong 2010 | 62; 82% (of 60) female; mean age 70.5 years | Unstable (dorsal angulation > 20 degrees and radial shortening > 5 mm) extra‐articular fracture of the distal radius | Fracture less than 2 weeks old. Closed reduction | Bier's block; 3 percutaneous K‐wires ("tripod" construct described in report); removable palmar splint for resting purpose; immediate gentle mobilisation under instructions of a physiotherapist was allowed; wires were removed "after fracture consolidation" | Closed reduction under haematoma block, Jones's methods used for reduction, below elbow plaster. No details on duration of immobilisation |
| Zyluk 2007 | 124; 82% (of 60) female; mean age 61 years | Displaced distal radius fracture. AO A2, A3, B1, B2, C1, C2a and Frykman I‐VII | Timing not stated. Closed reduction | Regional block; percutaneous fixation with 2 to 4 k‐wires introduced via the radial styloid so that they pierce the opposite cortex. In some cases, one K‐wire was introduced "from the base of the arm". Wires were buried and a plaster immobilising "only the wrist" was applied. Wires removed under local anaesthesia in clinic at 6 weeks | Closed reduction under local anaesthesia and immobilisation in a "Vienna" type above elbow plaster/backslab, shortened to below elbow at 2 weeks. Mobilised at 5 weeks |
a AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF)
Where known, the majority of participants were female in Azzopardi 2005; Gupta 1999; Rodriguez‐Merchan 1997; Shankar 1992; Wong 2010 and Zyluk 2007; and male in Delgado 2009 and Mardani 2011. Where stated, mean age in the trials ranged from 40 years in Delgado 2009 and 71.5 years in Azzopardi 2005. Six trials only included or involved extra‐articular fractures (Azzopardi 2005; Gupta 1999; Mardani 2011; Stoffelen 1998; Venkatesh 2016; Wong 2010), whereas Rodriguez‐Merchan 1997; Shankar 1992) included intra‐articular fractures only. The involvement of the articular surface of the distal radius fracture was not clear in Delgado 2009 and Verhulst 1990. Zyluk 2007 included both extra‐articular and intra‐articular fractures.
Each trial employed a different pinning technique or duration of immobilisation, or both. Various numbers of pins were used to transfix the distal radius fragment to the radial shaft in eight trials: Mardani 2011 and Verhulst 1990 used one pin; Venkatesh 2016 used one or two pins; Azzopardi 2005 and Gupta 1999 used two pins; Rodriguez‐Merchan 1997 and Wong 2010 used three pins; and Zyluk 2007 used two to four4 pins. In Shankar 1992, two pins were placed obliquely through the distal radial fragments across the inferior radio‐ulnar joint and fixed in the ulna. Stoffelen 1998 used Kapandji's triple intrafocal technique, where three pins introduced at the fracture site and driven into the radial shaft supported the fracture fragment. Stoffelen 1998, Wong 2010 and Zyluk 2007 also differed from the other trials in that there was a difference in the duration of plaster immobilisation between the two intervention groups. Wong 2010 opted for immediate mobilisation of the pinning group, a removable palmar splint being used for resting.
Four trials (Azzopardi 2005, Mardani 2011, Shankar 1992 and Venkatesh 2016) specified that closed reduction casting was performed under general anaesthetic in the casting arm, with the rest of the trials specifying either local anaesthesia (Rodriguez‐Merchan 1997; Zyluk 2007), hematoma block (Wong 2010) or giving no details of anaesthetic/analgesic technique (Delgado 2009; Gupta 1999, Stoffelen 1998 and Verhulst 1990). Two trials specified that closed reduction and casting was performed under fluoroscopic control (Azzopardi 2005; Shankar 1992).
Delgado 2009, which was reported only in an abstract, provided no details on wiring technique nor cast type and no duration of immobilisation for either group.
Any method of percutaneous pinning versus any other method of percutaneous pinning
Six trials made one of five comparisons of different methods of percutaneous wiring. Kapandji intrafocal pinning featured in five trials (Fikry 1998; Gravier 2006; Lenoble 1995; Saddiki 2012; Strohm 2004) but not in Shannon 2003.
Kapandji intrafocal pinning (two or three wires) versus trans‐styloid fixation (two wires)
One trial compared Kapandji fixation with two or three Kirschner wires followed by immediate mobilisation versus trans‐styloid fixation with two Kirschner wires and plaster cast immobilisation for about 45 days in 120 adults with a closed extra‐articular or simple (two part only) intra‐articular, dorsally displaced distal radius fracture with posteromedial fragment (Lenoble 1995). Wire removal was around 45 days in both groups. Baseline characteristics were provided only for the 96 participants assessed at final follow‐up; of these, most were female (65/96, 68%) with a mean age of 57 years.
Modified Kapandji (dorsal Kapandji wires and trans‐styloid fixation) pinning versus Kapandji intrafocalpinning
A single‐centre study conducted in France compared a modified Kapandji technique that included insertion of a lateral 2/100 diameter transfocal styloid pin versus intrafocal Kapandji pinning (Gravier 2006). Further details of the interventions are provided in Characteristics of included studies. A posterior back slab was then applied for three weeks followed by passive rehabilitation. Pins were removed on day 45 postoperatively. The full report of the trial reported the randomisation of 85 study participants, who were mostly female (57/85, 67%) with a mean age 55 years, had dorsally displaced extra‐articular fractures of the distal radius, with or without an associated ulnar styloid fracture. An earlier abstract reported a population of 95 participants, with an average age of 49 years.
Kapandji intrafocalpinning (three wires) versus Py's isoelastic pinning (two wires)
Two trials undertaken in France compared Kapandji intrafocal pinning with three Kirschner wires versus with Py's isoelastic pinning, where two wires were inserted across the fracture and along the medullary canal, in 207 people with dorsally displaced distal radius fractures (Fikry 1998; Saddiki 2012). In Fikry 1998, both groups had immobilisation in a forearm cast for four weeks with pin removal at eight weeks. In Saddiki 2012, participants were in plaster for three weeks with wires removed at six weeks. Saddiki 2012 included 97 participants with either intra‐ or extra‐articular dorsally displaced distal radius fracture requiring surgical treatment. Though some involvement of the articular surface of the distal radius was possible in Fikry 1998, dislocated radiocarpal joint and "comminuted" fractures were excluded.The demographics differed between the two trials. Of the 88 participants followed‐up in Fikry 1998, their mean age was 34 years and 25% were female. The mean age of participants in Saddiki 2012 was 63 years and 86% were female.
Modified Kapandji intrafocal pinning (three wires) versus Willenegger pinning (two wires)
In Strohm 2004, one participant group was treated with a modified Kapandji method, involving intrafocal pinning with two Kirschner wires through the fracture gap with a third wire inserted via the radial styloid, followed by six weeks of immobilisation in a volar splint. Physiotherapy, with the volar splint removed, was started at three weeks. The other participant group received Willenegger pinning, where two wires introduced via the styloid process were inserted across the fracture, followed by immobilisation in a forearm cast for six weeks. The 100, mainly female (85%), participants with an average age of 65 years had Colles' type fractures, either extra‐ and intra‐articular (AO types A2, A3 or C1).
"Spring‐loaded intramedullary" pinning (not Py) versus "traditional transcortical" pinning (three wires used for both)
Shannon 2003, a single‐centre trial from Ireland, reported their study in a conference abstract only and we have been unable to obtain further information. They compared the insertion of three k‐wires using "a novel spring‐loaded intramedullary technique", where the k‐wires were engaged in the opposite cortex and driven down the medullary canal, versus insertion in "the traditional transcortical fashion". All fractures were reduced, and three 1.6 mm K‐wires were used. No details on the immobilisation or rehabilitation regimen were provided. The 46 participants were mainly female (37/46, 80%) with a mean age of 58 years. Fractures were described as "unstable" fractures of the distal radius; the AO classification was used but the population was not described.
Any technique or type of material or device used for percutaneous pinning versus any other technique or type of material or device at surgery or postoperatively
Biodegradable pins or wires versus metal pins or wires
Two trials undertaken in European teaching hospitals (Casteleyn 1992; Korner 1999) evaluated the use of biodegradable pinning in 70 people with extra‐articular and intra‐articular fractures. None of the 30 mainly female (77%) participants (mean age 61 years, range 22 to 85) of Casteleyn 1992 had radiocarpal joint fractures, with only Frykmann type I, II, V and VI fractures included, balanced across randomisation groups. No details of participant age or sex were available for Korner 1999, though it is stated that the intervention group included AO type A2, A3 and B1 fractures, with no detail of fracture characteristics for the comparison group. Different pinning techniques were used in the two trials: Kapandji's intrafocal pinning with two wires was used in Casteleyn 1992, while two pins or Kirschner wires were inserted via the radial styloid and across the fracture (modified Willenegger method) in Korner 1999. There was no cast immobilisation in Casteleyn 1992; there was no mention of postsurgical care in Korner 1999. Korner 1999 was available only in abstract form.
Pinning with buried wires versus exposed percutaneous wires
Three single‐centre trials, conducted in hospitals in Ireland and the UK, evaluated the technique of burying wires versus leaving them exposed in 168 people with isolated distal radius fractures requiring Kirchner wire fixation (Hargreaves 2004; Murphy 2008; Waheed 2004). Only Hargreaves 2004 was reported in a full‐text article. Participants in Hargreaves 2004 included both children and adults (mean age 35 years) of which 45% were female. All participants in Waheed 2004 were adults (mean age 57 years) of which 73% were female. There was minimal information on fracture type; notably two participants in Hargreaves 2004 had open fractures. Murphy 2008 provided no details on the population and neither Murphy 2008 nor Waheed 2004 described the surgery or interventions. Usually fixation was by two crossed K‐wires in Hargreaves 2004, which provided a detailed account of the interventions and other care, including the administration of a single dose of antibiotics at induction of anaesthesia. Wire removal was probably at around six weeks for all three trials.
Any type or duration of postoperative immobilisation versus any other type or duration of immobilisation including none
Cast immobilisation of wrist in dorsiflexion versus palmar flexion after intrafocal pinning
Snow 2007 compared cast immobilisation with the wrist in 30 degrees dorsiflexion versus 30 degrees palmar flexion after closed manipulation and percutaneous intrafocal Kirschner wire fixation in 63 participants whose fracture had redisplaced within 14 days. Fractures were extra‐articular or intra‐articular and participants were mainly female (82.5%) with a mean age of 60 years. After removal of the K‐wires at the outpatient clinic at three weeks, participants were then placed back into their selected casts for a further two weeks. The postoperative care of both groups was identical.
Duration of postoperative immobilisation after percutaneous pinning
The duration of immobilisation after percutaneous pinning was tested by three single‐centre trials, two of which were quasi‐randomised, in 170 people with extra‐articular or intra‐articular distal radius fractures (Allain 1999; Milliez 1992; Soleiman pour 2011). Allain 1999 and Milliez 1992 compared one week versus six weeks cast immobilisation in 120 people and Soleiman pour 2011 compared one week versus four weeks immobilisation in 50 people. The participants of Allain 1999 and Milliez 1992 were mainly female (74% overall) with a mean age of 55 years. Soleiman pour 2011 included more males (54% of the population) but did not report on age. Trans‐styloid fixation with two Kirschner wires was used in Allain 1999 and Kapandji intrafocal pinning with three Kirschner wires was used in Milliez 1992; in both these trials, the early mobilisation group was advised against heavy work and lifting heavy loads but did not appear to receive any other specific instructions to those given after cast removal in both groups. Although there was mention of Kapanji in the text, Soleiman pour 2011 did not specify the surgical technique used nor provide information on the postsurgical regimen.
Any method or timing of pin or wire removal versus any other method or timing of pin or wire removal including no removal
There were no included trials for this topic.
Outcomes
The outcomes collected by individual trials are listed in the 'Outcomes' section of Characteristics of included studies, where these are grouped under '(1) Functional', which includes function assessed via PROMs, return to former activities, grip strength and range of motion, and composite measures such as the Gartland and Werley score; '(2) Clinical', which includes pain and complications; '(3) Anatomical'; and, exceptionally, '(4) Other'. In the following, we focused on function measured via PROM scores, complications, grip strength and quality of life as these were the outcomes chosen for presentation in 'Summary of findings' tables.
Length of final follow‐up ranged from around six weeks in five trials (Gravier 2006; Hargreaves 2004; Murphy 2008; Shannon 2003; Waheed 2004) to two years (Lenoble 1995; Verhulst 1990), but was of variable duration in Fikry 1998, Korner 1999, Strohm 2004 and Zyluk 2007.
The three trials measuring a PROM for function all recorded DASH scores (Delgado 2009; Saddiki 2012; Zyluk 2007). All trials reported to some extent on complications except Gravier 2006 and Venkatesh 2016. For most trials, the number of participants incurring a complication in each group was not reported and could not be deduced with confidence from the reported individual complications because of concerns over unit of analysis issues and/or incomplete data collection and reporting. Grip strength was reported in 13 trials, and would have been measured in several others as it formed part of composite scores. Just two trials reported on heath‐related quality of life: SF‐36 was reported by Azzopardi 2005, and the WHO quality of life by Wong 2010.
Excluded studies
Twelve studies were excluded for reasons stated in Characteristics of excluded studies. Six studies were found not to be randomised trials (Biedermann 2001; Delattre 1994; Galli 2002; Gunay 2015; Seifert 1998; Tomaszuik 2017); two trials were abandoned (Harper 2000; ISRCTN37842313); three trials did not test an appropriate comparison (Chung 2019; IRCT2016061828510N1; NCT02353338) and we judged that further clarification or a trial report of IRCT2013120814271N2, which was sparsely and unclearly reported in a trial registration document, was unlikely to materialise.
Ongoing studies
Details of the five ongoing studies are given below.
DRAFFT 2: pragmatic multicentre trial comparing percutaneous pinning plus cast immobilisation versus moulded cast immobilisation in 506 participants (probably actual recruitment). Recruitment was completed in March 2019. Trial completion, which included a 12‐month follow‐up, is scheduled for September 2020.
IRCT20160508027797N4: trial registered in March 2019 comparing 3‐pin percutaneous pinning with cast immobilisation in 60 participants (stated target). With a follow‐up of 42 weeks, it is likely this trial is still ongoing.
IRCT2016112727797N1: this completed trial compared three crossed pinning and four‐pin radioulnar transfixation methods in 50 participants. This small trial testing a new comparison in this review was published in mid August 2019, after the cut‐off time for inclusion (Hosseinzadeh 2019).
NCT03311633: trial registered in October 2017 comparing three versus six weeks of percutaneous pinning in 60 participants (stated target). All will receive six weeks cast immobilisation. The expected date of trial completed is February 2020.
RBR‐8mq8bs: trial registered in August 2017 compared pinning with buried wires versus exposed percutaneous wires in 200 participants (stated target). Correspondence on 30 August 2019 from the lead author indicated that the trial report was poised for submission.
Studies awaiting assessment
Details of the three trials pending assessment are given below.
Kanakeshwar 2017: this published abstract reported a comparison of three versus two k‐wires in 145 participants. We have received no response to our request for clarification of trial methods, including interventions, and results.
Mirhamidi 2013: a published journal article reported this trial of Kapandji pinning versus extrafocal pinning in 45 participants. We have received no response to our request for clarification of trial methods, including treatment allocation and further data.
Russe 2000: this multicentre trial incompletely reported in four abstracts, each with different numbers of participants, compared bioabsorbable versus metal wires. Although we received information in 2006 that it was a randomised trial with 115 participants, we received no further response to requests for further information and data.
Risk of bias in included studies
The 'Risk of bias' judgements on 12 items for the individual trials are summarised in Figure 3 and described in the 'Risk of bias' tables in the Characteristics of included studies. Frequently assessments resulted in an 'Unclear' (?) verdict; this often reflected a lack of information upon which to judge the item (Figure 4). However, we usually took a lack of information on blinding to imply that there was no blinding.
3.
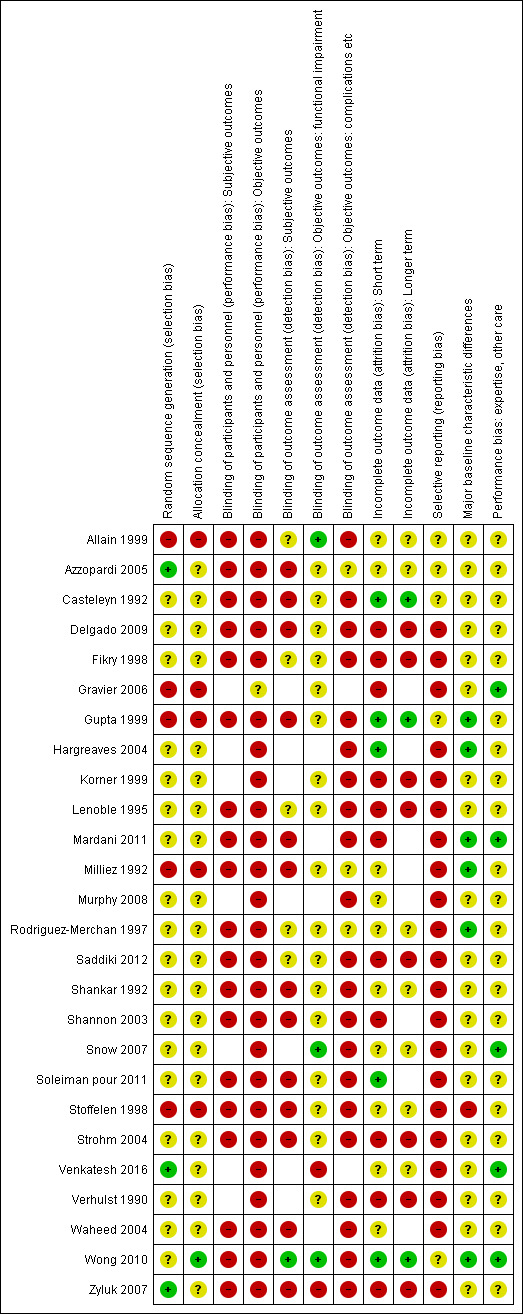
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
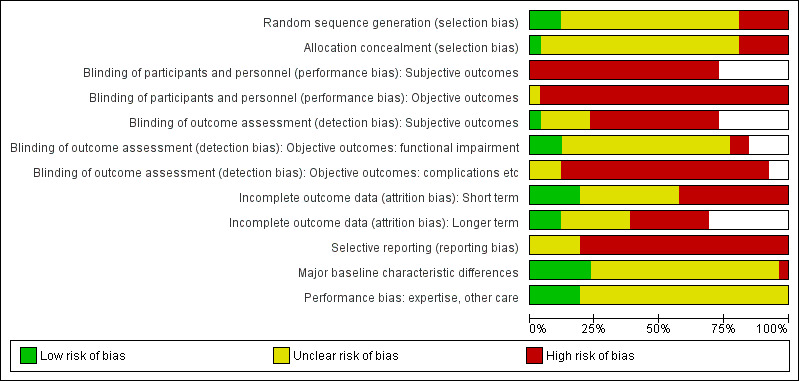
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
All trials were at high risk of bias, invariably performance bias that for most trials reflected the impracticality of blinding care providers or participants to the treatment allocation, and generally detection bias, although a few trials succeeded in blinding of some outcome assessment. As detailed below, most trials were rated at high risk of selective reporting bias.
Allocation
Five trials judged at high risk of selection bias, both relating to random sequence generation and allocation concealment used quasi‐randomised methods based on admission sequence or alternation (Gravier 2006; Gupta 1999; Milliez 1992; Stoffelen 1998), or participant chart numbers (Allain 1999). The three trials judged at low risk of random sequence generation bias, described tossing a coin (Azzopardi 2005), computer generation (Venkatesh 2016) and drawing of lots by the participant (Zyluk 2007). The other 18 trials did not describe their method of sequence generation and were rated at unclear bias for this item.
Only Wong 2010, which described the use of sequentially numbered opaque sealed envelopes, was judged at low risk of selection bias relating to allocation concealment. Of the other 20 trials rated at unclear risk of bias for this item, six mentioned the use of envelopes but did not provide sufficient details to confirm secure allocation concealment. This included Rodriguez‐Merchan 1997, which while describing the use of blinded consecutively numbered envelopes, did not describe whether these were sealed. The other five trials referred to sealed envelopes (Casteleyn 1992; Hargreaves 2004), closed envelopes (Shannon 2003; Snow 2007) or gave no details (Zyluk 2007). Fourteen trials gave no details of their method for safeguarding allocation concealment; although blinded randomisation was claimed by Strohm 2004.
Blinding
Blinding of participants and personnel was generally not practical and not claimed for these trials. All 19 trials reporting subjective outcomes were judged at high risk of performance bias for these outcomes. Aside from Gravier 2006, which was judged at unclear risk for radiological assessment only, the other 25 trials were judged at high risk of performance bias for objective outcomes.
Total blinding of outcome assessment is impractical for trials testing surgical interventions but it is possible for some outcomes and more so at longer term follow‐up. Of the 19 trials reporting on subjective outcomes, only Wong 2010 was considered at low risk of detection bias as there appeared to be effective blinding in place. We rated 13 trials at high risk of detection bias for these outcomes and the other five trials at unclear risk, generally as their outcome scores seemed less susceptible to bias (Allain 1999; Fikry 1998; Lenoble 1995; Rodriguez‐Merchan 1997; Saddiki 2012).
Of the 22 trials reporting on measures of functional impairment, three were rated at low risk of detection bias as there was blinded assessment of these outcomes (Allain 1999; Snow 2007; Wong 2010). We rated 17 trials at unclear risk of bias as we considered these outcomes were less susceptible to bias despite the lack of blinding. However, two trials were judged at high risk of bias (Venkatesh 2016; Zyluk 2007).
The risk of detection bias for complications was considered high for 21 trials and unclear for three trials, where the susceptibility for bias appeared less for different reasons. Notably, Gravier 2006 and Venkatesh 2016 did not report on complications.
Incomplete outcome data
Overall, participant flow was poorly reported; where reported, loss to follow‐up, sometimes including exclusions because of treatment failure, ranged from none to excesses of 20% in Fikry 1998 and Lenoble 1995; 32% in Saddiki 2012; and 52% in Zyluk 2007. The risk of attrition bias at short‐ and long‐term follow‐up was judged the same for those 18 trials for which short‐ and long‐term follow‐up data were available. Risk of attrition bias was judged at low risk in five trials (Casteleyn 1992; Gupta 1999; Hargreaves 2004; Soleiman pour 2011; Wong 2010); at high risk in 11 trials (Delgado 2009; Fikry 1998; Gravier 2006; Korner 1999; Lenoble 1995; Mardani 2011; Saddiki 2012; Shannon 2003; Strohm 2004; Verhulst 1990; Zyluk 2007) and at unclear risk in the remaining 10 trials. Aside from high loss to follow‐up, incomplete data and data inconsistencies or discrepancies were common reasons for rating trials at high risk of attrition bias.
Selective reporting
We judged 20 trials at high risk of selective reporting bias and six at unclear risk (Allain 1999; Azzopardi 2005; Casteleyn 1992; Gupta 1999; Wong 2010; Zyluk 2007). We found no published protocol for any trial. Trial registrations, both prospective, were found for just two trials (Azzopardi 2005; Snow 2007). As well as lack of information on the intended outcomes prior to starting the trial, missing outcomes, incomplete and inadequate reporting of results, extra outcomes not listed in methods, disparities between methods and results such as length of follow‐up or variable follow‐up were assessed as indicative of a high risk of selective reporting bias. No trial reported reasons for changes to protocol.
Other potential sources of bias
We assessed this in terms of bias resulting from major imbalances in key baseline characteristics and performance bias, particularly resulting from lack or potential lack of comparability in clinician's experience with the interventions under test.
We judged 19 trials at unclear risk of bias relating to important imbalances in baseline characteristics because of the absence of data on these, incompletely reported characteristics data for key characteristics or because of small imbalances that potentially could have affected the results. We judged that Stoffelen 1998 was at high risk for this item because of the major between‐group differences reported for sex and high‐energy injuries. We judged six trials at low risk of bias for this item (Gupta 1999; Hargreaves 2004; Mardani 2011; Milliez 1992; Rodriguez‐Merchan 1997; Wong 2010).
Comparability of care programmes, comprising interventions and related aspects other than the trial interventions (such as type of anaesthesia, timing of the interventions, comparability of the experience of the health professionals applying the interventions (operator bias), and rehabilitation) was hard to assess. We judged that five trials were at low risk of bias for this item (Gravier 2006; Mardani 2011; Snow 2007; Venkatesh 2016; Wong 2010) and the remaining 21 trials at unclear risk, predominantly because of incomplete information. We noted that distinctive differences between the two groups, where an intervention other than the trial intervention was used exclusively in one group, can change the actual comparison under test but was not considered a risk of bias. For instance, in Lenoble 1995, the Kapandji pinning group had immediate mobilisation but not the trans‐styloid fixation group; in Strohm 2004, physiotherapy was provided after three weeks of immobilisation to the modified Kapandji pinning group only.
Effects of interventions
See: Table 1
Any method of percutaneous pinning versus nonsurgical treatment involving plaster or brace use
Closed reduction and percutaneous pinning was compared with nonsurgical treatment usually comprising closed reduction and plaster immobilisation alone in 11 trials involving 917 participants (Azzopardi 2005; Delgado 2009; Gupta 1999; Mardani 2011; Rodriguez‐Merchan 1997; Shankar 1992; Stoffelen 1998; Venkatesh 2016; Verhulst 1990; Wong 2010; Zyluk 2007). Table 3 summarises the participants, fracture type, timing and details of the interventions for the 11 trials. Details on length of participant follow‐up and losses to follow‐up for all trials are summarised in Table 4. Nine trials (776 participants) applied across‐fracture pinning whereas Stoffelen 1998 used Kapandji's triple intrafocal technique with early mobilisation in 98 participants. Delgado 2009 provided no details on wiring technique. The anaesthetic/analgesic technique in the nonsurgical arm also varied, with four trials employing general anaesthesia. Results for outcomes were often incomplete for many of the trials and opportunities to pool data were limited. There were no data available to carry out our prespecified subgroup analyses by age and gender and type of fracture.
3. Percutaneous pinning versus nonsurgical treatment: length and completeness of follow‐up.
| Study IDa | Length of follow‐up in months | Completeness of follow‐up |
| Mardani 2011 | 3 | No participant flow diagram. Some loss, as the authors described mean postoperative visits of 4.4 for group 1 and 3.6 for group 2 out of a possible 5. |
| Gupta 1999 | 6 | No losses (reported) |
| Shankar 1992 | 6 | No losses (inferred) |
| Venkatesh 2016 | 6 | 10 participants lost to follow‐up (5 from each group) were excluded from the analysis. |
| Zyluk 2007 | 6 | 64 (31 from the pinning and 33 from the nonsurgical group) |
| Azzopardi 2005 | 12 | 9 (3 deaths and 6 lost to follow‐up). No information on which group they were from |
| Delgado 2009 | 12 | Not known |
| Rodriguez‐Merchan 1997 | 12 | No losses (inferred) |
| Stoffelen 1998 | 12 | No losses (inferred) |
| Verhulst 1990 | 24 | Not known |
| Wong 2010 | 19.5 (13 to 24) | 2 participants were excluded from the analysis; these were two deaths from heart disease "which was not related to postoperative complications"; hence they appeared to be from the surgery group. |
a Ordered by length of follow‐up
Primary outcomes
Patient‐reported function
DASH, a PROM score for upper‐limb function, was used in the two trials, Delgado 2009 and Zyluk 2007. Delgado 2009 reported similar functional results in the two groups, probably at 12 months. Zyluk 2007 reported median DASH scores (scale used 30 to 150; higher scores = worse outcome) and ranges at 1.5, 3 and 6 months for 60 participants. Zyluk 2007 indicated that the difference in scores, which favoured the pinning group, was not statistically significant at 1.5 months (median 85 versus 97) but was at six months (48 versus 64); very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision (see Analysis 1.1). In addition to the uncertainty surrounding these findings, it is not certain whether the difference between the two groups in DASH scores was clinically important. Based on a scoring scheme of 0 to 100 (worst outcome), a minimal clinically important difference (MCID) of 15 is recommended in DASH/QuickDASH; this translates to 18 for a scale of 30 to 150, which is greater than the 16 difference in median scores at six months. Zyluk 2007 also collapsed the DASH scores (range 30 to 150) into three categories (normal: 30 to 40, satisfactory: 41 to 60 and reduced function: > 60). At six months, there were fewer participants in the pinning group with reduced function: 8/30 versus 21/30, RR 0.38, 95% CI 0.20 to 0.72; very low‐quality evidence downgraded two levels for very serious risk of bias, one level for serious imprecision and one level for serious indirectness given this improper categorisation of the DASH scores (Analysis 1.2).
1.1. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 1 DASH scores (30 to 150; worst disability).
| DASH scores (30 to 150; worst disability) | |||
|---|---|---|---|
| Study | Pinning | Cast only | Statistically significant? |
| At 1.5 months | |||
| Zyluk 2007 | Median, range, no. 85, 30‐125, 30 |
Median, range, no. 97, 50‐142, 30 |
No |
| At 3 months | |||
| Zyluk 2007 | Median, range, no. 67, 41‐101, 30 |
Median, range, no. 84, 40‐128, 30 |
No |
| At 6 months | |||
| Zyluk 2007 | Median, range, no. 48, 32‐94, 30 |
Median, range, no. 64, 40‐119, 30 |
Yes |
1.2. Analysis.
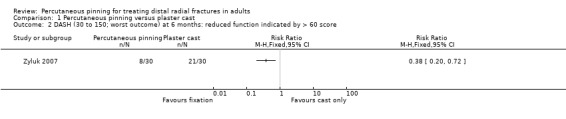
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 2 DASH (30 to 150; worst outcome) at 6 months: reduced function indicated by > 60 score.
Complications
With the exception of Delgado 2009, which claimed to have recorded complications but did not report them, and Venkatesh 2016, which did not mention any complications in their report, the other included trials reported to some extent on complications. The available data are presented in Analysis 1.3. Null events have also been entered when reported. Only data on individual complications were reported. All evidence for individual complications was considered very low quality, downgraded two levels for very serious risk of bias and one or two levels for serious or very serious imprecision.
1.3. Analysis.
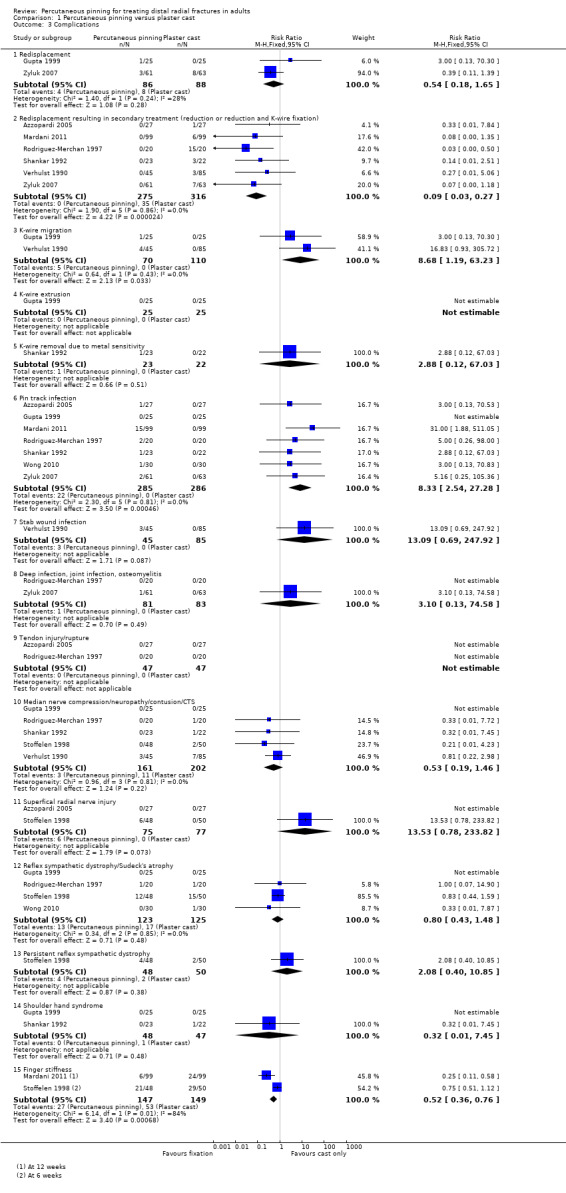
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 3 Complications.
We were not able to report on the overall numbers of participants who incurred one of more complications that would typically received substantive treatment. The main complications that would have contributed to this are redisplacement requiring secondary treatment, pin track infection that would have been treated with antibiotics and usually early wire removal, and CRPS (referred to as reflex sympathetic dystrophy in the majority of older studies). We didn't include finger stiffness during cast use. However, finger stiffness after cast removal at six weeks in Stoffelen 1998 and persistent stiffness at 12 weeks in Mardani 2011 would likely to have required additional intervention in the form of physiotherapy and is included. Hence, complications that would typically have received substantive treatment are reported as follows.
Redisplacement resulting in secondary treatment (consisting of reduction or reduction and K‐wire fixation) only occurred in the nonsurgical treatment group: 0/275 versus 38/316 (12%); RR 0.09, 95% CI 0.03 to 0.27; 6 studies. The percentages ranged from 3.3% (3/85) in Verhulst 1990 to 75% (15/20) in Rodriguez‐Merchan 1997. This last trial included intra‐articular fractures only.
Self‐evidently, pin tract infection requiring antibiotics and, where reported, wire removal only occurred in the pinning group: 22/285 (7.7%) versus 0/286; RR 8.33, 95% CI 2.54 to 27.28; 7 studies). The percentages ranged from 0% in Gupta 1999 to 15% (15/99) in Mardani 2011. There was one case of persistent deep infection in Zyluk 2007,
The incidence of RSD was similar in the two groups: 13/123 versus 17/125, RR 2.08, 95% CI 0.40 to 10.85; 4 studies. There was a high incidence (12/48 versus 15/50) of short‐term RSD in Stoffelen 1998; this persisted in six participants overall (4/48 versus 2/50). There was one case of shoulder hand syndrome in Shankar 1992.
More participants in the cast only treatment group had persistent finger stiffness at six weeks in Stoffelen 1998 and at three months in Mardani 2011: 29/147 versus 53/149; RR 0.52, 95% CI 0.36 to 0.76; 2 studies.
Other pinning ‐ or typically surgery‐related complications were: K‐wire migration, which occurred in 5/70 (7%) participants (data from Gupta 1999 and Verhulst 1990); one case of K‐wire removal due to metal sensitivity in Shankar 1992; three cases of stab wound infection in Verhulst 1990; and six cases of superficial radial nerve injury, one of which persisted, in the pinning group of Stoffelen 1998. Stoffelen 1998 suggested these nerve injuries may have resulted from injuries caused by the radially‐sited pin. There was no report of tendon rupture or injury in either treatment group; which was confirmed in two studies (Azzopardi 2005; Rodriguez‐Merchan 1997).
Although there were fewer complications relating to the median nerve, such as carpal tunnel syndrome, reported in the pinning group, the evidence is very low‐quality downgraded two levels for serious risk of bias and two levels for very serious imprecision: 3/161 versus 11/202; RR 0.53, 95% CI 0.19 to 1.46; 5 studies.
Secondary outcomes
Return to work or former activities
All 43 participants, who were all heavy labour workers in Delgado 2009 returned to the same work activity. Two people, both in the nonsurgical treatment group, out of 11 former labourers in Rodriguez‐Merchan 1997, did not return to their former work (Analysis 1.4). The evidence for this outcome is very low quality, downgraded two levels for very serious risk of bias and two levels for very serious imprecision. Verhulst 1990 (number of participants to which this applied unknown, maximum 130) reported duration of incapacity to work did not differ between the two groups at the 5% significance level. Wong 2010, which measured functional status relating to employment as part of the Mayo Wrist Score, found no difference (reported P = 0.914) between the two groups in this item at final (mean 19.5 months) follow‐up; it was clear that the majority of the 60 participants had 'returned to regular employment' or equivalent.
1.4. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 4 Non return to work (labourers).
Grip strength
Grip strength was reported in seven trials but no pooling of data was possible (Azzopardi 2005; Delgado 2009; Rodriguez‐Merchan 1997; Shankar 1992; Stoffelen 1998; Wong 2010; Zyluk 2007). Zyluk 2007 reported medians and ranges for global grip strength and global grip strength relative to the other wrist at 1.5, 3 and 6 months for 60 participants; see Analysis 1.5; Analysis 1.6. Zyluk 2007 indicated that the difference in grip strength, which favoured the pinning group, was not statistically significant at 1.5 months (median grip strength 7.2 kg versus 6.0 kg; relative grip strength 31% versus 27%) but was at six months (median grip strength 18.2 kg versus 16.0 kg; relative grip strength 72% versus 61%); very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision). Results at six months also favoured the pinning group in Shankar 1992 which reported on the numbers of participants with under half grip strength at six months: 0/23 versus 11/22; RR 0.04, 95% CI 0.00 to 0.67; very low‐quality evidence downgraded two levels for very serious risk of bias, one level for serious imprecision and one level for indirectness in terms of the outcome measure (Analysis 1.7). At 12 months, grip strength presented as a percentage of the grip strength of the unaffected arm, was similar in Azzopardi 2005 (mean 77%, SD 21% versus 72%, SD 17%, reported P = 0.54) and greater in Rodriguez‐Merchan 1997 (85% versus 65%; P not reported). Both Delgado 2009 (43 participants) and Stoffelen 1998 (98 participants) reported similar grip strength results in the two groups at 12 months without providing data. Wong 2010 found no significant differences at follow‐up averaging 19.5 months: MD ‐0.50 kg; CI ‐3.90 to 2.90; very low‐quality evidence downgraded one levels for serious risk of bias and two levels for very serious imprecision (Analysis 1.8).
1.5. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 5 Global grip strength (kg).
| Global grip strength (kg) | |||
|---|---|---|---|
| Study | Pinning | Cast only | Statistically significant? |
| At 1.5 months | |||
| Zyluk 2007 | Median, range, no. 7.2 kg, 0‐56 kg, 30 |
Median, range, no. 6.0 kg, 2‐15 kg, 30 |
No |
| At 3 months | |||
| Zyluk 2007 | Median, range, no. 15.0 kg, 10‐46 kg, 30 |
Median, range, no. 10.0 kg, 2‐20 kg, 30 |
Yes |
| At 6 months | |||
| Zyluk 2007 | Median, range, no. 18.2 kg, 12‐48 kg, 30 |
Median, range, no. 16.0 kg, 6‐26 kg, 30 |
Yes |
1.6. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 6 Global grip strength [probably] relative to other side (%).
| Global grip strength [probably] relative to other side (%) | |||
|---|---|---|---|
| Study | Pinning | Cast only | Statistically significant? |
| At 1.5 months | |||
| Zyluk 2007 | Median, range, no. 31%, 0‐86%, 30 |
Median, range, no. 27%, 10‐55%, 30 |
No |
| At 3 months | |||
| Zyluk 2007 | Median, range, no. 61%, 41‐88%, 30 |
Median, range, no. 41%, 7‐86%, 30 |
Yes |
| At 6 months | |||
| Zyluk 2007 | Median, range, no. 72%, 57‐94%, 30 |
Median, range, no. 61%, 30‐124%, 30 |
Yes |
1.7. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 7 Under half grip strength at 6 months.
1.8. Analysis.
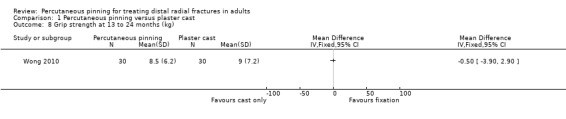
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 8 Grip strength at 13 to 24 months (kg).
Range of motion
At six months, all components of wrist movement were significantly better in the pinning group in Gupta 1999 (Analysis 1.9), as were the components of wrist movement in Shankar 1992 (statistically significant differences were reported for extension and ulnar deviation). In contrast, Venkatesh 2016 found no differences between the two groups; the differences in the means for all six components of wrist motion ranged from 1 to 3.5 degrees. Zyluk 2007 reported there were no statistically significant differences between the two groups in flexion and extension range of motion and pronation and supination range of motion at 6 weeks, 3 and 6 months; Analysis 1.10 showed the available median and range data for 6 weeks and 6 months. At 12 months, Azzopardi 2005 reported no statistically significant differences in the components of wrist movement, except for ulnar deviation (93% versus 76% of normal; reported P = 0.009). The overall range of wrist motion at 12 months was reported to be better in the pinning group in Rodriguez‐Merchan 1997 (80% versus 60% of normal). Delgado 2009 stated that flexion‐extension arc loss compared with the contralateral wrist was less in the nonsurgical group at 3 and 6 months, but similar in both groups at 12 months; no supporting data were available in the abstract report. There were no differences in range of motion between treatment groups at longer follow‐up in Wong 2010 (mean 19.5 months) (Analysis 1.11). Verhulst 1990 also reported that range of motion along the three axes showed no statistically significant differences between the two groups at two years. Overall, the quality of the evidence for range of motion was very low at short‐, medium‐ and long‐term follow‐up; this reflected the serious limitation in the data including downgrading one level for serious risk of bias and two levels for very serious imprecision.
1.9. Analysis.
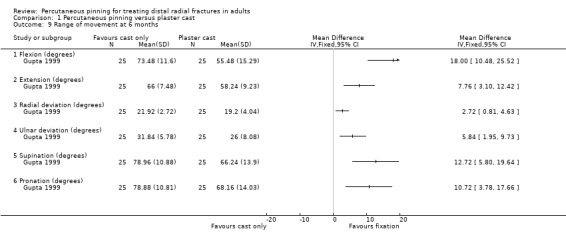
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 9 Range of movement at 6 months.
1.10. Analysis.
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 10 Range of motion (data from Zyluk 2007).
| Range of motion (data from Zyluk 2007) | |||
|---|---|---|---|
| Study | Pinning | Cast only | Statistically significant? |
| Flexion and extension at 1.5 months | |||
| Zyluk 2007 | Median, range, no. 88º, 64º to 126º, 30 |
Median, range, no. 81º, 17º to 130º, 30 |
No |
| Flexion and extension at 6 months | |||
| Zyluk 2007 | Median, range, no. 143º, 109º to 190º, 30 |
Median, range, no. 133º, 81º to 170º, 30 |
No |
| Pronation and supination at 1.5 months | |||
| Zyluk 2007 | Median, range, no. 110º, 11º to 164º, 30 |
Median, range, no. 117º, 9º to 160º, 30 |
No |
| Pronation and supination: at 6 months | |||
| Zyluk 2007 | Median, range, no. 142º, 84º to 164º, 30 |
Median, range, no. 138º, 61º to 175º, 30 |
No |
1.11. Analysis.
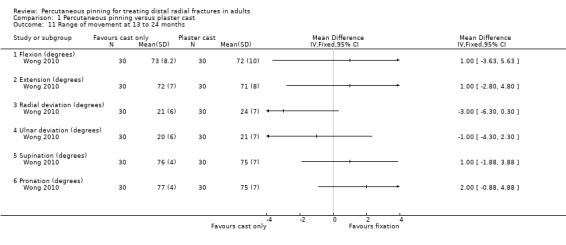
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 11 Range of movement at 13 to 24 months.
Health‐related quality of life
The evidence for this outcome, reported by two trials, is very low‐quality, being downgraded one level for serious risk of bias and two levels for very serious imprecision. Azzopardi 2005 found no significant differences between the two groups in the physical or mental score domains of the Short‐Form 36 at four months (Analysis 1.12). Wong 2010 reported no between‐group differences in WHOQoL‐BREF scores, either relating to a single question on the participant's view of their quality of life (Analysis 1.13) or for four domains (physiological, psychological, social, environment), at an average of 19.5 months follow‐up. Azzopardi 2005 reported there were no significant differences in either unilateral (reported P = 0.43) or bilateral (reported P = 0.74) activities of daily living scores at 12 months.
1.12. Analysis.
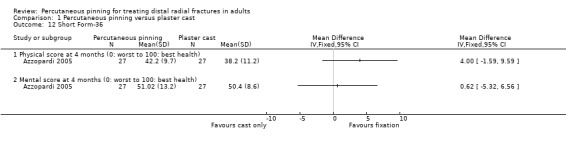
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 12 Short Form‐36.
1.13. Analysis.
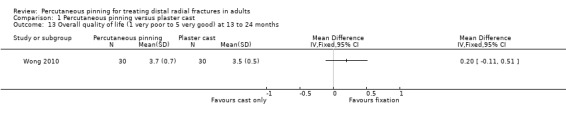
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 13 Overall quality of life (1 very poor to 5 very good) at 13 to 24 months.
Pain
There were no statistically significant differences between the two groups in pain scores at one year (0.7 versus 1.2 on a 0 (no pain) to 10 (worst pain) point scale; reported P = 0.16) in Azzopardi 2005 or in the numbers of participants with occasional pain at long‐term follow‐up in Rodriguez‐Merchan 1997 (2/20 versus 4/20; RR 0.50, 95% CI 0.10 to 2.43; very low‐quality evidence downgraded one level for serious risk of bias and two levels for very serious imprecision; Analysis 1.14). Although Delgado 2009 implied comparable pain scores at 12 months (2.9 in the surgery group versus 2.1 in the nonsurgical treatment group), these data were incomplete and there were no details of the pain scale used. Wong 2010, which measured pain as part of the Mayo Wrist Score, found no difference (reported P = 0.873) between the two groups in this item at final (mean 19.5 months) follow‐up; the mean scores implied that the majority appeared to have mild or occasional pain over the past four weeks.
1.14. Analysis.
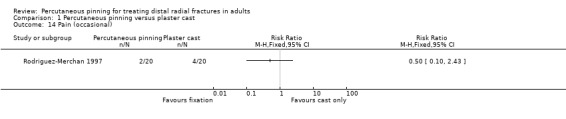
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 14 Pain (occasional).
Composite and mainly physician‐reported functional scoring systems
The composite and mainly physician‐reported functional scoring systems used by five of the trials (Gupta 1999; Rodriguez‐Merchan 1997; Shankar 1992, Venkatesh 2016; Zyluk 2007) were all derived from Gartland and Werley's scheme (Gartland 1951), which also rates deformity and various complications. Fewer participants had fair or poor outcome in the pinning group based on these measures: 29/128 versus 52/127; RR 0.53, 95% CI 0.37 to 0.78; 255 participants, 5 studies; I2 = 13%; very low‐quality evidence downgraded two levels for very serious risk of bias, one level for serious imprecision and one level for serious indirectness given nonvalidated categorisation of the score (Analysis 1.18). Stoffelen 1998 and Wong 2010 used the Cooney or Mayo Wrist Score, which included pain, functional status (employment), range of motion and grip strength (range 0 to 100, higher scores mean better outcome). There was minimal difference between the two groups in the numbers with a fair or poor outcome in Stoffelen 1998; very low‐quality evidence for the same reasons as above (Analysis 1.18). Wong 2010 reported the Mayo wrist score at final follow‐up (mean 19.5 months; range 13 to 24 months) and also found no difference between the treatment groups; very low‐quality evidence downgraded one level for risk of serious bias and two levels for very serious imprecision (Analysis 1.19).
1.18. Analysis.
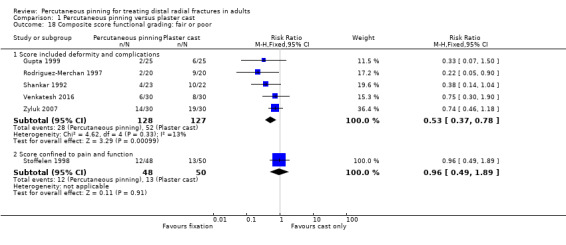
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 18 Composite score functional grading: fair or poor.
1.19. Analysis.
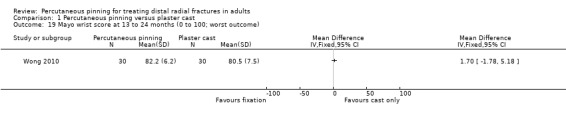
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 19 Mayo wrist score at 13 to 24 months (0 to 100; worst outcome).
Satisfaction
Mardani 2011 reported on patient‐reported satisfaction at 12 weeks based on criteria set in the Saito score. They found more participants in the surgery group had an excellent outcome (no pain, no disability and no limitation of motion): 93/99 versus 81/99, RR 1.15, 95% CI 1.03 to 1.28; very low‐quality evidence downgraded two levels for very serious risk of bias; two levels for serious imprecision and one level for serious indirectness relating to the interpretation of the outcome (Analysis 1.15). None reported a poor outcome. Wong 2010 found no difference between the two groups in patient satisfaction at 13 to 24 months; very low‐quality evidence downgraded one level for serious risk of bias; two levels for serious imprecision and one level for serious indirectness relating to the crude categorisation of the outcome measure (Analysis 1.16).
1.15. Analysis.
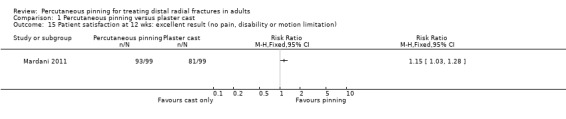
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 15 Patient satisfaction at 12 wks: excellent result (no pain, disability or motion limitation).
1.16. Analysis.
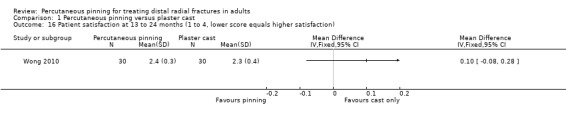
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 16 Patient satisfaction at 13 to 24 months (1 to 4, lower score equals higher satisfaction).
Radiographic outcomes
Four trials (Azzopardi 2005; Gupta 1999; Venkatesh 2016; Wong 2010) presented complete results for dorsal angulation, radial angulation and radial length and two trials (Venkatesh 2016; Wong 2010) for ulnar variance. Although we considered it is useful to present anatomical results, we decided against pooling these data because of the likely differences in their measurement. The data suggest better anatomical results in favour of the pinning group for all four measures but the differences in radiographic measurements recorded in most cases were small, may reflect measurement error, and are of uncertain clinical significance. Mardani 2011 and Rodriguez‐Merchan 1997 did not report on measurable radiographic parameters. Healing of all fractures was confirmed in Gupta 1999 and Shankar 1992 and is likely in the other trials. Loss of reduction and need for secondary procedures due to loss of position is captured under complications, as detailed above (Analysis 1.3).
Recording of anatomical results in the remaining trials was incomplete. Delgado 2009 reported that "radiographic correction" was anatomical in 38% of cases in the nonsurgical group and 80% in the pinning group. Losses in radial angulation, radial length and dorsal angulation from the reduced position were reported as being significantly greater in the nonsurgical treatment group in Shankar 1992. In contrast, Stoffelen 1998 did not report superior anatomical results for the Kapandji's pinning group. Whilst radial shift (lateral) was less in the pinning group (mean values: 0.5 mm versus 1.5 mm), radial shortening was greater (mean values: 2 mm versus < 1 mm). Stoffelen 1998 claimed that these differences were statistically significant. Stoffelen 1998 made no specific mention of the apparently greater, though indicated as not statistically different, difference in dorsal angulation between the two groups. Zyluk 2007 reported comparable results for the comparison groups in dorsal angulation, radial length and radial inclination at final follow‐up at six months.
Resource outcomes
None of the trials reported on economic outcomes. Mardani 2011 reported a mean number of postoperation visits was 4.4 in the pinning group and 3.6 in the cast‐only group. However, this was out of five scheduled visits and so can be considered more a measure of compliance.
Any method of percutaneous pinning versus any other method of percutaneous pinning
Six trials made one of five comparisons of different methods of percutaneous wiring. Kapandji intrafocal pinning featured in five trials (Fikry 1998; Gravier 2006; Lenoble 1995; Saddiki 2012; Strohm 2004) but not in Shannon 2003. None of the comparisons were sufficiently similar to pool data across comparisons.
Kapandji intrafocal pinning (two or three wires) versus trans‐styloid fixation (two wires)
In Lenoble 1995, Kapandji fixation followed by immediate mobilisation was compared with trans‐styloid fixation followed by around six weeks of plaster cast immobilisation in 120 people. However, results were presented only for the 96 participants available for follow‐up at 24 months. As well as not reporting a PROM for function, return to prior activities, quality of life, or patient satisfaction, Lenoble 1995 did not provide standard deviations for continuous outcomes such as grip strength. There was very low‐quality evidence for all reported outcomes; this was downgraded two levels for very serious risk of bias and one level for serious imprecision, reflecting the small sample size and low number of events.
Lenoble 1995 did not report the numbers of participants who had one or more complications. There was a higher number of reported complications in the Kapandji group, but all of the confidence intervals for the individual complications crossed the line of no effect (Analysis 2.1). In particular, there were more Kapandji group participants with persistent symptoms related to the superficial radial nerve (8/54 versus 3/42; RR 2.07, 95% CI 0.59 to 7.34); an identical result applied for bone‐scan confirmed RSD. Lenoble 1995 suggested that the radial nerve complications were related to Kirschner wire removal; these are closer to the sensory branches of the radial nerve in Kapandji pinning. The redisplaced fracture in the trans‐styloid fixation group was treated with an external fixator. There were no cases of tendon or vascular injury or median nerve dysfunction. All four cases of pin‐track infection were superficial.
2.1. Analysis.
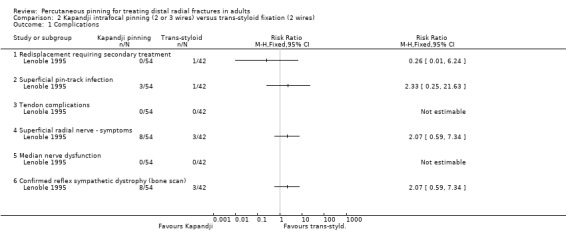
Comparison 2 Kapandji intrafocal pinning (2 or 3 wires) versus trans‐styloid fixation (2 wires), Outcome 1 Complications.
Lenoble 1995 reported there was no statistically significant difference between the two groups in either grip or pinch strength, both presented as a percentage of the uninjured wrist (one‐year results: mean grip strength 84% versus 83%; mean pinch strength 89% versus 87%). More participants of the Kapandji group were reported to suffer pain but the difference between the groups in the mean visual analogue scale values was not statistically significant (two‐year results: 7.6 versus 6.9 on a 0 (no pain) to 100 (unbearable pain) point scale). Wrist mobility was reported to be significantly better in the Kapandji group up until the removal of the K‐wires at six to eight weeks (all values as percentage of uninjured arm, flexion: 59% versus 46%; extension: 58% versus 44%; radial deviation: 60% versus 38%; ulnar deviation: 61% versus 52%; pronation: 80% versus 62%; supination: 76% versus 64%) but not subsequently. Lenoble 1995 considered that better early range of motion results for the Kapandji group related to the early mobilisation of this group and occurred at the cost of increased pain.
Similar long‐term results for anatomical outcomes in the two groups were evident from graphs presented in the trial report. The mean ulnar variance, which was positive in both groups, was approximately one millimetre greater in the Kapandji group. One of the six over‐reduced (by 15 degrees in the anterior direction) fractures in the Kapandji group developed a more extreme anterior tilt whilst the two over‐reduced fractures in the trans‐styloid group did not worsen. The four cases of DISI (dorsal intercalated segment instability) and seven cases of VISI (volar intercalated segment instability) were reported as being "evenly" distributed through the two groups.
Modified Kapandji (dorsal Kapandji wires and trans‐styloid fixation) pinning versus Kapandji intrafocal pinning
This comparison was made in Gravier 2006, a quasi‐randomised trial that reported only on radiological outcomes in 78 participants at a follow‐up of 45 days. Although the authors claimed final follow‐up was a minimum of six months, these results were not reported. The available complete radiographic data (dorsal and radial angulation) are presented in Analysis 3.1; this is very low‐quality evidence, downgraded two levels for very serious risk of bias and two levels for very serious imprecision, reflecting the small sample size and wide confidence intervals. Although the authors reported on "variance radio‐ulnaire", this was in degrees whereas ulnar variance is typically reported in millimetres; as we are uncertain what this measurement represents, we have not presented these data. Gravier 2006 also reported percentage loss of anatomical reduction at 45 days but did not provide sufficient data for us to evaluate this intermediate outcome.
3.1. Analysis.
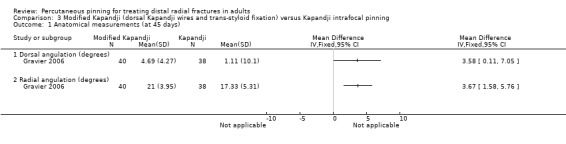
Comparison 3 Modified Kapandji (dorsal Kapandji wires and trans‐styloid fixation) versus Kapandji intrafocal pinning, Outcome 1 Anatomical measurements (at 45 days).
Kapandji intrafocal pinning (three wires) versus Py's isoelastic pinning (two wires)
Table 5 provides details of the two trials that compared Kapandji intrafocal versus Py's isoelastic pinning in 207 people with dorsally displaced distal radius fractures (Fikry 1998; Saddiki 2012). As well as demographic differences between the two trials, it is notable that Saddiki 2012 included both intra‐ or extra‐articular dorsally displaced distal radius fractures, while Fikry 1998 excluded "comminuted" fractures.
4. Kapandji intrafocal pinning (3 wires) versus Py's isoelastic pinning (2 wires).
| Trial | Participants | Fracture typea | Kapandji pinning | Py's pinning | Cast use and wire removal |
| Fikry 1998 | 110, demographics and results only for 88 who completed follow‐up: 25% female, mean age 34 years, range 18‐66 | Dorsally displaced distal radius fractures, with or without separation of ulnar styloid fragment. Comminuted fractures were excluded. Classification not given | 3 K‐wires inserted at fracture site. Image intensifier used | 2 K‐wires inserted through radial epiphysis, across fracture and along medullary canal up to radial head. Image intensification not used: grinding of wire against the cortical bone used to determine wire placement | Wires cut below the skin, hidden ends covered by a small bore drainage tube. Forearm plaster cast for 4 weeks; received instruction for shoulder and finger exercises. Pins removed at around 8 weeks under local or regional anaesthesia |
| Saddiki 2012 | 97, 86% female, mean age 63 years | Extra‐articular fractures: associated with fracture of the ulnar styloid process = Gerard‐Marchand (GM) fracture, not associated with fracture of the ulnar styloid process = Pouteau‐Colles (PC) fracture. Intra‐articular fractures: simple: fractures with postero‐medial fragment (PMF), complex: T‐shaped intra‐articular fractures (sagittal and/or frontal)b . |
3 K‐wires used to perform intrafocal pinning. After intraoperative reduction by close manipulation, the first K‐wire was introduced laterally while the two others were inserted postero‐laterally and postero‐medially, at a minimum 40º angle relative to the vertical axis of the radius. | 2 K‐wires featuring a spatulated tip for easier progression along the medullary canal of the radius were introduced into the epiphysis of the distal radius after reduction by close manipulation, the first one being inserted from the tip of the radial styloid and the second one from inside the Lister’s tubercle. The entry point of each K‐wire was radiographically controlled. Retrograde pinning of the radius was then performed up to the sub‐chondral bone of the radial head. | Plaster splint for 3‐weeks immobilisation, then a removable splint in all cases allowing the start of daily self‐rehabilitation exercises. Pin removal after 6 weeks postoperatively |
a Available information reported b Results reported per fracture category (incomplete)
Fikry 1998 compared Kapandji intrafocal pinning with Py's isoelastic pinning in 110 people but presented results only for the 88 participants available at 27 months follow‐up. Saddiki 2012 reported on 97 participants of whom 64 (66%) were available at final follow‐up of 12 months. The results for the two trials are presented together in the analyses but pooling was possible for individual complications only. Notably, both trials recorded components of the Jakim radio‐clinical score (Jakim 1991), but data for pooling were not available from Saddiki 2012, which reported incomplete data by fracture subgroup. Additionally, there were contradictions between text and table for the Jakim 1991 scores in the report of Fikry 1998: thus, we have presented two sets of values in the analyses showing mean Jakim scores: the first set of values are those in the report; in the second set, the results for the two groups are reversed, based on the assumption that the rows in the table were presented in the reverse order. Neither trial reported return to former activities, quality of life, separate pain measures or patient satisfaction.
There is very low‐quality evidence, downgraded two levels for very serious risk of bias and one level for serious imprecision reflecting a wide confidence interval, from Saddiki 2012 of no or very little difference between the two groups in function assessed using the DASH (0 to 100; higher scores mean worse function): MD 4.80, 95% CI ‐5.40 to 15.00; 64 participants (Analysis 4.1). Given the estimated condition‐specific minimum detectable change for DASH is 14, these results indicated no clinically important difference between the two groups (Kleinlugtenbelt 2018). A similar finding of no between‐group difference applied to the subjective (pain and function) scores, rated with the Jakim scoring system, for Fikry 1998 as shown in Analysis 4.2.
4.1. Analysis.
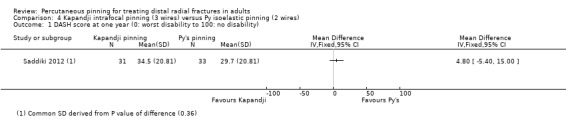
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 1 DASH score at one year (0: worst disability to 100: no disability).
4.2. Analysis.
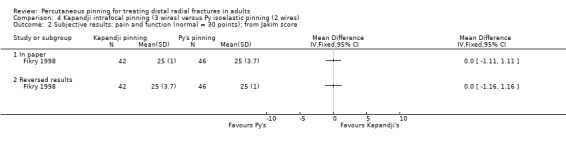
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 2 Subjective results: pain and function (normal = 30 points); from Jakim score.
Neither trial presented the numbers of participants who had one or more complications and clear unit of analysis issues, as exemplified in Saddiki 2012 which implied that pin migration had been sometimes associated with superficial infection and prevented the estimation of this outcome from the sum of the individual complications. Nonetheless, there were more complications after Kapandji intrafocal pinning with very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision (Analysis 4.3). In particular, there were more Kapandji group participants with secondary displacement (8/73 versus 3/79, RR 2.9, 95% CI 0.81 to 10.46) and wire displacement (10/73 versus 0/79, RR 11.87, 95% CI 1.57 to 89.61). Similar numbers of participants suffered tendon rupture or RSD in the two groups. Fikry 1998 reported all participants with ruptured tendons had successful secondary tendon transfer, and all those with RSD required prolonged rehabilitation. Saddiki 2012 did not report on the subsequent treatment of these complications but reported a remedial operation for severe malunion in one person, with group not stated. Fikry 1998 suggested that the excess of secondary fracture displacements and wire displacements in the Kapandji group was linked to posterior comminution of the original fracture. The iatrogenic fracture in the Py's pinning group of Fikry 1998 was successfully treated by a plate.
4.3. Analysis.
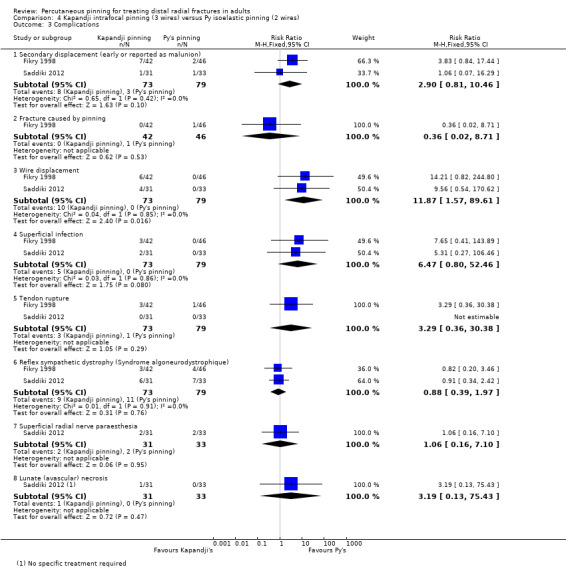
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 3 Complications.
Data unavailability and discrepancies means that we are very uncertain of the relative effects of the two pinning methods on clinical outcomes. Mobility and grip strength were reported as part of the objective category (mobility, grip strength and deformity) of the Jakim scoring system in Fikry 1998, which found higher scores of uncertain clinical relevance in the Kapapandji pinning group (according to the table) or in the Py pinning group (according to their conclusions). The clinical importance of the 3‐point difference shown in Analysis 4.4 is unknown. Saddiki 2012 reported there was no statistically significant between‐group difference in grip strength at one year: 75.6% versus 79.8% of the healthy side, reported P > 0.05. Range of motion results were reported only by type of fracture in Saddiki 2012, which did not provide denominators.
4.4. Analysis.
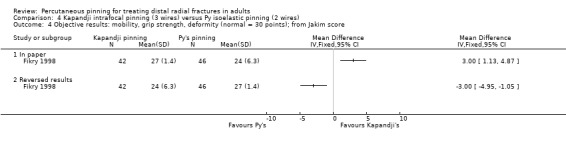
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 4 Objective results: mobility, grip strength, deformity (normal = 30 points); from Jakim score.
Fikry 1998 reported the overall (combined function and radiological results) Jakim scores (0 to 100; best outcome), which favoured either the Kapandji or the Py pinning group; again, the clinical importance of the 6‐point difference is not known (Analysis 4.5). Fikry 1998 also graded overall outcome into four categories (excellent, good, fair, poor). There was very low‐quality evidence, downgraded two levels for very serious risk of bias and two levels for very serious imprecision of little between‐group difference, in unsatisfactory outcome, based on the numbers of participants with fair or poor overall outcome: 7/42 versus 6/46; RR 1.28, 95% CI 0.47 to 3.50 (Analysis 4.6).
4.5. Analysis.
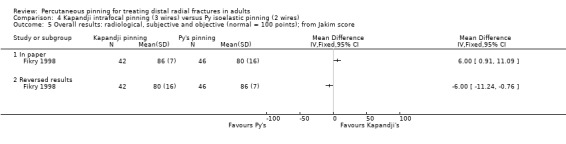
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 5 Overall results: radiological, subjective and objective (normal = 100 points); from Jakim score.
4.6. Analysis.
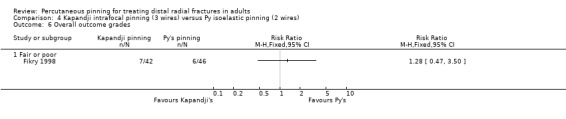
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 6 Overall outcome grades.
Anatomical results including dorsal and radial angulation, radial length, articulation of the radio‐ulnar joint, incongruity ("step‐off") and signs of arthritis were scored according to Jakim's scoring system (Jakim 1991). Fikry 1998 claimed a superior result for the Py's pinning group, but again the mean values for the radiological section presented in the report showed the converse (Analysis 4.8). As noted above, more secondary displacement of fractures occurred in the Kapandji group (Analysis 4.3). Saddiki 2012 found small but unimportant differences between the two groups in radial and dorsal angulation at one year (Analysis 4.8).
4.8. Analysis.
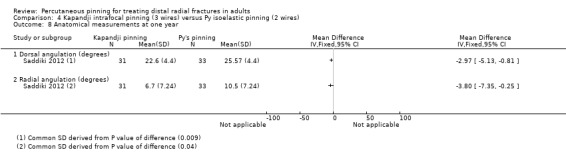
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 8 Anatomical measurements at one year.
Modified Kapandji intrafocal pinning (three wires) versus Willenegger pinning (two wires)
In Strohm 2004, modified Kapandji intrafocal pinning was compared with Willenegger pinning (two wires in the radial styloid) in 100 participants. The length of follow‐up was variable with a mean of 10 months (range six to 20). Nineteen participants were lost to follow‐up (two died) and only the remaining 81 were included by the authors in their analysis. Strohm 2004 did not report function using a PROM nor separate results for return to function, mobility, grip strength, quality of life, pain, satisfaction or radiographic findings. There was very low‐quality evidence for all reported outcomes; this was downgraded two levels for very serious risk of bias and one level for serious imprecision, reflecting the small sample size and low number of events. Additionally, the quality of the evidence for the Martini score was downgraded one level for serious indirectness in view of the difficulties in interpreting this nonvalidated composite measure.
Strohm 2004 reported that the modified Kapandji group had significantly higher modified Martini scores (this composite score included pain, subjective assessment, strength, work and sports, wrist and forearm mobility, radiological results and complications) at a median of 10 months follow‐up: 34 versus 28 on a scale 0 (worst) to 38 (best); reported P < 0.005). Strohm 2004 reported that 14 participants incurred complications but did not present separate data for the two groups. There were no significant differences between the two groups in the numbers of people with individual complications (Analysis 5.1). Two participants of the Willenegger group had an undefined "conversion procedure". Both cases of RSD resolved after treatment; and both cases of carpal tunnel syndrome were operated on. Strohm 2004 reported there was no significant difference between the two groups in operating times, but that imaging time was significantly less in the modified Kapandji group (89.5 versus 156 seconds; reported P < 0.004).
5.1. Analysis.
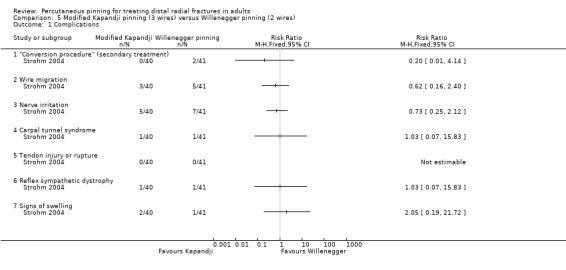
Comparison 5 Modified Kapandji pinning (3 wires) versus Willenegger pinning (2 wires), Outcome 1 Complications.
"Spring‐loaded intramedullary" pinning (not Py) versus "traditional transcortical" pinning (three wires used for both)
Shannon 2003 compared three wire pinning inserted either using "a novel spring‐loaded intramedullary technique" or in "the traditional transcortical fashion" in 46 participants, who were followed up for a minimum of six weeks. Return to prior activities, clinical outcomes, quality of life, pain or patient satisfaction were not reported. Based on an undefined "Wrist Fracture Score", Shannon 2003 reported only that functional outcome "was similar in both groups at 6 weeks". The only reported complications were two early fixation failures in the "spring‐loaded group", both in class AO C3 fractures: 2/24 versus 0/20; RR 4.60, 95% CI 0.23 to 90.84; very low‐quality evidence, downgraded two levels for very serious risk of bias and two levels for very serious imprecision (Analysis 6.1). Radiographic parameters (mean dorsal angle and mean loss of radial length) at six weeks were incompletely reported and could not be analysed.
6.1. Analysis.
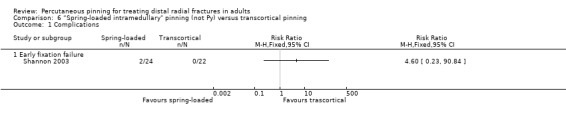
Comparison 6 “Spring‐loaded intramedullary" pinning (not Py) versus transcortical pinning, Outcome 1 Complications.
Any technique or type of material or device used for percutaneous pinning versus any other technique or type of material or device at surgery or postoperatively
Biodegradable pins or wires versus metal pins or wires
Table 6 provides brief details of the two trials that compared biodegradable pins with Kirschner wires in 70 people with extra‐articular or intra‐articular distal radial fractures (Casteleyn 1992; Korner 1999). Despite the differences in the pinning techniques used in the trials, the results for the two trials are presented together in the analyses but no pooling has been performed. Both trials were incompletely reported and neither recorded PROMS for function, return to former activities, clinical outcomes, quality of life, pain or patient satisfaction. Length of follow‐up was one year in Casteleyn 1992 with function data unavailable for 1 of 15 and range of motion or anatomical data for 2 of 15 participants from each comparison group at final follow‐up. Korner 1999 assessed 17 of 19 participants in the biodegradable pins group and 19 of 21 in the Kirschner wire group at a median of 25.4 months.
5. Biodegradable pins or wires versus metal pins or wires; participant characteristics and interventions.
| Trial | Participants | Fracture typea | Type of surgery | Biodegradable pinning | Metal pinning | Cast use and wire removal |
| Casteleyn 1992 | 30, 77% female; mean age 61, range 22 to 85 years | Extra‐ and intra‐articular; Frykman I, II, V, VI (involving the DRUJ)b | Closed reduction using finger traps. All fractures pinned percutaneously (stab incisions) using Kapandji's iintrafocal technique | Holes predrilled; 2 PGA (polyglycodic acid ‐ biodegradable) rods inserted and gently tapped home | Two 2 mm Kirscher‐wires inserted into fracture gap (one dorsally and the other laterally) and drilled into the opposite cortex of the radial shaft. Then cut to be under the skin | No cast applied. Wire removal at 6 weeks |
| Korner 1999 | 60, no other details | Extra‐ and intra‐articular; mention of AO A2, A3 and B1c | Mention of Willenegger: probably 2 pins placed through radial styloid and across fracture | Biodegradable pins | Kirschner wires | No details |
a Available information reported b DRUJ: distal radial ulnar joint c AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF)
In both trials, there was an excess of complications in the biodegradable pinning group (see Analysis 7.1); very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision. Difficulties during pin insertion in Korner 1999 (8/19 versus 0/21; RR 18.70, 95% CI 1.15 to 303.59) contributed to the longer duration of the operation in the biodegradable pinning group (mean duration: 40.5 versus 23.5 minutes). Secondary surgery was performed for the two tendon ruptures (Kirschner wiring group) and for one of the two cases of carpal tunnel syndrome; both of the latter were attributed to swelling brought on by the biodegradable rod. Additionally, one person in the biodegradable pinning group in Korner 1999 had a secondary displacement requiring revision. Painful scars, sinus (a cavity in bone or other tissue) formation and severe osteolytic reactions (9/15 versus 0/15; RR 19.00, 95% CI 1.20 to 299.63) were also related to the degradation and resorption of the biodegradable material in Casteleyn 1992. A histological examination carried out in one participant showed a nonspecific foreign‐body reaction with abundant giant cells. Most of the severe osteolytic reactions were most obvious around three to six months postoperatively, but had resolved or regressed by one year. Casteleyn 1992 pointed out that the main putative advantage of biodegradable implants, namely, that they do not need to be removed, was offset by the "management cost and inconvenience of the late inflammatory reactions".
7.1. Analysis.
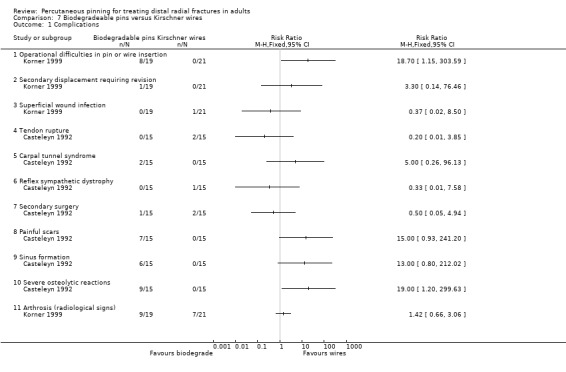
Comparison 7 Biodegradeable pins versus Kirschner wires, Outcome 1 Complications.
Casteleyn 1992 assessed functional ability using an own physician‐reported scoring system that was not described in detail. Functional recovery was reported to have been quicker in the Kirschner wiring group of Casteleyn 1992, although the eventual difference at one year between the two groups (based on a 5‐point scale) was reported as not being statistically significant, and no participant had any restriction in activities of daily living (very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious indirectness).
Range of motion was satisfactory in both groups; with no statistically significant difference between them (see Analysis 7.2), with very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision. Korner 1999 did not report whether the greater loss in range of motion in the biodegradable pinning group was statistically significant: mean loss compared to contralateral wrist: 30 versus 20 degrees (extension/flexion); 35 versus 30 degrees (pronation/supination).
7.2. Analysis.
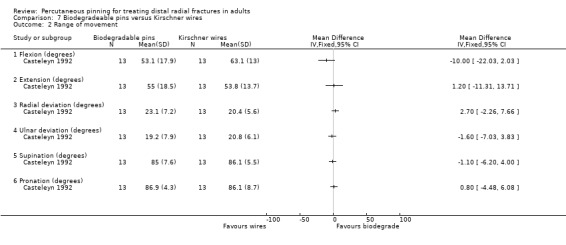
Comparison 7 Biodegradeable pins versus Kirschner wires, Outcome 2 Range of movement.
Final anatomical results were considered satisfactory in both groups in Casteleyn 1992, which found no statistically significant differences between the two groups (seeAnalysis 7.3).
7.3. Analysis.
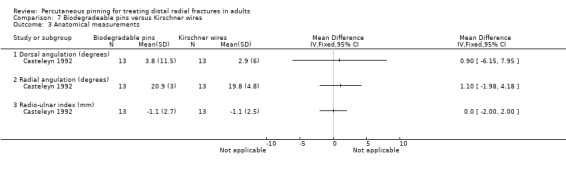
Comparison 7 Biodegradeable pins versus Kirschner wires, Outcome 3 Anatomical measurements.
Pinning with buried wires versus exposed percutaneous wires
Table 7 provides brief details of the three trials that evaluated the technique of burying wires versus leaving them exposed in 168 people with isolated distal radius fractures requiring Kirchner wire fixation (Hargreaves 2004; Murphy 2008; Waheed 2004). Details of antibiotic administration and wound care were provided only in Hargreaves 2004 (see Characteristics of included studies). Length of follow‐up was at around six weeks, coinciding with the return to hospital for wire removal in all three trials. None of the studies reported on participant flow, although it is probable that all participants were followed up. All three trials were incompletely reported and none recorded function, return to former activities, clinical outcomes or quality of life.
6. Buried versus exposed percutaneous wires: participants and interventions.
| Trial | Participants | Fracture typea | Type of surgery | Buried wiring | Exposed wiring | Cast use and wire removal |
| Hargreaves 2004 | 56, 45% female; mean age 35, range 7 to 81 years | 54 closed and 2 open | Closed reduction and 2 crossed wires. Open reduction in 9 cases | Buried deep to the skin; closed with sutures. Wire ends bent over | Exposed, skin released round wire. Wire ends bent over | Forearm cast applied. Removal at 6 weeks |
| Murphy 2008 | 60, no other details | Displaced | No details | Buried | Exposed | No details. Probably removed at 6 weeks |
| Waheed 2004 | 52; 73% female; mean age 57, range 19 to 84 years | No details | No details | Buried | Wires left protruding | Cast and wires removed mean 5.8 weeks at outpatients |
a Available information reported
The available data for complications are presented in Analysis 8.1, with very low‐quality evidence downgraded two levels for very serious risk of bias and one level for serious imprecision. No pooling was possible for overall or individual outcomes. Hargreaves 2004 found more wires were infected in the exposed wires group (2/27 versus 10/29; RR 0.21, 95% CI 0.05 to 0.89); Murphy 2008 reported slightly more superficial infection in the exposed group at two weeks but not at six weeks; and Waheed 2004 reported there was no difference in infection between the two groups (reported P = 0.15). Although Waheed 2004 did not state to which group(s) the four infections occurred, exploratory analysis based on the P value indicated that it was likely that all four probably occurred in the exposed wires group. Hargreaves 2004 reported that all five wires removed early were in the exposed wires group. Murphy 2008 reported one case of superficial radial nerve damage that followed removal of a buried wire; and confirmed the absence of tendon injury.
8.1. Analysis.
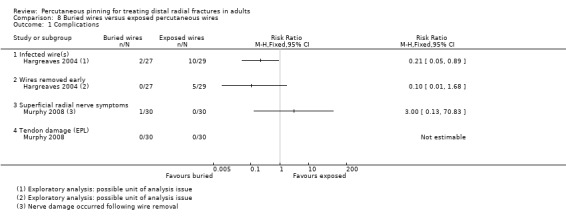
Comparison 8 Buried wires versus exposed percutaneous wires, Outcome 1 Complications.
More intensive treatment for removing buried wires were reported in Hargreaves 2004 (need for removal of wires in theatre: day surgery versus clinic) and Waheed 2004 (need for local anaesthesia): 44/52 versus 6/56; RR 7.36, 95% CI 3.54 to 15.31; 108 participants; 2 studies; low‐quality evidence downgraded two levels for serious risk of bias (Analysis 8.2).
8.2. Analysis.
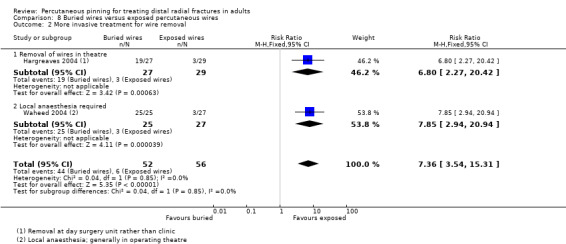
Comparison 8 Buried wires versus exposed percutaneous wires, Outcome 2 More invasive treatment for wire removal.
Waheed 2004 reported, without providing data, no between‐group differences in pain during fixation or in patient satisfaction.
Any type or duration of postoperative immobilisation versus any other type or duration of immobilisation including none
Cast immobilisation of wrist in dorsiflexion versus palmar flexion after intrafocal pinning
Snow 2007 evaluated the effect of immobilising the wrist in 30 degrees dorsiflexion versus 30 degrees palmar flexion after closed manipulation and percutaneous Kirschner wire fixation in 63 participants whose fracture had redisplaced within 14 days. Snow 2007 did not report on the number of participants assessed at final follow‐up at 17 weeks; it is probable though all were present except for three participants excluded post‐randomisation because of bilateral fractures. This trial did not report a PROM for function, return to prior activities, quality of life, pain or patient satisfaction. There was very low‐quality evidence for all reported outcomes; this was downgraded two levels for very serious risk of bias and one level for serious imprecision, reflecting the small sample size and wide confidence intervals.
The available data for complications are presented in Analysis 9.1. The only notable difference between the two groups was the greater number of referrals for physiotherapy in the palmar flexion group (2/27 versus 10/33; RR 0.24, 95% CI 0.06 to 1.02). There were high rates of malunion (defined as > 10 degrees of dorsal angulation, > 15 degrees of volar tilt or > 3 mm of radial shortening compared with the opposite side) in both groups: 5/33 versus 4/27; RR 0.98, 95% CI 0.29 to 3.29. One participant with malunion in the palmar flexion group had distal radial osteotomy at 17 months. Overall, there was very low‐quality evidence of no or minimal between‐group differences at 6 or 17 weeks follow‐up in grip strength (Analysis 9.2), pinch strength (Analysis 9.3), and range of motion (Analysis 9.4), all which were reported as percentages of the contra‐lateral side; or the time taken in seconds to perform the Roylan nine‐peg dexterity test (Analysis 9.5). There was no evidence of differences between the two groups in the four reported radiographic outcomes (dorsal angle, radial angle, radial height and length) at 17 weeks as shown in Analysis 9.6.
9.1. Analysis.
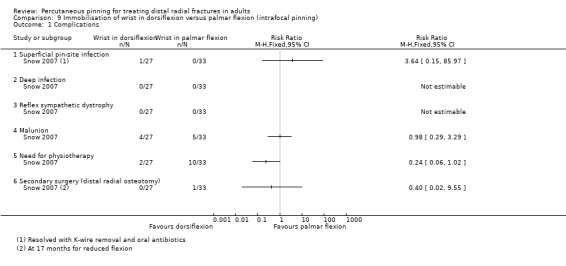
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 1 Complications.
9.2. Analysis.
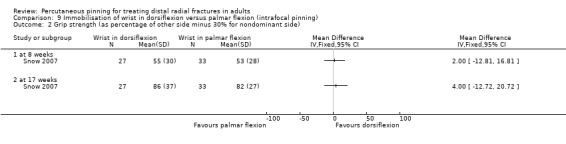
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 2 Grip strength (as percentage of other side minus 30% for nondominant side).
9.3. Analysis.
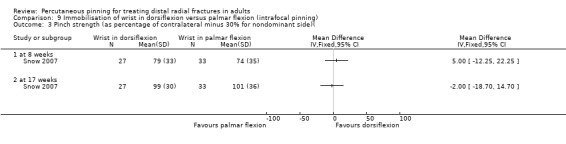
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 3 Pinch strength (as percentage of contralateral minus 30% for nondominant side)(.
9.4. Analysis.
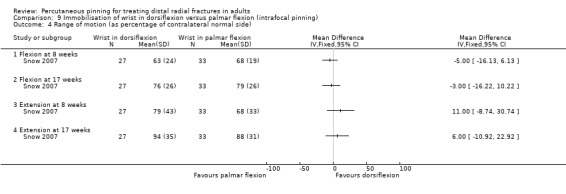
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 4 Range of motion (as percentage of contralateral normal side).
9.5. Analysis.
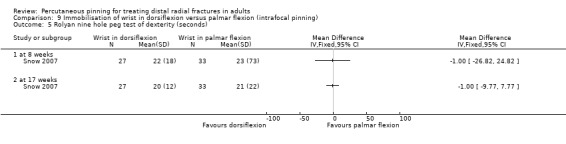
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 5 Rolyan nine hole peg test of dexterity (seconds).
9.6. Analysis.
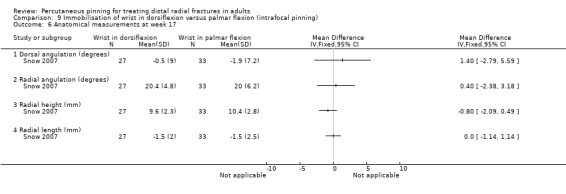
Comparison 9 Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning), Outcome 6 Anatomical measurements at week 17.
Duration of postoperative immobilisation after percutaneous pinning
Table 8 provides brief details of the three trials that compared one week plaster cast immobilisation versus either four weeks in 50 people in Soleiman pour 2011 or six weeks in 120 people in Allain 1999 and Milliez 1992. Length of follow‐up was one year in Allain 1999 and three months for Milliez 1992 and Soleiman pour 2011. None of the trials reported a PROM for function or quality of life. Data for pooling were available for a few individual complications. Given the clear clinical heterogeneity, particularly in terms of the different methods of percutaneous pinning (trans‐styloid fixation in Allain 1999, Kapandji intrafocal pinning in Milliez 1992 and undetermined method in Soleiman pour 2011), and differences in duration and type of immobilisation, we pooled results for these outcomes on an exploratory basis.
7. Duration of postoperative immobilisation after percutaneous pinning: participants and interventions.
| Trial | Participants | Fracture typea | Type of surgery | Short‐term immobilisation | Longer‐term immobilisation | Immobilisation type |
| Allain 1999 | 60, 75% female; mean age 55, range 18‐87 years |
AO types A2, A3, C1, C2b | Trans‐styloid fixation: 2 wires removed at 45 days |
1 week | 6 weeks | Short arm circular cast |
| Milliez 1992 | 60, 73% female, age 55, range 19‐91 years |
Closed displaced intra‐ and extra‐articular | Kapandji intrafocal pinning, 3 wires removed at 6 weeks |
1 week | 6 weeks | Plaster splint |
| Soleiman pour 2011 | 50, 46% female; no details on age | displaced metaphyseal distal radius fractures and non‐comminuted intra‐articular fractures |
Not specified, Kapandji mentioned | 1 week | 4 weeks | Not specified |
a Available information reported b AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF)
The available data for complications are presented in Analysis 10.1. Although overall complications were more frequently present in the early mobilisation groups of each trial, unit of analysis issues precluded meta‐analysis of this outcome. There is very low‐quality evidence, downgraded two levels for very serious risk of bias and two levels for very serious imprecision reflecting very few events, for little or no difference between the two groups in the incidence of individual complications: displaced wires or pins (6 cases in all); treatment failure (3 cases); superficial pin‐track infection (4 cases); tendon rupture (6 cases); nerve damage (4 cases); RSD (4 cases); and non‐union (3 cases) (see Analysis 10.1). In Allain 1999, surgery was necessary for the one person in the 6‐week group who had two tendon ruptures, and an anterior approach for removal of a migrated wire was required in another person in the same group. Three of the four reported cases of persistent radial nerve paraesthesia or hypoaesthesia appeared only after Kirschner wire removal. In Milliez 1992, all reported complications occurred in the early mobilisation group with the exception of one case of RSD. One of the five instances of wire displacement in the early mobilisation group in Milliez 1992 was associated with a tendon rupture, one resulted from poor technique (wiring was done by "junior" operators) and two from osteoporotic bone. In Milliez 1992, the wrists of three people with "treatment failure" were immobilised (one due to the need for tendon repair and two due to the risk of secondary displacement). Soleiman pour 2011 reported no cases of pin displacement, similar rates of pin site infection and non‐union, being the only study to report any cases of the latter.
10.1. Analysis.
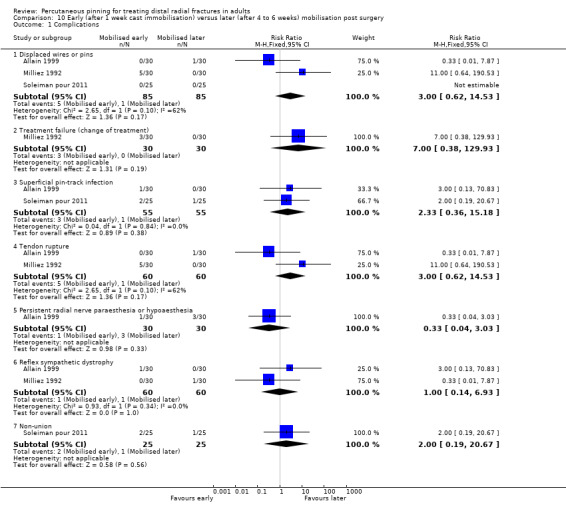
Comparison 10 Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery, Outcome 1 Complications.
Allain 1999 reported no significant between‐group differences in the mean duration of sick leave (which was seven weeks) for the 39 of 60 trial participants who worked, in the numbers reporting functional discomfort during domestic chores (14 of 60), or in the numbers of "sportsmen" with residual discomfort (3 of 22). One participant from the 6‐week group (who developed RSD) was the only one of 19 working people who had not returned to work by three months in Milliez 1992. Without providing numerical data for the 60 participants followed up, Soleiman pour 2011 stated that participants of the shorter immobilisation group returned to their daily activities "faster", with no differences at three months.
Very few data were available for presentation in the analyses of the clinical outcomes of grip strength and range of movement. At final follow‐up, one year in Allain 1999 and three months in Milliez 1992, the differences in grip strength, which favoured the early mobilisation group in both trials, were reported not to be statistically significant: 25 kg versus 21 kg in Allain 1999; and 55% versus 44% of the normal side in Milliez 1992. At one year follow‐up, the mean values for the six parameters of range of movement in Allain 1999 (60 participants) were between 0 and 4 degrees higher in the early mobilisation group; only the difference in ulnar deviation was reported as being statistically significant (39 versus 37 degrees; reported P = 0.03). The clinical importance of these small differences is uncertain. Milliez 1992, with 60 participants, found no statistically significant differences in flexion (74% versus 77% of the normal side) or extension (72% versus 74% of the normal side), although the results were marginally better in the group immobilised for six weeks; pronation and supination were reported as unrestricted. Soleiman pour 2011 reported there was no significant difference in "range of motion improvement" between the two groups at final follow‐up at three months but provided data only for six weeks (1.5 months) that illustrate a faster restoration of range of movement in the early mobilisation group (Analysis 10.2).
10.2. Analysis.
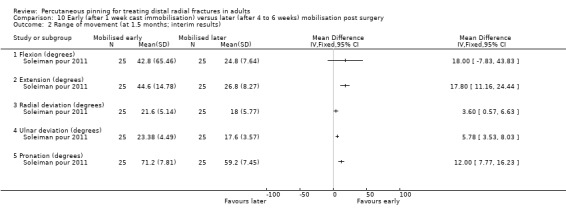
Comparison 10 Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery, Outcome 2 Range of movement (at 1.5 months; interim results).
In Milliez 1992, as calculated from percentages in the trial report, fewer people in the early mobilisation groups had some degree of pain at final follow‐up (4/27 versus 10/30; RR 0.44, 95% CI 0.16 to 1.25; very low‐quality evidence downgraded two levels for very serious risk of bias and two levels for imprecision (Analysis 10.3)). Allain 1999 reported no statistically significant difference between groups in the mean pain scores (13 versus 12.5 (15 = no pain)) and use of analgesics.
10.3. Analysis.
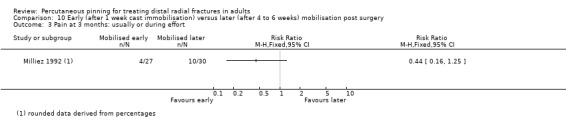
Comparison 10 Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery, Outcome 3 Pain at 3 months: usually or during effort.
Based on data from Milliez 1992, there was very low‐quality evidence of no or little between‐group difference in the numbers of people who were disappointed with their outcome: 3/30 versus 1/30; RR 3.00, 95% CI 0.33 to 27.23 (Analysis 10.4).
10.4. Analysis.
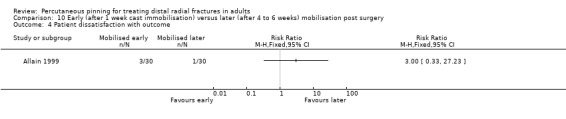
Comparison 10 Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery, Outcome 4 Patient dissatisfaction with outcome.
Similar radiological results in the two treatment groups were reported by Allain 1999 and Milliez 1992. As it is likely that the unusually small "standard deviations" provided in Allain 1999 were standard errors, the results for dorsal and radial angulation and radial length, with standard deviations calculated from the supposed standard errors, are shown in Analysis 10.5; these results are compatible with the claim of no statistical significance. The three radiological parameters listed in Milliez 1992 are defined in Characteristics of included studies. However, the results at three months seem to be for dorsal angulation (volar tilt: 6.9 versus 6.5 degrees), radial inclination (21.4 versus 21.3 degrees) and ulnar variance (1.4 mm versus 1.1 mm).
10.5. Analysis.
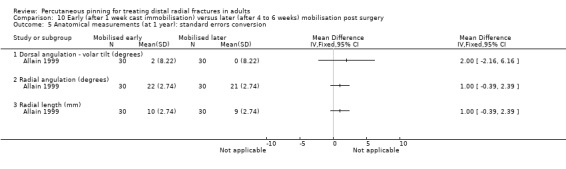
Comparison 10 Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery, Outcome 5 Anatomical measurements (at 1 year): standard errors conversion.
Discussion
This review covers one of the most commonly used surgical interventions for one of the most commonly sustained adult fractures. This review update doubled the number of trials from 13 in 2007 to 26. These involved a total of 1946 generally female and older adults with dorsally displaced and potentially or evidently unstable distal radial fractures.
Summary of main results
The 10 different treatment comparisons made across 26 trials are summarised below.
Percutaneous pinning versus plaster cast immobilisation alone
This comparison was tested in 11 heterogeneous trials involving 917 participants in dorsally displaced distal radius fractures. The majority of participants were female in six trials and male in two trials. As shown in Table 3, the trials employed different pinning techniques and durations of immobilisation. Nine trials (776 participants) applied across‐fracture pinning, Stoffelen 1998 used Kapandji's triple intrafocal technique with early mobilisation in 98 participants, and Delgado 2009 (43 participants) provided no details of the wiring technique. The anaesthetic/analgesic technique in the nonsurgical arm also varied, with four trials employing general anaesthesia. Two trials specified that casting was performed under fluoroscopic control; the latter was not reported in the remaining nine trials.
The main evidence for this comparison is summarised in Table 1. All quality of the evidence for all outcomes was rated at very low, invariably reflecting serious risk of bias and imprecision, which indicates our uncertainly in the results. Thus, we are uncertain if percutaneous pinning compared with plaster cast alone makes any difference to patient‐reported function, measured using the DASH, at six weeks or six months (incomplete data from one trial, 60 participants). There is no evidence available for patient‐reported function in the long term, that is over 12 months follow‐up.
There are no data available for overall numbers of participants incurring complications or complications requiring substantive treatment. The evidence for individual complications is also incomplete. We considered the evidence for four common individual complications requiring substantive treatment: treated redisplacement, pin tract infection, Complex Regional Pain Syndrome Type 1 (CRPS‐1), and persistent finger stiffness. Redisplacement resulting in secondary treatment, reported in six studies, occurred on average in 12% (range 3.3% to 75%) of participants treated nonsurgically in cast alone, whereas pin tract infection, requiring antibiotics and, often, early wire removal, reported in seven studies, occurred on average in 7.7% (range 0% to 15%) of participants treated by percutaneous pinning. There was one reported case of persistent deep infection.
We are uncertain whether pinning affects the incidence of RSD (or CRPS‐1); although reported in four studies, the results were dominated by the very high (28%) incidence of reported short‐term RSD in one study. Although two studies found finger stiffness after cast removal was less common after surgery (20% versus 36%), the severity and treatment implications were not stated. Reported events for other outcomes were generally few and reported in single trials only. These were mainly surgery‐related. The exception was complications relating to the median nerve, such as carpal tunnel syndrome, which were fewer in the pinning group but overall uncommon, amounting to 14 cases in 383 participants (3.7%) reported in five studies.
Based on incomplete data or qualitative statements from only four studies, we are uncertain of the effects of pinning on grip strength at 12 months. We are uncertain if percutaneous pinning compared with plaster cast alone makes any difference to patient‐reported quality of life at four months; this was assessed in one study only.
Similar uncertainties, and the underlying incompleteness, apply to the evidence for other outcomes reported in one or more of the 11 studies: return to work; grip strength at different follow‐up times; range of movement at different follow‐up times; pain; composite and mainly physician‐reported functional scoring systems such as that of Gartland and Werley; patient satisfaction; and final radiographic outcomes.
Any method of percutaneous pinning versus any other method of percutaneous pinning
Kapandji intrafocal pinning (two or three wires) versus trans‐styloid fixation (two wires)
This comparison was tested in one study of 120 participants that reported results for the 96 people available for follow‐up. Those in the Kapandji pinning group were mobilised immediately whereas those in the trans‐styloid group had cast immobilisation for around six weeks. No PROM outcome for function, return to former activities, quality of life or patient satisfaction was recorded and all clinical and anatomical outcomes were incompletely reported. The number of participants with one or more complications was not available. The very low‐quality evidence and few events from one trial only means that we are uncertain of the finding of a slightly higher number of cases of superficial radial nerve symptoms and of bone‐scan confirmed RSD in the Kapandji pinning group. There is very low‐quality evidence of no difference between the two pinning methods at 12 months in grip and pinch strength, in pain or in radiological outcomes.
Kapandji intrafocal pinning versus modified Kapandji (dorsal Kapandji wires and trans‐styloid fixation)
This comparison was tested in one study(Gravier 2006) of 85 participants, that reported only on limited radiographic outcomes at 45 days post‐surgery. Any reported differences in measured dorsal and radial angulation were small and not clinically meaningful. This very low‐quality evidence gives no basis for drawing conclusions on the relative effects of the two modes of fixation.
Kapandji intrafocal pinning (three wires) versus Py's isoelastic pinning (two wires)
This comparison was tested in two poorly reported studies, with marked differences in their population characteristics. Outcome data were available for a maximum of 152 participants of the 207 recruited into the two trials. Neither trial reported return to former activities, pain, quality of life or patient satisfaction and pooled data were available only for a few individual complications. There is very low‐quality evidence of no difference between the two groups in function at 12 months measured via the DASH or at an average of 27 months measured via the subjective component of the Jakim score. Although the number of participants with one or more complications could not be determined, there is very low‐quality evidence of a higher incidence of some complications, such as secondary displacement and wire migration, after Kapandji pinning. There is incomplete and, for one trial, contradictory evidence on clinical outcomes, primarily grip strength and range of motion.
Modified Kapandji intrafocal pinning (three wires) versus Willenegger pinning (two wires)
This comparison was tested in one incompletely reported study for which data for presentation in the analyses were available only for individual complications in 81 participants. There is very low‐certainly evidence of little difference between the two pinning methods in individual complications. The incomplete data for a nonvalidated composite score hinders interpretation of the reported superior mean score in the modified Kapandji pinning group at final follow‐up, based on very low‐quality evidence.
"Spring‐loaded intramedullary" pinning (not Py) versus "traditional transcortical" pinning (three wires used for both)
This comparison was tested in one study involving 46 participants that was reported in a conference abstract only. We reluctantly included this study as it met the inclusion criteria but, in particular, there was incomplete information on the interventions and outcome measurement, and the usable data for the few reported outcomes were limited to two early fixation failures (8.3%) in the spring‐loaded intramedullary pinning group. Aside from the very low quality of the available evidence, there is nothing that we could conclude about this comparison.
Any technique or type of material or device used for percutaneous pinning versus any other technique or type of material or device at surgery or postoperatively
Biodegradable pins or wires versus metal pins or wires
The use of biodegradable pins was tested in two small trials using two very different pinning techniques. There was insufficient evidence to detect a difference in anatomical and clinical outcomes between the two groups and neither trial recorded PROMs for function, return to former activities, clinical outcomes, quality of life, pain or patient satisfaction. Although there is very low quality of evidence, what is apparent are the extra demands at surgery of insertion of biodegradable pins, and the excess of complications associated with biodegradable material. Thus, the recommendation, expressed by both studies, not to use biodegradable pins for these fractures (in adults) seems justified. This conclusion could be have been moderated by further evidence from a multicentre trial involving 115 participants in 'Studies awaiting assessment' (Russe 2000); however, further information about this study, which was last reported 19 years ago in a series of abstracts, is unlikely to now emerge.
Pinning with buried wires versus exposed percutaneous wires
The technique of burying wires versus leaving them exposed was tested in three small poorly‐reported trials involving 168 participants. Our attempts to obtain more information, including the full reports of Murphy 2008; and Waheed 2004, were unsuccessful. None of the trials reported on function, clinical outcomes, return to former activities or quality of life. We are uncertain of the finding of a lower risk of superficial infection with buried wires reported in one trial; and possible in the other two trials (very low‐quality evidence). There is low‐quality evidence that burying of wires may be associated with a higher risk of requiring more invasive treatment for wire removal, such as it being performed in an operating theatre rather than a clinic, or requiring the use of local anaesthetic generally in an operating theatre.
Any type or duration of postoperative immobilisation versus any other type or duration of immobilisation including none
Cast immobilisation in wrist dorsiflexion versus wrist palmar flexion after intrafocal pinning
This comparison was tested in one study reporting limited results for 60 people whose fracture had redisplaced within 14 days and treated via intrafocal pinning. Snow 2007 did not report on function, return to prior activities, quality of life, pain or patient satisfaction. This study found more participants were referred for physiotherapy in the palmar flexion group (very low‐quality evidence), but otherwise there is very low‐quality evidence of little or no differences between the two groups for other complications. The same applied to clinical outcomes (grip and pinch strength, range of wrist motion, time to complete a dexterity test) at 6 and 17 weeks and radiographic outcomes at 17 weeks. Overall, we are uncertain of the findings of this incompletely reported trial; this uncertainty means that no recommendation for or against placing the wrist in dorsiflexion can be made.
Duration of postoperative immobilisation after percutaneous pinning
Three small incompletely‐reported trials, two of which were quasi‐randomised, compared cast immobilisation for one week (early mobilisation) versus four or six weeks after percutaneous pinning in 170 people. While the pinning technique was undefined in one trial, the other two trials used very different pinning techniques, respectively trans‐styloid fixation and Kapandji intrafocal fixation, an important source of clinical heterogeneity. There are insufficient data for subgroup analysis to check this, and indeed only data for individual complications were available for pooling, which was done on an exploratory basis in light of the clinical heterogeneity. None of the three trials reported a PROM for function or quality of life.
The very low‐quality evidence means we are uncertain of the potential finding of more complications following early mobilisation. The latter is based primarily on a higher number of individual complications only, in Milliez 1992, where there were five cases (17% of 30) each of displaced wires and of tendon rupture, and three cases (10%) of treatment failure in the early mobilisation group after Kapandji pinning. There is very low‐quality evidence of no clinically important between‐group differences in grip strength or range of movement at final follow‐up (3 or 12 months). There is incomplete and very low‐quality evidence of little or no between‐group difference in return to work or former activities. There is very low‐quality evidence of little or no between‐group difference in pain, participant dissatisfaction with outcome or anatomical results at final follow‐up.
Any method or timing of pin or wire removal versus any other method or timing of pin or wire removal including no removal
There are no included trials on this topic.
Overall completeness and applicability of evidence
Completeness of the evidence
This updated review includes 26 trials that recruited 1946 people with distal radius fractures. This is double the number of trials and just over double the number of participants since the first version of the review (Handoll 2007). Despite this increase, the incompleteness of the available evidence in terms of outcomes reported and available data remains noteworthy. In particular, there were minimal data available for the six included studies reported in conference abstracts only; one of these provided the sole evidence for one comparison (Shannon 2003). We were unsuccessful in obtaining further information on these six trials and, overall, our requests for additional unpublished information were successful for two trials only (Gupta 1999; Zyluk 2007). The included trials tested 10 treatment comparisons, with 11 trials comparing pinning with nonsurgical treatment alone in 917 people. The maximum number of participants in any pooled analysis for this review was 591 (64% of 917) for redisplacement requiring secondary treatment for the pinning versus nonsurgical treatment comparison. Notably, only 3 of 26 trials reported on a validated patient‐reported outcome measure of function (DASH) and, of these, only Saddiki 2012 (88 participants) provided data that could be presented in a forest plot (Analysis 4.1).
Results of our formal assessment of applicability
To inform consideration of applicability of the evidence from individual trials, we provide quite extensive details in the Characteristics of included studies on the study populations and interventions; as well as providing summaries of these in additional tables by comparison. Additionally, Table 9 shows our assessments for each trial of four aspects of relevance to ascertaining external validity: definition of the study population, description of the interventions, definition of the main outcome measures and length of follow‐up. Incomplete descriptions of study inclusion criteria (15 trials) and interventions (8 trials) are clearly unhelpful. It is notable that two trials where the study population was not clearly defined ('No') and five of the six trials where the interventions were not sufficiently described ('No') were for trials that were only reported in conference abstracts. The main outcome measures were sufficiently described in only nine trials. In eight trials, only three of which were reported in conference abstracts only, the description of the main outcomes was considered wholly insufficient ('No') to be certain what was being measured. The eight trials that had only short‐term follow‐up, thus within three months of randomisation, were considered not to have appropriate timing of outcome measurement. This is particularly the case for five trials that reported outcomes at around six weeks, corresponding to the time of wire removal (Gravier 2006; Hargreaves 2004; Murphy 2008; Shannon 2003; Waheed 2004).
8. Assessment of items relating to applicability of trial findings.
| Study ID | Clearly defined study population? | Interventions sufficiently described? | Main outcomes sufficiently described? | Appropriate timing of outcome measurement? (Yes = ≥ 12 months) |
| Allain 1999 | Yes | Yes | Partial: Inadequate definition of discomfort during activity; and CRPS‐1c | Yes: 12 months |
| Azzopardi 2005 | Yes | Yes | Yes | Yes: 12 months |
| Casteleyn 1992 | Partial: Insufficient information on exclusions | Yes | Partial: Poor description of functional grading tool | Yes: 12 months |
| Delgado 2009a | Partial: “Heavy‐labour workers with unstable fractures of distal radius.” Only description of participants | No: No details of type of wiring or cast or duration of immobilisation | Partial: Some outcomes types reported, not defined | Yes: 12 months |
| Fikry 1998 | Yes: However, a young population. | Yes | No: Not defined, just reported. | Yes: 20 months minimum |
| Gravier 2006 | Partial: Insufficient information on displacement criteria for surgery. | Yes | No: Radiological outcomes only; lack of clarity over one outcome | No: 45 days (data for) |
| Gupta 1999 | Partial: Colles radius fracture in the skeletally mature | Yes | Partial: Inadequate description of assessment of outcomes, including complications. Crude categorisation of functional scores | Partial: 6 months |
| Hargreaves 2004 | Partial: No clear information on indications for surgery. | Yes | Yes | No: 6 weeks |
| Korner 1999a | No: Distal radius fracture only. | No: Composition of bioabsorbable pins not given; no detail on modification to Wellenegger technique | No: Not defined, just reported. Inadequate descriptions of redisplacement and arthrotic findings | Partial: although median follow‐up was 25.4 months, some participants may not have been followed up for 12 months. |
| Lenoble 1995 | Yes | Yes | Yes | Yes: 24 months |
| Mardani 2011 | Partial: Not explicitly dorsally displaced fractures though implied by the study context i.e. reference to dorsal tilt and dorsal comminution | No: Inadequate information about the pin configuration, no broad description of the technique or approach used. Also no descriptors of cast positions or type or mode of application, bar “short” and "long arm cast”. No information as to if or when intraoperative imaging was used. Care provider was the same surgeon in both cases, assuming the lead surgeon but not clear | Partial: Primary outcome not determined. Lack of definition of finger stiffness. Loss to follow‐up taken to be a positive outcome, when 5 visits were scheduled for all participants. Failure of treatment (need for further intervention) not treated as an outcome parameter |
No: 3 months |
| Milliez 1992 | Yes | Partial: Not enough detail on early mobilisation | Partial: Inadequate description of CRPS‐1 and crude measure of pain. | No: 3 months |
| Murphy 2008a | Partial: “Patients with a distal radial fracture managed with percutaneous wire fixation and casting only.” Minimal information available. By deduction, probably adults | No: Interventions were only mentioned and not described. Care providers not defined | Partial: Outcome assessment appeared limited to K‐wire‐related complications – some detail provided on assessment | No: 6 weeks |
| Rodriguez‐Merchan 1997 | Partial: Other than fracture type, no exclusion criteria although age range provided. | Yes | Partial: Inadequate description of assessment of clinical union, radiographic measures, and complications | Yes: 12 months |
| Saddiki 2012 | Yes | Yes | Yes | Yes: 12 months |
| Shankar 1992 | Partial: Intra‐articular fractures but limited description of population. | Yes | Yes: Descriptions given of outcome measures, although crude measurement of subjective outcomes within composite outcome measure | Partial: 6 months |
| Shannon 2003a | No: “Patients with unstable fractures of the distal radius were entered into the study.” No report of exclusion criteria or description of fracture type |
No: Minimal information on intervention technique | No: No definition of outcome measures | No: 6 weeks |
| Snow 2007 | Yes | Yes | Yes: Main outcome criteria (radiographic) were clearly defined | Partial: 17 weeks (4.25 months) |
| Soleiman pour 2011 | Partial: No indication of participant age. No indication of type of pinning | Partial: No detail on method(s) of pinning nor immobilisation; no identification of care providers | Partial: Insufficient definitions provided for outcomes; but only part translation obtained | No: 3 months |
| Stoffelen 1998 | Yes | Yes | Yes: Descriptions given of outcome measures, although crude measurement of subjective outcomes within composite outcome measure | Yes: 12 months |
| Strohm 2004 | Partial: Colles type and AOb classification but no exclusions | Yes | Partial: Inadequate description of assessment of outcomes, including complications | Partial: range 6 to 20 months |
| Venkatesh 2016 | Partial: Not explicitly dorsally displaced fractures though implied by the study context i.e. reference to Colles' fracture, application of Colles' cast and restoration of volar tilt | Yes | Yes | Partial: 6 months |
| Verhulst 1990a | Partial: Colles' radius fracture only | Partial: No information on postoperative care or type of immobilisation | Partial: Inadequate descriptions of outcome assessment | Yes: 2 years |
| Waheed 2004a | Partial: “Patients with a distal radial fracture managed with percutaneous wire fixation and casting only.” | No: Intervention not described | No: No definition of outcome measures | No: Most likely up to 6 weeks |
| Wong 2010 | Yes | Yes | Yes | Yes: minimum 13 months |
| Zyluk 2007 | Partial: Inclusion criteria inadequately defined: “displaced isolated fractures”. Exclusion criteria listed: “… excluded people who did not meet the prerequisites, i.e. they had a fracture without displacement, open, with associated injuries of other structures, or did not agree to participate in the study”. | Yes | No: "The rate of secondary displacement was considered a primary outcome measure.” Secondary displacement not defined, some participants with secondary displacement excluded from analysis (if they required further surgery within 2 weeks) whereas others were analysed (if treated operatively and did not require further surgery). | Partial: 6 months |
a Reported in conference abstracts only b AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF) c CRPS‐1: Complex Regional Pain Syndrome type 1
Applicability of the evidence
Population
Where stated, the predominantly female sex of most trial populations indicates these were generally representative of the people who sustain these injuries in industrialised societies. Some exceptions include those trials which included more male participants, such as Delgado 2009 which focused on heavy labour workers in Spain. Assessment of applicability based on the fracture population was less straightforward, although it seems likely that the fractures were predominantly dorsally displaced. The variety of fracture classification systems, with associated issues of reliability and validity, complicates this area (Jupiter 1997). For example, the two fracture classifications used by trials in this review (the AO and Frykman) place different emphases on various fracture patterns and anatomical components. Studies have revealed unsatisfactory interobserver reliability and intraobserver reproducibility for both classification systems (Andersen 1996; Kreder 1996b), and neither was useful for predicting clinical outcome (Flinkkila 1998). Four trials in this review stipulated criteria for anatomical displacement of the fracture for trial entry. However, Kreder 1996a found quite broad margins of error ("tolerance limits") for anatomical measurements in general. This, along with other factors, such as variations in anatomical reference points, again hinders treatment comparison, both in terms of defining the population and when used as criteria for determining secondary displacement. A recent Delphi study including 43 expert surgeons indicated some agreement on radiological thresholds for surgical intervention; these were greater in people over 75 years (Johnson 2019a). However, pre‐injury function was the most important factor influencing the surgeon's decision, which is harder to assess in this review.
Fracture instability was the inherent or explicit criterion for many of the included trials, but it is not established how best to predict this. A study of 4024 patients concluded that patient age, metaphyseal comminution of the fracture and ulnar variance were the most important factors in predicting instability of distal radial fractures (MacKenney 2006). This unvalidated prognostic model is too complex for use in routine clinical practice (Downing 2008). Ultimately, a direct association between radiological parameters and patient‐rated outcome has yet to be established (Downing 2008; Johnson 2019b).
Interventions
Where details are provided, the variation in the interventions for the different comparisons is evident from the Additional Tables. For the pinning versus cast‐only comparison, this variation is notable not only for the primary interventions but also the co‐interventions, where described. While we anticipate most interventions are available and in use somewhere in the world, likely exceptions in terms of general applicability are the spring‐loaded intramedullary pinning technique tested in Snow 2007, the pinning technique described in Wong 2010, and the use of biodegradable pins or wires. Usually cast immobilisation was applied to both groups for comparable durations, around five to eight weeks, of the trials in this comparison. Notable exceptions were the pinning group of Wong 2010, where there was immediate mobilisation of the pinning group with a removable palmar splint used for resting, and Stoffelen 1998 where cast immobilisation was one week in the pinning group compared with six weeks in the nonsurgical group.
Outcomes
Although some improvement was notable in more recently conducted trials, overall there was inadequate assessment of outcome, particularly of patient‐reported function and long‐term outcome. Our restructuring of Types of outcome measures in this update gives prominence to validated patient‐rated assessment of function instruments such as the Patient‐Rated Wrist Evaluation (PRWE) and the Disability of the Arm, Shoulder, and Hand questionnaire (DASH) (MacDermid 2000). This helped highlight the fact that only three trials reported this outcome. Trials, however, continue to report nonvalidated and composite outcome measures, such as those based on the Gartland and Werley scoring system (Gartland 1951), which combine aspects of function, pain, deformity and complications. These are particularly crude indicators of outcome and considerable caution is needed in their interpretation, even more so when the scores have been categorised.
Lastly, only two trials reported on health‐related quality of life.
Quality of the evidence
Where data were available, the quality of the evidence for all outcomes in all comparisons was either low or, most often, very low.
We downgraded all evidence for risk of bias, which we considered either serious or very serious. In particular, this reflects the susceptibility to performance and detection biases for most outcomes where blinding to the allocated intervention is not possible. Other common sources of bias were selection bias (allocation concealment was deemed secure in just one trial) and selective reporting bias.
For some outcomes, we downgraded the quality for indirectness. This was always in relation to outcome assessment or presentation, such as inappropriate categorisation of outcomes.
We did not downgrade specifically for inconsistency. This reflected on the lack of data for pooling of most outcomes and the fact that the quality of the evidence had already been downgraded to very low in relation to risk of bias and imprecision.
Downgrading for imprecision was common. This reflected wide confidence intervals but also the problems relating to small sample sizes and typically small numbers of events. The need to be wary of the results from small single‐centre trials was often behind a decision to downgrade for imprecision. However, we did not apply this indiscriminately but considered the results of single trials in the context of properties of the measure used, including distinguishing between binary and continuous measures, and if there was other corroborative evidence.
We did not downgrade for publication bias. Constructing funnel plots to explore the possibility of publication bias was not viable, given the few trials. This does not, however, mean that we can discount the possibility of publication bias. The fact that nearly a quarter of the included trials were incompletely published as conference abstracts also points to significant concerns about publication bias.
Potential biases in the review process
Before we started our review update, we revised our protocol methods, including restructuring Types of outcome measures and identifying the main outcomes for presentation in 'Summary of Findings' tables, in accordance with the contemporary standards. We have noted the main changes in methods from the last version of the review under Differences between protocol and review.
Although we avoided bias by prespecifying our main outcomes, the selection of these, which is formally but not rigidly set at a maximum of seven, was a source of extensive discussion. Missing a key outcome of primary importance to people incurring these fractures is of concern. Potentially, one such outcome is return to former activities. However, this outcome is problematic because it can be measured in various ways, such as return to former work, duration of sick leave, and return and time to return to former daily activity. Additionally, the activity in question may only apply to a subgroup of participants or be influenced by different factors and circumstance such as, for employment, insurance and workers' compensation schemes, statutory sick‐pay and transport. We thus decided against including this as a main outcome.
Our search for trials was comprehensive, and screening and study selection were performed systematically and according to protocol. As shown by our identification of six unpublished trials, the possibility of other unpublished trials, such as conference abstracts, remains. However, based on our experiences of failing to obtain more data on unpublished trials, we anticipate the potential contribution of these, if included, to the evidence is likely to be very limited and thus we do not think this is an important source of bias. Our attempts to obtain further data and information on published trials were also largely unsuccessful. It is inevitable then that our appraisal of the evidence is one that reflects the quality of the reporting and not necessarily the actual conduct of the included trials.
Whether from the lack of information on or clear differences in trial characteristics, some of our decisions to place trials under the same comparison and then, where outcomes were in common, pool data can be open to question. There were insufficient data for subgroup analyses to examine decisions based on key characteristics. As well as being cautious in our interpretation of the evidence, we have provided tables of key characteristics of participants and interventions for each comparison that allow the reader to judge the key areas of clinical heterogeneity. We were mindful of potential unit of analysis problems and took a cautious approach particularly when presenting data for the numbers of participants with any complication. The possibility that participants could have more than one complication is illustrated by Strohm 2004, which reported that 14 of the 81 participants at follow‐up incurred complications but provided data for individual complications by group; these totaled 29 complications.
Lastly, GRADE is a blunt instrument and the downgrading by whole levels can rapidly result in a very low‐quality rating depicting "uncertainty about the estimate". This indeed applied to the evidence available for our largest comparison (pinning versus cast‐only). Although it is possible that our judgements were too severe, it is also the case that definitive evidence was not available for any of the comparisons.
Agreements and disagreements with other studies or reviews
In Why it is important to do this review, we pointed to the findings of DRAFFT in support for updating this review on percutaneous pinning: "Contrary to the existing literature, and against the rapidly increasing use of locking plate fixation, this trial found no difference in functional outcome in patients with dorsally displaced fractures of the distal radius treated with Kirschner wires or volar locking plates. Kirschner wire fixation, however, is cheaper and quicker to perform." (DRAFFT 2014). DRAFFT was aimed at dorsally displaced distal radius fractures in adults whose fractures, including simple articular fractures, could be reduced by closed manipulation. Based on English Hospital Episode Statistics data, Costa 2016 reported that in the five years prior to DRAFFT, 75% of patients were treated with plate fixation versus 12% with percutaneous pinning. Subsequent to the trial publication, the reported figures were 48% having plate fixation and 42% having K‐wire fixation; the proportion of other procedures stayed the same.
DRAFFT was a pragmatic trial where the size and number of wires, the insertion technique and the configuration of wires was left to the discretion of the surgeon as per their normal practice. This reliance on methods which the surgeon is familiar and experienced with is appropriate where, as seen in our review, there is a lack of evidence to inform the selection of any particular pinning technique. DRAFFT 2014 provided information on number of wires used (predominantly two or three); wire size (almost all 1.6 mm); and the technique: Kapandji (27%); 'interfragmentary' (38%) and mixed techniques (35%). The latter data illustrate that both Kapandji and transfixation approaches are still distinct methods employed in UK practice, but also that surgeons often also use combination techniques.
We do not think it serves any purpose to compare this unique review with other systematic reviews covering the management of fractures of the distal radius. A summary of the characteristics of 41 systematic reviews published up to May 2017 on treatments for fractures of the distal radius provided in Table 1 of Belloti 2019, confirms that our review is the only one looking at all aspects of percutaneous pinning. Mellstrand Navarro 2019, a more recently published broad intervention systematic review that confines its focus to the 'elderly' (study populations of mean age 60 years), included only two trials comparing pinning versus plaster cast alone (Azzopardi 2005; Wong 2010).
Authors' conclusions
Implications for practice.
Overall, there is insufficient evidence from RCTs or quasi‐RCTs to inform on the role of percutaneous pinning or associated treatment decisions such as method of pinning, burying or not of wire ends, and wrist position and duration of immobilisation subsequent to pinning. In the context of this insufficiency, it is as ever important to note that the lack of evidence of an effect (or difference) should not be taken as evidence of no effect. The very low‐quality and incomplete nature of the evidence means it is uncertain whether pinning improves outcome compared with wrist immobilisation alone for many dorsally displaced fractures. Percutaneous pinning can help to maintain reduced positions and, therefore, reduce radiographic deformity as well as avoiding treatment failure leading to secondary fixation when compared with plaster cast immobilisation alone. However, there is no available evidence that this improves patient‐reported function and complications of percutaneous pinning are also frequent. Therefore, uncertainty remains about the indications for percutaneous pinning. This uncertainty applies to other comparisons. However, despite the very low quality of the evidence, we suggest that the excess of biodegradable pin‐related complications, coupled with the extra demands at surgery, are likely to outweigh the putative advantages of biodegradable pins compared with metal wires or pins.
Implications for research.
The need for high‐quality evidence, primarily from sufficiently powered multicentre randomised trials, to help address key treatment uncertainties about these fractures has been heeded since the last update of this review. The findings of the UK DRAFFT study (DRAFFT 2014) gave a new urgency for large multicentre RCTs comparing reduction and percutaneous pinning with reduction and plaster cast treatment alone. In the UK, this role is currently being fulfilled by DRAFFT 2, which recruited 506 participants by 27 March 2019 and, at the time of publication of this review, is completing collection of one‐year follow‐up data. Given this timing, we propose that it is appropriate to wait on the results of this trial as these, placed in the context of the trial inclusion criteria, are likely to help inform the direction of future research. This is a pragmatic trial, where the size and number of wires, insertion technique and configuration of wires was left at the discretion of the surgeon, as per their normal practice. A similar approach was taken in relation to immediate postsurgical care. Collection of data on the pinning techniques utilised is likely to reveal the extent of variation in practice in the UK, as well as those most commonly used. Given this timing, we propose that it is appropriate to wait on the results of this trial as these, placed in the context of the trial inclusion criteria, are likely to help further inform the direction of any future research. Nevetheless , this large study pertains to one country only, whereas practice also varies worldwide; contributions to the evidence base of this review from 11 countries reveals common areas of research uncertainty and interest, but also differences, perhaps reflecting differences in clinical practice. Analysis of the variation of practice from large prospective patient cohorts, such as fracture registries from differing settings, can also contribute to the identification of priority questions for the management of distal radius fractures, including in relation to percutaneous pinning. Furthermore, though large, well‐conducted randomised clinical trials are best placed to established the comparative effectiveness of interventions, other questions such as those pertaining to the prediction of instability, prognosis and long‐term outcomes, are suited to alternative research study designs.
The lack of consistency and poor reporting of outcomes highlighted in this review, as well as the paucity of patient‐reported outcomes, reinforces the need for an established core outcome set for the treatment of these injuries as has been developed for other conditions (COMET initiative). A universally accepted core outcome set would increase consistency across studies, allowing more trials to be included in future meta‐analyses, and help reduce the incidence of selective reporting. As modern core outcome sets are patient‐centred and consensus‐driven, future studies of distal radius treatment would also be more likely to include outcomes most relevant to patients with this injury.
What's new
| Date | Event | Description |
|---|---|---|
| 11 October 2019 | New citation required and conclusions have changed |
|
| 11 October 2019 | New search has been performed | In this update, published in Issue 2, 2020, the following changes were made: 1. The Background was revised and updated. 2. The Methods were updated including a revised types of outcome measures section and 'Risk of bias' assessment. 3. The search was updated to June 2019. 4. Overall, 44 articles for 28 studies were identified. Of these, 13 studies were included, seven were excluded, five were placed in ongoing trials and three await classification. 5. Quality of the evidence was assessed using GRADE; one 'Summary of findings' table was added and the Discussion revised and updated. 6. Changes were made to the conclusions in line with the GRADE assessment. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 25 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Joanne Elliott and Maria Clarke for help with developing search strategies and providing the results from these, and facilitating translations. We are grateful to Meisam Abdar, Christopher Carroll and Sonia Stewart for their help with translation.
We are grateful to Manesh Vaghela and Rajan Madhok for their contribution to the previous version of the review.
We thank Liz Bickerdike, Teemu Karjalainen and Cecilia Mellstrand Navarro for their helpful comments and suggestions at external review of the review update and Maria Clarke and Joanne Elliott for their feedback and help during editorial processing of the review update.
We are very grateful to those trialists, in particular Rakesh Gupta, who provided clarification and further information on their trials.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies (January 2006 to June 2019)
CENTRAL (CRS Web)
1 January 2006 to 11 June 2019
1 MESH DESCRIPTOR Radius Fractures AND CENTRAL:TARGET 2 “Colles’Fracture”:MH AND CENTRAL:TARGET 3 MESH DESCRIPTOR Wrist Injuries EXPLODE ALL WITH QUALIFIERS SU AND CENTRAL:TARGET 4 (((distal and (radius or radial)) or wrist or colles or smith*) and fracture*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 5 #1 OR #2 OR #3 OR #4 6 MESH DESCRIPTOR Fracture Fixation EXPLODE ALL AND CENTRAL:TARGET 7 MESH DESCRIPTOR Bone Nails EXPLODE ALL AND CENTRAL:TARGET 8 MESH DESCRIPTOR Bone Wires EXPLODE ALL AND CENTRAL:TARGET 9 (percutaneous or pin or pins or pinned or pinning or rod or rods or cross‐pin* or wire* or wiring or Kirschner or K‐wir* or Kapandji or Py or trans‐styloid):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 10 #6 OR #7 OR #8 OR #9 11 #5 AND #10
MEDLINE (Ovid Online)
1 January 2006 to 12 June 2019
1 Radius Fractures/ 2 Colles' Fracture/ 3 Wrist Injuries/su 4 (((distal adj3 (radius or radial)) or wrist or colles or smith*2) adj3 fracture*).ti,ab. 5 or/1‐4 6 Fracture fixation/ or Fracture fixation, internal/ 7 Bone nails/ or Bone wires/ 8 (percutaneous or pin*1 or pinned or pinning or rod or rods or cross‐pin* or wire* or wiring or Kirschner or K‐wir* or Kapandji or Py or trans‐styloid).tw. 9 6 or 7 or 8 10 Randomized controlled trial.pt. 11 Controlled clinical trial.pt. 12 randomized.ab. 13 placebo.ab. 14 Drug therapy.fs. 15 randomly.ab. 16 trial.ab. 17 groups.ab. 18 or/10‐17 19 exp Animals/ not Humans/ 20 18 not 19 21 5 and 9 and 20
Embase (Ovid Online)
1 January 2006 to 12 June 2019
1 Radius fracture/ 2 Wrist fracture/ 3 Colles fracture/ 4 Wrist injury/su [Surgery] 5 or/1‐4 6 Fracture fixation/ or Wire fixation/ 7 Bone pin/ 8 Kirschner wire/ or Bone wire/ or Surgical wire/ 9 (percutaneous or pin*1 or pinned or pinning or rod or rods or cross‐pin* or wire* or wiring or Kirschner or K‐wir* or Kapandji or Py or trans‐styloid).tw. 10 or/6‐9 11 5 and 10 12 Randomized controlled trial/ 13 Clinical trial/ 14 Controlled clinical trial/ 15 Randomization/ 16 Single blind procedure/ 17 Double blind procedure/ 18 Crossover procedure/ 19 Placebo/ 20 Prospective study/ 21 ((clinical or controlled or comparative or placebo or prospective* or randomi#ed) adj3 (trial or study)).tw. 22 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. 23 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. 24 (cross?over* or (cross adj1 over*)).tw. 25 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. 26 RCT.tw. 27 or/12‐26 28 11 and 27
WHO ICTRP
distal AND radi* AND fracture AND fix* OR distal AND radi* AND fracture AND pin* OR distal AND radi* AND fracture AND rod* OR distal AND radi* AND fracture AND wire OR distal AND radi* AND fracture AND percutaneous
colles AND fracture AND fix* OR colles AND fracture AND pin* OR colles AND fracture AND rod* OR colles AND fracture AND wire OR colles AND fracture AND percutaneous
wrist AND fracture AND fix* OR wrist AND fracture AND pin* OR wrist AND fracture AND rod* OR wrist AND fracture AND wire OR wrist AND fracture AND percutaneous
ClinicalTrials.gov
distal AND radial AND fracture AND (fix OR pin OR rod OR wire OR percutaneous)
distal AND radius AND fracture AND (fix OR pin OR rod OR wire OR percutaneous)
colles AND fracture AND (fix OR pin OR rod OR wire OR percutaneous)
wrist AND fracture AND (fix OR pin OR rod OR wire OR percutaneous)
Conference proceedings
AAOS 2015 & 2016
Searched: 19 August 2016: https://aaos.scientificposters.com/epsSearchAAOS.cfm
AAOS 2017 & 2019
Searched July 2019 Hand and wrist sections oral and poster Trauma sections oral and poster AAOS 2019 was searched by Maria Clarke: 0 for review
American Orthopaedic Trauma Association
https://ota.org/education/meetings‐and‐courses/abstracts (August 2019); up to 2018 conference.
Search terms for 3 consecutive searches: Random* AND Distal* AND Radi*; Random* AND Colles* AND Fracture*; Random* AND Wrist* AND Fracture*
Bone and Joint Journal Orthopaedic Proceedings
Searched: April 2018
Title "distal radius fract*" and full text or abstract or title "random*" limited to Orthopaedic Proceedings Title "distal radial fract*" and full text or abstract or title "random*" limited to Orthopaedic Proceedings Title "wrist fract*" and full text or abstract or title "random*" limited to Orthopaedic Proceedings
BSSH
Looked through all meeting programmes (format changed in Autumn 2016)
Autumn 2016: https://bssh.meeting.org.uk/programme/programme.php?m=Autumn2016 Spring 2017: https://bssh.meeting.org.uk/programme/programme.php?m=Spring2017 Autumn 2017: https://bssh.meeting.org.uk/programme/programme.php?m=Autumn2017 Spring 2018: https://bssh.meeting.org.uk/programme/programme.php?m=Spring2018 Autumn 2018: https://bssh.meeting.org.uk/programme/programme.php?m=Autumn2018 Spring 2019: https://bssh.meeting.org.uk/programme/programme.php?m=Spring2019
British Trauma Society
Abstracts available for 2014, 2015, 2016 and 2018. No RCTs found: searched a) 'random', then b) 'RCT'.
FESSH
Federation of the European Societies for Surgery of the Hand (FESSH) XXI meeting June 2016 (published in Journal of Hand Surgery. European Volume 2016 41 Suppl 1: FESSH 2016), search 'random'
Federation of the European Societies for Surgery of the Hand (FESSH) XXII and EFSHT XII combined meeting June 2017 (published in Journal of Hand Surgery. European Volume 2017 42E Suppl 1: FESSH 2017), search 'random'
Federation of the European Societies for Surgery of the Hand (FESSH) XXIII meeting June 2018 (published in Journal of Hand Surgery. European Volume 2018 43 Issue 2 suppl: FESSH 2018), search 'random'
SICOT
Search term: 'random'; then 'RCT' (tally not kept) http://www.sicot.org/montreal (2018) http://www.sicot.org/cape‐town (2017) http://www.sicot.org/rome (2016) http://www.sicot.org/guangzhou (2015) http://www.sicot.org/rio‐2014 (2014)
Appendix 2. Report of search results in previous version of the review (Handoll 2007)
The search for trials predated the development of this review, which is essentially a reworked update of part of a previously published review (Handoll 2003a) covering all surgical intervention for these fractures. We have not documented the numbers of references retrieved by electronic searches; updates of MEDLINE, Embase and CINAHL are now generated on a weekly basis. Of 24 potentially eligible studies put forward for study selection, 13 were included, five were excluded and six are in 'Studies awaiting assessment'.
Aside from the two most recently published trials (Azzopardi 2005; Strohm 2004), the other included trials were present in the review covering all surgical intervention for these fractures (Handoll 2003a). Azzopardi 2005 appeared as an ongoing trial in Handoll 2003a.
Appendix 3. Former 'Types of outcome measures' section
This section is copied from Handoll 2007.
Types of outcome measures
Our primary outcome of choice is the number of people with an uncomplicated and speedy restoration of a pain‐free fully‐functioning wrist and arm with acceptable anatomic restoration and appearance. However, compatible with the general assessment and presentation of outcome within the orthopaedic literature, we reported outcomes in the following four categories.
Primary outcomes (1) Functional outcome and impairment
Patient functional assessment instruments such as Short Form‐36 (SF‐36), the Disability of the Arm, Shoulder, and Hand questionnaire (DASH) and the Patient‐Rated Wrist Evaluation (PRWE) (MacDermid 2000)
Return to previous occupation, including work, and activities of daily living
Grip strength
Pain
Range of movement (wrist and forearm mobility): range of movement for the wrist is described in terms of six parameters: flexion (ability to bend the wrist downwards) and extension (or upwards); radial deviation (ability to bend the wrist sideways on the thumb side) and ulnar deviation (on the little finger side); and pronation (ability to turn the forearm so that the palm faces downwards) and supination (palm faces upwards)
(2) Clinical outcome
Residual soft tissue swelling
Early and late complications associated with distal radial fractures or their treatment, including reflex sympathetic dystrophy (RSD) and post traumatic osteoarthritis
Cosmetic appearance
Patient satisfaction with treatment or outcome
Secondary outcomes (3) Anatomical outcome (anatomical restoration and residual deformity) Radiological parameters include radial length or shortening and shift, dorsal angulation, radial inclination or angle, ulnar variance, and for intra‐articular fractures: step off and gap deformity of the articular surface (Fernandez 1996; Kreder 1996a). Composite measures include malunion and total radiological deformity. Definitions of four of the most commonly reported radiological parameters are presented in Table 1.
(4) Resource use Hospital stay, number of outpatient attendances and other costs.
Timing of outcome assessment Results were collected for the final follow‐up time for which these were available. Where reported, interim trial results were also checked to see if a marked and important difference in the timing of recovery had occurred.
Data and analyses
Comparison 1. Percutaneous pinning versus plaster cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DASH scores (30 to 150; worst disability) | Other data | No numeric data | ||
| 1.1 At 1.5 months | Other data | No numeric data | ||
| 1.2 At 3 months | Other data | No numeric data | ||
| 1.3 At 6 months | Other data | No numeric data | ||
| 2 DASH (30 to 150; worst outcome) at 6 months: reduced function indicated by > 60 score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Complications | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Redisplacement | 2 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.18, 1.65] |
| 3.2 Redisplacement resulting in secondary treatment (reduction or reduction and K‐wire fixation) | 6 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.27] |
| 3.3 K‐wire migration | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.68 [1.19, 63.23] |
| 3.4 K‐wire extrusion | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 K‐wire removal due to metal sensitivity | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 67.03] |
| 3.6 Pin track infection | 7 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [2.54, 27.28] |
| 3.7 Stab wound infection | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.09 [0.69, 247.92] |
| 3.8 Deep infection, joint infection, osteomyelitis | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 74.58] |
| 3.9 Tendon injury/rupture | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Median nerve compression/neuropathy/contusion/CTS | 5 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.46] |
| 3.11 Superfical radial nerve injury | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.53 [0.78, 233.82] |
| 3.12 Reflex sympathetic dystrophy/Sudeck's atrophy | 4 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.48] |
| 3.13 Persistent reflex sympathetic dystrophy | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.40, 10.85] |
| 3.14 Shoulder hand syndrome | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.45] |
| 3.15 Finger stiffness | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.36, 0.76] |
| 4 Non return to work (labourers) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Global grip strength (kg) | Other data | No numeric data | ||
| 5.1 At 1.5 months | Other data | No numeric data | ||
| 5.2 At 3 months | Other data | No numeric data | ||
| 5.3 At 6 months | Other data | No numeric data | ||
| 6 Global grip strength [probably] relative to other side (%) | Other data | No numeric data | ||
| 6.1 At 1.5 months | Other data | No numeric data | ||
| 6.2 At 3 months | Other data | No numeric data | ||
| 6.3 At 6 months | Other data | No numeric data | ||
| 7 Under half grip strength at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Grip strength at 13 to 24 months (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Range of movement at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Radial deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Ulnar deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Supination (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Pronation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Range of motion (data from Zyluk 2007) | Other data | No numeric data | ||
| 10.1 Flexion and extension at 1.5 months | Other data | No numeric data | ||
| 10.2 Flexion and extension at 6 months | Other data | No numeric data | ||
| 10.3 Pronation and supination at 1.5 months | Other data | No numeric data | ||
| 10.4 Pronation and supination: at 6 months | Other data | No numeric data | ||
| 11 Range of movement at 13 to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Radial deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Ulnar deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Supination (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Pronation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Short Form‐36 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Physical score at 4 months (0: worst to 100: best health) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mental score at 4 months (0: worst to 100: best health) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Overall quality of life (1 very poor to 5 very good) at 13 to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Pain (occasional) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Patient satisfaction at 12 wks: excellent result (no pain, disability or motion limitation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 Patient satisfaction at 13 to 24 months (1 to 4, lower score equals higher satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17 Deformity (clinical and radiological) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 Angulated malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Articular incongruity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Articular step off > 2 mm | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Residual deformity ‐ prominence of ulnar styloid | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Composite score functional grading: fair or poor | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Score included deformity and complications | 5 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.37, 0.78] |
| 18.2 Score confined to pain and function | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.89] |
| 19 Mayo wrist score at 13 to 24 months (0 to 100; worst outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20 Anatomical measurements | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Dorsal angulation (degrees) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Radial angulation (degrees) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Radial length (mm) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Ulnar variance (mm) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.17. Analysis.
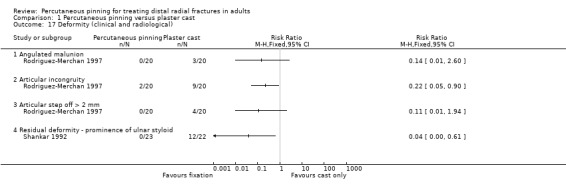
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 17 Deformity (clinical and radiological).
1.20. Analysis.
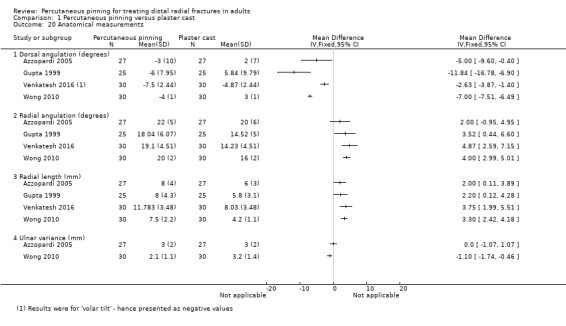
Comparison 1 Percutaneous pinning versus plaster cast, Outcome 20 Anatomical measurements.
Comparison 2. Kapandji intrafocal pinning (2 or 3 wires) versus trans‐styloid fixation (2 wires).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Redisplacement requiring secondary treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Superficial pin‐track infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tendon complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Superficial radial nerve ‐ symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Median nerve dysfunction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Confirmed reflex sympathetic dystrophy (bone scan) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Modified Kapandji (dorsal Kapandji wires and trans‐styloid fixation) versus Kapandji intrafocal pinning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anatomical measurements (at 45 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Dorsal angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Radial angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DASH score at one year (0: worst disability to 100: no disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Subjective results: pain and function (normal = 30 points); from Jakim score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 In paper | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Reversed results | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Secondary displacement (early or reported as malunion) | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.81, 10.46] |
| 3.2 Fracture caused by pinning | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.71] |
| 3.3 Wire displacement | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.87 [1.57, 89.61] |
| 3.4 Superficial infection | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.47 [0.80, 52.46] |
| 3.5 Tendon rupture | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.36, 30.38] |
| 3.6 Reflex sympathetic dystrophy (Syndrome algoneurodystrophique) | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.39, 1.97] |
| 3.7 Superficial radial nerve paraesthesia | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.16, 7.10] |
| 3.8 Lunate (avascular) necrosis | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.13, 75.43] |
| 4 Objective results: mobility, grip strength, deformity (normal = 30 points); from Jakim score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 In paper | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Reversed results | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Overall results: radiological, subjective and objective (normal = 100 points); from Jakim score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 In paper | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Reversed results | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Overall outcome grades | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Fair or poor | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Radiological results (normal = 40 points); from Jakim score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 In paper | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Reversed results | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Anatomical measurements at one year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Dorsal angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Radial angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.7. Analysis.
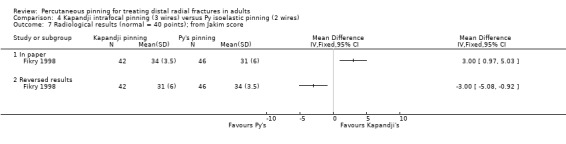
Comparison 4 Kapandji intrafocal pinning (3 wires) versus Py isoelastic pinning (2 wires), Outcome 7 Radiological results (normal = 40 points); from Jakim score.
Comparison 5. Modified Kapandji pinning (3 wires) versus Willenegger pinning (2 wires).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 "Conversion procedure" (secondary treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Wire migration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Nerve irritation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Carpal tunnel syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Tendon injury or rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Reflex sympathetic dystrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Signs of swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. “Spring‐loaded intramedullary" pinning (not Py) versus transcortical pinning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Early fixation failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Biodegradeable pins versus Kirschner wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Operational difficulties in pin or wire insertion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Secondary displacement requiring revision | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Superficial wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tendon rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Carpal tunnel syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Reflex sympathetic dystrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Secondary surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Painful scars | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Sinus formation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Severe osteolytic reactions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Arthrosis (radiological signs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Range of movement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Radial deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Ulnar deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Supination (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Pronation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Anatomical measurements | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Dorsal angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Radial angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Radio‐ulnar index (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Buried wires versus exposed percutaneous wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Infected wire(s) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Wires removed early | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Superficial radial nerve symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tendon damage (EPL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 More invasive treatment for wire removal | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.36 [3.54, 15.31] |
| 2.1 Removal of wires in theatre | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.80 [2.27, 20.42] |
| 2.2 Local anaesthesia required | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.85 [2.94, 20.94] |
Comparison 9. Immobilisation of wrist in dorsiflexion versus palmar flexion (intrafocal pinning).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Superficial pin‐site infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Reflex sympathetic dystrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Need for physiotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Secondary surgery (distal radial osteotomy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Grip strength (as percentage of other side minus 30% for nondominant side) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 at 17 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pinch strength (as percentage of contralateral minus 30% for nondominant side)( | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 at 17 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Range of motion (as percentage of contralateral normal side) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Flexion at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Flexion at 17 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Extension at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Extension at 17 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Rolyan nine hole peg test of dexterity (seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 at 17 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Anatomical measurements at week 17 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Dorsal angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Radial angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Radial height (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Radial length (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Early (after 1 week cast immobilisation) versus later (after 4 to 6 weeks) mobilisation post surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Displaced wires or pins | 3 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.62, 14.53] |
| 1.2 Treatment failure (change of treatment) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.93] |
| 1.3 Superficial pin‐track infection | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.36, 15.18] |
| 1.4 Tendon rupture | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.62, 14.53] |
| 1.5 Persistent radial nerve paraesthesia or hypoaesthesia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.03] |
| 1.6 Reflex sympathetic dystrophy | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.93] |
| 1.7 Non‐union | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.67] |
| 2 Range of movement (at 1.5 months; interim results) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Radial deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Ulnar deviation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Pronation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pain at 3 months: usually or during effort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Patient dissatisfaction with outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Anatomical measurements (at 1 year): standard errors conversion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Dorsal angulation ‐ volar tilt (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Radial angulation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Radial length (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allain 1999.
| Methods | Randomised by odd or even chart number Assessor blinding: yes Intention‐to‐treat analysis: likely Loss to follow‐up: none | |
| Participants | Teaching (probably) hospital, France 60 participants Inclusion criteria: dorsally displaced extra‐articular or non‐comminuted intra‐articular fracture of the distal radius. Skeletally mature Exclusion criteria: open fracture, anteriorly displaced fracture, fracture dislocation, > 2 articular fragments, styloid fragment too small to allow fixation with trans‐styloid K‐wires, multiple trauma, previous fracture of wrist or elbow Classification: AO (types A2, A3, C1, C2) (extra‐ and intra‐articular) Sex: 45 female Age: mean 55 years, range 18 ‐ 87 years Assigned: 30/30 [1 week/6 weeks] Assessed: 30/30 (at 1 year) | |
| Interventions | Timing of intervention: timing of operation not stated Closed reduction and operation under regional or general anaesthesia. Trans‐styloid fixation: 2 K‐wires inserted (2 cm skin incision) through radial styloid and across fracture and fixed into the opposite cortex of the proximal radius. (1) Short arm circular cast for 1 week. No heavy work before 6 weeks but daily activities recommended (2) Short arm circular cast for 6 weeks In both groups, hand was raised to prevent pain and oedema for the first few days, and fingers and elbow mobilised immediately post‐surgery. Wrist mobilisation encouraged after cast removal but no physiotherapist involvement. Wires removed at 45 days | |
| Outcomes | Length of follow‐up: 1 year; also assessed postoperatively and 4 weeks and 45 days (1) Functional: time to return to work; return to sporting activities (without discomfort); functional discomfort during domestic chores; grip strength; pain (VAS 0 to 15 cm: no pain); use of analgesics; range of movement (flexion, extension, radial and ulnar deviation, pronation, supination) (2) Clinical: complications: superficial infection (no deep infection), pin migration, RSD, radial nerve paraesthesia or hypoaesthesia (persistent; 3 of the 4 participants did not have this before K‐wire removal), tendon rupture. Patient satisfaction (3) Anatomical: X‐ray postoperatively, 45 days (before and after K‐wire removal) and 1 year. Radial angle, radio‐ulnar index (ulnar variance: no data), volar tilt, radial length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "prospectively randomised, on the basis of an even chart number, to a group..." Comment: quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised: predictable allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Participants were not blinded. The risk of bias was probably high early on (pain at 4 weeks) but much reduced at one year. Hence 'unclear risk' rating |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Low risk | Quote: "All the patients were independently reviewed, after a 1‐year follow‐up, by a single physician, who did not take part in the treatment of the fractures and did not know the duration of the period of postoperative immobilization." Comment: Blinded assessment thus low risk |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications and outcomes occurring before 1 year was not blinded. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Quote: "No patient was lost to follow‐up". Comment: However, incomplete data for continuous outcomes (no standard deviations) |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | Quote: "No patient was lost to follow‐up". Comment: However, incomplete data for continuous outcomes (no standard deviations) |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or prospective trial registration. Authors did not declare primary outcome but the outcomes recorded in methods were reported in results. |
| Major baseline characteristic differences | Unclear risk | Small trial. Some small imbalance that could affect results: more males (9 versus 6), more with active professional activities (21 versus 18) and sportsmen (12 versus 10), and on average younger (mean 53 versus 57 years) in the one‐week group. Thus, 'unclear risk' |
| Performance bias: expertise, other care | Unclear risk | Similar mobilisation procedures (no physiotherapist) but lack of information on care provider expertise and on use of analgesia |
Azzopardi 2005.
| Methods | Randomised by the tossing of a coin Assessor blinding: no, except radiological outcome Intention‐to‐treat analysis: claimed but the treatment groups of the six people lost to follow‐up were not identified Loss to follow‐up: 9 (3 deaths and 6 lost to follow‐up) | |
| Participants | Hospital, UK 57 participants Inclusion criteria: unstable extra‐articular fracture of the distal radius: AO‐A3 or Frykman I and II. Informed consent Exclusion criteria: age < 60 years, dementia or psychiatric illness, previous ipsi‐ or contra‐lateral wrist fracture, intra‐articular fracture, open fracture, volar angulated fracture (Smith's fracture), stable fracture with dorsal angulation < 20 degrees and minimal dorsal comminution Classification: AO type A3 or Frykman I and II (extra‐articular) Sex: (of 54) 48 female Age: (of 54) mean 71.5 years, range 60 ‐ 80 years Assigned: 30/27 [X‐pins/POP] Assessed: ?/? (at 1 year); numbers not reported | |
| Interventions | Timing of intervention: timing of operation not stated
Closed reduction under general anaesthesia and fluoroscopic guidance
(1) Percutaneous pinning: using 2 crossed, smooth K‐wires, 1.6 mm diameter, inserted through small stab incisions under fluoroscopic guidance. One wire through the styloid process, the other through Lister's tubercle or the dorso‐ulnar border of distal fragment. Both wires engaging opposite cortex. Blunt dissection to bone. Pins left protruding percutaneously and wrist immobilised in "well‐moulded" short‐arm cast
(2) Nonsurgical treatment: three‐point fixation obtained in a "well‐moulded" short‐arm cast. Wires and plasters were removed after 5 weeks. |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 1, 2 and 5 weeks and 4 months. (1) Functional: activities of daily living (unilateral and bilateral tasks); SF‐36 health status; grip strength; pain (VAS 0 to 10 cm: worst pain); range of movement (flexion, extension, radial and ulnar deviation, pronation, supination) (2) Clinical: complications: remanipulation after redisplacement, pin track infection (wire removal), tendon or neurovascular injury (none) (3) Anatomical: X‐ray at all follow‐up times. Dorsal angulation, radial angle, radial length, ulnar variance | |
| Notes | Study entry in the National Research Register UK indicated inclusion criteria of 50 years (not 60 years) and neurovascularly intact, with an intended study size of 100 participants. Correspondence with lead trialist (response 24 May 2006) did not yield further data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were then randomly allocated by tossing a coin to either the closed reduction or the percutaneous pinning groups." |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Quote: "The functional outcome was assessed by an orthopaedic specialist physiotherapist (TC) who was not blinded to the method of treatment since the pin‐track scars could be easily observed." However, these outcomes appeared less susceptible to bias related to lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | Unclear risk | There was no assessor blinding but very few complications. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Claimed to perform intention‐to‐treat analysis but no participant flow diagram and failed to confirm how many were available at short‐term follow‐up |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | Claimed to perform intention‐to‐treat analysis but no participant flow diagram and failed to say to which groups the 6 lost to follow‐up at 1 year follow‐up belonged |
| Selective reporting (reporting bias) | Unclear risk | Trial registration document available but a few differences: e.g. trial start date; 11 activities of daily living but 10 in paper; timing of SF‐36 data collection |
| Major baseline characteristic differences | Unclear risk | Participant characteristics were balanced but the 3 deaths in the pinning group were not included. |
| Performance bias: expertise, other care | Unclear risk | Quote: "All procedures were carried out under general anaesthesia within 24 hours of the injury and were performed or supervised by the senior author (SE)." Care programmes appeared similar but no information on rehabilitation, including advice & instructions |
Casteleyn 1992.
| Methods | Randomised using sealed cards Assessor blinding: not stated Intention‐to‐treat analysis: likely Loss to follow‐up: 2 (deaths) | |
| Participants | Teaching hospital, Belgium 30 participants Inclusion criteria: closed wrist fracture: Frykman type I, II, V or VI. Informed consent Exclusion criteria: no other injuries Classification: Frykman (I, II, V, VI) (extra‐ and intra‐articular involving the DRUJ) Sex: 23 female Age: mean 61 years, range 22 ‐ 85 years Assigned: 15/15 [biodegradable pins/K‐wires] Assessed: 14/14 (13/13 anatomical and ROM) (1 year) | |
| Interventions | Timing of intervention: timing of operation not stated
Closed reduction using finger traps and operation under regional or general anaesthesia. All fractures pinned percutaneously (stab incisions) using Kapandji's intrafocal technique
(1) Biodegradable pins: holes pre‐drilled, depth measured to select correct length of rod. Two PGA (polyglycodic acid ‐ biodegradable) rods inserted and gently tapped home
(2) Kirschner wires: two 2 mm K‐wires inserted into fracture gap (one dorsally and the other laterally) and drilled into the opposite cortex of the radial shaft. Then cut to be under the skin. Removed after 6 weeks using local anaesthesia No cast applied and participants encouraged to move their wrist freely from first postoperative day |
|
| Outcomes | Length of follow‐up: 1 year; also assessed day 1 postoperatively and 1, 3 and 6 months (1) Functional: overall functional score (subjective and objective evaluation; own system); range of movement (flexion, extension, radial and ulna deviation, pronation, supination) (2) Clinical: complications: rupture of EPL tendon, RSD (1 case that resolved by 1 year), secondary surgery, painful scars, sinus formation, CTS, osteolytic reaction (3) Anatomical: X‐rays at all follow‐up times. Dorsal angulation, radial angle, radial shortening & radio‐ulnar index. Fracture union | |
| Notes | Preoperative and post‐reduction position not given. The possibility that the subcutaneous protrusion of the ends of the pins in the Kapandji pinning technique could increase the specific complications of biodegradable implants was raised in the report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation to either PGA‐rod or K‐wire treatment was made using sealed cards". Comment: insufficient details on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No blinding but these outcomes appeared less susceptible to bias related to lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded. |
| Incomplete outcome data (attrition bias) Short term | Low risk | Four losses balanced between groups. In each group, one death and one non‐attender at one year follow‐up; both of the latter reported "complete functional recovery" over the telephone. |
| Incomplete outcome data (attrition bias) Longer term | Low risk | Four losses balanced between groups. In each group, one death and one non‐attender at one year follow‐up; both of the latter reported "complete functional recovery" over the telephone. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or prospective trial registration. Authors did not declare primary outcome but the outcomes recorded in methods were reported in results. |
| Major baseline characteristic differences | Unclear risk | No major imbalances in participant characteristics. However, PGA‐rod group participants were on average 6 years older. |
| Performance bias: expertise, other care | Unclear risk | No information on surgeon expertise (the PGA rods were acknowledged to be more difficult to use) or information on rehabilitation (no cast applied). Care programmes otherwise appeared similar. |
Delgado 2009.
| Methods | Method of randomisation: not stated Assessor blinding: not known Intention‐to‐treat analysis: not known Loss to follow‐up: not known | |
| Participants | Teaching (probably) hospital, Spain
43 participants
Inclusion criteria: heavy labour workers with unstable fractures of distal radius (intra‐articular fracture mentioned in abstract report) Exclusion criteria: not known Classification: not known Sex: 9 female Age: mean 40 years, range 22 to 65 years Assigned: 24/20 [K‐wire/cast only] (19 participants were reported in the cast group in the 2010 abstract report of the trial) Assessed: not known (minimum 1 year) |
|
| Interventions | Timing of intervention: not known (1) Indirect reduction and percutaneous fixation with K‐wires and cast immobilisation (2) Indirect reduction and cast immobilisation No details on wiring type, cast or duration of immobilisation |
|
| Outcomes | Length of follow‐up: minimum 1 year, also 3, 6 and 12 months (1) Functional: DASH score, return to work (and previous activity level), mobility (range of flexion‐extension), strength (isokinetics; grip mentioned in methods, pinch in results) (2) Clinical: pain score (scale not presented) (3) Anatomical: radiographic parameters and consolidation |
|
| Notes | Abstracts only. Attempted to contact authors in April 2017 with no response Two study reports (2007 and 2009) presented two comparison groups whereas a third study report presented in 2010, data from which have some inconsistencies with the other 2 reports, introduced a third comparison group (external fixation and k‐wires) (Delgado 2010). There are discrepancies in the reported participant numbers in the cast group (20 or 19), resulting in a total of 44 rather than 43 overall. None of the reported results was suitable for data entry: SDs, or data by which to calculate SDs, were not presented for continuous outcomes and the percentages provided for re‐operations and other dichotomous outcomes did not compute to whole numbers. We have used only data that were consistently reported in the two earlier reports for this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Title described: “A prospective randomised study to compare...”. No details of method or sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Title described: “A prospective randomised study to compare...”. No details of allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No mention of blinding. In this setting, blinding highly unlikely to be achievable |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No mention of blinding. In this setting, blinding highly unlikely to be achievable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No mention of blinding. In this setting, blinding highly unlikely to be achievable |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No mention of blinding; however, knowledge of allocation unlikely to be influential for detection bias for these outcomes and at longer follow‐up. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No mention of blinding. Complications not included in report; however, in this setting, blinding is highly unlikely to be achievable and lack of blinding may result in bias for these outcomes. |
| Incomplete outcome data (attrition bias) Short term | High risk | Discrepancies in participant numbers recruited and randomised. No participant flow and no follow‐up or response rates. Data discrepancies in later abstract (Delgado 2010) |
| Incomplete outcome data (attrition bias) Longer term | High risk | As above |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Only presented in conference proceeding abstracts. Two study reports (2007 and 2009) presented two comparison groups whereas the last study report (Delgado 2010) introduced a third comparison group (external fixation and k‐wires). Additionally, there are discrepancies in the participant numbers reported and probable mistakes in the reporting, including the DASH scores for the pinning group. |
| Major baseline characteristic differences | Unclear risk | Baseline characteristics per group not reported |
| Performance bias: expertise, other care | Unclear risk | No information on care programmes or care provider expertise |
Fikry 1998.
| Methods | Method of randomisation not stated Assessor blinding: not reported Intention‐to‐treat analysis: unlikely, 22 who were lost to follow‐up were excluded. Loss to follow‐up: 22 | |
| Participants | Hospital, Morocco 110 participants Inclusion criteria: dorsally displaced distal radius fractures, with or without separation of ulnar styloid fragment Exclusion criteria: comminuted fracture, dislocated radiocarpal fracture, associated carpal injuries, injuries to elbow or forearm on same side, major open injury. Participants not willing or able to follow rehabilitation. Classification: not given (extra‐articular; some intra‐articular fractures were possible) Sex: (of 88) 22 female Age: (of 88) mean 34 years, range 18 ‐ 66 years Assigned: [Kapandji/Py ] not reported Assessed: 42/46 (at 27 months) | |
| Interventions | Timing of intervention: timing of operation not stated
K‐wire insertion was probably percutaneous. Pins inserted under general or regional anaesthesia. Reduction was done during the operation.
(1) Kapandji intrafocal pinning. 3 K‐wires inserted at fracture site. Image intensifier used
(2) Py's isoelastic pinning. 2 K‐wires inserted through radial epiphysis, across fracture and along medullary canal up to radial head. Image intensification not used: grinding of wire against the cortical bone used to determine wire placement Where present, ulnar styloid fractures were transfixed using a nylon suture. |
|
| Outcomes | Length of follow‐up: 20 to 52 months (mean 27 months) (1) Functional: part of scoring system (Jakim 1991): subjective (pain and function), objective (grip, mobility, deformity) and overall grades (2) Clinical: complications: secondary displacement, fracture caused by wiring (the participant had a plate inserted), displaced wires (4 of 6 removed early), superficial infection, tendon rupture, RSD, arthritis (no data) (3) Anatomical: X‐ray at final follow‐up. Radial length, radial angle, volar angle, loss of reduction, arthritic change, step‐off, radioulnar joint articulation within overall scale (Jakim 1991) | |
| Notes | Paper in French. Translated into English by Sonia Stewart and Linda Digance. Conclusions given in summary for the Jakim scores cited the superiority of Py's method; these are not consistent with the data in Table III in the paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Translation: "The choice of technique was chosen randomly." Comment: no information available to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Translation: "The choice of technique was chosen randomly." Comment: no information available to make judgement |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Participants were not blinded; however, the difference in surgical techniques was unlikely to have affected assessment of pain at 20 months or more. Hence 'unclear risk' rating |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No mention of blinding but unclear whether objective outcomes at 20 months or over would have been affected by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded and is more susceptible to bias, including in the absence of thresholds for deformity. |
| Incomplete outcome data (attrition bias) Short term | High risk | 22 participants (20%) were lost to follow‐up (at 20 months or more) ‐ baseline allocation of these is not available. |
| Incomplete outcome data (attrition bias) Longer term | High risk | 22 participants (20%) were lost to follow‐up (at 20 months or more) ‐ baseline allocation of these is not available. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Authors appeared to have collected long‐term outcome data at a set time: range of recall 20 to 52 months. This seems like a post hoc decision and could be a source of bias. No listing of outcomes in methods. Inconsistencies in the reporting of Jakim scores |
| Major baseline characteristic differences | Unclear risk | A reasonable balance in age for those followed up but no baseline data for all participants |
| Performance bias: expertise, other care | Unclear risk | Uncertain if difference in anaesthesia or operator experience. Rehabilitation appears comparable. |
Gravier 2006.
| Methods | Randomised by alternation on admission Allocation not concealed Assessor blinding: not reported (two independent assessors for radiological outcomes) Intention‐to‐treat analysis: potential problems Loss to follow‐up: 7 reported in main article. The data from these participants were excluded from the main analysis. |
|
| Participants | Hospital, France 85 participants Inclusion criteria: Acute (emergency hospitalisation for a recent trauma) dorsally‐displaced extra‐articular fracture of the distal radius. Consent obtained. Age over 18 and under 80 years. Exclusion criteria: Intra‐articular fracture (an associated fracture of the ulnar styloid process was not an exclusion criterion). Classification: none stated: extra‐ and intra‐articular and direction of displacement part of trial inclusion criteria Sex: 57 female Age: mean 54.9 years, range 28 to 78 years Assigned: 44/41 [modified Kapandji/Kapandji] Assessed: 40/38 (45 days) |
|
| Interventions | Timing of intervention: timing of operation not stated. Closed reduction by manipulation before pinning.
(1) Modified Kapandji pinning. Following reduction, one or two dorsal intrafocal pins were placed percutaneously then one lateral transfocal pin was added. For the latter, an incision approximately 1 cm was made with respect to the styloid and after careful dissection a 2/100 diameter pin was placed with an ascending direction of approximately 45 degrees with respect to the plane of articulation.
(2) Kapandji pinning. Following reduction, one or two dorsal intrafocal pins were placed percutaneously, then one lateral pin completed stabilisation of the assembly. In both groups: If present, the tip of the styloid process was repaired under radiographic guidance. After a final anterior‐posterior (AP) and lateral x‐ray, the incision was closed with a rapidly resorbed thread. At the end of the procedure, a "posterior antebrachial‐palmar gutter" (posterior back slab) cast was applied for 3 weeks. Participants were reviewed at day 21 postoperatively for a first consultation and check x‐ray. The plaster was removed and the first sessions of passive rehabilitation were prescribed. The removal of the pins was performed under local anaesthetic in theatre on day 45 postoperatively following a second check x‐ray. |
|
| Outcomes | Length of follow‐up: day 21 and day 45 post‐surgery. Authors stated minimum follow‐up was 6 months, but only reported results for 45 days. (1) Functional: none recorded (2) Clinical: none recorded (3) Anatomical: X‐rays immediately post‐surgery and at 45 days. Dorsal angulation (volar tilt), radial angle (radial inclination), "variance radio‐ulnaire" (we are unsure about this parameter as it was reported in degrees, whereas ulnar variance is usually measured in mm). Satisfactory reduction post‐surgery and at 45 days (it is not clear whether this was assessed before or after wire removal). | |
| Notes | Part translation by Christopher Carroll, received 30 January 2017 Earlier abstract indicated there were 95 in the trial, with a slightly younger population (mean 49 years) (Gravier 2005). Communication from author indicated this abstract was an earlier report of the trial (July 2006); however, the limitations of the study meant that we decided to stick with the trial report in terms of recruitment numbers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "This was determined randomly by alternation according to the protocol upon admission of the patient." [translation] Comment: quasi‐randomised |
| Allocation concealment (selection bias) | High risk | As above. Predictable sequence ‐ no allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Neither participants nor care providers (surgeon(s)) were blinded. Outcomes were radiographic ‐ performance bias not possible from participants |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Assessors not blinded – but independent assessors means that it may not have been an important source of bias. Intra‐observer correlation was measured; levels of agreement (r values) were high. |
| Incomplete outcome data (attrition bias) Short term | High risk | 85 participants were initially included but 7 were lost to follow‐up. Data on those 7 were not used as part of the analysis. Although the 7 of 85 participants lost to follow‐up were similarly distributed between the two groups, the abstract published in 2005 of the same study described 95 participants: “Group K included 49 patients, mean age 45 years. Group KM included 46 patients, mean age 54 years” (Gravier 2005). The difference between the two groups should not be more than 1 person if alternation had been correctly applied. |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. No clinical outcomes. Minumum six month follow‐up mentioned, results for six months not reported |
| Major baseline characteristic differences | Unclear risk | Insufficient information to judge this. No details on fracture type. Intervention group had more males and younger participants; unclear if imbalance significant enough to affect results |
| Performance bias: expertise, other care | Low risk | Similar care pathways apart from intervention studied. Reported types of anaesthesia balanced between groups. It is probably safe to assume similar expertise in the two interventions by the three surgeons involved. |
Gupta 1999.
| Methods | Randomised by alternation of consenting patients Assessor blinding: not reported Intention‐to‐treat analysis: likely Loss to follow‐up: none | |
| Participants | Teaching hospital, India 50 participants Inclusion criteria: Colles' fracture, fused epiphysis, patient consent Exclusion criteria: not given Classification: Frykman: all extra‐articular Sex: 37 female Age: mean 56 years; range 22 ‐ 80 years Assigned: 25/25 [X‐pins/POP] Assessed: 25/25 (at 6 months) | |
| Interventions | Timing of intervention: timing of operation not stated Closed reduction under traction (1) Percutaneous pinning: crossed pin fixation using K‐wires under local anaesthesia: first wire inserted through tip of radial styloid, second wire through the dorso‐ulnar corner of the distal radius. Then below elbow plaster cast, with wrist at approximately 10 degrees extension and neutral deviation, for 6 weeks. Wires removed at fracture union (2) Nonsurgical: plaster cast. Wrist in palmar flexion and ulnar deviation for 3 weeks and then, after cast change, in neutral position for 3 weeks | |
| Outcomes | Length of follow‐up: 6 months; also assessed at "regular" but unspecified intervals including around 8 weeks (1) Functional: overall grading (Sarmiento 1980 ‐ actually Sarmiento 1975); range of movement (flexion, extension, radial deviation, ulnar deviation, pronation, supination) (2) Clinical: complications: pin track infection, K‐wire extrusion, redisplacement (also K‐wire migration); none of: CTS, shoulder hand syndrome, Sudeck's atrophy, tendon rupture (3) Anatomical: X‐ray at reduction, around 8 weeks and 6 months. Radial shortening, dorsal angulation, radial angulation. Overall grading (Stewart 1984) | |
| Notes | Information on method of randomisation and loss to follow‐up (none), type of fracture, baseline characteristics and standard deviations for continuous outcomes obtained from the trialist in 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote (from email from lead trialist): "Alternative patients who agreed to participate" Comment: quasi‐randomised trial |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised: predictable allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded. Although subjective, outcome only part of a combined functional outcome score |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No mention of blinding but unclear whether objective outcome measures of functional impairment at 6 months would have been affected by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded. |
| Incomplete outcome data (attrition bias) Short term | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) Longer term | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or prospective trial registration. Authors did not declare primary outcome nor describe outcomes in any detail. However, SDs and other information obtained from trial author |
| Major baseline characteristic differences | Low risk | No major imbalances in participant characteristics; all extra‐articular fractures |
| Performance bias: expertise, other care | Unclear risk | No information on clinician/surgeon expertise or information on rehabilitation Care programmes otherwise appeared similar but only the pinning group appeared to have received local anaesthetic. |
Hargreaves 2004.
| Methods | Randomised using sealed envelopes Assessor blinding: not known Intention‐to‐treat analysis: not known Loss to follow‐up: not known | |
| Participants | Teaching hospital, UK
56 participants (99 wires)
Inclusion criteria: patients with an isolated distal radial fracture that required Kirschner wire fixation. Open and closed fractures were included. Age range showed children were included. Exclusion criteria: not known Classification: closed 54, open 2 Sex: 25 female Age: mean 35.4, range 7 to 81, number of children not known Assigned: 29 (50 wires)/27 (49 wires) [percutaneous wires/buried wires] Assessed: not known (at 6 weeks) |
|
| Interventions | Timing of intervention: not known (1) Buried wires: At end of procedure, one or usually two wires were buried deep to the skin, which was closed with 4/0 Ethilon. Wires were bent over to prevent wire migration. Wires mostly removed in the Day Surgery Unit (2) Percutaneous wires: At end of procedure one or usually two wires were left percutaneously, with skin completely released around the wire to prevent tethering. Wires were bent over to prevent wire migration. Wires mostly removed in outpatients Common treatment: This is described in detail. Single dose of antibiotics administered. Closed reduction attempted first; if successful, wires inserted via small stab incisions followed by blunt dissection to bone. Usually two wires inserted, one near Lister's tubule and second in the radial styloid. Where open reduction, it was through the 3/4 extensor compartment interval. Supplementary bone graft in 9 cases. Wounds dressed, forearm cast applied. Wires removed at 6 weeks in Day Surgery Unit or in outpatients. All patients referred for hand therapy, for assessment and treatment with mobilisation and scar desensitisation. |
|
| Outcomes | Length of follow‐up: 6 weeks, also assessed at 2 and 6 weeks (1) Functional: none (2) Clinical: place of wire removal (Day Surgery Unit/clinic), number of wires infected (Oppenheim score), number of wires removed early (before 6 weeks) (3) Anatomical: none |
|
| Notes | Authors contacted 2006 and 2017. No information available in addition to the published report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization by means of a sealed envelope system at the time of surgery.” Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details (e.g. no mention of opaque envelopes) to judge if allocation was adequately concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Surgeons not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Detection of pin tract infection not blinded |
| Incomplete outcome data (attrition bias) Short term | Low risk | Probably no loss to follow‐up given the short‐term follow‐up |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. Potential unit of analysis problem (participants recruited but wires reported). No collection of functional outcomes |
| Major baseline characteristic differences | Low risk | No important or major differences in baseline characteristics |
| Performance bias: expertise, other care | Unclear risk | Surgery performed by six different surgeons (five trainees and one consultant) – no indication of experience of the trainees Other interventions including open reduction and use of bone graft similar in the two groups |
Korner 1999.
| Methods | Method of randomisation not stated Assessor blinding: not stated Intention‐to‐treat analysis: likely but incomplete information Loss to follow‐up: 4 | |
| Participants | Teaching hospital, Germany 40 participants Inclusion criteria: distal radius fracture Exclusion criteria: not given Sex: not given Age: not given Classification: AO (at least A2, A3, B1: intra‐articular and extra‐articular) Assigned: 19/21 [biodegradable pins/K‐wires] Assessed: 17/19 (median 25.4 months) | |
| Interventions | Timing of intervention: not stated (1) Biodegradable pins (2) Kirschner wires Pins and K‐wires inserted according to Willenegger (probably Willenegger 1959: two pins placed through radial styloid and across fracture) "in a modified manner" There was no information on post‐surgical care. | |
| Outcomes | Length of follow‐up: median 25.4 months (1) Functional: ROM (flexion/extension, supination/pronation) (2) Clinical: complications: operational difficulties with pin or wire insertion, superficial wound infection, secondary displacement requiring revision, arthrosis. Duration of operation (3) Anatomical: X‐ray at final follow‐up. Redisplacement requiring revision, radiological signs of arthrosis | |
| Notes | Report only available in poster abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective randomised study" Comment: no information available to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "prospective randomised study" Comment: no information available to make judgement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Care providers (surgeon(s)) not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Outcome assessors were not blinded but these outcomes are less susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded. |
| Incomplete outcome data (attrition bias) Short term | High risk | No participant flow for short‐term outcomes. Outcome data are very incomplete and percentages imprecise. |
| Incomplete outcome data (attrition bias) Longer term | High risk | No participant flow. Outcome data are very incomplete. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration; only published as a conference abstract. No subjective outcomes; very incomplete reporting of outcome |
| Major baseline characteristic differences | Unclear risk | No information available |
| Performance bias: expertise, other care | Unclear risk | Same method of insertion of pins and wires but otherwise no information on care programmes or surgeon expertise |
Lenoble 1995.
| Methods | Method of randomisation not stated: "random selection in the operating theatre" Assessor blinding: not reported Intention‐to‐treat analysis: problems. Baseline characteristics not presented for all trial participants Lost to follow‐up: 24 (15 lost, 6 missing data, 3 died) at follow‐up | |
| Participants | 2 teaching hospitals, France 120 participants Inclusion criteria: closed dorsally displaced extra‐ or intra‐articular distal radial fractures with posteromedial fragment, skeletally mature Exclusion criteria: anteriorly displaced fractures, open fractures, fracture dislocations, multiple trauma, previous fracture of wrist or elbow, fractures with > 2 intra‐articular fragments, comminuted fractures that could not be stabilised by K‐wire fixation Classification: Frykman (1/2, 3/4, 5/6, 7/8), Castaing, AO (extra‐articular: A2, A3; and intra‐articular: C1,C2) Sex: (of 96) 65 female Age: (of 96) mean 57 years; range 18 ‐ 88 years Assigned: ?/? [Kapandji/Trans‐styloid]; numbers not reported Assessed: 54/42 (at 2 years) | |
| Interventions | Timing of intervention: not stated Regional or general anaesthesia used, with radiological control of reduction and K‐wire positioning. Used 1.8 mm K‐wires (1) Kapandji fixation. Closed manual reduction. intrafocal placement by hand of 2 or 3 K‐wires via 1 cm incisions (vessels, nerves and tendons were retracted), advanced to impact into opposite cortex, and buried under skin. Immediate mobilisation. Wires removed 45 days (40 to 60 days) under local or regional anaesthesia (2) Trans‐styloid fixation. Closed reduction by traction, 2 K‐wires inserted percutaneously through the radial styloid anteriorly and posteriorly to reach the opposite cortex, using powered drilling. Wrist immobilisation in short arm plaster for 45 days (37 to 54 days). Wires removed 45 days (40 to 60 days) under local or regional anaesthesia | |
| Outcomes | Length of follow‐up: 24 months; also 45 days and 3, 6 and 12 months (1) Functional: grip and pinch strength; pain (VAS 0 to 100: unbearable pain); range of movement (flexion, extension, radial and ulnar deviation, pronation, supination) (2) Clinical: complications: redisplacement, pin track infection (all superficial), RSD, radial nerve symptoms (2 developed RSD), tendon injury (none), vascular complications (none), median nerve dysfunction (none), DISI or VISI (carpal instability), osteoarthritis (no new) (3) Anatomical: X‐ray at pre‐reduction, postoperatively, and each follow‐up time. Radial and palmar tilt, ulnar variance, radial shortening and step deformity. Over‐reduction in anterior direction | |
| Notes | The aim of the trial was to assess the results of the two most commonly used methods in France for dorsally displaced distal radial fractures, at that time. Hence, differences in procedures, especially mobilisation, between the interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The choice of method of K‐wire fixation was determined by simple random selection in the operating theatre". Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No blinding but pain may not have been susceptible to bias ‐ the pattern was similar over the five follow‐up visits. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No blinding but outcomes less susceptible to bias |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No blinding. These outcomes were of greater susceptibility to detection bias. |
| Incomplete outcome data (attrition bias) Short term | High risk | Quote: “15 were loss to follow‐up, three died before completion of the study, and six were excluded because of missing data.” Comment: Losses to follow‐up were not reported by allocation group. A 'per protocol' analysis was done (participants with missing data were excluded from the analysis). Additionally, incomplete data for continuous outcomes (no standard deviations) and some complications not reported by treatment group |
| Incomplete outcome data (attrition bias) Longer term | High risk | Quote: “15 were loss to follow‐up, three died before completion of the study, and six were excluded because of missing data.” Comment: Losses to follow‐up were not reported by allocation group. A 'per protocol' analysis was done (participants with missing data were excluded from the analysis). Additionally, incomplete data for continuous outcomes (no standard deviations) and some complications not reported by treatment group |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Authors did not declare primary outcome and some results were not fully reported: range of motion after 6 to 8 weeks, and grip & pinch at 24 months |
| Major baseline characteristic differences | Unclear risk | Baseline characteristics not provided for all participants (only 80%: 96 of 120). The Kapandji group had more participants with fractures in the dominant limb (52% versus 36%) but we considered that it was unlikely to have a major impact on the study results. |
| Performance bias: expertise, other care | Unclear risk | No mention of rehabilitation (advice and exercises) or surgeon expertise with treatment methods |
Mardani 2011.
| Methods | Method of randomisation: not stated
Assessor blinding: no
Intention‐to‐treat analysis: no. If participants lost reduction and required further intervention, they were excluded from the study. Loss to follow‐up: no participant flow diagram. There was some loss to follow‐up as the authors described mean postoperative visits of 4.4 for group 1 and 3.6 for group 2 out of a possible 5. |
|
| Participants | Teaching hospital, Iran 198 participants Inclusion criteria: musculoskeletally mature patients with displaced but stable distal radius fracture with congruous joint with less than 2 mm joint gap [Fernandez classification type 1: could be Colles or Smith fractures], patient consent Exclusion criteria: patients with open physis, open fracture, dorsal comminution, dorsal tilt more than 20 degrees, history of previous wrist or forearm fractures, congenital or other forearm or other anomalies, previous history of wrist operation, history of psychiatric problems, and fractures in other parts of injured upper limb Classification: Fernandez type 1 (extra‐articular) Sex: 87 female Age: mean 50.8 years, range 16 to 75 Assigned: 99/99 [Pinning and short arm cast/long arm cast] Assessed: ?/? (at 12 weeks); numbers not reported | |
| Interventions | Timing of intervention: timing not stated All had general anaesthesia and closed reduction. (1) Percutaneous pinning with smooth and without threaded 1.5 mm or 2 mm pin and then immobilised with short arm cast. The pin was shortened, curved and then remained out of the skin and the splint of the near pin was removed for monitoring pin tract infection. (2) Long arm cast applied by the same orthopaedist If reduction was unacceptable (criteria supplied), the fractures were re‐reduced and the participant was excluded from the study. Otherwise, the participants were asked to attend clinic at 1, 3, 6, 8 and 12 weeks for follow‐up. If they had acceptable reduction, the splints were opened in 6th or 8th weeks and pins were removed at outpatients and wrist physiotherapy started. |
|
| Outcomes | Length of follow‐up: 12 weeks, also 1, 3, 6 and 8 weeks
(1) Functional: Saito score (Fujii 2002) (2) Clinical: complications, pin tract infection, Saito score, subjective patient satisfaction (3) Anatomical: loss of reduction qualified by four reduction criteria (radial shortening less than 5 mm; radial inclination more than 15 degrees; volar tilt between 0‐15 degrees; and joint gap less than 2 mm) (4) Other: attendance of the 5 follow‐up visits |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After taking consent, patients were divided into two groups randomly.” No detail of randomisation/sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After taking consent, patients were divided into two groups randomly.” No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No report of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective outcome was the satisfaction scale (Saito chart) “which includes subjective satisfaction of each patient”. This subjective score is highly likely to have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Loss of radiographic reduction, mean postoperative visits, pin tract infection frequency. Neither participants nor personnel were blinded. |
| Incomplete outcome data (attrition bias) Short term | High risk | No participant flow diagram. There was some loss to follow‐up as the authors described mean postoperative visits of 4.4 for group 1 and 3.6 for group 2 out of a possible 5. No reasons for missing data available Also, results of participants with loss of reduction who required further intervention prior to discharge (and possibly at follow‐up) seem to have been excluded from analysis. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration No breakdown of the Saito score components in the results Additional outcome (finger stiffness) reported in the results |
| Major baseline characteristic differences | Low risk | Age and gender appear balanced. Type of fracture was consistent (Fernandez type 1 inclusion criterion). Additional potential sources of bias largely excluded “All other patients with open physis, open fracture, dorsal comminution, dorsal tilt more than 20 degree[s], history of previous wrist or forearm fractures, congenital or other forearm or other anomalies, previous history of wrist operation, history of psychiatric problems, and fractures in other parts of injured upper limb were excluded.” |
| Performance bias: expertise, other care | Low risk | Same surgeon, other care was comparable for groups. |
Milliez 1992.
| Methods | Randomised by admission sequence (alternation?) Assessor blinding: not reported Intention‐to‐treat analysis: potential problems Loss to follow‐up: 3 due to treatment failure | |
| Participants | Teaching hospital, France 60 participants Inclusion criteria: closed displaced distal radial fracture, intra‐ and extra‐articular; patient consent Exclusion criteria: age < 16 years, previous fracture, ipsilateral limb injury, dependent, disrupted volar radial cortex, anterior displacement, immediate vascular or nerve complications Classification: not given Sex: 44 female Age: mean 55 years, range 19 ‐ 91 years Assigned: 30/30 [1 week/6 weeks] Assessed: 27/30 (at 3 months) | |
| Interventions | Timing of intervention: timing of operation not stated Closed manipulation. Kapandji wiring: 3 pin K‐wire fixation under X‐ray control using the Kapandji intrafocal method. Regular incisions under tourniquet control. Pins cut level with skin and covered (1) Plaster splint 1 week, then early mobilisation (carrying object < 1 kg) (2) Plaster splint 1 week, then 5 weeks cast immobilisation Pins removed at 6 weeks | |
| Outcomes | Length of follow‐up: 3 months; also assessed at postoperative visit, 1, 3 and 6 weeks and 2 months (1) Functional: return to work (19 participants), grip strength, pain (none, during effort, during usual activities), range of movement (flexion, extension) (2) Clinical: complications: treatment failure, wire displacement, tendon injury or rupture, RSD (3) Anatomical: X‐ray postoperatively and 3 months. Bascule sagittale (dorsal angulation), index radio‐cubital inferieur (change in ulnar variance), bascule frontal (change in radial angulation) | |
| Notes | Paper in French ‐ translation obtained Potential for performance bias and iatrogenic complications arising from K‐wire fixation by junior operators. Only percentages were given for the pain categories: these did not yield exact numbers and thus there is some question regarding how these were derived in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from translation: "Randomisation done by regularly incorporating patients to one group or the other according to the chronology of their admission to the hospital". Comment: quasi‐randomised |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded. Subjective outcome: pain |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of participants. Subjective outcome: pain |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Outcome assessors were not blinded but these outcomes are less susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | Unclear risk | Recording of complications was not blinded. However, the type of complications reported are less susceptible to bias. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | No participant flow diagram and no explicit confirmation of loss to follow‐up; some percentages do not compute to whole numbers. Three post‐randomisation exclusions in the early mobilisation group for treatment failure but acknowledged |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Although the outcomes recorded in methods were reported in the results, there was incomplete reporting of outcome, including no SDs, P values or confidence intervals, and short follow‐up. |
| Major baseline characteristic differences | Low risk | Baseline characteristics were balanced for key characteristics: age, sex, basic fracture type (extra‐ and intra‐articular), side of injury (left/right) and whether injury was an accident at work. |
| Performance bias: expertise, other care | Unclear risk | No mention of instructions or advice during plaster cast immobilisation. No information on expertise of care provider. Although common to both groups, all operations were by less experienced (junior) surgeons. |
Murphy 2008.
| Methods | Method of randomisation not stated Assessor blinding: not known Intention‐to‐treat analysis: not known Loss to follow‐up: not known | |
| Participants | Teaching hospital, Ireland 60 participants Inclusion criteria: patients with displaced distal radius fractures "requiring K wiring" Exclusion criteria: not known Classification: not known Sex: not known Age: not known Assigned: 30/30 [buried/exposed] Assessed: not known (2 and 6 weeks) |
|
| Interventions | Timing of intervention: not known
(1) Buried Kirschner wiring (2) Exposed Kirschner wiring Semi‐closed procedure. No details of type of wiring, use of cast or timing of wire removal (probably at 6 weeks). No information on antibiotic administration or wound care |
|
| Outcomes | Length of follow‐up: 6 weeks (assessed at 2 and 6 weeks) (1) Functional: none (2) Clinical: complications: pin site infection (0‐6 point scale), EPL tendon damage, superficial radial nerve damage (3) Anatomical: none |
|
| Notes | Abstract only. Attempted to contact authors with no response (April 2017) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No report of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No blinding reported |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Missing data/loss to follow‐up not reported |
| Selective reporting (reporting bias) | High risk | No trial registration or publicly available protocol. Reported only in an abstract – incomplete data on outcomes reported (adverse events). No functional data reported |
| Major baseline characteristic differences | Unclear risk | No baseline characteristics reported |
| Performance bias: expertise, other care | Unclear risk | This information was not reported. |
Rodriguez‐Merchan 1997.
| Methods | Randomised using blinded consecutively numbered envelopes Assessor blinding: not stated Intention‐to‐treat analysis: likely Loss to follow‐up: none, probably | |
| Participants | Teaching hospital, Spain 40 participants Inclusion criteria: comminuted unstable fracture of distal radius [dorsal angulation >/= 10 degrees or radial shortening >/= 3 mm, or both] as a result of a fall, Frykman III to VIII (intra‐articular) Exclusion criteria: < 45 or > 65 years Classification: Frykman (III to VIII) Sex: 29 female Age: mean 57 years; range 46 ‐ 65 years Assigned: 20/20 [X‐pins/POP] Assessed: 20/20 (at 1 year) | |
| Interventions | Timing of intervention: probably reduction on first day, surgery on next day
(1) Closed reduction under general anaesthesia or brachial block. Percutaneous pinning with fluoroscopic assistance using 3 K‐wires. Two 0.45 mm K‐wires inserted from radial styloid proximally towards the ulna and one inserted from the ulnar side proximally towards the radius. Forearm cast applied. Pins and cast removed after 7 weeks
(2) Closed manipulation under local anaesthesia, split below‐elbow cast (20 degrees palmar flexion, 10 degrees ulnar deviation) (remanipulation at 1 week if dorsal angulation > 10 degrees, radial shortening > 3 mm. New cast applied). Patients given instructions to mobilise their fingers. Cast removed after 7 weeks. If fracture healed, arm was then bandaged in crepe and participants given instructions for mobilisation. |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 1, 3 and 7 weeks (1) Functional: probably at 1 year but not stated clearly. Overall grading (Horne 1990 ‐ actually Stewart 1985 modification of Gartland 1951) based on subjective and objective scores. Return to work (subgroup of participants), also grip strength, range of movement (overall), pain, deformity (2) Clinical: complications: remanipulation (at 1 week), pin track infection, joint infection or osteomyelitis, median nerve injury, Sudeck’s atrophy, tendon injuries, non‐union, angulated malunion (3) Anatomical: measured at post‐reduction and all other follow‐up times. Dorsal angulation, radial angulation, radial length | |
| Notes | There is no specific mention in the trial report of incorporation of the wire or pin ends into the plaster. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients .... were entered into a prospective randomized study (blinded consecutively numbered envelopes)". Comment: no information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients .... were entered into a prospective randomized study (blinded consecutively numbered envelopes)". Comment: insufficient mention of safeguards (i.e. sealed envelopes) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Participants were not blinded but we are unsure whether the limited subjectively reported outcome part of the composite score would have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Not blinded but unclear whether objective outcomes at 1 year would have been affected by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | Unclear risk | Recording of complications was not blinded but differential surveillance and recording resulting from lack of blinding are less likely. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | No participant flow or explicit confirmation of no loss to follow‐up. |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | No participant flow or explicit confirmation of no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Overall, the results were incompletely and inadequately reported. |
| Major baseline characteristic differences | Low risk | Balanced for age, sex, dominance, and labourer/non‐labourer. Although not split by fracture classification, all fractures met criteria for unstable fractures as defined by authors. |
| Performance bias: expertise, other care | Unclear risk | Difference in anaesthesia for reduction. No mention of instructions or advice during plaster cast immobilisation. No information on expertise of care provider(s) |
Saddiki 2012.
| Methods | Method of randomisation not stated Assessor blinding: neither participants nor personnel were blinded. Intention‐to‐treat analysis: not stated Loss to follow‐up: 18 lost at 3 months and 33 (8 of whom had died of unrelated causes) at 12 months | |
| Participants | Teaching hospital, France 97 participants Inclusion criteria: adult patients aged over 18 years with intra‐ or extra‐articular dorsally displaced distal radius fracture requiring surgical treatment Exclusion criteria: open fractures, fracture dislocations, palmarly displaced distal radius fractures, previous history of trauma to the upper limbs and "protected or minor individuals" (presumably those requiring protection, including children) Classification: four types of dorsally displaced distal radius fracture were identified: extra‐articular fractures: associated with fracture of the ulnar styloid process = Gerard‐Marchand (GM) fracture, not associated with fracture of the ulnar styloid process = Pouteau‐Colles (PC) fracture; intra‐articular fractures: simple: fractures with postero‐medial fragment (PMF), complex: T‐shaped intra‐articular fractures (sagittal and/or frontal). Results (incomplete) reported according to fracture type and independent of fracture type Sex: 83 female Age: mean 63 years Assigned: 49/48 [Kapandji/Py ] Assessed: 31/33 (at 12 months) | |
| Interventions | Timing of intervention: timing of operation not stated
In both groups, pins were inserted through a small incision taking care to retract the tendons of the extensor compartments for dorsal wires and the sensory branch of the radial nerve for lateral wires.
(1) Kapandji’s (intrafocal pinning): three '15/10◦' K‐wires were used to perform intrafocal pinning. After intraoperative reduction by close manipulation, the first K‐wire was introduced laterally while the two others were inserted postero‐laterally and postero‐medially, at a minimum 40º angle relative to the vertical axis of the radius.
(2) Py’s (isoelastic pinning): two K‐wires featuring a spatulated tip for easier progression along the medullary canal of the radius were introduced into the epiphysis of the distal radius after reduction by close manipulation, the first one being inserted from the tip of the radial styloid and the second one from inside the Lister’s tubercle. The entry point of each K‐wire was radiographically controlled. Retrograde pinning of the radius was then performed up to the sub‐chondral bone of the radial head. Two '18/10◦' K‐wires were systematically used in this series. Each K‐wire positioning and orientation was assessed radiographically. A frontal and sagittal radiographic control was systematically performed at the end of the surgery. A plaster splint was then applied for a 3‐week immobilisation period followed by a removable splint in all cases allowing the participant to start daily self‐rehabilitation exercises prior to the pin removal after 6 postoperative weeks. |
|
| Outcomes | Length of follow‐up: 12 months; also assessed at 3 and 6 weeks, and 3 months (1) Functional: DASH score, range of motion, grip strength (2) Clinical: complications, Jakim radio‐clinical score (Jakim 1991) (3) Anatomical: frontal radial tilt, sagittal radial tilt, radial length and ulnar variance, Jakim radio‐clinical score (Jakim 1991) | |
| Notes | It is not clear what the descriptors '18/10◦' and '15/10◦' stand for. However, it is possible that they represented the % chromium and nickel composition of the stainless steel wires: thus, 18% chromium and 10% nickel is known as '18/10 stainless'. SDs were not reported but could be derived from exact P values for DASH scores and two anatomical outcomes: frontal radial tilt, sagittal radial tilt. The data for range of motion, Jakim score and DASH were split according to fracture type. However, standard deviations (SD) and the numbers of participants in each subgroup (fracture type) were not provided and thus these data could not be used. Data split by treatment group for DASH at one year were available and thus presented in the analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Ninety seven patients were included randomly in one of two groups…”. No information on sequence generation or method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Ninety seven patients were included randomly in one of two groups…”. No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Subjective outcome was the DASH. Outcome assessors were not blinded; however, this type of outcome (patient‐reported) is less susceptible to outcome bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Outcome assessors were not blinded; however, this type of outcome is less susceptible to outcome bias. The exception is the Jakim radio‐clinical scoring system; however, the primary outcome measure was purely radiographic. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded. |
| Incomplete outcome data (attrition bias) Short term | High risk | 18% of participants did not attend at 3 months. The number of participants lost to follow‐up from each group at 3 months was not given. |
| Incomplete outcome data (attrition bias) Longer term | High risk | 34% of participants did not attend at 1 year, which is a high loss to follow‐up and could have affected the results despite the number of losses per group being similar (18 (37%) versus 15 (32%)). |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Choice of primary outcome was unusual (subscale of a composite clinician derived score). Reporting of conference abstract and final publication were reasonably consistent, with very minor differences. However, incompletely reported with no SDs and subgrouped by fracture type but with no reporting of the numbers in each category |
| Major baseline characteristic differences | Unclear risk | Differences in age and gender were present but were not major. Authors did not report the baseline distributions in fracture type of the four types they identified in their 'Patients and methods' section. |
| Performance bias: expertise, other care | Unclear risk | Data on surgeon or clinician experience with either technique or seniority not given. Nor are there data on timing of the procedures. The authors stated that in their practice the 2 techniques were used “indifferently by operators”, apart from complex cases. However, complex cases were included in the study. |
Shankar 1992.
| Methods | Method of randomisation not stated Assessor blinding: unlikely, not reported Intention‐to‐treat analysis: not known Loss to follow‐up: none, inferred | |
| Participants | Hospital, UK 45 participants Inclusion criteria: comminuted Colles' fracture, Frykman fracture IV‐VIII (intra‐articular) Exclusion criteria: not given Classification: Frykman (IV‐VIII) (intra‐articular) Sex: 40 female Age: range 17 ‐ 88 years (mostly "elderly") Assigned: 23/22 [X‐pins/POP] Assessed: 23/22 [6 months] | |
| Interventions | Timing of intervention: not stated. All procedures carried out under general anaesthesia and image intensifier control
(1) Percutaneous pinning: two 1.6 mm percutaneous Kirschner wires inserted from radial side into the medial cortex of distal ulna. The pins were left protruding 1.5 cm and incorporated into a plaster cast (slight palmar flexion and ulnar deviation). Wires and plaster removed at 5 to 6 weeks
(2) Nonsurgical treatment: forearm plaster cast (slight palmar flexion, ulnar deviation and pronation), for 5‐6 weeks All participants were admitted for overnight limb elevation. All had active physiotherapy after removal of plaster cast (and wires). |
|
| Outcomes | Length of follow‐up: 6 months; also 5‐6, 10 and 16 weeks (1) Functional: overall grading ‐ modified McBride scoring system (residual deformity, subjective (pain, limitations), objective, complications ‐ derived from Gartland and Werley 1951 (Gartland 1951), grip strength, range of movement (flexion, extension, radial and ulnar deviation, pronation, supination) (2) Clinical: complications: remanipulation, carpal tunnel syndrome, shoulder hand syndrome, residual deformity (ulnar styloid prominence), pin track infection, premature removal of pins at 3 weeks due to metal sensitivity, non‐union (3) Anatomical: X‐ray postoperatively, 10 and 16 weeks. Radial angle and length, dorsal and volar angle, and union | |
| Notes | The pinning technique was stated as being based on that described by DePalma 1952 but there were significant differences. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were allocated randomly upon admission to one of two groups.” Comment: No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were allocated randomly upon admission to one of two groups.” Comment: No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded. Subjective outcome: part of overall grading scheme |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded but, although limited, the subjectively reported outcome part of the composite score is likely to have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Not blinded but assessment of objective outcomes of functional impairment at 6 months would have been less susceptible to risk of bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded and may be susceptible to risk of bias. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | No participant flow or explicit confirmation of no loss to follow‐up. Incomplete data for continuous outcomes (no standard deviations) |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | No participant flow or explicit confirmation of no loss to follow‐up. Incomplete data for continuous outcomes (no standard deviations) |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Authors did not declare primary outcome. While the outcomes recorded in methods were reported in results, the results were incomplete and the categorisation of the McBride scoring system was not described beforehand. |
| Major baseline characteristic differences | Unclear risk | Age and sex distribution claimed to be "similar in both the groups" but pinning group had 2 and nonsurgical group 4 participants older than 80 years. Uncertain if this would have affected the results. Although all were intra‐articular fractures, there was no information on fracture distribution. |
| Performance bias: expertise, other care | Unclear risk | Similar anaesthesia and intent for rehabilitation. No information on surgeon and other care provider expertise |
Shannon 2003.
| Methods | Method of randomisation: randomisation was by closed envelope. No information on sequence generation Assessor blinding: not known Intention‐to‐treat analysis: not known Loss to follow‐up: not known | |
| Participants | Hospital, Ireland
46 participants
Inclusion: patients with unstable fractures of the distal radius Exclusion: not known Classification: AO Classification (not reported) Sex: 37 female Age: mean 58 years, range 17–87 Assigned: 24/22 [novel intermedullary spring loaded technique/transcortical K‐wire insertion] Assessed: 24/22 (minimum of 6 weeks) |
|
| Interventions | Timing of intervention: not known (1) K‐wires inserted using a novel intramedullary spring loaded technique: the wires were engaged into the opposite cortex and driven down the medullary canal (spring‐loaded) (2) Percutaneous stabilisation using K‐wires inserted in the traditional transcortical fashion All fractures were reduced and three 1.6 mm K‐wires were inserted using one of two techniques. |
|
| Outcomes | Length of follow‐up: minimum 6 weeks (1) Functional: "Wrist Function Score" (unclear which one) (2) Clinical: complication (fixation failure) (3) Anatomical: radiographic mean dorsal angle, mean loss of radial length |
|
| Notes | Abstract only. Authors contacted in 2006 and again April and July 2017. No information in addition to the published abstract was obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated using closed envelopes into one of two groups." No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated using closed envelopes into one of two groups." Insufficient detail to judge whether allocation was concealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding mentioned |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding mentioned. Not possible to blind surgeons |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding mentioned |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | No blinding mentioned |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No blinding mentioned |
| Incomplete outcome data (attrition bias) Short term | High risk | Participant flow not stated. Standard deviations not reported |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Abstract report only with incompletely reported results |
| Major baseline characteristic differences | Unclear risk | No information provided |
| Performance bias: expertise, other care | Unclear risk | No information on care pathways or surgeon experience with intervention |
Snow 2007.
| Methods | Method of randomisation using closed envelopes
Assessor blinding: assessor of function blinded; radiographic assessor not blinded; assessment of complications not blinded Intention‐to‐treat analysis: not reported, unlikely. Three participants excluded after randomisation (bilateral fractures) Loss to follow‐up: not reported. No flow chart and no information on loss to follow‐up given or imputable from results/tables |
|
| Participants | Hospital, UK
63 participants
Inclusion criteria: failure to hold fracture position in a typical Colles‐type cast and re‐displacement (following reduction in Accident and Emergency) with dorsal angulation greater than 10 degrees recognised within 14 days
Exclusion criteria: patients were excluded if there was volar comminution, a displaced intra‐articular fracture, if there was a delay in recognising displacement of greater than 14 days; who were unable physically or mentally to undertake regular follow‐up and perform functional assessment, or with previous malunion to the affected wrist and with injury to the opposite wrist. Classification: AO A3.2, A3.3, C2.1, C2.2 Sex: (of 63) 52 female Age: (of 63) mean 60 years, range 17‐84 Assigned: 27/33 [Dorsiflexion/ Palmar flexion] Assessed: ?/? (at 17 weeks); numbers not reported |
|
| Interventions | Timing of intervention: displacement recognised later than 14 days The fractures were initially reduced by closed manipulation and percutaneous Kirschner wires were "inserted as described by Clancey" (seems misreferenced in paper). One 1.6 mm wire was inserted at the tip of the radial styloid process just dorsal to the first extensor compartment and another was inserted into the dorsal ulnar corner of the distal part of the radius between the fourth and fifth extensor compartments. The surgeon was given the option to insert a third wire for intra‐articular fractures and when deemed necessary. The wires were cut, bent, and left protruding from the skin. Meticulous care was taken to avoid skin tethering. A full plaster was then applied to wrist. The K‐wires were removed in the outpatient clinic at 3 weeks and participants were then placed back into their selected casts for a further 2 weeks. The postoperative care of both groups was identical. (1) Group 1 had their wrists placed in 30 degrees of dorsiflexion, (2) Group 2 had their wrists placed in 30 degrees of palmar flexion. The angulation of the plaster was checked with a goniometer. |
|
| Outcomes | Length of follow‐up: 17 weeks, also 1, 3, 5 and 8 weeks (1) Functional: power, pinch grip strength (Jamar dynamometer), flexion and extension (goniometer), Rolyan nine‐peg test of dexterity (2) Clinical: pin site infection, deep infection, reflex sympathetic dystrophy (3) Anatomical: dorsal angulation, radial inclination, radial height, and radial length | |
| Notes | This trial was in Studies awaiting classification in Handoll 2007, where it was noted that a pre‐publication report had been received, sent by the 'Research Lead' in the recruiting hospital, after completion of the first version of the review. One of the tables in the draft report provided SDs for anatomical outcomes; these were not presented in the final report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was undertaken in theatre using a closed envelope system.” Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was undertaken in theatre using a closed envelope system.” Insufficient information on whether envelopes were sequentially numbered, opaque and sealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Low risk | Quote: “Functional review was carried out by an occupational therapist (who was blinded as to the patient group) at 5, 8 and 17 weeks post‐operation.” Functional assessor blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Assessment of complications not blinded and likely to be susceptible to bias |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Three participants excluded after randomisation (bilateral fractures) – but acknowledged. No flow chart and no information on loss to follow‐up given or imputable from results/tables. No absolute numbers of participants retained, hence missing data could not be checked |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | As above |
| Selective reporting (reporting bias) | High risk | Registration was available. Additional multiple follow‐up times for outcome measures than those reported in registration. Different exclusion criteria Radiographic outcomes reported using measurements that were not prespecified |
| Major baseline characteristic differences | Unclear risk | There were more severe fractures in group 2 (10 C2.2 vs 5 in group 1). Authors stated “Group I consisted of 27 patients and group 2 consisted of 33 patients. The groups were similar in age, gender, types of injury, initial displacement, and fracture type.” However, they did not provide absolute numbers apart from fracture type. |
| Performance bias: expertise, other care | Low risk | No information on surgeons or other care provider expertise. However, the intervention in this case is very simple and well standardised (apply a cast in a position, aided by goniometer to control angles), so level of expertise should not have introduced significant bias. In addition, “(t)he postoperative care of both groups was identical.” |
Soleiman pour 2011.
| Methods | Method of randomisation not stated Assessor blinding: unlikely, not reported Intention‐to‐treat analysis: not reported Loss to follow‐up: none, stated | |
| Participants | Hospital, Iran
50 participants treated with percutaneous pinning
Inclusion criteria: dorsally displaced metaphyseal distal radius fractures and non‐comminuted intra‐articular fractures treated by percutaneous pinning (method of pinning not stated although Kapanji mentioned in text) Exclusion criteria: open fracture, comminuted intra‐articular fractures, nerve injury, fracture not amenable to percutaneous pinning Classification: none given; mention of extra‐articular and intra‐articular fractures Sex: 23 female Age: no information Assigned: 25/25 [1 week/4 weeks] Assessed: 25/25 [3 months] |
|
| Interventions | Timing of intervention: timing of operation not stated Type of pinning not stated. passing mention of Kapandji pinning. Type of immobilisation not stated (1) Immobilisation for 1 week (2) Immobilisation for 4 weeks No other information on aftercare, exercises or wire removal | |
| Outcomes | Length of follow‐up: 3 months; also 1.5 months
(1) Functional: grip and pinch strength, range of movement (flexion, extension, radial and ulnar deviation, pronation). Return to daily activities: eating, wearing clothes, combing, closing buttons, writing
(2) Clinical: pain (no details), complications: pin track infection, pin loosening, non‐union (3) Anatomical: none |
|
| Notes | Article in Persian. Sent email requesting translation and method of randomisation on 05/12/2016. Repeated 16/12/2016 but just asked for randomisation method. Part translation obtained from Meisam Abdar on 11/01/2017; via Joanne Elliott, requested methods of pinning (Kapandji mentioned) Meisam Abdar confirmed that they didn't mention what type of pinning had been used and that "Kapandji" was mentioned in the introduction and the translation of the sentence was " one of the methods for treatment of distal radius fracture is Kapandji method". Meisam sent an email on 12/01/2017 to the "corresponding author and asked what kind of pinning did they use in Persian. If they answer me, I will forward for you.". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a randomised clinical trial, 50 patients … who were treated by percutaneous pinning were selected and divided into two groups of immobility for one week (group 1) and immobility for four weeks (group 2)." [translation] Comment: No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “with simple randomisation” [translation] Comment: No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded. No quantitative data for pain; this was still likely to have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Not blinded but assessment of objective outcomes of functional impairment at 1.5 and 3 months should have been less susceptible to risk of bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded and may be susceptible to risk of bias. |
| Incomplete outcome data (attrition bias) Short term | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Incomplete data reporting. Authors reported interim (1.5 months) statistically significant range of motion results but not the final results. |
| Major baseline characteristic differences | Unclear risk | No information on which to judge this. Authors stated there were no differences between the two groups without data. |
| Performance bias: expertise, other care | Unclear risk | No information on which to judge this. Especially, there was a lack of information on the method of percutaneous pinning. |
Stoffelen 1998.
| Methods | Method of randomisation: alternation Assessor blinding: unlikely, not reported Intention‐to‐treat analysis: imbalance of 2 in the numbers allocated to each group indicated a potential problem. Loss to follow‐up: none, inferred | |
| Participants | Teaching hospital, Belgium 98 participants Inclusion criteria: extra‐articular distal radius fractures with dorsal displacement ‐ Colles' type (Frykman I and II) (extra‐articular) Exclusion criteria: children, age > 80 years, bilateral fractures, severe injuries to ipsilateral or contralateral extremity, multiple injuries, severe brain injury Classification: Frykman (I and II) Sex: 41 female (but discrepancies between reports) Age: mean 58 years Assigned: not clearly reported but stated as 48/50 [X‐pins/POP] Assessed: 48/50 (at 1 year) | |
| Interventions | Timing of intervention: not stated (1) Percutaneous triple intrafocal Kapandji pinning (distal fragment was not transfixed; pins acted as a buttress to articular surface) followed by 1 week of plaster immobilisation until pain subsided. (By deduction: pins in place for 6 weeks) (2) Closed reduction, followed by an above‐elbow plaster cast for 3 weeks, then below‐elbow plaster cast for 3 weeks | |
| Outcomes | Length of follow‐up: 1 year; also assessed at 6 weeks, and 3 and 6 months (1) Functional: overall grading (Cooney score) including pain, functional status, range of movement, grip strength, finger and hand flexibility and function (2) Clinical: complications: nerve injuries (6 weeks): median nerve contusions, superficial radial nerve injuries; nerve injuries at 6 months (1 in the K‐wire group), reflex sympathetic dystrophy (temporary, and persisting at 1 year), diminished finger function (3) Anatomical: X‐ray at all follow‐up times, radial shortening, dorsal tilt, radial angle, lateral shift | |
| Notes | Reference (no. 26 in one paper) to the Cooney scoring system was incorrect. Unacknowledged duplicate publication. No response from trialist to request sent on 27/07/2000 for clarification of baseline characteristics (which differed between the trial reports) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were alternately treated". Comment: quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were alternately treated". Comment: quasi‐randomised |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded. Subjective outcome: part of overall grading scheme |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded but, although limited, the subjectively reported outcome part of the composite score is likely to have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Not blinded but assessment of objective outcomes of functional impairment would have been less susceptible to risk of bias in the long term. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded and may be susceptible to risk of bias. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Participant flow not provided and no mention of loss to follow‐up. Also, imbalance in numbers in each group (50 versus 48), was not correct for strict alternation. |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | Participant flow not provided and no mention of loss to follow‐up. Also imbalance in numbers in each group (50 versus 48), was not correct for strict alternation. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Incompletely reported data (no SDs) and no cross‐referencing between the trial publications |
| Major baseline characteristic differences | High risk | Gender imbalance but not sure if 8% versus 31% or 87% versus 31% were males in two groups. Major imbalance also reported for numbers with high velocity injuries |
| Performance bias: expertise, other care | Unclear risk | No mention of instructions or advice during plaster cast immobilisation. No information on expertise of care provider(s) |
Strohm 2004.
| Methods | Method of randomisation not stated (claimed to be blinded) Assessor blinding: unlikely, not reported Intention‐to‐treat analysis: not known, baseline data not given for all participants Loss to follow‐up: 19 (2 deaths) | |
| Participants | Teaching hospital, Germany 100 participants Inclusion criteria: Colles' type fracture, open or closed, AO types A2, A3 and C1, informed consent Exclusion criteria: not given Classification: AO (types A2, A3 and C1) (extra‐ and intra‐articular); also classification of soft tissue damage Sex: 85 female Age: mean 65 years, range 15 ‐ 92 years Assigned: 50/50 [Kapandji/Willenegger] Assessed: 40/41 (median 10 months) | |
| Interventions | Timing of intervention: all acute treatment with surgery provided straightaway
Closed reduction performed under image intensification. Brachial plexus block or general anaesthesia on an outpatient basis. All wires were 1.8 or 2.0 mm.
(1) Modified Kapandji fixation. After reduction, intrafocal placement by hand, using drill sleeve, of 2 K‐wires via stab incisions. Then a third wire inserted through a stab insertion over the radial styloid process in a conventional manner (through the fracture). Wires bent and buried under skin. Limb immobilised for 3 weeks in a volar splint. Then for the next 3 weeks: physiotherapy consisting of active and passive exercises for the wrist and forearm performed with the splint only removed for physiotherapy
(2) Willenegger fixation. After reduction, 2 small stab incisions made over the styloid process of the radius and 2 K‐wires inserted through and advanced proximally, and anchored in the opposite cortex of the radius. Wire ends bent over and buried. Wrist immobilised for 6 weeks in a functional position (15 degrees of dorsiflexion in below‐the‐elbow cast) Standard postoperative care used for the two wiring methods. Wires removed under local anaesthesia after 6 weeks |
|
| Outcomes | Length of follow‐up: median 10 months (range 6 to 20 months)
(1) Functional: modified Martini grading system (pain, subjective assessment, strength, work and sports, range of movement, radiological outcomes, complications: 0: worst to 38: best)
(2) Clinical: complications: nerve irritation, signs of swelling, wire migration, "conversion procedure" (other operation), RSD, carpal tunnel syndrome, tendon injury or rupture
(3) Anatomical: X‐ray times not known. Part of Martini grading system: radial shortening, "tilting of joint surface at the radiocarpal joint", and radiocarpal joint subluxation and arthrodesis Operating time and imaging time were also recorded. |
|
| Notes | Three participants (2 versus 1) were graded as having "a deep and contaminated wound, contusion of the skin and muscles from outside by direct injury, imminent compartment syndrome, and a moderately severe to severe fracture type". Trialist had indicated his intention to reply to queries sent in March 2006; no subsequent contact. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The type of treatment was assigned by means of prospective, blinded randomization". Comment: No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The type of treatment was assigned by means of prospective, blinded randomization". Comment: No information on allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not clear if blinded. Subjective outcome: part of overall grading scheme |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded but, although limited, the subjectively reported outcome part of the composite score is likely to have been susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Not blinded but assessment of objective outcomes of functional impairment (in the overall score) at 6 months would have been less susceptible to risk of bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded and may be susceptible to risk of bias. |
| Incomplete outcome data (attrition bias) Short term | High risk | Comparable (10 (20%) versus 9 (18%)) loss to follow‐up in each group but still quite high. Incomplete data for continuous outcomes (no standard deviations) and number of participants with one or more complications not given |
| Incomplete outcome data (attrition bias) Longer term | High risk | Comparable (10 (20%) versus 9 (18%)) loss to follow‐up in each group but still quite high. Incomplete data for continuous outcomes (no standard deviations) and number of participants with one or more complications not given |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration. Incompletely reported data (no SDs); main outcome defined and data collected but reported by fracture type. Variable length of follow‐up (6 to 20 months) |
| Major baseline characteristic differences | Unclear risk | Baseline data only provided for the 81 (of 100) followed up. Age and fracture type balanced in those followed up |
| Performance bias: expertise, other care | Unclear risk | No information on surgeon expertise (although collected) and other care |
Venkatesh 2016.
| Methods | Method of randomisation: "computer generated simple randomization protocol" Assessor blinding: not reported Intention‐to‐treat analysis: unlikely, 10 participants lost to follow‐up were excluded from the analysis Loss to follow‐up: 10 | |
| Participants | Teaching hospital, India
70 participants
Inclusion criteria: all patients with radiologically confirmed extra‐articular fractures of distal radius (AO types 23‐A2, 23‐A3), medically fit, willing for the procedure and consented to be part of the study, above the age of 18 years and presenting with injuries not older than 2 weeks Exclusion criteria: patients with intra‐articular fractures involving radio‐carpal joint, open fractures of distal radius, distal radius fracture associated with neurovascular deficit, fractures in children and patients found to be at poor anaesthetic risk Classification: extra‐articular fractures of distal radius (AO types 23‐A2, 23‐A3) Sex: (of 70) not reported Age: (of 70) mean 47.75 years, range 24 to 73 years Assigned: 35/35 [K‐wiring/cast] Assessed: 30/30 (at 6 months) |
|
| Interventions | Timing of intervention: injuries not older than 2 weeks (1) Percutaneous K‐wire fixation: Once acceptable reduction was achieved [as above],1.5 mm K‐wires were passed through the radial styloid process piercing the far medial cortex of proximal fragment under C arm guidance. An additional K‐wire was passed through the ulnar side of the radius engaging the opposite cortex when deemed necessary by the operating surgeon. Once satisfactory reduction under C arm was confirmed, K‐Wires were bent and cut, sterile gauze applied beneath the pin. Plaster cast was applied extending from below elbow to metacarpal heads with wrist in neutral. Participants treated with percutaneous K wiring were given Intravenous cefaperazone sulbactum 1.5 grams twice daily for 3 days, followed by oral cefixime 200 mg twice daily. After six weeks, K‐wires and cast were removed. (1) Closed reduction and cast application: The fracture reduction was carried out under a short general anaesthesia. A plaster cast was applied extending from below the elbow to the metacarpal heads, maintaining the wrist in palmar flexion and ulnar deviation (Colles cast) in the closed reduction group. | |
| Outcomes | Length of follow‐up: 6 months (1) Functional: demerit scoring system of Gartland and Werley, range of motion (2) Clinical: none recorded (3) Anatomical: the radiological outcome was assessed based on the values of volar inclination, radial inclination and radial height. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Sampling was done by simple randomization of the cases done according to a computer generated simple randomization protocol." |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Nothing to suggest personnel or assessors were blinded. Participants could not be blinded. Gartland and Werley score was predominately physician‐rated so reported score susceptible to bias. Range of motion and radiographic parameters were also reported as absolute values. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | High risk | Nothing to suggest personnel or assessors were blinded. Participants could not be blinded. Gartland and Werley score was predominately physician‐rated so reported score susceptible to bias. Range of motion and radiographic parameters were also reported as absolute values. |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | “Five patients in each group were lost to follow up and hence the final study was conducted with 30 patients in each group and a total of 60 patients.” We do not know when these 10 participants were lost to follow‐up. The researchers proceeded to do complete case analysis, excluding all data from the 10 participants. |
| Incomplete outcome data (attrition bias) Longer term | Unclear risk | Maximum follow‐up 3 months (not applicable) |
| Selective reporting (reporting bias) | High risk | No prospective trial registration or published protocol found. Some discrepancies between methods and results in reported outcomes. Gartland and Werley categories were reported rather than scores. |
| Major baseline characteristic differences | Unclear risk | Baseline characteristics per group not given |
| Performance bias: expertise, other care | Low risk | Comparable treatment pathways, bar intervention |
Verhulst 1990.
| Methods | Method of randomisation not stated (unexplained imbalance in numbers in the 2 treatment groups) Assessor blinding: not reported Intention‐to‐treat analysis: not known Loss to follow‐up: not stated | |
| Participants | Hospital, Belgium 130 participants Inclusion criteria: Colles' type fracture Exclusion criteria: not given Classification: not given Sex: not given Age: "elderly population" Assigned: 45/85 [K‐wire/POP] Assessed: ?/? (at 2 years); numbers not reported | |
| Interventions | Timing of intervention: not stated Closed reduction (1) Percutaneous pinning: reduction and fixation of reduced fragments using a single Kirschner wire inserted percutaneously (stab incision) through tip of radial styloid. Immobilised for 4 weeks (mean). Pin removed at 6 weeks (mean) (2) Nonsurgical treatment: reduction and immobilisation for 5 weeks (mean) | |
| Outcomes | Length of follow‐up: 2 years; also assessed at 6 weeks and 2 months (1) Functional: time to return to normal activities and work; range of movement (along 3 axes) (2) Clinical: complications: re‐reduction, wire migration, superficial stab wound infection, CTS, wrist deformity, time of immobilisation (3) Anatomical: X‐ray at above times. Radial shortening, radial and dorsal angulation, radial width | |
| Notes | Abstract only. No indication of criteria for mobilisation. Cast immobilisation assumed. Numbers derived from percentages | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomised study" Comment: no information available to make judgement but no explanation for imbalance (45 versus 85) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "prospective, randomised study" Comment: no information available to make judgement but no explanation for imbalance (45 versus 85) |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Care providers (surgeon(s)) not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Unclear risk | Outcome assessors were not blinded but these outcomes are less susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | Recording of complications was not blinded. |
| Incomplete outcome data (attrition bias) Short term | High risk | No participant flow for short‐term outcomes but unlikely to be an issue. However, outcome data were incomplete. |
| Incomplete outcome data (attrition bias) Longer term | High risk | Loss to follow‐up provided but distribution (range) of follow‐up not given and outcome data were incomplete. |
| Selective reporting (reporting bias) | High risk | No published protocol or prospective trial registration; only published as a conference abstract. No subjective outcomes; incomplete reporting of outcome |
| Major baseline characteristic differences | Unclear risk | No information available |
| Performance bias: expertise, other care | Unclear risk | No information on care programmes or surgeon expertise |
Waheed 2004.
| Methods | Method of randomisation not stated Assessor blinding: not known Intention‐to‐treat analysis: not known Loss to follow‐up: not known | |
| Participants | Hospital, Ireland
52 participants
Inclusion criteria: patients with a distal radial fracture managed with percutaneous wire fixation and casting only
Exclusion criteria: not known Classification: Frykman’s Sex: 38 female [discrepancy between total number of participants and sex distribution] Age: mean 56.6, range 19 ‐ 84 years Assigned: 25/27 [buried wires/protruding wires] Assessed: not known |
|
| Interventions | Timing of intervention: not known
(1) Wires buried (2) Wires left exposed (protruding) All had percutaneous wire fixation and casting. No details of type of wiring, cast or intended duration of immobilisation. Cast and wire fixation were removed at a mean of 5.8 weeks in an outpatient setting. No information on antibiotic administration or wound care |
|
| Outcomes | Length of follow‐up: mean 5.8 weeks (1) Functional: none (2) Clinical: superficial Infection, pain during the period of wire fixation or pain during the removal of wires (not reported) on a visual analogue scale, participants requiring local anaesthesia in the operating theatre for removal, patient satisfaction (3) Anatomical: none |
|
| Notes | Abstract only. Attempted to contact authors in March 2006 and in February 2017 and April 2017 with no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “We prospectively randomised 52 consecutive patients...”; “Patients ... were randomly allocated”. No details of method reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No report of blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No report of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No report of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No report of blinding |
| Incomplete outcome data (attrition bias) Short term | Unclear risk | Participant flow not provided |
| Selective reporting (reporting bias) | High risk | No trial registration or publicly available protocol. Incompletely reported in abstract only |
| Major baseline characteristic differences | Unclear risk | Reported no between‐group difference in distribution of fracture types classified according to Frykman, but incomplete reporting of data (abstract only). No information on demographics per group |
| Performance bias: expertise, other care | Unclear risk | No information to judge this |
Wong 2010.
| Methods | Method of randomisation (sequence generation) not stated. “Patients were then randomly allocated by opening sequentially numbered opaque sealed envelopes."
Assessor blinding: blinded for functional and radiological outcomes
Intention‐to‐treat analysis: yes. "All patients included in the outcome analysis remained in their primary randomisation group regardless of secondary procedures". However, two participants were excluded from all analyses due to their deaths caused by heart disease. Loss to follow‐up: 2 participants excluded from the analysis; both died from heart disease "which was not related to postoperative complications"; thus they appeared to be from the surgery group. |
|
| Participants | Teaching hospital, China 62 participants Inclusion criteria: patients presenting with an unstable, dorsally angulated, extra‐articular fracture of the distal radius. Dorsal angulation greater than 20 degrees and radial shortening greater than 5 mm. Exclusion criteria: patients younger than 65 years old; patients with intra‐articular involvement, compound fractures, concomitant fractures elsewhere, palmar angulated fractures, minimally displaced fractures or fractures with dorsal tilting less than 20 degrees and fractures more than 2 weeks old; patients who had dementia or psychiatric illness Classification: Frykman’s I (36 fractures) or II (24 fractures) (extra‐articular) Sex: (of 60) 49 Age: mean 70.5 years, range 65 to 76 years Assigned: 32/30 Assessed: 30/30 (at 12 months) | |
| Interventions | Timing of intervention: fracture less than 2 weeks old K‐wire insertion was probably percutaneous. Pins inserted under general or regional anaesthesia. Reduction was done during the operation. (1) K‐wire group: operation done under Bier's block; with the distal radius in the reduced position, the surgeon then inserted three percutaneous K‐wires under fluoroscopic guidance through three small stab incisions ("tripod" construct described in report); wires were not buried; no plaster of Paris was applied to augment the fracture stability but the occupational therapist made a removable palmar splint for resting purpose. Immediate gentle mobilisation under instructions of a physiotherapist was allowed. Wires were removed after fracture consolidation. (2) Cast group: closed reduction under haematoma block, Jones's methods used for reduction, below‐elbow plaster of Paris was applied. | |
| Outcomes | Length of follow‐up: mean 19.5 (range 13–24) months; also at 1, 2, 4 and 6 weeks, 3, 6 and 12 months (1) Functional: Mayo wrist score, WHO quality of life, range of motion, grip strength (2) Clinical: healing rate, healing time, and complications (pin track infection, complex regional pain syndrome; no tendon, nerve or vessel injuries), subjective patient satisfaction (3) Anatomical: dorsal angulation, radial inclination and radial length |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were then randomly allocated by opening sequentially numbered opaque sealed envelopes." |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel performing intervention(s) not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants and personnel performing intervention not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “The functional outcome was assessed by an orthopaedic specialist physiotherapist, who was considered as blinded to the procedure …..The assessor of the radiological outcomes was blinded to the method of the treatment and the functional outcomes after removal of the plaster of Paris, the K‐wires and the splint.” |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | Low risk | Quote: “The functional outcome was assessed by an orthopaedic specialist physiotherapist, who was considered as blinded to the procedure …..The assessor of the radiological outcomes was blinded to the method of the treatment and the functional outcomes after removal of the plaster of Paris, the K‐wires and the splint.” |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No suggestion that personnel diagnosing complications were blinded |
| Incomplete outcome data (attrition bias) Short term | Low risk | Intention‐to‐treat analysis and researchers stated no loss to follow‐up. However, two participants were excluded from the analysis because of death from heart disease "which was not related to postoperative complications"; hence, unlikely to be related to the intervention. |
| Incomplete outcome data (attrition bias) Longer term | Low risk | As above |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol identified. Authors did not declare primary outcome but the outcomes recorded in methods were reported in results. |
| Major baseline characteristic differences | Low risk | “The groups had comparable preoperative demographics” as described in the table. |
| Performance bias: expertise, other care | Low risk | Quote: "All patients in each group were treated by an orthopaedic specialist who had more than 10 years’ experience to standardise the method. For the ‘Cast’ group, one surgeon, who used the same method of moulding the plaster of Paris, applied all plasters. In the ‘K‐wire’ group, another surgeon with a similar length of experience as the surgeon in the ‘Cast’ group did all the operations." |
Zyluk 2007.
| Methods | Method of randomisation: drawing of lots by participant Assessor blinding: no Intention‐to‐treat analysis: not reported, unlikely Loss to follow‐up: 64 | |
| Participants | Teaching hospital, Poland 124 participants Inclusion criteria: displaced distal radius fracture Exclusion criteria: fracture without displacement, open, with associated injuries of other structures, or did not agree to participate in the study Classification: AO A2, A3, B1, B2, C1, C2 and Frykman I‐VII Sex: (of 60) 49 female Age: (of 60) mean 61 years (range 21 to 82 years) Assigned of 124: 61/63 [K‐wire/cast] Assessed: 30/30 (at 6 months) | |
| Interventions | Timing of intervention: timing of operation not stated (1) Manipulation and percutaneous K‐wire group: operation under regional block anaesthesia and X‐ray control. Manual fracture reduction and percutaneous fixation with two to four k‐wires introduced via the radial styloid so that they pierced the opposite cortex. In some cases, one K‐wire was introduced "from the base of the arm". Wires were buried under the skin and a plaster immobilising "only the wrist" was applied. Participants discharged the next day. K‐wires removed after 1.5 months on an outpatient basis under local anaesthesia (2) Nonsurgical treatment group: manipulation under local anaesthesia and immobilisation in a "Vienna" type above elbow plaster/backslab, shortened to below elbow at 2 weeks. Follow‐up at one week for X‐ray and if position was lost were referred for surgery. X‐rayed and mobilised at 5 weeks No participants had physical therapy. | |
| Outcomes | Length of follow‐up: 6 months, also 1.5 and 3 months (1) Functional: DASH questionnaire, McCabe scale, Gartland‐Werley score, Castaing scale, wrist range of motion, finger loss of flexion, total grip strength (2) Clinical: rate of secondary displacement (displacement not defined), complications: not reported (3) Anatomical: dorsal or volar tilt, radial angulation and radial length on X‐ray | |
| Notes | Trial report in Polish. Google translated. Method of randomisation: information received from Andrzej Zyluk (7 June 2016): "All 60 patients were randomly assigned to receive conservative (n = 30) or operative by K‐wire fixation (n = 30) treatment by drawing slips of paper marked 1 (conservative) or 2 (operative) from a sealed envelope in the presence of a witness." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots performed by participant Quote: “The choice of treatment was random and depended on the result of the draw. The method of randomisation: before the decision on the method of treating a patient asked to tap into the envelope between two strips of paper marked 1 (conservative therapy) or 2 (surgery).” |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Neither participants nor personnel blinded. Multiple scores and scales reported. Castaign and Gartland‐Werley scores are clinician‐based/reported composite scoring systems. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | As above |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of assessors. Castaign and Gartland‐Werley scores are clinician based/reported composite scoring systems. |
| Blinding of outcome assessment (detection bias) Objective outcomes: functional impairment | High risk | No blinding of assessors. Though range of motion and grip strength are less susceptible to this type of bias, Castaign and Gartland‐Werley scores are clinician‐based/reported composite scoring systems, susceptible to bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes: complications etc | High risk | No blinding of assessment of complications |
| Incomplete outcome data (attrition bias) Short term | High risk | Loss to follow‐up in the short term was not reported separately. However, it was not clear whether there was intention‐to‐treat analysis regarding 5 participants who did not accept surgery and were thus treated conservatively. The 8 participants who had treatment for secondary displacement (% calculated out of 30 not 63 in report) were excluded. |
| Incomplete outcome data (attrition bias) Longer term | High risk | “In the group treated surgically, not all stages of the study 31 patients completed, and conservatively treated group 33, the total 64 patients (52%). Flow diagram Figure 1. All stages of research completed by 60 patients, 30 treated surgically and 30 conservatively treated and this group is the subject of this study.” (Google translation) Though balanced, proportions of missing data are very high. Reasons for loss to follow‐up not available |
| Selective reporting (reporting bias) | High risk | No prospective trial registration or published protocol identified. The outcomes recorded in methods were reported in results at the specified times but some reservations in the treatment of the results (borderline concern) |
| Major baseline characteristic differences | Unclear risk | Baseline data reported only for 60 of the 124 participants. Confounders bar fracture type not reported by group. Some imbalance of fracture type across the two groups that could have affected outcome, especially in view of large proportions lost to follow‐up |
| Performance bias: expertise, other care | Unclear risk | There was inadequate information on who performed the interventions; the casting was performed under local anaesthetic and the K‐wring under "spinal"; this could mean different settings (outpatient/emergency department versus operating theatre) and therefore differing levels of expertise. |
<: less than >: more than AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF) AP: anterior‐posterior CTS: carpal tunnel syndrome DASH: Disability of the Arm, Shoulder and Hand DISI: dorsal intercalated segment instability EPL: extensor pollicis longus (tendon): hypoaesthesia: decrease in sensation K‐wires: Kirschner wires paraesthesia: numbness, tingling, "pins and needles" sensation PGA: polyglycodic acid POP: plaster of Paris ROM: range of movement (wrist and forearm) RSD: reflex sympathetic dystrophy SD: standard deviation SF‐36: Short‐Form‐36 VAS: visual analogue scale VISI: volar intercalated segment instability WHO: World Health Organisation X‐pins: crossed percutaneous pinning
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Biedermann 2001 | Not a randomised comparison as confirmed by Dr Biederman |
| Chung 2019 | This multicentre randomised trial compared three surgical techniques (volar plate fixation; external fixation; percutaneous pinning). A fourth group comprised a cohort of "elderly patients" who chose not to have surgery. Thus, this was not a randomised comparison of percutaneous pinning versus closed reduction and cast. |
| Delattre 1994 | Not a randomised comparison. Mainly retrospective |
| Galli 2002 | It is not clear whether this was a prospective, and if so, a randomised comparison. There was no response received from the trialists. |
| Gunay 2015 | Retrospective comparison |
| Harper 2000 | The contact person for this trial, Prof Harper, revealed that this trial, previously listed in Ongoing studies, was abandoned. |
| IRCT2013120814271N2 | "Completed trial" available only as a sparse population trial registration document, registered 7 Januaury 2014, sponsor "Vice chancellor for research, Tabriz University of Medical Sciences", Iran. Reported as involving 60 participants aged over 60 years. Comparison: Percutaneous pinning ("Fractured bones will be fixed with some pins inserted through the skin. These pins are made by the German Hoffman corporation") versus Pin‐in Plaster group ("In addition to pinning by the same pins used for percutaneous pinning group, plastering will be applied to fix the fracture site"). No trial report has been identified for this trial and we received no response to requests for information, including on the comparison, from the investigators: request for information sent to Alireza Rouhani on 30/03/2016; repeated request to Fatemeh Martexapour on 26/04/2016. It seems very unlikely that clarification on the interventions or a full report of this trial will become available. |
| IRCT2016061828510N1 | This trial compared open pinning fixation of the ulnar styloid fracture versus no fixation. Surgery and long‐arm casting applied to both groups. Although the type of surgery is unclear, the mention of open pinning for the ulnar styloid fracture indicated that it wasn't percutaneous pinning. Trial excluded because it evaluated the use of supplementary percutaneous pinning in addition to another method of surgical fixation. Additionally, the trial is excluded because the intervention was targeting an associated injury (ulnar styloid fracture) and not the primary injury (distal radius fracture). |
| ISRCTN37842313 | Trial abandoned due to poor recruitment |
| NCT02353338 | Ongoing (start date: May 2015) randomised trial comparing surgical versus nonsurgical management: "the attending surgeon will determine the optimal fracture fixation method for the fracture pattern". Thus, this is a mixed surgery trial and not specifically focused on percutaneous pinning. |
| Seifert 1998 | Very unlikely to be a randomised comparison. Trial report in German was checked by Jan Rasmus Kuester who indicated that it was "rather retrospective" but wrote to the authors for confirmation. No further information received |
| Tomaszuik 2017 | Very unlikely to be a randomised comparison. There was no mention of randomisation or prospective allocation of the 77 participants into three groups: open reduction and volar plate versus closed reduction and percutaneous pining versus closed reduction and plaster cast only. |
Characteristics of studies awaiting assessment [ordered by study ID]
Kanakeshwar 2017.
| Methods | "Double blinded randomised comparative study" Recruitment: January 2015 to December 2015 |
| Participants | 145 patients above 60 years with dorsally displaced extra‐articular distal radius fractures |
| Interventions | Timing of intervention: timing of operation not stated (1) 3 K‐wires (2) 2 K‐wires |
| Outcomes | Length of follow‐up: mean 12 months Functional outcome: DASH score, wrist range of movements Radiological outcome: radial height, the radial inclination and the volar tilt |
| Notes | Trial methods, including interventions, and results insufficiently reported in conference abstract. Contacted authors 15 August 2018; received no response |
Mirhamidi 2013.
| Methods | Prospective trial where they apparently "randomly selected the method of fixation". However, the methods are unclear. |
| Participants | Teaching hospital, Iran
45 participants (all were skeletally mature)
Inclusion criteria: extra‐articular distal radius fracture
Exclusion criteria: comminuted fractures, open fractures, multiple fractures and intra‐articular extension Sex: 20 female Age: mean 46 years (range 17 to 84 years) Assigned: 22/23 [Kapandji/'extra‐focal'] |
| Interventions | Timing of intervention: timing of operation not stated
Closed reduction and pinning under general or local anaesthesia. Use of stab wounds. Fluoroscopy used in both groups
(1) Kapandji pinning. Use of 3 K‐wires inserted through the fracture site. Sugar‐tongue splint immobilisation for 4 weeks, then partial mobilisation with splint use for 14 days
(2) Extra‐focal pinning. Three K‐wires inserted across the fracture site. Immobilisation in long arm cast for 6 weeks Wires removed under local anaesthesia at 6 weeks and routine physiotherapy started |
| Outcomes | Length of follow‐up: 6 months, also 3 months (1) Functional: Gartland‐Werley score, grip strength (2) Clinical: complications, specifically for reflex sympathetic dystrophy (3) Anatomical: ulnar variance, palmar tilt, radial length and inclination on X‐ray |
| Notes | No response to email requesting method of randomisation and other data on 05 December 2016; repeated 12/12/2016 |
Russe 2000.
| Methods | Randomised trial using a computer program |
| Participants | 115 participants (4 clinics) Inclusion: extra‐articular (AO classification A2.1 to A3.2) and intra‐articular (B1.1 to B1.3) Sex: 76 female Age: mean 68 years Assigned: 58/57 [resorbable pins/K‐wires] |
| Interventions | Modified Kapandji technique (1) Bioresobable pins (Poly‐D, L‐Lactid) (2) K‐wires, removed at 5 weeks All were immobilised with a dorsovolar lower arm plaster splint, changed after 1 week to a circular plaster. Cast and wires removed at 5 weeks |
| Outcomes | Length of follow‐up: 1 year; also 5 weeks and 6 months (1) Functional: subjective appraisal (pain, activity and movement limitation), range of movement (2) Clinical: complications, swelling, foreign body reactions (3) Radiological outcomes |
| Notes | Trial was incompletely reported only as a series of conference abstracts with different numbers of participants: 115 (4 centres) recruited April 1995 to October 1999; 25 participants recruited October 1998 to February 1999; 30 (1 clinic at Bochum) recruited 1st January 1995 to 15 March 1996; and 30 (no details). Translations from German by Sonia Stewart On 16 August 2006, Professor Andreas David confirmed it was a multicentre randomised trial with 115 participants, with a protocol in German. No response to queries sent to Professor David in September 2006 and February 2017 |
AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF) DASH: Disability of the Arm, Shoulder and Hand
Characteristics of ongoing studies [ordered by study ID]
DRAFFT 2.
| Trial name or title | DRAFFT2: Distal Radius Acute Fracture Fixation Trial 2 |
| Methods | Randomisation method: secure, centralised online randomisation service. Randomisation sequence, stratified on the basis of centre, intra‐articular extension of the fracture and age of the participant (above or below 50 years) Assessor blinding: participants not blinded, treating clinical team not blinded; the outcome data were being collected directly from the participants themselves. Analysis: intention‐to‐treat Loss to follow‐up: trial investigators accepted that it is likely that some data may not be available due to the voluntary withdrawal of participants, lack of completion of individual data items or general loss to follow‐up. |
| Participants | The main trial planned to recruit from a minimum of 24 trauma centres across the UK.
Planned sample size: 476, actual recruitment 506 (completed 27/03/2019) ‐ information from trial website; 890 listed in updated trial registration document
Inclusion criteria: patients with a dorsally displaced fracture of the distal radius (fracture within 3 cm of the radio‐carpal joint), aged 16 years or over, able to give informed consent, treating Consultant Surgeon believes that patient would benefit from manipulation of the fracture Exclusion criteria: injury is more than two weeks old, the fracture extends more than 3 cm from radio‐carpal joint, open fracture with a Gustilo grading greater than 1, the articular surface of the fracture (specifically the radio‐carpal joint) cannot be reduced by indirect techniques or there is evidence that the patient would be unable to adhere to trial procedures or complete questionnaires, such as cognitive impairment |
| Interventions | Timing of intervention: less than two weeks from the time of injury. This trial will compare two techniques for holding the position of the bone fragments following a manipulation of a dorsally displaced fracture of the distal radius. (1) Surgical fixation with K‐wires. After the skin has been covered in antiseptic, the sharp wires are passed through the skin over the back of the wrist and directly into the bone in order to hold the bone fragments in the correct position. The principles of K‐wire fixation are also inherent in the technique, although there are several different options for the positioning of wires. The size and number of wires, the insertion technique and the configuration of wires will be left entirely to the discretion of the surgeon as per their normal practice. A plaster cast will be applied at the end of the procedure, as per standard surgical practice, but this does not need to be specifically moulded as the wires themselves hold the bone in position. Relevant information with regards the initial technique used and any subsequent interventions, such as metal work removal, cast replacement or removal will be recorded. (2) 'Plaster casting’: This technique involves the application of a plaster cast which is shaped (moulded) over the skin to hold the bone fragments in position.The principles of applying a moulded plaster cast are inherent in the technique, although in this pragmatic trial, the type of casting material, extent of the cast and the details of the moulding technique will be left to the discretion of the treating surgeon as per their usual technique. All participants randomised into the two groups were set to receive the same standardised, written physiotherapy advice detailing the exercises they need to perform for rehabilitation following their injury. |
| Outcomes | Length of follow‐up: 12 months, also 3 and 6 months Primary outcome: Patient Rated Wrist Evaluation Secondary: EQ‐5D‐5L, complications, radiographic evaluation (intraoperatively and at 6 weeks), healthcare resource use |
| Starting date | Trial start date: 01/07/2016 to 31/08/2020 (trial registration document) |
| Contact information | Dr Robin Lerner Nuffield Department Of Orthopaedics, Rheumatology and Muscoloskeletal Sciences, University of Oxford, Oxford, OX3 9DU, United Kingdom Phone: +44 1865 227912 Email: drafft2@ndorms.ox.ac.uk https://www.ndorms.ox.ac.uk/clinical‐trials/current‐trials‐and‐studies/drafft‐2 |
| Notes | Registration date: 03/08/2016 (last update 09/05/2019)
Ethics approval: 06/10/2016; ref: 16/SC/0462
Funding: The NIHR Health Technology Assessment programme, UK HTA Project Reference: 15/27/01 According to trial website, the results are due to be published in September 2020 (site accessed 30/08/2019) |
IRCT20160508027797N4.
| Trial name or title | Outcomes of unstable intra‐articular distal radius fracture using closed reduction, percutaneous pinning and plaster cast compared with closed reduction and plaster cast in patient older than 65 years |
| Methods | Parallel group randomised Randomisation method/sequence: coin toss Blinding: Claims to be "Single blinded" and "Patients will not be aware of the treatment they are taking, and this will be blinded." Seems unlikely given that informed consent for trial participation is also claimed Analysis: not stated |
| Participants | Single centre, Babol University of Medical Sciences, Iran Planned sample size: 60 Inclusion criteria: age 65 to 95 years, patients with closed fractures and intra‐articular distal radius that needed to be fixed by surgery, informed consent of patient in the study Exclusion criteria: surgery on wrist, severe underlying disease, lack of timely referral for follow‐up therapy to the orthopaedic clinic |
| Interventions | Timing of intervention: not specified (1) Percutaneous pining preceded by closed reduction of fracture under general anaesthesia in the first 24 hours. Pinning method: 3 pins 2 mm diameter; one pin entered via the radial styloid volar radius; one on dorsal side and one on the ulnar. C‐arm fluoroscopy control (interpretation of the statement provided may be partly incorrect). No mention of cast immobilisation. Labelled as 'control group' by authors. (2) Nonsurgical treatment group: closed reduction of fracture under general anaesthesia within the first 24 hours then cast applied: "ray casting is conducted at [as] casting method does not need to roll the fabric." |
| Outcomes | Length of follow‐up: 42 weeks after surgery Primary: range of motion of wrist, through the examination with handheld goniometer, radiographic parameters immediately after surgery, then 3, 6 and 42 weeks after surgery Secondary: pain severity (visual analogue scale) at 42 weeks, pin track infection at 3 weeks |
| Starting date | 30/12/2018 (date of first enrolment) |
| Contact information | Dr Masoud Bahrami Ferydoni, Babol University of Medical Sciences, Babol, Iran Telephone: +98 11 3225 6285 Email: Drbahrami865@gmail.com Affiliation: Babol University of Medical Sciences |
| Notes | Registration date: 03‐11‐2019 Funding: Babol University of Medical Sciences |
IRCT2016112727797N1.
| Trial name or title | Comparison of radiologic changes of unstable fractures of the distal radius bone using of three cross pin with 4 pin trans‐radioulnar |
| Methods | Randomisation method: "In this study, 50 patients have been assigned to two groups of intervention and control by using random numbers table". Assessor blinding: not blinded Analysis: no details Loss to follow‐up: no details |
| Participants | Recruitment centre: Babol Shahid Beheshti Hospital, Iran Planned sample size: 50 (recruited) Inclusion criteria: age 18‐65 years; patients with closed fractures and intra‐articular distal radius that needed to be fixed surgery; also informed consent of patient in the study Exclusion criteria: surgery on wrist; severe underlying disease |
| Interventions | Timing of surgery: not stated (1) Cross‐pin ulnar corner fixator: "Pin‐third of the ulnar angle into the distal radius". (2) Trans‐ulnoradial pinning: "Pin‐third the size of 1.5 in the distal is obliquely driven to the fracture site and the fourth pin of size 2 in proximal of to the fracture site is driven cross‐ulnar into the radius bone and it will be ended in the cortex of the radius bone." No details of immobilisation or rehabilitation |
| Outcomes | Length of follow‐up: 24 weeks Primary outcomes (post‐surgery; 6, 12 and 24 weeks): range of motion of wrist – handheld goniometer; radiological parameters Secondary: pain severity VAS (24 weeks); pin tract infection (6 weeks) |
| Starting date | Not known. Ethics obtained: 03/08/2015 |
| Contact information | Dr Danyal Hosseinzadeh Babol University of Medical Sciences, Ganjafrooz street, Babol, Iran Phone: +98 119832197667 Email: danyal_h1984@yahoo.com |
| Notes | Registration date: 27/11/2016
Funding: Vice Chancellor of Research and Technology, Babol University of Medical Sciences Trial published 19 August 2019; located on 30 August 2019. All data above were taken from the trial registration document. |
NCT03311633.
| Trial name or title | Distal radius fracture: comparison between three and six weeks of percutaneous fixation |
| Methods | Randomisation method: no details Assessor blinding: not blinded Analysis: Information available in trial registration document. This included sample size based on PRWE scale: "Using a mean difference formula with a standard deviation of 5 and an expected magnitude of the differences of at least 4 points on the PRWE scale, with a confidence interval of 95%, a power β of 80%, with a statistically significant P = ˂ 0.05, adding 20% of error. A sample of 30 participants was obtained per group." Loss to follow‐up: no details |
| Participants | Recruitment centre: Universidad Autonoma de Nuevo Leon, Monterrey, Nuevo Leon, Mexico, 66460 Planned sample size: 60 (recruited) Inclusion criteria: patients 18 to 90, distal radius fracture type A or B of AO classification managed with closed reduction and percutaneous pinning, signed informed consent Exclusion criteria: associated ipsilateral fractures in the upper extremity, fractures attended and fixed at another institution, external fixation, previous skin conditions (infection, ulcers), limitation of wrist mobility prior to injury |
| Interventions | Timing of surgery: not stated
(1) Three‐week percutaneous pinning + short cast immobilisation for six weeks (2) Six‐week percutaneous pinning + short cast immobilisation for six weeks No details of rehabilitation |
| Outcomes | Length of follow‐up: 14 weeks
Primary outcome: pain VAS (0 to 10: worst pain) at 14 weeks Secondary outcomes: PRWE at 14 weeks; wrist mobility (flexion, extension, pronation, supination, cubital and radial deviation) using a goniometer; grip strength at 14 weeks using a hydraulic dynamometer; skin condition at 6 weeks |
| Starting date | 29/09/2017; trial recruitment ends: November 2019 |
| Contact information | Principal Investigator: Dr Carlos Acosta‐Olivo, Universidad Autonoma de Nuevo Leon, Monterrey, Nuevo Leon, Mexico, 66460
Email: dr.carlosacosta@gmail.com Also: Dr Yadira Tamez‐Mata, MD Email: dra.yadiratamez@gmail.com |
| Notes | Registration date: 18/10/2017 (updated: 22/08/2019)
Funding: not stated Estimated study completion date is February 2020 (checked 30/08/2019) |
RBR‐8mq8bs.
| Trial name or title | Buried or exposed K‐wires in distal radius fractures ‐ what causes more infection? A randomized clinical trial |
| Methods | "Prospective randomised clinical trial, parallel, open, with two arms." Randomisation method or sequence: not stated Blinding: not blinded ("open") Analysis: not stated. |
| Participants | Recruitment centre: Hospital Maradei ‐ Belém, PA, Brazil Planned sample size: 200 Inclusion criteria: patients who are 18 years or over with distal third radius fractures treated with closed reduction and percutaneous fixation with 2 1.5 mm Kirschner wires Exclusion criteria: open fractures; immunosuppressed patients; needing more than two Kirschner wires to fix the fracture; open reduction; associated fractures |
| Interventions | Timing of intervention: not specified Surgical treatment for distal radius fracture fixation using two 1.5 mm Kirschner wires (no details of pinning method) (1) K‐wires left percutaneously (2) K‐wires buried deep to the skin (referred to as the control group) No details of immobilisation or rehabilitation |
| Outcomes | Length of follow‐up: not stated explicitly; 6 weeks? Primary outcome: infection (prevalence, detected according to the Oppenheim classification) Secondary outcome: range of motion of the metacarpophalangeal joint using a goniometer, at two and six weeks postoperatively |
| Starting date | Planned dates of recruitment: 09/11/2017 to 09/05/2018 |
| Contact information | João Alberto Ramos Maradei Pereira, Belém, Brazil Telephone: +55(91) 98889 0712 Email: jamaradei@me.com Affiliation: Universidade Federal do Pará |
| Notes | Registration date: 15/08/2017 (updated: 13/04/2018) Funding: Hospital Maradei ‐ Belém, PA, Brazil and Universidade Federal do Pará ‐ Belém, PA, Brazil Response by João Alberto Ramos Maradei Pereira to an email enquiring on current status received on 30/08/2019: '"trial was completed and article almost ready to [for] publication. I will submit it to Bone & Joint Journal next week." |
AO: Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation (or ASIF) EQ‐5D‐5L: health‐related quality of life questionnaire developed by the Euroqol Group with 5 levels for each of the 5 dimensions PRWE: Patient Rated Wrist Evaluation (score) VAS: visual analogue score
Differences between protocol and review
Most of the changes to methods for the 2019 update reflect the uptake of new methodology and reporting as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). These include 'Risk of bias' assessment, more explicit reporting of data analysis and collection, assessment of the quality of the evidence using GRADE and implementation, with some restructuring, of subsection headings such as those in the Background, Data collection and analysis and Discussion. Other changes are described below.
Terminology
Previously, this review described treatment such as plaster cast immobilisation as 'conservative' treatment. Although this remains a common descriptor, we have generally, unless quoted or pertinent to the context, replaced this with 'nonsurgical'.
Primary focus on dorsally displaced fractures
Although this review included all fractures of the distal radius, we clarified in the Objectives and Types of participants that our primary focus was on dorsally displaced fractures, which are the predominant category of fractures of relevance.
Interventions selected by surgeons
We clarified in Types of interventions that trials comparing surgical versus nonsurgical treatment, where the type of surgery was chosen by the surgeon, would be excluded even if separate data were available for those participants receiving percutaneous pinning.
Outcome measures
We restructured Types of outcome measures under primary and secondary outcomes, stipulated what constituted short‐, medium‐ and long‐term follow‐up, and identified seven main outcomes for presenting in a 'Summary of Findings' table. The previous version of this section is shown in Appendix 3.
Search platform
In the 2018 search update, the CENTRAL search was run in the CRS Online platform to enable the search to be restricted by issue.
Contributions of authors
Work on all aspects of this review update was shared by Alexia Karantana (AK), who took the lead, and Helen Handoll (HH). Ammar Sabouni (AS) participated in trial screening and processing, inputted data into tables, and commented on the final drafts. Alexia Karantana is the guarantor of the review.
Contributions of editorial base
Xavier Griffin: edited the review and approved the final version for publication. Liz Bickerdike (Cochrane Editorial & Methods Department): acted as a methodological editor. Joanne Elliott: coordinated the editorial process, advised on content and edited the review. Maria Clarke: ran the search update and edited the Search methods section.
Sources of support
Internal sources
Teesside University, Middlesbrough, UK.
University of Nottingham, Nottingham, UK.
University of Manchester, Manchester, UK.
External sources
National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group, UK.
Declarations of interest
AK: None known. HH: None known. AS:None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Allain 1999 {published data only}
- Allain J, Guilloux P, Mouel S, Goutallier D. Trans‐styloid fixation of fractures of the distal radius. A prospective randomized comparison between 6‐ and 1‐week postoperative immobilization in 60 fractures. Acta Orthopaedica Scandinavica 1999;70(2):119‐23. [DOI] [PubMed] [Google Scholar]
Azzopardi 2005 {published and unpublished data}
- Azzopardi T. No further information provided [personal communication]. Email to: H Handoll 24 May 2006.
- Azzopardi T, Ehrendorfer S, Coulton T, Abela M. Unstable extra‐articular fractures of the distal radius: a prospective, randomised study of immobilisation in a cast versus supplementary percutaneous pinning. Journal of Bone and Joint Surgery ‐ British Volume 2005;87(6):837‐40. [DOI] [PubMed] [Google Scholar]
- Ehrendorfer S. Management of distal radius fracture. In: The National Research Register, Issue 2, 2000. Oxford: Update Software (archived).
Casteleyn 1992 {published data only}
- Casteleyn PP, Handelberg F, Haentjens P. Biodegradable rod fixation of wrist fractures [abstract]. Orthopaedic Transactions 1993;17(4):1036. [Google Scholar]
- Casteleyn PP, Handelberg F, Haentjens P. Biodegradable rods versus Kirschner wire fixation of wrist fractures. A randomised trial. Journal of Bone and Joint Surgery ‐ British Volume 1992;74(6):858‐61. [DOI] [PubMed] [Google Scholar]
- Casteleyn PP, Handelberg F, Haentjens P, Opdecam P. Biodegradable rod fixation of wrist fractures [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 1993;75 Suppl 2:196. [DOI] [PubMed] [Google Scholar]
Delgado 2009 {published data only}
- Delgado PJ, Abad J, Fuentes A, Lopez‐Oliva F, Sanz L. Treatment of distal radius fractures due to high‐energy injuries: a prospective randomised comparative study [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2010;92‐B(Suppl IV):579. [Google Scholar]
- Delgado PJ, Miranda M, Abad JM, Forriol F, Lopez‐Oliva F. Unstable intraarticular distal radius fractures. A prospective, randomised study to compare cast immobilisation versus percutaneous fixation [abstract]. EFORT: 8th.Congress of the European Federation of National Associations.of Orthopaedics and Traumatology; 2007 May 11‐15; Florence. 2007.
- Delgado PJ, Miranda M, Abad JM, Forriol F, Lopez‐Oliva F. Unstable intraarticular distal radius fractures. A prospective, randomised study to compare cast immobilisation versus percutaneous fixation [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2009;91‐B(Suppl I):119. [Google Scholar]
Fikry 1998 {published data only}
- Fikry T, Fadili M, Harfoui A, Dkhissi M, Zryouil B. Metaphysis fracture of the distal radius: Kapandji's or Py's pinning? [Fractures metaphysaires du radius distal: embrochage de Kapandji ou de Py?]. Annales de Chirurgie de la Main et du Membre Superieur 1998;17(1):31‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gravier 2006 {published data only}
- Argenson JN. Status of an abstract [personal communication]. Email to: H Handoll 4 July 2006.
- Gravier R, Flecher X, Parratte S, Rapaie P, Argenson JN. Trans‐styloid and intrafocal pinning for extra‐articular extension fractures of the distal radius: prospective randomized postoperative comparison with simple intra‐focal pinning [Brochage mixte trans‐styloïdien et intrafocal dans les fractures extra‐articulaires en extension du radius distal]. Revue de Chirurgie Orthopédique et Réparatrice de l'appareil Moteur 2006;92(7):657‐62. [DOI] [PubMed] [Google Scholar]
- Gravier R, Flecher X, Parratte S, Rapaie P, Argenson JN. Trans‐styloid and intrafocal pinning of Pouteau‐Colles fractures: advantage in terms of postoperative stability compared with simple intrafocal pinning [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2005;87 Suppl 2:130. [Google Scholar]
Gupta 1999 {published and unpublished data}
- Gupta R. Informatoin on methods and results [personal communication]. Email to: H Handoll 4 April 2006.
- Gupta R, Raheja A, Modi U. Colles' fracture: management by percutaneous crossed‐pin fixation versus plaster of Paris cast immobilization. Orthopedics 1999;22(7):680‐2. [PubMed] [Google Scholar]
Hargreaves 2004 {published and unpublished data}
- Hargreaves D. No further information available [personal communication]. Email to: H Handoll 20 March 2006.
- Hargreaves DG, Drew SJ, Eckersley R. Kirchner wire pin tract infection rates: a randomized controlled trial between percutaneous and buried wires. Journal of Hand Surgery ‐ British Volume 2004;29(4):374‐6. [DOI] [PubMed] [Google Scholar]
Korner 1999 {published data only}
- Korner J, Verheyden P, Nocker C, Josten C. The use of biodegradable pins in the operative treatment of distal radial fractures [abstract]. Final program of the 21st Triennial World Congress SICOT 99; 1999 Apr 18; Sydney. Sydney: SICOT, 1999:239.
Lenoble 1995 {published data only}
- Lenoble E, Dumontier C, Goutallier D, Apoil A. Fracture of the distal radius. A prospective comparison between trans‐styloid and Kapandji fixations. Journal of Bone and Joint Surgery ‐ British Volume 1995;77(4):562‐7. [PubMed] [Google Scholar]
Mardani 2011 {published data only}
- Mardani Kivi M, Asadi K, Hashemi Motlagh K, Shakiba M. Distal radius fracture, a comparison between closed reduction and long arm cast vs. closed reduction and percutaneous pinning and short arm cast. Shiraz.E‐Medical Journal 2011;12(3):155‐61. [Google Scholar]
Milliez 1992 {published data only}
- Milliez PY, Dallaserra M, Defives T, Ayoubi L, Thomine JM. Effect of early mobilization following Kapandji's method of intrafocal wiring in fractures of the distal end of the radius. Results of a prospective study of 60 cases [Influence de la mobilisation precoce apres embrochage intra‐focal selon Kapandji dans les fractures de l'extremite inferieure du radius. Resultats d'une etude prospective sur 60 cas]. International Orthopaedics 1992;16(1):39‐43. [DOI] [PubMed] [Google Scholar]
Murphy 2008 {published data only}
- Murphy M, Flannery O, Kenny P, Keogh P, Lui D, McHugh G, et al. Outcome following buried versus exposed Kirschner wiring of distal radius fractures: a randomised controlled trial [abstract]. Bone and Joint Journal 2010;92(Suppl IV):581‐2. [Google Scholar]
- Murphy M, Flannery O, McHugh G, Lui D, Kenny P, Keogh P, et al. Outcome following buried versus exposed Kirschner wiring of distal radius fractures: a randomised controlled trial [abstract]. Bone and Joint Journal 2010;92(Suppl I):50‐1. [Google Scholar]
- Murphy M, Flannery O, McHugh G, Lui D, Orakzai S, Kenny P, et al. Outcome following buried vs exposed Kirschner wiring of distal radius fractures; a randomised controlled trial [abstract]. Irish Journal of Medical Science 2008;177(Suppl 7):S213. [Google Scholar]
Rodriguez‐Merchan 1997 {published data only}
- Rodriguez‐Merchan EC. Plaster cast versus percutaneous pin fixation for comminuted fractures of the distal radius in patients between 46 and 65 years of age. Journal of Orthopaedic Trauma 1997;11(3):212‐7. [DOI] [PubMed] [Google Scholar]
Saddiki 2012 {published data only}
- Saddiki R, Harisboure, Hemery X, Ohl X, Kabbaj R, Dehoux E. Comparative studies of the Py and Kapandji techniques for fractures of the distal radius with posterior displacement [abstract]. Bone and Joint Journal 2011;93(Suppl IV):526. [Google Scholar]
- Saddiki R, Ohl X, Hemery X, Vitry F, Dehoux E, Harisboure A. Dorsally displaced distal radius fractures: comparative study of Py's and Kapandji's techniques. Orthopaedics & Traumatology, Surgery & Research 2012;98(1):61‐7. [DOI] [PubMed] [Google Scholar]
Shankar 1992 {published data only}
- Shankar NS, Craxford AD. Comminuted Colles' fractures: a prospective trial of management. Journal of the Royal College of Surgeons of Edinburgh 1992;37(3):199‐202. [PubMed] [Google Scholar]
Shannon 2003 {published data only}
- Shannon FJ, Thornes B, Awan N, Burke T. Fractures of the distal radius treated with 'spring loaded' wiring technique. A randomised prospective study [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2003;85 Suppl 2:137. [Google Scholar]
Snow 2007 {unpublished data only}
- Fahmy N. Function vs position of the wrist following distal radial fracture. In: The National Research Register, Issue 3, 2000. Oxford: Update Software (archived).
- ISRCTN48558975. Function versus position of the wrist following distal radial fracture. www.isrctn.com/ISRCTN48558975 (first received 30 September 2004).
- Johnson D. Provision of pre‐publication report [personal communication]. Email to: H Handoll 2 October 2006.
- Snow M, Kelly M, Jeyam M, Fahmy N. Function versus position: a randomised controlled trial of interfocal Kirschner wiring of unstable distal radial fractures. European Journal of Trauma (in press). [DOI] [PubMed]
- Snow M, Kelly M, Jeyam M, Fahmy N. Function versus position: a randomized controlled trial of interfocal Kirschner wiring of unstable distal radial fractures. European Journal of Trauma & Emergency Surgery 2007;33(1):81‐6. [DOI] [PubMed] [Google Scholar]
Soleiman pour 2011 {published data only}
- Soleiman Pour J, Ghorbanzadeh M, Ganjpor Sales J, Eslamian F. Comparison of outcomes and complications of 1‐ and 4‐ week cast immobilization after distal radius surgery. Journal of Kerman University of Medical Sciences 2011;18(1):49‐54. [Google Scholar]
Stoffelen 1998 {published data only}
- Stoffelen DV, Broos PL. Closed reduction versus Kapandji‐pinning for extra‐articular distal radial fractures. Journal of Hand Surgery ‐ British Volume 1999;24(1):89‐91. [DOI] [PubMed] [Google Scholar]
- Stoffelen DV, Broos PL. Kapandji pinning or closed reduction for extra‐articular distal radius fractures. Journal of Trauma 1998;45(4):753‐7. [DOI] [PubMed] [Google Scholar]
- Stoffelen DVC, Broos PL. Conservative treatment versus Kapandji‐pinning for extra‐articular distal radius fractures. Osteosynthese International 1999;7 Suppl 1:87‐93. [Google Scholar]
Strohm 2004 {published data only}
- Strohm P. Notice of intention to respond further [personal communication]. Email to: H Handoll 23 March 2006.
- Strohm PC, Muller CA, Boll T, Pfister U. Two procedures for Kirschner wire osteosynthesis of distal radial fractures: a randomized trial. Journal of Bone and Joint Surgery ‐ American Volume 2004;86(12):2621‐8. [DOI] [PubMed] [Google Scholar]
Venkatesh 2016 {published data only}
- Venkatesh RB, Maranna GK, Narayanappa RKB. A comparative study between closed reduction and cast application versus percutaneous K‐wire fixation for extra‐articular fracture distal end of radius. Journal of Clinical and Diagnostic Research 2016;10:RC05‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhulst 1990 {published data only}
- Verhulst D, Pimontel P, Puylaert D, Gijs T, Bogehams J. Single Kirschner wire fixation of Colles' fractures [abstract]. Orthopaedic Transactions 1990;14(3):654. [Google Scholar]
Waheed 2004 {published data only}
- Waheed K, Mulhall K, Mwaura B, Kaar K. Percutaneous wire fixation of distal radial fractures: is it preferable to bury the wires? [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2004;86 Suppl 2:125‐6. [Google Scholar]
Wong 2010 {published data only}
- Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra‐articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. Journal of Hand Surgery: European Volume 2010;35(3):202‐8. [DOI] [PubMed] [Google Scholar]
Zyluk 2007 {published data only}
- Zyluk A. Response to request for translation (if possible) and information on your distal radius fracture trial [personal communication]. Email to: H Handoll 7 June 2016.
- Zyluk A, Janowski P. A comparison of the results of the conservative vs operative by percutaneous Kirschner‐wiring treatment of fractures of the distal radius. Chirurgia Narządów Ruchu i Ortopedia Polska 2007;72(5):327‐34. [PubMed] [Google Scholar]
References to studies excluded from this review
Biedermann 2001 {published and unpublished data}
- Biedermann M. Confirmed it was not an RCT [personal communication]. Email to: H Handoll 25 July 2002.
- Biedermann M, Gerber BE. Minimal invasive stabilisation of distal radius fractures with an 1 K‐wire technique [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2001;83 Suppl 2:258. [Google Scholar]
Chung 2019 {published and unpublished data}
- Chung KC, Malay S, Shauver MJ, Kim HM, Group Wrist. Assessment of distal radius fracture complications among adults 60 years or older: a secondary analysis of the WRIST randomized clinical trial. JAMA Network Open 2019;2(1):e187053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01589692. A clinical trial for the surgical treatment of elderly distal radius fractures (WRIST) (version 14). clinicaltrials.gov/ct2/show/NCT01589692 (first received 2 May 2012).
- Wrist and Radius Injury Surgical Trial (WRIST) Study Group. Reflections 1 year into the 21‐Center National Institutes of Health‐funded WRIST study: a primer on conducting a multicenter clinical trial. Journal of Hand Surgery: American Volume 2013;38(6):1194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delattre 1994 {published data only}
- Delattre O, Saillant G, Lemoine J, Benazet JP, Roy‐Camille R. Reduction and osteosynthesis with pin fixation of wrist fractures. A comparative study between Kapandji's and Py's techniques [Reduction et synthese par brochage des fractures du poignet. Etude comparative entre la technique de Kapandji et la technique de Py]. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur 1994;80(2):94‐107. [PubMed] [Google Scholar]
- Delattre O, Saillant G, Lemoine J, Benazet JP, Roy‐Camille R. Reduction and pin fixation for fractures of the distal radius: a comparative study between the Kapandji and Py techniques [abstract]. Orthopaedic Transactions 1996;20(2):492. [Google Scholar]
- Delattre O, Saillant G, Lemoine J, Benazet JP, Roy‐Camille R. Reduction and pinning of wrist fractures: comparative study of the Kapandji and Py techniques [abstract]. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur 1996;82(7):672. [PubMed] [Google Scholar]
Galli 2002 {published data only}
- Galli S, Filippo G, Bonaspetti G, Zarattini G, Pazzaglia UE. Long arm cast versus percutaneous pinning in the treatment of distal radial fractures [abstract]. Journal of Hand Surgery ‐ British Volume 2002;27 Suppl 1:6‐7. [Google Scholar]
Gunay 2015 {published data only}
- Gunay C, Oken OF, Yavuz OY, Gunay SH, Atalar H. Which modality is the best choice in distal radius fractures treated with two different Kirschner wire fixation and immobilization techniques?. Ulusal Travma ve Acil Cerrahi Dergisi [Turkish Journal of Trauma & Emergency Surgery] 2015;21(2):119‐26. [DOI] [PubMed] [Google Scholar]
Harper 2000 {published and unpublished data}
- Harper WM. A randomised prospective trial comparing MUA and POP with K wire fixation in Colles fractures. nihr.ac.uk/404/ (accessed prior to 2 February 2020).
- Harper WM. Trial was abandoned [personal communication]. Email to: H Handoll October 2001.
IRCT2013120814271N2 {published data only}
- IRCT2013120814271N2. Comparison of treatment outcome of distal extra‐articular radius fracture treatment with percutaneous pinning and pin‐in plaster in patients older than 60 years old. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013120814271N2 (first received 07 January 2007).
IRCT2016061828510N1 {published data only}
- IRCT2016061828510N1. The effect of ulnar styloid fracture fixation in treatment of radius fracture. en.irct.ir/trial/23119 (first received 13 July 2016).
ISRCTN37842313 {published data only}
- ISRCTN37842313. A randomised controlled trial comparing Kirschner wire stabilisation versus manipulation under anaesthesia (MUA) plaster treatment for displaced radial fractures. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN37842313 (first received 29 September 2006).
NCT02353338 {unpublished data only}
- NCT02353338. Distal radial fractures in the late middle aged (RIST) (version 6). clinicaltrials.gov/ct2/show/study/NCT02353338 (first received 2 February 2015).
Seifert 1998 {published data only}
- Seifert J, Muller C, Schauwecker HH. Studying minimally invasive osteosynthesis methods for distal radius fractures. Intra‐focal vs. conventional wire osteosynthesis [Untersuchungen zu minimalinvasiven osteosyntheseverfahren bei distalen radiusfrakturen. Intrafokale versus konventionelle bohrdrahtosteosynthese.]. Unfallchirurgie 1998;24(1):18‐24. [PubMed] [Google Scholar]
Tomaszuik 2017 {published data only}
- Tomaszuk M, Kiryluk J, Tomaszuk A, Popko J. Evaluation of treatment of low‐energy distal radial fractures in postmenopausal women. Ortopedia Traumatologia Rehabilitacja 2017;19(1):55‐65. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kanakeshwar 2017 {published data only}
- Kanakeshwar RB, Dheenadyalan J, Rajasekaran S. A double blinded randomised comparative study of 2 pins versus 3 pins for the fixation of dorsally displaced extra‐articular distal radius fractures in the elderly [abstract]. artsloi.sicot.org/capetown/Abstract‐Book‐Short‐Free‐Papers.pdf (accessed 14 March 2018).
Mirhamidi 2013 {published data only}
- Mirhamidi SM, Bayat FM. A prospective comparison between Kapandji and percutaneous extra‐focal fixation in extra articular distal radius fractures. International Journal of Clinical and Experimental Medicine 2013;6(2):133‐9. [PMC free article] [PubMed] [Google Scholar]
Russe 2000 {published and unpublished data}
- David A. Confirmed that it was a multicentre trial [personal communication]. Email to: H Handoll 16 August 2006.
- David A, Muller D, Eitenmuller J, Muhr G. One‐year results of the osteosythesis of distal radius fractures with resorbable pins [abstract] [Einjahresergebnisse der osteosynthese distaler radiusfrakturen mit resorbierbaren stiften]. Hefte zur Zeitschrift der Unfallchirurg 1998;265:203‐6. [Google Scholar]
- Muller D, David A, Pommer A, Muhr G. Osteosynthesis of typical radius fracture with new resorbable polyactide pins [abstract] [Osteosynthese der radiusfraktur loco typico mit neuen resorbierbaren polyactidstiften]. Hefte zur Zeitschrift der Unfallchirurg 1997;268:567‐8. [Google Scholar]
- Russe K, Heyde VDD, David A. The osteosynthetic management of distal lower arm fractures ‐ comparison of bioresorbable material and Kirschner wire [abstract] [Die osteosynthetische versorgung der distalen unterarmfrakturen ‐ vergleich von bioresorbierbarem material und Kirschner draht]. Hefte zur Zeitschrift der Unfallchirurg 2000;275:305‐6. [Google Scholar]
- Russe K, Pommer A, Heyde VDD, David A. The bioresorbable osteosynthesis of distal radius fractures [abstract] [Die bioresorbierbare osteosynthese der distalen radiusfraktur]. Hefte zur Zeitschrift der Unfallchirurg 2000;282:122‐3. [Google Scholar]
References to ongoing studies
DRAFFT 2 {unpublished data only}
- Achten J, Sones W, Dias J, Hedley H, Cook JA, Dritsaki M, et al. Surgical fixation with K‐wires versus plaster casting in the treatment of dorsally displaced distal radius fractures: protocol for Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT 2). BMJ Open 2019;9(3):e028474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. DRAFFT 2: Distal Radius Acute Fracture Fixation Trial 2. www.nets.nihr.ac.uk/projects/hta/152701 (accessed 28 August 2016).
- Anonymous. DRAFFT2: Distal Radius Acute Fracture Fixation Trial 2. drafft2.octru.ox.ac.uk/ (accessed 30 September 2019).
- ISRCTN11980540. Comparison of surgical procedures for patients with a fracture of the wrist. isrctn.com/ISRCTN11980540 (first received 02 August 2016).
IRCT20160508027797N4 {published data only}
- IRCT20160508027797N4. Comparison outcome of unstable fractures of the distal radius bone between the two surgical methods. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20160508027797N4 (first received 11 March 2019).
IRCT2016112727797N1 {unpublished data only}
- Hosseinzadeh D, Janmohammadi N, Ganji SME, Frydoni MB. Comparison of radiographic results between three crossed pinning and four‐pin radioulnar transfixation methods in the treatment of unstable distal radius fracture. Journal of Evolution of Medical and Dental Sciences 2019;8(33):2587‐91. [DOI: 10.14260/jemds/2019/566] [DOI] [Google Scholar]
- IRCT2016112727797N1. Comparison of radiologic changes of unstable fractures of the distal radius bone using of three cross pin with 4 pin trans‐radioulnar. en.irct.ir/trial/22701 (accessed 26 April 2018).
NCT03311633 {unpublished data only}
- NCT03311633. Complications in distal radius fracture (version 2). clinicaltrials.gov/ct2/show/NCT03311633 (first received 17 October 2017).
RBR‐8mq8bs {published data only}
- Maradei‐Pereira JAR. Information on the trial status [personal communication]. Email to: H Handoll 30 August 2019.
- Pereira JARM. Buried or exposed pins in wrist fractures ‐ what causes more infection? A randomized clinical trial. ensaiosclinicos.gov.br/rg/RBR‐8mq8bs/ (first received 15 August 2017).
Additional references
Altissimi 1986
- Altissimi M, Antenucci R, Fiacca C, Mancini GB. Long‐term results of conservative treatment of fractures of the distal radius. Clinical Orthopaedics and Related Research 1986;206:202‐10. [PubMed] [Google Scholar]
Andersen 1996
- Andersen DJ, Blair WF, Steyers CM Jr, Adams BD, El‐Khouri GY, Brandser EA. Classification of distal radius fractures: an analysis of interobserver reliability and intraobserver reproducibility. Journal of Hand Surgery ‐ American Volume 1996;21(4):574‐82. [DOI] [PubMed] [Google Scholar]
AO 2018
- Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation compendium ‐ 2018. Journal of Orthopaedic Trauma 2018;32(Suppl 1):S1‐S170. [DOI] [PubMed] [Google Scholar]
Atkins 1989
- Atkins RM, Duckworth T, Kanis JA. Algodystrophy following Colles' fracture. Journal of Hand Surgery ‐ British Volume 1989;14(2):161‐4. [DOI] [PubMed] [Google Scholar]
Beaton 2005
- Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item‐reduction approaches. Journal of Bone and Joint Surgery ‐ American Volume 2005;87(5):1038‐46. [DOI] [PubMed] [Google Scholar]
Belloti 2019
- Belloti JC, Okamura A, Scheeren J, Faloppa F, Ynoe de Moraes V. A systematic review of the quality of distal radius systematic reviews: methodology and reporting assessment. PLOS One 2019;14(1):e0206895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belsole 1993
- Belsole RJ, Hess AV. Concomitant skeletal and soft tissue injuries. Orthopedic Clinics of North America 1993;24(2):327‐31. [PubMed] [Google Scholar]
Chung 1998
- Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. Journal of Hand Surgery ‐ American Volume 1998;23(4):575‐87. [DOI] [PubMed] [Google Scholar]
Colles 1814
- Colles A. On the fracture of the carpal extremity of the radius (reprinted from Edinburgh Medical Surgery Journal 1814;10:181). Clinical Orthopaedics and Related Research 2006;445:5‐7. [DOI] [PubMed] [Google Scholar]
Cooney 1980
- Cooney WP 3rd, Dobyns JH, Linscheid RL. Complications of Colles' fractures. Journal of Bone and Joint Surgery ‐ American Volume 1980;62(4):613‐9. [PubMed] [Google Scholar]
Cooney 1993
- Cooney WP. Fractures of the distal radius. A modern treatment‐based classification. Orthopedic Clinics of North America 1993;24(2):211‐6. [PubMed] [Google Scholar]
Costa 2016
- Costa ML, Jameson SS, Reed MR. Do large pragmatic randomised trials change clinical practice?: assessing the impact of the Distal Radius Acute Fracture Fixation Trial (DRAFFT). Bone and Joint Journal 2016;98‐B(3):410‐3. [DOI] [PubMed] [Google Scholar]
Cummings 1985
- Cummings SR, Kelsey JL, Nevitt MC, O'Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiologic Reviews 1985;7:178‐208. [DOI] [PubMed] [Google Scholar]
Delgado 2010
- Delgado PJ, Abad J, Fuentes A, Lopez‐Oliva F, Sanz L. Treatment of distal radius fractures due to high‐energy injuries: a prospective randomised comparative study [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2010;92‐B(Supp IV):579. [Google Scholar]
DePalma 1952
- DePalma AF. Comminuted fractures of the distal end of the radius treated by ulnar pinning. Journal of Bone and Joint Surgery ‐ American Volume 1952;24(3):651‐62. [PubMed] [Google Scholar]
Downing 2008
- Downing ND, Karantana A. A revolution in the management of fractures of the distal radius?. Journal of Bone and Joint Surgery. British Volume 2008;90(10):1271‐5. [DOI] [PubMed] [Google Scholar]
DRAFFT 2014
- Costa ML, Achten J, Parsons NR, Rangan A, Griffin D, Tubeuf S, et al. Percutaneous fixation with Kirschner wires versus volar locking plate fixation in adults with dorsally displaced fracture of distal radius: randomised controlled trial. BMJ 2014;349:g4807. [DOI: 10.1136/bmj.g4807] [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol Group 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
Fernandez 1996
- Fernandez DL, Jupiter JB. Fractures of the Distal Radius. A Practical Approach to Management. 1st Edition. New York: Springer‐Verlag, 1996. [Google Scholar]
Fernandez 1999
- Fernandez DL, Palmer AK. Fractures of the distal radius. In: Green DP, Hotchkiss RN, Pederson WC editor(s). Green's Operative Hand Surgery. 4th Edition. New York: Churchill Livingstone, 1999:929‐85. [Google Scholar]
Flinkkila 1998
- Flinkkila T, Raatikainen T, Hamalainen M. AO and Frykman's classifications of Colles' fracture. No prognostic value in 652 patients evaluated after 5 years. Acta Orthopaedica Scandinavica 1998;69(1):77‐81. [DOI] [PubMed] [Google Scholar]
Frykman 1967
- Frykman G. Fractures of the distal radius including sequelae ‐ shoulder‐hand‐finger syndrome, disturbance in the distal radio‐ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthopaedica Scandinavica Supplementum 1967;108:3‐153. [DOI] [PubMed] [Google Scholar]
Fujii 2002
- Fujii K, Henmi T, Kanematsu Y, Mishiro T, Sakai T, Terai T. Fractures of the distal end of radius in elderly patients: a comparative study of anatomical and functional results. Journal of Orthopaedic Surgery 2002;10(1):9‐15. [DOI] [PubMed] [Google Scholar]
Gartland 1951
- Gartland JJ, Werley CW. Evaluation of healed Colles' fractures. Journal of Bone and Joint Surgery. American Volume 1951;33(4):895‐910. [PubMed] [Google Scholar]
Gravier 2005
- Gravier R, Flecher X, Parratte S, Rapaie P, Argenson JN. Trans‐styloid and intrafocal pinning of Pouteau‐Colles fractures: advantage in terms of postoperative stability compared with simple intrafocal pinning [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 2005;87 Suppl 2:130. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2003a
- Handoll HHG, Madhok R. Surgical interventions for treating distal radial fractures in adults. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003209] [DOI] [PubMed] [Google Scholar]
Handoll 2003b
- Handoll HHG, Madhok R. Closed reduction methods for treating distal radial fractures in adults. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hosseinzadeh 2019
- Hosseinzadeh D, Janmohammadi N, Ganji SME, Frydoni MB. Comparison of radiographic results between three crossed pinning and four‐pin radioulnar transfixation methods in the treatment of unstable distal radius fracture. Journal of Evolution of Medical and Dental Sciences 2019;8(33):2587‐91. [DOI: 10.14260/jemds/2019/566] [DOI] [Google Scholar]
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C, Upper Extremity Collaborative Group (UECG). Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Huetteman 2019
- Huetteman HE, Shauver MJ, Malay S, Chung TT, Chung KC. Variation in the treatment of distal radius fractures in the United States: 2010 to 2015. Plastic & Reconstructive Surgery 2019;143(1):159‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakim 1991
- Jakim I, Pieterse HS, Sweet MB. External fixation for intra‐articular fractures of the distal radius. Journal of Bone and Joint Surgery ‐ British Volume 1991;73(2):302‐6. [DOI] [PubMed] [Google Scholar]
Jebsen 1969
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation 1969;50(6):311‐9. [PubMed] [Google Scholar]
Johnson 2019a
- Johnson N, Leighton P, Pailthorpe C, Dias J, Distal Radius Fracture Delphi Study Group. Defining displacement thresholds for surgical intervention for distal radius fractures – a Delphi study. PLOS One 2019;14(1):e0210462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2019b
- Johnson NA, Dias J. The current evidence‐based management of distal radial fractures: UK perspectives. Journal of Hand Surgery. European Volume 2019;44(5):450‐5. [DOI] [PubMed] [Google Scholar]
Jupiter 1997
- Jupiter JB, Fernandez DL. Comparative classification for fractures of the distal end of the radius. Journal of Hand Surgery ‐ American Volume 1997;22(4):563‐71. [DOI] [PubMed] [Google Scholar]
Kapandji 1988
- Kapandji IA, Epinette J‐A. Colles' fracture: treatment by double intrafocal wire fixation. In: Razemon JP, Fisk GR editor(s). The Wrist. Edinburgh: Churchill Livingstone, 1988:65‐73. [Google Scholar]
Kleinlugtenbelt 2018
- Kleinlugtenbelt YV, Krol RG, Bhandari M, Goslings JC, Poolman RW, Scholtes VAB. Are the Patient‐Rated Wrist Evaluation (PRWE) and the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaires used in distal radial fractures truly valid and reliable?. Bone and Joint Research 2018;7(1):36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knirk 1986
- Knirk JL, Jupiter JB. Intra‐articular fractures of the distal end of the radius in young adults. Journal of Bone and Joint Surgery ‐ American Volume 1986;68(5):647‐59. [PubMed] [Google Scholar]
Kosinski 2007
- Kosinski M, Ware JE, Turner‐Bowker DM, Gandek G. User's Manual for the SF‐12v2 Health Survey: with a Supplement Documenting SF‐12 Health Survey. Lincoln RI: QualityMetric Inc., 2007. [Google Scholar]
Kreder 1996a
- Kreder HJ, Hanel DP, McKee M, Jupiter J, McGillivary G, Swiontkowski MF. X‐ray film measurements for healed distal radius fractures. Journal of Hand Surgery ‐ American Volume 1996;21(1):31‐9 [published erratum in Journal of Hand Surgery ‐ American volume 1996; 21(3):532]. [DOI] [PubMed] [Google Scholar]
Kreder 1996b
- Kreder HJ, Hanel DP, McKee M, Jupiter J, McGillivary G, Swiontkowski MF. Consistency of AO fracture classification for the distal radius. Journal of Bone and Joint Surgery. British Volume 1996;78(5):726‐31. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lethaby 2013
- Lethaby A, Temple J, Santy Tomlinson J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD004551.pub3] [DOI] [PubMed] [Google Scholar]
MacDermid 1998
- MacDermid JC, Turgeon T, Richards RS, Beadle M, Roth JH. Patient rating of wrist pain and disability: a reliable and valid measurement tool. Journal of Orthopaedic Trauma 1998;12(8):577‐86. [DOI] [PubMed] [Google Scholar]
MacDermid 2000
- MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form‐36, Disability of the Arm, Shoulder and Hand questionnaire, Patient Rated Wrist Evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. Journal of Hand Surgery ‐ American Volume 2000;25(2):330‐40. [DOI] [PubMed] [Google Scholar]
Macey 1995
- Macey AC, Burke FD, Abbott K, Barton NJ, Bradbury E, Bradley A, et al. British Society for Surgery of the Hand. Outcomes of hand surgery. Journal of Hand Surgery. British Volume 1995;20(6):841‐55. [DOI] [PubMed] [Google Scholar]
MacIntyre 2016
- MacIntyre NJ, Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. Journal of Hand Therapy 2016;29(2):136‐45. [DOI] [PubMed] [Google Scholar]
MacKenney 2006
- MacKenney PJ, McQueen MM, Elton R. Predict of instability in distal radial fractures. Journal of Bone and Joint Surgery ‐ American Volume 2006;88(9):1944‐51. [DOI] [PubMed] [Google Scholar]
Mellstrand Navarro 2019
- Mellstrand Navarro C, Brolund A, Ekholm C, Heintz E, Hoxha Ekstrom E, Josefsson PO, et al. Treatment of radius or ulna fractures in the elderly: a systematic review covering effectiveness, safety, economic aspects and current practice. PLOS One 2019;14(3):e0214362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosenthal 2019
- Mosenthal WP, Boyajian HH, Ham SA, Conti Mica MA. Treatment trends, complications, and effects of comorbidities on distal radius fractures. Hand (New York) 2019;14(4):534‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 1991
- Muller M, Allgower M, Schneider R, Willenegger H. Manual of Internal Fixation: Techniques Recommended by the AO‐ASIF Group. 3rd Edition. Berlin: Springer‐Verlag, 1991. [Google Scholar]
O'Neill 2001
- O'Neill TW, Cooper C, Finn JD, Lunt M, Purdie D, Reid DM, et al. Incidence of distal forearm fracture in British men and women. Osteoporosis International 2001;12(7):555‐8. [DOI] [PubMed] [Google Scholar]
Rayhack 1993
- Rayhack JM. The history and evolution of percutaneous pinning of displaced distal radius fractures. Orthopedic Clinics of North America 1993;24(2):287‐300. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rundgren 2018
- Rundgren J, Enocson A, Mellstrand Navarro C, Bergstrom G. Responsiveness of EQ‐5D in patients with a distal radius fracture. Hand 2018;13(5):572‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahlin 1990
- Sahlin Y. Occurrence of fractures in a defined population: a 1‐year study. Injury 1990;21(3):158‐60. [DOI] [PubMed] [Google Scholar]
Sarmiento 1975
- Sarmiento A, Pratt GW, Berry NC, Sinclair WF. Colles' fractures. Functional bracing in supination. Journal of Bone and Joint Surgery ‐ American Volume 1975;57(3):311‐7. [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14.2.1 The GRADE approach. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Singer 1998
- Singer BR, McLauchlan GJ, Robinson CM, Christie J. Epidemiology of fractures in 15,000 adults: the influence of age and gender. Journal of Bone and Joint Surgery ‐ British Volume 1998;80(2):243‐8. [DOI] [PubMed] [Google Scholar]
Smith 1988
- Smith RJ, Floyd WE. Smith's and Barton's fractures. In: Barton N editor(s). Fractures of the Hand and Wrist. Edinburgh: Churchill Livingstone, 1988:252‐66. [Google Scholar]
Stewart 1984
- Stewart HD, Innes AR, Burke FD. Functional cast‐bracing for Colles' fractures. A comparison between cast‐bracing and conventional plaster casts. Journal of Bone and Joint Surgery ‐ British Volume 1984;66(5):749‐53. [DOI] [PubMed] [Google Scholar]
Stewart 1985
- Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury 1985;16(5):289‐95. [DOI] [PubMed] [Google Scholar]
Taleisnik 1984
- Taleisnik J, Watson HK. Midcarpal instability caused by malunited fractures of the distal radius. Journal of Hand Surgery ‐ American Volume 1984;9(3):350‐7. [DOI] [PubMed] [Google Scholar]
Van Staa 2001
- Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001;29(6):517‐22. [DOI] [PubMed] [Google Scholar]
Waljee 2011
- Waljee JF, Kim HM, Burns PB, Chung KC. Development of a brief, 12‐item version of the Michigan Hand Questionnaire. Plastic and Reconstructive Surgery 2011;128(1):208‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey Manual and Interpretation Guide. Boston MA: New England Medical Center, The Health Institute, 1993. [Google Scholar]
Willenegger 1959
- Willenegger A, Guggenbuhla A. Operative treatment of certain cases of distal radius fracture [Article in German]. Helvetica Chirurgica Acta 1959;26:81‐94. [PubMed] [Google Scholar]
References to other published versions of this review
Handoll 2007
- Handoll HHG, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006080.pub2] [DOI] [PubMed] [Google Scholar]


