Abstract
Background & Aims
Alagille syndrome is a genetic disorder characterized by cholestasis, ocular abnormalities, characteristic facial features, heart defects, and vertebral malformations. Most cases are associated with mutations in JAGGED1 (JAG1), which encodes a Notch ligand, although it is not clear how these contribute to disease development. We aimed to develop a mouse model of Alagille syndrome to elucidate these mechanisms.
Methods
Mice with a missense mutation (H268Q) in Jag1 (Jag1+/Ndr mice) were outbred to a C3H/C57bl6 background to generate a mouse model for Alagille syndrome (Jag1Ndr/Ndr mice). Liver tissues were collected at different timepoints during development, analyzed by histology, and liver organoids were cultured and analyzed. We performed transcriptome analysis of Jag1Ndr/Ndr livers and livers from patients with Alagille syndrome, cross-referenced to the Human Protein Atlas, to identify commonly dysregulated pathways and biliary markers. We used species-specific transcriptome separation and ligand-receptor interaction assays to measure Notch signaling and the ability of JAG1Ndr to bind or activate Notch receptors. We studied signaling of JAG1 and JAG1Ndr via NOTCH 1, NOTCH2, and NOTCH3 and resulting gene expression patterns in parental and NOTCH1-expressing C2C12 cell lines.
Results
Jag1Ndr/Ndr mice had many features of Alagille syndrome, including eye, heart, and liver defects. Bile duct differentiation, morphogenesis, and function were dysregulated in newborn Jag1Ndr/Ndr mice, with aberrations in cholangiocyte polarity, but these defects improved in adult mice. Jag1Ndr/Ndr liver organoids collapsed in culture, indicating structural instability. Whole-transcriptome sequence analyses of liver tissues from mice and patients with Alagille syndrome identified dysregulated genes encoding proteins enriched at the apical side of cholangiocytes, including CFTR and SLC5A1, as well as reduced expression of IGF1. Exposure of Notch-expressing cells to JAG1Ndr, compared with JAG1, led to hypomorphic Notch signaling, based on transcriptome analysis. JAG1-expressing cells, but not JAG1Ndr-expressing cells, bound soluble Notch1 extracellular domain, quantified by flow cytometry. However, JAG1 and JAG1Ndr cells each bound NOTCH2, and signaling from NOTCH2 signaling was reduced but not completely inhibited, in response to JAG1Ndr compared with JAG1.
Conclusions
In mice, expression of a missense mutant of Jag1 (Jag1Ndr) disrupts bile duct development and recapitulates Alagille syndrome phenotypes in heart, eye, and craniofacial dysmorphology. JAG1Ndr does not bind NOTCH1, but binds NOTCH2, and elicits hypomorphic signaling. This mouse model can be used to study other features of Alagille syndrome and organ development.
Keywords: Notch, Jagged1, Alagille, Heart, Liver, Kidney, Vertebrae, Development
Notch signaling is a highly conserved cell-contact–dependent signaling pathway used reiteratively in many developmental processes. Mutations in the Notch pathway lead to numerous diseases,1 including Alagille syndrome (ALGS1; Online Mendelian Inheritance in Man/OMIM no. 118450, and ALGS2, OMIM no. 610205).2 ALGS is an autosomal dominant genetic disorder that in more than 90% of patients is caused by mutations in JAGGED1 (JAG1),3,4 while about 1% harbor NOTCH2 mutations.5 Alagille syndrome often presents early in life with severe liver and heart defects,6 but also affects vertebrae, eyes, and craniofacial morphology.
How Alagille JAG1 mutations affect signaling through different Notch receptors is poorly understood. The JAG1 ligand is expressed on a signal-sending cell that activates signaling upon contact with a Notch receptor on a juxta-posed signal-receiving cell. Missense mutations in ALGS are enriched in the receptor-binding DSL and DOS domains1 of JAG1, and analysis of the crystallized JAG1 receptor-binding domain and an extracellular portion of NOTCH17 shows that the previously described Jag1Ndr mutation8 maps to this interaction domain, but how the JAG1Ndr mutation mechanistically affects Notch signaling through receptors other than NOTCH1 remains to be established.
Deletions in a single JAG1 allele are sufficient to cause ALGS, suggesting haploinsufficiency is the disease-causing mechanism. This is also supported by various mouse models, based on targeting Jag1 and/or Notch2 (for review, see 1). Mouse models for ALGS liver disease include conditional Jag1 ablation in portal vein mesenchyme,9 and Jag1/Notch2 compound heterozygous mice.10,11 However, the first model does not mimic the full syndrome, and the Jag1/Notch2 model biases our understanding of ALGS towards Jag1/Notch2-regulated conditions, though NOTCH2 mutations are observed in only a fraction of ALGS cases.12 Also, NOTCH2-related ALGS2 presents differently from JAG1-related ALGS1; for example, patients with NOTCH2 mutations less frequently display heart defects.13 Jag1+/dDSL mice on a C57bl6 background display bile duct paucity but are not jaundiced,14 and it is unknown whether these mice recapitulate other major features of ALGS. Thus, the link between missense Jag1 mutations and ALGS has not yet been possible to address in vivo.
In this report, we show that a missense mutation in Jag1 (H268Q; Nodder, Jag1Ndr 8) generates a mouse model for ALGS, mimicking disease pathology in eye, craniofacial morphology, heart, and liver. By investigating liver development at different stages, and using liver organoids from Jag1Ndr/Ndr mice, we show that while biliary differentiation is delayed, biliary morphogenesis and maintenance are disrupted. In line with dysregulated morphogenesis, whole transcriptome analysis of ALGS liver biopsies and Jag1Ndr/Ndr livers confirms dysregulated expression of cell polarity genes, but not of key regulators of bile duct differentiation at postnatal or adult stages. At the molecular level, the JAG1Ndr mutation generates a hypomorphic ligand that is unable to bind to specific Notch receptors: JAG1Ndr binds NOTCH2, but not NOTCH1, and to a lesser degree NOTCH3. Collectively, we show that a missense mutation in Jag1 is sufficient to invoke an ALGS phenotype in mice, and provide the first evidence that a Jag1 missense mutation can impact differentially on different Notch receptor interactions.
Methods
Mouse Maintenance, Breeding, and Genetics
Jag1+/Ndr mice have been described previously,8 and for the present study were maintained in a mixed C3H/C57bl6 genetic background. For details, see Supplementary Materials.
Measurement of Craniofacial Proportions
The distance from the eye to the snout tip and from the snout/forehead bridge to the snout tip were measured using ImageJ in images of E15.5 embryos taken from the animal’s right side. All measurements were performed by experimenters blinded to the genotype.
Antibodies, Immunohistochemistry, and Staining
Fourteen-μm cryosections of liver were stained using routine staining protocols. For antibodies and staining details, see Supplementary Materials.
Bile Duct Quantification
Bile ducts in 10–100 portal triads were quantified per stage in Jag1+/+ and Jag1Ndr/Ndr mice. For details, see Supplementary Materials.
Blood Chemistry Analysis
Plasma and serum were sent to the Swedish University of Agricultural Sciences for analysis of blood chemistry. For details, see Supplementary Materials.
Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed, as described.15 For primers see Supplementary Materials.
Liver Organoid Cell Culture
Liver organoids were isolated and cultured, as described,16 in the presence of R-spondin.
Collection of Human Samples for RNA Sequencing
Human liver needle biopsies were collected for clinical purposes, and a small part (3–5 mm x 1 mm) was snap-frozen and stored at -80°C. Diagnosis details are in Supplementary Materials.
Tissue Dissection, Homogenization, RNA Extraction, and cDNA Library Preparation
Liver was homogenized and RNA from liver or cells was extracted using Direct-zol RNA MiniPrep (cat. no. R2050; Zymo Research, Irvine, CA) or the RNeasy Mini Kit (cat. no. 74104; Qiagen, Hilden, Germany).
cDNA libraries for all samples were created using the TruSeq RNA Sample Prep Kit v2–48, Set A (cat. no. RS-122-2001; Illumina, San Diego, CA) and Set B (cat. no. RS-122-2002; Illumina). For specifics, see Supplementary Materials.
Alignment, Analysis of Technical Performance, and Bioinformatics
The cDNA libraries were sequenced on a HiSeq 2000 with a 50–52 read length, single-end, for different samples.17 Bioinformatics and sequencing details are provided in Supplementary Materials.
Human Protein Atlas Cross-referencing Enrichment of Bile Duct Genes
Proteins expressed in bile ducts were identified using the Human Protein Atlas (HPA, http://www.proteinatlas.org/),18 using the following search string: Field: Tissue expression (IHC), Tissue: Liver, Cell Type: Bile duct cells, Expression: High or Medium AND Field: Tissue expression (IHC), Tissue: Liver, Cell Type: Bile duct cells, Expression: Not detected or low. Supplementary Tables 5–8. For details, see Supplementary Materials.
Bile Duct Orientation by ZO-1 Staining in Adult Mice
ZO-1 orientation analysis was carried out in 6–12 well-formed/functional bile ducts per animal (n=3).
IGF1 ELISA
IGF1 in serum was detected using ELISA according to manufacturer’s instructions (cat. no. EMIGF1; Thermo Fisher Scientific, Waltham, MA).
Cell Lines and Cell Culture
Mouse C2C12 control and C2C12-FLNotch1 and human HEK-293-Flp-In cells8: HEK293-Flp control (Flp Ctrl), HEK293-Flp-Jag1WT (Flp JAG1+), HEK293-Flp-Jag1Ndr (Flp JAG1Ndr) were used. For culture conditions and luciferase experiments, see Supplementary Materials.
Notch ECD Uptake Experiments
NOTCH1-Fc, NOTCH2-Fc, and NOTCH3-Fc (R&D Systems, Minneapolis, MN) was coupled to Alexa 488 anti-Fc (Invitrogen, Carlsbad, CA). Flp Ctrl, Flp JAG1+, and Flp JAG1Ndr cells were treated with the tagged proteins for 1 hour at 37°C. Cells were stained for confocal imaging or trypsinized for fluorescence-activated cell sorter fluorescence-activated cell sorting (FACS) analysis, as described in Supplementary Materials.
Statistical Analysis
Differences between control and experimental conditions were tested using t test, 1-way ANOVA, or 2-way ANOVA. For specifics, see Supplementary Materials.
Results
Jag1Ndr/Ndr Mice Recapitulate Alagille Syndrome
We previously described a mouse Jag1 mutation (H268Q) in the second epidermal growth factor (EGF)-like repeat of JAG1,8 a region enriched for missense mutations in ALGS.1 This allele is nicknamed Nodder (Jag1Ndr) because of a head-nodding phenotype in heterozygous C3H mice. Jag1Ndr/Ndr mice are embryonic lethal on this genetic background8 and, because the phenotype of other Jag1 heterozygous mice depends on genetic background,14,19 we asked whether mixed Jag1Ndr/Ndr mice bypass C3H lethality and recapitulate ALGS.
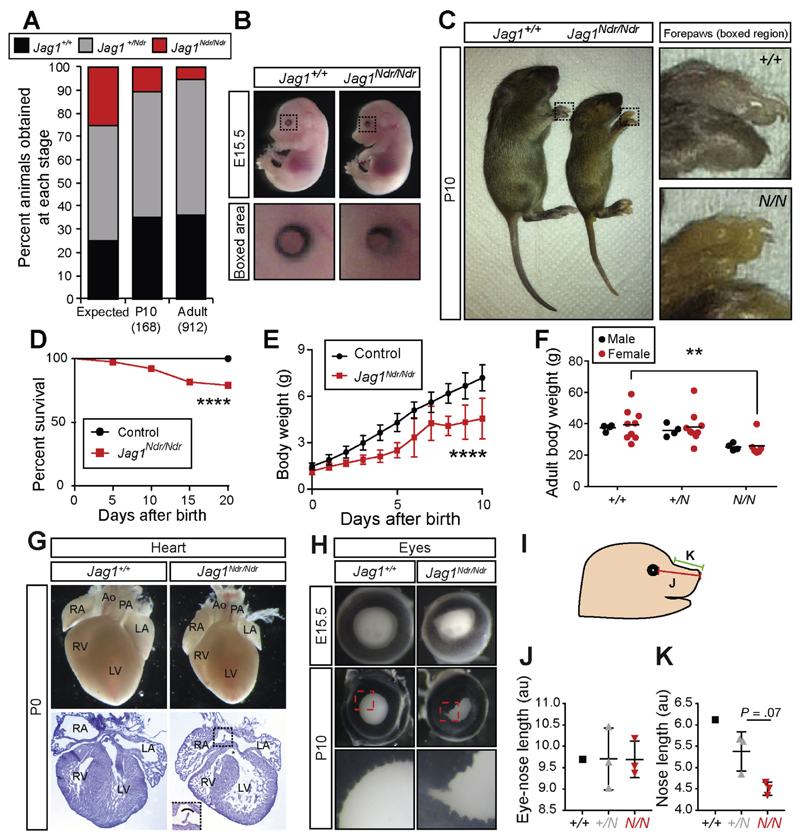
On a mixed C3H/C57bl6 background, viability was considerably improved: Jag1Ndr/Ndr embryos were recovered at a rate of 20% at embryonic day (E) 15.5, 10% from postnatal day (P) 0, and 5% in adults (Figure 1A, Supplementary Table 1, and data not shown). At E15.5, Jag1Ndr/Ndr embryos appeared grossly normal, and only exhibited a mild iris dysmorphology (Figure 1B). In contrast, postnatal Jag1Ndr/Ndr pups were jaundiced (Figure 1C), excreted yellow stools (data not shown), exhibited partial post-natal mortality (Figure 1D), and failed to thrive (Figure 1E). Adult Jag1Ndr/Ndr mice were 30% smaller than Jag1+/+ and Jag1+/Ndr mice (Figure 1F).
Figure 1.
Jag1Ndr/Ndr C3H/C57bl6 mice survive to adulthood with Alagille-like phenotypes. (A) Jag1+/Ndr mice were mated to generate Jag1+/+, Jag1+/Ndr and Jag1Ndr/Ndr offspring. At P10 and adult stages, fewer than the expected 25% of Jag1Ndr/Ndr mice were observed. (B, C) At E15.5 Jag1Ndr/Ndr mice appear grossly normal, with a mild eye defect (B), and by P10 are smaller and jaundiced (C). (D) After birth, 20% of Jag1Ndr/Ndr mice die within the first 20 days. (E) At birth, Jag1Ndr/Ndr mice are of normal size, but fail to gain weight as rapidly, a difference that is significant from P2, and (F) persistently weigh less than wild types. (G) Jag1Ndr/Ndr hearts are somewhat smaller than wild type hearts, likely corresponding to the smaller size of Jag1Ndr/Ndr mice. Hematoxylin staining of cryosections reveals ventricular (asterisk) and atrial (boxed) septation defects. (H) Iris dysmorphologies are manifested in Jag1Ndr/Ndr mice as early as E15.5. (I) Craniofacial proportions were measured in photos of E15.5 embryos, measuring the distance from (J) the eye to the tip of the snout and (K) the snout bridge to the tip of the snout, revealing a tendency towards altered proportions. For J and K, 3 animals were measured for Jag1+/Ndr and Jag1Ndr/Ndr, but only 1 Jag1+/+. Error bars indicate s.d.; **P <.01, ****P <.0001.
ALGS is diagnosed based on the presence of cholestasis, ocular abnormalities, characteristic facial features, heart defects, and vertebral malformations.6 The heart defects range from pulmonary artery stenosis to tetralogy of Fallot, a severe defect encompassing pulmonary stenosis, overriding aorta, ventricular septal defect, and right ventricular hypertrophy.20 Both atrial and ventricular septation defects were present in E15.5 and P0 Jag1Ndr/Ndr mice (Figure 1G and Supplementary Figure 1A).
Patients with ALGS display posterior embryotoxon,12 a malformation attributed to neural crest defects,21 and a smaller cornea.22 The first obvious phenotype in Jag1Ndr/Ndr mice was bilateral iris deformation with dorsal constriction at E13.5, which progressed to severe deformities and occasionally micropthalmia by P10 (Figure 1B,H, and Supplementary Figure 1B). Jag1Ndr/Ndr lenses were similar to wild types in size at E15.5 and were only slightly smaller at P10 (Supplementary Figure 1C,D), while 30% of adult Jag1Ndr/Ndr mice exhibited micropthalmia (data not shown).
Craniofacial alterations, including a broad prominent forehead, deep-set eyes, and a pointy chin, are seen in 77%–96% of patients.12 Jag1Ndr/Ndr mice similarly displayed a tendency toward altered craniofacial proportions with a wild type eye-nose length (Figure 1I,J), but a reduced snout length (bridge to tip, Figure 1I,K), supporting a role for Jag1 in craniofacial development, in line with previous reports.23 Alcian blue/Alizarin red staining of cartilage and bone at P0 and P10 did not reveal obvious vertebral malformations (data not shown), indicating that butterfly vertebrae12 are probably not present in Jag1Ndr/Ndr mice.
In conclusion, Jag1Ndr/Ndr mice recapitulate cardinal features of ALGS, including ocular, craniofacial, and cardiac defects. Jaundice indicates liver dysfunction, and we therefore next asked whether Jag1Ndr/Ndr mice display ductopenia.
Jag1Ndr/Ndr Mice Exhibit Early Life Biliary Dysmorphogenesis and Dysfunction With Later Rescue
A crippling ALGS symptom is cholestatic liver disease, with conjugated hyperbilirubinemia and decreased liver function, which histologically is associated with paucity of intrahepatic bile ducts. Thus, liver transplantation is frequently required. The pathomechanisms for ductopenia are poorly understood and it is unclear why, in some patients, cholestasis diminishes with time.24,25 Because some Jag1Ndr/Ndr mice survive to adulthood, the model provides an opportunity to elucidate disease development across different stages.
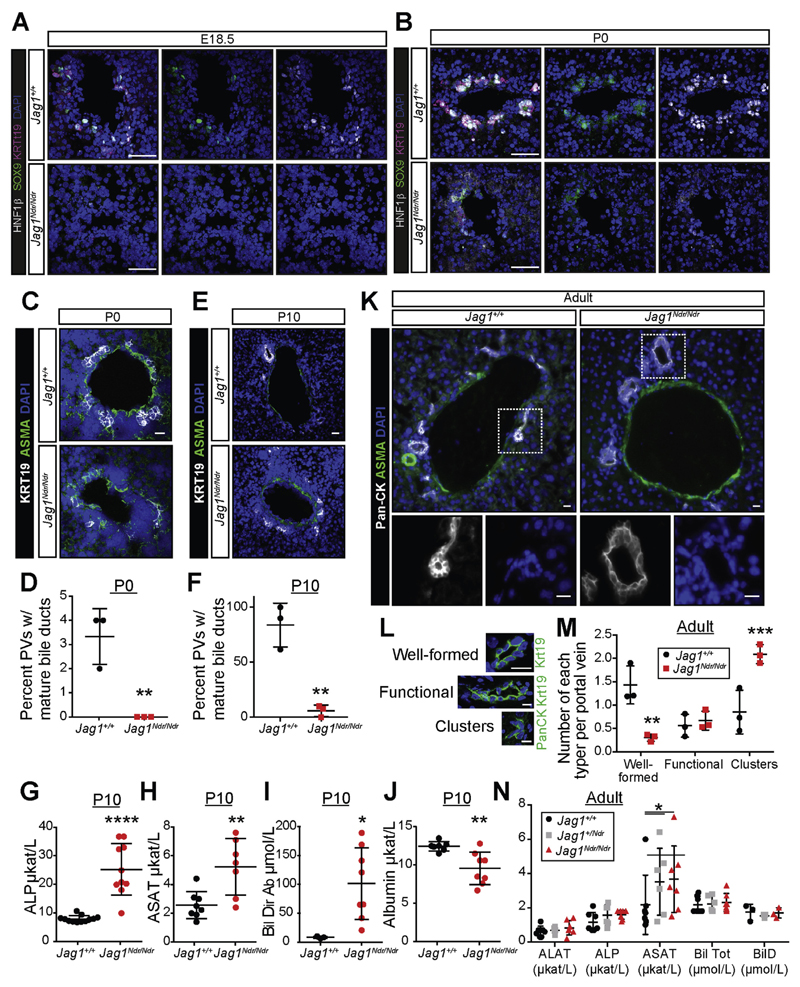
Jag1Ndr/Ndr mice displayed strong jaundice at neonatal stages (Figure 1C), whereas surviving adult Jag1Ndr/Ndr mice did not display jaundice, nor excrete yellow feces (data not shown). To determine whether Jag1Ndr/Ndr mice manifest a transient biliary phenotype, we analyzed biliary histology and marker expression in portal regions during development. Both Sox9 (a Notch target gene that regulates bile duct development26) and Hnf1β were present in Jag1+/+ periportal areas, but absent in Jag1Ndr/Ndr mice at E18.5. At p0, faintly positive cells were detected around portal tracts in Jag1Ndr/Ndr mice, at levels far weaker than the clusters of Sox9/Hnf1β-positive cells undergoing lumen formation in Jag1+/+ livers. At this stage, Jag1Ndr/Ndr hilar portal regions had no morphologically discernible mature bile ducts, while 3.3% of Jag1+/+ hilar portal veins had adjoining mature bile ducts (Figure 2C,D). The majority of P0 portal veins in Jag1Ndr/Ndr livers contained either no KRT19+ cells or disorganized clusters of KRT19+ cells (Figure 2B–D, Supplementary Figure 2A). At P10, bile ducts were rarely found in Jag1Ndr/Ndr livers, while portal veins in Jag1+/+ livers manifested 1 or 2 adjacent mature bile ducts (Figure 2E,F; Supplementary Figure 2B,C). Hepatoblast and hepatocyte marker expression levels were unaltered (data not shown), but serum biochemistry at P10 confirmed that Jag1Ndr/Ndr liver function was severely compromised (Figure 2G–J, Supplementary Figure 2D–F).
Figure 2.
Postnatal Jag1Ndr/Ndr mice display ductopenia, which is rescued in adults. (A, B) HNF1β, SOX9, and KRT19 staining show a marked absence of biliary cells at E18.5 (A) and weak staining at P0 (B) near the hilum in Jag1Ndr/Ndr liver. (C, D) KRT19+ cell clusters appear around ASMA+ periportal regions near the hilum of wild type Jag1+/+ mice at P0, but are absent in Jag1Ndr/Ndr mice. (E, F) By P10, clusters of biliary cells have lumenized to form ducts in Jag1+/+ mice, but not in Jag1Ndr/Ndr mice. Jag1Ndr/Ndr mice display increased (G) alkaline phosphatase (ALP), (H) aspartate aminotransferase (ASAT), (I) direct bilirubin (Bil Dir), and (J) decreased albumin. (K) At adult stages, lumenized bile ducts are present in both Jag1+/+ and Jag1Ndr/Ndr mice, though classification (L) of structures shows (M) significantly more clusters in Jag1Ndr/Ndr mice and fewer well-formed bile ducts. (N) Nevertheless, markers of liver function demonstrate a rescue of bile duct function in adult Jag1Ndr/Ndr mice in most serum chemistry markers. A small difference in aspartate aminotransferase levels persists. Error bars indicate s.d.; *P <.05, **P <.01, ***P <.001, ****P <.0001. Scale bars: (A, B) 50 μm, (C) 20 μm, (I, J) 10 μm.
In contrast, lumenized bile ducts could be found in both Jag1+/+ and Jag1Ndr/Ndr adult mice, though with disrupted morphology in Jag1Ndr/Ndr livers (Figure 2K–M, Supplementary Figure 2G,H). We classified and quantified pan-cytokeratin+ and KRT19+ bile ducts as “well-formed” (1 layer of biliary cells, a round lumen), “functional” (1 or more layers of biliary cells, a discernible lumen) or “clusters” (clusters of biliary cells, no discernible lumen) (Figure 2L). Adult Jag1Ndr/Ndr mice harbor fewer “well-formed” bile ducts and instead contain “clusters” of biliary cells (Figure 2M, Supplementary Figure 2G). However, there was no significant difference between Jag1+/+ and Jag1Ndr/Ndr mice when grouping well-formed and functional bile ducts (Supplementary Figure 2H). Serum analysis confirmed a full functional recovery in adult Jag1Ndr/Ndr mice (Figure 2N, Supplementary Figure 2I,J), with only a small difference in aspartate aminotransferase levels still detectable.
In contrast to the transient biliary phenotype, there was a persistent absence of hepatic arteries in Jag1Ndr/Ndr mice (Figure 2K, Supplementary Figure 2K,L). In conclusion, the Jag1Ndr/Ndr mice display a biliary phenotype that is severe at early postnatal stages but that improves during adulthood.
Disrupted Bile Duct Morphogenesis and Delayed Differentiation
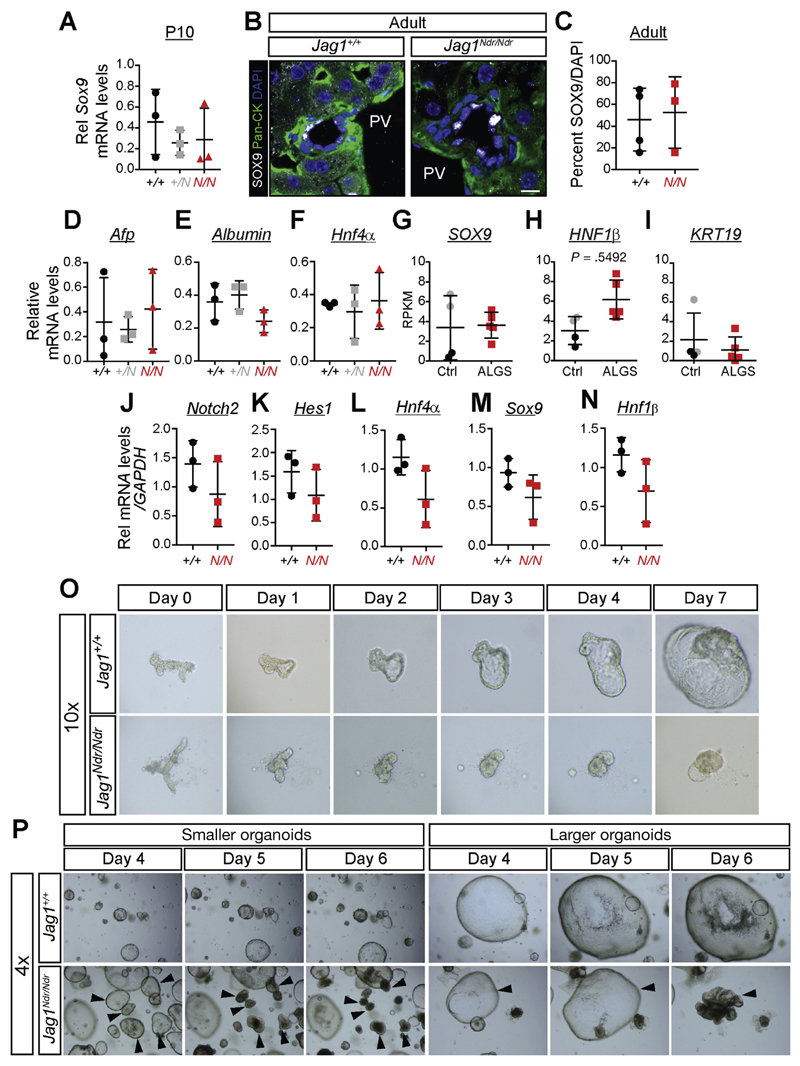
It is unclear whether the ALGS biliary defects are because of disrupted morphogenesis,11 differentiation defects,27 or both.28 To address this question, we analyzed the expression of key genes regulating differentiation. Expression of Sox9, and Hnf4α, a transcription factor required for hepatocyte differentiation,29 as well as alfa-fetoprotein (a marker of hepatoblasts) and albumin (a marker for hepatocyte function), was unaffected in P10 and adult Jag1Ndr/Ndr mice (Figure 3A–F, data not shown). Similarly, SOX9 mRNA levels were not affected in ALGS liver biopsies (Figure 3G), nor were the well-characterized biliary markers HNF1β or KRT19 (Figure 3H,I). This is in contrast to the early absence of SOX9- and HNF1β-positive cells at E18.5 and P0 (Figure 2A,B).
Figure 3.
Jag1Ndr/Ndr biliary cells express the expected markers but display structural instability. Sox9 levels are unchanged at P10 at the mRNA level (A), and at adult stages at protein levels (B, C). qPCR for (D) alpha-fetoprotein, (E) albumin, and (F) Hnf4α show no significant differences in Jag1Ndr/Ndr mice at P10. Similarly, RNA sequencing of ALGS livers shows no difference in (G) SOX9, (H) HNF1β, or (I) KRT19 levels. Organoids derived from adult Jag1Ndr/Ndr livers expressed normal levels of (J) Notch2, (K) Hes1, (L) Hnf4α, (M) Sox9, and (N) Hnf1β as assessed by qPCR, but (O) grew slowly and (P) sometimes spontaneously collapsed. Collapse was not related to organoid size because both smaller and larger organoids collapsed. No differences were significant. Scale bar: (B) 10 μm.
To assess adult bile duct development and morphology, we used a recently developed model for long-term in vitro expansion of bile duct-derived progenitor cells.16,30 Bile duct fragments were hand-picked and cultured in vitro as liver organoids, forming readily from both adult control and Jag1Ndr/Ndr mice. Notch2, Hes1, Hnf4α, Sox9, and Hnf1β mRNA expression were not altered (Figure 3J–N), further supporting that differentiation was delayed, but not completely inhibited (see Figure 2). However, liver organoids from Jag1Ndr/Ndr mice grew less well than Jag1+/+ organoids (Figure 3O). Importantly, a number of Jag1Ndr/Ndr organoids collapsed in culture after 5–6 days (Figure 3P), demonstrating structural instability. Jag1Ndr/Ndr biliary cells from adult mice are therefore similar to Jag1+/+ biliary cells in terms of cell identity, but exhibit differences in structural stability. In conclusion, the data argue for morphologic as well as differentiation defects.
Novel Biomarkers for Alagille Syndrome Reveal Dysregulation of Apical Proteins
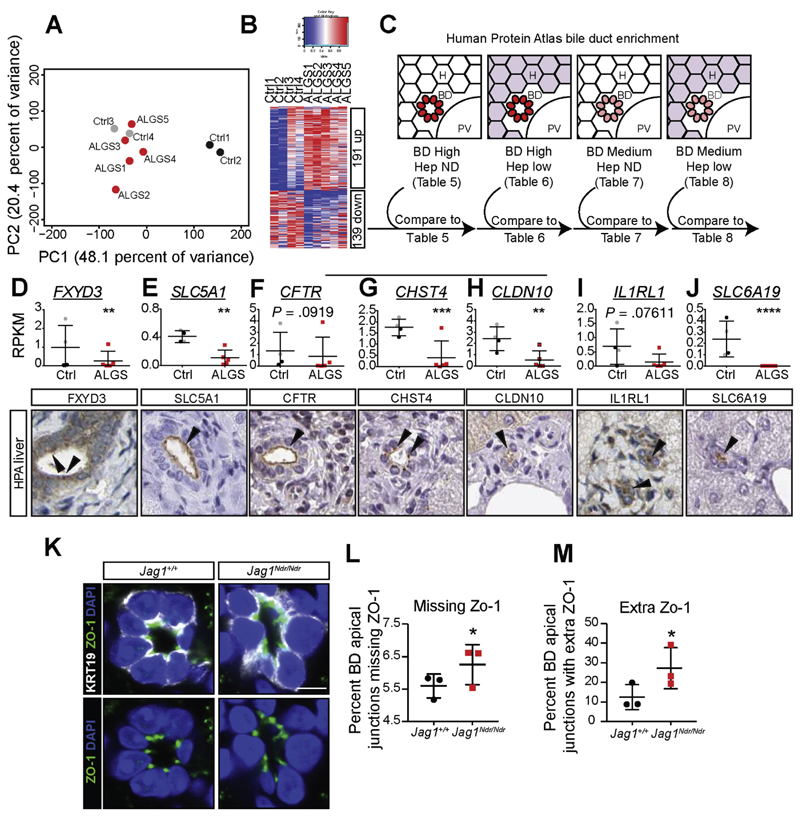
To further assess the molecular basis for ALGS, we performed genome-wide transcriptome studies of liver biopsies from 5 patients with ALGS. Control samples from pediatric patients with liver disease and/or cholangiopathies allow us to detect genes specifically dysregulated in ALGS, rather than cholestasis pathways or general liver disease mechanisms. Principal component analysis (PCA) showed that the 5 ALGS liver transcriptomes clustered with the 2 cholangiopathy samples: autoimmune hepatitis with primary sclerosing cholangitis, and progressive familial intrahepatic cholestasis type 2. In contrast, the transcriptomes from 2 patients with autoimmune hepatitis segregated more distinctly (Figure 4A, Supplementary Table 2). There were 191 up-regulated and 139 down-regulated genes (adjusted P value <.1, fold change >1.5, Figure 4B, Supplementary Tables 3 and 4, Supplementary Figure 3).
Figure 4.
RNA sequencing of ALGS liver reveals a specific decrease in apical markers of biliary cells. (A) Principle component analysis (PCA) of RNA sequencing of liver biopsies from patients with ALGS or control patients. A comparison with non-cholestatic control samples (Ctrl 1 and 2) and with cholestatic control samples (Ctrl 3 and 4) shows that ALGS samples cluster with cholestatic liver samples. (B) Heatmap shows 191 significantly up-regulated and 139 down-regulated genes in ALGS samples. (C) Dysregulated genes were compared with protein lists generated using the HPA (www.proteinatlas.org)18 for genes with high/medium protein expression in biliary cells, and undetected/low expression in hepatocytes (Supplementary Tables 5–8). This pipeline identified transcripts whose proteins were highly enriched at the apical side of bile ducts, including (D) FXYD3, and (E) SLC5A1. Manual comparison of the top 30 down-regulated genes in ALGS further revealed apically enriched proteins: (F) CFTR, (G) CHST4, (H) CLDN10, (I) IL1RL1, and (J) SLC6A19. (K) TJP1/ZO-1 is not down-regulated but is aberrantly localized, with some junctions (L) missing ZO-1, and other cell junctions with (M) extra ZO1 punctae. Error bars indicate s.d.; **corrected P-value (False Discovery Rate) <.01, ***False Discovery Rate <.001, ****False Discovery Rate <10 -7. Scale bar: 5 μm.
The transcriptome data are derived from bulk liver. We therefore devised a strategy to cross-reference the transcriptome data with protein expression patterns from the HPA (http://www.proteinatlas.org), a map of the human proteome,18 allowing us to identify genes encoding proteins expressed in bile ducts (Figure 4C, Supplementary Tables 5–8). This strategy identified the well-established biliary markers HNF1β, KRT19, and SOX9, confirming strategy validity and specificity, and HPA data showed the expected biliary expression (Supplementary Figure 4A).
Comparison of HPA bile duct-enriched proteins to ALGS transcriptomes revealed 5 up-regulated and 7 down-regulated novel bile duct markers in ALGS (Figure 4D,E, Supplementary Figure 4B–L). Of these, FXYD domain containing ion transport regulator 3 (FXYD3, Figure 4D) and Solute carrier family 5 (sodium/glucose cotransporter) member 1 (SLC5A1, Figure 4E) showed the highest significance, and encode proteins enriched at the apical surface of bile ducts. Manual comparison of the protein localization of the top 30 down-regulated genes to the HPA revealed 5 additional bile duct-specific genes with apical cholangiocyte staining including Cystic Fibrosis Transmembrane Conductance Regulator (CFTR, Figure 4F), Carbohydrate Sulfotransferase 4 (CHST4, Figure 4G), Claudin 10 (CLDN10, Figure 4H), Interleukin 1 receptor-like 1 (IL1RL1, Figure 4I) and Solute Carrier Family 6 (Neutral Amino Acid Transporter) member 19 (SLC6A19, Figure 4J). Given this link to aberrant cell polarity, we assessed the distribution of Zona occludens 1 (ZO-1, a.k.a. TJP-1), a marker of apical junctions in cholangiocytes that is not down-regulated in ALGS or in Jag1Ndr/Ndr mice (data not shown). Even in the best-formed bile ducts in Jag1Ndr/Ndr mice, ZO-1 was mis-localized, confirming polarity defects (Figure 4K–M). In conclusion, the most highly down-regulated biliary genes encode proteins enriched at the apical surface of bile ducts, corroborating morphogenesis disruption in ALGS.
Igf1 is Down-regulated in Patients With ALGS and Jag1Ndr/Ndr Mice
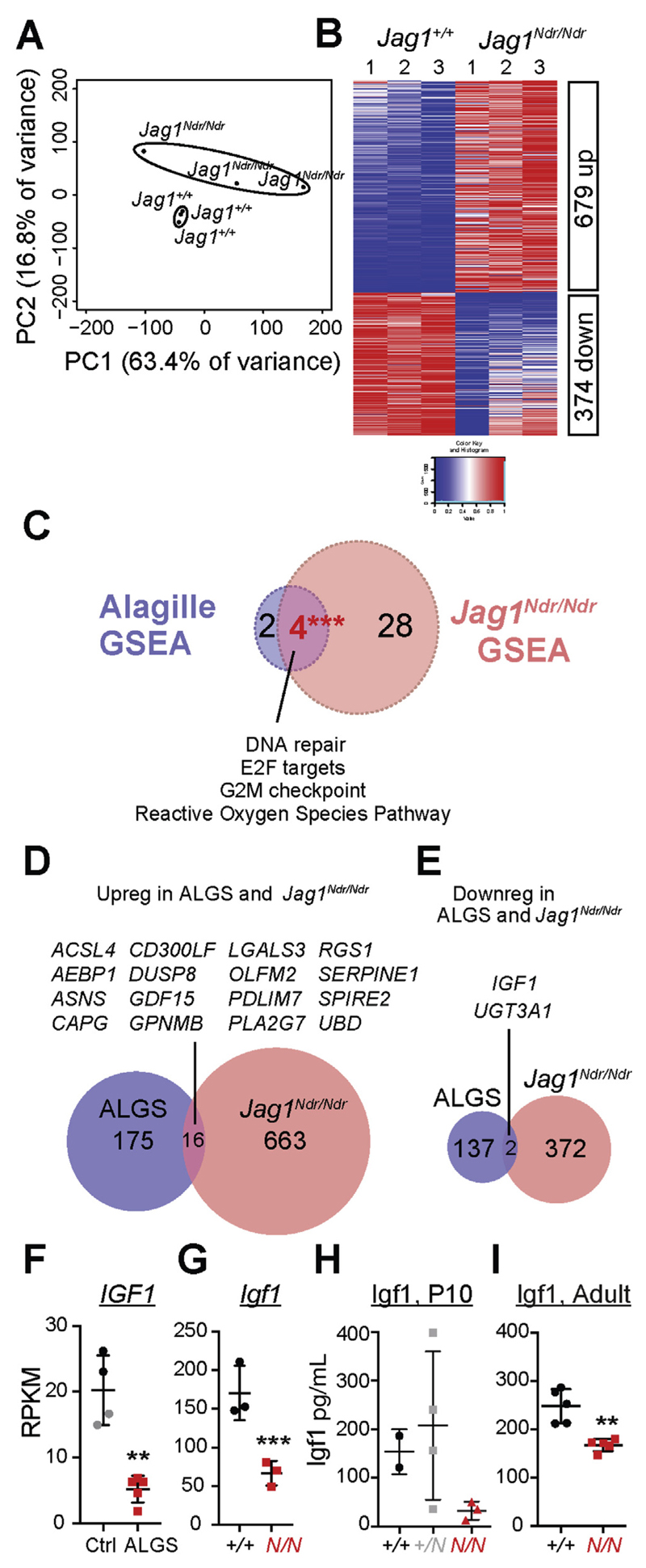
We next compared the transcriptomic changes in Jag1Ndr/Ndr livers to ALGS livers. RNA sequencing of Jag1Ndr/Ndr and Jag1+/+ livers yielded distinct transcriptional profiles (Figure 5A, Supplementary Table 9), with 679 up-regulated and 374 down-regulated genes (Figure 5B, Supplementary Tables 10 and 11, and Supplementary Figure 5).
Figure 5.
IGF1 is dysregulated in Jag1Ndr/Ndr and Alagille liver. (A) PCA of RNA sequencing reveals that Jag1+/+ and Jag1Ndr/Ndr liver transcriptomes cluster distinctly. (B) Heatmap shows 679 significantly up-regulated and 374 down-regulated genes in Jag1Ndr/Ndr livers. (C) Comparison of Gene Set Enrichment Analyses (GSEA), of livers from Jag1Ndr/Ndr mice and patients with ALGS (Supplementary Tables 12–15) shows extensive overlap. (D) Comparison of significantly dysregulated genes shows (D) 16 genes up-regulated and (E) 2 genes down-regulated in both Jag1Ndr/Ndr mice and ALGS, including Igf1. Igf1 mRNA levels are highly down-regulated in Alagille livers (F) and Jag1Ndr/Ndr livers (G). IGF1 protein levels were confirmed to be down-regulated in serum of (H) P10 and (I) adult mice. Error bars indicate s.d.; **P <.01, ***P <.001. (In F, and G, P-values are corrected P-values).
We assessed changes in signaling pathways and major cellular programs using gene set enrichment analyses (GSEA), which identified 35 sets significantly enriched in Jag1Ndr/Ndr livers at False Discovery Rate <25% (Figure 5C, Supplementary Tables 12 and 13, GSEA in Supplementary Figure 6). In contrast, GSEA for the ALGS transcriptome showed 6 enriched gene sets (Supplementary Tables 14 and 15, Supplementary Figure 7).
We next examined genes that were up- or down-regulated in both ALGS livers and in Jag1Ndr/Ndr livers. Sixteen genes were up-regulated (Figure 5D) and 2 were down-regulated (Figure 5E) in both transcriptomes. This relatively small degree of overlap is likely explained by the use of different controls in the 2 experiments (non-ALGS liver pathologies for patient data, and Jag1+/+ for mouse data), and innate differences between humans and mice, as well as in age. To test whether the use of different controls revealed different aspects of disease, we compared ALGS liver transcriptomes with the cholangiopathic and non-cholangiopathic controls separately (Supplementary Figure 8A–C). A greater numbers of enriched gene sets and dysregulated genes were detected when ALGS livers were compared with non-cholangiopathic livers (Supplementary Figure 8D–G, Supplementary Tables 16–19), than when ALGS livers were compared with cholangiopathic livers (Supplementary Figure 8H–K, Supplementary Tables 20–23), indicating general cholangiopathic transcriptomic responses are additionally revealed when non-cholestatic patients are included as controls. This is corroborated by the higher general overlap of this dataset with the Jag1Ndr/Ndr results (Supplementary Figure 8D–K). In all analyses, Igf1 (Insulin like growth factor 1) emerges as a target of particular interest.
Down-regulation of Igf1 in ALGS and in Jag1Ndr/Ndr mice is in line with a previous report showing that IGF1 is not up-regulated upon administration of growth hormone to patients with ALGS and growth deficiencies.31 The reduced expression of Igf1 in patients (Figure 5F) and in Jag1Ndr/Ndr mice (Figure 5G), which we confirmed with ELISA of serum from P10 and adult Jag1Ndr/Ndr mice (Figure 5H,I), is also likely to explain the Jag1Ndr/Ndr growth defects (Figure 1C,E,F).
JAG1Ndr Elicits Hypomorphic Notch Signaling
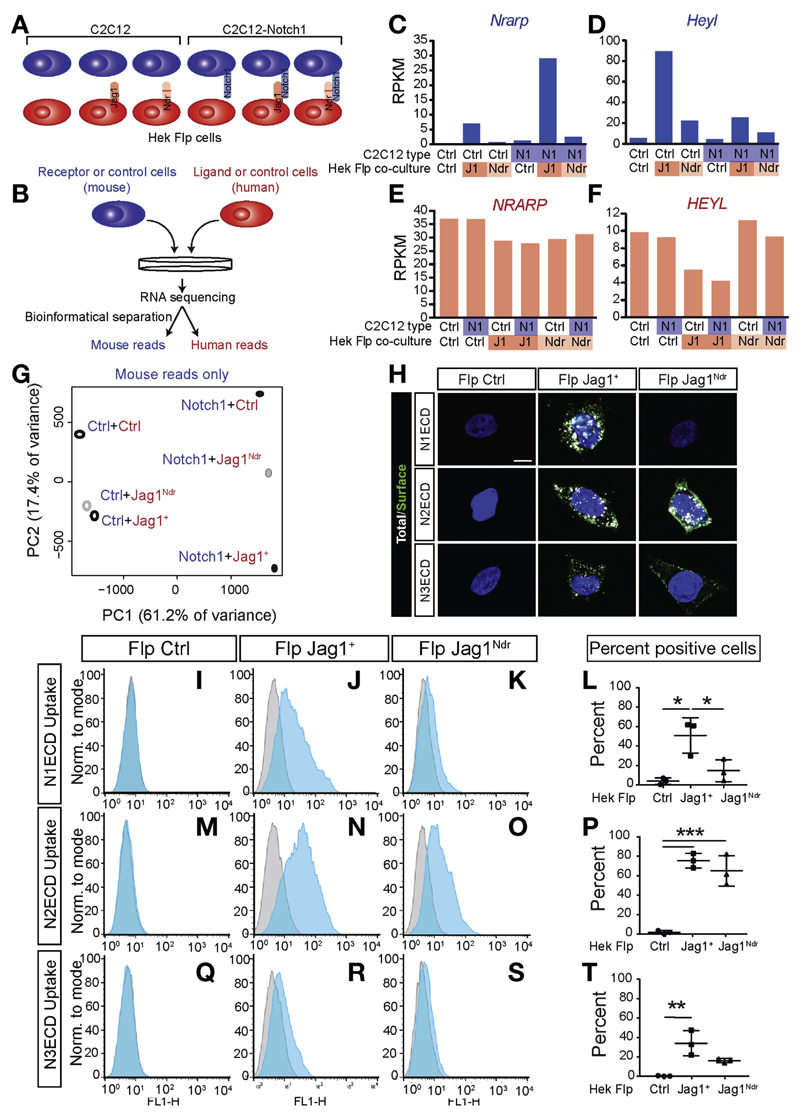
To address how the JAG1Ndr mutation influences signaling downstream of Notch, we analyzed the genome-wide transcriptomic response in NOTCH-expressing cells following activation by JAG1+ or JAG1Ndr. To specifically monitor the Notch response, we co-cultured mouse receptor cells (C2C12) with human ligand cells (HEK293 Flp) (Figure 6A). The transcriptome of C2C12 cells was bioinformatically separated from the HEK293 transcriptome based on species-specific sequencing (S3, Figure 6B, Supplementary Figure 9), which separates more than 99% of a mixed transcriptome to the correct species.17 Notch target gene responses were specifically detectable in the Notch receptor-expressing (mouse) cell reads in conditions with Notch activation (Figure 6C–F, Supplementary Figure 10A–D).
Figure 6.
JAG1Ndr is a Notch signaling hypomorph with receptor-selective binding. (A) Scheme depicting co-culture combinations. Control or NOTCH1-overexpressing C2C12 cells (mouse cells, blue) were co-cultured with Flp Ctrl, Flp JAG1+, or Flp JAG1Ndr cells (human cells, red). (B) After 6 hours, RNA was extracted for species-specific RNA sequencing (S3). Bioinformatic analyses separates mouse from human reads. The Notch target genes, Nrarp and Heyl, are (C, D) up-regulated in mouse receptor cells upon simulation with Flp JAG1+ cells, (E, F) but not in human ligand cells. (G) PCA for the mouse transcriptome shows that control and NOTCH1-overexpressing C2C12 cell lines both respond to JAG1+ stimulation with a similar downwards shift, reflecting Notch activation. JAG1Ndr is only capable of inducing part of this response in Notch1-overexpressing C2C12 cells, but behaves similar to JAG1+ in its activation of C2C12 cells. (H–T) JAG1Ndr does not bind NOTCH1 but does bind NOTCH2 and NOTCH3. Flp Ctrl, Flp JAG1+, or Flp JAG1Ndr cells were treated with fluorescently tagged extracellular domain of NOTCH1, 2, or 3 (N1-3ECD, white). After 1 hour of uptake, cells were fixed and anti-Fc was used to detect non-endocytosed, cell surface N1-3ECD (green), or cells were subjected to FACS analysis. (H) Flp Ctrl cells do not bind N1-3ECD. Flp JAG1+ cells bind and internalize NOTCH1, 2, and 3. Flp JAG1Ndr cells do not bind NOTCH1, but do bind NOTCH2 and NOTCH3. FACS analysis of N1ECD uptake by (I) Flp Ctrl cells, (J) Flp JAG1+, or (K) Flp JAG1Ndr cells, quantified in (L). FACS analysis of N2ECD uptake by (M) Flp Ctrl cells, (N) Flp JAG1+, or (O) Flp JAG1Ndr cells, quantified in (P). FACS analysis of N3ECD uptake by (Q) Flp Ctrl cells, (R) Flp JAG1+, or (S) Flp JAG1Ndr cells, quantified in (T). Quantifications (L, P, T) show Overton cumulative histogram subtractions. Error bars indicate s.d.; *P <.05, **P <.01, ***P <.001. C–G represent results from 1 experiment. Scale bar: (H) 10 μm.
We co-cultured ligand-expressing HEK293 Flp cells with control C2C12 cells (which express Notch2 and Notch3 at twice the levels of Notch1 and with almost undetectable Notch4 [Supplementary Figure 10E]), or with NOTCH1-overexpressing C2C12 cells, to examine whether NOTCH1 modified the transcriptomic response induced by ligand-expressing cells. PCA of the mouse transcriptomes showed that the JAG1Ndr-induced transcriptome is intermediate between response to control cells, and response to JAG1+-expressing cells (Figure 6G, Supplementary Table 24). However, in C2C12 cells, which predominantly express Notch2 and Notch3, the JAG1Ndr-induced transcriptome clustered with the JAG1+-induced transcriptome, whereas for NOTCH1-overexpressing C2C12 cells, the JAG1Ndr transcriptome lay closer to the Ctrl-induced transcriptome. This suggests that JAG1Ndr signals weakly or not at all through NOTCH1, in line with our previous report.8 In addition, Notch target genes showed weak (10%–80%) up-regulation by the JAG1Ndr ligand (Supplementary Figure 10F). In sum, the JAG1Ndr allele is hypomorphic at the global transcriptome level with regard to its ability to elicit Notch signaling.
JAG1Ndr Induces Receptor-selective Binding
The differentially hypomorphic signaling elicited by JAG1Ndr suggested that JAG1Ndr-mediated signaling through Notch receptor paralogs may be altered. Because the H268Q mutation resides in the Notch receptor-interacting domain, we tested whether JAG1Ndr exhibited receptor paralog-specific binding. Flp JAG1+ or Flp JAG1Ndr ligand-expressing cells were treated with fluorescently tagged soluble NOTCH1-3 receptor extracellular domain peptides (N1-3ECD, Figure 6H). Immunocytochemistry for the NECD-Fc was performed, without permeabilization, to detect extracellular ECDs (Figure 6H). N2ECD and N3ECD were bound by Flp JAG1+ and Flp JAG1Ndr cells, whereas N1ECD only interacted with Flp Jag1+ (Figure 6H); the latter in keeping with our previous report.8 FACS analysis showed that N1ECD was internalized by 50% of Flp JAG1+ cells, while Flp JAG1Ndr cells did not significantly internalize N1ECD (Figure 6I–L, Supplementary Figure 10G). In contrast, N2ECD was internalized by 80% of Flp JAG1+ cells, and by 70% of Flp JAG1Ndr cells (Figure 6M–P). However, the amount of N2ECD internalized by Flp JAG1Ndr cells was lower than by Flp JAG1+ cells (Supplementary Figure 10H). N3ECD was internalized by 35% of Flp Jag1+ cells, and by 20% of Flp Jag1Ndr cells (Figure 6Q–T, Supplementary Figure 10I). Because N2ECD internalization was reduced, we next tested the extent of activation of cells expressing Notch2 receptors, in response to co-culture with cells expressing Flp JAG1+ or Flp JAG1Ndr. Co-culture with Flp JAG1Ndr cells resulted in reduced Notch activation (as defined by 12XCSL-luciferase activation), as compared with co-culture with Flp JAG1+ cells (Supplementary Figure 10J). In conclusion, Flp JAG1Ndr exhibits a selective loss of interaction with NOTCH1, but the interaction with NOTCH2 and NOTCH3 is partially retained, although NOTCH2-mediated signaling elicited by Flp JAG1Ndr is reduced.
Discussion
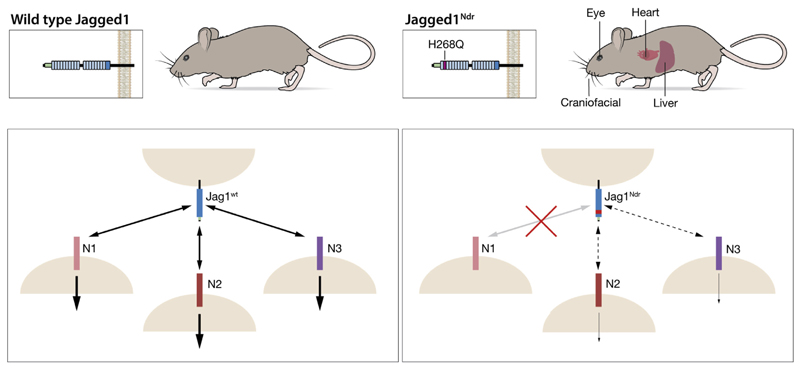
ALGS is usually caused by mutations in the JAG1 gene, but how dysregulated Notch signaling links to phenotypic consequences has been enigmatic. In this report, we provide evidence that a Jag1 missense mutation (Jag1H268Q) recapitulates Alagille symptoms in a number of organs. Jag1H268Q elicits a reduced Notch transcriptomic response and Notch receptor-selective binding (schematically depicted in Figure 7).
Figure 7.
Schematic summary of phenotypes and signaling aberrations in Jag1Ndr/Ndr mice. The location of the JAG1Ndr mutation, organs with phenotypes described here, and the interaction and signaling dysregulation for individual Notch receptors are depicted.
Disturbed Morphogenesis in Jag1Ndr Bile Ducts
The Jag1Ndr/Ndr mouse demonstrates that a Jag1 missense mutation can recapitulate ALGS. Other models based on loss-of-function Jag1 and/or Notch2 alleles do not display the entire spectrum of disease phenotypes. The Jag1Ndr/Ndr mouse displays Alagille-like phenotypes in several organs, including heart, lens, and craniofacial structures, as well as liver, and thus represents a clinically relevant mouse model for ALGS. An important distinction must be made, however, with regards to genetics: ALGS in humans is generally caused by heterozygosity for JAG1, while in mice the phenotype arises in the homozygous Jag1Ndr/Ndr state.
The fact that Jag1Ndr/Ndr mice survive to adulthood provides an opportunity to explore liver pathology over a lifetime. Interestingly, despite neonatal ductopenia, the number of bile ducts increases in the adult – although with aberrant morphology. In keeping with this, cholestasis was pronounced in pups, while adults display a full recovery regarding cholestasis, suggesting compensatory mechanisms rescue ductopenia in Jag1Ndr/Ndr mice. To what extent recuperation of the liver occurs also in ALGS is contested because some patients recover from cholestasis with time32 while in others biliary breakdown continues.24,25 Some patients display regenerating liver nodules with normal bile duct numbers.24,33,34 The RNA sequencing of ALGS liver samples (Figure 4) showed some heterogeneity, and in light of the variable liver disease severity and progression or reversal, additional analyses on bigger cohorts of patients will be necessary to determine whether there is a unifying molecular mechanism leading to bile duct abnormalities and whether diseases progression can be predicted based on transcriptomic data. The notion of a transient liver phenotype is also of interest for therapy; if cholestasis could be temporarily treated, then harnessing endogenous repair mechanisms could reduce the need for liver transplantation. In this context, the Jag1Ndr/Ndr mouse may serve as an important tool to explore novel therapeutic strategies, to test if transplanted cells can give rise to bile ducts, or which treatments induce repair mechanisms.
The Jag1Ndr/Ndr mice also shed light on the nature of the ALGS biliary pathology, attributed to disrupted morphogenesis of the bile ducts,11 defective bile duct maintenance, or differentiation defects.27,28 Our data indicate that morphogenesis and maintenance of bile ducts are affected, in addition to differentiation. This notion is supported by profound changes in gene expression in ALGS affecting cell polarity, at postnatal and adult stages. Further support for disturbed morphogenesis comes from the liver organoid data, in which Jag1Ndr/Ndr organoids initiated growth but collapsed a few days later, supporting structural rather than developmental defects. A previous report showed that liver organoids from human Alagille livers showed no phenotype in the undifferentiated state, but underwent collapse and apoptosis upon R-spondin withdrawal.30
Dysregulated cell polarity by disrupted Notch signaling has been shown in other polarized structures. Removal of CSL, the canonical Notch transcription factor, in embryonic stem cells disrupts neural rosettes,35 a lumenized and polarized colony of neural cells modeling the neural tube in vitro. Similarly, in the zebrafish lateral line, Notch is required for apical constriction of proneuromast rosettes,36 and regulates apical junction-associated genes, which together with our results, indicates a more general interaction between Notch and the cell polarity machinery.
The Jag1Ndr Mutation Causes Hypomorphic Signaling and Receptor Selectivity
Our data lend support to the hypomorphic view of ALGS mutations. The transcriptome data from co-cultured ligand- and receptor-expressing cells indicate that Jag1Ndr induces a hypomorphic Notch signaling response.
The finding that the H268Q mutation yields a JAG1 ligand that selectively loses its ability to interact with and activate NOTCH1, while maintaining interaction with NOTCH2 and 3, provides a novel facet of Notch signaling. Modifications of NOTCH receptors by Fringe fine-tunes their interaction with JAG or DLL ligands, but that a ligand mutation is sufficient to select for NOTCH2 and 3, but not NOTCH1, interaction is unprecedented. The H268Q missense mutation falls in the second EGF repeat, a region involved in Notch receptor activation,37 close to a number of patient-specific JAG1 mutations,1 and close to 3 interacting amino acids in NOTCH1, in EGF9, and EGF10,7 2 of which are conserved in NOTCH2 (L368 and P391); whereas the Valine 392 in NOTCH1 is instead a Leucine in NOTCH2. Whether this difference will explain the differential effect of the JAGGED1 H268Q mutation on NOTCH1 and 2 activation, however, remains to be tested. This difference cannot explain differences in binding to NOTCH3 because all 3 amino acids are conserved in NOTCH3 (Supplementary Materials).
The Recovery of Jag1Ndr/Ndr Mice Suggests Endogenous Mechanisms can Rescue Alagille Syndrome
Why certain patients with ALGS recover liver biliary function while others progress to liver transplantation is currently unknown. Jag1Ndr/Ndr pups display a severe biliary phenotype that is functionally rescued in adults. This is in contrast to Jag1+/dDSL mice, which display ALGS-like liver phenotypes in a C57bl6 background, but are not reported to improve with age.14
Sox9 is expressed not only in cholangiocytes, but also in stem-like hepatocytes upon insult,9,38,39 which can trans-differentiate into cholangiocytes. A similar rescue in adult albumin-Cre Hnf6flox/flox Rbpjflox/flox mice40 suggests Notch-independent mechanisms induce ductular reaction and hepatocyte trans-differentiation. The recovery in Jag1Ndr/Ndr mice may also be because of hepatocyte trans-differentiation, which can be induced with Notch activation.28 Thus, although Notch is not required for rescue, it may be sufficient. Transient activation of Notch may therefore – in principle – be feasible as therapy, though the role of Notch signaling in cancer suggests this could be associated with significant risks.41,42 The loss of Igf1 also presents an interesting therapeutic target because Igf1 stimulates cholangiocyte proliferation.43
While Jag1Ndr/Ndr mice recovered biliary function they did not recover hepatic artery numbers, suggesting that the absence of proper portal triad vascular architecture does not preclude recovery of a functional biliary tree. Thus, cholangiocytes are not completely dependent on the presence of a hepatic artery, which would otherwise be suggested by the biliary breakdown induced by hepatic artery ligation, and which would preclude cell replacement therapy. Instead, our results suggest cell replacement therapies to replace absent cholangiocytes may be feasible, even in the absence of normal hepatic vasculature.
Our data, showing a selective loss of primarily Notch1-mediated signaling, may be at apparent odds with the fact that ALGS can be caused by NOTCH2 mutations,12 and that compound heterozygous Jag1/Notch2 mice are also growth delayed, display jaundice, and recapitulate hepatic, cardiac, renal, and ocular defects.10 Moreover, Notch2 is required for bile duct morphogenesis and differentiation in vivo, 44–46 while Notch1 is dispensable.44 However, the mildly reduced Jag1Ndr-Notch2 signaling described here may be sufficient to cause a pathologic outcome in keeping with the dose-sensitive nature of the Notch signaling pathway.
In sum, the Nodder mouse provides a clinically relevant model for ALGS and allows for the first time a Jag1 missense mutation to be linked both to phenotypic traits typical for the disease and to dysregulated Notch signaling, manifested by hypomorphic signaling and receptor-selectivity.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2017.11.002.
Editor’s Notes.
Background and Context
Alagille syndrome is caused by mutations in JAGGED1, but it is unclear how specific mutations impact on Notch signaling and affect development, and how faithfully Jag1 mutant models mimic the human condition.
New Findings
A receptor-selective missense mutation in mouse Jag1 (H268Q) recapitulates Alagille syndrome phenotypes in mice. RNA seq of mouse liver samples and samples from patients, showed that apical polarity of bile ducts is severely disrupted.
Limitations
The mouse model is based on homozygous mutation of Jag1, while human patients are heterozygous for JAG1 mutations.
Impact
This new mouse model will allow development and testing of new therapies for Alagille syndrome, as well as a new model in which to study how Notch signaling controls development of organs affected by Alagille syndrome.
Acknowledgments
Funding
U.L. acknowledges support from the Swedish Research Council (project grant and the Linnaeus Center DBRM), European Research Council Marie Curie ITN-FP7 NotchIT (IVC), the Swedish Cancer Society, Hjärnfonden, Knut och Alice Wallenbergs Stiftelse, and ICMC (the Integrated Cardio Metabolic Center). E.R.A. and members of Andersson lab were supported by a Center of Innovative Medicine (CIMED) Grant, the Daniel Alagille Award, KI Funding, and the Alex and Eva Wallström Foundation. S.H. was supported by Wera Ekströms Stiftelse and a grant for KI-MU exchange (see below), and grants in ERA lab. M.S. was supported by an OSK Huttunen post doc fellowship. J.M. was supported by a WennerGren Fellowship and grants in ERA lab. V.B. and S.H. were supported by “KI-MU” program (CZ.1.07/2.3.00/20.0180) co-financed from European Social Fund and the state budget of the Czech Republic.
The funders had no role in study design, analysis, or interpretation of data.
Abbreviations used in this paper
- ALGS
Alagille syndrome
- ECD
extracellular domain
- EGF
epidermal growth factor
- FACS
fluorescence-activated cell sorting
- GSEA
gene set enrichment analyses
- HPA
Human Protein Atlas
- PCA
principal component analysis
- qPCR
quantitative real-time polymerase chain reaction
Footnotes
Author contributions: E.R.A., U.L.: Author study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; study supervision. V.B.: Critical revision of the manuscript for important intellectual content; obtained funding; technical or material support; study supervision. I.V.C., S.H.: Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. D.R., Y.L.T., R.S., E.V., H.S.: Analysis and interpretation of data; statistical analysis. M.S.: Acquisition of data; analysis and interpretation of data; statistical analysis; study supervision. J.N., A.E.: Acquisition of data; analysis and interpretation of data. A.H., J.M.: Acquisition of data; analysis and interpretation of data; statistical analysis. M.H.: Technical and material support; critical revision of the manuscript for important intellectual content. B.F., E.E., A.N., K.C., H.C., A.P.: Critical revision of the manuscript for important intellectual content; intellectual development of project, and material support (samples and/or methods).
Deposited data: GSE104876 is the reference series for this manuscript (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104876). GSE104873: RNA seq of livers from patients with Alagille syndrome (Figures 4 and 5) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104873). GSE104874: Species specific transcriptomic data (mouse) of mouse C2C12 cells cocultured with human JAG1- or JAG1Ndr-expressing cells (Figure 5) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104874). GSE104875: RNA Seq of Jag1Ndr/Ndr and Jag1+/+ liver (Figure 6) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104875).
Conflicts of interest
The authors disclose no conflicts. A separate project in ERA lab is funded by ModeRNA.
References
- 1.Mašek J, Andersson ER. The developmental biology of genetic Notch disorders. Development. 2017;144:1743–1763. doi: 10.1242/dev.148007. [DOI] [PubMed] [Google Scholar]
- 2.Grochowski CM, Loomes KM, Spinner NB. Jagged1 (JAG1): structure, expression, and disease associations. Gene. 2016;576:381–384. doi: 10.1016/j.gene.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 4.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 5.McDaniell R, Warthen DM, Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alagille D, Odièvre M, Gautier M, et al. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. doi: 10.1016/s0022-3476(75)80706-2. [DOI] [PubMed] [Google Scholar]
- 7.Luca VC, Kim BC, Ge C, et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;1:1–24. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson EM, Lanner F, Das D, et al. Control of Notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123:2931–2942. doi: 10.1242/jcs.073239. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann JJ, Zovein AC, Koh H, et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 11.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinner NB, Leonard LD, Krantz ID. Alagille Syndrome. University of Washington; Seattle: 2013. [PubMed] [Google Scholar]
- 13.Kamath BM, Bauer RC, Loomes KM, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet. 2012;49:138–144. doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakurdas SM, Lopez MF, Kakuda S, et al. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi) Hepatology. 2016;63:550–565. doi: 10.1002/hep.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin S, Mutvei AP, Chivukula IV, et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene. 2013;32:4892–4902. doi: 10.1038/onc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chivukula IV, Ramsköld D, Storvall H, et al. Decoding breast cancer tissue-stroma interactions using species-specific sequencing. Breast Cancer Res. 2015;17:109. doi: 10.1186/s13058-015-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan AE, Li R, Hawes NL, et al. Genetic background modifies inner ear and eye phenotypes of Jag1 heterozygous mice. Genetics. 2007;177:307–311. doi: 10.1534/genetics.107.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElhinney DB, Krantz ID, Bason L, et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation. 2002;106:2567–2574. doi: 10.1161/01.cir.0000037221.45902.69. [DOI] [PubMed] [Google Scholar]
- 21.Williams AL, Bohnsack BL. Neural crest derivatives in ocular development: discerning the eye of the storm. Birth Defects Res C Embryo Today. 2015;105:87–95. doi: 10.1002/bdrc.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BJ, Fulton AB. The genetics and ocular findings of Alagille syndrome. Semin Ophthalmol. 2007;22:205–210. doi: 10.1080/08820530701745108. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys R, Zheng W, Prince LS, et al. Cranial neural crest ablation of Jagged1 recapitulates the craniofacial phenotype of Alagille syndrome patients. Hum Mol Genet. 2012;21:1374–1383. doi: 10.1093/hmg/ddr575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jinguji M, Tsuchimochi S, Nakajo M, et al. Scintigraphic progress of the liver in a patient with Alagille syndrome (arteriohepatic dysplasia) Ann Nucl Med. 2003;17:693–697. doi: 10.1007/BF02984977. [DOI] [PubMed] [Google Scholar]
- 25.Sparks EE, Perrien DS, Huppert KA, et al. Defects in hepatic Notch signaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis Model Mech. 2011;4:359–367. doi: 10.1242/dmm.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoniou A, Raynaud P, Cordi S, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 28.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Ning G, Duncan SA. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- 30.Huch M, Gehart H, Van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucuvalas JC, Horn JA, Carlsson L, et al. Growth hormone insensitivity associated with elevated circulating growth hormone-binding protein in children with Alagille syndrome and short stature. J Clin Endocrinol Metab. 1993;76:1477–1482. doi: 10.1210/jcem.76.6.8501153. [DOI] [PubMed] [Google Scholar]
- 32.Riely CA, Cotlier E, Jensen PS, et al. Arteriohepatic dysplasia: A benign syndrome of intrahepatic cholestasis with multiple organ involvement. Ann Intern Med. 1979;91:520–527. doi: 10.7326/0003-4819-91-4-520. [DOI] [PubMed] [Google Scholar]
- 33.Torizuka T, Tamaki N, Fujita T, et al. Focal liver hyperplasia in Alagille syndrome: assessment with hepatoreceptor and hepatobiliary imaging. J Nucl Med. 1996;37:1365–1367. [PubMed] [Google Scholar]
- 34.Tuset E, Ribera JM, Doménech E, et al. Pseudotumorous hyperplasia of the caudate lobe of the liver in a patient with Alagille syndrome. Med Clin (Barc) 1995;104:420–422. [PubMed] [Google Scholar]
- 35.Main H, Radenkovic J, Jin SB, et al. Notch signaling maintains neural rosette polarity. PLoS One. 2013;8:e62959. doi: 10.1371/journal.pone.0062959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlovskaja-Gumbrienė A, Yi R, Alexander R, et al. Proliferation-independent regulation of organ size by Fgf/Notch signaling. Elife. 2017;6:e21049. doi: 10.7554/eLife.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovall RA, Blacklow SC. Mechanistic insights into notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- 38.Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanimizu N, Nishikawa Y, Ichinohe N, et al. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem. 2014;289:7589–7598. doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter TJ, Vanderpool C, Cast AE, et al. Intrahepatic bile duct regeneration in mice does not require Hnf6 or notch signaling through Rbpj. Am J Pathol. 2014;184:1479–1488. doi: 10.1016/j.ajpath.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 42.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling–are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 43.Alvaro D, Metalli VD, Alpini G, et al. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Geisler F, Nagl F, Mazur PK, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 45.Jeliazkova P, Jörs S, Lee M, et al. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 46.Falix FA, Weeda VB, Labruyere WT, et al. Hepatic Notch2 deficiency leads to bile duct agenesis perinatally and secondary bile duct formation after weaning. Dev Biol. 2014;396:201–213. doi: 10.1016/j.ydbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.