Abstract
BACKGROUND:
Genetic determinants may underlie the susceptibility of red blood cells (RBCs) to hemolyze in vivo and during routine storage. This study characterized the reproducibility and dynamics of in vitro hemolysis variables from a subset of the 13,403 blood donors enrolled in the RBC-Omics study.
STUDY DESIGN AND METHODS:
RBC-Omics donors with either low or high hemolysis results on 4°C-stored leukoreduced (LR)-RBC samples from enrollment donations stored for 39 to 42 days were recalled 2 to 12 months later to donate LR-RBCs. Samples of stored LR-RBCs from the unit and from transfer bags were evaluated for spontaneous and stress-induced hemolysis at selected storage time points. Intradonor reproducibility of hemolysis variables was evaluated in transfer bags over two donations. Hemolysis data at serial storage time points were generated on LR-RBCs from parent bags and analyzed by site, sex, race/ethnicity, and donation frequency.
RESULTS:
A total of 664 donors were successfully recalled. Analysis of intradonor reproducibility revealed that osmotic and oxidative hemolysis demonstrated good and moderate reproducibility (Pearson’s r = 0.85 and r = 0.53, respectively), while spontaneous hemolysis reproducibility was poor (r = 0.40). Longitudinal hemolysis in parent bags showed large increases over time in spontaneous (508.6%) and oxidative hemolysis (399.8%) and smaller increases in osmotic (9.4%) and mechanical fragility (3.4%; all p < 0.0001).
CONCLUSION:
Spontaneous hemolysis is poorly reproducible in donors over time and may depend on site processing methods, while oxidative and osmotic hemolysis were reproducible in donors and hence could reflect consistent heritable phenotypes attributable to genetic traits. Spontaneous and oxidative hemolysis increased over time of storage, whereas osmotic and mechanical hemolysis remained relatively stable.
Current guidelines regulating storage of red blood cell (RBC) components are uniform for all donors, despite genetic and metabolic variation among donors known to affect hemoglobin (Hb) production and stability, RBC membrane function, iron absorption and metabolism, and hemolysis during storage.1–7 Spontaneous storage hemolysis of RBCs, measured as the cell free Hb concentration at the end of component storage (with a cutoff < 1%), is one of the primary criteria, in addition to posttransfusion autologous radiolabeled RBC recovery and survival studies, for the US Food and Drug Administration (FDA) premarketing approvals to companies producing new blood bags, storage solutions, and so forth. Spontaneous end-of-storage hemolysis and posttransfusion recovery and survival studies are also the basis for FDA-defined limits for the duration of allowed storage of RBC components after postproduction manipulations such as gamma irradiation and pathogen reduction. In other countries, notably the EU member states and Canada, the routine quality control (QC) process by the organization that produces the leukoreduced (LR)-RBCs includes the hemolysis level (with a cutoff of <0.8%) at outdate (performed generally on 1% of inventory).
In addition to spontaneous hemolysis, stored RBCs exhibit membrane damage, blebbing, and loss of deformability during storage.3,8–10 Hemolysis before or after transfusion of damaged or senescent RBCs has been shown to severely disrupt nitric oxide (NO) bioavailability at the endothelium via accelerated NO deoxygenation reactions with free plasma Hb.3,11 This process contributes to endothelial dysfunction, adhesion molecule expression, platelet (PLT) and hemostatic activation, and reactive oxygen species generation. In addition to immediate effects on vascular function and blood flow, hemolysis has been shown to exert downstream effects by activating PLTs and the hemostatic system and by heme- and non-transferrin-bound iron-mediated modulation of the innate immune system that may contribute to infectious and noninfectious complications.12,13 Independent of transfusion, it has been shown that hemolysis is an important pathologic mechanism in many genetic diseases, including sickle cell disease, thalassemia, and paroxysmal nocturnal hemoglobinuria.14–17
It has been hypothesized that transfusion of RBCs with extended storage durations may be associated with an increased risk of cardiovascular events, multiorgan failure, and mortality in susceptible transfusion recipients, although this has not borne out in a series of randomized control trials.10,18–20 Studies of RBC recovery and survival after cold storage have demonstrated variability among donors that is reproducible over time in both human studies and murine models.6,17,21–24 These studies have suggested that the propensity of RBCs to hemolyze under stress, including cold storage, is heritable and that the intrinsic rate of hemolysis in vitro and in vivo is stable within individuals over time but highly variable among individuals and among different demographic subgroups.4,17,23,25 For example, in sickle cell disease, males have significantly higher rates of in vivo hemolysis compared to females26 and stored RBCs from healthy donors male RBCs show higher rates of spontaneous or stress-induced hemolysis than female RBCs.22,23,25
In an effort to further characterize donor differences in predisposition to storage-induced RBC hemolysis, the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology Donor Evaluation Study (REDS)-III program launched the Red Blood Cell-Omics (RBC-Omics) study.22 The RBC-Omics study is investigating the overarching hypothesis that genetic and metabolomic variation in donors underlies the variable propensity of RBCs to hemolyze in vitro during routine LR-RBC storage or in response to stressors of hemolysis. Specifically, the study was designed to characterize the rates and reproducibility of storage-induced RBC hemolysis propensity in large groups of US blood donors representing different racial/ethnic, sex, age, and previous donation frequency categories, to understand whether hemolytic propensity during storage is influenced by donor demographics and previous donation patterns and whether donor-specific hemolysis associations are reproducible over time and associated with genetic and metabolomics findings. We previously demonstrated that male sex, Asian or African racial background, and older age are significant modifiers of hemolysis during cold storage of RBCs.22 In this current analysis of the RBC-Omics cohort, the intradonor reproducibility in hemolytic variables over time was studied, and the donor characteristics associated with changes in hemolytic variables during the course of 42-day course of LR-RBC unit storage were investigated. We report that osmotic and oxidative stress–induced hemolysis measured at the end of storage are reproducible within donors over time; however, there are sex, age, and racial-ethnic differences in hemolytic responses, including spontaneous storage hemolysis and changes in osmotic, mechanical, and oxidative stress-induced hemolysis in LR-RBCs, throughout the period of routine storage.
MATERIALS AND METHODS
Human subjects research compliance
RBC-Omics was conducted under regulations applicable to all human subject research supported by federal agencies as well as requirements for blood product manipulation specified and approved by the FDA. The data coordinating center (RTI International) of REDS-III was responsible for the overall compliance of human subjects regulatory protocols including institutional review board approval from each participating blood center, from the REDS-III Central Laboratory (Vitalant Research Institute), and from the data coordinating center. By consenting to the RBC-Omics study, subjects agreed to be recontacted by study personnel within the following year to donate a study-dedicated LR-RBC unit in a “recall” phase of the study. Subjects who met recall criteria and agreed to participate in the recall phase of the study signed a new consent form during the REDS-III RBC-Omics study return visit and agreed to make a whole blood donation from which the LR-RBC component would be used for research.
Donor recruitment and data linkages
Donor selection and recruitment for RBC-Omics was performed at four large blood centers: the American Red Cross (ARC, Farmington, CT), the Institute for Transfusion Medicine (ITxM, Pittsburgh, PA), Blood Center of Wisconsin (BCW, Milwaukee, WI), and Blood Centers of the Pacific (BCP, San Francisco, CA). Overall, 98% (13,403) of the whole blood donations provided by 13,758 participating donors were evaluable for storage hemolysis variables (Fig. 1). Demographic data, including race/ethnicity, were collected directly from enrollment interviews and recorded in the RBC-Omics study management system database. Additional demographic data, including weight, height, and the date of birth, as well as the donation history, were derived from the blood centers’ routine donor/donation databases and linked through donor ID, donation date, and donation identification number. Donors were categorized into self-reported racial/ethnic groups: non-Hispanic white, Hispanic white, non-Hispanic African American, or non-Hispanic Asian. Donors with multiple races and Hawaiian American, Native American, and other donors were grouped as “other.” In addition, a group of 1976 high-intensity donors (also called super donors) was recruited among those meeting the specific criteria of nine or more successful RBC equivalent donations (double-RBC donations counted as two RBC-equivalent donations) in the preceding 24-month period, excluding the enrollment donation, without a low Hb deferral.
Fig. 1.
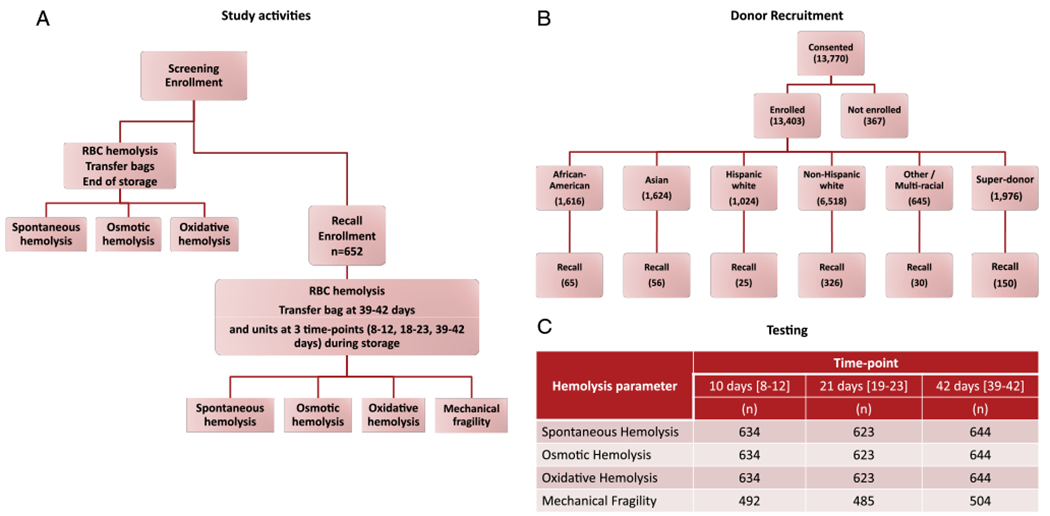
RBC-Omics subject enrollment, characteristics recall, and hemolysis variable testing. (A) Flow chart of the RBC-Omics study cohort and donor testing for hemolysis; (B) donor enrollment and recall statistics stratified by self-identified group; (C) count of number of recalled participants successfully completing each of the hemolytic tests at the three time points. [Color figure can be viewed at wileyonlinelibrary.com]
Of the 13,403 enrolled donors, 12,753 had spontaneous hemolysis measures, 12,799 had osmotic hemolysis measures, and 10,476 had oxidative hemolysis measures (which were added 6 months after study accrual was launched) and consented to be recontacted. Criteria for selection of up to 800 donors for recall were based on the goals of recalling donors who had at least one hemolysis measurement that was at the low or high end of the frequency distribution for that variable, as well as selecting a racially diverse recall cohort. Specific criteria to fulfill these goals evolved over the course of the recall phase of the study. We first identified subjects above the 95th percentile or below the 5th percentile of spontaneous or oxidative hemolysis measures; we subsequently did the same for osmotic hemolysis. Because African American, Asian, and Hispanic donors were underrepresented in the upper tails of all three distributions, we selected all such donors from the upper distribution tails while randomly selecting subsets of other racial groups in the upper tails and randomly selecting all racial groups in the lower tails. Due to a lack of correlation among the three hemolysis measures, most subjects in the tails of one distribution were not in the tails of the others, thus providing a wide distribution of each hemolysis variable in the overall recalled cohort. Subjects were invited by mail and telephone to participate in the recall study.
Blood collection and LR-RBC component processing
Component processing procedures employed during the recall phase of the study were equivalent to those in the initial enrollment phase as standardized operation procedures were used for this study.22,27 In both phases, whole blood units were collected and processed according to each blood center’s standard operating procedures. An aliquot of whole blood derived from the donation was collected into a 10-mL EDTA retention tube for the determination of complete blood count and selected hematology variables using automatic cell counters. Each RBC component was filtered to generate a LR-RBC unit in AS-1 or −3 (AS-1 in one center or AS-3 in the other three centers). Three blood centers (BCP, BCW, and ARC) performed prestorage LR immediately after the LR-RBC component was manufactured. One blood center (ITxM) delayed LR until after negative donor screening results were received and until after the LR-RBC unit was transferred to the central transfusion service, which was generally 48 to 72 hours after collection. At all centers, a 10- to 15-mL aliquot from each LR-RBC unit was sterile docked into a customized “transfer bag” (Haemonetics), which had been manufactured specifically for this study using the same materials and volume-to-surface ratio as the parent LR-RBC storage bags.27 In contrast to the initial phase during which the LR-RBC units (referred to as “parent” units) were released for distribution for transfusion to patients, the parent units and the transfer bags in the recall phase were sent to an RBC-Omics testing laboratory (University of Pittsburgh, Pittsburgh, PA; or Vitalant Research Institute, San Francisco, CA) for storage under routine blood bank conditions (1-6°C).
The methods to store LR-RBC samples and the assays used to measure the hemolytic propensity of RBCs were assessed to ensure the reproducibility of the study results. Study procedures were piloted and assessed to optimize consistency in assay results across laboratories and technicians and over time.28 This included monthly proficiency assessments based on distribution of identical LR-RBC component derived samples in study transfer bags that were sent to the two testing laboratoriess and monitoring of study data in real time to ensure that results generated across the testing laboratories fell within expected ranges. This QC and quality assurance program was implemented to guarantee the validity of the results obtained by two testing laboratories evaluating the spontaneous and stress-induced hemolysis of RBCs during storage.
Evaluation of hemolytic propensity in stored RBCs
As was done in the initial enrollment phase, transfer bags from the recall study subjects were accessed at the end of the storage period (39-42 days postcollection) when the content of each transfer bag was transferred into a 15-mL conical tube, from which two aliquots (1 mL) were collected for the hemolytic assays. One aliquot was used for the quantification of spontaneous storage hemolysis and the other for the stress-induced hemolysis assays, as detailed in our previous publication.22 Parent LR-RBC units acquired for research purposes from the recall donations were stored under blood bank storage conditions for 42 days and were accessed at three time points during storage period: 1) between 8 and 12 days, 2) between 18 and 23 days, and 3) between 39 and 42 days postcollection. At each time point, 15 mL of the LR-RBC component was removed for hemolysis testing. Repeated parent unit sampling was performed through one of the two sampling ports on Days 8 to 12 and 18 to 23, respectively, using a plasma transfer set (Baxter Item 4C2240), while sampling on Days 39 to 42 was performed through the LR-RBC unit main port.
Hemolysis assays included spontaneous, oxidative, and osmotic stress-induced hemolysis assays (the latter done after cell washing) as previously described.22 In addition, a mechanical fragility assay was performed on RBCs from the end of storage transfer bags and serial samples derived from the parent unit.
Mechanical fragility assay
Red blood cells were washed and suspended with PBS to a final hematocrit level of 3.5 ± 0.5%. Aliquots of 200 μL were transferred into a 96-well plate. Mechanical fragility was evaluated by adding one stainless-steel bead (3/32″, Small Parts, US) into each well and then shaking the plate for 3 hours using a titer plate shaker (ThermoFisher Scientific, US; instrument’s speed set to 6.5).29,30 Control samples were rocked in the same manner without the presence of a stainless steel bead. After being shaken, each RBC sample was transferred into a V-shaped 96-well plate, which was centrifuged (1520 × g, 10 min, 20° C) and sample supernatants were collected for measuring the Hb concentration (μmol/L). The mechanical fragility index was calculated as:
where Hbbead corresponds to the concentration of free Hb (μmol/L) from RBC supernatants rocked with a bead, Hbcontrol is the concentration of free Hb (μmol/L) from RBC supernatants rocked without a bead, and Hbtotal is the total amount of Hb (μmol/L) of each RBC sample.
Statistical analyses
Correlation between end-of-storage hemolysis results obtained on transfer bags versus parent bags
A comparison of each of the four hemolytic assays (spontaneous, osmotic, oxidative, and mechanical) was performed on end of storage samples derived from the transfer bags and the parent bags. Pearson’s correlation was used to compare the results.
Reproducibility of hemolysis variables over time
Evaluation of the reproducibility between screening and recall phases of the study for the spontaneous, osmotic, and oxidative hemolysis variables was performed by comparing end-of-storage transfer bag results from the initial enrollment (first time point) and recall phases (second time point) for each of the recalled donors. Correlation was evaluated by linear regression with screening and recall phases coded as variables and degree of agreement quantified with Pearson’s correlation.
Analysis of hemolysis measures across time points
The kinetics of hemolysis measures on Days 10, 21, and 42 were analyzed using time (days) as a numerical variable in computer software (R Statistical Software for Windows, Version 3.3.1). Linear mixed models were used to estimate the mean change in hemolysis variables by duration of storage while accounting for the correlation across time points for individual subjects. Linear mixed models were fitted for each of the hemolytic variables, with time points as fixed effects and participants as random effects. The estimation of effect size on hemolysis changes per day was used to estimate average hemolysis increase per day. Expected difference between Day 10 and Day 42 was estimated by multiplying the beta estimates by number of days of storage. Percentage increase was calculated by dividing the estimated difference between Day 10 and Day 42 by the mean of Day 10 hemolysis.
Generalized estimating equation models were fitted for each of the hemolytic variables and each of the explanatory covariates to assess the difference in hemolysis between male and female, among different race/ethnicity groups, and among blood donation centers, while accounting for the time course-dependent autocorrelation structure. Univariate generalized estimating equation models were fitted using an autoregressive model as the correlation structure to estimate the effect each of the explanatory variable has on hemolysis.
Differences in hemolysis over time due to sex, race/ethnicity, and prior donation history
Frequency plots for each of the hemolysis measurement were generated for each racial/ethnic and prior donation intensity category and across time using computer software (ggplot2 packages, Version 2.1.0, in R Statistical Software for Windows, Version 3.3.1).
RESULTS
RBC-Omics recalled donor recruitment and demographics
Between December 2013 and October 2015, a total of 13,770 blood donors consented to participate in the screening phase of the REDS-III RBC-Omics study and 13,403 were enrolled.22,27 Out of those, 12,753 donors provided sufficient samples and data for evaluation of spontaneous storage hemolysis, 12,799 were evaluated for osmotic hemolysis, and 10,476 were evaluated for oxidative hemolysis.31 Of those who agreed to be recontacted, 664 selected donors were successfully recalled and consented, providing 652 LR-RBC components for evaluation (Fig. 1). The demographics of recalled donors, including their sex, age, and race/ethnicity distributions, are compared to characteristics of screening phase donors in Table 1. Donors from both sexes were well represented in the recall phase of the study (46.1% females and 53.9% males).
TABLE 1.
Demographics of donors enrolled in the screening versus recall phase of the RBC-Omics study*
| Total | 13,403 | 652 |
|---|---|---|
| Sex | ||
| Female | 6,737 (50.26) | 301 (46.17) |
| Male | 6,666 (49.74) | 351 (53.83) |
| Race/ethnicity | ||
| White | 8,494 (63.37) | 476 (73.01) |
| African American non-Hispanic | 1,616 (12.06) | 65 (9.97) |
| Asian non-Hispanic | 1,624 (12.12) | 56 (8.59) |
| Hispanic | 1,024 (7.64) | 25 (3.83) |
| Other | 645 (4.81) | 30 (4.60) |
| HUB | ||
| ARC | 3,292 (24.56) | 132 (20.25) |
| BCW | 3,355 (25.03) | 188 (28.83) |
| BCP | 3,564 (26.59) | 146 (22.39) |
| ITxM | 3,192 (23.82) | 186 (28.53) |
| Number of Prior Donations | ||
| 0 | 3,998 (29.83) | 85 (13.04) |
| 1-2 | 3,076 (22.95) | 115 (17.64) |
| 3-4 | 1,770 (13.21) | 104 (15.95) |
| 5-6 | 1,260 (9.40) | 91 (13.96) |
| 7-10 | 2,410 (17.98) | 184 (28.22) |
| >10 | 889 (6.63) | 73 (11.20) |
Data are reported as number (%).
Reproducibility of hemolysis variables in RBCs stored in parent units versus transfer bags and in transfer bags across two time points
As seen in Fig. 2, osmotic (r = 0.9754) and mechanical (r = 0.904) hemolysis variables in Day 39 to Day 42 samples from the parent LR-RBC components were highly correlated with results from Day 39 to Day 42 transfer bags. There was a moderate correlation for spontaneous (r = 0.6642) and oxidative (r = 0.7966) hemolysis, with a shift toward higher levels in transfer bags.
Fig. 2.

Correlation between hemolysis variables in parent versus transfer bags at 39 to 42 days using samples from recalled donors. Scatter plots shown for percent spontaneous storage hemolysis (A), percent osmotic hemolysis (B), percent oxidative hemolysis (C), and percent mechanical hemolysis (D). Percent hemolysis of blood samples derived from parent unit is shown on the x-axis and that from transfer bag on y-axis. Pearson correlation statistics, p values, and sample sizes are shown on each panel.
We compared the amount of spontaneous and stress–induced oxidative and osmotic hemolysis obtained on RBCs from transfer bags accessed at 39 to 42 days of storage from the screening phase with that obtained from transfer bags from the recall phase for each donor enrolled in the recall study (Fig. 3). Spontaneous hemolysis exhibited the lowest correlation (r = 0.4031) between screening and recall. A moderate correlation (r = 0.5332) was found for oxidative hemolysis and a strong correlation was found for osmotic hemolysis (r = 0.857). Compared to the screening population, the recall population was enriched for donors reproducibly exhibiting the lowest and highest oxidative and osmotic hemolysis percentage across screening and recall phase (Fig. S1, available as supporting information in the online version of this paper).
Fig. 3.

Intraassay correlations for screening and recall results from transfer bags.Scatter plots shown for percent spontaneous storage hemolysis (A), percent oxidative hemolysis (B), and percent osmotic hemolysis (C), with hemolysis of blood samples derived from transfer bag from the enrollment donation shown on the x-axis and that of recall donation shown on the y-axis. Pearson correlation statistics and p values are shown on each panel.
Evolution of RBC hemolysis variables over time of storage and by donor characteristics
Spontaneous, oxidative, osmotic, and mechanical hemolysis were measured on RBCs sampled from the stored parent LR-RBC units on Post-collection Days 8 to 12, 18 to 23, and 39 to 42 (Fig. 4). Analysis of longitudinal hemolysis in parent bags from Day 10 to Day 42 revealed significant increases over time for spontaneous (508.6%) and oxidative hemolysis (399.8%) and a small but significant increase for osmotic (9.4%) across all sites (p < 0.0001 for each of these variables). LR-RBC units collected and processed by ITxM demonstrated higher spontaneous hemolysis over time of storage compared to those collected by the ARC site (p < 0.0001;Table 2). Mechanical hemolysis increased over time at two of the three evaluable sites (ARC and ITxM, p < 0.0001) with similar rates of increase at these two sites (3.4% from Day 10 to Day 42 of storage).
Fig. 4.
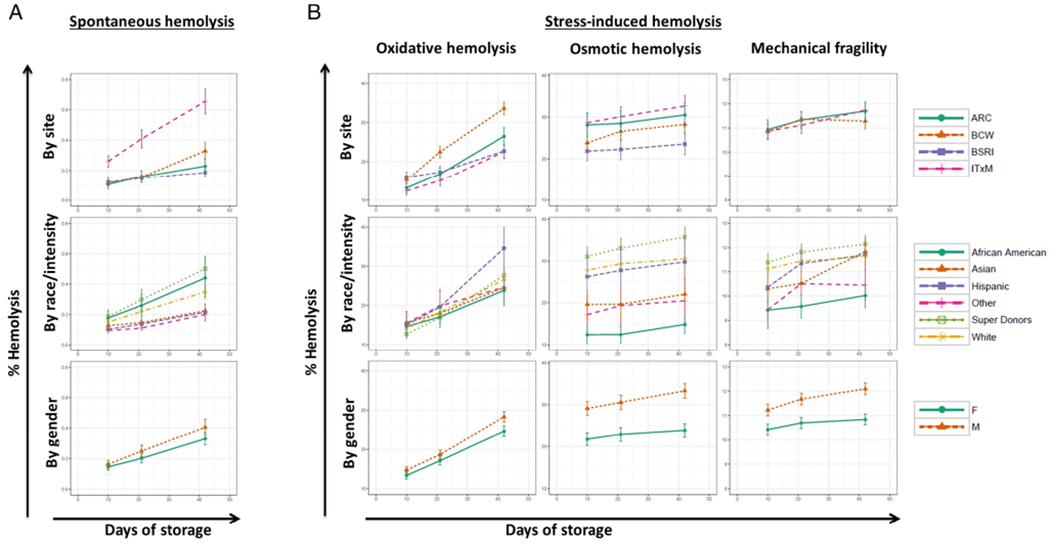
Hemolysis variables over time of storage in the recall population by site, sex, race/ethnicity, and donation frequency. Line plots showing the mean and confidence interval of percent spontaneous storage hemolysis (A) and percent stress-induced hemolysis (B) for oxidative hemolysis, osmotic hemolysis, and mechanical hemolysis, stratified by site (Row 1), by race/ethnicity (Row 2), or by sex (Row 3). Mechanical hemolysis measurements from BCP were excluded from plotting due to difference in assay performance at the laboratory testing for BCP donations. Measurements were taken at three time points (Postcollection Days 10 [range, 8-12 days], 20 [range, 18-23 days], and 42 [range, 39-42 days]).
TABLE 2.
Results of the generalized estimating equation model assessing the difference in hemolysis in male versus female, among race/ethnicity groups versus white donors, and among blood centers compared to the ARC site*
| Spontaneous hemolysis |
Oxidative hemolysis |
Osmotic hemolysis |
Mechanical hemolysis |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Pr(>|W|) | Estimate | Pr(>|W|) | Estimate | Pr(>|W|) | Estimate | Pr(>|W|) | |
| Sex | ||||||||
| Males | 0.046 | 0.0519 | 2.35 | 0.0008 | 8.30 | <0.0001 | 1.04 | <0.0001 |
| Group | ||||||||
| Super donors | 0.094 | 0.0072 | −0.29 | 0.7415 | 4.40 | 0.0019 | 0.42 | 0.0450 |
| Other | −0.103 | <0.0001 | −0.02 | 0.9930 | −10.38 | <0.0001 | −1.31 | 0.0045 |
| Hispanic | −0.089 | 0.0001 | 3.48 | 0.0696 | −0.73 | 0.8146 | −0.31 | 0.3166 |
| Asian | −0.073 | 0.0017 | −0.34 | 0.7972 | −8.26 | <0.0001 | −0.36 | 0.4417 |
| African American | 0.058 | 0.1939 | −1.14 | 0.3913 | −15.52 | <0.0001 | −1.67 | < 0.0001 |
| Hub | ||||||||
| BCW | 0.048 | 0.0691 | 4.92 | < 0.0001 | −3.09 | 0.0742 | −0.20 | 0.3798 |
| BCP | −0.015 | 0.3999 | −0.38 | 0.7201 | −6.13 | 0.0010 | NA | NA |
| ITxM | 0.287 | <0.0001 | −2.27 | 0.0257 | 1.50 | 0.4217 | −0.03 | 0.9125 |
Reference groups are female for sex, white for groups, and ARC for hubs.
Table 2 shows multiple observations; oxidative hemolysis was significantly higher at BCW (p < 0.0001) and oxidative hemolysis was lower at BCP (p = 0.0010) compared to ARC. High-intensity donors exhibited the largest increase in spontaneous hemolysis compared to white donors (p = 0.0072) while Hispanic, Asian, and others exhibited lower spontaneous hemolysis compared to white donors (p = 0.0017, p = 0.0001, and p < 0.0001, respectively). African American donors exhibited higher spontaneous hemolysis than Asian donors (p = 0.0030), while Asian and African American donors exhibited the lowest osmotic stress-induced hemolysis compared to white donors (p < 0.0001). African American donors also exhibited the lowest mechanical stress-induced hemolysis (p < 0.0001), while high-intensity donors exhibited the highest mechanical stress-induced hemolysis (p = 0.0450) over time of storage, both compared to white donors. Males consistently exhibited higher hemolysis across all time points than females with highly significant differences for oxidative, osmotic, and mechanical hemolysis (p = 0.0008, p < 0.0001, and p < 0.0001, respectively).
DISCUSSION
The RBC-Omics study is the first to characterize spontaneous and stress-induced hemolysis in a diverse population of US blood donors across multiple race/ethnicity and donation frequency groups and across multiple blood collection organizations. Donor enrollment, blood collections and RBC processing by four different hubs allowed for the representation of different LR-RBC component manufacturing and processing methods and detailed characterization of hemolysis phenotypes in diverse populations at four US locations.
In this recall phase of the RBC-Omics study, we characterized differences in spontaneous and stress-induced RBC hemolytic assay results in LR-RBC units and transfer bags from 652 recalled RBC-Omics donors. We demonstrated good correlations between transfer bags and corresponding parent units for stress-induced osmotic and oxidative hemolysis at the end of storage. Hemolysis results were generated from stored transfer bags from 652 recalled donors and results were compared to corresponding findings obtained from LR-RBCs stored in transfer bags prepared at the time of the enrollment visit months before the recall donation.22 Results at end of storage in transfer bags were correlated to results at end of storage in parent LR-RBC units and demonstrated good correlations for stress-induced osmotic and oxidative hemolysis, with poor correlations for spontaneous hemolysis. Finally, kinetics of RBC hemolysis over time of storage (from Day 10 through Day 42 of storage) were measured by accessing LR-RBC units from the recall donors at three time points during storage.
The observation that osmotic and oxidative hemolysis results were reproducible between the screening and recall phases suggests that osmotic and oxidative fragility are intrinsic to each person’s RBCs (washed cells) in healthy subjects, and although environmental factors may contribute, this may have a genetic basis.32 In contrast, spontaneous storage hemolysis results from the screening and recall phase showed a poor correlation. Interestingly, spontaneous hemolysis was highest in LR-RBC units prepared at ITxM, where processing methods were different including delayed leukoreduction after several days of storage. This finding suggested that spontaneous storage hemolysis may reflect differences in LR-RBC manufacturing and handling to a greater extent than stress hemolysis variables, although further analyses are warranted to determine if other nongenetic factors may influence spontaneous hemolysis during RBC storage such as environmental factors at the different geographic locations.
The finding that spontaneous hemolysis increased in RBC units manufactured with delayed leukoreduction could be interpreted to indicate that it is important to perform leukoreduction as early as possible after RBC component production. However, before this firm conclusion can be drawn, it will be important to investigate any association of delayed leukoreduction to clinical outcomes in recipients using the REDS-III linked donor/recipient database. If a link is demonstrated, it may lead to changes in manufacturing methods. Additionally, the finding that storage hemolysis may be impacted by manufacturing methods suggested that while spontaneous hemolysis is used by some blood banks for QC purposes, it may not be reliably used as a measure of a hemolysis phenotype that would be determined by genetic traits. The finding that oxidative hemolysis was higher in RBCs prepared by BCW than in those prepared by ARC suggests that preparation methods may impact oxidative hemolysis; indeed, BCW uses AS-1 while all other sites are using AS-3 for the preparation of RBCs.
Differences in longitudinal storage hemolysis findings were associated with frequent prior blood donations, ethnic groups, and sex, which supports the hypothesis that genetic and biological variables among blood donors can modulate RBC hemolytic propensity during the course of unit storage. The associations derived from serial samples from stored LR-RBC components confirm and extend our previously reported findings based on end-of-storage LR-RBC samples from the screening phase of RBC-Omics. While spontaneous storage and oxidative hemolysis values changed during storage, osmotic and mechanical stress-induced hemolysis were more stable during storage and hence may reveal more intrinsic RBC properties. Subsequent studies should define whether intrinsic differences in RBC membrane stability could predict the recovery and survival of stored RBCs in the patient circulation.
The REDS-III investigators are exploring the possibility that differences in in vitro hemolysis correlate with Hb increments in recipients of LR-RBC transfusions from RBC-Omics donors.33 In considering this issue, our data indicate that osmotic hemolysis may represent the best in vitro hemolysis variable to study, since osmotic hemolysis was the most reproducible across screening and recall phases. Hence, this variable may be more a characteristic of a donor rather than particular to a donation from that donor. Further evidence supporting the heritability of the osmotic hemolysis phenotype is provided by preliminary evidence from the RBC-Omics Genome Wide Association (GWA) study demonstrated that a larger number of genetic loci are associated with this phenotypic variation compared to spontaneous and oxidative hemolysis.32,34 Consequently, recipients of all donations from a donor for whom osmotic hemolysis was measured as part of the screening phase of this study can be imputed to have received a unit with a fixed osmotic hemolysis value, regardless of when it was donated during the 4 years (2013-2016) represented in the linked REDS-III donor-recipient database. Furthermore, since osmotic hemolysis was relatively stable over the course of storage of the recalled donors’ units (estimated to increase by 9.4% from Day 10 to Day 42), an analysis of recipient outcomes relative to this donor variable may not be highly influenced by the duration of LR-RBC storage before transfusion. Taken together, these factors allow for evaluation of a larger number of recipient transfusions from RBC-Omics donors than otherwise possible if the analysis was restricted to the enrollment donation–derived LR-RBC components.
Discovering polymorphisms associated with better storage and higher posttransfusion RBC recovery may lead to the development of new blood donor selection strategies to increase transfusion efficacy and reduce transfusion complications from hemolyzed or damaged RBCs in units subjected to extended storage. We are now completing analysis of metabolomics data from 600 samples from a subset 200 recalled donors described in this report to determine the biochemical correlates of our hemolysis findings and to correlate with genetic data from these donors. These analyses should yield valuable information as to the potential mechanistic basis for donor-specific differences in LR-RBC storage and functional properties.
Supplementary Material
Fig. S1. Distribution of hemolysis at day 42 of storage in the overall screening population, and within the recall population during recall versus screening. Bar graphs represent the frequency of donors exhibiting various levels of (A) spontaneous storage hemolysis, (B) oxidative hemolysis, and (C) osmotic hemolysis. Red bars are for recall donors at recall. Blue bars are for recall donors at screening. Green bars are for the overall screening population.
ACKNOWLEDGMENTS
The authors express their gratitude Dr Simone Glynn of NHLBI for her outstanding support throughout this study, to the RBC-Omics research staff at all participating blood centers and testing laboratories for their exceptional performance and contribution to this project, and to all blood donors who agreed to participate in this study.
The authors acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI Contracts NHLBI HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and -00009I.
ABBREVIATIONS:
- ARC
American Red Cross
- BCP
Blood Centers of the Pacific
- BCW
Blood Center of Wisconsin
- ITxM
Institute for Transfusion Medicine
- LR
leukoreduced
Footnotes
CONFLICT OF INTEREST
AEM receives research grant funding from Novo Nordisk. The remaining authors have disclosed no relevant conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Bordbar A, McCloskey D, Zielinski DC, et al. Personalized whole-cell kinetic models of metabolism for discovery in genomics and pharmacodynamics. Cell Syst 2015;1:283–92. [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. Biomedical excellence for safer transfusion scientific problems in the regulation of red blood cell products. Transfusion 2012;52:1827–35. [DOI] [PubMed] [Google Scholar]
- 3.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion 2012;52:1388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion 2016;56:1274–86. [DOI] [PubMed] [Google Scholar]
- 5.Tzounakas VL, Kriebardis AG, Papassideri IS, et al. Donor-variation effect on red blood cell storage lesion: a close relationship emerges. Proteomics Clin Appl 2016;10:791–804. [DOI] [PubMed] [Google Scholar]
- 6.Vostal JG, Buehler PW, Gelderman MP, et al. Proceedings of the Food and Drug Administration’s public workshop on new red blood cell product regulatory science 2016. Transfusion 2018;58:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion 2014;54:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasse M, McIntyre L, English SW, et al. Effect of blood donor characteristics on transfusion outcomes: a systematic review and meta-analysis. Transfus Med Rev 2016;30:69–80. [DOI] [PubMed] [Google Scholar]
- 9.Chasse M, Tinmouth A, English SW, et al. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med 2016;176:1307–14. [DOI] [PubMed] [Google Scholar]
- 10.Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion 2016;56:1462–8. [DOI] [PubMed] [Google Scholar]
- 11.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol 2009;16:515–23. [DOI] [PubMed] [Google Scholar]
- 12.Rapido F The potential adverse effects of haemolysis. Blood Transfus 2017;15:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest 2017;127:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest 2012;122:1205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez C, Saravia C, Gomez A, et al. Mechanisms of genetically-based resistance to malaria. Gene 2010;467:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osei-Hwedieh DO, Kanias T, Croix CS, et al. Sickle cell trait increases red blood cell storage hemolysis and post-transfusion clearance in mice. EBioMedicine 2016;11:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander PE, Barty R, Fei Y, et al. Transfusion of fresher vs older red blood cells in hospitalized patients: a systematic review and meta-analysis. Blood 2016;127:400–10. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DJ, McQuilten ZK, Nichol A, et al. Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med 2017;377:1858–67. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–8. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Schubert P, Devine DV. Proteomic analysis of red blood cells from donors exhibiting high hemolysis demonstrates a reduction in membrane-associated proteins involved in the oxidative response. Transfusion 2017;57: 2248–56. [DOI] [PubMed] [Google Scholar]
- 22.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017; 1:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016;56:2571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess JR. Scientific problems in the regulation of red blood cell products. Transfusion 2012;52:1827–35. [DOI] [PubMed] [Google Scholar]
- 25.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang 2011;100:418–21. [DOI] [PubMed] [Google Scholar]
- 26.Raslan R, Shah BN, Zhang X, et al. Hemolysis and hemolysis-related complications in females versus males with sickle cell disease. Am J Hematol 2018. doi: 10.1002/ajh.25258. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat and tears: red blood cell-Omics study objectives, design, and recruitment activities. Transfusion 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone M, Keating SM, Kanias T, et al. Implementation of quality assessment and quality control procedures in a large scale study of red blood cell storage hemolysis and function. Transfusion 2019;59:57–66. [DOI] [PubMed] [Google Scholar]
- 29.Kanias T, Wang L, Lippert A, et al. Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis. Transfusion 2013;53:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raval JS, Waters JH, Seltsam A, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang 2010;99:325–31. [DOI] [PubMed] [Google Scholar]
- 31.Kanias T, Stone M, Page G, et al. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion 2019;59:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page GK, Lanteri MC, Guo Y, et al. GWAS of osmotic hemolysis in 12,352 healthy blood donors identifies red cell genetic variants associated with steady state hemolysis in patients with sickle cell disease. Blood 2017;130:1117. [Google Scholar]
- 33.Karafin MS, Bruhn R, Westlake M, et al. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion 2017;57:2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y, Endres-Dighe S, Seielstad M, et al. Development and evaluation of a transfusion medicine genome wide SNP array. Transfusion 2019;59:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution of hemolysis at day 42 of storage in the overall screening population, and within the recall population during recall versus screening. Bar graphs represent the frequency of donors exhibiting various levels of (A) spontaneous storage hemolysis, (B) oxidative hemolysis, and (C) osmotic hemolysis. Red bars are for recall donors at recall. Blue bars are for recall donors at screening. Green bars are for the overall screening population.


