Abstract
Background
Superoxide dismutase (SOD) proteins, as one kind of the antioxidant enzymes, play critical roles in plant response to various environment stresses. Even though its functions in the oxidative stress were very well characterized, the roles of SOD family genes in regulating alkaline stress response are not fully reported.
Methods
We identified the potential family members by using Hidden Markov model and soybean genome database. The neighbor-joining phylogenetic tree and exon-intron structures were generated by using software MEGA 5.0 and GSDS online server, respectively. Furthermore, the conserved motifs were analyzed by MEME online server. The syntenic analysis was conducted using Circos-0.69. Additionally, the expression levels of soybean SOD genes under alkaline stress were identified by qRT-PCR.
Results
In this study, we identified 13 potential SOD genes in soybean genome. Phylogenetic analysis suggested that SOD genes could be classified into three subfamilies, including MnSODs (GmMSD1–2), FeSODs (GmFSD1–5) and Cu/ZnSODs (GmCSD1–6). We further investigated the gene structure, chromosomal locations and gene-duplication, conserved domains and promoter cis-elements of the soybean SOD genes. We also explored the expression profiles of soybean SOD genes in different tissues and alkaline, salt and cold stresses, based on the transcriptome data. In addition, we detected their expression patterns in roots and leaves by qRT-PCR under alkaline stress, and found that different SOD subfamily genes may play different roles in response to alkaline stress. These results also confirmed the hypothesis that the great evolutionary divergence may contribute to the potential functional diversity in soybean SOD genes. Taken together, we established a foundation for further functional characterization of soybean SOD genes in response to alkaline stress in the future.
Keywords: Soybean, Phylogenetic analysis, Evolution, Alkaline, Expression patterns
Introduction
Abiotic stresses, such as salt, cold and drought, are the main causes that affect plant growth and production. These abiotic stresses disrupt the equilibrium of oxidative reaction, increase the toxic reactive oxygen species (ROS) generation and create oxidative stress (Gill & Tuteja, 2010; Shokri-Gharelo & Noparvar, 2018). To adapt to these toxic ROS, plants have developed a series of enzymatic defense systems, such as catalase (CAT) system, glutathione S-transferase system, glutathione reductase system and superoxide dismutase (SOD) system (Finn et al., 2016). The SOD enzymes, as one of the antioxidant enzymes, play significantly roles in plant against oxidative stress (Geng et al., 2018). The toxic superoxide anion can be reduced by SOD dismutation to molecular oxygen and hydrogen peroxide in plant cells under oxidative stress (Quan et al., 2008).
Previous studies revealed that SOD genes were divided into three subfamilies (Kliebenstein, Monde & Last, 1998; Zhou et al., 2017). However, others showed that SOD genes could be classified into four subfamilies: MnSOD, FeSOD, Cu/ZnSOD and NiSOD (Gopavajhula et al., 2013). MnSOD, FeSOD and Cu/ZnSOD occur in almost all plants, whereas Ni-containing SOD was found in Streptomyces (Dupont et al., 2008). In Arabidopsis thaliana, eight SOD genes have been divided into three subfamilies: one MnSOD (MSD1), three FeSODs (FSD1–3) and Cu/ZnSODs (CSD1–3) subfamily, based on their types of prosthetic metals (Kliebenstein, Monde & Last, 1998). Frequently, different SOD subfamily genes are distributed to different cellular compartments (Bueno et al., 1995; Corpas et al., 2006). For example, MnSOD subfamily genes were mainly observed in mitochondria and also localized in different types of peroxisomes. FeSODs were reported to localize in chloroplasts cytoplasm. Cu/ZnSOD mainly localized in chloroplasts as well as in peroxisomes or cytoplasm. Studies have reported that these subfamily genes play essential roles in response to various environmental stresses. In addition, the plants improve stress tolerance mainly by decreasing the oxidative stress and enhancing the antioxidative defense capacity. For example, overexpression of OsCu/ZnSOD gene improved saline-sodic stress resistance in rice by increasing the detoxification capacity of ROS and reducing salt-induced oxidative damage. Ectopic expression of MnSOD gene in tomato confers tolerance to salt and oxidative stress (Guan et al., 2017). Furthermore, The FeSOD gene from Arabidopsis not only increased oxidative stress to transgenic maize, but also played important roles in early chloroplast development (Myouga et al., 2008). However, the previous studies mainly focused on the mechanism of SODs protection in plants against salt, drought and heat stresses, few studies have been reported on the SOD gene family in response to alkaline stress, especially in soybean.
Alkaline stress affects plant cells mainly by HCO3− , CO32− and high pH, which impose more serious damages than other stresses. Alkaline stress (HCO3− and CO32−) can inhibit the absorption of NO3−, Cl− and Fe2+, and breaks the ionic balance. The photosynthetic rate, sugar production, N metabolism and amino acid production were also significantly inhibited under alkaline stress (at high pH) (Hu et al., 2015). Further, alkaline stress has affected 434 million ha of land and limited the crop productivity worldwide (Chen et al., 2018), although single form of soybean SOD gene has been identified under various stresses (Gopavajhula et al., 2013; Wang et al., 2016). It is necessary to explore the roles of soybean SOD family under environmental stresses, especially under alkaline stress.
In the present study, to comprehensively explore the soybean SOD family, we identified 13 SOD genes from Glycine max database by using the HMM profile. We further determined their evolutionary relationship, gene structure, conserved domain, chromosomal locations and promoter cis-elements. Finally, we identified their expression patterns under alkaline, salt and cold stresses. We further suggested that soybean SOD genes possibly participate in responding to alkaline using qRT-PCR analysis.
Materials and Methods
Identification of SOD family genes in soybean genome
To identify all potential family members of SOD in soybean, the known soybean SOD amino acid sequences were used as queries to establish a Hidden Markov model (Gopavajhula et al., 2013), and searched in soybean genome database by using the HMM profile (build 2.3.2) (Finn, Clements & Eddy, 2011). The Pfam and SMART database were used to remove incomplete domains and overlapping genes (Finn et al., 2016). The molecular weight and isoelectric point values of SOD proteins were predicted using online software ExPASy (http://au.expasy.org/tools/pi_tool.html) (Artimo et al., 2012).
Bioinformatics analysis of SOD family genes
The neighbor-joining phylogenetic tree was generated by using software MEGA 5.0 (Kumar et al., 2008). The exon-intron structures were analyzed by the GSDS online server (http://gsds.cbi.pku.edu.cn/) (DuanMu et al., 2015). The conserved motifs were analyzed by MEME online server (http://meme-suite.org/) (Bailey et al., 2009). The multiple sequence alignments were performed using Clustal X program (Larkin et al., 2007). The syntenic analysis was conducted using Circos-0.69 (http://circos.ca/) (Krzywinski et al., 2009). The synonymous (Ks) and nonsynonymous (Ka) substitution rates of syntenic paralogs were calculated using DnaSP software (version 5.10.01) (Krzywinski et al., 2009). The PLANT CARE online software was used to analyses the cis-acting elements of promoters (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al., 2002).
Expression patterns of SOD family genes based on transcriptome sequencing data
To examine the expression profiles of soybean SOD family genes in different tissues, the expression profile data (GSE29163) was obtained from NCBI. The transcriptome data of soybean was downloaded from the NCBI database under cold (GSE117686), salt (GSE57252) and water-deficit (GSE49537) stresses. As there is limited information exist on soybean in response to alkaline stress, we downloaded wild soybean transcriptome data under alkaline stress. The hierarchical clustering trees of SOD family genes were generated using TM4: MeV4.9 software (Saeed et al., 2006).
Plant material, growth condition and alkaline stress treatment
The soybean (DN50) seeds were treated with 75% ethanol for 1 min and washed with sterile water before germination 2 days. Then, seedlings were grown in Hoagland nutrient solutions at 70–80% relative humidity, 22–28 °C room temperature and 8 h dark/16 h light. Twelve days after sowing, soybean seedlings were transferred into Hoagland solutions with 50 mm NaHCO3 for 0, 6 and 12 h (DuanMu et al., 2015). The roots and leaves were harvested as three biological replicates.
Transcript data analysis of SOD family genes under alkaline stress
Total RNA was extracted from soybean using the plant total RNA isolation kit (TIANGEN, Beijing, China), and the cDNAs were synthesized using the TransScript All-in-One First-Strand cDNA synthesis SuperMix for qPCR kit (TransGen Biotech, Beijing, China). qRT-PCR assays were performed using UtraSYBR Mixture (low ROX) and ABI 7500 sequencer. The GmGADPH was used as internal control (Huis, Hawkins & Neutelings, 2010). The SOD genes and GmGADPH primers are listed in supplementary Table S1. The three biological replicates were obtained and expression levels were calculated using 2−ΔΔCt method and Student’s t-test (Livak & Schmittgen, 2001).
Results
Identification and phylogenetic analysis of SOD genes in soybean
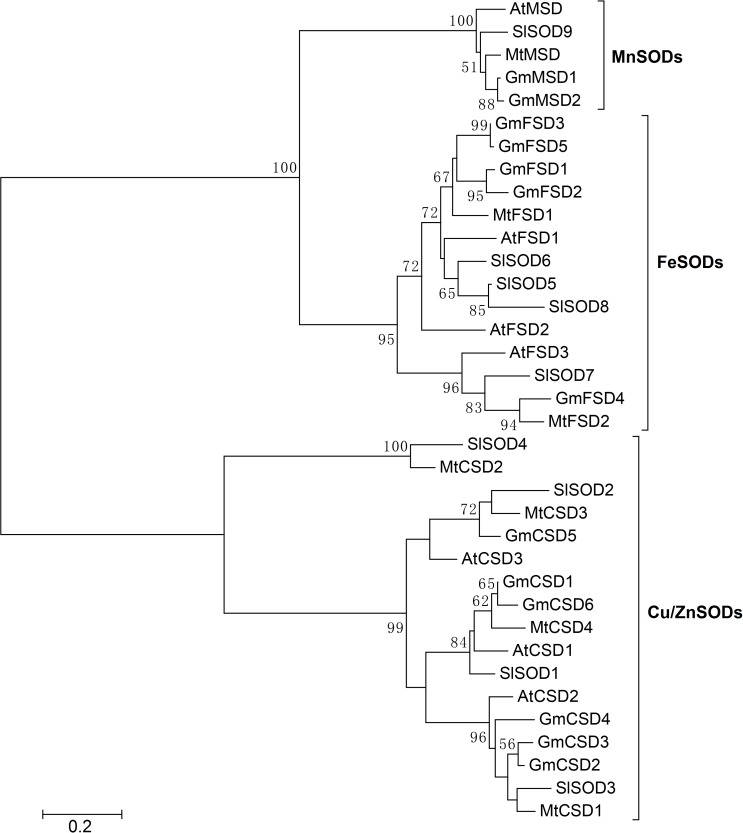
In soybean, ten SOD isoenzymes were identified in different tissues (Wang et al., 2016). For example, one MnSOD isoenzyme was mainly detected in stems and seeds. Four Cu/ZnSOD isoenzymes were detected in roots, leaves, stems and seeds. While, only five FeSOD isoenzymes was mainly detected in leaves. To further explore the SOD genes in G. max, we used SOD amino acid as query sequences to search the G. max database from NCBI. A total of 19 SOD candidate proteins were obtained. All candidate proteins were subjected to Pfam and SMART database to remove incomplete domains and overlapping genes. As a result, we obtained 13 non-redundant SOD genes in soybean. To confirm the classification and evolutionary relationships of soybean SOD family members, the full-length protein sequences of GmSODs were used to construct a neighbor-joining phylogenetic tree with Arabidopsis, Medicago truncatula and Solanum lycopersicum SOD family members. The results revealed that the soybean SOD family members were classified into three subfamilies, including MnSODs (GmMSD1–2), FeSODs (GmFSD1–5) and Cu/ZnSODs (GmCSD1–6), which is consistent with previous studies (Fig. 1). In addition, compared with previous study (Wang et al., 2016), we identified three more SOD genes in soybean.
Figure 1. Phylogenetic analysis of SOD genes in soybean, Arabidopsis, Medicago truncatula and Solanum lycopersicum.
The phylogenetic tree was constructed by the neighbor-joining method using MEGA 5.0. The bootstrap values were 1,000 replications for major branches. SOD family genes have been divided into three subfamilies.
As shown in Table 1, these GmSOD family members were chosen for further protein information analyses, including the exon numbers, length of CDS, protein sequence lengths, molecular weights (MW), and theoretical isoelectric points (pI) values. The protein sequence length ranged from 152 (GmCSD1 and GmCSD6) to 314 (GmFSD1) amino acids (aa). The MW varied from 15.19 (GmCSD6) to 35.86 (GmFSD1) kDa and the pI values ranged from 5.22 (GmMSD1) to 8.57 (GmFSD2). The Cu/ZnSODs subfamily members had a lower protein sequence length and MW than others. The MnSODs subfamily members appeared with higher pI values. This finding indicated that three subfamilies may displayed a great diversity in soybean.
Table 1. Protein information of SOD genes in soybean.
| Gene ID | Gene name | Exon number | Length of CDS (bp) |
Amino acid residues |
MW (kDa) | pI | Chromosome | Domain |
|---|---|---|---|---|---|---|---|---|
| Glyma.04G221300 | GmMSD1 | 6 | 723 | 240 | 26.54 | 8.57 | 4 | alpha-hairpin domain, C-terminal domain |
| Glyma.06G144500 | GmMSD2 | 6 | 726 | 241 | 26.70 | 8.56 | 6 | alpha-hairpin domain, C-terminal domain |
| Glyma.02G087700 | GmFSD1 | 9 | 945 | 314 | 35.86 | 5.59 | 2 | alpha-hairpin domain, C-terminal domain |
| Glyma.10G117100 | GmFSD2 | 9 | 933 | 310 | 35.26 | 5.22 | 10 | alpha-hairpin domain, C-terminal domain |
| Glyma.10G193500 | GmFSD3 | 9 | 735 | 244 | 27.51 | 5.45 | 10 | alpha-hairpin domain, C-terminal domain |
| Glyma.20G050800 | GmFSD4 | 9 | 918 | 305 | 35.10 | 6.40 | 20 | alpha-hairpin domain, C-terminal domain |
| Glyma.20G196900 | GmFSD5 | 9 | 747 | 248 | 27.84 | 5.60 | 20 | alpha-hairpin domain, C-terminal domain |
| Glyma.03G242900 | GmCSD1 | 7 | 459 | 152 | 15.23 | 5.59 | 3 | Copper/zinc superoxide dismutase (SODC) |
| Glyma.11G192700 | GmCSD2 | 8 | 630 | 209 | 21.64 | 5.87 | 11 | Copper/zinc superoxide dismutase (SODC) |
| Glyma.12G081300 | GmCSD3 | 8 | 615 | 204 | 20.80 | 5.79 | 12 | Copper/zinc superoxide dismutase (SODC) |
| Glyma.12G178800 | GmCSD4 | 8 | 552 | 183 | 18.62 | 6.28 | 12 | Copper/zinc superoxide dismutase (SODC) |
| Glyma.16G153900 | GmCSD5 | 7 | 504 | 167 | 17.17 | 7.19 | 16 | Copper/zinc superoxide dismutase (SODC) |
| Glyma.19G240400 | GmCSD6 | 7 | 459 | 152 | 15.19 | 5.27 | 19 | Copper/zinc superoxide dismutase (SODC) |
Phylogenetic and gene structure analysis of SOD genes
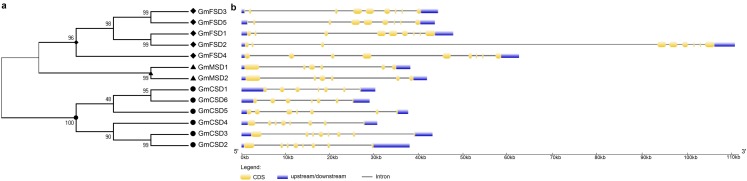
To further investigate the evolutionary relationship of SOD genes in soybean, we constructed a neighbor-joining phylogenetic rootless tree with the full-length protein sequences of GmSODs. The results suggested that three SOD subfamilies have a distant evolutionary relationship (Fig. 2A). However, we found that each SOD subfamilies have high bootstrap support pairs, such as GmFSD3 and GmFSD5, GmMSD1 and GmMSD2, GmCSD2 and GmCSD3. This indicated that each soybean SOD subfamilies was evolutionarily conserved, even though three subfamilies displayed a distant evolutionary relationship.
Figure 2. Phylogenetic and exon-intron structure analyses of SOD genes.
(A) The phylogenetic tree was produced by the neighbor-joining method using MEGA 5.0. The bootstrap values were 1,000 replications for major branches. (B) Exon-intron structure analysis of soybean SOD genes by using GSDS online tools. The CDSs, untranslated regions and introns are described by yellow boxes, light blue boxes and black lines, respectively.
The diversity of gene structure is mainly influenced by the evolution of multigene families (Mercereau-Puijalon, Barale & Bischoff, 2002; Pellicer et al., 2018). To further explore the structural diversity of SOD genes, the characteristics of exon-intron structures were analyzed by the GSDS online server. As shown in Fig. 2B, different subfamilies displayed variation in exon-intron structures. For example, the MnSODs subfamily comprised of six exons as well as exhibited a similarity in genomic structure. The FeSODs subfamily members consisted of nine exons. We also noticed that GmFSD2 exhibited a longer genomic structure than 10 KB. However, three Cu/ZnSODs subfamily members (GmCSD1, GmCSD5 and GmCSD6) possessed seven exons, while others appeared with eight exons. These results further confirmed that the different SOD subfamilies diverged greatly in soybean.
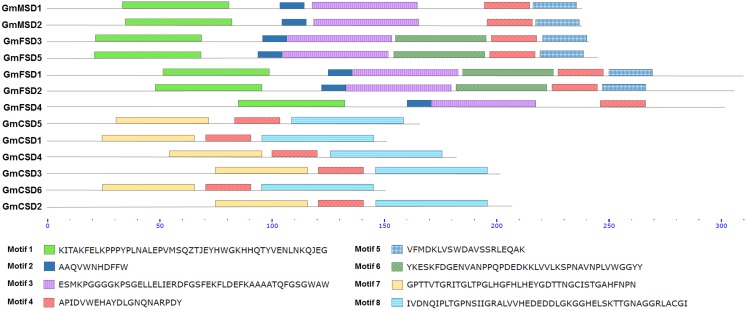
Conserved domain analysis of SOD proteins
For identification of the conserved domains of SOD proteins in soybean, the conserved motifs were analyzed by the MEME online server. Our results showed that eight predicted conserved motifs were identified (Fig. 3). Among them, all proteins in Cu/ZnSODs subfamilies contained motifs 4, 7 and 8. Motifs 1, 2, 3, 4 and 5 were detected in MnSODs subfamily members. Motifs 1, 2, 3, 4, 5 and 6 were discovered in all FeSODs subfamily members, except GmFSD2. It is noteworthy that motif 1 was detected in the N-terminal domain (alpha-hairpin domain) of MnSODs and FeSODs subfamily members. Besides, motifs 2, 3, 4 and 5 were also detected in the C-terminal domain. The motifs 4, 7 and 8 were also detected in the copper/zinc SOD domain of Cu/ZnSODs subfamily members (Figs. S1 and S2). These results indicated that the MnSODs and FeSODs subfamily members showed high similarities in conserved sequences. However, the Cu/ZnSODs subfamilies displayed wide divergence with other two subfamilies, which further verify the potential diversity of functional divergence in soybean SOD genes from different subfamilies.
Figure 3. Conserved domain analysis of SOD family proteins.
The conserved motifs were predicted using the MEME online server. The different conserved motifs were marked by different colors. The protein sequences of eight different motifs commonly observed by MEME online server.
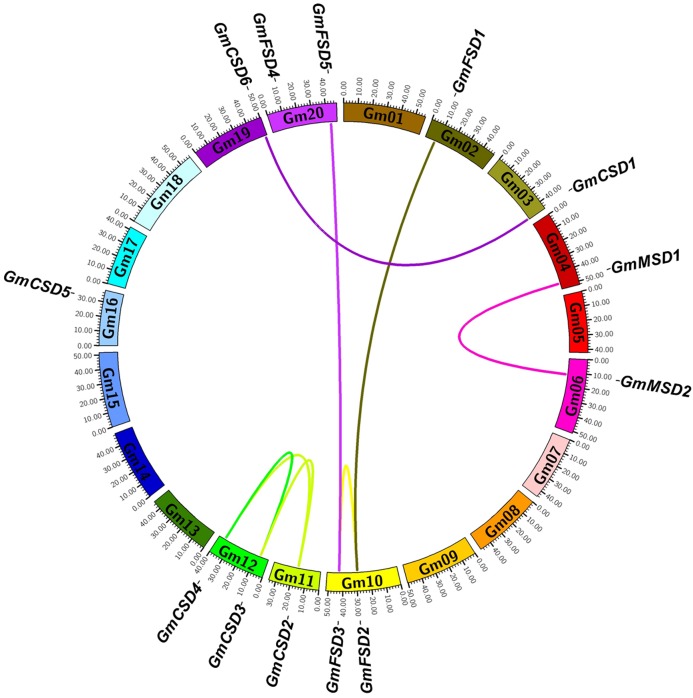
Chromosomal locations and syntenic analysis
To determine the genomic distribution of SOD genes, we identified their gene locations on the chromosomes and potential genome duplication events using the syntenic analysis. The thirteen SOD genes were randomly distributed among ten chromosomes, and each chromosome had one or two genes (Fig. 4). Gene duplication plays important role in plant functional diversity and evolutionary mechanism (Bowers et al., 2003). To detect the potential genome duplication events, a total of eight pairs of SOD syntenic paralogs were found in soybean genome, except GmCSD5. The results indicated that the soybean SOD family existed a high gene family expansion. Meanwhile, we found that the duplication pairs also exhibited a high evolutionary relationship in phylogenetic analysis (Fig. 2A).
Figure 4. Syntenic analysis of SOD family genes in soybean.
The chromosomes are indicated as a circle. The duplication pairs are connected by lines.
The non-synonymous/synonymous substitution (Ka/Ks) rates were used to detect the positive or negative selection of gene family expansion (Kaehler, Yap & Huttley, 2017) . To further investigate whether selective pressure was associated with the SOD family genes, the Ka/Ks rates were calculated for the full-length CDS sequences of eight duplication pairs (Table S2). Previous studies reported that Ka/Ks < 1 indicates a negative selection and Ka/Ks > 1 indicates a positive selection (Wang et al., 2005). The results showed that the Ka/Ks rates for six SOD duplication pairs were more than 1. However, two SOD duplication pairs were less than 1. This finding indicated that the soybean SOD genes had experienced positive and negative selection pressure after the duplication events.
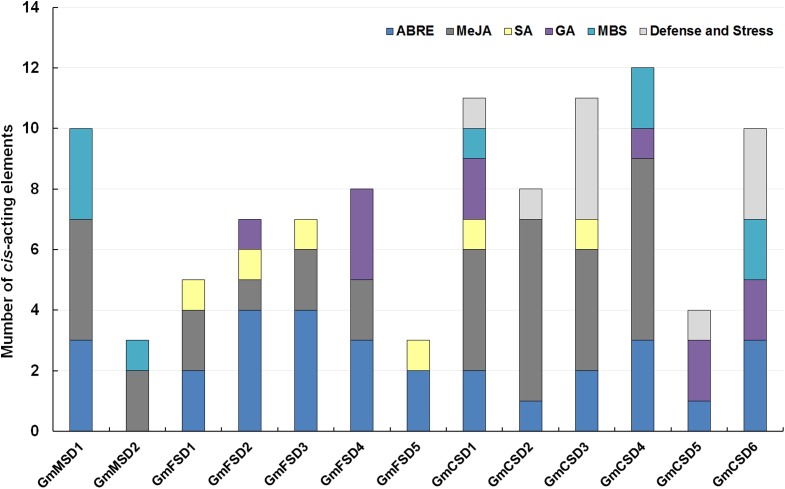
Analysis of cis-acting elements of SOD gene promoters
The cis-acting elements play significant roles in determine the regulatory roles under various stresses (Kimotho, Baillo & Zhang, 2019). In this study, to explore the potential roles of the soybean SOD genes under environmental stresses, we analyzed the SOD gene promoters coupled with 3 KB genomic sequence upstream regions of the ATG using PlantCARE online tool (Sun et al., 2014). The SOD family genes possessed a variety of putative abiotic stress and hormone-related responsive elements, including ABA (ABRE), Methyl jasmonate (MeJA), Salicylic acid, Gibberellin, MBS (Drought) and Defense and Stress responsive elements (Fig. 5; Table S3). Among them, almost all SOD family genes contained ABA-responsive elements, except GmMSD2. Ten of SOD family genes had MeJA-responsive elements. However, the GmMSD2 and GmFSD5 only contained two responsive elements, respectively. We also found that only Cu/ZnSODs subfamily genes contained Defense and Stress responsive elements. Collectively, the results suggested that SOD family genes might be related to the responses of abiotic stresses and hormone stimuli. Also, these genes might have potential functional diversity because they contained different responsive elements.
Figure 5. Analysis of cis-acting elements of putative SOD promoters related to stress responses.
The different cis-elements are present with different colors.
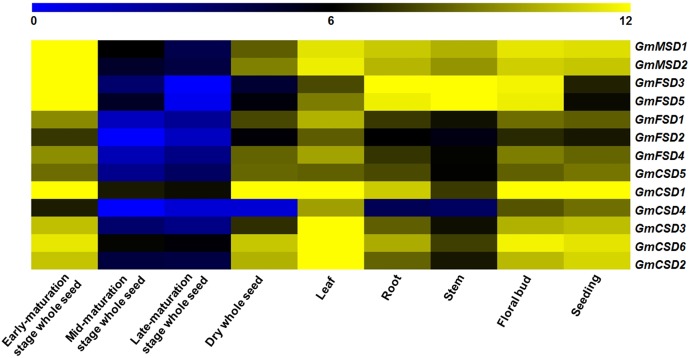
Expression profiles of SOD genes in soybean tissues
To investigate the potential function of SOD genes in soybean growth and development, we analyzed their expression in nine tissues using RNA-seq data (Chen et al., 2012). The results showed that the numbers of expressed soybean SOD genes in different tissues exhibited higher variations (Fig. 6). For example, GmMSD1, GmMSD2, GmFSD3, GmFSD5 and GmCSD1 showed higher expressions in early-maturation stage whole seed. GmCSD1, GmCSD2, GmCSD3 and GmCSD6 had higher expression levels in leaf. In addition, the GmCSD1 displayed the higher expression level in five tissues, which is consistent with the cucumber CSD1 gene studies (Zhou et al., 2017). The GmFSD2 showed an extremely lower expression levels than others in nine tissues. However, almost all the soybean SOD genes had the lowest expression level in mid- and late-maturation stage whole seed. In conclusion, these results indicated that soybean SOD genes showed special tissue expression profiles, indicating their potential divergent functions in soybean growth and development.
Figure 6. Expression patterns of SOD family genes in different soybean tissues.
The RNA-seq data (GSE29163) were downloaded from the NCBI. All expression data were analyzed by Log2 scaled. The heat map was generated using TM4: MeV4.9 software. The color scale represents the expression values: blue indicates low levels and yellow represents high levels.
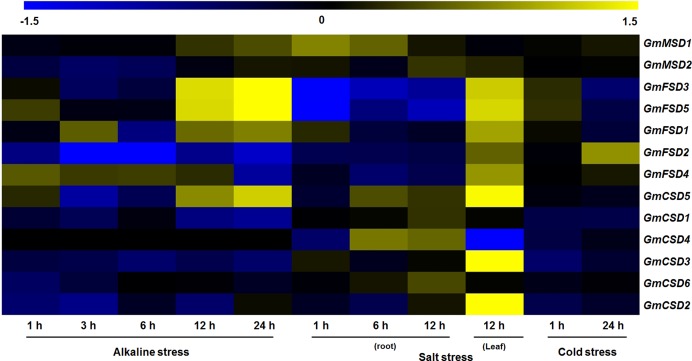
Expression analysis of soybean SOD genes in response to abiotic stresses
Previous studies have showed that the SOD genes play significant roles in plant responses to various stresses (Jiang et al., 2019). To investigate the potential roles of soybean SOD genes in various abiotic stress responses, we identified their expression patterns under alkaline, salt and cold stresses using transcriptome sequencing data. However, there is limited information exist on soybean in response to alkaline stress, we downloaded wild soybean transcriptome data under alkaline stress. As shown in Fig. 7, the heat map revealed that four and six of soybean SOD genes were differentially expressed (|Log2 fold change| > 1, P < 0.05) under alkaline and salt stresses, respectively. However, only GmFSD2 displayed slightly up-regulation under cold stress. Among them, we found that GmFSD3, GmFSD5 and GmCSD5 were all up-regulated under alkaline and salt stresses, indicating that they might act as positive regulators. It is worth noting that GmFSD3 and GmFSD5 showed differential expression in soybean leaves and roots under salt stress. This finding implied that these two genes may be involved in different signaling pathways in leaves and roots under salt stress.
Figure 7. Expression analysis of soybean SOD genes in response to various abiotic stresses.
The wild soybean transcriptome sequencing data were used to investigate the expression pattern of soybean SOD genes under alkaline stress. Expression of soybean SOD genes were downloaded from the soybean transcriptome sequencing data under salt (GSE57252) and cold (GSE57252) stresses. The heat map was generated using TM4: MeV4.9 software. The color scale represents the expression values: blue indicates low levels and yellow represents high levels (|Log2 fold change| > 1, P < 0.05).
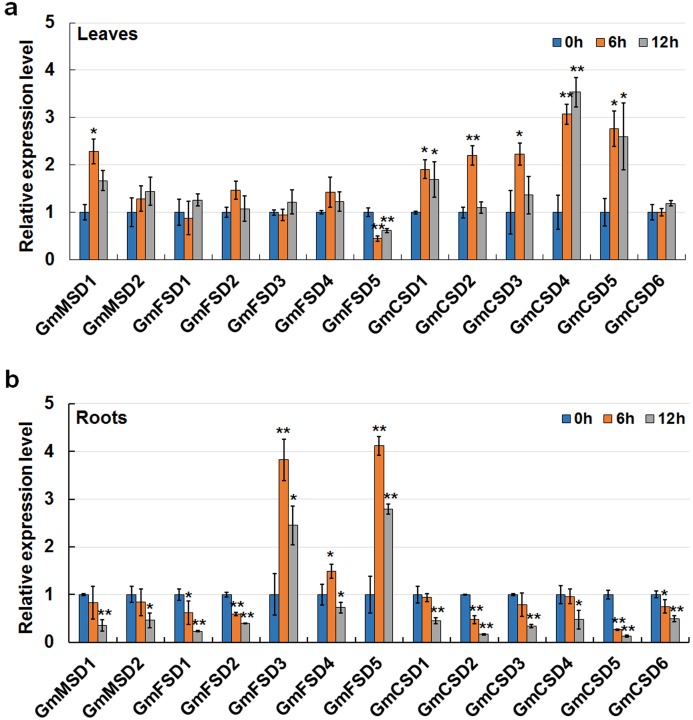
Expression analysis of SOD genes in response to alkaline treatment
Saline-alkali soil is considered as a major threat to crop growth and yields. However, only a few studies have focused on the mechanisms of plants respond to alkaline stress. In this study, to further confirm the soybean SOD genes in response to alkaline treatment, we detected their expression patterns in roots and leaves under 50 mm NaHCO3 treatment by using qRT-PCR. As shown in Fig. 8A total of six genes were up-regulated expressed and only GmFSD5 gene was down-regulated in soybean leaves under alkaline treatment. Three genes (GmFSD3, GmFSD4 and GmFSD5) were significantly induced in soybean roots under alkaline treatment, whereas others showed down-regulated expression, especially at 12 h. Among them, GmFSD5 had contrary expression pattern in leaves and roots, indicating this gene may be involved in different pathways.
Figure 8. Expression analysis of soybean SOD genes in response to alkaline stress in leaves (A) and roots (B) using qRT-PCR assays.
Twelve days soybean seedlings were treated with 50 mm NaHCO3 for 0, 6 and 12 h. The mean values were from three independent biological replicates. Statistical analyses were performed by Student’s t-test (*P < 0.05 and **P < 0.01).
In addition, we found that FeSODs and Cu/ZnSODs subfamily genes displayed different roles in roots and leaves after alkaline treatment. For example, only three FeSODs subfamily genes (GmFSD3, GmFSD4 and GmFSD5) were significantly induced (P < 0.05 or P < 0.01) in roots. Five Cu/ZnSODs subfamily genes (GmCSD1/2/3/4/5) were up-regulated in leaves. However, almost all FeSODs subfamily genes didn’t express significantly in leaves, except GmFSD5 which was down-regulated expressed. To conclude, qRT-PCR confirmed the results that soybean SOD genes possibly participate in responses to alkaline.
Discussion
Antioxidant enzymes SOD play significantly roles in plant response to abiotic stresses by reducing the molecular oxygen and hydrogen peroxide in plant cells. The plant SOD family genes have been identified and characterized in some species at the genome-wide level (Feng et al., 2016; Verma, Lakhanpal & Singh, 2019). However, the roles of soybean SOD family genes in regulating various abiotic stresses, especially alkaline stress, are rarely reported. Hence, our research conducted a comprehensive exploration of soybean SOD family, and mainly identified potential roles of soybean SOD genes in response to alkaline stress.
The SOD family genes were reported to be divided into three subfamilies, and each subfamily genes frequently located in different cellular compartments (Feng et al., 2016). This indicated that SOD family genes may display evolutionarily divergence. Here, we found several evidences to confirm the divergent evolution in soybean SOD family. Firstly, according to the results of the phylogenetic tree, the soybean SOD family genes were divided into three subfamilies (Fig. 1). In addition, each subfamily varied markedly in protein sequence length and theoretical pI values (Table 1). Secondly, the different subfamilies displayed different exon-intron structures and exon numbers (Fig. 2B). For example, MnSODs and FeSODs subfamily genes contained six and nine exons, respectively. However, Cu/ZnSODs subfamily genes possessed seven to eight exons. These results are in line with previous reports (Song et al., 2018), and further confirmed that three subfamilies may displayed a great diversity in soybean. Thirdly, the conserved motifs further revealed the varied widely from different subfamilies, especially MnSODs and Cu/ZnSODs subfamily (Fig. 3), which consistent with previous study (Wang et al., 2017). In conclusion, the above results confirmed SOD subfamilies displayed evolutionary divergence in soybean. Consistently, previous study showed that Cu/Zn-SODs evolved independently in Gossypium hirsutum (Wang et al., 2017). And MnSODs and FeSODs have evolved with common ancestral enzymes. This evidence could explain the similar motif patterns were found in MnSODs and FeSODs subfamilies (Fig. 3).
On the other hand, the great evolutionary divergence may contribute to the potential functional diversity in soybean SOD family. Among them, only Cu/ZnSODs subfamily genes contained Defense and Stress responsive elements. The GmMSD2 and GmFSD5 exhibited completely different abiotic stresses and hormone-related responsive elements (Fig. 5; Table S3). Moreover, the numbers of expressed soybean SOD genes in different tissues showed a lot of variations (Fig. 6). For example, four Cu/ZnSOD genes (GmCSD1, GmCSD2, GmCSD3 and GmCSD6) had higher expression levels in leaf. GmCSD1 displayed the higher expression levels in five tissues, while GmFSD2 showed opposite expression levels. In line with this, VvCSD2, VvCSD4 and VvCSD5 exhibited different expression patterns as compared with VvFSD1 and VvFSD2 in all the tested tissues in Grapevine (Hu et al., 2019). Also, they displayed different tissue expression profiles and cis-acting elements, indicating their potential divergent functions in soybean growth and responses to environmental stresses.
Even though the soybean SOD family displayed a great evolutionary divergence, however the duplication events also were detected in each SOD subfamilies. A total of eight pair of duplication genes were found in soybean genome by using syntenic analysis (Fig. 4). The duplication pairs also existed a high evolutionary relationship in phylogenetic analysis (Fig. 2A). In plants, the duplication events help plants to adapt to diverse environments via gene generation, loss and rearrangement (Faillace et al., 2019; Shang et al., 2013). Thus, our results indicated that soybean SOD family genes possess gene duplication to adapt to environmental conditions. And SOD genes have experienced positive and negative selection pressure after the duplication events according to the Ka/Ks rates (Table S2).
Three subfamilies of SODs have been reported to eliminate toxic ROS caused by abiotic stresses (Wu et al., 1999). To further explore the roles of SOD genes in soybean in relation with environment stress responses, we examined the expression patterns using transcriptome sequencing data, including salt, cold and alkaline stresses (Fig. 7). The results revealed that four and six of soybean SOD genes were significantly induced under alkaline and salt stresses, respectively. Similarly, studies have reported that SOD genes played positive roles in other plants (Feng et al., 2016). Whereas, almost all the expression levels of soybean SOD genes displayed no significant change under cold stress, except for a slightly up-regulation (|Log2 fold change| > 1) of GmFSD2. This indicated that the soybean SOD genes may play different roles under cold stress, which also consistent with previous research (Song et al., 2018). In addition, we also examined the expression patterns of soybean SOD genes under water-deficit stress (Fig. S3). The results showed that few soybean SOD genes showed significant up-regulated expressions, while five genes were down-regulated. In conclusion, these results suggested that soybean SOD genes may play different roles under various environment stresses.
Alkaline stress can impact crop growth and yields via disturbance in ionic balance and inhibition in organic synthesis. In this study, we focus on the potential roles of soybean SOD genes in response to alkaline treatment in roots and leaves by using qRT-PCR (Fig. 8). Firstly, qRT-PCR confirmed the results that soybean SOD genes may play positive roles in response to alkaline stress. For example, six and three soybean SOD genes were significantly induced (P < 0.05 or P < 0.01) in leaves and roots under alkaline treatment, respectively. Secondly, we found that different subfamilies might display different roles in leaves and roots under alkaline stress. For example, FeSODs and Cu/ZnSODs subfamily genes showed opposite expression patterns. Three FeSOD genes (GmFSD3, GmFSD4 and GmFSD5) were significantly induced in roots but not in leaves. In line with this, five Cu/ZnSODs subfamily genes (GmCSD1/2/3/4/5) were only up-regulated in leaves. These results also consistent with the expression patterns in leaves and roots under salt stress (Fig. 7). In addition, these results also confirmed the hypothesis that the great evolutionary divergence may contribute to the potential functional diversity in soybean SOD genes.
Conclusions
In conclusion, we identified 13 potential soybean SOD genes, which could be classified into three subfamilies: MnSODs (GmMSD1–2), FeSODs (GmFSD1–5) and Cu/ZnSODs (GmCSD1–6). We confirmed that SOD subfamilies displayed a great evolutionary divergence in soybean by gene structure, conserved domains and phylogenetic analysis. Furthermore, we identified that SOD genes showed special tissue expression profiles, indicating their potential divergent functions in soybean growth and development. Cis-acting elements analysis and expression profiles under environmental stresses suggested that SOD family genes might be related to the responses of abiotic stresses and hormone stimuli. Moreover, we focused on the SOD genes in response to alkaline stress by qRT-PCR, and found that different SOD subfamily genes could be involved in different roles in response to alkaline stress. Taken together, we established a foundation for further functional characterization of soybean SOD genes in response to alkaline stress in the future.
Supplemental Information
The copper/zinc SOD domain of Cu/ZnSODs subfamily members are marked with blue box. The alpha-hairpin domain and C-terminal domain of MnSODs and FeSODs subfamily members are marked with red and black boxes, respectively.
The motifs of SOD families in soybean are marked with black box.
The color scale represents the expression values: blue indicates low levels and yellow represents high levels.
Funding Statement
This work was supported by the Heilongjiang Natural Science Foundation (C2017039), the Project of Heilongjiang Education Department (12531178), Fundamental Research Funds for the Heilongjiang Provincial Universities (KJCXZD201717) and the PhD Research Fund (XKB201914). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Lijie Yu, Email: yulijie1961@126.com.
Chao Chen, Email: chchao@hrbnu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Wenxiu Lu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Huizi Duanmu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Yanhua Qiao performed the experiments, prepared figures and/or tables, and approved the final draft.
Xiaoxia Jin performed the experiments, prepared figures and/or tables, and approved the final draft.
Yang Yu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Lijie Yu performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Chao Chen conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Artimo et al. (2012).Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, De Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research. 2012;40(W1):W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey et al. (2009).Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37(Suppl. 2):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers et al. (2003).Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422(6930):433–438. doi: 10.1038/nature01521. [DOI] [PubMed] [Google Scholar]
- Bueno et al. (1995).Bueno P, Varela J, Gimeenez-Gallego G, Del Rio LA. Peroxisomal copper, zinc superoxide dismutase (characterization of the isoenzyme from watermelon cotyledons) Plant Physiology. 1995;108(3):1151–1160. doi: 10.1104/pp.108.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen C, Chen R, Wu S, Zhu D, Sun X, Liu B, Li Q, Zhu Y. Genome-wide analysis of Glycine soja ubiquitin (UBQ) genes and functional analysis of GsUBQ10 in response to alkaline stress. Physiol Plant. 2018;164(3):268–278. doi: 10.1111/ppl.12719. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2012).Chen H, Wang F-W, Dong Y-Y, Wang N, Sun Y-P, Li X-Y, Liu L, Fan X-D, Yin H-L, Jing Y-Y, Zhang X-Y, Li Y-L, Chen G, Li H-Y. Sequence mining and transcript profiling to explore differentially expressed genes associated with lipid biosynthesis during soybean seed development. BMC Plant Biology. 2012;12(1):122. doi: 10.1186/1471-2229-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas et al. (2006).Corpas FJ, Fernandez-Ocana A, Carreras A, Valderrama R, Luque F, Esteban FJ, Rodriguez-Serrano M, Chaki M, Pedrajas JR, Sandalio LM, Del Rio LA, Barroso JB. The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant and Cell Physiology. 2006;47(7):984–994. doi: 10.1093/pcp/pcj071. [DOI] [PubMed] [Google Scholar]
- DuanMu et al. (2015).DuanMu H, Wang Y, Bai X, Cheng S, Deyholos MK, Wong GK-S, Li D, Zhu D, Li R, Yu Y, Cao L, Chen C, Zhu Y. Wild soybean roots depend on specific transcription factors and oxidation reduction related genesin response to alkaline stress. Functional & Integrative Genomics. 2015;15(6):651–660. doi: 10.1007/s10142-015-0439-y. [DOI] [PubMed] [Google Scholar]
- Dupont et al. (2008).Dupont CL, Neupane K, Shearer J, Palenik B. Diversity, function and evolution of genes coding for putative Ni-containing superoxide dismutases. Environmental Microbiology. 2008;10(7):1831–1843. doi: 10.1111/j.1462-2920.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- Faillace et al. (2019).Faillace GR, Turchetto-Zolet AC, Guzman FL, De Oliveira-Busatto LA, Bodanese-Zanettini MH. Genome-wide analysis and evolution of plant thaumatin-like proteins: a focus on the origin and diversification of osmotins. Molecular Genetics and Genomics. 2019;294(5):1137–1157. doi: 10.1007/s00438-019-01554-y. [DOI] [PubMed] [Google Scholar]
- Feng et al. (2016).Feng K, Yu J, Cheng Y, Ruan M, Wang R, Ye Q, Zhou G, Li Z, Yao Z, Yang Y, Zheng Q, Wan H. The SOD gene family in tomato: identification, phylogenetic relationships, and expression patterns. Frontiers in Plant Science. 2016;7(131):1279. doi: 10.3389/fpls.2016.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, Clements & Eddy (2011).Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research. 2011;39(Suppl. 2):W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn et al. (2016).Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research. 2016;44(D1):D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng et al. (2018).Geng A, Wang X, Wu L, Wang F, Wu Z, Yang H, Chen Y, Wen D, Liu X. Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicology and Environmental Safety. 2018;158:266–273. doi: 10.1016/j.ecoenv.2018.03.050. [DOI] [PubMed] [Google Scholar]
- Gill & Tuteja (2010).Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gopavajhula et al. (2013).Gopavajhula VR, Chaitanya KV, Ali Khan PA, Shaik JP, Reddy PN, Alanazi M. Modeling and analysis of soybean (Glycine max. L.) Cu/Zn, Mn and Fe superoxide dismutases. Genetics and Molecular Biology. 2013;36(2):225–236. doi: 10.1590/S1415-47572013005000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan et al. (2017).Guan Q, Liao X, He M, Li X, Wang Z, Ma H, Yu S, Liu S. Tolerance analysis of chloroplast OsCu/Zn-SOD overexpressing rice under NaCl and NaHCO3 stress. PLOS ONE. 2017;12(10):e0186052. doi: 10.1371/journal.pone.0186052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2019).Hu X, Hao C, Cheng ZM, Zhong Y. Genome-wide identification, characterization, and expression analysis of the grapevine superoxide dismutase (SOD) family. International Journal of Genomics. 2019;2019:7350414. doi: 10.1155/2019/7350414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2015).Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huis, Hawkins & Neutelings (2010).Huis R, Hawkins S, Neutelings G. Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.) BMC Plant Biology. 2010;10(1):71. doi: 10.1186/1471-2229-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2019).Jiang W, Yang L, He Y, Zhang H, Li W, Chen H, Ma D, Yin J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum) PeerJ. 2019;7(2):e8062. doi: 10.7717/peerj.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler, Yap & Huttley (2017).Kaehler BD, Yap VB, Huttley GA. Standard codon substitution models overestimate purifying selection for nonstationary data. Genome Biology and Evolution. 2017;9:134–149. doi: 10.1093/gbe/evw308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimotho, Baillo & Zhang (2019).Kimotho RN, Baillo EH, Zhang Z. Transcription factors involved in abiotic stress responses in Maize (Zea mays L.) and their roles in enhanced productivity in the post genomics era. PeerJ. 2019;7(1):e7211. doi: 10.7717/peerj.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, Monde & Last (1998).Kliebenstein DJ, Monde RA, Last RL. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiology. 1998;118(2):637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski et al. (2009).Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Research. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2008).Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics. 2008;9(4):299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin et al. (2007).Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lescot et al. (2002).Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mercereau-Puijalon, Barale & Bischoff (2002).Mercereau-Puijalon O, Barale JC, Bischoff E. Three multigene families in Plasmodium parasites: facts and questions. International Journal for Parasitology. 2002;32(11):1323–1344. doi: 10.1016/S0020-7519(02)00111-X. [DOI] [PubMed] [Google Scholar]
- Myouga et al. (2008).Myouga F, Hosoda C, Umezawa T, Iizumi H, Kuromori T, Motohashi R, Shono Y, Nagata N, Ikeuchi M, Shinozaki K. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell. 2008;20(11):3148–3162. doi: 10.1105/tpc.108.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer et al. (2018).Pellicer J, Hidalgo O, Dodsworth S, Leitch IJ. Genome size diversity and its impact on the evolution of land plants. Genes. 2018;9(2):88. doi: 10.3390/genes9020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan et al. (2008).Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. Journal of Integrative Plant Biology. 2008;50(1):2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Saeed et al. (2006).Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods in Enzymology. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- Shang et al. (2013).Shang H, Li W, Zou C, Yuan Y. Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr.: chromosomal location, structure, phylogeny, and expression patterns. Journal of Integrative Plant Biology. 2013;55(7):663–676. doi: 10.1111/jipb.12085. [DOI] [PubMed] [Google Scholar]
- Shokri-Gharelo & Noparvar (2018).Shokri-Gharelo R, Noparvar PM. Molecular response of canola to salt stress: insights on tolerance mechanisms. PeerJ. 2018;6(4):e4822. doi: 10.7717/peerj.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2018).Song J, Zeng L, Chen R, Wang Y, Zhou Y. In silico identification and expression analysis of superoxide dismutase (SOD) gene family in Medicago truncatula. 3 Biotech. 2018;8(8):348. doi: 10.1007/s13205-018-1373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2014).Sun X, Luo X, Sun M, Chen C, Ding X, Wang X, Yang S, Yu Q, Jia B, Ji W, Cai H, Zhu Y. A glycine soja 14-3-3 protein GsGF14o participates in stomatal and root hair development and drought tolerance in Arabidopsis thaliana. Plant and Cell Physiology. 2014;55(1):99–118. doi: 10.1093/pcp/pct161. [DOI] [PubMed] [Google Scholar]
- Verma, Lakhanpal & Singh (2019).Verma D, Lakhanpal N, Singh K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genomics. 2019;20(1):227. doi: 10.1186/s12864-019-5593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang W, Zhang X, Deng F, Yuan R, Shen F. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genomics. 2017;18(1):376. doi: 10.1186/s12864-017-3768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang X, Zhang H, Gao Y, Zhang W. Characterization of Cu/Zn-SOD enzyme activities and gene expression in soybean under low nitrogen stress. Journal of the Science of Food and Agriculture. 2016;96(8):2692–2697. doi: 10.1002/jsfa.7387. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2005).Wang W, Zheng H, Yang S, Yu H, Li J, Jiang H, Su J, Yang L, Zhang J, McDermott J, Samudrala R, Wang J, Yang H, Yu J, Kristiansen K, Wong GK, Wang J. Origin and evolution of new exons in rodents. Genome Research. 2005;15(9):1258–1264. doi: 10.1101/gr.3929705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (1999).Wu G, Wilen RW, Robertson AJ, Gusta LV. Isolation, chromosomal localization, and differential expression of mitochondrial manganese superoxide dismutase and chloroplastic copper/zinc superoxide dismutase genes in wheat. Plant Physiology. 1999;120(2):513–520. doi: 10.1104/pp.120.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2017).Zhou Y, Hu L, Wu H, Jiang L, Liu S. Genome-wide identification and transcriptional expression analysis of cucumber superoxide dismutase (SOD) family in response to various abiotic stresses. International Journal of Genomics. 2017;2017:7243973. doi: 10.1155/2017/7243973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The copper/zinc SOD domain of Cu/ZnSODs subfamily members are marked with blue box. The alpha-hairpin domain and C-terminal domain of MnSODs and FeSODs subfamily members are marked with red and black boxes, respectively.
The motifs of SOD families in soybean are marked with black box.
The color scale represents the expression values: blue indicates low levels and yellow represents high levels.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.