Abstract
INTRODUCTION:
To determine if sleep-disordered breathing (SDB), daytime sleepiness, insomnia and sleep duration predict seven-year neurocognitive (NC) decline in U.S. Hispanic/Latinos (N=5,247).
METHODS:
The exposures were baseline SDB, daytime sleepiness, insomnia and sleep duration. The outcomes were change in episodic learning and memory (B-SEVLT-sum and SEVLT-Recall), language (WF; word fluency), processing speed (DSS; Digit Symbol Substitution), and a cognitive impairment screener (SIS; Six-item Screener).
RESULTS:
Mean age was 63±8 years, 55% females with 7.0% Central American, 24.5% Cuban, 9.3% Dominican, 35.9% Mexican, 14.4% Puerto Rican, and 5.1% South American background. Long sleep (>9 hours), but not short sleep (<6 hours), was associated with decline (Standard Deviation units) in episodic learning and memory (βSEVLT-Sum=−0.22 (se=0.06); p<0.001; βSEVLT-Recall=−0.13 (se=0.06); p<0.05), verbal fluency (βWF=−0.20 [se=0.06]; p<0.01), and SIS (βSIS=−0.16 [se=0.06]; p<0.01), but not processing speed, after adjusting for covariates. SDB, sleepiness and insomnia were not associated with NC decline.
CONCLUSION:
Long sleep duration predicted seven-year cognitive decline.
Keywords: Cohort studies, Sleep, Neurocognitive decline, Risk factors in epidemiology, Hispanic/Latino
INTRODUCTION
Sleep disturbances are linked with worse neurocognitive function and possibly a higher risk for Alzheimer’s disease (AD).[1, 2] For example, self-reports of short and long sleep durations are associated with increased incident dementia.[2] However, most epidemiological data do not account for important sleep confounders including sleep-disordered breathing (SDB). Similarly, chronic insomnia, a common sleep disorder seen in up to 15% of the U.S. adult population is associated with worse neurocognitive function in some, but not all studies. Recent meta-analyses link SDB, insomnia and self-reported sleep duration to increased AD risk.[2] Importantly, there is limited knowledge about the sleep disturbances associated with neurocognitive decline in Hispanic/Latinos, one of the largest US minority groups.[3] Latinos have up to a 4-fold risk for Alzheimer’s disease and related dementias (ADRD) compared to non-Hispanic whites.[4, 5] Our published data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the largest study of U.S. Hispanic/Latino adults (N≈16,000) across four urban sites, demonstrated a high prevalence of sleep disorders[6] associated with stress, acculturation and cardiometabolic diseases.[7–9] This is a prospective analysis of Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA), which is an ancillary study to HCHS/SOL that aims to determine the factors that predict neurocognitive disorders and decline in middle-aged to older Latino adults. We previously described cross-sectional associations between SDB with worse memory, language and executive function and showed [10] a curvilinear (inverted U-shaped) association between self-reports of sleep duration and neurocognitive function.[11] That is, participants with 7.8 hours ± 1.7 hours of sleep duration had better neurocognitive scores compared to those with short and long sleep durations.[11] The current HCHS/SOL and SOL-INCA study evaluates SDB, daytime sleepiness, insomnia and self-reported sleep duration as predictors of average 7-year neurocognitive change. We hypothesized that SDB, daytime sleepiness, insomnia and self-reported short and long sleep durations are associated with more pronounced average 7-year neurocognitive decline.
METHODS
Population.
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL)[12, 13] is a multicenter community-based prospective cohort study that aims to determine the prevalence and risk factors for chronic diseases (e.g. cardiovascular disease) in a diverse sample of U.S. Hispanic/Latino adults. The initial sample of 16,145 Hispanic/Latino aged 18–74 years had their baseline examination (2008 to 2011) in four U.S. field centers (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA). Eligible participants were self-identified as Hispanic/Latino background, based on questionnaires. The cohort was obtained through a stratified two-stage area probability sample of household addresses selected in each of the four field centers.[12, 13] The largest groups were Central American (n=1,732), Cuban (n=2,348), Dominican (n=1,473), Mexican (n=6,472), Puerto-Rican (n=2,728), and South American (n=1,072). The baseline evaluation gathered information on sleep disorders, demographics, socioeconomic status, lifestyle habits (smoking, physical activity), medical history and biological measures (e.g., anthropometrics, blood draw, oral glucose tolerance test, among others). Neurocognitive function was obtained at baseline (n=9,623) in participants 45–75 years of age. Detailed HCHS/SOL sampling procedures are published [12, 13] and available: https://sites.cscc.unc.edu/hchs/.
The SOL-INCA is an ancillary study, designed to examine the prevalence and determinants of neurocognitive decline and disorders in HCHS/SOL. SOL-INCA occurred concurrent to HCHS/SOL visit-2 (2015 to 2018). SOL-INCA repeated the same neuropsychological tests from HCHS/SOL baseline (Visit-1), and introduced new neuropsychological test as described below. SOL-INCA uses the complex design features of HCHS/SOL, which include a multistage sampling strategy with stratification and clustering, and probability weights that account for non-response and attrition, used to ensure valid generalizations to the HCHS/SOL targeted populations.[14] All participants provided informed consent and the study was approved by the institutional review board for each institution.
Outcomes: Neurocognitive function and score analysis:
The neurocognitive tests administered at baseline were the: (1) Six-Item Screener (SIS; mental status); (2) Spanish English Verbal Learning Test (SEVLT; verbal episodic learning and memory); (3) Controlled Oral Word Association (or Word Fluency; WF; verbal fluency) Test of the Multilingual Aphasia Examination; and (4) Digit Symbol Subtest (DSS; processing speed) of the Wechsler Adult Intelligence Scale-Revised.[14] To evaluate neurocognitive decline, SOL-INCA repeated the above neurocognitive battery at Visit-2 and also administered the Trail Making Test parts A and B (TMT). [15, 16]
Main exposures: Questionnaires:
The sleep heart health study Sleep Habits questionnaire evaluates weekday and weekend bedtime and wake time, napping behaviors, as well as related SDB symptoms such as snoring and witnessed apneas.[6] The following questions were used to determine sleep duration in our target population: What time do you usually go to bed? and What time do you usually wake up? Average sleep duration was computed as the weighted average of weekday and weekend sleep (5/7 weekday + 2/7 weekend). [8] We also categorized sleep duration into short sleep duration (<6 hours), intermediate sleep duration (6–9 hours) and long sleep duration (>9 hours). We used these cut-offs based on the distribution of sleep duration and previous literature on sleep duration and adverse health outcomes.[3, 11] HCHS/SOL also obtained the Epworth Sleepiness Scale (ESS),[17] a widely-used tool with a validated Spanish version that assesses the likelihood of falling asleep in eight common situations. The insomnia questions obtained at Baseline (2008–2011) were adapted from the Women’s Health Initiative Insomnia Rating Scale (WHIIRS),[7] which has a total of 20 points derived from five items scored from 0–4 each. The five items are intended to assess sleep latency, sleep maintenance insomnia, early morning awakening, and overall sleep quality. These are used as a continuous variable ranging from 0 – 20 and a binary variable of insomnia (yes vs. no) based on a score ≥10. All questionaires were administered in both English and Spanish versions, based on participant’s preference.
Information about sleep-disordered breathing [6] was collected using the ARES Unicorder 5.2; B-Alert (Carlsbad, CA). Sleep records were scored at the HCHS/SOL Sleep Reading Center. Respiratory events were identified as a 50% or greater reduction in airflow lasting greater than or equal to 10 seconds with desaturations greater than or equal to 3% and defined as the respiratory event index (REI). Sleep disordered breathing was used as a continuous variable and dichotomized with an REI≥15.
Cardiovascular risk factors:
We use the Global Vascular Risk Score (GVRS) and its component individual risk factors. The GVRS was developed to predict 10-year risk of a stroke and other vascular events using important demographic, anthropometric, behavioral, and vascular risk factors. GVRS includes ethnicity/race, and includes waist circumference, health behaviors and peripheral vascular disease. A detailed discussion of the GVRS, the methods for its derivation, and its component indicators is provided in Sacco et al (2009).[18] A GVRS value of 8.2 indicated a 10% chance for remaining stroke, myocardial infarction, or vascular death free over a 10-year period.
Covariates:
Age in years measured at baseline, time lapse between sleep assessment and SOL-INCA visit measured in days, depressive symptoms using the Center for Epidemiologic Studies Depression scale (CESD-10),[19] and self-reported frequency of sleep medication use in the past 4 weeks (0=None – 4 =5+ times a week). All fully adjusted models also control for Field Center site.
Analytic Subpopulation.
SOL-INCA enrolled 6,377 eligible HCHS/SOL participants. For the current study, we excluded n=398 participants who did not participate in the baseline sleep module, and 182 participants who reported a stroke or transient ischemic attack at baseline. We also excluded n=206 individuals with low mental status scores (SIS<=3) at Baseline. Finally, we excluded n=344 participants with missing values on any of the covariates of interest. The analytic sample size was 5,247. Excluded participants had similar sex and education distributions but were older (63-years vs. 65 years; p<0.001) than those in the analytic sample.
Statistical Analyses.
First, descriptive statistics to characterize the SOL-INCA target population are provided in Table 1. Second, all SOL-INCA cognitive outcomes were z-scored (generated using ([Yi-Mean Yi]/Standard Deviation) for analyses to facilitate comparison of the estimated associations across tests. To examine cognitive performance at SOL-INCA, we fit survey linear regression models to independently test the associations between each sleep risk factor and standardized cognitive outcomes in cross-sectional and prospective analysis. Two regression models were fit for each outcome to test (1) sex, age, education, and time lapse between sleep assessment and SOL-INCA visit; and (2) model 1 with further adjustments for baseline GVRS, depression symptoms, reported frequency of sleep medication, and field center.
Table 1.
Demographics, vascular risk score, and sleep characteristics of the HCHS/SOL and SOL-INCA.
| %(SE) | |
|---|---|
| Education, years | |
| <12 | 37.8 (1.1) |
| 12 | 21.5 (0.9) |
| 12+ | 40.8 (1.1) |
| Sex | |
| Female | 54.8 (1.0) |
| Sleep medication frequency | |
| None per week | 83.6 (0.8) |
| <1 per week | 3.1 (0.4) |
| 1–2 per week | 3.2 (0.3) |
| 3–4 week | 2.2 (0.3) |
| 5 or more per week | 8.0 (0.6) |
| Sleepiness (ESS≥10) | |
| Severe Sleepiness | 20.2 (0.9) |
| Sleep Duration (in hours) | |
| Short Sleep: <6 | 6.6 (0.5) |
| Intermediate Sleep: 6–9 | 78.6 (0.8) |
| Long Sleep: ≥9 | 14.8 (0.7) |
| REI | |
| ≥15 | 17.2 (0.8) |
| Sleep phenotype-Sleep Duration/Insomnia | |
| Short sleep/no insomnia | 3.5 (0.4) |
| Short sleep with insomnia | 3.1 (0.3) |
| Intermediate sleep/no insomnia | 53.9 (1.0) |
| Intermediate sleep with insomnia | 24.7 (0.8) |
| Long sleep/no insomnia | 8.9 (0.6) |
| Long sleep with insomnia | 5.8 (0.5) |
| Sleep Phenotype - ESS/REI | |
| ESS= 0; REI<15 | 8.9 (0.5) |
| ESS= 0; REI ≥15 | 1.2 (0.3) |
| ESS =1–9; REI <15 | 58.4 (1.0) |
| ESS 1–9; REI ≥15 | 11.3 (0.6) |
| ESS ≥10;REI <15 | 15.5 (0.8) |
| ESS ≥10; REI ≥15 | 4.5 (0.4) |
| Sleep Phenotype – Sleep Duration/REI | |
| Short Sleep; REI <15 | 5.1 (0.4) |
| Short Sleep; REI ≥15 | 1.5 (0.2) |
| Average Sleep; REI<15 | 65.5 (1.0) |
| Average Sleep; REI ≥15 | 13.4 (0.8) |
| Long Sleep; REI<15 | 12.1 (0.7) |
| Long Sleep; REI≥15 | 2.4 (0.3) |
|
Mean (SD) |
|
| REI-Continuous | 8.7 (13.4) |
| CESD-10 | 7.2 (6.3) |
| GVRS | 7.1 (0.9) |
| Days Sleep to INCA | 2542.1 (436.0) |
* GVRS=Global Vascular Risk Score; CESD=Center for Epidemiologic Studies Depression; REI= Respiratory Event Index. ESS= Epworth Sleepiness Scale. SD=Standard Deviation
SE=Standard Error based on the sampling weights and complex study design.
Third, change scores for repeated cognitive tests were calculated using survey linear regression to predict cognitive performance at SOL-INCA as a function of baseline cognitive performance, adjusting for age, education, and lapsed time (in days) between cognitive assessments.[21] Test specific standardized measures of change were subsequently calculated using T2 - T2pred/SEE where T2 was the respondent cognitive score at SOL-INCA, T2pred their predicted score and SEE is the standard error of the regression estimator. Subsequently, we used survey linear regression models to independently examine the associations between each sleep risk factor and the standardized measures of cognitive change. The z-scores that represented change in performance over time were calculated using a regression-based approach that accounted for age and education and therefore these variables were not included as covariates in the analytic models. Two regression models were fit for each change outcome to test (1) sex, and time lapse between sleep assessment and SOL-INCA visit; and (2) model 1 with further adjustments for baseline GVRS, depression symptoms, reported frequency of sleep medication, and field center. We do not adjust for age or education as these measures were used in the derivation of the cognitive change outcomes.
Post-hoc estimates of crude and adjusted marginal means and their 95% confidence intervals were calculated and graphed to facilitate visualization of associations across predictors and cognitive outcomes (Figures 1 and 2 for cognitive performance and change, respectively). ANOVA-based contrasts were used to test differences between sleep risk groups. Estimates of marginal differences and their 95% confidence intervals are posted in supplemental Figures 1 and 2 for cognitive performance and cognitive change, respectively.
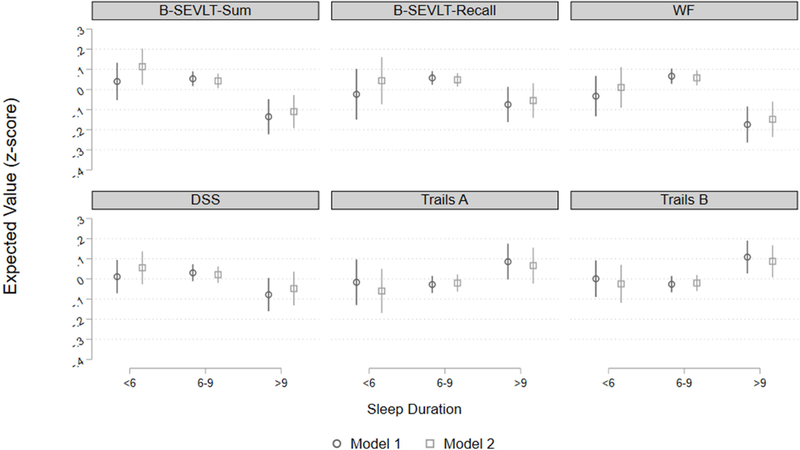
Figure 1. Cognitive performance (z-score) by sleep duration groups among middle-aged and older Latinos.
Model 1: Adjusted for sex, age, education, time lapse between sleep assessment and SOL-INCA visit; Model 2: Model 1 and global vascular risk score, depressive symptoms, report of sleep medication use frequency, and field center; B-SEVLT: Brief Spanish English Verbal Fluency Test; WF: Word Fluency; DSS: Digit Symbol Substitution. For Trails A & B higher values indicate worse function.
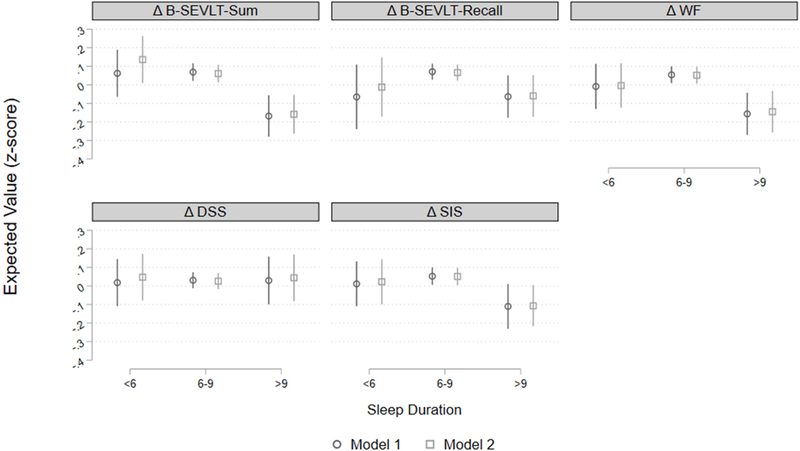
Figure 2. Cognitive decline (z-score) by sleep duration groups among middle-aged and older Latinos.
Model 1: Adjusted for sex, time lapse between sleep assessment and INCA visit; Model 2: Model 1 and global vascular risk score, depressive symptoms, report of sleep medication use frequency, and field center; B-SEVLT: Brief Spanish English Verbal Fluency Test; WF: Word Fluency; DSS: Digit Symbol Substitution; SIS: Six Item Screener.
We previously observed that short and long sleep duration, combined with insomnia was associated with higher rates of prevalent diabetes among Hispanic/Latino adults.[7] Therefore, we evaluated synergisms between sleep disorders by cross-classifying categories of SDB (respiratory event index), daytime sleepiness (Epworth score), insomnia (WHIIRS) and sleep duration (<6 hours, 6–9 hours, >9 hours) and tested their associations with neurocognitive change (Table 4) to examine whether and to what extent the combination of sleep disorders are linked to neurocognitive decline. To test these associations, we used the same regression procedures as detailed in step three above.
Table 4.
Associations between sleep phenotypes and 7-year cognitive change among middle-aged and older Hispanic/Latinos
| Δ B-SEVLT-Sum | Δ B-SEVLT-Recall | Δ WF | Δ DSS | Δ SIS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | |
| Sleep Duration/Insomnia Phenotypes | ||||||||||
| Short sleep/No insomnia | −0.034 | 0.007 | −0.280 | −0.251* | −0.171 | −0.158 | −0.028 | −0.001 | −0.104 | −0.105 |
| 0.086 | 0.085 | 0.145 | 0.127 | 0.095 | 0.092 | 0.091 | 0.090 | 0.104 | 0.104 | |
| Short sleep with insomnia | −0.052 | 0.116 | −0.093 | 0.028 | 0.007 | 0.028 | −0.028 | 0.039 | −0.047 | −0.011 |
| 0.103 | 0.101 | 0.093 | 0.092 | 0.089 | 0.091 | 0.099 | 0.101 | 0.077 | 0.081 | |
| Intermediate sleep/No insomnia | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Intermediate sleep with insomnia | −0.108* | −0.051 | −0.173*** | −0.120* | −0.076 | −0.040 | −0.027 | 0.001 | −0.125** | −0.106* |
| 0.046 | 0.049 | 0.047 | 0.050 | 0.055 | 0.061 | 0.042 | 0.046 | 0.045 | 0.053 | |
| Long/No insomnia | −0.404*** | −0.386*** | −0.282*** | −0.277*** | −0.253** | −0.244** | −0.044 | −0.026 | −0.274** | −0.272*** |
| 0.073 | 0.068 | 0.070 | 0.071 | 0.079 | 0.079 | 0.089 | 0.087 | 0.087 | 0.082 | |
| Long with insomnia | −0.101 | −0.027 | −0.103 | −0.048 | −0.204 | −0.154 | −0.040 | 0.010 | −0.106 | −0.083 |
| 0.082 | 0.085 | 0.103 | 0.102 | 0.111 | 0.109 | 0.103 | 0.101 | 0.084 | 0.088 | |
| Epworth score (ESS)/REI Phenotype | ||||||||||
| ESS=0;REI<15 | 0.002 | −0.038 | 0.009 | −0.025 | 0.007 | 0.003 | −0.033 | −0.039 | 0.012 | 0.026 |
| 0.066 | 0.062 | 0.063 | 0.062 | 0.067 | 0.067 | 0.065 | 0.063 | 0.062 | 0.062 | |
| ESS=0;REI≥15 | −0.015 | −0.016 | 0.243 | 0.228 | 0.305* | 0.326* | −0.124 | −0.107 | 0.182 | 0.133 |
| 0.138 | 0.130 | 0.134 | 0.136 | 0.127 | 0.126 | 0.153 | 0.153 | 0.149 | 0.162 | |
| ESS=1–9;REI<15 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| ESS=1–9;REI≥15 | −0.057 | −0.076 | −0.058 | −0.077 | −0.045 | −0.060 | −0.169* | −0.151* | 0.018 | −0.001 |
| 0.063 | 0.063 | 0.059 | 0.059 | 0.067 | 0.067 | 0.067 | 0.066 | 0.062 | 0.063 | |
| ESS=≥10; REI<15 | −0.058 | −0.032 | 0.013 | 0.034 | −0.125* | −0.120* | −0.019 | −0.016 | 0.029 | 0.026 |
| 0.058 | 0.059 | 0.057 | 0.057 | 0.056 | 0.056 | 0.051 | 0.053 | 0.056 | 0.056 | |
| ESS=≥10;REI≥15 | −0.004 | 0.006 | 0.049 | 0.043 | 0.124 | 0.135 | 0.008 | 0.036 | 0.125 | 0.120 |
| 0.127 | 0.121 | 0.120 | 0.116 | 0.133 | 0.132 | 0.116 | 0.114 | 0.142 | 0.134 | |
| Sleep Duration/REI phenotype | ||||||||||
| Short sleep; REI<15 | 0.026 | 0.093 | −0.128 | −0.082 | −0.127 | −0.113 | 0.025 | 0.047 | −0.059 | −0.055 |
| 0.073 | 0.073 | 0.106 | 0.098 | 0.074 | 0.074 | 0.066 | 0.066 | 0.080 | 0.081 | |
| Short sleep;REI≥15 | −0.105 | 0.003 | −0.173 | −0.105 | 0.158 | 0.142 | −0.248 | −0.160 | 0.152 | 0.187 |
| 0.167 | 0.163 | 0.201 | 0.193 | 0.147 | 0.137 | 0.186 | 0.178 | 0.116 | 0.118 | |
| Intermediate sleep; REI<15 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Intermediate sleep;REI≥15 | −0.019 | −0.036 | 0.021 | 0.001 | 0.043 | 0.034 | −0.057 | −0.035 | 0.080 | 0.063 |
| 0.062 | 0.062 | 0.061 | 0.061 | 0.066 | 0.065 | 0.060 | 0.060 | 0.056 | 0.057 | |
| Long sleep; REI<15 | −0.210*** | −0.188** | −0.103 | −0.090 | −0.196** | −0.185* | 0.014 | 0.033 | −0.147* | −0.137* |
| 0.063 | 0.058 | 0.069 | 0.066 | 0.072 | 0.072 | 0.077 | 0.076 | 0.061 | 0.059 | |
| Long sleep; REI≥15 | −0.324 | −0.329 | −0.272 | −0.289 | −0.259 | −0.237 | −0.261 | −0.230 | −0.353 | −0.388 |
| 0.181 | 0.179 | 0.156 | 0.158 | 0.189 | 0.186 | 0.166 | 0.165 | 0.244 | 0.231 | |
Model 1: Sex and time between INCA and sleep study adjustment; Model 2: Model 1 and global vascular risk score, depressive symptoms, report of sleep medication use frequency, and field center; B-SEVLT: Spanish English Verbal Fluency Test; WF: Word Fluency; DSS: Digit Symbol Substitution; SIS: Six Item Screener; REI: Respiratory Event Index. Sleep Duration: Short is <6; Average is 6–9; and Long is >9. se= standard error
We conducted three sets of sensitivity analyses to check the robustness of our results. We re-estimated all models detailed in steps two and three above to (1) control for APOE4 (APOE4 carriers vs. non-carriers) using the subsample of participants consenting to providing genetic data (n=3,577); (2) exclude participants with elevated self-reported use of sleep medication (i.e. 5+ times a week); and (3) using chained multiple imputation techniques to impute missing values on the model covariates for individuals excluded from our primary analyses due to missing data. [20–22] Our findings were robust to all three sensitivity specifications and the primary results remained qualitatively unchanged across all three sensitivity tests.
RESULTS
Demographic and clinical characteristics of the target population are presented in Table 1. The mean age at INCA was 63±8 years. More than half were females and a large proportion had less than a high school education (37.8%). Seventeen percent of the population had a respiratory event index ≥15 (SDB), 1-in-5 had daytime sleepiness (Epworth score ≥10), a third had insomnia (WHIIRS≥10), and fifteen-percent had long (>9 hours) sleep duration.
Neurocognitive performance.
We found no significant associations between SDB (respiratory event index) or severe sleepiness (Epworth score ≥10) and cognitive performance obtained after 7-year average follow-up (Table 2). Insomnia (WHIIRS≥10) was associated with worse performance on memory, processing speed and attention in age, sex, education and time from sleep assessment to INCA assessment adjusted models. These associations were no longer significant after further adjusting for vascular risk, depression symptoms, and field center. Longer sleep (>9 hours), compared to intermediate sleep duration (6–9 hours), was consistently associated with worse performance across all considered cognitive domains (Figure 1 and supplemental Figure 1). The associations were only slightly attenuated by controlling for the covariates in the fully adjusted model for the memory, phonemic fluency, and executive function. The associations between long sleep duration and cognitive performance on the DSS and Trails A were no longer significant by adjusting for the covariates.
Table 2.
Associations between baseline sleep characteristics and cognitive performance seven years after baseline in HCHS/SOL and SOL-INCA.
| B-SEVLT-Sum | B-SEVLT-Recall | WF | DSS | Trails A | Trails B | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | |
| Respiratory-Event Index (3% desaturation) | ||||||||||||
| REI < 15 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| REI≥15 | 0.025 | 0.036 | 0.028 | 0.035 | 0.016 | 0.032 | −0.008 | 0.005 | −0.010 | −0.014 | −0.051 | −0.051 |
| 0.040 | 0.038 | 0.039 | 0.037 | 0.044 | 0.044 | 0.034 | 0.034 | 0.037 | 0.035 | 0.039 | 0.039 | |
| Epworth sleepiness scale | ||||||||||||
| <10 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| ≥10 | −0.063 | −0.028 | −0.014 | 0.015 | −0.042 | −0.025 | −0.027 | −0.016 | 0.024 | 0.014 | −0.008 | −0.021 |
| 0.034 | 0.033 | 0.033 | 0.034 | 0.035 | 0.036 | 0.033 | 0.033 | 0.038 | 0.038 | 0.034 | 0.033 | |
| Women’s Health Initiative Insomnia Rating Scale | ||||||||||||
| No insomnia | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Insomnia | −0.109*** | −0.027 | −0.130*** | −0.051 | −0.053 | 0.021 | −0.053 | 0.018 | 0.038 | −0.019 | 0.052 | −0.021 |
| 0.032 | 0.033 | 0.030 | 0.034 | 0.033 | 0.033 | 0.028 | 0.028 | 0.034 | 0.035 | 0.031 | 0.032 | |
| Sleep Duration | ||||||||||||
| <6 hours | −0.014 | 0.071 | −0.081 | −0.004 | −0.099 | −0.048 | −0.019 | 0.034 | 0.011 | −0.040 | 0.027 | −0.004 |
| 0.050 | 0.049 | 0.065 | 0.060 | 0.055 | 0.055 | 0.041 | 0.040 | 0.058 | 0.057 | 0.048 | 0.049 | |
| 6–9 hours | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| >9 hours | −0.189*** | −0.153*** | −0.132** | −0.103* | −0.240*** | −0.206*** | −0.109* | −0.069 | 0.113* | 0.087 | 0.135** | 0.108* |
| 0.045 | 0.042 | 0.045 | 0.044 | 0.048 | 0.046 | 0.045 | 0.044 | 0.051 | 0.050 | 0.045 | 0.043 | |
Model 1: Adjusted for sex, age, education, and time lapse between sleep assessment and SOL-INCA visit
Model 2: Model 1 and global vascular risk score, depressive symptoms, report of sleep medication use frequency, and field center.
B-SEVLT: Spanish English Verbal Fluency Test; WF: Word Fluency; DSS: Digit Symbol Substitution; REI: Respiratory Event Index; For Trails A & B higher values indicate worse function. se= standard error
Neurocognitive change.
We found no significant associations between baseline SDB (respiratory event index), daytime sleepiness (Epworth score), insomnia (WHIIRS) and 7-year cognitive change (Table 3). Long sleep duration (vs. intermediate sleep) was consistently associated with decline in memory, phonemic fluency and mental status, but not with processing speed (DSS) (Figure 2 and supplemental Figure 2). Controlling for the GVRS, depression symptoms, sleep medication use, and field center did not change the magnitude and significance of the derived associations.
Table 3.
Associations between sleep characteristics and 7-year cognitive change among middle-aged and older Hispanic/Latinos
| Δ B-SEVLT-Sum | Δ B-SEVLT-Recall | Δ WF | Δ DSS | Δ SIS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | b/se | |
| Respiratory-Event Index (3% desaturation) | ||||||||||
| REI <15 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| REI ≥15 | −0.025 | −0.036 | −0.011 | −0.028 | 0.047 | 0.040 | −0.111* | −0.090 | 0.051 | 0.034 |
| 0.056 | 0.055 | 0.054 | 0.053 | 0.058 | 0.057 | 0.054 | 0.054 | 0.059 | 0.060 | |
| Epworth sleepiness scale | ||||||||||
| <10 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| ≥10 | −0.052 | −0.021 | 0.016 | 0.039 | −0.077 | −0.068 | 0.006 | 0.013 | 0.042 | 0.038 |
| 0.051 | 0.052 | 0.051 | 0.052 | 0.051 | 0.052 | 0.046 | 0.048 | 0.052 | 0.050 | |
| Women’s Health Initiative Insomnia Rating Scale | ||||||||||
| No Insomnia | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Insomnia | −0.045 | 0.024 | −0.094* | −0.038 | −0.051 | −0.007 | −0.032 | 0.003 | −0.072 | −0.044 |
| 0.042 | 0.046 | 0.042 | 0.046 | 0.045 | 0.052 | 0.037 | 0.041 | 0.038 | 0.050 | |
| Sleep Duration | ||||||||||
| <6 hours | −0.007 | 0.075 | −0.136 | −0.078 | −0.063 | −0.055 | −0.013 | 0.021 | −0.041 | −0.029 |
| 0.068 | 0.068 | 0.091 | 0.083 | 0.066 | 0.065 | 0.067 | 0.067 | 0.067 | 0.069 | |
| 6–9 hours | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| - | - | |||||||||
| >9 hours | −0.237*** | 0.219*** | −0.134* | −0.126* | 0.211** | −0.197** | −0.002 | 0.018 | −0.163* | −0.158** |
| 0.060 | 0.056 | 0.062 | 0.060 | 0.064 | 0.063 | 0.070 | 0.069 | 0.066 | 0.06 | |
Model 1: Adjusted for sex and time lapse between sleep assessment and SOL-INCA visit; Model 2: Model 1 and global vascular risk score, depressive symptoms, report of sleep medication use frequency, and field center; B-SEVLT: Spanish English Verbal Fluency Test; WF: Word Fluency; DSS: Digit Symbol Substitution; SIS: Six Item Screener; REI: Respiratory Event Index. se= standard error
Sleep phenotypes based on the cross-classification of sleep disorders with sleep duration showed that individuals with insomnia (WHIIRS ≥10) and intermediate sleep duration (6–9 hours) declined in memory [β= −0.12 (SE=0.05); p<0.05] and mental status (SIS) [β =−0.11 (SE=0.05); p<0.05], but not in other cognitive domains, after adjustment for model covariates. Long sleep (>9 hours) without insomnia (WHIIRS<10) was associated with decline in memory [βSEVLT-Sum= −0.39 (SE=0.07); p<0.001; βSEVLT-Recall= −0.28 (SE=0.07); p<0.001], phonemic fluency [β =−0.24 (SE=0.08); p<0.01] and mental status (SIS) [β =−0.27 (SE=0.08); p<0.001] but not with processing speed (DSS). Other sleep phenotypes were not consistently associated with neurocognitive performance or change (Table 4).
Discussion
Long sleep duration (>9 hours) was associated with seven-year neurocognitive decline in middle-aged to older Hispanics/Latinos. Cross-sectional and prospective studies describe U-shaped associations between sleep duration with neurocognitive impairment, decline and dementia. [3, 23] However, in our sample only long sleep (>9 hours), but not short sleep (<6 hours), predicted neurocognitive decline after accounting for confounders. In addition, SDB based on the respiratory event index ≥15 and daytime sleepiness with the Epworth sleepiness scale ≥10 did not predict neurocognitive decline.
In the Atherosclerosis Risk in Communities (ARIC) Study, SDB (measured with in-home polysomnography) was not associated with 15-year neurocognitive decline.[24] In the Osteoporotic Fractures in Men Study, neurocognitive decline was observed among participants with worse sleep quality (assessed with polysomnography), while in a different study long sleep duration (>9 hours) was associated with neurocognitive decline. [25, 26] However, a meta-analysis of 14 studies (six of them prospective) showed that SDB was associated with a 26% increased risk of neurocognitive decline and dementia.[27] The mechanisms by which SDB confers neurocognitive impairment is unclear, but other studies suggest that metrics of SDB severity, such oxygen desaturations in sleep, had stronger associations with neurocognitive impairment than the apnea-hypopnea index or the respiratory-event index. Daytime sleepiness, measures with the Epworth sleepiness scale, is a commonly used questionnaire of self-reported SDB that has been associated with neurocognitive decline, dementia. [28] Some suggest that daytime sleepiness could explain associations between SDB and neurocognitive decline, however, this finding was not observed in HCHS/SOL and SOL-INCA. Interestingly, participants with insomnia who also reported that 6 to 9 hours of sleep duration, but not short sleep duration (<6 hours), decline in memory function and the neurocognitive impairment screener. It is plausible that quantitative measures of sleep quality (macro- and micro-sleep architecture), rather than sleep duration, could be better predictors of neurocognitive decline in some participants with insomnia.[29, 30] Most studies of SDB involved participants that on average were older than HCHS/SOL participants. While direct comparison is not possible, our sleep, neurocognitive measures and follow-up time differ from prior studies, which could partly explain the observed null findings observed with SDB and daytime sleepiness. Importantly, our analyses included multiple dimensions of sleep with simultaneous measures of SDB, which overcomes limitations in previous epidemiological studies. [3, 23, 31]
Our findings suggest that individuals who report long sleep duration are at higher risk for neurocognitive decline. Self-reported long sleep duration has been associated with neurocognitive dysfunction in various cross-sectional analyses, [11, 32, 33] but there is limited data from prospective studies,[33] particularly among individuals from diverse Hispanic/Latino backgrounds.[31, 33] Similar to our study, a cohort of 2,855 participants from Korea, showed that long-sleep duration with normal neurocognitive function at baseline had a 70% increased chance of decline after 4-years of follow-up. However, long sleep duration did not predict neurocognitive decline among mild cognitive impairment (MCI) identified at baseline.[31] A meta-analysis of prospective studies showed a 58% increase in the risk of neurocognitive decline in executive function, working and verbal memory in older adults reporting >9 hours of sleep duration.[34, 35] In older Spaniards, long sleep duration had over a two-fold risk of dementia after 3-years of follow-up and a 58% risk of dementia-specific mortality after 13-years. [36, 37] Similar results were described in a community-based cohort of older adults from Japan.[38]
Possible mechanisms explaining long sleep duration and neurocognitive decline.
Longer habitual sleep duration could be an early marker of dementia with possible sleep cycle changes or increased sleep need manifested as longer bed times.[39] Proper sleep may facilitate β amyloid clearance from the brain; however, this remains undefined in long sleepers.[40] Additionally, long sleep duration is associated with cerebrovascular disease,[41] a known contributor to neurocognitive impairment and decline. While a specific pathway between long sleep duration and decline is not clear, long sleep duration has been associated with increased white matter hyperintensity volumes [42] and subclinical brain infarction, affecting brain regions (e.g. the prefrontal cortex), and potentially neurocognitive function, through vascular damage.[43] Interestingly, a meta-analysis showed that long sleep duration, but not short sleep, was associated with increased inflammatory markers that increase cardiovascular disease and vascular risk factors. [44] In addition, it has been suggested that long sleep duration is associated with a later “chronotype,” or nocturnal preference for wakefulness, which does not allow for proper realignment of the endogenous circadian rhythms.[45] Circadian misalignment can lead to fragmented sleep and decreased slow wave sleep, potentially contributing to long habitual sleep duration as consequence of greater sleep need.[45] Future studies should consider how circadian rhythms influence sleep duration and sleep quality.[46] While the specifics of the interplay between sleep and neurodegeneration merit further study, self-reported long sleep duration may be a marker of poor sleep efficiency, fragmentation or circadian misalignment, which may promote neurocognitive decline and impairment. [45] Interestingly, sleep disturbances could explain the pathological changes observed in mild cognitive impairment (MCI) and early AD.[47] For example, history of sleep disruption was associated with increased neurofibrillary tangles in the brainstem nuclei known to regulate sleep-wake process, in a post-mortem sample of patients with early AD (Braak stage I/II). In a different study, older adults with MCI had increased sleep symptoms, as well as, decreased objectively measured sleep continuity and more fragmented sleep independent of neurocognitive performance, suggesting that sleep disruption could be an early marker of neurodegeneration. [48, 49]
A strength of our study is the large sample of middle-aged and older Hispanic/Latinos, who are underrepresented in studies of sleep risk factors for neurocognitive decline. In addition, we systematically assessed demographic, behavioral, vascular risk factors, along with concomitant SDB, daytime sleepiness and insomnia. The study also had several limitations. First, there are a limited number of measures available for SDB assessment from the home sleep apnea test, preventing evaluation of arousal, sleep architecture and central sleep apnea. Second, there could be reporting bias since sleep duration is self-reported. However, in a study of older men, participants with long sleep duration compared to intermediate sleep, did spend more time asleep and in bed quantified with actigraphy and polysomnography.[50] Of importance, long sleep duration can be a symptom of poor sleep efficiency (decreased sleep quality or continuity).[39] Long time between going to bed and getting up could be either habitual long sleep duration or poor sleep efficiency. Follow-up studies should evaluate the efficiency and quality of sleep, as an important measure associating sleep and neurocognitive outcomes.[39]
Third, HCHS/SOL was designed to address gaps of U.S. Hispanic/Latino health. However, factors such as low health literacy, low education and the implementation of cultural specific protocols may serve as unknown confounders. Importantly, the chosen questionnaires are available and validated in Spanish and English. Finally, confounding through unmeasured factors is possible in any observational study such as this one.
Conclusion
In the largest study of U.S. Hispanic/Latinos, long sleep duration was associated to worse neurocognitive performance and predicted 7-year neurocognitive decline. Longer habitual self-reported sleep duration could be either a risk factor or harbinger of neurocognitive decline.
Supplementary Material
Research in Context.
Systematic review: The authors reviewed the literature using the PubMed MeSH terms, “sleep” and “cognition” and “prospective studies.” We reviewed the abstract of 146 studies and identified four relevant publications. Two studies did not find associations between sleep disorders and cognitive change. One study observed associations between worse sleep quality and cognitive decline among older adults with polysomnography. In another study, long sleep duration (>9 hours) was associated with decline. Most studies did not adjust for sleep disordered breathing, a known confounder of impaired cognition, and did not have simultaneous information on daytime sleepiness and insomnia within a single cohort. No studies used data from a representative sample of U.S. Hispanic/Latinos.
Interpretation: Our findings identified important sleep correlates of neurocognitive decline.
Future directions: Quantifying the role of sleep disorders and cerebrovascular disease as a pathway to neurocognitive decline. Perform chronotype and genetic studies that can clarify causal associations with neurocognitive decline.
Acknowledgements:
The authors thank the staff and participants of HCHS/SOL and SOL-INCA staff for their important contributions. Investigators website - http://www.cscc.unc.edu/hchs/
Funding/Support:
This work is support by National Institute on Aging (R01AG048642, RF1AG054548, R01AG061022, and R21AG056952). Dr. González also receives additional support from P30AG005131 and P30AG059299. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
Role of Funding Source: This work was supported by the National Institute on Aging and National Heart Lung Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no conflicts of interest that could inappropriately influence, or be perceived to influence, this work.
References:
- [1].Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Associations Between Sleep-Disordered Breathing, Nocturnal Hypoxemia, and Subsequent Cognitive Decline in Older Community-Dwelling Men: The Osteoporotic Fractures in Men Sleep Study 2015;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, et al. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 2017;40. [DOI] [PubMed]
- [3].Ramos AR, Gardener H, Rundek T, Elkind MS, Boden-Albala B, Dong C, et al. Sleep disturbances and cognitive decline in the Northern Manhattan Study 2016;87:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haan MN, Mungas DM, González HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- [5].Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in US race/ethnic populations. Alzheimers Dement 2016. [DOI] [PubMed]
- [6].Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic community health study/study of Latinos 2014;189:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cespedes EM, Dudley KA, Sotres-Alvarez D, Zee PC, Daviglus ML, Shah NA, et al. Joint associations of insomnia and sleep duration with prevalent diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J Diabetes 2016;8:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel SR, Sotres-Alvarez D, Castaneda SF, Dudley KA, Gallo LC, Hernandez R, et al. Social and Health Correlates of Sleep Duration in a US Hispanic Population: Results from the Hispanic Community Health Study/Study of Latinos. Sleep 2015;38:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alcantara C, Patel SR, Carnethon M, Castaneda S, Isasi CR, Davis S, et al. Stress and Sleep: Results from the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. SSM Popul Health 2017;3:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ramos AR, Tarraf W, Rundek T, Redline S, Wohlgemuth WK, Loredo JS, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology 2015;84:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ramos AR, Tarraf W, Daviglus M, Davis S, Gallo LC, Mossavar-Rahmani Y, et al. Sleep Duration and Neurocognitive Function in the Hispanic Community Health Study/Study of Latinos. Sleep 2016;39:1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos 2010;20:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology 2010;20:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol 2015;30:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crowe SFJJocp. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test 1998;54:585–91. [DOI] [PubMed] [Google Scholar]
- [16].Besha XS, Spencer RJ, Bieliauskas LAJIJoN. PPVT-I administration rules significantly shorten PPVT-III/IV administration 2017;127:412–6. [DOI] [PubMed] [Google Scholar]
- [17].Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- [18].Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, et al. Improving global vascular risk prediction with behavioral and anthropometric factors: the multiethnic NOMAS (Northern Manhattan Cohort Study) 2009;54:2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wassertheil-Smoller S, Arredondo EM, Cai J, Castaneda SF, Choca JP, Gallo LC, et al. Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2014;24:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].White IR, Royston P, Wood AMJSim. Multiple imputation using chained equations: issues and guidance for practice 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- [21].Royston P, White IRJJSS. Multiple imputation by chained equations (MICE): implementation in Stata 2011;45:1–20. [Google Scholar]
- [22].Azur MJ, Stuart EA, Frangakis C, Leaf PJJIjomipr. Multiple imputation by chained equations: what is it and how does it work? 2011;20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen J-C, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, et al. Sleep duration, cognitive decline, and dementia risk in older women 2016;12:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive sleep apnea and 15-year cognitive decline: the atherosclerosis risk in communities (ARIC) study 2016;39:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ESJJotAGS. Sleep duration in midlife and later life in relation to cognition 2014;62:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL, et al. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 2015;38:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leng Y, McEvoy CT, Allen IE, Yaffe KJJn. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis 2017;74:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsapanou A, Gu Y, O’Shea D, Eich T, Tang MX, Schupf N, et al. Daytime somnolence as an early sign of cognitive decline in a community-based study of older people. Int J Geriatr Psychiatry 2016;31:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BSJJopr. Insomnia and risk of dementia in older adults: systematic review and meta-analysis 2016;77:109–15. [DOI] [PubMed] [Google Scholar]
- [30].Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJJBsm. Changes in cognitive performance are associated with changes in sleep in older adults with insomnia 2016;14:295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH, et al. Sleep and cognitive decline: A prospective nondemented elderly cohort study 2018;83:472–82. [DOI] [PubMed] [Google Scholar]
- [32].Kim H-B, Myung S-K, Lee S-M, Park YC, Neuroepidemiology KM-ASGJ. Longer duration of sleep and risk of cognitive decline: a meta-analysis of observational studies 2016;47:171–80. [DOI] [PubMed] [Google Scholar]
- [33].Wennberg AM, Wu MN, Rosenberg PB, Spira AP. Sleep disturbance, cognitive decline, and dementia: a review. Seminars in neurology: Thieme Medical Publishers; 2017. p. 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med 2016;17:87–98. [DOI] [PubMed] [Google Scholar]
- [35].van Oostrom SH, Nooyens ACJ, van Boxtel MPJ, Verschuren WMM. Long sleep duration is associated with lower cognitive function among middle-age adults - the Doetinchem Cohort Study. Sleep Med 2018;41:78–85. [DOI] [PubMed] [Google Scholar]
- [36].Benito-Leon J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology 2014;83:1530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009;16:990–7. [DOI] [PubMed] [Google Scholar]
- [38].Ohara T, Honda T, Hata J, Yoshida D, Mukai N, Hirakawa Y, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community 2018;66:1911–8. [DOI] [PubMed] [Google Scholar]
- [39].Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita YJSMR. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression 2018;39:25–36. [DOI] [PubMed] [Google Scholar]
- [40].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain 2013;342:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Song Q, Liu X, Zhou W, Wang L, Zheng X, Wang X, et al. Long sleep duration and risk of ischemic stroke and hemorrhagic stroke: the Kailuan Prospective Study 2016;6:33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ramos AR, Dong C, Rundek T, Elkind MS, Boden-Albala B, Sacco RL, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study 2014;23:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ramos AR, Dib SI, Wright CB. Dementia Vascular. Curr Transl Geriatr Exp Gerontol Rep 2013;2:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Irwin MR, Olmstead R, Carroll JEJBp. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med Rev 2018;40:127–34. [DOI] [PubMed] [Google Scholar]
- [46].Landry GJ, Liu-Ambrose T. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci 2014;6:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Palmer K, Mitolo M, Burgio F, Meneghello F, Venneri A. Sleep Disturbance in Mild Cognitive Impairment and Association With Cognitive Functioning. A Case-Control Study. Front Aging Neurosci 2018;10:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hayes TL, Riley T, Mattek N, Pavel M, Kaye JA. Sleep habits in mild cognitive impairment. Alzheimer Dis Assoc Disord 2014;28:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD, et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 2018;66:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patel SR, Blackwell T, Ancoli-Israel S, Stone KL, Osteoporotic Fractures in Men-Mr OSRG. Sleep characteristics of self-reported long sleepers. Sleep 2012;35:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.