Summary
The RNA modification N6-methyladenosine (m6A) modulates mRNA fate and thus affects many biological processes. We analyzed m6A across the transcriptome following infection by dengue virus (DENV), Zika virus (ZIKV), West Nile virus (WNV), and hepatitis C virus (HCV). We found that infection by these viruses in the Flaviviridae family alters m6A modification of specific cellular transcripts, including RIOK3 and CIRBP. During viral infection, the addition of m6A to RIOK3 promotes its translation, while loss of m6A in CIRBP promotes alternative splicing. Importantly, viral activation of innate immune sensing or the endoplasmic reticulum (ER) stress response contributes to the changes in m6A in RIOK3 and CIRBP, respectively. Further, several transcripts with infection-altered m6A profiles, including RIOK3 and CIRBP, encode proteins that influence DENV, ZIKV, and HCV infection. Overall, this work reveals that cellular signaling pathways activated during viral infection lead to alterations in m6A modification of host mRNAs to regulate infection.
Graphical Abstract

eTOC Blurb
Here, Gokhale, McIntyre et al. identify m6A changes in cellular mRNAs following Flaviviridae infection and demonstrate that infection-activated pathways contribute to these changes. They show that altered m6A modification in RIOK3 and CIRBP mRNAs influence their translation and splicing, respectively and that RIOK3, CIRBP, and other m6A-altered factors regulate infection.
Introduction
Transcriptional and post-transcriptional regulatory mechanisms influence gene expression in cells following infection by viruses, including those in the Flaviviridae family. The Flaviviridae family of positive sense RNA viruses includes dengue virus (DENV), Zika virus (ZIKV), West Nile virus (WNV), and hepatitis C virus (HCV), all of which cause significant mortality and morbidity worldwide (Holbrook, 2017; Thrift et al., 2017). Previous studies have shown broad changes in cellular transcript levels during Flaviviridae infection that highlight a complex relationship between viral infection and gene expression, whereby the host attempts to resist infection by up- or down-regulating relevant genes while viruses co-opt host transcription to facilitate replication and avoid host defenses (Fink et al., 2007; Kumar et al., 2016; Rosenberg et al., 2018; Sessions et al., 2013; Su et al., 2002; Zanini et al., 2018). Differential expression of proviral and antiviral host factors is therefore an important determinant of the outcome of Flaviviridae infection.
Host gene expression during Flaviviridae infection can be tuned by post-transcriptional RNA controls (De Maio et al., 2016; Luna et al., 2015; Schwerk et al., 2015). One of these controls is the chemical modification of RNA (Gilbert et al., 2016). The most prevalent internal modification of mRNA is N6-methyladenosine (m6A). The regulation of m6A in RNA is controlled by specific cellular proteins. The METTL3-METTL14-WTAP “writer” complex catalyzes the methylation of adenosine residues in mRNA, targeting the consensus motif DRA*CH (where D=G/A/U, R=G/A, H=U/A/C, and * denotes modified A) in mRNA for methylation; however how specific DRACH motifs are selected for modification is still not well understood (Meyer and Jaffrey, 2017; Shi et al., 2019; Yang et al., 2018). “Reader” RNA-binding proteins recognize m6A to modulate mRNA metabolism, including mRNA splicing, nuclear export, stability, translation, and structure (Meyer and Jaffrey, 2017; Shi et al., 2019; Yang et al., 2018). By regulating specific transcripts, m6A affects many important biological processes (Gonzales-van Horn and Sarnow, 2017; Meyer and Jaffrey, 2017; Shi et al., 2019; Yang et al., 2018).
Viral infection can be influenced by m6A modification of either viral or host transcripts. Transcripts from both DNA and RNA viruses can be methylated, and m6A in these RNAs has various proviral and antiviral functions (Courtney et al., 2017; Gokhale and Horner, 2017; Gokhale et al., 2016; Hao et al., 2019; Imam et al., 2018; Kennedy et al., 2016; Lichinchi et al., 2016a; Lichinchi et al., 2016b; McIntyre et al., 2018; Rubio et al., 2018; Tirumuru et al., 2016; Tsai et al., 2018; Winkler et al., 2019; Ye et al., 2017). m6A in specific cellular transcripts is also important during viral infection (Liu et al., 2019b; Rubio et al., 2018; Winkler et al., 2019). For example, m6A regulates the antiviral IFNB1 transcript (Rubio et al., 2018; Winkler et al., 2019). However, the role of m6A in cellular mRNA during viral infection is still not well understood, in part because of difficulties in accurately and quantitatively mapping the modification. While several viruses alter m6A modification in cellular mRNAs (Hesser et al., 2018; Lichinchi et al.; Lichinchi et al., 2016b; Tan et al., 2018), the scale of these changes has likely been overestimated (McIntyre et al., 2019). Moreover, there are almost no data on common m6A changes in host mRNA across multiple viruses, and the functional consequences of m6A changes in cellular mRNA during viral infection have also not been examined. Therefore, identifying both m6A changes during viral infection and the consequences of these changes on cellular mRNA are important for understanding post-transcriptional regulation of the host response to infection.
Here, we studied the effect of DENV, ZIKV, WNV, and HCV infection on the m6A epitranscriptome. We found that infection by all four viruses led to altered m6A modification of a set of specific cellular transcripts and that activation of innate immunity and endoplasmic reticulum (ER) stress responses by infection contribute to differential m6A modification and changes in translation or splicing of these transcripts. Importantly, transcripts with altered m6A encode proteins that regulate infection, indicating that post-transcriptional gene regulation of mRNA by m6A has the potential to affect host response and viral replication.
Results
Flaviviridae infection alters m6A modification of specific cellular transcripts.
Flaviviridae infection changes the expression of proviral and antiviral gene products (Fink et al., 2007; Kumar et al., 2016; Rosenberg et al., 2018; Sessions et al., 2013; Su et al., 2002; Zanini et al., 2018). Since m6A can modulate RNA fate, and therefore protein expression, we hypothesized that altered m6A modification would influence expression of host genes that regulate viral infection. We therefore measured changes in the m6A modification of host transcripts during Flaviviridae infection using methylated RNA immunoprecipitation and sequencing (MeRIP-seq) (Figure 1A). For MeRIP-seq, we used an anti-m6A antibody to enrich m6A-modified RNA fragments prior to RNA sequencing of both the input and immunoprecipitated (IP) fractions (Dominissini et al., 2012; Meyer et al., 2012). We note that this antibody also recognizes the similar modification N6,2’-O-dimethyladenosine (m6Am), which in mRNA is only found in the 5’ cap (Linder et al., 2015; Mauer and Jaffrey, 2018). We performed MeRIP-seq on RNA from human Huh7 liver hepatoma cells, which are permissive for all four viruses. At 48 hours post-infection with DENV, ZIKV, WNV, or HCV, 60–90% of cells stained positive for viral antigen (Figure S1A). We first identified gene expression changes in response to infection. We analyzed differential expression of genes between infected samples and uninfected controls using the input fractions from MeRIP-seq and found 50 genes that were differentially expressed (DESeq2, adjusted p < 0.05, |Log2Fold Change (FC)| ≥ 2) across all four viruses (Figure S1B–C, Table S1). We found that several pathways were similarly altered by all four viruses (Figure S1D), including innate immunity (such as NF-κB, TNF, and MAPK signaling) and the ER stress response. These results, which we validated by RT-qPCR (Figure S1E), are similar to what has been reported for individual Flaviviridae (Carletti et al., 2019; Fink et al., 2007; Kumar et al., 2016; Rosenberg et al., 2018; Sessions et al., 2013; Su et al., 2002; Zanini et al., 2018).
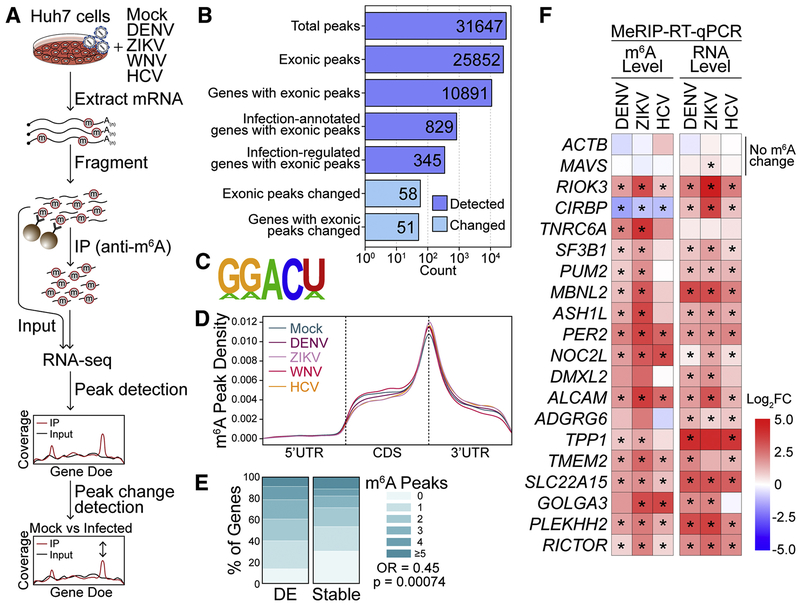
Figure 1: Flaviviridae infection alters m6A modification of specific transcripts.
(A) Schematic of the MeRIP-seq protocol used to identify differential m6A methylation following infection of Huh7 cells with DENV, ZIKV, WNV, and HCV. RNA was harvested at 48 hours post-infection (hpi) and experiments were performed in triplicate. (B) The number of peaks and genes with m6A peaks detected in ≥ 2 mock- or virus-infected samples (dark blue; MACS2 q-value < 0.05) and peaks that change during infection (light blue, |peak – gene Log2FC| ≥ 1, adjusted p < 0.05). “Infection-annotated genes:” genes with known annotations for the Reactome Pathways ‘Infectious Disease’, ‘Unfolded Protein Response’, ‘Interferon Signaling’, or ‘Innate Immune Signaling’ in the database used by fgsea. “Infection-regulated genes:” genes that show a Log2FC in gene expression ≥ 2 in RNA expression between mock- and virus- infected samples (adjusted p < 0.05). (C) The most significantly enriched motif in the MeRIP fractions across all samples (HOMER, p = 1e-831). (D) Metagene plot of “methylated” DRACH motifs (detected in a peak in at least two replicates) across transcripts in mock- and virus- infected cells. (E) The percent of genes with m6A peaks that changed expression with infection (|Log2FC| ≥ 2, adjusted p < 0.05, N = 137) and genes that remained stable (|Log2FC| < 0.5, adjusted p > 0.05, N = 7627) for transcripts with mean expression ≥ 50 reads. (F) (Left) MeRIP-RT-qPCR analysis of relative m6A level of transcripts with infection-altered m6A modification or controls (ACTB and MAVS) in DENV, ZIKV, and HCV-infected (48 hpi) Huh7 cells. (Right) RNA expression of these transcripts relative to GAPDH. Values in heatmap are the mean of 3 independent experiments. * p < 0.05, by unpaired Student’s t test. See also Figure S1 and Table S1 and S2.
We then predicted m6A-modified regions within mRNAs by calling peaks in IP over input RNA-seq coverage across transcripts using MACS2, a ChIP-seq peak caller commonly used to detect m6A peaks from MeRIP-seq data (McIntyre et al., 2019; Zhang et al., 2008). We detected a total of 31,647 peaks, with 25,852 exonic peaks corresponding to 10,891 genes across all uninfected and infected samples (Figure 1B). The known m6A motif DRACH (in particular, GGACU), was enriched under the identified peaks (Figure 1C). As expected, detected peaks were most common at the end of the coding sequence and beginning of the 3’ untranslated region (UTR) (Figure 1D) (Meyer and Jaffrey, 2017). We did not observe a change in the distribution of m6A across transcript regions with DENV, ZIKV, WNV, or HCV infection (Figure 1D). This is in contrast to a previous report that suggested ZIKV infection led to increased methylation in the 5’ UTRs of cellular transcripts (Lichinchi et al., 2016b); however, we also did not detect a difference in m6A distribution in 5’ UTRs following ZIKV infection on reanalysis of that published data using two different peak callers: MACS2 or MeTDiff (Figure S1F) (Cui et al., 2018). Further, following viral infection, we found only subtle changes in the overall level of m6A relative to unmodified adenosine in purified mRNA, as analyzed by liquid chromatography tandem-mass spectrometry (LC-MS/MS) of digested nucleotides, and no change in the expression of cellular m6A machinery, as analyzed by immunoblotting (Figure S1G–H). Indeed, since the expression of the methylation machinery was not changed by infection, we would not predict broad, unidirectional changes in the abundance or distribution of m6A in cellular mRNAs.
However, functional annotation of the m6A-modified genes expressed in the infected samples did reveal an enrichment for genes with roles in infection. In total, 829 methylated genes were annotated as involved in the Reactome Pathways of “Infectious Disease”, “Unfolded Protein Response”, “Interferon Signaling”, or “Innate Immune System” (“Infection-annotated genes”; see Methods; Figure 1B). Further, 345 methylated genes were differentially expressed between infected and uninfected samples (“Infection-regulated genes”; Figure 1B). Indeed, mRNAs that changed expression with infection (p adj < 0.05, |Log2FC| ≥ 2, mean expression ≥ 50) were more likely to have at least one m6A site than those that did not change expression (p adj > 0.05, |Log2FC| < 0.5, mean expression ≥ 50; Fisher’s exact test p = 0.00074, odds ratio = 0.64) (Figure 1E). These results support previous reports that transcripts that undergo dynamic regulation tend to contain more m6A sites than stable housekeeping mRNAs (Schwartz et al., 2014) and suggest that m6A may regulate genes implicated in infection.
We next determined changes in m6A from differences in IP enrichment relative to gene expression with infection by all four viruses. We detected shared m6A changes in 58 exonic peaks in 51 genes following infection, most of which showed increases in m6A and occurred in the 3’ UTR or coding sequence (Figure 1B, Table S2). While differentially expressed genes were enriched for pathways with known roles in infection (Figure S1D), genes that showed changes in methylation did not show enrichment for functional categories relevant to infection. We and others previously showed that MeRIP-RT-qPCR with primers under the changed m6A peaks can detect relative changes in m6A (Engel et al., 2018; McIntyre et al., 2019). Therefore, we used this method to orthogonally validate 18 of the predicted m6A changes following infection. In these and subsequent analyses, we focused on m6A changes following DENV, ZIKV, and HCV infection. Of these 18 transcripts, 16 showed a significant change in m6A relative to any change in gene expression with at least two viruses, and 9 of those showed a significant change with all three viruses. Most non-significant m6A changes trended towards the change predicted by MeRIP-seq (Figure 1F). ACTB and MAVS mRNAs, both predicted to be stably methylated during infection, indeed showed no m6A changes (Figure 1F).
For our predictions of pan-viral m6A changes using MeRIP-seq (above), we compared all infected to all uninfected replicates for increased statistical power (McIntyre et al., 2019). However, to also detect any peak changes unique to single viruses, we used the same computational approach described above (Table S2). MeRIP-RT-qPCR for these putative virus-specific peaks (two per virus) showed similar changes in relative m6A at those peaks with infection by all three viruses tested, rather than individual virus-mediated changes, in the same direction as predicted by MeRIP-seq (Figure S1I). This suggests that m6A regulation can occur through common processes activated by viral infection. Together, our data reveal that hundreds of transcripts differentially expressed during Flaviviridae infection contain m6A and that infection alters m6A modification of specific host transcripts.
Flaviviridae infection alters m6A modification of RIOK3 and CIRBP mRNA through distinct pathways.
We focused on two specific transcripts that gain or lose m6A (RIOK3 and CIRBP respectively) during infection by all viruses for further analysis. RIOK3 encodes a serine/threonine kinase that may regulate antiviral signaling (Feng et al., 2014; Takashima et al., 2015; Willemsen et al., 2017), while CIRBP encodes a stress-induced RNA-binding protein (Liao et al., 2017). Following viral infection, RIOK3 mRNA gains an m6A peak in the 3’ UTR near the stop codon (Figure 2A), and CIRBP mRNA loses an m6A peak in the coding sequence of its last exon (Figure 2B). The RIOK3 and CIRBP peaks span four and three DRACH motifs, respectively. Both peaks appear in published datasets; the RIOK3 peak in mouse liver tissue (Zhou et al., 2018), and the CIRBP peak in HepG2 cells (Huang et al., 2019; Zhong et al., 2018). We performed MeRIP-RT-qPCR on RNA from cells infected with DENV, ZIKV, and HCV to validate these m6A changes. MeRIP-RT-qPCR confirmed that the relative m6A modification of RIOK3 was significantly increased and that of CIRBP decreased after infection, while RIOK3 and CIRBP mRNA levels both increased (Figure 1F and 2C). These m6A changes in RIOK3 and CIRBP were also apparent in chromatin-associated RNA following ZIKV infection, suggesting that the regulation of m6A at these sites occurs co-transcriptionally (Ke et al., 2017; Slobodin et al., 2017) (Figure S2A). In uninfected cells, both RIOK3 and CIRBP transcripts are bound by the m6A-binding protein YTHDF1 (Figure S2B–C). However, DENV, ZIKV, and HCV infection increased YTHDF1 association with RIOK3 and decreased its association with CIRBP, suggesting that YTHDF1 recognizes the altered m6A status of RIOK3 and CIRBP transcripts following infection (Figure S2D).
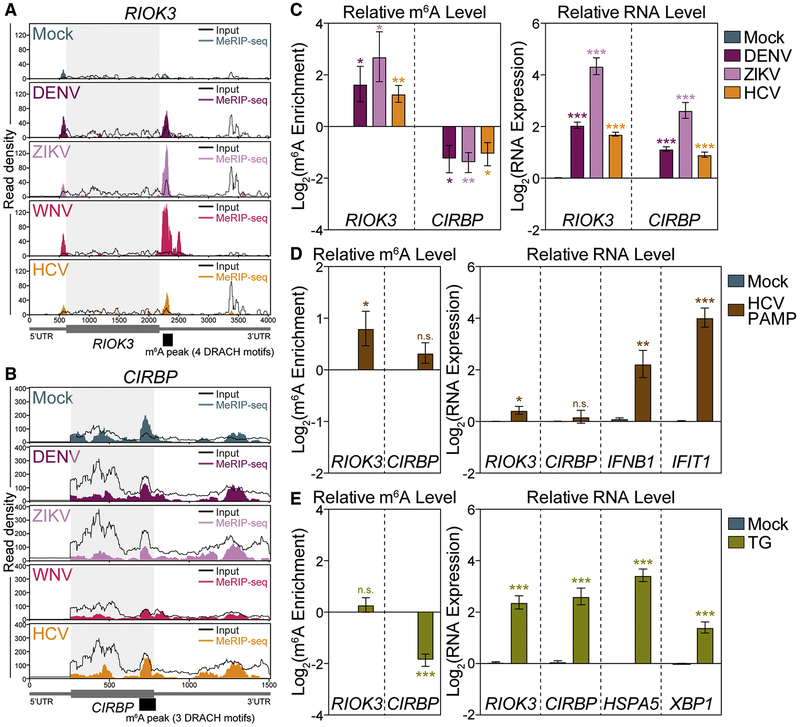
Figure 2: Flaviviridae infection alters m6A modification of RIOK3 and CIRBP mRNA through distinct cellular pathways.
(A and B) Coverage plot of MeRIP (color) and input (black) reads in (A) RIOK3 and (B) CIRBP transcripts in Huh7 cells infected with the indicated virus (48 hpi), as determined by MeRIP-seq. Representative of three biological replicates. Infection-altered m6A peaks are indicated in black under the transcript map. (C) (Left) MeRIP-RT-qPCR analysis of relative m6A level of RIOK3 and CIRBP in mock- and virus-infected (48 hpi) Huh7 cells. (Right) RNA expression of RIOK3 and CIRBP relative to HPRT1. (D) (Left) MeRIP-RT-qPCR analysis of relative m6A level of RIOK3 and CIRBP in mock- and HCV PAMP- transfected (8 h) Huh7 cells. (Right) RNA expression of RIOK3, CIRBP, as well as positive control transcripts IFNB1 and IFIT1 relative to HPRT1. (E) (Left) MeRIP-RT-qPCR analysis of relative m6A level of RIOK3 and CIRBP in mock- and thapsigargin-treated (TG; 16 h) Huh7 cells. (Right) RNA expression of RIOK3, CIRBP, and positive control transcripts HSPA5 and XBP1 relative to HPRT1. Values are the mean ± SEM of 6 (C-D), or 5 (E) biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired Student’s t test. n.s. = not significant. See also Figure S2 and Table S2 and S3.
We next investigated whether cellular pathways stimulated by viral infection (Figure S1D) contribute to the virally induced m6A changes in RIOK3 and CIRBP. Flaviviridae infection drives signaling cascades that lead to the induction of interferon-β (IFN) and antiviral IFN-stimulated genes (ISGs) by IRF3 (Horner and Gale, 2013; Munoz-Jordan and Fredericksen, 2010; Suthar et al., 2013). In infected Huh7 IRF3 KO cells (Vazquez et al., 2019), the increase in RIOK3 m6A with infection was attenuated (from ~4- to ~1.5-fold) compared to parental cells (Figure S2E and 2C). However, DENV and ZIKV infection of IRF3 KO cells still reduced the relative m6A enrichment of CIRBP, consistent with that seen following infection of the parental cells (Figure S2E and 2C) (Vazquez et al., 2019). This suggests that IRF3 activation contributes to increased RIOK3 m6A modification, while not affecting the m6A-status of CIRBP. To determine if innate immune activation in the absence of replicating virus alters m6A modification of RIOK3 and CIRBP, we measured the relative m6A levels of RIOK3 and CIRBP mRNA by MeRIP-RT-qPCR following transfection of Huh7 cells with an HCV immunostimulatory RNA (HCV PAMP) (Saito et al., 2008). HCV PAMP induced expression of IFNB1 and the ISG IFIT1 and also increased m6A modification of RIOK3, but did not decrease CIRBP methylation (Figure 2D). Importantly, we found that the increase in RIOK3 m6A following HCV PAMP was dependent on the m6A methyltransferases METTL3 and METTL14 as HCV PAMP did not increase m6A modification of RIOK3 following depletion of METTL3 and METTL14 (Figure S2F). IFN-β treatment, which activates the IFN response, also led to a slight but significant increase in the relative m6A enrichment of RIOK3 but not CIRBP (Figure S2G). These data indicate that signaling through innate immune sensing and response pathways promotes the m6A modification of RIOK3 mRNA following infection.
We next sought to define the signaling pathways that lead to reduced m6A modification of CIRBP mRNA. We and others have shown that Flaviviridae infection activates the ER stress response (Figure S1D) (Blazquez et al., 2014; Carletti et al., 2019; Chan, 2014; Neufeldt et al., 2018). To test whether ER stress alters the m6A modification of CIRBP or RIOK3, we measured their relative m6A levels following treatment of cells with thapsigargin (TG; Figure 2E), an ER Ca2+ ATPase inhibitor that induces an ER stress response (Lee et al., 2012). TG increased the mRNA level of both RIOK3 and CIRBP, and that of the positive controls HSPA5 and XBP1, by about 4-fold (Figure 2E). Further, TG reduced m6A modification of CIRBP, similar to what we observed with viral infection, while not changing the relative m6A level of RIOK3 (Figure 2E). Together, these data reveal that innate immune and ER stress signaling, both of which are activated during Flaviviridae infection, can divergently influence the m6A methylation program and can separately affect m6A modification of specific transcripts.
To define the mRNAs that have altered m6A in response to innate immune or ER stress signaling, we also performed MeRIP-seq analysis on mRNA from Huh7 cells treated with HCV PAMP or TG. Both of these treatments led to m6A peak changes in a subset of mRNAs (Figure S2H, Table S3). The m6A peaks detected in these data did not necessarily correspond to peaks called in the infection data (Table S2), likely because the reproducibility of individual MeRIP-seq peaks is low (McIntyre et al., 2019). Therefore, we calculated differences in m6A enrichment with HCV PAMP and TG at the 31,467 regions previously identified as m6A peaks in the infection data (|Log2FC > 1| and threshold of p<0.1). We observed five infection-altered peaks that were also changed by TG, including the CIRBP peak, and three infection-altered peaks also changed with HCV PAMP (Figure S2I). All of these changes were in the same direction as observed with infection. The infection-induced m6A peak in RIOK3 did show an increase in m6A enrichment at the same region with HCV PAMP but it was not statistically significant, perhaps because the m6A changes observed with HCV PAMP were smaller than those observed with infection (Figure 2C–D). These results reveal that innate immune and ER stress signaling drive a portion of the m6A changes we observed during Flaviviridae infection.
m6A modification enhances RIOK3 protein expression during infection
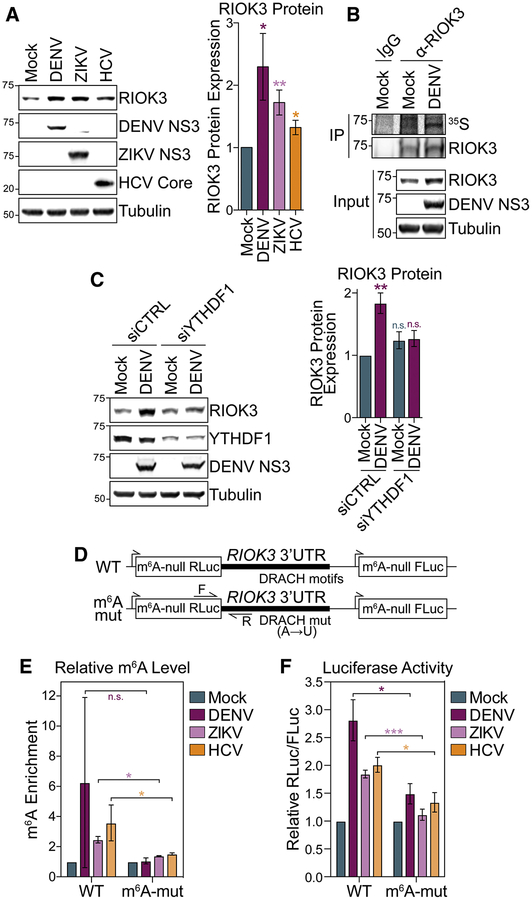
We next investigated the function of m6A in RIOK3 mRNA during infection. Consistent with our finding that DENV, ZIKV, and HCV infection all increased RIOK3 mRNA levels (Figure 2C), RIOK3 protein expression also increased following infection (Figure 3A). m6A can alter mRNA nuclear export, stability, and translation, all of which could regulate protein expression (Meyer and Jaffrey, 2017; Yang et al., 2018). We found no significant change in the nuclear export or mRNA stability of RIOK3 during infection (Figure S3A–B). However, we did detect increased nascent translation of RIOK3 in DENV-infected cells compared to uninfected cells as measured by 35S labeling of nascent proteins followed by RIOK3 protein immunoprecipitation, suggesting that RIOK3 translation was increased by infection (Figure 3B). This is consistent with our finding that during infection RIOK3 mRNA has increased binding to the m6A reader protein YTHDF1, which can promote translation of bound mRNAs under specific conditions (Figure S2D) (Han et al., 2019; Shi et al., 2018; Wang et al., 2019; Wang et al., 2015). To directly test whether YTHDF1 promotes RIOK3 translation, we measured RIOK3 protein levels following DENV infection in cells depleted of YTHDF1. We found that YTHDF1 depletion prevented the DENV-induced increase in RIOK3 protein expression (Figure 3C). RIOK3 translation increased during Flaviviridae infection, even though these viruses generally inhibit global cellular translation and induce the phosphorylation of the eukaryotic translation initiation factor eIF2a (Figure S3C) (Arnaud et al., 2010; Garaigorta and Chisari, 2009; Roth et al., 2017; Stern-Ginossar et al., 2019; Wek, 2018). Therefore, our results suggest that m6A modification of RIOK3 could allow this transcript to be efficiently translated during infection in a YTHDF1-dependent manner, despite global inhibition of translation.
Figure 3: m6A promotes RIOK3 protein expression.
(A) (Left) Representative immunoblot of RIOK3 protein expression in mock- and virus-infected (48 hpi) Huh7 cells. (Right) Quantification of RIOK3 protein expression relative to tubulin. (B) Immunoprecipitation (IP) of RIOK3 from mock- and DENV-infected (48 hpi) Huh7 cells labeled with 35S for 3 hours. IP fractions were analyzed by autoradiography (35S) and immunoblotting. Representative of 3 biological replicates. (C) (Left) Representative immunoblot of RIOK3 protein expression in mock- and DENV-infected (48 hpi) Huh7 cells treated with non-targeting control (CTRL) or YTHDF1 siRNA. (Right) Quantification of RIOK3 protein expression relative to tubulin. (D) Schematic of WT and mutant m6A-null Renilla luciferase (RLuc) RIOK3 3’ UTR reporters that also express m6A-null Firefly luciferase (FLuc) from a separate promoter. RT-qPCR primers (F and R) are indicated with arrows. (E) MeRIP-RT-qPCR analysis of relative m6A level of stably expressed WT and m6A-mut RIOK3 3’ UTR reporter RNA in mock- and virus-infected (48 hpi) Huh7 cells. (F) Relative luciferase activity (RLuc/FLuc) in mock- and virus-infected (48 hpi) Huh7 cells stably expressing WT and m6A-mut RIOK3 3’ UTR reporters. Relative luciferase activity in uninfected cells was set as 1 for each reporter. Values are the mean ± SEM of 6 (A), 4 (C), 2 (E), or 5 (F) biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired Student’s t test. n.s. = not significant. See also Figure S3.
To directly test whether m6A can promote RIOK3 protein expression during infection, we generated Huh7 cell lines stably expressing a luciferase reporter which contains the wild type (WT) RIOK3 3’ UTR, or an analogous 3’ UTR sequence in which all putative m6A sites were abrogated by A→T mutations (m6A-mut), downstream of a Renilla luciferase gene in which all DRACH motifs were ablated (m6A-null) (Figure 3D). These constructs also expressed a m6A-null Firefly luciferase gene whose expression is not regulated by m6A. The WT RIOK3 reporter had increased m6A modification compared to the m6A-mut RIOK3 reporter following viral infection, as measured by MeRIP-RT-qPCR using primers that specifically amplified reporter RNA (Figure 3E). Therefore, the RIOK3 3’ UTR sequence is sufficient for m6A addition following infection. Importantly, the relative luciferase activity of the WT RIOK3 reporter was significantly increased compared to the m6A-mut reporter following viral infection (Figure 3F). Taken together, these data reveal that m6A modification of the 3’ UTR of RIOK3 during infection promotes its translation during infection.
m6A modification promotes alternative splicing of CIRBP mRNA during infection
We then analyzed the function of reduced m6A modification in CIRBP mRNA following infection. Neither the nuclear export nor the stability of CIRBP mRNA were affected following infection, suggesting that the loss m6A in CIRBP does not regulate these processes (Figure S4A–B). Based on our RNA-seq data, CIRBP encodes at least 2 isoforms: (1) the dominant, short isoform (CIRBP-S) which encodes a 172 aa, 18 kDa protein and (2) a long isoform in which an intron immediately downstream of the infection-altered m6A peak and upstream of the stop codon is retained (CIRBP-L), resulting in a 297 aa, 32 kDa protein (Figure 4A; retained intron referred to as alternatively spliced region (ASR)). Interestingly, analysis of our RNA-seq data using MAJIQ (Vaquero-Garcia et al., 2016) to identify local splice variants suggested decreased retention of this intron during infection, which we confirmed in infected cells using RT-qPCR (Figure 4B). We observed a similar reduction of intron retention following TG treatment, which we had found also reduces CIRBP m6A modification (Figure 4C and 2F). Indeed, both viral infection and TG treatment significantly reduced the protein level of CIRBP-L containing the retained intron, without affecting expression of CIRBP-S (Figure 4D–E). To test whether reduction of m6A modification at the m6A peak in CIRBP might affect alternative splicing of this transcript, we generated a splicing reporter wherein the m6A-null Renilla luciferase gene was fused to the WT genomic sequence of CIRBP from exon 5 onwards (WT CIRBP) and a corresponding reporter in which the putative m6A sites in the identified CIRBP m6A peak were synonymously mutated (m6A-mut CIRBP) (Figure 4F). Using RT-qPCR, we found that the m6A-mut reporter had reduced intron retention compared to the WT reporter, revealing that the loss of m6A in CIRBP regulates its alternative splicing and reduces the expression of the long isoform (Figure 4G).
Figure 4: m6A promotes alternative splicing of CIRBP.
(A) Schematic of CIRBP transcript isoforms with a focus on the alternatively spliced region (ASR). RT-qPCR primer locations are indicated with arrows (FC-RC: control CIRBP amplicon; F-RL: long isoform specific; F-RS: short isoform specific. (B) RT-qPCR analysis of short (S) and long (L) CIRBP RNA isoforms in mock- and virus-infected (48 hpi) Huh7 cells relative to control CIRBP amplicon. (C) RT-qPCR analysis of S and L CIRBP RNA isoforms in mock- and TG-treated (16 h) Huh7 cells. (D) (Left) Representative immunoblot of short (CIRBP-S) and long (CIRBP-L) CIRBP protein isoforms in mock- and virus-infected (48 hpi) Huh7 cells. (Right) Quantification of CIRBP protein isoform expression relative to tubulin. (E) (Left) Representative immunoblot analysis of CIRBP protein isoforms in mock- and TG-treated (500nM, 16 h) Huh7 cells. HSPA5 and GADD34 are positive controls. (Right) Quantification of CIRBP protein isoform expression relative to tubulin. (F) Schematic of WT and m6A-mut CIRBP splicing reporters. RT-qPCR primer locations (Fluc-Rluc: control; F-RL: long isoform specific; F-Rs: short isoform specific) are indicated with arrows. (G) RT-qPCR analysis of CIRBP splicing reporter isoform expression (S and L) relative to control RLuc amplicon in Huh7 cells transfected with WT and m6A-mut constructs. Values are the mean ± SEM of 3 (B, D, E, G) or 5 (C) biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired Student’s t test. n.s. = not significant. See also Figure S4.
To understand the purpose of alternative isoform usage of CIRBP during infection, we measured the polysome occupancy of the two CIRBP isoforms in response to infection. As expected, due to the global translation suppression known to occur during DENV (Roth et al., 2017), the size of the 80S peak was increased and polysomal peaks were decreased in DENV-infected cells (Figure S4C). CIRBP-L was not found in heavy polysome fractions in either uninfected or DENV-infected cells, suggesting that this transcript is inefficiently translated (Figure S4D). In contrast, CIRBP-S was found in heavy polysome fractions, but this association was reduced during DENV infection (Figure S4D). This suggests that CIRBP-S has reduced translation during infection. Given that the protein expression of CIRBP-S is not significantly reduced during infection (Figure 4D), reducing the expression of the inefficiently translated CIRBP-L isoform may represent a mechanism to ensure consistent production of CIRBP protein during viral infection.
m6A-altered genes regulate Flaviviridae infection
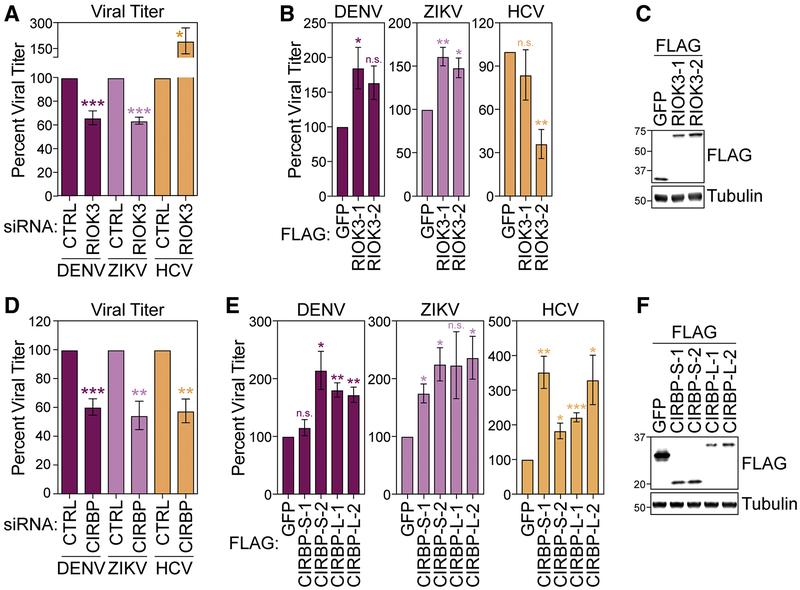
Having found that both RIOK3 and CIRBP transcripts have altered m6A modification during infection, we tested whether their encoded protein products affect Flaviviridae infection. We depleted RIOK3 and CIRBP in Huh7 cells, infected these cells with DENV, ZIKV, or HCV, and then measured viral titer in the supernatant. siRNA treatment reduced both RIOK3 and CIRBP mRNA levels by ~70% and did not affect cell viability (Figure S5A–B). We found that RIOK3 depletion significantly reduced the production of infectious DENV and ZIKV particles but increased the production of infectious HCV particles (Figure 5A). Consistent with these data, RIOK3 stably overexpressed in two different clonal cell lines had the opposite effect on DENV, ZIKV, and HCV infectious particle production (Figure 5B–C). This suggests that RIOK3 promotes DENV and ZIKV infection but inhibits HCV infection. However, the depletion of both the large and small isoforms of CIRBP, as well as only the large isoform of CIRBP, reduced the production of infectious DENV, ZIKV, and HCV (Figure 5D and S5C–D), while overexpression of both the short and long isoforms of CIRBP in two different clonal cell lines each increased infection by these viruses (Figure 5E–F). This suggests that both CIRBP isoforms are proviral during DENV, ZIKV, and HCV infection. Interestingly, CIRBP-S protein resides primarily in the nucleus, while CIRBP-L is predominantly cytoplasmic, irrespective of viral infection, which implicates distinct spatial regulation of proviral activity by CIRBP isoforms (Figure S5E).
Figure 5: RIOK3 and CIRBP regulate Flaviviridae infection.
(A) Focus-forming assay (FFA) of supernatants from DENV, ZIKV, or HCV-infected (72 hpi) Huh7 cells treated with non-targeting control (CTRL) or RIOK3 siRNA. (B) FFA of supernatants from DENV, ZIKV, or HCV-infected (72 hpi) Huh7 cells stably overexpressing FLAG-GFP or FLAG-RIOK3 (2 independent clones). (C) Immunoblot analysis of cell lines in (B). (D) FFA of supernatants harvested from DENV, ZIKV, or HCV-infected (72 hpi) Huh7 treated with CTRL or CIRBP siRNA. (E) FFA of supernatants from DENV, ZIKV, or HCV-infected (72 hpi) Huh7 cells stably overexpressing FLAG-GFP or the short (FLAG-CIRBP-S) or long (FLAG-CIRBP-L) isoforms of CIRBP (2 independent clones). (F) Immunoblot analysis of cell lines in (C). Values are the mean ± SEM of 4 (A and D), or 3 (B, E, G) biological replicates. Viral infections were performed at a multiplicity of infection of 0.2. * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired Student’s t test. n.s. = not significant. See also Figure S5.
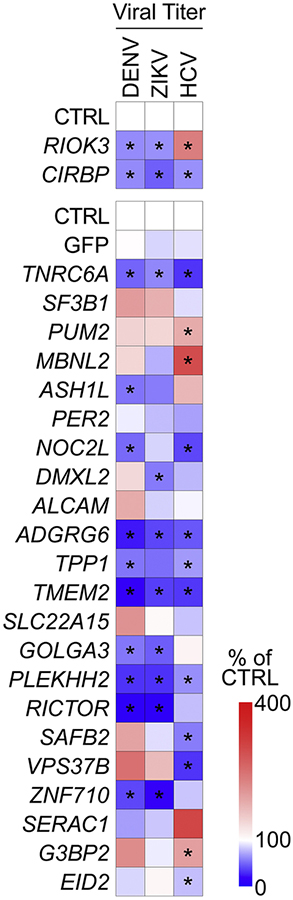
We then performed a targeted siRNA screen to test whether other transcripts with infection-altered m6A modification affect Flaviviridae infection. We depleted transcripts in which we had identified m6A changes during infection (Figure 1F and S1I)), infected these cells with DENV, ZIKV, or HCV, and measured cell viability, relative RNA depletion levels, and the production of infectious virions in the supernatant (Figure 6 and S6A–C). We focused only on those transcripts that were depleted by at least 40% in our further analysis (21 out of 24 tested). For these, we found that 18/21 (86%) regulate at least 1 virus, while 10/21 (48%) affect at least 2, and 6/21 (29%) regulate all three viruses. For each virus, ~50% of m6A-altered transcripts that we tested significantly increased or decreased infection. This indicates that m6A can, as a general principle, tune the outcome of infection by modifying specific transcripts that regulate infection.
Figure 6: Genes with infection-induced m6A alterations regulate Flaviviridae infection.
Heatmap of viral titers of supernatants harvested from DENV, ZIKV, or HCV-infected cells (48 hpi) treated with the indicated siRNAs. Data are presented as percentage of titer of each virus relative to cells treated with CTRL siRNA. Colors represent the mean of 3 biological replicates. Viral infections were performed at a multiplicity of infection of 0.2. * p < 0.05 by unpaired Student’s t test. See also Figure S6.
Discussion
Here, we identify changes in m6A methylation of cellular mRNAs during infection by viruses in the Flaviviridae family. We observed that infection by DENV, ZIKV, WNV, and HCV leads to changes in m6A of a specific set of cellular transcripts, including some that encode factors that modulate Flaviviridae infection. We found that virus-induced pathways, including innate immune signaling and ER stress signaling, contribute to altered m6A of several of these transcripts. Taken together, this work suggests that m6A changes induced through cellular signaling pathways influence Flaviviridae infection.
We identified hundreds of m6A-modified transcripts that were differentially expressed during infection or that were annotated as part of cellular pathways relevant for infection. These findings suggest that m6A has the potential to post-transcriptionally regulate many genes during infection. Here, we focused on specific transcripts with virus-induced m6A changes; we identified 58 peak changes in 51 transcripts following infection by DENV, ZIKV, WNV, and HCV. As our m6A change analysis pipeline controls for changes in gene expression (McIntyre et al., 2019), these data should represent true changes in m6A modification rather than changes in the expression of m6A-modified transcripts. While changes in both m6A modification and the expression of m6A-modified transcripts are biologically relevant, identifying bona fide m6A alterations during viral infection will allow us to understand how m6A modification of cellular mRNA is regulated.
We found that the changes in m6A methylation of RIOK3, CIRBP, and several other transcripts are driven by innate immune induction and the cellular response to ER stress, respectively. This suggests that these signals, and likely other infection-induced pathways, can be integrated into differential m6A methylation activity and ultimately affect m6A modification of cellular mRNAs. While expression changes in m6A machinery affect m6A modification during cancer and infection (Barbieri et al., 2017; Li et al., 2017b; Lin et al., 2016; Rubio et al., 2018; Vu et al., 2017; Winkler et al., 2019), this machinery did not change expression with Flaviviridae infection, pointing to a different mechanism for altered m6A modification. Going forward, identifying the molecular mechanisms through which these signaling pathways lead to differential m6A will be an important advance in understanding how the m6A machinery acts on specific sites.
Our data suggest that virus-induced m6A changes occur in nascent mRNA, which supports the hypothesis that m6A is added co-transcriptionally and does not dynamically change after export to the cytoplasm (Ke et al., 2017). At least three processes could modulate the selective m6A modification of specific transcripts during transcription. First, novel interactions of the m6A writers METTL3 and METTL14 with viral-induced or stress-regulated RNA-binding proteins could target these writers to specific mRNAs and lead to m6A changes during infection. For example, RBM15/15B and VIRMA can target the m6A methyltransferase complex to Xist long non-coding RNA or to the 3’ UTRs of mRNA, respectively (Patil et al., 2016; Yue et al., 2018). Second, the writers could be recruited to nascent mRNAs by the histone modification H3K36me3 which marks transcriptionally active loci and recruits METTL14 (Huang et al., 2019). Intriguingly, in HepG2 cells, the CIRBP locus is marked by H3K36me3 and its transcript contains an m6A peak at the same site that we identified in Huh7 cells (Huang et al., 2019). This suggests that infection- or ER stress-induced depletion of H3K36me3 marks at the CIRBP locus could result in reduced m6A of CIRBP by METTL3 and METTL14. Third, changes in transcription rates, which have been inversely correlated with m6A deposition in mRNA, could also contribute to m6A modification of specific transcripts during infection (Slobodin et al., 2017). Further, viral infection can affect RNA structure in cellular transcripts; it is possible that altered mRNA structure could result in divergent m6A modification of cellular transcripts during infection (Mizrahi et al., 2018). Perturbing cellular homeostasis by infection therefore has the potential to reveal new insights into the regulation of m6A modification of cellular transcripts.
We hypothesize that during viral infection, m6A regulation of RNA metabolism leads to rapid, tunable changes in mRNA and protein abundance of host factors. While m6A can affect mRNA nuclear export and stability, Flaviviridae infection did not affect these processes for either RIOK3 or CIRBP mRNA. Instead, we found that m6A changes promote translation of RIOK3 and alternative splicing of CIRBP. m6A promotes translation of modified mRNAs in multiple contexts by mediating interactions with m6A-binding proteins including YTHDF1 (Edupuganti et al., 2017; Han et al., 2019; Huang et al., 2018; Li et al., 2017a; Lin et al., 2016; Meyer et al., 2015; Shi et al., 2017; Shi et al., 2018; Wang et al., 2019; Wang et al., 2015). Similarly, the interaction of YTHDF1 with RIOK3 mRNA during infection promoted RIOK3 translation even in the context of eIF2α phosphorylation and suppression of global translation (Arnaud et al., 2010; Garaigorta and Chisari, 2009; Roth et al., 2017). For CIRBP, the loss of m6A following viral infection led to reduced expression of its long isoform. m6A regulates splicing by modulating mRNA interactions with several m6A-binding splicing factors, which suggests that the loss of m6A in CIRBP regulates alternative splicing through changes in its interactions with splicing factors (Alarcon et al., 2015; Liu et al., 2015; Liu et al., 2017b; Louloupi et al., 2018; Xiao et al., 2016; Ye et al., 2017; Zhao et al., 2014; Zhou et al., 2019). Interestingly, CIRBP-L is not translated as efficiently as CIRBP-S; therefore, reducing the relative abundance of the long isoform might be an expeditious mechanism to maintain abundant CIRBP protein levels during cellular stress. How m6A regulates the fate of other mRNAs with altered modification is still unknown, but it is possible that m6A post-transcriptionally affects the abundance of their protein products or splicing isoforms, similar to how it regulates RIOK3 and CIRBP.
RIOK3 promoted DENV and ZIKV infection, but inhibited HCV. Interestingly, RIOK3 can both positively and negatively regulate innate immune responses, by either stimulating the interaction between TBK1 and IRF3 or by phosphorylating and inactivating MDA5 (Feng et al., 2014; Shan et al., 2009; Takashima et al., 2015; Willemsen et al., 2017). The differences in the effects of RIOK3 on DENV, ZIKV, and HCV infection could reflect the different strategies used by these viruses to inhibit host immune responses (Chen et al., 2017; Gack and Diamond, 2016; Gokhale et al., 2014). Further, Willemsen et al. found that while RIOK3 enhanced innate immune activation, it also promoted influenza A virus infection, implying that RIOK3 could have roles in infection beyond innate immunity (Willemsen et al., 2017).
Both CIRBP isoforms were proviral for DENV, ZIKV, and HCV. The biological functions of the individual CIRBP isoforms, which we found have different subcellular localizations, remain unknown. CIRBP can modulate the translation of pro-inflammatory factors and have anti-apoptotic effects in response to various stresses (Liao et al., 2017). During infection, reduction in the long isoform of CIRBP through loss of m6A could inhibit infection, suggesting that this loss of m6A during infection is part of the host response to infection. Alternatively, reduction of the poorly translated long isoform of CIRBP mRNA may be a normal part of the cellular stress response to ensure robust production of CIRBP protein, which can then be coopted by Flaviviridae members to facilitate their replication.
Overall, transcripts with altered m6A modification during Flaviviridae infection encoded proteins that influenced the outcome of infection. For each virus, approximately half of the factors tested showed either proviral or antiviral effects, while 86% affected the titer of at least one virus. These data suggest that m6A itself does not represent a simple proviral or antiviral mechanism during infection, but rather distinctly modulates specific transcripts that ultimately affect the outcome of infection by different members of the Flaviviridae family.
The scale of m6A epitranscriptomic changes with virus infection varies greatly among previous reports (Hesser et al., 2018; Lichinchi et al., 2016a; Rubio et al., 2018; Tan et al., 2018; Winkler et al., 2019). Although we identified altered m6A in 58 peaks in 51 transcripts during infection, inherent variance in transcript coverage in MeRIP-seq data means that many replicates are necessary for statistically significant detection of m6A changes (McIntyre et al., 2019). In particular, this means that our analysis (n=3 per virus), may underestimate the total number of virus-specific, altered m6A peaks. Additionally, we used a more conservative statistical approach than many previous studies to reveal only the most robust peak changes (McIntyre et al., 2019). The changes detected in MeRIP-seq peaks were validated using MeRIP-RT-qPCR; however, these data do not provide the precise ratio of modified to unmodified copies of a transcript or the exact nucleotides that are modified. Biochemical assays like SCARLET or new sequencing methods will be necessary to resolve this question (Liu et al., 2019a; Saletore et al., 2012).
In summary, we found that Flaviviridae infection leads to m6A changes in transcripts that can influence viral infection. We identified innate immune activation and the ER stress response as signals that can modulate m6A levels in specific cellular mRNAs. Our work indicates that post-transcriptional regulation of specific transcripts by m6A and other RNA modifications can be an important determinant of the outcome of infection. Indeed, viral infection alters the abundance of several other epitranscriptomic modifications on cellular RNA (McIntyre et al., 2018), revealing that we are only beginning to understand how RNA modifications affect viral infection.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stacy M. Horner (stacy.horner@duke.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture
Huh7 and Huh-7.5 cells (gift of Dr. Michael Gale Jr., University of Washington (Sumpter et al., 2005)), Huh7 IRF3 KO cells (Vazquez et al., 2019), 293T cells (ATCC: CRL-3216) Vero cells (ATCC: CCL-81), C6/36 (ATCC: CRL-1660) were grown in Dulbecco’s modification of Eagle’s medium (DMEM; Mediatech) supplemented with 10% fetal bovine serum (HyClone), 25 mM HEPES (Thermo Fisher), and 1X non-essential amino acids (Thermo Fisher), referred to as complete DMEM (cDMEM). Huh7 and Huh-7.5 cells were verified using the Promega GenePrint STR kit (DNA Analysis Facility, Duke University), and cells were verified as mycoplasma free by the LookOut Mycoplasma PCR detection kit (Sigma-Aldrich).
Viruses
Infectious stocks of a cell culture-adapted strain of genotype 2A JFH1 HCV were generated and titered in Huh-7.5 cells by focus-forming assay (FFA), as described (Aligeti et al., 2015). DENV2-NGC (Sessions et al., 2009), ZIKV-PR2015 (Quicke et al., 2016), and WNV-NY2000 (Diamond et al., 2003) stocks were prepared in C6/36 insect cells and titered in Vero cells, as described. For viral infections, cells were incubated in a low volume of cDMEM containing virus at a multiplicity of infection (MOI) of 1 for 2–3 hours (except when otherwise stated), following which cDMEM was replenished. Cells were infected for 48 hours unless otherwise described. To quantify virus, cellular supernatants were analyzed by FFA.
METHOD DETAILS
MeRIP-seq
Huh7 cells seeded in 15 cm plates were infected with DENV, ZIKV, WNV, or HCV (MOI 1) or left uninfected (mock-infected). At 48 hours post-infection, total RNA was extracted using TRIzol (Thermo Fisher) and treated with TURBO DNase I (Thermo Fisher). mRNA was purified from 200 μg total RNA from each sample using the Dynabeads mRNA purification kit (Thermo Fisher) and concentrated by ethanol precipitation. mRNA was fragmented using the RNA Fragmentation Reagent (Thermo Fisher) for 15 minutes and purified by ethanol precipitation. MeRIP was performed using EpiMark N6-methyladenosine Enrichment kit (NEB) according to the manufacturer’s recommendations with the following modifications. Briefly, 25 μL Protein G Dynabeads (Thermo Fisher) per sample were washed three times in MeRIP buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.1% NP-40), and incubated with 1 μL anti-m6A antibody for 2 hours at 4°C with rotation. After washing three times with MeRIP buffer, anti-m6A conjugated beads were incubated with purified mRNA with rotation at 4°C overnight in 300 μL MeRIP buffer with 1 μL RNase inhibitor (recombinant RNasin; Promega). 10% of the mRNA sample was saved as the input fraction. Beads were then washed twice with 500 μL MeRIP buffer, twice with low salt wash buffer (50 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.1% NP-40), twice with high salt wash buffer (500 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.1% NP-40), and once again with MeRIP buffer. m6A-modified RNA was eluted twice in 100 μL of MeRIP buffer containing 5 mM m6A salt (Santa Cruz Biotechnology) for 30 minutes at 4°C wi th rotation. Eluates were pooled and concentrated by ethanol purification. RNA-seq libraries were prepared from both eluate and 10% input mRNA using the TruSeq mRNA library prep kit (Illumina), subjected to quality control (MultiQC), and sequenced on the HiSeq 4000 instrument.
MeRIP-RT-qPCR
For MeRIP-RT-qPCR, total RNA was harvested from uninfected and infected Huh7 and Huh7 IRF3 KO cells seeded in 10 cm plates or 6-well plates at 48 hours post-infection. For ER-stress induction, cells seeded in 6-well plates were treated with 500 nM thapsigargin (Tocris) for 16 hours. For interferon treatment, cells seeded in 6-well plates were incubated with 100 U/mL human IFN-β (PBL Assay Science) for 24 hours. HCV PAMP was prepared by in vitro transcription, as described (Beachboard et al., 2019; Saito et al., 2008). 2.5 μg of HCV PAMP RNA was transfected into cells seeded in 6-well plates for 8 hours using the Mirus mRNA transfection kit. At the indicated time points for each experiment, RNA was extracted and MeRIP-RT-qPCR was performed like MeRIP-seq with some differences. Specifically, total RNA was prepared from cells using TRIzol, and diluted to equivalent concentrations. Then, 20–50 μg total RNA was fragmented for 3 minutes, purified by ethanol precipitation, and resuspended in 30 μL water. 0.1 fmol of positive control (m6A-modified Gaussia luciferase RNA) and negative control (unmodified Cypridina luciferase RNA) spike-ins supplied with the EpiMark N6-methyladenosine Enrichment kit were added to each sample. Following MeRIP as described above, eluates were concentrated by ethanol precipitation. 1 μL input and the entire IP fractions were reverse transcribed using the iScript cDNA synthesis kit (BioRad) and subjected to RT-qPCR. Primer sequences are supplied in Table S4. Relative m6A level for each transcript was calculated as the percent of input in each condition normalized to that of the respective positive control spike-in. Fold change of enrichment was calculated with mock samples normalized to 1.
RT-qPCR
The iScript cDNA synthesis kit (Bio-Rad) was used for reverse transcription of total RNA samples. RT-qPCR was performed using the Applied Biosystems QuantStudio 6 Flex real-time PCR instrument. To measure relative abundance of CIRBP isoforms, total RNA was reverse transcribed with the Superscript III enzyme (Invitrogen) using a gene specific primer. RT-qPCR was performed using specific primers that detect CIRBP isoforms. The expression of each isoform was normalized to invariant region of CIRBP. Primer sequences are provided in Table S4.
Immunoblotting
Cell lysates were prepared in a modified RIPA buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 0.5% sodium deoxycholate, and 1% Triton X-100) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail II (Millipore), and clarified by centrifugation. Protein concentration was determined by Bradford assay (Bio-Rad). 5–15 μg of protein was resolved by SDS/PAGE and transferred to nitrocellulose membranes using the Trans-Blot Turbo System (Bio-Rad). Membranes were blocked in 5% milk in phosphate buffered saline with 0.1% Tween (PBS-T) and incubated with the relevant primary antibodies. After washing three times with PBS-T, membranes were incubated with species-specific horseradish peroxidase-conjugated antibodies (Jackson ImmunoResearch, 1:5000) or fluorescent antibodies (LI-COR, IRDye 800, 1:5000). Chemiluminescence (Clarity ECL, Bio-Rad) or fluorescence was detected on a LI-COR Odyssey Fc instrument and analyzed using the ImageStudio software. The following antibodies were used for immunoblot: anti-METTL3 (Novus Biologicals, 1:1000), anti-METTL14 (Sigma-Aldrich, 1:5000), anti-FTO (Abcam, 1:1000), anti-YTHDF1 (Proteintech, 1:1000), anti-YTHDF2 (Proteintech, 1:1000), anti-YTHDF3 (Sigma-Aldrich, 1:1000), anti-ALKBH5 (Sigma-Aldrich, 1:1000), anti-WTAP (Proteintech, 1:1000) anti-FLAG M2 (Sigma-Aldrich, 1:5000), anti-tubulin (Sigma-Aldrich, 1:5000), anti-HCV NS5A (clone 9E10, gift of Charles Rice, Rockefeller University (Lindenbach et al., 2005), 1:1000), anti-RIOK3 (Proteintech, 1:1000), anti-CIRBP (Proteintech 1:1000), anti-DENV NS3 (GeneTex, 1:1000), anti-ZIKV NS3 (GeneTex, 1:1000), anti-HCV NS4A (Genscript custom (Horner et al., 2011)), 1:1000), anti-eIF2α (Cell Signaling, 1:1000), anti-phospho-eIF2α (Cell Signaling, 1:1000), anti-GADD34 (Proteintech, 1:1000), anti-HSPA5 (Cell Signaling, 1:1000), anti-H2A.X (Cell Signaling, 1:1000), anti-U170K serum (gift of Dr. Jack Keene, Duke University, (Query and Keene, 1987), 1:1000)
FLAG-YTHDF RNA immunoprecipitation
Generation of Huh7 cells stably expressing FLAG-GFP or FLAG-YTHDF1 was described previously (Gokhale et al., 2016). Cells seeded in 6-well plates were infected with DENV, ZIKV, or HCV (MOI 1). At 48 hours post-infection cells were harvested by trypsinization and lysed in polysome lysis buffer (100 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7.0], 0.5% NP-40), supplemented with protease inhibitor cocktail (Sigma-Aldrich) and RNase inhibitor (RNasin), and cleared by centrifugation. Protein was quantified by Bradford assay, and 200 μg ribonucleoprotein complexes were immunoprecipitated with M2 anti-FLAG conjugated magnetic beads (Sigma-Aldrich) overnight at 4°C with rotatio n in NT2 buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40). Beads were washed five times in ice-cold NT2 buffer. Protein for immunoblotting was eluted from ten percent of beads by boiling in 2X Laemmli sample buffer (Bio-Rad). RNA was extracted from ninety percent of beads using TRIzol reagent (Thermo Fisher). Equal volumes of eluted RNA were used for cDNA synthesis, quantified by RT-qPCR, and normalized to RNA levels in input samples. Fold enrichment was calculated with FLAG-GFP and mock samples set as 1.
siRNA treatment and viral infectivity assays
Cells seeded in 24-well plates were transfected with siRNA against intended targets (Qiagen, sequences provided in Table S4) using Lipofectamine RNAiMAX (Thermo Fisher) according to the manufacturer’s recommendation. At 24 hours post-transfection, cells were infected with DENV, ZIKV, and HCV (MOI 0.2). At 48 (targeted siRNA screen) or 72 (RIOK3 and CIRBP depletion) hours post-infection, virus titer in the supernatant was measured by FFA. Serial dilutions of supernatants were used to infect naïve Vero (DENV and ZIKV) or Huh-7.5 (HCV) cells in triplicate wells of a 48-well plate. At 72 hours post-infection, cells were fixed in cold 1:1 methanol:acetone and immunostained with 4G2 antibody purified in the lab from a hybridoma (for DENV and ZIKV, 1:2000), or anti-HCV NS5A (1:2000). Following binding of horseradish peroxidase conjugated secondary antibody (1:1000; Jackson ImmunoResearch), infected foci were visualized with the VIP Peroxidase Substrate Kit (Vector Laboratories) and counted at 40X magnification. Titer was calculated using the following formula: (dilution factor × number of foci × 1000) / volume of infection (μl), resulting in units of focus forming units / mL (FFU/mL). Depletion of siRNA targets was confirmed by RT-qPCR (primer sequences in Table S4). Cellular viability after siRNA treatment was measured by the Cell-Titer Glo assay (Promega) according to the manufacturer’s recommendation.
For testing the effect of YTHDF1 on RIOK3 translation, cells plated in 6-well plates were transfected with siRNAs against YTHDF1 (Qiagen, Table S4) at 24 and 48 hours following seeding. 24 hours after the second round of transfection, cells were infected DENV, and lysates were harvested at 48 hours post-transfection and subjected to immunoblotting.
Quantification of infection by immunofluorescence
To measure percent of cells infected following viral infection, Huh7 cells seeded in 96-well plates were infected with DENV, ZIKV, WNV, or HCV (MOI 1). Cells were fixed in cold 1:1 methanol:acetone at the indicated hours post-infection, and immunostained with 4G2 antibody (DENV, ZIKV, WNV) or anti-HCV NS5A. Following binding of AlexaFluor 488-conjugated secondary antibody (Thermo Fisher) and nuclear staining with Hoechst (Thermo Fisher), cells were imaged using the Cellomics Arrayscan VTI robotic microscope at the Duke Functional Genomics Core Facility. The percentage of infected cells was determined by measuring cells stained for viral antigen relative to the total number of nuclei.
Immunofluorescence assay for CIRBP localization
Huh7 cells stably expressing FLAG-tagged CIRBP-S and CIRBP-L were plated in 4-well chamber slides (Millipore) and infected with the indicated virus (MOI 1). At 48 hours post-infection, cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton-X 100 (Sigma-Aldrich), and immunostained with anti-FLAG (Sigma-Aldrich, 1:1000) antibody, or antibody against viral antigens (4G2 for DENV and ZIKV (1:1000); anti-NS5A (1:1000) for HCV). Following treatment with AlexaFluor dye-conjugated secondary antibodies (Thermo Fisher) and the nuclear stain Hoescht, coverslips were mounted with ProLong Gold (Thermo Fisher) and imaged on a Leica DM4 B fluorescence microscope using a 63X objective. Images were processed with the Fiji software (Schindelin et al., 2012).
Cell fractionation
Fractionation of cells to isolate chromatin-associated RNA was performed as described (Ke et al., 2017). Briefly, cells were collected from 10 cm plates by trypsinization, lysed in 200 μL cytoplasmic lysis buffer (10 mM Tris-HCL [pH 7.4], 150 mM NaCl, 0.15% NP-40) on ice for 5 minutes, and passed through 500 μl 24% sucrose cushion by centrifugation at 12000 xG for 10 minutes at 4°C. The supernatant (cytoplasmic fraction) was then removed and the nuclear pellet was rinsed twice with cold phosphate buffered saline (PBS). The nuclear pellet was resuspended in 100 μL ice cold glycerol buffer (20 mM Tris-HCL [pH 7.4], 75 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 125 μM PMSF, 50% glycerol). 100 μL nuclear lysis buffer (10 mM HEPES [pH 7.4], 1 mM DTT, 7.5 mM MgCl2, 0.2 mM EDTA, 300 mM NaCl, 1 M urea, 1% NP-40) was added to the suspension, followed by brief vortexing, and incubation on ice for 2 minutes. Samples were centrifuged for 2 minutes at 4°C at 12 000 xG and the supernatant (nuclear fraction) was removed. The chromatin pellet was rinsed twice with cold PBS, resuspended in 50 μL DNase I buffer with 2 U Turbo DNase I (Invitrogen), and incubated at 37°C for 30 minutes. RNA was then extracted from the chromatin fraction using TRIzol reagent and subjected to MeRIP-RT-qPCR. The cytoplasmic, nuclear, and chromatin fractions were subjected to immunoblotting to analyze fractionation.
For nuclear/cytoplasmic fractionation to investigate mRNA export, uninfected and infected (MOI 1) cells grown in 10 cm plates were harvested by trypsinization and lysed in 200 μL lysis buffer (10mM Tris-HCl [pH 7.4], 140 mM NaCl, 1.5 mM MgCl2, 10 mM EDTA, 0.5% NP-40) on ice for 5 minutes. Following centrifugation at 12000 xG at 4°C for 5 minutes, the supernatant (cytoplasmic fraction) was collected, and the nuclear pellet was rinsed twice with lysis buffer. RNA was extracted from cytoplasmic and nuclear pellets using TRIzol reagent and analyzed by RT-qPCR.
Measurement of RNA stability
Cells plated in 24-well plates were infected with the indicated virus (MOI 1). At 36 hours post-infection, media was changed to cDMEM containing 1 μM Actinomycin D (Sigma-Aldrich). RNA was extracted from cells at the indicated time points post-treatment using TRIzol reagent and analyzed by RT-qPCR. Data were normalized as the percent of RNA remaining at each time point after treatment, relative to that at the time of treatment.
Polysome profiling
Mock- and DENV-infected (MOI 1) Huh7 cells plated in 10 cm plates were harvested by trypsinization at 48 hours post infection following a 10 min pulse with cycloheximide (0.2 mM; Sigma-Aldrich) and were lysed in cytoplasmic lysis buffer (200 mM KCl, 25 mM HEPES pH 7.0, 10 mM MgCl2, 2% n-Dodecyl β-D-maltoside (DDM; Chem-Impex), 0.2 mM cycloheximide (Sigma-Aldrich), 1 mM DTT, 40 U RNaseIn) for 15 mins on ice. Following clarification, lysates were ultracentrifuged on 15–50% sucrose gradients prepared in polysome gradient buffer (200 mM KCl, 25 mM HEPES pH 7.0, 15 mM MgCl2, 1 mM DTT, 0.2 mM cycloheximide) at 35,000 xG for 3.5 hours at 4 C. Following ultracentrifugation, 16 fractions were collected from each sample using a BioComp Piston Gradient Fractionator instrument fitted with a TRIAX flow cell to measure absorbance. RNA was extracted from each fraction using TRIzol LS reagent (Thermo Fisher), and RNA quality was checked on a 1% agarose gel. Following cDNA synthesis using the iScript cDNA synthesis kit, RT-qPCR was performed using primers specific for the long and short isoforms of CIRBP.
RIOK3 and CIRBP cloning and stable cell lines
All primer sequences used for cloning are provided in Table S4. RIOK3 (NM_003831.4), as well as both long (NM_001300829) and short (NM_001280) isoforms of CIRBP, were cloned by PCR (HiFi PCR premix, Clontech) from cDNA from Huh7 cells prepared with the Superscript III RT kit (Thermo Fisher) using the oligo(dT)20 primer. PCR products were inserted into pLEX-FLAG lentiviral vector between the NotI and XhoI sites using the InFusion HD cloning kit (Takara Bio) to generate constructs with N-terminal FLAG tags. Lentivirus was produced from 293T cells transfected with pLEX vectors and packaging plasmids psPAX2 and pMD2.G (provided by Duke Functional Genomics Facility). Huh7 cells were transduced by these lentiviruses and stable cell lines expressing FLAG-RIOK3, FLAG-CIRBP-S, and FLAG-CIRBP-L were selected using puromycin (2 μg/mL; Sigma-Aldrich). Single cell clones were obtained by serial dilution and verified by immunoblotting. Cell lines were maintained in cDMEM containing 1 μg/mL puromycin.
Reporter cloning and luciferase assays
All primer and gBlock sequences are provided in Table S4. To generate m6A-null RIOK3 reporters, the Renilla and Firefly luciferase genes in psiCheck2 plasmid (Promega) were first replaced by constructs with synonymous mutations in putative m6A sites (obtained as IDT gBlocks). The wild type RIOK3 3’ UTR was cloned from Huh7 cDNA (NM_003831.4) and inserted after the m6A-null Renilla luciferase gene in the multiple cloning site of psiCheck2 between XhoI and NotI using the InFusion HD kit. m6A-mut RIOK3 3’ UTR (in which all putative m6A sites were mutated from A to T) was obtained as a gBlock and also inserted between these restriction sites. WT and m6A-mut RIOK3 reporter plasmids along with the pcDNA-Blast plasmid (Kennedy et al., 2015) were linearized using BamHI and BglII respectively, purified by ethanol precipitation and co-transfected into Huh7 cells in 6-well plates (90 ng reporter, 10 ng pcDNA-Blast) using FuGENE 6 transfection reagent (Promega). Cells were selected with blasticidin (0.2 μg/mL; Thermo Fisher) and single cell clones stably expressing WT and m6A-mut reporters were isolated. For MeRIP-RT-qPCR of reporter RNA, WT and m6A-mut expressing cells were plated in 6-well plates, infected with the indicated virus (MOI 1), and RNA was extracted using TRIzol at 48 hours post-infection. Following MeRIP as described, RT-qPCR was performed to discriminate reporter RNA using a forward primer within the Renilla luciferase gene and a reverse primer in the RIOK3 3’ UTR. For luciferase assays, WT and m6A-mut expressing cells in 24-well plates were infected with the indicated virus (MOI 1) and dual luciferase assay (Promega) was performed at 48 hours post-infection according to the manufacturer’s instructions. Data was normalized as the value of Renilla luminescence divided by Firefly luminescence, and values for mock-infected cells were set as 1.
To generate CIRBP splicing reporters, CIRBP exon 5 – 3’ UTR (Hg38;chr19:127553–1273172) was amplified by PCR from genomic DNA. A fragment of m6A-null Renilla luciferase beyond the NruI site and up to the stop codon was amplified by PCR with overlapping ends with Renilla luciferase (5’; before the NruI site) and the CIRBP fragment (3’). These fragments were inserted into NruI-XhoI digested psiCheck2 m6A-null plasmid using the InFusion HD kit. m6A-mut CIRBP reporter was generated by mutating the essential C in the m6A site synonymously to T using two rounds of site-directed mutagenesis with the QuikChange Lightning kit (Agilent).
35S pulse-labeled immunoprecipitation
Huh7 cells seeded in 10 cm plates were infected with DENV (MOI 1) or left uninfected. At 45 hours post-infection, media was removed and 3 mL warm methionine/cysteine-free DMEM was added to plates. After 15 minutes of incubation, 3 mL methionine/cysteine-free DMEM containing 100 mCi 35S (Perkin Elmer) was added. Cells were harvested at 3 hours post-treatment and lysed in RIPA buffer. 300 μg protein was incubated with 4 μg anti-RIOK3 antibody (Proteintech) or normal rabbit IgG (Cell Signaling) in 300 μL RIPA buffer overnight at 4°C with rotation. Antibody-protein complexes were then incubated with 40 μL pre-washed protein G Dynabeads (Thermo Fisher) for 2 hours. Protein was eluted from beads in 2X Laemmli buffer. Eluates were resolved by SDS/PAGE. Gels were fixed in solution containing 50% methanol and 10% acetic acid, dried, and subjected to autoradiography on film.
LC-MS/MS for m6A/A determination
mRNA was purified from 200 μg total RNA extracted from uninfected and infected Huh7 cells (MOI 1, 48 hours post-infection) using one round of polyA selection (Dynabeads mRNA purification kit; Thermo Fisher) and one round of rRNA depletion (NEBNext rRNA depletion kit, NEB). After ethanol precipitation, purified mRNA was digested into mononucleotides with nuclease P1 (Sigma-Aldrich, 2 U) in buffer containing 25 mM NaCl and 2.5 mM ZnCl2 for 2 hours at 37 C, followed by incubation with Antarctic Phosphatase (NEB, 5 U) for an additional 2 hours at 37 C. Nucleosides were separated and quantified using UPLC-MS/MS as previously described, except acetic acid was used in place of formic acid (Basanta-Sanchez et al., 2016).
QUANTIFICATION AND STATISTICAL ANALYSIS
Western blot images were acquired and analyzed using Licor Image Studio. Microscopy pictures were processed in Fiji. Figure panels were processed and organized using Adobe Illustrator CC. RT-qPCR and MeRIP-RT-qPCR data was analyzed using Microsoft Excel. Graphpad Prism 8 was used to generate graphs, to determine the mean, standard deviation or standard error, and to perform statistical analyses, as described in the figure legends.
Data analysis for MeRIP-seq and RNA-seq
Reads were aligned using STAR (Dobin et al., 2013) to the human reference genome (hg38), combined with the appropriate virus genome for each infected sample. Differential gene expression between infected and uninfected samples was compared using DESeq2 (Love et al., 2014). UpSet plots of the intersects between genes regulated with individual viruses were generated using UpSetR (Conway et al., 2017). Gene ontology for RNA-seq changes in Figure S1D was analyzed using gProfiler, with redundant GO terms collapsed using REVIGO (Reimand et al., 2016; Supek et al., 2011). For gProfiler, upregulated genes with Log2FC ≥ 2 and adjusted p-value < 0.05 with all viruses were considered. There were very few consistently downregulated genes at Log2FC ≤ −2 (particularly for ZIKV), so we expanded our set to genes with smaller Log2FC ≤−0.5, downregulated by DENV, HCV, and WNV infection. For REVIGO, we allowed similarity of up to 0.5, with semantic similarity calculated using SimRel. Adjusted p-values were provided for the REVIGO calculations. Gene set enrichment analyses using fgsea in R showed similar differentially regulated pathways as gProfiler (Sergushichev, 2016). “Infection-annotated” genes and peaks were summarized for Figure 1B based on gene inclusion in “Infectious disease”, “Unfolded Protein Response (UPR)”, “Interferon Signaling”, and “Innate Immune System” Reactome pathways from fgsea.
We called m6A peaks from MeRIP-seq using MACS2 (Zhang et al., 2008) and used all peaks detected in at least two replicates for further analysis. Motif enrichment was calculated using HOMER for Figure 1C (Heinz et al., 2010). Metagene plots for methylated DRACH motifs were plotted using a custom script. DRACH motifs were considered methylated if detected under m6A peaks in at least 2 biological replicates. Relative positions of m6A peaks within genes are based on the transcripts with the highest mean coverage per gene, as calculated with kallisto (Bray et al., 2016).
We identified m6A peaks changes using a generalized linear model (adapted from (Park et al., 2014)), and the QNB program (Liu et al., 2017a). In brief (see Park et al., 2014 or McIntyre et al., 2019 for more details), a generalized linear model following the equation
was fit with the following parameters for each peak i and sample j: XIP = 1 for immunoprecipitated samples and 0 for input samples, and XVIR = 1 for infected samples and 0 for mock. A library size parameter was included for normalization (N) with edgeR (Robinson et al., 2010). The full model was compared to a reduced model without the infection:IP interaction term using a likelihood ratio test of the difference between deviances, implemented through DESeq2 (Love et al., 2014) or edgeR. To control for changes in gene expression, changes in gene expression were subtracted from changes in IP peak reads for significantly modified peaks from DESeq2, edgeR, and QNB, with a threshold for absolute difference in Log2 fold change of ≥ 1. Significant peaks were further filtered for location within exons, DRACH motif content, and mean input read counts of ≥ 10 to produce the final set of 58 peak changes.
Peaks of interest were plotted for visual evaluation using CovFuzze (https://github.com/al-mcintyre/CovFuzze) (Imam et al., 2018).
DATA AND CODE AVAILABILITY
The raw data from MeRIP-seq analysis of uninfected and infected Huh7 cells have been deposited and are available through GEO (accession numbers: GSE130891 and GSE138730).
Supplementary Material
Table S1. Gene expression changes with Flaviviridae infection, Related to Figure 1.
Table S3. m6A peaks and peak changes with HCV PAMP and TG treatment, Related to Figure 2.
Table S4. List of oligonucleotides and siRNAs used in this study, Related to STAR Methods.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-METTL3 | Novus Biologicals | Cat# H00056339-B01P; RRID:AB_2687437 |
| Anti-METTL14 | Sigma-Aldrich | Cat# HPA038002; RRID:AB_10672401 |
| Anti-WTAP | Proteintech | Cat# 60188–1-Ig; RRID:AB_10859484 |
| Anti-FTO | Abcam | Cat# ab92821; RRID:AB_10565042 |
| Anti-ALKBH5 | Sigma-Aldrich | Cat# HPA007196; RRID:AB_1850461 |
| Anti-YTHDF1 | Proteintech | Cat# 17479–1-AP; RRID:AB_2217473 |
| Anti-YTHDF2 | Proteintech | Cat# 24744–1-AP; RRID:AB_2687435 |
| Anti-YTHDF3 | Sigma-Aldrich | Cat# SAB2102736; RRID:AB_10599885 |
| Anti-FLAG | Sigma-Aldrich | Cat# F7425; RRID:AB_439687 |
| Anti-FLAG-HRP conjugated | Sigma-Aldrich | Cat# A8592; RRID:AB_439702 |
| Anti-Tubulin | Sigma-Aldrich | Cat# T5168; RRID:AB_477579 |
| Anti-DENV NS3 | GeneTex | Cat# GT2811; RRID:AB_2538763 |
| Anti-ZIKV NS3 | GeneTex | Cat# GTX133320 |
| Anti-HCV NS4A | Genscript custom antibody (Horner et al., 2011) | N/A |
| Anti-DENV/ZIKV E (4G2) | Made in lab from hybridoma | ATCC Cat# HB-112; RRID:CVCL_J890 |
| Anti-HCV NS5A | 9E10, gift from Dr. Charles Rice (Lindenbach et al., 2005) | N/A |
| Anti-RIOK3 | Proteintech | Cat# 13593–1-AP; RRID:AB_2178105 |
| Anti-CIRBP | Proteintech | Cat# 10209–2-AP; RRID:AB_2080263 |
| Anti-eIF2α | Cell Signaling Tech. | Cat# 9722; RRID:AB_2230924 |
| Anti-Phospho-eIF2α | Cell Signaling Tech. | Cat# 3398; RRID:AB_2096481 |
| Anti-HSPA5 | Cell Signaling Tech. | Cat# 3177; RRID:AB_2119845 |
| Anti-GADD34 | Proteintech | Cat# 10449–1-AP; RRID:AB_2168724 |
| Anti-H2A.X | Cell Signaling Tech. | Cat# 9718; RRID:AB_2118009 |
| Anti-U170K serum | Gift of Dr. Jack Keene (Query et al., 1987) | N/A |
| Normal rabbit IgG | Cell Signaling Tech. | Cat# 2729; RRID:AB_1031062 |
| Anti-mouse HRP Secondary | Jackson ImmunoResearch | Cat# 115-035-003; RRID:AB_10015289 |
| Anti-rabbit HRP Secondary | Jackson ImmunoResearch | Cat# 111-035-003; RRID:AB_2313567 |
| Anti-mouse IRDye 800 | LI-COR Biosciences | Cat# 926–32212; RRID:AB_621847 |
| Anti-rabbit IRDye 800 | LI-COR Biosciences | Cat# 926–32211; RRID:AB_621843 |
| Anti-mouse AlexaFluor 488 | Thermo Fisher Sci. | Cat# A11001; RRID:AB_2534069 |
| Bacterial and Virus Strains | ||
| Dengue virus (DENV; New Guinea C) | Sessions et al., 2009 | N/A |
| Zika virus (ZIKV, Puerto Rico 2015, PRVABC59) | Quicke et al., 2016 | N/A |
| West Nile virus (WNV; New York-2000) | Diamond et al., 2003 | N/A |
| Hepatitis C virus (HCV; JFH-1 strain, culture adapted) | Aligeti et al., 2015 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Thapsigargin | Tocris | Cat# 1138; CAS: 67526-95-8 |
| N6-methyladenosine 5’ monophosphate salt | Santa Cruz Biotech. | Cat# sc-215524; CAS: 81921-35-9 |
| Human IFN-β | PBL Assay Science | Cat# 11415–1 |
| TRIzol | Thermo Fisher Sci. | Cat# 15596026 |
| TRIzol LS | Thermo Fisher Sci. | Cat# 10296010 |
| NP-40 | Thermo Fisher Sci. | Cat# 85124 |
| n-Dodecyl-β-D-maltoside (DDM) | Chem-Impex | Cat# 21950 |
| Puromycin | Sigma-Aldrich | Cat# P8833 |
| Cycloheximide | Sigma-Aldrich | Cat# 7698 |
| Blasticidin | Thermo Fisher Sci. | Cat# R21001 |
| Recombinant RNaseIN RNase inhibitor | Promega | Cat# N2511 |
| Protease inhibitor | Sigma-Aldrich | Cat# P8340 |
| Phosphatase inhibitor | Thermo Fisher Sci. | Cat# 78426 |
| NotI-HF | New England Biolabs | Cat# R3189 |
| PmeI | New England Biolabs | Cat# R0560 |
| XhoI | New England Biolabs | Cat# R0146 |
| NruI-HF | New England Biolabs | Cat# R3192 |
| BamHI-HF | New England Biolabs | Cat# R3136 |
| BglII | New England Biolabs | Cat# R0144 |
| Hoescht 33342 | Thermo Fisher Sci. | Cat# 62249 |
| Actinomycin D | Sigma-Aldrich | Cat# A9415 |
| 2X Laemmli sample buffer | Bio-Rad | Cat# 161–0737 |
| Nuclease P1 | Sigma-Aldrich | Cat# N8630 |
| Antarctic phosphatase | New England Biolabs | Cat# M0289 |
| Protein G Dynabeads | Thermo Fisher Sci. | Cat# 10004D |
| FLAG M2 conjugated beads | Sigma-Aldrich | Cat# M8823; RRID: RRID:AB_2637089 |
| 35S | PerkinElmer | Cat# NEG772007MC |
| Opti-MEM I reduced serum medium | Thermo Fisher Sci. | Cat# 31985070 |
| Methionine/cysteine-free DMEM | Sigma-Aldrich | Cat# D0422 |
| Critical Commercial Assays | ||
| N6-methyladenosine enrichment kit | New England Biolabs | Cat# E1610S |
| Dynabeads mRNA purification kit | Thermo Fisher Sci. | Cat# 61006 |
| NEBNext rRNA depletion kit | New England Biolabs | Cat# E6310S |
| Power SYBR Green PCR master mix | Thermo Fisher Sci. | Cat# 4367659 |
| Dual luciferase reporter assay system | Promega | Cat# E1960 |
| CellTiter-Glo luminescent cell viability assay | Promega | Cat# G7571 |
| Protein assay dye-reagent concentrate | Bio-Rad | Cat# 5000006 |
| iScript cDNA synthesis kit | Bio-Rad | Cat# 1708891BUN |
| Superscript III enzyme | Thermo Fisher Sci. | Cat# 18080044 |
| InFusion HD cloning kit | Takara Bio | Cat# 639650 |
| Quik-change Lightning SDM kit | Agilent | Cat# 210518 |
| RNA fragmentation reagent | Thermo Fisher Sci | Cat# AM8740 |
| Trans-IT mRNA transfection reagent | Mirus | Cat# MIR2225 |
| FuGENE 6 transfection reagent | Promega | Cat# E2691 |
| Lipofectamine RNAiMAX transfection reagent | Thermo Fisher Sci. | Cat# 13778150 |
| CloneAmp HiFi PCR premix | Clontech | Cat# 639298 |
| VIP peroxidase substrate kit | Vector Laboratories | Cat# SK-4600 |
| TURBO DNase | Thermo Fisher Sci | Cat# AM2239 |
| Deposited Data | ||
| MeRIP-seq of mRNA from DENV, ZIKV, WNV, and HCV infected (MOI 1, 48 h) and uninfected Huh7 cells | This study | GEO: GSE130891 |
| MeRIP-seq of mRNA from HCV PAMP treated (8 h), TG treated (16 h) and untreated Huh7 cells | This study | GEO: pending |
| Experimental Models: Cell Lines | ||
| Huh7 | Gift of Dr. Michael Dale, Jr. (Sumpter et al., 2005) | RRID: RRID:CVCL_0336 |
| Huh7.5 | Gift of Dr. Michael Dale, Jr. (Sumpter et al., 2005) | RRID: RRID:CVCL_7927 |
| 293T | ATCC | ATCC Cat# CRL-3216; RRID:CVCL_0063 |
| Vero | ATCC | ATCC Cat# CCL-81; RRID:CVCL_0059 |
| C6/36 | ATCC | ATCC Cat# CRL-1660; RRID:CVCL_Z230 |
| Huh7 IRF3 KO | Vazquez et al., 2019 | N/A |
| Huh7 FLAG-GFP | Gokhale et al., 2016 | N/A |
| Huh7 FLAG-YTHDF1 | Gokhale et al., 2016 | N/A |
| Huh7 FLAG-RIOK3–1 | This study | N/A |
| Huh7 FLAG-RIOK3–2 | This study | N/A |
| Huh7 FLAG-CIRBP-S-1 | This study | N/A |
| Huh7 FLAG-CIRBP-S-2 | This study | N/A |
| Huh7 FLAG-CIRBP-L-1 | This study | N/A |
| Huh7 FLAG-CIRBP-L-2 | This study | N/A |
| Huh7 m6A-null RLuc – RIOK3 3’UTR WT | This study | N/A |
| Huh7 m6A-null RLuc – RIOK3 3’UTR m6A-mut | This study | N/A |
| Oligonucleotides | ||
| Oligonucleotides for RT-qPCR | Table S4 | N/A |
| Oligonucleotides and gBocks for Cloning | Table S4 | N/A |
| Oligonucleotides for siRNA | Table S4 | N/A |
| Recombinant DNA | ||
| pLEX-RIOK3 | This study | N/A |
| pLEX-CIRBP-S | This study | N/A |
| pLEX-CIRBP-L | This study | N/A |
| psiCheck2 m6A-null RIOK3–3’UTR WT | This study | N/A |
| psiCheck2 m6A-null RIOK3–3’UTR m6A-mut | This study | N/A |
| psiCheck2 m6A-null RLuc-CIRBP-splicing WT | This study | N/A |
| psiCheck2 m6A-null RLuc-CIRBP-splicing m6A-mut | This study | N/A |
| pcDNA-Blast | (Kennedy et al., 2015) | N/A |
| psPAX2 | Duke Functional Genomics Core Facility | Addgene plasmid # 12260; RRID:Addgene_12260 |
| pMD2.G | Duke Functional Genomics Core Facility | Addgene Plasmid #12259; RRID:Addgene_12259 |
| Software and Algorithms | ||
| ImageStudio | LI-COR Biosciences | RRID:SCR_013715; http://www.licor.com/bio/products/software/image_studio_lite |
| Fiji | (Schindelin et al., 2012) | RRID:SCR_002285 https://fiji.sc |
| Prism 8.0 | Graphpad | RRID:SCR_002798; http://www.graphpad.com |
| STAR | (Dobin et al., 2013) | v2.5.0a, https://github.com/alexdobin/STAR |
| MACS2 | (Zhang et al., 2008) | v2.1.1.20160309, https://github.com/taoliu/MACS |
| DESeq2 | (Love et al., 2014) | v1.20.0, https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| edgeR | (Robinson et al., 2010) | v3.22.3, https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| QNB | (Liu et al., 2017a) | v1.1.11, https://cran.r-project.org/src/contrib/Archive/QNB/ |
| CovFuzze | (Imam et al., 2018) | v0.1.3, https://github.com/al-mcintyre/CovFuzze |
| gProfiler | (Reimand et al., 2016) | ve95_eg42_p13_f6e58b9, https://biit.cs.ut.ee/gprofiler/gost |
| REVIGO | (Supek et al., 2011) | http://revigo.irb.hr/ |
| HOMER | (Heinz et al., 2010) | v4.9.1, http://homer.ucsd.edu/homer/motif/ |
| fgsea | (Sergushichev, 2016) | v1.8.0, https://bioconductor.org/packages/release/bioc/html/fgsea.html |
| UpSetR | (Conway et al., 2017) | v1.3.3, https://CRAN.R-project.org/package=UpSetR |
Highlights.
Flaviviridae infection alters m6A modification of specific cellular mRNAs.
Innate immune and ER stress signaling contribute to altered m6A modification.
Gain of m6A regulates RIOK3 translation and loss of m6A influences CIRBP splicing.
m6A-altered mRNAs encode factors that affect Flaviviridae infection.
Acknowledgements
We thank Moonhee Park, Xinhe Yin, Dr. Olga Ilkayeva, Dr. Christopher Nicchitta, and Dr. Heather Vincent for experimental help and advice, colleagues who provided reagents (see Methods), NEB for gift of anti-m6A antibodies, the Metabolomics Core at the Duke Molecular Physiology Institute, the Duke Functional Genomics Core Facility, the Epigenomics Core and the Scientific Computing Unit at Weill Cornell, and Dr. Kate Meyer and Horner lab members for manuscript discussion. This work was supported by funds from Burroughs Wellcome Fund (S.M.H.) and National Institutes of Health: R01AI125416, R21AI129851 (S.M.H. and C.E.M.), 5P30AI064518 (S.M.H.), R01MH117406 (C.E.M.), and R03HL135475 (C.L.H). Other funding sources include: American Heart Association (N.S.G. Pre-doctoral Fellowship, 17PRE33670017), National Science and Engineering Research Council of Canada (A.B.R.M. PGS-D funding), Bert L. and N. Kuggie Vallee Foundation, WorldQuant Foundation, Pershing Square Sohn Cancer Research Alliance, NASA (NNX14AH50G), and startup funds from the UNC Lineberger Comprehensive Cancer Center (H.M.L. and M.D.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
C.E.M. is a cofounder and board member for Biotia and Onegevity Health, and an advisor or compensated speaker for Abbvie, Acuamark Diagnostics, ArcBio, Bio-Rad, DNA Genotek, Genialis, Genpro, Illumina, NEB, QIAGEN, Whole Biome, and Zymo Research.
Supplemental Information
Supplemental information Figures S1-S6.
References
- Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, and Tavazoie SF (2015). HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligeti M, Roder A, and Horner SM (2015). Cooperation between the Hepatitis C Virus p7 and NS5B Proteins Enhances Virion Infectivity. J Virol 89, 11523–11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud N, Dabo S, Maillard P, Budkowska A, Kalliampakou KI, Mavromara P, Garcin D, Hugon J, Gatignol A, Akazawa D, et al. (2010). Hepatitis C virus controls interferon production through PKR activation. PLoS One 5, e10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, et al. (2017). Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta-Sanchez M, Temple S, Ansari SA, D’Amico A, and Agris PF (2016). Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Res 44, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachboard DC, Park M, Vijayan M, Snider DL, Fernando DJ, Williams GD, Stanley S, McFadden MJ, and Horner SM (2019). The small GTPase RAB1B promotes antiviral innate immunity by interacting with TNF receptor-associated factor 3 (TRAF3). J Biol Chem 294, 14231–14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez AB, Escribano-Romero E, Merino-Ramos T, Saiz JC, and Martin-Acebes MA (2014). Stress responses in flavivirus-infected cells: activation of unfolded protein response and autophagy. Front Microbiol 5, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. [DOI] [PubMed] [Google Scholar]
- Carletti T, Zakaria MK, Faoro V, Reale L, Kazungu Y, Licastro D, and Marcello A (2019). Viral priming of cell intrinsic innate antiviral signaling by the unfolded protein response. Nat Commun 10, 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW (2014). Unfolded protein response in hepatitis C virus infection. Front Microbiol 5, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu Z, Wang M, and Cheng A (2017). Innate Immune Evasion Mediated by Flaviviridae Non-Structural Proteins. Viruses 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JR, Lex A, and Gehlenborg N (2017). UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney DG, Kennedy EM, Dumm RE, Bogerd HP, Tsai K, Heaton NS, and Cullen BR (2017). Epitranscriptomic Enhancement of Influenza A Virus Gene Expression and Replication. Cell Host Microbe 22, 377–386 e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Zhang L, Meng J, Rao MK, Chen Y, and Huang Y (2018). MeTDiff: A Novel Differential RNA Methylation Analysis for MeRIP-Seq Data. IEEE/ACM Trans Comput Biol Bioinform 15, 526–534. [DOI] [PubMed] [Google Scholar]
- De Maio FA, Risso G, Iglesias NG, Shah P, Pozzi B, Gebhard LG, Mammi P, Mancini E, Yanovsky MJ, Andino R, et al. (2016). The Dengue Virus NS5 Protein Intrudes in the Cellular Spliceosome and Modulates Splicing. PLoS Pathog 12, e1005841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Marri A, Mahan D, and Engle M (2003). B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 77, 2578–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, Wang SY, Baltissen MPA, Jansen P, Rossa M, et al. (2017). N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24, 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M, Eggert C, Kaplick PM, Eder M, Roh S, Tietze L, Namendorf C, Arloth J, Weber P, Rex-Haffner M, et al. (2018). The Role of m(6)A/m-RNA Methylation in Stress Response Regulation. Neuron 99, 389–403 e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, De Jesus PD, Su V, Han S, Gong D, Wu NC, Tian Y, Li X, Wu TT, Chanda SK, et al. (2014). RIOK3 is an adaptor protein required for IRF3-mediated antiviral type I interferon production. J Virol 88, 7987–7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J, Gu F, Ling L, Tolfvenstam T, Olfat F, Chin KC, Aw P, George J, Kuznetsov VA, Schreiber M, et al. (2007). Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis 1, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, and Diamond MS (2016). Innate immune escape by Dengue and West Nile viruses. Curr Opin Virol 20, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaigorta U, and Chisari FV (2009). Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe 6, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert WV, Bell TA, and Schaening C (2016). Messenger RNA modifications: Form, distribution, and function. Science 352, 1408–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale NS, and Horner SM (2017). RNA modifications go viral. PLoS Pathog 13, e1006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale NS, McIntyre AB, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, et al. (2016). N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe 20, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale NS, Vazquez C, and Horner SM (2014). Hepatitis C Virus. Strategies to Evade Antiviral Responses. Future Virol 9, 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-van Horn SR, and Sarnow P (2017). Making the Mark: The Role of Adenosine Modifications in the Life Cycle of RNA Viruses. Cell Host Microbe 21, 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Liu J, Chen C, Dong L, Liu Y, Chang R, Huang X, Liu Y, Wang J, Dougherty U, et al. (2019). Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Hao S, Chen H, Chen Z, Zhang Y, Wang J, Wang H, Zhang B, Qiu J, Deng F, et al. (2019). N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res 47, 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesser CR, Karijolich J, Dominissini D, He C, and Glaunsinger BA (2018). N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog 14, e1006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook MR (2017). Historical Perspectives on Flavivirus Research. Viruses 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner SM, and Gale M Jr. (2013). Regulation of hepatic innate immunity by hepatitis C virus. Nat Med 19, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner SM, Liu HM, Park HS, Briley J, and Gale M Jr. (2011). Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A 108, 14590–14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. (2018). Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Zhou K, Wu T, Zhao BS, Sun M, Chen Z, Deng X, Xiao G, Auer F, et al. (2019). Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567, 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam H, Khan M, Gokhale NS, McIntyre ABR, Kim GW, Jang JY, Kim SJ, Mason CE, Horner SM, and Siddiqui A (2018). N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc Natl Acad Sci U S A 115, 8829–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, Geula S, Hanna JH, Black DL, Darnell JE Jr., and Darnell RB (2017). m(6)A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31, 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EM, Bogerd HP, Kornepati AV, Kang D, Ghoshal D, Marshall JB, Poling BC, Tsai K, Gokhale NS, Horner SM, et al. (2016). Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host Microbe 19, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EM, Whisnant AW, Kornepati AV, Marshall JB, Bogerd HP, and Cullen BR (2015). Production of functional small interfering RNAs by an amino-terminal deletion mutant of human Dicer. Proc Natl Acad Sci U S A 112, E6945–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Belcaid M, and Nerurkar VR (2016). Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci Rep 6, 26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Mendez R, Heng HH, Yang ZQ, and Zhang K (2012). Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am J Transl Res 4, 102–113. [PMC free article] [PubMed] [Google Scholar]
- Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. (2017a). Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. (2017b). FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell 31, 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tong L, Tang L, and Wu S (2017). The role of cold-inducible RNA binding protein in cell stress response. Int J Cancer 141, 2164–2173. [DOI] [PubMed] [Google Scholar]
- Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, Mason CE, and Rana TM (2016a). Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nature microbiology 1, 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, and Rana TM (2016b). Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Choe J, Du P, Triboulet R, and Gregory RI (2016). The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 62, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, et al. (2005). Complete replication of hepatitis C virus in cell culture. Science 309, 623–626. [DOI] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, and Jaffrey SR (2015). Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Begik O, Lucas MC, Ramirez JM, Mason CE, Wiener D, Schwartz S, Mattick JS, Smith MA, and Novoa EM (2019a). Accurate detection of m(6)A RNA modifications in native RNA sequences. Nat Commun 10, 4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang SW, Huang Y, and Meng J (2017a). QNB: differential RNA methylation analysis for count-based small-sample sequencing data with a quad-negative binomial model. BMC Bioinformatics 18, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Dai Q, Zheng G, He C, Parisien M, and Pan T (2015). N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, and Pan T (2017b). N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45, 6051–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, You Y, Lu Z, Yang J, Li P, Liu L, Xu H, Niu Y, and Cao X (2019b). N (6)-methyladenosine RNA modification-mediated cellular metabolism rewiring inhibits viral replication. Science 365, 1171–1176. [DOI] [PubMed] [Google Scholar]
- Louloupi A, Ntini E, Conrad T, and Orom UAV (2018). Transient N-6-Methyladenosine Transcriptome Sequencing Reveals a Regulatory Role of m6A in Splicing Efficiency. Cell Rep 23, 3429–3437. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna JM, Scheel TK, Danino T, Shaw KS, Mele A, Fak JJ, Nishiuchi E, Takacs CN, Catanese MT, de Jong YP, et al. (2015). Hepatitis C virus RNA functionally sequesters miR-122. Cell 160, 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, and Jaffrey SR (2018). FTO, m(6) Am, and the hypothesis of reversible epitranscriptomic mRNA modifications. FEBS Lett 592, 2012–2022. [DOI] [PubMed] [Google Scholar]
- McIntyre ABR, Gokhale NS, Cerchietti L, Jaffrey SR, Horner SM, and Mason CE (2019). Limits in the detection of m6A changes using MeRIP/m6A-seq. BiorXiv, doi: 10.1101/657130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre W, Netzband R, Bonenfant G, Biegel JM, Miller C, Fuchs G, Henderson E, Arra M, Canki M, Fabris D, et al. (2018). Positive-sense RNA viruses reveal the complexity and dynamics of the cellular and viral epitranscriptomes during infection. Nucleic Acids Res 46, 5776–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, and Jaffrey SR (2017). Rethinking m(6)A Readers, Writers, and Erasers. Annu Rev Cell Dev Biol 33, 319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, and Jaffrey SR (2015). 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 163, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, and Jaffrey SR (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi O, Nachshon A, Shitrit A, Gelbart IA, Dobesova M, Brenner S, Kahana C, and Stern-Ginossar N (2018). Virus-Induced Changes in mRNA Secondary Structure Uncover cis-Regulatory Elements that Directly Control Gene Expression. Mol Cell 72, 862–874 e865. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan JL, and Fredericksen BL (2010). How flaviviruses activate and suppress the interferon response. Viruses 2, 676–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeldt CJ, Cortese M, Acosta EG, and Bartenschlager R (2018). Rewiring cellular networks by members of the Flaviviridae family. Nat Rev Microbiol 16, 125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Deering RP, Lu Y, Tivnan P, Lianoglou S, Al-Shahrour F, Ebert BL, Hacohen N, Leslie C, Daley GQ, et al. (2014). Musashi-2 controls cell fate, lineage bias, and TGF-beta signaling in HSCs. J Exp Med 211, 71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, and Jaffrey SR (2016). m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query CC, and Keene JD (1987). A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell 51, 211–220. [DOI] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. (2016). Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 20, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, and Vilo J (2016). g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res 44, W83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg BR, Depla M, Freije CA, Gaucher D, Mazouz S, Boisvert M, Bedard N, Bruneau J, Rice CM, and Shoukry NH (2018). Longitudinal transcriptomic characterization of the immune response to acute hepatitis C virus infection in patients with spontaneous viral clearance. PLoS Pathog 14, e1007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H, Magg V, Uch F, Mutz P, Klein P, Haneke K, Lohmann V, Bartenschlager R, Fackler OT, Locker N, et al. (2017). Flavivirus Infection Uncouples Translation Suppression from Cellular Stress Responses. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio RM, Depledge DP, Bianco C, Thompson L, and Mohr I (2018). RNA m(6) A modification enzymes shape innate responses to DNA by regulating interferon beta. Genes Dev 32, 1472–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Owen DM, Jiang F, Marcotrigiano J, and Gale M Jr. (2008). Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletore Y, Meyer K, Korlach J, Vilfan ID, Jaffrey S, and Mason CE (2012). The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol 13, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. (2014). Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep 8, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerk J, Jarret AP, Joslyn RC, and Savan R (2015). Landscape of post-transcriptional gene regulation during hepatitis C virus infection. Curr Opin Virol 12, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergushichev AA (2016). An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. BiorXiv, doi: 10.1101/060012. [DOI] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, et al. (2009). Discovery of insect and human dengue virus host factors. Nature 458, 1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions OM, Tan Y, Goh KC, Liu Y, Tan P, Rozen S, and Ooi EE (2013). Host cell transcriptome profile during wild-type and attenuated dengue virus infection. PLoS Negl Trop Dis 7, e2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Wang P, Zhou J, Wu D, Shi H, and Huo K (2009). RIOK3 interacts with caspase-10 and negatively regulates the NF-kappaB signaling pathway. Mol Cell Biochem 332, 113–120. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, and He C (2017). YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wei J, and He C (2019). Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol Cell 74, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zhang X, Weng YL, Lu Z, Liu Y, Lu Z, Li J, Hao P, Zhang Y, Zhang F, et al. (2018). m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodin B, Han R, Calderone V, Vrielink J, Loayza-Puch F, Elkon R, and Agami R (2017). Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell 169, 326–337 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Ginossar N, Thompson SR, Mathews MB, and Mohr I (2019). Translational Control in Virus-Infected Cells. Cold Spring Harb Perspect Biol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, et al. (2002). Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A 99, 15669–15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter R Jr., Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, and Gale M Jr. (2005). Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79, 2689–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Bosnjak M, Skunca N, and Smuc T (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6, e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Aguirre S, and Fernandez-Sesma A (2013). Innate immune sensing of flaviviruses. PLoS Pathog 9, e1003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima K, Oshiumi H, Takaki H, Matsumoto M, and Seya T (2015). RIOK3-mediated phosphorylation of MDA5 interferes with its assembly and attenuates the innate immune response. Cell Rep 11, 192–200. [DOI] [PubMed] [Google Scholar]
- Tan B, Liu H, Zhang S, da Silva SR, Zhang L, Meng J, Cui X, Yuan H, Sorel O, Zhang SW, et al. (2018). Viral and cellular N(6)-methyladenosine and N(6),2’-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nature microbiology 3, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift AP, El-Serag HB, and Kanwal F (2017). Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol 14, 122–132. [DOI] [PubMed] [Google Scholar]
- Tirumuru N, Zhao BS, Lu W, Lu Z, He C, and Wu L (2016). N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K, Courtney DG, and Cullen BR (2018). Addition of m6A to SV40 late mRNAs enhances viral structural gene expression and replication. PLoS Pathog 14, e1006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero-Garcia J, Barrera A, Gazzara MR, Gonzalez-Vallinas J, Lahens NF, Hogenesch JB, Lynch KW, and Barash Y (2016). A new view of transcriptome complexity and regulation through the lens of local splicing variations. Elife 5, e11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez C, Tan CY, and Horner SM (2019). Hepatitis C virus infection is inhibited by a non-canonical antiviral signaling pathway targeted by NS3-NS4A. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. (2017). The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu X, Huang M, Liu J, Gu Y, Ma L, Zhou Q, and Cao X (2019). Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun 10, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, and He C (2015). N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC (2018). Role of eIF2alpha Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen J, Wicht O, Wolanski JC, Baur N, Bastian S, Haas DA, Matula P, Knapp B, Meyniel-Schicklin L, Wang C, et al. (2017). Phosphorylation-Dependent Feedback Inhibition of RIG-I by DAPK1 Identified by Kinome-wide siRNA Screening. Mol Cell 65, 403–415 e408. [DOI] [PubMed] [Google Scholar]
- Winkler R, Gillis E, Lasman L, Safra M, Geula S, Soyris C, Nachshon A, Tai-Schmiedel J, Friedman N, Le-Trilling VTK, et al. (2019). m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol 20, 173–182. [DOI] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. (2016). Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell 61, 507–519. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, and Yang YG (2018). Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res 28, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen ER, and Nilsen TW (2017). Kaposi’s Sarcoma-Associated Herpesvirus Utilizes and Manipulates RNA N(6)-Adenosine Methylation To Promote Lytic Replication. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, et al. (2018). VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini F, Pu SY, Bekerman E, Einav S, and Quake SR (2018). Single-cell transcriptional dynamics of flavivirus infection. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. (2014). FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24, 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM, Dolan K, Zhu X, Hubert N, Tao Y, et al. (2018). Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m(6)A mRNA Methylation. Cell Rep 25, 1816–1828 e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Shu XE, Mao Y, Liu XM, Yuan X, Zhang X, Hess ME, Bruning JC, and Qian SB (2018). N(6)-Methyladenosine Guides mRNA Alternative Translation during Integrated Stress Response. Mol Cell 69, 636–647 e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, He C, Parisien M, and Pan T (2019). Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the Low-Complexity Protein hnRNPG. Mol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gene expression changes with Flaviviridae infection, Related to Figure 1.
Table S3. m6A peaks and peak changes with HCV PAMP and TG treatment, Related to Figure 2.
Table S4. List of oligonucleotides and siRNAs used in this study, Related to STAR Methods.
Data Availability Statement
The raw data from MeRIP-seq analysis of uninfected and infected Huh7 cells have been deposited and are available through GEO (accession numbers: GSE130891 and GSE138730).