Abstract
It is estimated that in 2017 there were 451 million people with diabetes worldwide. These figures are expected to increase to 693 million by 2045; thus, innovative preventative programs and treatments are a necessity to fight this escalating pandemic disorder. Caveolin-1 (CAV1), an integral membrane protein, is the principal component of caveolae in membranes and is involved in multiple cellular functions such as endocytosis, cholesterol homeostasis, signal transduction, and mechanoprotection. Previous studies demonstrated that CAV1 is critical for insulin receptor-mediated signaling, insulin secretion, and potentially the development of insulin resistance. Here, we summarize the recent progress on the role of CAV1 in diabetes and diabetic complications.
1. Introduction
Caveolae are specialized, bulb-shaped, 50-100 nm wide cholesterol-rich lipid rafts found in the plasma membrane of most cell types [1]. They were once thought to be simple membrane structures but are now considered to be a more complex bona fide organelle. Caveolar density differs with cell type; caveolae are abundant in adipocytes, vascular endothelial cells, smooth muscle cells, fibroblasts, and epithelial cells. In adipocytes, caveolae constitute one-third of the membrane area [2]. Caveolae increase significantly the cellular surface area and are implicated in several essential cellular functions such as endocytosis, transcytosis, maintenance of plasma membrane integrity, lipid homeostasis, signal transduction, and mechanoprotection [3]. Despite caveolae's diverse functions, their physiological role in different cell types is not fully understood.
Caveolae are mostly made up of caveolins (1/2/3); a ∼22 kDa protein termed caveolin-1 (CAV1) oligomerizes in the endoplasmic reticulum (∼14–16 monomers form a ∼350 kDa oligomer) [4]. At the Golgi body, CAV1 oligomers interact with cholesterol molecules and then the newly formed complexes are transported to the cellular membrane where additional cytosolic adaptor proteins named avins contribute to caveolar formation. CAV1 mediates the recruitment of Cavin proteins (CAVIN1/2/3/4) to the caveolae [5–7]. Each caveola has around 140-150 CAV1 molecules [8]. Caveolar vesicles are also highly organized and enriched in saturated phospholipids, sphingolipids, plasmenylethalomines, and cholesterol [9]. Apart from caveolae, oligomerized CAV1 scaffolds exist in the plasma membrane and partake in CAV1-dependent signal regulation as well [10]. CAV1 molecules reside not only in the cell membrane but also in other membranous structures such as mitochondria and secretory vesicles (e.g., insulin granules) [11]. Caveolins interact with various signaling proteins via their N-termini termed the caveolin-scaffolding domain (CSD, 82-101 aa) [12]. Transport of caveolae-mediated vesicles (non-clathrin-mediated) follows predominantly the transcytotic route to transport solutes from the blood to underlying tissues [5]. Src kinases mediate CAV1 phosphorylation at Tyrosine 14 (P-Y14) and of dynamin-2, both required for caveolae-mediated endocytosis and trafficking [13]. Depletion of CAV1 and the resultant reduction in the number of caveolae have been recently associated with a broad range of disease states such as cancer and cardiovascular and pulmonary diseases [14–16].
In order to define the relationship between CAV1 and diabetes, in this review, we cover the state-of-the-art development and progress on CAV1 and diabetes, altogether describing the role of CAV1 in insulin secretion, insulin signaling, insulin resistance, oxidative stress, diabetic complications, diabetic drug effects on CAV1, and future therapeutic perspectives in the hope of supporting clinical applications of CAV1.
2. Role of Caveolin-1 in Insulin Secretion and Development of Diabetes
In pancreatic β-cells, CAV1 plays a role in insulin receptor- (IR-) mediated signaling, insulin secretion, and possibly in diabetes. Under physiological low glucose conditions, CAV1 forms a complex with insulin granule proteins including the Rho GTPase cell division cycle 42 (cdc42), vesicle-associated membrane protein 2 (VAMP2), and the guanine nucleotide exchange factor 7 (βPIX). Glucose stimulus mediates CAV1 dissociation and complex disassembly and promotes insulin secretion [17]. In pancreatic cell lines INS-1 and MIN6, CAV1 siRNA knockdown resulted in a significant increase in insulin secretion under physiological glucose levels [17, 18].
Further studies indicated that CAV1 depletion in vivo enhances insulin secretion. Relative to littermate controls, CAV1 knockout (KO) mice suffer from hyperinsulinemia under fasting conditions and when fed with high-fat diet (HFD) [19]. Recently, the mechanisms associated with CAV1 silencing and β-cell homeostasis, survival, and insulin secretion have been revealed. Zeng et al. found that in pancreatic β-cells, lipotoxicity is moderated when CAV1 is depleted due to the activation of AKT (RAC-alpha serine threonine protein kinase) and ERK1/2 (extracellular-signal-regulated kinase) signaling pathways, which in turn downregulate the expression of cell cycle arrest proteins and upregulate the expression of cell cycle activators [20].
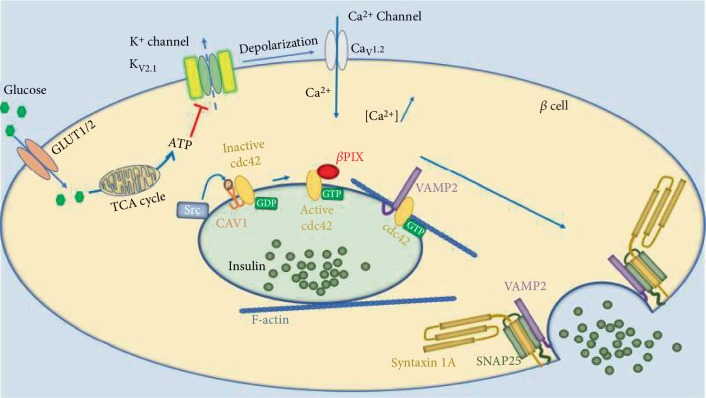
The molecular mechanism underlying insulin secretion is described in Figure 1 (best reviewed in [21]). At high glucose conditions, the increased ATP/ADP ratio results in the closure of the ATP-sensitive K+ channel Kv2.1, which in turn prompts the opening of the voltage-dependent Ca2+ channel Cav1.2 [22]. The increase in cytoplasmic Ca2+ ion concentration triggers the activation of exocytotic machinery [23]. The process is initiated by the dissociation of the CAV1-cdc42-GDP complex through active Src kinase-mediated CAV1 P-Y14 phosphorylation [17, 24, 25]. The released inactive cdc42-GDP binds to βPIX resulting in conformational changes that activate cdc42-GTP [17, 26]. On a side note, it has been proposed that YES, a Src family kinase, might be the kinase that is either phosphorylating CAV1 or βPIX and thus plays an important role in insulin secretion in MIN6 cells. Cdc42 is now active, and it interacts with insulin secretory granule-bound VAMP2 molecules, which are then targeted to fusion with the plasma membrane through the indirect interaction between cdc42, VAMP2, F-actin (filamentous actin), Syntaxin 1A, and SNAP-25 (synaptosomal-associated protein 25) modulations [27, 28].
Figure 1.
Insulin secretion mechanism and the role of CAV1. At high glucose conditions, the increased ATP/ADP ratio results in the closure of the ATP-sensitive K+ channel Kv2.1, which in turn prompts the opening of the voltage-dependent Ca2+ channel Cav1.2. The increased cytoplasmic Ca2+ triggers the activation of exocytotic machinery. The process is initiated by the dissociation of the CAV1-cdc42-GDP complex through CAV1Tyr14 phosphorylation. The released inactive cdc42-GDP binds to βPIX resulting in an active cdc42-GTP, which interacts with VAMP2-bound insulin secretory granules. These vesicles are then targeted to fusion with the plasma membrane through the interaction between cdc42, VAMP2, F-actin, Syntaxin 1A, and SNAP-25 modulations. Abbreviations: β cell—pancreatic β cell line; GLUT—glucose transporter; TCA cycle—tricarboxylic acid cycle or Krebs cycle; cdc42—cell division cycle 42; βPIX—guanine nucleotide exchange factor 7; VAMP2—vesicle-associated membrane protein 2; F-actin—filamentous actin; SNAP-25—synaptosomal-associated protein 25.
In sum, CAV1 facilitates insulin secretion through at least interacting with cdc42 and being an integral part of the insulin secretion vesicles.
3. Role of Caveolin-1 in Insulin Signaling and Insulin Resistance
Caveolae have an abundant number of intramembrane signaling proteins with lipid modification docked within them, allowing signaling cascade interaction [29]. CAV1 is central to the adjustment of these processes. It recruits and regulates various signaling proteins through the interaction of the CSD. CAV1 can negatively modulate signal transduction pathways such as those involving transforming growth factor-beta receptor 1, nitric oxide synthases, G proteins, protein kinase A, ERK1/2, and phosphatidylinositol-3-kinase- (PI3K-) AKT [30–32] or enhance the signal of downstream effector proteins such as IR, estrogen receptor, protein lipase C, and protein lipase D [19, 33–36].
3.1. Role of Caveolin-1 in Insulin Signaling
Multiple lines of accumulated evidence indicate a vital role of caveolae in regulating not only insulin secretion but also insulin signaling [33]. In the plasma membrane, structural studies showed that IR is mainly localized and highly enriched in caveolae with very little receptors outside of caveolae [37]. Furthermore, rat hepatocytes fed with HFD had caveolae that contained more IR than those fed with regular chow [38]. In pancreatic β cells, IR signaling was impaired by cholesterol depletion or by expression of a dominant-negative CAV1 mutant, further demonstrating that CAV1 plays an essential role for proper insulin response. Similarly, CAV1-/- mice that lack caveolae in all tissues (except tissues expressing caveolin-3) have a reduced amount of IR in adipocytes and exhibit the same characteristics observed in humans suffering from congenital generalized human Lipodystrophy type 3 (CGL3). CAV1-null mice on normal diet show no response to insulin stimulation due to the IR protein levels decreased by 90% even with normal mRNA expression, which suggest that CAV1 stabilizes IR in the plasma membrane [19].
In fact, CAV1 was found to directly interact through the CSD with the IR β-subunit in rat adipose tissue [39]. In vitro studies showed that insulin stimulation of adipocytes promotes CAV1 Y14 phosphorylation and activation by IR kinase activity. Zimnicka et al. used advanced quantitative live-cell imaging to show that endocytosis and trafficking of caveolae are associated with conformational changes triggered by CAV1 Y14 phosphorylation. The latter spatially broadens CAV1 molecules within the oligomeric caveolar coat due to repulsion of adjacent negatively charged N-terminal phosphotyrosine residues. This spreading promotes caveolar inward bulging and endocytosis [40]. After CAV1 activation by IR, the cascade of signal transduction leads to the translocation of glucose transporter GLUT4 within caveolae [38, 41]. CAV1 is also implicated in the endocytosis of GLUT4 in insulin-stimulated adipocytes [42]. Equally, CAV1 silencing showed a reduction in insulin-induced GLUT4 recruitment to the cellular membrane and IR activation in adipocytes [43].
Insulin binding to IR causes a conformational change leading to activation of the intrinsic receptor tyrosine kinase that can phosphorylate IR on 13 potential tyrosines [44]. Signaling events downstream of IR autophosphorylation can be divided into two major pathways; the PI3K/AKT pathway, which controls the majority of insulin's metabolic actions, such as glucose uptake and glycogen storage, and the mitogenic or MAPK (mitogen-activated protein kinase) pathway, which governs cell growth and differentiation along with PI3K.
Insulin signaling follows different pathways and outcomes depending on the cell type [45]. Whether it is inducing gluconeogenesis in skeletal muscle cells or inhibiting lipolysis in adipocytes, the activation of IR starts in the caveolae and ends with it. The series of signaling events triggered by IR autophosphorylation is mediated by SHC and GRB2 (mitogenic pathway) or insulin receptor substrates (IRS) and SH2B2/APS (metabolic pathway). SHC and IRS interact with IR via their SH2 domain (Src homology 2) and are themselves tyrosine phosphorylated by IR [46, 47]. This IRS phosphorylation appears to be malfunctioning in human adipocytes and skeletal muscle obtained from patients with type 2 diabetes mellitus (T2DM) [48].
Autophosphorylation of IR also causes its internalization into an endosome mediated by an activated CAV1-P-Y14, which downregulates IR signaling activity [49]. Activated receptors get concentrated within the trafficked caveolar endosomes, and IR's tyrosine kinases can now phosphorylate substrates deemed inaccessible when caveolae were still attached to the cell surface. Endosomal proton pumps acidify the lumen, which leads to the insulin-IR uncoupling, and IR will either be degraded or dephosphorylated and recycled to the plasma membrane [50].
As aforementioned, CAV1—as an integral part of caveolae—is responsible for concentrating insulin receptors and some of the downstream effector proteins in caveolae and mediating their signaling effect, internalizing IR and then recycling it back to the cellular surface.
3.2. Role of Caveolin-1 in Insulin Resistance
In vivo disruption of caveolae results in insulin resistance. In humans, homozygous mutation in the CAV1 gene causes CGL3, where affected patients have postprandial hyperinsulinemia, severe insulin resistance, hypertriglyceridemia, and lipodystrophy [51].
It was suggested earlier that adipose tissues show hypoxic areas causing the observed inflammatory response in the tissue [52], which may induce the development of T2DM as previously described. Recently, Varela-Guruceaga et al. investigated the role of CAV1 in hypoxia-induced insulin resistance in adipocytes. They observed a disruption in CAV1 localization in the plasma membrane of adipocytes, complete abolishment of the insulin-stimulated P-Y14 residues of CAV1, and a decrease in GLUT4 translocation to the plasma membrane suggesting CAV1 relevance in hypoxia-induced insulin resistance [53].
In fact, long-term exposure of adipocytes in culture to high glucose concentration increased CAV1 and IR expression and IR/IRS1/PI3K dephosphorylations and thus impairment of insulin signaling and insulin insensitivity [54]. In a different study, where adipocytes were induced by TNF-α to mimic a state of insulin resistance, live-cell experiments showed that IR was found to dynamically segregate from CAV1 in caveolae into glycosphingolipid GM3- (monosialodihexosylganglioside- or NANA-Gal-Glc-ceramide-) enriched microdomains compared to untreated adipocytes [55]. These results, taken together, suggest that during insulin resistance, the dissociation of IR from CAV1 leads to impaired insulin signaling and thus decreased GLUT4 translocation to the membrane, which reduces glucose uptake by the cell (see Figure 2). Blockade of the caveolae-mediated endocytosis of IR increased insulin signaling significantly in human adipocytes in vitro [56]. Mimicking this blockade can be a novel target for the treatment of T2DM insulin resistance.
Figure 2.
During normal insulin sensitivity, IR binds and activates CAV1 and then undergoes a series of autophosphorylations and activates the IRS/PI3K/AKT signaling pathway, which eventually leads to GLUT4 translocation in CAV1-mediated vesicles to the surface. During insulin resistance, while CAV1 and IR expression is increased, IR dissociates from CAV1 and moves into GM3-rich lipid rafts leading to impaired insulin signaling and decreased GLUT4 translocation to the membrane and causing a reduction in glucose uptake by the cell. Abbreviations: IR—insulin receptor; AKT—RAC-alpha serine threonine protein kinase; PI3K—phosphatidylinositol-3-kinase; IRS1—insulin receptor substrate 1; GLUT4—glucose transporter 4.
4. Role of Caveolin-1 in Inflammation and Obesity
CAV1 expression in the adipose tissue is augmented in obese patients with or without T2DM. It was suggested in earlier reports that this could be due to the increased transport of fatty acids to the plasma membrane [57]. A recent study by Ghorpade et al. demonstrated that silencing of DPP-4 (dipeptidyl peptidase-4) in hepatocytes in vitro decreased insulin resistance and inflammation hallmarks in visceral adipose tissue. CAV1 siRNA had the same effect in adipose tissue macrophages (ATMs). CAV1 is, in fact, a mediator of the inflammatory effects of DPP-4 and plasma factor Xa (FXa) on lymphocytes [58]. Ghorpade et al. proposed a mechanism where obesity triggers a Ca2+-CaMKII-ATF4 (calcium-calmodulin-dependent protein kinase II-activating transcription factor 4) pathway in the liver, leading to DDP-4 secretion, which then stimulates a CAV1-IRAK1-TAK1 (interleukin-1 receptor-associated kinase1-TGFβ activated kinase 1) pathway in ATMs, promoting ERK1/2-NFκB-mediated inflammation. Visceral adipose tissue inflammation aggravates glucose intolerance, weakened insulin signaling, and hyperinsulinemia. The authors concluded that targeting this pathway might be more beneficial than using oral hypoglycemics [59].
Multiple lines of evidence have linked caveolae to the regulation of cholesterol homeostasis. Initially, caveolins are cholesterol-binding proteins and are involved in cellular sterol transport [60]. Furthermore, cellular cholesterol concentration is central for caveolae formation [61]. Depleting the cell membrane from its cholesterol has shown disappearance of surface caveolae structures, marked changes in CAV1 distribution, and impairment in cellular signaling [62]. CAV1-null mice had lower fat mass at older age, were resistant to HFD-induced obesity, had lower hepatic lipid accumulation, and had decreased metabolic flexibility compared to wild-type mice [63, 64]. These mice exhibit increased levels of serum triglycerides and free fatty acids and reduced levels of the cell surface fatty-acid binding protein (CD36) [65, 66]. Mechanistically, it was suggested that adipocyte lipid droplet size is diminished due to a defective fatty acid uptake through CD36 in adipocytes and fibroblasts [67]. More recent work showed that in human subjects enrolled in a trial of 8 weeks of diet-induced obesity, adipocyte expansion response correlated with initial CAV1 expression in the collected subcutaneous adipose tissue. Combining this result with other in vivo and in vitro work, the authors showed that CAV1 expression is crucial to increase caveolar density, improve adipocyte ability in accommodating larger lipid droplets, and promote cell expansion through ameliorated insulin response and improved glucose utilization [68].
Obesity causes adipocytes to become dysfunctional and secrete adipokines that recruit macrophages to the adipose tissue. These proinflammatory macrophages secrete cytokines such as TNF-α (tumor necrosis factor-alpha) and IL-6 (interleukin 6) [69] causing a chronic low-grade state of inflammation, which is linked to several diseases such as insulin resistance, diabetes, hyperinsulinemia, dyslipidemia, and vascular abnormalities [70]. Mechanistically, TNF-α signaling pathway and circulating free fatty acids cause ER stress responses that inhibit many of the insulin signaling downplayers such as IRS1/2, AKT, glycogen synthase, and glycogen phosphorylase, thus blocking glucose uptake and glycogen storage. Insulin sensitivity also correlated with P-Y14-CAV1 levels, which suggests a role for CAV1 in maintaining active and insulin-sensitive glucose uptake [71]. An in vitro study showed that overexpressing CAV1 in murine macrophages dramatically inhibited TNF-α and IL-6 production and increased the anti-inflammatory interleukin 10 secretion [72]. In vivo, CAV1 expression in monocytes from diabetic patients decreased, while TLR4 and TNF-α secretion increased and even more significantly in diabetic patients with neuropathy, suggesting, a role of CAV1 in regulating TLR4-mediated inflammatory cascade in T2DM [73].
The CAV1 gene encompasses 36 kb and contains 3 exons producing 8 transcripts. The default transcript, which is representative of the biology and highly conserved, encodes an active 178 aa protein. Multiple long noncoding RNAs (lncRNAs) are transcribed upstream or downstream of the CAV1 gene region with no known effect yet on CAV1 gene regulation or expression. One particular lncRNA (AC006159.1) could be of interest for future investigation as it is transcribed from the reverse strand of the CAV1 intronic region.
CAV1 can be regulated by different microRNAs. It has been demonstrated that miR-124a activates CAV1 in podocytes, miR-375 downregulates CAV1 gene expression [74], and miR-204 can target CAV1 mRNA and decreases its expression in endothelial cells [75]. Trajkovski et al. identified CAV1 as a target gene for miR103 and miR107. Their inactivation upregulates CAV1 in adipocytes, thus improving IR maintenance, enhancing insulin signaling, reducing adipocyte size, and improving glucose uptake. The authors concluded that miR103/107 is important for insulin sensitivity and suggested it as a target for the treatment of T2DM [76].
CAV1 SNP variants have been suggested to be linked to metabolic syndrome (MetS), a major risk factor for diabetes and coronary artery disease. Several CAV1 single-nucleotide polymorphisms (SNP) were found to associate with MetS: rs926198 in Caucasians and Hispanics [77], rs3807989 in the Chinese Han population [78], and rs1997623 in Kuwaiti children as shown by our previous study [79]. The forest plot in Figure 3 shows an association between LHDLC and MetS with the heterozygous genotype CA and the A-allele, meaning that a carrier of the A genotype has a higher chance of developing MetS in the studied population.
Figure 3.

Forest plot representing relative odds ratio of MetS and LHDLC and their association with CAV1 rs1997623. CA—heterozygous; CC—wild type; A—A-allele; C—C-allele. The width of the horizontal lines represents the 95% confidence intervals for each measurement. Abbreviations: MetS—metabolic syndrome; LHDLC—low HDL cholesterol.
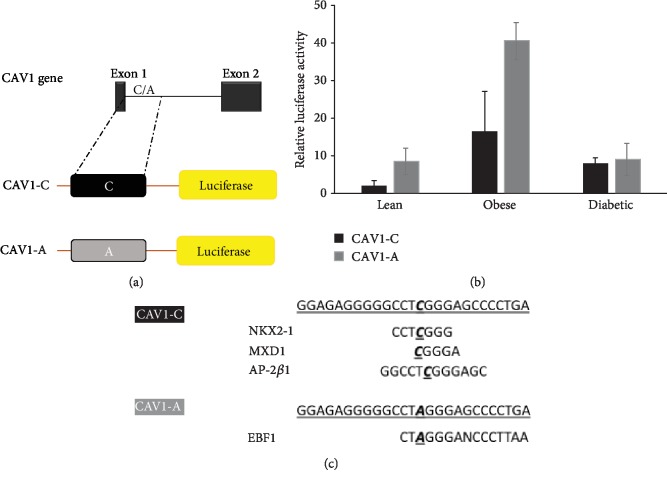
In order to study the role of the CAV1 gene in MetS, we used the pRMT reporter vector (origene) to test the effect of rs1997623 polymorphism on promoter activity using luciferase as a reporter gene. We cloned 650 bp of the intronic region downstream of exon 1 (Figure 4(a)) containing the most common variant base C (CAV1-C) or the less common variant base A (CAV1-A) upstream of the luciferase gene. The plasmids were transfected into human primary adipocytes from lean, obese, and diabetic subjects. Predicted binding sites for transcription factors depending on sequence using the online tool Promo [80] are shown in Figure 4(c). Here, we show our primary results where luciferase activity is increased using the CAV1-A plasmid compared to CAV1-C in the obese adipocytes and not in the lean or diabetic adipocytes (Figure 4(c)). This result could be due to the possible loss of NKX2-1 (NK2 Homeobox 1), MXD1 (MAX Dimerization Protein 1), and AP-2β1 (Adaptor-Related Protein Complex 2 Subunit Beta 1) binding or the newly gained EBF1 (EBF Transcription Factor 1) binding that is affecting reporter gene transcription. It is noteworthy to mention that EBF1 is implicated in adipogenesis and the regulation of lipid metabolism by PPARα (peroxisome proliferator-activated receptor alpha), while MXD1 is implicated in the MAPK-ERK pathway, apoptosis, and autophagy. CAV1 is known to play a role in the aforementioned metabolic pathways; further investigation is needed to possibly correlate MetS, CAV1, and these transcription factors. Another possibility could be that the rs1997623 variant affects an intronic regulator element lying downstream of exon 1 causing the increase in the luciferase activity in obese adipocytes and possibly be the reason behind the increased MetS and lower salivary HDL in Kuwaiti children harboring this variant.
Figure 4.
(a) Variants CAV1-C and CAV1-A cloning into the pRMT-Luc reporter vector. (b) Relative luciferase activity for pRMT-CAV1-C-Luc and pRMT-CAV1-A-Luc in lean, obese, and diabetic adipocytes. (c) Predicted binding sites for transcription factors depending on the CAV1-C and the sequence presenting rs1997623 SNP (CAV1-A). Abbreviations: NKX2-1—NK2 Homeobox 1; MXD1—MAX Dimerization Protein 1; AP-2β1—Adaptor-Related Protein Complex 2 Subunit Beta 1; EBF1—EBF Transcription Factor 1.
5. Role of Caveolin-1 in Oxidative Stress
The expression of CAV1 is involved in cellular proliferation, senescence, differentiation, adhesion, and migration; nevertheless, the specific roles of CAV1 through these pathways remain unclear and they might change depending on the cell type [81–84]. In human breast cancer cells [85], CAV1 overexpression has antiproliferative activity and CAV1-null mutation in mice displayed epithelial and vascular hyperplasia [86]. CAV1 negatively regulates the ERK1/2 and PI3K/AKT signaling pathway [87], which leads to the upregulation of the cell cycle inhibitors p53 and p21 and the downregulation of the cell cycle promoter cyclin D1 causing a G0/G1 cell cycle arrest [88]. On the other hand, Fernandez et al. noticed that CAV1-null mice displayed weakened hepatic regeneration and impaired survival after partial hepatectomy. Hepatocytes were senescent and had drastically reduced lipid storage. Remarkably, glucose treatment of CAV1 KO mice increased survival and restored cell cycle progression. Therefore, the authors conclude that CAV1 is essential for liver regeneration and regulation of lipid metabolism [89].
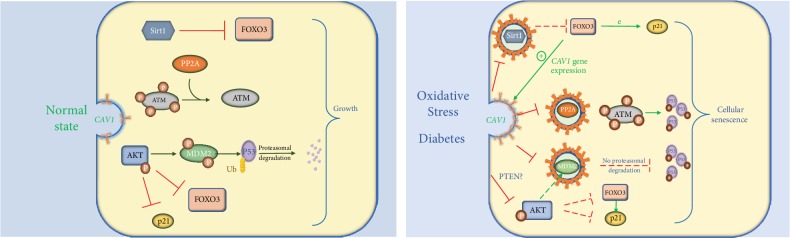
Our previous results and others showed that CAV1 expression level increases in diabetes, oxidative stress, and senescent cells. We have found that CAV1 sequesters MDM2 (E3 ubiquitin ligase mouse double minute 2 homolog), which ubiquitinates p53. The latter would be targeted for proteasomal degradation resulting in cellular proliferation [90]. This sequestration causes p53 and p21 accumulation and the cells become senescent [84]. Furthermore, CAV1 inhibits Sirtuin 1 (Sirt1), which is a histone deacetylase that controls a plethora of physiological processes, including senescence. Oxidative stress induces seizing of Sirt1 into caveolae and the interaction of Sirt1 with CAV1 through its CSD, leading to inhibition of Sirt1 activity and activation of p53/senescence signaling [91]. Under physiological conditions, Sirt1 deacetylates forkhead box protein O3 (FOXO3), attenuates FOXO-induced apoptosis, and stops FOXO-p21-dependent cell cycle arrest [92]. Interestingly, transcription of the CAV1 gene is directly stimulated by FOXO3 in a cell-cycle-independent manner; thus, the three factors CAV1, SIRT1, and FOXO3 are functioning in a feedback loop regulating cell cycle arrest [93–95]. CAV1 also activates ATM (ataxia telangiectasia-mutated) likely by sequestering the catalytic subunit of PP2A (protein phosphatase 2A) into caveolar structures, thereby stimulating engagement of the ATM-p53-p21 pathway [96] (Figure 5).
Figure 5.
Simplified presentation of the role of CAV1 under oxidative stress and diabetes. During normal CAV1 expression, Sirt1 and p-AKT block p21 activator FOXO3. PP2A dephosphorylates ATM, thus attenuating p53 activation. Active AKT induces MDM2 activation, thus leading to the induction of p53 proteasomal degradation and ultimately normal cellular growth. During diabetes, CAV1 expression increases and caveolae sequester Sirt1, PP2A, and MDM2 preventing their actions, hence leading to the release of FOXO3, phosphorylation of p53 by ATM, and cell cycle arrest. CAV1 decreases p-AKT possibly by interacting with PTEN. The decrease in p-AKT reduces MDM2 activation and attenuates the deactivation of FOXO3 and p21, which eventually leads to cellular senescence. Abbreviations: Sirt1—Sirtuin 1; FOXO3—forkhead box protein O3; ATM—ataxia telangiectasia-mutated; PP2A—protein phosphatase 2A; AKT—RAC-alpha serine threonine protein kinase; MDM2—E3 ubiquitin ligase mouse double minute 2 homolog; p53—tumor protein p53.
On the other hand, during normal or tumorigenic states, activated AKT phosphorylates MDM2 on two serine residues [97, 98], which promotes MDM2 nuclear localization and the subsequent p53 ubiquitination [99]. Post injury and in physiological conditions, high PTEN (phosphatase and tensin homolog) inhibits the PI3K/AKT signaling pathway and limits the proliferation of fibrotic tissue. Protein expression of both CAV1 and PTEN was diminished in lung fibroblasts in patients with idiopathic pulmonary fibrosis and in cardiac fibroblasts relative to control patients [100, 101]. Moreover, CAV1-null mice demonstrate low expression of PTEN compared to wild-type mice. CAV1 reconstitution in the knockout fibroblasts exhibited an elevated association of PTEN with the lipid bilayer and decreased PI3K/AKT signaling. Likewise, phosphorylated AKT is increased in liver and adipose tissue treated with CAV1 siRNA [59]. Altogether, CAV1 negatively modulates AKT signaling leading to decreased proliferation most likely by affecting PTEN expression levels. How CAV1 might be regulating PTEN is yet to be discovered.
6. Caveolin-1 in Chronic Complications of Diabetes
6.1. Caveolin-1 and Cardiovascular Disease in Diabetes
6.1.1. Vascular Complications
Decreased insulin sensitivity strongly disturbs endothelial function and is the major contributor to the progression of diabetic macrovascular complications [102]. Endothelial dysfunction is categorized by impaired vasodilation in conduit and resistance arteries, which contributes to the genesis of hypertension, atherosclerosis, and coronary artery disease [13, 40, 103]. Studies in CAV1-deficient mice revealed that CAV1 can downregulate eNOS (endothelial nitric oxide synthase), which is primarily responsible for the generation of nitric oxide (NO) in the vascular endothelium, thus increasing paracellular permeability [104, 105]. Insulin exerts its vascular effects by activating eNOS and the release of NO [106].
The most important mechanisms controlling vascular function are gap junction communication between vascular cells and endothelial production of vascular modulators (NO), vasodilator prostaglandins such as prostacyclin (PGI2), and endothelium-dependent hyperpolarization (EDH) factors [107]. These mechanisms range from diameter and structure regulation to the protection against atherosclerosis. PGI2 is a vasodilator and a platelet activation inhibitor, NO and EDH factors regulate vascular tone of large conduit vessels and small resistance vessels, respectively, and their imbalance is at the basis of cardiovascular diseases [108].
Endothelial cells lacking CAV1 exhibit a rise in PGI2 expression indicating that CAV1 might be implicated in PGI2 expression regulation [109]. The key constituents of NO production and gap junctions, eNOS and connexins (Cx), respectively, are contained in caveolae and physically associate with CAV1 in human endothelial cells [110, 111]. In vivo and in vitro evidence show that NO increase weakens gap junction communication through channels with Cx37 [112, 113], and vice-versa, loss of connexins affects eNOS expression and function and disruption of caveolae affects both NO signaling and gap junction function [114]. In fact, CAV1 is essential for proper vascular connexins' localization (Cx37, Cx40, and Cx43) to the membrane in the aorta and superior mesenteric artery, formation of functional myoendothelial junctions, and adequate vasodilatory responses [110].
(1) CAV1 and Coronary Disease. Ablation of the CAV1 gene aggravates cardiac dysfunction and decreases survival in mice exposed to myocardial ischemia. Mechanistically, CAV1-/- mice subjected to coronary artery ligation display abnormalities in β-adrenergic signaling [115]. Recently, the cardioprotective ischemic preconditioning (IPC) was found to involve increased NO production, Cx43 phosphorylation, and chemical gap junction uncoupling [116]. Diabetes attenuates IPC by inhibiting CAV1; Gupta et al. were able to restore the protective IPC and increase NO production in rat diabetic heart [117].
In unstimulated endothelial cells, CAV1 inhibits NO production by binding to eNOS and suppressing its activity in the caveolae [118]. After stimulation, Ca2+-CaM binds to eNOS and CAV1 detaches to permit full activation of eNOS [119]. If CAV1 is knocked out, there is a loss of proper regulation of eNOS function [86]. Hyperlipidemia enhances CAV1/eNOS interaction and reduces endothelial NO production, thus contributing to endothelial dysfunction and atherosclerotic plaque formation [120, 121].
CAV1 expression is higher in EDH-mediated coronary microvessels than in NO-mediated conduit arteries in the control group. On the other hand, CAV1 was unaltered in coronary arterioles after ischemia. These results indicate that there are significant compensatory interactions between eNOS and CAV1 in diabetes in vivo and that EDH is involved in coronary vasodilatation after ischemia [122]. Nevertheless, excessive NO production can induce superoxide release and nitrooxidative stress and consequently counter NO bioavailability [123]. Hence, CAV1 can possibly maintain the endothelial function through its ability to regulate NO production under normal physiological conditions. In normal conditions, insulin activates eNOS located in the caveolae through the AKT pathway causing its phosphorylation on ser1177. In vitro hyperglycemia [124] and T2DM in humans result in the O-linked N-acetylglycosylation of Ser1177 [125], thus interfering with the signaling mechanisms of eNOS and attenuating NO production.
Insulin resistance, obesity, and MetS increase oxidized LDL levels in plasma. The latter was found to cause caveolar cholesterol depletion leading to decreased eNOS inactivation, while HDL maintains the caveolar lipid structure and preserves the capacity for eNOS to be activated. HDL has another antiatherogenic property by binding to SR-BI (scavenger receptor BI) in caveolae and inducing eNOS phosphorylation at ser1177 [126]. Alternatively, CAV1-deficient mice have elevated plasma HDL levels [66]. In fact, CAV1 associates to and enhances the internalization and degradation of ABCA1 (ATP-binding membrane cassette transporter A1) [127], a key protein in HDL synthesis. This is one of the mechanisms by which decreasing CAV1 expression can produce antiatherogenic effects.
As aforementioned, CAV1-null mice have impaired cholesterol homeostasis, insulin resistance, elevated NO production, and defects in cardiopulmonary and vascular function [19]. CAV1 and ApoE double-knockout mice are strongly protected against atherosclerotic lesions compared to ApoE-/- mice, despite elevated hypercholesterolemia and hypertriglycemia. The reexpression of CAV1 in the endothelium fully recovers atherosclerosis in ApoE-/- CAV1-/- mice [66]. Increased NO production due to CAV1 removal has long been thought to be the reason behind the atheroprotection observed in CAV1-/- mice. Surprisingly, Ramirez et al. very recently showed that this protection is done independently of eNOS activation and NO production. They have crossed CAV1-/- with eNOS-/- mice into a proatherogenic background and found that CAV1 controls proatherogenic responses of endothelial cells to disturbed blood flow, including lipid trafficking, extracellular matrix (ECM) remodeling, and inflammatory signaling in early-stage atherosclerotic lesions. CAV1 also promotes proatherogenic matrix deposition leading to endothelial cell activation in atheroprone regions of the aorta [128]. Hence, these data indicate that CAV1-based therapeutics should be further studied to alleviate the burden of diabetic complications.
(2) CAV1 and Cerebrovascular Disease. Diabetes increases the risk of cerebral ischemia either by ischemic stroke or cardiovascular diseases [129]. CAV1 KO mice ischemic brains showed impaired angiogenesis and increased apoptotic cell death [130]. CAV1 was later found to be downregulated in focal cerebral ischemia and reperfusion injury. In fact, CAV1 regulates the blood-brain barrier (BBB) permeability through modifying matrix metalloproteinases' (MMP) activity responsible for ECM destructuring [131]. CAV1 decreased cerebral infarct volume, facilitated angiogenesis and neurogenesis, and promoted neurological recovery by upregulating the VEGF signaling pathway in models of middle cerebral artery occlusion [132]. CAV1 expression is regulated by cystatin C during the maintenance of BBB integrity after ischemic brain injury. Lentiviral overexpression of CAV1 inhibited tight junction degradation and attenuated cerebral edema in rats [133]. In humans, cerebral edema is a dreaded complication of diabetic ketoacidosis in children [134]. These studies identify new potential therapeutic strategies for stroke.
(3) CAV1 and Peripheral Artery Disease. Angiogenesis is impaired in diabetes; CAV1-null mice fail to recover functional vasculature in hindlimbs after induced ischemia [135]. Recently, endothelial CAV1 was found to be central for EDH-mediated vasodilatation and ischemic angiogenesis through mediating protein S-nitrosylation by reactive nitrogen species (RNS) in a mouse model of hindlimb ischemia [136].
Type 1 diabetes (T1DM) and T2DM induce oxidative stress and increase CAV1 expression, which inhibits NO production and thus vasodilation, while CAV1-null mice showed improved arterial relaxation [135]. The decrease in NO bioavailability causes impairment of endothelium-dependent relaxations. However, EDH appears to compensate at least in part for this dysfunction in human subcutaneous arterioles with T2DM [137].
6.1.2. Myocardial Impairment
Diabetic patients are at high risk of developing ventricular hypertrophy, and heart failure is the leading cause of death in diabetes. For a long time, it was thought that only caveolin-3 is expressed in the heart; however, CAV1 was later found to exist and function in cardiac myocytes [138], which explains why systemic CAV1-null mice exhibit striking biventricular hypertrophy with severely reduced systolic and diastolic heart function [139]. Although these mice show improved vascular responses [86], disruption of CAV1 leads to enhanced nitrosative stress [139] and eventual development of cardiomyopathy and pulmonary hypertension due to persistent eNOS activation, NO release, and p42/44 MAP kinase activation [140, 141]. Reexpression of CAV1 in the endothelium rescues the vascular and cardiac defects in CAV1-null mice [142], and phosphorylation of CAV1 was found to contribute to cardiac protection in isoflurane-induced mice [139].
Meta-analysis of prospective cohort and case-control studies of diabetes and risk of atrial fibrillation (AF) showed that diabetic patients have an increased risk of 40% to develop this major cause of thromboembolic stroke [143]. CAV1 is downregulated in atrial specimens of 13 patients with AF. Human atrial fibroblasts treated with CAV1 peptides abolished the profibrotic cytokine transforming growth factor-beta 1- (TGF-β1-) induced collagen production and decreased MMP2 and 9 expressions. Thus, the authors concluded that CAV1 is an important anti-AF signaling mediator by conferring its antifibrotic effects in atrium [144].
6.2. Caveolin-1 and Diabetic Nephropathy
Diabetic nephropathy (DN) is characterized structurally by mesangial expansion of the glomeruli, basement membrane thickening, and nodular glomerulosclerosis. Subsequently, interstitial fibrosis with tubular atrophy develops, along with arteriolar wall thickening, macrophage and T-lymphocyte infiltration, podocyte damage, and decreased endothelial cell fenestrations. Functionally, DN is characterized by glomerular hyperfiltration and increased albumin excretion, and in later stages by increasing proteinuria and declining glomerular filtration rate [145]. Many factors trigger these pathological changes such as hyperglycemia, mechanical stretch, oxidative stress, angiotensin II, chemokines, and profibrotic cytokines such as CTGF (connective tissue growth factor) and TGF-β1.
Caveolins play an important role in the regulation of diverse signaling pathways implicating the aforementioned triggers. Moriyama et al. assessed CAV1 expression in multiple glomerular diseases such as DN in humans. They have found augmented CAV1 expression and caveolae number in glomerular endothelial cells, which correlated with the degree of albuminuria [146].
In primary renal cells, CAV1 was found to be important for the trafficking of SGLT1 and SGLT2 (sodium/glucose cotransporters) to the cellular membrane, thus increasing glucose uptake by renal cells, indicating the importance of CAV1 in the pathology of glucose uptake in the kidney [147]. Caveolae and CAV1 enhance the biosynthesis and build-up of ECM in mesangial cells in response to elevated glucose and mechanical strain by promoting many profibrotic pathways [148–150]. CAV1 KO provided substantial protection from ECM accumulation and albuminuria in T1DM mice. They were considerably guarded from the rise of glomerular collagen I, CTGF, fibronectin, and TGF-β1 [151]. CAV1 was found to be necessary for glucose-induced generation of reactive oxygen species (ROS). Furthermore, caveolae are required for the activation of PKCβ (phosphor kinase C beta), upstream of ROS, and eventually upregulating DN-associated TGF-β1 [152].
In the kidney, CAV1 mediates Ang II (angiotensin II) uptake in proximal tubules. This was functionally associated with diminished sensitivity to Ang II infusion in CAV1-null mice [153]. CAV1 acts as a chaperone for AT1R (Ang II type 1 receptor), helping its shuttling to the plasma membrane [154]. Nephrin is an important protein that signals apoptosis and consequently decreases renal cellular death; its dephosphorylation was detected in DN and was found to associate with podocyte impairment and loss [155]. In rats injected with Ang II, nephrin dephosphorylation was linked to CAV1 P-Y14 activation [156].
As in the other endothelial tissues, NO and eNOS were found to play a central role in the pathogenesis of DN. Indeed, CAV1 plays a pivotal role in the eNOS-mediated decrease of renal NO levels, which is possibly responsible for the progression of DN in T1DM and T2DM rat models [157].
There is a causal association of elevated homocysteine levels with the development of T2DM [158]. Recently Pushpakumar et al. showed that homocysteinylation of eNOS and disruption of caveolin-mediated regulation leads to ECM remodeling and hypertension in mice. H2S treatment attenuated renovascular damage by modulating eNOS and CAV1 interaction and thus antagonizing ECM protein accumulation and smooth muscle cell proliferation [159].
Combined data show further that targeting CAV1 can be a very effective therapeutic agent against DN.
6.3. Caveolin-1 and Diabetic Retinopathy
Diabetes causes several eye disorders such as diabetic retinopathy (DR), diabetic macular edema (DME), glaucoma, and cataracts. Early DR is characterized by the breakdown of the blood-retinal barrier (BRB). Diffuse retinal vascular permeability causes BRB loss and denotes a significant mechanism quickening the diabetes-mediated retinal changes [160]. In later stages, DME is caused by abundant vascular leakage, which leads to blindness in patients with DR, principally in T2DM patients with nonproliferative DR [161].
CAV1 constitutes a part of the rod cell outer segment, hence the possible role of CAV1 in ocular diseases [162]. In the retina, caveolar transcytosis and size were upregulated in streptozotocin- (STZ-) induced diabetic rats [10]. CAV1 was also found to be upregulated in rat diabetic retina [163]. During inflammatory conditions such as DR, CAV1 expression is dramatically increased in retinal Müller glial cells. Likewise, caveolae are increased in number and show bipolar localization in the pericytes (smooth muscle cells) and endothelial cells of the retinal neurovascular unit possibly promoting transcellular permeability [164].
Intravenous administration of advanced glycation end product- (AGE-) modified proteins in normoglycemic rats caused pathophysiological characteristics of DR such as retinal basement membrane thickening and loss of pericytes [165]. AGE-modified proteins can be internalized by caveolae in cultured retinal endothelial cells [166]. When injected into nondiabetic rats, they induce BRB loss, which was found to correlate with increased caveolar formation in the endothelium [167]. These results suggest that the augmented caveolae-mediated transendothelial permeability is a significant BRB failure player. Gu et al. in 2017 suggested that the increased CAV1 expression can potentiate Toll-like receptor signaling and proinflammatory cytokine release, which contribute to the BRB breakdown [164]. Unexpectedly, increased endothelial permeability was seen in CAV1 knockout mice [168]. This paradox can be explained by the vasodilation induced by the release of eNOS due to CAV1 loss, thus causing an augmentation in hydraulic pressure [169].
Proliferative vitreoretinopathy (PVR) is the foremost complication of severe DR and other ocular diseases. Its pathophysiology maps a very complex pathway ensuing a proliferative response within the retina [164]. CAV1 was recently found highly expressed in the proliferative membranes of mice eyes with PVR, and CAV1 reduction increased retinal pigment epithelium cell migration abilities. This indicates that CAV1 might be a potential therapeutic target in preventing proliferative membrane development in PVR [162].
Collectively, CAV1 needs to be overexpressed in knockout cells and decreased during inflammation for the protection against neovascularization or vitreoretinopathy. Further investigation in diabetic models is needed to delineate the effect of altering expression levels of CAV1.
6.4. Caveolin-1 and Diabetic Neuropathy
CAV1 is expressed in the neurons and glial cells of the central nervous system [170]. Classical caveolar endocytosis does not happen in neurons; there is a limited number of flask-shaped caveolae in the neuronal endothelium [171]. Nevertheless, targeted overexpression of CAV1 in neurons improves signaling, branching, and hippocampus-dependent learning and memory [172, 173]. During myelination, CAV1 is upregulated [174] and regulates cell signaling pathways in glial cells [175]. Chronic hyperglycemia decreases CAV1 expression in Schwann cells (SC) [176], while antisense downregulation of CAV1 in SC increased neuregulin-induced demyelination in hyperglycemic conditions (HGC) [177]. Furthermore, heightened brain lesion volume, neuroinflammation, and accelerated neurodegeneration were observed in CAV1-null mice [178, 179]. A relationship between CAV1 and Huntington disease was reported [180], and CAV1 was found to be a risk gene for schizophrenia [181]. All this implies that CAV1 may have a role in neural development. Several epidemiological data found an association between diabetes and lower cognitive test performance [182]. Experimental findings have shown significant cognitive impairment in STZ-induced diabetic rats [183]. CAV1 expression is impaired during prolonged HGC in cell lines and brain neurons of diabetic rats [184–186]. However, a link between CAV1 and diabetic neuropathies is not well established.
Another debated topic is the implication of diabetes in the etiology of sporadic Alzheimer's disease. CAV1-null mice exhibited several motor and behavioral anomalies and fast tau-related neurodegeneration such as Alzheimer's disease [172, 187]. Tau are microtubules binding phosphoproteins abundant in neurons [188]. Hyperphosphorylated tau is found in T2DM rat brains [189]. Wu et al. have found that chronic HGC reduce CAV1 expression and that CAV1-small interfering RNA (siRNA) increase tau phosphorylation and activate mTOR/S6K (mammalian target of rapamycin/S6 kinase) signaling in diabetic rat brain neurons. Likewise, overexpression of CAV1 decreases HGC-induced tau hyperphosphorylation in the hippocampus primary neurons [184]. Recently, lower CAV1 expression and higher amyloid-β levels were found in frozen brain sections of T2DM patients versus healthy subjects. The authors replicated the work in mice and found that the depletion of CAV1 in diabetic mice brains promotes neuropathology and impairments in learning and memory. CAV1 overexpression in these mice restored the learning deficiency [190]. Therefore, CAV1 might be an effective therapeutic target for diabetes-related cognitive decline.
Painful diabetic peripheral neuropathy (DPN) is a common complication of T2DM affecting almost 50% of diabetic patients and is very challenging to treat [155]. CAV1 seems to have a regulatory role in DPN development as diabetic CAV1-null mice developed a higher deficit in motor nerve conduction velocity and thermal and mechanical sensitivity when compared with diabetic wild-type mice [191]. Yang et al. showed that the knockdown or blocking of CAV1 in the murine brain reversed behavioral and neuronal sensitization of induced pain, while the overexpression of CAV1 caused pain behavior in the unaffected mouse paw. This modulation of neuropathic pain is done via regulation of NR2B (NMDA receptor 2B subunit) and subsequent activation of ERK/CREB signaling (cAMP response-element binding), suggesting a potent CAV1-based therapy for chronic neuropathic pain [192]. It was further demonstrated that the CAV1-NR2B pathway is activated by microglial JAK2-STAT3 signaling (Janus kinase 2-signal transducer and activator of transcription 3), which contributes to diabetic neuropathic pain. Pain was also relieved by administering AG490 (JAK2 inhibitor) to the rats [193]. Collectively these data render promising DPN therapies based on the JAK2-STAT3-CAV1-NR2B signaling pathway.
7. Caveolin-1 and Antidiabetic Drugs
The interaction of CAV1 with antidiabetic drugs (summarized in Table 1) has been mainly investigated in the context of different types of cancers. Currently GLP-1R agonist, metformin, sulfonylureas, DPP-4, and SGPTL2 inhibitors are the major class of oral drugs used for glycemic control in T2DM. Glucagon-like receptor (GLP-1R) agonists function by stimulating the adenylyl cyclase pathway that leads to increased synthesis and release of insulin. Previous studies have shown that membrane localization and binding activity of GLP-1R require its interaction with CAV1, and this is in turn influenced by specific mutations within the putative region of CAV1 or GLP-1R [194]. The overexpression of CAV1 breast cancer-associated mutant, P132L, results in the attenuation of GLP-1 binding activity, regulating the downstream cellular trafficking and signaling activity of GLP-1R [194]. Metformin functions by diminishing the hepatic glucose production and intestinal glucose absorption. Metformin causes an increase in the AMP/ATP ratio and adenosine monophosphate-activated protein kinase (AMPK) activation. Knockdown of CAV1 in bovine aortic endothelial cells has been reported to diminish VEGF-dependent AMPK activation [195]. Accordingly, CAV1 was found to contribute to the inhibitory action of metformin on insulin growth factor 1 (IGF-1) activity in non-small-cell lung cancer cells where their sensitivity to metformin was dependent on CAV1 expression and CAV1 was required to induce AMPK phosphorylation and the increase in the AMP/ATP ratio [196]. Metformin induces CAV1 expression [197], which in return increases clinical efficacy of drug delivery (trastuzumab emtansine) in breast cancer cells [198]. Sulphonylureas on the other hand increase insulin release by blocking KATP channels [199]. Glimepiride, a second generation sulphonylurea, alters plasma membrane dynamics resulting in CAV1 tyrosine phosphorylation [200]. CAV1 coimmunoprecipitates with Kir6.1 (a subunit of the KATP channel) in rat aortic cells and with Kir6.2 in the pancreatic βTC-6 cell line. βTC-6-CAV1-depleted cells maintained a high rate of insulin secretion after KCl, but not after glucose and glimepiride stimulation. These results combined suggest that CAV1 might play a role in sulfonylurea-stimulated insulin secretion maybe through regulation of KATP channels [201, 202]. Dipeptidyl peptidase-4 (DPP-4) inhibitors are yet another class of antidiabetic drugs that act by increasing intestinal incretins GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide), leading to reduced glucagon release and increased insulin secretion. DPP-4 interacts with CAV1 in antigen-presenting cells [203] resulting in CAV1 phosphorylation and subsequent activation of the NFκB pathway [58]. CAV1 has been reported to be a drug target for linagliptin. This DPP-4 inhibitor increases eNOS availability by blocking its binding with CAV1 [204] leading to enhanced NO production in mesenteric arteries from type 1 diabetic rats [205]. The interaction of CAV1 with the sodium-glucose cotransporter 1 and 2 (SGLT1 and SGLT2) inhibitors is not well documented in the literature. SGLT inhibitors improve glycemic control by inhibiting the renal glucose reabsorption allowing excess sugar to be removed through urine. As aforementioned, cAMP-stimulated SGLT trafficking in renal proximal tubules depends on CAV1 expression and intact actin filaments. Coinhibition of both CAV1 and F-actin blocks SGLT-mediated glucose uptake [147]. Another study showed that CAV1 increases the expression, activity, and transport of SGLT1 to the cell membrane in Xenopus oocytes [206]. However, a new study showed that SGLT1 internalization is lipid raft-dependent but CAV1-independent in HEK and COS cells [207].
Table 1.
Antidiabetic drugs and their relationship with CAV1.
| Class | Effect on insulin | Drugs modulating CAV1 | Relation with CAV1 | Model/cell lines | References |
|---|---|---|---|---|---|
| Glucagon-like receptor (GLP-1R) agonists | Increase insulin synthesis and release | Not known | GLP-1R is internalized via CAV1-dependent mechanism | HEK and MIN6 cells | [194] |
| Metformin | Decreases hepatic glucose production and intestinal glucose absorption | Metformin | Increases CAV1 expression | Calu-1/6 and MCF-7 | [196, 197] |
| Sulphonylureas | Stimulate insulin secretion | Glimepiride | Induce CAV1 tyrosine phosphorylation | Primary rat adipocytes | [200] |
| Dipeptidyl peptidase-4 (DPP-4) inhibitors | Slow the inactivation and degradation of GLP-1 and GIP | Linagliptin | Block CAV1 binding to eNOS | NOD mice aorta and HEK cells | [204, 205] |
| Sodium-glucose transport (SGLT) inhibitors | Inhibit renal glucose reabsorption | Not known | SGLT needs CAV1 for trafficking and expression? | Primary rabbit proximal tubule cells, Xenopus oocytes | [147, 206, 207] |
Though CAV1 tends to improve glycemic control and associated complications via its interaction with various drug targets and downstream signaling molecules, its potential use as an antidiabetic drug requires further in-depth studies.
8. Future Perspectives
By virtue of its ability to affect numerous cellular pathways, CAV1 represents a challenging but interesting therapeutic target for micro- and macrovascular complications of diabetes. Since CAV1 can regulate several signaling cascades, targeting CAV1 to reduce inflammation and oxidative stress can be more efficient than aiming at the different pathways separately. Adding to this, the fact that CAV1 deletion is not lethal in mice, CAV1-based therapies can be a very enticing intervention for treating diabetic complications.
In the context of atherosclerosis, Sharma et al. have previously investigated the therapeutic effect of a cell-permeable CAV1-derived peptide, CavNOxin, on the development of atherosclerosis in T1DM model (STZ, ApoE-/-) and western diet-induced T2DM model mice. CavNOxin controlled oxidative stress and lowered diabetes-induced atherosclerotic plaque up to 84% in vivo. Mechanistically, CavNOxin attenuated oxidative stress markers, blocked leukocyte-endothelial interactions, and inhibited the expression of proinflammatory mediators, while preserving caveolar structures. Hence, CavNOxin should be further studied as a pharmacological therapy to alleviate the burden of diabetic macrovascular complications in both T1DM and T2DM [208].
Apropos of the treatment of nephropathy, many groups treated diabetic mice with different drugs that affect CAV1 expression, CAV1 activation (P-Y14), or caveolae formation such as salidroside, daidzein, curcumin, cyclodextrin, CSD, and PP2; they all mitigated nephropathy by at least decreasing proteinuria and ECM accumulation. For further information about these CAV1-based therapies, please read the beautiful review written by Krieken and Krepinsky [209]. An additional biomolecule called taxifolin (or dihydroquercetin (DHQ)) might have a promising effect on DN. Recent work showed that DHQ has protective kidney properties similar to losartan (angiotensin II inhibitor) including attenuating albuminuria, hyperglycemia, and lipid metabolism disorders, and modifying renal lesions in DN possibly by suppressing ROS and NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome [210]. Another study found that DHQ attenuates DN in STZ diabetic rats; CAV1 and p-NFκB expression was reduced upon DHQ administration, which suggests that DHQ might have its beneficial effects on DN by downregulating CAV1 [211].
With respect to ischemic stroke, several natural compounds, including calycosin-7-O-β-D-glucoside, baicalin, Momordica charantia polysaccharide, chlorogenic acid, lutein, and lycopene, have shown potential for targeting the RNS/CAV1/MMP signaling pathway (see the review in [212] and references therein).
In regard to ocular diseases, Jiang et al. in 2017 found that CAV1 deficiency exacerbates ocular neovascularization, which ultimately causes vision loss in several pathological visual diseases such as DR. The authors used cavtratin, a lipophilic peptide of CAV1 CSD, to inhibit neovascularization. They found that the combined administration of cavtratin and anti-VEGF-A (the usual treatment for DR) inhibited neovascularization more effectively than monotherapy. Cavtratin exerts its effect by downregulating eNOS expression and the platelet-derived growth factor-B (PDGF-B) in vivothrough the JNK pathway [213]. These two factors play important roles in pathological angiogenesis [214, 215]. This CAV1 peptide may thus have promising therapeutic applications for the treatment of DR by targeting angiogenic factors. Conversely, the in vivo silencing of CAV1 by siRNA in the retina can suppress neovascularization and pathological BRB breakdown in ischemia-induced retinal disease [216]. It is noteworthy to mention that thiazolidinediones or peroxisome proliferator-activated receptor-gamma (PPARγ) agonists that are used to treat T2DM, can suppress retinal neovascularization, attenuate retinal vascular inflammation and BRB breakdown, and promote neuroprotection in the retina [217, 218]. PPARγ activation induces CAV1 [219] and has all the hallmarks of CAV1 action such as the previously mentioned decrease of insulin resistance, adipocyte regulation, and antiproliferative action [220]. A very recent study found that rosiglatizone can protect BBB by attenuating inflammation through a CAV1-depedent pathway. It is yet to be studied in the state of diabetes [221].
Decreasing CAV1 expression in a targeted manner is an important issue to consider as different conditions need different CAV1 protein levels. For instance, CAV1 expression in the brain decreases with age and the effect of silencing CAV1 might be detrimental to cognitive function [179]. Taking this into consideration, engineering a drug based on CAV1 should be formulated in a way to be impermeable through the blood-brain barrier if the desired outcome is downregulating the expression of CAV1 such as in the case of atherosclerosis and nephropathy.
Acknowledgments
This work is funded by the Kuwait Foundation for the Advancement of Sciences (KFAS) grant number RA HM 2018-039.
Conflicts of Interest
The authors have no conflict of interest.
Authors' Contributions
Dania Haddad and Ashraf Al Madhoun contributed equally to this work.
References
- 1.Bastiani M., Parton R. G. Caveolae at a glance. Journal of Cell Science. 2010;123:3831–3836. doi: 10.1242/jcs.070102. [DOI] [PubMed] [Google Scholar]
- 2.Thorn H., Stenkula K. G., Karlsson M., et al. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Molecular Biology of the Cell. 2003;14(10):3967–3976. doi: 10.1091/mbc.e03-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shvets E., Ludwig A., Nichols B. J. News from the caves: update on the structure and function of caveolae. Current Opinion in Cell Biology. 2014;29:99–106. doi: 10.1016/j.ceb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Song K. S., Tang Z., Li S., Lisanti M. P. Mutational analysis of the properties of caveolin-1. Journal of Biological Chemistry. 1997;272(7):4398–4403. doi: 10.1074/jbc.272.7.4398. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary N., Gomez G. A., Howes M. T., et al. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biology. 2014;12(4, article e1001832) doi: 10.1371/journal.pbio.1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon K.-A., Zajicek H., Li W.-P., et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. The EMBO Journal. 2009;28(8):1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiani M., Liu L., Hill M. M., et al. MURC/cavin-4 and cavin family members form tissue-specific caveolar complexes. The Journal of Cell Biology. 2009;185(7):1259–1273. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelkmans L., Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436(7047):128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 9.Parton R. G., del Pozo M. A. Caveolae as plasma membrane sensors, protectors and organizers. Nature Reviews Molecular Cell Biology. 2013;14(2):98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi T., Kitahara Y., Harada Y., Harada S., Takamoto M., Ishibashi T. Immunologic features of mice with streptozotocin-induced diabetes: depression of their immune responses to sheep red blood cells. Diabetes. 1980;29(7):516–523. doi: 10.2337/diab.29.7.516. [DOI] [PubMed] [Google Scholar]
- 11.Lajoie P., Nabi I. R. Lipid rafts, caveolae, and their endocytosis. International Review of Cell and Molecular Biology. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Couet J., Lisanti M. P. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. Journal of Biological Chemistry. 1996;271(46):29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shajahan A. N., Timblin B. K., Sandoval R., Tiruppathi C., Malik A. B., Minshall R. D. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. Journal of Biological Chemistry. 2004;279(19):20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y., Lee S.-J., Minshall R. D., Choi A. M. K. Caveolin-1: a critical regulator of lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2011;300(2):L151–L160. doi: 10.1152/ajplung.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ketteler J., Klein D. Caveolin-1, cancer and therapy resistance. International Journal of Cancer. 2018;143(9):2092–2104. doi: 10.1002/ijc.31369. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Rojo M. A., Ramm G. A. Caveolin-1 function in liver physiology and disease. Trends in Molecular Medicine. 2016;22(10):889–904. doi: 10.1016/j.molmed.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Nevins A. K., Thurmond D. C. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. The Journal of Biological Chemistry. 2006;281(28):18961–18972. doi: 10.1074/jbc.M603604200. [DOI] [PubMed] [Google Scholar]
- 18.Bae G. D., Park E.-Y., Kim K., Jang S.-E., Jun H.-S., Oh Y. S. Upregulation of caveolin-1 and its colocalization with cytokine receptors contributes to beta cell apoptosis. Scientific Reports. 2019;9(1, article 16785) doi: 10.1038/s41598-019-53278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen A. W., Razani B., Wang X. B., et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. American Journal of Physiology-Cell Physiology. 2003;285(1):C222–C235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 20.Zeng W., Tang J., Li H., et al. Caveolin-1 deficiency protects pancreatic β cells against palmitate-induced dysfunction and apoptosis. Cellular Signalling. 2018;47:65–78. doi: 10.1016/j.cellsig.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Thurmond D. C. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. Journal of Cell Science. 2009;122(7):893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia F., Gao X., Kwan E., et al. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. Journal of Biological Chemistry. 2004;279(23):24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- 23.Henquin J. C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 24.Cheng H., Straub S. G., Sharp G. W. G. Inhibitory role of Src family tyrosine kinases on Ca2+-dependent insulin release. American Journal of Physiology-Endocrinology and Metabolism. 2007;292(3):E845–E852. doi: 10.1152/ajpendo.00103.2006. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Seitz R., Lisanti M. P. Phosphorylation of caveolin by src tyrosine kinases. The α-isoform of caveolin is selectively phosphorylated by v-Src in vivo. Journal of Biological Chemistry. 1996;271(7):3863–3868. doi: 10.1074/jbc.271.7.3863. [DOI] [PubMed] [Google Scholar]
- 26.Kepner E. M., Yoder S. M., Oh E., et al. Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion. American Journal of Physiology. Endocrinology and Metabolism. 2011;301(6):E1072–E1080. doi: 10.1152/ajpendo.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel S., Noda M., Cerione R. A., Sharp G. W. G. A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry. 2002;41(30):9663–9671. doi: 10.1021/bi025604p. [DOI] [PubMed] [Google Scholar]
- 28.Li G., Rungger-Brändle E., Just I., Jonas J. C., Aktories K., Wollheim C. B. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Molecular Biology of the Cell. 1994;5(11):1199–1213. doi: 10.1091/mbc.5.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couet J., Li S., Okamoto T., Ikezu T., Lisanti M. P. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. Journal of Biological Chemistry. 1997;272(10):6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 30.Razani B., Zhang X. L., Bitzer M., von Gersdorff G., Böttinger E. P., Lisanti M. P. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. Journal of Biological Chemistry. 2001;276(9):6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 31.Huang C., Hepler J. R., Chen L. T., Gilman A. G., Anderson R. G. W., Mumby S. M. Organization of G proteins and adenylyl cyclase at the plasma membrane. Molecular Biology of the Cell. 1997;8(12):2365–2378. doi: 10.1091/mbc.8.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razani B., Lisanti M. P. Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. American Journal of Physiology-Cell Physiology. 2001;281(4):C1241–C1250. doi: 10.1152/ajpcell.2001.281.4.C1241. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M., Toya Y., Schwencke C., Lisanti M. P., Myers M. G., Ishikawa Y. Caveolin is an activator of insulin receptor signaling. Journal of Biological Chemistry. 1998;273(41):26962–26968. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 34.Schlegel A., Wang C., Pestell R. G., Lisanti M. P. Ligand-independent activation of oestrogen receptor α by caveolin-1. Biochemical Journal. 2001;359(1):203–210. doi: 10.1042/bj3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang I. H., Kim J. H., Lee B. D., et al. Localization of phospholipase C-gamma1 signaling in caveolae: importance in EGF-induced phosphoinositide hydrolysis but not in tyrosine phosphorylation. FEBS Letters. 2001;491(1-2):4–8. doi: 10.1016/s0014-5793(01)02165-2. [DOI] [PubMed] [Google Scholar]
- 36.Czarny M., Lavie Y., Fiucci G., Liscovitch M. Localization of phospholipase D in detergent-insoluble, caveolin-rich membrane domains: modulation by caveolin-1 expression and caveolin-1182–101. Journal of Biological Chemistry. 1999;274(5):2717–2724. doi: 10.1074/jbc.274.5.2717. [DOI] [PubMed] [Google Scholar]
- 37.Gustavsson J., Parpal S., Karlsson M., et al. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. The FASEB Journal. 1999;13(14):1961–1971. [PubMed] [Google Scholar]
- 38.Hahn-Obercyger M., Graeve L., Madar Z. A high-cholesterol diet increases the association between caveolae and insulin receptors in rat liver. Journal of Lipid Research. 2009;50(1):98–107. doi: 10.1194/jlr.M800441-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Nystrom F. H., Chen H., Cong L. N., Li Y., Quon M. J. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Molecular Endocrinology. 1999;13(12):2013–2024. doi: 10.1210/mend.13.12.0392. [DOI] [PubMed] [Google Scholar]
- 40.Zimnicka A. M., Husain Y. S., Shajahan A. N., et al. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Molecular Biology of the Cell. 2016;27(13):2090–2106. doi: 10.1091/mbc.E15-11-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson M., Thorn H., Parpal S., Strålfors P., Gustavsson J. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. The FASEB Journal. 2002;16(2):249–251. doi: 10.1096/fj.01-0646fje. [DOI] [PubMed] [Google Scholar]
- 42.Yuan T., Hong S., Yao Y., Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Research. 2007;17(9):772–782. doi: 10.1038/cr.2007.73. [DOI] [PubMed] [Google Scholar]
- 43.González-Muñoz E., López-Iglesias C., Calvo M., Palacín M., Zorzano A., Camps M. Caveolin-1 loss of function accelerates glucose transporter 4 and insulin receptor degradation in 3T3-L1 adipocytes. Endocrinology. 2009;150(8):3493–3502. doi: 10.1210/en.2008-1520. [DOI] [PubMed] [Google Scholar]
- 44.Tavaré J. M., Denton R. M. Studies on the autophosphorylation of the insulin receptor from human placenta. Analysis of the sites phosphorylated by two-dimensional peptide mapping. Biochemical Journal. 1988;252(2):607–615. doi: 10.1042/bj2520607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen M. C., Shulman G. I. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X. J., Rothenberg P., Kahn C. R., et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 47.Sasaoka T., Ishihara H., Sawa T., et al. Functional importance of amino-terminal domain of Shc for interaction with insulin and epidermal growth factor receptors in phosphorylation-independent manner. Journal of Biological Chemistry. 1996;271(33):20082–20087. doi: 10.1074/jbc.271.33.20082. [DOI] [PubMed] [Google Scholar]
- 48.Danielsson A., Ost A., Lystedt E., et al. Insulin resistance in human adipocytes occurs downstream of IRS1 after surgical cell isolation but at the level of phosphorylation of IRS1 in type 2 diabetes. The FEBS Journal. 2005;272(1):141–151. doi: 10.1111/j.1432-1033.2004.04396.x. [DOI] [PubMed] [Google Scholar]
- 49.Di Guglielmo G. M., Drake P. G., Baass P. C., Authier F., Posner B. I., Bergeron J. J. Insulin receptor internalization and signalling. Molecular and Cellular Biochemistry. 1998;182(1-2):59–63. [PubMed] [Google Scholar]
- 50.Bevan A. P., Drake P. G., Bergeron J. J., Posner B. I. Intracellular signal transduction: the role of endosomes. Trends in Endocrinology and Metabolism. 1996;7(1):13–21. doi: 10.1016/1043-2760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim C. A., Delépine M., Boutet E., et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. The Journal of Clinical Endocrinology & Metabolism. 2008;93(4):1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 52.Ye J., Gao Z., Yin J., He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 53.Varela-Guruceaga M., Milagro F. I., Martínez J. A., de Miguel C. Effect of hypoxia on caveolae-related protein expression and insulin signaling in adipocytes. Molecular and Cellular Endocrinology. 2018;473:257–267. doi: 10.1016/j.mce.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Palacios-Ortega S., Varela-Guruceaga M., Martínez J. A., de Miguel C., Milagro F. I. Effects of high glucose on caveolin-1 and insulin signaling in 3T3-L1 adipocytes. Adipocyte. 2016;5(1):65–80. doi: 10.1080/21623945.2015.1122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabayama K., Sato T., Saito K., et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brännmark C., Palmér R., Glad S. T., Cedersund G., Strålfors P. Mass and information feedbacks through receptor endocytosis govern insulin signaling as revealed using a parameter-free modeling framework. The Journal of Biological Chemistry. 2010;285(26):20171–20179. doi: 10.1074/jbc.M110.106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catalán V., Gómez-Ambrosi J., Rodríguez A., et al. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clinical Endocrinology. 2008;68(2):213–219. doi: 10.1111/j.1365-2265.2007.03021.x. [DOI] [PubMed] [Google Scholar]
- 58.Ohnuma K., Yamochi T., Uchiyama M., et al. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Molecular and Cellular Biology. 2005;25(17):7743–7757. doi: 10.1128/MCB.25.17.7743-7757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghorpade D. S., Ozcan L., Zheng Z., et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555(7698):673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fielding C. J., Fielding P. E. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2003;1610(2):219–228. doi: 10.1016/S0005-2736(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 61.Hailstones D., Sleer L. S., Parton R. G., Stanley K. K. Regulation of caveolin and caveolae by cholesterol in MDCK cells. Journal of Lipid Research. 1998;39(2):369–379. [PubMed] [Google Scholar]
- 62.Parpal S., Karlsson M., Thorn H., Strålfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. The Journal of Biological Chemistry. 2001;276(13):9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 63.Razani B., Combs T. P., Wang X. B., et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. The Journal of Biological Chemistry. 2002;277(10):8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 64.Wernstedt Asterholm I., Mundy D. I., Weng J., Anderson R. G. W., Scherer P. E. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metabolism. 2012;15(2):171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heimerl S., Liebisch G., Le Lay S., et al. Caveolin-1 deficiency alters plasma lipid and lipoprotein profiles in mice. Biochemical and Biophysical Research Communications. 2008;367(4):826–833. doi: 10.1016/j.bbrc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Frank P. G., Lee H., Park D. S., Tandon N. N., Scherer P. E., Lisanti M. P. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(1):98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 67.Ring A., Le Lay S., Pohl J., Verkade P., Stremmel W. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochimica et Biophysica Acta. 2006;1761(4):416–423. doi: 10.1016/j.bbalip.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Briand N., Prado C., Mabilleau G., et al. Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes. 2014;63(12):4032–4044. doi: 10.2337/db13-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsui Y., Tomaru U., Miyoshi A., et al. Overexpression of TNF-α converting enzyme promotes adipose tissue inflammation and fibrosis induced by high fat diet. Experimental and Molecular Pathology. 2014;97(3):354–358. doi: 10.1016/j.yexmp.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 70.Oh D.-J., Kim H. R., Lee M.-K., Woo Y. S. Profile of human β-defensins 1,2 and proinflammatory cytokines (TNF-α, IL-6) in patients with chronic kidney disease. Kidney & Blood Pressure Research. 2013;37(6):602–610. doi: 10.1159/000355740. [DOI] [PubMed] [Google Scholar]
- 71.Palacios-Ortega S., Varela-Guruceaga M., Algarabel M., Milagro F. I., Martínez J. A., de Miguel C. Effect of TNF-alpha on caveolin-1 expression and insulin signaling during adipocyte differentiation and in mature adipocytes. Cellular Physiology and Biochemistry. 2015;36(4):1499–1516. doi: 10.1159/000430314. [DOI] [PubMed] [Google Scholar]
- 72.Wang X. M., Kim H. P., Song R., Choi A. M. K. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. American Journal of Respiratory Cell and Molecular Biology. 2006;34(4):434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu T., Meng Q., Ji J., Zhang L., Lou X. TLR4 and caveolin-1 in monocytes are associated with inflammatory conditions in diabetic neuropathy. Clinical and Translational Science. 2017;10(3):178–184. doi: 10.1111/cts.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poy M. N., Hausser J., Trajkovski M., et al. miR-375 maintains normal pancreatic α- and β-cell mass. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsunoda T., Takagi T. Estimating transcription factor bindability on DNA. Bioinformatics. 1999;15(7-8):622–630. doi: 10.1093/bioinformatics/15.7.622. [DOI] [PubMed] [Google Scholar]
- 76.Trajkovski M., Hausser J., Soutschek J., et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 77.Baudrand R., Goodarzi M. O., Vaidya A., et al. A prevalent caveolin-1 gene variant is associated with the metabolic syndrome in Caucasians and Hispanics. Metabolism. 2015;64(12):1674–1681. doi: 10.1016/j.metabol.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen S., Wang X., Wang J., et al. Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis. 2016;246:148–156. doi: 10.1016/j.atherosclerosis.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nizam R., Al-Ozairi E., Goodson J. M., et al. Caveolin-1 variant is associated with the metabolic syndrome in Kuwaiti children. Frontiers in Genetics. 2018;9:p. 689. doi: 10.3389/fgene.2018.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farré D., Roset R., Huerta M., et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Research. 2003;31(13):3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quest A. F. G., Lobos-González L., Nuñez S., et al. The caveolin-1 connection to cell death and survival. Current Molecular Medicine. 2013;13(2):266–281. doi: 10.2174/156652413804810745. [DOI] [PubMed] [Google Scholar]
- 82.Williams T. M., Sotgia F., Lee H., et al. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. The American Journal of Pathology. 2006;169(5):1784–1801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J., Lee P., Galbiati F., Kitsis R. N., Lisanti M. P. Caveolin-1 expression sensitizes fibroblastic and epithelial cells to apoptotic stimulation. American Journal of Physiology Cell Physiology. 2001;280(4):C823–C835. doi: 10.1152/ajpcell.2001.280.4.C823. [DOI] [PubMed] [Google Scholar]
- 84.Bitar M. S., Abdel-Halim S. M., Al-Mulla F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. American Journal of Physiology Endocrinology and Metabolism. 2013;305(8):E951–E963. doi: 10.1152/ajpendo.00189.2013. [DOI] [PubMed] [Google Scholar]
- 85.Lee H., Park D. S., Razani B., Russell R. G., Pestell R. G., Lisanti M. P. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (-/-) null mice show mammary epithelial cell hyperplasia. The American Journal of Pathology. 2002;161(4):1357–1369. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Razani B., Engelman J. A., Wang X. B., et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. Journal of Biological Chemistry. 2001;276(41):38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 87.Park J. H., Han H. J. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. American Journal of Physiology-Cell Physiology. 2009;297(4):C935–C944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- 88.Galbiati F., Volonté D., Liu J., et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Molecular Biology of the Cell. 2001;12(8):2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernandez M. A., Albor C., Ingelmo-Torres M., et al. Caveolin-1 is essential for liver regeneration. Science. 2006;313(5793):1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 90.Fuchs S. Y., Adler V., Buschmann T., Wu X., Ronai Z. Mdm2 association with p53 targets its ubiquitination. Oncogene. 1998;17(19):2543–2547. doi: 10.1038/sj.onc.1202200. [DOI] [PubMed] [Google Scholar]
- 91.Volonte D., Zou H., Bartholomew J. N., Liu Z., Morel P. A., Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) Journal of Biological Chemistry. 2015;290(7):4202–4214. doi: 10.1074/jbc.m114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seoane J., Le H.-V., Shen L., Anderson S. A., Massagué J. Integration of Smad and Forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117(2):211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 93.van den Heuvel A. P. J., Schulze A., Burgering B. M. T. Direct control of caveolin-1 expression by FOXO transcription factors. Biochemical Journal. 2005;385(3):795–802. doi: 10.1042/BJ20041449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nho R. S., Peterson M., Hergert P., Henke C. A. FoxO3a (Forkhead Box O3a) deficiency protects idiopathic pulmonary fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS One. 2013;8(4, article e61017) doi: 10.1371/journal.pone.0061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sisci D., Maris P., Grazia Cesario M., et al. The estrogen receptor α is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. Cell Cycle. 2013;12(21):3405–3420. doi: 10.4161/cc.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volonte D., Kahkonen B., Shapiro S., Di Y., Galbiati F. Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. The Journal of Biological Chemistry. 2009;284(9):5462–5466. doi: 10.1074/jbc.C800225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng J., Tamaskovic R., Yang Z., et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. Journal of Biological Chemistry. 2004;279(34):35510–35517. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 98.Milne D., Kampanis P., Nicol S., et al. A novel site of AKT-mediated phosphorylation in the human MDM2 onco-protein. FEBS Letters. 2004;577(1-2):270–276. doi: 10.1016/j.febslet.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 99.Mayo L. D., Donner D. B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia H., Khalil W., Kahm J., Jessurun J., Kleidon J., Henke C. A. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. The American Journal of Pathology. 2010;176(6):2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Y., Chu M., Hong J., Shang J., Xu D. Hypoxia induces cardiac fibroblast proliferation and phenotypic switch: a role for caveolae and caveolin-1/PTEN mediated pathway. Journal of Thoracic Disease. 2014;6(10):1458–1468. doi: 10.3978/j.issn.2072-1439.2014.08.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Potenza M., Gagliardi S., Nacci C., Carratu M., Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Current Medicinal Chemistry. 2009;16(1):94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 103.Sowers J. R. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schubert W., Frank P. G., Woodman S. E., et al. Microvascular hyperpermeability in caveolin-1 (-/-) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. Journal of Biological Chemistry. 2002;277(42):40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 105.Komarova Y., Malik A. B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annual Review of Physiology. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 106.Liu J., Liu Z. Muscle insulin resistance and the inflamed microvasculature: fire from within. International Journal of Molecular Sciences. 2019;20(3):p. 562. doi: 10.3390/ijms20030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities—from bench to bedside. European Heart Journal. 2014;35(45):3180–3193. doi: 10.1093/eurheartj/ehu427. [DOI] [PubMed] [Google Scholar]
- 108.Urakami-Harasawa L., Shimokawa H., Nakashima M., Egashira K., Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. The Journal of Clinical Investigation. 1997;100(11):2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo A., Lee M. Y., Yang K., Gross R. W., Sessa W. C. Caveolin-1 regulates lipid droplet metabolism in endothelial cells via autocrine prostacyclin-stimulated, cAMP-mediated lipolysis. The Journal of Biological Chemistry. 2018;293(3):973–983. doi: 10.1074/jbc.RA117.000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saliez J., Bouzin C., Rath G., et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117(8):1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 111.Yang K.-C., Rutledge C. A., Mao M., et al. Caveolin-1 modulates cardiac gap junction homeostasis and arrhythmogenecity by regulating cSrc tyrosine kinase. Circulation: Arrhythmia and Electrophysiology. 2014;7(4):701–710. doi: 10.1161/CIRCEP.113.001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKinnon R. L., Bolon M. L., Wang H.-X., et al. Reduction of electrical coupling between microvascular endothelial cells by NO depends on connexin37. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297(1):H93–H101. doi: 10.1152/ajpheart.01148.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Payne G. W., Madri J. A., Sessa W. C., Segal S. S. Histamine inhibits conducted vasodilation through endothelium-derived NO production in arterioles of mouse skeletal muscle. The FASEB Journal. 2004;18(2):280–286. doi: 10.1096/fj.03-0752com. [DOI] [PubMed] [Google Scholar]
- 114.Looft-Wilson R. C., Billaud M., Johnstone S. R., Straub A. C., Isakson B. E. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012;1818(8):1895–1902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jasmin J. F., Rengo G., Lymperopoulos A., et al. Caveolin-1 deficiency exacerbates cardiac dysfunction and reduces survival in mice with myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2011;300(4):H1274–H1281. doi: 10.1152/ajpheart.01173.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rong B., Xie F., Sun T., Hao L., Lin M.-J., Zhong J.-Q. Nitric oxide, PKC-ε, and connexin43 are crucial for ischemic preconditioning-induced chemical gap junction uncoupling. Oncotarget. 2016;7(43):69243–69255. doi: 10.18632/oncotarget.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gupta I., Goyal A., Singh N., Yadav H., Sharma P. Hemin, a heme oxygenase-1 inducer, restores the attenuated cardioprotective effect of ischemic preconditioning in isolated diabetic rat heart. Human & Experimental Toxicology. 2017;36(8):867–875. doi: 10.1177/0960327116673169. [DOI] [PubMed] [Google Scholar]
- 118.Bernatchez P., Sharma A., Bauer P. M., Marin E., Sessa W. C. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. The Journal of Clinical Investigation. 2011;121(9):3747–3755. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michel J. B., Feron O., Sacks D., Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. Journal of Biological Chemistry. 2004;272(25):15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 120.Feron O., Dessy C., Moniotte S., Desager J. P., Balligand J. L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. The Journal of Clinical Investigation. 1999;103(6):897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yue L., Bian J.-T., Grizelj I., et al. Apolipoprotein E enhances endothelial-NO production by modulating caveolin 1 interaction with endothelial NO synthase. Hypertension. 2012;60(4):1040–1046. doi: 10.1161/HYPERTENSIONAHA.112.196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yada T., Shimokawa H., Tachibana H. Endothelium-dependent hyperpolarization-mediated vasodilatation compensates nitric oxide-mediated endothelial dysfunction during ischemia in diabetes-induced canine coronary collateral microcirculation in vivo. Microcirculation. 2018;25(5, article e12456) doi: 10.1111/micc.12456. [DOI] [PubMed] [Google Scholar]
- 123.Hofmann A., Gosemann J.-H., Takahashi T., Friedmacher F., Duess J. W., Puri P. Imbalance of caveolin-1 and eNOS expression in the pulmonary vasculature of experimental diaphragmatic hernia. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2014;101(4):341–346. doi: 10.1002/bdrb.21117. [DOI] [PubMed] [Google Scholar]
- 124.Du X. L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. The Journal of Clinical Investigation. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Federici M., Menghini R., Mauriello A., et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106(4):466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 126.Mineo C., Shaul P. W. Regulation of eNOS in caveolae. In: Jasmin J. F., Frank P. G., Lisanti M. P., editors. Caveolins and Caveolae. Vol. 729. New York, NY, USA: Springer; 2012. pp. 51–62. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 127.Lu R., Tsuboi T., Okumura-Noji K., Iwamoto N., Yokoyama S. Caveolin-1 facilitates internalization and degradation of ABCA1 and probucol oxidative products interfere with this reaction to increase HDL biogenesis. Atherosclerosis. 2016;253:54–60. doi: 10.1016/j.atherosclerosis.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 128.RamÍrez C. M., Zhang X., Bandyopadhyay C., et al. Caveolin-1 regulates atherogenesis by attenuating Low-Density Lipoprotein transcytosis and vascular inflammation Independently of endothelial nitric oxide synthase activation. Circulation. 2019;140(3):225–239. doi: 10.1161/CIRCULATIONAHA.118.038571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luitse M. J. A., Biessels G. J., Rutten G. E. H. M., Kappelle L. J. Diabetes, hyperglycaemia, and acute ischaemic stroke. The Lancet Neurology. 2012;11(3):261–271. doi: 10.1016/S1474-4422(12)70005-4. [DOI] [PubMed] [Google Scholar]
- 130.Jasmin J. F., Malhotra S., Singh Dhallu M., Mercier I., Rosenbaum D. M., Lisanti M. P. Caveolin-1 deficiency increases cerebral ischemic injury. Circulation Research. 2007;100(5):721–729. doi: 10.1161/01.RES.0000260180.42709.29. [DOI] [PubMed] [Google Scholar]
- 131.Gu Y., Zheng G., Xu M., et al. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. Journal of Neurochemistry. 2012;120(1):147–156. doi: 10.1111/j.1471-4159.2011.07542.x. [DOI] [PubMed] [Google Scholar]
- 132.Chen Z., Hu Q., Xie Q., et al. Effects of treadmill exercise on motor and cognitive function recovery of MCAO mice through the caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochemical Research. 2019;44(4):930–946. doi: 10.1007/s11064-019-02728-1. [DOI] [PubMed] [Google Scholar]
- 133.Choi K.-H., Kim H.-S., Park M.-S., et al. Overexpression of caveolin-1 attenuates brain edema by inhibiting tight junction degradation. Oncotarget. 2016;7(42):67857–67867. doi: 10.18632/oncotarget.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muir A. B., Quisling R. G., Yang M. C. K., Rosenbloom A. L. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004;27(7):1541–1546. doi: 10.2337/diacare.27.7.1541. [DOI] [PubMed] [Google Scholar]
- 135.Sonveaux P., Martinive P., DeWever J., et al. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circulation Research. 2004;95(2):154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 136.Ito A., Shiroto T., Godo S., et al. Important roles of endothelial caveolin-1 in endothelium-dependent hyperpolarization and ischemic angiogenesis in mice. American Journal of Physiology. Heart and Circulatory Physiology. 2019;316(4):H900–H910. doi: 10.1152/ajpheart.00589.2018. [DOI] [PubMed] [Google Scholar]
- 137.Mokhtar S. S., Vanhoutte P. M., Leung S. W. S., et al. Endothelium dependent hyperpolarization-type relaxation compensates for attenuated nitric oxide-mediated responses in subcutaneous arteries of diabetic patients. Nitric Oxide. 2016;53:35–44. doi: 10.1016/j.niox.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 138.Cho W. J., Chow A. K., Schulz R., Daniel E. E. Caveolin-1 exists and may function in cardiomyocytes. Canadian Journal of Physiology and Pharmacology. 2010;88(1):73–76. doi: 10.1139/Y09-114. [DOI] [PubMed] [Google Scholar]
- 139.Patel H. H., Tsutsumi Y. M., Head B. P., et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. The FASEB Journal. 2007;21(7):1565–1574. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 140.Zhao Y.-Y., Liu Y., Stan R.-V., et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen A. W., Park D. S., Woodman S. E., et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. American Journal of Physiology-Cell Physiology. 2003;284(2):C457–C474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 142.Hnasko R., Lisanti M. P. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Molecular Interventions. 2003;3(8):445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- 143.Huxley R. R., Filion K. B., Konety S., Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. The American Journal of Cardiology. 2011;108(1):56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yi S. L., Liu X. J., Zhong J. Q., Zhang Y. Role of caveolin-1 in atrial fibrillation as an anti-fibrotic signaling molecule in human atrial fibroblasts. PLoS One. 2014;9, article e0224190 doi: 10.1371/journal.pone.0085144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lim A. K. Diabetic nephropathy – complications and treatment. International Journal of Nephrology and Renovascular Disease. 2014;7:361–381. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moriyama T., Tsuruta Y., Shimizu A., et al. The significance of caveolae in the glomeruli in glomerular disease. Journal of Clinical Pathology. 2011;64(6):504–509. doi: 10.1136/jcp.2010.087023. [DOI] [PubMed] [Google Scholar]
- 147.Lee Y. J., Kim M. O., Ryu J. M., Han H. J. Regulation of SGLT expression and localization through Epac/PKA-dependent caveolin-1 and F-actin activation in renal proximal tubule cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2012;1823(4):971–982. doi: 10.1016/j.bbamcr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 148.Peng F., Zhang B., Wu D., Ingram A. J., Gao B., Krepinsky J. C. TGFβ-induced RhoA activation and fibronectin production in mesangial cells require caveolae. American Journal of Physiology-Renal Physiology. 2008;295(1):F153–F164. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng F., Wu D., Ingram A. J., Zhang B., Gao B., Krepinsky J. C. RhoA activation in mesangial cells by mechanical strain depends on caveolae and caveolin-1 interaction. Journal of the American Society of Nephrology. 2007;18(1):189–198. doi: 10.1681/ASN.2006050498. [DOI] [PubMed] [Google Scholar]
- 150.Zhang B., Peng F., Wu D., Ingram A. J., Gao B., Krepinsky J. C. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cellular Signalling. 2007;19(8):1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 151.Guan T. H., Chen G., Gao B., et al. Caveolin-1 deficiency protects against mesangial matrix expansion in a mouse model of type 1 diabetic nephropathy. Diabetologia. 2013;56(9):2068–2077. doi: 10.1007/s00125-013-2968-z. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y., Peng F., Gao B., Ingram A. J., Krepinsky J. C. High glucose-induced RhoA activation requires caveolae and PKCβ1-mediated ROS generation. American Journal of Physiology. Renal Physiology. 2012;302(1):F159–F172. doi: 10.1152/ajprenal.00749.2010. [DOI] [PubMed] [Google Scholar]
- 153.Li X. C., Gu V., Miguel-Qin E., Zhuo J. L. Role of caveolin 1 in AT1a receptor-mediated uptake of angiotensin II in the proximal tubule of the kidney. American Journal of Physiology. Renal Physiology. 2014;307(8):F949–F961. doi: 10.1152/ajprenal.00199.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wyse B. D., Prior I. A., Qian H., et al. Journal of Biological Chemistry. 2003;278(26):23738–23746. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- 155.Tesfaye S., Boulton A. J. M., Dyck P. J., et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Denhez B., Lizotte F., Guimond M.-O., Jones N., Takano T., Geraldes P. Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. Journal of Biological Chemistry. 2015;290(1):350–358. doi: 10.1074/jbc.M114.612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arya A., Yadav H. N., Sharma P. L. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Molecular and Cellular Biochemistry. 2011;354(1-2):57–66. doi: 10.1007/s11010-011-0805-6. [DOI] [PubMed] [Google Scholar]
- 158.Huang T., Ren J. J., Huang J., Li D. Association of homocysteine with type 2 diabetes: a meta-analysis implementing Mendelian randomization approach. BMC Genomics. 2013;14(1):p. 867. doi: 10.1186/1471-2164-14-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pushpakumar S., Kundu S., Sen U. Hydrogen sulfide protects hyperhomocysteinemia-induced renal damage by modulation of caveolin and eNOS interaction. Scientific Reports. 2019;9(1, article 2223) doi: 10.1038/s41598-018-38467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frank R. N. Diabetic retinopathy. The New England Journal of Medicine. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 161.Fong D. S., Aiello L. P., Ferris F. L., Klein R. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–2553. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 162.Nagasaka Y., Kaneko H., Ye F., et al. Role of caveolin-1 for blocking the epithelial-mesenchymal transition in proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science. 2017;58(1):221–229. doi: 10.1167/iovs.16-20513. [DOI] [PubMed] [Google Scholar]
- 163.Klaassen I., Hughes J. M., Vogels I. M. C., Schalkwijk C. G., Van Noorden C. J. F., Schlingemann R. O. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Experimental Eye Research. 2009;89(1):4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 164.Gu X., Reagan A. M., McClellan M. E., Elliott M. H. Caveolins and caveolae in ocular physiology and pathophysiology. Progress in Retinal and Eye Research. 2017;56:84–106. doi: 10.1016/j.preteyeres.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu X., Li Z., Luo D., et al. Exogenous advanced glycosylation end products induce diabetes-like vascular dysfunction in normal rats: a factor in diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology. 2003;241(1):56–62. doi: 10.1007/s00417-002-0575-7. [DOI] [PubMed] [Google Scholar]
- 166.Stitt A. W., Burke G. A., Chen F., McMullen C. B., Vlassara H. Advanced glycation end-product receptor interactions on microvascular cells occur within caveolin-rich membrane domains. The FASEB Journal. 2000;14(15):2390–2392. doi: 10.1096/fj.00-0289fje. [DOI] [PubMed] [Google Scholar]
- 167.Stitt A. W., Bhaduri T., McMullen C. B., Gardiner T. A., Archer D. B. Advanced glycation end products induce blood–retinal barrier dysfunction in normoglycemic rats. Molecular Cell Biology Research Communications. 2000;3(6):380–388. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- 168.Schubert W., Frank P. G., Razani B., Park D. S., Chow C.-W., Lisanti M. P. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. The Journal of Biological Chemistry. 2001;276(52):48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 169.Rosengren B.-I., Rippe A., Rippe C., Venturoli D., Swärd K., Rippe B. Transvascular protein transport in mice lacking endothelial caveolae. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(3):H1371–H1377. doi: 10.1152/ajpheart.01364.2005. [DOI] [PubMed] [Google Scholar]
- 170.Virgintino D., Robertson D., Errede M., et al. Expression of caveolin-1 in human brain microvessels. Neuroscience. 2002;115(1):145–152. doi: 10.1016/s0306-4522(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 171.Shieh J. C., Schaar B. T., Srinivasan K., Brodsky F. M., McConnell S. K. Endocytosis regulates cell soma translocation and the distribution of adhesion proteins in migrating neurons. PLoS One. 2011;6(3, article e17802) doi: 10.1371/journal.pone.0017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Head B. P., Hu Y., Finley J. C., et al. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. The Journal of Biological Chemistry. 2011;286(38):33310–33321. doi: 10.1074/jbc.M111.255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mandyam C. D., Schilling J. M., Cui W., et al. Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biological Psychiatry. 2017;81(2):101–110. doi: 10.1016/j.biopsych.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mikol D. D., Scherer S. S., Duckett S. J., Hong H. L., Feldman E. L. Schwann cell caveolin-1 expression increases during myelination and decreases after axotomy. Glia. 2002;38(3):191–199. doi: 10.1002/glia.10063. [DOI] [PubMed] [Google Scholar]
- 175.Silva W. I., Maldonado H. M., Velázquez G., García J. O., González F. A. Caveolins in glial cell model systems: from detection to significance. Journal of Neurochemistry. 2007;103(s1) Suppl 1:101–112. doi: 10.1111/j.1471-4159.2007.04712.x. [DOI] [PubMed] [Google Scholar]
- 176.Tan W., Rouen S., Barkus K. M., et al. Nerve growth factor blocks the glucose-induced down-regulation of caveolin-1 expression in Schwann cells via p75 neurotrophin receptor signaling. The Journal of Biological Chemistry. 2003;278(25):23151–23162. doi: 10.1074/jbc.M212986200. [DOI] [PubMed] [Google Scholar]
- 177.Yu C., Rouen S., Dobrowsky R. T. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56(8):877–887. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Niesman I. R., Schilling J. M., Shapiro L. A., et al. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. Journal of Neuroinflammation. 2014;11(1, article 39) doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Head B. P., Peart J. N., Panneerselvam M., et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5(12, article e15697) doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trushina E., Singh R. D., Dyer R. B., et al. Mutant huntingtin inhibits clathrin-independent endocytosis and causes accumulation of cholesterol in vitro and in vivo. Human Molecular Genetics. 2006;15(24):3578–3591. doi: 10.1093/hmg/ddl434. [DOI] [PubMed] [Google Scholar]
- 181.Allen J. A., Yadav P. N., Setola V., Farrell M., Roth B. L. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Translational Psychiatry. 2011;1(8, article e33) doi: 10.1038/tp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wennberg A. M. V., Gottesman R. F., Kaufmann C. N., et al. Diabetes and cognitive outcomes in a nationally representative sample: the National Health and Aging Trends Study. International Psychogeriatrics. 2014;26(10):1729–1735. doi: 10.1017/S1041610214001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang X., Zhao L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochemical and Biophysical Research Communications. 2016;473(2):428–434. doi: 10.1016/j.bbrc.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 184.Wu J., Zhou S.-L., Pi L.-H., et al. High glucose induces formation of tau hyperphosphorylation via Cav-1-mTOR pathway: a potential molecular mechanism for diabetes-induced cognitive dysfunction. Oncotarget. 2017;8(25):40843–40856. doi: 10.18632/oncotarget.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hayashi T., Juliet P. A. R., Miyazaki A., Ignarro L. J., Iguchi A. High glucose downregulates the number of caveolae in monocytes through oxidative stress from NADPH oxidase: implications for atherosclerosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2007;1772(3):364–372. doi: 10.1016/j.bbadis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 186.Carrillo-Sepulveda M. A., Matsumoto T. Phenotypic modulation of mesenteric vascular smooth muscle cells from type 2 diabetic rats is associated with decreased caveolin-1 expression. Cellular Physiology and Biochemistry. 2014;34(5):1497–1506. doi: 10.1159/000366354. [DOI] [PubMed] [Google Scholar]
- 187.Trushina E., Du Charme J., Parisi J., McMurray C. T. Neurological abnormalities in caveolin-1 knock out mice. Behavioural Brain Research. 2006;172(1):24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 188.Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jung H.-J., Kim Y.-J., Eggert S., Chung K. C., Choi K. S., Park S. A. Age-dependent increases in tau phosphorylation in the brains of type 2 diabetic rats correlate with a reduced expression of p62. Experimental Neurology. 2013;248:441–450. doi: 10.1016/j.expneurol.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 190.Bonds J. A., Shetti A., Bheri A., et al. Depletion of caveolin-1 in type 2 diabetes model induces Alzheimer’s disease pathology precursors. The Journal of Neuroscience. 2019;39(43):8576–8583. doi: 10.1523/JNEUROSCI.0730-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McGuire J. F., Rouen S., Siegfreid E., Wright D. E., Dobrowsky R. T. Caveolin-1 and altered neuregulin signaling contribute to the pathophysiological progression of diabetic peripheral neuropathy. Diabetes. 2009;58(11):2677–2686. doi: 10.2337/db09-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang J.-X., Hua L., Li Y.-Q., et al. Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit. The Journal of Neuroscience. 2015;35(1):36–52. doi: 10.1523/JNEUROSCI.1161-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li C.-D., Zhao J.-Y., Chen J.-L., et al. Mechanism of the JAK2/STAT3-CAV-1-NR2B signaling pathway in painful diabetic neuropathy. Endocrine. 2019;64(1):55–66. doi: 10.1007/s12020-019-01880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Syme C. A., Zhang L., Bisello A. Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Molecular Endocrinology. 2006;20(12):3400–3411. doi: 10.1210/me.2006-0178. [DOI] [PubMed] [Google Scholar]
- 195.Levine Y. C., Li G. K., Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. The Journal of Biological Chemistry. 2007;282(28):20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 196.Salani B., Maffioli S., Hamoudane M., et al. Caveolin-1 is essential for metformin inhibitory effect on IGF1 action in non-small-cell lung cancer cells. The FASEB Journal. 2012;26(2):788–798. doi: 10.1096/fj.11-192088. [DOI] [PubMed] [Google Scholar]
- 197.Salis O., Bedir A., Ozdemir T., Okuyucu A., Alacam H. The relationship between anticancer effect of metformin and the transcriptional regulation of certain genes (CHOP, CAV-1, HO-1, SGK-1 and Par-4) on MCF-7 cell line. European Review for Medical and Pharmacological Sciences. 2014;18(11):1602–1609. [PubMed] [Google Scholar]
- 198.Chung Y. C., Chang C. M., Wei W. C., Chang T. W., Chang K. J., Chao W. T. Metformin-induced caveolin-1 expression promotes T-DM1 drug efficacy in breast cancer cells. Scientific Reports. 2018;8(1, article 3930) doi: 10.1038/s41598-018-22250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.De Wet H., Proks P. Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochemical Society Transactions. 2015;43(5):901–907. doi: 10.1042/BST20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Müller G., Schulz A., Wied S., Frick W. Regulation of lipid raft proteins by glimepiride- and insulin-induced glycosylphosphatidylinositol-specific phospholipase C in rat adipocytes. Biochemical Pharmacology. 2005;69(5):761–780. doi: 10.1016/j.bcp.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 201.Puddu A., Salani B., Cordera R., Viviani G. L., Maggi D. Caveolin-1 is essential for glimepiride-induced insulin secretion in the pancreatic βTC-6 cell line. Biochemical and Biophysical Research Communications. 2008;375(2):235–237. doi: 10.1016/j.bbrc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 202.Sampson L. J., Hayabuchi Y., Standen N. B., Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circulation Research. 2004;95(10):1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 203.Ohnuma K., Dang N. H., Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends in Immunology. 2008;29(6):295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 204.Vellecco V., Mitidieri E., Gargiulo A., et al. Vascular effects of linagliptin in non-obese diabetic mice are glucose-independent and involve positive modulation of the endothelial nitric oxide synthase (eNOS)/caveolin-1 (CAV-1) pathway. Diabetes, Obesity and Metabolism. 2016;18(12):1236–1243. doi: 10.1111/dom.12750. [DOI] [PubMed] [Google Scholar]
- 205.Salheen S. M., Panchapakesan U., Pollock C. A., Woodman O. L. The dipeptidyl peptidase-4 inhibitor linagliptin preserves endothelial function in mesenteric arteries from type 1 diabetic rats without decreasing plasma glucose. PLoS One. 2015;10(11, article e0143941) doi: 10.1371/journal.pone.0143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elvira B., Honisch S., Almilaji A., et al. Up-regulation of Na+-coupled glucose transporter SGLT1 by caveolin-1. Biochimica et Biophysica Acta, Biomembranes. 2013;1828(11):2394–2398. doi: 10.1016/j.bbamem.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 207.Ghezzi C., Calmettes G., Morand P., Ribalet B., John S. Real-time imaging of sodium glucose transporter (SGLT1) trafficking and activity in single cells. Physiological Reports. 2017;5(3, article e13062) doi: 10.14814/phy2.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sharma A., Sellers S., Stefanovic N., et al. Direct endothelial nitric oxide synthase activation provides atheroprotection in diabetes-accelerated atherosclerosis. Diabetes. 2015;64(11):3937–3950. doi: 10.2337/db15-0472. [DOI] [PubMed] [Google Scholar]
- 209.Van Krieken R., Krepinsky J. C. Caveolin-1 in the pathogenesis of diabetic nephropathy: potential therapeutic target? Current Diabetes Reports. 2017;17(3):p. 19. doi: 10.1007/s11892-017-0844-9. [DOI] [PubMed] [Google Scholar]
- 210.Ding T., Wang S., Zhang X., et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53. doi: 10.1016/j.phymed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 211.Zhao Y., Huang W., Wang J., Chen Y., Huang W., Zhu Y. Taxifolin attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. American Journal of Translational Research. 2018;10:1205–1210. [PMC free article] [PubMed] [Google Scholar]
- 212.Chen H.-S., Chen X., Li W.-T., Shen J.-G. Targeting RNS/caveolin-1/MMP signaling cascades to protect against cerebral ischemia-reperfusion injuries: potential application for drug discovery. Acta Pharmacologica Sinica. 2018;39(5):669–682. doi: 10.1038/aps.2018.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jiang Y., Lin X., Tang Z., et al. Critical role of caveolin-1 in ocular neovascularization and multitargeted antiangiogenic effects of cavtratin via JNK. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(40):10737–10742. doi: 10.1073/pnas.1706394114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gratton J. P., Lin M. I., Yu J., et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4(1):31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 215.Bucci M., Gratton J. P., Rudic R. D., et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature Medicine. 2000;6(12):1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 216.Tian X.-F., Xia X.-B., Xu H.-Z., Xiong S.-Q., Jiang J. Caveolin-1 expression regulates blood-retinal barrier permeability and retinal neovascularization in oxygen-induced retinopathy. Clinical & Experimental Ophthalmology. 2012;40(1):e58–e66. doi: 10.1111/j.1442-9071.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- 217.Higuchi A., Ohashi K., Shibata R., Sono-Romanelli S., Walsh K., Ouchi N. Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(1):46–53. doi: 10.1161/ATVBAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Normando E. M., Davis B. M., De Groef L., et al. The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson’s disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathologica Communications. 2016;4(1, article 86) doi: 10.1186/s40478-016-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen X., Whiting C., Borza C., et al. Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Molecular and Cellular Biology. 2010;30(12):3048–3058. doi: 10.1128/MCB.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Panigrahy D., Singer S., Shen L. Q., et al. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. The Journal of Clinical Investigation. 2002;110(7):923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao Y., Wei X., Song J., Zhang M., Huang T., Qin J. Peroxisome proliferator-activated receptor γ agonist rosiglitazone protects blood-brain barrier integrity following diffuse axonal injury by decreasing the levels of inflammatory mediators through a caveolin-1-dependent pathway. Inflammation. 2019;42(3):841–856. doi: 10.1007/s10753-018-0940-2. [DOI] [PubMed] [Google Scholar]