Abstract
Objectives
To assess the association between blood circulating vitamin D levels and colorectal cancer risk in the Asian population.
Design
This is a systematic review and dose-response meta-analysis of observational studies that investigated the relationship between blood circulating vitamin D levels and colorectal cancer risk in the Asian population.
Data sources
Relevant studies were identified through a literature search in Medline, Embase and Web of Science from 1st January 1980 to 31st January 2019. Eligibility criteria: original studies published in peer-reviewed journals investigating the association between blood circulating vitamin D levels and the risk of colorectal cancer and/or adenoma in Asian countries.
Data extraction and synthesis
Two authors independently extracted data and assessed the quality of included studies. Study-specific ORs were pooled using a random-effects model. A dose-response meta-analysis was performed with generalised least squares regression. We applied the Newcastle-Ottawa Scale quality assessment to evaluate the quality of the selected studies.
Results
The eight included studies encompassed a total of 2916 cases and 6678 controls. The pooled ORs of colorectal cancer for the highest versus lowest categories of blood circulating vitamin D levels was 0.75 (95% CI 0.58 to 0.97) up to 36.5 ng/mL in the Asian population. There was heterogeneity among the studies (I 2=53.9%, P heterogeneity=0.034). The dose-response meta-analysis indicated a significant linear relationship (P non-linearity=0.11). An increment of 16 ng/mL in blood circulating vitamin D level corresponded to an OR of 0.79 (95% CI 0.64 to 0.97).
Conclusions
The results of this meta‐analysis indicate that blood circulating vitamin D level is associated with decreased risk of colorectal cancer in Asian countries. The dose-response meta-analysis shows that the strength of this association among the Asian population is similar to that among the Western population. Our study suggests that the Asian population should improve nutritional status and maintain a higher level of blood circulating vitamin D.
Keywords: colorectal cancer, colorectal adenoma, vitamin D, 25-hydroxyvitamin D, Asia, meta-analysis
Strengths and limitations of this study.
Our study seeks to extend previous work by including a number of new studies and by distinguishing the Asian population explicitly.
The number of included studies is not sufficient to provide a robust estimate, so the results should be interpreted in the context of the limitations of the available data.
Heterogeneous definitions of blood circulating vitamin D categories were used across studies. The variability in definitions could limit comparability between studies.
Our study included seven case-control studies; the study design implies that the measurement of blood circulating vitamin D is measured in individuals already diagnosed with colorectal cancer. Results from case-control studies need to be interpreted cautiously because of the potential for reverse causation.
Time of blood sampling in relation to outcome ascertainment also varied among studies. Such cross-sectional measurements may not accurately reflect an individual’s vitamin D status across time.
INTRODUCTION
Colorectal cancer is the third most commonly diagnosed cancer and second in terms of mortality. With over 1.8 million new cases, and 881 000 deaths worldwide in 2018, it accounts for about 1 in 10 cancer cases and deaths.1 Some Asian countries where the incidence of colorectal cancer was historically low, such as Japan, Israel, Singapore, China and the Philippines, have experienced rising incidence rates over the past decades. In 2012, Japan (Miyagi Prefecture Cancer Registry) presented the highest colorectal cancer incidence in the world for men (62 per 100 000 persons) and women (37 per 100 000 persons).2 Observational studies have identified several risk factors associated with an increased incidence of colorectal cancer including lifestyle factors (eg, obesity, physical inactivity, smoking and heavy alcohol use) and non-modifiable factors (eg, ageing, personal and family history of colorectal cancer or adenoma).3 Other observational studies conducted in Western countries suggest blood circulating 25-hydroxyvitamin D (25(OH)D) (vitamin D) has a protective role in the development of colorectal cancer.4–8 Some meta-analyses have consistently reported that there was an inverse association between plasma vitamin D concentration in the blood and incidence of, and mortality from, colorectal cancer.9–15
The prevalence of vitamin D deficiency has increased in recent decades.16 17 In a recent population-based study of Asian adults, approximately 75% had suboptimal vitamin D concentrations.18 The Endocrine Society Clinical Practice Guideline defines vitamin D deficiency as 25(OH)D level <20 ng/mL and insufficiency as 21 to 29 ng/mL.19 Feldman et al 20 reported antineoplastic actions of vitamin D, particularly in colorectal cancer.9 Touvier et al 12 reported that improving vitamin D levels could be beneficial in reducing colorectal cancer incidence. Data from a cohort of healthy women showed that plasma vitamin D levels were inversely related to the occurrence and death from colorectal cancer.21 In the Nurses' Health Study, total circulating vitamin D was associated with a lower risk of colorectal cancer in white women.22 A recent international study of 17 cohorts in the Western population found that vitamin D deficiency was associated with increased colorectal cancer risk, and vitamin D above sufficiency levels was associated with 19% to 27% lower risk.23 Compared with Western countries, there was an inconsistent conclusion about the relationship between blood circulating vitamin D level and colorectal cancer risk in studies of Asian countries,24–30 given that lifestyles, and ethnic and environmental factors are different between Asian and Western countries.
We hypothesised that the association between blood circulating vitamin D and colorectal cancer in Asian countries is distinct from Western countries. Thus, this review aimed to summarise epidemiological evidence regarding blood circulating vitamin D level and colorectal cancer risk in Asian countries. This study underlines the public health importance of attaining and maintaining an optimal vitamin D status in the Asian population and may help to guide clinical and nutritional practice in Asians countries.
METHODS
We performed the systematic review according to a predetermined protocol and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines.31 Two reviewers (LZ and ZQ) independently undertook the literature search, assessment for eligibility, data extraction and qualitative assessment. Any inconsistencies between the two reviewers were reviewed by a third reviewer (YJ) and resolved by consensus.
Eligibility criteria
Participants: Our study uses the list of sovereign states and dependent territories in Asia by the United Nations (https://unstats.un.org/unsd/methodology/m49/) to draw participants from 48 countries located in five regions (central Asia, eastern Asia, southern Asia, south-eastern Asia and western Asia). Asians and people of Asian origin who live in Western countries were excluded.
Exposure: The exposure is blood circulating 25(OH)D level which is commonly measured to assess and monitor vitamin D status in individuals. Most studies only report the total level and do not distinguish D2 and D3 forms of the vitamin. In our meta-analysis, we consider the total level of vitamin D as the exposure.
Comparators (controls): In order to be eligible for inclusion, studies must compare outcomes in a group of exposed individuals with the highest category of blood circulating vitamin D level and a group of unexposed individuals with the lowest category of blood circulating vitamin D level.
Outcome: Studies included in the review have a diagnosis of colorectal cancer or colorectal adenoma, clinically confirmed by colonoscopy or pathology.
Study design: We target observational studies (case-control, cross-sectional and cohort). English language studies conducted post-1980 were considered eligible. Animal studies were excluded.
Studies were excluded if they: (1) Were reviews, editorials, case reports or guideline articles. (2) Did not explicitly state the blood circulating vitamin D level and its association with colorectal cancer risk. (3) Allowed controls to have a previous disease history of cancer. (4) Focused on the Western population or Asian population living in Western countries. (5) Investigated the blood circulating vitamin D level and its association with survival of colorectal cancer. By consensus among all three reviewers (LZ, ZQ and YJ), if data sources were duplicated in more than one study, only the original study was included in the meta-analysis.
Search strategy
We conducted a literature search using Medline, Embase and Web of Science, and retrieved all relevant articles that reported the associated plasma or serum vitamin D level and the risk of colorectal neoplasia in Asian countries, published from 1st January 1980 to 31st January 2019. Medical Subject Heading terms were used in conjunction with the following keywords for our search: (colorectal neoplasm or colon neoplasm or colorectal cancer or colon cancer) AND (25-OH-D or cholecalciferol or calcidiol or calcitriol or 25-hydroxyvitamin D or hydroxycholecalciferols or 25-hydroxyvitamin D3 or 1-alpha-hydroxylase or vitamin D) AND (Asia* or Afghanistan or Armenia or Azerbaijan or Bahrain or Bangladesh or Bhutan or Brunei or Cambodia or China or Cyprus or Georgia or India or Indonesia or Iran or Iraq or Israel or Japan or Jordan or Kazakhstan or Kuwait or Kyrgyzstan or Laos or Lebanon or Malaysia or Maldives or Mongolia or Myanmar or Burma or Nepal or North Korea or Oman or Pakistan or Palestine or Philippines or Qatar or Russia or Saudi Arabia or Singapore or South Korea or Sri Lanka or Syria or Taiwan or Tajikistan or Thailand or Timor-Leste or Turkey or Turkmenistan or United Arab Emirates or Uzbekistan or Vietnam or Yemen). Full search strings are presented in online supplementary table S1. References from relevant articles, editorials, conference abstracts, letters and reviews were thoroughly reviewed to identify additional studies. Full manuscripts of every article with a relevant title and abstract were then reviewed for eligibility.
bmjopen-2019-030513supp001.pdf (5.9MB, pdf)
Data extraction and qualitative assessment
Two reviewers (LZ and ZQ) independently extracted the following study-level characteristics from each eligible study: first author, year of publication, type of study, country where the study was conducted, selection criteria, the numbers of cases and controls (for case-control studies or cross-sectional studies) and the numbers of total participants and incident cases (for cohort studies), population characteristics (sex and age), follow-up period (for cohort studies), sample size, levels of vitamin D in both case and control groups, measures and ranges of vitamin D, adjusted variables, and risk estimates with corresponding 95% CI for each category. For studies that reported both crude and adjusted estimates of the association between blood circulating vitamin D and risk of colorectal cancer or adenoma, we used the adjusted estimates for the meta-analysis. For studies that reported several adjusted estimates of association, we used the estimates adjusted for the most variables.
We applied the Newcastle-Ottawa Scale (NOS) quality assessment tool to evaluate the quality of the selected observational studies. This tool was used to measure the key aspects of the methodology in selected studies with regard to design quality and the risk of biassed estimates based on three design criteria: (1) Selection of study participants. (2) Comparability of study groups. (3) Assessment of outcome and exposure with a star system (with a maximum of 9 stars). We judged studies that received a score of 7–9 stars to be at low risk of bias, studies that scored 4–6 stars to be at medium risk, and those that scored three or less to be at high risk of bias. A funnel plot was used to assess the publication bias. Any disagreement on the data extraction and quality assessment of the studies were resolved through comprehensive discussion (LZ, ZQ and YJ).
Statistical analysis
Study-specific OR estimates were combined using a random-effects model, that considers within-study and between-study variations. Corresponding 95% CIs were extracted directly from articles where available, with adjusted ORs extracted preferentially over unadjusted ORs. The dose-response analysis was used to assess the relationship between blood circulating vitamin D level and colorectal cancer risk using the generalised least squares method to resolve the inconsistency issue of different vitamin D levels in included studies.32 33 For the dose-response meta-analysis of blood circulating vitamin D levels, we used a method proposed by previous studies to compute the trend from the correlated log OR estimates across categories of vitamin D levels.34 This analytical method collected the distribution of cases and controls, median values of blood circulating vitamin D levels, and corresponding OR estimates in each category for each study. The assigned value of the lowest category was designated as a reference level. If the study did not provide median values of blood vitamin D, the midpoint of the upper and lower boundaries in each category was assigned. For the open-ended exposure categories, the length of the open-ended interval was assumed to be the same as that of the adjacent interval. We examined a potential non-linear dose-response relationship between blood circulating vitamin D level with colorectal cancer risk by modelling vitamin D levels using random-effects restricted cubic splines with three knots at centiles 25%, 50% and 75% of the distribution (spline model). A p value for non-linearity was calculated by testing the null hypothesis that the regression coefficient of the second spline was equal to zero by Wald-type test of non-linear hypotheses.34 A small p value (<0.05) of the Wald-type test indicates departure from linearity. The non-linear dose-response relationship was confirmed by several representative point values and the risk estimates of a subgroup analysis based on the range of exposure.
The statistical heterogeneity among studies was evaluated using Cochran’s Q test and I2 statistic, with values of 25%, 50% and 75% representing low, moderate and high heterogeneity, respectively.35 The criterion for identifying heterogeneity was a p value less than 0.05 for the Q test. If substantial heterogeneity was detected, we performed univariate meta-regression analyses to explore the proportion of between-study variance explained by study quality, participant characteristics and study characteristics. We were unable to perform a multivariate meta-regression analysis as only a small number of included studies reported information for all study-level factors. We performed subgroup analyses comparing pooled association estimates and heterogeneity with stratification by participant’s sex, outcome type, subregion of Asia, blood sample type and range of vitamin D levels (the range is the difference in the midpoint between the highest and lowest categories of blood circulating vitamin D in each study). An estimation of publication bias was evaluated by Begg’s funnel plot, in which the SE of log (OR) of each study was plotted against its log (OR). An asymmetrical plot suggests possible publication bias. Egger's linear regression test assessed funnel plot asymmetry, a statistical approach to identify funnel plot asymmetry on the natural logarithm scale of the ORs. All statistical analyses were performed using Stata V.14.2 (StataCorp, College Station, Texas). All p values were two-sided, and p<0.05 was considered as statistically significant.
RESULTS
Selection of studies
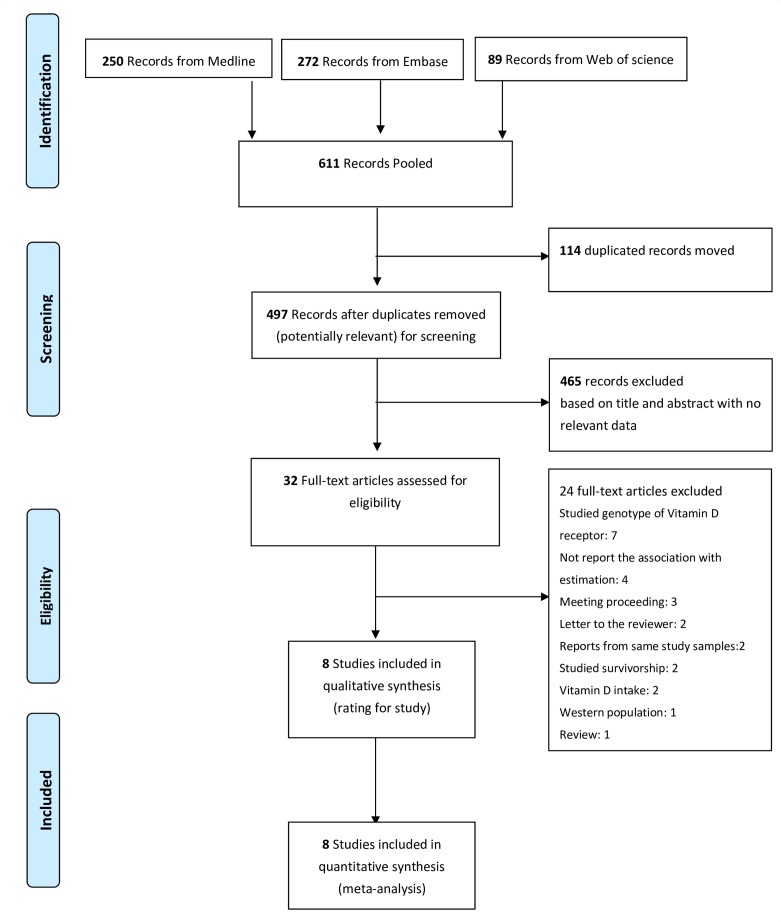
A detailed PRISMA flow diagram31 of literature search and inclusion criteria is shown in figure 1. A total of 611 studies was initially identified with this literature search (250 from Medline, 272 from Embase and 89 from Web of Science), but 114 studies were excluded due to duplication and 465 were excluded after screening the titles and abstracts. Twenty-four other studies were excluded after full-text review (details shown in online supplementary table S2). Finally, a total of eight studies were identified as eligible for meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection for meta-analysis.
Study characteristics
The eight studies included had a total of 2916 cases and 6678 controls (tables 1 and 2). These studies were published between 2007 and 2018—seven from eastern Asia (four from Japan,24 25 27 30 two from Korea26 28 and one from China36) and one from western Asia.29 Regarding study design, seven were case-control24–29 36 and one was a nested case-cohort study.30 Of the eight studies, four provided the main end point of colorectal cancer24 29 30 36 and the remaining four provided the main end point of colorectal adenoma.25–28
Table 1.
Summary of characteristics for eight studies included in the meta-analysis
| Author (year) | Study design | Country | Study period | Outcome | Outcome ascertainment | Sex | Sample size (n); characteristics | Blood sample type | Vitamin D testing method | |||
| Case or control | No. | Male (%) | Mean age (SD)/median age (range) | |||||||||
| Budhathoki et al (2018)30 | Nested case-cohort | Japan | 1990–2009 | Colorectal cancer | NA | All* | Case | 637 | 52 | 56.2 (7.5) | Plasma | Chemiluminescent enzyme immunoassay |
| Control | 4044 | 34 | 53.7 (7.9) | |||||||||
| Choi et al (2015)37 | Case-control | Korea | 2011–2012 | Colorectal adenoma | Colonoscopy | All | Case | 112 | 51 | 60.3 (5.3) | Serum | Chemiluminescent enzyme immunoassay |
| Control | 112 | 51 | 59.5 (5.4) | |||||||||
| Men | Case | 57 | 100 | NA | Serum | |||||||
| Control | 57 | 100 | NA | |||||||||
| Women | Case | 55 | 0 | NA | Serum | |||||||
| Control | 55 | 0 | NA | |||||||||
| Hong et al (2012)26 | Case-control | Korea | 2009–2010 | Colorectal adenoma | Colonoscopy | All | Case | 143 | 68 | 58.7 (6.0) | Serum | Direct competitive electrochemiluminescence immunoassay |
| Control | 143 | 68 | 58.7 (6.0) | |||||||||
| Otani et al (2007)24 | Case-control | Japan | 1990–2003 | Colorectal cancer | Pathologically confirmed | All | Case | 323 | 52 | NA | Plasma | Competitive protein-binding assay |
| Control | 621 | 52 | NA | |||||||||
| Men | Case | 163 | 100 | 56.9 | ||||||||
| Control | 324 | 100 | 56.9 | |||||||||
| Women | Case | 160 | 0 | 56.5 | ||||||||
| Control | 297 | 0 | 56.4 | |||||||||
| Takahashi et al (2010)25 | Case-control | Japan | 1997–2004 | Colorectal adenoma | Colonoscopy | Men | Case | 656 | 100 | 52.0 (1.4) | Plasma or Serum | Radioimmunoassay method |
| Control | 648 | 100 | 51.8 (1.5) | |||||||||
| Yamaji et al (2012)27 | Case-control | Japan | 2004–2005 | Colorectal adenoma | Colonoscopy | All | Case | 737 | 67 | NA | Plasma | Radioimmunoassay method |
| Control | 703 | 65 | NA | |||||||||
| Ying et al (2015)36 | Case-control | China | 2010–2014 | Colorectal cancer | Colonoscopy | All | Case | 212 | NA | 63(56-73) | Plasma | Direct competitive ELISA |
| Control | 212 | NA | 63(57-73) | |||||||||
| Yurekli et al (2015)29 | Case-control | Turkey | 2012 | Colorectal cancer | Colonoscopy | All | Cases | 96 | 67 | 57.8 (10.0) | Serum | NA |
| control | 195 | 54 | 51.2 (12.9) | |||||||||
All*: all participants, including men and women
NA, not available; SD, standard deviation.
Table 2.
Summary of risk estimates for eight studies included in the meta-analysis
| Author (year) | Sex | Vitamin D level cut points (ng/ml) |
Vitamin D medium level (ng/ml) |
Number of cases | Number of controls | N | OR | LCI | UCI | Adjusted variables |
| Budhathoki et al (2018) 30 | All* | First Q, 12.5–16.5 ng/mL | 14.7 | 134 | 1004 | 1138 | 1 | Age, sex, BMI, smoking, alcohol use, physical activity, family history of cancer and history of diabetes | ||
| Second Q, 17.6–21.6 ng/mL | 19.9 | 165 | 1000 | 1165 | 1.08 | 0.84 | 1.39 | |||
| Third Q, 21.2–25.6 ng/mL | 21.3 | 160 | 1016 | 1176 | 0.96 | 0.74 | 1.26 | |||
| Fourth Q, 25.8–33.0 ng/mL | 26.5 | 178 | 1024 | 1202 | 0.95 | 0.73 | 1.23 | |||
| Choi et al (2015)37 | All | First Q, 10.3 ng/mL | 10.3 | 37 | 28 | 65 | 1 | Age, sex, BMI, alcohol drinking, smoking status, folate intake; women: additional menopausal status and hormone replacement use | ||
| Second Q, 14.3 ng/mL | 14.3 | 26 | 28 | 54 | 0.72 | 0.31 | 1.66 | |||
| Third Q, 17.9 ng/mL | 17.9 | 28 | 28 | 56 | 0.73 | 0.29 | 1.82 | |||
| Fourth Q, 24.2 ng/mL | 24.2 | 21 | 28 | 49 | 0.49 | 0.19 | 1.27 | |||
| Men | First Q, 11.1 ng/mL | 11.1 | 15 | 14 | 29 | 1 | ||||
| Second Q, 14.6 ng/mL | 14.6 | 13 | 14 | 27 | 1.31 | 0.36 | 4.82 | |||
| Third Q, 17.7 ng/mL | 17.7 | 17 | 15 | 32 | 1.26 | 0.4 | 8.12 | |||
| Fourth Q, 22.7 ng/mL | 22.7 | 12 | 14 | 26 | 1.8 | 0.19 | 8.12 | |||
| Women | First Q, 9.5 ng/mL | 9.5 | 16 | 14 | 30 | 1 | ||||
| Second Q, 13.5 ng/mL | 13.5 | 22 | 14 | 36 | 0.89 | 0.26 | 3.1 | |||
| Third Q, 18.4 ng/mL | 18.4 | 8 | 14 | 22 | 0.54 | 0.13 | 2.16 | |||
| Fourth Q, 25.4 ng/mL | 25.4 | 9 | 13 | 22 | 0.22 | 0.04 | 1.15 | |||
| Hong et al (2012) 26 | All | First Q, <14.3 ng/mL | 10.1 | 31 | 42 | 73 | 1 | Age, sex, BMI, smoking, alcohol drinking, physical activity, corrected calcium level | ||
| Second Q, 14.3–18.5 ng/mL | 16.4 | 29 | 42 | 71 | 0.87 | 0.43 | 1.74 | |||
| Third Q, 18.6–23.8 ng/mL | 21.2 | 41 | 31 | 72 | 0.4 | 0.2 | 0.82 | |||
| Fourth Q, ≥23.9 ng/mL | 29.1 | 42 | 28 | 70 | 0.38 | 0.18 | 0.8 | |||
| Otani et al (2007) 24 | Men | First Q, <22.9 ng/mL | 18.3 | 43 | 74 | 117 | 1 | Age, sex, BMI, smoking, alcohol consumption, physical exercise, vitamin supplement use, family history of colorectal cancer, total energy intake, dietary fibre intake, folate intake, calcium intake, vitamin D intake, n-3 fatty acid intake, red meat intake, fish intake | ||
| Second Q, 22.9–27.5 ng/mL | 25.2 | 40 | 85 | 125 | 0.76 | 0.42 | 1.4 | |||
| Third Q, 27.6–32.0 ng/mL | 29.8 | 36 | 85 | 121 | 0.76 | 0.39 | 1.5 | |||
| Fourth Q, >32.1 ng/mL | 36.5 | 44 | 80 | 124 | 0.73 | 0.35 | 1.5 | |||
| Women | First Q, <18.7 ng/mL | 15.2 | 41 | 77 | 118 | 1 | ||||
| Second Q, 18.7–22.2 ng/mL | 20.5 | 34 | 73 | 107 | 1 | 0.55 | 1.9 | |||
| Third Q, 22.3–26.9 ng/mL | 24.6 | 44 | 71 | 115 | 1.2 | 0.65 | 2.3 | |||
| Fourth Q, >27.0 ng/mL | 31.6 | 41 | 76 | 117 | 1.1 | 0.5 | 2.3 | |||
| Takahashi et al (2010) 25 | Men | First Q, <22 ng/mL | 20 | 128 | 142 | 270 | 1 | BMI, hospital, rank in the self-defence forces, smoking, alcohol drinking, parental history of colorectal cancer, physical activity, type of blood sample, the month of blood drawing | ||
| Second Q, 23–25 ng/mL | 24 | 162 | 156 | 318 | 1.21 | 0.86 | 1.7 | |||
| Third Q, 26–29 ng/mL | 27.5 | 223 | 208 | 431 | 1.21 | 0.87 | 1.69 | |||
| Fourth Q, >30 ng/mL | 33 | 143 | 142 | 285 | 1.25 | 0.85 | 1.84 | |||
| Yamaji et al (2012) 27 | All | First Q, 14–19 ng/mL | 16.5 | 145 | 129 | 274 | 1 | Age, sex, BMI, screening periods, the season of blood collection, smoking, alcohol drinking, family history of colorectal cancer, non-steroidal anti-inflammatory drug use, height, daily energy intake | ||
| Second Q, 20–23 ng/mL | 21.5 | 132 | 128 | 260 | 0.86 | 0.6 | 1.24 | |||
| Third Q, 24–26 ng/mL | 25 | 157 | 145 | 302 | 0.91 | 0.64 | 1.29 | |||
| Fourth Q, 27–31 ng/mL | 29 | 175 | 144 | 319 | 1.03 | 0.73 | 1.46 | |||
| Fifth Q, 31–34 ng/mL | 32.5 | 128 | 157 | 285 | 0.64 | 0.45 | 0.92 | |||
| Ying et al (2015) 36 | All | First Q, <7.29 ng/mL | 3.65 | 80 | 53 | 133 | 1 | Age, sex, BMI, smoking, drinking, history of diabetes, hypertension, vitamin D binding protein | ||
| Second Q, 7.29–14.61 ng/mL | 10.95 | 49 | 53 | 102 | 0.62 | 0.35 | 1.12 | |||
| Third Q, 14.61–28.84 ng/mL | 21.73 | 46 | 53 | 99 | 0.67 | 0.38 | 1.20 | |||
| Fourth Q, ≥28.84 ng/mL | 35.96 | 37 | 53 | 90 | 0.53 | 0.29 | 0.98 | |||
| Yurekli et al (2015) 29 | All | First Q, <8.6 ng/mL | 7.1 | 24 | 48 | 72 | 1 | Age, sex, BMI, smoking, alcohol intake | ||
| Second Q, 8.6–10.1 ng/mL | 9.35 | 20 | 53 | 73 | 0.48 | 0.24 | 0.98 | |||
| Third Q, 10.1–14.5 ng/mL | 12.3 | 25 | 48 | 73 | 0.51 | 0.25 | 1.03 | |||
| Fourth Q, ≥14.5 ng/mL | 18.9 | 27 | 46 | 73 | 0.69 | 0.36 | 1.36 |
All*: all participants, including men and women
BMI, body mass index; LCI, 95% CI’s lower CI; OR, Odds Ratio; UCI, 95% CI’s upper CI.
Meta-analysis and dose-response analysis
The multivariable-adjusted ORs for each study and the combination of all eight studies for the highest versus lowest categories of blood circulating vitamin D levels are shown in figure 2. The mean blood circulating vitamin D level of the included study population was 20.21 ng/mL, with an SD of 7.92 ng/mL, minimal concentration of 3.65 ng/mL and maximal concentration of 36.5 ng/mL. Results from the studies on blood circulating vitamin D levels in relation to colorectal cancer risk were inconsistent, with both inverse and positive associations reported. The pooled ORs of colorectal cancer for the highest versus lowest categories of blood circulating vitamin D level was 0.75 (95% CI 0.58 to 0.97), indicating higher blood circulating vitamin D level had a significant inverse association with risk of colorectal cancer. There was statistically significant heterogeneity among the studies (I 2=53.9%, p=0.034).
Figure 2.

Meta-analysis of association between blood circulating vitamin D and the risk of colorectal cancer. The adjusted ORs of colorectal cancer for the highest versus lowest categories of blood vitamin D level from each study were included. The size of each square is proportional to the weight of the study. The range is the difference in the midpoint between the highest and lowest categories of blood vitamin D.
We evaluated the non-linear dose-response relationship between blood circulating vitamin D levels and colorectal cancer risk. A 16 ng/mL increment (about 2 SDs=15.84 ng/mL) in blood circulating vitamin D levels conferred an OR of 0.79 (95% CI 0.64 to 0.97), which meant the risk of colorectal cancer decreased by 21% for every 16 ng/mL increment in blood vitamin D, and the reduction was statistically significant. A moderate heterogeneity existed (I 2=53.9%, P heterogeneity=0.034) in the overall analysis of blood circulating vitamin D levels, without a significant non-linear dose-response relationship (P non-linearity=0.11), suggesting that the non-linear dose-response relationship does not depart from linearity. Similar trends were observed with linear and spline models (figure 3).
Figure 3.
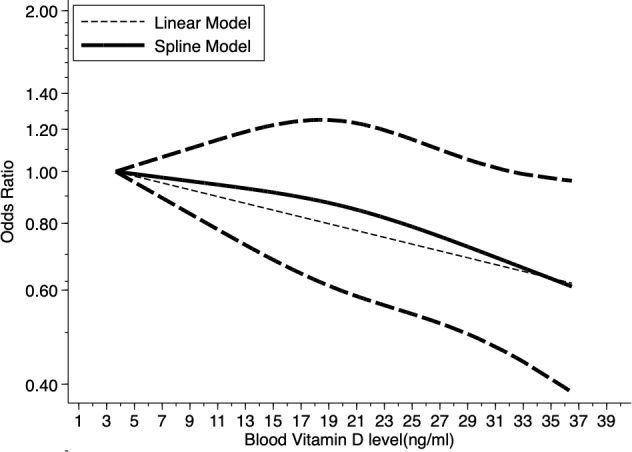
Dose-response relationship between blood vitamin D levels and the ORs of colorectal cancer. Adjusted OR and 95% CIs (dashed lines) are reported. Blood vitamin D levels were modelled with a linear and spline model in a random-effects meta-regression model. The median value of the lowest reference interval (3.65 ng/mL) was used to estimate all ORs. The vertical axis is on a log scale. Lines with long dashes represent the pointwise 95% CIs for the fitted spline model with a non-linear trend (solid line, P non-linearity=0.11). Lines with short dashes represent the linear trend.
Subgroup analysis
When we stratified the analysis according to blood sample type, the pooled ORs of serum sample and plasma for the highest versus lowest categories of blood circulating vitamin D levels were 0.52 (95% CI 0.34 to 0.80) and 0.77 (95% CI 0.59 to 1.00), respectively. The results showed that there was a substantial risk reduction (48%) for blood serum vitamin D levels associated with the risk of colorectal cancer. There was no evidence of significant statistical heterogeneity among studies (I 2=22.67%, p=0.21). We then performed a subgroup analysis of blood circulating vitamin D range in each study. The pooled ORs was 0.93 (95% CI 0.70 to 1.25) for studies with range ≤15 ng/mL and 0.62 (95% CI 0.47 to 0.83) for studies with range >15 ng/mL. There was no evidence of statistical heterogeneity among studies (I 2=28.48%, p=0.08). When stratified by outcome, the pooled ORs were 0.67 (95% CI 0.40 to 1.14) for studies when the outcome was colorectal adenoma and 0.83 (95% CI 0.66 to 1.06) when the outcome was colorectal cancer, respectively, with no statistically significant heterogeneity among studies (I 2=59.48%, p=0.73). When we stratified the studies by sex, the pooled ORs were 0.59 (95% CI 0.13 to 2.74) for studies with estimates for women and 1.13 (95% CI 0.81 to 1.58) for men, respectively, with no statistically significant heterogeneity among studies (I 2=45.89%, p=0.70). We also stratified according to the geographical region of the population. The pooled ORs were 0.75 (95% CI 0.57 to 1.00) for eastern Asia and 0.69 (95% CI 0.36 to 1.34) for western Asia. There was no statistically significant heterogeneity among studies (I 2=59.79%, p=0.87). The subgroup meta-analyses are shown in online supplementary figure S1 and summarised in table 3.
Table 3.
Summary of subgroup analysis for the associations of blood circulating vitamin D and the risk of colorectal cancer and adenoma by outcome, sex, the subregion of Asia, blood sample type and range.
| Subgroup analysis | Number of estimates | Random effect The summary OR (95% CI) |
Ratio of ORs (95% CI) | I 2 (%)* | P value* |
| Outcome | |||||
| Colorectal adenoma | 4 | 0.67 (0.40 to 1.14) | |||
| Colorectal cancer | 4 | 0.83 (0.66 to 1.06) | 1.11 (0.54 to 2.28) | 59.48 | 0.73 |
| Sex | |||||
| Women | 2 | 0.59 (0.13 to 2.74) | |||
| Men | 3 | 1.13 (0.81 to 1.58) | 1.46 (0.03 to 63.5) | 45.89 | 0.70 |
| Subregion | |||||
| Eastern Asia | 7 | 0.75 (0.57 to 1.00) | |||
| Western Asia | 1 | 0.69 (0.36 to 1.34) | 0.92 (0.28 to 2.99) | 59.79 | 0.87 |
| Blood sample type | |||||
| Serum | 3 | 0.52 (0.34 to 0.80) | |||
| Plasma | 4 | 0.77 (0.59 to 1.00) | 1.49 (0.73 to 3.03) | 22.67 | 0.21 |
| Range | |||||
| ≤15 ng/mL | 4 | 0.93 (0.70 to 1.25) | |||
| >15 ng/mL | 4 | 0.62 (0.47 to 0.83) | 0.65 (0.39 to 1.07) | 28.48 | 0.08 |
*I2 and p value related to subgroup differences.
I2, per cent residual variation due to heterogeneity.
Qualitative assessment and publication bias
The NOS tool was used to conduct a qualitative assessment of the selected studies to review the quality of the studies and detect possible bias. Of the eight studies, five were at low risk of bias (8–9) stars.24–27 30 Three studies were at medium risk (5–7 stars) mainly due to bias from representativeness of cases or controls, control definition and response rate.29 36 37 (shown in online supplementary table S3.) The funnel plot and Egger’s statistical test indicated no evidence of publication bias in the studies included in the meta-analysis (p=0.338) (online supplementary figure S2.)
DISCUSSION
Colorectal cancer is one of the cancers with high morbidity and mortality in the world. The one-carbon metabolism pathway requires adequate vitamin D, and this raises the possibility that vitamin D may have an essential role in the risk of colorectal cancer. Many epidemiological studies from Europe and USA believe that increasing the concentration of circulating vitamin D can reduce the morbidity and mortality of colorectal cancer.38 However, the association between blood circulating vitamin D levels and the risk of colorectal cancer in the Asian population is still under debate due to a lack of sufficient evidence. This systematic review highlights the inconsistencies among studies addressing the role of blood circulating vitamin D and colorectal cancer risk in the Asian population. Our systematic review identified eight studies consisting of 2916 cases and 6678 controls that addressed the relationship between blood circulating vitamin D levels and colorectal cancer risk. Our meta-analysis found 25% reduced risk of colorectal cancer for the highest versus lowest categories of blood circulating vitamin D levels (OR=0.75, 95% CI 0.58 to 0.97) up to 36.5 ng/mL, that indicated higher blood circulating vitamin D level has a significant inverse association with risk of colorectal cancer in the Asian population. Our meta-analysis results showed that the negative correlation between vitamin D and the risk of colorectal cancer is similar to that of European and American population studies11 12 21 22 39–42 and consistent with the result of a meta-analysis by Ekmekcioglu C et al,13 that found a pooled relative risk of 0.62 (95% CI 0.56 to 0.70) for colorectal cancer when comparing individuals with the highest category of 25(OH)D with those in the lowest.
Our results found a 16 ng/mL increment in blood circulating vitamin D levels with an OR of 0.79 (95% CI 0.64 to 0.97), which meant the risk of colorectal cancer decreased by 21% for every 16 ng/mL increment in blood vitamin D, and the reduction was statistically significant. Our study also suggested a linear dose-response relationship (P non-linearity=0.11), that was consistent with several studies conducted in Western populations revealing a similar protective dose-response association of the blood circulating vitamin D and colorectal cancer risk. For example, a meta-analysis reported a 26% lower risk of colorectal cancer per 10 ng/mL increment in blood circulating vitamin D levels.11 Most experts define vitamin D deficiency as a vitamin D level of less than 20 ng/mL.43 44 Vitamin D concentration of 21–29 ng/mL can be considered to indicate a relative insufficiency of vitamin D, and a level of 30 ng/mL or higher can be considered to indicate sufficient vitamin D.45 With the use of such definitions, it has been estimated that many people have vitamin D deficiency or insufficiency.43 44 Previous research has implied an association between vitamin D deficiency and an increased incidence of bone fractures.46 There is also data showing vitamin D deficiency to be associated with cancer,47–49 diabetes,50 cognitive impairment51 and all-cause mortality.52 Among patients with colorectal cancer, the prevalence of vitamin D deficiency was much higher (nearly 90%) than among patients with other chronic diseases.53 Humans obtain vitamin D from exposure to sunlight, from natural diets, fortified diets, supplementation, and so on.46 Dose response between vitamin D concentrations and colorectal cancer risk may be different between Asian and Western populations due to ethnic, anthropometric, dietary and environmental factors.12 Lifestyle and diet can promote the development of early onset colon lesions by regulating growth factors that interact with inflammatory pathways.41 An association between vitamin D status and reduced risk of colorectal cancer has been found in ethnically diverse populations.5 Vitamin D interacts with calcium to enhance the reduction of colon cancer risk.54–56 Studies have shown that vitamin D and calcium may interact and that both are needed in reducing cancer risk.57 However, even after adjusting for calcium intake in some studies,6 58 vitamin D was associated with a lower risk. The independent effects of vitamin D are supported, but the combined effects of vitamin D and calcium may be greater than the sum of their independent effects.59 Vitamin A has an antagonistic effect on vitamin D58 and taking both at the same time can lead to decreased calcium absorption. Still, vitamin A is often combined with vitamin D in supplements.
Vitamin D supplementation significantly reduced total cancer mortality but did not reduce total cancer incidence.60 Patients with low-risk prostate cancer under active surveillance may benefit from vitamin D3 supplementation at 4000 IU/d.61 Among patients with metastatic colorectal cancer, the addition of high-dose vitamin D3, versus standard-dose vitamin D3, to standard chemotherapy resulted in a difference in median progression-free survival that was not statistically significant, but with a significantly improved supportive effect.62 Epidemiological evidence links the incidence of colorectal cancer to lifestyle, smoking, physical activity, alcohol consumption and sleep.63 It has also been linked to reduced fruit and vegetable consumption and increased consumption of red meat. Dairy products, fish and other foods, and cooking methods also play an essential role.64 In addition, some drugs such as non-steroidal anti-inflammatory drugs and cyclo-oxygenase inhibitors are also involved.65 Women on oestrogen therapy, for example, did not reduce their risk of colorectal cancer by taking vitamin D and calcium supplements.66 Increased dietary fibre intake reduces the risk of colorectal cancer and obesity; and low physical activity may reduce plasma 25(OH)D concentration, thereby increasing the risk of colorectal cancer.42 For every 1 kg/m2 increase in BMI of patients with colorectal cancer, serum vitamin D level decreased significantly (0.46 ng/mL).67 Most variation in vitamin D levels usually comes from exposure to the sun, which is an essential source of vitamin D for people who get more from fish, even in Japan.68
This meta-analytical comparison revealed a statistically significant beneficial effect of blood circulating vitamin D for colorectal cancer. However, the diversity of the studies and the presence of moderate heterogeneity in the overall analysis of blood circulating vitamin D levels (I 2=53.9%, P heterogeneity=0.034) may preclude making meaningful conclusions from the pooled estimate because it may not reflect the true underlying effect. The subgroup analysis did not explain much of the heterogeneity.
Potential reasons for the heterogeneity in the strength of the association may include the following. First, food consumption and vitamin supplements varied according to the specific dietary habits and lifestyle in each Asian subregion. A systemic review studied correlations between various diet types, food or nutrients, and colorectal cancer risk among Asians, and suggested that red meats, processed meats, preserved foods, saturated/animal fats, cholesterol, high-sugar foods, spicy foods, tubers or refined carbohydrates have a positive association with colorectal cancer risk.69 Besides diet, other personal and lifestyle factors (eg, exposure to sunlight, obesity, smoking and drinking habit) may alter the strength of the association and contribute to the heterogeneity of the association in Asian countries. The numbers of studies from the different Asian subregions included in our meta-analysis were imbalanced, so we were cautious in interpreting results. For example, our meta-analysis included seven studies from eastern Asia, only one from western Asia, and none from central, southern or south-eastern Asia. Our study revealed no evidence of publication bias, and most of the studies included in our meta-analysis verified the diagnosis of colorectal cancer for cases. Histological confirmation of cancer diagnoses for cases was an optimal validation for the case-control design in our meta-analysis.
Possible confounders for the association between colorectal cancer and blood circulating vitamin D levels include sex, age, family history of colorectal cancer, smoking, alcohol drinking, body mass index and diabetes. Most studies in our meta-analysis provided risk estimates that were adjusted for age,24 26–30 36 sex,24 26 27 29 30 36 body mass index,24–30 36 smoking,24–30 36 drinking,24–26 29 36 physical activity,30 alcohol consumption24–30 and family history of colorectal cancer.24 25 29 Fewer were adjusted for folate,24 37 energy intake,24 27 hypertension,36 vitamin D binding protein,36 blood collection24 27 or for vitamin supplement use.24 For these studies, the observed reduced risk of colorectal cancer associated with vitamin D levels is likely confounded by one or more of these factors.
In the overall analysis for both adenoma and carcinoma that is part of our subgroup analysis, we report a statistically significant association; yet, in the stratified analysis by colorectal adenoma or colorectal cancer separately, the association was not statistically significant. The results, however, show that associations were in the same direction (ORs<1 indicating an inverse relationship). Uncontrolled confounders (eg, dietary sources of vitamin D, consumption of fish/fibres containing 25(OH)D, exposure to the sun, folate/calcium intake, vitamin D supplement use, etc) in the original studies that are part of our meta-analysis may be responsible for these differences. Of note, only one of the eight studies in our meta-analysis adjusted for these confounders.24 The presence of negative confounders in original colorectal cancer studies (ORs are closer to 1), and positive confounders in original colorectal adenoma studies (ORs are further from 1) as well as some effect modification may also be responsible for the statistically significant difference noted. We used a random effects model in our analysis to reduce this effect. Further investigation of the subgroup analysis show that the weight of the studies could also be contributory. Two studies25 30 contribute a large weight in the subgroup meta-analysis, but a smaller weight in the overall analysis.
Our analysis had the following limitations. First, the number of included studies is not sufficient to provide a robust estimate of the association of blood circulating vitamin D levels and colorectal cancer risk, so the analysis and results should be interpreted in the context of the limitations of the available data. Second, heterogeneous definitions of blood circulating vitamin D categories were used across studies. The variability in definitions could limit comparability between studies. Third, our study included seven case-control studies; the study design implied that the measurement of blood circulating vitamin D is in individuals already diagnosed with colorectal cancer. The results from this study need to be interpreted cautiously because of the potential for reverse causation. The time of blood sampling in relation to outcome ascertainment also varied among studies. Such cross-sectional measurements may not accurately reflect an individual’s vitamin D status across time. Fourth, some studies included in our meta-analysis did not adjust for potentially relevant confounders which may have led to residual confounding and may explain some of the observed heterogeneity. Fifth, the difference in the method used for measuring blood circulating vitamin D levels may also be a source of heterogeneity between the included studies. Sixth, in the dose-response analysis, the literature selected listed the median vitamin D value, instead of the original vitamin D value, which could also lead to inaccurate results. Seventh, although we assessed the quality of the observational studies in this meta-analysis with the NOS quality assessment tool, Bae70 suggested that it is more reasonable to control for quality level by performing subgroup analysis according to study design rather than by using the NOS tool. In this context, however, we did not perform subgroup analysis according to study design since the included eight studies were case-control and nested case-cohort studies. Finally, a recent meta-analysis investigated the association between blood circulating vitamin D levels and survival of colorectal cancer and found that the pooled HRs (95% CIs) were 0.68 (0.55 to 0.85) and 0.67 (0.57 to 0.78), respectively, for overall and colorectal cancer-specific survival comparing highest versus lowest categories of blood vitamin D.15 Our study did not explore the association of blood circulating vitamin D levels and colorectal cancer mortality, however, and this association in Asian countries is one we would encourage future studies to examine.
CONCLUSION
Blood circulating vitamin D level is inversely associated with colorectal cancer prevalence in the Asian population. Our findings on the inverse association between blood circulating vitamin D and the risk of colorectal cancer in the Asian population suggest the need for Asians to improve their nutritional status and maintain higher blood circulating vitamin D levels. This meta-analysis provides valuable information for future research on the association between blood circulating vitamin D and colorectal cancer risk in the Asian population. A multinational, population-based study in Asian countries may resolve the issue of heterogeneity and generate detailed information on blood circulating vitamin D levels and the risk of colorectal cancer. Further studies may also focus on evaluating the association of vitamin D levels with colorectal cancer mortality.60
Supplementary Material
Footnotes
Contributors: LZ: Study concept and design; acquisition of data; statistical analysis; interpretation of data;drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. HZ: Interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. YZ: Interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. CH: Drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. AA: Drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. XQ: Interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. PJ: Interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. YJ: Study concept and design; acquisition and interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript. ZQ: Study concept and design; acquisition of data; interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript.
Funding: This study was supported by the National Key R&D Program of China (No. 2018YFC1315000/2018YFC1315003), the State Key Laboratory of Urban and Regional Ecology of China (SKLURE2018-2-5), the National Health Commission Key Laboratory of Birth Defects Prevention, and the Key Laboratory of Population Defects Intervention Technology of Henan Province (ZD201905). Details of the role of the study sponsor: LZ is supported by Peking Union Medical College Hospital 100 Elite Scholarship and Melbourne International Research Scholarship, The University of Melbourne. Peng Jia, Director of the International Initiative on Spatial Lifecourse Epidemiology (ISLE), thanks Lorentz Center, the Netherlands Organization for Scientific Research, the Royal Netherlands Academy of Arts and Sciences, the Chinese Center for Disease Control and Prevention, and the West China School of Public Health in Sichuan University for funding the ISLE and supporting ISLE’s research activities.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethics approval is not required for this study because it is a systematic review.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not applicable as no data sets were generated and/or analysed for this study.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Ward EM, et al. . Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 3. Johnson CM, Wei C, Ensor JE, et al. . Meta-Analyses of colorectal cancer risk factors. Cancer Causes Control 2013;24:1207–22. 10.1007/s10552-013-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. . Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ 2010;340:b5500 10.1136/bmj.b5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woolcott CG, Wilkens LR, Nomura AMY, et al. . Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: the Multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2010;19:130–4. 10.1158/1055-9965.EPI-09-0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu K, Feskanich D, Fuchs CS, et al. . A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 2007;99:1120–9. 10.1093/jnci/djm038 [DOI] [PubMed] [Google Scholar]
- 7. Sy AM, Bautista JEK. Association between serum vitamin D levels and colonic carcinomatous polyps. J Gastrointest Cancer 2013;44:481–5. 10.1007/s12029-013-9533-3 [DOI] [PubMed] [Google Scholar]
- 8. Heath AK, Hodge AM, Ebeling PR, et al. . Circulating 25-hydroxyvitamin D concentration and risk of breast, prostate, and colorectal cancers: the Melbourne Collaborative cohort study. Cancer Epidemiol Biomarkers Prev 2019;28:900–8. 10.1158/1055-9965.EPI-18-1155 [DOI] [PubMed] [Google Scholar]
- 9. Grant WB, Garland CF. Reviews: a critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer 2004;48:115–23. 10.1207/s15327914nc4802_1 [DOI] [PubMed] [Google Scholar]
- 10. Yin L, Grandi N, Raum E, et al. . Meta-Analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113–25. 10.1111/j.1365-2036.2009.04022.x [DOI] [PubMed] [Google Scholar]
- 11. Ma Y, Zhang P, Wang F, et al. . Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 2011;29:3775–82. 10.1200/JCO.2011.35.7566 [DOI] [PubMed] [Google Scholar]
- 12. Touvier M, Chan DSM, Lau R, et al. . Meta-Analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2011;20:1003–16. 10.1158/1055-9965.EPI-10-1141 [DOI] [PubMed] [Google Scholar]
- 13. Ekmekcioglu C, Haluza D, Kundi M. 25-Hydroxyvitamin D status and risk for colorectal cancer and type 2 diabetes mellitus: a systematic review and meta-analysis of epidemiological studies. Int J Environ Res Public Health 2017;14:127 10.3390/ijerph14020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JE, Li H, Chan AT, et al. . Circulating levels of vitamin D and colon and rectal cancer: the physicians' health study and a meta-analysis of prospective studies. Cancer Prev Res 2011;4:735–43. 10.1158/1940-6207.CAPR-10-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maalmi H, Walter V, Jansen L, et al. . Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients 2018;10:896 10.3390/nu10070896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6. 10.1093/ajcn/87.4.1080S [DOI] [PubMed] [Google Scholar]
- 17. Hilger J, Friedel A, Herr R, et al. . A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014;111:23–45. 10.1017/S0007114513001840 [DOI] [PubMed] [Google Scholar]
- 18. Man R, Li L-J, Cheng C-Y, et al. . Prevalence and determinants of suboptimal vitamin D levels in a multiethnic Asian population. Nutrients 2017;9:313 10.3390/nu9030313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 20. Feldman D, Krishnan AV, Swami S, et al. . The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- 21. Chandler PD, Buring JE, Manson JE, et al. . Circulating vitamin D levels and risk of colorectal cancer in women. Cancer Prev Res 2015;8:675–82. 10.1158/1940-6207.CAPR-14-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song M, Konijeti GG, Yuan C, et al. . Plasma 25-hydroxyvitamin D, vitamin D binding protein, and risk of colorectal cancer in the nurses' health study. Cancer Prev Res 2016;9:664–72. 10.1158/1940-6207.CAPR-16-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCullough ML, Zoltick ES, Weinstein SJ, et al. . Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2019;111:158–69. 10.1093/jnci/djy087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otani T, Iwasaki M, Sasazuki S, et al. . Plasma vitamin D and risk of colorectal cancer: the Japan public health Center-Based prospective study. Br J Cancer 2007;97:446–51. 10.1038/sj.bjc.6603892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi R, Mizoue T, Otake T, et al. . Circulating vitamin D and colorectal adenomas in Japanese men. Cancer Sci 2010;101:1695–700. 10.1111/j.1349-7006.2010.01575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong SN, Kim JH, Choe WH, et al. . Circulating vitamin D and colorectal adenoma in asymptomatic average-risk individuals who underwent first screening colonoscopy: a Case–Control study. Dig Dis Sci 2012;57:753–63. 10.1007/s10620-011-1926-1 [DOI] [PubMed] [Google Scholar]
- 27. Yamaji T, Iwasaki M, Sasazuki S, et al. . Association between plasma 25-hydroxyvitamin D and colorectal adenoma according to dietary calcium intake and vitamin D receptor polymorphism. Am J Epidemiol 2012;175:236–44. 10.1093/aje/kwr295 [DOI] [PubMed] [Google Scholar]
- 28. Choi YJ, Kim YH, Cho CH, et al. . Circulating levels of vitamin D and colorectal adenoma: a case-control study and a meta-analysis. World J Gastroenterol 2015;21:8868–75. 10.3748/wjg.v21.i29.8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yurekli OT, Solakoglu T, Atalay R, et al. . Association between serum vitamin D and parathyroid hormone levels in Turkish patients with colonic polyps. Acta Gastroenterol Belg 2015;78:206–11. [PubMed] [Google Scholar]
- 30. Budhathoki S, Hidaka A, Yamaji T, et al. . Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: large case-cohort study within Japan public health Center-Based prospective study cohort. BMJ 2018;360 10.1136/bmj.k671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 33. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized Dose–response data. Stata J 2006;6:40–57. 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 34. Orsini N, Li R, Wolk A, et al. . Meta-Analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JPT, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ying H-Q, Sun H-L, He B-S, et al. . Circulating vitamin D binding protein, total, free and bioavailable 25-hydroxyvitamin D and risk of colorectal cancer. Sci Rep 2015;5:7956 10.1038/srep07956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi YJ, Kim YH, Cho CH, et al. . Circulating levels of vitamin D and colorectal adenoma: a case-control study and a meta-analysis. World J Gastroenterol 2015;21:8868–77. 10.3748/wjg.v21.i29.8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wesa KM, Segal NH, Cronin AM, et al. . Serum 25-hydroxy vitamin D and survival in advanced colorectal cancer: a retrospective analysis. Nutr Cancer 2015;67:424–30. 10.1080/01635581.2015.998838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wactawski-Wende J, Kotchen JM, Anderson GL, et al. . Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96. 10.1056/NEJMoa055222 [DOI] [PubMed] [Google Scholar]
- 40. Lappe JM, Travers-Gustafson D, Davies KM, et al. . Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586–91. 10.1093/ajcn/85.6.1586 [DOI] [PubMed] [Google Scholar]
- 41. Di Rosa M, Malaguarnera M, Zanghì A, et al. . Vitamin D3 insufficiency and colorectal cancer. Crit Rev Oncol Hematol 2013;88:594–612. 10.1016/j.critrevonc.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 42. Dou R, Ng K, Giovannucci EL, et al. . Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br J Nutr 2016;115:1643–60. 10.1017/S0007114516000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic Proceedings 2006;81:353–73. 10.4065/81.3.353 [DOI] [PubMed] [Google Scholar]
- 44. Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. . Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28. 10.1093/ajcn/84.1.18 [DOI] [PubMed] [Google Scholar]
- 45. Dawson-Hughes B, Heaney RP, Holick MF, et al. . Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–6. 10.1007/s00198-005-1867-7 [DOI] [PubMed] [Google Scholar]
- 46. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 47. van Schoor NM, Visser M, Pluijm SMF, et al. . Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 2008;42:260–6. 10.1016/j.bone.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 48. Garland CF, Garland FC, Gorham ED, et al. . The role of vitamin D in cancer prevention. Am J Public Health 2006;96:252–61. 10.2105/AJPH.2004.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo H, Guo J, Xie W, et al. . The role of vitamin D in ovarian cancer: epidemiology, molecular mechanism and prevention. J Ovarian Res 2018;11:71 10.1186/s13048-018-0443-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. González-Molero I, Rojo-Martínez G, Morcillo S, et al. . Vitamin D and incidence of diabetes: a prospective cohort study. Clinical Nutrition 2012;31:571–3. 10.1016/j.clnu.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 51. Annweiler C, Milea D, Whitson HE, et al. . Vitamin D insufficiency and cognitive impairment in Asians: a multi-ethnic population-based study and meta-analysis. J Intern Med 2016;280:300–11. 10.1111/joim.12491 [DOI] [PubMed] [Google Scholar]
- 52. Schottker B, Jorde R, Peasey A, et al. . Vitamin D and mortality: meta-analysis of individual participant data from a large Consortium of cohort studies from Europe and the United States. BMJ 2014;348:g3656 10.1136/bmj.g3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCullough ML, Robertson AS, Rodriguez C, et al. . Calcium, vitamin D, dairy products, and risk of colorectal cancer in the cancer prevention study II nutrition cohort (United States). Cancer Causes Control 2003;14:1–12. 10.1023/A:1022591007673 [DOI] [PubMed] [Google Scholar]
- 54. Kallay E, Bajna E, Wrba F, et al. . Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect Prev 2000;24:127–36. [PubMed] [Google Scholar]
- 55. Cross HS, Huber C, Peterlik M. Antiproliferative effect of 1,25-dihydroxyvitamin D3 and its analogs on human colon adenocarcinoma cells (Caco-2): influence of extracellular calcium. Biochem Biophys Res Commun 1991;179:57–62. 10.1016/0006-291X(91)91333-8 [DOI] [PubMed] [Google Scholar]
- 56. Cross HS, Pavelka M, Slavik J, et al. . Growth control of human colon cancer cells by vitamin D and calcium in vitro. J Natl Cancer Inst 1992;84:1355–7. 10.1093/jnci/84.17.1355 [DOI] [PubMed] [Google Scholar]
- 57. Grau MV, Baron JA, Sandler RS, et al. . Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst 2003;95:1765–71. 10.1093/jnci/djg110 [DOI] [PubMed] [Google Scholar]
- 58. Oh K, Willett WC, Wu K, et al. . Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol 2007;165:1178–86. 10.1093/aje/kwm026 [DOI] [PubMed] [Google Scholar]
- 59. Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res 2009;29:3687–98. [PubMed] [Google Scholar]
- 60. Keum N, Lee DH, Greenwood DC, et al. . Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol 2019;30:733–43. 10.1093/annonc/mdz059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marshall DT, Savage SJ, Garrett-Mayer E, et al. . Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab 2012;97:2315–24. 10.1210/jc.2012-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ng K, Nimeiri HS, McCleary NJ, et al. . Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the sunshine randomized clinical trial. JAMA 2019;321:1370–9. 10.1001/jama.2019.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Yu Q, Zhu Z, et al. . Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol 2015;32 10.1007/s12032-014-0434-5 [DOI] [PubMed] [Google Scholar]
- 64. Winkels RM, Heine-Bröring RC, van Zutphen M, et al. . The colon study: colorectal cancer: longitudinal, observational study on nutritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer 2014;14:374 10.1186/1471-2407-14-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Watson AJM. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol 2006;57:107–21. 10.1016/j.critrevonc.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 66. Ding EL, Mehta S, Fawzi WW, et al. . Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of women's health Initiative randomized trial. Int J Cancer 2008;122:1690–4. 10.1002/ijc.23311 [DOI] [PubMed] [Google Scholar]
- 67. Kure S, Nosho K, Baba Y, et al. . Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2765–72. 10.1158/1055-9965.EPI-09-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giovannucci E. Epidemiological evidence for vitamin D and colorectal cancer. J Bone Miner Res 2007;22:V81–5. 10.1359/jbmr.07s206 [DOI] [PubMed] [Google Scholar]
- 69. Azeem S, Gillani SW, Siddiqui A, et al. . Diet and Colorectal Cancer Risk in Asia--a Systematic Review. Asian Pac J Cancer Prev 2015;16:5389–96. 10.7314/APJCP.2015.16.13.5389 [DOI] [PubMed] [Google Scholar]
- 70. Bae J-M. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health 2016;38:e2016014 10.4178/epih.e2016014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-030513supp001.pdf (5.9MB, pdf)