Abstract
Following a request from the EU Commission, the Panel on Plant Health has addressed the pest categorisation of non‐EU isolates of potato virus S (PVS). The information currently available on geographical distribution, biology, epidemiology, potential entry pathways, potential additional impact compared to the current situation in the EU, and availability of control measures of non‐EU isolates of PVS has been evaluated with regard to the criteria to qualify as potential Union quarantine pest. Because non‐EU isolates of PVS are absent from the EU, they do not meet one of the requirements to be regulated as an RNQP (presence in the EU); as a consequence, the Panel decided not to evaluate the other RNQP criteria for these isolates. Populations of PVS can be subdivided into two strains: the ordinary strain (PVS‐O) with a worldwide distribution (including the EU), and the Andean strain (PVS‐A) which is absent from the EU or considered to have at most a limited distribution in the EU. Two additional divergent isolates (PVS‐A/PVS‐O recombinants and PVS‐arracacha) have also been categorised. Non‐EU isolates of PVS‐A are expected to have an additional impact as compared to the PVS isolates currently present in the EU, and therefore meet all the criteria to qualify as potential Union quarantine pests; the magnitude of the additional impact is, however, unknown. Non‐EU isolates of PVS‐A/PVS‐O recombinants and of PVS‐arracacha also meet these criteria, with the exception of the criterion regarding the potential additional consequences in the EU territory for which the Panel was unable to conclude. Non‐EU PVS‐O isolates are not expected to have an additional impact in the EU as compared to EU isolates and therefore do not meet the corresponding criterion.
Keywords: European Union, Non‐EU isolate, pest risk, plant health, plant pest, PVS, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocanthus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 5) Potato virus T |
| 2) Andean potato mottle virus | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| 3) Arracacha virus B, oca strain | |
| 4) Potato black ringspot virus | |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Pseudopityophthorus minutissimus (Zimmermann) | Pseudopityophthorus pruinosus (Eichhoff) |
| Dendrolimus sibiricus Tschetverikov | Scaphoideus luteolus (Van Duzee) |
| Diabrotica barberi Smith and Lawrence | Spodoptera eridania (Cramer) |
| Diabrotica undecimpunctata howardi Barber | Spodoptera frugiperda (Smith) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera litura (Fabricus) |
| Diabrotica virgifera zeae Krysan & Smith | Thrips palmi Karny |
| Diaphorina citri Kuway | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Heliothis zea (Boddie) | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | |
| Liriomyza sativae Blanchard | |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
European Food Safety Authority (EFSA) is asked to develop pest categorisations for non‐EU isolates of seven potato viruses, i.e. Potato leaf roll virus and potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc), which are defined by their geographical origin outside the European Union (EU). As such, isolates of these viruses occurring outside the EU territory are considered as non‐EU isolates. Accordingly, a plant infected with one of these viruses originating in a non‐EU country is considered to be infected with a non‐EU isolate. All seven viruses are important pathogens of potato and, therefore, there is no uncertainty about the fact that non‐EU isolates have an impact on potato crops in absolute terms. However, EU isolates of these viruses already have an impact in the EU; consequently, the Panel decided to evaluate whether the non‐EU isolates would have an additional impact compared to the current situation, upon introduction and spread in the EU. This interpretation was agreed with the European Commission.
This scientific opinion presents the pest categorisation of non‐EU isolates of potato virus S (PVS). Non‐EU isolates of PVS are listed in the Appendices of the Terms of Reference (ToR) to be subject to pest categorisation to determine whether they fulfil the criteria of a quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Because non‐EU isolates of PVS are absent from the EU, they do not meet one of the requirements to be regulated as an RNQP (presence in the EU); as a consequence, the Panel decided not to evaluate the other RNQP criteria for these isolates.
Despite the fact that Solanum phureja is considered by some authorities as an invalid taxon that should be renamed Solanum tuberosum Phureja Group,4 the Panel considered the uncertainty on this aspect high enough and decided, in line with the EPPO Global Database, to separately address S. phureja as a distinct entity regulated within the ‘potato and other tuber‐forming Solanum species’ in Directive 2000/29/EC.
The new Plant Health Regulation (EU) 2016/20315, on the protective measures against pests of plants, will be applying from December 2019. The regulatory status sections (Section 3.3) of the present opinion are still based on Council Directive 2000/29/EC, as the document was adopted in September 2019.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on potato virus S (PVS) was conducted in the ISI Web of Science bibliographic database. The scientific name of the pest was used as search term. Relevant papers were reviewed with a focus on potential differences between isolates and strains. Further references and information were obtained from experts, as well as from citations in the reviewed papers and grey literature. The search was continued until no further information could be found or until the collected information was considered sufficient to perform the pest categorisation; consequently, the presented data are not necessarily exhaustive.
2.1.2. Database search
Information on hosts, vectors and distribution at species level, was retrieved from CABI Crop Protection Compendium (CABI cpc) and relevant publications. Additional data on isolates distribution were obtained from the literature.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted to identify interceptions of non‐EU isolates of PVS. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States (MSs) and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for non‐EU isolates of PVS, following the guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018) and in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
General information on PVS will be provided at species level. Further information will be added at the level of strains and/or non‐EU isolates when available and/or applicable.
This work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest (RNQP) in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a RNQP. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential RNQP were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel.
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
2.3. Nomenclature
Virus nomenclature is reported using the latest release of the official classification by the International Committee on Taxonomy of Viruses (ICTV, Release 2018b.v1, https://talk.ictvonline.org/taxonomy/). Virus names are not italicised throughout this opinion, corresponding to ICTV instructions.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
3.1.1.1.
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes. PVS is a well‐known virus and the definition of ‘non‐EU isolates’, as used in the present opinion has been clarified (see Section 1.2).
Potato virus S (PVS) is a well‐characterised virus in the genus Carlavirus, family Betaflexiviridae (Adams et al., 2011). It has a single‐stranded positive‐sense RNA genome and complete and/or partial genomic sequences are available for a number of isolates.
3.1.2. Biology of the pest
PVS is not known to be transmitted by pollen or true seeds (Horvath, 1973; Goth and Webb, 1974). It is transmitted by vegetative propagation (via tubers) and can be transmitted mechanically, e.g. by contaminated tools and wounds (CABI, 2019). The Panel does not expect significant differences between PVS strains and/or isolates for these general properties.
In addition, some isolates in Europe and North America have been reported to be non‐persistently transmitted by aphids (see Table 2), while others are not (Bode and Weidemann, 1971; MacKinnon, 1973; Wardrop et al., 1989). The studies of Fletcher (1996) and Slack (1983) indicated that only PVS‐A isolates are transmitted by aphids. However, in these older reports, genomic data are lacking to unambiguously assign isolates to either PVS‐A or PVS‐O. Recently, Santillan et al. (2018) confirmed these early indications by showing that Myzus persicae (Sulzer) transmitted all nine tested PVS‐A isolates, but failed to transmit the three tested PVS‐O isolates. Additionally, Santillan et al. (2018) provided some evidence that PVS‐A isolates may occur in higher concentrations in infected potatoes, which might favour an increased efficiency of aphid transmission.
Table 2.
Aphid‐mediated transmission of strains and other isolates of PVS
| PVS | Aphid‐transmission | Rationale and/or uncertainty |
|---|---|---|
| Strain | ||
| PVS‐A | Yes | PVS‐A isolates, identified based on genomic data, reported to be non‐persistently transmitted by Myzus persicae (Santillan et al., 2018 (nine isolates)). Additionally, a PVS‐A isolate from Chile is reported to be transmitted by M. persicae (Thomas et al., 1980; Dolby and Jones, 1988; Santillan et al., 2018). It is not known whether all isolates of PVS‐A are aphid‐transmitted |
| PVS‐O | Cannot be excluded |
PVS‐O isolates, identified based on genomic data, reported not to be transmitted by Myzus persicae (Santillan et al., 2018 (three isolates)) However, some PVS isolates for which the strain was not specified have been reported to be non‐persistently transmitted by aphid species, such as Aphis fabae (CABI, 2019), Aphis nasturtii (CABI, 2019), Myzus persicae (Bode and Weidemann, 1971; MacKinnon, 1973; Slack, 1983; Wardrop et al., 1989; Fletcher, 1996) and Rhopalosiphum padi (CABI, 2019) and therefore aphid transmission of PVS‐O isolates cannot be excluded |
| Other isolate | ||
| PVS‐A/PVS‐O recombinants | Cannot be excluded | Not reported, but other isolates of PVS are transmitted by aphids |
| PVS‐arracacha | Cannot be excluded | Transmission of PVS isolate from arracacha by Myzus persicae and Macrosiphum euphorbiae failed (De Souza et al., 2018), but other isolates of PVS are transmitted by aphids |
Whether these differences in aphid transmissibility are general features applying to all isolates of PVS‐A and PVS‐O is unknown. There is no information of aphid transmissibility of PVS‐A/PVS‐O recombinants and of PVS‐arracacha.
Table 2 summarises the evidence on vector transmission of strains and other isolates of PVS with the associated rationale and/or uncertainties.
3.1.3. Intraspecific diversity
Viruses generally exist as quasispecies, which means that they accumulate as a cluster of closely related sequence variants in a single host (Andino and Domingo, 2015). This is likely due to competition among the genomic variants that are generated as a consequence of the error‐prone viral replication (higher in RNA than in DNA viruses) and the ensuing selection of the most fit variants in a given environment (Domingo et al., 2012). This genetic variability may have consequences on the virus’ biological properties (e.g. host range, transmissibility and pathogenicity) as well as on the reliability of detection methods, especially when they target variable genomic regions.
This pest categorisation focuses on taxonomic levels below the species level, i.e. on isolates and strains, which are defined in this opinion as follows:
Isolate: virus population as present in a plant;
Strain: group of isolates sharing biological, molecular and/or serological properties (Garcıa‐Arenal et al., 2001).
ICTV does not address taxonomic levels below the species level and, therefore, the names of strains are based on reports in literature. In the past, the term ‘strain’ has also often been used as a synonym for ‘isolate’. As a consequence of this inconsistent use of terminology, the literature is often unclear.
Studies showing an unambiguous relationship between specific virus genotypes (isolates/strains) and biological properties are limited. Moreover, the interpretation of such data may be hampered because discrimination between strains based on biological data is not always supported by genomic data. Historically, for many viruses, including PVS, strains have been distinguished based on differences in reactions on a set of indicator plants. This differentiation became further established by serology, especially by using monoclonal antibodies specifically selected to discriminate between the earlier distinguished strains. However, with the advent of molecular techniques, it appeared that phylogenetic analyses of isolates based on genomic data do not always support the previous biological or serological strain differentiation. Moreover, the discrimination between strains might be further complicated by the existence of recombinant isolates, hampering an unambiguous assignment of isolates to recognised strains. This implies that there is frequent uncertainty about the interpretation of (older) data on strain differentiation and on their geographical distribution.
For PVS two strains have been distinguished, the Ordinary strain (PVS‐O) and Andean strain (PVS‐A) (see Table 3). Currently, these strains are distinguished based on their molecular properties (Duan et al., 2018; Santillan et al., 2018). It should be stressed that this strain differentiation based on genome sequence analysis deviates from the criterion originally put forward to distinguish PVS‐O and PVS‐A, i.e. their ability (or inability) to cause systemic infections in Chenopodium spp. (Hinostroza‐Orihuela, 1973). Later publications show that this biological property does not allow for a reliable differentiation between the two strains (Cox and Jones, 2010; Lambert et al., 2012; Santillan et al., 2018).
Table 3.
Overview of reported strains and other isolates of PVS
| PVS | Acronym | Other information | Key references |
|---|---|---|---|
| Strain | |||
| Andean strain | PVS‐A | Including Southern potato latent virus (Kobayashi et al., 1985), PVS‐RVC, PVS‐Antioquia (Gutierrez et al., 2013; Vallejo et al., 2016; Duan et al., 2018; Santillan et al., 2018), Pepino latent virus infecting potato (Dolby and Jones, 1988) and PVS‐BB‐AND (De Sousa Geraldino Duarte et al., 2012; Santillan et al., 2018) | CABI (2019), Santillan et al. (2018) |
| Ordinary strain | PVS‐O | Including PVS‐WaDef (Lin et al., 2009), PVS‐Exodus (Dolby and Jones, 1987) and PVS‐Leona (Matoušek et al., 2005) | CABI (2019), Santillan et al. (2018) |
| Other isolate | |||
| Recombinant isolates | PVS‐A/PVS‐O recombinants | Including PVS‐Vltava from the Czech Republic (AJ863510) and a similar recombinant isolate from Peru (D00461) | MacKenzie et al. (1989), Matoušek et al. (2005), De Sousa Geraldino Duarte et al. (2012), Santillan et al. (2018) |
| PVS isolate from Arracacia xanthorrhiza | PVS‐arracacha | NCBI GenBank accession number KY451037 | De Souza et al. (2018) |
In addition to these two strains, a few other PVS isolates have been reported (see Table 3). In post‐entry quarantine (Japan, 1983) southern potato latent virus was reported in a potato cultivar from Peru. It was later shown to be an isolate of PVS‐A (Kobayashi et al., 1985). Similarly, Gutierrez et al. (2013) reported a novel PVS isolate (PVS‐RVC) in S. phureja, a tuber‐forming Solanum species grown in the Andes region. Vallejo et al. (2016) reported a related PVS isolate in the same host. Based on phylogenetic analyses using complete genome sequences, these isolates from Colombia were shown to belong to PVS‐A (Duan et al., 2018; Santillan et al., 2018). Therefore, all these isolates will be categorised within PVS‐A.
In the Czech Republic, a PVS isolate was reported from Solanum tuberosum cv. Vltava (PVS‐Vltava) (Matoušek et al., 2000). Biological data suggested that it belongs to PVS‐O (Matoušek et al., 2000); later, a molecular analysis performed by Salari et al. (2011) showed that it belongs to PVS‐A. A phylogenetic analysis of the coat protein gene by Vallejo et al. (2016) showed that PVS‐Vltava is related to a PVS isolate from Peru (host unknown) (MacKenzie et al., 1989). Recently, Santillan et al. (2018) concluded that PVS‐Vltava is a recombinant between PVS‐A and PVS‐O. It will therefore be categorised separately here.
De Souza et al. (2018) reported the characterisation of a carlavirus in arracacha (Arracacia xanthorrhiza) from Peru which was located in a distinct branch from PVS‐O and PVS‐A in a phylogenetic analysis. The partial replicase sequence identified this isolate as PVS (88% amino acid identity). The coat protein sequence showed 79% amino acid identity with PVS, which is just below the species demarcation criterion of carlaviruses (80%). Upon mechanical inoculation, symptoms of this carlavirus in Chenopodium spp. resembled those of PVS‐A. Taking these elements in consideration, the virus has been tentatively assigned to the PVS species and named PVS‐arracacha. However, its taxonomic status is not precisely established and it cannot be excluded that PVS‐arracacha could represent a separate species when more data become available. It will be categorised separately in the present opinion.
In view of this recent discovery, it cannot be excluded that additional divergent isolates that do not fit in the PVS‐A and PVS‐O strains may exist, particularly in South America.
3.1.4. Detection and identification of the pest
3.1.4.1.
Are detection and identification methods available for the pest?
Yes. Methods are available for detection and identification of PVS at the species and strain level, and therefore for the identification of non‐EU isolates. Genomic data are available for the design of diagnostic tests for PVS‐A/PVS‐O recombinants and PVS‐arracacha isolates.
As mentioned in the pest categorisation of non‐EU viruses and viroids of potato (EFSA PLH Panel, 2020), virus detection and identification is complicated by several recurrent uncertainties. ICTV lists species demarcation criteria, but it is not always clear whether these are met in diagnostic tests. Furthermore, in the absence or near absence of information on genetic variability, it is not possible to guarantee that a given test will detect all variants of a species. On the contrary, generic tests may detect closely related viruses in addition to the target species. This implies that the reliability of a test depends on its validation for the intended use. For initial screening, it is important to prevent false‐negative results, which means that the following performance characteristics are most relevant: analytical sensitivity, inclusivity of analytical specificity (coverage of the intra‐species variability) and selectivity (matrix effects). For identification, it is important to prevent false positives and, therefore, the possible occurrence of cross‐reactions should be determined, i.e. the exclusivity of the analytical specificity (the resolution should be sufficient to discriminate between related species).
PVS is a well‐known virus for which detection methods are available. Bioassays associated with ELISA are available for the detection and identification of PVS.
Wang et al. (2016) described a RT‐PCR assay to distinguish between PVS‐A and PVS‐O isolates. The exclusivity and inclusivity of this test are not fully established. Currently, no specific tests are available for the detection and identification of PVS‐A/PVS‐O recombinants and PVS‐arracacha isolates. However, genomic data are available (Gutierrez et al., 2013; De Souza et al., 2018; Duan et al., 2018) for the design of diagnostic PCR primers that could be used for detection and identification purposes.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
PVS occurs worldwide wherever potato is grown (Jeffries, 1998). Recent phylogenetic analyses show a clear separation between the geographical distribution of PVS isolates at the strain level (Duan et al., 2018; Santillan et al., 2018). PVS‐O isolates are reported from all continents (Salari et al., 2011; Duan et al., 2018; Santillan et al., 2018) while PVS‐A isolates are only reported from Asia, Oceania and South America (Cox and Jones, 2010; Duan et al., 2018; Khassanov and Vologin, 2018).
A PVS‐A/PVS‐O recombinant has been reported from Peru (MacKenzie et al., 1989; Vallejo et al., 2016).
PVS‐arracacha has been reported from Peru (De Souza et al., 2018), but similar isolates could be more widespread in the Andes region where arracacha is widely grown.
3.2.2. Pest distribution in the EU
3.2.2.1.
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
Yes. PVS‐O isolates are present in the EU. The PVS‐A/PVS‐O recombinant isolate Vltava has been reported once from the Czech Republic.
No. PVS‐A isolates are considered absent or present with limited distribution in the EU. The PVS‐arracacha isolate is not known to be present in the EU.
As indicated in the previous section, isolates belonging to the PVS‐O strain are reported worldwide, including several EU Member States (Germany, Hungary, the Netherlands and United Kingdom) (Duan et al., 2018; Santillan et al., 2018).
There are few reports of PVS‐A in the EU. One report from the United Kingdom on PVS‐A isolates concerned potato breeding lines and cultivars imported from Germany and the Netherlands (Dolby and Jones, 1987). Furthermore, the United Kingdom reported the interception of PVS‐A in ware potatoes from Germany and in cuttings of Solanum muricatum from Spain, both in 2000 (Europhyt reports 11265 and 11336). These reports carry some uncertainty because the virus isolates involved have only been characterised using bioassay and ELISA. Following eradication efforts and given the lack of recent reports on the presence of PVS‐A isolates, it is considered no longer present or present with only limited distribution in the EU.
The PVS‐arracacha isolate reported from Peru (De Souza et al., 2018) is not known to be present in the EU.
The PVS‐A/PVS‐O recombinant isolate Vltava has been reported once from the Czech Republic (Matoušek et al., 2000; Salari et al., 2011; De Sousa Geraldino Duarte et al., 2012; Santillan et al., 2018). Given that there are no further reports of PVS‐A/PVS‐O recombinant isolates, they are considered to have at most a limited distribution in the EU. However, this assessment is uncertain in the absence of specific surveys.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Non‐EU isolates of PVS are specifically listed in Council Directive 2000/29/EC and are regulated in Annex IAI (See Table 4).
Table 4.
Non‐EU isolates of PVS in Council Directive 2000/29/EC
| Annex I, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned |
|---|---|
| Section I | Harmful organisms not known to occur in any part of the community and relevant for the entire community |
| (d) | Viruses and virus‐like organisms |
| 2. |
Potato viruses and virus‐like organisms such as: (g) non‐European isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
3.3.2. Legislation addressing potato
Table 5 reports on the articles in Council Directive 2000/29/EC which address potato or tuber‐forming species of Solanum L. PVS may also infect other hosts; references to the corresponding legislation is reported in Table 6 (see Section 3.5).
Table 5.
Overview of the regulation in Annexes III, IV and V of Council Directive 2000/29/EC that applies to potato or tuber‐forming Solanum species
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
|---|---|---|
| Description | Country of origin | |
| 10. | Tubers of Solanum tuberosum L., seed potatoes | Third countries other than Switzerland |
| 11. | Plants of stolon‐ or tuber‐forming species of Solanum L. or their hybrids, intended for planting, other than those tubers of Solanum tuberosum L. as specified under Annex III A (10) | Third countries |
| 12. | Tubers of species of Solanum L., and their hybrids, other than those specified in points 10 and 11 | Without prejudice to the special requirements applicable to the potato tubers listed in Annex IV, Part A Section I, third countries other than Algeria, Egypt, Israel, Libya, Morocco, Syria, Switzerland, Tunisia and Turkey, and other than European third countries which are either recognised as being free from Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al., in accordance with the procedure referred to in Article 18(2), or in which provisions recognised as equivalent to the Community provisions on combating Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. in accordance with the procedure referred to in Article 18(2), have been complied with |
| Annex IV, Part A | Special requirements which shall be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within all Member States | |
| Section I | Plants, plant products and other objects originating outside the Community | |
| Plants, plant products and other objects | Special requirements | |
| 25.1 | Tubers of Solanum tuberosum L., originating in countries where Synchytrium endobioticum (Schilbersky) Percival is known to occur |
Without prejudice to the prohibitions applicable to the tubers listed in Annex III(A) (10), (11) and (12), official statement that: (a) the tubers originate in areas known to be free from Synchytrium endobioticum (Schilbersky) Percival (all races other than Race 1, the common European race), and no symptoms of Synchytrium endobioticum (Schilbersky) Percival have been observed either at the place of production or in its immediate vicinity since the beginning of an adequate period; or (b) provisions recognised as equivalent to the Community provisions on combating Synchytrium endobioticum (Schilbersky) Percival in accordance with the procedure referred to in Article 18(2) have been complied with, in the country of origin |
| 25.2. | Tubers of Solanum tuberosum L. |
Without prejudice to the provisions listed in Annex (A) (10), (11) and (12) and Annex IV(A)(I) (25.1), official statement that: (a) the tubers originate in countries known to be free from Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al.; or (b) provisions recognised as equivalent to the Community provisions on combating Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. in accordance with the procedure referred to in Article 18(2), have been complied with, in the country of origin |
| 25.3. | Tubers of Solanum tuberosum L., other than early potatoes, originating in countries where Potato spindle tuber viroid is known to occur | Without prejudice to the provisions applicable to the tubers listed in Annex III(A) (10), (11) and (12) and Annex IV(A)(I) (25.1) and (25.2), suppression of the faculty of germination |
| 25.4. | Tubers of Solanum tuberosum L., intended for planting |
Without prejudice to the provisions applicable to the tubers listed in Annex III(A)(10), (11) and (12) and Annex IV(A)(I) (25.1), (25.2) and (25.3), official statement that the tubers originate from a field known to be free from Globodera rostochiensis (Wollenweber) Behrens and Globodera pallida (Stone) Behrens and (aa) either, the tubers originate in areas in which Ralstonia solanacearum (Smith) Yabuuchi et al. is known not to occur; or (bb) in areas where Ralstonia solanacearum (Smith) Yabuuchi et al. is known to occur, the tubers originate from a place of production found free from Ralstonia solanacearum (Smith) Yabuuchi et al., or considered to be free thereof, as a consequence of the implementation of an appropriate procedure aiming at eradicating Ralstonia solanacearum (Smith) Yabuuchi et al. which shall be determined in accordance with the procedure referred to in Article 18(2) and (cc) either the tubers originate in areas where Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen are known not to occur; or (dd) in areas where Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen are known to occur, — either the tubers originate from a place of production which has been found free from Meloidogyne chitwoodi Golden et al. (all populations), and Meloidogyne fallax Karssen based on an annual survey of host crops by visual inspection of host plants at appropriate times and by visual inspection both externally and by cutting of tubers after harvest from potato crops grown at the place of production, or — the tubers after harvest have been randomly sampled and, either checked for the presence of symptoms after an appropriate method to induce symptoms, or laboratory tested, as well as inspected visually both externally and by cutting the tubers, at appropriate times and in all cases at the time of closing of the packages or containers before marketing according to the provisions on closing in Council Directive 66/403/EEC of 14 June 1996 on the marketing of seed potatoes (1) and no symptoms of Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen have been found. |
| 25.4.1. | Tubers of Solanum tuberosum L., other than those intended for planting | Without prejudice to the provisions applicable to tubers listed in Annex III(A) (12) and Annex IV(A)(I) (25.1), (25.2) and (25.3), official statement that the tubers originate in areas in which Ralstonia solanacearum (Smith) Yabuuchi et al. is not known to occur. |
| 25.4.2. | Tubers of Solanum tuberosum L. |
Without prejudice to the provisions applicable to tubers listed in Annex III(A) (10), (11) and (12) and Annex IV(A)(I) (25.1), (25.2), (25.3), (25.4) and (25.4.1), official statement that: (a) the tubers originate in a country where Scrobipalpopsis solanivora Povolny is not known to occur; or (b) the tubers originate in an area free from Scrobipalpopsis solanivora Povolny, established by the national plant protection organisation in accordance with relevant International Standards for Phytosanitary Measures |
| 25.5. | Plants of Solanaceae, intended for planting, other than seeds, originating in countries where Potato stolbur mycoplasm is known to occur | Without prejudice to the provisions applicable to tubers listed in Annex III(A) (10), (11), (12) and (13), and Annex IV(A)(I) (25.1), (25.2), (25.3) and (25.4), official statement that no symptoms of Potato stolbur mycoplasm have been observed on the plants at the place of production since the beginning of the last complete cycle of vegetation |
| Section II | Plants, plant products and other objects originating in the Community | |
| Plants, plant products and other objects | Special requirements | |
| 18.1. | Tubers of Solanum tuberosum L., intended for planting |
Official statement that: (a) the Union provisions to combat Synchytrium endobioticum (Schilbersky) Percival have been complied with; and (b) either the tubers originate in an area known to be free from Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. or the Union provisions to combat Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. have been complied with; and (d) (aa) either, the tubers originate in areas in which Ralstonia solanacearum (Smith) Yabuuchi et al. is known not to occur; or (bb) in areas where Ralstonia solanacearum (Smith) Yabuuchi et al. is known to occur, the tubers originate from a place of production found free from Ralstonia solanacearum (Smith) Yabuuchi et al., or considered to be free thereof, as a consequence of the implementation of an appropriate procedure aiming at eradicating Ralstonia solanacearum (Smith) Yabuuchi et al.; and (e) either, the tubers originate in areas in which Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen are known not to occur, or in areas where Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen are known to occur: — either, the tubers originate from a place of production which has been found free from Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen based on an annual survey of host crops by visual inspection of host plants at appropriate times and by visual inspection both externally and by cutting of tubers after harvest from potato crops grown at the place of production, or — the tubers after harvest have been randomly sampled and, either checked for the presence of symptoms after an appropriate method to induce symptoms or laboratory tested, as well as inspected visually both externally and by cutting the tubers, at appropriate times and in all cases at the time of closing of the packages or containers before marketing according to the provisions on closing in Council Directive 66/403/EEC, and no symptoms of Meloidogyne chitwoodi Golden et al. (all populations) and Meloidogyne fallax Karssen have been found. |
| 18.1.1. | Tubers of Solanum tuberosum L., intended for planting, other than those to be planted in accordance with Article 4.4(b) of Council Directive 2007/33/EC | Without prejudice to the requirements applicable to the tubers of Solanum tuberosum L., intended for planting in Annex IV, Part A, Section II (18.1), official statement that the Union provisions to combat Globodera pallida (Stone) Behrens and Globodera rostochiensis (Wollenweber) Behrens are complied with. |
| 18.2 | Tubers of Solanum tuberosum L., intended for planting, other than tubers of those varieties officially accepted in one or more Member States pursuant to Council Directive 70/457/EEC of 29 September 1970 on the common catalogue of varieties of agricultural plant species (1) |
Without prejudice to the special requirements applicable to the tubers listed in Annex IV(A)(II) (18.1), official statement that the tubers: — belong to advanced selections such a statement being indicated in an appropriate way on the document accompanying the relevant tubers, — have been produced within the Community, and — have been derived in direct line from material which has been maintained under appropriate conditions and has been subjected within the Community to official quarantine testing in accordance with appropriate methods and has been found, in these tests, free from harmful organisms. |
| 18.3 | Plants of stolon or tuber‐forming species of Solanum L., or their hybrids, intended for planting, other than those tubers of Solanum tuberosum L. specified in Annex IV(A)(II) (18.1) or (18.2), and other than culture maintenance material being stored in gene banks or genetic stock collections |
(a) The plants shall have been held under quarantine conditions and shall have been found free of any harmful organisms in quarantine testing; (b) the quarantine testing referred to in (a) shall: (aa) be supervised by the official plant protection organisation of the Member State concerned and executed by scientifically trained staff of that organisation or of any officially approved body; (bb) be executed at a site provided with appropriate facilities sufficient to contain harmful organisms and maintain the material including indicator plants in such a way as to eliminate any risk of spreading harmful organisms; (cc) be executed on each unit of the material; — by visual examination at regular intervals during the full length of at least one vegetative cycle, having regard to the type of material and its stage of development during the testing programme, for symptoms caused by any harmful organisms, — by testing, in accordance with appropriate methods to be submitted to the Committee referred to in Article 18: — in the case of all potato material at least for:Andean potato latent virus, — Arracacha virus B. oca strain, — Potato black ringspot virus, — Potato spindle tuber viroid, — Potato virus T, — Andean potato mottle virus, — common potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leaf roll virus, — Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al., — Ralstonia solanacearum (Smith) Yabuuchi et al., — in the case of true seed potato of least for the viruses and viroid listed above;(dd) by appropriate testing on any other symptom observed in the visual examination in order to identify the harmful organisms having caused such symptoms;(c) any material, which has not been found free, under the testing specified under (b) from harmful organisms as specified under (b) shall be immediately destroyed or subjected to procedures which eliminate the harmful organism(s);(d) each organisation or research body holding this material shall inform their official Member State plant protection service of the material held. |
| 18.3.1. | Seeds of Solanum tuberosum L., other than those specified in point 18.4. |
Official statement that: The seeds derive from plants complying, as applicable, with the requirements set out in points 18.1., 18.1.1, 18.2 and 18.3; and (a) the seeds originate in areas known to be free from Synchytrium endobioticum (Schilbersky) Percival, Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al., Ralstonia solanacearum (Smith) Yabuuchi et al. and Potato spindle tuber viroid; or (b) the seeds comply with all of the following requirements: (i) they have been produced in a site where, since the beginning of the last cycle of vegetation, no symptoms of disease caused by the harmful organisms referred to in point (a) have been observed; (ii) they have been produced at a site where all of the following actions have been taken:separation of the site from other solanaceous plants and other host plants of Potato spindle tuber viroid;prevention of contact with staff and items, such as tools, machinery, vehicles, vessels and packaging material, from other sites producing solanaceous plants and other host plants of Potato spindle tuber viroid, or appropriate hygiene measures concerning staff or items from other sites producing solanaceous plants and other host plants of Potato spindle tuber viroid to prevent infection;only water free from all harmful organisms referred to in this point is used. |
| 18.4 | Plants of stolon, or tuber‐forming species of Solanum L., or their hybrids, intended for planting, being stored in gene banks or genetic stock collections | Each organisation or research body holding such material shall inform their official Member State plant protection service of the material held. |
| 18.5. | Tubers of Solanum tuberosum L., other than those mentioned in Annex IV(A)(II)(18.1), (18.1.1), (18.2), (18.3) or (18.4) |
There shall be evidence by a registration number put on the packaging, or in the case of loose‐loaded potatoes transported in bulk, on the vehicle transporting the potatoes, that the potatoes have been grown by an officially registered producer, or originate from officially registered collective storage or dispatching centres located in the area of production, indicating that the tubers are free from Ralstonia solanacearum (Smith) Yabuuchi et al. and that (a) the Union provisions to combat Synchytrium endobioticum (Schilbersky) Percival, and (b) where appropriate, the Union provisions to combat Clavibacter michiganensis ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al., and (c) the Union provisions to combat Globodera pallida (Stone) Behrens and Globodera rostochiensis (Wollenweber) Behrens are complied with. |
| Annex IV, Part B | Special requirements which shall be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within certain protected zones | ||
|---|---|---|---|
| Plants, plant products and other objects | Special requirements | Protected zone(s) | |
| 20.1. | Tubers of Solanum tuberosum L., intended for planting |
Without prejudice to the provisions applicable to the plants listed in Annex III(A) (10), (11), Annex IV(A)(I) (25.1), (25.2), (25.3), (25.4), (25.5), (25.6), Annex IV(A)(II) (18.1), (18.2), (18.3), (18.4), (18.6), official statement that the tubers: (a) were grown in an area where Beet necrotic yellow vein virus (BNYVV) is known not to occur; or (b) were grown on land, or in growing media consisting of soil that is known to be free from BNYVV, or officially tested by appropriate methods and found free from BNYVV; or (c) have been washed free from soil. |
F (Britanny), FI, IRL, P (Azores), UK (Northern Ireland) |
| 20.2. | Tubers of Solanum tuberosum L., other than those mentioned in Annex IV(B) (20.1) |
(a) The consignment or lot shall not contain more than 1% by weight of soil, or (b) the tubers are intended for processing at premises with officially approved waste disposal facilities which ensures that there is no risk of spreading BNYVV. |
F (Britanny), FI, IRL, P (Azores), UK (Northern Ireland) |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
|---|---|
| Part A | Plants, plant products and other objects originating in the Community |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community and which must be accompanied by a plant passport |
| 1.3. | Plants of stolon‐ or tuber‐forming species of Solanum L. or their hybrids, intended for planting. |
| Section II |
Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for certain protected zones and which must be accompanied by a plant passport valid for the appropriate zone when introduced into or moved within that zone Without prejudice to the plants, plant products and other objects listed in Part I |
| 1.5. | Tubers of Solanum tuberosum L., intended for planting. |
| Part B | Plants, plant products and other objects originating in territories, other than those territories referred to in Part A |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community |
| 4. | Tubers of Solanum tuberosum L. |
Table 6.
Natural hosts of PVS. Data regarding natural hosts was retrieved from the CABI cpc and literature up to 13 August 2019
| PVS | Hostsa | Rationale and/or uncertainty | Regulationb |
|---|---|---|---|
| Strain | |||
| PVS‐A | Literature: Arracacia xanthorrhiza (Santillan et al., 2018), Solanum curtilobum (Santillan et al., 2018), S. muricatum (Santillan et al., 2018), S. phureja (Vallejo et al., 2016), S. tuberosum (Santillan et al., 2018) | Natural and experimental hosts in different botanical families (Santillan et al., 2018). Additional natural hosts may exist | Solanum sp.: IIIA 10,11,12; IVAI 25.1, 25.2, 25.3, 25.4, 25.4.1, 25.4.2, 25.5, 25.6, 25.7, 25.7.1, 25.7.2, 28.1, 36.2, 45.3, 48; IVAII 18.1, 18.1.1, 18.2, 18.3, 18.3.1, 18.4, 18.5, 18.6, 18.6.1, 18.7, 26.1, 27; IVBI 20.1, 20.2; VAI 1.3, 2.4; VAII 1.5; VBI 1, 3, 4. |
| PVS‐O | Literature: Amaranthus hybridus (Hosseini and Salari, 2017), Chenopodium album (Hosseini and Salari, 2017), C. botrytis (Hosseini and Salari, 2017), S. lycopersicum (Predajn et al., 2017), S. nigrum (Hosseini and Salari, 2017), S. tuberosum (Santillan et al., 2018) | Natural and experimental hosts in different botanical families (Santillan et al., 2018). Additional natural hosts may exist | |
| Other isolate | |||
| PVS‐A/PVS‐O recombinants | S. tuberosum (Matoušek et al., 2000) | Limited information. Additional natural hosts may exist | Solanum sp.: IIIA 10,11,12; IVAI 25.1, 25.2, 25.3, 25.4, 25.4.1, 25.4.2, 25.5, 25.6, 25.7, 25.7.1, 25.7.2, 28.1, 36.2, 45.3, 48; IVAII 18.1, 18.1.1, 18.2, 18.3, 18.3.1, 18.4, 18.5, 18.6, 18.6.1, 18.7, 26.1, 27; IVBI 20.1, 20.2; VAI 1.3, 2.4; VAII 1.5; VBI 1, 3, 4 |
| PVS‐arracacha | Literature: Arracacia xanthorrhiza (De Souza et al., 2018) | Chenopodium spp. are reported as experimental hosts. Not known whether potato is a host | – |
Natural hosts including potato i.e. Solanum tuberosum and tuber‐forming Solanum species.
Including regulation of hosts without information of the strain(s) involved.
3.3.3. Legislation addressing the organisms that vector PVS (Directive/2000/29/EC)
Some non‐EU isolates of PVS (see Section 3.1.2) are reported to be transmitted by aphid vectors, which are not subject to specific regulation.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
Table 6 provides information on reports of natural hosts (including potato) of PVS strains and other isolates including the associated uncertainties and regulation. Solanum betaceum is reported as a host of PVS without information of the strain(s) involved (CABI, 2019).
3.4.2. Entry
3.4.2.1.
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes. Non‐EU isolates of PVS may enter the EU territory via plants for planting, i.e. seed potatoes (tubers) and/or microplants. Additional pathways include: ware potatoes (i.e. tubers intended for consumption or processing), plants for planting and fruits of other hosts, and viruliferous aphid vectors.
The following pathways can be considered for entry of non‐EU isolates of PVS into the EU: potato plants for planting (seed potatoes, microplants), ware potatoes (i.e. tubers intended for consumption or processing), plants for planting of other natural hosts and viruliferous aphid vectors (see Table 7 for the major pathways).
Table 7.
Identified major pathways for potential entry of non‐EU isolates of PVS and the extent to which these pathways are addressed by current legislation
| PVS | Potato plants for plantinga | Ware potatoesa | Plants for planting of other hostsa , b | Uncertainties |
|---|---|---|---|---|
| Strain | ||||
| PVS‐A | Pathway closed: plants for planting of potato are banned from countries where PVS‐A is reported | Pathway closed: import of ware potatoes is banned from countries where PVS‐A is reported | Pathway partially regulated: regulated and unregulated hosts |
Geographic distribution Existence of other natural hosts Relevance of vectors |
| PVS‐O | Pathway partially regulated: plants for planting of potato can be imported from Canada and Switzerland | Pathway partially regulated: import of ware potatoes is allowed from Algeria, Bosnia‐Herzegovina, Egypt, Israel, Libya, Morocco, Serbia, Syria, Switzerland, Tunisia and Turkey | Pathway partially regulated: regulated and unregulated hosts |
Existence of other natural hosts Existence and relevance of vectors |
| Other isolate | ||||
| PVS‐A/PVS‐O recombinants | Pathway closed: import of plants for planting of potato is banned from countries where PVS‐A/PVS‐O recombinants are reported | Pathway closed: import of ware potatoes is banned from countries where PVS‐A/PVS‐O recombinants are reported | Pathway closed: not known to have non‐Solanum natural hosts |
Geographic distribution Existence of other natural hosts Existence and relevance of vectors |
| PVS‐arracacha | Pathway closed: import of plants for planting of potato is banned from countries where PVS‐arracacha is reported | Pathway closed: import of ware potatoes is banned from countries where PVS‐arracacha is reported | Pathway open: unregulated host |
Existence of other natural hosts Existence and relevance of vectors |
‘ Pathway open’: no regulation or ban that prevents this pathway, ‘Pathway closed’ (as opposed to ‘pathway open’): ban that prevents entry. ‘Pathway possibly open’: no direct evidence of the existence of the pathway (not closed by current legislation), but existence cannot be excluded based on comparisons with the biology of closely related viruses (in the same genus or family). ‘Pathway regulated’: regulations exist that limit the probability of entry along the pathway, but there is not a complete ban on imports. ‘Pathway partially regulated’: pathway consists of several sub‐pathways, some are open, while others are closed (e.g. regulation for some hosts, but not for others; a ban exists for some non‐EU MSs but not for all). ‘Not a pathway’: no evidence supporting the existence of the pathway.
Plants for planting, including seeds and pollen, of other hosts which are listed in Table 6.
PVS is transmitted by vegetative propagation and therefore seed potatoes and more generally, potato plants for planting, are considered the most important pathway for entry. The potential pathways for entry of non‐EU isolates via seed potatoes of S. tuberosum and plants for planting of other tuber‐forming Solanum species and their hybrids are addressed by the current EU legislation (table 5; (EU) 2000/29 Annex IIIA, 10 and 11), which sets that import is not allowed from third countries except Switzerland. However, import of seed potatoes from Canada into Greece, Spain, Italy, Cyprus, Malta and Portugal is allowed by a derogation (2011/778/EU, 2014/368/EU, document C (2014) 3878). PVS‐O is present in Canada and Switzerland. By definition, the PVS isolates present in these countries are considered to be non‐EU isolates. Therefore, the pathway of plants for planting is considered partially regulated for PVS. When considering the various strains and other isolates separately, only PVS‐O is known to be present in the two countries for which derogations apply. Therefore, the potato plants for planting pathway is considered partially regulated for non‐EU isolates of PVS‐O but closed by legislation for non‐EU isolates of PVS‐A, PVS‐A/PVS‐O recombinants and for PVS‐arracacha.
Entry of ware potatoes is addressed by the current EU legislation (table 5, Annex IIIA, 12). Import of ware potatoes is prohibited from third countries other than Algeria, Egypt, Israel, Libya, Morocco, Syria, Switzerland, Tunisia and Turkey and from European non‐EU countries which are not free from Clavibacter michiganensis spp. sepedonicus or in which provisions on combating Clavibacter michiganensis spp. sepedonicus have not been complied with. The latter exemption currently applies to Serbia and Bosnia‐Herzegovina. PVS is or should be considered present in these specified countries given its worldwide distribution. By definition, the PVS isolates present in these countries are considered non‐EU isolates. They can in principle enter the EU via the ware potato pathway as there are no specific measures in place that mitigate the risk of entry. As reported in the pest categorisation of non‐EU viruses and viroids of potato (EFSA PLH Panel, 2020), the majority of the imported ware potatoes comes from Egypt and Israel (47 and 47.2%, respectively). Note that as long as ware potatoes are used for the intended use (consumption or processing), the ability of the non‐EU isolates of PVS to establish is very low. In addition, there are specific measures in place (Annex IV 25.3) for countries where potato spindle tuber viroid is known to occur (according to EPPO: Egypt, Israel and Turkey) aimed at mitigating the risk of establishment by suppression of the faculty of germination of ware potatoes, other than early potatoes, from these countries. When considering the various strains and other isolates separately, only PVS‐O is known to be present in the countries for which derogations apply. Therefore, the ware potato pathway is considered partially regulated for non‐EU isolates of PVS‐O but closed by legislation for non‐EU isolates of PVS‐A, PVS‐A/PVS‐O recombinants and for PVS‐arracacha.
PVS has a limited number of natural hosts other than potato (see Section 3.5). The non‐Solanum hosts (arracacha, Chenopodium album, C. botrytis, Amaranthus hybridus) are not regulated. It is, however, unclear whether there is a trade of plants for planting of these species. If so, these alternative hosts could provide an additional pathway. When considering separately the various strains and other isolates, and their host range, the pathway is considered partially regulated for non‐EU isolates of PVS‐A and PVS‐O, open for PVS‐arracacha which has non‐regulated hosts, but closed for non‐EU PVS‐A/PVS‐O recombinants which are not known to have non‐Solanum natural hosts. This assessment is affected by uncertainties on trade and host range.
Viruliferous aphid vectors are a pathway of entry for some non‐EU isolates of PVS (see Table 2). Since the relevant aphid species are not subject to specific regulation, this pathway is open for non‐EU isolates of PVS‐A and possibly open for non‐EU isolates of the other categorised strains/isolates. PVS is transmitted by aphids in a non‐persistent way, which implies that viruliferous aphids will lose the ability to transmit the virus within a short period. Therefore, this pathway is considered as minor and is not listed in Table 7.
Import of fruits can be an additional pathway for entry of non‐EU isolates of PVS. However, the lack of seed transmission (see Section 3.1.2) reduces the relevance of this potential pathway. Aphid vectors can probe the infected fruits and acquire the virus for later transmission. Fruits of Solanum betaceum (natural host of PVS without lineage specification) and Solanum lycopersicum (natural host of PVS‐O) can be imported from a range of countries where PVS isolates have been reported. Overall, this pathway is considered to be open for non‐EU isolates of PVS‐O and possibly open for non‐EU isolates of the other categorised strains/isolates. However, given the relatively unlikely series of events involved (aphids feeding on imported fruits followed by moving to susceptible plants) and the absence of seed transmission, this pathway is considered as minor and is not listed in Table 7.
Table 8 reports two interceptions of PVS by EU member states during the period between 1995 and 8 August 2019. Only interceptions involving consignments imported from outside the EU were considered.
Table 8.
Interceptions of PVS by EU MSs on imported material from outside the EU. Data retrieved from the Europhyt database on 8 August 2019
| PVS | Europhyt interception ID | Year of interception | Origin | Plant species on which it has been intercepted |
|---|---|---|---|---|
| PVS (6 accessions) | 8510 | 1999 | USA | Solanum sp.a |
| PVS (4 accessions) | 11780 | 2000 | USA | Solanum tuberosum a |
Intercepted during post‐entry quarantine testing.
3.4.3. Establishment
3.4.3.1.
Is the pest able to become established in the EU territory?
Yes. Non‐EU isolates of PVS are likely to become established in the EU territory, as EU isolates and the main hosts are already present in the EU.
3.4.3.2. EU distribution of main host plants
Potato is widely grown in the EU, as reported in the pest categorisation of non‐EU viruses and viroids of potato (EFSA PLH Panel, 2020).
3.4.3.3. Climatic conditions affecting establishment
Except for those conditions affecting survival of the host plants, no eco‐climatic constrains exist for the PVS isolates categorised here. Therefore, it is expected that these isolates are able to establish wherever their hosts may live. Potato is widely cultivated in the EU and therefore the Panel considers that climatic conditions will not impair the ability of the viruses addressed here to establish in the EU. However, it must be taken into consideration that virus impact, accumulation and distribution within natural hosts are dependent on environmental conditions. The same applies to expression of symptoms, vector populations and virus transmission being affected by climatic conditions.
3.4.4. Spread
3.4.4.1.
Is the pest able to spread within the EU territory following establishment? How?
Yes. Non‐EU isolates of PVS can spread via plants for planting, by mechanical transmission and, at least, isolates of the PVS‐A strain can additionally be spread by aphid vectors.
Some non‐EU isolates of PVS can be transmitted by aphids (see Section 3.1.2), including Myzus persicae (Sulzer), which is widespread in and outside the EU (see Figure 1).
Figure 1.
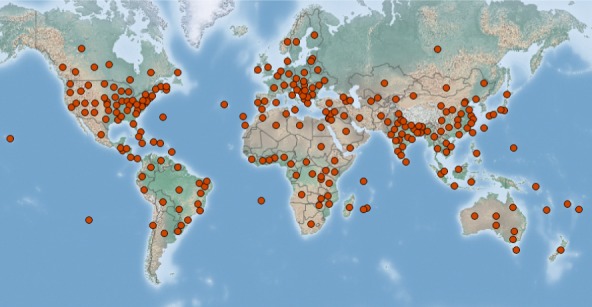
Global distribution map of Myzus persicae (Sulzer). Extracted from CABI cpc on 8 August 2019
3.5. Impacts
3.5.1.
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes. Non‐EU isolates of PVS‐A can be expected to have an additional impact on the EU territory, although the magnitude of the impact is uncertain.
No. Non‐EU isolates of PVS‐O are not known to differ from PVS‐O isolates already present and no additional impact is therefore expected on the EU territory.
Unable to conclude. The lack of conclusive data on the biological properties of PVS‐A/PVS‐O recombinants and PVS‐arracacha, does not allow the Panel to reach a conclusion on a potential additional impact on the EU territory.
As mentioned in the pest categorisation of non‐EU viruses and viroids of potato (EFSA PLH Panel, 2020), symptoms caused by viruses are influenced by different factors, such as the isolate of the virus, the host and variety, and environmental conditions. A causal relation between a virus and reported symptoms is not always clear, for example, in the case of mixed infections. Mixed infections are especially common in vegetative‐propagated crops such as potato and the presence of additional viruses might increase or attenuate the observed symptoms. Therefore, reports on the symptomatology of individual viruses might not be conclusive, leading to uncertainties on the causal relation between a virus and the symptoms reported.
Table 9 reports on the expected additional impact of non‐EU isolates of PVS in comparison to the PVS isolates already present in the EU. PVS is considered to have an impact at the species level and various control measures are already implemented (e.g. certification of plants for planting). To determine whether non‐EU isolates would have an additional impact, a comparison of biological properties was made between non‐EU isolates of PVS and isolates already present in the EU. No information on yield and quality losses is available at strain or isolate level.
Table 9.
Expected additional impact and rationale of non‐EU isolates of PVS on the EU territory
| PVS | Additional impact on the EU territory? | Rationale and/or uncertainty |
|---|---|---|
| Strains | ||
| PVS‐A | Yes | In comparison to PVS‐O isolates, PVS‐A isolates are reported to reach higher concentrations in plants and are more stable in plant sap, which would favour aphid transmission. PVS‐A isolates have been shown to be more readily transmitted by aphids (Santillan et al., 2018) so that faster epidemic progression is expected. However, the magnitude of the additional impact over the present situation is uncertain |
| PVS‐O | No | PVS‐O isolates occur worldwide and there is no evidence for differences in molecular or biological properties between EU and non‐EU PVS‐O isolates. Therefore, non‐EU PVS‐O isolates are not expected to have an additional impact over the present situation |
| Other isolates | ||
| PVS‐A/PVS‐O recombinants | Unable to conclude | In the absence of information on the biology and, in particular, on biological differences with the EU isolates of PVS (recombinant or not), the Panel is unable to conclude whether PVS‐A/PVS‐O recombinant isolates would have additional impact in the EU |
| PVS‐arracacha | Unable to conclude | Reported once in Arracacia xanthorrhiza, without information on biology (De Souza et al., 2018); it is unknown whether potato is a host and if other natural hosts exist. In the absence of such information, the Panel is unable to conclude whether PVS‐arracacha isolates would have additional impact in the EU, as compared to the present situation |
3.6. Availability and limits of mitigation measures
3.6.1.
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes. See Section 3.3 for measures already implemented in the current legislation. Additional measures could be implemented to further regulate the identified pathways or to limit entry, establishment or spread of non‐EU isolates of PVS.
3.6.2. Identification of additional measures
Phytosanitary measures are currently applied to potato and other hosts (see Sections 3.3 and 3.5). Potential additional measures to mitigate the risk of entry of the isolates categorised in this opinion may include:
Repel import derogations for potato plants for planting;
Set specific phytosanitary requirements addressing the isolates categorised in this opinion for imported seed potatoes and/or ware potatoes;
Extension of phytosanitary measures to specifically include hosts other than potato;
Banning import of non‐potato hosts plants for planting from countries where isolates other than PVS‐O isolates are present;
Extension of certification schemes and testing requirements to non‐Solanum natural hosts;
Extension of plant passport requirements to specifically include hosts other than stolon‐ and tuber‐forming Solanum species.
In addition, non‐EU isolates of PVS may enter in the EU through viruliferous aphids. Measures against aphids may include chemical treatment of consignments identified as potential entry pathways.
3.6.2.1. Additional control measures
Table 10 reports on the potential additional control measures to reduce the likelihood of entry, establishment and/or spread of the categorised non‐EU isolates of PVS. The additional control measures are selected form a longer list reported in EFSA PLH Panel (2018). Control measures are measures that have a direct effect on pest abundance.
Table 10.
Selected additional control measures to consider to reduce the likelihood of pest entry, establishment and/or spread of non‐EU isolates of PVS
| Information sheet (with hyperlink to information sheet if available) | Control measure summary | Risk component | Rationale |
|---|---|---|---|
| http://doi.org/10.5281/zenodo.1175887 | Description of possible exclusion conditions that could be implemented to isolate the crop from pests and if applicable relevant vectors. E.g. a dedicated structure such as glass or plastic greenhouses | Spread | Growing plants in insect proof greenhouses may prevent infestation by viruliferous aphid vectors. This measure would not be applicable for potato, with the exception of early stages of seed potato production. Production of seed potatoes in areas with low aphid pressure (e.g. high altitude) would minimise the risk of infestation |
| http://doi.org/10.5281/zenodo.1175910 |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage The treatments addressed in this information sheet are: a) fumigation; b) spraying/dipping pesticides; c) surface disinfectants; d) process additives; e) protective compounds |
Entry |
a), b) and c) could remove viruliferous aphid vectors PVS is transmitted by aphids in a non‐persistent way, which implies that viruliferous aphids will lose the ability to transmit the virus within a short period. Therefore, the additional effect on preventing entry is minimal |
| http://doi.org/10.5281/zenodo.1175929 | The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories (e.g. boxes, pots, pallets, palox, supports, hand tools). The measures addressed in this information sheet are: washing, sweeping and fumigation | Spread | Cleaning tools may limit the spread via mechanical transmission. Cutting tubers was associated with PVS transmission |
| http://doi.org/10.5281/zenodo.1181436 | Roguing is defined as the removal of infested plants and/or uninfested host plants in a delimited area, whereas pruning is defined as the removal of infested plant parts only, without affecting the viability of the plant | Establishment and spread | Roguing of infested plants is efficient, in particular to prevent spread of PVS via contact. Pruning is not effective to remove a virus from infected plants |
| http://doi.org/10.5281/zenodo.1181717 |
Crop rotation, associations and density, weed/volunteer control are used to prevent problems related to pests and are usually applied in various combinations to make the habitat less favourable for pests The measures deal with (1) allocation of crops to field (over time and space) (multicrop, diversity cropping) and (2) to control weeds and volunteers as hosts of pests/vectors |
Spread and impact | Viruses are maintained by vegetative propagation and, therefore, control of volunteers is important. Control of weed hosts may be of relevance |
| Timing of planting and harvesting | The objective is to produce phenological asynchrony in pest/crop interactions by acting on or benefiting from specific cropping factors such as: cultivars, climatic conditions, timing of the sowing or planting and level of maturity/age of the plant seasonal timing of planting and harvesting | Spread and impact | Relevant to prevent transmission by aphid vectors |
| Chemical treatments on crops including reproductive material | Chemical treatments on crops may prevent infestations by vectors and seed transmission | Spread and impact | Desiccation/removal of the foliage reduces the risk of transmission via aphid vectors and may prevent transport to the tubers of infected plants |
| Post‐entry quarantine and other restrictions of movement in the importing country |
This information sheet covers post‐entry quarantine of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; prohibition of import of relevant commodities into the domestic country Relevant commodities are plants, plant parts and other materials that may carry pests, either as infection, infestation or contamination |
Entry and spread | Identifying virus‐infected plants and banning their movement limit the risks of entry and spread in the EU |
3.6.2.2. Additional supporting measures
Table 11 reports on the possible additional supporting measures which are selected from the list reported in EFSA PLH Panel (2018). Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
Table 11.
Selected supporting measures in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Supporting measure summary | Risk component | Comments |
|---|---|---|---|
| http://doi.org/10.5281/zenodo.1181430 |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5) The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques |
Entry and spread |
Visual inspection may detect potentially infected material Only applicable when visible symptoms on leaves and/or propagating tissues occur, which is dependent on the isolate, host/cultivar, and environmental conditions |
| http://doi.org/10.5281/zenodo.1181213 | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests | Entry and spread | Laboratory testing may detect/identify non‐EU isolates of PVS on sampled material |
| http://doi.org/10.5281/zenodo.1180845 | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by a National Plant Protection Organization in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries | Entry and spread | Certified and approved premises may guarantee the absence of the harmful viruses imported for research and/or breeding purposes |
| http://doi.org/10.5281/zenodo.1180597 | ISPM 5 defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’ (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest‐free production place, site or area | Spread | Buffer zones may contribute to reduce the spread of non‐EU isolates of PVS after entry in the EU |
| Phytosanitary certificate and plant passport | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) a) export certificate (import) b) plant passport (EU internal trade) | Entry and spread | |
| Certification of reproductive material (voluntary/official) | Certification of reproductive material when not already implemented would contribute to reduce the risk associated with spread | Spread | |
| Surveillance | Official surveillance may contribute to early detection of non‐EU isolates of PVS, favouring immediate adoption of control measures if they come to establish | Spread |
3.6.2.3. Biological or technical factors limiting the effectiveness of measures to prevent the entry, establishment and spread of the pest
Symptomless infections for some of the non‐EU isolates of PVS in some hosts;
Uneven virus distribution or low concentrations limiting the reliability of the detection;
Absence of a validated diagnostic protocol allowing the typing of PVS strains/isolates.
3.7. Uncertainty
The Panel identified the following knowledge gaps and uncertainties:
Identity and biology
Lack of information to support the assignment of isolates to PVS‐A or PVS‐O in reports without genomic data;
Limited biological data, in particular at strain level, i.e. on host range and aphid transmission, pathogenicity in potato;
Lack of information on whether identified biological differences are general features of PVS strains or apply only to a fraction of the isolates in a given strain;
Uncertainty on the existence of other non‐EU isolates of PVS that have not yet been identified yet and might have an additional impact on the EU territory.
Pest distribution
Uncertainty on the geographical distribution and prevalence of the categorised strains/isolates of PVS because of the absence of systematic surveys.
Regulatory status
The concept of ‘non‐EU isolates’ leaves some room for interpretation, which may create confusion or difficulties when enforcing the legislation (see Section 1.2).
Entry, establishment and spread in the EU (host range, entry, establishment, spread)
Uncertainty on the host range of the categorised strains/isolates of PVS;
Uncertainty on the ability and efficiency of aphid vectors to transmit non‐EU isolates of PVS.
Impact
Uncertainty on the magnitude of the impact of non‐EU isolates and whether this impact would exceed that of the isolates already present in the EU.
4. Conclusions
The information currently available on geographical distribution, biology, epidemiology, potential additional impact over the present situation and potential entry pathways of non‐EU isolates of potato virus S (PVS) has been evaluated with regard to the criteria to qualify as potential Union quarantine pest. The conclusions of the Panel are summarised in Table 12.
Table 12.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column) for non‐EU isolates of PVS
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1) |
The identity of PVS is well established Methods are available for detection and identification of PVS at species and strain level, but not for the specific identification of PVS‐A/PVS‐O recombinants and PVS‐arracacha isolates. Genomic data are available for the design of diagnostic tests |
Uncharacterised PVS isolates may exist Uncertainty on the exclusivity and inclusivity of the strain‐typing test |
| Absence/presence of the pest in the EU territory (Section 3.2) |
PVS‐O isolates occur worldwide and are present in the EU PVS‐A isolates and PVS‐arracacha are not known to be present in the EU A PVS‐A/PVS‐O recombinant has been reported once in the Czech Republic |
Unreported or more widespread presence of isolates other than PVS‐O in the EU |
| Regulatory status (Section 3.3) | Non‐EU isolates of PVS are currently regulated in Annex IAI | Interpretation of the concept of ‘non‐EU isolate’ |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) |
Non‐EU isolates of PVS are able to enter into the EU The pathways of plants for planting of potato and of ware potatoes are considered partially regulated for non‐EU isolates of PVS‐O, but closed by legislation for non‐EU isolates of PVS‐A, PVS‐A/PVS‐O recombinants and for PVS‐arracacha The pathway of plants for planting of other hosts is partially regulated for non‐EU isolates of PVS‐O and PVS‐A, open for PVS‐arracacha and closed by current legislation for non‐EU PVS‐A/PVS‐O recombinants The minor pathways of viruliferous aphids and import of fruits of hosts species are open for non‐EU isolates of PVS‐O and possibly open for non‐EU isolates of the other categorised strains/isolates If non‐EU isolates of PVS were to enter the EU territory, they could become established and spread |
For all strains/isolates, uncertainties on:Geographical distributionExistence of other natural hostsExistence and/or relevance of vectors Trade of plants for planting of non‐Solanum hosts |
| Potential for consequences in the EU territory (Section 3.5) |
There are no indications that non‐EU isolates of PVS‐O differ from PVS‐O isolates already present in the EU and no additional impact is therefore expected from non‐EU isolates of the PVS‐O strain PVS‐A isolates are expected to have an additional impact over the present situation because they are expected to more readily spread as compared to PVS isolates already present in the EU For non‐EU PVS‐A/PVS‐O recombinants and PVS‐arracacha, the Panel was unable to conclude on potential additional consequences in the EU territory due to limited information |
Uncertainty on the magnitude of impact of non‐EU isolates |
| Available measures (Section 3.6) | Phytosanitary measures are available to reduce the likelihood of entry and spread of non‐EU isolates of PVS in the EU | No uncertainty |
| Conclusion on pest categorisation (Section 4) |
Non‐EU isolates of PVS‐O do not meet one of the criteria evaluated by EFSA to be regarded as a potential Union quarantine pest, since they are not expected to have an additional impact in the EU Non‐EU PVS‐A isolates meet the criteria evaluated by EFSA to qualify as a potential Union quarantine pest With the exception of the criterion regarding the potential consequences in the EU territory for which the Panel is unable to conclude (see Section 3.5), non‐EU isolates of PVS‐A/PVS‐O recombinants and of PVS‐arracacha meet all the other criteria evaluated by EFSA to qualify as potential Union quarantine pests |
|
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
The main knowledge gaps or uncertainties identified concern: — Lack of information on the biology of the categorised strains/isolates (e.g. host range, vector transmission, pathogenicity) — Existence of other harmful non‐EU isolates — Possible unreported presence in the EU of PVS‐A and PVS‐arracacha isolates— Volume of trade and countries of origin of plants for planting of non‐potato hosts— Uncertainty on magnitude of impact of non‐EU isolates of PVS |
|
Non‐EU PVS‐A isolates meet all the criteria to qualify as potential Union quarantine pests and, in particular, could potentially have an additional impact over the current situation because they are expected to be more readily spread.
With the exception of the criterion regarding the potential consequences in the EU territory for which the Panel is unable to conclude (see Section 3.5), non‐EU isolates of PVS‐A/PVS‐O recombinants and of PVS‐arracacha meet all the other criteria to qualify as potential Union quarantine pests.
Non‐EU isolates of PVS‐O do not meet one of the criteria evaluated by EFSA to be regarded as a potential Union quarantine pest, since they are not expected to have an additional impact in the EU.
The Panel wishes to stress that these conclusions are associated with uncertainties because of limited information on distribution, biology and impact of PVS isolates at strain level. In particular, the magnitude of the potential additional impact over the present situation is generally unknown. Furthermore, other potentially harmful non‐EU isolates of PVS might exist that have not been discovered yet.
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PVS
potato virus S
- PVS‐A
PVS‐Andean strain
- PVS‐O
PVS‐ordinary strain
- PZ
Protected Zone
- RNQP
Regulated Non‐Quarantine Pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Isolate
Virus population as present in a plant
- Measures
Control (of a pest) is defined in ISPM 5 (FAO 2017) as “Suppression, containment or eradication of a pest population” (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
- Strain
Group of isolates sharing biological, molecular and/or serological properties
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Candresse T, Lacomme C, Bottex B, Oplaat C, Roenhorst A, Schenk M and Di Serio F, 2020. Scientific Opinion on the pest categorisation of potato virus S (non‐EU isolates). EFSA Journal 2020;18(1):5855, 38 pp. 10.2903/j.efsa.2020.5855
Requestor: European Commission
Question Number: EFSA‐Q‐2019‐00508
Panel Members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Acknowledgments: This opinion was prepared in cooperation with the National Plant Protection Organization, Netherlands Food and Consumer Product Safety Authority under the tasking grant (GP/EFSA/ALPHA/2017/04).
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © CABI
Adopted: 26 September 2019
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
References
- Adams MJ, Candresse T, Hammond J, Kreuze JF, Martelli GP, Namba S, Pearson MN, Ryu KH, Saldarelli P and Yoshikawa N, 2011. Betaflexiviridae In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (eds.).Virus Taxonomy, 9th Report of the International Committee on Taxonomy of Viruses. pp. 920–941. [Google Scholar]
- Andino R and Domingo E, 2015. Viral quasispecies. Virology, 479–480, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode O and Weidemann HL, 1971. Untersuchungen zur blattlaustibertragbarkeit von Kartoffel ‐M‐ und ‐S‐ virus. Potato Research, 14, 119–129. [Google Scholar]
- CABI , 2019. Datasheet potato virus S. Available online: https://www-cabi-org/cpc/datasheet/43662 [Accessed 13 August 2019] [Google Scholar]
- Cox BA and Jones RA, 2010. Genetic variability in the coat protein gene of potato virus S isolates and distinguishing its biologically distinct strains. Archives of Virology, 155, 1163–1169. [DOI] [PubMed] [Google Scholar]
- De Sousa Geraldino Duarte P, Galvino‐Costa SB, de Paula Ribeiro SR and Figueira Ados R, 2012. Complete genome sequence of the first Andean strain of potato virus S from Brazil and evidence of recombination between PVS strains. Archives of Virology, 157, 1357–1364. [DOI] [PubMed] [Google Scholar]
- De Souza J, Gamarra H, Müller G and Kreuze J, 2018. First report of potato virus S naturally infecting arracacha (Arracacia xanthorrhiza) in Peru. Plant Disease, 102, 460. [Google Scholar]
- Dolby CA and Jones RAC, 1987. Occurrence of the Andean strain of potato virus S in imported potato material and its effects on potato cultivars. Plant Pathology, 36, 381–388. [Google Scholar]
- Dolby CA and Jones RAC, 1988. The relationship between the Andean strain of potato virus S and pepino latent virus. Annals of Applied Biology, 112, 231–234. [Google Scholar]
- Domingo E, Sheldon J and Perales C, 2012. Viral quasispecies evolution. Microbioly and Molecular Biology Reviews, 76, 159–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Zhan F, Du Z, Ho SYW and Gao F, 2018. Europe was a hub for the global spread of potato virus S in the 19th century. Virology, 525, 200–204. [DOI] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2020. Pest categorisation of non‐EU viruses and viroids of potato. EFSA Journal 2020;18(1):5853, 134 pp. 10.2903/j.efsa.2020.5853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf.
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No 5. Glossary of phytosanitary terms. Available online: https://www.ippc.int/en/publications/622/
- Fletcher JD, 1996. Potato virus SA ‐ characteristics of an isolate from New Zealand. New Zealand Journal of Crop and Horticultural Science, 24, 335–339. [Google Scholar]
- Garcıa‐Arenal F, Fraile A and Malpica JM, 2001. Variability and genetic structure of plant virus populations. Annual Review of Phytopathology, 39, 157–186. [DOI] [PubMed] [Google Scholar]
- Goth RW and Webb RE, 1974. Lack of potato virus S transmission via true seed in solanum tuberosum. Phytopathology, 65, 1347–1349. [Google Scholar]
- Gutierrez PA, Alzate JF and Marin‐Montoya MA, 2013. Complete genome sequence of a novel potato virus S strain infecting Solanum phureja in Colombia. Archives Virology, 158, 2205–2208. [DOI] [PubMed] [Google Scholar]
- Hinostroza‐Orihuela AM, 1973. Some properties of potato virus S isolated from Peruvian potato varieties. Potato Research, 16, 244–250. [Google Scholar]
- Horvath J, 1973. Seed transmission experiments of potato virus M and potato virus S in Lycopersicon species. Acta agronomica Academiae Scientiarum Hungaricae, 22, 390–392. [Google Scholar]
- Hosseini SA and Salari K, 2017. Detection and molecular characterisation of potato virus S of weed reservoirs in Iran. Archives of Phytopathology and Plant Protection, 50, 828–838. [Google Scholar]
- Jeffries CJ, 1998. FAO‐IPGRI Technical guidelines for the safe movement of germplasm no19 potato_IPGRI.
- Khassanov VT and Vologin SG, 2018. Occurrence of the ordinary and the andean strains of potato virus S infecting potatoes in the eastern region of Kazakhstan. Plant Diseases, 10, 2052. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kimura S, Nishio T, Motojima S and Matsunami M, 1985. Carlavirus isolated form potato. Research bulletin of the Plant Protection Service. (Japanese, English abstract) pp. 41–46. [Google Scholar]
- Lambert SJ, Scott JB, Pethybridge SJ and Hay FS, 2012. Strain characterization of potato virus S isolates from Tasmania, Australia. Plant Disease, 96, 813–819. [DOI] [PubMed] [Google Scholar]
- Lin Y‐H, Druffel KL, Whitworth J, Pavek MJ and Pappu HR, 2009. Molecular characterization of two potato virus S isolates from late‐blight‐resistant genotypes of potato (Solanum tuberosum). Archives of Virology, 154, 1861–1863. [DOI] [PubMed] [Google Scholar]
- MacKenzie DJ, Tremaine JH and Stace‐Smith R, 1989. Organization and interviral homologies of the 3’‐terminal portion of potato virus S RNA. Journal of General Virology, 70(Pt. 5), 1053–1063. [DOI] [PubMed] [Google Scholar]
- MacKinnon JP, 1973. Detection, spread, and aphid transmission of potato virus S. Canadian Journal of Botany, 52, 461–465. [Google Scholar]
- Matoušek J, Schubert J, Dědič P and Ptáček J, 2000. A broad variability of potato virus S (PVS) revealed by analysis of virus sequences amplified by reverse transcriptase ‐ polymerase chain reaction. Canadian Journal of Plant Pathology, 22, 29–37. [Google Scholar]
- Matoušek J, Schubert J, Ptáček J, Kozlová P and Dědič P, 2005. Complete nucleotide sequence and molecular probing of potato virus S genome. Acta Virologica, 49, 195–205. [PubMed] [Google Scholar]
- Predajn L, Šoltys K, Kraic J, Mihálik D and Glasa M, 2017. First report of potato virus S infecting tomato in Slovakia. Journal of Plant Pathology, 99, 811. [Google Scholar]
- Salari K, Massumi H, Heydarnejad J, Hosseini Pour A and Varsani A, 2011. Analysis of Iranian Potato virus S isolates. Virus Genes, 43, 281–288. [DOI] [PubMed] [Google Scholar]
- Santillan FW, Fribourg CE, Adams IP, Gibbs AJ, Boonham N, Kehoe MA, Maina S and Jones RAC, 2018. The biology and phylogenetics of potato virus S isolates from the Andean region of South America. Plant Disease, 102, 869–885. [DOI] [PubMed] [Google Scholar]
- Slack SA, 1983. Identification of an isolate of the Andean strain of potato virus S in North America. Plant Disease, 67, 786–789. [Google Scholar]
- Thomas W, Mohamed NA and Fry ME, 1980. Properties of a carlavirus causing a latent infection of pepino (Solanum muricatum). Annals of Applied Biology, 95, 191–196. [Google Scholar]
- Vallejo CD, Gutiérrez SP and Marín MM, 2016. Genome characterization of a potato virus S (PVS) variant from tuber sprouts of Solanum phureja Juz. et Buk. Agronomía Colombiana, 34, 51–60. [Google Scholar]
- Wang J, Meng F, Chen R, Liu J, Nie X and Nie B, 2016. RT‐PCR differentiation, molecular and pathological characterization of Andean and ordinary strains of Potato virus S in China. Plant Dis., 100, 1580–1585. [DOI] [PubMed] [Google Scholar]
- Wardrop EA, Gray AB, Singh RP and Peterson JF, 1989. Aphid transmission of potato virus S. American Potato Journal, 66, 449–459. [Google Scholar]


