Abstract
Diagnosing, surveilling, and understanding the biological consequences of clonal haematopoiesis poses a clinical challenge for both patients and clinicians. The relationship between peripheral blood cytopenias and myeloid neoplasms—such as myelodysplastic syndrome—is an area of active research, and understanding of clonal haematopoiesis has developed markedly on the basis of findings concerning somatic mutations in genes known to be associated with myelodysplastic syndrome. These findings have raised the conundrum of how to appropriately define and follow myelodysplastic syndrome precursor states, such as clonal haematopoiesis of indeterminate potential (CHIP) and clonal cytopenias of undetermined significance (CCUS). Identifying these conditions could allow earlier diagnosis of myelodysplastic syndrome, modify surveillance for myelodysplastic syndrome, and possibly guide therapies, but this information also comes at a cost to patients that might or might not be justified by our present understanding of clonal haematopoiesis. When faced with a diagnosis of clonal haematopoiesis, some patients and providers might be content to let the events unfold naturally, whereas others may insist on intense follow-up and early interventions. This Viewpoint assesses recent developments in clonal haematopoiesis and the related implications for affected patients and their providers.
Introduction
A patient who presents to the clinic for investigation of peripheral blood cytopenia brings dilemmas of diagnosis, monitoring, and therapy. Refractory cytopenia in the context of a normocellular or hypercellular bone marrow can often raise concern for myelodysplastic syndromes and related disorders.1,2 Myelodysplastic syndrome encompasses a heterogeneous collection of clonal haematopoietic malignancies affecting a predominantly older population. The disorder is characterised by poor overall survival due to ineffective haematopoiesis, progressive cytopenia, and transformation to acute myeloid leukemia.3 Extremely rare in patients younger than 50 years, the prevalence of myelodysplastic syndrome increases with age. Between 30 000 and 40 000 cases are diagnosed per year,4 with a median age at diagnosis of about 70 years.5 The diagnosis of myelodysplastic syndrome has become more complex since myelodysplastic syndrome precursor states and other causes of unexplained cytopenias have been defined. The advent of next-generation sequencing technology has provided additional diagnostic information, which could allow important clinical insights, such as early identification of predisposition states to myelodysplastic syndrome and increased accuracy in diagnosis of myelodysplastic syndrome.6,7 However, this added ability to assess a patient for evidence of clonal haematopoiesis poses inherent challenges of how to allow these results to affect the patient’s health—both physical and emotional.
Patients with unexplained cytopenias are increasingly undergoing molecular testing by next-generation sequencing of peripheral blood or bone marrow to diagnose possible myelodysplastic syndrome precursor states. These tests can detect mutations in individuals without morphological or cytogenetic evidence of myeloid neoplasm or myelodysplastic syndrome, and new entities have therefore been defined to categorise these patients appropriately. Some of these precursor states could evolve to frank malignancy, making it important to define a path that explains these conditions and allows proper understanding of and context for a patient’s health.5,8,9
Genesis and evolution of clonal haematopoiesis
Clinicians need to understand the biological explanation of positive next-generation sequencing results to guide their next steps, and patients also deserve and often require explanations to contextualise their new diagnosis. In truth, the acquisition of somatic mutations is an unavoidable consequence of cell division. Even though fewer than three somatic mutations occur per cell division, mutations can accumulate quickly in rapidly dividing haematopoietic progenitor pools.10–13 The human haematopoietic system is one of the most proliferative tissues in the human body. Haematopoietic stem cells have an enormous task of providing nearly 1012 cells every day,14 and thus the acquisition of somatic mutations with time is inevitable. The most rapidly dividing, mutation-prone haematopoietic progenitors lack the potential for self-renewal; therefore, any such mutation-carrying clone will usually disappear as a consequence of terminal differentiation and senescence. Occasionally, mutations can occur in self-renewing haematopoietic stem cells and be retained in the haematopoietic pool. As expected, these so-called single nucleotide variants accumulate with time and become relatively ubiquitous as people age beyond the fifth decade of life.15
Fortunately, because of their mostly random occurrence, most mutations affect non-coding regions and thus remain functionally silent (passenger mutations). However, somatic mutations occasionally fall within coding or regulatory regions of the genome and affect genes crucial to cell fate determination, proliferation, or self-renewal, resulting in selective growth advantage and clonal expansion (driver mutations). Uncontrolled proliferation and incomplete maturation frequently result in substantial expansion of the clones and attrition of the healthy haematopoietic counterparts, leading to a clinically apparent haematological malignancy, such as myelodysplastic syndrome. In the past decade, several groups8,9,16,17 have reported that mostly minor clones (marked by acquired mutations in genes frequently associated with haematological malignancies) are present in the blood of ageing individuals with no haematologic phenotype. The terms clonal haematopoiesis of indeterminate potential (CHIP) and age-related clonal haematopoiesis have been introduced to describe this intriguing and multifaceted condition.5,18
Assessment of clonality for CHIP
It is now widely accepted that cancers arise from a single progeny, as a result of uncontrolled growth of its daughter cells or clones. The clonal nature of cancer was first described in the 1960s using X-chromosome inactivation studies.19,20 Most contemporary methods of clonality detection are based on genetic analysis and include gene rearrangements (T-cell and B-cell receptors), structural and numerical chromosomal changes, small copy number variants, and somatic point mutations. All these methods differ in terms of sensitivity and specificity, directly related to detection limits and number of markers used in each assay. In general, broad panels—such as whole genome or whole exome sequencing—provide a high sensitivity for the detection of clonal haematopoiesis given the extent of the genome tested.21 Such broad approaches, although useful in research studies, are clinically impractical because of their cost, analytical challenges, and the difficulty of interpretation. By contrast, targeted panels, which are frequently limited to less than 100 cancer-relevant genes, are often more specific, affordable, and easier to interpret than their broader counterparts. Most clinically available panels for haematological malignancies also include genes frequently mutated in CHIP, such as DNMT3A, TET, ASXL1, TP53, JAK2, and around 25 other genes.22
The recent interest in clonal haematopoiesis stems from the broad application of next-generation sequencing in individuals without apparent haematological disease, and from the use of peripheral blood cells as a source of constitutional DNA. This approach resulted in incidental identification of somatic mutations in genes known to be frequently mutated in haematological malignancies. Thus, in most recent studies, clonal haematopoiesis in individuals with unremarkable haemograms was defined as a limited expansion of haematopoietic clones in peripheral blood, marked by the presence of somatic single nucleotide variants or small insertions or deletions (indels). This point is important to emphasise, because our references to clonal haematopoiesis will be based largely on the presence of somatic single nucleotide variants and indels, rather than on cytogenetic alterations determined by conventional cytogenetic studies and frequently used to diagnose haematological malignancies.
Definition of clonal haematopoiesis
Unfortunately, the term clonal haematopoiesis, outside its undisputable association with haematological malignancies, misses a degree of precision in its application. Clonal haematopoiesis is frequently defined as non-reactive, relative expansion of haematopoietic clones—regardless of magnitude—detected by any means and at any point in time. Although this definition could allow potential categorisation for some patients, the defining characteristics of clinically relevant clonal haematopoiesis remain unclear. Several questions need to be addressed as clinicians continue to apply incomplete knowledge of clonal haematopoiesis to the bedside. First, relative clonal dominance may be a consequence of genuine, uncontrolled expansion of cells due to clonal growth advantage or stem-cell attrition, which is frequently seen with ageing. A reliable method is needed to differentiate these two scenarios and their respective biological consequences.
Second, the minimum size of biologically relevant clones is yet to be established definitively. Initially, the proposed 2% variant allele frequency for the definition of CHIP was based on the lower limit of reliable detection of small somatic variants using whole exome sequencing.5 This variant allele frequency is reasonable in these conditions, but it might not fully detail the biological and clinical relevance of this value. We discuss the nuances of variant allele frequency at diagnosis later in this Viewpoint. Additionally, the development of ultra-deep, error-corrected targeted sequencing approaches that are capable of detecting mutations in less than 0·5% of cells revealed the presence of miniscule clones in more than 95% of older individuals (aged >60–70 years). These clones were evenly distributed among haematopoietic lineages in peripheral blood and remained stable for decades, suggesting both an haematopoietic stem cell origin and insufficient expansion potential.23 Third, it is unclear whether qualitative or quantitative (or both) characteristics of CHIP clones result in similar phenotypes across patients, or whether there are more diverse biological effects. To illustrate, cardiovascular complications have been associated with CHIP, and the likelihood of these events is increased when clonal monocytes are present in a patient’s peripheral blood; this increase could in part be related to the qualitative characteristics of the clone effect, but malignant potential is probably related to quantitative burdens.24–27 Furthermore, an exploration into phenotypes has shown that some mutations can result in spontaneous expansion and transformation, whereas others can require additional cell-extrinsic stressors such as chemotherapy, radiation, or environmental toxins, to produce the disease.28 Finally, there is the question of clonal persistence. Are all detectable clones permanent or transient? This last question has particular relevance to the establishment of a clinical monitoring plan once the diagnosis of clonal haematopoiesis has been made.
Classification of clonal haematopoiesis with and without cytopenias: axiom or linguistic mélange of four-letter acronyms?
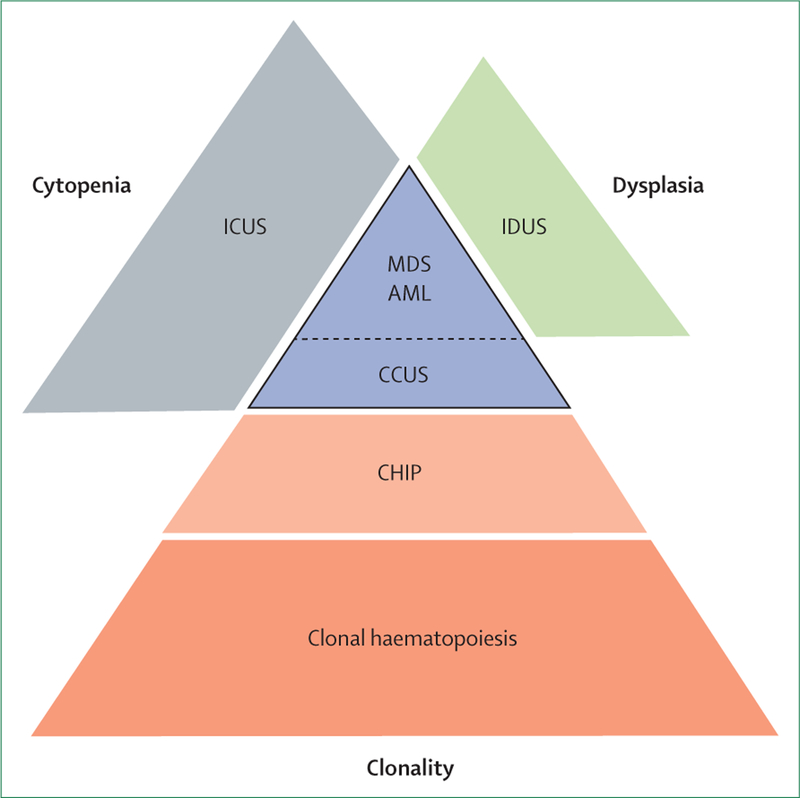
It has been postulated that ageing haematopoietic stem cells acquire random somatic mutations, which could lead to clonal expansion, acquisition of secondary hits, and ultimate transformation to clinically apparent disease. In fact, nearly 90% of patients with myelodysplastic syndrome have identifiable somatic mutations in haematopoietic cells.29 Moreover, mutations in putative cancer drivers such as DNMT3A, TET2, and ASXL1 encompass more than 90% of clonal haematopoiesis and are among the most frequently affected genes in myelodysplastic syndrome. Even though clonal haematopoiesis is frequent, less than 1% of people with clonal haematopoiesis progress to clinically apparent disease. Thus, the presence of somatic mutations in haematopoietic cells might be of clinical importance or might represent an incidental finding in older patients with marginally abnormal haemograms that do not fulfill minimal diagnostic criteria for myelodysplastic syndrome.30 What we do not yet know with precision is whether these incidental findings might evolve into clinically apparent disease. The associated uncertainly could lead to great anxiety in some patients and providers. The most recent attempts to classify these somewhat overlapping and potentially clinically important premalignant conditions encompass three major clinical and pathological findings: presence of clonal markers, dysplasia, and peripheral blood cytopenias (figure 1).
Figure 1: Relationship between cytopenia, dysplasia, and clonality in precursor states and myelodysplastic syndrome.
Figure showing clinical and pathologic overlap of various diseases, and a hierarchy whereby patients can acquire increasing depth of cytopenia, dysplasia, or even additional clonality on the path to myelodysplastic syndrome. ICUS=idiopathic cytopenia of unknown significance. MDS=myelodysplastic syndrome. IDUS=idiopathic dysplasia of unknown significance. AML=acute myeloid leukaemia. CCUS=clonal cytopenia of unknown significance. CHIP=clonal haematopoiesis of indeterminate potential.
Despite subtle differences, which are perhaps more pertinent to research than clinical practice, the terms CHIP and age-related clonal haematopoiesis can be used interchangeably and denote the expansion of haematopoietic clones, harbouring specific—probably disruptive—and recurrent genetic variants in individuals with normal haemograms and without clear diagnosis of haematological malignancies.5,18,31,32
Patients with cytopenias can be categorised (panel 1) as either idiopathic cytopenia of unknown significance (ICUS), or clonal cytopenia of unknown significance (CCUS). Peripheral blood cytopenias are defined on the basis of standard laboratory values (haemoglobin <130 g/L [males], <120 g/L [females]; absolute neutrophil count <1·8 × 109/L; platelets <150 × 109/L).33 ICUS is defined as any degree of cytopenia in one or more lineages that persists for at least 6 months, does not fulfill the minimal diagnostic criteria for myelodysplastic syndrome, and cannot be explained by other haematological or non-haematological conditions.34–36 A consensus panel of experts has proposed terminology for and classification of premalignant clonal conditions, which is useful for classification.36 Patients with ICUS in whom clonal abnormalities have been identified can be classified as patients with CCUS.31 This term is reserved only for patients with non-myelodysplastic syndrome or non-acute myeloid leukaemia, for defining cytogenetic abnormalities.
Panel 1: Definitions of the precursor states to guide diagnosis.
Clonal haematopoiesis of indeterminate potential
Presence of at least one somatic mutation that is relevant clinically and otherwise found in myelodysplastic syndrome (or other myeloid neoplasms)
Absence of persistent cytopenia
Exclusion of myelodysplastic syndrome and all other haematopoietic neoplasms (and other diseases) as the causal underlying condition
Idiopathic cytopenia of undetermined significance
Presence of relevant cytopenia in one or more lineage for at least 6 months
Not explained by any other disease
Diagnostic criteria of myeloid neoplasm not fulfilled
Clonal cytopenia of undetermined significance
Presence of one or more somatic mutations otherwise found in patients with myeloid neoplasms in bone marrow or peripheral blood cells with an allele burden of more than 2%
Presence of persistent cytopenia (≥4 months) in one or more peripheral blood cell lineages
Diagnostic criteria of myeloid neoplasm not fulfilled
Exclusion of all other causes of cytopenia and molecular aberration
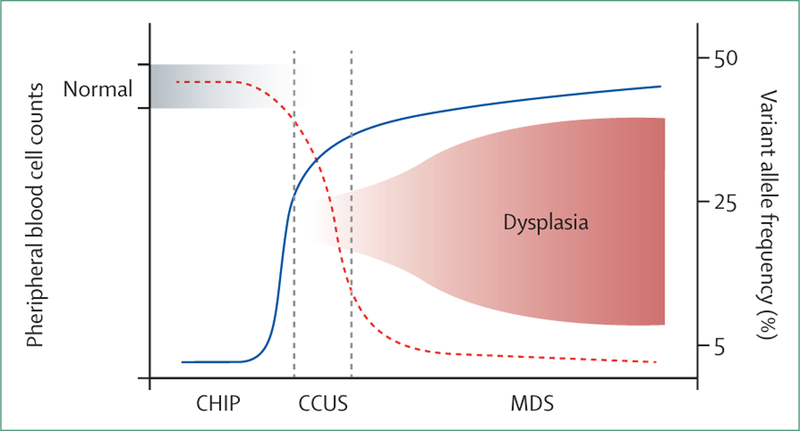
There is no standardised variant allele frequency cut-off for CCUS, and some authors propose the same as for CHIP (>2%). In our opinion, CCUS should imply that cytopenias are solely driven by the clonal process resulting in ineffective haematopoiesis. Since this causation cannot be fully explained by the presence of a minute clone, we propose to apply the variant allele frequency cut-off of the dominant clone (>20%) for CCUS diagnosis. This value is based on previously published data showing the variant allele frequency distribution in patients with CCUS with 95% cumulative progression to clinically apparent myeloid malignancy within 10 years.7 In addition, using this higher variant allele frequency cut-off would possibly separate cytopenias that are due to the clonal process (such as CCUS) from other cytopenias that co-occur with incidental and inconsequential small CHIP clones (figure 2). Given a nearly 100% 10-year probability of CCUS progression to myeloid malignancies, perhaps the term unknown significance is not the most fitting and this condition ought to be placed in an early myeloid neoplasm category. These thresholds may allow for improvements in monitoring patients and in risk assessment, especially for those at presumed highest risk for earlier progression and evolution.
Figure 2: Peripheral blood cell count, variant allele frequency, and dysplasia as a continuum from asymptomatic CHIP to clinically obvious myelodysplastic syndrome.
Clonal haematopoiesis of indeterminate potential (CHIP) is characterised by normal peripheral blood counts, low level clonal expansion (low variant allele frequency), and no evidence of dysplasia. With time, somatic mutations present in CHIP clones result in clonal outgrowth of cells (variant allele frequency >20–30%), with abnormal differentiation leading to peripheral blood cytopenias without morphological evidence of dysplasia (clonal cytopenia of unknown significance, CCUS) followed by clinically apparent myelodysplastic syndrome (MDS) with dysplastic features. Dashed red line indicates blood count; solid blue line indicates variant allele frequency.
Idiopathic dysplasia of undetermined significance (IDUS) is defined as the presence of dysplasia in peripheral blood or bone marrow, the absence of cytopenias, no evidence of clonality, and no obvious cause.31 Even though the offending condition may not be obvious at first, IDUS is usually a reactive process rather than primary marrow disorder.
Assessment of clonality in diagnosis
Since the first systematic reports in the late 1970s, numerical and structural chromosomal abnormalities and the use of various cytogenetic techniques have remained central to diagnostic testing, risk stratification, and therapeutic decision making. Beginning in 2001 (in recognition of chromosome 5q deletion syndrome), and then more broadly in 2007, WHO classification recognised cytogenetics as an essential diagnostic tool.3 The diagnosis of some types of acute myeloid leukaemia with recurrent cytogenetic abnormalities—such as inv(16), t(8;21), or t(15;17)—can now be made without referring to the blast count. Similarly, myelodysplastic syndrome can be diagnosed in patients with cytopenia who have myelodysplastic syndrome-specific chromosomal aberrations without obvious dysplasia, or even by SF3B1 without extensive ring sideroblasts.30 Next-generation sequencing techniques have now made their way to the clinic and are not only widely applied for diagnostic and prognostic purposes, but also essential in the implementation of novel targeted therapies. This wide application has been fueled further by continually shrinking costs of disease-specific targeted panels, which are now less expensive than traditional metaphase karyotyping.
Unilineage or multilineage peripheral blood cytopenia often result from a wide range of haematological or non-haematological disorders. These include some haematological cancers and bone marrow failure syndromes, autoimmune conditions, viral infections, systemic diseases, medication toxicity, and vitamin deficiencies.37 The term unexplained cytopenia is used to define a condition that is characterised by peripheral blood cytopenia whose origin is not attributable to causes that can be detected with conventional tests or to any concomitant diseases.38
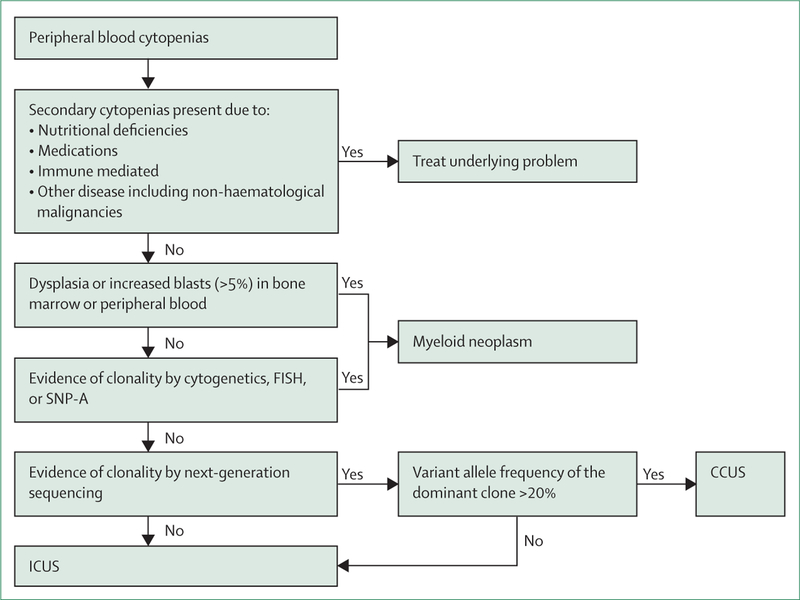
The current diagnostic approach to a suspected myeloid neoplasm with myelodysplasia includes morphological studies of peripheral blood and bone marrow aspirate smears, bone marrow biopsy, and cytogenetic studies aimed at identifying selected chromosomal abnormalities or genetic lesions that WHO classification recognises to be of diagnostic value.37,39 Tremendous progress in discovery of the genes associated with human disease combined with parallel sequencing of different genomic regions have resulted in wide use of next-generation sequencing in diagnostic schema for myeloid malignancies. The results of next-generation sequencing are now incorporated in the National Comprehensive Cancer Network guidelines, which provide the list of gene mutations likely to be somatic and disease-related, and therefore give presumptive evidence of myelodysplastic syndrome.39 Although helpful under some circumstances, the incorporation of next-generation sequencing results into a diagnostic process must be done with caution. Practising clinicians must be aware of the possible limitations of a test that is meant to complement proper diagnostic assessment, rather than act as a stand-alone diagnostic test (figure 3).
Figure 3: Clinical diagnostic testing for peripheral blood cytopenias.
The flowchart represents our approach to the diagnosis of precursor conditions. We consider variant allele frequencies greater than 20% to be most akin to clonal cytopenia of unknown significance (CCUS). FISH=fluorescence in-situ hybridisation. SNP-A=single nucleotide polymorphism array. ICUS=idiopathic cytopenia of unknown significance.
Diagnostic handling of CHIP
As with any new evolution in clinical classification systems, inherent limitations become apparent. It is in this way that clinicians must fully comprehend the consequences of labelling patients with precursor states. The prospective Myelodysplastic Syndrome Natural History Study40 is currently enrolling patients with ICUS to address this very issue. Although underdiagnosis might not be a relevant problem because disease will declare itself with time, overdiagnosis can be anxiety provoking in patients and clinicians alike, and could result in serious consequences if acted on prematurely. Thus, we believe that healthy individuals with unremarkable haemograms should not be tested for clonal haematopoiesis. We recognise that this condition is frequently discovered incidentally, in research studies, cell-free DNA or solid tumour biopsies, and commercial DNA testing. Once discovered, we do not recommend monitoring for changes in clonal dynamics in asymptomatic individuals, especially given that there are no approved or investigational therapeutic interventions proven to change the natural history of CHIP in otherwise healthy individuals.
Variant allele frequency
The analysis of molecular reports must take into account not only the binary information of the presence or absence of particular mutations, but also the percentage of affected cells (clonal burden). Variant allele frequency represents the percentage of mutated DNA molecules relative to the total DNA input. Most cancer-associated somatic mutations affect only one allele (heterozygous), and thus a variant allele frequency of 50% implies that 100% of tumour cells carry somatic mutations. Variant allele frequency depends on the size of the clone, tumour heterogeneity, the amount of non-clonal healthy cells (eg, non-clonal lymphocytes or stromal cells from bone marrow biopsy), and the co-occurrence of numerical chromosomal alterations. Particularly close attention should be paid to variant allele frequency in diagnostic investigation of unexplained cytopenias, because association between somatic mutations and cytopenias does not always prove causally significant. This could be one of the hardest principles for both patients and providers. For example, the presence of anaemia and DNMT3A mutation with variant allele frequency of 5% is probably very different from the same mutation at variant allele frequency of 40% in a patient receiving transfusions. For patients receiving transfusions, anaemia is probably due to clonal processes affecting 80% of haematopoietic cells, leading to ineffective erythropoiesis, but when transfusion is not involved, other causes of anaemia should be investigated thoroughly. Thus, we propose to incorporate a higher variant allele frequency cutoff (>20%) than is often used to distinguish CCUS from other conditions (panel 2). Another example could be the presence of unexplained anaemia and a small JAK2 Val617Phe clone. Most clinicians would agree that the two are probably independent processes and that alternative explanations of anaemia should be considered.
Panel 2: Recommendations for diagnosis and monitoring of precursor states.
Diagnosis
For diagnosis of clonal haematopoiesis of indeterminate potential (CHIP), the current cutoff for variant allele frequency of the dominant clone is more than 2%
We propose to apply a cutoff of more than 20% variant allele frequency of the dominant clone for diagnosis of clonal cytopenias of undetermined significance
Monitoring
Bone marrow and peripheral blood counts twice per year after CHIP diagnosis, with bone marrow evaluation dictated by change in peripheral counts
If diagnostic criteria for myelodysplastic syndrome or acute myeloid leukaemia are not met and cytopenia persists, we recommend monitoring haemograms at least once every 3–6 months
We propose next-generation sequencing testing once per year for symptomatic patients to observe changes in clonal burden
We do not recommend further testing or excessive monitoring for changes in clonal dynamics in haematologically asymptomatic individuals
Driver versus passenger mutations
Given the vast number of somatic alterations detectable by next-generation sequencing, discriminating between leukaemia-initiating driver mutations and incidental passenger mutations lacking functional consequences can be extremely challenging. This judgment relies heavily not only on the sequencing method, but also on the analytical pipeline, filtering strategies, variant annotations, and in silico prediction of pathogenicity. In reality, only a small proportion of somatic gene alterations passes these strict criteria and is reported by molecular laboratories. Even these highly refined lists frequently contain what are currently termed variants of unknown significance. With few exceptions of clearly deleterious recurring hot-spots (eg, JAK2 Val617Phe, SF3B1 Lys700Glu) or canonical truncating mutations (ASXL1 or CALR), most loss-of-function mutations are scattered across the affected genes.29 The limitations of currently available prediction algorithms and scarcity of experimental data make the distinction between pathological driver mutations and silent passenger mutations extremely challenging. To address this important and constantly evolving problem, attempts to standardise the interpretation and reporting of sequence variants have been undertaken.41
Somatic versus germline
When reviewing the results of next-generation sequencing panel testing, some germline variants could be present in somatic reports. How these are codified and interpreted can vary by report and can be a potential source of confusion. Thus, most molecular laboratories are slowly starting to incorporate concurrent DNA testing from non-haematopoietic tissues (eg, skin fibroblasts) as germline controls. In the absence of germline control, variant allele frequency could occasionally help to distinguish between germline and somatic mutations, especially if clones constitute less than 80% of tested tissue (variant allele frequency <40%). Unfortunately, in some conditions, the vast majority of nucleated haematopoietic cells are derived from a single pathological clone, which frequently makes this distinction infeasible. Moreover, mutations in certain genes (eg, RUNX1, DDX41, ETV6) can be present as inherited germline variants or as acquired somatic events. Interpretation of dedicated germline sequencing should be done according to guidelines for variant classification from the American College of Medical Genetics and Genomics.42,43 A detailed review of inherited haematopoietic disorders extends beyond the scope of this Viewpoint and can be found elsewhere.44
Following the CHIPs
Malignant consequences of CHIP
With regards to the risk of malignant consequences, CHIP can apply the multiple hit theory in cancer evolution.45 People with CHIP generally have a single somatic mutation and do not have an overt malignancy. The mutations found in people with CHIP are also common in myeloid malignancies, including acute myeloid leukaemia, myelodysplastic syndrome, myelo-proliferative neoplasms, and some lymphomas.46 In most cases, transformation to malignancy requires the sequential acquisition of multiple mutations.
Therapy-related myeloid neoplasms represent a specific clinical scenario in which chemotherapy or radiation can select for a mutant haematopoietic stem-cell clone, increasing the risk that this clone will acquire additional mutations and progress to malignancy.47,48 Individuals with CHIP who are treated for solid tumours have an elevated risk of therapy-related myeloid neoplasms and increased overall mortality.26,28,49
Individuals with CHIP have approximately ten times the risk of developing a haematological malignancy compared with people without CHIP, with the risk increasing with the size of the clone.8,9 Overall, the risk of transformation to malignancy is approximately 0·5–1% per year, which is roughly the same as the risk of transformation of monoclonal gammopathy of undetermined significance to multiple myeloma.45
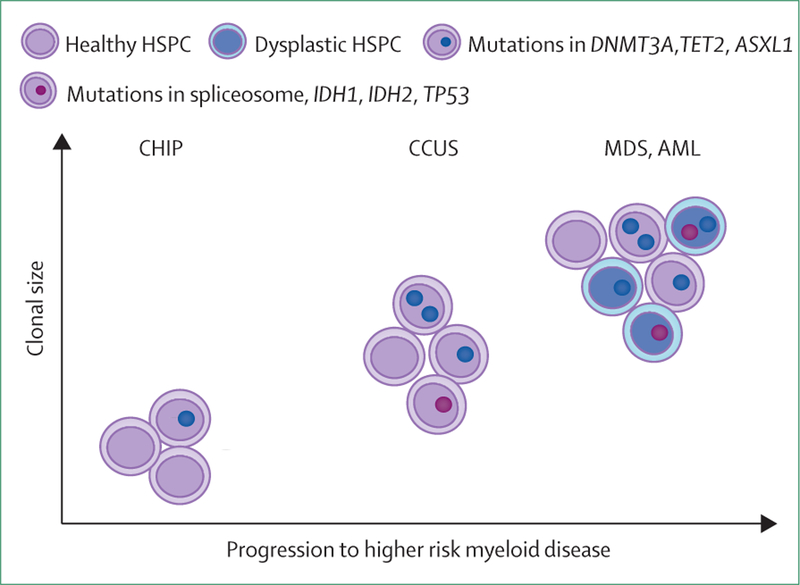
The risk of transformation to acute myeloid leukaemia has been evaluated specifically in large, retrospective cohort studies. Specific features predicted the risk of developing acute myeloid leukaemia to be three to five times higher than in individuals without CHIP. In particular, mutations in TP53 and genes encoding splicing factors were associated with an elevated risk of developing leukaemia (figure 4).50,51
Figure 4: Features of clonal haematopoiesis of indeterminate potential (CHIP) considered at high risk for transformation to haematological malignancy.
The evolution of CHIP to clinically apparent haematological malignancies is represented by clonal expansion or variant allele frequency (y axis), by acquisition of additional somatic mutations, somatic mutations in specific genes (TP53, IDH1, IDH2, spliceosome machinery), or multiple mutations in epigenetic modifiers (DNMT3A, TET2), and by increased morphological features of dysplasia. HSPC=hematopoietic stem and progenitor cell. CHIP=clonal haematopoiesis of indeterminate potential. CCUS=clonal cytopenia of unknown significance. MDS=myelodysplastic syndrome. AML=acute myeloid leukaemia.
Non-malignant consequences of CHIP
CHIP is associated with increased overall mortality.8,9 An increased risk of haematological malignancies alone does not explain this mortality risk, because overall blood cancers are relatively rare. In large genetic studies, CHIP has been associated with myocardial infarction, with a hazard ratio greater than many of the established risk factors for cardiovascular disease, such as blood pressure, cholesterol levels, and smoking.9 CHIP approximately doubles the risk of myocardial infarction,25 and studies indicate that CHIP plays a direct functional role in the pathogenesis of atherosclerosis.24,52 Altered inflammatory response in the blood cells of patients with CHIP has also been shown to influence a wide range of disease biology, particularly in diseases of ageing that are linked to inflammation.24,52–54 These associations could lead to providers outside of the discipline of haematology ordering more next-generation sequencing testing to determine whether CHIP could be contributing to the underlying pathophysiology. This could present further challenges, not only with interpretation of next-generation sequencing results, but also with subsequent requests for haematological evaluation of clinically insignificant conditions.
Monitoring
CHIP, ICUS, and CCUS are all currently considered to be premalignant conditions that can progress to myelodysplastic syndrome, acute myeloid leukaemia, or other haematological malignancies. The main implication of making these diagnoses is that the monitoring and clinical follow-up will change so that malignancy, if it occurs, will be diagnosed efficiently in a patient (table). Caution is required, however, as progression to malignancy is not a foregone conclusion and each precursor state does not carry the same level of risk.55 Clinical scenarios at increased risk for early progression and malignant evolution must be highlighted to a patient (figure 4). Additionally, the form of monitoring should be age-dependent. A patient younger than age 60 years, with more life years ahead, should be surveyed once per year, whereas a patient aged 80 years with comorbid conditions could require discussions for their expectations, but might have less use of monitoring. Precursor states also need to be factored in if a patient with CHIP requires chemotherapy for another malignancy. Care should be taken to avoid prescription of medications that could further predispose patients to acquiring additional somatic mutations that could further escalate the patient’s evolution to acute myeloid leukaemia.
Table:
Characteristic features of precursor states and proposed monitoring guideline
| Myelodysplastic syndrome | Idiopathic cytopenia of unknown significance | Clonal haematopoiesis of indeterminate potential | Clonal cytopenia of unknown significance | |
|---|---|---|---|---|
| Cytopenias | + | + | − | + |
| Dysplasia | + | − | − | − |
| Clonality | + | − | + | + |
| Risk of transformation to AML | ++ | ↓↓↓ | ↓(↓) | ↓↑ |
| Monitoring | Per consensus guidelines by disease stage | No need to reassess next-generation sequencing; monitoring 2–4 times per year CBC | Observation; routine CBC for health maintenance | Observation; monitoring 2–4 times per year; supportive care |
The proposed guidelines are based on currently available data for surveillance of these conditions; certainly all monitoring plans should be guided by a patient’s clinical scenario including age, expectation, specific mutations, and comorbid conditions. CBC=cell blood count. + sign=present. − sign= absent. Arrows indicate increase or decrease.
The clinical course of ICUS is variable and unpredictable. In a subset of patients, progression to myelodysplastic syndrome or acute myeloid leukemia is observed after a variable time period.56 In some patients with ICUS, a smaller clone carrying typical chromosome abnormalities (otherwise found in myelodysplastic syndrome or acute myeloid leukemia) is initially detected by a modality such as fluorescence in-situ hybridisation.57 It is important to repeat cytogenetic and molecular studies during follow-up in patients with ICUS, IDUS, and CCUS, especially when clinical signs of clinical progression are found.58
CHIP at the bedside
Providers of clinical care value the capacity to contextualise disease manifestations (and the patients they affect) into categories to help to create a path forward. The aforementioned aid this goal and have the added use of predicting with greater accuracy which patients might develop myeloid neoplasms.7 Additionally, these entities in many ways justify the use of next-generation sequencing at diagnosis. This form of molecular genetic testing promotes and confirms the diagnosis of a clonal disorder in a patient with unexplained cytopenia. Furthermore, a negative test result can also influence diagnostic assessment, because of the high negative predictive values of a normal result in ruling out a disorder such as myelodysplastic syndrome.59
In clinical practice, using molecular genetic testing and applying these acronyms at the individual patient level is complex.59,60 During the past few years, the emerging concept of premyelodysplastic syndrome conditions has received attention and acceptance from the medical community, owing to fact that the clinical implications of such conditions are becoming clear. It is clinically appropriate, in our opinion, to use the panels in patients with cytopenia to rule in, rule out, or predict myeloid neoplasms. This practice has increased such that it is now considered standard care by nearly all haematologists and oncologists. Overall, the recommendation is to follow premyelodysplastic syndrome conditions proactively and based on the risk of evolution, as best estimated by the clinician for the patient at the time. However, the frequency of next-generation sequencing repeat monitoring (beyond marrows and blood counts) has not been established clinically. We favour once per year to avoid an overdiagnosis burden (panel 2). In the future, it is likely that our colleagues in cardiology, rheumatology, or other providers specialising in diseases with inflammation will send these panels looking for causality of the comorbid condition. We do not recommend further testing or close monitoring in haematologically asymptomatic individuals. Bone marrow biopsy with repeat next-generation sequencing analysis should be done in individuals with CHIP who develop unexplained cytopenias. If diagnostic criteria for myelodysplastic syndrome or acute myeloid leukaemia are not met and cytopenia persists, we recommend monitoring haemograms at least once every 6 months (as recommended by the myelodysplastic syndrome National Comprehensive Cancer Network guidelines).39
Conclusions
Clonal haematopoiesis is an exciting area of research, leading to better knowledge and further diagnosis of precursor status for patients. However, comprehension of clonal haematopoiesis is ongoing, and as yet, we sometimes struggle to interpret clonal haematopoiesis results with complete diagnostic and prognostic certainty. When faced with uncertainty, some patients and providers will be content to let events unfold beyond their control. However, other patients (or physicians) might feel compelled to be more proactive and pursue either more intense monitoring or, more worryingly, earlier intervention without evidence. This approach could lead to earlier detection and treatment of malignancy or to unnecessary and potentially harmful overtreatment. Ongoing and future studies will help to refine understanding of clonal haematopoiesis and of its implications.
Search strategy and selection criteria.
We identified references for this Viewpoint through searches of PubMed databases and abstracts of the American Society for Hematology and the American Society of Clinical Oncology, using the search terms “CHIP”, “CCUS”, “ICUS”, and “clonal haematopoiesis”. We included articles that were published from Jan 1, 2010, to August 1, 2019. We considered results from all papers published in English only when drafting the manuscript, but included what we currently view as many of the most seminal papers to date for this Viewpoint in the reference list.
Acknowledgments
This work was supported by grants from the US National Institute of Health: K08HL136894 (LPG), R21HL143096 (LPG), 1R03HL136797 (AED), and CA006973 (AED).
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.DeZern AE, Sekeres MA. The challenging world of cytopenias: distinguishing myelodysplastic syndromes from other disorders of marrow failure. Oncologist 2014; 19: 735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gondek LP, DeZern AE. I walk the line: how to tell MDS from other bone marrow failure conditions. Curr Hematol Malig Rep 2014; 9: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–51. [DOI] [PubMed] [Google Scholar]
- 4.Cogle CR, Iannacone MR, Yu D, et al. High rate of uncaptured myelodysplastic syndrome cases and an improved method of case ascertainment. Leuk Res 2014; 38: 71–75. [DOI] [PubMed] [Google Scholar]
- 5.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015; 126: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejar R, Levine R, Ebert BL. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol 2011; 29: 504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 2017; 129: 3371–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014; 371: 2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017; 355: 1330–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015; 347: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomasetti C, Vogelstein B, Parmigiani G. Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc Natl Acad Sci USA 2013; 110: 1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rate Lynch M., molecular spectrum, and consequences of human mutation. Proc Natl Acad Sci USA 2010; 107: 961–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milholland B, Dong X, Zhang L, Hao X, Suh Y, Vijg J. Differences between germline and somatic mutation rates in humans and mice. Nat Commun 2017; 8: 15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012; 150: 264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014; 20: 1472–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busque L, Patel JP, Figueroa ME, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet 2012; 44: 1179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlush LI. Age-related clonal hematopoiesis. Blood 2018; 131: 496–504. [DOI] [PubMed] [Google Scholar]
- 19.Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science 1965; 150: 67–69. [DOI] [PubMed] [Google Scholar]
- 20.Fialkow PJ, Gartler SM, Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci USA 1967; 58: 1468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017; 130: 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program 2018; 2018: 264–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haemopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun 2016; 7: 12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017; 377: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia 2016; 30: 1916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol 2017; 35: 1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson CJ, Kennedy JA, Nikiforow S, et al. Donor-engrafted CHIP is common among stem cell transplant recipients with unexplained cytopenias. Blood 2017; 130: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillis NK, Ball M, Zhang Q, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol 2017; 18: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014; 28: 241–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–405. [DOI] [PubMed] [Google Scholar]
- 31.Valent P, Orazi A, Steensma DP, et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes (MDS) and potential pre-MDS conditions. Oncotarget 2017; 8: 73483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeZern AE, Malcovati L, Ebert BL. CHIP, CCUS, and other acronyms: definition, implications, and impact on practice. Am Soc Clin Oncol Educ Book 2019; 39: 400–10. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg PL, Tuechler H, Schanz J, et al. Cytopenia levels for aiding establishment of the diagnosis of myelodysplastic syndromes. Blood 2016; 128: 2096–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valent P, Horny HP. Minimal diagnostic criteria for myelodysplastic syndromes and separation from ICUS and IDUS: update and open questions. Eur J Clin Invest 2009; 39: 548–53. [DOI] [PubMed] [Google Scholar]
- 35.Valent P, Bain BJ, Bennett JM, et al. Idiopathic cytopenia of undetermined significance (ICUS) and idiopathic dysplasia of uncertain significance (IDUS), and their distinction from low risk MDS. Leuk Res 2012; 36: 1–5. [DOI] [PubMed] [Google Scholar]
- 36.Valent P, Akin C, Arock M, et al. Proposed terminology and classification of pre-malignant neoplastic conditions: a consensus proposal. EBioMedicine 2017; 26: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malcovati L, Hellström-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 2013; 122: 2943–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104: 2263–68. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 60–87. [DOI] [PubMed] [Google Scholar]
- 40.Sekeres MA, Gore SD, Stablein DM, et al. The National MDS Natural History Study: design of an integrated data and sample biorepository to promote research studies in myelodysplastic syndromes. Leuk Lymphoma 2019; published online Jan 1. DOI: 10.1080/10428194.2019.1616186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 2017; 19: 4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guidugli L, Johnson AK, Alkorta-Aranburu G, et al. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia 2017; 31: 1226–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy AL, Shimamura A. Genetic predisposition to MDS: clinical features and clonal evolution. Blood 2019; 133: 1071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol 2018; 15: 219–33. [DOI] [PubMed] [Google Scholar]
- 46.Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haemopoiesis to secondary leukaemia. Nat Rev Cancer 2017; 17: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter MJ, Shen D, Ding L, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 2012; 366: 1090–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015; 518: 552–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, Wang F, Kantarjian H, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol 2017; 18: 100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 2018; 559: 400–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai P, Mencia-Trinchant N, Savenkov O, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med 2018; 24: 1015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017; 355: 842–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Zhao K, Shen Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015; 525: 389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cull AH, Snetsinger B, Buckstein R, Wells RA, Rauh MJ. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol 2017; 55: 56–70.e13. [DOI] [PubMed] [Google Scholar]
- 55.Kwok B, Hall JM, Witte JS, et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 2015; 126: 2355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malcovati L, Cazzola M. The shadowlands of MDS: idiopathic cytopenias of undetermined significance (ICUS) and clonal hematopoiesis of indeterminate potential (CHIP). Hematology Am Soc Hematol Educ Program 2015; 2015: 299–307. [DOI] [PubMed] [Google Scholar]
- 57.Petrova-Drus K, Hasserjian R, Pozdnyakova O, et al. Clinicopathologic evaluation of cytopenic patients with isolated trisomy 8: a detailed comparison between idiopathic cytopenia of unknown significance and low-grade myelodysplastic syndrome. Leuk Lymphoma 2017; 58: 569–77. [DOI] [PubMed] [Google Scholar]
- 58.Neukirchen J, Lauseker M, Hildebrandt B, et al. Cytogenetic clonal evolution in myelodysplastic syndromes is associated with inferior prognosis. Cancer 2017; 123: 4608–16. [DOI] [PubMed] [Google Scholar]
- 59.Steensma DP. How I use molecular genetic tests to evaluate patients who have or may have myelodysplastic syndromes. Blood 2018; 132: 1657–63. [DOI] [PubMed] [Google Scholar]
- 60.Brunner AM, Steensma DP. Recent advances in the cellular and molecular understanding of myelodysplastic syndromes: implications for new therapeutic approaches. Clin Adv Hematol Oncol 2018; 16: 56–66. [PMC free article] [PubMed] [Google Scholar]