Abstract
Bats are an important group of mammals, frequently foraging in farmland and potentially exposed to pesticides. This statement considers whether the current risk assessment performed for birds and ground dwelling mammals exposed to pesticides is also protective of bats. Three main issues were addressed. Firstly, whether bats are toxicologically more or less sensitive than the most sensitive birds and mammals. Secondly, whether oral exposure of bats to pesticides is greater or lower than in ground dwelling mammals and birds. Thirdly, whether there are other important exposure routes relevant to bats. A large variation in toxicological sensitivity and no relationship between sensitivity of bats and bird or mammal test‐species to pesticides could be found. In addition, bats have unique traits, such as echolocation and torpor which can be adversely affected by exposure to pesticides and which are not covered by the endpoints currently selected for wild mammal risk assessment. The current exposure assessment methodology was used for oral exposure and adapted to bats using bat‐specific parameters. For oral exposure, it was concluded that for most standard risk assessment scenarios the current approach did not cover exposure of bats to pesticide residues in food. Calculations of potential dermal exposure for bats foraging during spraying operations suggest that this may be a very important exposure route. Dermal routes of exposure should be combined with inhalation and oral exposure. Based on the evidence compiled, the Panel concludes that bats are not adequately covered by the current risk assessment approach, and that there is a need to develop a bat‐specific risk assessment scheme. In general, there was scarcity of data to assess the risks for bat exposed to pesticides. Recommendations for research are made, including identification of alternatives to laboratory testing of bats to assess toxicological effects.
Keywords: Pesticides, bats, chiroptera, risk assessment, effects, exposure
Summary
Bats are an important and large group of mammals, in terms of ecosystem services and conservation status. They help to regulate arthropod populations, including pest species in farmland, forests and urban areas. The 53 European bat species provide an important component of vertebrate diversity, all being protected under the agreement ‘Conservation of Populations of European Bats’ (Annex I to the Agreement on the Conservation of Populations of European Bats, EUROBATS, 1991) under ‘The Convention on Migratory Species (UNEP/CMS)’ (also known as the Bonn Convention). Bats are not specifically mentioned under the current risk assessment for pesticides methodology, and recent research has raised concern regarding whether this methodology is sufficiently protective of bats. The objective of the current statement is therefore to elaborate on whether the current risk assessment for birds and ground dwelling mammals exposed to pesticides is also protective of bats.
It is clear from a literature survey that bats forage frequently in intensively used farmland, which could lead to potential exposure to pesticide residues via oral, dermal and inhalation routes. In addition, they have some particular traits that may increase their vulnerability to pesticide effects and exposure. For example, bats have very high energy requirements compared to terrestrial mammals due to their flight activity. This leads to high food intake rates and subsequently to potentially high residue intake with their food. Bats use fat storage for seasonal and daily patterns of torpor and arousal, which might result in high internal exposure peaks if lipophilic substances are accumulated in the fat. Bats are typical k‐selected species with a low fertility and high parental investment, resulting in a low potential for recovery of perturbed populations. Self‐ and social grooming represent a way through which pesticide residues, which collected on the fur and the wing membranes after dermal exposure may be ingested. Bats have a very large wing surface with high degree of vascularisation and extremely thin epidermis, which indicates that if exposed dermally there is potential for this to be a very important exposure route. Bats also use echolocation to locate their food, which provides a further potential vulnerability to neurotoxic substances.
In order to conclude whether or not bats are covered by the current risk assessment scheme for birds and mammals, the Panel considered the following three main questions: (a) Are bats toxicologically more or less sensitive than the most sensitive birds and mammals? (b) Is the oral exposure of bats to pesticides greater or lower compared to ground dwelling mammals and to birds? (c) Are there other important exposure routes that may be relevant to bats?
Toxicology
The available toxicity data for bats is not sufficient to draw a firm conclusion on whether bats are more or less sensitive to pesticides than ground dwelling mammals. However, in the restricted data set, a great variation in toxicity compared to standard test species was observed Therefore, given the current state of knowledge, it must be concluded that toxicological sensitivity of bats cannot be predicted by current acute and chronic toxicity studies on birds and mammals. Other studies indicated that due to the specific physiology and behaviour of bats other acute and chronic endpoints may also need to be considered, such as effects on echolocation and torpor, which both can lead to mortality of individuals in the wild. Investigation of disruption of metabolic pathways and enzyme‐specific targets modifications, by ex vivo and in vitro tests, should be considered for susceptibility determination of non‐target species (such as bats) in order to avoid in vivo testing.
Oral exposure
The oral exposure of bats is partly covered in the screening step of the current risk assessment for birds and mammals. However, at the first step of refinement (i.e. first‐tier assessment), terrestrial insectivorous mammals do not cover them, and only 4 out of 44 first‐tier scenarios for insectivorous birds cover bats. Furthermore, it is noted that bats might not be covered in those four scenarios if the exposure assessment is refined.
Studies show that lipophilic pesticides can be transferred to the young bats via maternal milk. This exposure route for pups can exceed the exposure of adults by a factor of more than 3. Therefore, the transfer of pesticides via this route should be considered in any future bat risk assessment.
Therefore, it was concluded that specific risk assessment scenarios for oral exposure need to be developed for bats taking into consideration the different feeding guilds of bats. This includes establishing standard residues per unit dose (RUD) values for the food items eaten by bats. While standard RUD values are available for ground‐dwelling and leaf‐dwelling arthropods, there is no generic RUD value currently available for flying insects. Residues data in flying insects were available from an EFSA supporting publication. This data set was analysed and several factors were identified which potentially have an influence on the insect residue values and might explain the high variability in measured residue values. These factors relate to the sampling method used, toxicological profile of the substance, insect behaviour and insect size.
The current standard estimates for drinking water exposure for birds and terrestrial mammals cover the exposure of bats via drinking water.
Other exposure routes
Explorative exposure calculation for inhalation and dermal exposure suggests that the risk from inhalation exposure is less than from oral exposure and that dermal exposure can be much greater than oral or inhalation exposure. However, the uptake via the lung membrane may be different to the skin and direct effects on the respiratory membrane of the lungs may also be more severe than effects on the skin. If bats are exposed dermally during and immediately after spraying operations, the exposure levels are likely to be extremely high and the probability of exposure would need to be considered in any bat risk assessment for pesticides. Furthermore, it is reasonable to assume that the different exposure routes (oral, dermal and inhalation) would be additive if bats are foraging in the field when a pesticide is applied in the evening.
Overall conclusion
The Panel concludes that bats are not adequately covered by the current risk assessment scheme and there is a need to develop a bat‐specific risk assessment scheme. Based on the current assessment, this should include a focus on (a) oral exposure via residues in insects and grooming, (b) dermal exposure and (c) exposure of pups via milk. It is important to highlight that any risk assessment scheme should consider the total body burden from all exposure routes as bats foraging in the field will be exposed to residues in insects, and via dermal and inhalation routes.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The pesticide regulation (Regulation (EU) No 1107/2009) requires the protection of non‐target organisms (no unacceptable effects on non‐target species including the ongoing behaviour of those species and its impact on biodiversity and the ecosystem). The risk assessment should cover i.a. all terrestrial vertebrate groups (as laid down in the requirements for data, Regulation (EU) No 283/2013). The risk assessment is conducted for birds and mammals based on standardised scenarios for indicator species.
A concern has been raised regarding whether the current risk assessment methodology is sufficiently protective of bats which may forage in agricultural landscapes (Stahlschmidt and Brühl, 2012). The current risk assessment scheme only considers birds and ground dwelling mammals and it is not immediately clear whether this risk assessment is protective of bats. The ecology and characteristics of bats could potentially result in a higher risk than that for birds and ground dwelling mammals. Moreover, there are 53 species of European bat species which are protected in the agreement “Conservation of Populations of European Bats” (Annex I to the Agreement on the Conservation of Populations of European Bats, EUROBATS, 1991, 1) under “The Convention on Migratory Species (UNEP/CMS)” (also known as the Bonn Convention).2 For these reasons it is considered vital that a consideration is given for the need of a specific risk assessment covering bat species.
The objective of the ‘PPR Panel statement on potential coverage of bats by the risk assessment for birds and mammals′ is to establish whether the current risk assessment for birds and ground dwelling mammals exposed to pesticides is also protective of bats. The main focus will be on the risk resulting from oral exposure of bats to pesticides. Other exposure routes such as inhalation and dermal exposure are currently not assessed for birds and mammals. An analysis should be provided to determine whether bats could be specifically at risk from inhalation and dermal exposure routes in addition to oral exposure to pesticides. A consideration should be given as to whether there are gaps in the current knowledge of bat ecology, in the estimation of exposure of bats to plant protection products and to identify possible areas for future research.
If it becomes evident from the PPR statement that bat species are not sufficiently covered by the current risk assessment schemes, then it is proposed to develop in a next step a scientific opinion that collates the necessary information to make proposals for a risk‐assessment scheme to cover bat species.
Ecological/biological and exposure information on bats which was retrieved in a preparatory literature review (see below) should be used to investigate whether bat species are already sufficiently covered by the current risk assessment schemes for birds and mammals. Additional information can be added to the literature review if justified.
The following points will be specifically investigated in the current statement:
Explore whether available toxicological endpoints derived from tests with other vertebrate species might be useful to predict the toxicity of pesticides to bats.
Conduct an assessment of exposure and toxicity of pesticides for bats in comparison with the outcome of current risk assessment schemes for birds and mammals. This should be performed preferably based upon explicit exposure and effect assessment goals for bats.
Provide an analysis of the relative importance of potential exposure via non‐oral routes (dermal and inhalation) in comparison with oral exposure for the overall risk of bats exposed to pesticides.
Conclude on the possible coverage of bats by the current bird and mammal pesticide risk assessment schemes. This should also include an analysis of ecological vulnerability based on life history traits and bat population biology, linked to exposure probabilities.
If identified, illustrate knowledge gaps and possible research priorities to adequately address the risk for bats exposed to pesticides in agricultural landscapes and make recommendations for further actions.
Interpretation of the terms of references
The scientific statement dealt only with insectivorous bat species, which account for all European bat species except Rousettus aegyptiacus. The latter is a frugivorous pteropodid occurring, within the EU, on Cyprus, where its population experienced a concerning decline in the last few years (Del Vaglio et al. 2011). Moreover, overseas territories of some European countries host considerably diverse bat assemblages, often featuring frugivorous species. Frugivorous bats differ from insectivorous species in terms of physiology, ecology and behaviour; so, a separate risk assessment would be needed to cover these species.
Although pesticide applications in forestry are not dealt with specifically, it is acknowledged that forests make important roosting and foraging habitats in bats. However, most of the points raised in the current document also apply to pesticide use in forestry.
The document focused on spray applications as the exposure via spray treatment is the most relevant for bats. Exposure from seed treatment, granules and other types of applications are not considered.
The underlying assumption of the exposure assessments in this document is that individual bats are effectively exposed (e.g. bats do eat contaminated arthropods or do fly close to the spray boom). This is in accordance with the current EFSA Birds & Mammals Guidance document (2010) with its toxicity‐exposure ratio (TER) approach, as well as the fact that lower tiers of the exposure assessment (i.e. the screening step and first tier) are intended to be conservative. However, this does not reflect the current proposals for exposure assessment in more recent documents (e.g. EFSA Guidance on bee risk assessment, Scientific opinion on amphibians & reptiles risk assessment) where the probability of exposure concentrations in space and time is more explicitly considered in higher tiers.
2. General biology and ecology of bats and differences to other mammalian species relevant for risk assessment
A more comprehensive description of bat natural history (morphology, physiology, ecology, behaviour) and conservation biology is provided in Appendix A. Here, the main points are summarised extensively covered therein and their relevance to pesticide risk assessment for bats are highlighted.
2.1. Diversity and evolutionary history
Among mammals, bats stand out for their remarkable species richness (over 1,300 species), being second only to rodents (Altringham, 2011). Bats belong to the order Chiroptera, which is characterised by the unique occurrence of powered flight, accomplished thanks to the modification of forelimbs into wings. The first bats appeared on the scene ca. 52 million years ago, and their evolutionary lineage arouse within the mammalian group named Laurasiatheria. The order comprises species whose body mass spans from ca. 2 g to over 1 kg
Bats have evolved a nocturnal behaviour probably in response to the evolutionary pressure exerted by diurnal avian predators, and most of them broadcast ultrasonic pulses to echolocate, which allows them to orientate and detect targets, including food, in complete darkness.
2.2. Life history in bats and other mammals
Bats are so‐called K‐strategists and often give birth to one, more rarely two young per year. The offspring are large at birth and are milked for several weeks, after which their body size equals that of their mother. A comparison among life history traits of bats, shrews and rodents (families Muridae and Cricetidae) reveals that female bats mature sexually later, give birth to fewer, heavier young, and have a longer interbirth interval, a lower birth rate, and longer gestation and lactation than females of rodents and shrews. At weaning, bats are also heavier than rodents – but not shrews – relative to their body size; shrews are heavier than rodents.
Relative to ground dwelling small mammals, bats also live much longer, on average ca. 15 years, and up to 41 years (Myotis brandtii; Podlutsky et al., 2005). Genetic, physiological and environmental factors, plus some ecological variables, are responsible for the outstanding longevity of bats (Wilkinson and South, 2002; Nabholz et al., 2008; Munshi‐South and Wilkinson, 2010; Seim et al., 2013; Foley et al., 2018).
The exceptional longevity of bats makes them potentially susceptible to long‐term contamination by pesticides, and their low fertility and high parental investment exposes them to high risks of population decline following contamination events.
2.3. Morphology
The most peculiar feature of a bat's body plan is the wing, which is made of the elongated forelimb whose skeleton supports a double layer of skin, the patagium (e.g. Altringham, 2011). Wing shape differs across species in relation to their foraging mode and flying style (e.g. Norberg and Rayner, 1987). Wings account for ca. 85% of the bat's body surface (Bassett and Studier, 1988). Because of their large surface and structure and their high degree of exposure to the environment during flight, wings play a major role in controlling heat and water exchange (Thomson and Speakman, 1999), also contributing substantially to total gas (CO2 and O2) exchange (Makanya and Mortola, 2007). The large wing surface of bats and its high degree of vascularisation set the scene for a prime role of this structure within the context of dermal exposure to pesticides; yet, this aspect warrants a quantitative assessment.
Relative to other mammals, bats also stand out for the peculiar shape of their ears and the presence, in several species, of noseleaves. Ear shape and size are related to detection of faint echoes for the functioning of bat biosonar (Altringham, 2011), or, in species that hunt by passive listening, to detect the rustling sound produced by prey moving on the ground (Arlettaz et al., 2001; Holderied et al., 2011). Noseleaves occur in most bats that broadcast ultrasound through the nostrils. They represent an acoustic lens that concentrates energy on a focal area to improve echolocation effectiveness (Vanderelst et al., 2010).
2.4. Physiology
Powered flight
Powered flight in bats is expensive – it may cost twice the energy needed for running (Thomas, 1987) – but it is also outstandingly efficient in terms of cost of transport, which is five times lower than that faced by a non‐flying mammal of equivalent size (Altringham, 2011). In this way, bats may cover long distances (Popa‐Lisseanu et al., 2009), access three‐dimensional landscapes, feed on airborne insects even at high altitudes (Voigt et al., 2018), escape predators effectively, overcome unsuitable habitat, and, in some cases, migrate over long distances to search for suitable climatic conditions and food in different seasons (Altringham, 2011). In the absence of sufficient fossil evidence, the stages that led to powered flight in modern bats may only be speculated (Bishop, 2008).
Echolocation
Widespread among bats, echolocation occurs in over 85% of living bat species. Apart from rare exceptions (genus Rousettus), bats generate the sound needed to echolocate through the larynx. Echolocation often relies on the time delay between an emitted pulse and the return of the corresponding echo to the emitter. Some bats adapted to hunt in clutter detect ‘acoustic glints’ generated by the interaction between the long constant‐frequency components of the calls they broadcast and the fluttering of objects they insonify, such as a moth's wing stroke (Fenton et al., 2012). These bats also possess an ‘acoustic fovea’ most sensitive to an individual‐specific frequency value, so that when flying, they will lower the frequency of their calls to compensate for the Doppler upwards shift induced by their own movement, and tune the echo in to their preferred frequency value (Schuller and Pollak, 1979). Echolocation provides a wealth of information about the surrounding world, since calls often cover a broad frequency spectrum, and just like in the case of visible light, different frequencies will provide different responses, overall showing ‘echo colours’ that inform the emitter about specific properties of the target, such as size and texture (Smith, 2008). Echolocation is a flexible tool that shows substantial variation both across species and within them, so that a certain individual may alter call design to best perform in differently structured habitats (e.g. Russo et al., 2018a,b,c).
Echolocation is a vital sense to bats, and its impairment implies serious spatial disorders, and, inevitably, fatal consequences. The insecticide imidacloprid may affect adversely spatial memory of bats through neural apoptosis in hippocampal CA1 and medial entorhinal cortex areas (Hsiao et al., 2016).
Metabolic rate
In bats, basic metabolic rate (BMR) varies according to body mass, but also other factors such as food habits, altitude and occurrence on islands vs mainland (McNab, 2015). Insectivorous bats (such as the European bats) tend to have slightly lower than expected BMR values relative to bats feeding on vertebrates or plants, whose BMR values are equal or slightly greater than those predicted (McNab, 1982). Insects in temperate regions are an ephemeral resource, so a lower BMR may, from this perspective, be adaptive. Active metabolic rates in bats are considerably high because powered flight is an energetically expensive activity (Thomas, 1987).
Torpor and hibernation
Bats are endotherms, but behave as facultative heterotherms to overcome energetically critical periods, such as winter for temperate insectivorous species, when food is too scarce to allow for active foraging. Bats may downregulate their body temperature to approach ambient temperature, a condition called torpor which makes it possible to save considerable amounts of energy (Altringham, 2011). Torpor is often adopted by active bats during daytime roosting: this is defined as ‘daily torpor’ because it lasts less than 24 h and is followed by nocturnal foraging. In bat hibernation, which takes place in the cold months of the year, torpor spans across several consecutive days to several weeks and energy is provided by metabolised fat accumulated in the active months (Ruf and Geiser, 2015). Prolonged torpor makes it possible to save enormous amounts of energy when it is prolonged for days, weeks or even months, i.e. during hibernation, which takes place in winter months. Torpid bats reduce body temperature, respiratory rate, heart rate, oxygen consumption and metabolic rate, and restrict blood flow to the main organs (Altringham, 2011). Arousal from torpor is sustained by the massive heat release caused by depletion of brown adipose tissue (BAT) which allows conversion of energy reserves into heat (Cannon and Nedergaard, 2004).
The massive fat consumption bats face in winter may expose them to the mobilisation of accumulated lipophilic pesticides during hibernation and increase the risk of direct mortality, yet this aspect warrants appropriate investigation.
Life cycle and reproduction
It is referred, here, to the general life cycle of a bat species from a temperate region. Bats typically enter hibernation in late autumn and become active again around mid‐spring, but significant changes may occur according to the local climate, species, individual status, etc. Mating occurs since the end of summer and may extend over the hibernation period. Several mating strategies occur among the bat species so far studied worldwide, and a useful synthesis is offered in Altringham (2011).
The typical reproductive cycle of bats of temperate regions is monoestry, which leads to one, more rarely two young per year (Altringham, 2011). In most cases, European bats show a delayed fertilisation mechanism, i.e. mating takes place in late summer, autumn or winter, when females store the sperms in their genital tract; females ovulate only once they arouse from hibernation to allow for an optimal matching between the time of female reproduction and the seasonal abundance of insect food.
With reference to risks for bats arising from pesticide uses, it is important to remark that considerable amounts of lipophilic pesticides may concentrate in milk and be consumed by the young during lactation. For example, after an application of the insecticide methamidophos to potato fields and apple orchards in Germany, high mortality was recorded among juvenile Myotis myotis, and pesticide residues were likely transferred to them via the milk after their mothers had fed on contaminated insects (Hofmann and Heise, 1991).
2.5. Ecology and behaviour
Foraging ecology and behaviour
European bats show a range of species‐specific foraging strategies that allow them to get access to habitats characterised by a different degree of structural complexity, or ‘clutter’. The ability of a given species to exploit a certain habitat structure for foraging depends on a combination of wing morphology and echolocation call design.
Food intake in bats is quite high, and peaks in especially demanding phases of the life cycle, such as lactation, when females may consume up to ca. 130% of their body mass in insects per foraging night (Kurta et al., 1989a).
A high food intake means that pesticide residues present in insects may be ingested in large amounts and lead to a considerable risk of high oral exposure. Bats are typically active at night. Some species, such as pipistrelles, start foraging soon after (or occasionally before) sunset and are especially active during the first hours of the evening, exploiting the abundance peak in crepuscular insects (Jones and Rydell, 1994). Others are active all night because their prey (such as moths or diurnal insects that are gleaned when resting on the substrate) show no temporal decline later in the evening (Jones and Rydell, 1994). In addition to oral exposure, bats can be exposed dermally if pesticides are sprayed after sunset. There is no evidence that bats avoid operating machinery, and in some cases bats have been seen foraging near tractors (D. Russo, pers. obs.). Artificial lighting associated with pesticides applications made in the evening might even be attractive to some bat species such as pipistrelles, common in farmland, that forage near lamps to exploit the insects attracted by the latter (Arlettaz et al., 2000).
Habitat use
While some bat species are strictly associated with one or few habitat types (Biscardi et al., 2007; Nardone et al., 2015), others are more flexible and show generalist habits (Russo and Jones, 2003). Farmed landscapes provide foraging opportunities for many bat species (Boughey et al., 2011). While traditional or organic farming, often associated with structural heterogeneity and a high connectivity, is important to sustain diverse bat assemblages (Wickramasinghe et al., 2003; Davy et al., 2007), based on habitat selection and dietary studies (Appendix C), there is strong evidence that bats also forage frequently in intensive farmland across Europe (e.g. Heim et al., 2016; Stahlschmidt et al., 2017) and consume agricultural pests in large numbers (Aizpurua et al., 2018).
Roosting ecology and behaviour
Bats spend over half of their lives in the roost, where they are sheltered from adverse weather and predation, and where they carry out many key activities of their life cycles including food digestion, hibernation, mating, parturition and nursing. Roost type changes according to species, sex, age and physiological phase (e.g. hibernation, mating or reproduction) or age. Many species of temperate bats overwinter in natural (caves) or artificial underground sites, while only some also reproduce there; the others may use overground, in buildings or tree cavities. Much of the evidence concerning contamination of bats in their roost regards the effects of roofing timber treatments with pesticides such as lindane, pentachlorophenol (PCP) and pyrethroids, which may have adverse consequences for bats (Mitchell‐Jones et al., 1989; Swanepoel et al., 1999; Voigt et al., 2016). In specific cases, there might be some chance of direct contamination through inhalation or dermal contact when bats roost in farmland structures, like rural buildings (barns, stables) or cavities in trees (e.g. in treelines or hedgerows). The probability of exposure is particularly high when bats roost in shallow cracks of exterior walls (Marnell and Presetnik, 2010), or in shallow tree cavities such as underneath flaking bark (Russo et al., 2017a). Because bats often use a given roost for several weeks or months and may reutilise it over years, slow poisoning of synanthropic bats in the roost, where they might not die immediately but suffer sublethal effects over time, is possible (Voigt et al., 2016). The probability of exposure to pesticides may be more pronounced when bats use night‐roosts to pause from nocturnal activity, in case pesticides are spread in the evening, because in such cases bats may roost in exposed outbuildings such as woodsheds or porches (Knight and Jones, 2009) or even hang from trees (Russo et al., 2002).
Drinking behaviour
Bats from temperate regions drink regularly to compensate for the significant amounts of water that they lose by transpiration through their body surface, especially via the respiratory system and the large surfaces of wing membranes (Korine et al., 2016). Drinking water constitutes one of the potential routes of bat exposure to pesticides. In fact, bats drink at a range of water sites (Korine et al., 2016), including those found in farmland such as irrigation canals or small ponds which are potentially contaminated with pesticides.
Other behavioural aspects relevant to pesticide contamination
Bats often form very numerous colonies both during hibernation and reproduction, which may often comprise thousands and up to several million individuals (Betke et al., 2008). In such situations, many individuals congregate in clusters to achieve social thermoregulation or buffer themselves from temperature shifts. Clusters may include several species, and in reproductive colonies, comprise also young. Roosting bats spend considerable time self‐grooming to keep their bodies clean and reduce parasite load (Ter Hofstede and Fenton, 2005). Moreover, social grooming – the cleaning of an individual's fur by a conspecific – is also frequent, and its role is likely that of strengthening social bonds within colonies and pacifying the subject groomed. Both self‐ and social grooming represent a way through which pesticide residues collected on the fur and the wing membranes may be ingested. Hairless juveniles in clusters or clinging to their mother's fur might also be affected by the tight contact with adults whose fur has been exposed to pesticide residues during foraging. One of the techniques used to cull vampire bats employs the coating of some individuals with anticoagulants and release them so that, once returned to the roost, they will contaminate others through mutual contact in clusters and social grooming (Linhart et al., 1972; Johnson et al., 2014). The topical treatment of vampire bats for culling demonstrates that contaminants, including pesticides, may easily spread to most or all colony members from just a few contaminated subjects.
2.6. Ecosystem services
Bats are important providers of ecosystem services worldwide, including pollination, seed dispersal and insect consumption (Kunz et al., 2011). While the first two services are restricted to tropical regions, arthropod consumption also occurs in temperate regions, including Europe. There is growing evidence that bats consume considerable numbers of pest species (Aizpurua et al., 2018; Russo et al., 2018a,b,c) and that this may produce tangible benefits to agriculture (Maine and Boyles, 2015; Puig‐Montserrat et al., 2015). Bat insectivory is worth 3.7 billion USD per year in North America (Boyles et al., 2011), and implies significant cost savings also in other regions (Taylor et al., 2018). No economic assessment of bat insectivory is available for Europe, where this service is likely to be as important to agriculture as seen elsewhere.
2.7. Conservation status and protection goals
Although recent estimates of positive population trends in European bat populations have led to some optimism (Van der Meij et al., 2015), the situation of many bat species is still of concern, and, at the national scale, bats are still on the decline in several EU countries. European bat species are threatened by several factors, including roost loss (Mitchell‐Jones et al., 2007; Marnell and Presetnik, 2010; Stone et al., 2015a; Voigt et al., 2016), alteration or disappearance of foraging habitat (Racey and Entwistle, 2003), agricultural intensification including pesticide use (Stahlschmidt and Brühl, 2012; Park, 2015; Stahlschmidt et al., 2017), intensive forestry (Russo et al., 2016a), large‐scale wildfires (Bosso et al., 2018), light pollution (Stone et al., 2015b), alien species (Welch and Leppanen, 2017) and windfarm development (Arnett et al., 2016).
Bats are important contributors to European vertebrate biodiversity and provide key ecosystem services (Kunz et al., 2011; Russo et al., 2018a,b,c; see chapter 2.6). All European bat species and their habitats are protected according to the 92/43/EEC ‘Habitats’ Directive (EEC, 1992) and listed in Annex IV under ‘animal and plant species of community interest in need of strict protection’. Moreover, several bat species are listed in Annex II, giving ‘animal and plant species of community interest whose conservation requires the designation of special areas of conservation’.
Another main conservation framework for European bats is offered by the UNEP EUROBATS ‘Agreement on the Conservation of Populations of European Bats’, which came into force in 1994 (FCO, 1994) and to date includes 36 out of 63 range states. Paragraph 8 of Article III of the EUROBATS ‘Agreement on the Conservation of Populations of European Bats’ states that Parties ‘shall, wherever appropriate, consider the potential effects of pesticides on bats when assessing pesticides for use’. More recently, EUROBATS Resolution 8.13 ‘Insect Decline as a Threat to Bat Populations in Europe’ has also requested Parties, besides avoiding ‘the use of pesticides, particularly those problematic for bats and their food resources, in and around areas which are important for bat conservation’, also to ‘consider bats in pesticide risk assessments’ (available at: https://www.eurobats.org/node/1422#3).
EU Regulation 1107/2009 (EU, 2009) sets the general protection goals for all non‐target organisms exposed to pesticides and should therefore in principle also cover bats. An authorised pesticide ‘shall have no unacceptable effects on the environment, having particular regard to […] its impact on non‐target species, including on the ongoing behaviour of those species and its impact on biodiversity and the ecosystem’. Accordingly, the data requirements for pesticides (EU, 2013) demands that presented information shall permit also an assessment of short‐ and long‐term risks to terrestrial wildlife. Effects on biodiversity and the ecosystem, including potential indirect effects via alteration of the food web shall be considered.
After the hazard characterisation and in order to conclude on the acceptability of observed effects in respect to the protection goals, the decision‐making in risk assessment follows the uniform principles for pesticide evaluation and authorisation (EU Regulation No 546/2011; EU, 2011). As for all other mammals, bats are not specifically mentioned, but are subsumed under ‘other non‐target terrestrial vertebrates’. ‘Where there is a possibility of birds and other non‐target terrestrial vertebrates being exposed, no authorisation shall be granted if the acute and short‐term toxicity‐exposure ratio for birds and other non‐target terrestrial vertebrates is less than 10 on the basis of LD50 or the long‐term toxicity‐exposure ratio is less than 5 […]’.
Following this decision scheme, a specific level of protection is envisaged for non‐target terrestrial vertebrates, which was agreed during the development of the relevant guidance document (EFSA 2009): ‘There was a consultation of Member States on the level of protection that should be provided. The outcome was that there should be no visible mortality and no long‐term repercussions for abundance and diversity. A high level of certainty was desired. Because of uncertainties around the methodology on determining visible mortality and to achieve a high certainty that there are no long‐term repercussions for abundance and diversity, surrogate protection goals were defined. These surrogate protection goals were defined as no acute mortality and no reproductive effects. The risk assessment scheme was designed in such a way that acute mortality and reproductive effects from pesticide exposure are unlikely’.
Summarising, the legislation and guidance in place establish that all birds and mammals, including bats, are not exposed to residues levels which would cause mortality or reproductive effects. However, no calibration of the assessment scheme and the respective assessment factors generally chosen for birds and mammals (TER ≥ 10 for acute effects and ≥ 5 for long‐term impacts) has been undertaken to specifically ensure the protection of bat populations in the field.
Considering the particular characteristics of this group presented above, it needs to be critically explored whether the current risk assessment in place for birds and ground dwelling mammals is protective of bats.
3. Comparison of toxicity and exposure estimates between birds/ground dwelling mammals and bats
The current risk assessment for birds and mammals is based on the TER for the acute and long‐term (reproductive) risk. If the TER is below a certain threshold, then it is assumed that the protection goals are not met. The risk assessment follows a tiered approach with a conservative screening and first‐tier assessment. The risk assessment can be refined with further data generated to allow a more realistic exposure and effects estimate for concerned focal species. The comparative assessment presented below is focused only on the screening step and first‐tier assessments for birds and mammals as in higher tiers specific data for focal species need to be generated.
The first aspect relates to the exposure assessment. It can be assumed that bats are not covered by the current exposure assessment if the exposure estimates for bats are greater than the exposure estimates for terrestrial mammals and birds.
A second aspect in the risk assessment is the toxicological sensitivity of bats compared to terrestrial mammals and birds. If there is evidence of bats being less or equally sensitive to pesticides, then the standard toxicity endpoints are also applicable to bats (i.e. bats are covered by the standard toxicity endpoints). However, if bats are systematically more sensitive, then an extrapolation factor may be applied to the toxicity endpoint in order to cover toxicity to bats. This is, however, only feasible when the degree of toxicity of the assessed pesticides to birds and mammals correlates to the degree of toxicity to bats (i.e. a pesticide that is more toxic to mammals compared to other pesticides would be also more toxic to bats). If no correlation is detectable, then the application of an extrapolation factor would not deliver the appropriate toxicological endpoint.
A third aspect is the assessment factor associated with the risk quotient (e.g. TER values) that needs to be reached to demonstrate the fulfilment of the protection goals. The magnitude of this assessment factor (e.g. 5 for long‐term effects) relates to the span between the step in the tiered approach where the assessment is currently performed and the reference tier (e.g. the populations of mammals in the field). The risk assessment factor to be applied to the risk quotient needs to be calibrated with the reference tier (or the surrogate reference tier if needed) and covers the vulnerability of, e.g. the populations in the field.
This chapter is split in two major sections. The first section summarises available information on toxicology and bioaccumulation, and draws conclusions on possible coverage of toxicological sensitivity of bats with current toxicological endpoints used in the risk assessment for terrestrial mammals. The second section is focused on the different routes of exposure and compares potential exposure of bats with the exposure estimates in the standard risk assessment for birds and terrestrial mammals. A conclusion is drawn on the coverage of bat exposure by the current exposure estimated for birds and terrestrial mammals.
3.1. Toxicological coverage of bats by the acute and long‐term endpoints for mammals
3.1.1. Bioaccumulation of PPPs in bats
Bioaccumulative substances which fulfil the Persistent, Bioaccumulative and Toxic (PBT) criteria should not be approved any more according to EU Regulation 1107/2009. However, here, it is investigated whether bioaccumulation may affect bats more than birds and other mammals. Bioaccumulation occurs if the metabolisation and excretion rate is lower than the intake rate of Plant Protection Products (PPPs). This usually occurs as a consequence of the high lipophilicity of active substances and their distribution in the fatty tissues of the animals. In hibernating mammals, a lot of attention has been put on the analysis of the fat composition and especially on the role of the brown fat and the white fat as energy sources.
In three bat species, the big brown bat (Eptesicus fuscus), the little brown bat (Myotis lucifugus) and the eastern pipistrelle (Pipistrellus subflavus), brown fat represents a small proportion of the total amount of fat (2.8‐3% for these three American species). White fat, by contrast, represents 13–19% of the total body weight and by far the major part of the total lipid content. Brown fat contains 23–30% lipids, while white fat contains ca. 90% of lipids. Brown fat content will decrease very rapidly during arousal of hibernating bats, providing high‐energy triglycerides. On the contrary, white fat shrinks slowly over hibernation time (Clark and Krynitsky, 1983). According to Jonasson and Willis (2011), the overwinter decline in fat reserves can account for ca. 29% of body weight loss in little brown bats (M. lucifugus)
Very limited information is available on bioaccumulation of PPPs or any organic compound in bats. Therefore the following paragraph includes examples of compounds which are not approved as pesticides under EU Regulation 1107/2009. Most studies rely on the analysis of residues in tissues of bats exposed in their natural habitat. In Indiana (Eidels et al., 2012), analytical investigations were conducted in free‐ranging bats (either distressed or dying) between 2005 and 2007. Among them, 40 were analysed for whole‐body insecticide residues. Organochlorine insecticides were detected in all bats (100%, range 0.01–5.15 mg/kg), organophosphates were detected in 10 bats (25%, range 0.03–0.81 mg/kg), pyrethroids were quantified in 5 bats (12.5%, range 0.004–0.37 mg/kg) and carbamates (carbaryl) was detected in only 1 bat (limit of quantification 0.025 mg/kg). Unfortunately, these analyses were conducted on whole body samples. Nevertheless, the authors suggested that, despite wide use at the time the study was conducted, several insecticides such as diazinon and pyrethroids were only detected in a limited number of individuals due to their relative short half‐life in the body (based on rodent or other mammalian data). Former on‐site studies with the big brown bat (E. fuscus), the little brown bat (M. lucifugus) or the eastern pipistrelle (P. subflavus) showed that brown fat contained the highest concentrations of Dichlorodiphenyldichloroethylene (DDE) lipid weight, followed by white fat. During the course of hibernation, with the decline in the total amount of fat, DDE concentration markedly increased in the body. The authors stated that a similar trend could be observed in the brain, thereby leading to toxicity and death (Clark and Krynitsky, 1983). For the examples found, there is a clear positive trend between the log Kow and the degree of bioaccumulation of organic compounds and there is evidence to suggest that bats accumulate more Dichlorodiphenyltrichloroethane (DDT) than birds and that this trend is even stronger for substances with higher log Kow (Streit et al., 1995). A review was published on organic contaminants in bats (Bayat et al., 2014) discussing former and current compounds of concern. There is substantial evidence that insectivorous bats accumulate insecticide residues such as pyrethroids and organophosphates (Bayat et al., 2014). Among the risk factors for accumulation, diet (insectivores) and location of roosting and foraging sites appear to be the major determinants. Some studies (Nagel and Disser, 1990) included in this review indicate that juveniles may accumulate more compounds, from milk transfer, as well as adult males may display higher concentrations than females, believed to eliminate some contaminants through the milk.
Few experimental data have been published on the kinetics and degree of pesticide accumulation in bats. Shore et al. (1991) exposed common pipistrelles (Pipistrellus pipistrellus) to PCP and/or permethrin. Common pipistrelles were exposed for 32 days. After termination of the experiments, bats were euthanised and different tissues and organs were sampled for analysis. The highest concentrations of PCP were found in brown fat and white fat, followed by liver and kidney. There was a significant positive association between PCP accumulation and total lipid weight of the carcass, confirming the high potency of PCP for bioaccumulation. Permethrin, however, was never detected.
To conclude, bioaccumulation of lipophilic compounds has been demonstrated or observed in bats. No evidence was found comparing bioaccumulation potential in bats and other mammals. According to the limited evidence accumulation appears to be even more important in bats than in birds, this trend increases with increasing log Kow in organochlorines.
3.1.2. Toxicity
A comprehensive review of the literature available on toxicity data in bats was conducted. Details on the literature review can be found in Appendix B. Each relevant reference was considered for inclusion in this statement as a reference toxicity value but most studies failed to reach this step due to either poor design, lack of information, inability to determine an actual exposure dose or duration of study not comparable with current guidelines for bird and mammal testing. A table comparing toxicity values of bats with standard toxicity measures for mice, rats and birds can be found in Appendix D.
Older studies conducted with pesticides such as DDT, fenthion or zinc phosphide (see e.g. Hurley and Fenton, 1980), provided limited data on the acute toxic dose tested (concentration in a sprayed solution, formulation, etc.) and it was not possible to determine the actual exposure dose and/or the actual route of exposure for all substances. In the study of Hurley and Fenton (1980), DDT was applied topically (dusted on the back of isolated bats). Doses of 2.5, 5, 10, 15 or 20 mg/bat were applied. All bats treated with the high dose of 20 mg died after showing typical signs of organochlorine toxicity (tremors, seizures), while none of the others did. Unfortunately, the body weight of the animals was not mentioned in the paper. The dermal LD50 might therefore range between 680 mg/kg body weight (bw) and 1300 mg/kg bw assuming a body weight between 11 and 22 g for adult bats. This is somewhat lower than the reported dermal LD50 in rats and mice (ca. 2,000–3,000 mg/kg bw) but would still be covered by the current assessment factors used.
An acute toxicity study (Clark, 1986) was conducted in the big brown bat (E. fuscus) with methyl‐parathion and the oral LD50 was estimated to be 372 mg/kg bw. Although, as the authors stated, this value is quite high compared with other toxicity values in rodents (e.g. 44 mg/kg bw for mice as cited in this paper), it should be mentioned that the bats showing severe loss of coordination were not able to right themselves for 24 h, while mice recovered within 2 h only (Clark, 1986; see Table D.1 in Appendix D for more comprehensive comparison of toxic endpoints between bats, mice, rats and birds).
Table D.1.
Overview on active substances for which toxicity endpoints are available for bats and standard mammalian test species (mouse, rats) and birds. The numbers behind the endpoints refer to source of the endpoint listed below the table
| Chemical | Bat | Mouse | Rat | Bird | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Exposure | Endpoint | Toxic value | Exposure | Endpoint | Toxic value | Exposure | Endpoint | Toxic value | Exposure | Endpoint | Toxic value | |
| DDE | Myotis lucifugus | Dietary (40 days) | NOEC1, # (behaviour) | 150 mg/kg diet | Dietary | NOAEL2 (increased liver weight, CYP450 induction) | 35 mg/kg bw per day | Dietary | NOAEL3 (reduced bw gain) | 28 mg/kg bw per day | NA | NA | NA |
| LOAEL2 | 62.5 mg/kg bw per day | ||||||||||||
| LOAEL3 (death) | 35 mg/kg bw per day | ||||||||||||
| DDT | Myotis lucifugus | Acute dermal | LD100 4 | 20 mg/bat ca 680–1,300 mg/kg bw | Acute dermal | LD50 5 | 250–500 mg/kg bw | Acute dermal | LD50 6 | 2,500–3,000 mg/kg bw | NA | NA | NA |
| Eptesicus fuscus | Acute oral | LD50 7 | 26 mg/kg bw | Acute oral | LD50 5 | 150–300 mg/kg bw | Acute oral | LD50 5 | 100 mg/kg bw | Acute oral | LD50 8 | 841 mg/kg bw | |
| Dieldrin | Pipistrellus pipistrellus | Dermal, 100 days | LD50 9, * | 0.05 mg/cm2 | Dermal, 100 days | LD50 9, * | 1.5 mg/cm2 | NA | NA | NA | NA | NA | NA |
| Deltamethrin | Artibeus lituratus | Oral, 7 days | LOAEL10 (Muscular and hepatic toxicity, reduced bw and food consumption) | 0.02 mg/kg diet ca 0.03 mg/kg bw | Oral, 28 days | LOAEL11 (reduced bw, mortality) | 28 mg/kg bw per day | Oral, 7 days | LOAEL11 (Reduced bw, decreased food consumption) | 13 mg/kg bw per day | Oral, 8 days | NOEC11 | 1,000 mg/kg |
| Permethrin | Myotis sodalis | Acute oral | LD50 12 | 38–45,502–533 mg/kg bw** | NA | NA | NA | Acute oral | LD50 12 | 806 mg/kg bw | Acute oral | LD5012 | > 2,000 mg/kg |
| Fenthion | Myotis lucifugus | Acute dermal | LD50 4 | > 28 mg/bat ca > 3,500 mg/kg bw | Acute dermal | LD50 13 | 500 mg/kg bw | NA | NA | NA | Acute dermal | LD50 13 | 44 mg/kg bw |
| Chlorpyrifos | Eptesicus fuscus | Acute oral | BMDL10 14 | 3.7 mg/kg bw (ChE inhibition)–6.2 mg/kg bw (impaired flight) | Acute oral | LD50 15 | 64 mg/kg bw | Acute oral | LD50 14 | 66 mg/kg bw | Acute oral | LD50 15 | 13.3 mg/kg bw |
| Methyl parathion | Myotis lucifugus | Acute oral | LD50 16 | 372 mg/kg bw | Acute oral | LD50 16 | 44.4 mg/kg bw | Acute oral | LD50 17 | 3 mg/kg bw | Acute oral | LD50 17 | 5.3 mg/kg bw |
| LD50 17 | 26 mg/kg bw | ||||||||||||
| Imidacloprid | Hipposideros terasensis | Oral, 5 days | LOAEL21 (behaviour, flight path) | 20 mg/kg bw per day | Chronic oral (90 days) | NOAEL22 | 86 mg/kg bw per day | Chronic oral (2 years) | NOAEL22 | 17 mg/kg bw per day | Short‐term oral (14 days) | NOAEL22 | 31 mg/kg bw per day |
| PCB (Aroclor 1254) | Myotis lucifugus | Chronic oral (40 days) | NOEC1, # (behaviour) | 15 mg/kg per day diet | Short–term oral (14 days) | NOEL 18 | 7.5 mg/kg bw per day | NA | NA | NA | NA | NA | NA |
| Sodium cyanide | Myotis lucifugus | Acute oral | LD50 19 | 8.4 mg/kg bw | Acute oral | LD5019 | 8.7 mg/kg bw | NA | NA | NA | NA | NA | NA |
| Zinc phosphide | Myotis lucifugus | Acute dermal | LD50 4 | >1,500 mg/bat ca > 187,000 mg/kg | NA | NA | NA | Acute dermal | LD5020 | 1,000 mg/kg bw | NA | NA | NA |
bw: body weight; NOAEL: no‐observed‐adverse‐effect‐level; LOAEL: lowest‐observed‐adverse‐effect level; LD100: lethal dose, 100%; LD50: lethal dose, 50%; CYP450: cytochrome 450; ChE: Cholinesterase; NOEL: no observable effect level; NA: not available.
Clark, 1981.
Orberg and Lundberg, 1974.
NCI, 1978.
Hurley and Fenton.
HSDB DDT.
Extoxnet.
Luckens and Davis.
Hudson et al.
Shore et al., 1996.
Oliveira et al., 2017.
EFSA deltamethrin.
McFarland.
NIOSH.
Eidels et al.
EFSA chlorpyrifos.
Clark, 1986.
EFSA Parathion.
Toxnet PCB.
Clark 1991.
EFSA zinc phosphide.
HSIAO et al., 2016.
EFSA Imidacloprid.
Dieldrin was applied on the wood in roosting boxes. The endpoint is given in mg Dieldrin applied per cm2.
Permethrin LD50 of 38–45 mg/kg bw was for the permethrin formulation and the LD50 of 502–533 mg/kg bw was for technical permethrin (ranges of LD50s are given because two different methods were applied for calculating the LD50s).
NOEC values are based on behaviour.
Studies conducted by gavage with permethrin in Indiana bats (Myotis sodalis), using a formulation (cis 51:trans 49, Pounce® 38.4%) or analytical grade active substance (cis 52:trans 48, 99.4%), dissolved in distilled water or corn oil, respectively, showed major differences in toxicity (McFarland, 1998). LD50 of the formulation was 38 mg a.s./kg bw per day while the analytical grade active substance had an LD50 of 502 mg a.s./kg bw per day and impaired flight was observed for a dose of 16% of the LD50. The commercial product used in this study contained some aromatic hydrocarbons such as xylene and benzene (unspecified proportions) which probably enhanced the toxicity of permethrin by increasing the bioavailability of the active substance as evidenced by higher concentrations of permethrin in all tissues of the bats exposed to Pounce (McFarland, 1998; see Table D.1 in Appendix D for more comprehensive comparison of toxic endpoints between bats, mice, rats and birds).
Three studies are considered below, because they are more recent and also because their design and results can provide some valuable information with respect to bat sensitivity to pesticides.
Oliveira et al. (2017) conducted a study in fruit bats exposed to deltamethrin applied to their diet. The animals were exposed to contaminated fruits daily for 7 days. Many parameters were recorded, including food consumption, body weight, biochemical biomarkers indicative of oxidative stress such as catalase, superoxide dismutase, but also lipid peroxidation or protein oxidation, energy reserves and liver damage. Two doses of deltamethrin were applied (respectively, 0.02 mg/kg diet and 0.04 mg/kg diet) and a negative control group was included. The results show statistically significant dose‐dependent responses in most parameters. As the authors point out, several of these responses were associated with increased oxidative stress. Lipidosis was observed in liver cells, associated with increased AST/ALT (transaminases) activities, indicating potential liver damage (steatosis). A mean deltamethrin daily dose could be determined from this study. Based on body weight change, a no observable effect level (NOEL) of 0.03 mg/kg bw per day could be estimated from the concentrations tested. This value should just be considered as indicative, because it is estimated it based on mean values of all parameters (body weight, food consumption, deltamethrin concentration). There are no comparable studies on other mammals. Nevertheless, from studies with longer exposure duration and other endpoints, it would appear that the toxicity of deltamethrin is much higher to bats than it is to laboratory mammals (rats, mice, rabbits, dogs) or birds (toxicity endpoints above 1 mg a.s./kg bw per day) (EC review report for the active substance deltamethrin: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.ViewReview&id=60, accessed on 14/05/2019).
Similarly, Eidels et al. (2016) conducted a study in big brown bats (E. fuscus) to investigate the sublethal effects of chlorpyrifos. Briefly, adult female brown bats were orally dosed by gavage with either 0, 10, 30 or 60 mg/kg bw. chlorpyrifos (single exposure, 24 h observation). A second comparable trial used lower doses (0, 5, 10, 15, 20, 25 mg/kg bw). In addition, a short‐term toxicity test was conducted with 0 or 60 mg/kg bw followed by a 1, 3, 7 or 14 day observation period, using 5 bats per duration‐group. All animals were sacrificed at variable time points in the study to measure brain acetylcholinesterase (AChE) activity. Body weight measurement and behavioural evaluation was also conducted in all bats, including overall appearance, tremors/clonic movements, righting reflex, crawling ability and flying ability. From these results, the authors determined a Benchmark dose (10) using standard models from the US‐EPA. Mortality was reported in the short‐term trial (14 days): 4 out of 20 bats died within 3 days, the last one died on day 7. There was a significant dose‐dependent decrease in both brain and plasma AChE activity after 24 h of chlorpyrifos exposure. Some bats exhibited a decrease in body weight during the acute toxicity test, but all surviving bats in the short‐term study exhibited a significant body weight gain. Body temperature was monitored throughout both trials as heterothermy is a constant feature of bat species and the ability to regulate body temperature is a critical factor for wintering bats. The major variable in body temperature fluctuation appeared to be the diurnal cycle and external temperature, but some interactions with chlorpyrifos were observed. The authors concluded that more attention should be paid to this effect in further studies. Based on behavioural evaluation, the BMDL10 (benchmark dose 10, lower limit of the confidence interval) could be determined at 3.5, 6.6 and 5.3 mg/kg bw for impaired flight, impaired movement or tremors, respectively. The BMDL10 was 3.5 mg/kg bw for brain AChE activity after 24 h (short‐term study). These values are in the range of other toxicity values obtained for chlorpyrifos in other laboratory experiments. Available data for chlorpyrifos (EFSA, 2011) indicate a NOEL of 2.88 mg/kg bw in Mallard ducks (reproduction), an acute LD50 of 13.3 mg/kg bw in common quail and a NOEL of 0.1 mg/kg bw in rats (2‐year study, AChE inhibition).
Recently, neurological effects of imidacloprid on bats have been demonstrated (Hsiao et al., 2016). Three Formosan leaf‐nosed bats (Hipposideros terasensis) were orally dosed with imidacloprid (20 mg/kg bw per day) for 5 days. Every day, bats were placed in flight chambers and their flight paths were recorded (up to 3 min of flight time per session), analysed for stereotypy and other abnormalities. All flight sessions were performed at the same time of the day in complete darkness. The results clearly show that non‐treated bats always presented a standard flight path, very similar to those recorded before the exposure period. Conversely, imidacloprid‐treated bats showed significantly different flight paths. Their paths were shorter and very different from their usual flight paths recorded during the pretreatment period. Immunostaining of brain regions showed evidence of neural impairment in areas important for echolocation, suggesting that imidacloprid administered at 20 mg/kg bw per day‐for 5 days could interfere with the spatial memory of echolocation. This value can be compared with the NOEL values available for neurotoxicity in other mammalian studies. The acute no‐observed‐adverse‐effect‐level (NOAEL) for neurotoxicity is 42 mg/kg bw per day (reduced motor activity in dogs, 1‐year study), the subchronic neurotoxicity NOAEL value is 9.3 mg/kg bw per day (13 weeks in rats) and the developmental NOAEL is 30 mg/kg‐bw per day in rats and 24 mg/kg bw per day in rabbits (EFSA, 2008).
The limited available literature on toxicity of PPPs in bats is not sufficient to draw conclusions on the sensitivity of bats relative to other birds or mammal species for a wide range of active substances.
In a recent report to the Canadian Wildlife Federation (Mineau & Callaghan, 2018), the authors discussed the potential immunological effects of some neonicotinoids in bats. A study conducted in rats (Mohany et al., 2012) and also discussed by (Gibbons et al., 2014) showed some indications of imidacloprid to affect the immune system of rats. However, the study was not conducted according to standard guidelines for immunotoxicity studies. One of the uncertainties of the study was the fact that it was performed with Confidor®, the commercial product containing 20% imidacloprid, neglecting the influence of other ingredients on the results.
Only limited information on bat sensitivity to pesticides could be found in the literature. Most studies retrieved were focused on insecticides, and for the ones that could be fully discussed, they showed that bat species could be either more sensitive (pyrethroids, imidacloprid), equally sensitive (chlorpyrifos) or less sensitive (parathion) than rodent species. Therefore, it is not possible to draw a conclusion on bat susceptibility to pesticides relative to other bird or mammal species, and there is probably no relationship between toxicity to bats and other mammals or birds on which a predictive assessment could be made. In lieu of direct toxicity testing, the Panel suggests the development of an adverse outcome pathway approach, whenever possible. The development of ex vivo and in vitro tools was successfully applied to the investigation of species susceptibility to anticoagulant rodenticides for instance. In this case, liver Vitamin K epoxide reductase (VKOR, the target enzyme) activity was investigated in several species or in resistant vs susceptible strains of rats (Hodroge et al. 2011), showing important differences, which could be related to differences in susceptibility in vivo. Similarly, Boitet et al. (2018) showed that differences in cytochrome 450 (CYP)‐associated metabolism of difenacoum explained the differences in susceptibility between two strains of resistant rats with the same mutated target enzyme. The development of genetic tools identifying either the VKOR polymorphism or metabolic differences in CYP expression (Boitet et al., 2018) offers promising techniques to investigate species susceptibility without experimental in vivo testing. Investigation of disruption of metabolic pathways and enzyme‐specific targets modifications, which can be partially achieved ex vivo and in vitro, should be considered for both active substances and PPPs for non‐target species (such as bats) susceptibility determination.
3.2. Coverage of bats by the current exposure estimates for birds and terrestrial mammals
3.2.1. Exposure routes
The working group investigated the different exposure routes for bats (see Table 1). Oral exposure from uptake of contaminated food items and drinking water were calculated and compared with the exposure estimates for birds and terrestrial mammals. Dermal and inhalation exposure was also estimated and their relative importance in the overall exposure was assessed. The available data were not sufficient to provide a quantitative estimate for oral exposure via grooming and for dermal exposure from residues on leaves or on soil for bats which glean insects from the canopy or from the soil surface. For exposure of bat pups via residues in milk, only an explorative calculation could be provided. Although it was not possible to provide a quantitative assessment, it does not mean that exposure via these routes is negligible. For example, distribution of toxic substances by grooming behaviour is used to control vampire bats (Linhart et al., 1972; Johnson et al., 2014) and residues in food items transfer to milk of lactating bats and can lead to mortality of pups (Hofmann and Heise, 1991).
Table 1.
Overview on the most important exposure routes for bats
| Oral exposure | Dermal exposure | Inhalation exposure |
|---|---|---|
| Residues in food items | Direct overspray (flying through spray cloud during foraging) | Inhalation of droplets and vapour when foraging in the field or field margins |
| Residues in water taken up orally | Contact with residues on leaves (gleaners) | |
| Grooming | Contact with residues on soil (gleaners) | |
| Pups via drinking milk | Contact with residues on the surface of roosting sites or conspecifics carrying residues on the fur |
The oral, dermal and inhalation exposure routes in Table 1 which are shaded in grey are addressed in more detail in the document below. For the other exposure routes, the information retrieved was not sufficient to provide quantitative exposure estimates for bats. The exposure estimates for dermal, inhalation exposure and oral exposure of pups via milk are explorative and for the purpose of deriving an indication on the relative importance of these exposure routes. They are less robust than the oral exposure estimates for residues in food items and water.
3.2.2. Oral exposure estimation
Oral exposure by residues in food items
The oral exposure calculations for bats follow the same methodology as in the EFSA Birds and Mammals Guidance Document (EFSA, 2009). The text below provides a short description of the oral exposure assessment according to EFSA (2009).
The risk assessment is based on TERs. TER thresholds are defined in the pesticide regulation. TER values < 10 for the acute risk and TER values < 5 for the long‐term risk to birds and mammals indicate that the current protection goals for birds and mammals are not met.
Equations for TER calculations:
with
LD50 = lethal dose 50, expressed in mg/kg bw, derived from studies where birds and mammals are dosed once by gavage
NOAEL = No‐observed‐adverse‐effect level, expressed in mg/kg bw per day, derived from bird reproduction studies and from two‐generation rat studies and mammalian developmental toxicity studies.
DDD = daily dietary dose expressed in mg/kg bw per day
Formula from the Birds and Mammals Guidance Document for calculating the daily dietary dose (DDD):
Acute risk assessment for single application:
Long‐term (reproductive) RA for single application3:
with
SV = shortcut value (mg/kg bw per day)
FIR = food intake rate (g fresh weight/day)
bw = body weight (g)
RUD = pesticide residues per unit dose in food items (fresh weight) (mg/kg fresh weight, normalised to an application rate of 1 kg/ha)
The DDD (single application) must be multiplied with an appropriate multiple application factor (MAF) for uses with multiple applications. For the purpose of comparing the oral exposure estimates for bats with birds and terrestrial mammals, it is not necessary to apply the MAF and hence it was left out from the exposure calculations.
with:
FIR = food intake rate (g fresh weight/day)
DEE = daily energy expenditure of the indicator species (kJ/day)
FE = food energy (kJ/g dry weight)
MC = moisture content (%)
AE = assimilation efficiency (%)
The FIRs and residues in food items are two key parameters in the exposure equation. Differences in FIRs and composition of food items can lead to differences in oral exposure of bats, birds and terrestrial vertebrates. These two parameters were investigated in more detail below.
Food intake rates in bats:
One of the key parameters for estimating oral exposure via food intake is the daily energy requirement or metabolic rates. Articles were found in the literature review where metabolic rates of bats were estimated. Most of the studies were related to basal metabolic rates (see Appendix A, Table A.2). However, the energy requirements of active and free‐ranging bats are more relevant for estimating oral exposure from food uptake than basal metabolic rates. Highest energy requirements were found in pregnant and lactating female bats (Kurta et al., 1989a,b; McLean and Speakman, 1999). The time of pregnancy and lactation overlaps with the time where most pesticides are applied (spring and early summer). Oral exposure of pregnant and lactating female bats is therefore considered as a relevant scenario for the risk assessment and is the basis of the calculations presented below. Data on energy requirements of lactating female bats were available for M. lucifugus (little brown bat) and Plecotus auritus (brown long‐eared bat).
Table A.2.
Basal metabolic rate (BMR) values used for the analyses presented in Fristoe et al. (2015) and Menzies et al. (2016), and body mass for 64 bat species. Body mass values were taken from the above‐mentioned articles or from the EOL website (https://eol.org). The reference column shows the articles from where the original values were taken
| Species | Mass (g) | BMR (mL O2/h) | References |
|---|---|---|---|
| Anoura caudifer | 11.5 | 42.7 | McNab (1969) |
| Anoura latidens | 13.6 | 36.9 | Soriano et al. (2002) |
| Artibeus concolor | 19.7 | 39.8 | McNab (1969) |
| Artibeus jamaicensis | 45.2 | 76.8 | McNab (1969) |
| Artibeus lituratus | 70.1 | 108.0 | McNab (1969) |
| Carollia perspicillata | 14.9 | 43.1 | McNab (1969) |
| Chrotopterus auritus | 96.1 | 141.3 | McNab (1969) |
| Cynopterus brachyotis | 37.4 | 47.5 | McNab (1989) |
| Desmodus rotundus | 29.4 | 34.7 | McNab (1969) |
| Diaemus youngi | 36.6 | 37.3 | McNab (1969) |
| Diphylla ecaudata | 27.8 | 38.6 | McNab (1969) |
| Dobsonia anderseni | 241.4 | 174.0 | McNab and Bonaccorso (2001) |
| Dobsonia praedatrix | 179.5 | 142.5 | McNab and Bonaccorso (2001) |
| Eonycteris spelaea | 51.6 | 48.0 | McNab (1989) |
| Erophylla bombifrons | 16.1 | 17.7 | Rodríguez‐Durán (1995) |
| Glossophaga longirostris | 13.5 | 26.5 | Arends (1995) |
| Glossophaga soricina | 6.8 | 17.7 | Cruz‐Neto and Abe (1997) |
| Hipposideros galeritus | 8.5 | 9.4 | McNab (1989) |
| Histiotus velatus | 11.2 | 10.0 | McNab (1969) |
| Leptonycteris curasoae | 24 | 34.1 | Arends (1995) |
| Macroderma gigas | 107 | 94.2 | Baudinette et al. (2000), 23) |
| Miniopterus schreibersii | 10.91 | 26.0 | Baudinette et al. (2000) |
| Molossus molossus | 15.6 | 22.5 | McNab (1969) |
| Monophyllus redmani | 8.7 | 11.1 | Rodríguez‐Durán (1995) |
| Mormoops blainvillei | 8.6 | 8.0 | Rodríguez‐Durán (1995) |
| Natalus tumidirostris | 5.4 | 8.3 | Genoud et al. (1990) |
| Noctilio albiventris | 27 | 31.6 | McNab (1969) |
| Noctilio leporinus | 61 | 70.8 | McNab (1969) |
| Nyctimene albiventer | 30.9 | 26.4 | McNab and Bonaccorso (2001) |
| Peropteryx macrotis | 5.1 | 11.8 | Genoud et al. (1990) |
| Phyllostomus discolor | 33.5 | 47.9 | McNab (1969) |
| Phyllostomus elongatus | 35.6 | 38.8 | McNab (1969) |
| Phyllostomus hastatus | 84.2 | 100.2 | McNab (1969) |
| Pteronotus quadridens | 4.9 | 6.1 | Rodríguez‐Durán (1995) |
| Rhinonicteris aurantia | 8.27 | 16.2 | Baudinette et al. (2000) |
| Rhinophylla pumilio | 9.5 | 18.6 | McNab (1969) |
| Saccopteryx bilineata | 8.2 | 15.3 | Genoud and Bonaccorso (1986) |
| Sturnira erythromos | 15.9 | 39.9 | Soriano et al. (2002) |
| Sturnira lilium | 21.9 | 53.2 | McNab (1969) |
| Tonatia bidens | 27.4 | 55.1 | McNab (1969) |
| Uroderma bilobatum | 16.2 | 31.6 | McNab (1969) |
| Antrozous pallidus | 20.8 | 21.20 | Licht and Leitner (1967) |
| Chalinolobus gouldii | 14.5 | 25.20 | Hosken and Withers (1997) |
| Eptesicus fuscus | 23.0 | 17.00 | Willis et al. (2005) |
| Eptesicus serotinus | 18.2 | 43.10 | Menzies et al. (2016) |
| Glossophaga soricina | 10.0 | 21.60 | McNab (1969) |
| Hipposideros armiger | 50.0 | 32.86 | Liu and Karasov (2011) |
| Macroglossus minimus | 16.0 | 21.00 | Bartels et al. (1998) |
| Miniopterus schreibersii | 11.2 | 25.42 | Speakman et al. (2003) |
| Mops condylurus | 26.6 | 31.09 | Maloney et al. (1999) |
| Myotis lucifugus | 10.0 | 9.30 | Kurta and Kunz (1988) |
| Myotis myotis | 28.6 | 25.00 | Hanus (1959) |
| Myotis thysanodes | 6.0 | 17.40 | O'Farrell and Studier (1970) |
| Nyctalus noctula | 27.8 | 39.69 | Speakman et al. (2003) |
| Nyctimene albiventer | 30.0 | 26.30 | Geiser (1988) |
| Nyctophilus bifax | 9.9 | 13.10 | Stawski and Geiser (2011) |
| Nyctophilus geoffroyi | 7.0 | 10.99 | Hosken and Withers (1997) |
| Nyctophilus gouldii | 11.3 | 10.39 | Geiser and Brigham (2000) |
| Pipistrellus pipistrellus | 5.0 | 12.10 | Speakman et al. (2003) |
| Plecotus auritus | 7.8 | 11.20 | McLean and Speakman (1999) |
| Rhinolophus ferrumequinum | 22.9 | 46.50 | Speakman et al. (2003) |
| Rhinolophus megaphyllus | 9.8 | 25.84 | Willis (2008) |
| Tadarida brasiliensis | 12.5 | 15.30 | Licht and Leitner (1967) |
| Tadarida teniotis | 35 | 31.52 | Speakman et al. (2003) |
Plecotus auritus occurs in Europe while M. lucifugus occurs in the US. However, the measurements in the study with Myotis were more accurate than in the study with Plecotus, where energy requirements could only be indirectly measured for lactating bats. Furthermore, M. lucifugus is smaller than P. auritus and hence represents better the energy requirements for smaller European bat species such as P. pipistrellus. Therefore, it was decided to use the data for M. lucifugus to calculate the SVs for the comparison with ground dwelling mammals and birds (Table 2).
Table 2.
Energy requirements of lactating Myotis lucifugus and Plecotus auritus and the corresponding food intake rates and weight of arthropods needed to cover the energy demand of the bats. The food intake rate used in the calculations is highlighted in bold
| Myotis lucifugus Average weight lactating bat: 7.9 g Energy requirement: 41.3 kJ/day per bat | Plecotus auritus Average weight lactating bat: 9.72 g Energy requirement: 45.3 kJ/day per bat | ||
|---|---|---|---|
|
FIR/bw (arthropods required/average bat weight) FIR calculated according to EFSA 2009, Appendix G |
Arthropods (g) (wet weight) |
FIR/bw (arthropods required/average bat weight) FIR calculated according to EFSA 2009, Appendix G |
Arthropods (g) (wet weight) |
| 0.848 | 6.702 | 0.756 | 7.352 |
FIR: food intake rate; bw: body weight.
Insect residue data
In order to estimate the oral exposure, it is necessary to know the amount of residues of active substances (a.s.) in the food items consumed. The residue values used in the birds and mammals risk assessment according to EFSA 2009 are based on a large data set. The residues values are normalised to an application rate of 1 kg a.s./ha and expressed in mg a.s./kg food per kg a.s./ha. These normalised residues values are called residues per unit dose (RUD) and need to be multiplied with the actual application rate when conducting the risk assessment for a particular use. In the standard risk assessment for birds and mammals, RUD values for ground dwelling and canopy dwelling insects are used. These RUDs are also applicable for exposure estimates for bats gleaning insects from the ground and from leaves. However, most bat species feed on flying insects and hence RUD values for flying insects would be most appropriate for the exposure estimate for aerial hawkers. No generic RUD value is currently available for flying insects and it was investigated further if data from insect residues studies could be used to derive a RUD value for flying insects.
Residues data in flying insects were available from an EFSA supporting publication (by Lahr et al., 2018). The studies were evaluated by the authors of the supporting publication as useful for risk assessment ‐except the study with fenoxycarb (by Stahlschmidt and Brühl, 2012) which was considered as less reliable because of lack of replicates, no checking of stability of the samples and no reporting of meteorological data. However, the methodology used for collecting the insects and handling of insects as well as the analytical methods was appropriate and the recovery of residues was checked with spiked insects. The residues measured in insects were higher in the study by Stahlschmidt and Brühl (2012) than in the other available studies. Therefore, it is unlikely that the meteorological conditions in the study were such that they would have caused a rapid decline of residues. The number of replicates was not sufficient to meet the quality criteria which were originally defined for refinement of the risk assessment for individual substances. However, in the context of deriving an overall estimate on residues levels in flying insects for different compounds, locations and crops, it was considered as useful to retain the study for the oral exposure calculations.
Overall 17 measurements of residues in flying insects from 9 different studies were included in the database. Seven of the measurements were from three studies with insecticides and 10 measurements from seven studies with fungicides. No studies were available for herbicide residues in flying insects. Two studies were conducted in oilseed rape and wheat and all other studies were conducted in orchards and vineyards.
The available studies give an indication of factors which can influence the residues levels found in flying insects (see Appendix E). One important factor is the sampling method applied. For example, the RUD from insects caught in malaise traps was 1.6 mg a.s./kg food per kg a.s./ha while from car netting it was 28.5 mg a.s./kg food per kg a.s./ha. A pronounced difference in RUDs was also observed in studies where a similar crop and sampling method was applied and the investigated substances had a very different toxicological profile: with quite low RUDs for fast‐acting acutely toxic insecticides and high RUDs for insecticides with a different mode of action. This can be explained by rapid immobilisation of insects exposed to fast‐acting highly acutely toxic substances. As a consequence, only less exposed insects and hence carrying less residues are able to reach the light traps. A difference in RUDs is also observed in relation to different insect size, with smaller insects having greater RUDs than larger insects due to the larger surface to volume ratio. In some of the studies, there were differences observed in RUDs among plots and differences in RUDs of active substances applied in the same product. In order to cover all the different factors, it would be ideal to have a data base with residues from flying insects for a broad range of substances and crops for which an appropriate sampling method was applied to obtain a representative sample of insects eaten by bats. For example, bats from the feeding guild of aerial hawkers mainly feed on small flying insects while gleaners take larger insects from the vegetation or from the ground.
The number of available studies is low in order to account for all the sources of variation and also to account for the specific groups of insects eaten by bats. Therefore, it was decided to provide an overview on the resulting RUD values depending on the choices made to include or exclude certain types of residues studies with flying insects.
Figure 1.
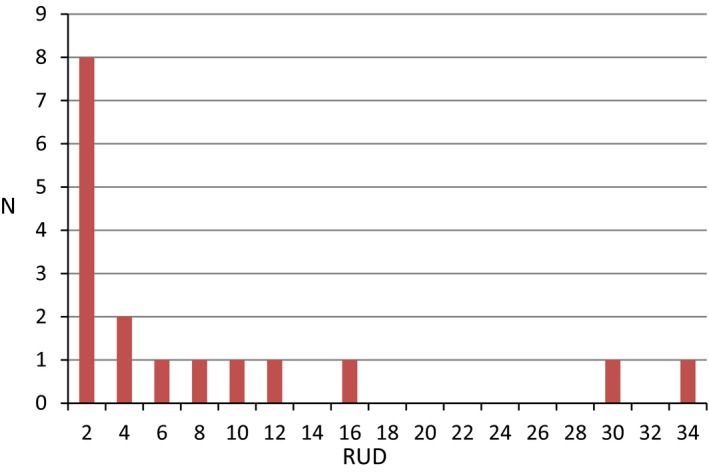
Number of RUD values grouped in ranges of 2 starting with the range of 0‐2 and ending with the range of 32–34
The RUDs are very unevenly distributed with a majority of values in the range of 0–2 mg a.s./kg food per kg a.s./ha, half of the values evenly distributed between 2–16 and few very high values. However, the high RUD values are from measurements which can be considered most relevant for the risk assessment as they represent well the insects potentially eaten by bats (insects were caught with light traps in the study with fenoxycarb). Furthermore, the active substance in that particular study was of low acute toxicity to insects and hence it is not biased towards low residue load.
The following table provides an overview on RUDs of flying insects as food for bats depending on the choices made for inclusion of residues studies (Table 3).
Table 3.
RUD values (for flying insects as food for bats (in mg a.s./kg food per kg a.s./ha) based on different choices for selecting residues studies. The arithmetic means and 90%tile RUD values are highlighted in bold as mean values and 90%tiles are applied in the standard long‐term and acute risk assessments for birds and mammals
| All residue data N = 17 | All residue data without fast‐acting actives N = 13 | Only Light trap data N = 7* | Only Light trap data without fast‐acting actives N = 3** | |
|---|---|---|---|---|
| Arithmetic Mean | 7.31 | 8.97 | 9.14 | 18.8 |
| Geomean | 3.58 | 4.49 | 4.49 | 16.26 |
| Median | 2.52 | 4.85 | 3.38 | 14.67 |
| 90%tile | 20.2 | 25.74 | 21.92 | 29.17 |
| Maximum | 32.8 | 32.8 | 32.8 | 32.8 |
| Minimum | 0.78 | 0.78 | 0.99 | 8.93 |
a.s.: active substance; RUD: residues per unit dose.
From three studies.
From one study.
The acute risk assessment for birds and mammals is based on the 90th percentile RUD values and the long‐term risk assessment is based on arithmetic mean RUD values. The data set for flying insects is very limited and it is also not following a normal distribution. It is questionable whether a meaningful 90th percentile can be derived from the available data. Therefore, it is suggested to focus on the studies which are most relevant in terms of representativeness of insects eaten by bats, i.e. RUDs from studies where light traps were used and considering small insects. The residues in flying insects might be underestimated for slow‐acting actives if based on the current data set for light traps which includes also highly acutely toxic actives. It is therefore recommended to base the comparative exposure assessment between bats and birds and mammals on the highest observed RUD value of 32.8 mg a.s./kg per kg a.s./ha.
Comparison of oral exposure based on shortcut values
The current risk assessment for birds and mammals (EFSA, 2009) is built on a tiered approach starting with a screening step followed by a first‐tier assessment and higher tier refinements of the risk assessment. The screening step assessment is conducted for indicator species representing a worst‐case scenario covering all feeding guilds, i.e. if an active substance passes the screening step then the risk to all birds and mammals is low. If a substance does not pass the screening step, then the risk assessment proceeds to the first‐tier assessment which makes use of scenarios for different crops and their growth stages and generic focal species. For example, if registration is requested for use on bulbs and onion like crops for the crop stage corresponding to growth stage BBCH ≥ 40 the exposure and risk is assessed for three avian (granivorous, omnivorous and insectivorous) and three mammalian (herbivorous, omnivorous and insectivorous) generic focal species which gives in total six scenarios for this crop growth stage (see Annex 1 of EFSA, 2009). The SVs calculated for bats in Table 4 were compared to the SVs used in the screening step and first‐tier assessments for birds and mammals. For the comparison with first‐tier SVs, only insectivorous birds and mammals were considered because of their similarity with bats in terms of food composition.
Table 4.
Results of shortcut value (SV) calculations for Myotis lucifugus. RUD: Residues per unit dose (in mg a.s./kg per kg a.s/ha); FIR: food intake rate (in g fresh weight/day); bw: body weight (in g)
| Arthropod feed items which could be taken up by different bat feeding guilds | RUD | Myotis lucifugus (FIR/bw = 0.848) | ||
|---|---|---|---|---|
| Mean | 90%tile* | SV long‐term (based on mean RUD) | SV acute (based on 90% tile or highest RUD value) | |
| Ground dwelling invertebrates (EFSA GD) | 7.5 | 13.8 | 6. 4 | 11.7 |
| Leaf dwelling insects (EFSA GD) | 21.0 | 54.1 | 17.8 | 45.9 |
| Flying insects (RUD from Lahr et al., 2018) | 18.8 | 32.8* | 15.9 | 27.8* |
| 50% flying/50% gleaning from leaves | 19.9 | 43.45* | 16.8 | 36.9* |
a.s.: active substance; GD: Guidance Document.
For flying insects, the maximum RUD value of 32.8 mg a.s./kg per kg a.s./ha (see text above) is used instead of the 90% tile RUD.
Conclusions from the comparison with shortcut values calculated for bats with the shortcut values for birds and terrestrial mammals used in the screening and first‐tier risk assessment (the conclusions refer to coverage of bats in terms of exposure assessment):
-
1
Screening step risk assessment
The screening step exposure assessment by birds and/or by mammals (comparison with the acute and long‐term SV for M. lucifugus) covers the exposure of bats, except for the following scenarios where some or all feeding guilds of bats are not covered:
Acute risk assessment
Bare soil and hop: Bats feeding on flying insects are not covered and ground dwelling arthropods are covered in the exposure estimates. Only the SV for bats feeding on flying insects and ground dwelling arthropods are considered for the comparison, since there are no or few plants from which leaf dwelling insects could be foraged on.
Grassland: Bats feeding on leaf dwelling insects and for a mix of 50% leaf dwelling and 50% flying insects are not covered in the exposure estimates. The exposure of bats feeding exclusively on flying insects is covered.
Long‐term risk assessment
Bare soil and hop: The exposure of bats feeding on flying insects are not covered and bats feeding on ground dwelling insects are covered by the exposure calculation for birds and mammals in the screening step. Only the SV for bats feeding on flying insects and ground dwelling insects were considered for the comparison, since there are no or only few plants from which leaf dwelling insects could be foraged on.
Grassland: Bats feeding on leaf dwelling insects and bats feeding on a mix of 50% leaf dwelling and 50% flying insects are not covered, bats feeding exclusively on flying insects are covered in terms of exposure.
The screening step scenarios are based on granivorous and herbivorous bird and mammal species, except for orchards for which insectivorous birds are considered in the screening step.
Therefore, if the risk assessment is refined for the species considered in the screening step, then it may be that the risk to insectivorous species is not covered any more. Therefore, the next step in the risk assessment, the first‐tier assessment, includes scenarios for insectivorous bird and mammal species.
-
2
First‐tier risk assessment
Ground dwelling insectivorous mammals
The first‐tier exposure assessment for insectivorous mammals (assuming 100% insect diet) in shrews and woodmouse does not cover the exposure of bats. The maximum SV for acute risk are the SV of 7.6 (shrew) and 6.1 (woodmouse) and the maximum SV for long‐term risk are the SV of 4.2 (shrew) and 3.3 (woodmouse) which are all below the SVs calculated for bats (see Table 4).
Insectivorous birds
A comparison with insectivorous bird species in the first‐tier assessment was conducted. There are 44 scenarios with different insectivorous bird species, feeding on different arthropods guilds, in different crops and at different crop stages included in the first‐tier assessments (see Appendix A of EFSA, 2009). The shortcut values from each of the scenario were compared to the shortcut values for bats feeding on ground dwelling arthropods, leaf dwelling insects, flying insects and a 50% mix of flying and leaf dwelling insects.
Acute risk assessment
Of the 44 exposure assessment scenarios, 29 cover the exposure of bats when feeding on ground dwelling arthropods, but only 4 scenarios cover the exposure of bats feeding on leaf dwelling insects, flying insects or on a 50% mix of flying and leaf dwelling insects. This means that 34% of the exposure scenarios available in the acute first‐tier risk assessment for insectivorous birds do not cover the exposure of bats feeding on ground dwelling arthropods and 91% of these scenarios do not cover bats feeding on leaf dwelling insects, flying insects or a 50% mix of flying and leaf dwelling insects, i.e. the exposure via food is underestimated for these species.
Long‐term risk assessment
Twenty‐eight of the 44 available exposure scenarios in the first‐tier risk assessment step for insectivorous birds result in exposure that are higher or comparable to that of bats feeding on ground dwelling arthropods, but only 4 out of 44 scenarios cover bats feeding on leaf dwelling insects, flying insects or on a 50% mix of flying and leaf dwelling insects. This means that 36% of the scenarios for the assessment of the reproductive risk for insectivorous birds do not cover bats feeding on ground dwelling arthropods and 91% of the available scenarios do not cover bats feeding on leaf dwelling insects, flying insects or a 50% mix of flying and leaf dwelling insects.
Conclusion
The current exposure assessment for birds and mammals cover the exposure of bats at the screening step – with some exceptions for bare soil, hop and grassland. This means that all calculated screening steps for birds and mammals in the scenarios bare soil, grassland and hops according to the current scheme would indicate a low risk, while the risk to insectivorous bats is higher. All calculations are based on the estimated exposure for bats as given in Table 4.
In the first‐tier assessment, the exposure calculations do not cover the exposure for bats if the available scenarios for insectivorous ground dwelling mammals is used. Considering birds, 29 (acute) and 28 (long‐term) scenarios out of 44 scenarios cover the exposure of bats feeding on ground dwelling arthropods. Only 4 out of 44 scenarios for insectivorous birds cover the exposure of bats feeding on flying insects, leaf dwelling insects or a 50% mixture of these. This is the case for acute and for long‐term risk assessment. It is noted that the exposure for bats might not be covered anymore in those four scenarios if the first‐tier risk assessment is refined. Ideally for every combination of crop and BBCH growth stage for which registration is sought, the exposure of the four feeding guilds of bats is covered by one (or more) scenarios.
Overall, it is concluded that specific risk assessment scenarios for oral exposure need to be developed for bats taking into consideration their different feeding guilds. The current methodology used for exposure assessment calculations for birds and mammals is in principle also applicable to bats, but with bat‐specific parameters for bat‐specific FIRs. Future research should focus particularly on enhancing the database for flying insects’ residues and the type of insects eaten by the different feeding guilds of bats.
Oral exposure by residues in drinking water
Bats must satisfy part of their water demand by drinking. Information is available that bats may also drink from small water sources such as cattle troughs or puddles in the field (Korine et al., 2016). Therefore, the exposure for bats via drinking water was compared with the standard assessments for birds and terrestrial mammals.
The standard risk assessment for birds and mammals (EFSA, 2009) includes an assessment for uptake of residues by drinking water from leaf whorls of some crops and from puddles formed in the field. Exposure to residues in larger water bodies is not assessed, as experience has shown that this is unlikely to pose a risk to birds and mammals.
The generic species considered for the drinking water assessment are small granivorous birds and mammals. Granivorous species have a higher drinking water demand compared to insectivorous or herbivorous species because seeds contain lower amounts of water compared to food items such as insects, fruits or other green plant matter.
Only limited data are available where the drinking water demand of bats was investigated. Lactating bats are expected to have a high water demand. Information of the water demand of lactating bats is available for big brown bats E. fuscus (study of Kurta et al., 1990) and for little brown bats M. lucifugus (study of Kurta et al., 1989b). The two species are from North America but they are considered as representative with regard to their water demand for European species as they share the same features as European species in terms of body size, insect food, habitats (temperate climates). Bat species living in arid and semi‐arid climates (e.g. Mactrotus californicus) can have a much lower drinking water demand (Geluso, 1978; Bell et al., 1986). However, species adapted to arid conditions do not occur in Europe and hence are not considered further in the comparison with birds and terrestrial mammals.
Most of the water demand of bats is covered by the water content in their insect food. About 20–22% (E. fuscus) and 23–26% (M. lucifugus) of the total water intake is from drinking. The amount of water uptake (in L/kg bw per day) was calculated for E. fuscus and M. lucifugus based on the data from Kurta et al. (1989b, 1990) (see Table 5 below)
Table 5.
Drinking water demand (L/kg bw per day) for the bat species Eptesicus fuscus and Myotis lucifugus. Data on the percentage water taken up by drinking compared to the total water intake taken from Kurta et al. (1989b, 1990)
| Eptesicus fuscus | Myotis lucifugus | |||||
|---|---|---|---|---|---|---|
| Bats | BW (g) | Drinking water (mL/day) | Drinking water (L/kg bw per day) | BW (g) | Drinking water (mL/day) | Drinking water (L/kg bw per day) |
| Pregnant | 20.84 | 1.85 | 0.088 | 9.02 | 1.60 | 0.177 |
| Lactating | 17.37 | 1.61 | 0.093 | 7.88 | 1.62 | 0.206 |
BW: body weight.
The drinking water uptake for granivorous birds and mammals used in the standard risk assessment is 0.46 L/kg bw per day for birds and 0.24 L/kg bw per day for mammals. The drinking water uptake of E. fuscus and M. lucifugus were in the range of 0.088–0.206 L/kg bw per day (see Table 5) which is similar or lower than the drinking water uptake considered in the birds and mammal risk assessment. Only little information is available on drinking water demands of bats which adds some uncertainty to the calculations. However, the available studies are of high quality and the species tested are considered as being representative also for European species. Furthermore, the data are for pregnant and lactating bats which are most likely the life stages with the highest drinking water demand. Therefore, on balance it is concluded that the available information is sufficient to conclude that bats are likely covered in terms of exposure by the current assessment for birds and terrestrial mammals. Bats exposure might not be covered any more in case that the assessment of risks resulting from drinking water uptake is refined based on a change of focal species or proportion of time spent in the treated field (PT). However, such refinement of the risk assessment is rare (see EFSA, 2009).
3.2.2.1. Oral exposure by grooming
Grooming is a common behaviour in bats which could lead to additional oral exposure if a bat was dermally exposed. This is relevant for the individual and also for the colony exposure via social grooming. Social grooming is a natural mechanism that is used to control vampire bats whereby individual bats are dermally dosed and released to bring back the toxin to the colony. The toxin is then spread via grooming among the individuals in the bat colony. For example, vampire bats (Desmodus rotundus) were housed in captivity and treated topically with diphacinone in order to investigate the dispersion of this rodenticide via social grooming and the presence of residues in dead bats. The experiment was conducted on 20 bats (10 females and 10 males) (Burns and Bullard, 1979 was excreted via maternal milk.
When born, young bats are very small; Jones et al. (2009) gives the weight of a new born common pipistrelle as being 1.28 g. Therefore, it can be calculated that in the case of the study by Nagel and Disser (1990), the oral exposure for new born young would be equivalent to the adult exposure multiplied by 0.83 times the ratio of adult to young body weight. P. pipistrellus adult body weight is 5.0 g (see Table 6 in Section 3.2.3). We can calculate 0.83 x (5.0/1.28) = 3.24. This means that a factor of 3.24 would need to be applied to the oral exposure of adults via contaminated insects in this case.
Table 6.
Parameter estimates with references for the dermal exposure model for examples of three foraging ecotypes of bats
| Parameter | Pipistrellus pipistrellus | Plecotus auritus | Myotis myotis |
|---|---|---|---|
| Foraging type | Hawker | Hawker/Foliage Gleaner | Ground Gleaner |
| Body weight (g) | 51 | 121 | 291 |
| Body diameter (m) | 0.03* | 0.07* | |
| Wingspan (m) | 0.180–0.2402 | 0.255‐0.3002 | 0.350‐0.4302 |
| Flight speed (m/s) | Older studies conducted with this species done in captivity show a low flight speed (2.48 m/s ± 0.233) but this may not represent what is observed in the field. Based on more recent work4 using real‐world data on a similar bat species, Pipistrellus kuhlii, which is only slightly larger, commuting flight was significantly faster than foraging flight (9·3 vs. 6·7 m/s). Further data on European pipistrelle bats show that flight speed decreases from 4 to 7 m/s during search flight to 2–4 m/S during approach flight5. Finally, another study measured an average flight speed of 5.4 m/s in edge habitat during foraging6 | Plecotus are typically slow when foraging (2.35 m/s in P. auritus 7), but they fly faster when commuting (up to 4.5 m/s8) | Not much information is available. In a study done in captivity where bats were presented with prey items on the ground, they ‘flew in large circles over the feeding area at average heights between 0.6 m and 1.4 m and average speeds between 3.0 m and 4.2 m/s’.9 Commuting speeds are certainly higher than in the other two species since M. myotis is much larger |
| Angle of wing strokes | 54.6°–83.8°3 | Stroke angle of ca. 91°7 | Not available – may be close to Plecotus but smaller |
Estimated by the working group.
AnAge website (http://genomics.senescence.info)
Eurobats website (http://www.eurobats.org)
Hughes, P., & Rayner, J. M. V. (1993). The flight of pipistrelle bats Pipistrellus pipistrellus during pregnancy and lactation. Journal of Zoology, 230(4), 541–555.
Grodzinski, U., Spiegel, O., Korine, C., & Holderied, M. W. (2009). Context‐dependent flight speed: evidence for energetically optimal flight speed in the bat Pipistrellus kuhlii? Journal of Animal Ecology, 78(3), 540–548.
Kalko, E. K. V. (1995). Insect pursuit, prey capture and echolocation in pipistrelle bats. Anim. Behav. 50, 861–880.
Seibert, A. M., Koblitz, J. C., Denzinger, A., & Schnitzler, H. U. (2013). Scanning behavior in echolocating common pipistrelle bats (Pipistrellus pipistrellus). PloS one, 8(4), e60752.
Norberg, U. M. (1976). Aerodynamics, kinematics, and energetics of horizontal flapping flight in the long‐eared bat Plecotus auritus. Journal of Experimental Biology, 65(1), 179–212.
Baagøe, H. J. (1987). The Scandinavian bat fauna: adaptive wing morphology and free flight in the field. Recent advances in the study of bats, 57–74.
Budenz, T., Denzinger, A., & Schnitzler, H. U. (2018). Reduction of emission level in approach signals of greater mouse‐eared bats (Myotis myotis): No evidence for a closed loop control system for intensity compensation. PloS one, 13(3), e0194600.
In another study, after an application of methamidophos to potato fields and apple orchards in Germany, high mortality was recorded among juvenile M. myotis, and pesticide residues were likely transferred to them via the milk after their mothers had fed on contaminated insects (Hofmann and Heise, 1991).
Transfer in maternal milk of bats is also recorded in other studies (e.g. Clark and Lamont, 1976) and for other compounds including anticoagulants (Clark and Lamont, 1976; Dennis and Gartrell, 2015). Therefore, the transfer of pesticides via this route needs to be considered in any future bat risk assessment.
In case that no physiological difference between rats and bats is known or assumed as regards species specific mechanism for transfer of pesticides into maternal milk, information from standard regulatory multigeneration toxicity studies with rats (OECD 416) can be potentially considered to support bat pups risk assessment. It needs to be investigated further on whether bat pups are exposed more to residues via milk as they rely solely on milk until they reach almost the size of an adult whereas rat pups start feeding on other items already when they did not reach the size of adults. It indeed appears that transfer to milk rather depends on kinetic factors of the substance like lipid solubility, molecular size, protein binding, oral bioavailability or half‐live of the molecule. Transfer of substance via milk however does not ultimately mean that there is a concern for pups, as this depends on the toxicity of the substance.
Rat pups open their eyes 10–14 days post‐partum. Until this time, they are only lactated and do not feed on their own. Potential effects on pups body weight, not observed at delivery but measured in the first days post partum (until days 10–14), are likely due to the effect of the compound transferred via maternal milk. If effects are observed after 14 days in rats, then it can be expected that similar effects will also occur in bats. Since bat pups are likely to be exposed more via milk, the effects could be more severe for bats.
In terms of estimating exposure for risk assessment purpose, the rat multigeneration study does not provide sufficient information. In rat multigeneration studies, residues in rat milk are not analytically investigated on routine basis. However, additional information on content of the pesticide in the milk could be retrieved from metabolism studies on livestock (lactating ruminants like goats and cows) when those studies are triggered in the pesticide approval process. In these studies, radioactive labelled material is fed to animals and the presence of radioactivity in milk gives indication as regards to a possible transfer to milk. Radioactivity found in milk may be subject to further identification in case of sufficient levels of milk radioactivity (levels > 0.01 mg/kg). It is noted that the aim of these studies is mainly the investigation of metabolites.
3.2.3. Dermal exposure
Dermal exposure may be of importance to bats as a combination of behavioural and physiological features specific to them. As noted in the introduction, they have a very large and highly vascularised wing surface. Makanya and Mortola (2007) studying fruit bats found that the body skin epidermis was thick (61 ± 3 μm). In contrast, the wing epidermis was thin at 9.8 ± 0.7 μm. The rate of oxygen consumption of the wings alone and of the whole animal measured under light anaesthesia at ambient temperatures of 24°C and 33°C, averaged 6% and 10% of the total oxygen consumption, respectively, with similar values for CO2 production. Thus, permeability of the wing membrane must be considered to be high, and it is assumed that any dermal exposure to pesticides will result in 100% penetration. The assumption of 100% penetration seems reasonable since 70% is used as the highest default value used for in human dermal exposure estimations (when data from experimental studies on dermal absorption with the pesticide under consideration are not available, see EFSA, 2017) for a much thicker and complex epidermis with more layers, and 100% was also suggested for dermal exposure of amphibians and reptiles (EFSA PPR Panel, 2018).
Behaviourally, for this section, it is considered that a primary foraging bout typical of small hawkers which will occur at dusk and early evening (Jones and Rydell, 1994). This main foraging bout of a bat such as a common pipistrelle may last for e.g. 2 h (Bartonička et al., 2008), during which time the bat may consume a very large proportion of its daily food intake. If this foraging bout takes place during a spraying operation, then the bat may be exposed directly to the pesticide as it flies in and around the spray cloud. The extent to which the bat is dermally exposed will be determined by the time spent in the air with different pesticide concentrations, and the amount of the pesticide in that air that is intercepted by the bat. There is no evidence to suggest that bats avoid machinery. Some species are even attracted to light (e.g. Arlettaz et al. 2000; Stone et al., 2015b), and hawkers might be attracted to insects that are stirred up by machinery, as seen for other insectivorous predators in farmland (Catry et al., 2014). Therefore, it cannot be ruled out that some bats will be attracted to spraying operations, and be exposed dermally.
Integrating the bat foraging behaviour with the concentrations of pesticides in air following spraying to calculate exposure accurately would be a complex task. The path of the bat as it forages behind a spraying operation is unknown and although the pesticide concentrations in the air above the crop with time behind the sprayer could be calculated, this is also quite complex. Both these two curves would need to be described, and the sum of products of the two curves would give the exposure. Figure 2 shows two hypothetical cases, giving the pesticide concentrations in air with distance and two possible patterns of bat flight (A and B). Flight pattern A is with variable distances from the spray boom (most of the time with a large distance from the spray boom and only short times close to the spray boom). Flight pattern B is for a constant distance from the spray boom. Flight pattern A is closer to real behaviour of bats than flight pattern B but needs integration of varying exposure over time. In this example, flight pattern B has 5 times the exposure of flight pattern A, assuming we use the product of the time and air concentration for the whole curve. Note that this is in contrast with the model used to calculate values for Table 6, where we assume 1 min exposure at distance zero only.
Figure 2.
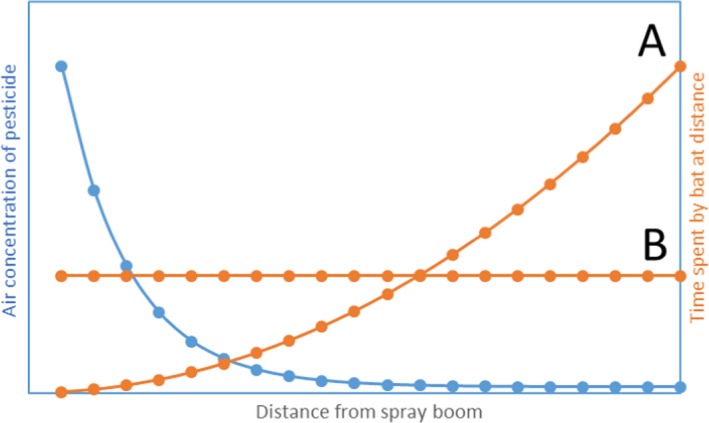
Hypothetical curves for air concentration of pesticide behind the boom with distance and time spent by bats at distance behind the boom. A and B are the curves for two different flight patterns of bats. A flies most of the time at a larger distance from the spray boom and only for short times close to the spray boom while B flies continuously at a certain distance from the spray boom
Since the calculation of the necessary curves for air concentration and bat time spent with distance from boom is beyond the scope of this statement, to provide some indication of the potential for concern three simple scenarios have been calculated, designed to represent possible conditions immediately behind the spray boom in a field crop (flight pattern B). The calculations were conducted based on the assumptions that bats are exposed at this concentration for 1 min per hour and 119 min unexposed (i.e. not on the graph in Figure 2), or for a full 2‐h exposure.
3.2.3.1. Calculation of exposure of bats when foraging during spraying operations
To do this, a simple model was created representing the volume of air that a bat is assumed to fly through, which is the basis for assessing potential dermal exposure.
To approximate the volume of air, the front surface area of the bat as it moves forward through the air was calculated. It is assumed that the rapid wing‐beat of the bats will effectively expose the large wing surface to the full volume of the wing stroke, and therefore, only the front surface area needs to be calculated. Surface area is given by twice the area of the segment of the circle formed by the stroke of the wing (wa) of length (wl), plus the area of a circle formed by the body diameter (bd).
Figure 3.
Scheme of surface area/air volume covered by bats flying through a spray cloud. (wa = stroke angle of wing, wl = wing length, bd = body diameter)
Note that this model does not take the area of the ears of the bat nor the tail of the bat into account, both of which would add surface area and in the case of the tail may considerably increase the front profile as it is used as a ‘scoop’ for flying insects.
The volume of air in litres passed through (v) is assumed to be the surface area multiplied by the speed s (m/sec) and by the number of seconds spend in the spray cloud (t).
The dermal exposure (de in mg/kg bw) is then calculated by defining the interception rate of the bat in terms of the proportion of pesticide coming into contact with the surface of the bat (i), and the concentration of active substance in the air volume (ac).
Here, it is assumed for simplicity in all cases that i is 1.0.
It is assumed that the pesticide concentration in the air (ac) can be calculated in three different ways. The simplest is just to assume an even distribution of active substance in the volume of air above the surface of the field, i.e. divide the application rate/m2 by the volume of air between the boom (0.5 m above the ground) and the surface of 1‐square metre of crop. This is referred to as the even distribution method, whereby the bats fly through a constant concentration with height in the spray cloud. This method is named ‘Even Distribution Method’.
The second method used was the terrestrial investigation model (TIM) model (US‐EPA, 2015), which results in a lower ac of 12% of the concentration calculated by the even distribution method. This method calculates the air concentration after spraying at ground level, assuming 90‐second exposure. This is referred to as the ‘TIM method’.
The third method was to assume a constant proportion based on a drift calculation at 1 m from the spray point. Assuming Rautmann et al. (2001) figures, this gives 2.77% of the application rate per unit area (this is the spray drift value for arable crops, for orchards this value would be higher). This calculation uses the width of the bat but not the volume, and is therefore width multiplied by distance covered multiplied by 2.77% of the application rate per unit area. This method is further referred to as ‘drift area method’.
We used all three methods of calculation for the largest and smallest of the three types of bats (hawker, hawker/gleaner and ground gleaner) considered for testing (see Table 6), and two different assumptions of speed and two of time in the spray cloud. These assumptions were 2.0 m/s (second), 6.7 m/s and 1 min and 2 h. These extremes are based on the fastest and slowest flight and assuming 2 h of activity directly in the spray cloud, or 1 min in the spray cloud, both providing a cumulative exposure. In all cases, the application rate was assumed to be 0.025 kg a.s./ha. Bat parameters used in each case are given by Table 6. The results are given in Table 7.
Table 7.
Calculation of dermal exposure in mg/kg bw under different assumptions of flight speed, time in spray cloud, species and for the Even Distribution, TIM Method, and Drift Area models for a hawker (Pipistrellus pipistrellus) and a ground gleaner bat (Myotis myotis). See text for further details. The following parameters were used for the calculations: P. pipistrellus – body weight of 5 g, body diameter of 0.03 m, wing length of 0.105 m (wing span of 0.24 m/2 minus half of body diameter of 0.015 m) and wing angle of 60°, M. myotis: body weight of 29 g, body diameter of 0.07 m, wing length of 0.17 m (wing span of 0.43 m/2 minus half of body diameter of 0.035 m) and wing angle of 90°. Flight speeds of 2 m/sec and 4 m/sec were assumed for slow and fast flying bats, respectively. The Hawker/Foliage Gleaner was ignored being intermediate in all cases between the other two species
| Time in the spray cloud | Flight speed | Dermal exposure (mg/kg bw) | ||
|---|---|---|---|---|
| Hawker | Even* | TIM* | Area | |
| 1 min | Fast | 2,490 | 311 | 798 |
| Slow | 1,470 | 184 | 399 | |
| 2 h | Fast | 352,863 | 44,108 | 95,731 |
| Slow | 176,430 | 22,054 | 47,865 | |
| Ground Gleaner | ||||
| Even* | TIM* | Area | ||
| 1 min | Fast | 2,037 | 255 | 234 |
| Slow | 1,018 | 127 | 117 | |
| 2 h | Fast | 244,524 | 30,566 | 56,007 |
| Slow | 122,673 | 15,334 | 16,718 | |
bw: body weight.
At 100% interception.
A typical bat foraging bout at the start of the foraging period is 2 h. If flying around the spraying equipment foraging on disturbed insects, then an estimate of 1 min in the spray cloud does not seem unreasonable (being 0.833% of the time of a foraging period).
In all cases, the model predicting the lowest exposure was the TIM Method, with a slow‐flying ground gleaner receiving 127 mg/kg bw. A slow hawker would receive 184 mg/kg bw and a fast one 311 mg/kg bw. Even using the TIM model and despite the high uncertainty of these estimates, these calculations suggest that there is the potential for dermal exposure to be a very significant pesticide exposure route for bats.
The working group did not calculate a value for a non‐arable crop, e.g. orchard spraying, which would be needed for any future risk assessment.
3.2.4. Inhalation exposure
The calculations below are based on the respiratory rates of three different bat species. The breathing rates and tidal volumes were available for flying and resting bats (see Table 8 below). In order to estimate the inhalation exposure of bats foraging in the field, it is suggested to use the breathing rates and tidal volumes of flying bats. Inhalation exposure of resting bats is also calculated for information and comparison.
Table 8.
Respired volumes calculated for different bat species during flight and rest. Rows with calculations for flying bats are shaded in grey
| Species | Body mass (g) | f (breath/min) | Tidal volume (mL) | Respired volume mL per min | Respired volume in l per min and kg bw | Activity |
|---|---|---|---|---|---|---|
| Phyllostomus hastatus | 105.9 | 500 | 1.75 | 875 | 8.262511804 | Flying1 |
| Noctilio albiventris | 40 | 125 | 0.25 | 31.25 | 0.78125 | Resting2 |
| Pteropus gouldii | 733 | 44.75 | 9.8 | 438.55 | 0.598294679 | Resting3 |
| Pteropus gouldii | 870 | 180.9 | 40.6 | 7344.54 | 8.442 | Flying3 |
The inhalation exposure was calculated according to the following formula:
With:
IE = inhalation exposure in mg/kg bw
Cair = concentration of spray in air in mg/L
Vr = Volume of air respired in L/kg bw
Tv = Tidal volume in L
t = time in minutes
f = frequency of breathing (breath per minute)
bw = bodyweight in kg
The concentration in air in the calculations presented below are based on the even distribution of spray under the boom and soil surface (same as done for dermal exposure) and on the air concentration calculated for droplets in the TIM model. A correction factor of 0.28 is applied to the air concentration to account for the proportion of inhaled droplets as suggested in the US‐EPA TIM (Tables 9 and 10).
Table 9.
Pesticide concentrations in air calculated according to even distribution of spray and according to the TIM model (US‐EPA) and the corresponding inhalation exposure for 1 min of inhalation. Rows with calculations for flying bats are shaded in grey and calculations for resting bats are without shading. Please see the text above for more details
| Species | Air concentration according to even distribution of 5 µg/L = 0.005 mg/L* | Air concentration droplets according to TIM in mg/L (C air drops for applied rate of 0.025 kg/ha) | Air concentration of TIM in mg/L** | Exposure of bats in mg/kg bw for 1 min of inhalation according to TIM (droplets) | Exposure of bats in mg/kg bw for 1 min of inhalation for the even distribution of concentration (5 µg/L as in the dermal exposure calculation)** |
|---|---|---|---|---|---|
| Phyllostomus hastatus | 0.005 | 0.000625 | 0.000175 | 0.00144594 | 0.0116 |
| Noctilio albiventris | 0.005 | 0.000625 | 0.000175 | 0.000136719 | 0.0011 |
| Pteropus gouldii | 0.005 | 0.000625 | 0.000175 | 0.000104702 | 0.0008 |
| Pteropus gouldii | 0.005 | 0.000625 | 0.000175 | 0.00147735 | 0.0118 |
bw: body weight.
The same as used in the dermal exposure calculation.
Please see Section 3.2.3.1 for details; the air concentrations were the same as for dermal exposure and corrected by a factor of 0.28 to account for the fraction of droplets inhaled (only small droplets can be inhaled).
Table 10.
Inhalation exposure for bats after 2 h of breathing air with a certain pesticide concentration. Rows with calculations for flying bats are shaded in grey and for resting bats are without shading. For further details, see Table 9 and the text above
| Species | Exposure in mg/kg bw for 2 h of inhalation according to TIM (droplets) | Exposure in mg/kg bw for 2 h of inhalation for the even distribution of concentration (5 µg/L as in the dermal exposure calculation) |
|---|---|---|
| Phyllostomus hastatus | 0.1735 | 1.388 |
| Noctilio albiventris | 0.0164 | 0.131 |
| Pteropus gouldii | 0.0125 | 0.101 |
| Pteropus gouldii | 0.1773 | 1.418 |
bw: body weight.
The bat exposure via inhalation is much lower compared to the values which were calculated for the dermal exposure (a factor of > 10,000). The oral exposure from uptake of residues in insects would be in the range of 0.29–1.15 mg/kg bw per day (depending on the feeding guild) for an application rate of 0.025 kg/ha as assumed in the dermal and inhalation exposure estimates. The inhalation exposure calculated for an exposure of 1 min is about 5–100 times lower than oral exposure and the 2 h inhalation exposure calculation is in the same range as the oral exposure from residues uptake via contaminated food items covering the daily energy requirement.
This suggests that the risk from inhalation exposure is for bats less than from dermal exposure and that inhalation exposure might be in a similar range as oral exposure. However, inhalation exposure to vapour was not considered in the calculations. For volatile substances, this could significantly increase inhalation exposure.
The different exposure routes are not directly comparable in terms of potential effects. The uptake via oral route, dermal route and inhalation can lead to different levels of internal concentrations because of differences in the membrane barriers. Substances which are taken up orally will not immediately reach the target organs as they first pass the liver and might undergo a detoxification mechanism or activation in case of more toxic metabolites. Effects on uptake via the lung membrane may be different than those via the skin and direct effects on the respiratory membrane of the lungs may also be more severe than effects on the skin.
Furthermore, it may be reasonable to assume that the different exposure routes (oral, dermal and inhalation) additive if bats are foraging in the field when a pesticide is applied in the evening and this should be considered in the risk assessment.
4. Uncertainty analysis
A high variation in toxicological sensitivity of bats in comparison to birds and ground dwelling mammals was observed. Bats were 1,000 times more sensitive (in the case of the active substance deltamethrin) to 100 times less sensitive (in the case of fenthion) than the standard mammalian and bird test species. This add substantial uncertainty if the same endpoint from the standard test species would be used in a risk assessment for bats.
Table 11 provides an overview on the main sources of uncertainty in the exposure calculations for bats. The potential over‐ or underestimation of exposure is only described qualitatively since the information available for a quantitative analysis of uncertainty is not available.
Table 11.
Uncertainty analysis for exposure estimations
| Source of uncertainty | Potential to over‐ (−)*or underestimate the exposure (+)** | Explanation |
|---|---|---|
| Uncertainty analysis for oral exposure estimates | ||
| Food intake rates (FIR) used in the oral exposure estimates | −/+ | The food intake rate is based on a small bat species which can be considered representative for small European bats. Some European bats such as Pipistrellus pipistrellus are smaller and may have higher FIRs but the majority of European bats are larger. The FIR was measured in lactating bats and hence covers the life stage with highest FIRs. Further studies measuring FIR or energy requirements of bats may broaden the database and the resulting FIRs may change to higher or lower values but the magnitude of change is likely to be low |
| Residue per Unit Dose (RUD) for residues in insect food items | −−/+ |
The residues for flying insects were taken from a study where insects most relevant as food items for bats were collected. The study was conducted with a substance which has no acute toxic mode of action for insects and as such may be well representative for substances with low toxicity to insects. However, the data are from one study only and hence broadening the database may lead to a lowering or increase of the RUD For substances which are highly acutely toxic to insects, the real residues are most likely lower as the insects with high pesticide load will not be able to fly anymore and hence will not be available to bats feeding on flying insects |
| Drinking water demand | −/+ | The drinking water demand was taken from a study with lactating females of a small bat species. The life stage with highest drinking water demand is therefore covered. However, it is only one study and if the database would be broadened the values for the drinking water demand could be increased or reduced but the magnitude of this change is likely to be low |
| Oral exposure by grooming | −−−/+++ | Grooming is a potential source of oral exposure from residues collected on the fur and wings of bats. The available data were not sufficient to provide an estimate of oral exposure from grooming. There is indication that this exposure route could be substantial since a method to control vampire bats is even derived from the natural behaviour of social grooming |
| Oral exposure of pups based on a measured excretion of 83% of residues in milk | −− | The estimate of exposure of pups from uptake of residues in milk is based on an excretion of 83% of residues via milk. This measurement is from one study only. Broadening the database may lead to an increase or decrease of this value |
| Dermal exposure estimates | ||
| Duration of exposure of 1 min | +++ | If bats forage in a field where a pesticide is applied then the duration of exposure is likely to be greater than for 1 min |
| Duration of exposure of 2 h | −−− | A typical foraging flight of an aerial hawker lasts for 2 h. These 2 h could be spent in the vicinity of the treated field. However, it is unlikely that it will be exposed continuously for 2 h at high air concentrations |
| Distance to spray boom | −−− | In the dermal exposure estimates, it is assumed that the bat is very close to the sprayer. In reality, the bat will be only for a short time close to the sprayer each time when it is passing by the machinery during the foraging flight |
| Pesticide concentrations in air | −−/++ | The calculation of more accurate air concentrations and the path of the bat takes through the spray cloud may lead to higher or lower exposure |
| All spray droplets in the volume of air passed through by the bat ends up on the bat surface (calculation methods 1 and 2) | − | In reality, not all spray droplets in the volume of air the bat is passing through will end up on the surface of a bat |
| All spray droplets in the drift at 1 m away from the sprayer end up on the upper surface of the bat over the distance covered during bat flying time (calculation method 3) | +/− | In reality, not all spray droplets in the area covered by the bat flight will end up on the bat surface but on the other hand bats may be exposed to some droplets from the spray which would deposit further away than 1 m from the sprayer |
| Inhalation exposure | ||
| Duration of exposure of 1 min | +++ | If bats forage in a field where a pesticide is applied, then the duration of exposure is likely to be greater than for 1 min |
| Duration of exposure of 2 h | −−− | A typical foraging flight of an aerial hawker lasts for 2 h. These 2 h could be spent in the vicinity of the treated field. However, it is unlikely that it will be exposed continuously for 2 h at high air concentrations |
| Distance to spray boom | −−− | In the dermal exposure estimates, it is assumed that the bat is very close to the sprayer. In reality, the bat will be only for a short time close to the sprayer each time when it is passing by the machinery during the foraging flight |
| Pesticide concentrations in air | −−/++ | The calculation of more accurate air concentrations and the path of the bat takes through the spray cloud may lead to greater or lower exposure |
| Respired volumes | −/+ | Data on respiratory volumes were available for four bat species. The bats were large in comparison to European bat species. However, there was no indication that smaller bats would have greater respired volumes per kg bw and the available data for the four species were consistent. Therefore, it is not expected that respired volumes of European bats would be significantly different than the ones used in the exposure calculations |
| Inhalation of vapour not considered | +++ | Exposure to vapour can be significant for volatile substances and this may significantly increase inhalation exposure compared to inhalation of droplets |
’−’, ‘−−’, ‘−−−’ Low, medium or high potential to make the true exposure lower.
‘+’, ‘++’, ‘+++’ Low, medium or high potential to make the true exposure higher.
5. Conclusions
Habitat use
Farmed landscapes provide foraging opportunities for many bats and there is strong evidence that bats forage frequently in intensive farmland across Europe and consume agricultural pests by large numbers.
Given the role bats play as suppressors of arthropods in farmland, forests and even urban areas, preserving insectivorous bats in temperate regions is seen as a major goal to keep benefiting of this important ecosystem service. In Europe, bats also make an outstanding contribution to vertebrate diversity. Most importantly, they are strictly protected across the EU.
Biology
Bats have exceptional longevity making them potentially susceptible to long‐term contamination by pesticides. They also have a low fertility and high parental investment that exposes them to high risks of population decline following perturbation events. They are therefore k‐selected specialist with potentially high vulnerability to stressors.
Bats use fat storage to support both daily and seasonal patterns of torpor and arousal; therefore, they are potentially at risk from internal exposure to pesticides as a result of rapid mobilisation of these fat reserves and the pesticides contained therein either on a daily or seasonal basis.
Pesticide residues may be ingested by the young during lactation. Since the young have a very high relative energy need, there is potential for this oral exposure route to be very important in bats.
Exposure
Food intake in bats is quite high, and peaks in especially demanding phases of the life cycle, such as lactation, when females may consume up to ca. 130% of their body mass in insects. A high FIR means that if food items contain pesticide residues, the oral exposure route is important.
Drinking water constitutes one of the potential routes of bat exposure to pesticides. In fact, bats drink at a range of water sites, including those found in farmland, such as irrigation canals or small ponds, which are potentially contaminated with pesticides. Exposure via contaminated water therefore is relevant.
While oral exposure via food and water are also considered under the Birds and Mammals Guidance Document, there are other routes of exposure that need to be considered for bats that are not part of the current birds and mammals RA. These are:
Grooming – both self‐ and social grooming represent a way through which pesticide residues collected on the fur and the wing membranes may be ingested. Hairless juveniles in clusters or clinging to their mother's fur might also be affected by the tight contact with adults whose fur has been exposed to pesticide residues during foraging.
Dermal exposure during foraging – Bats have a very large wing surface with high degree of vascularisation and extremely thin epidermis that may lead to a high level of dermal absorption. This indicates that if exposed dermally there is potential for this to be a very important exposure route. Therefore, there is a need to consider dermal exposure.
Inhalation exposure – if bats are foraging during spray applications, then they are also exposed via inhalation.
Dermal exposure during roosting – in specific cases, there might be some risk of direct contamination through inhalation or dermal contact when bats roost in farmland in rural buildings (e.g. barns, stables) in the proximity of the spray cloud or in treelines or hedgerows. The risk may be more pronounced when bats use night‐roosts to pause from nocturnal activity in areas where pesticides are sprayed.
Overall – for evening applications, the bats foraging in the field will be exposed via different exposure routes (oral, dermal, inhalation) simultaneously.
Toxicology
It is important to know whether toxicity to bats can be inferred by studies done on mammals and birds. There is insufficient data available on toxicity of pesticides to bats to draw firm conclusions. However, using the data available, there probably is no relationship between toxicity to bats and other mammals or birds on which a predictive assessment could be made Even within the same group of pesticides, there was no clear pattern relating toxicity of test species to bats.
There is evidence that echolocation and patterns of torpor can be affected by exposure to pesticides, both being potentially lethal to individuals in the wild. Therefore, bat‐specific endpoints may need to be used. However, currently there is a lack of information to address effects on these bat‐specific features.
Conclusions on coverage of bats by current risk assessment for birds and mammals
The oral exposure of bats is covered in the screening step assessment of the current assessment for birds and mammals with some exceptions for bare soil, hop and grassland. However, bats are not covered by exposure scenario of the terrestrial insectivorous mammals for the first‐tier assessment. Only few (4 out of 44) of the first‐tier scenarios for insectivorous birds cover the exposure for bats. It is noted that the exposure of bats might be underestimated even in those 4 scenarios if the exposure assessment is refined. Therefore, it is concluded that specific risk assessment scenarios for oral exposure need to be developed for bats taking into consideration the different feeding guilds of bats. This includes establishing standard pesticide residues values (RUD) for the food items eaten by bats. While standard RUD values are available for ground dwelling and leaf dwelling arthropods, there is no generic RUD value currently available for flying insects. Residues data in flying insects were available from an EFSA supporting publication (by Lahr et al., 2018). This data set was analysed and several factors were identified which potentially have an influence on the insect residues values and might explain the high variability in measured residues values. These factors are related to the sampling method used, toxicological profile of the substance and insect size.
Furthermore, it is frequent that risk assessments for birds and mammals under regulation 1107/2009 are refined in higher tier assessment steps. Often these are based on the refinement of ecological parameters, such as the proportion of diet obtained in the treated area, for key focal species. The studies used to derive these focal species are performed during the day and do not account for animals present at dusk. The assessment of the vulnerability of bat species against real focal species has not been considered in the context of this statement. For the reasons discussed previously, owing to the specific biology and ecology of bats, it could be expected that higher tier refinements for birds and mammals are not protective of bat species. However, to reach a definitive conclusion further experimental investigation would need to be performed.
The current standard estimates for drinking water exposure for birds and terrestrial mammals cover the exposure of bats via drinking water.
The inhalation exposure calculated for an exposure of 2 h is in the same range as the oral exposure from residues uptake via contaminated food items covering the daily energy requirement and it is a factor of more than 1,000 less than what is calculated for dermal exposure. This suggests that the risk from inhalation exposure for bats is less than from dermal exposure and that inhalation exposure may be in the same range as oral exposure. However, inhalation exposure to vapour was not considered in the calculations. This could significantly increase inhalation exposure for volatile substances. The uptake via the lung membrane may be different to that via the skin and direct effects on the respiratory membrane of the lungs may also be more severe than effects on the skin. Furthermore, it is reasonable to assume that the different exposure routes (oral, dermal and inhalation) are additive if bats are foraging in the field when a pesticide is applied in the evening.
If bats are exposed dermally during and immediately after spraying operations, exposure levels are likely to be extremely high and would need to be considered in any bat risk assessment for pesticides.
One measure to mitigate the risk from dermal exposure is to avoid evening applications of pesticides. The exact timing of such a restriction would need to carefully consider the behaviour of different bat species occurring in a certain region.
Overall, considering the low fertility and specific exposure routes of bats, the Panel concludes that bats are not adequately protected following the current birds and mammals guidance for regulatory assessment of pesticides.
6. Recommendations
In preparing these recommendations, the Panel is fully aware that due to the protected status of bats in Europe, experimentation on these species to generate ecotoxicological data is generally not possible. There is a need for a broad investigation of patterns of bat exposure to pesticides, which should focus on the routes described below.
6.1. Knowledge gaps
Dermal exposure during spraying operations in both rotational and permanent crops may have considerable importance, yet no study has addressed this important topic. For instance, bats are unlikely to avoid machinery, instead they might be attracted to the insects stirred up during agricultural operations, including pesticide spraying. Moreover, the artificial lighting adopted at night may concentrate phototactic insects such as moths and small dipterans, and, in turn, opportunistic bat species that tolerate illumination such as pipistrelles. These aspects warrant investigation.
Dermal exposure at roosting sites may be an issue for bat colonies that roost in buildings occurring in agricultural fields potentially exposed to pesticide drift, and probably even more for bat colonies that roost in the cavities of trees bordering fields or occurring as isolated trees in farmland. In such cases, suitable roosts might in fact, act as ‘ecological traps’ leading to occasional high mortality.
Assessing oral exposure requires an in‐depth knowledge of the generic residue per unit dose (RUD) values for flying insects as currently there are adequate RUDs only for ground‐ and canopy‐dwelling insects. The available database for flying insects residues is limited and should be broadened to derive robust RUDs specific for the type of insects eaten by the different feeding guilds of bats. It is also recommended establishing density, composition and pesticide content of insects on which bats feed in areas as the ones described above. The aim is to obtain insight into the temporal and spatial distribution of insects, and their pesticide contents, on which the bats feed.
Exposure of young bats to pesticides in maternal milk is of importance since mothers feed their young intensively on milk for ca. 1 month, thus representing a potentially high risk for pups.
Grooming also represents a potential way of oral exposure: in daytime roosting, bats scratch or lick their own fur (self‐grooming) and also chew or lick another individual's body (social grooming). Social grooming is especially important for bats because these mammals are highly gregarious and often cluster together by hundreds or thousands, not rarely forming heterospecific associations. Grooming may be an important way through which bats ingest pesticide residues they carry on the fur, but there is a lack of a quantitative assessment of the time spent grooming for most European species and an evaluation of the amount of pesticide that bats might ingest in this way.
Species susceptibility determination: the limited toxicity data available showed no evidence of a relationship between toxicity of PPPs to bats and other mammal or bird species. In lieu of direct toxicity studies, the Panel suggests the development of mechanism of action and adverse outcome pathway approaches. This should probably rely on two complementary approaches. First, elucidation of metabolic pathways, investigating for instance genetic and metabolic differences in phase I (e.g. CYP enzymes) and II xenobiotic metabolising enzymes, which affect species‐specific patterns of PPP toxicokinetics. Second, identification of molecular and functional targets for PPPs and their species‐specific variability should also be considered for assessing potential toxicodynamic susceptibilities and resistances to PPPs. Both approaches should rely primarily on ex vivo and in vitro techniques.
Both passive and active surveillance are urgently needed to assess the amount of residue in bats. Due to the protected status of bats, surveillance cannot rely on the killing of bats for research, rather passive surveillance might focus on individual bats found dead (e.g. from rehabilitation centres or windfarm fatalities), and active surveillance could focus on non‐invasive sampling such as collection of droppings.
Species‐specific variation – Different bat species might differ in the degree to which they are exposed to pesticides, for example in relation to their foraging style, habitat selection patterns or diet composition, or exhibit different resistance to the adverse effects of pesticide exposure. Any associated population effect might influence interspecific interactions such as competition patterns and have community‐scale consequences. In assessing such patterns, a combination of field observations (e.g. spatial use analysis, diet assessment) and modelling is recommended.
6.2. Development of a bat risk assessment guidance
6.2.1. Short‐term developments
Before guidance for risk assessment for bats is developed, a definition of clear Protection Goals is recommended, including the Specific Protection Goals (SPGs) for bats in accordance with the procedures described in EFSA (2010 and 2016). In operationalising the SPGs, the appropriate exposure estimate and its timeframe should be made explicit, for example using the approach of the Ecotoxicologically Relevant Concentration (ERC) developed in Boesten et al. (2007). This approach needs to take into account bat ecology and behaviour.
Bats are not covered by the current exposure assessment scheme for birds and mammals and there is a need to develop a bat‐specific risk assessment scheme. Based on the current analysis, this should include a focus on (a) oral exposure via residues in insects and grooming, (b) dermal exposure and (c) exposure of pups via milk. It is important to highlight that any risk assessment scheme should consider the total body burden from all exposure routes as bats foraging in the field will be exposed to residues in insects and also via dermal and inhalation routes.
It is recommended that the current higher tier risk assessment methodologies are further developed and adapted to cover bats.
For exposure assessment, it is recommended to consider explicitly the probability of occurrence in time and space, i.e. it acknowledges that there are also spatial units (i.e. fields, habitats), or periods in time (e.g. years, lactating periods) during which exposure to the relevant substance does not take place, and it is also explicitly stated for which percentile of the statistical population of Ecotoxicologically Relevant Exposure Quantities the protection goal should be met. In this way the variety of exposure values encountered in reality is better reflected and a better estimate of the wished degree of protection can be made. To achieve this exposure assessment goal, approaches as in the scientific opinion on Amphibians and Reptiles risk assessment (EFSA PPR Panel et al., 2018) and Boesten (2018) could be applied to create a systematic inventory of all relevant exposure items, incl. the exposure pathways.
6.2.2. Long‐term developments
In order to calibrate any future risk assessment methodology against protection goals, generic field studies in combination with suitable and validated population models would be needed. Consequently, it is recommended that such data or models are developed.
Although not the focus of the current statement, in order to properly protect bats from the effects of pesticide usage, it would be necessary to ensure that indirect effects are covered. Mitigation measures could be employed within farmlands to ensure that there are sufficient non‐treated areas to provide a source of insects.
As noted above, the risk to bats can be reduced (but not eliminated) by preventing spray applications during the foraging time for bats. For example, mortality of juvenile bats was observed after evening applications of methamidophos but no mortality was observed the years before during day applications (Hofmann and Heise, 1991). It is recommended to consider other possible mitigation measures to protect bats from exposure to pesticides.
We recommend gathering data on spatial and temporal foraging behaviour to link exposure to specified levels of pesticides in the air (dermal and inhalation exposure) or insects (oral exposure). In the long term, if population level effects are considered in the risk assessment it is necessary to integrate bat behaviour with landscape structure and environmental fate, such as including field size and distribution, spatial and temporal patterns of spray applications and bat utilisation of those areas.
Glossary and Abbreviations
- AChE
acetylcholinesterase
- AE
assimilation efficiency
- a.i.
active ingredient
- ALT
alanine aminotransferase
- AR
aspect ratio
- a.s.
active substance
- AST
aspartate aminotransferase
- BAT
brown adipose tissue
- BBCH
(Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie), the BBCH scale defines the Principal Plant Growth Stages
- BMD
benchmark dose. The benchmark dose is the minimum dose of a substance that produces a clear, low level health risk, usually in the range of a 1–10% change in a specific toxic effect such as cancer induction (e.g. BMD10 is a 10% change)
- BMR
basic metabolic rate
- bw
body weight
- CYP
cytochrome 450 ‐
- DDD
daily dietary dose
- DEE
daily energy expenditure of the indicator species
- ERC
Ecotoxicologically Relevant Concentration
- FE
food energy
- FIR
food intake rate
- k‐selected species
k‐selected species produce relatively low numbers of offspring and individual offspring tend to be quite large. K‐selected species are also characterised by extended parental care and long life spans.
- LD50
lethal dose, 50%
- LD100
lethal dose, 100%
- LOAEL
lowest‐observed‐adverse‐effect level
- log Kow
logarithm of the octanol–water partition coefficient
- MAF
multiple application factor
- MC
moisture content
- mtDNA
mitochondrial DNA
- NOAEL
no‐observed‐adverse‐effect‐level
- NOEL
no observable effect level
- PBT
Persistent, Bioaccumulative and Toxic
- PCP
pentachlorophenol
- PPP
Plant Protection Product
- PT
proportion of an animal's daily diet obtained in habitat treated with pesticide
- RA
risk assessment
- RUD
residues per unit dose
- SPG
Specific Protection Goal
- SV
shortcut value
- TER
toxicity‐exposure ratio
- Torpor
Torpor is a physiological state of an animal which is characterised by decreased physiological activity, reduced body temperature and metabolic rate. Torpor enables animals to survive periods of reduced food availability (e.g. during winter ‘hibernation’)
- TWA
time weighted average
- UNEP/CMS
The Convention on Migratory Species
- VKOR
Vitamin K epoxide reductase
- WL
wing loading
Appendix A – General biology and ecology of bats and differences to other mammalian species relevant for risk assessment
1.
General biology and ecology of bats and differences to other mammalian species relevant for risk assessment
A.1. Diversity and evolutionary history
All bats belong to an order of placental mammals named ‘Chiroptera’, from the Greek words for ‘hand’, ‘cheir’, and ‘wing’, ‘pteron’, which highlights the order's main feature, i.e. the presence of wings arising from the forelimb's adaptation to flight. While other mammals, such as flying squirrels and colugos, may glide (Jackson, 2000), bat's unique wings make them the only mammalian group capable of powered flight. Bats may fly over long distances, and due to their outstanding dispersal ability, not surprisingly they are often the only native mammals on remote islands (Conenna et al., 2017).
Among mammals, bats stand out for their remarkable species richness (over 1,300 species), being second only to rodents (Altringham, 2011). Although all species share the same body plan, physiological, ecological and behavioural adaptations have allowed bats to conquer all continents except Antarctica, succeeding in a wide array of habitats, from rainforests to the deserts, from temperate forests to human‐altered environments such as farmland and even urban areas.
Another remarkable feature of bats is their nocturnal behaviour, which is thought to constitute an evolutionary response to predation by diurnal avian predators that rely on vision (Mikula et al., 2016). Although all bats may show sporadic daytime flights, the few exceptions that do so frequently are typically restricted to oceanic islands that have never been reached by such predators (e.g. Russo et al., 2011a); even more rarely, daytime flight may take place at mainland sites where the risk of being caught is negligible compared to the advantage of foraging before dark (Russo et al., 2011b). The most remarkable adaptation to the bat's nocturnal lifestyle is the biosonar, or echolocation; the majority of living bats broadcast ultrasonic pulses and listen for their echoes to probe the space around them for orientation, navigation, and foraging (Fenton, 2013).
The oldest fossil remains of bats date back to the Eocene (over 50 million years ago), and testify that bats have been through a long evolutionary history. The 52.5 million‐year old Onychonycteris finneyi, the oldest bat known to date (Simmons et al., 2008), from the sedimentary rocks of the Green River Formation (US), possessed some primitive characters no longer recorded in living bats, such as the presence of claws on all fingers, yet it basically shows the same body plan shared by all modern bat species. Although, based on its skeletal morphology, O. finneyi has been suggested not to be able to echolocate (Simmons et al., 2008) in agreement with the hypothesis that bats evolved flight before echolocation, this interpretation has been questioned by Veselka et al. (2010) who suggested that this species was actually able to echolocate based on the fact that its stylohyal bone may have articulated with the tympanic bone, a condition typical of echolocating bats. Only slightly more recent (50 million years), Icaronycteris index, from the Polecat Bench Rocks of Wyoming were agile flyers and already efficient echolocators.
There is now strong evidence that bats are monophyletic. They belong to a diverse group of mammals called Laurasiatheria, comprising a diverse array of mammalian groups (Tsagkogeorga et al., 2013). Bats have long been classified into two suborders, Megachiroptera (megabats) and Microchiroptera (microbats), which although today is no longer considered valid is still useful. The former, comprising some of the largest bat species, are the so‐called flying foxes, forming a group of 200 species from the family Pteropodidae, and confined to the Palaeotropics. They are often of a large size and lacking the biosonar with the only exception of Rousettus aegyptiacus and Rousettus amplexicaudatus, which click their tongues to generate echolocation calls. All remaining bat species, the Microchiroptera, are mostly small, occur over most of the globe and possess laryngeal echolocation. This traditional separation was highly intuitive, yet sometimes misleading because some ‘microbats’ are actually larger than the smallest ‘megabats’. Molecular studies (Teeling et al., 2005) have shown that some of the microbat families (Rhinolophidae, Rhinopomatidae, Hipposideridae and Megadermatidae) are phylogenetically closer to the megabats than to the remaining microbats: Microchiroptera are therefore paraphyletic. In the latest classification, the Pteropodidae and the above‐mentioned four ‘microbat’ families constitute the suborder Yinpterochiroptera, while all other species form the suborder Yangochiroptera.
A.2. Life history in bats and other mammals
The body size of bats shows a remarkable range, spanning from the 2‐g bumblebee bat (Craseonycteris thonglongyai) to the largest flying foxes weighing over one kg, and showing a wingspan > 1.5 m (Altringham, 2011). However, most species are small: for example, most European bat species weigh < 20 g (Dietz and Kiefer, 2016). Yet, bats stand out of the crowd of living ‘small mammals’ such as shrews or rodents thanks to their unique life histories. This has important conservation consequences, because it is these features that render bats especially vulnerable to habitat changes and other threats posed by humans. In a nutshell, bats may be defined as k‐strategists, because they are long‐lived and in the course of their lives produce few, large offspring that are milked for several weeks, after which their body size practically equals that of their mother.
To provide a comparison between bats and other common terrestrial small mammals, we examined 10 life‐history traits, selecting for analysis 227 species of bats (order Chiroptera), 433 species of rodents (order Rodentia, families Muridae and Cricetidae) and 72 species of shrews (order Soricomorpha, family Soricidae). The mammal taxa considered have several characteristics in common, including similar body sizes, broad geographic distribution, high metabolic rates, and in many cases, the use of torpor as an energy‐saving strategy (Barclay and Harder, 2003). Life‐history data were extracted from the AMNIOTE database (Myhrvold et al. 2015). An approach similar to adopted by Barclay and Harder (2003) was used for statistical comparisons. A general linear model was used where ‘mammal order’ was the main treatment and ‘adult body mass’ entered as a covariate to adjust for the effects of interspecific differences in body size on life history traits. While the ‘adult body mass’ did not refer to a specific sex, Barclay and Harder (2003) used female mass only. This represents a negligible difference in the approach used in the two studies since most species considered show limited or no sexual dimorphism. Unlike Barclay and Harder (2003), the analysis was not restricted to insectivorous bats only, added rodents and, in most cases, used larger sample sizes. Despite such discrepancies in the approach we adopted, the findings are very similar to those presented in Barclay and Harder (2003). Data were log‐converted when the distribution of residuals departed significantly from the normal distribution.
In general, the analysis (Table A.1) confirms that bats on one side, and shrews and rodents on the other, are located at opposite ends of the fast‐slow continuum of life history traits that characterise mammals (Read and Harvey, 1989; Barclay and Harder, 2003). It also shows that body size has a significant effect in most cases. As discussed ahead, it was found that bats are the most long‐lived of the three groups, followed by rodents and shrews, and that reproduction is, in general, much slower than in the other mammalian groups considered (see Table A.1), namely:
In bats, females mature later than in rodents/shrews, taking on average slightly more than 1 year (similar to what shown in Barclay and Harder 2003), while female rodents mature earlier than shrews. Males also take more time to reach sexual maturity in bats than in the other groups; shrews take longer than rodents.
Bats produce fewer offspring than rodents and shrews (see also Barclay and Harder, 2003), and litter size in shrews is slightly larger than in rodents.
On average, bats give birth once a year, i.e. less frequently than shrews (2.7/year) and rodents (3.5/year). Birth rate differs significantly across the three groups considered.
Bats have a gestation, respectively, 4.0 and 4.7 longer than rodents and shrews, whereas the duration of gestation in the remaining two groups shows no significant difference. This difference is greater than that found by Barclay and Harder (2003) in their ‘bats vs. shrews’ comparison.
Lactation takes almost three times longer in bats than in the other two groups, which do not differ from each other (see also Barclay and Harder, 2003).
The body mass of bats at birth relative to that of adult conspecifics is over twice that of the other orders, which do not differ from each other. The magnitude of this difference is similar to that found between bats and shrews by Barclay and Harder (2003).
At weaning, bats are also heavier than rodents but not shrews relative to their body size; shrews are heavier than rodents. The lack of difference between bats and shrews was also found by Barclay and Harder (2003), and given the greater litter size of shrews, highlights that (assuming all juveniles survive to weaning) shrews wean litter > three times heavier than those of bats.
Bats have a longer interval between consecutive births (almost 1 year) than the other two groups, while rodents have a shorter interval than shrews.
Table A.1.
Comparison of 10 life‐history traits among bats (order Chiroptera), rodents (order Rodentia, families Muridae and Cricetidae) and shrews (order Soricomorpha, family Soricidae). Data extracted from the AMNIOTE database (Myhrvold, N. P., Baldridge, E., Chan, B., Sivam, D., Freeman, D. L., & Ernest, S. M. (2015). An amniote life‐history database to perform comparative analyses with birds, mammals, and reptiles. Ecology, 96(11), 3109–3109.). Statistical comparisons were made with a General Linear Model where ‘order’ was used as the main treatment and ‘adult body mass’ as a covariate to adjust for the effects of interspecific differences in body size on life history traits. In the ‘mean’ column, same letters label means that do not differ from one another
| Trait | N | mean | SD | Range | F | P |
|---|---|---|---|---|---|---|
| Female sexual maturity (days) | ||||||
| Bats | 227 | 372.9a | 183.3 | 53.6–831.0 | ||
| Rodents | 433 | 81.1b | 45.2 | 23.8–438.9 | ||
| Shrews | 72 | 210.9c | 122.8 | 36.0–417.4 | ||
| body mass | 42.2 | < 0.001 | ||||
| order | 627.7 | < 0.001 | ||||
| Male sexual maturity (days) | ||||||
| Bats | 127 | 473.5a | 160.3 | 98.0–775.8 | ||
| Rodents | 146 | 76.1b | 37.3 | 36.0–335.0 | ||
| Shrews | 37 | 154.8c | 128.3 | 36.0–365.0 | ||
| body mass | 3.5 | n.s. | ||||
| order | 530.8 | < 0.001 | ||||
| Litter size (n individuals) | ||||||
| Bats | 647 | 1.2a | 0.4 | 1.0–3.1 | ||
| Rodents | 783 | 3.6b | 1.5 | 1.0–8.9 | ||
| Shrews | 188 | 4.0c | 1.6 | 1.0–9.0 | ||
| body mass | 56.7 | < 0.001 | ||||
| order | 1943.2 | < 0.001 | ||||
| Number of litters per year | ||||||
| Bats | 378 | 1.3a | 0.4 | 0.8–3.0 | ||
| Rodents | 382 | 3.5b | 1.5 | 1.0–10.0 | ||
| Shrews | 82 | 2.7c | 1.6 | 1.0–9.8 | ||
| body mass | 8.3 | < 0.005 | ||||
| order | 683.8 | < 0.001 | ||||
| Maximum longevity (years) | ||||||
| Bats | 263 | 14.7a | 0.3–41.0 | |||
| Rodents | 491 | 4.0b | 0.1–13.6 | |||
| Shrews | 98 | 2.4c | 0.1–4.0 | |||
| body mass | 3.9 | 0.048 | ||||
| Order | 229.7 | < 0.001 | ||||
| Duration of gestation (days) | ||||||
| Bats | 291 | 110.2a | 48.0 | 22.9 – 260.2 | ||
| Rodents | 439 | 27.0b | 6.5 | 13.5 – 53.3 | ||
| Shrews | 76 | 23.6c | 4.3 | 17.0 – 30.4 | ||
| body mass | 64.4 | < 0.001 | ||||
| Order | 1863.5 | < 0.001 | ||||
| Days till weaning | ||||||
| Bats | 264 | 66.9a | 59.1 | 15.0–668.8 | ||
| Rodents | 437 | 24.1b | 7.6 | 11.8–68.7 | ||
| Shrews | 71 | 23.6c | 4.2 | 17.3–41.5 | ||
| body mass | 125.7 | < 0.001 | ||||
| Order | 491.2 | < 0.001 | ||||
| Birth/adult body mass (g) | ||||||
| Bats | 285 | 0.2a | 0.1 | 0.0–0.5 | ||
| Rodents | 388 | 0.1b | 0.0 | 0.0–0.2 | ||
| Shrews | 75 | 0.1b | 0.0 | 0.0–0.1 | ||
| body mass | 91.0 | < 0.001 | ||||
| Order | 426.2 | < 0.001 | ||||
| Weaning/adult body mass (g) | ||||||
| Bats | 120 | 0.7a | 0.2 | 0.2–1.3 | ||
| Rodents | 306 | 0.4b | 0.2 | 0.0–1.5 | ||
| Shrews | 65 | 0.8a | 0.2 | 0.5–1.1 | ||
| body mass | 19.2 | < 0.001 | ||||
| Order | 133.2 | < 0.001 | ||||
| Interbirth interval (fraction of year) | ||||||
| Bats | 89 | 1.0a | 0.1 | 0.4–1.0 | ||
| Rodents | 236 | 0.1b | 0.1 | 0.0–0.6 | ||
| Shrews | 30 | 0.1c | 0.1 | 0.1–0.3 | ||
| body mass | 16.1 | < 0.001 | ||||
| Order | 1643.4 | < 0.001 |
A.2..1.
A.2.1. A focus on longevity
A first outstanding feature of bats is their longevity. Relative to ground dwelling small mammals (Table A.1, rodents: 4 years; shrews: 2.4 years), bats may live much longer, on average ca. 15 years (Table A.1), despite their high active metabolic rate: the longevity record is held by Brandt's bat (Myotis brandtii), which in the wild may live up to 41 years (Podlutsky et al., 2005). It is important to consider, however, that longevity data are available only for a limited number of species, that data quality differ among those, and that records originate from recaptures of adult individuals banded as juveniles (as with the above‐mentioned M. brandtii) or, in a few cases, from captive bats.
Why are bats so long‐lived? Based on the analysis of 64 bat species, Wilkinson and South (2002) established that hibernation, body mass and occasional cave use increase longevity, while reproductive rate has an opposite effect. Occasional cave roosting may protect bats from predators, while hibernation may reduce the risk associated with extreme weather or starvation in the cold months, when resources are scarce (Munshi‐South and Wilkinson, 2010). Caloric restriction such as that occurring during hibernation may also prolong life span.
According to the disposable soma theory, allocating resources to reproduction rather than somatic maintenance and repair leads to physical degradation and ageing (Kirkwood, 2002). Bats that hibernate limit the cost of somatic maintenance for up to several months per year, so they save extra energy that can be allocated to reproduction (Munshi‐South and Wilkinson, 2010). The low reproductive rate of bats, compared to the greater rate known for other small, shorter lived mammals, allows bats to save resources that are allocated to somatic maintenance, prolonging individual lifespan at the expense of reproduction (Munshi‐South and Wilkinson, 2010). Bat species that exhibit higher reproductive rates have shorter lifespan (Wilkinson and South, 2002), and within species, early breeding may be associated to shorter individual lives (Rhinolophus ferrumequinum; Ransome, 1995).
Moreover, bat mitochondria produce smaller amounts of free radicals per unit of oxygen used compared to other small mammals. For example, in the mouse‐eared bat Myotis lucifugus (living up to 34 years), the amount of hydrogen peroxide produced in the mitochondria per unit of oxygen consumed is 1/2 to 1/3 those recorded, respectively, for the short‐tailed shrew Blarina brevicauda (maximum lifespan of 2 years) and the white‐footed mouse Peromyscus leucopus (maximum lifespan of 3 years), despite all these species show similar activity levels of the antioxidant superoxide dismutase enzyme (Brunet‐Rossini, 2004). According to mitochondrial theories of ageing, mitochondrial DNA (mtDNA) in long‐lived animals should undergo less frequent mutations than in short‐lived species to counter cumulative damage caused by reactive oxygen species, which seems to be the case with bats, whose mtDNA substitution rates are on average two times lower than in rodents, despite the smaller body size of the former (Nabholz et al., 2008).
Finally, the genome and transcriptome of M. brandtii show significant sequence alterations in growth hormone and insulin‐like growth factor 1 receptors, a feature possibly common to other bat species, which might partly account for the outstanding longevity of this species (Seim et al., 2013). Telomere shortening, induced by cell reproduction is associated with the degradation processes typical of ageing, but at least in the especially long‐lived Myotis bat species, highly efficient genetic mechanisms counter this process. Based on long‐term mark‐recapture field data, Foley et al. (2018) found that telomeres shorten with age in R. ferrumequinum and Miniopterus schreibersii, yet not in Myotis myotis or Myotis bechsteinii. Despite the absence of telomerase in Myotis bats, telomere maintenance is carried out by 21 genes that are differentially expressed and are either enriched for DNA repair or are linked to alternative telomere‐lengthening mechanisms.
A.3. Morphology
A bat's body plan for certain aspects reminds that of other mammals, with a noticeable exception, i.e. that forelimbs are modified into wings employed to attain powered flight. Hindlimbs are small to favour flight, and are rotated backwards. The tendons of the foot confer allow the bats to hang upside down with no efforts thanks to a special locking mechanism. Skull structure in laryngeally echolocating bats shows structural characteristics that are strictly related to echolocation (Veselka et al., 2010). Moreover, craniofacial and tooth morphology are linked with biting style and dietary habits (e.g. Dumont et al., 2005; Santana et al., 2012)
A.3.1. Wing structure and its physiological and ecological implications
The bat wing is made of the elongated forelimb (humerus, radius and hand bones, metacarpals and phalanges) whose skeleton supports a double layer of skin named patagium. While the arm and forearm bones sustain the medial section of the leading edge of the wing, the elongated metacarpals and phalanges of fingers 2–5 support the wing surface. The thumb is small, clawed and protrudes from the wing's upper edge. In Pteropodidae, the second digit bears a vestigial claw on the leading edge of the wing. The patagium typically includes the hindlimbs (which in comparison with a typical mammal body plan are rotated backwards and less developed) and the tail. The portion of patagium comprising the tail is called uropatagium, which, along with the wing surface, may be used by insectivorous bats to catch their prey in flight. Hindlimbs may be moved in flight and used to alter wing shape dynamically. Further details are provided in Altringham (2011).
Wings account for ca. 85% of the bat's body surface (Bassett and Studier, 1988). The two layers of skin the patagium is made of comprise a central connective core with collagen and elastic fibres where a rich network of blood vessels, nerves and skeletal muscle fibres is found (Gupta, 1967). Like in birds, the patagium's stratum corneum contains cerebrosides to increase pliability needed during flight (Ben‐Hamo et al., 2016). Qualitative and quantitative differences in the stratum corneum's lipid composition play a role in changing the rate of cutaneous water loss (Muñoz‐Garcia et al., 2012). Because of their large surface and structure and their high degree of exposure to the environment during flight, wings play a major role in controlling heat and water exchange (Thomson and Speakman, 1999). For the same reason, unlike most other mammals, whose small surface‐to‐volume ratio and skin thickness limit gas exchange, in bats the wing's contribution to total gas (CO2 and O2) exchange is in fact substantial (Makanya and Mortola, 2007). For instance, in epauletted fruit bats (Epomophorus wahlbergi), the rate of oxygen consumption of the wings vs. that of whole individual under light anaesthesia, averaged, respectively, 6% (at 24°C ambient temperature) and 10% (at 33°C) of the total, and similar values were recorded for carbon dioxide (Makanya and Mortola, 2007).
The shape of bat wings changes across species and is tightly linked with foraging habitat structure, diet and foraging style (Altringham, 2011), so bat ecomorphological characteristics are powerful predictors of species ecology. The two basic descriptors of bat wing shape are wing loading (WL) and aspect ratio (AR). WL (the ratio between a bat's body mass and its flight membrane area) expresses how large the wing surface is relative to the bat's body size (e.g. Norberg and Rayner, 1987). AR expresses wing shape (wing span2/area; Farney & Fleharty, 1969): fast fliers possess high AR values, i.e. long, narrow wings, while low AR values (short, broad wings) are typical of forest species. Bats that have high WL and AR values such as the European free‐tailed bat and the three species of noctule found in the Old Continent show long, narrow wings and possess a fast, energetically convenient flight, so they can cover long distances and exploit open spaces by hunting on the wing (aerial hawking). On the other hand, these bats are poor manoeuvres in narrow space, such as that typical of dense vegetation. The opposite situation is found in bats showing low WL and AR values, whose wings are typically short and broad, which are best suited to manoeuvre in dense vegetation such as the forest, yet their flight is slow and energetically costly. Intermediate situations are observed in bat species that mostly exploit edge habitats, such as pipistrelles (Pipistrellus spp.), which typically hunt on the wing along forest edges and other linear structures (Verboom and Huitema, 1997), but may fly frequently in open spaces or in moderately dense vegetation (Kalko and Schnitzler, 1993).
The large wing surface of bats and its high degree of vascularisation set the scene for a prime role of this structure within the context of dermal exposure to pesticides, yet this aspect warrants a quantitative assessment.
A.3.2. Ears and noseleaves
As discussed ahead, most living bats possess a biosonar, i.e. a sensorial modality based on the production of ultrasonic echolocation calls to probe the surrounding space in full darkness. This sophisticated system requires remarkable morphological adaptations, such as external ears (pinnae) showing complex shapes and often including ridges, plus, in many species, a tragus, i.e. a cartilaginous structure projecting upwards from the base of the ear. Such structures are needed to increase hearing sensitivity and directionality, which is of prime importance for bats as they need to detect the faint echoes of the echolocation calls that they emit (Altringham 2011). Elongated pinnae such as those of long‐eared bats (genus Plecotus) are used to listen to prey‐generated sound and hunt by passive listening, for instance when feeding on ultrasound‐sensitive prey such as tympanate moths (Anderson and Racey, 1991). Species that glean non‐flying arthropods from off the ground, where they would be difficult to detect through active echolocation, also use passive listening to identify prey and therefore possess long pinnae, such as those of the greater mouse‐eared bat (M. myotis), often preying upon carabids (Arlettaz et al., 2001), or the scorpion‐hunter Otonycteris hemprichii (Holderied et al., 2011).
Although many bat species emit sound through the mouth, some do so through the nostrils, and in such cases, bats often exhibit noseleaves (there are exceptions, however, such as Plecotus spp. and Barbastella barbastellus, which lack them but still broadcast sound through their nostrils). Noseleaves surround the nostrils, are present in bat species that broadcast long, constant‐frequency echolocation calls such as rhinolophids, and work as an acoustic lens that concentrates energy on a focal area increasing the difference in the energy this reflects relative to the periphery (Vanderelst et al., 2010).
A.4. Physiology
A.4.1. Powered flight
Although powered flight in bats is energetically expensive – it may cost twice the energy needed for running (Thomas, 1987) – it is outstandingly efficient in terms of cost of transport, which is five times lower than that faced by a non‐flying mammal of equivalent size (Altringham, 2011). Flight allows bats to cover long distances: among European bats, nightly linear displacements > 10 km are not rare (e.g. Biscardi et al. 2007), and greater noctules Nyctalus lasiopterus may cover up to 130 km on a single night (Popa‐Lisseanu et al., 2009). Thanks to powered flight, bats have access to three‐dimensional landscapes, and insectivorous species may feed on airborne insects even at very high altitudes (Voigt et al., 2018). The ability of covering long distances also enables bats to cope with resources that are scattered and ephemeral, which a non‐flying mammal would, in most cases, miss: other advantages comprise escaping predators effectively, overcoming unsuitable habitat, and, in some cases, migrating over long distances to search for suitable climatic conditions and food in different seasons (Altringham 2011). Flight has surely played a major role in allowing bats to colonise practically the whole Earth except the poles.
How bats evolved powered flight is still unclear. The common view is that flight originated through a complex transition history from a quadrupedal arboreal mammal that possessed the ability of gliding (Bishop, 2008). Molecular studies show that the Prx1 gene (expressing a transcription factor that is involved in limb bone growth) is upregulated in the cartilage and perichondrium of distal limb in bats. Hence, an alteration of this gene may have played a major role in specialising the bat forelimb from a non‐flying ancestor (Cretekos et al., 2008). Both palaeontological and molecular data suggest that the ancestor of modern bats appeared on the scene at the Mesozoic–Tertiary interface (ca. 64 million years ago) in the northern hemisphere (Teeling et al., 2005), but the oldest fossils available are ‘only’ 52 million years old, and judging from wing morphology, those bats already possessed powered flight (Simmons et al., 2008). Therefore, based on current knowledge and in the absence of sufficient fossil evidence, the steps that led to powered flight in modern bats can only be a matter of speculation (Bishop, 2008).
A.4.2. Echolocation
Along with flight, echolocation is one of the most astonishing bat features. Widespread among Chiroptera, it occurs in over 85% of living bat species, i.e. in all those formerly classified as ‘Microchiroptera’ (possessing laryngeal echolocation) plus one megabat genus (Rousettus aegyptiacus) which generates sound by clicking the tongue (Jones and Teeling, 2006). Here, the focus is on laryngeal echolocators. Echolocation is a clear adaptation to being active in the dark, when vision alone would be of little use. Echolocating bats broadcast ultrasonic calls apart from rare exceptions that emit audible sounds (e.g. the European free‐tailed bat Tadarida teniotis; e.g. Russo and Jones, 2002). Bats broadcast series of short (often a few ms) pulses characterised by a high amplitude, yet, being ultrasonic, these undergo rapid atmospheric attenuation (Lawrence and Simmons, 1982). The echoes reflected by the surrounding objects are detected by the bat, which in most cases assesses the time delay occurring between call emission and echo reception to determine target distance. For this system to work, however, no overlap between the outgoing call and the incoming echo is possible, which explains why echolocation calls are so short (e.g. Fenton et al., 2012). Some bats (e.g. rhinolophids, featuring five species in Europe) also rely on the detection of ‘acoustic glints’ generated by the interaction between the long constant‐frequency components of the calls they broadcast and the fluttering of objects they insonify, such as a moth's wing stroke, which generate distinct patterns (Fenton et al., 2012). These bats are adapted to hunt in clutter, so they overcome the problem of acoustic clutter produced by the structurally complex environment they hunt in by recognising the above‐described patterns. These bats also possess an ‘acoustic fovea’ most sensitive to an individual‐specific frequency value, so that when flying, they will lower the frequency of their calls to compensate for the Doppler upwards shift induced by their own movement, and tune the echo in to their preferred frequency value (Schuller and Pollak, 1979). Consequently, the frequency of the incoming echo will be higher than that of the outgoing call, and this separation by frequency, rather than time, still allows the assessment of time delay that the other bats use, even if in this case temporal overlap occurs between call and echo.
Echolocation provides a wealth of information about the surrounding world, since calls often cover a broad frequency spectrum, and just like in the case of visible light, different frequencies will provide different responses, overall showing ‘echo colours’ that inform the emitter about specific properties of the target, such as size and texture (Smith, 2008).
Different bat species show different echolocation call designs depending on the sensorial tasks they face when commuting or foraging. Species that cope with the acoustic clutter typical of dense vegetation such as the forest (e.g. the Natterer's bat Myotis nattereri) often show brief broad‐band echolocation calls whose rich array of frequencies provides many details of their complex surroundings. To the opposite end of the ecological scenario, species that fly in open space (e.g. the common noctule, Nyctalus noctula) typically broadcast calls comprising a narrow range of frequencies, and these are typically lower than in clutter specialists to mitigate atmospheric attenuation: this design is clearly adapted to maximise echolocation's operational range at the expense of discrimination power. Species that exploit a broad range of habitats and often forage along vegetation edges, such as pipistrelles, show calls that combine both designs, which confers on them sufficient flexibility to deal with different spatial contexts (e.g. Russo et al., 2018a,b,c).
Although call structure is, broadly speaking, adapted to different environmental conditions, bats are multiple habitat specialists that commute in a range of habitats, so they may change call design to increase echolocation performances. Therefore, a species that flies in open space such as a noctule, once in cluttered space will shorten call duration and broaden frequency bandwidth, whereas clutter specialists may to some extent show opposite adjustments when flying in open space. Interpulse interval (the time between two consecutive calls) may also change, decreasing in cluttered space (where the echo‐acoustic view needs to be refreshed more frequently), increasing in the open (e.g. Russo et al., 2018a,b,c).
Call structure, duration and interpulse intervals also change according to the task a bat is accomplishing – searching for, approaching or attempting to catch prey, in which case a terminal, or ‘feeding’ buzz, is broadcast (Ratcliffe et al., 2013). The latter is made of a rapid sequence of broad, short calls that are emitted by a bat locking in on a prey item. Buzzes are also emitted by bats when drinking or landing on the ground (e.g. Russo et al., 2007, 2016b).
Further sources of echolocation call variation may be associated with a bat's sex, age, geographic origin or even colony membership, or with different environmental conditions. A comprehensive review of call variation is given by Russo et al. (2018a,b,c).
Echolocation is a vital sense to bats, and its impairment implies serious spatial disorders, and, inevitably, fatal consequences. This may be relevant for pesticides, for example, exposure to imidacloprid may affect adversely the spatial memory of bats through neural apoptosis in hippocampal CA1 and medial entorhinal cortex areas (Hsiao et al., 2016).
Echolocation is the prime sensory modality bats use to find their way in the dark, detect obstacles and food. However, during long‐distance navigation bats are likely to employ also other senses, such as vision (Smith and Goodpaster, 1958; Griffin, 1970; Davis and Barbour, 1970; Tsoar et al. 2011) to identify distant landmarks and follow large‐scale cognitive maps. Bats have also been found to be sensitive to the Earth's magnetic field, i.e. they use a magnetic compass but this is sun‐calibrated recording sunset information when they leave the roost (Holland et al., 2010).
A.4.3. Metabolic rate
Table A.2 shows values of basal metabolic rate (BMR) for 64 bat species differing largely in terms of body mass, geographic origin and food habits. The average BMR value for such species is 37.4 ± 33.7 mL O2/h (range 6.1–174.0 mL O2/h). BMR varies according to a range of factors: for instance, in 30 species of neotropical bats from the family Phyllostomidae, body mass explained most (ca. 80%) of BMR variation, whereas the combination of body mass, food habits, altitude and occurrence on islands vs mainland explained ca. 99% of it (McNab, 2015). Insectivorous bats tend to have BMR values that are slightly lower than those predicted for mammals of their size according to the allometric relationship linking BMR and body size, while bats feeding on vertebrates or plants have BMR values equal or slightly greater than those predicted in this way (McNab, 1982). Insects in temperate regions are an ephemeral resource, so a lower BMR may, from this perspective, be adaptive.
It should also be considered that insectivorous bats from temperate regions are often torpid at rest so in such cases they do not show a significant difference with ambient temperature and hence cannot be assigned a ‘basal metabolic rate’ value, yet they may be assigned a ‘standard’ resting metabolic rate at some fixed value of body temperature (McNab, 1997).
Active metabolic rates in bats are considerably high because powered flight is an energetically expensive activity, in fact twice as costly as running for mammals that move on the ground (Thomas, 1987). In nectar feeding glossophagine bats, metabolic flight power was estimated as 15 times the predicted BMR (Winter and Von Helversen, 1998) – a high figure, yet still 20–25% less than the costs incurred by small birds.
A.4.4. Torpor and hibernation
Bats are endotherms, accomplishing high physiological performances by maintaining they body temperature at a constant level independent of ambient temperatures when active. Endothermy has a cost, however, and when ambient temperatures are low, much energy is needed to keep the body warm and counter the heat lost through the body surface. The amount of heat lost in such circumstances is greater for smaller animals such as bats because of their higher surface to volume ratio. Flight is energetically costly, so active bats also spend massive amounts of energy for foraging, commuting or migrating. Moreover, being small, bats have limited space in their bodies to store fat reserves, and the many insectivorous species rely on highly fluctuating and seasonally variable arthropods as their food source. To cope with this otherwise energetically unsustainable situation, bats behave as facultative heterotherms, i.e. when needed, they downregulate their body temperature to set point values that approach those of ambient temperature. This condition, called torpor, makes it possible to save considerable amounts of energy (Altringham, 2011).
Torpor is often adopted by active bats (in late spring, summer and much of autumn, depending on the climate of the region in question) during daytime roosting. In such cases, it is defined as ‘daily torpor’ because it lasts < 24 h and is associated with nocturnal foraging, while in bat hibernation, torpor periods span across several consecutive days to several weeks and the main source of energy is offered by fat accumulated in the active months (Ruf and Geiser, 2015). In daily torpor, bats arouse in order to leave the roost for their nocturnal foraging. Not all bats can utilise torpor in daytime, depending on their physiological status: torpor may, in fact, slow down physiological activities that require a high body temperature, such as pregnancy or lactation, so in summer, reproductive females use torpor much less than non‐reproductive ones or males (McAllan and Geiser, 2014; Russo et al., 2017a). By the end of summer, it is the turn of males to avoid torpor because they need to maintain high levels of activity for mating. Torpor also represents an emergency strategy useful when food becomes suddenly scarce in the active season, such as on an unusually cold summer night, when being torpid is more convenient than foraging (Altringham, 2011).
Torpor makes it possible to save enormous amounts of energy when it is prolonged for days, weeks or even months, i.e. during hibernation, which takes place in winter months. Especially for insectivorous bat species that occur in temperate regions, winter is in fact a very sensitive time of the year, when ambient temperatures are low and insects are seasonally scarce. Put it simply, hibernation is, therefore, a prolonged form of torpor. Hibernation is not continuous, however, and bats may arouse to drink, urinate or defecate, sleep, mate or even forage on a warm evening. Winter foraging can be especially frequent in regions characterised by a mild climate (Williams et al., 2010).
Torpid bats still regulate their body temperature, so when ambient temperatures drop to values close to 0°C, which might lead to death, the bat will arouse from torpor and become normothermic. Basically, a torpid bat will reduce: (a) body temperature to as little as 1–2°C above ambient temperature; (b) breath rate (interrupting breathing for up to ca. 1.5 h); and (c) heart rate (from 200 to 300 beats at rest to 10 beats); oxygen consumption (which becomes 140 times lower than in a normothermic condition) and metabolic rate; and blood flow, which is restricted to the main organs and does not reach the limbs (Altringham, 2011).
Bats enter hibernation in response to low ambient temperatures; in such phase, they rely on the fat reserves stored during the months of activity to support the highly reduced energy expenditure faced during prolonged torpor. Arousal is spontaneous and sustained by the massive heat release caused by consumption of brown adipose tissue (BAT), concentrated on the back of the bat, whose specific metabolic dynamics allow conversion of energy reserves into heat (Cannon and Nedergaard, 2004). Shivering also takes place to speed up the arousal process, which may last up to ca. 30 min. Arousals are highly costly: a bat arousing itself every second week during hibernation will consume up to 85% of the fat needed to survive winter (Thomas, 1995). Therefore, disturbance to hibernation colonies takes a heavy toll on hibernating bats and their likelihood to survive winter.
Although an extensive use of torpor is typical of insectivorous bats from temperate regions, which need to cope with an ephemeral food resource and seasonally harsh climate, torpor and hibernation also occur frequently in many tropical and subtropical species, suggesting that heterothermy is an ancestral trait common to all bats (Geiser and Stawski, 2011).
The massive fat consumption bats face in winter may expose them to the mobilisation of lipophilic pesticides during hibernation and increase the risk of direct mortality, an aspect warranting appropriate investigation.
A.4.5. Life cycle and reproduction
We will refer to the general life cycle of a bat species from a temperate region. Bats typically enter hibernation in late autumn and become active again around mid‐spring, but significant changes may occur according to the local climate, species, individual status, etc. Mating occurs since the end of summer and may extend over the hibernation period. Several mating strategies occur among the bat species so far studied worldwide, and a useful synthesis is offered in Altringham (2011). Among European species, polygyny is widespread, but this may be more complex and multifaceted than expected. For instance, a deeper insight into the mating system of some species, such as R. ferrumequinum, has revealed that females may mate with the same males over the years and that intra‐lineage polygyny occurs, i.e. relatives in the maternal line such as mothers and daughters, tend to share breeding partners (Rossiter et al., 2005). In some species, a ‘lek’ system exists, with males advertising themselves by calling from the roost (Nyctalus noctula: Sluiter and Van Heerdt, 1964) or performing songflights (Pipistrellus pipistrellus and Pipistrellus pygmaeus: Lundberg and Gerell, 1986; Jones, 1997). In other species (Plecotus spp., Myotis spp., B. barbastellus and Eptesicus spp.), numerous bats (whose sex ratio is strongly biased towards males), often from several species, swarm at night in underground sites, perhaps where males lek to attract females, and mating takes place (Glover and Altringham 2008; Altringham, 2011). The distances bats cover to reach swarming sites may be considerable (Parsons et al., 2003), and swarming sites represent hot spots of genetic diversity often overlooked, yet vital, in conservation plans (Kerth et al., 2003a; Veith et al., 2004).
The typical reproductive cycle of bats of temperate regions is monoestry, which leads to one, more rarely two young per year (Altringham, 2011). In most cases, European bats show a delayed fertilisation mechanism, i.e. mating takes place in late summer, autumn or winter, when females store the sperms in their genital tract; females ovulate only once they arouse from hibernation, so mating and fertilisation may occur months apart. This mechanism allows for an optimal matching between the time of female reproduction, when they face a high energy demand, and the seasonal abundance of insect food, which peaks right at the time when young are delivered and milked. All European bat species exhibit delayed fertilisation except one, Miniopterus schreibersii, in which fertilisation is not delayed and embryonic development starts immediately after it, but the embryo implants in the uterus only after several months so that embryo's further development, parturition and lactation take place in summer, when sufficient food is available. Delayed implantation is a common strategy in other mammal orders, e.g. ungulates (Aitken, 1974) and carnivores (Mead, 1989).
Lactation lasts for about a month, after which the young attains a body size close to that of adults, which means that in the final part of lactation, the mothers need to supply milk to young that are almost their own size (Kunz et al., 1995b). Before weaning, the young are unable to forage independently. With reference to pesticide risk, it is important to remark that considerable amounts of lipophilic pesticides may concentrate in milk and be consumed by the young during lactation. For example, after an application of methamidophos to potato fields and apple orchards in Germany, high mortality was recorded among juvenile M. myotis, and pesticide residues were likely transferred to them via the milk after their mothers had fed on contaminated insects (Hofmann and Heise, 1991).
A.5. Ecology and behaviour
A.5.1. Foraging ecology and behaviour
European bats show a range of species‐specific foraging strategies that allow them to get access to habitats characterised by a different degree of structural complexity, or ‘clutter’. The ability of a given species to exploit a certain habitat structure for foraging depends on a combination of wing morphology and echolocation call design.
Echolocation call design is tailored to exploit different habitat structures, which explains why it differs among species (e.g. Jones and Holderied 2007; Russo et al., 2018a,b,c). The species that hunt in open space broadcast echolocation calls at lower frequencies, and exhibit narrower frequency bandwidths, than those hunting in cluttered habitat. Calls made of low frequencies and showing a narrow bandwidth probe long distances but provide low resolution, so they perform well in open habitat but cannot cope with the complexity of dense vegetation, whereas calls with high frequencies and broader bandwidth undergo strong atmospheric attenuation, so that their operational range is limited, but provide many details about the surroundings, which is vital in structurally complex environments.
Finally, rhinolophids (Rhinolophus spp.) often adopt a ‘sit‐and‐wait’ strategy, known as perch‐hunting, in forest habitats or at the interface between forest and open habitat (Jones and Rayner, 1989): the bat hangs to a small branch, explores the surroundings with its specialised, high‐frequency biosonar (mostly relying on Doppler‐shift compensated calls that detect flutter) and as soon as prey is detected, takes off to seize it. Prey is typically dismembered and eaten while perching (Jones and Rayner, 1989).
A limited number of studies have assessed food intake in insectivorous bats, and although not everyone agrees on the estimates available in the literature, there is no doubt that bats consume many arthropods per night, and obviously this has major consequences in terms of oral exposure to pesticides.
One way to establish daily food intake is to measure it from captive subjects, yet this is likely to provide only a gross underestimate of the actual energy needs of free‐living bats. Based on studies done in captivity on North American bats, a single individual would eat the equivalent of up to 25% of its body mass in insects per night (Brisbin, 1966; Neuhauser and Brisbin, 1969; O'Farrell et al., 1971; Coutts et al., 1973). Estimates for free‐living bats show much higher food intakes, yet these vary depending on the bat's physiological status, and tend to be highest for females at their lactation peak. By analysing the turnover of doubly labelled water, a lactating M. lucifugus is estimated to consume up to 130% ca. of its body mass in insects (Kurta et al., 1989a,b), while in lactating Tadarida brasiliensis this figure would be as high as ca. 70% ca. (Kunz et al., 1995a). Since large prey are dismembered and their wings, heads and legs often discarded, the number of prey items eaten is actually larger than that estimated from the biomass consumed only. Other experimental evidence points at intake rates of wet food as high as 0.5 g/g body mass per day. For instance, Anthony and Kunz (1977) compared pre‐feeding vs. post‐feeding body weights of M. lucifugus (ca. 10 g body mass) returning to their roosts after two main nocturnal foraging bouts and established that pregnant females ate, on average, 2.5 g of prey per night, while this figure reached 3.7 g in lactating females and was 1.8 g in juveniles.
When prey is abundant, bats may show impressively high foraging rates. These are often estimated from the numbers of feeding buzzes (sequences of rapidly emitted echolocation calls that precede prey capture), but feeding buzzes represent foraging attempts, whether successful or not, so they may overestimate the actual number of arthropods caught. For instance, in three 5‐min foraging bouts, a daytime foraging P. pygmaeus broadcast 18.0 ± 5.8 feeding buzzes/min (Russo et al., 2011b). Assuming 50% foraging success, this would translate into > 1600 insects caught in 3 hours, which would account for a significant biomass despite the fact that in that case bats preyed upon small dipterans (Coelosia truncata), but of course such estimates should consider the actual foraging success, the seasonally changing individual‐specific energy requirements, variability in prey availability, and satiation.
From direct observations of foraging behaviour in Myotis daubentonii, Encarnação and Dietz (2006), a species scooping small prey (often chironomids) from the water surface over rivers, lakes and canals estimated that, using a high (92%) capture‐success model, daily insect intake of females was 8.0 g during pregnancy and 4.9 g during post‐lactation, corresponding to 5.0 and 3.0 kJ of energy taken daily per body mass gram. Males caught ca. 3.6 g insects per day in late spring, but this amount raised to 8.0 g at the peak of spermatogenesis, respectively, providing 2.6 and 5.7 kJ/(g body mass × day).
A.5.2. Habitat use
For the reasons mentioned above, different guilds may be recognised among bat species according to the structure of the habitat they forage in and the foraging mode they adopt, which are in turn consequences of their wing morphology and echolocation call design. Denzinger and Schnitzler (2013) divided bats into: (a) ‘open space aerial foragers’, hunting on the wing in open space; (b) ‘edge space aerial foragers’, mostly foraging along vegetation edges such as treelines, hedgerows or forest margins; (c) edge space trawling foragers, scooping prey from water surface (insects and in some cases fish); and day) narrow space foragers, hunting in cluttered vegetation. The latter can be further divided according to their foraging strategy into narrow space (active or passive) gleaners and narrow space flutter detecting foragers. Gleaners may detect prey using active echolocation or passively by listening to prey‐generated sound, while flutter detectors evaluate flutter information encoded in the echoes of their long, constant‐frequency calls corresponding to modulation in sound frequency and amplitude generated by prey wingbeat.
Within species, some flexibility in choosing habitats of a given structure may be possible, such as in pipistrelles, which are often observed foraging along vegetation edges but may also hunt in open space. Flexibility is mostly associated with habitat‐dependent, intraspecific variation in echolocation call structure (Russo et al., 2018a,b,c).
Bats often travel long distances from their roosts to the foraging sites. Distances of several kilometres on a single night are common in European bat species, sometimes up to > 20 km (Biscardi et al. 2007). Thanks to this remarkable mobility, bats may reach, and exploit, a range of foraging sites, and in many cases behave as multiple habitat specialists, like other terrestrial vertebrates (e.g. Law and Dickman, 1998). The degree of specialisation in a given habitat type is, however, variables, from generalist species such as Kuhl's or common pipistrelles (e.g. Russo and Jones, 2003), which can forage in a broad range of habitats, to the highly specialised foraging style of, e.g. trawling bat species such as Myotis capaccinii or M. daubentonii, which forage almost exclusively in riparian sites (Biscardi et al., 2007; Nardone et al., 2015).
Many European bat species forage in farmland (see Appendix C), and those that have been documented to do so are probably only a fraction of the actual number, as shown by recent diet analysis using state‐of‐art molecular techniques (Russo et al., 2018a,b,c). Farmed landscapes provide an array of habitat structures that span from open spaces (e.g. a maize field) to structurally complex woody habitat (orchards, olive groves, or residual woodland patches). Such landscapes are also rich with ecotones (treelines, hedgerows, edges between woodland patches and pastures or open fields) that may be exploited by edge foragers (Boughey et al., 2011), while canals and ponds may be used by trawling species such as M. daubentonii and, especially if bordered with vegetation, support many insects and thus significant bat activity (e.g. Russ and Montgomery, 2002). Besides hosting species that forage on the wing, gleaners and flutter‐detection foragers are also present in farmland. For example, intensive apple orchards are used by gleaning M. myotis (Drescher, 2004), while the ecotone between woodland and pastures often constitutes an important foraging habitat for perch‐hunting R. ferrumequinum (Duvergé and Jones, 2003).
Bats are very sensitive to habitat fragmentation, and in farmland they often need linear landscape elements such as hedgerow networks not only to forage but also to cross otherwise hostile landscapes (Russo et al., 2002; Boughey et al., 2011; Frey‐Ehrenbold et al., 2013; Monck‐Whipp et al., 2018). Structural heterogeneity such as that of traditional farmland, offering high connectivity, is important to sustain diverse bat assemblages (Wickramasinghe et al., 2003; Davy et al., 2007). The higher activity recorded at organic sites compared to conventional sites in some studies (e.g. Wickramasinghe et al. 2007) is likely to be due to a combination of multiple‐scale factors that are different to disentangle in analyses. However, the importance of intensive farmland should not be dismissed, since there is overwhelming evidence that bats also forage frequently in such habitat in a range of landscapes across Europe (e.g. Heim et al., 2016; Stahlschmidt et al., 2017; Appendix C). Bats that forage in intensive agricultural landscapes tend to narrow their trophic niche concentrating on agricultural pests, as seen in M. schreibersii (Aizpurua et al., 2018), which potentially implies a high risk of exposure to pesticides.
A.5.3. Roosting ecology and behaviour
Bats spend over half of their lives in the roost, where they are sheltered from adverse weather and predation, and where they carry out many key activities of their life cycles including food digestion, hibernation, mating, parturition and nursing. Roost characteristics such as roost structure and microclimate largely vary across species and geographic ranges, but the same individual may typically change roost according to its physiological requirements (e.g. hibernation, mating or reproduction) or age. Tree‐dwelling bats are typically less faithful to roosting sites and may switch between trees often, even on a daily basis (e.g. Russo and Jones, 2003; Russo et al., 2017a). Bats are often highly social and form colonies that can be very numerous, but at night their social groups typically split to reach foraging sites, so most social interactions too occur within roosts. Bats use a considerable range of roost types across the globe, but those of temperate regions may be broadly classified as underground sites (natural or artificial), overground sites (buildings) and tree cavities. A thorough description of roost structure and roosting behaviour lies beyond the scopes of this work, and much information is available in the literature (see e.g. Kunz, 1982; Kunz et al., 2003; Barclay and Kurta, 2007). Much of the evidence concerning contamination of bats in their roost regards the effects of roofing timber treatments such as lindane, pentachlorophenol, and pyrethroids, which may have adverse consequences for bats (Mitchell‐Jones et al., 1989; Swanepoel et al., 1999; Voigt et al., 2016). It is important, however, to stress that roosts are often found in farmland, and in specific cases there is some risk of direct contamination through inhalation or dermal contact when roosts are found where pesticides are spread, for example in rural buildings (barns, stables) or cavities in trees (e.g. in treelines or hedgerows), particularly when bats roost in shallow cracks of exterior walls (Marnell and Presetnik, 2010), or in shallow tree cavities such as underneath flaking bark (Russo et al., 2017a). Because bats often use a given roost for several weeks or months and may reutilise it over years, slow poisoning of synanthropic bats in the roost, where they might not die immediately but suffer sublethal effects over time, is possible (Voigt et al., 2016). The risk may be more pronounced when bats use night‐roosts to pause from nocturnal activity, in case pesticides are spread in the evening, because in such cases bats may roost in exposed outbuildings such as woodsheds or porches (Knight and Jones, 2009) or even hang from trees (Russo et al., 2002).
A.5.4. Drinking behaviour
Bats from temperate regions drink regularly to compensate for the significant amounts of water that they lose by transpiration through their body surface, especially via the respiratory system and the large surfaces of wing membranes.
In some cases, morphological or physiological adaptations may limit water loss, such as specific features of the lipid matrix in the epidermis’ stratum corneum (Muñoz‐Garcia et al., 2012), but drinking is definitely the principal way bats cope with dehydration, as much as they even arouse from hibernation to drink (Speakman and Racey, 1989; Boyles et al., 2006). Factors such as the disappearance of ephemeral water sites in response to climate change (Korine et al., 2016) or the influence of artificial illumination at drinking sites (Russo et al., 2017b, 2018a) are therefore seen as considerable threats to bats. Bats also take key minerals from drinking water, such as the calcium needed by lactating females to replete the amount mobilised to allow skeletal development in pups (e.g. Bernard and Davison, 1996), or sodium, especially in tropical climates, where this element is limiting (Bravo et al., 2010). Frugivorous bats may also exploit some water minerals to neutralise the otherwise adverse effects of certain secondary plant metabolites occurring in the fruits they feed upon when large amounts of the latter are taken at times of great energy demand (Voigt et al., 2008).
Drinking water constitutes one of the potential routes of bat exposure to pesticides. In fact, bats drink at a range of water sites (Korine et al., 2016), including those found in farmland such as irrigation canals or small ponds which are potentially contaminated with pesticides.
A.5.5. Other behavioural aspects relevant to pesticide contamination
Bats are highly social mammals and exhibit a complex social behaviour, a multifaceted communication and an array of social systems, the description of which are outside the scope of this work. While some aspects have been covered elsewhere in this document, comprehensive reviews on bat social behaviour are provided by Kerth (2008), Altringham (2011), Carter and Wilkinson (2013) and Chaverri et al. (2018). Here, the focus is on clustering behaviour and social grooming, which may have implications for contamination by pesticides.
Bats often form very numerous colonies both during hibernation and reproduction, which may often comprise thousands and up to several million individuals (Betke et al., 2008). In such situations, it is common to observe many individuals associated in clusters. When bats are normothermic, such as reproductive females in summer, clustering is needed to accomplish social thermoregulation and helps reduce the costs of homeothermy by protecting the individual roosting environment from changes in ambient temperature (e.g. Willis and Brigham, 2007; Russo et al., 2017a,b). Several bat species also cluster in hibernation, in which cases they maintain higher body temperatures than solitary hibernators and tend to select cooler caves to hibernate (Altringham, 2011). Clusters may be formed by one or more species, and the tight contact between individuals provides a tremendous opportunity for intra‐ and interspecific social interactions (Ancillotto et al., 2012, 2014). Newborns often cling to the mother's fur or form tight aggregations within the cluster of adults (Ancillotto et al., 2014).
When roosting, bats spend a substantial amount of time self‐grooming, i.e. scratching and licking their fur and wing membrane to keep their bodies clean and reduce parasite load (Ter Hofstede and Fenton, 2005). Moreover, social grooming – the cleaning of an individual's fur by a conspecific – is also frequent, and its role is likely that of strengthening social bonds within colonies and pacifying the subject groomed. Although this behaviour is especially frequent in vampire bats Desmodus rotundus, probably in relation to their unique habit of sharing food among colony members (Wilkinson, 1986; Carter and Leffer, 2015), it is also commonly observed in European insectivorous bats (Kerth et al., 2003b; Ancillotto et al., 2012, 2014). At their activity peak within the roost, Pipistrellus kuhlii and Hypsugo savii may self‐groom considerably (mean% time spent self‐grooming, respectively, 22.8% and 18.5%) and social grooming is also frequent (7.8% and 6.9%, respectively) (L. Ancillotto and D. Russo, unpublished). Both self‐ and social grooming represent a way through which pesticide residues collected on the fur and the wing membranes may be ingested. Hairless juveniles in clusters or clinging to their mother's fur might also be affected by the tight contact with adults whose fur has been exposed to pesticide residues during foraging.
In vampire bats, social grooming has been largely used to cull them in regions where they carry diseases to livestock such as rabies and represent a cause of economic loss and a health hazard. One of the techniques used to cull them relies on coating some individuals with anticoagulants and releasing them so that, once returned to the roost, they will contaminate others through mutual contact in clusters and social grooming (Linhart et al., 1972; Johnson et al., 2014). Vampire bats form multi‐specific colonies, so this culling approach has brought about adverse consequences for bat conservation, also killing non‐target species besides the focal one (Johnson et al., 2014). The topical treatment of vampire bats for culling demonstrates that contaminants, including pesticides, may easily spread to most or all colony members from just a few contaminated subjects.
A.6. Conservation
Conservation
Although recent estimates of positive population trends in European bat populations have led to some optimism (Van der Meij et al., 2015), the situation of many bat species is still concerning, and at the national scale, bats are still on the decline in several EU countries.
Bats are adversely affected by many pressures associated with human action on the environment. Bats depend on roost availability, so detrimental effects are linked to human disturbance at underground (Mitchell‐Jones et al., 2007) or aboveground (Marnell and Presetnik, 2010) roosts, destruction or renovation of roost buildings (Voigt et al., 2016), deliberate exclusion of colonies from human‐made structures (Stone et al., 2015a), or intensive forest management, which reduces the availability of dead or veteran trees bearing cavities suitable for roosting (Russo et al., 2016a,b).
Foraging habitats are often destroyed, fragmented or polluted. Bats are highly sensitive to the quality of riverine systems, where many species forage, and pollution and destruction of riparian vegetation may alter food availability (De Conno et al., 2018). Forests are also important for foraging, so deforestation, habitat fragmentation (Russo et al., 2016a,b) and large‐scale wildfires (Bosso et al., 2018) all affect bats negatively. Loss of structural and temporal heterogeneity in farmland and the spread of pesticides are seen as main factors threatening the bat species that occur in agricultural land (Park, 2015). Light pollution also poses a significant threat: while artificial lighting may favour a few opportunistic species that take advantage of insects concentrating near street lamps, it is repulsive to many others, excluding them from roosting, foraging and drinking sites or severing their commuting routes (Stone et al., 2015b). Invasive species and domestic cats also put bats at risk and locally kill them by large numbers (Ancillotto et al., 2013; Welch and Leppanen, 2017).
Finally, although wind energy development is seen as one of the actions needed to mitigate the negative consequences of climate change on the planet and its biodiversity, wind turbines located along commuting routes, at foraging sites or near roosts may kill impressive numbers of bats (Arnett et al., 2016).
Ecosystem services
Bats are important providers of ecosystem services worldwide, including pollination, seed dispersal, and insect consumption (Kunz et al., 2011). While the first two services are restricted to tropical regions, arthropod consumption also occurs in temperate regions, including Europe. Insect consumed by bats often comprise agricultural or forest pests, and modern molecular approaches to assessing bat diet have revealed that even species that were not suspected to play this role, in fact, eat considerable numbers of pest species (Russo et al., 2018a,b,c). Schreiber's bat Miniopterus schreibersii is, from this viewpoint, a good example: this cave‐dwelling species was often thought to be associated with natural habitats, but in fact, feeds opportunistically on over 20 major agricultural pests across Europe (Aizpurua et al., 2018). Insect pests increase considerably in plots from which bats are experimentally excluded (Maine and Boyles, 2015), and bat boxes set in agricultural land may help suppress large numbers of pest insects, see e.g. P. pygmaeus roosting in bat boxes in the Ebre Delta, where this bat acts as an important consumer of the rice borer moth Chilo suppressalis (Puig‐Montserrat et al., 2015). The effects of bat foraging on pests are only partly understood, and it is thought that besides direct pest suppression, bats might also exert indirect effects on ultrasound‐sensitive prey such as tympanate moths. The latter perform evasive manoeuvres when they detect bat echolocation calls, which may imply a decrease in the pest's success in key vital activities such as foraging, mating or egg laying, thus reducing crop damage (Russo et al., 2018a,b,c).
The economic benefit provided by bat insectivory to agriculture is overwhelming: in North America alone, this accounts for 3.7 billion USD per year (Boyles et al., 2011). In South Africa, bat predation services avoid costs between 9% and 23% of the current estimated cost of damage that stinkbugs cause every year to macadamia orchards, i.e. 613 USD/ha (Taylor et al., 2018). Although no economic assessment is available for Europe, there is consensus over the fact that its magnitude must be similar to, if not greater than the benefits brought about by bat predation in the regions for which estimates are available.
In Europe, bats also make an outstanding contribution to vertebrate diversity, so they are strictly protected across the EU. The main legal framework under which all EU bat species and their habitats are protected is the 92/43/EEC ‘Habitats’ Directive. The Directive, enforced in all EU countries, establishes that all bat species are of ‘community importance’ and that protection of 13 of them requires the designation of Special Areas of Conservation.
Besides prohibiting the killing of bats, the Directive states that disturbing them is also forbidden, particularly during the period of breeding, rearing, hibernation and migration, and that their roosts are also strictly protected since it is illegal to deteriorate or destroy breeding sites or resting places (Art. 12). The Directive also puts special emphasis on the conservation of many natural and semi‐natural habitats across Europe that are also important for bats for roosting and foraging. Another main conservation framework for European bats is offered by EUROBATS, the Agreement on the Conservation of Populations of European Bats, which came into force in 1994 and to date includes 36 out of 63 range states. Its aims are conserving all European species by promoting appropriate legislation, raising awareness about the importance of preserving bats, and favouring the promotion of conservation measures and international cooperation. Due to the migratory habits of several bat species and the necessity to adopt a broad geographic scope to achieve sound conservation, EUROBATS strongly encourages cooperation of parties and non‐party range states not only across Europe but also in Northern Africa and the Middle East.
Appendix B – Literature review
1.
A literature review was undertaken following a structured, stepwise process to retrieve information to investigate whether bat species are already sufficiently covered by the current risk assessment schemes for birds and mammals. Information was retrieved on topics as laid down in the Terms of Reference.
The literature searches were performed in the following databases: Web of Science (platform encompassing many databases), PubMed and Scopus. Furthermore, the EFSA library was used to obtain additional sources such as reports on specific topics related to bats. All searches were limited to studies published in English, French, German and Dutch, which are in concordance with the languages spoken by the reviewers. No further limits to study design and year of publication were applied due to the small amount of sources available on bats.
For all topics covered in the literature review, relevant sources were selected from the databases using the keywords listed in Table B.1, which cover the topics of the Terms of Reference. For every search that was run, a keyword was combined with the following search string:
Table B.1.
Overview on the number of hits in the literature search and the number of articles included after first screening
| Keywords | Date on which the search was conducted | Total amount of hits in all three databases | Total amount of included articles after first screening |
|---|---|---|---|
| Population trends/colony size/population ecology | 23/8/2018 | 3,475 | 33* |
| Biocides/timber treatment/wood treatment | 30/7/2018 | 17 | 0 |
| Dermal/skin | 10/8/2018 | 107 | 11 |
| Energy | 25/6/2018 | 328 | 14 |
| Food/Feed/Diet | 14/8/2018 | 224 | 74 |
| Gas exchange/Air exchange | 30/8/2018 | 214 | 15 |
| Grooming | 10/8/2018 | 228 | 29 |
| Habitat | 06/6/2018 | 610 | 57 |
| Metabolism | 25/9/2018 | 1,149 | 97 |
| Metals | 30/7/2018 | 1,501 | 60 |
| Pesticides | 30/7/2018 | 1,249 | 90 |
| Physiology | 6/6/2018 | 429 | 80 |
| Risk assessment | 30/7/2018 | 354 | 30 |
| Veterinary drugs/veterinary medicines/veterinary pharmaceuticals | 30/7/2018 | 9,394 | 0 |
Relevant sources on these topics were identified by using the EFSA library in order to obtain reports on general information on population trends and ecology. Articles identified in the Web of Science, PubMed and Scopus databases were not included for the data extraction as these articles did not provide relevant information.
(Chiroptera OR Bat OR Bats OR Pteropodidae OR Rousettus OR Rhinolophidae OR Rhinolophus OR Vespertilionidae OR Myotis OR Nyctalus OR Eptesicus OR Vespertilio OR Pipistrellus OR Plecotus OR Barbastella OR Miniopteridae OR Miniopterus OR Molossidae OR Tadarida OR Megabat OR Megabats OR Serotine OR Pipistrelle OR Barbastelle)
The searches were run between June and September 2018. The total amount of hits for every search for all databases used (this is including duplicates), as well as the number of relevant articles after the first screening are summarised below in Table B.1.
As a first step, the title and abstracts of papers were scanned for relevance to exclude obviously irrelevant sources. Articles were considered relevant when the keywords were mentioned in the title and/or abstract and the source related to bats. Relevant articles after first screening were further analysed with a full‐text screening. Articles were considered relevant in the full‐text screening when they related to the topics of the Terms of Reference. In this step, additional relevant papers were retrieved from the reference lists of the screened articles. Articles identified in searches on ecological parameters (e.g. diet and habitat) were only classified as relevant if they concerned European bat species. For all other topics, studies on non‐European bats were considered relevant as well.
Information was retrieved from 254 articles and 33 additional sources (such as reports and book chapters obtained from the EFSA library) and collected in excel data collection sheets. These sheets were designed to summarise all information on the desired topics. Different sheets were developed for the extraction on different topics:
Chemical exposure
In which information was summarised for every article on the bat species studied, the country where the study was conducted, study type (field or laboratory study), the chemical tested, exposure route (only for laboratory studies, e.g. dermal or dietary), exposure time (only for laboratory studies), measured endpoints and main findings.
Biological and ecological traits
In which information was collected on the following parameters: diet (foraging habitat, foraging behaviour/strategy, diet composition, time of emergence), body size, body weight, roosting sites, life history traits, energy requirements, metabolism, reproduction (lactation, pups, gestation/birth).
Appendix C – Evidence of bat foraging in farmland across Europe
1.
Table C.1.
Evidence of bat foraging in farmland across Europe classified according to species, agricultural type and management
| Species | Country | Agricultural type | Degree of intensification/description of sites | Reference |
|---|---|---|---|---|
| Rhinolophus euryale | Italy | Olive groves | Patches of olive groves managed traditionally interspersed with broadleaf forest | Russo et al. (2002) |
| Rhinolophus ferrumequinum | Greece | Organic and conventional olive groves |
Organic: Olive (Olea europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Italy | Olive groves | Olive groves: Olive Olea europea L. groves | Russo and Jones (2003) | |
| Rhinolophus hipposideros | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Rhinolophus mehelyi | Spain | Traditional ‘dehesas’ | Open oak forest managed traditionally for agriculture and livestock breeding | Russo et al. (2005a,b) |
| Rhinolophus total | UK | Conifer plantation | Not described | Vaughan et al. (1997) |
| Greece | Organic and conventional olive groves | Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) | Davy et al. (2007) | |
| Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray | Davy et al. (2007) | |||
| Barbastella barbastellus | Germany | Meadow, forest edge (species was only observed 3× in total in the study) |
Meadow: agricultural grasslands with differing management intensities Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Eptesicus nilssonii | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: Apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Sweden | Farmland | Not described | Rydell (1986) | |
| Finland | Grassland (grouped data; including meadows, arable land and pasture) | Agricultural land covered approximately 8% of the total study site. Intensification degree not described | Wermundsen and Siivonen (2008) | |
| Eptesicus serotinus | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: Apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Orchard | Orchard: mature commercial apple orchard (Braeburn variety). With approximate size of 4 ha. The entire 4 ha of the orchard were sprayed with Reldan (Dow AgroSciences) at a rate of 337 g a.i./ha against on one occasion (20 May 2009) and with Insegar (Syngenta Agro) at 150 g a.i./ha on two occasions (1 and 15 July 2009) | Stahlschmidt and Brühl (2012) | |
| UK | Arable land | Not described | Vaughan et al. (1997) | |
| UK | Pasture (organic and conventional), Arable land (conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| The Netherlands | Agricultural fields (only type of land assessed in the study) | Old agricultural landscape type with many linear elements. Meadows and fields are separated by a network of hedgerows and tree lanes. Agricultural intensification not specified | Verboom and Huitema (1997) | |
| Italy | Arable land | Arable land: generally characterised by a relatively complex mosaic of fields separated by tree lines, hedges, canals, etc. | Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Poland | Arable land | Arable fields: large‐scale arable fields as a main land use type. Degree of intensification is not described | Pluciński et al. (2015) | |
| Poland | Arable land, meadows/pastures, Orchards | Arable land: Land outside settlements, used for growing any crop including rye, barley, wheat, rapeseed, maize, beet, potatoes, cucumbers or carrots (including fallows) | Ciechanowski (2015) | |
| Meadow/Pasture: Grassland outside settlements used for hay production (mowed) or livestock grazing | ||||
| Orchards: Area used for growing vegetables, flowers or fruit, with at least 50% planted by fruit trees or bushes | ||||
| Eptesicus/Nyctalus total | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: Apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest. Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Hypsugo savii | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Italy | Chestnut woodlands, arable land, olive grove, conifer plantations |
Chestnut woodlands: Sweet chestnut Castanea sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees Arable land: generally characterised by a relatively complex mosaic of fields separated by tree lines, hedges, canals, etc. Olive groves: Olive O. europea L. groves Conifer plantation: Conifer (Pinus spp.) plantations |
Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Myotis bechsteinii | Germany | Forest edge, meadow, vineyard, vegetable field |
Meadow: agricultural grasslands with differing management intensities Forest edge: cereal sampling site that were situated 100 m away from a forest. Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| UK | Pasture (conventional) | Not described | Wickramasinghe et al. (2003) | |
| Myotis blythii | Switzerland | Meadows, pasture fields, orchards |
Meadows: freshly cut, or among hedgerows in meadows Pasture: along hedgerows in pasture fields Orchards: intensively cultivated |
Arlettaz (1996) |
| Myotis brandtii/mystacinus | Germany | Forest edge, meadows, vineyards, orchards, vegetable fields, cereal fields |
Meadows: agricultural grasslands with differing management intensities Orchards: Apple orchard Forest edges: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Finland | Grassland (grouped data; including meadows, arable land and pasture) | Agricultural land covered approximately 8% of the total study site. Intensification degree not described. | Wermundsen and Siivonen (2008) | |
| Myotis capaccinii | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practise ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Myotis daubentonii | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities. Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| UK | Arable land, conifer plantation | Not described | Vaughan et al. (1997) | |
| UK | Pasture (organic and conventional), Arable land (conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Myotis emarginatus | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Myotis myotis | Germany | Forest edge, meadow, vineyard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Meadows, fields | Not described | Zahn et al. (2005) | |
| Switzerland | Meadows, pasture fields, orchards |
Meadows: freshly cut, or among hedgerows in meadows. Pasture: along hedgerows in pasture fields Orchards: intensively cultivated |
Arlettaz (1996) | |
| Portugal | Agricultural fields | Agricultural field: sites with short ground vegetation. Degree of intensification not specified | Zahn et al. (2007) | |
| Italy (South Tyrol) | Apple orchards | Orchards: Intensively cultivated | Drescher (2004) | |
| Myotis mystacinus | Germany | Orchard | Orchard: mature commercial apple orchard (Braeburn variety). With approximate size of 4 ha. The entire 4 ha of the orchard were sprayed with Reldan (Dow AgroSciences) at a rate of 337 g a.i./ha against on one occasion (20 May 2009) and with Insegar (Syngenta Agro) at 150 g a.i./ha on two occasions (1 and 15 July 2009) | Stahlschmidt and Brühl (2012) |
| UK | Pasture (organic and conventional), arable land (conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Myotis nattereri | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Orchard | Orchard: mature commercial apple orchard (Braeburn variety). With approximate size of 4 ha. The entire 4 ha of the orchard were sprayed with Reldan (Dow AgroSciences) at a rate of 337 g a.i./ha against on one occasion (20 May 2009) and with Insegar (Syngenta Agro) at 150 g a.i./ha on two occasions (1 and 15 July 2009) | Stahlschmidt and Brühl (2012) | |
| Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) | |
| Myotis total | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) | |
| Myotis and Plecotus spp. | UK | Arable land, conifer plantations | Not described | Vaughan et al. (1997) |
| Nyctalus leisleri | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| UK | Arable land, conifer plantation | Not described | Vaughan et al. (1997) | |
| UK | Pasture (organic and conventional), Arable land (conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Italy | Chestnut woodlands, olive grove (only 1 pass recorded) | Chestnut woodlands: Sweet chestnut C. sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees | Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Nyctalus noctula | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Orchard | Orchard: mature commercial apple orchard (Braeburn variety). With approximate size of 4 ha. The entire 4 ha of the orchard were sprayed with Reldan (Dow AgroSciences) at a rate of 337 g a.i./ha against on one occasion (20 May 2009) and with Insegar (Syngenta Agro) at 150 g a.i./ha on two occasions (1 and 15 July 2009) | Stahlschmidt and Brühl (2012) | |
| Germany | Arable fields | Arable fields: cultivated with the locally prevailing crop types corn (N = 27), canola (N = 34) and wheat (N = 32) | Heim et al. (2016) | |
| UK | Arable land, conifer plantations | Not described | Vaughan et al. (1997) | |
| UK | Pasture (organic and conventional), Arable land (conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Poland | Arable land | Arable fields: large‐scale arable fields as a main land use type. Degree of intensification is not described | Pluciński et al. (2015) | |
| Poland | Arable land, meadows/pastures, Orchards |
Arable land: land outside settlements, used for growing any crop including rye, barley, wheat, rapeseed, maize, beet, potatoes, cucumbers or carrots (including fallows) Meadow/Pasture: grassland outside settlements used for hay production (mowed) or livestock grazing Orchards: area used for growing vegetables, flowers or fruit, with at least 50% planted by fruit trees or bushes |
Ciechanowski (2015) | |
| Nyctalus sp. | Greece | Organic olive groves | Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) | Davy et al. (2007) |
| Pipistrellus kuhlii | Greece | Organic and conventional olive groves | Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) | Davy et al. (2007) |
| Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray | ||||
| Italy | Chestnut woodlands, arable land, olive grove, conifer plantations |
Chestnut woodlands: sweet chestnut C. sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees Arable land: generally characterised by a relatively complex mosaic of fields separated by tree lines, hedges, canals, etc. Olive groves: Olive Olea europea L. groves Conifer plantation: Conifer (Pinus spp.) plantations |
Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Pipistrellus nathusii | Germany | Meadow, vineyard, orchard, vegetable fields, cereal fields, forest edge |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Arable fields | Arable fields: cultivated with the locally prevailing crop types corn (N = 27), canola (N = 34) and wheat (N = 32) | Heim et al. (2016) | |
| Poland | Arable land | Arable fields: large‐scale arable fields as a main land use type. Degree of intensification is not described | Pluciński et al. (2015) | |
| UK | Organic pasture | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Pipistrellus kuhlii/nathusii | Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) |
| Pipistrellus pipistrellus | Germany | Meadow, vineyard, orchard, vegetable fields, cereal fields, forest edge |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification of agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Orchard | Orchard: mature commercial apple orchard (Braeburn variety). The approximate size of the orchard was 4 ha. The entire 4 ha of the orchard were sprayed with Reldan (Dow AgroSciences) at a rate of 337 g a.i./ha against the woolly apple aphid (Eriosoma lanigerum) on one occasion (20 May 2009) and with Insegar (Syngenta Agro) against the codling moth (Cydia pomonella) at 150 g a.i./ha on two occasions (1 and 15 July 2009) | Stahlschmidt and Brühl (2012) | |
| Germany | Arable fields | Arable fields: cultivated with the locally prevailing crop types corn (N = 27), canola (N = 34) and wheat (N = 32) | Heim et al. (2016) | |
| UK | Arable land, conifer plantations | Not described | Vaughan et al. (1997) | |
| Italy | Chestnut woodlands, arable land, olive grove, conifer plantations |
Chestnut woodlands: sweet chestnut C. sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees Arable land: generally characterised by a relatively complex mosaic of fields separated by tree lines, hedges, canals, etc. Olive groves: Olive Olea europea L. groves Conifer plantation: Conifer (Pinus spp.) plantations |
Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Poland | Arable land | Arable fields: large‐scale arable fields as a main land use type. Degree of intensification is not described | Pluciński et al. (2015) | |
| Poland | Arable land (although the availability of this habitat was very high, only a few bat passes recorded), meadows/pastures, Orchards |
Arable land: Land outside settlements, used for growing any crop including rye, barley, wheat, rapeseed, maize, beet, potatoes, cucumbers or carrots (including fallows) Meadow/Pasture: grassland outside settlements used for hay production (mowed) or livestock grazing Orchards: area used for growing vegetables, flowers or fruit, with at least 50% planted by fruit trees or bushes |
Ciechanowski (2015) | |
| The Netherlands | Agricultural fields (only type of land assessed in the study) | Old agricultural landscape type with many linear elements. Meadows and fields are separated by a network of hedgerows and tree lanes. Agricultural intensification not specified | Verboom and Huitema (1997) | |
| UK | Pasture (organic and conventional), Arable land (organic and conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| Pipistrellus pygmaeus | Germany | Meadow, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest. Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Germany | Cultivated and managed land | Intensification not mentioned. Land cover of cultivated and managed areas was 35.5% of the whole area | Kusch and Schmitz (2013) | |
| Germany | Arable fields | Arable fields: cultivated with the locally prevailing crop types corn (N = 27), canola (N = 34) and wheat (N = 32) | Heim et al. (2016) | |
| Italy | Chestnut woodlands, arable land, conifer plantations |
Chestnut woodlands: sweet chestnut C. sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees Arable land: generally characterised by a relatively complex mosaic of fields separated by tree lines, hedges, canals, etc. Conifer plantation: Conifer (Pinus spp.) plantations |
Russo and Jones (2003) | |
| Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) | |
| Poland | Arable land | Arable fields: large‐scale arable fields as a main land use type. Degree of intensification is not described | Pluciński et al. (2015) | |
| Poland | Arable land (although the availability of this habitat was very high, only a few bat passes recorded), meadows/pastures, orchards |
Arable land: land outside settlements, used for growing any crop including rye, barley, wheat, rapeseed, maize, beet, potatoes, cucumbers or carrots (including fallows) Meadow/Pasture: grassland outside settlements used for hay production (mowed) or livestock grazing Orchards: area used for growing vegetables, flowers or fruit, with at least 50% planted by fruit trees or bushes |
Ciechanowski (2015) | |
| Czech Republic | Pastures/meadows | Not described | Bartonicka and Rehak (2010) | |
| UK | Pasture (organic and conventional), arable land (organic and conventional) | Organic fields according to Soil Association and UK Register of Organic Food Standard | Wickramasinghe et al. (2003) | |
| UK | Arable land, conifer plantations | Not described | Vaughan et al. (1997) | |
| Spain | Rice paddies | Large‐scale rice cultivations at the Delta de l'Ebro, where bats play a considerable role of pest suppression of the rice borer moth (Chilo suppressalis) | Flaquer et al. (2006); Puig‐Montserrat et al. (2015) | |
| Pipistrellus total | Germany | Meadow, vineyard, orchard, vegetable fields, cereal fields, forest edge |
Meadow: agricultural grasslands with differing management intensities Orchard: Apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Plecotus auritus | Germany | Forest edge, meadow, vineyard, orchard, vegetable fields, cereal fields |
Meadow: agricultural grasslands with differing management intensities Orchard: Apple orchard Forest edge: cereal sampling site that were situated 100 m away from a forest Degree of intensification for agricultural fields is not mentioned |
Stahlschmidt et al. (2017) |
| Finland | Grassland (grouped data; including meadows, arable land and pasture) | Agricultural land covered approximately 8% of the total study site. Intensification degree not described | Wermundsen and Siivonen (2008) | |
| Plecotus sp. | Italy | Organic and conventional rice farms |
Organic rice farms covered an overall surface of 310 ha and are characterised by rice paddies not treated with synthetic pesticides and certified as organic according to the Italian law Conventional rice farms covered a surface of 272 ha and rice paddies are regularly treated with pesticides (i.e. alpha‐cypermethrin, oxadiazon, glyphosate, triciclazol) |
Toffoli and Rughetti (2017) |
| Vespertilio murinus | Switzerland/Germany | Open (agricultural) fields | Not defined | Safi et al. (2007) |
| Vespertilionidae total | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Miniopterus schreibersii | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
| Italy | Chestnut woodland, olive groves, conifer plantation |
Chestnut woodlands: Sweet chestnut C. sativa Miller woodlands managed for chestnut production; traditional form of chestnut woodland management, often characterised by mature trees Olive groves: Olive Olea europea L. groves Conifer plantation: Conifer (Pinus spp.) plantations |
Russo and Jones (2003) | |
| Southern Europe, 16 sampling sites along a W‐E transect | Broad range of agricultural landscapes, also including intensive agriculture | Clear evidence that bats forage in farmland – 44 pest prey found it diet, 22 of which categorised as major pests. Trophic spectrum narrows along with an increase in intensive agriculture within 30 km around each sampling site | Aizpurua et al. (2018) | |
| Tadarida teniotis | Greece | Organic and conventional olive groves |
Organic: Olive (O. europea) plantations, not chemically treated. Two of six sites practice ‘organic’ pest control (scent and sticky traps) Conventional: O. europea plantations comparable to the organic groves in age, density of trees and altitude, but treated yearly with an insecticide spray |
Davy et al. (2007) |
a.i.: active ingredient.
Appendix D – Toxicity endpoint comparison
1.
References used in the Table D.1
Clark DR Jr, Hill EF, Henry PFP, 1991. Comparative sensitivity of little brown bats (Myotis lucifugus) to acute dosages of sodium cyanide. Bat Research News, 32, 68.
Clark DR, 1981. Effects of DDE and PCB (Aroclor 1260) on experimentally poisoned female little brown bats (Myotis lucifugus): Lethal brain concentrations. Journal of Toxicology and Environmental Health, Part A Current Issues, 7, 925–934. https://doi.org/10.1080/15287398109530035
Clark DR, 1986. Toxicity of methyl parathion to bats: Mortality and coordination loss. Environmental Toxicology and Chemistry: An International Journal, 5, 191–195. https://doi.org/10.1002/etc.5620050210
EFSA conclusions: chlorpyrifos, deltamethrin, imidacloprid, parathion, zinc phosphide.
Eidels RR, Sparks DW, Whitaker JO and Sprague CA, 2016. Sub‐lethal effects of chlorpyrifos on big brown bats (Eptesicus fuscus). Archives of environmental contamination and toxicology, 71, 322. https://doi.org/10.1007/s00244-016-0307-3
Extoxnet: http://extoxnet.orst.edu/pips/ddt.htm
Hsiao C‐J, Lin C‐L, Lin T‐Y, Wang S‐E and Wu C‐H, 2016. Imidacloprid toxicity impairs spatial memory of echolocation bats through neural apoptosis in hippocampal CA1 and medial entorhinal cortex areas. NeuroReport, 27, 462–468. http://doi.org/10.1097/WNR.0000000000000562
Hudson RH, Tucker RK and Haegele K, 1984. Handbook of Acute Toxicity of Pesticides to Wildlife. Resource Publication 153. U.S. Dept. of Interior, Fish and Wildlife Service, Washington, DC.
Hurley S and Fenton MB, 1980. Ineffectiveness of fenthion, zinc phosphide, DDT and two ultrasonic rodent repellers for control of populations of little brown bats (Myotis lucifugus). Bulletin of environmental contamination and toxicology, 25, 503–507.
Luckens MM and Davis WH, 1964. Bats: sensitivity to DDT. Science, 146, 948–948.
McFarland CA, 1998. Potential agricultural insecticide exposure of Indiana bats (Myotis sodalis) in Missouri (Doctoral dissertation, University of Missouri‐Columbia). From O'Shea (2009).
McFarland, CA (1998). Potential agricultural insecticide exposure of Indiana bats (Myotis sodalis) in Missouri (Doctoral dissertation, University of Missouri‐Columbia).
NCI1978 from TOXICOLOGICAL PROFILE FOR DDT, DDE, and DDD made by U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry.
NIOSH https://pubchem.ncbi.nlm.nih.gov/compound/fenthion#section=NIOSH-Toxicity-Data&fullscreen=true
Oliveira JM, Losano NF, Condessa SS, de Freitas RMP, Cardoso SA, Freitas MB, & de Oliveira LL, 2017. Exposure to deltamethrin induces oxidative stress and decreases of energy reserve in tissues of the Neotropical fruit‐eating bat Artibeus lituratus. Ecotoxicology and Environmental Safety, 148, 684–692. http://doi.org/10.1016/j.ecoenv.2017.11.024
Orberg and Lundberg 1974 DDT, p,p’ from TOXICOLOGICAL PROFILE FOR DDT, DDE, and DDD made by U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry.
Shore RF, Myhill DG and Wright J, 1996. A comparison of the toxicity to laboratory mice and pipistrelle bats Pipistrellus pipistrellus of exposure to remedially‐treated timber. Environmental Toxicology and Pharmacology, 2, 125–129. https://doi.org/10.1016/s1382-6689(96)00042-7
TOXNET: PCB [Aroclor 1254] https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~zO48tW:3
Appendix E – Overview on factors influencing the residues of pesticides in insects
1.
Overview on factors influencing the residues of pesticides in insects:
-
1
Sampling method
| Bitertanol | Malaise traps | RUD: 1.6 |
| Car netting | RUD: 28.5 |
Less insects were caught in the malaise traps. The study authors considered the malaise traps as more selective than the car netting. No details are provided in the study report on the malaise trap method.
-
2
Insect size
| Fenoxycarb | Large moths 10–20 mm | RUD: 8.9 |
| Small moths < 10 mm | RUD: 32.8 | |
| Other small insects 3–10 mm | RUD: 14.6 |
Insects were caught during the night with light traps in an apple orchard after treatment, treatment took place in July 1 and 15 and lasted from 16:00 to 20:00
-
3
Variation in different plots
| Fluopyram | Plot 1 | RUD: 0.78 |
| Plot 2 | RUD: 1.4 | |
| Plot 3 | RUD: 4.85 |
Insects were caught in malaise traps on three different vineyards. Methodology and crop (grapes) were identical.
-
4
Unknown substance related properties (different degradation or uptake by insects?)
| Fluopyram vs Prothioconazole | RUD: 7.4 vs 2.5 |
The combination product Propulse (Fluopyram + Prothioconazole) was applied in oilseed rape. Insects were caught in malaise traps
-
5
Difference in crop
| Fluopyram applied | in grapes | RUD: 0.78–1.4–4.85 |
| in oilseed rape | RUD: 7.4 |
Application of Fluopyram 500 SC in grape and application of Propulse in oilseed rape.
The same methodology was used in both studies. One explanation could be that there are different crop interception values and the insects might be less exposed in vineyards than in oilseed rape. However, the differences could also be explained by other factors such as insect size, variation in plots and application methods.
-
6
Difference in toxicity
| Fenoxycarb | RUD: 8.9–14.6–32.8 |
| Abamectin | RUD: 0.99–3.39 |
| Emamectin | RUD: 1.4–1.8 |
Light trapping was applied in all three studies. The main difference was in the acute toxicity of Fenoxycarb which is an insect growth regulator with very low acute toxicity to insects while Abamectin and Emamectin are very acutely toxic and fast‐acting insecticides. Therefore insects which were exposed more and carried higher residues of Abamectin or Emamectin might not have been able to fly anymore and thus only insects which were exposed less and carried only low residues levels could have reached the light traps.
Suggested citation: EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernández‐Jerez A, Adriaanse P, Aldrich A, Berny P, Coja T, Duquesne S, Gimsing AL, Marina M, Millet M, Pelkonen O, Pieper S, Tiktak A, Tzoulaki I, Widenfalk A, Wolterink G, Russo D, Streissl F and Topping C, 2019. Scientific statement on the coverage of bats by the current pesticide risk assessment for birds and mammals. EFSA Journal 2019;17(7):5758, 81 pp. 10.2903/j.efsa.2019.5758
Requestor: PPR Panel
Question Number: EFSA‐Q‐2018‐00615
Acknowledgments: EFSA wishes to acknowledge the contribution of the hearing expert Carsten Brühl, the trainees Joanke Van Dijk, Lucie Ctvertckova and EFSA staff Rachel Sharp and Stefania Barmaz to this scientific statement.
Figure 1: © Stockphoto
Adopted: 20 June 2019
Notes
Agreement on the Conservation of Populations of European Bats, EUROBATS, 1991 http://www.eurobats.org/official_documents/agreement_text [Accessed: 22 June 2018]
Convention on the Conservation of Migratory Species of Wild Animals; Bonn, Germany, 23 June 1979. Available online: http://www.cms.int [Accessed: 22 June 2018)
The value to be used for the time‐weighted average factor (TWA) depends on whether the toxicity endpoint could be caused by short‐term exposure or only by a long‐term exposure. For further details, see EFSA (2009). For the comparative exposure calculations in the current document, no TWA factor was applied as the exposure estimate was not related to a specific effect endpoint.
References
- Aitken RJ, 1974. Delayed implantation in roe deer (Capreolus capreolus). Journal of Reproduction and Fertility, 39, 225–233. [DOI] [PubMed] [Google Scholar]
- Aizpurua O, Budinski I, Georgiakakis P, Gopalakrishnan S, Ibañez C, Mata V and Zrncic V, 2018. Agriculture shapes the trophic niche of a bat preying on multiple pest arthropods across Europe: Evidence from DNA metabarcoding. Molecular Ecology, 27, 815–825. [DOI] [PubMed] [Google Scholar]
- Altringham JD, 2011. Bats: from evolution to conservation. Oxford University Press, Oxford. [Google Scholar]
- Ancillotto L, Serangeli MT and Russo D, 2012. Spatial proximity between newborns influences the development of social relationships in bats. Ethology, 118, 331–340. [Google Scholar]
- Ancillotto L, Serangeli MT and Russo D, 2013. Curiosity killed the bat: domestic cats as bat predators. Mammalian Biology‐Zeitschrift für Säugetierkunde, 78, 369–373. [Google Scholar]
- Ancillotto L, Allegrini C, Serangeli MT, Jones G and Russo D, 2014. Sociality across species: spatial proximity of newborn bats promotes heterospecific social bonding. Behavioral Ecology, 26, 293–299. [Google Scholar]
- Anderson ME and Racey PA, 1991. Feeding behaviour of captive brown long‐eared bats, Plecotus auritus. Animal Behaviour, 42, 489–493. [Google Scholar]
- Anthony EL and Kunz TH, 1977. Feeding strategies of the little brown bat, Myotis lucifugus, in southern New Hampshire. Ecology, 58, 775–786. [Google Scholar]
- Arends A, 1995. Basal rates of metabolism of nectarivorous bats (Phyllostomidae) from a semiarid thorn forest in Venezuela. Journal of Mammalogy, 76, 947–956. [Google Scholar]
- Arlettaz R, 1996. Foraging behaviour of the gleaning bat Myotis nattereri (Chiroptera, Vespertilionidae) in the Swiss Alps. Mammalia, 60, 181–186. [Google Scholar]
- Arlettaz R, Godat S and Meyer H, 2000. Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biological Conservation, 93, 55–60. [Google Scholar]
- Arlettaz R, Jones G and Racey PA, 2001. Effect of acoustic clutter on prey detection by bats. Nature, 414, 742. [DOI] [PubMed] [Google Scholar]
- Arnett EB, Baerwald EF, Mathews F, Rodrigues L, Rodríguez‐Durán A, Rydell J and Voigt CC, 2016. Impacts of wind energy development on bats: a global perspective. In Bats in the Anthropocene: Conservation of bats in a changing world. Springer, Cham: pp. 295–323. [Google Scholar]
- Barclay RMR and Harder LD, 2003. Life histories of bats: life in the slow lane. InBat ecology. Edited byT.H. Kunz and M.B.Fenton. University of Chicago Press, Chicago; pp. 209–253. [Google Scholar]
- Barclay RM and Kurta A, 2007. Ecology and behavior of bats roosting in tree cavities and under bark. Bats in forests: conservation and management (MJ LACKI, JP HAYES, and A. KURTA, eds.). Johns Hopkins University Press, Baltimore, Maryland, 17‐59.
- Bartels W, Law BS and Geiser F, 1998. Daily torpor and energetics in a tropical mammal, the northern blossom‐bat Macroglossus minimus (Megachiroptera). Journal of Comparative Physiology B, 168, 233–239. [DOI] [PubMed] [Google Scholar]
- Bartonička T, Řehák Z and Andreas M, 2008. Diet composition and foraging activity ofPipistrellus pygmaeusin a floodplain forest. Biologia, 63, 1–7. [Google Scholar]
- Bartonicka T and Rehak Z, 2010. Roost switching and activity pattern of the soprano pipistrelle Pipistrellus pygmaeus revealed by radio‐tracking.
- Bassett JE and Studier EH, 1988. Methods of determining water balance in bats In: Kunz TH. (ed.). Ecological and Behavioural Methods for the Study of Bats. Smithsonian Institution Press, Washington, DC: pp. 373–386. [Google Scholar]
- Baudinette RV, Churchill SK, Christian KA, Nelson JE and Hudson PJ, 2000. Energy, water balance and the roost microenvironment in three Australian cave‐dwelling bats (Microchiroptera). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 170, 439–446. [DOI] [PubMed] [Google Scholar]
- Bayat S, Geiser F, Kristiansen P and Wilson SC, 2014. Organic contaminants in bats: Trends and new issues. Environment International, 63, 40–52. 10.1016/j.envint.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Bell GP, Bartholomew GA and Nagy KA, 1986. The roles of energetics, water economy, foraging behavior, and geothermal refugia in the distribution of the bat, Mactrotus californicus . Journal of Comparative Physiology B, 156, 441–450. [Google Scholar]
- Ben‐Hamo M, Muñoz‐Garcia A, Larrain P, Pinshow B, Korine C and Williams JB, 2016. The cutaneous lipid composition of bat wing and tail membranes: a case of convergent evolution with birds. Proceedings of the Royal Society B: Biological Sciences, 283, 20160636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard RTF and Davison A, 1996. Does calcium constrain reproductive activity in insectivorous bats? Some empirical evidence for Schreibers’ long‐fingered bat (Miniopterus schreibersii). South African Journal of Zoology, 31, 218–220. [Google Scholar]
- Betke M, Hirsh DE, Makris NC, McCracken GF, Procopio M, Hristov NI and Crampton S, 2008. Thermal imaging reveals significantly smaller Brazilian free‐tailed bat colonies than previously estimated. Journal of Mammalogy, 89, 18–24. [Google Scholar]
- Biscardi S, Russo D, Casciani V, Cesarini D, Mei M and Boitani L, 2007. Foraging requirements of the endangered long‐fingered bat: The influence of micro‐habitat structure, water quality and prey type. Journal of Zoology, 273, 372–381. [Google Scholar]
- Bishop KL, 2008. The evolution of flight in bats: narrowing the field of plausible hypotheses. The Quarterly Review of Biology, 83, 153–169. [DOI] [PubMed] [Google Scholar]
- Bosso L, Ancillotto L, Smeraldo S, D'Arco S, Migliozzi A, Conti P and Russo D, 2018. Loss of potential bat habitat following a severe wildfire: a model‐based rapid assessment. International Journal of Wildland Fire, 27, 756–769. [Google Scholar]
- Boesten JJTI, Kopp H, Adriaanse PI, Brock TCM and Forbes VE, 2007. Conceptual model for improving the link € between exposure and effects in the aquatic risk assessment of pesticides. Ecotoxicology and Environmental Safety, 66, 291–308. [DOI] [PubMed] [Google Scholar]
- Boesten JJTI, 2018. Conceptual considerations on exposure assessment goals for aquatic pesticide risks at EU level. Pest Managagement Science, 74, 264–274. [DOI] [PubMed] [Google Scholar]
- Boitet M, Hammed A, Chatron N, Debaux JV, Benoit E and Lattard V, 2018. Elevated difenacoum metabolism is involved in the difenacoum‐resistant phenotype observed in Berkshire rats homozygous for the L120Q mutation in the vitamin K epoxide reductase complex subunit 1 (Vkorc1) gene. Pest Management Science, 74, 1328–1334. [DOI] [PubMed] [Google Scholar]
- Boughey KL, Lake IR, Haysom KA and Dolman PM, 2011. Improving the biodiversity benefits of hedgerows: how physical characteristics and the proximity of foraging habitat affect the use of linear features by bats. Biological Conservation, 144, 1790–1798. [Google Scholar]
- Boyles JG, Dunbar MB and Whitaker JO Jr, 2006. Activity following arousal in winter in North American vespertilionid bats. Mammal Review, 36, 267–280. [Google Scholar]
- Boyles JG, Cryan PM, McCracken GF and Kunz TH, 2011. Economic importance of bats in agriculture. Science, 332, 41–42. [DOI] [PubMed] [Google Scholar]
- Bravo A, Harmsa KE and Emmons LH, 2010. Puddles created by geophagous mammals are potential mineral sources for frugivorous bats (Stenodermatinae) in the Peruvian Amazon. Journal of Tropical Ecology, 26, 173–184. [Google Scholar]
- Brisbin IL Jr, 1966. Energy‐utilization in a captive hoary bat. Journal of Mammalogy, 47, 719–720. [Google Scholar]
- Brunet‐Rossini AK, 2004. Testing the free radical theory of aging in bats. Annals of the New Your Academy of Science, 1019, 506–508. [DOI] [PubMed] [Google Scholar]
- Burns RJ and Bullard RW, 1979. Diphacinone residue from whole bodies of vampire bats: a laboratory study. Bulletin of the. Pan American Health Organization (PAHO), 13(4), 365–369. [PubMed] [Google Scholar]
- Cannon B and Nedergaard JAN, 2004. Brown adipose tissue: function and physiological significance. Physiological Reviews, 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Carter G and Leffer L, 2015. Social grooming in bats: are vampire bats exceptional? PLoS ONE, 10, e0138430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GG and Wilkinson GS, 2013. Cooperation and conflict in the social lives of bats Bat evolution, ecology, and conservation. Springer, New York, NY: pp. 225–242. [Google Scholar]
- Catry I, Franco AM and Moreira F, 2014. Easy but ephemeral food: exploring the trade‐offs of agricultural practices in the foraging decisions of Lesser Kestrels on farmland. Bird Study, 61, 447–456. [Google Scholar]
- Chappell MA and Roverud RC, 1990. Temperature effects on metabolism, ventilation, and oxygen extraction in a Neotropical bat. Respiration Physiology, 81, 401–412. [DOI] [PubMed] [Google Scholar]
- Chaverri G, Ancillotto L and Russo D, 2018. Social communication in bats. Biological Reviews. [DOI] [PubMed] [Google Scholar]
- Ciechanowski M, 2015. Habitat preferences of bats in anthropogenically altered, mosaic landscapes of northern Poland. European Journal of Wildlife Research, 61, 415–428. [Google Scholar]
- Clark DR, 1986. Toxicity of methyl parathion to bats: Mortality and coordination loss. Environmental Toxicology and Chemistry, 5, 191–195. 10.1002/etc.5620050210 [DOI] [Google Scholar]
- Clark DR jr and Krynitsky AJ, 1983. DDE in brown and white fat of hibernating bats. Environmental Pollution (Series A), 31, 287–299. 10.1016/0143-1471(83)90065-x [DOI] [Google Scholar]
- Clark DR Jr and Lamont TG, 1976. Organochlorine residues in females and nursing young of the big brown bat (Eptesicus fuscus). Bulletin of Environment Contamination and Toxicology, 15, 1–8. 10.1007/BF01686188 [DOI] [Google Scholar]
- Conenna I, Rocha R, Russo D and Cabeza M, 2017. Insular bats and research effort: a review of global patterns and priorities. Mammal Review, 47, 169–182. [Google Scholar]
- Coutts RA, Fenton MB and Glen E, 1973. Food intake by captive Myotis lucifugus and Eptesicus fuscus (Chiroptera: Vespertilionidae). Journal of Mammalogy, 54, 985–990. [Google Scholar]
- Cretekos CJ, Wang Y, Green ED, Martin JF, Rasweiler JJ and Behringer RR, NISC Comparative Sequencing Program , 2008. Regulatory divergence modifies limb length between mammals. Genes & Development, 22, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Neto A and Abe A, 1997. Metabolic rate and thermoregulation in the nectarivorous bat, Glossophaga soricina (Chiroptera, Phyllostomatidae). Revista Brasileira de Biologia, 57, 203–209. [Google Scholar]
- Davis RW and Barbour WH, 1970. Bats of America. The University Press of Kentucky, Lexington: p. 286. [Google Scholar]
- Davy C, Russo D and Fenton M, 2007. Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean island: consequences for conservation. Journal of Zoology, 273, 397–405. [Google Scholar]
- De Conno C, Nardone V, Ancillotto L, De Bonis S, Guida M, Jorge I and Russo D, 2018. Testing the performance of bats as indicators of riverine ecosystem quality. Ecological Indicators, 95, 741–750. [Google Scholar]
- Del Vaglio MA, Nicolau H, Bosso L and Russo D, 2011. A first assessment of feeding habits in the fruit bat Rousettus aegyptiacus on Cyprus island. Hystrix, the Italian Journal of Mammalogy, 22, 281–289. [Google Scholar]
- Dennis GC and Gartrell BD, 2015. Nontarget mortality of New Zealand lesser short‐tailed bats (Mystacina tuberculata) caused by diphacinone. Journal of Wildlife Diseases, 51, 177–186. 10.7589/2013-07-160 [DOI] [PubMed] [Google Scholar]
- Denzinger A and Schnitzler HU, 2013. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Frontiers in Physiology, 4, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz C and Kiefer A, 2016. Bats of Britain and Europe. Bloomsbury, 400 pp. [Google Scholar]
- Drescher C, 2004. Radiotracking of Myotis myotis (Chiroptera, Vespertilionidae) in South Tyrol and implications for its conservation. Mammalia, 68, 387–395. [Google Scholar]
- Dumont ER, Piccirillo J and Grosse IR, 2005. Finite‐element analysis of biting behavior and bone stress in the facial skeletons of bats. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists, 283, 319–330. [DOI] [PubMed] [Google Scholar]
- Duvergé PL and Jones G, 2003. Habitat use by greater horseshoe bats. Conservation and Conflict: Mammals and Farming in Britain, 64–81. [Google Scholar]
- EEC , 1992. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal L 206, 22/07/1992 P. 0007 ‐ 0050
- EFSA (European Food Safety Authority), 2008. Conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid. EFSA Journal 2008;6(7):128r, 77 pp. 10.2903/j.efsa.2008.128r. Available online: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2008.128r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance Document on Risk Assessment for Birds & Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Conclusion on the peer review of the pesticide risk assessment of the active substance chlorpyrifos. EFSA Journal 2011;9(1):1961, 14 pp. 10.2903/j.efsa.2011.1961. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2011.1961 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Buist H, Craig P, Dewhurst I, Hougaard Bennekou S, Kneuer C, Machera K, Pieper C, Court Marques D, Guillot G, Ruffo F and Chiusolo A, 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 60 pp. 10.2903/j.efsa.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) Ockleford C, Adriaanse P, Berny P, Brock T, Duquesne S, Grilli S, Hernandez‐Jerez AF, Bennekou SH,Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Wolterink G, Aldrich A, Berg C, Ortiz‐Santaliestra M, Weir S, Streissl F and Smith RH, 2018. Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA Journal 2018:16(2):5125, 301 pp. 10.2903/j.efsa.2018.5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels RR, John O Whitaker J, Lydy MJ and Sparks DW, 2012. Screening of Insecticides in Bats from Indiana. Proceedings of the Indiana Academy of Science, 121, 133–142. [Google Scholar]
- Eidels RR, Sparks DW, Whitaker JO and Sprague CA, 2016. Sub‐lethal Effects of Chlorpyrifos on Big Brown Bats (Eptesicus fuscus). Archives of Environmental Contamination and Toxicology, 71, 322–335. 10.1007/s00244-016-0307-3 [DOI] [PubMed] [Google Scholar]
- Encarnação JA and Dietz M, 2006. Estimation of food intake and ingested energy in Daubenton's bats (Myotis daubentonii) during pregnancy and spermatogenesis. European Journal of Wildlife Research, 52, 221–227. [Google Scholar]
- EU (European Union), 2009. Regulation (EC) No. 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. 21 October 2009. Official Journal of the European Union L 309, 24 November 2009, 50 pp.
- EU (European Union), 2011. Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products OJ L 155, 11.6.2011, p. 127–175.
- EU (European Union), 2013. Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market OJ L 93, 3.4.2013, p. 1–84.
- EUROBATS , 1991. Agreement on the Conservation of Populations of European Bats, UNEP/Eurobats, 1991, Available online: http://www.eurobats.org/official_documents/agreement_text [Accessed: 22 June 2018]
- Farney J and Fleharty ED, 1969. Aspect Ratio, Loading, Wing Span, and Membrane Areas of Bats. Journal of Mammalogy, 50, 362–367. [Google Scholar]
- FCO Foreign and Commonwealth Affairs , 1994. Agreement on the Conservation of Bats in Europe. Entered into force on 16 January 1994. HMSO 7 pp. Available online: https://www.eurobats.org/sites/default/files/documents/pdf/Agreementtexts/FCO_Agreement_Text_engl.pdf
- Fenton MB, 2013. Questions, ideas and tools: lessons from bat echolocation. Animal Behaviour, 85, 869–879. [Google Scholar]
- Fenton MB, Faure PA and Ratcliffe JM, 2012. Evolution of high duty cycle echolocation in bats. Journal of Experimental Biology, 215, 2935–2944. [DOI] [PubMed] [Google Scholar]
- Flaquer C, Torre I and Ruiz‐Jarillo R, 2006. The value of bat‐boxes in the conservation of Pipistrellus pygmaeus in wetland rice paddies. Biological Conservation, 128, 223–230. [Google Scholar]
- Foley NM, Hughes GM, Huang Z, Clarke M, Jebb D, Whelan CV, Petit EJ, Touzalin F, Farcy O, Jones G, Ransome RD, Kacprzyk J, O'Connell MJ, Kerth G, Rebelo H, Rodrigues L, Puechmaille SJ and Teeling EC, 2018. Growing old, yet staying young: The role of telomeres in bats’ exceptional longevity. Science Advances, 4, eaao0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey‐Ehrenbold A, Bontadina F, Arlettaz R and Obrist MK, 2013. Landscape connectivity, habitat structure and activity of bat guilds in farmland‐dominated matrices. Journal of Applied Ecology, 50, 252–261. [Google Scholar]
- Fristoe TS, Burger JR, Balk MA, Khaliq I, Hof C and Brown JH, 2015. Metabolic heat production and thermal conductance are mass‐independent adaptations to thermal environment in birds and mammals. Proceedings of the National Academy of Sciences, 112, 15934–15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F, 1988. Reduction of metabolism during hibernation and daily torpor in mammals and birds: temperature effect or physiological inhibition? Journal of Comparative Physiology B, 158, 25–37. [DOI] [PubMed] [Google Scholar]
- Geiser F and Brigham RM, 2000. Torpor, thermal biology, and energetics in Australian long‐eared bats (Nyctophilus). Journal of Comparative Physiology B, 170, 153–162. [DOI] [PubMed] [Google Scholar]
- Geiser F and Stawski C, 2011. Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integrative and Comparative Biology, 51, 337–348. [DOI] [PubMed] [Google Scholar]
- Geluso KN, 1978. Urine concentrating ability and renal structure of insectivorous bats. Journal of Mammalogy, 59, 312–323. [PubMed] [Google Scholar]
- Genoud M and Bonaccorso F, 1986. Temperature regulation, rate of metabolism, and roost temperature in the greater white‐lined bat Saccopteryx bilineata (Emballonuridae). Physiological and Biochemical Zoology, 59, 49–54. [Google Scholar]
- Genoud M, Bonaccorso F and Anends A, 1990. Rate of metabolism and temperature regulation in two small tropical insectivorous bats (Peropteryx macrotis and Natalus tumidirostris). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 9, 229–234. [Google Scholar]
- Gibbons D, Morrissey C and Mineau P, 2014. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research International, 22, 103–118. 10.1007/s11356-014-3180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover AM and Altringham JD, 2008. Cave selection and use by swarming bat species. Biological Conservation, 141, 1493–1504. [Google Scholar]
- Griffin DR, 1970. Migrations and homing of bats In: Wimsatt WA. (ed). Biology of bats. Academic Press, New York: pp. 233–265. [Google Scholar]
- Gupta BB, 1967. The histology and musculature of plagiopatagium in bats. Mammalia, 31, 313–321. [Google Scholar]
- Hanus K, 1959. Body temperatures and metabolism in bats at different environmental temperatures. Physiol Bohemoslov, 8, 250–259. [Google Scholar]
- Heim O, Schröder A, Eccard J, Jung K and Voigt CC, 2016. Seasonal activity patterns of European bats above intensively used farmland. Agriculture, Ecosystems & Environment, 233, 130–139. [Google Scholar]
- Hodroge A, Longin‐Sauvageon C, Fourel I, Benoit E and Lattard V, 2011. Biochemical characterization of spontaneous mutants of rat VKORC1 involved in the resistance to antivitamin K anticoagulants. Archives of Biochemistry and Biophysics, 515, 14–20. [DOI] [PubMed] [Google Scholar]
- Hofmann K and Heise G, 1991. Vergiftung junger Mausohren (Myotis myotis) durch Pflanzenschutzmittel. Nyctalus (NF), 4, 85–87. [Google Scholar]
- Holderied M, Korine C and Moritz T, 2011. Hemprich's long‐eared bat (Otonycteris hemprichii) as a predator of scorpions: whispering echolocation, passive gleaning and prey selection. Journal of Comparative Physiology A, 197, 425–433. [DOI] [PubMed] [Google Scholar]
- Holland RA, Borissov I and Siemers BM, 2010. A nocturnal mammal, the greater mouse‐eared bat, calibrates a magnetic compass by the sun. Proceedings of the National Academy of Sciences of the United States of America, 107, 6941–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken DJ and Withers PC, 1997. Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. Journal of Comparative Physiology B, 167, 71–80. [DOI] [PubMed] [Google Scholar]
- Hsiao CJ, Lin CL, Lin TY, Wang SE and Wu CH, 2016. Imidacloprid toxicity impairs spatial memory of echolocation bats through neural apoptosis in hippocampal CA1 and medial entorhinal cortex areas. NeuroReport, 27, 462–468. 10.1097/WNR.0000000000000562 [DOI] [PubMed] [Google Scholar]
- Hurley S and Fenton MB, 1980. Ineffectiveness of fenthion, zinc, phosphide, DDT and two ultrasonic rodent repellers for control of populations of little brown bats (Myotis lucifugus). Bulletin of Environmental Contamination and Toxicology, 25, 503–507. [DOI] [PubMed] [Google Scholar]
- Jackson SM, 2000. Glide angle in the genus Petaurus and a review of gliding in mammals. Mammal Review, 30, 9–30. [Google Scholar]
- Johnson N, Aréchiga‐Ceballos N and Aguilar‐Setien A, 2014. Vampire bat rabies: ecology, epidemiology and control. Viruses, 6, 1911–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson KA and Willis CKR, 2011. Changes in Body Condition of Hibernating Bats Support the Thrifty Female Hypothesis and Predict Consequences for Populations with White‐Nose Syndrome. PLoS ONE, 6, e21061–e21068. 10.1371/journal.pone.0021061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G and Rydell J, 1994. Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Philosophical Transactions of The Royal Society B Biological Sciences, 346, 445–455. [Google Scholar]
- Jones G, 1997. Acoustic signals and speciation: the roles of natural and sexual selection in the evolution of cryptic species. In Advances in the Study of Behavior (Vol. 26, pp. 317‐354). Academic Press.
- Jones G and Holderied MW, 2007. Bat echolocation calls: Adaptation and convergent evolution. Proceedings of the Royal Society B: Biological Sciences, 274, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G and Rayner JM, 1989. Foraging behavior and echolocation of wild horseshoe bats Rhinolophus ferrumequinum and R. hipposideros (Chiroptera, Rhinolophidae). Behavioral Ecology and Sociobiology, 25, 183–191. [Google Scholar]
- Jones G and Teeling EC, 2006. The evolution of echolocation in bats. Trends in Ecology & Evolution, 21, 149–156. [DOI] [PubMed] [Google Scholar]
- Jones KE, Bielby J, Cardillo M, Fritz SA, O'Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda‐Emonds ORP, Gittleman JL, Mace GM and Purvis A, 2009. PanTHERIA: a species‐level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology, 90, 2648. [Google Scholar]
- Kalko EK and Schnitzler HU, 1993. Plasticity in echolocation signals of European pipistrelle bats in search flight: implications for habitat use and prey detection. Behavioral Ecology and Sociobiology, 33, 415–428. [Google Scholar]
- Kerth G, 2008. Causes and consequences of sociality in bats. AIBS Bulletin, 58, 737–746. [Google Scholar]
- Kerth G, Kiefer A, Trappmann C and Weishaar M, 2003a. High gene diversity at swarming sites suggest hot spots for gene flow in the endangered Bechstein's bat. Conservation Genetics, 4, 491–499. [Google Scholar]
- Kerth G, Almasi B, Ribi N, Thiel D and Lüpold S, 2003b. Social interactions among wild female Bechstein's bats (Myotis bechsteinii) living in a maternity colony. Acta Ethologica, 5, 107–114. [Google Scholar]
- Knight T and Jones G, 2009. Importance of night roosts for bat conservation: roosting behaviour of the lesser horseshoe bat Rhinolophus hipposideros . Endangered Species Research, 8, 79–86. [Google Scholar]
- Korine C, Adams R, Russo D, Fisher‐Phelps M and Jacobs D, 2016. Bats and Water: Anthropogenic Alterations Threaten Global Bat Populations. Pp. 215‐241 in (Voigt C.C., Kingston T. eds.). Bats in the Anthropocene: Conservation of Bats in a Changing World‐ Springer Open.
- Kunz TH, 1982. Roosting ecology of bats. In Ecology of bats. Springer, Boston, MA: pp. 1–55. [Google Scholar]
- Kunz TH, Whitaker JO and Wadanoli MD, 1995a. Dietary energetics of the insectivorous Mexican free‐tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia, 101, 407–415. [DOI] [PubMed] [Google Scholar]
- Kunz TH, Oftedal OT, Robson SK, Kretzmann MB and Kirk C, 1995b. Changes in milk composition during lactation in three species of insectivorous bats. Journal of Comparative Physiology B, 164, 543–551. [DOI] [PubMed] [Google Scholar]
- Kunz TH, Lumsden LF and Fenton MB, 2003. Ecology of cavity and foliage roosting bats. Bat Ecology, 1, 3–89. [Google Scholar]
- Kunz TH, Braun de Torrez E, Bauer D, Lobova T and Fleming TH, 2011. Ecosystem services provided by bats. Annals of the New York Academy of Sciences, 1223, 1–38. [DOI] [PubMed] [Google Scholar]
- Kurta A and Kunz TH, 1988. Roosting metabolic rate and body temperature of male little brown bats (Myotis lucifugus) in summer. Journal of Mammalogy, 69, 645–651. [Google Scholar]
- Kurta A, Bell GP, Nagy KA and Kun TH, 1989a. Energetics of pregnancy and lactation in freeranging little brown bats (Myotis lucifugus). Physiological Zoology, 62, 804–818. [Google Scholar]
- Kurta A, Bell GP, Nagy KA and Kunz TH, 1989b. Water balance of free‐ranging little brown bats (Myotis lucifugus) during pregnancy and lactation. Canadian Journal of Zoology, 67, 2468–2472. [Google Scholar]
- Kurta A, Kunz TH and Nagy KA, 1990. Energetics and water flux of free‐ranging big brown bats (Eptesicus fuscus) during pregnancy and lactation. Journal of Mammalogy, 71, 59–65. [Google Scholar]
- Kusch J and Schmitz A, 2013. Environmental factors affecting the differential use of foraging habitat by three sympatric species of Pipistrellus. Acta Chiropterologica, 15, 57–67. [Google Scholar]
- Lahr J, Krämer W, Mazerolles V, Poulsen V, Jölli D, Müller M, McVey E, Wassenberg J, Derkx R, Brouwer A, Deneer D, Beltman W, Lammertsma D, Jansman H and Buij R, 2018. Data collection for the estimation of ecological data (specific focal species, time spent in treated areas collecting food, composition of diet), residue level and residue decline on food items to be used in the risk assessment for birds and mammals. EFSA supporting publication 2018:EN‐1513. 159 pp. [Google Scholar]
- Law BS and Dickman CR, 1998. The use of habitat mosaics by terrestrial vertebrate fauna: implications for conservation and management. Biodiversity and Conservation, 7, 323–333. [Google Scholar]
- Lawrence BD and Simmons JA, 1982. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. The Journal of the Acoustical Society of America, 71, 585–590. [DOI] [PubMed] [Google Scholar]
- Licht P and Leitner P, 1967. Physiological responses to high environmental temperatures in three species of microchiropteran bats. Comparative Biochemistry and Physiology, 22, 371–387. [Google Scholar]
- Linhart SB, Flores Crespo R and Mitchell GC, 1972. Control of vampire bats by topical application of an anticoagulant, chlorophacinone. Boletin de la Oficina Sanitaria Panamericana, 6, 31–38. [PubMed] [Google Scholar]
- Liu JN and Karasov WH, 2011. Hibernation in warm hibernacula by free‐ranging Formosan leaf‐nosed bats, Hipposideros terasensis, in subtropical Taiwan. Journal of Comparative Physiology B, 181, 125–135. [DOI] [PubMed] [Google Scholar]
- Lundberg K and Gerell R, 1986. Territorial advertisement and mate attraction in the bat Pipistrellus pipistrellus . Ethology, 71, 115–124. [Google Scholar]
- Maine JJ and Boyles JG, 2015. Bats initiate vital agroecological interactions in corn. Proceedings of the National Academy of Sciences, 112, 12438–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanya AN and Mortola JP, 2007. The structural design of the bat wing web and its possible role in gas exchange. Journal of Anatomy, 211, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney SK, Bronner GN and Buffenstein R, 1999. Thermoregulation in the Angolan free‐tailed bat Mops condylurus: a small mammal that uses hot roosts. Physiological and Biochemical Zoology, 72, 385–396. [DOI] [PubMed] [Google Scholar]
- Marnell F and Presetnik P, 2010. Protection of overground roosts for bats. UNEP/EUROBATS Secretariat. [Google Scholar]
- McAllan BM and Geiser F, 2014. Torpor during reproduction in mammals and birds: dealing with an energetic conundrum. American Zoologist, 54, 516–532. [DOI] [PubMed] [Google Scholar]
- McFarland CA, 1998. Potential agricultural insecticide exposure of Indiana bats (Myotis sodalis) in Missouri. Doctoral dissertation. University of Missouri‐Columbia; p. 512 pp. [Google Scholar]
- McLean JA and Speakman JR, 1999. Energy budgets of lactating and non‐reproductive brown long‐eared bats (Plecotus auritus) suggest females use compensation in lactation. Functional Ecology, 13, 360–372. [Google Scholar]
- McNab BK, 1969. The economics of temperature regulation in neotropical bats. Comparative Biochemistry and Physiology, 31, 227–268. [DOI] [PubMed] [Google Scholar]
- McNab BK, 1982. Evolutionary alternatives in the physiological ecology of bats. In Ecology of bats. Springer, Boston, MA: pp. 151–200. [Google Scholar]
- McNab B, 1989. Temperature regulation and rate of metabolism in three Bornean bats. Journal of Mammalogy, 70, 153–161. [Google Scholar]
- McNab BK, 1997. On the utility of uniformity in the definition of basal rate of metabolism. Physiological Zoology, 70, 718–720. [DOI] [PubMed] [Google Scholar]
- McNab BK, 2015. Behavioral and ecological factors account for variation in the mass‐independent energy expenditures of endotherms. Journal of Comparative Physiology B, 185, 1–13. [DOI] [PubMed] [Google Scholar]
- McNab B and Bonaccorso F, 2001. The metabolism of New Guinean pteropodid bats. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 171, 201–214. [DOI] [PubMed] [Google Scholar]
- Mead RA, 1989. The physiology and evolution of delayed implantation in carnivores Carnivore behavior, ecology, and evolution. Springer, Boston, MA: pp. 437–464. [Google Scholar]
- Menzies AK, Webber QM, Baloun DE, McGuire LP, Muise KA, Coté D and Willis CK, 2016. Metabolic rate, latitude and thermal stability of roosts, but not phylogeny, affect rewarming rates of bats. Physiology & Behavior, 164, 361–368. [DOI] [PubMed] [Google Scholar]
- Mikula P, Morelli F, Lučan RK, Jones DN and Tryjanowski P, 2016. Bats as prey of diurnal birds: a global perspective. Mammal Review, 46, 160–174. [Google Scholar]
- Mineau P and Callaghan C, 2018. Neonicotinoid insecticides and bats: an assessment of the direct and indirect risks. Canadian Wildlife Federation., 87, pp. [Google Scholar]
- Mitchell‐Jones AJ, Cooke AS, Boyd IL and Stebbings RE, 1989. Bats and remedial timber treatment chemicals a review. Mammal Review, 19, 93–110. [Google Scholar]
- Mitchell‐Jones AJ, Bihari Z, Masing M and Rodrigues L, 2007. Protecting and managing underground sites for bats. UNEP/EUROBATS.
- Mohany M, El‐Feki M, Refaat I, Garraud O and Badr G, 2012. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. The Journal of Toxicological Sciences, 37, 1–11. [DOI] [PubMed] [Google Scholar]
- Monck‐Whipp L, Martin AE, Francis CM and Fahrig L, 2018. Farmland heterogeneity benefits bats in agricultural landscapes. Agriculture, Ecosystems & Environment, 253, 131–139. [Google Scholar]
- Muñoz‐Garcia A, Ro J, Reichard JD, Kunz TH and Williams JB, 2012. Cutaneous water loss and lipids of the stratum corneum in two syntopic species of bats. Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology, 161, 208–215. [DOI] [PubMed] [Google Scholar]
- Munshi‐South J and Wilkinson GS, 2010. Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Research Reviews, 9, 12–19. [DOI] [PubMed] [Google Scholar]
- Myhrvold NP, Baldridge E, Chan B, Sivam D, Freeman DL and Ernest SM, 2015. An amniote life‐history database to perform comparative analyses with birds, mammals, and reptiles. Ecology, 96, 3109–3109. [Google Scholar]
- Nabholz B, Glémin S and Galtier N, 2008. Strong variations of mitochondrial mutation rate across mammals‐the longevity hypothesis. Molecular Biology and Evolution, 25, 120–130. [DOI] [PubMed] [Google Scholar]
- Nagel A and Disser J, 1990. Rückstände von Chlorkohlenwasserstoff‐Pestiziden in einer Wochenstube der Zwergfledermaus (Pipistrellus pipistrellus). Zeitschrift fur Saugetierkunde, 55, 217–225. [Google Scholar]
- Nardone V, Cistrone L, Di Salvo I, Ariano A, Migliozzi A, Allegrini C and Russo D, 2015. How to be a male at different elevations: Ecology of intra‐sexual segregation in the trawling bat Myotis daubentonii . PLoS ONE, 10, e0134573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauser HN and Brisbin IL, 1969. Energy utilization in a captive silver‐haired bat. Bat Research News, 10, 30–31. [Google Scholar]
- Norberg UM and Rayner JMV, 1987. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 316, 335–427. [Google Scholar]
- O'Farrell MJ and Studier EH, 1970. Fall metabolism in relation to ambient temperatures in three species of Myotis. Comparative Biochemistry and Physiology, 35, 697–703. [Google Scholar]
- O'Farrell MJ, Studier EH and Ewing WG, 1971. Energy utilization and water requirements of captive Myotis thysanodes and Myotis lucifugus (Chiroptera). Comparative Biochemistry and Physiology Part A: Physiology, 39, 549–552. [DOI] [PubMed] [Google Scholar]
- Oliveira JM, Losano NF, Condessa SS, de Freitas RMP, Cardoso SA, Freitas MB and de Oliveira LL, 2017. Exposure to deltamethrin induces oxidative stress and decreases of energy reserve in tissues of the Neotropical fruit‐eating bat Artibeus lituratus . Ecotoxicology and Environmental Safety, 148, 684–692. 10.1016/j.ecoenv.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Park KJ, 2015. Mitigating the impacts of agriculture on biodiversity: bats and their potential role as bioindicators. Mammalian Biology, 80, 191–204. [Google Scholar]
- Parsons KN, Jones G, Davidson‐Watts I and Greenaway F, 2003. Swarming of bats at underground sites in Britain—implications for conservation. Biological Conservation, 111, 63–70. [Google Scholar]
- Pluciński T, Żmihorski M and Pluciński P, 2015. Impact of night‐time crop harvesting on bat activity in agricultural landscape. Zoology and Ecology, 25, 1–7. [Google Scholar]
- Podlutsky AJ, Khritankov AM, Ovodov ND and Austad SN, 2005. A new field record for bat longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 60, 1366–1368. [DOI] [PubMed] [Google Scholar]
- Popa‐Lisseanu AG, Bontadina F and Ibáñez C, 2009. Giant noctule bats face conflicting constraints between roosting and foraging in a fragmented and heterogeneous landscape. Journal of Zoology, 278, 126–133. [Google Scholar]
- Puig‐Montserrat X, Torre I, López‐Baucells A, Guerrieri E, Monti MM, Ràfols‐García R and Flaquer C, 2015. Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mammalian Biology‐Zeitschrift für Säugetierkunde, 80, 237–245. [Google Scholar]
- Racey PA and Entwistle AC, 2003. Conservation ecology of bats In: Kunz TH. and Fenton MB. (eds.). Bat Ecology. The University of Chicago Press, Chicago: p. 2003. [Google Scholar]
- Ratcliffe JM, Elemans CP, Jakobsen L and Surlykke A, 2013. How the bat got its buzz. Biology Letters, 9, 20121031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann D, Streloke M and Winkler R, 2001. New basic drift values in the authorisation procedure for plant protection products. In: Forster R and Streloke M (eds.)., Workshop on Risk Assessment and Risk Mitigation Measures in the Context of the Authorization of Plant Protection Products (WORMM). Mitt Biol Bundesanst Land‐Forstwirtsch Berlin‐Dahlem, 383, 133‐141.
- Read AF and Harvey PH, 1989. Life history differences among the eutherian radiations. Journal of Zoology, 219, 329–353. [Google Scholar]
- Rodríguez‐Durán A, 1995. Metabolic rates and thermal conductance in four species of neotropical bats roosting in hot caves. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 110, 347–355. [DOI] [PubMed] [Google Scholar]
- Rossiter SJ, Ransome RD, Faulkes CG, Le Comber SC and Gareth J, 2005. Mate fidelity and intra‐lineage polygyny in greater horseshoe bats. Nature, 437, 408. [DOI] [PubMed] [Google Scholar]
- Ruf T and Geiser F, 2015. Daily torpor and hibernation in birds and mammals. Biological Reviews, 90, 891–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ JM and Montgomery WI, 2002. Habitat associations of bats in Northern Ireland: implications for conservation. Biological Conservation, 108, 49–58. [Google Scholar]
- Russo D and Jones G, 2002. Identification of twenty‐two bat species (Mammalia: Chiroptera) from Italy by analysis of time‐expanded recordings of echolocation calls. Journal of Zoology, 258, 91–103. [Google Scholar]
- Russo D and Jones G, 2003. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: conservation implications. Ecography, 26, 197–209. 10.1034/j.1600-0587.2003.03422.x [DOI] [Google Scholar]
- Russo D, Jones G and Migliozzi A, 2002. Habitat selection by the Mediterranean horseshoe bat, Rhinolophus euryale (Chiroptera: Rhinolophidae) in a rural area of southern Italy and implications for conservation. Biological Conservation, 107, 71–81. [Google Scholar]
- Russo D, Cistrone L and Jones G, 2005a. Spatial and temporal patterns of roost use by tree‐dwelling barbastelle bats Barbastella barbastellus . Ecography, 28, 769–776. [Google Scholar]
- Russo D, Almenar D, Aihartza J, Goiti U, Salsamendi E and Garin I, 2005b. Habitat selection in sympatric Rhinolophus mehelyi and R. euryale (Mammalia: Chiroptera). Journal of Zoology, 266, 327–332. [Google Scholar]
- Russo D, Jones G and Arlettaz R, 2007. Echolocation and passive listening by foraging mouse‐eared bats Myotis myotis and M. blythii . Journal of Experimental Biology, 210, 166–176. [DOI] [PubMed] [Google Scholar]
- Russo D, Maglio G, Rainho A, Meyer CF and Palmeirim JM, 2011a. Out of the dark: diurnal activity in the bat Hipposideros ruber on São Tomé island (West Africa). Mammalian Biology‐Zeitschrift für Säugetierkunde, 76, 701–708. [Google Scholar]
- Russo D, Cistrone L, Garonna AP and Jones G, 2011b. The early bat catches the fly: daylight foraging in soprano pipistrelles. Mammalian Biology, 1, 87–89. [Google Scholar]
- Russo D, Billington G, Bontadina F, Dekker J, Dietz M, Gazaryan S and Ruczyński I, 2016a. Identifying key research objectives to make European forests greener for bats. Frontiers in Ecology and Evolution, 4, 87. [Google Scholar]
- Russo D, Ancillotto L, Cistrone L and Korine C, 2016b. The buzz of drinking on the wing in echolocating bats. Ethology, 122, 226–235. [Google Scholar]
- Russo D, Cistrone L, Budinski I, Console G, Della Corte M, Milighetti C, Di Salvo I, Nardone V, Brigham RM and Ancillotto R, 2017a. Sociality influences thermoregulation and roost switching in a forest bat using ephemeral roosts. Ecology and Evolution, 7, 5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D, Cistrone L, Libralato N, Korine C, Jones G and Ancillotto L, 2017b. Adverse effects of artificial illumination on bat drinking activity. Animal Conservation, 20, 492–501. [Google Scholar]
- Russo D, Ancillotto L, Cistrone L, Libralato N, Domer A, Cohen S and Korine C, 2018a. Effects of artificial illumination on drinking bats: a field test in forest and desert habitats. Animal, Conservation. [Google Scholar]
- Russo D, Bosso L and Ancillotto L, 2018b. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: research frontiers and management implications. Agriculture, Ecosystems & Environment, 266, 31–38. [Google Scholar]
- Russo D, Ancillotto L and Jones G, 2018c. Bats are still not birds in the digital era: echolocation call variation and why it matters for bat species identification. Canadian Journal of Zoology, 96, 63–78. [Google Scholar]
- Rydell J, 1986. Foraging and diet of the northern bat Eptesicus nilssoni in Sweden. Ecography, 9, 272–276. 10.1111/j.1600-0587.1986.tb01219.x [DOI] [Google Scholar]
- Safi K, König B and Kerth G, 2007. Sex differences in population genetics, home range size and habitat use of the parti‐colored bat (Vespertilio murinus, Linnaeus 1758) in Switzerland and their consequences for conservation. Biological Conservation, 137, 28–36. [Google Scholar]
- Santana SE, Grosse IR and Dumont ER, 2012. Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution: International Journal of Organic. Evolution, 66, 2587–2598. [DOI] [PubMed] [Google Scholar]
- Schuller G and Pollak G, 1979. Disproportionate frequency representation in the inferior colliculus of Doppler‐compensating greater horseshoe bats: evidence for an acoustic fovea. Journal of Comparative Physiology, 132, 47–54. [Google Scholar]
- Seim I, Fang X, Xiong Z, Lobanov AV, Huang Z, Ma S, Feng Y, Turanov AA, Yabing Zhu Y, Lenz TL, Gerashchenko MV, Fan D, Yim SH, Yao X, Jordan D, Xiong Y, Ma Y, Lyapunov AN, Chen G, Kulakova OI, Sun Y, Lee SG, Bronson RT, Moskalev AA, Sunyaev SR, Zhang G, Krogh A, Wang J and Gladyshev VN, 2013. Genome analysis reveals insights into physiology and longevity of the Brandt's bat Myotis brandtii. Nature Communications, 4, 2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore RF, Myhill DG, French MC, Leach DF and Stebbings RE, 1991. Toxicity and tissue distribution of pentachlorophenol and permethrin in pipistrelle bats experimentally exposed to treated timber. Elsevier, Environmental Pollution, 73, 101–118. [DOI] [PubMed] [Google Scholar]
- Simmons NB, Seymour KL, Habersetzer J and Gunnell GF, 2008. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature, 451, 818. [DOI] [PubMed] [Google Scholar]
- Sluiter JW and Van Heerdt PF, 1964. Seasonal habits of the noctule bat (Nyctalus noctula). Archives Néerlandaises de Zoologie, 16, 423–439. [Google Scholar]
- Smith C, 2008. The Biology of Sensory Systems. UK, John Wiley and Sons Ltd. [Google Scholar]
- Smith E and Goodpaster W, 1958. Homing in non‐migratory bats. Science, 127, 644.13529030 [Google Scholar]
- Soriano P, Ruiz A and Arends A, 2002. Physiological responses to ambient temperature manipulation by three species of bats from Andean cloud forests. Journal of Mammalogy, 83, 445–457. [Google Scholar]
- Speakman JR and Racey PA, 1989. Hibernal ecology of the pipistrelle bat: energy expenditure, water requirements and mass loss, implications for survival and the function of winter emergence flights. Journal of Animal Ecology, 58, 797–813. [Google Scholar]
- Speakman JR, Thomas DW, Kunz TH and Fenton MB, 2003. Physiological ecology and energetics of bats. Bat Ecology, 430–490. [Google Scholar]
- Stahlschmidt P and Brühl CA, 2012. Bats at risk? Bat activity and insecticide residue analysis of food items in an apple orchard. Environmental Toxicology and Chemistry, 31, 1556–1563. 10.1002/etc.1834 [DOI] [PubMed] [Google Scholar]
- Stahlschmidt P, Hahn M and Brühl CA, 2017. Nocturnal Risks‐High Bat Activity in the Agricultural Landscape Indicates Potential Pesticide Exposure. Frontiers in Environmental. Science, 5, 10.3389/fenvs.2017.00062 [DOI] [Google Scholar]
- Stawski C and Geiser F, 2011. Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 301, R542–R547. [DOI] [PubMed] [Google Scholar]
- Stone E, Zeale MR, Newson SE, Browne WJ, Harris S and Jones G, 2015a. Managing conflict between bats and humans: the response of soprano pipistrelles (Pipistrellus pygmaeus) to exclusion from roosts in houses. PLoS ONE, 10, e0131825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Harris S and Jones G, 2015b. Impacts of artificial lighting on bats: a review of challenges and solutions. Mammalian Biology, 80, 213–219. [Google Scholar]
- Streit B, Winter S and Nagel A, 1995. Bioaccumulation of selected organochlorines in bats and tits: Influence of chemistry and biology. Environmental Science and Pollution Research International, 2, 194–199. 10.1007/BF02986762 [DOI] [PubMed] [Google Scholar]
- Swanepoel RE, Racey PA, Shore RF and Speakman JR, 1999. Energetic effects of sublethal exposure to lindane on pipistrelle bats (Pipistrellus pipistrellus). Environmental Pollution, 104, 169–177. [Google Scholar]
- Taylor PJ, Grass I, Alberts AJ, Joubert E and Tscharntke T, 2018. Economic value of bat predation services–a review and new estimates from macadamia orchards. Ecosystem Services, 30, 372–381. [Google Scholar]
- Teeling EC, Springer MS, Madsen O, Bates P, O'brien SJ and Murphy WJ, 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science, 307, 580–584. [DOI] [PubMed] [Google Scholar]
- Ter Hofstede HM and Fenton MB, 2005. Relationships between roost preferences, ectoparasite density, and grooming behaviour of neotropical bats. Journal of Zoology, 266, 333–340. [Google Scholar]
- Thomas DW, 1995. Hibernating Bats Are Sensitive to Nontactile Human Disturbance. Journal of Mammalogy, 76, 940–946. [Google Scholar]
- Thomas SP, 1981. Ventilation and oxygen extraction in the bat Pteropus gouldii during rest and steady flight. Journal of Experimental Biology, 94, 231–250. [Google Scholar]
- Thomas SP, 1987. The physiology of bat flight. In Fenton MB, Racey P and Rayner J MV (eds.). Recent Advances in the Study of Bats, pp. 75–99.
- Thomas SP, Follette DB and Farabaugh AT, 1991. Influence of air temperature on ventilation rates and thermoregulation of a flying bat. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 260, R960–R968. [DOI] [PubMed] [Google Scholar]
- Thomson SC and Speakman JR, 1999. Absorption of visible spectrum radiation by the wing membranes of living pteropodid bats. Journal of Comparative Physiology B, 169, 187–194. [DOI] [PubMed] [Google Scholar]
- Toffoli R and Rughetti M, 2017. Bat activity in rice paddies: Organic and conventional farms compared to unmanaged habitat. Agriculture Ecosystems & Environment, 249, 123–129. 10.1016/j.agee.2017.08.022 [DOI] [Google Scholar]
- Tsoar A, Nathan R, Bartan Y, Vyssotski A, Dell'Omo G and Ulanovsky N, 2011. Large‐scale navigational map in a mammal. PNAS, 108, E718–E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagkogeorga G, Parker J, Stupka E, Cotton JA and Rossiter SJ, 2013. Phylogenomic analyses elucidate the evolutionary relationships of bats. Current Biology, 23, 2262–2267. [DOI] [PubMed] [Google Scholar]
- US‐EPA (US Environmental Protection Agency), 2015. Technical description and user's guidance document for the terrestrial investigation model (TIM) Version 3.0 BETA, March 25, 2015. Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency, Washington, DC.
- Van der Meij T, Van Strien AJ, Haysom KA, Dekker J, Russ J, Biala K and Limpens H, 2015. Return of the bats? A prototype indicator of trends in European bat populations in underground hibernacula. Mammalian Biology‐Zeitschrift für Säugetierkunde, 80, 170–177. [Google Scholar]
- Vanderelst D, De Mey F, Peremans H, Geipe I, Kalko E and Firzlaff U, 2010. What noseleaves do for FM bats depends on their degree of sensorial specialization. PLoS ONE, 5, e11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan N, Jones G and Harris G, 1997. Habitat Use by Bats (Chiroptera) Assessed by Means of a Broad‐Band Acoustic Method. Journal of Applied Ecology, 34, 716–730. [Google Scholar]
- Veith M, Beer N, Kiefer A, Johannesen J and Seitz A, 2004. The role of swarming sites for maintaining gene flow in the brown long‐eared bat (Plecotus auritus). Heredity, 93, 342. [DOI] [PubMed] [Google Scholar]
- Verboom B and Huitema H, 1997. The importance of linear landscape elements for the pipistrelle Pipistrellus pipistrellus and the serotine bat Eptesicus serotinus . Landscape Ecology, 12, 117–125. 10.1007/bf02698211 [DOI] [Google Scholar]
- Veselka N, McErlain DD, Holdsworth DW, Eger JL, Chhem RK, Mason MJ and Fenton MB, 2010. A bony connection signals laryngeal echolocation in bats. Nature, 463, 939. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Capps KA, Dechmann DKN, Michener RH and Kunz TH, 2008. Nutrition or detoxification: why bats visit mineral licks of the Amazonian rainforest. PLoS ONE, 3, e2011 10.1371/journal.pone.0002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CC, Phelps KL, Aguirre LF, Schoeman MC, Vanitharani J and Zubaid A, 2016. Bats and buildings: the conservation of synanthropic bats. In Bats in the Anthropocene: conservation of bats in a changing world. Springer, Cham: pp. 427–462. [Google Scholar]
- Voigt CC, Currie SE, Fritze M, Roeleke M and Lindecke O, 2018. Conservation Strategies for Bats Flying at High Altitudes. BioScience, 68, 427–435. [Google Scholar]
- Welch JN and Leppanen C, 2017. The threat of invasive species to bats: a review. Mammal Review, 47, 277–290. [Google Scholar]
- Wermundsen T and Siivonen Y, 2008. Foraging habitats of bats in southern Finland. Acta Theriologica, 53, 229–240. 10.1007/bf03193119 [DOI] [Google Scholar]
- Wickramasinghe LP, Harris S, Jones G and Vaughan N, 2003. Bat activity and species richness on organic and conventional farms: impact of agricultural intensification. Journal of Applied Ecology, 40, 984–993. [Google Scholar]
- Wilkinson GS, 1986. Social grooming in the common vampire bat Desmodus rotundus. Animal Behaviour, 34, 1880–1889. [Google Scholar]
- Wilkinson GS and South JM, 2002. Life history, ecology and longevity in bats. Aging Cell, 1, 124–131. [DOI] [PubMed] [Google Scholar]
- Williams C, Salter L and Jones G, 2010. The winter diet of the lesser horseshoe bat (Rhinolophus hipposideros) in Britain and Ireland. Hystrix, the Italian Journal of Mammalogy, 22. [Google Scholar]
- Willis CK, 2008. Do roost type or sociality predict warming rate? A phylogenetic analysis of torpor arousal. Hypometabolism in Animals: Hibernation, Torpor and Cryobiology. Interpak Books. South Africa, Pietermaritzburg: pp. 373–384. [Google Scholar]
- Willis CK and Brigham RM, 2007. Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity‐dwelling bat. Behavioral Ecology and Sociobiology, 62, 97–108. [Google Scholar]
- Willis CK, Lane JE, Liknes ET, Swanson DL and Brigham RM, 2005. Thermal energetics of female big brown bats (Eptesicus fuscus). Canadian Journal of Zoology, 83, 871–879. [Google Scholar]
- Winter Y and Von Helversen O, 1998. The energy cost of flight: do small bats fly more cheaply than birds? Journal of Comparative Physiology B, 168, 105–111. [DOI] [PubMed] [Google Scholar]
- Zahn A, Haselbach H and Güttinger R, 2005. Foraging activity of central European Myotis myotis in a landscape dominated by spruce monocultures. Mammalian Biology‐Zeitschrift für Säugetierkunde, 70, 265–270. [Google Scholar]
- Zahn A, Rodrigues L, Rainho A and Palmeirim JM, 2007. Critical times of the year for Myotis myotis, a temperate zone bat: Roles of climate and food resources. Acta Chiropterologica, 9, 115–125. [Google Scholar]


