Abstract
Following a request from the European Commission, the Panel on Nutrition, Novel Foods and Food Allergens (NDA) revised its 2009 Opinion on the appropriate age for introduction of complementary feeding of infants. This age has been evaluated considering the effects on health outcomes, nutritional aspects and infant development, and depends on the individual's characteristics and development. As long as foods have an age‐appropriate texture, are nutritionally appropriate and prepared following good hygiene practices, there is no convincing evidence that at any age investigated in the included studies (< 1 to < 6 months), the introduction of complementary foods (CFs) is associated with adverse health effects or benefits (except for infants at risk of iron depletion). For nutritional reasons, the majority of infants need CFs from around 6 months of age. Infants at risk of iron depletion (exclusively breastfed infants born to mothers with low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born small‐for‐gestational age or with high growth velocity) may benefit from earlier introduction of CFs that are a source of iron. The earliest developmental skills relevant for consuming pureed CFs can be observed between 3 and 4 months of age. Skills for consuming finger foods can be observed in some infants at 4 months, but more commonly at 5–7 months. The fact that an infant may be ready from a neurodevelopmental perspective to progress to a more diversified diet before 6 months of age does not imply that there is a need to introduce CFs. There is no reason to postpone the introduction of potentially allergenic foods (egg, cereals, fish and peanut) to a later age than that of other CFs as far as the risk of developing atopic diseases is concerned. Regarding the risk of coeliac disease, gluten can be introduced with other CFs.
Keywords: complementary food, introduction, timing, infant, health outcome, development, systematic review
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1686/full
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4969/full
Summary
Following a request from the European Commission, the Panel on Nutrition, Novel Foods and Food Allergens (NDA Panel) revised its Scientific Opinion of 2009 on the appropriate age for introduction of complementary feeding of infants.
This request arises in the context of the information regarding the use of processed cereal‐based foods and baby foods. This information is required for a future delegated act of the European Commission on these foods foreseen in Regulation (EU) No 609/2013 on food intended for infants and young children. This Regulation revises the legal framework set out in Directive 2009/39/EC on foodstuffs intended for particular nutritional uses and the specific Directives adopted under this framework, including Directive 2006/125/EC on processed cereal‐based foods and baby foods for infants and young children. This Directive required the mandatory indication of a statement on the appropriate age from which processed cereal‐based foods and baby foods may be used, that shall be not less than four months for any products.
The Panel specified upfront in a protocol the strategy and methodology to collect and evaluate scientific data on possible relationships between the timing of introduction of complementary foods (CFs) and a number of (health) outcomes. This protocol was released for public consultation and published, alongside a report on how comments received during the public consultation were taken into account in the final protocol. A draft of this Scientific Opinion was also released for public consultation and revised according to the comments received, where appropriate. The comments that were received were addressed in detail in a technical report that is published together with this Scientific Opinion.
The Panel considers that exclusive breastfeeding is nutritionally appropriate up to 6 months of age for the majority of healthy infants born at term from healthy well‐nourished mothers.
The purpose of this Scientific Opinion is to assess the scientific evidence in relation to whether there are:
any developmental factors relevant for the introduction of CFs,
any adverse health effects associated with the introduction of CFs before 6 months of age, and
any benefits associated with the introduction of CFs before 6 months of age.
Out of the scope of this Scientific Opinion are:
public health recommendations for the introduction of CFs; this task is outside the remit of the European Food Safety Authority (EFSA) but it is the role of public health authorities in Member States;
the effects of the duration of exclusive breastfeeding on the selected health outcomes, as the assessment is performed irrespective of whether infants were initially exclusively breastfed or formula fed;
the health benefits of breastfeeding itself (for the infant/child and the mother);
the effects on health outcomes of introduction of CFs solely after 6 months of age, as there is a nutritional requirement for CFs for the majority of exclusively breastfed infants by 6 months onwards;
the effects of the amount, order of introduction, variety, composition and texture of CFs;
the role of aspects, such as social interactions and the cultural context, on the appropriate age of introduction of CFs;
risks related to, e.g. chemical or microbiological contaminants or pesticides.
The definition of CFs differs in different publications. In the context of this Scientific Opinion, complementary feeding is defined as the period when CFs are given together with either breast milk or formula or both. CFs in this Scientific Opinion comprise foods other than breast milk, formula, water or vitamins that are given to infants and can be beverages, spoon‐fed pureed foods, spoon‐fed lumpy foods or finger foods, either prepared at home or produced commercially. This definition is in line with that used by some other bodies, such as the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), the UK Scientific Advisory Committee on Nutrition (SACN), the United States Department of Agriculture (USDA) and the American Academy of Pediatrics (AAP) but differs from the one that has been used by, for example the World Health Organization (WHO), which included formula in the definition of CFs.
In the interpretation of the Terms of Reference, the choice has been made by the Panel to limit the assessment to health effects associated with the timing of introduction of CFs or specific foods before the age of 6 months. This led to the exclusion of studies that had been considered by other bodies in their assessments done in different contexts than this Scientific Opinion. This is, for example, the case for some studies that investigated the introduction of some allergenic foods, such as fish, egg and peanut, or of gluten after 6 months of age.
The appropriate age of introduction of CFs is influenced not only by nutritional considerations, but also by effects on health outcomes and by infant development. Considering the influence of various factors, the Panel considers that it is likely that there is an appropriate age range rather than a single appropriate age for the introduction of CFs.
The Panel undertook a systematic literature search of intervention and observational studies for the assessment of the association between the timing of introduction of CFs and health outcomes, while an extensive literature search was carried out specifically for developmental determinants of the introduction of CFs. The Panel also appraised the risk of bias (RoB) of the studies included from the systematic search, thus classifying them as low, intermediate or high RoB (Tiers 1, 2 or 3).
Studies considered pertinent for this assessment were those in infants and children, generally healthy at the time of introduction of CFs, either born at term or preterm. The study groups had to be alike in terms of the type of milk feeding (breast milk or formula or mixed, with no additional behavioural interventions), i.e. the study groups had to differ only in the timing of the introduction of CFs. The selected papers were studies in which at least one group was introduced to CFs before 6 months of age. Studies on a specific CF item or food group were also considered for certain health outcomes (e.g. gluten in relation to the risk of coeliac disease). The list of outcomes to be evaluated was defined in the protocol, based on the previous EFSA Scientific Opinion of 2009, and expanded when evidence was available. Endpoints for which only one study was available were not included. In the systematic review, the Panel has assessed 283 studies that reported on the relationship between the timing of introduction of CFs (or specific foods for some outcomes) in relation to (1) body weight and growth, including body mass index (BMI), risk of developing overweight and obesity, as well as body composition, (2) risk of developing atopic diseases or symptoms of atopic diseases, such as asthma‐like symptoms, eczema, allergic rhinitis and symptomatic food allergy, (3) risk of developing coeliac disease and type 1 diabetes mellitus, (4) blood pressure, (5) infections, (6) sleep, (7) infant and child development, (8) nutrient status (i.e. iron) and (9) food preferences and eating behaviours later in life. For these outcomes, whenever enough data were available, forest plots were created, and pooled estimates were calculated from the individual studies, with associated 95% confidence and prediction intervals, using random effects meta‐analyses. Evidence was discussed separately for infants born at term and those born preterm.
Developmental skills relevant for the progression from a liquid to a diversified diet
For the assessment of the oral–motor developmental readiness of infants to receive CFs, the Panel conducted an extensive literature search to retrieve studies, review papers and text books that provided information on when certain milestones indicative of the oral–motor readiness to receive CFs are reached in the normally developing term infant.
One determinant of the appropriate age range of introduction of CFs is the infant's anatomical, physiological and oral–motor readiness to receive foods other than breast milk or formula. Gastrointestinal and renal functions are not limiting factors with respect to the timing of introduction of CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding. The changes that are required for progressing from a liquid to a semi‐solid and solid diet are: (1) anatomical changes in the oral cavity, (2) the disappearance or diminishing of reflexes present at birth that coordinate suckling, swallowing and respiration, and protect the infant from aspiration and choking (i.e. the extrusion reflex of the tongue), in favour of more voluntary movements and (3) the development of gross motor skills (head and trunk control to allow an improved movement of the jaw) and fine motor skills (lip, tongue and jaw movements).
The age range at which infants attain these developmental milestones shows considerable variation within and between populations, presumably reflecting the infant's innate developmental trajectory combined with the opportunities and experiences provided by the carer.
The earliest gross motor skills indicative of developmental readiness for spoon‐feeding of pureed foods (i.e. holding the head in midline when in supine position and to control its head well when pulled to sitting or at aided sitting) can be observed between 3 and 4 months of age. At this age, it can be assumed that the rooting and the extrusion reflexes may have also diminished in some infants. The gross motor skill indicative of developmental readiness for self‐feeding finger foods (i.e. sitting without support) can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age. In preterm infants, the necessary developmental milestones for feeding are also reached around the same age range (post‐term), depending on the severity of illness experienced during the neonatal period, the degree of prematurity and any sequelae.
Nutritional need for the introduction of CFs
Most infants do not need CFs for nutritional reasons up to around 6 months of age, with the exception of some infants at risk of iron depletion who may benefit from earlier introduction of CFs that are a source of iron. From the systematic review, the Panel concludes that there is high confidence in the evidence that the introduction of CFs at 4 months of age compared with 6 months of age reduces the risk of iron depletion at 6 months of age in exclusively breastfed infants at risk of iron depletion. However, the effect on iron depletion is not an effect of introducing CFs per se, but an effect of introducing CFs that are a source of iron. Infants that may benefit from an early introduction of CFs that are a source of iron are exclusively breastfed infants born to mothers with a low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born small‐for‐gestational age, or with a high growth velocity.
Adverse health effects or benefits associated with the introduction of CFs before 6 months of age
There is no convincing evidence for adverse health effects of introducing CFs at any of the ages investigated in the included studies. In the studies rated as Tiers 1 and 2, the definition of ‘early introduction of CFs’ ranged from < 1 month to < 6 months. In most instances, < 3 or < 4 months of age was investigated as ‘early introduction’ without precise information on the earliest age at which infants in the study were introduced to CFs. The Panel applied a weight of evidence approach to derive its conclusions and grade the confidence in the evidence.
The Panel concludes (high level of confidence) (1) that there was no effect of introduction of CFs at 3–4 months of age, compared with 6 months of age, on body weight, body length, head circumference, BMI and body composition; (2) that there is no effect of the introduction of gluten at 4 months of age compared with 6 months of age on the risk of developing coeliac disease; and (3) that there is no evidence for an effect or an association between the timing of introduction of CFs in mixed fed populations and iron status at 10–12 months of age.
The Panel concludes (moderate level of confidence) that there is no evidence for an association between the timing of introduction of CFs and body weight (between < 2 and < 6 months vs thereafter), body length (between 2–3 and < 6 months vs thereafter), BMI (between ≤ 2 and ≤ 5 months vs thereafter), body composition (< 4 months vs ≥ 4 to > 6 months) and coeliac disease (for gluten, between ≤ 3 and ≤ 4 months vs thereafter). The Panel also concludes (moderate level of confidence) that there is no evidence for an effect or an association between the timing of introduction of CFs and overweight (between ≤ 2 and < 4 months vs > 2 to > 6 months), obesity (between < 1 and < 4 months vs ≥ 3 to ≥ 6 months), atopic diseases (at 3–4 vs 6 months), asthma‐like symptoms (at 3–4 vs 6 months for CFs, < 3.75–5.5 months vs thereafter for cereals and < 5.25 to ≤ 6 months vs >5.25 to 8.5 months for fish), eczema (between < 3 and ≤ 6 months vs thereafter), allergic rhinitis (at 3–4 vs 6 months), symptomatic food allergy (at 3–4 vs 6 months), type 1 diabetes mellitus (gluten and CFs, between < 3 and < 5 months vs thereafter), blood pressure (between < 3 and < 5 months vs thereafter) and infections in general (between 3–4 months and < 6 months vs at 6 and > 6 months).
The Panel considers that the confidence level in the evidence was low to very low for a number of outcomes related to atopic diseases (and introduction of specific foods) as well as for gastrointestinal and lower respiratory tract infections, sleep, and infant and child development.
For some outcomes, the evidence was inconsistent and therefore the confidence in the evidence was not graded (i.e. timing of introduction of peanut and peanut allergy, upper respiratory tract infections, and food preferences and eating habits (introduction of CFs and fruit and vegetables)).
Even though there is no convincing evidence for a harmful effect of CF introduction at any age that was studied on the selected health outcomes, the Panel emphasises that foods given to infants should be presented in an age‐appropriate texture (to prevent aspiration and choking), are nutritionally appropriate and are prepared according to good hygiene practices. Also, the fact that, based on the available evidence, CFs could be introduced before 6 months of age does not imply that this is necessary or desirable.
In the following the main findings are summarised:
Specific allergenic foods
In relation to the introduction of allergenic foods (egg, cereals, fish and peanut) into an infant's diet, the Panel concludes that allergenic foods can be introduced in the same way as other CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding. There is no evidence to support postponing the introduction of potentially allergenic foods to a later age than the introduction of other CFs.
-
○
Hen's egg and egg allergy
With respect to egg introduction, the data pointed towards a favourable effect of its introduction between around 3–4 months compared with 6 months of age on the risk of developing egg allergy. However, the confidence in the evidence is low to moderate and is, therefore, insufficient to support introducing egg at around 3–4 months of age in all infants for the prevention of egg allergy. In the available studies, no serious adverse reactions occurred with consumption of cooked egg, while anaphylactic reactions were observed when the intervention consisted of pasteurised raw egg powder. As far as the risk of allergy is concerned, cooked egg can be introduced into the diet of infants when other CFs are introduced.
-
○
Peanut and peanut allergy
There is evidence that peanut introduction during the first year of life (either at 4–10 months or at 4–6 months) compared with peanut avoidance up to 5 years of age reduces the risk of developing peanut allergy. However, the evidence is insufficient to conclude whether, when comparing infants introduced to peanut ≤ 6 months of age with those introduced > 6 months (but still within the first year of life, which is the subject of this mandate), a similar effect occurs. As the evidence was inconsistent, no level of confidence was assigned.
Overweight and obesity
There is no evidence that the timing of introduction of CFs is associated with higher risk of developing overweight and obesity (moderate confidence in the evidence). This finding is supported by the results on body weight, BMI and fat mass (moderate to high confidence in the evidence, depending on the outcome).
Coeliac disease and type 1 diabetes mellitus
If gluten is introduced, there is no evidence for beneficial or adverse health effects of gluten introduction < 6 months of age compared with thereafter with respect to the risk of developing coeliac disease or type 1 diabetes mellitus, nor is there evidence that (any) continued breastfeeding could modify the effect of gluten introduction at that age (moderate to high level of confidence in the evidence, depending on the age of introduction of CFs investigated). As far as the risk of developing coeliac disease or type 1 diabetes mellitus is concerned, gluten can be introduced to an infant's diet when other CFs are introduced. Time to onset of coeliac disease or type 1 diabetes mellitus in relation to the timing of introduction of CFs was not considered.
Infections
When hygiene conditions are satisfactory,1 there is no evidence that the introduction of CFs < 6 months of age compared with thereafter is associated with an increased risk of (1) gastrointestinal infections (low level of confidence in the evidence), (2) lower respiratory tract infections (moderate level of confidence in the evidence) or (3) infections in general (moderate level of confidence in the evidence). The evidence for upper respiratory tract infections is inconsistent and insufficient to draw conclusions.
Sleep‐related endpoints
Even though the statistical analyses of the effect of the age of introduction of CFs on sleep‐related endpoints was significant (low level of confidence), the Panel considered that the size of the effect was not biologically relevant.
Preterm infants
The available evidence on preterm infants is limited and comprised only one study in the main line of evidence. From this study, there is no evidence for an effect of introduction of CFs at 4 months post‐term compared with 6 months post‐term on body weight, body length and head circumference (low level of confidence in the evidence).
Conclusions
The appropriate age range of introduction of CFs has been evaluated taking into account effects on health outcomes, nutritional aspects and infant development.
The available data do not allow the determination of a single age for the introduction of CFs for infants living in Europe. The appropriate age range depends on the individual's characteristics and development, even more so if the infant was born preterm.
As long as the foods are given in an age‐appropriate texture, are nutritionally appropriate and prepared according to good hygiene practices, there is no convincing evidence that the introduction of CFs is associated with either adverse or beneficial health effects (except for infants at risk of iron depletion) at any age investigated in the included studies (< 1 month to < 6 months for earlier introduction).
For nutritional reasons, the majority of infants need CFs from around 6 months of age. For preterm infants, this refers to post‐term age. Infants at risk of iron depletion (exclusively breastfed infants born to mothers with low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born small‐for‐gestational age or with high growth velocity) may benefit from introduction of CFs that are a source of iron before 6 months of age.
The earliest developmental skills relevant for the consumption of spoon‐fed pureed CFs can be observed between 3 and 4 months of age. Skills necessary for consuming self‐fed finger foods can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age. For preterm infants, this refers to post‐term age.
The fact that an infant may be ready from a neurodevelopmental point of view to progress from a liquid to a more diversified diet before 6 months of age does not imply that there is a need to introduce CFs.
There is no reason to postpone the introduction of potentially allergenic foods (egg, cereals, fish and peanut) to a later age than that of other CFs as far as the risk of developing atopic diseases is concerned. Regarding the risk of coeliac disease, gluten can be introduced with other CFs.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Directive 2009/39/EC2 of the European Parliament and of the Council on foodstuffs intended for particular nutritional uses lays down general compositional and information requirements of such foods that are specially designed to meet the particular nutritional requirements of the persons to whom they are intended, including those ‘of infants and young children in good health’.
Directive 2006/125/EC3 has established compositional and labelling requirements for processed cereal‐based foods and baby foods for infants and young children which are defined in the legislation as “foodstuffs for particular nutritional use fulfilling the particular requirements of infants and young children in good health (…) and are intended for the use by infants while they are being weaned, and by young children as a supplement to their diet and/or for their progressive adaptation to ordinary food”.
The Directive defines ‘infants’ as “children under the age of 12 months” and ‘young children’ as “children aged between one and three years”.
In particular, Article 8(1)(a) of Directive 2006/125/EC requires the mandatory indication of a statement as to the appropriate age from which processed cereal‐based food and baby food may be used. According to this provision the stated age shall be not less than four months for any products. The product, if its use is recommended from four months, may indicate that it is suitable from that age unless independent persons having qualifications in medicine, nutrition or pharmacy, or other professionals responsible for maternal and child care, advise otherwise. This requirement is in line with EFSA's scientific opinion on the appropriate age for introduction of complementary feeding of infants.
Regulation (EU) No 609/20134 of the European Parliament and of the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control revises the legal framework applicable to foods for particular nutritional uses as set out in Directive 2009/39/EC and the specific Directives adopted under this framework, including Directive 2006/125/EC.
The Regulation includes in its scope processed cereal‐based food and baby food, maintains the definitions as laid down in Directive 2006/125/EC for them. With respect to labelling, presentation and advertising Article 9(5) of the Regulation generally requires amongst others that the food governed by this legislation “shall provide information for the appropriate use of such food”.
In addition to the general requirements of Regulation (EU) No 609/2013 the Commission is required to lay down by the means of delegated act specific compositional and information requirements for processed cereal‐based food and baby food, taking into account relevant technical and new scientific evidence and knowledge available.
In the context of the information to be provided regarding the use of processed cereal‐based and baby food, questions have been raised on the appropriate age for introduction of complementary feeding of infants.
Taking into account the abovementioned, it is considered necessary, at this stage to request EFSA to update the conclusions of its scientific opinion on the appropriate age for introduction of complementary feeding of infants in light of more recent scientific evidence.
1.1.2. Terms of reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002, the European Commission asks EFSA to:
Update EFSA's scientific opinion on the appropriate age for introduction of complementary feeding of infants in light of more recent scientific evidence and knowledge available.
1.2. Previous assessments
In its previous Scientific Opinion (EFSA NDA Panel, 2009), the Panel concluded that ‘the introduction of complementary food into the diet of healthy term infants in the European Union (EU) between the age of 4 and 6 months is safe and does not pose a risk of adverse health effects’. The Panel also concluded that ‘available data on the risk of coeliac disease and type 1 diabetes mellitus (T1DM) support also the timing of the introduction of gluten‐containing food (preferably while still breastfeeding) not later than 6 months of age’. These conclusions were based on data from high‐income countries, and primarily on observational data in exclusively breastfed infants, healthy and born at term. The list of endpoints, discussed narratively in the Scientific Opinion in relation to exclusive breastfeeding and/or age of introduction of complementary foods (CFs), were nutrient requirement, growth, neurodevelopment, digestive system, renal function, development of food preferences, and risk of obesity, type 2 diabetes mellitus, atopic diseases, coeliac disease, T1DM, infectious morbidity and caries.
The Panel was also aware of the following position statements or reports. In the UK, the Scientific Advisory Committee on Nutrition (SACN) and the Committee on Toxicity of Chemicals in food, Consumer Products and the Environment (COT) published statements on health benefits and risks of introduction of peanut and hen's egg into the infant diet before 6 months and on the timing of introduction of gluten into the infant diet (SACN‐COT, 2011, 2018). Their main conclusions were that the ‘evidence that the introduction of hen's egg before 6 months might be beneficial was limited’. The committees concluded as well that ‘there were insufficient data to demonstrate that the introduction of peanut or hen's egg into the infant diet between four and six months of age reduced the risk of developing food allergy to any greater extent than introduction from around six months’. The committees also concluded that ‘currently available evidence on the timing of introduction of gluten into the infant diet and subsequent risk of coeliac disease and [type 1 diabetes mellitus T1DM] is insufficient to support recommendations about the appropriate timing of introduction of gluten into the infant diet beyond 3 completed months of age, for either the general population or high‐risk sub‐ populations’. They also considered that the evidence was insufficient to support the introduction of gluten into the infant's diet not later than 6 completed months of age with the objective of reducing the risk of developing coeliac disease and T1DM.
The Panel was also aware that the SACN report on feeding in the first year of life covers aspects of infant feeding other than complementary feeding, such as the adequate duration of breastfeeding (SACN, 2018). Its main conclusions in relation to the timing of introduction of complementary foods (CFs) were that ‘(a) observed relationships between the timing of introduction of complementary foods and obesity were in most prospective studies attributed to rapid early weight gain rather than early introduction of complementary foods, (b) there is insufficient evidence to demonstrate that introduction of peanut, hen's egg, gluten or fish before 6 months of age reduces the risk of developing food allergy as compared to the introduction at around 6 months of age, (c) there is high quality evidence that the timing of introduction of gluten is not related to the risk of developing coeliac disease, (d) there is low quality evidence that fish introduction before 6 to 12 months of age [i.e. from evidence covering different ages of introduction between < 6 and 12 months of age] is associated with a reduced risk of developing allergic rhinitis and sensitisation, (e) there is no “critical window” for introducing complementary foods that is related to later food acceptance’.
The Panel took note of the position papers of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) on complementary feeding and on gluten introduction and risk of coeliac disease (Szajewska et al., 2016; Fewtrell et al., 2017). Regarding specifically the introduction of CFs, their main conclusions were that ‘complementary foods (solids and liquids other than breast milk or infant formula) should not be introduced before 4 months but should not be delayed beyond 6 months’. Regarding the age of introduction of allergenic foods, their main conclusions were that ‘allergenic foods may be introduced when complementary food is commenced any time after 4 months’. In addition, ESPGHAN considered that ‘infants at high risk of peanut allergy […] should have peanut introduced between 4 and 11 months, following evaluation by an appropriate trained specialist’ and ‘gluten may be introduced between 4 and 12 months’. ESPGHAN indicated that ‘although breastfeeding should be promoted for its other well‐established health benefits, neither any breastfeeding nor breastfeeding during gluten introduction has been shown to reduce the risk of coeliac disease’.
The World Health Organization (WHO) report on Feeding and Nutrition of Infants and Young Children (WHO Regional Office for Europe, 2003) concluded, based on a narrative description of the evidence, that ‘complementary foods should be introduced at about 6 months of age. Some infants may need complementary foods earlier, but not before 4 months of age’.
The United States Department of Agriculture (USDA) and the Department of Health and Human Services launched the Pregnancy and Birth to 24 months project, which involved conducting a series of systematic reviews about the timing of introduction of complementary feeding in healthy term infants. They concluded that there was moderate evidence that there was no relationship between the introduction of CFs at 4–5 months compared with 6 months and weight, length, overweight and obesity, and body composition. However, limited evidence was found that introducing CFs before 4 months compared with later could increase the odds of overweight and obesity (English et al., 2019a). For outcomes on atopic diseases, Obbagy et al. (2019a) reported that there was moderate evidence for no association between the age of CF introduction and the risk of developing food allergy, atopic dermatitis, or childhood asthma. Limited to strong evidence (depending on the specific food studied) suggested that the risk of food allergy and atopic dermatitis did not increase by introducing allergenic foods after 4 months of age but within the first year of life, although it may prevent peanut and egg allergy. For bone health and developmental milestones, only three articles were available (English et al., 2019a; Obbagy et al., 2019b). Hence, the authors concluded that insufficient evidence was available to draw conclusions on the relationships, or to grade the confidence in the evidence. For micronutrient status, Obbagy et al. (2019c) found moderate evidence that introducing CFs at 4 months of age compared with 6 months does not affect iron status, derived from evidence generated in high‐income countries.
The Panel also took note of a recent report (Greer et al., 2019) of the American Academy of Pediatrics (AAP). This report concluded that ‘there is no evidence that delaying the introduction of allergenic foods, including peanut, egg, and fish, beyond 4 to 6 months prevents atopic disease’. It also concluded that ‘there is now evidence that the early introduction of infant‐safe forms of peanuts reduces the risk of peanut allergies. Data are less clear for timing of introduction of egg’.
The National Institute of Allergy and Infectious Diseases in the United States provided guidelines on early introduction of peanut into the diet of infants who were at three risk levels (Togias et al., 2017). To reduce the risk of peanut allergy, it was recommended to introduce peanut‐containing foods from 4 to 6 months of age into the diet of infants with severe eczema, egg allergy or both. Moreover, it was suggested to introduce peanut‐containing foods around 6 months of age into the diet of infants with mild‐to‐moderate eczema, and freely into the diet of infants without eczema or any food allergy.
1.3. Definitions
Complementary feeding means the period when CFs are given together with either breast milk or formula or both (EFSA NDA Panel, 2009). This definition is in line with the terms of reference received from the European Commission and is also in line with the definition used by other bodies (e.g. ESPGHAN (Fewtrell et al., 2017), SACN (SACN, 2018), USDA (Obbagy et al., 2019b) or the AAP (AAP, 2014). It differs from the definition of WHO which defined ‘complementary feeding’ as ‘the process starting when breast milk alone is no longer sufficient to meet the nutritional requirements of infants, and therefore other foods and liquids are needed, along with breast milk’.5 The Panel understands that these ‘other foods’ in this last definition may also comprise formula.
CFs in this Scientific Opinion comprises, therefore, all liquid, semisolid and solid foods other than breast milk, formula, water or vitamins that are given to infants. CFs can be beverages, spoon‐fed pureed foods, spoon‐fed lumpy foods or finger foods (EFSA NDA Panel, 2009), depending on the age of the infant. They can be either prepared at home or produced commercially.
Weaning in this Scientific Opinion means the time period of gradual reduction of frequency and volume of breast milk or formula which starts with the first introduction of CFs and gradually leads to a dietary pattern customary in the infant's family during the second year of life (EFSA NDA Panel, 2009).
Breastfeeding may be exclusive, predominant, full, mixed or partial. Exclusive breastfeeding means that no other food or liquid is given besides breast milk and medicines or vitamin drops. It is predominant if, in addition to breast milk, the infant receives ‘non‐milk liquids’ (i.e. other than breast milk or formula) like water or energy‐free ‘teas’. Exclusive and predominant breastfeeding together are called full breastfeeding. Mixed breastfeeding means that, in addition to breast milk, the infant receives formula. Partial breastfeeding is breastfeeding together with CFs (EFSA NDA Panel, 2009). The Panel notes that different definitions may be found in the literature.
Appropriate, according to the Oxford English Dictionary, means suitable for a given circumstance.
The Panel notes that, from a scientific point of view, the assessment of the appropriate age range of introduction of CFs (which is the subject of this mandate) is not an assessment of the optimal duration of exclusive breastfeeding.
1.4. Need for complementary foods for infants
The following Section summarises the knowledge that is available on the nutritional adequacy of exclusive breastfeeding in the first months of life in healthy infants born at term from healthy well‐nourished mothers.
1.4.1. Nutritional adequacy of exclusive breastfeeding
Breast milk composition changes with gestational and post‐natal age, from the start to the end of a feed, and follows a diurnal pattern.
1.4.1.1. Energy and protein
Energy content of breast milk is fairly stable over the first year of life (Nommsen et al., 1991; Nielsen et al., 2011; Gidrewicz and Fenton, 2014). It is sufficient to meet the energy requirements of exclusively breastfed infants during the first six months of life (Butte et al., 2002; Nielsen et al., 2011). This consideration is based on (1) the comparison of energy intakes from breast milk (using age‐specific volume intakes corrected for insensible water losses6) to data on total energy expenditure and energy deposition related to growth and accretion of fat and protein (Butte et al., 2002) and (2) data on adequate growth of infants exclusively breastfed up to 6 months of age (Nielsen et al., 2011).
Measured content of true protein of term breast milk was observed to decrease in the first few weeks of life (Gidrewicz and Fenton, 2014) and to be fairly stable thereafter up to 12 months of age (Nommsen et al., 1991). The protein content of breast milk fulfils the protein requirements of infants, as derived from factorial estimates of requirements for maintenance and deposition (EFSA NDA Panel, 2013). In addition, weight and length gain of exclusively breastfed healthy term infants who received a protein supplement from 4 to 6 months of age was similar to a control group exclusively breastfed for 6 months in a randomised controlled trial (RCT), despite a 20% higher protein intake (Dewey et al., 1996).
The Panel considers that the energy and the protein contents of breast milk are sufficient to cover the nutritional needs of infants up to 6 months of age.
1.4.1.2. Minerals, vitamins and fatty acids
The iron concentration of breast milk decreases with the duration of lactation, and is unaffected by maternal iron status and diet (EFSA NDA Panel, 2015a). The healthy term infant of a well‐nourished mother is born with a store of iron (body content about 75 mg/kg body weight), which can be increased by about 30–35 mg through delayed clamping of the umbilical cord (i.e. > 2 min after birth). According to the review by Chaparro (2008), this store is sufficient to supply the iron needed for the formation of haemoglobin (Hb) and myoglobin concomitant with growth until about 6 months of age in most fully breastfed infants (EFSA NDA Panel, 2015a).
However, some infants who are at risk of iron depletion, e.g. infants born to mothers with a low iron status, infants with early umbilical cord clamping (< 1 min after birth), infants born preterm, infants born small‐for‐gestational age (SGA) and infants with a high growth velocity, may need additional iron before 6 months of age. This was investigated in three RCTs (Dewey et al., 1998; Dewey et al., 2004; Jonsdottir et al., 2012), performed in healthy term exclusively breastfed infants, both SGA and appropriate‐for gestational age (AGA), at some degree at risk of iron depletion. A meta‐analysis of these trials done by EFSA (Appendix A.48) showed that the risk of iron depletion (serum ferritin (SF) concentrations < 12 μg/L) at 6 months of age was statistically significantly lower when CFs were introduced at 4 months of age (Section 15.3). It should be emphasised that iron depletion is a risk factor for iron‐deficiency anaemia which is associated with deleterious effects (e.g. delayed attention, poor recognition memory, long‐lasting poor cognitive and behavioural performance) (Geng et al., 2015; Lynch et al., 2018).
Zinc concentrations in breast milk sharply decline over the early months of lactation and are not associated with maternal zinc status, her dietary zinc intake or zinc supplementation (EFSA NDA Panel, 2014b). However, there are no reports describing zinc deficiency in term breastfed infants up to 6 months of age in well‐nourished populations. Zinc concentration in breast milk is considered to be adequate for the majority of healthy term breastfed infants up to six months of life (EFSA NDA Panel, 2013) and thus is not a determinant for the need to introduce CFs.
There is a general agreement that breast milk does not contain sufficient vitamin D to prevent rickets in the breastfed infant. The vitamin D content of breast milk is, however, not a determinant for the need to introduce CFs, because infants in the EU are routinely supplemented with vitamin D (daily supplement of 10 μg to all infants is recommended by ESPGHAN (Braegger et al., 2013)).
The vitamin A concentration in breast milk is dependent on the maternal vitamin A status and decreases with prolonged lactation (EFSA NDA Panel, 2015b). There is no indication that vitamin A insufficiency occurs in exclusively breastfed infants in well‐nourished populations (Butte et al., 2002), in which the vitamin A content of breast milk is thus not a determinant for the need to introduce CFs.
Breast milk has a low phylloquinone content, which can increase the risk of vitamin K deficiency bleeding. Administration of phylloquinone at a pharmacological dose is usual practice for prevention of haemorrhagic disease in newborn infants (EFSA NDA Panel, 2013, 2017) and phylloquinone content of breast milk is thus not a determinant for the need to introduce CFs.
Concentrations of most B vitamins, iodine and selenium and certain fatty acids, for example docosahexaenoic acid (DHA) in breast milk are directly influenced by current maternal intake and are, in well‐nourished populations, not determinants for the need to introduce CFs. However, there are case reports of infants from mothers with undetected pernicious anaemia or adhering to a strict vegan diet without taking supplements that show that clinical symptoms of cobalamin deficiency may occur in exclusively breastfed infants (Dror and Allen, 2008; EFSA NDA Panel, 2015c).
The Panel concludes that the micronutrient and fatty acid contents of breast milk are not determinants for the need to introduce CFs. However, the Panel considers that the iron status of the infants may be a determinant for the need to introduce CFs.
1.4.1.3. Growth of exclusively breastfed infants
Compared to formula fed infants, infants breastfed for at least 12 months grow more rapidly in the first 2–3 months and less rapidly (particularly in weight) from 3 to 12 months of age (Dewey, 1998). The growth pattern of breastfed infants is generally considered a healthier growth pattern. Indeed, many studies have shown that a high growth velocity during infancy is associated with an increased risk of non‐communicable diseases such as obesity and cardiovascular diseases later in life (Singhal, 2017).
In a systematic review, Kramer and Kakuma (2012) did not find any differences in measures of growth of infants exclusively breastfed for 6 months compared with shorter durations of exclusive breastfeeding. In addition, the RCT by Jonsdottir et al. (2012) compared the effects on growth of exclusive breastfeeding for 6 months, with exclusive breastfeeding for 4 months followed by complementary feeding in addition to breast milk. Infants in both groups grew at the same rate between 4 and 6 months of age. In a follow‐up study, there were no differences in anthropometric outcomes between both groups up to 29–38 months of age (Jonsdottir et al., 2014).
Several longitudinal or cross‐sectional studies that assessed growth of exclusively breastfed infants for more than 6 months of age are available in low‐income settings (Sidhu et al., 1981; Khan, 1984; Kumari et al., 1985; Rao and Kanade, 1992) and high‐income settings (French, 1967; Ahn and MacLean, 1980; Salmenpera et al., 1985). Most of them showed a decline in the rate of weight and/or length gain after the age of 6 months (French, 1967; Sidhu et al., 1981; Khan, 1984; Kumari et al., 1985; Rao and Kanade, 1992). However, many studies have methodological limitations (e.g. small number of infants, lack of adjustment for confounding factors, high attrition rate) and/or were performed in low‐income settings, thereby preventing firm conclusions being drawn on the adequacy of exclusive breastfeeding for more than 6 months in infants living in Europe.
The Panel concludes that exclusive breastfeeding for a duration of 6 months allows a normal growth pattern in most healthy term infants.
1.4.2. Nutritional adequacy of exclusive breastfeeding: overall conclusions
The Panel concludes that exclusive breastfeeding is nutritionally adequate up to 6 months for the majority of healthy infants born at term from healthy well‐nourished mothers. However, some infants at risk of iron depletion may benefit from the introduction of CFs that are a source of iron, before 6 months of age in addition to breastfeeding (see Sections 1.4.1.2 and 15.3).
1.5. Interpretation of the Terms of Reference
The appropriate age of introduction of CFs is influenced not only by nutritional considerations, but also by effects on health outcomes and by infant development. Aspects, such as social interactions and the cultural context, may also play a role but are not within the remit of the mandate. Considering the influence of various factors, the Panel considers it likely that there is an appropriate age range rather than a single appropriate age for the introduction of CFs. Taking into consideration the conclusions from Section 1.4.2 and the considerations above, EFSA interprets this mandate as follows:
To evaluate the appropriate age range for introduction of CFs to healthy infants, by answering the following questions:
Are there any developmental factors relevant for the introduction of complementary foods (CFs);
Is there evidence (based on a systematic literature review, Section 4 and following) to indicate that there would be (an) adverse (health) effect(s) for the child to have CFs introduced before the age of 6 months (selection of the age limit of 6 months based on conclusions of Section 1.4.2)?
Is there evidence (based on a systematic literature review, Section 4 and following) to indicate that there would be (a) benefit(s) for the child to have CFs introduced before the age of 6 months (selection of the age limit of 6 months based on conclusions of Section 1.4.2)?
Out of the scope of this mandate are:
public health recommendations for the introduction of CFs; this task is outside the remit of EFSA but it is the role of public health authorities in Member States;
the effects of the duration of exclusive breastfeeding on the selected health outcomes, as the assessment is performed irrespective of whether infants were initially exclusively breastfed or formula fed;
the health benefits of breastfeeding itself (for the infant/child and the mother);
the effects on health outcomes of introduction of CFs solely after 6 months of age, as there is a nutritional requirement for CFs for the majority of exclusively breastfed infants from around 6 months onwards;
the effects of the amount, order of introduction, variety, composition and texture of CFs;
the role of aspects, such as social interactions and the cultural context, on the appropriate age of introduction of CFs;
risks related to e.g. chemical or microbiological contaminants or pesticides.
1.6. General considerations on the outcomes assessed
(Health) outcomes that were considered in the systematic literature review (Section 4 and following) were identified a priori, in particular based on the Panel's previous Scientific Opinion (EFSA NDA Panel, 2009), and listed in a protocol for this assessment (EFSA, 2017b). The conceptual framework for this assessment is outlined in Figure 1.
Figure 1.
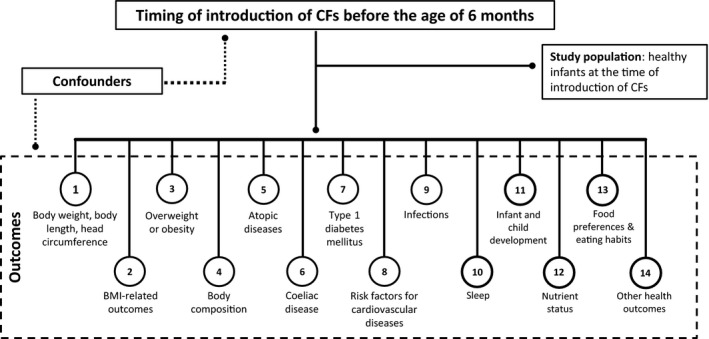
Conceptual framework for the systematic review on the appropriate age range of introduction of complementary foods (CFs) into an infant's diet
- BMI: body mass index; CF: complementary food.
Each outcome covered several endpoints (e.g. weight‐for‐age and weight‐for‐length). Compared to the protocol, a dedicated Section on BMI was created (Section 5), additional outcomes were considered when relevant studies were identified e.g. sleep (in a dedicated Section) or juvenile arthritis. The risk of type 2 diabetes mellitus was not discussed as no relevant data were identified on this outcome.
No limit on the length of follow‐up between timing of introduction of CFs and the age at outcome assessment was applied during the literature selection, with the exception of the following:
-
−
studies on growth in which the endpoint was measured at 6 months of age only, which were excluded (see Section 4.2 for reasons);
-
−
studies on infections with an age at outcome assessment beyond 1 year of age (see Section 12.2 for reasons);
-
−
studies on nutrient status with an age at outcome assessment beyond 1 year of age (see Section 15.2 for reasons).
-
−
studies investigating outcomes at time points for which a relationship with the timing of introduction of CFs is unlikely considering the influence of the background diet on the outcome (e.g. kidney function at 6 years of age).
No exclusion criterion was applied in relation to the method of measurement of the outcome during the literature selection. The reliability of the different methods was considered in the appraisal of the risk of bias (RoB) (Appendix B). One exception was applied to a study that measured F2‐isoprostane concentrations in spot urine samples (and not in 24‐hour urine) as a marker of oxidative damage to lipids (Frederiksen et al., 2015). Spot urine samples are not considered an appropriate sampling unit for this outcome (EFSA NDA Panel, 2018).
Studies which reported on the attainment of individual developmental milestones in months were not considered in the systematic review (see Section 14.2 for reasons). However, they are discussed in the Section on the extensive literature search (Section 3).
The Panel notes that the studies selected for this assessment were heterogeneous with respect to the length of follow‐up and the way in which the (health) outcomes were assessed.
2. Data and methodologies
A protocol was developed for this systematic review. It was subjected to public consultation (from 16 February to 23 March 2017) and amended as appropriate. The final version of the protocol described the methodology for data retrieval, study appraisal, data extraction and possible synthesis (EFSA, 2017b). It was published alongside a technical report on how the comments received during the public consultation were taken into account in the final protocol (EFSA, 2017a). Protocol amendments are listed in the following sections and Section 2.3. The EFSA guidance on the ‘Application of systematic review methodology to food and feed safety assessments to support decision making’ was applied for this assessment (EFSA, 2010).
2.1. Data
For all the (health) outcomes mentioned in Section 1.6, data selection and methodology followed the approach of a systematic literature review. For developmental readiness of term infants, in particular motor developmental milestones (called ‘neuromuscular development’ in the protocol), an extensive literature review was undertaken (as meta‐analyses were not envisaged). The differences in the various steps between these two approaches (systematic or extensive) are explained in the following sections. For developmental readiness of preterm infants (Section 18.1), data came from a narrative review (in the following not further addressed).
2.1.1. Eligibility criteria for the systematic literature search
2.1.1.1. Inclusion
Study populations and exposures considered pertinent
Papers that were selected were only those investigating infants (i.e. aged 0 to < 1 year), children or adults, males and females, who were generally healthy at the time when they were introduced to CFs as infants and were either born at term or preterm (i.e. born at less than 37 weeks of gestation). These were considered pertinent study populations by the Panel for this assessment.
The study groups of the selected papers had to be alike in terms of the type of milk feeding (breast milk or formula7 or mixed, with no additional behavioural interventions), i.e. the study groups had to differ only in the timing of introduction of CFs. In order to be included in this review, at least one study group had to have been introduced to CFs before 6 months of age (protocol amendment 2).
Introduction of CFs thus occurred with different types of milk feeding in the included studies, which compared:
-
−
groups of exclusively breastfed infants introduced to CFs at different time points up to 6 months of age;
-
−
groups of exclusively formula fed infants introduced to CFs at different time points up to 6 months of age;
-
−
groups of infants receiving various types of background milk feeding (i.e. breast milk, formula, mixed) and introduced to CFs at different time points up to 6 months of age.
Introduction of a specific CF item or food group, irrespective of the introduction of other CFs, was also considered as providing potentially relevant information for some of the outcomes discussed in this assessment and mentioned in Section 1.6. Thus, studies which compared the early (before 6 months of age) vs later introduction of a specific CF item or food group were included if investigating the following outcomes:
-
−
Atopic diseases: The specific foods considered were cereals (in particular wheat), egg, fish (as defined in the papers, i.e. generally undefined), peanut, soy (not in the form of formula), which are among the major food allergens relevant in children (EFSA NDA Panel, 2014a);
-
−
Coeliac disease and T1DM: The specific food (item) considered was gluten and gluten‐containing foods, as coeliac disease is triggered by the ingestion of gluten, found in wheat, barley and rye. For T1DM, gluten was considered relevant as the previous assessment of the Panel included specific conclusions on T1DM and gluten;
-
−
Eating behaviours/food preferences: The specific foods considered were fruit and vegetables.
The studies were included irrespective of:
-
−
the income of the population in the country in which the study was done, except for the outcome ‘infections’ as mentioned above;
-
−
the age of assessment of the exposure, i.e. timing of introduction of CFs. This was, however, considered in the appraisal of the RoB (Section 2.2.2).
Study designs and publication types considered pertinent
Articles were included if describing investigations based on the following study designs in humans:8
-
−
intervention (experimental) studies;
-
−
longitudinal prospective observational cohort studies;
-
−
nested case–control studies with prospective data collection;
-
−
letters to the editor, in a limited number of cases, i.e. if they provided sufficiently detailed information for assessment of the RoB and for data analysis (protocol amendment 4);
-
−
retrospective studies9 were included to assess the totality of the evidence in the context of a weight of evidence approach. The weight of evidence approach was not described in the protocol but was deemed necessary for transparent evidence integration (protocol amendment 8).
2.1.1.2. Exclusion
Study populations and exposures not considered pertinent
Human studies were not considered pertinent if they:
-
−
focused on the duration of breastfeeding only or on the comparison of breastfeeding with formula feeding: e.g. studies that compared breastfeeding vs formula feeding independently of CF introduction, studies that compared the introduction of CFs at the same age in breastfed versus formula fed infants, or studies that investigated the nutritional content of breast milk or formula, the duration or promotion of any breastfeeding or the duration of exclusive breastfeeding without reporting on the timing of introduction of CFs;
-
−
had an unclear definition of CFs, or defined CFs as including formula (Section 1.3), investigated the timing of introduction before 6 months of a specific food item/group not listed above (e.g. cow's milk for all outcomes, as the Panel considered that the effect of formula based on intact cow's milk protein and dairy products could not be disentangled);
-
−
investigated the introduction of CFs (in general or specific foods) at ages only after 6 months (see above and protocol amendment 2);
-
−
investigated texture (e.g. lumpy food introduction) or food diversity or preparation methods (e.g. home‐cooked vs commercial baby foods) or composition of CFs or weaning methods (e.g. baby‐led weaning);
-
−
investigated growth or iron status in populations with high prevalence of undernutrition, wasting and/or stunting, in populations under clinical care or with diseases/disorders/medication use known to affect nutritional status (e.g. malaria and iron status);
-
−
investigated the outcome ‘infections’ in low‐income and lower‐middle‐income countries in settings with poor hygiene conditions (i.e. situations in which it is difficult to disentangle the relative effect of co‐exposures on the incidence of respiratory and gastrointestinal infections from the effect of the timing of introduction of CFs on these outcomes; see Section 12.2 for reasons); low‐income and lower‐middle‐income countries were identified according to the World Bank criteria, comparing the year in which the studies were conducted with the historical data of the World Bank per country.10
Study design and publication types not considered pertinent
The following design and publication types were not considered pertinent:
-
−
in vitro studies;
-
−
animal studies;
-
−
case‐only studies (i.e. on a relevant (health) outcome but composed of cases only, e.g. time to onset of coeliac disease or T1DM);
-
−
publication types not providing sufficiently detailed information for assessment of the RoB and for data analysis or synthesis e.g. editorials or abstracts;
-
−
narrative reviews;
-
−
systematic reviews with or without meta‐analyses, and grey literature (i.e. conference abstracts, posters, dissertations, scientific reports). These were excluded from the assessment as such, and used only for hand search for peer‐reviewed studies in their list of references;
-
−
evidence‐based guidelines comprising evidence‐based and practice‐based recommendations. Although a specific search and a quality assessment of evidence‐based guidelines were required from an external contractor in the protocol (EFSA, 2017b), they were finally not used for this assessment (protocol amendment 4), in view of the large body of evidence coming from the peer‐reviewed articles. However, some of these guidelines are mentioned in Section 1.2.
Additional exclusion criteria (protocol amendment 3):
Additional exclusions, not stated in the protocol (EFSA, 2017b), occurred at the 2nd step of the full‐text screening (Section 2.1.1.2). The Panel estimated that the possible bias introduced by deciding on the exclusion of the following studies based on the knowledge of the evidence (and not a priori before study retrieval) was limited:
-
−
Studies on growth in which the endpoint was measured in the first 6 months of life only (and not after) (see Section 4.2 for reasons);
-
−
Studies on infections with an age at outcome assessment after 1 year of age and that did not cover the period during which CFs were introduced (see Section 12.2 for reasons);
-
−
Studies investigating outcomes at time points for which a relationship with the timing of introduction of CFs is unlikely considering the influence of the background diet on the outcome (e.g. kidney function at 6 years of age);
-
−
Studies on nutrient status with an age at outcome assessment after 1 year of age, e.g. Hb concentrations at 6 years (see Section 15.2 for reasons);
-
−
Studies on nutrient status focussing on nutrients either non‐critical for the European population of infants and young children or more influenced by other factors than the timing of introduction of CFs (see Section 15.2 for reasons);
-
−
Studies on nutrient status reporting only on mean blood concentrations of biomarkers with no consideration of the proportion of subjects below a certain cut‐off for nutrient sufficiency (see Section 15.2 for reasons);
-
−
Studies on neurodevelopmental milestones reported in months or weeks only (see Section 14.2 for reasons);
-
−
Studies in children reporting on bone mineral content (BMC) measurements not adjusted for bone area (see Section 7.2 for reasons);
-
−
Studies on sensitisation to aeroallergens (see Section 8.2);
-
−
Studies with inappropriate statistical analysis so that the results cannot be interpreted (e.g. matched (nested) case–control studies in which the matching factor was related to the exposure, but the matching was not taken into account in the analysis);
-
−
Studies with undefined units of measurement.
Studies were excluded at the level of title or abstract screening, at the level of the first step of the full‐text screening or at the second step of the full‐text screening (Section 2.1.1.2, based on the criteria of protocol amendments 2 and 3). Annex C provides a list of 230 excluded references with the reasons for exclusion at step 2 of the full‐text screening. These 230 references are composed of 221 references that were excluded overall and 9 references that were excluded from the assessment of certain outcomes, but included otherwise (Heinig et al., 1993; Cohen et al., 1994; Bainbridge et al., 1996; Mehta et al., 1998; Wilson et al., 1998; Kalanda et al., 2006; Hetzner et al., 2009; Jonsdottir et al., 2012; Noppornlertwong and Tantibhaedhyangkul, 2016).
2.1.2. Eligibility criteria for the extensive literature search (developmental readiness)
2.1.2.1. Inclusion
Study populations considered pertinent and endpoints related to developmental readiness of term infants
Age of achievement of motor development milestones in (generally healthy) infants in relation to the introduction of CFs before 6 months of age was considered by the Panel as the relevant topic for this search.
The Panel was in particular interested in:
-
−
when the extrusion reflex disappears,
-
−
when the child is able to transport foods with the tongue to the back of the mouth,
-
−
when the child gains some head control or postural control,
-
−
when the child is able to sit with some support.
Publication types
-
−
studies (whatever the design) described in peer‐reviewed articles;
-
−
reviews (either narrative or systematic);
-
−
reports or books, when accessible.
2.1.2.2. Exclusion
The following exclusion criteria were applied.
Study populations not considered pertinent
-
−
studies on subjects with a disease/disability, with no results from a healthy control group.
Study design and publication types
-
−
in‐vitro studies;
-
−
animal studies;
-
−
publication types not providing sufficiently detailed information, e.g. commentaries.
2.1.3. Considerations on the included data
The Panel notes that the studies selected were heterogeneous with respect to the length of follow‐up, the methods and criteria used for the assessment of (health) outcomes, the study design, the way in which the exposure was assessed (i.e. the timing of introduction of CFs), the classification into exposure groups, the study settings and the populations investigated (Figure 2).
Figure 2.
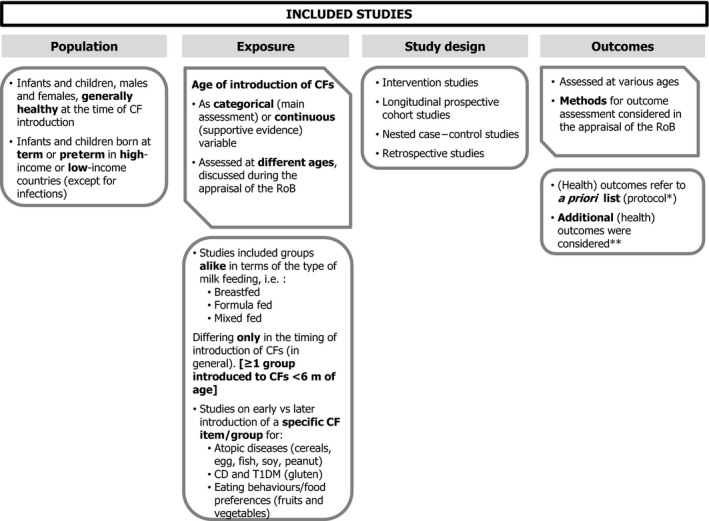
Characteristics of the included human studies (body of evidence) from the systematic literature search
Heterogeneity is discussed per outcome/endpoint from Section 4 onwards.
2.2. Methodologies
Six literature searches were undertaken:
-
−
four of them were systematic literature searches (see below);
-
−
one was an additional quality check by EFSA based on artificial intelligence (see below);
-
−
one was an extensive literature search by EFSA (on developmental readiness of term infants).
The general methodological approach regarding the systematic review (for the outcomes described in Section 1.6) was presented in broad terms in the protocol (EFSA, 2017b). The practical steps are described in the following sections and summarised in Figure 3. Some of them were not applied for the extensive review (developmental readiness of term infants) and this will be explained in the individual steps described below. Step f of the weighing and grading of the confidence in the evidence was not initially described in the protocol (protocol amendment 8).
Figure 3.
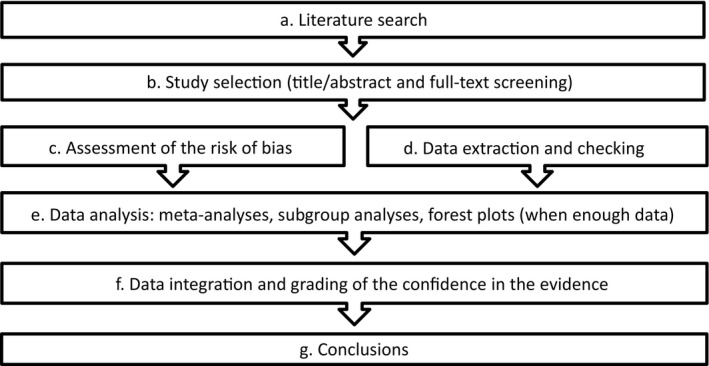
Methodological steps followed for the systematic review
2.2.1. Literature searches and study selection
2.2.1.1. Literature searches and study selection for the systematic review
Sources of information and publication date for published articles
Three databases were screened for the systematic literature searches, i.e. PubMed, Web of Science Core Collection and the Cochrane Library were searched for articles published since 1990. Data published before 1990 were obtained from hand searching in the reference lists of systematic reviews, grey literature and of the included primary studies. Thus, publication dates of the included studies ranged between 1973 and 2018.
Sources of information and publication date for grey literature (used for hand search, Section 2.1.1.2)
-
−
five databases in addition to Google were used: the National Technical Information Service (NTIS11), the System for Information on Grey Literature in Europe,12 CAB Abstracts, Open Access Theses and Dissertations,13 the US National Guideline Clearinghouse;
-
−
published since 2011 (conference abstracts or posters or dissertations) or most up‐to‐date versions (scientific reports and evidence‐based guidelines).
Language
For the systematic searches, no language limits were applied. Studies described in articles not published in English were screened/extracted/appraised either based on the information provided by an EFSA staff member proficient in that language or based on the information provided by on‐line translation tools. Eight studies in a language other than English, i.e. Chinese (Huang et al., 2013; Zheng et al., 2016), German (Forster et al., 1990), Japanese (Takahashi et al., 1999), Portuguese (Gomes et al., 2010) and Spanish (Bascunan Gamboa et al., 2012; Cu et al., 2015; Sandoval Jurado et al., 2016) were included.
Search strings
-
−
for the first search, the search strings were created by an external contractor and are presented in the protocol and the report of the contractor (EFSA, 2017b; Pallas Health Research and Consultancy, 2019);
-
−
for the other systematic searches, they were created by the information specialist of EFSA and are presented in Appendix D.
Dates and objectives of each of the searches
-
a
Initial literature search by the external contractor and quality check by EFSA
-
−
For peer‐reviewed articles, an external contractor conducted the initial search in May 2017, specifically on the 5th for Web of Science Core Collection) and 8th (PubMed and Cochrane Library) (Pallas Health Research and Consultancy, 2019);
-
−
For grey literature, the contractor conducted the search in June/July 2017 (Pallas Health Research and Consultancy, 2019).
The number of papers (on prospective or retrospective studies) that were finally included by EFSA from this search is given in Table 1.
Table 1.
Literature search flow
| Contractor search | Shiny R quality check in contractor excluded | Hand search in contractor excluded | Complementary search by EFSA | Upgrade contractor search by EFSA | Upgrade complementary search by EFSA | Hand search by EFSA | TOTAL | Developmental readiness | |
|---|---|---|---|---|---|---|---|---|---|
| Initial hits | 7,280 | 1,037 | n/a | 4,681 | 4,249 + 661b | 1,877 + 446b | n/a | 19,194a | 1,412 |
| Included full‐text screening step 1 | 352 | 107 | n/a | 477 | 271 + 32b | 115 + 22b | n/a | 1,376 | 84 |
|
Included in full‐text screening step 2 |
162 | 56 | 29 | 102 | 140 + 0b | 21 + 0b | 87 | 597 | 27 |
| Duplicates | n/a | −2 | n/a | −50 | −41 | −2 | n/a | −95 | n/a |
| Excluded full‐text screening step 2 (without duplicates) | −44 | −24 | −10 | −26 | −65 | −12 | −40 | −221 | −12 |
| Included papers on prospective studies | 111 | 14 | 7 | 20 | 22 | 3 | 24 | 201 |
15 + 8 hand search + 2 from SR |
| Included papers on retrospective studies | 7 | 18 | 12 | 6 | 12 | 4 | 23 | 82 | n/a |
n/a: not applicable; SR: systematic review.
Excludes the 1,037 references re‐screened using Shiny R;
number of publications retrieved in the update of the search (limited to RCTs) performed during the time period when the draft Scientific Opinion was subjected to public consultation.
The following steps were undertaken by EFSA, after the initial literature search by the contractor:
-
−
full‐text screening step 2 (see below) and further exclusion, based on the criteria listed in Section 2.1.1.2;
-
−
appraisal of the internal validity of the included studies, data extraction, presentation and synthesis (Sections 2.2.2);
-
−
retrieval of relevant retrospective studies (protocol amendment 4) initially excluded by the contractor in line with the protocol). This retrieval was done by searching through the list of excluded papers provided by the contractor;
-
−
additional quality check: the 7,280 references excluded by the contractor were screened again by EFSA using a tool based on machine learning (artificial intelligence), i.e. ‘ShinyR tool14 for the automation of systematic review’ that is available online in Zenodo15 or in the web platform R4EU ‐ Open Analytics.16 This led to the identification of 1,037 references, which were screened first based on their title and abstract, and then on their full texts by EFSA staff members using single screening (i.e. not duplicate screening). The number of papers re‐included is given in Table 1.
-
b
Complementary search
The information specialist of EFSA developed the search strings (Appendix D.1), and this complementary search by EFSA on 16 October 2017 retrieved:
-
−
studies that included terms related to exclusive breastfeeding in the abstract (as such could have been considered not relevant), but that discussed complementary feeding in the full text;
-
−
studies that were missing from the initial search (e.g. papers on timing of introduction of CFs and outcomes assessed in ‘pre‐school children’).
The number of papers (on prospective or retrospective studies) included by EFSA from this search is given in Table 1.
-
c
Upgrade of searches a and b
Both the initial and the complementary searches (‘a’ and ‘b’) were updated and upgraded by EFSA on 2 October 2018 (Appendix D.2), to retrieve papers published since, respectively, May and October 2017. The search for grey literature was not updated.
Refined search strings (compared to those used in the initial search by the contractor) were developed by the information specialist of EFSA (protocol amendment 1):
-
−
the search strings for countries were removed;
-
−
some relevant terms for the population were added;
-
−
the previous restriction on some study designs was removed (e.g. cross‐sectional studies).
The results (number of hits) presented in Appendix D.2 included almost all those from the search of the contractor as no time limit was applied to the search. Duplicates were removed before the start of the screening process.
The upgraded searches were updated on 10 May 2019 to retrieve RCTs (protocol amendment 1) published since October 2018. Again, no time limit was applied, and duplicates were removed before screening. Search strings were those already used in the upgrade of searches a and b and are given in Appendix D.2.
The number of papers (on prospective or retrospective studies) that were included by EFSA from the upgraded and the updated searches is given in Table 1.
-
d
Hand search
EFSA staff hand‐searched through the bibliography of:
-
−
the studies included from all the searches described above,
-
−
the systematic reviews (those performed by USDA (English et al., 2019b, English et al., 2019a; Obbagy et al., 2019c; Obbagy et al., 2019a; Obbagy et al., 2019b) and published shortly before the launch of the public consultation on this Scientific Opinion were searched during the public consultation and relevant papers were added thereafter),
-
−
the theses found through the search of grey literature undertaken by the external contractor.
The number of additional papers that were included via hand‐search is given in Table 1.
Study selection
For all searches (‘a’ to ‘c’ mentioned above), the study selection process was based on title and abstract and full‐text screenings.
-
−
For the initial systematic search by the external contractor (‘a’ mentioned above):
-
o
the study selection process (title/abstract screening and full‐text screening) is described in a report (Pallas Health Research and Consultancy, 2019);
-
o
the outcome was provided as EndNote® databases to EFSA; a second step of full‐text screening was applied by EFSA based on additional exclusion criteria (see below);
-
o
an additional quality check by EFSA was performed using an artificial intelligence tool (see above).
-
o
-
−
For the other systematic searches (‘b’ and ‘c’ mentioned above):
-
o
the screening of the title and abstract was done in duplicate by EFSA staff members;
-
o
a full‐text screening in two steps was undertaken:
-
o
-
■
the first step was based on the initial inclusion/exclusion criteria listed a priori in the protocol (EFSA, 2017b); it was done in duplicate and led to the exclusion of the studies irrelevant for this assessment;
-
■
the second step was based on the additional exclusion criteria described in Section 2.1.1.2 (protocol amendment 3); it was done by single screening (i.e. not in duplicate) and led to the further exclusion of papers (Annex C).
All systematic searches undertaken by EFSA (‘b’ to ‘c’ mentioned above) were screened in DistillerSR (Evidence Partners, Ottawa, Canada) and possible conflicts during the screening were discussed and resolved by EFSA staff.
2.2.1.2. Literature searches and study selection for the extensive review
Sources of information and publication date for published articles
Two literature databases were used for the extensive literature search, i.e. PubMed and Web of Science, without limiting the search with respect to publication dates.
Language
For the extensive literature search, only papers in English were selected.
Search strings
For the extensive literature search, search strings were created by the information specialist of EFSA and are presented in Appendix D.3, with the number of hits.
Dates and objectives of each of the searches
Specifically, for the aspects related to neuromotor developmental readiness of term infants, EFSA undertook an extensive literature search on 6 February 2019 in PubMed and Web of Science, for primary research studies and narrative or systematic reviews.
This led to the inclusion of 15 papers discussed in Section 3.3 (see final body of evidence further below and Table 1). These papers did not go through the steps described in the following sections, i.e. appraisal of the RoB, data extraction, data synthesis or grading the confidence in the evidence, as no meta‐analysis was envisaged. EFSA staff also hand‐searched through the bibliography of the included papers. The number of additional papers that were included via hand‐search is given in Table 1.
Study selection
The study selection process was based on title and abstract and full‐text screenings. The screening of title and abstract was done in duplicate by several EFSA staff members, and the full‐text screening was done by a single EFSA staff member.
References were screened in DistillerSR (Evidence Partners, Ottawa, Canada) and possible conflicts during the title and abstract screening were discussed and resolved by EFSA staff.
2.2.1.3. Final body of evidence
The overall number of hits for the different steps of all these searches is provided as Table 1.
The total number of papers included in the systematic review on the relationship between the timing of introduction of CFs and health outcomes is 283.
The 201 papers on 131 prospective studies17 included:
21 papers on 13 RCTs;
-
169 papers on 107 prospective cohort studies, of which:
-
o
131 referred to 72 individual cohort studies with a specified name;
-
o
38 referred to 35 individual cohort studies without a specified name;
-
o
9 papers on 9 nested case–control studies;
2 papers on 2 pooled analyses of prospective studies.
The 82 papers on 79 retrospective studies included:
12 papers on cross‐sectional baseline analyses of 9 otherwise prospective studies (RCTs or prospective cohort studies);
29 papers on cross‐sectional studies;
37 papers on case‐control studies;
3 papers on retrospective cohort studies;
1 paper on a prospective cohort study in which the timing of introduction of CFs was assessed after the outcome.
This number is higher than that initially predicted in the protocol (EFSA, 2017b).
The total number of papers included from the extensive literature search in this assessment on motor development of term infants was 15. Another 8 publications were added by hand search and 2 papers were used that were originally retrieved through the systematic search. Thus, the total number of references discussed in relation to motor development was 25.
The total number of papers discussed in this Scientific Opinion is therefore 308.
2.2.2. Assessment of the internal validity of studies included in the systematic review
Purpose and software
The appraisal of the included studies consisted of the assessment of their internal validity, i.e. their RoB. This was documented in the web‐based systematic review software Distiller SR (Evidence Partners, Ottawa, Canada).
Study designs for which this step applied
The studies with the designs initially included based on the protocol (EFSA, 2017b) were those that went through the appraisal step: intervention studies (mainly RCTs), prospective cohort studies, pooled analyses of prospective studies and nested case–control studies.
The retrospective studies initially not included in the protocol but finally considered in this assessment (Section 2.2.1; protocol amendment 4) were not appraised. They were considered as being, by design, of high RoB (Tier 3).
Assessment at outcome level
For a study investigating several outcomes in relation to the age of introduction of CFs (e.g. symptomatic food allergy and weight), each outcome of the study was appraised individually, possibly leading to different assessments of the RoB.
Tool used and rating scale
The appraisal was based on the tool proposed by the US National Toxicology Program (NTP) Office of Health Assessment and Translation (OHAT) for conducting a literature‐based health assessment (NTP, 2015), as mentioned in the protocol (EFSA, 2017b). The original set of questions proposed in the tool by NTP (2015) was reduced to those deemed most appropriate to the present assessment, as envisaged in the OHAT handbook (i.e. questions on reporting bias and on whether selection of study participants resulted in appropriate comparison groups in observational studies were dropped). The questions were answered on a four‐level rating scale (low, probably low, probably high and high RoB). The protocol stipulated that judgements to the RoB questions should be combined into an overall RoB judgement (Tier of RoB), using an algorithm. The algorithm used for this assessment is described below.
Criteria to answer the individual questions and to combine them into an overall RoB judgement
The outline of the criteria used to answer each question is included in Appendix C. The rating for each question per study and outcome is presented in Annex B for RCTs and observational studies.
Four key questions were identified and used to conclude on the final RoB Tier (Table 2). Regarding the question on exposure to complementary feeding (detection bias) (EFSA, 2017b), the experts considered it relevant to formulate a specific question on compliance for intervention studies (Table 2; question 1).
Table 2.
Four key questions which answers were combined into an overall judgement of the risk of bias (Tier of RoB)
| Number | Key questions |
|---|---|
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
The remaining questions (on concealed allocation and on blinding for RCTs, on other risks of bias for RCTs and prospective observational studies) that were considered in the protocol for the appraisal step were rated for completeness but did not influence the final overall allocation of a study to a RoB Tier.
In order for a study to be classified as Tier 1 (low RoB related to the outcome of interest), the publication must have been rated as ‘definitely low’ or ‘probably low’ RoB for all key questions. For a study to be classified as Tier 2 (intermediate RoB), one of the key questions, and for Tier 3 (high RoB), two of the key questions must have been rated as ‘definitely high’ or ‘probably high’ RoB.
Reviewers undertaking the appraisal
For the first half of the studies selected from the first two searches (Section 2.2.1.1), the appraisal was done in the full setting of the working group (WG) on Infant Nutrition, i.e. by each WG member present during the meetings. This resulted in an agreed rating of the individual RoB domains.
The appraisal for the remaining studies was done in parallel groups composed of half of the WG members, based on the experience gained.
Studies retrieved through the updated search (Section 2.2.1.2) were appraised by EFSA scientific staff members based on the same criteria established by the WG for the initial appraisal.
Insufficient information for appraisal
In case insufficient information was provided in a publication to allow an appropriate assessment of the RoB, the WG endeavoured to retrieve additional information:
-
–
for example, additional information on the study methodology provided in other related publications or from original questionnaires, when publicly available.
-
–
for RCTs or very large prospective studies for which information was missing on one or more items considered among the key questions, the authors of a limited number of papers (Brophy et al., 2009; Jonsdottir et al., 2012; Palmer et al., 2013; Vriezinga et al., 2014; Perkin et al., 2016) were contacted (protocol amendment 5).
If the information remained insufficient for an assessment, the ‘probably high RoB’ category was chosen by default.
2.2.3. Data extraction, presentation and synthesis in the systematic review
2.2.3.1. Data extraction
Data extraction was done in Microsoft Excel®. Data were extracted by one EFSA staff member and checked by a second EFSA staff member. The Microsoft Excel® files show all comparisons of age of introduction of CFs in a harmonised way, i.e. earlier introduction compared to later introduction.
Prospective (observational or intervention) studies
Most included studies considered the timing of introduction of CFs as categorical variable. In the following sections, studies in which the timing of introduction of CFs was used as a continuous variable in the analysis are identified as such in the text (the absence of such indication in the following sections means that the discussed study considered the timing of introduction as categorical).
The types of data extracted are listed in Table 3, and the detailed data are in Annex A (Microsoft Excel®).
Table 3.
Type of data extracted and used for data presentation and synthesis
| Type of data extracted | |
|---|---|
| Identification number of the comparison | Endpoint (e.g. attained BMI, WAZ) |
| Bibliography | Allergy to (e.g. egg, fish, peanut), if relevant |
| Inclusion (or not) in main analysis | Age at outcome assessment |
| Inclusion (or not) in subgroup analysis | Point estimate |
| Tier | Lower bound of the confidence interval (as reported in the paper or calculated by EFSA) |
| Study design | Upper bound of the confidence interval (as reported in the paper or calculated by EFSA) |
| Study name | Unit/Type (e.g. OR, RR) |
| Country (abbreviation) | Adjusted (yes/no) |
| At‐risk group (yes/no) | Remarks |
| Heredity of allergy (yes/no, mixed population, unclear) | Statistical significance (significant/not significant) |
| Allergic symptoms at introduction of CFs (yes/no/unclear) | ‘Reverse causality addressed through’ (e.g. sensitivity analysis) |
| Characteristics of the population (e.g. children with heredity of T1DM) | ‘Earlier introduction associated with’ (in case of significant result) |
| Specific study group (e.g. breastfed, preterm) | Exposure assessment time point (e.g. multiple ≤ 6 months, as classified for appraisal, see Appendix B) |
| E1 (age of introduction of CFs when used as categorical variable, group 1) | Exposure assessment method (e.g. interview, questionnaire) |
| E2 (age of introduction of CFs when used as categorical variable, group 2) | Outcome assessment (e.g. parent's report of symptoms) |
| Age of introduction of CFs as a continuous variable (yes/no) | Reference data/cut‐offs/method used (e.g. BMI ≥ P99 of CDC 2000) |
| N1 (number of subjects, group 1) | Food (e.g. CFs in general, egg, fish, gluten) |
| N2 (number of subjects, group 2) | Specific food (e.g. egg yolk if ‘food’ is egg) |
| Total N (total number of subjects of the comparison) | Amount (when available) |
| Section in opinion | Comparator (in RCTs) |
| Outcome (e.g. BMI, weight) | List of confounders (included, considered, not considered) |
CDC 2000: growth charts by CDC released in 2000; BMI: body mass index; CDC: US Centers for Disease Control and Prevention; CF: complementary food; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; T1DM: type 1 diabetes mellitus; WAZ: weight‐for‐age z‐score.
-
–
In cases where several models were reported in a paper, specifically an unadjusted model and several adjusted models with different sets of confounders, data from the fully adjusted model were extracted.
-
–
In cases where data were reported in a paper for the ‘full’ study population as well as for subgroups, the data from the ‘full’ study population was extracted. An exception to this was if papers reported separately results for breastfed and formula fed infants: the data from such subgroups were extracted and used in the subgroup analyses and dedicated forest plots described in the following sections (Appendix A).
-
–
For RCTs for which different types of analyses may be described (e.g. intention‐to‐treat (ITT), full analysis set (FAS), per protocol (PP)), the results of the most complete analyses (in most cases the FAS) were extracted. However, PP analyses may also be discussed in the following sections whenever needed.
Retrospective studies
The type of data extracted for retrospective studies was simplified compared to the list for prospective studies and is given in Table 4. The detailed data are in Annex A as Microsoft Excel® file.
Table 4.
Type of data extracted and used for data presentation and synthesis
| Type of data extracted | |
|---|---|
| Identification number of the comparison | Total N (total number of subjects of the comparison) |
| Bibliography | Section in opinion |
| Inclusion (or not) in main analysis | Outcome (e.g. overweight, blood pressure) |
| Study design | Endpoint (e.g. attained BMI, WAZ) |
| Study name | Allergy to (e.g. egg, fish, peanut), if relevant |
| Country (abbreviation) | Food (e.g. CFs in general, egg, fish, gluten) |
| At‐risk group (yes/no) | Specific food (e.g. egg yolk if ‘food’ is egg) |
| Characteristics of the population (e.g. children with heredity of T1DM) | Age at outcome assessment |
| Specific study group (e.g. breastfed, preterm) | Point estimate |
| E1 (age of introduction of CFs when used as categorical variable, group 1) | Lower bound of the confidence interval (as reported in the paper or calculated by EFSA) |
| E2 (age of introduction of CFs when used as categorical variable, group 2) | Upper bound of the confidence interval (as reported in the paper or calculated by EFSA) |
| Age of introduction of CFs as a continuous variable (yes/no) | Unit/Type (e.g. OR) |
| N1 (number of subjects, group 1) | Adjusted (yes/no) |
| N2 (number of subjects, group 2) | Statistical significance (significant/not significant) |
BMI: body mass index; CF: complementary food; OR: odds ratio; T1DM: type 1 diabetes mellitus; WAZ: weight‐for‐age z‐score.
2.2.3.2. Data analysis and subgroup analyses and data presentation
Forest plots and estimates from meta‐analyses
Data were visualised in forest plots whenever more than two studies were available for an endpoint. These forest plots are included in Appendix A.
In all forest plots representing RCTs and prospective observational studies (mostly prospective cohort studies), individual age comparisons from the included studies were organised in strata (subgroups) according to the following order:
-
–
First, study design (i.e. separating RCTs from prospective observational studies);
-
–
Second, RoB Tier: Tier 1 and Tier 2 studies were grouped together, separately from Tier 3 studies;
-
–
Then, alphabetical order of the name of the first author.
Retrospective studies (Tier 3 by design) were represented in separate forest plots, in line with the approach outlined above to separate studies by their study design.
This allows an assessment if, for a given endpoint, the response is consistent or changes according to study design or RoB Tier.
In addition to the name of first author of the paper, the publication date, the study design and the RoB Tier, the forest plots display the following information:
-
–
The point estimate for each age comparison with its 95% confidence interval (CI).
-
–
The forest plots indicate whether each comparison was adjusted for the four to five confounders considered by the Panel as most relevant for each outcome (Appendices A and C). The forest plot also includes information if a comparison from an observational study was completely unadjusted for confounders. This is indicated by an ‘N’.
-
–
The forest plots also display the country as abbreviation, the age at outcome assessment, the age categories of introduction of CFs and additional information whenever needed (e.g. reference population on which z‐scores are based).
-
–
Whenever a single publication or several publications on the same cohort provided results for different ages at outcome assessment or different relevant populations, the results displayed in the forest plots were those which referred to the latest age at outcome assessment in the lowest RoB Tier and the most complete analysis set, unless the comparison was from an unadjusted analysis of an observational study. A similar approach was followed for the assessment of individual studies when no meta‐analysis was possible.
-
–
For studies on atopic‐disease‐related endpoints that reported on several interrelated endpoints (e.g. wheeze and asthma) or presented results for the same endpoints assessed in different ways (e.g. parents’ report of a physician's diagnosis and parents’ report of symptoms), the disease‐related endpoint (e.g. asthma) and the most reliable outcome assessment (e.g. parents’ report of a physician's diagnosis) (based on Appendix C) were included in the forest plots. A similar approach was followed for the assessment of individual studies when no meta‐analysis was possible.
For comparisons not included in forest plots, the reader is still able to obtain information on the time course of the effect/association and on all endpoints assessed in a study in Annex A as Microsoft Excel® file.
Whenever possible, a pooled estimate of observed effect measures from individual studies (e.g. mean difference, OR etc.) was calculated using a random effects model carried out in the meta package of the R software (version R 3.5.0). Associated 95% CIs and prediction intervals were estimated for each stratum (as defined above) and represented in the forest plots.18
Heterogeneity index
The value of the I2 together with its 95% CI is shown in the forest plots (Appendix A).19
The Panel notes that, when the number of comparisons/studies in meta‐analyses is low, as is the case for several meta‐analyses conducted by the Panel, the uncertainty associated with the I2 estimate can be ‘large’; therefore the I2 has to be interpreted with caution (Ioannidis et al., 2007). With respect to the interpretation of I2, the Panel followed the classification proposed by Higgins and Green (2011), also taken over by NTP (2015), i.e. 0–40% heterogeneity might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; 75–100% represents considerable heterogeneity.
For grading the confidence in the evidence (Section 2.2.3.3), the Panel focused on the estimate of I2 (and not the 95% CI) as the most likely value of I2.
Calculations, estimations and methodological approaches
A number of calculations and estimations were made by EFSA to produce the forest plots in case of missing summary statistics:
-
–
When the articles did not report point estimates (e.g. OR) and associated 95% CIs, the point estimates and 95% CIs were either calculated based on the information reported numerically in the papers (e.g. number of subjects) or extracted by EFSA from graphs using an on‐line tool.20
-
–
Some studies reported point estimates without measures of spread. If an exact p‐value was provided in these studies, 95% CIs were calculated from p‐values. Otherwise, standard deviations (SDs) were imputed from other similar studies on the same endpoint. This was done for two papers on weight‐for‐age z‐scores (WAZ) (Haschke and van't Hof, 2000; Gaffney et al., 2012), two papers on length(height)‐for‐age z‐scores (L(H)AZ) (Dewey et al., 1999; de Beer et al., 2015) and two papers on BMI‐for‐age z‐scores (BMIZ) (Haschke and van't Hof, 2000; Zheng et al., 2015).
The following methodological approaches were taken:
-
–
The effect measures of the individual studies considered for a given endpoint were pooled and their 95% CIs estimated applying the Hartung and Knapp modification (Knapp and Hartung, 2003) to the DerSimonian and Laird approach (DerSimonian and Laird, 1986), a different approach to that originally described in the protocol (EFSA, 2017b) (protocol amendment 6). This method was used, as the DerSimonian and Laird approach without modification does not preserve the type 1 error rate21 in situations where the number of comparisons/studies included in a meta‐analysis is low and heterogeneity is high (Veroniki et al., 2016; Jackson et al., 2017). This was a situation that was present for a number of meta‐analyses performed by the Panel. The Hartung and Knapp modification was applied as it has been suggested that it may perform better in many situations and across types of outcomes (IntHout et al., 2014). However, it is not without criticism. Especially, it has been suggested to produce narrower 95% CIs than the DerSimonian and Laird approach in some instances, especially when τ2 22 is zero (Jackson et al., 2017). This is contrary to what is intended by the use of this modification.
-
–
Therefore, sensitivity analyses were conducted, for all endpoints for which a random effects meta‐analysis could be done, to check the relative performance of the DerSimonian and Laird approach with and without the Hartung and Knapp modification. In addition, the performance of another between‐study variance estimator, proposed by Paule and Mandel (Paule and Mandel, 1989), again with and without the Hartung and Knapp modification was tested in the sensitivity analyses. When the Hartung and Knapp modification was not used, Wald‐type CIs using a t‐distribution with k‐1 degrees of freedom were derived. The results are reported in Annex E. Indeed, in some instances applying the Hartung and Knapp modification led to narrower CIs as compared with not using this modification. This was the case for:
-
o
attained body weight (Appendix A.3): subgroups of 1) RCTs rated as Tiers 1 and 2 (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 38%]) and 2) prospective cohort studies rated as Tiers 1 and 2 (4 studies, τ2 = 0, I2 = 0% [95% CI 0 to 2%]);
-
o
attained body length/height (Appendix A.8): subgroups of 1) RCTs rated as Tiers 1 and 2 (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 0%]) and 2) prospective cohort studies rated as Tiers 1 and 2 (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 34%]);
-
o
attained body length by feeding mode (Appendix A.9): subgroup of formula fed infants (4 studies, τ2 = 0, I2 = 0% [95% CI 0 to 25%]);
-
o
attained head circumference (HC) (Appendix A.10): subgroups of RCTs rated as Tiers 1 and 2 (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 0%]);
-
o
odds of developing (at least) overweight (Appendix A.16): subgroup of prospective cohort studies rated as Tier 3 (10 studies; τ2 = 0.01, I2 = 61% [95% CI 23 to 81%]);
-
o
asthma‐like symptoms and fish – general population (Appendix A.24): subgroup of prospective cohort studies rated as Tiers 1 and 2 (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 15%]);
-
o
eczema and fish – general population (Appendix A.31): subgroup of prospective cohort studies rated as Tiers 1 and 2 (2 studies, τ2 = 0, I2 = 0%);
-
o
risk of iron depletion in exclusively breastfed infants at 6 months of age (3 studies, τ2 = 0, I2 = 0% [95% CI 0 to 4%]) (Appendix A.48).
-
o
In none of the above‐mentioned cases, the results with respect to their statistical significance changed according to the method applied. Considering that the Hartung and Knapp modification in these instances did not perform well, the results of the DerSimonian and Laird approach without the modification is indicated underneath the respective forest plots and used for reporting in the Scientific Opinion. In all other cases, the results of the Hartung and Knapp modification are reported. The Paule and Mandel approach gave similar results to the DerSimonian and Laird approach and they were therefore not considered further in the discussion of results. In some instances,23 the DerSimonian and Laird approach showed statistically significant results, while when the Hartung and Knapp modification was applied, the findings became non‐significant. However, considering the increased false positive rate of the unmodified DerSimonian and Laird approach, the results with the Hartung and Knapp modification were considered.
-
–
Prediction intervals (95% level) were estimated following the DerSimonian and Laird approach and using a t‐distribution with k‐2 degrees of freedom, whenever more than 2 studies were available per subgroup.
-
–
Some studies reported on more than two comparisons for the ages of introduction of CFs (e.g. a study comparing < 4 months vs >6 months, 4–5 months vs > 6 months, 5–6 months vs > 6 months). In these cases, the correlation among comparisons including a common reference category was considered by combining the estimates to obtain a single comparison for each study (e.g. ≤ 6 months vs > 6 months) (Higgins and Green, 2011), whenever possible.24 Sensitivity analysis showed that this had little impact on the estimate and the associated CIs of the meta‐analysis. Detailed information for each comparison remains available in Annex A (Microsoft Excel®).
Sensitivity and subgroup analyses
The possibility of sensitivity and subgroup analyses was mentioned in the protocol with some examples (EFSA, 2017b). Sensitivity analyses which were conducted were as follows:
-
–
applying alternative approaches to the estimation of CIs (see above);
-
–
for some forest plots (Appendix A), to test the influence of a specific study on the pooled estimates and heterogeneity.
The protocol also stipulated that the following sensitivity analyses were to be conducted: (1) a ‘sensitivity analysis to assess the impact of imputed summary data’ and (2) a sensitivity analysis on the inclusion of studies with high participant attrition (or with other missing data)’.
As the number of studies for which imputation was made by EFSA was low, such a sensitivity analysis was not undertaken. In addition, most included studies had high attrition, and attrition/exclusion was considered among the key questions in the assessment of the RoB (Section 2.2.2 and Appendix C). Therefore, this impacted on the attribution of studies to Tiers of RoB (according to which data were pooled in meta‐analyses, as explained above).
Enough studies were available for subgroup analyses regarding type of ‘milk’ feeding at the time of introduction of CFs (exclusively breastfed vs exclusively formula fed infants), for five endpoints: WAZ, attained body weight, BMIZ, L(H)AZ, attained body length. This was done on studies of RoB Tiers 1 and 2 only and was irrespectively of the study design.
Unplanned subgroup analyses were undertaken by EFSA:
-
–
for atopic diseases, data from populations at‐risk and the general population were analysed separately (protocol amendment 7): This was done to investigate whether different associations were observed for the general population and for the at‐risk population (a specific subpopulation of the general population). If associations were indeed not different, the Panel considered that data generated in at‐risk populations could be generalised to the whole population of infants living in Europe.25 Therefore, if not stated otherwise in the text, the conclusions of the Panel on atopic‐diseases apply to the whole population of infants living in Europe, which is the target of this mandate.
-
–
for coeliac disease, data were analysed according to age of introduction of CFs: This was done to investigate whether there is a differential effect of introducing gluten < 4 months of age and between 4 and 6 months of age on the risk of coeliac disease as concluded by the Panel in its previous Scientific Opinion (EFSA NDA Panel, 2009).
Limitations of the meta‐analyses
The Panel notes that for some meta‐analyses, the number of studies that could be considered by subgroup/stratum was low. However, the Panel wishes to highlight that the pooled estimates were calculated with the objective to summarise the data and describe the direction of the effect or association (if any observed). The uncertainty that is associated with meta‐analyses with a low number of studies, especially when heterogeneity is high, is expressed in the wide CIs around the point estimates. This uncertainty was addressed through the grading of the confidence in the evidence (Section 2.2.3.3). In addition, whenever the meta‐analysis in a subgroup/stratum was based on two studies only, both the results of the meta‐analysis and the individual studies are discussed.
Publication bias
Publication bias26 was assessed by EFSA in the body of evidence by generating funnel plots27 whenever ≥ 10 comparisons/studies were available (Higgins and Green, 2011). Funnel plots are shown in Annex D. The reference line that was used in the funnel plots was the value of the pooled estimate of the random effects meta‐analysis.
Whenever the funnel plots indicated asymmetry from visual inspection, the Egger's regression test (Egger et al., 1997; Sterne and Egger, 2005) and the trim‐and‐fill analysis (Duval and Tweedie, 2000) were used. In case of results suggesting asymmetry obtained from the aforementioned analyses (i.e. for odds of developing (at least) overweight, odds of developing obesity, BMIZ (Sections 5 and 6)), contours of statistical significance were overlaid on the funnel plot (Peters et al., 2008) (data not shown).28
2.2.3.3. Reporting, evidence integration and grading of the confidence in the evidence
Reporting of the ages of introduction of CFs
Ages at introduction of CFs reported in this Scientific Opinion should be interpreted bearing in mind the uncertainties that are associated with the ages that are described in the included papers. For RCTs, the ages reported in the Scientific Opinion are those when infants were randomised to start consuming CFs. However, variability is to be expected as to when infants were actually introduced to CFs or were able to consume the assigned CFs after randomisation. This may well span over some weeks. Therefore, reported ages for RCTs should not be interpreted as a single time point, but rather as a time span of one month. For example, introduction of CFs at 4 months should be read as introduction during the fifth month of life and an introduction of CFs at 3–4 months of life as an introduction during the fourth and fifth months of life. With respect to the reporting of observational studies, it should be noted that the uncertainty in the ages that are described in the included papers is usually higher than those reported for RCTs. This is owing to the mostly retrospective assessment of the timing of introduction of CFs and the lack of exact definition of the ages that are reported in the papers. Therefore, the Panel considered it valid to summarise the timing of introduction of CFs in individual studies, in particular RCTs, into an overarching age range of introduction of CFs in these studies (e.g. an introduction of CFs at 3–4 months and one at 4 months is summarised into an age of 3–4 months).
Preterm and term infants
The Panel decided to present and discuss in separate sections the results on infants born at term from the results on preterm infants, because of their differences in developmental steps and nutritional requirements (Section 18). Papers that did not specify if the included children were born at term or not or included both populations (‘mixed populations’) were grouped with papers on children born at term.
Main and supportive lines of evidence
In the following sections, for each outcome, the main line of evidence is discussed first. It consists of RCTs and prospective observational studies rated as Tiers 1 and 2. Within this line of evidence, endpoints for which forest plots could be created are discussed first. The conclusions by the Panel are based on the results of the meta‐analyses and not the individual studies, unless only two studies were available in a subgroup/stratum (Section 2.2.3.2 above). Studies providing information on p‐values without point estimates are discussed individually.
The supportive line of evidence consists of:
RCTs and prospective observational studies rated as Tier 3;
Retrospective studies (i.e. case–control studies, studies in which the exposure was assessed at the same time as the outcome or thereafter, and retrospective cohort studies; all Tier 3 because of the study design, as mentioned in previous sections);
Studies in which the timing of introduction of CFs was used as a continuous variable in the analyses (whatever RoB Tier);
Studies on sensitisation to food allergens (i.e. considered supportive compared with data on symptomatic food allergy), coeliac disease autoimmunity (i.e. considered supportive compared with data on coeliac disease) and islet autoimmunity (i.e. considered supportive compared with data on type 1 diabetes mellitus);
Studies on the timing of introduction of CFs in cases and controls.
The Panel considers that the evidence from studies in the supportive line of evidence is insufficient by itself to draw conclusions on an appropriate age range of introduction of CFs. This is either because of the high RoB (Tier 3) or because they do not directly address the research question, i.e. studies in which the timing of introduction of CFs is used as a continuous variable in the analyses do not allow to conclude on an appropriate age range of introduction of CFs, and also studies on sensitisation or coeliac disease and islet autoimmunity do not allow to draw conclusions on the disease. Therefore, the evidence from studies in the supportive line of evidence is only used in conjunction with evidence from the main line of evidence (see below for the approach for grading the evidence).
The Panel considers that endpoints investigated in single studies (pertaining to the main or supportive lines of evidence) or only in studies in the supportive line of evidence could not be used to establish the appropriate age range of introduction of CFs. Thus, they are mentioned in the following sections but are not considered further by the Panel.
This hierarchy of the available evidence is described in Figure 4.
Figure 4.
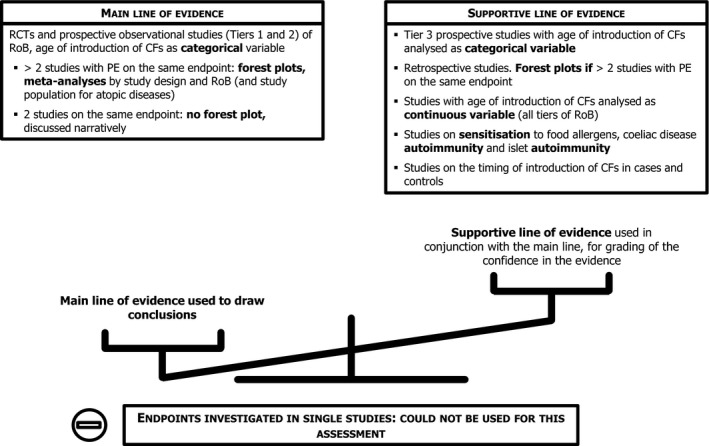
Hierarchy of the available evidence discussed in the systematic review
- CF: complementary food; PE: point estimate; RCT: randomised controlled trial; RoB: risk of bias.
Evidence integration and level of confidence
The determination of the level of confidence followed an approach that was inspired by the approach proposed by OHAT (NTP, 2015). Evidence derived from RCTs was initially attributed a high confidence level (i.e. ++++), evidence derived from prospective observational studies was considered to provide a moderate confidence level (i.e. +++) and the evidence derived from retrospective studies was considered to have a low confidence level (i.e. ++). Whenever a study was most likely underpowered for an endpoint and did not show a statistically significant association or effect, the Panel excluded it from the integration of the evidence, because its findings were not considered reliable.
The initial level of confidence in the evidence could then be downgraded:
for the RoB (i.e. prospective observational studies rated as Tier 3).
for inconsistency in the findings; whenever a meta‐analysis was available (Appendix A), substantial inconsistency was considered to be present when heterogeneity as identified by I2 exceeded 75%29 and could not be explained. Whenever I2 exceeded 75% and, from visual inspection of the forest plot, this was most likely attributable to a single study, sensitivity analyses were performed (protocol amendment 7) by removing the study from the analysis to investigate its impact on I2 and the results of the meta‐analysis. This was done for (i) one prospective cohort study on BMIZ (Section 5.3), (ii) one retrospective study on obesity (Section 6.3) and (iii) one prospective cohort study on coeliac disease (Section 9.3).
for serious imprecision (i.e. a wide 95% CI; to assess imprecision, results from the meta‐analysis were prioritised over results from individual studies). For odds/risk/hazard ratios (OR, RR, HR), the Panel considered that the estimate was imprecise if the upper bound of the CI divided by the lower bound of the CI was higher than 10. For other kinds of measurements (e.g. BMIZ), the Panel considered an estimate imprecise when the CIs were wide and if the lower or the upper bound of the 95% CI was indicative of biological relevance of the finding.
for limitations in the generalisability of the findings, i.e. (i) when lines of evidence consisted only of studies in exclusively breastfed infants or only of studies in exclusively formula fed infants (unless there was evidence that the background milk feeding was not an effect modifier); (ii) for atopic diseases, lines of evidence consisting only of studies performed in countries with a prevalence of the respective disease that is different from Europe and this difference could not be explained.
for evidence of publication bias.
if the main line of evidence contained only one study or, for endpoints related to atopic diseases, only one study per population group (i.e. lack of replication) and when (1) the supportive line of evidence was non‐existent, (2) consisted of only one study or (3) provided inconsistent findings.
The confidence could also be upgraded when
the effect or association in the line of evidence was large (the magnitude of the effect was defined as large when the RR or the OR exceeded 2 or was less than 0.5) and
when an indication for a dose‐response was available.
Aspects other than those listed above were also considered (e.g. discrepancies in the findings of the FAS analysis and the PP analysis), if they increased or decreased the confidence in a finding.
Confidence levels were truncated ++++ at the upper end and at + at the lower end.
Three conclusions were possible when findings were statistically non‐significant or of no biological relevance:
‘no effect’: for conclusions derived from RCTs with a high level of confidence in the evidence;
‘no evidence for an effect’: for conclusions derived from RCTs with a very low, low or moderate level of confidence in the evidence;
‘no evidence for an association’: for conclusions derived from prospective observational studies, irrespective of the level of confidence in the evidence.
In case there was insufficient evidence to conclude if the timing of introduction of CFs was associated with an outcome, no level of confidence was derived.
For all outcomes considered in the following sections, a paragraph on grading the confidence in the evidence lists the main considerations in relation to imprecision, inconsistency, generalisability, and publication bias, and is followed by the overall conclusions of the Panel.
In the overall conclusions of the Panel, for most outcomes, RCTs were considered separately from prospective cohort studies, as the ages of introduction of CFs were different between these study designs. This allowed a higher confidence to be attributed to the conclusions derived in relation to the ages of introduction of CFs studied in RCTs, while the confidence in the evidence for the age ranges studied in prospective cohort studies was lower. The results from RCTs and prospective cohort studies were integrated only when the ages of introduction of CFs in both study designs overlapped. The overall conclusions of the Panel per outcome refer to the main line of evidence only (and not the supportive line).
The approach for integrating the evidence and grading the confidence in the evidence is described in Figure 5.
Figure 5.
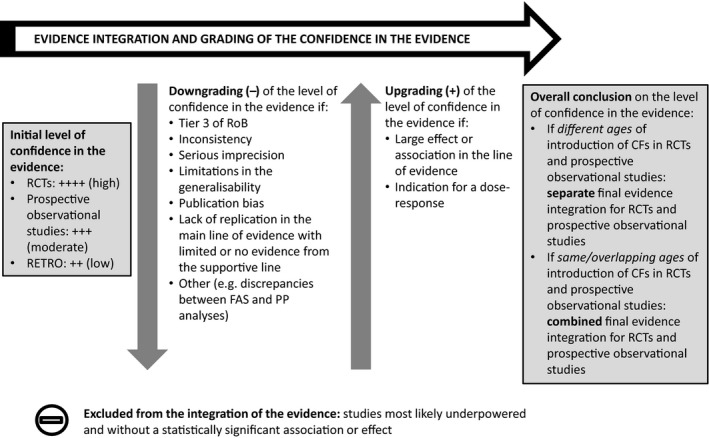
Approach for integrating the evidence and grading the confidence in the evidence
- CF: complementary food; FAS: full‐analysis set; PP: per protocol; RCT: randomised controlled trial; RETRO: retrospective studies; RoB: risk of bias.
2.3. Protocol amendments
As previously discussed, the following amendments to the protocol (EFSA, 2017b) have been made:
Upgrade and update of the literature search: the search strings were upgraded for the updated search that was performed before the release for public consultation; the additional literature search before the final adoption of the Scientific Opinion was limited to RCTs.
Eligibility criteria: consideration of studies in which at least one group was introduced to CFs before 6 months of age (Sections 1.4 and 2.1.1.1), instead of studies involving infants not older than 12 months of age at introduction of CFs as mentioned in the protocol.
Eligibility criteria: additional exclusion criteria (Section 2.1.1.2).
Eligibility criteria: in a few cases, letters to the editor were included, if they provided sufficiently detailed information for assessment of the RoB and for data analysis or synthesis (instead of excluding all letters to the editor as mentioned in the protocol). Retrospective studies were also included. Evidence‐based guidelines comprising evidence‐based and practice‐based recommendations collected and assessed by the external contractor, were finally not used for this assessment.
Missing data: gathering missing data for an appropriate assessment of the RoB was initially not mentioned in the protocol but was done in some cases described in Section 2.2.2 by contacting authors.
Data analysis: CIs of the pooled estimates were calculated based on the Hartung and Knapp modification to the DerSimonian and Laird approach (instead of using just the DerSimonian and Laird approach originally mentioned in the protocol) and sensitivity analyses were carried out.
Unplanned subgroup or sensitivity analyses: (i) for populations at‐risk of different atopic diseases and for the general population; and (ii) for assessing the impact of one potentially influential study on the I2 and the results of a meta‐analysis (in case I2 exceeded 75%).
Final step in the assessment: weighing the evidence and grading of the confidence in the evidence.
3. Assessment of the developmental readiness of the term infant to consume CFs
Developmental readiness can be defined as the physiological maturation necessary for an infant to metabolise ‘non‐milk foods’ i.e. other than breast milk or formula, and the neurodevelopmental changes necessary for safe and effective progression from suckling to spoon‐ and self‐feeding, including the infant's apparent emerging interest in non‐milk foods and feeding. Developmental skills necessary to consume CFs will differ depending on the texture of the food. The skills needed for spoon‐feeding of pureed CFs will appear earlier than the ones required for self‐feeding and therefore, will be used to define the lower bound of the age range of developmental readiness.
3.1. Gastrointestinal function
The human gut is anatomically and functionally mature at birth in the healthy term infant, although the secretion and activity of gastric and pancreatic enzymes are not developed to adult levels (EFSA Scientific Committee, 2017). These functions mature at very different rates (EFSA Scientific Committee, 2017) and the ingested foods appear to play a part in triggering the maturation of gastric and pancreatic enzymes (WHO, 1989).
The Panel notes that gastrointestinal function is not a limiting factor with respect to the timing of introduction of CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding.
3.2. Renal function
The renal control of water balance is not fully developed at birth. The rate of renal water excretion is influenced by the solute load to be excreted.30 As renal concentrating capacity is limited in the neonatal period (Joppich et al., 1977), a high solute load could result in rapid and profound alteration in water balance.
The renal concentrating ability was reported to increase rapidly in healthy term infants within the first month of life, with average individual maximum values for osmolality in urine samples of 515 mOsm/L on day 3, 663 mOsm/L on day 6 and 896 mOsm/L in the first month of life. Thereafter, the increase is attenuated, with average individual maximum values for osmolality reached at 10–12 months of 1,118 mOsm/L and of 1,362 mOsm/L at 14–18 years of age (Polacek et al., 1965).
The Panel notes that renal function is not a limiting factor with respect to the timing of introduction of CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding.
3.3. Neuromuscular coordination and neurodevelopment
At term, healthy infants are able to coordinate efficient suckling, swallowing and respiration (Bu'Lock et al., 1990; Morris and Klein, 2000) as a result of five feeding reflexes that develop prenatally: swallowing, sucking, gag, phasic bite and rooting. Changes in these reflexes over time combined with anatomical changes in the infant jaw and tongue facilitate the subsequent progression to solid foods (Stevenson and Allaire, 1991).
At birth, the tongue occupies most of the oral cavity (Bogaerts et al., 2012); the soft palate, the pharynx and the larynx are in close proximity. This protects airways from aspiration of liquids. However, it also limits the movements of the tongue with no room for up‐down or lateral movements and chewing (Morris and Klein, 2000). During the first months of life, with head and neck growth, the oral cavity and upper pharynx enlarge (Stevenson and Allaire, 1991; Arvedson and Lefton‐Greif, 1996) and free space for more refined tongue movements and for the infant to receive foods other than liquids (Morris and Klein, 2000). Initial suckling with peristaltic tongue movements decreases and is replaced by sucking with increasing voluntary up and down movements of the tongue. This is less automatic and requires more neurological control. These changes have been estimated to initiate at around 3–4 months of age (Arvedson and Lefton‐Greif, 1996; Morris and Klein, 2000). However, the Panel was unable to retrieve empirical data for this estimation.
While the swallowing reflex persists, other reflexes disappear or diminish. The gag reflex becomes less intense and is elicited over a smaller area of the tongue after about 6 months of age, whilst rooting disappears after about 3–4 months (Stevenson and Allaire, 1991). Another reflex that young infants’ exhibit and that disappears with time is the tongue‐thrust reflex, also called the extrusion reflex, when an object touches the infants’ tongue or lip. As a reaction, the tongue moves forward and pushes any material, including food, that is placed on the infant's tongue outwards (Rogers and Arvedson, 2005). It has been estimated that this reflex diminishes and finally disappears between around 4 and 6 months of age (Sheppard and Mysak, 1984; Rogers and Arvedson, 2005). However, the Panel was unable to retrieve empirical data for this estimation.
Another aspect to consider is that, in order to efficiently accept spoon‐fed foods, the infant has to be able to move the upper lip down to wipe the food from the spoon with the lips (instead of suckling it off the spoon) (Stevenson and Allaire, 1991; Ayano et al., 2000). It also must possess the necessary oral‐motor functions that permit the tongue to receive food on its surface, form a bolus, lift it up and press it against the hard palate to transport it to the back of the mouth where the swallow reflex is triggered. This is a complex motion that requires oral structures to move independently instead of moving together as in suckling (Ayano et al., 2000; Bogaerts et al., 2012). A prerequisite for these skills to emerge is that the child has gained oral stability to control the jaw, the tongue and the lips. This develops alongside head and trunk stability and control (Ogg, 1975; Morris and Klein, 2000; Redstone and West, 2004).
The spectrum of head control ranges from basic head control when the infant is able to position the head in the body midline, to full head and trunk control that is present when the infant is able to sit by itself without any support. Intermediate measures of a developing, but not fully achieved, head and trunk control are, for example, sitting balance (e.g. sitting with some support) and the ability of the infant to bring the hands to the midline (Arvedson and Lefton‐Greif, 1996).
The age at which infants attain different developmental milestones shows considerable variation within and between populations, presumably reflecting the infant's innate developmental trajectory combined with the opportunities and experiences provided by the caregiver (Lee and Galloway, 2012).
Also, feeding skills are acquired and consolidated over a period of time, so that the initial amount of food that is consumed by an infant when complementary feeding is started is small and increases over time with increasing feeding skills and repeated experiences. In an observational study in 39 healthy term infants who had spoon‐fed pureed food introduced between 4 and 8 months of age, it took on average 5.7 (SD 2.1) weeks (range 2–10 weeks) for them to consolidate their feeding skills, regardless of the age at which CFs were first given, or whether the infant was breastfed or bottle‐fed (van den Engel‐Hoek et al., 2014).
3.3.1. Gross and fine motor skills relevant for spoon‐feeding pureed CFs
The Panel considers the infant's ability of holding the head in midline when in supine position and to control its head well when pulled to sitting or at aided sitting to be the earliest gross motor skills indicative of an infant's developmental readiness to consume spoon‐fed pureed CFs.
Table 5 gives an overview about the achievement of these and related milestones in the studies that were retrieved through the extensive literature search.
Table 5.
Attainment of the gross motor developmental milestones indicative of an infant's developmental readiness to consume spoon‐fed pureed CFs
| Skill | Age | Result | N | Country | Study design | Author |
|---|---|---|---|---|---|---|
| Head in midline in supine position | 3 m | 60% could keep head in midline to a limited extent | 8 | SE | Cross‐sectional | Hedberg et al. (2005) |
| Head in midline in supine position | 3 and 4 m | Increased frequency of headline posture in midline | 13 | BR | Longitudinala | Lima‐Alvarez et al. (2014) |
| Head in midline in supine position | 4 m | 60% could keep the head adequately in the midline and another 20% could keep it in the midline to a limited extent | 8 | SE | Cross‐sectional | Hedberg et al. (2005) |
| Head in midline in supine position | 5 m | 100% could keep the head adequately in the midline | 8 | SE | Cross‐sectional | Hedberg et al. (2005) |
| Control of head movements in supine position | 3 and 4 m | Increased proportion of midline‐to‐side and side‐to‐side movements | 13 | BR | Longitudinal | Lima‐Alvarez et al. (2014) |
| Control of head movements in supine position | 4 m | Increased peak velocity of head movements | 13 | BR | Longitudinal | Lima‐Alvarez et al. (2014) |
| Head control when pulled to sitting | 3–4 m | Mean age when milestone reached: 3.25 (SD 0.72) m | 13,076 | JP | Longitudinal | Yokoyama et al. (2011) |
| Head control when pulled to sitting | 4 m | 33% had a good head control | 51 | AU | Longitudinal | Pin et al. (2009) |
| Head balance at aided sitting | 4 m | 100% of infants had adequate head control at aided sitting | 8 | SE | Cross‐sectional | Hedberg et al. (2005) |
AU: Australia; BR: Brazil; JP: Japan; m: months; SE: Sweden.
Studied at birth, 1, 2, 3 and 4 months of age.
Information on the development of fine motor skills relevant for spoon‐feeding of pureed food was available from only one longitudinal study (Carruth and Skinner, 2002). In this study, infants were able to use the tongue to move food to the back of the mouth and swallow it at a mean age of 4.95 (SD 1.27) months with a range of 2.0–7.5 months. The Panel notes that the development of this skill is also indicative that the extrusion reflex had already disappeared at that age. In the same study, infants were able to keep food in their mouth without the need to be re‐fed at a mean age of 5.72 (SD 1.58) months with a range of 0.5–10.5 months and to use the upper lip to remove food from the spoon at a mean age of 7.73 (SD 2.23) months with a range of 4.0–16.0 months.
The Panel notes that the earliest gross motor skills indicative of developmental readiness for spoon‐feeding of pureed CFs (i.e. holding the head in midline when in supine position and to control its head well when pulled to sitting or at aided sitting) can be observed between 3 and 4 months of age. From the limited evidence that is available, fine motor skills indicative of developmental readiness for spoon‐feeding of pureed foods and full disappearance of the extrusion reflex occur on average later.
3.3.2. Gross and fine motor skills relevant for self‐feeding of finger foods
The Panel considers the infant's ability to sit alone is indicative of an infant having achieved the developmental readiness to consume self‐fed finger foods.
Table 6 gives an overview about the achievement of this milestone in the studies that were retrieved through the extensive literature search.
Table 6.
Attainment of the gross motor developmental milestone indicative of an infant's developmental readiness to consume self‐fed finger foods
| Skill | Result | N | Country | Study design | Author |
|---|---|---|---|---|---|
| Sitting in lap without support |
Mean age: 5.54 m SD 2.08 m |
98 | US | Longitudinal | Carruth and Skinner (2002) |
| Sitting alone |
Mean age: 5.4 m Range 3.8–9.2 m |
816 | GH, IN, NO, OM, USa | Longitudinal | WHO Multicentre Growth Reference Study Group (2006) |
| Sitting alone | Mean age: 5.6–6.0 m depending on the group of infants investigated | 105 | US | Longitudinal | Heinig et al. (1993) |
| Sitting alone |
Median age: 6 m Range: 4–9 m |
189 | CN | Retrospective | Wang et al. (2019) |
| Sitting alone |
Median age: 6.3 m IQR: 6.0–7.2 m |
542b | IT | Longitudinal | Agostoni et al. (2009) |
| Sitting alone |
Mean age: 6.66 m SD 1.03 m |
13,076 | JP | Longitudinal | Yokoyama et al. (2011) |
| Sitting up from lying position |
Mean age: 6.9 m SD 1.3 m |
140c | HN | Longitudinal | Dewey et al. (2001) |
| Sitting up from lying position |
Mean age: 7.8 m SD 1.6 m |
108d | HN | Longitudinal | Dewey et al. (2001) |
CN: China; GH: Ghana; HN: Honduras; IN: India; IQR: interquartile age; IT: Italy; JP: Japan; m: months; NO: Norway; OM: Oman, US: United States.
Gross motor milestones were not assessed in the Brazilian study site.
Infants in the control group of a randomised controlled trial.
Appropriate and small‐for‐gestational age infants.
Small‐for‐gestational age infants.
With respect to fine motor skills necessary for self‐feeding of finger foods one study (Törölä et al., 2012) reported that in 11 term infants emerging chewing (i.e. lateral and diagonal movements) appeared at a median age of 5 (range 5–‐8) months, while chewing (i.e. rotatory movements) occurred later: diagonal rotatory movements were observed at a median age of 7 (range 7–10) months and circulatory movements at a median age of 8 (range 7–11) months.
In the Gateshead Millennium Study, a population‐based cohort study, 56% of infants (340 out of 602) were reported to be reaching for food before 6 months of age (Wright et al., 2011). During this time period, it is also expected that reaching movements become more organised and mature (von Hofsten, 1991). In addition, this may be interpreted as an apparent interest in food, even though the Panel acknowledges that this could be also an expression of interest in the environment.
The Panel notes that the gross motor skill indicative of developmental readiness for self‐feeding finger foods (i.e. sitting without support) can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age. From the limited evidence that is available, fine motor skills indicative of developmental readiness for self‐feeding finger foods may occur at the same time or slightly later.
3.4. Developmental readiness of the term infant to receive CFs: conclusions
The Panel considers that the gastrointestinal and renal functions are not limiting factors with respect to the timing of introduction of CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in such feeding.
The Panel further considers that there is a large biological variability when infants develop the necessary neuromotor skills for progressing from a liquid to a diet including pureed CFs and finger foods, depending on the individual infant. Furthermore, they are acquired and consolidated over a period of time with practice. From the neurodevelopmental data, it is not possible to define a precise age when introduction of CFs is appropriate.
The Panel concludes that the earliest gross motor skills indicative of developmental readiness for spoon‐feeding of pureed CFs (i.e. holding the head in midline when in supine position and to control its head well when pulled to sitting or at aided sitting) can be observed between 3 and 4 months of age. At this age, it can be assumed that the rooting and the extrusion reflexes may have also diminished in some infants.
The Panel also concludes that the gross motor skill indicative of developmental readiness for self‐feeding finger foods (i.e. sitting without support) can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age.
4. Assessment of the data on body weight, body length/height and head circumference in individuals born at term or mixed populations
4.1. Body weight, body length/height and head circumference: final body of evidence
The 42 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.1. These included two publications that were considered together (Kramer et al., 1985a,b) and one publication that covered four studies (Moschonis et al., 2017).
These publications reported on results from 42 studies:
5 RCTs (2 rated as Tier 1, 3 rated as Tier 2);
30 prospective cohort studies and 2 pooled analyses of prospective studies (3 rated as Tier 1, 15 rated as Tier 2 and 16 rated as Tier 3; 2 studies were allocated two different Tiers depending on the endpoint that was assessed);
6 retrospective studies (all Tier 3).
In these studies, 18 different endpoints related to body weight, body length and HC were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, for the main endpoints, results are summarised in the forest plots in Appendices 1, A.1., A.2., A.3., A.4., A.5., A.6., A.7., A.9.–A.10 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
4.2. Body weight, body length/height and head circumference: endpoint and study selection
The first anthropometric measure to be impacted in the presence of nutritional imbalances is body weight, whilst body length/height and HC change at later stages. In the absence of evidence of an effect of the timing of introduction of CFs on body weight endpoints, body length/height and HC are unlikely to be altered. In addition, measurement errors for body length/height and HC are higher compared to body weight (Harrison et al., 2001). Therefore, the emphasis of the assessment is put on body weight endpoints.
The interpretation of the biological relevance of mean differences in anthropometric outcomes depends on the age at outcome assessment and the characteristics of the reference group to which the other groups are compared. For example, a 1 kg difference in weight at 12 months of age might be of relevance while it may be minor at, e.g. 10 years of age. This difference compared to a relatively underweight reference group will have a different meaning than if compared to a relatively overweight reference group. The advantage of the use of z‐scores is that the age at outcome assessment (and gender) is already considered, which makes comparisons across measurement time points possible. However, the use of different reference populations between studies to transform absolute measurements into z‐scores (e.g. WHO, US Centers for Disease Control and Prevention (CDC) or national growth standards) limits the comparability of results between these studies. Despite this, the Panel decided to give priority to results reported as z‐scores.
Conditional body weight gain, expressed in z‐scores, takes into account that, on average, children with a relatively higher or lower body weight at an initial time point will tend to have a body weight closer to the median at a subsequent time point (regression to the mean). It also allows more readily comparisons of outcomes of different studies. On the contrary, the interpretation of absolute body weight gain is hampered by the different time periods in which body weight gain is measured, and the use of different metrics (grams over the whole period, g/month). Absolute body weight gains expressed in grams per month also assume a linear growth rate of a child, which is, however, not observed biologically. This use of different metrics was also observed for absolute body length gain.
The biological relevance of differences in z‐scores is judged compared to a difference of 0.5 z‐scores (SCF, 2003), which is considered to be biologically relevant for anthropometric outcomes.31 A difference of 3 g/day in weight gain over a 3‐ to 4‐month period was suggested by the AAP (1988) to constitute a biologically relevant difference.
With respect to the minimum study duration, only studies that provided results beyond the age of 6 months were included in this assessment, considering that they provide more reliable estimates of associations that may persist beyond infancy.
Regarding possible reverse causality of observational studies, infants growing faster or are heavier may be introduced to CFs earlier. Thus, this may (partially) explain an association at later time points between age of introduction of CFs and anthropometric outcomes. This aspect has been addressed in the assessment of the RoB related to confounders (Section 2.2.2; key question 3), by considering whether a previous outcome measurement was taken into account as a covariate in the analysis.
4.3. Body weight: summary of the evidence
This section discusses firstly WAZ and attained body weight and the related subgroup analyses, secondly weight‐for‐length(height)‐z‐scores (WL(H)Z), thirdly endpoints for which the results are not shown in forest plots, i.e. conditional body weight gain, absolute body weight gain, rapid body weight gain (either because of the availability of only two studies with point estimates or because of lack of comparability of result metrics) and finally miscellaneous endpoints.
4.3.1. WAZ and attained body weight
Main line of evidence (12 studies)
For WAZ or attained body weight, the evidence derived from the five RCTs (Cohen et al., 1995a; Mehta et al., 1998; Dewey et al., 1999; Jonsdottir et al., 2014; Perkin et al., 2016) did not show an effect of the introduction of CFs at 3–4 months of age compared with introduction at 6 months on these endpoints assessed up to the age of 3 years (Appendices A.1 and A.3). This is true for the pooled estimate as well as for the results of the individual studies. Heterogeneity was not important (I2 = 0% for both WAZ and attained body weight).
Seven prospective cohort studies were reported in eight papers (Forsyth et al., 1993; Wilson et al., 1998; Haschke and van't Hof, 2000; Grote et al., 2011; Imai et al., 2014; Noppornlertwong and Tantibhaedhyangkul, 2016; Eriksen et al., 2017). These investigated the timing of introduction of CFs at various ages, four of them investigated introduction at ages below 3 or 4 months vs later. They did not show a biologically relevant association of the age of introduction of CFs with WAZ and attained body weight assessed up to the age of 7 years (Appendices A.1 and A.3). This is true for the pooled estimate as well as for the results of the individual studies. Heterogeneity was substantial for WAZ (I2 = 74%) and not important for attained body weight (I2 = 0%).
Subgroup analyses were made on WAZ and attained body weight, in exclusively breastfed or formula fed infants (Section 2.2.3.2): there was no evidence for an association in any of these two groups, for these two endpoints (Appendices A.2 and A.4).
The Panel notes, from the five RCTs and seven prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and weight assessed up to the age of 7 years.
Supportive line of evidence (15 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (9 studies, Tier 3)
There was no evidence for an association from the meta‐analysis of four studies (Heinig et al., 1993; Kalanda et al., 2006; Huh et al., 2011; Gaffney et al., 2012) that investigated the association between the introduction of CFs, mostly below 3 or 4 months of age vs thereafter (three out of four studies), on WAZ assessed up to 3 years of age. Heterogeneity was moderate to substantial (I2 = 54%) (Appendix A.1).
The only studies for which the overall pooled estimate and associated 95% CI departed from the ‘null’ effect were the three prospective cohort studies that investigated attained body weight (Hodgson, 1978; Huh et al., 2011; Atkins et al., 2016). In the meta‐analysis of five group comparisons from these three studies, there was an association between earlier introduction (< 1.5 to < 6 months) of CFs, compared with later introduction, and a higher attained body weight up to the age of 3 years (mean difference 391 (95% CI 211 to 570) g) (Appendix A.3). All estimates were unadjusted and therefore it is likely that the association observed in the meta‐analysis is overestimated. Heterogeneity was not important (I2 = 0%). In addition, three studies (Warrington and Storey, 1988; WHO Working Group on Infant Growth, 1994; Morgan et al., 2004) did not report point estimates, but did not find statistically significant associations between the introduction of CFs and attained body weight or WAZ at 12, 18 and 24 months of age.
Retrospective studies (1 study, Tier 3)
In one cross‐sectional analysis of baseline data of a prospective cohort study (Sloan et al., 2008), a higher WAZ at 14 months of age with a borderline biological relevance was observed in infants introduced to CFs before 4 months of age compared with thereafter (adjusted mean difference 0.45 (95% CI 0.21 to 0.94) z‐scores) (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (5 studies)
The prospective cohort studies described in Kramer et al. (1985a), Vail et al. (2015) and Butte et al. (2000) (Tiers 2 and 3) as well as a cross‐sectional study (Zhu et al., 2015) and a retrospective cohort study (Klag et al., 2015) (both Tier 3) did not observe statistically significant associations between the timing of introduction of CFs and attained body weight at 2 years and WAZ at 1, 2 and 6 years (Annex A as Microsoft Excel® file).
The Panel notes that, in the supportive line of evidence, the meta‐analysis of five group comparisons from three prospective cohort studies (Tier 3) as well as a retrospective study indicate a significant association between ‘early’ introduction of CFs and a higher body weight in childhood (in total four studies). However, the meta‐analysis of the four prospective cohort studies (Tier 3) on WAZ and the results of the remaining individual studies (in total 10 studies) in the supportive line of evidence are consistent with the findings of the main line of evidence.
4.3.2. Other body weight‐related endpoints
Main line of evidence
For WL(H)Z (4 studies) assessed up to 4 years of age, the RCT (Perkin et al., 2016) and the meta‐analysis of the three prospective cohort studies (Grote et al., 2011; van Rossem et al., 2013; Eriksen et al., 2017) did not show an association with early introduction of CFs (in two studies: before 3 or 4 months of age). Heterogeneity was moderate to substantial (I2=51%) (Appendix A.5).
For conditional body weight gain (2 studies) assessed up to 3 years of age, the two prospective cohort studies (Griffiths et al., 2009; de Beer et al., 2015) did not show a biologically relevant association between the age of introduction of CFs before 4 months compared with thereafter and the outcome (Annex A as Microsoft Excel® file).
For absolute body weight gain (5 studies) assessed in different time spans, results of the five prospective studies (including one RCT) (Cohen et al., 1995a; Simondon and Simondon, 1997; Imai et al., 2014; Mäkelä et al., 2014; Noppornlertwong and Tantibhaedhyangkul, 2016) are not directly comparable (as explained in Section 4.2). They are therefore not summarised graphically. There were no statistically significant findings comparing various time points of introduction of CFs (Annex A as Microsoft Excel® file).
For rapid body weight gain (2 studies), defined as change in z‐score above 0.67, the prospective cohort study by Azad et al. (2018) showed higher odds of rapid body weight gain from 0 to 12 months, with introduction of CFs below 4 months compared with after 6 months (adjusted odds ratio (aOR) 1.43 (95% CI 1.01 to 2.01)) and also at 4–5 months compared with after 6 months (aOR 1.86 (95% CI 1.36 to 2.56)). On the contrary, Layte et al. (2014) showed no evidence for an association between rapid body weight gain from 0.75 to 3 years and introduction of CFs before 4 months of age compared with later (Annex A as Microsoft Excel® file).
For WAZ gain (1 study) between birth and 12 months, in the study by Azad et al. (2018), there was no biologically relevant association between the timing of introduction of CFs and WAZ gain (Annex A as Microsoft Excel® file).
The Panel notes that only one study (Tier 2) in the main line of evidence shows a relevant association between the timing of introduction of CFs before 6 months of age compared with thereafter and rapid weight gain in the first year of life, while the 12 other studies (Tiers 1 and 2) do not observe biologically relevant associations between the timing of introduction of CFs and the endpoints investigated.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
For WL(H)Z (3 studies, Tiers 2 and 3), the findings from two prospective studies (WHO Working Group on Infant Growth, 1994; Butte et al., 2000) and a cross‐sectional study (Zhu et al., 2015) that analysed the timing of introduction of CFs as a continuous variable are consistent with the results of the main line of evidence (Annex A as Microsoft Excel® file).
For conditional body weight gain (2 studies, Tier 3), the findings from a prospective study (Wright et al., 2004) are consistent with the results of the main line of evidence, while in one cross‐sectional analysis of baseline data of a prospective cohort study (Sloan et al., 2008), a higher conditional body weight gain between 2 and 14 months of age, with a borderline biological relevance, was observed in infants introduced to CFs before 4 months of age compared with thereafter (adjusted mean difference: 0.49 (95% CI 0.26 to 0.93) z‐scores) (Annex A as Microsoft Excel® file).
For absolute body weight gain (6 studies, 5 Tier 3 and 1 Tier 2), three prospective studies (Heinig et al., 1993; Baker et al., 2004; Morgan et al., 2004) found earlier introduction of CFs (< 3 to < 6 months vs thereafter) to be associated both with higher and lower weight gain that was not considered by the Panel to be of biological relevance (ranging from mean differences of −200 g to 167 g for 15‐ and 12‐month time periods, respectively). The prospective study that analysed the timing of introduction of CFs as a continuous variable (Haschke and van't Hof, 2000) (Tier 2), and a retrospective cohort study (Klag et al., 2015), are consistent with the main line of evidence, as well as one cross‐sectional analysis of baseline data of a prospective cohort study (Kim and Peterson, 2008) in which the statistically significantly higher body weight gain between birth and 9 months (adjusted mean difference: 47 (95% CI 15 to 78) g) was not of biological relevance (Annex A as Microsoft Excel® file).
For rapid body weight gain (1 study, Tier 3), the results of a cross‐sectional analysis of baseline data of an RCT (Mihrshahi et al., 2011) did not show an association between early introduction of CFs (< 3 vs ≥ 3 months) and rapid body weight gain from birth to 4–7 months of age (Annex A as Microsoft Excel® file).
For WAZ gain (1 study, Tier 3), Klag et al. (2015) in a retrospective cohort study did not observe a statistically significant association between the timing of introduction of CFs used as a continuous variable in the analysis and WAZ gain between birth and 12 months (Annex A as Microsoft Excel® file).
The Panel notes that the results in the supportive line of evidence are mostly consistent with those in the main line of evidence: 12 (10 Tier 3 and 2 Tier 2) out of 13 studies do not observe biologically relevant differences in the endpoints investigated, while one study (Tier 3) finds a borderline biologically relevant higher conditional weight gain between 2 and 14 months of age to be associated with introduction of CFs before 4 months of age compared with thereafter.
Endpoints investigated in single studies
Other endpoints investigated that were related to weight were: WL(H)Z‐trajectories and WAZ‐trajectories (Grote et al., 2011) (main line of evidence); proportion of children who had started CFs < 4 months of age in WAZ and WL(H)Z tertiles (Sit et al., 2001) (supportive line of evidence). These were assessed in single studies only. Therefore, they cannot be used to establish the appropriate age range of introduction of CFs (Section 2.2.3.3).
4.4. Body length/height: summary of the evidence
This section discusses first L(H)AZ and attained body length/height and the related subgroup analyses, then absolute body length gain for which no forest plot could be made (Section 2.2.3.2), and finally miscellaneous endpoints.
4.4.1. L(H)AZ and attained body length/height
Main line of evidence (11 studies)
For L(H)AZ or attained body length/height, the evidence derived from the five RCTs (Cohen et al., 1995a; Mehta et al., 1998; Dewey et al., 1999; Jonsdottir et al., 2014; Perkin et al., 2016) did not show an effect of the introduction of CFs at 3–4 months of age compared with the introduction at 6 months on these endpoints assessed up to around 3 years of age (Appendices A.6 and A.8). This is true for the pooled estimate as well as for the results of the individual studies. Heterogeneity was not important (I2 = 0% for both L(H)AZ and attained body length).
The six prospective cohort studies, investigating various ages of introduction of CFs (in three studies: before 3 or 4 months of age), did not show biological relevant associations of earlier introduction of CFs with L(H)AZ or attained body length/height up to the age of 9 years ((Haschke and van't Hof, 2000; Grote et al., 2011; Imai et al., 2014; de Beer et al., 2015; Noppornlertwong and Tantibhaedhyangkul, 2016; Eriksen et al., 2017) as well as Moschonis et al. (2017) for the EDEN32 study and the Avon Longitudinal Study of Parents and Children (ALSPAC)). This is true for each individual comparison and for the results of the meta‐analyses. Heterogeneity was moderate to substantial for L(H)AZ (I2 = 55%) and not important for attained body length/height (I2 = 0%).
Subgroup analyses were made on L(H)AZ and attained body length/height, in exclusively breastfed or formula fed infants (Section 2.2.3.2): there was no evidence for an association in any of these two groups, for these two endpoints (Appendices A.7 and A.9).
The Panel notes, from the five RCTs and the six prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and body length/height assessed up to the age of 9 years.
Supportive line of evidence (10 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
The meta‐analyses of the four prospective cohort studies reported in three publications rated as Tier 3 on L(H)AZ (Heinig et al., 1993; Huh et al., 2011; Moschonis et al., 2017) and the meta‐analysis on the two prospective cohort studies on attained body length/height (Huh et al., 2011; Atkins et al., 2016), which investigated various ages of introduction of CFs, were consistent with the findings in the main line of evidence (Appendices A.6 and A.8). Heterogeneity was not important (L(H)AZ I2 = 0%, attained body length/height I2 = 0%). Individually, the two studies on attained body length/height that were combined in the meta‐analysis found that exclusively breastfed infants, but not formula fed infants, that were introduced to CFs < 4 months of age compared with thereafter (Huh et al., 2011) and infants introduced to CFs < 6 months of age compared with thereafter (Atkins et al., 2016) were taller at 3 years and 20 months of age, respectively. However, the estimates were unadjusted and therefore it is likely that the associations that were observed were overestimated.
In addition, Morgan et al. (2004) and WHO Working Group on Infant Growth (1994) that did not provide a point estimate reported that the timing of introduction of CFs were not associated with attained body length at 18 months and L(H)AZ at 9, 10, 11 and 12 months of age, respectively.
Also the prospective cohort studies by Vail et al. (2015) and Butte et al. (2000), and the cross‐sectional study by Zhu et al. (2015) (all Tier 3) that analysed the timing of introduction of CFs as a continuous variable, found no evidence for an association (Annex A as Microsoft Excel® file).
The Panel notes that the results of the ten studies (Tier 3) in the supportive line of evidence are consistent with those in the main line of evidence.
4.4.2. Absolute length gain
Main line of evidence (3 studies)
The results of the three individual prospective studies, including one RCT (Cohen et al., 1995a; Simondon and Simondon, 1997; Noppornlertwong and Tantibhaedhyangkul, 2016), are not directly comparable (as explained in Section 4.2). Therefore, they are not summarised graphically in Appendix A. They did not find statistically significant associations between the timing of introduction of CFs (investigating various time points of introduction) and length gain assessed in different time spans up to 12 months of age (Annex A as Microsoft Excel® file).
The Panel notes, from the RCT and two prospective cohort studies (all Tier 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and length gain assessed in different time spans up to the age of 12 months.
Supportive line of evidence (3 studies)
The prospective cohort study (Tier 3) by Heinig et al. (1993) was consistent with the findings in the main line of evidence (Annex A as Microsoft Excel® file).
Morgan et al. (2004) (Tier 3) reported a statistically significantly lower body length gain between 3 and 18 months of age associated with introduction of CFs < 3 months of age compared with thereafter (Annex A as Microsoft Excel® file). However, infants introduced to CFs < 3 months of age were longer at baseline than those introduced later, and the lower length gain led to a comparable body length in both groups of infants at 18 months of age. Therefore, the Panel considers this finding not to be of biological relevance.
Haschke and van't Hof (2000) (Tier 2) that analysed the timing of introduction of CFs as a continuous variable reported a statistically significantly lower body length gain between 1 and 24 months to be associated with earlier introduction of CFs (adjusted mean difference: −0.05 (95% CI −0.09 to −0.01) mm/month per month of earlier introduction of CFs) (Annex A as Microsoft Excel® file). Between 1 and 12 months, differences in body length gain were not statistically significant and the point estimate was on the other side of the ‘null’ line, which may indicate that the lower body length gain primarily occurred between 12 and 24 months of age and thus may not be a result that could be directly attributed to the timing of introduction of CFs.
The Panel notes that the studies in the supportive line of evidence (2 Tier 3 and 1 Tier 2) show divergent results. However, these are considered by the Panel as being either of no biological relevance or unlikely to be a direct result of the timing of introduction of CFs. Therefore, the Panel considers the results of the studies in the supportive line of evidence to be consistent with the findings from the main line of evidence.
Endpoints investigated in single studies
Other investigated endpoints related to length were: conditional body length gain (de Beer et al., 2015) and L(H)AZ‐trajectories (Grote et al., 2011) (main line of evidence) (Annex A as Microsoft Excel® file). These were assessed in single studies only. Therefore, they cannot be used to establish the appropriate age range of introduction of CF.
4.5. Head circumference: summary of the evidence
This section discusses the endpoints related to HC, mostly investigated in RCTs, i.e. attained HC, HC‐for‐age z‐scores (HCZ), and finally, miscellaneous endpoints.
Main line of evidence (4 studies)
For HCZ, individual RCTs (Jonsdottir et al., 2014; Perkin et al., 2016) showed no statistically significant effect of the timing of introduction of CFs at 3–4 months of age compared with the introduction at ≥6 months on this endpoint assessed up to 3 years of age (Annex A as Microsoft Excel® file).
For attained HC, the evidence derived from the three RCTs (Mehta et al., 1998; Jonsdottir et al., 2014; Perkin et al., 2016) did not show an effect of the timing of introduction of CFs at 3–4 months of age compared with the introduction at 6 months on this endpoint assessed up to 3 years of age. This is true for the results of the meta‐analysis and the individual studies (Appendix A.10). Heterogeneity was not important (I2 = 0%). The results of the only prospective cohort study (Noppornlertwong and Tantibhaedhyangkul, 2016) that investigated introduction of CFs at 4–6 months of age vs > 6 months and attained HC at the age of 12 months, are consistent with the above.
For HC gain, one study (Noppornlertwong and Tantibhaedhyangkul, 2016) did not show statistically significant differences in HC gain between 4 and 12 months of age (Annex A as Microsoft Excel® file).
The Panel notes, from the three RCTs and the one prospective cohort study (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and HC assessed up to 3 years of age.
Supportive line of evidence (1 study)
For HC gain, the prospective study by Morgan et al. (2004) (Tier 3) found a statistically significant lower HC gain between 3 and 18 months of age associated with introduction of CFs < 3 months of age compared with thereafter (Annex A as Microsoft Excel® file). However, infants introduced to CFs < 3 months of age had a higher HC at baseline than those introduced later, and the lower HC gain led to a comparable HC in both groups of infants at 18 months of age. Therefore, the Panel considers this finding not to be of biological relevance.
The Panel notes that the association observed in the study (Tier 3) in the supportive line of evidence is not of biological relevance. The Panel also notes that the result of the study in this line of evidence is consistent with the findings in the main line of evidence.
4.6. Body weight, body length/height and head circumference: conclusions and grading of the confidence in the evidence
Imprecision: The results of the meta‐analyses did not indicate imprecision.
Inconsistency: The evidence is consistent across populations and the results of the studies in the supportive line of evidence (20 studies for body weight, 10 for body length/height and 1 for HC) were consistent with the main line. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: For all outcomes, RCTs in exclusively breastfed and exclusively formula fed infants were available. Subgroup analyses (independent of study design) did not show different effects on WAZ, attained body weight, L(H)AZ or attained body length/height, of the timing of introduction of CFs in exclusively breastfed and in exclusively formula fed infants (Appendices A.2, A.4, A.7 and A.9). Therefore, the Panel considers that the evidence from RCTs can be generalised to the whole population of infants living in Europe. As a representative number of populations were studied in the prospective cohort studies, the Panel considers that their results can also be generalised.
Publication bias: From visual inspections of the funnel plots on WAZ, attained body weight and L(H)AZ, there was no convincing evidence for publication bias (Annexes D.1, D.2 and D.3). For the other endpoints, publication bias could not be evaluated, because of the insufficient number of studies.
The Panel concludes from the RCTs, that there is no effect of introduction of CFs at 3–4 months vs 6 months of age on body weight (5 RCTs), body length/height (5 RCTs) and HC (3 RCTs) assessed up to around 3 years of age (high level of confidence in the evidence).
The Panel concludes from prospective cohort studies (Tiers 1 and 2), covering a broader range of ages of introduction of CFs than the RCTs, that there is no evidence for an association between the age of introduction of CFs and body weight (12 studies) and body length/height (9 studies) (moderate level of confidence in the evidence). For the assessment of body weight, the ages of introduction of CFs ranged between < 2 months and < 6 months for early introduction and > 2 months and ≥ 6 months for later introduction. For the assessment of body length/height, early introduction ranged from 2–3 months to < 6 months, and later introduction from > 4 to ≥ 6 months. The latest age of outcome assessment was 7 years for body weight and 9 years for body length/height.
The prospective cohort study available on HC was integrated with the RCTs as the ages that were compared were already covered by the RCTs.
5. Assessment of the data on BMI and related endpoints in individuals born at term or mixed populations
5.1. BMI: final body of evidence
The 40 publications that were considered in the assessment of data on BMI in individuals born at term or mixed populations are given in Appendix B.2 (2 publications were considered together (Kramer et al., 1985a,b)).
These publications reported on results of 36 studies:
2 RCTs (Tier 1);
26 prospective cohort studies (5 rated as Tier 1, 12 rated as Tier 2 and 12 rated as Tier 3; three studies were attributed two different Tiers);
8 retrospective studies (Tier 3).
In line with the reasons given in Section 5.2 for the selection of papers that reported on different ages at assessment of an endpoint in the same study, the results provided by de Beer et al. (2015) were used for the Amsterdam Born Children and their Development (ABCD) study (instead of those provided by Sirkka et al. (2018)) and the results provided by Vogelezang et al. (2018) for the Generation R study (instead of those provided by Durmuş et al. (2014)).
In the included studies, nine different endpoints related to BMI were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file, including the ones by Sirkka et al. (2018) and Durmuş et al. (2014). In addition, results are summarised in the forest plots in Appendices A.11–A.13 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
5.2. BMI: endpoint and study selection
Previous considerations (Section 4.2) on advantages and limitations of using z‐scores compared to absolute (attained) measurements in the context of endpoints related to body weight or body length, as well as previous considerations on biological relevance of differences in z‐scores, are also true in the context of studies on BMI, discussed in the following sections.
BMI‐related outcomes (i.e. a continuous outcome) are discussed separately from the dichotomised outcome of overweight or obesity. Even though the definition of overweight and obesity is based on BMI, the dichotomisation may lead to different findings compared with results obtained from an analysis of the outcome on a continuous scale (i.e. BMI). Therefore, results are not necessarily comparable.
Regarding possible reverse causality of observational studies, the same considerations and approach for the assessment of the RoB described above for weight endpoints (Section 4.2) were also relevant for BMI and related endpoints.
5.3. BMI: summary of the evidence
This section discusses first BMIZ and attained BMI for which forest plots could be made, then the subgroup analysis for BMIZ, and finally miscellaneous endpoints.
Main line of evidence (13 studies)
For BMIZ or attained BMI, the evidence derived from the two RCTs in exclusively breastfed infants (Jonsdottir et al., 2014; Perkin et al., 2016) did not show an effect of the timing of introduction of CFs at 3–4 months of age compared with the introduction at 6 months on these endpoints assessed up to 3 years of age, neither from the meta‐analysis nor individually. Heterogeneity was not important (I2 = 0% both for BMIZ and attained BMI) (Appendices A.11 and A.13).
For BMIZ, the nine prospective cohort studies (Burdette et al., 2006; Grote et al., 2011; Huh et al., 2011; de Beer et al., 2015; Fairley et al., 2015; Leary et al., 2015; Zheng et al., 2015; Azad et al., 2018; Vogelezang et al., 2018), the majority of which investigated the timing of introduction of CFs below the age of 3 or 4 months vs later, did not show a biologically relevant association between the age of introduction of CFs and BMIZ assessed up to 15 years of age. This is true for the result of the meta‐analysis and for each individual comparison. Heterogeneity was not important to moderate (I2 = 35%).
For attained BMI at the age of 2 years, neither the results of the two individual prospective studies nor the result of the meta‐analysis (Grote et al., 2011; Wen et al., 2014) was statistically significant, comparing introduction < 3 and < 2 months with later, respectively. This was also true for the individual studies. Heterogeneity was not important (I2 = 0%). In addition, Agras et al. (1990) did not report a statistically significant association between the introduction of CF ≤ 5 months of age compared with thereafter on attained BMI at 6 years of age (results only presented as correlation coefficients, thus not included in the meta‐analysis).
A subgroup analysis was performed for BMIZ, in exclusively breastfed or formula fed infants (Section 2.2.3.2): there was no evidence for an association in either of these two groups (Appendix A.12).
The Panel notes, from the two RCTs and 11 prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and BMI assessed up to 10 years of age.
Supportive line of evidence (17 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (7 studies, Tier 3)
The meta‐analysis of five studies (Wilson et al., 1998; Haschke and van't Hof, 2000; Iguacel et al., 2018; Schmidt Morgen et al., 2018) on BMIZ assessed up to 11 years of age did not show a statistically significant association with the timing of introduction of CFs before 3.5 or 4 months of age compared with thereafter (mean difference –0.06 (95% CI −0.37 to 0.25) z‐scores) (Appendix A.11). However, heterogeneity was important (I2 = 95%). When the study by Haschke and van't Hof (2000), that showed results considerably different from the other studies in that Tier (the reasons for which cannot be explained), was removed in a sensitivity analysis, heterogeneity became non‐important to moderate (I2 = 33%), the pooled point estimate shifted to the other side of the line of the ‘null’ effect and the 95% CI was reduced (i.e. mean difference 0.05 (95% CI −0.03 to 0.13) z‐scores).
Equally, the meta‐analysis of the four comparisons from the three studies on attained BMI assessed up to around 10 years of age (Veena et al., 2010; Huh et al., 2011; Imai et al., 2014) did not show a statistically significant association between the timing of introduction of CFs (in two studies < 4 months compared with later) and this endpoint (Appendix A.13). Heterogeneity was substantial (I2 = 63%).
Retrospective studies (3 studies, Tier 3)
One cross‐sectional study (Brambilla et al., 2016) and one prospective cohort study (in which the timing of introduction of CFs was assessed after the outcome) (Lin et al., 2013) did not find an association between the timing of introduction of CFs at various ages and BMIZ assessed up to 14 years of age.
Vafa et al. (2012) found a statistically significantly higher attained BMI in 7‐year‐old children introduced to CFs ≤ 4 months of age compared with thereafter (adjusted mean difference 0.88 (95% CI 0.26 to 1.50) kg/m2).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (7 studies)
Three such studies on BMIZ (two prospective cohort studies (Schack‐Nielsen et al., 2010; Vail et al., 2015) and one cross‐sectional study (Zhu et al., 2015), all Tier 3) did not find biologically relevant associations between the timing of introduction of CFs and the outcome assessed up to 42 years of age (Annex A as Microsoft Excel® file).
The four studies that analysed the timing of introduction of CFs as a continuous variable, did not find a statistically significant association between the timing of introduction of CFs and attained BMI assessed up to 4 years of age (one prospective cohort study rated as Tier 1 (Lande et al., 2005), two prospective cohort studies rated as Tier 2 (Kramer et al., 1985a; Robinson et al., 2009) and one cross‐sectional analysis of baseline data of a prospective cohort study (Tier 3) (Zive et al., 1992)) (Annex A as Microsoft Excel® file).
The Panel notes that, in the supportive line of evidence, the results of the meta‐analyses of the prospective cohort studies (7 studies, Tier 3) as well as 9 of the 10 remaining individual studies (3 rated as Tiers 1 and 2; 6 Tier in 3) are consistent with the findings in the main line of evidence. Only one cross‐sectional study (Tier 3) observed a higher attained BMI at 7 years to be associated with the introduction of CFs ≤ 4 months compared with later. Overall, the Panel considers that the results in the supportive line of evidence is consistent with those in the main line of evidence.
Endpoints investigated in single studies
Other investigated endpoints related to BMI were: BMIZ trajectories (Grote et al., 2011), BMI trajectory class membership (Garden et al., 2012), and ‘high’ BMI (Caleyachetty et al., 2013) (main line of evidence); % expected weight (Poskitt and Cole, 1978), waist circumference (Schack‐Nielsen et al., 2010) and the Shukla index (Thorogood et al., 1979) (supportive line of evidence). These were assessed in single studies only. Therefore, they cannot be used to establish the appropriate age range of introduction of CFs (Section 2.2.3.3).
5.4. BMI: conclusions and grading of the confidence in the evidence
Imprecision: The results of the meta‐analyses did not indicate imprecision.
Inconsistency: The evidence is consistent across populations and, overall, the supportive line of evidence (16 out of 17 studies) is consistent with the main line of evidence. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: The study population of both RCTs consisted only of exclusively breastfed infants. Individually, these studies cannot be generalised to formula fed or mixed fed infants. However, considering that subgroup analyses on exclusively breastfed and exclusively formula fed infants did not show different effects of the timing of introduction of CFs on BMI in those infants (Appendix A.12) and that observational studies with a variety of different background milk feedings were consistent with the findings of the RCTs, the Panel considers that the results of these two RCTs can be generalised to the whole population of infants living in Europe. In the prospective cohort studies, a representative number of populations were studied. Therefore, the Panel considers that results from these studies can also be generalised.
Publication bias: Even though the funnel plot for BMIZ appeared to be asymmetrical (Annex D.4), none of the statistical methods (Egger's test, trim‐and‐fill analysis and contour plots) applied suggested asymmetry (data not shown). Therefore, the Panel considers that publications bias is unlikely. For attained BMI, publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the two RCTs (Tier 1) that there is no effect of introduction of CFs at 3–4 months of age compared with 6 months of age on BMI assessed up to 3 years of age (high level of confidence in the evidence).
The Panel concludes from 11 prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs and BMI (moderate level of confidence in the evidence). The ages of introduction ranged between ≤ 2 months and ≤ 5 months for early introduction and > 2 months and ≥ 6 months for later introduction. The latest age of outcome assessment was 10 years.
6. Assessment of the data on obesity and overweight in individuals born at term or mixed populations
6.1. Obesity and overweight: final body of evidence
The 55 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.3. One publication covered four studies (Moschonis et al., 2017).
These papers reported on the results of 50 studies:
1 RCT (Tier 1);
29 prospective cohort studies (2 rated as Tier 1, 12 rated as Tier 2 and 15 rated as Tier 3);
20 retrospective studies (all Tier 3).
In line with the reasons given in Section 2.2.3.2 for the selection of papers that reported on different ages at assessment of an endpoint in the same study, the results provided by Massion et al. (2016) were used for the Millennium Cohort Study (MCS) (instead of those provided by Hawkins et al. (2009)) and the results provided by Moss and Yeaton (2014) for the Early Childhood Longitudinal Study, Birth Cohort (ECLS‐B) (instead of those provided by Gibbs and Forste (2014), Flores and Lin (2013b) and Gooze et al. (2011)).
In the included studies, nine different endpoints related to obesity and overweight were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file, including the ones by Hawkins et al. (2009), Gibbs and Forste (2014), Flores and Lin (2013b) and Gooze et al. (2011). In addition, results are summarised in the forest plots in Appendices A.14–A.17 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
6.2. Obesity and overweight: endpoint and study selection
Different reference populations were used in the included studies, e.g. from the WHO, the CDC, the International Obesity Task Force (IOTF) or national growth standards, as well as different cut‐offs (percentiles, z‐scores) to define overweight and obesity. The Panel considers that this limits the comparability of results between studies. In the following sections, the disease outcome, i.e. odds/risk of developing obesity, is discussed first.
The studies that were considered by the Panel for the section on overweight included studies that investigated the odds of developing at least overweight (i.e. combining overweight and obese children) and studies that assessed the odds of developing overweight separately from the odds of developing obesity (i.e. separated overweight from obese children).
Regarding possible reverse causality of observational studies, the same considerations and approach for the assessment of the RoB described above for weight endpoints were also relevant for obesity and overweight outcomes (Section 4.2).
6.3. Obesity: summary of the evidence
Main line of evidence (6 studies)
The main line of evidence consists of six prospective cohort studies and no RCT (Reilly et al., 2005; Brophy et al., 2009; Neutzling et al., 2009; Huh et al., 2011; Layte et al., 2014; Zheng et al., 2015).
For these studies, which mainly investigated introduction of CFs at below 3 or 4 months of age, the result of the meta‐analysis did not show a statistically significant association between the age of introduction of CFs and the odds of developing obesity up to 11 years of age. Heterogeneity was moderate (I2 = 50%) (Appendix A.14).
The Panel notes, from the six prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and the odds of developing obesity up to 11 years of age.
Supportive line of evidence (10 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (2 studies, Tier 3)
The meta‐analysis from the three comparisons of two studies (Moss and Yeaton, 2014; Barrera et al., 2016) did not show an association between the timing of introduction of CFs < 4 months of age compared with thereafter and the odds of developing obesity up to 6 years of age (Appendix A.14). Heterogeneity was not important (I2 = 0%). Also, individually the results of these studies did not show an association between the timing of introduction of CFs and obesity.
Retrospective studies (4 studies, Tier 3)
The meta‐analysis of one case–control study (Zhou et al., 2011) and three cross‐sectional studies (Birbilis et al., 2013; Vehapoglu et al., 2014; Sandoval Jurado et al., 2016) did not show an association between the timing of introduction of CFs (in three studies < 4 months of age compared with thereafter or with > 6 months of age), and the odds of developing obesity up to 14 years of age (Appendix A.15). However, heterogeneity was important (I2 = 81%). When the study by Zhou et al. (2011), that showed results that were considerably different from the other studies (which cannot be explained), was removed in a sensitivity analysis, heterogeneity became moderate (I2 = 46%); the results remained non‐statistically significant.
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (4 studies)
The prospective cohort studies Mäkelä et al. (2014) (Tier 1) and Schack‐Nielsen et al. (2010) (Tier 3) as well as the cross‐sectional studies by Gillman et al. (2001) and Sinigaglia et al. (2016) (both Tier 3) did not observe statistically significant associations between the timing of introduction of CFs and the odds of developing obesity assessed up to 42 years of age.
Difference in the timing of introduction of CFs between cases and controls (2 studies)
Two studies (both Tier 3) investigated the timing of introduction of CFs between obese and control subjects. One prospective cohort study (Flores and Lin, 2013a) found no statistically significant differences in the timing of introduction of CFs between 4‐year‐old children with severe obesity and their non‐severely obese counterparts, while one case–control study (Zhou et al., 2011) found that 3‐to 6‐year‐old obese children had significantly higher odds of having been introduced to CFs < 4 months of age than normal‐weight controls (OR 6.58 (95% CI 2.71 to 15.93)). This analysis was unadjusted and therefore is likely to overestimate the association.
The Panel notes that, in the supportive line of evidence, results of the two meta‐analyses of prospective cohort and retrospective studies are consistent with the findings in the main line of evidence (in total 6 studies). This is also true for the results of the four studies in which the timing of introduction of CFs was used as a continuous variable in the analysis. The results of the two studies investigating the difference in the introduction of CFs between cases and controls are inconsistent.
Endpoints investigated in single studies
One endpoint related to obesity was investigated in a single study in the supportive line of evidence only (i.e. %obese (Wolman, 1984) (Annex A as Microsoft Excel® file). Therefore, it cannot be used to establish the appropriate age range of introduction of CFs (Section 2.2.3.3).
6.4. Overweight: summary of the evidence
Main line of evidence (10 studies)
The RCT (Jonsdottir et al., 2014) that was available was relatively small in sample size, reflected in the wide 95% CI associated with the point estimate. No statistically significant effect of the timing of introduction of CFs (4 vs 6 months) on the odds of developing overweight up to 3 years of age was observed. However, this study was most likely underpowered for the outcome and its non‐statistically significant findings were therefore not further used by the Panel for drawing conclusions (Appendix A.16).
For the 10 prospective cohort studies ((Neutzling et al., 2009; Rossiter and Evers, 2013; Durmuş et al., 2014; Wen et al., 2014; Fairley et al., 2015; Zheng et al., 2015; Massion et al., 2016; Azad et al., 2018) as well as Moschonis et al. (2017) for the studies EDEN and ALSPAC), the results of the meta‐analysis did not show a statistically significant association between the timing of introduction of CFs (mostly < 4 vs ≥ 4 months) and the odds of developing overweight up to 13 years of age. Heterogeneity was substantial (I2 = 66%).
The Panel notes, from the ten prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and the odds of developing overweight up to 13 years of age.
Supportive line of evidence (30 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (10 studies, Tier 3)
The meta‐analysis of 10 studies reported in 9 publications ((Abraham et al., 2012; Moss and Yeaton, 2014; Hollis et al., 2016; Aris et al., 2018; Bell S et al., 2018; Pluymen et al., 2018; Schmidt Morgen et al., 2018; Sirkka et al., 2018) as well as Moschonis et al. (2017) for the studies Greek EuroPrevall and Generation XXI) showed increased odds of developing overweight up to 17 years of age associated with earlier introduction of CFs (mostly < 4 months vs later) (OR 1.28 (95% CI 1.18 to 1.39)) (Appendix A.16). The 95% prediction interval crossed the null line (0.98–1.67). Heterogeneity was moderate to substantial (I2 = 59%).
Retrospective studies (10 studies, Tier 3)
From the meta‐analysis of the 10 retrospective studies (Nascimento Simon et al., 2009; Jimenez‐Cruz et al., 2010; Magalhaes et al., 2012; Birbilis et al., 2013; Lin et al., 2013; Rathnayake et al., 2013; Cu et al., 2015; Škledar and Milošević, 2015; Sun et al., 2016; Papoutsou et al., 2018), there was no evidence for an association between the timing of introduction of CFs (at various ages) and the odds of developing overweight up to 14 years of age (Appendix A.17). Heterogeneity was moderate to substantial (I2 = 51%).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (6 studies)
The prospective cohort studies by Mäkelä et al. (2014) (Tier 1) and Schack‐Nielsen et al. (2010) (Tier 3) as well as the cross‐sectional studies by Butte (2009) and Gillman et al. (2001) (both Tier 3) did not find an association between the timing of introduction of CFs and the odds of developing overweight up to 42 years of age.
However, in the prospective cohort study by Seach et al. (2010) (Tier 3), a one month earlier introduction of CFs was associated with higher odds of developing overweight up to 10 years of age (aOR 1.11 (95% CI 1.03 to 1.19)). In the cross‐sectional study by Hediger et al. (2001) (Tier 3), a one month earlier introduction of CFs was associated with a higher odds of developing overweight up to 3–5 years of age (aOR 1.0006 (95% CI 1.0003 to 1.001)). The Panel considers that the observed OR is unlikely to be of biological relevance.
Difference in the timing of introduction of CFs between cases and controls (4 studies)
Four studies (all Tier 3) investigated the difference in the timing of introduction of CFs in overweight and normal weight subjects (Jiang et al., 2009; Gomes et al., 2010; Gungor et al., 2010; Flores and Lin, 2013b). The case–control study by Jiang et al. (2009) found that 1‐ to 3‐year‐old overweight children had statistically significantly higher odds of having been introduced of CFs < 4 months of age (aOR 1.76 (95% CI 1.15 to 3.64)) than controls. The retrospective cohort study by Gungor et al. (2010) found that 6‐ to 8‐year‐old overweight children had been introduced to CFs statistically significantly earlier than their controls (mean difference: −1.39 (95% CI −2.46 to −0.32) months). This analysis was unadjusted and therefore is likely to overestimate the association. The prospective cohort study (Flores and Lin, 2013b) and the other case–control study (Gomes et al., 2010) found no statistically significant differences in the timing of introduction of CFs between overweight cases and controls at 4 years and 2.2–6.8 years of age, respectively.
The Panel notes that the results within the supportive line of evidence (30 studies) are inconsistent.
Endpoints investigated in single studies
Another endpoint related to overweight was investigated in a single study only, i.e. %overweight (Burdette et al., 2006) (main line of evidence). Therefore, it cannot be used to establish the appropriate age range of introduction of CFs (Section 2.2.3.3).
6.5. Obesity and overweight: conclusions and grading of the confidence in the evidence
The RCT of the main line of evidence was most likely underpowered for this outcome and was therefore not considered further in the grading of the confidence in the evidence.
Imprecision: The results of the meta‐analyses did not indicate imprecision.
Inconsistency: The evidence is consistent across populations and endpoints in the main line of evidence (12 studies in total (4 studies reported both on obesity and overweight)). For obesity, the results in the supportive line of evidence (10 studies) are consistent with the findings in the main line of evidence, while, for overweight, the results within the supportive line of evidence (30 studies) are inconsistent. As there was enough evidence in the main line of evidence and results were consistent across lines of evidence for the disease endpoint (i.e. obesity), the Panel did not downgrade the confidence in the evidence for the inconsistency observed in the supportive line of evidence for overweight. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: A variety of populations and settings were covered in the available studies. Therefore, the Panel does not have concerns with respect to the generalisability of the findings to the whole population of infants living in Europe.
Publication bias: From visual inspections of the funnel plot on obesity, there was no convincing evidence for publication bias (Annex D.5). The funnel plot on overweight (Annex D.6) was asymmetrical as indicated by the Egger test and the trim‐and‐fill analysis, but the contour plot did not suggest that the asymmetry was due to publication bias (data not shown).
The Panel concludes from the prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs and obesity (6 studies) or overweight (10 studies) (moderate level of confidence). The ages of introduction of CFs ranged between < 1 month and < 4 months for early introduction and > 2 months and ≥ 6 months for later introduction. The latest age of outcome assessment was 13 years.
7. Assessment of the data on body composition in individuals born at term or mixed populations
7.1. Body composition: final body of evidence
The 21 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.4. These included two publications that were considered together (Kramer et al., 1985a,b) and one publication that reported on four prospective cohort studies (Moschonis et al., 2017).
These publications reported on results from 19 studies:
2 RCTs (1 rated as Tier 1, 1 rated as Tier 2);
13 prospective cohort studies and 1 pooled analysis of prospective studies (1 rated as Tier 1, 8 rated as Tier 2 and 6 rated as Tier 3; one study was assigned to two different Tiers, depending on how the outcome was measured);
3 retrospective studies (all Tier 3);
Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, results are summarised in the forest plots in Appendices A.18 and A.20 of this Scientific Opinion.
In these studies, 25 different endpoints related to body composition were investigated.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
7.2. Body composition: endpoint and study selection
Body composition measurements performed by either dual‐energy X‐ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) (fat mass, fat‐free mass, lean body mass and regional fat distribution) were preferred by the Panel over skinfold thickness (SFT) measurements expressed in millimetres, thus are described first in a separate subsection. The reliability of the outcome measurements was considered in the assessment of the RoB, i.e. DXA lower RoB than BIA.
BMC measurements not adjusted for bone area were not considered as an outcome for this assessment, owing to the lack of comparability in growing children that are of different size.
7.3. Fat mass: summary of the evidence
This section discusses first fat mass, then fat mass z‐score and percentage of fat mass for which no forest plot could be made (Section 2.2.3.2), and finally miscellaneous endpoints.
Main line of evidence
For fat mass (2 studies), neither the RCT (Mehta et al., 1998) nor the prospective cohort study (Burdette et al., 2006) showed an association between early introduction of CFs (3–4 vs 6 months and < 4 vs ≥ 4 months of age) and this endpoint (Appendix A.18).
For fat mass z‐scores (3 studies), the meta‐analysis of three prospective cohort studies (Durmuş et al., 2014; de Beer et al., 2015; Leary et al., 2015) did not show statistically significant associations with the age of introduction of CFs ranging from ≤ 2 to < 5 months of age versus later. Heterogeneity was not important (I2 = 0%) (Appendix A.19). The latest age at outcome assessment was 15 years.
For percentage of fat mass (2 studies), the RCT mentioned above (Mehta et al., 1998) also did not find a significant effect of the timing of introduction of CFs at 3–4 months, compared with 6 months of age, on this endpoint at 12 months of age. For the ALSPAC study when the outcome was assessed by DXA, Moschonis et al. (2017) did not report statistically significant associations between the introduction of CFs < 4 months of age and at 4–5 months of age, each compared with 5–6 months of age, and the percentage of fat mass at 13 years.
For high fat mass (1 study), the prospective cohort study by Burdette et al. (2006) did not find an association between the introduction of CFs < 4 months compared with thereafter and high fat mass (defined as the age and sex‐specific 75th percentile of the cohort at 5 years of age).
The Panel notes that the six studies in the main line of evidence (Tiers 1 and 2; some investigating several endpoints) showed consistently no association between the timing of introduction of CFs and fat mass assessed up to the age of 15 years.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
For fat mass (5 studies, reported in 2 papers), Moschonis et al. (2017) (Tier 3, covering four prospective cohort studies, including ALSPAC33), comparing < 4 and 4–5 vs 5–6 months of age (outcome assessed by BIA) (Appendix A.18), as well as one prospective cohort study that analysed the timing of introduction of CFs as a continuous variable (Robinson et al., 2009) (Tier 2), did not show an association between the timing of introduction of CFs and this endpoint.
For fat mass z‐scores, no studies were available in the supportive line of evidence.
For percentage of fat mass (1 study), the prospective cohort study by Wilson et al. (1998) (Tier 3) found a higher percentage of fat mass at 7 years of age to be associated with introduction of CF < 3.5 months vs thereafter (adjusted mean difference of 2% points (95% CI 1.42 to 2.58)). The Panel considers that this difference is unlikely to be of biological relevance.
For high fat mass (1 study), the results of the retrospective cohort study by Magalhaes et al. (2012), comparing an introduction of CFs ≤ 3 with 4–6 months of age did not find a significant association between the timing of introduction of CFs and the outcome. The outcome in this study was assessed at 4–7 years of age and was defined as the age‐ and sex‐specific 85th percentile of the cohort.
The Panel notes that the results in the supportive line of evidence (seven studies, some investigating several endpoints) are consistent with those in the main line of evidence.
Endpoints investigated in single studies
Other investigated endpoints related to fat (or fat‐free) mass were: lean mass z‐scores (Leary et al., 2015), lean mass (Mehta et al., 1998), fat‐free mass z‐score (de Beer et al., 2015), android:gynoid fat ratio z‐score (Durmuş et al., 2014), preperitoneal abdominal fat area z‐score (Durmuş et al., 2014), fat mass index z‐score and fat‐free mass index z‐score (Vogelezang et al., 2018) (main line of evidence); and high fat from the android region (Magalhaes et al., 2012) (supportive line of evidence). These were assessed in single studies only. Therefore, they cannot be used to establish the appropriate age range of introduction of CFs (Section 2.2.3.3).
7.4. Skinfold thickness: summary of the evidence
This section discusses first SFT and the related forest plot, then miscellaneous endpoints.
For skinfold thickness, the included studies provided data on SFT measured in one site in the body or a combination of two to four sites (i.e. subscapular, triceps, subscapular + suprailiac, triceps + biceps, triceps + subscapular, triceps + subscapular + suprailiac, triceps + biceps + subscapular + suprailiac SFT, expressed in millimetres). As these measures cannot be directly compared, no meta‐analysis was performed (Appendix A.20).
Main line of evidence (2 studies)
The RCT (Perkin et al., 2016) did not find statistically significant differences between the introduction of CFs at 3–4 months of age compared with 6 months of age on triceps and subscapular SFT at 3 years of age. The prospective cohort study (Durmuş et al., 2012) that investigated the association between various combinations of SFT measurements at 2 years of age and the timing of introduction of CFs before 4 months or at 4–5 months vs after 5 months of age, did not find a statistically significant association in most of the comparisons made.
The Panel notes, from the RCT and the prospective cohort study (Tiers 1 and 2) in the main line of evidence, that there is no association between the timing of introduction of CFs and SFT assessed up to 3 years of age.
Supportive line of evidence (4 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
This line is composed of the prospective cohort studies by Huh et al. (2011) (Tier 3) and by Kramer et al. (1985a) (in which the age of introduction of CFs was used as a continuous variable in the analysis, Tier 2), as well the cross‐sectional study by Patterson et al. (1986) and the cross‐sectional analysis of baseline data of a prospective cohort study by Zive et al. (1992) (both Tier 3). There was no evidence for an association between the timing of introduction of CFs and SFT measurements (assessed up to 4 years of age) in these studies.
The Panel notes that results of the four studies in the supportive line are consistent with the findings of the main line of evidence.
Endpoints investigated in single studies
Other endpoints related to SFT were assessed in the line of supportive evidence in single studies only and were SFT gain (Morgan et al., 2004), %difference in SFT (Caleyachetty et al., 2013). These cannot be used to establish the appropriate age range of introduction of CFs and were not considered further.
7.5. Bone health: summary of the evidence
Endpoints related to bone health were: areal BMC (aBMC), bone mineral density (BMD) and bone area. These were assessed in a single study only (van den Hooven et al., 2016) (Tier 1). Therefore, they cannot be used to establish the appropriate age range of introduction of CFs.
7.6. Body composition: conclusions and grading of the confidence in the evidence
Imprecision: The results of the individual studies did not indicate imprecision.
Inconsistency: The evidence is consistent across populations and endpoints. The results of the studies in the supportive line of evidence (11 studies) are consistent with the findings of the main line of evidence.
Generalisability: The two available RCTs (one on fat mass and one on SFT) were conducted in exclusively breastfed and formula fed infants, and their findings were consistent. Therefore, the Panel does not have concerns with respect to the generalisability of the findings to the whole population of infants living in Europe. Even though the number of prospective cohort studies is limited, they were performed in three different countries and covered a sufficient number of populations. Therefore, the Panel considers that results from these studies can also be generalised.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the two RCTs that there is no effect of introduction of CFs at 3–4 months of age compared with 6 months of age on fat mass or SFT (high level of confidence in the evidence).
The Panel concludes from prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs, covering a broader range of ages of introduction than the RCTs, on fat mass or SFT (6 studies) (moderate level of confidence in the evidence). The early introduction of CFs was defined in all of these studies as < 4 months of age and later introduction as ≥ 4 months to > 6 months. The latest age of outcome assessment was 13 years.
8. Assessment of the data on atopic diseases in individuals born at term or mixed populations
8.1. Atopic diseases: final body of evidence
The 92 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.5. These included two publications that were considered together (Kajosaari, 1991, 1994).
These publications reported on results from 79 studies:
6 RCTs (5 rated as Tier 1, 1 rated as Tier 2 and 1 rated as Tier 3; 1 study was allocated two different Tiers depending on the outcome that was assessed);
45 prospective cohort studies, 5 nested case–control studies (one study was analysed as prospective cohort study and as a nested case–control study), 2 observational analyses of an RCT and 1 pooled analysis of prospective studies (7 rated as Tier 1, 22 rated as Tier 2 and 28 rated as Tier 3; one study was assigned to three Tiers and three studies to two different Tiers, depending on the outcome that was assessed);
21 retrospective studies (all Tier 3).
In these studies, six different outcomes (each possibly covering several endpoints) related to atopic‐diseases were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, for the main endpoints, results are summarised in the forest plots in Appendices A.21–A.39 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
8.2. Atopic diseases: endpoint and study selection
As previously explained (Section 2.1.1), the Panel investigated the association between atopic disease‐related endpoints and the timing of introduction of CFs in general, as well as of egg, cereals (in particular wheat), fish (as defined in the papers, i.e. generally undefined), peanut and soy (not in the form of infant formula).
When assessing the timing of introduction of individual foods, the comparator can be either continued breast or formula feeding or CFs other than the one under investigation or mixed feeding regimens. This aspect will be discussed in each of the subsections on individual foods.
For food allergy, the Panel decided to draw its conclusions from the disease‐related endpoint, i.e. symptomatic food allergy. Data on sensitisation to allergens are used only as supportive evidence to the results from the studies on symptomatic food allergy, as positive results are associated with a higher risk of allergy but alone are not predictive of the disease (Chokshi and Sicherer, 2016). Also, the Panel decided to consider only sensitisation to food allergens and not to aeroallergens (Section 2.1.1.2).
Information in the individual publications was often insufficient to ascertain whether the diagnostic criteria used for the diagnosis of asthma and atopic dermatitis were able to distinguish cases of these diseases from cases of wheeze or eczema due to other causes. Thus, the Panel clustered:
-
–
under ‘asthma‐like symptoms’, the endpoints ‘wheeze’, ‘asthma’ and associated endpoints as investigated in the individual studies;
-
–
under ‘eczema’, the endpoints ‘symptomatic eczema’ and ‘atopic dermatitis’ as investigated in the individual studies.
An important consideration in the evaluation of the effect or association between the timing of introduction of CFs and an atopic‐disease‐related outcome is reverse causality which may be either due to the presence of an atopic family history on the one hand, and to the presence of allergic symptoms before the introduction of CFs on the other hand. In both cases, parents may decide to anticipate or postpone the introduction of CFs (depending on feeding recommendations given) while, at the same time, these children may already be at a higher risk of developing the disease, independent of the timing of introduction of CFs. This aspect was considered in the assessment of the RoB (Appendix B).
Populations considered as being at risk of the disease were those with a first‐degree family history of the disease (i.e. presence of symptomatic allergy in at least one of the following: father, mother or siblings) or already showing atopic symptoms other than those related to the disease under investigation (e.g. children with eczema in a study investigating symptomatic food allergy), while the general population comprises at‐risk and not‐at‐risk populations. At‐risk populations and the general population were considered separately in this assessment, as potentially differential effects or associations could be observed in these two populations. However, the Panel notes that the above definition of at‐risk infants is not comprehensive, as children without a first‐degree family history of the disease and without the presence of atopic symptoms may also develop atopic diseases.
In line with the previously described approach of focussing on the most complete datasets for the step of the data extraction (Section 2.2.3.1), for atopic‐disease‐related endpoints, the most comprehensive population within the general population and within the at‐risk population was used (e.g. results from the overall population were retained instead of results obtained in a subgroup of children without atopic symptoms before the introduction of CFs, if there was evidence that results in the overall population were not influenced by reverse causality). Also, the most reliable outcome assessment was kept for studies reporting results for several inter‐related endpoints. For example, asthma diagnosed by a physician was retained in the assessment rather than the caregivers’ reports of symptoms indicative of asthma.
Eczema and asthma‐like symptoms were the most frequently investigated endpoint in prospective observational studies, while symptomatic food allergy was the most investigated endpoint in the RCTs included.
In line with Section 2.2.3.2, all conclusions on atopic‐disease related endpoints refer to the general population of infants living in Europe.
8.3. Outcome cluster of atopic diseases: summary of the evidence
8.3.1. Timing of introduction of CFs in general
Main line of evidence
General population (1 study): In the RCT in exclusively breastfed infants (Perkin et al., 2016), no effect of introducing CF at 3–4 months compared with 6 months on the odds of developing an atopic disease up to 3 years of age was observed (Annex A as Microsoft Excel® file).
At‐risk population (2 studies): The prospective cohort study (Pöysä et al., 1991) did not find an association between the introduction of CFs < 3 months of age, compared with later, on the odds of developing an atopic disease up to 9–10 years of age. However, there was a high imprecision in the estimate (Annex A as Microsoft Excel® file). Also, Sandini et al. (2011) reported non‐significant findings, but without a point estimate, for CF introduction < 4 months of age compared with 4–6 months of age on the odds of developing an atopic disease up to 2 or 5 years of age.
The Panel notes for the general population that the RCT (Tier 1) available in the main line of evidence did not observe an effect of introducing CFs at 3–4 months of age compared with 6 months of age on the odds of developing an atopic disease up to 3 years of age in exclusively breastfed infants.
The Panel notes for the at‐risk population that the two prospective cohort studies (Tier 2) in the main line of evidence, one with a high imprecision and one without point estimate, did not show an association between the timing of introduction of CFs and odds of developing an atopic disease up to 9–10 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (2 studies, Tier 3)
General population (1 study): The prospective cohort study by Keijzers et al. (2018) (Tier 3) did not observe a statistically significant association between introduction of CFs < 6 months of age compared with later and the odds of developing an atopic disease up to 5 years of age.
At‐risk population (1 study): The prospective cohort study in exclusively breastfed infants (Kajosaari, 1991, 1994) did not observe an association between the introduction of CFs at 3 months of age, compared with after 6 months, and the outcome assessed at 5 years of age (Annex A as Microsoft Excel® file).
Retrospective studies (2 studies, Tier 3)
General population: The cross‐sectional study by Hatakka et al. (2008) did not find a statistically significant association between introduction of CFs < 3 months vs thereafter and the odds of developing an atopic disease up to 1–6 years of age. One case–control study (Parihar et al., 1984) observed higher odds of atopic diseases assessed up to 2 years to be associated with the introduction of CFs < 3 months of age compared with thereafter (OR 7.37 (2.18 to 24.92)). This analysis was unadjusted and therefore is likely to overestimate the association (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (2 studies)
General population: The two studies that analysed the timing of introduction of CFs as a continuous variable (i.e. the prospective cohort study by Savilahti et al. (1987) (Tier 2) and the cross‐sectional study by Forster et al. (1990) (Tier 3) did not find a relationship between the timing of introduction of CFs and the outcome assessed at 2 and 1.5 years, respectively (Annex A as Microsoft Excel® file).
Difference in the timing of introduction of CFs between cases and controls (1 study)
General population: One case–control study (Yung et al., 2015) (Tier 3) did not find a statistically significant difference in the timing of introduction of CFs between on average 20‐month‐old cases with atopic diseases and controls (Annex A as Microsoft Excel® file).
The Panel notes for the general population that only one of the six studies in the supportive line of evidence found an association between the introduction of CFs < 3 months vs later and higher odds of developing an atopic disease in an unadjusted analysis, while the five others did not find an association. The Panel considers that the results of the supportive line of evidence in the general population are consistent with those the main line of evidence.
The Panel notes for the at‐risk population that the result of the single study in the supportive line of evidence is consistent with the findings in the main line of evidence.
8.3.2. Timing of introduction of specific foods
Only single studies were available to assess the timing of introduction of specific foods, i.e. egg (Halpern et al., 1973) and cereals (Jonsson et al., 2017) (both supportive line of evidence) (Annex A as Microsoft Excel® file). Therefore, these cannot be used to establish the appropriate age range of introduction of CFs and were not considered further.
8.3.3. Outcome cluster of atopic diseases: conclusions and grading of the confidence in the evidence
Imprecision: Contrary to the RCT in the general population, the results of one of the two prospective cohort studies in at‐risk populations showed a high imprecision. The other one did not provide a point estimate to allow a judgement to be made. Therefore, the Panel downgraded by one category the confidence in the evidence derived from the cohort studies in at‐risk populations.
Inconsistency: The limited evidence that is available is consistent between the at‐risk population and the general population and the results of the six out of seven studies in the supportive line of evidence were consistent with the main line of evidence.
Generalisability: The study population of the RCT available in the general population consisted only of breastfed infants. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel downgraded by one category the confidence in the evidence derived from the RCT. With respect to the prospective cohort studies, the Panel notes that even though only one single study in a small population was available in the main line of evidence in an at‐risk population, the Panel did not have concerns with respect to generalisability of the findings to the whole population of infants living in Europe, considering that the evidence was consistent across populations, taking into account also the supportive line of evidence.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Other: The Panel noted the limited evidence available for assessment in the main line of evidence (one study in the general population and one in an at‐risk population). As six out of seven studies in the supportive line of evidence were consistent with the findings in the main line of evidence, the Panel decided not to downgrade the level of confidence in the main line of evidence for this low number of studies.
The Panel concludes from the RCT (Tier 1) that there is no evidence for an effect of introduction of CFs at 3–4 months vs 6 months of age on the odds of developing an atopic disease up to 3 years of age (moderate confidence in the evidence).
The Panel concludes from the two prospective cohort studies (Tier 2) that there is no evidence for an association between the introduction of CFs at < 3 months of age vs thereafter and at < 4 vs 4–6 months and the odds of developing an atopic disease up to 9–10 years of age (low confidence in the evidence).
8.4. Asthma‐like symptoms: summary of the evidence
8.4.1. Timing of introduction of CFs in general
Main line of evidence
General population (5 studies): The RCT (Perkin et al., 2016) did not show an effect of introduction of CFs at 3–4 months compared with 6 months of age on the odds of developing asthma‐like symptoms up to 3 years of age.
The result of the meta‐analysis of the two prospective cohort studies that provided information that could be used for this analysis (Zutavern et al., 2004; Lossius et al., 2018) was not statistically significant. Heterogeneity was not important (I2 = 27%). The imprecision around the pooled estimate was serious (Appendix A.21). However, also individually, these studies did not show an association between the timing of introduction of CFs (≤ 3 months and < 6 months vs thereafter, respectively) and the outcome. Equally, the studies by Wilson et al. (1998) and Nwaru et al. (2013a) that did not provide data in a form that could be incorporated into the meta‐analysis did not observe an association between the timing of introduction of CFs and the outcome. The latest age at outcome assessment was 7 years.
At‐risk populations (3 studies): The meta‐analysis of the two prospective cohort studies (Marini et al., 1996; Mihrshahi et al., 2007) did not show an association between the introduction of CFs < 4 and < 3 months, respectively, compared with thereafter, and the outcome assessed up to 5 years of age. Heterogeneity was not important (I2 = 0%) (Appendix A.22). The imprecision around the pooled estimate was serious. However, individually these two studies also did not observe an association. In addition, Sandini et al. (2011) did not find an association between CF introduction < 4 months of age compared with 4–6 months and the odds of developing asthma‐like symptoms up to 2 or 5 years of age, but did not provide a point estimate.
The Panel notes for the general population that, from one RCT and the four prospective cohort studies (Tiers 1 and 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of CFs and the odds of developing asthma‐like symptoms up to 7 years of age.
The Panel notes for the at‐risk population that, from the three prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of CFs and the odds of developing asthma‐like symptoms up to 5 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (9 studies, Tier 3)
General population (7 studies): The meta‐analysis of five studies (Fergusson et al., 1983; Larsson et al., 2008; Snijders et al., 2008; Zutavern et al., 2008; Hetzner et al., 2009) comparing various time points of introduction of CFs in relation to the odds of developing asthma‐like symptoms up to 9 years of age was not statistically significant. Heterogeneity was not important (I2 = 32%) (Appendix A.21). In addition, Morgan et al. (2004) and Kurukulaaratchy et al. (2004), that did not provide a point estimate, reported non‐significant findings in relation to the outcome at 1.5 and 10 years of age, respectively, comparing introduction of CFs < 3 with > 3 months of age.
At‐risk populations (2 studies): The meta‐analysis of the two prospective cohort studies reported in three publications (Van Asperen et al., 1984; Kajosaari, 1991, 1994) did not find an association between the timing of introduction of CFs (≤ 4 vs > 4 months and 3 vs > 6 months, respectively) and the outcome assessed up to 5 years of age. Heterogeneity was not important (I2 = 0%) (Appendix A.22). The imprecision around the pooled estimate was serious. However, individually these two studies also did not observe an association.
Retrospective studies (1 study, Tier 3)
General population: The case–control study by Karunasekera et al. (2001) did not find a relationship between the timing of introduction of CFs before and after 3 months of age and the odds of developing asthma‐like symptoms up to 1–10 years of age (Annex A as Microsoft Excel® file).
Difference in the timing of introduction of CFs between cases and controls (1 study)
General population: A nested case–control study (Hesselmar et al., 2010) (Tier 1) found no statistically significant difference between the timing of introduction of CFs in 1.5‐year‐old cases with asthma‐like symptoms and controls (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.4.2. Timing of introduction of CFs in general and asthma‐like symptoms: conclusions and grading of the confidence in the evidence
Imprecision: The imprecision in the results of the meta‐analyses of the prospective cohort studies in the main line of evidence in the general as well as the at‐risk populations was serious. Therefore, the Panel downgraded by one category the confidence in the evidence.
Inconsistency: The evidence is consistent across populations and the results of the studies in the supportive line of evidence (nine for the general population and two for at‐risk populations) were consistent with the main line. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: The study population of the RCT consisted only of breastfed infants. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel downgraded by one category the confidence in the evidence derived from this RCT. With respect to prospective cohort studies, the Panel did not have any concerns with respect to their generalisability, as a representative number of populations were studied.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the RCT (Tier 1) that there is no evidence for an effect of introduction of CFs at 3–4 months of age compared with 6 months of age on the odds of developing asthma‐like symptoms up to 3 years of age (moderate level of confidence in the evidence).
The Panel concludes from the seven prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs, covering a range of ages from ≤ 3 months to < 6 months that was compared mostly with thereafter, and the odds of developing asthma‐like symptoms up to 7 years of age (low level of confidence in the evidence).
8.4.3. Timing of introduction of egg
Main and supportive lines of evidence
General population (2 studies): Neither the prospective cohort study in the main line of evidence (Nwaru et al., 2013b) (Tier 2) nor the one in the supportive line of evidence (Tromp et al., 2011) (Tier 3) found an association between the timing of introduction of egg (comparing introduction at < 5 and ≤ 6 months to thereafter, respectively) and the odds of developing asthma‐like symptoms up to 10 years of age (Annex A as Microsoft Excel® file).
At‐risk populations (2 studies): Neither the RCT by Palmer et al. (2017) nor the prospective cohort study by Nwaru et al. (2013b) (both Tier 2; main line of evidence) showed an effect or association between the timing of introduction of egg (comparing introduction at 4–6.5 with ≥ 10 months and < 5 months with thereafter, respectively) and the odds of developing asthma‐like symptoms up to 10 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the prospective cohort study (Tier 2) in the main line of evidence did not show an association between the timing of introduction of egg and the odds of developing asthma‐like symptoms up to 10 years of age. The study in the general population in the supportive line of evidence is consistent with this finding.
The Panel notes for the at‐risk population that the results of one RCT and one prospective cohort study (Tier 2) in the main line of evidence did not show an effect or association between the timing of introduction of egg and the odds of developing asthma‐like symptoms up to 10 years of age.
8.4.4. Timing of introduction of egg and asthma‐like symptoms: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision of results.
Inconsistency: The evidence is consistent across populations and the results of the study in the supportive line of evidence is consistent with the main line.
Generalisability: The RCT was performed in Australia, a country that has an unexplained higher prevalence of allergy than Europe. Further the study used pasteurised raw egg powder as an intervention product, which is not the form that would be used when egg is introduced to infants. Therefore, the Panel downgraded the confidence level in the evidence twice for the RCT. With respect to prospective cohort studies, even though only one study in an at‐risk population was available in the main line of evidence, the Panel did not have concerns with respect to generalisability of the findings to the whole population of infants living in Europe, considering the consistency across populations, taking into account also the study in the supportive line of evidence.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Other: The confidence in the evidence was downgraded by one category because of the overall limited evidence that was available for the outcome in the main and the supportive lines of evidence.
The Panel concludes, from one RCT and one prospective cohort study (Tier 2), that there is no evidence for an effect or association between the introduction of egg, at 4–6.5 months vs ≥ 10 months of age and < 5 months vs ≥ 5 months, and the odds of developing asthma‐like symptoms up to 10 years of age (low level of confidence in the evidence).
8.4.5. Timing of introduction of cereals
Main line of evidence
General population (3 studies): From the meta‐analysis of three prospective cohort studies (Zutavern et al., 2004; Nwaru et al., 2013a,b), there was no evidence for an association between the timing of introduction of cereals (comparing various time points between 3.75 and 5.5 months with thereafter) and the odds of developing asthma‐like symptoms up to 10 years of age. Heterogeneity was moderate (I2 = 48%) (Appendix A.23).
At‐risk populations (1 study): In one study (Nwaru et al., 2013b), no association between the timing of introduction of cereals and the odds of developing asthma‐like symptoms up to 10 years of age was observed, comparing an introduction of cereals < 3.75 months with thereafter (Annex A as Microsoft Excel® file).
The Panel notes for the general population that, from the three prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of cereals and the odds of developing asthma‐like symptoms up to 10 years of age.
The Panel notes for the at‐risk population that, from the prospective cohort study (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of cereals and the odds of developing asthma‐like symptoms up to 10 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
General population (1 study): The prospective cohort study (Tromp et al., 2011) (Tier 3) did not find an association with the outcome assessed at 4 years of age comparing introduction of cereals ≤ 6 months with thereafter (Appendix A.23).
The Panel notes for the general population that the result of the study in the supportive line of evidence is consistent with the findings in the main line of evidence.
8.4.6. Timing of introduction of cereals and asthma‐like symptoms: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision of results.
Inconsistency: The evidence is consistent across populations and the results of the single study in the supportive line of evidence is consistent with the main line. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability of the results of the prospective cohort studies.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the three prospective cohort studies (Tier 2) that there is no evidence for an association between the age of introduction of cereals, covering a range of ages from < 3.75 months to ≤ 5.5 months vs thereafter, and the odds of developing asthma‐like symptoms up to 10 years of age (moderate confidence in the evidence).
8.4.7. Timing of introduction of fish
Main line of evidence
General population (3 studies): From the meta‐analysis of three prospective cohort studies (Zutavern et al., 2004; Virtanen et al., 2010; Nwaru et al., 2013b), there was no evidence for an association between the timing of introduction of fish (mostly comparing introduction < 5–6 months of age with > 5–6 months to > 8.5 months) and the odds of developing asthma‐like symptoms up to 10 years of age. Heterogeneity was not important (I2 = 0%) (Appendix A.24).
At‐risk population (1 study): One study (Nwaru et al., 2013b) found no association between the timing of introduction of fish before and after 5.25 months of age and the development of asthma‐like symptoms up to 10 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population that, from the three prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of fish and the odds of developing asthma‐like symptoms up to 10 years of age.
The Panel notes for the at‐risk population that, from the prospective cohort study (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of fish and the odds of developing asthma‐like symptoms up to 10 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (1 study, Tier 3)
General population (1 study): The prospective cohort study by Kiefte‐de Jong et al. (2012) found that introduction of fish before 6 months of age compared with introduction between 6 and 12 months of age was associated with higher odds of asthma‐like symptoms at 4 years of age (aOR 1.53 (1.07 to 2.19)) (Appendix A.24).
The Panel notes for the general population that the result of the study in the supportive line of evidence is not consistent with those in the main line of evidence.
8.4.8. Timing of introduction of fish and asthma‐like symptoms: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision of results.
Inconsistency: The evidence is consistent across populations in the main line of evidence. The result of the prospective cohort study in the supportive line of evidence is inconsistent with the main line. However, as there was enough evidence in the main line, the Panel did not downgrade the confidence in the evidence for this inconsistent finding. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability of the results of the prospective cohort studies.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the three prospective cohort studies (Tier 2) that there is no evidence for an association between the age of introduction of fish, covering a range of ages from < 5–6 months for earlier introduction and > 5–6 months to > 8.5 months for later introduction, and the odds of developing asthma‐like symptoms up to 10 years of age (moderate confidence in the evidence).
8.4.9. Timing of introduction of soy and peanut
Only one study (Tromp et al., 2011) (supportive line of evidence) investigated the relationship between the timing of introduction of soy and peanut and the odds of developing asthma‐like symptoms. Therefore, this study cannot be used to establish the appropriate age range of introduction of CFs and was not considered further.
8.5. Eczema: summary of the evidence
8.5.1. Timing of introduction of CFs in general
Main line of evidence
General population (5 studies): From the meta‐analysis of five prospective cohort studies (Fergusson et al., 1981; Forsyth et al., 1993; Zutavern et al., 2004; Chuang et al., 2011; Roduit et al., 2012) (Appendix A.25), there was no evidence for an association between the timing of introduction of CFs, in most cases ≤ 3–4 months vs thereafter, and the odds of developing eczema up to 5.5 years of age. Heterogeneity was moderate (I2 = 46%).
At‐risk population (8 studies): The meta‐analysis of the six prospective cohort studies (Fergusson et al., 1981; Ruiz et al., 1992; Marini et al., 1996; Schoetzau et al., 2002; Mihrshahi et al., 2007; Roduit et al., 2012) showed no association between the introduction of CFs (mostly before 3 or 4 months vs later) and the odds of developing eczema assessed up to 5 years of age. Heterogeneity was moderate to substantial (I2 = 53%) (Appendix A.26). In addition, Sandini et al. (2011) and Moore et al. (1985), who did not provide point estimates, did not find an association between CF introduction < 4 months of age compared with 4–6 months and < 3 months of age compared with later, and the odds of developing eczema up to 2 or 5 years and 1 year of age, respectively.
The Panel notes for the general population that, from the five prospective cohort studies in the main line of evidence (Tiers 1 and 2), there is no evidence for an association between the timing of introduction of CFs and the odds of developing eczema up to 5.5 years of age.
The Panel notes for the at‐risk population that, from the eight prospective cohort studies (Tiers 1 and 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of CFs and the odds of developing eczema up to 5 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (14 studies, Tier 3)
General population (11 studies): The results of these studies are heterogeneous. From the meta‐analysis of nine studies (Hide and Guyer, 1981; Dunlop et al., 2006; Filipiak et al., 2007; Larsson et al., 2008; Snijders et al., 2008; Zutavern et al., 2008; Sariachvili et al., 2010; Huang et al., 2013; Taylor‐Robinson et al., 2016), there was no evidence for an association between the timing of introduction of CFs (comparing various time points, but mostly below 3 or 4 months vs later) and the odds of developing eczema up to 9 years of age (Appendix A.25). Heterogeneity was substantial to considerable (I2 = 75%) and cannot be explained. In addition, Morgan et al. (2004) and Nwaru et al. (2013a), who did not provide a point estimate, did not observe an association between the introduction of CFs before around 3 months of age compared with later and the odds of developing eczema up to 1.5 and 5 years of age, respectively (Annex A as Microsoft Excel® file).
At‐risk populations (3 studies): The meta‐analysis of three prospective studies (reported in 4 publications) (Van Asperen et al., 1984; Kajosaari, 1991, 1994; Ranucci et al., 2018) did not show an association between the timing of introduction of CFs (comparing various time points) and the outcome investigated up to 5 years of age. Heterogeneity was not important (I2 = 0%) (Appendix A.26).
Retrospective studies (6 studies, Tier 3)
General population (5 studies): The results of these studies are also heterogeneous. The meta‐analysis of the three case–control studies (Haileamlak et al., 2005; Sahakyan et al., 2006; Turati et al., 2016) together with the two cross‐sectional studies (Zheng et al., 2016; Lee et al., 2017) did not show evidence for an association between the timing of introduction of CFs (comparing introduction < 4 months with later) and the odds of developing eczema up to 7 years of age (Appendix A.27). Heterogeneity was moderate to substantial (I2 = 51%).
At‐risk population (1 study): In the cross‐sectional study by Suryati et al. (2006), no association between the timing of introduction of CFs (< vs ≥ 4 months) and the odds of developing eczema up to 1–5 years of age was observed (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (2 studies)
General population (2 studies): In the cross‐sectional study by Takahashi et al. (1999) (Tier 3), no association was observed between the timing of introduction of CFs and the outcome at 1–2 years of age. Illi et al. (2004), in a prospective cohort study (Tier 3), did not find an association between the timing of introduction of CFs and the cumulative odds for developing eczema up to 2 years of age, but did not provide a point estimate. (Annex A as Microsoft Excel® file).
Difference in the timing of introduction of CFs between cases and controls (3 studies)
General population (3 studies): Sariachvili et al. (2010) (Tier 3), in a nested case–control study, found a lower likelihood that 4‐year‐old cases with eczema had been introduced to CFs before 4 months of age (OR 0.60 (95% CI 0.43 to 0.84)). However, the analysis was unadjusted and therefore is likely to overestimate the association. In the nested case–control study by Hesselmar et al. (2010) (Tier 1) the timing of introduction of CFs was not statistically significantly different between 18‐months‐old cases and controls. Also, in the case–control study by Kramer and Moroz (1981) (Tier 3), no statistically significant differences in the timing of introduction of CFs were observed between 1‐ to 20‐year‐old cases and their controls (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.5.2. Timing of introduction of CFs in general and eczema: conclusions and grading of the confidence in the evidence
Imprecision: There was no imprecision associated with the results of the meta‐analyses.
Inconsistency: The evidence is consistent across populations and the results of the studies in the supportive line of evidence (21 studies for the general population, 4 in at‐risk populations), are consistent with the results of the studies in the main line of evidence. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to the generalisability of the findings to the whole population of infants living in Europe.
Publication bias: From the visual inspection of the funnel plots of studies performed in the general population and at‐risk populations (Annexes D.7 and D.8), there was no convincing evidence of asymmetry.
The Panel concludes from the 11 prospective cohort studies (two in common in the general and at‐risk populations; Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs, covering a range of ages from < 3 months to ≤ 6 months vs thereafter and the odds of developing eczema up to 5.5 years of age (moderate confidence in the evidence).
8.5.3. Timing of introduction of egg
Main line of evidence
General population (2 studies): The two prospective cohort studies (Fergusson et al., 1990; Nwaru et al., 2013b) did not observe a relationship between the timing of introduction of egg < 5 months compared with later and the odds of developing eczema up to 10 years of age in the general population (Fergusson et al., 1990 did not provide a point estimate) (Appendix A.28 and Annex A as Microsoft Excel® file).
At‐risk populations (5 studies): Neither the meta‐analysis nor the results of the two RCTs considered individually (Palmer et al., 2017; Tan et al., 2017) (Tier 1) showed an effect of egg introduction at around 4–6 months of age, compared with an introduction at 8–10 months of age, on the odds of developing eczema up to 1 year of age. This is also true for the meta‐analysis and for the results of the two individual prospective cohort studies (Ruiz et al., 1992; Nwaru et al., 2013b) (Tier 2) that investigated egg introduction ≤ 6 and < 5 months vs later, respectively. The latest age at outcome assessment was 10 years. The pooled estimates of the two meta‐analyses were associated with a serious imprecision. Heterogeneity was substantial (I2 = 69%) and moderate (I2 = 37%), respectively (Appendix A.29).
In addition, the prospective cohort study by Fergusson et al. (1981) (Tier 2) (same study as Fergusson et al. (1990)), who did not provide a point estimate, showed no evidence for an association between egg introduction ≤ 4 months compared with later and the odds of developing eczema up to 2 years (Annex A as Microsoft Excel® file).
The Panel notes for the general population that, from the two prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of egg and the odds of developing eczema up to 10 years of age.
The Panel notes for the at‐risk population that, from the two RCTs and three prospective cohort studies (Tiers 1 and 2) in the main line of evidence, there is no evidence for an effect or association between the timing of introduction of egg and the odds of developing eczema up to 10 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (3 studies, Tier 3)
General population (3 studies): The result of the meta‐analysis of three prospective cohort studies (Zutavern et al., 2006; Filipiak et al., 2007; Elbert et al., 2017) comparing egg introduction before vs after 6 months of age was not statistically significant. Eczema was investigated up to 10 years of age. Heterogeneity was moderate (I2 = 45%) (Appendix A.28).
At‐risk populations (1 study): One prospective cohort study that investigated the association between egg introduction and eczema in the general population (Filipiak et al., 2007), provided also results on an at‐risk population. It did not observe an association between egg introduction before and after 6 months of age and the odds of developing eczema up to 4 years of age (Annex A as Microsoft Excel® file).
Retrospective studies (2 studies, Tier 3)
General population (1 study): In a cross‐sectional analysis of baseline data of a prospective cohort study (Peters et al., 2015), no statistically significant differences were observed between various time points of egg introduction (i.e. < 4 vs 4–6, 4–6 vs 7–9 and 4–6 vs 10–12 months), except for those introduced to egg at 4–6 months of age compared with those introduced after 12 months of age. The odds of eczema were statistically significantly lower in the earlier group (aOR 0.5 (95% CI 0.33 to 0.74)) (Annex A as Microsoft Excel® file).
At‐risk populations (1 study): In the cross‐sectional study by Suryati et al. (2006), no association was observed between egg introduction < 4 months compared with ≥4 months of age and the odds of developing eczema up to 1–5 years of age. However, imprecision was serious in this study (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of egg was used as a continuous variable in the analysis, irrespective of the study design (1 study)
General population (1 study): The cross‐sectional study by Takahashi et al. (1999) (Tier 3) observed lower odds of eczema at 1–2 years to be associated with earlier introduction of egg (aOR for one month of earlier introduction 0.94 (95% CI 0.89 to 0.99)) (Annex A as Microsoft Excel® file).
The Panel notes for the general population that, in the supportive line of evidence, two retrospective studies observed lower odds of eczema to be related to earlier introduction of egg, while in three prospective cohort studies no association was observed between the timing of introduction of egg and this outcome.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.5.4. Timing of introduction of egg and eczema: conclusions and grading of the confidence in the evidence
Imprecision: There was serious imprecision associated with the pooled estimate of the meta‐analyses of the two RCTs and the two prospective cohort studies in at‐risk populations. Therefore, the Panel downgraded by one category the confidence in the evidence in these lines of evidence.
Inconsistency: The findings were consistent across populations in the main lines of evidence. There were six studies in the supportive line of evidence (five in the general population, two in at‐risk populations; one in common in both groups). While in the at‐risk population the findings in the supportive line of evidence were consistent with the main line of evidence, the results of studies in the general population in the supportive line were inconsistent. However, as there was enough evidence available in the main line of evidence, the Panel did not downgrade the confidence in the evidence for this finding. For all meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: The two RCTs in at‐risk populations were performed in Australia, a country that has an unexplained higher prevalence of allergy than Europe. Further, the study used pasteurised raw egg powder as an intervention product, which is not the form that would be used when egg is introduced to infants. Therefore, the Panel downgraded the confidence level in the evidence twice for these RCTs. With respect to prospective cohort studies, a representative number of populations were studied. Therefore, the Panel considers that their results can be generalised to the whole population of infants living in Europe.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the two RCTs and the three prospective cohort studies (Tiers 1 and 2) that there is no evidence for an effect or association between the timing of introduction of egg, covering a range of ages from ≤ 4 months to ≤ 6 months compared with thereafter and the odds of developing eczema up to 10 years of age (very low to low confidence in the evidence).
8.5.5. Timing of introduction of cereals
Main line of evidence
General population (3 studies): Neither the meta‐analysis nor the results of the two prospective cohort studies considered individually (Zutavern et al., 2004; Nwaru et al., 2013b) showed an association between cereal introduction about ≤ 4 months of age compared with thereafter and the odds of developing eczema up to the age of 10 years. The pooled estimate of the meta‐analysis was associated with serious imprecision. Heterogeneity was substantial (I2 = 71%) (Appendix A.30). In addition, in the prospective cohort study by Fergusson et al. (1990) that did not provide a point estimate, no evidence for an association between cereal introduction ≤ 4 months compared with later and the odds of developing eczema up to 10 years was observed (Annex A as Microsoft Excel® file).
At‐risk populations (2 studies): Two prospective cohort studies (Fergusson et al., 1981; Nwaru et al., 2013b) (Fergusson et al. (1981) report on the same study as Fergusson et al. (1990)) did not show an association between the timing of introduction of cereals (≤ 4 months of age vs thereafter) and the odds of developing eczema up to 10 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population that, from the three prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of cereals and the odds of developing eczema up to 10 years of age.
The Panel notes for the at‐risk population that, from the two prospective cohort studies (Tier 2) in the main line of evidence, there is no evidence for an association between the timing of introduction of cereals and the odds of developing eczema up to 10 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (4 studies, Tier 3)
General population (4 studies): The result of the meta‐analysis of the three prospective cohort studies (Zutavern et al., 2006; Filipiak et al., 2007; Elbert et al., 2017), that investigated the association between cereal introduction ≤ 4 months and ≤ 6 months of age vs thereafter and the odds of developing eczema up to 10 years of age, was not statistically significant. Heterogeneity was not important (I2 = 17%) (Appendix A.30). In addition, Nwaru et al. (2013a), that did not provide a point estimate, did not find an association between cereal introduction before 5 months vs thereafter on the odds of developing eczema up to 5 years of age (Annex A as Microsoft Excel® file).
At‐risk population (1 study): The prospective cohort study that investigated the outcome in the general population, investigated it also in an at‐risk population (Filipiak et al., 2007). This study did not find an association between the introduction of cereals ≤ 4 months of age vs thereafter and the odds of developing eczema up to 4 years (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.5.6. Timing of introduction of cereals and eczema: conclusions and grading of the confidence in the evidence
Imprecision: The imprecision associated with the results of the meta‐analysis of the two studies in the general population was serious. Therefore, the Panel decided to downgrade by one category the confidence in the evidence.
Inconsistency: The evidence is consistent across populations. The findings in the supportive line of evidence (four studies in the general population, including one also in an at‐risk population) were consistent with the findings in the main line of evidence. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: A representative number of populations has been studied. Therefore, the Panel did not have any concerns with respect to the generalisability of the findings to the whole population of infants living in Europe.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the three prospective cohort studies (Tiers 2) that there is no evidence for an association between the introduction of cereals ≤ 4 months compared with thereafter and the odds of developing eczema up to 10 years of age (low confidence in the evidence).
8.5.7. Timing of introduction of fish
Main line of evidence
General population (2 studies): The meta‐analysis of two prospective cohort studies (Zutavern et al., 2004; Nwaru et al., 2013b) did not show an association between fish introduction ≤ 5–6 months of age compared with thereafter and the odds of developing eczema up to the age of 10 years. Heterogeneity was not important (I2 = 0%) (Appendix A.31).
At‐risk population (1 study): The prospective cohort study (Nwaru et al., 2013b) did not find an association between introduction of fish before 5.25 months of age compared with thereafter and the odds of developing eczema up to 5 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population, from the two prospective cohort studies (Tier 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of fish and the odds of developing eczema up to 10 years of age.
The Panel notes for the at‐risk population, from the prospective cohort study (Tier 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of fish and the odds of developing eczema up to 5 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (4 studies, Tier 3)
General population (4 studies): The meta‐analysis of three prospective cohort studies (Zutavern et al., 2006; Filipiak et al., 2007; Alm et al., 2009) did not show an association between fish introduction ≤6 months of age compared with thereafter and the odds of developing eczema up to the age of 4 years. Heterogeneity was not important (I2 = 0%) (Appendix A.31). In addition, Nwaru et al. (2013a), that did not provide a point estimate, did not find an association between introduction of fish before 6 months of age compared with after 9 months of age and the odds of developing eczema up to 5 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.5.8. Timing of introduction of fish and eczema: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: The evidence is consistent across populations. The results of the supportive line of evidence in the general population (4 studies; no studies in at‐risk populations) were consistent with the findings of the studies in the main line of evidence. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: Both prospective cohort studies in the general or at‐risk populations were conducted in the UK. Owing to the limited number of populations studied, generalisability is uncertain. Therefore, the Panel downgraded by one category the evidence.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the two prospective cohort studies (Tiers 2) that there is no evidence for an association between the introduction of fish at ≤ 5–6 months compared with later and the odds of developing eczema up to 10 years of age (low confidence in the evidence).
8.5.9. Timing of introduction of soy or peanut
Only one prospective cohort study reported in two publications (Tromp et al., 2011; Elbert et al., 2017) (supportive line of evidence) investigated the relationship between the timing of introduction of soy and peanut and the odds of developing eczema. Therefore, this study cannot be used to establish the appropriate age range of introduction of CFs.
8.6. Allergic rhinitis: summary of the evidence
8.6.1. Timing of introduction of CFs in general
Main line of evidence
General population (1 study): The RCT (Perkin et al., 2016) did not show an effect of introduction of CFs at 3–4 months of age compared with introduction at 6 months of age on the odds of developing allergic rhinitis up to 3 years of age in exclusively breastfed infants (Appendix A.32).
At‐risk population (2 studies): The prospective cohort study rated as Tier 2 (Marini et al., 1996) did not show a relationship between the timing of introduction of CFs (≤ 4 months vs later) and the odds of developing allergic rhinitis up to 1–3 years of age. In addition, Sandini et al. (2011) (Tier 2) did not find an association between CF introduction < 4 months of age compared with 4–6 months and the odds of developing allergic rhinitis up to 2 or 5 years of age, but did not provide a point estimate. (Annex A as Microsoft Excel® file).
The Panel notes for the general population, from the RCT (Tier 1) in the main line of evidence, that there is no evidence for an effect of the timing of introduction of CFs on the odds of developing allergic rhinitis up to 3 years of age.
The Panel notes for the at‐risk population, from the two prospective cohort studies (Tier 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and the odds of developing allergic rhinitis up to 5 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (6 studies, Tier 3)
General population (5 studies): The meta‐analysis of the four prospective cohort studies (Wright et al., 1994; Strachan et al., 1996; Larsson et al., 2008; Zutavern et al., 2008) that investigated various time points with respect to the timing of introduction of CFs (from ≤ 1 to ≤ 6 months vs thereafter) did not show an association between the timing of introduction of CFs and the odds of developing allergic rhinitis up to 16 years of age. Heterogeneity was not important (I2 = 1%) (Appendix A.32). In addition, Nwaru et al. (2013a), that did not provide a point estimate, reported non‐significant findings, comparing an introduction of CFs ≤ 4 months with thereafter, in relation to the odds of developing allergic rhinitis up to 5 years of age.
At‐risk population (1 study): The prospective cohort study (Van Asperen et al., 1984) did not show an association between the timing of introduction of CFs (≤ 4 months vs later) and the odds of developing allergic rhinitis up to around 1.5 years of age (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.6.2. Timing of introduction of cereals or fish
Only one study reported in two publications (Virtanen et al., 2010; Nwaru et al., 2013a) (supportive line of evidence) investigated the relationship between the timing of introduction of cereals or fish and the odds of developing allergic rhinitis. Therefore, this study cannot be used to establish the appropriate age range of introduction of CFs.34
8.6.3. Allergic rhinitis: conclusions and grading of the confidence in the evidence
Imprecision: There was no imprecision associated with the results of the studies. However, as the single prospective cohort study in the main line of evidence was small (n = 62) and the second one did not provide a point estimate, the Panel was still concerned about the precision of the result. Therefore, the confidence in the evidence was downgraded by one category.
Inconsistency: The evidence is consistent across populations and the supportive line of evidence (five studies in the general population, one in an at‐risk population) was consistent with the main line of evidence.
Generalisability: The study population of the RCT consisted only of breastfed infants. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel downgraded by one category the confidence in the evidence derived from the RCT. With respect to the prospective cohort studies, the Panel did not have concerns with respect to the generalisability of its findings, considering that the supportive line of evidence in which a number of populations were studied was consistent with the findings in the main line of evidence.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the RCT (Tier 1) that there is no evidence for an effect of the introduction of CFs at 3‐4 months of age compared with 6 months of age on the odds of developing allergic rhinitis up to 3 years of age (moderate confidence in the evidence).
The Panel concludes from the two prospective cohort studies (Tier 2) that there is no evidence for an association between the introduction of CFs ≤ 4 months of age compared with thereafter or compared with 4–6 months of age and the odds of developing allergic rhinitis up to 5 years of age (low confidence in the evidence).
8.7. Symptomatic food allergy: summary of the evidence
8.7.1. Timing of introduction of CFs in general
Main line of evidence
General population (3 studies): The RCT (Perkin et al., 2016) did not show an effect of the timing of introduction of CFs in general and the risk of symptomatic food allergy in exclusively breastfed infants at 1 or 3 years of age in the FAS (RR 0.80 (95% CI 0.51 to 1.25) (Appendix A.33). In the PP analysis, introduction of CFs at 3–4 months of age compared with an introduction at 6 months of age was associated with a statistically significant reduction in the risk of developing symptomatic food allergy (RR 0.33 (95% CI 0.13 to 0.83). However, this could be mainly attributed to the effect of the early introduction of egg and peanut on symptomatic egg and peanut allergy, respectively, in this study (discussed in a separate Section) and not to the timing of introduction of CFs per se.
The meta‐analysis of the nested case–control study (Grimshaw et al., 2013) and the prospective cohort study (Luccioli et al., 2014) did not show a significant association between the timing of introduction of CFs (≤ 3 and ≤ 4 months compared with thereafter) and the outcome assessed up to 6 years of age. The pooled estimate obtained from the meta‐analysis was associated with a serious imprecision. Heterogeneity was considerable (I2 = 78%) (Appendix A.32). However, the Panel considers that this could be explained by the different methods for assessing symptomatic food allergy (i.e. double‐blind placebo‐controlled food challenge vs parents’ report of a doctor's diagnosis) and the different age at outcome assessment (i.e. 1 vs 6 years). Individually, one study (Grimshaw et al., 2013) found higher odds of developing symptomatic food allergy in infants introduced to CFs earlier (aOR 4.08 (95% CI 1.47 to 11.34)), but the other larger study (Luccioli et al., 2014) did not find a statistically significant association.
At‐risk population (2 studies): The prospective cohort study that investigated the outcome in the general population, also investigated it in an at‐risk population (Luccioli et al., 2014). The study did not observe an association between the introduction of CF ≤ 3 months compared with thereafter and the change for developing symptomatic food allergy, assessed at 6 years of age. In addition, Sandini et al. (2011) did not find an association between CF introduction < 4 months of age compared with 4–6 months and the odds of developing symptomatic food allergy up to 2 or 5 years of age, but did not provide a point estimate. (Annex A as Microsoft Excel® file).
Supportive line of evidence
The Panel notes for the general population, from one RCT and two prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an effect or association between the timing of introduction of CFs and the odds of developing symptomatic food allergy up to 6 years of age.
The Panel notes for the at‐risk population, from the two prospective cohort studies (Tier 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of CFs and the odds of developing symptomatic food allergy up to 6 years of age.
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (2 studies, Tier 3)
General population (2 studies): The meta‐analysis of two prospective cohort studies (Venter et al., 2009; Kim et al., 2011) that compared the introduction of CFs < 4 and < 6 months of age with thereafter did not show a statistically significant association between the timing of introduction of CFs and the odds of developing symptomatic food allergy investigated up to 3 years of age (Appendix A.33). The pooled estimate obtained from the meta‐analysis was associated with serious imprecision. Heterogeneity was not important (I2 = 0%). Individually, Venter et al. (2009) showed significantly lower odds of symptomatic food allergy to be associated with the introduction of CFs < 4 months of age (OR 0.51 (95% CI 0.28 to 0.92)). However, this analysis was unadjusted and therefore is likely to overestimate the association. Kim et al. (2011) did not find an association between the introduction of CFs < 6 months of age compared with thereafter on the odds of developing symptomatic food allergy up to 1 year of age.
Retrospective studies (2 studies, Tier 3)
General population (1 study): The case–control study by Bascunan Gamboa et al. (2012) did not observe an association between the introduction of CFs < 6 months of age compared with later and the odds of developing symptomatic food allergy up to 6–24 months of age (Annex A as Microsoft Excel® file).
At‐risk populations (1 study): One cross‐sectional analysis of baseline data of a prospective cohort study (Koplin et al., 2010) did not find an association between the timing of introduction of CFs (< vs ≥ 4 months) and the odds of developing symptomatic egg allergy up to 1 year of age (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (1 study, Tier 3)
General population (1 study): The case–control study by Alkazemi et al. (2018) did not observe an association between the timing of introduction of CFs and the odds of developing symptomatic food allergy assessed up to 13 years of age (Annex A as Microsoft Excel® file).
Sensitisation to food allergens (10 studies)
General population (6 studies): The results of the RCT (Perkin et al., 2016) (Tier 1) with respect to sensitisation to food allergens is consistent with the results on symptomatic food allergy. The meta‐analysis of the four prospective cohort studies rated as Tiers 1 and 2 (Snijders et al., 2008; Zutavern et al., 2008; Joseph et al., 2011; Nwaru et al., 2013c) did not show statistically significant results (Appendix A.34). Heterogeneity was substantial to considerable (I2 = 79%) and cannot be explained. The results of the single prospective cohort study rated as Tier 3 (Venter et al., 2009) showed lower odds of sensitisation at 3 years to be associated with an introduction of CF < 4 months of age compared with thereafter (OR 0.33 (95% CI 0.11 to 0.86)). This analysis was unadjusted and therefore is likely to overestimate the association. Finally, with respect to the difference in the timing of introduction of CFs in cases sensitised to food allergens and controls, four studies that investigated this outcome did not find statistically significant differences (Kucukosmanoglu et al., 2008; Hesselmar et al., 2010; McGowan et al., 2015; Hua et al., 2017) (two Tier 1 and two Tier 3) (Annex A as Microsoft Excel® file).
At‐risk populations (4 studies): The meta‐analysis of four comparisons from three prospective cohort studies (Mihrshahi et al., 2007; Joseph et al., 2011; Nwaru et al., 2013c), that investigated the introduction of CFs before 3 or 4 months of age compared with thereafter, did not show a statistically significant association between the timing of introduction of CFs and sensitisation to food allergens. Heterogeneity was moderate to substantial (I2 = 59%) (Appendix A.35). Equally, the case–control study by Sicherer et al. (2010) (Tier 3) in which the timing of introduction of CFs was used as a continuous variable in the analysis did not find an association between the timing of introduction of CFs and sensitisation to peanut protein at 3–15 months of age (Annex A as Microsoft Excel® file).
The Panel considers that, given that symptomatic food allergy was not investigated as an outcome in the prospective cohort studies, the findings of these studies with respect to sensitisation are difficult to interpret.
Difference in the timing of introduction of CFs in cases and controls
General population (3 studies): One nested case–control study (McGowan et al., 2015) (Tier 2) reported that 5‐year old cases with symptomatic food allergy were introduced to CFs statistically significantly earlier (median ages of introduction in cases and controls: 18 weeks vs 20 weeks, p = 0.04). The Panel notes that the difference in the timing of introduction of CFs between cases and controls is small and is unlikely to represent a true relationship between the timing of introduction of CFs and symptomatic food allergy. In addition, the analysis was unadjusted. Another nested case–control study (Hesselmar et al., 2010) (Tier 1) did not observe statistically significant differences in the timing of introduction of CFs between 1.5‐year‐old cases and controls. Also, in the case–control study by DesRoches et al. (2010) (Tier 3), the timing of introduction of CFs in 18‐month‐old peanut allergy cases compared with controls was not statistically significant (Annex A as Microsoft Excel® file).
At‐risk populations (1 study): In a nested case–control study (McGowan et al., 2015) (Tier 2) in which cases with symptomatic food allergy and controls were selected from a population with heredity of atopic diseases, no statistically significant differences were observed in the timing of introduction of CFs between cases with symptomatic food allergy and controls (Annex A as Microsoft Excel® file).
The Panel notes for the general population that the results of three out of four studies on symptomatic food allergy in the supportive line of evidence are consistent with the findings in the main line of evidence, as are the results related to the difference in the timing of introduction of CFs between cases and controls (three studies) and the results on sensitisation of the RCT. The results of the prospective cohort studies with respect to sensitisation cannot be interpreted in the absence of results on symptomatic food allergy in these studies.
The Panel notes for the at‐risk population that the results in the supportive line of evidence are consistent with those in the main line of evidence.
8.7.2. Timing of introduction of CFs in general and symptomatic food allergy: conclusions and grading of the confidence in the evidence
Imprecision: The imprecision associated with the results of the meta‐analysis of the two observational studies on symptomatic food allergy in the general population was serious. Therefore, the Panel downgraded by one category the evidence.
Inconsistency: The evidence is consistent across populations and the results of the supportive line of evidence are overall consistent with those of the main line of evidence. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: The study population of the RCT consisted only of breastfed infants. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel downgraded by one category the confidence in the evidence derived from the RCT. There was no concern with respect to generalisability for the prospective cohort studies.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the RCT (Tier 1) that there is no evidence for an effect of introduction of CFs at 3–4 months of age compared with 6 months of age on the odds of developing symptomatic food allergy up to 3 years of age (moderate confidence in the evidence).
The Panel concludes from the two prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs ≤ 3 and ≤ 4 months vs thereafter and the odds of developing symptomatic food allergy up to 6 years of age (low confidence in the evidence).
8.7.3. Timing of introduction of egg
Main line of evidence
General population (3 studies)
Two RCTs (Perkin et al., 2016; Bellach et al., 2017) and one prospective cohort study (Tham et al., 2017) were available in this line of evidence. The use of different comparators in the two RCTs precluded pooling of the results (Annex A as Microsoft Excel® file).
In the RCT by Perkin et al. (2016) (Tier 1) in exclusively breastfed infants conducted in the UK, introduction of egg at 3–4 months (intervention) was compared with continued exclusive breastfeeding and an introduction of egg at 6 months of age (control), in relation to the prevalence of egg allergy at 1 or 3 years of age as diagnosed by a double‐blind placebo‐controlled food challenge. In this study, infants were recruited from the general population. According to the protocol, egg was to be administered as boiled hen's egg in an amount of 2 × 2 g of egg protein per week, equivalent to around 30 g of egg without shell (equivalent to 1 (very) small egg).
Compliance with the protocol in the intervention group was defined as consumption of at least 3 g allergen protein/week for at least five weeks between 3 and 6 months of age. There was a considerable number of infants (56.9%) who did not reach the minimum targeted amount of consumption of cooked hen's egg, hence were excluded from the PP analysis in the intervention group. Adherence to the protocol in the control group was much higher and only 7.9% of the infants were excluded from the PP analysis. The Panel notes that the differential adherence rates to the protocol and subsequent exclusions from analysis in the intervention and control groups might have led to the violation of the principle of randomisation, and thus to a potentially biased result, in the PP analysis.
In the PP population, a statistically significantly lower risk of developing symptomatic egg allergy was found at 1 or 3 years of age in the intervention group compared with the control group (RR 0.25 (95% CI; 0.08 to 0.82)). This did not reach statistical significance in the FAS (RR 0.69 (95% CI; 0.40 to 1.18)). One possible explanation is that the significant results in the PP analysis were due to reverse causality, i.e. those infants who did not consume egg had developed or were developing egg allergy. It is also possible that those infants who did not consume egg in sufficient amounts were unable to handle the texture and their non‐adherence was unrelated to the outcome. In this case, their inclusion in the FAS analysis would have diluted the overall findings. The authors of the study addressed this question by comparing the prevalence of egg allergy of the non‐compliant infants in the intervention group with infants in the control group and did not find statistically significant differences between these two groups (6.0% vs 5.5%; p = 0.79). This increases the confidence that the findings in the PP analysis were not due to the exclusion of children with egg allergy who could not consume the food. However, overall the Panel considers that the confidence in the finding is reduced by the inconsistent results in the PP and FAS analyses of this RCT.
The Panel also notes that there was limited evidence for an inverse dose‐response relationship when considering the amount of egg that was consumed by infants, and that the introduction of cooked egg at home did not result in any cases of anaphylaxis.
In the RCT by Bellach et al. (2017) conducted in Germany (Tier 1), the timing of introduction of egg between 4 and 6 months with regular consumption up to 12 months of age (intervention) was compared with egg avoidance (control), in relation to the prevalence of egg allergy at 12 months of age diagnosed by a double‐blind placebo‐controlled food challenge or an open food challenge. In this study, infants were recruited from the general population. Only infants who were not sensitised to hen's egg (i.e. who had specific immunoglobulin E (sIgE) concentrations < 0.35 kUA/L) were included. Egg was administered as a pasteurised raw egg white powder mixed with solid infant foods in an amount of 3 × 2.5 g of egg protein per week, equivalent to around 58 g of egg without shell (equivalent to 1 large egg).
The originally planned sample size of the trial to have 80% power to detect a 50% reduction (from 12% to 6%) in sensitisation to egg was 788 infants. Recruitment was stopped early when 383 infants had been included in the trial. The authors report that this decision was taken based on three reasons: 1) following an interim analysis performed by an independent statistician, 2) the high level of egg sensitisation and allergy in the infants screened for inclusion (5.3% were diagnosed with egg allergy of which 2/3 reacted with an anaphylactic reaction during challenge) and 3) the frequency of allergic symptoms that occurred during the course of the trial (7.1% (n = 13/184; 3 of which had egg allergy) in the intervention group and 0.5% (n = 1/199) in the placebo group). Results were presented in the FAS population. The PP analysis was not considered by the Panel as it excluded all infants who became allergic to egg during the intervention. This analysis was therefore not an informative analysis. No statistically significant differences between the intervention and the control groups were observed. However, as recruitment stopped early, the study was underpowered to detect a statistically significant effect. The point estimate in the FAS analysis indicated a higher risk to be associated with early introduction (RR 3.3 (95% CI; 0.35 to 31.32)).
The Panel notes that this study was designed to investigate the effect of egg introduction at 4–6 months of age compared with egg avoidance in infants who were not sensitised to egg at baseline. Therefore, it is not comparable to the other available evidence and the seemingly inconsistent findings may be explained by these factors. In addition, it was underpowered.
Allergic reactions to the study powder were reported in 7.1% of the intervention and in 0.5% of the control group. Two of the three children who were diagnosed with egg allergy in the intervention group had an anaphylactic reaction to the study powder at home.
In the population‐based birth cohort study (Tham et al., 2017) (Tier 2) conducted in Singapore on 1,152 singleton infants of Chinese, Malay or Indian ethnicity, symptomatic food allergy was defined as a convincing history of IgE‐mediated reaction to a food. Data were available at 12 months of age from 854 infants, at 18 months from 799 children and at 2 years from 796 children. The prevalence of egg allergy in the study population was 1.7% at 12 months, 1.1% at 18 months and 0.8% at 24 months. Only a few infants were introduced to egg before 6 months of age, i.e. 21, 19 and 19 of those assessed at the different time points mentioned above and none developed egg allergy. No OR could be calculated owing to the zero events in the group introduced to egg before 6 months of age. When EFSA used the Fisher's exact test on the data reported above, there was no statistically significant difference. However, considering the low event rate, the study was most likely underpowered to detect statistically significant differences. Therefore, the Panel notes that the non‐statistically significant findings of this study may not be reliable.
At‐risk populations (3 studies)
Three RCTs from two different research groups (Tier 1 (Palmer et al., 2013; Palmer et al., 2017), Tier 2 (Tan et al., 2017)) are included in this line of evidence.
The study populations consisted of infants with moderate‐to‐severe eczema (SCORAD ≥ 15) (Palmer et al., 2013), infants with atopic mothers (Palmer et al., 2017) and non‐sensitised infants (skin prick test (SPT) wheal < 2 mm) with at least one first‐degree relative with an atopic disease (Tan et al., 2017). In all trials, the introduction of egg between around 4–6 months was compared to an introduction of around 8–10 months. Foods other than egg were self‐selected. Egg was administered as pasteurised whole egg powder (raw in Palmer et al. (2013) and Palmer et al. (2017); unspecified in Tan et al. (2017)). The amount was equivalent to around 48 g of egg without shell per week (equivalent to 1 medium egg per week (daily consumption of 0.9 g of egg protein in Palmer et al. (2013)) and to around 19–22 g of egg without shell per week (equivalent to half a medium egg) in Palmer et al. (2017) and Tan et al. (2017) (daily consumption of 350–400 mg of egg protein).
In both trials by Palmer et al. (2013) and Palmer et al. (2017), recruitment had to be stopped early because of funding constraints and, therefore, they were individually not sufficiently powered to detect an effect. The study by Tan et al. (2017) was powered for sensitisation. Symptomatic food allergy was a secondary outcome.
In the meta‐analysis of all three trials (Appendix A.36), a statistically significantly lower risk of developing symptomatic egg allergy at 1 year of age was observed when comparing introduction of egg between 4 and 6 months of age with after 8–10 months of age (RR 0.69 (95% CI 0.51 to 0.93)). Heterogeneity was not important (I2 = 0%). The 95% prediction interval crossed the line of ‘null’ effect. However, the Panel notes the uncertainty around the estimation of a prediction interval when only three studies are available (Section 2.2.3.2).
Palmer et al. (2013) reported that 31% (n = 15/49) of the infants had a reaction to the egg powder used in the study, ten of those reacted at the first exposure, including one case of anaphylaxis. Palmer et al. (2017) reported that 6.1% (n = 25/407) had a confirmed allergic reaction to the egg powder used in the study with no case of anaphylaxis. In Tan et al. (2017), 4.4% of the infants had mild to moderate reactions to egg within one week of starting the intervention with no case of anaphylaxis. Palmer et al. (2017) also reported that 92% (n=60/65) of the infants who had a reaction to the pasteurised raw egg challenge tolerated baked or cooked egg in the diet.
The Panel notes for the general population that, in the main line of evidence, there is limited evidence from one RCT (Tier 1) conducted in Europe that the introduction of cooked egg at 3–4 months of age compared with 6 months may reduce the risk of symptomatic egg allergy at 3 years of age.
The Panel notes for the at‐risk population that, in the main line of evidence, there is evidence from three RCTs (Tiers 1 and 2) that egg introduction between 4 and 6 months of age may be associated with a lower risk of developing symptomatic egg allergy at 1 year of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Retrospective studies (1 study, Tier 3)
General population (1 study): In a cross‐sectional analysis of baseline data of a prospective cohort study performed in Australia (Koplin et al., 2012) diagnosis of egg allergy was based on an open food challenge. Children were also considered to be egg allergic and were not offered a food challenge if parents reported a definite reaction to egg, the children had a positive SPT and egg was avoided in the infants’ diet. Children who tolerated one raw egg during the food challenge were given single servings of one whole raw egg for 7 days to exclude egg allergy. There was no statistically significant difference in the odds of egg allergy at 11–15 months between infants that were introduced to egg between 4–6 months of age and those introduced between 7–9 months of age as well as those introduced between 10–12 months of age. However, infants introduced to egg between 4‐6 months of age had statistically significantly lower odds of egg allergy than those introduced to egg after 12 months of age (including those infants who had not yet been exposed to egg): aOR 0.23 (95% CI 0.15 to 0.35). The p‐for‐trend was statistically significant.
At‐risk population (1 study): For the same study described above also results in an at‐risk population (i.e. children with a SPT wheal size ≥ 1 mm) were available (Koplin et al., 2010). There was no statistically significant difference in the odds of egg allergy at 11–15 months of age between infants that were introduced to egg between 4–6 months of age and those introduced between 7–9 months of age (aOR: 0.77 (95% CI 0.48 to 1.25)). There was also no statistically significant difference in the 4‐ to 6‐month‐group compared with the 10‐ to 12‐month group (aOR 0.63 (95% CI 0.38 to 1.00)). However, infants introduced to egg at 4–6 months compared with those introduced to egg after 12 months of age (including those infants who had not yet been exposed to egg) had statistically significantly lower odds of symptomatic food allergy (aOR 0.29 (95% CI 0.15 to 0.56)). The analysis was adjusted for allergic symptoms occurring before the introduction of egg. The p‐for‐trend was statistically significant (Annex A as Microsoft Excel® file).
Sensitisation to food allergens (7 studies)
General population (4 studies): Four studies (Gabet et al., 2016; Perkin et al., 2016; Bellach et al., 2017; Tran et al., 2017) (Tiers 1 and 2), including two RCTs, investigated sensitisation to egg protein, except (Gabet et al., 2016) that investigated sensitisation to egg, cow's milk, wheat, fish, peanut, sesame, mustard, soy, shrimp, beef and kiwi together (Appendix A.37). The results for sensitisation of Perkin et al. (2016) and Bellach et al. (2017) are consistent with the findings on symptomatic food allergy. In the study by Perkin et al. (2016) again the PP analysis showed a statistically significantly lower risk of developing sensitisation in the intervention group compared with controls, while this was not the case for the FAS analysis. The two prospective cohort studies (Gabet et al., 2016; Tran et al., 2017) (Tiers 1 and 2) did not find an association between the timing of introduction of egg (≤ 6 months vs thereafter) and sensitisation assessed up to 18 months of age. However, the Panel notes that their results are difficult to interpret in the absence of results on symptomatic food allergy in the same studies.
At‐risk populations (3 studies): The result of the meta‐analysis of the three RCTs (Palmer et al., 2013; Palmer et al., 2017; Tan et al., 2017) (Tiers 1 and 2), described in the main line of evidence (symptomatic food allergy) and that also investigated sensitisation, was not statistically significant (Appendix A.38). However, the study that was powered to detect an effect on sensitisation (Tan et al., 2017), showed statistically significantly reduced odds of sensitisation in the group that was introduced to egg at 4 months of age compared with the group introduced at > 8 months (OR 0.46 (95% CI 0.22 to 0.95)). The Panel notes that the other two studies (Palmer et al., 2013; Palmer et al., 2017) that were included in the meta‐analysis were individually underpowered to detect significant findings. In addition, the higher uncertainty around the heterogeneity estimate led to a wider 95% CI than in the meta‐analysis on symptomatic food allergy. Therefore, the Panel considers that it is difficult to interpret whether or not the findings of these studies in relation to sensitisation are consistent with their results on symptomatic food allergy.
The Panel notes for the general population that the results of the retrospective study are consistent with the findings in the main line of evidence. Results of the studies investigating sensitisation are consistent within the two RCTs that investigated both symptomatic egg allergy and sensitisation, and cannot be interpreted for the two prospective cohort studies in the absence of results on symptomatic food allergy in these studies.
The Panel notes for the at‐risk population that the results of the retrospective study are consistent with the findings in the main line of evidence. The results of the studies investigating sensitisation cannot be interpreted.
8.7.4. Timing of introduction of egg and symptomatic food allergy: conclusions and grading of the confidence in the evidence
For the grading in the confidence of the evidence, the main and supportive lines of evidence were further subdivided into:
Main‐A: the RCT by Perkin et al. (2016) (Tier 1) conducted in the general population comparing egg introduction (cooked egg) at 3–4 months of age to continued exclusive breastfeeding and egg introduction at 6 months of age.
Main‐B: the RCT by Bellach et al. (2017) (Tier 1) conducted in the general population comparing egg introduction (pasteurised raw egg white powder) at 4–6 months of age with egg avoidance in infants not sensitised to egg at baseline.
Main‐C: the prospective cohort study by Tham et al. (2017) (Tier 2) in the general population comparing egg < 6 months of age with thereafter.
Main‐D: the three RCTs conducted in high‐risk populations in Australia (Palmer et al., 2013; Palmer et al., 2017; Tan et al., 2017) comparing egg introduction (pasteurised (raw) egg powder) at 4–6 months with > 8–10 months.
S‐A and B: the cross‐sectional analysis of baseline data of the HealthNuts study performed in Australia (Koplin et al., 2010; Koplin et al., 2012).
The results of the evaluation of inconsistency, generalisability, imprecision, magnitude of the effect, dose‐response and ‘other’ are summarised in Table 7.
Table 7.
Grading of the confidence in the evidence for symptomatic food allergy and timing of introduction of eggf
| Certainty assessment | Characteristics | No of subjects | Effect in the LoE | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Line of evidence and initial rating | No studies | Design | RoB | Inconsistency | Generalisability | Imprecision | Magnitude | Dose response | Other | Comparator, population | Early CF | Late CF | Age | Analysis | Early | Late | Relative | Certainty in the LoE | Certainty across LoE |
|
1 (main‐A) ++++ |
1 | RCT | ↔ | o | ↓ | ↓ | ↑ | ↑ | ↓a |
EBF, EU, gen pop |
3–4 m | 6 m | 1 or 3 y | FAS | 21/569 (3.7%) | 32/596 (5.4%) |
RR 0.69 (0.40–1.18) |
+++ | ++ to +++ g |
| PP | 3/214 (1.4%) | 29/525 (5.5%) |
RR 0.25 (0.08–0.82) |
||||||||||||||||
|
2 (main‐B) ++++ |
1 | RCT | ↔ | o | ↓↓ | ↓↓ | ↑ | o | ↔ |
CF, EU, gen pop |
4–6 m | No egg | 1 y | FAS | 3/142 (2.1%) | 1/156 (0.6%) |
RR 3.3 (0.35–31.3) |
+ d | |
|
3 (main‐C) +++ |
1 | PC | ↔ | o | ↓↓ | ↓ | ↔ | o | ↔ |
CF, SG, gen pop |
< 6 m | > 6 m | 2 y | n/a |
0/19 (0%) |
6/777 (0.8%) |
n/a | + e | |
|
4 (main‐D) ++++ |
3 | RCT | ↔ | ↔ | ↓↓ | ↔ | ↔ | ↔ | ↔ |
CF, AU, at‐risk |
4–6 m | > 8–10 m | 1 y | FAS |
48/542 (8.9%) |
70/536 (13.1%) |
RR 0.69 (0.51–0.93) |
++ | |
|
4 (S‐A) + |
1 | CS | ↔ | o | ↓ | ↔ | ↔ | o | ↑b |
CF, AU, at‐risk |
4–6 m | 7–12 m | 1 y | n/a | 27/485 (5.6%) | 147/1663 (8.8%) |
OR 0.61 c (0.40‐0.93) |
+ | + |
|
4 (S‐B) + |
1 | CS | ↔ | o | ↓ | ↔ | ↔ | o | ↑b |
CF, AU, gen pop |
4–6 m | 7–12 m | 1 y | n/a | n/a | n/a | n/a | + | |
AU: Australia; CS: cross‐sectional; CF: complementary food; EBF: exclusively breastfed; FAS: full analysis set; gen pop: general population LoE: line of evidence; m: months; n/a: not applicable; OR: odds ratio; PP: per‐protocol analysis; RCT: randomised controlled trial; RR: risk ratio; SG: Singapore; y: year(s); ↓: downgrade; ↑: upgrade; ↔: no concern/impact; o: not evaluable.
Because of inconsistency between FAS and PP analysis.
Significant p‐for‐trend across different age categories of introduction of egg.
Unadjusted (calculated based on the raw data of events per group).
This line of evidence is not considered to be inconsistent with the findings in the lines of evidence 1 and 3, as differential findings could be explained. In addition, the study was underpowered. Therefore, this line of evidence was not considered in the grading of the confidence of the evidence.
The study in this line of evidence was most likely underpowered and therefore this line of evidence was not considered in the grading of the confidence of the evidence.
Results for sensitisation were consistent with the results on symptomatic food allergy within each of the RCTs in the general population and could not be interpreted for two additional studies (Gabet et al., 2016; Tran et al., 2017) in the general population in the absence of results on symptomatic food allergy in these studies. Also, the results on sensitisation of the meta‐analysis on the three RCTs considered in the line of evidence 4 in at‐risk populations cannot be interpreted. Therefore, the studies on sensitisation were not used in the grading of the confidence in the evidence.
Derived as a range from the certainty in the lines of evidence 4 (++) and 1 (+++).
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Safety: In the studies, there were some anaphylactic reactions associated with the consumption of pasteurised raw egg powders as intervention products. In the trial in which cooked egg was given to infants, no such reactions were observed. The Panel considers that, as far as the odds/risk of allergy is concerned, cooked egg can be introduced into the diet of infants when other CFs are introduced.
The Panel concludes from four RCTs (Tiers 1 and 2) that introduction of egg at 3–4 months of age compared with 6 months of age may reduce the risk of developing egg allergy (low to moderate level of confidence).
8.7.5. Timing of introduction of cereals
Main line of evidence
General population (1 study): The RCT (Perkin et al., 2016) did not find a statistically significant effect of the timing of introduction of wheat on the risk of wheat allergy at 1 or 3 years of age comparing the introduction at 3‐4 months of age with 6 months of age in exclusively breastfed infants (Annex A as Microsoft Excel® file).
No studies were available in at‐risk populations.
The Panel notes for the general population that, from the RCT (Tier 1) in the main line of evidence, there is no evidence for an effect of the timing of introduction of wheat in exclusively breastfed infants on the risk of developing wheat allergy up to 3 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (1 study, Tier 3)
General population (1 study): The prospective cohort study (Poole et al., 2006) reported lower odds of wheat allergy at 4 years of age with introduction of wheat, rye, oats and barley ≤ 6 months of age compared with thereafter (aOR 0.26 (95% CI 0.08 to 0.85)) (Annex A as Microsoft Excel® file).
Retrospective studies (1 study, Tier 3)
General population (1 study): The cross‐sectional study (Kumar et al., 2010) found higher odds of wheat allergy at 0.2–21 years to be associated with introduction of wheat or rice before 6 months of age compared with thereafter (aOR 1.6 (95% CI 1.004 to 2.5)) (Annex A as Microsoft Excel® file).
Sensitisation to food allergens (2 studies)
General population (2 studies): The RCT by Perkin et al. (2016) (Tier 1) reported a statistically significant lower risk of sensitisation to wheat protein to be associated with introduction of wheat at 3–4 months of age compared with 6 months of age at 1 year (RR 0.40 (95% CI 0.17 to 0.95)), but not at 3 years of age. The prospective cohort study by Nwaru et al. (2013c) (Tier 1) reported that introduction of wheat before 5 months of age compared with thereafter was not associated with sensitisation to wheat protein at 5 years of age (Annex A as Microsoft Excel® file). However, this finding in the last study is difficult to interpret in the absence of data on symptomatic food allergy in the same study.
At‐risk populations (1 study): The same prospective cohort study (Nwaru et al., 2013c) (Tier 1) described above assessed sensitisation to wheat protein as an outcome in an at‐risk population. It found lower odds of wheat protein sensitisation at 5 years of age to be associated with introduction of wheat < 5.1 months of age compared with > 6.6 months of age (aOR 0.76 (95% CI 0.58 to 0.99)) (Annex A as Microsoft Excel® file). However, this finding is difficult to interpret in the absence of data on symptomatic food allergy in the same study.
The Panel notes for the general population that the results of the two studies on symptomatic wheat allergy in the supportive line of evidence are inconsistent. The Panel also notes that the results of the RCT with respect to sensitisation are inconsistent with those on symptomatic wheat allergy and that the results of the prospective cohort study in relation to sensitisation cannot be interpreted in the absence of data on symptomatic wheat allergy in the same study.
8.7.6. Timing of introduction of cereals and symptomatic food allergy: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: Only one study was available in the main line of evidence. The findings of the two studies on symptomatic food allergy in the supportive line of evidence are inconsistent, as is the finding on sensitisation of the RCT that is not consistent with the results on symptomatic wheat allergy of the same RCT. For the decision on the grading of the confidence in the evidence in relation to these inconsistent findings, see ‘other’.
Generalisability: The study population of the RCT consisted of only breastfed infants. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel downgraded by one category the confidence in the evidence derived from this RCT.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Other: The Panel downgraded by one category the evidence, because of the limited number of studies that were available in the main line of evidence that were not supported by the findings in the supportive line of evidence.
The Panel concludes from the RCT (Tier 1) that there is no evidence for an effect of introduction of cereals at 3–4 months of age compared with 6 months of age on the risk of developing wheat allergy, assessed up to 3 years of age (low confidence in the evidence).
8.7.7. Timing of introduction of fish
The timing of introduction of fish and symptomatic fish allergy was investigated in a single study (Perkin et al., 2016) (main line of evidence). Therefore, this study cannot be used to establish an appropriate age range of introduction of CFs. This means that the studies in the supportive line of evidence on sensitisation (Nwaru et al., 2013c; Gabet et al., 2016; Perkin et al., 2016) cannot not be used either.
8.7.8. Timing of introduction of peanut
Main line of evidence
General population (1 study): In the FAS analysis, Perkin et al. (2016) (Tier 1) did not observe an effect of introduction of peanut at 3–4 months of age compared with 6 months in exclusively breastfed infants on the risk of symptomatic peanut allergy, assessed up to 3 years of age (RR 0.49 (95% CI 0.20 to 1.19)) (Annex A as Microsoft Excel® file). In the PP analysis, it was observed that introduction of peanut at 3–4 months of age reduced the risk of developing symptomatic peanut allergy compared with an introduction at 6 months of age (0% vs 2.5%, p = 0.003; RR not calculable due to zero events in the early introduction group). There was limited evidence for an inverse dose‐response relationship when taking into account the amount of peanut consumed.
At‐risk populations (1 study): In the study by Du Toit et al. (2015), infants between 4 and 10 months with severe eczema or egg allergy or both were randomly assigned either to peanut consumption (that was started depending on the age of the infant at enrolment between 4 and 10 months of age) or to peanut avoidance up to 5 years of age. At 5 years of age, the early introduction group had statistically significantly reduced odds in developing peanut allergy (in the ITT analysis: OR 0.16 (95% CI 0.01 to 0.32; PP: 0.02 (0.002–0.12)). As such, this study did not meet the inclusion criteria set by the Panel for the systematic review, because the early introduction group covered a time span beyond the first six months of life.
However, in a letter of response to a publication by Greenhawt et al. (2017), Lawson et al. (2017) (Tier 2) (Annex A as Microsoft Excel® file) provided further data that were used by the Panel to evaluate whether the introduction of peanut before the age of 6 months of age was associated with the development of peanut allergy.
In infants who were introduced to peanut ≤ 6 months of age, the odds of developing peanut allergy up to the age of 5 years was significantly reduced compared with those who avoided peanut up to that age (OR 0.17 (95% CI 0.06 to 0.47)). When performing a comparison in the intervention arm of the trial between infants that were introduced to peanut ≤ 6 months and those introduced at 7–10 months of age, there was no statistically significant difference between these two groups in the odds of developing peanut allergy. However, it should be noted that both reanalyses were observational and not based on the original randomised group.
The Panel notes for the general population that, in the main line of evidence, there is limited evidence from one RCT (Tier 1) that the introduction of peanut at 3–4 months of age compared with introduction at 6 months of age may reduce the risk of developing peanut allergy.
The Panel notes for the at‐risk population that in the main line of evidence there is limited evidence from one RCT that the introduction of peanut between 4 and 10 months or between 4 and 6 months compared with after 5 years reduces the risk of developing peanut allergy. However, this was not the case for introduction of peanut ≤ 6 months of age compared with 7–10 months.
Supportive line of evidence
Sensitisation to food allergens (1 study)
The result for sensitisation in the study by Perkin et al. (2016) is consistent with the findings in relation to symptomatic peanut allergy (Annex A as Microsoft Excel® file).
8.7.9. Timing of introduction of peanut and symptomatic food allergy: conclusions
The Panel considers that there is evidence that the introduction of peanut between 4 and 10 months or between 4 and 6 months of age in at‐risk infants compared with after 5 years reduces the risk of developing symptomatic peanut allergy. However, the evidence is insufficient to conclude whether a similar effect occurs when comparing infants introduced to peanut ≤ 6 months of age compared with > 6 months, but still within the first year of life, owing to the inconsistent evidence between the study in the general population and the study in an at‐risk population. Therefore, no level of confidence was assigned.
8.7.10. Atopic diseases: conclusions
For egg, the Panel concludes that there is evidence that its introduction at 3–4 months of age compared with 6 months of age may reduce the risk of developing egg allergy (low to moderate confidence in the evidence). In the studies that investigated egg allergy, there were some anaphylactic reactions associated with the consumption of pasteurised raw egg powders as intervention products. In the trial in which cooked egg was given to infants, no such reactions were observed.
For peanut, there is evidence that the introduction of peanut between 4 and 10 months or between 4 and 6 months of age in at‐risk infants compared with after 5 years reduces the risk of developing peanut allergy. However, the evidence is insufficient to conclude whether a similar effect occurs when comparing infants introduced to peanut ≤ 6 months of age with those introduced later within the first year of life (no level of confidence assigned).
For CFs in general, fish and cereals, there is no evidence for an association between the timing of their introduction and the odds for developing atopic diseases. The confidence in the evidence ranges from low to moderate, depending on the outcome, the food and the age range studied.
The Panel also concludes that, as far as the odds/risk of developing allergy is concerned, cooked egg, fish, peanut and cereals can be introduced to the diet of infants when other CFs are introduced; there is no need to postpone their introduction.
9. Assessment of the data on coeliac disease in individuals born at term or mixed populations
9.1. Coeliac disease: final body of evidence
The 17 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.6.
These publications reported on results from 15 studies:
1 RCT (Tier 1);
7 prospective cohort studies, 2 nested case–control studies (one study was analysed both as a prospective cohort study and a nested case–control study; 6 rated as Tier 1, 1 rated as Tier 2 and 1 rated as Tier 3);
6 retrospective studies (all Tier 3).
In these studies, three different endpoints were investigated. All results of the studies are given in Annex A as Microsoft Excel® file. In addition, for the main endpoints, results are summarised in the forest plots in Appendices A.39–A.41 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
9.2. Coeliac disease: endpoint and study selection
Studies were included if cases of coeliac disease were identified following the criteria established by the Guidelines of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) (Husby et al., 2012) for the diagnosis of coeliac disease. In children that show symptoms indicative of coeliac disease and have high anti‐tissue transglutaminase type 2 antibody (IgA‐tTGA) titres (> 10 times the upper limit of normal), the diagnosis is based on the additional presence of an elevated titre of endomysial antibodies in a blood sample drawn at an occasion separate from the initial one and the presence of haplotypes in the human leucocyte antigen (HLA) region associated with the risk of developing coeliac disease (HLA‐DQ2 or HLA‐DQ8), with no need for a small bowel biopsy. Under all other circumstances, the diagnosis is confirmed by a small bowel biopsy.
Coeliac disease autoimmunity was defined in most of the studies as IgA‐tTGA concentrations above a pre‐defined cut‐off in children not fulfilling the above‐mentioned criteria for diagnosing coeliac disease based on IgA‐tTGA concentrations. All studies were used in the assessment, irrespective of the cut‐offs used. The upper limit of normal concentration of antibodies depends on the test kit that is used.
No distinction was made in the assessment between study populations at risk of disease or not, as there is a strong genetic predisposition to coeliac disease. Individuals having neither HLA‐DQ2 nor HLA‐DQ8 are unlikely to have or develop coeliac disease (Husby et al., 2012). Selecting the general population as study population rather than subjects with a positive HLA‐DQ2 or HLA‐DQ8 status will dilute the effect or association but will not lead to differential results. Therefore, results of all study populations were combined. Individuals that are positive for HLA‐DQ2 or HLA‐DQ8 (and therefore at risk of the disease) will only develop coeliac disease if they are exposed to gluten. The present assessment focussed on an evaluation of the risk of developing coeliac disease in infants introduced to gluten at different time points (with at least one time point < 6 months of age). Time to onset of the disease or the effect of complete gluten avoidance was not investigated.
The Panel decided to draw its conclusions from the disease‐related endpoint, i.e. coeliac disease. Data on coeliac disease autoimmunity are used only as supportive evidence to the results from the studies on coeliac disease, as positive results are associated with a higher risk of developing coeliac disease but alone are not predictive of the disease (see above).
For this outcome, only studies were available that investigated the timing of introduction of gluten (at various ages between < 3 and < 6 months), but not of CFs in general.
9.3. Coeliac disease: summary of the evidence
Main line of evidence (5 studies)
The RCT by Vriezinga et al. (2014) (Appendix A.39) conducted in a population at risk of coeliac disease did not find a statistically significant effect of the introduction of gluten (200 mg/day of vital wheat gluten with lactose, equivalent to 100 mg of immunologically reactive gluten35 , 36) at 4 months vs 6 months of age, on the hazard of developing coeliac disease up to 3 years of age. The authors explained that the amount of gluten that was administered is sufficient to cause histologic lesions (i.e. villous atrophy) in patients with coeliac disease.
From the meta‐analysis of four prospective cohort studies (Norris et al., 2005; Welander et al., 2010; Størdal et al., 2013; Andren Aronsson et al., 2015), there is no evidence for an association between the timing of introduction of gluten or gluten‐containing foods and the hazard of coeliac disease studied up to 12 years of age (Appendix A.39). Heterogeneity was substantial (I2 = 73%).
This heterogeneity was mainly caused by one study (Norris et al., 2005) which showed results that were substantially different from those of the other available studies, and this could not be explained. When Norris et al. (2005) was removed from the meta‐analysis in a sensitivity analysis (data not shown), heterogeneity was reduced to 18% with no substantial change to the point estimate (HR 0.91 instead of 0.94) with a narrower 95% CI (still not statistically significant).
An unplanned subgroup analysis (data not shown) was performed to investigate whether introduction of gluten below 3 or 4 months of age compared with around 4–6 months of age would have a different effect than introduction around 4–6 months of age compared with thereafter. This was done following the conclusion of the Panel in the previous Scientific Opinion (EFSA NDA Panel, 2009) that ’introduction of gluten < 4 months might increase the risk of coeliac disease […], whilst the introduction of gluten between 4 and 6 months while still breastfeeding might decrease the risk’.
-
−
The meta‐analysis comparing those who were introduced to gluten ≤ 3 or 4 months of age with those introduced around 4–6 months of age did not show an association between the timing of introduction of gluten and the outcome (HR 1.47 (95% CI 0.05 to 14.91)). Heterogeneity was substantial (I2 = 83%) and imprecision around the point estimate was serious. When the study by Norris et al. (2005) was removed from the meta‐analysis, heterogeneity reduced to 24%, the point estimate shifted to the other side of the line of the ‘null’ effect and the 95% CI was reduced (HR 0.85 (95% CI 0.37 to 1.96)).
-
−
Equally, the meta‐analysis comparing those who were introduced to gluten around 4–6 months of age with those introduced later did not show an association between the timing of introduction of gluten and the outcome (HR 0.85 (95% CI 0.45 to 1.62)). Heterogeneity was moderate to substantial (I2 = 60%). When the study by Norris et al. (2005) was removed from the meta‐analysis, heterogeneity reduced to 41%, without substantial effects on the point estimate and the 95% CI (HR 0.92 (95% CI 0.57 to 1.46)).
Two studies reported on breastfeeding at the time of gluten introduction before 6 months of age and the risk of developing coeliac disease. Based on data reported in Szajewska et al. (2015), the RCT by Vriezinga et al. (2014) did not find an effect of breastfeeding during gluten introduction at 4 months of age compared with 6 months of age (RR 1.31 (95% CI 0.77 to 2.23)) on the risk of developing coeliac disease (secondary observational analysis). Also, the prospective cohort study by Størdal et al. (2013) (Tier 1), including 45,156 infants in the analysis, did not observe an association between continued breastfeeding at the time of gluten introduction ≤ 6 months of age and the risk of developing coeliac disease.
The assessment of the effect of breastfeeding while introducing gluten over a wider age range (> 6 months) as investigated in the systematic review by Szajewska et al. (2015) and in the position paper by ESPGHAN (Szajewska et al., 2016) is not part of the current mandate and was not considered further.
The Panel notes that, from the RCT and the meta‐analysis of four prospective cohort studies (Tiers 1 and 2) in the main line of evidence, there is no evidence for an association between various timings of introduction of gluten or gluten‐containing foods and the hazard of developing coeliac disease up to 12 years of age. There are also no differential effects of gluten introduction < 4 months of age and between 4 and 6 months of age, or gluten introduction < 6 months of age while still breastfeeding.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Retrospective studies (4 studies, Tier 3)
The meta‐analysis of the four case–control studies (Auricchio et al., 1983; Greco et al., 1988; Peters et al., 2001; Ivarsson et al., 2002) did not find a statistically significant association between the various timings of introduction of gluten‐containing foods (ranging from ≤ 2 to ≤ 4 months) and the odds of developing coeliac disease up to around 6 years of age (Appendix A.40). Heterogeneity was not important (I2 = 26%).
Studies in which the timing of introduction of gluten was used as a continuous variable in the analysis, irrespective of the study design (2 studies)
One prospective cohort study by Andren Aronsson et al. (2016) (Tier 1) and one case–control study (Myleus et al., 2012) (Tier 3) did not observe statistically significant associations between the timing of introduction of gluten‐containing foods and the odds of developing coeliac disease up to 2 years of age (Annex A as Microsoft Excel® file).
Coeliac disease autoimmunity (5 studies)
The findings of the RCT (Vriezinga et al., 2014) on coeliac disease autoimmunity were consistent with the findings on the disease endpoint (Appendix A.41).
From the meta‐analysis of four prospective cohort studies (Norris et al., 2005; Jansen et al., 2014; Andren Aronsson et al., 2015; Chmiel et al., 2015) (Tiers 1 and 2), there is no evidence for an association between various timings of introduction of gluten‐containing foods (ranging from < 3 months to ≤ 6 months compared with thereafter) and the hazard of developing coeliac disease autoimmunity up to around 9 years of age. Heterogeneity was moderate to substantial (I2 = 56%). Two of the studies also investigated coeliac disease and their findings on coeliac disease autoimmunity were consistent with those on the disease endpoint.
Difference in the timing of introduction of gluten in cases and controls (3 studies)
Two nested case–control studies (Andren Aronsson et al., 2016; Savilahti et al., 2018) (Tiers 1 and 3, respectively) and one case–control study performed in cases with coeliac disease and their healthy siblings (Ascher et al., 1997) (Tier 3), did not find statistically significantly different timings of introduction of gluten or gluten‐containing foods in coeliac disease cases and controls aged 2, 5 and 8 years, respectively (Annex A as Microsoft Excel® file).
The Panel notes that the results in the supportive line of evidence are consistent with those in the main line of evidence.
9.4. Coeliac disease: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: The results were consistent across populations and the results of the supportive line of evidence (six studies on coeliac disease, five on coeliac disease autoimmunity and three on the timing of introduction of gluten in cases and controls) were consistent with the results in the main line of evidence. For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability, as a variety of populations were studied.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the RCT (Tier 1) that there is no effect of the introduction of gluten at 4 months of age compared with 6 months of age and the hazard of developing coeliac disease up to 3 years of age (high confidence in the evidence).
The Panel concludes from the four prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of gluten ≤ 3 or 4 months of age compared with thereafter and the hazard of developing coeliac disease up to 12 years of age (moderate confidence in the evidence).
In its previous Scientific Opinion, the Panel considered that introduction of gluten < 4 months of age might increase the risk of coeliac disease, whereas introduction between 4 and 6 months of age while still breastfeeding might decrease the risk of coeliac disease. With the data that have become available on coeliac disease since the publication of the last Scientific Opinion, these conclusions are no longer supported.
10. Assessment of the data on type 1 diabetes mellitus in individuals born at term or mixed populations
10.1. Type 1 diabetes mellitus: final body of evidence
The 23 publications that were considered in the assessment in individuals born at term or mixed populations are given in Appendix B.7.
These publications reported on results from 18 studies:
7 prospective cohort studies and 1 nested case–control study (6 rated as Tier 1, 2 rated as Tier 2);
10 retrospective studies (all Tier 3).
In these studies, three different endpoints were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, for the main endpoints, results are summarised in the forest plots in Appendices A.42–A.45 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
10.2. Type 1 diabetes mellitus: endpoint and study selection
Studies were included if the diagnosis of T1DM in the studies was based on well‐established criteria for diagnosing the disease at the time of the study (e.g. WHO (2006) or recommendations from the American Diabetes Association which are updated regularly).
With respect to the endpoint on islet autoimmunity, studies were included if the outcome assessment was based on the presence of elevated titres of at least one autoantibody in two consecutive samples. Chmiel et al. (2015) presented results for the hazard of having elevated titres for one and for two autoantibodies. In this case, the results related to the hazard of having an elevated titre of one autoantibody was considered in the analysis for comparability reasons.
No distinction was made in the assessment between study populations at risk of disease or not. The Panel decided to draw its conclusions from the disease‐related endpoint, i.e. T1DM. Data on islet autoimmunity are used only as supportive evidence to the results from the studies on T1DM, as positive results are associated with a higher risk of developing T1DM but alone are not predictive of the disease.
10.3. Type 1 diabetes mellitus: summary of the evidence
10.3.1. Timing of introduction of CFs in general
Main line of evidence (4 studies)
From the meta‐analysis of six age comparisons from four prospective cohort studies (Savilahti and Saarinen, 2009; Frederiksen et al., 2013; Lund‐Blix et al., 2015; Hakola et al., 2018), there is no evidence for an association between various timings of introduction of CFs (ranging from < 3 months to < 5 months compared with thereafter) and the hazard of developing T1DM up to 15 years of age (Appendix A.42). Heterogeneity was substantial (I2 = 65%).
The Panel notes that, from the meta‐analysis of four prospective cohort studies (Tiers 1 and 2) in the main line of evidence, there is no evidence for an association between various timings of introduction of CFs and the hazard of developing T1DM up to 15 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Retrospective studies (7 studies, Tier 3)
From the meta‐analysis of the six case–control studies (Kostraba et al., 1993; Meloni et al., 1997; EURODIAB Substudy 2 Study Group, 2002; Stene et al., 2003; Rosenbauer et al., 2008; Rabiei and Reza, 2012) in children with ages ranging from 2 to 18 years, there is no evidence for an association between various timings of introduction of CFs (ranging from < 3 months to < 6 months compared with thereafter) and the odds of developing T1DM. Heterogeneity was substantial: I2 = 79%, which cannot be explained (Appendix A.43).
In another case–control study that did not provide a point estimate (hence is not present in the forest plot), Visalli et al. (2003) did not find a significant association between the timing of introduction of CFs (< 3 vs > 3 months) and the odds of developing T1DM up to the age of 6‐18 years.
Islet autoimmunity (2 studies)
Neither the prospective cohort study by Hakola et al. (2018) nor that by Lund‐Blix et al. (2015) (both Tier 1) found a statistically significant association between the timing of introduction of CFs (< 3 months and < 5 months vs thereafter, respectively) and the hazard of developing islet autoimmunity up to 15 years of age (Annex A as Microsoft Excel® file). Both studies also investigated T1DM and their results are consistent for the two endpoints investigated.
Difference in the timing of introduction of CFs in cases and controls (2 studies)
The case–control study by Liese et al. (2012) did not find statistically significant differences between the timing of introduction of CFs in on average 15‐year‐old T1DM cases and controls, while in the case–control study by Perez‐Bravo et al. (1996) (both Tier 3) on average 15‐year‐old cases were introduced to CFs earlier (mean difference: −1.01 (95% CI −1.83 to −0.2) months) (Annex A as Microsoft Excel® file). Both analyses were unadjusted and therefore are likely to overestimate the association.
The Panel notes that the findings of 10 out of 11 studies in the supportive line of evidence are consistent with the findings in the main line of evidence.
10.3.2. Timing of introduction of CFs in general and type 1 diabetes mellitus: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: The results were consistent across populations and across lines of evidence (4 studies in the main line and 11 studies in the supportive line). For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability, as a variety of populations were studied.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the four prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of CFs and the hazard of developing T1DM up to 15 years of age (moderate confidence in the evidence).
The age of introduction investigated varied between studies, ranging from < 3 months of age to < 5 months of age for ’early’ introduction, compared with thereafter.
10.3.3. Timing of introduction of gluten
Main line of evidence (5 studies)
From the meta‐analysis of the five prospective cohort studies (Welander et al., 2010; Frederiksen et al., 2013; Chmiel et al., 2015; Lund‐Blix et al., 2015; Hakola et al., 2018), there is no evidence for an association between various timings of introduction of gluten or gluten‐containing foods (ranging from < 3 months to < 5 months compared with thereafter) and the hazard of developing T1DM up to 16 years of age (Appendix A.44). Heterogeneity was moderate (I2 = 40%).
There were too few studies to assess whether gluten introduction < 4 months of age had a different effect than gluten introduction at 4–6 months of age, as purported by the Panel in its previous Scientific Opinion (EFSA NDA Panel, 2009).
There were also no data to evaluate a potential differential effect of continued breastfeeding while introducing gluten < 6 months of age. Considering breastfeeding while introducing gluten at any age, as done in two studies within the body of evidence (Welander et al., 2010; Frederiksen et al., 2013), is not part of the current mandate and was not considered further.
The Panel notes, from the meta‐analysis of the five prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that there is no evidence for an association between the timing of introduction of gluten or gluten‐containing foods and the hazard of developing T1DM up to 16 years of age.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Islet autoimmunity (7 studies, Tiers 1 and 2)
From the meta‐analysis from seven prospective cohort studies (Norris et al., 2003; Wahlberg et al., 2006; Couper et al., 2009; Chmiel et al., 2015; Lund‐Blix et al., 2015; Hakola et al., 2018; Uusitalo et al., 2018), there is no evidence for an association between various timings of introduction of gluten or gluten‐containing foods (ranging from < 3 months to ≤ 6 months) and the hazard of developing islet autoimmunity up to 20 years of age (Appendix A.45). Heterogeneity was moderate to substantial (I2 = 50%). All these studies (some with a different author name) were already included in the assessment of T1DM, except for Couper et al. (2009) and Uusitalo et al. (2018), and their findings with respect to islet autoimmunity are consistent with the results on T1DM.
Difference in the timing of introduction of gluten in cases and controls (1 study)
The case–control study in siblings by Bezzera Alves et al. (2012) did not find a statistically significant difference in the timing of introduction of gluten between on average 9‐year‐old cases with T1DM and controls.
10.3.4. Timing of introduction of gluten and type 1 diabetes mellitus: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: The results were consistent across populations and across lines of evidence (five studies in the main line and eight studies in the supportive line). For the meta‐analysis conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability, as a variety of populations were studied.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the five prospective cohort studies (Tiers 1 and 2) that there is no evidence for an association between the age of introduction of gluten and the hazard of developing T1DM up to 16 years of age (moderate confidence in the evidence).
The age of introduction of gluten that was investigated varied between studies, ranging from < 3 months of age to < 5 months of age for ’early’ introduction, compared with thereafter.
In its previous Scientific Opinion, the Panel considered that introduction of gluten < 4 months of age might increase the risk of T1DM, whereas introduction between 4 and 6 months of age while still breastfeeding might decrease the risk of T1DM. However, in the present assessment there were insufficient data to investigate whether the introduction of gluten < 4 months of age could have a different effect on the risk of developing T1DM than gluten introduction between 4 and 6 months of age. There were no data to evaluate whether gluten introduction < 6 months of age while still breastfeeding has a different effect than gluten introduction < 6 months of age while not breastfeeding.
11. Assessment of the data on risk factors for cardiovascular diseases in individuals born at term or mixed populations
11.1. Risk factors for cardiovascular diseases: final body of evidence
The eight publications that were considered in the assessment of risk factors for cardiovascular diseases in individuals born at term or mixed populations are given in Appendix B.8.
These publications reported on results from six studies:
4 prospective cohort studies (1 Tier 1, 3 Tier 2);
2 retrospective studies (all Tier 3).
For these outcomes, 15 different endpoints were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, for blood pressure, results are summarised in the forest plots in Appendices A.46 and A.47 of this Scientific Opinion.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
11.2. Risk factors for cardiovascular diseases: endpoint and study selection
A sufficient number of studies was available to draw conclusions only for systolic and diastolic blood pressure expressed in mmHg. All other endpoints were generally studied in single studies and were not further considered.
11.3. Blood pressure: summary of the evidence
Main line of evidence (4 studies)
From the meta‐analysis of four prospective cohort studies (Wilson et al., 1998; Martin et al., 2004; de Jonge et al., 2013; de Beer et al., 2016), there was a statistically significant association between earlier timings of introduction of CFs (< 3 to < 6 months, compared with thereafter) and higher systolic blood pressure at 5 to about 7 years of age (Appendix A.46). Heterogeneity was not important (I2 = 0%). However, the Panel considers that the observed small mean difference of 0.6 mmHg (95% CI 0.2 to 1 mmHg) between the groups with earlier and later introduction of CFs is unlikely to affect the risk of cardiovascular diseases later in life.
The findings on diastolic blood pressure from the same cohort studies were similar (Appendix A.47). The mean difference that was observed between the groups with earlier and later introduction of CFs was 0.5 mmHg (95% CI 0.2 to 0.8 mmHg), which is again considered unlikely to affect the risk of cardiovascular diseases later in life.
The Panel notes, from the four prospective cohort studies (Tiers 1 and 2) in the main line of evidence, that even though the statistical analysis of the association between the age of introduction of CFs (< 3 to < 6 months, compared with thereafter) and blood pressure was significant, the size of the effect was not biologically relevant.
11.4. Blood pressure: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: Only studies in the main line of evidence were available. There was no inconsistency. For all the meta‐analyses conducted in the main line of evidence, I2 was below 75%.
Generalisability: There were no concerns with respect to generalisability, as a variety of populations were studied.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the four prospective cohort studies (Tiers 1 and 2) that even though the statistical analysis of the association between the age of introduction of CFs (< 3 to < 6 months, compared with thereafter) and blood pressure assessed up to 7 years of age was significant (moderate confidence in the evidence), the size of the effect was not biologically relevant.
12. Assessment of the data on infections in individuals born at term or mixed populations
12.1. Infections: final body of evidence
The 12 publications that were considered in the assessment of data on infections in individuals born at term or mixed populations are given in Appendix B.9.
These publications reported on results from 11 studies:
3 RCTs (3 rated as Tier 2);
7 prospective cohort studies (3 rated as Tier 1, 1 rated as Tier 2 and 3 rated as Tier 3);
1 retrospective study (Tier 3).
In these studies, 13 different endpoints were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
12.2. Infections: endpoint and study selection
Endpoints considered were gastrointestinal infections, upper and lower respiratory tract infections and infections of all types.
In the RCT by Perkin et al. (2016), the endpoints designated as diarrhoea and vomiting were assessed as undesirable events and were not intended to comprise gastrointestinal infections only. Even though, it could be assumed that an infection would have been the most likely cause of diarrhoea and vomiting in infants, the different findings in relation to diarrhoea (i.e. no effect of the timing of introduction of CFs) and vomiting (i.e. earlier introduction of CFs related to a higher incidence of vomiting) in this study, indicate that at least vomiting cannot be interpreted as a symptom of an infection in this instance, as vomiting of infectious origin is usually accompanied by diarrhoea. Therefore, the results for vomiting in the study by Perkin et al. (2016) were not considered further.
Studies, in which the incidence of infections was assessed > 1 year of age only and which did not cover the period during which CFs were introduced were not considered in the assessment owing to the implausible association between the timing of introduction of CFs and infections that occur several months later (Section 1.6).
Studies conducted in low‐income and lower‐middle‐income countries were excluded for this outcome, in line with the protocol for this review (EFSA, 2017b) owing to the difficulties of disentangling the effects on infections of poor food hygiene, suboptimal nutritional status and/or the nutritional inadequacy of CFs in these countries from the timing of introduction of CFs (Section 2.1.1.2). The Panel, however, decided to consider further two RCTs performed in Honduras (Cohen et al., 1994; Dewey et al., 1999), a lower‐middle‐income country, as the two studies were well controlled to exclude the interference of bad hygiene and nutritionally inadequate food, i.e. the CFs administered were provided by the investigators to the mothers of the study participants.
12.3. Gastrointestinal infections: summary of the evidence
Main line of evidence (5 studies)
Gastrointestinal infections were investigated during the time period of 4–6 months of age in exclusively breastfed infants in three RCTs (Cohen et al., 1994; Dewey et al., 1999; Perkin et al., 2016), all of which compared the introduction of CFs at 3‐4 months with 6 months of age (Annex A as Microsoft Excel® file). Results of these trials are difficult to compare, as they used different outcome measures to assess gastrointestinal infections and reported the results in different metrics.
Dewey et al. (1999) found that the percentage of days with diarrhoea between 4 and 6 months was higher in exclusively breastfed infants than in those who had received CFs at 4 months of age (5.4 ± 8.5 vs 2.8 ± 5.4%, p < 0.05 using a (not further specified) non‐parametric test). However, imprecision was serious. The Panel notes that the findings by Dewey et al. (1999) are contradictory to what is usually observed in assessment for the benefit of more prolonged exclusive breastfeeding (Kramer and Kakuma, 2012).
Cohen et al. (1994) and Perkin et al. (2016) did not find statistically significant differences in morbidity scores based on the number of days with diarrhoea (point estimate not reported; Cohen et al. (1994)) or absolute difference in number of days with diarrhoea (Perkin et al., 2016).
Two prospective cohort studies (Forsyth et al., 1993; Noppornlertwong and Tantibhaedhyangkul, 2016) were also available (Annex A as Microsoft Excel® file). Forsyth et al. (1993) did not find significant differences in the number of infants with one or more diarrhoea or vomiting episodes at 4–6, 6–9 and 9–12 months of age, comparing infants introduced to CFs < 2 months of age or between 2 and 3 months of age vs thereafter.
Noppornlertwong and Tantibhaedhyangkul (2016) (Tier 2) did not find a difference in gastrointestinal infections from 5 to 15 months of age in exclusively formula fed infants introduced to CFs at 4–6 months vs 6 months. However, this study in 41 infants is likely to have been underpowered for this outcome. Therefore, its non‐statistically significant finding was not considered further by the Panel.
The Panel notes from the three RCTs and one prospective cohort study (Tiers 1 and 2) from which conclusions could be drawn in the main line of evidence that there is no evidence that the introduction of CFs at various ages < 6 months of age increases gastrointestinal infections.
Supportive line of evidence (4 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (2 studies, Tier 3)
The pooled analysis by Morgan et al. (2004) found no evidence for an association between the timing of introduction of CFs (< vs > 3 months of age) and gastroenteritis assessed up to 18 months of age.
Wright et al. (2004) observed higher odds of diarrhoea during the time period of 1.5–4 months (aOR 1.65 (95% CI 1.09 to 2.50)) in infants introduced to CFs < 3 months of age compared with thereafter. The association between the timing of introduction of CFs and episodes of diarrhoea for which a medical doctor needed to be consulted was, however, not statistically significant (Annex A as Microsoft Excel® file).
Retrospective studies (1 study, Tier 3)
In a cross‐sectional analysis of baseline data of the Millennium Cohort Study (Quigley et al., 2009), the authors estimated the monthly risk of hospitalisation for diarrhoea between birth and 8 months of age, depending on whether or not CFs had been introduced in that month. They did not find an association between the introduction to CFs and the odds of the outcome (Annex A as Microsoft Excel® file).
Studies in which the timing of introduction of CF was used as a continuous variable in the analysis, irrespective of study design (1 study)
The prospective cohort study by Lopez‐Alarcon et al. (1997) (Tier 1) did not find an association between the timing of introduction of CFs and the odds of diarrhoea between birth and 6 months of age (Annex A as Microsoft Excel® file).
The Panel notes that the results of the four studies in the supportive line of evidence are mostly consistent with the findings in the main line of evidence. One study found the introduction of CFs < 3 months compared with thereafter to be associated with higher odds of diarrhoea between 1.5 and 4 months of age. However, there was no difference in this study in relation to more severe diarrhoea which required the consultation of a medical doctor.
12.4. Upper respiratory tract infections: summary of the evidence
Main line of evidence (5 studies)
Upper respiratory tract infections (URTIs) were investigated in the same RCTs in exclusively breastfed infants (Tier 2) and during the same time span (i.e. 4–6 months) as described above (Annex A as Microsoft Excel® file). Perkin et al. (2016) reported statistically significant increased odds of URTI at 5 and 6 months of age (OR 1.33 (95% CI 1.06 to 1.68) and 1.45 (1.15–1.83),37 respectively) in the group of infants introduced to CFs at 3–4 months of age compared with those introduced at 6 months of age. No statistically significant differences were observed at 4 months or at 7 months. Dewey et al. (1999) reported that nasal discharge, expressed in % of days, in the early introduction group was not statistically significantly different from that in the group introduced at 6 months of age. Cohen et al. (1994) reported no statistically significant difference in the incidence of URTI at 4–6 months in the group introduced to CFs at 4 months compared with the group that was introduced at 6 months of age.
The same two prospective cohort studies described above (Forsyth et al., 1993; Noppornlertwong and Tantibhaedhyangkul, 2016) (Tiers 1 and 2) also investigated URTI (Annex A as Microsoft Excel® file).
Forsyth et al. (1993) reported statistically significant differences in the number of infants with one or more episodes of URTI between 3 and 6 months of age, comparing infants with various background milk feedings introduced to CFs < 2 months of age (52% with URTI), at 2–3 months of age (46.9% with URTI) and thereafter (36.6% with URTI), after adjustment for confounders. There were no statistically significant differences in URTI in other time spans investigated, i.e. between 0 and 3, 6 and 9, and 9 and 12 months of age.
Noppornlertwong and Tantibhaedhyangkul (2016) did not find a difference in the number of respiratory tract infections (undefined) from 5 to 15 months of age in exclusively formula fed infants introduced to CFs between 4–6 months and 6 months. However, the study in 41 infants is likely to have been underpowered for this outcome. Therefore, its non‐statistically significant finding was not considered further by the Panel.
No studies were available in the supportive line of evidence.
The Panel notes that the results of the three RCTs and one prospective cohort study (Tiers 1 and 2) in the main line of evidence from which conclusions could be drawn in relation to URTI are inconsistent.
In the two studies which observed an effect or association between the introduction of CFs (at 3–4 months of age compared with 6 months, and < 2 and 2–3 months of age compared with >3 months) and the endpoint, the difference in the incidence of URTI was limited to a time period of 2–3 months, considered transitory and of low biological significance by the Panel.
12.5. Lower respiratory tract infections: summary of the evidence
Main line of evidence (1 study)
The RCT by Perkin et al. (2016) did not find a statistically significant difference in the incidence of lower respiratory tract infections (LRTI) between the groups in which CFs were introduced at 3–4 months and that introduced at 6 months (Annex A as Microsoft Excel® file).
The Panel notes that there is no evidence for an association between the timing of introduction of CFs and LRTI from the RCT in the main line of evidence.
Supportive line of evidence (3 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (2 studies, Tier 3)
No association between the timing of introduction of CFs (< vs > 3 months) and the odds of developing LRTI was observed in the studies by Wright et al. (2004) and by Morgan et al. (2004) (Annex A as Microsoft Excel® file).
Retrospective studies (1 study, Tier 3)
In a cross‐sectional analysis of baseline data of the Millennium Cohort Study (Quigley et al., 2009), the authors estimated the monthly risk of hospitalisation for LRTI between birth and 8 months depending on whether or not CFs had been introduced in that month (Annex A as Microsoft Excel® file). They did not find an association between introduction to CFs and the odds of the outcome.
The Panel notes that the results of the studies in the supportive line of evidence (3 studies) are consistent with the main line.
12.6. Infections in general: summary of the evidence
Main line of evidence (1 study, Tier 1)
One prospective cohort study (Størdal et al., 2017) performed in Norway in a large population of 57,007 partially breastfed infants was available: the timing of introduction of CFs was unrelated to the risk of hospitalisation for infections in the time period 0–18 months (Annex A as Microsoft Excel® file).
The Panel notes that there is no evidence for an association between the timing of introduction of CFs and hospitalisation for infections in the study (Tier 1) in the main line of evidence from a large cohort.
Supportive line of evidence (1 study, Tier 3)
A prospective cohort study (Heinig et al., 1993), performed in a group of exclusively breastfed infants and in another group of exclusively formula fed infants, did not show statistically significant differences in the incidence of infections between infants introduced to CFs < and > 6 months of age in either group. No point estimate was provided. Infections in this study were defined as respiratory illness, diarrhoea, otitis media, unexplained fevers, vomiting, chicken pox and other non‐respiratory and presumably viral infections.
The Panel notes that the findings of the study in the supportive line of evidence are consistent with the main line of evidence. This study investigated the incidence of infections in general, but not related to hospitalisation.
12.7. Infections: conclusions and grading of the confidence in the evidence
An overview of the considerations made with respect to the grading of the confidence in the evidence is given in Table 8. The lines of evidence are divided into:
Table 8.
Grading of the confidence in the evidence for infections and timing of introduction of CFs
| Certainty assessment | Characteristics | Effect in the LoE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Line of evidence and initial rating | No studies | Design | RoB | Inconsistency | Generalisability | Imprecision | Magnitude | Dose response | Other | Comparator, population | Early CF | Late CF | Age | Relative | Certainty in the LoE | Certainty across LoE |
| Gastrointestinal infections | ||||||||||||||||
|
1 (main‐A) ++++ |
3 | RCT | ↔ | ↓ | ↓d | o | ↔ | o | ↔ | EBF | 3‐4 m | 6 m | 4–6 m | No evidence for increased risk | ++ |
++ (derived from RCTs) |
|
2 (main‐B) +++ |
1 | PC | ↔ | o | ↔ | ↔ | ↔ | o | ↔ | Mixed | < 3 m | > 3 m | 4–6 m | No evidence for effect | +++ | |
|
3 (S‐A) +++ |
4 | PC, CSa | ↓ | ↓ | ↔ | ↔ | ↔ | o | ↔ | Mixed | n/a | n/a | infancy | No evidence for effect | + | |
| Upper respiratory tract infections | ||||||||||||||||
|
1 (main‐A) ++++ |
3 | RCT | n/a | n/a | n/a | n/a | n/a | n/a | n/a | EBF | 3–4 m | 6 m | 4–6 m | Inconsistent | n/a | |
|
2 (main‐B) +++ |
1 | PC | n/a | n/a | n/a | n/a | n/a | n/a | n/a | Mixed | < 3 m | > 3 m | 4–6 m | Inconsistentb | n/a | |
| Lower respiratory tract infections | ||||||||||||||||
|
1 (main‐A) ++++ |
1 | RCT | ↔ | o | ↓d | o | ↔ | o | ↓c | EBF | 3–4 m | 6 m | 4–6 m | No evidence for effect | ++ | ++ |
|
2 (S‐A) +++ |
2 | PC | ↓ | ↔ | ↔ | ↔ | ↔ | o | ↔ | Mixed | < 3 m | > 3 m | infancy | No evidence for effect | ++ | |
| Undefined infections | ||||||||||||||||
|
1 (main‐A) ++++ |
1 | RCT | ↔ | o | ↓d | o | ↔ | o | ↔ | EBF | 3–4 m | 6 m | 4–6 m | No evidence for effect | +++ | +++ |
|
2 (main‐B) +++ |
1 | PC | ↔ | o | ↔ | ↔ | ↔ | o | ↔ | PBF | < 6 m | > 6 m | 0–18 m | No evidence for effect | +++ | |
|
3 (S‐A) +++ |
1 | PC | ↓ | o | ↓d | ↔ | ↔ | o | ↔ | EBF | < 6 m | > 6 m | 4–6 m | No evidence for effect | + | |
CS: cross‐sectional; CF: complementary food; EBF: exclusively breastfed; LoE: line of evidence; m: months; n/a: not applicable; PBF: partially breastfed; PC: prospective cohort; RCT: randomised controlled trial; RoB: risk of bias; ↓: downgrade; ↑: upgrade; ↔: no concern/impact; o: not evaluable.
(a) Baseline data of the Millennium Cohort Study. (b): Only one time period out of many. (c): Limited evidence in the main line of evidence. (d): Studied on exclusively breastfed infants only.
Main‐A: RCTs
Main‐B: prospective cohort studies
S‐A: supportive line of evidence.
As the evidence in relation to URTI was inconsistent, the confidence in the evidence was not graded for this outcome.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from the three RCTs and the one prospective cohort study (Tiers 1 and 2) that there is no evidence for an effect or association between the introduction of CFs, ranging from < 3 months to 3–4 months compared with > 3 months and 6 months of age, and an increased risk of gastrointestinal infections (low level of confidence in the evidence).
The Panel concludes from one RCT (Tier 2) that there is no evidence for an effect of the introduction of CFs at 3–4 months compared with 6 months of age on LRTI (low level of confidence in the evidence).
The Panel concludes from one large prospective cohort study (Tier 1) that there is no evidence for an association between the odds of developing infections (in general) and the timing of introduction of CFs, ranging from 3–4 months to < 6 months compared with 6 months of age and thereafter (moderate level of confidence).
The Panel concludes that the evidence with respect to URTI is inconsistent.
The conclusions apply to settings with satisfactory hygiene conditions.38
13. Assessment of the data on sleep‐related endpoints in individuals born at term or mixed populations
13.1. Sleep‐related endpoints: final body of evidence
The five publications that were considered in the assessment of sleep‐related endpoints in individuals born at term or mixed populations are given in Appendix B.10.
These publications reported on results from five studies:
2 RCTs (all Tier 2);
2 prospective cohort studies and 1 pooled analysis of prospective studies (all Tier 3).
In these studies, five different endpoints were investigated. Results of all the studies are given in Annex A as Microsoft Excel® file.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
13.2. Sleep‐related endpoints: endpoint selection
Included studies covered a variety of endpoints that are not directly comparable, i.e. night‐time sleep duration, sleep time, 24‐h sleep duration, night wakings and sleep problems. Thus, the findings were not represented in a forest plot and no pooled estimate was calculated.
13.3. Sleep‐related endpoints: summary of the evidence
Main line of evidence (2 studies)
The RCT by Perkin et al. (2018) found a statistically significant effect of introduction of CFs at 3–4 months of age compared with 6 months of age on night time sleep duration assessed by a validated questionnaire (to assess sleep over the past week) in exclusively breastfed infants. Infants introduced to CFs at 3–4 months of age slept on average about 7 min longer during the night (95% CI: 2 to about 13 min) over the whole course of the study from birth to 3 years of age (adjusted FAS analysis). A peak was observed at 6 months of age at which infants introduced to CFs at 3–4 months of age slept on average about 17 min longer during the night (95% CI: about 8 to 25 min) than their counterparts introduced to CFs at 6 months of age.
In the same RCT, infants introduced to CFs at 3–4 months of age were also reported to have fewer night wakings (mean % difference: −9.1 (95% CI −4 to −14%), adjusted complete case analysis). The infants introduced to CFs at 3–4 months of age also showed lower odds of both ‘very serious’ and ‘small’ sleep problems (as perceived by the parents when answering the question ‘do you consider your child's sleep as a problem?’), in comparison to infants introduced to CFs at 6 months of age (OR 0.83 (95% CI 0.71 to 0.95) and 0.55 (0.38–0.82), respectively, in an unadjusted FAS analyses).
The Panel notes that the observed differences in sleep duration or night wakings in the RCT by Perkin et al. (2016) were small in relation to an overall night time sleep duration of around 10–11 h at 6 months of age (Dias et al., 2018) and that the severity of sleep problems was based on the perception of the parents. The Panel considers that the results on these three endpoints are unlikely to be of biological relevance.
The RCT by Bainbridge et al. (1996), in which rice cereal was added to formula in the bottle in exclusively formula fed infants at 4 months vs 6 months of age, showed that infants receiving the rice cereal at 4 months slept on average 60 min longer during the night at 6 months of age (95% CI: −34 to 154 min). This was assessed as the time that had passed between the last bottle at night and the first in the morning. The result was not statistically significant. However, the study population included 38 infants only and thus the trial was most likely underpowered for this outcome. Therefore, the Panel did not consider further the non‐statistically significant findings of this study.
The Panel notes that from the RCT (Tier 2) from which conclusions could be drawn in the main line of evidence that even though the statistical analyses of the effect of the age of introduction of CFs (3–4 vs 6 months) on sleep‐related endpoints was significant, the size of the effect was not biologically relevant.
Supportive line of evidence
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Prospective cohort studies (3 studies, Tier 3)
The prospective studies showed:
-
−
either a longer night sleep duration (Morgan et al., 2004), i.e. on average 12 min at 9 and 18 months for those introduced to CFs at ≤ 3 vs > 3 months of age;
-
−
or a shorter 24‐h sleep duration (Nevarez et al., 2010), i.e. on average about 24 min at 1 year and 13 min at 2 years for those introduced to CFs at ≤ 4 vs > 4 months of age;
-
−
or no association between the timing of introduction of CFs and 24‐h sleep duration at 6 months of age (Nevarez et al., 2010) or with sleep time in breastfed infants at 9 months of age (Heinig et al., 1993).
The Panel notes that the findings in the supportive line of evidence are inconsistent.
13.4. Sleep‐related endpoints: conclusions and grading of the confidence in the evidence
The non‐statistically significant results of one of the two RCTs available were not further considered, as this RCT was likely underpowered for the assessment of sleep.
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: There was only one study in the main line of evidence. The results of the studies in the supportive line of evidence were inconsistent within this line. For the decision with respect to downgrading, see ‘other’.
Generalisability: The study population of the remaining RCT consisted of breastfed infants only. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel decided to downgrade the confidence in the evidence derived from this RCT.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Other: The Panel downgraded the confidence in the evidence by one category, because of the limited number of studies that were available and because the results of the single RCT in the main line of evidence were not supported by the findings in the supportive line of evidence.
The Panel concludes from the RCT that even though the statistical analyses of the effect of the age of introduction of CFs (3–4 vs 6 months) on sleep‐related endpoints was significant (low level of confidence in the evidence), the size of the effect is not biologically relevant.
14. Assessment of the data on infant and child development in individuals born at term or mixed populations
14.1. Infant and child development: final body of evidence
The three publications that were considered in the assessment of infant and child development in individuals born at term or mixed populations are given in Appendix B.11.
These publications reported on results of three studies:
1 RCT (Tier 1);
1 prospective cohort study (Tier 3);
1 retrospective study (Tier 3).
In these studies, 11 endpoints were investigated. Results of the studies are given in Annex A as Microsoft Excel® file.
With respect to the interpretation of the age of introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
14.2. Infant and child development: endpoint and study selection
The assessment of this outcome was based on studies that used validated tools for assessing infant and child development on the infant and child itself, i.e. Brigance Screens II by Jonsdottir et al. (2013) and the adapted, but validated, Kaufman Assessment Battery for Children II by Veena et al. (2010). Tools that evaluated parental concerns about the developmental status of their child, i.e. the Parent's Evaluation of Developmental Status (PEDS) (Jonsdottir et al., 2013) were not used.
Studies which only reported on the attainment of individual developmental milestones in months (Heinig et al., 1993; Michels et al., 2017) were not further considered in the assessment owing to the wide biological variability when infants achieve certain milestones (WHO Multicentre Growth Reference Study Group, 2006). Therefore, any potential effect of the timing of introduction of CFs is expected to be lower than the biological variability observed for this outcome.
Endpoints/constructs which were investigated in single studies only, i.e. fine and gross motor skills alone (Jonsdottir et al., 2013) and socio‐emotional skills (Metwally et al., 2016) were not considered further as they cannot be used to determine an appropriate age range of introduction of CFs.
14.3. Infant and child development: summary of the evidence
Main line of evidence (1 study, Tier 1)
In the RCT by (Jonsdottir et al., 2013), there was no effect of introduction of CFs at 4 months of age compared with 6 months of age on the risk of developmental delay at about 3 years of age. Risk of developmental delay was defined based on the age‐appropriate cut‐off value of the total score of the Brigance Preschool Screens II. Skills that were assessed were: personal data response, identification of body parts, gross motor skills, identification of objects, repetition of sentences, visual motor skills, number concepts, building tower with blocks, matching colours, picture vocabulary and using plurals correctly.
The Panel notes from the RCT in the main line of evidence that there is no evidence for an effect of the timing of introduction of CFs on child development.
Supportive line of evidence (1 study, Tier 3)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
The prospective cohort study by Veena et al., 2010, in which the timing of introduction of CFs was analysed as a continuous variable, did not show an association between the timing of introduction of CFs and child development at 9.7 years of age. Child development in this study covered language development, learning ability, memory span, pattern reasoning, language production, visuospatial problem solving and visual‐motor processing speed.
The Panel notes that result of the prospective cohort study in the supportive line of evidence is consistent with the findings in the main line of evidence.
14.4. Infant and child development: conclusions and grading of the confidence in the evidence
Imprecision: There were no concerns with respect to imprecision.
Inconsistency: There was one study in the main line of evidence. The result of the study in the supportive line of evidence was consistent with the main line of evidence.
Generalisability: The study population of the RCT consisted of breastfed infants only. The Panel considers that the results of this study cannot be generalised to formula fed infants and thus to the whole population of infants living in Europe. Therefore, the Panel decided to downgrade by one category the confidence in the evidence derived from the RCT.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
Other: The Panel decided to downgrade by one category the confidence in the evidence, because of the limited evidence that was available.
The Panel concludes from the RCT that there is no evidence for an effect of the timing of introduction of CFs at 4 months vs 6 months of age on child development assessed at 3 years of age (low level of confidence in the evidence).
15. Assessment of the data on indicators of nutrient status in individuals born at term or mixed populations
15.1. Nutrient status: final body of evidence
The seven publications considered in the assessment of nutrient status in individuals born at term or mixed populations are given in Appendix B.12.
These publications reported on results from seven studies:
4 RCTs (1 rated as Tier 1, 3 rated as Tier 2);
2 prospective cohort studies (Tier 2);
1 retrospective study (Tier 3).
Results of all the studies are given in Annex A as Microsoft Excel® file. In addition, results are summarised in the forest plots in Appendix A.48 of this Scientific Opinion.
With respect to the interpretation of the age of introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
15.2. Nutrient status: endpoint and study selection
For the outcome on nutrient status, the Panel decided to limit the assessment to the nutrients which had been considered critical nutrients in infants and young children in Europe in a previous Scientific Opinion (EFSA NDA Panel, 2013), i.e. iron, vitamin D, iodine (in some subpopulations), docosahexaenoic acid (DHA) and alpha‐linolenic acid (ALA). The Panel also considered that vitamin D status is more influenced by vitamin D supplementation (programmes) and sunlight exposure than by the timing of introduction of CFs. Therefore, vitamin D was not considered further.
In addition, to evaluate nutrient status, reliable biomarkers of status of the respective nutrients need to be available. Pertinent studies were those which investigated whether the timing of introduction of CFs influences the proportion of subjects that are below a certain cut‐off for nutrient sufficiency. Studies comparing mean blood concentrations of biomarkers are difficult to interpret and have, therefore, not been considered in this section.
Finally, studies pertinent for this assessment were only available for iron status. All studies in the main line of evidence assessed iron depletion, defined in all the studies as serum ferritin (SF) < 12 μg/L. Therefore, the Panel concentrated in the following on the assessment of iron depletion. The proportion of children with anaemia or iron‐deficiency anaemia will be reported for each study whenever this information is available. Study populations receiving iron supplements in addition to CFs were not considered pertinent for the assessment, as term infants living in Europe are not routinely supplemented with iron. Therefore, the aim of the assessment is to investigate the effect of the timing of introduction of CFs per se and not concomitant with iron supplementation. For considerations of the Panel regarding the inclusion of RCTs performed in Honduras discussed further below, please see also Section 12.2.
Studies or time points in studies in which nutrient status was assessed after the age of 1 year only were not considered in the assessment owing to the implausible association between the timing of introduction of CFs and nutrient status after the complementary feeding period. Within this first year of life, results for the first and second half‐year are discussed separately in the following sections.
15.3. Iron status: summary of the evidence
Main line of evidence (5 studies)
Iron status at 6 months of age in exclusively breastfed infants (3 studies)
Three RCTs (Dewey et al., 1998; Dewey et al., 2004; Jonsdottir et al., 2012) were available that investigated the effect of introduction of CFs at 4 months compared with exclusive breastfeeding up to 6 months of age on iron depletion.
Two RCTs were conducted in Honduras (Dewey et al., 1998; Dewey et al., 2004) and their study population consisted of term infants that were SGA (Dewey et al., 2004) and a mixture of term infants born SGA and AGA (Dewey et al., 1998). In these two studies, infants consumed commercial baby foods provided by the investigators. Iron depletion occurred in around 23% of SGA infants introduced to CFs at 6 months of age versus around 8% of those introduced at 4 months in the study by Dewey et al. (2004). Hb concentrations < 100 g/L were observed in 21% and 2% of these infants, respectively. In the population of term infants that were a mixture of SGA and AGA, iron depletion occurred in 16% of infants introduced to CFs at 6 months of age compared with 7% of those introduced at 4 months of age (Dewey et al., 1998). Hb concentrations < 110 g/L and < 103 g/L were observed in 66% and 32% of infants introduced to CFs at 6 months of age and in 55% and 25% of infants introduced to CFs at 4 months of age.
The third RCT that was available was conducted in Iceland on healthy term infants (Jonsdottir et al., 2012). In this study, 10% of infants exclusively breastfed for 6 months were iron depleted at 6 months of age, versus 4% of those introduced to CFs at 4 months of age. Iron‐deficiency, defined by the authors as mean corpuscular volume (MCV) 74 fl and SF < 12 μg/L, occurred in 8% vs 4% of infants and iron‐deficiency anaemia (defined by the authors as Hb < 105 g/L, MCV 74 fl and SF < 12 μg/L) in 2% of infants in each group. However, this study was performed before recommendations for delaying umbilical cord clamping (that increases iron stores of the infant at birth, see Section 1.4.1.2) were routinely implemented in Iceland (Thorsdottir, personal communication).
Individually, these studies did not show a statistically significant effect of the timing of introduction of CFs on iron depletion. However, in the meta‐analysis (Appendix A.48), a statistically significant reduction of risk of iron depletion at 6 months of age was observed in the group of exclusively breastfed infants who had been introduced to CFs at 4 months of age, i.e. RR 0.38 (95% CI: 0.18 to 0.80).39 The 95% prediction interval crossed the line of the ‘null’ effect. Heterogeneity was not important (I2 = 0%). The Panel notes that the study population of the three RCTs consisted of infants who were to some degree at risk of iron depletion, either because they were SGA or because delayed umbilical cord clamping was not routine practice or both.
The Panel notes, from three RCTs (Tiers 1 and 2) in the main line of evidence, that introduction of CFs at 4 months of age compared with 6 months of age in exclusively breastfed infants to some degree at risk of iron depletion reduces the risk of iron depletion at 6 months of age.
Iron status in the second half of the first year of life (between 7 and 12 months of age) (2 studies)
One RCT (Kattelmann et al., 2001) in exclusively formula fed infants did not show an effect of introduction of CFs at 3‐4 months vs 6 months of age on iron depletion (12% and 9% in the early and late introduction groups, respectively) or anaemia (defined by the authors as Hb < 110 g/L; 10.5% vs 11%) at 1 year of age.
The prospective cohort study (Libuda et al., 2016) in a population of breastfed and formula fed infants did not show an association between the timing of introduction of CFs < vs > 5 months of age and iron status at 10 months of age (assessed by the authors using the odds of having a concentration of SF < 12 μg/L and odds of having Hb < 105 g/L and SF < 12 μg/L).
The Panel notes that, from the RCT and the prospective cohort study (both Tier 2) in the main line of evidence, that there is no evidence for an effect or association between the timing of introduction of CFs and iron depletion at 10‐12 months of age in formula fed infants and infants with a variety of background ‘milk’ feedings.
Supportive line of evidence (2 studies): iron status in the second half of the first year of life (between 7 and 12 months of age)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Retrospective studies (1 study, Tier 3)
The cross‐sectional study by Hong et al. (2017), in a population of infants breastfed or formula fed, showed lower odds of iron‐deficiency (defined by the authors as SF < 12 μg/L, MCV < 70 fl and transferrin saturation (TfS) < 10%) at around 12 months of age in those infants introduced to CFs before 6 months of age compared with thereafter (OR 0.49 (95% CI 0.33 to 0.72)) (Annex A as Microsoft Excel® file). This analysis was unadjusted and therefore is likely to overestimate the association.
Studies in which the timing of introduction of CFs was used as a continuous variable in the analysis, irrespective of the study design (1 study, Tier 2)
The prospective cohort study by Meinzen‐Derr et al. (2006) in breastfed infants did not show an association between introduction of CFs and iron status at 9 months of age (assessed by the authors as the odds of Hb < 100 g/L) (Annex A as Microsoft Excel® file).
The Panel notes that the results of studies in the supportive line of evidence are not directly comparable, because different biomarkers have been used. Therefore, they cannot be evaluated with respect to their consistency with the main line of evidence.
15.4. Iron status: conclusions and grading of the confidence in the evidence
Magnitude of the effect and imprecision: The magnitude of the effect was large (see Section 2.2.3.3). There were no concerns with respect to imprecision. For the meta‐analysis of the three RCTs in exclusively breastfed infants that assessed the risk of developing iron depletion at 6 months of age, the 95% prediction interval crossed the line of ‘null’ effect. However, given the uncertainty that is associated with the estimation of the prediction interval when only three studies are available, the Panel decided that this finding was not sufficient to downgrade the confidence in the evidence.
Inconsistency: There was no inconsistency in the main line of evidence for studies that assessed the risk of developing iron depletion at 6 months of age (exclusively breastfed infants) and those in the second half of the first year of life (7–12 months of age) (formula fed and mixed fed infants). Studies in the supportive line of evidence cannot be interpreted with respect to their consistency as different biomarkers were used.
Generalisability: All RCTs that investigated iron status at 6 months of age were conducted in exclusively breastfed infants who were to some degree at risk of iron depletion, i.e. because infants were SGA, or because delayed umbilical cord clamping was not routine practice or both. The Panel considers that the results of the meta‐analysis on these studies can be generalised to the whole population of infants at risk of iron depletion living in Europe (i.e. exclusively breastfed infants born to mothers with a low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born SGA, or with a high growth velocity). They cannot be used to establish an effect of the timing of introduction of CFs on iron status in formula fed infants.
Similarly, results obtained in exclusively formula fed or mixed fed populations cannot be used to establish an effect on iron status in exclusively breastfed infants. Therefore, the two studies investigating iron status in the second half of the first year of life (between 7 and 12 months of age) that were performed in formula fed infants or infants with a variety of background milk feedings cannot be used to draw conclusions on how the risk of iron depletion in exclusively breastfed infants evolves after the age of 6 months.
Publication bias: Publication bias could not be evaluated, because of the insufficient number of studies available.
The Panel concludes from three RCTs (Tiers 1 and 2) that introduction of CFs at 4 months of age compared with introduction at 6 months of age reduces the risk of iron depletion (SF < 12 μg/L) at 6 months of age in exclusively breastfed infants at risk of iron depletion (high level of confidence in the evidence).
The Panel notes that the effect on iron depletion is not an effect of introduction of CFs per se, but an effect of introduction of CFs that are a source of iron. Infants that may benefit from an early introduction of CFs that are a source of iron are exclusively breastfed infants born to mothers with a low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born SGA, or with a high growth velocity. The Panel also notes that iron depletion is a risk factor for iron‐deficiency anaemia which is associated with deleterious effects (e.g. delayed attention, poor recognition memory, long‐lasting poor cognitive and behavioural performance) (Section 1.4.2).
The Panel concludes, from one RCT and one prospective cohort study (both Tier 2), that there is no evidence for an effect or association between the timing of introduction of CFs (at 3–4 months of age and < 5 months of age vs 6 months and >5 months of age, respectively) and iron depletion at 10‐12 months of age in formula and mixed fed infants (high level of confidence in the evidence).
16. Assessment of the data on food preferences and eating behaviours in individuals born at term or mixed populations
16.1. Food preferences and eating behaviours: final body of evidence
The 17 publications considered in the assessment of food preferences and eating behaviours in individuals born at term or mixed populations are given in Appendix B.13. These included two publications that reported on the same four studies (de Lauzon‐Guillain et al., 2013; Jones et al., 2015).
These publications reported on results from 18 studies:
1 RCT (Tier 3);
13 prospective cohort studies (2 rated as Tier 1, 8 rated as Tier 2 and 4 rated as Tier 3; 1 study was allocated two different Tiers depending on the endpoint that was assessed);
4 retrospective studies (all Tier 3).
In these studies, 19 different endpoints were investigated. Results of the studies are given in Annex A as Microsoft Excel® file.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
16.2. Food preferences and eating behaviours: endpoint and study selection
A variety of endpoints was investigated in the included studies, which were not directly comparable. Thus, the results could not be represented in forest plots. However, studies that investigated similar concepts of eating behaviours were clustered together, i.e.:
Food approach,40 covering the following endpoints that were assessed in the studies: food responsiveness41 and enjoyment of food.
Food avoidance,42 covering the following endpoints that were assessed in the studies: satiety responsiveness, slowness in eating and food fussiness. The latter covers also the following related endpoints: composite food acceptance scores, picky eating behaviour, acceptance of new foods, food neophobia and feeding difficulties.
Eating patterns, covering the following endpoints that were assessed in the studies: positive eating pattern, food diversity of ‘healthy’ foods, number of food groups consumed per day and proportion of daily energy intake from ultra‐processed foods.
Vegetable and fruit intake assessed either in absolute terms or as frequency of their consumption or as odds of consuming less than one serving per day.
The following endpoints were considered in single studies only and were not considered further: ‘food intake at the midday meal’ (Cohen et al., 1995b).
In the following Sections, the timing of introduction of CFs (in general) is discussed first followed by the timing of introduction of vegetables and fruit.
16.3. Food preferences and eating behaviours: summary of evidence
16.3.1. Timing of introduction of CFs in general
Main line of evidence (4 studies)
Measures of food approach
In the prospective cohort study by Möller et al. (2013), no association between the timing of introduction of CFs < 4 months of age compared with 6 months of age and food responsiveness and enjoyment of food assessed at 5 years of age was observed (Annex A as Microsoft Excel® file).
Measures of food avoidance
In two prospective cohort studies, no association between the introduction of CFs < 4 months of age as compared with introduction at 6 months of age or thereafter on food fussiness (de Barse et al., 2017) and slowness in eating (Möller et al., 2013) assessed at 4 and 5 years of age, respectively, was observed (Annex A as Microsoft Excel® file).
Möller et al. (2013) found a lower satiety responsiveness at 5 years of age to be associated with introduction of CFs < 4 months of age as compared with introduction at 6 months. Hollis et al. (2016), in a prospective cohort study, reported higher feeding difficulties (undefined) to be associated with introduction of CFs at 4–6 months of age compared with ≥ 6 months of age.
Eating patterns
In the prospective cohort study by Abraham et al. (2012), 1.5‐ to 2‐year‐old children introduced to CFs < 3 months compared with those introduced at 4–5 months and compared with those introduced > 6 months were less likely to show a positive eating pattern (Annex A as Microsoft Excel® file).
Vegetable and fruit intake
Introduction of CFs < 4 months compared with 6 months of age was associated with a statistically significantly higher fruit intake (on average 16 g difference), but not vegetable intake, at 5 years of age (Möller et al., 2013). The Panel notes that the difference in fruit intake observed at 5 years of age (i.e. 16 g/day) is small and unlikely to be of biological relevance.
The Panel notes from the four prospective cohort studies (Tier 2) in the main line of evidence that introduction of CFs below 3 or 4 months of age compared with 6 months of age and thereafter is associated with some less desirable eating behaviours (i.e. lower satiety responsiveness, higher feeding difficulties and lower likelihood to have a positive eating pattern), while other, potentially also less desirable,43 eating behaviours (i.e. food responsiveness, enjoyment of food, food fussiness and slowness in eating) are not associated with the timing of introduction of CFs.
The Panel notes from the one prospective cohort study (Tier 2) in the main line of evidence that even though introduction of CFs < 3 months of age (compared with 4–5 and > 6 months of age) was statistically significantly associated with a higher fruit intake at 5 years of age, the observed difference is not biologically relevant. The Panel also notes from this study that there is no association between the timing of introduction of CFs and vegetable intake.
Supportive line of evidence (7 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Measures of food approach
In the prospective cohort study (Tier 1) that used the timing of introduction of CFs as a continuous variable in the analysis (Brown and Lee, 2012) earlier introduction of CFs was not associated with food responsiveness at 1.5–2 years of age.
Measures of food avoidance
There was no association between the timing of introduction of CFs with food fussiness (defined in different ways, see Section 16.2) in the following studies:
-
−
the RCT (Tier 3) by Cohen et al. (1995b) at 9 and 12 months of age comparing CF introduction at 4 with 6 months of age;
-
−
the prospective cohort study (Tier 3) by Emmett et al. (2018) at 3 years of age comparing CF introduction < 3 with > 5 months of age;
-
−
the prospective cohort study (Tier 2) that used the timing of introduction of CFs as continuous variable in the analysis (Lange et al., 2013). In this study, the outcome was assessed two months after each infant had started complementary feeding;
-
−
the cross‐sectional study (Tier 3) by Bell LK et al. (2018) at 2–3 years of age comparing CF introduction < 3 with 4–5 and ≥ 6 months of age (Annex A as Microsoft Excel® file).
The only study that found an association between the introduction of CFs and food fussiness was the cross‐sectional analysis of baseline data of a prospective cohort study by Shim et al. (2011), in which the introduction of CFs < 6 months compared with thereafter was associated with higher odds of developing food fussiness (in particular of not readily accepting new foods and consuming a limited variety of foods) at 2–3 years of age.
With respect to satiety responsiveness, the prospective cohort study (Tier 1) that used the timing of introduction of CFs as continuous variable in the analysis (Brown and Lee, 2012) found that earlier introduction of CFs was associated with a lower satiety responsiveness at 1.5–2 years of age.
Eating patterns
A prospective cohort study (Tier 3) (Bielemann et al., 2018) found that the proportion of daily energy intake from ultra‐processed foods was higher in around 7‐year‐old children when they had been introduced to CFs < 3 months of age as compared with thereafter (Annex A as Microsoft Excel® file).
Vegetable and fruit intake
In the cross‐sectional analysis of baseline data of a prospective cohort study (Tier 3), Okubo et al. (2016) did not observe an association between the introduction of CFs < 5 months of age compared with thereafter on the frequency of vegetable and fruit consumption at 1.5–2 years of age.
The Panel notes that consistent with the main line of evidence, in the supportive line of evidence, there is no association between the timing of introduction of CFs and food responsiveness, and the frequency of vegetable and fruit consumption. This is also true for four of the five studies that investigated food fussiness; one study found introduction of CFs < 6 months of age to be associated with higher odds of developing food fussiness. Finally, the study that assessed eating patterns found in line with the main line of evidence a less favourable eating pattern (i.e. a higher proportion of daily energy intake from ultra‐processed foods) to be associated with introduction of CFs < 3 months of age compared with thereafter.
16.3.2. Timing of introduction of vegetables and fruit
Main line of evidence (5 studies, Tiers 1 and 2)
Measures of food avoidance
The prospective cohort study by de Barse et al. (2017) found lower food fussiness at 4 years of age to be associated with introduction of vegetables at 4–5 months of age compared with ≥ 6 months. A similar point estimate was found for the comparison of introduction of CFs < 4 months of age with ≥ 6 months, but this comparison did not reach statistical significance. The Panel notes that this last comparison may, however, have been underpowered to detect significant differences. Timing of introduction of fruit was not associated with food fussiness in this study.
Eating patterns
Jones et al. (2015) reported on two prospective cohort studies rated as Tier 2 (i.e. ALSPAC and EDEN). For the ALSPAC study, vegetable introduction at 4–5 months of age compared with ≥ 6 months of age was associated with higher ‘healthy plate variety scores’ at 4 years of age (adjusted mean difference 0.09 (95% CI 0.04 to 0.14)). The Panel notes that considering that the ‘healthy plate variety scores’ used in this publication had a maximum score of 5, the observed differences are unlikely to be of biological relevance. In the EDEN study, in which the latest age at outcome assessment was 3 years, no association between the timing of introduction of vegetables and the ‘healthy plate variety score’ was observed. Also, there was no association between the timing of introduction of fruit and the ‘healthy plate variety score’.
Vegetable and fruit intake
In four prospective cohort studies reported in three papers ((Burnier et al., 2011; Grimm et al., 2014) and de Lauzon‐Guillain et al. (2013) for the studies ALSPAC and EDEN), there was no association between the timing of introduction of vegetables, comparing various ages of introduction, and the frequency of vegetable consumption. The latest age at outcome assessment was 13 years.
In three of these studies ((Grimm et al., 2014) and de Lauzon‐Guillain et al. (2013) for the studies ALSPAC and EDEN), the timing of introduction of fruit was investigated. There were no statistically significant associations between the timing of introduction of fruit and the frequency of fruit consumption.
The Panel notes from one prospective cohort study (Tier 2) in the main line of evidence that there is an association between the timing of introduction of vegetables, but not fruit, < 6 months of age compared with thereafter and a lower food fussiness at 4 years of age.
The Panel also notes that in the main line of evidence there is no biologically relevant association between the timing of introduction of vegetables and fruit and eating patterns (2 prospective cohort studies, Tier 2) and the frequency of consumption of vegetables and fruit (4 and 3 prospective cohort studies, respectively, Tiers 1 and 2).
Supportive line of evidence (4 studies)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
Measures of food approach
In the prospective cohort study in which the timing of introduction of CFs was used as a continuous variable in the analysis (Lange et al., 2013) (Tier 2), earlier introduction of vegetables was associated with a lower food fussiness as indicated by a higher acceptance of new vegetables, but earlier introduction of fruit was not related to a higher acceptance of new fruit. The outcome was assessed two months after each infant had been introduced to the food groups.
Eating patterns
Jones et al. (2015) reported for the prospective cohort studies rated as Tier 3 (i.e. Generation XXI and Greek EuroPrevall) that there was no association between the timing of introduction of vegetables or fruit at 4–5 months compared with 5–6 and >6 months of age and ‘healthy plate variety scores’ at 4 and 3 years of age, respectively.
Vegetable and fruit intake
In the studies Generation XXI and Greek EuroPrevall (Tier 3) reported in de Lauzon‐Guillain et al. (2013), there was no association between the timing of introduction of vegetables or fruit (< 5 months compared with 5–6 and > 6 months) and the frequency of vegetable and fruit consumption at 4 and 2 years, respectively.
In the cross‐sectional study by Cooke et al. (2004) in which the timing of introduction of CFs was used as a continuous variable in the analysis, it was reported that earlier fruit introduction was associated with a higher frequency of fruit consumption at 2–6 years of age. No association was found for vegetables.
The Panel notes that the results with respect to food fussiness (i.e. earlier introduction of vegetables associated with lower food fussiness, no association for fruit) are consistent with the findings in main line of evidence, as are the results with respect to eating patterns. The results related to the association between the introduction of vegetables and fruit and the frequency of vegetable and fruit consumption are inconsistent within the supportive line of evidence.
16.4. Food preferences and eating behaviours: conclusions
The Panel considers that the evidence in relation to the outcome on food preference and eating behaviours is inconsistent. Therefore, the confidence in the evidence was not graded.
17. Assessment of the data on other health outcomes
Other outcomes that were investigated in the studies retrieved in the systematic review were non‐alcoholic fatty liver disease (Ayonrinde et al., 2017), juvenile idiopathic arthritis (Ellis et al., 2012; Kindgren et al., 2017) (investigating the timing of introduction of CFs and gluten, respectively), dental caries (Tanaka et al., 2013), thyroid disease (Fort et al., 1990) and Crohn's disease and ulcerative colitis (Strisciuglio et al., 2017). These outcomes were investigated in single studies only. Therefore, they were not considered further.
18. Assessment of the data on the timing of introduction of CFs in individuals born preterm
18.1. Developmental readiness of the preterm infant to consume CFs
Available developmental data on the introduction of CFs in term infants (Section 3) cannot be directly translated to preterm infants. These represent a more heterogeneous population than infants born at term as they vary in the stage of development at birth (gestational age ranging from 23 to 36 weeks), their postnatal course (illness, nutrient intake and mode/type of feeding) and sequelae. All these factors may influence their developmental readiness to consume CFs, and also their nutritional requirements.
18.1.1. Gastrointestinal function
The human gut is anatomically mature by around 25 weeks gestation and enteral feeding appears to play a part in triggering the maturation of gastric and pancreatic enzymes (WHO, 1989) and gut motility (Berseth, 1992).
Intestinal permeability, a biomarker of the gastrointestinal barrier function, measured using sugar absorption tests in 116 preterm infants (gestational age 26–36 weeks) and 16 term infants was not related to gestational age or birthweight but was higher during the first two days of life than 3–6 days later (van Elburg et al., 2003). It was higher in preterm infants than in healthy term infants only if measured within two days of birth, suggesting rapid postnatal adaptation of the small intestine in preterm infants.
The Panel considers that the available data suggest that gastrointestinal function is not a limiting factor with respect to the timing of introduction of CFs once the preterm infant has the necessary neuromotor skills and the infant has developed an apparent interest in non‐milk foods and feeding.
18.1.2. Renal function
While renal glomerular and tubular function are influenced by gestational age, there is a high capacity for postnatal maturation, regardless of the degree of prematurity (Gubhaju et al., 2014).
D'Souza et al. (1985) measured serum electrolytes and osmolality at term equivalent and 4 months post‐term in 50 infants born at 28–32 weeks gestation with birthweight < 1,501 g. All infants received formula, and 26 infants received CFs between 2 weeks and 4 months post‐term. There were no significant differences in serum electrolytes or serum osmolality between those receiving formula alone or formula with CFs, suggesting that renal function was sufficient to process the protein load imposed by CFs combined with formula.
The Panel considers that the available data suggest that renal function is not a limiting factor with respect to the timing of introduction of CFs once the preterm infant has the necessary neuromotor skills and the infant has developed an apparent interest in non‐milk foods and feeding.
18.1.3. Neuromuscular coordination and neurodevelopment
The skills necessary for an infant to coordinate efficient suckling, swallowing and respiration, which are essential for safe oral milk feeding, start to appear from around 32 to 34 weeks of gestation (Mizuno and Ueda, 2003), but this will vary depending on the degree of prematurity and related illness.
A prospective study (Törölä et al., 2012), comparing the feeding development (assessed by video recording in regular intervals from 37 weeks post‐conceptional age onwards until chewing skills appeared) of 19 preterm infants (birthweight 670–1,020 g, gestational age ranging from 23 to 30 weeks) and 11 term infants, concluded that most of the preterm infants showed a disorganised sucking pattern as long as suckling was present, and still used the sucking pattern for 1–3 months following introduction of CFs before they started to munch. Therefore, preterm infants were considered to have more feeding problems than term infants, as feeding was prolonged and messy. However, these infants were introduced to CFs considerably earlier than term infants (1–4 months post‐term age vs 4–7 months).
Emerging chewing skills (i.e. lateral and diagonal jaw movements and lateral tongue movements), emerged at 2.5–7 months post‐term age in preterm infants. Chewing skills (i.e. diagonal rotatory and circular rotatory jaw movements) appeared at 5–10 months post‐term age. There were no statistically significant differences in the attainment of these skills in preterm and term infants. However, munching was learned earlier in preterm infants (median 3 months post‐term age (range 1.5–5) vs 5 months (4–8) in the term infants).
Also, the age at which preterm infants attain gross motor developmental milestones may be delayed compared to term infants, even when accounting for the degree of prematurity (van Haastert et al., 2006). This is also likely to vary according to the severity of illness experienced during the neonatal period and any sequelae.
These findings suggest that there is a wide range of ages at which preterm infants develop the necessary neuromotor skills for progressing from a liquid to a solid diet.
The Panel considers that the available data do not suggest a precise age at which CFs should be introduced to preterm infants from the perspective of neuromuscular development. The skills necessary to consume small amounts of pureed foods will differ from those required to consume more textured, lumpy or finger foods. It has been suggested that, as a guide, most preterm infants may be developmentally ready to receive pureed CFs at 3 months (13 weeks, post‐term), having gained sufficient head control (that is a prerequisite for improved jaw control, see Section 3). However, this must be adapted for individual infants (Palmer and Makrides, 2012).
18.2. Preterm infants: final body of evidence
The four publications considered in the assessment of outcomes in preterm infants are given in Appendix B.15.
These publications reported results from four studies:
1 RCT (Tier 1);
1 prospective cohort study and 1 pooled analysis of prospective studies (both Tier 3);
1 retrospective study (Tier 3).
Results of the studies are given in Annex A as an Microsoft Excel® file. Available data was insufficient to present in forest plots.
With respect to the interpretation of the age at introduction of CFs as reported in the following, please refer to Section 2.2.3.3.
18.3. Preterm infants: endpoint selection
Endpoints already investigated in individuals born at term or mixed populations were considered for preterm infants (Appendix B.15). Results for infections (diarrhoea, LRTI and hospital admission) reported in Gupta et al. (2017) were not considered as this study was conducted in a lower‐middle‐income country (according to the criterion explained in Section 2.1.1.2). There was insufficient data on endpoints investigated in preterm infants to create forest plots. Owing to the low number of studies available for the assessment, all studies were evaluated, even if an outcome was addressed in a single study only. Contrary to term infants, study populations of preterm infants receiving iron supplementation were considered pertinent for the endpoint ‘iron status’, as recommendations in Europe exist to provide preterm infants with supplemental iron from 2 to 6 weeks of life onwards (Agostoni et al., 2010).
BMC measurements not adjusted for bone area were not considered as an outcome for this assessment, owing to the lack of comparability in growing children that are of different size.
The four studies considered were conducted in preterm infants with the following gestational ages and birthweights:
-
−
The RCT by Gupta et al. (2017) included infants < 34 weeks of gestation, of which the average gestational age was 31.7 (SD 1.4) weeks and the birthweight 1,479 (SD 308) g among infants in the early introduction group (4 months) and 31.5 (SD 1.7) weeks and 1492 (SD 344) g among infants in the late introduction group (6 months). In the two groups, 28.4% and 27.4%, respectively, were SGA.
-
−
The pooled analysis by Morgan et al. (2004) included infants < 37 weeks of gestation with a birthweight < 2,000 g.
-
−
The prospective cohort study by Spiegler et al. (2015) included infants at 22 + 0 to 36 + 6 weeks of gestation with a birthweight < 1,500 g.
-
−
The retrospective study by Yrjänä et al. (2018) included infants born below 37 weeks of gestation with a birthweight between 440 and 4,915 g.
All time points that are reported in the following subsections are post‐term ages of infants.
18.4. Preterm infants: summary of the evidence
18.4.1. Body weight, body length/height, head circumference, and BMI‐related endpoints
Main line of evidence (1 study, Tier 1)
In the RCT (Gupta et al., 2017), there was no statistically significant difference in WAZ, attained body weight, L(H)AZ, attained body length, HCZ, attained HC, BMIZ and attained BMI at 12 months of age comparing an introduction of CFs at 4 months of age vs introduction at 6 months (Annex A as Microsoft Excel® file).
Supportive line of evidence (2 studies, Tier 3)
The reasoning behind the use of the data comprised in the supportive line of evidence is explained in Section 2.2.3.3.
The results of the pooled analysis of prospective studies by Morgan et al. (2004) did not find a significant association between the timing of introduction of CFs and attained body weight and attained body length at 18 months of age and body weight gain, body length gain and HC gain between 3 and 9 months of age, comparing introduction of CFs before with after 3 months of age (Annex A as Microsoft Excel® file).
The other prospective cohort study by Spiegler et al. (2015) used the age of introduction of CFs as a continuous variable in the analysis and found earlier introduction of CFs to be significantly associated with higher body weight (WAZ or attained body weight) and height (L(H)AZ or attained height) at 2 years of age.
18.4.2. Body composition
Main line of evidence (1 study, Tier 1)
Gupta et al. (2017) did not find an effect of the timing of introduction of CFs (at 4 months vs 6 months of age) on fat mass endpoints, i.e. fat mass and % fat mass, and on lean mass endpoints (i.e. lean mass plus BMC), and on BMD (Annex A as Microsoft Excel® file).
Supportive line of evidence (1 study, Tier 3)
Fat mass endpoints or BMD were not investigated in the supportive line of evidence. In the pooled analysis by Morgan et al. (2004), subscapular SFT gain and triceps SFT gain between 3 and 9 months of age were not associated with the timing of introduction of CFs (comparing introduction of CFs before with after 3 months of age) (Annex A as Microsoft Excel® file).
18.4.3. Atopic diseases (asthma‐like symptoms, eczema and symptomatic food allergy)
No study was available for this outcome in the main line of evidence.
Supportive line of evidence (2 studies, Tier 3)
Both studies that were available assessed atopic‐diseases in children in the general population (i.e. no at‐risk population).
Morgan et al. (2004) found no evidence for an association between the timing of introduction of CFs before vs after 3 months of age and the odds of developing asthma‐like symptoms and eczema up to 18 months of age (Annex A as Microsoft Excel® file).
In a case–control study (Yrjänä et al., 2018), 2‐year‐old children who had developed symptomatic food allergy had been introduced to CFs significantly later compared with controls (median age of 2.3 vs 1.4 months post‐term). This was not the case for children who had developed eczema, compared with controls.
18.4.4. Risk factors for cardiovascular diseases
Main line of evidence (1 study, Tier 1)
No significant difference between complementary feeding groups (i.e. introduced at 4 months vs 6 months) was shown in the RCT by Gupta et al. (2017) in relation to concentrations of total cholesterol, high‐density lipoprotein (HDL)‐, low‐density lipoprotein (LDL)‐ and very‐low‐density lipoprotein (VLDL) cholesterol, and triglycerides, as well as systolic and diastolic blood pressure (Annex A as Microsoft Excel® file).
No study was available for this outcome in the supportive line of evidence.
18.4.5. Infant and child development
Main line of evidence (1 study, Tier 1)
The RCT (Gupta et al., 2017) did not show differences in the risk of delay with respect to motor development or mental development (using the Developmental Assessment Scale for Indian Infants, a validated adaptation of the Bayley‐II scales) between the group introduced to CFs at 4 months of age and the one introduced at 6 months.
18.4.6. Infections
No study was available for this outcome in the main line of evidence.
Supportive line of evidence (1 study, Tier 3)
Morgan et al. (2004) did not find an association between the timing of introduction of CFs before vs after 3 months of age and the odds of developing gastrointestinal infections and LRTI (Annex A as Microsoft Excel® file).
18.4.7. Sleep
No study was available for this outcome in the main line of evidence.
Supportive line of evidence (1 study, Tier 3)
In Morgan et al. (2004), there was no difference in night time sleep duration at 9 and 18 months of age comparing introduction of CFs before with after 3 months of age (Annex A as Microsoft Excel® file).
18.4.8. Nutrient status (iron status)
Main line of evidence (1 study, Tier 1)
The RCT (Gupta et al., 2017) did not show any difference in the odds of developing iron depletion (SF < 12 μg/L), concomitant with iron supplementation, assessed at 12 months of age between the group introduced to CFs at 4 months of age and the one introduced at 6 months (91.4% in the ‘early’ introduction group vs 88.9% in the ‘late’ group were supplemented with iron).
18.5. Timing of introduction of CFs in preterm infants: conclusions
Only one study was available in the main line of evidence. This was an RCT (Tier 1) that was performed in India (Gupta et al., 2017). The study population consisted mostly of infants on vegetarian diets. The mortality rate in the population was high, i.e. 34% died before hospital discharge and another 10% died before the age of 4 months. Surviving infants showed severe growth failure and the prevalence of iron depletion was high, despite the recommended use of iron supplements (Embleton and Fewtrell, 2017). The Panel considers that taken together the generalisability of the findings of this study to the whole population of preterm infants born in Europe is uncertain.
Only for body weight, body length and HC enough studies were available to grade the confidence in the evidence. There was no imprecision in the results of the RCT in the main line of evidence. The results of the studies in the supportive line of evidence were inconsistent. Therefore, the Panel downgraded by one category the confidence in the evidence. As explained above, generalisability of the RCT to the European setting is uncertain. Therefore, the evidence was downgraded by another category.
The Panel concludes from the RCT, that there is no evidence for an effect in preterm infants of introduction of CFs at 4 months of age post‐term compared with 6 months of age post‐term on body weight, body length and HC assessed at 1 year of age (low level of confidence in the evidence).
The Panel considers that no conclusions can be drawn for the other endpoints (i.e. BMI, fat mass, lean mass, SFT, BMD, asthma‐like symptoms, eczema, food allergy, concentrations of blood lipids, blood pressure, infant and child development, infections, sleep and iron depletion) that were assessed in the available studies, because endpoints were either investigated in single studies only or only in studies in the supportive line of evidence.
19. Integration of results
Complementary feeding has been defined, for this assessment, as the period when CFs are given together with either breast milk or formula or both. CFs comprise foods other than breast milk, formula, water or vitamins that are given to infants and can be beverages, spoon‐fed pureed foods, spoon‐fed lumpy foods or finger foods, either prepared at home or produced commercially. Therefore, the definition of complementary feeding in this Scientific Opinion does not include formula (Section 1.3) and the assessment has been made irrespective of whether infants had been breastfed or formula fed (Section 2.1.1). Subgroup analyses on exclusively breastfed or formula fed infants were made, in order to explore if the background milk feeding could have an influence on the results of the studies, but the number of available studies and assessed endpoints in such analyses was limited (Section 2.2.3.2 and Appendix A). The Panel wishes to emphasise that, from a scientific point of view, an assessment of the appropriate age range of introduction of CFs (which is the subject of this mandate) is not an assessment of the optimal duration of exclusive breastfeeding (Section 1.3).
The Panel wishes to clarify that, in this Scientific Opinion, introduction of CFs was defined as ‘early’ or ‘delayed’ when it occurred before or after 6 months of age, respectively. The rationale for this was that, for most healthy infants born at term to healthy well‐nourished mothers, breast milk alone will provide sufficient nutrients up to 6 months of age (Section 1.4.1). Owing to this definition of ‘early’ introduction of CFs, some studies, including some well‐known RCTs, in particular on the introduction of allergenic foods (e.g. introduction of fish, egg or peanut) and of gluten, that have been used by other bodies in their assessment on a similar topic, did not meet the inclusion criteria for this Scientific Opinion (Section 2.1.1.2).
In the context of complementary feeding, the appropriate age of introduction of CFs is influenced not only by nutritional considerations, but also by effects on health outcomes and by infant development.
As indicated in the interpretation of the Terms of Reference, the questions that the Panel set out to answer were:
Are there any developmental factors relevant for the introduction of CFs, based on an extensive literature review?
Are there any adverse health effects associated with the introduction of CFs before 6 months of age, based on a systematic literature review?
Are there any benefits associated with the introduction of CFs before 6 months of age, based on a systematic literature review?
In the systematic literature review undertaken for the (health) outcomes investigated, the Panel created forest plots and undertook meta‐analyses (whenever possible), identified main and supportive lines of evidence and graded the confidence in the evidence (Section 2). An overview is presented in Figure 6.
Figure 6.
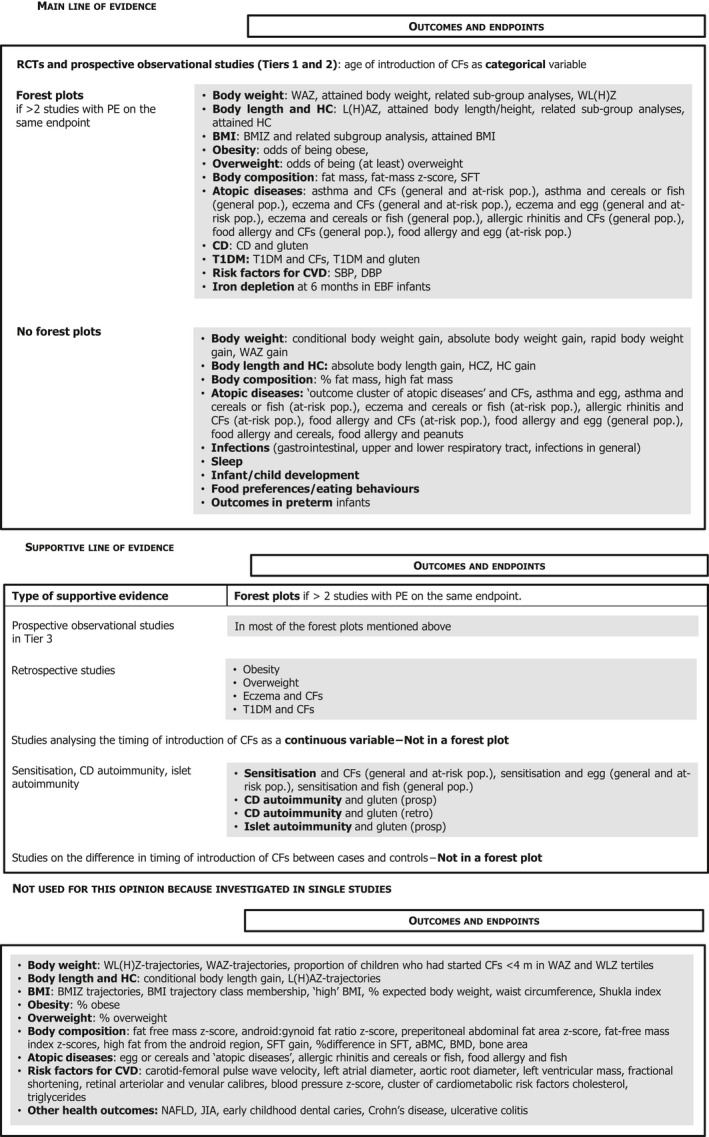
Overview of the endpoints considered in main and supportive lines of evidence and those that could not be used for this assessment
- aBMC: areal bone mineral content; BMD: bone mineral density; BMI: body mass index; BMIZ: body mass index‐for‐age z‐score; CD: coeliac disease; CF: complementary food; CVD: cardiovascular disease; DBP: diastolic blood pressure; EBF: exclusively breastfed; HC: head circumference; HCZ: head circumference‐for‐age z‐score; JIA: juvenile idiopathic arthritis; L(H)AZ: length(height)‐for‐age z‐score; m: months; NAFLD: non‐alcoholic fatty liver disease; PE: point estimate; RCT: randomised controlled trial; SBP: systolic blood pressure; SFT: skinfold thickness; T1DM: type 1 diabetes mellitus; WAZ: weight‐for‐age z‐score; WLZ: weight length z‐score WL(H)Z: weight‐length(height)‐for‐age z‐score.
Developmental readiness of the infant to consume CFs
Developmental readiness can be defined as the physiological maturation necessary for an infant to metabolise ‘non‐milk foods’, i.e. other than breast milk or formula, and the neurodevelopmental changes necessary for safe and effective progression from suckling to spoon‐ and self‐feeding, including the infant's apparent emerging interest in non‐milk foods and feeding (Section 3). Gastrointestinal and renal functions are not limiting factors with respect to the timing of introduction of CFs once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding.
A number of changes are required for progressing from a liquid to a semi‐solid and solid diet:
-
−
anatomical changes in the oral cavity,
-
−
the disappearance or diminishing of reflexes present at birth that coordinate suckling, swallowing and respiration and protect the infant from aspiration and choking (i.e. the extrusion reflex of the tongue), in favour of more voluntary movements,
-
−
and the development of gross motor skills (head and trunk control to allow an improved movement of the jaw) and fine motor skills (lip, tongue and jaw movements).
Developmental skills necessary to consume CFs will differ depending on the texture of the food. The skills needed for spoon‐feeding of pureed foods will appear earlier than the ones required for self‐feeding and therefore, will be used to define the lower bound of the age range of developmental readiness.
The age range at which infants attain developmental milestones shows considerable variation within and between populations, presumably reflecting the infant's innate developmental trajectory combined with the opportunities and experiences provided by the carer.
The infant's ability of holding the head in midline when in supine position and to control its head well when pulled to sitting or at aided sitting are considered by the Panel as the earliest gross motor skills that are indicative of an infant's developmental readiness to consume spoon‐fed pureed CFs. These earliest skills can be observed between 3 and 4 months of age. At this age, it can be assumed that the rooting and the extrusion reflexes may have also diminished in some infants. The skills needed for consuming self‐fed finger foods (i.e. sitting without support) can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age. In preterm infants, the necessary developmental milestones for feeding are also reached around the same age range (post‐term), depending on the severity of illness experienced during the neonatal period, the degree of prematurity and any sequelae.
Most infants do not need CFs for nutritional reasons up to around 6 months of age, with the exception of some infants at risk of iron depletion who may benefit from earlier introduction of CFs that are a source of iron. From this systematic review, the Panel concludes that there is high confidence in the evidence that an introduction of CFs at 4 months of age compared with 6 months reduces the risk of iron depletion at 6 months of age in exclusively breastfed infants at risk of iron depletion. However, the effect on iron depletion is not an effect of introducing CFs per se, but an effect of introducing CFs that are a source of iron. Infants that may benefit from an early introduction of CFs that are a source of iron are exclusively breastfed infants born to mothers with a low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born SGA, or with a high growth velocity.
Summary of the conclusions on adverse health effects or benefits associated with the introduction of CFs before 6 months of age
Term infants
In the systematic review (undertaken according to the criteria described in Section 2), the Panel has assessed 283 studies that reported on the relationship between the timing of introduction of CFs (or of specific foods, for some outcomes) and (health) outcomes. These were: (1) body weight and growth, including BMI, risk of developing overweight and obesity, as well as body composition, (2) risk of developing atopic diseases or symptoms of atopic diseases, such as asthma‐like symptoms, eczema, allergic rhinitis and symptomatic food allergy, (3) risk of developing coeliac disease and type 1 diabetes mellitus, (4) blood pressure, (5) infections, (6) sleep, (7) infant and child development, (8) nutrient status (i.e. iron) and (9) food preferences and eating behaviours in later life.
The Panel applied a weight of evidence approach to derive its conclusions and grade the confidence in the evidence (Section 2.2.3.3). The Panel concludes that there is no convincing evidence for adverse health effects of introducing CFs at any of the ages that were studied. In the studies rated as Tiers 1 and 2, these ages ranged from < 1 month to < 6 months for ‘early’ introduction and, mostly, thereafter for ‘late’ introduction.
Table 9 gives an overview about the conclusions of the Panel and the confidence in the evidence upon which these conclusions are based. For the same outcome, the conclusions in relation to the confidence in the evidence may be different for the different age ranges of introduction of CFs that were studied in RCTs or prospective observational studies.44
Table 9.
Level of confidence in the evidence (per outcome and study design) and related conclusions
| Confidence in the evidence | Outcome | ‘Early’ introduction’ | ‘Late’ introduction | Conclusionsa |
|---|---|---|---|---|
| Term infants | ||||
| High | Body weight | 3–4 m | 6 m | No effect |
| Body length/height | 3–4 m | 6 m | No effect | |
| Head circumference | 3–4 m | 6 m | No effect | |
| BMI | 3–4 m | 6 m | No effect | |
| Body composition | 3–4 m | 6 m | No effect | |
| Coeliac disease and gluten (hazard) | 4 m | 6 m | No effect | |
| Iron depletion 6 m (EBF infants) | 4 m | 6 m | Reduction of the risk of iron depletion | |
| Iron depletion 7‐12 mb (mixed feeding) | 3–4 m | 6 m | No evidence for an effect or association | |
| Moderate | Body weight | < 2 to < 6 m | Thereafter | No evidence for association |
| Body length/height | 2–3 to < 6 m | Thereafter | No evidence for association | |
| BMI | ≤ 2 to ≤ 5 m | Thereafter | No evidence for association | |
| Overweight | ≤ 2 to < 4 m | > 2 to > 6 m | No evidence for association | |
| Obesity | < 1 to < 4 m | ≥ 3 to ≥ 6 m | No evidence for association | |
| Body composition | < 4 m | ≥ 4 to > 6 m | No evidence for association | |
| Atopic diseases | 3–4 m | 6 m | No evidence for effect | |
| Asthma‐like symptoms and CFs | 3–4 m | 6 m | No evidence for effect | |
| Asthma‐like symptoms and cereals | < 3.75 to ≤ 5.5 m | Thereafter | No evidence for association | |
| Asthma‐like symptoms and fish | < 5.25 to ≤ 6 m | > 5.25 to > 8.5 m | No evidence for association | |
| Eczema and CFs | < 3 to ≤ 6 m | Thereafter | No evidence for association | |
| Allergic rhinitis and CFs | 3–4 m | 6 m | No evidence for effect | |
| Symptomatic food allergy and CFs | 3–4 m | 6 m | No evidence for effect | |
| Coeliac disease and gluten (hazard) | ≤3 to ≤4 m | Thereafter | No evidence for association | |
| T1DM and CFs | < 3 to < 5 m | Thereafter | No evidence for association | |
| T1DM and gluten | < 3 to < 5 m | Thereafter | No evidence for association | |
| Blood pressure | < 3 to < 5 m | Thereafter | Size of the effect not biologically relevant | |
| Infections in general | 3–4 and < 6 m | 6 and > 6 m | No evidence for association | |
| Low to moderate | Egg allergy and egg | 3–4 m | 6 m | Reduction of the risk of egg allergy |
| Low | Atopic diseases | < 3 m | > 3 m | No evidence for association |
| Asthma‐like symptoms and CFs | ≤ 3 to < 6 m | Thereafter | No evidence for association | |
| Asthma‐like symptoms and egg | < 5 and 4–6.5 m | ≥ 5 and ≥ 10 m | No evidence for effect | |
| Asthma‐like symptoms and egg | < 5 m | Thereafter | No evidence for association | |
| Eczema and egg | ≤ 4 to ≤ 6 m | Thereafter | No evidence for association | |
| Eczema and cereal | ≤ 4 m | Thereafter | No evidence for association | |
| Eczema and fish | ≤ 5–6 m | Thereafter | No evidence for association | |
| Allergic rhinitis and CF | ≤ 4 m | Thereafter | No evidence for association | |
| Symptomatic food allergy and CFs | ≤ 3–4 m | Thereafter | No evidence for association | |
| Wheat allergy and cereals | 3–4 m | 6 m | No evidence for effect | |
| GI infections | < 3 and 3–4 m | > 3 m and 6 m | No evidence for association | |
| LRT infections | < 3 and 3–4 m | > 3 m and 6 m | No evidence for association | |
| Sleep | 3–4 m | 6 m | Size of the effect not biologically relevant | |
| Infant and child development | 4 m | 6 m | No evidence for effect | |
| Very low | Eczema and egg | 4–6 m | 8‐10 m | No evidence for effect |
| n/a c | Peanut allergy and peanut | ≤ 6 m | > 6 m | Insufficient evidence to conclude |
| URT infections | < 3 and 3–4 m | > 3 and 6 m | Insufficient evidence to conclude | |
| Food preferences and eating behaviours and CFs/fruit/vegetables | Multitude of ages investigated | Insufficient evidence to conclude | ||
| Preterm infants | ||||
| Low | Weight | 4 m | 6 m | No evidence for effect |
| Length | 4 m | 6 m | No evidence for effect | |
| Head circumference | 4 m | 6 m | No evidence for effect | |
| n/a c | BMI, fat mass, lean mass, SFT, bone mineral density, asthma‐like symptoms, eczema, symptomatic food allergy, concentrations of blood lipids, blood pressure, infant and child development, infections, sleep and iron depletion | n/a | n/a | Insufficient evidence to conclude |
BMI: body mass index; CF: complementary food; EBF: exclusively breastfed; GI: gastrointestinal; LRT: lower respiratory tract; m: months; SFT: skinfold thickness; T1DM: type‐1 diabetes mellitus; URT: upper respiratory tract.
Conclusions were derived as follows: ‘no effect’: high confidence in the evidence derived from (an) RCT(s); ‘no evidence for an effect’: moderate, low or very low confidence in the evidence derived from (an) RCT(s); ‘no evidence for an association’: evidence derived from observational studies (see Section 2.2.3.3).
second half of the first year of life (7–12 months).
not graded because the evidence was insufficient to conclude.
In relation to the delayed introduction of allergenic foods into an infant's diet, the Panel finds no evidence to support postponing the introduction of potentially allergenic foods to a later age than the introduction of other CFs. The Panel also finds no evidence that the introduction of gluten < 6 months of age compared with thereafter increases the risk of developing coeliac disease or T1DM. As far as the risk of developing coeliac disease or type 1 diabetes mellitus is concerned, gluten can be introduced to an infant's diet when other CFs are introduced. Time to onset of coeliac disease or T1DM in relation to the timing of introduction of CFs was not considered.
Even though there is no convincing evidence for a harmful effect of CF introduction at any age studied on the selected health outcomes, it needs to be emphasised that foods that are given to infants should be presented in an age‐appropriate texture (to prevent choking), are nutritionally appropriate and are prepared according to good hygiene practices. Also, the fact that, based on the available evidence, CFs could be introduced at an early age does not mean that this is necessary or desirable.
Preterm infants
For preterm infants, as a specific subpopulation of the general population, one RCT (Tier 1) (Gupta et al., 2017) was available in the main line of evidence. However, given the specific population of preterm infants that was studied, i.e. mostly infants on a vegetarian diet, a population with a high mortality rate, severe growth failure and high prevalence of iron depletion (despite recommendations for use of iron supplements), the Panel considers that generalisability of the findings of this study to the general population of preterm infants born in Europe is uncertain. Based on this RCT, the Panel concludes that there is no effect of the introduction of CFs at 4 vs 6 months of post‐term age on body weight, body length and HC (low level of confidence in the evidence). No conclusions could be drawn on the other endpoints that were assessed in the available studies, as these were investigated only in single studies or in studies in the supportive line of evidence.
Main reasons for the conclusions of the Panel (see above) for selected outcomes
Hen's egg and egg allergy 45
The Panel concludes that introduction of hen's egg at around 3–4 months compared with 6 months of age may reduce the risk of egg allergy. However, given that all included studies have limitations, the confidence in the evidence is low to moderate and is, therefore, insufficient to support introducing egg at around 3–4 months of age in all infants for the prevention of egg allergy.
This conclusion is based on the following evidence:
There is only one RCT performed in Europe in the general population (Perkin et al., 2016) (EAT study) that compared introduction of cooked egg at 3–4 months of age to exclusively breastfed infants with introduction at 6 months of age. The FAS analysis in this study did not show a statistically significant difference in the risk of egg allergy at 1 or 3 years of age, while the PP analysis found an average of 75% (95% CI 18 to 92%) reduced risk of egg allergy. However, more than 50% of children were excluded from the intervention group, compared with only around 7% in the control group, which may have violated the principle of randomisation. Given that the authors of the study presented results comparing the prevalence of egg allergy in the non‐compliant intervention group with that in the control group, and that the prevalence was not different, the Panel considers that the findings in the PP analysis were unlikely caused by reverse causality (i.e. by excluding egg allergic children or those becoming allergic who cannot consume the food). The findings of this study are strengthened by the fact that there was limited evidence for a dose–response relationship when considering the amount of egg that was consumed by infants. The Panel has, however, reservations in relation to the generalisability of the results of the study to the whole population of infants living in Europe as the study population consisted of exclusively breastfed infants. It is unknown whether breastfeeding could be an effect modifier and whether effects could be different in formula or mixed fed infants.
In at‐risk populations, three RCTs were available, which were conducted by two research groups in Australia (Palmer et al., 2013; Palmer et al., 2017; Tan et al., 2017) (STAR, STEP and BEAT studies), a country with a high prevalence of egg allergy. Even though two of them were underpowered for the outcome and the third one was powered for sensitisation, the meta‐analysis of the three trials performed by the Panel showed a statistically significantly reduced risk of egg allergy (on average 31% (95% CI 7 to 49%)) associated with introduction of egg in a pasteurised raw form between around 4 and 6 months of age, compared with after 8–10 months of age. The comparison group continued the usual diet until egg was introduced, therefore, combining a variety of possible feeding modes, not only breast milk or formula feeding. The Panel considers that the generalisability to the whole population of infants living in Europe is hampered (1) because of the form of the egg that was used (i.e. pasteurised raw egg powder) that is not the form that would be used in the normal diet of an infant and (2) because the studies were conducted in at‐risk populations in a country with a prevalence of egg allergy that is higher than in Europe which cannot be explained. Finally, there was a cross‐sectional analysis of baseline data of an Australian population‐based cohort study (HealthNuts study (Koplin et al., 2010; Koplin et al., 2012)) available in the supportive line of evidence, whose results were consistent with the findings of the RCTs in Australia.
In addition, regarding safety, there were some anaphylactic reactions associated with the consumption of pasteurised raw egg powders as intervention products. In the trial in which cooked egg was given to infants, no such reactions were observed.
Peanut and peanut allergy
There is evidence from an RCT (Du et al., 2015) (LEAP study) conducted in an at‐risk population that the introduction of peanut between 4 and 10 months compared with peanut avoidance up to 5 years of age reduces the risk of developing peanut allergy.
The same RCT that investigated the effect of early egg introduction on egg allergy (Perkin et al., 2016) (EAT study) also assessed the effect of introducing peanut at 3–4 months of age compared with introduction at 6 months of age. Like the results for egg, the FAS analysis for peanut did not show an effect of early peanut introduction on the outcome, while in the PP analysis a statistically significant reduction of the risk of peanut allergy was observed.
A re‐analysis of data from the LEAP study performed by EFSA, based on the publication by Lawson et al. (2017), compared those infants who were introduced to peanut ≤ 6 months of age with those who avoided peanut up to the age of 5 years. This analysis found that those introduced to peanut ≤ 6 months of age had a reduced risk of peanut allergy compared with those who avoided peanut up to 5 years. However, there were no statistically significant differences in the risk of peanut allergy between those introduced to peanut ≤ 6 months and those introduced between 7 and 10 months of age. This observational re‐analysis was not based on the original randomised groups.
The Panel considers that there is evidence that peanut introduction during the first year of life (either at 4–10 months or at 4–6 months) compared with peanut avoidance up to 5 years of age reduces the risk of peanut allergy. However, the evidence is insufficient to conclude whether, when comparing infants introduced to peanut ≤ 6 months of age with those introduced > 6 months (but still within the first year of life, which is the subject of this mandate), a similar effect occurs. Therefore, the confidence in the evidence was not graded.
Overweight and obesity
There is no evidence that the timing of introduction of CFs is associated with higher risk of developing overweight and obesity (moderate confidence in the evidence). This finding is supported by the available data on weight, BMI and fat mass (moderate to high confidence in the evidence, depending on the outcome).
Coeliac disease and type 1 diabetes mellitus
In its previous Scientific Opinion (EFSA NDA Panel, 2009), the Panel considered that introduction of gluten before 4 months of age might increase the risk of coeliac disease and T1DM, whereas introduction between 4 and 6 months of age while still breastfeeding might decrease the risk of coeliac disease and T1DM.
-
o
Coeliac disease
Based on the findings of an RCT conducted in seven EU Member States plus Israel (Vriezinga et al., 2014) (PreventCD study) and a meta‐analysis of four prospective cohort studies rated as Tiers 1 and 2 performed by the Panel, there is no evidence for an effect or association between the timing of introduction of gluten and the risk of developing coeliac disease (moderate to high level of confidence in the evidence, depending on the age of introduction of CFs investigated). This is true overall and when the analysis was stratified by age of introduction, comparing early introduction (mostly < 4 months of age) with a middle age category (4 to 6 months) and the middle age category with late introduction (mostly > 6 months of age).
The previous conclusion (EFSA NDA Panel, 2009), that (any) continued breastfeeding could modify the effect of gluten introduction < 6 months of age, is not confirmed in an observational analysis of the RCT (Vriezinga et al. (2014) reported in Szajewska et al. (2015)) and a large prospective cohort study (Størdal et al., 2013) (Section 9).
-
o
Type 1 diabetes mellitus
For T1DM, the meta‐analysis of the four prospective cohort studies rated as Tiers 1 and 2 did not show an association between the age of introduction of CFs or gluten ranging from < 3 to < 5 months of age compared with thereafter and the outcome assessed (moderate confidence in the evidence).
There were insufficient data to investigate whether the introduction of gluten < 4 months of age could have different effects on the risk of developing T1DM than gluten introduction between 4 and 6 months of age as purported by the Panel in its previous Scientific Opinion (EFSA NDA Panel, 2009). There were no data to evaluate whether gluten introduction < 6 months of age while still breastfeeding has a different effect than gluten introduction < 6 months of age in infants not breastfed at the time of this introduction.
Infections
There is no evidence that, when hygiene conditions are satisfactory,46 the introduction of CFs < 6 months of age compared with thereafter is associated with an increased risk of (1) gastrointestinal infections (low level of confidence in the evidence), (2) LRTI (moderate level of confidence in the evidence) or (3) infections in general (including hospital admissions for infections; moderate level of confidence in the evidence). The evidence for URTI is inconsistent and insufficient to draw conclusions. Therefore, the confidence in the evidence was not graded. It should, however, be noted that URTI are less severe than gastrointestinal infections, LRTI or infections requiring hospital admissions. In addition, the Panel considers that the criteria for diagnosis of URTI are less reliable and less specific than for other infections, which introduces considerable uncertainty into the assessment of the outcome. Also, the difference in the incidence of URTI was transitory and limited to a time period of 2–3 months.
Sleep‐related endpoints
Two RCTs (Tiers 1 and 2) were available, including one (Bainbridge et al., 1996) most likely underpowered for this outcome and with non‐statistically significant results. This RCT was therefore not considered further by the Panel. The other (Perkin et al., 2016) (EAT study) provided findings considered by the Panel of no biological relevance: the difference of on average 7 min in night time sleep over the duration of the study (peaking at on average about 17 min at 6 months of age) is small compared to the average night time sleep at that age (around 10 h). The confidence in the evidence was low.
Food preferences and eating behaviours
The evidence for an association between the timing of introduction of CFs and food preferences and eating behaviours is inconsistent. Therefore, the confidence in the evidence was not graded. While earlier introduction of CFs is associated with less desirable eating behaviours (i.e. lower satiety responsiveness, higher feeding difficulties and lower likelihood to have a positive eating pattern), there is no relationship between the timing of introduction of CFs with other (potentially also less desirable) aspects of eating behaviours (i.e. food responsiveness, enjoyment of food, food fussiness and slowness in eating, vegetable or fruit intake).
This inconsistency is also observed for the assessment of the timing of introduction of vegetables and fruit for which an association between the introduction of vegetables, but not fruit, < 6 months of age and a lower food fussiness was observed. No biologically relevant association between the timing of introduction of vegetables and fruit and eating patterns as well as the frequency of consumption of vegetables and fruit were observed.
Uncertainties in the body of evidence
The following sources of uncertainties in the body of evidence were identified. Some of them were addressed in the appraisal of the RoB.
Classification into exposure groups
The classification into exposure groups may be influenced by recall bias, which increases with time elapsed since the exposure and depends on whether the information was interviewer‐elicited or not. This was discussed during the appraisal of the RoB (Section 2.2.2 and Appendix C).
The classification into exposure groups may also be affected by the lack of a definition provided to caregivers about what the ‘introduction’ of CFs means, e.g. first tastes vs regular consumption, and what to be considered as ‘CFs’ or ‘solids’.
It can be assumed that the timing of introduction of CFs in general may be remembered more precisely by caregivers than the introduction of more specific food items, especially when asked at more distant time points.
In addition, residual uncertainty remains on how the age of introduction of CFs was defined in the individual publications, unless the age was reported in weeks. For example, an introduction at 5 months of age could mean that the infant is introduced during the fifth month of life or in the month following the 5‐month birthday (i.e. the sixth month of life) (see also Section 2.2.3.3.). In some publications, introduction of CFs is reported as before or after a certain cut‐off (e.g. < vs > 3 months) without information to which group infants introduced at the precise age of the cut‐off (e.g. infants introduced to CFs at 3 months of age) were attributed. In addition, the same cut‐offs used in the studies may have been expressed in different ways (e.g. < 4 months equivalent to ≤ 3 months). However, given the uncertainties around the classification into age groups of introduction of CFs, no efforts of harmonisation (in the text of this Scientific Opinion and related Annexes) have been made by the Panel. Age groups reported in this Scientific Opinion are those provided in the individual papers.
Lack of information on the diet of children between the introduction of CFs and the outcome measurement
There is a general lack of information on the diet of children between the introduction of CFs and the outcome assessment. The lack of such information is of particular importance for (health) outcomes for which the evidence was solely available from observational studies.
It is difficult to judge whether any differences observed in the outcomes may be attributed solely to the age at which CFs were introduced or whether other influencing factors, such as the composition of the diet, may have contributed to the effect (that could not be accounted for by adjusting the analysis for confounders). This was discussed during the appraisal of the RoB (Section 2.2.2. and Appendix C).
Generalisability
The available data originate mainly from large prospective cohort studies that were initially representative of the population to be studied. However, in many studies, a substantial number of subjects had missing data on the exposure and/or the outcome. This resulted in a selective subgroup of the original populations that could be included in the analyses.
When comparisons between the characteristics of the included and excluded children are available, the caregivers of included children generally had a higher education or socioeconomic status and breastfed for longer compared to those not included in the analyses. In addition, subjects that agree to take part in a study tend to have a higher socioeconomic status than those who do not participate.
Whether the loss of subjects or the exclusion of subjects could have influenced the internal validity of the study was discussed during the appraisal of the RoB (Section 2.2.2. and Appendix C).
Whether or not results could be generalised to the whole population of infants living in Europe was considered in the grading of the confidence in the evidence.
20. Conclusions
General considerations
The conclusions are intended for infants living in Europe.
Complementary feeding in this Scientific Opinion means the period when CFs are given together with either breast milk or formula or both. CFs comprise, therefore, foods other than breast milk, formula, water or vitamins that are given to infants and can be beverages, spoon‐fed pureed foods, spoon‐fed lumpy foods or finger foods, either prepared at home or produced commercially.
This Scientific Opinion is a scientific assessment of the available evidence and should not be interpreted as providing public health recommendations for the timing of introduction of CFs. This task is outside the remit of EFSA but it is within the remit of public health authorities in Member States.
From a scientific point of view, the assessment of the appropriate age range of introduction of CFs (which is the subject of this mandate) is not an assessment of the optimal duration of exclusive breastfeeding.
The appropriate age range of introduction of CFs was evaluated in this Scientific Opinion by considering developmental, nutritional, and health outcomes. Other aspects of complementary feeding that were not part of this assessment may need to be considered when discussing an appropriate age range of introduction of CFs, for example social interactions and the cultural context.
Most infants do not need CFs for nutritional reasons up to around 6 months of age, with the exception of some infants at risk of iron depletion who may benefit from earlier introduction of CFs that are a source of iron. Infants at risk of iron depletion are exclusively breastfed infants born to mothers with a low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born SGA, or with a high growth velocity.
The earliest skills considered relevant for the consumption of spoon‐fed pureed CFs (i.e. holding the head in midline when in supine position and good head control when pulled to sitting or at aided sitting together with a diminishing in the rooting and extrusion reflexes) can be observed between 3 and 4 months of age. Skills necessary for the consumption of self‐fed finger foods (i.e. sitting without support) can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age.
There is no convincing evidence for adverse health effects of introduction of CFs in term infants at any of the ages investigated by the studies assessed in this Scientific Opinion, as long as the foods are given in an age‐appropriate texture, are nutritionally appropriate and prepared according to good hygiene practices. Equally, there is no convincing evidence for any benefit of introducing CFs < 6 months of age, except for infants at risk of iron depletion. In the studies that were assessed, the age group for ‘early’ CF introduction ranged from < 1 month of age to < 6 months of age but was in most instances defined as either < 3 or < 4 months of age47 without precise information on the earliest age of introduction. The outcomes that have been studied in relation to CFs were body weight, body length, HC, BMI, risk of developing overweight or obesity, body composition, risk of developing atopic diseases, including asthma‐like symptoms, eczema, allergic rhinitis, and symptomatic food allergy, risk of developing coeliac disease or T1DM, blood pressure, infections, sleep, infant and child development, iron status and food preferences and eating behaviours.
Once the infant has the necessary neuromotor skills and has developed an apparent interest in non‐milk foods and feeding, allergenic foods can be introduced in the same way as other CFs.
The evidence for preterm infants in relation to introduction of CFs is limited. From the available data, there is no evidence for an effect of introduction of CFs at 4 months (post‐term) compared with 6 months (post‐term) on body weight, body length and head circumference.
Specific considerations
There is some evidence that the introduction of hen's egg at around 3–4 months of age compared with 6 months of age may reduce the risk of developing egg allergy. However, the Panel notes that the confidence in the evidence is insufficient to support introducing egg at around 3–4 months of age in all infants for the prevention of egg allergy. In the available studies, no serious adverse reactions occurred with consumption of cooked egg, while anaphylactic reactions were observed when the intervention consisted of pasteurised raw egg powder. As far as the risk of developing allergy is concerned, cooked egg can be introduced into the diet of infants when other CFs are introduced.
There is evidence that peanut introduction during the first year of life (either at 4–10 months or at 4–6 months) compared with peanut avoidance up to 5 years of age reduces the risk of peanut allergy. However, the evidence is insufficient to conclude whether, when comparing infants introduced to peanut ≤ 6 months of age with those introduced > 6 months (but still within the first year of life, which is the subject of this mandate), a similar effect occurs. As far as the risk of developing allergy is concerned, peanut can be introduced into the diet of infants when other CFs are introduced.
There is no evidence for an association between various timings of introduction of gluten or gluten‐containing foods and the risk of developing coeliac disease. Regarding the risk of coeliac disease, gluten can be introduced to an infant's diet when other CFs are introduced.
The evidence suggests that, when hygiene conditions are satisfactory,48 there is no evidence for an adverse effect of introduction of CFs before 6 months of age on gastrointestinal infections, LRTI and infections in general (including hospital admission for infections). The evidence with respect to URTI is insufficient.
Overall conclusions
The appropriate age range of introduction of CFs has been evaluated taking into account effects on health outcomes, nutritional considerations and infant development.
The available data do not allow the determination of a single age for the introduction of CFs for infants living in Europe. The appropriate age range depends on the individual's characteristics and development, even more so if the infant was born preterm.
As long as the foods are given in an age‐appropriate texture, are nutritionally appropriate and prepared according to good hygiene practices, there is no convincing evidence that the introduction of CFs is associated with either adverse or beneficial health effects (except for infants at risk of iron depletion) at any age investigated in the included studies (< 1 months to < 6 months for earlier introduction).
For nutritional reasons, the majority of infants need CFs from around 6 months of age. For preterm infants, this refers to post‐term age.
Infants at risk of iron depletion (exclusively breastfed infants born to mothers with low iron status, or with early umbilical cord clamping (< 1 min after birth), or born preterm, or born SGA or with high growth velocity) may benefit from introduction of CFs that are a source of iron before 6 months of age.
The earliest developmental skills relevant for the consumption of spoon‐fed pureed CFs can be observed between 3 and 4 months of age. Skills necessary for consuming self‐fed finger foods can be observed in some infants at 4 months, but more commonly between 5 and 7 months of age. For preterm infants, this refers to post‐term age.
The fact that an infant may be ready from a neurodevelopmental point of view to progress from a liquid to a more diversified diet before 6 months of age does not imply that there is a need to introduce CFs.
Glossary
- Conditional weight gain
difference between the actual weight z‐scores and the ones predicted by weight at an earlier time point, adjusted for regression to the mean.
- FAS analysis
the analysis set that contains all children with at least one outcome measurement irrespective of whether they had consumed the food under investigation in the pre‐specified amounts or not. This type of analysis is more indicative of results that could be obtained in the whole population where the adherence pattern might be similar (e.g. the proportion of infants who have difficulty consuming the food at an early age could be assumed to be similar in the whole population and the study population).
- Shukla index
(actual weight/actual height)/(50th percentile weight‐for‐age/50th percentile height‐for‐age) × 100
- Percentage expected weight‐for‐age
(actual weight of the child/50th percentile weight at the age when the child's height was on the 50th percentile of reference standards) × 100
- PP analysis
the analysis set that includes only those children who had followed the protocol, i.e. complied with the a priori assumption of the amount and settings in which the food is assumed to show an effect and provided a full data set with respect to outcome measurements and compliance measurements. This analysis is more indicative of whether the food has an effect under the assumptions that had been made a priori.
- Vital gluten
are a by‐product of starch isolation obtained during wet milling, in which flour is separated into starch and proteins (including gluten).
Abbreviations
- AAP
American Academy of Pediatrics
- ABCD (study)
Amsterdam born children and their development (study)
- aBMC
areal bone mineral content
- ABIS (study)
alla barn i sydöstra sverige (study) [all babies in Southeast Sweden]
- ACF
age at complementary feeding
- ADA
American Diabetes Association
- ADJ
adjusted
- AGA
appropriate‐for‐gestational age
- ALA
alpha‐linolenic acid
- ALSPAC (study)
Avon longitudinal study of parents and children (study)
- AM
Armenia
- aOR
adjusted odds ratio
- at‐risk pop
at‐risk population
- AU
Australia
- BE
Belgium
- BEAT (study)
beating egg allergy trial (study)
- BF
breastfed
- BIA
bioelectrical impedance analysis
- BioIC
automated microfluidic‐based immunoassay system
- BMC
bone mineral content
- BMD
bone mineral density
- BMI
body mass index
- BMIZ
body mass index‐for‐age z‐score
- BR
Brazil
- CA
Canada
- CAPS (study)
childhood asthma prevention study
- CBGS (study)
Cambridge baby growth study
- CC
case–control study
- CD
coeliac disease
- CDC
US Centers for Disease Control and Prevention
- CF
complementary food
- CH
Switzerland
- CHILD (study)
Canadian healthy infant longitudinal development (study)
- CI
confidence interval
- CLARITY (study)
childhood arthritis risk factor identification study
- CLG
conditional length gain
- CN
China
- COT
UK Committee on Toxicity of Chemicals in Food, Consumer products and the environment
- CS
cross‐sectional study
- CSA
cross‐sectional analysis
- CV
continuous variable
- CVD
cardiovascular disease
- CWG
conditional weight gain
- DAISY (study)
diabetes autoimmunity study in the young (study)
- DARLING (study)
Davis area research on lactation, infant nutrition and growth (study)
- DBP
diastolic blood pressure
- DBH (study)
dampness in building and health (study)
- DE
Germany
- DHA
docosahexaenoic acid
- DIPP (study)
type 1 diabetes prediction and prevention (study)
- DK
Denmark
- DNBC (study)
Danish national birth cohort (study)
- DXA
dual‐energy X‐ray absorptiometry
- EAT (study)
enquiring about tolerance (study)
- EBF
exclusively breastfed
- ECLS‐B (study)
early childhood longitudinal study‐birth cohort (study)
- EDEN (study)
étude des déterminants pré et postnatals précoces du développement et de la santé de l'enfant (study) [study on the pre‐ and early postnatal determinants of child health and development]
- EFHL (study)
environments for healthy living (study)
- ENID (study)
early nutrition and immune development (study)
- ES
Spain
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FAIR (study)
food allergy and intolerance research (study)
- FAS
full analysis set
- FC
food challenge
- FF
formula fed
- FI
Finland
- FR
France
- gen pop
general population
- GH
Ghana
- GINI (study)
German infant nutritional intervention program (study)
- GNN (study)
German neonatal network (study)
- GM
Gambia
- GR
Greece
- GUS (study)
growing up in Scotland (study)
- GUSTO (study)
growing up in Singapore towards healthy outcomes (study)
- Hb
haemoglobin
- HC
head circumference
- HCZ
head circumference‐for‐age z‐score
- HDL
high‐density lipoprotein
- HEAP (study)
hen's egg allergy prevention (study)
- HLA
human leucocyte antigen
- HN
Honduras
- HR
Croatia
- HR
hazard ratio
- HU
Hungary
- ID
identification
- IDEFICS (study)
identification and prevention of dietary‐ and lifestyle‐ induced health effects in children and infants (study)
- IE
Ireland
- IFPS (study)
infant feeding practices study
- Ig
immunoglobulin
- IL
Israel
- IN
India
- InFANT (study)
Melbourne infant feeding, activity and nutrition trial (study)
- IOTF
International Obesity Task Force
- IQR
interquartile range
- IR
Iran
- IS
Iceland
- IT
Italy
- ITT
intention‐to‐treat (analysis)
- IOTF
international obesity task force
- JIA
juvenile idiopathic arthritis
- JP
Japan
- KNHANES (study)
Korea national health and nutrition examination survey (study)
- KOALA (study)
kind, ouders en gezondheid: aandacht voor leefstijl en aanleg (study)
- KR
South Korea
- kUA
kilounits of allergen(‐specific IgE)
- LDL
low‐density lipoprotein
- LEAP (study)
learning early about peanut allergy (study)
- L(H)AZ
length‐(height)‐for‐age z‐score
- LISA (study)
Einfluss von Lebensbedingungen und Verhaltensweisen auf die Entwicklung von Immunsystem und Allergien (study) [influences of lifestyle related factors on the human immune system and development of allergies in childhood]
- LoE
line of evidence
- LRTI
lower respiratory tract infection
- LSAC (study)
longitudinal study of Australian children
- LT
Lithuania
- LU
Luxembourg
- LV
Latvia
- m
Months
- MACS (study)
Melbourne atopy cohort study
- MAS (study)
multicenter allergy study
- MCS (study)
millennium cohort study
- MCV
mean corpuscular volume
- MD
mean difference
- MIDIA (study)
miljøårsaker til type 1‐diabetes (study) [environmental causes of type 1 diabetes]
- mmHg
millimetre mercury
- MoBa (study)
den norske mor og barn‐undersøkelsen (study) [the Norwegian mother and child cohort]
- mOsm
Milliosmole
- MW
Malawi
- MX
Mexico
- n/a
not applicable
- NAFLD
non‐alcoholic fatty liver disease
- NHANES (study)
US national health and nutrition examination survey
- NL
the Netherlands
- NO
Norway
- NOURISH (study)
nourishing our understanding of role modelling to improve support and health (study)
- NTIS
National Technical Information Service
- NTP
US National Toxicology Program
- NZ
New Zealand
- OHAT
US Office of Health Assessment and Translation
- OM
Oman
- OMCHS (study)
Osaka maternal and child health study
- OPALINE (study)
observatory of food preferences in infants and children (study)
- OR
odds ratio
- PA
pooled analysis
- PARIS (study)
pollution and asthma risk: an infant study
- PASTURE (study)
protection against allergy–study in rural environments (study)
- PATCH (study)
prediction of allergies in Taiwanese children (study)
- PBF
partially breastfed
- PC
prospective cohort study
- PE
point estimate
- PE (in forest plots)
pooled estimate
- PEDS
Parent's Evaluation of Developmental Status
- PI
prediction interval
- PIAMA (study)
prevention and incidence of asthma and mite allergy (study)
- PIFA (study)
prevalence of infant food allergy study
- PINGU (study)
polyunsaturated fatty acids in child nutrition ‐ a German multimodal optimisation study
- PIPA (study)
prebiotics in prevention of atopy (study)
- PIPO (study)
prospective cohort on the influence of perinatal factors on the occurrence of asthma and allergies (study)
- PreventCD (study)
prevent coeliac disease (study)
- PP
per protocol (analysis)
- PRPD
parents’ report of physician's diagnosis
- PRS
parents’ report of symptoms
- PT
Portugal
- QLSCD (study)
Québec longitudinal study of child development
- RC
retrospective cohort study
- RCT
randomised controlled trial
- RETRO
retrospective study
- RoB
risk of bias
- RR
risk ratio
- SACN
UK Scientific Advisory Committee on Nutrition
- SACN (study)
study of children's activity and nutrition
- SBP
systolic blood pressure
- SCC
sibling case–control study
- SCORAD
scoring atopic dermatitis
- SD
standard deviation
- SE
Sweden
- SEARCH CC (study)
search for diabetes in youth case–control (study)
- SEATON (study)
study of eczema and asthma to observe the influence of nutrition
- SF
serum ferritin
- SFT
skinfold thickness
- SG
Singapore
- SGA
small‐for‐gestational age
- sIg
specific immunoglobulin
- SKOT (study)
smabørns kost og trivsel (study) [small children's diet and well‐being]
- SMILE (study)
study of mothers and infants’ life events affecting oral health
- SPT
skin prick test
- STAR (study)
solids timing for allergy research (study)
- STEP (study)
starting time of egg protein (study)
- STEPS (study)
steps to healthy development (study)
- STRONG Kids (study)
synergistic theory and research on obesity and nutrition group kids (study)
- SWS (study)
Southampton women's survey (study)
- T
Term
- T1DM
type 1 diabetes mellitus
- TBCS (study)
Taiwan birth cohort study
- TEDDY (study)
the environmental determinants of diabetes in the young (study)
- TfS
transferrin saturation
- TH
Thailand
- TR
Turkey
- tTG (=TGC=TGM2)
tissue transglutaminase
- tTGA
tTG autoantibodies
- TW
Taiwan
- UK
United Kingdom
- URECA (study)
urban environment and childhood asthma (study)
- URTI
upper respiratory tract infection
- US
United States
- USDA
United States Department of Agriculture
- VLDL
very‐low‐density lipoprotein
- WAZ
weight‐for‐age z‐score
- WHEALS (study)
Wayne county health, environment, allergy and asthma longitudinal study
- WG
working group
- WHO
World Health Organization
- WL
weight‐for‐length
- WL(H)Z
weight‐for‐length(height) z‐score
- y
year(s)
- Y6FU
year 6 follow‐up (of the infant feeding practices study IFPS II)
Appendix A – Data analysis and synthesis in forest‐plots
1.
Interpretation of the graphical representation:
In the following appendices (and in line with the explanations given in Section 2.2.3.2), results are presented in forest‐plots, i.e. graphical representations in which:
-
−
the point estimate, i.e. the mean difference/odds ratio (OR)/risk ratio (RR)/hazard ratio (HR), of each comparison from each study is represented by a small black dot;
-
−
the 95% confidence interval (CI) around this point estimate is represented by a horizontal line;
-
−
the vertical line is the line of the ‘null effect’;
-
−
the size of a grey square represents the weight given to the related individual study estimate (indicated in the last column), when calculating a pooled estimate over several studies;
-
−
the pooled estimate is shown as a diamond, and the width of each diamond represents its 95% CI calculated based on the DerSimonian and Laird approach with the Hartung and Knapp modification (with some exceptions; see below);
-
−
below each pooled estimate and 95% CI, whenever more than two comparisons were available, the 95% prediction interval based on a t‐distribution with k‐2 degrees of freedom is depicted by a black line;
-
−
the heterogeneity index I2 is shown together with its 95% CI, the latter only whenever more than two comparisons were available; the p‐value provided is related to the χ2 test of heterogeneity.
Different possible sources of heterogeneity are indicated in the plots in addition to the age of introduction to complementary feeding, i.e. the specific study population, age at outcome assessment, reference data (e.g. reference population used to calculate z‐scores, cut‐off for the definition of binary endpoints of interest), the main confounders for the outcome of interest (identified by the Panel) or whether the analysis was unadjusted.
Structure of this appendix
The following plots are organised following the order in the core text of the opinion.
Below eight forest plots49 results of the meta‐analysis performed using the DerSimonian and Laird approach without the Hartung and Knapp modification are shown for some subgroups. The forest plot on ‘iron depletion in exclusively breastfed infants at 6 months of age’ is as a whole based on the DerSimonian and Laird approach without the Hartung and Knapp modification. This has been done following the sensitivity analysis performed by the Panel that indicated that the Hartung and Knapp modification did not perform well in those cases (see Section 2.2.3.2). Therefore, the Panel considered the results without the modification to be more reliable.
A.1. WAZ comparing early introduction with later introduction of CFs
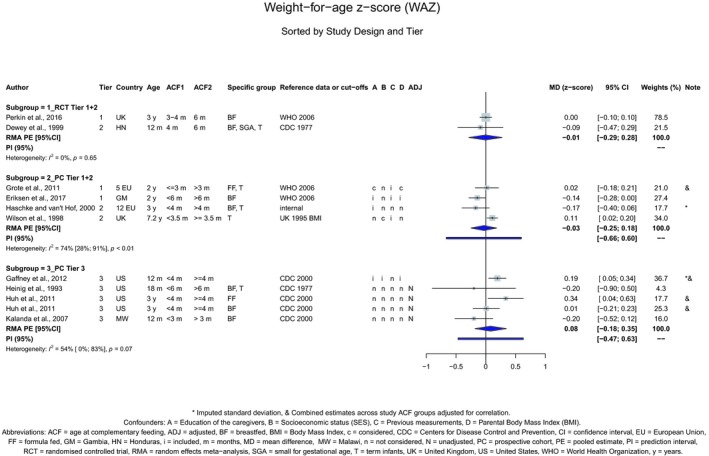
A.2. WAZ by feeding mode (exclusive breastfeeding or formula feeding) for studies rated as Tiers 1 and 2 comparing early introduction with later introduction of CFs
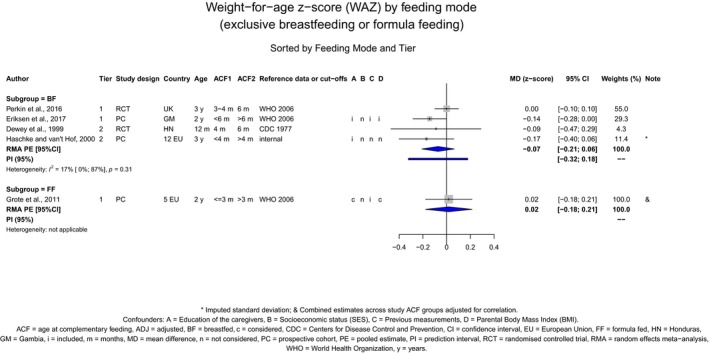
A.3. Attained body weight comparing early introduction with later introduction of CFs
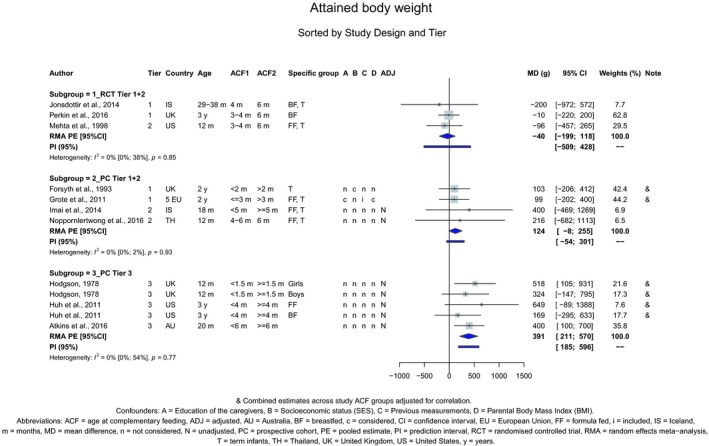
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup of RCTs rated as Tiers 1 and 2: PE = −40; 95% CI [−217; 136]
-
o
subgroup of prospective cohort studies rated as Tiers 1 and 2: PE = 124; 95% CI [−80; 327]
A.4. Attained body weight by feeding mode (exclusive breastfeeding or formula feeding) for studies rated as Tiers 1 and 2 comparing early introduction with later introduction of CFs
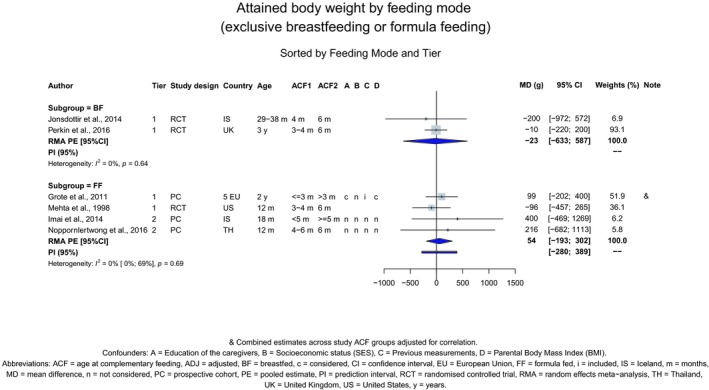
A.5. WL(H)Z comparing early introduction with later introduction of CFs
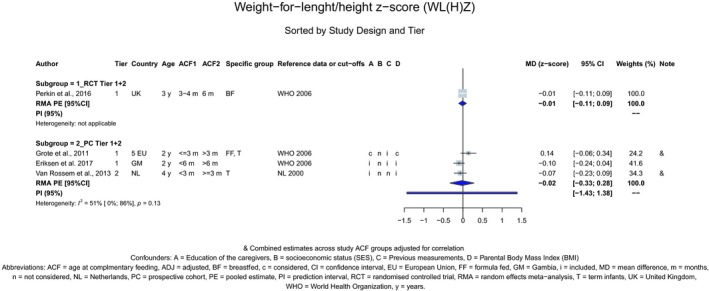
A.6. L(H)AZ comparing early introduction with later introduction of CFs
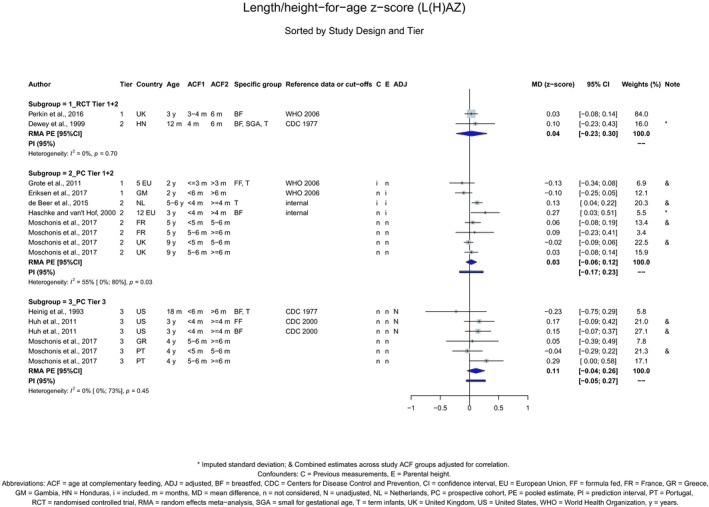
A.7. L(H)AZ by feeding mode (exclusive breastfeeding or formula feeding) for studies rated as Tiers 1 and 2 comparing early introduction with later introduction of CFs
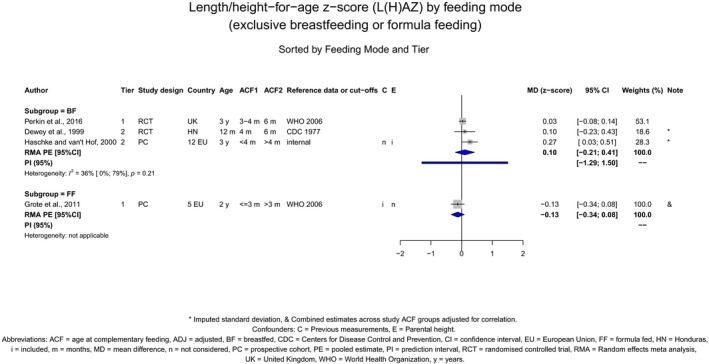
A.8. Attained body length/height comparing early introduction with later introduction of CFs
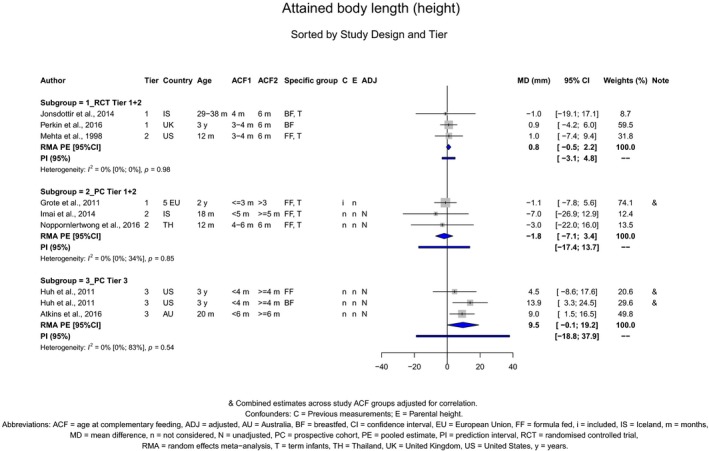
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup of RCTs rated as Tiers 1 and 2: PE: 0.8; 95% CI [−3.4; 5.0]
-
o
subgroup of prospective cohort studies rated as Tiers 1 and 2: PE = −1.8; 95% CI [−7.9; 4.2]
A.9. Attained body length/height by feeding mode (exclusive breastfeeding or formula feeding) for studies rated as Tiers 1 and 2 comparing early introduction with later introduction of CFs
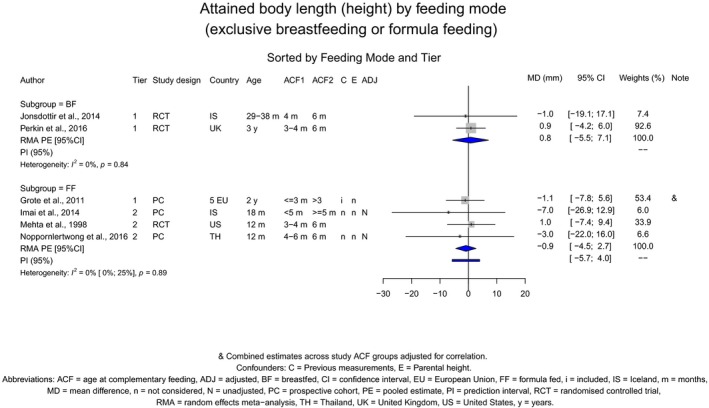
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup of formula fed infants PE = −0.9; 95% CI [−5.8; 4.0]
A.10. Attained head circumference comparing early introduction with later introduction of CFs
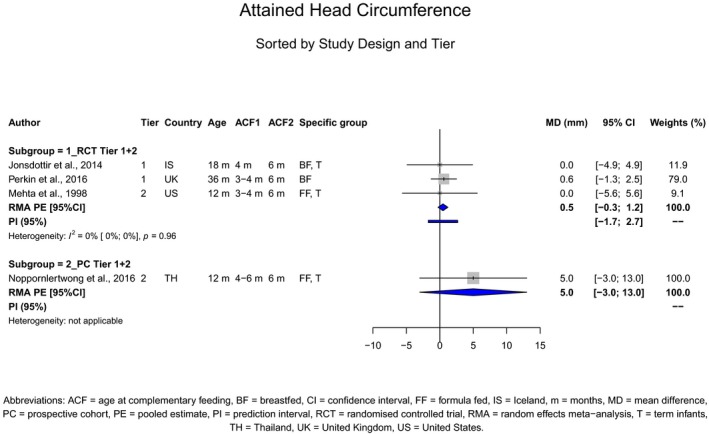
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroups of RCTs rated as Tiers 1 and 2 PE = 0.5; 95% CI [−1.2; 2.2]
A.11. BMIZ comparing early introduction with later introduction of CFs
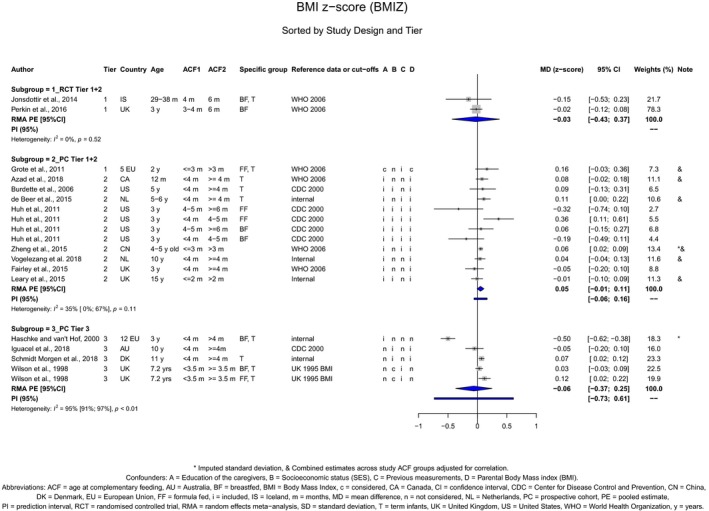
A.12. BMIZ by feeding mode (exclusive breastfeeding or formula feeding) for studies rated as Tiers 1 and 2 comparing early introduction with later introduction of CFs
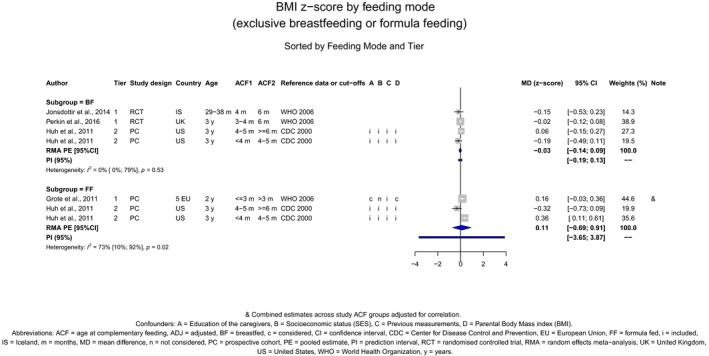
A.13. Attained BMI comparing early introduction with later introduction of CFs
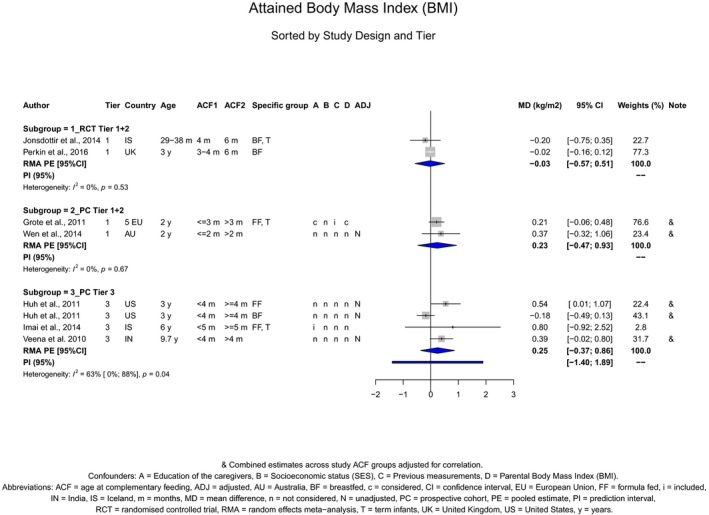
A.14. Odds of developing obesity comparing early introduction with later introduction of CFs
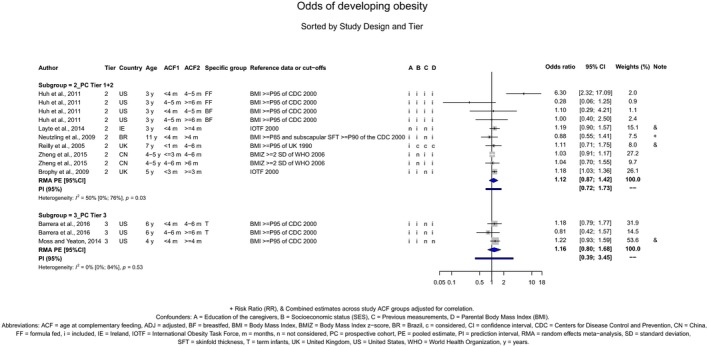
A.15. Odds of developing obesity comparing early introduction with later introduction of CFs, retrospective studies
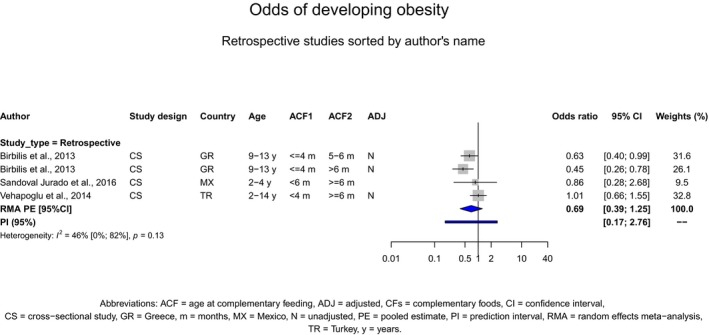
A.16. Odds of developing (at least) overweight comparing early introduction with later introduction of CFs
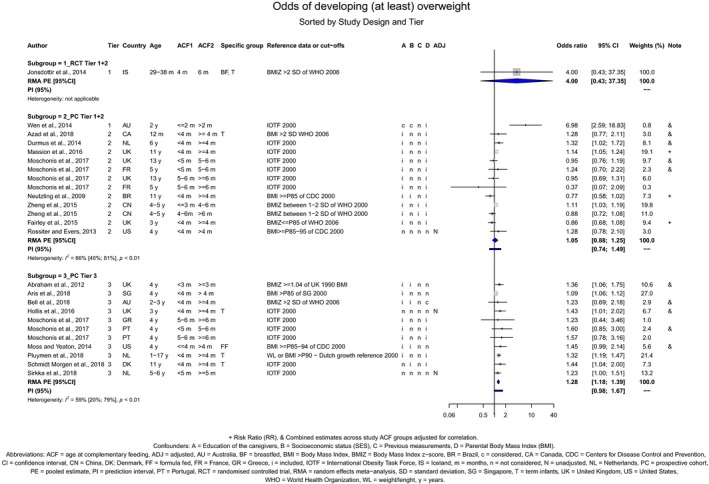
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup prospective cohort studies rated as Tier 3: PE = 1.28; 95% CI [1.14; 1.42]
A.17. Odds of developing (at least) overweight comparing early introduction with later introduction of CFs, retrospective studies
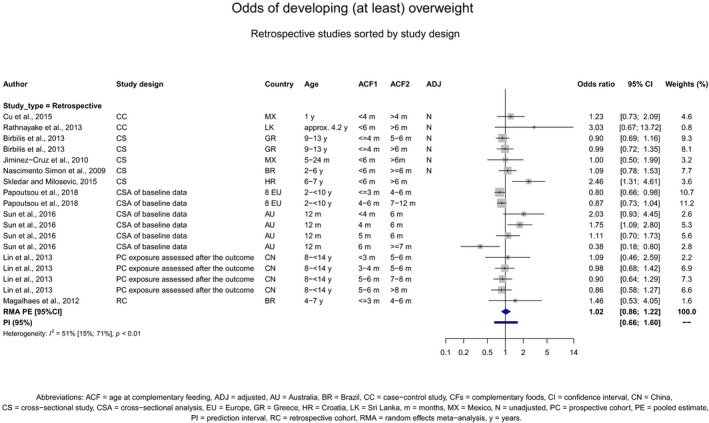
A.18. Fat mass comparing early introduction with later introduction of CFs
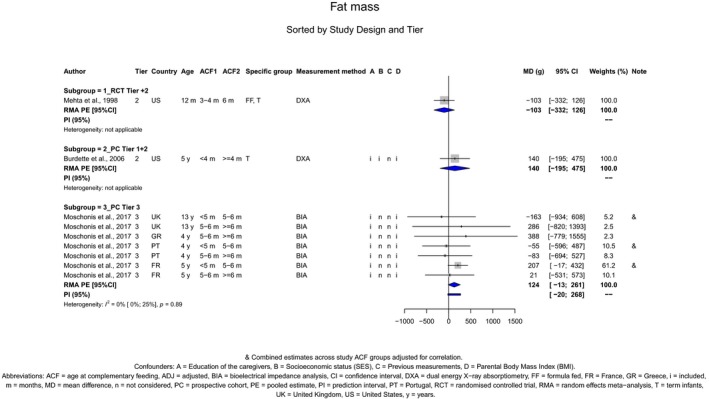
A.19. Fat mass z‐score comparing early introduction with later introduction of CFs
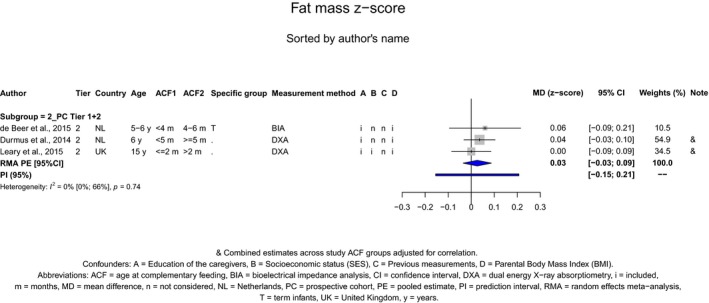
A.20. Skinfold thickness comparing early introduction with later introduction of CFs
A.21. Asthma‐like symptoms and CFs – general population – comparing early introduction with later introduction
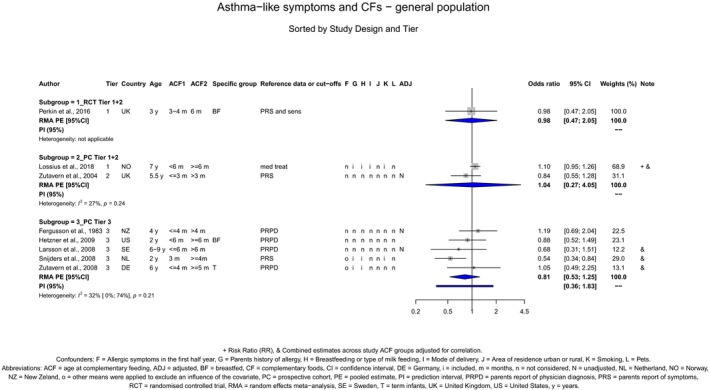
A.22. Asthma‐like symptoms and CFs – at‐risk population – comparing early introduction with later introduction

A.23. Asthma‐like symptoms and cereals – general population – comparing early introduction with later introduction
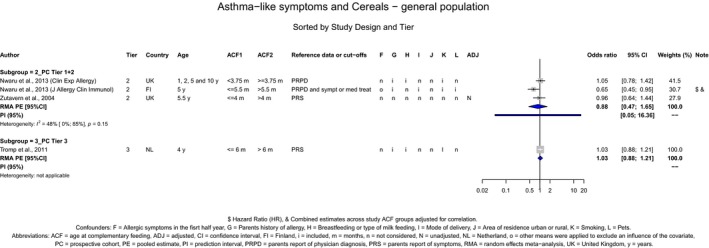
A.24. Asthma‐like symptoms and fish – general population – comparing early introduction with later introduction
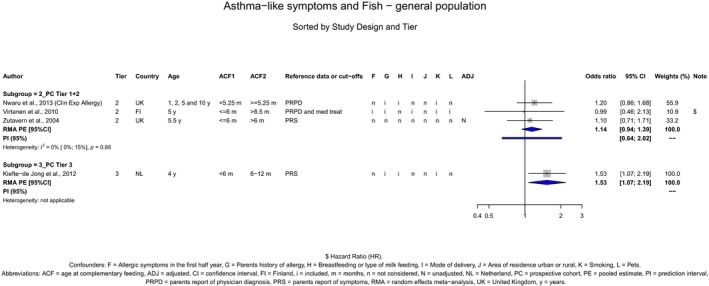
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup of prospective cohort studies rated as Tiers 1 and 2: PE = 1.14; 95% CI [0.88; 1.46]
A.25. Eczema and CFs – general population – comparing early introduction with later introduction
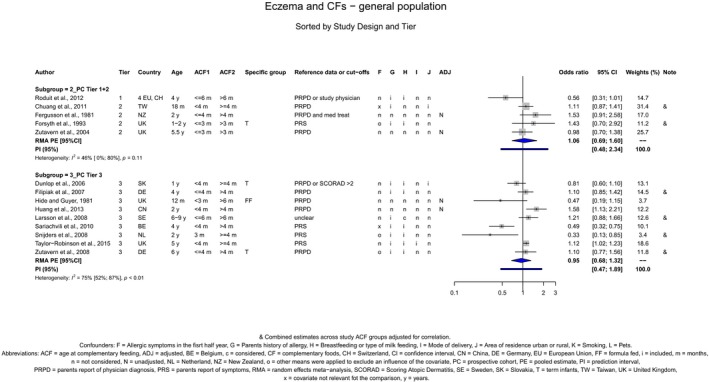
A.26. Eczema and CFs – at‐risk population – comparing early introduction with later introduction
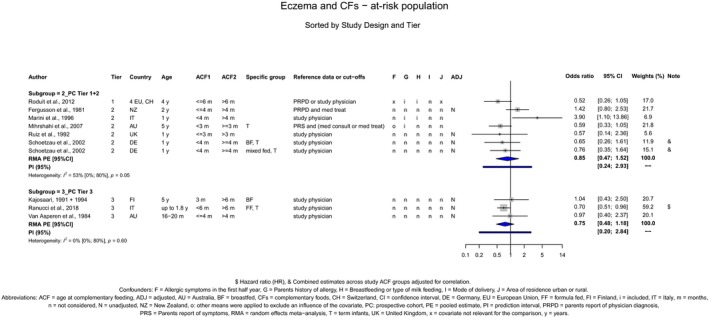
A.27. Eczema and CFs – general population – comparing early introduction with later introduction, retrospective studies
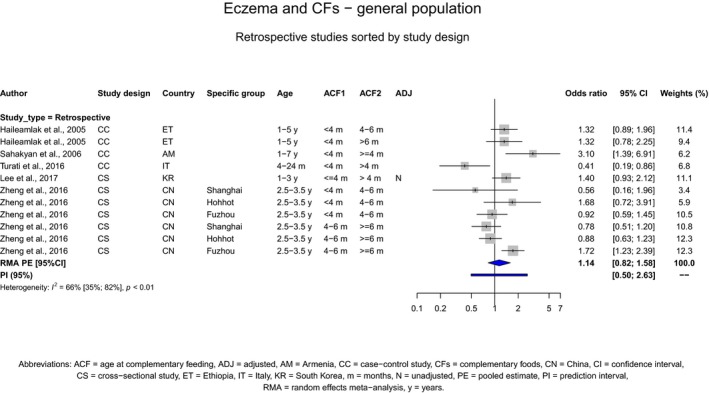
A.28. Eczema and egg – general population – comparing early introduction with later introduction

A.29. Eczema and egg – at‐risk population – comparing early introduction with later introduction
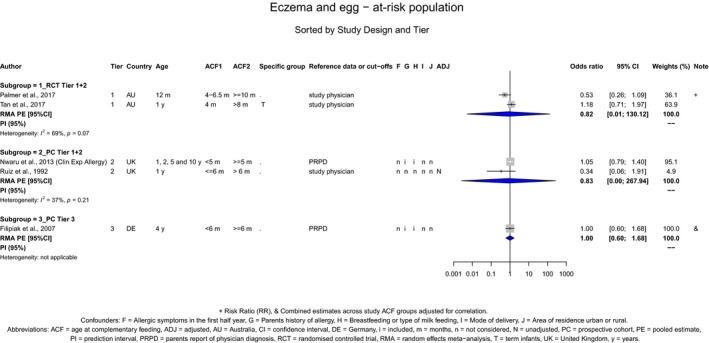
A.30. Eczema and cereals – general population – comparing early introduction with later introduction
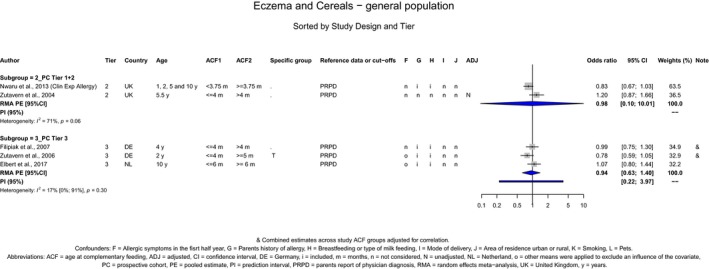
A.31. Eczema and fish – general population – comparing early introduction with later introduction
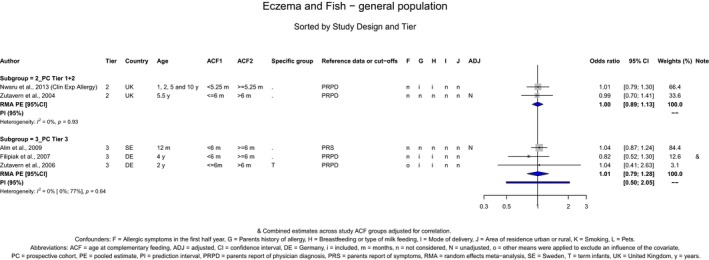
Random effects meta‐analysis calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification:
-
o
subgroup of prospective cohort studies rated as Tiers 1 and 2: PE = 1; 95% CI [0.81; 1.22]
A.32. Allergic rhinitis and CFs – general population – comparing early introduction with later introduction
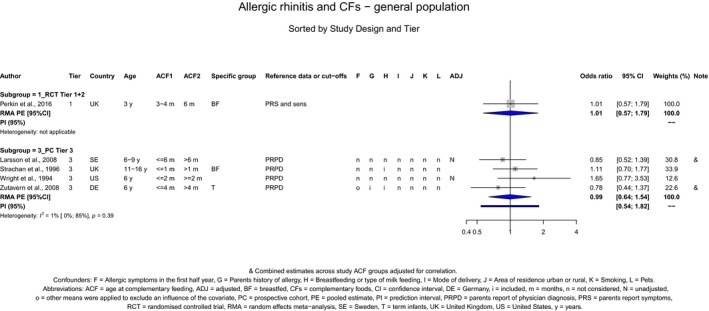
A.33. Symptomatic food allergy and CFs – general population – comparing early introduction with later introduction
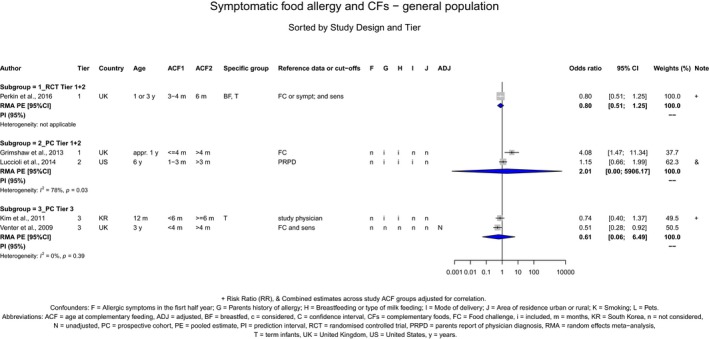
A.34. Sensitisation and CFs – general population – comparing early introduction with later introduction
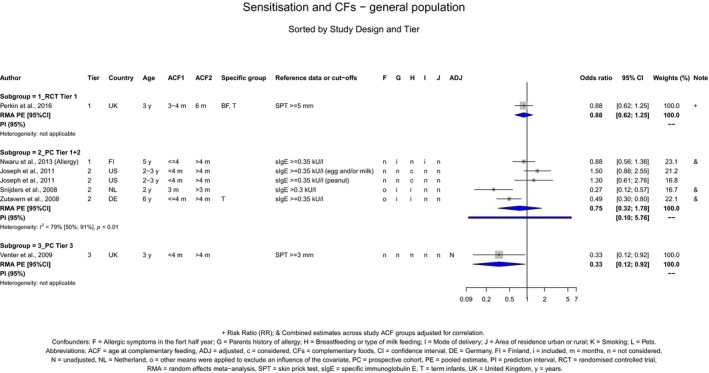
A.35. Sensitisation and CFs – at‐risk population – comparing early introduction with later introduction

A.36. Symptomatic food allergy and egg – at‐risk population – comparing early introduction with later introduction
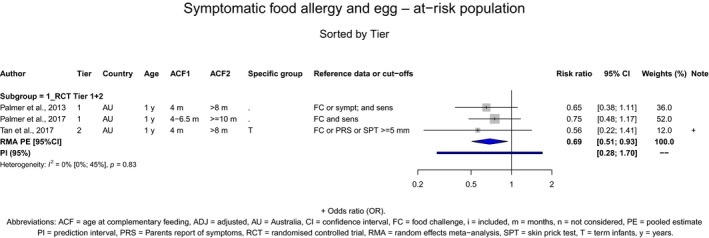
A.37. Sensitisation and egg – general population – comparing early introduction with later introduction
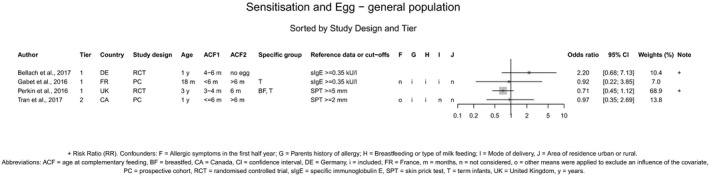
A.38. Sensitisation and egg – at‐risk population – comparing early introduction with later introduction
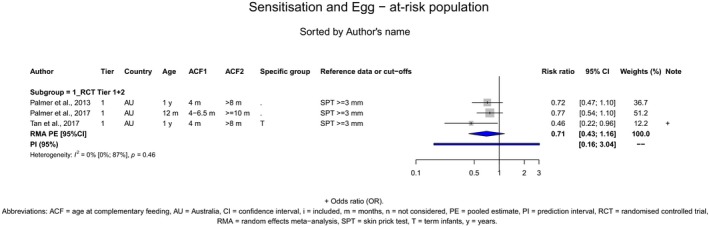
A.39. Coeliac disease and gluten comparing early introduction with later introduction
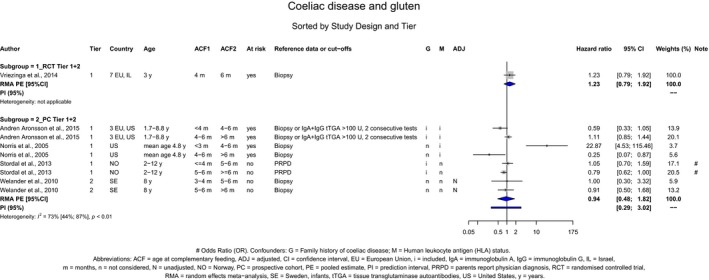
A.40. Coeliac disease and gluten comparing early introduction with later introduction, retrospective studies
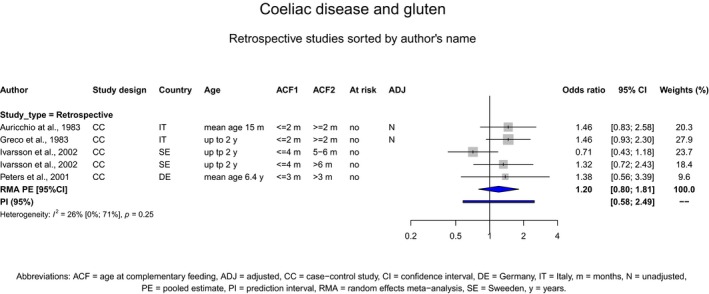
A.41. Coeliac disease autoimmunity and gluten comparing early introduction with later introduction
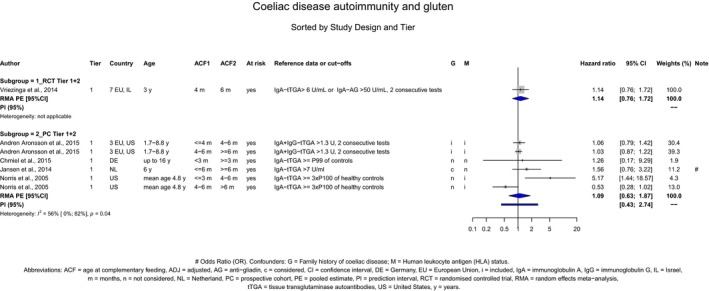
A.42. Type 1 diabetes mellitus and CFs comparing early introduction with later introduction
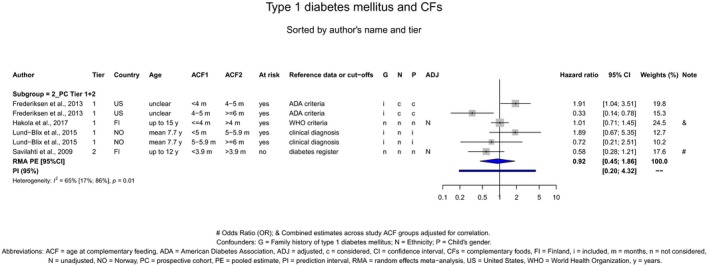
A.43. Type 1 diabetes mellitus and CFs comparing early introduction with later introduction, retrospective studies
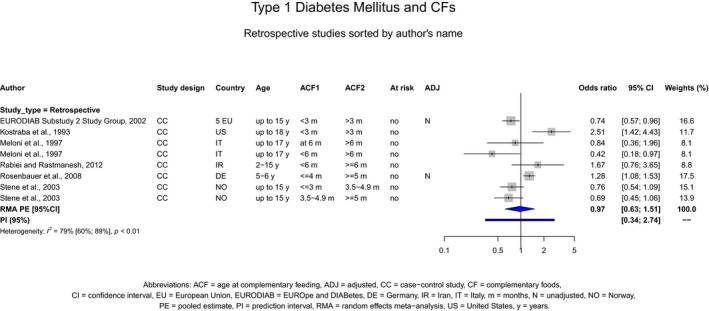
A.44. Type 1 diabetes mellitus and gluten comparing early introduction with later introduction
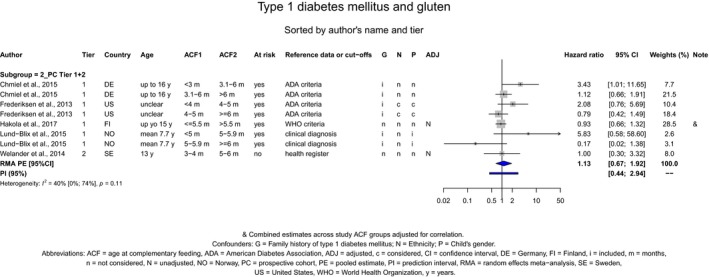
A.45. Islet autoimmunity and gluten comparing early introduction with later introduction of CFs
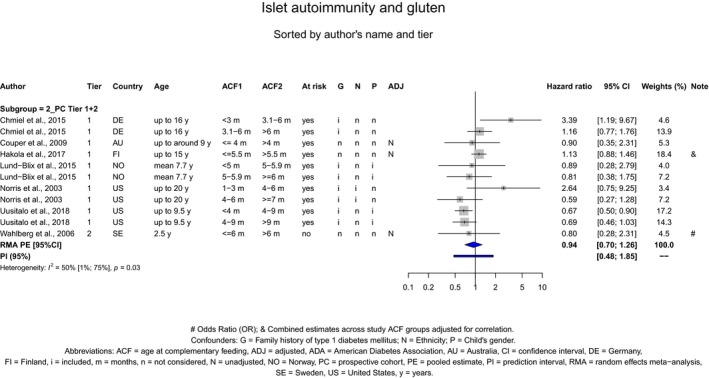
A.46. Systolic blood pressure comparing early introduction with later introduction of CFs

A.47. Diastolic blood pressure comparing early introduction with later introduction of CFs
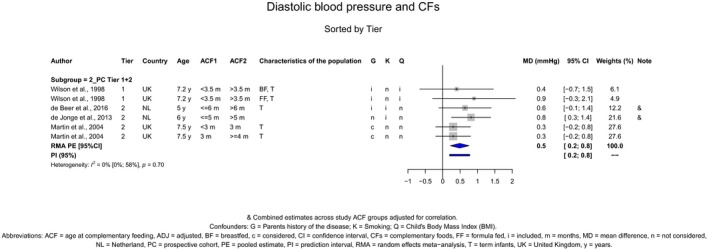
A.48. Risk of iron depletion at 6 months of age (SF < 12 μg/L) in exclusively breastfed infants comparing early introduction with later introduction of CFs*
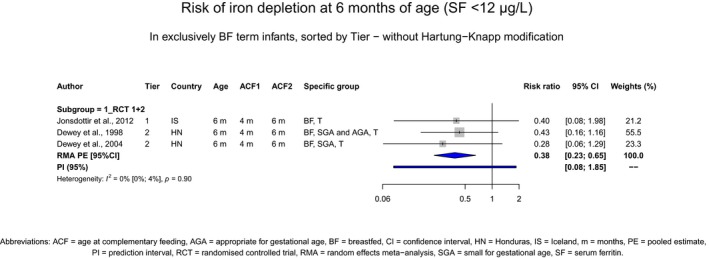
*The meta‐analysis was calculated using the DerSimonian and Laird approach without the Hartung and Knapp (H&K) modification.
Appendix B – Publications considered in the assessment
B.1. Body weight, body length/height and head circumference – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoints assessed | Additional comments |
|---|---|---|---|---|---|
| Cohen et al. ( 1995a ) | 2 | RCT | n/a |
Attained body length Attained body weight Length gain Weight gain |
No PE No PE No PE No PE |
| Dewey et al. ( 1999 ) | 2 | RCT | n/a |
L(H)AZ WAZ |
|
| Jonsdottir et al. ( 2014 ) | 1 | RCT | n/a |
Attained HC Attained body length Attained body weight HCZ L(H)AZ WAZ |
No PE No PE No PE |
| Mehta et al. ( 1998 ) | 2 | RCT | n/a |
Attained HC Attained body length Attained body weight |
|
| Perkin et al. ( 2016 ) | 1 | RCT | Enquiring About Tolerance (EAT) |
Attained HC HCZ Attained body length Attained body weight L(H)AZ WAZ WL(H)Z |
|
| Azad et al. ( 2018 ) | 2 | PC | Canadian Healthy Infant Longitudinal Development (CHILD) |
WAZ gain Rapid/high weight gain |
|
| Atkins et al. ( 2016 ) | 3 | PC | Melbourne Infant Feeding, Activity and Nutrition Trial (InFANT) |
Attained body length Attained body weight |
|
| Baker et al. ( 2004 ) | 3 | PC | Danish National Birth Cohort (DNBC) | Weight gain | No PE |
| Butte et al. ( 2000 ) | 2 | PC | n/a |
WAZ WL(H)Z L(H)AZ |
|
| de Beer et al. ( 2015 ) | 2 | PC | Amsterdam Born Children and their Development (ABCD) |
CLG CWG L(H)AZ |
|
| Eriksen et al. ( 2017 ) | 1 | PC | Early Nutrition and Immune Development (ENID) |
WAZ WL(H)Z |
|
| Forsyth et al. ( 1993 ) | 1 | PC | Dundee Infant Feeding Study | Attained body weight | |
| Gaffney et al. ( 2012 ) | 3 | PC | Infant feeding practices study (IFPS) II and year 6 follow‐up (Y6FU) | WAZ | |
| Griffiths et al. ( 2009 ) | 2 | PC | Millennium Cohort Study (MCS) | CWG | |
| Griffiths et al. ( 2010 ) | 3 | PC | MCS | Rapid/high weight gain | No PE |
| Grote et al. ( 2011 ) | 1 | PC | n/a |
Attained body length Attained body weight L(H)AZ L(H)AZ‐trajectories WAZ WAZ‐trajectories WL(H)Z WL(H)Z‐trajectories |
No PE No PE No PE |
| Haschke and van't Hof ( 2000 ) | 2 | PC | Euro‐Growth Study |
L(H)AZ Length gain WAZ Weight gain |
CV CV |
| Heinig et al. ( 1993 ) | 3 | PC | Davis Area Research on Lactation, Infant Nutrition and Growth (DARLING) |
L(H)AZ Length gain WAZ Weight gain |
|
| Hodgson ( 1978 ) | 3 | PC | n/a | Attained body weight | |
| Huh et al. ( 2011 ) | 3 | PC | ProjectViva |
Attained body length Attained body weight L(H)AZ WAZ |
|
| Imai et al. ( 2014 ) | 2 | PC | n/a |
Attained body length Attained body weight Weight gain |
|
| Kalanda et al. ( 2006 ) | 3 | PC | n/a | ||
| Kalies et al. ( 2005 ) | 3 | PC | Einfluss von Lebensbedingungen und Verhaltensweisen auf die Entwicklung von Immunsystem und Allergien (LISA) | Rapid/high weight gain | |
| Kramer et al. ( 1985b ); Kramer et al. ( 1985a ) | 2 | PC | n/a | Attained body weight | CV, no PE |
| Layte et al. ( 2014 ) | 2 | PC | n/a | Rapid/high weight gain | |
| Mäkelä et al. ( 2014 ) | 2 | PC | Steps to Healthy Development (STEPS) | Weight gain | |
| Morgan et al. ( 2004 ) | 3 | PA | n/a |
Attained body length Attained body weight HC gain Weight gain Length gain |
No PE No PE |
| Moschonis et al. ( 2017 ) | 3 | PC | Greek EuroPrevall | L(H)AZ | |
| 3 | PC | Generation XXI | L(H)AZ | ||
| 2 | PC | Etude des déterminants pré et postnatals précoces du développement et de la santé (EDEN) | L(H)AZ | ||
| 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) | L(H)AZ | ||
| Noppornlertwong and Tantibhaedhyangkul ( 2016 ) | 2 | PC | n/a |
Attained body weight Weight gain Attained body length Length gain Attained HC HC gain |
|
| Simondon and Simondon ( 1997 ) | 2 | PC | n/a |
Length gain Weight gain |
|
| Vail et al. ( 2015 ) | 3 | PC | Cambridge Baby Growth Study (CBGS) |
L(H)AZ WAZ |
CV CV |
| van Rossem et al. ( 2013 ) | 2 | PC | Generation R | WL(H)Z | |
| Warrington and Storey ( 1988 ) | 3 | PC | n/a | Attained body weight | No PE |
| WHO Working Group on Infant Growth ( 1994 ) | 3 | PA | WHO Growth Reference Study |
L(H)AZ WAZ WL(H)Z |
No PE No PE No PE |
| Wilson et al. ( 1998 ) | 2 | PC | Dundee Infant Feeding Study | WAZ | |
| Wright et al. ( 2004 ) | 3 | PC | Millennium Baby Study | CWG | No PE |
| Kim and Peterson ( 2008 ) | 3 | RETRO: CSA of baseline data of a PC | Early Childhood Longitudinal Study‐Birth Cohort (ECLS‐B) | Weight gain | |
| Klag et al. ( 2015 ) | 3 | RETRO: RC | Moms2Moms |
WAZ Weight gain WAZ gain |
CV |
| Mihrshahi et al. ( 2011 ) | 3 | RETRO: CSA of baseline data of an RCT | Nourishing Our Understanding of Role Modelling to Improve Support and Health (NOURISH) | Rapid/high weight gain | |
| Sit et al. ( 2001 ) | 3 | RETRO: CS | n/a | Proportion of children who had started CFs < 4 m in WAZ and WLZ tertiles | |
| Sloan et al. ( 2008 ) | 3 | RETRO: CSA of baseline data of a PC | n/a |
WAZ CWG |
|
| Zhu et al. ( 2015 ) | 3 | RETRO: CS | National Children's Study Formative Research in Anthropometry |
WAZ WL(H)Z L(H)AZ |
CV |
CLG: conditional length gain; CS: cross‐sectional study; CSA: cross‐sectional analysis; CWG: conditional weight gain; CV: timing of introduction of CF used as a continuous variable in the analysis, HC: head circumference; HCZ: head circumference‐for‐age z‐score; L(H)AZ: length (height)‐for‐age z‐score; m: months; n/a: not available; PA: pooled analysis; PC: prospective cohort study; PE: point estimate; RC: retrospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; WAZ: weight‐for‐age z‐score; WHO: World Health Organization; WL(H)Z: weight‐for‐length (height) z‐score.
B.2. BMI and related endpoints – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoints assessed | Additional comments |
|---|---|---|---|---|---|
| Jonsdottir et al. ( 2014 ) | 1 | RCT | n/a |
Attained BMI BMIZ |
No PE |
| Perkin et al. ( 2016 ) | 1 | RCT | Enquiring About Tolerance (EAT) |
Attained BMI BMIZ |
|
| Agras et al. ( 1990 ) | 1 | PC | n/a | Attained BMI | No PE |
| Azad et al. ( 2018 ) | 2 | PC | Canadian Healthy Infant Longitudinal Development (CHILD) | BMIZ | |
| Burdette et al. ( 2006 ) | 2 | PC | n/a | BMIZ | |
| Caleyachetty et al. ( 2013 ) | 2 | PC | Mysore Parthenon Study | High BMI | CV |
| de Beer et al. ( 2015 ) | 2 | PC | Amsterdam Born Children and their Development (ABCD) | BMIZ | |
| Durmuş et al. ( 2014 ) | 2 | PC | Generation R | BMIZ | Data from Vogelezang et al. (2018) considered |
| Fairley et al. ( 2015 ) | 2 | PC | Born in Bradford | BMIZ | |
| Garden et al. ( 2012 ) | 2 | PC | Childhood Asthma Prevention Study (CAPS) | BMI trajectory class membership | No PE |
| Grote et al. ( 2011 ) | 1 | PC | n/a |
Attained BMI BMIZ BMIZ trajectories |
No PE |
| Haschke and van't Hof ( 2000 ) | 3 | PC | Euro‐Growth Study |
BMI gain BMIZ |
CV |
| Huh et al. ( 2011 ) | 3 | PC | ProjectViva | Attained BMI | |
| 2 | BMIZ | ||||
| Iguacel et al. ( 2018 ) | 3 | PC | Longitudinal Study of Australian Children (LSAC) | BMIZ | |
| Imai et al. ( 2014 ) | 3 | PC | n/a | Attained BMI | |
| Kramer et al. ( 1985b ); Kramer et al. ( 1985a ) | 2 | PC | n/a | Attained BMI | No PE, CV |
| Lande et al. ( 2005 ) | 1 | PC | n/a | Attained BMI | No PE, CV |
| Leary et al. ( 2015 ) | 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) | BMIZ | |
| Poskitt and Cole ( 1978 ) | 3 | PC | n/a | % expected weight | No PE, CV |
| Robinson et al. ( 2009 ) | 2 | PC | Southampton Women's Survey (SWS) | Attained BMI | No PE, CV |
| Salahuddin et al. ( 2017 ) | 3 | PC | Early Childhood Longitudinal Study‐Birth Cohort (ECLS‐B) | BMI trajectory class membership | |
| Schack‐Nielsen et al. ( 2010 ) | 3 | PC | Copenhagen Perinatal Cohort |
BMIZ Waist circumference |
CV CV |
| Schmidt Morgen et al. ( 2018 ) | 3 | PC | Danish National Birth Cohort (DNBC) | BMIZ | |
| Sirkka et al. ( 2018 ) | 3 | PC | ABCD | BMIZ | Data from de Beer et al. (2015) considered |
| Thorogood et al. ( 1979 ) | 1 | PC | n/a | Shukla index | No PE, CV |
| Vail et al. ( 2015 ) | 3 | PC | Cambridge Baby Growth Study (CBGS) | BMIZ | CV |
| Veena et al. ( 2010 ) | 3 | PC | Mysore Parthenon Study | Attained BMI | |
| Vogelezang et al. ( 2018 ) | 2 | PC | Generation R | BMIZ | |
| Wen et al. ( 2014 ) | 1 | PC | Healthy Beginnings | Attained BMI | |
| Wilson et al. ( 1998 ) | 3 | PC | Dundee Infant Feeding Study | BMIZ | No PE |
| Zheng et al. ( 2015 ) | 2 | PC | Jiaxing Birth Cohort | BMIZ | |
| Brambilla et al. ( 2016 ) | 3 | RETRO: CS | New Millennium Baby Study | BMIZ | |
| Kramer ( 1981 ) | 3 | RETRO: CS | n/a | Relative weight | No PE, CV |
| Lin et al. ( 2013 ) | 3 | RETRO: PC with exposure assessed after outcome | Hong Kong Children of 1997 | BMIZ | |
| Magalhaes et al. ( 2012 ) | 3 | RETRO: RC | n/a | High waist circumference | |
| Patterson et al. ( 1986 ) | 3 | RETRO: CS | n/a | Relative weight | No PE, CV |
| Vafa et al. ( 2012 ) | 3 | RETRO: CS | n/a |
Attained BMI BMI class membership |
No PE |
| Zhu et al. ( 2015 ) | 3 | RETRO: CS | National Children's Study Formative Research in Anthropometry | BMIZ | No PE, CV |
| Zive et al. ( 1992 ) | 3 | RETRO: CSA of baseline data of a PC | Study of Children's Activity and Nutrition (SACN) | Attained BMI | No PE, CV |
BMI: body mass index; BMIZ: body mass index‐for‐age z‐score; CS: cross‐sectional study; CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; PC: prospective cohort study; PE: point estimate; RCT: randomised controlled trial; RETRO: retrospective study; RC: retrospective cohort study; RoB: risk of bias.
B.3. Obesity and overweight – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoints assessed | Additional comments |
|---|---|---|---|---|---|
| Jonsdottir et al. ( 2014 ) | 1 | RCT | n/a | Odds/risk of developing (at least) overweight | |
| Abraham et al. ( 2012 ) | 3 | PC | Growing‐up in Scotland (GUS) | Odds/risk of developing (at least) overweight | |
| Aris et al. ( 2018 ) | 3 | PC | Growing Up in Singapore Towards healthy Outcomes (GUSTO) | Odds/risk of developing (at least) overweight | |
| Azad et al. ( 2018 ) | 2 | PC | Canadian Healthy Infant Longitudinal Development (CHILD) | Odds/risk of developing (at least) overweight | |
| Barrera et al. ( 2016 ) | 3 | PC | Infant feeding practices study (IFPS) II and year 6 follow‐up (Y6FU) | Odds/risk of developing obesity | |
| Bell S et al. ( 2018 ) | 3 | PC | Study of Mothers and Infants Life Events Affecting Oral Health (SMILE) | Odds/risk of developing (at least) overweight | |
| Brophy et al. ( 2009 ) | 2 | PC | Millennium Cohort Study (MCS) | Odds/risk of developing obesity | |
| Burdette et al. ( 2006 ) | 2 | PC | n/a | % overweight | |
| Durmuş et al. ( 2014 ) | 2 | PC | Generation R | Odds/risk of developing (at least) overweight | |
| Fairley et al. ( 2015 ) | 2 | PC | Born in Bradford | Odds/risk of developing (at least) overweight | |
| Flores and Lin ( 2013a ) | 3 | PC | Early Childhood Longitudinal Study‐Birth Cohort (ECLS‐B) | Timing of CF introduction in cases and controls (severe obesity) | No PE |
| Flores and Lin ( 2013b ) | 3 | PC | ECLS‐B | Odds/risk of developing (at least) overweight | |
| Timing of CF introduction in cases and controls (overweight) | No PE | ||||
| Gibbs and Forste ( 2014 ) | 3 | PC | ECLS‐B | Odds/risk of developing obesity | Data from Moss and Yeaton (2014) considered |
| Gooze et al. ( 2011 ) | 3 | PC | ECLS‐B | Odds/risk of developing obesity | Data from Moss and Yeaton (2014) considered |
| Hawkins et al. ( 2009 ) | 2 | PC | MCS | Odds/risk of developing (at least) overweight | Data from Massion et al. (2016) were considered |
| Hollis et al. ( 2016 ) | 3 | PC | Southampton Women's Survey (SWS) | Odds/risk of developing (at least) overweight | |
| Huh et al. ( 2011 ) | 2 | PC | Project Viva | Odds/risk of developing obesity | |
| Layte et al. ( 2014 ) | 2 | PC | n/a | Odds/risk of developing obesity | |
| Mäkelä et al. ( 2014 ) | 1 | PC | Steps to Healthy Development (STEPS) | Odds/risk of developing (at least) overweight | CV |
| Odds/risk of developing obesity | CV | ||||
| Massion et al. ( 2016 ) | 2 | PC | MCS | Odds/risk of developing (at least) overweight | |
| Moschonis et al. ( 2017 ) | 3 | PC | Greek EuroPrevall | Odds/risk of developing (at least) overweight | |
| 3 | PC | Generation XXI | Odds/risk of developing (at least) overweight | ||
| 2 | PC | Etude des déterminants pré et postnatals précoces du développement et de la santé (EDEN) | Odds/risk of developing (at least) overweight | ||
| 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) | Odds/risk of developing (at least) overweight | ||
| Moss and Yeaton ( 2014 ) | 3 | PC | ECLS‐B | Odds/risk of developing (at least) overweight | |
| Odds/risk of developing obesity | |||||
| Neutzling et al. ( 2009 ) | 2 | PC | n/a | Odds/risk of developing (at least) overweight | |
| Odds/risk of developing obesity | |||||
| Pluymen et al. ( 2018 ) | 3 | PC | Prevention and Incidence of Asthma and Mite Allergy (PIAMA) | Odds/risk of developing (at least) overweight | |
| Reilly et al. ( 2005 ) | 2 | PC | ALSPAC | Odds/risk of developing obesity | |
| Rios‐Castillo et al. ( 2015 ) | 3 | PC | n/a | Timing of CF introduction in cases and controls (overweight) | |
| Rossiter and Evers ( 2013 ) | 2 | PC | Better Beginnings, Better Futures | Odds/risk of developing (at least) overweight | |
| Schack‐Nielsen et al. ( 2010 ) | 3 | PC | Copenhagen Perinatal Cohort | Odds/risk of developing (at least) overweight | CV |
| Odds/risk of developing obesity | CV | ||||
| Schmidt Morgen et al. ( 2018 ) | 3 | PC | Danish National Birth Cohort (DNBC) | Odds/risk of developing (at least) overweight | |
| Seach et al. ( 2010 ) | 3 | PC | Melbourne Atopy Cohort Study (MACS) | Odds/risk of developing (at least) overweight | CV |
| Sirkka et al. ( 2018 ) | 3 | PC | Amsterdam Born Children and their Development (ABCD) | Odds/risk of developing (at least) overweight | |
| Wen et al. ( 2014 ) | 1 | PC | Healthy Beginnings | Odds/risk of developing (at least) overweight | |
| Wolman ( 1984 ) | 3 | PC | n/a | % obese | No PE |
| Zheng et al. ( 2015 ) | 2 | PC | Jiaxing Birth Cohort | Odds/risk of developing (at least) overweight | |
| Odds/risk of developing obesity | |||||
| Birbilis et al. ( 2013 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| 3 | Odds/risk of developing obesity | ||||
| Butte ( 2009 ) | 3 | RETRO: CS | Viva La Familia | Odds/risk of developing (at least) overweight | CV |
| Cu et al. ( 2015 ) | 3 | RETRO: CC | n/a | Odds/risk of developing (at least) overweight | |
| Gillman et al. ( 2001 ) | 3 | RETRO: CS | Growing Up Today | Odds/risk of developing (at least) overweight | No PE, CV |
| Odds/risk of developing obesity | No PE, CV | ||||
| Gomes et al. ( 2010 ) | 3 | RETRO: CC | n/a | Timing of introduction of CF in cases and control (overweight) | |
| Gungor et al. ( 2010 ) | 3 | RETRO: CC | n/a | Timing of introduction of CF in cases and control (overweight) | |
| Hediger et al. ( 2001 ) | 3 | RETRO: CS | National Health and Nutrition Examination Survey (NHANES) III | Odds/risk of developing (at least) overweight | CV |
| Jiang et al. ( 2009 ) | 3 | RETRO: CC | n/a | Odds of having been introduced to CF < 4 months ‐ overweight | |
| Jimenez‐Cruz et al. ( 2010 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| Lin et al. ( 2013 ) | 3 | RETRO: PC with exposure assessed after outcome | Hong Kong Children of 1997 | Odds/risk of developing (at least) overweight | |
| Magalhaes et al. ( 2012 ) | 3 | RETRO: RC | n/a | Odds/risk of developing (at least) overweight | |
| Nascimento Simon et al. ( 2009 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| Papoutsou et al. ( 2018 ) | 3 | RETRO: CSA of baseline data of a PC | Identification and prevention of dietary‐ and lifestyle‐induced health effects in children and infants (IDEFICS) | Odds/risk of developing (at least) overweight | |
| Rathnayake et al. ( 2013 ) | 3 | RETRO: CC | n/a | Odds/risk of developing (at least) overweight | |
| Sandoval Jurado et al. ( 2016 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| Sinigaglia et al. ( 2016 ) | 3 | RETRO: CS | n/a | Odds of being normal weight | CV |
| Škledar and Milošević ( 2015 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| Sun et al. ( 2016 ) | 3 | RETRO: CSA of baseline data | HealthNuts | Odds/risk of developing (at least) overweight | |
| Vehapoglu et al. ( 2014 ) | 3 | RETRO: CS | n/a | Odds/risk of developing (at least) overweight | |
| Zhou et al. ( 2011 ) | 3 | RETRO: CS | n/a | Odds/risk of developing obesity | |
| Odds of having been introduced to CF < 4 months ‐ obesity |
CC: case–control study; CF: complementary food; CS: cross‐sectional study; CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; PC: prospective cohort study; PE: point estimate; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias.
B.4. Body composition – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoints assessed | Additional comments |
|---|---|---|---|---|---|
| Mehta et al. ( 1998 ) | 2 | RCT | n/a |
% fat mass Fat mass Lean mass |
|
| Perkin et al. ( 2016 ) | 1 | RCT | Enquiring About Tolerance (EAT) |
Subscapular SFT Triceps SFT |
|
| Burdette et al. ( 2006 ) | 2 | PC | n/a |
Fat mass High fat mass |
|
| Caleyachetty et al. ( 2013 ) | 2 | PC | Mysore Parthenon Study | % difference in triceps + subscapular SFT | CV |
| de Beer et al. ( 2015 ) | 2 | PC | Amsterdam Born Children and their Development (ABCD) |
Fat free mass z‐score Fat mass z‐score |
|
| Durmuş et al. ( 2012 ) | 2 | PC | Generation R |
Subscapular + suprailiac SFT Triceps + biceps + subscapular + suprailiac SFT |
|
| Durmuş et al. ( 2014 ) | 2 | PC | Generation R |
Android:gynoid fat ratio z‐score Fat mass z‐score Preperitoneal abdominal fat area z‐score |
|
| Ejlerskov et al. ( 2015 ) | 3 | PC | Smabørns Kost og Trivsel (SKOT) | Fat mass index | |
| Huh et al. ( 2011 ) | 3 | PC | ProjectViva | Triceps + subscapular SFT | |
| Kramer et al. ( 1985b ); Kramer et al. ( 1985a ) | 2 | PC | n/a | Triceps + subscapular + suprailiac SFT | 1985a: no PE, 1985a and b: CV |
| Leary et al. ( 2015 ) | 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) |
Fat mass z‐score Lean mass z‐score |
|
| Morgan et al. ( 2004 ) | 3 | PA | n/a |
Subscapular SFT gain Triceps SFT gain |
|
| Moschonis et al. ( 2017 ) | 3 | PC | Greek EuroPrevall | Fat mass | |
| 3 | PC | Generation XXI | Fat mass | ||
| 2 | PC | Etude des déterminants pré et postnatals précoces du développement et de la santé (EDEN) | Fat mass | ||
| 2 | PC | ALSPAC |
Fat mass %fat mass |
||
| Robinson et al. ( 2009 ) | 2 | PC | Southampton Women's Survey (SWS) |
Fat mass Fat mass index |
No PE, CV |
| van den Hooven et al. ( 2016 ) | 1 | PC | Generation R |
aBMC BMD Bone area |
|
| Vogelezang et al. ( 2018 ) | 2 | PC | Generation R |
Fat mass index z‐score Fat‐free mass index z‐score |
|
| Wilson et al. ( 1998 ) | 3 | PC | Dundee Infant Feeding Study | % fat mass | |
| Magalhaes et al. ( 2012 ) | 3 | RETRO: RC | n/a |
High fat mass High fat in the android region |
|
| Patterson et al. ( 1986 ) | 3 | RETRO: CS | n/a | Triceps SFT | No PE, CV |
| Zive et al. ( 1992 ) | 3 | RETRO: CSA of baseline data of a PC | Study of Children's Activity and Nutrition (SACN) | Triceps + subscapular SFT | No PE, CV |
aBMC: areal bone mineral content; BMD: bone mineral density; CS: cross‐sectional study; CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; PA: pooled analysis; PC: prospective cohort study; PE: point estimate; RC: retrospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; SFT: skinfold thickness.
B.5. Atopic diseases – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | At‐risk group | Food | Outcome | Endpoint | Additional comments |
|---|---|---|---|---|---|---|---|---|
| Bellach et al. ( 2017 ) | 1 | RCT | Hen's Egg Allergy Prevention (HEAP) | No | Egg | Food allergy | Symptomatic food allergy | |
| Sensitisation | sIgE | |||||||
| Halpern et al. ( 1973 ) | 3 | RCT | n/a | No | Egg | Atopic disease | Atopic disease | |
| Palmer et al. ( 2013 ) | 1 | RCT | Western Australian Solids Timing for Allergy Research (STAR) | Yes | Egg | Food allergy | Symptomatic food allergy | |
| Sensitisation | SPT | |||||||
| Palmer et al. ( 2017 ) | 2 | RCT | Starting Time of Egg Protein (STEP) | Yes | Egg | Asthma‐like symptoms | Wheeze | |
| 1 | Eczema | Symptomatic eczema | ||||||
| 1 | Eczema | Atopic dermatitis | ||||||
| 1 | Food allergy | Symptomatic food allergy | ||||||
| 1 | Sensitisation | SPT | ||||||
| Perkin et al. ( 2016 ) | 1 | RCT | Enquiring About Tolerance (EAT) | No | CF, cereals, egg, peanut, fish | Food allergy | Symptomatic food allergy | No PE |
| Egg | Sensitisation | SPT | ||||||
| CF | Allergic rhinitis | Allergic rhinitis | ||||||
| CF | Atopic disease | Atopic disease | ||||||
| CF | Asthma‐like symptoms | Wheeze | ||||||
| Tan et al. ( 2017 ) | 1 | RCT | Beating Egg Allergy Trial (BEAT) | Yes | Egg | Eczema | Symptomatic eczema | |
| Food allergy | Probable food allergy | |||||||
| Sensitisation | SPT | |||||||
| Alm et al. ( 2009 ) | 3 | PC | Infants of Western Sweden | No | Fish | Eczema | Symptomatic eczema | |
| Chuang et al. ( 2011 ) | 2 | PC | Taiwan Birth Cohort Study (TBCS) | No | CF | Eczema | Atopic dermatitis | |
| Dunlop et al. ( 2006 ) | 3 | PC | n/a | No | CF | Eczema | Atopic dermatitis | |
| Elbert et al. ( 2017 ) | 3 | PC | Generation R | No | Peanut | Food allergy | Symptomatic food allergy | |
| Peanut | Sensitisation | SPT | ||||||
| Cereals, egg, peanut | Eczema | Symptomatic eczema | ||||||
| Fergusson et al. ( 1981 ) | 2 | PC | Christ‐church Child Developmental Study | No/Yes | CF, | Eczema | Symptomatic eczema | |
| Cereals, egg | No PE | |||||||
| Fergusson et al. ( 1990 ) | 2 | PC | Christ‐church Child Developmental Study | No | Cereals, egg | Eczema | Symptomatic eczema | No PE |
| Fergusson et al. ( 1983 ) | 3 | PC | Christ‐church Child Developmental Study | No | CF | Asthma‐like symptoms | Asthma | |
| Filipiak et al. ( 2007 ) | 3 | PC | German Infant Nutritional Intervention Program (GINI) | No/Yes | CF, cereals, egg, fish | Eczema | Atopic dermatitis | |
| Eczema | Symptomatic eczema | |||||||
| Forsyth et al. ( 1993 ) | 2 | PC | Dundee Infant Feeding Study | No | CF | Eczema | Symptomatic eczema | |
| Gabet et al. ( 2016 ) | 1 | PC | Pollution and Asthma Risk: an Infant Study (PARIS) | No | Egg, fish | Sensitisation | sIgE | |
| Grimshaw et al. ( 2013 ) | 1 | NCC | Prevalence of Infant Food Allergy Study (PIFA) (UK Euro‐Prevall) | No | CF | Food allergy | Symptomatic food allergy | |
| Timing of introduction of CF in cases and controls | No PE | |||||||
| Hesselmar et al. ( 2010 ) | 1 | NCC | ALLERGY‐FLORA | No | CF | Asthma‐like symptoms | Timing of introduction of CFs in cases and controls | No PE |
| Eczema | Timing of introduction of CFs in cases and controls | No PE | ||||||
| Food allergy | Timing of introduction of CFs in cases and controls | No PE | ||||||
| Sensitisation | Timing of introduction of CFs in cases and controls | No PE | ||||||
| Hetzner et al. ( 2009 ) | 3 | PC | Early Childhood Longitudinal Study‐Birth Cohort (ECLS‐B) | No | CF | Asthma‐like symptoms | Asthma | |
| Hide and Guyer ( 1981 ) | 3 | PC | n/a | No | CF | Eczema | Symptomatic eczema | |
| Hua et al. ( 2017 ) | 3 | PC | Prediction of Allergies in Taiwanese Children (PATCH) | No | Aller‐genic foods (fruit, egg, fish, shell fish, peanut) | Eczema | Atopic dermatitis | |
| CF | Sensitisation | BioIC | ||||||
| CF | Sensitisation | Timing of introduction of CFs | ||||||
| Huang et al. ( 2013 ) | 3 | PC | n/a | No | CF | Eczema | Symptomatic eczema | |
| Illi et al. ( 2004 ) | 3 | PC | Multicenter Allergy Study (MAS) | No | CF | Eczema | Atopic dermatitis | No PE |
| Jonsson et al. ( 2017 ) | 2 | PC | FARM‐FLORA | No | Cereals | Atopic disease | Atopic disease | CV |
| Joseph et al. ( 2011 ) | 2 | PC | Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS) | No/Yes | CF | Sensitisation | sIgE | |
| Kajosaari and Saarinen ( 1983 ) | 3 | PC | n/a | Yes | CF | Eczema | Atopic dermatitis | |
| Food allergy | Symptomatic food allergy | |||||||
| Kajosaari ( 1991 , 1994 ) | 3 | PC | n/a | Yes | CF | Asthma‐like symptoms | Asthma | |
| Atopic disease | Atopic disease | |||||||
| Eczema | Atopic dermatitis | |||||||
| Keijzers et al. ( 2018 ) | 3 | PC | Environments for Healthy Living (EFHL): Griffith Birth Cohort | No | CF | Atopic disease | Atopic disease | |
| Kiefte‐de Jong et al. ( 2012 ) | 3 | PC | Generation R | No | Fish | Asthma‐like symptoms | Wheeze in the past 12 months | |
| Kim et al. ( 2011 ) | 3 | PC | n/a | No | CF | Food allergy | Symptomatic food allergy | |
| Kurukulaaratchy et al. ( 2004 ) | 3 | PC | Isle of Wight Birth Cohort | No | CF | Asthma‐like symptoms | Early‐onset persistent wheeze | No PE |
| Late‐onset persistent wheeze | No PE | |||||||
| Larsson et al. ( 2008 ) | 3 | PC | Dampness in Building and Health (DBH) | No | CF | Allergic rhinitis | Allergic rhinitis | |
| Allergic rhinitis | Allergic rhinitis symptoms | |||||||
| Asthma‐like symptoms | Asthma | |||||||
| Eczema | Symptomatic eczema | |||||||
| Lawson et al. ( 2017 ) | 2 | Observational analysis of RCT | Learning Early About Peanut Allergy (LEAP) | Yes | Peanut | Food allergy | Symptomatic food allergy | |
| Lossius et al. ( 2018 ) | 1 | PC | Den norske mor og barn‐undersøkelsen (MoBa) | No | CF | Asthma‐like symptoms | Asthma | |
| Luccioli et al. ( 2014 ) | 2 | PC | Infant feeding practices study (IFPS) II and year 6 follow‐up (Y6FU) | No/Yes | CF | Food allergy | Diagnosed food allergy | |
| Marini et al. ( 1996 ) | 2 | PC | n/a | Yes | CF | Allergic rhinitis | Allergic rhinitis | |
| Asthma‐like symptoms | Recurrent wheeze | |||||||
| Eczema | Atopic dermatitis | |||||||
| McGowan et al. ( 2015 ) | 2 | NCC | Urban Environment and Childhood Asthma (URECA) | Yes | CF | Food allergy | Timing of introduction of CFs in cases and controls | |
| Sensitisation | Timing of introduction of CFs in cases and controls | |||||||
| Mihrshahi et al. ( 2007 ) | 2 | PC | Childhood Asthma Prevention Study (CAPS) | Yes | CF | Asthma‐like symptoms | Asthma | |
| Eczema | Symptomatic eczema | |||||||
| Sensitisation | SPT | |||||||
| Moore et al. ( 1985 ) | 2 | PC | n/a | Yes | CF | Eczema | Symptomatic eczema | No PE |
| Morgan et al. ( 2004 ) | 3 | PA | n/a | No | CF | Asthma‐like symptoms | Asthma | No PE |
| Asthma‐like symptoms | Wheeze | No PE | ||||||
| Eczema | Symptomatic eczema | No PE | ||||||
| Nwaru et al. ( 2010 ) | 2 | PC | Type 1 Diabetes Prediction and Prevention (DIPP) nutrition | No | CF, cereals | Sensitisation | sIgE |
Data from Nwaru et al. (2013c) (Allergy) considered |
|
Nwaru et al. ( 2013c ) (Allergy) |
1 | PC | DIPP nutrition | No/Yes | CF, cereals, fish | Sensitisation | sIgE | |
|
Nwaru et al. ( 2013a ) (J Allergy Clin Immunol) |
3 | PC | DIPP nutrition | No | CF, cereals, fish | Allergic rhinitis | Allergic rhinitis | |
| Asthma‐like symptoms | Asthma | |||||||
| Eczema | Atopic dermatitis | No PE | ||||||
|
Nwaru et al. ( 2013b ) (Clin Exp Allergy) |
2 | PC | Study of Eczema and Asthma To Observe the influence of Nutrition (SEATON) | No/Yes | Cereals, egg, fish | Asthma‐like symptoms | Wheeze in the past 12 months | |
| Asthma‐like symptoms | Wheeze without cold in the past 12 months | |||||||
| Asthma‐like symptoms | Asthma | |||||||
| Eczema | Symptomatic eczema | |||||||
| Poole et al. ( 2006 ) | 3 | PC | Diabetes Auto‐immunity Study in the Young (DAISY) | No | Cereals | Food allergy | Probable food allergy | |
| Pöysä et al. ( 1991 ) | 2 | PC | n/a | Yes | CF | Atopic disease | Atopic disease | |
| Ranucci et al. ( 2018 ) | 3 | Observational analysis of an RCT | Prebiotics in Prevention of Atopy (PIPA) | Yes | CF | Eczema | Atopic dermatitis | |
| Roduit et al. ( 2012 ) | 1 | PC | Protection Against Allergy–Study in Rural Environments (PASTURE) | No/Yes | CF | Eczema | Atopic dermatitis with onset after 1 year | |
| Ruiz et al. ( 1992 ) | 2 | PC | n/a | Yes | CF, egg | Eczema | Atopic dermatitis | |
| Sandini et al. ( 2011 ) | 2 | PC | n/a | Yes | CF | Atopic disease | Atopic disease | No PE |
| Food allergy | Symptomatic food allergy | No PE | ||||||
| Eczema | Atopic dermatitis | No PE | ||||||
| Asthma‐like symptoms | Asthma | No PE | ||||||
| Allergic rhinitis | Allergic rhinitis | No PE | ||||||
| Sariachvili et al. ( 2010 ) | 3 | NCC | Prospective Cohort on the Influence of Perinatal Factors on the Occurrence of Asthma and Allergies (PIPO) | No | CF | Eczema | Symptomatic eczema | |
| Eczema | Odds of having been introduced to CF < 4 months | |||||||
| Savilahti et al. ( 1987 ) | 2 | PC | n/a | No | CF | Atopic disease | Atopic disease | No PE, CV |
| Schoetzau et al. ( 2002 ) | 2 | PC | GINI | Yes | CF | Eczema | Atopic dermatitis | |
| Snijders et al. ( 2008 ) | 3 | PC | Kind, ouders en gezondheid: aandacht voor leefstijl en aanleg (KOALA) | No | CF | Asthma‐like symptoms | Recurrent wheeze | |
| Eczema | Symptomatic eczema | |||||||
| Eczema | Atopic dermatitis | |||||||
| Sensitisation | sIgE | |||||||
| Strachan et al. ( 1996 ) | 3 | PC | Sheffield child development study | No | CF | Allergic rhinitis | Allergic rhinitis | |
| Taylor‐Robinson et al. ( 2016 ) | 3 | PC | Millennium Cohort Study (MCS) | No | CF | Eczema | Symptomatic eczema | |
| Tham et al. ( 2017 ) | 2 | PC | Growing Up in Singapore Towards healthy Outcomes (GUSTO) | No | Egg | Food allergy | Symptomatic food allergy | No PE |
| Tran et al. ( 2017 ) | 2 | PC | Canadian Healthy Infant Longitudinal Develop‐ment (CHILD) Study | No | Egg | Sensitisation | SPT | |
| Tromp et al. ( 2011 ) | 3 | PC | Generation R | No | Cereals, egg, peanut, soy | Asthma‐like symptoms | Wheeze | |
| Eczema | Symptomatic eczema | |||||||
| Van Asperen et al. ( 1984 ) | 3 | PC | n/a | Yes | CF | Allergic rhinitis | Allergic rhinitis | |
| Asthma‐like symptoms | Wheeze | |||||||
| Eczema | Symptomatic eczema | |||||||
| Venter et al. ( 2009 ) | 3 | PC | Food Allergy and Intolerance Research (FAIR) | No | CF | Food allergy | Symptomatic food allergy | |
| Sensitisation | SPT | |||||||
| Venter et al. ( 2016 ) | 1 | NCC | FAIR | No | CF | Food allergy | Symptomatic food allergy | CV |
| Virtanen et al. ( 2010 ) | 2 | PC | DIPP nutrition | No | Cereals, fish | Allergic rhinitis | Allergic rhinitis | |
| Asthma‐like symptoms | Persistent asthma | |||||||
| Wilson et al. ( 1998 ) | 2 | PC | Dundee Infant Feeding Study | No | CF | Asthma‐like symptoms | Asthma | No PE |
| Asthma‐like symptoms | Wheeze | No PE | ||||||
| Wright et al. ( 1994 ) | 3 | PC | Tucson Children's Respiratory Study | No | CF | Allergic rhinitis | Allergic rhinitis | |
| Zutavern et al. ( 2004 ) | 2 | PC | n/a | No | CF, cereals, fish | Asthma‐like symptoms | Pre‐school wheeze without cold in the past 12 months | |
| Asthma‐like symptoms | Transient wheeze without cold in the past 12 months | |||||||
| Eczema | Symptomatic eczema | |||||||
| Zutavern et al. ( 2006 ) | 3 | PC | Einfluss von Lebensbe‐dingungen und Verhaltens‐weisen auf die Entwick‐lung von Immun‐system und Allergien (LISA) | No | CF, cereals, egg, fish | Eczema | Atopic dermatitis | |
| Eczema | Symptomatic eczema | |||||||
| 2 | CF | Sensitisation | sIgE | |||||
| Zutavern et al. ( 2008 ) | 3 | PC | LISA | No | CF | Allergic rhinitis | Allergic rhinitis | |
| Allergic rhinitis | Allergic rhinitis symptoms | |||||||
| Asthma‐like symptoms | Asthma | |||||||
| Asthma‐like symptoms | Asthma symptoms | |||||||
| Eczema | Symptomatic eczema | |||||||
| Sensitisation | sIgE | |||||||
| Alkazemi et al. ( 2018 ) | 3 | RETRO:CC | n/a | No | CF | Food allergy | Symptomatic food allergy | |
| Bascunan Gamboa et al. ( 2012 ) | 3 | RETRO:CC | n/a | No | CF | Food allergy | Symptomatic food allergy | No PE |
| DesRoches et al. ( 2010 ) | 3 | RETRO:CC | n/a | No | CF | Food allergy | Timing of introduction of CFs in cases and controls | |
| Forster et al. ( 1990 ) | 3 | RETRO:CS | n/a | No | CF | Atopic disease | Atopic disease | No PE, CV |
| Haileamlak et al. ( 2005 ) | 3 | RETRO:CC | n/a | No | CF | Eczema | Atopic dermatitis | |
| Hatakka et al. ( 2008 ) | 3 | RETRO: CS | n/a | No | CF | Atopic disease | Atopic disease | |
| Karunasekera et al. ( 2001 ) | 3 | RETRO:CC | n/a | No | CF | Asthma‐like symptoms | Asthma | |
| Koplin et al. ( 2010 ) | 3 | RETRO: CSA of baseline data of a PC | HealthNuts | Yes | CF, egg | Food allergy | Symptomatic food allergy | |
| Koplin et al. ( 2012 ) | 3 | RETRO: CSA of baseline data of a PC | HealthNuts | No | egg | Food allergy | Symptomatic food allergy | |
| Kramer ( 1981 ) | 3 | RETRO:CC | n/a | No | CF | Eczema | Timing of introduction of CFs in cases and controls | No PE |
| Kucukosmanoglu et al. ( 2008 ) | 3 | RETRO:CC | n/a | No | CF | Sensitisation | Timing of introduction of CFs in cases and controls – sensitisation (egg) | |
| Kumar et al. ( 2010 ) | 3 | RETRO:CS | n/a | No | Cereals, allergenic foods (egg, peanut, tree nut, shellfish, fish, sesame) | Food allergy | Symptomatic food allergy | |
| Lee et al. ( 2017 ) | 3 | RETRO:CS | Korea National Health and Nutrition Examination Survey (KNHANES) IV and V | No | CF | Eczema | Atopic dermatitis | |
| Nathan et al. ( 2012 ) | 3 | RETRO: CC | n/a | No | CF | Asthma‐like symptoms | Asthma | |
| Parihar et al. ( 1984 ) | 3 | RETRO:CC | n/a | No | CF | Atopic disease | Atopic disease | |
| Peters et al. ( 2015 ) | 3 | RETRO: CSA of baseline data of a PC | HealthNuts | Yes | egg | Eczema | Symptomatic eczema | |
| Sahakyan et al. ( 2006 ) | 3 | RETRO:CC | n/a | No | CF | Eczema | Atopic dermatitis | |
| Sicherer et al. ( 2010 ) | 3 | RETRO: CC | n/a | Yes | CF | Sensitisation | Sensitisation (peanut) | CV |
| Suryati et al. ( 2006 ) | 3 | RETRO:CS | n/a | Yes | CF, egg | Eczema | Atopic dermatitis | |
| Takahashi et al. ( 1999 ) | 3 | RETRO:CS | n/a | No | CF, egg | Eczema | Atopic dermatitis | |
| Turati et al. ( 2016 ) | 3 | RETRO:CC | n/a | No | CF | Eczema | Atopic dermatitis | |
| Yung et al. ( 2015 ) | 3 | RETRO:CC | n/a | No | CF | Atopic disease | Timing of introduction of CFs in cases and controls | |
| Zheng et al. ( 2016 ) | 3 | RETRO:CS | n/a | No | CF | Eczema | Symptomatic eczema |
BioIC: automated microfluidic‐based immunoassay system; CC: case–control study, CF: complementary food; CS: cross‐sectional study; CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; NCC: nested case–control study; PC: prospective cohort study; PE: point estimate; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; sIgE: specific immunoglobulin E; SPT: skin prick test.
B.6. Coeliac disease – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | At‐risk group | Food | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|---|
| Vriezinga et al. ( 2014 ) | 1 | RCT | Prevent Coeliac Disease (PreventCD) | Yes | Gluten |
Coeliac disease Coeliac disease autoimmunity |
|
| Andren Aronson et al. ( 2015 ) | 1 | PC | The Environmental Determinants of Diabetes in the Young (TEDDY) | Yes | Gluten |
Coeliac disease Coeliac disease autoimmunity |
|
| Andren Aronson et al. ( 2016 ) | 1 | NCC | Swedish TEDDY | Yes | Gluten |
Coeliac disease Timing of introduction of gluten in cases and controls |
CV |
| Chmiel et al. ( 2015 ) | 1 | PC | BABYDIET + BABYDIAB | Yes | Gluten | Coeliac disease autoimmunity | |
| Hummel et al. ( 2007 ) | 1 | PC | BABYDIAB | Yes | Gluten | Coeliac disease autoimmunity | |
| Jansen et al. ( 2014 ) | 1 | PC | Generation R | Yes | Gluten | Coeliac disease autoimmunity | |
| Norris et al. ( 2005 ) | 1 | PC | Diabetes Autoimmunity Study in the Young (DAISY) | Yes | Gluten |
Coeliac disease Coeliac disease autoimmunity |
|
| Savilahti et al. ( 2018 ) | 3 | NCC | n/a | Yes | Gluten | Timing of introduction of gluten in cases and controls | |
| Størdal et al. ( 2013 ) | 1 | PC | Den norske mor og barn‐undersøkelsen (MoBa) | No | Gluten | Coeliac disease | |
| Welander et al. ( 2010 ) | 2 | PC | Alla Barn i Sydöstra Sverige (ABIS) | No | Gluten | Coeliac disease | |
| Ziegler et al. ( 2003 ) | 1 | PC | BABYDIAB | Yes | Gluten | Coeliac disease autoimmunity | |
| Ascher et al. ( 1997 ) | 3 | RETRO: SCC | n/a | Yes | Gluten | Timing of introduction of gluten in cases and controls | |
| Auricchio et al. ( 1983 ) | 3 | RETRO: CC | n/a | Yes | Gluten | Coeliac disease | |
| Greco et al. ( 1988 ) | 3 | RETRO: CC | n/a | Yes | Gluten | Coeliac disease | |
| Ivarsson et al. ( 2002 ) | 3 | RETRO: CC | n/a | No | Gluten | Coeliac disease | |
| Myleus et al. ( 2012 ) | 3 | RETRO: CC | n/a | No | Gluten | Coeliac disease | CV |
| Peters et al. ( 2001 ) | 3 | RETRO: CC | n/a | No | Gluten | Coeliac disease |
CC: case–control study; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; NCC: nested case–control study; PC: prospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; SCC: sibling case–control study (i.e. diseased cases and their healthy siblings).
B.7. Type 1 diabetes mellitus – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | At‐risk group | Food | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|---|
| Chmiel et al. ( 2015 ) | 1 | PC | BABYDIET + BABYDIAB | Yes | Gluten | Islet autoimmunity | |
| Multiple islet autoimmunity | |||||||
| T1DM | |||||||
| Couper et al. ( 2009 ) | 1 | PC | Australian BabyDiab | Yes | Gluten, non‐gluten cereal | Islet autoimmunity | |
| Frederiksen et al. ( 2015 ) | 1 | PC | Diabetes Autoimmunity Study in the Young (DAISY) | Yes | CF, gluten | T1DM | |
| Hakola et al. ( 2018 ) | 1 | PC | Type 1 Diabetes Prediction and Prevention Study (DIPP) nutrition | Yes | CF, gluten | Advanced islet autoimmunity | |
| CF, gluten | T1DM | ||||||
| Lund‐Blix et al. ( 2015 ) | 1 | PC | Miljøårsaker til type 1‐diabetes (MIDIA) | Yes | Gluten | Islet autoimmunity | |
| CF, gluten | T1DM | ||||||
| Norris et al. ( 2003 ) | 1 | PC | DAISY | Yes | Gluten | Islet autoimmunity | |
| Savilahti and Saarinen ( 2009 ) | 2 | NCC | n/a | No | CF | T1DM | |
| Uusitalo et al. ( 2018 ) | 1 | PC | Environmental Determinants of Diabetes in the Young (TEDDY) | Yes | Gluten | Islet autoimmunity | |
| Virtanen et al. ( 2006 ) | 1 | PC | DIPP nutrition | Yes | CF, gluten | Advanced islet autoimmunity | Data from Hakola et al. (2018) considered |
| Virtanen et al. ( 2011 ) | 1 | PC | DIPP nutrition | Yes | CF, gluten | Islet autoimmunity | Data from Hakola et al. (2018) considered |
| Wahlberg et al. ( 2006 ) | 2 | PC | Alla Barn i Sydöstra Sverige (ABIS) | No | Gluten | Islet autoimmunity | |
| Welander et al. ( 2010 ) | 2 | PC | ABIS | No | Gluten | T1DM | |
| Ziegler et al. ( 2003 ) | 1 | PC | BABYDIAB | Yes | Gluten | Islet autoimmunity | |
| Bezzera Alves et al. ( 2012 ) | 3 | RETRO: SCC | n/a | No | Gluten | Timing of introduction of gluten in cases and controls | |
| EURODIAB Substudy 2 Study Group ( 2002 ) | 3 | RETRO: CC | EURODIAB substudy 2 | No | CF | T1DM | |
| Kostraba et al. ( 1993 ) | 3 | RETRO: CC | n/a | No/Yes | CF | T1DM | |
| Liese et al. ( 2012 ) | 3 | RETRO: CC | Search for Diabetes in Youth’ case‐control (SEARCH CC) study | No | CF | Timing of introduction of CFs in cases and controls | |
| Meloni et al. ( 1997 ) | 3 | RETRO: CC | n/a | No | CF | T1DM | |
| Perez‐Bravo et al. ( 1996 ) | 3 | RETRO: CC | n/a | No | CF | Timing of introduction of CFs in cases and controls | |
| Rabiei and Reza ( 2012 ) | 3 | RETRO: CC | n/a | No | CF | T1DM | |
| Rosenbauer et al. ( 2008 ) | 3 | RETRO: CC | n/a | No | CF | T1DM | |
| Stene et al., ( 2003 ) | 3 | RETRO: CC | n/a | No | CF | T1DM | |
| Visalli et al. ( 2003 ) | 3 | RETRO: CC | n/a | No | CF | T1DM | No PE |
CC: case–control study; CF: complementary food; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; NCC: nested case–control study; PC: prospective cohort study; PE: point estimate; RETRO: retrospective study; RoB: risk of bias; SCC: sibling case–control (i.e. diseased cases and their healthy siblings); T1DM: type 1 diabetes mellitus.
B.8. Risk factors for cardiovascular diseases – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|
| de Beer et al. ( 2016 ) | 2 | PC | Amsterdam Born Children and their Development (ABCD) |
Systolic blood pressure Diastolic blood pressure |
|
| de Jonge et al. ( 2013 ) | 2 | PC | Generation R |
Systolic blood pressure Diastolic blood pressure Carotid‐femoral pulse wave velocity Aortic root diameter Fractional shortening Left atrial diameter Left ventricular diameter Left ventricular mass |
|
| Gishti et al. ( 2014 ) | 2 | PC | Generation R |
Total cholesterol z‐scores HDL‐cholesterol z‐scores LDL‐cholesterol z‐scores Triglycerides z‐scores Cluster of cardiometabolic risk factors |
|
| Gishti et al. ( 2016 ) | 2 | PC | Generation R |
Retinal arteriolar calibers Retinal venular calibers |
|
| Martin et al. ( 2004 ) | 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) |
Systolic blood pressure Diastolic blood pressure |
|
| Wilson et al. ( 1998 ) | 1 | PC | Dundee Infant Feeding Study |
Systolic blood pressure Diastolic blood pressure |
|
| Behairy et al. ( 2017 ) | 3 | RETRO: CS | n/a |
HDL‐cholesterol LDL‐cholesterol |
|
| Brambilla et al. ( 2016 ) | 3 | RETRO: CS | n/a |
Systolic blood pressure z‐scores Diastolic blood pressure z‐scores |
CS: cross‐sectional study; HDL: high density lipoproteins; LDL: low density lipoproteins; n/a: not applicable; PC: prospective cohort study; RETRO: retrospective study.
B.9. Infections – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Outcome assessed | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|
| Cohen et al. ( 1994 ) | 2 | RCT | n/a |
GI infections RT infections |
Diarrhoea URTI URTI with fever |
|
| Cohen et al. ( 1995a ) | 2 | RCT | n/a |
GI infections RT infections Infections |
Diarrhoea URTI Fever |
|
| Dewey et al. ( 1999 ) | 2 | RCT | n/a |
GI infections RT infections |
Diarrhoea URTI |
|
| Perkin et al. ( 2016 ) | 2 | RCT | Enquiring About Tolerance (EAT) |
GI infections RT infections |
Diarrhoea Vomiting URTI LRTI |
|
| Forsyth et al. ( 1993 ) | 1 | PC | Dundee Infant Feeding Study |
GI infections RT infections |
Diarrhoea or vomiting URTI |
|
| Heinig et al. ( 1993 ) | 3 | PC | Davis Area Research on Lactation, Infant Nutrition and Growth (DARLING) | Infections | Respiratory illness, diarrhoea, otitis media, unexplained fevers, vomiting, chicken pox and other non‐respiratory, presumably viral infections | |
| Lopez‐Alarcon et al. ( 1997 ) | 1 | PC | n/a | GI infections | Diarrhoea | CV |
| Morgan et al. ( 2004 ) | 3 | PA | n/a |
GI infections RT infections |
Gastroenteritis LRTI |
|
| Noppornlertwong and Tantibhaedhyangkul ( 2016 ) | 2 | PC | n/a |
GI infections RT infections |
Not specified | |
| Størdal et al. ( 2017 ) | 1 | PC | Den norske mor og barn‐under‐søkelsen (MoBa) | Infections | Hospitalisation for infection | |
| Wright et al. ( 2004 ) | 3 | PC | Millennium Baby Study |
GI infections RT infections |
Diarrhoea LRTI URTI |
|
| Quigley et al. ( 2009 ) | 3 | RETRO: CSA of baseline data of a PC | Millennium Cohort Study (MCS) |
Diarrhoea LRTI |
CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; GI: gastrointestinal; LRTI: lower respiratory tract infections; n/a: not applicable; PA: pooled analysis; PC: prospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; RT: respiratory tract; URTI: upper respiratory tract infections.
B.10. Sleep‐related endpoints – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|
| Bainbridge et al. ( 1996 ) | 2 | RCT | n/a | Night time sleep duration | |
| Perkin et al. ( 2018 ) | 2 | RCT | Enquiring About Tolerance (EAT) |
Night time sleep duration Night wakings Sleep problems |
|
| Heinig et al. ( 1993 ) | 3 | PC | Davis Area Research on Lactation, Infant Nutrition and Growth (DARLING) | Sleep time (unspecified) | |
| Morgan et al. ( 2004 ) | 3 | PA | n/a | Night time sleep duration | |
| Nevarez et al. ( 2010 ) | 3 | PC | Project Viva | 24‐h sleep duration |
n/a: not applicable; PA: pooled analysis; PC: prospective cohort study; RCT: randomised controlled trial; RoB: risk of bias.
B.11. Infant and child development – individuals born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier |
Study design |
Study name | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|
| Jonsdottir et al. ( 2013 ) | 1 | RCT | n/a |
Risk of developmental delay Gross motor skills Fine motor skills |
|
| Veena et al. ( 2010 ) | 3 | PC | Mysore Parthenon study |
Language development Learning ability Memory span Pattern Reasoning Language production Visuo‐spatial problem solving Visual‐motor processing speed |
CV |
| Metwally et al. ( 2016 ) | 3 | RETRO: CS | n/a | Odds of being below average of the socio‐emotional composite score socio‐emotional composite score of Bayley III |
CS: cross‐sectional study; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not applicable; PC: prospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias.
B.12. Nutrient status – infants born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|
| Dewey et al. ( 1998 ) | 2 | RCT | n/a |
SF < 12 μg/L Hb < 110 g/L |
Same RCT as Cohen et al. (1995a), reported in previous sections |
| Dewey et al. ( 2004 ) | 2 | RCT | n/a | SF < 12 μg/L | Same RCT as Dewey et al. (1999), reported in previous sections |
| Kattelmann et al. ( 2001 ) | 2 | RCT | n/a |
SF < 12 μg/L Hb < 110 g/L |
Same RCT as Mehta et al. (1998), reported in previous sections |
| Jonsdottir et al. ( 2012 ) | 1 | RCT | n/a | SF < 12 μg/L | |
| Libuda et al. ( 2016 ) | 2 | PC | Polyunsaturated fatty acids in child nutrition—A German multimodal optimisation study (PINGU) | SF < 12 μg/LSF < 12 +Hb < 105 g/L | |
| Meinzen‐Derr et al. ( 2006 ) | 2 | PC | n/a | Hb < 100 g/L | CV |
| Hong et al. ( 2017 ) | 3 | RETRO: CS | n/a | SF < 12 ng/mL, MCV < 70 fl and TfS < 10% |
CS: cross‐sectional study; CV: timing of introduction of CF used as a continuous variable in the analysis; Hb: haemoglobin; MCV: mean corpuscular volume; n/a: not applicable; PC: prospective cohort study; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias; SF: serum ferritin; TfS: transferrin saturation.
B.13. Food preferences and eating behaviours – infants born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Food | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|
| Cohen et al. ( 1995b ) | 3 | RCT | n/a | CF |
Composite food acceptance scores Food intake at midday meal Number of food groups consumed per day |
|
| Abraham et al. ( 2012 ) | 2 | PC | Growing Up in Scotland (GUS) | CF | Positive eating pattern | |
| Bielemann et al. ( 2018 ) | 3 | PC | Pelotas Birth Cohort | CF | Proportion of daily energy intake from ultra‐processed foods | |
| Brown and Lee ( 2012 ) | 1 | PC | n/a | CF |
Food responsiveness Satiety responsiveness |
CV |
| Burnier et al. ( 2011 ) | 1 | PC | Québec Longitudinal Study of Child Development (QLSCD) | Vegetables | Vegetable intake | |
| de Barse et al. ( 2017 ) | 2 | PC | Generation R |
CF Fruit Vegetables |
Food fussiness | |
| de Lauzon‐Guillain et al. ( 2013 ) | 2 | PC | Avon Longitudinal Study of Parents and Children (ALSPAC) |
Fruit Vegetables |
Fruit intake Vegetable intake |
No PE |
| 2 | Étude des déterminants pré et post natals précoces de la santé et de développement de l'enfant (EDEN) | |||||
| 3 | Generation XXI | |||||
| 3 | Greek EuroPrevall | |||||
| Emmett et al. ( 2018 ) | 3 | PC | ALSPAC | CF | Picky eating behaviour | |
| Grimm et al. ( 2014 ) | 2 | PC | Infant feeding practices II study and its year 6 follow‐up (IFPS II/Y6FU) |
Fruit Vegetables |
Fruit and vegetable intake | |
| Hollis et al. ( 2016 ) | 2 | PC | Southampton Women's Survey (SWS) | CF | Feeding difficulties | |
| Jones et al. ( 2015 ) | 2 | PC | ALSPAC |
Fruit Vegetables |
Food diversity of ‘healthy’ foods | |
| 2 | EDEN | |||||
| 3 | Generation XXI | |||||
| 3 | Greek EuroPrevall | |||||
| Lange et al. ( 2013 ) | 2 | PC | Observatory of Food Preferences in Infants and Children (OPALINE) |
CF Fruit Vegetables |
Acceptance of new foods, fruit and vegetables | CV and no PE |
| Möller et al. ( 2013 ) | 2 | PC | Amsterdam Born Children and their Development (ABCD) | CF |
Enjoyment of food Food responsiveness Fruit intake Satiety responsiveness Slowness in eating Vegetable intake |
|
| Bell LK et al. ( 2018 ) | 3 | RETRO: CS | How and what parents feed their children: an international study | CF | Food neophobia/acceptance of new foods | CV |
| Cooke et al. ( 2004 ) | 3 | RETRO: CS | n/a |
Fruit Vegetables |
Frequency of fruit consumption Frequency of vegetable consumption |
|
| Okubo et al. ( 2016 ) | 3 | RETRO: CSA of baseline data of a PC | Osaka Maternal and Child Health Study (OMCHS) | CF |
Odds of consuming < 1 serving of fruit per day Odds of consuming < 1 serving of vegetables per day |
|
| Shim et al. ( 2011 ) | 3 | RETRO: CSA of baseline data of a PC |
Synergistic Theory and Research on Obesity and Nutrition Group Kids (STRONG Kids) |
CF | Food neophobia/acceptance of new foods |
CF: complementary food; CS: cross‐sectional study; CSA: cross‐sectional analysis; CV: timing of introduction of CF used as a continuous variable in the analysis; n/a: not available; PC: prospective cohort study; PE: point estimate; RCT: randomised controlled trial; RETRO: retrospective study; RoB: risk of bias.
B.14. Other health outcomes – infants born at term or mixed populations (sorted by study design and author)
| Bibliography | RoB Tier | Study design | Study name | Food | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|
| Ayonrinde et al. ( 2017 ) | 3 | PC | Western Australian Pregnancy Cohort (Raine Cohort) | CF | Non‐alcoholic fatty liver disease | |
| Kindgren et al. ( 2017 ) | 2 | NCC | Alla Barn i Sydöstra Sverige (ABIS) | Gluten | Juvenile idiopathic arthritis | |
| Tanaka et al. ( 2013 ) | 3 | PC | Osaka Maternal and Child Health Study (OMCHS) | CF | Early childhood dental caries | |
| Ellis et al. ( 2012 ) | 3 | RETRO: CC | Childhood Arthritis Risk factor Identification Study (CLARITY) | CF | Juvenile arthritis – Timing of introduction of CFs in cases and controls | |
| Fort et al. ( 1990 ) | 3 | RETRO: CC | n/a | CF | Autoimmune thyroid disease – Timing of introduction of CFs in cases and controls | |
| Strisciuglio et al. ( 2017 ) | 3 | RETRO: CC | n/a | Gluten |
Crohn's disease Ulcerative colitis |
CC: case–control study; CF: complementary food; n/a: not applicable; NCC: nested case–control study; PC: prospective cohort study; RETRO: retrospective study; RoB: risk of bias.
B.15. Outcomes in individuals born preterm (sorted by study design and author)
| Bibliography | Tier | Study design | Study name | Food | Endpoint assessed | Additional comments |
|---|---|---|---|---|---|---|
| Gupta et al. ( 2017 ) | 1 | RCT | n/a | CF |
WAZ Attained body weight L(H)AZ Attained body length (height) HCZ Attained HC BMIZ Attained BMI BMD Lean + BMC mass % fat mass Fat mass Total cholesterol HDL‐cholesterol LDL‐cholesterol VLDL‐cholesterol Triglycerides Systolic blood pressure Diastolic blood pressure Iron status |
|
| Morgan et al. ( 2004 ) | 3 | PA | n/a | CF |
Attained body weight Weight gain Attained body length Length gain HC gain Subscapular SFT gain Triceps SFT gain Asthma‐like symptoms Eczema Sleep GI infections LRTI |
No PE No PE No PE No PE No PE No PE |
| Spiegler et al. ( 2015 ) | 3 | PC | German Neonatal Network (GNN) | CF |
WAZ Attained body weight L(H)AZ Attained body length (height) |
CV |
| Yrjänä et al. ( 2018 ) | 3 | RETRO: CC | n/a | CF | Eczema ‐ Timing of introduction of CFs in cases and controls | No PE (eczema) |
| Food allergy ‐ Timing of introduction of CFs in cases and controls |
BMC: bone mineral content; BMD: bone mineral density; BMI: body mass index; BMIZ: body mass index‐for age z‐scores; CC: case–control study; CF: complementary food; CV: timing of introduction of complementary foods used as a continuous variable in the analysis; GI: gastrointestinal; HC: head circumference; HCZ: head circumference‐for‐age z‐scores; HDL: high density lipoprotein; LDL: low density lipoprotein; L(H)AZ: length (height)‐for‐age z‐scores; LRTI: lower respiratory tract infections; n/a: not applicable; PA: pooled analysis; PC: prospective cohort study; PE: point estimate; RCT: randomised controlled trial; RETRO: retrospective study; SFT: skin fold thickness; VLDL: very low density lipoprotein; WAZ: weight‐for‐age z‐scores.
Appendix C – Specific items considered in the appraisal of studies
1.
The approach followed by the Panel for the assessment of the risk of bias (RoB) was described in brief in the protocol (EFSA, 2017b) and in details in Section 2.2.2 of this scientific opinion.
The specific items considered by the Panel are described below, as well as how they were judged according to a four‐level rating scale.
1. Timing of introduction of CFs for observational studies (detection bias)
Definitely low RoB
Assessment of the timing of introduction of CFs at multiple time points or diaries in the first six months of life with complete information on how the assessment was done.
Probably low RoB
-
Assessment of the timing of introduction of CFs
-
o
at a single time point during the first six months of life (irrespective of the method used)
-
o
during the second half of infancy (irrespective of the method used)
-
o
between or at 1 and 2 years of age if elicited by a face‐to‐face interview.
-
o
Probably high RoB
-
Assessment of the timing of introduction of CFs
-
o
between or at 1 and 2 years of age if obtained through a caregiver‐completed questionnaire or by a phone interview
-
o
Insufficient information available for a judgment.
Definitely high RoB
Assessment of the timing of introduction of CFs >2 years of age (irrespective of the method used).
2. Assessment of compliance with the intervention (RCTs)
Definitely low RoB
Assessment of the compliance with the intervention at several time points using reliable methods (e.g. home visits by trained research staff, diaries, number of sachets or amount of intervention products returned).
Probably low RoB
Assessment of the compliance with the intervention with some information on the modalities missing (the extent of missing information determining the low or high RoB decisions).
Probably high RoB
This category was not used.
Definitely high RoB
Compliance not assessed.
3. Outcome assessment for those outcomes addressed in the opinion 50 (detection bias)
3.1. Outcomes that involve anthropometric measurements (i.e. body weight, body length/height, HC, BMI, overweight and obesity)
Definitely low RoB
Measurements performed for the purpose of the study by trained personnel with either full information on the equipment used (using appropriate equipment) or reference to specific (standard) procedure that were followed (e.g. WHO manual).
Probably low RoB
Measurements performed for the purpose of the study by trained personnel, but with some information missing (e.g. type of scale) or reference to not further defined standard procedures.
Probably high RoB
Measurements not performed for the purpose of the study (e.g. transcripts from health cards of measurements taken at last family doctor's or paediatrician's visit).
Insufficient information available for a judgement.
Definitely high RoB
Self‐ or caregivers’ reports of self‐measurements.
3.2. Body composition
Fat mass
Definitely low RoB
Measurements taken by DXA.
Probably high RoB
Measurements taken by BIA.
Probably or definitely high RoB
These categories were not used for this outcome.
Skinfold thickness
Definitely low RoB
Triplicate measurements performed by trained personnel with calibrated calipers following standard procedures.
Probably low RoB
At least two measurements performed by trained personnel.
Probably high RoB
Single measurements by trained personnel.
Insufficient information available for a judgement.
Definitely high RoB
Measurements by untrained individuals.
3.3. Atopic diseases
Asthma‐like symptoms, eczema and allergic rhinitis
Definitely low RoB
Diagnosis made by the study physician for the purpose of the study using pre‐defined criteria.
Probably low RoB
Caregivers’ reports of family doctors’/physicians’ diagnoses plus the use of medication or other treatments for the disease.
Probably high RoB
Caregivers’ reports of family doctors’/physicians’ diagnoses.
Caregivers’ reports of symptoms.
Definitely high RoB
This category was not used.
Symptomatic food allergy and food sensitisation
Definitely low RoB
Diagnoses based on double‐blind placebo‐controlled food challenge (a minor number of cases of food allergy diagnosed by other means were acceptable).
Sensitisation (sIgE, skin prick test (SPT)) measured by well‐accepted standard methods using cut‐offs usually used in clinical practice.
Probably low RoB
Diagnoses based on open food challenge.
Caregivers’ report of convincing symptoms of food allergy (e.g. vomiting, eczema) after ingestion of the food plus other supporting evidence (e.g. food avoidance, positive SPT).
Sensitisation measured by well‐accepted standard methods but using cut‐offs that are not usually used in clinical practice.
Probably high RoB
Caregivers’ reports of family doctors’/physicians’ diagnoses.
Caregivers’ reports of symptoms.
Definitely high RoB
Unclear assessment and/or criteria.
3.4. Coeliac disease
Definitely low RoB
Assessment according to the ESPHGAN diagnostic criteria (Husby et al., 2012).
Coeliac disease autoimmunity assessed by tissue transglutaminase (tTG = TGC = TGM2) autoantibody measurements using well‐accepted standard methods (NB: independent of the cut‐off used, usually IgA antibodies to tTG are measured, in case of IgA deficiency IgG can be used – some studies used a combination).
Probably low RoB
Caregivers’ reports of coeliac disease diagnoses
Combination of various method to obtain information on coeliac disease diagnosis.
Probably or definitely high RoB
These categories were not used.
3.5. Type 1 diabetes mellitus
Definitely low RoB
Based on well accepted criteria for diagnosis of type 1 diabetes mellitus (e.g. criteria of WHO or the American Diabetes Association).
Diabetes autoimmunity assessed by islet autoantibodies using well‐accepted standard methods (NB: independent of the cut‐off used, and the autoantibodies assessed).
Probably low, high or definitely high RoB
These categories were not used.
3.6 Blood pressure
Definitely low RoB
Automatic measurements with an average of at least two readings taken, including a full description of how measurements were taken (e.g. after rest in supine position).
Probably low RoB
Automatic measurements, but with details of the exact procedures lacking.
Manual measurements.
Probably and definitely high
These categories were not used.
3.7. Infections
Definitely low RoB
This category was not used.
Probably low RoB
Hospitalisation for infection.
Caregivers’ recording of symptoms plus confirmation by research staff or medical doctors.
For diarrhoea, daily records of stool consistency and frequency.
Probably high RoB
Caregivers’ report of symptoms.
Insufficient information.
Definitely high RoB
This category was not used.
3.8. Sleep‐related endpoints
Probably and definitely low RoB
These categories were not used.
Probably high RoB
Measurements based on a validated sleep questionnaire that assessed sleep over the past week (this was not considered sufficiently precise for the purpose of the present assessment).
Definitely high RoB
Caregivers’ reports.
3.9. Infant and child development
Probably low RoB
Assessments based on validated tools to assess infant and child development.
Definitely low, probably high or definitely high RoB
These categories were not used.
3.10. Nutrient status
Definitely low RoB
Measurements performed according to standard criteria.
Probably low or high or definitely high RoB
These categories were not used.
3.11. Food preferences and eating behaviours
Definitely low RoB
This category was not used.
Probably low RoB
Use of validated questionnaires.
Use of non‐validated tools but caregivers were provided with a detailed description and examples how behaviours should be rated.
Use of 24‐h dietary recalls.
Use of food frequency questionnaires to assess frequency of consumption/number of servings.
Use of validated food frequency questionnaires to assess amount of consumption for which details on their validation is publicly available and could be assessed.
Probably high RoB
Use of validated food frequency questionnaires to assess amount of consumption for which details on their validation are not publicly available and could not be assessed.
Use of non‐validated tools for which caregivers were not provided with a description or examples how behaviours should be rated.
Definitely high RoB
This category was not used.
4. Assessment of the appropriate adjustment for confounders in observational studies for those outcomes addressed in the opinion 50 (confounding bias)
Definitely low RoB
Consideration of (most) of the main confounders identified for each outcome, plus consideration of other relevant confounders, including a detailed description on how confounders were assessed and subsequently selected for inclusion in the analysis.
Probably low RoB
Consideration of some of the main confounders and some other relevant confounders.
Lack of adjustment for confounders when the result of the analyses was not statistically significant under the assumption that adjustment for confounders would yield results even closer to the null effect.
Probably high RoB
Lack of the majority of main confounders.
Insufficient information for a judgement.
Definitely high RoB
Lack of adjustment for confounders, if the results were statistically significant.
The main confounders (selected based on expert knowledge) considered to determine the RoB were the following:
4.1. Body weight
Socioeconomic status
Education of the caregiver.
4.2. Body length/height
Parents’ height.
4.3. BMI; overweight and obesity; fat, fat‐free and lean mass
Maternal BMI
Previous measurements of related outcomes, e.g. growth rates during infancy (reverse causality).
4.4. Atopic diseases
Allergic symptoms before or at the timing of introduction of CFs (reverse causality, always), unless this item was addressed in another way (e.g. through sensitivity analysis)
Parental history of allergy (always), unless this item was addressed in another way (i.e. children selected based on the parental history of allergy)
Smoking (for respiratory outcomes only)
Furry pets (for respiratory outcomes only).
4.5. Autoimmune disease (coeliac disease, type 1 diabetes)
Family history (if children were recruited from the general population)
Gender
Ethnicity (if children were recruited from the general population).
4.6. Blood pressure
Child's body weight/BMI
Family history of hypertension/parents’ blood pressure
Smoking during pregnancy (not passive smoking after birth, in the same room).
4.7. Infections (low income countries excluded)
Socioeconomic status
Education of the caregiver
Smoking (respiratory infections only)
Number of siblings
Day‐care attendance
Breastfeeding vs formula feeding.
4.8. Sleep‐related endpoints
Socioeconomic status
Smoking
Breastfeeding vs formula feeding.
4.9. Infant and child development
Socioeconomic status
Education of the caregiver
Gestational age.
4.10. Nutrient status
Education of the caregiver
Gestational age
Breastfeeding vs formula feeding.
4.11. Food preferences and eating behaviours
Socioeconomic status
Education of the caregiver
Caregivers’ age
Number of siblings.
5. Randomisation in RCTs (selection bias)
Definitely low RoB
Use of appropriate methods for randomisation (e.g. computer‐generated random numbers).
Probably low RoB
This category was not used.
Probably high RoB
Insufficient information on randomisation (e.g. statement that subjects were randomly allocated to groups without further information).
Definitely high RoB
Inappropriate methods for randomisation (e.g. division of subjects based on birth dates).
6. Concealed allocation in RCTs (selection bias)
Definitely low RoB
Use of appropriate methods for ensuring concealed allocation (e.g. web‐based central randomisation, telephone randomisation, sealed non‐transparent envelopes).
Probably low RoB
This category was not used.
Probably high RoB
Insufficient information available.
Definitely high RoB
This category was not used.
7. Blinding in RCTs (performance bias)
Definitely low RoB
Intervention and control products did not differ in appearance (including packaging), smell and taste.
No breaking of the blinding during the study.
Detailed explanation provided how blinding was ensured even if blinding had to be broken for a subject (based on criteria pre‐defined in the study protocol).
Probably low RoB
Blinding of outcome assessors for studies in which blinding of the exposure was not possible (e.g. timing of introduction of CFs).
Probably high RoB
No blinding of outcome assessors in studies in which blinding of the exposure was not possible (e.g. timing of introduction of CFs).
Definitely high RoB
No blinding in studies in which blinding of both the exposure and the outcome assessment was possible.
8. Attrition/exclusion from analysis (attrition/exclusion bias)
Definitely low RoB
No attrition or exclusion from analysis.
Probably low RoB
Comparison of characteristics of subjects that were included with those that were excluded from the analysis, and no appreciable differences were observed with respect to characteristics which could be related both to exposure and outcome.
Limited number of subjects excluded (based on expert judgment).
Time‐to‐event analyses.
Probably high RoB
No comparison of characteristics of subjects that were included with those that were excluded from the analysis presented.
Appreciable differences in the characteristics of subjects included and excluded from analysis.
Definitely high RoB
Substantial number of subjects excluded from analysis without any comparison of characteristics.
9. Other risks of bias
In this category, the appropriateness of the statistical analysis was assessed, as was any selective reporting or any other threats to internal validity (e.g. changes in feeding recommendations during the course of the study which might have shifted the exposure category of some of the infants in the study).
For most of the studies, this item was rated as probably low RoB.
Definitely high RoB was used in case of unadjusted analyses with statistically significant findings owing to the inappropriate statistical analysis.
Appendix D – Search strings of the literature searches undertaken by EFSA
D.1. Original search performed by the contractor (5 and 8 May 2017)
The search strings are published in the report by the contractor (Pallas Health Research and Consultancy, 2019).
D.2. Original complementary search (16 October 2017)
Cochrane
| ID | Search | Items found |
|---|---|---|
| #1 | [mh ^Infant] or infan*:ti,ab,kw or young child*:ti,ab,kw or baby:ti,ab,kw or babies:ti,ab,kw or early childhood:ti,ab,kw or weanling*:ti,ab,kw or “first year of life”:ti,ab,kw or “early life”:ti,ab,kw | 59,821 |
| #2 | (Exclusiv*:ti,ab,kw or fully:ti,ab,kw or full:ti,ab,kw) near/5 (breastfeed*:ti,ab,kw or breast feed*:ti,ab,kw or breastfed:ti,ab,kw or “breast fed”:ti,ab,kw or lactat*:ti,ab,kw) | 927 |
| #3 | (Exclusiv*:ti,ab,kw or fully:ti,ab,kw or full:ti,ab,kw) and [mh “breast feeding”] | 564 |
| #4 | (full:ti,ab,kw or fully:ti,ab,kw or exclusiv*) and (“breast milk”:ti,ab,kw or “human milk”:ti,ab,kw or “maternal milk”:ti,ab,kw or “mother's own milk”:ti,ab,kw) and (fed:ti,ab,kw or feeding*:ti,ab,kw or diet:ti,ab,kw or intake:ti,ab,kw) | 464 |
| #5 | #2 or #3 or #4 | 1,234 |
| #6 | (time:ti,ab,kw or timing:ti,ab,kw or moment:ti,ab,kw or duration:ti,ab,kw or age:ti,ab,kw or month:ti,ab,kw or months:ti,ab,kw or early:ti,ab,kw or week:ti,ab,kw or weeks:ti,ab,kw or year:ti,ab,kw or years:ti,ab,kw or day:ti,ab,kw or days:ti,ab,kw or [mh “Time factors”] or [mh ^”Age Factors”]) | 690,281 |
| #7 | #1 and #5 and #6 | 1,052 |
| #8 | #7 Publication Year from 1990 | 1,007 |
Pubmed
| ID | Search | Items found |
|---|---|---|
| #14 | Search #13 AND (“1990”[Date ‐ Publication] : “3000”[Date ‐ Publication]) | 4,381 |
| #13 | Search #11 NOT #12 | 4,739 |
| #12 | Search (Afghanistan*[tiab] OR Benin*[tiab] OR Burkina Faso[tiab] OR Burund*[tiab] OR Central African Republic[tiab] OR Republique Centrafricaine[tiab] OR Chad*[tiab] OR Comoros[tiab] OR Congo*[tiab] OR Eritrea[tiab] OR Ethiopi*[tiab] OR Gambia[tiab] OR Guinea[tiab] OR Guinean[tiab] OR Guinée[tiab] OR Guinea‐Bissau[tiab] OR Guinea Bissau[tiab] OR Guinée Bissau[tiab] OR Haiti*[tiab] OR Korea*[tiab] OR Liberia*[tiab] OR Madagascar*[tiab] OR Malawi*[tiab] OR Mali[tiab] OR Malian[tiab] OR Mozambiqu*[tiab] OR Nepal[tiab] OR Niger[tiab] OR Rwand*[tiab] OR Senegal*[tiab] OR “Sierra Leone”[tiab] OR Somali*[tiab] OR Sudan*[tiab] OR Tanzani*[tiab] OR Togo[tiab] OR Togolese[tiab] OR Ugand*[tiab] OR Zimbabw*[tiab] OR Armenia*[tiab] OR Banglades*[tiab] OR Bhutan[tiab] OR Bolivia[tiab] OR “Cabo Verde”[tiab] OR “Cape Verde”[tiab] OR Cambodia*[tiab] OR Cameroon*[tiab] OR Congo[tiab] OR “Cote D'Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Djibout*[tiab] OR Egypt*[tiab] OR “El Salvador”[tiab] OR Ghana*[tiab] OR Guatemala[tiab] OR Honduras[tiab] OR India*[tiab] OR Indonesia*[tiab] OR Keny*[tiab] OR Kiribati[tiab] OR Kyrgyzstan*[tiab] OR “Kyrgyz Republic”[tiab] OR Lao*[tiab] OR Lesotho*[tiab] OR Mauritania*[tiab] OR Mauritius[tiab] OR Mauritian*[tiab] OR Micronesi*[tiab] OR Mongolia*[tiab] OR Morocc*[tiab] OR Burma[tiab] OR Myanmar[tiab] OR Nicaragua*[tiab] OR Nigeria*[tiab] OR Pakistan*[tiab] OR “Papua New Guinea”[tiab] OR Philippine*[tiab] OR Samoa[tiab] OR “São Tomé and Principe”[tiab] OR “São Tomé e Príncipe”[tiab] OR Solomon Island*[tiab] OR Sri Lanka[tiab] OR Sudan*[tiab] OR Swazi*[tiab] OR Syria*[tiab] OR Tajikistan*[tiab] OR Timor‐Leste[tiab] OR Tonga[tiab] OR Tunisia*[tiab] OR Uzbekistan*[tiab] OR Vanuatu*[tiab] OR Vietnam*[tiab] OR “West Bank”[tiab] OR Gaza[tiab] OR Yemen*[tiab] OR Zambia*[tiab]) NOT ((Afghanistan*[tiab] OR Benin*[tiab] OR Burkina Faso[tiab] OR Burund*[tiab] OR Central African Republic[tiab] OR Republique Centrafricaine[tiab] OR Chad*[tiab] OR Comoros[tiab] OR Congo*[tiab] OR Eritrea[tiab] OR Ethiopi*[tiab] OR Gambia[tiab] OR Guinea[tiab] OR Guinean[tiab] OR Guinée[tiab] OR Guinea‐Bissau[tiab] OR Guinea Bissau[tiab] OR Guinée Bissau[tiab] OR Haiti*[tiab] OR Korea* | 519,702 |
| [tiab] OR Liberia*[tiab] OR Madagascar*[tiab] OR Malawi*[tiab] OR Mali[tiab] OR Malian[tiab] OR Mozambiqu*[tiab] OR Nepal[tiab] OR Niger[tiab] OR Rwand*[tiab] OR Senegal*[tiab] OR “Sierra Leone”[tiab] OR Somali*[tiab] OR Sudan*[tiab] OR Tanzani*[tiab] OR Togo[tiab] OR Togolese[tiab] OR Ugand*[tiab] OR Zimbabw*[tiab] OR Armenia*[tiab] OR Banglades*[tiab] OR Bhutan[tiab] OR Bolivia[tiab] OR “Cabo Verde”[tiab] OR “Cape Verde”[tiab] OR Cambodia*[tiab] OR Cameroon*[tiab] OR Congo[tiab] OR “Cote D'Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Djibout*[tiab] OR Egypt*[tiab] OR “El Salvador”[tiab] OR Ghana*[tiab] OR Guatemala[tiab] OR Honduras[tiab] OR India*[tiab] OR Indonesia*[tiab] OR Keny*[tiab] OR Kiribati[tiab] OR Kyrgyzstan*[tiab] OR “Kyrgyz Republic”[tiab] OR Lao*[tiab] OR Lesotho*[tiab] OR Mauritania*[tiab] OR Mauritius[tiab] OR Mauritian*[tiab] OR Micronesi*[tiab] OR Mongolia*[tiab] OR Morocc*[tiab] OR Burma[tiab] OR Myanmar[tiab] OR Nicaragua*[tiab] OR Nigeria*[tiab] OR Pakistan*[tiab] OR “Papua New Guinea”[tiab] OR Philippine*[tiab] OR Samoa[tiab] OR “São Tomé and Principe”[tiab] OR “São Tomé e Príncipe”[tiab] OR Solomon Island*[tiab] OR Sri Lanka[tiab] OR Sudan*[tiab] OR Swazi*[tiab] OR Syria*[tiab] OR Tajikistan*[tiab] OR Timor‐Leste[tiab] OR Tonga[tiab] OR Tunisia*[tiab] OR Uzbekistan*[tiab] OR Vanuatu*[tiab] OR Vietnam*[tiab] OR “West Bank”[tiab] OR Gaza[tiab] OR Yemen*[tiab] OR Zambia*[tiab]) AND (Europe[MeSH] OR Europe*[tw] OR Scandinavia* [tw] OR Mediterranean[tw] OR Baltic[tw] OR Andorra*[tw] OR Azerbaijan*[tw] OR Albania*[tw] OR Armenia*[tw] OR Austria*[tw] OR Belarus*[tw] OR Byelarus*[tw] OR Bosni*[tw] OR Herzegovin*[tw] OR Croat*[tw] OR Cyprus[tw] OR Cypriot*[tw] OR Czech[tw] OR Belgi*[tw] OR Bulgaria*[tw] OR Denmark[tw] OR Danish[tw] OR Estonia*[tw] OR Finland[tw] OR Finnish[tw] OR France*[tw] OR French*[tw] OR Georgia*[tw] OR German*[tw] OR Greece[tw] OR Greek[tw] OR Hungar*[tw] OR Iceland*[tw] OR Ital*[tw] OR Sicil*[tw] OR Sardinia*[tw] OR Latvi*[tw] OR Liechtenstein*[tw] OR Lithuania*[tw] OR Luxembourg*[tw] OR Macedonia*[tw] OR Malta[tw] OR Maltese[tw] OR Moldova*[tw] OR Monaco[tw] OR Montenegr*[tw] OR Netherlands[tw] OR Dutch[tw] OR Norway[tw] OR Norwegian*[tw] or Svalbard*[tw] OR Poland*[tw] OR Polish*[tw] OR Portugal[tw] OR Portuguese[tw] OR Romania*[tw] OR Roumania*[tw] OR Rumania*[tw] OR San Marino[tw] OR Serb*[tw] OR Slovak*[tw] OR Slovenia*[tw] OR Spain*[tw] OR Spanish*[tw] OR Sweden[tw] OR Swedish[tw] OR Switzerland[tw] OR Swiss[tw] OR Great Britain*[tw] OR British*[tw] OR Channel Islands*[tw] OR Guerns*[tw] OR England*[tw] OR English*[tw] OR Hebrid*[tw] OR Ireland*[tw] OR Irish*[tw] OR Scotland*[tw] OR Scotch*[tw] OR Scottish*[tw] OR Wales*[tw] OR Welsh*[tw] OR United Kingdom*[tw] OR UK[tw] OR Gibraltar[tw] OR Ukrain*[tw] OR Vatican[tw] OR Yugoslavia*[tw])) | ||
| #11 | Search #9 NOT #10 | 5,787 |
| #10 | Search Animals[Mesh] NOT Humans[Mesh] | 4,382, 326 |
| #9 | Search #7 NOT #8 | 5,800 |
| #8 | Search (Editorial[ptyp] OR Letter[ptyp]) | 1,403, 312 |
| #7 | Search #1 AND #5 AND #6 | 5,824 |
| #6 | Search (time[tiab] OR timing[tiab] OR moment[tiab] OR duration[tiab] OR age[tiab] OR month[tiab] OR months[tiab] OR early[tiab] OR week[tiab] OR weeks[tiab] OR year[tiab] OR years[tiab] OR day[tiab] OR days[tiab] OR “Time factors”[Mesh] OR “Age Factors”[Mesh:NoExp]) | 8,822,572 |
| #5 | Search #2 OR #3 OR #4 | 8,051 |
| #4 | Search Exclusiv*[tiab] AND (breastfeed*[tiab] OR breast feed*[tiab] OR breastfed[tiab] OR “breast fed”[tiab] OR lactat*[tiab] OR “breast feeding”[Mesh]) | 7,044 |
| #3 | Search (full[tiab] OR fully[tiab] OR exclusiv*[tiab]) AND (“breast milk”[tiab] OR “human milk”[tiab] OR “maternal milk”[tiab] OR “mother's own milk”[tiab]) AND (fed[tiab] OR feeding*[tiab] OR diet[tiab] OR intake[tiab]) | 2,047 |
| #2 | Search “fully breastfeeding”[tiab] OR “fully breastfeeding”[tiab] OR fully breastfed*[tiab] OR fully breastfed*[tiab] OR “full breastfeeding”[tiab] OR “full breastfeeding”[tiab] | 394 |
| #1 | Search “Infant”[mh:noexp] OR infan*[tiab] OR young child*[tiab] OR baby[tiab] OR babies[tiab] OR early childhood[tiab] OR weanling*[tiab] OR “first year of life”[tiab] OR “early life”[tiab] | 1,031, 125 |
Web of Science. Core Collection
| ID | Search | Items found |
|---|---|---|
| #10 |
#9 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1990‐2017 |
3,410 |
| #9 |
#6 NOT #7 Refined by: [excluding] DOCUMENT TYPES: (LETTER OR EDITORIAL MATERIAL) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
3,425 |
| #8 |
#6 NOT #7 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
3,456 |
| #7 |
TS=((Afghanistan* OR Benin* OR Burkina Faso OR Burund* OR Central African Republic OR Republique Centrafricaine OR Chad* OR Comoros OR Congo* OR Eritrea OR Ethiopi* OR Gambia* OR Guinea OR Guinée OR Guinea‐Bissau OR Guinea Bissau OR Guinée Bissau OR Haiti* OR Korea* OR Liberia* OR Madagascar* OR Malawi* OR Mali OR Malian OR Mozambiqu* OR Nepal* OR Niger OR Rwand* OR Senegal* OR “Sierra Leone” OR Somali* OR Sudan* OR Tanzani* OR Togo OR Togolese OR Ugand* OR Zimbabw*) OR (Armenia* OR Banglades* OR Bhutan OR Bolivia OR “Cabo Verde” OR “Cape Verde” OR Cambodia* OR Cameroon* OR Congo OR “Cote D'Ivoire” OR “Ivory Coast” OR Djibout* OR Egypt* OR “El Salvador” OR Ghana* OR Guatemala OR Honduras OR India* OR Indonesia* OR Keny* OR Kiribati OR Kyrgyzstan* OR “Kyrgyz Republic” OR Lao* OR Lesotho* OR Mauritania* OR Mauritius OR Mauritian* OR Micronesi* OR Mongolia* OR Morocc* OR Burma OR Myanmar OR Nicaragua* OR Nigeria* OR Pakistan* OR “Papua New Guinea” OR Philippine* OR Samoa OR “São Tomé and Principe” OR “São Tomé e Príncipe” OR Solomon Island* OR Sri Lanka OR Sudan* OR Swazi* OR Syria* OR Tajikistan* OR Timor‐Leste OR Tonga OR Tunisia* OR Uzbekistan* OR Vanuatu* OR Vietnam* OR “West Bank” OR Gaza OR Yemen* OR Zambia*) NOT ((Afghanistan* OR Benin* OR Burkina Faso OR Burund* OR Central African Republic OR Republique Centrafricaine OR Chad* OR Comoros OR Congo* OR Eritrea OR Ethiopi* OR Gambia* OR Guinea OR Guinée OR Guinea‐Bissau OR Guinea Bissau OR Guinée Bissau OR Haiti* OR Korea* OR Liberia* OR Madagascar* OR Malawi* OR Mali OR Malian OR Mozambiqu* OR Nepal* OR Niger OR Rwand* OR Senegal* OR “Sierra Leone” OR Somali* OR Sudan* OR Tanzani* OR Togo OR Togolese OR Ugand* OR Zimbabw*) OR (Armenia* OR Banglades* OR Bhutan OR Bolivia OR “Cabo Verde” OR “Cape Verde” OR Cambodia* OR Cameroon* OR Congo OR “Cote D'Ivoire” OR “Ivory Coast” OR Djibout* OR Egypt* OR “El Salvador” OR Ghana* OR Guatemala OR Honduras OR India* OR Indonesia* OR Keny* OR Kiribati OR Kyrgyzstan* OR “Kyrgyz Republic” OR Lao* OR Lesotho* OR Mauritania* OR Mauritius OR Mauritian* OR Micronesi* OR Mongolia* OR Morocc* OR Burma OR Myanmar OR Nicaragua* OR Nigeria* OR Pakistan* OR “Papua New Guinea” OR Philippine* OR Samoa OR “São Tomé and Principe” OR “São Tomé e Príncipe” OR Solomon Island* OR Sri Lanka OR Sudan* OR Swazi* OR Syria* OR Tajikistan* OR Timor‐Leste OR Tonga OR Tunisia* OR Uzbekistan* OR Vanuatu* OR Vietnam* OR “West Bank” OR Gaza OR Yemen* OR Zambia*) AND (Europe* OR Scandinavia* OR Mediterranean OR Baltic OR Andorra* OR Azerbaijan* OR Albania* OR Armenia* OR Austria* OR Belarus* OR Byelarus* OR Bosni* OR Herzegovin* OR Croat* OR Cyprus OR Cypriot* OR Czech OR Belgi* OR Bulgaria* OR Denmark OR Danish OR Estonia* OR Finland OR Finnish OR France* OR French* OR Georgia* OR German* OR Greece OR Greek OR Hungar* OR Iceland* OR Ital* OR Sicil* OR Sardinia* OR Latvi* OR Liechtenstein* OR Lithuania* OR Luxembourg* OR Macedonia* OR Malta OR Maltese OR Moldova* OR Monaco OR Montenegr* OR Netherlands OR Dutch OR Norway OR Norwegian* or Svalbard* OR Poland* OR Polish* OR Portugal OR Portuguese OR Romania* OR Roumania* OR Rumania* OR San Marino OR Serb* OR Slovak* OR Slovenia* OR Spain* OR Spanish* OR Sweden OR Swedish OR Switzerland OR Swiss OR Great Britain* OR British* OR Channel Islands* OR Guerns* OR England* OR English* OR Hebrid* OR Ireland* OR Irish* OR Scotland* OR Scotch* OR Scottish* OR Wales* OR Welsh* OR United Kingdom* OR UK OR Gibraltar OR Ukrain* OR Vatican OR Yugoslavia*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
1,221,223 |
| #6 |
#5 AND #4 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
4,300 |
| #5 |
TS=(time OR timing OR moment OR duration OR age OR month OR months OR early OR week OR weeks OR year OR years OR day OR days) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
12,919,619 |
| #4 |
#3 OR #2 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
6,170 |
| #3 |
TS=((full OR fully OR exclusiv*) NEAR (“breast milk” OR “human milk” OR “maternal milk” OR breastmilk OR “mother* own milk”) AND (fed OR feeding* OR diet OR intake)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
761 |
| #2 |
TS=((Exclusiv* OR fully OR full) NEAR/5 (breastfeed* OR “breast feed*” OR breastfed OR “breast fed*” OR lactat*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,815 |
| #1 |
TS= (infan* OR “young child*” OR baby OR babies OR “early childhood” OR weanling* OR “first year of life” OR “early life”) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
539,234 |
D.3. Update and upgrade of the literature searches (2 October 2018)
D.3.1. Update and upgrade of the search performed by the contractor
Cochrane Library
| ID | Search | Items found |
|---|---|---|
| #1 | [mh ^Infant] OR [mh “child, preschool”] OR infan*:ti,ab,kw OR young child*:ti,ab,kw OR baby:ti,ab,kw OR babies:ti,ab,kw OR “early childhood”:ti,ab,kw OR weanling*:ti,ab,kw OR “first year of life”:ti,ab,kw OR “early life”:ti,ab,kw OR ((“pre school”:ti,ab,kw OR preschool:ti,ab,kw OR kindergar*:ti,ab,kw) AND (child*:ti,ab,kw)) OR “preschool aged”:ti,ab,kw OR “preschool age”:ti,ab,kw OR “pre school age”:ti,ab,kw OR “pre school aged”:ti,ab,kw OR “kindergarten age”:ti,ab,kw OR “kindergarten aged”:ti,ab,kw | 79,467 |
| #2 | [mh ^”Infant Nutritional Physiological Phenomena”] OR [mh ^”Infant Food”] OR [mh Weaning] OR diet:ti,ab,kw OR nutrition:ti,ab,kw OR food*:ti,ab,kw OR feeding:ti,ab,kw OR wean*:ti,ab,kw OR beikost:ti,ab,kw OR “partial breastfeeding”:ti,ab,kw OR “partial breastfeeding”:ti,ab,kw OR “non‐exclusive breastfeeding”:ti,ab,kw OR “non‐exclusive breastfeeding”:ti,ab,kw OR “mixed breastfeeding”:ti,ab,kw OR “mixed breastfeeding”:ti,ab,kw OR fruit*:ti,ab,kw OR vegetable*:ti,ab,kw OR cereal*:ti,ab,kw OR wheat:ti,ab,kw OR gluten:ti,ab,kw OR egg*:ti,ab,kw OR peanut*:ti,ab,kw OR fish:ti,ab,kw OR shellfish:ti,ab,kw OR porridge:ti,ab,kw OR rice:ti,ab,kw OR meat:ti,ab,kw OR bread:ti,ab,kw OR juice:ti,ab,kw OR corn:ti,ab,kw OR IYCF:ti,ab,kw OR puree*:ti,ab,kw OR solid*:ti,ab,kw OR “spoon‐fed”:ti,ab,kw OR spoonfed:ti,ab,kw OR meal*:ti,ab,kw | 108,124 |
| #3 | (introduction:ti,ab,kw,kw OR introduce*:ti,ab,kw,kw OR introducing:ti,ab,kw,kw OR start:ti,ab,kw,kw OR beginning:ti,ab,kw,kw OR milestone*:ti,ab,kw,kw) NEAR (time:ti,ab,kw OR timing:ti,ab,kw OR moment:ti,ab,kw OR duration:ti,ab,kw OR age:ti,ab,kw OR month:ti,ab,kw OR months:ti,ab,kw OR early:ti,ab,kw OR week:ti,ab,kw OR weeks:ti,ab,kw OR year:ti,ab,kw OR years:ti,ab,kw OR day:ti,ab,kw OR days:ti,ab,kw) | 15,912 |
| #4 | #1 AND #2 AND #3 | 673 |
Systematic reviews (SR): 31
Clinical trials (CT), filtered from 1990: 611
642 imported into a library (1 clinical question not imported)
Pubmed
| ID | Search | Items found |
|---|---|---|
| #18 | Search (#17) AND (“1990”[Date ‐ Publication] : “3000”[Date ‐ Publication]) | 8,789 |
| #17 | Search #15 NOT #16 | 9,807 |
| #16 | Search Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Clinical Conference[ptyp] OR Comment[sb] OR “pubmed books”[Filter] | 3,377,353 |
| #15 | Search #13 NOT #14 | 10,636 |
| #14 | Search ”Animals”[Mesh] NOT (“Humans”[Mesh] AND “Animals”[Mesh]) | 4,499,401 |
| #13 | Search #8 AND #10 AND #11 AND #12 | 11,246 |
| #12 | Search “Infant Nutritional Physiological Phenomena”[Mesh:NoExp] OR “Infant Food”[Mesh:NoExp] OR “Weaning”[Mesh] OR wean*[tiab] OR diet[tiab] OR nutrition*[tiab] OR food*[tiab] OR feeding[tiab] OR beikost[tiab] OR “partial breastfeeding”[tiab] OR “partial breastfeeding”[tiab] OR “non‐exclusive breastfeeding”[tiab] OR “non‐exclusive breastfeeding”[tiab] OR “mixed breastfeeding”[tiab] OR “mixed breastfeeding”[tiab] OR fruit*[tiab] OR vegetable*[tiab] OR cereal*[tiab] OR wheat[tiab] OR gluten[tiab] OR egg*[tiab] OR peanut*[tiab] OR fish[tiab] OR shellfish[tiab] OR porridge[tiab] OR rice [tiab] OR meat[tiab] OR bread[tiab] OR juice[tiab] OR corn[tiab] OR puree*[tiab] OR IYCF[tiab] OR solid*[tiab] OR “spoon‐fed”[tiab] OR “spoonfed”[tiab] OR meal*[tiab] | 1,721,416 |
| #11 | Search time[tiab] OR timing[tiab] OR moment[tiab] OR duration[tiab] OR age[tiab] OR month[tiab] OR months[tiab] OR early[tiab] OR week[tiab] OR weeks[tiab] OR year[tiab] OR years[tiab] OR day[tiab] OR days[tiab] | 8,740,036 |
| #10 | Search introduction[tiab] OR introduce*[tiab] OR introducing[tiab] OR start*[tiab] OR beginning[tiab] OR milestone*[tiab] | 1,296,548 |
| #8 | Search ”Infant”[mh:noexp] OR “Child, Preschool”[Mesh] OR infan*[tiab] OR young child*[tiab] OR baby[tiab] OR babies[tiab] OR early childhood[tiab] OR weanling*[tiab] OR “ first year of life”[tiab] OR “early life”[tiab] OR ((“pre school”[tiab] OR preschool[tiab] OR kindergar*[tiab]) AND (child*[tiab])) OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab] | 1,461,295 |
Web of Science. Core Collection
| ID | Search | Items found |
|---|---|---|
| #6 |
#5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1990‐2018 |
5,324 |
| #5 |
#3 AND #2 AND #1 Refined by: DOCUMENT TYPES: (ARTICLE OR REVIEW OR REPRINT OR PROCEEDINGS PAPER OR CORRECTION OR BOOK REVIEW) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,329 |
| #4 |
#3 AND #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,466 |
| #3 |
TS=((introduction OR introduce* OR introducing OR start OR beginning OR milestone*) NEAR (time OR timing OR moment OR duration OR age OR month OR months OR early OR week OR weeks OR year OR years OR day OR days)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
489,108 |
| #2 |
TS=(diet OR nutrition OR food* OR wean* OR feeding OR beikost OR IYCF OR “partial breastfeeding” OR “partial breastfeeding” OR “non‐exclusive breastfeeding” OR “non‐exclusive breastfeeding” OR “mixed breastfeeding” OR “mixed breastfeeding” OR fruit* OR vegetable* OR cereal* OR wheat OR gluten OR egg* OR peanut* OR fish OR shellfish OR porridge OR rice OR meat OR bread OR juice OR corn OR puree* OR solid* OR “spoon‐fed” OR meal*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
4,066,938 |
| #1 |
TS=(infan* OR “young child*” OR baby OR babies OR “early childhood” OR weanling* OR “first year of life” OR “early life” OR ((“pre school” OR preschool OR kindergar*) NEAR (child* OR “age” OR “aged”))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
626,719 |
D.3.2. Update and upgrade of the complementary search
Cochrane Library
| ID | Search | Items found |
|---|---|---|
| #1 | [mh ^Infant] OR [mh “child, preschool”] OR infan*:ti,ab,kw OR young child*:ti,ab,kw OR baby:ti,ab,kw OR babies:ti,ab,kw OR “early childhood”:ti,ab,kw OR weanling*:ti,ab,kw OR “first year of life”:ti,ab,kw OR “early life”:ti,ab,kw OR ((“pre school”:ti,ab,kw OR preschool:ti,ab,kw OR kindergar*:ti,ab,kw) AND (child*:ti,ab,kw)) OR “preschool aged”:ti,ab,kw OR “preschool age”:ti,ab,kw OR “pre school age”:ti,ab,kw OR “pre school aged”:ti,ab,kw OR “kindergarten age”:ti,ab,kw OR “kindergarten aged”:ti,ab,kw | 79,467 |
| #2 | (Exclusiv*:ti,ab,kw or fully:ti,ab,kw or full:ti,ab,kw) near/5 (breastfeed*:ti,ab,kw or breast feed*:ti,ab,kw or breastfed:ti,ab,kw or “breast fed”:ti,ab,kw or lactat*:ti,ab,kw) | 1,813 |
| #3 | (Exclusiv*:ti,ab,kw or fully:ti,ab,kw or full:ti,ab,kw) and [mh “breast feeding”] | 609 |
| #4 | (full:ti,ab,kw or fully:ti,ab,kw or exclusiv*) and (“breast milk”:ti,ab,kw or “human milk”:ti,ab,kw or “maternal milk”:ti,ab,kw or “mother's own milk”:ti,ab,kw) and (fed:ti,ab,kw or feeding*:ti,ab,kw or diet:ti,ab,kw or intake:ti,ab,kw) | 566 |
| #5 | #2 OR #3 OR #4 | 2,044 |
| #6 | (time:ti,ab,kw or timing:ti,ab,kw or moment:ti,ab,kw or duration:ti,ab,kw or age:ti,ab,kw or month:ti,ab,kw or months:ti,ab,kw or early:ti,ab,kw or week:ti,ab,kw or weeks:ti,ab,kw or year:ti,ab,kw or years:ti,ab,kw or day:ti,ab,kw or days:ti,ab,kw or [mh “Time factors”] or [mh ^“Age Factors”]) | 816,392 |
| #7 | #1 AND #5 AND #6 | 1,639 |
SR: 81
CT from 1990: 1,501
Protocols (8) not imported
Pubmed
| ID | Search | Items found |
|---|---|---|
| #12 | Search (#11) AND (“1990”[Date ‐ Publication] : “3000”[Date ‐ Publication]) | 5,708 |
| #11 | Search #9 NOT #10 | 6,095 |
| #10 | Search Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Clinical Conference[ptyp] OR Comment[sb] OR “pubmed books”[Filter] | 3,377,353 |
| #9 | Search #8 NOT #7 | 6,363 |
| #8 | Search #1 AND #5 AND #6 | 6,378 |
| #7 | Search ”Animals”[Mesh] NOT (“Humans”[Mesh] AND “Animals”[Mesh]) | 4,499,401 |
| #6 | Search (time[tiab] OR timing[tiab] OR moment[tiab] OR duration[tiab] OR age[tiab] OR month[tiab] OR months[tiab] OR early[tiab] OR week[tiab] OR weeks[tiab] OR year[tiab] OR years[tiab] OR day[tiab] OR days[tiab] OR “Time factors”[Mesh] OR “Age Factors”[Mesh:NoExp]) | 9,319,237 |
| #5 | Search #2 OR #3 OR #4 | 8,749 |
| #4 | Search Exclusiv*[tiab] AND (breastfeed*[tiab] OR breast feed*[tiab] OR breastfed[tiab] OR “breast fed”[tiab] OR lactat*[tiab] OR “breast feeding”[Mesh]) | 7,687 |
| #3 | Search (full[tiab] OR fully[tiab] OR exclusiv*[tiab]) AND (“breast milk”[tiab] OR “human milk”[tiab] OR “maternal milk”[tiab] OR “mother's own milk”[tiab]) AND (fed[tiab] OR feeding*[tiab] OR diet[tiab] OR intake[tiab]) | 2,171 |
| #2 | Search ”fully breastfeeding”[tiab] OR “fully breastfeeding”[tiab] OR fully breastfed*[tiab] OR fully breastfed*[tiab] OR “full breastfeeding”[tiab] OR “full breastfeeding”[tiab] | 388 |
| #1 | Search ”Infant”[mh:noexp] OR “Child, Preschool”[Mesh] OR infan*[tiab] OR young child*[tiab] OR baby[tiab] OR babies[tiab] OR early childhood[tiab] OR weanling*[tiab] OR “ first year of life”[tiab] OR “early life”[tiab] OR ((“pre school”[tiab] OR preschool[tiab] OR kindergar*[tiab]) AND (child*[tiab])) OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab] | 1,461,295 |
Web of Science. Core Collection
| ID | Search | Items found |
|---|---|---|
| #13 |
#12 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1990‐2018 |
5,077 |
| #12 |
#10 AND #9 AND #1 Refined by: DOCUMENT TYPES: (ARTICLE OR CORRECTION OR REVIEW OR RETRACTED PUBLICATION OR PROCEEDINGS PAPER) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
5,084 |
| #11 |
#10 AND #9 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
5,174 |
| #10 |
TS=(time OR timing OR moment OR duration OR age OR month OR months OR early OR week OR weeks OR year OR years OR day OR days) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
14,434,421 |
| #9 |
#8 OR #7 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
7,402 |
| #8 |
TS=((full OR fully OR exclusiv*) NEAR (“breast milk” OR “human milk” OR “maternal milk” OR breastmilk OR “mother* own milk”) AND (fed OR feeding* OR diet OR intake)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
890 |
| #7 |
TS=((Exclusiv* OR fully OR full) NEAR/5 (breastfeed* OR “breast feed*” OR breastfed OR “breast fed*” OR lactat*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1975‐2018 |
6,994 |
| #6 |
#5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=1990‐2018 |
5,324 |
| #5 |
#3 AND #2 AND #1 Refined by: DOCUMENT TYPES: (ARTICLE OR REVIEW OR REPRINT OR PROCEEDINGS PAPER OR CORRECTION OR BOOK REVIEW) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,329 |
| #4 |
#3 AND #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,466 |
| #3 |
TS=((introduction OR introduce* OR introducing OR start OR beginning OR milestone*) NEAR (time OR timing OR moment OR duration OR age OR month OR months OR early OR week OR weeks OR year OR years OR day OR days)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
489,108 |
| #2 |
TS=(diet OR nutrition OR food* OR wean* OR feeding OR beikost OR IYCF OR “partial breastfeeding” OR “partial breastfeeding” OR “non‐exclusive breastfeeding” OR “non‐exclusive breastfeeding” OR “mixed breastfeeding” OR “mixed breastfeeding” OR fruit* OR vegetable* OR cereal* OR wheat OR gluten OR egg* OR peanut* OR fish OR shellfish OR porridge OR rice OR meat OR bread OR juice OR corn OR puree* OR solid* OR “spoon‐fed” OR meal*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
4,066,938 |
| #1 |
TS=(infan* OR “young child*” OR baby OR babies OR “early childhood” OR weanling* OR “first year of life” OR “early life” OR ((“pre school” OR preschool OR kindergar*) NEAR (child* OR “age” OR “aged”))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
626,719 |
D.4. Search strings of the extensive literature search undertaken by EFSA on motor development (developmental readiness of the infant to receive CFs)
Sources of information
| Source of information | Platform | Date range | Date of search |
|---|---|---|---|
| PubMed | PubMed | Inception–Present | 6/2/2019 |
| Science Citation Index Expanded (SCI‐EXPANDED) | Web of Science. Core Collection | 1975–present | |
| Social Sciences Citation Index (SSCI) | 1975–present | ||
| Arts & Humanities Citation Index (A&HCI) | 1975–present | ||
| Conference Proceedings Citation Index‐ Science (CPCI‐S) | 1990–present | ||
| Conference Proceedings Citation Index‐ Social Science & Humanities (CPCI‐SSH) | 1990–present | ||
| Book Citation Index– Science (BKCI‐S) | 2005–present | ||
| Book Citation Index– Social Sciences & Humanities (BKCI‐SSH) | 2005–present | ||
| Emerging Sources Citation Index (ESCI) | 2005–present |
Search strings
Pubmed
| ID | Search | Items found |
|---|---|---|
| #20 | Search ((#18) NOT (“Animals”[Mesh] NOT “humans”[Mesh]))) NOT ((rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR murine[ti] OR rodent[ti] OR rodents[ti] OR hamster[ti] OR hamsters[ti] OR pig[ti] OR pigs[ti] OR porcine[ti] OR rabbit[ti] OR rabbits[ti] OR animal[ti] OR animals[ti] OR dogs[ti] OR dog[ti] OR cats[ti] OR cow[ti] OR bovine[ti] OR sheep[ti] OR ovine[ti] OR monkey[ti] OR monkeys[ti] OR horse[ti] OR horses[ti]) NOT medline[sb]) | 1,083 |
| #19 | Search (rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR murine[ti] OR rodent[ti] OR rodents[ti] OR hamster[ti] OR hamsters[ti] OR pig[ti] OR pigs[ti] OR porcine[ti] OR rabbit[ti] OR rabbits[ti] OR animal[ti] OR animals[ti] OR dogs[ti] OR dog[ti] OR cats[ti] OR cow[ti] OR bovine[ti] OR sheep[ti] OR ovine[ti] OR monkey[ti] OR monkeys[ti] OR horse[ti] OR horses[ti]) NOT medline[sb] | 107,607 |
| #18 | Search #16 NOT (“Animals”[Mesh] NOT “humans”[Mesh]) | 1,084 |
| #17 | Search ”Animals”[Mesh] NOT “humans”[Mesh] | 4,544,991 |
| #16 | Search #15 OR #13 OR #10 OR #8 | 1,119 |
| #15 | Search ((((((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab]))) OR “Child Development”[Mesh:NoExp])) AND (((“Deglutition”[Mesh] OR deglutition*[tiab] OR swallow*[tiab]) AND (“Physiology”[Mesh] OR physiolog*[tiab])) OR “Deglutition/physiology”[Majr]) | 158 |
| #14 | Search ((“Deglutition”[Mesh] OR deglutition*[tiab] OR swallow*[tiab]) AND (“Physiology”[Mesh] OR physiolog*[tiab])) OR “Deglutition/physiology”[Majr] | 3,831 |
| #13 | Search (((((((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab]))) OR “Child Development”[Mesh:NoExp])) AND (“Head Movements”[Mesh] OR ((head[tiab] OR heads[tiab]) AND (Hold*[tiab] OR held[tiab] OR movement*[tiab])) OR ((head[ti] OR heads[ti]) AND control*[ti]) OR head control*[tiab] OR “Postural Balance”[Mesh] OR postural balance*[tiab] OR postural equilibr*[tiab] OR body equilibr*[tiab] OR body balance[tiab])) AND (“Motor Skills”[Mesh] OR “Motor Activity”[Mesh:noexp] OR “Psychomotor Performance”[Mesh:NoExp] OR motor skill*[tiab] OR motor milestone*[tiab] OR motor activit*[tiab] OR motor performance*[tiab] OR motor function*[tiab] OR neurodevelopmental skill*[tiab] OR motor abilit*[tiab] OR neurodevelopmental milestone*[tiab] OR neurodevelopmental activit*[tiab] OR neurodevelopmental performance*[tiab] OR neurodevelopmental function*[tiab] OR psychomotor skill*[tiab] OR psychomotor milestone*[tiab] OR psychomotor activit*[tiab] OR psychomotor performance*[tiab] OR psychomotor function*[tiab] OR psychomotor abilit*[tiab]) | 513 |
| #12 | Search ”Motor Skills”[Mesh] OR “Motor Activity”[Mesh:noexp] OR “Psychomotor Performance”[Mesh:NoExp] OR motor skill*[tiab] OR motor milestone*[tiab] OR motor activit*[tiab] OR motor performance*[tiab] OR motor function*[tiab] OR neurodevelopmental skill*[tiab] OR motor abilit*[tiab] OR neurodevelopmental milestone*[tiab] OR neurodevelopmental activit*[tiab] OR neurodevelopmental performance*[tiab] OR neurodevelopmental function*[tiab] OR psychomotor skill*[tiab] OR psychomotor milestone*[tiab] OR psychomotor activit*[tiab] OR psychomotor performance*[tiab] OR psychomotor function*[tiab] OR psychomotor abilit*[tiab] | 206,728 |
| #11 | Search ”Head Movements”[Mesh] OR ((head[tiab] OR heads[tiab]) AND (Hold*[tiab] OR held[tiab] OR movement*[tiab])) OR ((head[ti] OR heads[ti]) AND control*[ti]) OR head control*[tiab] OR “Postural Balance”[Mesh] OR postural balance*[tiab] OR postural equilibr*[tiab] OR body equilibr*[tiab] OR body balance[tiab] | 43,898 |
| #10 | Search ((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Gagging”[Mesh] OR ((extrusion[tiab] OR gag[tiab] OR gagging[tiab] OR tongue*[tiab] OR “Tongue”[Mesh] OR oralpharyn*[tiab]) AND (reflex*[tiab] OR “Reflex”[Mesh] OR “push out”[tiab] OR pushing[tiab])) OR (oral*[ti] AND reflex*[ti]) OR oral reflex*[tiab] OR tongue thrust*[tiab] OR tongue push*[tiab]) | 325 |
| #9 | Search ”Gagging”[Mesh] OR ((extrusion[tiab] OR gag[tiab] OR gagging[tiab] OR tongue*[tiab] OR “Tongue”[Mesh] OR oralpharyn*[tiab]) AND (reflex*[tiab] OR “Reflex”[Mesh] OR “push out”[tiab] OR pushing[tiab])) OR (oral*[ti] AND reflex*[ti]) OR oral reflex*[tiab] OR tongue thrust*[tiab] OR tongue push*[tiab] | 2,095 |
| #8 | Search (((((((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab]))) OR “Child Development”[Mesh:NoExp])) AND ((“Deglutition”[Mesh] OR deglutition*[tiab] OR swallow*[tiab]))) AND ((“Feeding Behavior”[Mesh:noexp] OR ((feed*[tiab] OR eat*[tiab] OR alimentar*[tiab]) AND (behav*[tiab] OR skill*[tiab])))) | 189 |
| #7 | Search (“Feeding Behavior”[Mesh:noexp] OR ((feed*[tiab] OR eat*[tiab] OR alimentar*[tiab]) AND (behav*[tiab] OR skill*[tiab]))) | 136,822 |
| #6 | Search (“Deglutition”[Mesh] OR deglutition*[tiab] OR swallow*[tiab]) | 31,928 |
| #5 | Search ((((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab]))) OR “Child Development”[Mesh:NoExp] | 607,960 |
| #4 | Search ”Child Development”[Mesh:NoExp] | 42,995 |
| #3 | Search ((“Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab])) AND (“Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab]) | 602,540 |
| #2 | Search ”Growth and Development”[Mesh] OR “growth and development” [Subheading] OR develop*[tiab] OR grow*[tiab] | 6,122,998 |
| #1 | Search ”Infant”[Mesh] OR infan*[tiab] OR child*[tiab] OR baby[tiab] OR babies[tiab] OR “first year of life”[tiab] OR “early life”[tiab] OR “preschool aged”[tiab] OR “preschool age”[tiab] OR “pre school age”[tiab] OR “pre school aged”[tiab] OR “kindergarten age”[tiab] OR “kindergarten aged”[tiab] | 2,130,451 |
Web of Science. Core Collection
| ID | Search | Items found |
|---|---|---|
| #15 | #13 NOT #14 | 640 |
| #14 |
TI=(rat OR rats OR mouse OR mice OR murine OR rodent OR rodents OR hamster OR hamsters OR pig OR pigs OR porcine OR rabbit OR rabbits OR animal OR animals OR dogs OR dog OR cats OR cow OR bovine OR sheep OR ovine OR monkey OR monkeys OR horse OR horses) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
2,546,480 |
| #13 |
#12 OR #10 OR #7 OR #5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
653 |
| #12 |
#11 AND #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
84 |
| #11 |
TS=((deglutition* OR swallow*) AND physiolog*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
1,720 |
| #10 |
#9 AND #8 AND #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
238 |
| #9 |
TS=((motor OR neurodevelopmental OR psychomotor) NEAR (skill* OR milestone* OR activit* OR performance* OR ability* OR function*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
136,076 |
| #8 |
TS=(((“head” OR heads) NEAR (hold* OR held OR control* OR movement*)) OR ((postural OR body) NEAR (balance* OR equilibr*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
51,476 |
| #7 |
#6 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
190 |
| #6 |
TS=(((extrusion OR gag OR “gagging” OR tongue* OR oral OR oralpharyn*) NEAR (reflex* OR “push out” OR “pushing”)) OR “tongue thrust*” OR “tongue push*”) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
1,151 |
| #5 |
#4 AND #3 AND #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
168 |
| #4 |
TS=((feed* OR eat* OR alimentar*) AND (behav* OR skill*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
181,405 |
| #3 |
TS=(Deglutition* OR swallow*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
32,197 |
| #2 |
TS=(develop* OR grow*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
10,703,331 |
| #1 |
TS=(infan* OR child* OR baby OR babies OR “first year of life” OR “early life” OR ((“pre school” OR preschool OR kindergar*) NEAR (“age” OR “aged”))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
2,045,346 |
Annex A – Outcome of the data extraction from the included prospective and retrospective studies
1.
Description: The annex is an Microsoft Excel® file that provides the full details of the data extracted from the included papers on prospective (intervention or observational) studies (Table A.1) and retrospective studies (Table A.2). The methodology applied for the data extraction is described in Section 2.2.3 and the assessment of the extracted data is provided in Sections 4–18 and related Appendices.
Annex B – Result of the assessment of the risk of bias per question and outcome for randomised controlled trials and prospective observational studies
1.
Description: The annex is an Microsoft Excel® file that provides the results of the assessment of the internal validity undertaken per question/item considered and outcome. The methodology applied is described in Section 2.2.2. The colour code used in the Annex is as follows: dark green for definitely low RoB, light green for probably low RoB, light red for probably high RoB and dark red for definitely high RoB.
Annex C – List of papers excluded at full‐text screening (step 2) of the searches
1.
Description: The annex is an Microsoft Excel® file that comprises the list of papers excluded at the second step of the full‐text screening (Section 2.2.1), either in full or for some outcomes only. It also includes publications from cohorts for which for the same endpoint and for the same outcome assessment another publication had already been considered in the review (Section 2.1.1.2). It shows the name of first author, the country, the study design, the outcome and the reasons for exclusion.
Annex D – Funnel plots for the assessment of publication bias
1.
Description: The annex is a PDF file that provides the funnel plots that were used in the assessment of publication bias (Section 2.2.3.2).
Annex E – Sensitivity analyses on the use of different between‐study variance estimators in the random effects meta‐analyses
1.
Description: The annex is a PDF file that provides the result of a sensitivity analysis per outcome, study design and Tier of RoB, showing the confidence intervals calculated based on the DerSimonian and Laird approach or based on the Paule and Mandel approach, with or without the Hartung and Knapp modification, as described in Section 2.2.3.2.
Supporting information
Outcome of the data extraction from the included prospective and retrospective studies
Result of the assessment of the risk of bias per question and outcome for randomised controlled trials and prospective observational studies
List of papers excluded at full‐text screening (step 2) of the searches
Funnel plots for the assessment of publication bias
Sensitivity analyses on the use of different between‐study variance estimators in the random effects meta‐analyses
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Castenmiller J, de Henauw S, Hirsch‐Ernst K‐I, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Bresson J‐L, Fewtrell M, Kersting M, Przyrembel H, Dumas C, Titz A and Turck D, 2019. Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant's diet. EFSA Journal 2019;17(9):5780, 241 pp. 10.2903/j.efsa.2019.5780
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00482
Panel members: Jacqueline Castenmiller, Stefaan de Henauw, Karen‐Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri, Dominique Turck and Marco Vinceti.
Acknowledgements: The Panel wishes to thank the following EFSA statutory staff members (Declarations of interest available upon request) for the support provided to this scientific output: Federica Barrucci, Andrea Bau, Laura Ciccolallo, Agnès de Sesmaisons‐Lecarré , Lucia Fabiani, Leonard Matijević, Irene Muñoz Guajardo, and Ermolaos Ververis. The Panel also wishes to thank the following EFSA non‐statutory staff members* for the support provided to this scientific output: Mathias Amundsen, Claudia Denami, Pauline Lachevre, Federico Morreale, Charlotte Salgaard Nielsen and Qinqing Sun.
Interests and EFSA funding: All authors of this Scientific Opinion have submitted Declarations of Interest (DoI) which have been evaluated in accordance with EFSA's policy on Independence and Competing Interest Management (http://www.efsa.europa.eu/en/corporate/pub/policyonindependence, https://www.efsa.europa.eu/en/corporate/pub/independencepolicy17). The DoIs of members of the NDA Panel and of the Working Group are available in the ‘DoI database’ on EFSA's website (https://ess.efsa.europa.eu/doi/doiweb/doisearch/). In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to an expert of the Working Group until end of October 2018. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the discussion and in the drafting phase of the scientific output but was not allowed to take up the role of rapporteur within that time frame. Any competing interests are recorded in the respective minutes of the meetings of the NDA Panel (http://www.efsa.europa.eu/en/events/advanced-search) and of the Working Group (https://www.efsa.europa.eu/sites/default/files/wgs/nutrition/ndainfantnutrition.pdf). EFSA statutory staff members are also subject to DoI submission and screening requirements and are assessed in line with the above policy under the EU Staff Regulations and its implementing rules. DoIs of the Heads of Department and Heads of Unit responsible for managing EFSA's scientific and operational services are also available on EFSA's website. Allowances and indemnities received by experts for their involvement in EFSA's work are outlined in the EFSA experts’ compensation guide (http://www.efsa.europa.eu/sites/default/files/Experts_compensation_guide.pdf).
EFSA is fully funded by the general budget of the European Union or of Member States participating to its governance bodies and receives no fees or contributions from private sources.
* Non‐statutory staff members (i.e. trainees, visiting scientists and interim staff) are not covered by the EU staff regulations and are therefore not subject to DoIs submission.
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1686/full
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4969/full
Notes
Studies in low‐income and lower‐middle‐income countries that were conducted in poor hygiene conditions were excluded.
Directive 2009/39/EC of the European Parliament and of the Council of 6 May 2009 on foodstuffs intended for particular nutritional uses (recast), OJ L 124, 20.5.2009, p. 21–29.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children, OJ L 339, 6.12.2006, p. 16–35.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009; OJ L 181, 29.6.2013, p. 35–56.
Losses via transepidermal diffusion and via the respiratory tract.
The terms ‘background milk feeding’ are used in the following sections, even though the Panel is aware that formula is not ‘milk’ from a legal perspective.
Randomised controlled trials (RCTs) may provide evidence of causality. Observational studies after adjustments for confounders can indicate the presence of an association. Associations shown in an observational study should also be interpreted in the light of possible reverse causality (Sections 4.2, 5.2, 6.2 and 8.2).
i.e. case–control studies, sibling case–control studies, cross‐sectional studies, cross‐sectional analyses of otherwise prospective studies, retrospective cohorts and a prospective cohort in which exposure was assessed after the outcome.
Topic was used as feature space and the best prediction was obtained using the following ensemble: RF rose, NN rose, svm Linear rose, svm Poly rose, svm Poly smote, svm Linear smote, NN smote, svm Radial smote and GBM smote.
EFSA. (2018, June 26). Shiny R tool for the automation of systematic reviews (Version v3). Zenodo. https://doi.org/10.5281/zenodo.1299654
In some cases, several papers on the same study were available that investigated different outcomes and ages at which the outcome was assessed.
The pooled estimate of a random effects meta‐analysis represents the estimated weighted average of the effect/associations observed in the different sub‐populations and settings investigated. It should not be interpreted as an estimation of the ‘true mean effect’ (which is estimated by a fixed effect meta‐analysis). The 95% CI provides information about the uncertainty around the weighted average. The prediction interval illustrates the range in which results of future studies in similar populations and settings could fall with a certain probability.
I2 is a measure of inconsistency in study results that cannot be attributed to sampling error.
The probability of rejecting the null hypothesis if it is true is > 5%.
Estimate of the between‐study variance.
(1) attained body length: prospective cohort studies in Tier 3; (2) symptomatic food allergy and CFs (general population): prospective cohort studies in Tier 3, (3) sensitisation and CFs (at‐risk populations): prospective cohort studies Tiers 1 and 2, and (4) sensitisation and egg (at‐risk populations): RCTs Tiers 1 and 2.
This was not possible when the reference category was the ‘middle’ category. In this case, the individual comparisons were included in the analysis.
If no other limitations prevented the generalisation of results.
i.e. the selective publication of positive findings.
Funnel plots show the effect estimate from individual studies (plotted on the x‐axis) against a measure of variability represented on the y‐axis, such as the standard error. Smaller and less precise studies tend to scatter at the bottom of the ‘inverted funnel’.
If studies are missing in the area of non‐statistical significance (i.e. p ≥ 0.05), publication bias is a possible explanation for the asymmetry that is observed; if studies are missing in the area of statistical significance (i.e. p < 0.05) factors other than publication bias are the more likely explanation for asymmetry.
Lower bound for considering heterogeneity as considerable.
Renal solute load is derived from exogenous and endogenous sources. The former comes mainly from electrolyte intake, while the latter results from metabolism, particularly nitrogenous end‐products related to protein metabolism.
Even though this value is an arbitrary value.
Etude des déterminants pré et post natals précoces de la santé et de développement de l'enfant.
In this study fat mass was measured both by DXA and BIA.
Only studies that investigated the timing of introduction of fish at at‐least one time point before 6 months of age were pertinent for the present assessment, in line with the interpretation of the Terms of Reference, as explained previously (Section 2.1.1.1.). This led to the exclusion of studies related to the timing of introduction of fish that had been considered by other bodies (e.g. SACN) in their assessment undertaken in a different regulatory context from this opinion. Therefore, the assessment by the Panel is not necessarily comparable to assessments that have been performed by other bodies in this respect.
Vital gluten is a by‐product of starch isolation obtained during wet milling, in which flour is separated into starch and proteins (including gluten).
Translation of this amount into an amount of food is difficult owing to the different gluten content of flours, depending on the type of cereal, the variety and environmental factors that may influence the percentage of storage proteins of a grain (such as growing season or region) as well as the gluten that is added for technological reasons. For example, for durum wheat pasta, protein content usually varies between 10 and 15%, of which around 50% are gluten (Atwell and Finnie, 2016). Using these assumptions, 200 mg vital gluten are contained in 2–4 g of durum wheat pasta.
Calculation performed by EFSA based on data read from a graph.
Studies in low‐income and lower‐middle‐income countries that were conducted in poor hygiene conditions were excluded.
Calculated using the DerSimonian and Laird approach without the Hartung and Knapp modification (see Section 2.2.3 for reasons).
In practical terms, higher scores are indicative of children who like eating.
The desire of the child to eat in response to food stimuli regardless of how hungry it is.
In practical terms, higher scores are indicative of children who do not like eating.
Depending on the direction of the association.
Confidence in evidence derived from RCTs is generally higher than the one derived from prospective observational studies.
The RCT by Bellach et al. (2017) (HEAP) study was not used in the integration of the evidence, as its specific study design did not address the question to be answered in this opinion. The study by Tham et al. (2017) was also not used further, because of the high likelihood that this study was underpowered for this outcome. (see Section 8.7.3).
Studies in low‐income and lower‐middle‐income countries that were conducted in poor hygiene conditions were excluded.
For observational studies, the exact ages that were covered in the investigated age categories were in most instances not well defined (see Section 19). Therefore, there is uncertainty around the classification into age categories of introduction of CFs as defined in the included papers.
Studies in low‐income and lower‐middle‐income countries that were conducted in poor hygiene conditions were excluded.
(1) Attained body weight: subgroups of RCTs in Tiers 1 and 2 and prospective cohort studies in Tiers 1 and 2; (2) attained body length/height: subgroups of RCTs in Tiers 1 and 2, and prospective cohort studies in Tiers 1 and 2; (3) attained body length/height by feeding mode: subgroup of formula fed infants; (4) attained HC: subgroups of RCTs in Tiers 1 and 2; (5) odds of developing (at least) overweight: subgroup of prospective cohort studies in Tier 3; (6) asthma‐like symptoms and fish – general population: subgroup of prospective cohort studies in Tiers 1 and 2; (7) eczema and fish – general population: subgroup of prospective cohort studies in Tiers 1 and 2; (8) risk of iron depletion at 6 months of age in exclusively breastfed infants.
Outcomes assessed in single studies only and which have not been used in the assessment are not listed.
References
- AAP (American Academy of Pediatrics), 1988. Clinical testing of infant formulas with respect to nutritional suitability for term infants. Report prepared under FDA contract 223‐86‐2117.
- AAP (American Academy of Pediatrics), 2014. Complementary feeding. In: Pediatric nutrition. American Academy of Pediatrics, IL, USA. pp. 123–142.
- Abraham EC, Godwin J, Sherriff A and Armstrong J, 2012. Infant feeding in relation to eating patterns in the second year of life and weight status in the fourth year. Public Health Nutrition, 15, 1705–1714. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Zuccotti GV, Radaelli G, Besana R, Podesta A, Sterpa A, Rottoli A, Riva E and Giovannini M, 2009. Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: a randomized, prospective, double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition, 89, 64–70. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellof M, Embleton ND, Fusch C, Genzel‐Boroviczeny O, Goulet O, Kalhan SC, Kolacek S, Koletzko B, Lapillonne A, Mihatsch W, Moreno L, Neu J, Poindexter B, Puntis J, Putet G, Rigo J, Riskin A, Salle B, Sauer P, Shamir R, Szajewska H, Thureen P, Turck D, van Goudoever JB, Ziegler EE and Nutrition ECo , 2010. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 50, 85–91. [DOI] [PubMed] [Google Scholar]
- Agras WS, Kraemer HC, Berkowitz RI and Hammer LD, 1990. Influence of early feeding style on adiposity at 6 years of age. Journal of Pediatrics, 116, 805–809. [DOI] [PubMed] [Google Scholar]
- Ahn CH and MacLean WC Jr, 1980. Growth of the exclusively breastfed infant. American Journal of Clinical Nutrition, 33, 183–192. [DOI] [PubMed] [Google Scholar]
- Alkazemi D, Albeajan M and Kubow S, 2018. Early infant feeding practices as possible risk factors for immunoglobulin E‐mediated food allergies in Kuwait. International Journal of Pediatrics, 2018, 1701903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Goksor E and Wennergren G, 2009. Early introduction of fish decreases the risk of eczema in infants. Archives of Disease in Childhood, 94, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andren Aronsson C, Lee HS, Liu E, Uusitalo U, Hummel S, Yang J, Hummel M, Rewers M, She JX, Simell O, Toppari J, Ziegler AG, Krischer J, Virtanen SM, Norris JM, Agardh D and the TEDDY Study Group , 2015. Age at gluten introduction and risk of celiac disease. Pediatrics, 135, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andren Aronsson C, Lee HS, Koletzko S, Uusitalo U, Yang J, Virtanen SM, Liu E, Lernmark A, Norris JM, Agardh D and the TEDDY Study Group , 2016. Effects of gluten intake on risk of celiac disease: a case‐control study on a Swedish birth cohort. Clinical Gastroenterology and Hepatology, 14, 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris IM, Bernard JY, Chen LW, Tint MT, Pang WW, Soh SE, Saw SM, Shek LP, Godfrey KM, Gluckman PD, Chong YS, Yap F, Kramer MS and Lee YS, 2018. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: significance of parental overweight status. International Journal of Obesity, 42, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvedson JC and Lefton‐Greif MA, 1996. Anatomy, physiology, and development of feeding. Seminars in Speech and Language, 17, 261–268. [DOI] [PubMed] [Google Scholar]
- Ascher H, Krantz I, Rydberg L, Nordin P and Kristiansson B, 1997. Influence of infant feeding and gluten intake on coeliac disease. Archives of Disease in Childhood, 76, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins LA, McNaughton SA, Campbell KJ and Szymlek‐Gay EA, 2016. Iron intakes of Australian infants and toddlers: findings from the Melbourne Infant Feeding, Activity and Nutrition Trial (InFANT) Program. British Journal of Nutrition, 115, 285–293. [DOI] [PubMed] [Google Scholar]
- Atwell WA and Finnie S, 2016. Wheat flour. American Association of Cereal Chemists (AACC) International, St. Paul, MN, USA, 164 pp. [Google Scholar]
- Auricchio S, Follo D, Ritis Gd, Giunta A, Marzorati D, Prampolini L, Ansaldi N, Levi P, Dall'Olio D, Bossi A, Cortinovis I and Marubini E, 1983. Does breast feeding protect against the development of clinical symptoms of celiac disease in children? Journal of Pediatric Gastroenterology and Nutrition, 2, 428–433. [DOI] [PubMed] [Google Scholar]
- Ayano R, Tamura F, Ohtsuka Y and Mukai Y, 2000. The development of normal feeding and swallowing: Showa University study of the feeding function. International Journal of Orofacial Myology, 26, 24–32. [PubMed] [Google Scholar]
- Ayonrinde OT, Oddy WH, Adams LA, Mori TA, Beilin LJ, de Klerk N and Olynyk JK, 2017. Infant nutrition and maternal obesity influence the risk of non‐alcoholic fatty liver disease in adolescents. Journal of Hepatology, 67, 568–576. [DOI] [PubMed] [Google Scholar]
- Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, Taylor MS, Lefebvre DL, Sears MR, Subbarao P and the Child Study Investigators , 2018. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics, 142, pii: e20181092. [DOI] [PubMed] [Google Scholar]
- Bainbridge RR, Mimouni FB, Landi T, Crossman M, Harris L and Tsang RC, 1996. Effect of rice cereal feedings on bone mineralization and calcium homeostasis in cow milk formula fed infants. Journal of the American College of Nutrition, 15, 383–388. [DOI] [PubMed] [Google Scholar]
- Baker JL, Michaelsen KF, Rasmussen KM and Sorensen TI, 2004. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. American Journal of Clinical Nutrition, 80, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Barrera CM, Perrine CG, Li R and Scanlon KS, 2016. Age at introduction to solid foods and child obesity at 6 years. Childhood Obesity, 12, 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascunan Gamboa KA, Weisstaub Nuta SG, Chamorro Melo RA, Guzman MA and Araya Quezada M, 2012. [Association of dietary patterns and food allergy in infants during the first year of life]. Archivos Argentinos de Pediatria, 110, 375–380. [DOI] [PubMed] [Google Scholar]
- Behairy OG, Abul Fadl AM, Arafa OS, Abul Fadl A and Attia MA, 2017. Influence of early feeding practices on biomarkers of cardiovascular disease risk in later life. Egyptian Pediatric Association Gazette, 65, 114–121. [Google Scholar]
- Bell LK, Jansen E, Mallan K, Magarey AM and Daniels L, 2018. Poor dietary patterns at 1‐5 years of age are related to food neophobia and breastfeeding duration but not age of introduction to solids in a relatively advantaged sample. Eating Behaviors, 31, 28–34. [DOI] [PubMed] [Google Scholar]
- Bell S, Yew SSY, Devenish G, Ha D, Do L and Scott J, 2018. Duration of breastfeeding, but not timing of solid food, reduces the risk of overweight and obesity in children aged 24 to 36 months: findings from an Australian cohort study. International Journal of Environmental Research and Public Health, 15, pii E599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellach J, Schwarz V, Ahrens B, Trendelenburg V, Aksunger O, Kalb B, Niggemann B, Keil T and Beyer K, 2017. Randomized placebo‐controlled trial of hen's egg consumption for primary prevention in infants. Journal of Allergy and Clinical Immunology, 139, 1591–1599. [DOI] [PubMed] [Google Scholar]
- Berseth CL, 1992. Effect of early feeding on maturation of the preterm infant's small intestine. Journal of Pediatrics, 120, 947–953. [DOI] [PubMed] [Google Scholar]
- Bezzera Alves JG, Figueiroa JN, Meneses J and Alves GV, 2012. Breastfeeding protects against type 1 diabetes mellitus: a case‐sibling study. Breastfeeding Medicine, 7, 25–28. [DOI] [PubMed] [Google Scholar]
- Bielemann RM, Santos LP, Costa CDS, Matijasevich A and Santos IS, 2018. Early feeding practices and consumption of ultraprocessed foods at 6 y of age: Findings from the 2004 Pelotas (Brazil) Birth Cohort Study. Nutrition, 47, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbilis M, Moschonis G, Mougios V, Manios Y and the Healthy Growth Study Group , 2013. Obesity in adolescence is associated with perinatal risk factors, parental BMI and sociodemographic characteristics. European Journal of Clinical Nutrition, 67, 115–121. [DOI] [PubMed] [Google Scholar]
- Bogaerts M, Deggoujf N, Huart C, Hupin C, Laureyns G, Lemkens P, Rombaux P, Ten Bosch J and Gordts F, 2012. Physiology of the mouth and pharynx, Waldeyer's ring, taste and smell. B‐ent, 8 (Suppl 19), 13–20. [PubMed] [Google Scholar]
- Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, Turck D and van Goudoever J, 2013. Vitamin D in the healthy European paediatric population. Journal of Pediatric Gastroenterology and Nutrition, 56, 692–701. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bedogni G, Pietrobelli A, Cianfarani S and Agostoni C, 2016. Predictors of blood pressure at 7–13 years: The “New Millennium Baby” study. Nutrition, Metabolism & Cardiovascular Diseases, 26, 706–712. [DOI] [PubMed] [Google Scholar]
- Brophy S, Cooksey R, Gravenor MB, Mistry R, Thomas N, Lyons RA and Williams R, 2009. Risk factors for childhood obesity at age 5: analysis of the Millennium Cohort Study. BMC Public Health, 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A and Lee M, 2012. Breastfeeding during the first year promotes satiety responsiveness in children aged 18‐24 months. Pediatric Obesity, 7, 382–390. [DOI] [PubMed] [Google Scholar]
- Bu'Lock F, Woolridge MW and Baum JD, 1990. Development of co‐ordination of sucking, swallowing and breathing: ultrasound study of term and preterm infants. Developmental Medicine and Child Neurology, 32, 669–678. [DOI] [PubMed] [Google Scholar]
- Burdette HL, Whitaker RC, Hall WC and Daniels SR, 2006. Breastfeeding, introduction of complementary foods, and adiposity at 5 y of age. American Journal of Clinical Nutrition, 83, 550–558. [DOI] [PubMed] [Google Scholar]
- Burnier D, Dubois L and Girard M, 2011. Exclusive breastfeeding duration and later intake of vegetables in preschool children. European Journal of Clinical Nutrition, 65, 196–202. [DOI] [PubMed] [Google Scholar]
- Butte NF, 2009. Impact of infant feeding practices on childhood obesity. Journal of Nutrition, 139, 412S–416S. [DOI] [PubMed] [Google Scholar]
- Butte N, Wong WW, Hopkinson JM, Smith EO and Ellis KJ, 2000. Infant feeding mode affects early growth and body composition. Pediatrics, 106, 1355–1366. [DOI] [PubMed] [Google Scholar]
- Butte N, López‐Alarcón M and Garza C, 2002. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. World Health Organization. 57 pp.
- Caleyachetty A, Krishnaveni GV, Veena SR, Hill J, Karat SC, Fall CH and Wills AK, 2013. Breastfeeding duration, age of starting solids and high BMI risk and adiposity in Indian children. Maternal & Child Nutrition, 9, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth BR and Skinner JD, 2002. Feeding behaviors and other motor development in healthy children (2–24 months). Journal of the American College of Nutrition, 21, 88–96. [DOI] [PubMed] [Google Scholar]
- Chaparro CM, 2008. Setting the stage for child health and development: prevention of iron deficiency in early infancy. Journal of Nutrition, 138, 2529–2533. [DOI] [PubMed] [Google Scholar]
- Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG and Winkler C, 2015. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetologica, 52, 621–624. [DOI] [PubMed] [Google Scholar]
- Chokshi NY and Sicherer SH, 2016. Interpreting IgE sensitization tests in food allergy. Expert Review of Clinical Immunology, 12, 389–403. [DOI] [PubMed] [Google Scholar]
- Chuang CH, Hsieh WS, Chen YC, Chang PJ, Hurng BS, Lin SJ and Chen PC, 2011. Infant feeding practices and physician diagnosed atopic dermatitis: a prospective cohort study in Taiwan. Pediatric Allergy and Immunology, 22, 43–49. [DOI] [PubMed] [Google Scholar]
- Cohen RJ, Brown KH, Canahuati J, Rivera LL and Dewey KG, 1994. Effects of age of introduction of complementary foods on infant breast milk intake, total energy intake, and growth: a randomised intervention study in Honduras. Lancet, 344, 288–293. [DOI] [PubMed] [Google Scholar]
- Cohen RJ, Brown KH, Canahuati J, Rivera LL and Dewey KG, 1995a. Determinants of growth from birth to 12 months among breastfed Honduran infants in relation to age of introduction of complementary foods. Pediatrics, 96, 504–510. [PubMed] [Google Scholar]
- Cohen RJ, Rivera LL, Canahuati J, Brown KH and Dewey KG, 1995b. Delaying the introduction of complementary food until 6 months does not affect appetite or mother's report of food acceptance of breastfed infants from 6 to 12 months in a low income, Honduran population. Journal of Nutrition, 125, 2787–2792. [DOI] [PubMed] [Google Scholar]
- Cooke LJ, Wardle J, Gibson EL, Sapochnik M, Sheiham A and Lawson M, 2004. Demographic, familial and trait predictors of fruit and vegetable consumption by pre‐school children. Public Health Nutrition, 7, 295–302. [DOI] [PubMed] [Google Scholar]
- Couper JJ, Beresford S, Hirte C, Baghurst PA, Pollard A, Tait BD, Harrison LC and Colman PG, 2009. Weight gain in early life predicts risk of islet autoimmunity in children with a first‐degree relative with type 1 diabetes. Diabetes Care, 32, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cu L, Villarreal E, Rangel B, Galicia L, Vargas E and Martinez L, 2015. Risk factors for overweight and obesity in infants. Revista Chilena De Nutricion, 42, 139–144. [Google Scholar]
- de Barse LM, Jansen PW, Edelson‐Fries LR, Jaddoe VWV, Franco OH, Tiemeier H and Steenweg‐de Graaff J, 2017. Infant feeding and child fussy eating: The Generation R Study. Appetite, 114, 374–381. [DOI] [PubMed] [Google Scholar]
- de Beer M, Vrijkotte TG, Fall CH, van Eijsden M, Osmond C and Gemke RJ, 2015. Associations of infant feeding and timing of linear growth and relative weight gain during early life with childhood body composition. International Journal of Obesity, 39, 586–592. [DOI] [PubMed] [Google Scholar]
- de Beer M, Vrijkotte TG, Fall CH, van Eijsden M, Osmond C and Gemke RJ, 2016. Associations of infant feeding and timing of weight gain and linear growth during early life with childhood blood pressure: findings from a prospective population based cohort study. PLoS ONE, 11, e0166281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge LL, Langhout MA, Taal HR, Franco OH, Raat H, Hofman A, van Osch‐Gevers L and Jaddoe VW, 2013. Infant feeding patterns are associated with cardiovascular structures and function in childhood. Journal of Nutrition, 143, 1959–1965. [DOI] [PubMed] [Google Scholar]
- de Lauzon‐Guillain B, Jones L, Oliveira A, Moschonis G, Betoko A, Lopes C, Moreira P, Manios Y, Papadopoulos NG, Emmett P and Charles MA, 2013. The influence of early feeding practices on fruit and vegetable intake among preschool children in 4 European birth cohorts. American Journal of Clinical Nutrition, 98, 804–812. [DOI] [PubMed] [Google Scholar]
- DerSimonian R and Laird N, 1986. Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- DesRoches A, Infante‐Rivard C, Paradis L, Paradis J and Haddad E, 2010. Peanut allergy: is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? Journal of Investigational Allergology and Clinical Immunology, 20, 289–294. [PubMed] [Google Scholar]
- Dewey K, 1998. Growth characteristics of breastfed compared to formula fed infants. Biology of the Neonate, 74, 94–105. [DOI] [PubMed] [Google Scholar]
- Dewey K, Cohen R, Rivera L, Canahuati J and Brown K, 1996. Do exclusively breastfed infants require extra protein? Pediatric Research, 39, 303–307. [DOI] [PubMed] [Google Scholar]
- Dewey K, Cohen RJ, Rivera LL and Brown KH, 1998. Effects of age of introduction of complementary foods on iron status of breastfed infants in Honduras. American Journal of Clinical Nutrition, 67, 878–884. [DOI] [PubMed] [Google Scholar]
- Dewey K, Cohen RJ, Brown KH and Rivera LL, 1999. Age of introduction of complementary foods and growth of term, low‐birth‐weight, breastfed infants: a randomized intervention study in Honduras. American Journal of Clinical Nutrition, 69, 679–686. [DOI] [PubMed] [Google Scholar]
- Dewey K, Cohen RJ, Brown KH and Rivera LL, 2001. Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: results of two randomized trials in Honduras. Journal of Nutrition, 131, 262–267. [DOI] [PubMed] [Google Scholar]
- Dewey K, Cohen R and Brown K, 2004. Exclusive breastfeeding for 6 months, with iron supplementation, maintains adequate micronutrient status among term, low‐birthweight, breastfed infants in Honduras. Journal of Nutrition, 134, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Dias CC, Figueiredo B, Rocha M and Field T, 2018. Reference values and changes in infant sleep‐wake behaviour during the first 12 months of life: a systematic review. Journal of Sleep Research, 27, e12654. [DOI] [PubMed] [Google Scholar]
- Dror DK and Allen LH, 2008. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutrition Reviews, 66, 250–255. [DOI] [PubMed] [Google Scholar]
- D'Souza SW, Vale J, Sims DG and Chiswick ML, 1985. Feeding, growth, and biochemical studies in very low birthweight infants. Archives of Disease in Childhood, 60, 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G and the LEAP Study Team , 2015. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England Journal of Medicine, 372, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop AL, Reichrtova E, Palcovicova L, Ciznar P, Adamcakova‐Dodd A, Smith SJ and McNabb SJ, 2006. Environmental and dietary risk factors for infantile atopic eczema among a Slovak birth cohort. Pediatric Allergy and Immunology, 17, 103–111. [DOI] [PubMed] [Google Scholar]
- Durmuş B, Ay L, Duijts L, Moll HA, Hokken‐Koelega AC, Raat H, Hofman A, Steegers EA and Jaddoe VW, 2012. Infant diet and subcutaneous fat mass in early childhood: the Generation R Study. European Journal of Clinical Nutrition, 66, 253–260. [DOI] [PubMed] [Google Scholar]
- Durmuş B, Heppe DH, Gishti O, Manniesing R, Abrahamse‐Berkeveld M, van der Beek EM, Hofman A, Duijts L, Gaillard R and Jaddoe VW, 2014. General and abdominal fat outcomes in school‐age children associated with infant breastfeeding patterns. American Journal of Clinical Nutrition, 99, 1351–1358. [DOI] [PubMed] [Google Scholar]
- Duval S and Tweedie R, 2000. Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Outcome of a public consultation on a draft protocol for a systematic review on health outcomes related to the age of introduction of complementary food for the scientific assessment of the appropriate age of introduction of complementary feeding into an infant's diet EFSA Supporting publication 2017:EN‐1275, 18 pp. 10.2903/sp.efsa.2017.en-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Protocol for a systematic review on health outcomes related to the age of introduction of complementary food for the scientific assessment of the appropriate age of introduction of complementary feeding into an infant's diet. EFSA Journal 2017;15(8):4969, 20 pp. 10.2903/j.efsa.2017.4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014a. Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA Journal 2014;12(11):3894, 286 pp. 10.2903/j.efsa.2014.3894 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014b. Scientific Opinion on Dietary Reference Values for zinc. EFSA Journal 2014;12(10):3844, 76 pp. 10.2903/j.efsa.2014.3844 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015a. Scientific Opinion on Dietary Reference Values for iron. EFSA Journal 2015;13(10):4254, 120 pp. 10.2903/j.efsa.2015.4254 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015b. Scientific Opinion on Dietary Reference Values for vitamin A. EFSA Journal 2015;13(3):4028, 84 pp. 10.2903/j.efsa.2015.4028 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015c. Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA Journal 2015;13(7):4150, 64 pp. 10.2903/j.efsa.2015.4150 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2017. Scientific Opinion on the Dietary Reference Values for Vitamin K. EFSA Journal 2017;15(5):4780, 78 pp. 10.2903/j.efsa.2017.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2018. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health (Revision 1). EFSA Journal 2018;16(1):5136, 21 pp. 10.2903/j.efsa.2018.5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017. Guidance on the risk assessment of substances present infood intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M and Minder C, 1997. Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov KT, Christensen LB, Ritz C, Jensen SM, Molgaard C and Michaelsen KF, 2015. The impact of early growth patterns and infant feeding on body composition at 3 years of age. British Journal of Nutrition, 114, 316–327. [DOI] [PubMed] [Google Scholar]
- Elbert NJ, Kiefte‐de Jong JC, Voortman T, Nijsten TEC, de Jong NW, Jaddoe VWV, de Jongste JC, Gerth van Wijk R, Duijts L and Pasmans S, 2017. Allergenic food introduction and risk of childhood atopic diseases. PLoS ONE, 12, e0187999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JA, Ponsonby AL, Pezic A, Chavez RA, Allen RC, Akikusa JD and Munro JE, 2012. CLARITY ‐ ChiLdhood Arthritis Risk factor Identification sTudY. Pediatric Rheumatology Online Journal, 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embleton ND and Fewtrell M, 2017. Complementary feeding in preterm infants. Lancet Global Health, 5, e470–e471. [DOI] [PubMed] [Google Scholar]
- Emmett PM, Hays NP and Taylor CM, 2018. Antecedents of picky eating behaviour in young children. Appetite, 130, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English LK, Obbagy JE, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS and Stoody EE, 2019a. Complementary feeding and developmental milestones: a systematic review. American Journal of Clinical Nutrition, 109 (Suppl 1), 879s–889s. [DOI] [PubMed] [Google Scholar]
- English LK, Obbagy JE, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS and Stoody EE, 2019b. Timing of introduction of complementary foods and beverages and growth, size, and body composition: a systematic review. American Journal of Clinical Nutrition, 109 (Suppl 1), 935s–955s. [DOI] [PubMed] [Google Scholar]
- Eriksen K, Johnson W, Sonko B, Prentice A, Darboe M and Moore S, 2017. Following the World Health Organization's recommendation of exclusive breastfeeding to 6 months of age does not impact the growth of rural Gambian infants. Journal of Nutrition, 147, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EURODIAB Substudy 2 Study Group , 2002. Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care, 25, 1755–1760. [DOI] [PubMed] [Google Scholar]
- Fairley L, Santorelli G, Lawlor DA, Bryant M, Bhopal R, Petherick ES, Sahota P, Greenwood DC, Hill AJ, Cameron N, Ball H, Barber S and Wright J, 2015. The relationship between early life modifiable risk factors for childhood obesity, ethnicity and body mass index at age 3 years: findings from the Born in Bradford birth cohort study. BMC Obesity, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Beautrais AL, Shannon FT and Taylor B, 1981. Eczema and infant diet. Clinical Allergy, 11, 325–331. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ and Shannon FT, 1983. Asthma and infant diet. Archives of Disease in Childhood, 58, 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ and Shannon FT, 1990. Early solid feeding and recurrent childhood eczema: a 10‐year longitudinal study. Pediatrics, 86, 541–546. [PubMed] [Google Scholar]
- Fewtrell M, Bronsky J, Campoy C, Domellof M, Embleton N, Fidler Mis N, Hojsak I, Hulst JM, Indrio F, Lapillonne A and Molgaard C, 2017. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 64, 119–132. [DOI] [PubMed] [Google Scholar]
- Filipiak B, Zutavern A, Koletzko S, von Berg A, Brockow I, Grubl A, Berdel D, Reinhardt D, Bauer CP, Wichmann HE, Heinrich J and the GINI Study Group , 2007. Solid food introduction in relation to eczema: results from a four‐year prospective birth cohort study. Journal of Pediatrics, 151, 352–358. [DOI] [PubMed] [Google Scholar]
- Flores G and Lin H, 2013a. Factors predicting severe childhood obesity in kindergarteners. International Journal of Obesity, 37, 31–39. [DOI] [PubMed] [Google Scholar]
- Flores G and Lin H, 2013b. Factors predicting overweight in US kindergartners. American Journal of Clinical Nutrition, 97, 1178–1187. [DOI] [PubMed] [Google Scholar]
- Forster J, Dungs M, Wais U and Urbanek R, 1990. [Atopy‐suggesting symptoms in the first 2 years of life. Effect of gestational age, nutrition and social class]. Klinische Pädiatrie, 202, 136–140. [DOI] [PubMed] [Google Scholar]
- Forsyth JS, Ogston SA, Clark A, Florey CD and Howie PW, 1993. Relation between early introduction of solid food to infants and their weight and illnesses during the first two years of life. BMJ, 306, 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, Moses N, Fasano M, Goldberg T and Lifshitz F, 1990. Breast and soy‐formula feedings in early infancy and the prevalence of autoimmune thyroid disease in children. Journal of the American College of Nutrition, 9, 164–167. [DOI] [PubMed] [Google Scholar]
- Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, Rewers M and Norris JM, 2013. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatrics, 167, 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen BN, Seifert J, Kroehl M, Lamb MM, Milne GL, Rewers M and Norris JM, 2015. Timing of solid food introduction is associated with urinary F2‐isoprostane concentrations in childhood. Pediatric Research, 78, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JG, 1967. Relationship of morbidity to the feeding patterns of Navajo children from birth through twenty‐four months. American Journal of Clinical Nutrition, 20, 375–385. [DOI] [PubMed] [Google Scholar]
- Gabet S, Just J, Couderc R, Seta N and Momas I, 2016. Allergic sensitisation in early childhood: Patterns and related factors in PARIS birth cohort. International Journal of Hygiene and Environmental Health, 219, 792–800. [DOI] [PubMed] [Google Scholar]
- Gaffney KF, Kitsantas P and Cheema J, 2012. Clinical practice guidelines for feeding behaviors and weight‐for‐age at 12 months: a secondary analysis of the Infant Feeding Practices Study II. Worldviews on Evidence‐Based Nursing, 9, 234–242. [DOI] [PubMed] [Google Scholar]
- Garden FL, Marks GB, Simpson JM and Webb KL, 2012. Body mass index (BMI) trajectories from birth to 11.5 years: relation to early life food intake. Nutrients, 4, 1382–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, Shao J and Lozoff B, 2015. Impact of fetal‐neonatal iron deficiency on recognition memory at 2 months of age. Journal of Pediatrics, 167, 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs BG and Forste R, 2014. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatric Obesity, 9, 135–146. [DOI] [PubMed] [Google Scholar]
- Gidrewicz DA and Fenton TR, 2014. A systematic review and meta‐analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics, 14, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Rifas‐Shiman SL, Camargo CA Jr, Berkey CS, Frazier AL, Rockett HR, Field AE and Colditz GA, 2001. Risk of overweight among adolescents who were breastfed as infants. JAMA, 285, 2461–2467. [DOI] [PubMed] [Google Scholar]
- Gishti O, Gaillard R, Durmus B, Hofman A, Duijts L, Franco OH and Jaddoe VW, 2014. Infant diet and metabolic outcomes in school‐age children. The Generation R Study. European Journal of Clinical Nutrition, 68, 1008–1015. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Duijts L, Franco OH, Hofman A, Ikram MK and Gaillard R, 2016. Influence of breastfeeding on retinal vessel calibers in school‐age children. The Generation R Study. European Journal of Clinical Nutrition, 70, 72–77. [DOI] [PubMed] [Google Scholar]
- Gomes S, Espanca R, Gato A and Miranda C, 2010. [Obesity in preschool age ‐ too early to be too heavy!]. Acta Medica Portuguesa, 23, 371–378. [PubMed] [Google Scholar]
- Gooze RA, Anderson SE and Whitaker RC, 2011. Prolonged bottle use and obesity at 5.5 years of age in US children. Journal of Pediatrics, 159, 431–436. [DOI] [PubMed] [Google Scholar]
- Greco L, Auricchio S, Mayer M and Grimaldi M, 1988. Case control study on nutritional risk factors in celiac disease. Journal of Pediatric Gastroenterology and Nutrition, 7, 395–399. [DOI] [PubMed] [Google Scholar]
- Greenhawt M, Fleischer DM, Chan ES, Venter C, Stukus D, Gupta R and Spergel JM, 2017. LEAPing through the looking glass: secondary analysis of the effect of skin test size and age of introduction on peanut tolerance after early peanut introduction. Allergy, 72, 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer FR, Sicherer SH and Burks AW, 2019. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics, 143, pii: e20190281. [DOI] [PubMed] [Google Scholar]
- Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ and Dezateux C, 2009. Effects of infant feeding practice on weight gain from birth to 3 years. Archives of Disease in Childhood, 94, 577–582. [DOI] [PubMed] [Google Scholar]
- Griffiths LJ, Hawkins SS, Cole TJ, Dezateux C and the Millennium Cohort Study Child Health Group , 2010. Risk factors for rapid weight gain in preschool children: findings from a UK‐wide prospective study. International Journal of Obesity, 34, 624–632. [DOI] [PubMed] [Google Scholar]
- Grimm KA, Kim SA, Yaroch AL and Scanlon KS, 2014. Fruit and vegetable intake during infancy and early childhood. Pediatrics, 134 (Suppl 1), S63–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw KE, Maskell J, Oliver EM, Morris RC, Foote KD, Mills EN, Roberts G and Margetts BM, 2013. Introduction of complementary foods and the relationship to food allergy. Pediatrics, 132, e1529–e1538. [DOI] [PubMed] [Google Scholar]
- Grote V, Schiess SA, Closa‐Monasterolo R, Escribano J, Giovannini M, Scaglioni S, Stolarczyk A, Gruszfeld D, Hoyos J, Poncelet P, Xhonneux A, Langhendries JP, Koletzko B and the European Childhood Obesity Trial Study Group , 2011. The introduction of solid food and growth in the first 2 y of life in formula fed children: analysis of data from a European cohort study. American Journal of Clinical Nutrition, 94, 1785S–1793S. [DOI] [PubMed] [Google Scholar]
- Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, Moore L, Singh G, Hoy WE and Black MJ, 2014. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. American Journal of Physiology‐Renal Physiology, 307, F149–F158. [DOI] [PubMed] [Google Scholar]
- Gungor DE, Paul IM, Birch LL and Bartok CJ, 2010. Risky vs rapid growth in infancy: refining pediatric screening for childhood overweight. Archives of Pediatrics & Adolescent Medicine, 164, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal R, Aggarwal KC, Chellani H, Duggal A, Arya S, Bhatia S, Sankar MJ, Sreenivas V, Jain V, Gupta AK, Deorari AK, Paul VK and the investigators of the CF trial , 2017. Complementary feeding at 4 versus 6 months of age for preterm infants born at less than 34 weeks of gestation: a randomised, open‐label, multicentre trial. Lancet Global Health, 5, e501–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert IC, de Vries LS, Helders PJM and Jongmans MJ, 2006. Early gross motor development of preterm infants according to the Alberta Infant Motor Scale. Journal of Pediatrics, 149, 617–622. [DOI] [PubMed] [Google Scholar]
- Haileamlak A, Dagoye D, Williams H, Venn AJ, Hubbard R, Britton J and Lewis SA, 2005. Early life risk factors for atopic dermatitis in Ethiopian children. Journal of Allergy and Clinical Immunology, 115, 370–376. [DOI] [PubMed] [Google Scholar]
- Hakola L, Takkinen HM, Niinistö S, Ahonen S, Nevalainen J, Veijola R, Ilonen J, Toppari J, Knip M and Virtanen SM, 2018. Infant feeding in relation to the risk of advanced islet autoimmunity and type 1 diabetes in children with increased genetic susceptibility: a cohort study. American Journal of Epidemiology, 187, 34–44. [DOI] [PubMed] [Google Scholar]
- Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S and Reisch JS, 1973. Development of childhood allergy in infants fed breast, soy, or cow milk. Journal of Allergy and Clinical Immunology, 51, 139–151. [DOI] [PubMed] [Google Scholar]
- Harrison D, Harker H, Heese HD, Mann MD and Berelowitz J, 2001. Errors in anthropometric measurements in neonates and infants. Curationis, 24, 23–27. [DOI] [PubMed] [Google Scholar]
- Haschke F and van't Hof MA, 2000. Euro‐Growth references for breastfed boys and girls: influence of breastfeeding and solids on growth until 36 months of age. Euro‐Growth Study Group. Journal of Pediatric Gastroenterology and Nutrition, 31 Suppl 1, S60–S71. [DOI] [PubMed] [Google Scholar]
- Hatakka K, Piirainen L, Pohjavuori S, Poussa T, Savilahti E and Korpela R, 2008. Allergy in day care children: prevalence and environmental risk factors. Acta Paediatrica, 98, 817–822. [DOI] [PubMed] [Google Scholar]
- Hawkins SS, Cole TJ, Law C and the Millennium Cohort Study Child Health Group , 2009. An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. Journal of Epidemiology and Community Health, 63, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg A, Carlberg EB, Forssberg H and Hadders‐Algra M, 2005. Development of postural adjustments in sitting position during the first half year of life. Developmental Medicine and Child Neurology, 47, 312–320. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Overpeck MD, Kuczmarski RJ and Ruan WJ, 2001. Association between infant breastfeeding and overweight in young children. JAMA, 285, 2453–2460. [DOI] [PubMed] [Google Scholar]
- Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B and Dewey KG, 1993. Intake and growth of breastfed and formula fed infants in relation to the timing of introduction of complementary foods: the DARLING study. Davis Area Research on Lactation. Infant Nutrition and Growth. Acta Paediatrica, 82, 999–1006. [DOI] [PubMed] [Google Scholar]
- Hesselmar B, Saalman R, Rudin A, Adlerberth I and Wold A, 2010. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatrica, 99, 1861–1867. [DOI] [PubMed] [Google Scholar]
- Hetzner NM, Razza RA, Malone LM and Brooks‐Gunn J, 2009. Associations among feeding behaviors during infancy and child illness at two years. Maternal and Child Health Journal, 13, 795–805. [DOI] [PubMed] [Google Scholar]
- Hide DW and Guyer BM, 1981. Clinical manifestations of allergy related to breast and cows’ milk feeding. Archives of Disease in Childhood, 56, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J and Green S, 2011. Cochrane handbook for systematic reviews of interventions ‐ Version 5.1.0. The Cochrane Collaboration, Available online: http://www.cochrane-handbook.org
- Hodgson SV, 1978. Early infant feeding and weight gain. Journal of the Royal College of General Practitioners, 28, 280–281. [PMC free article] [PubMed] [Google Scholar]
- Hollis JL, Crozier SR, Inskip HM, Cooper C, Godfrey KM, Robinson SM and the Southampton Women's Survey Study Group , 2016. Age at introduction of solid foods and feeding difficulties in childhood: findings from the Southampton Women's Survey. British Journal of Nutrition, 116, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Chang JY, Shin S and Oh S, 2017. Breastfeeding and red meat intake are associated with iron status in healthy Korean weaning‐age infants. Journal of Korean Medical Science, 32, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua MC, Yao TC, Chen CC, Tsai MH, Liao SL, Lai SH, Chiu CY, Su KW, Yeh KW and Huang JL, 2017. Introduction of various allergenic foods during infancy reduces risk of IgE sensitization at 12 months of age: a birth cohort study. Pediatric Research, 82, 733–740. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang FY, Hang JQ, Zhu J, Wang R, Chen PF and Gu WL, 2013. [Cohort study of 684 pairs of mother‐and‐child allergic diseases]. Zhonghua Er Ke Za Zhi, 51, 168–171. [PubMed] [Google Scholar]
- Huh SY, Rifas‐Shiman SL, Taveras EM, Oken E and Gillman MW, 2011. Timing of solid food introduction and risk of obesity in preschool‐aged children. Pediatrics, 127, e544–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel S, Hummel M, Banholzer J, Hanak D, Mollenhauer U, Bonifacio E and Ziegler AG, 2007. Development of autoimmunity to transglutaminase C in children of patients with type 1 diabetes: relationship to islet autoantibodies and infant feeding. Diabetologia, 50, 390–394. [DOI] [PubMed] [Google Scholar]
- Husby S, Koletzko S, Korponay‐Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Mäki M, Ribes‐Koninckx C, Ventura A and Zimmer KP, 2012. ESPGHAN Working Group on Coeliac Disease; ESPGHAN Gastroenterology Committee, European Society for Paediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. Journal of Pediatric Gastroenterology and Nutrition, 54, 136–160. [DOI] [PubMed] [Google Scholar]
- Iguacel I, Chung A, Gearon E, Moreno LA, Peeters A and Backholer K, 2018. Influence of early‐life risk factors on socioeconomic inequalities in weight gain. Journal of Public Health, 40, e447–e445. [DOI] [PubMed] [Google Scholar]
- Illi S, von Mutius E, Lau S, Nickel R, Gruber C, Niggemann B, Wahn U and the Multicenter Allergy Study Group , 2004. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy and Clinical Immunology, 113, 925–931. [DOI] [PubMed] [Google Scholar]
- Imai CM, Gunnarsdottir I, Thorisdottir B, Halldorsson TI and Thorsdottir I, 2014. Associations between infant feeding practice prior to six months and body mass index at six years of age. Nutrients, 6, 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP and Borm GF, 2014. The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Medical Research Methodology, 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA and Evangelou E, 2007. Uncertainty in heterogeneity estimates in meta‐analyses. BMJ, 335, 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson A, Hernell O, Stenlund H and Persson LA, 2002. Breast‐feeding protects against celiac disease. American Journal of Clinical Nutrition, 75, 914–921. [DOI] [PubMed] [Google Scholar]
- Jackson D, Law M, Rucker G and Schwarzer G, 2017. The Hartung‐Knapp modification for random‐effects meta‐analysis: A useful refinement but are there any residual concerns? Statistics in Medicine, 36, 3923–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MA, Tromp II, Kiefte‐de Jong JC, Jaddoe VW, Hofman A, Escher JC, Hooijkaas H and Moll HA, 2014. Infant feeding and anti‐tissue transglutaminase antibody concentrations in the Generation R Study. American Journal of Clinical Nutrition, 100, 1095–1101. [DOI] [PubMed] [Google Scholar]
- Jiang J, Rosenqvist U, Huishan W, Koletzko B, Guangli L, Jing H and Greiner T, 2009. Relationship of parental characteristics and feeding practices to overweight in infants and young children in Beijing, China. Public Health Nutrition, 12, 973–978. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Cruz A, Bacardi‐Gascon M, Pichardo‐Osuna A, Mandujano‐Trujillo Z and Castillo‐Ruiz O, 2010. Infant and toddlers’ feeding practices and obesity amongst low‐income families in Mexico. Asia Pacific Journal of Clinical Nutrition, 19, 316–323. [PubMed] [Google Scholar]
- Jones L, Moschonis G, Oliveira A, de Lauzon‐Guillain B, Manios Y, Xepapadaki P, Lopes C, Moreira P, Charles MA and Emmett P, 2015. The influence of early feeding practices on healthy diet variety score among pre‐school children in four European birth cohorts. Public Health Nutrition, 18, 1774–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir OH, Thorsdottir I, Hibberd PL, Fewtrell MS, Wells JC, Palsson GI, Lucas A, Gunnlaugsson G and Kleinman RE, 2012. Timing of the introduction of complementary foods in infancy: a randomized controlled trial. Pediatrics, 130, 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir OH, Thorsdottir I, Gunnlaugsson G, Fewtrell MS, Hibberd PL and Kleinman RE, 2013. Exclusive breastfeeding and developmental and behavioral status in early childhood. Nutrients, 5, 4414–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir OH, Kleinman RE, Wells JC, Fewtrell MS, Hibberd PL, Gunnlaugsson G and Thorsdottir I, 2014. Exclusive breastfeeding for 4 versus 6 months and growth in early childhood. Acta Paediatrica, 103, 105–111. [DOI] [PubMed] [Google Scholar]
- Jonsson K, Barman M, Brekke HK, Hesselmar B, Johansen S, Sandberg AS and Wold AE, 2017. Late introduction of fish and eggs is associated with increased risk of allergy development ‐ results from the FARMFLORA birth cohort. Food & Nutrition Research, 61, 1393306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppich R, Kollmann D, Ingrisch U and Weber P, 1977. Urinary cyclic AMP and renal concentrating capacity in infants. European Journal of Pediatrics, 124, 113–119. [DOI] [PubMed] [Google Scholar]
- Joseph CL, Ownby DR, Havstad SL, Woodcroft KJ, Wegienka G, MacKechnie H, Zoratti E, Peterson EL and Johnson CC, 2011. Early complementary feeding and risk of food sensitization in a birth cohort. Journal of Allergy and Clinical Immunology, 127, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajosaari M, 1991. Atopy prophylaxis in high‐risk infants. Prospective 5‐year follow‐up study of children with six months exclusive breastfeeding and solid food elimination. Advances in Experimental Medicine and Biology, 310, 453–458. [PubMed] [Google Scholar]
- Kajosaari M, 1994. Atopy prevention in childhood: the role of diet. Prospective 5‐year follow‐up of high‐risk infants with six months exclusive breastfeeding and solid food elimination. Pediatric Allergy and Immunology, 5, 26–28. [DOI] [PubMed] [Google Scholar]
- Kajosaari M and Saarinen UM, 1983. Prophylaxis of atopic disease by six months’ total solid food elimination. Evaluation of 135 exclusively breastfed infants of atopic families. Acta Paediatrica Scandinavica, 72, 411–414. [DOI] [PubMed] [Google Scholar]
- Kalanda BF, Verhoeff FH and Brabin BJ, 2006. Breast and complementary feeding practices in relation to morbidity and growth in Malawian infants. European Journal of Clinical Nutrition, 60, 401–407. [DOI] [PubMed] [Google Scholar]
- Kalies H, Heinrich J, Borte N, Schaaf B, von Berg A, von Kries R, Wichmann HE, Bolte G and Group LS, 2005. The effect of breastfeeding on weight gain in infants: results of a birth cohort study. European Journal of Medical Research, 10, 36–42. [PubMed] [Google Scholar]
- Karunasekera KA, Jayasinghe JA and Alwis LW, 2001. Risk factors of childhood asthma: a Sri Lankan study. Journal of Tropical Pediatrics, 47, 142–145. [DOI] [PubMed] [Google Scholar]
- Kattelmann KK, Ho M and Specker BL, 2001. Effect of timing of introduction of complementary foods on iron and zinc status of formula fed infants at 12, 24, and 36 months of age. Journal of the American Dietetic Association, 101, 443–447. [DOI] [PubMed] [Google Scholar]
- Keijzers G, Sweeny A, Crilly J, Good N, Cameron CM, Mihala G, Scott R and Scuffham PA, 2018. Parental‐reported allergic disorders and emergency department presentations for allergy in the first five years of life; a longitudinal birth cohort. BMC Pediatrics, 18, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MU, 1984. Breastfeeding, growth and diarrhoea in rural Bangladesh children. Human Nutrition. Clinical Nutrition, 38, 113–119. [PubMed] [Google Scholar]
- Kiefte‐de Jong JC, de Vries JH, Franco OH, Jaddoe VW, Hofman A, Raat H, de Jongste JC and Moll HA, 2012. Fish consumption in infancy and asthma‐like symptoms at preschool age. Pediatrics, 130, 1060–1068. [DOI] [PubMed] [Google Scholar]
- Kim J and Peterson KE, 2008. Association of infant child care with infant feeding practices and weight gain among US infants. Archives of Pediatrics & Adolescent Medicine, 162, 627–633. [DOI] [PubMed] [Google Scholar]
- Kim J, Chang E, Han Y, Ahn K and Lee SI, 2011. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatric Allergy and Immunology, 22, 715–719. [DOI] [PubMed] [Google Scholar]
- Kindgren E, Fredrikson M and Ludvigsson J, 2017. Early feeding and risk of juvenile idiopathic arthritis: a case control study in a prospective birth cohort. Pediatric Rheumatology Online Journal, 15, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klag EA, McNamara K, Geraghty SR and Keim SA, 2015. Associations between breast milk feeding, introduction of solid foods, and weight gain in the first 12 months of life. Clinical Pediatrics, 54, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G and Hartung J, 2003. Improved tests for a random effects meta‐regression with a single covariate. Statistics in Medicine, 22, 2693–2710. [DOI] [PubMed] [Google Scholar]
- Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, Tey D, Slaa M, Thiele L, Miles L, Anderson D, Tan T, Dang TD, Hill DJ, Lowe AJ, Matheson MC, Ponsonby AL, Tang ML, Dharmage SC and Allen KJ, 2010. Can early introduction of egg prevent egg allergy in infants? A population‐based study. Journal of Allergy and Clinical Immunology, 126, 807–813. [DOI] [PubMed] [Google Scholar]
- Koplin JJ, Dharmage SC, Ponsonby AL, Tang ML, Lowe AJ, Gurrin LC, Osborne NJ, Martin PE, Robinson MN, Wake M, Hill DJ, Allen KJ and the HealthNuts Investigators , 2012. Environmental and demographic risk factors for egg allergy in a population‐based study of infants. Allergy, 67, 1415–1422. [DOI] [PubMed] [Google Scholar]
- Kostraba JN, Cruickshanks KJ, Lawler‐Heavner J, Jobim LF, Rewers MJ, Gay EC, Chase HP, Klingensmith G and Hamman RF, 1993. Early exposure to cow's milk and solid foods in infancy, genetic predisposition, and risk of IDDM. Diabetes, 42, 288–295. [PubMed] [Google Scholar]
- Kramer MS, 1981. Do breastfeeding and delayed introduction of solid foods protect against subsequent obesity? Journal of Pediatrics, 98, 883–887. [DOI] [PubMed] [Google Scholar]
- Kramer MS and Kakuma R, 2012. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews, CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS and Moroz B, 1981. Do breastfeeding and delayed introduction of solid foods protect against subsequent atopic eczema? Journal of Pediatrics, 98, 546–550. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Barr RG, Leduc DG, Boisjoly C and Pless IB, 1985a. Infant determinants of childhood weight and adiposity. Journal of Pediatrics, 107, 104–107. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Barr RG, Leduc DG, Boisjoly C, McVey‐White L and Pless IB, 1985b. Determinants of weight and adiposity in the first year of life. Journal of Pediatrics, 106, 10–14. [DOI] [PubMed] [Google Scholar]
- Kucukosmanoglu E, Yazi D, Yesil O, Akkoc T, Gezer M, Bakirci N, Bahceciler NN and Barlan IB, 2008. Prevalence of egg sensitization in Turkish infants based on skin prick test. Allergologia et Immunopathologia, 36, 141–144. [PubMed] [Google Scholar]
- Kumar R, Caruso DM, Arguelles L, Kim JS, Schroeder A, Rowland B, Meyer KE, Schwarz KE, Birne JS, Ouyang F, Pongracic JA and Wang X, 2010. Early life eczema, food introduction, and risk of food allergy in children. Pediatric Allergy, Immunology, and Pulmonology, 23, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Pruthi PK, Mehra R and Sehgal H, 1985. Breast feeding: physical growth during infancy. Indian Journal of Pediatrics, 52, 73–77. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy RJ, Matthews S and Arshad SH, 2004. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics, 113, 345–350. [DOI] [PubMed] [Google Scholar]
- Lande B, Andersen LF, Henriksen T, Baerug A, Johansson L, Trygg KU, Bjorneboe GE and Veierod MB, 2005. Relations between high ponderal index at birth, feeding practices and body mass index in infancy. European Journal of Clinical Nutrition, 59, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Lange C, Visalli M, Jacob S, Chabanet C, Schlich P and Nicklaus S, 2013. Maternal feeding practices during the first year and their impact on infants’, acceptance of complementary food. Food Quality and Preference, 29, 89–98. [Google Scholar]
- Larsson M, Hagerhed‐Engman L, Sigsgaard T, Janson S, Sundell J and Bornehag CG, 2008. Incidence rates of asthma, rhinitis and eczema symptoms and influential factors in young children in Sweden. Acta Paediatrica, 97, 1210–1215. [DOI] [PubMed] [Google Scholar]
- Lawson K, Bahnson HT, Brittain E, Sever M, Du Toit G, Lack G, Keet C, Greenhawt M, Fleischer D, Chan ES, Venter C, Stukus D, Gupta R and Spergel J, 2017. Letter of response to Greenhawt et al. ‘LEAPing through the looking glass: secondary analysis of the effect of skin test size and age of introduction on peanut tolerance after early peanut introduction’. Allergy, 72, 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layte R, Bennett A, McCrory C and Kearney J, 2014. Social class variation in the predictors of rapid growth in infancy and obesity at age 3 years. International Journal of Obesity, 38, 82–90. [DOI] [PubMed] [Google Scholar]
- Leary SD, Lawlor DA, Davey Smith G, Brion MJ and Ness AR, 2015. Behavioural early‐life exposures and body composition at age 15 years. Nutrition & Diabetes, 5, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM and Galloway JC, 2012. Early intensive postural and movement training advances head control in very young infants. Physical Therapy, 92, 935–947. [DOI] [PubMed] [Google Scholar]
- Lee KS, Rha YH, Oh IH, Choi YS, Kim YE and Choi SH, 2017. Does breastfeeding relate to development of atopic dermatitis in young Korean children?: Based on the fourth and fifth Korea National Health and Nutrition Examination Survey 2007‐2012. Allergy, Asthma & Immunology Research, 9, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libuda L, Hilbig A, Berber‐Al‐Tawil S, Kalhoff H and Kersting M, 2016. Association between full breastfeeding, timing of complementary food introduction, and iron status in infancy in Germany: results of a secondary analysis of a randomized trial. European Journal of Nutrition, 57, 523–531. [DOI] [PubMed] [Google Scholar]
- Liese AD, Puett RC, Lamichhane AP, Nichols MD, Dabelea D, Lawson AB, Porter DE, Hibbert JD, D'Agostino RB Jr and Mayer‐Davis EJ, 2012. Neighborhood level risk factors for type 1 diabetes in youth: the SEARCH case‐control study. International Journal of Health Geographics, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima‐Alvarez CD, Tudella E, van der Kamp J and Savelsbergh GJ, 2014. Early development of head movements between birth and 4 months of age: a longitudinal study. Journal of Motor Behavior, 46, 415–422. [DOI] [PubMed] [Google Scholar]
- Lin SL, Leung GM, Lam TH and Schooling CM, 2013. Timing of solid food introduction and obesity: Hong Kong's “children of 1997” birth cohort. Pediatrics, 131, e1459–e1467. [DOI] [PubMed] [Google Scholar]
- Lopez‐Alarcon M, Villalpando S and Fajardo A, 1997. Breastfeeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. Journal of Nutrition, 127, 436–443. [DOI] [PubMed] [Google Scholar]
- Lossius AK, Magnus MC, Lunde J and Stordal K, 2018. Prospective cohort study of breastfeeding and the risk of childhood asthma. Journal of Pediatrics, 195, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccioli S, Zhang Y, Verrill L, Ramos‐Valle M and Kwegyir‐Afful E, 2014. Infant feeding practices and reported food allergies at 6 years of age. Pediatrics, 134 (Suppl 1), S21–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund‐Blix NA, Stene LC, Rasmussen T, Torjesen PA, Andersen LF and Ronningen KS, 2015. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA Study. Diabetes Care, 38, 257–263. [DOI] [PubMed] [Google Scholar]
- Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather‐Tait S, Hurrell RF, McArdle HJ and Raiten DJ, 2018. Biomarkers of nutrition for development (BOND)‐iron review. Journal of Nutrition, 148, 1001S–1067S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes TC, Vieira SA, Priore SE, Ribeiro AQ, Lamounier JA, Franceschini SC and Sant'Ana LF, 2012. Exclusive breastfeeding and other foods in the first six months of life: effects on nutritional status and body composition of Brazilian children. ScientificWorldJournal, 2012, 468581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä J, Vaarno J, Kaljonen A, Niinikoski H and Lagström H, 2014. Maternal overweight impacts infant feeding patterns–the STEPS Study. European Journal of Clinical Nutrition, 68, 43–49. [DOI] [PubMed] [Google Scholar]
- Marini A, Agosti M, Motta G and Mosca F, 1996. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow‐up. Acta Paediatrica. Supplement, 414, 1–21. [DOI] [PubMed] [Google Scholar]
- Martin RM, Ness AR, Gunnell D, Emmett P, Davey Smith G and the ALSPAC Study Team , 2004. Does breastfeeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation, 109, 1259–1266. [DOI] [PubMed] [Google Scholar]
- Massion S, Wickham S, Pearce A, Barr B, Law C and Taylor‐Robinson D, 2016. Exploring the impact of early life factors on inequalities in risk of overweight in UK children: findings from the UK Millennium Cohort Study. Archives of Disease in Childhood, 101, 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, O'Connor G, Kattan M, Gern J and Wood RA, 2015. Influence of early‐life exposures on food sensitization and food allergy in an inner‐city birth cohort. Journal of Allergy and Clinical Immunology, 135, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KC, Specker BL, Bartholmey S, Giddens J and Ho ML, 1998. Trial on timing of introduction to solids and food type on infant growth. Pediatrics, 102, 569–573. [DOI] [PubMed] [Google Scholar]
- Meinzen‐Derr JK, Guerrero ML, Altaye M, Ortega‐Gallegos H, Ruiz‐Palacios GM and Morrow AL, 2006. Risk of infant anemia is associated with exclusive breastfeeding and maternal anemia in a Mexican cohort. Journal of Nutrition, 136, 452–458. [DOI] [PubMed] [Google Scholar]
- Meloni T, Marinaro AM, Mannazzu MC, Ogana A, La Vecchia C, Negri E and Colombo C, 1997. IDDM and early infant feeding. Sardinian Case‐Control Study. Diabetes Care, 20, 340–342. [DOI] [PubMed] [Google Scholar]
- Metwally AM, Salah El‐Din EM, Shehata MA, Shaalan A, El Etreby LA, Kandeel WA, Shaaban SY and Rabah TM, 2016. Early life predictors of socio‐emotional development in a sample of Egyptian infants. PLoS ONE, 11, e0158086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels KA, Ghassabian A, Mumford SL, Sundaram R, Bell EM, Bello SC and Yeung EH, 2017. Breastfeeding and motor development in term and preterm infants in a longitudinal US cohort. American Journal of Clinical Nutrition, 106, 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihrshahi S, Ampon R, Webb K, Almqvist C, Kemp AS, Hector D, Marks GB and the CAPS Team , 2007. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clinical and Experimental Allergy, 37, 671–679. [DOI] [PubMed] [Google Scholar]
- Mihrshahi S, Battistutta D, Magarey A and Daniels LA, 2011. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatrics, 11, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K and Ueda A, 2003. The maturation and coordination of sucking, swallowing, and respiration in preterm infants. Journal of Pediatrics, 142, 36–40. [DOI] [PubMed] [Google Scholar]
- Möller LM, de Hoog ML, van Eijsden M, Gemke RJ and Vrijkotte TG, 2013. Infant nutrition in relation to eating behaviour and fruit and vegetable intake at age 5 years. British Journal of Nutrition, 109, 564–571. [DOI] [PubMed] [Google Scholar]
- Moore WJ, Midwinter RE, Morris AF, Colley JR and Soothill JF, 1985. Infant feeding and subsequent risk of atopic eczema. Archives of Disease in Childhood, 60, 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JB, Lucas A and Fewtrell MS, 2004. Does weaning influence growth and health up to 18 months? Archives of Disease in Childhood, 89, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE and Klein MD, 2000. Pre‐feeding skills. A comprehensive resource for mealtime development. Second Edition, Therapy Skill Builders, USA: 798 pp. [Google Scholar]
- Moschonis G, de Lauzon‐Guillain B, Jones L, Oliveira A, Lambrinou CP, Damianidi L, Lioret S, Moreira P, Lopes C, Emmett P, Charles MA and Manios Y, 2017. The effect of early feeding practices on growth indices and obesity at preschool children from four European countries and UK schoolchildren and adolescents. European Journal of Pediatrics, 176, 1181–1192. [DOI] [PubMed] [Google Scholar]
- Moss BG and Yeaton WH, 2014. Early childhood healthy and obese weight status: potentially protective benefits of breastfeeding and delaying solid foods. Maternal and Child Health Journal, 18, 1224–1232. [DOI] [PubMed] [Google Scholar]
- Myleus A, Hernell O, Gothefors L, Hammarstrom ML, Persson LA, Stenlund H and Ivarsson A, 2012. Early infections are associated with increased risk for celiac disease: an incident case‐referent study. BMC Pediatrics, 12, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento Simon VG, Pacheco de Souza JM and Buongermino de Souza S, 2009. Breastfeeding, complementary feeding, overweight and obesity in pre‐school children. Revista de Saude Publica, 43, 60–69. [DOI] [PubMed] [Google Scholar]
- Nathan AM, de Bruyne J, Khalid F and Arumugam K, 2012. Caesarean section and asthma in Malaysian children: a case‐control study. Asian Pacific Journal of Allergy & Immunology, 30, 204–208. [PubMed] [Google Scholar]
- Neutzling MB, Hallal PR, Araujo CL, Horta BL, Vieira Mde F, Menezes AM and Victora CG, 2009. Infant feeding and obesity at 11 years: prospective birth cohort study. International Journal of Pediatric Obesity, 4, 143–149. [DOI] [PubMed] [Google Scholar]
- Nevarez MD, Rifas‐Shiman SL, Kleinman KP, Gillman MW and Taveras EM, 2010. Associations of early life risk factors with infant sleep duration. Academic Pediatrics, 10, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SB, Reilly JJ, Fewtrell MS, Eaton S, Grinham J and Wells JC, 2011. Adequacy of milk intake during exclusive breastfeeding: a longitudinal study. Pediatrics, 128, e907–e914. [DOI] [PubMed] [Google Scholar]
- Nommsen LA, Lovelady CA, Heinig MJ, Lonnerdal B and Dewey KG, 1991. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. American Journal of Clinical Nutrition, 53, 457–465. [DOI] [PubMed] [Google Scholar]
- Noppornlertwong C and Tantibhaedhyangkul R, 2016. Comparison of growth, infections and feeding habits among formula fed infants starting complementary feeding at 4 to 6 months old with those starting at 6 months old. Southeast Asian Journal of Tropical Medicine & Public Health, 47, 506–513. [PubMed] [Google Scholar]
- Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA and Rewers M, 2003. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA, 290, 1713–1720. [DOI] [PubMed] [Google Scholar]
- Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS and Rewers M, 2005. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA, 293, 2343–2351. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2015. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. Office of Health Assessment and Translation (OHAT), 98 pp.
- Nwaru BI, Erkkola M, Ahonen S, Kaila M, Haapala AM, Kronberg‐Kippila C, Salmelin R, Veijola R, Ilonen J, Simell O, Knip M and Virtanen SM, 2010. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics, 125, 50–59. [DOI] [PubMed] [Google Scholar]
- Nwaru BI, Takkinen HM, Niemela O, Kaila M, Erkkola M, Ahonen S, Haapala AM, Kenward MG, Pekkanen J, Lahesmaa R, Kere J, Simell O, Veijola R, Ilonen J, Hyoty H, Knip M and Virtanen SM, 2013a. Timing of infant feeding in relation to childhood asthma and allergic diseases. Journal of Allergy and Clinical Immunology, 131, 78–86. [DOI] [PubMed] [Google Scholar]
- Nwaru BI, Craig LC, Allan K, Prabhu N, Turner SW, McNeill G, Erkkola M, Seaton A and Devereux G, 2013b. Breastfeeding and introduction of complementary foods during infancy in relation to the risk of asthma and atopic diseases up to 10 years. Clinical and Experimental Allergy, 43, 1263–1273. [DOI] [PubMed] [Google Scholar]
- Nwaru BI, Takkinen HM, Niemela O, Kaila M, Erkkola M, Ahonen S, Tuomi H, Haapala AM, Kenward MG, Pekkanen J, Lahesmaa R, Kere J, Simell O, Veijola R, Ilonen J, Hyoty H, Knip M and Virtanen SM, 2013c. Introduction of complementary foods in infancy and atopic sensitization at the age of 5 years: timing and food diversity in a Finnish birth cohort. Allergy, 68, 507–516. [DOI] [PubMed] [Google Scholar]
- Obbagy JE, English LK, Wong YP, Butte NF, Dewey KG, Fleischer DM, Fox MK, Greer FR, Krebs NF, Scanlon KS and Stoody EE, 2019a. Complementary feeding and food allergy, atopic dermatitis/eczema, asthma, and allergic rhinitis: a systematic review. American Journal of Clinical Nutrition, 109 (Suppl 1), 890s–934s. [DOI] [PubMed] [Google Scholar]
- Obbagy JE, English LK, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS and Stoody EE, 2019b. Complementary feeding and bone health: a systematic review. American Journal of Clinical Nutrition, 109 (Suppl 1), 7, 872s–878s. [DOI] [PubMed] [Google Scholar]
- Obbagy JE, English LK, Psota TL, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS and Stoody EE, 2019c. Complementary feeding and micronutrient status: a systematic review. American Journal of Clinical Nutrition, 109 (Suppl 1), 852s–871s. [DOI] [PubMed] [Google Scholar]
- Ogg HL, 1975. Oral‐pharyngeal development and evaluation. Physical Therapy, 55, 235–241. [DOI] [PubMed] [Google Scholar]
- Okubo H, Miyake Y, Sasaki S, Tanaka K and Hirota Y, 2016. Feeding practices in early life and later intake of fruit and vegetables among Japanese toddlers: the Osaka Maternal and Child Health Study. Public Health Nutrition, 19, 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas Health Research and Consultancy , 2019. Extensive literature search as preparatory work for a systematic review on health outcomes related to the age of introduction of complementary food for the scientific assessment of the appropriate age of introduction of complementary feeding into an infant's diet. EFSA Supporting publication 2019:EN‐1276, 55 pp. 10.2903/sp.efsa.2019.EN-1276 [DOI] [Google Scholar]
- Palmer DJ and Makrides M, 2012. Introducing solid foods to preterm infants in developed countries. Annals of Nutrition and Metabolism, 60 (Suppl 2), 31–38. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, Loh R and Prescott SL, 2013. Early regular egg exposure in infants with eczema: A randomized controlled trial. Journal of Allergy and Clinical Immunology, 132, 387–392. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Sullivan TR, Gold MS, Prescott SL and Makrides M, 2017. Randomized controlled trial of early regular egg intake to prevent egg allergy. Journal of Allergy and Clinical Immunology, 139, 1600–1607. [DOI] [PubMed] [Google Scholar]
- Papoutsou S, Savva SC, Hunsberger M, Jilani H, Michels N, Ahrens W, Tornaritis M, Veidebaum T, Molnar D, Siani A, Moreno LA, Hadjigeorgiou C and the IDEFICS consortium , 2018. Timing of solid food introduction and association with later childhood overweight and obesity: The IDEFICS study. Maternal & Child Nutrition, 14, 10.1111/mcn.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar H, Kumar L, Puri R and Kumar V, 1984. The incidence of allergic diseases and feeding patterns in children upto 2 years of age. Indian Journal of Pediatrics, 51, 7–12. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Typpo JT, Typpo MH and Krause GF, 1986. Factors related to obesity in preschool children. Journal of the American Dietetic Association, 86, 1376–1381. [PubMed] [Google Scholar]
- Paule RC and Mandel J, 1989. Consensus values, regressions, and weighting factors. Journal of Research of the National Institute of Standards and Technology, 94, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Bravo F, Carrasco E, Gutierrez‐Lopez MD, Martinez MT, Lopez G and de los Rios MG, 1996. Genetic predisposition and environmental factors leading to the development of insulin‐dependent diabetes mellitus in Chilean children. Journal of Molecular Medicine, 74, 105–109. [DOI] [PubMed] [Google Scholar]
- Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J, Flohr C, Lack G and the EAT Study Team , 2016. Randomized trial of introduction of allergenic foods in breastfed infants. The New England Journal of Medicine, 374, 1733–1743. [DOI] [PubMed] [Google Scholar]
- Perkin MR, Bahnson HT, Logan K, Marrs T, Radulovic S, Craven J, Flohr C and Lack G, 2018. Association of early introduction of solids with infant sleep: a secondary analysis of a randomized clinical trial. JAMA Pediatrics, 172, e180739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters U, Schneeweiss S, Trautwein EA and Erbersdobler HF, 2001. A case‐control study of the effect of infant feeding on celiac disease. Annals of Nutrition and Metabolism, 45, 135–142. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR and Rushton L, 2008. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology, 61, 991–996. [DOI] [PubMed] [Google Scholar]
- Peters RL, Allen KJ, Dharmage SC, Lodge CJ, Koplin JJ, Ponsonby AL, Wake M, Lowe AJ, Tang MLK, Matheson MC, Gurrin LC and the HealthNuts Study Team , 2015. Differential factors associated with challenge‐proven food allergy phenotypes in a population cohort of infants: a latent class analysis. Clinical and Experimental Allergy, 45, 953–963. [DOI] [PubMed] [Google Scholar]
- Pin TW, Darrer T, Eldridge B and Galea MP, 2009. Motor development from 4 to 8 months corrected age in infants born at or less than 29 weeks’ gestation. Developmental Medicine and Child Neurology, 51, 739–745. [DOI] [PubMed] [Google Scholar]
- Pluymen LPM, Wijga AH, Gehring U, Koppelman GH, Smit HA and van Rossem L, 2018. Early introduction of complementary foods and childhood overweight in breastfed and formula fed infants in the Netherlands: the PIAMA birth cohort study. European Journal of Nutrition, 57, 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacek E, Vocel J, Neugebauerova L, Sebkova M and Vechetova E, 1965. The osmotic concentrating ability in healthy infants and children. Archives of Disease in Childhood, 40, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M and Norris JM, 2006. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics, 117, 2175–2182. [DOI] [PubMed] [Google Scholar]
- Poskitt EM and Cole TJ, 1978. Nature, nurture, and childhood overweight. British Medical Journal, 1, 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöysä L, Korppi M, Remes K and Juntunen‐Backman K, 1991. Atopy in childhood and diet in infancy. A nine‐year follow‐up study. I. Clinical Manifestations. Allergy Proceedings, 12, 107–111. [DOI] [PubMed] [Google Scholar]
- Quigley MA, Kelly YJ and Sacker A, 2009. Infant feeding, solid foods and hospitalisation in the first 8 months after birth. Archives of Disease in Childhood, 94, 148–150. [DOI] [PubMed] [Google Scholar]
- Rabiei S and Reza R, 2012. The association of type 1 diabetes mellitus with nutrition style through the first 2 years of life in Iranian children. Journal of US‐China Medical Science, 9, 56–60. [Google Scholar]
- Ranucci G, Buccigrossi V, Borgia E, Piacentini D, Visentin F, Cantarutti L, Baiardi P, Felisi M, Spagnuolo MI, Zanconato S, Baraldi E, Giaquinto C and Guarino A, 2018. Galacto‐oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: a randomized clinical trial. Nutrients, 10, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S and Kanade AN, 1992. Prolonged breastfeeding and malnutrition among rural Indian children below 3 years of age. European Journal of Clinical Nutrition, 46, 187–195. [PubMed] [Google Scholar]
- Rathnayake KM, Satchithananthan A, Mahamithawa S and Jayawardena R, 2013. Early life predictors of preschool overweight and obesity: a case‐control study in Sri Lanka. BMC Public Health, 13, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redstone F and West JF, 2004. The importance of postural control for feeding. Pediatric Nursing, 30, 97–100. [PubMed] [Google Scholar]
- Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A and the ALSPAC Study Team , 2005. Early life risk factors for obesity in childhood: cohort study. British Medical Journal, 330, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios‐Castillo I, Cerezo S, Corvalan C, Martinez M and Kain J, 2015. Risk factors during the prenatal period and the first year of life associated with overweight in 7‐year‐old low‐income Chilean children. Maternal & Child Nutrition, 11, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Marriott LD, Crozier SR, Harvey NC, Gale CR, Inskip HM, Baird J, Law CM, Godfrey KM, Cooper C and the Southampton Women's Survey Study Group , 2009Variations in infant feeding practice are associated with body composition in childhood: a prospective cohort study. Journal of Clinical Endocrinology and Metabolism, 94, 2799–2805. [DOI] [PubMed] [Google Scholar]
- Roduit C, Frei R, Loss G, Buchele G, Weber J, Depner M, Loeliger S, Dalphin ML, Roponen M, Hyvärinen A, Riedler J, Dalphin JC, Pekkanen J, von Mutius E, Braun‐Fahrlander C, Lauener R and the Protection Against Allergy–Study in Rural Environments study group , 2012. Development of atopic dermatitis according to age of onset and association with early‐life exposures. Journal of Allergy and Clinical Immunology, 130, 130–136. [DOI] [PubMed] [Google Scholar]
- Rogers B and Arvedson J, 2005. Assessment of infant oral sensorimotor and swallowing function. Mental Retardation and Developmental Disabilities Research Reviews, 11, 74–82. [DOI] [PubMed] [Google Scholar]
- Rosenbauer J, Herzig P and Giani G, 2008. Early infant feeding and risk of type 1 diabetes mellitus‐a nationwide population‐based case‐control study in pre‐school children. Diabetes/Metabolism Research and Reviews, 24, 211–222. [DOI] [PubMed] [Google Scholar]
- Rossiter MD and Evers SE, 2013. Infant feeding practices and children's weight status. Canadian Journal of Dietetic Practice and Research, 74, 107–113. [DOI] [PubMed] [Google Scholar]
- Ruiz RG, Kemeny DM and Price JF, 1992. Higher risk of infantile atopic dermatitis from maternal atopy than from paternal atopy. Clinical and Experimental Allergy, 22, 762–766. [DOI] [PubMed] [Google Scholar]
- SACN (Scientific Advisory Committee on Nutrition), 2018. Feeding in the first year of life. 290 pp.
- SACN‐COT (Scientific Advisory Committee on Nutrition ‐ Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment), 2011. Joint statement: Timing of introduction of gluten to the infant diet. 18 pp.
- SACN‐COT (Scientific Advisory Committee on Nutrition ‐ Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment), 2018. Statement on the introduction of peanut and hen's egg into the infant diet. 22 pp.
- Sahakyan A, Armenian HK, Breitscheidel L, Thompson ME and Enokyan G, 2006. Feeding practices of babies and the development of atopic dermatitis in children after 12 months of age in Armenia: is there a signal? European Journal of Epidemiology, 21, 723–725. [DOI] [PubMed] [Google Scholar]
- Salahuddin M, Perez A, Ranjit N, Hoelscher DM and Kelder SH, 2017. The associations of large‐for‐gestational‐age and infant feeding practices with children's body mass index z‐score trajectories: the Early Childhood Longitudinal Study, Birth Cohort. Clinical Obesity, 7, 307–315. [DOI] [PubMed] [Google Scholar]
- Salmenpera L, Perheentupa J and Siimes MA, 1985. Exclusively breastfed healthy infants grow slower than reference infants. Pediatric Research, 19, 307–312. [DOI] [PubMed] [Google Scholar]
- Sandini U, Kukkonen AK, Poussa T, Sandini L, Savilahti E and Kuitunen M, 2011. Protective and risk factors for allergic diseases in high‐risk children at the ages of two and five years. International Archives of Allergy and Immunology, 156, 339–348. [DOI] [PubMed] [Google Scholar]
- Sandoval Jurado L, Jiménez Báez MV, Olivares Juárez S and de la Cruz Olvera T, 2016. Breastfeeding, complementary feeding and risk of childhood obesity. Atencion Primaria, 48, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariachvili M, Droste J, Dom S, Wieringa M, Hagendorens M, Stevens W, van Sprundel M, Desager K and Weyler J, 2010. Early exposure to solid foods and the development of eczema in children up to 4 years of age. Pediatric Allergy and Immunology, 21, 74–81. [DOI] [PubMed] [Google Scholar]
- Savilahti E and Saarinen KM, 2009. Early infant feeding and type 1 diabetes. European Journal of Nutrition, 48, 243–249. [DOI] [PubMed] [Google Scholar]
- Savilahti E, Tainio VM, Salmenpera L, Siimes MA and Perheentupa J, 1987. Prolonged exclusive breast feeding and heredity as determinants in infantile atopy. Archives of Disease in Childhood, 62, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savilahti EM, Ilonen J, Kukkonen AK, Savilahti E and Kuitunen M, 2018. Celiac disease by the age of 13 years is not associated with probiotics administration in infancy. Journal of Pediatric Gastroenterology and Nutrition, 66, 937–940. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2003. Report on the revision of essential requirements of infant formulae and follow‐on formulae. 213 pp.
- Schack‐Nielsen L, Sorensen T, Mortensen EL and Michaelsen KF, 2010. Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. American Journal of Clinical Nutrition, 91, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt Morgen C, Angquist L, Baker JL, Andersen AN, Sorensen TIA and Michaelsen KF, 2018. Breastfeeding and complementary feeding in relation to body mass index and overweight at ages 7 and 11 y: a path analysis within the Danish National Birth Cohort. American Journal of Clinical Nutrition, 107, 313–322. [DOI] [PubMed] [Google Scholar]
- Schoetzau A, Filipiak‐Pittroff B, Franke K, Koletzko S, Von Berg A, Gruebl A, Bauer CP, Berdel D, Reinhardt D, Wichmann HE and the German Infant Nutritional Intervention Study Group , 2002. Effect of exclusive breastfeeding and early solid food avoidance on the incidence of atopic dermatitis in high‐risk infants at 1 year of age. Pediatric Allergy and Immunology, 13, 234–242. [DOI] [PubMed] [Google Scholar]
- Seach KA, Dharmage SC, Lowe AJ and Dixon JB, 2010. Delayed introduction of solid feeding reduces child overweight and obesity at 10 years. International Journal of Obesity, 34, 1475–1479. [DOI] [PubMed] [Google Scholar]
- Sheppard JJ and Mysak ED, 1984. Ontogeny of infantile oral reflexes and emerging chewing. Child Development, 55, 831–843. [PubMed] [Google Scholar]
- Shim JE, Kim J, Mathai RA and the STRONG Kids Research Team , 2011. Associations of infant feeding practices and picky eating behaviors of preschool children. Journal of the American Dietetic Association, 111, 1363–1368. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Wood RA, Stablein D, Lindblad R, Burks AW, Liu AH, Jones SM, Fleischer DM, Leung DY and Sampson HA, 2010. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. Journal of Allergy and Clinical Immunology, 126, 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu LS, Grewal R and Bhatnagar DP, 1981. A study of physical growth in breastfed and bottle‐fed male infants. Indian Journal of Pediatrics, 48, 75–79. [DOI] [PubMed] [Google Scholar]
- Simondon KB and Simondon F, 1997. Age at introduction of complementary food and physical growth from 2 to 9 months in rural Senegal. European Journal of Clinical Nutrition, 51, 703–707. [DOI] [PubMed] [Google Scholar]
- Singhal A, 2017. Long‐term adverse effects of early growth acceleration or catch‐up growth. Annals of Nutrition and Metabolism, 70, 236–240. [DOI] [PubMed] [Google Scholar]
- Sinigaglia OE, Rios EM, Campos M, Diaz B and Palacios C, 2016. Breastfeeding practices, timing of introduction of complementary beverages and foods and weight status in infants and toddlers participants of a WIC clinic in Puerto Rico. Springerplus, 5, 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkka O, Vrijkotte T, Halberstadt J, Abrahamse‐Berkeveld M, Hoekstra T, Seidell J and Olthof M, 2018. Prospective associations of age at complementary feeding and exclusive breastfeeding duration with body mass index at 5–6 years within different risk groups. Pediatric Obesity, 13, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit C, Yeung DL, He M and Anderson GH, 2001. The growth and feeding patterns of 9 to 12 month old Chinese Canadian infants. Nutrition Research, 21, 505–516. [Google Scholar]
- Škledar MT and Milošević M, 2015. Breastfeeding and time of complementary food introduction as predictors of obesity in children. Central European Journal of Public Health, 23, 26–31. [DOI] [PubMed] [Google Scholar]
- Sloan S, Gildea A, Stewart M, Sneddon H and Iwaniec D, 2008. Early weaning is related to weight and rate of weight gain in infancy. Child: Care. Health and Development, 34, 59–64. [DOI] [PubMed] [Google Scholar]
- Snijders BE, Thijs C, van Ree R and van den Brandt PA, 2008. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics, 122, e115–e122. [DOI] [PubMed] [Google Scholar]
- Spiegler J, Eisemann N, Ehlers S, Orlikowsky T, Kannt O, Herting E, Gopel W and the GNN Team , 2015. Length and weight of very low birth weight infants in Germany at 2 years of age: does it matter at what age they start complementary food? European Journal of Clinical Nutrition, 69, 662–667. [DOI] [PubMed] [Google Scholar]
- Stene LC, Joner G and the Norwegian Childhood Diabetes Study Group , 2003. Use of cod liver oil during the first year of life is associated with lower risk of childhood‐onset type 1 diabetes: a large, population‐based, case‐control study. American Journal of Clinical Nutrition, 78, 1128–1134. [DOI] [PubMed] [Google Scholar]
- Sterne JAC and Egger M, 2005. Regression methods to detect publication and other bias in meta‐analysis. In: Rothstein HR, Sutton AJ. and Borenstein M. (eds.). Publication bias in meta‐analysis: Prevention, assessment, and adjustments. Wiley, Chichester, England, pp. 99–110.
- Stevenson RD and Allaire JH, 1991. The development of normal feeding and swallowing. Pediatric Clinics of North America, 38, 1439–1453. [DOI] [PubMed] [Google Scholar]
- Størdal K, White RA and Eggesbø M, 2013. Early feeding and risk of celiac disease in a prospective birth cohort. Pediatrics, 132, e1202–e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Størdal K, Lundeby KM, Brantsaeter AL, Haugen M, Nakstad B, Lund‐Blix NA and Stene LC, 2017. Breastfeeding and infant hospitalization for infections: large cohort and sibling analysis. Journal of Pediatric Gastroenterology and Nutrition, 65, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP, Taylor EM and Carpenter RG, 1996. Family structure, neonatal infection, and hay fever in adolescence. Archives of Disease in Childhood, 74, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strisciuglio C, Giugliano F, Martinelli M, Cenni S, Greco L, Staiano A and Miele E, 2017. Impact of environmental and familial factors in a cohort of pediatric patients with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition, 64, 569–574. [DOI] [PubMed] [Google Scholar]
- Sun C, Foskey RJ, Allen KJ, Dharmage SC, Koplin JJ, Ponsonby AL, Lowe AJ, Matheson MC, Tang ML, Gurrin L, Wake M and Sabin M, 2016. The impact of timing of introduction of solids on infant body mass index. Journal of Pediatrics, 179, 104–110. [DOI] [PubMed] [Google Scholar]
- Suryati R, Akib AAP, Boediman I and Latief A, 2006. The prevalence of atopic dermatitis history in asthmatic children. Paediatrica Indonesiana, 46, 164–169. [Google Scholar]
- Szajewska H, Shamir R, Chmielewska A, Piescik‐Lech M, Auricchio R, Ivarsson A, Kolacek S, Koletzko S, Korponay‐Szabo I, Mearin ML, Ribes‐Koninckx C, Troncone R and the PreventCD Study Group , 2015. Systematic review with meta‐analysis: early infant feeding and coeliac disease–update 2015. Alimentary Pharmacology and Therapeutics, 41, 1038–1054. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Shamir R, Mearin L, Ribes‐Koninckx C, Catassi C, Domellof M, Fewtrell MS, Husby S, Papadopoulou A, Vandenplas Y, Castillejo G, Kolacek S, Koletzko S, Korponay‐Szabo IR, Lionetti E, Polanco I and Troncone R, 2016. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 62, 507–513. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hayasawa H and Tomita M, 1999. A predictive model for affect of atopic dermatitis in infancy by neural network and multiple logistic regression. Arerugi (Japanese Journal of Allergology), 48, 1222–1229. [PubMed] [Google Scholar]
- Tan J, Valerio C, Barnes EH, Turner PJ, Van Asperen PA, Kakakios AM, Campbell DE and the Beating Egg Allergy Trial Study Group , 2017. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. Journal of Allergy and Clinical Immunology, 139, 1621–1628. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Miyake Y, Sasaki S and Hirota Y, 2013. Infant feeding practices and risk of dental caries in Japan: the Osaka Maternal And Child Health Study. Pediatric Dentistry, 35, 267–271. [PubMed] [Google Scholar]
- Taylor‐Robinson DC, Williams H, Pearce A, Law C and Hope S, 2016. Do early‐life exposures explain why more advantaged children get eczema? Findings from the U.K. Millennium Cohort Study. British Journal of Dermatology, 174, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham EH, Lee BW, Chan YH, Loo EXL, Toh JY, Goh A, Teoh OH, Yap F, Tan KH, Godfrey KM, Chong MFF, Van Bever HPS, Chong YS and Shek LP, 2017. Low food allergy prevalence despite delayed introduction of allergenic foods ‐ data from the GUSTO cohort. Journal of Allergy and Clinical Immunology. Practice, 6, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood M, Clark R, Harker P and Mann JI, 1979. Infant feeding and overweight in two Oxfordshire towns. Journal of the Royal College of General Practitioners, 29, 427–430. [PMC free article] [PubMed] [Google Scholar]
- Togias A, Cooper SF, Acebal ML, Assa'ad A, Baker JR Jr, Beck LA, Block J, Byrd‐Bredbenner C, Chan ES, Eichenfield LF, Fleischer DM, Fuchs GJ 3rd, Furuta GT, Greenhawt MJ, Gupta RS, Habich M, Jones SM, Keaton K, Muraro A, Plaut M, Rosenwasser LJ, Rotrosen D, Sampson HA, Schneider LC, Sicherer SH, Sidbury R, Spergel J, Stukus DR, Venter C and Boyce JA, 2017. Addendum guidelines for the prevention of peanut allergy in the United States: summary of the National Institute of Allergy and Infectious Diseases‐sponsored expert panel. Journal of the Academy of Nutrition and Dietetics, 117, 788–793. [DOI] [PubMed] [Google Scholar]
- Törölä H, Lehtihalmes M, Yliherva A and Olsén P, 2012. Feeding skill milestones of preterm infants born with extremely low birth weight (ELBW). Infant Behavior & Development, 35, 187–194. [DOI] [PubMed] [Google Scholar]
- Tran MM, Lefebvre DL, Dai D, Dharma C, Subbarao P, Lou W, Azad MB, Becker AB, Mandhane PJ, Turvey SE, Sears MR and the Child Study Investigators , 2017. Timing of food introduction and development of food sensitization in a prospective birth cohort. Pediatric Allergy and Immunology, 28, 471–477. [DOI] [PubMed] [Google Scholar]
- Tromp II, Kiefte‐de Jong JC, Lebon A, Renders CM, Jaddoe VW, Hofman A, de Jongste JC and Moll HA, 2011. The introduction of allergenic foods and the development of reported wheezing and eczema in childhood: the Generation R study. Archives of Pediatrics & Adolescent Medicine, 165, 933–938. [DOI] [PubMed] [Google Scholar]
- Turati F, Bertuccio P, Galeone C, Pelucchi C, Naldi L, Bach JF, La Vecchia C, Chatenoud L and the HYGIENE Study Group , 2016. Early weaning is beneficial to prevent atopic dermatitis occurrence in young children. Allergy, 71, 878–888. [DOI] [PubMed] [Google Scholar]
- Uusitalo U, Lee HS, Andren Aronsson C, Vehik K, Yang J, Hummel S, Silvis K, Lernmark A, Rewers M, Hagopian W, She JX, Simell O, Toppari J, Ziegler AG, Akolkar B, Krischer J, Virtanen SM, Norris JM and the TEDDY Study Group , 2018. Early infant diet and islet autoimmunity in the TEDDY Study. Diabetes Care, 41, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafa M, Moslehi N, Afshari S, Hossini A and Eshraghian M, 2012. Relationship between breastfeeding and obesity in childhood. Journal of Health, Population and Nutrition, 30, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vail B, Prentice P, Dunger DB, Hughes IA, Acerini CL and Ong KK, 2015. Age at weaning and infant growth: primary analysis and systematic review. Journal of Pediatrics, 167, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asperen PP, Kemp AS and Mellis CM, 1984. Relationship of diet in the development of atopy in infancy. Clinical Allergy, 14, 525–532. [DOI] [PubMed] [Google Scholar]
- van den Engel‐Hoek L, van Hulst KC, van Gerven MH, van Haaften L and de Groot SA, 2014. Development of oral motor behavior related to the skill assisted spoon feeding. Infant Behavior & Development, 37, 187–191. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Gharsalli M, Heppe DH, Raat H, Hofman A, Franco OH, Rivadeneira F and Jaddoe VW, 2016. Associations of breastfeeding patterns and introduction of solid foods with childhood bone mass: The Generation R Study. British Journal of Nutrition, 115, 1024–1032. [DOI] [PubMed] [Google Scholar]
- van Elburg RM, Fetter WP, Bunkers CM and Heymans HS, 2003. Intestinal permeability in relation to birth weight and gestational and postnatal age. Archives of Disease in Childhood. Fetal and Neonatal Edition, 88, F52–F55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossem L, Kiefte‐de Jong JC, Looman CW, Jaddoe VW, Hofman A, Hokken‐Koelega AC, Mackenbach JP, Moll HA and Raat H, 2013. Weight change before and after the introduction of solids: results from a longitudinal birth cohort. British Journal of Nutrition, 109, 370–375. [DOI] [PubMed] [Google Scholar]
- Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Hill JC, Kurpad AV, Muthayya S, Karat SC, Nalinakshi M and Fall CH, 2010. Infant feeding practice and childhood cognitive performance in South India. Archives of Disease in Childhood, 95, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehapoglu A, Yazici M, Demir AD, Turkmen S, Nursoy M and Ozkaya E, 2014. Early infant feeding practice and childhood obesity: the relation of breastfeeding and timing of solid food introduction with childhood obesity. Journal of Pediatric Endocrinology and Metabolism, 27, 1181–1187. [DOI] [PubMed] [Google Scholar]
- Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, Arshad SH and Dean T, 2009. Factors associated with maternal dietary intake, feeding and weaning practices, and the development of food hypersensitivity in the infant. Pediatric Allergy and Immunology, 20, 320–327. [DOI] [PubMed] [Google Scholar]
- Venter C, Maslin K, Dean T and Arshad SH, 2016. Does concurrent breastfeeding alongside the introduction of solid food prevent the development of food allergy? Journal of Nutritional Science, 5, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D and Salanti G, 2016. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Research Synthesis Methods, 7, 55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg‐Kippila C, Hakulinen T, Ahonen S, Uusitalo L, Niinisto S, Veijola R, Simell O, Ilonen J and Knip M, 2006. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA‐conferred susceptibility to type 1 diabetes. Diabetologia, 49, 1512–1521. [DOI] [PubMed] [Google Scholar]
- Virtanen SM, Kaila M, Pekkanen J, Kenward MG, Uusitalo U, Pietinen P, Kronberg‐Kippila C, Hakulinen T, Simell O, Ilonen J, Veijola R and Knip M, 2010. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. British Journal of Nutrition, 103, 266–273. [DOI] [PubMed] [Google Scholar]
- Virtanen SM, Takkinen HM, Nevalainen J, Kronberg‐Kippila C, Salmenhaara M, Uusitalo L, Kenward MG, Erkkola M, Veijola R, Simell O, Ilonen J and Knip M, 2011. Early introduction of root vegetables in infancy associated with advanced ß‐cell autoimmunity in young children with human leukocyte antigen‐conferred susceptibility to Type 1 diabetes. Diabetic Medicine, 28, 965–971. [DOI] [PubMed] [Google Scholar]
- Visalli N, Sebastiani L, Adorisio E, Conte A, De Cicco AL, D'Elia R, Manfrini S, Pozzilli P and the IMDIAB Group , 2003. Environmental risk factors for type 1 diabetes in Rome and province. Archives of Disease in Childhood, 88, 695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelezang S, Santos S, van der Beek EM, Abrahamse‐Berkeveld M, Duijts L, van der Lugt A, Felix JF and Jaddoe VWV, 2018. Infant breastfeeding and childhood general, visceral, liver, and pericardial fat measures assessed by magnetic resonance imaging. American Journal of Clinical Nutrition, 108, 722–729. [DOI] [PubMed] [Google Scholar]
- von Hofsten C, 1991. Structuring of early reaching movements: a longitudinal study. Journal of Motor Behavior, 23, 280–292. [DOI] [PubMed] [Google Scholar]
- Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, Kolacek S, Koletzko S, Korponay‐Szabo IR, Mummert E, Polanco I, Putter H, Ribes‐Koninckx C, Shamir R, Szajewska H, Werkstetter K, Greco L, Gyimesi J, Hartman C, Hogen Esch C, Hopman E, Ivarsson A, Koltai T, Koning F, Martinez‐Ojinaga E, te Marvelde C, Pavic A, Romanos J, Stoopman E, Villanacci V, Wijmenga C, Troncone R and Mearin ML, 2014. Randomized feeding intervention in infants at high risk for celiac disease. The New England Journal of Medicine, 371, 1304–1315. [DOI] [PubMed] [Google Scholar]
- Wahlberg J, Vaarala O, Ludvigsson J and the ABIS‐study group , 2006. Dietary risk factors for the emergence of type 1 diabetes‐related autoantibodies in 21/2 year‐old Swedish children. British Journal of Nutrition, 95, 603–608. [DOI] [PubMed] [Google Scholar]
- Wang P, Hao M, Han W and Yamauchi T, 2019. Factors associated with nutritional status and motor development among young children. Nursing & Health Science, Apr 15, 10.1111/nhs.12604 [DOI] [PubMed] [Google Scholar]
- Warrington S and Storey DM, 1988. Comparative studies on Asian and Caucasian children. 2: Nutrition, feeding practices and health. European Journal of Clinical Nutrition, 42, 69–79. [PubMed] [Google Scholar]
- Welander A, Tjernberg AR, Montgomery SM, Ludvigsson J and Ludvigsson JF, 2010. Infectious disease and risk of later celiac disease in childhood. Pediatrics, 125, e530–e536. [DOI] [PubMed] [Google Scholar]
- Wen LM, Baur LA, Rissel C, Xu H and Simpson JM, 2014. Correlates of body mass index and overweight and obesity of children aged 2 years: findings from the healthy beginnings trial. Obesity (Silver Spring), 22, 1723–1730. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1989. Infant feeding ‐ the physiological basis. Bulletin of the World Health Organization, supplement to volume 67, 115 pp. [PMC free article] [PubMed]
- WHO (World Health Organization), 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. 50 pp.
- WHO Multicentre Growth Reference Study Group , 2006. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatrica. Supplement, 450, 86–95. [DOI] [PubMed]
- WHO Regional Office for Europe (World Health Organization Regional Office for Europe), 2003. Feeding and nutrition of infants and young children. Guidelines for the WHO European region, with emphasis on the former Soviet countries. 288 pp.
- WHO Working Group on Infant Growth (World Health Organization Working Group on Infant Growth), 1994. An evaluation of infant growth: a summary of analyses performed in preparation for the WHO Expert Committee on Physical Status, the Use and Interpretation of Anthropometry. 85 pp.
- Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C and Howie PW, 1998. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. British Medical Journal, 316, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman PG, 1984. Feeding practices in infancy and prevalence of obesity in preschool children. Journal of the American Dietetic Association, 84, 436–438. [PubMed] [Google Scholar]
- Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W and Taussig LM, 1994. Epidemiology of physician‐diagnosed allergic rhinitis in childhood. Pediatrics, 94, 895–901. [PubMed] [Google Scholar]
- Wright CM, Parkinson KN and Drewett RF, 2004. Why are babies weaned early? Data from a prospective population based cohort study. Archives of Disease in Childhood, 89, 813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CM, Cameron K, Tsiaka M and Parkinson KN, 2011. Is baby‐led weaning feasible? When do babies first reach out for and eat finger foods? Maternal & Child Nutrition, 7, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y, Sugimoto M, Miyake Y, Sono J, Mizukami K, Kaprio J and Silventoinen K, 2011. Motor development of triplets: a Japanese prospective cohort study. Twin Research and Human Genetics, 14, 185–191. [DOI] [PubMed] [Google Scholar]
- Yrjänä JM, Koski T, Törölä H, Valkama M and Kulmala P, 2018. Very early introduction of semisolid foods in preterm infants does not increase food allergies or atopic dermatitis. Annals of Allergy, Asthma & Immunology, 121, 353–359. [DOI] [PubMed] [Google Scholar]
- Yung J, Yuen JW, Ou Y and Loke AY, 2015. Factors associated with atopy in toddlers: a case‐control study. International Journal of Environmental Research and Public Health, 12, 2501–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JS, Liu H, Zhao YM, Li J, Chen Y, Zhu S, Chen H, Huang T and Li D, 2015. Complementary feeding and childhood adiposity in preschool‐aged children in a large Chinese cohort. Journal of Pediatrics, 166, 326–331. [DOI] [PubMed] [Google Scholar]
- Zheng XQ, Zhu GW, Zheng ZQ, Yang Y, Gong CD, Deng SS, Wu QL and Peng YM, 2016. [Effects of infant feeding practice on eczema during early childhood in Shanghai, Hohhot, and Fuzhou]. Zhonghua Er Ke Za Zhi, 54, 908–912. [DOI] [PubMed] [Google Scholar]
- Zhou L, He G, Zhang J, Xie R, Walker M and Wen SW, 2011. Risk factors of obesity in preschool children in an urban area in China. European Journal of Pediatrics, 170, 1401–1406. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hernandez LM, Dong Y, Himes JH, Hirschfeld S and Forman MR, 2015. Longer breastfeeding duration reduces the positive relationships among gestational weight gain, birth weight and childhood anthropometrics. Journal of Epidemiology and Community Health, 69, 632–638. [DOI] [PubMed] [Google Scholar]
- Ziegler AG, Schmid S, Huber D, Hummel M and Bonifacio E, 2003. Early infant feeding and risk of developing type 1 diabetes‐associated autoantibodies. JAMA, 290, 1721–1728. [DOI] [PubMed] [Google Scholar]
- Zive MM, McKay H, Frank‐Spohrer GC, Broyles SL, Nelson JA and Nader PR, 1992. Infant‐feeding practices and adiposity in 4‐y‐old Anglo‐ and Mexican‐Americans. American Journal of Clinical Nutrition, 55, 1104–1108. [DOI] [PubMed] [Google Scholar]
- Zutavern A, von Mutius E, Harris J, Mills P, Moffatt S, White C and Cullinan P, 2004. The introduction of solids in relation to asthma and eczema. Archives of Disease in Childhood, 89, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutavern A, Brockow I, Schaaf B, Bolte G, von Berg A, Diez U, Borte M, Herbarth O, Wichmann HE, Heinrich J and the LISA Study Group , 2006. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics, 117, 401–411. [DOI] [PubMed] [Google Scholar]
- Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, Kraemer U, Herbarth O, Behrendt H, Wichmann HE, Heinrich J and the LISA Study Group , 2008. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics, 121, e44–e52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome of the data extraction from the included prospective and retrospective studies
Result of the assessment of the risk of bias per question and outcome for randomised controlled trials and prospective observational studies
List of papers excluded at full‐text screening (step 2) of the searches
Funnel plots for the assessment of publication bias
Sensitivity analyses on the use of different between‐study variance estimators in the random effects meta‐analyses


