Abstract
Introduction:
Antiarrhythmic drugs have not proven to significantly improve overall survival after out-of-hospital cardiac arrest (OHCA) from shock-refractory ventricular fibrillation/pulseless ventricular tachycardia (VF/VT). How this might be influenced by the route of drug administration is not known.
Methods:
In this pre-specified analysis of a randomized, placebo-controlled clinical trial, we compared differences in survival to hospital discharge in adults with shock-refractory VF/VT OHCA who were randomized by emergency medical services (EMS) personnel to an antiarrhythmic drug versus placebo in the Resuscitation Outcomes Consortium Amiodarone, Lidocaine or Placebo Study (ALPS), when stratified by the intravenous (IV) versus intraosseous (IO) route of administration.
Results:
Of 3,019 randomized patients with known vascular access site, 2,358 received ALPS drugs IV and 661 patients by IO route. IO and IV groups differed in sex, time-to-EMS arrival, and some CPR characteristics, but were similar in others, including time-to-IV/IO-drug receipt. Overall hospital discharge survival was 23%. Compared to placebo, discharge survival was significantly higher in recipients of IV amiodarone ((adjusted risk ratio (RRadj) 1.26 (95% confidence interval (CI) 1.06, 1.50); adjusted absolute survival difference 5.5% (95% CI 1.5, 9.5)) and IV lidocaine (RRadj 1.21 (95% CI 1.02, 1.45)); absolute survival difference 4.7% (95% CI 0.7, 8.8)); but not in recipients of IO amiodarone (RRadj 0.94 (95% CI 0.66, 1.32)) or IO lidocaine (RRadj 1.03 (95% CI 0.74, 1.44)). Survival to hospital admission also increased significantly when drugs were given IV but not IO, and favored improved neurological outcome at discharge. There were no outcome differences between IV and IO placebo, indicating the access route itself did not demarcate patients with poor prognosis. The study was underpowered to assess IV/IO-drug interactions, which were not statistically significant.
Conclusions:
We found no significant effect modification by drug administration route for amiodarone or lidocaine compared to placebo during OHCA. However, point estimates for the effects of both drugs compared to placebo were greater for the IV than IO route across all outcomes and beneficial only for IV. Given that the study was underpowered to statistically assess interactions, these findings signal the potential importance of the drug administration route during resuscitation that merits further investigation.
Keywords: cardiac arrest, cardiac arrhythmia, amiodarone, antiarrhythmic agent, vascular access
INTRODUCTION
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death and a significant public health problem worldwide. Its treatment consists of a sequence of time-sensitive interventions known as the chain of survival. Antiarrhythmic medications are frequently administered when OHCA is caused by shock-refractory ventricular fibrillation or pulseless ventricular tachycardia (VF/VT). While showing promise when the OHCA is witnessed, these drugs have not been proven to improve overall survival in patients with witnessed and unwitnessed VF/VT arrest.1 Whether this apparent inefficacy is influenced by their route of administration - intravenous (IV) versus intraosseous (IO) - is not known.
American Heart Association (AHA) resuscitation guidelines preferentially recommend drug administration by peripheral IV, but endorse use of an IO route when IV access cannot be readily obtained.2 Technological advances over the last two decades have made it easier to access the IO space in adults. Studies suggest this approach is faster and more likely to be successful in achieving vascular access than IV cannulation.3, 4, 5 As a result, Emergency Medical Services (EMS) systems have increasingly incorporated IO vascular access into their resuscitation protocols, with some in fact prioritizing this approach over IV cannulation. However, little is known about the pharmacokinetics of antiarrhythmic drugs administered IO during the low flow state of cardiac arrest and ongoing cardiopulmonary resuscitation (CPR). Recent studies have challenged whether resuscitation medications, particularly epinephrine, are as effective when administered IO rather than IV during CPR.6, 7, 8 The effectiveness of IV vs IO administration of antiarrhythmic agents during resuscitation, particularly in context of a placebo control, has not been previously addressed and could have important implications for patient care.
In this pre-specified analysis of a randomized, placebo-controlled trial, we compared survival to hospital discharge in adults with non-traumatic, shock-refractory VF/VT OHCA who were randomized to antiarrhythmic drugs versus placebo in the Resuscitation Outcomes Consortium (ROC) Amiodarone, Lidocaine or Placebo Study (ALPS), stratified by the route of drug administration (IV versus IO) during CPR. We hypothesized a beneficial association with survival from amiodarone and lidocaine, compared to placebo, when an active drug was administered IV during active resuscitation, but an attenuated association when given IO.
METHODS
The ALPS trial has been previously described,1,9 and anonymized data and materials are publicly available at the National Institutes of Health Biologic Specimen and Data Repository Information Coordinating Center website (https://biolincc.nhlbi.nih.gov/home/). Adults with non-traumatic OHCA presenting as shock-refractory VF/VT were randomized, double-blind by paramedics from 55 emergency medical services (EMS) agencies across 10 North American sites to receive amiodarone, lidocaine or placebo. The trial was conducted under exception from informed consent in emergency research in compliance with all applicable regulatory requirements. This included institutional and independent data safety monitoring board oversight, as well as the Federal Food and Drug Administration (FDA). It was principally supported by the National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research, with provision of study drug by Baxter Healthcare (who otherwise played no role in the trial). Eligible patients had established IV or IO access and confirmed persistent (non-terminating) or recurrent (only transiently terminated) VF/VT after ≥1 defibrillation shocks at the time of drug administration. Selection of IV or IO access was at the discretion of providers and local EMS protocols and was defined as the specified route by which study drug was administered. This approach varied according to clinical practice across sites. In some cases IO represented the primary attempt at vascular access; whereas in others deployed when IV access was unsuccessful or deemed unlikely to be successful. A set of 3 syringes each containing the same study drug (150 mg amiodarone, 60 mg lidocaine or matching placebo) for IV or IO administration was placed in indistinguishable sealed kits. Kits were randomly assigned to patients in a ratio of 1:1:1. Two syringes were initially administered by bolus injection. This was subsequently followed, if necessary, by the third syringe for persistent or recurring VF/VT, in addition to all other standard advanced life support measures as required. Open label use of lidocaine or amiodarone was not permitted during prehospital care. All trial interventions were completed before hospital arrival; subsequent hospital care was monitored but not standardized by the trial protocol. The main trial and its pre-specified sub-analyses were approved by respective institutional review boards in participating communities.
Outcome
The primary study outcome was survival to hospital discharge between active drugs and placebo when administered IV and when given IO. Secondary outcomes included survival to hospital admission and survival with favorable neurological function at hospital discharge, defined as a modified Rankin scale (mRS) ≤ 3 (range 0 (no symptoms) to 6 (death)), with 3 or less indicating the ability to conduct daily activities independently or with minimal assistance. Primary and secondary outcomes were also assessed comparing IV against IO placebo, to distinguish effects associated with active drugs versus placebo at those sites from those that might be associated with the selected route of vascular access itself, as were adverse effects associated with IV vs IO treatment.1,9 Primary and secondary outcome differences between IV and IO groups were expressed in terms of relative risk (or risk ratio) with 95% confidence intervals (CI)) and also in absolute differences (95% CI) permitting comparison with how results were expressed in the primary trial.
Data Collection and Analysis
Data from prehospital patient care reports, electronically collected CPR process measures and hospital records were collected and abstracted without specific knowledge of treatment assignment or route of prehospital vascular access.1,9 Data collection used a standardized data dictionary and a common web-based electronic data entry platform hosted at the study’s Data Coordinating Center. The route of access was a pre-defined data element and was ascertained by review of the EMS record. If patients received both IV and IO access, outcomes were assigned according to the route by which study drug (amiodarone, lidocaine or placebo) was administered.
Statistics
Primary and secondary outcomes were evaluated across treatment groups stratified by route of vascular access. We used multivariable linear regression with robust standard errors to adjust for potential confounding when comparing the association of study drug with outcome according to vascular route. Collected data that served as covariates in the adjusted analyses included age, sex, location of arrest, presumed etiology, witnessed status (bystander and EMS), provision of bystander CPR; emergency call to first EMS arrival, to arrival of advanced life support personnel, and to receipt of study drug (IV or IO). In addition, multivariable models were specifically adjusted for clustering of potentially correlated data by study site using the fixed effects approach10 based on (“dummy”) indicator variables for study sites. Unadjusted analyses were performed on all patients with observed outcomes (> 99% of cases) and in patients in whom all covariate data were known; adjusted analyses were restricted to the latter population of patients with complete covariate data. The proportion of patients missing complete covariate data was relatively small (5%). We also evaluated a statistical interaction by IV vs IO drug treatment on primary and secondary outcomes by adding a multiplicative term (route of access X study drug) to the multivariable model, recognizing that the study was not originally designed nor sufficiently powered for determining interactions. Such a design would require 6,000 patients to detect an interaction between drug route and outcome with 80% power based on the current study’s estimates of survival. Results of analyses were not adjusted for multiple comparisons. Two-sided p values ≤ 0.05 were considered statistically significant.
RESULTS
Study Population
In all, 3,026 patients with OHCA due to shock refractory VF/VT were randomly assigned to receive amiodarone (n=974), lidocaine (n=991), or placebo (1054); the route of vascular access for study drug administration was known for 3,019 patients and not known in 7 patients who were excluded from the analysis. Of these, 2,358 patients (78%) received their study drug IV (explicitly specified as an upper extremity vein in 1,989 (84%)), and 661 patients (22%) by an IO route (explicitly specified as a tibial IO in 615 patients (93%)) (Figure 1). Follow-up for the primary and secondary study outcomes was complete in >99% of these patients. Utilization of IV versus IO access varied by study site and local practice. IO drug administration ranged from 1–53% across sites; half of sites contributed 89% of patients who received study drugs by IO access (Supplemental Figure 1).
Figure 1:
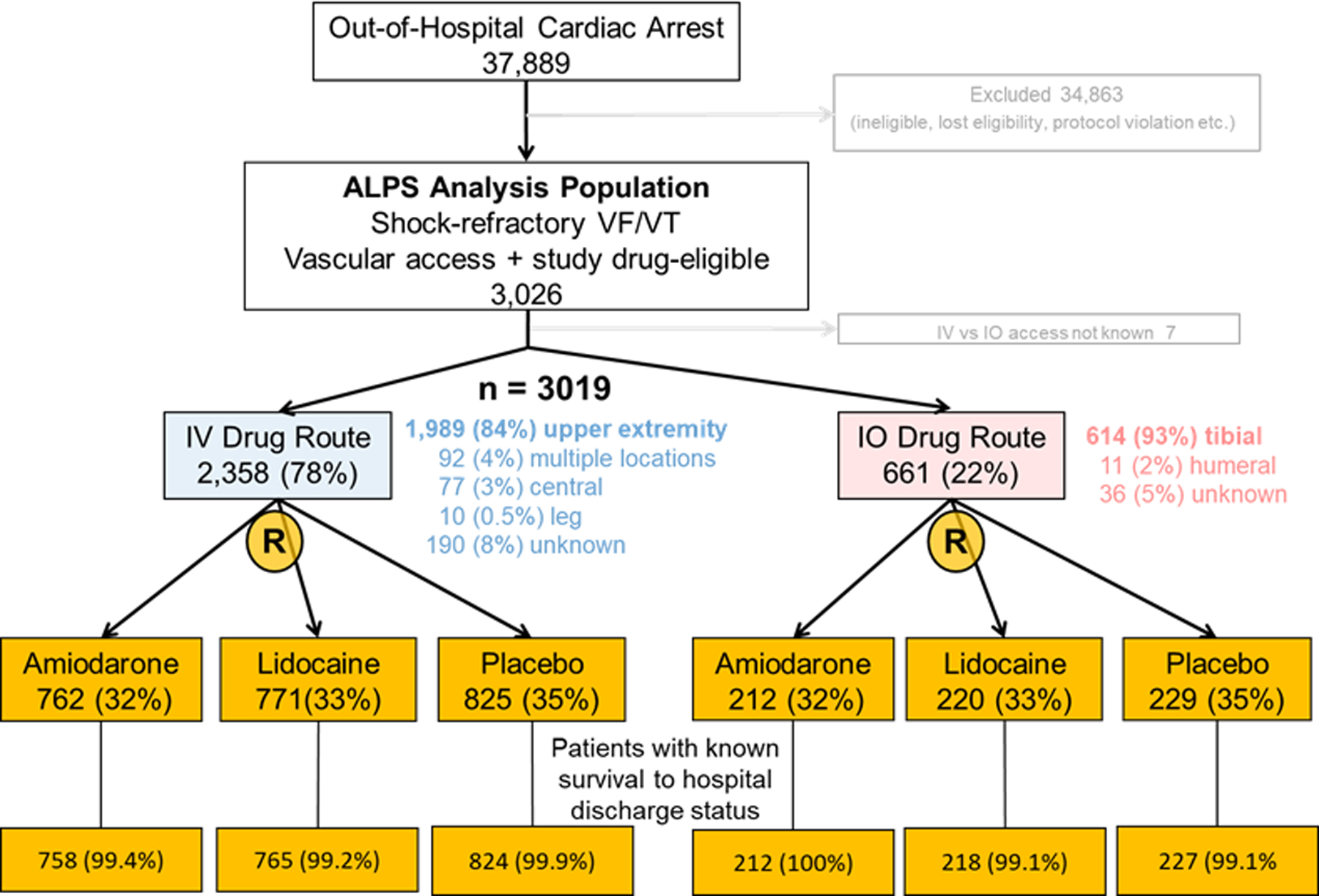
Stratification of patients who were eligible for study drug by intravenous or intraosseous route of vascular access, and their randomized treatment assignment to amiodarone, lidocaine or placebo.
A comparable proportion of patients in IV and IO groups were randomized to treatment with lidocaine, amiodarone or placebo, nearly identical to the proportion randomized to each of these study drug arms in the overall ALPS population (Figure 1).1 A complete set of covariate data for adjusted analyses was available in approximately 95% of the study population: in 2,876 patients with known survival to hospital admission outcome, 2,861 for survival to discharge and in 2,857 patients with known neurological outcome.
Patients comprising IV and IO groups did not significantly differ in age, the location of their arrest, frequency of bystander or EMS witnessed arrest, receipt of bystander shock or CPR, CPR compression rate, duration of peri-shock pauses, successful advanced airway placement, the time intervals (mean ± standard deviation) from the emergency call to IV or IO vascular access (14.2 ± 5.6 versus 13.9 ± 5.8 minutes, respectively) and to study drug administration (19.3 ± 7.4 vs 19.4 ± 7.3 minutes), nor receipt of other resuscitation drugs (Tables 1–2). Differences were observed between IV and IO groups in sex, the time interval from emergency call to EMS vehicle arrival and to arrival of paramedics with advanced life support capabilities, chest compression depth and CPR fraction (i.e. the proportion of time that chest compressions were administered during pulseless periods). Epinephrine was commonly administered before study drug (Table 2), whose time of administration was similar between IV and IO groups and concordant with the same IV or IO route as study drug administration (data not shown).
Table 1.
Prehospital characteristics of the study population.
| Characteristic | IV Route (n = 2358) |
IO Route (n = 661) |
|---|---|---|
| Age – years, mean ± sd [n=2351, 660] | 62.7 ± 14.3 | 62.3 ± 14.8 |
| Placebo [n=823, 229] | 62.7 ±14.4 | 60.5 ±15.3 |
| Lidocaine [n=770, 220] | 62.2 ±14.6 | 63.5 ±15.1 |
| Amiodarone [n=758, 212] | 63.2 ±13.9 | 63.2 ±14 |
| Male sex – n (%) [total n=2357, 661]* | 1935 (82.1%) | 481 (72.8%) |
| Placebo [n=825, 229] | 682 (82.7%) | 158 (69%) |
| Lidocaine [n=771, 220] | 647 (83.9%) | 167 (75.9%) |
| Amiodarone [n=761, 212] | 606 (79%) | 156 (73.6%) |
| Public location – n (%) [total n=2355, 661] | 739 (31.4%) | 188 (28.4%) |
| Placebo [n=822, 229] | 246 (29.9%) | 66 (28.8%) |
| Lidocaine [n=771, 220] | 245 (31.8%) | 67 (30.5%) |
| Amiodarone [n=762, 212] | 248 (32.5%) | 55 (25.9%) |
| EMS witnessed– n (%) [total n = 2345, 655] | 121/2345 (5.2%) | 34/655 (5.2%) |
| Placebo [n=820, 228] | 44 (5.4%) | 10 (4.4%) |
| Lidocaine [n=676, 219] | 34 (4.4%) | 10 (4.6%) |
| Amiodarone [n=758, 208] | 43 (5.7%) | 14 (6.7%) |
| Bystander witnessed – n (%) [total n=2345, 655] | 1530 (65.2%) | 409 (62.4%) |
| Placebo [n=820, 228] | 547 (66.7%) | 136 (59.6%) |
| Lidocaine [n=676, 219] | 496 (64.7%) | 139 (63.5%) |
| Amiodarone [n=568, 208] | 487 (64.2%) | 134 (64.4%) |
| Bystander shock – n. (%) [total n = 2322, 644] | 123 (5.3%) | 47 (7.3%) |
| Placebo [n=812, 225] | 42 (5.2%) | 15 (6.7%) |
| Lidocaine [n=755, 213] | 36 (4.8%) | 15 (7%) |
| Amiodarone [n=755, 206] | 45 (6%) | 17 (8.3%) |
| Bystander CPR n (%) [total n = 2323, 645] | 1322 (56.9%) | 375 (58.1%) |
| Placebo [n=812, 225] | 461 (56.8%) | 131 (58.2%) |
| Lidocaine [n=756, 213] | 428 (56.6%) | 121 (56.8%) |
| Amiodarone [n=755, 207] | 433 (57.4%) | 123 (59.4%) |
| Call to 1st EMS arrival min, mean ± sd [n=2327, 654]* | 5.8 ± 2.6 | 5.4 ± 2.2 |
| Placebo [n=811, 227] | 5.9 ± 2.7 | 5.5 ± 2.3 |
| Lidocaine [n=762, 218] | 5.7 ± 2.5 | 5.1 ± 2 |
| Amiodarone [n=754, 209] | 5.8 ± 2.6 | 5.7 ± 2.3 |
| Call to ALS arrival min, mean ± sd [n=2345, 658]* | 8.3 ± 4.7 | 6.8 ± 4.1 |
| Placebo [n=822, 229] | 8.4 ± 4.8 | 6.6 ± 3.2 |
| Lidocaine [n=766, 219] | 8.2 ± 4.3 | 6.5 ± 3.9 |
| Amiodarone [n=757, 210] | 8.2 ± 5.1 | 7.4 ± 5.1 |
Abbreviations: ALS - Advanced Life Support, Call – emergency call activating the EMS system (911), EMS - Emergency Medical Services, IO – intraosseous vascular access, IV – intravenous vascular access, sd -standard deviation
The [n =] in the gray rows of the table refers to the number of patients in the IV and IO groups, respectively, in whom data were available for the indicated measure.
p<0.001 for differences in sex, and call interval to EMS and ALS arrival (respectively) between IV and IO treatment groups.
Table 2.
Resuscitation characteristics of the study population
| Characteristic | IV Route (n = 2358) |
IO Route (n = 661) |
|---|---|---|
| Call to IV or IO access mean minutes ± sd [n=2294, 652] | 14.2 ± 5.6 | 13.9 ± 5.8 |
| Placebo [n= 805, 228] | 14.4 ± 5.8 | 13.7 ± 4.7 |
| Lidocaine [n=752, 219] | 14 ± 5.4 | 13.6 ± 5.4 |
| Amiodarone [n=737, 205] | 14.1 ± 5.6 | 14.6 ± 7.1 |
| Call to Study Drug*, mean minutes ± sd [n=2213, 617] | 19.3 ± 7.4 | 19.4 ± 7.3 |
| Placebo [n=772, 213] | 19.3 ± 7.5 | 19 ± 6.7 |
| Lidocaine [n=731, 209] | 19.3 ± 7.6 | 19.3 ± 7.6 |
| Amiodarone [n=710, 195] | 19.2 ± 7 | 19.8 ± 7.6 |
| Arrest to Study Drug†, mean minutes± sd [n=114, 33] | 12 ± 6.4 | 11.7 ± 5.7 |
| Placebo [n=42, 10] | 12.7 ± 6.8 | 9.5 ± 4.8 |
| Lidocaine [n=31, 10] | 12.1 ± 7 | 12.3 ± 5.5 |
| Amiodarone [n=41, 13] | 11.2 ± 5.6 | 13.1 ± 6.5 |
| Chest compression rate, mean cpm ± sd [n=2173, 619] | 110 ± 10.9 | 110 ± 11.3 |
| Placebo [n=760, 210] | 110 ± 10.9 | 110 ± 11.9 |
| Lidocaine [n=712, 212] | 110 ± 10.8 | 110 ± 10.6 |
| Amiodarone [n=701, 197] | 109 ± 10.9 | 109 ± 11.3 |
| Chest compression depth, mean mm ± sd [n=1118, 326]‡ | 51.7 ± 10.3 | 49.7 ± 9.0 |
| Placebo [n=392, 108] | 52.3 ± 10.3 | 50.4 ± 7.6 |
| Lidocaine [n=382, 119] | 51.8 ± 11.4 | 48.7 ± 9.4 |
| Amiodarone [n=344, 99] | 51 ± 9 | 50.2 ± 9.9 |
| Chest compression fraction, mean % ± sd [n=2201, 623]‡ | 0.83 ± 0.10 | 0.85 ± 0.09 |
| Placebo [n=774, 211] | 0.83 ± 0.10 | 0.84 ± 0.09 |
| Lidocaine [n=718, 212] | 0.83 ± 0.09 | 0.85 ± 0.09 |
| Amiodarone [n=709, 200] | 0.83 ± 0.10 | 0.84 ± 0.10 |
| Pre-shock pause, mean secs ± sd [n=2112, 594] | 10.3 ± 9.6 | 9.8 ± 9.3 |
| Placebo [n=737, 202] | 10.5 ± 9.2 | 9 ± 8.1 |
| Lidocaine [n=691, 200] | 10.3 ± 9 | 9.7 ± 9.8 |
| Amiodarone [n=684, 192] | 10.1 ± 10.7 | 10.7 ± 10.8 |
| Post-shock pause, mean secs ± sd [n=2099, 592] | 6.2 ± 32.4 | 6.7 ± 37.0 |
| Placebo [n=733, 200] | 7.6 ± 52.7 | 5.9 ± 9 |
| Lidocaine [n=688, 200] | 5.5 ± 13.6 | 4.7 ± 5.5 |
| Amiodarone [n=678, 192] | 5.3 ± 8.2 | 9.7 ± 64.2 |
| Prehospital advanced airway success, n (%) [total n=2358, 661] | 2007 (85.1%) | 555 (84.0%) |
| Placebo [n=825, 229] | 699 (84.7%) | 189 (82.50%) |
| Lidocaine [n=771, 220] | 668 (86.6%) | 186 (84.5%) |
| Amiodarone [n=762, 212] | 649 (84%) | 180 (84.9%) |
| Receipt of epinephrine, n (%) [total n = 2358, 661] | 2329 (98.7%) | 653 (98.7) |
| Placebo [n=825, 229] | 817 (99%) | 224 (97.8%) |
| Lidocaine [n=771, 220] | 761 (98.7%) | 218 (99.1%) |
| Amiodarone [n=762, 212] | 751 (98.6%) | 211 (99.5%) |
| Receipt of magnesium, n (%) [total n = 2358, 661] | 201 (8.5%) | 64 (9.6%) |
| Placebo [n=825, 229] | 88 (10.7%) | 31 (13.5%) |
| Lidocaine [n=771, 220] | 56 (7.3%) | 12 (5.5%) |
| Amiodarone [n=762, 212] | 57 (7.5%) | 21 (9.9%) |
Abbreviations: cpm – compressions per minute, IO – intraosseous vascular access, IV – intravenous vascular access, sd -standard deviation
The [n =] in the gray rows of the table refers to the number of patients in the IV and IO groups, respectively, in whom data were available for the indicated measure
Patients with cardiac arrest before arrival of Emergency Medical Services
Patients with cardiac arrest after arrival of Emergency Medical Services
p<0.001 for differences in chest compression depth and CPR compression fraction (respectively) between IV and IO treatment groups
Hospital-initiated treatments, including targeted temperature management, subsequent defibrillator implantation and the proportion of patients in whom care was limited or withdrawn during their stay were balanced across IV and IO treatment groups. A lower proportion of IO than IV recipients underwent coronary angiography with a percutaneous coronary intervention during the first 24 hours of hospitalization (Supplemental Table 1).
Active Drug vs Placebo Outcome
Among the 3,019 randomized ALPS patients with a known IV or IO site of vascular access, overall survival to hospital discharge was 23%. Among IV recipients, unadjusted survival was higher among those randomized to amiodarone than placebo (25.9% vs 20.6% of patients, respectively, p=0.014 and approached statistical significance in recipients of IV lidocaine compared to placebo (24.6% vs 20.6% of patients, p=0.06). This corresponded to an unadjusted risk ratio (in the full study population and in the population sample with complete covariate data, respectively) of 1.25 (95% confidence interval (CI) 1.05, 1.50) and 1.28 (95% CI 1.06, 1.54) when IV amiodarone was compared against placebo; and 1.19 (95% CI 0.99, 1.43) and 1.25 (95% CI 1.03, 1.50) when IV lidocaine was compared against placebo (Table 3). Conversely, there was no significant difference in unadjusted survival to hospital discharge between either amiodarone or lidocaine compared to placebo when given IO (Table 3).
Table 3.
Unadjusted and adjusted primary and secondary study outcomes in the full study population and in patients within that population with complete covariate data (complete data sample).
| Outcome (full study population) | Relative risk % (95% CI) | Overall p for IV/IO interaction§ | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Outcome (full study population) Unadjusted outcome (complete data sample) |
Adjusted Outcome‡ (complete data sample) | |||||||
| Vascular Access* [n in each treatment arm]† |
Placebo n (%) |
Lidocaine n (%) |
Amiodarone n (%) |
Amiodarone vs Placebo | Lidocaine vs Placebo | Amiodarone vs Placebo | Lidocaine vs Placebo | |
| Survival to Hospital Admission (Full study population n= 3019; Complete data sample n = 2876) | ||||||||
|
IV 1070/2353 (45.5%) [n=825, 768, 760] |
328 (39.8%) | 372 (48.4%) | 370 (48.7%) | 1.22 (1.09, 1.37) | 1.21 (1.09, 1.36) | 1.23 (1.11, 1.37) | 1.24 (1.11, 1.38) | 0.11 |
| IV/IO interaction (p) | 0.15 | 0.85 | ||||||
|
IO 259/660 (39.2%) [n= 228, 220, 212] |
89 (39%) | 95 (43.2%) | 75 (35.4%) | 0.91 (0.71, 1.16) | 1.11 (0.89, 1.39) | 0.95 (0.75, 1.21) | 1.20 (0.97, 1.49) | |
| Survival to hospital discharge (Full study population n=3004; Complete data sample n=2861) | ||||||||
|
IV 554/2347 (23.6%) [n= 824, 765, 758] |
170 (20.6%) | 188 (24.6%) | 196 (25.9%) | 1.25 (1.05, 1.50) | 1.19 (0.99, 1.43) | 1.26 (1.06, 1.50) | 1.21 (1.02, 1.45) | 0.32 |
| IV/IO interaction (p) | 0.22 | 0.48 | ||||||
|
IO 137/657 (20.9%) [n= 227, 218, 212] |
51 (22.5%) | 45 (20.6%) | 41 (19.3%) | 0.86 (0.60, 1.24) | 0.92 (0.64, 1.31) | 0.94 (0.66, 1.32) | 1.03 (0.74, 1.44) | |
| Survival with mRS ≤ 3 at hospital discharge (Full study population n=2999; Complete data sample n=2857) | ||||||||
|
IV 431/2342 (18.4%) [n= 823, 764, 755] |
137 (16.6%) | 142 (18.6%) | 152 (20.1%) | 1.21 (0.98, 1.49) | 1.12 (0.90, 1.38) | 1.24 (1.02, 1.52) | 1.17 (0.95, 1.44) | 0.47 |
| IV/IO interaction (p) | 0.31 | 0.48 | ||||||
|
IO 97/657 (14.8%) [n= 227, 218, 212] |
37 (16.3%) | 30 (13.8%) | 30 (14.2%) | 0.87 (0.56, 1.36) | 0.84 (0.54, 1.32) | 0.94 (0.61, 1.43) | 0.96 (0.63, 1.46) | |
Abbreviations: CI - confidence interval, IO - intraosseous vascular access, IV - intravenous vascular access, mRS - modified Rankin scale
Full study population refers to all patients with a known survival outcome (>99% of cases); complete data sample refers to patients in whom all covariate data were known (~95% of cases).
The numerator of the fractions shown in the first column corresponds to the combined number of patients in placebo, lidocaine and amiodarone groups who achieved the described endpoint, and the denominator to the total number of patients who received that (IV or IO) route of treatment.
The [n =] refers to the number of patients in the placebo, lidocaine and amiodarone groups, respectively, in whom data were available for the indicated endpoint.
Adjusted for age, sex, cardiac cause, public location, EMS witnessed, bystander witnessed, bystander CPR, EMS arrival time, ALS arrival time, time to study drug, and study site
Comparing the overall interaction between IV and IO treatment for amiodarone versus placebo and lidocaine versus placebo in the adjusted outcome analysis. The corresponding p values for the overall interaction for unadjusted outcomes in the full study population and in the sample with complete data were 0.08 and 0.09 respectively for survival to hospital admission; 0.18 and 0.18 for survival to hospital discharge; and 0.29 and 0.36 for survival with mRS ≤ 3 at hospital discharge.
Adjusted analyses mirrored these findings. IV drug administration was associated with a significantly higher adjusted survival to hospital discharge compared to placebo both for amiodarone recipients (adjusted relative risk 1.26 (95% CI 1.06, 1.50), or an adjusted absolute survival difference of 5.5% (95% CI 1.5, 9.5)) and lidocaine recipients (relative risk 1.21 (95% CI 1.02, 1.45), absolute survival difference 4.7% (95% CI 0.7, 8.8)). In contrast, there were no significant differences in adjusted survival outcome between amiodarone and placebo (relative risk 0.94 (95% CI 0.66, 1.32), or an absolute survival difference of −1.8% (95% CI −9.2, 5.6)) nor between lidocaine and placebo (relative risk 1.03 (95% CI 0.74, 1.44), absolute survival difference 0.3% (95% CI −7.4, 7.9)) when administered IO (Table 3 and Figure 2). However, a statistically significant interaction between route of vascular access and survival after study drug was not evident (p= 0.32).
Figure 2.
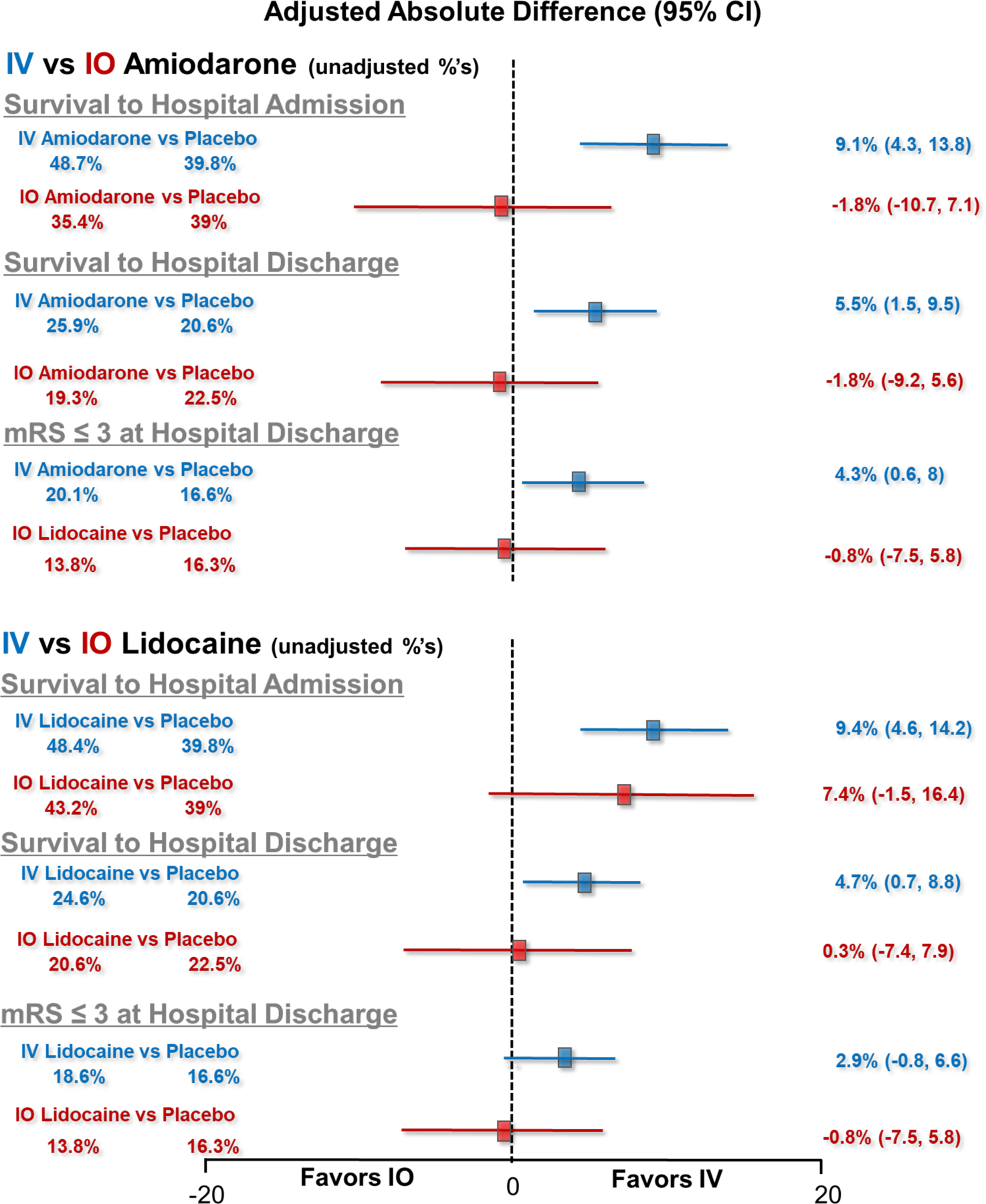
Unadjusted and adjusted absolute differences in survival to hospital admission, survival to hospital discharge and functional status (modified Rankin Scale ≤3) at hospital discharge. The left side of the figure describes the unadjusted percentages of patients who achieved the described endpoints. The forest plot and numerical values on the right of each figure depict the adjusted absolute differences (with 95% confidence intervals) in the described endpoints. The upper forest plot depicts outcomes comparing IV amiodarone vs placebo and IO amiodarone vs placebo. The lower plot depicts outcomes comparing IV lidocaine vs placebo and IO lidocaine vs placebo. Significant differences in survival to hospital admission, to hospital discharge and favoring improved functional status at hospital discharge were observed in association with IV administration of amiodarone and lidocaine compared to placebo, but not when these drugs were administered IO. A statistically significant interation between the route of drug administration and outcome was not found.
Secondary Outcomes
In both unadjusted and adjusted analyses, amiodarone and lidocaine were each associated with a significantly greater likelihood of survival to hospital admission than placebo when administered IV but not when given IO (Table 3, Figure 2). A concordant trend toward a more favorable neurological recovery at hospital discharge with IV but not IO administration of these drugs was also observed, but the adjusted difference for this endpoint between IV lidocaine and placebo was less robust than for IV amiodarone versus placebo, but was not statistically significant (p=0.13). A significant interaction between the route of vascular access and study drug was also not observed for either secondary outcome (p=0.11, p=0.47 respectively).
IV vs IO placebo
In addition to comparing active drugs (amiodarone or lidocaine) against placebo when given IV and IO, placebo was also compared directly against itself by the route of administration to distinguish whether receipt of IV or IO access might itself bear an association with outcome. There was no association between receipt of placebo by IV versus IO access and primary or secondary study outcomes. This included survival to hospital discharge (20.6% versus 22.5% for IV versus IO recipients respectively, adjusted relative risk 0.93 (95% CI 0.71, 1.21), p=0.6); admission alive to hospital (39.8% vs 39%, adjusted relative risk 1.02 (95%CI 0.85, 1.23), p=0.83) and survival with favorable neurological status at discharge (16.6% vs 16.3%, adjusted relative risk 0.92 (95% CI 0.66, 1.27), p=0.6). Similarly, there were no significant adjusted absolute risk differences for these comparisons between IV and IO placebo (data not shown).
Adverse events
Expected adverse effects, including acute thrombophlebitis, anaphylaxis, seizure activity or need for acute cardiac pacing was overall small and did not differ between IV versus IO access groups among patients randomized to amiodarone, lidocaine or placebo (Supplemental Table 2).
DISCUSSION
In this pre-specified analysis of a randomized, placebo-controlled clinical trial of antiarrhythmic drug treatment for refractory VF/VT OHCA, we found that amiodarone and lidocaine were each associated with significantly increased survival to hospital admission and discharge compared to placebo, and favored an improved neurological outcome at hospital discharge when the drugs were administered IV. Conversely, we did not observe significant differences in these endpoints between amiodarone or lidocaine compared to placebo when administered IO. Though underpowered for and mitigated by inconclusive evidence for an interaction between the mode of drug delivery and outcome, the consistency of these findings across multiple outcome measures and drugs signals the potential importance of the route of vascular access for drug treatment in OHCA and how this might influence treatment effectiveness.
Previous studies
The pharmacokinetic profiles and acute effects of parenteral antiarrhythmic drugs used for the treatment of OHCA have mainly been described after IV administration.11,12,13,14 However, successful IV cannulation can be challenging when the quality of patients’ peripheral vasculature is poor or access compromised by poor perfusion. In such instances, IO access provides an alternate means of vascular access via non-collapsible venous plexi within the bone marrow space; the proximal tibia ultimately draining to the popliteal vein, and the proximal humerus to the axillary vein.15,16 Comparable concentrations and physiologic responses to a number of drugs given by an IO or IV route can be achieved under normal circulatory conditions,17 but with less certainty in animal models under conditions of hypovolemic and electrically induced VF/VT cardiac arrest that required CPR.18, 19, 20, 21, 22, 23, 24, 25, 26 The critical question is whether IV and IO approaches to vascular access are equally effective in patients with clinical OHCA?
Thus far clinical studies have not resolved this issue, having reported both similar as well as concerning disparities in outcomes between IV and IO drugs such as epinephrine when administered to patients during OHCA.6, 7, 8, 27 None to date has specifically addressed the clinical use of IO antiarrhythmic drugs during cardiac arrest, nor in context of a placebo control. As such, the observations that emerge from the present study have importance in adding new knowledge and potential concerns about the “equivalence” of IO as compared with IV access in patients with OHCA and whether drug effectiveness could be also affected by the choice of vascular access.7
Notably, the parent trial (ALPS) from which this pre-designated analysis was drawn found no significant improvement in survival between amiodarone or lidocaine and placebo in its overall population of randomized patients (comprised of IV and IO drug recipients).1 As a result, there is waning enthusiasm in the resuscitation community for the use of these drugs altogether in cardiac arrest based on this “negative” trial. Findings from the current study differentiated the potential effects of these drugs depending on how they were given during the parent trial. Such stratification demonstrated a significant improvement in survival over placebo associated with IV administration of amiodarone or lidocaine (resulting in an absolute improvement in survival of 5.5% and 4.7%, corresponding to risk ratios of 1.26 and 1.21, respectively). This survival benefit might have been offset by the null association when these medications were given IO, producing a smaller and statistically insignificant net survival difference in the combined population of treated patients.1 From this perspective, the actual life-saving potential of amiodarone and lidocaine may have been concealed by the manner in which they were administered in the parent trial and potentially correctable in clinical practice.
Mechanisms
The relationship between the effectiveness of a drug and its route of administration is complex. If the differences in outcome observed in the present study are actual, it is possible that IO drug administration may be attenuated by local absorption related to the physical and chemical characteristics of the agent. For example, lidocaine is well recognized as an effective local anesthetic and is commonly used to minimize pain during IO drug administration28 suggesting some degree of local absorption of the drug in the periosteum; whereas amiodarone being more lipophilic29 would be expected to be even more highly absorbed in fatty marrow. A reduction in drug dose resulting from such absorption when given IO might be manifested in an attenuated antiarrhythmic drug effect compared to IV administration. Even if attenuated differentially by periosteum or marrow absorption, the net effect was nonetheless sufficient to mitigate the apparent effectiveness of both drugs by comparison with placebo when given IO.
Alternatively, the anatomic site of administration (upper versus lower body) could itself play a role in a drug’s effective delivery to the heart during active CPR, independent of whether it is given IV or IO. Experimental work has shown drugs achieve a delayed time-to-peak as well as lower peak concentrations in the heart when given via an IV route that reaches the heart via the inferior vena cava (IVC) rather than the superior vena cava (SVC) during active CPR.30 These pharmacokinetic differences were also seen in an animal model of cardiac arrest when epinephrine was given by tibial IO compared to a peripheral IV route, but notably not when comparing humeral IO with peripheral IV administration.31 This phenomenon, which is not observed during spontaneous circulation, may be attributable to the presence of venous valves in the region of the SVC as contrasted with their absence in the IVC. Closure of these valves in response to the high intrathoracic pressures achieved during the compression phase of CPR can minimize regurgitant blood flow that might otherwise oppose venous blood return and drug delivery from upper extremity veins draining to the heart via the SVC during CPR.32 Such is not the case for lower extremity vessels draining to the heart via the IVC, where, lacking such valves, venous blood return and drug delivery to the heart can be impeded during CPR.33 This phenomenon could explain the apparent effectiveness of IO administered drugs observed under normal circulatory conditions, in contrast to cardiac arrest with ongoing CPR. If true, the preferential selection of an upper extremity for drug administration (such as the proximal humerus or sternum when other IV access is not feasible) might address the possible limitations associated with a lower extremity site (such as the tibial IO) observed in this study during active CPR.34, 35 Other considerations might also apply. For example, blood flow to bone marrow (and other peripheral body compartments) may be diminished during circulatory shock or in response to vasopressors, requiring a fluid bolus and/or continuously pressurized infusion to facilitate egress of an IO administered drug into the central circulation during CPR.36 Unrecognized misplacement of the IO access device could have also contributed to poor drug delivery by this route. Such factors, whether alone or taken in combination, could potentially account for the attenuated clinical effects associated with receipt of an active antiarrhythmic drug IO that were observed in this study.
These potential mechanisms, while speculative, lend biological support and plausibility to the findings from this and previous cited studies as to whether and why differences in outcome might be expected between IV and IO drug administration during ongoing CPR.
LIMITATIONS
The study results are consistent across primary and secondary outcomes with its a-priori hypothesis, but should be interpreted cautiously. Though pre-specified and derived from a blinded, placebo-controlled trial of antiarrhythmic drug treatment, the route of drug administration was not randomized, and a small proportion of patients had incomplete data. Thus, unmeasured confounders could also explain the results. The study was not designed nor sufficiently powered to detect interactions and none was found, rendering an effect modification attributable to the IV versus IO route of drug administration inconclusive. There are nonetheless valid reasons to consider an association between access route and clinical outcome, apart from risk of a Type II (false negative) error due to an underpowered study that might have obscured a true interaction. Among these, the analysis itself was pre-specified, its findings are biologically plausible, supported by other work challenging the efficacy of IO drug administration and were consistent across multiple pre-designated outcomes for two different drugs in both unadjusted and adjusted analyses. The evaluation was also conducted in context of a placebo control. The resulting null association across all study endpoints when comparing receipt of placebo by an IV versus IO route also suggests that neither the underlying morbidity of the patient nor other factors that might have been involved in the site’s selection rendered the access route itself a surrogate marker of outcome. Furthermore, while strengthening study findings, the presence of a significant statistical interaction would not obviate the need for their prospective confirmation.
The study also did not evaluate other aspects of drug delivery such as administration techniques (rapidity of study drug injection, whether accompanied by fluid boluses or a pressurized infusion), which may have differentially affected route-specific drug effects Nor were there sufficient patients in tibial IO versus humeral IO treatment arms to test the mechanistic hypothesis related to the location of vascular access proposed in this analysis. In addition, the commercially available formulation of amiodarone (branded Nexterone) used in ALPS is electrophysiologically identical to amiodarone, but differs from the more widely used generic product with regard to its solvent properties (Captisol versus polysorbate 80), which may have resulted in differing IV/IO performance. These limitations should be balanced against the study’s strengths, including its pre-specified design, prospective data collection, multicenter performance, relatively large size and unique inclusion of a placebo control in its assessment of IV vs IO outcomes.
IMPLICATIONS
The findings from this study are observational and not clinically definitive, but nonetheless provocative. They suggest that amiodarone and lidocaine might each be life-saving drugs in patients with shock-refractory OHCA, though only when given intravenously. Confirming this hypothesis, could have an important public health impact, if, as in the present study, it might be found to spare 1 additional life for every 20 patients treated for shock-refractory OHCA.
CONCLUSION
In this subgroup analysis of the ALPS trial, we found no significant effect modification by drug administration route for amiodarone or lidocaine compared to placebo. However, point estimates for the effects of both drugs compared to placebo were greater for the IV than IO route for all outcomes in both unadjusted and adjusted analyses. This included a significant improvement in adjusted survival to hospital admission, survival to hospital discharge and favored an improved neurological outcome at hospital discharge, but only when these drugs were given IV. Given that the study was underpowered to statistically assess interactions, these findings signal the potential importance of the drug administration route during resuscitation and an opportunity to improve outcome from OHCA that merits further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
This study was presented in abstract form at the American Heart Association Resuscitation Science Symposium Sessions, November 2018 and the National Association of EMS Physicians Annual Meeting in January 2019.
We wish to acknowledge and thank all the participating EMS personnel, agencies and medical directors, as well as all hospitals that cared for patients, collected and contributed data for this study along with site investigators and staff. Participating sites and leadership included:
Alabama Resuscitation Center, University of Alabama at Birmingham, Birmingham, AL: Jeffrey D. Kerby, MD, PhD, Principal Investigator; Henry E. Wang, MD, MS, Principal Investigator
University of British Columbia, Vancouver, BC: James Christenson, MD, Principal Investigator
Dallas Center for Resuscitation Research, University of Texas Southwestern Medical Center, Dallas, TX: Ahamed H. Idris, MD, Principal Investigator
Milwaukee Resuscitation Research Center, Medical College of Wisconsin, Milwaukee, WI: Tom P. Aufderheide, MD, Principal Investigator
Ottawa/OPALS RCC, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario: Ian Stiell, MD, Principal Investigator
Portland Resuscitation Outcomes Consortium, Oregon Health and Science University, Portland, OR: Mohamud R. Daya, MD, MS, Principal Investigator
UCSD-San Diego Resuscitation Research Center, University of California at San Diego, San Diego, CA: Daniel Davis, MD, Principal Investigator
Seattle-King County Center for Resuscitation Research at the University of Washington, University of Washington, Seattle, WA: Peter J. Kudenchuk, MD, Principal Investigator
Toronto Regional Resuscitation Research Out of Hospital Network (Toronto Regional RescuNET), University of Toronto, Toronto, Ontario, Canada: Laurie J. Morrison, MD, MSc, FRCPC, Principal Investigator
Steering Committee
Chair: Myron Weisfeldt, MD, Johns Hopkins University School of Medicine, Baltimore, MD
Co-Chair-Cardiac: Joseph P. Ornato, MD, Virginia Commonwealth University Health System, Richmond, VA
National Heart, Lung, and Blood Institute, Bethesda, MD: George Sopko, MD, MPH; Debra Egan, MPH; David Lathrop, PhD; Patrice Desvigne Nickens, MD; Colin Wu, PhD; Phyllis Mitchell, PhD; Monica Shah, MD; Ellen Rosenberg, BSN, MHA; Gail Pearson, MD
Clinical Trial Center, University of Washington, Seattle, WA: Susanne May, PhD; Graham Nichol, MD, MPH; Judy Powell, BSN; Rob Schmicker, MS; Brian Leroux, PhD; Siobhan Brown, PhD; Heather Herren, RN, MPH; Katy Sims, RN, MN; Scott Emerson, PhD; Amy Gest, MPA; Gerald van Belle, PhD; Jonas Carson; Wienwipa Kirdpoo, BS; Ben Bergsten-Buret; Richard Moore, BS; Jackie Berhorst; David Prince, MS; Cesar Torres; Erin Case; Danielle Guffey, MS; Brittany Sanchez; Leila Zelnick, MS; Sean M. Devlin, PhD; Lois Van Ottingham, BSN; Gena Sears, BSN
SOURCES OF FUNDING
The Resuscitation Outcomes Consortium was supported by a series of cooperative agreements to nine regional clinical centers (spanning 10 North American communities) and one Data Coordinating Center (HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas Southwestern Medical Center/Dallas, HL077908-University of California San Diego) from the National Heart, Lung, and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Non-Standard Abbreviations and Acronyms
- AHA
American Heart Association
- ALPS
Amiodarone, Lidocaine or Placebo Study
- CI
confidence interval
- CPR
cardiopulmonary resuscitation
- EMS
Emergency Medical Services
- FDA
Federal Food and Drug Administration
- IO
intraosseous
- IV
intravenous
- IVC
inferior vena cava
- mRS
modified Rankin Scale
- OHCA
out-of-hospital cardiac arrest
- ROC
Resuscitation Outcomes Consortium
- RRadj
Adjusted relative risk
- SVC
superior vena cava
- VF/VT
Ventricular fibrillation or pulseless ventricular tachycardia
Footnotes
DISCLOSURES
None
References
- 1.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW et al. Amiodarone, lidocaine or placebo in out of hospital cardiac arrest. N Engl J Med 2016;374:1711–1722. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Part 7.2 Management of cardiac arrest. Circulation 2005;112 (issue no. 24, suppl): IV58–IV66. [DOI] [PubMed] [Google Scholar]
- 3.Leidel BA, Kirchhoff C, Bogner V, Stegmaier J, Mutschler W, Kanz KG, Braunstein V. Is the intraosseous access route fast and efficacious compared to conventional central venous catheterization in adult patients under resuscitation in the emergency department? Patient Saf Surg 2009;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs S, LaCovey D, Paris P. A prehospital model of intraosseous infusion. Ann Emerg Med 1991;20;371–374. [DOI] [PubMed] [Google Scholar]
- 5.Reades R, Studnek JR, Vandeventer S, Garrett J. Intraosseous versus intravenous vascular access during out of hospital cardiac arrest: A randomized controlled trial. Ann Emerg Med 2011;58:509–16. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein BA, Stubbs BA, Rea T, Kudenchuk PJ. Intraosseous compared to intravenous drug resuscitation in out of hospital cardiac arrest. Resuscitation 2017;117:91–96. [DOI] [PubMed] [Google Scholar]
- 7.Kawano T, Brunae B, Scheuermeyer FX, GIbo K, Fordyce CB, Lin S, Stenstrom R, Schlamp R, Jenneson S, Christenson J. Intraosseous vascular access is associated with lower survival and neurologic recovery among patients with out of hospital cardiac arrest. Ann Emerg Med 2018;71:588–596. [DOI] [PubMed] [Google Scholar]
- 8.Mody P, Brown SP, Kudenchuk PJ, Chan PS, Khera R, Ayers C, Pandey A, Kern KB, deLemos JA, Link MS et al. Intraosseous versus intravenous access in patients with out of hospital cardiac arrest. Resuscitation 2019;134:69–75. [DOI] [PubMed] [Google Scholar]
- 9.Kudenchuk PJ, Brown SP, Daya M, Morrison LJ, Grunau BE, Rea T, Aufderheide T, Powell J, Leroux B, Vaillancourt C et al. Resuscitation Outcomes Consortium Amiodarone, Lidocaine or Placebo Study (ROC-ALPS): Rationale and methodology behind an out-of-hospital cardiac arrest antiarrhythmic drug trial. Am Heart J 2014;167:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner JC, Luo Z, Roman LA. Fixed effects, random effects and GEE: what are the differences? Statistics in Medicine 2009;28:221–239. [DOI] [PubMed] [Google Scholar]
- 11.Collinsworth KA, Kalman SM, Harrison DC. The clinical pharmacology of lidocaine as an antiarrhythmic drug. Circulation 1974;50:1217–1229. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt DJ, Bolognini V, Koch-Weser J, Harmatz JS. Phamacokinetic approach to the clinical use of lidocaine intravenously. JAMA 1976;236:273–277. [PubMed] [Google Scholar]
- 13.Riva E, Gerna M, Laini R, GIani P, Volpi A, Maggioni A. Pharmacokinetics of amiodarone in man. J Cardiovasc Pharm 1982;4:264–269. [DOI] [PubMed] [Google Scholar]
- 14.Cushing DJ, Adams MP, Cooper WD, Kowey PR, Lipicky RJ. Bioequivalence of two intravenous amiodarone formulations in healthy participants. J Clin Pharm 2009;49:407–415. [DOI] [PubMed] [Google Scholar]
- 15.Laroche M Intraosseous circulation from physiology to disease. Joint Bone Spine 2002;69:262–269. [DOI] [PubMed] [Google Scholar]
- 16.Paxton JH. Intraosseous vascular access. Trauma 2012;14:195–232. [Google Scholar]
- 17.Orlowski JP, Porembka DT, Gallagher JM, Lockrem JD, VanLente F. Comparison study of intraosseous, central intravenous and peripheral intravenous infusions of emergency drugs. Am J Dis Child 1990;144:112–117. [DOI] [PubMed] [Google Scholar]
- 18.Burgert J, Gegel B, Loughren M, Cerenuga T, Desai M, Schlicher M, O’Sullivan J, Lewis P, Johnson D. Comparison of tibial intraosseous, sternal intraosseous, and intravenous routes of administration on pharmacokinetics of epinephrine during cardiac arrest: a pilot study. Am Assoc Nurse Anesth J 2012;80(August (4 Suppl.)):S6–10. [PubMed] [Google Scholar]
- 19.Mader TJ, Coute Ryan A, Kellog AR, Harris JL. Coronary perfusion pressure response to high dose intraosseous versus standard dose intravenous epinephrine administration after prolonged cardiac arrest. Open J Emerg Med 2014;2:1–7. [Google Scholar]
- 20.Hampton K, Wang E, Argame JI, Bateman T, Craig W, Johnson D. The effects of tibial intraosseous versus intravenous amiodarone administration in a hypovolemic cardiac arrest porcine model. Am J Disaster Med 2016;11:253–260. [DOI] [PubMed] [Google Scholar]
- 21.Hoskins SL, do Nascimento P Jr, Lima RM, Espana-Tenorio JM, Kramer GC. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation. 2012;83:107–112. [DOI] [PubMed] [Google Scholar]
- 22.Zuercher M, Kern KB, Indik JH, Loedl M, Hilwig RW, Ummenhofer W, Berg RA, Ewy GA. Epinephrine improves 24-hour survival in a swine model of prolonged ventricular fibrillation demonstrating that early intraosseous is superior to delayed intravenous administration. Anesth Analg. 2011;112:884–890. [DOI] [PubMed] [Google Scholar]
- 23.Spivey WH, Crespo SG, Fuhs LR, Schoffstall JM. Plasma catecholamine levels after intraosseous epinephrine administration in a cardiac arrest model. Ann Emerg Med 1992;21:127–131. [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan M, Martinez A, Long A, Johnson M, Blouin D, Johnson AD, Burgert JM. Comparison of the effects of sternal and tibial intraosseous administered resuscitative drugs on return of spontaneous circulation in a swine model of cardiac arrest. Am J Dis Med J 2016;11:175–182 [DOI] [PubMed] [Google Scholar]
- 25.Wong MR, Reggio MT, Morocho FR, Holloway MM, Garcia-Blanco JC, Jenkins C, Johnson AD. Effects of introsseous epinephrine in a cardiac arrest swine model. J Surg Res 2016;201:327–333. [DOI] [PubMed] [Google Scholar]
- 26.Burgert JM, Austin PN, Johnson A. An evidence based review of epinephrine administered via the intraosseous route in animal models of cardiac arrest. Mil Med 2014:179:99–104. [DOI] [PubMed] [Google Scholar]
- 27.Clemency B, Tanaka K, May P, Innes J, Zagroba S, Blaszak J, Hostler D, Cooney D, McGee K, Lindstrom H. Intravenous vs intraosseous access and return of spontaneous circulation during out of hospital cardiac arrest. Am J Emerg Med 2017;35:222–226. [DOI] [PubMed] [Google Scholar]
- 28.Ilicki J, Scholander J. Lidocaine can reduce the pain of intra-osseous fluid infusion. Crit Care 2016;230:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyss PA, Moor MJ, Bickel MH. Single dose kinetics of tissue distribution, excretion and metabolism of amiodarone in rats. J Pharmacol Exper Therap 1990;254:502–507. [PubMed] [Google Scholar]
- 30.Dalsey WC, Barsan WG, Joyce SM, Hedges JR, Lukes SJ. Comparison of superior vena caval and inferior vena caval access using a radioisotope techniqueduring normal perfusion and cardiopulmonary resuscitation. Ann Emerg Med 1984;13:881–884. [DOI] [PubMed] [Google Scholar]
- 31.Burgert JM, Johnson AD, O’Sullivan JC, Blalock WJ, Duffield BC, Albright BP, Herzog CC, Moore MS, Dempster KS, Rauch JW. Pharmacokinetic effects of endotracheal, intraosseous and intravenous epinephrine in a swing model of traumatic cardiac arrest. Am J Emerg Med 2019; doi: 10.1016/j.ajem.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Niemann JT, Rosborough J, Hausknecht M, Garner D, Criley JM. Blood flow without cardiac compression during closed chest CPR. Crit Care Med 1981;9:380–381. [DOI] [PubMed] [Google Scholar]
- 33.Fisher J, Vaghaiwalla F, Tsitlik, Levin H, Brinker J, Weidfeldt M, Yin F. Determinants and clinical significance of jugular venous valve competence. Circulation 1982;65:188–196. [DOI] [PubMed] [Google Scholar]
- 34.Burgert JM, Johnson AD, Garcia-Blanco J, Fulton LV, Loughren MJ. The resuscitative and pharmacokinetic effects of humeral intraosseous vasopressin in a swine model of ventricular fibrillation. Prehosp Disaster Med 2017;32:1–6. [DOI] [PubMed] [Google Scholar]
- 35.Burgert JM, Martinez A, O’Sullivan M, Blouin D, Long A, Johnson AD. Sternal route more effective than tibial route for intraosseous amiodarone administration in a swine model of ventricular fibrillation. Prehosp Emerg Care 2018;22:266–275. [DOI] [PubMed] [Google Scholar]
- 36.Voelkel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, Wenzel V, Lindner KH. Comparison of epinephrine with vasopressin on bone marrow blood flow in animal model of hypovolemic shock and subsequent cardiac arrest. Crit Care Med 2001;29;1587–1592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


