Teylan et al. reveal slower cognitive decline in primary age-related tauopathy (PART) than in Alzheimer’s disease across multiple neuropsychological domains, lending further support to the hypothesis that PART has distinct clinical features compared to Alzheimer’s disease.
Keywords: primary age-related tauopathy, Alzheimer’s disease, cognitive decline, neuropsychology, neuropathology
Abstract
Primary age-related tauopathy is increasingly recognized as a separate neuropathological entity different from Alzheimer’s disease. Both share the neuropathological features of tau aggregates and neuronal loss in the temporal lobe, but primary age-related tauopathy lacks the requisite amyloid plaques central to Alzheimer’s disease. While both have similar clinical presentations, individuals with symptomatic primary age-related tauopathy are commonly of more advanced ages with milder cognitive dysfunction. Direct comparison of the neuropsychological trajectories of primary age-related tauopathy and Alzheimer’s disease has not been thoroughly evaluated and thus, our objective was to determine how cognitive decline differs longitudinally between these two conditions after the onset of clinical symptoms. Data were obtained from the National Alzheimer’s Coordinating Center on participants with mild cognitive impairment at baseline and either no neuritic plaques (i.e. primary age-related tauopathy) or moderate to frequent neuritic plaques (i.e. Alzheimer neuropathological change) at subsequent autopsy. For patients with Alzheimer’s disease and primary age-related tauopathy, we compared rates of decline in the sum of boxes score from the CDR® Dementia Staging Instrument and in five cognitive domains (episodic memory, attention/working memory, executive function, language/semantic memory, and global composite) using z-scores for neuropsychological tests that were calculated based on scores for participants with normal cognition. The differences in rates of change were tested using linear mixed-effects models accounting for clinical centre clustering and repeated measures by individual. Models were adjusted for sex, age, education, baseline test score, Braak stage, apolipoprotein ε4 (APOE ε4) carrier status, family history of cognitive impairment, and history of stroke, hypertension, or diabetes. We identified 578 participants with a global CDR of 0.5 (i.e. mild cognitive impairment) at baseline, 126 with primary age-related tauopathy and 452 with Alzheimer’s disease. Examining the difference in rates of change in CDR sum of boxes and in all domain scores, participants with Alzheimer’s disease had a significantly steeper decline after becoming clinically symptomatic than those with primary age-related tauopathy. This remained true after adjusting for covariates. The results of this analysis corroborate previous studies showing that primary age-related tauopathy has slower cognitive decline than Alzheimer’s disease across multiple neuropsychological domains, thus adding to the understanding of the neuropsychological burden in primary age-related tauopathy. The study provides further evidence to support the hypothesis that primary age-related tauopathy has distinct neuropathological and clinical features compared to Alzheimer’s disease.
Introduction
Primary age-related tauopathy (PART), a recently defined neuropathological category, is increasingly recognized in the scientific and medical community as distinct from Alzheimer’s disease (Jellinger, 2019). PART and Alzheimer’s disease share the neuropathological feature of tau deposits and the subsequent neurodegeneration of the temporal lobe; however, amyloid deposits, which are characteristic of the pathophysiology of Alzheimer’s disease, are absent in PART. The two conditions also have different clinical manifestations. PART is more commonly observed in older age groups and is less likely to lead to more severe cognitive dysfunction (Crary et al., 2014; Crary, 2016; Kryscio et al., 2016; Besser et al., 2017).
Thus far, few studies have examined other, more detailed, differences in the clinical manifestations of PART compared with the better studied entity of Alzheimer’s disease. In fact, antemortem diagnostic criteria have not yet been established for PART, and little is known about how PART is perceived in clinical practice. One study examined the presumptive primary and contributing diagnoses of cognitive impairment in individuals who met neuropathological criteria for PART at autopsy and found that while clinicians recognized a distinction in the clinical presentation between PART and Alzheimer’s disease, a clinical diagnosis of Alzheimer’s disease was given more than 50% of the time to PART participants (Teylan et al., 2019).
Determining the cognitive presentation as a result of the neuropathological burden occurring in PART, in comparison to Alzheimer’s disease, will increase our understanding of the condition and potentially aid in developing clinical diagnostic criteria. One study examining Mini-Mental State Examination scores suggested that cognition is relatively more preserved in PART compared with Alzheimer’s disease, even at higher Braak stages (Crary et al., 2014). Another showed that language/semantic memory is relatively preserved in PART compared with Alzheimer’s disease (Besser et al., 2019). Both of these studies examined their sample at a single time point within a year or two of death.
Otherwise, differences in neuropsychological outcome have not been well addressed, particularly with regards to cognitive changes over time. Bell et al. (2019) found significantly slower rates of decline on measures of memory, language, and visuospatial performance; however, their analysis was on a small cohort recruited from a single site (PART n = 34; Alzheimer’s disease n = 116). Thus, we sought to determine how the trajectories of neuropsychological test scores of individuals with mild cognitive impairment (MCI) differ for individuals with underlying PART neuropathological features compared with those of individuals with underlying Alzheimer’s disease neuropathological change in a large autopsy sample from across the USA. This is of scientific interest, as it increases our understanding of the cognitive consequences of these different neuropathological conditions. It is also of clinical interest, as it informs the prognosis that would be given to patients presenting with MCI, assuming that PART and Alzheimer’s disease could eventually be distinguishable antemortem.
Materials and methods
Data source
Data were obtained from the National Alzheimer’s Coordinating Center (NACC). The NACC is the repository for data collected at the Alzheimer’s Disease Centers (ADCs) located across the USA and is funded by the National Institute on Aging (NIA). The ADCs contribute standardized clinical data to the Uniform Data Set (UDS) and neuropathological evaluations obtained at autopsy to the Neuropathology Data Set. Each local ADC obtained written informed consent from participants enrolled to their centre, and study protocols were approved by the ADCs’ institutional review boards. Consent for autopsy was obtained for the subset of participants who contribute to the Neuropathology Data Set. Research using the NACC database was approved by the University of Washington Institutional Review Board. The UDS and Neuropathology Data Set data have been described in detail previously (Morris et al., 2006; Beekly et al., 2007; Weintraub et al., 2009, 2018; Besser et al., 2018).
Study sample
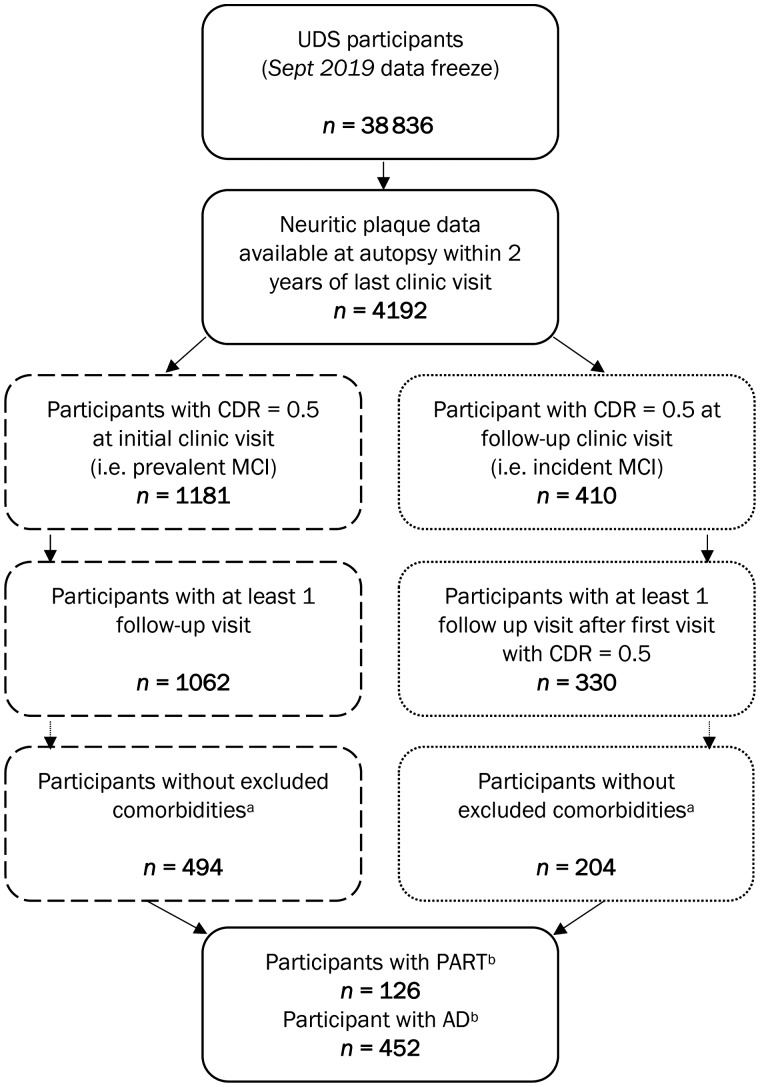
The analytical sample was extracted from the September 2018 data freeze, which included 4192 participants with clinical UDS data within 2 years from death and neuritic plaque burden assessed at autopsy (Fig. 1). We restricted our sample to participants who had prevalent MCI, which we defined as participants with a CDR® Dementia Staging Instrument (CDR) global score of 0.5 at their initial UDS visit (n = 1181), or participants with incident MCI who had CDR = 0.5 at a follow-up UDS visit (n = 410). Participants were excluded if they did not have at least two visits after their first visit with MCI (i.e. their baseline visit), resulting in 1062 prevalent MCI and 330 incident MCI cases. Given the paucity of studies to date, we focused on PART in its purest form by applying a number of exclusions to ensure that we were assessing PART, and not other pathologies or diseases that might obscure our findings and impede our ability to make definitive conclusions. Hence, we also excluded participants who had (i) neuropathological evidence of frontotemporal lobar degeneration (FTLD), Lewy bodies, amyotrophic lateral sclerosis (ALS), prion disease, or argyrophilic grains at autopsy; (ii) clinical evidence of dementia with Lewy bodies (DLB), Parkinson’s disease, Down syndrome, Huntington’s disease, prion disease, corticobasal degeneration (CBD), or progressive supranuclear palsy (PSP) at their most recent UDS visit prior to autopsy; or (iii) sparse neuritic plaques at autopsy. Limbic-predominant age-related TDP-43 encephalopathy (LATE) was not excluded. After applying these criteria, we combined the prevalent and incident MCI cases, resulting in a sample of 126 PART (i.e. no neuritic plaques) and 452 Alzheimer’s disease (i.e. moderate to frequent neuritic plaques) participants.
Figure 1.
Sample size flow chart. aExcluding neuropathological evidence of FTLD, Lewy bodies, ALS, prion disease, or argyrophilic grains, clinical evidence of DLB, Parkinson disease, Down syndrome, Huntington disease, prion disease, corticobasal syndrome, or progressive supranuclear palsy. bExcluding participants with sparse neuritic plaques; PART defined as no neuritic plaques; Alzheimer’s disease defined as moderate or frequent neuritic plaques.
Cognitive measurements
Participants were assessed using the CDR and the UDS version 2 neuropsychological test battery (Weintraub et al., 2009). CDR is a required assessment at each UDS visit, and thus all eligible visits were included in the analysis of changes in CDR over time. The UDS version 2 battery includes the Logical Memory Immediate and Delayed Recall tests, Digit Span Forward and Backward tests, the Boston Naming Test, animal and vegetable naming tests, the Wechsler Adult Intelligence Scale-Revised Digit Symbol test (WAIS-R Digit Symbol), and Trail Making tests A and B. Z-scores for each test were calculated by subtracting the score from the mean test score and dividing it by the standard deviation of all UDS initial visit scores among cognitively normal subjects (i.e. CDR = 0). Tests were grouped by cognitive domains (i.e. episodic memory, attention/working memory, language/semantic memory, executive function; Table 1), which were established by Hayden et al. (2011) using factor analysis, and z-scores for the tests within a domain were then averaged to calculate a domain z-score. A global composite score was created by averaging the domain z-scores. The UDS neuropsychological test battery was revised, and version 3 was implemented at the ADCs in March 2015 (Weintraub et al., 2018). Models examining the cognitive domain z-scores excluded visits in which a participant was tested using the UDS version 3 battery.
Table 1.
Cognitive domains assessed in UDS neuropsychological test battery and sample size by domain z-score
| Domain | Neuropsychological test | Sample size, n (%) | ||
|---|---|---|---|---|
| Total n = 578 | PART n = 126 | AD n = 452 | ||
| Global composite | All tests | 368 (63.7) | 85 (67.5) | 283 (62.6) |
| Episodic memory | Logical Memory Immediate Recall | 477 (82.5) | 106 (84.1) | 371 (82.1) |
| Logical Memory Delayed Recall | ||||
| Attention/working memory | Digit Span Forward - Correct Trails | 487 (84.3) | 105 (83.3) | 382 (84.5) |
| Digit Span Forward - Length | ||||
| Digit Span Backward - Correct Trails | ||||
| Digit Span Backward - Length | ||||
| Semantic memory/ language | Animal Naming | 475 (82.2) | 102 (81.0) | 373 (82.5) |
| Vegetable Naming | ||||
| Boston Naming Test | ||||
| Executive function | WAIS-R Digit Symbol | 382 (66.1) | 88 (69.8) | 294 (65.0) |
| Trail Making A | ||||
| Trail Making B | ||||
AD = Alzheimer’s disease.
Statistical analysis
To identify differences between groups and potential confounders, chi-square, Fisher’s exact tests, and t-tests were applied to compare the demographic characteristics, clinical and neuropathological features, and cognitive scores at the baseline visit for the PART and Alzheimer’s disease groups. Rate of cognitive decline was estimated by using linear mixed models with random effects for repeated measures by participant and for clustering of participants by ADC. Time was measured as years since baseline visit, or more specifically, initial UDS visit for prevalent MCI cases and first UDS visit with CDR = 0.5 for incident MCI cases. Separate unadjusted and adjusted models were run for CDR sum of boxes (CDR-SB), each domain score, and the global composite score. The adjusted models included covariates that are known confounders in Alzheimer’s disease and related dementias research or were found to have different distributions between our PART and Alzheimer’s disease samples. Covariates included in the adjusted models were sex, age at baseline visit, education in years, baseline test score, Braak stage at autopsy, APOE ε4 carrier status, family history of cognitive impairment, and history of stroke, hypertension, or diabetes. Several sensitivity analyses were run to examine factors that may have influenced our results. These findings are addressed below, and the corresponding table is available in the Supplementary material. All analyses were run using SAS version 9.4.
Data availability
The data that support the findings of this study are available on request from NACC at https://www.alz.washington.edu/.
Results
We identified 126 PART and 452 Alzheimer’s disease autopsy-confirmed participants with CDR = 0.5 at their baseline visit and longitudinal cognitive measures. These two groups did not differ significantly by age at baseline, at last UDS visit, or at death, but participants with PART were found to have an older age of onset of cognitive decline compared to Alzheimer’s disease (Table 2). PART and Alzheimer’s disease groups were not significantly different by years between visits, sex, years of education, race, history of depression, or history of traumatic brain injury. Alzheimer’s disease participants were followed for more visits on average (Alzheimer’s disease 4.9 visits; PART 4.5 visits) than those with PART, which also translates into longer follow-up time (i.e. 4.6 versus 4.0 years, respectively). Alzheimer’s disease participants were more likely to have a higher Braak stage, with 27.7% of the sample having Braak stage V and 41.4% with Braak stage VI, compared to only one PART participant with either Braak stage V or VI. Forty-nine per cent of the Alzheimer’s disease group were APOE ε4 carriers compared to only 14% of the PART sample, and family history of cognitive impairment was also higher in the Alzheimer’s disease group (62% Alzheimer’s disease versus 44% PART). History of stroke, hypertension, and diabetes, as well as neuropathological features of vascular brain injury (i.e. presence at autopsy of haemorrhage, microbleed, infarct/lacune, or microinfarct), were more common in the PART group (Table 2).
Table 2.
Demographic, clinical, and neuropathological characteristics by PART versus Alzheimer’s disease at baseline visit
| Characteristic | PART | AD | P-value |
|---|---|---|---|
| Sample size | 126 | 452 | |
| Number of visits, mean (SD) | 4.5 (2.2) | 4.9 (2.2) | 0.047 |
| Follow up years, mean (SD) | 4.0 (2.5) | 4.6 (2.4) | 0.03 |
| Years between visits, mean (SD) | 1.2 (0.4) | 1.2 (0.5) | 0.62 |
| Mode of onset of cognitive symptomsa, n (%) | <0.001 | ||
| No impairment in cognition | 28 (22.6) | 21 (4.7) | |
| Gradual | 89 (71.8) | 414 (92.0) | |
| Subacute | 3 (2.4) | 7 (1.6) | |
| Abrupt | 3 (2.4) | 8 (1.8) | |
| Other | 1 (0.8) | 0 (0.0) | |
| Age, onset of cognitive decline, mean (SD) | 79.8 (13.4) | 75.8 (11.3) | 0.007 |
| Age, at baseline visit, mean (SD) | 80.1 (11.6) | 78.2 (10.0) | 0.09 |
| Age, at last UDS visit, mean (SD) | 85.4 (12.2) | 83.3 (10.1) | 0.09 |
| Age, at death, mean (SD) | 86.1 (12.2) | 84.1 (10.1) | 0.09 |
| Male sex, n (%) | 68 (54.0) | 231 (51.1) | 0.57 |
| Education, mean (SD) | 15.2 (2.7) | 15.6 (3.0) | 0.11 |
| Non-white race, n (%) | 8 (6.4) | 24 (5.4) | 0.65 |
| Braak stage, n (%) | <0.001 | ||
| None | 16 (12.9) | 0 (0.0) | |
| I | 28 (22.6) | 5 (1.1) | |
| II | 43 (34.7) | 26 (5.8) | |
| III | 13 (10.5) | 36 (8.0) | |
| IV | 23 (18.6) | 73 (16.2) | |
| V | 0 (0.0) | 125 (27.7) | |
| VI | 1 (0.8) | 187 (41.4) | |
| Thal phaseb, n (%) | <0.001 | ||
| None | 40 (56.3) | 0 (0.0) | |
| 1 | 11 (15.5) | 3 (1.3) | |
| 2 | 6 (8.5) | 11 (4.8) | |
| 3 | 7 (9.9) | 38 (16.5) | |
| 4 | 4 (5.6) | 66 (28.6) | |
| 5 | 3 (4.2) | 113 (48.9) | |
| APOE ε4 carrier, n (%) | 16 (13.7) | 204 (48.8) | <0.001 |
| Family history of cognitive impairment, n (%) | 50 (43.5) | 256 (62.0) | <0.001 |
| History of stroke, n (%) | 21 (17.1) | 35 (7.8) | 0.002 |
| History of hypertension, n (%) | 85 (69.7) | 244 (54.3) | 0.002 |
| History of depression, n (%) | 45 (36.0) | 164 (36.8) | 0.87 |
| History of diabetes, n (%) | 21 (17.2) | 34 (7.6) | 0.001 |
| History of traumatic brain injury, n (%) | 13 (10.7) | 53 (11.8) | 0.72 |
| Vascular brain injury at autopsyc, n (%) | 60 (47.6) | 161 (35.8) | 0.02 |
P-value for Fisher’s exact test reported because 40% of the cells have expected counts <5. Mode of onset is assessed in a different manner from CDR global score, being based on the clinician judgement of symptoms.
Thal phase was not assessed in 43.7% of PART and 48.9% of Alzheimer’s disease participants.
Vascular brain injury is a binary variable that includes the presence of haemorrhage, microbleed, infarct/lacune, or microinfarct.
AD = Alzheimer’s disease; SD = standard deviation.
Despite restricting our sample to participants with MCI at baseline, indicated by a CDR of 0.5, we found that there was a significant difference in baseline CDR-SB between PART and Alzheimer’s disease (Table 3). Alzheimer’s disease participants performed worse than PART participants at baseline for CDR-SB. The Alzheimer’s disease group performed worse than those with PART in episodic memory, attention/working memory, semantic memory/language, and the global composite z-score. There was no difference at baseline between the two groups in executive function.
Table 3.
Baseline scores by PART versus Alzheimer’s disease in participants with MCI (CDR = 0.5) at baseline
| Score | Baseline scores | ||||
|---|---|---|---|---|---|
| PART | AD | P-value | |||
| n | Mean (SD) | n | Mean (SD) | ||
| CDR-SB | 126 | 1.30 (1.07) | 452 | 1.89 (1.21) | <0.001 |
| Global composite | 85 | −0.50 (0.71) | 283 | −0.84 (0.65) | <0.001 |
| Episodic memory | 106 | −0.46 (1.13) | 371 | −1.35 (1.22) | <0.001 |
| Attention/working memory | 105 | −0.21 (0.89) | 382 | −0.42 (0.83) | 0.04 |
| Executive function | 88 | −0.74 (0.89) | 294 | −0.89 (0.94) | 0.16 |
| Semantic memory/language | 102 | −0.65 (0.85) | 373 | −0.95 (0.85) | 0.002 |
AD = Alzheimer’s disease.
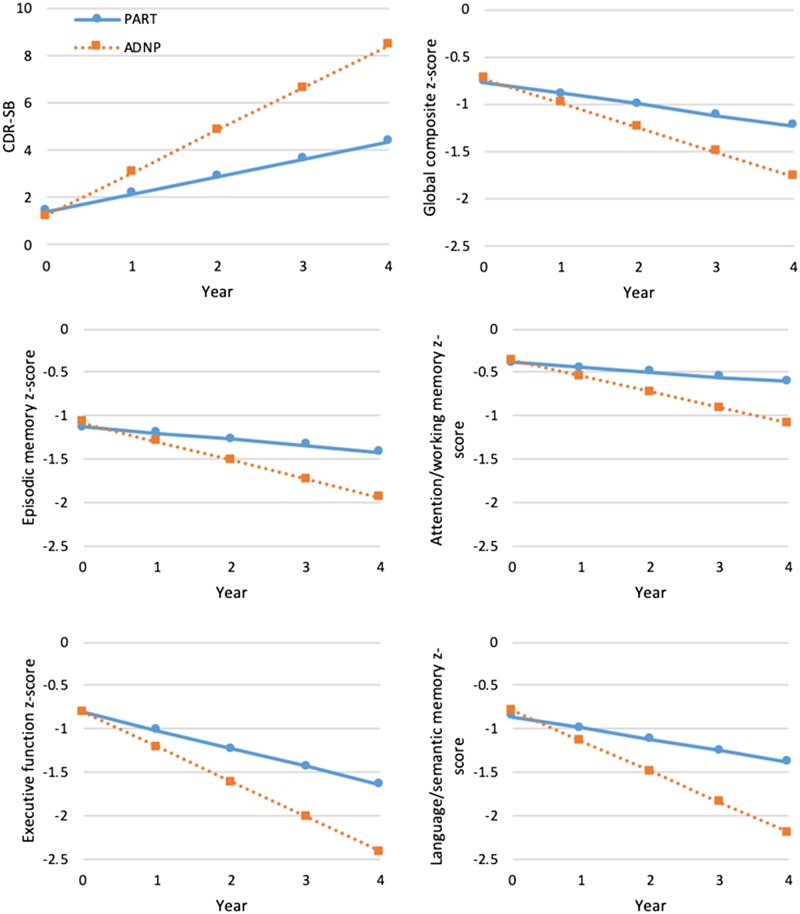
Approximately 74% of the Alzheimer’s disease group progressed to dementia by their last UDS visit (i.e. CDR ≥ 1), compared to 37% of PART participants (not shown in tables); however, this difference may be partially due to the fact that participants with Alzheimer’s disease had slightly longer follow-up than those with PART. Unadjusted models examining the annual rate of cognitive change reveal that both the PART and Alzheimer’s disease groups significantly declined in all cognitive measures (Table 4). The difference in slopes between the two groups was statistically significant for all cognitive measures, with participants with PART declining at a slower rate than those with Alzheimer’s disease. After adjusting for covariates, the results of the multivariable models were consistent with the unadjusted models (Table 5). PART and Alzheimer’s disease participants showed a significant decline for all domains, global composite, and CDR-SB; however, Alzheimer’s disease had a significantly steeper decline in all scores compared to PART (Fig. 2).
Table 4.
Unadjusted models by PART versus Alzheimer’s disease in participants with MCI (CDR = 0.5) at baseline
| Score | Number of participant visits | Annual rate of change (unadjusted) a | Annual difference between PART and AD b | ||
|---|---|---|---|---|---|
| PART | AD | ||||
| β est (95% CI) | β est (95% CI) | β est (95% CI) | P-value | ||
| CDR-SB | 2798 | 0.71 (0.49, 0.93) | 1.81 (1.63, 1.99) | 1.10 (0.89, 1.31) | <0.001 |
| Global composite | 1437 | −0.11 (−0.15, −0.08) | −0.25 (−0.28, −0.22) | −0.13 (−0.17, −0.10) | <0.001 |
| Episodic memory | 1989 | −0.07 (−0.12, −0.01) | −0.19 (−0.23, −0.16) | −0.13 (−0.19, −0.06) | <0.001 |
| Attention/working memory | 2058 | −0.06 (−0.10, −0.03) | −0.17 (−0.19, −0.15) | −0.11 (−0.14, −0.07) | <0.001 |
| Executive function | 1500 | −0.20 (−0.26, −0.14) | −0.39 (−0.42, −0.35) | −0.19 (−0.25, −0.12) | <0.001 |
| Semantic memory/language | 1991 | −0.13 (−0.16, −0.10) | −0.34 (−0.38, −0.30) | −0.21 (−0.26, −0.16) | <0.001 |
Interpretation: a negative β estimate and a 95% CI that does not include zero indicates significant decline with the exception of CDR-SB, where a positive β estimate and a 95% CI that does not include zero indicates significant decline.
Interpretation: a negative difference indicates PART is declining at a slower rate than Alzheimer’s disease except for CDR-SB where a positive difference indicates PART is declining at a slower rate than Alzheimer’s disease.
AD = Alzheimer’s disease; CI = confidence interval.
Table 5.
Adjusted models PART versus Alzheimer’s disease in participants with MCI (CDR = 0.5) at baseline
| Score | Number of participant visits | Annual rate of change (adjusted) a | Annual difference between PART and AD b | ||
|---|---|---|---|---|---|
| PART | AD | ||||
| β est (95% CI) | β est (95% CI) | β est (95% CI) | P-value | ||
| CDR-SB | 2424 | 0.74 (0.49, 0.98) | 1.80 (1.63, 1.96) | 1.06 (0.83, 1.29) | <0.001 |
| Global composite | 1306 | −0.12 (−0.16, −0.07) | −0.26 (−0.29, −0.23) | −0.14 (−0.19, −0.09) | <0.001 |
| Episodic memory | 1808 | −0.07 (−0.13, −0.01) | −0.21 (−0.26, −0.17) | −0.14 (−0.22, −0.06) | <0.001 |
| Attention/working memory | 1879 | −0.06 (−0.09, −0.02) | −0.18 (−0.21, −0.16) | −0.13 (−0.17, −0.09) | <0.001 |
| Executive function | 1365 | −0.21 (−0.28, −0.13) | −0.40 (−0.45, −0.36) | −0.19 (−0.28, −0.11) | <0.001 |
| Semantic memory/language | 1815 | −0.13 (−0.16, −0.10) | −0.35 (−0.39, −0.31) | −0.22 (−0.27, −0.17) | <0.001 |
Interpretation: a negative β estimate and a 95% CI that does not include zero indicates significant decline with the exception of CDR-SB, where a positive β estimate and a 95% CI that does not include zero indicates significant decline.
Interpretation: a negative difference indicates PART is declining at a slower rate than AD except for CDR-SB where a positive difference indicates PART is declining at a slower rate than AD.
Adjusting for sex, age at baseline, education, baseline test score Braak stage, APOE ε4 carrier status, family history of cognitive impairment, history of stroke, hypertension, or diabetes.
AD = Alzheimer’s disease; CI = confidence interval.
Figure 2.
Adjusted mean rate of change for primary age-related tauopathy versus Alzheimer’s disease. Plots correspond with results shown in Table 5. Slopes are significantly different between PART and Alzheimer’s disease for all plots (P < 0.001).
We examined whether the slopes of decline estimated in the models for the PART and Alzheimer’s disease groups were differentially influenced by our inclusion of both prevalent and incident MCI cases. Participants with prevalent MCI had a CDR = 0.5 at their initial UDS visit, and it is possible that there could be unmeasured differences between the time that they spent in the MCI state before enrolling in the UDS. In a sensitivity analysis we ran separate adjusted models for CDR-SB in the prevalent and incident MCI samples. We found that the prevalent MCI sample had a steeper decline compared to those with incident MCI. However, the difference in slopes between the PART and Alzheimer’s disease groups were comparable (Supplementary Table 1). We also ran a sensitivity analysis in which we substituted vascular brain injury determined at autopsy for history of hypertension, stroke, or diabetes, as a covariate in the multivariable model. All comparisons between the PART and Alzheimer’s disease groups remained significant (Supplementary Table 2), and the covariate for vascular brain injury did not significantly influence the rate of decline in CDR-SB, global composite z-score, and all cognitive domain z-scores (P-values for all six models were >0.31).
As described earlier, there is a significant difference in the distribution of Braak stage when comparing PART to Alzheimer’s disease (Table 2). To investigate how adjusting for Braak stage may be driving the difference in rate of decline between groups, we ran the same regression models as the main analysis removing Braak stage as covariate (Supplementary Table 3), and found that there was generally no difference in the estimated slopes or the difference in slopes between PART and Alzheimer’s disease. If the variation in the distribution of Braak stage is not driving the faster rate of decline, the presence of amyloid plaques in the Alzheimer’s disease group may explain these findings. To investigate this, we ran the same regression models in the main analysis (Table 5) and Supplementary Table 3 on a subset of Alzheimer’s disease participants who had Thal phase assessed, stratifying the group into those with Thal phase 1–3 compared to Thal phase 4–5. Of the 452 Alzheimer’s disease participants, 231 (51.1%) had Thal phase assessed. Of that subset, 52 (22.5%) had a Thal phase of 1, 2, or 3, and the remaining 179 participants had either Thal phase 4 or 5. The results of these analyses show that Alzheimer’s disease participants with Thal phase 4–5 decline significantly faster in executive function compared to Thal phase 1–3. While not statistically significant, we also see that those with Thal phase 4–5 have a steeper slope for episodic memory, attention/working memory, and semantic memory/language. The results were nearly identical between the models that adjusted for Braak stage, and the models that did not. As this sensitivity analysis uses a subset of our main Alzheimer’s disease group, these models might have limited statistical power to detect significant differences for these domains; however, they suggest that higher Thal phase contributes to a faster rate of decline in Alzheimer’s disease.
Participants had been assessed for the abundance of neuritic plaques [i.e. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) score], and identified as PART if they had no neuritic plaques. Thal phase, by contrast, refers to any amyloid deposits (diffuse plaques and neuritic plaques) and stages the spatial distribution of amyloid deposition in the brain. Among those with PART who were assessed for Thal phase, 24 participants had Thal phase 1, 2, or 3, and seven had Thal phase 4 or 5 (Table 2). Because of the small sample size, comparable models to those that examined the relationship between Thal phase and cognitive trajectory in Alzheimer’s disease (i.e. Supplementary Table 4) did not meet the minimal statistical conditions to properly run.
Discussion
We sought to assess cognitive trajectories of neuropsychological test scores for participants with MCI and to compare the rate of decline in these tests for participants with PART against those with Alzheimer’s disease. We found that individuals with PART had significantly slower rates of decline in all cognitive domains, in a global composite score, and in the CDR-SB compared to those with Alzheimer’s disease. This study thus corroborates other studies showing that PART is generally more benign than Alzheimer’s disease and is less likely to lead to more severe cognitive dysfunction (Crary et al., 2014; Crary, 2016; Kryscio et al., 2016; Besser et al., 2017; Bell et al., 2019).
The current study adds to the understanding of the symptomatic presentation of PART, particularly the neuropsychological changes over time. Two prior studies examined the association between different degrees of PART pathology and neuropsychological test scores. PART participants with higher neurofibrillary tangle burden were shown to have more rapid decline in language/semantic memory, episodic memory, and attention (Jefferson-George et al., 2017). Similarly, Josephs et al. (2017) showed an association between higher Braak stage and poorer performance on tests of working memory and visuospatial reasoning in participants with PART.
This study also provides further details on the differences in the clinical manifestations of PART compared to Alzheimer’s disease, which is more widely studied and understood. Crary et al. (2014) demonstrated that Mini-Mental State Examination scores are relatively more preserved in PART compared with Alzheimer’s disease, even at higher Braak stages. Another study showed that people with PART had sparing of language/semantic memory compared to individuals with Alzheimer’s disease neuropathology (Besser et al., 2019). This was true for individuals with milder degrees of cognitive change (CDR = 0.5 or 1) and more severe cognitive change (CDR = 2 or 3). There were less consistent differences in other domains. Specifically, there was relative sparing of episodic memory for individuals with PART who had milder impairment. While this was not seen in those with PART who had more severe cognitive impairment, there was relative sparing of attention/working memory in this group.
Crary et al. (2014) and Besser et al. (2019) both examined cognitive performance at a single time point within a year or two of death. The current study builds on these prior studies by examining the trajectory of cognitive changes. Longitudinal data are more likely to detect subtle changes or differences, especially early in cognitive dysfunction, than are one-time measurements (Riley et al., 2011; Knopman and Caselli, 2012). This may explain, in part, why the two studies discussed above, which cross-sectionally examined cognitive performance, showed differences in fewer domains of neuropsychological function. By contrast, the current study, using more sensitive longitudinal data, showed differences in all domains between persons with PART and persons with Alzheimer’s disease neuropathology. This raises the question of whether PART and Alzheimer’s disease have different clinical features or whether PART is merely a slower variant of Alzheimer’s disease. Although the current study did find declines in all cognitive domains for PART, the rates of decline for two of the domains (attention and episodic memory) were minimal. Although not conclusive, these findings suggest a different pattern of cognitive decline for PART.
In a similar fashion, one recent study evaluated individuals with long-lasting (defined as >4 years since symptom onset) amnestic MCI who had slow rates of cognitive progression. Amyloid PET images of these participants revealed that the majority had absent or low amyloid load. A subset of the participants who were evaluated by amyloid PET also received a lumbar puncture to assess CSF biomarker concentrations. For this sample, amyloid PET results were consistent with CSF amyloid-β1-42 protein concentrations. Based on these results, the authors hypothesized that many of these slowly progressing cases of amnestic MCI might have had a variety of underlying pathological conditions other than Alzheimer’s disease neuropathology, suggesting alternatives like argyrophilic grain disease, age-related TAR DNA-binding protein 43 (TDP-43) proteinopathy, or PART (Cerami et al., 2018).
In the current study, there was an increased proportion of subjects with cerebrovascular disease (whether defined by risk factors or at autopsy) among the PART participants (Table 2). As PART is a more benign disease than Alzheimer’s disease, part of the cognitive decline in PART may be being driven by the co-existent cerebrovascular disease. It is notable, however, that neither vascular brain injury nor the risk factors (hypertension, stroke, diabetes) appeared as significant predictors of cognitive decline in the multivariable models. The relationship between PART and cerebrovascular disease appears to be an important topic for future research.
Baseline scores were different between PART and Alzheimer’s disease for most domains (Table 3), with PART participants having better function. Nonetheless, adjusting for the baseline scores did not change the significance of the differences in trajectories of test scores for the two groups (Tables 4 and 5).
Before drawing conclusions from the data, the study limitations need to be addressed. First, study participants were more highly educated and more likely to be white than the general population, limiting the generalizability of the findings. Second, 55% of the participants were 80 years old or older at their baseline visit. This might limit the generalizability of the study findings to younger subjects. However, as has been shown in prior studies, PART is primarily a disease of older ages (Crary et al., 2014; Crary, 2016; Kryscio et al., 2016; Besser et al., 2017), and the age distributions of this study were similar for individuals with PART and with Alzheimer’s disease neuropathology. Third, our inclusion of participants with MCI at their initial visit may have introduced unaccounted underlying differences, as these participants may have had MCI for varying time periods prior to enrolment in the UDS. Our sensitivity analysis found that the rate of decline was slower for those with incident MCI compared to those with prevalent MCI in both the PART and Alzheimer’s disease groups; however, the differences in slopes between the PART and Alzheimer’s disease groups were comparable between those with prevalent and incident MCI.
Fourth, data were missing for some of the neuropsychological test scores, which reduced the sample size for the models assessing the trajectory of the cognitive domain z-scores. All 578 participants had CDR-SB data at all visits. Based on this denominator, the percentage of participants excluded from the models because of missing domain z-scores ranged from 16% for attention/working memory to 36% for the global composite score (Table 1). Additionally, 3.2% of the PART sample and 3.6% of the Alzheimer’s disease group did not contribute to the models examining the trajectory of the cognitive domain z-scores because they were tested with the UDS version 3 battery. In both these instances, the proportion of participants excluded from the models was not meaningfully different for those with PART compared to those with Alzheimer’s disease. Higher proportions of missing data and the lower sample size would primarily be expected to result in wider confidence intervals and loss of statistical power to detect differences. Thus, we may not be able to detect certain significant differences between the PART and Alzheimer’s disease groups. Fifth, Braak stage is an incomplete measure of tau burden and it would be beneficial to have more in-depth, quantitative measures of tau burden. However, these require additional special staining, which is labour intensive, not currently routinely done at most centres, and not available in the NACC neuropathology database (Neltner et al., 2012; Walker et al., 2017). Finally, the domain of attention/working memory is a broad construct. The specific tests used in the UDS neuropsychological battery are Digit Span Forward and Backward, which may not represent all aspects of this domain (Tulsky and Price, 2003; Kessels et al., 2008; Hayden et al., 2011).
Despite these limitations, this study has major strengths. It uses multi-institutional data on a large group of individuals from across the USA, and the neuropsychological tests are administered in standardized fashion at all ADCs. Likewise, neuropathological changes are assessed at autopsy using standardized techniques and reporting methods (Montine et al., 2016). Thus, these data allow us to draw reasonable conclusions regarding the differences in trajectories of neuropsychological function in individuals who present with MCI but who differ in whether they have PART or Alzheimer’s disease underlying neuropathological features.
Our study focused on participants with MCI. Future studies examining PART in comparison to Alzheimer’s disease will need to examine cognitive changes over time while participants are still clinically asymptomatic, as well as differences in decline between the two groups in those with dementia. Additionally, our study adjusted for the presence of APOE ε4 alleles in the multivariable models. APOE ε4 is a substantial contributor to cognitive impairment across various dementias, and the differences seen in this analysis may be driven by the different distributions of APOE ε4 carriers in the PART sample compared to Alzheimer’s disease. Future studies with sufficient sample sizes should stratify the analysis by APOE ε4 carriers versus non-carriers to examine whether the relationship is modified by the presence of APOE ε4.
In conclusion, this study has shown that individuals with MCI and underlying PART neuropathological features experience a slower rate of decline in summary scores and in individual neuropsychological domains compared to individuals who have MCI with Alzheimer’s disease neuropathological features. This study provides further evidence that the clinical manifestation of PART has generally milder cognitive consequences than that of Alzheimer’s disease. Assuming that PART and Alzheimer’s disease could eventually be distinguishable antemortem, their differing rates of decline would factor into prognoses assigned to people with these conditions who present with MCI.
Supplementary Material
Acknowledgements
We are grateful for the research volunteers, their families, and our colleagues at the ADCs and NACC. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). J.F.C. is supported by National Institutes of Health Grants R01 NS095252 (PI), R01 AG054008 (PI), RF1 AG06096 (PI), R01 AG062348 (PI), Alzheimer's Association NIRG-15-363188 (PI), and the Tau Consortium (PI).
Funding
The NACC database and this study was supported by NIA/NIH Grant U01 AG016976.
Competing interests
The authors report no competing interests.
Glossary
Abbreviations
- ADC
Alzheimer’s Disease Center
- CDR
CDR® Dementia Staging Instrument, global score
- CDR-SB
CDR® Dementia Staging Instrument, sum of boxes score
- MCI
mild cognitive impairment
- NACC
National Alzheimer’s Coordinating Center
- NIA
National Institute on Aging
- PART
primary age-related tauopathy
- UDS
Uniform Data Set
References
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, et al. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord 2007; 21: 249–58. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004; 18: 270–7. [PubMed] [Google Scholar]
- Bell WR, An Y, Kageyama Y, English C, Rudow GL, Pletnikova O, et al. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer's disease. Alzheimers Dement 2019; 15: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, Crary JF, Mock C, Kukull WA. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 2017; 89: 1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, et al. The Revised National Alzheimer's Coordinating Center's Neuropathology Form-available data and new analyses. J Neuropathol Exp Neurol 2018; 77: 717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, Mock C, Teylan MA, Hassenstab J, Kukull WA, Crary JF. Differences in Cognitive impairment in primary age-related tauopathy versus Alzheimer disease. J Neuropathol Exp Neurol 2019; 78: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami C, Dodich A, Iannaccone S, Magnani G, Santangelo R, Presotto L, et al. A biomarker study in long-lasting amnestic mild cognitive impairment. Alzheimers Res Ther 2018; 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: the exception that proves the rule? J Neurol Neuromed 2016; 1: 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Jones RN, Zimmer C, Plassman BL, Browndyke JN, Pieper C, et al. Factor structure of the National Alzheimer’s Coordinating Centers uniform dataset neuropsychological battery: an evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord 2011; 25: 128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson-George KS, Wolk DA, Lee EB, McMillan CT. Cognitive decline associated with pathological burden in primary age-related tauopathy. Alzheimers Dement 2017; 13: 1048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Primary age-related tauopathy (PART) and Alzheimer's disease (AD). Alzheimers Dement 2019; 15: 720. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 2017; 133: 705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC, van den Berg E, Ruis C, Brands A. The backward span of the Corsi Block-Tapping task and its association with the WAIS-III digit span. Assessment 2008; 15: 426–34. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Caselli RJ. Appraisal of cognition in preclinical Alzheimer’s disease: a conceptual review. Neurodegener Dis Manag 2012; 2: 183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Abner EL, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, et al. Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis 2016; 3: 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, Cairns NJ, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer's disease. Alzheimers Dement 2016; 12: 164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006; 20: 210–6. [DOI] [PubMed] [Google Scholar]
- Neltner JH, Abner EL, Schmitt FA, Denison SK, Anderson S, Patel E, et al. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J Neuropathol Exp Neurol 2012; 71: 1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KP, Jicha GA, Davis D, Abner EL, Cooper GE, Stiles N, et al. Prediction of preclinical Alzheimer’s disease: longitudinal rates of change in cognition. J Alzheimer's Dis 2011; 25: 707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teylan M, Besser LM, Crary JF, Mock C, Gauthreaux K, Thomas NM, et al. Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer's neuropathology. Lab Invest 2019; 99: 1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: development and cross-validation of a six-factor model of cognitive functioning. Psychol Assess 2003; 15: 149–62. [DOI] [PubMed] [Google Scholar]
- Walker L, McAleese KE, Johnson M, Khundakar AA, Erskine D, Thomas AJ, et al. Quantitative neuropathology: an update on automated methodologies and implications for large scale cohorts. J Neural Transm 2017; 124: 671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer Disease Centers' neuropsychological test battery in the uniform data set (UDS). Alzheimer Dis Assoc Disord 2018; 32: 10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009; 23: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from NACC at https://www.alz.washington.edu/.