Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State Belgium and co‐rapporteur Member State Greece for the pesticide active substance alpha‐cypermethrin are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of alpha‐cypermethrin as an insecticide on cereals, winter oilseed rape, lettuces, leafy brassica and on cucumber and courgette in permanent glasshouses. The reliable end points, appropriate for use in regulatory risk assessment and the proposed maximum residue levels (MRLs), are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: alpha‐cypermethrin, peer review, risk assessment, pesticide, insecticide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Alpha‐cypermethrin is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Belgium, and co‐rapporteur Member State (co‐RMS), Greece, received an application from BASF for the renewal of approval of the active substance alpha‐cypermethrin. In addition, BASF submitted applications for maximum residue levels (MRLs), as referred to in Article 7 of Regulation (EC) No 396/2005. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Greece), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on alpha‐cypermethrin in the renewal assessment report (RAR), which was received by EFSA on 7 May 2017. The RAR included a proposal to set MRLs, submitted under Article 7 of Regulation (EC) No 396/2005. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, BASF, for comments on 9 August 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 16 October 2017.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether alpha‐cypermethrin can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council and give a reasoned opinion concerning MRL applications as referred to in Article 10(1) of Regulation (EC) No 396/2005.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of alpha‐cypermethrin as an insecticide on cereals, winter oilseed rape, lettuces, leafy brassica and on cucumber and courgette in permanent glasshouses, as proposed by the applicant. An MRL was assessed for the use in leafy brassica. Full details of the representative uses and the proposed MRL can be found in Appendix A of this report.
Data were submitted to conclude that the uses of alpha‐cypermethrin result in a sufficient insecticidal efficacy against the target organisms according to the representative uses proposed at the European Union (EU) level.
In the area of identity, physical and chemical properties and analytical methods, data gaps were identified for an analytical method for the determination of the octanol water partition coefficient of two metabolites, for additional validation data of several analytical methods used in data generation studies and for a monitoring method in body fluids.
In the area of mammalian toxicology, two data gaps were identified for further investigations of the endocrine disrupting potential and for further assessment of the toxicological profile of metabolites (including enantiomer [1S cis αR]).
In the residue section, the consumer dietary risk assessment cannot be finalised as for the provisional residue definitions for risk assessment in plants and animal commodities and the data gaps identified for additional residue trials on cucumbers, kales, lettuces and barley. No chronic intake concern was identified using the MRL proposals for the representative uses and for animal commodities (theoretical maximum daily intake (TMDI): 67% of acceptable daily intake (ADI), Dutch child). An acute intake concern was however identified for cucumbers (international estimated short‐term intake (IESTI): 131% of acute reference dose (ArfD), Dutch child), courgettes (IESTI: 104% of ARfD, UK toddler), kales (IESTI: 2541% of ARfD, Dutch child) and for lettuces (IESTI: 1248% of ARfD, German child).
No MRL can be proposed for alpha‐cypermethrin for the whole subgroup of leafy brassica in view of the data gap identified for two additional residue trials and four residue trials on kales and compliant, respectively, with the northern European Union (NEU) and southern European Union (SEU) outdoor GAPs to be extrapolated to the whole subgroup of leafy brassica. Moreover, as outcome of the renewal review, specifically as for the lowered toxicological reference values, a prioritisation of the initiation of the existing MRLs review of cypermethrins is recommended in view of indication of possible consumer intake concerns for a number of commodities.
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at EU level, with the notable exception that a data gap was identified for information on the effect of water treatment processes on the nature of residues of both the active substance and its identified metabolites potentially present in surface, when surface water is abstracted for drinking water. This gap leads to the consumer risk assessment from the consumption of drinking water being not finalised for all the representative uses. Another data gap was identified for the formation of water photolysis products > 5% applied radioactivity (AR) at two consecutive sampling dates.
In the area of ecotoxicology, for the representative outdoor uses, a high risk to aquatic organisms was concluded for a number of surface water scenarios even when considering risk mitigation measures (data gap). A high in‐field risk to non‐target arthropods was concluded for the uses to lettuce and leafy brassicas (data gap). Risk mitigation measures are needed to reach a low risk to aquatic organisms, honeybees and non‐target arthropods.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Belgium and co‐RMS Greece received an application from BASF for the renewal of approval of the active substance alpha‐cypermethrin. In addition, BASF submitted an application for maximum residue levels (MRLs) as referred to in Article 7 of Regulation (EC) No 396/2005.3 Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Greece), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on alpha‐cypermethrin in the RAR, which was received by EFSA on 7 May 2017 (Belgium, 2017). The RAR included a proposal to set MRLs, submitted under Article 7 of Regulation (EC) No 396/2005.
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, BASF, for consultation and comments on 9 August 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 16 October 2017. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS on 1 December 2017. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment and on the proposed MRLs took place with Member States via a written procedure in July 2018.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of alpha‐cypermethrin as an insecticide on cereals, winter oilseed rape, lettuces, leafy brassica and on cucumber and courgette in permanent glasshouses, as proposed by the applicant. An MRL was assessed in leafy brassica. A list of the relevant end points for the active substance and the formulation and the proposed MRLs is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2018), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (1 December 2017);
the evaluation table (24 July 2018);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Belgium, 2018), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Alpha‐cypermethrin is the ISO common name for the racemate comprising (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate or racemate comprising (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate (IUPAC).
The unresolved isomeric mixture of this substance has the ISO common name cypermethrin.
The representative formulated product for the evaluation was ‘BAS 310 55 I’, a microemulsion (ME) containing 50 g/L alpha‐cypermethrin.
The representative uses evaluated were field spray applications as insecticide in cereals, winter oilseed rape, lettuce, leafy brassica and spray applications in permanent glasshouses in cucumber and courgette in the EU. Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the uses of alpha‐cypermethrin result in a sufficient insecticidal efficacy against the target organisms according to the representative uses proposed at EU level following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b), SANCO/825/00‐rev. 8.1 (European Commission, 2010).
Alpha‐cypermethrin consists of two of the eight stereo isomers that comprise cypermethrin (i.e. one of the two enantiomeric pairs of the cis‐cypermethrin isomers: the 1R‐cis‐alpha‐S isomer and its enantiomer 1S‐cis‐alpha‐R). As for alpha‐cypermethrin, also other subsets of cypermethrin isomers have their own ISO common names: beta‐cypermethrin, theta‐cypermethrin and zeta‐cypermethrin.
The proposed specification for alpha‐cypermethrin was based on batch data from industrial scale production and on quality control data. The proposed minimum purity of the technical material was 980 g/kg. Hexane was considered relevant impurity with a maximum level of 1 g/kg. It should be noted that the minimum purity of the first inclusion was 930 g/kg and also that a FAO specification under the new procedure exists for alpha‐cypermethrin: 454/TC (2013), with a minimum content of alpha‐cypermethrin of 930 g/kg, relevant for the TCs originating from BASF and Tagros, Bharat Rasayan, Meghmani Organics, Gharda Chemicals and Heranba Industries. The minimum purity meets the requirements of the FAO Specification.
Based on the batch data and the impurity profiles, it is proposed to update the reference specification according to the renewal data, because the initial reference specification has not been considered as fully covered by the toxicological/ecotoxicological batches, while the specification proposed for renewal is considered covered.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of alpha‐cypermethrin or the representative formulation. The main data regarding the identity of alpha‐cypermethrin and its physical and chemical properties are given in Appendix A.
Adequate methods of analysis, including CIPAC methods are available for the determination of the active substance in the technical material and in the representative formulation and for the determination of the relevant impurity. Data gaps were however identified for additional validation data for several methods used in data generation and for the determination of the log Pow of two metabolites included in the risk assessment.
The residue definition for monitoring for food and feed of plant and animal origin was set to cypermethrin including other mixtures of constituent isomers (sum of isomers). The QuEChERS multiresidue method with liquid chromatography with tandem mass spectrometry (LC–MS/MS) is available for monitoring the enantiomeric pairs of cypermethrin diastereomers cis‐I, cis‐II (alpha‐cypermethrin), trans‐III and trans‐IV individually, with limit of quantifications (LOQs) of 0.01 mg/kg for cis‐I and trans‐III and 0.00695 mg/kg for cis‐II and 0.00429 mg/kg for trans‐IV, in all commodity groups.
Monitoring the compounds of the residue definition for animal matrices can be done by the QuEChERs multi‐residue method approach, with the exception of fat, where the DFG S19 module was used for extraction, using LC–MS/MS. The enantiomeric pairs of the diastereomers cis‐I, cis‐II (alpha‐cypermethrin), trans‐III and trans‐IV can be determined individually with LOQs of 0.01 mg/kg for cis‐I and trans‐III and 0.00695 mg/kg for cis‐II and 0.00429 mg/kg for trans‐IV, in meat, liver, kidney, fat, egg and milk.
The residue definition for monitoring in the environmental matrices was defined as alpha‐cypermethrin. Alpha‐cypermethrin (the enantiomeric pair of the diastereomer cis‐II) can be determined in soil by LC–MS/MS with a LOQ of 0.001 mg/kg. It should be noted that the method can be used also for monitoring the enantiomeric pairs cis‐I, trans‐III and trans‐IV and also the metabolites cis‐ and trans‐DCVA and 3‐PBA with LOQs of 0.001 mg/kg for each analyte.
Residues of alpha‐cypermethrin in drinking water and surface water can be monitored by LC–MS/MS with a LOQ of 0.825 ng/L. It should be emphasised that the methods available can also determine cis‐I, trans‐III and trans‐IV, and metabolites PBA, DCVA (cis‐ and trans‐isomers) and POAL, with corresponding LOQs of 0.675 ng/L for cis‐I, 0.652 ng/L for trans‐III, 0.847 ng/L for trans‐IV and 50 ng/L for each metabolite, respectively. Monitoring alpha‐cypermethrin residues in air is possible with gas chromatography–mass spectrometry (GC–MS) with a LOQ of 0.06 μg/m3.
Residues of alpha‐cypermethrin in the body fluids can be determined by LC–MS/MS with a LOQ of 0.035 mg/L, but the method is able to determine also cis‐I, trans‐III and trans‐IV with LOQs of 0.05 mg/L for cis‐I and for trans‐III and 0.021 mg/L for trans‐IV. The residue definition for monitoring in body fluids was defined as 4‐OH‐PBA sulfate and DCVA glucuronide, as a consequence, a data gap was identified for an analytical method for the determination of the components of the residue definition.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: SANCO/221/2000‐rev. 10‐final (European Commission, 2003), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012), Guidance on Dermal Absorption (EFSA PPR Panel, 2012) and Guidance on the Application of the CLP Criteria (ECHA, 2017).
Alpha‐cypermethrin was discussed at the Pesticides Peer Review Experts’ Meeting 175 in April 2018.
With regard to the proposed new technical specification, only hexane is a toxicologically relevant impurity (due to its hazardous reproductive toxicity properties). It is of no concern at the proposed level in the new technical specification. On this basis, the batches used for most of the toxicological studies (of lower purity and containing more impurities) can be considered as supportive of the new technical specification. The analytical methods used in the toxicological studies were concluded as appropriately validated and/or fit for purpose.
For alpha‐cypermethrin, the available toxicokinetic data suggested a saturation effect at high dose. Supported by the available data with cypermethrin, an oral absorption value of 40% was concluded (bridging was supported). After a wide distribution in well‐perfused organs as well as in fat, alpha‐cypermethrin is preferentially metabolised by hydrolytic cleavage of the ester bound and hydroxylation with cleavage of the ether bridge, and almost completely excreted at 48 h mostly via faeces and urine. The main metabolites identified were 4‐OH‐PBA sulfate and DCVA glucuronide. Regarding in vitro comparative metabolism, the predominant pathway was oxidation in rat and hydrolysis in human, no unique human metabolite is expected.
Alpha‐cypermethrin is toxic if swallowed and harmful if inhaled. Alpha‐cypermethrin is of low acute dermal toxicity. The acute test results allow the hazard identification of alpha‐cypermethrin as a toxicant for the central nervous system and as a local irritant for the airways (and classified as STOT SE 3 H335 May cause respiratory irritation). Alpha‐cypermethrin was not a skin sensitiser, irritant or phototoxic in the available studies.
In short‐term dietary studies, alpha‐cypermethrin causes primarily neurotoxicity in rats, mice and dogs, as well as axonal degeneration in rats and hepatotoxicity in mice at higher doses. The lowest no observed adverse effect level (NOAEL) is 2 mg/kg body weight (bw) per day (1‐year dog) based on paresthesia, decreased prostate and testes weight. On the basis of the observed neurotoxic effects in the 90‐day dog studies and DNT rat study, the classification STOT RE 1 (H372) 4 is proposed for alpha‐cypermethrin.
In the 14‐day rat inhalation study, the NOAEL is 0.029 mg/L, i.e. 7.83 mg/kg bw per day (highest dose tested). Since the vehicle used was kerosene and the observed systemic effects are considered related to the inhalation of kerosene, this study is considered only as complementary.
Alpha‐cypermethrin can be considered as unlikely to be genotoxic based on the available guidelines studies. Considering the positive results in a non‐guideline repeat dose toxicity study in rabbits with a subsequent assessment of micronuclei formation in blood (Belgium, 2018), the experts agreed that further scientific valid data should be provided in order to clarify these results and the mode of action for micronucleus formation and its possible link (causal or not) with inflammatory events (data gap).
For the long‐term toxicity, two rat studies with cypermethrin and two mouse studies (one with cypermethrin and one with alpha‐cypermethrin) were taken into account. The relevant long‐term NOAEL for the rat is 0.5 mg/kg bw per day based on increased urea level and changes in kidneys (weight), while for the mouse it is 3 mg/kg bw per day based on clinical signs and decreased body weight gain (study performed with alpha‐cypermethrin). In the absence of treatment‐related tumours, alpha‐cypermethrin was concluded unlikely to be carcinogenic.
For the multigeneration rat studies (one with alpha‐cypermethrin, considered complementary, and one with cypermethrin considered acceptable), the parental NOAEL is 10 mg/kg bw per day based on decreased body weight and food consumption, the offspring NOAEL is 10 mg/kg bw per day based on decreased litter weight and histopathological findings in the liver, lung, lymph nodes and thymus; and the reproductive NOAEL is 10 mg/kg bw per day based on decreased survival of pups at birth.
For the developmental rat study, the maternal NOAEL is 3 mg/kg bw per day based on decreased body weight gain, and the developmental NOAEL is 9 mg/kg bw per day based on decreased fetal body weight. For the developmental rabbit study, the maternal NOAEL is 15 mg/kg bw per day based on decreased food consumption and body weight gain, and the developmental NOAEL is 30 mg/kg bw per day (top dose).
With regard to the neurotoxicity assessment, the acute NOAEL is 4 mg/kg bw based on the increased neurologic reactivity and fibre degeneration in sciatic nerves, while the 90‐day NOAEL is 36.1 mg/kg bw per day (top dose). For the developmental neurotoxicity study with alpha‐cypermethrin, the maternal NOAEL is 2 mg/kg bw per day based on decreased body weight gain at the end of the gestation period, and the developmental NOAEL is <0.25 mg/kg bw per day based on clinical signs in pups. Regarding the potential link between pyrethroids and neurodegenerative diseases, the experts agreed that no robust animal or epidemiological studies exist indicating a causal relationship between Parkinson's disease and exposure to pyrethroids, including alpha‐cypermethrin.
Concerning immunotoxicity, alpha‐cypermethrin did not exhibit evidence of immunotoxicity in a 4‐week rat study.
Alpha‐cypermethrin is not classified or proposed to be classified as carcinogenic or toxic for reproduction category 2, on this basis, the conditions of the interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine‐disrupting (ED) properties are not met. On the basis of the available regulatory studies and literature findings, it was acknowledged that alpha‐cypermethrin has endocrine‐mediated activity but the potential for endocrine disruption could not be concluded upon (data gap).
The metabolites including hydroxylated derivatives of alpha‐cypermethrin and their conjugates are considered unlikely to be genotoxic or to be more toxic than the parent. The metabolites including DCVA derivatives are also considered unlikely to be more toxic than the parent since DCVA glucuronide is a major rat metabolite.
Based on current available information in the dossier for alpha‐cypermethrin, the conclusion for all metabolites bearing the 3‐phenoxybenzoyl moiety such as 4‐OH‐PBA, 4‐OH‐PBA sulfate, 3‐PBA, 3‐PBAldehyde was that they could be initially considered unlikely to be of higher toxicity than the parent. Grouping was considered acceptable based on structural similarities and metabolic pathway considerations. The conclusion on the toxicological properties for this group was based on the fact that 4‐OH‐PBA sulfate was also identified as a major rat metabolite and could be initially considered covered by the toxicity profile of the parent. For 3‐PBAldehyde, it could be considered unlikely to be more toxic than alpha‐cypermethrin based on acute toxicity, genotoxicity and repeat‐dose toxicity studies. However, it was noted during the experts’ meeting that further data including genotoxicity, were submitted for some common metabolites with lambda‐cyhalothrin. These data have been submitted for the assessment of the confirmatory data on lambda‐cyhalothrin; however, they are not yet peer‐reviewed by EFSA and a conclusion on these metabolites (3‐PBA and 4‐OH‐PBA) cannot be currently drawn (data gap)5. It is therefore further noted that the conclusions on this group of metabolites might need to be revised once confirmatory data on lambda‐cyhalothrin are peer reviewed.
For the metabolite 3‐OH‐benzoic acid, the limited data available are insufficient to conclude on its toxicological profile and relative toxicity in comparison with the parent (no further data are requested regarding the representative uses).
No specific toxicological investigations were provided for the individual isomers, in particular the enantiomer (1S cis αR) in order to allow a conclusion on their relative toxicity (data gap, see also Section 3).
The acceptable daily intake (ADI) and acute reference dose (ARfD) for alpha‐cypermethrin are both 0.00125 mg/kg bw per day based on the lowest observable adverse effect level (LOAEL) for pups in the DNT study and applying an increased uncertainty factor (UF) of 200. The acceptable operator exposure level (AOEL) and acute acceptable operator exposure level (AAOEL) are 0.0005 mg/kg bw per day based on the LOAEL for pups in the DNT study, applying an UF of 200 and a correction for oral absorption 40%. It is noted that in the Review Report (European Commission, 2004), the ADI was 0.015 mg/kg bw per day based on the 1‐year dog study, the ARfD was 0.04 mg/kg bw based on the rat acute neurotoxicity study (both with the application of an UF of 100), and the AOEL was 0.01 mg/kg bw based on the 90‐day dog study, applying an UF of 100 and a correction for an oral absorption value of 45%).
The RMS estimated non‐dietary exposure (i.e. operator, worker, bystander and resident) considering dermal absorption values of alpha‐cypermethrin in ‘BAS 310 55 I’ of 3% for the concentrate and of 1% for the dilution as input values.
Considering the representative uses with ‘BAS 310 55 I’ as an insecticide in a range of field crops such as cereals, oilseed rape, lettuces and leafy cabbages, the RMS estimated exposure for lettuces and leafy cabbages as the critical use for field crops based on the higher application rate (i.e. 20 g/ha per application). Operator exposure was below the AOEL (16% of the AOEL) with the use of personal protective equipment (PPE; gloves) according to the German Model. Worker exposure was below the AOEL even without the use of PPE (60% of the AOEL). Bystander and resident exposure was below the AOEL according to the original German Model (inhalation exposure to vapours is not taken into account). Children resident exposure would be above the AOEL if the default vapour concentration of 0.001 mg/m3 is taken into account (following UK approach and EFSA scheme). A refinement of the vapour concentration was presented by the applicant indicating that children resident exposure would be below the AOEL.
Considering the representative uses with ‘BAS 310 55 I’ as an insecticide in glasshouse crops (cucumbers and courgettes), the RMS estimated operator exposure according to the ECPA Southern European Greenhouse Model. The exposure was below the AOEL (79% AOEL) if PPE (gloves) is used. It is acknowledged that this model is not yet validated at EU level and further estimates according to available models like the Dutch Model was not provided leading to a data gap. Worker exposure was below the AOEL even without the use of PPE (60% of the AOEL). Bystander and resident exposure is not expected in permanent glasshouse structure.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of residue chemistry studies (OECD, 2009), the OECD publication on MRL calculations (OECD, 2011), the European Commission guideline document on MRL setting (European Commission, 2011) and the Joint Meeting on Pesticide Residues (JMPR) recommendations on livestock burden calculations (JMPR, 2004, 2007).
Alpha‐cypermethrin was discussed at the Pesticides Peer Review Meeting 176 in April 2018.
3.1. Representative use residues
The metabolism of alpha‐cypermethrin was investigated in cabbages and lettuces (leafy crops) and in wheat (cereals) after foliar spray applications. Significant isomerisation of cis‐II (alpha‐cypermethrin) to cis‐I, trans‐III and trans‐IV isomers of cypermethrin was observed mainly in cabbage leaves and to a lower extent in wheat green parts and straw, whilst no isomerisation was noted in wheat grain and in lettuces harvested at a short preharvest interval (PHI) (3 days), where alpha‐cypermethrin remained the predominant compound of the total residues (23% total radioactive residue (TRR) and up to 98% TRR, respectively). The sum of all cypermethrin isomers was predominant in all crops parts investigated, accounting for 23% TRR up to more than 98% TRR. Besides cypermethrin isomers, the metabolite cis‐ and trans‐DCVA was recovered mainly under its conjugated form and indicated the cleavage of the molecule with the formation of metabolites containing the counterpart 3‐phenoxybenzoyl moiety such as 3‐PBA, 3‐PBAldehyde and 4‐OH‐PBA. Both compounds and their derivatives were recovered at very minor levels, accounting for less than 10% TRR in the alpha‐cypermethrin metabolism studies whilst they occurred at significant proportions in some of the studies conducted with cypermethrin or cis‐cypermethrin. Metabolite DCVA is unlikely of greater toxicity than alpha‐cypermethrin while for the metabolites containing the 3‐phenoxybenzoyl moiety only preliminary conclusions have been possible with regard to their toxicity (see Section 2). Since similar metabolic patterns were depicted from the plant metabolism studies conducted, respectively, with cypermethrin and alpha‐cypermethrin with the parent compound being predominant in all crops parts, the bridging from cypermethrin to alpha‐cypermethrin plant metabolism was considered acceptable. As the metabolism of alpha‐cypermethrin was not investigated in fruit crops, the metabolism study conducted on apples with cis‐ and trans‐cypermethrin was considered in which cypermethrin isomers accounted for the major part of the total residues in apple fruit (50–82%TRR) and in leaves (48–60%TRR) with a significant shift of ‘Cis‐’ into ‘Trans‐’ isomers (33% and 15% in apple leaves and peel, respectively). The apple metabolism study as a stand‐alone study cannot be considered as acceptable to cover the representative use of alpha‐cypermethrin on fruit crops (cucumbers/courgettes). However, considering the whole plant metabolism data package for both alpha‐cypermethrin and cypermethrin, it is concluded that sufficient and acceptable metabolism studies are available to cover the representative uses on alpha‐cypermethrin.
The residue definition for monitoring was limited to ‘cypermethrin including other mixtures of constituent isomers (sum of isomers)’ as a valid marker of the total residues in all crop groups. For risk assessment, since metabolites cis‐ and trans‐DCVA and 3‐PBA were recovered at negligible proportions in the metabolism studies, the residue definition was agreed as ‘cypermethrin including other mixtures of constituent isomers (sum of isomers)’ considering the significant isomerisation of alpha‐cypermethrin vs the different isomers of cypermethrin that might not match exactly the isomeric ratios of cypermethrin. The residue definition for risk assessment should however be considered provisional pending finalisation of the assessment of the genotoxic potential of 3‐PBA and review of the preliminary conclusions in toxicology on the whole group of related metabolites bearing the 3‐phenoxybenzoyl moiety (besides 3‐PBA also e.g. PBAldehyde, 4‐OH‐PBA) once the confirmatory data on lambda‐cyhalothrin have been peer reviewed (see Section 2). It is also noted that the toxicity of alpha‐cypermethrin should be considered, taking into account that isomerisation of the alpha‐cypermethrin isomers to the other cypermethrin isomers may not occur, particularly in case of application at short preharvest intervals.
From the confined rotational crop study conducted with 14C‐cypermethrin at a 33 N rate, residue levels in rotational crops are expected to be below 0.01 mg/kg when the primary crops are treated according to the representative uses. Rotational crops field residue trials conducted with alpha‐cypermethrin on carrots, cabbages, lettuces and wheat following bare soil application at 1.3 to 3 N rate showed cypermethrin residues (cis‐I, cis‐II, trans‐III and trans‐IV isomers) below 0.01 mg/kg (for each isomer pair) in the edible parts of the rotational crops at normal harvest. A different residue definition for the rotational crops compared to the primary crops is not deemed necessary.
In a hydrolysis study simulating standard food processing conditions, alpha‐cypermethrin was hydrolytically stable under pasteurisation and baking/brewing/boiling and degrades into 3‐ phenoxybenzaldehyde (3‐PBAldehyde) and DCVA (up to 13% and 23% of applied radioactivity (AR), respectively) at sterilisation. Under all the conditions tested in this study unchanged parent compound was recovered in a range of 66–97% of AR. A specific residue definition for processed commodities is currently not proposed and the residue definition for primary crops may be used upon finalisation of the assessment of the toxicological relevance of metabolites with the 3‐phenoxybenzoyl moiety (3‐PBAldehyde). Processing residue trials determined the residues of cypermethrin (sum of isomers) in gherkins, oilseed rape and barley processed commodities.
Total cypermethrin (sum of isomers) were found to be stable for up to 12 months in lettuces, tomatoes, oilseed rape whole plant, cereal grain, cereal whole green plants and straw and in rape seed. Alpha‐cypermethrin residues were stable for 24 months in pineapple fruit, barley grain and bean seeds. The storage stability data in animal matrices demonstrated acceptable storage stability of alpha‐cypermethrin residues for up to 6 months in tissues (muscle, fat, liver and kidney), for 9 months in milk and for 1.5 months only in eggs.
Sufficient and acceptable residue trials compliant, respectively, with the northern European Union (NEU) and southern European Union (SEU) outdoor GAPs on oilseed rape and wheat (extrapolation to rye and triticale) have been submitted. As for cucumbers, based on the overdosed residues trials (> 25%) that were scaled down, an acute intake concern for both cucumbers and courgettes could not be excluded; sufficient residue trials on cucumbers and compliant with the indoor GAP are therefore required (data gap). Four residue trials and two residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAPs on leafy brassica are available. In accordance with the current extrapolation rules, two additional residue trials and four residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAPs are required to be extrapolated to the whole subgroup of leafy brassica (data gap). Based on the overdosed NEU and SEU residues trials on lettuces that were scaled down, an acute intake concern for lettuces could not be excluded and sufficient residue trials on lettuces and compliant, respectively, with the NEU and SEU GAPs are required (data gap). Seven residue trials on barley (extrapolation to oats) are compliant with the SEU GAP and one additional residue trial is therefore required to complete the residue data set (data gap). In all the residue trials, the residues of alpha‐cypermethrin were determined as total cypermethrin (sum of cypermethrin isomers) according to sufficiently validated analytical methods. The equivalence between the different formulations used in the residue trials was demonstrated from bridging studies conducted on fruit crops, potatoes and lettuces.
Metabolism studies on poultry, ruminants and fish were conducted with 14C‐alpha‐cypermethrin. In livestock, the parent compound was predominant in muscle (up to 66% TRR), fat (45–90% TRR), milk (52–81% TRR) and in egg yolk (34–42% TRR). DCVA was found at significant levels in poultry muscle (up to 24% TRR), liver (58% TRR) and in egg white (18% TRR); whilst DCVA glucuronide conjugate was only detected in high proportions in ruminant kidney (17% TRR). 3‐PBA and 3‐PBA glycine occurred mainly in ruminant kidney (17 and 38% TRR, respectively) and 3‐PBA glycine in milk (30% TRR). In fish, alpha‐ cypermethrin was major in muscle and skin (up to 78% TRR and 72% TRR, respectively) and the glucuronic acid conjugate of 4’‐OH‐alpha‐cypermethrin was significant in the liver (up to 25% TRR) only. A preferential isomerisation was observed mainly in ruminant matrices, which were enriched with the enantiomer (1S cis αR) of the cis‐II isomers (alpha‐cypermethrin). Although isomerisation of alpha‐cypermethrin (cis‐II) into the other cypermethrin isomers (cis‐I, trans‐III and trans‐IV) was not observed in animal matrices, their occurrence in animal matrices cannot be excluded, considering the significant isomerisation of alpha‐cypermethrin into the different isomers of cypermethrin that may occur in crops treated with alpha‐cypermethrin and potentially fed to animals. As the relative toxicity of the individual cypermethrin isomers and in particular the enantiomer (1S cis αR) was not provided, the impact of the change in the isomeric ratio in animal matrices on the consumer dietary risk assessment could not be addressed.
Although it is acknowledged that alpha‐cypermethrin is not the most relevant residue marker for the kidney and liver where DCVA, 3‐PBA and their conjugates are the predominant compounds of the residues, these compounds are however common to other cypermethrins and cannot therefore be included in the residue definition for monitoring. The agreed residue definition for monitoring is limited to ‘cypermethrin including other mixtures of constituent isomers (sum of isomers)’. For risk assessment, the residue definition is proposed as ‘cypermethrin including other mixtures of constituent isomers (sum of isomers)’ and is considered as provisional pending upon the assessment of the relative toxicity of the individual cypermethrin isomers (in particular the enantiomer (1S cis αR), the genotoxic potential of 3‐PBA and the review of the preliminary conclusions in toxicology on the whole group of related metabolites with the 3‐phenoxybenzoyl moiety (see Section 2). Feeding studies were also provided analysing total cypermethrin (sum of isomers) residues in ruminant and poultry matrices. MRLs were proposed at the LOQ for all the matrices.
No chronic intake concern was identified using the MRL proposals for the representative uses and for animal commodities (theoretical maximum daily intake (TMDI): 67% of ADI, Dutch child). An acute intake concern was however identified for cucumbers (IESTI: 131% of ARfD, Dutch child), courgettes (IESTI: 104% of ARfD, UK toddler), kales (IESTI: 2,541% of ARfD, Dutch child) and for lettuces (IESTI: 1,248% of ARfD, German child). Acute intakes of milk and milk products for vulnerable consumer groups are very close to the ARfD (> 99% of ARfD for infants), applying the provisional risk assessment residue definition. EFSA therefore emphasises the importance of reducing uncertainty in the current assessment by providing further information on the relative toxicity of individual cypermethrin isomers (in particular the enantiomer (1S cis αR)), on the genotoxic potential of metabolite 3‐PBA and on the review of the preliminary conclusions in toxicology on the whole group of related metabolites bearing the 3‐phenoxybenzoyl moiety once the confirmatory data on lambda‐cyhalothrin have been peer reviewed. The consumer dietary intake calculation is also provisional considering the data gaps identified for additional residue trials on cucumbers, kales, lettuces and barley.
The consumer risk assessment is also not finalised with regard to the unknown nature of residues that might be present in drinking water, consequent to water treatment processes (issue not finalised, see also Section 4).
Field residue trials on Phacelia and on oilseed rape were submitted to analyse the residues of alpha‐cypermethrin in flowers, pollen and nectar. In the residue trials on Phacelia (application at flowering), residues of alpha‐cypermethrin were analysed in honey and were not detected (< 0.003 mg/kg) whilst in the residue trials on oilseed rape, residues of alpha‐cypermethrin analysed in nectar and pollen showed a considerable decline after application, with residue levels ≤ 0.05 mg/kg in pollen and were not detected (< 0.003 mg/kg) in nectar. Considering that a low translocation of alpha‐cypermethrin residues in the different plant parts was observed in the plant metabolism and taking into account the lipophilic properties of the active substance, further residue trials for the determination of residues of alpha‐cypermethrin and its relevant metabolites in honey in regards to the other representative uses are not required to address the data requirement for the determination of residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom.
3.2. Maximum residue levels
The MRL request on leafy brassica was not fully supported by the available data. Four residue trials and two residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAPs on leafy brassica are available. In accordance with the current extrapolation rules, two additional residue trials and four residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAP are required (data gap) to be extrapolated to the whole subgroup of leafy brassica. No MRL can therefore be proposed for alpha‐cypermethrin for the whole subgroup of leafy brassica. Furthermore as outcome of the renewal review, specifically as for the lowered toxicological reference values, a prioritisation of the initiation of the existing MRLs review of cypermethrins is recommended. A screening assessment for all MRLs in place indicated a TMDI corresponding to > 3,300% of the ADI and a large exceedance of the ARfD for several commodities (highest IESTI: > 21,000% of ARfD for oranges). It should be noted that the assessment is unrefined. However, it can be reasonably expected that exceedance of the toxicological reference values will occur for a number of commodities also in a refined risk assessment.
4. Environmental fate and behaviour
Alpha‐cypermethrin was discussed at the Pesticides Peer Review TC 172 in April 2018.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, alpha‐cypermethrin exhibited low to moderate persistence, forming the major (> 10% AR) metabolites (cis‐)DCVA (max. 13.6% AR) and M310I017 (max. 8.4% AR), which exhibited low to moderate persistence and metabolite 3‐PBA (max. 5.4% AR), which exhibited very low to low persistence. Mineralisation of the cyclopropane and benzyl ring 14C radiolabel to carbon dioxide accounted for 41.4–51.4% AR after 120 days. The formation of unextractable residues (not extracted by acetonitrile/water) for these radiolabels accounted for 36.9–44.7% AR after 120 days. In anaerobic soil incubations, degradation of alpha‐cypermethrin was moderate, with the degradation pathway similar to that under aerobic conditions. The contribution of photolytic transformation processes on soil surfaces to the dissipation of alpha‐cypermethrin from the soil environment is not considered a significant degradation pathway. A data gap was identified for the missing correction of the DT50 values into days of natural summer sunlight at a latitude of 30°N–50°N.
Metabolite DCVA is common metabolite to beta‐cypermethrin and zeta‐cypermethrin and metabolite 3‐PBA is common metabolite to beta‐cypermethrin, zeta‐cypermethrin and gamma‐cyhalothrin for which published EFSA conclusions are available (EFSA 2009a, 2014b,c). Therefore, reliable peer‐reviewed agreed endpoints were added to the degradation endpoints of the present assessment for both the metabolites.
Alpha‐cypermethrin and the metabolite M310I017 exhibited immobility in soil, metabolites (cis‐)DCVA and 3‐PBA exhibited very high to medium soil mobility. Metabolite DCVA is a common metabolite to beta‐cypermethrin and zeta‐cypermethrin for which published EFSA conclusions are available (EFSA, 2009a, 2014c). Therefore, reliable peer‐reviewed agreed endpoints were added to the adsorption endpoints of the present assessment, however as for metabolite DCVA only the cis‐configuration is present in alpha‐cypermethrin, then only the adsorption endpoints available for the cis‐isomer were taken into account. Furthermore, it was agreed that there was a pH dependency of adsorption, and then soils with pH > 6.1 were used to derive adsorption endpoint to be used as an input parameter for modelling. For metabolite 3‐PBA, no chiral centres were present, and then adsorption endpoints available in the EFSA conclusions of zeta‐cypermethrin, gamma‐cyhalothrin and beta‐cypermethrin (EFSA, 2009a, 2014b,c) were combined with the data available in the present assessment. A pH dependency was observed, and then only endpoints from soils with pH‐H2O > 5.6 were considered to derive adsorption endpoint to be used as an input parameter for modelling.
Field study DegT50 values were derived following normalisation to FOCUS reference conditions (20°C and PF2 soil moisture) following the EFSA (2014a) DegT50 guidance. The field data endpoints were combined with lab values to derive modelling endpoints. It was agreed that, based on the available similarity assessment, the US field studies can be considered representative for EU conditions.
In laboratory incubations in dark aerobic natural sediment water systems, alpha‐cypermethrin exhibited low to medium persistence, forming the major metabolites 3‐PBA (max. 18% AR in water and max. 5.1 in sediment, exhibiting low persistence), (cis)‐DCVA (max. 47.3% AR in water and 19.5% AR in sediment, exhibiting low to moderate persistence). The unextractable sediment fraction (not extracted by acetonitrile/water) of cyclopropane and benzyl ring 14C radiolabel, accounted for 15.9–37.1% AR at study end (105 days). Mineralisation of these radiolabels accounted for 24.9–53.1% AR at the end of the study. Alpha‐cypermethrin was fast degraded in a laboratory sterile aqueous photolysis experiment, forming the major metabolites 3‐PBAldehyde (max. 12.9% AR), 3‐PBA (max. 22.5% AR) and DCVA (max. 43.7% AR). A data gap was identified because the formation of water photolysis products > 5% AR at two consecutive sampling dates could not be excluded based on the available information.
Chiral analysis showed that only the cis‐isomers of alpha‐cypermethrin and the metabolite DCVA were observed, no trans‐isomers were formed and no significant change of the enantiomeric ratio was observed. The conversion of cis‐ to trans‐isomers is a photochemical reaction and requires intense irradiation. It was agreed that no differentiation of the behaviour of alpha‐cypermethrin enantiomers should be considered in the exposure assessment.
The necessary surface water and sediment exposure assessments (Predicted environmental concentrations (PEC) calculations) were carried out for the metabolites 3‐PBA, (cis‐)DCVA, M310I017, and 3‐PBAldehyde using the FOCUS (2001) step 1 and step 2 approach (version 3.2 of the Steps 1‐2 in FOCUS calculator). For the active substance alpha‐cypermethrin, appropriate step 3 (FOCUS, 2001) and step 4 calculations were available.6 The step 4 calculations appropriately followed the FOCUS (2007) guidance, with no‐spray drift buffer zones of up to 20 m being implemented for the drainage scenarios (representing a 91–93% spray drift reduction). The SWAN tool (version 4.0.1) was appropriately used to implement these mitigation measures in the simulations.
For the representative protected uses in permanent glasshouses, the necessary surface water and sediment exposure assessments (PEC) were appropriately carried out using the FOCUS (2001) step 1 and step 2 approach (version 3.2 of the steps 1‐2 in FOCUS calculator), which was then modified by post‐processing the spray drift input results (option no runoff or drainage was selected) to obtain a 0.1% emission of alpha‐cypermethrin from greenhouses being redeposited on adjacent surface water bodies. This approach has been accepted by Member State experts as an assumption that can be used in EU level surface water exposure assessments for greenhouse uses and is referred to in FOCUS (2008) guidance as being appropriate, except when applications are made with ultra low volume application techniques when 0.2% emission is prescribed.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 for alpha‐cypermethrin. The potential for groundwater exposure from the representative uses by alpha‐cypermethrin above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all nine FOCUS groundwater scenarios for alpha‐cypermethrin and metabolites 3‐PBA, (cis‐)DCVA, and M310I017.
Calculations of PECsoil were conducted for alpha‐cypermethrin and metabolites M310I017, DCVA and 3‐PBA in the context of a risk envelope approach for a worst‐case application scenario applied to cabbage, covering all the representative uses.
The applicant did not provide appropriate information to address the effect of water treatments processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water. This has led to the identification of a data gap (see Section 7) and results in the consumer risk assessment not being finalised (see Section 9).
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a), SETAC (2001), EFSA (2009b), EFSA PPR Panel (2013) and EFSA (2013). According to Regulation (EU) No. 283/2013, data should be provided regarding the acute and chronic toxicity to honeybees and data to address the development of honeybee brood and larvae. As the European Commission (2002a) does not provide a risk assessment scheme which is able to use the chronic toxicity data for adult honeybees and the honeybee brood, when performing the risk assessment according to European Commission (2002a), the risk to adult honeybees from chronic toxicity and the risk to bee brood, could not be finalised due to the lack of a risk assessment scheme. Therefore, the EFSA (2013) was used for risk assessment in order to reach a conclusion for the representative uses.
Several aspects of the hazard and risk assessment were discussed at the Pesticides Peer Review Meeting 177 in April 2018.
Suitable toxicity data were available to assess the acute and reproductive risks to birds and wild mammals. For the risk via dietary exposure, a low acute and reproductive risk to birds and wild mammals was concluded for all representative uses based on the available screening level risk assessment. A study investigating the potential for bioconcentration in fish was available and discussed at the experts’ meeting. The experts raised several concerns but overall considered that the endpoint was sufficiently reliable for risk assessment given the margin of safety obtained in the risk assessment for fish‐eating birds and mammals. A low risk to birds and mammals from secondary poisoning, exposure to contaminated water and from metabolites was concluded for all representative uses.
Toxicity data and risk assessments were available to assess the risk to aquatic organisms from alpha‐cypermethrin and metabolites DCVA, 3‐PBA and 3‐PBAldehyde. No toxicity data were available for the metabolite M310I017. The available tier‐1 risk assessments indicated a high acute and chronic risk to fish and aquatic invertebrates for all representative uses including those in permanent glasshouses. A low risk to algae, aquatic plants and sediment dwelling organisms from the parent compound was concluded using FOCUS step 2 or 3 PEC values.
The higher tier risk assessments for fish and aquatic invertebrates were discussed and agreed at the experts’ meeting. For the refined risk assessment for fish, the experts did not agree with the use of time‐weighted average PEC values for the chronic risk assessment nor was the acute species sensitivity distribution (SSD) considered reliable. The use of a geometric mean of the acute toxicity data for fish was agreed to be suitable for and therefore used in tier‐2 risk assessments. On the basis of the available refined tier‐2 risk assessments, a low acute risk to fish was concluded for all representative uses. A chronic fish‐early life stage study performed with modified exposure was available. FOCUS surface water exposure profiles were not available for the representative outdoor uses; therefore it was not possible to use the refined endpoint in a tier‐2 risk assessment. The exposure profile in the modified exposure study was considered to sufficiently cover the predicted exposure profile for the representative use to permanent glasshouses. On the basis of the tier‐2 risk assessment, a low chronic risk to fish was concluded for the representative use in permanent glasshouses. Although no tier‐2 risk assessment was available, a low chronic risk to fish was concluded, for all representative outdoor uses, using FOCUS step 4 PEC values which accounted for risk mitigation measures.
Several reliable mesocosm studies were available and discussed at the expert meeting. The experts agreed that the data were only suitable to derive an ETO‐RAC (ecological threshold option for a regulatory acceptable concentration). A ERO‐RAC (ecological recovery option for a regulatory acceptable concentration) could not be derived from the available data as concerns were raised that recovery was not demonstrated within an acceptable time period. An assessment factor of 2 was agreed to be used with the ETO‐RAC value. Using the refined ETO‐RAC value in a higher tier risk assessment and considering risk mitigation measures such a no‐spray buffer zones together with drift reduction technology, a low risk was concluded for some scenarios for all representative outdoor uses (3/9 for cereals, 3/6 for winter oilseed rape, 4/7 lettuce/leafy brassicas at 10 g a.s./ha and 0/7 for lettuce/leafy brassicas at 20 g a.s./ha). A data gap was identified to address the risk to aquatic invertebrates for the scenarios where a high risk was indicated (see Appendix A and response to Experts’ consultation 5.7 in the evaluation table). Using the refined ETO‐RAC, a low risk to aquatic invertebrates was also concluded for the representative use to permanent glasshouses.
A low risk to aquatic organisms from the metabolites was concluded for all representative uses. In consideration of the structural similarity of the metabolite M310I017 with the parent, no increased toxicity is expected. As PECsw calculated using FOCUS Step 2 are lower than the parent ETO‐RAC, a low risk was also concluded for this metabolite.
Toxicity data were available for both honeybees and bumblebees. Tier‐1 risk assessments for honeybees, following both the European Commission (2002a) Guidance Document and also the EFSA (2013) Guidance Document, were available. Both risk assessments indicated a high risk to honeybees. Numerous higher tier semi‐field and field studies were available and discussed during the experts’ meeting. It was agreed that the data indicated a low risk to honeybees, for exposure via pollen and insect honeydew, could only be concluded for when applications are made after bee flight. Therefore, risk mitigation measures to ensure that applications are only made in the evening after bee flight were needed (see Section 8). A low risk to honeybees from exposure via residues in surface water and guttation fluid was also concluded. Given that the risk assessment for the active substance was based on field studies, it was concluded that the risk from metabolites in pollen and nectar is also addressed provided that the mitigation measures above are applied.
An acute contact and oral risk assessment for bumblebees, performed in accordance with EFSA (2013), was available. A low acute risk to bumblebees was indicated for the majority of the scenarios assessed (see Belgium, 2018). However, a high risk via contact exposure was indicated for the treated crop and weeds scenario for all representative outdoor uses. No data were available to perform a higher tier risk assessment and therefore a data gap was concluded.
A low risk to all types of wild bee was concluded for the representative uses restricted to permanent glasshouses. A high risk to pollinators introduced to glasshouses cannot be excluded with the available information; this should be considered at Member State level.
Suitable toxicity data were available for non‐target arthropods. On the basis of the tier‐1 and tier‐2 risk assessments, a high in‐field and off‐field risk was indicated. Several higher tier field studies were available performed at both in‐field and off‐field exposure rates; these were discussed in the context of the risk assessment for the representative uses during the expert meeting. For the in‐field risk assessment field studies performed in cereals in Germany and France were deemed as reliable and were concluded to show a low risk to in‐field population of non‐target arthropods for applications made to cereals and oilseed rape. However, the experts concluded that the studies were not suitable to cover the representative use to leafy brassicas and lettuce. Therefore, a high in‐field risk to non‐target arthropods was concluded for the representative uses to lettuce and leafy brassicas (data gap). A field study, performed in France, investigated the effects to non‐target arthropod populations at off‐field exposure rates. The experts agreed a no effect rate (NOER) could be derived from this study and a low risk to off‐field populations of non‐target arthropods was concluded for all representative uses provided that risk mitigation measures such as no‐spray buffer zones up to 15 m are used (see Section 8 and Appendix A). A low risk to non‐target arthropods was concluded for the representative uses restricted to permanent glasshouses.
A low risk to earthworms, other soil macroorganisms and soil microorganisms was concluded for the active substance and the soil metabolites (3‐PBA, DCVA and M310I017) for all representative uses.
A low risk to non‐target terrestrial plants and for organisms involved in sewage treatment processes was concluded for all representative uses.
The assessment of endocrine properties was discussed at the expert meeting where it was agreed that, pending on the outcome of the data gap in the mammalian toxicology (see Section 2), further consideration may be needed on potential endocrine effects in non‐target organisms.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Alpha‐cypermethrin |
Low to moderate persistence Biphasic kinetics DT50 3.8–48.1 days (DT90 45.7–329.4 days; laboratory conditions at 20°C, 40–50% MWHC soil moisture) EU and US field dissipation studies single first‐order and biphasic kinetics DT50 3.4‐46.1 days (DT90 13.4–250.5 day) |
Low risk to soil dwelling organisms. |
| 3‐PBA |
Very low to low persistence Single first order and biphasic kinetics DT50 0.38–5.0 days (DT90 1.3–16.0 days; laboratory conditions at 20°C, 40–50% MWHC soil moisture) |
Low risk to soil dwelling organisms. |
| (cis‐)DCVA |
Low to moderate persistence Single first order DT50 2.7–13.5 days (DT90 10.0–45.0 days; laboratory conditions at 20°C, 40–50% MWHC soil moisture) |
Low risk to soil dwelling organisms. |
| M310I017 |
Low to moderate persistence Single first order DT50 4.9–42.3 days (DT90 16.2–140.4 days; laboratory conditions at 20°C, 40–50% MWHC soil moisture) |
Low risk to soil dwelling organisms. |
DT50: period required for 50% dissipation; DT90: period required for 90% dissipation; MWHC: maximum water‐holding capacity.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Alpha‐cypermethrin | Harmful if inhaled |
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Alpha‐cypermethrin |
Immobile Kdoc 228,622–353,100 mL/g |
No | Yes | Yes |
| 3‐PBA |
Very high to medium mobility KFoc 46–215 mL/g |
No | No | Assessment not triggered |
| (cis‐)DCVA |
Very high to medium mobility KFoc 37–318 mL/g |
No | No | Assessment not triggered |
| M310I017 |
Immobile Kdoc 139,148–365,806 mL/g |
No | No | Assessment not triggered |
Kdoc: organic carbon linear adsorption coefficient; KFoc: Freundlich organic carbon adsorption coefficient; LD50: lethal dose, median; bw: body weight.
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Alpha‐cypermethrin |
Low risk to aquatic organisms for several exposure scenario provided that risk mitigation measures are used for all representative outdoor uses (3/9 for cereals, 3/6 for winter oilseed rape, 4/7 lettuce/leafy brassicas at 10 g a.s./ha and 0/7 for lettuce/leafy brassicas at 20 g a.s./ha). Low risk to aquatic organisms for the representative use to permanent glasshouses |
| 3‐PBA (soil, aqueous photolysis, water/sediment) | Low risk to aquatic organisms |
| 3‐PBAldehyde (aqueous photolysis) | Low risk to aquatic organisms |
| (cis‐)DCVA (soil, aqueous photolysis, water/sediment) | Low risk to aquatic organisms |
| M310I017 (soil) | Low risk to aquatic organisms |
a.s.: active substance.
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
7.1. Data gaps identified for the representative uses evaluated
Validation data of analytical method for determination of alpha‐alpha‐cypermethrin in 0.1% CMC drinking water (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 2).
Applicant to submit revalidation of method APL0445/01 (M3499‐01) (relevant for glasshouse uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 5).
Further validation data for the method used in Study SBGR. 82.298 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 5).
Appropriate revalidation of the GC‐ECD method used in Report No. SBGR.82.119 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 5).
log Pow for metabolites included in the risk assessment: on 3‐PBAldehyde and M310I017 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 5)
A method for monitoring the compounds of the residue definition for body fluids (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 5).
Considering the positive results in a non‐guideline repeat dose toxicity study in rabbits with a subsequent assessment of micronuclei formation in blood (Belgium, 2018), further scientific valid data should be provided in order to clarify these results and the mode of action for micronucleus formation and its possible link (causal or not) with inflammatory events (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Further investigations of the ED potential of alpha‐cypermethrin, at least with a male pubertal assay (including measurement of hormones) (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Further assessment of the genotoxic potential of the metabolites 3‐PBA and 4‐OH‐PBA (common with lambda‐cyhalothrin) (relevant to all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Non‐dietary exposure estimates according to the Dutch Model (relevant for glasshouse representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
The relative toxicity of the individual cypermethrin isomers, in particular the enantiomer (1S cis αR) to be addressed, or an argumentation to be provided, on how a sufficiently sound consumer dietary risk assessment can be conducted considering the change in isomer ratio in animal commodities (relevant for cereals, oilseed rape and leafy brassica; submission date proposed by the applicant: unknown; see Sections 2 and 3).
Sufficient residue trials on cucumbers compliant with the indoor GAP (relevant for the representative uses evaluated on cucumbers and courgettes; submission date proposed by the applicant: unknown; see Section 3).
Two residue trials and four residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAPs to be extrapolated to the whole subgroup of leafy brassica (relevant for the representative use evaluated on leafy brassica; submission date proposed by the applicant: unknown; see Section 3).
Sufficient residue trials on lettuces and compliant, respectively, with the NEU and SEU GAPs (relevant for the representative use evaluated on lettuces; submission date proposed by the applicant: unknown; see Section 3).
One residue trial on barley compliant with the SEU GAP (relevant for the representative uses evaluated on barley and oats; submission date proposed by the applicant: unknown; see Section 3).
Correction of the DT50 values for alpha‐cypermethrin into days of natural summer sunlight at a latitude of 30‐50°N in soil photolysis (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Formation of water photolysis products > 5% AR at two consecutive sampling (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Formation of water photolysis products > 5% AR at two consecutive sampling dates (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
The effect of water treatment processes on the nature of residues present in surface, when surface water is abstracted for drinking water (Article 4 (approval criteria for active substances) 3. (b) of Regulation (EC) No 1107/2009) has not been assessed. In the first instance, a consideration of the processes of ozonation and chlorination may be considered appropriate. If an argumentation is made that concentrations at the point of extraction for drinking water purposes will be low, this argumentation should cover metabolites predicted to be in groundwater and surface water, as well as the active substance (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Further information are required to address the risk to aquatic organisms in situations represented by the FOCUS surface water scenarios for which a high risk is indicated (relevant for the representative uses to cereals, winter oilseed rape, leafy brassicas and lettuce; submission date proposed by the applicant: unknown; see Section 5).
Further data are needed to address the high acute risk to bumblebees (relevant for the representative uses to cereals, winter oilseed rape, leafy brassicas and lettuce; submission date proposed by the applicant: unknown; see Section 5).
Further data are needed to address the risk to in‐field populations of non‐target arthropods (relevant for lettuce and leafy brassicas; submission date proposed by the applicant: unknown; see Section 5).
7.2. Data gaps identified for the maximum residue level applications
Two additional residue trials and four residue trials on kales and compliant, respectively, with the NEU and SEU outdoor GAPs are required to be extrapolated to the whole subgroup of leafy brassica (relevant for use in leafy brassica; see Section 3.2).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
8.1. Particular conditions proposed for the representative uses evaluated
Operators should use gloves to reduce exposure below the AOEL according to the German model for field uses and according to the ECPA greenhouse uses for glasshouse uses (see Section 2).
To protect aquatic organisms mitigation measures to reduce exposure to surface water are needed for all representative outdoor uses (see Section 5 and Appendix A).
To protect honeybees mitigation measures to ensure that applications are only made in the evening after bee flight are needed for all representative outdoor uses (see Section 5).
To protect non‐target arthropods mitigation measures to reduce exposure to off‐field areas are needed for all representative outdoor uses (see Section 5 and Appendix A).
8.2. Particular conditions proposed for the maximum residue level applications
No particular conditions are proposed for the MRL applications.
9. Concerns
9.1. Concerns for the representative uses evaluated
9.1.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20117 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The consumer dietary risk assessment cannot be finalised as for the provisional residue definitions for risk assessment in plants and animal commodities (pending finalisation of the assessment of the toxicity for the group of related metabolites bearing the 3‐phenoxybenzoyl moiety, the genotoxic potential of metabolites 3‐PBA and the relative toxicity of the individual cypermethrin isomers, in particular the enantiomer (1S cis αR) and the identified data gaps for additional residue trials on cucumbers, kales, lettuces and barley. Furthermore, an acute intake concern was identified for cucumbers (IESTI: 131% of ARfD, Dutch child), courgettes (IESTI: 104% of ARfD, UK toddler), kales (IESTI: 2541% of ARfD, Dutch child) and for lettuces (IESTI: 1248% of ARfD, German child) (see Section 3.1).
The consumer risk assessment is not finalised with regard to the unknown nature of residues that might be present in drinking water, consequent to water treatment following abstraction of surface water that might contain alpha‐cypermethrin and its metabolites (see Sections 3 and 4).
9.1.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
None identified for the representative uses.
9.1.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Cereals | Winter oilseed rape | Lettuce 2 × 10 g a.s./ha | Lettuce 1 × 20 g a.s./ha | Leafy brassicas 2 Lettuce 1 × 20 g a.s./ha 10 g a.s./ha | Leafy brassicas 1 × 20 g a.s./ha | Cucumber, Courgette Permanent glasshouse | |
|---|---|---|---|---|---|---|---|---|
| Operator risk | Risk identified | |||||||
| Assessment not finalised | ||||||||
| Worker risk | Risk identified | |||||||
| Assessment not finalised | ||||||||
| Resident/bystander risk | Risk identified | |||||||
| Assessment not finalised | ||||||||
| Consumer risk | Risk identified | |||||||
| Assessment not finalised | X1,2 | X1,2 | X1,2 | X1,2 | X1,2 | X1,2 | X1,2 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | |||||||
| Assessment not finalised | ||||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X | X | X | X | |||
| Assessment not finalised | ||||||||
| Risk to aquatic organisms | Risk identified | 6/9 FOCUSsw scenarios | 3/6 FOCUSsw scenarios | 3/7 FOCUSsw scenarios | X | 3/7 FOCUSsw scenarios | X | |
| Assessment not finalised | ||||||||
| Groundwater exposure to metabolites | Legal parametric value breacheda | |||||||
| Parametric value of 10 μg/Lb breached | ||||||||
| Assessment not finalised | ||||||||
a.s.: active substance; FOCUS: Forum for the Co‐ordination of Pesticide Fate Models and their Use.
Columns are grey if no safe use can be identified. The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2 Where there is no superscript number, see Sections 2, 3, 3.1, 3.2–6 for further information.
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
9.2. Issues related to the maximum residue level applications
9.2.1. Issues not finalised under the maximum residue level applications
The MRL request on leafy brassica was not supported by sufficient residue trials on kales with a possible extrapolation to the whole subgroup of leafy brassica.
9.2.2. Consumer risk identified under the maximum residue level applications
The consumer risk assessment for leafy brassica is not finalised as a MRL for the whole subgroup of leafy brassica cannot be set based on the available residue trials on kales (see data gap for additional residue trials on kales to support the NEU and SEU outdoor GAPs in Section 3.1)
Abbreviations
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- bw
body weight
- CIPAC
Collaborative International Pesticides Analytical Council Limited
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- ED
endocrine‐disrupting
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- IESTI
international estimated short‐term intake
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- Kdoc
organic carbon linear adsorption coefficient
- KFoc
Freundlich organic carbon adsorption coefficient
- LC50
lethal concentration, median
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LD50
lethal dose, median; dosis letalis media
- LOAEL
lowest observable adverse effect level
- LOQ
limit of quantification
- ME
microemulsion
- MS
mass spectrometry
- NEU
northern European Union
- NOAEL
no observed adverse effect level
- NOER
no effect rate
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PHI
preharvest interval
- Pow
partition coefficient between n‐octanol and water
- PPE
personal protective equipment
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- SSD
species sensitivity distribution
- SWAN
surface water assessment enabler
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2018.5403
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
|
alpha‐cypermethrin (1 R cis ·α S ) (1 S cis·α R ) |
racemate comprising (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate and (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C.N#C[C@H](OC(=O)[C@H]1[C@@H](/C=C(/Cl)Cl)C1(C)C)c1 cccc(Oc2ccccc2)c1 GUQZCTLEJXHSIH‐RZAVTOELSA‐N |
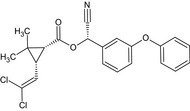
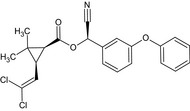
|
| cypermethrin |
(RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS;1RS,3SR)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/C1C(C(=O)OC(C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐UHFFFAOYSA‐N |
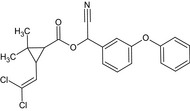
|
|
Cis ‐I (1 R cis α R and 1 S cis α S ) |
(R)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@H]1[C@@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐BJLQDIEVSA‐N (S)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@@H]1[C@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐QWFCFKBJSA‐N (enantiomer: GUQZCTLEJXHSIH‐JMSVTXOYSA‐N) |
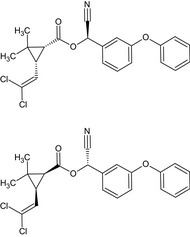
|
|
Trans ‐III (1 R trans α R and 1 S trans α S ) |
(R)‐α‐cyano‐3‐phenoxybenzyl (1R,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@@H]1[C@@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐HBFSDRIKSA‐N (S)‐α‐cyano‐3‐phenoxybenzyl (1S,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@H]1[C@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐NLWGTHIKSA‐N (enantiomer: GUQZCTLEJXHSIH‐RBTQXQDPSA‐N) |
|
|
Trans ‐IV (1 R trans α S and 1 S trans α R ) |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐GGPKGHCWSA‐N (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate Cl\C(Cl)=C/[C@H]1[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C KAATUXNTWXVJKI‐CMKODMSKSA‐N (enantiomer: GUQZCTLEJXHSIH‐TXXTYBRISA‐N) |
|
|
3‐PBAldehyde 3‐PBAld |
3‐phenoxybenzaldehyde O=Cc1 cc(Oc2ccccc2)ccc1 MRLGCTNJRREZHZ‐UHFFFAOYSA‐N |
|
| 3‐PBA |
3‐phenoxybenzoic acid O=C(O)c1 cc(Oc2ccccc2)ccc1 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |
|
| 4‐OH‐PBA |
3‐(4‐hydroxyphenoxy)benzoic acid O=C(O)c1 cc(Oc2ccc(O)cc2)ccc1 OSGCDVKVZWMYBG‐UHFFFAOYSA‐N |
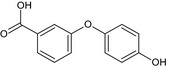
|
| 4‐OH‐PBA sulfate |
3‐[4‐(sulfooxy)phenoxy]benzoic acid OS(=O)(=O)Oc1 ccc(cc1)Oc1 cc(ccc1)C(=O)O VQSRFYGVVRDCSY‐UHFFFAOYSA‐N |
|
| 3‐OH‐benzoic acid |
3‐hydroxybenzoic acid OC(=O)c1cc(O)ccc1 IJFXRHURBJZNAO‐UHFFFAOYSA‐N |
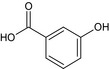
|
| 3‐PBA glycine |
N‐(3‐phenoxybenzoyl)glycine O=C(O)CNC(=O)c1cc(Oc2ccccc2)ccc1 IHTUCGBIFBJPEK‐UHFFFAOYSA‐N |
|
| cis ‐DCVA |
(1R,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropane‐1‐carboxylic acid—(1S,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropane‐1‐carboxylic acid (1/1) Cl\C(Cl)=C/[C@H]1[C@@H](C(=O)O)C1(C)C.O=C(O)[C@H]1[C@@H](/C=C(/Cl)Cl)C1(C)C QNOJQXYROGDZAX‐LNDXSTFSSA‐N |
|
| trans‐ DCVA |
(1R,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropane‐1‐carboxylic acid—(1S,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropane‐1‐carboxylic acid (1/1) Cl\C(Cl)=C/[C@@H]1[C@@H](C(=O)O)C1(C)C.O=C(O)[C@H]1[C@H](/C=C(/Cl)Cl)C1(C)C QNOJQXYROGDZAX‐RPBIHNRISA‐N |
|
| DCVA glucuronide |
1‐O‐{[(1RS,3RS; 1RS,3SR)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropyl]carbonyl}‐β‐D‐glucopyranuronic acid O=C(O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(=O)O)C1C(/C=C(/Cl)Cl)C1(C)C SCDVRNUOLGVBJK‐UUADDGCPSA‐N |
|
|
4’‐OH‐alpha‐cypermethrin M310I017 |
(R)‐cyano[3‐(4‐hydroxyphenoxy)phenyl]methyl (1S,3S)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate ‐ (S)‐cyano[3‐(4‐hydroxyphenoxy)phenyl]methyl (1R,3R)‐3‐(2,2‐dichlorovinyl)‐2,2‐dimethylcyclopropanecarboxylate (1:1) Cl\C(Cl)=C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccc(O)cc3)c2)C1(C)C.Oc1ccc(cc1)Oc1cccc(c1)[C@H](C#N)OC(=O)[C@H]1[C@@H](/C=C(/Cl)Cl)C1(C)C LKGAJGAUIXTWOT‐RZAVTOELSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; InChiKey: International Chemical Identifier Key; SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 06 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Barmaz S, Brancato A, Brocca D, Bura L, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, Ctverackova L, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans JO, Medina P, Mineo D, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Van Dijk J and Villamar‐Bouza L, 2018. Conclusion on the peer review of the pesticide risk assessment of the active substance alpha‐cypermethrin. EFSA Journal 2018;16(9):5403, 30 pp. 10.2903/j.efsa.2018.5403
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00715
Approved: 31 July 2018
Amended: 22 October 2018
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
It should be noted that harmonised classification and labelling is formally proposed and decided in accordance with Regulation (EC) No 1272/2008. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
During the experts’ meeting the RMS indicated that, according to preliminary assessment reports on metabolites 3‐PBA and 4‐OH‐PBA, the results are equivocal for genotoxicity. However, the confirmatory addendum on lambda‐cyhalothrin was not available during the meeting. Please refer to experts’ consultation 2.10 in the report of peer review 175 meeting (EFSA, 2018).
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2017. Renewal Assessment Report (RAR) on the active substance alpha‐cypermethrin prepared by the rapporteur Member State Belgium, in the framework of Commission Implementing Regulation (EU) No 844/2012, May 2017. Available online: http://www.efsa.europa.eu
- Belgium , 2018. Revised Renewal Assessment Report (RAR) on alpha‐cypermethrin prepared by the rapporteur Member State Belgium in the framework of Regulation (EC) No 1107/2009, March 2018. Available online: http://www.efsa.europa.eu
- ECHA (European Chemicals Agency), 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5. Available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- EFSA (European Food Safety Authority), 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Conclusion regarding the peer review of the pesticide risk assessment of the active substance zeta‐cypermethrin. EFSA Journal 2009;7(1):RN‐196, 119 pp. 10.2903/j.efsa.2009.196r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Conclusion on the peer review of the pesticide risk assessment of the active substance gamma‐cyhalothrin. EFSA Journal 2014;12(2):3560, 93 pp. 10.2903/j.efsa.2014.3560 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014c. Conclusion on the peer review of the pesticide risk assessment of the active substance beta‐cypermethrin. EFSA Journal 2014;12(6):3717, 90 pp. 10.2903/j.efsa.2014.3717 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance alpha‐cypermethrin. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2013. Scientific Opinion on the hazard assessment of endocrine disruptors: scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA Journal 2013;11(3):3132, 84 pp. 10.2903/j.efsa.2013.3132 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003
- European Commission , 2004. Review report for the active substance alpha‐cypermethrin. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 13 February 2004 in view of the inclusion of alpha‐cypermethrin in Annex I of Council Directive 91/414/EEC. SANCO/4335/2000 final, 13 February 2004, 76 pp.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. p.1–46
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.3, December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2008. Pesticides in air: considerations for exposure assessment. Report of the FOCUS Working Group on Pesticides in Air. EC Document Reference SANCO/10553/2006‐rev. 2, June 2008.
- JMPR (Joint Meeting on Pesticide Residues), 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


