Abstract
EFSA and EMA have jointly reviewed measures taken in the EU to reduce the need for and use of antimicrobials in food‐producing animals, and the resultant impacts on antimicrobial resistance (AMR). Reduction strategies have been implemented successfully in some Member States. Such strategies include national reduction targets, benchmarking of antimicrobial use, controls on prescribing and restrictions on use of specific critically important antimicrobials, together with improvements to animal husbandry and disease prevention and control measures. Due to the multiplicity of factors contributing to AMR, the impact of any single measure is difficult to quantify, although there is evidence of an association between reduction in antimicrobial use and reduced AMR. To minimise antimicrobial use, a multifaceted integrated approach should be implemented, adapted to local circumstances. Recommended options (non‐prioritised) include: development of national strategies; harmonised systems for monitoring antimicrobial use and AMR development; establishing national targets for antimicrobial use reduction; use of on‐farm health plans; increasing the responsibility of veterinarians for antimicrobial prescribing; training, education and raising public awareness; increasing the availability of rapid and reliable diagnostics; improving husbandry and management procedures for disease prevention and control; rethinking livestock production systems to reduce inherent disease risk. A limited number of studies provide robust evidence of alternatives to antimicrobials that positively influence health parameters. Possible alternatives include probiotics and prebiotics, competitive exclusion, bacteriophages, immunomodulators, organic acids and teat sealants. Development of a legislative framework that permits the use of specific products as alternatives should be considered. Further research to evaluate the potential of alternative farming systems on reducing AMR is also recommended. Animals suffering from bacterial infections should only be treated with antimicrobials based on veterinary diagnosis and prescription. Options should be reviewed to phase out most preventive use of antimicrobials and to reduce and refine metaphylaxis by applying recognised alternative measures.
Keywords: alternatives, antimicrobial consumption, antimicrobial resistance, control options, husbandry
Summary
Following a request from the European Commission, the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA) were asked to deliver a Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union (EU) and the resulting impacts on food safety, taking into account the impact on public health and animal health and welfare. EFSA and EMA were asked to review the measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU (Term of Reference (ToR) 1), to assess the impact of such measures regarding the occurrence of antimicrobial resistance (AMR) in bacteria from food‐producing animals and food (ToR 2), to review the recent scientific developments in the area of possible alternatives to the use of antimicrobials in animal husbandry in the EU (ToR 3), to assess the potential impact of such alternative measures on the occurrence of AMR in bacteria from food‐producing animals and food (ToR 4), and, finally, to recommend options to reduce antimicrobial use in animal husbandry in the EU, including consideration of the advantages and disadvantages of the different alternatives. Where a continued need is identified to use antimicrobials in the interests of animal health and welfare, the Opinion should recommend how such use can continue with the minimum possible risk to human health (ToR 5). In the framework of the mandate in general, the use of antimicrobials is only discussed in relation to food‐producing animals in the EU.
To assist in the formulation of this Opinion, the joint EFSA/EMA ad hoc Working Group (WG) on the reduction of the need to use antimicrobials in food‐producing animals (RONAFA) reviewed published information available on specific measures applied by the Member States (MSs), available data on the sale and use of antimicrobials in food‐producing animals, including circumstances and diseases where antimicrobials are most intensively used, AMR surveillance data and scientific publications. Additional information was also collected through questionnaires to stakeholders and one external expert, in the role of hearing expert. The focus was on cattle, pig and poultry production systems, but other food‐producing species were also considered where information was available.
For ToR 1 (review measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU), EFSA and EMA concluded that a wide range of control strategies to have been implemented in several EU MSs with the aim to combat AMR through reducing antimicrobial use in animal husbandry. Favourable results have been noted, especially in countries in northern Europe. The EC Guidelines for the prudent use of antimicrobials in veterinary medicine (PUAVM Guidelines), published in September 2015, provide practical guidance for the development and implementation of prudent use strategies. In successful programmes to reduce antimicrobial use, a multifaceted approach has been applied, reflecting the multiplicity of factors that influence antimicrobial use. Programmes have taken account of local livestock production systems and have involved all relevant stakeholders in their implementation. Some individual measures appear to have had a specific impact in driving a reduction in antimicrobial use in MSs where they have been applied: high‐level reduction targets supported in national strategies; farm‐level measurement of antimicrobial use and benchmarking; strengthening controls on group treatments, especially premixes; a requirement for antimicrobial susceptibility testing prior to use of high priority critically important antimicrobials (CIAs); and legislative and voluntary industry sector restrictions on the use of high priority CIAs. Supporting measures, such as provision of treatment guidelines and education, may have been important but have had less clear impacts.
For ToR 2 (assess the impact of such measures regarding the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food), EFSA and EMA concluded that assessing the impact of measures to reduce antimicrobial use on the occurrence of AMR in food‐producing animals and food is difficult for several reasons. For example, several measures may have been applied simultaneously, trends can only be observed where there is a sustained period of longitudinal, standardised monitoring data (which is not available from all MSs) and it is difficult to establish causality in such complex systems. Nevertheless, there are a few examples where specific measures to reduce antimicrobial use have been associated with a reduction in AMR in bacteria from food‐producing animals or foods thereof. Ecological studies have also demonstrated correlations between antimicrobial use and resistance in bacteria from food‐producing animals. Overall, it is reasonable to assume that a reduction in antimicrobial use will result in a general reduction in AMR in bacteria from food‐producing animals and food.
For ToR 3 (review the recent scientific developments in the area of possible alternatives to the use of antimicrobials in animal husbandry in the EU), all measures aimed at reducing the need to use antimicrobials were reviewed and discussed. In addition to recent scientific developments, animal husbandry measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU are also detailed. Furthermore, compounds that are presently used as alternatives to antimicrobials are also summarised.
Animal husbandry and disease prevention measures that can be implemented to improve animal health and welfare, and therefore reduce the need to use antimicrobials, can be divided into three main categories, including practices to reduce the introduction and spread of microorganisms between farms (primary prevention), to reduce transmission or spread within a farm (secondary prevention), and to increase the ability of animals to cope with these pathogens (tertiary prevention). Primary prevention includes external biosecurity, compartmentalisation and eradication measures. Secondary prevention includes internal biosecurity, production groupings, housing design, building and maintenance. Tertiary prevention includes housing, nutrition, stress reduction, vaccination and genetic selection; collectively and individually, these approaches can increase the ability of an animal's immune system to respond appropriately to an infectious challenge. Organic or similar alternative farming practices may improve housing and management conditions for animals and therefore contribute to secondary and tertiary prevention, while primary prevention may be compromised, for example, by increased levels of exposure to wildlife. In relation to reducing AMR, in the majority of the studies appraised, an association was observed between organic farming and reduced AMR. However, due to the limitations in the study design, methodologies for data analysis and biological relevance of the approach, in many of these studies, there is a potential for bias in the estimate of the association and effect of organic farming on AMR. Therefore, conclusive evidence of the impact of organic farming on reducing AMR cannot be established because of the high level of uncertainty in the appraised studies.
A literature search was undertaken to identify peer‐reviewed published articles on alternatives to antimicrobials, with the primary aim to select studies on the efficacy of the alternative measure on health parameters (e.g. reduced morbidity or mortality) and, preferably, reporting a comparison with an antimicrobial treatment. EFSA and EMA concluded that there are numerous published papers that discuss the potential of compounds and live microorganisms that may be used as alternatives to antimicrobials in livestock production. Only a limited number of studies provide robust scientific evidence that conclusively prove that the above agents are possible alternatives, positively affecting health parameters in animals. Some of the published papers describe the use of alternatives for the reduction of disease risk. The literature review has identified gaps in knowledge that limit the use of alternatives to antimicrobials in animal husbandry in the EU. For example, there are very few cases in which data on the same agent used as an alternative to antimicrobials are reported in more than one study, and most of these studies demonstrate the efficacy of these agents, but very few are clinical trials or provide robust data to demonstrate the efficacy according to the authorisation guidelines as feed additives or veterinary medicines. A positive impact on animal health parameters has been demonstrated for some of the alternatives considered. These include organic acids, probiotics, competitive exclusion, synbiotics, passive immunisation, bacteriophages, immunomodulators, Zinc oxide, clay minerals and teat sealants. Evidence on the efficacy of these alternatives, associated risks and specific knowledge gaps are listed in the Opinion.
For ToR 4 (to assess the potential impact of such alternative measures on the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food), EFSA and EMA concluded that due to the limitation in data availability, the potential impact of the alternative measures on the occurrence of AMR in bacteria from food‐producing animals and food cannot be conclusively established. Measures which reduce the need to use antimicrobials, such as improved biosecurity, control and/or eradication of infectious diseases and the alternatives identified above, are likely to reduce development of AMR indirectly. Some substances which are used as alternatives to antimicrobials (e.g. zinc oxide) may also increase selection pressure towards AMR, but this has not been investigated for other alternatives.
For ToR 5 (recommend options to reduce antimicrobial usage in animal husbandry in the EU, including consideration of the advantages and disadvantages of the different alternatives. Where a continued need is identified to use antimicrobials in the interests of animal health and welfare, recommend how such use can continue with the minimum possible risk to human health), the primary overarching objective of the recommended options is that an integrated, multifaceted approach is taken to reduce the use of antimicrobials in the livestock industry. This approach should be developed in national strategies implemented through action plans and harmonised systems for monitoring antimicrobial use and for surveillance of AMR across food‐producing animals and food derived thereof should be developed to evaluate the effectiveness of the measures taken. Recommended options (non‐prioritised) for reducing the use and need for antimicrobials include establishing targets for reduction of the use of antimicrobials, especially CIAs; development and use of on‐farm animal health management with professional input; increasing the responsibility taken by veterinarians for prescribing antimicrobials; increased oversight of preventive and metaphylactic antimicrobial use; training and education for veterinarians and for end users of antimicrobials, and raising public awareness; increasing the availability and use of rapid and reliable diagnostics and antimicrobial susceptibility tests, including at the farm level; improvement of husbandry and management procedures for disease prevention, control and eradication in livestock production, including vaccination; rethinking livestock production systems including reduced reliance on antimicrobial use and exploring further the potential of alternative production systems; and, finally, the development of treatments which are alternatives to antimicrobials. Considerations of the advantages and disadvantages of the recommended options have been provided, together with indications of the levels of responsibility (EU, national, local, etc.) for implementing these options. Of note is that all options listed should be assessed, and if necessary adjusted, in the light of local circumstances.
Antimicrobials remain a key tool for the treatment of infectious diseases in animals. In the treatment of livestock, there are three different circumstances for antimicrobial treatment: curative treatment, metaphylaxis and prevention. In all cases where administration of an antimicrobial is required, this should be prescribed following appropriate diagnosis by a veterinarian with a good knowledge of the disease epidemiology on the farm and immune status of the livestock. Approved treatment guidelines which give consideration to the responsible use of antimicrobials that are CIAs for human health should be followed.
Animals with clinical signs of a bacterial infection that is impacting on their health and welfare in many cases need curative treatment with antimicrobials. Metaphylaxis is a strategy frequently used in intensively reared animals and is appropriate when there is potential for high morbidity due to rapidly spreading disease. There should be an aim to refine and reduce the use of metaphylaxis based on identification of underlying risk factors and implementation of measures for their control. There should be an aim to phase out preventive use of antimicrobials, except in exceptional circumstances. This should be based on a structured review of such use in each sector/region and development of disease‐specific guidance.
Several knowledge gaps and uncertainties have been identified. Detailed knowledge of trends in AMR (human, veterinary, food) at both MS and local level is frequently lacking in several MSs. Inferences on the impacts of measures taken to reduce antimicrobial use would be facilitated by knowledge of antimicrobial use and AMR at an individual species and farm level. A number of treatments have been studied as alternatives to antimicrobials and some have shown the potential to be efficacious. There is a gap of knowledge in relation to their effectiveness in field conditions. National strategies and action plans on AMR do not exist or are not readily accessible for all MSs.
Recommendations for further research are diverse. For example, methodologies for AMR surveillance and monitoring antimicrobial use should be developed. Investigation is needed into the requirements for antimicrobial stewardship programmes and developing rapid diagnostic methods. As treatment options are evolving, the impact of different formulations and classed of antimicrobial and dosing regimens on the development of AMR should be assessed. In addition, development of improved vaccines against specific infections accounting for high antimicrobial use in farm production systems is needed. Additional research is needed to develop reliable alternatives to antimicrobials, investigating their mode of action and effectiveness in controlled and meaningful clinical trials. The potential of alternative farming systems on reducing AMR without compromising animal health and welfare should be further explored.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Combating antimicrobial resistance is a priority for the European Commission (EC) which launched in 2011 a 5‐year Action Plan against the rising threats from antimicrobial resistance (AMR), based on a holistic approach, in line with the ‘One Health’ initiative. The plan introduced a set of rigorous measures to fight against AMR.
Antimicrobials are necessary for treating many human and animal diseases. Any use of antimicrobials, either in human or veterinary medicine, might result in the development of AMR and has an impact on human and animal health, although the specific impact has not been quantified to date. The prudent use of antimicrobials in human and veterinary medicine is therefore a key element of the Action plan to contain resistance for the benefit of both animal and human health.
Antimicrobial agents have been used for many years in animal husbandry mostly for treatment and also for animal production purposes. Their use as feed additives for growth promotion has been banned in the European Union (EU) since 1 January 2006. The use of antimicrobial agents in animal husbandry is necessary for the treatment of animal disease. In certain cases, antimicrobials are used for prophylaxis.
Figures from the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Report 2014 (EMA ESVAC, 2016) of the sales of antimicrobial veterinary medicinal products in food‐producing species in 29 European countries accounting for approximately 95% of the food‐producing animal population in the EU/EEA area, show that a total of 8,936 tonnes of active ingredients of veterinary medicinal products were sold for use in livestock in the 29 reporting countries. The ESVAC report shows that during the last years some Member States (MSs) have introduced successful initiatives to reduce antimicrobial consumption. For 24 countries reporting sales data to ESVAC for the years 2011–2014, an overall decrease of 12% in sales (mg/PCU) was observed. Spain changed its system for collecting sales data in 2014, if Spain is included in the calculations the resulting decrease would be 2.4%. The report shows considerable variation in the use of antimicrobial agents between countries and it is of note that antimicrobial classes such as 3rd‐ and 4th‐generation cephalosporins, fluoroquinolones, aminoglycosides and polymyxins, which are classified as Critically Important Antimicrobials (CIAs) by the World Health Organisation (WHO), are sold, in substantive amounts for use in animals in some MSs. Tetracyclines were by far the most common class of antimicrobials used.
The use of antimicrobials in food production animals has come under considerable scrutiny, particularly in recent years. At the request of the European Commission, the European Food Safety Authority (EFSA) has published several opinions on this subject, sometimes independently and at other times in collaboration with the European Centre for Disease Prevention and Control (ECDC), the European Medicines Agency (EMA) and Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). EFSA and ECDC produce yearly the European Union Summary Report (EUSR) on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food. Following a request from the European Commission, EMA in collaboration with EFSA and ECDC provided a ranking of antibiotics taking into account the risk for public and animal health.
Further inter‐EU agency collaborations have resulted on the ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of AMR in bacteria from humans and food‐producing animals.
The use of antimicrobial agents in food‐producing animals has an impact on human health, although this cannot be quantified at present. Such problems were highlighted in the EFSA Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum β‐lactamases and/or AmpC β‐lactamases in food and food‐producing animals. Key conclusions from this report were that
‘since most ESBL‐ and AmpC‐producing strains carry additional resistances to other commonly‐used veterinary drugs, generic antimicrobial use is a risk factor for ESBL/AmpC and it is not restricted specifically to the use of cephalosporins. Prioritisation is complex, but it is considered that a highly effective control option would be to stop all uses of cephalosporins/systemically active 3rd– and 4th–generation cephalosporins, or to restrict their use (use only allowed under specific circumstances). As co‐resistance is an important issue, it is also of high priority to decrease the total antimicrobial use in animal production in the EU’.
Because of these concerns for public health, and the possible consequences for animal health and welfare, there is increasing focus on measures to reduce antimicrobial usage in animal husbandry by promoting prudent use initiatives, as well as exploring alternative management aspects to the use of antimicrobials in farms. In addition, there is great interest to deploy possible alternatives to the use of such agents in livestock production. Such measures range from changes in husbandry practices, improved biosecurity, to more direct interventions such as the use of vaccines, immune modulation, interventions aimed to influence gut microbiome, bacteriophage therapy and competitive exclusion, to name a few examples.
The European Commission requests jointly to EFSA and EMA, taking into account the impact on public health and animal health and welfare, to:
review the measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU;
assess the impact of such measures regarding the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food;
review the recent scientific developments in the area of possible alternatives to the use of antimicrobials in animal husbandry in the EU;
assess the potential impact of such alternative measures on the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food;
recommend options to reduce antimicrobial usage in animal husbandry in the EU, including consideration of the advantages and disadvantages of the different alternatives. Where a continued need is identified to use antimicrobials in the interests of animal health and welfare, recommend how such use can continue with the minimum possible risk to human health.
1.2. Interpretation of the Terms of Reference and modus operandi
The above terms of reference (ToR) have been further discussed and clarified by EFSA, EMA and the European Commission, the requestor of the mandate. Each individual ToR is further examined below and its interpretation in the framework of this Scientific Opinion is presented.
To assist in the formulation of this Opinion, a joint EFSA/EMA ad hoc Working Group (WG) on the reduction of the need to use antimicrobials in food‐producing animals (RONAFA) was convened. The RONAFA WG has reviewed published information available on specific measures applied by MSs, available data on the sale and use of antimicrobials in food‐producing animals, including circumstances and diseases where antimicrobials are most intensively used, AMR surveillance data and scientific publications. Additional information was also collected through questionnaires to stakeholders and one external expert, in the role of hearing expert. The focus was on cattle, pig and poultry (all poultry species) EU production systems, but other food‐producing species were also considered where information was available.
1.2.1. Terms of Reference 1 and 3
In the framework of this ToR and of this mandate in general, the use of antimicrobials is only discussed in relation to animal species used as food‐producing animals in the EU. No other animal species are considered.
Under ToR 1, the Opinion will review the measures that have been or are being applied to reduce the use of antimicrobials. The starting point, and main focus of the review, will be the measures that have been already identified in the EC Guidelines for the prudent use of antimicrobials in veterinary medicine (EC PUAVM Guidelines).1 , 2 Additionally, under ToR 3, the Opinion will review the measures aimed at reducing the need to use antimicrobials, including alternatives to antimicrobials and recent scientific developments. Development of new antimicrobials is not covered by this mandate and will not be discussed. The Opinion will cover measures related to the prudent use of antimicrobials and the selection of appropriate antimicrobials for treatment, as well as those measures aimed at preventing the establishment of infections in animals, including for example enhanced biosecurity, vaccination, etc. In particular, measures will be considered when they may have an implication on the level of AMR in zoonotic pathogenic or commensal bacteria, rather than on bacteria that are pathogenic only to animals.
1.2.2. Terms of Reference 2 and 4
As mentioned in the background of the mandate, the use of antimicrobial agents in food‐producing animals is recognised potentially to have an impact on human health. The overall aim of the mandate is to review available information and recommend the most appropriate options to reduce the need for and the use of antimicrobials in food‐producing animals in the EU, with the ultimate goal of protecting public health from AMR‐related risks acquired through the food‐borne route and food‐producing animals.
Due to data and time constrains, this assessment will not go further than measuring potential exposure, and there will not be any attempt to quantify the impact of the measures in terms of reduction of human disease or of occurrence of AMR bacteria in humans. The study of the association between the use of antimicrobials in animals and AMR in animals and humans is not in the scope of this assessment, and is an object of other activities of ECDC, EFSA and EMA. Under ToRs 2 and 4, the Opinion will assess the impact of the identified applied and alternative measures on the occurrence of AMR in bacteria in food‐producing animals and food thereof. This assessment will support the identification of those measures that are expected to have the highest impact.
In this respect, there are some limitations on what is possible considering the availability of EU data. The assessment will evaluate, in a qualitative way, the impact of the measures reviewed based on the data on the use of antimicrobials (e.g. ESVAC), and on the occurrence of resistance in bacteria which have been/are under official EU monitoring on a mandatory or voluntary basis (e.g. Salmonella spp., Campylobacter spp., indicator E. coli, indicator Enterococcus, meticillin‐resistant Staphylococcus aureus (MRSA)) in food‐producing animals and food where available. These zoonotic pathogens or commensals could be transmitted to humans and either cause disease or be the source of resistance genes. It is assumed that their levels in food‐producing animals and food thereof correlate with the exposure of humans to resistant bacteria/resistance genes. When the impact of the measures on the use of antimicrobials or on the occurrence of AMR is not clear or there are not sufficient data to assess it, data gaps and limitations will be indicated and explained.
It should be further understood that, due to the complexity of the factors contributing to AMR development (e.g. occurrence of cross‐ and co‐resistance, fitness costs and compensatory mechanisms, dissemination clonally and via transfer of mobile genetic elements) and because multiple measures may have been implemented simultaneously, it may be difficult to attribute any impacts directly to individual actions.
1.2.3. Term of Reference 5
The recommendations formulated in answer to ToR 5 will be focused on desired benefits in terms of public health. Advantages and disadvantages of the options proposed will be discussed.
The Opinion will identify the circumstances under which continued use of antimicrobials in food‐producing animals is necessary for animal health and welfare reasons, and discuss how such continued use can be made so to take into account the primary aim of enhancing the protection of public health.
1.2.4. Other issues
Ionophore compounds are used as coccidiostats in livestock species and, in dairy cattle, monensin is used for the prevention of ketosis. These compounds present an antimicrobial activity against Gram‐positive bacteria and have been used in the past as growth promoters in animal farming. Since ionophores are not used to treat infectious disease in humans, the use of these compounds will not be considered in the Opinion.
The effect of the use of antimicrobials in food‐producing animals on the development of AMR in the environment is also outside the remit of this Opinion, and thus will not be assessed.
Similarly, financial consideration related to the implementation of the measures discussed in this Opinion will not be considered.
1.3. Definitions
Definitions of antimicrobials, antimicrobial resistance and resistance genes, together with antimicrobial use terminology are provided in the Glossary.
Throughout the document, the term ‘antimicrobial’ has been used in place of ‘antibiotic’ or ‘antibacterial’.
1.4. Emergence and transmission of antimicrobial resistance, priority antimicrobials and microorganisms
Fundamental to the emergence and spread of bacteria exhibiting AMR are the processes of selection and dissemination.
1.4.1. Selection
Selection occurs when a single AMR bacterium in a population is provided with the opportunity to become more prevalent as a result of the killing or suppression of the previously dominant sensitive population. Such opportunities are afforded by selection following the application of an antimicrobial or antimicrobials to which the organism exhibits reduced susceptibility or clinical resistance. The single AMR organism then survives to reproduce, often in an exponential progression, until a new equilibrium is reached, thereby becoming more dominant organism within the bacterial population (Baquero, 2011). In the treated host, the selection process is driven by the drug pharmacokinetics and dosage regimen. The dosage regimen is defined by the dosage (mg/kg), the route of administration (formulation), the treatment interval and the treatment duration. Variation in the regimen creates different selection windows (time and concentration levels) of bacterial populations (microbiota) in different locations (digestive tract, skin, infected tissue, etc.). It is often claimed that AMR has not been a major issue for the treatment of animal infections, but quantitative surveillance data suggest otherwise (DeDonder et al., 2016) and such resistance to simple antimicrobials, especially in intestinal organisms, has been partly responsible for increased use of priority antimicrobials in food‐producing animals in recent years.
1.4.2. Dissemination
The dissemination of AMR genes is a consequence of a variety of interactions between many biological vehicles containing such genes and is summarised in Table 1.
Table 1.
Biological entities and factors contributing to the selection and dissemination of AMR (adapted from ECDC, EFSA and EMA, 2015)
| Biological entities | Description | Process of resistance transmission | Further considerations |
|---|---|---|---|
| AMR genes |
Number and size of genes coding for resistance Mechanism of resistance (mutation or gene located on mobile genetic element(s)) Functions encoded Copy number |
Vertical spread | Cross‐resistance |
| Genetic environment |
Chromosome Mobile genetic elements (transposons and/or plasmids) |
Bacterial multiplication Recombination events Conjugation Transformation Transduction |
Co‐resistance Fitness in cell |
| Bacterial cells |
Expressed resistance phenotype against antimicrobials Expressed phenotype in microenvironment |
Spread of bacterial population Carriage by different hosts (food‐producing animals, wildlife animals, human beings) Survival in the environment (e.g. water, soil, dust) |
Antimicrobial susceptibility pattern Growth rate Associated virulence characteristics Associated colonisation characteristics Host environment (e.g. farm, hospital) |
| Bacterial population/microbiota |
Bacterial species Diversity Connectivity Intrinsic and historical changes in antimicrobial susceptibility (pharmacodynamic variability) |
Spread between bacterial species Coevolution |
Level of antimicrobial concentrations |
| Host |
Frequency of drug exposure Pharmacokinetics variability |
Selection window in different body location |
Drug elimination in environment Bacterial transfer between hosts Bacterial emission in environment |
| Human and animal population |
Contact between individuals (animal/animal, human/human) Contact between animal/human |
Rate of transmission |
Animal end products Food from animal origin Animal excreta Human excreta |
| Environment |
Emission of excreta in environment Bacterial load |
Transmission in water Exposure of soils and vegetables Dust |
Mode of treatment Antimicrobial concentrations |
Aspects of the evolution and organisation of resistance mechanisms that may influence the likelihood of transfer of AMR from animals to humans were considered by the EMA Antimicrobial Advice ad hoc Expert Group (AMEG) (EMA, 2014) for the purposes of providing a categorisation of antimicrobials based on their risk to public health from AMR development following the use of in animals. Five factors were identified:
The presence of a chromosomal mutation or mutations contributing to the development of resistance to a clinically relevant antimicrobial – e.g. to quinolones and fluoroquinolones. Chromosomal mutations occur randomly and a single mutation may give rise to high‐level resistance, or a series of stepwise mutations may be required before resistance of clinical importance develops. Where mutations are stable, this fosters clonal spread of resistance. A single mutation can confer resistance to several substances within a related antimicrobial class (cross‐resistance).
Organisation of non‐chromosomal resistance genes into horizontally transferable elements (Carattoli, 2009), enabling localisation on deoxyribonucleic acid (DNA) outside the bacterial chromosome (e.g. conjugative or mobilisable plasmids, transposons, integron‐gene cassettes). Depending on the plasmid and the presence or absence of genes, transfer of genetic elements encoding for AMR may be transferred between related or distinct bacterial species.
The presence of a cluster of linked AMR genes, facilitating co‐selection of resistance to one substance during exposure to an unrelated substance. Co‐selection may involve resistance to heavy metals or, less commonly, tolerance to residual level of biocides such as triclosan or quaternary ammonium compounds such as benzalkonium chloride used in the food industry and on farms (Nhung et al., 2015; Wales and Davies, 2015).
The potential for transmission of resistance through zoonotic and commensal food‐borne bacteria. The gut microbiota is considered as the largest reservoir of transferable/mobilisable resistance genes, not only within livestock (Looft et al., 2012) but also in humans (Sommer et al., 2010) and bacteria present in the gut can act as donor, vector or recipients of AMR genes.
Other factors, such as the incorporation of plasmid‐ or transposon/integron‐mediated resistance into the bacterial chromosome, the presence of plasmid addiction systems and other mechanisms contributing to plasmid stability in bacterial cells all serve to maintain AMR genes within a bacterial population and lessen their chance of loss when antimicrobial selection pressure is withdrawn.
In addition to the factors listed above, that for the most part relate only to genetic mechanisms, there are many others that may affect the emergence and spread of AMR and the probability of transfer of AMR bacteria or the determinants therein from animals to humans. Such factors include dosage, including underdosage (Kohanski et al., 2010; van der Horst et al., 2011; Callens et al., 2012; Pardon et al., 2012a), dosing regimens, including volume of use (Chantziaras et al., 2014), administration route (Burow and Käsbohrer, 2016) and animal husbandry conditions (Catry et al., 2016). There are many pathways through which resistance can be transmitted between animals, humans and the environment (Landers et al., 2012). There are also examples of the transmission of AMR from humans to food‐producing animals (e.g. certain strains of MRSA), and thence back to animals, but such transfer happens predominantly via direct contact with living animals (Bal et al., 2016). Such transfer has not been considered in depth in the context of this Opinion as it has been reviewed elsewhere (Catry et al., 2010).
Antimicrobials are used in food‐producing animal production for treatment and prevention of a large number of infections and, although banned in the EU, in many countries outside the EU for growth promotion (Shea et al., 2004). The emergence of AMR bacteria and selection of resistance genes following the use of antimicrobials is widely acknowledged and all antimicrobials can select for resistance to varying degrees. Nevertheless, knowledge of the occurrence of AMR in food‐producing animals in relation to the quantitative impact of the use of different treatment regimens on the selection for resistance, together with information on the best choices of therapy to limit the development of AMR, remains incomplete, as do the relative contributions of antimicrobial use in both human and veterinary medicine (Aarestrup, 1999, 2000; Levy, 2014).
Dissemination of AMR from hospitals, or more generally sewage, into the environment via waste and the excrement of treated patients is increasingly perceived as a threat to public health, and a possible original source of AMR organisms and resistance genes that may be further disseminated by animals exposed to contaminated water or waste (Acar and Rostel, 2001). Manure from animal production may be an important route for contamination of the environment with AMR organisms.
Concern over AMR bacteria causing human infections that are difficult to treat has led to a proliferation of studies investigating resistance in livestock, food products, the environment and people, as well as in the mechanisms of transfer of the genetic elements of resistance between bacteria, and the routes, or risk pathways, by which the spread of AMR might occur. The possibility of transfer of genetic elements conferring resistance to antimicrobials between bacteria in mixed populations adds many additional and complex potential routes of spread. There is considerable evidence that transfer of AMR, such as that encoded by extended‐spectrum beta (β)‐lactamase (ESBL)‐related genes, from food‐producing animals to humans directly via the food chain is a likely route of spread (EFSA BIOHAZ Panel, 2011; Maciuca et al., 2015). Although undoubtedly important, the role of the environmental transmission of resistance is not considered relevant to this Opinion, which is targeted at strategies to reduce antimicrobial use in the food‐producing animal sector and will not be discussed further.
1.4.3. Priority organisms, priority antimicrobials and their use in animal husbandry, and resistance development
The following priority organisms, priority antimicrobials and their use in animal husbandry and resistance development have been highlighted in various studies and are addressed in this document.
1.4.3.1. Priority Organisms
Pathogenic bacteria
The pathogenic microorganisms of importance to public health in relation to food safety that have been primarily addressed are Salmonella spp., Campylobacter spp., and pathogenic Escherichia coli. For AMR in pathogenic E. coli from animals, there are insufficient data about its zoonotic potential for meaningful conclusions (van Hoek et al., 2016).
Commensals
The commensal indicator organisms considered are non‐pathogenic E. coli and Enterococcus spp.
1.4.3.2. Antimicrobials
The WHO has classified certain antimicrobial classes as ‘Critically Important Antimicrobials for human medicine’ (WHO, 2012). These include: cephalosporins (3rd‐ and 4th‐generation); quinolones (including fluoroquinolones), aminoglycosides, macrolides, penicillins and polymyxins. These antimicrobial classes have been used in some countries in the EU as first‐line treatment for a variety of infections in veterinary medicine. Using the WHO list as a basis, in 2013, the AMEG categorised the CIAs according to the risk to public health and advised that the fluoroquinolones and systemically administered 3rd‐ and 4th‐generation cephalosporins should only be used in veterinary medicine when there is no alternative available (Category 2, higher risk to public health) (EMA, 2013a). Although the above antimicrobial classes are considered of high importance, because of the issue of co‐resistance (see Glossary), resistance to other antimicrobial classes (e.g. tetracyclines) is discussed where considered relevant.
1.4.3.3. Combinations – organisms and priority resistances
ECDC, EFSA, EMA and SCENIHR (2009) selected the following combinations of microorganisms’ antimicrobials as the ones of major concern and relevance for public health:
Salmonella spp. – quinolone resistance;
Campylobacter spp. – quinolone resistance;
Salmonella spp. – cephalosporin resistance (3rd‐ and 4th‐generation);
Campylobacter spp. – macrolide resistance.
Following recent concerns about the appearance and spread of plasmid‐mediated resistance to colistin (see below), and its increasing importance in human medicine to treat carbapenem‐resistant infections, this substance has been reviewed by the AMEG (EMA, 2016b) and is now considered as an antimicrobial of high concern to be included in Category 2. Therefore, this report will also consider:
Enterobacteriaceae – transferable colistin resistance.
1.4.4. Key emerging issues in relation to the food‐borne/zoonotic transmission of AMR
Key emerging issues in relation to the food‐borne transmission of AMR that have been identified include:
transferable resistance to colistin mediated by mcr genes in livestock and humans;
the emergence over the last decade of multidrug‐resistant (MDR)/ciprofloxacin‐resistant (Cipr) isolates of Salmonella Stanley, S. Infantis, S. Kentucky, S. Heidelberg (USA), S. Enteritidis (Far East), S. Typhimurium (Africa) and the more recent emergence in the Netherlands of extended‐spectrum cephalosporin‐resistant (ESC) S. Heidelberg, which can cause human infections in food‐producing animals and poultry meat;
MDR monophasic Salmonella organisms which are now prevalent in pigs in most EU MSs (ECDC and EFSA, 2016);
the ongoing spread of livestock‐associated MRSA (LA‐MRSA) in certain high‐risk groups of people/workers in direct contact with live animals and the spread of MRSA in pigs and other species (Catry et al., 2010);
high to very high levels of resistance to fluoroquinolones and tetracyclines in isolates of Campylobacter spp. from humans and from broilers in several EU MSs in 2014 (ECDC and EFSA, 2016);
increasing levels of resistance to 3rd‐ and 4th‐generation ESBL‐producing organisms in community patients and livestock (EFSA BIOHAZ Panel, 2011).
This list is not comprehensive, and will undoubtedly change as new issues emerge. More details of the above issues are presented in Appendix A.
1.5. EU level surveillance and monitoring programmes for AMR and antimicrobial use
1.5.1. EU Member States – harmonised surveillance of AMR in food‐producing animals and food thereof
At the EU level, the monitoring and reporting of antimicrobial resistance in the main livestock animal species (cattle, pigs, poultry) and derived food is regulated by Commission Implementing Decision 2013/652/EU.3 This Decision aims at enlarging the scope of the monitoring and harmonising data collection between MSs. It establishes a list of combinations of bacterial species, food‐producing animal populations and food products, as well as technical requirements regarding the sampling framework, the panel of antimicrobials to be used for testing resistance, and indications on the laboratory analytical methods and data reporting. According to this Decision, representative isolates of Salmonella spp., Campylobacter jejuni, indicator commensal E. coli and ESBL‐, AmpC‐ or carbapenemase‐producing E. coli shall be collected by MSs, which can also voluntarily collect isolates of Campylobacter coli and indicator commensal Enterococcus faecalis and Enterococcus faecium. Isolates should be collected from caecal samples and carcasses, depending on the animal species, which include laying hens, broilers, fattening turkeys, fattening pigs and bovines under 1 year of age.
Data from routine monitoring are collected by the EU MSs on a rotating basis and according to a biannual schedule. They are reported to EFSA on a yearly basis, analysed and presented yearly in the EU Summary Report on AMR in zoonotic and indicator bacteria from humans, animals and food (ECDC and EFSA, 2016). ECDC contributes to this report by producing analysed data on resistance in Salmonella and Campylobacter from humans.
The first report on AMR in zoonotic and indicator bacteria from humans, animals and food in 2009 was published in 2011 (ECDC and EFSA, 2011). Resistance was commonly found in isolates from humans, animals and food, although disparities in resistance were frequently observed between MSs. High resistance levels were recorded to ampicillin, tetracyclines and sulfonamides in Salmonella isolates from humans, while resistance to 3rd‐generation cephalosporins and fluoroquinolones remained low. In Salmonella and indicator E. coli isolates from fowl, pigs, cattle and meat thereof, resistance to ampicillin, tetracyclines and sulfonamides was commonly detected, while resistance to 3rd‐generation cephalosporins was low. Moderate to high resistance to ciprofloxacin was observed in Salmonella and indicator E. coli isolates from fowl, broiler meat and pigs. In campylobacter isolates from both human cases and from fowl, broiler meat, pigs and cattle ciprofloxacin, nalidixic acid and tetracyclines resistance was high, while resistance to erythromycin was low.
In the latest report for 2014 (ECDC and EFSA, 2016), for non‐human cases, 28 MSs and three non‐MSs reported data on AMR in tested Salmonella spp. and Campylobacter spp., and commensal E. coli isolates from various poultry populations and/or related meat derived thereof, sampled through harmonised national schemes. Resistance was interpreted using EUCAST ECOFF values. Data on MRSA and on specific monitoring of E. coli ESBL‐/AmpC‐/carbapenemase‐producers were reported on a voluntary basis.
In contrast to previous reports, the 2016 report only included figures from poultry and meat thereof. With regard to Salmonella spp. and E. coli isolates from broilers, fattening turkeys and meat thereof, resistance to ampicillin, (fluoro)quinolones, tetracyclines and sulfonamides was frequently detected, whereas resistance to 3rd‐generation cephalosporins was uncommon. The occurrence of ESBL‐/AmpC‐producers, monitored for the first time among these bacteria, was low, and carbapenemase‐producers were not detected. A low level of resistance to colistin was observed in Salmonella spp. and E. coli from poultry and meat thereof. A minority of Salmonella isolates from animals belonging to a few serovars (notably Kentucky and Infantis) exhibited high‐level resistance to ciprofloxacin. Colistin‐resistant Salmonella isolates were found by several MSs originating from broilers, laying hens and fattening turkeys, but high‐level colistin resistance (minimum inhibitory concentration (MIC) > 16) was not reported.
With regard to isolates of Campylobacter spp. from broilers and broiler meat, a high resistance to ciprofloxacin and tetracyclines was observed, whereas much lower levels were recorded for erythromycin.
Generally, co‐resistance to CIAs was uncommon in animal isolates, but high MDR levels (sometimes very high or extremely high) were observed in some Salmonella serovars and in indicator and commensal E. coli.
Monitoring of trends has been possible in the last years for those MSs reporting consistently for several years. The implementation of Decision 2013/652/EU should enable trends, for the combinations of bacteria/antimicrobials for which data are collected, to be monitored.
In addition to compulsory surveillance of AMR carried out under Decision 2013/652/EU, various MSs within the EU have national surveillance programmes for monitoring AMR development in zoonotic and indicator bacteria from animals, and animal pathogens. These are discussed in Section 3.3.2.
Although there is information available from the above‐mentioned surveillance programmes on the occurrence of AMR in bacterial species in raw meat, it should be noted that data are lacking on the prevalence of AMR bacteria in ready‐to‐eat (RTE) foods, in particular those of animal origin such as cheese and meat products. Consequently, no information is available on the consumer exposure to this potential hazard.
Beyond the EU harmonised monitoring of AMR in food‐producing animals and food thereof under Decision 2013/652/EU, methodologies for sampling and testing for the presence of target and zoonotic pathogens/indicator organisms and antimicrobial susceptibility are not standardised across all MSs. This complicates intercountry comparisons.
1.5.2. Use of antimicrobials based on sales data ‐ ESVAC
As yet, there is no legal requirement at the EU level for the collection of data on the consumption of antimicrobials in animals, although this is envisaged in the draft veterinary medicinal products legislative proposal. Nevertheless, there are several publications of on‐farm use in different MSs (Bos et al., 2015; Postma et al., 2015a), and there has been considerable discussion on units of measurement for on‐farm use (Taverne et al., 2015). The ESVAC project collects and publishes data on the overall sales of veterinary antimicrobial agents, voluntarily submitted to EMA from 29 European countries. In October 2016, the sixth annual report of the series was published (EMA ESVAC, 2016). Data are collected at package level, using a standardised protocol, from different sources, which include marketing authorisation holders, wholesalers, feed mills, pharmacies and veterinarians.
The main indicator for reporting sales of antimicrobials is mg active ingredient sold per population correction unit (mg/PCU). The PCU is a technical unit of measurement mostly based on official EU/EEA data (Eurostat and TRACES) that is used to estimate sales corrected by the animal population (kg biomass at time of treatment) in individual countries. As the sales data cover all food‐producing species, when interpreting the measure it should taken into account that there are differences in species distributions and livestock systems between countries.
In the future, a goal of ESVAC is to provide a standardised measurement of consumption by species that takes into account differences in dosing regimens. This will facilitate comparison of consumption between countries. The technical units of measurement, Defined Daily Dose animal (DDDvet) and Defined Course Doses (DCDvet), were published in April 2016 and are available from the ESVAC web pages.
Figure 1 reports the total sales of veterinary antimicrobial agents for food‐producing animals related to years 2011–2014, as reported by EMA ESVAC (2016).
Figure 1.
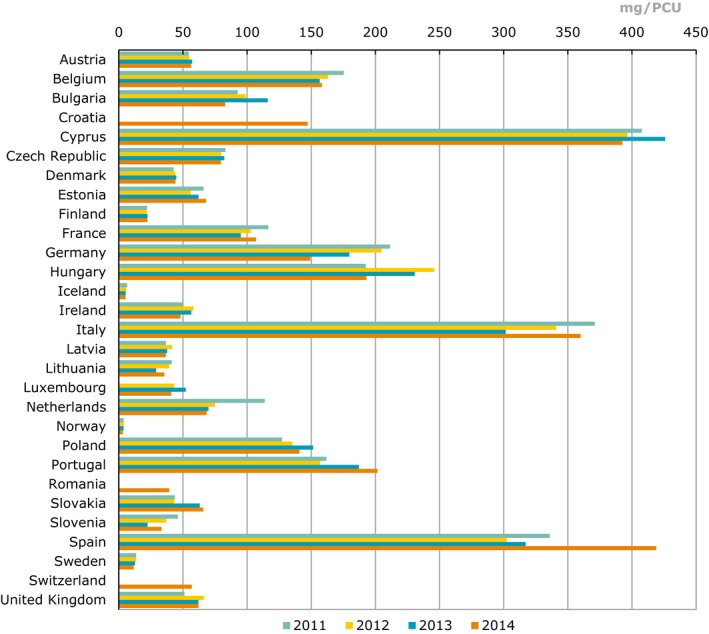
Total sales of veterinary antimicrobials agents for food‐producing species, in mg/PCU, from 2011 to 2014, for 29 European countries (EMA ESVAC, 2016)
- Correction of sales data and/or PCU data published in ESVAC 2013 report is described in section 1.6 (EMA ESVAC, 2015b). Under‐reported for Bulgaria for 2011 and 2012 as several wholesalers failed to report data. Strength reported as base for most VMPs for 2011‐2012 for the Czech Republic; for 2013 and 2014, strength reported as in the label of the VMPs. Strength reported as base for some VMPs for 2011–2012 for the Netherlands; for 2013 and 2014, strength reported as in the label of the VMPs. For Slovakia, for 2011 and 2012, the data only represents antimicrobial VMPs imported by wholesalers; for 2013 and 2014, data represents all sales from wholesalers to end users (veterinarians, pharmacies, producers of medicated feeding stuffs and farmers, obtained by import and from national manufacturers). For Spain, under‐reporting for the years 2011–2013 has been identified (underestimated). For the UK, high sales of certain tetracycline‐containing products late in 2010 were probably used in 2011 and thus the use has been underestimated for 2011.
Key points from EMA ESVAC (2016), which reports sales from the year 2014, include the following:
across the EU the estimated weight at treatment of livestock and of slaughter animals was (in 1,000 tonnes): pigs (19.6), cattle (18.9), poultry (8.1), sheep/goats (7.8), fish (2.3), horses (2.2), rabbits (0.2);
there was a large variation in the sales of antimicrobials across MSs, ranging from 3.1 to 418.8 mg/PCU. It is unlikely that this could be explained by differences in the composition of the animal population, or formulations and treatment regimens used, alone;
91.6% of overall antimicrobials sales were for oral administrations, which can be assumed largely for group treatment, with 42.1% of sales as premixes;
7.6% of sales were injectable preparations and 0.5% intramammaries. The most frequently used antimicrobials were tetracyclines (33.4%), penicillins (25.5%) and sulfonamides (11.0%);
of the CIAs, polymyxins (mostly colistin) account for 6.6% of sales, fluoroquinolones for 1.9%, and 3rd‐ and 4th‐generation cephalosporins for 0.2%. There were no formulations of 3rd‐ and 4th‐generation cephalosporins that were applicable for group treatments. For polymyxins, 99.8% of sales were formulations for oral group treatments. For fluoroquinolones, 76.0% of sales were of oral solutions, and 24.0% as injections (EMA ESVAC, 2016);
prescribing patterns for the different antimicrobial classes varied substantially between countries;
for 24 countries reporting sales data to ESVAC for the years 2011–2014, an overall decrease of 12% in sales (mg/PCU) was observed. Spain changed its system for collecting sales data in 2014, if Spain is included in the calculations, the resulting decrease would be 2.4%.
1.5.3. Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report
In January 2014, the first joint report from ECDC, EFSA and EMA on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals from 2011–2012 (the JIACRA report) was published (ECDC, EFSA and EMA, 2015).
The report utilised data from 2011 and 2012, from five different surveillance networks, collecting information about AMR in humans and food‐producing animals and food thereof from the EU MSs, Iceland, Norway, Croatia and Switzerland. The organisms studied were Salmonella spp., Campylobacter spp. and indicator E. coli. Depending on the organism, the antimicrobials surveilled were tetracyclines, cefotaxime (representative of 3rd‐generation cephalosporins), ciprofloxacin (fluoroquinolones), and erythromycin (macrolides). Information on antimicrobial sales and use came from relevant ESVAC reports.
The main findings were that consumption in food‐producing animals was lower or much lower than in humans in 15 of 26 countries, in three countries they were similar, and in eight countries consumption in food‐producing animals was higher or much higher than in humans. Overall, a positive association was observed between antimicrobial consumption in food‐producing animals and occurrence of resistance in bacteria from such animals. The strongest associations between consumption and resistance in food‐producing animals were detected for the antimicrobials studied in relation to indicator E. coli. Positive associations were similarly noted for Salmonella spp. and Campylobacter spp.
A positive association was observed between the total consumption of 3rd‐ and 4th‐generation cephalosporins in humans and the occurrence of resistance to 3rd‐generation cephalosporins. A positive association was similarly observed between the total consumption of fluoroquinolones in humans and the occurrence of fluoroquinolone resistance in E. coli from humans. No association was found between the consumption of fluoroquinolones in humans and the occurrence of fluoroquinolone resistance in Salmonella spp., S. Enteritidis and S. Typhimurium from cases of human infection.
For both cephalosporins and fluoroquinolones, positive associations were found between occurrence of resistance in indicator E. coli originating from food‐producing animals and the occurrence of resistance in E. coli from humans.
No associations were observed between the consumption of 3rd‐ and 4th‐generation cephalosporins in food‐producing animals and the occurrence of resistance to this subclass in selected bacteria from humans. No associations were observed between the consumption of fluoroquinolones in food‐producing animals and the occurrence of resistance in Salmonella spp. and Campylobacter spp. from cases of human infection. Positive associations were also noted for consumption of macrolides in food‐producing animals and the occurrence of resistance in Campylobacter spp. from cases of human infection, and for consumption of tetracyclines and the occurrence of resistance in Salmonella spp. and Campylobacter spp.
In the reported analyses, associations between the consumption of selected combinations of antimicrobials and the occurrence of resistance in bacteria were mostly, but not always, observed. In particular, it was noted that the epidemiology of resistance is complex, and several factors aside from antimicrobial consumption influence occurrence of resistance.
The RONAFA WG has noted that an updated JIACRA report is underway, with a publication target of mid‐2017.
1.6. Overview of measures in place at the EU and international level
1.6.1. Withdrawal of antimicrobial growth promoters
1.6.1.1. Introduction
Gut bacteria rapidly respond to antimicrobials such as antimicrobial growth promoters (AGPs) by activating systems to avoid the antimicrobial effects of the drugs, while presumptively attenuating their overall energetic metabolic status and the capacity to transport and metabolise bile acid, cholesterol, hormones and vitamins. Antimicrobials targeting specific pathogenic infections may alter gut microbial ecology and interactions with host metabolism more profoundly than previously assumed (Elena Perez‐Cobas et al., 2013).
Following widespread concern about the effects that the use of AGPs might have on the development and spread of resistance in livestock, the use of such compounds in food‐producing animals have been progressively banned in EU MSs. Following recommendations from a UK government committee which reported in 1969 (Swann et al., 1969), certain antimicrobials such as penicillin and tetracyclines were withdrawn from the list of approved AGPs in the UK and the EU in the seventies. Examples of compounds that were still in use after this restriction were mainly those with an anaerobic and Gram‐positive spectrum, among others macrolides (spiramycin, zinc bacitracin, tylosin), streptrogramins (virginamycin, conferring cross‐resistance to quinipristin/dalfopristin), glycopeptides (avoparcin, conferring cross‐resistance to vancomycin), flavomycin (flavophospholipol) and everninomycin (avilamycin). Carbadox and olaquindox, which are active against swine enteritis were also authorised for growth promotion (Casewell et al., 2003).
Enterococci resistant to the antimicrobial class of glycopeptides (avoparcin and vancomycin) (GRE) were retrieved with increasing frequency from patients in Scandinavian countries during the late eighties. Soon afterwards, vancomycin‐resistant enterococci (VRE) were found in farmed animals (Bates et al., 1993), and were recovered from food and faeces from poultry, which received the livestock analogue glycopeptide, avoparcin, as an AGP (Bates et al., 1993; Klare et al., 1995). Since then, several studies have been performed to establish the relationship between use of avoparcin as growth promoters in animals and VRE epidemiology (Hammerum et al., 2010). The gene responsible was the vanA gene. Several studies show the decline of GRE prevalence after the ban, but recent studies in Scandinavian countries that had banned the use of avoparcin showed that GRE are maintained in the microbiota of animals. The underlying reason was co‐selection by the linking of vanA gene with the ermB gene. The ermB gene causes resistance in Gram‐positive bacteria for macrolides, lincosamides and streptogramins (B), and this linking was responsible for the persistence of GRE through the use of macrolides (erythromycin, tylosin) as AGPs. In 1986, Sweden became the first EU MS to categorically ban the use of AGPs in livestock (Wierup, 2001). Avoparcin was banned in Denmark and Norway in 1995, in Germany in 1996 and in the rest of the EU in 1997. Supported by the report of an expert committee in 1998, ‘the Copenhagen recommendations, the EU followed from 1999 onwards the action which was already in place in certain Scandinavian countries to gradually (but eventually completely) restrict all use of AGPs by 1 January 2006. In this respect, the first EU legislation banning the use of AGPs was introduced in 2003 (Regulation (EC) No 1831/20034), and legislation banning the final four remaining AGPs (sodium monensin, sodium salinomycin, avilamycin and flavophospholipol) was completed in 2006.
1.6.1.2. Impacts of withdrawal on animal health
A major problem in assessing the impact of the withdrawal of AGPs has been the lack of knowledge with regard to the exact mechanism of AGPs. Assessments of the influence of AGPs have been performed, but case‐control studies are limited and biased since previous exposure during consecutive production rounds will have altered the herd related immunity, the microbial flora including AMR genes in the different production types (Heuer et al., 2002b). In addition, management interventions, including measures such as downtime for buildings and vaccination strategies will alter the infection status of the herds. Furthermore, some AGPs such as tylosin had some systemic antimicrobial effects and the distinction between growth promotion and prophylaxis/prevention has not always been clear (Dibner and Richards, 2005).
1.6.1.3. Positive impacts
Several positive results have been documented in various EU countries following the reduction in use of specific AGPs prior to the EU ban on the use of these substances in 2006. For example, from 1996 to 2008, there were major reductions in vancomycin‐resistant E. faecium from broilers and pigs in Denmark following decreased use of avoparcin (DANMAP, 2007). The reduction in use of avoparcin was therefore considered not only to dramatically reduce the food‐producing animal reservoir of enterococci‐resistant AGPs, but also to reduce the reservoir of genes that encode resistance to several clinically important antimicrobial agents in humans. One publication (Heuer et al., 2002a) presented evidence that VRE may still persist in the animal environment in the absence of the selective pressure exerted by avoparcin. The publication did not question that a quantitative reduction took place, but indicated that, qualitatively, VRE could still be isolated after the discontinuation of avoparcin use when a more sensitive isolation procedure was used and suggests that such reductions might reflect differences in isolation procedures.
Macrolide resistance, specifically to tylosin, which is used for therapy as well as having been used as an AGP, and also resistance to avilomycin, were reduced in E. faecium among broilers (WHO, 2003). This resulted in a concomitant overall reduction in resistance to other AGPs in farm animals and resistance to these substances in bacteria isolated from humans in various countries. In Sweden, a ban on AGPs in the 1980s resulted in an apparently transient increase in post‐weaning diarrhoea in piglets. To avoid post‐weaning diarrhoea in piglets and necrotic enteritis in poultry, dietary levels of protein were reduced and dietary fibre increased, resulting in improved animal health.5 An overall reduction in resistance to antimicrobial substances previously used as AGPs in farm animals and in humans has been reported in some Scandinavian countries – e.g. Denmark and Sweden (Grave et al., 2006; DANMAP, 2009a,b; SVA, 2009, 2010, 2011; DANMAP, 2011).
1.6.1.4. Negative impacts
Despite initial promising observations showing a decrease after the cessation of AGPs in confined studies (Boerlin et al., 2001), larger data sets after the ban were not initially indicative of a decrease in multiresistance in the livestock species of concern in the Netherlands (MARAN, 2012), although some reductions have been more recently reported (MARAN, 2015) in relation with change in the use of antimicrobials as veterinary drugs.
Casewell et al. (2003) considered that, following the ban of all food‐producing animal growth‐promoting antimicrobials by Sweden in 1986 and the EU ban on avoparcin in 1997 and bacitracin, spiramycin, tylosin and virginiamycin in 1999, the only attributable effect in humans some 3 years later was a diminution in acquired resistance in enterococci from human faecal carriers (Casewell et al., 2003). They noted that there had been an increase in human infection from VRE in Europe, which they concluded was probably related to the increase in use of vancomycin for the treatment of meticillin‐resistant staphylococci in humans. They concluded that the ban of AGPs revealed that these agents had important preventive activity and their withdrawal was associated with a deterioration in animal health, including increased diarrhoea, weight loss and mortality due to E. coli and Lawsonia intracellularis in early post‐weaning pigs, and clostridial necrotic enteritis in broilers. The conclusions reached by Casewell et al. (2003) have been criticised by several authors (Jensen et al., 2004b).
1.6.1.5. Further considerations
Following the withdrawal of AGPs for use in cattle, broilers and finisher pigs in Denmark in February 1998 and in weaner pigs in 1999, there was a substantive increase in the use of certain therapeutic antimicrobials in these animals, particularly tetracyclines in pigs, in the following 2 years. The occurrence of resistance to tetracyclines consequently increased (Geenen et al., 2011). Only in recent years has there been a visible decline in the therapeutic use of such antimicrobials and as yet the resultant impact on resistance levels has not been fully evaluated. The use of tetracyclines in Europe has been considered an important factor in the dissemination of LA‐MRSA ST398 in herds it is already present (Catry and Threlfall, 2009; Catry et al., 2010).
1.6.2. The European Commission's action plan to tackle AMR
The European Commission has developed its own 5‐year Action Plan to tackle AMR, which was published in 2011.6
This includes seven identified areas where measures are regarded as most needed:
making sure antimicrobials are used appropriately;
preventing microbial infections and their spread;
developing new effective antimicrobials or alternatives for treatment;
cooperating with international partners to contain the risks of AMR;
improving monitoring and surveillance;
promoting research and innovation;
improving communication, education and training.
In line with the first bullet above and in accordance with Action 3 of that plan, in September 2015, the European Commission published the PUAVM Guidelines.
1.6.3. EC's Prudent Use Guidelines
The EC PUAVM Guidelines provide practical guidance to all parties possibly involved in the development and implementation of prudent use strategies, including the MS authorities, veterinarians, farmers, industry, stakeholder associations and academia, and highlights how such strategies can contribute to containing the development of AMR. They provide a compendium of examples of initiatives taken by different actors in EU MSs to promote prudent use of antimicrobials in veterinary medicine.
The first part of the EC PUAVM Guidelines offers advice on general principles on responsible use of antimicrobials, on the considerations to make before using antimicrobials, including the choice of the classes of antimicrobials to use. They particularly focus on those measures aimed at the reduction of the use of antimicrobials. These measures are reviewed and discussed in Section 3 of this Opinion.
The second part of the EC PUAVM Guidelines is focused on measures aimed at preventing clinical and subclinical conditions that require treatment of animals with antimicrobials, and which therefore reduce the need to use antimicrobials. These measures are reviewed and discussed in Section 4 of this Opinion.
As mentioned above, the initial sections of the EC PUAVM Guidelines discuss issues to be considered before using antimicrobials in veterinary medicine, the use of which should be limited to ‘situations where they are necessary’. The EC PUAVM Guidelines highlight the importance of justifying treatments by veterinary diagnosis, the conditions for the application of metaphylactic treatments, the importance of avoiding preventive treatments, and the principles for the selection of the appropriate classes of antimicrobials to use and for the off‐label use. Special attention is given to considerations that should accompany the use of antimicrobials recognised as CIAs for human medicine. These general principles and associated measures are reviewed in Sections 3.2 and 3.3 of this Opinion.
A section of the EC PUAVM Guidelines is dedicated to the oral administration of antimicrobials to groups of animals through feed and drinking water, indicating that whenever possible individual treatment should be preferred to group or mass treatment, and providing general principles for group oral administration. Rules for production and administration or antimicrobials via the oral route and connected measures are reviewed in Section 3.2.3 of this Opinion.
The EC PUAVM Guidelines then review the roles and the responsibilities of all the different parties possibly contributing to the design and implementation of prudent use strategies, including prescribers of the antimicrobials, administrators of the treatments, pharmaceutical industry, pharmacists, retailers and wholesalers, feed business operators, food business operators, veterinary faculties and agricultural schools, veterinary professional associations, industry stakeholder associations, farmers’ associations, competent authorities and laboratories. A set of principles to be followed by the different actors above are proposed, highlighting that the primary responsibility for the prudent use lies with the prescriber and the administrator of antimicrobials. Subsequently, the EC PUAVM Guidelines discuss the importance of awareness campaigns, which ensure that all parties involved, including the veterinary sectors, stakeholders owners and consumers, are well informed. Section 3.3 of this Opinion reviews the measures taken at national level, and connected to the different parties involved, to reduce the use of antimicrobials, including national action plans, monitoring and surveillance, targets for the reduction of use and benchmarking of farms, measures aimed at encouraging the responsible use of antimicrobials, at preventing conflicts of interest of prescribers and at regulating the advertising of antimicrobials and the role of education. Section 3.4 reviews the role that the food industry, and in particular producers and retailers, can take in driving a reduction and a more prudent use of antimicrobials in food‐producing animals, and the role of consumers’ organisations.
The second part of the EC PUAVM Guidelines, focuses on the measures aimed at reducing the need to use antimicrobials in veterinary medicine, reviews measures such as the implementation of hygiene and biosecurity measures and of protocols for the prevention of infectious diseases, optimal husbandry systems, integrated production systems, the introduction of herd health plans, the use of vaccination and alternatives to antimicrobials, and of high‐quality feed and water. Species‐specific recommendations are presented, including, among food‐producing animals, pigs, poultry, ruminants, aquaculture and rabbits. Section 4 of this Opinion reviews and discusses the measures that can be used to reduce the need to use antimicrobials in food‐producing animals.
The EC PUAVM Guidelines stress the role of monitoring and surveillance on the use of antimicrobials and on AMR in zoonotic and indicator bacteria taken from food‐producing animals.
Finally, the EC PUAVM Guidelines support the development and implementation of holistic national strategies covering all aspect of AMR, including public health, animal health, environment, research, etc., and provide general principles for designing such strategies and examples of measures that could be part of them.
1.6.4. International control strategies
1.6.4.1. WHO/FAO/OIE
Building upon the World Health Assembly Resolution of 1998, which urged MSs to take action against antimicrobial resistance, the WHO, in 2001, published its global strategy for the containment of antimicrobial resistance.7 Since this time, the Food and Agriculture Organization (FAO), the World Organisation for Animal Health (OIE) and WHO have worked closely on multiple initiatives in relation to tackling the problem of AMR.
The OIE Terrestrial and Aquatic Animal Health Codes provide a broad framework to address AMR that is applicable worldwide. Standards are laid out for harmonisation of national AMR surveillance programmes and monitoring of antimicrobial use in food‐producing animals. The codes address the implementation of regulatory frameworks and responsibilities of the pharmaceutical industry, animal feed manufacturers and veterinary professionals in relation to stewardship. Further, they provide guidance on risk analysis for AMR arising from the use of antimicrobial in animals.
In 2003, the OIE published a series of five guidelines to reduce the occurrence of AMR in food‐producing animals on a world‐wide scale. Recommendations included standardisation and harmonisation of laboratory methodologies for the detection and quantification of resistance (White et al., 2001); monitoring the quantities of antimicrobials used in animal husbandry (Nicholls et al., 2001); risk analysis methodology for the potential impact on public health of AMR bacteria of animal origin (Vose et al., 2001); guidelines for the responsible and prudent use of antimicrobial agents in veterinary medicine (Anthony et al., 2001); and harmonisation of national AMR monitoring and surveillance programmes in animals and animal‐derived food (Franklin et al., 2001).
Codex Alimentarius is established by the FAO and WHO to develop standards on food safety for the purpose of the facilitation of trade. In regards to AMR, the Codex Alimentarius Commission (CAC) has produced a Code of Practice to minimise and contain AMR (CAC/RCP 61‐2005) and Guidelines for risk analysis of food‐borne AMR (CAC/GL 77‐2011). More recently, the Codex Secretariat, in collaboration with FAO and WHO, has invited the CAC to consider the following recommendations: (i) start new work on: a revision of the Code of Practice to minimise and contain AMR; (ii) establish a dedicated task force on AMR and identify appropriate host country(ies); (iii) request FAO/WHO to provide scientific advice on AMR, in collaboration with OIE; and (iv) request FAO and WHO to develop a capacity development programme to respond to identified needs in respect of AMR (Codex Alimentarius, 2016).
The WHO, in 2005, published its first list of CIAs for human medicine; this list had its third revision in 2011. The OIE's list of antimicrobial agents of veterinary importance was adopted in 2007. These two lists can be seen as complementary and provide a categorisation of antimicrobials that can be used to guide risk management decisions when balancing animal health needs and the potential risk to public health.
In 2010, recognising the need for a ‘One Health’ approach to health risks, the FAO, OIE and WHO established a tripartite agreement with AMR as one of its priority issues. On World Health Day in 2011, the WHO published a series of policy briefs, one of which specifically addressed the need to reduce the use of antimicrobials in food‐producing animals.8 Core actions included:
provide national leadership and promote intersectoral collaboration;
create and enforce an enabling regulatory framework;
strengthen surveillance and monitoring;
promote education and training on antimicrobial use in food‐producing animals; and
reduce the need or antimicrobials through better animal husbandry.
These key requirements have been taken forward in regional action plans aimed at tackling AMR.
In 2011, the WHO regional office for Europe published a booklet entitled ‘Tackling antibiotic resistance from a food safety perspective in Europe’ (WHO, 2011). The booklet sets out seven key themes to combat AMR under the headings ‘overall coordination’, ‘regulation’, ‘reduced need for and prudent use of antibiotics in animal husbandry’, ‘surveillance’, ‘advocacy and communication’, ‘training and capacity building’, and ‘knowledge gaps and research needs’.
In May 2015, the World Health Assembly endorsed a global action plan on AMR, subsequently adopted by FAO and OIE, which was developed from the many existing initiatives already enacted by governments and other organisations around the world (FAO, 2015; OIE, 2015, 2016). Its activities are grouped into five strategic objectives:
to improve awareness and understanding of AMR;
to strengthen the knowledge through surveillance and research;
to reduce the incidence of infection;
to optimise the use of antimicrobial agents; and
to ensure sustainable investment in countering AMR.
The OIE has taken forward the objectives of the Global Action Plan in its own Strategy on AMR and the prudent use of antimicrobials, in November 2016.9
The ‘One Health’ approach taken by the WHO's Global action plan was supported when, in September 2016, United Nations General Assembly adopted a political declaration aimed at combating AMR.10
In November 2016, the FAO has produced a booklet entitled ‘Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal production’, which contains several recommendations targeted at reducing AMR in animal production on a global scale (FAO, 2016).
1.6.4.2. Examples of regional activities outside the EU
The Strategic Action Plan to control AMR in the Asia‐Pacific region (Asia‐Pacific Economic Cooperation, APEC), 2011, provides a framework for the APEC economies, indicating that this should be implemented according to the local situation.11 For example, in those countries where AGPs are still used it is recommended that this should be regulated, with the best option being a ban. National systems for surveillance of AMR and use in food‐producing animals are promoted, particularly monitoring of use of quinolones and colistin. It is acknowledged that in many Asian countries antimicrobials are available without medical prescription and increased awareness of the problem of AMR through campaigns for the general public and healthcare professionals are promoted.
The Global Health Security Agenda is a collaboration led by the USA government working with other nations and international organisations to address global health challenges. In 2014, the Global Health Security Agenda (GHSA) AMR Action Package12 was launched with the aim of supporting the work of the WHO, OIE and FAO to coordinate development of the Global Action Plan. Since 2009 the USA and EU, more recently joined by Canada and Norway, have also cooperated through the Transatlantic Taskforce on Antimicrobial Resistance (TATFAR), with one of the goals being to enhance information sharing on appropriate therapeutic use of antimicrobials between veterinarians on an international scale.
Some examples of measures taken in the USA and Canada in regards to CIAs are given in Section 3.3.4.6 of the Opinion.
1.7. Circumstances and diseases of food animal production where antimicrobials are most intensively used
1.7.1. Introduction
The production circumstances and the potential diseases of food animal production where antimicrobials are most intensively used were defined by qualitative information provided by stakeholders (Federation of Veterinarians of Europe, FVE) through a questionnaire (see Section 2.1.3 and Annex A), and then confirmed through peer review papers and data collected in certain countries such as Denmark (DANMAP reports 2006–2015) and France (ANSES, 2015a) (see also Appendix B). Also, a report of the OIE that prioritised diseases for which vaccines could reduce antimicrobial use in animals was used (OIE, 2015).
For each species, this chapter provides, when appropriate, a summary of information related to (i) the main pathogens for which antimicrobials are used, (ii) examples of circumstances that lead to disease emergence and antimicrobial use and (iii) examples of AMR bacteria deriving from such use. It should be noted that the use of antimicrobials in food‐producing animals differs between MSs. This section refers to circumstances and production systems where antimicrobials are most intensively used, which may not reflect the use in all situations and MSs.
There are three main strategies for the use of antimicrobials for animals (Hughes et al., 2008). Antimicrobials may be administered to clinically diseased animals (therapeutic use), to healthy animals in the same group as diseased animals (metaphylaxis), or to healthy animals when the probability of becoming sick is considered high (prevention).
Apart from primary bacterial infections, it is important to stress that antimicrobials can in practice also be used to treat bacterial infections that are secondary infections following a primary viral infection. This is the reason why examples are reported below of viral diseases for which large amounts of antimicrobials are used. In some instances, antimicrobials may be inappropriately used for conditions of non‐bacterial aetiology.
1.7.2. Circumstances of use of antimicrobials in the different species
1.7.2.1. Poultry
As stated in the EC PUAVM Guidelines, routine group medication in poultry often occurs immediately before or after transport of day‐old chicks or possibly to address perceived potential losses of productivity.
Main pathogens/disorders for which antimicrobials are mostly used
According to the information received by the FVE, the circumstances and periods where antimicrobials are mostly used in poultry include:
-
broilers:
-
–
gastrointestinal disorders (such as coccidiosis, necrotic enteritis, dysbacteriosis);
-
–
respiratory diseases (including infections that are often followed by secondary infection with E. coli, such as infectious bronchitis, Newcastle disease, infectious laryingotracheitis);
-
–
locomotion‐related diseases (bacterial arthritis ‐ due to e.g. E. coli, Staphylococcus aureus or Enterococcus spp., and secondary bacterial infections connected with tenosynovitis, necrosis of the femur head);
-
–
septicaemia, omphalitis;
-
–
-
laying hens (much less use, in part due to the effects of withdrawal periods on eggs):
-
–
gastrointestinal disorders (such as enteritis caused by E. coli, avian intestinal spirochaetosis);
-
–
respiratory and locomotion‐related diseases (caused by E. coli and Mycoplasma);
-
–
secondary bacterial infections connected, for example, with red mite infestation;
-
–
taeniosis (in free range production systems);
-
–
-
turkeys:
-
–
respiratory diseases (caused by Ornithobacterium infection);
-
–
gastrointestinal disorders (caused by coccidiosis).
-
–
A report from France reflected this overview and confirmed that in France the pathogenic agents that particularly contribute to antimicrobial use in poultry are E. coli and Mycoplasma spp. for systemic and respiratory diseases and Clostridium perfringens for digestive diseases (ANSES, 2015a).
Hughes et al. (2008) provide an example of the use of antimicrobials in broiler chicken farms in the UK. On 714 broiler chicken farms in 2002–2003, 42.4% of the farms used antimicrobials therapeutically, 54% for prophylaxis/prevention and 24% for both reasons. Therapeutic use of antimicrobials was mostly related to respiratory and digestive diseases and coccidiosis.
Examples of circumstances that lead to disease emergence and antimicrobial use
Necrotic enteritis has been emerging in broiler chickens since the ban, in 2006, of AGPs (see above). Other factors are linked to antimicrobial use, such as wet litter (that can be a consequence of digestive disorders due, for example, to coccidiosis), the use of a live vaccine against infectious bursal disease (involving immunosuppression) and addition of finely ground wheat to feed (which may predispose to necrotic enteritis outbreaks (Annett et al., 2002), whereas whole or coarsely ground wheat or maize can be protective (M'Sadeq et al., 2015). Regarding preventive use of antimicrobials, the number of different hatcheries supplying the farm with chicks and the average slaughter weight of the flock (the longer time spent in the farm the higher the risk of contamination is perceived) are risk factors for antimicrobial use.
1.7.2.2. Pigs
Main pathogens/disorders for which antimicrobials are mostly used
According to the FVE report (see Annex A) and the EC PUAVM guidelines, the disorders for which antimicrobials are mostly used in pigs are listed below. Antimicrobials are administered orally via drinking water, in medicated feed or by injection:
suckling piglets: locomotory infections (arthritis), neurological disorders and diarrhoea (caused by E. coli);
weaners: diarrhoea, and respiratory diseases often associated with transport and stress when bringing together pigs originating from different farms or housing animals in holdings with inappropriate ventilation systems, and/or improper feeding strategies and insufficient biosecurity measures;
fatteners: respiratory (e.g. Porcine Respiratory Disease Complex) and digestive disorders (e.g. proliferative enteropathy by L. intracellularis, swine dysentery, ileitis, Salmonella spp.);
sows: urogenital disorders (e.g. leptospirosis), post‐partum dysgalactia syndrome, Actinobacillus pleuropneumoniae in gilts.
In Denmark, as reported in the Vetstat database (Dupont et al., 2016), the use of antimicrobials in intensive swine production is higher for weaner pigs compared to sows, piglets and finishers. Such use is mostly related to gastrointestinal disorders; for example, 58% of the standardised animal daily doses in Danish pig production in 2014 were for gastrointestinal diagnoses. Secondly, antimicrobials are used for respiratory problems. Such use was also observed in Spain (Moreno, 2014b). A different picture was observed in Sweden, with the highest consumption recorded for suckling piglets (Sjölund et al., 2015), and in Germany, with the treatment of respiratory diseases and then intestinal diseases in piglets and weaner pigs (van Rennings et al., 2015). Use of antimicrobials to prevent post‐weaning diarrhoea and respiratory infections has been reported in Belgium and Spain (Callens et al., 2012; Moreno, 2014a).
Swine dysentery is a mucohaemorrhagic colitis of pigs caused by Brachyspira hyodysenteriae, which may be present in apparently healthy herds (Hampson et al., 2016). The disease can be controlled by treatment with antimicrobial agents, with the pleuromutilins tiamulin and valnemulin being widely used. In recent years, the occurrence of B. hyodysenteriae with reduced susceptibility or full clinical resistance to these drugs has been increasing and clonal dissemination of some drug‐resistant strains has occurred (Rugna et al., 2015).
The overview in France is similar, as confirmed by ANSES (2015a).
Examples of circumstances that lead to disease emergence and antimicrobial use
Husbandry factors such as ineffective pen cleaning and disinfection and poor biosecurity measures are important contributors to dissemination of undesirable microorganisms on pig farms. Although wet feed increases the risk of pig infection by Listeria monocytogenes, it is a protective factor for Salmonella spp. Mixing batches of pigs presents a risk for the transmission of Salmonella spp. and Yersinia enterocolitica. However, small herds are more likely to be infected by Campylobacter spp. and Y. enterocolitica, Salmonella prevalence is increased in large herds if there is frequent mixing of batches but reduced by all‐in/all‐out batch rearing systems. Antimicrobial treatment during the finishing period increases the risk of transmission of Salmonella spp. (Fosse et al., 2009) as well as general AMR development (Holman and Chénier, 2015).
1.7.2.3. Ruminants
Main pathogens for which antimicrobials are mostly used
As mentioned in the EC PUAVM Guidelines, mass or group medication is rare in cattle, although veal calves commonly are subjected to group treatments with antimicrobials (Pardon et al., 2012a), and blanket intramammary treatment given to cows at drying off is important.
Based on the information from the FVE, the main specific disorders of cattle leading to antimicrobial use are the following:
-
dairy cattle:
-
–
mastitis (especially the dry cow treatment);
-
–
lameness/foot disease;
-
–
uterine problems (e.g. metritis);
-
–
surgery;
-
–
-
calves and veal:
-
–
respiratory diseases;
-
–
diarrhoea;
-
–
-
beef:
-
–
respiratory diseases (mainly at the beginning of the fattening period);
-
–
locomotory diseases (lameness, arthritis);
-
–
neonatal diarrhoea.
-
–
The overview reported above is confirmed by an ANSES study in France and by an epidemiological study conducted in France (Gay et al., 2012; ANSES, 2015a), where it was reported that the main reasons for antimicrobial use in adult ruminants concerned udder infections and, secondly, obstetric disorders. Danish and Swedish data have confirmed that mastitis is the primary reason for parenteral antimicrobial treatment (DANMAP, 2015; Public Health Agency of Sweden and SVA, 2015). For young animals, the primary reasons were respiratory diseases and secondary digestive disorders. In Belgium, the main indication for drug use in white veal calves was respiratory disease, and oral group treatments were used predominantly (96%) (Pardon et al., 2012a).
In veal calves, dysbacteriosis and other gastrointestinal conditions are by far the most important indication for antimicrobial therapy. Here, E. coli and Clostridium perfringens often are the bacteria that overgrow within the digestive tract (Pardon et al., 2012a).
Information received from FVE summarised the main specific disorders of sheep and goat as:
-
lambs in their first month of life:
-
–
enteritis/enterotoxaemia (‘watery mouth’);
-
–
Mannheimia spp. infections in case of motherless rearing;
-
–
Arthritis, especially in intensive goat farming);
-
–
-
growing fattening lambs:
-
–
respiratory diseases (e.g. Mannheimia spp. infections, especially during the end of housing period and first time on pasture);
-
–
lameness due to arthritis, including problems resulting of tick pyaemia or footrot;
-
–
infectious conjunctivitis;
-
–
-
ewes/does and adults:
-
–
bacterial abortion, e.g. Chlamyidia spp., Campylobacter spp., Listeria spp., Coxiella burnetii;
-
–
post‐partum disorders of the genital system;
-
–
diarrhoea due to clostridial infections;
-
–
bacterial mastitis and contagious agalactia;
-
–
lameness (e.g. footroot, scald, contagious ovine digital dermatitis);
-
–
tick‐borne fever;
-
–
listeriosis.
-
–
Examples of circumstances that lead to disease emergence and consequent antimicrobial use
The production of white veal requires a specific diet and housing conditions resulting in a controlled iron anaemic state and pale carcasses. In response to public concern about animal welfare, legal limits for haemoglobin, the provision of a minimum quality of solid feed to assure rumen development and group housing from the age of 8 weeks have been implemented; nevertheless, intensive antimicrobial use in veal calves remains a major issue (Pardon et al., 2014; Lava et al., 2016b). In beef cattle, sharing water between pens and mixing animals of different ages, especially in the same pen, increase the risk of respiratory disease (Hay et al., 2016).
Small ruminant management systems vary throughout Europe from intensive to extensive, with milk, meat and wool production types. Overall, the use of antimicrobials seems to be similar in both sheep and goats kept under the same conditions. Preventive or metaphylactic use of antibimicrobials, particularly by the oral route, is uncommon in sheep that are reared extensively, although there may be whole flock treatments with long‐acting tetracycline injections following bacterial abortion storms or oral treatment of newborn lambs to prevent watery mouth. This low level of use is reflected in lower levels of AMR in bacteria from sheep than in those from other food‐producing animals (Dargatz et al., 2015).
1.7.2.4. Horses
Information provided by the FVE highlighted the following uses of antimicrobials:
use within racing yards with young horses at risk of disease or respiratory infections limiting grade performance;
respiratory diseases in stable and studs with large number of horses or horses frequently travelling to competitions stabled with a variety of horses;
wounds;
intrauterine treatment of broodmares treated for hypofertility;
some specific infections, such as from Rhodococcus equi;
perioperative antimicrobials.
Treatment options for horses are influenced by the category of production, since horses can be kept as food‐producing, companion or sport animals. For instance, horses kept as companion and declared as not for food production, can be treated with a much wider range of veterinary medicines as residues of antimicrobials in food would not be a concern. Scicluna et al. (2013) found that respiratory diseases, skin diseases and reproductive disorders were the predominant indications for use.
1.7.2.5. Rabbits
Main pathogens for which antimicrobials are mostly used
Little quantitative data exist on the use of antimicrobials in rabbit production (Rosell and de la Fuente, 2016). The FVE answers to the questionnaire on the use of antimicrobials highlighted the following critical phases of rabbit production requiring most of the antimicrobial use:
breeding females: respiratory and genital infections due to Pasteurella multocida, metritis and mastitis due to staphylococcal bacteria and others;
small kits before weaning: enterotoxemia due to Clostridium spiriforme, colibacillosis, neonatal enteritis and staphylococcal infections;
fattening phase: major cause of death in young rabbits immediately after weaning is due to intestinal disorders such as Enzootic Rabbit Enterocolitis, Colibacillosis, proliferative enteropathy caused by Lawsonia intracellularis bacteria, coccidiosis caused by Eimeria spp.
Examples of circumstances that lead to diseases emergence and antimicrobial use in rabbits
A number of factors lead to a high use of antimicrobials in rabbit production (see FVE report, Annex A), including a lack of biosecurity (e.g. absence of the implementation of all‐in‐all‐out principles followed by adequate cleaning and disinfection), difficulties to ensure the right microclimatic conditions for every breeding phase, and pressure put on the reproductive phase (intensive breeding systems) or in the growth phase (early weaning, mixing).
1.7.2.6. Dysbacteriosis
A further factor leading to a large amount of antimicrobial consumption in young food‐producing animals is dysbacteriosis, which is a non‐specific enteritis following from a disturbance in the equilibrium of the gut microbiota, similar to small intestinal bacterial overgrowth in human medicine (Abu‐Shanab and Quigley, 2009).
In broilers, dysbacteriosis and necrotic enteritis are major indications for group treatments (Persoons et al., 2012). In veal calves and piglets, as well as in laying hens, colistin has often been used to prevent as well as treat digestive disorders (Timmerman et al., 2006; Pardon et al., 2012a). Given the potential side effects of antimicrobial agents as AGPs by modifying the intestinal gut through immune stimulation (Khadem et al., 2014) or food conversion through substrate modifications (Lin, 2014), and the potential harmful effects of antimicrobials to the equilibrium of microbiota in the gut, it is difficult to define to what extent antimicrobials are harmful, preventative, or restorative of the natural equilibrium between gastrointestinal microbial communities (Fasina et al., 2016).
This suggests that through better understanding of dysbacteriosis and its management, antimicrobial consumption for gastrointestinal diseases in young animals could be reduced, e.g. by improving nutrient digestibility or by using alternative products (e.g. probiotics, prebiotics, organic acids, heavy metals).
1.7.2.7. Bees
There are no authorised antimicrobial veterinary medicines for honey bees in the EU. Antimicrobials and chemotherapeutics can be used in the EU in apiculture under the ‘cascade’ as described in Article 11 of Directive 2001/82/EC,13 as amended by Directive 2004/28/EC. However, there may be practical difficulties with setting an appropriate withdrawal period. According to the FVE, veterinary bee experts believe that no antimicrobials should be used to treat honeybees. Rather, good beekeeping management of apiaries can be helpful against most infections. The following infections are sometimes treated with antimicrobials:
American foulbrood and European foulbrood, due to Paenibacillus larvae and Melissococcus pluton, respectively;
nosemosis type‐A and type‐C, due to Nosema apis and Nosema ceranae, respectively.
1.7.2.8. Fish
Large quantities of antimicrobials are used in aquaculture in some countries to prevent and treat bacterial infection. The potential bridging of aquatic and human pathogen resistomes is considered to lead to the emergence of new AMR bacteria and global dissemination of the organisms and their AMR genes into animal and human populations (Cabello et al., 2016).
European aquaculture includes more than 35 different species and takes a variety of production systems, such extensive or intensive, in natural settings or tanks, in fresh water or sea water, in cold, moderate or warm water, in flow‐through or recirculation systems, traditional or modern, classic or organic systems (see also Annex A).
Antimicrobials have been used extensively in salmon aquaculture, as therapeutic agents in the treatment of infections. There are very substantial differences in antimicrobial use between producer countries. Use of antimicrobials is extremely low in Norway, the largest producer of farmed Atlantic salmon in the world, in comparison to some competitor countries (Burridge et al., 2010) (see also Appendix C and Annex A).
Main pathogens for which antimicrobials are mostly used
Marine coldwater fish species (Atlantic salmon, trout and cod) represent the largest production sector, followed by fresh water species (trout and carp) and marine Mediterranean species (sea bass, sea bream and turbot).14 Farmed crustaceans and mollusc production seldom use antimicrobials. In aquaculture, most of the antimicrobials are given to treat bacterial and parasitic diseases and the use of antimicrobials varies between countries and production systems (Cabello et al., 2016). The most common route for the administration of antimicrobials is the oral route via medicated feed, and antimicrobials are mostly used in metaphylactic treatment. Information from FVE (see Annex A) highlighted the following critical phases of aquaculture production requiring most of the antimicrobial use:
salmon: fry in the fresh‐water phase (florfenicol and flumequine);
sea‐bass and sea‐bream: juvenile early life stages for tenacibaculosis, photobacteriosis and vibriosis;
trout: fry (early life stage) for rainbow trout fry syndrome (florfenicol,oxytetracycline), enteric redmouth diseases by Yersinia ruckeriii, furunculosis (sulfadiazine‐trimethoprim, florfenicol, oxytetracycline, 1st‐ and 2nd‐generation quinolones).
Examples of circumstances that lead to disease emergence and antimicrobial use
Norway has a very important production of marine cold‐water fish species. Different epidemics occurred since the 1970s, due to Vibrio anguillarum in the 1970s and, during the 1980s, cold‐water vibriosis (Vibrio salmonicida), which led to an increase use of antimicrobials. In time, these infections were more or less controlled by vaccination programmes and/or sanitary measures such as ‘all‐in‐all‐out’ and coordination areas to reduce infection pressure (Sapkota et al., 2008; Midtlyng et al., 2011) (see also Appendix C). Smolt infected with Aeromonas salmonicida subsp. salmonicida causing furunculosis was then imported. The disease spread quickly, and antimicrobial use again increased in 1990, with the use of antimicrobials increasing in tandem with the increase in production and the epidemics. Subsequently, the amounts of antimicrobials used significantly decreased, and by 2015, the consumption of antimicrobials was the lowest since 1975. During the same period, fish production increased by 400%. Appendix C reports historical description of the evolution of the aquaculture in Norway and related use of antimicrobials.
1.8. Examples of development of resistance, important to public health, resulting from antimicrobial use in food‐producing animals
Poultry
Enterobacteriaceae/cephalosporins
The use of ceftiofur for hatching eggs and day‐old chickens appears to have been a driver for the emergence of ESBL‐ and AmpC–producing Enterobacteriaceae with plasmidic MDR, but termination of such use can be followed by a rapid reduction in occurrence if high standards of hygiene that minimise perpetuation of these organisms on poultry farms are applied (Hiki et al., 2015; Hering et al., 2016). Such use in chickens and turkeys may have been responsible for the emergence of MDR strains of S. Heidelberg that have caused large numbers of human disease cases in the USA and Canada (Routh et al., 2015). In 2011, the CVMP produced a scientific evaluation of veterinary medicinal products containing 3rd‐ and 4th‐generation cephalosporins.15 This evaluation indicated in relation to cephalosporins and poultry:
‘The extent of use in EU is not known but there is anecdotal evidence for quite comprehensive use both in ovo and to 1‐day‐old chicken also in countries where there is no products authorised for use in poultry. Outside the EU such practice is common and treatment of one day‐old chicken with ceftiofur is authorised e.g. in the United States. Such use would entail a high risk for spread of ESBL to humans via food due to dissemination in poultry production pyramid. Treatment of eggs and/or one day‐olds in grandparent and parent flocks could lead to dissemination to a large number of animals in the following generation with spread to numerous farms in different countries. There is evidence of correlation between such use of cephalosporins and resistant infections in humans and poultry and poultry products are most frequently reported to carry ESBL and/or AmpC‐producing bacteria’.
In Europe, increasing proportions of human bloodstream infections caused by E. coli have been reported to be resistant to 3rd‐generation cephalosporins and a large proportion of cephalosporin‐resistant isolates causing human infections are considered to be derived from food‐producing animals (Vieira et al., 2011a). Based on data from the Netherlands (Overdevest et al., 2011), Collignon et al. (2013) extrapolated that, in the Netherlands, infections in humans with cephalosporin‐resistant E. coli derived from poultry sources have been associated with 21 additional deaths and resulted in 908 hospital bed‐days needed to treat persons with these cephalosporin‐resistant bloodstream infections. They concluded that if these values were extrapolated to all of Europe, 1,518 additional deaths and an associated increase of 67,236 days of hospital admissions would be counted as a result of cephalosporin and other antimicrobial drug use in poultry. This conclusion has been strongly criticised, as statistically validated information was not included in the paper, nor were the effects of other extraneous factors contributing to patient mortality considered.
A systematic review (Lazarus et al., 2015) concluded that evidence shows that a proportion of human extraintestinal ESBL‐producing E. coli infections originate from food‐producing animals, and in particular poultry. Evidence about transmission mechanisms showed to be contrasting. Some molecular epidemiology studies supported transmission through whole bacteria, especially in the Netherlands; some others, from different geographical regions, supported transmission through mobile genetic elements, while some others did not support those transmission mechanisms.
Campylobacter spp./fluoroquinolones
In 2014, five of 13 MSs reported ciprofloxacin resistance in more than 80% of isolates from cases of human infection, and one country reported resistance in 97.7% (ECDC and EFSA, 2016); in such settings, effective treatment options for human enteric Campylobacter infection are significantly reduced.
Salmonella spp./fluoroquinolones
Treatment failures, increased hospitalisation and higher risk of death have been reported for MDR S. Typhimurium definitive phage type (DT) 104 with additional resistance to quinolone antimicrobials (Helms et al., 2002, 2004; Threlfall, 2002; ECDC, EFSA, EMA and SCENIHR, 2009; WHO, 2011).
Pigs
MDR Salmonella spp. are prevalent in pigs in most countries, and are likely to have resulted from the regular use of in‐feed antimicrobial treatments that are used to prevent disease under less than optimal husbandry conditions (Molla et al., 2006). The success of Salmonella 4,[5],12:i:‐ (monophasic S. Typhimurium) in pig populations is likely be related to the selective advantage offered by MDR profiles associated with stable genetic elements, and also the presence of virulence features, within bacterial lineages that are well adapted to the porcine host. Such strains are prevalent in human infections as a result of the handling and consumption of contaminated pig meat (Mourao et al., 2014). Acquired tolerance to heavy metals such as copper and zinc also provide a selective advantage as both these elements are commonly used at high levels in pig feed (Mourao et al., 2015).
An increased frequency of ESBL‐producing E. coli can be found on pig farms with high use of 3rd‐ or 4th‐generation cephalosporins, typically as convenient low volume long‐acting injections used preventively to protect weaned pigs against farm‐resident Streptococcus suis infection. Transfer of either ESBL‐producing E. coli or plasmids between pigs and farmers can occur as a result of such practices (Hammerum et al., 2014). Management factors such as farm hygiene and husbandry measures can also relate to risk, but sometimes counter‐intuitively by increasing the risk for ESBL‐positive samples (Hering et al., 2014). An additional factor to be considered for pigs is that antimicrobials added to semen may present an unrecognised route for dissemination of antimicrobials and AMR‐contaminating organisms (Luis Yaniz et al., 2010).
A further issue is the ongoing spread of LA‐MRSA in certain high‐risk groups of workers in direct contact with animals, especially pigs. (See Appendix A.3).
Colistin, mcr‐1
Following its identification in food production animals in China in late 2015, mobile (transferable) colistin resistance mediated by the mcr‐1 gene has been documented in food‐producing animals in several EU/EEA countries, particularly in pigs but also in poultry and veal calves (see Appendix A). Human infections have also been noted. This is of great concern due to the rapidly increasing use of colistin in EU/EEA hospitals leading to increased selection pressure. The mcr‐1 gene has subsequently been identified in invasive human pathogens and as such is regarded as seriously decreasing the options for the treatment of infections with highly resistant Enterobacteriaceae (EMA, 2016b).
Ruminants
There appears to be a quantitative relationship between antimicrobial use and the occurrence of LA‐MRSA in veal calves (Dorado‐García et al., 2015b), and resistance in E. coli and Pasteurellaceae in different production types (Catry et al., 2016).
The use of diverse antimicrobials in veal production and the use of penicillins and 1st‐ and 2nd‐generation cephalosporins, alone or in combination with aminoglycosides, for mastitis prevention and treatment, with consequent possible exposure of calves via colostrum and feeding of waste milk from treated cows, are important issues. The latter problem has been specifically assessed recently by the EFSA BIOHAZ Panel (2017).
From 2000, in the USA and Canada, highly MDR strains of S. Newport have emerged, primarily in cattle, following routine use of ceftiofur for dairy cattle after calving. These bacteria were resistant to at least 11 antimicrobials, including extended‐spectrum cephalosporins. Resistance genes bla(CMY‐2’), flo(st’), strA, strB, sulII and tetA were located on self‐transmissible plasmids. Additional resistances were found in a proportion of isolates. The increase in bovine‐associated MDR S. Newport in people is cause for concern as it indicates an increased risk of human acquisition of the infection via the food chain (Poppe et al., 2006). MRSA has been found as a cause of mastitis in dairy cattle in some countries (Turkyilmaz et al., 2010), and although such organisms may be found on beef, their occurrence in beef cattle appears to be minimal; based on these findings, further contamination along the food chain, possibly by food handlers, may be involved (Weese et al., 2012).
1.9. Concluding remarks
Although resistance to antimicrobials is an inevitable consequence of the use of such compounds in both human and veterinary medicine, the relative contribution of such use to AMR development has not been quantified.
Genetic factors contributing to the spread of AMR organisms and genes therein from animals to humans and vice‐versa include the presence of chromosomal mutations contributing to the development of resistance to some clinically relevant antimicrobials, organisation of mainly non‐chromosomal resistance genes into horizontally transferable elements, and the presence of a cluster of linked AMR genes facilitating co‐selection of resistance to one substance during exposure to an unrelated substance.
The gut microbiota is regarded as the largest reservoir of antimicrobial transmissible resistance genes, not only within livestock but also in humans. Such bacteria can act as donor, vector or recipients of AMR genes.
Key emerging issues in relation to food‐borne/zoonotic transmission of AMR include transferable resistance to colistin mediated by mcr genes in livestock and humans, the emergence and spread of MDR/ciprofloxacin‐resistant (Cipr) isolates of various Salmonella serovars, the spread of monophasic MDR Salmonella spp. in pigs and in broilers in many EU countries, the ongoing spread of LA‐MRSA in certain high‐risk groups of workers, the ongoing spread of MRSA in pigs and other species, and increasing levels of resistance to 3rd‐ and 4th‐generation ESBL‐producing organisms in community patients and livestock.
The high to very high levels of resistance to fluoroquinolones and tetracyclines in isolates of Campylobacter spp. from cases of human infection and from broilers in several EU MSs in 2014, and increasing levels of resistance to ESBLs in community patients and livestock are also matters of concern.
The overall surveillance of the occurrence of AMR both in isolates from animals and foodstuffs and in isolates from human cases as reported annually in the EU Summary Report on AMR in zoonotic and indicator bacteria from humans, animals and food should enable trends to be monitored.
Data on overall sales of antimicrobials in MSs, as published by ESVAC, provide an analysis of use by country, although it is not possible to distinguish such use by food‐producing animal species.
Data based on the sales of antimicrobials for use in food‐producing animals in the different EU MSs show that that there are considerable differences between the amounts used (mg/PCU).
More than 90% of antimicrobials (kg) are administered as oral (group) treatments.
The JIACRA report published jointly by ECDC, EFSA and EMA has provided an integrated analysis of consumption of antimicrobial agents and occurrence of AMR in bacteria from humans and food‐producing animals in EU MSs in 2011–2012. Overall, a positive association was observed between antimicrobial consumption in food‐producing animals and occurrence of resistance in bacteria from such animals. A second JIACRA report is in preparation and is scheduled to be published in 2017.
The use of AGPs has been banned completely in the EU since 2006. Their use had already been phased out in certain countries prior to this date and an overall reduction in resistance in farm animals and in humans to substances previously used as AGPs has been reported in some Scandinavian countries. The impacts on animal and human health of the removal of AGPs are subject to debate.
The European Commission introduced a 5‐year Action Plan against AMR in 2011 and subsequently published guidelines on the prudent use of antimicrobials in veterinary medicine in 2015. These guidelines contain strategies for limiting the development of AMR, which can be implemented by stakeholders in EU MSs at all levels.
At international level, the WHO/OIE animal health codes address the regulatory frameworks relating to the control of AMR while Codex guidelines address AMR from the food safety perspective. The WHO and OIE publish lists of CIAs for human and veterinary medicine.
In 2015, the World Health Assembly endorsed a global action plan on AMR, which underscores the need for a ‘One Health’ approach and provides the framework for national action plans to combat AMR.
Antimicrobials are used for the treatment, metaphylaxis and prevention of infectious diseases in all food‐producing animal species in EU/EEA countries. The availability of antimicrobial treatment is regarded as essential for the health and welfare of the animals in question. The level of use varies between countries and also between production systems, livestock species, life stages and depending on disease expression.
1.10. Recommendations
Vigilance is required and should be in place at the EU level in respect of key emerging issues related to AMR in zoonotic pathogens, to ensure that appropriate control measures are rapidly taken to minimise or prevent the spread of such organisms.
Sales data of antimicrobials by animal species/production sector would be helpful in establishing more precise patterns of use (see also Section 3.3.2).
At the EU level, relevant indicators suitable for monitoring and detecting trends in the levels of key antimicrobial‐resistant microorganisms in humans, food‐producing animals and food derived thereof, and in antimicrobial consumption should be developed.
2. Data and methodologies
2.1. Data
2.1.1. AMR surveillance data
Where relevant, data obtained from the AMR monitoring at the EU and national level, have been referred to in the Opinion to describe the AMR situation and its evolution along the years, and/or showing the impact of measures taken to reduce the impact of the use of antimicrobials in AMR in food‐producing animals and certain foods thereof. It should be noted that other than for those aspects designed for the purpose of compulsory EU surveillance (Decision 2013/652/EC), national AMR surveillance programmes may use different methodologies for sampling, susceptibility testing and/or the interpretive criteria for defining resistance (see Section 3.3.2).
2.1.2. European and national data on sales of antimicrobials
Where relevant, data on sales of antimicrobials obtained from the ESVAC project have been referred to in the Opinion to describe the use of antimicrobials at national or EU level and its evolution through the years, and/or showing the impact of measures taken to reduce the use of antimicrobials in food‐producing animals. In addition, data on sales and use has been taken from national consumption surveillance reports (see Section 3.3.2). These may use different methodologies for data collection and units of use/consumption.
2.1.3. Data collected through questionnaires on the use of antimicrobials in food‐producing animals and measures to reduce the use
The experts of the RONAFA WG identified the need to collect information in relation to the use of antimicrobials in food‐producing animals, with particular focus on the cattle, pig and poultry (all poultry species) EU production systems, but also on other species, and on possible measures to reduce the use of antimicrobials and the need to use antimicrobials in food‐producing animals. In order to obtain this information from field experts, the WG approached the Federation of Veterinarians of Europe (FVE), stakeholder organisation representing the veterinary profession at the EU level. For this purpose, the RONAFA WG developed a questionnaire, to which FVE provided detailed feedback. The questionnaire included, for the different food‐producing animal species, questions in relation to the circumstances in which the greatest amounts of antimicrobials are used, and where it would be easier or more difficult to implement measures to reduce the need for antimicrobials, in relation to the use of vaccination and to the need for vaccines to directly or indirectly reduce the use of antimicrobials. It also included a request for examples of measures implemented and their impact in terms of use of antimicrobials, AMR, animal health and animal welfare.
In addition, to get more specific information in relation to the use of antimicrobials in aquaculture and the strategies implemented in Norway to reduce the use of antimicrobials in this sector and the results obtained, the RONAFA WG consulted one external expert, in the role of hearing expert.
The information provided in the answer to the above questionnaire and further information from the hearing expert has been used by the RONAFA WG to inform the assessment, and information has been considered and discussed as relevant in the Opinion. A copy of the questionnaire is available in Appendix D, and the report produced by FVE to respond to the questionnaire is available in Annex A. The information requested to and provided by the hearing expert is available in Appendix C.
2.1.4. Data collected through a questionnaire on agreements between producers and retailers
The experts of the RONAFA WG identified the need to collect some information in relation to the possible role and impact of the voluntary measures taken by producers and retailers, and agreements between these groups, on the use of antimicrobials in food‐producing animals in the EU. In order to obtain this information from stakeholders, the WG approached selected organisations representing these groups at the EU level, or other sectors tightly linked with them. For this purpose, the RONAFA WG developed a questionnaire, to which a number of stakeholders provided feedback. The questionnaire included the request to provide information on any guidance or schemes in place between retailers and producers, and related controls, which are in place at national or international level within the EU with regard to antimicrobial use in food‐producing animals. The information provided in the answer to the questionnaire has been used by the RONAFA WG to inform the assessment, and information has been considered and discussed as relevant in the Opinion. A copy of the questionnaire and a summary of the answers are available in Appendix E.
2.1.5. DG SANTE questionnaire
Within the framework of the European Commission's action plan on AMR, the Directorate on Health and Food Audits and Analysis (former Food and Veterinary Office, FVO) of the European Commission's Directorate‐General for Health and Food Safety (DG SANTE) is conducting a fact‐finding project on the prudent use of antimicrobials in animals in the EU. As part of this project, in 2015 a questionnaire (‘DG SANTE questionnaire’) was sent to the competent authorities of all EU MSs, Iceland, Norway and Switzerland, as well as representative stakeholders, to collect information on the distribution and use of antimicrobial veterinary medicinal products including medicated premixes, and also on how the MSs have put national and/or the EC PUAVM Guidelines into practice. The replies to this questionnaire16 provided by the respondents, with their kind permission, were used by the RONAFA WG as a tool for identifying material relevant to this Opinion. No attempt has been made to perform an analysis of the responses in this report.
2.2. Methodologies
2.2.1. Measurement of impact of measures
Measuring the impact of guidance on responsible use of antimicrobials, and/or specific management measures implemented, on the level of resistance to antimicrobials in bacteria isolated from food‐producing animals and food thereof is a difficult task for several reasons.
There are multiple steps between the formulation of guidelines and observation of any change in AMR patterns in bacteria in food‐producing animals and food. Guidelines have to be translated into effective actions, after which leverage may be applied by authorities to ensure that the actions are effectively implemented. Monitoring of use and surveillance programmes for AMR need to be in place in order to analyse the impact of the measures.
There is a delay before any impact of measures can be observed. The delay relates to the time for measures to be implemented and for the reduction in selection pressure to impact on AMR, depending on factors such as the stability of the resistance mechanisms involved, the microorganisms involved, the spread of resistance determinants among bacteria and among animal species, concomitant measures taken, etc.
In many MSs monitoring programmes have not been in place for sufficient time to reliably identify trends in AMR evolution.
Identifying causality in complex systems can be difficult. In some contexts, variables may be positively associated, while in some others they may not be associated, or negatively correlated depending on many factors.
-
The implementation of specific measures may for example lead to different results and response times in different animal species, production systems, environmental conditions, etc., depending for example on:
-
–
lifespan of animals;
-
–
characteristics of the production system (e.g. broiler chicken vs laying hens, poultry vs pigs, dairy cattle vs veal calves);
-
–
farm management;
-
–
antimicrobial use pattern (e.g. active principles used, route of administration, dosage, duration of treatment);
-
–
disease incidence.
-
–
The EC PUAVM Guidelines include a number of measures. It is probable that countries will have implemented a series of these measures all at the same time, and to different extents, and the impact of individual measures cannot be separated.
Other events can compete with implementation of measures. For example, in Denmark, an outbreak of Postweaning Multisystemic Wasting Syndrome (PMWS) in the Danish pig population may have been expected to result in a national increase in antimicrobial use in weaners. The effect on the use of antimicrobials was unclear even when data collected through a national register of antimicrobial use at the farm level represented more than half of pig farms (Vigre et al., 2010). In the UK, a slight increase in antimicrobial use in the poultry sector in 2013 was attributed possibly to feed quality issues resulting from a poor harvest during the associated period (BPC, 2016).
These difficulties result in the impossibility to quantitatively measure the impact of the measures reviewed on the use of antimicrobials, or on the need to use antimicrobials, and on the occurrence of AMR. Rather, this assessment evaluates the impact qualitatively.
2.2.2. Data gathering and literature searches
Several methods have been used to gather scientific publications, reports and official documents relevant for this opinion.
Relevant data from scientific publications, official EU publications, scientific documents of EU agencies (see for example Appendix F) and specific reports from MSs were used as appropriate. Reports, publications and other types of information available on websites from MSs’ competent authorities have been used to gather information and provide examples on strategies and measures put in place by the different EU MSs. This information might not be complete due, for example, to the lack of public availability, or to the language used in the publications.
In a number of areas it was considered important to review existing scientific literature to extract information to be presented and discussed in this opinion, especially because recent studies and scientific findings would provide insights into the possible alternatives to the use of antimicrobials in food‐producing animals. In such cases, specific literature searches were undertaken to identify relevant literature. This was used, together with additional scientific information known by experts, to support the expert review in these areas.
Detailed information on the literature searches performed is provided in Appendix G.
3. Assessment on measures taken to reduce the use of antimicrobials in animal husbandry in the EU and their impact on the occurrence of AMR in bacteria from food‐producing animals (ToRs 1 and 2)
3.1. Introduction
Section 3 of this opinion addresses the measures that have been taken to reduce the use of antimicrobials in food‐producing animals in the EU. It discusses measures that have been implemented according to the EU Regulations or Commission Decisions, measures taken by the individual MSs at the national level and, briefly, measures undertaken independently by food producers and retailers. The impact of the measures on antimicrobial use, and on AMR in bacteria from animals and food, where data are available, is noted.
3.2. EU regulatory measures
3.2.1. Veterinary medical product (VMP) authorisation procedures
The regulatory system for the authorisation of new antimicrobial veterinary medical products (VMPs) in the EU is science‐led. In accordance with Directive 2001/82/EC, before any substance can be used in a VMP intended for food‐producing animal species (FPS), maximum residue limits (MRLs) must be established which take account of potential adverse effects on public health due to residues of the substance in food products derived from treated animals. The effects of antimicrobial residues on the human gut flora are taken into consideration, both in terms of their impact on the colonisation barrier, and any increase in the population of resistant bacteria. In regards to the latter, an acceptable daily intake (ADI) is calculated based on the antimicrobial concentration, which leads to no observable increase in the population of resistant bacteria in the colon (VICH GL 3617). Withdrawal periods in compliance with MRLs are applied following the administration of VMPs to ensure that the consumer's intake of antimicrobial residues do not exceed the ADI. Thus it can be assumed that with the withdrawal period applied, residues of antimicrobial agents in food will not lead to an increase in the population of resistant bacteria in the human gastrointestinal tract that could adversely affect human health.
Before VMPs can be placed on the market in the EU, they require a Marketing Authorisation (MA). MA requirements include provision of data on minimal inhibitory concentration (MIC), i.e. pharmacodynamics, pharmacokinetics, justification for the dosing regimen and demonstration of effectiveness of the antimicrobial in clinical field trial(s). Detailed guidance on the data requirements is provided in the recently updated EMA/CVMP Guideline for the demonstration of efficacy for VMPs containing antimicrobial substances (EMA, 2016a). MA applicants should ensure that product indications are aligned with principles of the responsible use of antimicrobials, and are encouraged to take into account modern PK/PD principles in establishment of dosing regimens and to justify treatment duration taking into account the risk for development of AMR. Special consideration is given for metaphylaxis claims, which must be justified on epidemiological and clinical grounds. Furthermore, it is foreseen that prevention claims would only be approved when the risk for infection is very high and the consequences are severe (see Section 3.3.6).
In addition to provision of data on potential emergence of AMR in target pathogens, for all new MA applications concerning food‐producing species, except those for generic products, then certain data are required to consider the likelihood that use may lead to selection of resistance in zoonotic or commensal organisms that could be of concern to human health (VICH GL 2718). This guidance does not address all aspects of the risk pathway or make recommendations on the overall risk estimation. Further guidance is under development, which will expand upon this by considering the subsequent extent of human exposure to AMR genes and potential consequences to human health (EMA CVMP AWP, 2015).
Although an environmental risk assessment is required as part of a MA application, this does not consider the effect of the excreted antimicrobial residues on bacteria in the environment, or the contribution of resistance genes that are excreted from treated animals to the environmental resistome and their dissemination throughout the environment. The need to assess this risk is under consideration by CVMP, although further research into this complex topic is required (Sarmah et al., 2006; Wellington et al., 2013). Further elaboration is considered to be outside the scope of this Opinion.
Based on the regulator's assessment of the AMR risk, risk management measures, which are focused on responsible use warnings, can be implemented within the terms of the Marketing Authorisation and are documented in the product's Summary of the product characteristics (SPC). These address, for example:
advice to base decisions about the use of the product on susceptibility testing of bacteria isolated from the animal;
where this is not possible, advice to base therapy on local (regional, farm level) epidemiological information about susceptibility of target bacteria;
advice to use narrow‐spectrum antimicrobials by preference where susceptibility testing suggests the likely efficacy of this approach;
advice to follow the dosing directions in the SPC to avoid the development of antimicrobial resistance.
The SPC and product information (label, package information leaflet) are the tools through which risk management measures are conveyed to users of antimicrobial VMPs. In a survey conducted by the FVE (De Briyne et al., 2013), 72.5% of food‐producing animal practitioners responded that they always (14.8%), or in most cases (57.7%), take into account responsible use warnings in the SPC and/or package information leaflet when prescribing. It was noted that practitioners referred to labels and package leaflets rather than SPCs. Although product information can be seen as an effective means to communicate risk management measures to the veterinarian, and there is an expectation that the veterinarian complies with the SPC unless prescribing under the cascade the impact of these warnings on prescribing where this is not supported by additional national legislative control is more difficult to discern (see CIAs, below). Additional information on pharmacodynamic particulars (MIC, MBC, clinical break‐points) and pharmacokinetic properties may be useful in supporting appropriate authorised and off‐label use and is included in Section 5 of the SPC but rarely in the product information.
In addition to SPC responsible use warnings, which are generally applied by regulators during marketing authorisation application procedures, other, substance‐specific risk management measures could be considered which would have a bearing at the time of product development. These were discussed previously by the AMEG (Q.4, table 6 of the AMEG advise (EMA, 2014)) and include:
restrictions on preventive and metaphylactic use;
restriction from use as mass treatment, e.g. for herds and flocks; individual treatment only;
restrictions on indications;
restriction to use in certain species only;
limitation on route of administration and/or duration of treatment.
Although these risk management measures may be intrinsic to the product and should be taken into consideration as part of the AMR risk assessment at the time of authorisation, there is no comprehensive EU regulatory guidance on antimicrobial VMP risk management that can be used to guide development of new products.
In all cases, the approval of a Marketing Authorisation for a new antimicrobial VMP is dependent on the demonstration of a positive benefit‐risk evaluation for the product. The benefit‐risk balance considers the intended use and demonstrated indications for the product in respect to its overall safety, taking account of possible risk management measures (EMA, 2009). The CVMP has previously refused to approve an indication for a 3rd‐generation injectable cephalosporin product for the treatment of bovine respiratory disease (BRD). In this case, it was noted that increasing levels of cephalosporin resistance had been reported in E. coli from cattle and greater exposure to the antimicrobial could be anticipated following approval of the BRD indication. The risk to public health was assessed and considered to contribute, among other factors, to an overall negative benefit‐risk assessment for the indication (EMA CVMP, 2012b).
The AMEG (EMA, 2014) has proposed that new antimicrobial substances could be prohibited from use in food‐producing species prior to, or on consideration of, a hazard characterisation performed ahead of Marketing Authorisation application in order to preserve their efficacy for the treatment of infections in humans. Carbapenems are a last line therapy for treatment of serious infections in humans caused by MDR Gram‐negative bacteria. Although they are not authorised for use in animals, carbapenem resistance has been detected sporadically in Enterobacteriaceae from food‐producing animals in the EU. In 2013, EFSA published an opinion on carbapenem resistance in food‐producing animal ecosystems in which it was recommended that an effective control option to minimise spread of resistance via the food chain would be to continue to prohibit the use of carbapenems in food‐producing animals (EFSA BIOHAZ Panel, 2013).
It has been noted previously that a solution to the problem of transfer of AMR via the food chain would be to develop veterinary‐use antimicrobials that do not select for resistance to antimicrobials used in human medicine; as in human medicine, investment in new veterinary antimicrobials has stalled. An independent review identified that it is the expectation of more regulatory or policy instruments to restrict or ban the use of novel antimicrobials in animals has led to a halt in commercial investment (Tait et al., 2014). Industry identified that one of the most important solutions to overcoming the barriers to investment in new veterinary antimicrobials is a stable and predictable regulatory process without unnecessary restrictions on their use (du Marchie Sarvaas, 2015).
The proposed Regulation on veterinary medicinal products currently undergoing legislative process seeks to strengthen the regulatory framework for antimicrobial VMPs in line with Action 2 of the Commission's 5‐year Action Plan on AMR. Applicants must provide data on the risks to public and animal health from the use of antimicrobial VMPs and information on risk management measures. Where the risk to public health due to development of AMR is considered to outweigh benefits of the product to animal health this can be deemed as grounds for refusal of a Marketing Authorisation. Furthermore, the Commission also seeks to establish a list of antimicrobial VMPs, which cannot be used, or can only be used under certain conditions, outside the terms of the marketing authorisation.
There is no specific requirement linked to Marketing Authorisations for post‐marketing surveillance of AMR in zoonotic and commensal bacteria as a risk management measure. A recommendation from the AMEG was that at the time of approval of a new antimicrobial substances/classes, MA holders should have in place plans to monitor the susceptibility in zoonotic and indicator bacteria through approved programmes. Mandatory monitoring of AMR in zoonotic and commensal bacteria from animals is carried out in the EU under Directive 2003/99/EC17 and the priorities for monitoring from the public health point of view are laid out in Commission Implementing Decision 2013/652/EU (see Section 1.5.1). SAGAM concluded that commitments for Post‐Marketing Authorisation Resistance Surveillance (PMARS) could be required for products intended for food‐producing animals depending on the assessment of the public health risk, and that such activities should be complementary to mandatory EU monitoring programmes (EMEA CVMP SAGAM, 2008).
In accordance with Directive 2001/82/EC, generic products are exempt from the need to provide an assessment of the AMR risk warnings in regards to responsible use are normally extrapolated from those of the reference product; although these warnings may not take into account the evolution of microbial susceptibility to the substance in the years following its first authorisation. A study conducted in Denmark found that after the introduction of generic ciprofloxacin to the human market, the total consumption in primary healthcare increased significantly from 0.13 to 0.33 DDD/1,000 inhabitant‐days and this was positively correlated with resistance in E. coli obtained from urine samples (Jensen et al., 2010). The impact of the release of generics onto the veterinary market on the sales of specific antimicrobials should be investigated, as has been done in human medicine (Monnet et al., 2005; Jensen et al., 2010) and suggested in veterinary medicine (Toutain and Bousquet‐Melou, 2013). Under current legislation, the AMR risk for generic VMPs can only be reviewed as part of a ‘class’ referral procedure (see Section 3.2.2).
Concluding remarks
The risk to public health from AMR that is transmitted via food produce derived from animals treated with antimicrobial VMPs is assessed in the authorisation process according to guidance provided under VICH GL 27 and guidance in draft/development by the CVMP/AWP. Experience needs to be gained with this methodology.
During the process of VMP authorisation, provided that the overall benefit‐risk is assessed to be positive, the main tool for otherwise mitigating the AMR risk is the placing of warnings in the SPC. There is evidence that SPC‐based product information is a useful tool for communication of responsible use warnings to veterinarians.
New antimicrobial substances could be prohibited from use in food‐producing species prior to, or on consideration of, a hazard characterisation in order to preserve their efficacy for the treatment of infections in humans. A previous EFSA opinion recommended that carbapenems should be prohibited from use in food‐producing animals.
No new AMR risk assessment is required as part of the MA application for generic VMPs, and there is little published evidence of the impact of generic products on antimicrobial use.
Recommendations
Support should be given to the development of further regulatory guidance on (i) the assessment of the risk to public health from AMR due to the use of VMPs, and (ii) a framework for risk management measures that are proportionate and relevant to the risk assessment. The guidance should continue to be science‐led and transparent, in order to support a predictable regulatory environment.
A process should be developed for evaluation of the potential AMR risk from future veterinary use of human last‐resort antimicrobials, which might be restricted from use in veterinary medicine.
Regulatory authorities should take steps to maintain and improve access to SPCs so that more information is conveniently accessible to prescribers.
The impact of the release onto the market of generic VMPs on the sales of specific antimicrobials and potential to influence AMR development should be investigated.
3.2.2. Measures based on CVMP reflection papers and referrals
The Committee for Medicinal Products for Veterinary Use (CVMP), in collaboration with its Antimicrobials Working Party (AWP), has provided over the last decade a series of reflection papers addressing the use of certain antimicrobial classes in food‐producing animals in the EU, the development of resistance and its impact on human and animal health. Based on these papers, CVMP has made recommendations which have been followed up, according to priority, by ‘class referral’ procedures aimed at amending the SPCs of groups of related VMPs to ensure that they are in line with the CVMP's risk profiling and responsible use principles. Following a referral procedure, a Decision is issued by the EC, requiring MSs to implement the CVMP's recommendations.
The first of these reflection papers, on the use of (fluoro‐)quinolones, was published in 2006 (EMEA CVMP, 2006) and followed by a further paper in 2010 (EMA CVMP, 2010), which made recommendations on responsible use guidance to be included in the SPCs of these products. It indicated that:
’official and local antimicrobial policies should be taken into account when the product is used’;
’whenever possible, (fluoro)quinolones should only be used based on susceptibility testing’;
’use of the product deviating from the instructions given in the SPC may increase the prevalence of bacteria resistant to the (fluoro)quinolones due to the potential for cross resistance’;
and, additionally for fluoroquinolones, that:
’fluoroquinolones should be reserved for the treatment of clinical conditions which have responded poorly, or are expected to respond poorly, to other classes of antimicrobials’.
A referral for enrofloxacin products administered in water to poultry (EMA CVMP, 2014b) recommended removal of indications that were not consistent with responsible use (including treatment of Salmonella spp.) and included a condition (pending) for Marketing Authorisation Holders to review the dosing regimen in line with modern PK/PD principles.
In 2009, the CVMP published a reflection paper on the use of 3rd‐ and 4th‐generation cephalosporins in food‐producing animals in the EU (EMEA CVMP, 2009). A subsequent referral and Commission Decision issued in January 2012 required the addition of SPC warnings for systemically administered products equating to those that had been included in the SPCs for the fluoroquinolones (EMA CVMP, 2012a). In addition, due to concerns regarding misuse of the products for preventive group treatments in cattle, swine, horses, and particularly in day‐old chicks, and associated concerns over the human health risk due to selection of ESBLs (extended‐spectrum β‐lactamases), further warnings were added. These included a contraindication from use of the products in poultry and statements indicating that the products are intended for use in individual animals only, and should not be used for disease prevention.
The CVMP review of the use of macrolides, lincosamides and streptogramins identified that of most concern was emergence of resistance in Campylobacter spp. in poultry and pigs, although the outcome of public health risk assessments due to veterinary use is equivocal (EMA CVMP, 2011). The reflection paper proposed that the duration of treatment with such products should be limited to the minimum time needed for cure of the disease. This was followed up by a referral (2014) for tylosin products administered orally to pigs, which restricted the treatment duration to three weeks and deleted the indication for swine dysentery (Brachyspira hyodysenteriae) (EMA CVMP, 2014c).
Recognising the increasing importance of colistin to treat MDR Gram‐negative infections in humans, recommendations were made by the AMEG with the goal of reducing overall use of colistin in veterinary medicine (EMA, 2013a). Subsequently, a CVMP referral addressing orally administered colistin products in 2014 led to restrictions of the indications for such products and in the duration of treatment. Following the identification in 2015 of the mcr‐1 gene conferring resistance to colistin and the further increasing importance of colistin in human medicine, its continued use in veterinary medicine came under intense scrutiny. The AMEG's report was subsequently updated in 2016 with the recommendation that a target should be set to substantially reduce veterinary use of colistin in the EU, and to move the substance into the AMEG's category 2 (higher risk substances, to be used only when there is no other effective alternative) (EMA, 2016b).
The use of pleuromutilins in humans is limited to topical application (e.g. retapamulin); other substances in this class are under development for human use. Use in animals could select for pleuromutilin‐resistant staphylococci (e.g. via cfr genes), including LA‐MRSA. Potentially the spread of resistance genes could compromise treatment of MRSA infections in humans. The reflection paper on pleuromutilins recommended that prevention claims should be removed, except for use in well‐defined eradication programmes, and the duration of treatment limited to that for cure of disease. No measures have been implemented to date based on this reflection paper.
The Joint Scientific report of ECDC, EFSA and EMA on MRSA in livestock, companion animals and food (EMEA CVMP, 2009) advised that due to the multidrug resistant character of MRSA, effective control measures could not be limited to a specific antimicrobial class, but routine antimicrobial use should be regarded as a risk factor and measures should aim to reduce unnecessary use. It was suggested that biosecurity and hygiene would be useful measures and transmission via trade should be avoided. Systematic surveillance for MRSA in humans and animals in order to identify any trends in the spread and evolution of zoonotically acquired MRSA was also recommended.
Over the years, the CVMP has conducted numerous referrals for antimicrobial VMPs with emerging resistance in mind. In many cases, this has involved the review of supporting data resulting in removal of indications or adjustment of dosing regimens with the aim of improving rational antimicrobial use. It has to be taken into account that when indications or animal species are removed from the SPC(s) for one product/substance, this could result in use of a substance that is of greater importance to human health and over‐reliance on a narrower range of substances. In addition, the impact of increasing doses to address a change in the susceptibility profile of target pathogens may have un‐assessed impact on AMR in organisms of relevance to public health, and could increase overall antimicrobial use if not coupled with other measures. In addition, the paucity of new data to enable updating of dose regimens and safety risk assessments is frequently noted.
3.2.2.1. Impact of the measures
According to the responses to the DG SANTE questionnaire (see Section 2.1.5), as of 2015, the Commission Decisions implementing SPC warnings on responsible use of fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins had been enacted in almost all member states.
As the Decision on cephalosporins was only implemented at the start of 2012, it is too early to assess any impact on the basis of the ESVAC data. The Commission Decision on the fluoroquinolones was adopted in January 2010. Examination of data on the sales of fluoroquinolones across the EU suggests an increasing trend since 2011 (see Figure 2) (EMA ESVAC, 2016). The sales of fluoroquinolones (in mg/PCU) differ by more than 100‐fold between MSs. Spain and Poland, account for around 60% of the total sales (tonnes) of fluoroquinolones in the EU and have high sales in mg/PCU and therefore strongly influence the EU overall data. Neither of these MSs has seen a decline in the sales of fluoroquinolones since 2011. Reductions in sales of certain critically important antimicrobials (CIAs) have been seen in some MS/livestock sectors where legislative or voluntary actions have been taken at national level (see Section 3.3.4).
Figure 2.
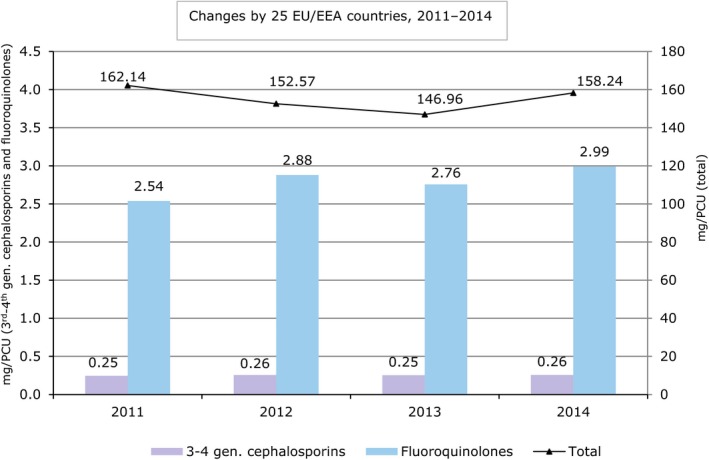
Changes in total sales and in sales of fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins, from 2011 to 2014, for 25 EU/EEA countries (Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden and United Kingdom) (source: EMA ESVAC, 2016)
Concluding remarks
The CVMP's reflection papers and referral procedures, followed by Commission Decision, have been effective in implementing responsible use warnings into the SPCs for certain CIAs.
It is too early to fully assess the impact of these measures on sales of these CIAs, but they may have limited impact compared to measures taken at national level.
Despite this, responsible use warnings implemented in SPCs at the EU level may help to support measures included in national action plans, such as the requirement to conduct susceptibility testing prior to use of certain CIAs.
3.2.3. Medicated feedstuffs and other oral administrations
3.2.3.1. Regulation of the manufacture and placing on the market of Medicated feedstuffs in the EU
The conditions for mixing veterinary medicines into feed, its marketing and use across the EU are regulated under EU Directive 90/167/EEC18. In accordance with Article 8, the supply of medicated feedingstuffs (MFS) to farmers may only be on prescription by a veterinarian; the MFS should not be used for more than one treatment under the same prescription and the prescription should not be valid for more than three months. In addition, the veterinarian must prescribe the MFS only in such quantities as are necessary for the purpose of the treatment and within maximum limits laid down by national legislation. Further to this, Article 9 states that MFS for the treatment of animals intended for human consumption must not be supplied in quantities greater than one month's requirements.
Medicated feed is usually produced by feed mills approved by the competent authority. In compliance with the Feed Hygiene Regulation 183/2005,19 they must follow certain standards in relation to manufacturing processes, and should take steps to avoid cross‐contamination and transfer of antimicrobial to subsequent batches. Under a derogation of the Directive 90/167/EEC, MSs may authorise farms to manufacture medicated feed from authorised premixes. This derogation is applied in Ireland, Italy, Sweden, United Kingdom and France, but not in all the EU MSs. Mixing ready‐to‐use oral VMPs into water and ‘top dressing’ of feed with oral powders by the farmer are not regulated by the Directive and therefore rules, for example relating to homogeneity of production, do not apply.
The EU legislative framework relating to medicated feed was evaluated by Civic Consulting of the Food Chain Evaluation Consortium in a report published in 2010 (EC, 2010). This report identified that there are few official statistics with regards to the production of MFS in the EU. Medicated feed is the most common route of oral administration of antimicrobials in many countries. According to Civic Consulting's survey of nine national feed manufacturers’ associations (conducted in 2009), the most frequently used VMPs for the production of feed were (in descending order) tetracyclines, sulfonamides/trimethoprim and macrolides. Use of medicated feed was most common in intensive production, especially of pigs. There was no generally valid economic rationale for farmers to prefer a specific way of administering oral VMPs, through either feed or water – cost varied depending on the pricing strategy of the manufacturer, active substance and member state. There was no indication that different ways of administering oral VMPs led to significant differences regarding user safety, public health (AMR was not directly addressed) and environmental safety if handling instructions were properly observed. If the latter cannot be guaranteed, then the claimed advantage of medicated feed is that it ensures homogeneity and stability of the active substance, reduces the risk of dosing errors and limits the number of people exposed to the concentrated VMP. Other factors mentioned that may influence the effectiveness of treatment relating to the route of administration included perceptions around effectiveness (e.g. sick pigs may continue to drink after they have stopped feeding) availability of equipment (e.g. dosing pumps for water systems) and storage constraints.
The importance of ensuring the homogeneity of antimicrobial distribution in medicated feed, and of use of appropriate drug delivery systems for administration in water are emphasised in the EC PUAVM Guidelines. In addition, factors such as the lower doses of antimicrobials used in medicated feed, the prolonged periods of administration (sometimes through habitual use rather than real need), cross‐contamination in feed mills, delivery vehicles and farm storage and the under‐dosing resulting from inappetance and weakness of sick animals should be taken into account (Van Miert, 1983; Almond and Monahan, 2000).
The EC concluded that Directive 90/167/EEC is out‐dated, and noted that due to divergences in national implementation, use of MFS varies greatly between member states. As part of an impact assessment that was conducted by the EC to support revision of this Directive, among other issues it was identified that there was inconsistency around national requirements relating to the quality of manufacture of MFS in terms of homogeneity of mixing and carry‐over of antimicrobials from medicated feed to plain feed. Lenient requirements in this regard can increase the risk for development of AMR due to subtherapeutic dosing and unnecessary exposure to antimicrobials. A study conducted in the Netherlands analysed samples of ‘flushing feeds’ and found that 87% contained levels of antimicrobial in the same range as levels previously used for growth promotion (Stolker et al., 2013). In addition, where requirements are overly strict, use of medicated feedstuff may be replaced by oral powders (top dressing) with the potential for even less precise dosing. Stakeholder consultation identified a desire for concrete EU‐harmonised measures to be taken.
A new Regulation on the manufacture, placing on the market and use of medicated feed is undergoing legislative process in the EU and will include measures to tackle AMR. These include addressing preventive use, establishing limits for ‘carry‐over’ and tightening of the provisions for prescribing and handling of medicated feed containing antimicrobials.
Within the EC's impact assessment20 it is commented that the evolution of the quantities of antimicrobials and the quantities of medicated feed used in the EU shows that the decision to use therapeutic antimicrobials is independent of the availability of medicated feed. Therefore, it is stated that specific restrictions on medicated feed do not lead to a reduction in the use of antimicrobials as products with alternative methods of administration are available in place. In Germany, production of medicated feedstuffs is very low due to their stringent regulation under pharmaceutical law, introduced in 2006. This did not lead to an overall decrease in antimicrobial use in Germany in the associated time period to 2008. In 2014, overall sales of oral formulations made up 94% of total antimicrobial sales (mg/PCU) in Germany, with oral powders and oral solutions being used in place of premixes (see Figure 3) (EMA ESVAC, 2016).
Figure 3.
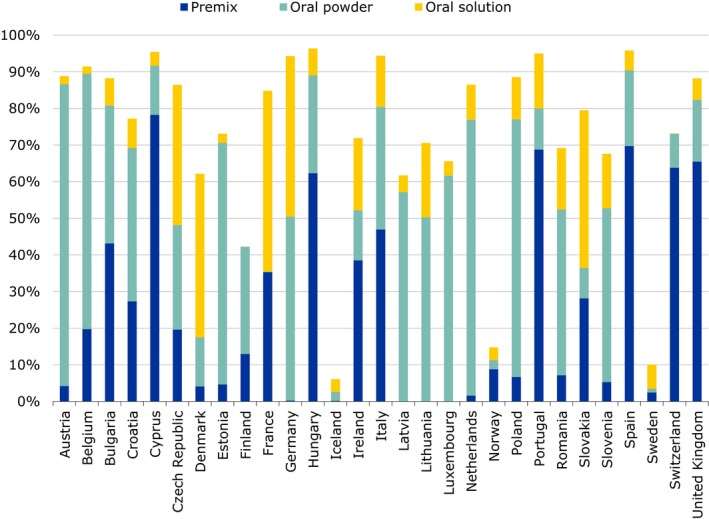
Oral solutions, oral powders and premixes as percentages of total sales, in mg per population correction unit (mg/PCU), of veterinary antimicrobial agents for food‐producing animals, including horses, in 29 European countries for 2014
Examples of orally administered antimicrobial formulations commonly available in the EU are given in Appendix H. Other than cephalosporins, all classes of antimicrobial that are categorised as veterinary CIAs by the OIE21 are available as oral formulations suitable for group treatments. Most classes are available as premix, oral powder and oral solutions for drinking water. Examination of the SPCs suggests that premix formulations of macrolides, pleuromutilins and aminoglycosides are authorised for long treatment durations of up to 3–4 weeks, primarily for treatment of enteric diseases (in various species) and pneumonia in pigs.
3.2.3.2. Findings from recent ESVAC data in relation to oral antimicrobial medication
According to the ESVAC data for 2014 (EMA ESVAC, 2016), across the 29 European countries included, oral formulations accounted for 91.6% of all antimicrobial use in food‐producing animals (mg/PCU). Premixes accounted for 42.1% of total antimicrobial sales, oral powders 31.7% and oral solutions 17.8%. Oral formulations of tetracyclines and penicillins alone constituted 32.6% and 22.8%, respectively, of the total antimicrobial sales for food‐producing animals.
There were no formulations of 3rd‐ and 4th‐generation cephalosporins applicable for oral group treatment. Although 76.0% of fluoroquinolones were sold as oral solutions, which are mostly used for group treatment, total fluoroquinolones, make up only 1.9% of administered antimicrobials used in food species. Polymyxins (mostly colistin, administered orally) accounted for 6.6% of administered antimicrobials.
There is much variability between EU countries in the proportions of premixes, oral powders and oral solutions sold, which may reflect species distributions and national policies. For example, in Germany, production of medicated feedstuffs is very low (< 0.5% of total mg/PCU) due to stringent regulation (see above). In Iceland and Norway, use of oral formulations overall is low (6% and 15% of total sales, respectively), reflecting the smaller population proportion of pigs and poultry in those countries (9% and 11% of the PCU); whereas in countries with a higher proportion of these species such as Spain (59%) and Portugal (55%) oral formulations predominate (96% and 95% total sales, respectively (EMA ESVAC, 2016)).
3.2.3.3. Issues associated with use of oral formulations and the development of AMR
There are particular difficulties that arise in the antimicrobial treatment of intensively reared livestock due to the potential for a high environmental pathogenic load and rapid spread of infection. Under these circumstances, herds/flocks, rather than individuals, have to be treated to control disease outbreaks and metaphylaxis is common place. Oral formulations are most often used for reasons of practicality of administration, particularly in pigs, poultry and veal calves. They are not suitable for use in adult ruminants due to inactivation of the antimicrobial by ruminal microorganisms and also because these animals tend to be reared under more extensive conditions. In accordance with modern principles, the antimicrobial dose is optimised in terms of the PK/PD relationship in order to maximise efficacy and limit the development of resistance in target pathogens. For concentration‐dependent and time‐dependent antimicrobials where Cmax/MIC and AUIC (area under the inhibitory curve) are critical indices, the oral route of administration is not ideal. For antimicrobials administered as mass medication, population PK/PD can be used when establishing dose regimens to further take account of the interanimal variability that occurs due to the impact of husbandry factors on antimicrobial intake. The duration of treatment is usually based on empirical clinical factors and will be affected by the ability of the animal to develop an immune response. In a review by (Martinez et al., 2012), it was concluded that in most situations it is the exposure achieved during the first dose that is relevant for determining therapeutic outcome of an infection, that therapeutic interventions should be initiated as soon as possible to minimise the bacterial burden at the infection site and that the duration of drug administration should be kept as brief as clinically appropriate to reduce the risk of selecting for resistant [target pathogen] strains. In human medicine, there is evidence that when immunity to infection can be expected, shorter treatment durations that reduce selection for resistance without interfering with clinical outcome are likely to be optimal (Geli et al., 2012). These requirements do not fit comfortably with administration of medicated feeding stuffs, which take time to be manufactured and delivered through storage bins, and when feed is supplied on a set‐timed basis. For this reason, in‐feed medication tends to be used for preventive and metaphylactic purposes, and duration of application may be prolonged to a few weeks (see Appendix H). Water medication is used more frequently in the early/acute stages of disease outbreak due to greater flexibility of dose adjustment and because sick animals may often continue to drink normally when their appetite is suppressed; such medication can be less convenient if appropriate administration equipment is unavailable (Pijpers et al., 1991).
Oral formulations have the disadvantage that they generally result in higher exposure to the antimicrobial of the gastrointestinal microbiome, which acts as a potential source of resistant bacteria and resistance determinants of significance to human health. This is especially the case for substances with low oral bioavailability; although the mode of excretion varies between drugs and parenterally administered drugs can exposed the intestinal microbiota if they are excreted in the gut lumen as active compounds (Toutain et al., 2016). The impact of the dosing regimens on selection of resistance in gut commensals is rarely investigated as part of the dossier for Marketing Authorisations. In rodents, experiments have clearly established resistance in E. coli to be substantially more efficiently established in the gut following the oral route of administration for different types of antimicrobials (Zhang et al., 2013). Wiuff et al. (2003) found no significant difference in resistance development in experimentally S. Enterica‐infected pigs between intramuscular administration of enrofloxacin and oral administration. Cattle treated for bovine respiratory disease showed a significantly higher percentage of resistant E. coli following oral administration compared to a subcutaneous treatment at day 14 after initiation of the trial (Checkley et al., 2010), although a dilution effect washed out this difference prior to slaughter. A recent systematic review (Burow et al., 2014) concluded that orally administered antimicrobials increase the risk of the development of resistance in E. coli from swine, although it was noted that more research is needed into the impact of dosage and the longitudinal effects of treatment. Carriage of MRSA in pigs in positive herds and MDR E. coli and Pasteurellaceae in cattle have been found to be associated with group antimicrobial treatments (Dorado‐García et al., 2015a; Catry et al., 2016).
3.2.3.4. Observations from MSs reported in literature
In a survey of farrow‐to‐finish pig farms in Spain, conducted in 2010, the most used antimicrobial per farm, administration route and production stage was colistin by feed, followed by β‐lactams by feed both during the growing and preweaning phases. Preventive treatments were employed on 96% of the farms, for which feed was the most frequently used administration route. Long treatment durations were noted for in‐feed treatments, ranging from three to 60 days. The mean number of days of exposure to antimicrobials (including zinc oxide) during the lifespan of a pig was 44 days and it was highlighted that feed is the route that produces the highest antimicrobial exposure (Moreno, 2014b). A survey was conducted in 2010 to investigate preventive and metaphylactic use of antimicrobials in Belgian fattening pig herds (Callens et al., 2012). Similarly, this showed that preventive group treatment was applied to 98% of the included herds and accounted for 93% of group treatment. The treatment index (the number of pigs per 1,000 that is treated daily with the animal daily dose, ADDpig) for oral therapy was 183.5, compared to 52.3 for injectable therapy, although compared to a previous study in 2003, a shift was noticed to use of long‐acting injectable treatments. The most frequently used group oral treatments were colistin (30.7%) (for post‐weaning E. coli infections) and amoxicillin (30.0%) (for prevention of streptococcal infections). Orally administered group treatments were under‐dosed in 47.3% of administrations. Persoons et al. (2012) noted the potential for under‐ and over‐dosing of oral treatments in broiler production. The authors noted that the findings indicated a need for clearer information about correct dosing and a reduction of group level preventive antimicrobial use. It was further noted by Callens et al. (2012) that human CIAs were used on a regular basis to treat fattening pigs, and that the shift from older orally administered compounds to newer injectable, long‐acting compounds should be assessed for the impact on AMR trends in organisms of relevance to public health.
3.2.3.5. Measures taken by individual MSs
In Sweden, only 10% of the overall antimicrobial consumption (in kg) is for group treatments (premix, oral powder, oral solution) (Public Health Agency of Sweden and SVA, 2015). Antimicrobials for oral medication of groups of animals are mostly used for pigs and by 2014 consumption (kg) had decreased by 55% since 2010. The Swedish national strategic programme against antimicrobial resistance focuses on reducing the need for antimicrobials by disease prevention and infection control and correct use of antimicrobials through diagnosis and prudent use. Programmes to eradicate swine dysentery are thought to have contributed to a 64% reduction (in kg) in the use of pleuromutilins between 2010 and 2014. A decrease in 55% in the use of macrolides over the same period is thought to reflect improved knowledge of the management of secondary infections in herds affected by PMWS and the introduction of vaccination programmes. The overall consumption of antimicrobials (mg/kg slaughtered pig) has been stable over the 5 years as the consumption of products for individual medication has increased (tetracyclines, trimethoprim‐sulfonamides) with benzylpenicillin by far the most commonly used substance. The shift from group medication towards medication of individual animals with narrow spectrum substances is consistent with prudent use principles, which have been developed in species‐specific treatment guidelines by the Swedish Veterinary Association and Medical Products Agency. Since 1990, the Swedish Veterinary Association adopted guidance for prescription of veterinary antimicrobials for group medication with particular emphasis on weaners (SVS, 1990). According to this guidance, prescription of antimicrobials for mixing in feed or water should be coupled with a thorough herd investigation and written recommendations on changes needed for prevention of recurrent disease.
According to the ANSES report on sales of veterinary antimicrobials in France (ANSES, 2013), premixes made up 36.9% of antimicrobial sales (tonnes) and 44.9% were oral powders and solutions. In terms of bodyweight treated, this equated to 16% for premixes and 50% for oral powders and solutions. Over the preceding 5 years, overall exposure to antimicrobials (as measured by Animal Level of Exposure to Antibiotic – ALEA – relating to the proportion of animals treated in relation to the total population) decreased by 15.7%, with exposure via premixes falling by 45.9%, and via oral powders and solutions by 13.9%. Exposure via injections increased by 9.4% over the same period. When analysing group treatments (premixes, oral powders and oral solutions) by ALEA, rabbits were the highest consumers (2.70), followed by veal calves (2.63), poultry (1.12) and pigs (0.96). Based on the ALEA, there was a decrease in exposure over the preceding 5 years of 30% for rabbits, 25% for pigs, 12.3% for poultry and 0.2% for cattle. ESVAC (EMA ESVAC, 2015b) notes that the greatest reduction in consumption has been in species sectors where voluntary actions have been initiated, but links the decrease in overall sales of antimicrobials in France on implementation of the French national action plan in 2011. This plan proposes measures for improved procedures for prescription, especially regarding the prescription without clinical examination of livestock and adherence to prescriptions by manufacturers of medicated feed. It proposes collection of more detailed information from manufacturers of medicated feed, particularly in relation to use of antimicrobials for preventive purposes.
In Denmark in 2013, sales of oral formulations made up 65% of total sales of antimicrobials (mg/PCU) according to ESVAC data. Pigs made up 43% of the live biomass (kg) and consumed 76% of antimicrobials (DANMAP, 2014). In June 2014, the Danish Food and Veterinary Administration introduced new legislation aimed at improving the effectiveness of mass medication for pigs. This requires veterinarians to take diagnostic samples for testing at an approved laboratory when prescribing orally administered antimicrobials for the treatment of gastrointestinal or respiratory infections. Treatment should then be re‐evaluated if necessary in light of the findings. VMPs can only be prescribed in connection with a visit to the farm and repeated visits are required for continued use. DANMAP 2015 reported that antimicrobial use for treatment of groups of pigs decreased by 17% between 2014 and 2015, but attributed this also to a reduction in the threshold levels for the Yellow Card initiative (DANMAP, 2016). A study of pig farms in Denmark showed an increase in the total amount of antimicrobial prescribed when moving from feed to water medication, but this may have been due to an increase in the number of animals treated due to the change from pen‐level to sector‐level treatment (Fertner et al., 2016).
In Belgium, AMCRA (the Center of Expertise on Antimicrobial Consumption and Resistance in Animals, supported by government food and medicines agencies, agricultural organisations, pharmaceutical industry and academia) has the objective to reduce the use of antimicrobials in medicated feed by 50% (compared to 2011 reference) by 2017, and to ensure that medicated feed is prescribed only by the veterinarian who establishes the health plan for the farm (AMCRA 2020 vision statement). In 2011, 90% of sales of antimicrobials (mg/PCU) in Belgium were oral formulations, although only 19% were premixes (ESVAC) and this may limit the impact of the measure. In 2013, AMCRA produced guidelines in which antimicrobials are colour‐coded according to their importance for human health. According to BelVetSac (2015), there has been a 15.9% reduction (mg/kg biomass) in antimicrobial use overall and 14.7% reduction in antibacterial premixes compared to 2011. More than 99.6% of antimicrobial premixes go into pig feed. In 2012, a temporary authorisation was introduced to allow use of zinc oxide for the treatment of post‐weaning diarrhoea in piglets and it is inferred that over 70% of piglets were treated. This has been linked to a substantial, although lesser than expected, reduction in the use of polymyxins (almost entirely colistin) over this period.
NEVEDI, the association of Dutch Feed Producers, voluntarily stopped producing antimicrobial medicated feed in 2011 due to concerns about difficulty in predicting carry‐over levels even when using GMP procedures.
In the Czech Republic a national legal provision (Act No 378/2007) stipulates that medicated feeds may be mixed in licensed feed mills only on the basis of a veterinary prescription, which must specify the animal species/category and treatment course, and provide exact information on the proper mixing and use of the medicated feed. Rules for self‐ and official checking of the quality of mixing, and rules/limits on cross‐contamination, are also set. Off‐label use of premixes is banned under the national legal provision. From 2007 there has been a reduction in use of antimicrobials in premixes from 25.4 tonnes (active substance) to 8.2 tonnes in 2015.22
Concluding remarks
Oral administration is of particular concern in terms of promoting the development of AMR due to the high exposure of gastrointestinal commensal bacteria, and the sometimes prolonged duration of treatment/exposure. Parenteral administration may also expose the intestinal microbiota if the antimicrobial is excreted in an active form into the gut lumen.
A new Regulation on the manufacture, placing on the market and use of medicated feed is undergoing legislative process in the EU.
According to ESVAC data for 2014, oral formulations accounted for 91.6% of all antimicrobial use in food‐producing animals in the EU (mg/PCU). Premixes accounted for 42.1% of sales and oral powders 31.7%. This demonstrates the importance of focusing reduction measures on oral formulations.
There is much variability between EU MSs in the proportions of premixes, oral powders and oral solutions sold, which may reflect species distributions and national policies.
Oral formulations of tetracyclines and penicillins constituted 32.6% and 22.8%, respectively, of the total antimicrobial sales for food‐producing animals.
In terms of the highest priority CIAs, there are no formulations of 3rd‐ and 4th‐generation cephalosporins authorised for oral group treatments. Fluoroquinolones, which are available for oral administration via drinking water made up only 1.9% of administered antimicrobials (mg/PCU). Polymyxins (colistin) are available as both premix and oral solution formulations for group treatment and made up 6.6% of antimicrobials used. Recommendations have been made recently to restrict its use (Section 3.2.2).
A shift has been noted in recent years from use of oral formulations to long‐acting injections (e.g. macrolides) in pigs.
For practical reasons, premix formulations are more often used for systematic prevention or for metaphylaxis of disease and treatment durations tend to be prolonged. Oral solutions tend to be used for shorter periods to treat acute disease outbreaks.
Oral formulations are mostly used for treatment of veal calves, pigs and broilers.
Factors such as the lower doses of antimicrobials used in medicated feed, the risk of under‐dosing, prolonged periods of administration and cross‐contamination in feed mills, delivery vehicles and farm storage may increase the risk of AMR development associated with in‐feed antimicrobials.
In Sweden, a reduction in the use of oral medication in pigs and shift to individual animal treatment has been achieved through a national programme focusing on infection prevention and control programmes, vaccination against endemic diseases, improved diagnosis and use of species‐specific treatment guidelines.
In France, measures in the national action plan including increased emphasis on clinical examination prior to prescribing and closer adherence to prescriptions by medicated feed manufacturers have been effective, alongside voluntary species sector initiatives.
Measures to improve diagnosis and veterinary oversight of prescribing of oral formulations for pigs have been implemented in Denmark, but it is too early to assess their impact.
Recommendations
Dosing regimens for older antimicrobials that may have been established prior to development of newer PK/PD concepts may need review. Attention should be paid to the justification of the treatment duration.
Further research is needed into the impact of dosing regimens and different formulations on development of AMR in commensal organisms.
-
Greater oversight of the use of antimicrobials in oral formulations should be considered. This includes:
-
–
The need for veterinary clinical examination of animals prior to each prescription.
-
–
Treatment should not be prolonged beyond clinical/bacteriological necessity.
-
–
Clear dosing instruction should be provided in the SPC and by the veterinarian to ensure accurate dosing.
-
–
Further relevant recommendations are included in the subsection on Prevention, Prophylaxis and Metaphylaxis (see Section 3.3.6).
3.3. Measures taken at national level
Measures taken at national level are mostly based around MSs’ individual strategies and action plans and are focused on:
Measures to limit the use of antimicrobials (e.g. targets, benchmarking, controlling distribution channels, taxation)
Measures encouraging the responsible use of antimicrobials (e.g. treatment guidelines, education)
Although national action plans and surveillance and monitoring of antimicrobial use and AMR do not, per se, reduce antimicrobial use or AMR, it is essential that a strategic approach is taken and that there are the means to evaluate the impact of the direct measures taken.
3.3.1. National Action Plans
When the WHO adopted its global action plan on AMR in May 2015, it indicated the expectation that countries would have in place their own multisectoral national action plans by 2017. The WHO in conjunction with the FAO and OIE have developed a manual to assist countries in preparing and updating their operational national action plans so that they support the strategic objectives of the global action plan (WHO FAO and OIE, 2016).
to improve awareness and understanding of AMR through effective communication, education and training
to strengthen the knowledge and evidence base through surveillance and research;
to reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures;
to optimise the use of antimicrobial agents in human, animal and plant health; and
to develop the economic case for sustainable investment, taking into account the needs of all countries, and to increase investment in new medicines, diagnostic tools, vaccines and other interventions.
Emphasis is placed upon the need for action plans to take a ‘One Health’ approach, supporting strong collaboration between the key sectors: human health, animal health, agriculture, finance, environment and consumers, in order to avoid disjointed outcomes. An assessment should be undertaken of the resources needed for implementation of the plans and political commitment is required to secure long‐term financial investment. It is suggested that plans are implemented incrementally and that they build step‐wise on any existing systems. A programme for monitoring and evaluation should be part of the plan.
The need for EU MSs to develop national strategies or action plans was supported in the EU Council conclusions of 29 May, 2012. Many MSs now have action plans which follow a ‘One Health’ approach, albeit with focus in specific sections on human or veterinary antimicrobial use.
The key elements included within most the EU national plans are:
Communication, education and training: this includes both training at the professional and animal keeper level, and raising of public awareness.
Ensuring effective monitoring and surveillance programmes for antimicrobial use and AMR in humans and animals.
Encouraging responsible antimicrobial use: development of national treatment guidelines; encouraging development and use of diagnostic tests; limiting preventive use; improving the oversight of prescribing for herd/flock treatments in particular
Limiting the use of CIAs
Improving animal health and disease prevention, e.g. through biosecurity, hygiene measures and vaccination.
Promoting research into AMR, development of novel antimicrobials and alternative treatments.
Establishing a multisectoral network to implement and monitor the action plan.
Strengthening international collaboration.
As advised in the EC PUAVM Guidelines, the national strategies and action plans should take into account national animal production and local patterns of antimicrobial availability and resistance. For example, in Denmark, the Joint Antibiotic Resistance Action Plan identifies a need to address MRSA in the pig population, proposing funding to be allocated to the identification of herd‐related risk factors to support a risk management strategy, and a coordination group to be established for zoonotic MRSA to improve communication to doctors and households in contact with pig herds. The Norwegian National Strategy against Antibiotic Resistance 2015–2020 has animal sector goals to prevent the establishment of LA‐MRSA in the pig population and to reduce ESBL‐producing bacteria in poultry to a minimum. Some national strategies include risk‐based targeting.
Recommendations
All MSs should have national strategies on AMR that are implemented through action plans.
National strategies/action plans should be developed taking into account the ‘One Health’ aspects of AMR to integrate actions on veterinary and human side. They should be tailored to the local circumstances (e.g. actual use of antimicrobials, AMR levels, animal species farmed and livestock production systems, environmental conditions, etc.).
All sectors throughout the food chain should be involved collaboratively in the development and implementation of national action plans
Action plans should be regularly reviewed and monitored for effectiveness. With time, the objectives should be tailored to address the national AMR situation.
Lessons should be learned from national examples of success in sustainably reducing antimicrobial use.
3.3.2. Monitoring and surveillance programmes for AMR and antimicrobial use
3.3.2.1. National AMR surveillance programmes
In addition to compulsory surveillance of AMR carried out in compliance with CD 2013/652/EU, various EU MSs have implemented national surveillance programmes for monitoring resistance in zoonotic and indicator bacteria from animals, and animal pathogens, e.g. Finland (FINRES‐Vet), France (Résapath), and Spain (VAV Network). Silley et al. (2011) highlight that changes introduced into surveillance schemes, particularly the use of epidemiological cut‐off values (ECOFF) values rather than clinical breakpoints, and lack of standardisation in definitions of ECOFFs, can lead to misinterpretation of trends in resistance development. Other than for those aspects designed for the purpose of compulsory EU surveillance, there is no uniform methodology across national AMR surveillance programmes for sampling, antimicrobial susceptibility testing (AST) or the interpretive criteria for defining resistance (Silley et al., 2012). Harmonisation of monitoring programmes has been identified previously as an urgent need for the EU to optimise use of data in risk assessment and development of risk management measures (Silley et al., 2011). Requirements for surveillance programmes have been published by the OIE in the Terrestrial Animal Health Code (chapter 6.7) and the need for national surveillance programmes was reinforced in the WHO global action plan (WHO, 2015).
Some EU MSs produce integrated reports that include resistance data from human and veterinary/food isolates and antimicrobial use data, e.g. Denmark (DANMAP), Germany (GERMAP), the Netherlands (NethMap, MARAN), Norway (NORM, NORM‐VET), Sweden (Swedres‐Svarm) and the UK (UK ‘One Health’ Report).
The UK ‘One Health’ Report, which was published for the second time in 2015, highlighted several limitations in the resistance data collated: differences in methodologies used for susceptibility testing and the lack of a standardised panel of antimicrobial agents. In addition, there were differences in the way in which data on antimicrobial use were collected between the human and animal sectors. It was noted that the variation in methods of data collection prevented meaningful comparisons and this highlighted the need for good collaboration in the development of surveillance programmes between human and animal sectors. In the UK surveillance programme, samples from animals are obtained both from clinical surveillance, as part of the National Control Programme for Salmonella in poultry and under EU harmonised monitoring (CD/2013/652/EU). For veterinary isolates from England, Scotland and Wales, susceptibility testing was performed either in accordance with EFSA's requirements for the EU harmonised programme, or according to British Society for Antimicrobial Chemotherapy (BSAC) methods. Both EFSA's ECOFFs and BSAC clinical breakpoints were used for interpretation and reporting.
In Denmark, DANMAP, was established in 1995 by the Danish Ministry of Health and the Ministry of Food, Agriculture and Fisheries. AMR is monitored in three categories of bacteria: human and animal pathogens, zoonotic bacteria and indicator bacteria that have been isolated from humans, food‐producing animals and food of animal origin. Samples are obtained from healthy animals at slaughter and diagnostic submissions, meat at the abattoir and from retail outlets, and from human clinical isolates. For DANMAP 2014, samples from broilers were collected at slaughter according to CD 2013/652/EU for testing of indicator E. coli, Campylobacter spp. and enterococci. Salmonella isolates were obtained from random sampling of healthy pigs at slaughter, but due to the low number of isolates from broilers and cattle, data on Salmonella from these species were not presented. Data were interpreted using the EUCAST ECOFFs in most instances. DANMAP also monitors consumption of antimicrobial agents in order to study associations between consumption and resistance and to identify transmission routes and requirements for further research. The report from DANMAP 2014 includes the findings of two studies: a report investigating the zoonotic link between carbapenemase‐ and ESBL‐producing E. coli from meat and human bloodstream infections, and a survey on the prevalence of LA‐MRSA in Danish pig herds and in humans. The findings from the DANMAP programme have been used to monitor the impacts of policy interventions (Hammerum et al., 2007). For this purpose, a sufficiently long period of surveillance using standardised methodology is required.
The Swedish report, Swedres‐Svarm, is prepared by the Public Health Agency of Sweden in collaboration with the National Veterinary Institute, with the aim of following a ‘One Health’ approach. Bacterial susceptibility data are collected from several sources including national monitoring programmes. For screening of indicator E. coli (including for ESBLs), samples are collected from healthy pigs, cattle and broilers at the abattoir. Samples from pigs and cattle are also screened for Campylobacter spp., while samples from broilers are collected under the Swedish Campylobacter programme. For Salmonella, clinical isolates in addition to isolates obtained under the Salmonella surveillance programme are tested. The ECOFF values issued by EUCAST or by EFSA are used for interpretation of MICs in most instances (Public Health Agency of Sweden and SVA, 2016). The report from 2014 provided a summary of a joint project conducted between 2009 and 2014 to investigate food as a potential source and dissemination route for ESBL‐producing E. coli to humans. The study used samples collected from food‐producing animals, foodstuffs, healthy humans, seriously ill patients and the environment. It was concluded that food on the Swedish market contributed little to the occurrence of ESBL‐producing E. coli in the healthcare sector, and that there were three separate populations of genes encoding ESBL: one in Swedish food and farm animals, one in imported food, and one in humans and the environment.
The DANMAP and Swedres‐Svarm reports demonstrate that data collected through integrated national AMR surveillance can be of considerable value for investigating sources of resistance and transmission between different reservoirs. Such links are more difficult to make on data collected at the EU‐wide level, and absence of truly integrated national surveillance programmes, especially those providing sufficient longitudinal monitoring for identification of trends, is a data gap in many EU MSs. As noted by the WHO, the purpose of integrated surveillance is to guide policy development across the sectors and ensure that control measures are implemented in areas most likely to have an impact on public health (WHO, 2011). Integration in the WHO‐net for antimicrobial resistance should be considered at a global level.
The considerable time gap between data collection and reporting, which could interfere with rapid implementation of investigations and interventions, is problematic. To overcome this hurdle, the process of collection, validation and reporting should be as real‐time as possible and at the level of detail required for meaningful action to be taken.
Concluding remarks
Beyond data collected under the EU harmonised monitoring (Decision 2013/652/EU), a lack of standardisation within surveillance programmes and harmonisation of national AMR surveillance programmes across the EU may limit their usefulness in risk assessment and for risk management across countries.
In individual countries integrated systems that allow analysis of data from humans, animals and food have been shown to be valuable for demonstrating dissemination of AMR between the different reservoirs, identifying research needs, supporting policy development and evaluating the effectiveness of interventions.
The absence of a truly integrated European network, especially providing significant longitudinal monitoring, is a data gap.
Recommendations
MSs should be strongly encouraged to develop AMR surveillance systems that allow integrated analysis of data from animals, food and humans and which give scope for the investigation of dissemination of AMR between the different reservoirs.
Building upon programmes already in place to comply with Directive 2003/99/EC, MSs should collaborate to develop AMR surveillance programmes that are harmonised across the EU.
Data should be as much as possible consultable via an open data policy to encourage research, including retrospective analysis.
3.3.2.2. Monitoring of antimicrobial use at national level, on farms and by prescriber
In Europe, 29 countries provided their antimicrobial sales data for 2014 to ESVAC. In addition to provision of data to the ESVAC project, the EC PUAVM Guidelines recommend that MSs collect data on use by animal species and age group. These databases are a vital tool that underpins the benchmarking of antimicrobial use, for measuring the effectiveness of reduction of use policies and ultimately, when analysed in conjunction with AMR monitoring data, for making inferences about the impact of antimicrobial use on AMR.
Denmark
Structured approaches to monitoring antimicrobial use and bacterial resistance were introduced in Denmark to overcome vested interests among farmers and veterinarians that previously incentivised liberal use of routine medication to enhance productivity in the face of intensified production and consequent endemic disease (Wielinga et al., 2014). In the early 1990s, Denmark documented a rapid increase in the use of antimicrobial agents and an increase in antimicrobial‐resistant bacteria causing disease in animals. The findings of high levels of vancomycin‐resistant Enterococcus (VRE) bacteria in chickens from farms using avoparcin as an AGP and the similar increase in VRE in humans led to the realisation among Danish authorities and farmers that a better understanding was needed of the effects of the use of antimicrobials in humans and animals. DANMAP (see above) was established to follow the impact of withdrawing antimicrobial growth promoters in the pig and poultry sectors between 1997 and 2000, but more broadly to monitor antimicrobial consumption and study its association with AMR in animals and humans. It was established as a collaborative effort of all stakeholders involved in the farm‐to‐fork chain, including commercial and public, and human and animal health sectors.
The veterinary medicines database VetStat was established in 2000 to monitor antimicrobial consumption and is hosted by the Danish Veterinary and Food Administration. Veterinarians, feed mills and pharmacists send information electronically to VetStat, which includes the supplier and prescribing veterinarian identity, farm‐identity, name of product, quantity prescribed and administered, animal species, age group and diagnostic group. Consumption is recorded as ‘defined animal daily doses’ (ADD), a statistical unit defined for each age‐group and species of animal, which is independent of the potency of the drug (Jensen et al., 2004a). Some caution needs to be exercised when interpreting the data as account may need to be taken of changes in animal populations over time. The development of this system was facilitated in Denmark by a well‐functioning and integrated infrastructure around livestock production.
By 2006, with 11 years of data, DANMAP was able to demonstrate a marked decline in the occurrence of vancomycin‐resistant Enterococcus faecium (VREF) in broiler chickens as a result of the ban on use of avoparcin as an AGP. Similarly a reduction of VREF in pigs was observed after macrolide use for growth promotion in this species was stopped, and by 2007 only three VREF isolates were identified out of 485 samples taken from humans since 2002. Likewise, the impact of the ban on virginimycin was shown on carriage of streptogramin‐resistant E. faecium in broilers and pigs (Hammerum et al., 2007). VetStat drew attention to the high use of fluoroquinolones in animal production, supporting a regulation to restrict its use, and of antimicrobials generally in pig production resulting in the development of guidelines on prescribing (see Section 3.3.4). The establishment of the DANMAP and VetStat systems has provided information to support veterinary risk assessment through centralised systems for monitoring AMR in food, animals and humans and for monitoring antimicrobial prescribing and use (Hammerum et al., 2007). By bringing transparency and independence to these processes, it has supported evidence‐based policy making which has been broadly accepted by stakeholders (Wielinga et al., 2014).
The Netherlands
In 2010, the independent Netherlands Veterinary Medicines Authority (SDa) was set up to collect data on antimicrobial consumption on farms, establish benchmark indicators for the individual major livestock sectors and analyse trends in consumption. The SDa is a public‐private partnership between government, stakeholders from the major livestock sectors (pigs, broilers, veal calves and dairy cattle) and the Royal Dutch Veterinary Association (KNMvD). Veterinarians enter prescription information in a Practice Management System and this is transferred to a central database. The information includes veterinarian and farm details, quantity supplied and animal species treated. Data are then transferred to databases held by private livestock quality assurance systems. The sectors calculate consumption as the number of days per year on a farm that animals have been administered antimicrobials. The data show that large differences in consumption exist between different livestock sectors and farm categories, highlighting that data are needed on consumption per species in order to adequately compare countries (Bos et al., 2013).
Belgium
In Belgium, Belpork is a non‐profit organisation that manages the quality label ‘Certus’ for pig producers. From 1 January 2014, Belpork launched an online antimicrobial monitoring programme ‘AB Register’ which covers 60% of the pig farms (75% of the pig population) in Belgium. Data are provided to the register by veterinarians, feed producers and pharmacists; pig producers are then able to access reports including a benchmarking of their antimicrobial use against other producers. Data analysis is to be provided by AMCRA, and will be linked to resistance surveillance. Through their specific scheme, the Certus quality label obliges the highest users to establish measures to reduce their use. A national data collection system for pigs, broiler chickens, layer hens and veal calves is expected to be in place in Belgium by mid‐2016. The input of the data in this system will be mandatory and provided by the veterinarians. The data will be used to benchmark at the level of the veterinary practice and at the farm level. The development of the national data collection system and the analyses of the data are financed by supplementary taxes on the sales of veterinary antimicrobials.
France
In France, the French agency for veterinary medicinal products (ANSES‐ANMV) began monitoring sales of veterinary antimicrobials in 1999. This surveillance is based on the recommendation of the OIE guideline on ‘monitoring of the quantities and patterns of use of antimicrobials agents used in food‐producing animals’ from the terrestrial animal health code. It is carried out in conjunction with the French Union for the Veterinary Medicinal Product and Reagent Industry (SIMV), based on the annual reporting of antimicrobial sales by the pharmaceutical industry and their estimate of use per target species. New legislation adopted in September 2014 by the French government introduced a mandatory reporting of antimicrobials sales by pharmaceutical companies and those entitled to dispense antimicrobials.
Spain
In Spain, an on‐line‐platform ESVAC‐ES (European Surveillance of Veterinary Antimicrobial Consumption, Espana) has been set up to collect prescription data from individual farms and veterinarians. This system will be mandatory. The data will be used to benchmark and to set annual targets for antimicrobial use in the different major livestock sectors, including species‐specific benchmark indicators that will differentiate between low, moderate, high and very high users (farmers) and prescribers (veterinarians).
Germany
In Germany a project was undertaken to investigate how best to record the quantity of antimicrobials used in food‐producing animals (German antimicrobial resistance strategy, DART).23 This information was used to formulate a mandatory requirement for recording use, with the aim to integrate these data in assessment of the risk of AMR development. In new legislation which came into force in 2014 under the amended German Medicinal Products Act, data on the use of antimicrobials on fattening farms are reported to the competent authority. Key figures on treatment rates are published in the Federal Gazette. A benchmarking system is in place whereby every six months livestock producers must compare their use against nationwide key figures and if necessary, in conjunction with a veterinarian take measures to reduce use. Competent authorities have regulatory powers if management measures on farm are ineffective. Further to this, the VetCAb project will examine a random sample of farms to identify the main diseases and livestock sectors where antimicrobials are being used to enable more focused reduction measures (DART 2020).24
The United Kingdom
In the UK, the industry body AHDB Pork with assistance from the government agency, the Veterinary Medicines Directorate (VMD), has developed an electronic medicines book to collect accurate on‐farm antimicrobial use data. The system allows farmers to fulfil their legal obligations to record medicines use, and will allow the VMD to collect data to meet the future EU monitoring requirements. In the longer term, the system will allow farmers to benchmark their use with anonymised data from similar units.
The analysis of links between consumption of antimicrobials and AMR may be facilitated by availability of data at antimicrobial class, animal species and bacterial species level. In addition, production classes may need to be taken into account to ensure effective intervention measures (EMA ESVAC, 2013). An important property of all data collection systems is that they use a descriptor of use that is transparent, accurately reflects patterns of use and allows comparisons to be made between countries and over time (Chauvin et al., 2001). A study by Dupont et al. (2016), showed that trends in consumption figures could be heavily influenced by use of different methods for assigning Animal Daily Dose (ADD) and the chosen method of population measurement. Postma et al. (2015a) established a ‘defined daily dose animal’ (DDDA) for antimicrobial products authorised for use in pigs in four EU countries. ESVAC has established principles for assignment of defined daily dose for animals (DDDvet) and defined course dose (DCDvet) values for antimicrobials for use in cattle, pigs and broilers and will start to publish use data using these measurements (EMA ESVAC, 2015a) (see section 2.1.1).
The Czech Republic
The Czech Republic has collected data on antimicrobial consumption under national legislation since 2000.22 The system provides long‐term stable data and allows analysis and identification of trends in the overall sales, as well as specific analyses at several levels (e.g. premixes). Data from wholesalers and feed mills are provided to ESVAC, other data are used for cross validation. Based on these data vets with highest prescription of CIAs were identified and targeted for inspections carried out in 2013 with aim to identify the underlying reasons for prescribing and to educate on more responsible prescription.
Concluding remarks
Systems for monitoring antimicrobial use that have been set up in some countries have usually been initiated by governments, and then successfully implemented through public‐private partnerships involving all stakeholders in the food production chain, including the animal health sector, industry and professional bodies and academia. Once established these systems have been made mandatory.
The availability of well organised livestock production quality systems has facilitated the implementation of monitoring systems in Denmark, Netherlands and Belgium.
Data collected at species level (at least) using a standardised unit of measurement are necessary to allow improved comparison of antimicrobial consumption between countries and trends over time.
Recommendations
A common protocol should be considered which would enable a comparison of antimicrobial consumption data between all countries and over time. A meaningful, harmonised statistic (e.g. DDDvet) should be used in the analysis of use data.
Data on antimicrobial use should be collected in timely fashion and preferably electronically from prescribing veterinarians, pharmacists, producers of medicated feed and others, as required, to enable a mechanism for external validation. Internal validation (QA) should also be undertaken.
Systems should be transparent with regular reporting of findings. Farmers, veterinarians and quality schemes should have access to view their own and/or aggregated data.
The minimum data collected should allow identification to the prescribing veterinarian, farm of use, animal species, formulation details and quantity to allow for benchmarking and tailoring of risk management measures. (See Section 3.3.3).
To be of most use for tailoring risk management measures, data should be collected at the level of animal production type and age group. Diagnostic information is also of value to help target future treatment and to assess impacts of measures on animal health and welfare. (See Section 3.3.3).
Systems for monitoring antimicrobial consumption should preferably be integrated with national (or even farm‐level) surveillance programmes for AMR. This would facilitate the establishment of an evidence base to identify the need for risk management measures/policies at the local level and to assess their effectiveness.
3.3.3. Targets for reduction of use and benchmarking of farms
The EC PUAVM Guidelines identify that some MSs have included within their national AMR strategies targets for the reduction of antimicrobial use.
The Netherlands
Between 1999 and 2007, the use of antimicrobials in farm animals in the Netherlands doubled (MARAN, 2011). In addition, concerns were being raised by the medical profession and in the media regarding the risk to public health from the livestock reservoir of resistant pathogens such as MRSA and ESBL‐producing bacteria, leading to a loss of public confidence in the livestock industry (Speksnijder et al., 2015c). This led the Dutch government to introduce between 2008 and 2011 a policy for reduction and more responsible use of antimicrobials in the livestock industry. The policy was established under an independent taskforce, the SDa (see earlier). The key elements of the policy were (1) benchmarking of antimicrobial use, (2) improvements of herd health with clear responsibilities for veterinarians and farmers, and (3) reduction targets for livestock production as a whole. These targets were, relative to the 2009 level of use:
2011: 20% reduction
2013: 50% reduction
2015: 70% reduction
The targets were not evidence‐based, and are not directed at livestock sectors and antimicrobial classes, but reflected the need for the Dutch government to take urgent action in the face of public pressure (Speksnijder et al., 2015c).
Sector‐specific benchmark levels for antimicrobial consumption are set by the SDa. In 2011, these targeted farms above the top 75th percentile, but they are re‐evaluated annually. Data collected in 2011 showed that although most farms had a low consumption of antimicrobials, the distribution was right‐skewed with a minority of farms having very high levels of use. Farms that exceed benchmark thresholds are required by their private quality assurance systems to reduce their antimicrobial consumption, and if use is persistently high this is brought to the attention of the Netherlands Food and Consumer Product Safety Authority. Further policy measures included the prohibition in law of preventive use of antimicrobials and an initiative for the KNMvD to develop treatment guidelines. A legal basis was created for mandatory susceptibility testing before using human CIAs.
According to the MARAN report (2015) sales of veterinary antimicrobials in Netherlands in 2014 decreased by 58.1% compared to the index year of 2009 (see also Figure 4). This is a substantial reduction, but it has to be noted that ESVAC data indicate that sales in 2009 were relatively high, at 165 mg/PCU, compared to other EU countries. Although use of antimicrobials in food‐producing animals in the Netherlands is still high compared to Denmark, it is now low compared to other countries with similar livestock production. Downward trends have been noted across all major species (pigs, broilers, dairy cows and veal calves). It has been acknowledged that a critical factor in the success of the Dutch policy was the rapid response of the livestock production industry and the KNMvD to the sense of urgency expressed by human health care bodies and subsequently at the political level. This supported a public‐private approach to implementing reduction measures, which was facilitated by the presence of already operational quality systems in the production chain (Dutch Ministry of Economic Affairs, 2014). Speksnijder noted that a Memorandum of Understanding signed by taskforce stakeholders in 2008 did not significantly reduce antimicrobial use, which did not occur until strict government targets were imposed.
Figure 4.
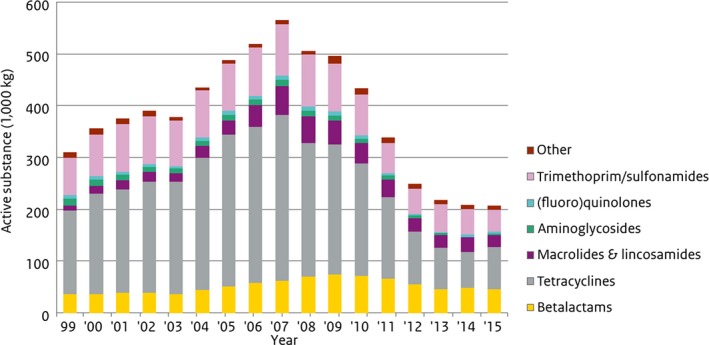
Antimicrobial veterinary medicinal product sales from 1999–2015 in kg (thousands) in the Netherlands (source: MARAN, 2016)
The outcomes show what can be possible, while still retaining a financially viable food industry (Speksnijder et al., 2015c). There was a reduction in 2014 of 4.4% compared to 2013; use has levelled off in most species except for poultry (increase) and dairy cattle (decrease), and it is acknowledged that to reach the 70% reduction target by 2015 will be a challenge.
The SDa (SDa, 2016a) analysed the relationship between antimicrobial use and AMR (from slaughterhouse sampling) based on monitoring data collected between 2009 and 2014. In regards to the prevalence of AMR in faecal E. coli samples, the following associations were shown:
Veal calves: 37% reduction in total antimicrobial use, 26% decline in AMR to one or more classes
Pigs: 54% reduction in total antimicrobial use, 22% decline in AMR to one or more classes
Broilers: 57% reduction in total antimicrobial use, 8% decline in AMR to one or more classes
Statistically significant correlations were found between the total amount of antimicrobials used and the prevalence of resistance in E. coli for pigs and veal calves. In many cases, antimicrobial‐specific resistance in E. coli was more strongly associated with total antimicrobial use than with antimicrobial‐specific use, and it is suggested that this could be due to co/cross‐resistance.
The SDa notes that it is not yet possible to identify a level of use (threshold) below which AMR would be reduced to background levels. It is also not yet possible to define a level of AMR in livestock, which is considered ‘acceptable’ in terms of the risk to public health and could be used as the basis for benchmark values. The SDa recommended that ideally AMR data collected at individual farm level on resistance and antimicrobial use should be combined and communicated back to the individual farmers and livestock sectors concerned.
Dierikx et al. (2016) investigated whether the 50% reduction in antimicrobial use in the Netherlands observed from 2009 to 2014 had led to a reduction in MRSA prevalence among Dutch pigs at slaughter. In 2005, 39% of pigs and 81% of slaughter batches were MRSA positive. The prevalence was still at least as high in samples taken in 2014, with all isolates belonging to the livestock‐associated CC398 strain.
A study conducted in the Netherlands (de Jong et al., 2013b) investigated the relationship between reduction in antimicrobial use and two welfare parameters in broilers: mortality and hock burn, using data collected under the EC Broiler Directive. There was no clear relationship and no difference between figures for mortality and hock burn in flocks that did not receive antimicrobial treatment and those that did. In a questionnaire sent to farmers, a large number indicated that the number of rejections at slaughter had increased, as had the number of chickens culled on the farm, as a result of the reduced use of antimicrobials.
The Council for Animal Matters in the Netherlands (RDA) discussed in a report published in 2016 (RDA, 2016) that there was yet insufficient scientific literature or field evidence to assess the impacts of the Dutch policy on antimicrobials on animal health and welfare. Some concerns were raised regarding possible increased mortality in veal calves and post‐calving mastitis in dairy cows, but it was stated that further information and objective indicators of animal welfare are needed before any the impacts of reduced antimicrobial use can be established.
Denmark
As a result of detailed monitoring of antimicrobial consumption via VetStat, the Danish Veterinary and Food Administration introduced in 2010 the Yellow Card initiative aimed at reducing antimicrobial consumption in the (major) pig sector by 10% by 2013. The system was directed at the pig farmers that used the highest amounts of antimicrobials, with targets initially set at twice the average use for each production age group (sow herds, weaners and finishers). This system established thresholds for animal daily doses (ADD), which if exceeded require a veterinarian to provide an action plan with concrete interventions to reduce antimicrobial consumption on the farm, and ultimately results in an injunction to allow unannounced inspection visits and prevent represcription and holding on the farm of antimicrobials administered via food and water. All costs of injunctions and inspections are born by the farmer.
Between 2009 and 2011, there was a 25% reduction in the total antimicrobial use per pig produced, explained mainly by a reduction in prescription of oral antimicrobials (tetracyclines, macrolides and pleuromutilins) for treatment of gastrointestinal disease in weaners and finishers (Jensen et al., 2014). It was hypothesised that the decrease in use of tetracyclines could be related to cessation of systematic metaphylaxis in some herds. The reduction in antimicrobial use showed no impact on the occurrence of chronic pleuritis, the most common lesion seen in the slaughterhouse (23%); although there was a short‐term increase in the prevalence of less common (< 1%) specific lesions. According to (DANMAP, 2014), there was a temporary decrease in productivity in some herds, but no increase in disease outbreaks. There was an increase in the use of vaccines, which could impact positively on the prevalence of chronic pneumonia (Alban et al., 2013). In 2012 and 2013, there was a partial reversal and antimicrobial consumption in pigs increased; however, by 2015 consumption had decreased again and was 22% lower than in 2009 (DANMAP, 2016). In 2014, Danish pig producers committed to a voluntary reduction in the use of tetracyclines of 50% by the end of 2015. Although this target was not met, use was reduced in 2015 by 24% compared to 2009 data (DANMAP, 2016).
Antimicrobial use in food‐producing animals in Denmark is low compared to other high pig producing countries in the EU (EMA ESVAC, 2016). Although the measures taken by the Danish government have clearly had an impact on antimicrobial consumption, there are financial costs associated with their implementation and administration and success may be dependent on the specific husbandry and organisation of the pig industry in Denmark. Most Danish pig farmers are members of one of two cooperative systems, which operate comprehensive documentation throughout the production chain, therefore, quality initiatives can be rapidly assimilated.
In addition, the amount of zinc oxide prescribed by veterinarians has increased markedly in Denmark From 2005 to 2011 a threefold increase in use of zinc and zinc oxide (ZnO) was reported to VetStat, whereas the number of pigs produced in Denmark increased by 14% (DANMAP, 2015). Since 2011, the consumption of ZnO has been relatively stable.
DANMAP 2014 reported that in indicator E. coli from pigs, trend analysis showed an increase in resistance to ampicillin from 23% in 2010 to 34% in 2014; while resistance to tetracyclines and sulfonamides showed no trend. Prior to this, DANMAP 2010 had reported an increase in resistance in E. coli to tetracyclines over the preceding decade corresponding to a dramatic increase in use of the substance in pigs from 2002 to 2007.
DANMAP 2014 also reported a high level of resistance in isolates of S. Typhimurium from pigs to ampicillin, sulfonamides and tetracyclines (ASuT), with 64% of isolates being MDR. Monophasic S. Typhimurium constitute a high proportion of the MDR isolates and have been increasing in pigs since 2010.
The prevalence of MRSA in pigs at slaughter was reported to have increased dramatically in 2012. A prevalence survey conducted in 2014 showed high prevalence of MRSA in breeding (63%) and slaughter herds (68%) compared to surveys in 2010 and 2011 when prevalence was 16% (DANMAP, 2015). The number of humans sampled positive for LA‐MRSA belonging to CC398 (see Appendix A), has increased rapidly since 2009, with 1,277 cases in 2014 compared with 643 cases in 2013; although this may reflect increased screening of hospital admissions, especially of people employed in the pig industry (DANMAP, 2015). The SSI reported that the number of human reports of MRSA CC398 recorded decreased to 1,172 in 2015, of which 18% were clinical cases and 12% had no direct contact with pigs.25
France
In 2012, the French National Action Plan for the Reduction of the Risk of Antibiotic Resistance in Veterinary Medicine (EcoAntibio 2017) was implemented, becoming ratified in legislation in 2014 (Law n°2014–1170).26 This plan aims to achieve a 25% reduction in antimicrobial use over 5 years. A package of measures has been proposed including creation of benchmark indicators for prescription and use of antimicrobials to be used on a voluntary basis, improving control over the prescribing of antimicrobials without prior clinical examination and revision of controls over commercial practices that can influence product procurement (sales discounts, etc.). After 2 years, there has been a decrease in antimicrobial use in France of 12.7%, in line with the EcoAntibio 2017 target. In 2013, total sales of antimicrobials amounted to 699 tones, the lowest since monitoring began. Between 2012 and 2013, exposure to antimicrobials decreased by 6.6% for cattle, 5.4% for poultry and 4% for pigs, but rose by 3.6% for rabbits.
Norway
In the second part of the 1990s the Norwegian husbandry organisations set a target to reduce antimicrobial sales by 25% over a 5‐year period from 1996 to 2000. This goal was supported by a campaign, which included conferences and meetings, and provision of comprehensive treatment guidelines aimed at areas of high antimicrobial use (e.g. bovine mastitis). From 1992 to 1996 there was a 17% reduction in antimicrobial use which was mostly attributed to improved diagnosis and prescribing (Grave et al., 1999). From 1995 to 2013, antimicrobial consumption in terrestrial food‐producing animals decreased by 41% (NORM/NORM‐VET, 2015). The Norwegian Strategy on Antibiotic Resistance 2015 to 2020 sets further targets to reduce use of antimicrobials in terrestrial food‐producing animals by 10% compared to 2013, and not to increase the (very low) use in fish farming.
In 2014, sales of antimicrobials in Norway for food‐producing animals were the lowest of all countries reporting to ESVAC at 3.1 mg/PCU (EMA ESVAC, 2016). The overall prevalence of resistance in E. coli from broilers was low‐moderate (14.6%) in 2014, reflecting the minimal use of antimicrobials in broiler chickens in Norway. The prevalence of resistance to sulfonamides and tetracyclines in E. coli from broilers has decreased since 2000 (NORM/NORM‐VET, 2015). ESBL‐producing E. coli were first identified in poultry in Norway in 2006 and became established despite the low overall antimicrobial use and no use of cephalosporins. It was determined that resistant bacteria were entering the national flock from imported parent stock and after this was addressed, resistant bacteria in parent birds declined from 30% in 2011 to 10% in 2014. In broilers, ESBL‐producing E. coli were detected in 35.5% of caecal samples when selective methods were used and it is concluded that it may be too early for impacts to be seen from the measures taken on parent stock. The incidence of quinolone resistance in indicator E. coli in broilers in Norway is low when non‐selective methods are used; although with selective methods, quinolone resistance is found in the majority of caecal samples. As quinolones are not used in poultry production in Norway, the reason for this is unknown. Of all MSs reporting to EFSA in 2014, Norway had the highest proportion (86%) of isolates of indicator E. coli from broilers that were fully susceptible to the panel of antimicrobials against which they were tested (ECDC and EFSA, 2016). The first cases of LA‐MRSA in Norwegian pigs were reported in 2013 and affected herds were destroyed. Animal populations in Norway are virtually free from Salmonella spp.
Belgium
In Belgium AMCRA is a collaboration between government agencies, agricultural and veterinary organisations, the pharmaceutical sector and academia. AMCRA members have agreed a ‘Plan for veterinary antibiotic policy until 2020’, which includes three targets for reduction in antimicrobial use by 2020 (compared with 2011):27
50% lower antimicrobial use, with objectives being defined for each species;
75% lower use of CIAs;
50% lower use of medicated feed.
Achievement of these targets will be supported by, among other measures: data collections systems to allow use of antimicrobials to be measured at farm level for each species (see Section 3.3.3); farm health plans developed under veterinary guidance to address antimicrobial use; benchmarking of use by farm and veterinarian, coupled with support to reduce use where necessary and withdrawal of preventive use.
This plan is based on self‐regulation, for instance by the different livestock sector quality labels. It will be supported by the authorities through further financing of AMCRA, by creating a legal basis and by controlling the implementation of this legislation (e.g. Sanitel‐Med) (see Section 3.3.4.5). There was a substantial decrease in antimicrobial use (mg/kg biomass) in 2012 and 2013; this trend was reversed in 2014, with a 1.1% increase in use, but was back on track in 2015 when, compared with the reference year of 2011 (AMCRA 2020), there was an overall cumulative reduction of 15.9% in antimicrobial use in mg/kg biomass (BelVetSac, 2015). No underlying reasons could be identified for the increase in use observed in 2014, but it was speculated that more strict regulation would be required to support the AMCRA measures (BelVetSac, 2015).
Concluding remarks
Public concern about rising levels of AMR and loss of consumer confidence in the livestock industry have acted as a driver in those countries that have been proactive about taking measures to control high antimicrobial use.
In the Netherlands and France, where high‐level overall percentage reduction targets were set, reductions in use were seen in all major livestock sectors.
In several MSs reductions towards targets have been seen within 2 years of their implementation.
Targets for reduction in antimicrobial use have been supported in most countries by a package of measures, which address the many different factors that can impact on antimicrobial use.
Benchmarking of antimicrobial use on farms has been a key tool to reduce antimicrobial use. Sector‐specific thresholds have been set so that farms are benchmarked against the distribution of antimicrobial use across the relevant animal production sector. Where benchmarks are exceeded, actions to address underlying problems on the farm have been supervised by either the veterinarian or through the quality schemes.
The successful implementation of measures to reduce antimicrobial use may be associated with the presence of well integrated quality schemes and with the husbandry systems in operation.
There are some examples where the reduction in antimicrobial use in the MSs appears to have been associated with decreased prevalence of certain types of AMR (e.g. analysis by the SDa in Netherlands (SDa, 2016b)). Nevertheless, there are other types of resistance for which prevalence is less clearly impacted with changes in antimicrobial use in the MS. This is likely to be a consequence of the multiple factors that influence maintenance of resistance mechanisms, such as fitness costs, co‐ and cross‐selection, success of particular clones and movement of animals between countries.
Overall, there has been very little research into the impacts of reducing antimicrobial use (beyond the withdrawal of AGPs) on animal health and welfare. In Denmark and Belgium, reduction in use of antimicrobials has been accompanied by an increase in the use of zinc oxide in the pig sector, which has prompted concerns about the environmental impact as well as the potential for co‐selection of MDR bacteria (see Section 4.4.15).
Recommendations
Targets are recommended as an effective motivator to reduce antimicrobial use in livestock.
High‐level targets are appropriate where antimicrobial use is considered high across several or all livestock sectors. The level and time frame should be set according to the circumstances of the country (baseline level of use, husbandry systems, infrastructure through which to implement reduction measures).
In order to attain a target, a package of reduction measures is likely to be needed, tailored to the underlying factors impacting use, e.g. benchmarking of use on farm; improved prescribing, especially around the use of CIAs; review of preventive antimicrobial use; use of farm health plans; sector‐specific treatment guidelines. See later sections.
All stakeholders should be involved in developing these measures: livestock industry (quality schemes); professional bodies and academia, with oversight from government as required.
Measures should preferably include sector‐specific benchmarking. For this purpose, thresholds/targets should be set according to levels of use in the individual sector and reviewed regularly. The process should be transparent with quality schemes and farmers receiving regular progress reports.
Alternative treatments, such as zinc oxide, that also have the potential to select for AMR should be included in monitoring systems and associated targets.
Data based on relevant and high‐quality monitoring, further research and analysis are needed to identify the impact of reductions in use on antimicrobial resistance both in animal and human populations.
Studies are required into the impact of reduction measures on animal health and welfare and quality of animal produce.
3.3.4. Treatment guidelines and measures to reduce the use of antimicrobials of critical importance for human medicine
As treatment guidelines place heavy emphasis on responsible use of CIAs, they are discussed alongside the measures taken to reduce the use of CIAs.
3.3.4.1. Treatment Guidelines
There is an increasing trend in veterinary medicine for the publication of treatment guidelines by veterinary associations or veterinary specialist societies. These are typically intended for national use and dedicated to specific animal species. In EU MSs, guidelines on antimicrobial prescribing have been published as booklets, tables and on‐line. Some guidelines are driven and endorsed by national competent authorities and/or national veterinary organisations. The EC PUAVM Guidelines recommend that prescribers use these types of guidelines to assist in selecting the appropriate antimicrobial and dosing regimens. At present, there is no standardised approach to the methods for developing treatment guidelines, which involve interplay between following approved indications for authorised VMPs, reference to published studies, traditional veterinary practices, pharmacokinetic/pharmacodynamic factors (e.g. target site concentrations) and consideration of MIC data for the various treatment options and pathogens involved. Many treatment guidelines do not include discussion of the criteria used as the basis for the treatment recommendations (e.g. for use of CIAs, broad‐spectrum or combination treatments).
Well‐researched treatment guidelines can assist veterinarians in making rational prescribing choices by being evidence‐based and by taking into account results of national or regional surveillance of antimicrobial resistance, and if needed they can be relatively easily updated to reflect changes in local resistance patterns. Furthermore, there are circumstances, especially for food‐producing animals, where prescribing choice cannot be guided by antimicrobial susceptibility testing, either because the causative organisms cannot be readily cultured or there is a lack of agreed international microbiology standards. Relevant examples of common pathogens include Lawsonia intracellularis, Brachyspira spp., Mycoplasma spp. and Ornithobacterium rhinotracheale. Here, treatment guidelines could assist veterinarians in their prescribing.
3.3.4.2. Antimicrobials of critical importance for human medicine
Antimicrobials have been classified based on the importance for human and animal medicine by several institutions or committees, such as the WHO, OIE and the AMEG.
The WHO has categorised all antimicrobial classes into three groups: critically important (CIAs), highly important (HIAs) and important (IAs) for human medicine, based on two criteria (Collignon et al., 2009; WHO, 2012).
These criteria are:
‘An antimicrobial agent which is the sole, or one of limited available, therapy to treat serious human disease.’
‘Antimicrobial agent is used to treat diseases caused by either: (1) organisms that may be transmitted to humans from non‐human sources or, (2) human diseases causes by organisms that may acquire resistance genes from nonhuman sources.’
The CIAs meet both criteria, the HIAs meet either criterion 1 or criterion 2 and the IAs meet neither of the criteria.
Within the CIAs, the classes are prioritised ‘to allow allocation of resources towards the agents for which management of the risks from antimicrobial resistance are needed most urgently’. The classes with the highest priority are fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins, glycopeptides and macrolides.
The AMEG has further categorised the WHO CIAs (and several HIAs) based on their risk to man due to resistance development following use in animals (EMA, 2014). The following three categories have been proposed by the AMEG:
Category 1 contains the antimicrobials used in veterinary medicine where the risk for public health is estimated low or limited;
Category 2 contains the antimicrobials used in veterinary medicine where the risk for public health is estimated as higher;
Category 3 contains the antimicrobials not approved for use in veterinary medicine.
The highest priority antimicrobial classes in the WHO CIAs are categorised by AMEG as category 1 (macrolides), category 2 (fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins for systemic use) and category 3 (glycopeptides; not authorised in veterinary medicine in the EU). The extended‐spectrum penicillins and aminoglycosides are provisionally placed in category 2 while undergoing further risk‐profiling. Polymyxins (colistin and polymyxin B) were originally classified by AMEG as category 1 but the classification of colistin was changed to category 2 after review in 2016 (EMA, 2016b).
The EC PUAVM Guidelines refer to CIAs as those identified in the advice provided by EMA (AMEG) to the Commission (EMA, 2014). For the general purposes of this report, the CIAs include those in the AMEG's category 2 and category 3, unless making direct reference to a document, which uses a different definition. At present, there are no category 3 substances that are included in table 1 of the Annex to Regulation (EU) No 37/201028 containing the MRLs, and therefore, these cannot be used in food‐producing animals.
3.3.4.3. Summary of measures relating to CIAs proposed in EC PUAVM Guidelines
In general, the EC PUAVM Guidelines advice that critically important antimicrobials should only be used when and where the veterinarian has determined that no effective non‐critically important alternative is available based on antimicrobial susceptibility testing and relevant epidemiological data.
In accordance with Directive 2001/82/EC, as for other veterinary medicinal products, cascade (off‐label) use of critically important antimicrobials should only be applied in order to prevent animal suffering. In those cases, the prescribing veterinarian should sufficiently justify and record the use, taking into consideration ethical and public health concerns.
The EC PUAVM Guidelines specifically advise to prohibit the use of 3rd‐ and 4th‐generation cephalosporins in poultry (including eggs). Fluoroquinolones are advised only to be used in poultry when treatment response to other antimicrobials is poor or expected to be poor, and only after susceptibility testing has been performed.
3.3.4.4. Use of CIAs within the EU
According to ESVAC data for 2014 (EMA ESVAC, 2016) the sales of 3rd‐ and 4th‐generation cephalosporins, fluoroquinolones and polymyxins made up 0.2%, 1.9% and 6.6% of the total sales of antimicrobial VMPs, respectively, in the 29 reporting EU MSs, with substantial variation between countries. Overall sales of 3rd‐ and 4th‐generation cephalosporins have remained relatively stable at the EU level from 2011 to 2014; whereas the sales of fluoroquinolones suggest an increasing trend (+18%) since 2011 (EMA ESVAC, 2016).
In a survey of EU veterinarians (De Briyne et al., 2014), the most frequently cited uses were: in pigs, fluoroquinolones and polymyxins for treatment of diarrhoea and fluoroquinolones for post‐partum dysgalactia syndrome; in cattle/calves, 3rd‐ and 4th‐generation cephalosporins for locomotor and uterine disorders and polymyxins and fluoroquinolones for diarrhoea.
Appendix I includes information on the classes of CIAs that are authorised for use in human medicine but not in veterinary medicine.
3.3.4.5. Measures taken in the MSs to reduce the use of CIAs, and their impact
Belgium
In Belgium, the Centre of Expertise on Antimicrobial Consumption and Resistance in Animals (AMCRA), as part of its prudent use campaign, in 2013 produced formularies containing guidance for the responsible use of antimicrobials in food‐producing animals (pigs, cattle and poultry) to be used on a voluntary basis.29 The formularies are species‐specific and contain a list of indications for which appropriate antimicrobials are listed together with the route of administration.30 The antimicrobials are classified into first, second and third choice, based on scientific literature, taking into account antimicrobial susceptibility, pharmacokinetics and –dynamics, efficacy and clinical signs of the animals. Within the groups of first, second and third choice a colour code is given to each antimicrobial (yellow, orange or red).
The colour‐coded classification for antimicrobials is based on the importance for human (WHO ranking) and veterinary (OIE ranking) medicine:31
Yellow substances are the substances of lowest importance to human medicine and are usually first and second choice substances for treatment;
Orange substances should only be used after additional laboratory testing (bacteriology, PCR, serology, etc.), and include macrolides, polymyxins and aminoglycosides, etc.;
Red substances are of highest importance to human medicine, are third choice for treatment, and include fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins. In principle, they should not be used for group or systematic treatments of farm animals, but if needed then only after additional laboratory testing and antibacterial susceptibility testing which shows that yellow and orange substances are not effective. Red substances are not allowed to be kept in stock by farmers, and can only be kept on the farms for a five day treatment.
In 2014, AMCRA released the AMCRA 2020 vision statement, which comprises 10 objectives and action points, one of which introduces a 75% reduction by 2020 in use of quinolones and 3rd‐ and 4th‐generation cephalosporins, compared to 2011. The Belgian Statutory Body of Veterinarians has modified the Deontological Code for Veterinarians in March 2015 to emphasise the prudent use of antimicrobials in general and specifically the use of CIAs (Nederlandstalige Gewestelijke Raad van de Orde der Dierenartsen, 2015).
Furthermore, a tax has to be paid on all VMPs based on the content of the active substance, and is multiplied by 1.5 for VMPs containing CIAs.32 These taxes will be used to finance a national data collection system (Sanitel‐Med) which will enable monitoring of (off‐label) use of antimicrobials and especially the use of CIAs in food‐producing species, and which ultimately will allow for benchmarking of farms and veterinarians.
Most actions taken in Belgium are relatively recent, with AMCRA having been founded only in 2012, whereas the most recent report on the consumption of veterinary antimicrobials in Belgium contains data on sales from 2015. Therefore, impact of the actions may not be fully visible yet. Table 2 shows the relative changes in sales of veterinary antimicrobials in Belgium over the period of 2011–2015 (BelVetSac, 2015).
Table 2.
The relative changes in sales of veterinary antimicrobials (kg) in Belgium over the period of 2011–2015 (BelVetSac, 2015)
| Overall sales | 2011 > 2012 | 2012 > 2013 | 2013 > 2014 | 2014 > 2015 |
|---|---|---|---|---|
| Quinolones (%) | +3.1 | −21.4 | +5.3 | +16.0 |
| Cephalosporins (%) | +2.7 | +0.7 | +4.1 | −5.1 |
| Polymyxins (mainly colistin sulfate) (%) | +5.9 | −18.3 | −28.1 | −16.0 |
| Overall sales (%) | −7.2 | −6.6 | +3.2 | −2.8 |
The decreased use of polymyxins was considered to coincide with the availability of zinc oxide as an alternative to colistin for the treatment of diarrhoea in piglets. The increased use of quinolones in 2015 was attributed solely to an increase in the use of flumequine which, based on the product concerned, was thought to be for poultry production. The BelVetSac report indicates that in comparison to 2011, the reduction in use of red molecules (fluoroquinolones and 3rd and 4th generation cephalosporins) by 2015 was only 6.4%, compared to the goal to achieve a 75% reduction by 2020. It was suggested that new requirements for veterinarians to perform antimicrobial susceptibility testing prior to use of CIAs, and to register use of all antimicrobials in the national database, might help to reinforce responsible use principles in future.
A study conducted in 2012–2013 on 47 farrow‐to‐finish pig farms in Belgium showed that 3rd‐ and 4th‐generation cephalosporins were the 4th ranking antimicrobials used, with a proportion of 10.8% of the total antimicrobial consumption (Sjölund et al., 2016). For fluoroquinolones and colistin this proportion was 5.3% and 17.5% (2nd ranking), respectively. Another study on 61 pig farms in Flanders, performed between December 2010 and May 2014, studied the potential reduction in antimicrobial consumption that could be achieved by optimisation of herd management, biosecurity status, vaccination strategy, anthelmintic therapy and advice on prudent use of antimicrobials (Postma et al., 2016b). The study farms showed a reduction in the consumption of CIAs between the first and third farm visit, likely due to the provision of specific guidance on use of these substances.
A study in 2012–2013 in adult dairy cattle in Flanders showed that on average the most used substance class was the 4th‐generation cephalosporins, 4.99 DDDA/1,000 cow‐days, with average consumption of 3rd‐generation cephalosporins calculated at 2.95 DDDA/1,000 cow‐days and of fluoroquinolones at 0.65 DDDA/1,000 cow‐days. The average consumption of CIAs on the farms was 8.59 DDDA/1,000 cow‐days, whereas the average consumption for non‐CIAs was 12.18 DDDA/1,000 cow‐days (Stevens et al., 2016) From the study there appeared to be a positive association between overall treatment duration of subclinical mastitis and the treatment incidence of CIAs. It was suggested that replacing intramammary dry cow treatments and (long‐acting) injectable products containing 3rd‐ and 4th‐generation cephalosporins (that have zero day withdrawal time for milk) could help reduce the use of the CIAs, but as yet there is no evidence to support this proposal.
Denmark
In 2002, Danish legislation restricted the use in production animals of injectable products containing fluoroquinolones to veterinarians only (Order(DK) 119/2002); in addition, in 2003 susceptibility testing before use of fluoroquinolones in production animals became mandatory, together with notification of the use of this class of antimicrobials to the authorities (Order(DK) 134/2003). This led to a steep decline in the use of fluoroquinolones from 2001 to 2003. Furthermore, in July 2010 a voluntary ban on the use of cephalosporins was carried out by the pig industry; the dairy cattle industry applied a similar voluntary ban in July 2014 (DANMAP, 2011, 2015).
Treatment guidelines, developed in collaboration between the authorities, universities, industry and the veterinary association and maintained by the Danish Veterinary and Food Administration (DVFA), recommending the appropriate antimicrobial treatment for common indications in pigs and cattle33 have been made available to veterinarians since 1996. (DANMAP, 2015). The DVFA published updated ‘dynamic evidence‐based’ prudent use treatment guidelines for pigs for use by veterinarians in 2010.34 These guidelines include recommendations for the use of antimicrobials for the major indications in pigs, based on ranking for efficacy, susceptibility, pharmacokinetics and human importance (according to the FDA and OIE guidelines). Substances are divided into green (recommended), yellow (can be used but better alternatives available) and red (not recommended because of either a very low susceptibility or high importance to human health). Fluoroquinolones and cephalosporins are included in the red substances. A concept for treatment guidelines for cattle was reviewed in 2008.
In 2013 and under the Second Veterinary Action Plan (2013–2016) the DVFA, together with the Danish Health and Medicines Authority and backed by the Danish Parliament, increased the taxes on sales of veterinary antimicrobials from 0.84% to 5.5% for veterinary antimicrobials other than simple and narrow spectrum penicillins and to 10.8% for 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones (DANMAP, 2015).
Perhaps as a result of their strict regulation, sales of fluoroquinolones in Denmark are very low (< 0.01 mg/PCU (EMA ESVAC, 2016)) ‐ no use of fluoroquinolones was reported for cattle in 2014 and 2015 and no fluoroquinolones were used in broilers from 2010 to 2014 (DANMAP, 2015, 2016).
The use of cephalosporins is minor compared to overall antimicrobial use in pigs, and since the voluntary ban in 2010 has been extremely low – 1 kg in 2015 (DANMAP, 2016). The use of intramammary products containing 3rd‐ and 4th‐generation cephalosporins has steadily but markedly decreased from 2007 to 2015 in cattle. This may be explained by the introduction of a milk quality campaign by the Danish cattle Association in 2010, and legal regulations on use of antimicrobial agents for mastitis treatment which require the use of narrow spectrum penicillins unless susceptibility testing indicates otherwise (see Figure 5) (DANMAP, 2016). Overall sales corrected for the biomass‐at‐risk (in mg/PCU) showed that the sales of 3rd‐ and 4th‐generation cephalosporins decreased by 30% from 2011 to 2014; compared to 2010, the decrease is 66% (EMA ESVAC, 2016).
Figure 5.
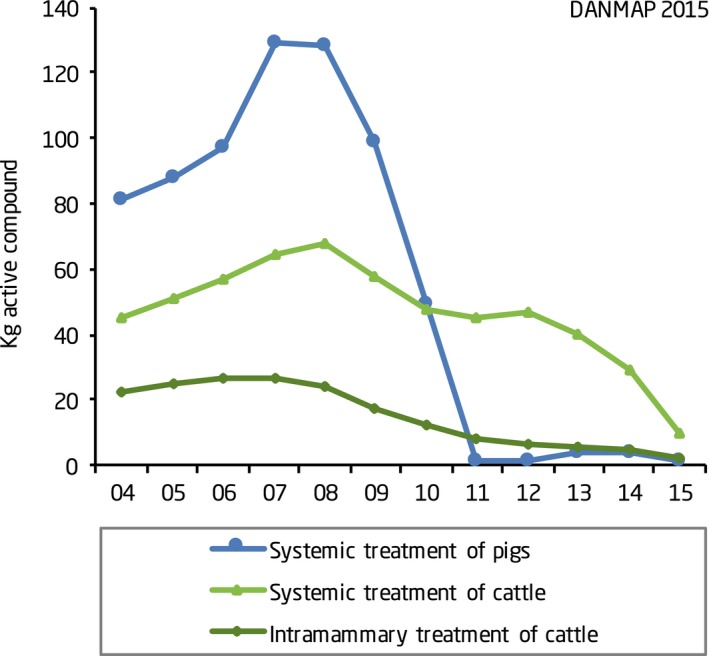
Consumption of 3rd‐ and 4th‐generation cephalosporins in pigs and cattle in Denmark (source: (DANMAP, 2016))
Resistance to ciprofloxacin in C. jejuni isolates from broilers increased steadily from 2002 to 2011, and despite the cessation of its use by the Danish poultry industry in 2009, resistance has since fluctuated – although it decreased from 26% in 2013 to 18% in 2014. From 2006 onwards, resistance to fluoroquinolones remained at a similar level in C. jejuni isolates from Danish cattle (16–21%), although there was a notable decrease in 2012.
Resistance to ciprofloxacin in E. faecalis and E. faecium in Danish broiler meat, broilers, beef, pigs and pork was very low in 2014 (0–3%). Resistance in indicator E. coli isolated from Danish broilers, cattle and pigs and Danish broiler meat, beef and pork varied between 0 and 12% for ciprofloxacin and nalidixic acid (with the highest levels in broilers) in 2014 (DANMAP, 2015). Resistance to ciprofloxacin and nalidixic acid in E. coli from Danish broilers in 2014 was 12% and 11%, respectively, and as in other Nordic countries, is considerably lower than that reported overall in EU MSs (66% and 63%, respectively) (ECDC and EFSA, 2016).
From 2011, there has been an ongoing reduction in occurrence of ESBL‐producing E. coli in Danish broiler meat and for the first time a decrease was found in imported broiler meat in 2014 (ceftriaxone resistance was 9% and 25%, respectively). This is most likely due to a stop in the use of 3rd‐generation cephalosporins in the top of the breeding pyramid abroad, resulting in a reduced transmission of ESBL‐producing E. coli from imported parent animals to Danish broilers (DANMAP, 2015). In addition, ceftriaxone resistance was very low (< 1%) in Danish and imported beef and pork during the last few years and in 2013 ESBL‐producing E. coli were found in six per cent of pigs at slaughter which was lower than in 2012, 2010 and 2009 and likely to be a result of the voluntary stop of the use of cephalosporins in the Danish pig production. This was also concluded by a study that found that the voluntary ban of the use of cephalosporins in Danish pig production significantly reduced the occurrence of extended‐spectrum cephalosporinase‐producing E. coli in pigs and pork (Agersø and Aarestrup, 2013). In 2015, the occurrence of E. coli resistant to 3rd‐generation cephalosporins from pigs increased compared to 2009 to 2013, although it was considered that the change in the methodology of testing introduced under Decision 2013/652/EU may have impacted the results (DANMAP, 2016).
Finland
Cascade use is not allowed under Finnish legislation for certain substances, among which are 3rd‐ and 4th‐generation cephalosporins (Mamma 847/2008).35 Finland has relative to other EU MSs, a very low use of 3rd‐ and 4th‐generation cephalosporins (< 0.02 mg/PCU), and of fluoroquinolones (0.18 mg/PCU). Sales of both classes have been fairly static since 2010 (EMA ESVAC, 2016).
Levels of resistance to ciprofloxacin and nalidixic acid in indicator E. coli from broilers in Finland in 2014 were very low at 4.6% resistance to both (ECDC and EFSA, 2016). For Campylobacter jejuni isolates from broilers, resistance to both substances was 25%, which is low compared to non‐Nordic countries. In pigs, data from 2013 also indicated low levels of resistant in indicator E. coli to ciprofloxacin (1.9%) and nalidixic acid (1.3%) (ECDC and EFSA, 2015).
No resistance was detected to the 3rd‐generation cephalosporin, cefotaxime, in E. coli from broilers in 2014 (ECDC and EFSA, 2016), and it was very low (0.6%) in E. coli isolates from pigs in 2013 (ECDC and EFSA, 2015).
France
The pig sector introduced a voluntary restriction of the use of 3rd‐ and 4th‐generation cephalosporins in pig production in 2010. Following on, the estimated exposure for pigs to ceftiofur and cefquinome decreased by 78.2% from 2010 to 2014 (Figure 6) (ANSES, 2015b).
Figure 6.
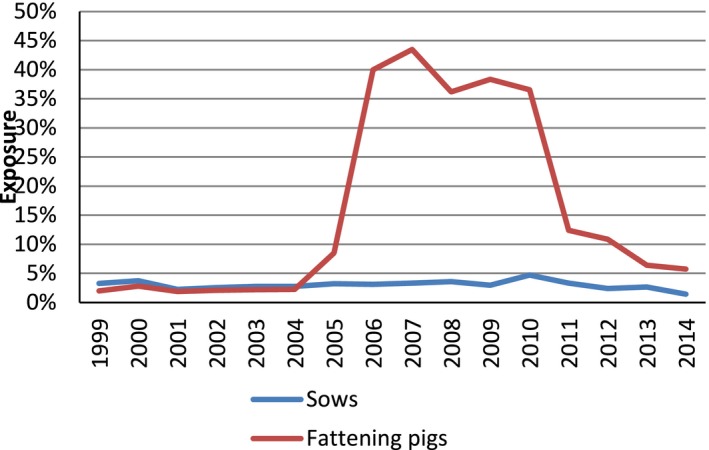
Change in the estimated number of pigs treated with cephalosporins (source: (ANSES, 2015b))
Resistance to ceftiofur in E. coli from pigs decreased from 2010 to 2014 (Figure 7) (ANSES, 2016).
Figure 7.
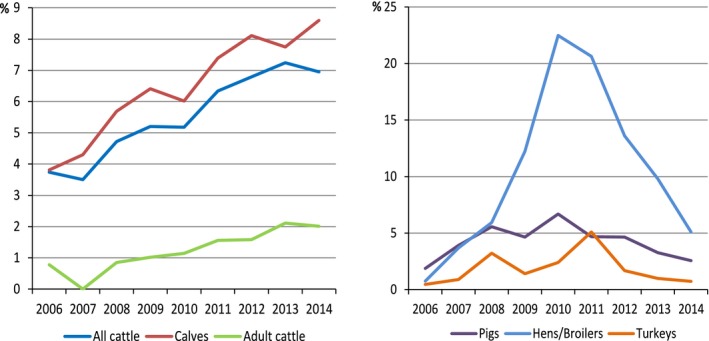
Evolution of proportions of E. coli isolates non‐susceptible to ceftiofur in cattle, pigs, poultry and turkey (2006–2014) (source: (ANSES, 2016))
In September 2014, the bill on the future of farming, food and forestry set a target of 25% reduction in the use of fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins, taking 2013 as the reference year.36 This new legislation controls and restricts the prescription of CIAs as well as discounts and promotions on antimicrobials. In March 2016, a decree (2016‐317) was issued which prohibited the use of critical antimicrobials (fluoro)quinolones, 3rd‐and 4th‐generation cephalosporins and certain other human‐only authorised substances) for preventive use, and only allowed their use for metaphylaxis or cure of disease following a clinical examination and laboratory testing showing the susceptibility of the identified bacterial strain to the antimicrobial concerned. Prescriptions are also limited to one month without re‐examination of the animal(s).
A study conducted mainly in 2013 on 60 farrow‐to‐finish pig farms in France showed that 3rd‐ and 4th‐generation cephalosporins formed 1.2% of the total antimicrobial consumption, fluoroquinolones formed 5.3% of total consumption and colistin was most used substance with 30.1% (Sjölund et al., 2016).
Italy
The Italian trade association, UNAITALIA, and the Italian health authorities and research organisations for animal health and food safety of the regions Lombardy and Emilia Romagna drafted a national plan for the responsible use of veterinary medicines in the fight against AMR in rabbit production, which was adopted in 2013.37 Farmers participate on a voluntary basis and part of the plan is that they will not use cephalosporins. In 2015, a similar plan followed for prudent use of veterinary medicines in poultry production.38
Netherlands
In the Netherlands, the Workgroup Veterinary Antimicrobial Policy (WVAB) is part of the Royal Dutch Association for Veterinary Medicine (KNMvD) and approves formularies and guidelines regarding the use of antimicrobials.39 Since 2011, formularies have been introduced for food‐producing animal species and are undergoing revision. The formularies indicate the preferred choice of antimicrobial for the relevant indications per species and per administration route, based on efficacy and limitation of antimicrobial resistance (especially ESBLs). These formularies are binding, with any deviations having to be well‐justified or otherwise potentially subject to disciplinary action (Dutch Ministry of Economic Affairs, 2014).
The WVAB has produced a classification of veterinary antimicrobial agents40 into first, second and third choice, and those antimicrobials that can be used in non‐food‐producing animals under the cascade regulation in case of veterinary emergency. The classification takes into account selection pressure for resistance factors. 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones are classified as the third choice antimicrobials. In principle, they should not be used, unless for treatment of individual animals for which, based on bacteriology and susceptibility tests, it is shown that no alternatives are available. The antimicrobials that are considered as last‐resort antimicrobials for human treatment (e.g. carbapenems, glycopeptides, tigecycline) should never be used to treat animals, not even under the cascade regulation.
In 2011, the Dutch Health Council published a report on the use of antimicrobials in animal husbandry and resistant bacteria in humans (Health Council of the Netherlands, 2011). In this report, it was recommended to prohibit as soon as possible the use of 3rd‐ and 4th‐generation cephalosporins in group treatment and dry cow treatment, and to stop the use of fluoroquinolones in the longer term. Therapeutic use in individual animals would remain possible in exceptional cases. Further advice was to phase out the use of colistin as group treatment, but considering that colistin is classed as a second choice antimicrobial and appropriate alternatives are lacking, the WVAB considered that this should be done over time.
Since 1 January 2013 veterinarians have been obliged by legislation to perform bacteriological and susceptibility testing before prescribing 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones.41 Various quality assurance systems anticipated this by restricting or prohibiting the use of these substances: the pig sector voluntarily restricted use of 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones and the dairy sector banned the use of 3rd‐ and 4th‐generation cephalosporins for dry cow treatment (see PUAVM Guidelines).
Since 2011, the Netherlands Veterinary Medicines Authority (SDa) monitors use of antimicrobials per species at the farm level by means of animal defined daily doses (DDDA), which are technical units of measurement that take into account the potency of the substance. The SDa set specific target values for the use of 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones: in all animal sectors these are set at ‘0’ defined daily doses per farm per animal year (SDa, 2013). In terms of weight, it appears that only about 5% of cephalosporins are used in the monitored sectors (see Table 4) the rest is used in, e.g. companion animals or small livestock sectors such as rabbits. (SDa, 2016b) About a third of all fluoroquinolones sold are used in the pigs, veal calves, cattle, broiler or turkey sectors (Table 3). In the monitored livestock, sectors use decreased by about 26%.
Table 4.
Examples of identified ‘at‐risk practices’ (source: ANSES (2014))
| Practices to be abandoned without delay | Practices to be abandoned over time | |
|---|---|---|
| Poultry |
Use of ceftiofur in hatcheries – now stopped Systematic administration of fluoroquinolones to broiler chicks in the first days of life |
Preventive treatment by oral route for pathogenic Escherichia coli in all poultry; Mycoplasma in chickens; Ornithobacterium in turkeys |
| Pigs | Preventive or metaphylactic treatments administered to lactating sows to prevent digestive problems in suckling piglets |
Preventive use of polypeptides and aminoglycosides for post‐weaning diarrhoea Preventive use of antimicrobials to control Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae in nucleus/breeder herds, and for disease control of swine dysentery |
| Ruminants |
Preventive group treatment against neonatal diarrhoea due to E. coli and Salmonella spp. infections and respiratory infections due to Mycoplasma and Pasteurellaceae Intramammary treatment at dry‐off in dairy cows |
Table 3.
Use of 3rd‐ and 4th‐generation cephalosporins, fluoroquinolones and polymyxins in several animal species in the Netherlands in 2012 (2013) and 2015; use is reported in DDDANAT
| Pigs | Veal calves | Cattle | Broilers | Turkeys | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substance | 2012 | 2015 | 2012 | 2015 | 2012 | 2015 | 2012 | 2015 | 2013 | 2015 |
| 3rd‐ and 4th‐generationa cephalosporins | 0.00 | b | 0.00 | b | 0.03 | 0.00 | b | b | b | b |
| Fluoroquinolonesa | 0.00 | 0.00 | 0.31 | 0.02 | 0.01 | 0.00 | 0.84 | 0.07 | 1.76 | 1.2 |
| Polymyxins | 0.58 | 0.38 | 0.73 | 0.19 | 0.05 | 0.01 | 0.88 | 0.06 | 0.18 | 0.63 |
0.00 means consumption was below 0.005 DDDANAT.
Means no consumption was reported.
Whereas use of fluoroquinolones has declined steadily, the use of polymyxins increased slightly from 2014 to 2015 data in all monitored species, except cattle where use is anyway minimal. Use of second and third choice antimicrobials has shifted to first‐choice antimicrobials in dairy cattle, particularly in dry cow treatment (SDa, 2016b).
Results on resistance to specific CIAs for 2014 (Figure 8) compared to 2009 (SDa, 2016a):
broilers: 84% reduction in resistance to cefotaxime, 19% reduction in resistance to ciprofloxacin;
pigs: 86% reduction in resistance to cefotaxime, 100% reduction in resistance to ciprofloxacin;
veal calves: 41% reduction in resistance to cefotaxime, 64% reduction in resistance to ciprofloxacin;
dairy cattle: 75% reduction in resistance to cefotaxime, no resistance to fluoroquinolones.
Figure 8.
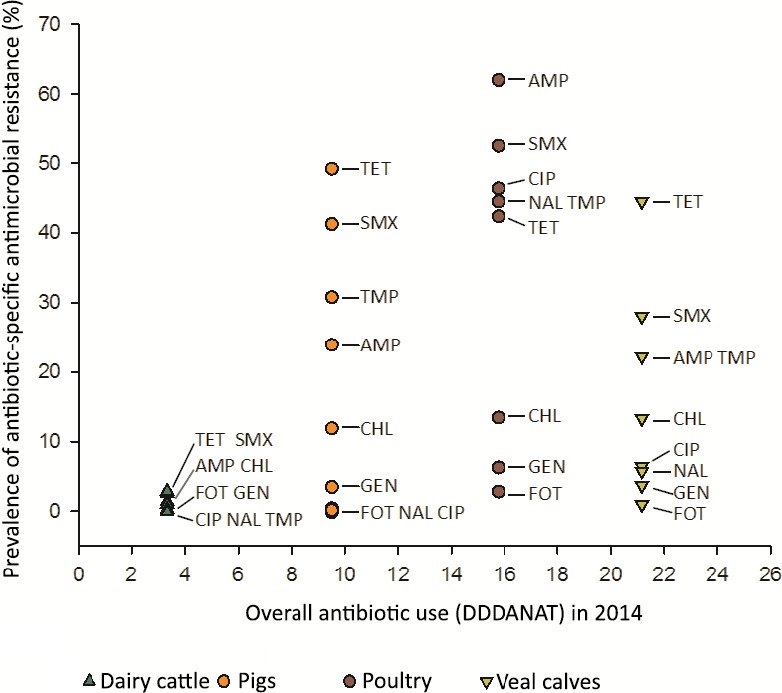
Ranking of the four livestock sectors based on total antimicrobial use and antimicrobial –specific resistance in 2014 (source: (SDa, 2016a))
The 2015 MARAN report (MARAN, 2015) concluded that reductions in total sales and sales of 3rd‐ and 4th‐generation cephalosporins were associated with a reduction of the general levels of antimicrobial resistance in isolates from food‐producing animals, and to a certain extent the levels of ESBLs. The 2014 data showed that the levelling off in reduction of antimicrobial sales was directly followed by stabilisation of resistance levels. The SDa found that AMR in isolates from poultry, especially to 3rd‐ and 4th‐generation cephalosporins, had decreased markedly in recent years, which was thought to be because these substances are no longer used in hatcheries and because of a decrease in the use of second choice broad‐spectrum penicillins in broiler farms (SDa, 2016a).
MARAN (2015) reported that although fluoroquinolone resistance in E. coli is decreasing, it is still common in broiler chickens – 46% of E. coli isolates were resistant to ciprofloxacin in 2014, compared to 50% in 2012 and 54% in 2013.
Sweden
The Swedish Veterinary Association has published a general guideline on prudent use.42 Specific guidelines for antimicrobial treatment in cattle, pigs, sheep, goats and horses highlight that CIAs should be considered to be second and third choice for treatment and should only be used when no other substances are available (SVS, 2013, 2014). As a follow up of these guidelines, the Swedish Medical products agency has published guidance on use of antimicrobials for pigs (201243) cattle and sheep (201344) and horses (201545). In general, all guidelines have a recommendation in place to restrict the use of CIAs, but the substances are still indicated as optional treatment for certain indications.
Following a regulation established in January 2013, a veterinarian in Sweden may only prescribe quinolones or 3rd‐ and 4th‐generation cephalosporins when microbiological and susceptibility testing show that there are no effective alternatives.46 , 47 Additional provisions allow use of these CIAs in food‐producing animals where susceptibility testing has been performed during the previous six months and where science and proven experience show that alternative treatments are not effective, although in this case microbiological testing should still be performed to identify the infectious agent. In an acute life, threatening condition treatment may be started while awaiting the results of tests.
Overall data corrected for the biomass‐at‐risk (in mg/PCU) showed that the sales of 3rd‐ and 4th‐generation cephalosporins in Sweden in 2014 were extremely low (0.003 mg/PCU) with reductions having started before the prescribing regulation was introduced in 2013. Sales of fluoroquinolones are also comparatively low, and have declined sharply since 2010, and more than halved during 2014 to 0.03 mg/PCU (EMA ESVAC, 2016).
A study conducted mainly in 2013 on 60 farrow‐to‐finish pig farms in Sweden showed that 3rd‐ and 4th‐generation cephalosporins were not used at all, whereas the proportion of fluoroquinolones of the total antimicrobial consumption expressed as treatment incidence was 2.1%, and of colistin 17.7% (Sjölund et al., 2016). Swedres‐Svarm data indicate that there was no consumption of 3rd‐generation cephalosporins in pigs in 2014 and 2015 and total sales of fluoroquinolones in pigs were 0.013 and 0.006 mg/kg pig slaughtered, respectively (Public Health Agency of Sweden and SVA, 2015, 2016). Sales of colistin in 2015 equalled 0.4 mg/kg pig slaughtered.
In the monitoring of indicator E. coli from healthy pigs in 2015, (according to CD 2013/652/EU), resistance to cefotaxime was found in 1% of isolates, in 3% of isolates to ciprofloxacin and 0% to colistin. In cattle, indicator E. coli showed resistance to colistin in 1% of isolates (non mcr‐1) and 0% for cefotaxime and ciprofloxacin. Selective screening for ESBL or plasmid‐mediated AmpC (pAmpC) showed 1% of the samples from pig intestine, < 1% from pig meat, and 0% of intestinal samples from cattle and cattle meat were positive for ESBL or pAmpC‐producing E. coli (Public Health Agency of Sweden and SVA, 2016). These low levels of resistance reflect the very low use of these CIAs in Sweden.
In slaughter chicken, sales of fluoroquinolones have been below 0.5 kg for the last few years, with no reported sales for either chickens, hens or turkeys for 2015, whereas cephalosporins have never been used. The level of resistance to quinolones in indicator E. coli isolated from broilers gradually increased until 2012, and decreased to 11% in 2014, with 0% of the isolates in 2014 showing resistance to cefotaxime, ceftazidime and colistin, and 11% to ciprofloxacin. In 2015, with selective screening, 39% if intestinal samples from chickens were positive for E. coli producing ESBLs or pAmpC (Public Health Agency of Sweden and SVA, 2015, 2016). Indicator E. coli isolated from turkeys in 2014 showed resistance to cefotaxime in 2% of the isolates, 2% to ceftazidime (no confirmed ESBL), 3% to ciprofloxacin and 0% to colistin. No samples were positive for E. coli producing ESBLs or pAmpC in the selective screening (Public Health Agency of Sweden and SVA, 2015).
United Kingdom
In 2012 the UK poultry meat sector introduced a voluntary ban on the use of 3rd‐ and 4th‐generation cephalosporins under its BPC Antibiotic Stewardship Scheme, together with a commitment to reducing the use of fluoroquinolones in day‐old broilers (BPC, 2016). In fact, in 2013 there was a general increase in antimicrobial use in the sector, which was attributed to poor harvest and hence poor feed quality, and fluoroquinolone use increased between 2012 and 2014. Scheme members revised their strategies and from 2014 to 2015, use of fluoroquinolones decreased by 48% in poultry overall. A 96% reduction was achieved in the chicken sector, but smaller reductions in other poultry sectors (e.g. turkeys) were attributed to the poor availability of alternative products. Resistance to ciprofloxacin and nalidixic acid in C. jejuni isolates from broilers increased from 31% in 2013 to 44% in 2014 and was 25% in E. coli isolates from broilers in 2014; no resistance to colistin, cefotaxime or ceftazidime was found in E. coli and Salmonella spp. from broilers (VMD, 2015).
The Czech Republic
Under national legislation (Decree No 344/2008) certain CIAs (currently 3rd‐ and 4th‐generation cephalosporins, fluoroquinolones, rifaximin, gentamicin and kanamycin) have been placed under a ‘prudent use regimen’ in the Czech Republic (Decree No 344/2008 Coll., paragraph 2, article 348). The ‘prudent use regimen’ stipulates the conditions for prescribing and handling these antimicrobials, including a requirement for susceptibility testing and absence of a first‐line treatment, with possible fines when the law is not followed. Targeted inspections of veterinarians with the highest rate of prescription are performed, and involve checks of the laboratory testing supporting the use of these antimicrobials. Results of inspections allow identification of gaps in the system, which have been minimised step‐wise (e.g. by improvement of laboratory diagnosis, establishment of the National target veterinary pathogen monitoring programme) to provide appropriate tools for decision‐making by veterinarians in practice.
3.3.4.6. Measures taken in third countries
In September 2005, the FDA withdrew approval for the use of enrofloxacin in poultry in the USA due to concerns over the human health hazard from fluoroquinolone‐resistant campylobacter infections. This decision followed an increase in the prevalence of fluoroquinolone resistance among human Campylobacter spp. isolates in the late 1990s that was temporally related to the approval of fluoroquinolones for use in poultry in 1995 (Nelson et al., 2007). Some fluoroquinolones are approved for use in pigs and cattle, although ‘extra‐label’ use has been prohibited in food‐producing animals since 1997 (FDA, 2015). Ciprofloxacin and nalidixic acid resistance in indicator E. coli and in non‐typhoidal Salmonella isolates from retail meats, slaughtered chickens, and caecal samples from chicken, turkeys, cattle, and pigs varied between 0% and 2.5% in 2013. Although resistance to ciprofloxacin was at its lowest level to date (11%) in C. jejuni isolates from retail chicken samples, the level of resistance in slaughter chicken samples has not declined (24% in 2013). This may be because fluoroquinolone‐resistant Campylobacter spp. isolates possess enhanced fitness, which allows them to persist in the absence of antimicrobial selection pressure (Han et al., 2012).
In 2012, the FDA issued an order prohibiting certain ‘extra‐label’ uses of products containing cephalosporins for use in certain food‐producing animals, due to concerns relating to the public health risk from food‐borne cephalosporin‐resistant bacteria.49 In particular, concerns related to use of ceftiofur in dairy cattle resulting in increased cephalosporin‐resistant bacteria in the dairy farm environment, administration at unapproved doses (e.g. via biobullets) and automated administration via egg injection at poultry hatcheries. Ceftriaxone resistance in indicator E. coli isolates from retail chickens decreased from 13% in 2011 to 4.4% in 2013, and in retail turkeys from 10% to 6.7%. Ceftriaxone resistance in E. coli isolates from ground beef in 2013 was 2.2%. On the other hand, ceftriaxone resistance in non‐typhoidal Salmonella isolates from retail chicken, ground turkey and ground beef in 2013 was 19.7%, 9.4% and 26.7%, respectively (FDA, 2015).
The 3rd‐generation cephalosporin, ceftiofur, is reported to be used off‐label in hatcheries in Canada, administered in ovo to control E. coli omphalitis in broiler chickens. This practice was voluntarily ceased by hatcheries in Quebec in 2005 to 2006 in response to public health concerns relating to reports of high rates of ceftiofur resistance in S. Heidelberg isolates from chicken meat. Following the withdrawal there was a sharp decline in the prevalence of ceftiofur resistance from 2004 to 2006 among S. Heidelberg isolates from retail chicken and from human infections. In 2007, there was a partial reintroduction of the use of ceftiofur by the hatcheries and simultaneous re–emergence of ceftiofur resistance, suggesting that the fluctuation was the result of exposure to ceftiofur in the chicken hatcheries (Dutil et al., 2010).
In Canada, in May 2014, the poultry industry formally eliminated the use of antimicrobials considered of very high importance to human medicine (including 3rd‐generation cephalosporins) in broiler chickens and turkeys. This policy was extended to the broiler breeder sector in May 2015. The 2014 elimination of 3rd‐generation cephalosporin use has corresponded with a significant decrease in the proportion of flocks reporting ceftiofur use at the hatcheries and with changes in resistance to third 3rd‐generation cephalosporins. For Salmonella spp., 3rd‐generation cephalosporin resistance trends have shown different regional patterns, but overall, a decreasing trend has been apparent. For E. coli, overall less 3rd‐generation cephalosporin resistance was observed in 2014 across the country and similar changes were observed following a voluntary end of ceftiofur use in Québec during the period of 2005–2006.50
Concluding remarks
Identification of the particular measures that have a significant impact on the consumption of CIAs specifically is not straightforward as often several measures are implemented simultaneously and many other factors play a role.
-
The following measures have been identified in relation to CIAs:
-
–
Emphasis of prudent use of CIAs in treatment guidelines.
-
–
Classification of 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones into so‐called third choice antimicrobials or red molecules/substances, which implies that in principle they can only be used for treatment of individual animals and only after antimicrobial susceptibility testing shows that no effective alternative antimicrobials are available.
-
–
Voluntary bans in animal production sectors on the use of CIAs.
-
–
Legislation requiring AST to show that effective alternatives are not available before prescribing CIAs.
-
–
Setting specific reduction or benchmark targets for the use of CIAs.
-
–
Higher (differential) tax to be paid on CIAs compared to other antimicrobials.
-
–
In some MSs, measures have only been implemented recently; in these cases it may be too early for an impact on CIA consumption and antimicrobial resistance to be seen.
The impact on sales of CIAs is variable, with some countries showing an increase in consumption of (specific) CIAs, but other countries having achieved significant reduction.
Where observed, the reduction in use of CIAs appears in general to have a reducing impact on AMR to CIAs; although it should be taken into account that several years may be needed before a trend can be reliably concluded upon. An exception would appear to be reduction in the use of fluoroquinolones and the occurrence of resistance in Campylobacter spp. where, even after several years, impacts are less tangible.
Some measures, e.g. treatment guidelines, have been implemented on a voluntary basis whereas other measures, such as increased tax, have a legal basis immediately. In other cases, measures first adopted voluntarily have later been introduced into legislation.
In particular the legal requirement for antimicrobial susceptibility testing prior to use of CIAs (and anticipation of this) appears to have a marked impact on the sales of CIAs.
In several countries animal production sectors have implemented a voluntary ban on the use of, e.g. 3rd‐ and 4th‐generation cephalosporins, which subsequently led to a decrease in consumption. This shows the importance of involving all stakeholders and creating a sense of ownership of the responsibilities surrounding AMR.
Recommendations
Development of sector‐specific treatment guidelines at national/regional level should be encouraged. Training in their use should be a key part of antimicrobial stewardship programmes.
A set of clear general criteria should be developed on which to base treatment guidelines. In addition to considering factors associated with clinical effectiveness, the risk to both animal and public health due to AMR should be taken into account.
Guidelines need to be updated regularly to take account of the evolution of AMR locally and in a public health context to reflect any change in the importance of a particular substance to human medicine.
Treatment guidelines should pay specific attention to the responsible use of CIAs, so that they are reserved for use after susceptibility testing and only where no alternative antimicrobial would be effective.
National targets for reduction in the use of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin should be considered.
Livestock sectors should also be encouraged to set specific targets for the reduction in the use of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin.
Consideration should be given to require mandatory bacteriology and antimicrobial susceptibility testing prior to the prescription of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin.
Consideration should be given to a legal prohibition of any use of 3rd‐ and 4th‐generation cephalosporins in poultry at the EU level.
Consideration should be given to strategies to replace the use of 3rd‐ and 4th‐generation cephalosporins (a) in cows, administered as blanket intramammary dry cow therapy; (b) administered as systemic injections due to the advantage of short/zero day milk withdrawal periods and as long‐acting formulations solely to reduce the need for animal handling.
As many MSs rely in their treatment guidelines on CIA lists developed by bodies such as WHO for determining the importance of antimicrobials to human health, it is important that a regularly reviewed list is available at the EU level (such as that developed by AMEG) to take account of the evolution of AMR and changing importance of different substances/classes to human medicine.
3.3.5. Off‐label and cascade use
In EU legislation, it is considered implicit that veterinarians should follow the SPC instructions for authorised veterinary medicines. Use outside of the SPC is commonly referred to as ‘off‐label’ use and is defined in the European Directive 2001/82/EC as amended by Directive 2004/28/EC:
‘The use of a veterinary medicinal product that is not in accordance with the summary of the product characteristics (SPC), including the misuse and serious abuse of the product.’
Acknowledging that approved indications for veterinary medicinal products might not cover completely all clinical needs, especially for minor species and rare diseases or minor uses (MUMS), then regulations are in place to allow use outside approved indications. Thus, there are clinical situations in which off‐label drug use is necessary. Furthermore, the availability of authorised veterinary antimicrobial products differs between countries for various reasons. Within the EU, this scenario is recognised in Article 11 of the Directive where a concept known as ‘the cascade principle’ was developed. The principle of the cascade is that if no suitable veterinary medicine is authorised in the member state to treat a condition, the veterinary surgeon responsible for the animal may, ‘by way of exception’ and ‘in particular to avoid causing unacceptable suffering’ treat the animal in accordance with the following sequence in descending order of priority:
a veterinary medicinal product authorised in the MS for use in another animal species or for a different condition in the same species;
-
if there is no such product, then either:
-
–
a medicine authorised for human use in the MS; or
-
–
a VMP authorised in another MS for use in the same species or another species;
-
–
if there is no product referred to above, a VMP prepared extemporaneously.
The use of the expressions, ‘by way of exception’, and ‘in particular to avoid unacceptable suffering’ allows legislators to indicate that off‐label use is restricted and that it is assumed that the legislation is used on only rare occasions; in veterinary practice there are many therapeutic gaps. At present, there is very little data available on the volume of off‐label antimicrobial use in the EU and therefore very little evidence on which to base an assessment of the risk due to AMR that this use actually poses to public health.
More common reasons for off‐label antimicrobial use include:
unintentional off‐label use (e.g. under‐dosing of in‐feed formulations, lack of harmonised SPCs);
unmet medical need (e.g. MUMS, surgical prophylaxis/prevention);
alternative routes of administration (e.g. inhalational, in ovo);
individual patient characteristics (e.g. neonates, difficult to handle animals);
complex conditions (e.g. dysbacteriosis);
practical considerations (e.g. preference for oral products, preference for shorter withdrawal periods);
alternative posologies;
combination treatments (e.g. to compensate for lack of diagnosis);
non‐antibacterial purposes (e.g. immunomodulatory effects).
Thus, there are examples of ‘appropriate’ off‐label antimicrobial use and ‘inappropriate’ or misuse of antimicrobials. Some examples of ‘appropriate’ off‐label antimicrobial include those with good documentation of a clinically relevant infection where either other approved antimicrobials will not suffice or other factors (PK/PD, dose, route‐of‐administration) will lead to an off‐label antimicrobial use providing more optimal evidence‐based patient therapy.
Unintentional off‐label use may occur, for example, with use of oral group treatments, which are frequently under‐ and sometimes over‐dosed (Timmerman et al., 2006; Callens et al., 2012; Persoons et al., 2012). This could be associated with misevaluation of bodyweight at the time of administration, failure to follow or lack of clear dosing instruction in the SPC, and may lead to ineffective treatment, increased risk of development of AMR and an overall increase in antimicrobial use.
If veterinary practitioners choose off‐label use of antimicrobials systematically as a substitute for good farm management, biosecurity, optimal hospital hygiene, or lack of a precise diagnosis, this could be classed as ‘misuse’.
Using antimicrobials off‐label for purposes other than antibacterial (e.g. AGPs) could be defined as serious ‘abuse’. Furthermore, practices that have been found to lead to the widespread dissemination of resistant bacteria or genes of public health concerns (e.g. in ovo use of cephalosporins in parent flocks or day‐old chicks) are increasingly being recognised as an ‘abuse’ of off‐label antimicrobials (see Section 3.3.4.4).
As noted above, some MSs have legislated to prohibit cascade use of certain CIAs (fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins).
A more detailed overview of off‐label use of antimicrobials in veterinary medicine by the CVMP is in progress.
Recommendations
Further research should be done into the nature and extent of off‐label use of antimicrobials in food‐producing animals in the EU, and the associated potential for impacts on AMR.
The regulatory system should encourage the availability and maintenance of a range of antimicrobial VMPs across all the EU MSs so that veterinarians can base antimicrobial prescribing on evidence‐based SPC guidance and principles of responsible use rather than therapeutic decisions being driven by local product availability.
When prescribing under the cascade, the risk to public health due to AMR should be taken into account alongside the need to protect animal welfare.
Evidence‐based treatment guidelines can support responsible off‐label use of antimicrobials by taking into account the local AMR situation and product availability in the member state in addition to the general clinical evidence base for such use. The potential impact on public health should be included in the risk assessment underlying this guidance.
3.3.6. Prophylaxis, Prevention, Metaphylaxis
The concept of prophylaxis appears to have first entered the scientific literature in relation to food‐producing animals in the 1950s, mostly in response to high mortality rates (King et al., 1955). Reference to metaphylaxis first appeared in the 1980s.
Different definitions exist in the scientific literature for the terms prophylaxis, prevention and metaphylaxis. For example, prophylactic use of antimicrobials is typically regarded as a herd management measure designed to maintain health (of an individual or group of animals) and prevent the occurrence of disease. Recently, the EMA guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances has been revised (EMA, 2016a). The term ‘prevention’ is defined as ‘the administration of a veterinary medicinal product (VMP) to healthy animals to prevent infection if the risk for infection is very high and the consequences severe’. EPRUMA uses the terms preventive treatment and prophylaxis synonymously, with the following definition, ‘Preventive treatment (Prophylaxis): treatment of an animal or a group of animals before clinical signs of disease, in order to prevent the occurrence of disease or infection’.51
The essence of most definitions is that prophylaxis/prevention involves the administration of an antimicrobial agent to ‘healthy’ individuals in order to ‘prevent’ an infection due to a perceived ‘risk’. Often the risk is neither defined nor quantified in the justification for preventive use.
For herd‐health purposes, preventive uses of antimicrobials have been based on:
traditional farm practices or attitudes;
reduced labour costs since less monitoring of animals is needed;
previous history of herd outbreaks;
-
herd management practices:
-
–
introduction of new animals into groups (e.g. ‘welcome shots’);
-
–
high stocking densities (i.e. increased ‘risk’ of disease);
-
–
scheduled events in the production animal cycle (e.g. dry‐off cow period, before transport);
-
–
stressful events (e.g. weaning, castration, dehorning, viral outbreaks).
-
–
Originally, the term ‘metaphylaxis’ was defined in the scientific literature as the same as prevention, where the only difference was that prevention is given to individuals and metaphylaxis is given to groups/flock/herd. Other working definitions have been specified and are more frequently utilised today. For example, the EMA guideline (EMA, 2016a) has defined metaphylaxis as the ‘Group treatment of all clinically healthy (but presumably infected) animals kept in close contact with animals showing clinical signs of a contagious disease. Metaphylaxis is always combined with the treatment of the diseased individuals …’. Different definitions from the scientific peer‐reviewed literature, include:
metaphylaxis is defined as the treatment of an entire group or population with an approved antimicrobial medication with the intent of controlling the incidence of acute onset disease in highly stressed, newly received animals (Urban‐Chmiel and Grooms, 2012);
metaphylaxis is indicated for ‘high‐risk individuals’, when the number of clinical cases within a group reaches a threshold, the remainder of the in‐contact animals are treated simultaneously in order to restrict the spread and impact of the disease (Lees and Shojaee Aliabadi, 2002).
According to Edwards (2010) and Smith et al. (2001), the criterion for whether to apply antimicrobial metaphylaxis to a particular group of animals is when morbidity exceeds 10% (known as the ‘attack rate’) for 2–3 consecutive days.
Both metaphylaxis and prevention have the potential for substantial consumption of antimicrobials since ‘healthy’ individuals usually outnumber sick individuals in any given scenario. In some livestock systems (e.g. poultry, fish), treatments can only be applied to a whole group of animals. Certain types of veterinary medicinal products (VMPs) used in food‐producing animals are suited for easier administration as either preventive treatment or metaphylaxis. For example, antimicrobials intended for oral use in drinking water and premixes for medicated feeds are used commonly for larger populations of food‐producing animals. According to (EMA ESVAC, 2016), in 2014 91.6% of overall antimicrobial sales were for oral administrations; data are not available on the proportion used for each of preventive, metaphylactic and curative use. A recent DG SANTE questionnaire confirmed that data on mass medication is not specifically collected in most EU member states.
3.3.6.1. Examples of prophylaxis/prevention and metaphylaxis from literature
In a Belgian study assessing antimicrobial consumption in veal calves, approximately 13.0% of antimicrobials were used preventively (immediately after arrival on farm) and 87.0% for metaphylactic use or as a curative measure (Pardon et al., 2012a). Antimicrobial use was higher than that in the pig and poultry industries and it was suggested that this may be due to the organisation of the veal industry in Belgium in which young calves are sourced from multiple farms and comingled after the stress of recent transportation, increasing disease risk. The authors concluded that antimicrobial use could be reduced by decreasing the number of oral antimicrobial group treatments and improving management practices.
In another Belgian survey conducted in 2003 and designed to quantify antimicrobial drug consumption in pigs, injectable antimicrobial drugs were found to be mainly administered for preventive treatments at birth and castration and included broad‐spectrum penicillins and cephalosporins. Group treatments for diarrhoea were mainly metaphylactic, using fluoroquinolones and aminoglycosides. Polymyxin E (colistin) was administered mainly to prevent post‐weaning diarrhoea (Timmerman et al., 2006). A later similar survey in Belgian pigs conducted in 2010, (Callens et al., 2012) identified an increase in group level treatments and that 93% of them were for preventive reasons and often lacked a precise diagnosis. Group treatments via oral administration accounted for higher antimicrobial exposure than via injectable administration (TIADDpig 183.5 and 52.3, respectively). The most frequently used antimicrobials at oral group level were colistin (30.7%), mainly to prevent post‐weaning E. coli infections, and amoxicillin (30.0%), as prevention against streptococcal infections. Of concern was a shift from oral group treatments with doxycycline and potentiated sulfonamides towards use of long‐acting injectable formulations, some of which included 3rd‐ and 4th‐generation cephalosporins. It was suggested that farmers on large production facilities often consider the preventive use of antimicrobials, in spite of the associated high cost, as a necessity to achieve less disease, lower mortality and better production results, as well as easier and less labour intensive to implement than treatment of clinically diseased animals after losses have occurred (Callens et al., 2012).
A survey of antimicrobial consumption in farrow‐to‐finish farms conducted in 2010 in Spain showed that 34.2% of records related to use for prophylaxis indications, decreasing from the preweaning phase (49.0%) and growing phase (41.6%) to the fattening phase (15.0%) (Moreno, 2014b). Preventive treatment was administered on 96% of farms during the 6‐month period of the survey, with digestive and respiratory disorders being the most common indications. Administration was via medicated feed in 70.5% of records with colistin, amoxicillin and zinc oxide being the most used substances. Therapeutic/metaphylactic use was indicated in 65.8% of records and in 65.5% of records was associated with parenteral administration. The substances mostly administered by parenteral route were β‐lactams and fluoroquinolones. It was shown in a wider survey that antimicrobial consumption in fattening pigs was higher on farms, which only finished pigs and this was attributed to a high turnover of animals coming from multiple sources and greater use of preventive treatments (Moreno, 2012).
In the UK, pig veterinarians considered that use of in‐feed antimicrobials was justifiable for prevention of disease. If management changes to allow withdrawal of preventive measures were not practical or not seen as economically feasible, then veterinarians expressed concerns about the impacts withdrawal of preventive use would have on animal welfare, and also perceived that there would be pressure to prescribe from farmers reluctant to suffer an economic loss (Coyne et al., 2016).
Sjölund et al. (2015) compared the herd level use of antimicrobials among farrow‐to‐finish farms in Belgium, France, Germany and Sweden. The treatment index was lowest in the Swedish herds where most treatments were therapeutic (curative) individual treatments; whereas in Belgium, Germany and France it was common practice in some herds to apply group treatments to suckling and weaned piglets at strategic time points when the pigs were thought most likely to contract disease. The authors noted that Sweden is free of porcine reproductive and respiratory syndrome (PRRS) and that more severe disease may be seen in pigs that have this infection concurrent to infections with Streptococcus suis or Mycoplasma hyopneumoniae. They commented that pig and herd density is lower in Sweden, and this could influence disease transmission within the region.
In Denmark, a trial was conducted to investigate the efficacy of oxytetracycline (OTC) in the treatment of Lawsonia intracellularis (LI) in pigs when administered at either the batch, pen or individual animal level (Larsen et al., 2016). Treatment was started when LI‐related diarrhoea was detected in the batch and batches were randomly assigned to a treatment strategy. For the batch‐treated group, 100% of pigs received oral treatment with OTC for 5 days. For the pen‐treated group, the same treatment was administered to the diarrhoeic pens only, and 87% of pigs received medication. The individually treated pigs were treated with OTC by injection when they showed signs of diarrhoea, and 55% of pigs were treated. The batch treatment was found to be the most effective in reducing high‐level LI‐shedding and diarrhoea, although the number of animals treated was the greatest.
The effectiveness of metaphylactic use of the macrolide, gamithromycin, to control bovine respiratory disease (BRD) was investigated in recently comingled cattle at rearing units across Europe (Baggott et al., 2011). Healthy animals were treated either with gamithromycin or placebo control when at least 5% of cattle in the same airspace had presented clinical signs of BRD. Treatment success (lack of clinical signs) was 86% in the treated group, compared to 61% in the control group (p = 0.0012); indicating that morbidity was reduced by 64%. Based on the data included in the publication, then the number of animals needed to treat (NNT) to prevent one clinical case was four under the conditions of this study (range 2.5 to 11 across the 5 sites). The authors commented that therapeutic treatment will generally result in the lowest level of antimicrobial use, but requires good stockmanship to detect BRD in its early stages, and adequate labour and handling facilities, in order to protect animal welfare. It has been suggested that the decision to treat should weigh up the risk of infection with the risk of use of an antimicrobial. Factors to take into consideration include knowledge of the specific diagnosis and virulence of the organisms based on previous experience of the disease on the holding and a prediction of the animal's immune status.
A further study was conducted to evaluate a selective approach to the use of metaphylaxis in feedlot calves in the Netherlands (Gonzalez‐Martin et al., 2011). In this study, it was shown that administration of metaphylaxis with florfenicol based on treatment of calves with a rectal temperature > 39.7°C was associated with lower antimicrobial use and less intensive handling of calves compared to mass metaphylaxis.
Commenting on the feedlot and veal calf industries in the USA, Ives and Richeson (2015) identified that a major obstacle to the successful management and control of bovine respiratory disease in these cattle populations is associated with the segmented infrastructure of the industry. For example, calves change ownership frequently and are therefore subject to multiple stressor such as transportation, relocation and comingling. This provides ample opportunity for pathogens associated with bovine respiratory disease to colonise the lower respiratory tract. Preconditioning programmes aimed at improving the health of calves at the time of transition by use of vaccination, castration and allowing adequate time for weaning/feeding training prior to shipping are recommended. Further, it was noted that the poor sensitivity of field diagnostics, and labour issues were additional reasons to use metaphylaxis (Ives and Richeson, 2015).
Antimicrobial dry cow therapy (ADCT) was introduced as part of the Five‐point Mastitis Control Plan in the 1950s and can be regarded as either preventive or metaphylactic use. The goal is to treat existing intramammary infections (IMI) during the dry period, and prevent new infections from establishing during this high‐risk stage. ADCT was often administered to the whole herd as a blanket treatment. In a recent survey of drying‐off practices on dairy farms in northern Germany (Bertulat et al., 2015), 79.6% of participating farms practised blanket ADCT. Since the prevalence of contagious mastitis pathogens has now decreased and due to concerns about AMR, this approach is now under question (Biggs et al., 2016). Selective ADCT is an alternative approach in which the risk of the presence of IMI is investigated for each cow using diagnostic tests such as bacteriology, California Mastitis Test and individual somatic cell counts (SCC). The decision to treat an individual animal is also made on the basis of the cow's history and knowledge of the herd. Use of selective as opposed to blanket ADCT has the potential to reduce antimicrobial use and negatively impact udder health depending on the SCC criteria used to select cows for ADCT (Scherpenzeel et al., 2016). In the Netherlands, preventive use of antimicrobials in dry cows has been prohibited since 2011. MARAN (2015) notes that there has been a reduction in the use of antimicrobials in dairy cattle and particularly a shift away from use of CIAs in ADCT in 2012 and 2013, and a reduction in ADCT in 2014. A survey of Dutch dairy farms conducted in 2013 found that udder health had not deteriorated compared to that seen in previous studies where herds were smaller and before the restriction in antimicrobial use (Santman‐Berends et al., 2016).
3.3.6.2. Actions taken in the Member States
In several MSs preventive use of antimicrobials is not allowed, or only in limited circumstances. In 1995, preventive use of antimicrobials became illegal under Danish law. Veterinary Advisory Service Contracts (VASC) between veterinarian and farmer were introduced in 1995, and became compulsory for large cattle and pig farms in 2010 (see Section 3.3.7). Under the VASC, when veterinarians prescribe group treatments for pigs for respiratory or gastrointestinal infections, by law they must verify the diagnosis by submission of samples to an approved laboratory. Between 2009 and 2011, coinciding with the introduction of the Yellow Card initiative (see Section 3.3.7), there was a 25% reduction in the total antimicrobial use per pig produced, explained mainly by a reduction in prescription of oral antimicrobials (tetracyclines, macrolides and pleuromutilins) for treatment of gastrointestinal disease in weaners and finishers. It was hypothesised that the decrease in use of tetracyclines could be related to cessation of systematic metaphylaxis in some herds (Jensen et al., 2014).
The German Medicinal Products Act, 2005,52 includes special provisions that require the use of antimicrobials in animals to be justified in compliance with Federal Veterinary Surgeons’ Association Guidelines for the prudent use of veterinary antimicrobial drugs. The guidelines state that the use of antimicrobials preventively in healthy (uninfected) animals should be avoided except in well‐founded cases, such as in association with surgery or in immunocompromised patients. For metaphylactic use, then it should be proven that the infection is expected in animals in the herd not yet showing signs and that illness would otherwise manifest soon.
In accordance with the Dutch government's policy on responsible use of antimicrobials in food‐producing animals,53 preventive use was ‘prohibited’ by the deletion of such indications from the label texts and SPCs of antimicrobial medicines, and a re‐emphasis that off‐label use should be limited to exceptional cases. In addition, third choice antimicrobials (3rd‐ and 4th‐generation cephalosporins, fluoroquinolones) may only be used on an individual animal basis and following susceptibility testing.
In Belgium AMCRA recommends that there should be no preventive use of antimicrobials, except associated with perioperative use and for dry cow management.
In Sweden about 90% of antimicrobial consumption is for treatment of individual animals, and 10% is for group treatments (Public Health Agency of Sweden and SVA, 2015). Products for group treatment are mostly used for pigs, and use has decreased by 55% since 2010. Consumption of pleuromutilins decreased by 64% in 2014 compared to 2010, and was thought to be due to an eradication and certification programme for swine dysentery. Over the same period, use of macrolides decreased by 55%; it is suggested that this may reflect better management of concomitant infections in farms affected by PWMS and introduction of vaccination. In pigs, there has been a shift from use of group medication to individual animal treatment and use of narrow spectrum agents such as benzylpenicillin.
The French Agency for Food, Environmental and Occupational Health and Safety provided an opinion in 2014 on the risk of emergence of AMR associated with modes of antibiotic use in animal health (ANSES, 2014). This report reviewed preventive, metaphylactic and curative use of antimicrobials in various animal sectors with the objective of identifying ‘at‐risk practices’ (i.e. those resulting in significant selection of AMR bacteria). The experts involved concluded that metaphylactic treatment is appropriate in so far as it can increase the benefit/risk compared to preventive treatment. In regards to use of preventive treatments, in many cases it was concluded that these could be abandoned either immediately, or over a period of time to allow the introduction of recognised alternative measures. It was recommended that the specific practices should be considered further by sector professionals. To allow for phasing out of preventive use, there is a need to identify sick animals rapidly in case of the need for treatment. In regards to metaphylaxis, it was recommended that appropriate indicators should be defined on which to base the decision‐making criteria for prescription. Furthermore, the development of early detectors of disease should be encouraged in order to improve the indicators.
It was proposed that there should be no systematic use of preventive and metaphylactic treatment, with their use being based on aetiological diagnosis and prescriptions limited in duration. Underlying factors were identified that lead to the use of the various at‐risk practices, such as technical, economic and sociological constraints, poor biosecurity and management practices.
Some examples of identified ‘at‐risk practices’ are given in Table 4.
Prudent‐use concepts for metaphylactic and preventive use of antimicrobials need to be developed. The different definitions that exists for prophylaxis/prevention and metaphylaxis can be seen as a source of confusion that further impedes this action. Thus, it is important to define the terms in a manner that provides truly biologically important separate definitions. Metaphylaxis can be differentiated from prevention using modern definitions that identify that a proportion of animals within the group have a clinical expression of the disease‐of‐interest. There are particular difficulties associated with antimicrobial treatment of intensively reared livestock due to the potential for a high environmental pathogenic load and rapid spread of infection. Under these circumstances, herds/flocks, rather than individuals, have to be treated to control disease outbreaks and metaphylaxis is common place. When no disease is typically present at the initiation of preventive use of antimicrobials, this use should, be linked to clear risk factors for an identified disease with serious animal health consequences.
Relevant differences between the present time and the 1950s to 1980s, when mass medication concepts were first described, are the development of modern herd health management practices and the better understanding of the common disease complexes that affect food‐producing animals (e.g. BRD). For example, there is a now a better understanding that typical pathogenesis involves the initiation of the disease from viruses and/or other stress factors and ends in an opportunistic bacterial infection. Thus, in a wider context there are now relevant biological ‘barriers of defence’ to prevent common bacterial production animal diseases that did not exist previously. These include:
improved animal genetics with improved vigour and growth rates;
better husbandry practices (e.g. biosecurity, improved hygiene, reduced transport and comingling);
better nutrition;
improved vaccines against both common viral and bacterial pathogens.
These measures are discussed further in Section 4 of this Opinion.
The likelihood that an infection in a group of animals either creates a disease outbreak, becomes endemic or dies out is dependent on a complex of factors, including:
level of herd immunity – Herd immunity occurs when a significant proportion of the population (or the herd) have been vaccinated or possess natural immunity (e.g. acquired or colostral immunity). This provides some protection for non‐immunised individuals, since it is more difficult for diseases to spread between individuals if a proportion are already immune, and thus the ‘chain‐of‐infection’ can be broken;
animal stress factors that promote immunosuppression (e.g. weaning, castration, dehorning);
animal husbandry practices that promote stress and/or introduction of contagious diseases (e.g. stocking density, transport of animals, comingling of animals from different sources, poor biosecurity measures, farm hygiene, changing feed compositions and high energy/low fibre feeds);
characteristics of the bacterial clone involved in the disease (e.g. virulence factors, antigenicity, previous exposure to the population).
These factors stated above can create major variations in herd population dynamics that lead to examples of the same bacterial contagious diseases causing little‐to‐major impacts on individual herds. Thus, in many circumstances, not all in‐contact animals are susceptible to the bacterial disease, due to an acquired immunity or previous immunisation. Therefore, not all contacts will become infected and the average number of secondary cases per infectious case will decrease or be highly variable between groups.
In the veterinary scientific literature, there is little specific guidance or critical evaluation as to which types of bacterial diseases would justify antimicrobial prevention vs metaphylaxis. Some general concepts can be proposed to justify antimicrobial use for prevention, for certain contagious bacterial diseases, including:
contagious bacterial diseases where it is known from the pathogenesis that it will rapidly progress (e.g. 24 h) to an epidemic and where mortality is a major outcome;
where waiting for the scenario when metaphylaxis using antimicrobials (i.e. with the disease clinically present) is applicable will negatively affect the outcome of the outbreak (especially mortality rate);
where there is not an effective vaccine available or other means to establish herd immunity, and there are no other recognised effective herd‐health control measures.
Examples of contagious bacterial diseases that could fulfil all these concepts include Streptococcus suis, and certain virulent forms of Actinobacillus pleuropneumoniae in pigs (MacInnes and Desrosiers, 1999; Halbur et al., 2000; Taylor, 2013; Varela et al., 2013). Note should also be taken of the recommendations at the end of this section.
The performance of surgical procedures (e.g. caesarean section, displaced abomasum) in farm animals is another scenario where antimicrobials are commonly administered for preventive use. The relative risk for surgical site infections is often assumed to be higher in farm animals than in human or companion animal surgery, because of the potentially unsanitary operating environment in the field, depressed patient immune function in the periparturient period and the high probability of post‐operative wound contamination (Dumas et al., 2016). Unfortunately, there are no prospective, blinded, randomised, controlled trials that have been conducted to investigate the necessity, efficacy and optimal dosing regimen of antimicrobials to prevent surgical site infections in farm animals. Dumas et al. (2016) considered the evidence relating to various risk factors such as the classification of the wound according to the level of bacterial contamination, potential pathogens, the surgical technique and estimated duration, host immune status and presence of intercurrent disease, and advised that surgeons should evaluate the risk of surgical site infection on a case‐by‐case basis.
Concluding remarks
Please also refer to the section of the report on Medicated Feedingstuffs and other oral administrations
For practical reasons metaphylaxis can be distinguished from prevention in that in metaphylactic treatment a proportion of animals within the group have clinical expression of disease, whereas in preventive treatment no clinical disease is present at the start of treatment.
Both preventive and metaphylactic treatment have potential for high antimicrobial consumption, although specific data are not collected in most EU countries. Depending on the disease morbidity, a high proportion of animals that would have remained healthy may be exposed to antimicrobials in order to prevent a smaller proportion of clinical cases.
There is evidence that both modes are widely practised in regions of the EU, especially for enteric and respiratory diseases in veal calves and in pig production.
Risk factors include transport stress, poor biosecurity, obtaining animals from multiple sources and comingling.
Prevention and metaphylaxis are used on some farms as a management tool to reduce labour and improve production.
Oral group treatments frequently used include CIAs, such as colistin and beta‐lactams. In recent years, there has been a shift towards use of long‐acting injections, including 3rd‐ and 4th‐generation cephalosporins and macrolides. There is evidence that requiring diagnosis prior to use of group treatments may decrease the systematic use of prevention and metaphylaxis.
Recommendations
Please also refer to the section of the report on Medicated Feedingstuffs and other oral administrations
There should be an aim at national and farm level to phase out preventive use of antimicrobials. This should be based on a structured review of such use at national or regional level by livestock sector professionals with the knowledge of local endemic disease epidemiology, underlying risk factors for disease and local husbandry systems. Related disease‐specific guidance should be developed.
-
In exceptional cases, if preventive use of antimicrobials can be justified, either to groups of animals or individuals, the following principles should apply (not all are applicable for individual animals):
-
–
Clear risk factors should be identified for a contagious bacterial infection that has serious disease consequences.
-
–
There should be a recent aetiological diagnosis on the farm of the potential pathogens involved and their antimicrobial susceptibility.
-
–
The prescribing veterinarian should have a good knowledge of the epidemiology of disease on the farm (e.g. virulence of organisms) and the risk factors for infection associated with the group, e.g. the immune status, management factors.
-
–
In the veterinarian's judgement, the alternative of waiting to initiate metaphylaxis would negatively affect the outcome (especially mortality).
-
–
Antimicrobials should be prescribed for a limited duration to cover the period of risk and there should be documented justification for such use.
-
–
Prevention should not be used systematically if the underlying risk factors could be controlled by recognised alternative measures (e.g. vaccination, nutrition, hygiene).
-
–
Specific principles for the main sectors/diseases should be developed at national or regional level with assistance from livestock sector experts.
-
–
When preventive treatment is applied to groups of animals, this should be focused on the subset of animals at highest risk.
-
–
-
There should be an aim at national and farm level to reduce and refine the use of metaphylaxis:
-
–
Livestock handlers should pay close attention to monitoring animals’ health and should have the skills and knowledge to detect disease early in order to allow movement away from use of systematic preventive treatments and towards metaphylactic use only.
-
–
The prescribing veterinarian should use appropriate diagnostic tests, have a good knowledge of the epidemiology of disease on the farm and the risk factors for infection associated with the group.
-
–
Consideration should be given to the impact of housing structures and food and water delivery systems on the overall numbers of animals that are exposed to group treatment and the possibility to control the spread of disease.
-
–
Metaphylaxis should not be used systematically if the underlying risk factors could be controlled by recognised alternative measures (e.g. vaccination, nutrition, hygiene).
-
–
Specific principles for the main sectors/diseases should be developed at national or regional level with assistance from livestock sector experts. These should characterise the early detectors of disease and establish criteria on which to base initiation of treatment. The associated risk factors for disease should be identified, together with alternative measures for their control.
-
–
Where antimicrobials are used repeatedly for preventive or metaphylactic use, then the underlying risk factors on the farm should be investigated by the responsible veterinarian and other professionals. Infection prevention and control measures on the farm should be reviewed and recognised alternative measures to reduce the need for antimicrobials should be implemented via the farm health plan.
Consideration should be given to collecting data on consumption of antimicrobials for preventive use at the EU, national and farm level for monitoring purposes.
Consideration should be given to a move away from use of blanket dry cow therapy to selective dry cow therapy regimens, where possible.
Further research is needed to identify the indicators and criteria that can be used for different species/diseases to identify when initiation of metaphylaxis can be expected to deliver maximum benefits and to limit unnecessary exposure of animals that would anyway remain healthy.
3.3.7. Prescribing, distribution and supply channels
3.3.7.1. EU Regulatory background
Articles 66 to 69 of the veterinary medicinal products Directive 2001/82/EC (as last amended), lay down the provisions that should be taken by member states in national legislation in relation to the retail supply, prescription and record keeping for veterinary medicinal products. In EU Member States, in general a veterinary prescription is required for dispensing veterinary medicinal products for food‐producing animals to the public. With regard to veterinary medicinal products for the treatment of bacterial infections, Member States may apply an exemption to the requirement for a veterinary prescription on condition that several discrete criteria are satisfied. In the 26 Member States that provided data for the ESVAC project in 2013 and according to the responses to the DG SANTE questionnaire, all antimicrobial veterinary medicinal products are ‘prescription only’ with minor exemptions reported in only two MSs.
3.3.7.2. Evidence for factors that influence prescribing
Several qualitative studies or surveys have been conducted to investigate the influences on prescribing behaviour. Among pig veterinarians in the UK, external pressures such as demand from clients, legislation, fears of litigation and public perception were considered to strongly influence prescribing behaviour (Coyne et al., 2014, 2016). A survey of cattle practitioners in Ireland indicated that non‐clinical issues such as actual or perceived client demand and concern over blame or the need to revisit if the animal did not improve, influenced the prescribing decision of the majority of respondents (Gibbons et al., 2013), and in a study of farm animal veterinarians in the Netherlands, belief in professional obligation to animal welfare, risk avoidance, shortcomings in advisory skills and financial dependency on clients, were among the influential determinants (Speksnijder et al., 2015a). Training to improve the advisory skills of veterinarians and educational campaigns aimed at both veterinarians and farmers could help to address some of these factors including the (perceived) pressure from farmers to prescribe antimicrobials.
In 2012, Postma et al. investigated by means of a questionnaire the opinions of veterinarians in Netherlands and Flanders regarding antimicrobial use in farm animals (Postma et al., 2016a). The study investigated the perceived effectiveness of the different measures to reduce antimicrobial use introduced in Netherlands and Belgium. In 2010, the Dutch government introduced a series of mandatory interventions to reduce antimicrobial use by 50%; whereas in Belgium, the Center of Expertise on Antimicrobial Consumption and Resistance in Animals (AMCRA) ‐ established in 2012 ‐ aims to reduce use through advice and awareness raising. In both countries, respondents reported to have become more aware of the issue; there were differences in their perceptions of the policy measures with 63.8% of Dutch respondents supporting the policy to halve antimicrobial use, compared to only 32.9% of Flemish veterinarians. Flemish veterinarians were more concerned about negative impacts on animal health and welfare and were significantly less concerned about prescribing antimicrobials to prevent or treat suspected bacterial infections compared to Dutch counterparts. Dutch veterinarians were significantly less likely to feel pressure from farmers to prescribe antimicrobials. The authors concluded that cultural differences and the diverging policies had led to different habits relating to antimicrobial use.
Accurate diagnosis is the cornerstone of responsible prescribing. An independent review commissioned by the UK government (O'Neill, 2015) highlighted that rapid, cheap and easy to use diagnostics could reduce the need for preventive use of antimicrobials on farms and aid selection of effective treatment. The EC PUAVM Guidelines emphasise that diagnosis should be based on clinical examination supported by appropriate pathological sampling, taken prior to treatment, and preferably after AST. A survey conducted by the FVE with responses from 3,004 (self‐selected) practitioners from across the EU indicated that the factors that most strongly influenced antimicrobial choice were results of susceptibility tests, own experience, the risk for AMR and ease of administration (De Briyne et al., 2013). Similarly, in the Netherlands and Flanders, personal experience, recommendations from experts/literature and results of AST were the most important factors influencing the choice of antimicrobial (Postma et al., 2016a). Among Irish cattle practitioners, the veterinarian's prior experience of a drug, ease of administration and frequency of treatment influenced the choice of antimicrobial prescribed (Gibbons et al., 2013). According to the FVE survey, among farm animal practitioners, AST was performed regularly by 44% of respondents, and usually where a treatment failure had occurred. Use was limited by the urgency of the situation, sampling difficulties, concerns regarding the clinical relevance of in vitro results and the cost. It was suggested that the responses indicate a need to improve susceptibility tests and services, with key factors being the availability of rapid and cheaper testing. Similarly, UK pig veterinarians reported that the time delay in obtaining results of AST was problematic and that an antimicrobial was often selected on presumed diagnosis in order not to compromise animal welfare (Coyne et al., 2016). When use of AST is strongly recommended in national guidelines it is used more regularly. In 2014, the Danish Veterinary and Food Administration introduced a legal requirement for veterinarians, when prescribing antimicrobials to be administered in feed or water to treat respiratory or gastrointestinal infections in pigs, to submit samples for testing to an approved laboratory (DANMAP, 2015). Care should be taken that AST on commensal, often multiresistant bacteria, does not lead to an alibi for the use of antimicrobials including CIA. Bacterial carriage must thus be differentiated from pathological disorders. External (e.g. cross laboratory findings, linking with outcomes) and internal validation (e.g. internal quality control organisms) procedures are required. If needed, quantitative or semiqualiltative bacterial culture should precede the susceptibility testing. Also, the influence of interpretation criteria leading to substantial fluctuations in so‐called clinical resistance percentage percentages (Goossens et al., 2013), are of great concern (e.g. CLSI vs EUCAST or VETCAST). Finally, a delay in laboratory result leads often to empiric broad‐spectrum treatment, and de‐escalation. This additional antimicrobial selection pressure, sometimes aggravating the multiplication of pathogens timely due to inactivity and therefore the disorder, could be reduced in the future by on side (on farm) rapid and accurate diagnostics. In other words, diagnostic testing protocols should be developed so that only clinically meaningful tests are performed.
The FVE survey showed that training, published literature and experience were very important in influencing prescribing decisions and 72.5% of respondents indicated that they take into account responsible use warnings on SPC/product information. The authors advised that articles in veterinary journals could be effective tools to influence prescribing behaviour although it was noted that materials should be tailored to local antimicrobial availability and resistance patterns, and that multifaceted approaches might be needed to reach all veterinarians.
National and regional treatment guidelines/formularies (see Section 3.3.4) did not rank highly in relative importance in influencing prescribing behaviour in the FVE survey and reasons for this may warrant further investigation. Ease of administration was an important in consideration of compliance with effective treatment regimens. The necessity for the prescriber to make treatment decisions based upon their expert knowledge of the animal/herd and without financial conflicts of interest is emphasised in the EC PUAVM Guidelines. In Finland, restrictions are in place to prevent veterinarians profiting from the sale of antimicrobials. Other MSs have gone further and ‘decoupled’ prescribing and dispensing such that antimicrobials can only be dispensed at a pharmacy on presentation of a prescription (Denmark, Spain, Sweden, Italy). In this case, generally provisions are made to allow the veterinarian to dispense restricted quantities of antimicrobial treatment in case of an emergency. Based on data from ESVAC, although in some member states where decoupling is in place the sales of antimicrobials (mg/PCU) are low (Norway, Sweden, Denmark), this is not the case in others (Spain, Italy) where the sales are some of the highest in the EU, although reducing since 2010 in Italy. In the FVE survey, profit margin and marketing factors were ranked by veterinarians themselves as the least important of the factors investigated for influence on prescribing behaviour. Likewise, Dutch and Flemish veterinarians believed that decoupling would not greatly impact antimicrobial use, but felt unable to earn a reasonable income without their pharmacy revenues. Hence conserving the pharmacy was seen as a strong motivator to reduce antimicrobial use (Postma et al., 2016a).
3.3.7.3. Measures that have been implemented in MSs and their impacts
In 1994, the Danish government introduced a law to restrict the quantity of medicines that veterinarians could supply and sell and the profit that could be made so that effectively the prescribing and dispensing of antimicrobials were decoupled. Together with prohibition of preventive use, these two measures led to a reduction in the volume of antimicrobials prescribed by veterinarians of 40% in 1 year54 and now almost all medicines used in the livestock sector are sold directly to the farmer from a pharmacy. More recent measures on prescribing are the need for a contract to be in place between the veterinarian and farmer, and benchmarking of the veterinarian's prescribing and consumption on individual pig farms under the Yellow Card system (see Section 3.3.3). The Veterinary Advisory Service Contract (VASC) became mandatory for large cattle and pig herds in 2010, and requires the veterinarian to perform regular advisory visits to the farm with a focus on prevention of disease and optimising antimicrobial use to limit development of resistance (DANMAP, 2015). It may allow the farmer additional rights with respect to antimicrobial administration. The Yellow Card intervention applies legal actions on pig farmers with high antimicrobial use. A study showed a temporal relationship between the announcement of the Yellow Card intervention in July 2010, when farmers in the top 20th percentile were sent a warning letter, and a subsequent 10% decrease in antimicrobial use already by the 3rd quarter of 2010 (Jensen et al., 2014). The study authors suggested that the decrease prior to enforcement of the Yellow Card could indicate that the risk of receiving a Yellow Card affected the veterinarian and farmers decision‐making. A further potential factor was the increase in vaccination during the spring and summer of 2010, specifically against PCV2 which was thought to contribute significantly to metaphylactic antimicrobial use for associated secondary infections. The decrease in antimicrobial use continued concurrently with enforcement of the Yellow Card intervention from December 2010 into 2011, supporting the hypothesis of a causal association. The changes in prescription patterns were explained by a change in the number of herds receiving regular monthly prescriptions, leading to the hypothesis that use of systematic metaphylaxis was discontinued in some herds. The authors concluded that client expectation may be of major importance for driving antimicrobial use. Figure 9 shows the antimicrobial consumption in the pig production in Denmark.
Figure 9.
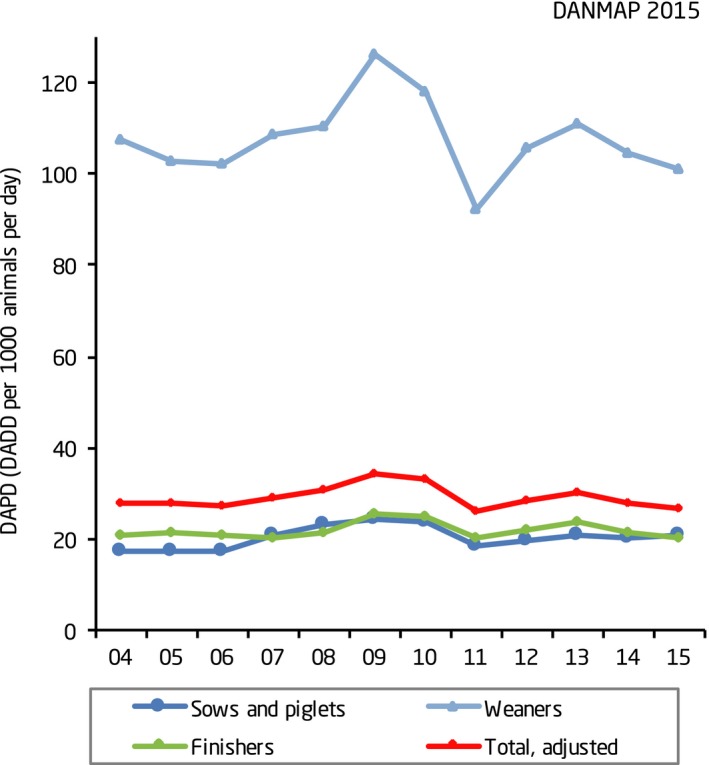
Antimicrobial consumption in the pig production, and the distribution on age groups, Denmark (source: (DANMAP, 2016) – see also original report)
The Berenschot report (Beemer et al., 2010) was commissioned by the Dutch Ministry of Agriculture to investigate the consequences of decoupling the prescription and sale of veterinary medicines by veterinarians. The report identified that most livestock farmers in Netherlands viewed antimicrobials as a cost‐effective management tool in the face of competition in the global meat production market. Most parties in the production chain, from farmer to consumer, benefit from the economic advantages of antimicrobial use, but the veterinarian has to also consider public health interests. Although decoupling would remove the economic incentive for the veterinarian, it was not considered to sufficiently strengthen the independence of the veterinarian from the livestock farmer for whom there is an economic incentive to use antimicrobials. The farmer might still exert pressure on the veterinarian to prescribe antimicrobials, or turn to source them through illegal channels. Therefore, it was suggested that instead of decoupling, the position of the veterinarian should be strengthened by taking measures to increase their professionalism when acting as gatekeepers of antimicrobials. Measures proposed included increasing the transparency in the prescribing and administering of antimicrobials, investing in a quality assurance system for veterinarians and promoting the implementation of alternative measures to antimicrobials to keep livestock production costs low.
In response to the increasing use of antimicrobials in farm animals in the Netherlands, the Dutch government introduced between 2008 and 2011 several policies for the reduction and more responsible use of antimicrobials in the livestock industry. These included a system of benchmarking, first for farms, and then, in 2011, of veterinarians’ prescribing. The latter is overseen by the Royal Dutch Veterinary Association (KNMvD). The benchmark indicator for veterinarians estimates the likelihood of exceeding the farm action benchmark threshold for all farms to which the veterinarian is contracted. Benchmarking of the veterinarian's prescribing allows them to compare their prescribing patterns with those of their colleagues, and farmers to make a considered choice over the veterinarian with whom they establish a contract (Bos et al., 2015). If benchmark values are exceeded, the KNMvD can take disciplinary action on individual veterinarians. The KNMvD has introduced a quality system for veterinarians which includes development of treatment guidelines and compulsory education.
In addition, in 2014 the Dutch government introduced new legislation that required that the administration of all veterinary antimicrobials should be by a veterinarian (the ‘UDD measure’). Under certain conditions, farmers may stock first‐choice antimicrobials for a single treatment in a limited proportion of animals. Among the conditions, the farmer must have a 1‐to‐1 relationship with a veterinarian and a Farm Health Plan and Farm Treatment Plan developed in collaboration with that veterinarian on the basis of their specific knowledge of the farm and in accordance with KNMvD formularies. Together with other measures, discussed later, sales of veterinary antimicrobials in Netherlands in 2014 decreased by 58.1% compared to the index year of 2009 (MARAN, 2015).
Concluding remarks
Veterinarians are influenced by complex and competing factors such as professional obligations to prevent animal suffering, a real or perceived demand from clients, legislative requirements and public perception when deciding to prescribe antimicrobials.
The choice of antimicrobial is influenced by factors including results of AST, training, prior experience, ease of administration. Use of diagnostic tests (e.g. AST) is limited by the urgency of the need to treat, concerns over the clinical relevance of the test and costs (see also Section 4.3, Diagnostic tools).
Livestock farmers view antimicrobials as a cost‐effective management tool. Veterinarians are financially dependent on their clients and in most MSs are allowed to profit from the sale of antimicrobials; these factors contribute to a financial conflict of interest when prescribing.
’Decoupling’ of prescription and dispensing of antimicrobials has been part of effective packages of measures to reduce antimicrobial use in some MSs, but decoupling is not always associated with low antimicrobial sales in those countries where it has been implemented.
Strengthening the role of the veterinarian in prescribing (requirements for prescribing in the context of 1:1 relationship with the farmer and farm health plans, benchmarking of prescribing) has contributed to part of an effective package of measures to reduce antimicrobial use.
Recommendations
Educational campaigns should be aimed at both veterinarians and farmers to raise awareness of the responsible use of antimicrobials.
Consideration should be given to improving the advisory skills of veterinarians with the aim of assisting them to address the actual or perceived pressure from farmers to prescribe antimicrobials and to improve the veterinarian‐client relationship (see Section 3.3.8).
The use of AST for target veterinary pathogens linked with infection and sampling prior start of the treatment should be promoted in national treatment guidelines, but with clear indications to avoid commensal bacteria being tested.
Diagnostic testing protocols could be developed so that the appropriate clinically meaningful tests are performed.
Steps should be taken to improve the availability of rapid, standardised and economical diagnostic tests (see Section 4.3).
MSs could explore conflicts of interest for veterinarians prescribing antimicrobials in their countries, and implement systems to address this.
Veterinarians could only be permitted to prescribe antimicrobials following sufficiently regular clinical examination of the animals to be treated and use of appropriate diagnostic testing. This could be enacted within the context of a farm health plan that has been drawn up between the farmer and the veterinary practice in a 1:1 relationship.
Consideration should be given to monitoring and benchmarking of antimicrobial prescribing by veterinarians.
Attention should be paid to prevention of sourcing and retailing of antimicrobial VMPs outside of legal channels.
3.3.8. Education and training
3.3.8.1. Introduction
Controlling AMR requires cooperation between public health, food and veterinary authorities, veterinarians, farmers and other parties, who all have a responsibility in this area. The responsibility for rational use of antimicrobials lies with the prescriber and the person administering the antimicrobials. Within the animal sector this means the veterinarian and the farmer.
3.3.8.2. Veterinarian
Undergraduate education
The EC PUAVM Guidelines advise that veterinary universities should ensure that sufficient attention is given to the problem of AMR and the prudent use of antimicrobials in their undergraduate programmes, and that knowledge relating to these areas is kept up to date. Undergraduate programmes should focus on developing learning materials and techniques relating to ways to improve and promote breeding and husbandry practices that promote animal health.
Educational programmes are frequently aimed at promoting good ‘antimicrobial stewardship’ – an approach aimed at promoting the responsible use of antimicrobials to preserve their future effectiveness. A recent survey has focused on the teaching of antimicrobial stewardship in undergraduate healthcare educational degree programmes including human and veterinary medicine, dentistry, pharmacy and nursing in the UK (Castro‐Sánchez et al., 2016). Antimicrobial stewardship includes subjects such as the selection of appropriate drugs, enhanced surveillance of prescribing and use, implementation of prescribing guidelines and policies, introduction of infection prevention and control strategies, and increased efforts on audit and education. In total, 109 courses were included of which six were veterinary courses. The majority of programmes taught antimicrobial stewardship principles (88/109, 80.7%) but among the six veterinary courses only one‐third addressed all those recommended. ‘Adopting necessary infection prevention and control precautions’ was the most frequently taught principle (83/88, 94.3%), followed by ‘Timely collection of microbiological samples for microscopy, culture and sensitivity’ (73/88, 82.9%) and ‘Minimisation of unnecessary antimicrobial prescribing’ (72/88, 81.8%). For veterinary medicine each of these principles accounted were included in 5 of the 6 (83%) undergraduate programmes. Overall, it was concluded that professionals receive disparate education on antimicrobial stewardship and that this could be improved by standardisation of curricula.
Pulcini and Gyssens (2013) considered how to best educate prescribers in antimicrobial stewardship practices in the human healthcare setting. They collated the key principles that should be addressed, which included topics such as antimicrobials and bacterial resistance; diagnosis, treatment and prevention of infection; record keeping and prescribing. Each topic was aligned with learning outcomes and competencies. It was noted that the undergraduate curriculum must build a sound knowledge base for later practice. In the UK, the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) has proposed to develop learning outcomes, i.e. statements that indicate what a student should know, understand and be able to do by the end of an educational programme, which could be used to develop a robust and transparent framework for curriculum development at all stages (HM Government, 2014). The French National Action Plan proposes that a module should be developed for veterinary students (by government departments, veterinary professional bodies and universities) which should integrate the theoretical teaching given earlier in the course with practical training in prescribing and bring to awareness guidelines on responsible use.
Postgraduate training
The EC PUAVM Guidelines propose that the veterinary professional associations and statutory bodies develop guidelines and provide training on the responsible use of antimicrobials. Until now, most initiatives on education in antimicrobial stewardship have been deployed in the postgraduate setting. In a number of EU countries training programmes for veterinary practitioners have been introduced by the national authorities, national veterinary associations or universities. Many training programmes are voluntary and are given as lectures alone without incorporation of active intervention. Veterinary Continuing Professional Development is not administered at the EU level – it remains under national responsibility and is not mandatory in all countries.
Although there has been much attention on antimicrobial stewardship programmes in the human healthcare setting (Goff, 2011; NICE, 2015), there has been little research into the needs or to support the effectiveness of antimicrobial stewardship programmes in the veterinary field. In a Dutch survey (Speksnijder et al., 2015b), it was found that veterinarians working with different animal species were comparable in their opinions towards the necessity to reduce veterinary antimicrobial use and the policy to halve veterinary antimicrobial consumption. Both veterinarians working with ruminants and veterinarians working with multiple animal species reported to feel more uncertain in acting independently from farmers’ and significant others’ demands for antimicrobials than veterinarians mainly working with intensively raised animals (pigs, poultry, veal calves). ‘Years of experience in practice’ was negatively related to feelings of uncertainty in acting independently. On the other hand, years of experience was also associated with being less concerned about the possible contribution of veterinary antimicrobial use to AMR, considering it more important to keep the right to prescribe and sell antimicrobials, and being less hesitant to apply antimicrobials to prevent infection. The results showed that younger veterinarians might require additional support to act independently from farmers. Additionally, experienced veterinarians could need to be educated about possible risks related to the overuse of antimicrobials.
A systematic review of the literature investigating interventions aimed at improving antimicrobial prescribing practices in human ambulatory care concluded that the effectiveness of an intervention depends to a large extent on the prescribing behaviour and barriers to change in the particular community (Arnold and Straus, 2005). Interventions, involving physicians, patients and public education were most successful in reducing antimicrobial prescribing. The NICE review (Robertson and Jochelson, 2006) noted that change is more likely to be embedded where individual interventions are supported by organisational and national level strategies.
Training and use of published literature are considered important sources of information influencing antimicrobial prescribing habits among farm animal practitioners (De Briyne et al., 2013). Further investigation is needed into the most effective means to provide training to veterinarians in AMS. A review by NICE (Robertson and Jochelson, 2006) investigated the barriers to the adoption of clinical guidelines by human medical physicians. The review concluded that passive forms of education (lectures, publications), although having a role as part of a multifaceted strategy, were less effective than active strategies (e.g. small scale interactive meetings) in getting clinicians to modify their practice. Due to its accessibility, e‐learning is becoming well‐established as part of a blended learning approach to postgraduate medical education. In the USA, the Centers for Disease Control and Prevention (CDC) have funded development of an open‐access web‐based multimedia suite of educational materials for veterinary students, practising veterinarians and other professionals.55 On‐line training ‘Antimicrobial resistance – theory and methods’ is available from the EU Reference Laboratory (EURL) for Antimicrobial resistance at the Danish Technical University.
Veterinary practice differs in many aspects from human healthcare, including among them the structural organisation of provision of care and the types of economic issues to be taken into consideration. It is therefore unclear as to how far the findings in regards to the implementation of antimicrobial stewardship programmes in human medicine could be extrapolated to the veterinary practice situation in the EU. Further research in this important area is warranted.
3.3.8.3. Farmers
In many countries farmers play an important and direct role in antimicrobial use decisions, sometimes through the ability to purchase and administer antimicrobials with minimal veterinary involvement. Diagnosis by the veterinarian must be a prerequisite in prescribing an antimicrobial but the practical dosing may be done by the farmer.
A survey of pig producers conducted in Spain in 2010 showed that they had an imperfect knowledge of the use of antimicrobials and regarded them as a cost‐effective tool for animal health and husbandry while having little concern about the impacts on public health (Moreno, 2014a). A small survey (n = 281) of pig farmers in five EU countries found that it would be beneficial to increase their awareness of the importance of AMR and its association with antimicrobial consumption as farmers did not appear to be concerned about antimicrobial use and this affected their perception of policy interventions to reduce use (Visschers et al., 2015).
Concerns over farmers’ compliance with dosing instructions has been shown to impact prescribing decisions. In the UK, pig veterinarians reported that they had used long‐acting formulations of CIAs based on concerns that farmers would be unable to deliver repeat injections of an alternative antimicrobial. It was concluded that producers might benefit from further education on the importance of completing the antimicrobial course, and offered practical solutions for the administration of repeated injections, in order to reduce the use of CIAs (Coyne et al., 2016).
Coyne et al. (2014) conducted focus group meetings with pig farmers and found that they considered the veterinarian to be the most trusted source of information on antimicrobials and held them responsible for prudent use. In the UK, a study investigated the factors that influenced decision‐making by dairy farmers around the use of antimicrobials (Jones et al., 2015). Again, the veterinarian was considered to be the most influential source of information on antimicrobial use and it was suggested that, taking into account the frequency of contact, policy‐makers should target veterinarians as the advisory group to deliver messages to farmers on the means and benefits from reducing antimicrobial use.
In the Netherlands, Lam et al. (2016) have developed a model to explain how modifying behaviour can be used as an approach to decreasing antimicrobial use in the dairy industry. The RESET model recognises that different people react to different stimuli and identifies five important cues to action: regulation, education, social pressure, economic incentives and tools. It explains how educating farmers about the potential effects of the use of certain antimicrobials on AMR contributed towards the reduction in use of 3rd‐ and 4th‐generation cephalosporins in the Netherlands, and recognises that other factors contributed to behavioural change – e.g. opportunities for farmers to express at public meetings the low antimicrobial use in their herd.
In Denmark, the government body, the DVFA, requires owners and their employees who administer medicines to food‐producing animals to complete an approved course in the management of veterinary medicines. This course gives theoretical an practical knowledge on the use of medicines in livestock and on the related legislation.
In the UK, the Responsible Use of Medicines in Agriculture Alliance (RUMA), an alliance of organisations from across the livestock sector, has developed sector‐specific guidelines for responsible Use of antimicrobials in poultry, pigs, cattle, sheep and fish. These guidelines place emphasis on the need for disease control and reinforce the message that antimicrobials should not be used to support failing management systems. They provide advice on strategies to reduce the need to use antimicrobials and on their correct administration, storage and recording of use. The study by Jones et al. (2015) found that around 53% of UK dairy farmers were aware of the RUMA guidelines, and 75% of these followed the guidelines either fully or partially. Denmark similarly has Guidelines on Good Antibiotic Practice aimed at farmers and with a focus on the prevention diarrhoea in pigs. AMCRA, in Belgium has developed recommendations for farmers on good farming practices and on prudent use of antimicrobials.
At European level, EPRUMA (The European Platform for the Responsible Use of Medicines in Animals) has published a best practice framework for the use of antimicrobials in food‐producing animals which follows similar lines to those in the member states and is based on the adage ‘As little as possible and as much as necessary’. Recently, this has been followed up with a ‘next level’ document which lists the building blocks which can be used to form a farm‐specific health plan.
3.3.8.4. Raising public awareness
The EC PUAVM Guidelines advice the importance of public awareness campaigns aimed not only at veterinary professionals and farmers, but also at consumers.
The ECDC hosts European Antibiotic Awareness Day (EAAD) annually on November 18th. Linked to this there are national initiatives in many EU MSs aimed at raising awareness to healthcare professionals and the wider public on the risks associated with inappropriate use of antimicrobials, and changing behaviour towards antimicrobial use.
In the UK, Public Health England launched the ‘Antibiotic Guardian’ campaign in 2014. The associated website includes resources for the public on AMR topics and invites visitors to take a pledge, tailored to their circumstances, to reduce their use of antimicrobials.
EFSA, EMA and ECDC all produce fact sheets and infographics which are available from their websites and explain the basics of AMR for the public audience.
The O'Neill report (O'Neill, 2016) points to the importance of public awareness campaigns in influencing consumer choice, which it suggests has in turn stimulated food producers and retailers to impose voluntary targets to reduce antimicrobial use in their supply chains (see Section 3.4).
Concluding remarks
The teaching of antimicrobial stewardship principles at undergraduate level is not consistent across EU veterinary faculties.
Postgraduate training in antimicrobial stewardship is provided by a combination of national authorities, veterinary associations and universities; it is not mandatory across the EU.
Multifaceted training strategies including active training elements may be more effective in getting clinicians to change their practices.
There is evidence that different demographics of veterinary professionals have different training needs, e.g. younger veterinarians may need training to support them to take prescribing decisions independently from farming clients.
There is evidence of a need to improve farmers’ awareness of the association between antimicrobial use, the development of AMR and its impacts on public health.
Farmers regard veterinarians as being responsible for the prudent use of antimicrobials and as an influential source of information on this topic.
Recommendations
The value of a core curriculum linking basic scientific knowledge through to the principles of antimicrobial stewardship and use of guidelines could be considered for application across all EU veterinary schools.
Consideration could be given to development of an EU‐wide Antimicrobial Stewardship programme for postgraduate veterinarians, with agreed core principles, learning outcomes and competencies.
All veterinary practitioners should be offered regular training in AMS, which could be accredited by national statutory bodies, with attainment recorded.
Further evaluation should be made of the effectiveness of different training methods, especially interactive e‐learning materials, in improving prescribing practice of postgraduate veterinarians.
Further research is needed into the specific elements required in an antimicrobial stewardship programme, for example, the need to address behavioural barriers that influence the veterinarian's prescribing and advisory skills.
Training should be provided to farmers to increase their awareness of (i) the association between antimicrobial use and the development of AMR, and its impacts on public health (ii) principles to reduce the need to use antimicrobials such as those promoted by EPRUMA.
Veterinarians should be encouraged to reinforce messages to the farming community on strategies to reduce antimicrobial use and on responsible use.
Antimicrobial stewardship is likely to be more successfully assimilated when education is introduced early, at the time when knowledge, attitude and behaviour of professionals are being shaped. It is therefore crucial to focus on an adapted undergraduate medical/professional curriculum that teaches the underlying theoretical principles that provide a sound knowledge base for the practical application in an antimicrobial stewardship programme: microbiology, infectious diseases and clinical pharmacology, with emphasis on the principles of prudent prescribing.
Increasing public awareness on AMR may help to address underlying barriers to change attitudes in the wider community on antimicrobial use; this may be supported through national level strategies.
In order to change attitudes and behaviours, education and awareness of AMR should be addressed to all levels of society including veterinarians, farmers and the public.
3.3.9. Taxes and other financial incentives
Vågsholm and Höjgård (2010) suggested that antimicrobial resistance is in economic terms a ‘negative externality’, or public cost that is not included in the price of antimicrobials. They suggested that if bacterial susceptibility is to be managed as a finite natural resource, then the incentive to use antimicrobials might be balanced by a tax based on the cost of the development of new substances. Hollis and Ahmed (2013) proposed that a ‘user fee’ should be placed on the use of antimicrobials on farms. It was thought that this would be easy to administer, deter farmers from low‐value use of antimicrobials and generate revenue that could be invested in the development of new antimicrobials. The O'Neill report (O'Neill, 2015) advised that taxation of antimicrobials would force farmers to pay for the societal cost of antimicrobial use and increase the economic incentive for them to use alternatives such as improved husbandry and vaccination. This report advised that the tax should be set at a level to stop unnecessary preventive use, but not the treatment of sick animals, and that it should be higher for antimicrobials that are likely to create resistance with greatest impact on human health.
In Denmark in 2013, a new risk management strategy was introduced which imposed differential taxes on medicines of 0.8% on simple penicillins, 5.5% on most other antimicrobial agents, 10.8% on CIAs but no tax on vaccines (DANMAP, 2015). The aim is to provide an incentive for the use of alternatives to antimicrobials, and where antimicrobial use is necessary, to encourage the choice in accordance with responsible use principles. The income from this tax (c. €1.1 M p.a.) contributes to the financing of responsible use policies.
In Belgium the development of the national data collection system (Sanitel‐Med) and the analyses of the data are financed by supplementary taxes on the sales of veterinary antimicrobials.
A study was conducted under the MINAPIG project to determine, among pig health experts from six EU countries, the ranking of alternative measures to the use of antimicrobial agents in terms of their feasibility, effectiveness and return on investment (Postma et al., 2015b). ‘Financial/tax’ (i.e. increased price of products, incentive/penalty) was ranked low in importance at 17 out of 19 alternatives, with internal biosecurity, vaccination, zinc/metals, feed quality and diagnostics/action plan as the top five.
Concluding remarks
Differential taxation of antimicrobials according to the importance of the class to public health has been used as an incentive to encourage responsible use, and to fund associated policies. This measure has low uptake among the EU MSs and its impact on disincentivising the use of CIAs is unknown.
3.3.10. Regulation of advertising of antimicrobial VMPs
The EU legislation specifies that the MSs shall prohibit the advertising to the general public of veterinary medicinal products that are available on veterinary prescription only; this includes all veterinary medicines intended for treatment of food‐producing animals and therefore antimicrobials. The EC PUAVM Guidelines advise that furthermore, advertising should be in line with the SPC, and highlight the risk of AMR and prudent use. Promotional campaigns which involve economic incentives for prescribers should be avoided.
This EU legislation has in general been implemented in national law in the MSs. Advertising by marketing authorisation holders can be distributed only to veterinarians for products that are ‘prescription‐only’. The French agency ANSES publishes a code of conduct for advertising in which a specific reference is made to antimicrobials (Point 3.1): each advertisement for an antimicrobial product shall mention that ‘this VMP is an antimicrobial and all use has an impact on development of antimicrobials resistance’. Furthermore the French association for the animal health industry (Syndicat de l'Industrie dumedicament et réactif Vétérinaires, SIMV) has put in place an advertisement observatory in order to self‐regulate its members’ use of advertising.
In the UK guidance provided by the VMD (VMGN No 4) states that promotion of antimicrobials should not encourage unnecessary use of these medicines and all advertising material should contain a strap line indicating that the prescription and use of the product should be in accordance with the responsible use of antimicrobials. The UK veterinary pharmaceutical industry body (NOAH) publishes a code of practice for the promotion of animal medicines which cautions against the offering of inducements to veterinarians in relation to antimicrobials and provides guidance on principles to be observed in relation to fluoroquinolones.
The impact of advertising by Marketing Authorisation holders on antimicrobial use in the veterinary sector has not been directly investigated; but it has to be acknowledged that its primary aim is to increase sales. Then again, a ban on advertising could reduce veterinarians’ awareness of the availability of different antimicrobial products, which will impede their ability to make fully informed prescribing decisions, and could encourage off‐label use.
Concluding remarks
Under EU legislation prescription, only veterinary medicines, including antimicrobials, may not be advertised to the general public.
In some countries, national competent authorities and pharmaceutical industry organisations provide further guidance to encourage promotion of responsible use within advertisements. Advertising is self‐regulated by the pharmaceutical industry in some EU MSs.
Recommendations
Pharmaceutical industry organisations and regulatory agencies should work together to provide guidance on advertising that informs veterinarians of the availability of antimicrobial products and give guidance on how they can be used responsibly.
A ban on advertising could reduce veterinarians’ awareness of the availability of different antimicrobial products, which will impede their ability to make fully informed prescribing decisions.
3.4. Measures taken by food producers and retailers
Certification schemes are often in place between farmers and retailers to provide the consumer with quality criteria, including, in some cases, reduced use of antimicrobials during the rearing of food‐producing animals. These quality criteria are defined by specific regulations, for example for organic foods, or result from private schemes between the food producers or retailers and farmers. These measures are aimed to provide the consumers with food of a quality standard that may be greater than the minimum requirement as defined by the EU legislation. The heightened concern and media attention associated with the possible relationship between the use of antimicrobials in animal production and the selection and spread of AMR has led to a number of consumer and lobbyist organisations pushing for an end to the use of antimicrobials in livestock for growth promotion (outside the EU where this is still allowed) and disease prevention or metaphylactic therapy where husbandry‐based alternatives to such regular treatments are possible.
This section presents examples of measures taken by stakeholders within the food sectors, other than farmers, breeders and competent authorities, to reduce the use of antimicrobials in animal husbandry, and is not intended to be a comprehensive list. Information from a questionnaire sent to selected stakeholders was also included (see Section 2.1.4 and Appendix E).
3.4.1. Organic and ‘antibiotic‐free’ production
Food animal product marketing based on antimicrobial use is a hot topic in the USA and is beginning to take off in some other countries, including some European countries. In this context, ‘antibiotic‐free’ and ‘organic’ farming practices are of interest with view to the content of this scientific opinion. In the EU, the general principles for organic livestock production are laid down by Regulations (EC) No 834/200756 and (EC) No 889/200857. According to these regulations, organic livestock farming should respect high animal welfare standards and meet animals’ species‐specific behavioural needs while animal health management should be based on disease prevention. This goal can be reached by selecting the appropriate breed and strain, choosing the appropriate stocking density, adequate housing and good hygienic conditions. The use of chemically synthesised allopathic veterinary medicinal products or antimicrobials is prohibited for any preventive treatments, with the exception of vaccination. These regulations establish that, when the animals are ill or injured, non‐chemically synthesised products and mineral trace elements shall be preferentially used. If these compounds are not effective in combating illness, chemically synthesised allopathic veterinary medicinal products or antimicrobials may be used under the responsibility of a veterinarian. The impact of organic farming practices on the use of antimicrobials and AMR is further discussed in Section 3.2.2.
Sales of chicken in the USA labelled as ‘antibiotic‐free’ rose by 34% in 2013–2014, driven by public concerns about antimicrobial use in food animal production. The term ‘antibiotic‐free’ is used in many different ways, ranging from non‐use of growth promoters in those countries that still allow this, to non‐use of specific listed antimicrobials, to non‐use in individual identified animals, even though other animals in the group or farm may have been treated, to non‐use in whole batches of animals. In the latter case, any use of therapeutic antimicrobials would lead to diversion of the product from the whole group to the non‐premium ‘standard’ or low cost markets (Saitone et al., 2015).
The ‘antibiotic‐free’ market is different from organic in that the feeding and production systems used may be standard and conventional, without outdoor access or other non‐medication related requirements and associated risks and benefits of organic production (Van Loo et al., 2012; Karreman and Fulwider, 2015; Salaheen et al., 2015) but with their own associated stress factors, which need to be effectively controlled (Komalova, 2013).
There is conflicting information on benefits in terms of public health associated with ‘antibiotic‐free production’ (Susick et al., 2012; Van Hoorebeke et al., 2012; Smith et al., 2013; Sanad et al., 2016), mainly resulting from background variability in prevalence of resistant organisms, types of resistance and multiple resistance and occurrence and/or clonal dissemination of some organisms, e.g. monophasic S. Typhimurium in pigs, independent of antimicrobial use (Rothrock et al., 2016). The strongest evidence of a protective association with ‘antibiotic‐free’ production may be in occurrence of LA‐MRSA in pig herds (Rinsky et al., 2013; Smith et al., 2013).
3.4.2. Retailer, producer and industry initiatives
Private, and usually confidential, agreements on food quality and safety between producers/retailers and farmers that are properly applied may be more efficient than legislative enforcements, as highlighted by the response of stakeholders to the questionnaire. Thus, food producers may sometimes require more stringent standards than the current legislative measures on the use of antimicrobials in animal husbandry. Most of these schemes are targeted at a reduction of antimicrobial residues in the product, rather than at a reduction of drug‐resistant bacteria/genes in the product. In the case of dairy industries, criteria on residues of antimicrobials are in place and each batch of milk is usually tested before its use. This is important for technological reasons, since the presence of compounds with antimicrobial activity interferes with the bacterial cultures used in dairy fermentations, e.g. for cheese or yoghurt. A second example involves certification schemes for monitoring antimicrobial use in pigs, poultry and fattening calves, based on systematic recording of antimicrobial use or deliveries recorded in the database of the certification body (QA Germany).
Some multinational food manufacturers, retailers and restaurant chains have recently announced new policies to serve meat from animals grown without use of medically important antimicrobials. This has led to a pressure on animal farming companies, mainly chicken producers, to rear the majority of animals for such influential customers (e.g. major supermarket chains) without supplementation by antimicrobials important to human medicine, and with antimicrobials use limited to treating clinical disease outbreaks.
Some animal farming companies or farm cooperatives use their labelling to communicate about products obtained without, or with limited use of, antimicrobials.
EPRUMA, a multistakeholder platform that includes several European organisations representing all the stakeholders of the animal health sector (e.g. veterinarians, farmers, food‐producing animal medicines manufacturers, professionals). Its goal is to promote the responsible use of medicines in animals in the EU, with particular attention to veterinary antimicrobials. As an example, RUMA, the UK associate partner of EPRUMA, as a consequence of publication of data on transferrable colistin resistance in E. coli and Salmonella spp., has proposed to voluntarily restrict the use of colistin in livestock.
Some agricultural allied industry and professional organisations contribute to reduction in the use of antimicrobials in livestock; for instance in Belgium, the professional association of compound feed manufactures increased their efforts to minimise antimicrobial consumption in specific target animal production sectors. Belgian compound feed manufacturers no longer accept prescriptions for antimicrobial medicated feed for pigs older than 15 weeks.
3.4.3. Consumer organisations
Because of the increased awareness of the risk posed by the use of antimicrobials in food animal production various organisations that represent consumer of products from the agricultural industry are pushing for stronger measures to end use of antimicrobials for routine disease prevention. An example is the ‘Recommendations to reduce the use of antibiotics in farm animals’,58 issued in 2014 by Consumers International. This document, supporting the conclusion of the Strategic and Technical Advisory Group on Antimicrobial Resistance of WHO, recommends action at governmental level to reduce the use of antimicrobials in animal production, by establishing or more effective enforcement of regulatory frameworks, strengthening surveillance and improving health management in food animal production systems. This consumer organisation aims its message at multinational food manufacturers, retailers and restaurant chains, recommending that they use their global purchasing power to encourage reduction of antimicrobial use through better animal husbandry. The organisation advises consumers to refrain from using meat that has been produced from animals that have received antimicrobials for growth promotion or routine disease prevention.
Concluding remarks
Media and consumer attention are powerful tools for motivating change, as these can exert economic pressure on producers via market expectations or requirements.
Recommendations
Since the meaning of ‘antibiotic‐free’ food of animal origin is not clear and varies according to different requirements in different countries, a harmonised definition of ‘antibiotic‐free’ food of animal origin (meat, milk, eggs and fish) should be made at the EU level. This quality criterion should be based on recognised standards and on transparent reporting.
4. Assessment of measures to reduce the need for antimicrobials in animal husbandry in the EU and their potential impact on AMR in bacteria from food‐producing animals and food (ToRs 3 and 4)
4.1. Introduction
In March 2016, the European Parliament and the Council adopted a new Regulation on transmissible animal diseases (‘Animal Health Law’). The purpose of the new Regulation is to lay down rules for the prevention and control of animal diseases that are transmissible to other animals or to people. This law establishes the first ever link between animal welfare and public health in EU law, and will be an important tool for fighting antimicrobial resistance in humans, animals and the environment.
Previously the Animal Health Strategy 2007–2013 was built upon the concept of ‘Prevention is better than cure’. The new legislation puts greater emphasis on the prevention of disease problems, requiring farmers and other animal owners and traders to apply the principles of good animal husbandry and to adopt a prudent and responsible approach to the use of veterinary medicines, such as antimicrobial agents.
As annex to the Regulation, the European Parliament, EC and European Council issued a joint statement calling on EU MSs to collect ‘relevant, comparable and sufficiently detailed data’ on the actual use of antimicrobial medicinal products in animals and to send these data to the EC, which should then publish them regularly.
The use of antimicrobials is greatest at critical stages of an animal's life, during periods when animals may have lower resistance to infections (for instance when immature animals first meet a disease challenge) and/or when they experience a higher exposure to microbes. These critical phases are influenced by the economics of the farming system, and by practical requirements in certain systems where large groups of young pigs, poultry, calves, sheep are introduced into the farm at particular times of the farming year cycle. In many cases, antimicrobials are used as a preventive option providing support to animals through the critical first exposure to specific pathogens. For example, diarrhoea at weaning in piglets, and mortality during the first week as a result of yolk sac infection in chicks.
Efficient and minimal utilisation of antimicrobials is the main purpose of the EC PUAVM Guidelines, with additional focus on potential adaptations of farming systems to improve these critical periods of life for the animals, as a means of improving animal health and welfare conditions thereby decreasing the need for antimicrobials.
Working on such a question is a difficult task for different reasons. Multiple parameters of a farming system are strongly tied to each other with a high interdependency between factors and influences ‐ one cannot move or delay one factor without adapting or changing others. An integrated approach is needed, with particular attention to disease prevention. Furthermore, the multiple influences and parameters refer to a wide range of different disciplines and expertises, including livestock production systems, including the ancillary areas such as housing, feeding, etc., systems; livestock diseases (infectious, production), veterinary epidemiology (understanding of disease transmission and risk); disease control under farm conditions (biosecurity, including bioexclusion and biocontainment); animal welfare and social sciences. Such complementary expertise is essential, for each species/farming system where antimicrobial use is significant, to ensure that the outputs are robust, specific and practical under commercial farming conditions.
In a study by Postma et al. (2015b), nineteen alternatives to using antimicrobial agents were ranked on perceived effectiveness, feasibility and return on investment by 111 pig health experts from Belgium, Denmark, France, Germany, Sweden and Switzerland. The top five measures in terms of perceived effectiveness were: (i) improved internal biosecurity; (ii) improved external biosecurity; (iii) improved climate/environmental conditions; (iv) high health/specific pathogen free (SPF)/disease eradication; and v) increased vaccination. Although eradication and SPF methods are perceived as being effective (Wierup, 2012), they are less popular than other less costly measures, despite providing a greater long‐term benefit (WHO, 1990; Lund et al., 2012; Alban et al., 2013; Wielinga et al., 2014; Postma et al., 2015b).
Potential strategies may not only be evaluated as regards their effectiveness in improving the health situation and therefore reducing the need for antimicrobial use, but also scrutinised as to which extent they affect the animals’ welfare state. For example, restricting the access to outdoor areas for external biosecurity reasons may at the same time impair opportunities to perform the species‐specific behaviour repertoire.
4.2. Animal management and husbandry procedures to reduce the need for antimicrobials in livestock production systems
This section of the Opinion focuses on prevention of disease as a tool to reduce the need for, and the use of, antimicrobials. Many different approaches, including farmer priorities, farm environment, and multiple integrated strategies will generally be required.
The need to use antimicrobials can be reduced dramatically through the application of good farm management and husbandry practices for terrestrial and aquatic animals. Roughly, these can be divided into three main categories, including practices:
to reduce the introduction and spread of microorganisms between farms (primary prevention);
to reduce their transmission or spread within a farm (secondary prevention); and
to increase the ability of animals to cope with these pathogens (tertiary prevention).
The chapter has been structured following these three categories. Eradication of an existing pathogen and associated disease from a herd, a region or a country would qualify as a means of reducing the need for medication of infected hosts, and is covered separately at the end of Section 4.2.1 (primary prevention) because it is an overarching disease management strategy involving many different actors/agencies. Herd health plans are important measures to reduce antimicrobial use and involve integrated aspects related to disease prevention and monitoring. A separate section on herd health plans is included after the section on tertiary prevention. Additional considerations on husbandry and management procedures are presented such as the need to rethink those particular farming systems which place much reliance on antimicrobial use, the need to raise farmer awareness on AMR issues and examples from successful countries.
4.2.1. Primary prevention (national, regional, local, farm level)
Biosecurity means ‘the sum of management and physical measures designed to reduce the risk of the introduction, development and spread of diseases to, from and within an animal population or an establishment zone, compartment, means of transport, premises or location’ (Regulation (EU) No 2016/42959) and ‘a set of preventive measures designed to reduce the risk of transmission of infectious diseases in crops and livestock, quarantined pests, invasive alien species, and living modified organisms’ (Koblentz, 2010).
External biosecurity (or bioexclusion) pertains to introduction of the pathogen to a population, and internal biosecurity (or biocontainment) centres on reducing the spread within a herd/flock.
4.2.1.1. External biosecurity, including introduction of animals
Most biosecurity measures are considered at the individual farm level, with the farm owner or manager being responsible for the planning, implementation and monitoring of the chosen measures. For epidemic notifiable infections, the competent authority is in charge of the necessary biosecurity measures nationally and internationally, and outbreak measures will include export bans and protection of all national clean farms from introduction of the epidemic agent, through quarantine, zoning, movement restrictions, surveillance, etc. around the infected farms. National biosecurity measures include import regulations and control of animals and animal products by the competent authority, as well as inspection, surveillance and control of markets, slaughterhouses, etc.
There are examples where industry has taken responsibility for national biosecurity measures. For instance, in Denmark the industry has taken on biosecurity tasks at the national level, by requesting washing and disinfection of lorries entering Denmark after having transported live animals to farms, slaughterhouses, etc., abroad. This would help protect Danish farms not only against introduction of epidemic infections such as African swine fever (ASF), classical swine fever (CSF) and foot‐and‐mouth disease (FMD), but also aims at protecting against introduction of infections endemic elsewhere but foreign to Denmark, such as porcine epidemic diarrhoea (PED). Introduction of a pathogen into a herd/flock can occur either via introduction of animals or by contact between animals and an external source of the pathogen such as wildlife at pasture (or elsewhere), contaminated area or fomites (including contaminated pens, outdoor areas, or utensils), a contaminated feed supply, water litter, enrichment materials and utilities for animal production, visitors etc. External biosecurity is thus any measure taken to prevent these opportunities for pathogen introduction. The risk of introduction may be relatively pathogen‐specific, such as introduction of Salmonella by contaminated feed, and most pathogens can be introduced by introduction of animals (Houe et al., 1996; Maes et al., 2000; Nielsen and Toft, 2011; Bae et al., 2013). Closed farming generally reduces transmission between herds (van Schaik et al., 1998). Although each specific pathogen calls for specific actions, the required measures are usually from a common pool. These are measures to reduce introduction of animals, contact with animals in other herds, contact with wildlife, environment and vermin, contact with visitors, pathogen occurrence in feed, semen, and with any utilities used on the farm, cleaning and disinfection of pens and outdoor places.
Biosecurity recommendations vary between animal species (Moore et al., 2008). Although introduction of live animals is a major source of infection, thorough cleaning and disinfection of animal transport vehicles is also important. This is especially important when eradicating infections where disease free replacement animals is a prerequisite for success (Mee et al., 2012; More et al., 2015).
Transport, mixing and introduction of animals is associated with an increased external biosecurity risk. This risk can be reduced through the following measures:
minimising the introduction of animals; if animals are to be introduced, minimising the number of herds from which they originate;
routine cleaning and disinfection of transport vehicles (trucks, trailers, boats, containers);
reduced mixing of animals from different batches;
isolation of animals found to be sick or diseased during transport or in a market. Further, in‐contact animals should be isolated until it has been clarified whether the sick or diseased animals were infectious. Human operators should not leave an animal area without changing boots or without thorough cleansing and disinfecting of boots. In‐contact clothing should be changed if possible;
when movement and other controls are placed by government following an outbreak of diseases, these movement restrictions should be rigorously implemented to protect the health of non‐diseased animals (and humans);
live animal distribution hubs, such as markets, offer the opportunity for pathogen transmission between animals from different farms. For this reason, these hubs should be avoided where possible (Marquetoux et al., 2016).
As a basic principle, if purchase cannot be avoided, the purchaser should have a sound understanding of the health status of the farm from which animals are being purchased. Further, farmers should have an understanding of the health status of neighbouring farms. Realistically, this information is only available in countries with robust programmes for disease control/eradication where objective health status can be assigned to individual farms. To protect the health status of the purchasing farm, the health status of the selling farm with respect to pathogens of interest should be at least equivalent or better. This approach is consistent with our knowledge of the importance of introduced animals in the spread of pathogens between farms. This requires independent, objective assessment of farm health status, and, in non‐regulatory programmes, a willingness among vendors to share such information with prospective purchasers. Appropriate public access to farm health status should be considered.
Farmers can reduce the chance of pathogen introduction by sourcing their young animals from a minimum number of rearing farms. For veal calves, this principle is more difficult to apply, as young calves of the same age generally originate from a number of dairy farms, often involving long distance transport (Dorado‐García et al., 2015b; Roelvink, 2015; Lava et al., 2016a). The use of litter, enrichment materials or feed ingredients originating from other farms increases the risk of disease introduction.
Contaminated surface water (for example, drinking water from ponds, rivers, puddles, uncovered troughs, surface water contaminated with water from human sewerage plants) can represent a potential risk of both primary infection (for example from water contaminated by waterfowl, conspecific faeces) or of transmission between animals within the same herd (for example transmission of bacterial infections within herds) (Collado et al., 2008; Çelik and Ünver, 2015). Provision of clean drinking water for animals which are housed may be possible, but for animals at pasture – particularly when on extensive pasture in open country, water will often be sourced from streams and rivers, waterholes or ponds – and control of the bacteriological quality of these sources of water can be difficult (Suthar et al., 2009).
Housing is an important part of ‘integrated biosecurity’ and disease prevention (Hinchliffe and Ward, 2014). Farm animal buildings should be designed in such a way that they minimise the influx of pathogens from outside. There are three main areas through which prevention could be supported.
Firstly, wild or pest animals should be kept out of the premises and away from livestock. This can be achieved through good initial design, and by effective maintenance of doors, walls, screens, meshes, drain covers and all measures designed to prevent access of animals (Laanen et al., 2013).
Secondly, it is important to have purpose designed biosecurity barriers for human access. This can include, if appropriate, boot changing facilities, foot dips, hand washes, sinks for human hygiene, separate toilet and washing facilities to support worker hygiene, separation of canteens or changing areas for staff to reduce risks of contamination, visitor book a and visitor exclusion arrangements (Chowdhury et al., 2012).
Finally, dust and pathogen laden air is exhausted from the building and should, where possible, be filtered, to reduce risk to transmission outside of the farm building (Anthony et al., 2014; Sanz et al., 2015).
Concluding remarks
The major route of pathogen introduction onto a farm is through contact with an infected animal, during transport and mixing or following introduction. Other routes of introduction are also important for some infectious agents.
Strategies are available to limit biosecurity risks during animal recruitment, and during the movement of people.
Housing, and effective management of hygiene and biosecurity in housed animals, is a critical component of primary prevention strategies.
Recommendations
Efforts are needed to maximise the application of primary methods of disease prevention, nationally, regionally and on‐farm.
Minimise the number of introduced live animals and, if animals are introduced, minimise the number of herds from which animals are introduced.
External biosecurity measures should focus on measures to reduce each route of transmission, both direct (e.g. animal‐to‐animal) or indirect (e.g. introduction via contaminated feed).
Farmers should have a sound understanding of the health status of farms from which animals are being purchased or of neighbouring farms, with respect to specific pathogens, to enable them to make sound management decisions. This information is available in countries with robust programmes for disease control/eradication where objective health status can be assigned to individual farms.
Distribution hubs for live animals should be avoided, where possible and the number of separate sources for replacement animals should be minimised.
Housing systems should be designed, built and maintained so to minimise the entry of pathogens.
4.2.1.2. Compartmentalisation (including internal trading, SPF systems)
As defined by the OIE, compartmentalisation is a procedure to establish subpopulations of distinct health status based on management and biosecurity factors. Using this approach, animals within a compartment should be contained in one or more establishments under a common biosecurity management system (OIE, 2012).
SPF is one example of compartmentalisation, and is used extensively, particularly in pig and chicken production. The SPF approach has been facilitated by the knowledge that the disease transmission potential of washed embryos is much less than that of either semen or live animals (Singh, 1987). In 1996, the first SPF nucleus pig herd was established by hysterectomy in Norway. Since 1997, more than thirty new SPF herds have been established with gilts recruited from the first SPF nucleus herd or from the two SPF nucleus herds established from the first one and maintained as closed herds. This closed SPF health and breeding pyramid is kept free from important animal diseases such as sarcoptic mange, swine dysentery and enzootic pneumonia, as well as zoonotic infections such as Salmonella spp. Yersinia enterocolitica and Campylobacter spp. (Nesbakken, 2012; Kolstoe et al., 2015). It is considered likely that the improved management and hygiene standards applied to pig farms in order to maintain SPF status and to control Salmonella infections will have had a beneficial impact on other infectious diseases (Wegener et al., 2003; Goldbach and Alban, 2006; Kolstoe et al., 2015).
Since 1971, the Danish pig industry has organised an extensive SPF system for pig farms, which is claimed to be the world's most comprehensive health programme for pigs. Today, more than 70% of Danish sows are housed in SPF production herds, while 98% of the sows in nucleus and multiplier herds are covered by the SPF system.60 The Danish SPF system includes a publicly available declaration of health status for individual herds, which allows pig buyers to plan their purchases to match the health status of their own herds for a series of swine pathogens: lice, mange, swine dysentery, atrophic rhinitis, pleuropneumonia (Serotype 1–10 and 12), Enzootic pneumonia (Mycoplasma) and PRRS (EU and US strains). Infections of public health relevance are also included: Salmonella spp., Yersinia spp., and E. coli (Oedema types). Comparisons of the occurrence of endemic porcine infections among Danish pigs from SPF herds and conventional herds have been published (Thomsen et al., 1992; Enøe et al., 2002; Zhuang et al., 2007). The system has proved its efficacy; less medication is needed and vaccination costs are reduced (ten Hooven, 2007).
Concluding remarks
The SPF system can be cost‐effective: less medication is needed and vaccination costs are reduced. It does depend on strict biosecurity and a closed herd policy or strict sourcing and transportation from controlled herds with similar health status. The impact of the SPF system will be greatest in farms towards the top of the breeding pyramid (for example, grandparent and parent broiler stock).
Recommendations
When seeking to minimise antimicrobial use, the concept of compartmentalisation, including SPF systems, could be more widely adopted.
4.2.1.3. Eradication
The 1997 Dahlem Workshop on the Eradication of Infectious Diseases, addressed four questions: (1) how is eradication to be defined and what are the biological criteria? (2) what are the criteria for estimating the cost and benefits of disease eradication? (3) what are the societal and political criteria for eradication? and (4) when and how should eradication programmes be implemented? (Dowdle, 1998). In most cases, ‘eradication’ relates to elimination of a pathogen from a holding, integrated food‐producing animal company or region, and cost is the major limitation.
The only disease to be successfully eradicated globally in animals, with the assistance of an attenuated vaccine, is Rinderpest, which the FAO declared eradicated on 14 October 2010. National eradication measures have been successfully applied for numerous other notifiable diseases where there is a strong regulatory incentive and usually state funding for control actions or compensation but without achieving total biological extinction of the organisms worldwide (Sutmoller et al., 2003; Pappas et al., 2006; Mariner et al., 2012).
Australia completely eradicated contagious bovine pleuropneumonia (CBPP) in 1973, using vaccination followed by stamping out,61 bovine brucellosis in 1989 using vaccination coupled with strict biosecurity measures, movement controls and repeated testing, and bovine tuberculosis in 1997 (More et al., 2015) by test and cull methods incorporating biosecurity, movement controls and elimination of non‐native wild maintenance hosts of tuberculosis. All three are notifiable diseases according to OIE. Australia has a number of advantages when it comes to successful disease eradication in that it relatively recently commenced farming livestock and it is a geographically isolated island where strict biosecurity arrangements can be maintained under control of a single government. In other countries CBBP remains a problem because of the frequent occurrence of subacute or subclinical infections, the persistence of chronic carriers after the clinical phase and the lack of extensive vaccine coverage. In addition, the absence of confirmatory diagnosis may lead to antimicrobial treatments being used in the case of CBPP outbreaks. In the case of bovine brucellosis, several countries in Northern and Central Europe, Canada, Japan, Australia and New Zealand are believed to be free from the agent62 for which a number of vaccines are available to aid eradication. Many other countries have reduced the tuberculosis prevalence of infected herds, but reservoirs of tuberculosis in wild‐living animals including bison and Elk in Canada, possum in New Zealand, badgers in the UK, France and Ireland, wild boar in Spain, deer in the USA, Germany, Austria, France and elsewhere appear to have hindered total eradication especially where societal acceptance of culling tuberculosis‐infected wild populations is not the predominant view.63 The different success rates for eradication programmes in different countries and with different diseases reflect the varying epidemiological features and constraints to eradication that may exist in different regions.
Fundamentally it is only in situations where biological extinction of the causative organism has been attained that intervention measures can cease entirely in the aftermath of an eradication programme therefore continued measures are necessary to instigate surveillance, prevent re‐emergence and re‐establishment of transmission (Dowdle, 1998).
To consider disease eradication as a possible control measure so as to reduce the use of antimicrobials the condition must either directly or indirectly be responsible for increased use of antimicrobials. In such cases, the eradication of a clinically relevant or production‐limiting disease will have economic benefits (Sasaki et al., 2015) and remove the need for medication to control such diseases. Medication is never totally successful in terms of microbiological cure or avoiding production losses and leads to a risk of selection for increasing resistance. This is followed by increased use of medication in terms of dose rate and time of administration and replacement of simple antimicrobials with more potent agents or combinations. To be a candidate for an eradication programme, the disease or condition needs to fulfil a number of criteria. Firstly sufficient information on the life cycle and transmission dynamics of the condition must be available. An effective, efficient and practical intervention (e.g. a vaccine) should likewise be available together with practical, sufficiently robust diagnostic tools with satisfactory sensitivity and specificity to interrupt the transmission pathways of the agent. Preferably there would be only one maintenance host unless all such hosts can be targeted with effective intervention tools and the disease cannot amplify in the environment. In addition, the level of societal and political commitment to the eradication measures are hugely important. The use of vaccination programmes or antimicrobial treatment before the introduction of an eradication campaign can help reduce the susceptible population (Dieste‐Pérez et al., 2016) and so‐called ‘marker’ vaccines, together with a companion Differentiating Infected from Vaccinated Animals (DIVA) test can be particularly supportive.
Certain fundamental principles need to be fulfilled in the preparatory stages and prior to the design for an eradication programme, such as the need for a thorough evaluation of the epidemiological situation in the region/country or area of focus (including identifying and controlling other relevant domestic and wild reservoirs), to minimise the risk of reintroduction of infection, correct choice of the relevant epidemiological unit, e.g. a largely closed integrated poultry company or closed primary pig breeding herds, and sound epidemiological parameters. Effective control of the disease may be essential as a preliminary step towards eradication with the intermediate target to attain a rapid decrease in the prevalence of infection. The authorities and other players involved in the programme need to be clear on their respective responsibilities and an appropriate legislative framework for the programme must be established together with an administrative team with sufficient and proportionate financial resources for the running of the programme. Training at the appropriate level is mandatory for all relevant parties with adequate time devoted to a quality assured programme to provide advice and guidance as necessary following an awareness campaign targeted at farmer participants. For a successful eradication programme, it is essential that all involved stakeholders clearly understand the tasks and duties involved, which therefore need to be clearly defined, and that they actively commit and contribute to the full implementation of all the measures of the programme.64
It is particularly useful if there are successful models of eradication in local or regional areas on which to base a wider eradication programme and from which to learn.
Some examples of successful approaches to eradication of infectious disease in food‐producing animals are provided below.
Pigs
Aujeszky's Disease has been successfully eradicated in a number of countries, e.g. the USA, Canada, New Zealand and within the EU in Denmark (which assisted neighbouring regions), Germany, Ireland, Sweden and others, most often using vaccination initially to reduce infection pressure followed by containment of herds that remained infected, and a DIVA Elisa to identify naturally but latently infected animals for removal65 (Müller et al., 2003; Pejsak and Truszczynski, 2006; SVA, 2016). The epidemiological and economical effectiveness of this methodology for regional eradication was documented in 1990–1996 by an EU‐supported project, involving regions in Denmark, Germany and parts of the Netherlands. The vaccination/test‐and‐removal programmes, which were carried out in northern Germany and southern parts of the Netherlands, were the first ever area‐wide attempts to eradicate the virus from large populations of endemically infected swine herds with the use of gene‐deleted vaccines (Willeberg et al., 1996).
Eradication of Mycoplasma pneumonia infection at herd level based on age‐segregation and medication of pigs is possible, but there is a permanent risk of reinfection (Maes et al., 2008). In one study of the potential for regional eradication of infection, all sow herds in a region were screened for M. hyopneumoniae. The ultimate goal was to eradicate M. hyopneumoniae from all member herds of a cooperative slaughterhouse (153 breeding herds, 85 farrow‐to‐finish herds and 150 specialised finishing herds). In addition to the results of testing herds, a decreasing prevalence of lung lesions at slaughter (from 5.2% to 0.1%) and lack of clinical breakdowns indicated that all member herds were finally free from M. hyopneumoniae by the end of the 3‐year eradication programme (Rautiainen et al., 2001). Medicated early weaning can be used to assist this process (Meszaros et al., 1985).
Eradication measures taken to maintain national freedom from PRRS in Sweden included stamping out, cleaning, disinfection and an empty period of 3 weeks before the herds were repopulated. This successful programme demonstrates the importance of early action after incursion of a pathogen into a region that was previously free of disease (Carlsson et al., 2009).
A programme to control atrophic rhinitis was initiated in Sweden in 1995. Nucleus and multiplying herds are screened and positive herds are cleared from infection. Since 2005, there have been no positive herds (SVA, 2016).
A programme of swine dysentery eradication, including introduction of a strict biosecurity regime to prevent transmission and focus on effective treatment of disease carriers to eliminate the spread of the organism, combined with thorough cleaning and disinfecting of all surrounding areas to prevent reinfection has been successful, without depopulating the whole farm, when carefully conducted (Fossi et al., 2001; Stojanov et al., 2015) or when medicated early weaning can be used (Meszaros et al., 1985). It is debateable whether eradication programmes that involve prolonged mass treatment of animals with antimicrobials are acceptable, especially as some strains of B. hyodysenteriae that are resistant to all permitted therapeutic options are emerging and depopulation‐repopulation options are available (Aarestrup et al., 2008).
Poultry
Although poultry are usually managed on an all‐in‐all‐out basis, primary breeding holdings may involve continuous production and breeding of replacements from infected stock. Eradication of various bacterial infections from the progeny of such birds has been attempted by injection or dipping of eggs with antimicrobials such as enrofloxacin or gentamicin has historically been carried out (McCapes et al., 1975; Ekperigin et al., 1983). Mycoplasma synoviae is a common respiratory pathogen in the poultry industry. Eradication of M. synoviae from broiler breeder flocks is important for reducing economic losses caused by M. synoviae‐associated diseases on broiler farms. Intensive and prolonged medication with antimicrobials, such as tilmicosin, chlortetracycline, doxycycline or enrofloxacin, has been used to eradicate M. synoviae from poultry breeding farms (Hong et al., 2015). The relative risks associated with use of intensive medication to assist elimination of pathogens compared with a permanent requirement for medication of resident pathogens have not been defined.
Eradication of Salmonella Gallinarum/Pullorum has been successfully achieved in many countries by repeated serological testing and removing reactors, plus eliminating vectors (rodents, red mites, etc.) (Barrow and Neto, 2011; Kozdruń et al., 2015). More recently, eradication programmes for zoonotic Salmonella serovars, particularly S. Enteritidis and S. Typhimurium, have been introduced in the EU and are now a statutory requirement for chicken and turkey breeding flocks (Regulation (EC) No 2160/200366). Prior to this, eradication by stamping out, or attempts at eradication by medication were more variable in different countries (McIlroy et al., 1989; Edel, 1994; Reynolds et al., 1997). Medication is no longer permitted for routine control of subclinical carriage of Salmonella spp. in the EU, but it is permitted in the case of infection in breeding flocks where culling would result in the loss of rare genetic material.
Cattle
Brucellosis in cattle has largely been eradicated from most countries in the EU using test and cull methods with or without the use of vaccination at various stages of the eradication programme. The disease has also been successfully eradicated in other areas outside the EU including the USA.
Eradication strategies, involving removal of carrier animals or depopulation/repopulation on a local or national scale with or without movement controls for major production limiting cattle diseases such as bovine viral diarrhoea (BVD), enzootic bovine leucosis (EBL), IBR and neosporosis can be successful, but depending on the disease, the extent and success of the eradication programme continued freedom from disease is dependent on cost‐effective checks before new animals enter the herd, good biosecurity and effective vaccination programmes (Lindberg and Alenius, 1999; Fray et al., 2000; Chi et al., 2002; Ackermann and Engels, 2006; Makoschey and Bielsa, 2007; Nuotio et al., 2007; Heffernan et al., 2008; Barrett et al., 2011; Stott et al., 2012; Schirrmeier, 2014). The processes and principles behind the establishment of control and eradication programmes on BVD, S. Dublin and paratuberculosis in Denmark have been outlined by Houe et al. (2014).
BVD virus is one of the major pathogens of importance for the bovine respiratory disease complex in feedlot/fattening cattle and in other intensive management systems such as calf rearing units (Campbell, 2004; Lovaas, 2006). BVD virus causes early immune suppression with the severity of disease varying with both virus strain and the involvement of secondary pathogens, both viral and bacterial, to which BVD‐infected animals will be more predisposed (Brownlie, 1990). Control of BVD, whether by vaccination or by eradication (Laureyns et al., 2010), should assist to prevent immune suppression and thereby, maintain an effective immune system to reduce the incidence of respiratory and digestive disorders that have required antimicrobial control particularly when calves or fattening stock come together from multiple sources such as occurs in veal production and in beef‐fattening units both of which production systems are significant users of antimicrobials.
The first eradication programmes in Europe were introduced during the 1990s in the Scandinavian countries based on the identification and elimination of persistently infected animals. These programmes have been very successful, and all of the Scandinavian countries are either free, or almost free from BVD virus. Today control programmes are underway in several European countries (Ståhl and Alenius, 2012).
Disadvantages of eradication to the cattle industry include cost of the eradication programme including the necessity to diagnose persistently infected animals and to kill them as soon after birth as possible. Once eradicated, continued surveillance for BVD virus and biosecurity to prevent ingress is necessary.
Concluding remarks
Eradication of endemic pathogens can reduce both the disease burden and the need for regular antimicrobial treatments.
Eradication of such specific conditions from breeding herds of food‐producing animals predisposing directly or indirectly for antimicrobial therapy is possible but can be costly in the short term. Ultimately, once eradication has been achieved, the cost‐benefit can be considerable.
Eradication is most easily achieved in poultry production systems, as ‘all‐in‐all‐out’ production facilitates a clean break between flocks.
Stamping out of infected herds/flocks is usually the most reliable method if the disease is newly introduced and regional herd prevalence is low. In specific cases, depending on key aspects of the infection, including its epidemiology, medication combined with vaccination, DIVA tests and/or selective removal of individual infected carrier animals may be an effective control option.
Eradication is unlikely to be successful in the long term if the disease is endemic and widespread or there is a need to source replacement animals from herds that may not be free of disease.
For diseases where the risk of transmission between herds s high, control/eradication should preferably be done on an area/region/country‐level.
Recommendations
More holistic research is needed to determine the cost‐effectiveness of disease eradication programmes both in terms of reduced animal and zoonotic disease and in terms of direct or indirect reduction of the use of antimicrobials and consequent development of AMR.
Blueprints should be developed for eradication of major endemic diseases of animals from herds or animal populations.
Lessons should be learned from those EU regions where a range of diseases have been successfully eradicated, to identify reasons for success.
Viral infections also should be targeted for eradication, although antimicrobial treatment in these situations may primarily be aimed at controlling secondary infections.
4.2.2. Secondary prevention (once a disease is present on the farm)
4.2.2.1. Internal biosecurity, reducing transmission within a farm
Once pathogens have been introduced, measures to eliminate or reduce transmission and spread within a herd or flock can impact production efficiency as well as disease occurrence. Internal biosecurity (or biocontainment) measures may also be pathogen‐ and species‐dependent, while the modes of transmission dictate the relevant measures to prevent transmission, which are commonly poorly applied compared to external biosecurity measures (Kruse et al., 2016; Mankad, 2016). Stocking density has an impact on disease occurrence of several pathogens (Maes et al., 2000), and segregation is effective against many pathogens. The type of segregation is pathogen dependent. For example, only a few viruses may be needed to establish an infection in a susceptible animal, whereas much more bacteria are needed for some infections. Because of differences between pathogens, their survival times, transmission pathways and infectious doses, on‐farm microbiological risk evaluations are becoming common tools to deal with a variety of infections (Rossiter et al., 1999; Nielsen and Nielsen, 2012; McAloon et al., 2015). Although several of these are related to infections triggering limited, if any, antimicrobial use, applications of on‐farm risk assessment and risk management are becoming more prevalent and can be further developed for a number of infections of high prevalence and associated with high antimicrobial consumption.
Diagnosis of specific pathogens is pivotal to the control of many infections, whereas it is used to a much lower extent for others. This is because faster or more accurate diagnosis at herd/flock or individual level can direct antimicrobial treatments in an appropriate direction (Pedersen et al., 2015). There are several issues to consider:
What is the purpose of the diagnosis? Is it to study or reduce spread of a pathogen? Is it to identify individuals or groups of individuals that may become diseased to prevent disease? Is it to treat the individual that is already suffering from disease? Is it to confirm that a specific pathogen is the likely cause? Or something different?
Should the diagnosis be done at the herd/flock or at the individual level? If the costs of diagnosis at the individual level is too high or if logistical challenges prevent a diagnostic procedure, then such a diagnosis may not be established, but a herd/flock diagnosis may be useful to guide antimicrobial treatment.
Prevention of disease, for example through attention to external biosecurity (primary prevention) or quarantine, is more effective than batch treatment (metaphylaxis) applied after infection is identified.
Decision‐making and risk‐management, i.e. can a timely diagnosis prevent the spread of pathogens if the individuals are segregated or if other risk‐based measures can be put in place, as alternatives to antimicrobial treatment?
If a pathogen is present in a herd or flock, the lack of accurate diagnostic tools may result in spread of the pathogen between animals (if the sensitivity is poor), thus requiring batch‐level medication to reduce the disease and retain production, or inefficient treatment (if the specificity is poor). For example, a recent Danish randomised clinical field trial indicated that batch‐level medication was more efficient in reducing diarrhoea and occurrence of Lawsonia intracellularis than treatment of pens or individual pigs (Larsen et al., 2016), thus highlighting the importance of within‐herd spread of the pathogen on disease occurrence and consequently on antimicrobial consumption, particularly if a purpose‐specific diagnosis is a challenge. Furthermore, multiple infectious agents are often present simultaneously, and if only one is treated due to a poor diagnostic workup, then treatment failure may occur. This leads to difficulties in choosing the optimal antimicrobial for treatment, and deciding whether antimicrobials need be part of the control measures at all.
In the future, it may be that automated detection (precision livestock farming methods) are installed in farm buildings to assist in early detection of disease through detection of changes in animal activity or behaviour patterns, and this may play a role in disease protection and ‘early disease detection’. The idea of very early disease detection through automated monitoring is being explored in a EU project EUPLF,67 and applications for early disease detection and triggering of management action by producers are already noted by Banhazi et al. (2012) and Fontana et al. (2015a), Fontana et al. (2015b).
Concluding remarks
On‐farm risk assessments can assist in secondary prevention, in identifying areas for focused action.
Accurate and timely diagnosis can focus the use of appropriate antimicrobial agents and support management strategies to limit spread of pathogens.
Recommendations
Diagnostic tests should be used to focus the use of antimicrobial agents and support management strategies to limit disease spread within a herd.
4.2.2.2. Production groupings
All‐in‐all‐out system is a process by which, after each batch of animals, the building or the epidemiological unit is cleaned and disinfected before new animals are introduced (Andres and Davies, 2015; Dorado‐García et al., 2015b). Individual animals or small groups such as breeding gilts on a multiplier farm are often introduced to a quarantine unit first. To be effective, a strict hygiene protocol needs to be in force separating the newcomers from the existing herd, until the newcomers can be assumed not to pose any risk. ‘All‐in‐all‐out’ production is common in poultry and pig production, and in other production types where batch production is feasible. In ruminant production systems, continuous production prevails. This type of production is therefore standard in some intensive production systems, whereas more extensive production systems with free‐ranging poultry or swine, or most farming systems with grazing ruminants, preclude ‘all‐in‐all‐out’ production with subsequent clearing, cleaning and disinfection.
The process should be applied on all finishing pig, broiler, layer and calf rearing units, for example, veal calves. In addition, it may be employed for certain more specialist stages of production in other systems such as on‐contract calf rearing farms where calves are reared for various systems, e.g. dairy heifer calf rearing, bull calf rearing for bull beef units.
In addition, the spread of pathogens present on farm can be reduced by simple hygiene measures, for example, minimal contacts between different age categories, changing management practices such as cross‐fostering in piglets, or the mixing of veal calves and finishing pigs from different groups or units (to balance feed intake or growth rate).
Concluding remarks
Internal biosecurity measures, including ‘all‐in‐all‐out’ systems, are important measures to reduce within farm disease transmission.
Recommendations
Biosecurity measures to reduce within‐farm spread of pathogens should be implemented. Potential risk areas can be identified using on‐farm risk assessments.
4.2.2.3. Housing design, building and maintenance
The design of livestock facilities plays an important role in the spread pathogens once they are on a farm (Hinchliffe and Ward, 2014).
Farm animal buildings should support good biosecurity by being well drained with means to removed slurry, faeces or waste water so that contaminated material does not accumulate, or is not easily accessible to animals (Cook and Nordlund, 2009). The quality and the amount of bedding are linked to drainage and moisture in the house. As an example of the importance given to this in an intensively farmed species, the Broiler Directive 2007/43/EC68 states (Clause 1) that ‘drinkers shall be positioned and maintained in such a way that spillage is minimised’, and (Clause 3) that ‘all chickens shall have permanent access to litter which is dry and friable on the surface’.
Similarly, proper ventilation will prevent accumulation of pathogens, aerial pollutants and dust to accumulate within the building to a degree likely to increase transmission of infection between the animals within the building (Anthony et al., 2014; Sanz et al., 2015).
Ventilation rates and the quality of air delivery and exchange in animal houses can be a significant factor in animal health and disease protection. Good air exchange and control of humidity, temperature and the removal of dusts, gaseous pollutants (carbon dioxide, carbon monoxide, ammonia, methane, hydrogen sulfide, nitrogen oxides) can have a key role on respiratory health in housed animals. As an example of the importance ascribed to ventilation, the Broiler Directive 2007/43/EC states (Clause 4) that ‘ventilation shall be sufficient to avoid overheating and, where necessary, in combination with heating systems to remove excessive moisture’. A balance between environmental concerns, and the need for effective and well managed bedding for animal comfort and health must be achieved. For all farm systems there is a need to balance the requirements of clean hygienic animal bedding and management, and the influence that feed and bedding have on animal health, welfare and disease, with the potential production of greenhouse gas emissions (GHGE) – and a number of EU recommendations and requirements – for example the report on Measures at Farm Level to reduce GHGE European Parliament 2014, and the Directive on National Emission Ceilings for certain pollutants (Directive 2001/81/EC).69
Routine cleaning of housing, particularly before the introduction of new stock, is recognised to be of significant protective value. As an example, the Broiler Directive formalises this as (Clause 10) ‘those parts of buildings, equipment or utensils which are in contact with the chickens shall be thoroughly cleaned and disinfected every time after final depopulation is carried out, before a new flock is introduced into the house. After the final depopulation of a house, all litter must be removed and clean litter must be provided’.
Provision of footbaths for cattle and sheep to treat or prevent foot problems (Speijers et al., 2010) will reduce the spread of pathogens on farm. Sick pens or other facilities for isolating sick animals should be part of the building and farm design, just like facilities for dealing with animals which need to be euthanised. Biosecure disposal of carcasses should be part of farm design and of farm operating procedures (Wilson et al., 2014).
Modifications to the bedding materials used in animal houses has focused on improving hygiene, animal comfort (i.e. material and amount of bedding), respiratory and other health risks (LeJeune and Kauffman, 2005; Westphal et al., 2011; Domer et al., 2012; Saastamoinen et al., 2015). Products are in development to reduce the production of ammonia in the environs of animals by drying the litter and reducing the bacteria therein that degrade urea and uric acid in urine. Ammonia is irritating and causes necrosis of tissues which may predispose to bacterial entry, for example Staphylococcus spp., Streptococcus spp. and E. coli bacteria through the teat canal and thus causing mastitis in cattle. The respiratory systems of horses, cattle, pigs, poultry, and domestic pets can be harmed by the presence of ammonia in the atmosphere in barns, stalls, pens and other enclosures. At high levels, inhaled ammonia can cause system damage and adversely affect weight gain and feed conversion. It is therefore desirable to reduce the quantity of ammonia to which farmed animals are exposed through appropriate housing, ventilation, drainage and bedding management.
Concluding remarks
Farm animal buildings can be designed and managed in a way that supports good internal biosecurity by the measures listed in the text.
Ventilation rates and the quality of air delivery and exchange, and the removal of pollutant gases including carbon dioxide, carbon monoxide, ammonia, methane, hydrogen sulfide, nitrogen oxides, and dust, in animal houses can be a significant factor in animal health and disease protection.
Recommendations
The design and management of farm animal buildings should support good biosecurity. The spread of pathogens on farm should be minimised by reducing the contact between animals and slurry, waste water or faeces, by the use of a proper ventilation system, by including sick pens to reduce the contact between healthy and sick animals.
Attention to effective air exchange and control of humidity, temperature and the removal of dusts, gaseous pollutants should be a focus of well managed farm buildings to reduce the effects of poor air quality on animal respiratory health.
Reductions in carbon dioxide, carbon monoxide, ammonia, methane, hydrogen sulfide, nitrogen oxides and dust through management of bedding and feeding practices should be promoted as a means to increase the respiratory health and to reduce infectious disease linked with poor air quality.
Innovative types of bedding, and innovative methods to reduce the production rate of ammonia in bedding may have a role in reducing the respiratory suppressive effects of high ammonia levels in animal housing. These methods should be promoted as a means to increase respiratory health.
4.2.3. Tertiary prevention (through a more resilient animal)
4.2.3.1. Overview
Tertiary prevention refers to strategies to improve animal welfare, and therefore their resilience or immune competence.
Resilience can be described as the adaptive capacity of animals that allows to react to changing environmental conditions or potentially stressful challenges with minimum loss of function. Within animals, often the term allostasis is used for the adaptive processes that actively maintain stability (homeostasis) through change (Sterling and Eyer, 1988).
If environmental perturbation or challenges are larger than can be coped with, the adaptive capacity of animal resilience is at stake. On the other hand, the adaptive capacity itself can be inadequate, e.g. as a result of chronic stress (McEwen and Stellar, 1993; McEwen, 1998). When stability cannot be achieved by failing allostatic systems, disease or even death may occur.
There are a number of strategies to promote increased resilience of animals including housing and appropriate nutrition, stress reduction, vaccination and genetic selection.
Concluding remarks
Housing, nutrition, stress reduction, vaccination and genetic selection each contribute to tertiary prevention. Collectively, these approaches can increase the ability of an animal's immune system to respond appropriately to an infectious challenge thus reducing the need for antimicrobial treatments.
Recommendations
The implementation of tertiary prevention measures should complement primary and secondary prevention in order to increase animal resilience.
4.2.3.2. Housing
Issues relating to housing have been addressed previously.
4.2.3.3. Nutrition
Different parameters are of importance concerning nutrition. In this paragraph, the attention is focused on commercial feed but most aspects are true for on‐farm feed.
The feed should ensure good nutritional balance and adequate feed formulation, following international standard of good manufacturing practice. Among parameters to take into account in relationship with health are the use of components that improve the microflora and avoid dysbiosis, the digestibility, the use of fibres in the feed to promote gut health, the stability in face of constantly varying least cost rations.
The composition and diversity of the intestinal microbiome are important factors for maintenance of immune homeostasis. Disruption of the microbial balance in the gut (dysbiosis) frequently leads to altered immune functions and increased risks of disease development (Schokker et al., 2014). Early life microbial colonisation of the gut appears to be an important factor in the programming of the immune system (Schokker et al., 2015a). Several extrinsic factors are known to influence this process, including prenatal and neonatal nutrition, management, use of antimicrobials, and behavioural learning curves (Schokker et al., 2015b).
Another aspect concerns feed hygiene. The implementation of best practices in the production of feed should ensure that all ingredients meet the required standards and that the manufacturing process does not allow the feed to be contaminated with deleterious agents, which could compromise the safety of the feed (cross‐contamination, quality of raw materials, for example concerning Salmonella contamination of soya or bedding materials as potential source of pathogens).
Since early exposure to antimicrobials can compromise the full development of the immune system (Schokker et al., 2014, 2015b), at the farm level, two aspects are worth enhancing.
The first one is the prevention of feeding calves with milk or colostrum containing residues of antimicrobials, such as colostrum of cows treated with antimicrobials at the start of their dry period, and milk of cows that have been treated during the lactation with antibimicrobial and milked during the withdrawal period. A recent opinion from the EFSA BIOHAZ Panel (2017) reviewed scientific information on the presence of residues in milk and colostrum, and indicated that with a dry period length according to the foreseen length in the SPC of the antimicrobial, the residue level in colostrum is judged to be low, while milk from cows receiving antimicrobial treatment during lactation contains substantial residues during the treatment and withdrawal period.
The second one is the good management of diet transitions at critical time points. Examples of this are the process of weaning in piglets, and the first few days following hatching of chicks.
Weaning in piglets is a process that does not just involve separation of the dam from the offspring, but also a substantial change in diet: piglets are abruptly denied access to milk and will have to rely on solid food. The process of change can be facilitated by management procedures and diet composition. One important aspect is to avoid an abrupt change: piglets should have access to solid feed well before they are being weaned. In contrast to what is generally assumed, van den Brand et al. (2014) suggest that this weaner diet should not be offered in small pellets, but rather in bigger pellets to facilitate intake. Increasing the length of the light period will facilitate uptake of solid feed (piglets will not look for feed in the dark).
Similar processes play a role following hatching of chicks, albeit it that the chick can still rely for a relatively short period on nutrition from the yolk sac. The timely availability of feed and water are still important, and recently poultry hatchery systems have been developed and are used in commercial practice to facilitate the transition from a dependency on the yolk to solid food (van de Ven et al., 2009; van der Pol et al., 2013).
Regarding diet composition one important aspect for young pigs as well as poultry is the level of crude protein (De Lange et al., 2010; Qaisrani et al., 2015). Crude protein cannot readily be digested yet, and serves as a substrate for pathogenic bacteria. The pathogens use the proteins for their own metabolism, producing ammonia and inflicting damage to the intestinal wall potentially causing infections. At the same time, the diets should contain all the essential amino acids for good health. Crude protein levels in the weaner diet have therefore gone down in recent years, whereas the quality of the protein sources for the diet has gone up; proteins from soy, fishmeal and potatoes are mainly included.
Additives are also used to promote health in young animals. Additional zinc in piglet diets is banned in some EU countries but not all. It has antimicrobial properties, but is a heavy metal with negative consequences for the environment (see Section 4.4.14). Organic acids are used in commercial pig and poultry diets. Butyric acid for example, will reduce the presence of pathogenic bacteria by creating an acid environment: the young gut still has to develop sufficiently high HCl concentrations following weaning/hatching. At the same time, it promotes enterocytes, which are cells in the mucosa responsible for absorption of nutrients by the gut wall. Butyric acid is a source of easy accessible energy, and its antimicrobial properties indirectly promote the recovery of damaged gut villi (Moquet et al., 2016). Finally pre‐ and probiotics, etheric oils and some herbal remedies may be additives which promote gut health (Pluske, 2013; Lindberg, 2014). Not all of them are as yet used in commercial diets (see also Section 4.4.1).
Concluding remarks
Good health in farm animals can be promoted through nutritional measures (e.g. diet transitions, feed hygiene).
Recommendations
Transition periods should be facilitated by providing diets, which promote the animal's ability to deal with change.
Weaning of piglets should be facilitated through proper management.
Weaning diets and early feed for chicks should be low in crude protein but contain sufficient essential amino acids and have high digestibility.
Feed additives for young animals should promote a fast development of optimal microbiota composition.
4.2.3.4. Reducing the level of stress
Cannon (1914) first described how animals cope when confronted with a stressful stimulus through the so‐called ‘fight‐or‐flight’ response. This involves autonomic and neuroendocrine elements, which mobilise the body's reserves so it can respond adequately to the challenge. Selye (1985) argued that more or less independent of the stressor, the physiological response of the organism can pass through three different stages.
The first stage constitutes an immediate alarm response, and is similar to the ‘fight‐or‐flight’ Cannon (1914) refers to. It includes various neurological and physiological responses, triggered by the hypothalamus signalling both the sympathetic nervous system and the pituitary. The former will stimulate the adrenal glands to release corticosteroids and increase metabolism to immediately provide energy. The latter will release adrenocorticotrophic hormone (ACTH) which affects the adrenal glands. These will release epinephrine and noradrenaline to prolong the fight‐or‐flight response. The second stage is one of continued arousal or ‘resistance’ to the stressor. A prolonged stressful situation will ultimately have a negative effect on homeostasis and harm internal organs leaving the organism vulnerable to disease. During the final stage, the body's energy reserves will become depleted and breakdown occurs.
The relationship between stress, the immune system and disease has been studied well in humans. In their review, Agarwal and Marshall (2001) address the adverse relationship between stress and human disease. They state that ‘although the effects of stress on health have been difficult to demonstrate’ and go on to say that ‘several investigators have demonstrated a significant interaction between psychological stress and health’. They refer to immune‐based diseases such as increased susceptibility to infections, atopic diseases and asthma, but human immunodeficiency virus (HIV) and cancer as examples involving an association between stress and health.
According to Martin et al. (2011) there are at least two hypotheses to explain a relationship between stressors and immune function. The first suggests that by suppressing the immune function when an individual is stressed, its resources are aimed at processes that are more crucial for survival. The second one proposes that stress hormones prevent immune cells from mounting misdirected attacks, as ‘without stress hormones, the body would over‐respond to benign substances and hence cause more damage than protection’ (Martin et al., 2011).
Knowledge on the relationship between stress and animal disease is accumulating. Kelley (1980) listed eight stressors that are present in modern livestock production units: heat, cold, crowding, mixing, weaning, limit‐feeding, insufficient bedding, noise and restraint. The author claims that all of these stressors have been shown to alter the immune system of animals. Many review papers have since been published (Dohms and Metz, 1991; Minton, 1994), and other stressors such as human–animal interaction (handling) (Hemsworth et al., 2000), enrichment (Beattie et al., 1995, 1996; De Jonge et al., 1996) and transportation (Garcia et al., 2016) have been added. The literature provides evidence for a bidirectional interaction between the immune and the nervous system, largely mediated by cytokines and without doubt related to animal stress. Gross and Siegel (1997) investigated poultry health and suggest that when stress levels are too high, viral diseases and other diseases which stimulate a lymphoid response are more prevalent. Yegani et al. (2005) suggests that stress is the number one cause of immunosuppression in birds. They argue that stressors leave birds more susceptible to infectious agents and propose that new poultry management guidelines need to be endorsed that reduce stress.
Our understanding regarding the main stressors mentioned above is summarised in the paragraphs below. They describe the nature of the stressor and their primary causes (besides the prime origin of depriving animals of their natural environment). To assess the level of stress, several indicators are available. Recent research focusses on animal‐based measures (ABMs) which are directly linked to an animal's response to adverse circumstances (as opposed to non‐animal‐based measures which describe the situation in which they are kept, and only indirectly represent the effect they may have on the animal) (Welfare Quality® consortium, 2009). ABM's include physiological parameters (such as the stress hormones referred to above), behavioural parameters (aggression, restlessness, but stereotypies, social withdrawal and apathy), as well as the actual appearance of the animal (skin lesions, clinical signs of ill health) (EFSA AHAW Panel, 2012a,b).
Thermal comfort
Livestock species have a thermal comfort zone: this is the ambient temperature range in which no extra effort or energy is needed to maintain normal body temperature. Related to this is the thermal neutral zone, in which normal body temperature is maintained with little effort. Below and above the thermal neutral zone animals have to make an increased effort, and with increasing discrepancy between ambient temperature and thermoregulatory capacity, the level of stress will increase.
Young (1981) reviewed the data on cold stress in farm animals. He suggests that pigs are rather sensitive to cold temperatures without deep bedding and are therefore usually kept in heated housing when raised in colder regions. In contrast, cattle can cope much easier with low temperatures, and rarely experience climatic conditions below their lower critical temperature. The animal's response to decreasing temperatures includes an increased metabolic rate, and hence an increased energy requirement for maintenance, and an increased rate of passage of digesta, which results in reduced digestive efficiency (Young, 1981). Behavioural responses include huddling, e.g. in pigs.
In their review on heat stress in lactating dairy cows, Kadzere et al. (2002) point out that cows can succumb to hyperthermia if they fail to maintain thermoneutrality. To avoid heat stress, cows revert to behavioural measures (seeking shade, drinking) and they sweat. Sweating is a major, if not the most important, thermoregulatory mechanism used to dissipate excess body heat (Kadzere et al., 2002). Due to high metabolic heat increment, high‐producing dairy cows may enter heat stress much earlier than their lower‐producing counterparts.
Pigs do not sweat, and have to revert to behavioural solutions to avoid overheating (Huynh et al., 2005). Pigs will avoid physical contact when lying, increase the contact of their body with the floor by lying laterally and generally occupy more space at higher temperatures compared to lower ambient temperatures (Aarnink et al., 2006; Spoolder et al., 2012). In the short term, they will increase their water intake, and if possible wet their skin by wallowing (Bracke and Spoolder, 2011). It can be argued that for a pig to wallow in its own faeces to cool down, as often happens when other ways of cooling down are not available, is a serious stressor given the animals’ normal tendency to avoid contact with its excrements. In the longer term, they will reduce their metabolism and reproductive performance (Einarsson et al., 1996).
Heat stress is one of the most important environmental stressors challenging poultry production worldwide (Lara and Rostagno, 2013). The negative effects of heat stress on broilers and laying hens range from reduced growth and egg production to decreased poultry and egg quality and safety. Mack et al. (2013) showed that heat stressed birds will spend less time feeding, more time drinking and panting, as well as more time with their wings elevated, less time moving or walking, and more time resting. In addition, as indicated in previous paragraphs, there is a well‐established relationship between heat stress on productivity and immune response in poultry (Payne, 1966; Quinteiro et al., 2010).
Stocking density
Crowding and restraint put pressure on animals. With decreasing levels of space per animal, animals will first respond behaviourally. There are several studies in pigs, which show that there will be a constantly increasing aggression level (Ewbank and Bryant, 1972; Turner et al., 2000). In pigs and poultry, the thermoregulatory behaviour of the animal will come under pressure, as there may be insufficient space to avoid physical contact with other animals during periods of high ambient temperature (Huynh et al., 2005; Bessei, 2006). Chronically reduced space allowances will have physiological consequences, such as reduced growth of pigs (Gonyou et al., 2006). It has been suggested that decreased space allowance will increase the prevalence of gastrointestinal disorders in veal calves and beef cattle (Cozzi et al., 2009; Brscic et al., 2011), result in dirtier animals and in a higher prevalence of mastitis in cows housed in straw yards, and increase the occurrence of agonistic behaviours and increase the resting time in cows housed in cubicles (Fregonesi and Leaver, 2002; Fregonesi et al., 2007).
Mixing
Mixing unfamiliar animals is stressful, as it requires them to establish a new social organisation. Rank order fights result in elevated stress hormone levels, injuries and even death. A poorly established social group may have individuals, which cannot adapt to the new situation and remain outside the group structure, resting in unfavourable parts of the pen or building and with limited access to resources. Cold, hunger and thirst may result from this. Pigs that were not mixed at weaning, and not mixed and transported at 25 kg showed a better immune response than mixed and transported control animals kept under the same environmental conditions (Ekkel et al., 1995). Mixing stress may also differentially affect animals of different social status. For example, lymphocyte proliferation and immunoglobulin G (IgG) were greater in dominant than in subordinate pigs (Tuchscherer et al., 1998; Salak‐Johnson and McGlone, 2007).
Weaning
The weaning process involves several abrupt changes in the lives of the young mammal, as well as its dam. Stressors associated with weaning include removal from the mother at a relatively young age compared with wild species, at a stage when maternal immunity is waning but intrinsic immunity is not yet well developed, change in diet, cessation of nursing, separation from the dam, switching to a new spatial environment and changes in the social environment. The social environment, the physical environment and the type of feed changes from one moment to the next. Effects may include reduced immune function, growth retardation and the performance of abnormal behaviours. Important diseases associated with weaning include diarrhoea in piglets (Prunier et al., 2010) and respiratory disorders in (beef) calves (Galyean et al., 1999). Measures to prevent weaning diarrhoea in piglets comprise appropriate weaning age (not before four weeks), avoidance of social mixing (Prunier et al., 2010), good hygiene (e.g. ‘all‐in‐all‐out’ management)(Madec et al., 1998) as well as provision of bedding (Munsterhjelm et al., 2009). In calves, preweaning management in terms of reducing cumulative effects of multiple stressors such as dehorning and castration around weaning, vaccination programmes and feeding concentrates preweaning reduces morbidity. Management practices reducing stress at weaning include the use of nose‐clips or fence‐line contact with the dam post‐weaning (Lorenz et al., 2011).
Feed restriction
Not all livestock is fed ad libitum. Pregnant sows and broiler breeders are fed restricted. Genetically they are ‘programmed’ for high feed intakes as they are supposed to produce offspring with high growth potential. However, for these animals, high intakes would mean excessive growth and associated anatomical–physical and physiological problems (Meunier‐Salaun et al., 2001; Mench, 2002). Quantitative food restriction can result in (chronic) hunger with apparent negative welfare consequences (increased motivation for food, foraging activity redirected towards non‐food substrates, development of stereotypies, increased aggression)(D'Eath et al., 2009). While it is generally accepted that chronic hunger is a stressor, the physiological implications and adverse effects on health are less well understood (D'Eath et al., 2009).
Handling
The impact of poor animal handling on stress in various livestock species has been well‐documented (Hemsworth et al., 1986; Grandin, 1987, 2008; Hemsworth and Coleman, 2011). It is obvious that handling which involves painful stimuli (slapping, kicking, electric prods) has a direct effect on pain and stress in the recipient animals. There is an indirect and more chronic effect, via fear of humans. Spoolder and Waiblinger (2009) have reviewed the literature on human–animal interactions in the pig industry, and discuss various studies in which fear responses were provoked through inappropriate handling (Hemsworth et al., 1981, 1986; Gonyou et al., 1986). These and a number of other studies suggest that negative behaviours (sudden movements, slapping, kicking, using electric goads) increase the latency for pigs to approach an observer in a standard test, as opposed to positive behaviours (calm movements, stroking, talking quietly). The same increase in approach latency due to poor handling can be observed in cows (Pajor et al., 2000; Rousing and Waiblinger, 2004) and chickens (Jones, 1986; Zulkifli and Azah, 2004). Stress through increased fear of humans has an effect on performance. Hemsworth and Coleman (2011) list a number of studies in which reduced growth rates were observed when pigs have been handled negatively. Further data from the Australian group suggest that reproductive performance in pigs can be negatively affected. Hemsworth et al. (1986) found pregnancy rate of gilts at 40–60 days post‐mating dropped significantly when handling practices were increasingly unpleasant. In dairy cows, Waiblinger et al. (2002) identified similar relationships in relation to milk yield. Finally, van der Mheen and Spoolder (2003) found that their ‘roughly’ handled pigs had similar growth rates to ‘calmly’ handled pigs, but less back fat. The latter were less active and the authors suggest that the calmly handled animals had a better energy conversion ratio. Spoolder and Waiblinger (2009) argue that pigs are very adaptable and will resort primarily to behavioural responses to cope with handling stress before physiology and (ultimately) performance and health are affected. They conclude that reduction in performance can therefore be considered a serious signal in relation to handling quality.
Enrichment
Most animals are naturally inquisitive and require stimuli from their environment to support that need.
Barren environments may result in behavioural abnormalities: stereotypies, self‐directed mutilations or damaging behaviours aimed at conspecifics (tail biting, feather pecking, aggression). According to Newberry (1995), environmental enrichment is defined as an improvement in the biological functioning of captive animals resulting from modifications to their environment. The functionality or adaptiveness of environmental enrichment may be assessed by the performance of normal behaviour patterns such as exploration, foraging, play and social interaction, the reduction of abnormal behaviours such as tail biting in pigs or by an improvement of the health status (van de Weerd and Day, 2009). For example, in pigs environmental enrichment in terms of provision of straw has been reported to reduce the incidence of injuries caused by tail biting (Moinard et al., 2003), gastric ulcers and lung damage (Guy et al., 2002; van Dixhoorn et al., 2016). Swiss ‘animal‐friendly’ farms (multiple areas, straw bedding, access to outdoor facilities) used less group‐based antimicrobial treatments than control farms (slatted floors) (Cagienard et al., 2005). More recently, cognitive enrichment in the form of equipment provoking attention and cognitive activity (call‐feeding‐station) improved IgG concentration, in vitro T‐cell proliferation and wound healing following biopsy (Ernst et al., 2006).
Transportation
Moving animals from one location to another, be it for slaughter or rehousing, involves several stressors. Broom (2000) lists several, most of them are related to the handling of livestock during loading and unloading (see above). He describes behavioural responses during transport, indicating that that some species will lie down quite readily (pigs and poultry) whereas others will remain standing while the situation is still disturbing (sheep and cattle). It may take several hours of acclimatisation before these animals will lie down. Lateral movements, sudden breaking or acceleration will all negatively affect the settling down of the animals. Although pigs usually lie down pretty quickly, they may stay standing if the journey is really rough (Bradshaw et al., 1996), indicating a level of discomfort related to the driving. It is well known that pigs may suffer from travel sickness and vomit during rough journey (Bradshaw et al., 1996). Transportation involves crowding, and if animals are mixed, it involves social unrest possibly resulting in aggression and injuries. The EFSA AHAW Panel (2011) reviewed the scientific information concerning transport of the main farm species, following an earlier thorough review by SCAHAW (2002). The opinion addresses several issues in addition to the stressors mentioned above: feed and water deprivation, and unfavourable climatic conditions being the main ones.
Concluding remarks
On‐farm stressors interfere with the normal behaviour of the animals and have been shown to alter the immune system of animals and susceptibility to diseases. Husbandry conditions have been shown to significantly influence resilience of the animals and this is mediated by direct impact of the housing environment as well as alterations of the immune system.
Recommendations
Husbandry conditions should be aimed at minimising chronic stress levels in livestock. Stocking densities, degree and frequency of mixing, nutritional aspects and the thermal environment should be optimised.
Handling and transportation of animals should be done in a calm and quiet way to reduce handling stress and associated negative effects on the immune system.
4.2.3.5. Vaccination
Veterinary vaccines play an important role for animal and public health, animal welfare and food production and present no hazard to consumers of produces from vaccinated animals. The goal of a vaccination programme is either to prevent or reduce infectious disease, and thereby promote optimal health of a single animal, a herd and the public. For production animals, prophylactic vaccines are typically used routinely against endemic diseases to reduce production losses, prevent secondary infections and to improve animal welfare. Vaccines may also be used strategically in eradication programmes, in which case an accompanying diagnostic test to differentiate infected from vaccinated animals (DIVA concept) is important in order to maintain surveillance for freedom of disease within the vaccinated population. For exotic infections, prophylactic emergency vaccinations may effectively be used to control outbreaks of disease or reduce risk of transmission from wildlife to production animals. In a few cases, such as rabies in humans and tetanus in horses, individual post‐exposure vaccination may be indicated as a therapeutic approach.
The choice of the type of vaccine developed to target a particular organism is based on the nature of the organism itself, its invasive properties and the immune response the organism generates. Consequently, a range of vaccine types are available including modified live vaccines such as attenuated and recombinant vector vaccines and killed/inactivated vaccines, subunit, conjugate and DNA vaccines. Delivery of vaccines can be by a wide variety of mucosal delivery routes: oral, nasal, oronasal, conjunctival through water, baits, spray, or using the more classical subcutaneous or intramuscular injection to bypass the difficulties of mucosal immune activation.
Vaccines are granted with a marketing authorisation based on their proven efficacy to induce protective immunity against infectious disease. Detailed and appropriately controlled studies have rarely been performed to document the effect of vaccination on antimicrobial use. A recent register‐based study on indoor herds with a minimum of 50 sows and weaner production revealed an association between herds purchasing swine vaccines against porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae in Denmark and increased amount of antimicrobials prescribed for weaners (Temtem et al., 2016). Limited data were available from each study herd. As a consequence, and as acknowledged by the authors, the analytical methods could not account for some key systematic differences between vaccinated and non‐vaccinated herds. It is suspected, for example, that herds with health problems are more likely to invest in vaccination than those without health problems. This absence of formal documentation of vaccine effect on reduced antimicrobial use is not an evidence of a small impact of vaccine‐mediated disease prevention on antimicrobial use in general. For antibacterial vaccines the implications for reduced antimicrobial use is straightforward when the disease‐causing bacteria are reduced. For antiviral vaccines the positive effect on antimicrobial use is mediated through prevention of the immunosuppressive effect of many viruses and the predisposition to secondary bacterial infections. Given the documented efficacy of vaccines in disease prevention, it is a reasonable assumption that both antibacterial and antiviral vaccination is an efficient and rational means to reduce the need for antimicrobials by improving animal health and welfare when infections are present. As shown in the study by Temtem et al. (2016) vaccines are, however, not a solve‐all solution to antimicrobial use and the numerous other recommendations in this report are likely to have a more sustained effect on antimicrobial use. There are recent expert‐based priority lists of veterinary infections where new or improved vaccines would significantly reduce the need for antimicrobial use70 and the response from the FVE to questions in preparation of this report (see Appendix D and Annex A). Furthermore, a number of important infectious diseases have been described in detail by the DISCONTOOLS expert opinions.71
Introduction to vaccine based health control
For a description and examples of different types of vaccines including 1st‐generation live and inactivated viral or bacterial vaccines, 2nd‐generation subunit vaccines, and 3rd‐generation genetically modified vector and DNA vaccines see Meeusen et al. (2007).
Live and modified (attenuated, or recombinant) live vaccines
Live vaccines are commonly capable of conferring long‐term immunity following a single dose or in combination with a booster dose when administered to susceptible animals, i.e. not protected by maternal immunity interfering with live vaccines or by antimicrobials preventing establishment of live bacterial vaccines. Risks associated with live vaccines include potential reversion to virulence in which case the vaccine will actually cause disease as exemplified with the reversion of attenuated Type 2 PPRS virus to virulence after introduction in Denmark in 1996 (Mortensen et al., 2002). Thus many vaccines use DNA technology to remove several key genes from the pathogen and thus effectively have more than one attenuating modification to the pathogen (e.g. the most recent modified live virus vaccine for BVD virus II has 2 separate modifications to the virus achieved by deletion of specific genome sequences that should effectively prevent reversion (EMA CVMP, 2014a)). In addition, in cases of immune impairment the use of live vaccines is not recommended. Furthermore, live vaccines must normally be kept at special temperatures (2–8°C, −20°C, −196°C) to maintain efficacy.
Inactivated vaccines
Inactivated vaccines do not carry any risk of infectious disease transmission, as they do not contain any live organisms. Most inactivated vaccines require an adjuvant formulation to activate an appropriate immune response and still generate weaker immune responses and require repeated doses to maintain immune memory compared to live vaccines.
Subunit vaccines include only selected antigens or specific epitopes from the pathogen that elicit protection after immunisation and are even more dependent on adjuvant formulation.
DIVA vaccines
The ability to identify and selectively delete genes from a pathogen has allowed the development of ‘marker vaccines’ that, combined with suitable diagnostic assays, allow differentiating infected from vaccinated animals (DIVA) by distinction of antibody responses induced by infection with the wild‐type virus or bacteria from those induced by the vaccine (no antibodies generated to deleted genes). This is an important development that will make it possible to vaccinate under regulatory control without impairing the sanitary status of the infected herd and which has proven useful to, e.g. accelerate the eradication of Aujeszky's disease in a number of countries for example Germany, the Netherlands, Italy, Spain, Portugal and Ireland (Pensaert et al., 1992, 2003; Müller et al., 2003).72 Therefore, in countries with infected pigs, where the eradication of Aujeszky's disease is planned, these marker vaccines are the vaccines of choice as demonstrated in 1996 during an EU‐supported project (Willeberg et al., 1996).
Autogenous vaccines
Autogenous vaccines are inactivated immunological veterinary medicinal products which are manufactured from pathogens and antigens obtained from an animal or animals from a holding and used for the treatment of that animal or the animals of the holding in the same locality. They are primarily used for pigs, poultry and fishes and are prepared from the pathogenic organism or organisms specific to the individual herd or flock after a problem has been identified and when no registered vaccines for the pathogen or the serotype in question are available or those vaccines have been shown not to be efficacious against the particular pathogen or serotype in the locality. The regulations for production and use of autogenous vaccines vary considerably between EU MSs although there is an ongoing Heads of Medicines Agencies (HMA) lead initiative to harmonise regulations. There are inherent conflicts between individual production of herd‐specific vaccines and good manufacturing practices (GMP) (or GMP‐like) requirements where only one batch production is allowed at any time in a production facility. Autogenous vaccines are quite widely used in many EU countries (Hera and Bures, 2003). Because autogenous vaccines take some considerable time to produce they are only useful in case of chronic or recurrent disease. Specificity of these tailored vaccines is an important characteristic of autogenous vaccines and they are used only in animals epidemiologically linked to the locality from which the original pathogen was taken. Each vaccine carries the risk of unwanted or adverse effects and they are consequently normally tested in a small number of the animals to be treated in order to ensure there are no untoward effects before wider use. A mild local reaction at the site of injection is a common occurrence. Controlled efficacy studies are lacking as each vaccine is produced for the individual herd or flock, although improved efficacy for the autogenous vaccine compared to the registered product has been reported, e.g. for PRRS virus in pigs (Geldhof et al., 2012) and Salmonella spp. in ducks (Youn et al., 2016).
In a 2011 study in the Netherlands, an average of 11.72% of sow farms used autogenous vaccines and 18.96% of the total sows were vaccinated with an autogenous vaccine. Autogenous vaccines were used for Streptococcus suis, Staphylococcus hyicus, Pasteurella multocida, Bordetella bronchiseptica, Actinobacillus pleuropneumoniae, Clostridium perfringens, Clostridium difficile and E. coli (van de Ven, 2013). Poultry autogenous vaccines are typically for E. coli and are quite widely used although no exact figures are available.
If common grounds for the continued access to autogenous vaccines cannot be found, there is a risk that the uncontrolled (and illegal) use of so‐called back feeding of faeces and/or intestines from piglets that have succumbed to a disease to sows in the same herd will escalate. This procedure has been widely and controversially used in the USA for the control of enteric infections such as transmissible gastroenteritis virus (TGEV) and PED virus, where existing vaccines are not effective (Schwartz et al., 2013). As reported in veterinary scientific meetings, back‐feeding is widely used in some European countries for the control of enteric disease syndromes with unknown aetiology in newborn piglets (neonatal porcine diarrhoea syndrome, NPDS). This practice may risk facilitating the evolution and species adaption of non‐virulent pathogens into virulent variants, and may contribute to increased transmission of known or unknown pathogens within the herd and facilitate airborne spread of, e.g. viruses from herd to herd due to the massive amount of viruses shed when thousands of animals are infected simultaneously (Chang et al., 2014; Jung and Saif, 2015).
Administration of vaccines
Proper storage and administration of vaccines is important in obtaining the full effect of vaccination. New developments in terms of needle‐free intradermal delivery of vaccines to obtain a better targeting of immune activating dendritic cells located in the epidermis appear promising, and although oral delivery of inactivated vaccines has been a target for vaccine research through decades, there are new developments in this area as well, although all registered vaccines for oral veterinary use are still live attenuated vaccines.
Research is still needed to support development of multivalent veterinary vaccines and to investigate the optimal vaccination protocols with combined (parallel) administration of existing veterinary vaccines. In theory, inactivated vaccines will have no or minimal immunomodulatory effects on efficacy of other vaccines administered in parallel, while even minor immunosuppressive effects of live attenuated vaccines may influence the efficacy of other vaccinations administered at the same time or in the following weeks. Such studies may be performed as large efficacy studies in the field, or more prudent by monitoring of validated immunological correlates of protection for each vaccine.
Passive immunisation
Vaccination of pregnant animals is frequently used as a means to protect the new born animal from specific diseases that occur early in life. Immunoglobulins form an important component of the immunological activity found in milk and colostrum and are central to the immunological link that occurs when the mother transfers passive immunity to the offspring. The mechanism of transfer varies among mammalian species, but access to colostrum of good quality is imperative for the vaccination of the mother to have preventive effects on the disease susceptibility of the offspring. Laying hens transfer immunoglobulins via egg yolk to their chickens. In contrast to vaccination, administration of immunoglobulins establishes instant immunity but with no induction of immunological memory.
Different options for alternative sources for immunoglobulins enabling passive immunisation of production animals at critical time points have been explored (Hedegaard and Heegaard, 2016) (See also Section 4.4.6). Such products include natural antibodies contained in slaughterhouse waste products reused in the form of spray‐dried blood plasma (Niewold et al., 2007) or purified plasma immunoglobulins (Hedegaard et al., 2016), or egg yolk immunoglobulins from hens specifically immunised against non‐poultry infections (Diraviyam et al., 2014). For immunoglobulin products derived from slaughterhouse waste products the risk of unwarranted spread of infectious agents with the biological material needs to be addressed in the production process.
Trained innate immunity
All classical vaccines rely on the principle of induction of a specific adaptive immune response to the vaccine target. An emerging topic in vaccinology is based on the recent appreciation of trained immunity or innate immune memory, where cells of the innate immune system achieve a temporarily improved functional state to more efficiently combat secondary infections after a challenge by a primary infection or vaccine (Netea et al., 2016). Trained immunity has now been demonstrated in plants, invertebrates, animals and humans and has the potential to improve the health status of particularly neonates where traditional adaptive immunity is difficult to achieve by other means than passive immunisation.
New technological developments in vaccine development and production
While traditional vaccine design has most often been developed from cultivated microbial agents and isolating the protective antigens, there are a number of recent technological developments that allow vaccines to be designed from a more rational reverse vaccinology approach. Here whole genome sequencing of microbial genomes, high‐throughput protective antibody discovery and sequencing of both B and T cell antigenic repertoires allow for a tailored vaccine antigen selection to only induce the appropriate protective immune responses (Rappuoli et al., 2016). Beyond the development of new technologies for rational antigen discovery, the advances in understanding the activation of innate and adaptive immune responses have spurred new hope for development of adjuvant formulations with a more focused immune activation following delivery of recombinant subunit antigens. Developments in live attenuated delivery platforms based on Vaccinia virus, Adenovirus or ribonucleic acid (RNA) alpha viruses allow for new vaccines with strong immune activation and a high safety profile.
Irrespective of any technological advances it must be remembered that vaccines are registered biological products and exchanging a component, e.g. an adjuvant or an antigen, with a superior modification cannot be done without filing a new registration for the full product to secure public and animal health. The expense and difficulty of this will inevitably keep many of the ‘old’ vaccines on the market for many years to come.
Increasing the availability of veterinary vaccines
EMA and its partners in the European Medicines Regulatory Network, the HMA, are implementing a joint action plan to help increase the availability of veterinary vaccines in the EU. This plan contains a number of initiatives to facilitate timely access to the EU market for new or improved veterinary vaccines.
Species‐specific considerations and examples
Pigs
Documented effects of vaccination on reduced antimicrobial use includes Lawsonia intracellularis (Bak and Rathkjen, 2009) where the antimicrobial reduction may be directly linked to reduced L. intracellularis treatment, and PCV2 (Raith et al., 2016) where antimicrobial reduction can be explained by reduced secondary bacterial infections. In other studies, it was not possible to document reduced antimicrobial use in spite of reduced pathology for, e.g. M. hyopneumoniae (Kristensen et al., 2014) and the above mentioned register‐based study (Temtem et al., 2016) even documented increased AM use in herds relying on vaccination to control M. hyopneumoniae and PCV2 infections. The indication associated with most antimicrobial use is post‐weaning diarrhoea (PWD) caused by enterotoxigenic E. coli (ETEC). There are several difficulties that such a vaccine must overcome: (i) there is a very short window for induction of immunity; (ii) the vaccine must provide mucosal immunity, (iii) the vaccine must work in the face of maternal immunity; and (iv) the vaccine must cover a range of serotypes. It is likely that a combination of appropriate maternal immunity, passive immunisation in the critical time through weaning, non‐specific enhancement of immune capacity and novel vaccines for the weaned piglets will be needed. In 2015, a live oral vaccine against F4 fimbriae‐positive ETEC was introduced in the EU (first introduced in Canada in 2008). F4+ ETEC is the most frequent cause of PWD, but further work is needed to introduce an F18 ETEC vaccine and to optimise delivery and functionality of oral live vaccines in the presence of maternally derived antibodies against the same fimbriae virulence factors (Melkebeek et al., 2013). PWD caused by L. intracellularis is a target for improved vaccine development although the available attenuated live oral vaccine is one of the few documented cases where vaccination significantly reduced antimicrobial use (Bak and Rathkjen, 2009). In spite of the vaccine availability, antimicrobial use for Lawsonia infections remains high as: (i) there are other pathogens involved in the PWD syndrome; (ii) the vaccine requires an antimicrobial‐free window for vaccination; and (iii) the vaccine does not prevent shedding of bacteria after infection (Riber et al., 2015). Other bacterial infections where research to improve or develop new vaccines should be a priority to reduce antimicrobial use are Streptococcus suis, Leptospira spp., Actinobacillus pleuropneumoniae, and Clostridium perfringens (type A).
Porcine Respiratory Disease Complex (PRDC) describes a multifactorial disease complex present in modern pig farming with viruses as primary agents of respiratory disease acting as facilitating agents for secondary bacterial infections. According to GAP analyses completed in the EU‐funded project DISCONTOOLS,73 available vaccines against microbes involved in PRDC are either lacking or would gain from an improved efficacy. PRRS has been shown to be able to be controlled and eradicated on a country basis in Sweden.74
New or improved vaccines should also be developed against rotavirus.
Poultry
There are many vaccines on the poultry market and, although not documented, it is generally accepted that they have had a positive impact on reducing the need for antimicrobial use. Nevertheless, autogenous vaccines are still widely used for, e.g. E. coli infections in poultry which is believed to be due to the variable efficacy of current vaccines against the wide strain variation. In line with this, both the OIE AHG report75 and the FVE report (see Annex A) highlight that although current vaccines have had a positive impact on antimicrobial use already, vaccines against colibacillosis can be improved to cover more strains. High antimicrobial use remains for Clostridium perfringens Type A (necrotic enteritis) where the passive immunity to broiler from vaccination of layers is short lived. Poultry vaccines for Ornithobacterium rhinotracheale and enterococci are needed, and treatment of secondary bacterial infections would be reduced with the development of more effective vaccines against coccidiosis (also for turkeys along with histomoniasis).
Although the prevalence of Salmonella spp. in poultry in Belgium (and the other MSs, e.g. Germany) has declined significantly with the implementation of a mandatory Salmonella control programme, including obligatory vaccination of laying hens against S. Enteritidis, some persistently infected flocks cannot successfully control the infection. This demonstrates that additional measures to vaccination are needed to control the infection (Dewaele et al., 2012). From a zoonotic perspective the effective prevention of salmonellosis in poultry is still an issue as the human seroincidence across 13 European countries was not correlated with the reported national incidence of Salmonella infections in humans, but was correlated with prevalence data of Salmonella spp. in laying hens (p < 0.001), broilers (p < 0.001), and to a lesser degree slaughter pigs (p = 0.03) (Mølbak et al., 2014).
Chickens are particularly susceptible to bacterial and viral infections in their first 10–14 days of life (the brooding period) where they are developing their adaptive immune, digestive and thermoregulatory systems. Kemmett et al. (2014) have shown that 70% of early dead birds showed signs of E. coli infection and early control of Salmonella infections is critical to decrease later contaminations (Van Immerseel et al., 2005). Viral infections may occur during this period and may contribute to the development of runting and stunting syndrome (RSS), a condition that is characterised by growth depression, decreased uniformity and poor economic performance (Kang et al., 2012). The multiplicity of agents, including astroviruses, reoviruses, parvoviruses, that have been involved in RSS makes it difficult to develop multivalent vaccines for these early infections of broilers. Alternatively, non‐specific stimulation of the immune system of the young chicks (trained immunity) may potentially increase their resistance to the pathogens occurring in that critical period. It may also potentially improve the efficacy of other vaccines administered at the hatchery.
Bovines
For cattle a wide range of vaccines are available which include mono‐ and polyvalent vaccines against virus, bacterial, fungal and/or parasitic diseases most often targeted at calves that may be particularly susceptible. As described previously, the highest antimicrobial use is related to bovine mastitis, the utility of vaccination against mastitis pathogens in cattle remains uncertain. In a trial to evaluate a polyvalent mastitis vaccine, Bradley et al. (2015) found no significant difference in the incidence of either clinical or subclinical mastitis in the first 120 days in milk. They did find, in comparison to unvaccinated animals in the same herd, that vaccinated cows were significantly less likely to experience severe clinical mastitis and produced significantly more milk and milk solids. Using the same vaccine, Landin et al. (2015) could not detect any beneficial effects on either udder health, milk production or survival in two commercial dairy herds with mastitis problems due to Staphylococcus aureus.
Vaccination may be useful to reduce the prevalence of disease and thus antimicrobial use in certain production systems such as veal production units. Vaccines against bovine respiratory syncytial virus (BRSV) and other infections of importance in the veal production are not optimal and other management measures may be more important to reduce antimicrobial use. Non‐specific immune stimulation of gamma‐delta T cells in calves has shown promise to increase the innate resistance to Salmonella‐induced diarrhoea (Hedges et al., 2016) and this concept should be further investigated as a means to improve health status of comingled calves in veal production.
Vaccination, while not essential, may play a significant role in the development of a disease eradication or control programme. For example Scandinavian countries successfully eradicated BLV, IBR, BVD without use of vaccines in the end of the 20th century. This may not be feasible in countries or areas with less favourable geographical conditions, but illustrates that in some cases an eradication programme may be a more sustainable solution than a vaccination programme (Moennig et al., 2005; Houe et al., 2006).
Small ruminants
Development of various types of vaccines for small ruminants has evolved very quickly in recent years, offering improved protection against diseases that cause major economic losses. These include bacterial abortion (abortion associated with Brucella melitensis, Campylobacter spp., Chlamydophila abortus, Coxiella burnetii, S. Abortusovis or S. Brandenburg), caseous lymphadenitis, clostridial diseases, colibacillosis, contagious echtyma, epididymitis caused by Brucella ovis, footrot, mammary gland diseases (contagious agalactia, mastitis), paratuberculosis and respiratory diseases caused by Mannheimia haemolytica or other Pasteurellaceae (Lacasta et al., 2015).
Aquaculture
Particularly in Norwegian Atlantic aquaculture vaccines have already been demonstrated to efficiently tackle some of the most commonly occurring bacterial diseases as described previously (Burridge et al., 2010; Midtlyng et al., 2011). Appendix C provides details on the use of vaccination within the strategy to reduce the use of antimicrobials in salmon farming in Norway. The Mediterranean saltwater production of European sea bass and Gilthead sea bream are not favoured by the low water temperature in the North Sea. Vaccines against Vibrio anguillarum, Photobacterium damsela and Tenacibauculum maritinum are used, but registration of antimicrobial use in Mediterranean European or North African aquaculture is incomplete. Vaccines (and antimicrobials) are used against a number of bacterial diseases in freshwater aquaculture where Flavobacterium psychrophilum poses a particular problem due to the size of affected fry when immune system is not yet developed, and Yersinia ruckeri where the emergence of a new biotype has challenged vaccine efficacy.
Fish appear to be particularly receptive for DNA vaccination and EMA has in April 2016 adopted a positive opinion for marketing authorisation of a DNA plasmid vaccine for active immunisation of Atlantic salmon against pancreas disease caused by salmonid alphavirus subtype 3 (SAV 3) (EMA CVMP, 2016) although there is no final decision on the license. In conclusion, although the Norwegian history of vaccination in salmon is a remarkable success, further development of vaccines for aquaculture is warranted.
Concluding remarks
Vaccines have contributed significantly to the control of infectious diseases in production animals.
Despite the use of vaccination, substantial levels of disease can still persist. Therefore, in some circumstances, regional disease eradication is preferred. In individual herds, primary, secondary and other tertiary measures to improve the animal health status may have a more sustained effect on reducing antimicrobial use than continued vaccination.
Antiviral vaccines contribute to reduced antimicrobial use through prevention of immune suppression and secondary bacterial infections.
There are new technical opportunities for improved vaccine design and efficacy.
Non‐specific stimulation of the immune system may have potential to increase resistance to complex diseases such as post‐weaning diarrhoea in pigs, calf diseases and runting and stunting syndrome in young chicks.
For pigs, one important infectious problem to be solved for reduced antimicrobial use is post‐weaning diarrhoea, but also improved vaccines against, e.g. porcine respiratory disease complex and Streptococcus suis are needed.
For poultry, the main infectious disease problem to be solved for reduced antimicrobial use is various manifestations of E. coli infections.
For bovines, the main infectious disease problems to be solved for reduced antimicrobial use are mastitis and viral diseases in veal production, but new (or re‐emerging) pathogens such as Mycoplasma bovis warrants further vaccine research.
Recommendations
Regulations for autogenous vaccines differ between the MSs and could be harmonised.
Research opportunities for further development of technology platforms for improved development, formulation and delivery of next‐generation vaccines and trained immunity should be promoted.
Development of improved vaccines against specific infections accounting for high antimicrobial use in farm production systems should be supported.
4.2.3.6. Animal genetics
To date, genetic selection has primarily focused on improving production, growth rate and meat quality traits. These breeding criteria have not fully taken account of animal health and welfare, and it is perceived that selection for these production traits may have resulted in animals with reduced abilities to cope with infectious hazards, and with increased susceptibility to a range of infectious diseases. For example, in most dairy cattle population, the primary selection objective has been milk production, whereas fertility and health traits have received less attention (Miglior et al., 2005). Cows with a higher genetic merit for milk yield are more susceptible to mastitis, cystic ovaries, and lameness (Heringstad et al., 2000).
Resistance and tolerance are the main strategies of the animal immune responses to infection. Resistance or resilience is the ability of the host to resist pathogens by preventing the pathogen from entering, or by inhibiting pathogen replication. Tolerance is the ability to minimise the damage caused by pathogen. Both strategies may be genetically controlled (Davies et al., 2009; Rodenburg and Turner, 2012), and can complement, but not replace, existing interventions to control infectious diseases. To date the relative contribution of resistance and tolerance to infection outcome is poorly understood (Lough et al., 2015).
The feasibility of selective breeding to increase disease resistance depends on the amount of variation in resistance shown by individual animals in a population, and the heritability of the traits, which provide disease resistance. Livestock show considerable variability in their responses to a wide range of disease challenges, and much of the variability is genetic (Bishop, 2015). Identifying animals with the innate ability to make superior immune responses can reduce disease occurrence (Mallard et al., 2015). High responders have an inherent ability to produce more balanced and robust immune responses compared with average or low responders. High responders may pass their superior immune response genes to future generation, through higher longevity or survival rates, thereby accumulating health benefits within the herd.
In dairy cattle, mastitis is a promising trait to be included in routine genetic evaluation, because it is the most recorded ‘production’ disease and has a high positive genetic correlation to other health traits. The heritability of mastitis incidence in dairy cattle is low (0.04), as is the heritability of somatic cell count (0.11 ± 0.04), the proxy for mastitis incidence, but the genetic correlation between mastitis incidence and somatic cell count is high at 0.70 (Mrode and Swanson, 1996). Other traits such as good udder conformation, and strongly attached fore udders have been associated with lower mastitis incidence (Sørensen et al., 2000). In August 2014, Canada implemented a national genetic evaluation for resistance to clinical mastitis (Miglior et al., 2014). The prediction of phenotype from genomic markers is possibly the field in which the most rapid advances are occurring with opportunities to improve the understanding of cow resistance to mastitis, as long as high‐quality phenotypic information is available. For mastitis, it is probable that most of the variation in cow mastitis resistance will be under the control of multiple genes. Thus it will be necessary to integrate a polygenic approach to genome investigation to provide benefits in explaining the overall impact of genetic variability; similar studies have proved invaluable in deciphering the genetic involvement for human diseases (Musliner et al., 2015; Szulkin et al., 2015).
Breeding for disease resistance or resilience is now widespread in the livestock industry, being applied on an extensive or nationwide basis as part of the selection index in a number of countries. The earliest and most notable examples are the disease recording routines in Scandinavian countries (Koeck et al., 2010), where selection is applied in cattle to increase milk production, reproductive performance, and resistance to mastitis, ketosis and ‘other’ diseases. Health data collected via veterinary systems are integrated into the national electronic database system, and used for genetic evaluation (Heringstad et al., 2000). Health data recorded by dairy producers can be used to establish an evaluation system for genetic selection for improved disease resistance (Koeck et al., 2012). Routine genetic evaluation for health traits based on producer‐recorded data have, not been implemented so far. One of the reasons is that it is difficult to get accurate health data from a large number of herds (Koeck et al., 2012).
Although genetic resistance to disease is regulated by multiple genes, controlling different processes of the host–pathogen interaction, the genetics of natural resistance is increasingly understood through identification and characterisation of candidate genes, microsatellite markers and comparative gene mapping, to develop more practical methods of application. Research is being undertaken to decipher host response to infectious pathogens, in view of possible genetic solutions to improve health (Doeschl‐Wilson et al., 2012; Guy et al., 2012), by characterising phenotypes and identifying biomarkers for genes and gene expression which are associated with an increased or impaired resistance and tolerance. With the sequencing of the genomes of some species now complete, new opportunities exist for identifying genes and DNA markers for disease resistance quantitative trait loci (QTLs). In the longer term, one objective would be to incorporate these genes or marker tests into existing selection programmes (Morris, 2007).
New gene editing approaches against specific diseases have been successfully investigated in several species. Gene editing is a type of genetic engineering in which DNA is inserted, replaced, or removed from a genome using specially engineered enzymes called restriction enzymes or molecular scissors. One example is in pigs against PRRS (Whitworth et al., 2016). Vaccines have been unable to control the disease and genetic selection programmes have failed to identify animals that are resistant to PRRS virus challenge. CD163 is the receptor for entry of PRRS virus into cells and therefore required for infection, and Whitworth et al. (2016) suggested that pigs with defective CD163 would be immune to PRRS virus, and these authors indicate that the use of such genome‐edited pigs could substantially reduce PRRS‐related economic losses.
Concluding remarks
Genetic selection has the potential to improve both innate and adaptive immune competence, thus potentially reducing the need to use antimicrobials.
Recommendations
Further research should be encouraged to develop genetic selection strategies, which take account of both welfare and animal health criteria, identifying animals with ability to mount superior immune responses.
Further integration of national database systems is needed, to facilitate research and breeding for improved disease resistance in farm animals.
Further research on new gene editing approaches against specific diseases should be encouraged.
Availability of data on production, health and welfare should be secured for future research on the associations between the respective parameters.
4.2.3.7. Herd health plans
Health planning is becoming an important element of many farming practices in pig, poultry, beef and dairy farming. Driven by market economics, the risks and consequences of poor health and suboptimal production become greater. Herd health plans make listed targets and goals for assessment, monitoring and ‘action’ with regard to health of the animals in a flock or herd. According to (Sibley, 2006), the listed goals or aims of health plans should be:
Specific. Use figures or observations that are specific to the farm, and can be related to the particular disease problems seen the particular farm.
Measurable. Use criteria that can be objectively measured, so that improvement or deterioration can be assessed and compared without dispute. Avoid subjective judgements such as ‘the cows look better this year’ or ‘lameness must be improved’.
Achievable. Gradual, sustained improvement will be more achievable and desirable than grandiose eradication campaigns that may be destined to fail. For example, eradicating mastitis completely will not be possible, nor will completely eliminating lameness, but setting achievable and realistic targets may result in significant reduction in these conditions.
Relevant. Prioritise objectives to those issues and areas that will bring most benefit. For example, attempting to eradicate subclinical infectious bovine rhinotracheitis from a herd with an 80% incidence of clinical mastitis and a 30% incidence of lameness may not be well received nor cost effective.
Time based. Set realistic objectives and targets with specific milestones so that progress can be monitored. Sometimes, things do not go to plan and reviews may be required more often than annually.
A herd health plan is the actual operational tool developed to achieve goals regarding herd health based on an overall strategy on herd health. The goal and targets for herd health can be set by many different stakeholders. Mostly the goals about herd health are set by the individual farmer (i.e. control production disease). The herd health plan is constructed for the farmer, who ideally defines targets, create action plans, monitor the health and review the plans, and modify the plans if needed (Sibley, 2000). Herd health plans are primarily used to plan for higher profitability, and have proven their value for this purpose (Young et al., 1985; Hogeveen et al., 1992). The goals can be set by the industry (i.e. voluntary eradication programmes) or by the authorities. Here, we focus on herd health plan as a tool to achieve a goal set by the authorities.
A study was recently conducted in the UK to identify dairy farmers’ drivers and barriers to reduce use of antimicrobials (Jones et al., 2015). It found that health planning was part of described processes being used by farmers in the study to understand their use of antimicrobials and to assist in planning to reduce their use of antimicrobials. Advice from their veterinarian, some of which was included in health planning, was instrumental in this, noting that farmers considered veterinarians to be their most influential source of information, and 97% of the interviewed farmers thought it important to keep medicine treatment records.
As noted previously, herd health plans are mandatory in some pig herds in Denmark, when antimicrobial use exceeds a certain level. Furthermore, the farmers’ access to antimicrobials is regulated through herd health plan and herd visits by the antimicrobial prescribing veterinarian in dairy production. The treatment pattern within a herd has been observed to change when a herd health plan is initiated that involves frequent visits by the veterinarian (Krogh and Enevoldsen, 2014). The results were interpreted as a change of treatment threshold in combination the educational focus resulting from the frequent contact between farmer and veterinarian. The veterinarian may safeguard against excessive use of antimicrobials and animal welfare related to mal‐ and mistreatment of diseased animals. Whereas herd health plans are mandatory, there are no specifications on specific targets that farmers should set.
In a review of the impacts of herd health planning, Tremetsberger and Winckler (2015) state that animal health and welfare planning aims to help maintain and promote animal health and welfare through interventions, applied in a structured way. The review examines scientific approaches to, and improvements achieved by, health and welfare planning in dairy herds, and discusses the economic, and non‐monetary benefits to farmers. These authors indicate that implementation of successful changes in management and housing is based on an assessment of the health and welfare status, on assessment of farm‐specific measures of management and housing, on continuous review, and on prompt adaptive action, through health planning. The authors state that health planning has the potential to result in monetary and non‐monetary benefits for the farmer, as costs of disease and impaired health can be examined in a health plan, and so rational decisions made on the efficiency of treatment, particularly in regard to mastitis and lameness. Non‐monetary factors such as job satisfaction are reported as motivating factors for farmers, linked to the use of herd health planning, enabling them to assess and adapt to planned management activities for improvement.
Herd health plans are probably most useful as advisory tools, because that is what they were developed for. The authorities can make them mandatory, but the value can be reduced if they become too prescriptive, because they will then lose their primary force. Tremetsberger and Winckler (2015) examined the impact on dairy herd health of a 1‐year duration programme of animal health and welfare planning, carried out on 34 farms. As well as written health plans, this study involved an integrated ‘participatory approach’ where discussion of farm‐specific aims and measures took place between the farmer and the research team/advisors. These authors found that, in dairy cows, the udder health and cleanliness of teats improved significantly 1 year after farms had implemented changes in husbandry practices linked to use of herd health plans, but did not find evidence that the use of management changes linked to health plans led to an improvement of leg health.
Herd health plans are operational in different forms, and the primary purpose of a specific herd health plan may differ greatly from that of another. The purposes can include monitoring of outcomes (health or disease), monitoring of risk mitigation, monitoring of treatments, or disease prevention broadly. In studies on Swedish farmers’ choices of mastitis control, an integrated risk‐benefit analysis is carried out by Hansson and Lagerkvist (2014). They describe planned management activity in 175 Swedish farmers related to: (i) grouping cows and applying milking order to prevent spread of existing infection, and (ii) working in a precautionary way to prevent mastitis occurring. These planned herd activities were linked to: (i) reactive management behaviour regarding detection of udder‐health problems in individual cows, and (ii) proactive management behaviour to prevent mastitis developing. This study suggests that farmers’ management behaviour depends on their individual attitudes related to grouping cows and applying milking order to prevent the spread of mastitis once infected cows were detected were stronger in the ‘risk domain’ than in the ‘benefit domain’, whereas planned management efforts to prevent cows from becoming infected in the first place were stronger in the ‘benefit domain’ than in the ‘risk domain’.
The use of the Herd health plan in the latter can for example be risk assessment as an educational tool, or as a communication tool between farmer and advisor. Herd health plan can also be used by different stakeholders, e.g. veterinary advisors and veterinary authorities. Central to herd health plans are that they centre on the herd, while the basis of the herd is the individual animal. Animals differ from herd to herd, and the herd is subject to the conditions given by the farm and the farmer. Consequently, the purposes and objectives can very well differ from one herd to another. Therefore, the outcomes are usually not comparable, and evaluation of herd health plan by a farmer is different to that of a herd health plan of a researcher.
Concluding remarks
Herd health plans can assist in disease control efforts at farm level since they are based on an integrated approach through monitoring of health, maximising passive and active immunity, monitoring of disease and disease prevention. Herd health plan can play a significant role in monitoring and responding to disease risks and in optimising on‐farm use of antimicrobials.
A herd health plan can serve as a tool to set targets and identify measures to improve the biosecurity and management measures, but requires a high level of commitment and agreement between the farmer, his/her veterinarian and other professional advisors.
Recommendations
Professional input into on‐farm animal health management, e.g. use of herd health plan should be encouraged.
Herd health plans need to be designed, implemented and followed up with care and with the support and involvement of the farm veterinarian and other professional advisors.
Herd health plans should be reviewed and revised on a regular basis to ensure that they are ‘living documents’ and are of active management value, resulting in real potential to improve animal healt hand reduce antimicrobial use, rather than simply ‘audit requirements’.
4.2.3.8. Rethinking of the livestock production systems
In some farming systems, much reliance is placed on the routine use of antimicrobials for disease prevention or for the treatment of avoidable outbreaks of disease, such that these systems would be unsustainable in the absence of antimicrobials. The stress associated with intensive, indoor, large scale production may lead to an increased risk of livestock contracting disease. One example relates to white veal production, which specialises in rearing calves on a low‐iron milk powder diet (Pardon et al., 2012b). In this industry, there is comingling of young, recently transported, highly stressed calves from multiple farms, sometimes from both domestic and foreign origins (Pardon et al., 2012a). In this industry, the disease risk is high, in particular bovine respiratory disease (Pardon et al., 2012b), and there is very high on‐farm use of antimicrobial agents (Pardon et al., 2012a; Filippitzi et al., 2014). The occurrence of high levels of MDR in isolates of pathogenic, commensal and zoonotic bacteria from veal calves has been noted (Pardon et al., 2014). There may be opportunities to substantially reduce on‐farm antimicrobial use within this industry, given country‐level differences in on‐farm antimicrobial use that have been observed (Pardon et al., 2012a; Lava et al., 2016a). A further example comes from the dairy industry with a focus for many years on the routine use of antimicrobial dry cow therapy that is intramammary antimicrobials administered at drying off to all quarters of all cows as a protective treatment against mastitic organisms. Given general concerns of antimicrobial use and resistance in the farming industries there has been a critical rethink of the blanket use of dry cow therapy in recent years. Emphasis is now placed on selective dry cow therapy, where antimicrobial therapy at drying off is limited to cows with a known infection risk (Biggs et al., 2016). In the Netherlands, for example, selective dry cow therapy has been mandated since 2013. This shift is not entirely straightforward. Recent research has highlighted an increase in both clinical and subclinical mastitis in low somatic cell count cows with this change (Scherpenzeel et al., 2014), with the selection criteria used when identifying cows to treat influencing the effect on udder health, antimicrobial use and farm economics (Scherpenzeel et al., 2016). Within this industry, there is increased use of internal teat sealant at the time of drying off, which has been shown to significantly decrease the risk of new intramammary infections during the dry period, compared with untreated cows (Rabiee and Lean, 2013).
Given the increased scrutiny on on‐farm antimicrobial use, there may be a need to critically evaluate the long‐term sustainability of all farming systems. Farming systems, and individual farms, should combine good husbandry, animal welfare and hygiene practices alongside an appreciation for the need to sustainably reduce use of antimicrobials. On many intensive pig and poultry farms, the approach generally applied to reduce disease exposure, and hence antimicrobial need, is to increase hygiene and ‘biosecurity’. Similarly, a reduction in livestock density, especially in critical areas such as calving pens and calf houses, is another means to reduce both disease incidence and the need for on‐farm antimicrobial use in farm animals.
Concluding remarks
Some farming systems are heavily reliant on on‐farm antimicrobial use and may not be sustainable in the long term.
Recommendations
Farming systems should combine good husbandry, animal welfare and hygiene practices alongside an appreciation for the need to sustainably reduce on‐farm use of antimicrobials.
Farming systems with heavy antimicrobial use should be critically reviewed, to determine whether/how such systems could sustainably reduce the use of on‐farm antimicrobials. If a sustainable reduction in the use of on‐farm antimicrobials is not achievable, these systems ideally be phased out.
4.2.3.9. Alternative food animal production systems
‘Alternative’ food animal production refers to farming practices that are different than those used in conventional farming. In this context, some systems have legally defined standards, such as ‘organic production’, while others are governed by private standards, such as ‘label‐rouge’.
‘Antibiotic‐free production’ is discussed in Section 3.4.1 but is a marketing label rather than a defined production system.
There are features of these production standards, which are likely to reduce the need for antimicrobial use during animal production. This is illustrated for organic production in the following section since there are fundamental differences between organic and conventional farming systems for example in terms of husbandry practices (e.g. space allowance, access to outdoor, type of feed ingredients) and restrictions related to antimicrobial use.
These practices may also be used in other systems to reduce the need for antimicrobial use.
4.2.3.10. Organic production
The intentions of organic livestock production have been formulated by the International Federation of Organic Agriculture Movements (IFOAM) and were originally regulated by Regulation (EEC) No 2092/9176, and later by the new Regulations (EC) No 834/2007 and 889/2008. Disease prevention in organic farming is based on several broad principles including that an animal is allowed to exhibit natural behaviour in more natural and less intensive conditions, is subject to reduced stress and is fed optimal (organic) feed, and that choice of breeds and lines should consider susceptibility/resistance to disease. The supposition is that this will result in an improved ability for organically farmed animals to withstand disease challenges, or that organic systems may not present some of the disease challenges seen in intensive production systems.
Animal health and welfare
With organic farming, the freedom to express some of the range of normal behaviours may be achieved through a focus on environmental enrichment, such as outdoor access (for example, to grass, grazing or foraging and rooting (for pigs) areas), and group housing.
In organic herds, control of animal health problems is affected by the restrictions in medicine use and preventive medication. This situation might be aggravated when there is risk of wildlife contact (Spoolder, 2007) and difficulties in cleaning and disinfection imposed by the access to outdoor areas and ban on the use of certain biocides (Gupta et al., 2015). Consequently, organically reared animals are more exposed to wildlife and pests (rodents) and at risk of contracting infectious diseases from the outside environment through airborne or soil borne organisms via insects or as a result of climatic variation, or through temperature and humidity alterations (Spoolder, 2007). It is possible (although not proven) that some organically managed animals develop more innate resistance to illness.
As discussed below, the standards associated with organic farming may but do not per se ensure either high levels of animal health and welfare or safer (from a public health perspective) livestock food products (Hovi et al., 2003; Lund, 2006; Vaarst et al., 2006; Fall et al., 2008).
As yet, there has been limited research on the health of organic pigs (Simoneit et al., 2012). Some studies report a higher prevalence of joint condemnations (Etterlin et al., 2014) and lameness (Krieter et al., 2004) in organic free‐ranging finisher pigs (pigs close to slaughter weight) with outdoor access compared to conventional finishers. Analysis of the prevalence of lesions in pigs at slaughter in Denmark (Alban et al., 2015) and Sweden (HS, 2010) show the overall prevalence of chronic pleuritis was higher in conventional than organic pigs; there was, however, no consistent pattern across the full range of different conditions reported.
Similarly, studies of the incidence of mastitis in dairy cattle have given varying results with the majority showing significantly lower incidences in organic systems (Hardeng and Edge, 2001; Ellis et al., 2007; Pol and Ruegg, 2007b), but with occasional reports to the contrary (Hovi and Roderick, 2000).
Blanco‐Penedo et al. (2012) carried out a study to evaluate and compare organic beef cattle farming in Spain with intensive and conventional farming systems. The authors concluded that organic and conventional beef cattle farms did not substantially differ with regard to animal health. The evaluation of condemned and rejected animals at slaughter showed that organic calves presented fewer condemnations compared with intensively reared calves.
Antimicrobial resistance
Considerable research has been conducted investigating the potential association between organic farming and reduced AMR, and in particular the hypothesis that organic farming may reduce the occurrence and/or prevalence of AMR bacteria isolated from food‐producing animals raised under such conditions.
In this Opinion, the above‐mentioned hypothesis has been tested using two sets of evidence: (i) existing published systematic reviews and (ii) an extensive literature review specifically performed by EFSA.
Existing systematic reviews
The review from Wilhelm et al. (2009) considered 47 studies, including 12 on the prevalence of AMR. The review concluded that reports of lower AMR prevalence in animals raised under organic conditions were more consistent in the USA as opposed to Europe. A number of methodological issues were identified, with the authors highlighting the need for more well‐designed and well‐executed studies, both at farm and post‐farm level.
Young et al. (2009) retrieved 38 studies and extracted data from 18 of these, comparing the prevalence of bacteria resistant to antimicrobials in organic and conventional poultry, swine and beef production. After pooling data with a meta‐analysis, bacterial prevalence was found to be higher in organic broiler chickens at slaughter, but not at retail. Bacteria isolated from conventional animal production exhibited a higher prevalence of resistance to antimicrobials; the recovery of some antimicrobial‐resistant strains was also identified in organic animal production, in situations with an apparent reduced antimicrobial selection pressure.
Finally, Smith‐Spangler et al. (2012) published a systematic review involving seven studies in humans and 223 studies of nutrient and contaminant levels in food. This review concluded that consumption of organic foods may reduce exposure to AMR bacteria. The design and statistical analyses in at least four of the five studies used to reach this conclusion were not aligned. In particular, a hierarchical sample design was used with several sampling units collected within each study unit (e.g. meat samples, drumstick samples, whole chicken carcases, etc.). The resulting clustering effect with multiple technical replicates from the same sample and multiple samples from the same store was not included in the statistical analyses, either in the original studies (Cui et al., 2005; Miranda et al., 2007; Miranda et al., 2008a,b), or in the systematic review. The fifth study included did not demonstrate any effect of farming type (Lestari et al., 2009).
Due to some methodological flaws identified in the three systematic reviews and the large uncertainties in the results, the RONAFA WG elected to perform an additional extensive literature review. Details of the search strategy, queried databases and selection criteria used to identify relevant studies are provided in Appendix G. In addition, all primary papers included in the three systematic reviews were screened.
Extensive literature review
The results of the extensive literature review are reported in Appendix J. A total of 125 items were identified with the search. Out of these, 33 papers were considered relevant. Nine additional papers were evaluated, as they were included in the three systematic reviews reported above. In total, 42 papers were evaluated.
In total, 16 papers refer to studies conducted within the EU, in EEA countries and in Switzerland and 26 studies in other countries.
Detailed information about the 42 studies is presented in Appendix J:
In Table J.1 studies are listed and their main characteristics described.
In Table J.2 some key limitations of the studies (i.e. confounders that need to be controlled/accounted for in the studies; other biases that could systematically impact on the study results; appropriateness of the methods used to analyse data; biological relevance of the study results) are addressed and findings are reported.
Table J.1.
Studies identified and their main characteristics
| Study ID | Citation | Species | Bacteria, matrix |
|---|---|---|---|
| 1 | Osterberg et al. (2016) | Pigs | E. coli, faeces |
| 2 | Gerzova et al. (2015) | Pigs | Faecal microbiota, faeces |
| 3 | Huijbers et al. (2015) | Broilers and humans working in farms | S. aureus and E. coli, broiler and human samples |
| 4 | Fraqueza et al. (2014) | Chickens | Campylobacter spp., caecal pools, carcasses |
| 5 | Cohen Stuart et al. (2012) | Chickens | E. coli, poultry products |
| 6 | Kola et al. (2012) | Chickens | E. coli producing ESBLs, meat |
| 7 | Garmo et al. (2010) | Dairy cows | S. aureus and coagulase‐negative staphylococci, milk |
| 8 | Miranda et al. (2008b)a | Pigs | E. coli, meat at the point of retail |
| 9 | Miranda et al. (2008a) | Poultry | E. coli, S. aureus, Listeria monocytogenes, meat at the point of retail |
| 10 | Schwaiger et al. (2010) | Laying hens | Salmonella spp., E. coli, Campylobacter spp., eggs, cloaca samples |
| 11 | da Miran et al. (2007)a | Chickens | Enterococcus spp., meat at the point of retail |
| 12 | Bennedsgaard et al. (2006) | Dairy cows | S. aureus, milk |
| 13 | Roesch et al. (2006) | Dairy cows | S. aureus, non‐aureus staphylococci, Streptococcus uberis, Str dysgalactiae, milk |
| 14 | Boutet et al. (2005)a | Dairy cows | Mastitis pathogens, milk |
| 15 | Heuer et al. (2001) | Broilers | Campylobacter spp., faeces |
| 16 | Kassem et al. (2016) | Chickens | Campylobacter spp., faeces |
| 17 | Lee et al. (2013) | Poultry | Salmonella spp., eggs |
| 18 | Zwonitzer et al. (2016) | Pigs | E. coli, manure |
| 19 | Tamang et al. (2015) | Pigs | Salmonella spp., faeces |
| 20 | Cicconi‐Hogan et al. (2014) | Dairy cows | S. aureus, coagulase‐negative staphylococci (CNS), milk |
| 21 | Mollenkopf et al. (2014) | Chickens | Salmonella spp., E. coli, Campylobacter spp.,, chicken products |
| 22 | Sapkota et al. (2014) | Chickens | Salmonella spp., litter/water/food samples |
| 23 | Sapkota et al. (2011) | Chickens | Enterococcus spp. |
| 24 | Millman et al. (2013) | Poultry | E. coli, poultry products |
| 25 | Park et al. (2012) | Dairy cows | Coagulase‐negative staphylococci, milk |
| 26 | Lestari et al. (2009) | Chickens | Salmonella spp., carcases at the point of retail |
| 27 | Nulsen et al. (2008)a | Pigs | E. coli, Enterococcus spp., faecal samples |
| 28 | Bombyk et al. (2008) | Dairy cows | Staphylococcus spp., milk |
| 29 | Bunner et al. (2007)a | Pigs | E. coli, faecal samples |
| 30 | Cho et al. (2007) | Dairy cows | E. coli, faecal samples, manure |
| 31 | Pol and Ruegg (2007a)a | Dairy cows | Gram‐positive mastitis pathogens |
| 32 | Siemon et al. (2007)a | Poultry | Salmonella spp., faecal droppings |
| 33 | Walk et al. (2007) | Dairy cows | E. coli, faeces |
| 34 | Gebreyes et al. (2006)a | Pigs | Salmonella spp., faecal samples and carcass swabs |
| 35 | Halbert et al. (2006) | Dairy cows | Campylobacter spp., faeces, environmental samples |
| 36 | Luangtongkum et al. (2006) | Chickens and turkeys | Campylobacter spp., intestinal tracts |
| 37 | Ray et al. (2006) | Dairy cows | Salmonella spp., environmental and faecal samples |
| 38 | Cui et al. (2005) | Chickens | Campylobacter and Salmonella spp., carcases at the point of retail |
| 39 | Price et al. (2005)a | Chickens | Campylobacter spp., poultry products |
| 40 | Sato et al. (2005) | Dairy cows | E. coli, faeces |
| 41 | Sato et al. (2004) | Dairy cows | Campylobacter spp., faeces |
| 42 | Tikofsky et al. (2003) | Dairy cows | S. aureus, milk |
Studies retrieved from one of the three systematic reviews considered (see Section 4.2.3.10).
Table J.2.
Findings and some key limitations of the studies identified
| Study ID | Observational study type | Location | Data analysis | Number of AM evaluated | Study findings | |
|---|---|---|---|---|---|---|
| Account for farm‐level clustering | Confounders/correlated risk factors controlled for | |||||
| 1 | Cross‐sectional | EU [Denmark, France, Italy, Sweden] | Yes | Not stated | Multiple antimicrobials tested | In each of the four countries, resistance to intestinal E. coli (ampicillin, streptomycin, sulfonamides, trimethoprim) was significantly lower in organic than conventional pigs. There were also large differences between countries within each production type |
| 2 | Cross‐sectional | EU [Denmark, France, Italy, Sweden] | No | Country | Multiple antimicrobials tested | There were no extensive differences between the abundance of AMR genes in samples from organic or conventional housed pigs. Samples from southern European countries exhibited significantly higher AMR gene abundance than those from northern Europe |
| 3 | Cross‐sectional (at two points in the production cycle) | EU [Netherlands] | Yes | Not stated | Single antimicrobial (beta‐lactams) | MRSA was not detected in broiler and human samples on organic farms. At 34 days of age, there was no difference by production system in the prevalence of ESBL/AmpC‐producing E. coli. On organic farms, there was an observed fall in the prevalence of ESBL/AmpC‐producing E. coli by day 68 |
| 4 | Cross‐sectional | EU [Portugal] | No | None | Multiple antimicrobials and MDR tested | AMR was common among Campylobacter spp.isolates from both organic and intensive poultry production systems. Isolates from all origins were resistant to fluoroquinolones and tetracyclines. For ciprofloxacin and ofloxacin and for MDR, isolates from extensive indoor chicken were significantly less resistant than that from organic and intensive production |
| 5 | Cross‐sectional | EU [Netherlands] | No | None | MDR tested | The majority of organic chicken meat samples were contaminated with ESBL‐ producing E. coli. Prevalence of ESBL‐producing microorganisms was 100% on conventional and 84% on organic samples (p < 0.001). Median loads of ESBL‐producing microorganisms were 80 (range < 20–1,360) in conventional, and < 20 (range 0–260) CFU/25 g in organic samples (p = 0.001). Co‐resistance rates of ESBL‐positive isolates were: co‐trimoxazole 56%, ciprofloxacin 14%, and tobramycin 2% (no significant differences between organic and conventional isolates). Tetracycline co‐resistance was more prevalent in conventional than in organic samples (73% vs 46%, p < 0.001) |
| 6 | Cross‐sectional | EU [Germany] | No | Type of meat, store and store chain | Single antimicrobial tested | No differences could be observed in the prevalence of ESBL producers between organic and conventional samples |
| 7 | Cross‐sectional | EEA [Norway] | No | parity distributions, proportion dried off quarters, AI vs natural mating, season for first AI | Single antimicrobial tested | There was no significant association between AMR and herd type. There was few S. aureus isolates exhibiting wresistance to penicillin in both management systems: 8.8% and 14% in conventional and organic farming, respectively. Penicillin resistance against coagulase‐negative staphylococci isolated from subclinically infected quarters was 48.5% in conventional herds and 46.5% in organic herds |
| 8 | Cross‐sectional | EU [Spain] | No | Not stated | Multiple antimicrobials tested | In comparison with meat from conventionally raised pigs, E. coli isolates from organic pork meat were significantly less resistant to several antimicrobials (ampicillin, doxycycline, sulfisoxazole) |
| 9 | Cross‐sectional | EU [Spain] | No | Not stated | Multiple antimicrobials and MDR tested | In comparison with meat from conventionally raised chickens, E. coli isolates from organic chicken meat were significantly less resistant to 7 of 10 antimicrobials tested. For S. aureus and Listeria monocytogenes, AMR was significantly higher only for doxycycline |
| 10 | Cross‐sectional | EU [Germany] | No | Not stated | MDR tested | AMR rates and mean inhibitory concentrations of bacteria isolated from organic systems had lower values than from conventional systems |
| 11 | Cross‐sectional | EU [Spain] | No | Not stated | Multiple antimicrobials tested | In comparison with meat from conventionally raised chickens, Enterococcus spp. isolates from organic chicken meat were significantly less resistant to several antimicrobials (ampicillin, chloramphenicol, doxycycline, ciprofloxacin, erythromycin, vancomycin) |
| 12 | Cross‐sectional & longitudinal | EU [Denmark] | Yes | Not stated | Single antimicrobial tested (penicillin) | No statistically significant differences were observed in the prevalence of S. aureus resistant to penicillin between herd groups |
| 13 | Cross‐sectional | EU region [Switzerland] | No | Not stated | Multiple antimicrobials tested | The frequency of AMR on organic farms was not different from conventional farms |
| 14 | Cross‐sectional | EU [Belgium] | No | Health status and farming practices info collected but not stated if they were taken into account in the analysis | Multiple antimicrobials tested | Lower levels of antimicrobial resistance were observed among major mastitis pathogens on organic compared to conventional farms. Marked differences were seen for S. uberis, S. aureus, S. dysgalactaie in favour of organic farming but not for coagulase‐negative staphylococci |
| 15 | Cross‐sectional | EU [Denmark] | No | None | Multiple antimicrobials tested | The prevalence of Campylobacter spp.isolates was higher in organic compared to either conventional or extensive indoor broiler rearing farms. In the present investigation, low numbers of AMR isolates (six of 62 isolates) hampered comparison of resistance patterns of C. jejuni as well as of C. coli isolates between the three rearing systems. Thus, no relation between resistance pattern and origin of the Campylobacter spp. isolates could be established |
| 16 | Cross‐sectional | Non‐EU [USA] | Yes | Not stated | Multiple antimicrobials tested | Evidence of association. On two organic farms compared to three conventional farms, isolates has significantly lower resistance to ciprofloxacin, erthyromycin and tylosin. On a third organic farm (excluded after external validation of results), a relatively high number of AMR Campylobacter spp. were isolated |
| 17 | Cross‐sectional | Non‐EU [South Korea] | No | Not stated | Multiple antimicrobialstested | Isolates of Salmonella Gallinarum from organic and conventional isolates showed similar antimicrobial susceptibilities. Overall resistance was very low for the majority of agents tested |
| 18 | Cross‐sectional | Non‐EU [US] | No | Not stated | Multiple antimicrobials tested | E. coli isolates from conventional systems were significantly more resistant to a range of antimicrobials, compared to conventional systems. Results show that E. coli isolates from conventional systems were significantly more resistant to amoxicillin, ampicillin, chlortetracycline, erythromycin, kanamycin, neomycin, streptomycin, tetracyclines, and tylosin (p < 0.001) |
| 19 | Cross‐sectional | Non‐EU [Korea] | No | Not stated | MDR tested | The prevalence of AMR in Salmonella spp. isolates was significantly higher in pigs from conventional than from organic farms. The prevalence of AMR, MDR phenotype, and resistance to tetracyclines, ampicillin, and gentamicin were significantly higher in swine Salmonella spp. isolates from conventional farms than those from organic farms |
| 20 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Single antimicrobial tested | The prevalence of meticillin‐resistant S. aureus (MRSA) and methicillin‐resistant CNS was low, and no more prevalent in bulk tank milk from either organic (2% resistant coagulase‐negative staphylococci) or conventional (5%) herds |
| 21 | Cross‐sectional | Non‐EU [USA] | No | GROCERY STORE, STORE CHAIN, AND PROCESSING PLANT | Multiple antimicrobials and MDR tested | Similar levels of commensal bacteria harbouring genes conferring resistance were found in organic and ‘antibiotic‐free’ labelled meat compared to meat from conventional farms. Fluoroquinolone‐resistant E. coli recovered using selective media were more common (p < 0.05) in conventional (18.9%) compared to organic (0) and ‘antibiotic‐free’ (2.1%) packages |
| 22 | Cross‐sectional | Non‐EU [USA] | Yes | Not stated | Multiple antimicrobials tested | Among S. Kentucky isolates (n = 41), per cent resistance was statistically significantly lower among isolates recovered from newly organic vs conventional poultry houses for: amoxicillin‐clavulanate (p = 0.049), ampicillin (p = 0.042), cefoxitin (p = 0.042), ceftiofur (p = 0.043) and ceftriaxone (p = 0.042). Per cent MDR (resistance to ≥ 3 antimicrobial classes) was also statistically significantly lower among S. Kentucky isolates recovered from newly organic poultry houses (6%) compared to those recovered from conventional houses (44%) (p = 0.015) |
| 23 | Cross‐sectional | Non‐EU [USA] | Yes | Not stated | Multiple antimicrobials tested | The percentages of AMR Enterococcus faecalis and AMRE. faecium were significantly lower (p < 0.05) among isolates from newly organic vs conventional poultry houses for two (erythromycin and tylosin) and five (ciprofloxacin, gentamicin, nitrofurantoin, penicillin, and tetracyclines) antimicrobials, respectively. Forty‐two per cent of E. faecalis isolates from conventional poultry houses were multidrug resistant (MDR; resistant to three or more antimicrobial classes), compared with 10% of isolates from newly organic poultry houses (p = 0.02); 84% of E. faecium isolates from conventional poultry houses were MDR, compared with 17% of isolates from newly organic poultry houses (p < 0.001) |
| 24 | Cross‐sectional | Non‐EU [USA] | No | Meat brand | MDR tested | No difference was observed in resistance levels for bacteria isolated from organic chicken and conventional products. The frequency of AMR E. coli tended to be only slightly lower for RWA (raised without antimicrobials), and organic chicken was statistically indistinguishable from conventional products that have no restrictions. Over half of all strains collected exhibited resistance to one or more antimicrobials: 55%, 58%, 60%, and 76% from conventional, RWA, organic, and kosher chicken samples, respectively |
| 25 | Cross‐sectional | Non‐EU [USA] | No | Not stated | MDR tested | During the transition from conventional to organic on two farms, there was a significant decrease in CNS isolates deemed resistant to β ‐lactam antimicrobials, but no significant changes in the sensitivity patterns of antimicrobials to Streptococcus spp. or S. aureus |
| 26 | Cross‐sectional | Non‐EU [USA] | No | Salmonella serovar, chicken type, chicken brand, supermarket chain, store, and sampling date | MDR tested | Salmonella Kentucky isolates from organic chicken samples were susceptible to 11 of the antimicrobials tested, whereas those from conventional chickens were only susceptible to 4 antimicrobials. Three S. Kentucky isolates from conventional chickens possessed multidrug resistance phenotype MDR‐AmpC |
| 27 | Cross‐sectional and Longitudinal | Non‐EU [New Zealand] | No | Not stated | MDR tested | Higher levels of AMR in E. coli and Enterococcus spp. were observed in pigs from conventional compared to organic farms. Isolates of E. coli from conventional pig farms were resistant to gentamicin (0.7%), neomycin (0.7%), ampicillin (2.7%), cotrimoxazole (11%), streptomycin (25%) and tetracyclines (60%). Enterococcus spp. isolates from the same farms were resistant to erythromycin (68%), tetracyclines (66%), streptomycin (54%) and virginiamycin (49%). By contrast, for the organic pig farm < or = 5% of either the E. coli or the Enterococcus spp. isolates were resistant to any of the antimicrobials tested |
| 28 | Cross‐sectional | Non‐EU [USA] | Yes | Not stated | Multiple antimicrobials tested | A larger proportion of isolates from organic rather than conventional farms were susceptible to erythromycin, pirlimycin and tetracyclines. For pirlimycin and tetracycline, different patterns of susceptibility were observed among Staphylococcus categories. This latter result suggests that multiple management practices, including some unrelated to antimicrobial usage, may contribute to the observed difference in susceptibility |
| 29 | Cross‐sectional | Non‐EU [USA] | Yes | Herd size | Multiple antimicrobials tested | Compared with antimicrobial free farms, conventional farms has significantly higher levels of resistance to ampillicin, sulfamethoxazole, tetracyclines and chloramphenicol. Cessation of antimicrobial usage did not appear to result in an immediate reduction in AMR |
| 30 | Cross‐sectional | Non‐EU [USA] | No | Not stated | MDR tested | Resistance to at least one antimicrobial was observed in 18 (62%) isolates from conventional farms and in 11 (48%) isolates from organic farms. The percentage of MDR was 17.2%, 4.3%, and 10% in isolates from conventional farms, organic farms, and exhibition barns at county fairs, respectively. No significant difference in recovery of MDR between isolates from conventional and organic farms was observed |
| 31 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Multiple antimicrobials tested | Variable results were obtained. This study demonstrated a dose–response effect for several antimicrobial drug exposures and the MIC of the studied pathogens (S. aureus & pirlimycin p = 0.04; CNS & erythromycin p = 0.038, penicillin p = 0.007 pirlimycin p < 0.001, tetracyclines p = 0.004 Streptococci: pirlimycin p < 0.001 and tetracycline p = 0.001) |
| 32 | Cross‐sectional | Non‐EU [USA] | No | Not stated | MDR tested | Salmonella prevalence was not different between conventional and organic farms. A higher frequency of resistance was observed on conventional farms. MDR (resistance to three or more classes of antimicrobials) was found in 69% of the isolates from conventional farms and 11% on pasture farms (p < 0.0001), with the predominant resistance type of AmCSSuTeAx (ampicillin, chloramphenicol, streptomycin, sulfasoxazole, tetracyclines, amoxicillin/clavulanic acid; 62%). About 5% of the pasture isolates showed the AmAxCFCRO (ampicillin, amoxicillin/clavulanic acid, cephalothin, ceftriaxone) MDR pattern |
| 33 | Cross‐sectional | Non‐EU [USA] | Yes | age of cattle | Multiple antimicrobials tested | Study discovered a significant association between low MDR, organic farms, and strains of the numerically dominant phylogroup B1. The results suggest that organic farming practices changes the frequency of AMR isolates, and also impacts the genetic composition of the resident E. coli flora in the overall population |
| 34 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Multiple antimicrobials and MDR tested | Salmonella prevalence was significantly higher among antimicrobial free compared to conventional farms. In general, antimicrobial resistance is more common in conventional farms. Frequency of resistance to most classes of antimicrobials (except tetracyclines) was significantly higher among conventional farms than antimicrobial‐free farms, with ORs ranging from 2.84 for chloramphenicol to 23.22 for kanamycin at the on‐farm level. A pentaresistance pattern with ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracyclines was strongly associated with antimicrobial‐free groups (OR = 5.4; p = 0.01). Distinct MDR isolates of Salmonella spp. were common in antimicrobial‐free herds |
| 35 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Multiple antimicrobials tested | Similar levels of AMR were found in organic and conventional farms. The proportion of AMR isolates was higher for conventional than for organic farms. |
| 36 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Multiple antimicrobials tested | The AMR rates were significantly different between the organic and conventional farms. Less than 2% of Campylobacter spp. isolates from organically raised poultry were resistant to fluoroquinolones, while 46% and 67% of Campylobacter spp.isolates from conventionally raised broilers and conventionally raised turkeys, respectively, were resistant to these antimicrobials. In addition, a high frequency of resistance to erythromycin (80%), clindamycin (64%), kanamycin (76%), and ampicillin (31%) was observed among Campylobacter spp. isolates from conventionally raised turkeys. None of the Campylobacter spp. isolates obtained in this study was resistant to gentamicin, while a large number of the isolates from both conventional and organic poultry operations were resistant to tetracyclines. MDR was observed mainly among Campylobacter spp. isolates from the conventional turkey operation (81%). Findings from this study clearly indicate the influence of conventional and organic poultry production practices on AMR of Campylobacter spp. isolates on poultry farms |
| 37 | Cross‐sectional | Non‐EU [USA] | Yes | Herd size and state | Multiple antimicrobials tested | Although not statistically significant, conventional farms tended to be more likely than organic farms to have at least one Salmonella isolate resistant to five or more antimicrobial drugs |
| 38 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Multiple antimicrobials tested | The prevalence and antimicrobial susceptibility of campylobacters and salmonellae varied by farming system. Organic chickens were more frequently contaminated with campylobacters and salmonellae; the pathogens from organic animal production were more susceptible to certain antimicrobials |
| 39 | Cross‐sectional | Non‐EU [USA] | No | Not stated | Single antimicrobials tested | Conventional products has significantly higher odds of carrying resistant strains of Campylobacter spp.isolates compared with antimicrobial‐free products |
| 40 | Cross‐sectional | Non‐EU [USA] | No | Animal age (cow vs calf) and season(September vs March) | Multiple antimicrobials tested | There was a significant association with herd type for 7 of 17 antimicrobials. After controlling for age, logistic regression analyses indicated that isolates from conventional dairy farms had significantly higher rates of resistance to ampicillin, streptomycin, kanamycin, gentamicin, chloramphenicol, tetracyclines, and sulfamethoxazole than did isolates from organic dairy farms. No significant differences were detected for the 10 other antimicrobials that were tested |
| 41 | Cross‐sectional | Non‐EU [USA] | Yes |
Animal age (cow vs calf) and season (September vs March) |
Multiple antimicrobials tested | Farm type (organic or conventional) was not a significant predictor of resistance to any of the four tested antimicrobials: ciprofloxacine, gentamicin, erythromycin or tetracyclines |
| 42 | Cross‐sectional | Non‐EU [USA] | Yes | Herd | Multiple antimicrobials tested | Significant difference observed with 7 of 9 antimicrobials. S. aureus isolates from both organic and conventional herds showed good susceptibility to most commonly used bovine mastitis antimicrobials isolates from organic herds were significantly more susceptible |
AMR: antimicrobial resistance; CIA: critically important antimicrobials; ESBL: extended‐spectrum beta‐lactamase; MDR: multidrug resistance; MIC: minimum inhibitory concentration; MRSA: meticillin‐resistant S. aureus; OR: odds ratio; RWA: raised without antimicrobials.
The key findings may be summarised as follows:
In the majority of studies, an association was observed between organic farming and reduced AMR.
-
These results need to be interpreted with considerable care, for a range of reasons:
-
–
These studies were observational, based on samples collected over a limited period of time. These were mainly cross‐sectional studies conducted on small numbers of organic and conventional farms. It is not possible to determine causation from such cross‐sectional observational studies.
-
–
In most studies, there was limited or no control of potential confounders/correlated risk factors, either during study design or data analysis. It may be very difficult to control for those factors most likely to confound the association between farming type and AMR, such as disease status and farmer characteristics, other than through controlled experimental studies.
-
–
Many of the studies were conducted outside the EU/EEA countries/Switzerland. It may be difficult to generalise these studies to a European situation.
-
–
Few studies appropriately accounted for farm‐level clustering during data analysis. In studies where farm‐level clustering has not appropriately been controlled, the association between farming type and AMR may be deemed statistically significant, when in fact it is not.
-
–
In many studies, consideration was given to the biological relevance of the study results. Emphasis should be placed on studies that simultaneously consider the presence of different acquired resistance determinants.
-
–
There is a need for further research, through studies that appropriately address the concerns raised previously, to rigorously evaluate the potential for organic farming to reduce AMR.
Longitudinal studies of livestock farms undergoing the transition to organic farming, or withdrawal of specific antimicrobial classes, would help elucidate the dynamics of antimicrobial resistance and the potential return of antimicrobial susceptibility in some bacteria (Tikofsky et al., 2003).
Concluding remarks
While the standards associated with organic farming may but do not per se ensure high levels of animal health and welfare, the prevalence of certain diseases associated with high antimicrobial use can be lower in organic farming.
Organic or similar alternative farming practices may improve housing and management conditions for animals and therefore contribute to secondary and tertiary prevention, while primary prevention may be compromised, for example by increased levels of exposure to wildlife).
In the majority of the studies appraised, an association was observed between organic farming and reduced AMR. However, due to the limitations in the study design, methodologies for data analysis and biological relevance of the approach, in many of these studies there is a potential for bias in the estimate of the association and effect of organic farming on AMR. Therefore, conclusive evidence of the impact of organic farming on reducing AMR cannot be established because of the high level of uncertainty in the appraised studies.
Recommendations
Some regulated production systems are able to operate with low antimicrobial use, and the farming practices which facilitate this should be further evaluated. Any associated animal health and welfare risks due to, e.g. higher risk for bacterial/parasitical/viral exposure also need to be considered.
Further research is needed, evaluating the potential for organic farming to reduce AMR. In future work, potential limitations of study design, study analysis and biological relevance should be addressed.
Table 5 summarises the animal management and husbandry procedures reducing the need for use of antimicrobials discussed above.
Table 5.
Animal management and husbandry procedures reducing the need for antimicrobial use
| Primary prevention (to reduce the introduction and spread of microorganisms between farms) | |
|---|---|
| External biosecurity, including introduction of animals | Routine cleaning and disinfection of facilities between batches of animals |
| Disinfection of vehicles for transport | |
| Reduced mixing of animals from different batches | |
| Isolation of sick or diseased animals and cleaning operator clothes and boots | |
| Movement restrictions following outbreaks | |
| Avoiding distribution hubs for live animals | |
| Understanding of health status of farms from which animals are being purchased or of neighbouring farms | |
| Control of surface waters, enrichment materials and feed from other farms | |
| Housing design to minimise entry of pathogens (doors and walls to prevent access of wild and pest animals, barriers for human access, facilities for human hygiene, air filters) | |
| Compartmentalisation (including internal trading, SPF systems) | SPF concept could be more widely adopted |
| Eradication | Eradication of specific diseases and endemic pathogens by all‐in all‐out (poultry), stamping out and medication combined with vaccination, DIVA tests and/or selective removal of individual infected carrier animals |
| Secondary prevention (to reduce transmission or spread within a farm) | |
| Internal biosecurity | On‐farm risk assessment to identify areas for focused action |
| Diagnostic test strategies to focus antimicrobial use and support management strategies | |
| Segregation | |
| Reducing stocking density | |
| Production groupings | All‐in‐all‐out methods, batching by age, multisite production |
| Housing design, building and maintenance | Reducing the contact between animals and slurry, waste water or faeces |
| Use of a proper ventilation system | |
| Routine cleaning of housing | |
| Include sick pens | |
| Reducing production of ammonia by maintaining dry litter and removal of pollutant gases | |
| Tertiary prevention (to increase the ability of animals to cope with these pathogens) | |
| Vaccination | Need to improve vaccines for bacterial conditions |
| For pigs, vaccines for post‐weaning diarrhoea, porcine respiratory disease complex and Streptococcus suis are needed | |
| For poultry, E. coli is the main bacterial organism of concern | |
| For bovines, vaccines for mastitis, viral diseases in veal production, Mycoplasma bovis are needed | |
| Animal genetics | Genetic selection to improve both the innate and adaptive immune competence |
| Reducing the level of stress | Ensuring thermal comfort |
| Reducing stocking density | |
| Reducing mixing of unfamiliar animals | |
| Ensure proper weaning | |
| Avoid feed restrictions | |
| Ensure proper animal handling | |
| Ensure proper enrichment | |
| Ensure proper conditions during transport (provision of feed and water, climatic conditions, avoid mixing and crowding) | |
| Nutrition | Promoting proper nutritional measures, e.g. transition period, weaning of piglets, feed additives for microbiota composition |
| Herd (flock) health plans | |
| Professional input into on‐farm animal health management, e.g. use of herd (flock) health plans | |
| Review and revision of health plans on a regular basis to ensure that they are ‘living documents’ and are of active management value to the producer rather than ‘audit requirements’ | |
| Additional considerations on husbandry and management procedures | |
| Rethinking of the livestock production systems | Review farming systems with heavy antimicrobial use to determine whether/how such systems could sustainably reduce the use of on‐farm antimicrobials |
| Potential of alternative farming principles | Some regulated production systems are able to operate with low antimicrobial use, and the farming practices which facilitate this should be further evaluated. Any associated animal health and welfare risks due to, e.g. higher risk for bacterial/parasitical/viral exposure also need to be considered |
| Raising awareness of AMR issues | Increasing producer awareness of ‘general’ AMR issues (public and animal health threats) and encouraging best practices among farmers |
| Raising education on factors influencing disease occurrence | |
4.3. Diagnostic tools to enable targeted treatments
Diagnostic sampling and biomarkers
In ambulatory care, both in human and veterinary medicine, only the minority of patients undergo sampling at first visit. In an FVE survey (De Briyne et al., 2013), 44% of farm animal practitioners responded that they performed AST regularly, usually where treatment failure had occurred, and commented that use was limited by urgency of the situation, sampling difficulties, clinical relevance of the results and costs. The authors noted that AST is carried out in both private and public laboratories, which may not be specialised in veterinary testing, and also that tests are generally not regulated in Europe.
In the UK, pig veterinarians commented that AST was rarely used as routine when treating endemic disease but was reserved more severe disease outbreaks or for when a novel pathogen was suspected. The delay in obtaining test results was identified as being problematic when immediate treatment was required to prevent animal suffering (Coyne et al., 2016). If done and following applied routine methodologies, the identification of a bacterium and its susceptibility profile (antibiogram) takes on average 36 h (O'Neill, 2016). This time frame is shortened by the introduction new techniques including of mass spectrometric assays (e.g. matrix‐assisted laser desorption ionisation‐time of flight mass spectrometry, MALDI‐TOF). Such techniques have increased the accuracy of bacterial species identification substantially in sample matrices that have a sterile or low commensal bacterial load such as the blood, cerebrospinal fluid, urinary tract, and lower respiratory tract. For large bacterial load environments like the digestive tract, the bacterial diagnosis is still difficult.
Practitioners do not have patient‐side tests that allow to distinguish between a bacterial and other diseases (viral, mycotic, parasitic, metabolic and toxins). A bacterial diagnosis currently relies on anamnesis, physical and further examinations and specific tests if required or affordable. Detection of bacteria in sterile organs is highly indicative for a bacterial infection, especially if a certain microbial qualitative threshold is agreed upon by specialists (Jourdain et al., 1997). Since bacteria are always present in the direct environment, for non‐sterile body compartments like the upper respiratory tract and the gut, identification alone is insufficient for the majority of bacterial diagnosis. In these circumstances, additional symptoms and investigations, e.g. detection of the presence of virulence markers, measured either qualitatively or quantitatively, are needed (Ryder et al., 2010).
In bovine medicine a widely applied patient‐side test that has been used for decades, is the California Mastitis Test (CMT) (Barnum and Newbould, 1961). Tests with moderate specificity have been used to distinguish clinical mastitis caused by Gram‐negative vs Gram‐positive infections. Patient‐side biomarker tests are used and are under further development to improve bacterial diagnosis in human and veterinary medicine. Routine practice is so far limited in human medicine to a restricted number of measurements, such as procalcitonin (PCT) and C‐reactive protein (CRP) (Sundén and Wullt, 2015).
Use of Clinical breakpoints in veterinary medicine
Clinical breakpoints assist the veterinarian in determining whether a specific antimicrobial with the given dosing regimen will be effective at treating a bacterial infection, based on knowledge of the MIC for the isolate. Although use of AST to facilitate responsible use of antimicrobials is promoted in the EC PUAVM, it has been noted that quality standards for AST in the EU are unharmonised and incomplete and that there is a lack of veterinary species‐specific clinical breakpoints77 (Silley et al., 2012; de Jong et al., 2014). Papich (2014) noted that use of inaccurate breakpoints may encourage ineffective and unnecessary antimicrobial treatments. In support of establishment of clinical breakpoints, and in order to monitor evoluation of resistance in target pathogens, there is a need for harmonised monitoring of target pathogens in animals, such as under the programme established by the European Animal Health Study Centre (CEESA) (De Jong et al., 2013a). The Czech Republic has started a National Target Veterinary Pathogens Surveillance Programme in 2015 (Pokludova et al., 2016). The programme aims to provide an overview of MICs, including trends, for selected veterinary pathogens of pigs, poultry and cattle, for use by veterinarians in practice and in training and seminars. Harmonisation of the laboratory methods and interpretative criteria are also key elements.
Traditional phenotyping of organisms
The detection of most bacteria relies on culture‐based methods developed over many years. For routine bacteriology and mycology, the identification and antimicrobial susceptibility profiling relies on a phenotypic culture and biochemical tests, and Kirby‐Bauer disk diffusion test, respectively. Semi‐ or fully‐automated systems have been developed for this process, and sometimes the disk diffusion is replaced by the golden standard dilution technique for AST, namely determination of the MIC.
For the majority of microorganisms, specific characterisation such as serotyping (immunoassays, e.g. ELISA) and protein electrophoresis (biochemistry) are being gradually replaced by genotyping.
Genotyping of pathogens
Molecular analysis with polymerase chain reaction (PCR) is needed to demonstrate the presence of virulence or resistance genes in organisms (genotyping), sometimes in combination with plasmid profiling to detect horizontal transferable mechanisms. With multiplex PCR and microarrays different genes can be simultaneously identified, and advanced PCR techniques enables to quantify genes to improve the relevance of found genes and species‐specific virulence factors (Aarts et al., 2006; Batchelor et al., 2008). These systems can have a little workload and can have very short turn on hands (minutes) and turnaround time (hours). Detection and accuracy relies on and is limited to a well‐managed and up‐to‐date database with DNA information the needs continuously updated to detect upcoming mutations and mobile genetic elements. The mobile colistin resistance gene mcr‐1, (see Appendix A) was not detected for 10 years due to absence of the specific sequence in applied PCRs (Poirel and Nordmann, 2016).
When evolutionary (phylogenetic) and relatedness studies at the bacterial species level are undertaken, 16S rRNA sequencing has been revolutionised, thereby improving turnaround time and comparability (next‐generation sequencing (NGS), see below). To type organisms at the bacterial strain level (clonal relatedness) many techniques have been used, e.g. like pulsed‐field gel electrophoresis (PFGE), multilocus sequence typing (MLST), multiple locus variable number of tandem repeats analysis (MLVA), or PCR‐ribotypage (the latter for instance for C. difficile) (Barbut et al., 1993). These latter systems have been both widely and successfully applied during outbreak investigations. The typeability, discriminatory power, interlaboratory exchangeability, turnaround time, ergonomics and cost vary across these systems according to the materials and methodology used and the bacterial strain collection of interest.
Whole‐genome sequencing (WGS) and NGS are becoming increasingly used in clinical microbiology both in the human and veterinary spheres. WGS on isolated bacteria can reduce the processing time significantly and NGS directly on clinical samples can reduce the time even further. Additionally, diagnosis might be more accurate using WGS/NGS as virulence factors and AMR genes can be detected (Hasman et al., 2013). Another advantage of WGS/NGS is that unknown bacterial species can be detected, and mixed cultures are easily unravelled. AMR in human invasive pathogens (Aanensen et al., 2016) and zoonotic agents (Leekitcharoenphon et al., 2016) have been studied in detail. It might be hard to determine the clinical relevance of all detected species, but this will probably improve in time (Bae et al., 2013). WGS has also been used to study the evolution and emergence of AMR (Köser et al., 2014), and might therefore aid the accuracy of continuous surveillance systems (Zankari et al., 2013; McDermott et al., 2016).
A pipeline for annotating antimicrobial resistance genes (ARGs) based on metagenomic assembly to investigate ARGs and their co‐occurrence with associated genetic elements within complex intestinal microbiomes has recently been described. Genetic elements found on the assembled genomic fragments include mobile genetic elements (MGEs) and metal resistance genes (MRGs). This methodology will facilitate the determination of ARG hosts and the shared resistome from metagenomic data sets and establishment of the relationship between ARGs, hosts, and environments (Ma et al., 2015).
If an appropriate centralised sequence reference centre is available, nanopore systems may allow real‐time sequencing to be applied to detect specific AMR genes. By this method results are obtained by leading the DNA/RNA strands through a tunnel at the nanolevel. Results for bacterial species (including mixed cultures) and presumptive resistance profiles may be available within 2–10 h (Cao et al., 2016).
Spectrometry
The most recent wave in routine microbiology is the implementation of MALDI‐TOF and related spectrometry assays for rapid diagnosis of pathogens. By comparisons of protein profiles with a databank, identification of the bacterial species from pure culture is possible within minutes (Dingle and Butler‐Wu, 2013), although standardisation by bacterial species needs to be done (Martiny et al., 2013). A standard culture is still required, but recent advances show promising results for direct identification on clinical samples (Nilsen, 2012; Johansson et al., 2014).
These assays are not restricted to a limited number of organisms (including non‐bacterial infective agents like yeasts), and some are able to identify clonal relatedness and antimicrobial resistance mechanisms (e.g. β‐lactamases including carbapenemases) (Burckhardt and Zimmermann, 2011; Hrabák et al., 2011; Sparbier et al., 2012; Jung et al., 2014). When spectrometry is combined with antimicrobial testing via stable isotopes, clearly this assay indicates a high potential for rapid susceptibility testing of virtual any class of antimicrobials (Jung et al., 2014; Lange et al., 2014; Liu et al., 2016). Hence, these techniques have a large potential for outbreak investigations, including for veterinary medicine.
New diagnostics at herd level
The value of rapid diagnostic testing at the individual, commercial and social level have recently been reviewed by a specialist panel in the UK (O'Neill, 2016). To distinguish animals that need treatment from those that do not during an epidemic is a further strategy to reduce the number of antimicrobial administrations.
Rapid molecular diagnostic (RMD) platforms for identification of pathogens and ARGs present may lead to better antimicrobial use (Evans et al., 2016). Techniques such as virulotyping, which will probably become standard practice as WGS replaces other microbial diagnostic and characterisation technologies, will become increasingly important for focusing decisions on medication and other disease control options (van Hoek et al., 2016).
The coupled reporting of both human and veterinary clinically laboratories across EU countries, in an automated and harmonised way taking healthcare setting and herd history into account, can substantially increase the ‘One Health’ epidemiology of AMR both for research and for clinical purposes.
New techniques such as microfluidic paper based assays that are laser driven are under development, e.g. for antibody detection, and might aid in patient‐side testing for livestock in the future (Arnold et al., 2007; Martinez et al., 2010). Since they assume to be an affordable point‐of‐care diagnostic, including both pathogen and resistance detection, they also might get introduced at the herd level.
Concluding remarks
The average time between sampling and result, including AST is, under routine circumstances, still several days.
Existing diagnostic methodologies are limited by the availability of specialised veterinary laboratory facilities, time taken to obtain results and concerns over costs and clinical relevance of the findings.
Integration in veterinary medicine of modern techniques could enable more rapid and precise diagnosis, allowing better targeted antimicrobial use.
New techniques (genotyping, spectrometric phenotyping, WGS) are proving fast and efficient in human medicine for sterile sample sites.
The largest challenge is the rapid identification of enzootic diseases, in particular gastrointestinal diseases at the herd level.
Recommendations
The development of genotyping techniques should be encouraged in veterinary medicine.
Steps should be taken to accelerate the availability of new technologies that enable economical, rapid on‐farm diagnosis, so that veterinarians only prescribe antimicrobials when needed and can select the correct treatment.
New diagnostic methodologies should be validated to the EU standards to improve reliability and confidence in results and to ensure consistency between laboratories. Appropriate quality assurance systems should be developed and utilised.
Diagnostic testing protocols should be improved so that the appropriate clinically meaningful tests are performed in relation to presenting syndromes.
Caution should be taken not to over‐diagnose certain endemic syndromes by inaccurate diagnosis and clinically irrelevant AST on indicator or other ubiquitous commensal bacteria.
Clinical breakpoints of resistance to specific antimicrobials for veterinary pathogens should be established and agreed at the European level. In support, development of target pathogen monitoring programmes should be encouraged.
The results of national AST for target pathogens could be made publically available as a resource to assist veterinarians in making rational prescribing choices and to feed into development of treatment guidelines.
4.4. Alternative measures
In recent years, a number of alternatives to antimicrobial products have been suggested for use in food‐producing animals. These include a range of different agents, such as probiotics, prebiotics, plant‐derived compounds, bacteriophages, antimicrobial peptides and immunomodulatory agents. Most alternatives are administered to animals via feed. In spite of the large number of published articles in which such agents have been put forward as possible alternatives to antimicrobials, only a minor proportion of these reports include in vivo studies where the proposed alternative has been assessed in comparison with antimicrobials.
A literature search was undertaken to identify peer‐reviewed published articles on alternatives to antimicrobials, as indicated in Section 2.2.2. Details of the search terms used, databases queried and selection criteria used are provided in Appendix G. Specific criteria were used to select the papers for further review from those identified from the literature search; the aim was to select studies on the efficacy of the alternative measure on health parameters (e.g. reduced morbidity or mortality) and, preferably, reporting a comparison of with an antimicrobial treatment. Reduction in human zoonotic pathogens (e.g. Salmonella spp. and Campylobacter spp.) was generally not considered further when discussing alternatives to antimicrobials.
The majority of the papers identified by the search failed to meet one or more of the inclusion criteria. Most of the articles focussed on the effect of the potential alternative to antimicrobials on performance and did not include health parameters as endpoints of the studies. In a few studies, a comparison with an antimicrobial treatment was reported, mainly in the context of antimicrobials used as growth promoters.
The retrieved articles report studies on agents that in the EU fall into three different categories:
products authorised as veterinary medicinal products (VMPs) defined in Directive 2001/82/EC as any substance or combination of substances presented for treating or preventing disease in animals or which may be administered to animals with a view to making a medical diagnosis or to restoring, correcting or modifying their physiological functions;
feed additives, defined in Regulation (EC) No 1831/200378 as products used in animal nutrition for purposes of improving the quality of feed and of food from animal origin, or to improve the animals’ performance and welfare, e.g. providing enhanced digestibility of the feed materials. Most, e.g. probiotics and enzymes, are included in the category ‘zootechnical additives’. Others, such as zinc and copper, fall into the category ‘nutritional additives’. The procedure for the authorisation of feed additives under Regulation (EC) No 1831/2003, as defined in the Regulation (EC) No 429/200879, requires the demonstration of safety for the consumer of food of animal products, for the animal target species, for the user/worker exposed to the additive and for the environment. Moreover, the efficacy of the additive shall be proved. The main difference between VMPs and feed additives lays on the health status of the target animal (i.e. feed additives are to be used on healthy animals while VMPs on sick or potentially sick animals);
products not authorised in the EU. Some of the papers selected during the literature search describe agents not authorised as feed additives or VMPs.
There are very few authorised VMPs that have been tested in controlled trials alongside antimicrobials, or which have similar medicinal claims. The best evidenced examples are products that are intended for use in dairy cattle herd management programmes to prevent the development of new intramammary infections during the dry period, or to reduce the risk of clinical mastitis in the periparturient period (see Section 4.4.10).
Similarly, very few authorised feed additives have been assessed for the efficacy to positively affect the animal welfare and health.
4.4.1. Organic acids
Organic acids and their salts have been used in poultry and pigs for decades and appear to be beneficial for feed and water hygiene, as well as for reduction of specific food‐borne human pathogens. Moreover, these compounds have been shown to positively affect growth performance (Broom, 2015; Khan and Iqbal, 2016). Different short‐chain fatty acids, medium‐chain fatty acids and other organic acids and their salts (e.g. formic acid, acetic acid, lactic acid, propionic acid, butyric acid, sorbic acid, benzoic acid) have been tested in animal nutrition (Rasschaert et al., 2016).
While the mode of action of organic acids for feed preservation and water hygiene mainly reflects pH reduction, their role in the gut is still not completely elucidated. Effects on the gastrointestinal tract, such as increased villus height and width of the duodenum, and deeper crypts in the jejunum were observed in chickens fed with formic acid or sorbic and citric acids (Khan and Iqbal, 2016). A reduction in the prevalence of some pathogens, such as Salmonella spp., Campylobacter spp. and E. coli, was reported by several authors when organic acids were supplemented to the diet of farmed animals. More knowledge is available on the mode of action of butyric acid. It has been shown to positively affect epithelial cells metabolism, proliferation and differentiation (Dalmasso et al., 2008). This organic acid shows anti‐inflammatory effects (Hodin, 2000) and strengthens the gut mucosal barrier (Mariadason et al., 1997; Schauber et al., 2003; Bordin et al., 2004; Peng et al., 2007).
A limited number of studies have addressed the use of organic acids as alternatives to therapeutic antimicrobial treatments and their effectiveness in reducing infections. Timbermont et al. (2009) investigated the efficacy of butyric acid and medium‐chain fatty acids (C6 to C12, mainly lauric acid) to control necrotic enteritis in chickens, in two trials using an experimental necrotic enteritis model. This study showed that butyric acid and medium‐chain fatty acids alone or in combination contribute to the prevention of necrotic enteritis in chickens. The efficacy of different preparations of organic acids (a blend of formic, acetic, propionic, sorbic, caprylic and capric acids) to prevent Clostridium perfringens‐induced clinical necrotic enteritis in chickens was studied by Geier et al. (2010). These authors did not observe any positive effects of the tested organic acid mixture for the protection against necrotic enteritis. In weaning piglets, the effect of organic acids on the reduction of post‐weaning diarrhoea and oedema disease was studied. For example, feed supplementation with benzoic acid reduced diarrhoea in weaning pigs, while morbidity and mortality were not significantly affected by the treatment (Papatsiros et al., 2013). Lückstädt (2006) has reviewed the use of organic acids on animal health and performances in aquaculture, reporting that in some species, such as shrimps and fish the supplementation with organic acids reduced infections. An example is the study of Ramli et al. (2005) on Tilapia farmed in tropical conditions. When fishes were challenged with Vibrio anguillarum, the formate supplementation was shown to increase the survival rate.
The results from the literature showed that organic acid supplementation had a beneficial effect on the performance of animals. Some organic acids are more effective against acid‐intolerant species such as E. coli, Salmonella spp. and Campylobacter spp. They improve nutrient digestibility, modifying the microbiota composition. In those cases where lack of consistency on beneficial effects was observed, it was related to experimental conditions such as feed composition, farm environment and heterogeneity of gut microbiota In a review on the effects of the use of organic acids on the prevention of enteric disease, nutrient digestibility, immunity and performance of chickens and laying hens (Khan and Iqbal, 2016) the authors concluded that organic acids irrespective of the type and levels used exerted a beneficial effect on the health and performance of poultry. Furthermore, they signalled that inconsistency in observations were related to experimental conditions such as feed composition, farm environment and heterogeneity of gut microbiota. Additional research is needed to elucidate the role and manage these factors during experimental trials.
4.4.2. Probiotics and live microorganisms
4.4.2.1. Probiotics
Probiotics were defined by the FAO/WHO in 2001 as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’. This definition has been recently reconfirmed by the International Scientific Association for Probiotics and Prebiotics (Hill et al., 2014).
Although probiotics have been demonstrated to positively affect the health of farmed animals, their mechanisms of action are not fully elucidated. The identification of the mode(s) of action is further complicated by the inclusion, under the term ‘probiotic’, of a variety of microbial cells, such as different bacterial species, vegetative cells or spores and yeasts. The potential mechanisms of action of probiotics were reviewed (Oelschlaeger, 2010; Reid et al., 2011; Saad et al., 2013; Vuong et al., 2016).
Over the last decades, several studies have been published on the effect of viable cultures of bacteria and yeast on the health and welfare of farmed and pet animals. The majority of these studies involved strains of lactic acid bacteria (e.g. Lactobacillus spp. and Enterococcus spp.), bifidobacteria, Bacillus spp. and Saccharomyces cerevisiae.
The endpoints of these studies can be categorised as follows:
improvement of growth performances;
reduction of zoonotic pathogen prevalence/shedding;
reduction of animal infection (e.g. necrotic enteritis in chickens or diarrhoea in pigs), decreased mortality and improved welfare;
immune modulatory activity.
The use of probiotics in animal nutrition as feed additives, with the purpose to improve growth performance and welfare of farmed and pet animals is subject to an authorisation process.
In cattle, studies on probiotics are focused mainly on the reduction of diarrhoea in calves. A mix of probiotic bacteria, belonging to Lactobacillus acidophilus, Bifidobacterium bifidum, and Enterococcus faecium species at a dose 107 CFU/day was shown to reduce the diarrhoea period in milk‐fed calves (Batista et al., 2008). Similar results were achieved by Kim et al. (2011) using a multispecies probiotic including Lactobacillus salivarius, Pediococcus acidilactici, L. plantarum, Bacillus subtilis, B. polymyxa, non‐pathogenic E. coli and Saccharomyces boulardii (dose of 109 CFU each of eight species/day per head). The calves supplemented with this probiotic had a reduced incidence rate of diarrhoea compared to the antimicrobial‐treated animals (neomycin sulfate in milk replacer and colistin 0.08% in calf starter). Furthermore, a meta‐analysis study assessed the effects of probiotics on the diarrhoea incidence in calves (Signorini et al., 2012). These authors considered 15 trials that evaluated calves fed with probiotics and control groups. In the pooled estimate, the relative risk to present diarrhoea was significantly lower in the animals fed with probiotics than in the non‐treated controls.
The effect of probiotics in reducing diarrhoea in weaned piglets was assessed in several studies. The efficacy of a multistrain probiotic product was assessed containing L. acidophilus, L. casei, L. delbrueckii subsp. lactis, Enterococcus faecium, B. bifidus and B. subtilis at a dose of 9.7 × 107 CFU/g, for reducing diarrhoea in comparison with two antimicrobials principles (tylosin and doxycycline–gentamicin) supplied to piglets during nursery phase. The probiotic was shown to be more efficacious than tylosin and to be as active as doxycycline–gentamicin (da Silva et al., 2007). Similarly, probiotic products composed by Bifidobacterium bifidum Enterococcus faecium Lactobacillus acidophilus and Lactobacillus plantarum, when added to diets for sows and piglets, showed the same effect on the incidence of diarrhoea compared to antimicrobial treatments (amoxicillin and colistin) (Silva et al., 2010). A study in the USA (Kritas and Morrison, 2005) was performed in a large pig nursery, where medication was routinely required to prevent disease and production losses, comparing antimicrobial treatments (neomycin for the first five to seven days post‐weaning; neomycin plus oxytetracycline for the next seven days, and thereafter, tylosin up to the age of 70 days) with probiotic supplementation (Bacillus licheniformis and B. subtilis at a dose of 109 CFU/kg of feed). No significant difference in mortality was observed between antimicrobial and probiotic groups, nor in growth parameters. In the absence of a control group, it is not possible to conclude, like the authors did, that the efficacy of probiotic treatments was similar to antimicrobials when used as growth promoters. In other studies, the effect of probiotics on diarrhoea in piglets was studied but not compared with antimicrobial treatments. Giang et al. (2010) and Jurado‐Gámez et al. (2013) tested different lactic acid bacteria and found reductions in the diarrhoea incidence (duration) in weaned piglets.
For chickens, the majority of the studies on probiotics were focused on the reduction of incidence of Clostridium perfringens infections. The effect of viable cultures of bacteria and yeast on C. perfringens‐induced necrotic enteritis has been reviewed by Caly et al. (2015). Supplementation of chicken feed with single or multistrain products was shown to be efficient in preventing or reducing necrotic enteritis in field studies. For example, chickens supplemented with Bacillus spores experienced a reduction in the colonisation and persistence of C. perfringens (La Ragione and Woodward, 2003). In another field trial, B. licheniformis supplementation reduced mortality in the group of chickens compared to non‐treated controls. Several studies report that lactic acid bacteria and enterococci inhibit C. perfringens in vitro (Caly et al., 2015) and in vivo. Cao et al. (2012) showed that Lactobacillus fermentum reduced the occurrence of C. perfringens‐induced ileal lesions and inflammation in young chickens. Conversely, (Geier et al., 2010) demonstrated that a mixture of Lactobacillus johnsonii and a non‐specified organic acid (OA) did not prevent necrotic enteritis caused by Cl. perfringens, while in the same experiment a significant reduction was observed when birds were treated with zinc bacitracin. Lactobacillus reuteri has been reported to be beneficial against Salmonella spp. and E. coli infection in chicks (Zhang et al., 2012). Metabolites produced by probiotic Lactobacilli have also been shown to promote improved health and productivity in laying hens (Loh et al., 2014).
Studies on fish, crustacea and mollusca aimed primarily at reducing the infection at the larval stage, were performed using as probiotic cultures bacterial strains isolated from the marine environment. In in vivo trials with rainbow trout, viable cells of bacteria isolated from the microbiota of fish were demonstrated to significantly decrease mortality due to Flavobacterium psychrophilum, the causative agent of coldwater disease. The two isolates were identified as member of the Enterobacter genus (Burbank et al., 2011). Two strains of Phaeobacter and Bacillus pumilus, isolated from oysters (Crassostrea virginica), were assessed as probiotic to improve the survival of oyster larvae (Kapareiko et al., 2011). The supplementation at a dose of 104 CFU/mL of water in laboratory experiments protected larval oysters against mortality resulting from challenge with oyster pathogens R. crassostreae and V. tubiashii and juvenile oysters against challenge with V. tubiashii. In shrimps (Penaeus monodon), the mortality of larvae due to Vibrio harveyi infection was significantly lower when viable cells of Bacillus spp., Pseudomonas spp. or Arthrobacter spp. were introduced in the larval rearing systems (Pai et al., 2010). In the species Litopenaeus stylirostris (shrimp), a strain of the Pseudoalteromonas genus, isolated from the marine environment significantly improved post‐larval survival when added to the rearing water (Pham et al., 2014).
4.4.2.2. Predatory bacteria
The predatory bacteria, which comprise members of the genus Bdellovibrio are Proteobacteria that for reproduction depends on the invasion of a Gram‐negative bacterial prey cell. The growth and multiplication occurs in the host cells, which is destroyed when new Bdellovibrio cells are released. Although recently there has been increased attention to predatory bacteria as an alternative approach to combat antimicrobial‐resistant bacterial infections (Kadouri et al., 2013), only limited knowledge is available on the use of predatory bacteria to control the zoonotic pathogens such as E. coli and Salmonella spp. Atterbury et al. (2011) demonstrated a significant reduction in Salmonella numbers in bird gut caecal contents and reduced abnormal caecal morphology (reduced caecal inflammation) in animals orally supplemented with Bdellovibrio bacteriovorus.
4.4.2.3. Competitive exclusion
Competitive exclusion was first defined by Lloyd et al. (1977), who indicated a range of complex biological effects including bacterial antagonism, bacterial interference, barrier effect, and colonisation resistance experienced following the supplementation of animals with complex microbial communities. The pioneering work of Nurmi and Rantala (1973) demonstrated efficacy of inoculating chicks with un‐manipulated intestinal bacteria from healthy adult birds for preventing infection when challenged with Salmonella spp. Most of the studies are focused on the reduction of human pathogen shedding, as reviewed in the systematic review‐meta‐analysis‐meta‐regression study on effectiveness of fourteen different competitive exclusion products in reducing Salmonella spp. colonisation in chickens (Kerr et al., 2013), rating complex undefined products most highly in terms of protective effect.
A more limited number of studies report effect on animal health. A product based on freeze‐dried intestinal microbiota from healthy adult chickens, containing Bacterioides spp., Citrobacter spp., Clostridium spp., Enterococcus spp., Escherichia spp., Eubacterium spp., Fusobacterium spp., Lactobacillus spp., Propionibacterium spp., Ruminococcus spp. and Streptococcus spp., has shown a reduction in C. perfringens colonisation in a chicken necrotic enteritis infection model (Hofacre et al., 1998a,b; Abudabos et al., 2015). A second competitive exclusion product, based on an undefined bacterial community derived from the gut of healthy chicken, demonstrated a reduction of necrotic enteritis in chickens. Birds treated on the day‐of‐hatch with these products experienced a reduced overall mortality and reduced incidence of intestinal lesions (Elwinger et al., 1992; Kaldhusdal et al., 2001). Craven et al. (1999) showed that a third competitive exclusion product was effective in reducing the caecal carriage of C. perfringens in chickens.
An effect on the reduction of AMR E. coli, was reported (Hofacre et al., 2002). One oral dose of a commercial competitive exclusion product significantly reduced the colonisation of the small intestine, large intestine, and caeca of broiler chicks by the highly AMR poultry pathogenic E. coli 078:K80 at seven and 14 days post‐challenge.
4.4.3. Bacteriophages
Bacteriophages or phages are obligate intracellular parasites and must enter a host bacterium to replicate. To initiate the viral infection bacteriophages recognise specific receptors on the surface of bacteria. As a consequence, the majority of bacteriophages are highly specific and show a narrow host range, generally related strain within a single bacterial species. A minority of lytic bacteriophages show a broad host range and effectively attack a range of bacterial species.
Bacteriophages as antimicrobial agents were first proposed by Félix d'Hérelle in 1917, but after the discovery of antimicrobials, which were demonstrated to be more effective against bacterial infections, the development of bacteriophage products did not proceed, except in the Soviet Union. The need for alternatives to antimicrobials has revived the interest on phages as agents for the treatment of bacterial infections in both humans and farmed animals.
The use of bacteriophages as biocontrol agents to reduce Salmonella spp., Listeria spp. and Campylobacter spp. persistence and shedding in chickens has been studied extensively (Grant et al., 2016). Zhang et al. (2015) reviewed the use of phages as a strategy in pigs for controlling the colonisation of zoonotic pathogens, namely Salmonella spp. and E. coli O157:H7.
More limited are scientific papers describing the effect of bacteriophages as therapeutic agents in farmed animals. Huff et al. (2005) showed that bacteriophages prevented and treated colibacillosis in poultry, when animals were challenged with a pathogenic strain of E. coli serotype O2. A significant reduction in the mortality rate was observed when the birds were treated with an aerosol spray of two bacteriophages against E. coli O2. In another study, when three bacteriophages propagated on E. coli were administered to chickens with diarrhoea from a natural infection, a reduction in mortality was observed (Li et al., 2012).
The role of bacteriophages in aquaculture was reviewed by Oliveira et al. (2012), who concluded that although beneficial effects could be demonstrated, particularly in invertebrates, more work is needed on effective methods of application in commercial systems. Most of the articles describe the bacteriophages and assess their activity in vitro, while only a few studies assess the effect of phages on reducing the infections. Cruz‐Papa et al. (2014), in a laboratory scale experiment, showed that Aeromonas hydrophila bacteriophage, injected intraperitoneally into the abdominal cavity, was able to decrease the amount of A. hydrophila in the blood of fish and mortality in Nile Tilapia (Oreochromis niloticus). The results were similar to those obtained using oxytetracycline. Prasad et al. (2011) tested the efficacy of bacteriophages to control Flavobacterium columnare, the aetiological agent of columnaris disease in walking catfish (Clarias batrachus). An intramuscular injection, or oral administration trough feed or exposure via water of the bacteriophage reduced significantly the pathogens counts in the sera, gill, liver and kidney of challenged fishes. In Oncorhynchus fontinalis, the supplementation of a bacteriophage delayed for 7 days the development of Aeromonas salmonicida furunculosis (Imbeault et al., 2006). Silva et al. (2016) demonstrated the efficacy of a bacteriophage to control of furunculosis caused by Aeromonas infection in juvenile forms of soles (Solea senegalensis). Moreover, bacteriophage therapy has been used as a treatment method for controlling MDR metallo‐β‐lactamse producing strain of P. aeruginosa infection in farmed catfish (Clarias gariepinus) (Khairnar et al., 2013).
Two studies in giant tiger prawn (Penaeus monodon) (Vinod et al., 2006; Karunasagar, 2012) showed that bacteriophages have a potential in the control of luminous vibriosis in aquaculture, by reducing the shrimp mortality caused by Vibrio harveyi.
The potential limitation of bacteriophage treatments for controlling pathogenic bacteria is the selection of phage‐resistant populations (Cota et al., 2015). This is a well‐described phenomenon, which involves several bacterial phage resistance mechanisms (e.g. restriction modification enzymes, abortive infections and CRISPR‐Cas systems) when bacteriophages have been used in animal farming. Barbosa et al. (2013) observed in shrimp hatcheries that the use of a single phage targeted to a strain of Vibrio spp. led these bacteria to rapidly develop phage resistance. In contrast, the use of three phages applied together avoided the development of such resistance.
During an EMA workshop on bacteriophages it was concluded that any medicine, including bacteriophages, before approval needs to have its efficacy and safety proven based on appropriately designed clinical trials, which is difficult for bacteriophages, so it can be concluded that there are technical difficulties of authorising such products (EMA, 2015).
4.4.4. Prebiotics
A prebiotic has been traditionally defined as a compound which is ‘a non‐digestible food ingredient that beneficially affects the host by selectively stimulating the growth of or limited number of bacteria in the colon, and thus improves host health’ (Roberfroid, 2007). More specifically, prebiotics select for microbiota already present within the gastrointestinal tract (GIT) that are considered beneficial to the host.
This appears to be mediated through changes in gastrointestinal microbiology, including enhanced numbers of favourable bacteria (lactobacilli, bifidobacteria) and/or decreased numbers of potentially pathogenic bacteria (E. coli, Clostridia, etc.) together with more favourable profiles of fermentation products (Lallès et al., 2007).
Candidate prebiotic compounds encompass numerous relatively non‐digestible oligosaccharides (NDOs) including, among others, fructo‐oligosaccharide products (FOS; oligofructose, inulin‐type fructans), trans‐galactooligosaccharides, glycooligosaccharides, maltooligosaccharides, xylooligosaccharides, yeast cell walls (mannan‐oligosaccharides), and gluco‐oliogosacharides.
Some work has been done with feeding these compounds as well as other carbohydrate sources such as high fibre containing ingredients to conventionally produced poultry (Hajati and Rezaei, 2010; Hume, 2011) and non‐conventionally produced poultry (such as through organic production) (Ricke, 2015). Some experiments studied the effect on growth promotion and immune response, but very few considered their preventive effect against bacterial diseases.
Supplementation of feed with mannan or fructo‐oligosaccharides (MOS; FOS) can improve the performance, immune competence, and intestinal bacterial balance in laying hens (Ghasemian and Jahanian, 2016). For example, FOS supplementation may increase ileal mucosa thickness and elevate the expression of certain cytokine genes. There may be alterations of leucocyte compositions and serum IgY levels in response to lipopolysaccharide (LPS) challenge, suggesting that FOS supplementation may be effective to induce protective outcomes in gut health and immunity of broiler chickens (Shang et al., 2015).
In poultry, the effects to control necrotic enteritis of two different water‐soluble carbohydrate extracts (Renga Renga lily extract and Acacia extract), and two commercially available prebiotic compounds, one containing an arabinogalactan product and one containing inulin, were compared with a Zn‐bacitracin treatment and a negative control (Vidanarachchi et al., 2013). The results did not demonstrate that supplementation with water‐soluble carbohydrates is effective for controlling necrotic enteritis.
Two battery experiments were conducted to evaluate the effect of a commercial yeast extract feed supplement on growth, haematological parameters and mortality (Huff et al., 2007). One‐week‐old turkeys were exposed to cold stress and challenged with E. coli via the respiratory route. The batteries of chicks differed by the age of their mothers (33 vs 40 week‐old‐hens). In each case, a group receiving a control basal diet was compared with a group receiving the same diet supplemented with two doses of the commercial yeast extract. The authors described that the results showed variation depending on the age of poults producing hens, and concluded that the age of the hens should be taken into consideration when assessing and using alternatives to antimicrobials. In another experiment, yeast β‐d‐glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens (Shao et al., 2016).
In piglets challenged with S. Typhimurium, mannan was not as effective as carbadox in reducing severe enteritis‐associated fever and inappetence after injection (Burkey et al., 2004).
A study was performed to evaluate the effects of probiotics, prebiotics and herbal extracts as alternatives to antimicrobial agents (as growth promoters) on the intestinal microbiology, diarrhoea incidence, and performance of weaning pigs. Two randomised complete block design experiments were carried out during 35 days to compare five treatments: control ‐ basal diet; antimicrobial ‐ basal diet plus Zn bacitracin and olaquindox (50 ppm of each); probiotic ‐ basal diet plus 1,300 ppm of probiotic (Bacillus subtilis and Bacillus licheniformis); prebiotic ‐ basal diet plus 3,000 ppm of mannanoligosaccharide; herbal extract ‐ basal diet plus 500 ppm of herbal extract (garlic, clove, cinnamon, pepper, thyme, cinnamaldehyde and eugenol). Faecal scores were evaluated daily to calculate diarrhoea incidence. A treatment effect was not observed on the diarrhoea incidence. The antimicrobial agents improved average daily gain of weaning pigs, compared to pigs fed the control diet. Daily gain of piglets receiving the diet supplemented with probiotic and herbal extracts was not improved when compared to animals fed the control diet. The performance of piglets fed prebiotic was similar to those fed antimicrobials, during 1–14 days of the experimental period but feed conversion was not improved (Utiyama et al., 2006).
Yeast derivate based on whole brewery yeast added to the creep feed of suckling and newly weaned piglets or to the creep feed of the piglets and the sow's diet may prevent post‐weaning diarrhoea and improve performance (Jensen et al., 2013b) as observed in a model using an E. coli challenge.
The direct effects of prebiotics on the innate immune system of fish have been reviewed and discussed (Song et al., 2014). Prebiotics, such as fructooligosaccharide, mannanoligosaccharide, inulin, or β‐glucan, enhance innate immune responses including: phagocytic activation, neutrophil activation, activation of the alternative complement system, increased lysozyme activity, and more. Many studies have indicated that immunosaccharides are beneficial to both finfish and shellfish by enhancing innate immune responses (Song et al., 2014; Hoseinifar et al., 2015a). A recent review discussed the interest of the oral adminsitration of a wide‐range of plant‐derived products as immunomodulators in aquaculture (Bulfon et al., 2015).
In Nile tilapia, an experimental study using shrimp shells derived chitosan enhanced several innate immunological parameters and increased resistance against A. hydrophila (Abu‐Elala et al., 2015). Yeast autolysate and linseed fibre had prebiotic effects when added to silver catfish diets, providing metabolic advantages and boosting the immune system (Adorian et al., 2016).
In shrimp (Penaeus monodon), studies were made to evaluate the immunomodulating effects of chitosan and levamisole. In a challenge experiment with Vibrio harveyi, a reduction of mortality was observed in the two treated groups while 100% mortality was observed in the non‐treated control group (Huxley et al., 2011).
4.4.5. Synbiotics
When combined with certain probiotic cultures, prebiotics may stimulate the growth and colonisation of these specific probiotic microorganisms in which case the resulting combination is referred to as synbiotic (Hume, 2011).
A study (Kehoe and Carlson, 2015) was performed on 36 young bull calves (3 days of life up to 35 days) fed with a milk replacer alone (control), with a blend of a non‐medicated mixture of feed additives and feed materials (animal plasma, yeast cell wall extracts, inulin, ascorbic acid, and direct‐fed microbials) and a medicated milk replacer (362.87 g/t of neomycin; 181.44 g/t of oxytetracycline). Both treatments diets resulted in improved growth and lower faecal scores (denoting harder faeces). The authors concluded that both treatment diets may beneficially affect gastrointestinal morphology, reduce scouring, and improve growth characteristics of neonatal dairy calves compared with unsupplemented diets.
The efficacy of E. coli probiotics, raw potato starch prebiotic, and their combination was determined in young pigs challenged with E. coli K88, compared to a group treated with in‐feed antimicrobials (chlortetracycline, penicillin and sulfamethazine) (Krause et al., 2010). A negative control group was not included in the experiment. The combination of the synbiotic treatment (prebiotic and probiotic) had a beneficial effect on piglet growth performance and resulted in a reduction of diarrhoea and increased microbial diversity within the gut compared to the other groups.
The potential of a prebiotic oligosaccharide lactulose, a probiotic strain of L. plantarum, or their synbiotic combination to control post‐weaning colibacillosis in pigs was evaluated using an ETEC K88 oral challenge, seven days after the treatment start (Guerra‐Ordaz et al., 2014). Inclusion of lactulose increased significantly the average daily gain (ADG), the number of lactobacilli and the percentage of butyric acid in the colon. The inclusion of the probiotic increased numbers of L. plantarum in the ileum and colon and in the total lactobacilli in the colon and showed a trend to reduce incidence of diarrhoea (p = 0.09). This finding suggests that there is a correlation between the counts and the incidence of diarrhoea. The positive effects of the two additives were combined in the synbiotic treatment, resulting in a complementary synbiotic with the potential to be used to control post‐weaning colibacillosis.
In an experiment (Wideman et al., 2015), a commercial prebiotic (mannan oligosaccharide β‐glucan yeast cell wall product) plus a probiotic (Bacillus subtilis C‐3102) was tested to prevent bacterial chondronecrosis with osteomyelitis (BCO) in chickens reared on wire flooring which consistently developed higher incidences of BCO than hatchmates reared on wood shavings litter. Adding the synbiotic to the feed delayed the age of onset and reduced the cumulative incidence of BCO on wire flooring when compared with chickens fed the control feed.
The effects of dietary supplements of galactooligosaccharides (GOS), Pediococcus acidilactici and P. acidilactici + GOS on innate immune response, skin mucus as well as disease resistance of rainbow trout (Oncorhynchus mykiss) fingerlings were investigated (Hoseinifar et al., 2015b). After 8 weeks of feeding, several innate immune (lysozyme, alternative complement and respiratory burst activities) and skin mucus parameters (bactericidal activity against Streptococcus faecium, Streptococcus iniae, Serratia marcescens, Staphylococcus aureus and E. coli and mucus protein content) were studied. The results indicated that the three supplemented diets significantly increased innate immune responses and skin mucus parameters in rainbow trout. The highest innate immune response, skin mucus activity as well as protein level was observed in synbiotic fed fish. Furthermore, at the end of the feeding experiment, some fish were intraperitoneally injected with Streptococcus iniae to determine their disease resistance. The mortality of fingerlings fed supplemented diet was significantly lower than fish from the control group.
4.4.6. Antibodies
Antibodies are specialised immune proteins produced because of the introduction of an antigen into the body, and which possesses the ability to combine with the very antigen that triggered its production. In human medicine, antibodies that bind to and inactivate a pathogen, its virulence factors, or its toxins were considered as one of the alternative approaches most likely to have major clinical impact.
Animal plasma is a by‐product from the abattoir, obtained from animal blood after separation by centrifugation with the use of an anticoagulant. Spray dried animal plasma (SDAP) has been produced by different processes from this plasma. The process preserves the biological activity of the proteins and the beneficial effects are related to the immunoglobulin content. For this reason, some SDAP sources have a standardised IgG content. These Igs may prevent viruses and bacteria from interacting with the gut wall, resulting in an improvement of gut function. In addition, sources of plasma that are enriched for some specific immunoglobulins have been obtained from pigs that have been vaccinated against specific pathogens for this purpose and are known as spray dried immune porcine plasma (SDIPP) (Torrallardona, 2010). Published reviews of trials testing spray dried plasma for piglets at weaning (Gallois et al., 2009; Torrallardona, 2010) suggest an immunomodulatory effect combined with the nutritional effect. The spray dried plasmas of porcine origin have a better efficacy which could be explained by the specificity of IgG. One major problem is the stability of Igs during the manufacturing process of SDP. The most critical step would be spray drying. Use of spray dried animal plasma in nutrition to positively improve growth and modulate immune response was investigated in calves (Quigley Iii and Drew, 2000), poultry (Beski et al., 2015), laying hens (Orda et al., 2012), fish (Gisbert et al., 2015). In a challenge study on piglets the use of plasma immunoglobulins reduced the shedding of E. coli F4+, but no effect on mortality or morbidity in piglets were observed (Hedegaard and Heegaard, 2016).
Research to identify pathogen‐specific antibacterials such as monoclonal antibodies (mAbs) has shown early promise. Advances in mAb discovery, engineering, and production have driven significant progress in developing mAb‐based antibacterials. These should provide clinicians with precision weapons to combat drug‐resistant bacterial infections (DiGiandomenico and Sellman, 2015).
The immunisation of hens provides an efficient method for antibody production (Gadde et al., 2015; Vega et al., 2015) (Alustiza et al., 2016). Chicken egg yolk immunoglobulin IgY is the major low molecular weight immunoglobulin in oviparous animals. Oral administration of IgY has attracted considerable attention as a means of controlling infectious diseases of bacterial and viral origin (Spillner et al., 2012). Thus, it is possible to immunise the hen against specific foreign pathogens thereby promoting the production of IgY with activity against these specific disease conditions. A review of the potential application of this technology has been described for the prevention of bovine, pig, poultry and shrimp diseases (Xu et al., 2011). A more detailed review of performance of IgY to prevent diarrhoea in neonatal and post‐weaning pigs such as pathogenic E. coli and viruses has been published recently, demonstrating positive results in some experimental studies (Li et al., 2015). Another review summarised the potential application for aquaculture (Baloch et al., 2015). The main limitations for the use of IgY in oral passive immunotherapies is their susceptibility to proteolysis. IgY is fairly resistant to digestion by intestinal proteases but its activity was decreased at low pH and lost at pH 3. Protection of IgY against peptic digestion and acidity within the stomach is possible through microencapsulation, e.g. within nanotubes (Alustiza et al., 2016). The cost of production of IgY and the need to develop bioprotection limits further commercial scale development.
4.4.7. Immunomodulators
Immunomodulators are substances or agents capable of adjusting the immune response to a desired level, as in immunostimulation, immunosuppression, or induction of immunological tolerance. Immunostimulants can be used to increase the non‐specific immune response and activate the specific defence mechanism. Immunostimulants and immunomodulators comprising a group of biological and synthetic compounds, such as levamisole, β‐glucan, peptidoglycan, chitin, chitosan yeast, lipopolysaccharide (LPS), and various plant and animal products have been found effective in preventing the diseases by enhancing the nonspecific cellular and humoral defence mechanisms (Awad and Austin, 2010).
They have received considerable attention in aquaculture in order to increase disease resistance in farmed fish. Several immunostimulants have been demonstrated to enhance the immune response and play a role in protection against disease in fish by injection or oral administration.
Lipopolysaccharide (LPS), also termed endotoxin, is a component of the outer cell wall membrane of Gram‐negative bacteria. LPS can induce multiple specific and non‐specific biological effects in all living system, including immunological responses (Swain et al., 2008).
LPS has been shown to enhance the resistance of fish against bacterial infection as shown in experimental disease such as Aeromonas hydrophila challenge in rainbow trout (Oncorhynchus mykiss) (Nya and Austin, 2010) or Edwardsiella ictaluri in striped catfish (Pangasianodon hypophthalmus) (Bich Hang et al., 2014).
In a study performed in striped catfish (Bich Hang et al., 2014), LPS or levamisole, a long‐established anthelmintic product with recognised immunopotentiation capability (Renoux, 1978), were intraperitoneally administered daily during 14 days. A control group received a saline solution. Then, a challenge test was performed with Edwardsiella ictaluri administered intramuscularly at 21 days. Animals were treated or not with doxycycline by oral route. In both treatments with or without doxycycline, a lower mortality was obtained in LPS and levamisole treated groups than in control group. Moreover, no differences on mortality were observed between fish treated with levamisole or LPS without doxycycline and control fish treated with doxycycline.
Levamisole has been reported to be able of enhancing resistance to pathogenic bacteria such as Aeromonas hydrophila in Cyprinus carpio (Gopalakannan and Arul, 2006) and Vibrio anguillarum in seawater teleost gilthead seabream (Sparus aurata L.) (Mulero et al., 1998).
Chitosan and levamisole were administered in feed to shrimp Penaeus monodon during 30 days (Huxley et al., 2011) and reduced mortality induced by intramuscular administration of Vibrio fisheri.
A Brevibacillus texasporus strain produces a group of small cationic peptides (BT) with immune modulatory properties. BT peptides were found to be highly efficacious against a natural outbreak of colibacillosis in broiler chickens based on improved performance and reduced mortality in comparison with unmedicated birds (Jiang et al., 2005). The BT peptides primed the caecal tissue for increased transcription of proinflammatory cytokines (interleukin 1β [IL‐1β], IL‐6, IL‐18, type I and II IFNs) and inflammatory chemokine (CxCLi2) in response to Salmonella Enteritidis challenge infection 1 and 7 days p.i. compared to the chickens fed the basal diet (Kogut et al., 2013).
A novel cytokine product has been approved as a VMP in the EU. This product is an immunoregulatory cytokine which increases the number of circulating neutrophils and restores normal neutrophil function to cattle during the periparturient period. In a large European field trial in which the product was used as part of a dairy herd management programme, the incidence of clinical mastitis in the 30 days following calving was reduced in the group treated with the cytokine compared to an untreated control group.80
4.4.8. Antimicrobial peptides (AMPs)
4.4.8.1. Bacteriocins
Bacteriocins are usually defined as ribosomally synthesised peptides produced by bacteria that inhibit the growth of other closely related bacteria. The bacteriocin definition describes these compounds as peptides: short chains of amino acid residues. Most known bacteriocins are around 20–60 amino acid residues in length, but there are bacteriocins which are significantly longer and therefore better described as proteins (Snyder and Worobo, 2014).
While traditionally defined as an antagonism of closely related species, many well‐characterised bacteriocins have relatively broad‐spectrum inhibitory activity. The bacteriocins of Gram‐negative bacteria have a narrower inhibitory spectrum than Gram‐positive counterparts. Most bacteriocins are produced by Gram‐positive organisms. Determinants for the spectrum of activity are often based on the mechanism of action for a particular bacteriocin: whether its activity requires binding to a specific cell surface receptor and the relative distribution of that receptor among bacterial species. Within the food industry, bacteriocins or other bacterially synthesised peptides have found the most widespread application among AMPs, while a very limited number of studies have been published to investigate the clinical efficacy of bacteriocins as purified or semipurified preparations on animals.
A study of the effect on poultry of an ultrafiltrate of the culture medium of a nisin‐producing Lactococcus lactis showed a significant beneficial effect on animal growth and intestinal microbiota composition (Józefiak et al., 2013). The same group investigated the effect of divercin on the composition of poultry intestinal microbiota (Józefiak et al., 2010) and during an experimental challenge against C. perfringens in poultry (Józefiak et al., 2012) with results partially dependent on the physical form of the applied compound.
Lantibiotics are small peptides that possess the post‐translationally modified amino acid residues lanthionine or β‐methyllanthionine. A multitude of studies have highlighted the in vitro potency of these compounds against nosocomial pathogens (Piper et al., 2009). Many lantibiotics exhibit activity against clinically relevant targets such as MRSA, VRE, Propionibacterium acne, Streptococcus mutans, Streptococcus pyogenes, S. pneumoniae, C. difficile, Listeria, and Bacillus species. There have been a number of encouraging studies to suggest that some lantibiotics can be effective in vivo (Field et al., 2015). Very few are under development for human or veterinary medicine (Dischinger et al., 2014). Bioengineering and the use of synthetic biology‐based approaches have been important for advancing the understanding of their mode of action and for the development of new ways to produce in vivo active compounds by enhancing the antimicrobial spectrum and physicochemical properties (including heat stability, solubility, diffusion and protease resistance, of these compounds) (Field et al., 2015; Sandiford, 2015).
4.4.8.2. Host defence peptides
Innate immunity constitutes an evolutionarily ancient scheme founded on a relatively generic, but nevertheless quite effective defence strategy. In addition to the immediate anatomical barriers of the organism, this intrinsic resistance system relies primarily on pattern recognition receptors and associated signalling pathways, specialised chemical mediators (cytokines), the complement cascade, leucocytes, and importantly host defence peptides (HDPs).
The list of natural compounds with antimicrobial activities is extensive, but largely includes three functional groups: (1) digestive enzymes targeting microbial structures (e.g. lysozyme, a 1,4‐β‐N‐acetylmuramidase that enzymatically cleaves a glycosidic linkage in the peptidoglycan component of bacterial cell wall), (2) peptides that bind essential elements such as zinc or iron (calprotectin and lactoferrin, respectively), and (3) peptides that disrupt the microbial membrane (Šíma et al., 2003).
Antimicrobial peptides (AMPs) are oligopeptides with a varying number (from five to over a hundred) of amino acids (Bahar and Ren, 2013). AMPs have a broad spectrum of targeted organisms ranging from viruses to parasites. More than 5,000 AMPs have been discovered or synthesised up to date (Zhao et al., 2013; Waghu et al., 2015). Despite their vast diversity, most AMPs work directly against microbes through a mechanism involving membrane disruption and pore formation, allowing efflux of essential ions and nutrients. There are intensive research activities in this field with the objectives to provide new drug and new treatment of infection. A review of knowledge and the potential application in veterinary medicine was recently published (Mojsoska and Jenssen, 2015). Unfortunately, advances towards this goal have proven disappointing, in part owing to limited understanding of relevant structure–activity and selective toxicity relationships in vivo, due to high amounts of drug needed for therapy, very high susceptibility to proteolytic degradation by microbial enzymes, toxicity, relatively short half‐life, and the difficulty of cost‐effective production of such peptides on a commodity scale (Yount and Yeaman, 2012).
Lysozyme, in vitro, is active against Gram‐positive bacteria. It is a naturally occurring enzyme found in secretions such as tears, saliva, and milk, animal tissues or eggs. It is considered as a part of the innate defence in mammals. A review of studies performed in pigs with lysozyme‐enriched feed show some positive results on growth rates similar to those obtained with antimicrobials used as growth promoters (Oliver and Wells, 2015). In an experimental study of E. coli infection in piglets, consumption of transgenic goat lysozyme‐enriched milk demonstrated positive clinical results by comparison with controls (Cooper et al., 2013).
4.4.9. Interferon
Interferons are proteins that inhibit the intracellular stages of viral reproduction and may also help stimulate more generalised immune responses (Shtrichman and Samuel, 2001). Some of the most effective antiviral preparations used in veterinary medicine are preparations based on recombinant alpha2b‐interferon, and these have been found to have significant antibacterial properties. Thus Interferon preparations have been used for the treatment and prevention of viral and viral‐bacterial infections, especially in studies in pigs (Cummins et al., 1999; Khmylov, 2015), but no data on the use to control bacterial infections in husbandry.
4.4.10. Teat sealants
Teat sealants are medicinal products intended for use in dairy cattle herd management programmes to prevent the development of new intramammary infections during the dry period, or to reduce the risk of clinical mastitis in the periparturient period. Internal teat sealant products containing bismuth subnitrate have been available in the EU for over a decade, and have well‐established use. A meta‐analysis (Rabiee and Lean, 2013) indicated that internal teat sealants reduced the risk of new intramammary infections (IMI) during the dry period by 73% compared with untreated cows and reduced the risk of clinical mastitis after calving by 29%. Similarly, a meta‐analysis by Halasa et al. (2009) showed a significant protection from teat sealants against new IMI during the dry period. Huxley et al. (2002) compared the efficacy of a bismuth subnitrate teat sealer to a long‐acting intramammary antimicrobial containing the 1st‐generation cephalosporin, cefalonium, in dairy cows without clinical mastitis and with somatic cell counts (SCC) < 200,000 cells/mL at drying off. Compared with the antimicrobial, quarters that were treated with the teat sealer developed significantly fewer new IMI caused by E. coli, all Enterobacteriaceae and all major pathogens combined.
The management of dairy cattle at drying off is important for animal health and welfare, and for preserving fertility and future milk production. Most programmes take a holistic approach addressing milking regimens, nutrition and environmental factors among others. In a recent survey of drying‐off practices on dairy farms in northern Germany (Bertulat et al., 2015), 79.6% of participating farms practised blanket ADCT. Taking into account guidelines on responsible use of antimicrobials, there is a move towards selective ADCT in which treatment of individual cows is based on their history, SCC and bacteriological examination.
In the meta‐analysis by Halasa et al., blanket antimicrobial dry cow therapy (ADCT) was shown to have significant protection against new IMI caused by Streptococcus spp., but no protection against coliform mastitis. Selective ADCT was more effective compared to no treatment, blanket dry cow therapy was more effective than selective therapy.
Teat disinfection before milking is considered to be a valuable practice to reduce introduction of microorganisms from the skin of the teat into the udder (Bohm and Kromker, 2016).
4.4.11. Botanicals
Some botanical products have been demonstrated to favourably affect the health of farmed animal species, by a variety of potential models of action, e.g. improving in the functional integrity of the enterocyte membrane, increasing in the villus height, and villus height: crypt depth ratio which is an indication of improved growth performance or influencing the production of organic acid by modulating the gut microbiota. A limited number of controlled trials to support efficacy have been published. Moreover most of the studies do not provide any data about the chemical composition of the tested plant products.
4.4.11.1. Plant feed supplementation
Although the term ‘phytobiotic’ comprises a wide range of substances of biological origin, formulation, chemical description and purity, these substances can be classified into four groups (Windisch and Kroismayr, 2006): (1) herbs (products from flowering, non‐woody and non‐persistent plants); (2) botanicals (entire or processed parts of a plant, e.g. roots, leaves, bark); (3) essential oils (EOs) (hydrodistilled extracts of volatile plant compounds); and (4) oleoresins (extracts based on non‐aqueous solvents).
Certain plant extracts, such as extracts from fenugreek seeds, can have health as well as environmental benefits for farm animals in terms of reduced production of greenhouse gases (Hossain et al., 2015). Some articles review some of the relevant information available on dietary fibres and protein fermentation and their interactive effects on the gut environment of pigs (Jensen et al., 2013a; Aumiller et al., 2015; Jha and Berrocoso, 2016).
The positive effects of phytobiotics on intestinal functions (digestibility, gut histology, microbiota composition) are linked mainly to the plant constituents including terpenoids (mono‐ and sesquiterpenes), steroids, alkaloids, flavonoids and glucosinolates.
In a study performed in a large pig fattening unit suffering from multimorbidity, the supplementation of oregano enhanced average daily gain, feed conversion rate and lower mortality of pigs compared to a negative control. Oregano has, however, been demonstrated to be less effective than doxycycline (Bilkei et al., 2011a).
A study was performed in order to prove the effects of garlic, horseradish or doxycycline in the prevention of post‐parturient diseases in sows and pre‐ and post‐weaning mortality of piglets. In this farm trial, horseradish as doxycycline significantly reduced the occurrence of post‐parturient disease complexes of sows and pre‐ and post‐weaning mortality of piglets compared to the untreated control group (Bilkei et al., 2011b).
During an outbreak of acute diarrhoea, a study showed that oregano leaves as an oral solution was as effective as neomycin administered orally on affected calves (Bampidis et al., 2006). In this investigation, the lack of control limits the interpretation of the results.
Tannins from different sources have been shown to have an antimicrobial activity (Schalbert, 1991). Despite tannins having been considered as antinutritional factors, these substances can be beneficial to poultry but several factors must be considered and evaluated for their use in feed (e.g. final concentration, structure of the compounds, feed preparation). Reports shows that tannins derived from chestnut and quebracho have in vitro antibacterial and antitoxin activities against Cl. perfringens and its toxins (Elizondo et al., 2010). The in vivo effects of chestnut (Tosi et al., 2013) and quebracho (Redondo et al., 2014) tannins were confirmed in broiler necrotic enteritis models reducing the incidence and severity of gross lesions and improving the productive performance of broiler chickens.
Berberine, a plant alkaloid that can be extracted from many types of plant, such as Hydrastis canadensis (goldenseal), Coptis chinensis (Coptis or goldenthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry) and Berberis aristata (tree turmeric), possesses antimicrobial activity against a variety of organisms including bacteria, viruses, fungi, protozoa and helminths. In Thailand, a farm study demonstrated that piglets receiving colistin or berberine had better average daily weight gains than those treated with halquinol, but the mortality rates of piglets in all groups was less than 2.0% and not significantly different between groups (Tummaruk et al., 2009).
4.4.11.2. Essential oils
Essential oils (EO) are compounds primarily obtained from aromatic plants, herbs, or spices (Yang et al., 2009). Many EOs contain multiple active components and these components are used primarily to protect the plants from damage caused by insects and bacteria. Each component may have a different mechanisms of action and can work synergistically (Senatore, 1996). It is difficult to predict the efficacy of EOs because the active components can vary depending on the method of extraction, geographical origin, plant genotype, the harvesting season, and length of storage (Wenk, 2006). EOs tend to be more effective against Gram‐positive than Gram‐negative bacteria (Burt, 2004). Bacterial tolerance to essential oils used as alternatives to antimicrobials can develop, so doses need to be optimised to reduce this (Melo et al., 2015).
A major issue with some EO's is the reduction of the antimicrobial activity when given in feed (Si et al., 2006), mainly associated with the volatility and poor solubility of the active components. EOs are absorbed quickly after oral, pulmonary, or dermal administration and then metabolised and typically eliminated by the kidneys in the form of glucuronides. Thus, their accumulation within the body is unlikely due to rapid clearance and short half‐lives (Kohlert et al., 2002). Nevertheless, more toxicological studies are needed to determinate the acute oral effects and dosage levels of EOs in animal diets.
In an environmentally controlled research facility, two experiments were conducted to determine the effect of phytobiotics (defined as a mixture of essential oils with anis oil, citrus oil, oregano oil and natural flavours) and organic acids on faecal scores (as an indicator of the incidence of diarrhoea) and growth performance of nursery pigs as an alternative to antimicrobials (Kommera et al., 2006). Results from this study showed that addition of phytobiotics to diets did not affect faecal scores (denoting consistency of stools), and that the growth of pigs fed the diets with phytobiotics or the combination of phytobiotics and organic acids did not differ from those treated with antimicrobials and not treated. The authors recommended further studies to investigate the effect of alternatives on growth performance in disease challenged conditions.
The protective effect of a commercial phytogenic feed additives containing oregano, anis and citrus oils on the resistance of rainbow trout Oncorhynchus mykiss after intraperitoneal administration of Aeromonas salmonicida was demonstrated (Menanteau‐Ledouble et al., 2015).
The activity (survival rate of P. monodon larvae, bacterial concentrations of larvae) of two essential oils (EOs) of Cinnamosma fragrans showed than one oil had a similar effect (p > 0.05) as an antimicrobial (erythromycin) (Randrianarivelo et al., 2010).
The positive effect and the limitations of different phytochemicals was reviewed and discussed in some papers for pigs (de Lange et al., 2010), poultry (Diarra and Malouin, 2014), fish (Chakraborty and Hancz, 2011; Reverter et al., 2014; Bulfon et al., 2015) and shrimps (Randrianarivelo et al., 2010). All of these reviews concluded that the beneficial properties and efficacy depend on the plant part, the method of preparation and the concentration of use. Although numerous studies have reported activities, there has been a lack of standardisation of protocol for preparation/extraction, dose‐safety and dose‐efficacy determination.
4.4.12. Biocides
Biocidal products (biocides) are defined by Directive 98/8/EC81 as ‘active substances and preparations containing one or more active substances, put up in the form in which they are supplied to the user, intended to destroy, deter, render harmless, prevent the action of, or otherwise exert a controlling effect on any harmful organism by chemical or biological means’.
Biocides are widely used in many applications, including animal husbandry and in the food industry, e.g. feed preservatives, teat cleaning, for the disinfection of production plants and of food containers, and the control of microbial growth in food and drinks. In the laboratory, resistance to biocides has been linked to the appearance of resistance to antimicrobials and a clear connection between exposure to biocides and activation of the expression of different genes (structural and regulatory) involved in AMR has been demonstrated in important bacterial pathogens (E. coli and Salmonella spp.) (Maillard et al., 2013). Co‐selection resulting from the use of biocides may involve resistance to heavy metals or, less commonly, tolerance to residual levels of biocides such as triclosan or quaternary ammonium compounds such as benzalkonium chloride used in the food industry and on farms (Maillard et al., 2013; Nhung et al., 2015; Wales and Davies, 2015).
In some situations, especially for topical therapy, and also for some bacterial or fungal tissue infections, biocides can be used instead of antimicrobials for actinobacillosis, actinomycosis or ringworm. Resistance to such biocides in much less likely to develop because of their modes of action (Pettey et al., 2015).
4.4.13. Clay
Clays have been incorporated in animal diets (10–20 g/kg) as a technological additive (lubricant or agglomerant) to improve feed manufacture. Clays are crystalline, hydrated aluminosilicate molecules composed of alkali and alkaline earth cations along with small amounts of various other elements. The best‐known are smectite, illite, kaolinite, biotite and clinoptilolite. The three‐dimensional structures creating internal voids and channels of clays are capable of trapping a wide variety of molecules. Clay minerals are regarded as a simple and effective tool for the prevention of the negative effects of many toxic compounds. Dietary supplementation with clays has been shown to improve weight gain and feed conversion in pigs. According a recent review (Subramaniam and Kim, 2015), several studies have indicated that feeding clay reduces the incidence, severity and duration of diarrhoea in pigs. For example, the use of kaolin reduce the incidence and expression of pig diarrhoea in an experimental challenge (Trckova et al., 2009). In a study with E. coli challenge in weaned piglets, the diarrhoea scores of groups receiving smectite, zeolite, kaolinite, alone or in combination were significantly reduced in comparison to those of a control group (Song et al., 2012). In an experimental E. coli challenge on post‐weaning pigs, two smectites and one zeolite clay effect were tested in comparison with a control untreated group (Almeida et al., 2013). One of the smectite clay has a significant effect on the piglet immune response parameters. In an experimental farm, incorporation of copper exchanged montmorillonite, in weaned piglet feed, had the same effect on reduction of diarrhoea and improvement of growth performance parameters as chlortetracycline (Song et al., 2013).
A study was performed to investigate the effect of different levels of dietary yellow loess (or red clay) as an alternative of oxytetracycline in rainbow trout. Fourteen days of challenge test with bacteria A. salmonicida showed significantly lower survival rate for control than those of fish fed other experimental diets (yellow loess or oxytetracycline) (Lee et al., 2015).
Because the mechanism of action of clays, a risk related to its potential interactions with other nutrient compounds of the diet exists. Another risk associated with clay is their potential toxicity as they have been mined from the environment with natural or anthropogenic occurrence of toxic compounds. The potential biological contamination from surface water, dust, soil, and other constituents of the environment in the mines during clay extraction, processing and storage must be assessed as described for kaolin (Trckova et al., 2004).
4.4.14. Minerals
Copper (Cu) and zinc (Zn) at doses greater than recommended nutritional levels are commonly used, particularly in pigs as alternatives to antimicrobials for some enteric conditions during food animal production. Resistance to copper can be conferred by a plasmid‐borne transferable copper resistance gene (tcrB) reported in E. faecium and E. faecalis, and a higher prevalence of tcrB‐positive enterococci in piglets fed elevated copper levels compared to that in piglets fed physiological copper levels suggests that supplementation of copper in swine diets selected for resistance (Amachawadi et al., 2011). The tcrB gene is harboured by a conjugative plasmid that also carries genes conferring resistance to tetracyclines and macrolides (Amachawadi et al., 2015); copper supplementation may therefore exert a selection pressure for AMR. In two opinions, the EFSA FEEDAP Panel concluded that a co‐selection in the gut bacteria for resistance to Cu and resistance to erythromycin could not be excluded (EFSA FEEDAP Panel, 2012b, 2016a). It was demonstrated for Gram‐positive bacteria (namely enterococci) but not for Gram‐negative bacteria. The relevance of this finding is still uncertain.
The use, particularly in pig and poultry production, of heavy metal‐containing compounds as nutritional feed additives or for growth promotion and therapy of intestinal disease potentially selects for microorganisms with reduced susceptibilities to heavy metals and coselects for resistance to antimicrobial which are often colocated on the same mobile genetic elements. These compounds are used at inhibitory rather than lethal concentrations, thus potentially allowing resistance to emerge within the alimentary tract and the immediate farm environment (Wales and Davies, 2015; Campos et al., 2016).
Zinc oxide (ZnO) is an authorised medicinal product in the EU, indicated for the prevention of post‐weaning diarrhoea in piglets. Several peer‐reviewed papers indicate that high dose supplementation of ZnO (1,000–3,000 mg/kg feed) reduces the incidence of intestinal disorders, mainly the post‐weaning diarrhoea, and improve growth performances (Li et al., 2001; Hojberg et al., 2005; Chai et al., 2014). This metal improves the intestinal morphology, increasing villus height and villus height:crypt depth ratio, and intestinal barrier function (Song et al., 2015). The effects of ZnO on the innate and adaptive gut associated immune system pigs has been investigated, demonstrating that this mineral supplementation positively affects the expression of genes involved in nonspecific defence mechanisms (Sargeant et al., 2010) and protects the intestinal barrier via mitogen‐activated protein kinases and TGF‐β1 signalling pathways (Liu et al., 2014).
There is an increasing concern that high doses of ZnO may lead to an increase prevalence of AMR bacteria in weaned piglets. An increased occurrence of tetracycline and sulfonamide‐resistant E. coli strains and of the genes tetA and sul1, coding for the resistance to these antimicrobials, was observed in pigs fed high zinc doses (Bednorz et al., 2013; Vahjen et al., 2015). Moreover, high dose zinc supplementation was shown increase the prevalence in pigs of MRSA (Slifierz et al., 2015). This is due to colocation on the same staphylococcal genomic island of the zinc resistance gene (czrC) and meticillin resistance gene (mecA) (Amachawadi et al., 2015). The use of ZnO in pig production is suspected of being involved in the emergence of epizootic strains of MDR Salmonella spp. (Wales and Davies, 2015; Schulte et al., 2016). There are further concerns about the impact of zinc on the environment.
Silver nanoparticles synthesised by plants have recently as been proposed as a possible solution to treatment of highly AMR microorganisms (Mapara et al., 2015).
4.4.15. Other alternatives
The application of light‐activated molecules (photoantimicrobials) has been proposed as an alternative to the therapeutic use of antimicrobials, although clinical trials proving its effectiveness are still rare (Wainwright et al., 2016). In veterinary medicine, this approach has been applied for example in the treatment of lymphadenitis abscesses in sheep, with encouraging results (Sellera et al., 2016).
4.4.16. Summary on the alternative measures
Evidence on the efficacy of the alternatives identified, associated risks and specific knowledge gaps are summarised in Table 6.
Table 6.
Alternative measures: summary on the evidence on the efficacy, associated risks and specific knowledge gaps
| Alternative | Target species | Reported data on potential efficacy | Risksa | Current regulatory framework applicable | Effects on nutrition and performances | Knowledge gaps | Comments |
|---|---|---|---|---|---|---|---|
| Probiotics | Calves | Reduction of diarrhoeal infections | Presence of virulence factors and/or AMR determinants in strains used as probiotics | Some of the strains are authorised as zootechnical feed additives | Demonstration of performance improvements for strains authorised as zootechnical feed additives under Regulation (EC) No 1831/2003 |
Mode of action of probiotics Dose response Limited controlled trials to support efficacy |
Data on efficacy as alternatives to antimicrobials are strictly strain dependent |
| Pigs | Reduction of diarrhoeal infections in weaned piglets | ||||||
| Chickens | Reduction of C. perfringens‐induced necrotic enteritis | ||||||
| Fish, crustacea and mollusca | Reduction of mortality due to bacterial infections, mainly at larval stage | ||||||
| Competitive exclusion products | Chickens | Reduction of C. perfringens‐induced necrotic enteritis |
Possible spread of viruses and pathogens Possible spread of AMR |
Not authorised under a specific EU regulatory framework |
Dose response limited controlled trials to support efficacy |
||
| Predatory bacteria | Chickens | Reduced colonisation with Salmonella spp. | Not authorised under a specific EU regulatory framework | Very limited data to support efficacy | Data on efficacy as alternatives to antimicrobials are strictly dependent to the strain of predatory bacterium used and to its host range | ||
| Bacteriophages | Chickens | Reduction of colibacillosis |
Emergence of phage resistant populations. Might carry AMR determinants Transduction of virulence genes in the target bacterial population |
Not authorised under a specific EU regulatory framework |
Long‐term efficacy Dose response Limited number of studies to support the efficacy |
Data on efficacy as alternatives to antimicrobials are strictly dependent to the strain of phage used and to its host range | |
| Aquaculture | Reduction of bacterial infections | ||||||
| Antibodies | All species | Reduction of diarrhoea in neonatal and post‐weaning pigs | Viruses and/or pathogen contamination of the products |
Few old veterinary biological products or Not authorised under a specific EU regulatory framework |
Dose response studies Limited controlled trials to support efficacy |
||
| Immunomodulators | Chickens | Reduction of colibacillosis in chickens | Toxicity residue |
Veterinary medicinal products or Not authorised under a specific EU regulatory framework |
Limited number of studies to support the efficacy | ||
| Fish | Reduction of bacterial infections | ||||||
| Cattle | Reduction of mastitis | ||||||
| Antimicrobial Peptides | Chickens | Antibacterial activity | Toxicity |
VPMs Not authorised under a specific EU regulatory framework |
Lack of controlled trials to support efficacy Pharmacolgy |
||
| Organic acids | Chickens | Reduction of necrotic enteritis in chickens and of diarrhoea in pigs | Some of the molecules are authorised as technological feed additivesb | Lack of controlled trials to support efficacy | |||
| Pigs | |||||||
| Clays | Pigs | Binder of toxins | Risk of contaminants | Authorised as feed additives | Binders, anticaking agents | Limited controlled trials to support efficacy | |
| Prebiotics | All animals |
Microbiota development Antitoxins |
Antinutritive or toxic residue | Some authorised as feed additives | Can be used as fibre sources, astringent substances, mucilaginous substances |
Mode of action Pharmacology Chemical composition Limited controlled trials to support efficacy |
Data on efficacy as alternatives to antimicrobials are strictly product/formulation dependent |
| Symbiotics |
Chickens Pigs Fish |
Reduction of bacterial infections | As for probiotics and prebiotics | As for probiotics and prebiotics | As for probiotics and prebiotics | As for probiotics and prebiotics | As for probiotics and prebiotics |
| Zinc oxide | Piglets | Prevention of post‐weaning diarrhoea in piglets |
Cross selection for AMR bacteria Environmental risk |
Authorised as veterinary medicinal products and as a feed additive | Nutritional additive | Limited controlled trials to support efficacy | |
| Botanicals | All animals |
Antibacterial activity Immunomodulation Microbiota development |
Toxicity residue |
Some are authorised as feed additives Lack of veterinary phytobiotics assessment guidelines as veterinary products |
Natural products Botanically defined |
Mode of action Pharmacology Chemical composition Limited controlled trials to support efficacy |
Data on efficacy as alternatives to antimicrobials are strictly product/formulation dependent |
| Teat sealants | Dairy cows | Reduction of intrammary infections | VMPs |
AMR: antimicrobial resistance; VMP: veterinary medicinal product.
An assessment of the risk related to the use of this potential alternative for the animals, the consumers of food of animal origin and the environment shall be performed, following the requirements of specific authorisation frameworks (e.g. VMP or Feed Additives).
Feed additives shall favourably affect animal production, performance or welfare, particularly by affecting the gastrointestinal flora, as per Regulation (EC) No 1831/2003.
When considering the information reported above for the different alternatives, a positive impact on animal health parameters has been demonstrated for the following alternatives:
Organic acids: in some studies they have shown to reduce the prevalence and spread of some food‐borne zoonotic bacteria such as Salmonella spp., Campylobacter spp. and E. coli when supplemented in the diet of food‐producing animals. A limited number of studies showed the effects in preventing necrotic enteritis in chicken and reducing diarrhoea in pigs and mortality in fish.
Probiotics: studies with single or multispecies probiotics have shown the prevention and/or reduction of diarrhoea in calves and piglets and of necrotic enteritis in poultry, and improved survival in fish and shellfish. Some of the compounds showed similar or better effect compared to treatment with certain antimicrobials.
Competitive exclusion: some studies have demonstrated the effects of competitive exclusion in reducing necrotic enteritis in chicken.
Synbiotics: some studies in pigs, poultry and fish have shown the positive impact on health parameters when combining treatment with certain probiotics and prebiotics compared to probiotics or prebiotics alone or, in some cases, in‐feed antimicrobials.
Passive immunisation: it can be used to treat diseases caused by infection and/or toxin and offer a way for the control of infectious disease.
Bacteriophages: they have been shown to reduce enteric colonisation and shedding of zoonotic pathogens such as Salmonella spp., Campylobacter spp. and Listeria spp. in pigs and poultry, and a limited number of studies showed a positive health impact by reducing diarrhoea and mortality in chickens and aquaculture species. A major limitation in using bacteriophages is that the target bacteria may develop resistance to them after use. There are also problems in regulatory aspects of their development and authorisation.
Immunomodulators: they have been particularly studied for use in aquaculture. For example, lipopolysaccharides, levamisole and chitosan have been shown to enhance the resistance of fish against some bacterial infections. The preventive administration of lipopolysaccharides has shown to reduce mortality similarly to the use of therapeutic antimicrobials within experimental infections. Peptides have been used in poultry with positive results.
Zinc oxide (ZnO): several peer‐reviewed papers indicate that high dose supplementation of ZnO reduces the incidence of intestinal disorders, mainly the post‐weaning diarrhoea, and improve growth performances. There are some concerns that at high doses ZnO may lead to increase in the prevalence of AMR bacteria in piglets, and also in regards to its impacts on the environment.
Clay minerals: they prevent the negative effects of many toxic compounds including some bacterial toxins.
Teat sealants: products containing bismuth subnitrate are authorised as veterinary medicinal products and have shown to protect against new intramammary infections, also in comparison to some antimicrobials.
A very limited number of the recent studies on alternatives to antimicrobials have been conducted in the EU. Possible reasons may be:
lack of funding on this specific research topic at both national and EU level;
the authorisation procedure of feed additives requires the demonstration of the capacity of the additive to favourably affect the performance or welfare of animals. This demonstration should be based on efficacy studies that are carried out with healthy animals. This requirement is a major limitation for companies when trying to develop products aiming to replace antimicrobials for disease control or treatment.
The literature review has identified gaps in knowledge that limit the use of alternatives to antimicrobials in animal husbandry in EU MSs:
there are very few cases in which data on the same agent used as an alternative to antimicrobials (e.g. an AMP or a probiotic culture) are reported in more than one study;
most of these studies demonstrate the efficacy of these agents, but very few are clinical trials or provide robust data to demonstrate the efficacy according to the methodology recommended in market authorisation criteria as feed additives or veterinary medicines.
Formulations of acids and probiotic or competitive exclusion products are very varied and it is difficult to find sufficient repeatable evidence of efficacy for a particular formulation and the variability hinders assessment of product classes as a whole. Several alternatives have an impact on the development of intestinal functions (absorption, integrity) and establishment of a microbiota in equilibrium with immune response.
The use of possible alternatives to the use of antimicrobials in animal husbandry in the EU can result in new risks in the food chain. For example:
Heavy metal products, particularly those containing salts of copper or zinc can select for both resistance to heavy metals and multiple AMR.
Use of ZnO in pig production is suspected of being involved in the emergence of epizootic strains of MDR Salmonella spp. and LA‐MRSA.
The use of animal‐derived products, such as antibodies, if not properly treated and used can result in the spread of bacterial and viral animal pathogens.
Bacteriophage therapy may lead to the selection of bacteriophage‐insensitive or bacteriophage‐resistant bacteria.
The EU regulations foresee two different classes of products: the zootechnical feed additives and medicinal products with substantial different functions and claims. The first category encompasses agents improving mainly the zootechnical performances, the second pharmacological actions. Some of the alternative products do not fall precisely in one of the two categories, e.g. probiotics aimed at disease risk reduction or competitive exclusion products. Botanicals can potentially be included in both categories or considered feed materials and may fall in the category feed with nutritional purposes, as defined in the EU.
Concluding remarks
There are numerous published papers that discuss the potential of compounds and live microorganisms that may be used as alternatives to antimicrobials in livestock production. A very limited number of the recent studies have been conducted in the EU.
Only a limited number of articles provide robust scientific evidence that conclusively prove that the above agents are successful alternatives, positively affecting health parameters in animals (e.g. reduced diarrhoea, general morbidity or mortality). Some of the published papers describe the use of alternatives for the reduction of disease risk.
Due to the limitation in data availability, the potential impact of the alternative measures on the occurrence of AMR in bacteria from food‐producing animals and food cannot be established.
Study design to assess the efficacy of alternatives to antimicrobials is a key issue. The endpoints differ according the type of claims. In many cases, alternatives have been compared with AGPs for which the endpoints are growth and feed efficiency, which are not particularly relevant in this context.
In only a few studies, the effects of the different alternatives to antimicrobial have been compared. Of note is that contradictory results on the efficacy were obtained for the same agent in different studies.
Development of an alternative for bacterial disease prevention preferably requires establishing the main mode of action according to the dose of use.
Recommendations
Additional research is needed to develop reliable alternatives to antimicrobials. Such research should be focused on both the identification of new alternatives or on increasing of the knowledge on already described alternatives.
Studies aimed to understand the mode of action and robust clinical field trials are considered necessary.
Robust validation protocols for assessing efficacy of potential alternatives are necessary.
The potential for selection for AMR and co‐selection for transferable resistance to other therapeutic antimicrobials should be considered as part of the authorisation process for alternative agents for disease control in food‐producing animals.
More research about the intestinal function and microbiota ecology should be supported to develop new feed additives and formulations adapted to animal requirements.
A framework at the EU level should be developed for the regulation of substances that reduce the need for antimicrobials and do not sit within the definition of a veterinary medicinal product or a feed additive, considering the possible use of specific claims such as the reduction of disease risk.
5. Recommended options to reduce antimicrobial use in animal husbandry in the EU, including consideration of the advantages and disadvantages of the different alternatives (ToR 5)
5.1. Recommended options
A series of recommended options are presented below, together with suggestions for the levels of responsibility necessary to ensure fulfilment. These options, which have not been prioritised, are focused on an anticipated benefit to public health through reducing the overall use of antimicrobials in livestock production. This has to be balanced against the need to protect animal health and welfare, and to ensure livestock productivity. In addition to eliminating antimicrobial use that does not directly protect animal health and to using all antimicrobials in a more targeted way, particular attention should be given to those critically important antimicrobials included in category 282 of the AMEG classification.
Even more importantly, measures must be implemented that improve animal health and welfare and thereby reduce the need for antimicrobials in the first place. Therefore, the primary overarching objective of the options listed below is that an integrated, multifaceted approach is taken to reducing the use of antimicrobials in the livestock industry.
Of note is that no single recommended option will be sufficient to make a lasting impact on the occurrence of AMR in livestock production and its subsequent impact on public health. All measures listed should be assessed, and if necessary prioritised in light of local circumstances.
Although the first two options below may not, per se, directly reduce antimicrobial use or AMR, it is essential that a strategic approach is taken and that there are the means to evaluate the impact of the direct measures taken.
5.1.1. Option 1: Development of national strategies implemented through action plans
All the MSs should have national strategies on AMR that are implemented through action plans.
National strategies and action plans should be developed by the MSs taking into account the ‘One Health’ aspects of AMR to integrate actions on the veterinary and human side. They should be designed considering the circumstances of the specific local situation (e.g. actual use of antimicrobials, AMR levels, animal species farmed and farming systems, environmental conditions).
-
To be effective and to facilitate implementation, all sectors throughout the food chain should be involved collaboratively in their development and implementation. The key areas to be addressed include:
-
–
communication, education and training;
-
–
monitoring and surveillance of antimicrobial use and AMR;
-
–
encouraging prudent responsible antimicrobial use;
-
–
identification and limiting the use of specific CIAs82 such as fluoroquinolones, 3rd‐and 4th‐generation cephalosporins and colistin;
-
–
improving animal health and disease prevention;
-
–
promoting research into AMR;
-
–
development of novel antimicrobials and alternative treatments;
-
–
international and cross‐border collaboration consistent with EC and global action plans.
-
–
Action plans should be regularly reviewed and monitored for effectiveness.
Consideration by: National governments, all stakeholders.
5.1.2. Option 2: Development of harmonised systems for monitoring antimicrobial use and surveillance of AMR integrating data from humans, food‐producing animals and food derived thereof
Systems should be established by all the MSs for the monitoring and transparent reporting of antimicrobial use and surveillance of AMR. Although this opinion is not targeted at the monitoring of antimicrobial use or resistance in the human sector, it is important that these systems should use protocols harmonised throughout the EU (as in Decision 2013/652/EU) and integrate data across sectors: humans, food‐producing animals and food derived thereof. Data should be as much as possible consultable via an open data policy to encourage research, including retrospective analysis.
Such national systems should be used to establish an evidence base to identify the need for risk management measures/policies at the local level, and to assess their effectiveness.
At the EU level, relevant indicators suitable for monitoring and detecting trends in the levels of key drug‐resistant microorganisms in humans, food‐producing animals and food derived thereof, and in antimicrobial consumption should be developed.
A ‘One Health’ approach should be taken by the EU MSs for the analysis of antimicrobial use and resistance data, integrating the evidence in relation to food‐producing animals with the evidence in relation to similar activities in the human sphere in order to identify any associations.
Individual livestock sectors should be strongly encouraged by national authorities to establish antimicrobial use data collection systems.
Systems should ideally measure farm‐level use, for each antimicrobial substance used, to the level of the livestock species and production stage. These systems, including the statistic used for analysis, should be harmonised, to allow benchmarking and comparison between farms, livestock sectors and countries. Opportunities for benchmarking and comparison will be greatly reduced if data are collected at higher levels of aggregation.
Consideration by: EC, national governments, academia.
5.1.3. Option 3: Establishing targets for reduction of the use of antimicrobials in food‐producing animals, especially CIAs
When antimicrobial use is considered high across several or all sectors, targets for reducing overall antimicrobial use should be established, at national level and with support from the government. These targets should be set according to national circumstances (baseline level of antimicrobial use, husbandry systems, infrastructure through which to implement reduction measures).
Consideration should be given to setting of national targets specifically for reduction in the use of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins, and colistin to be achieved without an increase in use of other antimicrobials.
A package of reduction measures should be proposed (linked to the national action plan) with the goal of achieving targets within a given time frame. National targets should be reviewed regularly.
Once a system is in place to monitor and record the use of antimicrobials in the different species and production stages on‐farm, then sector‐specific targets should be set and may be monitored by livestock sectors. Sector‐specific targets should be linked to benchmarking between farms and should be reviewed regularly.
When benchmark thresholds are exceeded, a plan of clear interventions to reduce antimicrobial use should be developed jointly by the farmer and his veterinarian and included in the farm health plan (see Option No. 4).
Recognising that voluntary actions can be very effective, livestock sectors should be encouraged to set specific targets for the reduction in the use of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin for their sector.
Consideration by: EC, national governments, livestock sectors within individual Member States.
5.1.4. Option 4: On‐farm animal health management with professional input
All farms should have a farm health plan developed in close collaboration between the farmer, the veterinarian and other professional advisors, as part of an integrated approach to on‐farm animal health.
The plan should outline a programme of disease prevention (see Option No 9), with meaningful and measurable the farm‐level outcomes.
The plan should include interventions aimed at reducing antimicrobial use guided by measurement and benchmarking.
Health plans should be reviewed and revised on a regular basis to ensure that they are ‘living documents’ and are of active management value, resulting in real potential to improve animal health and reduce antimicrobial use.
Consideration by: National governments, farmers and veterinarians within individual Member States.
5.1.5. Option 5: Increasing the responsibility taken by veterinarians for prescribing antimicrobials
Veterinarians should be responsible and accountable for their prescribing decisions.
Veterinarians should prescribe only within the context of a single prescribing veterinary practice (or veterinarian) per farm, following sufficiently regular clinical examination of animals to be treated and with knowledge of the disease epidemiology on that farm.
Consideration should be given at national level to possible conflicts of interest for veterinarians prescribing antimicrobials, such as financial incentives and perceived pressure from employers and clients. This should be addressed with relevant controls and training.
Prescribing should be based on appropriate use of diagnostic testing and treatment guidelines (see Options No. 6 and 7).
Consideration should be given to require mandatory bacteriology and antimicrobial susceptibility testing at the time of prescription of fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin.
Consideration should be given to monitoring and benchmarking of antimicrobial prescribing by veterinarians.
Consideration by: National governments, veterinarians and their statutory bodies within individual Member States, veterinary associations.
5.1.6. Option 6: Increased oversight of preventive and metaphylactic antimicrobial use
There should be an aim at national and farm level to phase out preventive use of antimicrobials. This aim should be based on a structured review of such use at national or regional level by livestock sector professionals with the knowledge of local endemic disease epidemiology, underlying risk factors for disease and local husbandry systems. Related disease‐specific guidance should be developed.
In exceptional cases, if preventive antimicrobial use can be justified, this should be prescribed on the basis of recent diagnostic testing, by a veterinarian with good knowledge of the epidemiology of disease on the farm, and for a limited duration. The justification for preventive use should be recorded (see Section 3.3.6 for specific recommendations).
There should be an aim at national and farm level to reduce and refine the use of metaphylaxis. Specific principles for the main sectors/diseases should be developed at national or regional level with assistance from livestock sector experts. These should characterise the early detectors of disease and establish criteria on which to base initiation of treatment. The associated risk factors for disease should be identified, together with recognised alternative measures for their control.
Where antimicrobials are used repeatedly for metaphylactic use, then the underlying risk factors on the farm should be investigated by the responsible veterinarian and other professionals. Infection prevention and control measures should be reviewed and recognised alternative measures to reduce the need for antimicrobials should be implemented via the farm health plan.
Consideration by: National governments, farmers, veterinarians, veterinary associations, livestock sector professionals.
5.1.7. Option 7: Training and education for veterinarians and for end users of antimicrobials, and raising public awareness
Antimicrobial stewardship programmes should be developed which address, in addition to scientific principles, other factors that influence prescribing (e.g. behavioural barriers to change, advisory skills). The programmes should be delivered using evidenced multifaceted training methods.
Consideration could be given to development of the EU‐wide core curricula on the underlying science and principles of antimicrobial stewardship for both undergraduate and postgraduate veterinarians.
Regular training in antimicrobial stewardship should be made available to all veterinarians.
Sector‐specific treatment guidelines should be developed at national/regional level and training in their use should be a key part of antimicrobial stewardship programmes.
Training should be provided to farmers to increase their awareness of: (i) the association between antimicrobial use and the development of AMR, and its impacts on public health; (ii) principles to reduce the need to use antimicrobials. Veterinarians should be encouraged to reinforce these messages to the farming community.
In order to change attitudes and behaviours, education and awareness of AMR should be addressed to all levels of society.
Consideration by: EC; National governments; veterinarians and their statutory bodies within individual Member States, veterinary associations, livestock sector organisations, consumer associations.
5.1.8. Option 8: Increasing the availability and use of rapid and reliable diagnostics and antimicrobial susceptibility tests, including at the farm level
Diagnostic testing protocols should be developed so that the appropriate clinically meaningful tests are performed in relation to presenting syndromes.
New diagnostic methodologies should be validated to the EU standards to improve reliability and confidence in results and to ensure consistency between laboratories. Appropriate quality assurance systems should be developed and utilised.
Steps should be taken to accelerate the availability of new technologies that enable economical, rapid on‐farm diagnosis, so that veterinarians only prescribe antimicrobials when needed and can select the correct treatment.
The use of AST should be strongly promoted in national treatment guidelines, especially before the administration of CIAs.
Implementation of monitoring and surveillance systems for target pathogen susceptibility should be encouraged.
Clinical breakpoints for resistance to specific antimicrobials for veterinary pathogens should be established and agreed at the European level.
Consideration by: EC; National governments; veterinarians within individual Member States; industry/private sector.
5.1.9. Option 9: Improvement of husbandry and management procedures for disease prevention, control and eradication of infectious diseases in livestock production, including vaccination
-
On‐farm management and husbandry procedures should be optimised for disease prevention, as follows:
-
–
Primary prevention, to limit the entry of pathogens onto a premise, with particular attention to external biosecurity and other relevant measures to reduce major transmission routes. The SPF concept could be widely adopted.
-
–
Secondary prevention, to reduce within‐farm transmission, including internal biosecurity measures and adequate cleaning and disinfection procedures.
-
–
Tertiary prevention, for animal robustness, to increase the ability of an animal's immune system to respond to an infection, including use of efficacious vaccines and the promotion of husbandry conditions beneficial for health and welfare.
-
–
Each of these principles of disease prevention is applicable both on‐farm, and within a network of farms, clustered either geographically or based on trading linkages, or throughout the value chain of a production species. Adverse effects on animal welfare should be considered before implementation.
Where feasible, programmes should be undertaken to eradicate key diseases that contribute substantially to antimicrobial use.
Development of improved vaccines against infections accounting for high antimicrobial use in farm production systems should be supported.
Regulations for autogenous vaccines differ between the MSs and could be harmonised.
Consideration by: National governments; farmers and veterinarians within individual Member States.
5.1.10. Option 10: Rethinking livestock production systems: reduced reliance on antimicrobial use and exploring further the potential of alternative production systems
A critical evaluation of farming systems with heavy antimicrobial use is required to determine whether/how the system could be sustainable with reduced use of antimicrobials. If not, the farming system under review should be investigated by national authorities.
Some regulated production systems are able to operate with low antimicrobial use, and the farming practices which facilitate this should be further evaluated. Any associated animal health and welfare risks due to higher risk for bacterial/parasitical/viral exposure may also need to be considered.
Consideration by: National governments; farmers and veterinarians within individual Member States.
5.1.11. Option 11: Development of treatments which are alternatives to antimicrobials
Although a number of treatments have been studied as alternatives to antimicrobials and some have shown the potential to be efficacious, additional research is needed. The research should be focused on the identification of new alternatives or on increasing the knowledge on already described alternatives.
Studies aimed to understand the mode of action are considered necessary, together with controlled and meaningful clinical trials. Robust validation protocols are needed to assess the efficacy of potential alternatives in field conditions.
The use of possible alternatives to the use of antimicrobials requires appropriate risk assessments to evaluate the potential new risks for the consumers of food of animal origin, for the target animal species and for the environment.
A framework at the EU level should be developed for the regulation of substances that reduce the need for or use of antimicrobials and do not sit within the definition of a veterinary medicinal product or a feed additive, considering the possible use of specific claims.
Consideration by: EC; National governments; industry, regulatory bodies.
5.2. Consideration of advantages and disadvantages of the recommended options
The possible advantages and disadvantages of each of these eleven options in the context of reducing the need for, and the use of, antimicrobials are considered in Table 7. Many of the recommended options would entail substantial financial investment at either the local, national or EU level. Financial considerations are not regarded as appropriate for this Opinion, which is based on scientific criteria, and are not discussed.
Table 7.
Possible advantages and disadvantages of each of the eleven options in the context of reducing the need for and the use of antimicrobials
| Recommended option | Advantages | Disadvantages |
|---|---|---|
| 1. Development of national strategies implemented through action plans |
|
|
| 2. Development of harmonised systems for monitoring antimicrobial use and surveillance of AMR integrating data from humans, food‐producing animals and food derived thereof |
|
|
| 3. Establishing targets for reduction of the use of antimicrobials in food‐producing animals, especially CIAs |
|
|
| 4. On‐farm animal health management with professional input |
|
|
| 5. Increasing the responsibility taken by veterinarians for prescribing antimicrobials |
|
|
| 6. Increased oversight of preventive and metaphylactic antimicrobial use |
|
|
| 7. Training and education for veterinarians and for end users of antimicrobials, and raising public awareness |
|
|
| 8. Increasing the availability and use of rapid and reliable diagnostics and antimicrobial susceptibility tests, including at the farm level |
|
|
| 9. Improvement of husbandry and management procedures for disease prevention, control and eradication of infectious diseases in livestock production, including vaccination |
|
|
| 10. Rethinking livestock production systems: reduced reliance on antimicrobial use and exploring further the potential of alternative production systems |
|
|
| 11. Development of treatments which are alternatives to antimicrobials |
|
|
AMR: antimicrobial resistance; CIA: critically important antimicrobial; EU: European Union; MS: Member State.
5.3. Data Gaps, data quality and uncertainties
National strategies and action plans on AMR do not exist or are not readily accessible for all the MSs. Likewise, for many MSs, there are few accessible publications reporting on the implementation of their action plans and impacts on antimicrobial use or AMR.
Other than surveillance data collected under Decision 2013/652/EU since 2013, there is a lack of harmonised longitudinal data on AMR in zoonotic and indicator organisms from livestock and food in EU MSs. This limits usefulness for analysing trends and complicates intercountry comparisons.
There is a lack of harmonised longitudinal data on AMR in animal pathogens.
Data on sales (use) of antimicrobials do not exist from all the EU MSs, and from many are only recently available. The sales data from the ESVAC project at present do not collect data at the species/production sector level or use a standardised measurement of consumption by species. This limits usefulness of the data for intercountry comparison due to differences in livestock profiles.
Few MSs provide reports that present data on antimicrobial use together with findings from AMR surveillance, and they are often not yet in a position to integrate the data for analysis.
Inferences on the impacts of measures taken to reduce antimicrobial use would be facilitated by knowledge of antimicrobial use and AMR at an individual species and farm level.
No official data could be found for the amount of antimicrobials used preventively.
A number of treatments have been studied as alternatives to antimicrobials and some have shown the potential to be efficacious. There is a gap of knowledge in relation to their efficacy in field conditions.
5.4. Examples of the implementation of an integrated approach to reduce the use of antimicrobials in food‐producing animals
Two examples of the implementation of an integrated approach to reduce the use of antimicrobials in food‐producing animals are presented in Appendix K:
management of mastitis in cattle;
pig farming in Denmark.
5.5. Circumstances where continued use of antimicrobials is needed
Antimicrobials remain a key tool for the treatment of infectious diseases in animals. In the treatment of livestock, it is generally understood that there are three different circumstances for antimicrobial administration:
-
(Curative) Treatment
Animals with clinical signs of a bacterial infection that is impacting on their health and welfare in many cases need to be treated with antimicrobials. Some examples of infections that commonly require antimicrobial treatment are given in Section 1.7.2 of this Opinion. It should furthermore be recognised that if infections are not treated, this can affect the quality of animal produce such that at harvest it might not be fit for human consumption.
-
Metaphylaxis
In the case of metaphylaxis, apparently healthy animals are treated simultaneously with clinically ill animals, with whom they have contact, as part of the same group. Metaphylaxis is a strategy frequently used in veterinary medicine, mostly in intensively reared animals, and is appropriate in circumstances where there is potential for high morbidity (and sometimes mortality) due to rapidly spreading contagious disease. Indications for metaphylaxis include enteric and respiratory diseases in pigs, veal calves, poultry and rabbits. More specific examples are given in Sections 1.7.2 and 3.3.6.
-
Prevention
Preventive treatment involves the administration of an antimicrobial to healthy animals before clinical signs of disease have developed in order to prevent the occurrence of disease or infection.
Both metaphylaxis and preventive antimicrobial treatments are frequently administered to groups of animals, often as oral formulations, which ESVAC data indicate account for more than 90% of antimicrobial consumption in livestock (EMA ESVAC, 2016). Evidence suggests that a significant proportion of these administrations is for prevention of diseases, such as respiratory disease in calves or post‐weaning diarrhoea in pigs, often in the absence of precise knowledge of the potential infection (Callens et al., 2012; Moreno 2014a). These diseases are commonly linked to management practices such as poor biosecurity, transportation, comingling and/or dietary change (Moreno, 2012; Pardon et al., 2012b). Furthermore, preventive administration of antimicrobials may be used as a management tool to reduce labour and for economic reasons (Callens et al., 2012; Coyne et al., 2016). Some MSs have already made good progress in reducing these types of group treatments through use of vaccination, disease eradication and husbandry changes (DANMAP, 2014; SVA, 2015).
Options for reducing the risk to human health
General options aimed at encouraging more responsible antimicrobial use are addressed primarily in Sections 3.3.4 to 3.3.8 of this Opinion. In all cases where administration of an antimicrobial is required, this should be prescribed following appropriate diagnosis by a veterinarian with a good knowledge of the disease epidemiology on the farm and immune status of the livestock. The diagnosis should include knowledge of the (potential) target pathogens and their antimicrobial susceptibility based on recent sampling or surveillance. Antimicrobials should be administered following the effective dosing regimen and only for as long as is required for clinical or bacteriological cure (as appropriate). Approved treatment guidelines which give consideration to the responsible use of antimicrobials that are CIAs for human health should be followed. In this respect, CIA lists should be regularly reviewed at the EU level to take account of the evolution of AMR and changing importance of different substances/classes to human medicine.
Metaphylaxis
There should be an aim at national and farm level to reduce and refine the use of antimicrobial metaphylaxis. Specific principles for the main sectors/diseases should be developed at national or regional level with assistance from livestock sector experts. These should characterise the early detectors of disease and establish criteria on which to base initiation of treatment. The associated risk factors for disease should be identified, together with recognised alternative measures for their control. Livestock handlers should closely monitor their animals’ health and should have the skills and knowledge to detect disease early in order to allow movement away from use of systematic preventive treatments and towards metaphylactic use only.
Where antimicrobials are used repeatedly for preventive or metaphylactic use, then the underlying risk factors on the farm should be investigated by the responsible veterinarian and other professionals. Infection prevention and control measures should be reviewed and recognised alternative measures to reduce the need for antimicrobials should be implemented via the farm health plan.
Prevention
There should be an aim at national and farm level to phase out preventive use of antimicrobials. This aim should be based on a structured review of such use at national or regional level by sector professionals with the knowledge of local endemic disease epidemiology, underlying risk factors for disease and local husbandry systems. Related disease‐specific guidance should be developed. It is acknowledged that preventive use may need to be continued in exceptional circumstances (see Section 3.3.6).
It is likely that preventive use would need to be continued for individual animals in exceptional circumstances (e.g. prior to major surgery such as caesarean section, where risk factors dictate).
5.6. The impact of reducing antimicrobial use on animal health and welfare
In several countries, substantial progress has now been made to reduce antimicrobial use both in defined livestock sectors and nationally. Given the critical role of antimicrobials in infection control, concern has been raised of potentially adverse impact of reducing antimicrobial use on animal health and welfare. This concern has been specifically addressed in recent research.
At a national level, Aarestrup et al. (2010) found that the substantial (higher than 50%) reduction in antimicrobial consumption during 1992–2008 in Denmark, both prior to and following a ban on the use of AGPs, did not have a negative impact on long‐term swine productivity. In later work, Alban et al. (2013) identified short‐term increases both in vaccine use and in the prevalence of specific lesions during meat inspection in Danish finisher pigs, following the introduction of the yellow card scheme in 2010. This study was conducted over only a short period, and therefore long‐term trends were not evaluated.
At the farm level, several studies have highlighted the link between farm characteristics, disease problems and high antimicrobial use (Laanen et al., 2013; Jensen et al., 2016). Until recently no prospective studies have been conducted, to critically evaluate the potential impact of changes to farm management on antimicrobial use. For this reason, the very recent work by Postma et al. (2016b) is particularly important. In this intervention study, conducted on 61 pig farms in Belgium, these authors sought to determine whether antimicrobial use could be reduced in pig production without jeopardising animal health and production. On each of these farms, farm‐specific action plans were developed following an initial assessment, focusing on optimal herd management, improved external and internal biosecurity and with guidance on prudent antimicrobial use. During the study period, antimicrobial use was significantly reduced (by 52% for the pigs from birth to slaughter, by 32% for breeding animals), including the use of critically important antimicrobials, while, on average, also achieving improved production results. The work was conducted using a team‐based approach, by farmers and their veterinarian and other professional advisors.
In conclusion, animal health can be maintained or improved, both nationally and on individual farms, in association with reduced antimicrobial use. This has recently been demonstrated using a team‐based approach, involving farmers and their veterinarian and other farm professionals, focusing on general herd management, improved biosecurity, optimised vaccination and prudent antimicrobial use. Further research should be made into the impact of different measures under different husbandry systems, and objective indicators of animal welfare are needed before the impacts of reduced antimicrobial use on these aspects can be fully established (RDA, 2016).
6. Conclusions
6.1. Answer to Term of Reference 1
‘Review the measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU’
A wide range of control strategies have been implemented in several EU MSs with the aim to combat antimicrobial resistance (AMR) through reducing antimicrobial use in animal husbandry.
Reductions in antimicrobial use have been achieved, especially in northern European countries, but in many other MSs it is too early to assess the impacts of their strategies.
The European Commission Guidelines for the prudent use of antimicrobials in veterinary medicine (PUAVM Guidelines), published in September 2015, provide practical guidance for the development and implementation of prudent use strategies and can be used as part of the basis for action plans to combat AMR.
National strategies and action plans have included the principles reported below, adapted to local circumstances.
-
Monitoring and surveillance programmes for AMR and antimicrobial use:
-
–
Systems combining AMR and antimicrobial use data have been used to identify where interventions are needed to reduce use, for supporting policy development and evaluating the effectiveness of measures applied. When AMR and use data from humans, animals and food have been integrated for analysis, they have been shown to be valuable for demonstrating the dissemination of AMR between different reservoirs.
-
–
Collection of antimicrobial use data at national level (for European Surveillance of Veterinary Antimicrobial Consumption, ESVAC) has been valuable to enable comparisons between the MSs, although analysis is limited by a lack of harmonisation and detail (e.g. data at species level) in the data.
-
–
Collection of antimicrobial use data at the production sector (species, production type) level has allowed focused measures to reduce antimicrobial use, and may have motivated livestock sectors to take action voluntarily.
-
–
Collection of farm‐level data on antimicrobial use has been an important driver for change, allowing critical assessment and benchmarking between farms and production systems.
-
–
-
Limiting the use of antimicrobials:
-
–
In the MSs where overall antimicrobial use is high, setting of overall percentage reduction targets at national level by governments has led to reductions in antimicrobial use across the major livestock sectors.
-
–
Benchmarking between farms has been a key tool to reduce antimicrobial use but requires the infrastructure to be in place to allow monitoring and analysis of antimicrobial use at the farm and production sector level.
-
–
-
Encouraging responsible antimicrobial use:
-
–
Sector‐specific evidence‐based treatment guidelines have been implemented to promote the use of antimicrobial susceptibility testing (AST) and emphasise the need to reserve those antimicrobials classed as highest priority critically important antimicrobials (CIAs) for human health for use in livestock only as a last resort.
-
–
Some MSs have made AST testing prior to use of fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins a legislative requirement.
-
–
Individual livestock sectors have adopted voluntary bans or restrictions on the use of 3rd‐ and 4th‐generation cephalosporins and fluoroquinolones in some MSs.
-
–
Several MSs have prohibited preventive use of antimicrobials, except in exceptional circumstances.
-
–
Taking account of the high consumption of antimicrobials in group treatments of intensively reared animals, some MSs require increased oversight of prescribing in these circumstances.
-
–
In order to address conflicts of interest for prescribing veterinarians, some MSs have ‘decoupled’ the prescription of antimicrobial medicines by the veterinarian from their dispensing.
-
–
In some MSs, veterinarians have increased responsibility and are only allowed to prescribe antimicrobials for food‐producing animals in the context of a farm health plan and a single prescribing veterinarian for each farm.
-
–
-
Communication, education and training:
-
–
Postgraduate training in antimicrobial stewardship is provided by a combination of national authorities, veterinary associations and universities in some MSs; such training is not mandatory across the EU.
-
–
Where farmers’ awareness of AMR has been increased through policy measures and best practice guidelines, they may have been more supportive of measures to reduce antimicrobial use.
-
–
Several MSs have run campaigns to increase public awareness of AMR. Media attention may also have helped to change attitudes towards antimicrobial use.
-
–
Overall,
In successful programmes to reduce antimicrobial use, a multifaceted approach has been applied, reflecting the multiplicity of factors that influence antimicrobial use. Programmes have taken account of local livestock production systems and have involved all relevant stakeholders in their implementation.
-
Some individual measures appear to have had a specific impact in driving a reduction in antimicrobial use in the MSs where they have been applied. These are:
-
–
high‐level reduction targets supported in national strategies;
-
–
farm‐level measurement of antimicrobial use and benchmarking;
-
–
strengthening controls on group treatments, especially premixes;
-
–
a requirement for antimicrobial susceptibility testing prior to use of high priority CIAs;
-
–
legislative and voluntary industry sector restrictions on the use of high priority CIAs.
-
–
Many of these measures have been made mandatory in successful programmes. Supporting measures, such as provision of treatment guidelines and education, may have been important but have had less clear impacts.
There is a limited evidence of either the positive or negative impact on animal health and welfare of national programmes of reduced antimicrobial use.
6.2. Answer to Term of Reference 2
‘Assess the impact of such measures regarding the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food’
-
Assessing the impact of measures mentioned under TOR 1 to reduce antimicrobial use on the occurrence of AMR in food‐producing animals and food is difficult for several reasons, key of which are:
-
–
Many MSs have implemented packages of measures, some of which have been applied simultaneously. It is therefore difficult to determine the specific impact of the individual measures to reduce antimicrobial use on AMR.
-
–
There is a lag period between reduction measures being applied, the selection pressure on organisms being lifted and collection and analysis of data on AMR. Sufficient time may not yet have elapsed to see the impacts.
-
–
Impacts can only be observed where there is a sustained period of longitudinal, standardised monitoring data.
-
–
It is difficult to establish causality in such complex systems.
-
–
There are a few examples where specific measures to reduce antimicrobial use have been associated with a reduction in AMR in bacteria from food‐producing animals or foods thereof. For example, cessation of use of 3rd‐ and 4th‐generation cephalosporins in the pig and poultry sectors has been associated with a reduction in the occurrence of extended‐spectrum β‐lactamase (ESBL)‐producing E. coli in animals and meat, although several years’ data are needed before a trend in AMR evolution can be reliably concluded upon.
Marked reductions in antimicrobial use achieved in some MSs have had lesser/no impact for certain resistances, e.g. fluoroquinolone resistance in Campylobacter spp., and multidrug‐resistance (MDR) in monophasic Salmonella Typhimurium.
Some ecological studies (e.g. JIACRA report, see Section 1.5.3) have demonstrated correlations between antimicrobial use and resistance in bacteria from food‐producing animals
Overall it is reasonable to assume that a reduction in antimicrobial use will result in a general reduction in AMR in bacteria from food‐producing animals and food.
6.3. Answer to Term of Reference 3
‘Review the recent scientific developments in the area of possible alternatives to the use of antimicrobials in animal husbandry in the EU’
As clarified in Section 1.2, under this Term of Reference all measures aimed at reducing the need to use antimicrobials were reviewed and discussed.
In addition to recent scientific developments, animal husbandry measures that have been, or are being taken, to reduce the use of antimicrobials in animal husbandry in the EU are also detailed under TOR 3. Furthermore, compounds that are presently used as alternatives to antimicrobials are also summarised.
Animal husbandry and disease prevention measures
-
The need to use antimicrobials can be reduced dramatically through the application of good farm management and husbandry practices for terrestrial and aquatic animals. Roughly, these can be divided into three main categories, including practices:
-
–
to reduce the introduction and spread of microorganisms between farms (primary prevention),
-
–
to reduce transmission or spread within a farm (secondary prevention), and
-
–
to increase the ability of animals to cope with these pathogens (tertiary prevention).
-
–
Primary prevention includes external biosecurity including introduction of infection by live animals, compartmentalisation and eradication of infectious diseases.
Secondary prevention includes: internal biosecurity, production groupings, housing design, building and maintenance.
-
Tertiary prevention includes: housing, nutrition, stress reduction, vaccination and genetic selection. Collectively and individually, these approaches can increase the ability of an animal's immune system to respond appropriately to an infectious challenge.
-
–
Vaccines have contributed significantly to the control of infectious diseases in production animals.
-
–
Non‐specific stimulation of the immune system may have the potential to increase resistance to complex diseases such as post‐weaning diarrhoea in pigs, calf diseases and runting and stunting syndrome in young chicks.
-
–
Genetic selection has the potential to improve both innate and adaptive immune competence.
-
–
Husbandry conditions have been shown to significantly influence resilience of the animals and this is mediated by direct impact of the housing environment as well as alterations of the immune system.
-
–
Good health in farm animals can be promoted through nutritional measures (e.g. diet transitions, feed hygiene).
-
–
Herd health plans can assist in disease control efforts at the farm level since they are based on an integrated approach through monitoring of health, maximising passive and active immunity, monitoring of disease and disease prevention. Herd health plans can play a significant role in monitoring and responding to disease risks and in optimising on‐farm use of antimicrobials.
A herd health plan can serve as a tool to set targets and identify measures to improve the biosecurity and management measures, but requires a high level of commitment and agreement between the farmer, his/her veterinarian and other professional advisors.
For diseases where the risk of transmission between herds is high, control/eradication should preferably be done on an area/region/country‐level.
Stamping out of infected herds/flocks is usually the most reliable method if the disease is newly introduced and regional herd prevalence is low. In specific cases, depending on key aspects of the infection, including its epidemiology, medication combined with vaccination, DIVA tests and/or selective removal of individual infected carrier animals may be an effective control option.
Alternative production systems
Organic or similar alternative farming practices may improve housing and management conditions for animals and therefore contribute to secondary and tertiary prevention, while primary prevention may be compromised, for example by increased levels of exposure to wildlife.
In the majority of the studies appraised, an association was observed between organic farming and reduced AMR. However, due to the limitations in the study design, methodologies for data analysis and biological relevance of the approach, in many of these studies there is a potential for bias in the estimate of the association and effect of organic farming on AMR. Therefore, conclusive evidence of the impact of organic farming on reducing AMR cannot be established because of the high level of uncertainty in the appraised studies.
Diagnostics
Some existing diagnostic methodologies are limited by the time taken to obtain results and concerns over costs and clinical relevance of the findings. The development of modern techniques could enable more rapid and precise diagnosis, allowing better targeted antimicrobial use.
Compounds and live microorganisms used as alternatives to antimicrobials
There are numerous published papers that discuss the potential of compounds and live microorganisms that may be used as alternatives to antimicrobials in livestock production. A very limited number of the recent studies have been conducted in the EU.
Only a limited number of studies provide robust scientific evidence that conclusively prove that the above agents are successful alternatives, positively affecting health parameters in animals. Some of the published papers describe the use of alternatives for the reduction of disease risk.
Study design to assess the efficacy of alternatives to antimicrobials is a key issue. The endpoints differ according to the type of claims. In many cases, alternatives have been compared with antimicrobial growth promoters (AGPs) for which the endpoints are the growth and feed parameters, which are not particularly relevant in this context.
A positive impact on animal health parameters has been demonstrated for some of the alternatives considered. These include: organic acids, probiotics, competitive exclusion, synbiotics, passive immunisation, bacteriophages, immunomodulators, Zinc oxide, clay minerals and teat sealants. Evidence on the efficacy of these alternatives, associated risks and specific knowledge gaps are listed in Section 4.4.
-
Gaps in knowledge limit the use of alternatives to antimicrobials in animal husbandry in the EU:
-
–
there are very few cases in which data on the same agent used as alternative to antimicrobials are reported in more than one study;
-
–
most of these studies demonstrate the efficacy of these agents, but very few are clinical trials or provide robust data to demonstrate the efficacy according to the methodology recommended in market authorisation criteria as feed additives or veterinary medicines.
-
–
6.4. Answer to Term of Reference 4
‘Assess the potential impact of such alternative measures on the occurrence of antimicrobial resistance in bacteria from food‐producing animals and food’
Due to the limitations in data availability, the potential impact of the alternative measures on the occurrence of AMR in bacteria from food‐producing animals and food cannot be conclusively established.
Measures which reduce the need to use antimicrobials, such as improved biosecurity, control and/or eradication of infectious diseases and the alternatives identified above, are likely to reduce development of AMR as discussed in the answer to Term of Reference 2.
Some substances which are used as alternatives to antimicrobials (e.g. zinc oxide) may also increase AMR selection pressure towards AMR, but this has not been investigated for other alternatives.
6.5. Answer to Term of Reference 5
‘Recommend options to reduce antimicrobial usage in animal husbandry in the EU, including consideration of the advantages and disadvantages of the different alternatives. Where a continued need is identified to use antimicrobials in the interests of animal health and welfare, recommend how such use can continue with the minimum possible risk to human health’
Recommended options to reduce antimicrobial use in food‐producing animals
The primary overarching objective of the recommended options is that an integrated, multifaceted approach is taken to reduce the use of antimicrobials in the livestock industry.
No single recommended option will be sufficient to make a lasting impact on the occurrence of AMR in livestock production and its subsequent impact on public health.
-
Recommended options (non‐prioritised) include:
-
–
Development of national strategies implemented through action plans.
-
–
Development of harmonised systems for monitoring antimicrobial use and surveillance of AMR integrating data from humans, food‐producing animals and food derived thereof.
-
–
Establishing targets for reduction of the use of antimicrobials in food‐producing animals, especially CIAs.
-
–
On‐farm animal health management with professional input.
-
–
Increasing the responsibility taken by veterinarians for prescribing antimicrobials.
-
–
Increased oversight of preventive and metaphylactic antimicrobial use.
-
–
Training and education for veterinarians and for end users of antimicrobials, and raising public awareness.
-
–
Increasing the availability and use of rapid and reliable diagnostics and antimicrobial susceptibility tests, including at the farm level.
-
–
Improvement of husbandry and management procedures for disease prevention, control and eradication of infectious diseases in livestock production, including vaccination.
-
–
Rethinking livestock production systems: reduced reliance on antimicrobial use and exploring further the potential of alternative production systems.
-
–
Development of treatments which are alternatives to antimicrobials.
-
–
All options listed should be assessed, and if necessary adjusted, in light of local circumstances.
Further details on these recommended options, including considerations of the advantages and disadvantages of the options above, have been provided (see Section 5).
Recommended conditions for use of antimicrobials in food‐producing animals
Antimicrobials remain a key tool for the treatment of infectious diseases in animals. In the treatment of livestock, there are three different circumstances for antimicrobial treatment: curative treatment, metaphylaxis and prevention.
In all cases where administration of an antimicrobial is required, this should be prescribed following appropriate diagnosis by a veterinarian with a good knowledge of the disease epidemiology on the farm and immune status of the livestock. Approved treatment guidelines which give consideration to the responsible use of antimicrobials that are CIAs for human health should be followed.
Animals with clinical signs of a bacterial infection that is impacting on their health and welfare in many cases need curative treatment with antimicrobials.
Metaphylaxis is a strategy frequently used in intensively reared animals and is appropriate when there is potential for high morbidity due to rapidly spreading disease. There should be an aim to refine and reduce the use of metaphylaxis based on identification of underlying risk factors and implementation of measures for their control.
There should be an aim to phase out preventive use of antimicrobials, except in exceptional circumstances. This should be based on a structured review of such use in each sector/region and development of disease‐specific guidance.
7. Further recommendations
Further research is needed:
to assess the impact of dosing regimens, different formulations and classes of antimicrobials on the development of AMR in commensal and zoonotic organisms. This is important, especially as there appears to have been a shift in recent years away from group oral treatments towards use of long‐acting parenteral formulations (e.g. of macrolides and cephalosporins);
to further develop rapid diagnostic methods;
to promote the development of harmonised methods to quantify antimicrobial use (farms, veterinarians, production systems, the MSs, etc.);
to assess the effectiveness of individual MSs strategies on antimicrobial use and AMR;
to assess the impact of the MSs antimicrobial use reduction programmes on animal health and welfare;
to clarify the nature and extent of off‐label use of antimicrobials and how this might contribute to the development of AMR;
to better understand drivers for antimicrobial prescribing by veterinarians at national level;
to assess the effectiveness of different training methods in improving prescribing practice of postgraduate veterinarians, and to define the specific elements required in an antimicrobial stewardship programme (e.g. behavioural barriers to change, advisory skills);
to expand the knowledge of the impact of genetic selection strategies which take account of both welfare and animal health criteria would be helpful in identifying animals with ability to mount superior immune responses;
to develop improved vaccines, including next‐generation vaccines, especially against specific infections accounting for high antimicrobial use in farm production systems, e.g. enteric and respiratory diseases;
to develop reliable alternatives to antimicrobials. The research should be focused on the identification of new alternatives and on increasing the knowledge on already described alternatives, including understanding their mode of action, in addition to controlled and meaningful clinical trials;
to further explore the potential of alternative farming systems on reducing AMR without compromising animal health and welfare;
to evaluate the potential for organic farming to reduce AMR. In future work, potential limitations of study design, study analysis and biological relevance should be addressed.
Glossary and Abbreviations
Glossary
In the context of this report the following definitions are used:
Acquired resistance:
A bacterial strain can acquire resistance by mutation, by the uptake of exogenous genes by horizontal transfer from other bacterial strains or by the activation/triggering of a genetic cascade, thereby inducing the expression of resistance mechanisms. Genes encoding enzymes that can modify the structure of an antimicrobial are commonly located on transferable genetic elements (i.e. plasmids, transposons, integrons) that may usually carry more than one resistance gene. Acquisition of resistance by mutation usually arises spontaneously due to point mutations that result, for instance, in changes in an antimicrobial target – e.g. chromosomal changes that result in resistance to quinolones and fluoroquinolones.
Unlike intrinsic resistance, traits associated with acquired resistance are found only in some strains or subpopulations of a particular bacterial species. Acquired resistance results from successful gene change and/or exchange that may involve: mutation or horizontal gene transfer via transformation, transduction or conjugation. The MIC of those strains with acquired resistance are higher than the range of MIC observed for strains of the wild‐type population.
Antimicrobial resistance gene(s):
The genetic entities within a cell that promote the expression of resistance to a specific compound or compounds, and are identified by molecular (i.e. genotypic) methods. At the molecular level, AMR can result from mutations within existing genes in the bacterial chromosome, resulting in changes in the chromosomal DNA of the bacterium, or by the acquisition of external elements promoting resistance to one or more compounds (i.e. by horizontal gene transfer) by a variety of biological process. Horizontal gene transfer (see below) is common between bacteria of the same species, but under ideal conditions may take place between distinct evolutionary lineages of different bacterial species. Both pathogenic bacteria and commensal bacteria may undergo chromosomal mutations and be subject to horizontal gene transfer. Such AMR genes may be present on or in mobile genetic elements such as plasmids, transposons, integrons, mobilisable cassettes and bacteriophages (see below).
Antimicrobial:
An active substance of synthetic or natural origin which destroys microorganisms, suppresses their growth or their ability to reproduce after administration for therapeutic indications in animals or humans. In this context, antivirals, antiparasitics (e.g. coccidiostats) and disinfectants are excluded from the definition (EMA, 2014).
Antimicrobial resistance (AMR):
Defined as the inability or reduced ability of an antimicrobial agent to inhibit the growth of a bacterium, which, in the case of a pathogenic organism, can lead to therapy failure. For this report AMR is discussed at what is regarded as ‘epidemiological cut off’ levels (ECOFFs) (EFSA, 2012). An ECOFF is established by an independent scientific committee for an antimicrobial compound (e.g. ampicillin) to distinguish the wild‐type and the non wild‐type populations of bacterial species (e.g. E. coli).
Clinical resistance:
Clinical resistance is defined by scientific committee (CLSI, EUCAST) on the basis of knowledge of drug pharmacology and clinical efficacy. A breakpoint is established to define an isolate with a high rate of failure in case of treatment of systemic infection. The antimicrobial treatment is unlikely to be successful because active concentrations obtained during treatment are insufficient to decrease the bacterial population in vivo and may be associated with therapeutic failure. Clinical resistance breakpoints may be above the ECOFF levels (see above).
Co‐resistance:
The presence of resistance to more than one class of antimicrobial in the same bacterial strain, as might occur when different resistance genes are found on the same plasmid.
Co‐selection of resistance:
The selection of multiple AMR genes when one of these is selected by the presence of a relevant antimicrobial. An example of this is the integron, which is a cassette of AMR genes that are under the control of a single promoter. As a result, these genes are expressed in a coordinated manner, although the furthest downstream gene may not be as efficiently expressed as the gene next to the promoter. These cassettes are commonly found in both Gram‐positive and Gram‐negative bacteria. Since they are a form of transposon they can become a part of the bacterial chromosome or plasmid and can then be transmitted among different bacterial strains.
Critically Important Antimicrobial (CIA):
Those antimicrobials which meet following criteria, as defined by WHO: Criterion 1: an antimicrobial agent which is the sole, or one of limited available therapy, to treat serious human disease, and Criterion 2: antimicrobial agent is used to treat diseases caused by either: (i) organisms that may be transmitted to humans from non‐human sources, or (ii) human diseases causes by organisms that may acquire resistance genes from nonhuman sources.
For the general purpose of this Opinion, the CIAs include those in the AMEG's category 2 and category 3 (EMA, 2014). Category 2 includes fluoroquinolones, 3rd and 4th generation cephalosporins and colistin, and, while undergoing risk‐profiling, the extended‐spectrum penicillins and aminoglycosides. Category 3 includes classes of the WHO's CIAs which are not approved for use in veterinary medicine.
Cross‐resistance:
A single resistance mechanism confers resistance to an entire class of antimicrobials. An example is the aminoglycoside‐modifying enzymes which may confer resistance to several members of the aminoglycoside family. Cross resistance can occur across different classes of agents ‐ a result of either overlapping drug targets, as is the case with macrolides and lincosamides, or a drug efflux pump with a broad range of activity (i.e. capable of exporting different classes of drugs).
Dysbacteriosis:
A non‐specific enteritis following from a disturbance in the equilibrium of the gut microbiota, similar to small intestinal bacterial overgrowth in human medicine.
Evidence of similarity of AMR: genes/mobile genetic elements/resistant bacteria:
Defined as similar AMR gene(s) detected in bacterial isolates of animal and human origin.
Horizontal transmission of an AMR gene:
An AMR gene can be transferred between bacterial cells from the same or different species through different process which are:
Conjugation: transfer of plasmids harbouring AMR gene(s)
Transduction: transfer by phages harbouring AMR gene(s)
Transformation: integration of naked DNA containing AMR gene(s).
Intrinsic resistance:
Intrinsic resistance is the innate ability of a bacterial species to resist activity of a particular antimicrobial agent through its inherent structural or functional characteristics, which allow tolerance of a particular drug or antimicrobial class. This can also be called ‘insensitivity’ since it occurs in organisms that have never been susceptible to that particular drug. Such natural insensitivity can be due to: lack of bacterial target, lack of affinity of the drug for the bacterial target, inaccessibility of the drug into the bacterial cell, extrusion of the drug by chromosomally encoded active exporters or innate production of enzymes that inactivate the drug.
Measurement of antimicrobial susceptibility:
Antimicrobial susceptibility of a strain is determined after isolation, purification and proper bacterial species identification. Strain susceptibility can be determined in the laboratory by measuring the minimal inhibitory concentration (MIC) according standardised conditions (medium, incubation time, atmosphere, inoculum size). The MIC is the concentration at which the bacterial growth is visibly inhibited under laboratory conditions and expressed in μg/mL (or mg/L).
Metaphylaxis (based on CVMP definitions):
Group treatment of all clinically healthy (but presumably infected) animals kept in close contact with animals showing clinical signs of a contagious disease. Metaphylaxis is always combined with the treatment of the diseased individuals.
Minimum inhibitory concentration (MIC):
Lowest concentration of an antimicrobial that will inhibit in vitro the visible growth of a microorganism after overnight incubation. MICs are expressed in μg/mL (or mg/L).
Mobile genetic elements:
Segments of DNA that encode enzymes and other proteins that mediate the movement of DNA within genomes (intracellular mobility) or between bacterial cells (intercellular mobility).
Mobile genetic element‐mediated transfer of AMR:
An AMR gene that is moved in different DNA structures by means of mobile genetic elements (integron, transposon).
Prevention (based on CVMP definitions):
The administration of an antimicrobial to healthy animals to prevent infection if the risk for infection is very high and the consequences severe.
For practical reasons metaphylaxis can be distinguished from prevention in that in metaphylactic treatment a proportion of animals within the group have clinical expression of disease, whereas in preventive treatment no clinical disease is present at the start of treatment.
Spectrum of activity of an antimicrobial:
The list of bacterial species which can be targeted by an antimicrobial defines its spectrum of activity. Bacterial species with an intrinsic resistance are out of the antimicrobial treatment indications. Antimicrobials are defined as possessing a narrow or large spectrum of activity according the number and diversity of bacterial species included in their spectrum of activity.
Some bacterial species are susceptible to a limited number of antimicrobials due to their pattern of intrinsic resistance.
Transmission of AMR through zoonotic and commensal food‐borne bacteria:
Horizontal or vertical transmission of AMR through food‐borne zoonotic pathogens (e.g. Salmonella spp., Campylobacter spp., Listeria spp., E. coli (pathogenic or commensal) through commensal food‐borne bacteria (e.g. E. coli, Enterococcus spp., Staphyloccus aureus).
Vertical transmission of an AMR gene:
The transfer of an AMR gene through the parent to the daughter bacterium, which then may become disseminated through a bacterial population.
Abbreviations
European, national and international agencies, committees, networks and publications thereof
- AHAW
EFSA Panel on Animal Health and Welfare
- AHDB
Agriculture and Horticulture Development Board
- AMCRA
Center of Expertise on Antimicrobial Consumption and Resistance in Animals
- AMEG
Antimicrobial Advice ad hoc Expert Group
- ANSES
French Agency for Food, Environmental and Occupational Health and Safety
- ANSES‐ANMV
French Agency for Veterinary Medicinal Products
- APEC
Asia‐Pacific Economic Cooperation
- AWP
Antimicrobials Working Party (EMA)
- BelVetSAC
Belgian Veterinary Surveillance of Antimicrobial Consumption
- BIOHAZ
EFSA Panel on Biological Hazards
- BPC
British Poultry Council
- BSAC
British Society for Antimicrobial Chemotherapy
- CAC
Codex Alimentarius Commission
- CBL
Dutch Food Retail Association
- CDC
Centers for Disease Control and Prevention
- CEESA
Executive Animal Health Study Centre
- CHMP
Committee for Medicinal Products for Human Use
- CLSI
The Clinical and Laboratory Standards Institute
- Codex Alimentarius
International ‘Food Code’ established by FAO and WHO Organization to develop and harmonise international food standards
- CVMP
Committee for Medicinal Products for Veterinary Use
- DANMAP
Danish Programme for surveillance of antimicrobial consumption and resistance in bacteria from animals, food and humans
- DART
German Antibiotics Resistance Strategy
- DG SANTE
European Commission's Directorate General for Health and Food Safety
- DVFA
Danish Veterinary and Food Administration
- EAAD
European Antibiotic Awareness Day
- EARS‐Net
European Antimicrobial Resistance Surveillance Network
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EMA
European Medicines Agency
- EPRUMA
European Platform for the Responsible Use of Medicines in Animals
- ESAC‐Net
European Surveillance of Antimicrobial Consumption Network
- ESVAC
European Surveillance of Veterinary Antimicrobial Consumption
- ESVAC‐ES
ESVAC‐España (Spain)
- EU‐PLF
EU Precision Livestock Farming
- EURL
European Union Reference Laboratory
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- EUSR
European Union Summary Report
- FAO
Food and Agriculture Organization
- FDA
Food and Drug Administration
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FVE
Federation of Veterinarians of Europe
- FVO
former Food and Veterinary Office
- FWD‐Net
European Food‐ and Waterborne Diseases and Zoonoses Network
- GHSA
Global Health Security Agenda
- HAI‐Net
Healthcare‐associated Infections Surveillance Network
- HMA
Heads of Medicines Agencies
- IFOAM
International Federation of Organic Agriculture Movements
- IWP
Immunologicals Working party (EMA)
- JIACRA
Joint Interagency Antimicrobial Consumption and Resistance Analysis (EU)
- KNMvD
Royal Dutch Veterinary Association
- MARAN
Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands
- MINAPIG
Project on alternatives to antimicrobial use in pig production
- MS(s)
Member State(s)
- NARMS
National Antimicrobial Resistance Monitoring System for Enteric bacteria
- NEVEDI
Dutch Feed Industry Association
- NOAH
National Office of Aanimal Health
- OIE
World Organisation for Animal Health
- PMARS
Post‐Marketing Authorisation Resistance Surveillance
- PUAVM
(EC Guidelines for) prudent use of antimicrobials in veterinary medicine
- RDA
Raad voor Dierenaangelegenheden (Council for Animal Affairs, the Netherlands)
- RONAFA
EFSA‐EMA ad hoc Working Group (WG) on the Reduction Of the Need for Antimicrobials in Food‐producing Animals
- RSPCA
Royal Society for the Prevention of Cruelty to Animals
- RUMA
Responsible Use of Medicines in Agriculture Alliance
- SACAR
Specialist Advisory Committee on Antimicrobial Resistance
- SAGAM
Scientific Advisory Group on Antimicrobials (EMA) (now replaced by the AWP)
- SCENIHR
Scientific Committee on Emerging and Newly Identified Health Risks
- SDA
Netherlands Veterinary Medicines Authority
- SIMV
French association for animal health industry
- SVA
National Veterinary Institute
- SVARM
Swedish Veterinary Antimicrobial Resistance Monitoring
- SVS
Swedish Veterinary Association
- Swedres
Swedish Antibiotic Utilisation and Resistance in Human Medicine
- TATFAR
Transatlantic Taskforce on Antimicrobial Resistance
- TRACES
Trade Control and Expert System
- VASC
Veterinary Advisory Service Contracts
- VETCAST
Veterinary Committee on Antimicrobial Susceptibility Testing
- VETSTAT
Danish system for surveillance of the veterinary use of drugs for production animals
- VICH
International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary medicinal Products
- VMD
Veterinary Medicines Directorate
- WHO
World Health Organization
- WVAB
Workgroup Veterinary Antimicrobial Policy
Antimicrobial resistance/heavy metal genes, resistance symbols and associated terminology
- ARG(s)
antimicrobial resistance gene(s)
- Cipr
ciprofloxacin‐resistant
- czrC
zinc resistance gene
- ermB
erythromycin resistance gene
- ESBL(s)
extended‐spectrum beta(β)‐lactamase(s)
- flo
florphenicol resistance gene
- mcr‐1,‐2
transmissible gene(s) encoding resistance to colistin
- mecA
meticillin resistance gene
- MGE (s)
mobile genetic element(s)
- MRG(s)
metal resistance gene(s)
- str(A, B)
streptomycin resistance gene(s)
- sul
sulfonamide resistance gene(s)
- tet (A,B,C)
tetracycline resistance gene(s)
- vanA
vancomycin resistance gene
Animal diseases, syndromes and associated terminology
- ABM(s)
animal‐based Measure(s)
- ACTH
adrenocorticotropic hormone
- ADCT
antimicrobial dry cow therapy
- ADD
animal daily dose
- ASF
African swine fever
- BCO
bacterial chondronecrosis with osteomyelitis
- BLV
bovine leukaemia virus
- BRD
bovine respiratory disease
- BRSV
bovine respiratory syncytial virus
- BVD
bovine viral diarrhoea
- BVDV
bovine viral diarrhoea virus
- CBPP
contagious bovine pleuropneumonia
- CMT
California Mastitis Test
- CSF
classical swine fever
- DCDvet
defined course dose (animal)
- DDD
defined daily dose
- DDDA
defined daily dose animal
- DDDvet
Defined daily dose for animals
- DIVA
(Vaccine) Differentiating infected from vaccinated animals
- EBL
enzootic bovine leukosis
- FMD
foot‐and‐mouth disease
- GHGE
greenhouse gas emissions
- IBR
infectious bovine rhinotracheitis
- IMI
intramammary Infection
- LI
Lawsonia intracelluaris
- MS
Mycoplasma synoviae
- NPDS
Neonatal porcine diarrhoea syndrome
- PCV2
porcine circovirus type 2
- PED
porcine epidemic diarrhoea
- PEDV
porcine epidemic diarrhoea virus
- PMWS
post‐weaning multisytemic wasting syndrome
- PRDC
porcine respiratory disease complex
- PRRS
porcine reproductive and respiratory syndrome
- PRRSV
porcine reproductive and respiratory syndrome virus
- PWD
Post‐weaning diarrhoea
- QTL(s)
quantitative trait locus(i)
- RSS
runting and stunting syndrome
- SAV 3
salmonid alphavirus subtype 3
- TGEV
transmissible gastroenteritis virus
Miscellaneous abbreviations
- ADI
acceptable daily intake
- AGP(s)
antimicrobial growth promoter(s)
- ALEA
animal level of exposure to antimicrobials
- AMR
antimicrobial resistance
- AMU
antimicrobial use
- AST
antimicrobial susceptibility testing
- AUIC
area under the inhibitory curve
- BT
A group of small cationic peptides produced by Brevibacillus texasporus
- CFU
colony‐forming units
- CIA(s)
critically important antimicrobial(s)
- CRP
C‐reactive protein
- DT
definitive phage type
- ECOFF
epidemiological cut‐off (value)
- EO
essential oils
- ETEC
enterotoxigenic Escherichia coli
- FOS
fructooligosaccharides
- FPS
food‐producing animal species
- GL
guideline
- GMP
good manufacturing practice
- GOS
galactooligosaccharides
- GRE
glycopeptide‐resistant enterococci
- HIV
human immunodeficiency virus
- Ig
immunoglobulin
- IgG
immunoglobulin G
- IgY
chicken egg yolk immunoglobulin
- LA‐MRSA
livestock‐associated MRSA (see below)
- LPS
lipopolysaccharide
- MA
marketing authorisation
- MFS
medicated feedstuffs
- mAbs
monoclonal antibodies
- MALDI‐TOF
matrix‐assisted laser desorption ionization‐time of flight mass spectrometry
- MBC
minimum bactericidal concentration
- MDR
multidrug‐resistant
- MIC
minimal inhibitory concentration
- MLST
multilocus sequence typing
- MLVA
multiple locus variable number tandem repeats analysis
- MOS
mannan‐oligosaccharides
- MRL
maximum residue limit
- MRSA
meticillin‐resistant Staphylococcus aureus
- MUMS
minor use or minor species
- NDOs
non‐digestible oligosaccharides
- NGS
next‐generation sequencing
- NNT
number of animals needed to treat
- PCR
polymerase chain reaction
- PCT
procalcitonin
- PCU
population correction unit
- PD
pharmacodynamics
- PFGE
pulsed‐field gel electrophoresis
- PK
pharmacokinetics
- PK/PD
pharmacokinetic/pharmacodynamic concept
- OTC
oxytetracycline
- QA
quality assurance
- RMD
rapid molecular diagnostic (test)
- RNA
ribonucleic acid
- RTE
ready‐to‐eat
- SCC
somatic cell counts
- SDAP
spray‐dried animal plasma
- SDIPP
spray‐dried immune porcine plasma
- SPC
Summary of the product characteristics
- SPF
Specific pathogen free
- spp.
Species (plural)
- subsp.
Subspecies
- ToR(s)
Term(s) of Reference
- VMP(s)
Veterinary medicinal product(s)
- VRE
Vancomycin‐resistant enterococci
- VREF
Vancomycin‐resistant Enterococcus faecium
- WG
Working group
- WGS
Whole genome sequencing
- ZnO
Zinc oxide
Appendix A – Emerging issues
A.1. Transferable resistance to colistin
In November 2015, transferable resistance to the polymyxin antimicrobial colistin, mediated by the mcr‐1 gene, was reported in E. coli from pigs in China and some other countries in the Far East and Europe (Liu et al., 2015). Subsequently mcr‐1 has been identified in retrospective studies of colistin‐resistant E. coli and Salmonella from many countries worldwide (Skov and Monnet, 2016). More recently, a novel mcr gene, mcr‐2, has been identified in passive screening of porcine and bovine colistin‐resistant E. coli isolates made in Belgium between 2011 and 2012 that did not show presence of mcr‐1. Of particular note is that this mcr gene was associated with a highly transmissible IncX4 plasmids (Xavier et al., 2016). The implications for human health of the spread of mcr genes in livestock‐associated organisms are being assessed. ECDC published a rapid risk assessment of the implications for human medicine of plasmid‐mediated colistin resistance (ECDC, 2016).
The EU Antimicrobial Advice ad hoc Expert Group (AMEG) recently noted that here are wide variations in the use of colistin in EU MSs adjusted for the biomass under exposure (kg livestock, expressed as PCU) between countries (EMA, 2016b). Countries with intensive livestock production can have a level of use below 1 mg/PCU (e.g. Denmark and the UK) or much higher, up to 20–25 mg/PCU (Italy and Spain). The AMEG have therefore recommended that for the ‘high and moderate consumers’ the target and desirable levels are set at 5 mg/PCU and 1 or below 1 mg/PCU, respectively, based on the observations on the level of use in other countries. The AMEG further recommended that more information should be gathered to determine the minimum level of colistin use that can be achieved while maintaining animal welfare and preventing the increased use of other CIAs.
A.2. The emergence of multidrug‐resistant (MDR)/ciprofloxacin‐resistant (Cipr) strains of Salmonella Stanley, S. Infantis, S. Kentucky, S. Heidelberg (USA), S. Enteritidis (Far East), S. Typhimurium (Africa) and the more recent emergence in the Netherlands of ESC S. Heidelberg which can cause human infections in food‐producing animals and poultry meat
MDR Salmonella spp. are prevalent in pigs in most countries, and are likely to have resulted from the regular use of in‐feed antimicrobial treatments that are used to prevent disease under suboptimal husbandry conditions (Molla et al., 2006). The international dissemination of S. 4,[5],12:i:‐ monophasic S. Typhimurium in pig populations is likely be related to the selective advantage offered by MDR profiles associated with stable genetic elements, also carrying virulence features, within bacterial lineages that are well adapted to the porcine host and are prevalent in human infections as a result of contaminated pig meat (EFSA BIOHAZ Panel, 2011; Mourao et al., 2014). Acquired tolerance to heavy metals such as copper and zinc provide a selective advantage as both these elements are commonly used at high levels in pig feed (Mourao et al., 2015) and are associated with the occurrence of MRSA (Amachawadi et al., 2015).
The industrialisation of the livestock industry and lack of effective control programmes in many countries have contributed to the rapid spread of AMR Salmonella spp. and contamination of farms and slaughter plants within a single company can result in very large outbreaks, e.g. MDR S. Heidelberg in the USA (Grinnell et al., 2013; Rothrock et al., 2015).
Salmonella Infantis is one of the most commonly isolated serovars and MDR strains have recently been emerging worldwide. Comparative analyses between pre‐emergent and the clonal emergent S. Infantis populations demonstrated the fixation of adaptive mutations in the DNA gyrase (gyrA) and nitroreductase (nfsA) genes, conferring resistance to quinolones and nitrofurans, respectively, and the carriage of an emergent‐specific plasmid, designated pESI. This self‐transferred episome is a mosaic megaplasmid (∼ 280 kb), which increases bacterial tolerance to environmental mercury (mer operon) and oxidative stress, and provides further resistance to tetracyclines, sulfamethoxazole and trimethoprim, most likely due to the presence of tetRA, sulI and dfrA genes, respectively. Moreover, pESI codes for the yersiniabactin siderophore system and two novel chaperone‐usher fimbriae. In vitro studies established that pESI conjugation into a plasmidless S. Infantis strain results in superior biofilm formation, adhesion and invasion into avian and mammalian host cells. In vivo mouse infections demonstrated higher pathogenicity and increased intestinal inflammation caused by an S. Infantis strain harbouring pESI compared with the plasmidless parental strain (Aviv et al., 2014).
MDR Salmonella Kentucky strains have been isolated from turkeys in Poland and across Europe since 2009. Multiple mutations within chromosomal genes gyrA and parC are responsible for high‐level ciprofloxacin resistance. One of the isolates was extended‐spectrum β‐lactamase‐ (ESBL) positive: the strain 1643/2010 carried a conjugative 167,779 bp plasmid of IncA/C family. The sequence analysis revealed that it carried a bla CTX‐M‐25 gene and an integron with another β‐lactamase encoding gene – bla OXA‐21. This is the first known report of a CTX‐M‐25 encoding gene both in Poland and in S. Kentucky world‐wide, as well as in the IncA/C plasmid. Analysis of the integron showed a novel arrangement of gene cassettes – aacA4, aacC‐A1 and bla OXA‐21 where the latter might result from an intergeneric gene transfer. The study confirmed that the S. Kentucky population isolated in Poland belongs to global epidemics of high‐level fluoroquinolone‐resistant clone ST198 that can carry rare β‐lactamase genes (Wasyl et al., 2015).
The recent identification in the Netherlands of clonal clusters shared by extended‐spectrum‐resistant (ESC) Salmonella Heidelberg strains in food‐producing animals and poultry meat that can cause human infections (Liakopoulos et al., 2016) underscores the risk for potential zoonotic or food‐borne transmission of these strains to humans. Although no human infections linked to these contaminated products have been yet documented in the Netherlands, the risk of potential zoonotic or food‐borne transmission of ESC‐resistant S. Heidelberg strains further highlights the necessity for active surveillance and intervention strategies by public health organisations.
A.3. The ongoing spread of LA‐MRSA in certain high‐risk groups of workers in direct contact with live animals and of MRSA in pigs and other species
A new zoonotic reservoir in food production animals has been found involving a specific clone, MRSA ST398, which spreads extensively in animals, and is found in retail meat. It poses a potential threat to public health, as people in contact with food production animals are at higher risk of colonisation. The most probable transmission route seems to be by contact (Huang et al., 2014), and dust in intensive animal housing is often contaminated with MRSA ST398 (Cole et al., 2000; Broens et al., 2008).
Although the clonal relationship between MRSA strains of CC398 is clear in livestock and people, this is less obvious in horses. Small companion animals typically share MRSA strains that seem to originate from and exchange with a human reservoir (Catry et al., 2010), as well as harbouring other resistant species of staphylococcus (Cohn and Middleton, 2010). Most isolates from clinically infected animals carry numerous genetic elements related to AMR and virulence genes, and a phi3 prophage encoding immune‐modulating proteins that is associated with animal‐to‐human transmission. Recent findings suggest clonal expansion and dissemination of a new subpopulation of CC398 isolates, responsible for invasive infections in various animals, with a considerable potential to colonise and infect humans; probably greater than that of the original pig/human‐adapted CC398 isolates (Mee‐Marquet et al., 2014). Recently, specific clones of MRSA have been reported as circulating between pigs and dairy cattle in Italy, raising the fear of still wider dissemination of such organisms between species if action is not taken (Feltrin et al., 2016).
LA‐MRSA suddenly emerged in pigs in 2005 (Voss et al., 2005) along with MDR strains of Streptococcus suis and ESBL‐producing E. coli selected by the use of ceftiofur (especially routine use of long‐acting injections to prevent meningitis in weaned pigs) and cefquinome in pigs. To date, LA‐MRSA is only prevalent in certain high‐risk groups of workers in direct contact with live animals and the spatial distribution of MRSA genotypes suggests interspecies transmission and colonisation of different populations, communities, animals and their products, although a relevant association between contamination of food products and consumers has yet to be confirmed (Stefani et al., 2012; Grema et al., 2015). The carrier rate of MRSA is high in food handlers in restaurants in some countries and this can lead to contamination of food (Alsamarai et al., 2015). Nevertheless, the proportion of human MRSA infections that are due to LA‐MRSA strains remains low overall in the EU and in most EU MSs. A survey from ECDC showed that, in 2007, LA‐MRSA strains represented on average less than 2% of MRSA clinical isolates and represented more than 4% of MRSA clinical isolates in only three MSs (Belgium, Germany and the Netherlands) (van Cleef et al., 2011). ECDC is currently finalising a similar survey with updated data for 2013.
A.4. High to very high levels of resistance to fluoroquinolones and tetracyclines in isolates of Campylobacter spp. from humans and from broilers in several EU Member States in 2014
In 2014, five of 13 MSs reported ciprofloxacin resistance in > 80% Campylobacter spp. isolates from cases of human infection and one country, in 97.7% (ECDC and EFSA, 2016); in such settings, effective treatment options for human enteric campylobacter infection are significantly reduced. High levels of tetracycline resistance were also observed (46.4% for C. jejuni and 53.8% for C. coli). For isolates of C. jejuni from broilers in 2014, overall resistance was very high for ciprofloxacin (69.8%), nalidixic acid (65.1%) and tetracycline (54.4%).
A.5. Increasing levels of resistance to 3rd‐ and 4th‐generation cephalosporins through production of extended‐spectrum β‐ lactamase (ESBL) enzymes, in bacteria from community patients and livestock
Since 2000 a variety of plasmid‐mediated β‐lactamases, have emerged in Gram‐negative bacteria, resulting in reduced susceptibility to broad‐spectrum β‐lactams. These β‐lactamases included both ESBLs and AmpC β‐lactamases (AmpC). The multiresistant nature of bacteria that produce ESBLs and AmpCs can affect the selection and timely administration of appropriate antimicrobials for community‐acquired and healthcare‐associated infections, since many first‐line antimicrobials are no longer active against them. Although person‐to‐person spread is recognised as the main method of spread of ESBL/AmpC‐β‐lactamase‐containing E. coli both in hospitals and the community, the primary reservoirs of such organisms are contentious. ESBL‐producing E. coli have been isolated from food‐producing animals in many European countries, particularly poultry and cattle, and farm animals are now recognised as important carriers of ESBL/AmpC‐producing E. coli and Salmonella spp. Similarly there have been an increasing number of reports of ESBL‐producing E. coli being isolated from foods of animal origin.
Appendix B – Circumstances and diseases of food animal production where antimicrobials are most intensively used according to a National report from France
Data recorded in France (ANSES, 2015a) allowed the specification of the antimicrobials that are mostly used in food animal production, divided by species and, within the same species, by production life stages. The bacteria responsible for each disease are also reported as well as the antimicrobials most commonly used to treat the specific disease.
B.1. Poultry
In France the pathogenic agents that particularly contribute to antimicrobial use in poultry are E. coli and Mycoplasma spp. for systemic and respiratory diseases and Clostridium perfringens for digestive diseases:
Certain strains of E. coli can be responsible of septicaemia, omphalitis/yolk sac infection in young chicks and serositis and arthritis in adult chickens. The serotypes that are mainly involved are O78:K80, O2:K1 and O1:K1. Antibiogram results and epidemiology of the disease within a flock will determine the antimicrobials used. The main antimicrobials used are amoxicillin, colistin, fluoroquinolones and trimethoprim‐sulfonamide, but uses may differ between countries.
Mycoplasma spp. are responsible for respiratory, articular or genital diseases. M. gallisepticum has been virtually eradicated from France and M. synoviae is common in Gallus gallus. It is mainly asymptomatic except in laying hens where it is responsible for infectious synovitis. In turkeys, it produces articular lesions, leading to cachexia and mortality. Antimicrobial treatment is largely based on tetracyclines and macrolides.
Clostridium perfringens is associated with other precipitating factors (high growth rate, variation in feed ingestion, quality or formulation, viral or coccidial presence) and can lead to a digestive disease due to toxin production. The main antimicrobials used are β‐lactams (1st‐generation cephalosporins) and macrolides.
Furthermore, some infections are specific to certain poultry production sectors:
Gallus gallus: in laying hens, Enterococcus faecalis is the main pathogen implicated in amyloid arthritis which is treated by β‐lactams or lincospectin. Enterococcus faecium is increasingly implicated in articular diseases associated with Staphylococcus spp. and E. coli in broiler chickens. Antimicrobials used are mainly β‐lactams, e.g. amoxicillin.
Turkeys: Ornithobacterium rhinotracheale is responsible for acute or chronic respiratory diseases leading to culling of animals. Treatment is usually with macrolides and tetracyclines. Some flagellate parasites, such as Histomonas, can cause digestive disease. In the absence of authorised efficient antiparasitic drugs, antimicrobials are used to try to reduce the impact of disease caused by such parasite, with a low level of success (reduced mortality).
Ducks: Pasteurella multocida and Riemerella induce acute respiratory diseases with high mortality. Treatment involves use of β‐lactams, quinolones, tetracyclines and macrolides. Medical prevention involves vaccination for Pasteurella and use of autogenous vaccine for Riemerella.
B.2. Pigs
As an example, according to (ANSES, 2015a), antimicrobial consumption in pigs can be differentiated regarding the stage of production:
-
Sows: sows usually suffer from three main types of infections:
-
–
Urogenital infection: e.g. leptospirosis, genital infection (caused, e.g. by E. coli), swine Erysipelas, etc.
-
–
Respiratory diseases: quite rare, mostly in association with viral infections, main pathogens are: Mycoplasma ssp., Actinobacillus/Haemophilus and Pasteurella spp., treated, respectively, by macrolides, amoxicillin or cotrimoxazole and tetracyclines or cotrimoxazole.
-
–
Locomotory infection: arthritis could occur mostly due to Mycoplasma hyosynoviae and treated by macrolides.
-
–
-
Suckling piglets: infections can alter different functions in piglets but the main impact is on the digestive function
-
–
Enteritis is the main manifestation of the digestive system, and the primary agents are enterotoxic collibacillae or others bacteria such as Clostridium perfringens type A toxin, Clostridium difficile and Enterococcus spp., all of which need different antimicrobial treatments.
-
–
Locomotory infection: arthritis could occur mostly due to Streptococcus spp. or Staphylococcus spp. that penetrate the body after injection, mutilation or wounds following biting or housing defects.
-
–
-
After weaning:
-
–
Digestive function: digestive infections are mostly due to colibacillae and two clinical forms are predominant: an enteric form and oedema disease, both of which can be found in the same farm simultaneously. Dietary and sanitary measures are the only measures to control diseases, but frequently antimicrobials are used (mostly colistin or aminoglycosides), while in some countries zinc oxide is used to control the diseases. Some complex outbreaks can necessitate the use of other AM after antibiogram establishment.
-
–
Systemic infections: at weaning Streptococcus suis is responsible for streptococcal diseases such as arthritis. After weaning, two mains forms are present: a septicaemic and a neurological form that can lead to death a few weeks after weaning if no serious curative measures are undertaken.
-
–
-
Fattening pigs:
-
–
Digestive tract: digestive infections frequently occur during fattening but E. coli is less frequently the causative agent compared to during maternity and post weaning. Instead, Lawsonia intracellularis and Brachyspira ssp. are mainly implied (both being sensitive to macrolides).
-
–
Respiratory tract: respiratory infections are very frequent at the fattening stage, mostly implicating housing conditions (e.g. ventilation) which may impact disease evolution in the herd. The main respiratory diseases are (i) athrophic rhinitis (Bordetellabronchiseptica ± Pasteurella multocida); (ii) pneumonia (with heavy consequences on animal health) mainly due to Mycoplasma hyopneumoniae (treated by macrolides or tetracyclines) and eventually complicated by viral infection (e.g. grippal or corona viruses); (iii) actinobacillosis (A. pleuropneumoniae), as being one of the most acute respiratory infections (housing conditions, e.g. ventilation may play an important role in the clinical expression, and antimicrobials often have to be used).
-
–
Locomotor system: arthritis can appear due to Mycoplasma hyorhinis, M. hyosynoviae, Haemophilus parasuis or swine erysipelas. Antimicrobials can be used but the articular localisation of the bacteria makes the healing difficult.
-
–
B.3. Ruminants
According to (ANSES, 2015a), antimicrobial use in ruminants can be differentiated according to the different species and different production stages.
Use for bovines is summarised as:
-
Adults:
-
–
Udder diseases are mainly due to Streptococcus spp., Staphylococcus and various other Gram‐negative organisms (E. coli, Klebsiella spp.,…). A high proportion of dairy cows are treated with antimicrobials within each production year.
-
–
Genital infections are quite heterogenous due to retained placentas, metritis and abortion (often due to Brucella, Salmonella spp., Listeria spp. or Coxiella burnetii). For this latter infection, antibioprevention with tetracyclines at the dry period have been used (now partly replaced by vaccination). Antimicrobial treatment can be local or systemic.
-
–
Locomotory infection is the third cause of veterinary intervention in herds and an important cause of antimicrobial use. The infections are mostly foot infections with Dichelobacter nodosus or Fusobacterium necrophorum.
-
–
-
Young bovines
-
–
Digestive tract: neonatal gastroenteritis is the main reason for antimicrobial use. The causative agents include bacteria (mostly pathogenic E. coli, but also Salmonella spp. or Clostridiae), virus, (rota/corona virus), cryptosporidiae and coccidiae.
-
–
The respiratory infections are heterogenous in clinical expression and aetiology. The main manifestation is infectious enzootic bronchopneumonia, where the aetiology is multifactorial (air quality, viruses, fungi and bacteria (Pasteurella spp. and Mycoplasma spp.). Biosecurity, housing conditions and finally antibioprevention are useful to control the disease.
-
–
In France, (ANSES, 2015a), reports suggest that sheep and goats present with the same aetiologic agents as cattle, specifically:
Infections with Mycoplasma spp. are frequent in sheeps leading to respiratory, mammary and sometimes articular (polyarthritis) infections. Alone or associated with Pasteurella spp., Mycoplasma can be linked with severe contagious infection (with up to 50% mortality) that requires symptomatic or preventive antimicrobial treatment (tylosin, marbofloxacin, danofloxacin, tetracyclines, etc.)
The frequency of Chlamydia infection in the abortion process often leads to the use of antibioprevention (tetracyclines) at the end of gestation in sheep
Locomotory infection requiring antimicrobials is quite low compared to bovines
Limited knowledge on the aetiology of neonatal diarrhoea leads to systematic oral antibioprevention including fluoroquinolones and 3rd‐ and 4th‐generation cephalosporins
B.4. Horses
According to (ANSES, 2015a), the main diseases responsible of antimicrobial use include:
Respiratory diseases such as strangles (Streptococcus equi subsp. equi) and pneumonia are often occurring and lead to antimicrobial use. Viral contagious infections such as flu, laryngitis of rhinopneumonia are treated by some veterinarians by preventive, metaphylactic or curative use of penicillin or trimethoprim + sulfonamide to prevent the spread of the diseases with a stable. Rhodococcosis is an acute respiratory disease of foals that necessitate the use of different antimicrobials such as rifampicin.
Systemic infections and septicaemia are the second main reason for antimicrobial prescription
Arthritis (that needs intra‐articular injection of antimicrobials), gynaecological and dermatological problems, and enteric infections in foals.
B.5. Rabbits
The main circumstances that lead to antimicrobial use in EU MSs with intensive rabbit production (e.g. France, Belgium, Italy, Germany, Hungary) are respiratory diseases (breeding females) and digestive problems (fattening rabbits), with four main bacteria involved (ANSES, 2015a):
Pasteurella multocida is involved in respiratory lesions and troubles and abces affecting breeder and fattening rabbits. It has an impact on growing rate, mortality, reproduction and condemnation rate.
Staphylococcus aureus, like pasteurellosis, is implied in respiratory lesions and abces (foot pad) with the same consequences.
Escherichia coli (particularly O103) and other enterobacteria are responsible for digestive problems in all stages of production, even though most occur in young animals in the week after birth or after weaning. Infections with Klebsiella spp. could occur around 3 weeks of age and salmonellosis could appear, more occasionally, but with acute consequences.
Clostridium perfringens is often associated to enterocolitis (even if aetiology has never been proved) in rearing rabbits (mostly at the end of fattening). It is actually the main cause, in France, of antimicrobial prescription in fattening rabbits.
Appendix C – Information in relation to use of antimicrobials in aquaculture and strategy to reduce the use of antimicrobials in this sector in Norway
Below the information requested to and information provided by Dr. Atle Lillehaug (hearing expert of the WG RONAFA) is reported.
C.1. Question 1
Could you please provide a presentation to the WG on the experience of the use of antimicrobials in aquaculture in Norway, on the strategies used in Norway to reduce the use of and need to use antimicrobials, and on the results obtained? Could you also please provide a short summary of this in writing?
Answer to Question 1
The Norwegian marine farming of salmonids started with pilots during the 1960s, and commercial industry developed during the 1970s and the following decades. Atlantic salmon is the main species, with a production of approximately 1.2 mio tons per year and approximately 70,000 tons of rainbow trout the last four years, including 2015. Figure D.1 outlines the typical salmon production cycle in Norway.
Figure D.1.
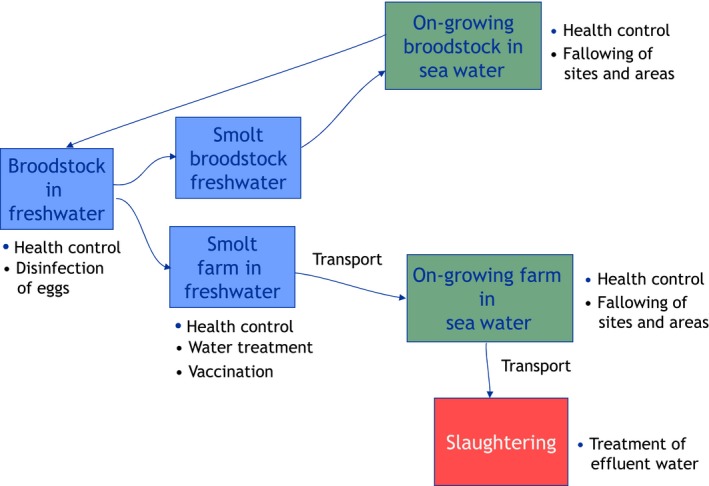
Scheme representing the salmon production cycle in Norway
From the mid‐1970s, the use of antimicrobials also increased, parallel to fish production, to a maximum of 49 tons in 1987. Vibriosis caused by Vibrio anguillarum was one cause of disease outbreaks, mainly in rainbow trout, and vaccines were introduced from 1977, and used to some extent, mainly by immersion (dipping). During the 1980s, cold‐water vibriosis (Vibrio salmonicida) developed to be a great problem for Atlantic salmon, causing significant mortalities and the all time high use of antibacterials in 1987. From the same year, experimental vaccines were tested (dipping method) in field trials, and implemented from the following year by the entire industry. Vaccination proved effective, and antibacterial went down the next 2 years.
When smolt infected with Aeromonas salmonicida subsp. salmonicida causing furunculosis were imported, the disease spread quickly, and in 1990 antimicrobial use again increased. Testing of vaccines demonstrated low protection of dip vaccines, and moderate effect of injection vaccines adjuvanted with aluminium salts. The latter were tested in field trials, and taken into use in commercial farms. Vaccines with oil‐adjuvants were developed during this period, giving a high level of protection, and from early 1990s, polyvalent vaccines against furunculosis, vibriosis and cold‐water vibriosis were introduced. Since then, close to all salmonids have been injection‐vaccinated with these products before transferred to seawater. The three diseases have almost been eliminated, except for a few cases of cold‐water vibriosis, probably caused by vaccination failure.
Later in the 1990s, the polyvalent vaccines have also been added antigens from Moritella viscosa, causing ‘winter ulcers’. The disease problems have been reduced, but not eliminated; low water temperatures and handling of fish probably contributing to disease outbreaks. Vaccine products for salmonids may also include Infectious pancreatic necrosis virus or Salmon pancreas disease virus.
Autogenous vaccines against yersiniosis caused by Yersinia ruckeri is used in farms having this specific problem.
Since 1996, the total volume of antibacterials used for aquaculture production in Norway has varied between approximately 500–1,500 kg per year, in 2015 the consumption was 301 kg, the lowest volume since 1975 (see Figure D.2). Temporary slight increases in the amount of antimicrobials used, e.g. in years 2006, 2009 and 2012 were due to specific outbreaks of fish diseases (e.g. cold‐water vibriosis in 2011–2012) and not to preventive use of antimicrobials, since the latter is not acceptable in Norway. During the same period, fish production has increased from 315,000 to 1.3 mio tons.
Figure D.2.
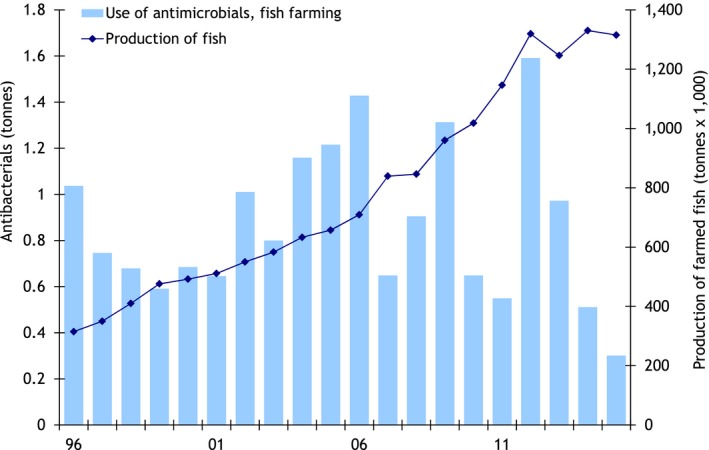
Fish production and use of antimicrobials in fish farming in Norway (1996–2015)
Other marine fish species have been produced (cod, halibut, others), and during the last years, ‘cleaner fish’ for salmon lice are produced in culture, causing some treatments. Vaccines are used to some extent in these fish species against vibriosis and atypical A. salmonicida. Antimicrobials have also been used in the production of ‘live start‐feed’, zooplankton/crustaceans, for marine fish larvae.
Today, specific bacterial infections during the marine production phase are under control by vaccination. More unspecific infections, caused by environmental factors, do not cause significant problems. Sea currents and relatively low water temperatures provide good environmental conditions for the grow‐out phase of salmonids, and law regulates maximum densities of biomass. Ulcer problems during winter constitute an exemption, avoiding handling of fish during periods they are vulnerable being important to avoid problems.
‘All‐in‐all‐out’ at farm and coordination‐area is an important strategy to avoid building up infection pressure; i.e. one generation of fish at one site (or neighbouring sites), and fallowing, cleaning and disinfection before the next generation. Other measures aimed at improving operating management have been consistently applied in Norway since the 1990s, allowing the reduction of spread of disease and use of antimicrobials in aquaculture, such as restrictions on the transport of live fish, improved biosecurity with all‐in‐all‐out systems, regulation of fish stocking density, optimisation of fish farm site placing with regards to water quality and water flow).
Even if 85% of the prescriptions for antibacterials are for the fresh‐water production period of fingerlings and smolt in tank systems, the volume of antimicrobials used in marine farming is higher than in freshwater farming, counting for around 75% of the weight of antimicrobials used. During the fresh‐water period, water flow‐through may be limited, due to the need to heat the water. Antibacterials used include mainly amphenicols and quinolones (90–100%) and tetracyclines. Disinfection of water intake, biological filters in water recirculation systems, and reduction of fish densities may reduce environmental induced infections.
Production of fry for marine fish (including cleaner fish) is also in land‐based tank systems, and problems with unspecific infections are similar to freshwater tank systems.
C.2. Question 2
Do you know of other examples of measures taken in other countries (for example for different species, under different climatic conditions and husbandry systems) to reduce the use of and the need to use antimicrobials in aquaculture?
Answer to Question 2
In the other European countries with marine production of salmonids of some extent, i.e. Scotland, Faroe Islands, Ireland, vaccination is used in a way similar to Norway. For example, the production in the Faroes was reported to be 88 000 tons in 2015, and antimicrobials were not reported to be used. I have not been able to trace statistics from the other two countries.
C.3. Question 3
Can you think at other possible strategies, including vaccination strategies, which would allow reducing the need to use antimicrobials in aquaculture?
Answer to Question 3
Vaccination is probably the best control measure against specific bacterial infections. In Norway, 100% of farmed salmonids are vaccinated, following to mandatory measures established by legislation. Vaccines used are all inactivated, injectable vaccines, which may be administered effectively and rapidly through automated systems.
When it comes to more unspecific infections relating to environmental conditions like unfavourable water quality, low water flow‐through and high fish densities, water treatment and fish densities should be addressed. Improved husbandry measures such as all‐in‐all‐out systems with fallowing and cleaning/disinfection should be used in all animal production systems.
The impact of the implementation of different measures and subsequent reduction of the use of antimicrobials in aquaculture might be also influenced by the local aquatic conditions, e.g. water temperature, in both fresh‐water and sea‐water farming.
Appendix D – Data collected through a questionnaire on the use of antimicrobials in food‐producing animals and measures to reduce the use
Below the questionnaire sent to FVE. The report produced by FVE to respond to the questionnaire is available in Annex A.
D.1. Species: Cattle
Please advise which ‘production systems/life stages’−‘syndrome/disease’ combinations use the greatest amount of antimicrobials in the target species.
For which of these combinations is it considered most difficult to implement measures aimed at reducing the need for antimicrobials?
For which of these combinations is it considered most easy to implement measures aimed at reducing the need for antimicrobials?
In relation to the above, do you have specific examples of where vaccination can be used to directly or indirectly reduce the use of antimicrobials?
In relation to the above, do you have other examples of where vaccines would be needed and could be used to directly or indirectly reduce the use of antimicrobials?
Stakeholders were previously requested by the AMEG to provide examples of the impact of risk management measures in regards to antimicrobial use on animal health, welfare and husbandry. Are you aware of any further specific examples where measures have successfully reduced the use of antimicrobials in cattle and, if available, the impact on the occurrence of resistance to such antimicrobials?
D.2. Species: Pigs
Please advise which ‘production systems/life stages’−‘syndrome/disease’ combinations use the greatest amount of antimicrobials in the target species.
For which of these combinations is it considered most difficult to implement measures aimed at reducing the need for antimicrobials?
For which of these combinations is it considered most easy to implement measures aimed at reducing the need for antimicrobials?
In relation to the above, do you have specific examples of where vaccination can be used to directly or indirectly reduce the use of antimicrobials?
In relation to the above, do you have other examples of where vaccines would be needed and could be used to directly or indirectly reduce the use of antimicrobials?
Stakeholders were previously requested by the AMEG to provide examples of the impact of risk management measures in regards to antimicrobial use on animal health, welfare and husbandry. Are you aware of any further specific examples where measures have successfully reduced the use of antimicrobials in pigs and, if available, the impact on the occurrence of resistance to such antimicrobials?
D.3. Species: Poultry
Please advise which ‘production systems/life stages’−‘syndrome/disease’ combinations use the greatest amount of antimicrobials in the target species.
For which of these combinations is it considered most difficult to implement measures aimed at reducing the need for antimicrobials?
For which of these combinations is it considered most easy to implement measures aimed at reducing the need for antimicrobials?
In relation to the above, do you have specific examples of where vaccination can be used to directly or indirectly reduce the use of antimicrobials?
In relation to the above, do you have other examples of where vaccines would be needed and could be used to directly or indirectly reduce the use of antimicrobials?
Stakeholders were previously requested by the AMEG to provide examples of the impact of risk management measures in regards to antimicrobial use on animal health, welfare and husbandry. Are you aware of any further specific examples where measures have successfully reduced the use of antimicrobials in poultry and, if available, the impact on the occurrence of resistance to such antimicrobials?
D.4. Species: other species
Please advise which ‘production systems/life stages’−‘syndrome/disease’ combinations use the greatest amount of antimicrobials in the following different categories of food‐producing animals/sectors: aquaculture, equids, rabbits, farmed game and bees.
For which of these combinations is it considered most difficult to implement measures aimed at reducing the need for antimicrobials?
For which of these combinations is it considered most easy to implement measures aimed at reducing the need for antimicrobials?
In relation to the above, do you have specific examples of where vaccination can be used to directly or indirectly reduce the use of antimicrobials?
In relation to the above, do you have other examples of where vaccines would be needed and could be used to directly or indirectly reduce the use of antimicrobials?
Stakeholders were previously requested by the AMEG to provide examples of the impact of risk management measures in regards to antimicrobial use on animal health, welfare and husbandry. Are you aware of any further specific examples where measures have successfully reduced the use of antimicrobials in the above animal species and, if available, the impact on the occurrence of resistance to such antimicrobials?
Appendix E – Data collected through a questionnaire on agreements between producers and retailers
Below the questionnaire sent to and information provided by selected organisations1 representing producers and retailers at the EU level, or other sectors tightly linked with them is reported.
E.1. Questions
Question 1
Please provide information on any guidance by retailers to producers, specific agreements or schemes in place between retailers and producers, and related controls, which are in place at the national or international level within the EU with regards to antimicrobials use in food‐producing animals.
Question 2
With regard to the above, what is your view on the impact that such guidelines/agreements have or may have in achieving a reduction of the use of antimicrobials in food‐producing animals?
E.2. Answers to Questions by FoodDrinkEurope
Answer to Question 1
Milk
Many schemes between dairy industry and farmers are in place, and checks are done at the entry of the dairy plant, so no need for a push at retail level.
Any positive testing that is done after collection of milk is discarded from any dairy manufacturing and anyway cultures used in dairy processing would not function if antibiotic residues were there.
Anyway antibiotics are only in use for medical treatment and the aim of the dairy sector is to further work to keep animals healthy as much as possible.
A position paper on animal welfare from the concerned sector might be interesting, please click:
Meat
Please see the method of food safety management concerning antibiotics for food‐producing animals in Germany:
https://www.q-s.de/veterinarians/antibiotics-monitoring-veterinarians.html
Meat
Prophylactic use of antibiotics is strongly discouraged by means of scientific argumentation on (long‐term) consequences of imprudent antibiotics use. This information is provided by Centers of Expertise and governmental institutions to veterinarians and farmers. Guidelines on the preferred antibiotic per type of therapeutic treatment are provided to veterinarians, focus is given primarily on social responsibility of the antibiotic of choice and secondly on efficiency of the treatment. From a legal point of view, all antibiotics use should be administered upon guidance of a veterinarian. Documents on antibiotics use have to be kept and are evaluated by government inspectors; sampling for control by analysis mainly occurs in slaughterhouses or on milk/eggs. A minority of retailers request certain management conditions in addition to existing national legislation. To date, these do not or hardly include prerequisites on the prudent use of antibiotics, but rather focus on, e.g. welfare issues. Still, such prerequisites (e.g. on animal density, chemical instead of surgical castration of piglets) may already indirectly contribute to a reduction in the need for antibiotics and thus actual antibiotics use. Compliance is usually evaluated by externally hired veterinarians.
Answer to Question 2
Meat
Unintentional ingestion of traces of antibiotics we do not consider a major source of emergence or spread of antimicrobial resistance.
Current legislatively imposed measures succeeded mainly in the prevention of antimicrobial residues in the edible parts or products of animal origin.
Generally speaking retailer‐ producer agreements, in contrast to legislative enforcements, may be more efficient.
E.3. Answers to Questions by Copa‐Cogeca
Answer to Questions 1 and 2
There are several private schemes in place in different Member States. Some examples of these schemes are the Certus quality system in Belgium and Cerdo Natur in Spain for pigs and pork meat or the Axfoundation in Sweden. In the case of the UK, it is mainly the industry who imposes such schemes.
However, the existing private schemes differ from one country to another and also within the same country, from one retailer to others.
At international level, Copa and Cogeca are aware of specific trade barriers established by third countries in the use of certain antibiotics (Russian microbiological and residue requirements provided in the Customs Union Decision). These specific trade requirements have led to longer withdrawal periods, rather than reductions on antimicrobial use.
EU farmers and agri‐cooperatives contribute to provide sufficient, safe, healthy and high‐quality food for a growing population. In addition to all these efforts, they are actively involved in tackling AMR. The main results achieved in this field have come from the engagement of the different actors in the food chain (i.e. EPRUMA at the EU level but also others at the national level).
Policies on tackling AMR should focus on promoting Responsible Use through platforms such as EPRUMA and monitoring of use of antibiotics in veterinary medicine through programmes such as European Surveillance of Veterinary Antimicrobial Consumption (ESVAC), as well as monitoring the development of resistance.
Copa and Cogeca would also like to highlight that the principles of the General Food Law mainly address food safety and issues linked to protecting consumers against misleading practices. Therefore, it is essential to draw a distinction between these requirements – a prerequisite for any agricultural and food product to be placed on the market – and those requirements related to the specific attributes of products or specific production process, which enhance the quality of products.
For instance, according to article 22 from Regulation 470/20092, on Circulation of foodstuffs, Member States may not prohibit or impede the import or the placing on the market of food of animal origin on grounds related to maximum residue limits or reference points for action where this Regulation and its implementing measures have been complied with.
Beyond the fact that there are very high legal requirements which all European products must meet, European quality schemes should be first and foremost a way for farmers and cooperatives to inform consumers about the specific characteristics of their products, if they fulfil requirements going beyond the ‘compulsory regulatory framework’. In conclusion, the main objective of these initiatives should be to reduce resistance to antibiotics, not simply to reduce antibiotic use. Care should be taken to ensure that any restrictions on the use of antibiotics in veterinary medicine do not adversely impact animal health and welfare.
E.4. Answers to Questions by EuroCommerce
Answer to Question 1
Some examples of quality schemes and partnerships in the supply chain are:
United Kingdom
Responsible Use of Medicines in Agriculture Alliance (RUMA) which is a group of the UK made up of farming organisations, retailers, vets and NGOs such as the RSPCA. They produce guidance for the responsible use of antimicrobials in various species. http://www.ruma.org.uk/antimicrobials.htm
Retailers specify Assured Food Standards (red tractor) as the basic standard for farm produce. AFS are also members of RUMA and cross index to their guidance in their specifications to farmers wanting to be certified under the scheme.
The Netherlands
-
CBL, the Dutch Food Retail Association (CBL) representing the food retailers and foodservice companies in The Netherlands, has formulated sustainability criteria for the generic pork and poultry meat assortment for the Dutch market. The ‘Sustainable Meat Initiative’ scheme is built on three pillars, which are all based on private standards and in particular GLOBALG.A.P. on the farm level:
-
–
Animal Health/Human Health: No human antibiotics and controlled reduction of antibiotics
-
–
Environment
-
–
Animal Welfare
-
–
Germany
Most retailers do not have individual requirements regarding antibiotics vis‐à‐vis their suppliers but apply the measures of QS functioning as a ‘bundler’ pooling all requirements as a service provider for participating retailers.
More information under: https://www.q-s.de/qs-scheme/monitoring-programms-meat-livestock-feed.html
Sweden
-
The member companies, of the Swedish Food Retailers Federation, are in the process of adopting the following statement, applying to purchasing requirements for the own brand products:
-
–
Antibiotics should not be used as a growth promotor
-
–
Antibiotics is only allowed after prescription from a veterinary
-
–
Ample documentation of all use of antibiotics, including via feed and water, should be done. Veterinarian should frequently review and sign this documentation.
-
–
If antibiotics to all animals of a certain age are used regularly, the motives for this should be documented, an investigation should be performed by a veterinarian and a programme for measures that deal with the health problems should be developed and implemented.
-
–
Answer to Question 2
EuroCommerce does not have any data or information on the impact of the existing guidelines and agreements. The quality schemes mentioned above could be better placed to provide a general view and input on this.
Specific agreements between food business operators in the supply chain are individual business decisions and include commercially sensitive information.
In addition, considering that at the level of the member states there are different actions ongoing in regards antimicrobial resistance and it will be challenging to assign impact to the guidelines/agreements vs those resulting from these various national/regional initiatives.
While we are in favour of guidelines these should aim at facilitating responsible use of antibiotics and remain voluntary. Stakeholders should be fully involved in the development of any such guidelines.
A higher impact is more likely if achieved through collaborative action involving the different sectors in the supply chain.
E.5. Answers to Questions by a.v.e.c.
Answer to Question 1
In general we are not aware of specific guidance of retailers for the producers of poultry meat, but we expect that there will be confidential agreements in place between retailers and their (preferred) suppliers.
The general rule is of course that producers need to produce in compliance with the law which means that the following conditions are standard between retailers and their suppliers of poultry meat.
Use of antibiotics: we are not aware of specific instructions from retailers for producers on the use of antibiotics. a.v.e.c. and EPB have a joint position on the use of antibiotics and antimicrobial resistance and it is clearly laid down that antibiotics should only be used upon veterinary diagnosis, testing by antibiogram and prescription and that the withdrawal period should be respected. In specific cases, the withdrawal period of antibiotics is prolonged to comply with the demand of the customer, but there is no indication that this will lead to a relevant reduction of antibiotic use.
Absence of residues: retailers accept the European MRL's and will reject consignments that are non‐compliant with the legal maximum residue levels. We are not aware of specific requirements from retailers that go beyond the legislation.
General conclusion, retailers are following the legal requirements on application to the animals by veterinary prescription, withdrawal periods and MRL's and do not impose or demand in general conditions going beyond the law.
In addition, I draw your attention to the national private schemes that are applicable in different Member States. A number of these schemes have aligned with an agreement on equivalence and/or compatibility. Representatives and experts of different stages in the poultry meat supply chain that may include also the retailers are defining together the standards and conditions in these schemes.
With the increased awareness about the impact of antibiotic use the private scheme owners have inserted in the schemes standards for recording antibiotic usage. Indeed, it is a regulatory requirement to record all medicine use and animal keepers must provide these data with the Food Chain Information document to the slaughterhouse.
At last, general guidelines for responsible use of antibiotics exists at the national and European level and retailers can access these so called (EP)RUMA guidelines. Many companies in the poultry industry have signed up to the antibiotic stewardship schemes.
Answer to Question 2
The awareness of the impact of antimicrobial resistance is high and the active monitoring and recording introduced in the different Member States have the effect that the use of antibiotics has gone down. We see that the national action plans introduced in different Member States on the initiative of the private sector and or the official bodies have caused a drop of antibiotic usage.
However, without having a comprehensive insight of the guidelines of retailers to producers we think that the impact of such guidelines should not be overestimated.
E.6. Answers to Questions by UECBV
Answer to Question 1
Denmark
Voluntary restrictions on the use of fluoroquinolones and third and fourth generation cephalosporins have been implemented in primary production in several countries. Retailers have been informed about measures to reduce and control antibiotic usage in primary production, but so far we have no knowledge of specific agreements between retailers and producers.
In the organic production, there are limitations on the use of antibiotics. Individual animals can lose the status as organic if it is treated several times with antibiotics during the lifetime.
In Denmark there is a small specialised production of pigs, produced without the use of antibiotics. It is not an antibiotic‐free production, since sick pigs will be treated. But the treated pigs will be marked, and cannot be sold for this product.
France
Antimicrobial resistance is a significant problem. Actions at the European level were taken, but also national plans have been put in place. From 1st April 2016, the preventive use in veterinary medicine of critical antibiotics (major antibiotics for human therapy) will be no longer allowed. Several Member States have launched national plans for reducing the risks of antibiotic resistance in veterinary medicine whose objective is: rational, careful and responsible use of veterinary antibiotics (like the ECOANTIBIO plan launched in 2012 by France). A European study has just shown that France, after the Netherlands, is the European country that has most reduced the use of veterinary antibiotics over the last 3 years.
Spain
Since 2014, there's Strategic Action Plan to reduce the risk of selection and dissemination of antibiotic resistance in Spain. The UECBV Spanish Members, ANPROGAPOR and ASOPROVAC are heavily involved among many other experts.
Six common strategic lines that correspond to the priority areas identified in the terms of reference are proposed for human and veterinary health:
Surveillance of antibiotic consumption and antimicrobial resistance.
Control of bacterial resistance.
Identification and spearheading of alternative and/or complementary measures of prevention and treatment.
Defining research priorities.
Training and information for healthcare professionals.
Communication and raising awareness in the population as a whole and in population subgroups.
There are many research projects ongoing in public or private sector. For instance ASOPROVAC is trying to lead an operational group to design and disseminate tools for the promotion of good practice in the use of antibiotics, limiting prophylactic use of antibiotics to cases with well‐defined clinical needs, etc.
Finally, we would like also to highlight that we will probably have in a few months a common system of electronic prescription on veterinary medicine that will allow greater control of their use, which itself presents problems of control and facilitates the appearance of AMR.
Answer to Question 2
Denmark
Market driven incentives to reduce antibiotic use can have an effect. But it is important to avoid creating incentives, where sick animals are not treated, with damaging effects for animal health. Antimicrobials are important drugs that can save animals and alleviate pain and suffering. Although infections can be controlled in many ways, treatment will be needed for some animals, to avoid negative consequences for animal health and welfare.
France
To change practices at the farm level, the best lever remains without doubt the consumer. At the moment, France, consumer associations put pressure on professionals to source from farms limiting the use of antibiotics.
Appendix F – Recent EFSA/EMA opinions and reflection papers, and other relevant reports from the EU agencies
F.1. Recent EFSA opinions
Two recent EFSA opinions discussing public health risk related to ESBL‐, AmpC and carbapenemase‐producing bacteria have discussed possible control options to reduce risks (EFSA BIOHAZ Panel, 2011, 2013). Various control options were identified, but in both opinions, due to a lack of comparative efficacy and efficiency of individual control options, a prioritisation of the options was deemed to be very difficult. In particular, the assessment of the impact of control measures aimed at controlling carbapenemase‐producing bacteria was particularly difficult, given the very low expected prevalence of those bacteria in the food chain.
In both the above opinions, the EFSA BIOHAZ Panel indicated that the decrease in the frequency of use of antimicrobials in animal production in the EU in accordance with EC PUAVM Guidelines is of high priority to control the emergence and spread of resistance and co‐resistance phenomena.
With regard to the reduction of use of the above specific antimicrobial classes in food‐producing animals, the EFSA BIOHAZ Panel indicated that that stopping all uses of cephalosporins/systemically active 3rd‐ and 4th‐generation cephalosporins, or restricting their use to specific circumstances, would be a highly effective option. Carbapenems are not licensed for use in food‐producing animals in the EU, and the continuation of this prohibition would be an effective measure to minimise the emergence and spread of resistance to these antimicrobials.
The need for implementing strict measures or prohibitions of off‐label use of those substances was indicated.
In the case of carbapenemases, it was suggested that, since carbapenam‐producing genes and genes encoding resistance to certain heavy metals such as zinc are sometimes linked, the use of compounds containing such elements should be minimised.
Together with the above control measures, other measures aimed at controlling the risks of spread of those bacteria through the food chain were discussed, such as biosecurity measures for the containment of resistant bacteria at the farm level, and other measures applied during transport of animals and products thereof, and post‐harvest hygienic and decontamination measures having effect at later stages of the food chain. Measures aimed at minimising risks of transmission of resistant bacteria to humans through pathways other than food were also considered.
As provided for in Regulation (EC) No 429/2008, strains of microorganisms intended for use as additives or as a source of feed additives shall not contribute further to the reservoir of AMR genes already present in the gut flora of animals and the environment. Consequently, all strains of bacteria shall be tested for resistance to antimicrobials in use in human and veterinary medicine. To allow differentiation between resistant and susceptible microorganisms, the FEEDAP Panel has produced the Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel, 2012a). Where resistance is detected, the genetic basis of the resistance and the likelihood of transfer of resistance to other gut inhabiting organisms shall be established. Strains of microorganisms carrying an acquired resistance to antimicrobial(s) shall not be used as feed additives, unless it can be demonstrated that resistance is a result of chromosomal mutation(s) and it is not transferable.
The FEEDAP Panel has produced several opinions in which concerns on the AMR of the microbial based feed additives has been identified (EFSA FEEDAP Panel, 2010, 2011, 2012c, 2014a,b,c, 2016b). The strains (one Bacillus toyonensis, one Enterococcus faecium, two Pediococcus pentosaceus, one Lactobacillus pentosus and one Lactobacillus brevis) were found to be resistant to one or more antimicrobials. In none of the cases was the resistance found to be intrinsic in the species. In only in one case (E. faecium), the genetic basis of the resistance could be identified and the concern related to its potential transfer to other microbes dismissed (EFSA FEEDAP Panel, 2010). In the remaining cases, the genetic basis of the resistance was not fully identified, and therefore, the extent of the risk of horizontal gene transfer to other bacteria in the food chain and in the environment could not be established. Up to date and based on these opinions, the EC has adopted Regulations denying the authorisation of four of these bacteria as feed additives (Toyocerin® (B. toyonensis),3 P. pentosaceus (NCIMB 30068) and P. pentosaceus (NCIMB 30044), 4 and L. pentosus (DSM 14025)5).
Additionally, within the frame of feed additives re‐evaluation, the FEEDAP Panel adopted an opinion on the use of cupric sulfate in which the influence of copper in animal nutrition on the development of AMR in bacteria was investigated (EFSA FEEDAP Panel, 2012b). It was stated that
‘The limited database available allowed the FEEDAP Panel to conclude that: (i) high copper concentrations in the microbial environment increase the number of copper‐resistant bacteria and (ii) copper resistance seems to be correlated with more frequent resistance to several antibiotics in certain bacterial species. A cotransfer of plasmid genes encoding for resistance to copper and erythromycin is plausible at least in Enterococcus faecium. The database does not allow any conclusion on a potential threshold concentration of copper in feeds, below which a significant increase in copper resistance could not be expected. The total pool of macrolide resistance in animals probably originates from antibiotic treatment and not from the use of high dietary copper. The extent to which copper‐resistant bacteria contribute to the overall antibiotic resistance situation cannot be quantified at present. More precise (and quantitative) conclusions will require further studies.’
These findings were confirmed in an opinion on the revision of maximum authorised levels of copper in feed adopted in July 2016 (EFSA FEEDAP Panel, 2016a).
F.2. Recent EMA opinions, guidelines and reflection papers
The EMA guidance and recommendations on antimicrobials, including opinions, strategy, guidelines and reflection papers can be found on EMA web pages.6
The latest of those documents are the draft guideline ‘Assessment of the risk to public health from antimicrobial resistance due to the use of an antimicrobial veterinary medicinal product in food‐producing animals (EMA/CVMP/AWP/706442/2013)’, the CVMP strategy on antimicrobials 2016–2020 (EMA/CVMP/209189/2015) and the reflection paper on risk of antimicrobial resistance transfer from companion animals (EMA/CVMP/AWP/401740/2013).
Annex 2 of the EC PUAVM Guidelines includes a summary of the EMA/CVMP recommendations on responsible use.
AMEG
Summary assessment and recommendations on Question 4
At the request of the EC, EMA provided advice on the risk mitigation options related to the use of antimicrobials that are CIAs in human medicine and are authorised as veterinary medicinal products (EMA, 2014).
The AMEG concluded that a number of risk management options have already been implemented at the EU/national level. Measuring the impact of individual risk management measures is difficult, but efforts should be made to evaluate such measures by means of agreed criteria.
Assessment of the EU‐wide impact of new risk management measures requires the development of internationally agreed systems that are capable of measuring their success or failure through adequate monitoring systems of antimicrobial sales/use and resistance.
Such monitoring systems may include:
Monitoring by ESVAC of changes in sales for fluoroquinolones and cephalosporins, and all antimicrobials as a means to measure impact of actions implemented.
More precise data by animal species/species categories in future ESVAC reports.
Prescribers should keep records of off‐label use to be provided at the request of the Authorities.
Further research on the off‐label use of antimicrobials in animals should take place, actions could be derived from the result of these research findings.
Regular joint analyses of the evolution of antimicrobial resistance and sale/use by the Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) EU expert group.
Reduce overall antimicrobial consumption.
Researching methodologies to evaluate the potential economic consequences involved for both human and animal health that would result from the introduction of new risk‐based measures.
Legal tools should be provided to restrict the cascade use depending on the outcome of an AMR risk assessment, in the framework of the medicines authorisation procedure.
The need for further risk management measures should be based on data obtained and on a dedicated risk assessment. Should future legislation on antimicrobial use be considered necessary following such risk assessments, then flexible tools should be in place to enable restriction of use (including use according to the cascade).
Finally, adherence to the latest guidelines and recommendations from international bodies, regulatory authorities and professional associations on responsible use are considered to be of primary importance, particularly in relation to the use of antimicrobials of critical importance for human health.
In light of the importance ascribed to co‐resistance, high priority should also be given to decreasing the total antimicrobial use in animal production in the EU.
F.3. Joint interagency opinions
ECDC EFSA EMA and SCENIHR (2009) jointly analysed available data on AMR in zoonotic agents and identified and described the agent/antimicrobial combinations of highest concern for public health, as mentioned in Section 1.4.3.3. The Joint Opinion confirms previous recommendations formulated by the four EU bodies, such as the promotion and implementation of the prudent use of antimicrobials in animals and of biosecurity measures, the need for training of veterinarians and farmers on the strategies to minimise AMR, the discouragement of the off‐label use of the antimicrobials, the restriction of the use of certain antimicrobials such as fluoroquinolones and cephalosporins to treat specific infections that respond poorly to other antimicrobials, and the monitoring of the development of the AMR in both humans and animals.
A brief summary of the JIACRA report (ECDC EFSA and EMA, 2015) is provided in Section 1.5.3.
F.4. Other relevant reports from the EU agencies
ECDC has intensively elaborated the topic of antimicrobial resistance and healthcare‐associated infections since its foundation in 2005 and published various surveillance and techncial reports. Aside from continuous surveillance programmes on human antimicrobial consumption (European Surveillance of Antimicrobial Consumption Network, ESAC‐Net) and on antimicrobial resistance in bacteria from humans (European Antimicrobial Resistance Surveillance Network, EARS‐Net, and Food‐ and Waterborne Diseases and Zoonoses network, FWD‐Net), the modules that form the Healthcare‐Associated Infections surveillance Network (HAI‐Net) have provided additional information on antimicrobial resistance and on antimicrobial use in hospitals and other healthcare settings: ECDC point prevalence surveys in European acute care hospitals (HAI‐Net PPS) and in long‐term care facilities such as nursing homes (HAI‐Net HALT), surveillance of infections acquired in intensive care units (HAI‐Net ICU), surveillance of surgical site infections (HAI‐Net SSI), surveillance of Clostridium difficile infections (HAI‐Net CDI). In addition, structure and process indicators for infection prevention and control and for antimicrobial stewardship in various healthcare settings have been developed are being implemented in the HAI‐Net modules.
Appendix G – Literature searches performed
G.1. Literature search on alternative measures
As indicated in Sections 2.2 and 4.4, a literature search was performed to inform the review of the alternatives available to the use of antimicrobials, including probiotics and other living organisms, prebiotics, botanicals, enzymes, bacteriocines, antibodies, organic acids, bacteriophages, metals and immunomodulators. The search string used for this literature search is reported in Table G.1. The search was conducted in the following databases: Web of Science™ Core Collection, BIOSIS Citation IndexSM, CAB Abstracts®, Chinese Science Citation DatabaseSM, Current Contents Connect®, Data Citation IndexSM, FSTA®, KCI‐Korean Journal Database, MEDLINE®, SciELO Citation Index, Zoological Record®. The search was conducted for the last 10 years (i.e. 2005–2016). A total of 1,358 references (26 January 2016) was retrieved and screened for studies of interest.
Table G.1.
Search string used for a literature search on alternatives to the use of antimicrobials (probiotics and other living organisms, prebiotics, botanicals, enzymes, bacteriocines, antibodies, organic acids, bacteriophages, metals and immunomodulators)
| Search terms | Field searched |
|---|---|
| antibiotic* OR antimicrobial* OR bacteria* | Title |
| AND | |
| animal* OR livestock OR cattle OR cow* or bovine* OR goat* OR sheep OR ovine* OR caprine* OR pig* OR swine OR poultry OR chicken* or hen* OR horse* OR soliped* OR fish* OR aquaculture OR bee* OR game* OR rabbit* OR pork | Topic |
| AND | |
| replace* OR alternative* OR prebiotic* OR probiotic* OR “feed additive*” OR bacteriophage* OR phage* OR enzyme* OR “organic acid*” OR “heavy metal*” OR competitive OR defensin* OR bacteriotherap* OR “faecal transplantation*” OR antibod* OR lysin* OR bacteriocin* OR lysozym* OR lactoferrin* OR immunomodul* OR immunemodul* OR immuno‐modul* OR immune‐modul* OR “immuno modul*” OR “immune modul*” OR immunostimul* OR immunestimul* OR immuno‐stimul* OR immune‐stimul* OR “immuno stimul*” OR “immune stimul*” | Title |
| AND | |
| disease* OR resistan* | Topic |
| AND | |
| antimicrobial* OR antibiotic* | Topic |
| NOT | |
| wom$n OR m$n OR children OR patient* | Topic |
An additional literature search was performed to complement the above search in relation to antimicrobial peptides available as alternatives to antimicrobials. The search string used for this literature search is reported in Table G.2. The search was conducted in the following databases: Web of Science™ Core Collection, BIOSIS Citation IndexSM, CAB Abstracts®, Chinese Science Citation DatabaseSM, Current Contents Connect®, Data Citation IndexSM, FSTA®, KCI‐Korean Journal Database, MEDLINE®, SciELO Citation Index, Zoological Record®. The search was conducted for the last 10 years (i.e. 2005–2016). A total of 167 references (26 January 2016) was retrieved and screened for studies of interest.
Table G.2.
Search string used for a literature search on alternatives to the use of antimicrobials (antimicrobial peptides)
| Search terms | Field searched |
|---|---|
| animal* OR livestock OR cattle OR cow* or bovine* OR goat* OR sheep OR ovine* OR caprine* OR pig* OR swine OR poultry OR chicken* or hen* OR horse* OR soliped* OR fish* OR aquaculture OR bee* OR game* OR rabbit* OR pork | Topic |
| AND | |
| “antimicrobial peptide*” OR “antibiotic peptide*” | Title |
| AND | |
| disease* OR resistan* | Topic |
| AND | |
| alternative* OR replace* | Topic |
| NOT | |
| wom$n OR m$n OR children OR patient* | Topic |
The results of the two above searches were merged into one single database, and all duplicates were removed, resulting in a database including 1,505 references. Titles and abstracts of the references were screened in order to identify relevant papers to be reviewed in detail. When screening the references, papers were considered relevant if:
they were about experimental studies comparing the effect of the above alternatives with the effect of antimicrobials, or reviews on the subject; or
they investigated the effect of the above alternatives on the health status of food‐producing animals (papers were excluded if they were pure assessment of the growth or production quality); or
they compared the effect of the above alternatives with the effect of antimicrobials for growth promotion but included as end points the assessment of some health parameters of the animals (e.g. lower mortality rates, lower morbidity rates for specific diseases, lower diarrhoea episodes, etc.); or
they investigated the effect of the above alternatives on the prevalence/concentration/spread of zoonotic bacteria or of resistant bacteria in food‐producing animals; and
they were carried out in farming conditions comparable to the EU ones.
After the screening, 130 references were identified as relevant, and reviewed in detail, to extract information useful to inform the expert review to draft Section 4.4 of this opinion.
G.2. Literature search on the association between AMR and organic farming compared to conventional farming
As indicated in Sections 2.2 and 4.2.3.10, a literature search was conducted to identify available evidence concerning the possible association of AMR and organic compared to conventional farming.
The searches were conducted using combinations of terms covering three main question components: (1) organic farming, (2) bacterial and antimicrobial resistance and (3) animal populations. The search string used is reported in Table G.3. The search was conducted in the following database: PubMed. The search was conducted for years 1975–2016. A total of 125 references (1 November 2016) was retrieved and screened for studies of interest.
Table G.3.
Search string used for a literature search on the association between AMR and organic farming compared to conventional farming)
| Search terms |
|---|
| (“Drug Resistance”[Mesh:NoExp] OR “Drug Resistance, Microbial”[Mesh:NoExp] OR “Drug Resistance, Multiple”[Mesh:NoExp] OR “Drug Resistance, Bacterial”[Mesh] OR AMR[tiab] OR ((drug[tiab] OR antimicrobial*[tiab] OR anti microbial*[tiab] OR microbial*[tiab] OR antibiotic*[tiab] OR Anti Infective*[tiab] OR antiinfective*[tiab] OR antibacteri*[tiab] OR “anti bacterial”[tiab] OR bacteriu*[tiab] OR bacteria*[tiab]) AND (resistan*[tiab] OR susceptibilit*[tiab]))) |
| AND |
| (“Organic Agriculture”[Mesh] OR “eco agriculture”[tiab] OR eco farm*[tiab] OR ((organic*[ti] OR ecological*[ti]) AND (product*[tiab] OR farm*[tiab] OR agriculture[tiab] OR “Animal Husbandry”[Mesh] OR husbandry[tiab] OR grown[tiab] OR grew[tiab] OR raise*[tiab]))) |
| AND |
| (“animal experimentation”[MeSH Terms] OR “models, animal”[MeSH Terms] OR “invertebrates”[MeSH Terms] OR “Animals”[Mesh:noexp] OR “animal population groups”[MeSH Terms] OR “chordata”[MeSH Terms:noexp] OR “chordata, nonvertebrate”[MeSH Terms] OR “vertebrates”[MeSH Terms:noexp] OR “amphibians”[MeSH Terms] OR “birds”[MeSH Terms] OR “fishes”[MeSH Terms] OR “reptiles”[MeSH Terms] OR “mammals”[MeSH Terms:noexp] OR “primates”[MeSH Terms:noexp] OR “artiodactyla”[MeSH Terms] OR “carnivora”[MeSH Terms] OR “cetacea”[MeSH Terms] OR “chiroptera”[MeSH Terms] OR “elephants”[MeSH Terms] OR “hyraxes”[MeSH Terms] OR “insectivora”[MeSH Terms] OR “lagomorpha”[MeSH Terms] OR “marsupialia”[MeSH Terms] OR “monotremata”[MeSH Terms] OR “perissodactyla”[MeSH Terms] OR “rodentia”[MeSH Terms] OR “scandentia”[MeSH Terms] OR “sirenia”[MeSH Terms] OR “xenarthra”[MeSH Terms] OR “haplorhini”[MeSH Terms:noexp] OR “strepsirhini”[MeSH Terms] OR “platyrrhini”[MeSH Terms] OR “tarsii”[MeSH Terms] OR “catarrhini”[MeSH Terms:noexp] OR “cercopithecidae”[MeSH Terms] OR “hylobatidae”[MeSH Terms] OR “hominidae”[MeSH Terms:noexp] OR “gorilla gorilla”[MeSH Terms] OR “pan paniscus”[MeSH Terms] OR “pan troglodytes”[MeSH Terms] OR “pongo pygmaeus”[MeSH Terms]) OR ((animals[tiab] OR animal[tiab] OR mice[Tiab] OR mus[Tiab] OR mouse[Tiab] OR murine[Tiab] OR woodmouse[tiab] OR rats[Tiab] OR rat[Tiab] OR murinae[Tiab] OR muridae[Tiab] OR cottonrat[tiab] OR cottonrats[tiab] OR hamster[tiab] OR hamsters[tiab] OR cricetinae[tiab] OR rodentia[Tiab] OR rodent[Tiab] OR rodents[Tiab] OR pigs[Tiab] OR pig[Tiab] OR swine[tiab] OR swines[tiab] OR piglets[tiab] OR piglet[tiab] OR boar[tiab] OR boars[tiab] OR “sus scrofa”[tiab] OR ferrets[tiab] OR ferret[tiab] OR polecat[tiab] OR polecats[tiab] OR “mustela putorius”[tiab] OR “guinea pigs”[Tiab] OR “guinea pig”[Tiab] OR cavia[Tiab] OR callithrix[Tiab] OR marmoset[Tiab] OR marmosets[Tiab] OR cebuella[Tiab] OR hapale[Tiab] OR octodon[Tiab] OR chinchilla[Tiab] OR chinchillas[Tiab] OR gerbillinae[Tiab] OR gerbil[Tiab] OR gerbils[Tiab] OR jird[Tiab] OR jirds[Tiab] OR merione[Tiab] OR meriones[Tiab] OR rabbits[Tiab] OR rabbit[Tiab] OR hares[Tiab] OR hare[Tiab] OR diptera[Tiab] OR flies[Tiab] OR fly[Tiab] OR dipteral[Tiab] OR drosophila[Tiab] OR drosophilidae[Tiab] OR cats[Tiab] OR cat[Tiab] OR carus[Tiab] OR felis[Tiab] OR nematoda[Tiab] OR nematode[Tiab] OR nematodes[Tiab] OR sipunculida[Tiab] OR dogs[Tiab] OR dog[Tiab] OR canine[Tiab] OR canines[Tiab] OR canis[Tiab] OR sheep[Tiab] OR sheeps[Tiab] OR mouflon[Tiab] OR mouflons[Tiab] OR ovis[Tiab] OR goats[Tiab] OR goat[Tiab] OR capra[Tiab] OR capras[Tiab] OR rupicapra[Tiab] OR rupicapras[Tiab] OR chamois[Tiab] OR haplorhini[Tiab] OR monkey[Tiab] OR monkeys[Tiab] OR anthropoidea[Tiab] OR anthropoids[Tiab] OR saguinus[Tiab] OR tamarin[Tiab] OR tamarins[Tiab] OR leontopithecus[Tiab] OR hominidae[Tiab] OR ape[Tiab] OR apes[Tiab] OR “pan paniscus”[Tiab] OR bonobo[Tiab] OR bonobos[Tiab] OR “pan troglodytes”[Tiab] OR gibbon[Tiab] OR gibbons[Tiab] OR siamang[Tiab] OR siamangs[Tiab] OR nomascus[Tiab] OR symphalangus[Tiab] OR chimpanzee[Tiab] OR chimpanzees[Tiab] OR prosimian[Tiab] OR prosimians[Tiab] OR “bush baby”[Tiab] OR bush babies[Tiab] OR galagos[Tiab] OR galago[Tiab] OR pongidae[Tiab] OR gorilla[Tiab] OR gorillas[Tiab] OR “pongo pygmaeus”[Tiab] OR orangutan[Tiab] OR orangutans[Tiab] OR lemur[Tiab] OR lemurs[Tiab] OR lemuridae[Tiab] OR horse[Tiab] OR horses[Tiab] OR equus[Tiab] OR cow[Tiab] OR calf[Tiab] OR bull[Tiab] OR chicken[Tiab] OR chickens[Tiab] OR gallus[Tiab] OR quail[Tiab] OR bird[Tiab] OR birds[Tiab] OR quails[Tiab] OR poultry[Tiab] OR poultries[Tiab] OR fowl[Tiab] OR fowls[Tiab] OR reptile[Tiab] OR reptilia[Tiab] OR reptiles[Tiab] OR snakes[Tiab] OR snake[Tiab] OR lizard[Tiab] OR lizards[Tiab] OR alligator[Tiab] OR alligators[Tiab] OR crocodile[Tiab] OR crocodiles[Tiab] OR turtle[Tiab] OR turtles[Tiab] OR amphibian[Tiab] OR amphibians[Tiab] OR amphibia[Tiab] OR frog[Tiab] OR frogs[Tiab] OR bombina[Tiab] OR salientia[Tiab] OR toad[Tiab] OR toads[Tiab] OR “epidalea calamita”[Tiab] OR salamander[Tiab] OR salamanders[Tiab] OR eel[Tiab] OR eels[Tiab] OR fish[Tiab] OR fishes[Tiab] OR pisces[Tiab] OR catfish[Tiab] OR catfishes[Tiab] OR siluriformes[Tiab] OR arius[Tiab] OR heteropneustes[Tiab] OR sheatfish[Tiab] OR perch[Tiab] OR perches[Tiab] OR percidae[Tiab] OR perca[Tiab] OR trout[Tiab] OR trouts[Tiab] OR char[Tiab] OR chars[Tiab] OR salvelinus[Tiab] OR minnow[Tiab] OR cyprinidae[Tiab] OR carps[Tiab] OR carp[Tiab] OR zebrafish[Tiab] OR zebrafishes[Tiab] OR goldfish[Tiab] OR goldfishes[Tiab] OR guppy[Tiab] OR guppies[Tiab] OR chub[Tiab] OR chubs[Tiab] OR tinca[Tiab] OR barbels[Tiab] OR barbus[Tiab] OR pimephales[Tiab] OR promelas[Tiab] OR “poecilia reticulata”[Tiab] OR mullet[Tiab] OR mullets[Tiab] OR eel[Tiab] OR eels[Tiab] OR seahorse[Tiab] OR seahorses[Tiab] OR mugil curema[Tiab] OR atlantic cod[Tiab] OR shark[Tiab] OR sharks[Tiab] OR catshark[Tiab] OR anguilla[Tiab] OR salmonid[Tiab] OR salmonids[Tiab] OR whitefish[Tiab] OR whitefishes[Tiab] OR salmon[Tiab] OR salmons[Tiab] OR sole[Tiab] OR solea[Tiab] OR lamprey[Tiab] OR lampreys[Tiab] OR pumpkinseed[Tiab] OR sunfish[Tiab] OR sunfishes[Tiab] OR tilapia[Tiab] OR tilapias[Tiab] OR turbot[Tiab] OR turbots[Tiab] OR flatfish[Tiab] OR flatfishes[Tiab] OR sciuridae[Tiab] OR squirrel[Tiab] OR squirrels[Tiab] OR chipmunk[Tiab] OR chipmunks[Tiab] OR suslik[Tiab] OR susliks[Tiab] OR vole[Tiab] OR voles[Tiab] OR lemming[Tiab] OR lemmings[Tiab] OR muskrat[Tiab] OR muskrats[Tiab] OR lemmus[Tiab] OR otter[Tiab] OR otters[Tiab] OR marten[Tiab] OR martens[Tiab] OR martes[Tiab] OR weasel[Tiab] OR badger[Tiab] OR badgers[Tiab] OR ermine[Tiab] OR mink[Tiab] OR minks[Tiab] OR sable[Tiab] OR sables[Tiab] OR gulo[Tiab] OR gulos[Tiab] OR wolverine[Tiab] OR wolverines[Tiab] OR mustela[Tiab] OR llama[Tiab] OR llamas[Tiab] OR alpaca[Tiab] OR alpacas[Tiab] OR camelid[Tiab] OR camelids[Tiab] OR guanaco[Tiab] OR guanacos[Tiab] OR chiroptera[Tiab] OR chiropteras[Tiab] OR bat[Tiab] OR bats[Tiab] OR fox[Tiab] OR foxes[Tiab] OR iguana[Tiab] OR iguanas[Tiab] OR xenopus laevis[Tiab] OR parakeet[Tiab] OR parakeets[Tiab] OR parrot[Tiab] OR parrots[Tiab] OR donkey[Tiab] OR donkeys[Tiab] OR mule[Tiab] OR mules[Tiab] OR zebra[Tiab] OR zebras[Tiab] OR shrew[Tiab] OR shrews[Tiab] OR bison[Tiab] OR bisons[Tiab] OR buffalo[Tiab] OR buffaloes[Tiab] OR deer[Tiab] OR deers[Tiab] OR bear[Tiab] OR bears[Tiab] OR panda[Tiab] OR pandas[Tiab] OR “wild hog”[Tiab] OR “wild boar”[Tiab] OR fitchew[Tiab] OR fitch[Tiab] OR beaver[Tiab] OR beavers[Tiab] OR jerboa[Tiab] OR jerboas[Tiab] OR capybara[Tiab] OR capybaras[Tiab]) NOT medline[sb]) |
Relevance screening was conducted by one reviewer person whereas quality assessment and data extraction were conducted by three reviewers. For relevance screening, only citations that described the AMR situation in organic vs conventional food animal production were included. Studies that did not compare the bacterial resistance to antimicrobials in the two types of production systems were excluded. Upon completion of relevance screening, relevant articles were procured and assessed for methodological soundness based on the criteria of farm clustering and multidrug susceptibility testing for acquired resistance. This criterion were used for description purposes and not as exclusion criteria.
A total of 125 items were identified with the search. Out of these, 33 papers were considered relevant. Nine additional papers were evaluated as they were included in the three systematic reviews reported above. In total, 42 papers were evaluated. They are summarised in Appendix J and in Section 4.2.3.10.
Appendix H – Examples of orally administered formulations commonly available in the EU (information based on product SPCs, non‐exhaustive list)
As indicated in Section 3.2.3.1, Table H.1 reports examples of orally administered antimicrobial formulations commonly available in the EU.
Table H.1.
Examples of orally administered formulations commonly available in the EU (information based on product SPCs, non‐exhaustive list)
| Class/substance | Indications (based on SPCs) | Route of administration | Dose duration (based on SPCs) |
|---|---|---|---|
|
Tetracyclines Oxytetracycline, chlortetracycline, doxycycline |
Treatment and metaphylaxis Pigs: respiratory infections, septicaemia, digestive infection Poultry: respiratory infections; septicaemia, digestive infection, infectious bursal disease Calves, Lambs, Kids: respiratory disease, septicaemia, digestive infection Salmon and trout: furunculosis, enteric redmouth |
Premix for MFS Oral powders, solutions for drinking water |
10 days 3–8 (5) days |
|
Macrolides Tylvalosin, tylosin, tilmicosin, erythromycin, lincomycin |
Treatment and metaphylaxis Pigs: enzootic pneumonia (Mycoplasma), PPE (ileitis); swine dysentery; enteritis Poultry: Mycoplasma infections, necrotic enteritis, ORT Rabbits: respiratory diseases |
Premix for MFS Oral powder/solutions for drinking water |
7 days – 3 weeks in feed, or until clinical signs resolve 3–5 days for powder in feed or drinking water |
|
Sulfonamides/Trimethoprim Sulfadiazine, sulfamethoxazole |
Treatment and metaphylaxis Pigs: infections in general Poultry: infections in general Calves, lambs, rabbits and fish |
Premix for MFS Oral solutions for drinking water |
5–10 days in feed 3–4 (4–7) days for drinking water |
|
Pleuromutilins Tiamulin, valnemulin |
Treatment and metaphylaxis Pigs: colitis; swine dysentery; PPE (ileitis); enzootic pneumonia (Mycoplasma) Poultry: respiratory diseases; Mycoplasma infections Rabbits: epizootic rabbit enterocolitis (ERE) |
Premix for MFS Oral powder Oral solutions for drinking water |
7 days to 4 weeks, or until clinical signs resolve Drinking water administration for 5 days |
|
Penicillins Penicillin V, Amoxicillin Ampicillin |
Treatment and metaphylaxis Pigs: respiratory diseases; Streptococcus suis Poultry: respiratory and intestinal infections Calves: respiratory and intestinal infections |
Premix for MFS, Oral powders for feed and drinking water |
5 days to 2 weeks (up to 6 weeks for Strep. suis) in feed Drinking water administrations for 5 days |
|
Aminoglycosides/aminocyclitols Apramycin, neomycin, streptomycin, spectinomycin |
Pigs: bacterial enteritis, enzootic pneumonia (incl Mycoplasma) Calves: colibacillosis, salmonellosis Lambs and Poultry: colibacillosis Poultry: Mycoplasma respiratory infections |
Premix for MFS, Oral solution for drinking water |
28 days in feed 5–7 days for drinking water |
|
Amphenicols florfenicol |
Treatment and metaphylaxis Pigs: respiratory disease Salmon & trout: furunculosis (Aeromonas salmonicida) |
Premix for MFS Oral powder in feed Oral solutions for drinking water |
5–10 days in feed 5 days for drinking water |
|
Polymyxins Colistin sulfate |
Treatment and metaphylaxis Pigs, calves, lambs, poultry: enteric E. coli infections |
Premix for MFS Oral solutions in drinking water |
3–7 days maximum |
|
Fluoroquinolones Enrofloxacin |
Treatment Pigs: respiratory and gastrointestinal infections, septicaemia Calves: respiratory and gastrointestinal infections |
Oral solutions for drinking water | 3–5 days |
Appendix I – Classes of CIAs that are authorised for use in human medicine but not in veterinary medicine
The AMEG was requested to provide a classification of CIAs according to their use in veterinary medicine and the associated risk for public health, the WHO list of CIAs was taken as the basis for the classification (EMA, 2014). The answers include a series of recommendations of classes of critically important antimicrobials that are authorised for use in human medicine but not in veterinary medicine as follows:
Carbapenems and other penems: use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance. The EFSA BIOHAZ Panel concluded that carbapenems should not be used for food‐producing animals; it also concluded that as ‘co‐resistance may be an important issue […] decreasing the frequency of use of antimicrobials in animal production in the EU in accordance with prudent use guidelines is also of high priority’(EFSA BIOHAZ Panel, 2013).
Ceftaroline and ceftobiprole: no specific concern identified yet.
Cyclic esters (e.g. fosfomycin): use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance.
Glycopeptides: use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance.
Glycylcyclines: see response to Question 1 (EMA, 2013b).
Lipopeptides: No specific concern identified yet.
Monobactams: use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance.
Oxazolidinones: use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance.
Penicillins: carboxy‐penicillins and ureido‐penicillins including β‐lactamase inhibitors combinations; use in veterinary medicine should be kept at an absolute minimum due to high risk for spread of resistance.
Riminofenazines: no specific concern identified yet.
Sulfones: no specific concern identified yet.
Drugs used solely to treat tuberculosis or other mycobacterial diseases: no specific concern identified yet.
The mentioned answer indicates that before considering applying for any marketing authorisation for products containing antimicrobials belonging to any of the above mentioned classes, the above concluding remarks should be taken into account and finalises indicating that the above list is not inclusive of all antimicrobials authorised in human medicine.
Appendix J – Results of a literature search on the association between AMR and organic farming compared to conventional farming
When reviewing evidence on a particular topic, it is acknowledged that not all the forms of evidence can be considered of equal value.83 Randomised controlled studies are considered as the most reliable evidence to confirm a hypothesis of association and, even better, causality. Cross‐sectional observational studies are on the other extreme of the scale. Although providing valuable evidence to generate hypotheses, these kind of designs are considered poor to perform causal inferences when a comparison group is missing (e.g. exposed vs non exposed) and confounding factors, i.e. variables that affect the relationship between the potential cause and the hypothesised effect, are not accounted for.
The studies included in the EFSA literature review are all observational studies, most based on a cross‐sectional study design. Therefore, methodologically speaking, they lack the scientific robustness necessary to address a causality question.
Limitations in the methods used to perform a study can lead to a risk of bias (systematic error) both in the estimate of an effect and/or of its precision (Viswanathan et al., 2013). In turn, this translates into uncertainty in the validity of the results that needs to be taken into account when drawing conclusions.
In this assessment, several aspects were considered critical in order to evaluate the reliability of the included studies. They can be classified into four broad categories:
confounders that need to be controlled/accounted for in the studies;
other biases that could systematically impact on the study results;
appropriateness of the methods used to analyse data;
biological relevance of the study results.
Confounding/correlated risk factors
Confounders are factors that, when not accounted for, introduce a risk of bias in the effect estimate between, in this case, farming type (organic or conventional, the exposure) and AMR (the outcome). These factors can make it difficult to recognise the effect of the different farm types (organic vs traditional) on antimicrobial resistance, since they significantly affect the outcome and its variability. Some examples of potential confounders include past or current disease status of animals on the farm, level of biosecurity on the farm and characteristics of the farmer (such as diligence in animal care, disease control, etc.). The possible impact of not accounting for confounders is the risk of introducing a bias (i.e. a systematic error) in the estimate of the effect of organic farming on antimicrobial resistance. Depending on the nature of the confounder, the bias may go in either direction, masking an effect when it exists (false negative) or amplifying the level of an effect when it is irrelevant (false positive).
Other factors that might introduce risk of bias
A range of other biases may impact on the study results. Organic farming is described in the EU legislation, with equivalent meaning through the EU/EEA countries/Switzerland. This may not be the case in third countries, where the legislative basis for organic farming may either be different or absent. In addition, the general systems of farming in Europe relevant to the development of AMR may differ from those of third countries. For both of these reasons, consideration should be given to the representativeness of each study population, and potential concerns when seeking to generalise the study results to the European context (our target population). Therefore, when a study is carried out outside the EU/EEA countries/Switzerland, differences in both the farming system and in the definition of ‘organic production’ might represent sources of bias if they are not properly accounted for.
Methodological issues
A number of methodological issues also need to be considered. Firstly, it is important to highlight the need to properly address the issue of clustering at any level of the sampling, e.g. when multiple samples are obtained from the same meat sample, and when multiple samples are obtained from the same herd or store. When a herd (clustering) effect is present, as would be expected with AMR, animals within a herd are more similar with respect to an outcome of interest than animals from different herds, i.e. the responses of herd mates are correlated. This is termed herd/flock‐level clustering. In these circumstances, it is incorrect to use statistical methods based on assumption of independence. If such tests are used, the estimated variances will be too low which may lead to false positive results when doing inferences (McDermott and Schukken, 1994). In the current studies, if clustering is not appropriately handled during data analysis, the association between farming type and AMR may be deemed statistically significant, when in fact it is not. Several other methodological issues are important. There is the potential effect of the multiplicity issue when simultaneously testing several endpoints (multiple tests performed on different parameters). In this case, it is well known that false positive could increase unless proper adjustments are used in the estimate of the type I error. From a statistical perspective, consideration should also be given to whether any dose–response relationship could affect the association between organic farming and AMR. The differential effect of farming types could occur, for example, only above certain level of usage of antimicrobials.
Biological relevance
A range of elements are considered when seeking to determine causality, including temporality (the cause A preceding the outcome B), the strength of the statistical association (influenced, as highlighted above, by issues relating to study design and data analysis), and biological plausibility. With respect to biological plausibility or relevance, a key factor is represented by acquired resistance for multiple agents and MDR (simultaneous presence of different acquired resistance determinants). The within‐organism repeated measurements is important because not all types of resistance are maintained for equally long periods of time in a given environment after the removal of the selecting antimicrobial drug (Dunlop et al., 1998; Price et al., 2005). Other factors relevant to biological relevance including acquired vs intrinsic antimicrobial resistance, selective vs non‐selective culturing, time lag effects following an antimicrobial selection pressure (persistence), and the distinction between zoonotic, commensal, clinical, and subclinical animal pathogens.
Tables J.1 and J.2 list the studies identified and summarise their main characteristics, findings, and some key limitations.
Appendix K – Case studies
The case studies below indicate how some of the recommended options proposed in this report have already been implemented to reduce antimicrobial use, and highlight the multifacteted approach.
K.1. Case study 1: Mastitis control
Recommended option 1. Development of national strategies implemented through action plans
National strategies for mastitis control have been in place for many years. The five‐point mastitis plan was devised in the 1960s, and remains the basis for contagious mastitis control. A further five points, specifically addressing the control of environmental mastitis, were added later (More, 2009).
There are many examples of national mastitis control programmes.
Recommended option 2. Development of harmonised systems for monitoring antimicrobial use and surveillance of AMR integrating data from humans, food‐producing animals and food derived thereof
Over the years, a range of methods to measure on‐farm antimicrobial use have been reported. There is now convergence towards the use of DDDvet/DCDvet and related measurements (Kuipers et al., 2015; Stevens et al., 2016). On‐farm antimicrobial use can now be compared, both in‐lactation and dry cow therapy, as can underlying drivers for use, including the incidence of clinical mastitis.
Recommended option 3. Establishing targets for reduction of the use of antimicrobials in food‐producing animals, especially CIAs
Several countries have introduced targets for reduction in the use of antimicrobials, including in dairy production (see Section 3.3.3). In Denmark, legal regulations introduced in 2010 on the use of antimicrobials for the treatment of mastitis require the use of narrow‐spectrum antimicrobials unless susceptibility testing indicates otherwise. This has led to a reduction in the use of 3rd and 4th generation cephalosporins as intramammary treatments (DANMAP, 2015). In 2014, the Danish cattle industry adopted a voluntary ban on the use of 3rd‐ and 4th‐generation cephalosporins.
Recommended option 4. On‐farm animal health management with professional input
In many countries, professional input is critical to the success of on‐farm mastitis control programmes. In the Netherlands, for example, farmers identified their veterinarian as the first person to approach when faced with an udder health problem (Jansen et al., 2009).
Recommended option 5. Increasing responsibility taken by veterinarians for prescribing antimicrobials
In several countries, a broad range of strategies have been introduced to encourage prudent decision‐making by veterinarians when prescribing antimicrobials, including dairy veterinarians (see Section 3.3.7).
Recommended option 6. Increased oversight of preventive and metaphylactic antimicrobial use
In recent years, there has been a critical rethink of the blanket use of dry cow therapy, a preventive strategy. The alternative, selective dry cow therapy, is now an important area of research. In the Netherlands, selective dry cow therapy has been mandated since 2013.
Recommended option 7. Training and education for veterinarians and for end users of antimicrobials, and raising public awareness
National mastitis control programmes generally emphasise considerable emphasis on prudent antimicrobial use. Antimicrobial benchmarking (of use by farmers, of prescribing patterns by veterinarians) has been introduced in several countries.
Treatment guidelines are available in several MSs. In the Nordic countries, selective dry cow therapy is preferred, if ADCT is used at all.
Recommended option 8. Increasing the availability and use of rapid and reliable diagnostics and antimicrobial susceptibility tests, including at the farm level
A range of diagnostic methods are used to assist with mastitis management. Somatic cell counts (SCC, a consequence of inflammation) are used to monitor subclinical mastitis in the herd (bulk milk) and individual cows (during milk recording and also using the California mastitis test). Bacteriology of milk samples, to identify the causative organism, is used to guide clinical decision‐making, including the appropriate selection of antimicrobials.
Recommended option 9. Improvement of husbandry and management procedures for disease prevention, control and eradication of infectious diseases in livestock production, including vaccination
Mastitis cannot be controlled in dairy farms without considerable attention to husbandry and management. The ten‐point plan for control of both contagious and environmental mastitis pathogens primarily considers husbandry and management, including the use of proper milking management methods, proper installation, function and maintenance of milking equipment, maintenance of an appropriate environment, good record keeping and setting goals for udder health status (Radostits et al., 2007).
There has been ongoing progress towards the development of a multivalent mastitis vaccine for cattle. Results have been promising, with vaccination contributing to a broader mastitis control programme (see Section 4.2.3.5).
Recommended option 10. Rethinking livestock production systems: reduced reliance on antimicrobial use and exploring further the potential of alternative production systems
In recent years, there has been a critical rethink of the blanket use of dry cow therapy, given general concerns of antimicrobial use and resistance in the farming industries. In the Netherlands, selective dry cow therapy has been mandated since 2013. Recent research has highlighted an increase in both clinical and subclinical mastitis in low‐SCC cows with this change (Scherpenzeel et al., 2014), with the selection criteria used when identifying cows to treat influencing the effect on udder health, antimicrobial use and farm economics (Scherpenzeel et al., 2016).
Recommended option 11. Development of treatments which are alternatives to antimicrobials
Several non‐antimicrobial products are used extensively as part of a mastitis control programme. The application of internal teat sealant at the time of drying off has been shown to significantly decrease the risk of new intramammary infections during the dry period, compared with untreated cows (Rabiee and Lean, 2013) (see Section 4.4.10). Antiseptic teat dip/spray is routinely used at each milking, to limit new infections.
K.2. Case study 2: Approaches taken to reduction of the use of antimicrobials in pig production in Denmark
Recommended option 1. Development of national strategies implemented through action plans
The Danish action plan for reduction and prudent use of antimicrobials in pigs was introduced in 2005. The main aims were to provide treatment guidelines for the most common diseases and to benchmark veterinarians’ antimicrobial use.8
The challenges in addressing antimicrobial consumption in the pig sector are noted in the 2010 Joint antibiotics and resistance plan. In particular, the plan announces initiatives in relation to investigating the spread of MRSA in Danish pig herds and reducing overall antimicrobial use and benchmarking use under the Yellow Card scheme.
Recommended option 2. Development of harmonised systems for monitoring antimicrobial use and surveillance of AMR integrating data from humans, food‐producing animals and food derived thereof
DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Program) was established in 1995 by the Danish Ministry of Health and the Ministry of Food, Agriculture and Fisheries to monitor antimicrobial use and AMR in bacteria from humans, animals and foods.
The VetStat database was introduced by the DVFA in 2000 to track antimicrobial use. The database captures all prescription data for antimicrobials from veterinarians, feed mills and pharmacists, at the level of farm, species and age group (Jensen et al., 2004a).
Recommended option 3. Establishing targets for reduction of the use of antimicrobials in food‐producing animals, especially CIAs
The DVFA introduced the ‘Yellow Card’ scheme in 2010 aimed at reducing antimicrobial consumption in pigs by 10% by 2013. The system establishes thresholds of use based on sector average consumption. If a farm exceeds these thresholds, the veterinarian should implement an action plan to address use, restrictions are placed on represcription and the farm may be subject to unannounced official inspections (DANMAP).
Susceptibility testing prior to the use of fluoroquinolones was made mandatory in 2003, and use must be reported.
In 2010, the pig industry adopted a voluntary ban on the use of 3rd‐ and 4th‐generation cephalosporins (DANMAP, 2011).
In 2013, differential taxes on antimicrobials were increased to disincentivise the use of CIAs in particular.
In 2014, the pig industry adopted a target to reduce the use of tetracyclines by 50% by the end of 2015.
Recommended option 4. On‐farm animal health management with professional input
Veterinary Advisory Service Contracts became compulsory for large pig farms in 2010. This establishes a contract between farmer and veterinarian and requires the veterinarian to perform regular visits to the farm to advise on disease prevention (DANMAP, 2015).
Recommended option 5. Increasing responsibility taken by veterinarians for prescribing antimicrobials
In 1994 a law was introduced restricting the amount of medicines that veterinarians could supply to their client, and the profit, so that prescribing and dispensing were effectively ‘decoupled’.
Under the VASC, veterinarians can only prescribe antimicrobials in conjunction with a visit to the farm, and for a limited treatment period (5/10 days at each visit) (DANMAP, 2015).
VetStat data are used to monitor and benchmark prescribing by veterinarians.
Recommended option 6. Increased oversight of preventive and metaphylactic antimicrobial use
Preventive use of antimicrobials was prohibited in law in 1995.
Voluntary discontinuation of all AGPs by Danish pig producers, 1998 (Hammerum et al., 2007).
Since 2014, veterinarians prescribing antimicrobials for group treatment of pigs via feed or water for respiratory or gastrointestinal disease must submit samples to an approved laboratory to verify the diagnosis (DANMAP, 2015).
Recommended option 7. Training and education for veterinarians and for end users of antimicrobials, and raising public awareness
Treatment guidelines have been available from the DVFA since 1996, and in 2010 updated ‘One Health’ evidence‐based prudent use guidelines for use of antimicrobials in pigs were published (DANMAP, 2011).
The DVFA requires animal owners who administer medicines to food‐producing animals to have completed an approved course in the management of medicines.
Recommended option 9. Improvement of husbandry and management procedures for disease prevention, control and eradication of infectious diseases in livestock production, including vaccination
Industry led initiatives on biosecurity have been implemented requiring disinfection of animal transporters on entry to Denmark.
From 2004 to 2014 the use of vaccination for the prevention of gastroenteric diseases (E. coli, Clostridium perfringens, Lawsonia intracellularis) has increased by +56%. Vaccination against respiratory diseases (swine influenza, Mycoplasma hyopneumoniae, Actinobacillosis pleuropneumoniae, Pasteurella multocida) has increased by +55% over the same period. The use of vaccines against PRRS, PCV2 and PPV has increased from 1.4 M doses in 2004 to 16.2 M doses in 2014 (DANMAP, 2015).
Recommended option 10. Rethinking livestock production systems: reduced reliance on antimicrobial use and exploring further the potential of alternative production systems
From 2002 to 2008 there has been a decline in the number of small pig herds in Denmark, which were shown to use larger quantities of antimicrobials, and an increase in the prevalence of larger herds (Vieira et al., 2011b).
Recommended option 11. Development of treatments which are alternatives to antimicrobials
The use of zinc oxide in pig production increased threefold between 2005 to 2011, to approximately 500 tonnes p.a. (DANMAP, 2015).
The reduction in on‐farm antimicrobial use has occurred coincident with a marked improvement in overall pig productivity, which suggests that changes in antimicrobial consumption have not had a negative impact on long‐term pig production in Denmark (Aarestrup et al., 2010a).
Appendix L – Divergent position
Keith Baptiste, Member of the EMA CVMP
After careful consideration of the drafted RONAFA report, the undersigned would formally request to make a ‘divergent opinion’ statement of the final RONAFA document with regards to the section on prophylaxis/metaphylaxis in Food animals.
3.3.6. Prophylaxis, Prevention, Metaphylaxis
This section comprises only a relatively minor contribution to the entire RONAFA report, despite the common use of antimicrobial prophylaxis and metaphylaxis in agriculture and the tonnes of antimicrobials consumed yearly.
The text appears fragmented and recommending metaphylaxis, whereas it is not clear as to how the conclusions are backed up with scientific evidence:
There are dozens of published randomized clinical trials (RCT), with and without negative control groups that have investigated different aspects of prophylaxis and metaphylaxis in food animals (e.g. morbidity, mortality, weight gain, etc...), and these have not been cited in the section.
It is unclear as to how the references cited in the section have been selected.
Summaries of European clinical trials on bovine respiratory disease are misrepresented, in that they in this author's interpretation did not demonstrate a consistent benefit of metaphylaxis on morbidity and mortality, in terms of relative risk.
Since multiple definitions of metaphylaxis are mentioned in this section of the RONAFA report, then it is unclear as to which definition are being referred to in the conclusions.
It is therefore the view of this undersigned that the text in this section does not provide an adequate, unbiased overview of the scientific literature on antimicrobial prophylaxis and metaphylaxis in agriculture, particularly on its effects on morbidity and mortality.
London, 8 December 2016
Keith Baptiste
Appendix M – Divergent position
Peter Hekman, Johan Schefferlie, Gábor Kulcsár, Members of the EMA CVMP
We, the undersigned, wish to express a divergent position on the EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety.
Although we do agree with the general tendency of the opinion and it's recommendations, we do not support the recommendation for the preventive use of antimicrobial substances in herds. This recommendation is made for exceptional cases and it contains a number of criteria. Examples of cases where preventive use is recommended, are Actinobacillus pleuropnemoniae and Streptococcus suis in pigs.
We object to this recommendation for the following reasons:
The recommendation is not in line with the CVMP Strategy on antimicrobials 2016–2020 that says that ‘Antimicrobials should never be used to compensate for the impact of husbandry systems or a lack of biosecurity’.
The recommendation is not in line with the Question and answer on the CVMP guideline on the SPC for antimicrobial products, that says that ‘prevention’ as a single and separate claim, will refer to the administration of an antimicrobial veterinary medicinal product to an individual healthy animal to prevent infection’. Clearly, this claim only applies to individual treatment, and does not cover preventive mass medication.
The preventive use of antibiotic substances in herds should not be encouraged, therefore it doesn't seem fit to recommend such use in a report that is aimed at the reduction of antibiotic use in animals.
If preventive mass medication is considered as exceptional indeed, then it may not need to be covered by the opinion at all.
This recommendation will legitimize marketing authorisations for veterinary medicinal products with preventive mass medication as an indication for use.
Actinobacillus pleuropneumoniae and Streptococcus suis are major pathogens in pigs. Therefore it is not understood how these examples can be considered as ‘exceptional’.
Given that the examples are major pathogens, we fear that the recommendation for the ‘exceptional’ preventive mass medication will be used commonly in practice.
One of the conditions mentioned is that ‘there are no other recognized effective herd‐health control measures’. However, it appears that the examples given would not fulfill this condition, as there are well‐known herd‐health control measures.9 , 10 , 11 , 12 , 13 The pathogens mentioned are often naturally present in swine and may cause disease following stress (e.g. due to other infections, housing and climate problems, bad management).
The recommendation may be used as an excuse to continue preventive mass medication, rather than taking other herd‐heath control measures.
The changes that are needed to reduce antimicrobial consumption will not take place as long as we defend, and even recommend, the continuation of preventive mass medication.
London, 8 December 2016
Peter Hekman, Johan Schefferlie, Gábor Kulcsár, Members of the EMA CVMP
Annex A – Antimicrobial use in food‐producing animals
Annex A – can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4666
Description: replies to EFSA/EMA questions on the use of antimicrobials in food‐producing animals in the EU and possible measures to reduce antimicrobial use – 29 February 2016, Federation of Veterinarians of Europe (FVE).
Supporting information
Antimicrobial use in food‐producing animals
Suggested citation: EMA (European Medicines Agency) and EFSA (European Food Safety Authority) , 2017. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). [EMA/CVMP/570771/2015]. EFSA Journal 2017;15(1):4666, 245 pp. doi: 10.2903/j.efsa.2017.4666
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00216
EMA CVMP Members: Zanda Auce, Keith Baptiste, J. Gabriel Beechinor, Hanne Bergendahl, Rory Breathnach, Jiří Bureš, João Pedro Duarte Da Silva, Judita Hederová, Peter Hekman, Cornelia Ibrahim, Emil Kozhuharov, Helen Jukes, Gábor Kulcsár, Eva Lander Persson, Johann M. Lenhardsson, Petras Mačiulskis, Ioannis Malemis, Ljiljana Markus‐Cizelj, Alia Michaelidou‐Patsia, Cristina Muñoz Madero, David Murphy, Martti Nevalainen, Paolo Pasquali, Jean‐Claude Rouby, Johan Schefferlie, Wilhelm Schlumbohm, Marc Schmit, Stephen Spiteri, Stanko Srčič, Lollita Taban, Toomas Tiirats, Bruno Urbain, Ellen‐Margrethe Vestergaard, Anna Wachnik‐Święcicka, Jason Weeks and Barbara Zemann.
EFSA BIOHAZ Panel members: Ana Allende, Declan Bolton, Marianne Chemaly, Robert Davies, Pablo Salvador Fernandez Escamez, Rosina Girones, Lieve Herman, Kostas Koutsoumanis, Roland Lindqvist, Birgit Nørrung, Antonia Ricci, Lucy Robertson, Giuseppe Ru, Moez Sanaa, Marion Simmons, Panagiotis Skandamis, Emma Snary, Niko Speybroeck, Benno Ter Kuile, John Threlfall and Helene Wahlström.
Divergent position: Part of this scientific opinion is not shared by the following members of the EMA CVMP: Keith Baptiste (see Appendix L), Peter Hekman, Johan Schefferlie and Gábor Kulcsár (see Appendix M).
Acknowledgements: The EMA CVMP and the EFSA BIOHAZ Panel wish to thank the following for the support provided to this scientific opinion: the EFSA Panel on Animal Health and Welfare (AHAW), which endorsed Section 4.2 of the scientific opinion; the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), which endorsed Section 4.4 of the scientific opinion; the EMA Antimicrobials Working Party (AWP); the EMA Immunologicals Working Party (IWP); the EMA members of the ad hoc Working Group (WG) drafting this scientific opinion: Keith Baptiste, Boudewijn Catry, Christian Friis, Helen Jukes (WG co‐chair), Cristina Muñoz Madero and Pascal Sanders; the EFSA members of the ad hoc Working Group (WG) drafting this scientific opinion: Pier Sandro Cocconcelli, Robert Davies, Christian Ducrot, Gregers Jungersen, Simon More and John Threlfall (WG co‐chair); Els Broens (EFSA working group member until 4 March 2016); EFSA hearing expert Atle Lillehaug; EFSA hearing expert Charlotte Dunoyer (representing the French Agency for Food, Environmental and Occupational Health & Safety, ANSES); EFSA hearing expert Maryline Kouba; EFSA staff Laura Martino and Irene Munoz Guajardo (AMU Unit) for their support in Section 4.2.3.10. The EMA CVMP and EFSA BIOHAZ Panel wish to acknowledge all the European competent institutions, the Member State bodies and other organisations that provided data for this scientific opinion.
Adopted: 1 December 2016 (EFSA BIOHAZ Panel), 8 December 2016 (EMA CVMP)
Notes
Commission Notice 2015/C 299/04. Guidelines for the prudent use of antimicrobials in veterinary medicine. OJ C 299, 11.9.2015, p. 7–26.
Commission Staff Working Document. Guidelines for the prudent use of antimicrobials in veterinary medicine. Practical examples. Available at: http://ec.europa.eu/health/antimicrobial_resistance/docs/2015_prudent_use_guidelines_annex_en.pdf
Commission Implementing Decision of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria (2013/652/EU). OJ L 303, 14.11.2013, p. 26–39.
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29–43.
Report from the Commission on Antimicrobial Feed Additives, Stockholm, 1997. Available at: http://www.government.se/legal-documents/1997/01/sou-1997132/
Available at: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf
Available at: http://www.un.org/pga/71/wp-content/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108E.pdf
Available at: http://publications.apec.org/publication-detail.php?pub_id=1577
Available at: http://www.cdc.gov/globalhealth/security/actionpackages/antimicrobial_resistance.htm
Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products. OJ L 311, 18.11.2001, p. 1–66. As last amended.
FEAP Annual Report 2015. Available at: http://www.feap.info/shortcut.asp?FILE=1402
Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/cephalosporins_35/WC500121720.pdf
Further information will be available in an overview report on this prudent use project, including an analysis of the questionnaire responses, expected to be published by DG SANTE in March 2017.
VICH GL 36 Studies to evaluate the safety of residues of veterinary drugs in human food: General approach to establish a microbiological ADI.
VICH GL 27 Guidance on preapproval information for registration of new veterinary medicinal products for food‐producing animals with respect to antimicrobial resistance.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40. As last amended.
Council Directive 90/167/EEC of 26 March 1990 laying down the conditions governing the preparation, placing on the market and use of medicated feedingstuffs in the Community. OJ L 92, 7.4.1990, p. 42–48.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1–22. As last amended.
Proposal for a Regulation of the European Parliament and of the Council on the manufacture, placing on the market and use of medicated feed and repealing Council Directive 90/167/EEC. Available at: http://ec.europa.eu/transparency/regdoc/rep/1/2014/EN/1-2014-556-EN-F1-1.Pdf
Available at: http://www.oie.int/doc/ged/D9840.PDF
The Czech Republic Institute for State Control of Veterinary Biologicals and Medicines, see: http://www.uskvbl.cz.
Available at: http://www.bmel.de/SharedDocs/Downloads/EN/Agriculture/GermanAntimicrobialResistanceStrategy.pdf?__blob=publicationFile
Available at: http://www.bmel.de/EN/Animals/AnimalHealth/_Texte/DART2020.html
EPI‐NEWS No 23 – 2016. Available at: http://www.ssi.dk/English/News/EPI-NEWS/2016/No%2023%20-%202016.aspx
The écoantibio 2017 plan. Reducing antibiotic use in veterinary medicine. Available at: http://agriculture.gouv.fr/telecharger/82562?token=89ecb14c82eb7e500b8180cb14401b00
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1–72. As last amended.
Available at: http://www.amcra.be/en/tag/formulaire
Available at: http://www.e-vademecum.be
Available at: http://www.amcra.be/fr/classification-des-antibiotiques
Legislation available at: http://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=nl&la=N&cn=2014020716&table_name=wet
Available at: https://www.ddd.dk/sektioner/hundkatsmaedyr/Documents/Retningslinjer%20for%20brug%20af%20antibiotika%20kv%E6g.pdf
Available at: http://www.finlex.fi/fi/laki/alkup/2008/20080847
Available at: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000029573022&categorieLien=id
Available at: http://wvab.knmvd.nl/over-de-WVAB
Available at: http://wvab.knmvd.nl/media/default.aspx/emma/org/10870194/160413%20wvab-richtlijn%203.1%20definitief.pdf
Available at: https://zoek.officielebekendmakingen.nl/stb-2012-616.html
Available at: http://www.jordbruksverket.se/download/18.566673b01501fd7f99dbcd9d/1443691866966/2015-032.pdf
Available at: http://www.jordbruksverket.se/download/18.53b6e8e714255ed1fcc4fd9/1385713779939/2013-042.pdf
Available at: http://www.uskvbl.cz/en/information/information-for-vets/antimicrobials-under-qprudent-useq
Available at: https://www.gpo.gov/fdsys/pkg/FR-2012-01-06/pdf/2012-35.pdf
Available at: http://www.ontlpc.ca/pdfs/CIPARSSurveillanceBulletin.pdf
Available at: http://www.epruma.eu/publications/factsheets/publication/32-epruma-document-on-veterinary-medicinal-product-terminology.html
Medicinal Products Act (The Drug Law), published on 12 December 2005, as last amended.
Available at: http://amrls.cvm.msu.edu
Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91. OJ L 189, 20.7.2007, p. 1–23. As last amended.
Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. OJ L 250, 18.9.2008, p.1–84. As last amended.
Available at: http://www.consumersinternational.org/media/1456844/antibiotics-in-farming-ci-recommendations-english.pdf
Regulation (EU) No 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
Available at: https://www.spf.dk/en-us/health/the-danish-spf-system
Available at: http://www.bovinetb.info/docs/tb_workingdoc2006_en.pdf
Available at: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.02_AUJESZKYS_DIS.pdf
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of Salmonella and other specified food‐borne zoonotic agents. OJ L 325, 12.12.2003, p. 1–15. As last amended.
See: http://www.eu-plf.eu
Council Directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. OJ L 182, 12.7.2007, p. 19–28. As last amended.
Directive 2001/81/EC of the European Parliament and of the Council of 23 October 2001 on national emission ceilings for certain atmospheric pollutants. OJ L 309, 27.11.2001, p. 22–30. As last amended.
Available at: http://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/SCAD/A_SCAD_Sept2015.pdf
Available at: http://www.agriculture.gov.ie/animalhealthwelfare/diseasecontrol/aujeszkyspigdiseaseprogramme/
Available at: http://www.sva.se/om-sva/publikationer/sjukdomsovervakning/surveillance-of-zoonotic--other-animal-disease-agents-in-sweden
Available at: http://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/SCAD/A_SCAD_Sept2015.pdf (see Annex 5)
Council Regulation (EEC) No 2092/91 of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. OJ L 198, 22.7.1991, p. 1–15.
See VetCAST at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/VetCAST/VetDocuments/Veterinary_Committee_on_AST_II_150507.pdf
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/veterinary/002763/WC500204000.pdf
Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market. OJ L 123, 24.4.98, p. 1.
For the purpose of this report, the CIAs include those in the AMEG's category 2 and category 3 (EMA, 2014). Category 2 includes fluoroquinolones, 3rd‐ and 4th‐generation cephalosporins and colistin, and, while undergoing risk‐profiling, the extended‐spectrum penicillins and aminoglycosides. Category 3 includes classes of the WHO's CIAs which are currently not approved for use in veterinary medicine.
The organisations contacted for this exercise are: AVEC, BEUC, COPA‐COGECA, EUROCOMMERCE, EUROCOOP, FESASS, FOOD DRINK EUROPE, IFOAM‐EU GROUP, INDEPENDENT RETAIL EUROPE, UECBV.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OJ L 152, 16.6.2009, p. 11–22. As last amended.
Commission Implementing Regulation (EU) 2015/1399 of 17 August 2015 concerning the denial of authorisation of the preparation of Bacillus toyonensis (NCIMB 14858T) (formerly Bacillus cereus var. toyoi NCIMB 40112/CNCM I‐1012) as a feed additive for cattle for fattening, rabbits for fattening, chickens for fattening, piglets (weaned), pigs for fattening, sows for reproduction and calves for rearing and the revocation of the authorisations of the preparation of Bacillus cereus var. toyoi (NCIMB 40112/CNCM I‐1012) as a feed additive for turkeys for fattening and rabbit breeding does, amending Regulations (EC) No 256/2002, (EC) No 1453/2004, (EC) No 255/2005 and (EC) No 1200/2005 and repealing Regulations (EC) No 166/2008, (EC) No 378/2009 and Implementing Regulation (EU) No 288/2013. OJ L 217, 18.8.2015, p. 1–4.
Commission Implementing Regulation (EU) No 754/2014 of 11 July 2014 concerning the denial of authorisation of Pediococcus pentosaceus (NCIMB 30068) and Pediococcus pentosaceus (NCIMB 30044) as feed additives. OJ L 205, 12.7.2014, p. 10–11.
Commission Implementing Regulation (EU) No 81/2012 of 31 January 2012 concerning the denial of authorisation of Lactobacillus pentosus (DSM 14025) as a feed additive. OJ L 29, 1.2.2012, p. 36–37.
Dixhoorn, I. D., Reimert, I., Middelkoop, J., Bolhuis, J. E., Wisselink, H. J., Koerkamp, P. W. G.,… & Stockhofe‐Zurwieden, N. (2016). Enriched Housing Reduces Disease Susceptibility to Co‐Infection with Porcine Reproductive and Respiratory Virus (PRRSV) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) in Young Pigs. PLoS One, 11(9).
Stygar, A.H., Niemi, J.K., Oliviero, C., Laurila, T., Heinonen, M. (2016) Economic value of mitigating Actinobacillus pleuropneumoniae infections in pig fattening herds. Agricultural Systems 144: 113–121.
Postma, M., Stärk, K. D., Sjölund, M., Backhans, A., Beilage, E. G., Lösken, S.,… & Nielsen, E. O. (2015). Alternatives to the use of antimicrobial agents in pig production: A multicountry expert‐ranking of perceived effectiveness, feasibility and return on investment. Preventive veterinary medicine, 118(4), 457–466.
Maes, D., Chiers, K., Haesebrouck, F., Laevens, H., Verdonck, M., & De Kruif, A. (2001). Herd factors associated with the seroprevalences of Actinobacillus pleuropneumoniae serovars 2, 3 and 9 in slaughter pigs from farrow‐to‐finish pig herds. Veterinary research, 32(5), 409–419.
Postma, M., Vanderhaeghen, W., Sarrazin, S., Maes, D., & Dewulf, J. (2016). Reducing Antimicrobial Usage in Pig Production without Jeopardizing Production Parameters. Zoonoses and Public Health.
References
- Aanensen DM, Feil EJ, Holden MT, Dordel J, Yeats CA, Fedosejev A, Goater R, Castillo‐Ramírez S, Corander J and Colijn C, 2016. Whole‐genome sequencing for routine pathogen surveillance in public health: a population snapshot of invasive Staphylococcus aureus in Europe. MBio, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarestrup FM, 1999. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. International Journal of Antimicrobial Agents, 12, 279–285. [DOI] [PubMed] [Google Scholar]
- Aarestrup FM, 2000. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS, Acta Pathologica, Microbiologica et Immunologica Scandinavica, 108, 0–48. [PubMed] [Google Scholar]
- Aarestrup FM, Oliver Duran C and Burch DG, 2008. Antimicrobial resistance in swine production. Animal Health Research Reviews, 9, 135–148. [DOI] [PubMed] [Google Scholar]
- Aarestrup FM, Jensen VF, Emborg H‐D, Jacobsen E and Wegener HC, 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. American Journal of Veterinary Research, 71, 726–733. [DOI] [PubMed] [Google Scholar]
- Aarnink A, Schrama J, Heetkamp M, Stefanowska J and Huynh T, 2006. Temperature and body weight affect fouling of pig pens. Journal of Animal Science, 84, 2224–2231. [DOI] [PubMed] [Google Scholar]
- Aarts HJ, Guerra B and Malorny B, 2006. Molecular methods for detection of antibiotic resistance In: Aaarestrup FM. (ed.). Antimicrobial Resistance in Bacteria of Animal Origin, ASM Press, Washington, DC: pp. 37–48. [Google Scholar]
- Abudabos A, Al‐Batshan H and Murshed M, 2015. Effects of prebiotics and probiotics on the performance and bacterial colonization of broiler chickens. South African Journal of Animal Science, 45, 419–428. [Google Scholar]
- Abu‐Elala NM, Mohamed SH, Zaki MM and Eissa AE, 2015. Assessment of the immune‐modulatory and antimicrobial effects of dietary chitosan on Nile tilapia (Oreochrmis niloticus) with special emphasis to its bio‐remediating impacts. Fish and Shellfish Immunology, 46, 678–685. [DOI] [PubMed] [Google Scholar]
- Abu‐Shanab A and Quigley EM, 2009. Diagnosis of small intestinal bacterial overgrowth: the challenges persist!. Expert Review of Gastroenterology and Hepatology, 3, 77–87. [DOI] [PubMed] [Google Scholar]
- Acar J and Rostel B, 2001. Antimicrobial resistance: an overview. Revue Scientifique Et Technique De L Office International Des Epizooties, 20, 797–810. [DOI] [PubMed] [Google Scholar]
- Ackermann M and Engels M, 2006. Pro and contra IBR‐eradication. Veterinary Microbiology, 113, 293–302. [DOI] [PubMed] [Google Scholar]
- Adorian TJ, Goulart FR, Mombach PI, de Menezes Lovatto N, Dalcin M, Molinari M, Lazzari R and da Silva LP, 2016. Effect of different dietary fiber concentrates on the metabolism and indirect immune response in silver catfish. Animal Feed Science and Technology, 215, 124–132. [Google Scholar]
- Agarwal S and Marshall G, 2001. Stress effects on immunity and its application to clinical immunology. Clinical and Experimental Allergy, 31, 25–31. [PubMed] [Google Scholar]
- Agersø Y and Aarestrup FM, 2013. Voluntary ban on cephalosporin use in Danish pig production has effectively reduced extended‐spectrum cephalosporinase‐producing Escherichia coli in slaughter pigs. Journal of Antimicrobial Chemotherapy, 68, 569–572. [DOI] [PubMed] [Google Scholar]
- Alban L, Dahl J, Andreasen M, Petersen J and Sandberg M, 2013. Possible impact of the “yellow card” antimicrobial scheme on meat inspection lesions in Danish finisher pigs. Preventive Veterinary Medicine, 108, 334–341. [DOI] [PubMed] [Google Scholar]
- Alban L, Petersen J and Busch M, 2015. A comparison between lesions found during meat inspection of finishing pigs raised under organic/free‐range conditions and conventional, indoor conditions. Porcine Health Management, 1(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JAS, Liu Y, Song M, Lee JJ, Gaskins HR, Maddox CW, Osuna O and Pettigrew JE, 2013. Escherichia coli challenge and one type of smectite alter intestinal barrier of pigs. Journal of Animal Science and Biotechnology, 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond G and Monahan K, 2000. Water medication with antibiotics. Proceedings of the North Carolina Healthy Hogs Seminar.
- Alsamarai AM, Abbas HM and Atia QM, 2015. Nasal carriage of methicillin resistant Staph aureus in food provider in restaurant at Samara city. World Journal of Pharmacy and Pharmaceutical Sciences (WJPPS), 4, 50–58. [Google Scholar]
- Alustiza F, Bellingeri R, Picco N, Motta C, Grosso MC, Barbero CA, Acevedo DF and Vivas A, 2016. IgY against enterotoxigenic Escherichia coli administered by hydrogel‐carbon nanotubes composites to prevent neonatal diarrhoea in experimentally challenged piglets. Vaccine, 34, 3291–3297. [DOI] [PubMed] [Google Scholar]
- Amachawadi RG, Shelton NW, Shi X, Vinasco J, Dritz SS, Tokach MD, Nelssen JL, Scott HM and Nagaraja TG, 2011. Selection of fecal enterococci exhibiting tcrB‐mediated copper resistance in pigs fed diets supplemented with copper. Applied and Environmental Microbiology, 77, 5597–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amachawadi RG, Scott HM, Vinasco J, Tokach MD, Dritz SS, Nelssen JL and Nagaraja TG, 2015. Effects of in‐feed copper, chlortetracycline, and tylosin on the prevalence of transferable copper resistance gene, tcrB, among fecal enterococci of weaned piglets. Foodborne Pathogens and Disease, 12, 670–678. [DOI] [PubMed] [Google Scholar]
- Andres VM and Davies RH, 2015. Biosecurity measures to control salmonella and other infectious agents in pig farms: a review. Comprehensive Reviews in Food Science and Food Safety, 14, 317–335. [Google Scholar]
- Annett C, Viste J, Chirino‐Trejo M, Classen H, Middleton D and Simko E, 2002. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathology, 31, 598–601. [DOI] [PubMed] [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2013. Résapath – Réseau d’épidémiosurveillance de l'antibiorésistance des bactéries pathogènes animales. Available online: https://www.anses.fr/fr/system/files/LABO-Ra-Resapath2012_0.pdf
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2014. Assessment of the risks of emergence of antimicrobial resistance associated with modes of antibiotic use in the field of animal health. Available online: https://www.anses.fr/en/system/files/SANT2011sa0071RaEN.pdf
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2015a. Résapath – Réseau d’épidémiosurveillance de l'antibiorésistance des bactéries pathogènes animales. Available online: https://www.anses.fr/en/system/files/LABO-Ra-Resapath2014.pdf
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2015b. Sales survey of Veterinary Medicinal Products containing Antimicrobials in France – 2014. Available online: https://www.anses.fr/en/system/files/ANMV-Ra-Antibiotiques2014EN.pdf
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2016. RESAPATH – French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin. 2014 Annual Report. Available online: https://www.anses.fr/en/system/files/LABO-Ra-Resapath2014EN.pdf
- Anthony F, Acar J, Franklin A, Gupta R, Nicholls T, Tamura Y, Thompson S, Threlfall EJ, Vose D, van Vuuren M and White DG, 2001. Antimicrobial resistance: responsible and prudent use of antimicrobial agents in veterinary medicine. Revue Scientifique et Technique, 20, 829–839. [DOI] [PubMed] [Google Scholar]
- Anthony TR, Altmaier R, Park JH and Peters TM, 2014. Modeled effectiveness of ventilation with contaminant control devices on indoor air quality in a swine farrowing facility. Journal of Occupational and Environmental Hygiene, 11, 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SR and Straus SE, 2005. Interventions to improve antibiotic prescribing practices in ambulatory care. The Cochrane Library. [DOI] [PMC free article] [PubMed]
- Arnold CB, Serra P and Pique A, 2007. Laser direct‐write techniques for printing of complex materials. Mrs Bulletin, 32, 23–31. [Google Scholar]
- Atterbury RJ, Hobley L, Till R, Lambert C, Capeness MJ, Lerner TR, Fenton AK, Barrow P and Sockett RE, 2011. Effects of orally administered bdellovibrio bacteriovorus on the well‐being and salmonella colonization of young chicks. Applied and Environmental Microbiology, 77, 5794–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller T, Mosenthin R and Weiss E, 2015. Potential of cereal grains and grain legumes in modulating pigs’ intestinal microbiota ‐ a review. Livestock Science, 172, 16–32. [Google Scholar]
- Aviv G, Tsyba K, Steck N, Salmon‐Divon M, Cornelius A, Rahav G, Grassl GA and Gal‐Mor O, 2014. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994. [DOI] [PubMed] [Google Scholar]
- Awad E and Austin B, 2010. Use of lupin, Lupinus perennis, mango, Mangifera indica, and stinging nettle, Urtica dioica, as feed additives to prevent Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 33, 413–420. [DOI] [PubMed] [Google Scholar]
- Bae J, Wieland B, Sait M, Longbottom D, Smith D, Alarcon P and Wheelhouse N, 2013. Risk factors associated with Lawsonia intracellularis in English pig farms. The Veterinary Journal, 197, 707–711. [DOI] [PubMed] [Google Scholar]
- Baggott D, Casartelli A, Fraisse F, Manavella C, Marteau R, Rehbein S, Wiedemann M and Yoon S, 2011. Demonstration of the metaphylactic use of gamithromycin against bacterial pathogens associated with bovine respiratory disease in a multicentre farm trial. Veterinary Record, 168, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar AA and Ren D, 2013. Antimicrobial peptides. Pharmaceuticals, 6, 1543–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak H and Rathkjen PH, 2009. Reduced use of antimicrobials after vaccination of pigs against porcine proliferative enteropathy in a Danish SPF herd. Acta Veterinaria Scandinavica, 51, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A, Coombs G, Holden M, Lindsay J, Nimmo G, Tattevin P and Skov R, 2016. Genomic insights into the emergence and spread of international clones of healthcare‐, community‐and livestock‐associated meticillin‐resistant Staphylococcus aureus: Blurring of the traditional definitions. Journal of Global Antimicrobial Resistance, 6, 95–101. [DOI] [PubMed] [Google Scholar]
- Baloch AR, Zhang XY and Schade R, 2015. IgY Technology in aquaculture ‐ a review. Reviews in Aquaculture, 7, 153–160. [Google Scholar]
- Bampidis VA, Christodoulou V, Florou‐Paneri P and Christaki E, 2006. Effect of dried oregano leaves versus neomycin in treating newborn calves with colibacillosis. Journal of Veterinary Medicine Series A: Physiology Pathology Clinical Medicine, 53, 154–156. [DOI] [PubMed] [Google Scholar]
- Banhazi TM, Lehr H, Black J, Crabtree H, Schofield P, Tscharke M and Berckmans D, 2012. Precision livestock farming: an international review of scientific and commercial aspects. International Journal of Agricultural and Biological Engineering, 5, 1–9. [Google Scholar]
- Baquero F, 2011. The 2010 Garrod Lecture: the dimensions of evolution in antibiotic resistance: ex unibus plurum et ex pluribus unum. Journal of Antimicrobial Chemotherapy, 66, 1659–1672. [DOI] [PubMed] [Google Scholar]
- Barbosa C, Venail P, Holguin AV and Vives MJ, 2013. Co‐evolutionary dynamics of the bacteria Vibrio sp. CV1 and phages V1G, V1P1, and V1P2: implications for phage therapy. Microbial Ecology, 66, 897–905. [DOI] [PubMed] [Google Scholar]
- Barbut F, Mario N, Delmée M and Gozian J, 1993. Genomic fingerprinting of Clostridium difficile isolates by using a random amplified polymorphic DNA (RAPD) assay. FEMS Microbiology Letters, 114, 161–166. [DOI] [PubMed] [Google Scholar]
- Barnum D and Newbould F, 1961. The use of the California mastitis test for the detection of bovine mastitis. The Canadian Veterinary Journal, 2, 83. [PMC free article] [PubMed] [Google Scholar]
- Barrett DJ, More SJ, Graham DA, O'Flaherty J, Doherty ML and Gunn HM, 2011. Considerations on BVD eradication for the Irish livestock industry. Irish Veterinary Journal, 64, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P and Neto OF, 2011. Pullorum disease and fowl typhoid—new thoughts on old diseases: a review. Avian Pathology, 40, 1–13. [DOI] [PubMed] [Google Scholar]
- Batchelor M, Hopkins KL, Liebana E, Slickers P, Ehricht R, Mafura M, Aarestrup F, Mevius D, Clifton‐Hadley FA, Woodward MJ, Davies RH, Threlfall EJ and Anjum MF, 2008. Development of a miniaturised microarray‐based assay for the rapid identification of antimicrobial resistance genes in Gram‐negative bacteria. International Journal of Antimicrobial Agents, 31, 440–451. [DOI] [PubMed] [Google Scholar]
- Bates J, Jordens Z and Selkon J, 1993. Evidence for an animal origin of vancomycin‐resistant enterococci. The Lancet, 342, 490–491. [DOI] [PubMed] [Google Scholar]
- Batista C, Coelho S, Rabelo E, Lana A, Carvalho A, Reis R and Saturnino H, 2008. Performance and health of calves fed milk without antimicrobials residue or milk from mastitis treated cows with or without probiotic. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 60, 185–191. [Google Scholar]
- Beattie V, Walker N and Sneddon I, 1995. Effects of environmental enrichment on behaviour and productivity of growing pigs. Animal Welfare, 4, 207–220. [Google Scholar]
- Beattie V, Walker N and Sneddon I, 1996. An investigation of the effect of environmental enrichment and space allowance on the behaviour and production of growing pigs. Applied Animal Behaviour Science, 48, 151–158. [Google Scholar]
- Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, Bethe A, Semmler T, Tedin K and Schierack P, 2013. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi‐resistant Escherichia coli in vivo. International Journal of Medical Microbiology, 303, 396–403. [DOI] [PubMed] [Google Scholar]
- Beemer F, Velzen G, Berg C, Zunderdorp M, Lambrechts E, Gier K and Oud N 2010. What would be the effects of decoupling the prescription and sale of veterinary medicines by veterinarians? (Berenschot report). Available online: http://www.fve.org/uploads/publications/docs/berenschot%20report_02_2010.pdf
- BelVetSac (Belgian Veterinary Surveillance of Antimicrobial Consumption), 2015. National consumption report 2014. Available online: http://www.belvetsac.ugent.be/pages/home/BelvetSAC_report_2014%20finaal.pdf
- Bennedsgaard TW, Thamsborg SM, Aarestrup FM, Enevoldsen C, Vaarst M and Christoffersen AB, 2006. Resistance to penicillin of Staphylococcus aureus isolates from cows with high somatic cell counts in organic and conventional dairy herds in Denmark. Acta Veterinaria Scandinavica, 48, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertulat S, Fischer‐Tenhagen C and Heuwieser W, 2015. A survey of drying‐off practices on commercial dairy farms in northern Germany and a comparison to science‐based recommendations. Veterinary Record Open, 2, e000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beski SSM, Swick RA and Iji PA, 2015. Subsequent growth performance and digestive physiology of broilers fed on starter diets containing spray‐dried porcine plasma as a substitute for meat meal. British Poultry Science, 56, 559–568. [DOI] [PubMed] [Google Scholar]
- Bessei W, 2006. Welfare of broilers: a review. Worlds Poultry Science Journal, 62, 455–466. [Google Scholar]
- Bich Hang BT, Phuong NT and Kestemont P, 2014. Can immunostimulants efficiently replace antibiotic in striped catfish (Pangasianodon hypophthalmus) against bacterial infection by Edwardsiella ictaluri? Fish and Shellfish Immunology, 40, 556–562. [DOI] [PubMed] [Google Scholar]
- Biggs A, Barrett D, Bradley A, Green M, Reyher K and Zadoks R, 2016. Antibiotic dry cow therapy: where next? Veterinary Record, 178, 93–94. [DOI] [PubMed] [Google Scholar]
- Bilkei G, Bille G, Bilkei V and Bilkei M, 2011a. Influence of phytogenic feed additives on production and mortality of pigs ‐ Part I: prophylactic effect of oregano in a pig fatting unit. Tierarztliche Umschau, 66, 157–162. [Google Scholar]
- Bilkei G, Bille G, Bilkei V and Bilkei M, 2011b. Influence of phytogenic feed additives on production and mortality of pigs ‐ Part II: effect of garlic (Allium sativum), horseradish (Aromatica rusticana) and doxycycline in prevention of postparturient diseases of the sows and pre‐ and postweaning mortality in piglets. Tierarztliche Umschau, 66, 253–257. [Google Scholar]
- Bishop S, 2015. Genetic resistance to infections in sheep. Veterinary Microbiology, 181, 2–7. [DOI] [PubMed] [Google Scholar]
- Blanco‐Penedo I, López‐Alonso M, Shore R, Miranda M, Castillo C, Hernández J and Benedito J, 2012. Evaluation of organic, conventional and intensive beef farm systems: health, management and animal production. Animal, 6, 1503–1511. [DOI] [PubMed] [Google Scholar]
- Boerlin P, Wissing A, Aarestrup FM, Frey J and Nicolet J, 2001. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in enterococci from pigs. Journal of Clinical Microbiology, 39, 4193–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm F and Kromker V, 2016. Current aspects of teat disinfection with special focus on premilking teat disinfection. Der Praktische Tierarzt, 97, 147–160. [Google Scholar]
- Bombyk RA, Bykowski AL, Draper CE, Savelkoul EJ, Sullivan LR and Wyckoff TJ, 2008. Comparison of types and antimicrobial susceptibility of Staphylococcus from conventional and organic dairies in west‐central Minnesota, USA. Journal of Applied Microbiology, 104, 1726–1731. [DOI] [PubMed] [Google Scholar]
- Bordin M, D'Atri F, Guillemot L and Citi S, 2004. Histone deacetylase inhibitors up‐regulate the expression of tight junction proteins. Molecular Cancer Research, 2, 692–701. [PubMed] [Google Scholar]
- Bos ME, Taverne FJ, van Geijlswijk IM, Mouton JW, Mevius DJ and Heederik DJ, 2013. Consumption of antimicrobials in pigs, veal calves, and broilers in the Netherlands: quantitative results of nationwide collection of data in 2011. PLoS ONE, 8, e77525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos ME, Mevius DJ, Wagenaar JA, van Geijlswijk IM, Mouton JW and Heederik DJ, 2015. Antimicrobial prescription patterns of veterinarians: introduction of a benchmarking approach. Journal of Antimicrobial Chemotherapy, dkv104. [DOI] [PubMed] [Google Scholar]
- Boutet P, Detilleux J, Motkin M, Deliège M, Piraux E, Depinois A, Debliquy P, Mainil J, Czaplicki G and Lekeux P, 2005. Comparaison du taux cellulaire et de la sensibilité antimicrobienne des germes responsables de mammite subclinique bovine entre les filières conventionnelles et biologique. Annales de Médecine Vétérinaire, 149, 173–182. [Google Scholar]
- BPC (British Poultry Council), 2016. Leading the way in the responsible use of antibiotics ‐ The BPC antibiotic stewardship scheme. Available online: http://www.britishpoultry.org.uk/wp-content/uploads/2016/04/The_BPC_Antibiotic_Stewardship_Scheme_April2016.pdf
- Bracke MBM and Spoolder HAM, 2011. Review of wallowing in pigs: implications for animal welfare. Animal Welfare, 20, 347–363. [Google Scholar]
- Bradley A, Breen J, Payne B, White V and Green M, 2015. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. Journal of Dairy Science, 98, 1706–1720. [DOI] [PubMed] [Google Scholar]
- Bradshaw RH, Parrott RF, Forsling ML, Goode JA, Lloyd DM, Rodway RG and Broom DM, 1996. Stress and travel sickness in pigs: effects of road transport on plasma concentrations of cortisol, beta‐endorphin and lysine vasopressin. Animal Science, 63, 507–516. [Google Scholar]
- Broens EM, Cleef BAGLv, Graat EAM and Kluytmans JAJW, 2008. Transmission of methicillin‐resistant Staphylococcus aureus from food production animals to humans: a review. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 3, 1–12. [Google Scholar]
- Broom D, 2000. Welfare assessment and welfare problem areas during handling and transport. Livestock Handling and Transport, 2, 43–61. [Google Scholar]
- Broom LJ, 2015. Organic acids for improving intestinal health of poultry. Worlds Poultry Science Journal, 71, 630–642. [Google Scholar]
- Brownlie J, 1990. The pathogenesis of bovine virus. Rev. sci. tech. Off. int Epiz, 9, 43–59. [DOI] [PubMed] [Google Scholar]
- Brscic M, Heutinck LFM, Wolthuis‐Fillerup M, Stockhofe N, Engel B, Visser EK, Gottardo F, Bokkers EAM, Lensink BJ, Cozzi G and van Reenen CG, 2011. Prevalence of gastrointestinal disorders recorded at postmortem inspection in white veal calves and associated risk factors. Journal of Dairy Science, 94, 853–863. [DOI] [PubMed] [Google Scholar]
- Bulfon C, Volpatti D and Galeotti M, 2015. Current research on the use of plant‐derived products in farmed fish. Aquaculture Research, 46, 513–551. [Google Scholar]
- Bunner CA, Norby B, Bartlett PC, Erskine RJ, Downes FP and Kaneene JB, 2007. Prevalence and pattern of antimicrobial susceptibility in Escherichia coli isolated from pigs reared under anti microbial‐free and conventional production methods. Javma‐Journal of the American Veterinary Medical Association, 231, 275–283. [DOI] [PubMed] [Google Scholar]
- Burbank DR, Shah DH, LaPatra SE, Fornshell G and Cain KD, 2011. Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus mykiss). Aquaculture, 321, 185–190. [Google Scholar]
- Burckhardt I and Zimmermann S, 2011. Using matrix‐assisted laser desorption ionization‐time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. Journal of Clinical Microbiology, 49, 3321–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey TE, Dritz SS, Nietfeld JC, Johnson BJ and Minton JE, 2004. Effect of dietary mannanoligosaccharide and sodium chlorate on the growth performance, acute‐phase response, and bacterial shedding of weaned pigs challenged with Salmonella enterica serotype Typhimurium. Journal of Animal Science, 82, 397–404. [DOI] [PubMed] [Google Scholar]
- Burow E and Käsbohrer A, 2016. Risk factors for antimicrobial resistance in Escherichia Coli in pigs receiving oral antimicrobial treatment: a systematic review. Microbial Drug Resistance, online ahead of print. [DOI] [PubMed] [Google Scholar]
- Burow E, Simoneit C, Tenhagen B‐A and Käsbohrer A, 2014. Oral antimicrobials increase antimicrobial resistance in porcine E. coli–a systematic review. Preventive Veterinary Medicine, 113, 364–375. [DOI] [PubMed] [Google Scholar]
- Burridge L, Weis JS, Cabello F, Pizarro J and Bostick K, 2010. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture, 306, 7–23. [Google Scholar]
- Burt S, 2004. Essential oils: their antibacterial properties and potential applications in foods ‐ a review. International Journal of Food Microbiology, 94, 223–253. [DOI] [PubMed] [Google Scholar]
- Cabello FC, Godfrey HP, Buschmann AH and Dölz HJ, 2016. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. The Lancet Infectious Diseases, 16, E127–E133. [DOI] [PubMed] [Google Scholar]
- Cagienard A, Regula G and Danuser J, 2005. The impact of different housing systems on health and welfare of grower and finisher pigs in Switzerland. Preventive Veterinary Medicine, 68, 49–61. [DOI] [PubMed] [Google Scholar]
- Callens B, Persoons D, Maes D, Laanen M, Postma M, Boyen F, Haesebrouck F, Butaye P, Catry B and Dewulf J, 2012. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Preventive Veterinary Medicine, 106, 53–62. [DOI] [PubMed] [Google Scholar]
- Caly DL, D'Inca R, Auclair E and Drider D, 2015. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Frontiers in Microbiology, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JR, 2004. Effect of bovine viral diarrhea virus in the feedlot. Veterinary Clinics of North America: Food Animal Practice, 20, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J, Cristino L, Peixe L and Antunes P, 2016. MCR‐1 in multidrug‐resistant and copper‐tolerant clinically relevant Salmonella 1, 4,[5], 12: i:‐and S. Rissen clones in Portugal, 2011 to 2015. Eurosurveillance, 21, 2–6. [DOI] [PubMed] [Google Scholar]
- Cannon WB, 1914. The emergency function of the adrenal medulla in pain and the major emotions. American Journal of Physiology‐Legacy Content, 33, 356–372. [Google Scholar]
- Cao L, Yang XJ, Li ZJ, Sun FF, Wu XH and Yao JH, 2012. Reduced lesions in chickens with Clostridium perfringens‐induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poultry Science, 91, 3065–3071. [DOI] [PubMed] [Google Scholar]
- Cao MD, Ganesamoorthy D, Elliott AG, Zhang HH, Cooper MA and Coin LJM, 2016. Streaming algorithms for identification of pathogens and antibiotic resistance potential from real‐time MinION (TM) sequencing. Gigascience, 5, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A, 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrobial Agents and Chemotherapy, 53, 2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson U, Wallgren P, Renström L, Lindberg A, Eriksson H, Thoren P, Eliasson‐Selling L, Lundeheim N, Nörregard E and Thörn C, 2009. Emergence of porcine reproductive and respiratory syndrome in Sweden: detection, response and eradication. Transboundary and Emerging Diseases, 56, 121–131. [DOI] [PubMed] [Google Scholar]
- Casewell M, Friis C, Marco E, McMullin P and Phillips I, 2003. The European ban on growth‐promoting antibiotics and emerging consequences for human and animal health. Journal of Antimicrobial Chemotherapy, 52, 159–161. [DOI] [PubMed] [Google Scholar]
- Castro‐Sánchez E, Drumright LN, Gharbi M, Farrell S and Holmes AH, 2016. Mapping antimicrobial stewardship in undergraduate medical, dental, pharmacy, nursing and veterinary education in the United Kingdom. PLoS ONE, 11, e0150056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catry B and Threlfall J, 2009. Critically important antimicrobial—or not? Clinical Infectious Diseases, 49, 1961–1962. [DOI] [PubMed] [Google Scholar]
- Catry B, Van Duijkeren E, Pomba MC, Greko C, Moreno MA, Pyorala S, Ruzauskas M, Sanders P, Threlfall EJ, Ungemach F, Torneke K, Munoz‐Madero C and Torren‐Edo J, 2010. Reflection paper on MRSA in food‐producing and companion animals: epidemiology and control options for human and animal health. Epidemiology and Infection, 138, 626–644. [DOI] [PubMed] [Google Scholar]
- Catry B, Dewulf J, Maes D, Pardon B, Callens B, Vanrobaeys M, Opsomer G, de Kruif A and Haesebrouck F, 2016. Effect of antimicrobial consumption and production type on antibacterial resistance in the bovine respiratory and digestive tract. PLoS ONE, 11, e0146488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik E and Ünver A, 2015. Isolation and identification of arcobacter spp. by multiplex PCR from water sources in Kars region. Current Microbiology, 71, 546–550. [DOI] [PubMed] [Google Scholar]
- Chai WD, Zakrzewski SS, Gunzel D, Pieper R, Wang ZY, Twardziok S, Janczyk P, Osterrieder N and Burwinkel M, 2014. High‐dose dietary zinc oxide mitigates infection with transmissible gastroenteritis virus in piglets. BMC Veterinary Research, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty SB and Hancz C, 2011. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Reviews in Aquaculture, 3, 103–119. [Google Scholar]
- Chang H, Liu H, Guo Z, Du J, Zhao J, Chen L, Yang X, Wang X, Yao H and Wang C, 2014. Investigation of etiology of massive infection with porcine pseudorabies virus in Henan and neighboring Provinces. Chinese Journal of Virology, 30, 441–449. [PubMed] [Google Scholar]
- Chantziaras I, Boyen F, Callens B and Dewulf J, 2014. Correlation between veterinary antimicrobial use and antimicrobial resistance in food‐producing animals: a report on seven countries. Journal of Antimicrobial Chemotherapy, 69, 827–834. [DOI] [PubMed] [Google Scholar]
- Chauvin C, Madec F, Guillemot D and Sanders P, 2001. The crucial question of standardisation when measuring drug consumption. Veterinary Research, 32, 533–543. [DOI] [PubMed] [Google Scholar]
- Checkley SL, Campbell JR, Chirino‐Trejo M, Janzen ED and Waldner CL, 2010. Associations between antimicrobial use and the prevalence of antimicrobial resistance in fecal Escherichia coli from feedlot cattle in western Canada. The Canadian Veterinary Journal, 51, 853. [PMC free article] [PubMed] [Google Scholar]
- Chi J, Weersink A, VanLeeuwen JA and Keefe GP, 2002. The economics of controlling infectious diseases on dairy farms. Canadian Journal of Agricultural Economics/Revue canadienne d'agroeconomie, 50, 237–256. [Google Scholar]
- Cho S, Fossler CP, Diez‐Gonzalez F, Wells SJ, Hedberg CW, Kaneene JB, Ruegg PL, Warnick LD and Bender JB, 2007. Antimicrobial susceptibility of Shiga toxin‐producing Escherichia coli isolated from organic dairy farms, conventional dairy farms, and county fairs in Minnesota. Foodborne Pathogens and Disease, 4, 178–186. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Sandberg M, Themudo G and Ersbøll A, 2012. Risk factors for Campylobacter infection in Danish broiler chickens. Poultry Science, 91, 2701–2709. [DOI] [PubMed] [Google Scholar]
- Cicconi‐Hogan KM, Belomestnykh N, Gamroth M, Ruegg PL, Tikofsky L and Schukken YH, 2014. Short communication: prevalence of methicillin resistance in coagulase‐negative staphylococci and Staphylococcus aureus isolated from bulk milk on organic and conventional dairy farms in the United States. Journal of Dairy Science, 97, 2959–2964. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius , 2016. Joint FAO/WHO Food Standards Programme ‐ 71st Session FAO Headquarters, Rome, Italy, 20‐23 June 2016. CODEX work on antimicrobial resistance. CX/EXEC 16/71/3 April 2016.
- Cohen Stuart J, van den Munckhof T, Voets G, Scharringa J, Fluit A and Hall ML, 2012. Comparison of ESBL contamination in organic and conventional retail chicken meat. International Journal of Food Microbiology, 154, 212–214. [DOI] [PubMed] [Google Scholar]
- Cohn LA and Middleton JR, 2010. A veterinary perspective on methicillin‐resistant staphylococci. Journal of Veterinary Emergency and Critical Care, 20, 31–45. [DOI] [PubMed] [Google Scholar]
- Cole D, Todd L and Wing S, 2000. Concentrated swine feeding operations and public health: a review of occupational and community health effects. Environmental Health Perspectives, 108, 685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado L, Inza I, Guarro J and Figueras MJ, 2008. Presence of Arcobacter spp. in environmental waters correlates with high levels of fecal pollution. Environmental Microbiology, 10, 1635–1640. [DOI] [PubMed] [Google Scholar]
- Collignon P, Powers JH, Chiller TM, Aidara‐Kane A and Aarestrup FM, 2009. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clinical Infectious Diseases, 49, 132–141. [DOI] [PubMed] [Google Scholar]
- Collignon P, Aarestrup FM, Irwin R and McEwen S, 2013. Human deaths and third‐generation cephalosporin use in poultry, Europe. Emerging Infectious Diseases, 19, 1339–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NB and Nordlund KV, 2009. The influence of the environment on dairy cow behavior, claw health and herd lameness dynamics. The Veterinary Journal, 179, 360–369. [DOI] [PubMed] [Google Scholar]
- Cooper CA, Garas Klobas LC, Maga EA and Murray JD, 2013. Consuming transgenic goats’ milk containing the antimicrobial protein lysozyme helps resolve diarrhea in young pigs. PLoS ONE, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota I, Sánchez‐Romero MA, Hernández SB, Pucciarelli MG, García‐del Portillo F and Casadesús J, 2015. Epigenetic control of Salmonella enterica O‐antigen chain length: a tradeoff between virulence and bacteriophage resistance. PLoS Genetics, 11, e1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne L, Pinchbeck G, Williams N, Smith R, Dawson S, Pearson R and Latham S, 2014. Understanding antimicrobial use and prescribing behaviours by pig veterinary surgeons and farmers: a qualitative study. Veterinary Record, 175, 593. [DOI] [PubMed] [Google Scholar]
- Coyne L, Latham S, Williams N, Dawson S, Donald I, Pearson R, Smith R and Pinchbeck G, 2016. Understanding the culture of antimicrobial prescribing in agriculture: a qualitative study of UK pig veterinary surgeons. Journal of Antimicrobial Chemotherapy, 71, 3300–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi G, Brscic M and Gottardo F, 2009. Main critical factors affecting the welfare of beef cattle and veal calves raised under intensive rearing systems in Italy: a review. Italian Journal of Animal Science, 8, 67–80. [Google Scholar]
- Craven SE, Stern NJ, Cox NA, Bailey JS and Berrang M, 1999. Cecal carriage of Clostridium perfringens in broiler chickens given Mucosal Starter Culture (TM). Avian Diseases, 43, 484–490. [PubMed] [Google Scholar]
- Cruz‐Papa DMAD, Candare CMG, Cometa GLS, Gudez D and Guevara A, 2014. Aeromonas hydrophila bacteriophage UP87: an alternative to antibiotic treatment for motile aeromonas septicemia in Nile Tilapia (Oreochromis niloticus). Philippine Agricultural Scientist, 97, 96–101. [Google Scholar]
- Cui S, Ge B, Zheng J and Meng J, 2005. Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Applied and Environment Microbiology, 71, 4108–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JM, Guthrie D, Hutcheson DP, Krakowka S and Rosenquist BD, 1999. Natural human interferon‐alpha administered orally as a treatment of bovine respiratory disease complex. Journal of Interferon and Cytokine Research, 19, 907–910. [DOI] [PubMed] [Google Scholar]
- D'Eath RB, Tolkamp BJ, Kyriazakis I and Lawrence AB, 2009. ‘Freedom from hunger’ and preventing obesity: the animal welfare implications of reducing food quantity or quality. Animal Behaviour, 77, 275–288. [Google Scholar]
- da Silva CA, Bridi AM, Castro‐Gomez RJH, da Silva CRB, Menegucci CG and de Carvalho BB, 2007. Probiotic and antibiotics on swine feeding during nursery phase. Semina: Ciências Agrárias, 28, 739–746. [Google Scholar]
- Dalmasso G, Nguyen HTT, Yan YT, Charrier‐Hisamuddin L, Sitaraman SV and Merlin D, 2008. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS ONE, 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2007. DANMAP 2006 – use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2009a. DANMAP 2008 ‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org. 1600‐2032
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2009b. DANMAP 2009 ‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2011. DANMAP 2010‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2014. DANMAP 2013 ‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2015. DANMAP 2014 ‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme), 2016. DANMAP 2015 ‐ use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032. Available online: http://www.danmap.org
- Dargatz DA, Marshall KL, Fedorka‐Cray PJ, Erdman MM and Kopral CA, 2015. Salmonella prevalence and antimicrobial susceptibility from the National Animal Health Monitoring System Sheep 2011 Study. Foodborne Pathogens and Disease, 12, 953–957. [DOI] [PubMed] [Google Scholar]
- Davies G, Genini S, Bishop S and Giuffra E, 2009. An assessment of opportunities to dissect host genetic variation in resistance to infectious diseases in livestock. Animal, 3, 415–436. [DOI] [PubMed] [Google Scholar]
- De Briyne N, Atkinson J, Pokludova L, Borriello SP and Price S, 2013. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Veterinary Record, 173, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Briyne N, Atkinson J, Pokludová L and Borriello S, 2014. Paper: antibiotics used most commonly to treat animals in Europe. Veterinary Record, 175, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong A, Thomas V, Klein U, Marion H, Moyaert H, Simjee S and Vallé M, 2013a. Pan‐European resistance monitoring programmes encompassing food‐borne bacteria and target pathogens of food‐producing and companion animals. International Journal of Antimicrobial Agents, 41, 403–409. [DOI] [PubMed] [Google Scholar]
- de Jong IC, Bondt N, Ge L, Puister L, van den Heuvel H and Lourens A, 2013b. Reduction in antibiotic usage in broilers: side effects and best practices. Report 678. Wageningen UR Livestock Research.
- de Jong A, Thomas V, Simjee S, Moyaert H, El Garch F, Maher K, Morrissey I, Butty P, Klein U and Marion H, 2014. Antimicrobial susceptibility monitoring of respiratory tract pathogens isolated from diseased cattle and pigs across Europe: the VetPath study. Veterinary Microbiology, 172, 202–215. [DOI] [PubMed] [Google Scholar]
- De Jonge FH, Bokkers E, Schouten W and Helmond F, 1996. Rearing piglets in a poor environment: developmental aspects of social stress in pigs. Physiology and Behavior, 60, 389–396. [DOI] [PubMed] [Google Scholar]
- De Lange C, Pluske J, Gong J and Nyachoti C, 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livestock Science, 134, 124–134. [Google Scholar]
- DeDonder K, Apley M, Li M, Gehring R, Harhay D, Lubbers B, White B, Capik S, KuKanich B, Riviere J and Tessman RK, 2016. Pharmacokinetics and pharmacodynamics of gamithromycin in pulmonary epithelial lining fluid in naturally occurring bovine respiratory disease in multisource commingled feedlot cattle. Journal of Veterinary Pharmacology and Therapeutics, 39, 157–166. [DOI] [PubMed] [Google Scholar]
- Dewaele I, Rasschaert G, Wildemauwe C, Van Meirhaeghe H, Vanrobaeys M, De Graef E, Herman L, Ducatelle R, Heyndrickx M and De Reu K, 2012. Polyphasic characterization of Salmonella Enteritidis isolates on persistently contaminated layer farms during the implementation of a national control program with obligatory vaccination: a longitudinal study. Poultry Science, 91, 2727–2735. [DOI] [PubMed] [Google Scholar]
- Diarra MS and Malouin F, 2014. Antibiotics in Canadian poultry productions and anticipated alternatives. Frontiers in Microbiology, 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J and Richards J, 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poultry Science, 84, 634–643. [DOI] [PubMed] [Google Scholar]
- Dierikx CM, Hengeveld PD, Veldman KT, de Haan A, van der Voorde S, Dop PY, Bosch T and van Duijkeren E, 2016. Ten years later: still a high prevalence of MRSA in slaughter pigs despite a significant reduction in antimicrobial usage in pigs the Netherlands. Journal of Antimicrobial Chemotherapy, 71, 2414–2418. [DOI] [PubMed] [Google Scholar]
- Dieste‐Pérez L, Frankena K, Blasco J, Muñoz P and de Jong M, 2016. Efficacy of antibiotic treatment and test‐based culling strategies for eradicating brucellosis in commercial swine herds. Preventive Veterinary Medicine, 126, 105–110. [DOI] [PubMed] [Google Scholar]
- DiGiandomenico A and Sellman BR, 2015. Antibacterial monoclonal antibodies: the next generation? Current Opinion in Microbiology, 27, 78–85. [DOI] [PubMed] [Google Scholar]
- Dingle TC and Butler‐Wu SM, 2013. MALDI‐TOF mass spectrometry for microorganism identification. Clinics in Laboratory Medicine, 33, 589–609. [DOI] [PubMed] [Google Scholar]
- Diraviyam T, Zhao B, Wang Y, Schade R, Michael A and Zhang X, 2014. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta‐analysis. PLoS ONE, 9, e97716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dischinger J, Basi Chipalu S and Bierbaum G, 2014. Lantibiotics: promising candidates for future applications in health care. International Journal of Medical Microbiology, 304, 51–62. [DOI] [PubMed] [Google Scholar]
- Doeschl‐Wilson AB, Bishop SC, Kyriazakis I and Villanueva B, 2012. Novel methods for quantifying individual host response to infectious pathogens for genetic analyses. Should we aim for genetic improvement in host resistance or tolerance to infectious disease?, 18. [DOI] [PMC free article] [PubMed]
- Dohms JE and Metz A, 1991. Stress‐mechanisms of immunosuppression. Veterinary Immunology and Immunopathology, 30, 89–109. [DOI] [PubMed] [Google Scholar]
- Domer DA, Erickson RL, Petty JM, Bergdall VK and Hickman‐Davis JM, 2012. Processing and treatment of corncob bedding affects cage‐change frequency for C57BL/6 mice. Journal of the American Association for Laboratory Animal Science, 51, 162–169. [PMC free article] [PubMed] [Google Scholar]
- Dorado‐García A, Dohmen W, Bos ME, Verstappen KM, Houben M, Wagenaar JA and Heederik DJ, 2015a. Dose‐response relationship between antimicrobial drugs and livestock‐associated MRSA in pig farming. Emerging Infectious Diseases, 21, 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado‐García A, Graveland H, Bos ME, Verstappen KM, Van Cleef BA, Kluytmans JA, Wagenaar JA and Heederik DJ, 2015b. Effects of reducing antimicrobial use and applying a cleaning and disinfection program in veal calf farming: experiences from an intervention study to control livestock‐associated MRSA. PLoS ONE, 10, e0135826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle WR, 1998. The principles of disease elimination and eradication. Bulletin of the World Health Organization, 76, 22. [PMC free article] [PubMed] [Google Scholar]
- du Marchie Sarvaas C, 2015. Investment in new veterinary antibiotics: barriers, consequences and solutions. International Animal Health Journal, 2, 26–28. [Google Scholar]
- Dumas S, French H, Lavergne S, Ramirez C, Brown L, Bromfield C, Garrett E, French D and Aldridge B, 2016. Judicious use of prophylactic antimicrobials to reduce abdominal surgical site infections in periparturient cows: part 1‐a risk factor review. Veterinary Record, 178, 654. [DOI] [PubMed] [Google Scholar]
- Dunlop RH, McEwen SA, Meek AH, Clarke RC, Black WD and Friendship RM, 1998. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow‐to‐finish farms in Ontario, Canada. Preventive Veterinary Medicine, 34, 283–305. [DOI] [PubMed] [Google Scholar]
- Dupont N, Fertner M, Kristensen CS, Toft N and Stege H, 2016. Reporting the national antimicrobial consumption in Danish pigs: influence of assigned daily dosage values and population measurement. Acta Veterinaria Scandinavica, 58, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch Ministry of Economic Affairs , 2014. Reduced and Responsible: use of antibiotics in food‐producing animals in the Netherlands.
- Dutil L, Irwin R, Finley R, Ng LK, Avery B, Boerlin P, Bourgault AM, Cole L, Daignault D, Desruisseau A, Demczuk W, Hoang L, Horsman GB, Ismail J, Jamieson F, Maki A, Pacagnella A and Pillai DR, 2010. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerging Infectious Diseases, 16, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC (European Commission), 2010. Evaluation of the EU Legislative Framework in the Field of Medicated Feed. Framework Contract for evaluation and evaluation related services ‐ Lot 3: Food Chain (awarded through tender no 2004/S 243‐208899) Final report. Available online: http://ec.europa.eu/food/safety/docs/animal-feed-medic-medicated_feed_report_20100224.pdf
- ECDC (European Centre for Disease Prevention and Control), 2016. Rapid Risk Assessment ‐ Plasmid‐mediated colistin resistance in Enterobacteriaceae. Available online: http://ecdc.europa.eu/en/publications/Publications/enterobacteriaceae-risk-assessment-diseases-caused-by-antimicrobial-resistant-microorganisms-europe-june-2016.pdf
- ECDC and EFSA (European Centre for Disease Prevention and Control, European Food Safety Authority), 2011. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in the European Union in 2009. EFSA Journal, 9(7), 2154. [Google Scholar]
- ECDC and EFSA (European Centre for Disease Prevention and Control, European Food Safety Authority), 2015. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA Journal, 13(2), 4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC and EFSA (European Centre for Disease Prevention and Control, European Food Safety Authority), 2016. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA Journal, 14(2), 4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC, EFSA, EMA and SCENIHR (European Centre for Disease Prevention and Control, European Food Safety Authority, European Medicines Agency, Scientific Committee on Emerging and Newly Identified Health Risks), 2009. Joint Opinion on antimicrobial resistance (AMR) focused on zoonotic infections.
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control, European Food Safety Authority, European Medicines Agency), 2015. First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals (JIACRA). EFSA Journal, 13(1), 4006. [Google Scholar]
- ECDC, EFSA and EMEA (European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency), 2009. Joint scientific report of ECDC, EFSA and EMEA on meticillin resistant Staphylococcus aureus (MRSA) in livestock, companion animals and food. EFSA‐Q‐2009‐00612 ((EFSA Scientific Report 2009) 301, 1‐10) and EMEA/CVMP/SAGAM/62464/2009
- Edel W, 1994. Salmonella enteritidis eradication programme in poultry breeder flocks in The Netherlands. International Journal of Food Microbiology, 21, 171–178. [DOI] [PubMed] [Google Scholar]
- Edwards T, 2010. Control methods for bovine respiratory disease for feedlot cattle. Veterinary Clinics of North America: Food Animal Practice, 26, 273–284. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Technical specifications for the analysis and reporting of data on antimicrobial resistance (AMR) in the European Union Summary Report. EFSA Journal 2012;10(2):2587, 53 pp. doi: 10.2903/j.efsa.2012.2587 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2011. Scientific Opinion concerning the welfare of animals during transport. EFSA Journal 2011;9(1):1966, 125 pp. doi: 10.2903/j.efsa.2011.1966. [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2012a. Statement on the use of animal‐based measures to assess the welfare of animals. EFSA Journal 2012;10(6):2767, 29 pp. doi: 10.2903/j.efsa.2012.2767 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2012b. Scientific Opinion on the use of animal‐based measures to assess welfare in pigs. EFSA Journal 2012;10(1):2512, 85 pp. doi: 10.2903/j.efsa.2012.2512 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum β‐lactamases and/or AmpC β‐lactamases in food and food‐producing animals. EFSA Journal 2011;9(8):2322, 95 pp. doi: 10.2903/j.efsa.2011.2322 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on Carbapenem resistance in food animal ecosystems. EFSA Journal 2013;11(12):3501, 70 pp. doi: 10.2903/j.efsa.2013.3501 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez‐Escamez PS, Girones R, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Threlfall J, Wahlström H, Bengtsson B, Bouchard D, Randall L, Tenhagen BA, Ter Kuile B, Verdon John Wallace E, Brozzi R, Guerra B, Liebana E, Stella P and Herman L, 2017. Scientific Opinion on the risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA Journal 2017;15(1):4665, 107 pp. doi: 10.2903/j.efsa.2017.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Scientific Opinion on the safety and efficacy of the product Cylactin® (Enterococcus faecium) as a feed additive for chickens for fattening. EFSA Journal 2010;8(7):1661, 13 pp. doi: 10.2903/j.efsa.2010.1661 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Scientific Opinion on the safety and efficacy of Lactobacillus pentosus (DSM 14025) as a silage additive for all animal species. EFSA Journal 2011;9(11):2449, 8 pp. doi: 10.2903/j.efsa.2011.2449 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. doi: 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Scientific Opinion on the safety and efficacy of copper compounds (E4) as feed additives for all animal species: cupric sulphate pentahydrate based on a dossier submitted by Manica S.p.A. EFSA Journal 2012;10(12):2969, 38 pp. doi: 10.2903/j.efsa.2012.2969 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Scientific Opinion on Toyocerin® (Bacillus cereus) as a feed additive for sows, piglets, pigs for fattening, cattle for fattening, calves for rearing, chickens for fattening and rabbits for fattening. EFSA Journal 2012;10(10):2924, 34 pp. doi: 10.2903/j.efsa.2012.2924 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014a. Scientific Opinion on the safety and efficacy of Pediococcus pentosaceus (NCIMB 30044) as a silage additive for all animal species. EFSA Journal 2014;12(3):3610, 12 pp. doi: 10.2903/j.efsa.2014.3610 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014b. Scientific Opinion on the safety and efficacy of Pediococcus pentosaceus (NCIMB 30068) as a silage additive for all animal species. EFSA Journal 2014;12(3):3609, 11 pp. doi: 10.2903/j.efsa.2014.3609 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014c. Scientific Opinion on the safety and efficacy of Toyocerin® (Bacillus toyonensis) as a feed additive for chickens for fattening, weaned piglets, pigs for fattening, sows for reproduction, cattle for fattening and calves for rearing and for rabbits for fattening. EFSA Journal 2014;12(7):3766, 17 pp. doi: 10.2903/j.efsa.2014.3766 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific opinion on the revision of the currently authorised maximum copper content in complete feed. EFSA Journal 2016;14(8):4563, 100 pp. doi: 10.2903/j.efsa.2016.4563 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Prod ucts or Substances usedin Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, De Lourdes Bastos M, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez Puente S, Lopez‐Alonso M, Mantovani A, Mayo B, Ramos F, Villa RE, Wallace RJ, Wester P, Brozzi R and Saarela M, 2016b. Scientific opinion on the safety and efficacy of Lactobacillus brevis NCIMB 42149 as a silage additive for all animal species. EFSA Journal 2016;14(11):4616, 10 pp. doi: 10.2903/j.efsa.2016.4616 [DOI] [Google Scholar]
- Einarsson S, Madej A and Tsuma V, 1996. The influence of stress on early pregnancy in the pig. Animal Reproduction Science, 42, 165–172. [Google Scholar]
- Ekkel ED, Vandoorn CEA, Hessing MJC and Tielen MJM, 1995. The specific‐stress‐free housing system has positive effects on productivity, health, and welfare of pigs. Journal of Animal Science, 73, 1544–1551. [DOI] [PubMed] [Google Scholar]
- Ekperigin H, Jang S and McCapes R, 1983. Effective control of a gentamicin‐resistant Salmonella arizonae infection in turkey poults. Avian Diseases, 27, 822–829. [PubMed] [Google Scholar]
- Elena Perez‐Cobas A, Jose Gosalbes M, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Daeumer C, Heinsen F‐A, Latorre A, Barbas C, Seifert J, dos Santos VM, Ott SJ, Ferrer M and Moya A, 2013. Gut microbiota disturbance during antibiotic therapy: a multi‐omic approach. Gut, 62, 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo AM, Mercado EC, Rabinovitz BC and Fernandez‐Miyakawa ME, 2010. Effect of tannins on the in vitro growth of Clostridium perfringens. Veterinary Microbiology, 145, 308–314. [DOI] [PubMed] [Google Scholar]
- Ellis KA, Innocent GT, Mihm M, Cripps P, McLean WG, Howard CV and Grove‐White D, 2007. Dairy cow cleanliness and milk quality on organic and conventional farms in the UK. Journal of Dairy Research, 74, 302–310. [DOI] [PubMed] [Google Scholar]
- Elwinger K, Schneitz C, Berndtson E, Fossum O, Teglof B and Engstom B, 1992. Factors affecting the incidence of necrotic enteritis, cecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Veterinaria Scandinavica, 33, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMEA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2009. Recommendation on the evaluation of the benefit ‐ Risk balance of veterinary medicinal products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500005264.pdf
- EMA (European Medicines Agency), 2013a. Use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health (EMA/755938/2012). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/07/WC500146813.pdf
- EMA (European Medicines Agency), 2013b. Use of glycylcyclines in animals in the European Union: development of resistance and possible impact on human and animal health (EMA/291760/2013). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/07/WC500146814.pdf
- EMA (European Medicines Agency), 2014. Answers to the requests for scientific advice on the impact on public health and animal health of the use of antibiotics in animals – Answer to the second request from the EC (ranking of antibiotics); Answer to the third request from the EC (new antibiotics); Answer to the fourth request from the EC (risk mitigation options) (EMA/381884/2014). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/07/WC500170253.pdf
- EMA (European Medicines Agency), 2015. Workshop on the therapeutic use of bacteriophages (EMA/389257/2015). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2015/07/WC500189409.pdf
- EMA (European Medicines Agency), 2016a. Guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances (EMA/CVMP/627/2001‐Rev.1). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500200984.pdf
- EMA (European Medicines Agency), 2016b. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health (EMA/CVMP/CHMP/231573/2016). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211080.pdf
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2010. Opinion following an Article 35 referral for all veterinary medicinal products containing quinolones including fluoroquinolones intended for use in food‐producing species. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/veterinary/referrals/Quinolones_containing_medicinal_products/vet_referral_000039.jsp&mid=WC0b01ac05805c5170
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2011. Reflection paper on the use of macrolides, lincosamides and streptogramins (MLS) in food‐producing animals in the European Union: development of resistance and impact on human and animal health. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/11/WC500118230.pdf
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2012a. Opinion following an Article 35 referral for all veterinary medicinal products containing systemically administered (parenteral and oral) 3rd and 4th generation cephalosporins intended for use in food producing species. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/veterinary/referrals/Cephalosporins/vet_referral_000056.jsp&mid=WC0b01ac05805c5170
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2012b. Refusal EPAR for Naxcel ‐ Type II variation (EMEA/V/C/000079/II/0013) ‐ EMA/CVMP/746112/2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/veterinary/000079/WC500142251.pdf
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2014a. CVMP assessment report for Bovela (EMEA/V/C/003703/0000). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/veterinary/003703/WC500182853.pdf
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2014b. Opinion following an Article 35 referral for all veterinary medicinal products containing enrofloxacin to be administered via the drinking water to chickens and/or turkeys. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/veterinary/referrals/Veterinary_medicines_containing_enrofloxacin_to_be_administered_via_the_drinking_water_to_chickens_and/or_turkeys/vet_referral_000079.jsp&mid=WC0b01ac05805c5170
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2014c. Opinion following an Article 35 referral for veterinary medicinal products containing tylosin to be administered orally via feed or the drinking water to pigs. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/veterinary/referrals/Veterinary_medicinal_products_containing_tylosin_to_be_administered_orally_via_feed_or_the_drinking_water_to_pigs/vet_referral_000098.jsp&mid=WC0b01ac05805c5170
- EMA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2016. CLYNAV ‐ Common name: Salmon pancreas disease vaccine (recombinant DNA plasmid) (EMA/CVMP/223807/2016). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/veterinary/002390/WC500205218.pdf
- EMA CVMP AWP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use, Antimicrobials Working Party), 2015. Guideline on the assessment of the risk to public health from antimicrobial resistance due to the use of an antimicrobial veterinary medicinal product in food‐producing animals. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500183774.pdf
- EMA ESVAC (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption), 2013. Revised ESVAC reflection paper on collecting data on consumption of antimicrobial agents per animal species, on technical units of measurement and indicators for reporting consumption of antimicrobial agents in animals (EMA/286416/2012‐Rev.1). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/12/WC500136456.pdf
- EMA ESVAC (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption), 2015a. Principles on assignment of defined daily dose for animals (DDDA) and defined course dose for animals (DCDA) (EMA/710019/2014). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184369.pdf
- EMA ESVAC (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption), 2015b. Sales of veterinary antimicrobial agents in 26 EU/EEA countries in 2013 (EMA/387934/2015). Fifth ESVAC report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2015/10/WC500195687.pdf
- EMA ESVAC (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption), 2016. Sales of veterinary antimicrobial agents in 29 EU/EEA countries in 2014 (EMA/61769/2016). Trends from 2011 to 2014. Sixth ESVAC report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2016/10/WC500214217.pdf
- EMEA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2006. Reflection paper on the use of fluoroquinolones in food producing animals ‐ Precautions for use in the SPC regarding prudent use guidance (EMEA/CVMP/416168/2006‐FINAL). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500005173.pdf
- EMEA CVMP (European Medicines Agency, Committee for Medicinal Products for Veterinary Use), 2009. Revised reflection paper on the use of 3rd and 4th generation cephalosporins in food producing animals in the European Union: development of resistance and impact on human and animal health (EMEA/CVMP/SAGAM/81730/2006‐Rev.1). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004307.pdf
- EMEA CVMP SAGAM (European Medicines Agency, Committee for Medicinal Products for Veterinary Use, Scientific Advisory Group on Antimicrobials), 2008. Reflection paper on Antimicrobial Resistance Surveillance as Post‐marketing Authorisation Commitment (EMEA/CVMP/SAGAM/428938/2007). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500005150.pdf
- Enøe C, Mousing J, Schirmer AL and Willeberg P, 2002. Infectious and rearing‐system related risk factors for chronic pleuritis in slaughter pigs. Preventive Veterinary Medicine, 54, 337–349. [DOI] [PubMed] [Google Scholar]
- Ernst K, Tuchscherer M, Kanitz E, Puppe B and Manteuffel G, 2006. Effects of attention and rewarded activity on immune parameters and wound healing in pigs. Physiology and Behavior, 89, 448–456. [DOI] [PubMed] [Google Scholar]
- Etterlin PE, Ytrehus B, Lundeheim N, Heldmer E, Österberg J and Ekman S, 2014. Effects of free‐range and confined housing on joint health in a herd of fattening pigs. BMC Veterinary Research, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Hujer AM, Jiang H, Hujer KM, Hall T, Marzan C, Jacobs MR, Sampath R, Ecker DJ and Manca C, 2016. Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clinical Infectious Diseases, 62, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank R and Bryant M, 1972. Aggressive behaviour amongst groups of domesticated pigs kept at various stocking rates. Animal Behaviour, 20, 21–28. [DOI] [PubMed] [Google Scholar]
- Fall N, Emanuelson U, Martinsson K and Jonsson S, 2008. Udder health at a Swedish research farm with both organic and conventional dairy cow management. Preventive Veterinary Medicine, 83, 186–195. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization), 2015. Report of the conference of FAO ‐ Thirty‐ninth Session ‐ Rome, 6‐13 June 2015 ‐ Resolution 4/2015: Antimicrobial Resistance. Available online: http://www.fao.org/3/a-mo153e.pdf
- FAO (Food and Agriculture Organization), 2016. Drivers, dynamics and epidemiology of antimicrobial resistance in animal production. Wall B.A., Mateus A., Marshall L., Pfeiffer D.U., Lubroth J., Ormel H.J., Otto P., Patriarchi A. Food and Agriculture Organization of the United Nations, Rome, Italy. Available online: http://www.fao.org/3/a-i6209e.pdf
- Fasina YO, Newman MM, Stough JM and Liles MR, 2016. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poultry Science, 95, 247–260. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration), 2015. NARMS Integrated Report: 2012‐2013 ‐ The National Antimicrobial Resistance Monitoring System: Enteric Bacteria. Available online: http://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM453398.pdf
- Feltrin F, Alba P, Kraushaar B, Ianzano A, Argudín MA, Di Matteo P, Porrero MC, Aarestrup FM, Butaye P and Franco A, 2016. A livestock‐associated, multidrug‐resistant, methicillin‐resistant Staphylococcus aureus clonal complex 97 lineage spreading in dairy cattle and pigs in Italy. Applied and Environmental Microbiology, 82, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertner M, Boklund A, Dupont N and Toft N, 2016. Changes in group treatment procedures of Danish finishers and its influence on the amount of administered antimicrobials. Preventive Veterinary Medicine, 126, 89–93. [DOI] [PubMed] [Google Scholar]
- Field D, Cotter PD, Hill C and Ross RP, 2015. Bioengineering lantibiotics for therapeutic success. Frontiers in Microbiology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippitzi M, Callens B, Pardon B, Persoons D and Dewulf J, 2014. Antimicrobial use in pigs, broilers and veal calves in Belgium. Vlaams Diergeneeskundig Tijdschrift, 83, 215–224. [Google Scholar]
- Fontana I, Tullo E, Butterworth A and Guarino M, 2015a. An innovative approach to predict the growth in intensive poultry farming. Computers and Electronics in Agriculture, 119, 178–183. [Google Scholar]
- Fontana I, Tullo E, Scrase A and Butterworth A, 2015b. Vocalisation sound pattern identification in young broiler chickens. Animal, 1–8. [DOI] [PubMed] [Google Scholar]
- Fosse J, Seegers H and Magras C, 2009. Prevalence and risk factors for bacterial food‐borne zoonotic hazards in slaughter pigs: a review. Zoonoses and Public Health, 56, 429–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossi M, Heinonen M, Pohjanvirta T, Pelkonen S and Peltoniemi O, 2001. Eradication of endemic Brachyspira pilosicoli infection from a farrowing herd: a case report. Animal Health Research Reviews, 2, 53–58. [PubMed] [Google Scholar]
- Franklin A, Acar J, Anthony F, Gupta R, Nicholls T, Tamura Y, Thompson S, Threlfall EJ, Vose D, van Vuuren M, White DG, Wegener HC and Costarrica ML, 2001. Antimicrobial resistance: harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal‐derived food. Revue Scientifique et Technique, 20, 859–870. [DOI] [PubMed] [Google Scholar]
- Fraqueza MJ, Martins A, Borges AC, Fernandes MH, Fernandes MJ, Vaz Y, Bessa RJB and Barreto AS, 2014. Antimicrobial resistance among Campylobacter spp. strains isolated from different poultry production systems at slaughterhouse level. Poultry Science, 93, 1578–1586. [DOI] [PubMed] [Google Scholar]
- Fray M, Paton D and Alenius S, 2000. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Animal Reproduction Science, 60, 615–627. [DOI] [PubMed] [Google Scholar]
- Fregonesi JA and Leaver JD, 2002. Influence of space allowance and milk yield level on behaviour, performance and health of dairy cows housed in strawyard and cubicle systems. Livestock Production Science, 78, 245–257. [Google Scholar]
- Fregonesi JA, Tucker CB and Weary DM, 2007. Overstocking reduces lying time in dairy cows. Journal of Dairy Science, 90, 3349–3354. [DOI] [PubMed] [Google Scholar]
- Gadde U, Rathinam T and Lillehoj HS, 2015. Passive immunization with hyperimmune egg‐yolk IgY as prophylaxis and therapy for poultry diseases–a review. Animal Health Research Reviews, 16, 163–176. [DOI] [PubMed] [Google Scholar]
- Gallois M, Rothkoetter HJ, Bailey M, Stokes CR and Oswald IP, 2009. Natural alternatives to in‐feed antibiotics in pig production: can immunomodulators play a role? Animal, 3, 1644–1661. [DOI] [PubMed] [Google Scholar]
- Galyean ML, Perino LJ and Duff GC, 1999. Interaction of cattle health/immunity and nutrition. Journal of Animal Science, 77, 1120–1134. [DOI] [PubMed] [Google Scholar]
- Garcia A, Sutherland M, Pirner G, Picinin G, May M, Backus B and McGlone J, 2016. Impact of providing feed and/or water on performance, physiology, and behavior of weaned pigs during a 32‐h transport. Animals, 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmo RT, Waage S, Sviland S, Henriksen BI, Osteras O and Reksen O, 2010. Reproductive performance, udder health, and antibiotic resistance in mastitis bacteria isolated from Norwegian Red cows in conventional and organic farming. Acta Veterinaria Scandinavica, 52, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay E, Cazeau G, Jarrige N and Calavas D, 2012. Utilisation des antibiotiques chez les ruminants domestiques en France: résultats d'enquêtes de pratiques auprès d’éleveurs et de vétérinaires. Bull Epid Santé Anim Alim, 53, 8–10. [Google Scholar]
- Gebreyes WA, Thakur S and Morrow WEM, 2006. Comparison of prevalence, antimicrobial resistance, and occurrence of multidrug‐resistant Salmonella in antimicrobial‐free and conventional pig production. Journal of Food Protection, 69, 743–748. [DOI] [PubMed] [Google Scholar]
- Geenen P, Blaak H, Koene M, Havelaar A, van Leverstein‐Hall M, Mulders M, Neeling Ad, Roda Husman Ad, Stobberingh E and Wagenaar J, 2011. Antimicrobial resistance transmissible from food animals to humans–a risk profile. Available online: http://www.rivm.nl/bibliotheek/rapporten/330334001.pdf
- Geier M, Mikkelsen L, Torok V, Allison G, Olnood C, Boulianne M, Hughes R and Choct M, 2010. Comparison of alternatives to in‐feed antimicrobials for the prevention of clinical necrotic enteritis. Journal of Applied Microbiology, 109, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Geldhof MF, Vanhee M, Van Breedam W, Van Doorsselaere J, Karniychuk UU and Nauwynck HJ, 2012. Comparison of the efficacy of autogenous inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) vaccines with that of commercial vaccines against homologous and heterologous challenges. BMC Veterinary Research, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli P, Laxminarayan R, Dunne M and Smith DL, 2012. “One‐size‐fits‐all”? Optimizing treatment duration for bacterial infections. PLoS ONE, 7, e29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzova L, Babak V, Sedlar K, Faldynova M, Videnska P, Cejkova D, Jensen AN, Denis M, Kerouanton A, Ricci A, Cibin V, Osterberg J and Rychlik I, 2015. Characterization of antibiotic resistance gene abundance and microbiota composition in feces of organic and conventional pigs from four EU Countries. PLoS ONE, 10, e0132892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemian M and Jahanian R, 2016. Dietary mannan‐oligosaccharides supplementation could affect performance, immunocompetence, serum lipid metabolites, intestinal bacterial populations, and ileal nutrient digestibility in aged laying hens. Animal Feed Science and Technology, 213, 81–89. [Google Scholar]
- Giang HH, Viet TQ, Ogle B and Lindberg JE, 2010. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livestock Science, 129, 95–103. [Google Scholar]
- Gibbons JF, Boland F, Buckley JF, Butler F, Egan J, Fanning S, Markey BK and Leonard FC, 2013. Influences on antimicrobial prescribing behaviour of veterinary practitioners in cattle practice in Ireland. Veterinary Record, 172, 14. [DOI] [PubMed] [Google Scholar]
- Gisbert E, Skalli A, Campbell J, Solovyev MM, Rodríguez C, Dias J and Polo J, 2015. Spray‐dried plasma promotes growth, modulates the activity of antioxidant defenses, and enhances the immune status of gilthead sea bream (Sparus aurata) fingerlings. Journal of Animal Science, 93, 278–286. [DOI] [PubMed] [Google Scholar]
- Goff DA, 2011. Antimicrobial stewardship: bridging the gap between quality care and cost. Current Opinion in Infectious Diseases, 24, S11–S20. [DOI] [PubMed] [Google Scholar]
- Goldbach SG and Alban L, 2006. A cost–benefit analysis of Salmonella‐control strategies in Danish pork production. Preventive Veterinary Medicine, 77, 1–14. [DOI] [PubMed] [Google Scholar]
- Gonyou HW, Hemsworth PH and Barnett JL, 1986. Effects of frequent interactions with humans on growing‐pigs. Applied Animal Behaviour Science, 16, 269–278. [Google Scholar]
- Gonyou HW, Brumm MC, Bush E, Deen J, Edwards SA, Fangman T, McGlone JJ, Meunier‐Salaun M, Morrison RB, Spoolder H, Sundberg PL and Johnson AK, 2006. Application of broken‐line analysis to assess floor space requirements of nursery and grower‐finisher pigs expressed on an allometric basis. Journal of Animal Science, 84, 229–235. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Martin JV, Elvira L, Lopez MC, Villalobos NP, Lopez‐Guerrero EC and Astiz S, 2011. Reducing antibiotic use: selective metaphylaxis with florfenicol in commercial feedlots. Livestock Science, 141, 173–181. [Google Scholar]
- Goossens M, Catry B and Verhaegen J, 2013. Antimicrobial resistance to benzylpenicillin in invasive pneumococcal disease in Belgium, 2003–2010: the effect of altering clinical breakpoints. Epidemiology and Infection, 141, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakannan A and Arul V, 2006. Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture, 255, 179–187. [Google Scholar]
- Grandin T, 1987. Animal handling. Veterinary Clinics of North America‐Food Animal Practice, 3, 323–338. [DOI] [PubMed] [Google Scholar]
- Grandin T, 2008. Humane livestock handling. Storey Publishing, LLC, North Adams, 227 pp. [Google Scholar]
- Grant AQ, Hashem F and Parveen S, 2016. Salmonella and Campylobacter: antimicrobial resistance and bacteriophage control in poultry. Food Microbiology, 53, 104–109. [DOI] [PubMed] [Google Scholar]
- Grave K, Lingaas E, Bangen M and Rønning M, 1999. Surveillance of the overall consumption of antibacterial drugs in humans, domestic animals and farmed fish in Norway in 1992 and 1996. Journal of Antimicrobial Chemotherapy, 43, 243–252. [DOI] [PubMed] [Google Scholar]
- Grave K, Jensen VF, Odensvik K, Wierup M and Bangen M, 2006. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Preventive Veterinary Medicine, 75, 123–132. [DOI] [PubMed] [Google Scholar]
- Grema HA, Geidam YA, Gadzama GB, Ameh JA and Suleiman A, 2015. Methicillin resistant Staphylococcus aureus (MRSA): a review. Advances in Animal and Veterinary Sciences, 3, 79–98. [Google Scholar]
- Grinnell M, Provo G, Marsden‐Haug N, Stigi KA, DeBess E, Kissler B, Crarey E, Tate H, Pringle J, Grass J, Folster J, Williams I, Gieraltowski L and Laufer AS, 2013. Outbreak of Salmonella Heidelberg infections linked to a single poultry producer‐13 states, 2012–2013. Mmwr‐Morbidity and Mortality Weekly Report, 62, 553–556. [PMC free article] [PubMed] [Google Scholar]
- Gross WB and Siegel PB, 1997. Why some get sick. Journal of Applied Poultry Research, 6, 453–460. [Google Scholar]
- Guerra‐Ordaz AA, González‐Ortiz G, La Ragione RM, Woodward MJ, Collins JW, Pérez JF and Martín‐Orúe SM, 2014. Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Applied and Environmental Microbiology, 80, 4879–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C, Vanathi M and Tandon R, 2015. Current concepts in operative room sterilisation. The Official Scientific Journal of Delhi Ophthalmological Society 25, 190–194. [Google Scholar]
- Guy JH, Rowlinson P, Chadwick JR and Ellis M, 2002. Health conditions of two genotypes of growing‐finishing pig in three different housing systems: implications for welfare. Livestock Production Science, 75, 233–243. [Google Scholar]
- Guy SZY, Thomson PC and Hermesch S, 2012. Selection of pigs for improved coping with health and environmental challenges: breeding for resistance or tolerance? Frontiers in Genetics, 3, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajati H and Rezaei M, 2010. The application of prebiotics in poultry production. International Journal of Poultry Science, 9, 298–304. [Google Scholar]
- Halasa T, Osteras O, Hogeveen H, van Werven T and Nielen M, 2009. Meta‐analysis of dry cow management for dairy cattle. Part 1. Protection against new intramammary infections. Journal of Dairy Science, 92, 3134–3149. [DOI] [PubMed] [Google Scholar]
- Halbert LW, Kaneene JB, Ruegg PL, Warnick LD, Wells SJ, Mansfield LS, Fossler CP, Campbell AM and Geiger‐Zwald AM, 2006. Evaluation of antimicrobial susceptibility patterns in Campylobacter spp isolated from dairy cattle and farms managed organically and conventionally in the midwestern and northeastern United States. Journal of the American Veterinary Medical Association, 228, 1074–1081. [DOI] [PubMed] [Google Scholar]
- Halbur P, Thanawongnuwech R, Brown G, Kinyon J, Roth J, Thacker E and Thacker B, 2000. Efficacy of antimicrobial treatments and vaccination regimens for control of porcine reproductive and respiratory syndrome virus and Streptococcus suis coinfection of nursery pigs. Journal of Clinical Microbiology, 38, 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerum AM, Heuer OE, Emborg H‐D, Bagger‐Skjot L, Jensen VF, Rogues A‐M, Skov RL, Agerso Y, Brandt CT and Seyfarth AM, 2007. Danish integrated antimicrobial resistance monitoring and research program. Emerging Infectious Diseases, 13, 1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerum AM, Lester CH and Heuer OE, 2010. Antimicrobial‐resistant enterococci in animals and meat: a human health hazard? Foodborne Pathogens and Disease, 7, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Hammerum AM, Larsen J, Andersen VD, Lester CH, Skytte TSS, Hansen F, Olsen SS, Mordhorst H, Skov RL, Aarestrup FM and Agerso Y, 2014. Characterization of extended‐spectrum beta‐lactamase (ESBL)‐producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third‐or fourth‐generation cephalosporins. Journal of Antimicrobial Chemotherapy, 69, 2650–2657. [DOI] [PubMed] [Google Scholar]
- Hampson DJ, La T, Phillips ND and Holyoake PK, 2016. Brachyspira hyodysenteriae isolated from apparently healthy pig herds following an evaluation of a prototype commercial serological ELISA. Veterinary Microbiology, 191, 15–19. [DOI] [PubMed] [Google Scholar]
- Han J, Wang Y, Sahin O, Shen Z, Guo B, Shen J and Zhang Q, 2012. A fluoroquinolone resistance associated mutation in gyrA Affects DNA supercoiling in Campylobacter jejuni. Frontiers in Cellular and Infection Microbiology, 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H and Lagerkvist CJ, 2014. Defining and measuring farmers’ attitudes to farm animal welfare. Animal Welfare, 23, 47–56. [Google Scholar]
- Hardeng F and Edge VL, 2001. Mastitis, ketosis, and milk fever in 31 organic and 93 conventional Norwegian dairy herds. Journal of Dairy Science, 84, 2673–2679. [DOI] [PubMed] [Google Scholar]
- Hasman H, Saputra D, Sicheritz‐Ponten T, Lund O, Svendsen CA, Frimodt‐Møller N and Aarestrup FM, 2013. Rapid whole genome sequencing for the detection and characterization of microorganisms directly from clinical samples. Journal of Clinical Microbiology, JCM, 02452‐02413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay K, Morton J, Clements A, Mahony T and Barnes T, 2016. Associations between feedlot management practices and bovine respiratory disease in Australian feedlot cattle. Preventive Veterinary Medicine, 128, 23–32. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands , 2011. Antibiotics in food animal production and resistant bacteria in humans. Health Council of the Netherlands, The Hague, 2011; Publication no. 2011/16. Available online: http://www.gezondheidsraad.nl/sites/default/files/201116E_Antibiotica_in_food_animal.pdf
- Hedegaard CJ and Heegaard PM, 2016. Passive immunisation, an old idea revisited: basic principles and application to modern animal production systems. Veterinary Immunology and Immunopathology, 174, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard CJ, Strube ML, Hansen MB, Lindved BK, Lihme A, Boye M and Heegaard PM, 2016. Natural Pig Plasma immunoglobulins have anti‐bacterial effects: potential for use as feed supplement for treatment of intestinal infections in pigs. PLoS ONE, 11, e0147373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges JF, Holderness J and Jutila MA, 2016. Adjuvant materials that enhance bovine γδ T cell responses. Veterinary Immunology and Immunopathology, 181, 30–38. [DOI] [PubMed] [Google Scholar]
- Heffernan C, Nielsen L, Thomson K and Gunn G, 2008. An exploration of the drivers to bio‐security collective action among a sample of UK cattle and sheep farmers. Preventive Veterinary Medicine, 87, 358–372. [DOI] [PubMed] [Google Scholar]
- Helms M, Vastrup P, Gerner‐Smidt P and Mølbak K, 2002. Excess mortality associated with antimicrobial drug‐resistant Salmonella Typhimurium. Emerging Infectious Diseases, 8, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms M, Simonsen J and Mølbak K, 2004. Quinolone resistance is associated with increased risk of invasive illness or death during infection with Salmonella serotype Typhimurium. Journal of Infectious Diseases, 190, 1652–1654. [DOI] [PubMed] [Google Scholar]
- Hemsworth PH, Barnett JL and Hansen C, 1981. The influence of handling by humans on the behavior, growth, and corticosteroids in the juvenile female pig. Hormones and Behavior, 15, 396–403. [DOI] [PubMed] [Google Scholar]
- Hemsworth PH, Barnett JL and Hansen C, 1986. The influence of handling by humans on the behavior, reproduction and corticosteroids of male and female pigs. Applied Animal Behaviour Science, 15, 303–314. [Google Scholar]
- Hemsworth P, Coleman G, Barnett J and Borg S, 2000. Relationships between human‐animal interactions and productivity of commercial dairy cows. Journal of Animal Science, 78, 2821–2831. [DOI] [PubMed] [Google Scholar]
- Hemsworth PH and Coleman GJ, 2011. Human–livestock interactions: the stockperson and the productivity and welfare of intensively farmed animals. 2nd Edition CAB International, Wallingford. [Google Scholar]
- Hera A and Bures J, 2003. Veterinary autogenous vaccines. Developments in Biologicals, 117, 19–25. [PubMed] [Google Scholar]
- Hering J, Hille K, Froemke C, von Muenchhausen C, Hartmann M, Schneider B, Friese A, Roesler U, Merle R and Kreienbrock L, 2014. Prevalence and potential risk factors for the occurrence of cefotaxime resistant Escherichia coli in German fattening pig farms‐A cross‐sectional study. Preventive Veterinary Medicine, 116, 129–137. [DOI] [PubMed] [Google Scholar]
- Hering J, Frömke C, von Münchhausen C, Hartmann M, Schneider B, Friese A, Roesler U, Kreienbrock L and Hille K, 2016. Cefotaxime‐resistant Escherichia coli in broiler farms–a cross‐sectional investigation in Germany. Preventive Veterinary Medicine, 125, 154–157. [DOI] [PubMed] [Google Scholar]
- Heringstad B, Klemetsdal G and Ruane J, 2000. Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries. Livestock Production Science, 64, 95–106. [Google Scholar]
- Heuer OE, Pedersen K, Andersen JS and Madsen M, 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Letters in Applied Microbiology, 33, 269–274. [DOI] [PubMed] [Google Scholar]
- Heuer OE, Pedersen K, Andersen J and Madsen M, 2002a. Vancomycin‐resistant enterococci (VRE) in broiler flocks 5 years after the avoparcin ban. Microbial Drug Resistance, 8, 133–138. [DOI] [PubMed] [Google Scholar]
- Heuer OE, Pedersen K, Jensen LB, Madsen M and Olsen J, 2002b. Persistence of vancomycin‐resistant enterococci (VRE) in broiler houses after the avoparcin ban. Microbial Drug Resistance, 8, 355–361. [DOI] [PubMed] [Google Scholar]
- Hiki M, Kawanishi M, Abo H, Kojima A, Koike R, Hamamoto S and Asai T, 2015. Decreased resistance to broad‐spectrum cephalosporin in escherichia coli from healthy broilers at farms in Japan after voluntary withdrawal of ceftiofur. Foodborne Pathogens and Disease, 12, 639–643. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ and Salminen S, 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology, 11, 506–514. [DOI] [PubMed] [Google Scholar]
- Hinchliffe S and Ward KJ, 2014. Geographies of folded life: how immunity reframes biosecurity. Geoforum, 53, 136–144. [Google Scholar]
- HM Government , 2014. UK 5 year antimicrobial resistance (AMR) strategy 2013 to 2018: annual progress report and implementation plan 2014.
- Hodin R, 2000. Maintaining gut homeostasis: the butyrate‐NF‐kappaB connection. Gastroenterology, 118, 798–801. [DOI] [PubMed] [Google Scholar]
- Hofacre CL, Froyman R, Gautrias B, George B, Goodwin MA and Brown J, 1998a. Use of aviguard and other intestinal bioproducts in experimental Clostridium perfringens‐associated necrotizing enteritis in broiler chickens. Avian Diseases, 42, 579–584. [PubMed] [Google Scholar]
- Hofacre CL, Froyman R, George B, Goodwin MA and Brown J, 1998b. Use of Aviguard, virginiamycin, or bacitracin MD against Clostridium perfringens‐associated necrotizing enteritis. Journal of Applied Poultry Research, 7, 412–418. [Google Scholar]
- Hofacre CL, Johnson AC, Kelly BJ and Froyman R, 2002. Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic‐resistant pathogenic Escherichia coli in day‐old broiler chickens. Avian Diseases, 46, 198–202. [DOI] [PubMed] [Google Scholar]
- Hogeveen H, Dykhuizen A and Sol J, 1992. Short‐and long‐term effects of a 2 year dairy herd health and management program. Preventive Veterinary Medicine, 13, 53–58. [Google Scholar]
- Hojberg O, Canibe N, Poulsen HD, Hedemann MS and Jensen BB, 2005. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Applied and Environmental Microbiology, 71, 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis A and Ahmed Z, 2013. Preserving antibiotics, rationally. New England Journal of Medicine, 369, 2474–2476. [DOI] [PubMed] [Google Scholar]
- Holman DB and Chénier MR, 2015. Antimicrobial use in swine production and its effect on the swine gut microbiota and antimicrobial resistance. Canadian Journal of Microbiology, 61, 785–798. [DOI] [PubMed] [Google Scholar]
- Hong Y‐H, Kwon J‐S, Lee H‐J, Song C‐S and Lee S‐W, 2015. Eradication of Mycoplasma synoviae from a multi‐age broiler breeder farm using antibiotics therapy. Poultry Science, 94, 2364–2368. [DOI] [PubMed] [Google Scholar]
- Hoseinifar SH, Esteban MA, Cuesta A and Sun YZ, 2015a. Prebiotics and fish immune response: a review of current knowledge and future perspectives. Reviews in Fisheries Science and Aquaculture, 23, 315–328. [Google Scholar]
- Hoseinifar SH, Mirvaghefi A, Amoozegar MA, Sharifian M and Esteban MT, 2015b. Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon synbiotic feeding. Fish and Shellfish Immunology, 45, 27–32. [DOI] [PubMed] [Google Scholar]
- Hossain M, Begum M, Nyachoti C, Hancock J and Kim I, 2015. Dietary fenugreek seed extract improves performance and reduces fecal E. coli counts and fecal gas emission in lactating sows and suckling piglets. Canadian Journal of Animal Science, 95, 561–568. [Google Scholar]
- Houe H, Uttenthal A, Pedersen K, Jensen AM and Bartlett P, 1996. Risk factors for a change in bovine virus diarrhoea seropositivity in 41 Danish dairy herds during two years. Proceedings of the 3. ESVV Symposium on Pestvirus Infections.
- Houe H, Lindberg A and Moennig V, 2006. Test strategies in bovine viral diarrhea virus control and eradication campaigns in Europe. Journal of Veterinary Diagnostic Investigation, 18, 427–436. [DOI] [PubMed] [Google Scholar]
- Houe H, Nielsen LR and Nielsen SS, 2014. Control and eradication of endemic infectious diseases in cattle. College Publications, London. [Google Scholar]
- Hovi M and Roderick S, 2000. Mastitis in organic dairy herds in England and Wales. Proc. Br. Mast. Conf. Axient Information Services, Crewe, UK. 86 pp.
- Hovi M, Sundrum A and Thamsborg S, 2003. Animal health and welfare in organic livestock production in Europe—current state and future challenges. Livestock Production Science, 80, 41–53. [Google Scholar]
- Hrabák J, Walková R, Študentová V, Chudáčková E and Bergerová T, 2011. Carbapenemase activity detection by matrix‐assisted laser desorption ionization‐time of flight mass spectrometry. Journal of Clinical Microbiology, 49, 3222–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HS (Hushållningssällskapet), 2010. Slaktkropparnas kvalitet i ekologisk uppfödning 2010 (Carcass quality in organic production 2010, in Swedish). Hushållningssällskapet, HS Consult, Uppsala, Sweden, pp. 43 Available online: http://hs-konsult.hush.se/dotnet/GetAttachment.aspx?siteid=80&id=12284 [Google Scholar]
- Huang E, Gurzau AE, Hanson BM, Kates AE, Smith TC, Pettigrew MM, Spinu M and Rabinowitz PM, 2014. Detection of livestock‐associated methicillin‐resistant Staphylococcus aureus among swine workers in Romania. Journal of Infection and Public Health, 7, 323–332. [DOI] [PubMed] [Google Scholar]
- Huff W, Huff G, Rath N, Balog J and Donoghue A, 2005. Alternatives to antibiotics: utilization of bacteriophage to treat colibacillosis and prevent foodborne pathogens. Poultry Science, 84, 655–659. [DOI] [PubMed] [Google Scholar]
- Huff GR, Huff WE, Rath NC, Santos FSdl, Farnell MB and Donoghue AM, 2007. Influence of hen age on the response of turkey poults to cold stress, Escherichia coli challenge, and treatment with a yeast extract antibiotic alternative. Poultry Science, 86, 636–642. [DOI] [PubMed] [Google Scholar]
- Hughes L, Hermans P and Morgan K, 2008. Risk factors for the use of prescription antibiotics on UK broiler farms. Journal of Antimicrobial Chemotherapy, 61, 947–952. [DOI] [PubMed] [Google Scholar]
- Huijbers PM, van Hoek AH, Graat EA, Haenen AP, Florijn A, Hengeveld PD and van Duijkeren E, 2015. Methicillin‐resistant Staphylococcus aureus and extended‐spectrum and AmpC beta‐lactamase‐producing Escherichia coli in broilers and in people living and/or working on organic broiler farms. Veterinary Microbiology, 176, 120–125. [DOI] [PubMed] [Google Scholar]
- Hume ME, 2011. Historic perspective: prebiotics, probiotics, and other alternatives to antibiotics. Poultry Science, 90, 2663–2669. [DOI] [PubMed] [Google Scholar]
- Huxley J, Green M, Green L and Bradley A, 2002. Evaluation of the efficacy of an internal teat sealer during the dry period. Journal of Dairy Science, 85, 551–561. [DOI] [PubMed] [Google Scholar]
- Huxley VAJ, Suthan P, Joseph MV and Lipton AP, 2011. Immunomodulatary potential of Chitosan and Levamisole against bacterial diseases of Penaeus monodon. Asian Journal of Bio Science, 6, 33–40. [Google Scholar]
- Huynh TTT, Aarnink AA, Gerrits WJJ, Heetkamp MJH, Canh TT, Spoolder HAM, Kemp B and Verstegen MWA, 2005. Thermal behaviour of growing pigs in response to high temperature and humidity. Applied Animal Behaviour Science, 91, 1–16. [Google Scholar]
- Imbeault S, Parent S, Lagace M, Uhland CF and Blais JF, 2006. Using Bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. Journal of Aquatic Animal Health, 18, 203–214. [Google Scholar]
- Ives SE and Richeson JT, 2015. Use of antimicrobial metaphylaxis for the control of bovine respiratory disease in high‐risk cattle. Veterinary Clinics of North America: Food Animal Practice, 31, 341–350. [DOI] [PubMed] [Google Scholar]
- Jansen J, Van den Borne B, Renes R, Van Schaik G, Lam T and Leeuwis C, 2009. Explaining mastitis incidence in Dutch dairy farming: the influence of farmers’ attitudes and behaviour. Preventive Veterinary Medicine, 92, 210–223. [DOI] [PubMed] [Google Scholar]
- Jensen VF, Jacobsen E and Bager F, 2004a. Veterinary antimicrobial‐usage statistics based on standardized measures of dosage. Preventive Veterinary Medicine, 64, 201–215. [DOI] [PubMed] [Google Scholar]
- Jensen VF, Neimann J, Hammerum AM, Mølbak K and Wegener HC, 2004b. Does the use of antibiotics in food animals pose a risk to human health? An unbiased review? Journal of Antimicrobial Chemotherapy, 54, 274–275. [DOI] [PubMed] [Google Scholar]
- Jensen US, Muller A, Brandt CT, Frimodt‐Møller N, Hammerum AM, Monnet DL and Group DS, 2010. Effect of generics on price and consumption of ciprofloxacin in primary healthcare: the relationship to increasing resistance. Journal of Antimicrobial Chemotherapy, dkq093. [DOI] [PubMed] [Google Scholar]
- Jensen AN, Hansen LL, Baggesen DL and Mølbak L, 2013a. Effects of feeding finisher pigs with chicory or lupine feed for one week or two weeks before slaughter with respect to levels of Bifidobacteria and Campylobacter. Animal, 7, 66–74. [DOI] [PubMed] [Google Scholar]
- Jensen KH, Damgaard BM, Andresen LO, Jørgensen E and Carstensen L, 2013b. Prevention of post weaning diarrhoea by a Saccharomyces cerevisiae‐derived product based on whole yeast. Animal Feed Science and Technology, 183, 29–39. [Google Scholar]
- Jensen VF, de Knegt L, Andersen VD and Wingstrand A, 2014. Temporal relationship between decrease in antimicrobial prescription for Danish pigs and the “Yellow Card” legal intervention directed at reduction of antimicrobial use. Preventive Veterinary Medicine, 117, 554–564. [DOI] [PubMed] [Google Scholar]
- Jensen VF, Sommer HM, Struve T, Clausen J and Chriel M, 2016. Factors associated with usage of antimicrobials in commercial mink (Neovison vison) production in Denmark. Preventive Veterinary Medicine, 126, 170–182. [DOI] [PubMed] [Google Scholar]
- Jha R and Berrocoso JFD, 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Animal Feed Science and Technology, 212, 18–26. [Google Scholar]
- Jiang YW, Sims MD and Conway DP, 2005. The efficacy of TAMUS 2032 in preventing a natural outbreak of colibacillosis in broiler chickens in floor pens. Poultry Science, 84, 1857–1859. [DOI] [PubMed] [Google Scholar]
- Johansson Å, Nagy E and Sóki J, 2014. Instant screening and verification of carbapenemase activity in Bacteroides fragilis in positive blood culture, using matrix‐assisted laser desorption ionization–time of flight mass spectrometry. Journal of Medical Microbiology, 63, 1105–1110. [DOI] [PubMed] [Google Scholar]
- Jones RB, 1986. The tonic immobility reaction of the domestic fowl ‐ a review. Worlds Poultry Science Journal, 42, 82–96. [Google Scholar]
- Jones P, Marier E, Tranter R, Wu G, Watson E and Teale C, 2015. Factors affecting dairy farmers’ attitudes towards antimicrobial medicine usage in cattle in England and Wales. Preventive Veterinary Medicine, 121, 30–40. [DOI] [PubMed] [Google Scholar]
- Jourdain B, Joly‐Guillou M‐L, Dombret M‐C, Calvat S, Trouillet J‐L, Gibert C and Chastre J, 1997. Usefulness of quantitative cultures of BAL fluid for diagnosing nosocomial pneumonia in ventilated patients. CHEST Journal, 111, 411–418. [DOI] [PubMed] [Google Scholar]
- Józefiak D, Sip A, Kaczmarek S and Rutkowski A, 2010. The effects of Carnobacterium divergens AS7 bacteriocin on gastrointestinal microflora in vitro and on nutrient retention in broiler chickens. Journal of Animal and Feed Sciences, 19, 460–467. [Google Scholar]
- Józefiak D, Sip A, Rutkowski A, Rawski M, Kaczmarek S, Wołuń‐Cholewa M, Engberg RM and Højberg O, 2012. Lyophilized Carnobacterium divergens AS7 bacteriocin preparation improves performance of broiler chickens challenged with Clostridium perfringens. Poultry Science, 91, 1899–1907. [DOI] [PubMed] [Google Scholar]
- Józefiak D, Kierończyk B, Juśkiewicz J, Zduńczyk Z, Rawski M, Długosz J, Sip A and Højberg O, 2013. Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS ONE, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Eberl T, Sparbier K, Lange C, Kostrzewa M, Schubert S and Wieser A, 2014. Rapid detection of antibiotic resistance based on mass spectrometry and stable isotopes. European Journal of Clinical Microbiology & Infectious Diseases, 33, 949–955. [DOI] [PubMed] [Google Scholar]
- Jung K and Saif LJ, 2015. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. The Veterinary Journal, 204, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado‐Gámez H, Ramírez C and Martínez J, 2013. In vivo evaluation of Lactobacillus plantarum as an alternative to antibiotics uses in piglets. Revista MVZ Córdoba, 18, 3648–3657. [Google Scholar]
- Kadouri DE, To K, Shanks RMQ, and Doi Y, 2013. Predatory Bacteria: A Potential Ally against Multidrug‐Resistant Gram‐Negative Pathogens. PloS one, 8, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadzere CT, Murphy MR, Silanikove N and Maltz E, 2002. Heat stress in lactating dairy cows: a review. Livestock Production Science, 77, 59–91. [Google Scholar]
- Kaldhusdal M, Schneitz C, Hofshagen M and Skjerv E, 2001. Reduced incidence of Clostridium perfringens‐associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Diseases, 45, 149–156. [PubMed] [Google Scholar]
- Kang K‐I, El‐Gazzar M, Sellers HS, Dorea F, Williams SM, Kim T, Collett S and Mundt E, 2012. Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathology, 41, 41–50. [DOI] [PubMed] [Google Scholar]
- Kapareiko D, Lim HJ, Schott EJ, Hanif A and Wikfors GH, 2011. Isolation and evaluation of new probiotic bacteria for use in shellfish hatcheries: II. Effects of a Vibrio sp. probiotic candidate upon survival of oyster larvae (Crassostrea Virginica) in pilot‐scale trials. Journal of Shellfish Research, 30, 617–625. [Google Scholar]
- Karreman HJ and Fulwider W, 2015. Animal well‐being in organic farms In: Grandin T. (ed.). Improvign Animal Welfare: A Practical Approach. 2nd Edition, CAB International, Wallingford: pp. 267–277. [Google Scholar]
- Karunasagar I, 2012. Alternatives to antibiotics in aquaculture. Improving biosecurity through prudent and responsible use of veterinary medicines in aquatic food production, 155.
- Kassem II, Kehinde O, Kumar A and Rajashekara G, 2016. Antimicrobial‐resistant campylobacter in organically and conventionally raised layer chickens. Foodborne Pathogens and Disease, online ahead of print. [DOI] [PubMed] [Google Scholar]
- Kehoe SI and Carlson DB, 2015. Influence of nonmedicated additives as alternatives to antibiotics on calf growth and health. Professional Animal Scientist, 31, 516–522. [Google Scholar]
- Kelley K, 1980. Stress and immune function: a bibliographic review. Annals of Veterinary Research, 11, 445–478. [PubMed] [Google Scholar]
- Kemmett K, Williams N, Chaloner G, Humphrey S, Wigley P and Humphrey T, 2014. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathology, 43, 37–42. [DOI] [PubMed] [Google Scholar]
- Kerr AK, Farrar AM, Waddell LA, Wilkins W, Wilhelm BJ, Bucher O, Wills RW, Bailey RH, Varga C, McEwen SA and Rajic A, 2013. A systematic review‐meta‐analysis and meta‐regression on the effect of selected competitive exclusion products on Salmonella spp. prevalence and concentration in broiler chickens. Preventive Veterinary Medicine, 111, 112–125. [DOI] [PubMed] [Google Scholar]
- Khadem A, Soler L, Everaert N and Niewold TA, 2014. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti‐inflammatory properties. British Journal of Nutrition, 112, 1110–1118. [DOI] [PubMed] [Google Scholar]
- Khairnar K, Raut MP, Chandekar RH, Sanmukh SG and Paunikar WN, 2013. Novel bacteriophage therapy for controlling metallo‐beta‐lactamase producing Pseudomonas aeruginosa infection in Catfish. BMC Veterinary Research, 9, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SH and Iqbal J, 2016. Recent advances in the role of organic acids in poultry nutrition. Journal of Applied Animal Research, 44, 359–369. [Google Scholar]
- Khmylov A, 2015. Interferon‐containing preparations. The end justifies the means.. Svinovodstvo (Moskva), 2, 53–54. [Google Scholar]
- Kim M‐K, Lee H‐G, Park J‐A, Kang S‐K and Choi Y‐J, 2011. Effect of feeding direct‐fed microbial as an alternative to antibiotics for the prophylaxis of calf diarrhea in Holstein calves. Asian‐Australasian Journal of Animal Sciences, 24, 643–649. [Google Scholar]
- King N, Edgington B, Ferguson L, Thomas D, Pounden W and Klosterman E, 1955. Preliminary results in the control and treatment of shipping fever complex in beef cattle. Journal of the American Veterinary Medical Association, 127, 320–323. [PubMed] [Google Scholar]
- Klare I, Heier H, Claus H, Böhme G, Marin S, Seltmann G, Hakenbeck R, Antanassova V and Witte W, 1995. Enterococcus faecium strains with vanA‐mediated high‐level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microbial Drug Resistance, 1, 265–272. [DOI] [PubMed] [Google Scholar]
- Koblentz GD, 2010. Biosecurity reconsidered calibrating biological threats and responses. International Security, 34, 96–132. [Google Scholar]
- Koeck A, Egger‐Danner C, Fuerst C, Obritzhauser W and Fuerst‐Waltl B, 2010. Genetic analysis of reproductive disorders and their relationship to fertility and milk yield in Austrian Fleckvieh dual‐purpose cows. Journal of Dairy Science, 93, 2185–2194. [DOI] [PubMed] [Google Scholar]
- Koeck A, Miglior F, Kelton D and Schenkel F, 2012. Health recording in Canadian Holsteins: data and genetic parameters. Journal of Dairy Science, 95, 4099–4108. [DOI] [PubMed] [Google Scholar]
- Kogut MH, Genovese KJ, He H, Swaggerty CL and Jiang Y, 2013. Modulation of chicken intestinal immune gene expression by small cationic peptides as feed additives during the first week posthatch. Clinical and Vaccine Immunology, 20, 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski MA, DePristo MA and Collins JJ, 2010. Sublethal antibiotic treatment leads to multidrug resistance via radical‐induced mutagenesis. Molecular Cell, 37, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlert C, Schindler G, März RW, Abel G, Brinkhaus B, Derendorf H, Gräfe EU and Veit M, 2002. Systemic availability and pharmacokinetics of thymol in humans. Journal of Clinical Pharmacology, 42, 731–737. [DOI] [PubMed] [Google Scholar]
- Kola A, Kohler C, Pfeifer Y, Schwab F, Kuhn K, Schulz K, Balau V, Breitbach K, Bast A, Witte W, Gastmeier P and Steinmetz I, 2012. High prevalence of extended‐spectrum‐beta‐lactamase‐producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. Journal of Antimicrobial Chemotherapy, 67, 2631–2634. [DOI] [PubMed] [Google Scholar]
- Kolstoe E, Iversen T, Østensvik Ø, Abdelghani A, Secic I and Nesbakken T, 2015. Specific pathogen‐free pig herds also free from campylobacter? Zoonoses and Public Health, 62, 125–130. [DOI] [PubMed] [Google Scholar]
- Komalova I, 2013. Wiener Schnitzel without antibiotics. Svinovodstvo (Moskva), 1, 9–11. [Google Scholar]
- Kommera SK, Mateo RD, Neher FJ and Kim SW, 2006. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian‐Australasian Journal of Animal Sciences, 19, 1784–1789. [Google Scholar]
- Köser CU, Ellington MJ and Peacock SJ, 2014. Whole‐genome sequencing to control antimicrobial resistance. Trends in Genetics, 30, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozdruń W, Czekaj H and Styś N, 2015. Avian zoonoses–a review. Bulletin of the Veterinary Institute in Pulawy, 59, 171–178. [Google Scholar]
- Krause DO, Bhandari SK, House JD and Nyachoti CM, 2010. Response of nursery pigs to a synbiotic preparation of starch and an anti‐escherichia coli K88 probiotic. Applied and Environmental Microbiology, 76, 8192–8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieter J, Schnider R and Tolle KH, 2004. Health conditions of growing‐finishing pigs in fully‐slatted pens and multi‐surface systems. DTW. Deutsche Tierarztliche Wochenschrift, 111, 462–466. [PubMed] [Google Scholar]
- Kristensen CS, Vinther J, Svensmark B and Bækbo P, 2014. A field evaluation of two vaccines against Mycoplasma hyopneumoniae infection in pigs. Acta Veterinaria Scandinavica, 56, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritas S and Morrison R, 2005. Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Veterinary Record‐English Edition, 156, 447–448. [DOI] [PubMed] [Google Scholar]
- Krogh MA and Enevoldsen C, 2014. Evaluation of effects of metritis management in a complex dairy herd health management program. Journal of Dairy Science, 97, 552–561. [DOI] [PubMed] [Google Scholar]
- Kruse AB, Johansen C, Alban L and Nielsen LR, 2016. Evaluation of biosecurity in 140 Danish pig herds using Biocheck. ugent® . Proceedings of the SVEPM2016.
- Kuipers A, Koops W and Wemmenhove H, 2015. Antibiotic use in dairy herds in the Netherlands from 2005 to 2012. Journal of Dairy Science, 99, 1632–1648. [DOI] [PubMed] [Google Scholar]
- La Ragione RM and Woodward MJ, 2003. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Veterinary Microbiology, 94, 245–256. [DOI] [PubMed] [Google Scholar]
- Laanen M, Persoons D, Ribbens S, de Jong E, Callens B, Strubbe M, Maes D and Dewulf J, 2013. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. The Veterinary Journal, 198, 508–512. [DOI] [PubMed] [Google Scholar]
- Lacasta D, Ferrer L, Ramos J, González J, Ortín A and Fthenakis G, 2015. Vaccination schedules in small ruminant farms. Veterinary Microbiology, 181, 34–46. [DOI] [PubMed] [Google Scholar]
- Lallès JP, Bosi P, Smidt H and Stokes CR, 2007. Weaning ‐ a challenge to gut physiologists. Livestock Science, 108, 82–93. [Google Scholar]
- Lam TJGM, Wessels RJ and Jansen J, 2016. RESET the mindset of antibiotic usage in dairy cows. Proceedings of the World Buiatrics Congress, Dublin, Ireland, 3–8 July 2016, 82–84.
- Landers TF, Cohen B, Wittum TE and Larson EL, 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Reports, 127, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin H, Mörk MJ, Larsson M and Waller KP, 2015. Vaccination against Staphylococcus aureus mastitis in two Swedish dairy herds. Acta Veterinaria Scandinavica, 57, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Schubert S, Jung J, Kostrzewa M and Sparbier K, 2014. Quantitative matrix‐assisted laser desorption ionization‐time of flight mass spectrometry for rapid resistance detection. Journal of Clinical Microbiology, 52, 4155–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L and Rostagno M, 2013. Impact of heat stress on poultry production. Animals, 3, 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen I, Nielsen SS, Olsen JE and Nielsen JP, 2016. The efficacy of oxytetracycline treatment at batch, pen and individual level on Lawsonia intracellularis infection in nursery pigs in a randomised clinical trial. Preventive Veterinary Medicine, 124, 25–33. [DOI] [PubMed] [Google Scholar]
- Laureyns J, Ribbens S and de Kruif A, 2010. Control of bovine virus diarrhoea at the herd level: reducing the risk of false negatives in the detection of persistently infected cattle. The Veterinary Journal, 184, 21–26. [DOI] [PubMed] [Google Scholar]
- Lava M, Pardon B, Schüpbach‐Regula G, Keckeis K, Deprez P, Steiner A and Meylan M, 2016a. Effect of calf purchase and other herd‐level risk factors on mortality, unwanted early slaughter, and use of antimicrobial group treatments in Swiss veal calf operations. Preventive Veterinary Medicine, 126, 81–88. [DOI] [PubMed] [Google Scholar]
- Lava M, Schüpbach‐Regula G, Steiner A and Meylan M, 2016b. Antimicrobial drug use and risk factors associated with treatment incidence and mortality in Swiss veal calves reared under improved welfare conditions. Preventive Veterinary Medicine, 126, 121–130. [DOI] [PubMed] [Google Scholar]
- Lazarus B, Paterson DL, Mollinger JL and Rogers BA, 2015. Do Human extraintestinal escherichia coli infections resistant to expanded‐spectrum cephalosporins originate from food‐producing animals? A systematic review. Clinical Infectious Diseases, 60, 439–452. [DOI] [PubMed] [Google Scholar]
- Lee SK, Chon JW, Song KY, Hyeon JY, Moon JS and Seo KH, 2013. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poultry Science, 92, 2789–2797. [DOI] [PubMed] [Google Scholar]
- Lee YK, Katya K, Yun HH, Yoon MY, Park JK, Sung JS, Shin HS and Bai SC, 2015. Evaluation of dietary yellow loess as an antibiotic replacer on growth, immune responses, serological characteristics and disease resistance in rainbow trout, Oncorhynchus mykiss. Aquaculture Nutrition, 22, 1018–1025. [Google Scholar]
- Leekitcharoenphon P, Raufu I, Nielsen MT, Lund BSR, Ameh JA, Ambali AG, Sørensen G, Le Hello S, Aarestrup FM and Hendriksen RS, 2016. Investigating Salmonella Eko from various sources in Nigeria by whole genome sequencing to identify the source of human infections. PLoS ONE, 11, e0156212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees P and Shojaee Aliabadi F, 2002. Rational dosing of antimicrobial drugs: animals versus humans. International Journal of Antimicrobial Agents, 19, 269–284. [DOI] [PubMed] [Google Scholar]
- LeJeune JT and Kauffman MD, 2005. Effect of sand and sawdust bedding materials on the fecal prevalence of Escherichia coli O157: H7 in dairy cows. Applied and Environmental Microbiology, 71, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestari SI, Han F, Wang F and Ge B, 2009. Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. Journal of Food Protection, 72, 1165–1172. [DOI] [PubMed] [Google Scholar]
- Levy S, 2014. Reduced antibiotic use in livestock: how Denmark tackled resistance. Environmental Health Perspectives, 122, A160–A165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BT, Van Kessel AG, Caine WR, Huang SX and Kirkwood RN, 2001. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Canadian Journal of Animal Science, 81, 511–516. [Google Scholar]
- Li H, Ma ML, Xie HJ and Kong J, 2012. Biosafety evaluation of bacteriophages for treatment of diarrhea due to intestinal pathogen Escherichia coli 3‐2 infection of chickens. World Journal of Microbiology & Biotechnology, 28, 1–6. [DOI] [PubMed] [Google Scholar]
- Li X, Wang L, Zhen Y, Li S and Xu Y, 2015. Chicken egg yolk antibodies (IgY) as non‐antibiotic production enhancers for use in swine production: a review. Journal of Animal Science and Biotechnology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakopoulos A, Geurts Y, Dierikx CM, Brouwer MSM, Kant A, Wit B, Heymans R, van Pelt W and Mevius DJ, 2016. Extended‐Spectrum Cephalosporin‐Resistant Salmonella enterica serovar Heidelberg Strains, the Netherlands. Emerging Infectious Diseases, 22, 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, 2014. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Frontiers in Microbiology, 5, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL and Alenius S, 1999. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Veterinary Microbiology, 64, 197–222. [DOI] [PubMed] [Google Scholar]
- Lindberg JE, 2014. Fiber effects in nutrition and gut health in pigs. Journal of Animal Science and Biotechnology, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Pieper R, Rieger J, Vahjen W, Davin R, Plendl J, Meyer W and Zentek J, 2014. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PloS One, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y‐Y, Wang Y, Walsh TR, Yi L‐X, Zhang R, Spencer J, Doi Y, Tian G, Dong B and Huang X, 2015. Emergence of plasmid‐mediated colistin resistance mechanism MCR‐1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases, 16, 161–168. [DOI] [PubMed] [Google Scholar]
- Liu C‐Y, Han Y‐Y, Shih P‐H, Lian W‐N, Wang H‐H, Lin C‐H, Hsueh P‐R, Wang J‐K and Wang Y‐L, 2016. Rapid bacterial antibiotic susceptibility test based on simple surface‐enhanced Raman spectroscopic biomarkers. Scientific Reports, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AB, Cumming RB and Kent RD, 1977. Prevention of Salmonella Typhimurium infection in poultry by pretreatment of chickens and poults with intestinal extracts. Australian Veterinary Journal, 53, 82–87. [DOI] [PubMed] [Google Scholar]
- Loh TC, Choe DW, Foo HL, Sazili AQ and Bejo MH, 2014. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Veterinary Research, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, Sul WJ, Stedtfeld TM, Chai B and Cole JR, 2012. In‐feed antibiotic effects on the swine intestinal microbiome. Proceedings of the National Academy of Sciences, 109, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I, Earley B, Gilmore J, Hogan I, Kennedy E and More SJ, 2011. Calf health from birth to weaning. III. Housing and management of calf pneumonia. Irish Veterinary Journal, 64, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough G, Kyriazakis I, Bergmann S, Lengeling A and Doeschl‐Wilson AB, 2015. Health trajectories reveal the dynamic contributions of host genetic resistance and tolerance to infection outcome. Proceedings of the Royal Society B: Biological Sciences B, 20152151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovaas B, 2006. BVD in the Feedlot, Part I. Extension, University of Minnesota; Available online: http://www.extension.umn.edu/agriculture/beef/components/pdfs/09-01-06-Lovaas.pdf [Google Scholar]
- Luangtongkum T, Morishita TY, Ison AJ, Huang S, McDermott PF and Zhang Q, 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Applied and Environment Microbiology, 72, 3600–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückstädt C, 2006. Use of organic acids as feed additives—sustainable aquaculture production the non‐antibiotic way. Int Aquafeed, 9, 21–26. [Google Scholar]
- Luis Yaniz J, Angeles Marco‐Aguado M, Angel Mateos J and Santolaria P, 2010. Bacterial contamination of ram semen, antibiotic sensitivities, and effects on sperm quality during storage at 15 degrees C. Animal Reproduction Science, 122, 142–149. [DOI] [PubMed] [Google Scholar]
- Lund V, 2006. Natural living – a precondition for animal welfare in organic farming. Livestock Science, 100, 71–83. [Google Scholar]
- Lund M, Hougaard JL, Biira J and Houe H (University of Copenhagen), 2012. Economic incentives for disease prevention in Danish pig and cattle herds. 70.
- M'Sadeq SA, Wu S, Swick RA and Choct M, 2015. Towards the control of necrotic enteritis in broiler chickens with in‐feed antibiotics phasing‐out worldwide. Animal Nutrition, 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Gao Y and Maresso AW, 2015. Escherichia coli free radical‐based killing mechanism driven by a unique combination of iron restriction and certain antibiotics. Journal of Bacteriology, 197, 3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes JI and Desrosiers R, 1999. Agents of the “suis‐ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 63, 83–89. [PMC free article] [PubMed] [Google Scholar]
- Maciuca IE, Williams NJ, Tuchilus C, Dorneanu O, Guguianu E, Carp‐Carare C, Rimbu C and Timofte D, 2015. High Prevalence OF Escherichia coli‐producing CTX‐M‐15 extended‐spectrum beta‐lactamases in poultry and human clinical isolates in Romania. Microbial Drug Resistance. [DOI] [PubMed] [Google Scholar]
- Mack LA, Felver‐Gant JN, Dennis RL and Cheng HW, 2013. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poultry Science, 92, 285–294. [DOI] [PubMed] [Google Scholar]
- Madec F, Bridoux N, Bounaix S and Jestin A, 1998. Measurement of digestive disorders in the piglet at weaning and related risk factors. Preventive Veterinary Medicine, 35, 53–72. [DOI] [PubMed] [Google Scholar]
- Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Vrijens B and De Kruif A, 2000. Herd factors associated with the seroprevalences of four major respiratory pathogens in slaughter pigs from farrow‐to‐finish pig herds. Veterinary Research, 31, 313–327. [DOI] [PubMed] [Google Scholar]
- Maes D, Segales J, Meyns T, Sibila M, Pieters M and Haesebrouck F, 2008. Control of Mycoplasma hyopneumoniae infections in pigs. Veterinary Microbiology, 126, 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard JY, Bloomfield S, Coelho JR, Collier P, Cookson B, Fanning S, Hill A, Hartemann P, McBain AJ, Oggioni M, Sattar S, Schweizer HP and Threlfall J, 2013. Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microbial Drug Resistance, 19, 344–354. [DOI] [PubMed] [Google Scholar]
- Makoschey B and Bielsa J, 2007. Europe's progress in IBR virus eradication. International Dairy Topics, 6, 13–14. [Google Scholar]
- Mallard BA, Emam M, Paibomesai M, Thompson‐Crispi K and Wagter‐Lesperance L, 2015. Genetic selection of cattle for improved immunity and health. Japanese Journal of Veterinary Research, 63, S37–S44. [PubMed] [Google Scholar]
- Mankad A, 2016. Psychological influences on biosecurity control and farmer decision‐making. A review. Agronomy for Sustainable Development, 36, 1–14. [Google Scholar]
- Mapara N, Sharma M, Shriram V, Bharadwaj R, Mohite K and Kumar V, 2015. Antimicrobial potentials of Helicteres isora silver nanoparticles against extensively drug‐resistant (XDR) clinical isolates of Pseudomonas aeruginosa. Applied Microbiology and Biotechnology, 99, 10655–10667. [DOI] [PubMed] [Google Scholar]
- MARAN , 2011. MARAN‐2009 ‐ Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands In 2009. Available online: http://edepot.wur.nl/165958
- MARAN , 2012. MARAN‐2012 ‐ Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2010/2011. Available online: https://www.wageningenur.nl/upload_mm/f/2/8/39a1adb8-497e-49d6-b696-9401f23089f5_MARAN2012.pdf
- MARAN , 2015. MARAN‐2015 ‐ Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2014. Available online: http://www.wageningenur.nl/upload_mm/2/2/2/0ab4b3f5-1cf0-42e7-a460-d67136870ae5_NethmapMaran2015.pdf
- MARAN , 2016. MARAN‐2016 ‐ Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2015. Available online: http://www.wur.nl/upload_mm/0/b/c/433ca2d5-c97f-4aa1-ad34-a45ad522df95_92416_008804_NethmapMaran2016+TG2.pdf
- Mariadason JM, Barkla DH and Gibson PR, 1997. Effect of short‐chain fatty acids on paracellular permeability in Caco‐2 intestinal epithelium model. American Journal of Physiology, 272, G705–G712. [DOI] [PubMed] [Google Scholar]
- Mariner JC, House JA, Mebus CA, Sollod AE, Chibeu D, Jones BA, Roeder PL, Admassu B and van't Klooster GG, 2012. Rinderpest eradication: appropriate technology and social innovations. Science, 337, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Marquetoux N, Stevenson MA, Wilson P, Ridler A and Heuer C, 2016. Using social network analysis to inform disease control interventions. Preventive Veterinary Medicine, 126, 94–104. [DOI] [PubMed] [Google Scholar]
- Martin L, Andreassi E, Watson W and Coon C, 2011. Stress and animal health: physiological mechanisms and ecological consequences. Nature Education Knowledge, 3. [Google Scholar]
- Martinez AW, Phillips ST, Whitesides GM and Carrilho E, 2010. Diagnostics for the developing world: microfluidic paper‐based analytical devices. Analytical Chemistry, 82, 3–10. [DOI] [PubMed] [Google Scholar]
- Martinez MN, Papich MG and Drusano GL, 2012. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrobial Agents and Chemotherapy, 56, 2795–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny D, Visscher A, Catry B, Chatellier S and Vandenberg O, 2013. Optimization of Campylobacter growth conditions for further identification by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS). Journal of Microbiological Methods, 94, 221–223. [DOI] [PubMed] [Google Scholar]
- McAloon CG, Whyte P, More SJ, O'Grady L and Doherty ML, 2015. Development of a HACCP‐based approach to control paratuberculosis in infected Irish dairy herds. Preventive Veterinary Medicine, 120, 152–161. [DOI] [PubMed] [Google Scholar]
- McCapes R, Yamamoto R, Ortmayer H and Scott W, 1975. Injecting antibiotics into turkey hatching eggs to eliminate Mycoplasma meleagridis infection. Avian Diseases, 506–514. [PubMed] [Google Scholar]
- McDermott JJ and Schukken YH, 1994. A review of methods used to adjust for cluster effects in explanatory epidemiologic studies of animal populations. Preventive Veterinary Medicine, 18, 155–173. [Google Scholar]
- McDermott PF, Tyson GH, Kabera C, Chen Y, Li C, Folster JP, Ayers SL, Lam C, Tate HP and Zhao S, 2016. Whole‐genome sequencing for detecting antimicrobial resistance in nontyphoidal salmonella. Antimicrobial Agents and Chemotherapy, 60, 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS and Stellar E, 1993. Stress and the individual: mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease: allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McIlroy S, McCracken R, Neill S and O'Brien J, 1989. Control, prevention and eradication of Salmonella enteritidis infection in broiler and broiler breeder flocks. The Veterinary Record, 125, 545–548. [DOI] [PubMed] [Google Scholar]
- Mee JF, Geraghty T, O'Neill R and More SJ, 2012. Bioexclusion of diseases from dairy and beef farms: risks of introducing infectious agents and risk reduction strategies. The Veterinary Journal, 194, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee‐Marquet NLvd, Corvaglia A, Haenni M, Bertrand X, Franck JB, Kluytmans J, Girard M, Quentin R and Francois P, 2014. Emergence of a novel subpopulation of CC398 Staphylococcus aureus infecting animals is a serious hazard for humans. Frontiers in Microbiology, 5, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen EN, Walker J, Peters A, Pastoret P‐P and Jungersen G, 2007. Current status of veterinary vaccines. Clinical Microbiology Reviews, 20, 489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkebeek V, Goddeeris BM and Cox E, 2013. ETEC vaccination in pigs. Veterinary Immunology and Immunopathology, 152, 37–42. [DOI] [PubMed] [Google Scholar]
- Melo ADB, Amaral AF, Schaefer G, Luciano FB, de Andrade C, Costa LB and Rostagno MH, 2015. Antimicrobial effect against different bacterial strains and bacterial adaptation to essential oils used as feed additives. Canadian Journal of Veterinary Research, 79, 285–289. [PMC free article] [PubMed] [Google Scholar]
- Menanteau‐Ledouble S, Krauss I, Santos G, Fibi S, Weber B and El‐Matbouli M, 2015. Effect of a phytogenic feed additive on the susceptibility of Onchorhynchus mykiss to Aeromonas salmonicida. Diseases of Aquatic Organisms, 115, 57–66. [DOI] [PubMed] [Google Scholar]
- Mench JA, 2002. Broiler breeders: feed restriction and welfare. Worlds Poultry Science Journal, 58, 23–29. [Google Scholar]
- Meszaros J, Stipkovits L, Antal T, Szabo I and Veszely P, 1985. Eradication of some infectious pig diseases by perinatal tiamulin treatment and early weaning. The Veterinary Record, 116, 8–12. [DOI] [PubMed] [Google Scholar]
- Meunier‐Salaun MC, Edwards SA and Robert S, 2001. Effect of dietary fibre on the behaviour and health of the restricted fed sow. Animal Feed Science and Technology, 90, 53–69. [Google Scholar]
- Midtlyng PJ, Grave K and Horsberg TE, 2011. What has been done to minimize the use of antibacterial and antiparasitic drugs in Norwegian aquaculture? Aquaculture Research, 42, 28–34. [Google Scholar]
- Miglior F, Muir B and Van Doormaal B, 2005. Selection indices in Holstein cattle of various countries. Journal of Dairy Science, 88, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Miglior F, Koeck A, Jamrozik J, Schenkel F, Kelton D, Kistemaker G and Van Doormaal B, 2014. Index for mastitis resistance and use of BHBA for evaluation of health traits in Canadian Holsteins. Interbull Bulletin, 48, 73–78. [Google Scholar]
- Millman JM, Waits K, Grande H, Marks AR, Marks JC, Price LB and Hungate BA, 2013. Prevalence of antibiotic‐resistant E. coli in retail chicken: comparing conventional, organic, kosher, and raised without antibiotics. F1000Research, 2, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton JE, 1994. Function of the hypothalamic‐pituitary‐adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. Journal of Animal Science, 72, 1891–1898. [DOI] [PubMed] [Google Scholar]
- Miranda JM, Guarddon M, Mondragon A, Vazquez BI, Fente CA, Cepeda A and Franco CM, 2007. Antimicrobial resistance in Enterococcus spp. strains isolated from organic chicken, conventional chicken, and turkey meat: a comparative survey. Journal of Food Protection, 70, 1021–1024. [DOI] [PubMed] [Google Scholar]
- Miranda JM, Guarddon M, Vazquez BI, Fente CA, Barros‐Velazquez J, Cepeda A and Franco CM, 2008a. Antimicrobial resistance in Enterobacteriaceae strains isolated from organic chicken, conventional chicken and conventional turkey meat: a comparative survey. Food Control, 19, 412–416. [DOI] [PubMed] [Google Scholar]
- Miranda JM, Vazquez BI, Fente CA, Barros‐Velazquez J, Cepeda A and Abuin CMF, 2008b. Antimicrobial resistance in Escherichia coli strains isolated from organic and conventional pork meat: a comparative survey. European Food Research and Technology, 226, 371–375. [Google Scholar]
- Moennig V, Houe H and Lindberg A, 2005. BVD control in Europe: current status and perspectives. Animal Health Research Reviews, 6, 63–74. [DOI] [PubMed] [Google Scholar]
- Moinard C, Mendl M, Nicol CJ and Green LE, 2003. A case control study of on‐farm risk factors for tail biting in pigs. Applied Animal Behaviour Science, 81, 333–355. [Google Scholar]
- Mojsoska B and Jenssen H, 2015. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals, 8, 366–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølbak K, Simonsen J, Jørgensen CS, Krogfelt KA, Falkenhorst G, Ethelberg S, Takkinen J and Emborg H‐D, 2014. Seroincidence of human infections with nontyphoid Salmonella compared with data from public health surveillance and food animals in 13 European countries. Clinical Infectious Diseases, 59, 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla B, Berhanu A, Muckle A, Cole L, Wilkie E, Kleer J and Hildebrandt G, 2006. Multidrug resistance and distribution of Salmonella serovars in slaughtered pigs. Journal of Veterinary Medicine Series B‐Infectious Diseases and Veterinary Public Health, 53, 28–33. [DOI] [PubMed] [Google Scholar]
- Mollenkopf DF, Cenera JK, Bryant EM, King CA, Kashoma I, Kumar A, Funk JA, Rajashekara G and Wittum TE, 2014. Organic or antibiotic‐free labeling does not impact the recovery of enteric pathogens and antimicrobial‐resistant escherichia coli from fresh retail chicken. Foodborne Pathogens and Disease, 11, 920–929. [DOI] [PubMed] [Google Scholar]
- Monnet DL, Ferech M, Frimodt‐Møller N and Goossens H, 2005. The more antibacterial trade names, the more consumption of antibacterials: a European study. Clinical Infectious Diseases, 41, 114–117. [DOI] [PubMed] [Google Scholar]
- Moore DA, Merryman ML, Hartman ML and Klingborg DJ, 2008. Comparison of published recommendations regarding biosecurity practices for various production animal species and classes. Journal of the American Veterinary Medical Association, 233, 249–256. [DOI] [PubMed] [Google Scholar]
- Moquet P, Onrust L, van Immerseel F, Ducatelle R, Hendriks W and Kwakkel R, 2016. Importance of release location on the mode of action of butyrate derivatives in the avian gastrointestinal tract. World's Poultry Science Journal, 72, 61–80. [Google Scholar]
- More SJ, 2009. Global trends in milk quality: implications for the Irish dairy industry. Irish Veterinary Journal, 62, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SJ, Radunz B and Glanville RJ, 2015. Lessons learned during the successful eradication of bovine tuberculosis from Australia. Veterinary Record, 177, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, 2012. Survey of quantitative antimicrobial consumption in two different pig finishing systems. Veterinary Record, 171, 325. [DOI] [PubMed] [Google Scholar]
- Moreno MA, 2014a. Opinions of Spanish pig producers on the role, the level and the risk to public health of antimicrobial use in pigs. Research in Veterinary Science, 97, 26–31. [DOI] [PubMed] [Google Scholar]
- Moreno MA, 2014b. Survey of quantitative antimicrobial consumption per production stage in farrow‐to‐finish pig farms in Spain. Veterinary Record Open, 1, e000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, 2007. A review of genetic resistance to disease in Bos taurus cattle. Veterinary Journal, 174, 481–491. [DOI] [PubMed] [Google Scholar]
- Mortensen S, Stryhn H, Søgaard R, Boklund A, Stärk KD, Christensen J and Willeberg P, 2002. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Preventive Veterinary Medicine, 53, 83–101. [DOI] [PubMed] [Google Scholar]
- Mourao J, Machado J, Novais C, Antunes P and Peixe L, 2014. Characterization of the emerging clinically‐relevant multidrug‐resistant Salmonella enterica serotype 4, 5,12:i:‐ (monophasic variant of S. Typhimurium) clones. European Journal of Clinical Microbiology and Infectious Diseases, 33, 2249–2257. [DOI] [PubMed] [Google Scholar]
- Mourao J, Novais C, Machado J, Peixe L and Antunes P, 2015. Metal tolerance in emerging clinically relevant multidrug‐ resistant Salmonella enterica serotype 4, 5,12: i:‐ clones circulating in Europe Joana. International Journal of Antimicrobial Agents, 45, 610–616. [DOI] [PubMed] [Google Scholar]
- Mrode R and Swanson G, 1996. Genetic and statistical properties of somatic cell count and its suitability as an indirect means of reducing the incidence of mastitis in dairy cattle. Animal Breeding Abstracts (United Kingdom).
- Mulero V, Esteban MA, Muñoz J and Meseguer J, 1998. Dietary intake of levamisole enhances the immune response and disease resistance of the marine teleost gilthead seabream (Sparus aurata L.). Fish and Shellfish Immunology, 8, 49–62. [Google Scholar]
- Müller T, Bätza HJ, Schlüter H, Conraths F and Mettenleiter T, 2003. Eradication of Aujeszky's disease in Germany. Journal of Veterinary Medicine, Series B, 50, 207–213. [DOI] [PubMed] [Google Scholar]
- Munsterhjelm C, Peltoniemi OAT, Heinonen M, Halli O, Karhapaa M and Valros A, 2009. Experience of moderate bedding affects behaviour of growing pigs. Applied Animal Behaviour Science, 118, 42–53. [Google Scholar]
- Musliner KL, Seifuddin F, Judy JA, Pirooznia M, Goes FS and Zandi PP, 2015. Polygenic risk, stressful life events and depressive symptoms in older adults: a polygenic score analysis. Psychological Medicine, 45, 1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederlandstalige Gewestelijke Raad van de Orde der Dierenartsen 2015. Code der plichtenleer.
- Nelson JM, Chiller TM, Powers JH and Angulo FJ, 2007. Fluoroquinolone‐resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clinical Infectious Diseases, 44, 977–980. [DOI] [PubMed] [Google Scholar]
- Nesbakken T, 2012. Specific Pathogen‐Free (SPF) pig herds also free from zoonotic agents? Proceedings of the International Conference Biological Food Safety and Quality.
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA and Xavier RJ, 2016. Trained immunity: a program of innate immune memory in health and disease. Science, 352, aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry RC, 1995. Environmental enrichment ‐ increasing the biological relevance of captive environments. Applied Animal Behaviour Science, 44, 229–243. [Google Scholar]
- Nhung NT, Thuy CT, Trung NV, Campbell J, Baker S, Thwaites G, Hoa NT and Carrique‐Mas J, 2015. Induction of antimicrobial resistance in escherichia coli and non‐typhoidal salmonella strains after adaptation to disinfectant commonly used on farms in Vietnam. Antibiotics, 4, 480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE (National Institute for Health and Care Excellence), 2015. Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. Available online: https://www.nice.org.uk/guidance/ng15/resources/antimicrobial-stewardship-systems-and-processes-for-effective-antimicrobial-medicine-use-1837273110469
- Nicholls T, Acar J, Anthony F, Franklin A, Gupta R, Tamura Y, Thompson S, Threlfall EJ, Vose D, van Vuuren M, White DG, Wegener HC and Costarrica ML, 2001. Antimicrobial resistance: monitoring the quantities of antimicrobials used in animal husbandry. Revue Scientifique et Technique, 20, 841–847. [DOI] [PubMed] [Google Scholar]
- Nielsen SS and Toft N, 2011. Effect of management practices on paratuberculosis prevalence in Danish dairy herds. Journal of Dairy Science, 94, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Nielsen LR and Nielsen SS, 2012. A structured approach to control of Salmonella Dublin in 10 Danish dairy herds based on risk scoring and test‐and‐manage procedures. Food Research International, 45, 1158–1165. [Google Scholar]
- Niewold T, Van Dijk A, Geenen P, Roodink H, Margry R and Van Der Meulen J, 2007. Dietary specific antibodies in spray‐dried immune plasma prevent enterotoxigenic Escherichia coli F4 (ETEC) post weaning diarrhoea in piglets. Veterinary Microbiology, 124, 362–369. [DOI] [PubMed] [Google Scholar]
- Nilsen E, 2012. Automated identification and susceptibility determination directly from blood cultures facilitates early targeted antibiotic therapy. Scandinavian Journal of Infectious Diseases, 44, 860–865. [DOI] [PubMed] [Google Scholar]
- NORM/NORM‐VET , 2015. NORM/NORM‐VET 2014. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø / Oslo. Available online: http://www.vetinst.no/overvaking/antibiotikaresistens-norm-vet/_/attachment/download/14b6b9e7-1338-4fa9-af87-08cddff97a08:ef8a65c3682e4ec702900c3b2945a0b35c32b2e3/2014_NORM%20NORM-VET.pdf
- Nulsen MF, Mor MB and Lawton DEB, 2008. Antibiotic resistance among indicator bacteria isolated from healthy pigs in New Zealand. New Zealand Veterinary Journal, 56, 29–35. [DOI] [PubMed] [Google Scholar]
- Nuotio L, Neuvonen E and Hyytiäinen M, 2007. Epidemiology and eradication of infectious bovine rhinotracheitis/infectious pustular vulvovaginitis (IBR/IPV) virus in Finland. Acta Veterinaria Scandinavica, 49, 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi E and Rantala E, 1973. New aspects of Salmonella infection in broiler production. Nature, 241, 210–211. [DOI] [PubMed] [Google Scholar]
- Nya EJ and Austin B, 2010. Use of bacterial lipopolysaccharide (LPS) as an immunostimulant for the control of Aeromonas hydrophila infections in rainbow trout Oncorhynchus mykiss (Walbaum). Journal of Applied Microbiology, 108, 686–694. [DOI] [PubMed] [Google Scholar]
- O'Neill J 2015. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste ‐ The Review on Antimicrobial Resistance ‐ December 2015. Available online: http://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf
- O'Neill J, 2016. Tackling drug‐resistant infections globally: final report and recommendations. The review on antimicrobial resistance. Chaired by Jim O'Neill. May 2016. Available online: http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
- Oelschlaeger TA, 2010. Mechanisms of probiotic actions–a review. International Journal of Medical Microbiology, 300, 57–62. [DOI] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2012. Checklist on the Practical Application of Compartmentalisation. Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/A_CMP_Checklist.pdf
- OIE (World Organisation for Animal Health), 2015. Resolution No 26: Combating Antimicrobial Resistance and Promoting the Prudent Use of Antimicrobial Agents in Animals. Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_RESO_AMR_2015.pdf
- OIE (World Organisation for Animal Health), 2016. Resolution No 36: Combating Antimicrobial Resistance through a One Health Approach: Actions and OIE Strategy. Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_RESO_AMR_2016.pdf
- Oliveira J, Castilho F, Cunha A and Pereira M, 2012. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquaculture International, 20, 879–910. [Google Scholar]
- Oliver WT and Wells JE, 2015. Lysozyme as an alternative to growth promoting antibiotics in swine production. Journal of Animal Science and Biotechnology, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orda J, Jamroz D, Wiliczkiewicz A, Skorupińska J and Kubizna JK, 2012. The influence of porcine blood by‐products on laying hen performance, egg quality, and yolk mineral content. Journal of Animal and Feed Sciences, 21, 107–121. [Google Scholar]
- Osterberg J, Wingstrand A, Jensen AN, Kerouanton A, Cibin V, Barco L, Denis M, Aabo S and Bengtsson B, 2016. Antibiotic resistance in escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE, 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, Heck M, Savelkoul P, Vandenbroucke‐Grauls C, van der Zwaluw K, Huijsdens X and Kluytmans J, 2011. Extended‐spectrum beta‐lactamase genes of escherichia coli in chicken meat and humans, the Netherlands. Emerging Infectious Diseases, 17, 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai SS, Anas A, Jayaprakash NS, Priyaja P, Sreelakshmi B, Preetha R, Philip R, Mohandas A and Singh ISB, 2010. Penaeus monodon larvae can be protected from Vibrio harveyi infection by pre‐emptive treatment of a rearing system with antagonistic or non‐antagonistic bacterial probiotics. Aquaculture Research, 41, 847–860. [Google Scholar]
- Pajor EA, Rushen J and de Passille AMB, 2000. Aversion learning techniques to evaluate dairy cattle handling practices. Applied Animal Behaviour Science, 69, 89–102. [DOI] [PubMed] [Google Scholar]
- Papatsiros V, Katsoulos P, Koutoulis K, Karatzia M, Dedousi A and Christodoulopoulos G, 2013. Alternatives to antibiotics for farm animals. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 8, 1–15. [Google Scholar]
- Papich MG, 2014. Pharmacokinetic–pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Veterinary Microbiology, 171, 480–486. [DOI] [PubMed] [Google Scholar]
- Pappas G, Papadimitriou P, Akritidis N, Christou L and Tsianos EV, 2006. The new global map of human brucellosis. The Lancet Infectious Diseases, 6, 91–99. [DOI] [PubMed] [Google Scholar]
- Pardon B, Catry B, Dewulf J, Persoons D, Hostens M, De Bleecker K and Deprez P, 2012a. Prospective study on quantitative and qualitative antimicrobial and anti‐inflammatory drug use in white veal calves. Journal of Antimicrobial Chemotherapy, 67, 1027–1038. [DOI] [PubMed] [Google Scholar]
- Pardon B, De Bleecker K, Hostens M, Callens J, Dewulf J and Deprez P, 2012b. Longitudinal study on morbidity and mortality in white veal calves in Belgium. BMC Veterinary Research, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon B, Catry B, Boone R, Theys H, De Bleecker K, Dewulf J and Deprez P, 2014. Characteristics and challenges of the modern Belgian veal industry. Vlaams Diergeneeskundig Tijdschrift, 83, 155–163. [Google Scholar]
- Park YK, Fox LK, Hancock DD, McMahan W and Park YH, 2012. Prevalence and antibiotic resistance of mastitis pathogens isolated from dairy herds transitioning to organic management. Journal of Veterinary Science, 13, 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, 1966. Practical aspects of environmental temperature for laying hens. World's Poultry Science Journal, 22, 126–139. [Google Scholar]
- Pedersen KS, Okholm E, Johansen M, Angen Ø, Jorsal SE, Nielsen JP and Bækbo P, 2015. Clinical utility and performance of sock sampling in weaner pig diarrhoea. Preventive Veterinary Medicine, 120, 313–320. [DOI] [PubMed] [Google Scholar]
- Pejsak ZK and Truszczynski M, 2006. Aujeszky's disease (Pseudorabies) In: Straw BE, Zimmerman JJ, D'Allaire S. and Taylor DJ. (eds.). Diseases of Swine. 9th Edition Blackwell Science, Oxford, UK: pp. 419–433. [Google Scholar]
- Peng L, He Z, Chen W, Holzman IR and Lin J, 2007. Effects of butyrate on intestinal barrier function in a Caco‐2 cell monolayer model of intestinal barrier. Pediatric Research, 61, 37–41. [DOI] [PubMed] [Google Scholar]
- Pensaert M, Gielkens A, Lomniczi B, Kimman T, Vannier P and Eloit M, 1992. Round table on control of Aujeszky's disease and vaccine development based on molecular biology. Veterinary Microbiology, 33, 53–67. [DOI] [PubMed] [Google Scholar]
- Pensaert M, Labarque G, Favoreel H and Nauwynck H, 2003. Aujeszky's disease vaccination and differentiation of vaccinated from infected pigs. Developments in Biologicals, 119, 243–254. [PubMed] [Google Scholar]
- Persoons D, Dewulf J, Smet A, Herman L, Heyndrickx M, Martel A, Catry B, Butaye P and Haesebrouck F, 2012. Antimicrobial use in Belgian broiler production. Preventive Veterinary Medicine, 105, 320–325. [DOI] [PubMed] [Google Scholar]
- Pettey JH, Mifflin MD and Olson RJ, 2015. Study of the acute effects of povidone‐iodine on conjunctival bacterial flora. Journal of Ocular Pharmacology and Therapeutics, 31, 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham D, Ansquer D, Chevalier A, Dauga C, Peyramale A, Wabete N and Labreuche Y, 2014. Selection and characterization of potential probiotic bacteria for Litopenaeus stylirostris shrimp hatcheries in New Caledonia. Aquaculture, 432, 475–482. [Google Scholar]
- Pijpers A, Schoevers E, Van Gogh H, Van Leengoed L, Visser I, Van Miert A and Verheijden J, 1991. The influence of disease on feed and water consumption and on pharmacokinetics of orally administered oxytetracycline in pigs. Journal of Animal Science, 69, 2947–2954. [DOI] [PubMed] [Google Scholar]
- Piper C, Cotter PD, Ross RP and Hill C, 2009. Discovery of medically significant lantibiotics. Current Drug Discovery Technologies, 6, 1–18. [DOI] [PubMed] [Google Scholar]
- Pluske JR, 2013. Feed‐and feed additives‐related aspects of gut health and development in weanling pigs. Journal of Animal Science and Biotechnology, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L and Nordmann P, 2016. Emerging plasmid‐encoded colistin resistance: the animal world as the culprit? Journal of Antimicrobial Chemotherapy, dkw074. [DOI] [PubMed] [Google Scholar]
- Pokludova L, Nedbalcova K, Dubska M, Cerny T, Bures J and Satran P, 2016. The Czech Republic national programme of monitoring antimicrobial resistance of pathogens with veterinary importance. 8th AAVM conference, Budapest, Hungary, August 2016.
- Pol M and Ruegg PL, 2007a. Relationship between antimicrobial drug usage and antimicrobial susceptibility of gram‐positive mastitis pathogens. Journal of Dairy Science, 90, 262–273. [DOI] [PubMed] [Google Scholar]
- Pol M and Ruegg PL, 2007b. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. Journal of Dairy Science, 90, 249–261. [DOI] [PubMed] [Google Scholar]
- Poppe C, Martin L, Muckle A, Archambault M, McEwen S and Weir E, 2006. Characterization of antimicrobial resistance of Salmonella Newport isolated from animals, the environment, and animal food products in Canada. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 70, 105–114. [PMC free article] [PubMed] [Google Scholar]
- Postma M, Sjolund M, Collineau L, Losken S, Stark KD, Dewulf J, Andreasen M, Backhans A, Belloc C and Emanuelson U, 2015a. Assigning defined daily doses animal: a European multi‐country experience for antimicrobial products authorized for usage in pigs. Journal of Antimicrobial Chemotherapy, 70, 294–302. [DOI] [PubMed] [Google Scholar]
- Postma M, Stärk KD, Sjölund M, Backhans A, Beilage EG, Lösken S, Belloc C, Collineau L, Iten D and Visschers V, 2015b. Alternatives to the use of antimicrobial agents in pig production: A multi‐country expert‐ranking of perceived effectiveness, feasibility and return on investment. Preventive Veterinary Medicine, 118, 457–466. [DOI] [PubMed] [Google Scholar]
- Postma M, Speksnijder D, Jaarsma A, Verheij T, Wagenaar J and Dewulf J, 2016a. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Veterinary Record, 179, 68. [DOI] [PubMed] [Google Scholar]
- Postma M, Vanderhaeghen W, Sarrazin S, Maes D and Dewulf J, 2016b. Reducing Antimicrobial Usage in Pig Production without Jeopardizing Production Parameters. Zoonoses Public Health, online ahead of print. [DOI] [PubMed]
- Prasad Y, Arpana Kumar D and Sharma AK, 2011. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. Journal of Environmental Biology, 32, 161–168. [PubMed] [Google Scholar]
- Price LB, Johnson E, Vailes R and Silbergeld E, 2005. Fluoroquinolone‐resistant Campylobacter isolates from conventional and antibiotic‐free chicken products. Environmental Health Perspectives, 113, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier A, Heinonen M and Quesnel H, 2010. High physiological demands in intensively raised pigs: impact on health and welfare. Animal, 4, 886–898. [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Sweden and SVA (National Veterinary Institute), 2015. Swedres‐Svarm 2014 – Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2014.pdf
- Public Health Agency of Sweden and SVA (National Veterinary Institute), 2016. Swedres‐Svarm 2015 – Consumption of antibiotics and occurrence of antibiotic resistance in Sweden 2015. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2015.pdf
- Pulcini C and Gyssens IC, 2013. How to educate prescribers in antimicrobial stewardship practices. Virulence, 4, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaisrani S, Van Krimpen M, Kwakkel R, Verstegen M and Hendriks W, 2015. Dietary factors affecting hindgut protein fermentation in broilers: a review. World's Poultry Science Journal, 71, 139–160. [Google Scholar]
- Quigley Iii JD and Drew MD, 2000. Effects of oral antibiotics or bovine plasma on survival, health and growth in dairy calves challenged with Escherichia coli. Food and Agricultural Immunology, 12, 311–318. [Google Scholar]
- Quinteiro WM, Ribeiro A, Ferraz‐de‐Paula V, Pinheiro ML, Sakai M, Sa LRM, Ferreira AJP and Palermo‐Neto J, 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 89, 1905–1914. [DOI] [PubMed] [Google Scholar]
- Rabiee A and Lean I, 2013. The effect of internal teat sealant products (Teatseal and Orbeseal) on intramammary infection, clinical mastitis, and somatic cell counts in lactating dairy cows: a meta‐analysis. Journal of Dairy Science, 96, 6915–6931. [DOI] [PubMed] [Google Scholar]
- Radostits O, Gay C, Hinchcliff K and Constable P, 2007. Veterinary medicine: a textbook of the diseases of cattle, sheep, goats, pigs and horses. Saunders Co, London. [Google Scholar]
- Raith J, Trauffler M, Firth C, Lebl K, Schleicher C and Köfer J, 2016. Influence of porcine circovirus type 2 vaccination on the level of antimicrobial consumption on 65 Austrian pig farms. The Veterinary Record, 178, 504. [DOI] [PubMed] [Google Scholar]
- Ramli N, Heindl U and Sunanto S, 2005. Effect of potassium‐diformate on growth performance of tilapia challenged with Vibrio anguillarum. Abstract, World Aquaculture Society, World Aquaculture 2005, May 9–13, 2005, Bali, Indonesia.
- Randrianarivelo R, Danthu P, Benoit C, Ruez P, Raherimandimby M and Sarter S, 2010. Novel alternative to antibiotics in shrimp hatchery: effects of the essential oil of Cinnamosma fragrans on survival and bacterial concentration of Penaeus monodon larvae. Journal of Applied Microbiology, 109, 642–650. [DOI] [PubMed] [Google Scholar]
- Rappuoli R, Bottomley MJ, D'Oro U, Finco O and De Gregorio E, 2016. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. The Journal of Experimental Medicine, 213, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert G, Michiels J, Tagliabue M, Missotten J, De Smet S and Heyndrickx M, 2016. Effect of organic acids on Salmonella shedding and colonization in pigs on a farm with high Salmonella prevalence. Journal of Food Protection, 79, 51–58. [DOI] [PubMed] [Google Scholar]
- Rautiainen E, Oravainen J, Virolainen J and Tuovinen V, 2001. Regional eradication of Mycoplasma hyopneumoniae from pig herds and documentation of freedom of the disease. Acta Veterinaria Scandinavica, 42, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KA, Warnick LD, Mitchell RM, Kaneene JB, Ruegg PL, Wells SJ, Fossler CP, Halbert LW and May K, 2006. Antimicrobial susceptibility of Salmonella from organic and conventional dairy farms. Journal of Dairy Science, 89, 2038–2050. [DOI] [PubMed] [Google Scholar]
- RDA (Raad voor Dierenaangelegenheden), 2016. Antibioicabeleid in de Dierhouderij – Effectten en Perspectieven. Available online: https://www.rijksoverheid.nl/binaries/rijksoverheid/documenten/rapporten/2016/03/01/antibioticabeleid-in-de-dierhouderij-effecten-en-perspectieven/antibioticabeleid-in-de-dierhouderij-effecten-en-perspectieven.pdf
- Redondo LM, Chacana PA, Dominguez JE and Fernandez Miyakawa ME, 2014. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Frontiers in Microbiology, 5, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R and Busscher HJ, 2011. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nature Reviews Microbiology, 9, 27–38. [DOI] [PubMed] [Google Scholar]
- Renoux G, 1978. Modulation of immunity by levamisole. Pharmacology & Therapeutics Part a‐Chemotherapy Toxicology and Metabolic Inhibitors, 2, 397–423. [Google Scholar]
- Reverter M, Bontemps N, Lecchini D, Banaigs B and Sasal P, 2014. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture, 433, 50–61. [Google Scholar]
- Reynolds D, Davies R, Richards M and Wray C, 1997. Evaluation of combined antibiotic and competitive exclusion treatment in broiler breeder flocks infected with Salmonella enterica serovar Enteritidis. Avian Pathology, 26, 83–95. [DOI] [PubMed] [Google Scholar]
- Riber U, Heegaard PM, Cordes H, Ståhl M, Jensen TK and Jungersen G, 2015. Vaccination of pigs with attenuated Lawsonia intracellularis induced acute phase protein responses and primed cell‐mediated immunity without reduction in bacterial shedding after challenge. Vaccine, 33, 156–162. [DOI] [PubMed] [Google Scholar]
- Ricke SC, 2015. Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poultry Science, 94, 1411–1418. [DOI] [PubMed] [Google Scholar]
- Rinsky JL, Nadimpalli M, Wing S, Hall D, Baron D, Price LB, Larsen J, Stegger M, Stewart J and Heaney CD, 2013. Livestock‐associated methicillin and multidrug resistant staphylococcus aureus is present among industrial, not antibiotic‐free livestock operation workers in North Carolina. PLoS ONE, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M, 2007. Prebiotics: the concept revisited. Journal of Nutrition, 137, 830S–837S. [DOI] [PubMed] [Google Scholar]
- Robertson R and Jochelson K, 2006. Interventions that change clinician behaviour: mapping the literature. National Institute of Clinical Excellence (NICE).
- Rodenburg T and Turner S, 2012. The role of breeding and genetics in the welfare of farm animals. Animal Frontiers, 2, 16–21. [Google Scholar]
- Roelvink J, 2015. Association between farm management practices and the development of disease in the digestive and respiratory tract in young stock on Dutch dairy farms.
- Roesch M, Perreten V, Doherr MG, Schaeren W, Schallibaum M and Blum JW, 2006. Comparison of antibiotic resistance of udder pathogens in dairy cows kept on organic and on conventional farms. Journal of Dairy Science, 89, 989–997. [DOI] [PubMed] [Google Scholar]
- Rosell J and de la Fuente L, 2016. Causes of mortality in breeding rabbits. Preventive Veterinary Medicine, 127, 56–63. [DOI] [PubMed] [Google Scholar]
- Rossiter C, Hutchinson L, Hansen D and Whitlock R, 1999. Johne's disease prevention/control plan for beef herds: manual for veterinarians. The Bovine Practitioner, 33, 1–22. [Google Scholar]
- Rothrock MJ Jr, Ingram KD, Gamble J, Guard J, Cicconi‐Hogan KM, Hinton A Jr and Hiett KL, 2015. The characterization of Salmonella enterica serotypes isolated from the scalder tank water of a commercial poultry processing plant: recovery of a multidrug‐resistant Heidelberg strain. Poultry Science, 94, 467–472. [DOI] [PubMed] [Google Scholar]
- Rothrock MJ, Hiett KL, Guard JY and Jackson CR, 2016. Antibiotic resistance patterns of major zoonotic pathogens from all‐natural, antibiotic‐free, pasture‐raised broiler flocks in the Southeastern United States. Journal of Environmental Quality, 45, 593–603. [DOI] [PubMed] [Google Scholar]
- Rousing T and Waiblinger S, 2004. Evaluation of on‐farm methods for testing the human‐animal relationship in dairy herds with cubicle loose housing systems ‐ test‐retest and inter‐observer reliability and consistency to familiarity of test person. Applied Animal Behaviour Science, 85, 215–231. [Google Scholar]
- Routh J, Pringle J, Mohr M, Bidol S, Arends K, Adams‐Cameron M, Hancock W, Kissler B, Rickert R and Folster J, 2015. Nationwide outbreak of multidrug‐resistant Salmonella Heidelberg infections associated with ground turkey: United States, 2011. Epidemiology and Infection, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugna G, Bonilauri P, Carra E, Bergamini F, Luppi A, Gherpelli Y, Magistrali C, Nigrelli A, Alborali G and Martelli P, 2015. Sequence types and pleuromutilin susceptibility of Brachyspira hyodysenteriae isolates from Italian pigs with swine dysentery: 2003–2012. The Veterinary Journal, 203, 115–119. [DOI] [PubMed] [Google Scholar]
- Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X and Tang Y‐W, 2010. Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real‐time cell analysis system. Journal of Clinical Microbiology, 48, 4129–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad N, Delattre C, Urdaci M, Schmitter J‐M and Bressollier P, 2013. An overview of the last advances in probiotic and prebiotic field. LWT‐Food Science and Technology, 50, 1–16. [Google Scholar]
- Saastamoinen M, Särkijärvi S and Hyyppä S, 2015. Reducing respiratory health risks to horses and workers: a comparison of two stall bedding materials. Animals, 5, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitone TL, Sexton RJ and Sumner DA, 2015. What happens when food marketers require restrictive farming practices? American Journal of Agricultural Economics, 97, 1021–1043. [Google Scholar]
- Salaheen S, Chowdhury N, Hanning I and Biswas D, 2015. Organic poultry production with natural feed supplements as antimicrobials zoonotic bacterial pathogens and mixed crop‐livestock farming. Poultry Science, 94, 1398–1410. [DOI] [PubMed] [Google Scholar]
- Salak‐Johnson JL and McGlone JJ, 2007. Making sense of apparently conflicting data: stress and immunity in swine and cattle. Journal of Animal Science, 85, E81–E88. [DOI] [PubMed] [Google Scholar]
- Sanad YM, Johnson K, Park SH, Han J, Deck J, Foley SL, Kenney B, Ricke S and Nayak R, 2016. Molecular characterization of salmonella enterica serovars isolated from a turkey production facility in the absence of selective antimicrobial pressure. Foodborne Pathogens and Disease, 13, 80–87. [DOI] [PubMed] [Google Scholar]
- Sandiford SK, 2015. Perspectives on lantibiotic discovery‐where have we failed and what improvements are required? Expert Opinion on Drug Discovery, 10, 315–320. [DOI] [PubMed] [Google Scholar]
- Santman‐Berends I, Swinkels J, Lam T, Keurentjes J and van Schaik G, 2016. Evaluation of udder health parameters and risk factors for clinical mastitis in Dutch dairy herds in the context of a restricted antimicrobial usage policy. Journal of Dairy Science, 99, 2930–2939. [DOI] [PubMed] [Google Scholar]
- Sanz S, Olarte C, Martínez‐Olarte R, Navajas‐Benito EV, Alonso CA, Hidalgo‐Sanz S, Somalo S and Torres C, 2015. Airborne dissemination of Escherichia coli in a dairy cattle farm and its environment. International Journal of Food Microbiology, 197, 40–44. [DOI] [PubMed] [Google Scholar]
- Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P and Lawrence R, 2008. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environment International, 34, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Sapkota AR, Hulet RM, Zhang G, McDermott P, Kinney EL, Schwab KJ and Joseph SW, 2011. Lower prevalence of antibiotic‐resistant Enterococci on U.S. conventional poultry farms that transitioned to organic practices. Environmental Health Perspectives, 119, 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota AR, Kinney EL, George A, Hulet RM, Cruz‐Cano R, Schwab KJ, Zhang G and Joseph SW, 2014. Lower prevalence of antibiotic‐resistant Salmonella on large‐scale U.S. conventional poultry farms that transitioned to organic practices. Science of the Total Environment, 476–477, 387–392. [DOI] [PubMed] [Google Scholar]
- Sargeant HR, McDowall KJ, Miller HM and Shaw MA, 2010. Dietary zinc oxide affects the expression of genes associated with inflammation: transcriptome analysis in piglets challenged with ETEC K88. Veterinary Immunology and Immunopathology, 137, 120–129. [DOI] [PubMed] [Google Scholar]
- Sarmah AK, Meyer MT and Boxall AB, 2006. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 65, 725–759. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sekiguchi S, Uemura R and Sueyoshi M, 2015. The effect of depopulation and restocking on reproductive and growth performances on Japanese commercial swine farms. Journal of Veterinary Medical Science, 78, 333–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Bartlett PC, Kaneene JB and Downes FP, 2004. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter spp. isolates from organic and conventional dairy herds in Wisconsin. Applied and Environment Microbiology, 70, 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Bartlett PC and Saeed MA, 2005. Antimicrobial susceptibility of Escherichia coli isolates from dairy farms using organic versus conventional production methods. Journal of the American Veterinary Medical Association, 226, 589–594. [DOI] [PubMed] [Google Scholar]
- SCAHAW (Scientific Committee on Animal Health and Welfare), 2002. Report of the Scientific Committee on Animal Health and Welfare ‐ The welfare of animals during transport (details for horses, pigs, sheep and cattle). European Commission – Health and Consumer Protection Directorate‐General. Directorate C–Scientific Opinions.
- Schalbert A, 1991. Antimicrobial properties of tannins. Phytochemistry, 30, 3875–3883. [Google Scholar]
- Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Luhrs H and Gudmundsson GH, 2003. Expression of the cathelicidin LL‐37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut, 52, 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpenzeel C, den Uijl I, Van Schaik G, Riekerink RO, Keurentjes J and Lam T, 2014. Evaluation of the use of dry cow antibiotics in low somatic cell count cows. Journal of Dairy Science, 97, 3606–3614. [DOI] [PubMed] [Google Scholar]
- Scherpenzeel C, den Uijl I, van Schaik G, Riekerink RO, Hogeveen H and Lam T, 2016. Effect of different scenarios for selective dry‐cow therapy on udder health, antimicrobial usage, and economics. Journal of Dairy Science, 99, 3753–3764. [DOI] [PubMed] [Google Scholar]
- Schirrmeier H, 2014. Three years of mandatory BVD control in Germany – Lessons to be learned. Proceedings of the XXVIII World Buiatrics Congress, Cairns 2014.
- Schokker D, Zhang J, Zhang LL, Vastenhouw SA, Heilig H, Smidt H, Rebel JMJ and Smits MA, 2014. Early‐life environmental variation affects intestinal microbiota and immune development in new‐born piglets. PLoS ONE, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D, Veninga G, Vastenhouw SA, Bossers A, deBree FM , Kaal‐Lansbergen L, Rebel JMJ and Smits MA, 2015a. Early life microbial colonization of the gut and intestinal development differ between genetically divergent broiler lines. Bmc Genomics, 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D, Zhang J, Vastenhouw SA, Heilig H, Smidt H, Rebel JMJ and Smits MA, 2015b. Long‐lasting effects of early‐life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE, 10, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte JN, Brockmann GA and Kreuzer‐Redmer S, 2016. Feeding a high dosage of zinc oxide affects suppressor of cytokine gene expression in Salmonella Typhimurium infected piglets. Veterinary Immunology and Immunopathology, 178, 10–13. [DOI] [PubMed] [Google Scholar]
- Schwaiger K, Schmied EM and Bauer J, 2010. Comparative analysis on antibiotic resistance characteristics of Listeria spp. and Enterococcus spp. isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, Germany. Zoonoses Public Health, 57, 171–180. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Henry S, Tokach L, Potter M, Davidson D, Egnor C and PFFJ L, 2013. Infective material, concepts and procedures for intentional sow herd exposure to Porcine Epidemic Diarrhea virus. Iowa State University, 8.
- Scicluna MT, Issel CJ, Cook FR, Manna G, Cersini A, Rosone F, Frontoso R, Caprioli A, Antonetti V and Autorino GL, 2013. Is a diagnostic system based exclusively on agar gel immunodiffusion adequate for controlling the spread of equine infectious anaemia? Veterinary Microbiology, 165, 123–134. [DOI] [PubMed] [Google Scholar]
- SDa (Netherlands Veterinary Medicines Authority), 2013. Het gebruik van fluorochinolonen en derde en vierde generatie cefalosporines in landbouwhuisdieren – Rapportage van het SDa expertpanel. Available online: http://www.autoriteitdiergeneesmiddelen.nl/Userfiles/rapportage-sda-ep-fluorochinolonen-en-3e-4e-generatie-cefalosporinen-7-maart-2013.pdf
- SDa (Netherlands Veterinary Medicines Authority), 2016a. Associations between antimicrobial use and the prevalence of resistant micro‐organisms – Is it possible to benchmark livestock farms based on resistance data? Available online: http://www.autoriteitdiergeneesmiddelen.nl/Userfiles/rapport%20ab%20en%20resistentie/def-engels-rapport-abgebruik-en-resistentie-0516.pdf
- SDa (Netherlands Veterinary Medicines Authority), 2016b. Het gebruik van antibiotica bij landbouwhuisdieren in 2015 ‐ Trends, benchmarken bedrijven en dierenartsen, en aanpassing benchmarkwaardensystematiek. Available online: http://www.autoriteitdiergeneesmiddelen.nl/Userfiles/AB%20gebruik%202015/def-sda-rapportage-antibioticumgebruik-2015.pdf
- Selye H, 1985. History and present status of the stress concept In: Monat A, Lazarus RS, (eds.). Stress and Coping, 2nd Edition Columbia University, New York: pp. 17–29. [Google Scholar]
- Sellera FP, Gargano RG, Libera AM, Benesi FJ, Azedo MR, de Sa LR, Ribeiro MS, da Silva Baptista M and Pogliani FC, 2016. Antimicrobial photodynamic therapy for caseous lymphadenitis abscesses in sheep: Report of ten cases. Photodiagnosis and Photodynamic Therapy, 13, 120–122. [DOI] [PubMed] [Google Scholar]
- Senatore F, 1996. Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L.) growing wild in Campania (Southern Italy). Journal of Agricultural and Food Chemistry, 44, 1327–1332. [Google Scholar]
- Shang Y, Regassa A, Kim JH and Kim WK, 2015. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poultry Science, 94, 2887–2897. [DOI] [PubMed] [Google Scholar]
- Shao Y, Wang Z, Tian X, Guo Y and Zhang H, 2016. Yeast β‐d‐glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. International Journal of Biological Macromolecules, 85, 573–584. [DOI] [PubMed] [Google Scholar]
- Shea KM, Comm Env H and Comm Infect D, 2004. Nontherapeutic use of antimicrobial agents in animal agriculture: implications for pediatrics. Pediatrics, 114, 862–868. [DOI] [PubMed] [Google Scholar]
- Shtrichman R and Samuel CE, 2001. The role of gamma interferon in antimicrobial immunity. Current Opinion in Microbiology, 4, 251–259. [DOI] [PubMed] [Google Scholar]
- Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R and Du Z, 2006. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. Journal of Applied Microbiology, 100, 296–305. [DOI] [PubMed] [Google Scholar]
- Sibley R, 2000. Planning health care on dairy farms. In Practice, 0263841X, 22. [Google Scholar]
- Sibley R, 2006. Developing health plans for the dairy herd. In Practice, 0263841X, 28. [Google Scholar]
- Siemon CE, Bahnson PB and Gebreyes WA, 2007. Comparative investigations of prevalence and antimicrobial resistance of Salmonella between pasture and conventionally reared poultry. Avian Diseases, 51, 112–117. [DOI] [PubMed] [Google Scholar]
- Signorini M, Soto L, Zbrun M, Sequeira G, Rosmini M and Frizzo L, 2012. Impact of probiotic administration on the health and fecal microbiota of young calves: a meta‐analysis of randomized controlled trials of lactic acid bacteria. Research in Veterinary Science, 93, 250–258. [DOI] [PubMed] [Google Scholar]
- Silley P, de Jong A, Simjee S and Thomas V, 2011. Harmonisation of resistance monitoring programmes in veterinary medicine: an urgent need in the EU? International Journal of Antimicrobial Agents, 37, 504–512. [DOI] [PubMed] [Google Scholar]
- Silley P, Simjee S and Schwarz S, 2012. Surveillance and monitoring of antimicrobial resistance and antibiotic consumption in humans and animals. Revue scientifique et technique (International Office of Epizootics), 31, 105–120. [DOI] [PubMed] [Google Scholar]
- Silva MLF, Lima JAdF, Cantarelli VdS, Amaral NdO, Zangerônimo MG and Fialho ET, 2010. Probiotics and antibiotics as additives for sows and piglets during nursery phase. Revista Brasileira de Zootecnia, 39, 2453–2459. [Google Scholar]
- Silva YJ, Moreirinha C, Pereira C, Costa L, Rocha RJM, Cunha A, Gomes NCM, Calado R and Almeida A, 2016. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS‐A. Aquaculture, 450, 225–233. [Google Scholar]
- Šíma P, Trebichavský I and Sigler K, 2003. Mammalian antibiotic peptides. Folia Microbiologica, 48, 123–137. [DOI] [PubMed] [Google Scholar]
- Simoneit C, Bender S and Koopmann R, 2012. Quantitative and qualitative overview and assessment of literature on animal health in organic farming between 1991 and 2011–Part I: general and cattle. vTI Agriculture and Forestry Research, 62, 97–104. [Google Scholar]
- Singh EL, 1987. The disease control potential of embryos. Theriogenology, 27, 9–20. [Google Scholar]
- Sjölund M, Backhans A, Greko C, Emanuelson U and Lindberg A, 2015. Antimicrobial usage in 60 Swedish farrow‐to‐finish pig herds. Preventive Veterinary Medicine, 121, 257–264. [DOI] [PubMed] [Google Scholar]
- Sjölund M, Postma M, Collineau L, Lösken S, Backhans A, Belloc C, Emanuelson U, Beilage EG, Stärk K and Dewulf J, 2016. Quantitative and qualitative antimicrobial usage patterns in farrow‐to‐finish pig herds in Belgium, France, Germany and Sweden. Preventive Veterinary Medicine, 130, 41–50. [DOI] [PubMed] [Google Scholar]
- Skov RL and Monnet DL, 2016. Plasmid‐mediated colistin resistance (mcr‐1 gene): three months later, the story unfolds. Eurosurveillance, 21, 30155. [DOI] [PubMed] [Google Scholar]
- Slifierz M, Friendship R and Weese J, 2015. Zinc oxide therapy increases prevalence and persistence of methicillin‐resistant staphylococcus aureus in pigs: a randomized controlled trial. Zoonoses and Public Health, 62, 301–308. [DOI] [PubMed] [Google Scholar]
- Smith R, Stokka G, Radostits O and Griffin D, 2001. Health and production management in beef feedlots. Herd health: food animal production medicine. 3rd Edition WB Saunders Co, Philadelphia: pp.581–633. [Google Scholar]
- Smith TC, Gebreyes WA, Abley MJ, Harper AL, Forshey BM, Male MJ, Martin HW, Molla BZ, Sreevatsan S, Thakur S, Thiruvengadam M and Davies PR, 2013. Methicillin‐resistant staphylococcus aureus in pigs and farm workers on conventional and antibiotic‐free swine farms in the USA. PLoS ONE, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Spangler C, Brandeau ML, Hunter GE, Bavinger C, Pearson M, Eschbach PJ, Sundaram V, Liu H, Schirmer P, Stave C, Olkin I and Bravata DM, 2012. Are organic foods safer or healthier than conventional alternatives? Annals of Internal Medicine, 157, 348‐U112. [DOI] [PubMed] [Google Scholar]
- Snyder AB and Worobo RW, 2014. Chemical and genetic characterization of bacteriocins: antimicrobial peptides for food safety. Journal of the Science of Food and Agriculture, 94, 28–44. [DOI] [PubMed] [Google Scholar]
- Sommer MO, Church GM and Dantas G, 2010. The human microbiome harbors a diverse reservoir of antibiotic resistance genes. Virulence, 1, 299–303. [DOI] [PubMed] [Google Scholar]
- Song M, Liu Y, Soares JA, Che TM, Osuna O, Maddox CW and Pettigrew JE, 2012. Dietary clays alleviate diarrhea of weaned pigs. Journal of Animal Science, 90, 345–360. [DOI] [PubMed] [Google Scholar]
- Song J, Li YL and Hu CH, 2013. Effects of copper‐exchanged montmorillonite, as alternative to antibiotic, on diarrhea, intestinal permeability and proinflammatory cytokine of weanling pigs. Applied Clay Science, 77–78, 52–55. [Google Scholar]
- Song SK, Beck BR, Kim D, Park J, Kim J, Kim HD and Ringø E, 2014. Prebiotics as immunostimulants in aquaculture: a review. Fish and Shellfish Immunology, 40, 40–48. [DOI] [PubMed] [Google Scholar]
- Song ZH, Xiao K, Ke YL, Jiao LF and Hu CH, 2015. Zinc oxide influences mitogen‐activated protein kinase and TGF‐beta 1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immunity, 21, 341–348. [DOI] [PubMed] [Google Scholar]
- Sørensen MK, Jensen J and Christensen LG, 2000. Udder conformation and mastitis resistance in Danish first‐lactation cows: heritabilities, genetic and environmental correlations. Acta Agric Scand (A), 50, 72–82. [Google Scholar]
- Sparbier K, Schubert S, Weller U, Boogen C and Kostrzewa M, 2012. Matrix‐assisted laser desorption ionization–time of flight mass spectrometry‐based functional assay for rapid detection of resistance against β‐lactam antibiotics. Journal of Clinical Microbiology, 50, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speijers M, Baird L, Finney G, McBride J, Kilpatrick D, Logue D and O'Connell N, 2010. Effectiveness of different footbath solutions in the treatment of digital dermatitis in dairy cows. Journal of Dairy Science, 93, 5782–5791. [DOI] [PubMed] [Google Scholar]
- Speksnijder D, Jaarsma A, Gugten A, Verheij T and Wagenaar J, 2015a. Determinants associated with veterinary antimicrobial prescribing in farm animals in the Netherlands: a qualitative study. Zoonoses and Public Health, 62, 39–51. [DOI] [PubMed] [Google Scholar]
- Speksnijder DC, Jaarsma DA, Verheij TJ and Wagenaar JA, 2015b. Attitudes and perceptions of Dutch veterinarians on their role in the reduction of antimicrobial use in farm animals. Preventive Veterinary Medicine, 121, 365–373. [DOI] [PubMed] [Google Scholar]
- Speksnijder DC, Mevius DJ, Bruschke CJM and Wagenaar JA, 2015c. Reduction of veterinary antimicrobial use in the Netherlands. The Dutch Success Model. Zoonoses and Public Health, 62, 79–87. [DOI] [PubMed] [Google Scholar]
- Spillner E, Braren I, Greunke K, Seismann H, Blank S and du Plessis D, 2012. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals, 40, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoolder HAM, 2007. Animal welfare in organic farming systems. Journal of the Science of Food and Agriculture, 87, 2741–2746. [Google Scholar]
- Spoolder HA and Waiblinger S, 2009. Pigs and humans. In: The welfare of pigs. Springer, 211–236.
- Spoolder HAM, Aarnink AAJ, Vermeer HM, van Riel J and Edwards SA, 2012. Effect of increasing temperature on space requirements of group housed finishing pigs. Applied Animal Behaviour Science, 138, 229–239. [Google Scholar]
- Ståhl K and Alenius S, 2012. BVDV control and eradication in Europe‐an update. [PubMed]
- Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H and MacKenzie FM, 2012. Meticillin‐resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. International Journal of Antimicrobial Agents, 39, 273–282. [DOI] [PubMed] [Google Scholar]
- Sterling P and Eyer J, 1988. Allostasis: A new paradigm to explain arousal pathology In: Fisher S, Reason J. (eds.). Handbook of life stress cognition and health. John Wiley and sons, Inc. Chichester, England, UK; New York, New York, USA, pp. 629–650. [Google Scholar]
- Stevens M, Piepers S, Supré K, Dewulf J and De Vliegher S, 2016. Quantification of antimicrobial consumption in adult cattle on dairy herds in Flanders, Belgium, and associations with udder health, milk quality, and production performance. Journal of Dairy Science, 99, 2118–2130. [DOI] [PubMed] [Google Scholar]
- Stojanov I, Milanov D, Dosen R, Marcic D, Prodanov‐Radulovic J, Pusic I and Polacek V, 2015. Swine Dysentery: Practical Observations, Control And Diagnostics.
- Stolker A, Manti V, Zuidema T, van Egmond H, Deckers E, Herbes R, Hooglugt J, Olde Heuvel E and de Jong J, 2013. Carry‐over of veterinary drugs from medicated to non‐medicated feeds in commercial feed manufacturing plants. Food Additives and Contaminants: Part A, 30, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Stott AW, Humphry RW, Gunn GJ, Higgins I, Hennessy T, O'Flaherty J and Graham DA, 2012. Predicted costs and benefits of eradicating BVDV from Ireland. Irish Veterinary Journal, 65, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam MD and Kim IH, 2015. Clays as dietary supplements for swine: a review. Journal of Animal Science and Biotechnology, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundén F and Wullt B, 2015. Predictive value of urinary interleukin‐6 for symptomatic urinary tract infections in a nursing home population. International Journal of Urology. [DOI] [PubMed] [Google Scholar]
- Susick EK, Putnam M, Bermudez DM and Thakur S, 2012. Longitudinal study comparing the dynamics of Clostridium difficile in conventional and antimicrobial free pigs at farm and slaughter. Veterinary Microbiology, 157, 172–178. [DOI] [PubMed] [Google Scholar]
- Suthar S, Chhimpa V and Singh S, 2009. Bacterial contamination in drinking water: a case study in rural areas of northern Rajasthan, India. Environmental Monitoring and Assessment, 159, 43–50. [DOI] [PubMed] [Google Scholar]
- Sutmoller P, Barteling SS, Olascoaga RC and Sumption KJ, 2003. Control and eradication of foot‐and‐mouth disease. Virus Research, 91, 101–144. [DOI] [PubMed] [Google Scholar]
- SVA (National veterinary institute), 2009. Swedish veterinary antimicrobial resistance monitoring 2008. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/svarm-2008-vers-19-maj.pdf. 0346‐2250
- SVA (National Veterinary Institute), 2010. Swedish Veterinary Antimicrobial Resistance Monitoring 2009. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/trycksaker/1/svarm-2009.pdf
- SVA (National Veterinary Institute), 2011. Swedish Veterinary Antimicrobial Resistance Monitoring 2010. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/trycksaker/svarm2010.pdf
- SVA (National veterinary institute), 2015. Swedres‐Svarm 2014. Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Available at: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2014.pdf
- SVA (National veterinary institute), 2016. Surveillance of infectious diseases in animals and humans in 2015. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/surveillance-2015-w.pdf
- SVS (Swedish Veterinary Association), 1990. SVS 1990, Riktlinjer för antibiotikainblandning i foder till svin (Guidelines on antimicrobials in feed for pigs). Svensk veterinärtidning, 42, 407–413. [Google Scholar]
- SVS (Swedish Veterinary Association), 2013. Riktlinjer för användning av antibiotika till produktionsdjur ‐ nötkreatur och gris. Available online: http://www.svf.se/Documents/S%C3%A4llskapet/Husdjurssektionen/SVS%20Riktlinjer%20f%C3%B6r%20anv%C3%A4ndning%20av%20antibiotika%20till%20produktionsdjur%202013.pdf
- SVS (Swedish Veterinary Association), 2014. Riktlinjer för antibiotikaanvändning till får och get. Available online: http://www.svf.se/Documents/S%C3%A4llskapet/Husdjurssektionen/SVS%20Riktlinjer%20antibiotika%20f%C3%A5r%20o%20get.pdf
- Swain P, Nayak SK, Nanda PK and Dash S, 2008. Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: a review. Fish and Shellfish Immunology, 25, 191–201. [DOI] [PubMed] [Google Scholar]
- Swann M, Baxter K and Field H, 1969. Report of the joint committee on the use of antibiotics in animal husbandry and veterinary medicine. Her Majesty's Stationary Office, London. [Google Scholar]
- Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, Kote‐Jarai Z, Amin Al Olama A, Benlloch S and Muir K, 2015. Prediction of individual genetic risk to prostate cancer using a polygenic score. The Prostate, 75, 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait J, Bruce A, Mittra J, Purves J and Scannell J, 2014. Independent review on antimicrobial resistance regulation/innovation interactions and the development of antimicrobial drugs and diagnostics for human and animal diseases: Main Report. 14th Dec., 2014. Available online: https://amr-review.org/sites/default/files/AMR_Final_Report_141214_0.pdf
- Tamang MD, Gurung M, Nam HM, Moon DC, Kim SR, Jang GC, Jung DY, Jung SC, Park YH and Lim SK, 2015. Prevalence and characterization of Salmonella in pigs from conventional and organic farms and first report of S. serovar 1,4,[5],12:i:‐ from Korea. Veterinary Microbiology, 178, 119–124. [DOI] [PubMed] [Google Scholar]
- Taverne F, Jacobs J, Heederik D, Mouton J, Wagenaar J and van Geijlswijk I, 2015. Influence of applying different units of measurement on reporting antimicrobial consumption data for pig farms. BMC Veterinary Research, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, 2013. Pig diseases. 9th Edition, 5m Publishing, Sheffield. [Google Scholar]
- Temtem C, Brinch Kruse A, Nielsen LR, Pedersen KS and Alban L, 2016. Comparison of the antimicrobial consumption in weaning pigs in Danish sow herds with different vaccine purchase patterns during 2013. Porcine Health Management, 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hooven M, 2007. Danish SPF system proves its efficacy. Pig Progress, 23, 24–25. [Google Scholar]
- Thomsen BL, Jorsal SE, Andersen S and Willeberg P, 1992. The Cox regression model applied to risk factor analysis of infections in the breeding and multiplying herds in the Danish SPF system. Preventive Veterinary Medicine, 12, 287–297. [Google Scholar]
- Threlfall EJ, 2002. Antimicrobial drug resistance in Salmonella: problems and perspectives in food‐and water‐borne infections. FEMS Microbiology Reviews, 26, 141–148. [DOI] [PubMed] [Google Scholar]
- Tikofsky LL, Barlow JW, Santisteban C and Schukken YH, 2003. A comparison of antimicrobial susceptibility patterns for Staphylococcus aureus in organic and conventional dairy herds. Microbial Drug Resistance, 9, 39–45. [DOI] [PubMed] [Google Scholar]
- Timbermont L, Lanckriet A, Gholamiandehkordi AR, Pasmans F, Martel A, Haesebrouck F, Ducatelle R and Van Immerseel F, 2009. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comparative Immunology, Microbiology and Infectious Diseases, 32, 503–512. [DOI] [PubMed] [Google Scholar]
- Timmerman T, Dewulf J, Catry B, Feyen B, Opsomer G, de Kruif A and Maes D, 2006. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Preventive Veterinary Medicine, 74, 251–263. [DOI] [PubMed] [Google Scholar]
- Torrallardona D, 2010. Spray dried animal plasma as an alternative to antibiotics in weanling pigs ‐ a review. Asian‐Australasian Journal of Animal Sciences, 23, 131–148. [Google Scholar]
- Tosi G, Massi P, Antongiovanni M, Buccioni A, Minieri S, Marenchino L and Mele M, 2013. Efficacy test of a hydrolysable tannin extract against necrotic enteritis in challenged broiler chickens. Italian Journal of Animal Science, 12, 386–389. [Google Scholar]
- Toutain PL and Bousquet‐Melou A, 2013. The consequences of generic marketing on antibiotic consumption and the spread of microbial resistance: the need for new antibiotics. Journal of Veterinary Pharmacology and Therapeutics, 36, 420–424. [DOI] [PubMed] [Google Scholar]
- Toutain P‐L, Ferran AA, Bousquet‐Melou A, Pelligand L and Lees P, 2016. Veterinary medicine needs new green antimicrobial drugs. Frontiers in Microbiology, 7, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trckova M, Matlova L, Dvorska L and Pavlik I, 2004. Kaolin, bentonite, and zeolites as feed supplements for animals: health advantages and risks. Veterinarni Medicina, 49, 389–399. [Google Scholar]
- Trckova M, Vondruskova H, Zraly Z, Alexa P, Hamrik J, Kummer V, Maskova J, Mrlik V, Krizova K, Slana I, Leva L and Pavlik I, 2009. The effect of kaolin feeding on efficiency, health status and course of diarrhoeal infections caused by enterotoxigenic Escherichia coli strains in weaned piglets. Veterinarni Medicina, 54, 47–63. [Google Scholar]
- Tremetsberger L and Winckler C, 2015. Effectiveness of animal health and welfare planning in dairy herds: a review. Animal Welfare, 24, 55–67. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M, Puppe B, Tuchscherer A and Kanitz E, 1998. Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Physiology and Behavior, 64, 353–360. [DOI] [PubMed] [Google Scholar]
- Tummaruk P, Tantilertcharoen R and Prapasarakul N, 2009. The use of herbal medicine as an alternative antimicrobial in the feed of post‐weaning piglets: a field trial. Journal of Applied Animal Science, 2, 23–31. [Google Scholar]
- Turkyilmaz S, Tekbiyik S, Oryasin E and Bozdogan B, 2010. Molecular epidemiology and antimicrobial resistance mechanisms of methicillin‐resistant staphylococcus aureus isolated from bovine milk. Zoonoses and Public Health, 57, 197–203. [DOI] [PubMed] [Google Scholar]
- Turner S, Ewen M, Rooke J and Edwards S, 2000. The effect of space allowance on performance, aggression and immune competence of growing pigs housed on straw deep‐litter at different group sizes. Livestock Production Science, 66, 47–55. [Google Scholar]
- Urban‐Chmiel R and Grooms D, 2012. Prevention and control of bovine respiratory disease. Journal of Livestock Science, 3, 27–36. [Google Scholar]
- Utiyama CE, Oetting LL, Giani PA, Ruiz UdS and Miyada VS, 2006. Effects of antimicrobials, prebiotics, probiotics and herbal extracts on intestinal micobiology, diarrhea incidence and performance of weanling pigs. Revista Brasileira De Zootecnia‐Brazilian Journal of Animal Science, 35, 2359–2367. [Google Scholar]
- Vaarst M, Bennedsgaard TW, Klaas I, Nissen T, Thamsborg SM and Østergaard S, 2006. Development and daily management of an explicit strategy of nonuse of antimicrobial drugs in twelve Danish organic dairy herds. Journal of Dairy Science, 89, 1842–1853. [DOI] [PubMed] [Google Scholar]
- Vågsholm I and Höjgård S, 2010. Antimicrobial sensitivity—a natural resource to be protected by a Pigouvian tax? Preventive Veterinary Medicine, 96, 9–18. [DOI] [PubMed] [Google Scholar]
- Vahjen W, Pietruszyńska D, Starke IC and Zentek J, 2015. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathogens, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Cleef B, Monnet DL, Voss A, Krziwanek K, Allerberger F, Struelens M, Zemlickova H, Skov RL, Vuopio‐Varkila J, Cuny C, Friedrich AW, Spiliopoulou I, Paszti J, Hardardottir H, Rossney A, Pan A, Pantosti A, Borg M, Grundmann H, Mueller‐Premru M, Olsson‐Liljequist B, Widmer A, Harbarth S, Schweiger A, Unal S and Kluytmans J, 2011. Livestock‐associated Methicillin‐Resistant Staphylococcus aureus in Humans, Europe. Emerging Infectious Diseases, 17, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven LJF, van Wagenberg AV, Koerkamp P, Kemp B and van den Brand H, 2009. Effects of a combined hatching and brooding system on hatchability, chick weight, and mortality in broilers. Poultry Science, 88, 2273–2279. [DOI] [PubMed] [Google Scholar]
- Van de Ven GFA 2013. The use of autogenous vaccines in the Dutch pig industry and suggestions for new legislation of autogenous vaccines. Master thesis. Available online: http://dspace.library.uu.nl/handle/1874/287206
- van de Weerd HA and Day JEL, 2009. A review of environmental enrichment for pigs housed in intensive housing systems. Applied Animal Behaviour Science, 116, 1–20. [Google Scholar]
- van den Brand H, Wamsteeker D, Oostindjer M, van Enckevort LCM, van der Poel AFB, Kemp B and Bolhuis JE, 2014. Effects of pellet diameter during and after lactation on feed intake of piglets pre‐ and postweaning. Journal of Animal Science, 92, 4145–4153. [DOI] [PubMed] [Google Scholar]
- van der Horst MA, Schuurmans JM, Smid MC, Koenders BB and ter Kuile BH, 2011. De novo acquisition of resistance to three antibiotics by Escherichia coli. Microbial Drug Resistance, 17, 141–147. [DOI] [PubMed] [Google Scholar]
- van der Mheen H and Spoolder HAM, 2003. Gently or roughly treated pigs. PraktijkRapport Varkens 19. Available online: http://library.wur.nl/WebQuery/wurpubs/fulltext/47152
- van der Pol CW, van Roovert‐Reijrink IAM, Maatjens CM, van den Brand H and Molenaar R, 2013. Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poultry Science, 92, 2145–2155. [DOI] [PubMed] [Google Scholar]
- van Dixhoorn ID, Reimert I, Middelkoop J, Bolhuis JE, Wisselink HJ, Koerkamp PWG, Kemp B and Stockhofe‐Zurwieden N, 2016. Enriched housing reduces disease susceptibility to co‐infection with porcine reproductive and respiratory virus (PRRSV) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) in Young Pigs. PLoS ONE, 11, e0161832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoek AH, Stalenhoef JE, van Duijkeren E and Franz E, 2016. Comparative virulotyping of extended‐spectrum cephalosporin‐resistant E. coli isolated from broilers, humans on broiler farms and in the general population and UTI patients. Veterinary Microbiology. [DOI] [PubMed] [Google Scholar]
- Van Hoorebeke S, Dewulf J, Van Immerseel F and Jorgensen F, 2012. Production systems for laying hens and broilers and risk of human pathogens In: Sandilands V. and Hocking PM. (eds.). Alternative systems for poultry ‐ health, welfare and productivity. CAB International, Wallingford, 77–96. [Google Scholar]
- Van Immerseel F, Methner U, Rychlik I, Nagy B, Velge P, Martin G, Foster N, Ducatelle R and Barrow P, 2005. Vaccination and early protection against non‐host‐specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiology and Infection, 133, 959–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo EJ, Alali W and Ricke SC, 2012. Food Safety and Organic Meats. Annual Review of Food Science and Technology, 3, 203–225. [DOI] [PubMed] [Google Scholar]
- Van Miert A, 1983. Medicated feed: problems and solutions In: Ruckebusch Y, Toutain PL. and Koritz GD. (eds.). Veterinary Pharmacology and Toxicology. Springer, Netherlands: pp. 752–757. [Google Scholar]
- van Rennings L, von Münchhausen C, Ottilie H, Hartmann M, Merle R, Honscha W, Käsbohrer A and Kreienbrock L, 2015. Cross‐sectional study on antibiotic usage in pigs in Germany. PLoS ONE, 10, e0119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik C, Dijkhuizen A, Benedictus G, Barkema H and Koole J, 1998. Exploratory study on the economic value of a closed farming system on Dutch dairy farms. Veterinary Record, 142, 240–242. [DOI] [PubMed] [Google Scholar]
- Varela NP, Gadbois P, Thibault C, Gottschalk M, Dick P and Wilson J, 2013. Antimicrobial resistance and prudent drug use for Streptococcus suis. Animal Health Research Reviews, 14, 68–77. [DOI] [PubMed] [Google Scholar]
- Vega C, Bok M, Saif L, Fernandez F and Parreño V, 2015. Egg yolk IgY antibodies: a therapeutic intervention against group A rotavirus in calves. Research in Veterinary Science, 103, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidanarachchi JK, Mikkelsen LL, Constantinoiu CC, Choct M and Iji PA, 2013. Natural plant extracts and prebiotic compounds as alternatives to antibiotics in broiler chicken diets in a necrotic enteritis challenge model. Animal Production Science, 53, 1247–1259. [Google Scholar]
- Vieira AR, Collignon P, Aarestrup FM, McEwen SA, Hendriksen RS, Hald T and Wegener HC, 2011a. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathogens and Disease, 8, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Pires SM, Houe H and Emborg HD, 2011b. Trends in slaughter pig production and antimicrobial consumption in Danish slaughter pig herds, 2002–2008. Epidemiology and Infection, 139, 1601–1609. [DOI] [PubMed] [Google Scholar]
- Vigre H, Dohoo IR, Stryhn H and Jensen VF, 2010. Use of register data to assess the association between use of antimicrobials and outbreak of Postweaning Multisystemic Wasting Syndrome (PMWS) in Danish pig herds. Preventive Veterinary Medicine, 93, 98–109. [DOI] [PubMed] [Google Scholar]
- Vinod MG, Shivu MM, Umesha KR, Rajeeva BC, Krohne G, Ka‐Unasagar I and Karunasagar I, 2006. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture, 255, 117–124. [Google Scholar]
- Visschers VHM, Backhans A, Collineau L, Iten D, Loesken S, Postma M, Belloc C, Dewulf J, Emanuelson U, Beilage EG, Siegrist M, Sjolund M and Staerk KDC, 2015. Perceptions of antimicrobial usage, antimicrobial resistance and policy measures to reduce antimicrobial usage in convenient samples of Belgian, French, German, Swedish and Swiss pig farmers. Preventive Veterinary Medicine, 119, 10–20. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Berkman ND, Dryden DM and Hartling L, 2013. Assessing risk of bias and confounding in observational studies of interventions or exposures: Further Development of the RTI item bank. AHRQ Publication No. 13‐EHC106‐EF. Agency for Healthcare Research and Quality (US), Rockville, MD: Available at: https://www.effectivehealthcare.ahrq.gov/ehc/products/414/1612/RTI-item-bank-bias-precision-130805.pdf [PubMed] [Google Scholar]
- VMD (Veterinary Medicines Directorate), 2015. UK‐VARSS 2014 – UK veterinary antibiotic resistance and sales surveillance report. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477788/Optimised_version_-_VARSS_Report_2014__Sales___Resistance_.pdf
- Vose D, Acar J, Anthony F, Franklin A, Gupta R, Nicholls T, Tamura Y, Thompson S, Threlfall EJ, van Vuuren M, White DG, Wegener HC and Costarrica ML, 2001. Antimicrobial resistance: risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. Revue Scientifique et Technique, 20, 811–827. [DOI] [PubMed] [Google Scholar]
- Voss A, Loeffen F, Bakker J, Klaassen C and Wulf M, 2005. Methicillin‐resistant Staphylococcus aureus in pig farming. Emerging Infectious Diseases, 11, 1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong CN, Chou W‐K, Hargis BM, Berghman LR and Bielke LR, 2016. Role of probiotics on immune function and their relationship to antibiotic growth promoters in poultry, a brief review. International Journal of Probiotics & Prebiotics, 11, 1–6. [Google Scholar]
- Waghu FH, Barai RS, Gurung P and Idicula‐Thomas S, 2015. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Research, 44, D1094–D1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiblinger S, Menke C and Coleman G, 2002. The relationship between attitudes, personal characteristics and behaviour of stockpeople and subsequent behaviour and production of dairy cows. Applied Animal Behaviour Science, 79, 195–219. [Google Scholar]
- Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP and Hamblin MR, 2016. Photoantimicrobials‐are we afraid of the light? The Lancet Infectious Diseases, online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales AD and Davies RH, 2015. Co‐Selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics, 4, 567–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST, Mladonicky JM, Middleton JA, Heidt AJ, Cunningham JR, Bartlett P, Sato K and Whittam TS, 2007. Influence of antibiotic selection on genetic composition of Escherichia coli populations from conventional and organic dairy farms. Applied and Environment Microbiology, 73, 5982–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasyl D, Kern‐Zdanowicz I, Domańska‐Blicharz K, Zając M and Hoszowski A, 2015. High‐level fluoroquinolone resistant Salmonella enterica serovar Kentucky ST198 epidemic clone with IncA/C conjugative plasmid carrying bla CTX‐M‐25 gene. Veterinary Microbiology, 175, 85–91. [DOI] [PubMed] [Google Scholar]
- Weese JS, Hannon SJ, Booker CW, Gow S, Avery BP and Reid‐Smith RJ, 2012. The prevalence of methicillin‐resistant staphylococcus aureus colonization in feedlot cattle. Zoonoses and Public Health, 59, 144–147. [DOI] [PubMed] [Google Scholar]
- Wegener HC, Hald T, Wong LF, Madsen M, Korsgaard H, Bager F, Gerner‐Smidt P and Mølbak K, 2003. Salmonella control programs in Denmark. Emerging Infectious Diseases, 9, 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welfare Quality® consortium , 2009. Welfare Quality® Assessment Protocol for Cattle, 2009, ISBN/EAN 978‐90‐78240‐04‐4. 180 pp.
- Wellington EM, Boxall AB, Cross P, Feil EJ, Gaze WH, Hawkey PM, Johnson‐Rollings AS, Jones DL, Lee NM and Otten W, 2013. The role of the natural environment in the emergence of antibiotic resistance in Gram‐negative bacteria. The Lancet Infectious Diseases, 13, 155–165. [DOI] [PubMed] [Google Scholar]
- Wenk C, 2006. Are herbs, botanicals and other related substances adequate replacements for antimicrobial growth promoters? In: Antimicrobial Growth Promoters: Where do we go from here?, 329–340. [Google Scholar]
- Westphal A, Williams ML, Baysal‐Gurel F, LeJeune JT and Gardener BBM, 2011. General suppression of Escherichia coli O157: H7 in sand‐based dairy livestock bedding. Applied and Environmental Microbiology, 77, 2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DG, Acar J, Anthony F, Franklin A, Gupta R, Nicholls T, Tamura Y, Thompson S, Threlfall EJ, Vose D, van Vuuren M, Wegener HC and Costarrica ML, 2001. Antimicrobial resistance: standardisation and harmonisation of laboratory methodologies for the detection and quantification of antimicrobial resistance. Revue Scientifique et Technique, 20, 849–858. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, Rowland RR, Ewen CL, Trible BR, Kerrigan MA, Cino‐Ozuna AG, Samuel MS, Lightner JE, McLaren DG and Mileham AJ, 2016. Gene‐edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nature Biotechnology, 34, 20–22. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1990. Guide to an SPF‐swine programme. Available online: http://apps.who.int/iris/bitstream/10665/60804/1/WHO_Zoon._90.165.pdf
- WHO (World Health Organization), 2003. Impacts of antimicrobial growth promoter termination in Denmark: the WHO international review panel's evaluation of the termination of the use of antimicrobial growth promoters in Denmark: Foulum, Denmark 6–9 November 2002. Available online: http://apps.who.int/iris/bitstream/10665/68357/1/WHO_CDS_CPE_ZFK_2003.1.pdf
- WHO (World Health Organization), 2011. Tackling antibiotic resistance from a food safety perspective in Europe. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/136454/e94889.pdf. 9289014210
- WHO (World Health Organisation), 2012. Critically important antimicrobials for human medicine ‐ 3rd revision 2011. Available online: http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf
- WHO (World Health Organization), 2015. Global action plan on antimicrobial resistance. Available online: http://www.who.int/drugresistance/global_action_plan/en/ [DOI] [PubMed]
- WHO FAO and OIE (World Health Organization, Food and Agriculture Organization, World Organisation for Animal Health), 2016. Antimicrobial resistance: a manual for developing national action plans. Available online: http://apps.who.int/iris/bitstream/10665/204470/1/9789241549530_eng.pdf
- Wideman RF Jr, Al‐Rubaye A, Kwon YM, Blankenship J, Lester H, Mitchell KN, Pevzner IY, Lohrmann T and Schleifer J, 2015. Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers. Poultry Science, 94, 25–36. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Jensen VF, Aarestrup FM and Schlundt J, 2014. Evidence‐based policy for controlling antimicrobial resistance in the food chain in Denmark. Food Control, 40, 185–192. [Google Scholar]
- Wierup M, 2001. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microbial Drug Resistance, 7, 183–190. [DOI] [PubMed] [Google Scholar]
- Wierup M, 2012. Principles and strategies for the prevention and control of infectious diseases in livestock and wildlife. In: Norrgren L. and Levengood J. (eds.). Ecology and Animal Health. The Baltic University Programme, Uppsala University, 203–211. [Google Scholar]
- Wilhelm B, Rajic A, Waddell L, Parker S, Harris J, Roberts KC, Kydd R, Greig J and Baynton A, 2009. Prevalence of zoonotic or potentially zoonotic bacteria, antimicrobial resistance, and somatic cell counts in organic dairy production: current knowledge and research gaps. Foodborne Pathogens and Disease, 6, 525–539. [DOI] [PubMed] [Google Scholar]
- Willeberg P, Leontides L, Ewald C, Mortensen S, McInerney JP, Howe KS and Kooij D, 1996. Effect of vaccination against Aujeszky's Disease compared with test and slaughter programme: epidemiological and economical evaluations. Acta Veterinaria Scandinavica, 90, 25–51. [PubMed] [Google Scholar]
- Wilson R, Holyoake P, Cronin G and Doyle R, 2014. Managing animal wellbeing: a preliminary survey of pig farmers. Australian Veterinary Journal, 92, 206–212. [DOI] [PubMed] [Google Scholar]
- Windisch W and Kroismayr A, 2006. The effects of phytobiotics on performance and gut function in monogastrics. Proceedings of the World nutrition forum: The future of animal nutrition, 85–90.
- Wiuff C, Lykkesfeldt J, Svendsen O and Aarestrup FM, 2003. The effects of oral and intramuscular administration and dose escalation of enrofloxacin on the selection of quinolone resistance among Salmonella and coliforms in pigs. Research in Veterinary Science, 75, 185–193. [DOI] [PubMed] [Google Scholar]
- Xavier B, Lammens C, Ruhal R, Kumar‐Singh S, Butaye P, Goossens H and Malhotra‐Kumar S, 2016. Identification of mcr‐2 in colistin‐resistant E. coli isolates not harbouring mcr‐1. Eurosurveillance, 21, pii=30280. [Google Scholar]
- Xu Y, Li X, Jin L, Zhen Y, Lu Y, Li S, You J and Wang L, 2011. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnology Advances, 29, 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Iji PA and Choct M, 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in‐feed antibiotics. World's Poultry Science Journal, 65, 97–114. [Google Scholar]
- Yegani M, Butcher G and Nilipour A, 2005. Immunosuppression‐threat to bird health and welfare. World Poultry, 21, 18–22. [Google Scholar]
- Youn S, Kwon Y, Song C, Lee H, Jeong O, Choi B, Jung S and Kang M, 2016. Increased efficacy of inactivated vaccine candidates prepared with Salmonella enterica serovar Typhimurium strains of predominant genotypes in ducks. Poultry Science, 95, 1764–1773. [DOI] [PubMed] [Google Scholar]
- Young BA, 1981. Cold stress as it affects animal production. Journal of Animal Science, 52, 154–163. [DOI] [PubMed] [Google Scholar]
- Young C, Eidman V and Reneau J, 1985. Animal health and management and their impact on economic efficiency. Journal of Dairy Science, 68, 1593–1602. [DOI] [PubMed] [Google Scholar]
- Young I, Rajic A, Wilhelm BJ, Waddell L, Parker S and McEwen SA, 2009. Comparison of the prevalence of bacterial enteropathogens, potentially zoonotic bacteria and bacterial resistance to antimicrobials in organic and conventional poultry, swine and beef production: a systematic review and meta‐analysis. Epidemiology and Infection, 137, 1217–1232. [DOI] [PubMed] [Google Scholar]
- Yount NY and Yeaman MR, 2012. Emerging themes and therapeutic prospects for anti‐infective peptides. 52, 337–360. [DOI] [PubMed] [Google Scholar]
- Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agerso Y, Lund O, Larsen MV and Aarestrup FM, 2013. Genotyping using whole‐genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. Journal of Antimicrobial Chemotherapy, 68, 771–777. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li R and Li J, 2012. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella‐induced pullorum disease in a chick model of infection. Research in Veterinary Science, 93, 366–373. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang Y, Zhou Y, Buckley T and Wang HH, 2013. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrobial Agents and Chemotherapy, 57, 3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Z, Cao Z, Wang L, Li X, Li S and Xu Y, 2015. Bacteriophages as antimicrobial agents against major pathogens in swine: a review. Journal of Animal Science and Biotechnology, 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wu H, Lu H, Li G and Huang Q, 2013. LAMP: a database linking antimicrobial peptides. PLoS ONE, 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Q, Barfod K, Wachmann H, Mortensen S, Lavritsen D, Ydesen B and Willeberg P, 2007. Risk factors for Actinobacillus pleuropneumoniae serotype 2 infection in Danish genetic specific pathogen‐free pig herds. The Veterinary Record, 160, 258–262. [DOI] [PubMed] [Google Scholar]
- Zulkifli I and Azah ASN, 2004. Fear and stress reactions, and the performance of commercial broiler chickens subjected to regular pleasant and unpleasant contacts with human being. Applied Animal Behaviour Science, 88, 77–87. [Google Scholar]
- Zwonitzer MR, Soupir ML, Jarboe LR and Smith DR, 2016. Quantifying attachment and antibiotic resistance of from conventional and organic swine manure. Journal of Environmental Quality, 45, 609–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antimicrobial use in food‐producing animals


