Knowledge of the spatio-temporal relationship between electrical excitation and mechanical contraction in the human heart is essential for understanding cardiac physiology and disease. However, electromechanical data from non-diseased human hearts are currently lacking. The present study is the first to combine Electrocardiographic Imaging (ECGI; a noninvasive method for cardiac electrophysiology mapping) with tagged magnetic resonance imaging (MRI) to study the electro-mechanics of healthy adult hearts in situ. This also represents the largest ECGI study of healthy adults to date. We provide three-dimensional data of the normal cardiac electrical and mechanical activation sequences, obtained from the same hearts under complete physiological conditions. These are important baseline data for studies and diagnosis of cardiac disorders and for constraining computer models of the human heart.
Twenty healthy adults were enrolled at Washington University in St. Louis. Healthy volunteer demographics are provided in Supplemental Table I. The study was approved by the Human Research Protection Office at Washington University in St. Louis. All participants provided written informed consent. Data are available upon reasonable request.
The ECGI method, developed and validated in our laboratory, was described previously1. A schematic of the procedure is presented in Supplemental Figure I. The method consists of recording approximately 250 simultaneous ECGs from the torso, using electrode strips. Heart-torso geometries of subjects were imaged using a navigated anatomic MRI sequence. Electrode positions were marked with MRI-visible capsules. ECG recordings were combined with the heart-torso geometries to construct epicardial potentials and unipolar epicardial electrograms. Local activation times were computed from electrograms using the minimum dV/dt during the QRS, and recovery times using the maximum dV/dt during the T wave. Activation-recovery intervals (ARIs), a surrogate of local action potential duration, were computed by subtracting the local activation time from the local recovery time. Activation and recovery maps were edited based on overall sequence and neighboring electrograms.
Tagged MR images were obtained and analyzed using previously described methods2. ECG-gated images were obtained in short-axis and long-axis views for a complete cardiac cycle beginning at end-diastole. Tagged and non-tagged images were acquired during the same breath hold to ensure similar anatomic positioning. Tag lines in the myocardium were tracked and 3D displacements were calculated from the movement of intramural tag surface intersection points during systole. StressCheck software (ESRD, Inc., St. Louis, MO) was used to compute strain values throughout the left ventricle (LV). Additional details of the ECGI and tagged MRI analyses are provided in the Supplemental Methods.
The epicardial electrical activation sequence is consistent among healthy adults in its overall pattern. In all subjects, the anatomical segment of earliest activation was between the lateral right ventricle (RV) and the anterior RV wall in mid or basal regions. Latest activation was in basal LV. The activation sequence of a representative subject is presented in Figure 1A and 1B. Repolarization was consistent with previous descriptions1. The mean epicardial ARI had a median value of 238 msec, in good agreement with the previously reported 235 msec1. Females had longer mean epicardial ARI than males (female median: 261 msec, male median: 229 msec, p = 0.001). Female ARIs were longer in both ventricles, with greater gender difference in the RV (Supplemental Table II). Representative ARI maps for a male and female are presented in Figure 1C. In contrast to previous pathological ECGI studies3–5, control subjects do not have indications of scar, prolonged repolarization, or steep gradients of repolarization. LV segment contractions were synchronous and reached peak strain approximately 430 msec after the onset of the QRS. A representative 3D electromechanical sequence is presented in Figure 1D; it is animated in the Supplemental Movie. Control ranges for ECGI indices of activation, scar, and repolarization are provided in Supplemental Table III.
Figure 1.
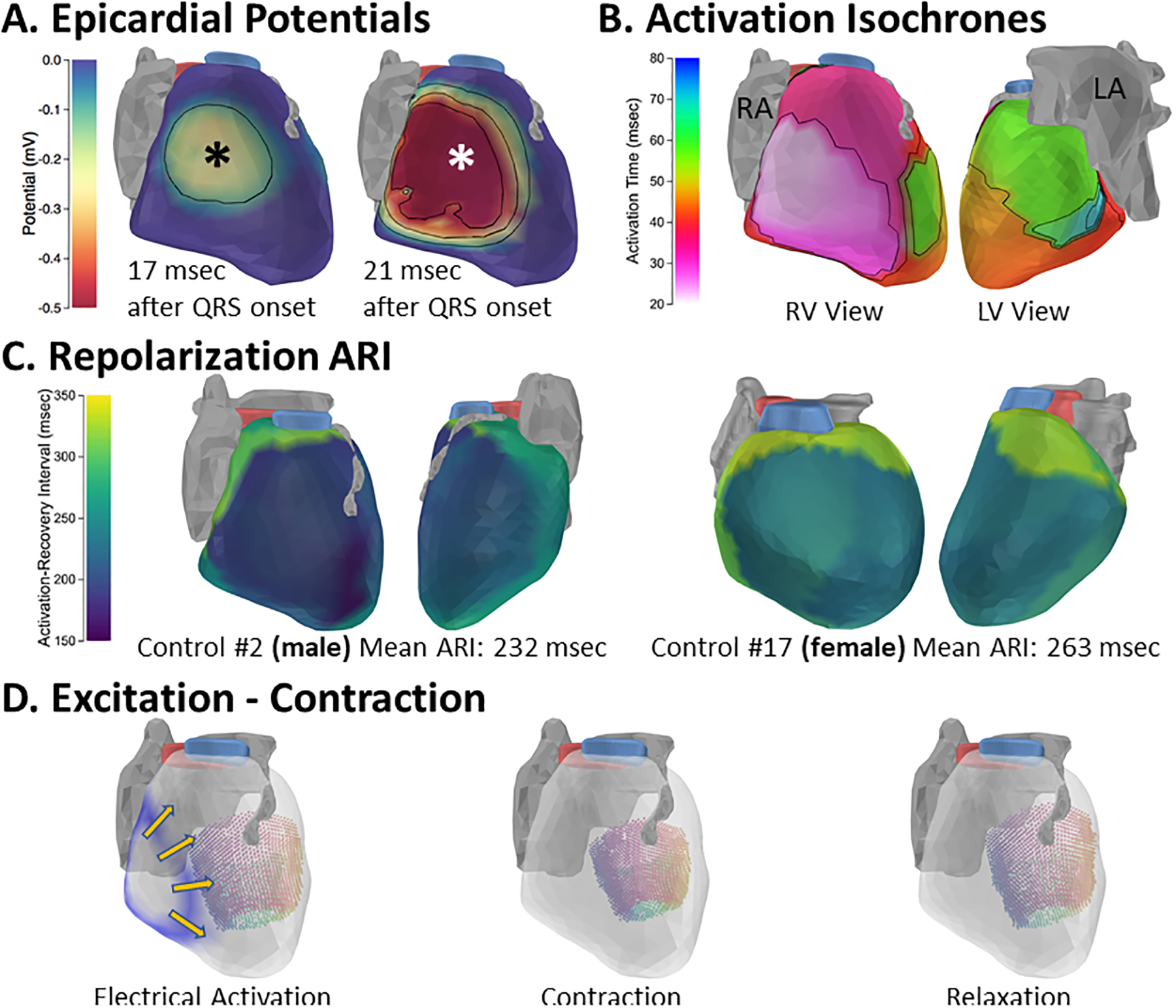
Excitation and contraction of the healthy adult human heart. A. Epicardial breakthrough. A potential minimum, indicating epicardial breakthrough, appears and expands in the mid lateral RV shortly after the QRS onset; the breakthrough center is indicated by asterisk. RV outflow tract is shown in blue and LV outflow tract in pink. Atria and LAD shown in gray. B. Electrical activation isochrones in the same subject. C. Activation-recovery interval maps from a representative male (left) and female (right) demonstrate the longer repolarization in females compared to males. D. Electromechanical sequences in a healthy adult heart. Electrical activation wave is shown in blue, with direction of propagation indicated by yellow arrows (left image). LV displacements during contraction of the same heart are depicted by colored points, with colors indicating wall segments. Point colors vary from septal (purple) to lateral (yellow). An animation of the electro-mechanical sequence is provided in the Supplemental Movie.
RA: right atrium; LA: left atrium. LAD: left anterior descending coronary artery
The population of this study is the largest group of healthy adults imaged with ECGI to date. Epicardial activation breakthrough locations were consistent with previous studies by Durrer and Wyndham, as well as with previous ECGI results1. Importantly, this population did not show activation or repolarization abnormalities observed in pathological ECGI studies3–5, which confirms the specificity of ECGI for diagnosis and study of these conditions. The larger size of this study compared to a previous ECGI study of healthy adults allowed us to quantify the difference in epicardial repolarization between males and females. The longer repolarization in females is consistent with ECG findings that women have longer QTc intervals than men and highlights the sensitivity of ECGI for measuring repolarization. Exploring the link between this repolarization prolongation and the higher susceptibility of women to torsade de pointes would be an interesting application of ECGI. Using tagged MRI, we provide a high-resolution 3D description of contraction of healthy hearts that is co-registered spatially and temporally with detailed electrical activation and repolarization maps. These data can be beneficial for constraining mathematical models and validating computer simulations of human cardiac electromechanics.
Supplementary Material
Acknowledgments:
The authors acknowledge Julia Meyer for her contribution to data collection and Nicole Joison for her contribution to the tagged MRI analysis.
Sources of Funding: This study was supported by NIH - National Heart, Lung and Blood Institute grants R01-HL-033343 and R01-HL-049054 (to YR) and by Washington University Institute of Clinical and Translational Sciences grant UL1-TR000448 from the National Center for Advancing Translational Sciences of the NIH. Dr. Rudy is the Fred Saigh Distinguished Professor at Washington University.
Non-standard Abbreviations and Acronyms
- ECGI
Electrocardiographic Imaging
- LV
Left Ventricle
- RV
Right Ventricle
- ARI
Activation-Recovery Interval
Footnotes
Disclosures: YR receives royalties from CardioInsight Technologies (CIT). CIT does not support any research conducted in Dr. Rudy’s laboratory.
References:
- 1.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci USA. 2006;103:6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupps BP, Taggar AK, Reynolds LM, Lawton JS, Pasque MK. Regional myocardial contractile function: multiparametric strain mapping. Interact Cardiovasc Thorac Surg. 2010;10:953–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Sacher F, Hoffmayer K, O’Hara T, Strom M, Cuculich P, Silva J, Cooper D, Faddis M, Hocini M, et al. Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation. 2015;131:1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews CM, Srinivasan NT, Rosmini S, Bulluck H, Orini M, Jenkins S, Pantazis A, McKenna WJ, Moon JC, Lambiase PD, et al. Electrical and Structural Substrate of Arrhythmogenic Right Ventricular Cardiomyopathy Determined Using Noninvasive Electrocardiographic Imaging and Late Gadolinium Magnetic Resonance Imaging. Circ Arrhythm Electrophysiol. 2017;10:e005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumar R, Silva JNA, Desouza KA, Abraham RL, Strom M, Sacher F, Van Hare GF, Haïssaguerre M, Roden DM, Rudy Y. Electrophysiologic substrate in congenital Long QT syndrome: noninvasive mapping with electrocardiographic imaging (ECGI). Circulation. 2014;130:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


