Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Belgium, and co‐rapporteur Member State, Greece, for the pesticide active substance mepanipyrim are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of mepanipyrim as a fungicide on table and wine grapes, and in field and protected strawberries and tomatoes. The reliable end points, appropriate for use in regulatory risk assessment are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: mepanipyrim, peer review, risk assessment, pesticide, fungicide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Mepanipyrim is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Belgium, and co‐rapporteur Member State (co‐RMS), Greece, received an application from K‐I Chemical Europe SA/NV for the renewal of approval of the active substance mepanipyrim. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS, the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on mepanipyrim in the renewal assessment report (RAR), which was received by EFSA on 3 May 2016. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, K‐I Chemical Europe SA/NV, for comments on 12 July 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 13 September 2016.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether mepanipyrim can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of mepanipyrim as a fungicide on table and wine grapes, field and protected strawberries and tomatoes, as proposed by the applicant. Full details of the representative uses can be found in Appendix A of this report.
The use of mepanipyrim according to the representative uses proposed at the European Union (EU) level (Southern zone) results in a sufficient fungicidal efficacy against grey mould.
In the section identity, physical chemical properties and analytical methods, a data gap was identified for a method of monitoring for residues in body fluids and tissues.
In the mammalian toxicology area, data gaps were identified in relation to the absence of comparative interspecies metabolism study in vitro, need for quantitative structure–activity relationship (QSAR) data and repeated dose toxicity data relevant to consumer exposure for the metabolite M31, and to address the toxicological relevance of two impurities present in the technical specification. The conditions of the interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine disrupting properties are not met for mepanipyrim. However, considering the effects observed in the available studies, the endocrine disrupting potential of mepanipyrim cannot be ruled out and further clarification is needed using mechanistic data. Since mepanipyrim was found to be phototoxic in vitro, and there is currently no validated test in vivo, the phototoxic potential of the substance could not be finalised. Operator and worker exposure were found to exceed the acceptable operator exposure level (AOEL) in some scenarios even when using personal protective equipment.
In the residue section, in addition to the request in the mammalian toxicology area to address the toxicological profile of metabolite M31, a data gap was identified for the investigation of the fate of M31 under standard processing conditions. Hence, the consumer risk assessment could not be finalised considering the outstanding data to finalise the residue definitions in primary crops and in processed commodities. Moreover, an additional indoor Good Agricultural Practice (GAP)‐compliant residue trial on tomatoes is required, and a data gap for the determination of mepanipyrim and M31 residues in pollen and bee products for human consumption resulting from residues taken up by honeybees at blossom from grapes, field grown strawberries and field grown tomatoes was not addressed (data gap).
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at the EU level for the representative uses. A data gap was identified for information on the effect of water treatment processes on the nature of residues potentially present in surface water, when surface water is abstracted for drinking water. This gap leads to the consumer risk assessment from the consumption of drinking water being not finalised for all the representative uses.
In the section on ecotoxicology, a critical area of concern has been identified for wild mammals, as high long‐term risk was identified for all uses of mepanipyrim. Further data gaps were identified in the area of bee risk assessment.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of up to 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Belgium and co‐RMS Greece received an application from K‐I Chemical Europe SA/NV for the renewal of approval of the active substance mepanipyrim. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Greece), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on mepanipyrim in the RAR, which was received by EFSA on 3 May 2016 (Belgium, 2016).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, K‐I Chemical Europe SA/NV, for consultation and comments on 12 July 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 13 September 2016. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 21 October 2016. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in April 2017.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative use of mepanipyrim as a fungicide on table and wine grapes, and in field and protected strawberries and tomatoes, as proposed by the applicant. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2017), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (21 October 2016);
the evaluation table (8 May 2017);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Belgium, 2017), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Mepanipyrim is the ISO common name for N‐(4‐methyl‐6‐prop‐1‐ynylpyrimidin‐2‐yl)aniline (IUPAC).
The representative formulated product for the evaluation was ‘Frupica 50 WP’, a wettable powder in sealed water soluble bag (WP‐SB), containing 500 g/kg mepanipyrim.
The representative uses evaluated were foliar spray applications for the control of grey mould Botryotinia fuckeliana (BOTRCI) in table and wine grapes, and in field and protected strawberries and tomatoes, in the Southern European zone. Full details of the GAPs can be found in the list of end points in Appendix A.
Data were submitted to conclude that the uses of mepanipyrim according to the representative uses proposed at the EU level result in a sufficient fungicidal efficacy against grey mould, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012) and SANCO/825/00‐rev. 8.1 (European Commission, 2010).
The new proposed reference specification for mepanipyrim is based on batch data from industrial scale production and also on QC data for the relevant impurity. The minimum purity of the technical material is 970 g/kg. There is no FAO specification available for mepanipyrim. Toluene is considered a relevant impurity, however of no toxicological concern at the level specified (maximum 5 g/kg). The batches used in the toxicological and ecotoxicological assessments support the proposed renewal specification. The initial reference specification for first approval was considered to be not entirely covered by the toxicological studies. As a consequence, it is recommended to update the reference specification of the first approval.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of mepanipyrim or the representative formulation. The main data regarding the identity of mepanipyrim and its physical and chemical properties are given in Appendix A.
The methods for the generation of pre‐approval data required for the risk assessment were adequately addressed. High‐pressure/high‐performance liquid chromatography‐ultraviolet (HPLC‐UV) methods are available for the determination of mepanipyrim in the technical material and in the representative formulation, and for the determination of the respective impurities in the technical material. CIPAC MT 198 can be used for the determination of toluene in the formulation.
Mepanipyrim residues can be monitored in food and feed of plant origin by the QuEChERS method using liquid chromatography with tandem mass spectrometry (LC–MS/MS) with limit of quantifications (LOQs) of 0.01 mg/kg in acidic, dry and high water content matrices and by gas chromatography (GC) with a LOQ of 0.01 mg/kg in oily matrices.
An analytical method for food of animal origin is not required due to the fact that no residue definition is proposed.
Adequate LC–MS/MS or GC–MS methods are available for monitoring residues of mepanipyrim in soil with a LOQ of 0.01 mg/kg. Mepanipyrim residues can be determined in drinking water by LC–MS/MS with a LOQ of 0.05 μg/L, while in surface water by GC–MS with a LOQ of 0.1 μg/L. Monitoring mepanipyrim in air can be done by GC–MS with a LOQ of 0.75 μg/m3.
A data gap was identified for a method for monitoring mepanipyrim in body fluids and tissues.
2. Mammalian toxicity
The toxicological profile of the active substance mepanipyrim and its metabolites was discussed at the Pesticides Peer Review Experts’ Meeting 151 and assessed based on the following guidance documents: SANCO/221/2000‐rev. 10‐final (European Commission, 2003), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012), Guidance on dermal absorption (EFSA PPR Panel, 2012) and Guidance on the application of the CLP Criteria (ECHA, 2015).
A number of significant impurities were reported for mepanipyrim. The toxicological assessment covers the technical specification. Toluene is a relevant impurity due to its hazard classification. However, the maximum toluene level proposed for the technical specifications is not of toxicological concern. The relevance of two other impurities reported cannot be assessed due to lack of adequate information regarding their toxicological profile (data gap).
Mepanipyrim absorption is rapid and extensive (higher than 80%). Mepanipyrim is mainly distributed in fat, skin, kidney, adrenals, thyroid and liver. More than 90% of mepanipyrim is excreted within 48 h, mostly through faeces and bile. The kinetics pattern between the low and high dose is similar, with excretion through urine being slightly delayed for the high dose. Mepanipyrim is extensively metabolised in the rat via oxidations, hydroxylations and glutathione substitutions. Unchanged parent is only observed in faeces. Comparative interspecies metabolism study in vitro has not been provided and consequently the kinetics investigation remains open (data gap – issue not finalised).
Low acute toxicity was observed when mepanipyrim was administered by the oral, dermal or inhalation routes; no skin irritation, very slight eye irritation and no potential for skin sensitisation were attributed to the active substance. Since mepanipyrim was found to be phototoxic in vitro, and there is currently no validated test in vivo, the phototoxic potential of the substance could not be finalised.
In the 90‐day rat study, critical effects observed were related to haematology (decrease of the mean corpuscular haemoglobin concentration (MCHC) and lymphocytes in males, increase of neutrophils in males), and clinical chemistry findings (increase of cholesterol, decrease of triglyceride and decrease of the non‐esterified fatty acids) leading to a no‐observed adverse effect level (NOAEL) of 6.95 mg/kg body weight (bw) per day. In the 13‐week study in mice, the NOAEL was 19 mg/kg bw per day due to liver hypertrophy. In dogs, the critical effects were liver hypertrophy and prostate atrophy in both 90‐day and 1‐year dog study leading to NOAELs of 7.5 mg/kg bw per day and 2.5 mg/kg bw per day, respectively. Liver hypertrophy was observed also in the 28‐day dermal study in rabbit with the NOAEL in the 300 mg/kg bw per day. The genotoxic potential of mepanipyrim was fully tested (Ames test, in vitro chromosomal aberrations (CA) in Chinese hamster ovary (CHO), and in vivo CA and micronucleus (MN) test) and the results were discussed in the experts’ meeting. Overall, it was agreed that mepanipyrim is unlikely to be genotoxic. The findings of the long‐term carcinogenicity rat study (2 years) were discussed in the experts’ meeting concluding on a long‐term low‐observed adverse effect level (LOAEL) of 2.45 mg/kg bw per day due to pancreas atrophy in males and non‐relevance of the hydrometra. The long‐term NOAEL set for the respective mice study was at 56 mg/kg bw per day. Based on the liver adenomas, cystadenomas and marginal uterine carcinomas observed in rats and the liver adenomas and carcinomas observed in mice, the experts proposed to maintain the harmonised classification of mepanipyrim for carcinogenicity category 2. Some experts considered that classification in the category 1B could also be justified. Regarding the mechanism of carcinogenicity the experts considered that in the absence of a genotoxic potential, an initiating potential of mepanipyrim is not considered plausible.
Two main two‐generation reproductive studies in rats were submitted for mepanipyrim. The LOAEL for parental and offspring's toxicity is set at 2.45 mg/kg bw per day based on the observed increased incidence of centrilobular hepatocytic fatty vacuolation. The NOAEL for reproduction is set at 46 mg/kg bw per day due to decrease of the fertility index. Two main developmental studies (one in rats and one in rabbits) were submitted for mepanipyrim. The NOAEL for maternal toxicity in rats set at 150 mg/kg bw per day based on a greater than 10% decrease of the bodyweight gain observed at the 750 mg/kg bw per day. The NOAEL for maternal toxicity in rabbits is set at 10 mg/kg bw per day based on few faeces in under‐tray. The overall NOAEL for development is set at 10 mg/kg bw per day based on resorptions and post‐implantations in rabbits observed at 30 mg/kg bw per day. The experts considered that there is no need for classification regarding developmental toxicity. The neurotoxicity of mepanipyrim was studied through an acute study. Clinical signs and decrease of rearing and activity counts were observed in the two higher doses in the absence of histopathological examination. An acute NOAEL for neurotoxicity was set at 80 mg/kg bw.
Mepanipyrim has a harmonised classification (and proposed by the peer review to be maintained) as carcinogenic category 2, in accordance with the provisions of Regulation (EC) No 1272/20083. As it is not classified or proposed to be classified as toxic for reproduction category 2, the conditions of the interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine disrupting (ED) properties are not met. However, considering the effects observed in the available studies (decreased fertility index, reduced implantation sites and litter size, increased incidences of uterine adenocarcinoma in rats, increased post implantation losses in rabbits, prostate effect in dogs) an ED potential could not be ruled out and further clarification is needed using mechanistic data (data gap). The issue could not be finalised.
The acceptable daily intake (ADI) and acceptable operator exposure level (AOEL) are set at 0.012 mg/kg bw per day based on the 2‐year and two generations LOAEL of 2.45 mg/kg bw per day and an uncertainty factor (UF) of 200 (two for the use of a LOAEL instead of a NOAEL and 100 as the standard UF). The acute reference dose (ARfD) and acute acceptable operator exposure level (AAOEL) are set at 0.1 mg/kg bw based on the NOAEL of 10 mg/kg bw per day from the rabbit developmental study and a UF of 100. The newly set reference values constitute a revision of those set during the first peer review (ADI = 0.024 mg/kg bw per day, ARfD = 0.30 mg/kg bw and AOEL = 0.07 mg/kg bw per day) (European Commission, 2004).
The RMS estimated non‐dietary exposure (i.e. operator, worker, bystander and resident) with dermal absorption values derived from an in vitro dermal absorption study on human skin, i.e. 0.4% for the concentrate, 6% for in‐use field dilutions for low volume applications and 13% for high volume applications. Using these dermal absorption values and based on the AOEL of 0.012 mg/kg bw per day, the operator exposure exceeds the AOEL, even when personal protective equipment (PPE) is used, in the cases of (a) vine crops high volume field application with tractor‐mounted broadcast air‐assisted sprayer (estimated as 141% of the AOEL in the less conservative case of German model with the use of PPE (gloves), coverall and sturdy footwear), and (b) of low volume hand‐held knapsack application, indoors, to strawberry or tomato (105% of the AOEL, Dutch indoor model, additional PPE including gloves). For all other scenarios, PPE should be used to ensure that operator exposure does not exceed the AOEL. Estimated worker exposure exceeds the AOEL, even with PPE, in the case of re‐entry in vine crops for harvesting and crop‐inspection (estimated as 115% and 130% of the AOEL, respectively). PPE should be used to ensure that workers exposure in tomatoes and strawberries does not exceed the AOEL. Bystanders’ and residents’ exposure is below AOEL in all cases.
The metabolite M31 was found in significant amounts in plant residues while no ground water metabolites were identified. In addition, the metabolites M33 and M36 were identified in lower levels (see Section 3); therefore, the toxicological profile of M31, M33 and M36 was discussed during the experts’ meeting. It was agreed that their genotoxic potential can be considered covered by the parent and by the metabolite M11, for which a full set of genotoxicity tests is available, as the structural differences are not considered related to alteration of the genotoxic potential of these chemicals. However, the same argument is not applicable for other toxic endpoints and consequently the reference values of the parent compound cannot be applied to M31. For this reason, QSAR data and repeated dose toxicity data should be provided for the metabolite M31 (main metabolite) (data gap). The same data would apply to metabolites M33 and M36.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of the residue chemistry studies (OECD, 2009), the OECD publication on the maximum residue level (MRL) calculations (OECD, 2011) the European Commission guideline document on the MRL setting (European Commission, 2011).
Mepanipyrim was discussed at the Pesticide Peer Review Expert Meeting 153 in February 2017.
Metabolism of mepanipyrim in primary crops was investigated upon foliar application on fruit (grapes, tomatoes and apples) with the parent compound 14C‐labelled either on the aniline or on the pyrimidine moiety. The experimental designs were in compliance with the representative uses for the total dose rates representing 1.9N rate when compared to the EU GAPs for strawberries and tomatoes and 2.5N for table grapes, while the harvest interval was longer (30–32 days for apples and grapes and 62 days for tomatoes) compared to the representative GAPs with 1‐day preharvest interval (PHI) for tomatoes and strawberries and 21 days for grapes. Although, a small deficiency of the metabolism study design was identified, it is not expected to influence the final outcome on the metabolic pattern of mepanipyrim. Therefore, the metabolism studies in plant are considered reliable.
The parent mepanipyrim was found to be the predominant compound of the total residues in all crops (23–70% total radioactive residues (TRRs)). M31 was recovered at significant levels in grapes only and mainly under its conjugated form (20–30% TRR) while it occurred at very low proportions in tomato and apple (≤ 1% TRR). Other minor metabolites (M33 and M36) were also identified but accounted for low levels (< 3% TRR) in the investigated crops. It is noted that the metabolic pattern of mepanipyrim in fruit crops was confirmed in the GAP‐compliant residue trials on grapes and strawberries where significant residue levels of M31 were recovered (0.22 and 0.32 mg/kg, respectively) while this compound was not detected in the tomato residue trials (< 0.01 mg/kg).
The residue definition for monitoring was defined as mepanipyrim only. For risk assessment and considering the toxicological profile of M31 was not fully addressed (see data gap in Section 2), the residue definition for risk assessment was proposed as mepanipyrim and M31 (free and conjugated). The way the risk assessment residue definition will be expressed, is pending upon the requested toxicity profile of M31. The proposed residue definitions are limited to fruit crops only.
Based on the confined rotational crop metabolism study conducted at the target application rate (1N), the same residue definitions as for the primary crops are applicable. No residues are expected to be present in rotational crops, provided that mepanipyrim is applied according to the representative uses.
Under the standard hydrolysis conditions representative of food processing, mepanipyrim residues were found to be stable. Moreover, in view of the significant residue levels of M31 recovered in the GAP‐compliant field residue trials on grapes and strawberry, the experts were of the opinion that the fate of M31 under the standard processing conditions should also be investigated (data gap). Since the nature of M31 under hydrolysis conditions was not addressed and considering the chemical structure of M31, further data should be submitted to exclude potential degradation of M31 leading to the formation of aniline. Meanwhile, the residue definition for processed commodities cannot be concluded on.
A sufficient number of residue trials are available, respectively, for table and wine grapes and for strawberries, while for tomatoes one additional residue trial compliant with the indoor GAP is requested (data gap). All the trials were analysed for mepanipyrim and M31 residues and are supported by validated analytical methods and acceptable storage stability data where residues of mepanipyrim and M31 are shown to be stable for at least 18 months in high acid‐ and high water‐ content commodities, in processed commodities and for 9 months in high starch commodities. Processing studies were submitted on strawberries, tomatoes and grapes, and processing factors were derived for several processed commodities. It is, however, highlighted that the validity of the derived processing factors should be reconsidered upon the outcome of the identified data gap to address the behaviour of M31 under the standard hydrolysis conditions for processing. Conversion factors from monitoring to risk assessment were derived from the residue trials of grapes (1.6) and strawberries (1.1) provided that the toxicological reference values set for the parent compound apply also to metabolite M31.
Having regard to the representative uses, a livestock exposure assessment is not triggered.
For the time being, the consumer risk assessment has to be regarded as provisional considering the outstanding data to finalise the residue definitions in primary crops and in processed commodities. Pending the outcome on the toxicological profile of M31, an indicative consumer risk assessment has been conducted for parent mepanipyrim by using the EFSA PRIMo rev.2 model. Long‐term or short‐term intake concerns were not identified for the consumers since the highest chronic and highest acute intakes accounted for 15% ADI (WHO Cluster diet B) and 76% ARfD (table grapes). Further, a preliminary consumer risk assessment has been conducted considering the exposure to the sum of mepanipyrim and M31 (free and conjugated). Assuming for M31 the same toxicity as for the parent and using the HR and STMR values derived from the residue field trials for table and wine grapes, strawberries and tomatoes, acute and chronic intakes concern were not identified (max international estimated short‐term intake (IESTI): 82% ARfD for table grapes and max international estimated daily intake (IEDI) 16% of ADI, FR all population). However, it should be highlighted that this risk assessment is provisional only.
It is noted that in the framework of the peer review of mepanipyrim the toxicological reference values were lowered (see Section 2) and the inclusion of M31 in the residue definition for risk assessment was proposed. Pending the final decision on the expression of the risk assessment residue definition, the established MRLs under Article 12 of Regulation (EC) No 396/2005 and the overall consumer exposure and risk assessment might need to be revised (EFSA, 2011).
The data requirement for determination of the residue levels of mepanipyrim and M31 in pollen and bee products for human consumption resulting from residues taken up by honeybees at blossom from grapes, field grown strawberries and field grown tomatoes was not addressed (data gap).
4. Environmental fate and behaviour
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, mepanipyrim exhibited moderate to high persistence. No major (> 10% applied radioactivity (AR)) metabolites were formed. Mineralisation of the pyrimidine ring 14C radiolabel to carbon dioxide accounted for 2.4–14.2% AR after 120 days and mineralisation of the phenyl ring 14C radiolabel to carbon dioxide accounted for 5.4% AR after 120 days. The formation of unextractable residues (not extracted by acetonitrile/water) for the pyrimidine ring 14C radiolabel accounted for 18.6–67.7% AR after 120 days and for the phenyl ring 14C radiolabel accounted for 26.0% AR after 120 days. In anaerobic soil incubations and in photolysis studies, degradation of mepanipyrim was slow and no major (> 10% AR) metabolites were formed.
Mepanipyrim exhibited medium to immobility in soil; adsorption is not expected to be pH dependent.
In satisfactory field dissipation studies carried out at four different sites: one in the Netherlands, one in France, one in Spain and one in Italy (spray application to the soil surface on bare soil plots in spring), mepanipyrim exhibited moderate to medium persistence. Field study DegT50 values for modelling were derived following normalisation to FOCUS reference conditions (20°C and pF2 soil moisture) following the EFSA (2014) DegT50 guidance. When deriving the modelling endpoint in the Spanish field study only three data points were available, and so this study was not considered when calculating the geometric mean DT50. Consequently, as only three normalised DT50 values were available from the field dissipation studies, following EFSA (2014) DegT50 guidance all the laboratory and field DT50 values were pooled to derive the geometric mean DT50 to be used in future modelling. The field data endpoints were not combined with lab values to derive modelling endpoints. Column leaching studies were carried out for mepanipyrim. Radioactivity in the leachates was very low (< 0.134% AR) and no metabolites were formed.
In laboratory incubations in dark aerobic natural sediment water systems, mepanipyrim exhibited moderate persistence; no major metabolites were formed. The unextractable sediment fraction (not extracted by acetonitrile/water) was the major sink for the pyrimidine ring 14C radiolabel, accounting for 84.3% AR at study end (100 days). Mineralisation of this radiolabel accounted for 5.5–14.6% AR at the end of the study. The rate of decline of mepanipyrim in a laboratory sterile aqueous photolysis experiment was slow relative to that occurred in the aerobic sediment water incubations. No chromatographically resolved component (excluding mepanipyrim) accounted for > 10% AR. A data gap was identified for studies on aerobic mineralisation in surface water. However, the available information on sediment water systems was sufficient for use in exposure modelling for the edge of field surface water bodies.
The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for mepanipyrim, using the FOCUS (2001) Step 1 and Step 2 approach (version 3.2 of the Steps 1‐2 in FOCUS calculator). Furthermore appropriate Step 3 (FOCUS, 2001) and Step 4 calculations were available.4 The Step 4 calculations appropriately followed the FOCUS (2007) guidance, with no‐spray drift buffer zones of up to 20 m being implemented for the drainage scenarios (representing a 91–93% spray drift reduction), and combined no‐spray buffer zones with vegetative buffer strips of up to 20 m (reducing solute flux in run‐off by 80% and erosion runoff of mass adsorbed to soil by 95%) being implemented for the run‐off scenarios. The SWAN tool (version 4.0.1) was appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that whilst run‐off mitigation is included in the Step 4 calculations available, the FOCUS (2007) report acknowledges that for substances with KFoc < 2,000 mL/g (i.e. mepanipyrim), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds. At Step 4, the deposition following volatilisation from plant surfaces was calculated using the EVA 2.0 model.
The representative protected uses (in strawberries and tomatoes) have been assessed as being covered by the exposure assessment performed for open field uses. Protected cropping systems were not considered limited to permanent high technology greenhouses.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 4.4.3 and MACRO 5.5.33. The potential for groundwater exposure from the representative uses by mepanipyrim above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all nine FOCUS groundwater scenarios.
The applicant did not provide appropriate information to address the effect of water treatments processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water. This has led to the identification of a data gap (see Section 7) and results in the consumer risk assessment not being finalised (see Section 9).
The PEC in soil, surface water, sediment, and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013). According to Regulation (EU) No 283/20135, data should be provided regarding the acute and chronic toxicity to honeybees and data to address the development of honeybee brood and larvae. As the European Commission (2002a) does not provide a risk assessment scheme which is able to use the chronic toxicity data for adult honeybees and the honeybee brood, when performing the risk assessment according to European Commission (2002a), the risk to adult honeybees from chronic toxicity and the risk to bee brood, could not be finalised due to the lack of a risk assessment scheme. Therefore, EFSA (2013) was used for risk assessment in order to reach a conclusion for the representative uses.
Based on the available data and risk assessment, a low acute risk via dietary exposure to birds and wild mammals was concluded for all representative uses of mepanipyrim. A low long‐term risk was also concluded for birds at the Tier I for all the representative uses. A high long‐term risk was identified at the Tier I for small herbivorous mammal (all representative uses) and for frugivorous mammal (uses on tomato). At the Pesticide Peer Review Meeting 154 (February 2017), the experts agreed that the available information was not sufficient for supporting the selection of any specific focal species. Therefore no refinement based on ecological data could be used in the risk assessment.
Residue data were available for tomato fruits. However, the incorporation of those data (n = 9) into the larger dataset already available for default residue per unit dose (RUD) estimation (n = 86) would not change the outcome of the risk assessment. Considering all of the above, a data gap was identified for the scenarios where high long‐term risk to wild mammals was identified at the tier I. A low risk for both birds and mammals was concluded from secondary poisoning and from exposure via contaminated water.
No specific PEC calculations were available for the uses in protected crops, where upward spraying is allowed. The RMS in the RAR has concluded that, in lack of specific calculations, PECsw (up to step 3) for open field downward applications cover for uses in permanent structure, but not necessarily for uses in non‐permanent structures. However, considering the kind of application in protected structure (hand‐held knapsack sprayer), EFSA concluded that the PECsw for open field uses would represent a worst case (see Section 4); therefore the risk assessment for all uses in protected structures is considered covered by the analogues uses in the open field. The Tier I acute regulatory acceptable concentration (RAC) for aquatic organisms was based on the effects to invertebrates, while the chronic RAC was based on the effects seen on fish. Based on PEC calculation with FOCUS Step 3, a high acute and chronic risk was identified in some scenarios for each of the representative uses of mepanipyrim. PEC calculated at the FOCUS Step 4, considering mitigation measures equivalent to 20 m no‐spray buffer (uses on vines) and 20 m no‐spray buffer and vegetated filter strip (uses on tomato and strawberries) were sufficient to demonstrate a low acute and chronic risk for all scenarios in all representative uses.
The RMS has assessed the risk to honeybees in accordance with both the European Commission (2002a) and EFSA (2013). A low acute risk (oral and contact) was concluded for all the representative uses of mepanipyrim. A low risk was also concluded for honeybee larvae. Based on Tier I calculations, a low chronic risk was also concluded for all representative uses of mepanipyrim, with the only exception of the treated crop scenario for side‐upwards application on strawberry in non‐permanent protected structures. However, considering that: (i) the trigger was only slightly breached; (ii) the exposure toxicity ratio (ETR) was based on a ‘greater than’ LDD50 value; (iii) only 4% mortality was observed at the highest tested dose determining the LDD50 value, a low risk was concluded also for the scenario where the trigger was breached. A low risk for honeybees (acute, chronic, and larvae) was concluded at the screening step for consumption of contaminated water. No assessment was available for effects on hypopharyngeal glands (HPG) (data gap). No assessment for accumulative effects was available. However, due to the lack of effects observed in the available chronic studies, accumulative effects are not likely to occur. No information was available regarding plant metabolites occurring in pollen and nectar. Therefore, a data gap was identified.
Acute (contact and oral) toxicity data were available for bumblebees. The RMS has performed the risk assessment in accordance with EFSA (2013). A low acute risk was concluded for contact exposure at the screening step (all uses). Based on Tier I calculations, a low acute oral risk was also concluded for all representative uses of mepanipyrim, with the exception of the treated crop scenario on strawberry (application in open field and in non‐permanent protected structures). However, the triggers were only slightly breached, and the LD50 was a ‘greater than’ value derived from a test where 0% mortality was recorded at the highest tested dose. For these reasons, a low oral acute risk to bumblebees was concluded for all representative uses of mepanipyrim.
No data were available for solitary bees.
Tier I data were available for six species of non‐target arthropods. The risk assessment based on mortality data from these studies was sufficient for demonstrating a low risk. However, as effects on reproduction were seen on Aphidius rhopalosiphi and other two non‐standard species, higher tier tests were carried out, including three extended laboratory studies, one semifield and one field study. Based on such higher tier data, the conclusion of low risk was further supported.
A low risk to earthworms, other soil macroorganisms, soil microorganisms and non‐target terrestrial plants was concluded for all the representative uses. A low risk is also concluded for biological methods of sewage treatment.
For the ecotoxicological assessments, no other studies were available to address the potential endocrine activity of mepanipyrim. Pending on the outcome of the data gap identified in Section 2, further ecotoxicological tests might be necessary to address the potential endocrine disrupting properties of mepanipyrim.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Mepanipyrim |
Moderate to high persistence Single first‐order and biphasic kinetics DT50 38.8–155.8 days (DT90 128.9–> 1,000 days; 20°C, 19.6–44.9% water content at pF2) Northern and southern European field dissipation studies Moderate to medium persistence Single first‐order and biphasic kinetics DT50 11.8–82.1 days (DT90 127–273 days) |
Low risk to soil organisms |
DT50: period required for 50% dissipation; DT90: period required for 90% dissipation.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Mepanipyrim | Rat LC50 inhalation > 0.59 mg/L air/4 h (nose only) (no classification required) |
LC50: lethal concentration, median.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Mepanipyrim |
Medium mobility to immobile KFOC 395–5,859 mL/g |
No | Yes | Yes |
KFOC: Freundlich organic carbon adsorption coefficient.
FOCUS scenarios or a relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Mepanipyrim | Low risk to organisms living in surface water when mitigation measures are in place |
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
A method for monitoring mepanipyrim in body fluids and tissues (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
The assessment of the toxicological relevance of two impurities in comparison to the toxicological profile of the parent should be provided (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Comparative interspecies metabolism study in vitro should be provided (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Further clarification of the endocrine disrupting potential using mechanistic data is needed due to the effects observed in the available studies (decreased fertility index, reduced implantation sites and litter size, increased incidences of uterine adenocarcinoma in rats, increased post implantation losses in rabbits, prostate effect in dogs) (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
QSAR data and repeated dose toxicity data relevant to consumer exposure should be provided for the metabolite M31 (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
An additional indoor GAP‐compliant residue trial on tomatoes (relevant for tomato indoor use evaluated; submission date proposed by the applicant: unknown; see Section 3).
The fate of M31 under the standard processing conditions should be further investigated (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
Determination of residues as proposed for risk assessment residue definition in pollen and bee products for human consumption, taken up by honeybees from crops at blossom (relevant for grapes, strawberries outdoor use and tomatoes outdoor use; submission date proposed by the applicant: unknown; see Section 3).
Further information on the effect of water treatment processes on the nature of residues potentially present in surface water, when surface water is abstracted for drinking water (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Studies on aerobic mineralisation in surface water should be provided (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Further information to refine the long‐term risk to wild mammals (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Based on EFSA (2013), suitable data to address the risk of sublethal effects (i.e. HPG development effects) to honeybees due to exposure to mepanipyrim (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
Information to assess the risk to honeybees due to plant metabolites occurring in pollen and nectar (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
PPE has to be used to mitigate the risk for the operators during application (a) of low volume on vines, and (b) outdoor on strawberry or tomato (see Section 2).
PPE has to be used during harvesting strawberry or tomato to mitigate the risk for the worker (see Section 2).
Measures equivalent to 20 m no‐spray buffer (uses on vines) and 20 m no‐spray buffer and vegetated filter strip (uses on tomato and strawberries) are needed for mitigating the risk to aquatic organisms (see Section 5).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20116 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
1
The need for further tests and risk assessment to unique human metabolites could not be finalised while an in vitro comparative metabolism study is not submitted (see Section 2).
-
2
Mepanipyrim was phototoxic in the in vitro study. The assessment of phototoxic and photomutagenic potential of mepanipyrim could not be finalised due to lack of methodology on addressing the in vivo potential as follow‐up of positive in vitro results (see Section 2).
-
3
The interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine disrupting properties are not met for mepanipyrim. However, considering the effects observed in the available studies, the endocrine disrupting potential of mepanipyrim cannot be ruled out and further clarification is needed using mechanistic data (see Section 2).
-
4
The consumer risk assessment could not be finalised considering the outstanding data to finalise the residue definitions for risk assessment in primary crops and in processed commodities and the required GAP‐compliant residue trial on protected tomatoes (see Section 3).
-
5
The consumer risk assessment from the consumption of water could not be finalised, whilst satisfactory information was not available to address the effect of water treatment processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water (see Section 4).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
6
A high long‐term risk was identified for wild mammals exposed to mepanipyrim via dietary exposure, for all the representative uses (see Section 5).
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Vines (low volume application) | Vines (high volume application) | Strawberry (open field) | Strawberry (protected crop) | Tomato (open field) | Tomato (protected crop) | |
|---|---|---|---|---|---|---|---|
| Operator risk | Risk identified | X | X | X | |||
| Assessment not finalised | |||||||
| Worker risk | Risk identified | X | X | ||||
| Assessment not finalised | |||||||
| Resident/bystander risk | Risk identified | ||||||
| Assessment not finalised | |||||||
| Consumer risk | Risk identified | ||||||
| Assessment not finalised | X4,5 | X4,5 | X4,5 | X4,5 | X4,5 | X4,5 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X6 | X6 | X6 | X6 | X6 | X6 |
| Assessment not finalised | |||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||||||
| Assessment not finalised | |||||||
| Risk to aquatic organisms | Risk identified | ||||||
| Assessment not finalised | |||||||
| Groundwater exposure to active substance | Legal parametric value breached | ||||||
| Assessment not finalised | |||||||
| Groundwater exposure to metabolites | Legal parametric value breached | ||||||
| Parametric value of 10 μg/La breached | |||||||
| Assessment not finalised | |||||||
Columns are grey if no safe use can be identified. The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2, 3, 4, 5, 6 for further information.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
Abbreviations
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- BOTRCI
Botryotinia fuckeliana
- bw
body weight
- CA
Chromosomal Aberration
- CHO
Chinese hamster ovary
- CIPAC
Collaborative International Pesticides Analytical Council Limited
- CLP
Classification, Labelling and Packaging
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- ED
endocrine disruption/endocrine disruptor
- EEC
European Economic Community
- ETR
exposure toxicity ratio
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- HPLC
high‐pressure liquid chromatography or high‐performance liquid chromatography
- HPG
hypopharyngeal glands
- HR
hazard rate
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- KFoc
Freundlich organic carbon adsorption coefficient
- LC50
lethal concentration, median
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LD50
lethal dose, median; dosis letalis media
- LDD50
lethal dietary dose; median
- LOAEL
lowest observable adverse effect level
- LOQ
limit of quantification
- MCHC
mean corpuscular haemoglobin concentration
- MN
Micronucleus
- MRL
maximum residue level
- MS
mass spectrometry
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PHI
preharvest interval
- PPE
personal protective equipment
- PPR
Pesticides Peer Review
- QSAR
quantitative structure–activity relationship
- RAC
regulatory acceptable concentration
- RAR
renewal assessment report
- RMS
rapporteur Member State
- RUD
residue per unit dose
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TRR
total radioactive residue
- UF
uncertainty factor
- UV
ultraviolet
- WHO
World Health Organization
- WP‐SB
wettable powder in sealed water soluble bag
Appendix A – List of end points for the active substance and the representative formulation
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4852
Appendix B – Used compound codes
| Code/trivial name | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| M11 |
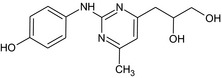
3‐[2‐(4‐Hydroxyanilino)‐6‐methyl‐4‐pyrimidinyl]‐1,2‐propanediol OCC(O)Cc2cc(C)nc(Nc1ccc(O)cc1)n2 |
|
| M31 |
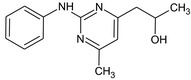
1‐(2‐Anilino‐6‐methyl‐4‐pyrimidinyl)‐2‐propanol CC(O)Cc2cc(C)nc(Nc1ccccc1)n2 |
|
| M33 |
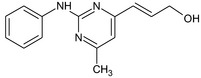
(2E)‐3‐(2‐Anilino‐6‐methyl‐4‐pyrimidinyl)‐2‐propen‐1‐ol OC\C=C\c2cc(C)nc(Nc1ccccc1)n2 |
|
| M36 |
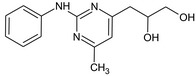
3‐(2‐Anilino‐6‐methyl‐4‐pyrimidinyl)‐1,2‐propanediol OCC(O)Cc2cc(C)nc(Nc1ccccc1)n2 |
|
| B‐11 |
3‐(2‐Anilino‐6‐methyl‐4‐pyrimidinyl)propanoic acid O=C(O)CCc2cc(C)nc(Nc1ccccc1)n2 |
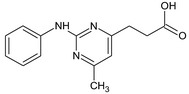
|
SMILES: simplified molecular‐input line‐entry system.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner Renata, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Conclusion on the peer review of the peer review of the pesticide risk assessment of the active substance mepanipyrim. EFSA Journal 2017;15(6):4852, 22 pp. 10.2903/j.efsa.2017.4852
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00834
Corrigendum: Appendix A has been republished to correct errors of an editorial nature in the original version.
Approved: 12 May 2017
Amended: 7 July 2017
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, 1–1355.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2016. Renewal Assessment Report (RAR) on the active substance mepanipyrim prepared by the rapporteur Member State Belgium, in the framework of Commission Implementing Regulation (EU) No 844/2012, May 2016. Available online: http://www.efsa.europa.eu
- Belgium , 2017. Revised Renewal Assessment Report (RAR) on mepanipyrim prepared by the rapporteur Member State Belgium in the framework of Commission Implementing Regulation (EU) No 844/2012, March 2017. Available online: http://www.efsa.europa.eu
- ECHA (European Chemicals Agency), 2015. Guidance on the application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 4.1, June 2015. Reference: ECHA‐15‐G‐05‐EN; ISBN: 978‐92‐9247‐413‐3. Available online: http://echa.europa.eu/documents/10162/13562/clp_en.pdf
- EFSA (European Food Safety Authority), 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for mepanipyrim according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(8):2342, 31 pp. 10.2903/j.efsa.2011.2342 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance mepanipyrim. Available online: http://www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2004. Review report for the active substance mepanipyrim. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 30 March 2004 in view of the inclusion of mepanipyrim in Annex I of Council Directive 91/414/EEC. SANCO/1412/2001‐Final, 29 March 2004, 27 pp.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. p. 1–46.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp, as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


