Abstract
The conclusions of EFSA following the peer review of the initial risk assessment carried out by the competent authority of the rapporteur Member State, the United Kingdom, for the pesticide active substance terbuthylazine are reported. The context of the peer review was that requested by the European Commission following the submission and evaluation of confirmatory data on groundwater metabolites. The conclusions were reached on the basis of the evaluation of the representative uses of terbuthylazine as a herbicide on maize and sorghum. The reliable endpoints concluded as being appropriate for use in regulatory risk assessment, derived from the available studies and literature in the dossier peer reviewed, are presented. Concerns are identified.
Keywords: terbuthylazine, peer review, confirmatory data, risk assessment, pesticide, herbicide
Summary
Terbuthylazine was approved in accordance with Regulation (EC) No 1107/2009 by Commission Implementing Regulation (EU) No 820/2011. It was a specific provision of the approval that the applicant was required to submit to the European Commission further studies on (1) the specification of the technical material, as commercially manufactured including information on the relevance of the impurities; (2) the equivalence between the specifications of the technical material, as commercially manufactured, and the specifications of the test material used in the toxicity studies; (3) groundwater exposure assessment for the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6; (4) the relevance of the metabolites MT1 (N‐tert‐butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine), MT 13 (4‐(tert‐butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol or 6‐hydroxy‐N 2‐ethyl‐N 4‐tert‐butyl‐1,3,5‐triazine‐2,4‐diamine), MT14 (4‐amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol or N‐tert‐butyl‐6‐hydroxy‐1,3,5‐triazine‐2,4‐diamine), and of the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6 with respect to cancer, if terbuthylazine is classified under Regulation (EC) No 1272/2008 as ‘suspected of causing cancer’, by 30 June 2012 for point (1) and (2), by 30 June 2013 for point (3) and within 6 months from the notification of the classification decision concerning terbuthylazine for point (4).
In accordance with the specific provision, the applicants, Syngenta Crop Protection AG and Oxon Italia SpA, submitted an updated dossier in June 2012 and June 2013, which was evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment report. In compliance with guidance document SANCO 5634/2009‐rev. 6.1, the RMS distributed the addendum to Member States, the applicant and the European Food Safety Authority (EFSA) for comments on 6 August 2015. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 18 November 2015. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and finalised the Technical Report in December 2015 (EFSA, 2016).
Concerning points (1) and (2), these points were considered addressed in the Technical Report (EFSA, 2016) and thus not further discussed in this conclusion. Concerning point (4), the Risk Assessment Committee (RAC) of the European Chemicals Agency (ECHA) confirmed in its Opinion of 5 June 2015 (ECHA, 2015) that terbuthylazine should not be classified with respect to cancer, and therefore, this point became obsolete. As indicated in the Technical Report (EFSA, 2016), EFSA and the RMS had different views on some issues of the risk assessment of confirmatory data for terbuthylazine, in particular on the relevance of groundwater metabolites. The RMS proposed that the reference values of parent terbuthylazine could be applied to the metabolites occurring in groundwater above 0.75 μg/L (LM2, LM3, LM4, LM5 and LM6) and that based on these values, the exposure of the consumers would be acceptable. EFSA on the other hand considers that further toxicological data is required to establish metabolite‐specific reference values to be used in consumer risk assessment (taking into account exposure from drinking water). The RMS had the opinion that in respect of both the levels of metabolites predicted to occur in groundwater and in comparison with the relatively low acceptable daily intake (ADI) for terbuthylazine, an acceptable risk to consumers is demonstrated and considers it unjustified to perform further animal studies to support the setting of metabolite‐specific reference values.
Given the divergence in opinion, the European Commission requested EFSA to organise a peer review of the evaluation of the confirmatory data and to conclude on whether the available data is sufficient to conclude on whether exposure to groundwater metabolites above 0.75 μg/L would pose an acceptable risk to consumers through consumption of drinking water.
In accordance with the guidance document on the assessment of the relevance of metabolites in groundwater (European Commission, 2003), no specific reference values for the consumer risk assessment can be derived for the groundwater metabolites LM2, LM3, LM4, LM5 and LM6 on the basis of the available data (data gap). Consequently, the risk assessment for consumers potentially exposed to these groundwater metabolites being predicted to occur in groundwater at concentrations above 0.75 μg/L could not be finalised.
The metabolites MT1, MT13 and MT14 have been concluded as relevant groundwater metabolites as their intakes through drinking water by infants are calculated to be 108% of the relevant ADI (it was agreed in the first peer review (EFSA, 2011) that the reference values of the parent terbuthylazine could be used for the consumer's risk assessment) and 117% of the ADI when consumption of food is also considered.
The information, data and assessments provided in relation to the confirmatory data requirements for environmental fate and behaviour satisfy the confirmatory data request made. The relevant metabolite desethyl‐terbuthylazine (MT1) (considering estimated intakes above toxicological reference values as well as herbicidal activity) is predicted to be above the parametric drinking water limit of 0.1 μg/L in geoclimatic conditions represented by the Hamburg, Kremsmunster, Okehamption and Piacenza FOCUS groundwater scenarios (80th percentile annual average recharge concentrations moving below the top 1 m). For the remaining four relevant FOCUS scenarios, the modelling indicated these concentrations being below the parametric drinking water limit. In the Italian field leaching studies, groundwater (saturated zone) concentrations of MT1 of up to 1.98 μg/L were measured, which is higher than the modelled concentration at Piacenza (0.2 μg/L), the FOCUS scenario in the geoclimatic situation closest to the Italian field leaching study sites investigated. In 20 out of 395 (or 5%) groundwater samples taken at eight sites in northern Italy where terbuthylazine had been applied at a rate of 856 g/ha (1.01N), MT1 concentrations exceeded 0.1 μg/L. This leads to a critical area of concern. There is also a critical area of concern regarding the relevant groundwater metabolites MT13 and MT14 (considering estimated intakes above toxicological reference values), predicted to be above the parametric drinking water limit of 0.1 μg/L in geoclimatic conditions represented by all eight pertinent FOCUS groundwater scenarios (80th percentile annual average recharge concentrations moving below the top 1 m). In 42 out of 144 (or 29%) groundwater samples taken at the eight sites in northern Italy where terbuthylazine had been applied, MT14 concentrations exceeded 0.1 μg/L, being up to 2.65 μg/L.
Background
Terbuthylazine was approved in accordance with Regulation (EC) No 1107/20091 by Commission Implementing Regulation (EU) No 820/20112. The European Food Safety Authority (EFSA) previously finalised a Conclusion on this active substance on 10 January 2011 (EFSA, 2011).
It was a specific provision of the approval that the applicant was required to submit to the European Commission further studies on (1) the specification of the technical material, as commercially manufactured including information on the relevance of the impurities; (2) the equivalence between the specifications of the technical material, as commercially manufactured, and the specifications of the test material used in the toxicity studies; (3) groundwater exposure assessment for the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6; (4) the relevance of the metabolites MT1 (N‐tert‐butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine), MT 13 (4‐(tert‐butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol or 6‐hydroxy‐N 2‐ethyl‐N 4‐tert‐butyl‐1,3,5‐triazine‐2,4‐diamine), MT14 (4‐amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol or N‐tert‐butyl‐6‐hydroxy‐1,3,5‐triazine‐2,4‐diamine), and of the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6 with respect to cancer, if terbuthylazine is classified under Regulation (EC) No 1272/20083 as ‘suspected of causing cancer’, by 30 June 2012 for point (1) and (2), by 30 June 2013 for point (3) and within 6 months from the notification of the classification decision concerning terbuthylazine for point (4).
In accordance with the specific provision, the applicant, Syngenta Crop Protection AG and Oxon Italia SpA, submitted an updated dossier in June 2012, which was evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment report (United Kingdom, 2015). In compliance with guidance document SANCO 5634/2009‐rev. 6.1 (European Commission, 2013), the RMS distributed the addendum to Member States, the applicant and EFSA for comments on 6 August 2015. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 18 November 2015. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and finalised the Technical Report in December 2015 (EFSA, 2016).
Concerning points (1) and (2) these points were considered addressed in the Technical Report (EFSA, 2016) and thus no further discussed in this conclusion. Concerning point (4), the Risk Assessment Committee (RAC) of the European Chemicals Agency (ECHA) confirmed in its Opinion of 5 June 2015 (ECHA, 2015) that terbuthylazine should not be classified with respect to cancer and therefore this point became obsolete. As indicated in the Technical Report (EFSA, 2016), EFSA and the RMS had different views on some issues of the risk assessment of confirmatory data for terbuthylazine, in particular on the relevance of groundwater metabolites. The RMS proposed that the reference values of parent terbuthylazine could be applied to the metabolites occurring in groundwater above 0.75 μg/L (LM1, LM2, LM3, LM4, LM5 and LM6) and that based on these values, the exposure of the consumers would be acceptable. EFSA on the other hand considers that further toxicological data is required to establish metabolite‐specific reference values to be used in consumer risk assessment (taking into account exposure from drinking water). The RMS had the opinion that in respect of both the levels of metabolites predicted to occur in groundwater and in comparison with the relatively low acceptable daily intake (ADI) for terbuthylazine, an acceptable risk to consumers is demonstrated and considers it unjustified to perform further animal studies to support the setting of metabolite‐specific reference values.
Given the divergence in opinion, the European Commission requested EFSA to organise a peer review of the RMS's evaluation of the confirmatory data and in particular to conclude on whether the available data is sufficient to conclude on whether exposure to groundwater metabolites above 0.75 μg/L would pose an acceptable risk to consumers through consumption of drinking water.
The addendum and the reporting table were discussed at the pesticides peer review meeting on mammalian toxicology in February 2017. Details of the issues discussed, together with the outcome of these discussions were recorded in the meeting report.
A final consultation on the conclusions arising from the peer review took place with Member States via a written procedure in April–May 2017.
The conclusions laid down in this report were reached on the basis of the peer review of the RMS's evaluation of the confirmatory data. A key supporting document to this conclusion is the peer review report, which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review. The peer review report (EFSA, 2017) comprises the following documents, in which all views expressed during the course of the peer review, including minority views, can be found:
the report of the scientific consultation with Member State experts;
the comments received on the draft EFSA conclusion.
Given the importance of the addendum to the assessment report (United Kingdom, 2015) and the peer review report, these documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated to have regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Terbuthylazine is the ISO common name for N 2‐tert‐butyl‐6‐chloro‐N 4‐ethyl‐1,3,5‐triazine‐2,4‐diamine (IUPAC).
The representative formulated products for the evaluation were ‘Gardo® Gold®’ (A9476C), a suspo‐emulsion (SE) containing 187.5 g/L terbuthylazine and 312.5 g/L S‐metolachlor, and ‘Terbuthylazine 500 g/L SC’, a suspension concentrate (SC) containing 500 g/L terbuthylazine, both registered under different trade names in Europe.
The representative uses evaluated comprise foliar spraying on maize and sorghum against annual and perennial monocotyledonous and dicotyledonous weeds. Full details of the Good Agricultural Practice (GAP) can be found in the list of end points in Appendix A.
Conclusions of the evaluation
The assessment of the information was presented in a confirmatory data addendum (United Kingdom, 2015). The conclusions laid down in this report were reached on the basis of the peer review of the RMS's evaluation of the confirmatory data submitted on whether the available data is sufficient to conclude on whether exposure to groundwater metabolites above 0.75 μg/L would pose an acceptable risk to consumers through consumption of drinking water.
For clarity, the assessment in relation to the confirmatory data for environmental fate and behaviour is presented as stand‐alone assessment including part of the results already agreed (EFSA, 2011, 2016).
Mammalian toxicity
The following guidance document was followed in the production of this conclusion: SANCO/221/2000‐rev.10‐final (European Commission, 2003).
Terbuthylazine has been discussed during the pesticides peer review meeting 151 on mammalian toxicology (PPR 151) in February 2017.
For terbuthylazine, the ADI is 0.004 mg/kg body weight (bw) per day, based on the no observed adverse effect level (NOAEL) of the 1‐year dog and 2‐year rat studies, and applying an uncertainty factor of 100. The acute reference dose (ARfD) is 0.008 mg/kg bw, based on the maternal NOAEL of 0.8 mg/kg bw per day from the developmental toxicity study in rabbits, and applying an uncertainty factor of 100 (EFSA, 2011).
In the previous conclusion (EFSA, 2011), it was concluded that the reference values of terbuthylazine are applicable to the metabolites desethyl‐terbuthylazine (MT1), hydroxy‐terbuthylazine (MT13) and desethyl‐hydroxy‐terbuthylazine (MT14).
On the basis of the agreed classification of terbuthylazine at EU level (ECHA, 2015), none of the groundwater metabolites is considered toxicologically relevant. In accordance with the guidance document (European Commission, 2003) and considering the available data (negative genotoxicity studies), the majority of the experts (PPR 151) agreed that more information on the repeat‐dose toxicity for the groundwater metabolites LM2, LM3, LM4, LM5 and LM6 is needed in order to conclude on the relevant reference values to be used for the consumer risk assessment which is triggered for these compounds being predicted to occur in groundwater at concentrations of above 0.75 μg/L for the representative uses being assessed (data gap).
The RMS disagrees and considers that, overall, the available information is sufficient to conclude on the acceptability of the groundwater metabolites.
Residues (Consumer risk assessment)
The following guidance document was followed in the production of this conclusion: SANCO/221/2000‐rev.10‐final (European Commission, 2003).
With regard to the metabolites desethyl‐terbuthylazine (MT1), hydroxy‐terbuthylazine (MT13) and desethyl‐hydroxy‐terbuthylazine (MT14) that have passed step 3 of the guidance (see table 1.2 in European Commission, 2003) a consumer exposure and risk assessment was conducted for the sum of these metabolites as they were predicted as co‐occurring above 0.1 μg/L in all eight FOCUS groundwater scenarios (see Section Environmental fate and behaviour), they are found among the residues on treated commodities (EFSA, 2011) and the same toxicological reference values are applicable to these metabolites.
The consumer exposure estimates are based on the default assumptions laid down in the WHO Guidelines (WHO, 2011) for drinking water quality for (a) a 60‐kg adult drinking 2 L of water per day, (b) a 10‐kg child drinking 1 L of water per day and (c) a 5‐kg bottle‐fed infant drinking 0.75 L of water per day.
The combined intake of MT1, MT13 and MT14 through drinking water, expressed as terbuthylazine equivalents, is estimated for (a) adults as 0.96 μg/kg bw, (b) for toddlers as 2.87 μg/kg bw and (c) for infants as 4.30 μg/kg bw corresponding to (a) 23.9%, (b) 71.7% and (c) 108% of the ADI of terbuthylazine applicable to the metabolites considering the most critical scenario (Thiva). For metabolite LM2, LM3, LM4, LM5 and LM6, the consumer exposure assessment cannot be finalised as these metabolites have not passed step 3 of the guidance (European Commission, 2003).
The metabolites MT1, MT13 and MT14 are major crop metabolites with MT1 and MT14 included in the residue definition for dietary risk assessment in addition to terbuthylazine because of significant residues of these metabolites (EFSA, 2011). Chronic dietary exposure from the representative uses was recalculated by EFSA with EFSA PRIMO rev. 2 and the critical result was selected for each of the population subgroups of infants, toddlers and adults considering comparable body weights as used in the drinking water exposure assessment. Dietary exposure considering residues at or below limit of quantification (LOQ) level in accordance with the residue definition for dietary risk assessment for maize and rotated cereals, root crops and oilseeds was reported as 12.6% (IE adult), 9.1% (FR toddler) and 9.0% (UK infant) of the ADI. Therefore, total intakes (from food and drinking water) for the population subgroups of infants, toddlers and adults can be estimated as corresponding to 117% (infant), 80.8% (toddler) and 36.5% (adult) of the ADI.
For the vulnerable population subgroup, infants where there is the possibility they could be exposed to residues from more than one exposure route, an exceedance of the ADI has been calculated. Considering the SANCO/221/2000‐rev.10‐final (European Commission, 2003) guidance, metabolites MT1, MT13 and MT14 should be considered relevant groundwater metabolites.
Environmental fate and behaviour
For clarity, the assessment in relation to the confirmatory data for environmental fate and behaviour is presented as stand‐alone assessment including part of the results already agreed (EFSA, 2011, 2003).
In soil laboratory incubations under aerobic conditions in the dark, terbuthylazine exhibits medium to high persistence forming the major (> 10% applied radioactivity (AR)) metabolites desethyl‐terbuthylazine (MT1, max. 25.1% AR) and hydroxy‐terbuthylazine (MT13, max. 34.5% AR). The persistence of these two metabolites ranged from moderate to high for desethyl‐terbuthylazine and high to very high for hydroxy‐terbuthylazine. Mineralisation of the triazine ring radiolabel to carbon dioxide accounted for 0.4–10.4% AR after 112–120 days. The formation of unextractable residues (not extracted using acetonitrile:water) for this radiolabel accounted for 17–31% AR after 112–120 days. In anaerobic soil incubations, terbuthylazine was essentially stable. In the available field dissipation studies (spray application to the soil surface on bare soil plots in late spring), the persistence of terbuthylazine was moderate to high (23 European sites) while that of desethyl‐terbuthylazine was low to high (10 European sites). Terbuthylazine and hydroxy‐terbuthylazine exhibited medium mobility in soils, while the mobility of desethyl‐terbuthylazine and desethyl‐hydroxy‐terbuthylazine (MT14) was high to very high and low to very high, respectively. There was no evidence that the adsorption of these metabolites was pH dependent. Leaching assessments (modelling) for terbuthylazine were completed assuming a pH dependence for adsorption as it is apparent that adsorption may be lower under alkaline soil conditions.
Terbuthylazine, desethyl‐terbuthylazine (MT1) and hydroxy‐terbuthylazine (MT13) did not leach in average concentrations exceeding 0.1 μg/L in any of the available lysimeter studies (n = 8 for terbuthylazine and desethyl‐terbuthylazine, n = 6 for hydroxy‐terbuthylazine), whereas the metabolite desethyl‐hydroxy‐terbuthylazine (MT14) leached in average annual concentration exceeding 0.1 μg/L in one of the three lysimeters analysing this metabolite. Moreover, five lysimeter studies identified a high leaching risk of six additional metabolites, which were not triggered as metabolites requiring further consideration via the standard laboratory route of degradation studies. Annual average leaching exceeding 0.1 μg/L was observed for LM3, LM4, LM5, LM6 (5 out of 5 lysimeters) and LM2 and LM1 (3 out of 5 lysimeters). In these five lysimeters, the application rate was 5–15% higher than the representative use. Nevertheless, measured concentration suggested that, had the application rate been similar to the representative use of 850 g/ha average, the leaching concentration of all metabolites would still exceed the 0.1 μg/L.
In the lysimeters, the concentration of LM1 was always < 0.75 μg/L (max 0.33 μg/L). Laboratory aerobic soil incubations were carried out for metabolites desethyl‐hydroxy‐terbuthylazine (MT14), LM1 (MT24), LM2 (MT28), LM3, LM4, LM5 (MT23) and LM6 generating the DT50 that can be found in Appendix A. The geomeans of these DT50 included in Appendix A were agreed as appropriate for use as input parameters in groundwater modelling. Batch soil adsorption studies were also provided for the lysimeter metabolites LM1 (MT24), LM2 (MT28), LM3, LM4, LM5 (MT23) and LM6 resulting in the soil mobility being characterised for all of them, as very high.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (2009) scenarios and the models PEARL 4.4.4 and PELMO 4.4.34 for the active substance terbuthylazine and the metabolites desethyl‐terbuthylazine (MT1), hydroxy‐terbuthylazine (MT13), desethyl‐hydroxy‐terbuthylazine (MT14), LM1 (MT24), LM2 (MT28), LM3, LM4, LM5 (MT23) and LM6. Only results from the PEARL 4.4.4 simulations are reported in Appendix A, PELMO 4.4.3 results were comparable but were lower at every FOCUS scenario.
For terbuthylazine, the potential for groundwater exposure from the representative uses above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all eight FOCUS groundwater scenarios. The potential for groundwater exposure by the metabolites desethyl‐terbuthylazine (MT1), hydroxy‐terbuthylazine (MT13) and desethyl‐hydroxy‐terbuthylazine (MT14) was, however, concluded to be high over a wide range of geoclimatic conditions represented by the FOCUS groundwater scenarios (see Table 3).
Table 3.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance | Ecotoxicological activity |
|---|---|---|---|---|---|
| Terbuthylazine |
Medium mobility 191–318 mL/g |
FOCUS: No Lysimeter: No The trigger value of 0.1 μg/L was not exceeded in the 8 lysimeters available |
Yes | Yes | A high risk to the aquatic environment was indicated in the risk assessment for surface water |
|
Desethyl‐terbuthylazine MT1 |
High to very high mobility 44–122 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger value of 0.1 μg/L was 4, (0.163–0.4 μg/L) Lysimeter: No The trigger value of 0.1 μg/L was not exceeded in the 8 lysimeters available |
Yes, so this groundwater metabolite is considered relevant | From the consumer exposure assessment point of view, the reference values of the parent are applicable to this metabolite. Intakes for MT1+MT13+MT14 account for 117% of the ADI (infant). Therefore, MT1 is considered a relevant metabolite in groundwater | The risk to aquatic organisms in surface water was assessed as low |
|
Hydroxy‐terbuthylazine MT13 |
Medium mobility 104–280 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 8 and 6, respectively Lysimeter: No The trigger value of 0.1 μg/L was not exceeded in the 6 lysimeters available |
No | From the consumer exposure assessment point of view, the reference values of the parent are applicable to this metabolite. Intakes for MT1+MT13+MT14 account for 117% of the ADI (infant). Therefore, MT13 is considered a relevant metabolite in groundwater | The risk to aquatic organisms in surface water was assessed as low |
|
Desethyl‐hydroxy‐terbuthylazine MT14 |
Low to very high mobility 22–1,010 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 7 and 0, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in 1 lysimeter and in 42 samples in field leaching study, concentrations up to 2.65 μg/L |
No | From the consumer exposure assessment point of view, the reference values of the parent are applicable to this metabolite. Intakes for MT1+MT13+MT14 account for 117% of the ADI (infant). Therefore, MT14 is considered a relevant metabolite in groundwater | The risk to aquatic organisms in surface water was assessed as low |
|
LM1 MT24 |
Very high mobility 30–37 mL/g |
FOCUS: No Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in 3 of 5 lysimeter |
No, based on the argumentation that it is a breakdown product of LM5 that did not exhibit pesticidal activity |
No No genotoxic potential |
The risk to aquatic organisms in surface water was assessed as low |
|
LM2 MT28 |
Very high mobility 6–13 mL/g pH dependent |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 7 and 0, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in 3 of 5 lysimeters |
No |
No genotoxic potential Reference values for consumer risk assessment could not be derived |
The risk to aquatic organisms in surface water was assessed as low |
| LM3 |
Very high mobility 3.3–4.2 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 7 and 0, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in all 5 lysimeters |
No |
No genotoxic potential Reference values for consumer risk assessment could not be derived |
The risk to aquatic organisms in surface water was assessed as low |
| LM4 |
Very high mobility 4–15 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 8 and 1, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in all 5 lysimeters |
No |
No genotoxic potential Reference values for consumer risk assessment could not be derived |
The risk to aquatic organisms in surface water was assessed as low |
|
LM5 MT23 |
Very high mobility 13–19 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 7 and 0, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in all 5 lysimeters |
No |
No genotoxic potential Reference values for consumer risk assessment could not be derived |
The risk to aquatic organisms in surface water was assessed as low |
| LM6 |
Very high mobility 13–14 mL/g |
FOCUS: Yes The number of FOCUS scenarios exceeding the trigger values of 0.1, 0.75 and 10 μg/L was 8, 8 and 0, respectively Lysimeter: Yes The trigger value of 0.1 μg/L was exceeded in all 5 lysimeters |
No |
No genotoxic potential Reference values for consumer risk assessment could not be derived |
The risk to aquatic organisms in surface water was assessed as low |
At least one FOCUS scenario or a relevant lysimeter.
It should be noted that for desethyl‐terbuthylazine and hydroxy‐terbuthylazine the high leaching risk calculated with the FOCUS scenario modelling tools was not consistent with the result from the individual lysimeter studies, which all suggested a low leaching risk of these two metabolites. However, because terbuthylazine has an extensive metabolic pathway, a limited number of applications was made (perennial use not investigated), and the observation that leachate concentrations of some of the metabolites were increasing in the second year, the pattern of leaching observed in these lysimeter studies does not provide a definitive picture of leaching that would enable the first tier FOCUSgw exposure estimates to be overruled.
A large number of groundwater monitoring data were presented in the original dossier, and the experts’ meeting (PRAPeR 84) discussed to what extent these monitoring data should be taken into account in the groundwater risk assessment. The experts considered that FOCUS scenario modelling results should not normally be overruled by using monitoring data unless there is a very strong justification. In the case of terbuthylazine, information on the extent of uses (both the proportion of the monitored area and the application rate) and the average travel time to the monitoring screen was generally not provided in many of the monitoring exercises presented in the dossier. Without this information, it becomes very difficult to justify that the monitoring data actually reflect a realistic use condition that will continue should terbuthylazine be continued to be approved, and so it is difficult to use in a regulatory context. However, among the available studies, the experts considered that two monitoring exercises provided data of sufficient quality and quantity and should be viewed alongside the FOCUS modelling results in order to establish the most representative picture possible of the overall leaching potential. The two monitoring studies comprised a targeted monitoring study in Germany (samples taken from shallow wells mostly situated less than 5 m below ground surface) and field leaching studies comprising eight field sites in northern Italy (samples taken from the saturated zone via piezometers, with an average depth to groundwater ranging from 1.1 to 6.2 m). As had been agreed previously in the context of the use of residue levels in samples taken from the saturated zone (EFSA, 2011), it was considered appropriate to compare regulatory triggers with concentrations measured in individual samples and not with the annual averages that are relevant when assessing concentrations in leachate recharge leaving the upper layers of the soil column. A summary of the monitoring and modelling results are presented in the following bullets and in Table 1.
Terbuthylazine : Both monitoring data and modelling results suggested that the potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L is expected to be low in geoclimatic and use situations represented by the monitoring study in northern Italy and Germany and eight FOCUS groundwater scenarios (Table 1)
-
Desethyl‐terbuthylazine : The potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L is expected to be:
-
1
— high in geoclimatic and use situations represented by four FOCUS groundwater scenarios.
-
2
— low in geoclimatic and use situations represented by four FOCUS groundwater scenarios, six of the eight monitoring sites in northern Italy and the monitoring in Germany.
-
1
While desethyl‐terbuthylazine was rarely detected in a German monitoring study (and never above 0.1 μg/L), the potential for leaching of desethyl‐terbuthylazine in Italian field sites seems to be higher than that of terbuthylazine (Table 1). The latter finding is consistent with the FOCUS scenarios suggesting that desethyl‐terbuthylazine would leach to a higher degree than terbuthylazine. At two of the eight Italian field sites, frequency of exceedence of 0.1 μg/L reached 12% and 14% indicating that leaching below the root zone in concentrations above 0.1 μg/L is likely to occur in some areas. However, when averaging all sites the frequency of exeedence of 0.1 μg/L was only 5% so it was concluded that the overall potential for groundwater exposure from the representative uses above the parametric drinking water limit of 0.1 μg/L is expected to be low under conditions represented by the two monitoring exercises (excluding two Italian sites) and the Chateaudun, Porto, Sevilla and Thiva FOCUS scenarios.
-
Desethyl‐hydroxy‐terbuthylazine : The potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L is expected to be
-
1
— high in geoclimatic and use situations represented by the monitoring study in northern Italy and eight FOCUS groundwater scenarios.
-
2
— low in geoclimatic and use situations represented targeted monitoring in Germany.
-
1
Consistent with the FOCUS modelling result, the monitoring data suggest that the potential for leaching of desethyl‐hydroxy‐terbuthylazine is higher than for desethyl‐terbuthylazine and terbuthylazine. However, it should be noted that groundwater concentrations exceeding 0.1 μg/L were not observed in the German monitoring study.
Hydroxy‐terbuthylazine : The potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L is expected to be
high in geoclimatic and use situations represented by the eight FOCUS groundwater scenarios.
low in geoclimatic and use situations represented by the monitoring study in northern Italy and Germany.
The FOCUS scenarios suggest that leaching of hydroxy‐terbuthylazine is approximately five times higher than that of desethyl‐hydroxy‐terbuthylazine. This is noted to be a relative tendency which (unlike the information for the other metabolites) is not reflected in the available monitoring data.
-
Metabolites LM2 (MT28), LM3, LM4, LM5 (MT23) and LM6 : The potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L is expected to be
-
1
— high in geoclimatic and use situations represented by the eight FOCUS groundwater scenarios.
-
2
— high in geoclimatic and use situations represented by the monitoring study in northern Italy and Germany where concentrations of all these metabolites were found in water samples from the saturated zone with maxima in the range 0.26–1.58 μg/L with metabolite LM6 being present at > 0.75 μg/L at three of the Italian field leaching study sites.
-
1
Table 1.
Results from the FOCUS modelling as well as the field leaching study in northern Italy and targeted monitoring data from Germany
| Terbuthylazine | Desethyl‐terbuthylazine MT1 | Hydroxy‐terbuthylazine MT13 | Desethyl‐hydroxy‐terbuthylazine MT14 | |
|---|---|---|---|---|
| FOCUS modelling results | ||||
| Number of scenarios > 0.1 μg/L | 0 | 4 | 8 | 8 |
| Number of scenarios < 0.1 μg/L | 8 | 4 | 0 | 0 |
| Monitoring data | ||||
| Northern Italy (8 field leaching studya, 395 samples) | ||||
| – Detection (% of analysed samples) | 16% | 32% | 1%3) | 40%c |
| – Detection > 0.1 μg/L (% of analysed samples) | 3% | 5% | 0%3) | 29%c |
| Germanyb (targeted monitoring, 25 wells, 29 samples) | ||||
| – Detection (% of analysed samples) | 7% | 7% | 3% | 14% |
| – Detection > 0.1 μg/L (% of analysed samples) | 0% | 0% | 0% | 0% |
| LM2 | LM3 | LM4 | LM5 | LM6 | |
|---|---|---|---|---|---|
| FOCUS modelling results | |||||
| Number of scenarios > 0.1 μg/L | 8 | 8 | 8 | 8 | 8 |
| Number of scenarios < 0.1 μg/L | 0 | 0 | 0 | 0 | 0 |
| Monitoring data | |||||
| Northern Italy (7 field leaching studya, 366 samples) | |||||
| – Detection (% of analysed samples) | 37% | 39% | 22% | 52% | 41% |
| – Detection > 0.1 μg/L (% of analysed samples) | 19% | 22% | 11% | 27% | 30% |
| Germanyb (targeted monitoring, 25 wells, 29 samples) | |||||
| – Detection (% of analysed samples) | na | 36% | na | 52% | 48% |
| – Detection > 0.1 μg/L (% of analysed samples) | na | 28% | na | 38% | 38% |
Na: not analysed.
The two sites receiving ‘basin irrigation’ are not included.
Monitored area being treated with terbuthylazine ranges between 8% and 80% (average 25%).
Only 144 samples were analysed for hydroxy‐terbuthylazine and desethyl‐hydroxy‐terbuthylazine.
Considering the consumer risk assessment to infants and the SANCO/221/2000‐rev.10‐final (European Commission, 2003) guidance, metabolites MT1, MT13 and MT14 should be considered relevant groundwater metabolites (see Residues section).
Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 2–4)
Table 2.
Soil
| Compound (name and/or code) | Persistence |
|---|---|
| Terbuthylazine |
Medium to high persistence Single first‐order DT50 65–167 days; 20°C, soil moisture 13–36% w/w) (Field dissipation studies: single first order DT50 10–148 days; 20°C, pF 2 soil moisture) |
| Desethyl‐terbuthylazine (MT1) |
Moderate to high persistence Single first‐order DT50 27–113 days (20°C, soil moisture 11–29% w/w) (Field dissipation studies: single first order DT50 2–223 days; 20°C, pF 2 soil moisture) |
| Hydroxy‐terbuthylazine (MT13) |
High to very high persistence Single first‐order DT50 207–> 1,000 days (20°C, soil moisture 11–29% w/w) |
DT50: period required for 50% dissipation.
Data gaps
This is a list of data gaps identified in the focussed peer review process of confirmatory data. Data gaps identified in the previously finalised EFSA conclusion on the active substance (EFSA, 2011) that were not part of the focussed peer review process of confirmatory data remain unchanged.
More information on the repeat‐dose toxicity for the groundwater metabolites LM2, LM3, LM4, LM5 and LM6 is needed in order to conclude on the relevant reference values to be used for the consumer risk assessment (relevant for all representative uses; date of submission: unknown; see mammalian toxicity Section).
Concerns
1. Issues that could not be finalised
An issue is listed as an issue that could not be finalised where there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20115, and where the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
-
1
As the toxicological data on metabolites LM2 (MT28), LM3, LM4, LM5 (MT23) and LM6 was insufficient to determine reference values, the risk assessment for consumers potentially exposed to these groundwater metabolites being predicted to occur in groundwater in concentrations above 0.75 μg/L could not be finalised. This leads to the groundwater (toxicological) relevance assessment for these metabolites being not finalised, in geoclimatic situations represented by 8/8 FOCUS groundwater scenarios.
2. Critical areas of concern
An issue is listed as a critical area of concern where there is enough information available to perform an assessment for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and where this assessment does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern where the assessment at the higher tier level could not be finalised due to lack of information, and where the assessment performed at the lower tier level does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
-
2
The potential for groundwater exposure by the herbicidally active and relevant groundwater metabolite: desethyl‐terbuthylazine (MT1) above the parametric drinking water limit of 0.1 μg/L is predicted to be high over a wide range of geoclimatic conditions. For the representative uses assessed, four out of the eight pertinent FOCUS groundwater scenarios exceeded the 0.1 μg/L limit. In 20 out of 395 (or 5%) of groundwater samples taken at eight sites in northern Italy where terbuthylazine had been applied at a rate of 856 g/ha (1.01N), desethyl‐terbuthylazine concentrations exceeded 0.1 μg/L.
-
3
The potential for groundwater exposure by the relevant groundwater metabolites that have passed beyond step 3 of the guidance (European Commission, 2003): hydroxy‐terbuthylazine (MT13) and desethyl‐hydroxy‐terbuthylazine (MT14) above the parametric drinking water limit of 0.1 μg/L is predicted to be high over a wide range of geo‐climatic conditions. For the representative uses assessed, all eight pertinent FOCUS groundwater scenarios exceeded the 0.1 μg/L limit. In 42 out of 144 (or 29%) of groundwater samples taken at eight sites in northern Italy where terbuthylazine had been applied at a rate of 856 g/ha (1.01N), desethyl‐hydroxy‐terbuthylazine (MT14) concentrations exceeded 0.1 μg/L.
-
4
Total intakes of metabolites MT1, MT13 and MT14 from food and drinking water for the vulnerable population subgroup infants were estimated as corresponding to 117% of the ADI and therefore, where there is the possibility that infants could be exposed to residues from more than one exposure route the toxicological reference value may be exceeded.
3. Overview of the concerns identified for each representative use considered
Table 4.
Overview of concerns
| Representative use | Maize | Sorghum | |
|---|---|---|---|
| Consumer risk | Risk identified | X | X |
| Assessment not finalised | X1 | X1 | |
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breached | X2,3 | X2,3 |
| Parametric value of 10 μg/La breached | |||
| Assessment not finalised | X1 | X1 | |
Abbreviations
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- bw
body weight
- CI
confidence interval
- DAR
draft assessment report
- DT50
period required for 50% dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification (determination)
- NOAEL
no observed adverse effect level
- RAC
Risk Assessment Committee
- RMS
rapporteur Member State
- SC
suspension concentrate
- SE
suspo‐emulsion
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- w/w
weight per unit weight
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation (relevant for the current assessment)
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4868
Appendix B – Used compound codes
1.
| Code/trivial namea | Chemical name/SMILES notation | Structural formula |
|---|---|---|
|
MT1 Desethyl‐terbuthylazine GS 26379 |
N‐tert‐Butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine Nc1nc(NC(C)(C)C)nc(Cl)n1 |
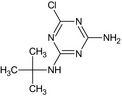
|
|
MT13 Hydroxy‐terbuthylazine or 2‐Hydroxy‐terbuthylazine GS 23158 |
4‐(tert‐Butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol or 6‐Hydroxy‐N 2‐tert‐butyl‐N 4‐tert‐butyl‐1,3,5‐triazine‐2,4‐diamine Oc1nc(NCC)nc(NC(C)(C)C)n1 |
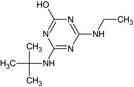
|
|
MT14 Desethyl‐hydroxy‐terbuthylazine or Desethyl‐2‐hydroxy terbuthylazine GS 28620 |
4‐Amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol or N‐tert‐Butyl‐6‐hydroxy‐1,3,5‐triazine‐2,4‐diamine Nc1nc(NC(C)(C)C)nc(O)n1 |
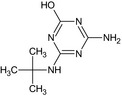
|
|
MT19 De‐t‐Butyl‐hydroxy‐terbuthylazine or Atrazine‐desisopropyl‐2‐hydroxy GS17792 |
4‐Amino‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol or N‐Ethyl‐6‐hydroxy‐1,3,5‐triazine‐2,4‐diamine Nc1nc(NCC)nc(O)n1 |
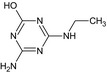
|
|
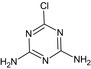
MT20 Diamino‐chlorotriazine or AtRazine‐desethyl desisopropyl GS28273 |
6‐Chloro‐1,3,5‐triazine‐2,4‐diamine Nc1nc(N)nc(Cl)n1 |
|
|
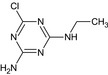
MT22 De‐t‐Butyl‐terbuthylazine or Atrazine‐desisopropyl‐2‐hydroxy |
6‐Chloro‐N‐ethyl‐1,3,5‐triazine‐2,4‐diamine Nc1nc(NCC)nc(Cl)n1 |
|
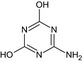
|
LM1 MT24 Amino‐dihydroxy‐triazine GS 35713 CSAA404936 |
6‐Amino‐1,3,5‐triazine‐2,4‐diol Oc1nc(N)nc(O)n1 |
|
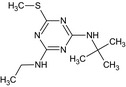
|
terbutryn MT26 GS 14260 |
N 2 ‐tert‐butyl‐N 4‐ethyl‐6‐methylthio‐1,3,5‐triazine‐2,4‐diamine CSc1nc(NCC)nc(NC(C)(C)C)n1 |
|
|
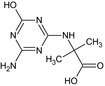
LM2 MT28 CSAA036479 CGA046571 |
N‐(4‐Amino‐6‐hydroxy‐1,3,5‐triazin‐2‐yl)‐2‐methylalanine Nc1nc(NC(C)(C)C(=O)O)nc(O)n1 |
|
|
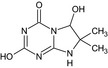
LM3 SM9 CSCD692760 SYN546009 |
2,6‐Dihydroxy‐7,7‐dimethyl‐6,8‐dihydroimidazo[1,2‐a][1,3,5]triazin‐4(6H)‐one O=C1N=C(O)N=C2NC(C)(C)C(O)N12 |
|
|
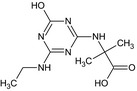
LM4 SM4 CSAA404949 GS40436 |
N‐[4‐(ethylamino)‐6‐hydroxy‐1,3,5‐triazin‐2‐yl]‐2‐methylalanine Oc1nc(NCC)nc(NC(C)(C)C(=O)O)n1 |
|
|
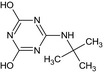
LM5 MT23 SM12 GS 16984 |
6‐(tert‐Butylamino)‐1,3,5‐triazine‐2,4‐diol Oc1nc(NC(C)(C)C)nc(O)n1 |
|
|
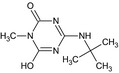
LM6 SM6 CSCD648241 SYN545666 |
4‐(tert‐Butylamino)‐6‐hydroxy‐1‐methyl‐1,3,5‐triazin‐2(1H)‐one O=C1N=C(NC(C)(C)C)N=C(O)N1C |
|
|
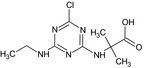
LM7 GS31398 |
N‐[4‐Chloro‐6‐(ethylamino)‐1,3,5‐triazin‐2‐yl]‐2‐methylalanine Clc1nc(NCC)nc(NC(C)(C)C(=O)O)n1 |
|
SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, Bura L, Chiusolo A, Marques DC, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Stanek A, Sturma J, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Conclusion on the peer review of the pesticide risk assessment for the active substance terbuthylazine in light of confirmatory data submitted. EFSA Journal 2017;15(6):4868, 20 pp. 10.2903/j.efsa.2017.4868
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00035
Approved: 23 May 2017
Notes
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 820/2011 of 16 August 2011 approving the active substance terbuthylazine, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 and Commission Decision 2008/934/EC Text with EEA relevance. OJ L 209, 17.8.2011, p. 18–23.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Simulations used a Q10 of 2.58 in line with EFSA (2008) and a Walker equation coefficient of 0.7.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA (European Chemicals Agency), 2015. Committee for Risk Assessment (RAC) Opinion proposing harmonised classification and labelling at EU level of terbuthylazine. [CLH‐0‐0000001412‐86‐66/F]. Adopted in 5 June 2015.
- EFSA (European Food Safety Authority), 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Conclusion on the peer review of the pesticide risk assessment of the active substance terbuthylazine. EFSA Journal 2011;9(1):1969, 133 pp. 10.2903/j.efsa.2011.1969 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for terbuthylazine in light of confirmatory data. EFSA supporting publication 2016:EN‐919, 54 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance terbuthylazine. Available online: http://www.efsa.europa.eu
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009‐rev. 6.1.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2009. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 1, 604 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.0, January 2011.
- United Kingdom , 2015. Addendum to the assessment report on terbuthylazine prepared by the rapporteur Member State the United Kingdom in light of confirmatory data, November 2015. Available online: http://www.efsa.europa.eu
- WHO , 2011. WHO Guidelines for drinking‐water quality. 4th Edition, ISBN 978 92 4 154815 1, 541 pp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


