Abstract
Hypertension is a major public health problem among the aging population worldwide. It causes cardiac remodeling, including hypertrophy and interstitial fibrosis, which leads to development of hypertensive heart disease (HHD). Although microRNA-21 (miR-21) is associated with fibrogenesis in multiple organs, its contribution to cardiac remodeling in hypertension is poorly understood. Circulating miR-21 level was higher in patients with HHD than that in the control subjects. It also positively correlated with serum myocardial fibrotic markers. MiR-21 expression levels were significantly upregulated in the mice hearts after angiotensin II (Ang II) infusion or transverse aortic constriction (TAC) compared with control mice. Expression level of programmed cell death 4 (PDCD4), a main target of miR-21, was significantly decreased in Ang II infused mice and TAC mice compared with control mice. Expression levels of transcriptional activator protein 1 (AP-1) and transforming growth factor-β1 (TGF-β1), which were downstream targets of PDCD4, were increased in Ang II infused mice and TAC mice compared with control mice. In vitro, mirVana-miR-21-specific inhibitor attenuated Ang II-induced PDCD4 downregulation and contributed to subsequent deactivation of AP-1/TGF-β1 signaling pathway in neonatal rat cardiomyocytes. Thus, suppression of miR-21 prevents hypertrophic stimulation-induced cardiac remodeling by regulating PDCD4, AP-1, and TGF-β1 signaling pathway.
Introduction
Hypertension is a major public health concern among the elderly population worldwide. It is associated with increased risk of adverse cardiovascular events [1]. An epidemiological report indicated that 31.1% of adults in the world (1.39 billion people) had hypertension in 2010 [2]. Hypertension increases the risk of developing hypertension-induced organ damages, such as hypertensive heart disease (HHD), hypertensive encephalopathy, and nephrosclerosis [3]. HHD is one of the most important hypertension-induced organ damages [4]. According to the Framingham Heart Study, 20 mmHg increase in systolic blood pressure contributes to 56% increased risk for heart failure [5]. Furthermore, it was reported that HHD is a common pathophysiology of heart failure with preserved ejection fraction [6, 7]. Hypertension causes cardiac remodeling characterized by cardiac fibrosis, which contributes to progression of heart failure [3, 4].
MicroRNAs (miRs) are small non-coding RNAs that regulate post-transcriptional gene expressions. They have been shown to play an important role in fibrogenic process in multiple organs [8]. In the present study, we focused on the fibrogenic function of miR-21, which is a ubiquitously expressed miR that is reported to have a pivotal role in development of tissue fibrosis [9]. Transforming growth factor-β1 (TGF-β1), a pleiotropic and multifactorial cytokine involved in many biological processes, plays a crucial role in the pathogenesis of cardiac remodeling in hypertension [10]. It has been demonstrated that miR-21 can promote TGF-β1 signaling [11, 12]. On the other hand, miR-21 has been found to be upregulated by TGF-β1 [13]. This interrelationship forms a positive feedback loop, which may exacerbate the fibrogenic process. Previous studies have also demonstrated the contribution of miR-21 in patients with aortic stenosis, hypertrophic cardiomyopathy, and dilated cardiomyopathy [14–16]. However, the association between miR-21 and cardiac remodeling in hypertension is still not clear.
We hypothesized that miR-21 deteriorates hypertension-induced cardiac remodeling by enhancing TGF-β1 signaling pathway through suppressing its target gene expression. In the present study, we investigated the following: (1) miR-21 expression levels in patients with HHD; (2) miR-21 expression levels and its downstream signaling in animal model of hypertrophic cardiac remodeling by transverse aortic constriction (TAC) or angiotensin II (Ang II) infusion; (3) the function of miR-21 in cardiac remodeling process in response to Ang II stimulation in vitro; (4) the therapeutic potential of miR-21 inhibitor in hypertrophic stimulation-induced cardiac remodeling in vitro.
Materials and methods
Human studies
The present study included 10 HHD patients with at least ten years of hypertension and 10 control patients who were assessed to rule out cardiomyopathy and heart failure, and had normal cardiac function (Table 1). Endomyocardial biopsies (EMBs) were collected from the patients who had left ventricular hypertrophy and suspected some types of cardiomyopathy. EMBs were taken from left ventricle with a total of 4 to 6 samples through the femoral arteries. EMBs were analyzed in 3 HHD patients who were excluded other cardiomyopathy based on EMBs and other clinical data, and 3 control patients who had transient left ventricular dysfunction and suspected myocarditis but were eventually ruled out cardiomyopathy. The final diagnosis of HHD was made by two expert cardiologists based on angiography, echocardiographic data, clinical background, and medical history. Written informed consent was obtained from all patients before the study. The protocol was performed in accordance to the Helsinki Declaration and was approved by the human investigations committee of Yamagata University School of Medicine.
Table 1. Clinical characteristics of 10 control subjects and 10 HHD patients.
| Variables | Control patients n = 10 | HHD patients n = 10 | P value |
|---|---|---|---|
| Age (years old) | 61 ± 8 | 58 ± 12 | ns |
| Male / Female | 6 / 4 | 7 / 3 | ns |
| BMI (kg/m2) | 23.7 ± 1.9 | 26.1 ± 5.9 | ns |
| Hypertension, n (%) | 3 (30) | 10 (100) | <0.05 |
| Diabetes mellitus, n (%) | 1 (10) | 6 (60) | <0.05 |
| Dyslipidemia, n (%) | 3 (30) | 4 (40) | ns |
| NYHA functional class III-IV, n (%) | 0 (0) | 4 (40) | <0.05 |
| Echocardiographic data | |||
| LVEDD (mm) | 47 ± 5 | 55 ± 9 | <0.05 |
| LVEF (%) | 68 ± 6 | 52 ± 14 | <0.05 |
| IVSD (mm) | 9 ± 2 | 14 ± 2 | <0.05 |
| LVPWD (mm) | 10 ± 1 | 13 ± 2 | <0.05 |
| Blood examination | |||
| eGFR (mL/min/1.73 m2) | 92.0 ± 18.9 | 56.3 ± 25.5 | <0.05 |
| BNP (pg/mL) | 36 (17–70) | 463 (143–713) | <0.05 |
| Medications | |||
| ACEIs and/or ARBs, n (%) | 3 (30) | 10 (100) | <0.05 |
| CCBs, n (%) | 1 (10) | 7 (70) | <0.05 |
| Diuretics, n (%) | 0 (0) | 6 (60) | <0.05 |
| Statins, n (%) | 3 (30) | 5 (50) | ns |
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; CCBs, calcium-channel blockers; eGFR, estimated glomerular filtration rate; HHD, hypertensive heart disease; IVSD, interventricular septum diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVPWD, left ventricular posterior wall diameter; NYHA, New York Heart Association.
Measurement of circulating miR-21 levels and biochemical assays
Blood samples were collected in the early morning within 24 hours after admission, centrifuged at 3000 g for 15 min at 4°C, and the obtained serum was stored at −80°C. Circulating miRs were isolated from 300 μL serum by using a NucleoSpin microRNA isolation kit (TaKaRa, Otsu, Japan).
Serum carboxy-terminal telopeptide of type I collagen (I-CTP) concentrations were determined by radioimmunoassay (Orion Diagnostica, Finland) [17]. Serum procollagen type III N-terminal propeptide (P3NP) levels were measured with enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA, USA).
Animal treatment regimens
Hypertension-induced cardiac remodeling models were established by Ang II infusions or TAC surgery in male C57BL/6J (8–10 weeks old) mice [18, 19]. Briefly, Ang II was infused with ALZET osmotic pumps (1.5 mg/kg/day) as we previously described [18]. Cardiac function, dimension, and blood pressure were assessed after 2 weeks from Ang II infusion. Blood pressure was measured using a non-invasive tail-cuff blood pressure system (Muromachi Kikai Co, Ltd, MK-2000ST NP-NIBP Monitor, Tokyo, Japan). The mice were sacrificed by intraperitoneal injection of a combination of ketamine (1g/kg) and xylazine (100 mg/kg), and the heart samples were obtained for the biochemical and histopathological study. TAC surgery was performed to induce chronic pressure overload as we previously described [20]. Briefly, mice were anesthetized by intraperitoneal injection with a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg). Animals were intubated and ventilated with a rodent ventilator (Harvard Apparatus, Holliston, MA, USA). The transverse aortic arch was ligated (7–0 prolene) between the right innominate and left common carotid arteries with a 27-gauge needle, and then the needle was promptly removed leaving a discrete region of stenosis. Cardiac remodeling was assessed after 4 weeks from surgery. All experimental procedures were performed according to the animal welfare regulations of Yamagata University School of Medicine, and the study protocol was approved by the Animal Subjects Committee of Yamagata University School of Medicine. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011).
Neonatal rat cardiomyocyte isolation, cell culture, and treatment
Isolation and culture of neonatal rat cardiomyocytes (NRCMs) were performed as we previously described [21]. Briefly, ventricles were obtained from 1- to 2-day-old Sprague-Dawley rat pups (mixed gender) after euthanasia by decapitation, and cardiomyocytes were isolated by digestion with collagenase. Cardiomyocytes were kept in serum-supplemented (10% fatal bovine serum, FBS) Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, MA, USA). Primary culture of cardiofibroblasts were obtained as previously described [22]. Briefly, ventricles of Sprague-Dawley rat pups were digested with collagenase, and resuspended in DMEM with 10% FBS. Cells were then seeded into 10-cm culture dishes and cultured at 37°C for 2h. Unattached cells were discarded, and attached cells were cultured in DMEM with 10% FBS. NRCMs were transfected with small interfering RNA (siRNA) specific for programmed cell death 4 (PDCD4) (Thermo Fisher Scientific), 10 nM mirVana hsa-miR-21 specific inhibitor (Thermo Fisher Scientific), or mirVana miRNA inhibitor Negative Control (Thermo Fisher Scientific) using Lipofectamine 3000 Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The medium was replaced with DMEM with 10% FBS after transfection for 4h. NRCMs were stimulated with 1 μM Ang II for 24 hours of serum starvation.
Western blotting
The total protein extracts were prepared with radio-immunoprecipitation assay (RIPA) buffer as we previously reported [21]. Equal amounts of protein were subjected to 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were probed overnight at 4°C with the following primary antibodies: PDCD4 (Santa Cruz, Dallas, TX, USA, sc-376430), c-Jun (Cell Signaling Technology, Danvers, MA, USA, #9165), TGF-β1 (Cell Signaling Technology, #3711), phospho-transforming growth factor-β-activated kinase 1 (p-TAK1) (Cell Signaling Technology, #4537), TAK1 (Cell Signaling Technology, #4505), β-tubulin (Cell Signaling Technology, #2146). Protein expression levels were normalized to that of β-tubulin.
RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from human endomyocardial biopsy specimens, mouse whole heart, and NRCMs using TRIzol reagent (Thermo Fisher Scientific) as we previously described [23]. For miRs screening assay, first strand cDNA of miRs was synthesized, and PCR reaction was performed using a miR-X miRNA qRT-PCR SYBR Kit (TaKaRa) according to the manufacturer’s instructions. For other studies, first strand cDNA was synthesized using a Superscript IV First-strand cDNA synthesis kit (Thermo Fisher Scientific) and quantitative RT-PCR (qRT-PCR) was performed with SYBR Green Real-Time PCR Master Mixes (Thermo Fisher Scientific) according to the manufacturer’s instructions. Gene expressions were normalized to U6 for miR assay and β-actin for other assays.
Histopathological examinations
Biopsy samples of human cardiomyocyte and mice heart samples were fixed with 4% formalin and embedded in paraffin. Sections of 3–5 μm thickness were stained with hematoxylin-eosin (HE) or Masson’s trichrome stain for histopathological analysis as we previously described [20]. The extent of myocardial interstitial fibrosis was evaluated using a microscope and attached software (BZ-X710; Keyence, Osaka, Japan).
Statistical analysis
All values are expressed as mean ± standard error of mean (SEM). Statistical differences among groups were evaluated with one-way analysis of variance (ANOVA) followed by Tukey-Kramer post hoc tests. Correlations between the circulating miRs levels and biomarkers of cardiac fibrosis were analyzed by using Pearson’s correlation coefficient. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed with a standard software package (JMP version 12; SAS institute, Cary, NC, USA).
Results
MiRs expression levels in patients with hypertensive heart disease
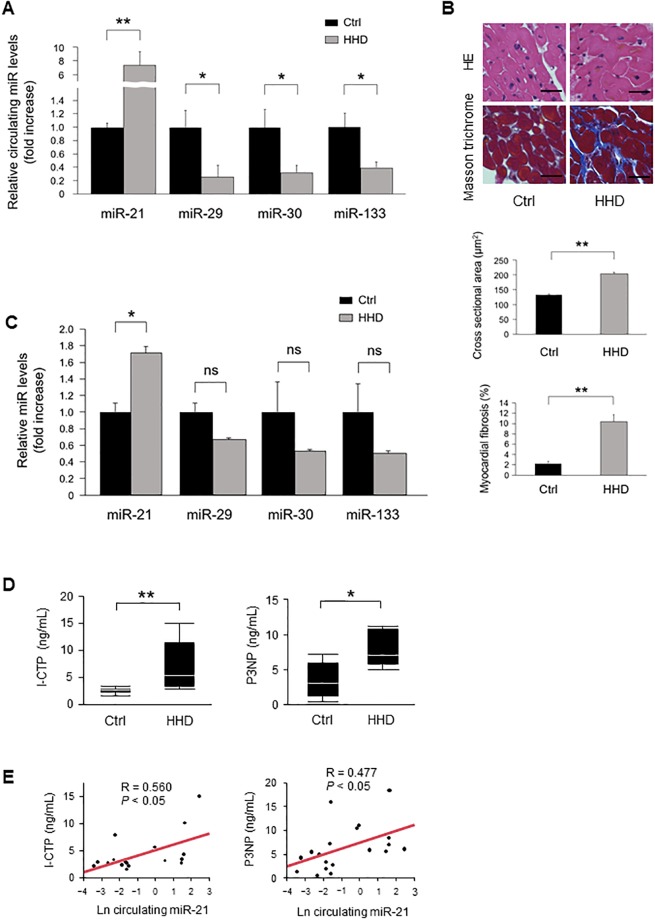
To investigate the expression levels of fibrosis-associated miRs according to previous report [24], we first measured the levels of circulating miR-21, miR-29, miR-30, and miR-133 in patients with HHD. Circulating miR-21 levels were significantly increased in patients with HHD compared with those of control subjects. On the other hand, circulating miR-29, miR-30, and miR-133 levels were significantly decreased in patients with HHD (Fig 1A). HE and Masson’s trichrome staining revealed that significant cardiac hypertrophy and fibrosis was observed in the heart section from patients with HHD (Fig 1B). MiR-21 levels were significantly increased in the heart samples of patients with HHD compared with those of the normal subjects. In contrast, miR-29, miR-30, and miR-133 levels tended to be decreased in patients with HHD, but the differences were not statistically significant (Fig 1C). We measured serum I-CTP and P3NP levels as markers of myocardial fibrosis [17, 25]. Serum I-CTP and P3NP levels were significantly higher in patients with HHD compared with those of control subjects (Fig 1D). As shown in Fig 1E, there were significant positive correlations between circulating miR-21 levels and serum I-CTP (R = 0.560) and P3NP (R = 0.477). However, there were no significant correlations between other miRs and I-CTP (miR-29: R = −0.215; miR-30: R = −0.068; miR-133: R = 0.268) and P3NP (miR-29: R = −0.302; miR-30: R = −0.263; miR-133: R = −0.138) levels.
Fig 1. Association between miRs expressions and cardiac remodeling in patients with HHD.
(A) Circulating miRs expressions in patients with HHD (n = 10 per group). (B) Representative images and analysis of cardiac remodeling by HE and Masson’s trichrome staining in heart samples from HHD patients and normal subjects (n = 3 per group). Scale bars = 20 μm. (C) Expression of miRs levels in heart samples from patients with HHD (n = 3 per group). (D) Serum I-CTP and P3NP levels in patients with HHD. (E) Circulating miR-21 levels were positively correlated with serum I-CTP and P3NP levels in patients with HHD. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01.
MiR-21 expression levels in Ang II infused mice and TAC mice
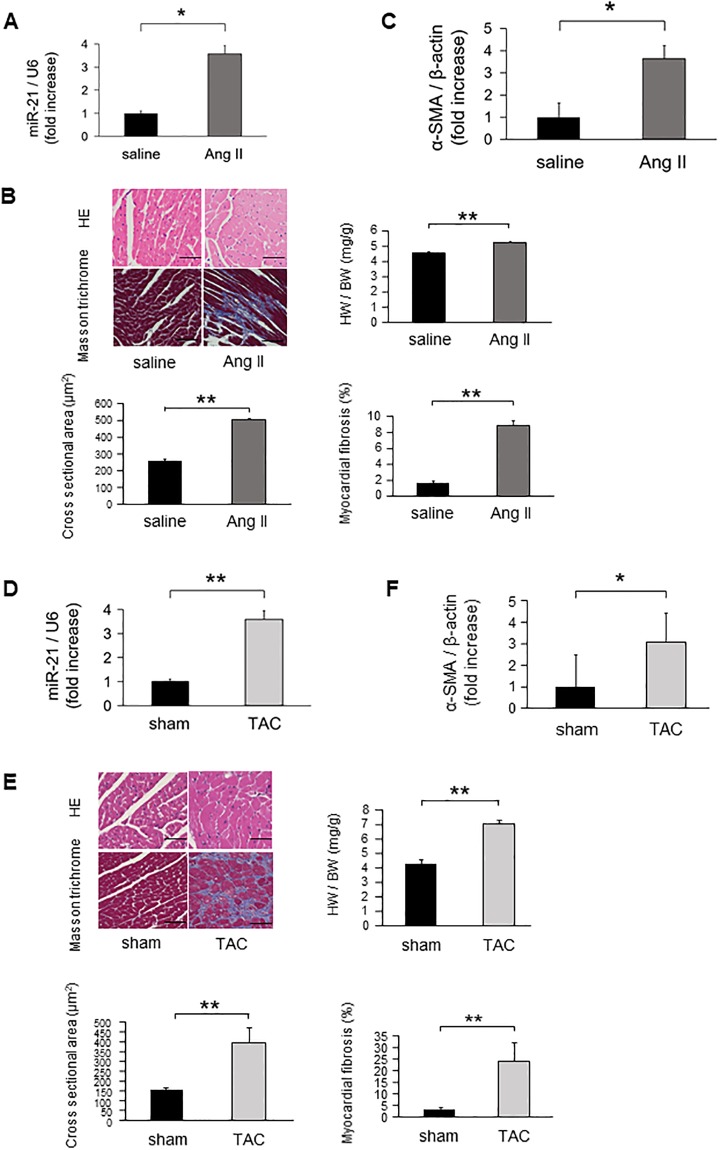
MiR-21 expression levels were significantly increased in Ang II infused mice hearts compared with those of sham mice (Fig 2A). Cardiac remodeling was detected by HE and Masson’s trichrome staining in Ang II infused mice hearts but not in sham mice (Fig 2B). Alpha smooth muscle actin (α-SMA) mRNA expression was significantly upregulated in the Ang II infused mice hearts compared with those of the sham mice (Fig 2C). Similarly, miR-21 expression levels were significantly increased in the heart of TAC mice compared with those of sham-operated mice (Fig 2D). Cardiac remodeling was also detected by HE and Masson’s trichrome staining in TAC mice but not in the sham-operated mice (Fig 2E). α-SMA mRNA expression was significantly upregulated in the TAC mice hearts compared with those of the sham-operated mice (Fig 2F). Echocardiographic and hemodynamic data of Ang II infused mice and TAC operated mice are shown in Table 2.
Fig 2. The expression levels of miR-21 on cardiac remodeling in Ang II infused and TAC mice models.
(A) MiR-21 expression levels in Ang II infused mice (n = 6 per group). (B) Representative images and analysis of cardiac remodeling by HE and Masson’s trichrome staining in left ventricular sections in Ang II infused mice hearts (n = 6 per group). Scale bars = 50 μm. (C) α-SMA expression in Ang II infused mice (n = 6 per group). (D) MiR-21 expression levels in the heart samples of TAC-operated mice (n = 6 per group). (E) Representative images and analysis of cardiac remodeling by HE and Masson’s trichrome staining in left ventricular sections of the TAC-operated mice hearts (n = 6 per group). Scale bars = 50 μm. (F) α-SMA expression in TAC-operated mice (n = 6 per group). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01.
Table 2. Echocardiographic and hemodynamic data of Ang II infused mice and TAC operated mice.
| Saline | Ang II | Sham | TAC | |
|---|---|---|---|---|
| LVEDD, mm | 3.19 ± 0.11 | 2.96 ± 0.08 | 3.06 ± 0.16 | 3.13 ± 0.15 |
| LVESD, mm | 1.79 ± 0.11 | 1.58 ± 0.09 | 1.66 ± 0.16 | 2.45 ± 0.14† |
| IVSD, mm | 0.76 ± 0.04 | 0.97 ± 0.03** | 0.66 ± 0.03 | 0.99 ± 0.02† |
| LVPWD, mm | 0.80 ± 0.03 | 0.94 ± 0.02** | 0.72 ± 0.03 | 1.06 ± 0.03† |
| FS, % | 45.9 ± 2.1 | 46.9 ± 1.6 | 45.6 ± 2.3 | 22.2 ± 2.0† |
| HR, bpm | 682 ± 22 | 628 ± 16 | 542 ± 23 | 554 ± 21 |
| SBP, mmHg | 100 ± 4 | 139 ± 3** | ||
| DBP, mmHg | 60 ± 6 | 79 ± 4* | ||
| MBP, mmHg | 71 ± 5 | 99 ± 4** |
Data are expressed as mean ± SEM; n = 6 each;
*P < 0.05 and
**P < 0.01 compared with saline infused mice;
†P < 0.01 compared with sham mice.
Ang II, angiotensin II; DBP, diastolic blood pressure; FS, fractional shortening; HR, heart rate; IVSD, interventricular septum diameter; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVPWD, left ventricular posterior wall diameter; MBP, mean blood pressure; SBP, systolic blood pressure; TAC, transverse aortic constriction.
Modulation of miR-21 altered PDCD4 expression in vivo
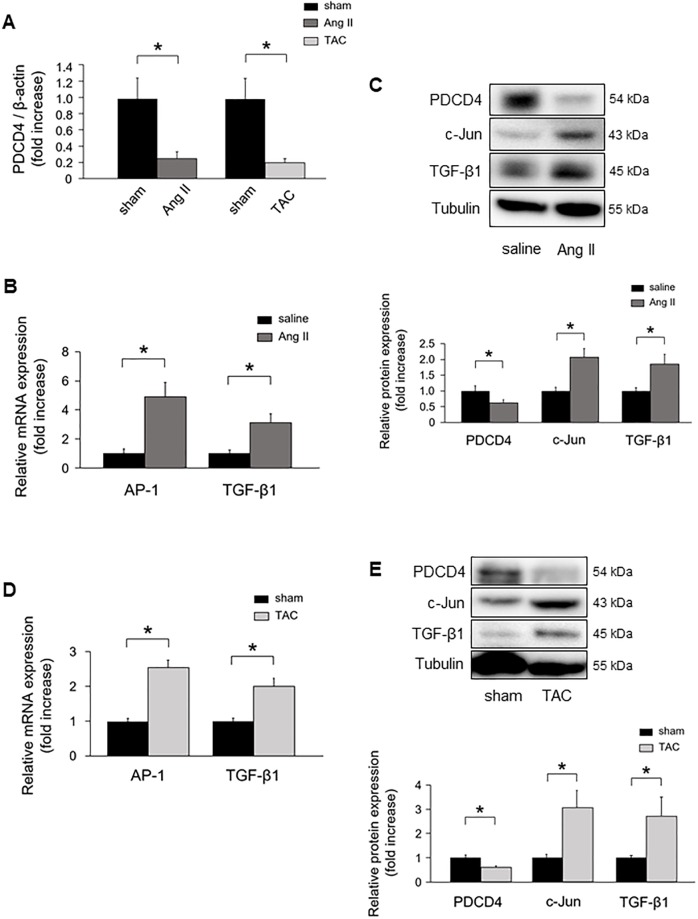
MiR-21 has been implicated in fibrosis by suppressing its downstream genes, such as PDCD4, smad family member 7 (Smad7), phosphatase and tensin homolog (PTEN), and sprouty 1 (Spry1) [12, 15, 26, 27]. We examined the mRNA expression levels of these targets using qRT-PCR in Ang II infused mice and TAC mice hearts. PDCD4 mRNA levels were significantly downregulated in Ang II infused mice hearts compared with saline infused mice. PDCD4 mRNA levels were significantly lower in TAC mice hearts than in sham mice (Fig 3A). Smad7 mRNA levels were significantly decreased in Ang II infused mice compared with saline infused mice, although there were no significant differences in PTEN and Spry1 mRNA levels between Ang II infused mice and saline infused mice (S1A Fig). There were no significant differences in Smad7, PTEN, and Spry1 mRNA levels between TAC mice and sham mice (S1B Fig).
Fig 3. PDCD4 expression and its downstream signaling in Ang II- and TAC-induced cardiac remodeling.
(A) PDCD4 mRNA expression in Ang II infused and TAC-operated mice (n = 6 per group). (B) AP-1 and TGF-β1 mRNA expressions in Ang II infused mice (n = 6 per group). (C) Protein expressions of PDCD4, c-Jun, and TGF-β1 in Ang II infused mice (n = 6 per group). (D) AP-1 and TGF-β1 mRNA expressions in TAC-operated mice (n = 6 per group). (E) Protein expressions of PDCD4, c-Jun, and TGF-β1 in TAC-operated mice (n = 6 per group). Representative images from at least six independent results are shown. Data are expressed as mean ± SEM. *P < 0.05.
Since PDCD4 mRNA levels were consistently decreased in Ang II infused mice and TAC mice hearts, we focused on PDCD4. We next investigated PDCD4 downstream target of transcription activator protein 1 (AP-1), a dimeric complex composed of c-Jun and c-Fos family, and TGF-β1 signaling pathway. AP-1 and TGF-β1 mRNA levels were significantly upregulated in Ang II infused mice hearts compared with those of saline infused mice (Fig 3B). PDCD4 protein expression was significantly decreased in Ang II infused mice hearts, whereas c-Jun and TGF-β1 protein levels were significantly increased compared with those of saline infused mice (Fig 3C). AP-1 and TGF-β1 mRNA levels were significantly increased in the hearts of TAC mice compared with those of sham mice (Fig 3D). Moreover, PDCD4 protein expression was significantly decreased in TAC mice hearts, whereas c-Jun and TGF-β1 protein levels were significantly increased compared with those of sham mice (Fig 3E).
The impact of miR-21 in cardiomyocytes under hypertrophic stimulation in vitro
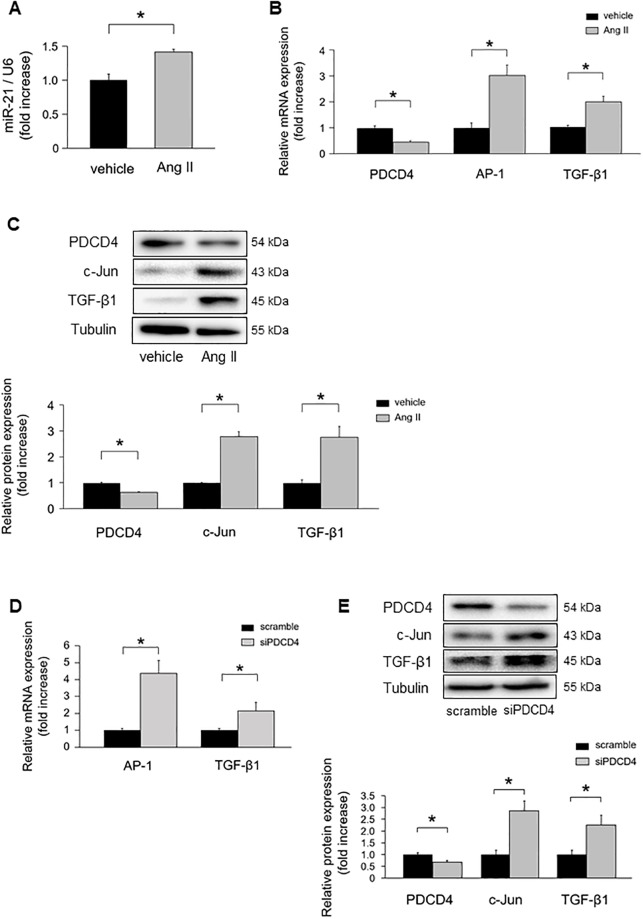
MiR-21 expression levels were significantly increased in NRCMs under Ang II stimulation (Fig 4A). PDCD4 mRNA expression was significantly downregulated in NRCMs under Ang II stimulation (Fig 4B). However, there were no significant differences in mRNA expression levels of Smad7, PTEN, and Spry1 in NRCMs (S2A Fig). The mRNA expression levels of PDCD4, Smad7, and Spry1 were significantly decreased in neonatal rat cardiofibroblasts under Ang II stimulation (S2B Fig). AP-1 and TGF-β1 mRNA levels were significantly upregulated in NRCMs under Ang II stimulation (Fig 4B). PDCD4 protein expressions were significantly decreased in NRCMs under Ang II stimulation, whereas its targets, c-Jun and TGF-β1 protein expression levels were significantly increased (Fig 4C).
Fig 4. MiR-21 and PDCD4 expressions in cardiomyocytes under Ang II stimulation.
(A) MiR-21 expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (B) PDCD4, AP-1, and TGF-β1 mRNA expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (C) Protein expression levels of PDCD4, c-Jun, and TGF-β1 in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (D) AP-1 and TGF-β1 mRNA expressions in cardiomyocytes transfected with siPDCD4 (n = 4–6 per group). (E) Protein expressions of PDCD4, c-Jun, and TGF-β1 in cardiomyocytes transfected with siPDCD4 (n = 4–6 per group). Representative images from at least four independent results are shown. Data are expressed as mean ± SEM. *P < 0.05.
To verify whether PDCD4 regulates AP-1 and subsequent downregulation of TGF-β1, we transfected siPDCD4 into NRCMs. PDCD4 knockdown significantly increased AP-1 and TGF-β1 mRNA levels (Fig 4D). Western blot analysis also revealed that c-Jun and TGF-β1 protein expression levels were significantly increased by knockdown of PDCD4 (Fig 4E).
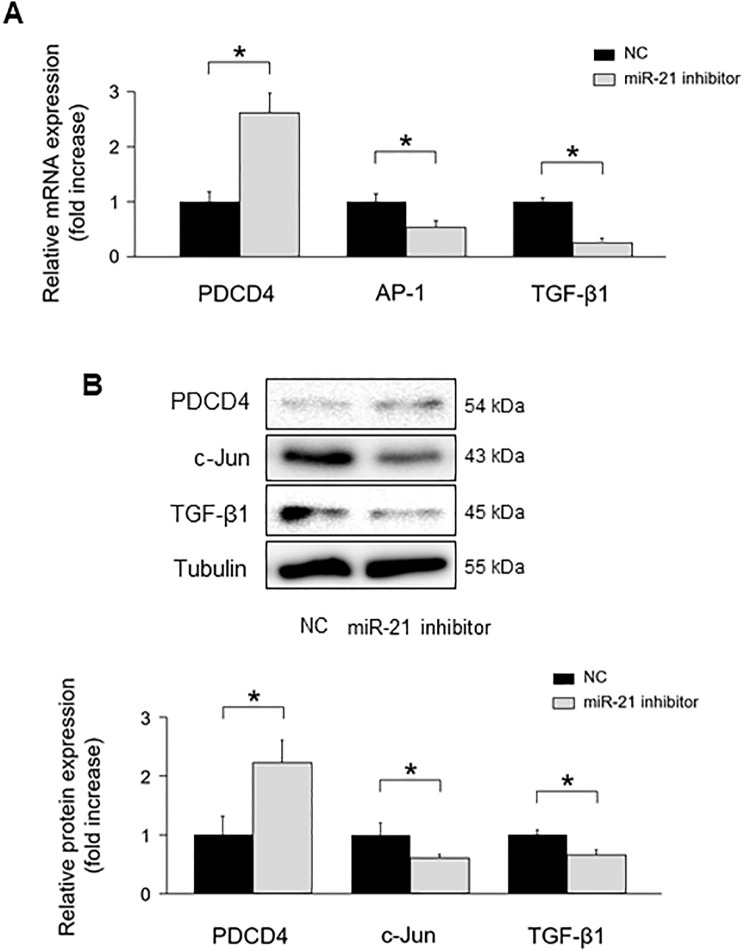
To verify whether miR-21 regulates PDCD4 expression in NRCMs, we transfected mirVana-miR-21-specific inhibitor into NRCMs. MiR-21 inhibitor significantly upregulated PDCD4 mRNA expressions compared with negative control (Fig 5A). AP-1 and TGF-β1 mRNA expressions were significantly downregulated in NRCMs with miR-21 inhibitor compared with those in the negative control. PDCD4 protein expression levels were significantly increased in NRCMs with miR-21 inhibitor compared with those in the negative control, whereas its targets, c-Jun and TGF-β1 protein expression levels were significantly decreased under miR-21 inhibitor transfection (Fig 5B).
Fig 5. The impact of miR-21 suppression on PDCD4, AP-1, and TGF-β1 signaling in cardiomyocytes.
(A) Effect of miR-21 suppression on PDCD4, AP-1, and TGF-β1 mRNA expressions in cardiomyocytes (n = 4–6 per group). (B) Effect of miR-21 suppression on PDCD4, c-Jun, and TGF-β1 protein expressions in cardiomyocytes (n = 4–6 per group). Representative images from at least four independent results are shown. Data are expressed as mean ± SEM. *P < 0.05.
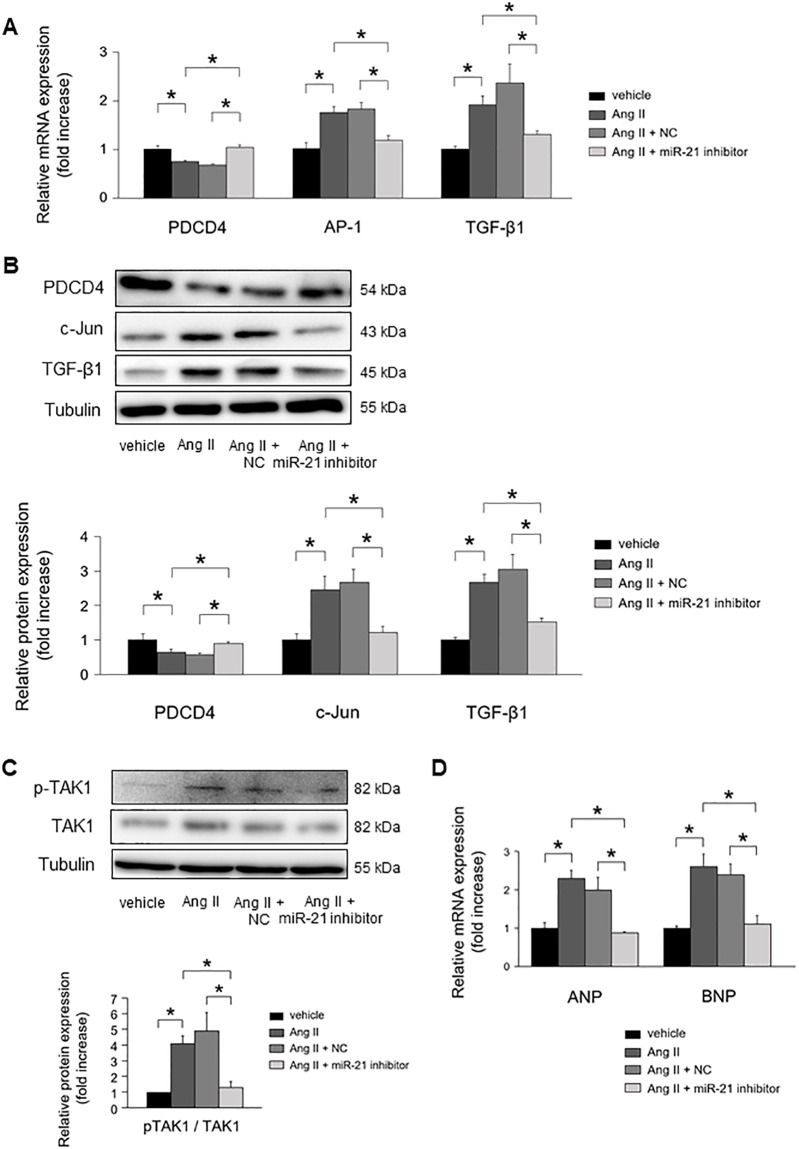
MiR-21 inhibitor attenuated Ang II-induced PDCD4 suppression (Fig 6A). As a result, subsequent Ang II-induced activation of AP-1 and TGF-β1 mRNA expressions were significantly suppressed by miR-21 inhibitor. PDCD4 protein expression levels were restored by inhibiting miR-21 expressions, whereas c-Jun and TGF-β1 protein expression levels were significantly suppressed (Fig 6B).
Fig 6. The impact of miR-21 suppression on cardiomyocyte remodeling under Ang II stimulation.
(A) Effect of miR-21 suppression on PDCD4, AP-1, and TGF-β1 mRNA expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (B) Effect of miR-21 suppression on PDCD4, c-Jun, and TGF-β1 protein expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (C) Effect of miR-21 suppression on TAK1 and pTAK1 expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). (D) Effect of miR-21 suppression on fetal gene expressions in cardiomyocytes under Ang II stimulation for 24 h (n = 4–6 per group). Representative images from at least four independent results are shown. Data are expressed as mean ± SEM. *P < 0.05.
MiR-21 inhibitor significantly suppressed the phosphorylation of TAK1, a key molecule for cardiac hypertrophy, in NRCMs under Ang II stimulation (Fig 6C). Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) mRNA expressions were significantly upregulated in NRCMs under Ang II stimulation. MiR-21 inhibitor significantly suppressed the mRNA expression of ANP and BNP in NRCMs under Ang II stimulation (Fig 6D).
Discussion
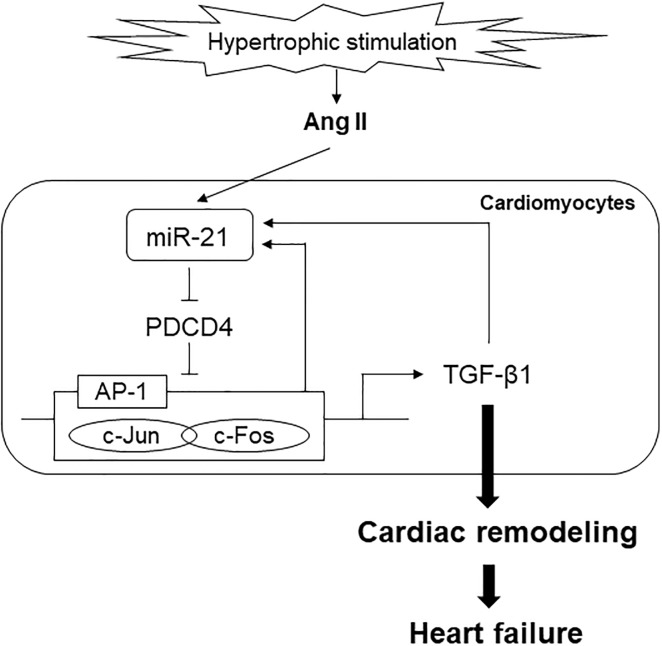
In the present study, we revealed that Ang II-induced TGF-β1 production was associated with miR-21/PDCD4/AP-1 signaling pathway. In patients with HHD, miR-21 expression levels were upregulated in the heart and blood samples. Furthermore, circulating miR-21 levels were positively correlated with serum markers of myocardial fibrosis. In cardiomyocytes, PDCD4 expression was downregulated among target genes of miR-21 under hypertrophic stimulation. Knockdown of miR-21 ameliorated AP-1 mediated TGF-β1 signaling through regulating PDCD4 in cardiomyocytes. To the best of our knowledge, this study is the first report to evaluate the association between miR-21 and cardiac remodeling in patients with HHD. A schema that includes the suggested pathway from the present study is shown in Fig 7.
Fig 7. A schema that includes the proposed pathway of miR-21 in cardiac remodeling.
The pathogenetic mechanism of HHD is thought to be related to cardiac remodeling, including cardiac fibrosis and hypertrophy [28]. Hence, regression of hypertension-induced cardiac remodeling can improve the prognosis of patients with hypertension. The contribution of miRs in the cardiomyopathies such as ischemic heart disease, hypertrophic cardiomyopathy, and dilated cardiomyopathy has been shown [29–31]. However, the impact of miR-21 as well as other miRs on the pathogenesis of HHD, which is one of the most important hypertension-induced organ damages is still unclear. In the present study, we showed that miR-21 expression levels were significantly increased in both serum and heart samples of patients with HHD compared with normal subjects. Moreover, there were significant positive correlations between circulating miR-21 levels and serum markers of myocardial fibrosis. These findings support the association between miR-21 and cardiac remodeling in patients with HHD.
It is well known that hypertension-derived mechanical stress induces Ang II synthesis, and subsequent activation of nuclear AP-1 leads to the upregulation of TGF-β1 [32]. TGF-β1 induces fibroblast-to-myofibroblast differentiation and extracellular matrix (ECM) production, which leads to cardiac fibrosis [33]. It was reported that TGF-β1 was increased with advancing of fibrosis in the hearts of patients who underwent cardiac surgery [34]. While mice with systemic overexpression of TGF-β1 showed cardiac fibrosis and hypertrophy, mice with systemic knock-out of TGF-β1 ameliorated Ang II-induced cardiac hypertrophy [35, 36]. TGF-β1 is a key mediator of the pathogenesis of cardiac remodeling under hypertrophic stimulation. Interestingly, activation of TGF-β1 signaling increases miR-21 expressions [13]. In contrast, several reports revealed that miR-21 can activate TGF-β1 signaling [11, 12]. This interrelationship forms interesting positive feedback loop. It has been reported that miR-21 expression levels were significantly upregulated in cardiac remodeling induced by pressure overload and Ang II administration [37, 38]. Consistently, our results in vivo study showed that miR-21 and TGF-β1 expression levels were significantly increased in Ang II infused mice and TAC mice, suggesting that an interrelationship between miR-21 and TGF-β1 may play an important role in hypertrophic stimulation-induced cardiac remodeling.
Elevated miR-21 expression levels were reported to be associated with organ fibrosis, such as lung, kidney, liver, and heart via promoting fibroblast activation [12, 16, 39, 40]. Several reports have shown the fibrogenic function of miR-21 in fibroblasts through modulation of its target genes, such as PDCD4, Smad7, PTEN, and Spry1 [12, 15, 26, 27]. Thus, although the functional role of miR-21 in fibroblast is well known, there were few studies assessing the functional role of miR-21 in cardiomyocyte. In the present study, we found that Ang II significantly downregulated PDCD4 mRNA expression in cardiomyocytes, although there were no significant differences in the mRNA expression levels of Smad7, PTEN, and Spry1. These results suggest that PDCD4 is more important among target genes of miR-21 under hypertrophic stimulation in cardiomyocyte.
PDCD4 is a well-known tumor suppressor and is involved in apoptosis. It was reported to be a powerful inhibitor of AP-1 [41]. On the other hand, activation of AP-1 upregulates miR-21 expressions [42]. In the present study, we showed that PDCD4 was significantly decreased and AP-1 was increased in Ang II infused mice and TAC mice. In addition, AP-1 mediated TGF-β1 expression was significantly upregulated under Ang II stimulation in vitro. Thus, there arises a possibility that miR-21 might enhance its own transcription through miR-21/PDCD4/AP-1 pathway and exacerbate the fibrogenic process in hypertrophic stimulation-induced cardiac remodeling.
Cardiomyocytes and fibroblasts cooperatively regulate cardiac cell signaling via paracrine mediators, which are involved in cardiac remodeling [43]. Bang et al. reported that cardiac fibroblast exosomal-derived miR-21-3p was involved in the crosstalk between cardiac fibroblasts and cardiomyocytes [44]. In addition, it has been reported that TGF-β1 was induced in response to hypertrophic stimuli not only in fibroblasts but also in cardiomyocytes, and acting in a paracrine and/or autocrine manner [45, 46]. However, although the effects of miR-21 inhibition on cardiac remodeling were demonstrated in fibroblast, its effects in cardiomyocyte were poorly understood. In the present study, knockdown of miR-21 expression rescued Ang II-induced PDCD4 suppression. Furthermore, knockdown of miR-21 significantly suppressed Ang II-induced AP-1 and TGF-β1 signaling in cardiomyocytes. These results suggest that inhibition of miR-21 prevents hypertrophic stimulation-induced cardiac fibrosis by suppressing miR-21/PDCD4/AP-1 feedback loop.
In addition to fibrogenic function of TGF-β1, Koitabashi et al. showed that suppression of myocyte-derived TGF-β1 ameliorated cardiac hypertrophy by inhibiting non-canonical pathways, in particular TAK1 [47]. Consistently, we showed that Ang II stimulation induced TAK1 activation in cardiomyocytes. Furthermore, we showed that inhibition of miR-21 expression suppressed TAK1 activity and subsequent fetal gene expressions in cardiomyocytes. Remarkably, Thum et al. demonstrated that silencing of miR-21 in vivo attenuated cardiac fibrosis and hypertrophy under pressure overload stimulation through deactivation of cardiac fibroblast [15]. Taking our results into consideration, this beneficial effect of miR-21 inhibitor in suppressing hypertrophic stimulation-induced cardiac remodeling might be attributed to not only cardiac fibroblast but also cardiomyocyte.
Consistent with our results, Li et al. showed that hypertensive patients had higher levels of circulating miR-21 than healthy controls [48]. However, they found that miR-21 can reduce blood pressure and attenuate cardiac hypertrophy in rats. Similarly, Patrick et al. reported that inhibition of miR-21 did not prevent pathological cardiac remodeling in response to TAC or Ang II stimulation, although miR-21 levels were upregulated [38]. In addition, some reports showed that inhibition of miR-21 exacerbated aldosterone-mediated cardiac remodeling and dysfunction [49, 50]. Even though elevated miR-21 levels seem to be associated with cardiac remodeling, the effect of inhibition of miR-21 on hypertrophic stimulation-induced cardiac remodeling is still controversial.
We need to point out several limitations of our study. First, 3 patients with hypertension were included in control group, although they had normal cardiac function and had no left ventricular hypertrophy. Second, because there were 6 patients with diabetes mellitus in HHD group, we could not completely rule out the possibility of the influence of diabetes mellitus on cardiac remodeling. Third, EMB study size was relatively small for investigating the impact of miR-21 on cardiac remodeling in patients with HHD. Fourth, since clinical data including hypertension was obtained on the basis of medical records or history of medical therapy, the duration of hypertension was different between HDD patients. Finally, we have not evaluated the effect of miR-21 inhibitor in vivo. Several studies demonstrated that inhibition of miR-21 suppressed cardiac remodeling by regulating cardiac fibroblast [15, 16, 26], although we confirmed the protective effect of miR-21 inhibitor in cardiomyocyte under hypertrophic stimulation in vitro.
Conclusions
MiR-21 was associated with hypertension-induced cardiac remodeling. Inhibition of miR-21 expressions can prevent cardiac remodeling by regulating PDCD4 and AP-1, TGF-β1 signaling pathway.
Supporting information
(A) Smad7, PTEN, and Spry1 mRNA levels in Ang II infused mice compared with those of saline infused mice (n = 6 per group). (B) Smad7, PTEN, and Spry1 mRNA levels in TAC mice compared with those of sham mice (n = 6 per group). Data are expressed as mean ± SEM. *P < 0.05.
(TIF)
(A) The mRNA expressions of Smad7, PTEN, and Spry1 after treatment with vehicle or Ang II for 24 h in NRCMs (n = 4–6 per group). (B) The mRNA expressions of PDCD4, Smad7, PTEN, and Spry1 after treatment with vehicle or Ang II for 24 h in cardiofibroblasts (n = 4–6 per group). Data are expressed as mean ± SEM. *P < 0.05.
(TIF)
(PPTX)
Acknowledgments
We thank Ms. Emiko Nishidate and Ms. Hiroko Sasaki for their excellent technical assistance and comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
- Initial: TN - Grant number: 18H06191 - Funder: JSPS KAKENHI - Website: https://www.jsps.go.jp/ - The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet (London, England). 2017;389(10064):37–55. Epub 2016/11/20. 10.1016/s0140-6736(16)31919-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–50. Epub 2016/08/10. 10.1161/CIRCULATIONAHA.115.018912 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. Epub 2018/08/31. 10.1093/eurheartj/ehy339 . [DOI] [PubMed] [Google Scholar]
- 4.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123(3):327–34. Epub 2011/01/26. 10.1161/CIRCULATIONAHA.108.845792 . [DOI] [PubMed] [Google Scholar]
- 5.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Annals of internal medicine. 2003;138(1):10–6. Epub 2003/01/07. 10.7326/0003-4819-138-1-200301070-00006 . [DOI] [PubMed] [Google Scholar]
- 6.Runte KE, Bell SP, Selby DE, Haussler TN, Ashikaga T, LeWinter MM, et al. Relaxation and the Role of Calcium in Isolated Contracting Myocardium From Patients With Hypertensive Heart Disease and Heart Failure With Preserved Ejection Fraction. Circulation Heart failure. 2017;10(8). Epub 2017/08/09. 10.1161/circheartfailure.117.004311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355(3):251–9. Epub 2006/07/21. 10.1056/NEJMoa052256 . [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. The FEBS journal. 2010;277(9):2015–21. Epub 2010/04/24. 10.1111/j.1742-4658.2010.07632.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, He Y, Li J. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr Pharm Des. 2015;21(17):2236–42. Epub 2014/12/30. 10.2174/1381612820666141226095701 . [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. Journal of molecular and cellular cardiology. 2011;51(4):600–6. Epub 2010/11/10. 10.1016/j.yjmcc.2010.10.033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu RH, Ning B, Ma XE, Gong WM, Jia TH. Regulatory roles of microRNA-21 in fibrosis through interaction with diverse pathways (Review). Molecular medicine reports. 2016;13(3):2359–66. Epub 2016/02/06. 10.3892/mmr.2016.4834 . [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Miao J, Luo J, Yuan Q, Cao H, Su W, et al. The feedback loop between miR-21, PDCD4 and AP-1 functions as a driving force for renal fibrogenesis. J Cell Sci. 2018;131(6). Epub 2018/01/24. 10.1242/jcs.202317 . [DOI] [PubMed] [Google Scholar]
- 13.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. Epub 2008/06/13. 10.1038/nature07086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang L, Ellims AH, Moore XL, White DA, Taylor AJ, Chin-Dusting J, et al. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. Journal of translational medicine. 2015;13:314 Epub 2015/09/26. 10.1186/s12967-015-0672-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4. Epub 2008/12/02. 10.1038/nature07511 . [DOI] [PubMed] [Google Scholar]
- 16.Lorenzen JM, Schauerte C, Hubner A, Kolling M, Martino F, Scherf K, et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur Heart J. 2015;36(32):2184–96. Epub 2015/04/23. 10.1093/eurheartj/ehv109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitahara T, Takeishi Y, Arimoto T, Niizeki T, Koyama Y, Sasaki T, et al. Serum carboxy-terminal telopeptide of type I collagen (ICTP) predicts cardiac events in chronic heart failure patients with preserved left ventricular systolic function. Circulation journal: official journal of the Japanese Circulation Society. 2007;71(6):929–35. Epub 2007/05/29. 10.1253/circj.71.929 . [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Shishido T, Kinoshita D, Watanabe K, Toshima T, Sugai T, et al. Cardiac Nuclear High-Mobility Group Box 1 Ameliorates Pathological Cardiac Hypertrophy by Inhibiting DNA Damage Response. JACC Basic to translational science. 2019;4(2):234–47. Epub 2019/05/08. 10.1016/j.jacbts.2018.11.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60(1):188–97. Epub 2012/05/23. 10.1161/HYPERTENSIONAHA.112.195511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netsu S, Shishido T, Kitahara T, Honda Y, Funayama A, Narumi T, et al. Midkine exacerbates pressure overload-induced cardiac remodeling. Biochem Biophys Res Commun. 2014;443(1):205–10. Epub 2013/12/03. 10.1016/j.bbrc.2013.11.083 . [DOI] [PubMed] [Google Scholar]
- 21.Otaki Y, Takahashi H, Watanabe T, Funayama A, Netsu S, Honda Y, et al. HECT-Type Ubiquitin E3 Ligase ITCH Interacts With Thioredoxin-Interacting Protein and Ameliorates Reactive Oxygen Species-Induced Cardiotoxicity. J Am Heart Assoc. 2016;5(1). 10.1161/JAHA.115.002485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Zhu Y, Li J, Liu J, Gao Y, Ha T, et al. Pellino1-mediated TGF-beta1 synthesis contributes to mechanical stress induced cardiac fibroblast activation. Journal of molecular and cellular cardiology. 2015;79:145–56. Epub 2014/12/03. 10.1016/j.yjmcc.2014.11.006 . [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Takeishi Y, Seidler T, Arimoto T, Akiyama H, Hozumi Y, et al. Adenovirus-mediated overexpression of diacylglycerol kinase-zeta inhibits endothelin-1-induced cardiomyocyte hypertrophy. Circulation. 2005;111(12):1510–6. Epub 2005/03/23. 10.1161/01.CIR.0000159339.00703.22 . [DOI] [PubMed] [Google Scholar]
- 24.van Rooij E, Olson EN. Searching for miR-acles in cardiac fibrosis. Circ Res. 2009;104(2):138–40. Epub 2009/01/31. 10.1161/CIRCRESAHA.108.192492 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour IN, Bress AP, Groo V, Ismail S, Wu G, Patel SR, et al. Circulating Procollagen Type III N-Terminal Peptide and Mortality Risk in African Americans With Heart Failure. Journal of cardiac failure. 2016;22(9):692–9. Epub 2016/01/02. 10.1016/j.cardfail.2015.12.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y, et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;42(6):2207–19. Epub 2017/08/18. 10.1159/000479995 . [DOI] [PubMed] [Google Scholar]
- 27.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82(1):21–9. Epub 2009/01/17. 10.1093/cvr/cvp015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber KT, Sun Y, Gerling IC, Guntaka RV. Regression of Established Cardiac Fibrosis in Hypertensive Heart Disease. American journal of hypertension. 2017;30(11):1049–52. Epub 2017/04/06. 10.1093/ajh/hpx054 . [DOI] [PubMed] [Google Scholar]
- 29.Li X, Yang Y, Wang L, Qiao S, Lu X, Wu Y, et al. Plasma miR-122 and miR-3149 Potentially Novel Biomarkers for Acute Coronary Syndrome. PLoS One. 2015;10(5):e0125430 Epub 2015/05/02. 10.1371/journal.pone.0125430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuster DW, Mulders J, Ten Cate FJ, Michels M, Dos Remedios CG, da Costa Martins PA, et al. MicroRNA transcriptome profiling in cardiac tissue of hypertrophic cardiomyopathy patients with MYBPC3 mutations. Journal of molecular and cellular cardiology. 2013;65:59–66. Epub 2013/10/03. 10.1016/j.yjmcc.2013.09.012 . [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Schotterl S, Backes D, Brunner E, Hahn JK, Ionesi E, et al. Inhibition of miR-208b improves cardiac function in titin-based dilated cardiomyopathy. Int J Cardiol. 2017;230:634–41. Epub 2017/01/10. 10.1016/j.ijcard.2016.12.171 . [DOI] [PubMed] [Google Scholar]
- 32.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63(3):423–32. Epub 2004/07/28. 10.1016/j.cardiores.2004.04.030 . [DOI] [PubMed] [Google Scholar]
- 33.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nature reviews Cardiology. 2013;10(1):15–26. Epub 2012/12/05. 10.1038/nrcardio.2012.158 . [DOI] [PubMed] [Google Scholar]
- 34.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–91. Epub 2003/02/26. 10.1161/01.cir.0000051865.66123.b7 . [DOI] [PubMed] [Google Scholar]
- 35.Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). American journal of physiology Heart and circulatory physiology. 2002;283(3):H1253–62. Epub 2002/08/16. 10.1152/ajpheart.00578.2001 . [DOI] [PubMed] [Google Scholar]
- 36.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109(6):787–96. Epub 2002/03/20. 10.1172/JCI14190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Molecular biology of the cell. 2008;19(8):3272–82. Epub 2008/05/30. 10.1091/mbc.E08-02-0159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–6. Epub 2010/10/28. 10.1172/JCI43604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun NN, Yu CH, Pan MX, Zhang Y, Zheng BJ, Yang QJ, et al. Mir-21 Mediates the Inhibitory Effect of Ang (1–7) on AngII-induced NLRP3 Inflammasome Activation by Targeting Spry1 in lung fibroblasts. Scientific reports. 2017;7(1):14369 Epub 2017/11/01. 10.1038/s41598-017-13305-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, et al. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288(52):37082–93. Epub 2013/11/08. 10.1074/jbc.M113.517953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003;22(24):3712–20. Epub 2003/06/13. 10.1038/sj.onc.1206433 . [DOI] [PubMed] [Google Scholar]
- 42.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D'Esposito M, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28(1):73–84. Epub 2008/10/14. 10.1038/onc.2008.370 . [DOI] [PubMed] [Google Scholar]
- 43.Pellman J, Zhang J, Sheikh F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: Mechanisms and model systems. Journal of molecular and cellular cardiology. 2016;94:22–31. Epub 2016/03/22. 10.1016/j.yjmcc.2016.03.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. Epub 2014/04/20. 10.1172/JCI70577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi N, Calderone A, Izzo NJ Jr., Maki TM, Marsh JD, Colucci WS. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest. 1994;94(4):1470–6. Epub 1994/10/01. 10.1172/JCI117485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40(2):352–63. Epub 1999/01/20. 10.1016/s0008-6363(98)00121-7 . [DOI] [PubMed] [Google Scholar]
- 47.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121(6):2301–12. Epub 2011/05/04. 10.1172/JCI44824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Zhang X, Wang F, Zhou L, Yin Z, Fan J, et al. MicroRNA-21 Lowers Blood Pressure in Spontaneous Hypertensive Rats by Upregulating Mitochondrial Translation. Circulation. 2016;134(10):734–51. Epub 2016/08/21. 10.1161/CIRCULATIONAHA.116.023926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ball JP, Syed M, Maranon RO, Hall ME, Kc R, Reckelhoff JF, et al. Role and Regulation of MicroRNAs in Aldosterone-Mediated Cardiac Injury and Dysfunction in Male Rats. Endocrinology. 2017;158(6):1859–74. Epub 2017/04/04. 10.1210/en.2016-1707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syed M, Ball JP, Mathis KW, Hall ME, Ryan MJ, Rothenberg ME, et al. MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. American journal of physiology Endocrinology and metabolism. 2018;315(6):E1154–e67. Epub 2018/08/29. 10.1152/ajpendo.00155.2018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Smad7, PTEN, and Spry1 mRNA levels in Ang II infused mice compared with those of saline infused mice (n = 6 per group). (B) Smad7, PTEN, and Spry1 mRNA levels in TAC mice compared with those of sham mice (n = 6 per group). Data are expressed as mean ± SEM. *P < 0.05.
(TIF)
(A) The mRNA expressions of Smad7, PTEN, and Spry1 after treatment with vehicle or Ang II for 24 h in NRCMs (n = 4–6 per group). (B) The mRNA expressions of PDCD4, Smad7, PTEN, and Spry1 after treatment with vehicle or Ang II for 24 h in cardiofibroblasts (n = 4–6 per group). Data are expressed as mean ± SEM. *P < 0.05.
(TIF)
(PPTX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.