Abstract
BACKGROUND:
Sickness during pregnancy is associated with an increased risk of offspring neurodevelopmental disorders. Rodent models have played a critical role in establishing causal relationships and identifying mechanisms of altered brain and behavior development in pups prenatally exposed to maternal immune activation (MIA). We recently developed a novel nonhuman primate model to bridge the gap between human epidemiological studies and rodent models of prenatal immune challenge. Our initial results demonstrated that rhesus monkeys given the viral mimic synthetic double-stranded RNA (polyinosinic:polycytidylic acid stabilized with poly-l-lysine) during pregnancy produce offspring with abnormal repetitive behaviors, altered communication, and atypical social interactions.
METHODS:
We utilized noninvasive infrared eye tracking to further evaluate social processing capabilities in a subset of the first trimester MIA-exposed offspring (n = 4) and control animals (n = 4) from our previous study.
RESULTS:
As juveniles, the MIA offspring differed from control animals on several measures of social attention, particularly when viewing macaque faces depicting the fear grimace facial expression. Compared with control animals, MIA offspring had a longer latency before fixating on the eyes, had fewer fixations directed at the eyes, and spent less total time fixating on the eyes of the fear grimace images.
CONCLUSIONS:
In the rhesus monkey model, exposure to MIA at the end of the first trimester results in abnormal gaze patterns to salient social information. The use of noninvasive eye tracking extends the findings from rodent MIA models to more human-like behaviors resembling those in both autism spectrum disorder and schizophrenia.
Keywords: Autism spectrum disorders, Immunology, Macaque, Nonhuman primate, Poly IC, Schizophrenia, Social attention
The prenatal environment and, in particular, the maternal immune system may have a profound effect on fetal neurodevelopment (1–4). In humans, exposure to infections during pregnancy may increase the risk of giving birth to a child who will later develop an autism spectrum disorder (ASD) or schizophrenia (SZ) (5–14). Animal models have demonstrated that exposing pregnant dams to prenatal challenges, such as influenza (15–18) or the bacterial endotoxin lipopolysaccharide (19–22), produce offspring with behavioral abnormalities and neuropathology relevant to both SZ and ASD. The diversity of infections associated with alterations in neurodevelopment suggests that the maternal immune response, rather than a specific pathogen, drives changes in fetal brain development. The emerging maternal immune activation (MIA) hypothesis has been directly tested in animal models by artificially activating the immune system of pregnant rodents with the viral mimic, synthetic double stranded RNA (polyinosinic: polycytidylic acid [poly IC]), a toll-like receptor-3 agonist that stimulates an inflammatory response in the absence of a specific pathogen (23). Rodent pups born to dams treated with poly IC at mid gestation demonstrate behavioral abnormalities, neuropathology, and altered gene expression relevant to both ASD and SZ [reviewed in (24–28)].
It is important to emphasize that sickness during pregnancy is not uncommon (29,30), and clearly, not all women who experience infection during pregnancy have children later diagnosed with a neurodevelopmental disorder (31). A number of factors, including genetic susceptibility, the intensity and timing of the infection, and exposure to additional postnatal challenges, all may influence the degree to which the prenatal immune challenge alters neurobehavioral development (32). Translational animal models provide a powerful tool to systematically examine how these factors contribute to offspring pathophysiology following MIA. In the rodent MIA model, the effects of poly IC on brain and behavior development appear to be mediated by the maternal cytokine response, in particular interleukin-6 (33,34), and exacerbated by postnatal environmental stressors (35).
While rodent models have provided compelling evidence for a causal relationship between MIA and aberrant brain and behavioral development in the offspring (36–40), there are limitations in relying solely on rodent models to study complex human brain disorders. Nonhuman primates, such as the rhesus macaque (Macaca mulatta), can provide a more direct comparison with human brain and behavior pathologies to determine the clinical relevance of the MIA model to human neurodevelopmental disorders (41–43). Previous primate models have documented changes in brain and behavior development of macaque offspring following third trimester exposure to influenza or lipopolysaccharide (18,19); however, the effects of MIA at earlier gestational time points have not been explored. We have developed a novel, nonhuman primate model of maternal immune activation using a modified form of poly IC (poly IC stabilized with poly-l-lysine [poly ICLC]) that is recognized by the primate immune system and induces a transient innate inflammatory response (44,45). Pregnant rhesus monkeys injected with poly ICLC at the end of either the first or second trimester produce offspring with abnormal motor stereotypies and repetitive behaviors (46). While both first and second trimester MIA offspring produced fewer affiliative vocalizations than control animals, only the first trimester MIA offspring showed signs of atypical social interactions with unfamiliar peers. Given that both ASD and SZ are characterized by changes in social cognition and emotion (47), as well as altered visual attention devoted to facial expressions (48,49), we initiated a series of noninvasive eye-tracking studies to provide further insight into the nature of the social impairments observed in the MIA offspring. Here, we present the initial results from these eye-tracking studies demonstrating abnormal patterns of social attention in the macaque offspring exposed to MIA in the first trimester.
METHODS AND MATERIALS
All experimental procedures were developed in collaboration with the veterinary, animal husbandry, and environmental enrichment staff at the California National Primate Research Center and approved by the University of California, Davis Institutional Animal Care and Use Committee. All attempts were made (in terms of social housing, enriched diet, use of positive reinforcement strategies, and minimizing the duration of daily training/testing sessions) to promote the psychological wellbeing of the animals that participated in this research. Additional methodological details are provided in Supplement 1.
Subjects and Living Conditions
Noninvasive eye tracking was used to evaluate social attention in a subset of juvenile macaque monkeys from our previous study (46) before puberty, when the animals were approximately 2.5 years of age. All control (CON) (n = 4) and first trimester MIA exposed male animals (n = 5) were selected to participate in the current series of experiments to follow-up on the social behavior differences described in our earlier report. One of the MIA animals did not habituate to the testing procedures and was therefore dropped from the study, yielding a final sample size of n = 4 for the MIA group. The MIA offspring were born to dams injected with .25 mg/kg synthetic double-stranded RNA (poly ICLC; Oncovir, Inc., Washington, DC) via intravenous injection while temporarily restrained by trained technicians at the end of the first trimester on gestational days 43, 44, and 46. CON offspring were born to dams injected with saline at these same time points or at the end of the second trimester (gestational days 100, 101, and 103) or had no manipulation at all during pregnancy. Preliminary analyses revealed that the behavioral profiles of the male saline-treated control monkeys (n = 1 first trimester, n = 2 second trimester) and the male untreated control monkey (n = 1) were very similar and thus pooled to form a single control group. While we detected no differences in first trimester, second trimester, and untreated control animals in our previous report (46), it is important to note that repeated, daily prenatal stress has been shown to alter neurobehavioral development in nonhuman primates (50–54). To minimize the influence of prenatal stress, MIA and saline-control dams were preselected for their willingness to receive intravenous injections and followed identical procedures for routine ultrasounds, housing, and prenatal care.
Noninvasive Eye Tracking
All data collection occurred while the animals sat in a modified primate chair with a slanted top (Crist Instrument Co., Inc., Damascus, Maryland). Visual stimuli were presented to each monkey using a personal computer running the Eprime 2.0 Professional software package (Psychology Software Tools, Pittsburgh, Pennsylvania). All gaze data were collected using the Eye-Trac 6 .NET User Interface program (Applied Science Laboratories Bedford, Massachusetts) on a separate personal computer.
Experiment 1: Facial Expressions
The animal viewed 40 color photographs of unfamiliar adult male and female macaque facial expressions that are used for social communication. These facial expressions included neutral (n = 10), lipsmack (n = 10), fear grimace (n = 10), and open-mouth threat (n = 10). Each face stimulus was presented for 5 seconds and was preceded and followed by a blank, black screen (5 seconds). The experimental animals saw the same 40 facial expression stimuli on each of the 5 days of this experiment but in a random order each day. Figure 1A shows a schematic of an eye-tracking trial and Figure 1B shows examples of facial expression categories.
Figure 1.
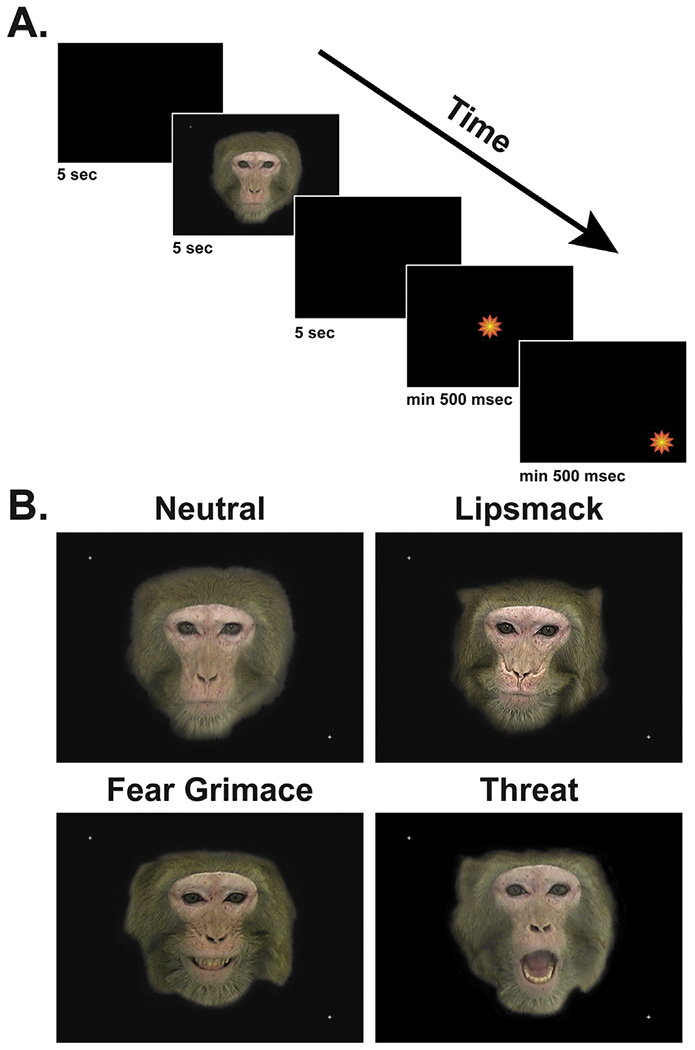
Schematic of a typical testing trial (A). A 5-second black screen preceded and followed each face stimulus. Animals were also required to fixate a center and peripheral pulsating star target for >500 msec to receive a juice reward and proceed to the next trial. Examples of the four face stimulus categories (neutral, lipsmack, fear grimace, and open-mouth threat) are also shown (B).
Experiment 2: Facial Expressions Embedded in Complex Scenes
Similar to experiments involving patients with ASD (55), the animals viewed 50 color photographs showing 10 different nature scenes. Each nature scene was seen five times during a testing session, once each with a neutral, lipsmack, fear grimace, or threat face embedded at a random spatial location and once without any embedded face (Figure 2). The experimental animals saw the same 50 images on each of the 5 days of this experiment but in a random order each day. These stimuli were presented in the same trial structure and for the same duration as the stimuli used for experiment 1.
Figure 2.

Four examples of visual stimuli used for experiment 2 are shown. Each background was downloaded from the internet and a neutral, lipsmack, fear grimace, or open-mouth threat facial expression was added at a pseudorandom spatial location (balanced across all stimuli) using Adobe Photoshop software.
Data Analysis
Each animal’s total fixation duration, total frequency of fixations, average fixation duration, average gaze dwell duration, and average pupil diameter were measured for each stimulus in experiments 1 and 2 using the ASL Results Plus software package (Applied Science Laboratories). This software also calculated the conditional probability of a fixation shifting from one area of interest (AOI) within an image to another. For experiment 1, eye-tracking parameters were computed for rectangular AOIs that encompassed the entire face or the eyes, nose, and mouth separately. For experiment 2, results were generated for AOIs that encompassed the small embedded face and the entire scene background.
Each dependent variable was summarized as an average across all four face categories or with each category considered separately. The data were first examined with Shapiro-Wilk tests and found to be largely not normally distributed. All measures were therefore log10(X + 1) transformed before parametric statistical analyses, but nontransformed values were used for all figures. Data were analyzed using anaylses of variance (ANOVAs) with groups as a between-subjects factor and day as within-subjects factors with repeated measures. A Huynh-Feldt correction was used to adjust the degrees of freedom if the group variance did not remain equal across days. Post hoc tests for significant group × day interactions included one-way ANOVAs and paired-sample t tests. Because the number of post hoc comparisons was five or fewer for each variable, we did not correct p values for multiple comparisons. For all analyses, alpha was set at p < .05. Because main effects of day did not indicate differences between the two experimental groups, discussion of those effects has been omitted from the following sections.
RESULTS
Experiment 1: Facial Expressions
When data were averaged across all facial expressions, only one difference between CON and offspring of dams treated with poly ICLC at the end of the first trimester (MIA1) was found. There was a significant group × day interaction for average fixation duration when animals looked at an AOI that encompassed the entire face (F1,6 = 2.892, p < .05, ηp2 = .325; Figure 3). The two groups did not differ significantly on any given day, but MIA1 showed a significant decrease in average fixation duration between day 1 and day 2 (t3 = 3.952; p < .05) with additional, but nonsignificant, decreases until day 4. By contrast, CON showed largely consistent levels of average fixation duration on the faces until a significant decrease was detected between day 4 and day 5 (t3 = 3.077; p = .05).
Figure 3.
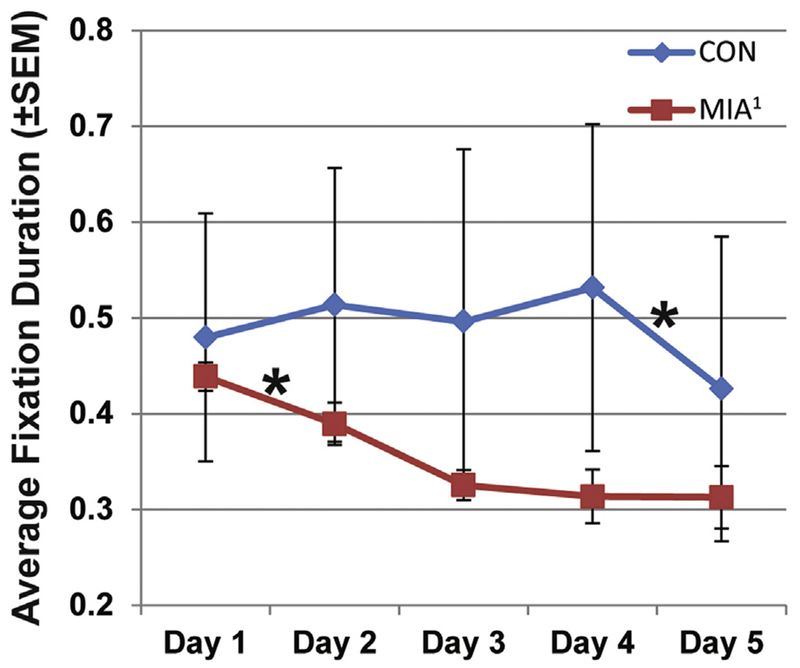
The average fixation duration directed at the face area of interest for control (CON) and offspring of dams treated with poly ICLC at the end of the first trimester (MIA1) animals, regardless of facial expression (i.e., all expressions averaged). MIA1 showed a significant decrease between day 1 and day 2 and then remained generally constant after that. Average fixation duration for CON animals remained constant until a significant decrease between day 4 and day 5. *p < .05.
Face categories were then analyzed separately. A majority of the differences between the groups were found for fear grimace expressions, particularly with visual attention directed toward the eyes AOI. CON directed a greater frequency (group main effect; F1,6 = 7.839, p < .05, ηp2 = .566; Figure 4A) and duration (group main effect; F1,6 = 10.117, p < .05, ηp2 = .628; Figure 4B) of fixations at the eyes of conspecifics displaying fear grimaces than MIA1. The average dwell duration on the eyes AOI for CON also increased significantly between day 1 and day 2, but such values for MIA1 did not change (group × day effect; F4,24 = 3.111, p < .05, ηp2 = .341; day 1 vs. day 2, t3 = 3.169, p = .05). In fact, by day 4, the average dwell duration directed at the eyes AOI for CON was significantly greater than MIA (one-way ANOVA; F1,6 = 9.135, p < .05).
Figure 4.
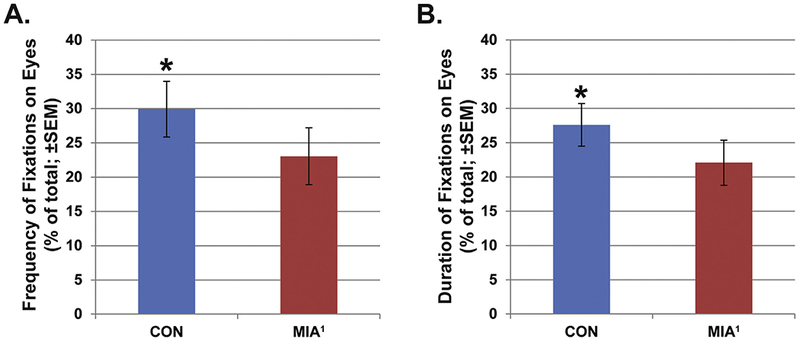
The total frequency (A) and duration (B) of fixations directed at the eyes area of interest of fear grimace expressions. Offspring of dams treated with poly ICLC at the end of the first trimester (MIA1) directed fewer fixations at the eyes and those fixations totaled less time than control (CON) animals. *p < .05.
The temporal sequence of fixations also differentiated the groups. The latency from the beginning of a trial until a fixation was directed at the eyes AOI was also shorter for CON relative to MIA1 (group main effect; F1,6 = 7.994, p < .05, ηp2 = .571; Figure 5A). By contrast, MIA1 had a shorter latency until a fixation was directed at the mouth AOI relative to CON (group main effect; F1,6 = 6.442, p < .05, ηp2 = .517; Figure 5B). A supplementary video is available online showing the difference in fixations directed at a composite image of all 10 fear grimace stimuli by CON and MIA1 animals. Still frames from the first 1500 msec (at 500 msec intervals) are shown in Figure 6 for each group. It was during this brief time frame at the beginning of a stimulus presentation when the groups seemed to differ most in visual attention to fear grimaces. Fixations for CON animals quickly converge on the face and around the eyes in particular within the first 500 msec and remain largely clustered there and on the mouth for the subsequent 1000 msec. By contrast, fixations for MIA1 animals also converge quickly on the face but are directed more quickly at the nose and mouth areas than the eyes. Over the next 1000 msec, fixations for MIA1 animals are far more scattered throughout the face and black background relative to CON.
Figure 5.
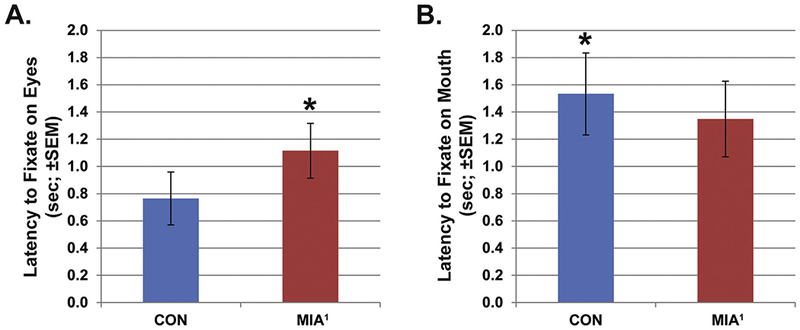
The average latency from the start of a trial until the first fixation was registered in the eyes area of interest (A) or the mouth area of interest (B) for fear grimace expressions. The offspring of dams treated with poly ICLC at the end of the first trimester (MIA1) animals showed a significantly longer latency before fixating on the eyes and significantly shorter latency before fixating the mouth relative to control (CON) animals. *p < .05.
Figure 6.
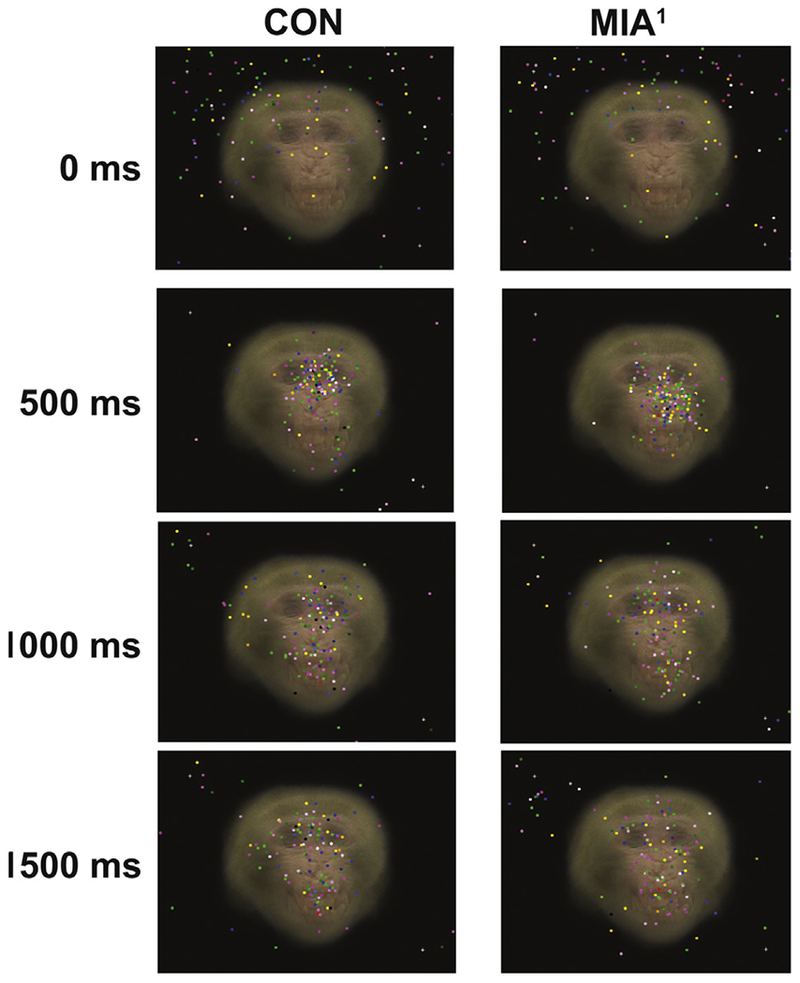
Each image shown is a composite of the 10 fear grimace facial expressions included in experiment 1. The 10 images were made semi-transparent and then overlaid on each other. The point of gaze for all offspring of dams treated with poly ICLC at the end of the first trimester (MIA1) or control (CON) animals on days 1 through 5 were then mapped onto these images at 0 msec, 500 msec, 1000 msec, and 1500 msec to show the general distribution of gaze for the two groups when viewing fear grimaces. Notice that CON animals largely direct their gaze at the eyes by 500 msec into the presentation. During the next 1000 msec, their gaze is split between the eyes and the mouth. By contrast, MIA1 animals largely direct their gaze at the nose at 500 msec, and their gaze generally disperses to many different parts of the face and background by 1500 msec into the presentation. A supplemental video is available online showing a comparison between the CON and MIA1 animals for the entire 5-second presentation of the fear grimace composite image.
Only two additional differences were found between the groups when facial expressions were analyzed separately. For open-mouth threat facial expressions, MIA1 directed a greater frequency of fixations at the nose AOI than CON (group main effect; F1,6 = 7.111, p < .05, ηp2 = .542). The probability of gaze shifting from one AOI to another also differentiated the groups. When macaques and humans view faces, gaze moves quickly to the eyes but then shifts to other parts of the face, particularly the mouth and nose, then back to the eyes. We found that for neutral facial expressions, the probability of gaze shifting from the eyes to the mouth was significantly greater for CON relative to MIA1 (group main effect; F1,6 = 10.585, p < .05, ηp2 = .638) and that difference was particularly strong on day 1 (group × day effect; F4,24 = 2.83, p < .05, ηp2 = .320; one-way ANOVA, F1,6 = 9.961, p < .05). No differences were found between the groups in all other comparisons, including average pupil diameter.
Consistency of Individual Differences
In addition to the abnormalities in visual attention described here, MIA1 monkeys also previously displayed a heightened tendency to approach, contact, and remain in close proximity to an unfamiliar conspecific relative to CON animals (46). This result was interpreted as MIA1 animals being unrestrained or inappropriately social in a context where normal animals typically chose to be more restrained or hesitant. We hypothesized that the lack of social restraint displayed by MIA1 animals in our previous study may be related to their abnormally low levels of visual attention directed at the eyes of a conspecific (frequency and duration of fixation) and their elevated latency to fixate on the eyes in the current study. To assess the generality and consistency of behavioral measures during a dyadic social interaction session with an unfamiliar conspecific and the eye-tracking study described here, we further analyzed a total of six measures that differentiated MIA1 and CON animals in these two experiments: 1) the frequency of fixations directed at the eyes of fear grimace expressions; 2) the duration of fixations directed at the eyes of fear grimace expressions; 3) the latency to fixate on the eyes of fear grimace expressions; 4) the combined duration of arms-reach proximity and contact with an unfamiliar conspecific; 5) the frequency of approaching to within arms-reach of an unfamiliar conspecific; and 6) the frequency of sitting in arms-reach proximity with an unfamiliar conspecific. The data for each of these measures were transformed into ranks (1 = lowest measure, 8 = highest measure) and subjected to Kendall’s coefficient of concordance (W) analysis. This non-parametric test assesses the agreement or consistency across multiple measures and is not impacted by tied ranks. This analysis was conducted for each group separately. The outcome of this test indicated substantial stability of individual responsiveness for MIA1 (W = .625, χ25 = 12.5, p < .05) but not for CON animals (W = .406, χ25 = 8.12, p = .150). This result indicates that MIA1 animals were consistently inappropriate (i.e., low visual attention to the eyes and high tendency to approach) across two very different social situations, a controlled setting where animals looked at pictures of fear grimace facial expressions and a semi-naturalistic context where animals encountered an unfamiliar conspecific. CON animals, by contrast, were not consistent across these two conditions, implying an ability to regulate their behavior differentially depending on the context (nonthreatening pictures of facial expressions vs. a potentially dangerous, unfamiliar conspecific). This finding indicates that MIA1 animals may lack an important ability to regulate their social behavior adaptively across contexts.
Experiment 2: Facial Expressions Embedded in Complex Scenes
Analysis of the background images without an embedded face did not reveal any significant differences between the groups or group × day interactions for the total number of fixations, total duration of fixations, average duration of fixations, average dwell duration, or average pupil diameter. Likewise, when faces were embedded into the backgrounds, there were also no significant differences between the groups or group × day interactions for any of these variables for AOIs that encompassed the entire background or the embedded face, both when all facial expressions were considered together or separately. There were no significant differences between the groups or group × day interactions for the conditional probability of shifting fixation between the background AOI and the face AOI.
DISCUSSION
We previously demonstrated that nonhuman primates prenatally exposed to MIA in the first trimester demonstrate abnormal behaviors, including deviation from species-typical social interactions (46). Here, we utilized noninvasive eye tracking to determine if these same MIA-exposed macaque offspring demonstrate abnormal attention to salient social information (i.e., still images of faces). Much like humans, macaque monkeys have evolved a sophisticated social communication system that includes a variety of facial expressions, body postures, and vocalizations (56,57). Among these social signals, the use of facial expressions is one of the most salient features of macaque social behavior and the most similar to our own social communication (58–60). Our experiments were therefore designed to maximize translational potential between the nonhuman primate model and gaze-processing abnormalities observed in human neurodevelopmental disorders.
We first measured gaze patterns when juvenile offspring of control dams (untreated and saline-treated) and age-/sex-matched offspring of dams treated with poly ICLC at the end of the first trimester viewed faces of unfamiliar conspecifics displaying four basic facial expressions used for macaque social communication. The expressions consisted of faces showing a neutral expression, faces depicting affiliative/submissive expressions (i.e., lipsmack), faces depicting submissive/fearful expressions (i.e., fear grimaces), and faces showing an open-mouth threat. We found several important differences in gaze between the control and MIA1 animals. When all expressions were considered as a single category, the MIA1 animals showed a significant drop in average fixation duration after the first presentation, while the control animals did not show such a drop until after their fourth presentation. When the animals looked at fear grimace expressions, MIA1 animals took longer to direct their attention at the eyes than control animals and ultimately looked less at the eyes (in terms of frequency and duration) than control animals. MIA1 animals looked more at the nose of open-mouth threat expressions relative to control animals. The MIA1 animals were also less likely to shift their gaze from the eyes to the mouth when looking at neutral expressions than control animals. In our second experiment, we tracked eye gaze as the animals looked at complex, naturalistic scenes with monkey faces embedded at random locations. Both groups were able to locate and fixate on the faces embedded in the scenes and showed similar levels of visual attention directed at the backgrounds and the embedded faces. Thus, differences in gaze patterns between the groups did not appear to reflect a global inability to locate and attend to social cues but rather specific abnormalities related to sustained social attention and the fine-scale analysis of salient social features, particularly when viewing fear grimace faces.
It is interesting that the observed differences in gaze allocation between the two groups were most pronounced for facial expressions of fear or submission. It has been hypothesized in the human literature that fearful faces require more cognitive processing than other faces that also signal threat, like angry facial expressions (61–63). The amygdala has been identified as a critical neural structure for evaluating the emotional content of human (64–68) and macaque faces (69,70) and plays an essential role in directing attention to the eye region to evaluate fearful faces (71). It is possible that maternal immune activation during the first trimester of fetal development disrupted fetal amygdala development during a critical period of growth. The end of the first trimester is a time when neurogenesis occurs rapidly in the macaque amygdala (72). This was precisely the time point when MIA1 mothers were exposed to poly ICLC in the present experiment. It is therefore plausible that maternal immune activation during the first trimester disrupted normal amygdala development in fetuses, which manifested later in offspring as a fairly selective deficit in visual attention when viewing faces used to convey fear or subordination in social settings. Histologic evaluation of brain pathology in the MIA-exposed offspring is underway and may provide additional insight into the behavioral deficits.
Given that impaired social functioning is a hallmark feature of both ASD and SZ, we would expect a valid animal model to also produce impairments in social processing that resemble features of these disorders. The lack of attention to the eye region exhibited by MIA1 animals represents a significant departure from social processing in normal macaques and typically developing humans (73–76) and parallels findings in both ASD and SZ clinical populations (48,49). While the developmental courses of ASD and SZ are quite different–ASD is diagnosed in early childhood (77), while the onset of psychotic symptoms in SZ typically occurs during the transition from adolescence to adulthood (78)–many clinically relevant behaviors are similar (79–81). The manifestations of ASD diagnostic social impairments (82) have been extensively studied, with numerous empirical studies documenting deficits in social interaction, theory of mind, and imitation. Many individuals with ASD also demonstrate abnormal fixation patterns when viewing faces, characterized by a lack of fixation in the eye region and a possible compensatory strategy of fixating on the mouth region (83–88). SZ is also characterized by persistent and severe social deficits, though these behavioral changes are first observable in adolescence and progressively become worse as one approaches illness onset (89–93). Individuals with SZ demonstrate deficits in theory of mind, face processing, and social perception (94,95), as well as impaired emotion recognition (96–99). Moreover, scan path analyses consistently demonstrate a restricted scanning strategy adopted by SZ patients when viewing pictures of faces, characterized by fewer fixations of increased duration, a shorter scan path length, and a marked avoidance of salient facial features (100–104). The gaze patterns demonstrated by the MIA-exposed macaque offspring resemble features of both ASD and SZ and are being further explored in ongoing experiments.
The data presented here indicate that MIA1 offspring consistently demonstrate aberrant behavior across two very different social situations: a controlled setting where animals looked at pictures (MIA1 offspring demonstrated abnormal attention to social information) and a semi-naturalistic context where animals encountered an unfamiliar conspecific (MIA offspring failed to show species-typical hesitation to approach a novel social partner) (46). The atypical gaze patterns demonstrated by the adolescent MIA1 offspring raise the possibility that the inappropriate social interactions demonstrated in our earlier studies may have been due to an inability to attend to and process salient social information conveyed in the face. This study lends support to the hypothesis that prenatal infection acts as a neurodevelopmental disease primer that is possibly relevant to a number of neurodevelopmental disorders (32). Additional brain imaging and histologic studies will be needed to further evaluate the relevance of the monkey MIA model to both ASD and SZ.
While the rhesus monkey provides an animal model that closely parallels human brain organization and cognitive and social functioning, there are ethical and pragmatic limitations in the development of a nonhuman primate model. The primary limitation of the current study is the sample size, and replication in a larger cohort will be essential. Moreover, we are at the earliest stages of evaluating prenatal immune challenge in the nonhuman primate model and have not yet evaluated additional environmental adversities that exacerbate (35) or therapeutic interventions that ameliorate (105) behavioral pathologies in mouse models. The use of noninvasive eye tracking in the nonhuman primate MIA model will provide a powerful tool to evaluate these novel preventative and therapeutic interventions in future studies and can improve translation from animal models to clinical populations.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a grant from the Simons Foundation (SFARI 9900060 to PHP) and by a gift from Ted and Ginger Jenkins. Additional support was provided by the Center for Neuroscience at the University of California, Davis, and the base grant (RR00169) of the California National Primate Research Center. SEPS is currently affiliated with Department of Immunology, Mayo Clinic, Rochester, Minnesota.
We thank the veterinary and animal services staff of the California National Primate Research Center for helping to refine the animal handling procedures described in this article. We also thank the four anonymous reviewers that provided positive and constructive comments on an earlier version of this paper. Poly-ICLC was kindly provided by Dr. Andres Salazar, M.D., Oncovir, Washington, DC.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.07.035.
REFERENCES
- 1.Brown AS (2012): Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 72: 1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. (2011): Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagberg H, Gressens P, Mallard C (2012): Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71:444–457. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA (2009): Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 163: 907–914. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES (2005): Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 162:767–773. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES (2000): Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: A prospective birth cohort study. Schizophr Bull 26:287–295. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA (2009): Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull 35:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS (2006): Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry 163:927–929. [DOI] [PubMed] [Google Scholar]
- 9.Buka SL, Cannon TD, Torrey EF, Yolken RH (2008): Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry 63:809–815. [DOI] [PubMed] [Google Scholar]
- 10.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. (2004): Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 61:774–780. [DOI] [PubMed] [Google Scholar]
- 11.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM (2014): Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 19:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. (2012): Neonatal levels of cytokines and risk of autism spectrum disorders: An exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol 252:75–82. [DOI] [PubMed] [Google Scholar]
- 13.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010): Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430. [DOI] [PubMed] [Google Scholar]
- 14.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. (2011): Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P (2002): Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry 7:633–640. [DOI] [PubMed] [Google Scholar]
- 16.Fatemi SH, Sidwell R, Kist D, Akhter P, Meltzer HY, Bailey K, et al. (1998): Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero. Brain Res 800:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. (2008): Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neuro-developmental disorders. Schizophr Res 99:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. (2010): Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 67:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL (2011): Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res 219:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM (2009): Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res 197:210–218. [DOI] [PubMed] [Google Scholar]
- 21.Fortier ME, Joober R, Luheshi GN, Boksa P (2004): Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res 38:335–345. [DOI] [PubMed] [Google Scholar]
- 22.Fortier ME, Luheshi GN, Boksa P (2007): Effects of prenatal infection on prepulse inhibition in the rat depend on the nature ofthe infectious agent and the stage of pregnancy. Behav Brain Res 181:270–277. [DOI] [PubMed] [Google Scholar]
- 23.TraynorTR Majde JA, Bohnet SG Krueger JM (2004): Intratracheal double-stranded RNA plus interferon-gamma: A model for analysis of the acute phase response to respiratory viral infections. Life Sci 74:2563–2576. [DOI] [PubMed] [Google Scholar]
- 24.Boksa P (2010): Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav Immun 24:881–897. [DOI] [PubMed] [Google Scholar]
- 25.Meyer U, Feldon J (2012): To poly(I:C) or not to poly(I:C): Advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology 62:1308–1321. [DOI] [PubMed] [Google Scholar]
- 26.Meyer U, Feldon J, Dammann O (2011): Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 69:26R–33R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson PH (2009): Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- 28.Meyer U, Feldon J (2009): Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res 204:322–334. [DOI] [PubMed] [Google Scholar]
- 29.Laibl VR, Sheffield JS (2005): Influenza and pneumonia in pregnancy. Clin Perinatol 32:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longman RE, Johnson TR (2007): Viral respiratory disease in pregnancy. Curr Opin Obstet Gynecol 19:120–125. [DOI] [PubMed] [Google Scholar]
- 31.Selten JP, Frissen A, Lensvelt-Mulders G, Morgan VA (2010): Schizophrenia and 1957 pandemic of influenza: Meta-analysis. Schizophr Bull 36:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer U (2014): Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75: 307–315. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher D, Norman AA, Woodard CL, Yang G, Gauthier-Fisher A, Fujitani M, et al. (2013): Transient maternal IL-6 mediates long-lasting changes in neural stem cell pools by deregulating an endogenous self-renewal pathway. Cell Stem Cell 13:564–576. [DOI] [PubMed] [Google Scholar]
- 34.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007): Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. (2013): Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339:1095–1099. [DOI] [PubMed] [Google Scholar]
- 36.Piontkewitz Y, Arad M, Weiner I (2011): Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry 70:842–851. [DOI] [PubMed] [Google Scholar]
- 37.Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013): Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH (2012): Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi L, Fatemi SH, Sidwell RW, Patterson PH (2003): Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K (2012): Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson KK, Platt ML (2012): Of mice and monkeys: Using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capitanio JP, Emborg ME (2008): Contributions of non-human primates to neuroscience research. Lancet 371:1126–1135. [DOI] [PubMed] [Google Scholar]
- 43.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, et al. (2014): Why primate models matter. Am J Primatol 76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, et al. (1975): A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis 132:434–439. [DOI] [PubMed] [Google Scholar]
- 45.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. (2011): Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 208:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH (2014): Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey off-spring. Biol Psychiatry 75:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King BH, Lord C (2011): Is schizophrenia on the autism spectrum? Brain Res 1380:34^1. [DOI] [PubMed] [Google Scholar]
- 48.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J (2002): Visual scanning of faces in autism. J Autism Dev Disord 32: 249–261. [DOI] [PubMed] [Google Scholar]
- 49.Toh WL, Rossell SL, Castle DJ (2011): Current visual scanpath research: A review of investigations into the psychotic, anxiety, and mood disorders. Compr Psychiatry 52:567–579. [DOI] [PubMed] [Google Scholar]
- 50.Schneider ML, Roughton EC, Koehler AJ, Lubach GR (1999): Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Dev 70:263–274. [DOI] [PubMed] [Google Scholar]
- 51.Schneider ML, Moore CF, Roberts AD, Dejesus O (2001): Prenatal stress alters early neurobehavior, stress reactivity and learning in non-human primates: A brief review. Stress 4:183–193. [DOI] [PubMed] [Google Scholar]
- 52.Schneider ML, Moore CF, Kraemer GW (2004): Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child Dev 75:96–109. [DOI] [PubMed] [Google Scholar]
- 53.Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, et al. (2004): Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicol Teratol 26:169–178. [DOI] [PubMed] [Google Scholar]
- 54.Converse AK, Moore CF, Moirano JM, Ahlers EO, Larson JA, Engle JW, et al. (2013): Prenatal stress induces increased striatal dopamine transporter binding in adult nonhuman primates. Biol Psychiatry 74:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riby DM, Hancock PJ (2009): Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. J Autism Dev Disord 39:421–431. [DOI] [PubMed] [Google Scholar]
- 56.Tomasello M, Call J (1997): Social knowledge and interaction In: Tomasello M, Call J, editors. Primate Cognition. New York: Oxford University Press, 191–230. [Google Scholar]
- 57.Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML (2013): Neuroethology of primate social behavior. Proc Natl Acad Sci USA 110(suppl 2):10387–10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deaner RO, Khera AV, Platt ML (2005): Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Curr Biol 15:543–548. [DOI] [PubMed] [Google Scholar]
- 59.Bower S, Suomi SJ, Paukner A (2012): Evidence for kinship information contained in the rhesus macaque (Macaca mulatta) face. J Comp Psychol 126:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrari PF, Paukner A, Ionica C, Suomi SJ (2009): Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr Biol 19:1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- 62.Bannerman RL, Milders M, de Gelder B, Sahraie A (2009): Orienting to threat: Faster localization of fearful facial expressions and body postures revealed by saccadic eye movements. Proc Biol Sci 276: 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whalen PJ (1998): Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci 7:177–188. [Google Scholar]
- 64.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. [DOI] [PubMed] [Google Scholar]
- 66.Adolphs R, Tranel D, Damasio H, Damasio AR (1995): Fear and the human amygdala. J Neurosci 15:5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. (1999): Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 37:1111–1117. [DOI] [PubMed] [Google Scholar]
- 68.Spezio ML, Huang PY, Castelli F, Adolphs R (2007): Amygdala damage impairs eye contact during conversations with real people. J Neurosci 27:3994–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG (2007): Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol 97:1671–1683. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK (2007): Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol 17:766–772. [DOI] [PubMed] [Google Scholar]
- 71.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR (2005): A mechanism for impaired fear recognition after amygdala damage. Nature 433:68–72. [DOI] [PubMed] [Google Scholar]
- 72.Kordower JH, Piecinski P, Rakic P (1992): Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res 68:9–15. [DOI] [PubMed] [Google Scholar]
- 73.Walker-Smith GJ, Gale AG, Findlay JM (1977): Eye movement strategies involved in face perception. Perception 6:313–326. [DOI] [PubMed] [Google Scholar]
- 74.Ekman P, Friesen WV (1975): Unmasking the Face: A Guide to Recognizing Emotions from Facial Cues. Oxford, UK: Prentice-Hall. [Google Scholar]
- 75.Machado CJ, Bliss-Moreau E, Platt ML, Amaral DG (2011): Social and nonsocial content differentially modulates visual attention and autonomic arousal in rhesus macaques. PloS One 6:e26598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leonard TK, Blumenthal G, Gothard KM, Hoffman KL (2012): How macaques view familiarity and gaze in conspecific faces. Behav Neurosci 126:781–791. [DOI] [PubMed] [Google Scholar]
- 77.Mandell DS, Novak MM, Zubritsky CD (2005): Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics 116:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziermans TB, Schothorst PF, Sprong M, van Engeland H (2011): Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res 126:58–64. [DOI] [PubMed] [Google Scholar]
- 79.Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J (2010): Comparison of social cognitive functioning in schizophrenia and high functioning autism: More convergence than divergence. Psychol Med 40:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasson NJ, Pinkham AE, Carpenter KL, Belger A (2011): The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J Neurodev Disord 3:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solomon M, Olsen E, Niendam T, Ragland JD, Yoon J, Minzenberg M, Carter CS (2011): From lumping to splitting and back again: Atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr Res 131:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 83.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. (2005): Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 8:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kliemann D, Dziobek I, Hatri A, Steimke R, Heekeren HR (2010): Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. J Neurosci 30:12281–12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klin A, Jones W, Schultz R, Volkmar F, Cohen D (2002): Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry 59:809–816. [DOI] [PubMed] [Google Scholar]
- 86.Neumann D, Spezio ML, Piven J, Adolphs R (2006): Looking you in the mouth: Abnormal gaze in autism resulting from impaired top-down modulation of visual attention. Soc Cogn Affect Neurosci 1:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spezio ML, Adolphs R, Hurley RS, Piven J (2007): Abnormal use of facial information in high-functioning autism. J Autism Dev Disord 37:929–939. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy DP, Adolphs R (2012): Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia 50:3313–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen DN, Frantom LV, Strauss GP, van Kammen DP (2005): Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophr Res 75:389–397. [DOI] [PubMed] [Google Scholar]
- 90.Ballon JS, Kaur T, Marks II, Cadenhead KS (2007): Social functioning in young people at risk for schizophrenia. Psychiatry Res 151:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chudleigh C, Naismith SL, Blaszczynski A, Hermens DF, Hodge MA, Hickie IB (2011): How does social functioning in the early stages of psychosis relate to depression and social anxiety? Early Interv Psychiatry 5:224–232. [DOI] [PubMed] [Google Scholar]
- 92.Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD (2007): Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull 33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amminger GP, Schafer MR, Klier CM, Schlogelhofer M, Mossaheb N, Thompson A, et al. (2012): Facial and vocal affect perception in people at ultra-high risk of psychosis, first-episode schizophrenia and healthy controls. Early Interv Psychiatry 6:450–454. [DOI] [PubMed] [Google Scholar]
- 94.Bora E, Pantelis C (2013): Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: Systematic review and meta-analysis. Schizophr Res 144:31–36. [DOI] [PubMed] [Google Scholar]
- 95.Ventura J, Wood RC, Jimenez AM, Hellemann GS (2013): Neurocognition and symptoms identify links between facial recognition and emotion processing in schizophrenia: Meta-analytic findings. Schizophr Res 151:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pinkham AE, Gur RE, Gur RC (2007): Affect recognition deficits in schizophrenia: Neural substrates and psychopharmacological implications. Expert Rev Neurother 7:807–816. [DOI] [PubMed] [Google Scholar]
- 97.Edwards J, Jackson HJ, Pattison PE (2002): Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin Psychol Rev 22:789–832. [DOI] [PubMed] [Google Scholar]
- 98.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ (2010): Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr Bull 36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoekert M, Kahn RS, Pijnenborg M, Aleman A (2007): Impaired recognition and expression of emotional prosody in schizophrenia: Review and meta-analysis. Schizophr Res 96:135–145. [DOI] [PubMed] [Google Scholar]
- 100.Loughland CM, Williams LM, Harris AW (2004): Visual scanpath dysfunction in first-degree relatives of schizophrenia probands: Evidence for a vulnerability marker? Schizophr Res 67:11–21. [DOI] [PubMed] [Google Scholar]
- 101.Williams LM, Loughland CM, Green MJ, Harris AW, Gordon E (2003): Emotion perception in schizophrenia: An eye movement study comparing the effectiveness of risperidone vs. haloperidol. Psychiatry Res 120:13–27. [DOI] [PubMed] [Google Scholar]
- 102.Loughland CM, Williams LM, Gordon E (2002): Schizophrenia and affective disorder show different visual scanning behavior for faces: A trait versus state-based distinction? Biol Psychiatry 52:338–348. [DOI] [PubMed] [Google Scholar]
- 103.Loughland CM, Williams LM, Gordon E (2002): Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res 55:159–170. [DOI] [PubMed] [Google Scholar]
- 104.Williams LM, Loughland CM, Gordon E, Davidson D (1999): Visual scanpaths in schizophrenia: Is there a deficit in face recognition? Schizophr Res 40:189–199. [DOI] [PubMed] [Google Scholar]
- 105.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. (2013): Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


