Abstract
Cascade testing for hereditary breast/ovarian cancer is an important public health priority. Increasing attention has been paid to the relevance of testing for men within BRCA1/2-positive families given that such testing may provide important information about their cancer risks, particularly for prostate cancer, and risks to their offspring. However, men are much less likely to seek genetic counseling and testing than their at-risk female relatives. To facilitate access to pre-test information and testing, we developed a web-based intervention (WI) for men that we are evaluating in a pilot randomized controlled trial (RCT). This paper describes three phases of research in the development of the WI: (1) formative (qualitative) research among men from BRCA1/2 families to assess needs and preferences for education; (2) a detailed description of the organization, format, and content of the WI; and (3) usability testing. We discuss the aims and hypotheses of the pilot RCT in which the WI is being compared to an enhanced usual care condition among at-risk men. We expect that the WI described here will foster informed decisions and lead to increased use of BRCA1/2 counseling and testing, potentially yielding improved cancer control outcomes for this understudied group, and for their at-risk relatives.
Keywords: BRCA1/BRCA2, Genetic counseling, Decision aid, Prostate cancer, Cascade testing
Introduction
Pathogenic variants (PVs) in the BRCA1 and BRCA2 (BRCA1/2) genes are the most common cause of hereditary breast and ovarian cancer (HBOC) and are associated with highly elevated lifetime risks of these cancers [1]. Genetic testing has been part of clinical care for high risk women for many years given the availability of effective surgical risk reduction procedures for breast and ovarian cancer, as well as breast cancer early detection [2, 3]. The potential relevance of this testing for men has been highlighted recently in the medical community [4, 5] the popular press [6], and by patient advocacy groups [7]. Although controversial, there has been a call to rename the HBOC syndrome (e.g., to “King syndrome” after the scientist who discovered BRCA1) because it perpetuates the misconception that the associated cancer risks affect only women [8].
Men with a BRCA1/2 PV (i.e., BRCA1/2 carriers) have an increased, although modest, absolute risk of breast and pancreatic cancer [9]. However, these risks are greatly increased over those observed in the general population. Although studies are investigating the role of targeted and platinum based therapies in BRCA1/2 carriers with pancreatic cancer, the mortality remains high [10]. Men with a PV in BRCA1 or BRCA2 also have an estimated 15–20% and 30–40% lifetime risk of prostate cancer, respectively [11]. Moreover, the prostate cancer phenotype in carriers is much more aggressive than that observed in average risk men. Compared to non-carriers, carriers are more likely to have prostate tumors that are faster growing and higher grade, and have poorer survival outcomes [12, 13]. Thus, if diagnosed with prostate cancer, carriers may choose active treatment, such as surgery or radiotherapy, rather than a program of active surveillance, even for low grade cancers [12]. For men with metastatic prostate cancer, who are eligible for genetic testing regardless of their family history [14], identification of PVs in BRCA1/2 and other DNA-repair genes may guide the use of targeted therapies, including PARP (poly ADP-ribose polymerase) or immune checkpoint inhibitors [15, 16].
For these reasons, male BRCA2 carriers are recommended to undergo annual prostate cancer screening with serum prostate specific antigen (PSA) testing and digital rectal exam (DRE) beginning at age 40–45, whereas BRCA1 carriers should consider this screening [14, 17]. Accumulating evidence from an international consortium study demonstrates that PSA testing in these high risk men detects clinically significant cancers and has a higher positive predictive value than in the general population [18]. This finding is relevant, because men at average risk may choose to opt out of prostate cancer screening after shared decision-making with their doctor [19].
One of the most efficient ways of identifying BRCA1/2 carriers is through the process of cascade testing. That is, once a PV is identified in an individual, genetic testing can be expanded step-wise to at-risk relatives, extending both horizontally and vertically through a pedigree [20]. Indeed, a primary motivator for undergoing BRCA1/2 testing is to obtain risk information for relatives, especially daughters [21–25]. Yet, in individuals unaffected with cancer, men appear to undergo genetic testing for BRCA1/2 PVs at about one-tenth the rate observed in women [26]. The main reasons for this finding may be that men are not informed by their female relatives that a PV exists in the family, psychological discomfort associated with being at-risk, lack of information about why testing is relevant to them, or lack of awareness about how to access genetic counseling and testing [23–27]. For some men, insurance coverage for testing, particularly those with Medicaid or Medicare, may also be a barrier [28]. Even when men are motivated to obtain more information, they report that BRCA1/2 education materials tailored to their concerns are lacking [23]. Overcoming these barriers is important because men’s interest in and uptake of testing is greatly increased when they are provided with adequate information [29], and when men attend genetic counseling, they test at the same rate as women [30]. Thus, it is vital for healthcare providers to be more proactive about identifying at-risk men and encouraging them to pursue genetic counseling and testing [31].
Given these considerations, there is an urgent need to make information about BRCA1/2 testing more accessible to men and to develop more efficient and effective alternatives to standard clinic-based genetic counseling. Indeed, the genetic counseling community at large has recognized the need for alternative forms of service delivery in order to meet increased demand and to accommodate patient preferences [32–34]. While most of these modes of service involve telemedicine (i.e., phone or video) consults for pre- and post-test genetic counseling, web-based platforms have recently been used to provide similar services [35–37].
To date, research is lacking about how to address barriers men may face related to BRCA1/2 genetic counseling and testing. To fill this gap, we developed and conducted usability testing on a brief, low cost, individually tailored web-based intervention (WI). The WI is designed to educate untested men from families with a BRCA1/2 PV about the personal relevance of such testing, and to give them the opportunity to proceed directly to genetic testing after viewing the intervention (i.e., without the need for additional pre-test education or formal genetic counseling). To accomplish these goals, we expanded upon an Australian print-based decision aid for men considering BRCA1/2 testing [38], and we were guided by our prior research assessing longitudinal outcomes following BRCA1/2 testing in men [30], our experience in developing novel interventions to expand genetic testing access, [34, 39–42] and prior literature. The purpose of this paper is to describe the results from our formative work, the development process and the content of the WI. We conclude with a discussion of the pilot randomized controlled trial (RCT) that is underway, as well as future research directions and implications.
Methods and Results
Conceptual Framework
The study is guided by the Informed Choice Model (ICM) [43, 44] and the Ottawa Decision Support Framework (ODSF) [45] (See Fig 1). The ICM defines an informed decision as one that is based on relevant knowledge, is consistent with the decision-maker’s attitudes, and which is subsequently implemented. In the context of genetic testing, a decision not to be tested can be an informed decision if it is made with adequate knowledge and is consistent with the individual’s (negative) attitudes toward testing. Similarly, a decision to be tested can be an uninformed decision if it is made without adequate knowledge or is inconsistent with the individual’s attitudes toward testing. In the proposed randomized controlled pilot trial, our premise is that men’s counseling and testing decisions are poorly informed due to lack of prior knowledge about BRCA1/2 and negative attitudes toward the relevance of testing in men. Based on the ICM, we predict that the WI will foster higher knowledge and more positive attitudes, resulting in reduced decisional conflict and higher genetic testing uptake. The ODSF identifies barriers to informed decision-making (e.g., poor knowledge, unclear preferences) that can serve as intervention targets, which we address through the provision of factual information and preference clarification.
Fig 1.
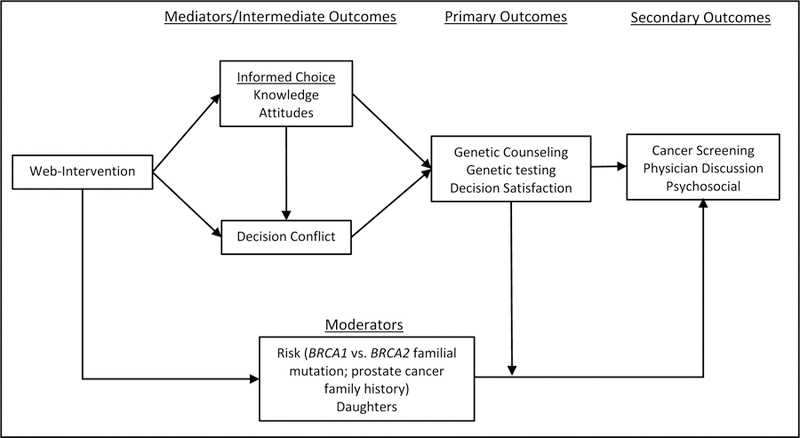
Conceptual Model
Phase I: Formative Research
This research was approved by the MedStar Health Research Institute-Georgetown University Oncology Institutional Review Board. The goal of this phase of the research was to conduct individual in-depth qualitative telephone interviews with male relatives of women with a PV in BRCA1 or BRCA2.
Study participants.
To be eligible for participation in this phase of our research, men had to be between the ages of 40–70 years old with no history of cancer (except non-melanoma skin cancer), English speaking, and able to provide informed consent. A research assistant contacted females with a BRCA1/2 PV from Georgetown Lombardi Comprehensive Cancer Center’s Familial Cancer Registry and requested contact information for eligible male first- and second-degree relatives. Women were asked to obtain permission from their male relatives before providing us with contact information. If they consented to recontact, we also approached males who were carriers of a familial BRCA1/2 PV from the Familial Cancer Registry or a prior genetic counseling study at our institution. All men approached for study participation were provided with multiple options and opportunities to opt out of the study before they were contacted for the telephone interview.
We enrolled men in this formative phase of the study after they provided informed consent. The sample of participants (n= 13) consisted of men who were untested (n=3) as well as those who were tested and received a positive (n=9) or true negative (n=1) BRCA1/2 PV result. All participants were Non-Hispanic White, 6 were Ashkenazi Jewish, and more than half had a BRCA2 familial mutation (n=8) and had children (n=9). The mean age was 55 years (range 41–70 years).
Procedures.
All participants completed an individualized telephone interview with a trained research assistant, after which they received a $25 gift card. With permission, the call was audio-recorded. Each interview lasted approximately 30 minutes.
Using an iterative process based on our prior research [46] and clinical experience, the study team developed a structured interview script for the research assistant to administer. For untested men, the first part of the interview contained 6 questions followed by probes related to the men’s reactions to learning about the presence of a BRCA1/2 PV in their family, whether they considered genetic testing and from whom they sought input about that decision, why they decided not to pursue testing, their understanding of the personal relevance for and effects of that information on themselves and their children, and their needs and decision-making around genetic counseling and testing. They were also asked to consider more broadly what factors may affect men’s desire or ability to obtain genetic counseling and testing. For men who were previously tested for a BRCA1/2 PV, we asked about their testing decision, including what information they had received prior to testing, who was instrumental in their choice to be tested, their main reason for getting tested, and with whom they have communicated their test results. Previously tested men were also asked about details related to the genetic counseling process, such as whether and by whom it was provided and what information was covered. Interviewers used specific probes to assess men’s understanding about prostate cancer risks and the effect of carrying a BRCA1/2 PV on cancer screening decisions.
For the second part of the interview, men were informed about the study’s aim to develop an educational WI for untested men. They were then asked 4 questions with probes about what they thought would be the most important points to cover in the WI, whether and how men would seek out such information, and ways in which men could be encouraged to pursue genetic counseling.
The audio recordings were transcribed and analyzed for major themes by two research assistants, including specific content that men would like to see included in the WI. By consensus, the study team agreed on the themes that were important to include in the WI. In addition, quotes from men that were representative of the group as well as those reflecting less common concerns were reviewed in conjunction with the themes to help construct a content outline for the WI.
Results.
Review of the interview data indicated that men believed that the topics in Table 1 were most important to include. Interestingly, a common but unsolicited theme emerged from the interviews related to insurance (n=6) and cost concerns (n=4). Responses also provided valuable information on what factors would encourage or discourage other men from getting tested. The majority of participants mentioned that fear (n=6), or misconceptions of how BRCA1/2 PVs apply to men and their cancer risk (n=5) would discourage other men from getting tested. Several men stated that having a family (children) or a personal or family history of cancer would encourage men to be tested (n=6). Men who were previously tested reflected on their primary reason for going through with testing, and several men stated that they received testing for the sake of their children and female relatives (n=6).
Table 1.
Percent of participants who endorsed coverage of specific topics in the WI
| Theme | % |
|---|---|
| Statistics on BRCA1/2 associated cancers | 70% (n=9) |
| Prevention and screening for associated cancers | 54% (n=7) |
| Information for children and relatives | 46% (n=6) |
| BRCA1/2 information and the specific impact on men | 31% (n=4) |
| At-risk ages and timeframes to be tested | 23% (n=3) |
Several general themes emerged in the interviews that men thought were important to include in the intervention. Participants felt it was necessary to include specific statistical information on cancer risks associated with BRCA1/2 PVs in men and the chances of inheriting the mutation (n=9). The information men recommended included a clear presentation of risks associated with different types of cancer (e.g., prostate cancer, male breast cancer, colorectal, pancreatic) as well as information about which cancers are not associated with a BRCA1/2 PV. Participants also wanted to have information for their female relatives and children (n=6). In addition, one participant thought it was important that the intervention include information on what age to talk to their children and what options are available for their children. Men felt it was important to discuss how test results would affect cancer screening, including what screening tests are available, and what age to start screening (n=7). General testing information was requested to explain the basic procedure (e.g., blood draw or mouth swab), and participants were interested in knowing with which medical professionals they should discuss genetic testing before pursuing it. Lastly, participants mentioned wanting a general summary page which included information to discuss with a primary care provider regarding test results and screening guidelines.
Phase II: Development of the Web Intervention (WI)
Based on data from the interviews, our prior research and the research of others, we developed a tailored web-based education and decision tool to be tested in the intervention arm of a pilot RCT with men who are untested and first-, second- or third-degree relatives of BRCA1/2 carriers. Content was generated by the research team who had expertise in genetic counseling (BNP), medical oncology (CI), genomics risk communication and related decision-making (BNP, KDG, SCO, MDS), and decision-making about prostate cancer screening (MDS, KLT). The team developed content from several resources, including: literature review, clinical experience, themes generated from the formative research (Phase I), and our previous decision support interventions for women who have undergone BRCA1/2 testing [47, 48] and for men weighing the pros and cons of prostate cancer screening [49–52].
Phase III: Usability testing
Eligibility.
Prior to beginning the pilot RCT of the WI, we sought user feedback on content and usability. For this phase of the research, eligible men were between the ages of 40–70 years old with no history of cancer (except for non-melanoma skin cancer), English speaking, and able to provide informed consent. Given the limited number of available index patients and thus a limited pool of men to enroll, we chose to select a subset of men who participated in the formative interviews for the usability testing. These men were highly interested and engaged, and we wanted their feedback about the degree to which the WI was responsive to the needs and concerns they raised. A research assistant sent out recruitment packets to the nine men from the formative interviews with an identified BRCA1/2 PV inviting them to review the website and provide feedback.
Procedures.
Men were enrolled in the study after providing informed consent. A research assistant conducted brief telephone interviews to collect demographic data and information to tailor participants’ websites. We informed participants that the intervention was intended for untested male relatives of a BRCA1/2 mutation carrier and therefore that the website would not be completely tailored. Following the telephone interview, men were sent an e-mail link to the website and asked to review the website as if they had not yet been tested. They were also mailed a paper version of their tailored website along with a brief paper follow-up survey to complete after their review of the intervention. Men were asked to provide any questions or feedback about the website on the print version. As displayed in Table 2, the website is tailored on the following factors: Gene containing the familial mutation (BRCA1 or BRCA2), closest relative that tested positive for the familial mutation (e.g. sister, mother, maternal uncle), number of sons and number of daughters, race, Ashkenazi Jewish heritage, and family history of prostate cancer. All participant websites had insurance status set to “unclear.”
Table 2.
Tailoring Variables
| • Familial gene variant (BRCA1 or BRCA2) |
| • Closest relative identified with PV variant |
| • Family history of prostate cancer (yes/no) |
| • Sex of biological children |
| • Plans for future children |
| • Race |
| • Ashkenazi Jewish ancestry (yes/no) |
| • Insurance status for testing (i.e., testing covered, not covered, unclear, no insurance, network testing or genetic counseling required) |
| • Panel testing candidate (family history suggestive of another hereditary cancer syndrome) (yes/no) |
Participants were mailed a $25 Amazon gift card after the research team received their comments on the paper version of the website and the completed follow-up survey. Participant responses were reviewed by several members of the research team.
Results.
Of the 9 men contacted, 3 consented and returned their paper version of the screens, and the follow-up survey. These men ranged in age from 56 to 69, were non-Hispanic White, and all had advanced degrees. Two had a BRCA2 mutation, and the other had a BRCA1 mutation. Only 1 of the 3 men had biological children, and 2 were of Ashkenazi Jewish descent.
Participants were asked to provide feedback in the following categories: Satisfaction, Acceptability, Website Usability, Website Credibility, Content-Specific Comprehension, and Technology Usage. Overall, all participants said they were very satisfied with the website, each stating that it provided important or helpful information about cancer risks. All participants said that they feel comfortable using a computer, and they all accessed the website on a computer. In terms of usability, all participants completed the website in a single session, and did not have difficulty with technical glitches, or with screens failing to load. They all thought that the length of the web-based program was appropriate; however, we did not ask how long it took them to review. The men agreed that the information provided on the website could be trusted.
The participants provided several suggestions for website improvement. One participant was frustrated that the “next screen” buttons were too low on each screen requiring the user to scroll down to advance to the next screen, so we made the necessary adjustments. For content comprehension, two men noted that that graphs were inconsistent, some figures lacked depth, and some of the stock photos did not fit the text. In light of this feedback, we made several changes to the graphs and figures. Another participant was concerned about the amount of statistics and risk figures that were unfamiliar to him. However, we selected information based on the available risk data supported by research evidence. All three participants provided small wording changes, and as a team we accepted many of these changes to improve the clarity of the website.
Description of the Final WI
The major content areas of the WI contain essential elements of informed consent for cancer genetic testing, as outlined in recommendations from the National Society of Genetic Counselors [53]. These content areas are as follows: BRCA Overview, Implications of a BRCA1/2 Mutation in Men, Implications for Children and Other Relatives, Genetic Counseling and Testing, and Making a Testing Decision. The content of WI is designed to be tailored on the factors listed in Table 2. An outline of the content is in Table 3. Participants can move sequentially to subsequent or previous screens. After reviewing the intervention sequentially once in its entirety (except for optional screens), participants are able to navigate freely between major content areas. Most screens contain brief bulleted text in addition to a photo, figure, or pictogram. Participants can click on words or terms to view glossary definitions. Of note, the WI can be viewed on a computer, tablet or mobile phone. Importantly, the final WI, including what is used in the pilot RCT, does not incorporate audio. While audio can be particularly valuable to low-literacy participants, our primary focus in developing the WI was to establish its preliminary efficacy. Subsequent revisions of this tool will incorporate audio in order to facilitate broader dissemination. Below we describe the WI content in more detail.
Table 3.
Web-Intervention Contents Outline
| Home |
| BRCA Overview |
| - What Is The BRCA(1/2) Gene? |
| - How Common Are BRCA Mutations? |
| - How Are BRCA Mutations Inherited? |
| - What Is My Risk for Carrying the BRCA(1/2) Mutation In My Family? |
| - Implications Of BRCA(1/2) Testing |
| Implications in Men |
| - Prostate Cancer |
| - Overview |
| - What Are The Risk Factors For Prostate Cancer? |
| - How Common Is Prostate Cancer? |
| - When Is Prostate Cancer Usually Diagnosed? |
| - What Screening Tests Are There for Prostate Cancer? |
| - What Can BRCA(1/2) Testing Tell Me About My Risk For Prostate Cancer? |
| - Prostate Cancer Risks In BRCA(1/2) Carriers |
| - Prostate Cancer In BRCA(1/2) Carriers |
| - How Would My Genetic Test Results Affect My Prostate Cancer Screening Recommendations? |
| - Optional: Benefits And Harms of Prostate Cancer Screening |
| - Optional: IMPACT Trial: Prostate Cancer Screening In High Risk Men |
| - Can Prostate Cancer Be Prevented? |
| - Would Knowing My BRCA(1/2) Mutation Status Impact My Prostate Cancer Treatment? |
| - Male Breast Cancer |
| - Overview And Risks |
| - How Would Genetic Testing Affect My Breast Cancer Screening Recommendations? |
| - Optional: Breast Self-Examination |
| - Optional: Clinical Breast Exam |
| - Optional: Mammography |
| - Are There Options For Reducing My Breast Cancer Risk |
| - Pancreatic Cancer |
| - Overview And Risks |
| - Are There Options For Pancreatic Cancer Screening? |
| - Colorectal Cancer |
| - Overview And Risks |
| - Colorectal Cancer Screening |
| - Other Cancers |
| - Overview |
| Implications for Children & Family |
| - Cancer Risks For My Children |
| - What Information Should I Provide To My Daughter? |
| - What Information Should I Provide To My Son? |
| - Chances Of Siblings And Other Relatives Carrying The Mutation |
| Genetic Counseling and Testing |
| - What Is Genetic Counseling? |
| - Is Genetic Counseling And Testing Available To Me Through This Study? |
| - What Information Is Covered In Pre-Test Genetic Counseling That Is Not Covered In This Web-Based Program? |
| - Will My Health Insurance Or Employment Be Affected If I Get Genetic Testing? |
| - Optional: What Federal And State Laws Protect Against Genetic Discrimination? |
| - What Is The Cost Of Testing? |
| - What Is Involved In The Process Of Genetic Testing? |
| Making a Testing Decision |
| - Is Genetic Testing Right For You? The Choice Is Yours. |
| - Worksheet For Making My Testing Decision |
| - Are You Interested In Obtaining BRCA(1/2) Genetic testing? |
| Resources |
| Glossary |
BRCA Overview
This section contains four screens and provides a general overview of the BRCA1 or BRCA2 gene, including its function, how mutations are inherited, and how rare they are in the general population. Of note, in the intervention, we decided to use the term “mutation” as our formative research indicated that this is an acceptable and well understood term by participants. Further, this term is defined in the WI when it is first presented. A participant’s tailored individual risk for carrying the familial mutation is provided and explained based on the closest relative that he identified as testing positive (e.g., mother or sister) (Supplementary Figure A1).
Implications of BRCA1/2 Testing in Men
This section details the cancers most commonly associated with a BRCA1/2 mutation in men. Following a general description of each cancer, screens provide tailored risk estimates in carriers relative to the general population by using interactive pictograms. Specifically, participants can click on a figure showing the average risk for the specific cancer in the general population, after which they can click a button to have the figure depict the increased risk of that cancer in a mutation carrier (Supplementary Figure A2). Information is also provided about the average age of diagnosis for carriers, and options for cancer screening. Information about cancer risk reduction is provided for prostate and male breast cancer. Currently, there is no approved or guideline recommended prevention for these cancers, but general information is provided (Supplementary Figure A3). For each cancer, men learn what the implications of their testing decision may be on understanding their risk of developing cancer, their cancer screening recommendations, or their treatment options (for prostate cancer) based on whether they test positive or negative for the familial mutation, or whether they choose not undergo BRCA1/2 testing (Supplementary Figure A4).
Information about prostate cancer is likely to have the most significant impact on men’s medical management, especially if they are diagnosed with prostate cancer in the future. Thus, the WI includes more screens dedicated to this cancer compared to other BRCA1/2-associated cancers (i.e., male breast, pancreatic). There is in depth information about general prostate cancer risk factors (i.e., age, race, diet, family history, gene mutation), prostate cancer screening and diagnosis (Supplementary Figure A5), current information on chemoprevention for prostate cancer, and information about potential treatment implications. Additionally, participants have the option to view additional screens about potential benefits and harms associated with prostate cancer screenings (e.g., overtreatment of slow growing cancer) (Supplementary Figure A6) as well as background about the IMPACT trial in high-risk men. The IMPACT study is a UK screening trial, “Identification of Men with a genetic Predisposition to Prostate Cancer: Targeted screening in men at high genetic risk and controls.” [54]
After the discussion on prostate cancer, the WI provides information on male breast cancer, pancreatic cancer, and colorectal cancer. The male breast cancer section includes optional pages on expert recommendations for breast cancer screening tests. These tests include: breast self-examination, clinical breast exams, and mammography [14]. A link is provided with instructions for men about how to do breast self-exams, including cartoon depictions of a man performing the exam [55]. Men are informed that mammography is not recommended by national guidelines, but they can ask their doctor about this test. The section concludes with one screen describing other potential cancers associated with the familial mutation (e.g., melanoma is associated with BRCA2). The entire section on associated cancer risks contains between 19–24 pages, depending on how much optional content men choose to view.
Implications for Children and Other Family Members
This section reviews the implications of a mutation for a participant’s children (Supplementary Figure A7) and other family members, and is tailored based on the children’s sex. The resources section provides links to websites for additional information. The final screen in this section contains information about implications for siblings and other relatives, and explains that a genetic counselor can help identify risks in these individuals. A clear recommendation is made that if the participant tests positive, he should notify his adult at-risk relatives so they can make decisions about testing. This section contains between 2–4 screens.
Genetic Counseling and Genetic Testing
After these sections, the intervention provides an overview of genetic counseling and testing options for the participant. Here we provide a brief explanation of what genetic counseling is and how it helps individuals make informed decisions about testing and managing cancer risks. However, we explain that the content of website is comparable to information received during genetic counseling. The WI explains that participants can proceed directly to BRCA1/2 testing coordinated through the study, which would be billed to the participant or his insurance company. Participants with a family history suggestive of other hereditary cancer syndromes will also see a screen about the option for multigene panel testing, which is increasingly offered to relatives instead of testing for only the familial PV or gene [56]. Men have the option of discussing this option in greater detail either during the pre- or post-test genetic counseling session. (We explain that participants can reflex to a panel at no additional cost within 90 days after receiving their BRCA1/2 test result.) The WI clarifies that genetic testing is optional, with no additional pre-test genetic counseling required, unless the participant’s insurance company requires genetic counseling. However, for men who do opt for testing, a post-test phone call with a board-certified, and if applicable, licensed, genetic counselor is required (Supplementary Figure A8). Importantly, all participants have the option of individual pre-test genetic counseling if they decide that they need additional information or have additional questions. This counseling can be requested from within the WI and is provided at no cost by phone by the genetic counselor. The subsequent screen explains what is covered during pre-test genetic counseling beyond the WI (Supplementary Figure A9).
The final few screens in this section address concerns related to genetic discrimination (e.g., in insurance and employment), costs of testing, and logistics of genetic testing. Optional screens contain more detailed information on protections against genetic discrimination, such as the Genetic Information Nondiscrimination Act of 2008 (GINA). Information about costs and logistics may be tailored based on the participant’s insurance (e.g., if genetic counseling by a credentialed provider is required and can be provided by the study or whether it needs to be done in-network). This section contains between 5–7 screens.
Making a Testing Decision
The final section of the WI is a value-clarification exercise to help men make their choice about whether or not they want to be tested for their familial BRCA1/2 mutation. The opening screen in this section provides six edited quotes from men who participated in the formative interviews, half from men who thought testing was helpful, and half from men who wondered whether testing was or would be helpful (Supplementary Figure A10). Subsequently, men can complete an interactive worksheet listing the pros and cons of getting tested (Supplementary Figure A11). On the worksheet, seven potential pros (e.g., “Getting tested may eliminate the need for my children to undergo genetic testing in the future.”) and seven potential cons (e.g., “Getting tested may cause me to be anxious or worry about my cancer risks.”) are listed, along with space for participants to list two of their own additional pros and two additional cons. After each statement, they are asked, “Is the statement important to you?” (yes or no). If men have already made up their mind about genetic testing (yes or no), they can skip the worksheet.
Next, participants are asked to indicate their choice about genetic testing. They are reminded that there is no charge for genetic counseling obtained through the study. They have four options from which to choose: (1) “I am undecided and have questions.” If participants select this option, they are contacted within two business days by a study research assistant, and can opt for genetic testing or to schedule a phone appointment with a study genetic counselor. (2) “I would like to have genetic counseling before genetic testing.” If they select this option, they are contacted within two business days to set up a telephone genetic counseling session. (3) “I would like to have genetic testing without genetic counseling.” Participants are contacted within two business days to discuss testing logistics with a research assistant, after which they are mailed materials to send DNA to the commercial testing lab, which is not affiliated with Georgetown University. They are reminded that post-test genetic counseling will be provided by telephone after genetic test results are available. Finally, (4) “I am not interested in genetic counseling or testing.”
The last screen in this section thanks men for their participation, provides telephone and email contact information for the study team, and provides a link for additional resources about BRCA1/2 mutations and genetic testing, including information for at-risk men and women (e.g., guidelines from the National Comprehensive Cancer Network on hereditary breast and ovarian cancer), support groups/advocacy for men and women (e.g., FORCE: Facing Our Risk of Cancer Empowered), genetic counseling (National Society of Genetic Counselors), and anti-genetic discrimination legislation (national and state).
Aims and Hypotheses of the Pilot Randomized Trial
The pilot RCT is designed to provide an initial evaluation of the efficacy and impact of the WI versus enhanced Usual Care (hereafter abbreviated as UC) among men from families with a BRCA1/2 PV who have not pursued genetic counseling and testing. Men randomized to the UC arm are provided with a brochure from FORCE entitled, “Information about BRCA Testing for Men” [57] which reviews cancer risks and the relevance of testing. They may also receive free pre- and post-test telephone genetic counseling through the study, along with coordination of genetic testing (as is offered to men in the WI). However, men in UC must obtain pre-test genetic counseling prior to testing. The primary aim of the trial is to evaluate the impact of WI vs. UC on: a) the uptake of genetic counseling and testing and b) informed decision-making regarding genetic testing. Specifically, we hypothesize that men randomized to the WI intervention will be more likely to complete genetic education/counseling and will be more likely to opt for genetic testing compared to UC participants. We also hypothesize that those randomized to the WI arm will make more well-informed testing decisions (characterized by higher knowledge, more positive attitudes and lower decisional conflict) compared to the UC group. In exploratory analyses, we will evaluate the behavioral and psychosocial impact of BRCA1/2 testing decisions. Our study measures and assessment points are displayed in Table 4.
Table 4.
Measures Used in the Randomized Trial
| Variables | Measures | Baseline | 1-Month | 6-Month |
|---|---|---|---|---|
| Demographics | Age, education, income, race, ethnicity, marital status, employment | X | ||
| Family/Medical History | Familial mutation, family cancer history, number of children | X | ||
| Knowledge | 10-items about BRCA knowledge [34, 61] | X | X | X |
| Perceived Risks | Perceived risk of BRCA mutation and colon, prostate, lung, pancreatic and breast cancer | X | ||
| Attitudes | Adapted version of the Multidimensional Measure of Informed Choice [43, 44, 62] | X | ||
| Decisional Conflict | 16-item Decisional Conflict Scale [63] | X | X | |
| Help/Harm of Genetic Testing | 11-items asking ways that BRCA1/2 testing could help or harm participant (e.g., “Undergoing BRCA1/2 gene testing may make me anxious or worried.”); Adapted from Bancroft et al. [64] | X | X | X |
| Reasons for not pursuing testing | 8-items assessing reasons for not pursuing testing (e.g. “Did not know testing was an option”) | X | X | X |
| Genetic Counseling and Testing | Assess genetic counseling/testing/test result. Assess counseling/testing discussions with physician | X | X | X |
| Testing Decision Satisfaction | Satisfaction with Decision Scale [65, 66] | X | ||
| Distress | Cancer distress: 15-item Impact of Event Scale [67]; Genetic testing distress: 21-item MICRA [68] | X | X | |
| Stress | 4-item Perceived Stress Scale [69] | X | X | |
| Cancer Worry | 4-items adapted from Lerman’s Cancer Worry Scale [70] | X | X | |
| Depression/Anxiety | PROMIS Depression and Anxiety Scales [71] | X | ||
| Global Health | PROMIS Global Health Scale [72] | X | ||
| Risk Management | Self-report of ever/last use of cancer screening tests; Assess if discussed management options with physician and physician recommendations | X | X | |
| WI Use/Process | WI Log-in data; Perceptions of how informative and helpful WI is, perception of WI quality. | X |
Discussion
Cascade testing for HBOC syndrome due to pathogenic variants in BRCA1 and BRCA2 is a highly efficient mechanism for identifying carriers, which has the potential to lower mortality from breast and ovarian cancer and result in cost savings [58, 59]. As a Centers for Disease Control and Prevention Tier 1 genomic application, identifying such carriers is an important public health priority [58, 60]. Historically, men in HBOC families are much less likely to undergo testing than their female counterparts. Efforts to increase men’s participation in BRCA1/2 genetic counseling and testing is important not only because it may inform medical decision-making in the men themselves, but also because it facilitates identification and eventual testing of additional at-risk female and male relatives. We designed a web-based intervention tailored to men with a BRCA1/2 PV in their family that has content similar to and potentially beyond what would be covered in a traditional genetic counseling session. In addition, unless required by their insurance company, men have the option to proceed directly to genetic testing without the need for pre-test interaction with a genetic counselor.
By developing a web-based tailored intervention for men at risk for BRCA1/2 mutations and testing it within a RCT, the pilot RCT will address key questions such as how men respond to online education, what effect it has on their knowledge and interest in testing, and how often men opt for additional pre-test genetic counseling prior to testing. If the study demonstrates that men in the WI pursue testing at a higher rate than men in usual care, as we predict, we will then need to validate these findings in a larger trial. We anticipate that our intervention will be highly scalable, adaptable to other populations (e.g., at-risk women, individuals at risk for other cancer predisposing gene variants, and probands), and could lower the costs of genetic counseling service delivery.
There are several limitations that need to be considered. We developed the content of the WI based largely on extant literature and our prior experience. Although we did seek the input of tested and untested men, our sample sizes for the formative interviews and usability testing were small and we had a low response rate. Similarly, we obtained only limited feedback about the finalized WI. The sociodemographics (e.g., race, ethnicity, level of education) of men who did participate in our research were homogenous and very few men had previously declined testing. Moreover, most men who participated previously underwent BRCA1/2 testing, which was likely accompanied by comprehensive pre- and post-test genetic counseling. Thus, we did not have substantial input from men who were not exposed to such information. In addition, the lack of audio narration in the WI limits its use by low-literate populations. We anticipate that the results of this preliminary trial will enable us to identify areas of the WI that should be modified to address concerns of a more diverse patient population, including racial and ethnic minorities, younger men, men who are ambivalent about getting tested, and men of low literacy. Prior to a larger trial, we will also explore mechanisms to make the WI more broadly applicable, such as using audio narration and integrating videos.
Despite these limitations, this study will have clinical significance through the development and evaluation of an exportable, low-cost intervention for men at the highest risk of testing positive for a BRCA1/2 PV. By fostering informed decisions, we expect that our WI will lead to increased use of BRCA1/2 counseling and testing, potentially yielding improved cancer control outcomes for this understudied group, and for their at-risk relatives. This pilot investigation will set the stage for additional research with a larger and more diverse population of at-risk men, with the ultimate goal of clinical dissemination.
Supplementary Material
Acknowledgements
The authors thank research assistants Yvette Noah and Samantha Philip. We thank Susan Marx for assistance with manuscript formatting. The study website was designed by Chadwick Cipiti Studios, Inc. Finally, we are grateful to all of the individuals who participated in the study.
Funding: The study was supported by Grant R21 CA185808 from the National Cancer Institute (Dr. Marc Schwartz, PI) and by the Jess and Mildred Fisher Center for Hereditary Cancer and Clinical Genomics Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Clinical trial registration number: NCT02957981
Conflict of Interest: Ms. Peshkin is a paid consultant for Clear Genetics, San Francisco, CA. Dr. Isaacs has received consulting fees from Pfizer and Astra Zeneca and is on the speaker’s bureau for Pfizer. Ms. Ladd, Ms. Segal, Ms. Jacobs, Drs. Taylor, Graves, O’Neill and Schwartz declare that they have no conflict of interest.
Ethical approval and informed consent: All procedures performed in studies involving human participants were in accordance with the ethical standards of Georgetown University (IRB 2017-0128) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Animal Studies: This article does not contain any studies with animals performed by any of the authors.
References
- 1.Rosenthal ET, Bernhisel R, Brown K, Kidd J, Manley S (2017) Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet 218-219: 58–68. 10.1016/j.cancergen.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J (2017) National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol 35(34): 3800–6. 10.1200/JCO.2017.73.6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Febbraro T, Robison K, Wilbur JS, et al. (2015) Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecol Oncol 138(1): 109–14. 10.1016/j.ygyno.2015.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ASCO Connection (2018) Prostate cancer (8 August 2018): molecular oncology tumor boards https://connection.asco.org/discussion/prostate-cancer-august-2018-molecular-oncology-tumor-boards
- 5.Healio.com (2018) Family history, disease stage key in determining need for inherited prostate cancer genetic testing 29 January https://www.healio.com/hematology-oncology/prostate-cancer/news/in-the-journals/%7b5f648cfd-bfd8-4c50-b684-b5b62a2c7d71%7d/family-history-disease-stage-key-in-determining-need-for-inherited-prostate-cancer-genetic-testing
- 6.Marcus AD (2018) The genetic test some men don’t know they need. The Wall Street Journal, August 7 https://www.wsj.com/articles/the-genetic-test-some-men-dont-know-they-need-1533651551,
- 7.Friedman S (2018) Solving for Y: reaching men about genetic testing for hereditary breast, ovarian, pancreatic, prostate and related cancers (HBOC). FORCE blog June 17, 2018 https://www.facingourrisk.org/get-involved/HBOC-community/BRCA-HBOC-blogs/FORCE/uncategorized/solving-for-y-genetic-testing-in-men/
- 8.Pritchard CC (2019) New name for breast-cancer syndrome could help to save lives. Nature 571(7763): 27–9 10.1038/d41586-019-02015-7 [DOI] [PubMed] [Google Scholar]
- 9.Liede A, Karlan BY, Narod SA (2004) Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 22(4): 735–42. 10.1200/JCO.2004.05.055 [DOI] [PubMed] [Google Scholar]
- 10.Kowalewski A, Szylberg L, Saganek M, Napiontek W, Antosik P, Grzanka D (2018) Emerging strategies in BRCA-positive pancreatic cancer. J Cancer Res Clin Oncol 144(8): 1503–7 10.1007/s00432-018-2666-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecarpentier J, Silvestri V, Kuchenbaecker KB, et al. (2017) Prediction of breast and prostate cancer risks in male BRCA1 and BRCA2 mutation carriers using polygenic risk scores. J Clin Oncol 35(20): 2240–50. 10.1200/JCO.2016.69.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro E, Goh C, Olmos D, et al. (2013) Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31(14): 1748–57. 10.1200/JCO.2012.43.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleicher S, Kauffman EC, Kotula L, Bratslavsky G, Vourganti S (2016) Implications of high rates of metastatic prostate cancer in BRCA2 mutation carriers. Prostate 76(13): 1135–45. 10.1002/pros.23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (2019) NCCN clinical practice guidelines in oncology (NCCN guidelines). Genetic/familial high-risk assessment: breast and ovarian Version 3.2019--January 18, 2019 https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- 15.Mateo J, Carreira S, Sandhu S, et al. (2015) DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373(18): 1697–708. 10.1056/NEJMoa1506859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schepisi G, Farolfi A, Conteduca V, et al. (2017) Immunotherapy for prostate cancer: Where we are headed. Int J Mol Sci 18(12): 10.3390/ijms18122627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri VN, Knudsen KE, Kelly WK, et al. (2018) Role of genetic testing for inherited prostate cancer risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol 36(4): 414–24. 10.1200/JCO.2017.74.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bancroft EK, Page EC, Castro E, et al. (2014) Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol 66(3): 489–99. 10.1016/j.eururo.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman DC, Curry SJ, Owens DK, et al. (2018) Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 319(18): 1901–13. 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 20.Schwiter R, Rahm AK, Williams JL, Sturm AC (2018) How can we reach at-risk relatives? Efforts to enhance communication and cascade testing uptake: a mini-reivew. Curr Genet Med Rep 6(2): 21–7. 10.1007/s40142-018-0134-0 [DOI] [Google Scholar]
- 21.Hallowell N, Arden-Jones A, Eeles R, et al. (2006) Guilt, blame and responsibility: men’s understanding of their role in the transmission of BRCA1/2 mutations within their family. Sociol Health Illn 28(7): 969–88. 10.1111/j.1467-9566.2006.00515.x [DOI] [PubMed] [Google Scholar]
- 22.Liede A, Metcalfe K, Hanna D, et al. (2000) Evaluation of the needs of male carriers of mutations in BRCA1 or BRCA2 who have undergone genetic counseling. Am J Hum Genet 67(6): 1494–504. 10.1086/316907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauscher EA, Dean M, Campbell-Salome GM (2018) “I am uncertain about what my uncertainty even is”: Men’s uncertainty and information management of their BRCA-related cancer risks. J Genet Couns 27(6): 1417–27. 10.1007/s10897-018-0276-y [DOI] [PubMed] [Google Scholar]
- 24.Stromsvik N, Raheim M, Oyen N, Gjengedal E (2009) Men in the women’s world of hereditary breast and ovarian cancer--a systematic review. Fam Cancer 8(3): 221–9. 10.1007/s10689-009-9232-1 [DOI] [PubMed] [Google Scholar]
- 25.Suttman A, Pilarski R, Agnese DM, Senter L (2018) “Second-class status?” Insight into communication patterns and common concerns among men with hereditary breast and ovarian cancer syndrome. J Genet Couns 27(4): 885–93. 10.1007/s10897-018-0214-z [DOI] [PubMed] [Google Scholar]
- 26.Childers KK, Maggard-Gibbons M, Macinko J, Childers CP (2018) National distribution of cancer genetic testing in the United States: Evidence for a gender disparity in hereditary breast and ovarian cancer. JAMA Oncol 4(6): 876–9. 10.1001/jamaoncol.2018.0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daly MB (2009) The impact of social roles on the experience of men in BRCA1/2 families: implications for counseling. J Genet Couns 18(1): 42–8. 10.1007/s10897-008-9183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FORCE (Facing Our Risk of Cancer Empowered) (2018) Paying for genetic services https://www.facingourrisk.org/understanding-brca-and-hboc/information/finding-health-care/paying_for_testing/basics/overview.php
- 29.Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C (2013) Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns 22(5): 603–12. 10.1007/s10897-013-9592-4 [DOI] [PubMed] [Google Scholar]
- 30.Graves KD, Gatammah R, Peshkin BN, et al. (2011) BRCA1/2 genetic testing uptake and psychosocial outcomes in men. Fam Cancer 10(2): 213–23. 10.1007/s10689-011-9425-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauscher EA, Dean M (2017) “I’ve just never gotten around to doing it”: Men’s approaches to managing BRCA-related cancer risks. Patient Educ Couns 101(2): 340–5 DOI 10.1016/j.pec.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 32.Buchanan AH, Rahm AK, Williams JL (2016) Alternate service delivery models in cancer genetic counseling: A mini-review. Front Oncol 6: 120 10.3389/fonc.2016.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon ES, Babu D, Laney DA (2018) The future is now: Technology’s impact on the practice of genetic counseling. Am J Med Genet C Semin Med Genet 178(1): 15–23. 10.1002/ajmg.c.31599 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. (2014) Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 32(7): 618–26. 10.1200/JCO.2013.51.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biesecker BB, Lewis KL, Biesecker LG (2018) Web-based platform vs genetic counselors in educating patients about carrier results from exome sequencing--Reply. JAMA Intern Med 178(7): 999 10.1001/jamainternmed.2017.8049 [DOI] [PubMed] [Google Scholar]
- 36.Caswell-Jin JL, Zimmer AD, Stedden W, Kingham KE, Zhou AY, Kurian AW (2019) Cascade genetic testing of relatives for hereditary cancer risk: Results of an online initiative. J Natl Cancer Inst 111(1): 95–8. 10.1093/jnci/djy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturm AC, Schmidlen T, Scheinfeldt L, et al. (2018) Early outcome data assessing utility of a post-test genomic counseling framework for the scalable delivery of precision health. J Pers Med 8(3): 10.3390/jpm8030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juan AS, Wakefield CE, Kasparian NA, Kirk J, Tyler J, Tucker K (2008) Development and pilot testing of a decision aid for men considering genetic testing for breast and/or ovarian cancer-related mutations (BRCA1/2). Genet Test 12(4): 523–32. 10.1089/gte.2008.0035 [DOI] [PubMed] [Google Scholar]
- 39.Butrick M, Kelly S, Peshkin BN, et al. (2015) Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med 17(6): 467–75. 10.1038/gim.2014.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Interrante MK, Segal H, Peshkin BN, et al. (2017) Randomized noninferiority trial of telephone vs in-person genetic counseling for hereditary breast and ovarian cancer: A 12-month follow-up. JNCI Cancer Spectr 1(1): pkx002 10.1093/jncics/pkx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peshkin BN, Demarco TA, Graves KD, et al. (2008) Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: rationale and development of a randomized controlled trial. Genet Test 12(1): 37–52. 10.1089/gte.2006.0525 [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MD, Peshkin BN, Isaacs C, et al. (2018) Randomized trial of proactive rapid genetic counseling versus usual care for newly diagnosed breast cancer patients. Breast Cancer Res Treat 170(3): 517–24. 10.1007/s10549-018-4773-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michie S, Dormandy E, Marteau TM (2002) The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns 48(1): 87–91. 10.1016/S0738-3991(02)00089-7 [DOI] [PubMed] [Google Scholar]
- 44.Michie S, Dormandy E, Marteau TM (2003) Informed choice: understanding knowledge in the context of screening uptake. Patient Educ Couns 50(3): 247–53. 10.1016/S0738-3991(03)00044-2 [DOI] [PubMed] [Google Scholar]
- 45.The Ottawa Hospital Research Institute (2015) Ottawa Decision Support Framework (ODSF) 22 June https://decisionaid.ohri.ca/odsf.html
- 46.Nusbaum R, Leventhal KG, Hooker GW, et al. (2013) Translational genomic research: Protocol development and initial outcomes following SNP testing for colon cancer risk. Transl Behav Med 3(1): 17–29. 10.1007/s13142-012-0149-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufman EM, Peshkin BN, Lawrence WF, et al. (2003) Development of an interactive decision aid for female BRCA1/BRCA2 carriers. J Genet Couns 12(2): 109–29. 10.1023/A:1022698112236 [DOI] [PubMed] [Google Scholar]
- 48.Ladd MK, Peshkin BN, Senter L, et al. (2018) Predictors of risk-reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer. Transl Behav Med 10.1093/tbm/iby101 [Epub ahead of print]: [DOI] [PMC free article] [PubMed]
- 49.Dorfman CS, Williams RM, Kassan EC, et al. (2010) The development of a web- and a print-based decision aid for prostate cancer screening. BMC Med Inform Decis Mak 10: 12 10.1186/1472-6947-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor KL, Davis JL 3rd, Turner RO, et al. (2006) Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 15(11): 2179–88. 10.1158/1055-9965.epi-05-0417 [DOI] [PubMed] [Google Scholar]
- 51.Taylor KL, Williams RM, Davis K, et al. (2013) Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 173(18): 1704–12. 10.1001/jamainternmed.2013.9253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams RM, Davis KM, Luta G, et al. (2013) Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ Couns 91(3): 329–36. 10.1016/j.pec.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riley BD, Culver JO, Skrzynia C, et al. (2012) Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns 21(2): 151–61. 10.1007/s10897-011-9462-x [DOI] [PubMed] [Google Scholar]
- 54.ClinicalTrials.gov (2019) The IMPACT study - identification of men with a genetic predisposition to prostate cancer: targeted screening in BRCA1/2 mutation carriers & controls https://clinicaltrials.gov/ct2/show/NCT00261456
- 55.United Breast Cancer Foundation (2016) Male self breast exam http://ubcf.org/male-self-breast-exam/
- 56.Thomas MH, Higgs LK, Modesitt SC, Schroen AT, Ring KL, Dillon PM (2019) Cases and evidence for panel testing in cancer genetics: Is site-specific testing dead? J Genet Couns [Epub 2 February]: 1–8. 10.1002/jgc4.1044 [DOI] [PubMed]
- 57.FORCE (Facing Our Risk of Cancer Empowered) (2018) If cancer runs in your family…information about BRCA testing for men https://www.facingourrisk.org/understanding-brca-andhboc/publications/documents/Info%20for%20Men%20Flyer%207.16.14.pdf
- 58.George R, Kovak K, Cox SL (2015) Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns 24(3): 388–99. 10.1007/s10897-014-9805-5 [DOI] [PubMed] [Google Scholar]
- 59.Tuffaha HW, Mitchell A, Ward RL, et al. (2018) Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med 20(9): 985–94. 10.1038/gim.2017.231 [DOI] [PubMed] [Google Scholar]
- 60.Dotson WD, Douglas MP, Kolor K, et al. (2014) Prioritizing genomic applications for action by level of evidence: a horizon-scanning method. Clin Pharmacol Ther 95(4): 394–402. 10.1038/clpt.2013.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erblich J, Brown K, Kim Y, Valdimarsdottir HB, Livingston BE, Bovbjerg DH (2005) Development and validation of a Breast Cancer Genetic Counseling Knowledge Questionnaire. Patient Educ Couns 56(2): 182–91. 10.1016/j.pec.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 62.Dormandy E, Hankins M, Marteau E (2006) Attitudes and uptake of a screening test: The moderating role of ambivalence. Psychol Health 21(4): 499–511. 10.1080/14768320500380956 [DOI] [Google Scholar]
- 63.O’Connor AM (1995) Validation of a decisional conflict scale. Med Decis Making 15(1): 25–30. 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 64.Bancroft EK, Castro E, Ardern-Jones A, et al. (2014) “It’s all very well reading the letters in the genome, but it’s a long way to being able to write”: Men’s interpretations of undergoing genetic profiling to determine future risk of prostate cancer. Fam Cancer 13(4): 625–35. 10.1007/s10689-014-9734-3 [DOI] [PubMed] [Google Scholar]
- 65.Holmes-Rovner M, Kroll J, Schmitt N, et al. (1996) Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making 16(1): 58–64. 10.1177/0272989X9601600114 [DOI] [PubMed] [Google Scholar]
- 66.Schwartz MD, Valdimarsdottir HB, Demarco TA, et al. (2009) Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol 28(1): 11–9. 10.1037/a0013147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horowitz M, Wilner N, Alvarez W (1979) Impact of Event Scale: a measure of subjective stress. Psychosom Med 41(3): 209–18. http://www.ncbi.nlm.nih.gov/pubmed/472086 [DOI] [PubMed] [Google Scholar]
- 68.Cella D, Hughes C, Peterman A, et al. (2002) A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol 21(6): 564–72. 10.1037/0278-6133.21.6.564 [DOI] [PubMed] [Google Scholar]
- 69.Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24(4): 385–96. http://www.jstor.org/stable/2136404 [PubMed] [Google Scholar]
- 70.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A (1991) Psychological side effects of breast cancer screening. Health Psychol 10(4): 259–67. http://www.ncbi.nlm.nih.gov/pubmed/1915212 [DOI] [PubMed] [Google Scholar]
- 71.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D (2011) Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 18(3): 263–83. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D (2009) Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 18(7): 873–80. 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


