Abstract
Objective
The concept of using naturally occurring compounds such as polyenylphosphatidylcholine (PPC) as an adjunctive therapy to treat non-alcoholic fatty liver disease (NAFLD) and alleviate or reverse hepatic steatosis appears a very attractive option for liver protection. We aim to evaluate if PPC adjunctive therapy can effectively improve the ultrasonographic features of NAFLD in routine clinical practice in Russian patients with cardiometabolic comorbidities.
Design
This 24-week, observational, prospective study was carried out in 174 medical sites across 6 federal districts of Russia. A total of 2843 adult patients with newly diagnosed NAFLD, who had a least one of four comorbidities, namely overweight/obesity, hypertension, type 2 diabetes mellitus and hypercholesterolaemia, and who received PPC as an adjunctive treatment to standard care, were enrolled. The assessment of liver ultrasonography was qualitative.
Results
Overall, 2263 (79.6%) patients had at least two metabolic comorbidities associated with NAFLD, and overweight/obesity was the most common comorbidity reported in 2298 (80.8%) patients. Almost all study participants (2837/2843; 99.8%) were prescribed 1.8 g of PPC administered three times daily. At baseline, the most frequently identified abnormalities on ultrasound were liver hyperechogenicity (84.0% of patients) and heterogeneous liver structure (62.9%). At 24 weeks, a significant (p<0.05) improvement in liver echogenicity and in liver structure was observed in 1932/2827 (68.3%) patients (95% CI 66.6% to 70.1%) and in 1207/2827 (42.7%) patients (95% CI 40.9% to 44.5%), respectively. The analysis of ultrasonographic signs by number of comorbidities revealed similar findings—liver echogenicity improved in 67.2%–69.3% and liver structure in 35.6%–45.3% of patients depending on the number of comorbidities.
Conclusion
This study showed that PPC adjunctive therapy may be useful in improving the ultrasonographic features of NAFLD in patients with associated cardiometabolic comorbidities. It also supports evidence regarding the role of PPC in the complex management of NAFLD.
Keywords: ultrasonography, fatty liver, nutritional supplementation
Summary box.
What is already known about this subject?
It is particularly important to identify patients with simple steatosis, in order to prevent progression of non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis and improve their long-term prognosis.
Ultrasonography is a very efficient and widely available technique for the detection of fatty liver, which compares favourably to alternative non-invasive techniques.
Administration of polyenylphosphatidylcholine (PPC) has been associated with liver-protective effects.
What are the new findings?
PPC administered as adjunctive therapy in patients with NAFLD with metabolic comorbidities improves the ultrasonographic features of NAFLD, particularly with regard to liver echogenicity and structure.
The improvement of ultrasonographic findings associated with PPC adjunctive therapy is consistent across different comorbidity subgroups.
How might it impact on clinical practice in the foreseeable future?
PPC has a promising role in the complex management of patients with NAFLD and metabolic comorbidities.
Patients with difficulties in adhering lifestyle changes might benefit the most from the marked improvement in liver hyperechogenicity resulting from PPC adjunctive therapy.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognised as a major public health problem affecting 20%–30% of the adult population worldwide.1 According to the latest guidelines established by the American Association for the Study of Liver Diseases (AASLD), NAFLD is defined as the presence of fat (≥5%) in the liver (hepatic steatosis) observed either by imaging or histology, after the exclusion of secondary causes of hepatic fat accumulation, such as significant alcohol consumption, long-term use of a steatogenic medication or monogenic hereditary disorders.2 Clinically, NAFLD encompasses a wide spectrum of histological alterations ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), characterised by inflammation and fibrosis.3 Therefore, NAFLD has been traditionally interpreted as a condition, which may progress to liver-related complications, such as cirrhosis, hepatocellular carcinoma and liver mortality.4 At the same time, it is well known that NAFLD substantially contributes to the risk of fatal cardiovascular events, and may, therefore, exacerbate overall mortality.5 6 Indeed, according to the Global Burden of Diseases, Injuries and Risk Factors Study, the largest significant increase in disability-adjusted life years from 2007 to 2017 was for liver cancer due to NASH (37.4%; 95% CI 32.8% to 42.9%).7
NAFLD can be diagnosed by various methods, including clinical, biochemical and imaging tests.8 9 Due to the differences in diagnostic tools and study populations, the prevalence of NAFLD varies greatly among different populations.10 In Russia, the prevalence of NAFLD in the adult population has been estimated as high as 37%.11
The gold standard for diagnosing, staging and grading NAFLD is liver biopsy.12 However, the invasiveness of this method, and its associated morbidity and mortality, especially in individuals with non-progressive fatty liver conditions, have led to the emergence of less invasive methods which include serum markers (both direct and indirect markers of fibrosis), medical imaging techniques (CT (CT), MRI (MRI) and ultrasonography), and transient elastography. All of these techniques have the potential to reduce the number of biopsies performed in a high-risk population.13 14
Liver ultrasonography is currently the most commonly used technique to assess the presence of fatty liver in clinical settings and population studies.8 It is an attractive diagnostic tool because it is readily available, inexpensive, well tolerated and is already extensively used in the diagnostic workup of patients with NAFLD.14 15
In addition to the clinical use of liver ultrasound in treatment decisions and in monitoring therapeutic responses, it has also been found that ultrasound-based grading of the severity of NAFLD is associated with abnormalities in the metabolic profiles of patients.15 Indeed, NAFLD is regarded as the liver manifestation of the metabolic syndrome, which includes hyperlipidaemia, glucose intolerance, obesity and systemic hypertension.6 16 17 Thus, NAFLD is a multisystem disease that requires a multidisciplinary treatment approach.18
Other than lifestyle modifications, a variety of pharmacological agents (eg, statins, thiazolidinediones, metformin and antioxidants such as vitamin E) have been studied in the treatment of NAFLD, with conflicting results.19 This area of drug development is particularly challenging due to the complexity of NAFLD pathophysiology, the difficulties in its diagnosis and therapy monitoring, as well as the heterogeneity of the patient population.20 Hence, any therapeutic intervention that targets fat accumulation in the liver and ameliorates hepatic histology would be of great benefit.19 21 22 In terms of influencing steatosis, essential phospholipids (EPLs) are currently considered as one of the most promising therapeutic agents.23 EPLs contain a highly purified extract of polyenylphosphatidylcholine (PPC) molecules from soybean. Because of their membranous, antioxidative and antifibrotic effects, administration of EPL in NAFLD is pathogenetically justified.22 Thus, the concept of using EPL to reduce hepatic steatosis appears a very attractive option for liver protection in patients with NAFLD with metabolic comorbidities.17 Moreover, EPLs were recommended to treat NAFLD in the Russian Guidelines for the Diagnosis and Management of NAFLD.23 24 Considering these factors, the aim of the present study was to evaluate if PPC administered as adjunctive therapy can effectively improve the ultrasonographic features of NAFLD in routine clinical practice in Russian patients with metabolic comorbidities.
Methods
Study population
MANPOWER was a 6-month, observational, multicentre, prospective study that was carried out in 174 medical sites across the six major federal districts of the Russian Federation. Patients who met the study eligibility criteria were enrolled by general practitioners and gastroenterologists between 28 September 2015 and 28 September 2016.
Outpatients, aged between 18 and 60 years, with newly diagnosed NAFLD (within 30 days prior to study entry) were included in the study. NAFLD diagnosis was established on the basis of clinical examination and laboratory tests, supported by one of the following diagnostic procedures: ultrasonography, elastography and/or liver biopsy. In addition, eligible patients had at least one of the following concomitant diseases: (1) high blood pressure diagnosed by a cardiologist, (2) type 2 diabetes mellitus (T2DM) diagnosed by an endocrinologist, (3) high serum cholesterol (defined as a total cholesterol level of ≥5.0 mmol/L) and/or (4) overweight/obesity (body mass index (BMI) ≥27 kg/m2). Subjects had also been receiving prescribed PPC therapy (Essentiale Forte N which contains 300 mg of EPL) as an adjunctive treatment to standard care. Major exclusion criteria encompassed the presence of other severe acute or chronic conditions (including other liver diseases and cancers), and treatment with other hepatoprotectors, intravenous PPC or other concomitant EPL within 30 days before study entry.
This study was conducted under real-life conditions of daily clinical practice and in accordance with the Declaration of Helsinki, the Good Clinical Practice guidelines and all applicable Russian laws and regulations.
Data collection
Individual patient data were collected using paper-based case report forms at three time points: at baseline, 12 and 24 weeks. At baseline, information on the nature and number of metabolic comorbidities, the severity and histological features of NAFLD, patients’ previous and current pharmacological treatments, and the prescribed dosage and duration of PPC therapy was collected. The general health examination was performed at the same time. The most recent measurements of biochemical parameters including fasting plasma glucose, blood lipids, hepatic function and urine routine analysis were also obtained. At 12 and 24 weeks of the study, in addition to liver blood test results, ultrasonography findings (if available), current pharmacological treatments, symptoms and reasons for study withdrawal were recorded.
The assessment of liver ultrasound imaging was qualitative; the following four ultrasonographic signs were under assessment: (1) diffuse liver hyperechogenicity, (2) heterogeneous structure of the liver, (3) indistinctness and/or underlined vascular pattern and (4) distal echo signal attenuation. Moreover, the liver was assessed for size, contour, echogenicity, structure, penetration of the ultrasound beam, that is, posterior beam attenuation and portal vessel wall distinction.
Endpoints
The main endpoint of interest in the present study was to assess the effectiveness of PPC adjunctive therapy in improving ultrasonographic findings in each comorbidity subgroup throughout the 24-week study period. Key secondary endpoint of this study was to evaluate the effectiveness of PPC adjunctive therapy in improving ultrasonographic findings, according to the number of metabolic comorbidities associated with NAFLD. Furthermore, the study aimed to describe the diagnosis of NAFLD based on all available clinical, biochemical and histopathological data.
Statistical analyses
Findings were reported for categorical variables using proportions, while mean, SD, median and IQR were used to describe continuous variables. The population set used for statistical analysis comprised all eligible enrolled patients who provided adequate ultrasonographic data. The Wilcoxon-Mann-Whitney test, the Student’s t-test and the analysis of variance test were applied in order to assess the significance of the difference between subgroups in continuous variables. The association between categorical variables was examined by Pearson’s χ2 test or Fisher’s exact two-tailed test, when appropriate. All statistical tests were two sided and were performed at a 0.05 significance level. Statistical analyses were performed using SAS V.9.3 (SAS Institute).
Results
Patient characteristics
Of the 2843 patients with newly diagnosed NAFLD included in the study, 1076 were males and 1767 females with a median age (IQR) of 48.7 years (40.8–55.0) and 51.8 years (45.5–55.9), respectively. Patients were recruited by 174 qualified general practitioners and gastroenterologists, from 18 cities located in 6 different regions of Russia (Northwestern Federal District, Volga Federal District, Southern Federal District, Ural Federal District, Siberian Federal District and Central Federal District). Sixteen subjects (0.56%) dropped out of the study due to logistic issues. Demographic and baseline characteristics of the study population are summarised in table 1. The great majority (2434/2843; 85.6%) of study participants were non-smokers, and only 201 patients (7.1%) reported alcohol consumption at least once a week. Results of the lipid and glycaemic profiles revealed that most of the subjects had an elevated total cholesterol level and impaired fasting glucose (table 1).
Table 1.
Demographic and baseline characteristics of the study population (n=2843)
| Baseline characteristic | Study population (n=2843) |
| Age—years | 48.7±8.6 (50.7; 43.6–55.6) |
| Male/female—n (%) | 1076 (37.8)/1767 (62.2) |
| Weight—kg | 91.0±14.1 (90.0; 82.0–99.5) |
| Body mass index—kg/m2 | 32.0±4.6 (31.8; 29.2–34.6) |
| Waist circumference—cm | 98.4±12.4 (98.0; 90.0–105.0) |
| Fasting plasma glucose—mmol/L | 5.6±1.4 (5.4; 4.8–6.0) |
| Total cholesterol—mmol/L | 6.3±1.2 (6.2; 5.5–7.1) |
| Serum triglycerides—mmol/L | 2.1±0.9 (1.9; 1.5–2.6) |
| Alanine aminotransferase—U/L | 50.2±33.2 (42.6; 29.0–64.0) |
| Aspartate aminotransferase—U/L | 43.6±25.7 (39.0; 27.0–54.3) |
| Gamma-glutamyl transferase—U/L | 51.7±37.6 (42.0; 30.0–61.0) |
| Ultrasound findings—n (%) | |
| Hepatic steatosis | 2747 (96.6) |
| Hepatomegaly | 1774 (62.4) |
| Increase in portal vein and/or splenic vein diameters | 120 (4.2) |
| Liver fibrosis | 71 (2.5) |
| Liver cirrhosis | 0 (0) |
| Comorbid condition | |
| According to the nature of the disease—n (%)* | |
| Overweight/obesity | 2298 (80.8) |
| Elevated cholesterol | 2122 (74.6) |
| Hypertension | 1642 (57.8) |
| Type 2 diabetes mellitus | 477 (16.8) |
| According to the no of diseases—n (%) | |
| 1 | 580 (20.4) |
| 2 | 1112 (39.1) |
| 3 | 869 (30.6) |
| 4 | 282 (9.9) |
Values are expressed as mean±SD (median; IQR), unless otherwise specified.
Percentages are calculated as n/N.
*Patients may have more than one comorbid condition.
A detailed description of the metabolic comorbidities and their treatment patterns among the study participants has already been published elsewhere.25 Briefly, overweight/obesity was the most common comorbid condition in the overall study population (80.8%), followed by hypercholesterolaemia (74.6%) and hypertension (57.8%). The majority of study participants (2263/2843; 79.6%) had at least two metabolic comorbidities associated with NAFLD (table 1). Angiotensin-converting enzyme inhibitors were the most commonly prescribed drugs for the treatment of patients with NAFLD with hypertension (administered in 588/1642 (35.8%) patients at baseline). Among patients with T2DM, biguanides were the most prescribed medications, administered in 241/477 (50.5%) patients of this subgroup at baseline. Similar treatment patterns were observed for patients with NAFLD with overweight/obesity and/or with high serum cholesterol at baseline. Overall, 898/2298 (39.1%) and 756/2122 (35.6%) patients with overweight/obesity and with hypercholesterolaemia did not receive any comorbidity-related medications, respectively. Furthermore, statins were prescribed in 698/2122 (32.9%) patients with high serum cholesterol and in 601/2298 (26.2%) patients with overweight/obesity.
Almost all study participants (2837/2843; 99.8%) were prescribed 1.8 g of PPC per os (600 mg administered three times daily).
Diagnosis of NAFLD
Several signs of chronic liver disease were detected at baseline clinical examination in 1429/2843 (50.3%) patients. Moreover, percussion revealed an enlargement of the liver in 1813 (63.8%) patients. Table 1 summarises the results of the liver function tests.
The abnormal clinical and laboratory findings were confirmed by ultrasound examination which was performed for all study participants. The ultrasound findings of NAFLD among study participants are depicted in table 1. At baseline, the most frequently identified abnormalities on ultrasound (figure 1) were liver hyperechogenicity (detected in 84.0% of patients) and heterogeneous liver structure (in 62.9%). An indistinct and/or an underlined vascular pattern was identified in 32.9%, and distal echo signal attenuation in 28.2% of patients.
Figure 1.
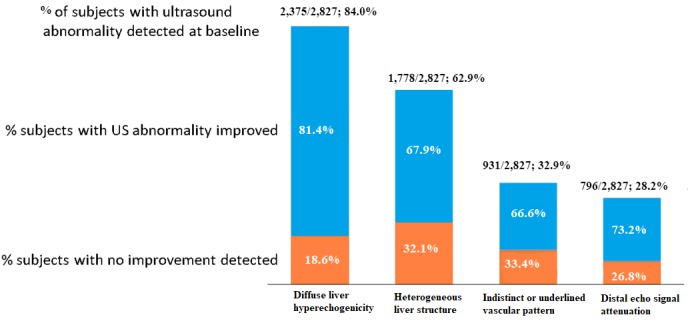
Abnormal ultrasound (US) findings detected at baseline, and their dynamic improvement at 24 weeks of the study (p<0.05 compared with baseline).
In addition to ultrasound examination, elastography and liver biopsy were also performed in 125 (4.4%) and 2 (0.07%) patients, respectively.
Staging of NAFLD showed that simple steatosis was the most frequently seen clinical form of NAFLD, diagnosed in 2128 (74.9%) patients. NASH was detected in 712 out of 2843 patients with NAFLD (25.0%), and only 3 (0.1%) patients suffered from fibrosis. Cirrhosis was not detected in any of the patients.
Changes in ultrasonography findings
Ultrasound examination at baseline detected diffuse liver hyperechogenicity in 86.0% of patients with NAFLD with T2DM (410/477), 84.7% of patients with high serum cholesterol (1798/2122), 84.5% of patients with hypertension (1388/1642) and 83.3% of patients with overweight/obesity (1915/2298). Similarly, a heterogeneous structure of the liver was also identified in 58.3%–64.2% of patients in each comorbidity subgroup. Figure 2 illustrates the ultrasonographic features of NAFLD at baseline, according to the nature of the comorbidity.
Figure 2.
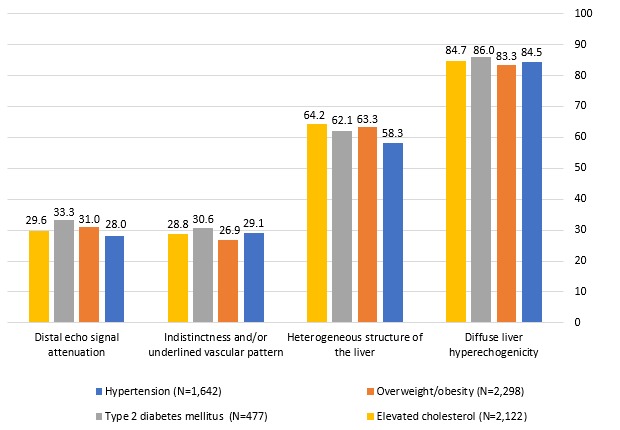
Ultrasonography findings at baseline, according to the comorbidity nature (data are expressed as a percentage of N).
At 24 weeks of the study, a notable improvement in liver echogenicity was detected in 1932/2827 (68.3%) patients (95% CI 66.6% to 70.1%; p<0.05 compared with baseline) and in 67.7%–68.8% of patients in the four comorbidity subgroups. A similar significant positive change was observed with respect to hepatic structure which improved considerably in 1207/2827 (42.7%) patients (95% CI 40.9% to 44.5%; p<0.05 compared with baseline) and in 40.6%–43.7% of patients in the comorbidity subgroups. Table 2 summarises the changes in ultrasound findings throughout the 24-week study period, according to the comorbidity nature. Concerning other ultrasonographic features, liver vascular patterns were improved in 620/2827 (21.9%) patients (95% CI 20.4% to 23.5%); echo signal attenuation in 582/2827 (20.6%) patients (95% CI 19.1% to 22.1%).
Table 2.
Proportion (%) of patients with improved or unchanged ultrasonographic findings after 24 weeks of PPC treatment, according to comorbidity nature
| Features—% | Hypertension (n=1635) | Overweight/obesity (n=2285) |
Type 2 diabetes mellitus (n=475) | High cholesterol (n=2119) |
||||
| Improved | No change | Improved | No change | Improved | No change | Improved | No change | |
| Diffuse liver hyperechogenicity | 67.7 | 32.3 | 68.8 | 31.2 | 68.2 | 31.8 | 67.8 | 32.2 |
| Heterogeneous structure of the liver* | 43.6 | 56.4 | 43.3 | 56.6 | 40.6 | 59.3 | 43.7 | 56.2 |
| Indistinctness and/or underlined vascular pattern | 24.8 | 75.2 | 23.1 | 76.9 | 24.8 | 75.2 | 24.4 | 75.6 |
| Distal echo signal attenuation | 21.7 | 78.3 | 22.5 | 77.5 | 22.7 | 77.3 | 21.5 | 78.5 |
*Worsening of ‘heterogeneous structure of the liver’ occurred in 0.1% of patients in each comorbidity subgroup.
PPC, polyenylphosphatidylcholine.
Considering only patients with ultrasound abnormalities detected at baseline, PPC adjunctive therapy was associated with a pronounced and consistent improvement of each ultrasound feature at both 12 and 24 weeks of the study, with the maximal improvement reported for liver hyperechogenicity in 81.4% of patients at 24 weeks (figure 1). At 12 weeks of the study, statistically significant (p<0.05 compared with baseline) improvements in liver echogenicity (in 69.6% of patients), liver structure (44.8%), vascular patterns (43.9%) and echo signal attenuation (40.6%) were also observed among patients with liver ultrasound abnormalities detected at baseline.
The analysis of ultrasonographic signs by number of comorbidities revealed similar findings. At baseline, in the subgroups of patients with either one, two, three or four concomitant diseases, diffuse liver hyperechogenicity was reported in 80.4%–89.7% of patients, whereas a heterogeneous structure of the liver was observed in 53.3%–65.7% of study participants. The ultrasonographic findings of NAFLD at baseline according to the number of comorbidities are displayed in figure 3. At 24 weeks of the study, there were marked improvements in liver echogenicity and liver structure in 67.2%–69.3% and in 35.6%–45.3% of patients in the subgroups with either one, two, three or four comorbidities, respectively. Overall, 73.5%–85.4% of patients showed no change in liver vascular patterns and in echo signal attenuation. Table 3 shows the proportion of patients with improved or unchanged ultrasonographic findings at 24 weeks of the study, according to the number of comorbidities.
Figure 3.
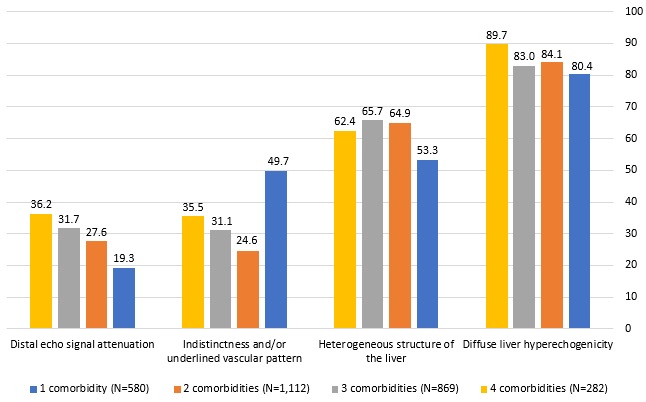
Ultrasonography findings at baseline, according to the number of comorbidities (data are expressed as a percentage of N).
Table 3.
Proportion (%) of patients with improved or unchanged ultrasonographic findings after 24 weeks of PPC treatment, according to the number of comorbidities
| Features—% | One comorbidity (n=570) |
Two comorbidities (n=1108) |
Three comorbidities (n=868) |
Four comorbidities (n=281) |
||||
| Improved | No change | Improved | No change | Improved | No change | Improved | No change | |
| Diffuse liver hyperechogenicity | 69.3 | 30.7 | 68.8 | 31.2 | 67.2 | 32.8 | 69.0 | 31.0 |
| Heterogeneous structure of the liver* | 35.6 | 64.4 | 45.3 | 54.5 | 45.3 | 54.7 | 38.8 | 60.8 |
| Indistinctness and/or underlined vascular pattern | 15.4 | 84.6 | 21.1 | 78.9 | 26.5 | 73.5 | 24.4 | 75.6 |
| Distal echo signal attenuation | 14.6 | 85.4 | 20.0 | 80.0 | 23.8 | 76.2 | 25.3 | 74.7 |
*Worsening of ‘heterogeneous structure of the liver’ occurred in 0.2% of patients with two comorbidities and in 0.4% of patients with four comorbidities.
PPC, polyenylphosphatidylcholine.
Discussion
The findings of this study suggest that PPC administered as adjunctive therapy in patients with NAFLD with metabolic comorbidities has a favourable effect on the ultrasonographic features of NAFLD, with improvement in diffuse liver hyperechogenicity reported in 81.4% of subjects treated with PPC. The analysis of liver function tests by nature and number of comorbidities also showed that PPC adjunctive therapy is associated with notable improvements in aspartate aminotransferase, alanine aminotransferase and gamma-glutamyl transferase. However, details on liver function test results are described in a separate paper.
Liver ultrasonography is becoming a common imaging modality to assess hepatic structural changes that are related to the metabolic abnormalities affecting the liver. In this study, patients had features of the metabolic syndrome including an unfavourable lipid and glycaemic profile, as well as a high range of BMI at baseline. The pathogenesis of NAFLD and metabolic syndrome seems to have common pathophysiological mechanisms, with insulin resistance being a key factor. In adipose tissue, insulin resistance decreases the inhibitory action of insulin on hormone sensitive lipase, thus enhancing triglyceride (TG) lipolysis and free fatty acid (FFA) release. This results in increased circulating levels of FFAs, which are then taken up by the liver.26 Subsequently, increased hepatocyte FFA content drives hepatic TG synthesis which is also favoured by insulin-mediated upregulation of lipogenic enzymes, such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and sterol regulatory element binding protein 1. The large pool of FFAs activates mitochondrial fatty acid β-oxidation by activation of PPAR-alpha and enhanced carnitine palmitoyltranferase.26 When FFA delivery to the liver or hepatic de novo synthesis exceeds TG export or oxidation, NAFLD may occur.26 27 NAFLD is also strongly associated with obesity characterised by an excess of intra-abdominal fat, which may be a key determinant in the pathogenesis of NAFLD, because of its strong association with insulin resistance and possibly as a source of FFAs.26 28 This is in line with the findings of our study in which overweight/obesity was the most common comorbid condition, reported in approximately 81% of the overall study population.
In general, liver echogenicity increases with the severity of NAFLD.12 The high echogenicity of NAFLD is correlated not only with the total lipid content but even more strongly with the histomorphological distribution of fat deposits.28 Thus, NAFLD detection by ultrasonography appears to be very useful for the diagnosis and evaluation of metabolic syndrome.28 Although clinical guidelines still consider liver biopsy as the gold standard for NAFLD diagnosis and evaluation, performing a liver biopsy is invasive and can cause severe complications (eg, pain, hypotension, infection, bleeding and bile leakage).2 29 Thus, liver biopsy is neither routinely feasible nor cost-effective in the diagnosis and monitoring of NAFLD.15 Even though liver ultrasonography is associated with some limitations (eg, lack of quantification of hepatic TG deposit, highly operator dependent), the potential role of liver ultrasonography in clinical settings and in population research is very important.8 17 30 In the current obesity epidemic, the prevalence of NAFLD is likely to increase, making it necessary to use practical tools for measuring the burden of disease and tracking time trends. In the clinical context, the number of patients at risk for NAFLD is also increasing.8 There is thus a pressing need to have readily available, accurate methods to assess the presence of fatty liver, and ultrasound compares favourably to alternative non-invasive techniques.8 In a meta-analysis of 49 studies evaluating the diagnostic accuracy of ultrasonography compared with histology, the overall sensitivity and specificity of ultrasonography for the detection of moderate to severe fatty liver were 84.8% and 93.6%, respectively.8 Liver enzymes, indirect markers of liver injury, have lower sensitivity and specificity than ultrasound.31 Other imaging techniques (ie, CT or MRI) have similar operating characteristics, but are more expensive, and CT involves radiation, and therefore, their widespread usefulness is limited.8 In our study, liver ultrasonography reflected the trend of change in radiographic patterns in every patient, and was beneficial in highlighting the improvement in the pathological findings of NAFLD.
According to the AASLD, the management of NAFLD should consist of treating the liver disease as well as the associated metabolic comorbidities, such as obesity, hyperlipidaemia, insulin resistance and T2DM.2 The significant progress that has been made in previous years in understanding the pathogenesis of NAFLD has led to an increase of medical therapies targeting various aspects of the fat accumulation and injury pathways. However, there is still no ‘gold-standard’ pharmacological therapy for NAFLD.32 Interestingly, current US guidelines on NAFLD treatment indicate that only patients with biopsy-proven NASH should be treated pharmacologically with either pioglitazone or with high-dose vitamin E.2 However, there have been lingering concerns about the long-term safety of both these agents. Bariatric surgery is another option that can be considered in otherwise obese individuals with NAFLD or NASH.2 Though the metabolic disturbances, as well as the long-term complications of NAFLD, are surmountable through lifestyle interventions (ie, dietary modifications, weight loss and exercise), poor implementation and reduced adherence to lifestyle intervention programmes would ultimately diminish their efficacy.32 33
In this connection, it is worth mentioning that even though the pathogenesis of NAFLD has not been fully elucidated (evolving from the ‘two-hit theory’ to a ‘multiple-hit model’), it is fair to say that no steatohepatitis may take place without a pre-existing simple hepatic steatosis.34 Therefore, in real-world clinical practice, it is important to detect patients with simple steatosis, or to put it in other words, patients with increased liver echogenicity at ultrasound examination in order to prevent progression to NASH and improve their long-term prognosis. Among existing therapeutic agents, EPLs have accumulated clear evidence against steatosis. Their positive influence on the grade of liver steatosis was highlighted in several randomised clinical trials based on histology, CT and ultrasound dynamic evaluation.23 Moreover, EPLs have shown, in in vitro and animal investigations, antioxidant, anti-inflammatory and lipid-regulating effects.35 In a preclinical study by Ling et al, 36 conducted in mice with NAFLD that underwent a 70% partial hepatectomy, a decreased hepatic phosphatidylcholine (PC)-to-phosphatidylethanolamine (PE) ratio was shown to be a major contributor to the pathogenesis of NAFLD. As such, hepatic PC/PE could be used as a predictor of survival following hepatectomy.36 This study also provided evidence that EPL supplementation can improve survival in the posthepatectomy setting by increasing hepatic PC/PE, which decreases inflammation and improves energy utilisation.36 These beneficial properties of EPL support the results of our study which highlighted the effectiveness of EPL in routine clinical practice in patients with NAFLD and concomitant metabolic comorbidities, by notable improvements of ultrasound signs.
One of the first clinical trials to show that EPL lead to improvement in histological steatosis was a randomised, placebo-controlled, double-blind study from Poland by Gonciarz et al 37 in 29 patients with NAFLD with T2DM. EPLs, given at 600 mg three times per day for 6 months, were associated with a marked histological improvement in 4/15 (26.7%) patients in the EPL group versus 1/14 (7.1%) in the placebo group. In addition, EPLs were significantly effective in reducing liver size (mean measurement of the right liver lobe: 14.6 cm at baseline vs 14.4 cm at 6 months of therapy in the placebo group, as compared with 14.9 cm at baseline vs 14.0 cm at 6 months in the EPL group; p<0.05), probably as a result of reducing liver fat content.37 Our study results were also in line with other randomised controlled trials. A placebo-controlled, double-blind study38 conducted in 36 Chinese patients with NAFLD with obesity reported about the positive influence of 1.8 g of EPL/day administered for 3 months on total cholesterol (which was decreased by 10%), TG (decreased by 9%) and transaminases (normalised in 87.5% of patients), supported by a significant (p<0.05) improvement of the fatty liver (as seen from CT).38 In another randomised, open-controlled study39 from China of 68 patients with NAFLD with T2DM (of which 34 received 500 mg of metformin +600 mg of EPL both administered thrice daily vs 34 treated with metformin only for 3 months), ultrasonographic appearance and total effective rate were significantly better (p<0.05) in the EPL+ metformin arm compared with the metformin only arm (total effective rate: 78.4% vs 54.1%, respectively).39 Likewise, in an open-label, controlled study by Yin and Kong,40 in which 185 patients were treated with standard diet +oral anti-diabetics+physical exercise, and 125 of these were additionally treated with 1.8 g of EPL per day administered for 3 months, the total effective rate, which was reflected by improvements of ultrasound findings and of lipid and liver panels, was 90.2% in EPL-treated patients versus 51.0% in the control group (p<0.05).40 In a more recent long-term trial from Russia conducted in 215 diabetic patients with NASH who were randomly allocated to either a basic treatment scheme including metformin taken at 1000 mg per day or the basic treatment scheme +1368 mg of PPC per day, ultrasonography performed after 6 months of treatment revealed that hepatic echotexture significantly improved in 101/152 (66.4%) patients in the PPC group (p=0.02).41 Seven years of PPC treatment led to a decrease in ultrasound signs of fatty liver in 93/114 (81.6%) and a more effective control of T2DM in 98/114 (86.0%) patients. Fibrosis progression also appeared significantly slower in those treated with PPC compared with the control group (p=0.03).41
The findings of this study were also similar to those of real-world studies. In an open-label study by Koga et al 42 from Japan in which 51 patients with NAFLD (including 39 with NAFLD due to obesity) received 500 mg of EPL given three times per day for 6 months, the continuous, EPL-induced improvement or normalisation of the ultrasonic picture was observed in 51% of patients (p<0.001 compared with baseline). Bright liver was reduced, and decrement of backward echocardiogram and the obscurity of the intrahepatic venogram were decreased.42 In another open-label, single-centre, 6-month study from India conducted in 28 patients with NAFLD and T2DM, liver ultrasonography revealed that the hepatic echotexture improved after PPC adjunctive therapy (700 mg three times per day) in 12/22 (54.5%) study subjects.43 More recently, in a prospective, multicentre, open-label study17 evaluating EPL (1.8 g per day given for 24 weeks, followed by 0.9 g per day for 48 weeks), in a cohort of 324 patients with NAFLD from the United Arab Emirates, as an adjuvant nutrient to the treatment of primary NAFLD (n=113) or NAFLD with comorbid disease (T2DM in 107 patients and hyperlipidaemia in 104 patients), improvement of ultrasonography findings (complete or partial) occurred in 29.2%, 23.4% and 20.2% of patients with NAFLD only, NAFLD with T2DM, and NAFLD with hyperlipidaemia, respectively.17
A recent review of 25 clinical studies evaluating EPL in fatty liver disease confirmed that EPL accelerate the improvement or normalisation of ultrasonographic features of NAFLD.22 According to Gundermann et al, this is due to the positive effects of phospholipids on membrane composition and functions. The therapeutic activity of EPL in liver diseases is not only attributed to the ability of PPC to be incorporated into damaged sections of cellular membranes, which improves hepatic regeneration and replaces endogenous, less unsaturated hepatic PC molecules, but also to its ability to increase membrane fluidity and functioning.22
Although several randomised controlled trials and single-arm studies from different countries showed the positive effects of EPL on ultrasonographic as well as histological outcomes, most published studies on EPL in NAFLD have their shortcomings, such as low number of enrolled patients and varying dosages of EPL.22 35 Thus, further randomised, double-blind trials with a higher number of patients who have comparable histology or imaging status, and understanding the reasons for not responding to PPC therapy could optimise the therapeutic utility of EPL in liver diseases of various origins.22 35
Our study had certain limitations. First, we could not assess the accuracy of liver ultrasonography, and we could not use the standard ultrasound-based four-grade scale (ie, normal, intermediate, moderate and severe) in the evaluation of the ultrasonographic features of NAFLD. Second, this study had limitations inherent to its observational nature, mainly patient selection bias and the influence of confounding factors. However, in order to minimise patient selection bias, each physician was requested to enrol a consecutive set of patients (16 patients per physician) that met the study specific eligibility criteria. Additionally, the possible influence of confounding factors on the outcomes of this study has been accounted for in the statistical analyses by use of multivariate analyses. Third, the diagnosis of NAFLD in this study was mainly based on ultrasonography, as liver biopsy and transient elastography were rarely carried out. As previously mentioned, it is not feasible to routinely perform liver biopsy due to risk of complications, sampling errors and its high cost; selection bias towards more active disease, overestimating the incidence of advanced fibrosis, may also exist.5 44 Likewise, although it would have been valuable if paired transient elastography was more commonly performed, this technique is not yet widely used in Russia, and since this was an observational study, the investigators had no control over the choice of the diagnostic methods used in the study centres. Nevertheless, these limitations were balanced by several key strengths. The study had a very large sample size of participants (n=2843) enrolled from several study centres (n=174) located throughout Russia. Thus, this study makes it possible to generalise the results to the general population of patients with newly diagnosed NAFLD and metabolic comorbidities in the Russian Federation. Additionally, since we had individual patient data, we were able to evaluate the ultrasonographic features of NAFLD in key patient subgroups (eg, according to the nature and number of associated metabolic comorbidities).
In conclusion, this study showed in routine clinical practice that PPC adjunctive therapy may be useful in improving the ultrasonographic features of NAFLD in patients with associated metabolic comorbidities. From a clinical perspective, the most important finding is the improvement in liver hyperechogenicity in more than 81% of patients after 6 months of PPC adjunctive therapy. This is especially relevant in groups of patients who have difficulties maintaining lifestyle changes. This study also adds valuable knowledge to the growing evidence on the role of EPL in the complex management of patients with NAFLD and metabolic comorbidities.
Footnotes
Contributors: IVM was the study’s national coordinator, who contributed to the design of the study and its supervision. All authors participated in the acquisition, analysis and interpretation of the data. CSP, ENS, LKP, AAS, EIV and KMS contributed to the initial manuscript drafting and finalisation. All authors have read and approved the final version of the manuscript.
Funding: This study was funded by Sanofi-Aventis. Medical writing support was provided by Thomas Rohban and Magalie El Hajj (Partner 4 Health, France) and was funded by Sanofi-Aventis.
Competing interests: KMS is a Sanofi employee. The other authors declare that they have no conflict of interest.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies (Protocol #13 dated 28 August 2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 3. Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol 2013;59:550–6. 10.1016/j.jhep.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 4. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 5. Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:475–85. 10.3748/wjg.v20.i2.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamazaki H, Tsuboya T, Tsuji K, et al. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care 2015;38:1673–9. 10.2337/dc15-0140 [DOI] [PubMed] [Google Scholar]
- 7. GBD DALYs and HALE Collaborators. global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2017;2018:1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–90. 10.1002/hep.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abangah G, Yousefi A, Asadollahi R, et al. Correlation of body mass index and serum parameters with ultrasonographic grade of fatty change in non-alcoholic fatty liver disease. Iran Red Crescent Med J 2014;16:e12669 10.5812/ircmj.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Wang J, Tang Y, et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS One 2017;12:e0174291 10.1371/journal.pone.0174291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivashkin VT, Drapkina OM, Mayev IV, et al. [The prevalence of non-alcoholic fatty liver disease in patients of outpatient practice in the Russian Federation: the results of the study DIREG 2]. Russian J Gastroenterol Hepatol Coloproctol 2015;25:31–8. [Google Scholar]
- 12. Liao Y-Y, Yang K-C, Lee M-J, et al. Multifeature analysis of an ultrasound quantitative diagnostic index for classifying nonalcoholic fatty liver disease. Sci Rep 2016;6:35083 10.1038/srep35083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology 2008;134:1670–81. 10.1053/j.gastro.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 14. Allan R, Thoirs K, Phillips M. Accuracy of ultrasound to identify chronic liver disease. WJG 2010;16:3510–20. 10.3748/wjg.v16.i28.3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuenza LR, Razon TLJ, Dayrit JC. Correlation between severity of ultrasonographic nonalcoholic fatty liver disease and cardiometabolic risk among Filipino wellness patients. J Cardiovasc Thorac Res 2017;9:85–9. 10.15171/jcvtr.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pastori D, Polimeni L, Baratta F, et al. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Digestive and Liver Disease 2015;47:4–11. 10.1016/j.dld.2014.07.170 [DOI] [PubMed] [Google Scholar]
- 17. Dajani AIM, Abu Hammour AM, Zakaria MA, et al. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab Journal of Gastroenterology 2015;16:99–104. 10.1016/j.ajg.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 18. Targher G, Byrne CD. Clinical review: nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab 2013;98:483–95. 10.1210/jc.2012-3093 [DOI] [PubMed] [Google Scholar]
- 19. Malinowski SS, Byrd JS, Bell AM, et al. Pharmacologic therapy for nonalcoholic fatty liver disease in adults. Pharmacotherapy 2013;33:223–42. 10.1002/phar.1190 [DOI] [PubMed] [Google Scholar]
- 20. Francque S, Vonghia L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv Ther 2019;36:1052–74. 10.1007/s12325-019-00898-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J 2006;82:315–22. 10.1136/pgmj.2005.042200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gundermann KJ, Gundermann S, Drozdzik M, et al. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol 2016;9:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivashkin VT, Mayevskaya MV, Pavlov CS, et al. Diagnostics and treatment of non-alcoholic fatty liver disease: clinical guidelines of the Russian Scientific Liver Society and the Russian gastroenterological association].. Russian J Gastroenterol Hepatol Coloproctol 2016;2:24–42. [Google Scholar]
- 24. Lazebnik LB, Radchenko VG, Golovanova EV, et al. Nonalcoholic fatty liver disease: diagnostic, symptoms, treatment. Guidelines were approved by the XV gastroenterological scientific society of Russia in 2015]. Eksp Klin Gastroenterol 2015;7:85–96. [PubMed] [Google Scholar]
- 25. Maev IV, Samsonov AA, Palgova LK, et al. Real-World comorbidities and treatment patterns among patients with non-alcoholic fatty liver disease receiving phosphatidylcholine as adjunctive therapy in Russia. BMJ Open Gastroenterol 2019;6:e000307 10.1136/bmjgast-2019-000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 27. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–89. 10.1002/hep.23280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eguchi Y, Eguchi T, Mizuta T, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol 2006;41:462–9. 10.1007/s00535-006-1790-5 [DOI] [PubMed] [Google Scholar]
- 29. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500. 10.1056/NEJM200102153440706 [DOI] [PubMed] [Google Scholar]
- 30. Cengiz M, Sentürk S, Cetin B, et al. Sonographic assessment of fatty liver: intraobserver and interobserver variability. Int J Clin Exp Med 2014;7:5453–60. [PMC free article] [PubMed] [Google Scholar]
- 31. Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–24. 10.1002/hep.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017;66:180–90. 10.1136/gutjnl-2016-312431 10.1136/gutjnl-2016-312431 [DOI] [PubMed] [Google Scholar]
- 33. Ahmed IA, Mikail MA, Mustafa MR, et al. Lifestyle interventions for non-alcoholic fatty liver disease. Saudi J Biol Sci 2019;26:1519–24. 10.1016/j.sjbs.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang Y-L, Chen H, Wang C-L, et al. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. WJG 2018;24:2974–83. 10.3748/wjg.v24.i27.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gundermann K-J, Kuenker A, Kuntz E, et al. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacological Reports 2011;63:643–59. 10.1016/S1734-1140(11)70576-X [DOI] [PubMed] [Google Scholar]
- 36. Ling J, Chaba T, Zhu L-F, et al. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012;55:1094–102. 10.1002/hep.24782 [DOI] [PubMed] [Google Scholar]
- 37. Gonciarz Z, Besser P, Lelek E, et al. Randomised placebo-controlled double blind trial on “essential” phospholipids in the treatment of fatty liver associated with diabetes. Méd Chir Dig 1988;17:61–5. [Google Scholar]
- 38. JH L, Chen XY, Zhong CF, et al. A randomized controlled study of essential phospholipids (Essentiale capsules) in the treatment of fatty liver]. Infect Dis Info 2000;13:180–1. [Google Scholar]
- 39. Sun C, Zheng X, Tan Z, et al. Clinical observation on polyene phosphatidyl choline and metformin in the treatment of type 2 diabetes and non-alcoholic fatty liver disease]. Clin Focus 2008;23:1272–3. [Google Scholar]
- 40. Yin D, Kong L. Observation for curative effect of Essentiale in treatment of fatty liver caused by diabetes mellitus. Med J Q Ilu 2000;15:277–8. [Google Scholar]
- 41. Sas E, Grinevich V, Efimov O, et al. 1366 beneficial influence of polyunsaturated phosphatidylcholine enhances functional liver condition and liver structure in patients with nonalcoholic steatohepatitis. Results of prolonged randomized blinded prospective clinical study. J Hepatol 2013;58:S549 10.1016/S0168-8278(13)61365-3 [DOI] [Google Scholar]
- 42. Koga S, Irisa T, Miyata Y, et al. Clinical progress of 51 fatty liver cases analyzed by liver function tests and ultrasonic screening and results of EPL administered cases]. Prog Med 1991;11:1891–9. [Google Scholar]
- 43. Poongothai S, Karkuzhali K, Prakash GSiva, et al. Effect of essentiale in diabetic subjects with Non - Alcoholic fatty liver. Int J Diabetes Dev Ctries 2005;25:12–19. 10.4103/0973-3930.26859 [DOI] [Google Scholar]
- 44. Wong GL-H, Chan HL-Y, Yu Z, et al. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol 2013;28:1842–8. 10.1111/jgh.12327 [DOI] [PubMed] [Google Scholar]


