Highlights
-
•
Though Parvimonas micra is not generally regarded as virulent, it may cause fulminant infection in people with weak immune system.
-
•
The diagnosis of P. micra infection is sometimes missed because of its slow growth during culturing. Molecular methods may be helpful for identification.
-
•
Our patient died due to disseminated infection. The organism was later identified as P. micra using 16S ribosomal RNA sequencing.
Keywords: Anaerobic infection, Gas gangrene, Iliopsoas abscess, Parvimonas micra, Spondylodiscitis
Abstract
An 83-year-old man visited an orthopedic hospital for his lower back pain. A compression fracture was noted in his second lumbar vertebra. He had taken pain medication for approximately five weeks, but the pain had worsened and he was unable to walk by himself. He was transferred to our hospital and diagnosed with lumbar spondylodiscitis, an iliopsoas abscess, gas gangrene of his left lower limb, and left massive pleural effusion. He was admitted to the intensive care unit. We drained the abscess and pleural effusion, provided continuous hemodiafiltration under ventilator control, and administered intravenous antibiotics. However, he died from sepsis and multiple organ failure three days following admission. Several days after his death, gram-positive cocci were identified in blood culture, pus from the abscess, and pleural exudate; although the causative organism could not be identified. Two weeks subsequent to his death, 16S ribosomal RNA gene sequencing identified Parvimonas micra in specimens taken from his body.
Introduction
Parvimonas micra is a gram-positive anaerobic coccus that is part of the normal bacterial flora in the oral cavity, gastrointestinal tract, and genital organs [1]. The number of case reports of P. micra infection causing spondylodiscitis [[1], [2], [3]], osteomyelitis [4,5], endocarditis [6], and meningitis [7] are increasing; however, cases may be underestimated because of its slow growth during culturing. P. micra is usually susceptible to penicillin, metronidazole, and clindamycin, but resistance to these antibiotics in the treatment of spondylodiscitis has also reported [1,2,6]. P. micra is an opportunistic pathogen with low virulence. Hence, it typically only causes pathology in individuals with predisposing conditions such as cancer or immunosuppression. The incidence of serious P. micra infection is, therefore, likely to increase in aging societies.
Here, we report a case of a fulminant P. micra infection in an 83-year-old man with prostatic cancer and diabetes mellitus.
Case report
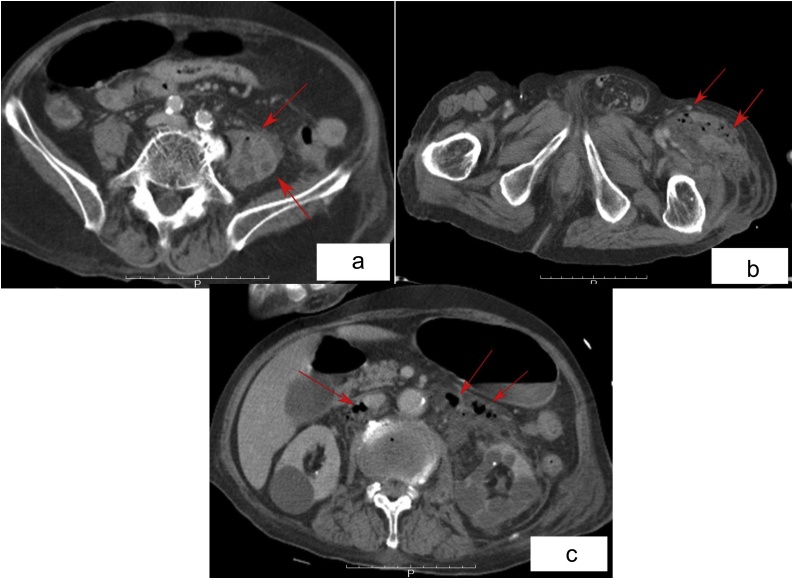
In July 2015, an 83-year-old man with a history of gradually progressive lower back pain was admitted to the emergency room at our hospital. He had been diagnosed with acompression fracture of his second lumbar (L2) vertebra by an orthopedic surgeon five weeks previously, and had been taking painkillers since the diagnosis. The patient had been diagnosed with prostatic cancer 12 years ago, which had been treated with hormones and radiation. Additionally, he was being treated for diabetes mellitus, hypertension, and hyperuricemia. On arrival to our hospital, he had a heart rate of 140 beats/min, a respiratory rate of 39 breaths/min, and a percutaneous arterial oxygen saturation of 89 % on oxygen therapy via nasal cannula at 2 L/min. In addition to his lower back pain, he complained of tenderness and a stabbing pain in his left thigh, whicn was accompanied by an erythematous rash. Blood test results revealed a white blood cell count of 30,600 cells/mm3 with 92.9 % neutrophils, hemoglobin of 10.9 g/dL, creatinine of 2.48 mg/dL, C-reactive protein of 42.72 mg/dL, and procalcitonin of 29.69 ng/mL. His glucose level was 156 mg/dL and his glycated hemoglobin level was 6.1 %, indicating good glycemic control. A computed tomography (CT) scan uncovered a massive pleural effusion in his left thoracic cavity, and fluid retention in his left iliopsoas muscle with air extending to his lower limb (Fig. 1a and b). The scan also revealed inflammation in the retroperitoneal cavity (Fig. 1c) that appeared as a diffuse low-density region in front of his lumbar vertebrae, suggestive of a lumbar vertebral infection. His Sequential Organ Failure Assessment (SOFA) score was 6. We maintained rapid infusion and administered pressor agents to stabilize his hemodynamics. After administering 1 g of intravenous meropenem, a thoracotomy tube was inserted into his left thoracic cavity to drain the pleural effusion. The effusion was exudative with a neutrophil count of 25,000 cells/μL. The abscess in his left thigh was incised and drained, and a catheter was inserted into the retroperitoneal cavity. The pus in the abscess was chocolate- colored and foul smelling. He was admitted to the intensive care unit and given continuous hemodiafiltration under ventilator control for management of sepsis, and 2 g of meropenem and 1200 mg of clindamycin daily. Despite the intensive treatment, he died of multiple organ failure on the third day of his hospitalization. A biopsy specimen from his left femoral muscle showed necrotizing fasciitis and myositis, with neutrophil infiltration and gram-positive bacteria. The anaerobic culture of his pleural effusion didn’t become positive until the next day of his death, and the blood culture needed more few days to grow. The species of bacteria could not be immediately identified, so samples were sent to Tokyo Medical University for gene sequencing. There, the organism was identified as P. micra after 16S ribosomal RNA (rRNA) was found to be a 99.9 % match. P. micra was identified from all specimens taken from the patient, including blood, pleural fluid and pus from the abscess.
Fig. 1.
a A computed tomography image showing abscess in the patient’s left iliopsoas muscle. b A computed tomography image showing abscess extended from the patient’s left iliopsoas muscle to the left lower limb. c A computed tomography image showing the air in the retroperitoneal cavity in front of the patient’s lumbar vertebrae at the level of L2-L3.
Discussion
P. micra was originally classified as Peptostreptococcus micros, reclassified as Micromonas micros in 1999, and reclassified again as Parvimonas micra in 2006 [1,3,5]. The bacterium has been increasingly recognized as the primary pathogenic agent in various invasive human infections including spondylodiscitis, endocarditis, and meningitis [6]. However, the diagnosis of P. micra infection is sometimes missed because of its slow growth during culturing [[1], [2], [3]]. Infection may be transmitted via oral secretions, and involvement of the digestive system or genitourinary tract may result in bacterial invasion and systemic infection. Though it is not generally regarded as virulent, there have been reports of severe spondylodiscitis due to P. micra occurring after dental treatment or spinal surgery [1,2,4]. The majority of spondylodiscitis cases are caused by aerobic bacteria. Anaerobes are isolated in less than 4 % of cases, but the incidence of spondylodiscitis due to anaerobes might be underestimated as they are difficult to culture [[1], [2], [3], [4]]. The patient in this report did not have a history of recent tooth extraction or periodontitis, but did have a history of prostatic cancer and diabetes mellitus. These conditions may have weakened his immune system and disrupted his host defenses, enabling P. micra to enter his bloodstream.
It is unclear why the laboratory in our institute did not identify the organism on culture as belonging to the Peptostreptococcus group, or why the bacteria caused such a fulminant infection. This case demonstrates that anaerobes of low virulence can become invasive as a result of patient factors. Uemura et al. [1] proposed that advanced age could be an important risk factor for P. micra-induced spondylodiscitis. They stressed the importance of obtaining culture results to determine antibiotic susceptibility so that appropriate antimicrobial therapy can be prescribed. In the present case, even if P. micra had been identified before the patient died, it is likely his management would have remained the same. Moreover, his prognosis would probably not have improved, as the infection was already advanced when he was admitted to the hospital. Nevertheless, in instances such as this, we consider it important to identify the causative pathogen, especially in a patient with conditions such as spondylodiscitis and endocarditis, which may require long-term antimicrobial therapy or early de-escalation of therapy.
Molecular-based methods such as 16S rRNA gene sequencing provides species-level identification and reveals antimicrobial susceptibility [6], thus providing a guide to treatment. These molecular methods will become increasingly important as the society ages and complicated and severe infections become more common.
Conclusions
Our patient died as a result of a P. micra infection, which was only diagnosed after his death. The 16S rRNA gene sequencing method was required to make the diagnosis. Culturing anaerobic bacteria is difficult, therefore, anaerobic bacterial infections are often missed. There is a need for greater awareness of the important role anaerobic bacteria play in infectious diseases.
Consent
Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
CRediT authorship contribution statement
Manami Miyazaki: Writing - original draft. Tomoya Asaka: Writing - review & editing, Supervision. Masaaki Takemoto: Writing - review & editing. Takaaki Nakano: Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgements
The authors would like to thank Dr. Okusu for the genetic diagnosis and advice given for this case report.
References
- 1.Uemura H., Hayakawa K., Shimada K., Tojo M., Nagamatsu M., Miyoshi-Akiyama T. Parvimonas micra as a causative organism of spondylodiscitis: a report of two cases and a literature review. Int J Infect Dis. 2014;23:53–55. doi: 10.1016/j.ijid.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Endo S., Nemoto T., Yano H., Kakuta R., Kanamori H., Inomata S. First confirmed case of spondylodiscitis with epidural abscess caused by Parvimonas micra. J Infect Chemother. 2015;21:828–830. doi: 10.1016/j.jiac.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Pilmis B., Israel J., Le Monnier A., Mizrahi A. Spondylodiscitis due to anaerobic bacteria about a case of Parvimonas micra infection. Anaerobe. 2015;34:156–157. doi: 10.1016/j.anaerobe.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Garcia Gonzalez M., Muniz Montes J.R., Garcia Rosado D., Bustabad Reyes S. Multifocal hematogenous vertebral osteomyelitis due to Parvimonas micra and a subsequent pleural effusion in a diabetic patient. Reumatol Clin. 2014;10:191–192. doi: 10.1016/j.reuma.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.George I.A., Pande A., Parsaei S. Delayed infection with Parvimonas micra following spinal instrumentation. Anaerobe. 2015;35:102–104. doi: 10.1016/j.anaerobe.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Gomez C.A., Gerber D.A., Zambrano E., Banaei N., Derensinski S., Blackburn B.G. First case of infectious endocarditis caused by Parvimonas micra. Anaerobe. 2015;36:53–55. doi: 10.1016/j.anaerobe.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Ko J.H., Beak J.Y., Kang C.I., Lee J.Y., Cho S.Y. Bacteremic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe. 2015;34:161–163. doi: 10.1016/j.anaerobe.2015.05.004. [DOI] [PubMed] [Google Scholar]